- Lupe_Grau Cancel

Laboratory Products for “Research Use Only” (RUO) – Often a Dangerous Claim
Manufactures use the “Research Use Only” (RUO) label to declare that their products should not be used in diagnostic procedures. This enables them to avoid the time-consuming and costly documentation required for conformity-assessed in vitro diagnostic medical devices (CE-IVDs). Nevertheless, some medical laboratories, for example, still use RUO products in diagnostic procedures, sometimes even with the knowledge of the manufacturers. This can have consequences – not just for manufacturers and operators, but for patients as well.
In this article, you will learn:
- What the “Research Use Only” label (RUO) means
- What the requirements for RUO products are
- How to avoid legal problems
- What alternatives there are to RUO products
1. “Research Use Only” – what does it mean?
Labeling products for “research use only” has far-reaching consequences. It means the products are barely subject to any regulatory controls under the IVDR. As a result, for a lot of manufacturers and operators, they are desirable alternatives to more costly and time-intensive conformity-assessed in-vitro diagnostic medical devices (CE-IVDs) that must comply with the applicable legal requirements.
a) Institutions affected
The following institutions, in particular, use RUO products:
- Medical laboratories can use RUO products, but this makes them the manufacturer with all the consequences this entails. You can find more information on “lab developed tests” in our article “ The E U Regulates Medical Laboratories. Are Laboratory Developed Tests Still Allowed? ”
- If medical laboratories use RUO products for purposes other than research then, in the worst case, this makes them liable for damages as well as criminally liable.
- Therefore, medical laboratories should inform themselves about the parameters for RUO products and possible alternatives .
- Manufacturers Manufacturers use RUO products as components for their IVDs. They should, therefore, make sure that they know all the requirements in detail before labeling a product as “RUO”.
b) Definition
There is no uniform definition of “research use only” products. In general, they can be understood to be what the name implies, i.e., products to be used for analysis that are intended to be used for scientific research purposes only.
They primarily differ from medical devices in that they cannot be used for medical purposes.
However, the understanding of “research use only” is different in Europe and the USA.
Definition in Europe
In Europe, the MEDDEV 2.14/2 guidance document (IVD Guidance: Research Use Only products – A guide for manufacturers and notified bodies) provides clues as to the definition of RUOs. This guidance was written within the framework of the now obsolete Directive 98/79/EC on in vitro diagnostic medical devices (IVDD) and, in the absence of an up-to-date replacement, it can still be considered the state of the art.
MEDDEV 2.14/2 states:
“for a product to be categorized as an RUO product it must have no intended medical purpose or objective."
Source: MEDDEV 2.14/2 rev.1
This means that an RUO product must not have even a rudimentary medical purpose.
However, in the case of tests developed in-house by a laboratory (LDTs), this restriction does not apply provided that the products are not sold to other companies. The guidance gives the following specific examples of LDTs that may be designated “research use only” under this requirement:
- PCR enzymes
- Gel component agars
The IVDR also addresses RUO products.
“device for performance study’ means a device intended by the manufacturer to be used in a performance study.
A device intended to be used for research purposes, without any medical objective, shall not be deemed to be a device for performance study; ”
Source: IVDR Art. 2(45)
Thus, the IVDR, like MEDDEV 2.14/1 (IVD Medical Device Borderline and Classification issues), draws a distinction between RUO products and “devices for performance studies.”
Again, the key aspect of the definition is the RUO product’s lack of medical purpose.
To be classed an RUO product, it is vital that the product does not serve a medical purpose. Even a suspected medical purpose is enough for a device to be no longer considered an RUO product.
(See MEDDEV 2.14/1 section 1.1 4.)
Definition in the USA
In 2013, the FDA published a guidance document on RUOs entitled “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only.”
This guidance defines RUO products as follows:
“ An RUO product is an IVD product that is in the laboratory research phase of development and is being shipped or delivered for an investigation that is not subject to part 812” [NB: Part 812 concerns the provision of devices for performance evaluation purposes as a preliminary step to IVDs]
Source: FDA guidance “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only”
Some examples of products that the FDA believes fall into this research phase of development are:
- Tests that are in development to identify test kit methodology, necessary components, and analytes to be measured
- Instrumentation, software, or other electrical/mechanical components under development to determine correct settings, subcomponents, subassemblies, basic operational characteristics, and possible use methods
- Reagents under development to determine production methods, purification levels, packaging needs, shelf life, storage conditions, etc.
Therefore, according to the FDA, a clearly visible RUO label must be affixed specifically to products that are in a research phase.
c) What are the consequences of using the “Research Use Only” label?
Normally, IVDs are subject to regulatory requirements (for example, according to the IVDR or FDA) based on their risk class.
However, RUO products do not fall within the definition of “in vitro diagnostic medical devices” given by the IVDR or the relevant FDA regulations . This means that these regulations do not apply to RUO products.
Definition: In vitro diagnostic medical devices (IVDs) in the EU
“‘In vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on one or more of the following:
(a) concerning a physiological or pathological process or state;
(b) concerning congenital physical or mental impairments;
(c) concerning the predisposition to a medical condition or a disease;
(d) to determine the safety and compatibility with potential recipients;
(e) to predict treatment response or reactions;
(f) to define or monitoring therapeutic measures.
Specimen receptacles shall also be deemed to be in vitro diagnostic medical devices;”
Source: Article 2 IVDR
Definition: In vitro diagnostic medical devices (IVDs) in the USA
“In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae. Such products are intended for use in the collection, preparation, and examination of specimens taken from the human body.”
Source: 21 CFR 809.3
Therefore, the requirements of the IVDR do not apply to RUO products. In the USA, they are exempt from cGMP and the FDA's quality regulations.
Depending on the product, they may still have to comply with requirements that are not specifically intended for IVDs (such as the REACH regulation for chemicals or the Machinery Directive ).
Since RUO products are thus subject to considerably fewer controls than IVDs, it is necessary to severely restrict their use.
Therefore, in particular they may not be used to:
- Make diagnoses
- Conduct performance studies
2. Use and misuse of “Research Use Only” labels
A) what should ruo products be used for.
As the name “research use only” indicates, products with RUO labeling are intended for research purposes only. RUO products are particularly attractive for the research sector due to the simplified process and lower hurdles for placing them on the market.
MEDDEV. 2.14/2 rev.1 provides a precise list of areas where RUO products may potentially be used:
- Basic research
- Pharmaceutical research
- Better identification and quantification of individual chemical substances or ligands in biological specimens
- In house manufacturing of so called “home brew kits” for research purposes
And of areas where the use of RUOs is expressly not permitted:
- Use of raw materials which are labeled “for “research use only” but which are incorporated into a finished product
- So called “research use” products being tested against a comparator IVD product that bears the CE mark
- Products for market studies/ feasibility studies
b) What RUO products are often used for
However, the low hurdles are also the reason why RUO products are often used for purposes they are not intended for. This poses significant dangers for manufacturers, operators and patients.
Sale of RUO products to medical laboratories
RUO products are sold by manufacturers to medical laboratories. Although doctors sometimes also conduct research, this is not really the main purpose of a medical laboratory. Therefore, when discussing sales with doctors, it should always be assumed that there is a medical reason behind the use of the product.
This means that anyone who knowingly sells RUO products to medical laboratories is potentially under suspicion of using the pretext “for research use only” to ignore an intended medical purpose and thus avoid responsibility for a medical device.
There are certainly laboratory products that clearly have no specific medical purpose, e.g.:
- Nutrient media
- Reaction vessels
- Washing solutions
These products are best labeled as “general laboratory supplies” rather than “RUO”.
Avoid reference to any specific diagnostic procedures in your advertising materials for products that clearly do not have a medical purpose. You should always stay on the technical or purely analytical level.
The issue with analyte specific reagents
Whether an RUO product contains analyte specific reagents, e.g., primary antibodies, FISH probes, PCR primers and probes, and sequencing panels, can be critical. In some cases, a medical purpose can be inferred just from the description of the product's performance.
This would be the case if a manufacturer of a RUO-labeled kit for the detection of viral genes specifies a number of copies per ml of blood that the kit can detect.
ASR in the USA
The FDA abbreviates the term “analyte specific reagents” to “ASR” and defines it as follows:
“Analyte specific reagents (ASR's) are antibodies, both polyclonal and monoclonal, specific receptor proteins, ligands, nucleic acid sequences, and similar reagents which, through specific binding or chemical reaction with substances in a specimen, are intended for use in a diagnostic application for identification and quantification of an individual chemical substance or ligand in biological specimens.”
Source: 21CFR864.4020 a)
In other words, US law says that, by definition, ASRs have a diagnostic purpose.
Exception: The sale of ASRs to IVD manufacturers as components for manufacturing kits or to non-clinical laboratories for research and development without compliance with regulatory requirements is permitted.
ASR in the EU EU law does not contain this exception. Nor does the term “analyte specific reagent” does appear in any of the applicable EU regulations. Therefore, such products may have a general laboratory purpose in the EU, depending on the justification. This means they do not fall under the IVDR if the manufacturer defines the intended purpose accordingly. However, if the manufacturer assigns a medical or diagnostic purpose to these products, the regulatory hurdles will very high once the IVDR comes into full effect (currently scheduled for May 26, 2022).
This means that the crucial factor is whether manufacturers have clearly defined the intended purpose and whether communication with customers (e.g., in advertising materials) is in line with this purpose.
Further information
You can find out more about the intended purpose of medical devices here: Intended purpose and intended use
Use of RUO products in medical laboratories
It is not just manufacturers for whom the sale of RUOs to medical laboratories represents a problem. The laboratories themselves may also not be acting in line with their status as operators and may, as a result, be liable under certain circumstances.
- Medical laboratories are free to develop in-house tests themselves. In such cases, RUO products are often used in diagnostic procedures. Even under the IVDD, MEDDEV 2.14/2 was critical of this. However, with the new In Vitro Diagnostic Medical Device Regulation (IVDR) , the EU is explicitly placing more restrictions on the routine use of such lab developed tests . Read more in our article The EU Is Regulating Medical Laboratories. Are Laboratory Developed Tests Still Allowed? .
- Due to the low regulatory hurdles, purchasing RUO products is very affordable. As a result, medical laboratories prefer them over expensive CE-IVD devices if they can achieve the same level of performance. Nevertheless, the use of RUO products for purposes other than research, even in cases where they provide similar results, is not permitted.

