Covidence website will be inaccessible as we upgrading our platform on Monday 23rd August at 10am AEST, / 2am CEST/1am BST (Sunday, 15th August 8pm EDT/5pm PDT)

The difference between a systematic review and a literature review
- Best Practice
Home | Blog | Best Practice | The difference between a systematic review and a literature review
Covidence takes a look at the difference between the two
Most of us are familiar with the terms systematic review and literature review. Both review types synthesise evidence and provide summary information. So what are the differences? What does systematic mean? And which approach is best 🤔 ?
‘ Systematic ‘ describes the review’s methods. It means that they are transparent, reproducible and defined before the search gets underway. That’s important because it helps to minimise the bias that would result from cherry-picking studies in a non-systematic way.
This brings us to literature reviews. Literature reviews don’t usually apply the same rigour in their methods. That’s because, unlike systematic reviews, they don’t aim to produce an answer to a clinical question. Literature reviews can provide context or background information for a new piece of research. They can also stand alone as a general guide to what is already known about a particular topic.
Interest in systematic reviews has grown in recent years and the frequency of ‘systematic reviews’ in Google books has overtaken ‘literature reviews’ (with all the usual Ngram Viewer warnings – it searches around 6% of all books, no journals).
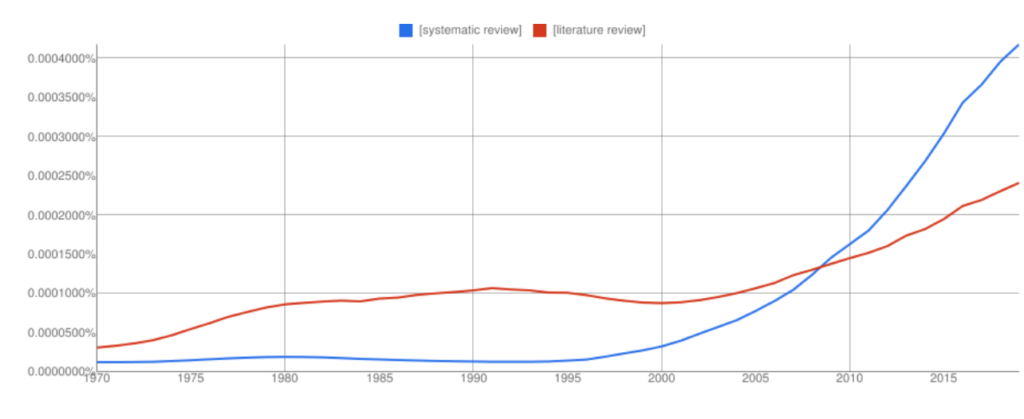
Let’s take a look at the two review types in more detail to highlight some key similarities and differences 👀.
🙋🏾♂️ What is a systematic review?
Systematic reviews ask a specific question about the effectiveness of a treatment and answer it by summarising evidence that meets a set of pre-specified criteria.
The process starts with a research question and a protocol or research plan. A review team searches for studies to answer the question using a highly sensitive search strategy. The retrieved studies are then screened for eligibility using the inclusion and exclusion criteria (this is done by at least two people working independently). Next, the reviewers extract the relevant data and assess the quality of the included studies. Finally, the review team synthesises the extracted study data and presents the results. The process is shown in figure 2 .

The results of a systematic review can be presented in many ways and the choice will depend on factors such as the type of data. Some reviews use meta-analysis to produce a statistical summary of effect estimates. Other reviews use narrative synthesis to present a textual summary.
Covidence accelerates the screening, data extraction, and quality assessment stages of your systematic review. It provides simple workflows and easy collaboration with colleagues around the world.
When is it appropriate to do a systematic review?
If you have a clinical question about the effectiveness of a particular treatment or treatments, you could answer it by conducting a systematic review. Systematic reviews in clinical medicine often follow the PICO framework, which stands for:
👦 Population (or patients)
💊 Intervention
💊 Comparison
Here’s a typical example of a systematic review title that uses the PICO framework: Alarms [intervention] versus drug treatments [comparison] for the prevention of nocturnal enuresis [outcome] in children [population]
Key attributes
- Systematic reviews follow prespecified methods
- The methods are explicit and replicable
- The review team assesses the quality of the evidence and attempts to minimise bias
- Results and conclusions are based on the evidence
🙋🏻♀️ What is a literature review?
Literature reviews provide an overview of what is known about a particular topic. They evaluate the material, rather than simply restating it, but the methods used to do this are not usually prespecified and they are not described in detail in the review. The search might be comprehensive but it does not aim to be exhaustive. Literature reviews are also referred to as narrative reviews.
Literature reviews use a topical approach and often take the form of a discussion. Precision and replicability are not the focus, rather the author seeks to demonstrate their understanding and perhaps also present their work in the context of what has come before. Often, this sort of synthesis does not attempt to control for the author’s own bias. The results or conclusion of a literature review is likely to be presented using words rather than statistical methods.
When is it appropriate to do a literature review?
We’ve all written some form of literature review: they are a central part of academic research ✍🏾. Literature reviews often form the introduction to a piece of writing, to provide the context. They can also be used to identify gaps in the literature and the need to fill them with new research 📚.
- Literature reviews take a thematic approach
- They do not specify inclusion or exclusion criteria
- They do not answer a clinical question
- The conclusions might be influenced by the author’s own views