3. Consequences of incorrect classification
Lack of controls can have a negative effect on quality. As a result, the relevant bodies (e.g., authorities during inspections) take a closer look at whether a product is actually intended for “research use only”.
Manufacturers should also be aware that simply sticking an RUO label on a product does not on its own mean that the product no longer has to comply with requirements for IVDs that would otherwise apply.
In its guidance document on RUO , the FDA writes that only the actual intended use qualifies a product as RUO – or doesn’t. The FDA also uses marketing materials or other general factors as evidence of the intended purpose.
"Because these products are exempt from most regulatory controls, it is important that they are not distributed for clinical diagnostic uses. Mere placement of an RUO or IUO label on an IVD product does not render the device exempt from otherwise applicable clearance, approval, or other requirements. FDA may determine that the device is intended for use in clinical diagnosis based on other evidence, including how the device is marketed. ”
Manufacturers and operators who misuse the RUO label could face severe penalties, as such behavior can cause serious harm to patients or even the general public.
a) Consequences for manufacturers and operators
Improperly selling IVDs with an RUO label or using RUO products for purposes other than research is not a trivial offense.
Manufacturers who demonstrably hide or aim to hide a diagnostic purpose behind the RUO label should expect legal consequences in Germany. The same applies for operators who misuse RUO products. There is the possibility of a fine or even prison sentences. In addition, there is potential liability for harm suffered by patients.
b) Consequences in the USA
There are also severe penalties in the USA. If an RUO label is deemed to have been incorrectly used for a product, the product would be considered misbranded under sections 502(a) and 502(o) of 21 US Code, 352(a), 352(o) [A1] and would be considered adulterated under section 501(f) of 21 US Code 351(f).
c) Consequences for patients
However, the consequences can be even worse for patients. After all, the regulatory requirements for IVDs aren’t just plucked out of thin air to annoy manufacturers and operators. The regulations are intended to protect patients against incorrect results and subsequent wrong decisions. False-negative results can lull patients into a false sense of security and an existing disease may worsen undetected. One example would be the metastasis of an undetected cancer due to a test not performing as intended.
Some incorrect diagnoses could even be so severe that they can cause the death of a lot of people: an undetected viral infection can cost many lives in the early stages of an epidemic or pandemic, as the coronavirus pandemic sadly demonstrated.
4. Alternatives to “research use only” products
To avoid legal problems and risks for third parties, manufacturers and users should use alternatives to RUO products in borderline cases.
These alternatives don’t always have to be CE-IVDs. Depending on the specific situation, the following alternatives can be considered based on the intended purpose:
a) Products for general laboratory use
According to the MEDDEV 2.14/1 (IVD Medical Device Borderline and Classification Issues) guidance, it is a product's characteristics that determine whether it can be classified as a product for general laboratory use or not.
- If, based on its characteristics, a product is not specifically intended to be used for in vitro diagnostic examinations, it is not an IVD.
- Manufacturers cannot label products for general laboratory use as IVDs.
RUO products used for a better identification and quantification of individual chemical substances or ligands in biological specimens
Source: MEDDEV 2.14/2
Such products must have a general use. However, use as an IVD does not have to be ruled out, provided the product is not made specifically for a particular test. According to MEDDEV 2.14/2, even the aforementioned analyte specific reagents (ASRs) without a medical purpose fall into this category.
There are several advantages to using products for general laboratory use instead of RUO products:
- The product does not fall under the IVD Directive or the IVDR, which saves you a lot of time and money.
- Laboratories that use these products for in-house procedures are not in danger of being accused of using RUO products in routine diagnostic procedures.
However, the disadvantage is that the medical laboratory is responsible for ensuring that the examination conforms with the IVDR. This can make the product less interesting because the regulatory requirements entail a lot of work.
b) Lab developed tests with class A CE-IVDs Manufacturers may sell general laboratory reagents, which can be authorized as IVDs under the IVDR, to medical laboratories.
In combination with the ASRs developed in-house, laboratories can validate and use these products as lab developed tests (LDTs).
Read our article on lab developed tests to find out what laboratories should be aware of.
c) “For performance evaluation only” as a preliminary stage for certified IVDs
The IVDR defines " device for performance studies ” as follows:
“‘Device for performance study’ means a device intended by the manufacturer to be used in a performance study.”
Source: IVDR 2017/746/EU
These devices must already be safe, as far as possible, and meet the relevant general safety and performance requirements.
5. Ways to protect yourself
Manufacturers, operators and patients can take the following steps to avoid legal and other negative consequences when using RUO products:
a) Manufacturers
In the case of manufacturers, it is particularly important that they narrowly define the intended purpose of their product.
Analyte specific reagents should only be labeled as RUO products for specific non-medical purposes.
Example: SARS-CoV-2 and its mutations: a test kit that uses specific primers and probes to distinguish the variants B.1.1.7 (alpha variant) and B.1.351 (beta variant) from the initial variant following a positive result may be an RUO product if it is only intended to be used to determine the prevalence of the variant in the population. A specific intended purpose in this case would be: “Intended solely for epidemiological research for the purpose of surveying the prevalence of SARS-CoV-2 variants in the general population.” If a medical laboratory subsequently, based on new findings, used this test to provide the best possible treatment for infection by a specific variant, this would be an off-label use. The laboratory would then be responsible for the test's conformity.
Provided the manufacturer did not advertise the product with this clinical benefit, it would be adequately protected.
b) Operators
Operators should record exactly what they use IVDs and RUO products for.
Medical laboratories are operators of medical devices and IVDs and, therefore, are responsible for only using medical devices according to their intended purpose and in accordance with the generally accepted rules of the technology. This is stipulated in Section 4 of the German Medizinprodukte-Betreiberverordnung (MPBetreibV (German)). To be on the safe side, laboratories should keep a record of which medical devices and IVDs are in operation and routine use. This record should include a reference to the applicable test procedure and the intended purpose of the IVD.
This record can also be used to identify investigational procedures for which there are no adequate CE-IVDs available on the market. The lack of alternatives would justify the use of RUOs (as lab developed tests) in validated processes it has developed in-house, provided that the laboratory checks and can demonstrate that the general safety and performance requirements and the additional requirements of Article 5(5) of the IVDR are met.
Read more about the requirements for LDTs in our article on the topic .
c) Patients
Patients lack the knowledge to recognize what is and isn’t an RUO on their own. They are often given little to no information about the test they are undergoing. So, patients should follow this basic rule: ask your doctor or pharmacist!
- Patients can ask for the complete test report from the laboratory so that they can get a second opinion in case of doubt. The report should also indicate which specific test was performed.
- Patients should inform themselves about how “well” or “poorly” a test works, as well as the benefit-risk ratio.
- In the future, patients and doctors will also be able to get information about medical devices from EUDAMED and use this information to decide whether or not the test was performed with certified and thus legally compliant IVDs.
6. Conclusion
In the opinion of the EU Commission and the FDA, products “for research use only" have no place in diagnostics. To be used for diagnostic purposes, products have to go through the necessary controls. But these controls do not apply to RUO products.
Anyone who ignores this prohibition and uses or sells RUO products for purposes other than pure research is playing with fire. Manufacturers and operators run the risk of legal trouble and could even endanger patients’ health. Therefore, RUO products should only be used for research purposes. For other uses, manufacturers and operators should use the alternatives mentioned.
Our tip is: if you, as a manufacturer or medical laboratory, find that an RUO product is particularly well-suited for in vitro diagnostics, consider whether further development and conformity assessment to make it an IVD is worthwhile. We will be happy to help you work out which of the three alternatives to RUOs mentioned above is the best alternative to your product as part of our IVD authorization strategy consultation. If necessary, we can also help you ensure your product development conforms with the regulations.

Dr. Diana Gabriel

A quick overview: Our
Starter-Kit
Always up to date: Our
Back To Top
Privacy settings
We use cookies on our website. Some of them are essential, while others help us improve this website and your experience.
Individual Cookie Settings
Only accept required cookies.
Privacy Notes Imprint
Here is an overview of all cookies use
Required Cookies
These cookies are needed to let the basic page functionallity work correctly.
Show Cookie Informationen
Hide Cookie Information
Provide load balancing functionality.
Provides functions across pages.
Hubspot Forms
Used for the google recaptcha verification for online forms.
Cookies for Statistics
Statistic cookies anonymize your data and use it. These information will help us to learn, how the users are using our website.
Google Analytics
Tracking and analys of traffic on our websites.
Cookies for Marketing
Marketing cookies from thrid parties will be used to show personal advertisment. They use them to track users outside of their own web page.
Keeping track of a visitor's identity. It is passed to HubSpot on form submission and used when deduplicating contacts. It contains an opaque GUID to represent the current visitor. It also introduces cookies from linked in for marketing reasons.
LinkedIn conversion tracking.
Cookies for external Content
Content for Videoplatforms und Social Media Platforms will be disabled automaticly. To see content from external sources, you need to enable it in the cookie settings.
Google Maps
Used to display google maps on our Websites. Google uses cookies to identify and track users.
Search Thermo Fisher Scientific
- Order Status
- Quick Order
- Check Order Status
- Aspire Member Program
- Connect: Lab, Data, Apps
- Custom Products & Projects
- Instrument Management
In Vitro Diagnostic Regulation (IVDR)—Frequently Asked Questions
What is the ivdr.
The In Vitro Medical Devices Regulation (EU) 2017/746 (IVDR) is a new regulation that will create a robust, transparent, and sustainable regulatory framework that “improves clinical safety and creates fair market access for manufacturers and healthcare professionals”(1).
The IVDR “brings EU legislation into line with technical advances, changes in medical science, and progress in law-making”(1). IVDR has binding legal enforcement throughout all EU member states, and it sets higher standards for quality and safety of IVD devices.
Certification and use of IVDR-compliant products
What is the thermo fisher scientific roadmap for ivdr.
The majority of our current CE-marked IVDs will transition to the IVDR.
How long does it take to transition an instrument or kit to the IVDR? What is the Thermo Fisher transition timeline?
The timeline is progressive (2) and depends on a product’s classification.

The new rules contain a number of changes, including the introduction of a risk-based classification system with four risk classes of in vitro diagnostic medical devices:
- Class A devices will be self-certified by their manufacturers unless they are sold as sterile
- Class B (moderate individual risk and/or low public health risk)
- Class C (high individual risk and/or moderate public health risk)
- Class D (high individual risk and high public health risk)
Examples of risk-based classification by product type
Instruments.
Instruments such as the QuantStudio 5 Dx and QuantStudio 7 Pro Dx real-time PCR systems are classified as Class A non-sterile
Reagents such as the MagMA Viral/Pathogen II Nucleic Acid Isolation Kit and the SpeciMAX Dx Stabilized Saliva Collection Kit are classified as Class A sterile
COVID-19 assays: TaqPath COVID-19 assays are in Class D ( Clinical Testing Solutions: Your Questions, Answered )
Non–COVID-19 assays : the majority are Class C and Class B
Devices in Classes B, C, and D will require conformity assessment by a Notified Body.
OEMpowered blog post: IVDD vs. IVDR: Classifications defined and compared
For questions related to specific products, please contact our team today .
Frequently asked questions for developers of laboratory developed tests (LDTs)
Changing needs for institutions developing tests under ivdr.
Customers producing LDTs have additional considerations. By including in-house assays in the scope of the IVDR, the aim of the EU Health Authorities is not only to restrict the use of in-house assays, but to equal the compliance between commercial tests and in-house assays.
In many cases, laboratories may need to transition from in-house assay use to CE-IVD-marked device use, due to Article 5 (3).
In circumstances where patient needs cannot be met by available commercial tests, in-house assays/LDTs may still benefit from an exemption to the full IVDR, by applying Article 5 only.
Which additional constraints are expected for in-house assays, as addressed by IVDR Article 5.5?
Some additional constraints and considerations include:
- The devices must not be transferred to another legal entity
- Appropriate quality management systems such as standard EN ISO 15189 or applicable national provisions
- Product may not be made on an industrial scale
- Justification by documenting why an equivalent device available on the market is not used
In-house assays may be more difficult to validate and to justify use, but will remain important for some healthcare institutions and rare conditions.
Is compliance to the IVDR Annex I GSPRs (General Safety & Performance Requirements) required?
Yes. The institution making an in-house assay (also defined as laboratory-developed test or LDT) must demonstrate and document its safety and performance.
This compliance requirement is generally welcomed as it may improve the quality of in-house products in line with CE-IVD products.
Who will verify compliance?
Competent authorities will verify compliance. Enforcement strategies between EU countries may vary.
When do these changes go into effect?
In 2022, the EU will begin to enforce the transition from the current In Vitro Diagnostic Medical Devices Directive 98/79/EC (IVDD) to the In Vitro Diagnostic Medical Devices Regulation (EU) 2017/746 (IVDR) for clinical diagnostic applications.
On May 26, 2022, the IVDR becomes effective, meaning after that date any new IVD devices placed on the market must be CE marked according to IVDR.
A recent amendment by the commission to the implementation of the IVDR allows some existing products (excluding Class A non-sterile devices) to continue to be placed on the market in compliance with the IVDD. The length of the extension is dependent on risk class.
What is the timeline for implementation of the IVDR Article 5.5 specific to in-house devices/laboratory-developed tests?
The new progressive transitional timeline for implementation of the IVDR Article 5.5 is:
26 May 2022:
- Compliance with the GSPRs in Annex I
- No transfer of devices between legal entities
- No manufacture on an industrial scale
- Competent authority oversight
26 May 2024:
- Appropriate QMS system: ISO 15189 " Medical Laboratories – Requirements for Quality and Compliance " and manufacturing process
- Review experience gained from clinical use
26 May 2028:
- Justification for use over commercially available tests
What is the process for producing the justification of an in-house device?
The considerations are very different depending on if there is or is not an equivalent IVDR-compliant device on the market.
Our IVDR transition team is here to help you with scenario planning specific to your needs. Contact our team today .
Laboratories complying with ISO 15189:2012 may find that they largely comply with Annex I of the IVDR.
Laboratories with no existing accreditation may find that compliance requires greater effort.
Note: Manufacturing process is not covered by ISO 15189 but required under Annex I.
Definitions and applications under Article 5
Manufacturing of in-house assays.
Putting together an assay from raw materials and components; making a new device from used CE IVDs; modifying a CE IVD to deviate from IFU; making a device from RUO reagents or instruments.
General Safety & Performance Requirements
Health(care) institution
Means an organization of which the primary purpose is the care or treatment of patients or the promotion of public health. It’s established in the EU. Includes private laboratories that support the healthcare system, even if they do not treat or care for patients directly.
In-house assays/in-house devices, also referred to as “homebrew assays” or “LDTs” (laboratory-developed tests)
In vitro diagnostics; regulated product with a restricted in-house assay; homebrew assays intended use and protocol for use labelled with “for in vitro diagnostic use”; and IVD accessories
Instructions for use; part of CE IVD products
Research use only; non-regulated product; no set intended use; protocol for use open for adaptation/change; labelled with “for research use only not for diagnostic use”
- https://ec.europa.eu/health/medical-devices-new-regulations/getting-ready-new-regulations_en
- https://ec.europa.eu/commission/presscorner/detail/en/qanda_21_5210
- https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0746

Purpose (+) Scope
This post is about the fundamental distinctions between an IVD product and a RUO product. The emphasis is placed on the regulatory requirements of the European Union (EU) and the United States (US).
in vitro Diagnostic (IVD) Medical Device
An “in vitro diagnostic (IVD) medical device” is defined generically as a device that, whether used alone or in combination, is intended to the manufacturer for the in vitro examination of specimens derived from the human body solely and principally to provide information for diagnostic, monitoring or compatibility purposes. IVDs can be reagents, calibrators, control materials, specimen receptacles, software, instruments, apparatus or other articles.
Usually, IVDs are classified based on their risk levels. Each risk class is linked to a given conformity assessment type who is itself linked to specific regulatory requirements.
Depending of the jurisdiction, the IVD classes may be covered by separate national regulations.
IVDs can be sterile, not sterile, intended to be used by lay people (self-testing devices) or not by health professionals within or outside a laboratory environment (near-patient testing devices).
Regulations, norms and standards (such as ISO 13485) apply for IVDs and they are, in most of the countries, subject to product registration. Extensive validations (such as scientific validity, analytical and clinical performances, stability studies, etc.) are required to be performed for IVDs and expected to be approved by certification body or regulatory agency. After approval, IVDs they must bear the IVD symbol (Fig. 1).

IVDs in the EU
In the EU, the IVDs are regulated by the Directive 98/79/EC (IVDD) . In May 2022, a new law governing the IVDs will be fully applicable: The Regulation (EU) 2017/746 (IVDR) . Passed the IVDR different transition periods, all IVDs in the European Economic Area (EEA) would need to comply with the requirements of the IVDR.
As a reminder, please note that all IVDs compliant with the EU requirements must carry the CE Mark (Fig. 2).
There no major differences between the definition of an IVD under the IVDD (Art.1.2.b) and the IVDR (Art.2(2)). Both texts define an IVD as the following:
“‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on one or more of the following:
(a) concerning a physiological or pathological process or state;
(b) concerning congenital physical or mental impairments;
(c) concerning the predisposition to a medical condition or a disease;
(d) to determine the safety and compatibility with potential recipients;
(e) to predict treatment response or reactions;
(f) to define or monitoring therapeutic measures.
Note that the IVDR specifies the following: “Specimen receptacles shall also be deemed to be in vitro diagnostic medical devices”.

IVDs in the U.S.
The US Food and Drug Administration (FDA) defines IVDs in Title 21 of the Code of Federal Regulation (CFR) Part 809.3 as “products that are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae. Such products are intended for use in the collection, preparation, and examination of specimens taken from the human body.”
IVDs are devices as defined in Section 210(h) of the Federal Food, Drug and Cosmetic Act (FD&C Act) and can be also biological devices subject to Section 351 of the Public Health Service Act (PHS Act).
It is important to know that IVD products must be labelled “For In Vitro Diagnostic Use” (as per 21 CFR 809.10(a)(4)) or carry the IVD symbol.
The FDA proposes on its website a whole overview of how it regulates IVD products. See section Recommended Reading .
Research Use Only (RUO) Products
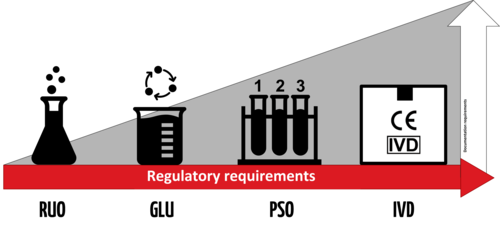
RUO means Research Use Only. It means that a given product is basically for that, for research such as basic laboratory research, performance investigation, design investigation, etc.
Norms and standards don’t apply here. Depending of the countries, the RUO products can be considered or not as ineffective IVDs. Therefore, depending of the countries and jurisdictions, the RUO products can be more or less regulated.
RUOs in the EU
RUO products are not subject to the IVDD not the IVDR. As they lack a clinical application, there are not qualified as medical devices by law.
The IVDD and the IVDR don’t define the term “Research Use Only” explicitly and no requirements are defined.
Manufacturers of RUO products should not apply the CE Mark of these products and clearly label them as “Research Use Only” and use the RUO label (Fig.3).
In the EU, a distinction is made between RUO products and IVDs for Performance Testing. IVDs introduced in European laboratories to establish their performance characteristics also are subject to the IVDD and IVDR. Such products cannot carry the CE Mark as their performance have not been established yet. IVDD and IVDR have specific performance documentation and notification requirements for these products.
For more information: The EU Commission has published a MEDDEV guidance concerning Research Use Only products. See section Recommended Reading. Note this guidance is not aligned with the IVDR and that a MDCG Guidance for RUO products is still under development at this moment.

RUO in the U.S.
The 21 CFR 809.10 and 21 CFR 864 define four types of IVDs: General Purpose Reagent (GPR), Investigational Use Only (IUO), Analyte Specific Reagent (ASR) and Research Use Only (RUO). This is why in the U.S., RUOs are also called RUO IVDs – In contrast, in EU, only the term RUO prevails.
As per the 21 CFR, RUO products are IVD products in the laboratory research phase of development and not represented as effective IVDs (21 CFR 809.10(c)(2)(i)). In essence, RUO products are reagents, instruments, or systems under development and evaluated for their potential use as IVDs (Evaluation of design, performance, usability, etc.).
RUO product are essentially unregulated in the U.S but must be labelled with the following statement: “For Research Use Only. Not for use in diagnostic procedures”. Labelling a product as such permits it to be used by researchers, who can evaluate usefulness for a specific diagnostic purpose. Beyond the labelling statement, FDA regulations do not mandate any other restrictions or limitations on RUO products, and RUO manufacturers do not have to register or list their RUO products with FDA or comply with manufacturing standards. RUO products can be offered for sale without any FDA clearance or approval.
RUO products can be also used in conducting nonclinical laboratory research with goals other than commercial IVD product development and are used in basic life science research and not intended for further clinical diagnostic use development. In this case, these RUOs are used to carry out research and are not, themselves, the object of the research.
As good marketing practices, RUO products must never be represented as effective IVD products. And no specific disease, condition, or diagnostic performance claims can be made for RUO products. In the other side, an IVD product that is inappropriately labelled as RUO may be also considered misbranded or adulterated due to the lack of premarket notification (510(k)) or premarket approval (PMA) if distributed/or labelled for clinical diagnostic purpose.
For more information, the FDA has published a guidance regarding IVD products labelled for Research Use Only. See section Recommended Reading .
Recommended Reading
Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only, FDA Guidance, 2013
IVD Guidance : Research Use Only products, MEDDEV. 2.14/2 rev.1, 2004
Overview of IVD Regulation, FDA website
Manufacturer IVD, EC website
Medical Devices – Sector, EC website
CFR Title 21
Directive 98/79/EC (IVDD)
Regulation (EU) 2017/746 IVDR: 02017R0746 — EN — 05.05.2017 — 000.003 (consolidated version)

Template – Product Qualification and Classification under the EU IVDR 2017/746 (v.1.0)

Checklist – ISO 13485 2016 Internal Audit (v.1.0)

Template – SOP Master Validation Test Plan (v.1.0)

Template – Technical Documentation Table of Content according to the IVDR (v1.0)

Template – SOP Technical Documentation according the IVDR (v1.0)

How to Create a Project Timeline in Simple Steps

How to create a Project Timeline with VisualMaker?

The IVD Product Types: RUO, IUO, GPR, ASR

Claims under the MDR and the IVDR (Art. 7/7)

The Investigational Device Exemption (IDE)

Conformity Assessment Options for Products Failing under the MDR
#regulatoryandmore
We use cookies to offer you a better browsing experience and analyse our website performance. We also use third-party cookies to further customise your experience showing relevant content while you are navigating on third-party platforms.

Our Priorities MedTech Europe strives to support our dynamic sector in meeting the needs of patients and health systems. To achieve this, we focus on engaging with healthcare stakeholders on key issues from regulations and market access to digital health and Brexit, among others.
- COVID-19 Information Hub
- Interactions with the Medical Community
- Access to Medical Technology
- Medical Technology Regulations
- Symbols for compliance with the MDR
- Symbols for compliance with the IVDR
- New IVD symbols for compliance with the IVDR
- Digital Health
- International
- Environmental and Social Sustainability
- Market Data
- Research and Innovation
- Innovative Health Initiative (IHI)
Sector Groups MedTech Europe sector groups bring together company experts to drive forward key healthcare domains, helping to address issues facing these sectors and shaping their future. We have dedicated groups focused on cardiovascular health, ophthalmology, diabetes, orthopaedics, and AMR/HAI.
- Antimicrobial Resistance (AMR) and Healthcare Associated Infections (HAIS)
- Cardiovascular
- Homecare & Community Care
- Orthopaedic

Real stories of people’s lives transformed by medtech.

Your platform for dialogue about medical technologies.
Search on this website
Research Use Only Products

What are Research Use Only (RUO) products? Research Use Only (RUO) products are a distinct category of in vitro diagnostics (IVDs) exclusively tailored for laboratory research. RUOs encompass specialised reagents, equipment, and materials crucial for scientific investigations, contributing significantly to the development of cutting-edge tools and solutions for research applications.
Research Use Only (RUO) products play a crucial role in medical research and innovative management of many patients. These specialised products, which include laboratory reagents and equipment, are exclusively designed for research in controlled laboratory environments. As essential tools for medical and scientific investigations, experimentation, and analysis, RUOs contribute to developing innovative solutions and advancements in medical research.
For example: RUO products can be used for Fundamental Research, in Pharmaceutical Research to find new drug compounds, and for a better identification and quantification of individual chemical substances. In diagnostics research, RUO products are essential to the development of new diagnostic assays and tools.
Unlike in vitro diagnostic medical devices (IVDs), RUOs are dedicated to facilitating research initiatives and are not intended for direct medical procedures with human patients. RUOs are not defined in the EU’s In Vitro Diagnostic Medical Devices Regulation 2017/746 (IVDR); they are regulated by the EU General Product Safety Regulation and other applicable EU legislations. Manufacturers of RUO products clearly label them as “Research Use Only” and use the RUO label.
From a production and specifications general perspective, the knowledge and processes needed to manufacture RUOs are very similar to those needed to manufacture CE marked IVDs. Many companies which operate in the IVD space will have RUO products in their portfolio. RUOs will generally have a similar chemical and physical composition compared to IVDs, but their intended purpose will be different. While RUO or IVDs might seem similar in their appearance and specifications, unambiguous and documented evidence associating the use of devices with in vitro diagnostic examination procedures is required to qualify a device as an IVD.
RUOs provide researchers and scientists – including those operating in medical laboratories – with valuable resources to advance in the understanding of disease, in drug discovery, in the development of new therapies and diagnostic tools. Laboratories or research consortia often collaborate with RUO manufacturers to tailor products to meet specific research needs and requirements, fostering a collaborative environment and contributing to the continuous evolution of research tools and solutions.
One critical application of RUO is to enable medical laboratories to develop in-house assays to e.g. diagnose rare and emerging conditions or to improve the current knowledge and management of specific diseases for which no adequate CE marked IVDs exist. This not only fulfils a critical and imminent healthcare need but is also a key stepping stone in the eventual development of IVDs. A poignant example of this was the development of COVID-19 assays during the early phase of the pandemic – initially, reference laboratories developed in house assays test for the SARS-CoV-2 virus, and shortly afterwards, commercial IVDs began to reach the market in order to fulfil a critical need during the global health crisis. However, it is worth noting that the use of in-house assays is regulated in IVDR and is subject to certain conditions.
In essence, RUO products provide researchers and physicians with the necessary tools to conduct experiments and studies, contributing to the overall progress in medical research. Their intended use in laboratory settings supports the development of new technologies and innovative solutions for various research applications.
Share this page
The MedTech Forum 2024
Join the conference on 22-24 May in Vienna
Sign up for your monthly newsletter
By clicking the Subscribe button, you give consent to MedTech Europe AISBL to use of the information you provided and send you content on the services you selected. We will ensure that the information is processed confidentially, and will only share it with third party providers that assist in providing these services. These providers may be located outside the EU; in this case, we will ensure that they are subject to a legal framework adequate in safeguarding your data, in compliance with European data protection law. You can unsubscribe, change your preferences or update your information at any time by clicking on the unsubscribe button available on all messages. For more information on how MedTech Europe will handle your personal data, please refer to our Privacy Policy . You can contact us at [email protected] for further questions related to your privacy and your rights.