🙋🏽 Ok, but what is a systematic literature review?
A quick internet search retrieves a cool 200 million hits for ‘systematic literature review’. What strange hybrid is this 🤯🤯 ?
Systematic review methodology has its roots in evidence-based medicine but it quickly gained traction in other areas – the social sciences for example – where researchers recognise the value of being methodical and minimising bias. Systematic review methods are increasingly applied to the more traditional types of review, including literature reviews, hence the proliferation of terms like ‘systematic literature review’ and many more.
Beware of the labels 🚨. The terminology used to describe review types can vary by discipline and changes over time. To really understand how any review was done you will need to examine the methods critically and make your own assessment of the quality and reliability of each synthesis 🤓.
Review methods are evolving constantly as researchers find new ways to meet the challenge of synthesising the evidence. Systematic review methods have influenced many other review types, including the traditional literature review.
Covidence is a web-based tool that saves you time at the screening, selection, data extraction and quality assessment stages of your systematic review. It supports easy collaboration across teams and provides a clear overview of task status.
Get a glimpse inside Covidence and how it works

Laura Mellor. Portsmouth, UK
Perhaps you'd also like....

Webinars from start to finish: Creating a Review
Creating a systematic review can be a complex process, but with the right resources, you can navigate it smoothly from start to finish. In the

Data Extraction Tip 5: Communicate Regularly
The Covidence Global Scholarship recipients are putting evidence-based research into practice. We caught up with some of the winners to discover the impact of their work and find out more about their experiences.

Data Extraction Tip 4: Extract the Right Amount of Data
Better systematic review management, head office, working for an institution or organisation.
Find out why over 350 of the world’s leading institutions are seeing a surge in publications since using Covidence!
Request a consultation with one of our team members and start empowering your researchers:
By using our site you consent to our use of cookies to measure and improve our site’s performance. Please see our Privacy Policy for more information.
5 differences between a systematic review and other types of literature review
September 26, 2017.

There are many types of reviews of the medical and public health evidence, each with its own benefits and challenges. In this blog post, we detail five key differences between a systematic review and other types of reviews, including narrative and comprehensive reviews.
First, we must define some terms. “Literature review” is a general term that describes a summary of the evidence on a certain topic. Literature reviews can be very simple or highly complex, and they can use a variety of methods for finding, assessing, and presenting evidence. A “systematic review” is a specific type of review that uses rigorous and transparent methods in an effort to summarize all of the available evidence with little to no bias. A good systematic review adheres to the international standards set forth in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 27-item checklist. 1 Reviews that are less rigorous are often called “narrative,” “comprehensive,” or simply “literature reviews.”
So, what are the 5 key differences between a systematic review and other types of review?
1. The goal of the review The goal of a literature review can be broad and descriptive (example: “ Describe the available treatments for sleep apnea ”) or it can be to answer a specific question (example: “ What is the efficacy of CPAP for people with sleep apnea? ”). The goal of a systematic review is to answer a specific and focused question (example: “ Which treatment for sleep apnea reduces the apnea-hypopnea index more: CPAP or mandibular advancement device? ”). People seeking to make evidence-based decisions look to systematic reviews due to their completeness and reduced risk of bias.
2. Searching for evidence Where and how one searches for evidence is an important difference. While literature reviews require only one database or source, systematic reviews require more comprehensive efforts to locate evidence. Multiple databases are searched, each with a specifically tailored search strategy (usually designed and implemented by a specialist librarian). In addition, systematic reviews often include attempts to find data beyond typical databases. Systematic reviewers might search conference abstracts or the web sites of professional associations or pharmaceutical companies, and they may contact study authors to obtain additional or unpublished data. All of these extra steps reflect an attempt to minimize bias in the summary of the evidence. 3. Assessing search results In a systematic review, the parameters for inclusion are established at the start of the project and applied consistently to search results. Usually, such parameters take the form of PICOs (population, intervention, comparison, outcomes). Reviewers hold search results against strict criteria based on the PICOs to determine appropriateness for inclusion. Another key component of a systematic review is dual independent review of search results; each search result is reviewed by at least two people independently. In many other literature reviews, there is only a single reviewer. This can result in bias (even if it is unintentional) and missed studies.
4. Summary of findings In a systematic review, an effort is usually made to assess the quality of the evidence, often using risk of bias assessment, at the study level and often across studies. Other literature reviews rarely assess and report any formal quality assessment by individual study. Risk of bias assessment is important to a thorough summary of the evidence, since conclusions based on biased results can be incorrect (and dangerous, at worst). Results from a systematic review can sometimes be pooled quantitatively (e.g., in a meta-analysis) to provide numeric estimates of treatment effects, for example.
5. Utility of results Due to the rigor and transparency applied to a systematic review, it is not surprising that the results are usually of higher quality and at lower risk of bias than results from other types of literature review. Literature reviews can be useful to inform background sections of papers and reports and to give the reader an overview of a topic. Systematic reviews are used by professional associations and government agencies to issue guidelines and recommendations; such important activities are rarely based on a non-systematic review. Clinicians may also rely on high quality systematic reviews to make evidence-based decisions about patient care.
Each type of review has a place in the scientific literature. For narrow, specific research questions, a systematic review can provide a thorough summary and assessment of all of the available evidence. For broader research questions, other types of literature review can summarize the best available evidence using targeted search strategies. Ultimately, the choice of methodology depends on the research question and the goal of the review.
[1] Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyse s: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097.

IMAGES
VIDEO