Research Use Only or IVD: What’s Right for Your Lab?
by Tina Sobania | Clinical , Molecular

Publish Date: September 13, 2018
There are many misconceptions in the clinical industry regarding laboratory quality control materials. With numerous products available and manufacturers using various labeling practices, how do you know what’s best for your laboratory?
To help clear up the confusion, we’re answering two important questions clinical laboratorians have about quality control products.
Are diagnostic system controls IVDs?
One common misconception is that materials used for quality control of diagnostic systems are not themselves in vitro diagnostics (IVDs). However, the U.S. Food & Drug Administration (FDA) has written regulations citing quality control material as medical devices. For example, 21 CFR 862.1660 , Mulit-Analyte Controls Unassayed under Clinical Chemistry, and more recently 21 CFR 866.3920 , classify Class II controls requiring FDA 510(k) review under microbiology.
It’s important to understand that if a manufacturer for controls of nucleic acid amplification states its product works with a specific instrument or assay in its labeling or marketing literature, the FDA considers the material to be a Class II IVD and requires a 510(k) review . The FDA has established special controls for this type of material to ensure the product is properly labeled, performs according to claims and remains stable. In addition, IVD material must be manufactured under the FDA’s current Good Manufacturing Practices (cGMP).
Should “Research Use Only” products be used for quality control?
The second misconception clinical laboratories should be aware of involves material labeled as Research Use Only (RUO). RUO labeling is intended for products that are still under development and are not commercially distributed. A developer would use this labeling to ship product for “investigation relating to product development” as explained by the FDA in guidance document, Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigation Use Only .
Another factor one must consider is products labeled RUO are not required to be manufactured in accordance with cGMP and FDA Quality System Regulation. Lack of manufacturing controls may be detrimental to the quality of the control material. As such, clinical laboratories using RUO quality control materials to ensure the quality of testing may be placing patients at unnecessary risk.
Key Takeaway
To maintain the highest possible quality of your diagnostic testing, it’s best to choose materials that have been manufactured by a cGMP compliant facility under the FDA QSR, and when necessary reviewed by the FDA. Materials clearly labeled as IVDs provide that assurance and lower your laboratory’s risk.
Follow the links below to find all the FDA regulations cited in this post.
- 21 CFR 862.1660 CFR – Code of Federal Regulations Title 21, Subchapter H – Medical Devices
- 21 CFR 866.3920 CFR – Code of Federal Regulations Title 21, Subchapter H – Medical Devices
- Distribution of In Vitro Diagnostic Products Labeled for Research Use Only of Investigation Use Only
Written by Tina Sobania
You may also like.

MLS and MLT Career Opportunities
As a manufacturer of quality controls used in clinical diagnostics, we see firsthand the difficulties that laboratory...

The Challenges of Diagnosing and Treating Secondary Infections
In the past two decades, there have been six major global outbreaks of infectious diseases. While these infections may...
Trackbacks/Pingbacks
- Put Your Best Plate Forward – #CreepyCultures is Back! – Microbiologics Blog - […] Read Next – Research Use Only or IVD: What’s Right for Your Lab? […]
- Clinical Case File: Group A Streptococcus – Microbiologics Blog - […] Read Next – Research Use Only or IVD: What’s Right for Your Lab? […]
- Dear Stanley: Molecular QC Best Practices – Microbiologics Blog - […] Read Next – Research Use Only or IVD: What’s Right for Your Lab? […]
- Our Top Posts from 2018 – Microbiologics Blog - […] 1. Research Use Only or IVD: What’s Right for Your Lab? […]
- LDT Oversight Deliberation Continues – Microbiologics Blog - […] Read Next – Research Use Only or IVD: What’s Right for Your Lab? […]

- Pharmaceutical
- Events and Webinars
- Uncategorized

1-800-599-2847 microbiologics.com [email protected]
QUICK LINKS
CATEGORIES RESOURCES ABOUT US CONTACT US SITE MAP PRIVACY POLICY
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Regulatory Information
- Search for FDA Guidance Documents
- Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only
GUIDANCE DOCUMENT

Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only Guidance for Industry and FDA Staff November 2013
FDA is issuing this guidance document to provide the current thinking of the Center for Devices and Radiological Health (CDRH) and the Center for Biologics Evaluation and Research (CBER) on when in vitro diagnostic (IVD) products are properly labeled “for research use only” (RUO) or “for investigational use only” (IUO).
Submit Comments
You can submit online or written comments on any guidance at any time (see 21 CFR 10.115(g)(5))
If unable to submit comments online, please mail written comments to:
Dockets Management Food and Drug Administration 5630 Fishers Lane, Rm 1061 Rockville, MD 20852
All written comments should be identified with this document's docket number: FDA-2011-D-0305 .
Structure and content of the EU-IVDR
Current status and implications for pathology
Struktur und Inhalt der EU-IVDR
Bestandsaufnahme und Implikationen für die Pathologie
- Review article
- Open access
- Published: 03 February 2023
- Volume 44 , pages 73–85, ( 2023 )
Cite this article
You have full access to this open access article
- Andy Kahles 1 ,
- Hannah Goldschmid 1 ,
- Anna-Lena Volckmar 1 ,
- Carolin Ploeger 1 ,
- Daniel Kazdal 1 ,
- Roland Penzel 1 ,
- Jan Budczies 1 ,
- Gisela Kempny 2 ,
- Marlon Kazmierczak 2 ,
- Christa Flechtenmacher 1 ,
- Gustavo Baretton 3 ,
- Wilko Weichert 4 ,
- David Horst 5 ,
- Frederick Klauschen 6 ,
- Ulrich M. Gassner 7 ,
- Monika Brüggemann 8 ,
- Michael Vogeser 9 ,
- Peter Schirmacher 1 &
- Albrecht Stenzinger 1
2851 Accesses
3 Citations
1 Altmetric
Explore all metrics
Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) was passed by the European Parliament and the Council of the European Union on 5 April 2017 and came into force on 26 May 2017. A new amending regulation, which introduces a phased implementation of the IVDR with new transitional provisions for certain in vitro diagnostic medical devices (IVDs) and a later date of application of some requirements for in-house devices for healthcare facilities, was adopted on 15 December 2021.
The combined use of CE-certified IVDs (CE-IVDs), in-house IVDs (IH-IVDs), and research use only (RUO) devices are a cornerstone of diagnostics in pathology departments and crucial for optimal patient care. The IVDR not only regulates the manufacture and placement on the market of industrially manufactured IVDs, but also imposes conditions on the manufacture and use of IH-IVDs for internal use by healthcare facilities.
Our work provides an overview of the background and structure of the IVDR and identifies core areas that need to be interpreted and fleshed out in the context of the legal framework as well as expert knowledge.
Conclusions
The gaps and ambiguities in the IVDR crucially require the expertise of professional societies, alliances, and individual stakeholders to successfully facilitate the implementation and use of the IVDR in pathology departments and to avoid aberrant developments.
Similar content being viewed by others

Regulation (EU) 2017/746 (IVDR): practical implementation of annex I in pathology
Andy Kahles, Hannah Goldschmid, … Albrecht Stenzinger

Medical device legislation and POCT

EU 2017/746 – In Vitro Diagnostic Medical Devices
Avoid common mistakes on your manuscript.
Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR), fully applicable since May 26, 2022, and the associated fulfillment of the conditions pose new challenges for health institutions and thus also for diagnostic facilities such as departments of pathology. This article is intended to provide an overview on the background and framework conditions as well as to identify areas of IVDR that need to be filled in and supplemented by specialist scientific expertise to achieve successful implementation and application in laboratories.
Regulation (EU) 2017/746 on in vitro diagnostic medical devices (hereinafter: IVDR) was adopted by the European Parliament and the Council of the European Union on April 5, 2017, and entered into force on May 26, 2017 [ 31 ]. This regulation repeals the EU Directive 98/79/EC on in vitro diagnostic medical devices (abbreviated IVDD for in vitro diagnostic medical devices directive) [ 21 ], which had been in force since 1998. Once adopted, an EU regulation is a valid and a binding legislative act that all EU member states must apply by in its entirety. Therefore, in contrast to the now superseded EU Directive, it does not first have to be transposed into national law [ 16 ]. Together with the IVDD, the German Medical Devices Act ( Medizinproduktgesetz , MPG), which serves as the German implementation of the IVDD, will thus also become invalid (for definitions of terms, see Table 1 ).
The introduction of the IVDR is intended to reduce the risk of national differences in the interpretation of the IVDD within the EU. The original version of the IVDR provided, after a transition period of 5 years, that all requirements of the IVDR for an in vitro diagnostic medical device must be fully met as of May 26, 2022. Exemptions and transition periods for economic operators are regulated by Article 110 of the regulation. Deviating from the initial deadline definition, the European Commission—following a request by an intergroup letter of the European Parliament and a decision of the Council of Health Ministers—proposed amended transitional provisions for certain in vitro diagnostic medical devices and introduced a later start date for some requirements for in-house devices for health institutions on October 14, 2021 (Table 2 ; [ 13 , 14 ]). This proposal was adopted on December 15, 2021 [ 15 ]. In this regard, the new amending regulation only refers to the phasing-in of the requirements and does not change any of the requirements in the original regulation.
In the preamble to the IVDR, the European Parliament and the Council of the European Union provide 101 recitals for the replacement of the 1998 IVD Directive 98/79/EC (IVDD) by IVD Regulation 2017/746 (IVDR), focusing on health protection through high patient and user safety and a functioning internal market through harmonization of legislation. Primarily, the IVDR regulates the placing on the market, the provision on the single European market, and a risk-based classification of the devices according to their intended purpose and the respective resulting requirements. These regulations mainly address economic operators, i.e., manufacturers, distributors, and importers of in vitro diagnostic medical devices. However, the IVDR also concerns the use of in vitro diagnostic medical devices in health institutions. Recital 29 of the IVDR emphasizes the special importance of health institutions and the in vitro diagnostic devices they develop themselves (hereinafter IH-IVD for in-house in vitro diagnostic devices; also referred to as laboratory developed tests [LDT]). Health institutions should continue to be able to manufacture, modify, and use devices in-house to meet the specific needs of patients. However, health protection is to be increased by now stricter requirements (recital 28). For the first time, the IVDR attempts to create harmonized conditions throughout the EU and imposes several requirements on health institutions that develop and use in-house IVDs.
Use of in vitro diagnostic medical devices in health institutions
Health institutions such as hospitals, institutes of pathology, medical laboratories, and health care centers use commercial IVDs as consumers in their diagnostic process chains (CE-certified IVDs [CE-IVDs], but also research use only [RUO] devices). They also develop, optimize, implement, and validate in-house diagnostic procedures and materials. For example, a study from a Belgian university hospital found that although almost all test results were obtained with CE-IVD-labeled procedures (98%), of the different IVD devices used, about half (47%) were developed in-house (IH-IVDs), with no commercial alternatives available for the majority (72%) [ 29 ].
Within a defined process chain from specimen collection or receipt to diagnostic findings, different types of in vitro diagnostic devices can be used (Fig. 1 a). Here, both commercial devices (CE-IVDs, RUO devices) and IH-IVDs are used or combined with one another (Fig. 1 b). Here, the complementary or combined use of industrially manufactured IVDs, in-house procedures, and materials of general laboratory use leads to a valid finding, which is necessary for optimal patient care (example in Fig. 2 ). The IVDR now not only regulates the manufacture and placing on the market of industrially manufactured in vitro diagnostic devices but also imposes conditions on the manufacture and use of IH-IVDs for internal use by health institutions. This includes the use of RUO devices (Fig. 1 b and 2 ) in diagnostics. The definition of “in vitro diagnostic medical device” has been slightly modified by the new IVDR and now explicitly covers stand-alone software (Table 1 ). However, a diagnostic procedure itself does not constitute an in vitro diagnostic device according to the definition of the IVDR.
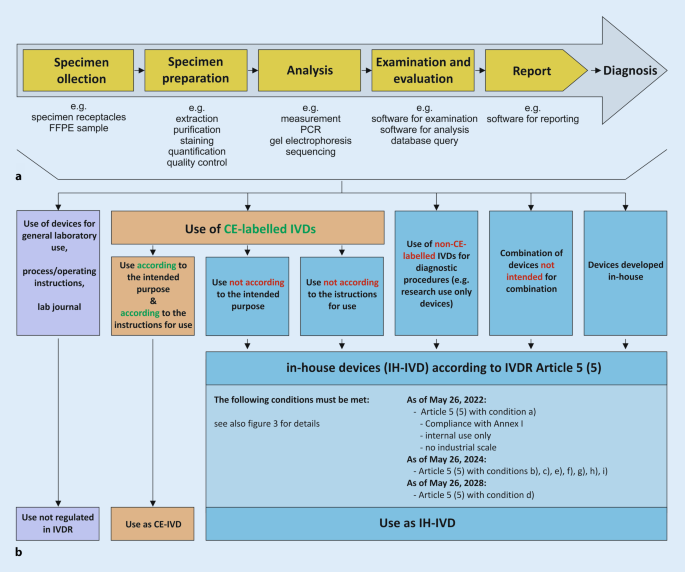
a Process chain in the analytics of health institutions. b Each link in the process chain ( a ) from specimen receipt to diagnostic findings may contain devices that can be assigned to either general laboratory use ( purple ), CE-marked in vitro diagnostic devices ( CE-IVD , orange ), or in-house developed IVDs ( IH-IVD , blue )
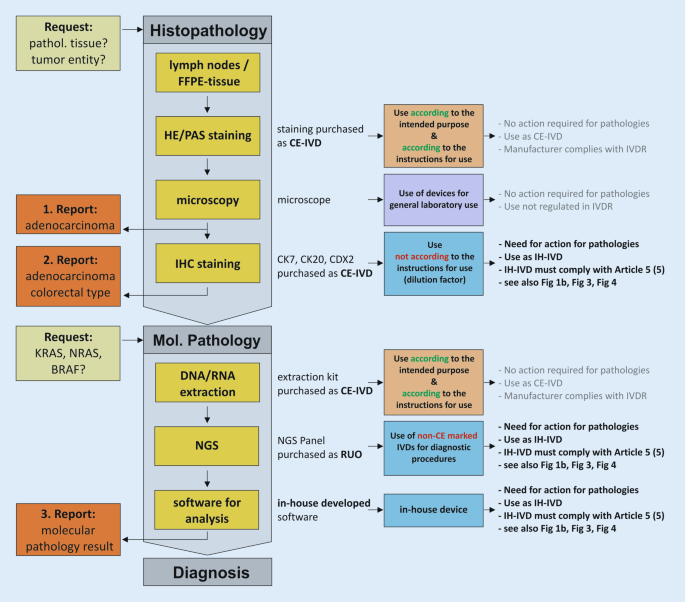
Exemplary process chain within pathology institutes from request to reporting to diagnosis using various CE-marked ( CE-IVD ) and in-house in vitro diagnostic devices ( IH-IVD ). FFPE formalin-fixed paraffin-embedded, IHC immunohistochemistry, NGS next-generation sequencing, RUO research use only, IVDR regulation on in vitro diagnostic devices
The new and stricter requirements for manufacturers to obtain approval for their IVDs and the lack of grandfathering for products that have already been approved are likely to have a direct impact on price developments. Devices that are relevant for patients but become uneconomical due to the increased requirements could even be withdrawn from the market altogether. For example, important but rarely used tests for patients with rare diseases could no longer be performed or would have to be compensated by self-developed tests. In July 2021, a survey of 115 manufacturers for the European market conducted by MedTech Europe, the European trade association representing the medical technology industries, revealed that they are unlikely to transfer all CE-IVD devices to the new regulation [ 20 , 28 ]. This would affect approximately one in five CE-IVDs currently available on the market, which would then no longer be available to health institutions for patient care or would have to be replaced by an in-house developed and validated IH-IVD. Devices stocked by health institutions may continue to be used until their expiration date [ 10 ].
According to recital 29 of the preamble to the IVDR, the development, manufacture, and use of in-house tests by health institutions should continue to be an option for addressing the specific needs of patients. This should continue to be possible without the involvement of conformity assessment bodies—the so-called notified bodies—and without the exclusive use of CE-marked devices. However, this regulation now aims to clarify and tighten the rules for IH-IVDs to ensure the “ highest level of health protection ” (recital 28). These recitals are specified in Article 5 (5) of the IVDR. This article imposes several conditions on health institutions for the manufacture and use of IH-IVDs. It also describes that all other requirements of the IVDR shall not apply to health institutions once the conditions set out therein have been met.
Use of in-house in vitro diagnostic medical devices in health institutions—Article 5 (5)
For optimal patient care, internally developed, optimized, and established IH-IVDs are used in departments of pathology and other health institutions (e.g., PCR-based analyses or immunohistochemical tests). Article 5 of the IVDR generally regulates the placing on the market and putting into service of in vitro diagnostic medical devices and specifically regulates in paragraph 5—in contrast to the previous IVD Directive 98/79—also in-house devices (IH-IVDs), which are “ manufactured and used only within health institutions established in the Union ” and are not manufactured on an industrial scale (Fig. 3 ). Thus, the introduction of the IVDR also has a major impact on health institutions that use RUO devices and adapted or in-house developed devices complementarily to CE-marked IVDs. For such in-house devices, the requirements of the IVDR do not apply provided that several conditions (a)–(i) defined in Article 5 (5) and the relevant general safety and performance requirements defined in Annex I are met.

Regulation 2017/746 on in vitro diagnostic devices (IVDR)—Article 5 (5) [ 31 ]
Article 5 (5)—condition a)
“a) the devices are not transferred to another legal entity”
Condition a) restricts the transfer of an internally developed IVD device. In-house devices may not be transferred to another legal entity. Although the physical devices themselves may not be transferred, this restriction does not affect the transfer or publication of development or testing protocols, documentation, or results necessary to maintain high quality in health institutions through professional exchange, interpretation, and scientific discussion.
Article 5 (5)—condition b)
“b) manufacture and use of the devices occur under appropriate quality management systems”
The manufacture and use of in-house devices in health institutions must occur “ under appropriate quality management systems. ” The “ appropriate quality management system ” is not conclusively defined by the IVDR; thus, it does not specify how a quality management system appropriate for the manufacture and use of IH-IVDs is defined for health institutions that manufacture IH-IVDs. Article 10 (8) can provide some guidance in this context. This article lists a minimum of aspects and criteria that a quality management system for manufacturers must consider and include. However, and importantly, Article 5 (5) excludes the application of all requirements of the IVDR for in-house devices in case of compliance with the conditions listed within Article 5 (5). Thus, the requirements of Article 10 (8) address economic operators and the manufacture of commercial IVDs and not health institutions with their manufacture of IH-IVDs or their use of RUO devices under Article 5 (5). The “ appropriate quality management system ” required for health institutions for the manufacture and use of in-house IVDs therefore leaves scope of action and room for interpretation.
Article 5 (5)—condition c)
“c) the laboratory of the health institution is compliant with standard EN ISO 15189 or where applicable national provisions, including national provisions regarding accreditation”
Condition c) in Article 5 (5) concretizes this scope of action for the laboratories of the health institutions in such a way that it designates the EN ISO 15189 standard or, if applicable, national regulations including national accreditation regulations. However, condition c) differs from the above-mentioned condition b) in that condition c) focuses on the laboratory of the health institution itself and not on the device with its manufacture and use. The EN ISO standard explicitly mentioned in condition c) is not to be seen as exclusive, but rather as an exemplary possibility of design. The EN ISO 15189 standard describes the requirements for quality and competence of medical laboratories. It depicts the requirements for the quality management system as well as the general requirements for laboratory operations. A comparative analysis of the requirements from Article 5 (5) with the EN ISO 15189 standard has shown only partial conformity with the IVDR for the manufacture of in-house IVDs [ 24 ]. Due to the strong focus on the IVD device itself, the requirements of the IVDR set out in Article 5 (5) go beyond the EN ISO 15189 standard.
Although a quality management system complying with this standard is a prerequisite, accreditation is not explicitly required. In Germany, many departments of pathology comply with the standard DIN EN ISO/IEC 17020 or are accredited as so-called inspection bodies according to this standard. The German accreditation body DAkkS (Deutsche Akkreditierungsstelle GmbH) describes this aspect on its homepage: “ The DIN EN ISO/IEC 17020 standard is relevant for the accreditations of the services offered in the field of pathology in patient care. All conformity assessment bodies in pathology are thus inspection bodies. The accreditation focuses on the expert assessment of the physician—on the diagnosis. The requirements of the ISO 15189 standard are also taken into account according to ILAC P15:07/2016 ” ([ 8 ], translation from German into English by the authors). Hereby, the accreditation body DAkkS emphasizes that the standard DIN EN ISO/IEC 15189 mentioned in the IVDR plays a special role in the field of pathology in Germany. Therefore, DAkkS also includes this standard in the assessment and accreditation of departments of pathology according to DIN EN ISO/IEC 17020.
It should therefore be noted that departments of pathology accredited to DIN EN ISO/IEC 15189 or DIN EN ISO/IEC 17020 meet condition c) of the IVDR. Facilities without accreditation are not required to be accredited to comply with the IVDR but must operate an appropriate quality management system.
Article 5 (5)—condition d)
“d) the health institution justifies in its documentation that the target patient group’s specific needs cannot be met, or cannot be met at the appropriate level of performance by an equivalent device available on the market”
Health institutions must provide justified documentation that “ the target patient group’s specific needs cannot be met, or cannot be met at the appropriate level of performance by an equivalent device available on the market, ” as stated in Article 5 (5)—condition d). The opening of the IVDR to health institutions by Article 5 (5) is severely curtailed in this condition by elevating commercial market products compared to in-house devices. Thus, in condition d), the IVDR privileges commercial IVD manufacturers by prohibiting the diagnostic use of in-house devices if an equivalent device is available on the market. If equivalent, the commercial device must be used. Of note, this condition (so-called industry privilege) represents a fundamental paradigm shift, as the standard of quality is no longer dominated by the academic-scientific side, but by industry standards. The “ equivalent device available on the market ” is not further defined. It is unclear whether a like CE device or even an RUO device precludes the diagnostic use of an IH-IVD.
This industry privilege implicitly requires health institutions that develop, establish, and use in-house devices to allocate additional time and human resources and represents an increased documentary burden. This requires a precise definition of the IH-IVD (intended purpose) and its areas of application (type of underlying tissue, sample size, etc.) that ensures comparability and emphasizes potential superiority over CE-IVDs. The intended purpose forms the basis for finding equivalence of CE-marked devices. Devices can only be “ equivalent ” if they have the same intended purpose. Since the terms “ equivalent ” and “ the target patient group’s specific needs ” are not defined, there is room for interpretation, which should be used adequately by diagnostic colleagues and professional associations.
The assessment of equivalence must be updated by means of documented and regular, but temporally undefined, market monitoring. As a result, an elaborately developed in-house test could very quickly lose its authorization for use, possibly already during the development process, as soon as an equivalent CE-IVD reaches market maturity. However, health institutions need not fear competitive lawsuits from manufacturers and distributors of commercial CE-IVDs as a result of this requirement, as they each serve different markets. Since the regularity of the equivalence assessment is not defined in the IVDR, individual intervals adapted to the devices can be defined and specified in the risk management plan.
The IVDR requires economic operators to register their CE-IVDs in detail in the European Database on Medical Devices (EUDAMED). EUDAMED aims to improve market surveillance by mapping the lifecycle of medical devices (and thus IVDs) in real time [ 9 ]. Thus, this database also assists health institutions in fulfilling condition d). However, health institutions themselves do not have to use the database.
The IVDR also does not explicitly describe how to proceed in the event of a potentially necessary, rapid, and short-term compensation of a CE-IVD by an IH-IVD in the event of supply or production bottlenecks, but this scenario would be a case of nonavailability with the regulations intended for this purpose.
Article 5 (5)—condition e)
“e) the health institution provides information upon request on the use of such devices to its competent authority, which shall include a justification of their manufacturing, modification, and use”
The documentation resulting from Article 5 (5) for the development and manufacture of in-house devices must be made available to the competent authority upon request (see also conditions g) and h); Fig. 4 ). Written documented information and justifications for the use, manufacture, and modification of the devices must be available for this purpose and must be available for review by the competent state authority. In Germany, the responsibility lies with the respective responsible regional state authority. It is important to note at this point that the competent authority does not correspond to the notified bodies, which are responsible for the conformity assessment of the devices of economic operators according to Article 48 with Annexes IX to XI.
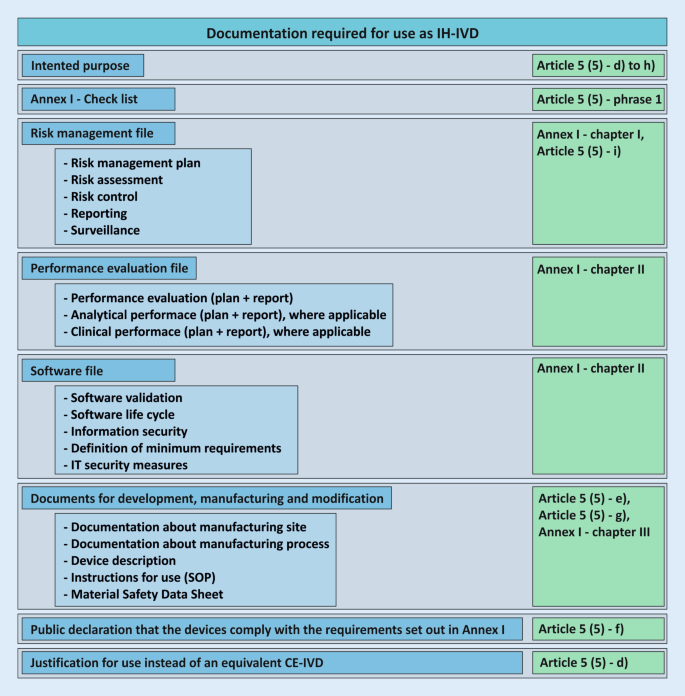
Required documentation for the use of an in-house in vitro diagnostic device ( IH-IVD ) in health institutions with reference to the regulation on in vitro diagnostic devices (IVDR; green ). These documents do not necessarily apply to all devices and may not be complete. SOP standard operating procedure
Article 5 (5)—condition f)
“f) the health institution draws up a declaration which it shall make publicly available, including:
(i) the name and address of the manufacturing health institution,
(ii) the details necessary to identify the devices,
(iii) a declaration that the devices meet the general safety and performance requirements set out in Annex I to this Regulation and, where applicable, information on which requirements are not fully met with a reasoned justification therefor”
Health institutions that manufacture in-house devices must issue a declaration similar to the former Medizinprodukte-Verordnung (German Medical Devices Regulation; MPV, § 5 [6]) that identifies them as a health institution (subitem i) and the respective device as such (subitem ii). In addition, the health institution declares in writing the conformity of the devices with the essential safety and performance requirements according to Annex I of the IVDR (subitem iii). Annex I requirements that cannot be met must be justified. These declarations must be made publicly available. The IVDR does not give a more precise indication of public accessibility; so, for example, an indication on the homepage would be possible.
Article 5 (5)—conditions g) and h)
“g) as regards class D devices in accordance with the rules set out in Annex VIII, the health institution draws up documentation that makes it possible to have an understanding of the manufacturing facility, the manufacturing process, the design and performance data of the devices, including the intended purpose, and that is sufficiently detailed to enable the competent authority to ascertain that the general safety and performance requirements set out in Annex I to this Regulation are met. Member States may apply this provision also to class A, B or C devices in accordance with the rules set out in Annex VIII”
“h) the health institution takes all necessary measures to ensure that all devices are manufactured in accordance with the documentation referred to in point (g)”
In Annex VIII the IVDR describes seven rules for manufacturers to classify IVDs into four classes of increasing individual and public risk (A to D) based on their intended purpose.
Health institutions thus classify their IH-IVDs based on their self-defined intended purpose. Most IH-IVDs used in pathology departments can be classified as class C (includes, e.g., devices used for cancer diagnosis or detection of an infectious agent with high individual but moderate public risk), class B (includes, e.g., devices that are controls with no qualitative or quantitative value or products that cannot be classified as classes A, C, and D), or class A (includes, e.g., devices for general laboratory use such as buffer solutions or histological stains).
Condition g) of Article 5 (5) tightens the documentation for devices classified in the highest risk class D (rules 1 and 2). Here, the documentation requirements listed in conditions d)–f) are extended and supplementary and more detailed documentation is required. The extended documentation enables the authority to ensure that the essential safety and performance requirements according to Annex I are fulfilled. EU member states may also extend the more stringent documentation requirement to in vitro diagnostic medical devices that are classified as class A, B, or C. The German legislator has done so, although the authorized Federal Ministry of Health has not yet made use of this possibility.
On the one hand, condition h) covers “ all devices, ” i.e., also those of classes A, B, or C, but on the other hand, it refers to the documents mentioned in condition g). However, as the Union legislator has obliged health institutions in condition g) only with regard to IH-IVDs of class D to prepare the documentation referred to therein, condition h) cannot result in an indirectly tightened documentation obligation also being constituted for in vitro diagnostic medical devices of classes A, B, or C, as long as the legislator does not extend this obligation to such devices as well. Condition h) is therefore only relevant for IH-IVDs classified in class D.
Article 5 (5)—condition i)
“i) the health institution reviews experience gained from clinical use of the devices and takes all necessary corrective actions”
Condition (i) requires health institutions to conduct a review of the clinical use experience of their IH-IVDs. Consequently, resulting necessary corrective actions must be derived and taken. This systematic process was already mandatory for health institutions by the former German Medizinprodukte-Verordnung (MPV, § 5 [6]). The process as such is also part of the standards DIN EN ISO/IEC 15189 for medical laboratories and DIN EN ISO/IEC 17020 for institutes of pathology.
Safety and performance requirements—Annex I
As of May 26, 2022, IH-IVDs of health institutions must comply with the general safety and performance requirements (Annex I of the IVDR) according to Article 5 (5). This Annex is divided into three chapters: (I) general requirements; (II) requirements regarding performance, design, and manufacturing; (III) requirements regarding information supplied with the device (Fig. 5 ). The purpose is to ensure and demonstrate that the devices (1) are fit for their intended purpose, that they (2) are safe and effective, and that they (3) do not endanger the health of users, third parties, or patients, and that any potential risk is under control and acceptable.
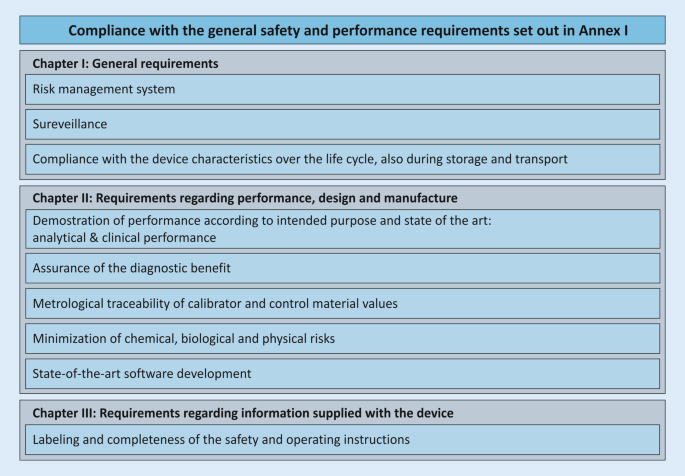
Elements from Annex I, which are necessary for the fulfillment of the essential safety and performance requirements. These elements do not necessarily apply to all devices and may not be complete
This annex describes the general safety and performance requirements for all IVD devices and applies to CE-IVDs placed on the market as well as to IH-IVDs of health institutions. However, not all requirements are always applicable to IH-IVDs, and the wording suggests that Annex I focuses more on economic operators and commercial devices. Health institutions should consider which requirements specifically apply to their IH-IVDs and create a checklist for their needs that will guide them through the annex. Condition f) of Article 5 (5) requires a publicly available statement from the health institution that its IH-IVDs comply with the requirements of Annex I. For devices that may be classified as class D under Annex VIII, condition g) of Article 5 (5) requires much more extensive documentation for this purpose.
Risk management and safety evaluation
An essential core point of Annex I is the definition and ongoing execution of a risk management system. This product-specific risk management system required by the IVDR is only partially covered by the ISO 15189 standard mentioned under condition c) and the systemic risk management system described there [ 24 ]. This notion also applies to the standard DIN EN ISO/IEC 17020. However, these standards provide a basis for a primarily institutional and general risk management system, which can be extended for the manufacture and use of IH-IVDs. The two standards ISO 22367 and ISO 14971, which specify a risk management process for medical laboratories, can provide assistance in this regard. The content of the ISO 22367 standard also covers, for example, the manufacture and use of in-house IVDs. According to the IVDR, a risk management system is only required for those diagnostic laboratory tests in which in-house devices are used.
Annex I requires health institutions to perform a comprehensive safety assessment for each IH-IVD to assess and minimize risks to the user, the patient, or, if applicable, third parties (in this case, trained laboratory personnel). It is important not only to identify risks, but also to derive their probabilities and the subsequent measures to avoid or control risks. Since the risks of the methodologies (polymerase chain reaction [PCR], real-time PCR [qPCR], next-generation sequencing [NGS], etc.) are likely to be similar irrespective of the exact molecular target, the development of in-house templates is possible here.
Performance evaluation
Annex I, chapter II, paragraph 9 contains the performance requirements for IVD devices to ensure that the devices are fit for their intended purpose. Here, the regulation lists performance parameters for analytical performance and clinical performance that are to be provided “ where applicable ” for the device. How such validation is to be performed is to be determined for each device and according to the generally accepted state of the art. In Article 56 and Annex XIII, the IVDR itself describes how a performance evaluation is to be performed for CE-IVDs to be placed on the market. These specifications can also be used as a reference for IH-IVDs. The standard DIN EN ISO 15189, which health institutions must comply with according to condition c) of Article 5 (5), the standard DIN EN ISO/IEC 17020, and the guideline for quality assurance of laboratory medical examinations issued by the German Federal Medical Association ( Richtlinie der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen —Rili-BÄK, [ 22 ]), also require that all procedures must be fully validated before being introduced into routine diagnostics [ 7 ]. In Germany, guidance at the national level is provided by the guidelines of the DAkkS Pathology/Neuropathology Sector Committee, which is responsible for interpreting accreditation requirements in the field of pathology. They include guidance for validations and verifications of sub-procedures in PCR-based molecular pathology [ 6 ] and immunohistology [ 5 ]. Across Europe, the Biomedical Alliance Europe is currently developing more detailed guidance on how to implement the IVDR for IH-IVDs as well [ 3 ]. In addition, for the field of NGS-based molecular pathology, reference can be made to the U.S. Food and Drug Administration (FDA) guidelines [ 17 ] and scientific publications [ 18 , 26 ], which reflect the state of the art and can serve as a template for an individual and device-specific validation plan. These documents provide technical and scientific guidance on how to meet the requirements for validation and verification of molecular pathology or immunohistochemistry assays. They also describe practical examples of implementation. For example, the comparison with commercial standards, external reference standards, and with orthologous validated or commercial methods is presented. For the verification of the correctness of IH-IVDs, e.g., (commercial) control samples or participation in interlaboratory comparisons are suitable. For further design and definition of the performance evaluation, the expertise of pathologists and the respective professional societies and working groups is essential. The important role of networks, in which, for example, validation strategies, protocols, and control samples can be exchanged, should also be emphasized.
Minimum requirements of an intended purpose
The intended purpose, Annex I, chapter III, 20.4.1 c), focuses on a clear definition of the device, the processes, its capabilities, and areas of application. It is used as a benchmark for evaluating the similarity or superiority of an IH-IVD over a CE-IVD. Also, the intended purpose specifies the scope of performance requirements. A precise definition of the clinical diagnostic question (testing for pathogenic changes, determination of tumor entity, genetic change sought, pathogen detection, etc.), the material used (formalin-fixed paraffin-embedded [FFPE], fresh material, amount of material used, etc.), and the metrics of the methodology used from the performance evaluation creates a concrete evaluation matrix with which different IVDs must be measured. The purpose, therefore, represents an important part of the decision regarding whether the establishment of an IH-IVD is necessary or whether a comparable CE-IVD must be resorted to.
Discussion and conclusion
The primary intention and rationale of the IVDR is to control the quality of diagnostic devices from commercial manufacturers to improve patient safety. In general, this intention is to be welcomed. Exemplifying that this stricter regulation can be necessary, a recent study by the Paul Ehrlich Institute in collaboration with researchers from other institutions reviewed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rapid antigen tests available in Europe and found poor sensitivity in about 21% [ 23 ]. Currently, manufacturers are still allowed to CE mark these tests themselves as IVDs (so-called self-certification) and place them on the market. In the future, an EU reference laboratory and a notified body will be required for certification, as these rapid tests fall into the highest risk class D according to the IVDR.
At the same time, the IVDR recognizes the fact of in-house developed tests (opening clause) that are used by health institutions. While this aspect is generally positive, the IVDR, via the industry privilege described above, inherently completes a paradigm shift from the previous quality specification by (non-industrial) academic experts to a quality benchmark according to industry standards. Health institutions provide diagnostic services to patients and represent them in terms of content as well as legal aspects. It is therefore of great importance that the above-mentioned points of Article 5 (5) are addressed and substantially shaped by stakeholders of the health institutions. The IVDR is intended to achieve standardization and quality monitoring without using the analogue of the Food and Drug Administration (FDA; for CE-IVDs) and Clinical Laboratory Improvement Amendments (CLIA; for IH-IVDs) on the national and European executive side, which are both large institutions that review and monitor the marketing and use of diagnostic tests in the US. The IVDR, therefore, delegates these tasks to notified bodies (for CE-IVDs) on the one hand and to (local) state authorities (for IH-IVDs) on the other. This constellation will likely lead to great heterogeneity and bottlenecks across the EU. In addition, the massively increased demand and simultaneous shortage of notified bodies pose great challenges for economic actors. There is concern that established CE-IVDs could be taken off the market, prices could increase, or new IVDs could only come onto the market as RUO devices.
Articles 98 and 99 of the IVDR describe and define the tasks of the Medical Device Coordination Group (MDCG). One of its tasks is the development of guidelines for the harmonized implementation of the IVDR in order to support stakeholders in the application of the regulation. A guidance document specific to IH-IVDs and their use by health institutions was published in early 2023 [ 11 ]. These guidance documents are not legally binding but represent a blueprint for the practical application of the IVDR to achieve effective and harmonized implementation of the regulations. Assistance for the implementation of the IVDR is provided by templates, checklists, and handouts of the Ad-Hoc Commission In-Vitro Diagnostics of the German Working Group of Scientific Medical Societies (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e. V., AWMF), which are freely available for use and modification [ 1 ].
Due to the definitional gaps and vagueness of the IVDR outlined above, there is also an opportunity for professional societies and associations to contribute their academic knowledge and expertise to the content of the IVDR, e.g., the AWMF [ 1 ], German Society for Pathology [ 25 ], Federal Association of German Pathologists [ 4 ], Dutch Society of Pathology [ 2 , 27 ], European Hematology Association [ 19 ], or the Biomedical Alliance Europe [ 3 ]). Only through this approach can a successful application and implementation of the IVDR succeed and substantial gaps in supply and inadequate pricing of diagnostics be avoided. In this context, IH-IVDs will continue to contribute to precise diagnostics and optimal patient care.
Conclusion for practice
Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) entered into force on May 26, 2017.
The new amending regulation, dated December 15, 2021, defines a phased introduction of the IVDR, for both commercial CE-IVDs and in-house IVDs (IH-IVDs) from health institutions.
The primary intention of the IVDR, namely to improve patient safety, is to be welcomed. Thus, EU-wide harmonized requirements are intended to improve the quality control and safety of diagnostic devices.
In this context, the IVDR recognizes the benefit and necessity of in-house developed tests (opening clause) used by health institutions.
The IVDR is primarily aimed at manufacturers of IVDs, but also has a drastic impact on pathology institutes.
The IVDR imposes several conditions on health institutions that develop and use in-house IVDs.
Due to definitional blanks and vagueness within the IVDR, there is an opportunity for professional societies and associations to contribute their academic knowledge and expertise to the content design of the IVDR and to shape the IVDR to fulfill its purpose.
Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (2021) Stellungnahme und Handreichungen der Ad-hoc-Kommission In-vitro-Diagnostika der AWMF zur Umsetzung der Verordnung (EU) 2017/746 (IVDR) im Hinblick auf In-vitro-Diagnostika aus Eigenherstellung. https://www.awmf.org/die-awmf/kommissionen/nutzenbewertung/ad-hoc-kommission-bewertung-von-medizinprodukten/ad-hoc-kommission-in-vitro-diagnostik.html . Accessed 1 Mar 2022
Bank PCD, Jacobs LHJ, Van Den Berg SA et al (2020) The end of the laboratory developed test as we know it? Recommendations from a national multidisciplinary taskforce of laboratory specialists on the interpretation of the IVDR and its complications. Clin Chem Lab Med. https://doi.org/10.1515/cclm-2020-1384
Article PubMed Google Scholar
Biomedical Alliance in Europe (2021) Implementation of the new EU regulation for in vitro diagnostic medical devices: a ticking time bomb for the diagnostic sector. https://ehaweb.org/assets/BioMed-Alliance-IVDR-statement-final.pdf . Accessed 13 Jan 2022
Buchmaier B (2020) Die neue Europäische Verordnung über In-vitro-Diagnostika (IVDR) – ein Zwischenstand vol 2.2020
Google Scholar
DAkkS (2016) Leitfaden des Sektorkomitees Pathologie/Neuropathologie für die Validierung von Untersuchungsverfahren in der Immunhistologie. Kennung: 71 SD 4 028
DAkkS (2016) Leitfaden des Sektorkomitees Pathologie/Neuropathologie für die Validierung von Untersuchungsverfahren in der Molekularpathologie. Kennung: 71 SD 4 037
DAkkS (2017) Anforderungen der DIN EN ISO/IEC 17020:2012 und technische Kriterien für deren Anwendung zur Akkreditierung in der Pathologie/Neuropathologie. Kennung: 71 SD 4 001
DAkkS (2022) Fachbereich Pathologie. https://www.dakks.de/de/pathologie.html . Accessed 13 Jan 2022
EUDAMED (2021) Europäische Datenbank für Medizinprodukte. https://ec.europa.eu/tools/eudamed/#/screen/home . Accessed 13 Jan 2022
European Commission (2020) Factsheet for healthcare professionals and health institutions. https://health.ec.europa.eu/system/files/2020-09/healthcareprofessionals_factsheet_en_0.pdf . Accessed 19 Jan 2023
Medical Device Coordination Group, Medical Device Coordination Group Document MDCG 2023-1 (2023), Guidance on the health institution exemption under Article 5(5) of Regulation (EU) 2017/745 and Regulation (EU) 2017/746. https://health.ec.europa.eu/system/files/2023-01/mdcg_2023-1_en.pdf . Accessed 19 Jan 2023
European Commission, Nando (new approach notified and designated organisations) information system. https://ec.europa.eu/growth/tools-databases/nando/ . Accessed 19 Jan 2023
European Commission German representation (2021) Öffentliche Gesundheit: Kommission schlägt schrittweise Einführung der neuen Verordnung über In-vitro-Diagnostika vor. https://germany.representation.ec.europa.eu/news/medizinprodukte-kommission-schlagt-schrittweise-einfuhrung-der-neuen-regeln-zu-vitro-diagnostika-vor-2021-10-14_de . Accessed 19 Jan 2023
European Commission (2021) Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending Regulation (EU) 2017/746 as regards transitional provisions for certain in vitro diagnostic medical devices and deferred application of requirements for in-house devices. Accessed 19 Jan 2023
European Commission (2021) Progressive roll-out of the In Vitro Diagnostic Medical Devices Regulation. Press release. https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6965 . Accessed 19 Jan 2023
European Union, Types of legislation. https://european-union.europa.eu/institutions-law-budget/law/types-legislation_en . Accessed 19 Jan 2023
FDA (2018) Considerations for design, development, and analytical validation of next generation sequencing (NGS)—based in vitro diagnostics (IVDs) intended to aid in the diagnosis of suspected germline diseases. https://www.fda.gov/media/99208/download . Accessed 13 Jan 2022 (Guidance for stakeholders and food and drug administration staff)
Jennings LJ, Arcila ME, Corless C et al (2017) Guidelines for validation of next-generation sequencing-based oncology panels: a joint consensus recommendation of the Association for Molecular Pathology and College of American Pathologists. J Mol Diagn 19:341–365
Lubbers BR, Schilhabel A, Cobbaert CM et al (2021) The new EU regulation on in vitro diagnostic medical devices: implications and preparatory actions for diagnostic laboratories. Hemasphere 5:e568
Article PubMed PubMed Central Google Scholar
MedTech Europe (2021) MedTech Europe survey report analysing the availability of in vitro diagnostic medical devices (IVDs) in May 2022 when the new EU IVD regulation applies. https://www.medtecheurope.org/wp-content/uploads/2021/09/medtech-europe-survey-report-analysing-the-availability-of-in-vitro-diagnostic-medical-devices-ivds-in-may-2022-when-the-new-eu-ivd-regulation-applies-8-september-2021.pdf . Accessed 13 Jan 2022
Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices
Richtlinie der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen (RiLi-BÄK-2019)
Scheiblauer H, Filomena A, Nitsche A et al (2021) Comparative sensitivity evaluation for 122 CE-marked rapid diagnostic tests for SARS-CoV‑2 antigen, Germany, September 2020 to April 2021. Euro Surveill 26(44):2100441
Article PubMed PubMed Central CAS Google Scholar
Spitzenberger F, Patel J, Gebuhr I, Kruttwig K, Safi A, Meisel C (2022) Laboratory-developed tests: design of a regulatory strategy in compliance with the international state-of-the-art and the regulation (EU) 2017/746 (EU IVDR [in vitro diagnostic medical device regulation]). Ther Innov Regul Sci 56(1):47–64
Stenzinger A, Weichert W (2020) Impact of the novel in vitro diagnostic regulation (IVDR) of the European Union on pathology laboratories. What’s important? Pathologe 41:129–133
Strom SP (2016) Current practices and guidelines for clinical next-generation sequencing oncology testing. Cancer Biol Med 13:3–11
Task Force IVDR (2020) Lab-developed tests—guidance on use of lab-developed tests as described in REGULATION (EU) 2017/746 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 5 April 2017on in vitro diagnostic medical devices. https://pathology.nl/wp-content/uploads/2021/07/Guidance_use_LDT_IVDR_taskforce_vs2.0-final-10122020.pdf . Accessed 13 Jan 2022
Verband der Diagnostica-Industrie VDGH (2021) Europaweite Studie: Versorgung mit Labordiagnostik ab Mai 2022 gefährdet – VDGH sieht sofortigen Handlungsbedarf für die EU-Kommission. https://www.vdgh.de/media/file/40121.PM_VDGH_Versorgung_mit_Labordiagnostik_gefaehrdet_130921.pdf . Accessed 13 Jan 2022
Vermeersch P, Van Aelst T, Dequeker EMC (2021) The new IVD regulation 2017/746: a case study at a large university hospital laboratory in Belgium demonstrates the need for clarification on the degrees of freedom laboratories have to use lab-developed tests to improve patient care. Clin Chem Lab Med 59(1):101–106
Article CAS Google Scholar
Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC (Text with EEA relevance)
Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU (Text with EEA relevance)
Download references
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany
Andy Kahles, Hannah Goldschmid, Anna-Lena Volckmar, Carolin Ploeger, Daniel Kazdal, Roland Penzel, Jan Budczies, Christa Flechtenmacher, Peter Schirmacher & Albrecht Stenzinger
Professional Association of German Pathologists, Bundesverband Deutscher Pathologen e. V., Berlin, Germany
Gisela Kempny & Marlon Kazmierczak
Department of Pathology, University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany
Gustavo Baretton
Institute of Pathology, Technical University of Munich, Munich, Germany
Wilko Weichert
Institute of Pathology, Charité—Universitätsmedizin Berlin, Berlin, Germany
David Horst
Institute of Pathology, Ludwig-Maximilian University of Munich, Munich, Germany
Frederick Klauschen
Faculty of Law, University of Augsburg, Augsburg, Germany
Ulrich M. Gassner
2nd Internal Medicine Department, Hematology Lab Kiel, University Hospital Schleswig-Holstein (UKSH), Kiel, Germany
Monika Brüggemann
Institute of Laboratory Medicine, Ludwig-Maximilian University of Munich, Munich, Germany
Michael Vogeser
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Andy Kahles or Albrecht Stenzinger .
Ethics declarations
Conflict of interest.
A.-L. Volckmar: personal fees from AstraZeneca outside the submitted work. D. Kazdal: personal fees from AstraZeneca, Bristol-Myers Squibb, Pfizer, Lilly, Agilent, and Takeda outside the submitted work. W. Weichert: advisory boards and invited speaker for Roche, MSD, BMS, AstraZeneca, Pfizer, Merck, Lilly, Boehringer, Novartis, Takeda, Bayer, Janssen, Amgen, Astellas, Illumina, Eisai, Siemens, Agilent, ADC, GSK, and Molecular Health; research support by Roche, MSD, BMS, and AstraZeneca. M. Brüggemann: advisory boards and invited speaker for Amgen, BD, Janssen, art tempi communications, Pfizer, Research support by Amgen. A. Stenzinger: advisory board/speaker: AGCT, Aignostics, Astra Zeneca, Bayer, BMS, Eli Lilly, Illumina, Incyte, Janssen, MSD, Novartis, Pfizer, Roche, Seattle Genetics, Takeda, Thermo Fisher; grants: Bayer, BMS, Chugai, Incyte. P. Schirmacher: Speakers bureau: Incyte, Roche, Janssen, Novartis, AstraZeneca, BMS, Eisai, Board: Incyte, Roche, AstraZeneca, BMS, MSD, Amgen, Janssen, Novartis, Bayer, Eisai, Grant support: Novartis, BMS, AstraZeneca, Illumina, Gilead, Falk. A. Kahles, H. Goldschmid, C. Ploeger, R. Penzel, J. Budczies, G. Kempny, M. Kazmierczak, C. Flechtenmacher, G. Baretton, D. Horst, F. Klauschen, U.M. Gassner and M. Vogeser declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
The supplement containing this article is not sponsored by industry.
Additional information

Scan QR code & read article online
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Kahles, A., Goldschmid, H., Volckmar, AL. et al. Structure and content of the EU-IVDR. Pathologie 44 (Suppl 2), 73–85 (2023). https://doi.org/10.1007/s00292-022-01176-z
Download citation
Accepted : 20 December 2022
Published : 03 February 2023
Issue Date : November 2023
DOI : https://doi.org/10.1007/s00292-022-01176-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diagnostic reagent kits
- Quality of healthcare
- Government regulation
- In-house production
- Laboratory-developed tests
- Find a journal
- Publish with us
- Track your research
Avance IVDr
Furthering human disease research
with powerful technology

Standardized nuclear magnetic resonance (NMR) spectroscopy platform enabling cost-effective, high-performance NMR pre-clinical screening and IVD-by-NMR discovery and validation (on RUO Level) of novel NMR assays. The new AVANCE IVDr system, presently for research use only, is a complete, proven and standardized platform for NMR pre-clinical research and screening, as well as for IVD-by-NMR research. It features high sensitivity and information-rich output at 600 MHz proton-NMR frequency, and incorporates advanced hardware, software, automation, spectral libraries and standard operating procedures (SOPs) for high-performance biofluid assay validation and pre-clinical screening. Customer benefits include higher information content and spectral feature differentiation compared to low-field NMR systems, as well as excellent reproducibility, high throughput and potentially dramatically lower cost per sample for better preparation and support of clinical screening and IVD-by-NMR discovery and validation (on RUO level).
- Minimal sample preparationn
- Non-destructive measurement
- Full automation in high throughput screening mode
- Highest reproducibility and data quality from sample to sample and instrument to instrument
- Quantification of large sets of metabolites with automated reporting Classification and discriminiation using statistical tools with automated reporting
- Low cost per sample and lowest cost per parameter, with small footprint to suit every lab
- No complex sample preparation or sample identification required
- Ease of use – no need for operator expertise in NMR
- Highest reproducibility and data quality under full automation – anytime & anywhere
- Handling of large data sets with simple retrieval of information
- Rapid transfer from spectra to relevant information
- Retrospective analysis
- Integration of spectra and results from different groups world wide

B.I. Methods

B.I.QUANT-UR
B.I. Quant-PS
B.I. BioBank QC

B.I. IEM Panel
Avance ivdr applications, applications, specifications.
High Performance and Throughput IVD-by-NMR Research Paving the way for worldwide epidemiological studies as well as for preclinical research. Dedicated to NMR-based pre-clinical in vitro screening and research, the AVANCE IVDr is optimized for ease-of-use and highest data quality, reliability and reproducibility as shown in the figure on the right side. The new standardized platform offers barcode analysis, control by a LIMS system, the high-throughput autosampler SampleJet™, remote access, and automatic analysis and customizable analytical result reporting. Based on Bruker-validated SOPs, the AVANCE IVDr platform enables the development of future potential diagnostic tools for body-fluids that can address a variety of medical questions. The SOPs guarantee the preparation of highly reproducible clinical data, enabling the exchange and validation of novel NMR assays between laboratories on a global basis. In a translational clinical research environment the results produced by these NMR assays can easily be transferred into potential clinical screening and future IVD use. This level of large-scale health-related NMR screening is paving the way for worldwide epidemiological studies as well as for pre-clinical in vitro research. The benefits have been significant: facilitated by the low cost per sample and the even lower cost per parameter as compared to established single parameter screening methods. Novel NMR methods for determining the cause of disease, delivering individualized patient treatment are now enabling many clinical researchers for developing strategies for prevention.

Scientific On-Demand Webinars
Testimonials.
More Information
- IVDr Research by NMR - Brochure.pdf
- T165319_IVDr-overview-brochure.pdf
- B.I.-Methods - Flyer.pdf
- B.I.QUANT-UR - Flyer.pdf
- B.I.BioBankTool Bruker IVDr BioBankTool - Application Note.pdf
- B.I.QUANT-PS Bruker IVDr Quantification in Plasma Serum - Application Note.pdf
- B.I.QUANT-UR - Application Note.pdf
- Enabling Tools on the IVDr Platform - Application Note.pdf
Service & Life Cycle Support for Magnetic Resonance and Preclinical Imaging
Bruker’s commitment to provide customers with unparalleled help throughout the buying cycle, from initial inquiry to evaluation, installation, and the lifetime of the instrument is now characterized by the LabScape service concept.
LabScape Maintenance Agreements, On-Site On-Demand and Enhance Your Lab are designed to offer a new approach to maintenance and service for the modern laboratory
- Download LabScape Brochure
Contact Information

Contact Sales

Contact Customer Support
In Vitro Diagnostic Regulation (IVDR)
All you need to know about the IVDR that comes into force on 26 May 2022
- May 20, 2022
- 4 min Reading time

As of May 26, 2022, the new European Union IVDR regulation regarding medical devices will enter into force , replacing the EU IVD Directive (I VDD ) . IVDR stands for I n V itro D iagnostic R egulation. I n vitro diagnostics can be described as used to identify illness by testing body samples like blood, tissue, or urine. These tests take place outside of the body. This new EU regulation aims to increase the safety and performance of these medical devices for both patients and users. Implementing this new regulation meant a lot of wor k including implementing many new procedures. You can find more information about IVDR , how it might affect you and how to be compliant .
You can watch the below 6 minutes long webinar where Tim Kool explains the key elements and implications of IVDR.
Main differences between IVDD and IVDR

IVDR is approximately four times longer than the IVDD, which can also be seen in the image above. There are several points that every IVD manufacturer should be aware of:
- Only manufacturers of non-sterile Class A devices will be able to self-certify , meaning that anywhere from 80-90% of the medical devices will require CE certification by a Notified Body.
- There are more post-market surveillance requirements in IVDR , and they are more rigorous than those in the IVDD.
- Provisions for software are also included in IVDR. Software a s part of an IVD, software as a medical device ( SaMD ) and apps are all regulated under IVDR .
For further reference, t here is also a more extensive explanation of the differences between IVDD and IVDR .
What does it mean for pathology professionals ?
Under the IVDD , product s used for Research Use Only (RUO) and Lab-Developed Tests (LDTs) were considered outside the scope of the directive. IVDR however also regulates LDTs. RUO products are still considered outside the scope of the IVDR, provided the product is used for research purposes only and not for the benefit of a specific patient. If they are placed on the market as IVD by the man u facturer , they come within the scope of the IVDR under LDTs.
This makes IVDR, to a certain extent, also applicable to entities using RUO products or creating LDTs in-house for diagnostic purposes .
LDTs under IVDR
Based on the guidance issued, the following products are as LDTs considered within the scope of IVDR:
- An in-house developed and produced test
- Tests that are labelled Research Use Only (RUO) but used for diagnostics
- A CE-marked test to which adjustments are made (intended purpose , or protocols)

IVDR requirements on LTDs
A developer of LDTs is considered a manufacturer of an IVD under IVDR, and specific requirements of the IVDR legislation apply to those health institutions. IVDR – Article 5(5) – defines the requirements that need to be fulfilled for these LDTs and the health institu tions that create and use them to comply with IVDR .
In order for LDTs not to be considered IVDs all of the following requirements need to be met :
- the LDTs are not transferred to another legal entity;
- manufacture and use of the LDTs occur s in a controlled manner, i.e. under control of a Quality Management System ;
- the laboratory of the health institution is compliant with standard EN ISO 15189 or where applicable national provisions, including national provisions regarding accreditation;
- the health institution justifies in its documentation that the target patient group's specific needs cannot be met , or not sufficiently with an existing device .
- the health institution provides information upon request on the use of such devices to its competent authority, which shall include a justification of their manufacturing, any modification s made, and use;
- the name and address of the manufacturing health institution,
- the details necessary to identify the devices ,
- a declaration that the devices meet the general safety and performance requirements of the IVDR , including information on which requirements are not fully met with a reasoned justification therefore.
If any of the requirements above is not applicable or cannot be met , and the tests are used for diagnostics purposes on human tissue , the full responsibilities of the IVDR apply.
We support your lab in advanced staining
We achieve standardisation by using one protocol as the way to achieve optimal quality. Simplify your lab accreditation & IVDR compliance with our quality-controlled system, designed and tested to perform optimally. Yet, when we speak of quality, it is not just about our technology and innovation. It is about who we collaborate with. NordiQC is an independent external quality assessment partner that allows us to provide you with a wide range of optimal only biomarkers. Their quality assurance sets the foundation for everything we do. You can therefore have the confidence, using optimal only biomarkers for the best profiling, receiving the optimal slide every time.
If you have any questions, please contact us at [email protected]. For further information please visit :
- European commission
- Guidance_use_LDT_IVDR_taskforce (pathology.nl)
- The end of the laboratory developed test as we know it? Recommendations from a national multidisciplinary taskforce of laboratory specialists on the interpretation of the IVDR and its complications (researchgate.net)
EMWEB0003-01
We use cookies that are necessary for the website to function as well as possible. By clicking accept all cookies, you agree to this.
Cookie preferences
Choose which cookie profile suits you best. We will handle your data with care. You can check our privacy statement for more information on how we manage your data.
- Visit GenomeWeb on Twitter
- Visit GenomeWeb on LinkedIn
Request an Annual Quote
FDA Releases Guidance on Research Use, Investigational Use Only IVDs
NEW YORK (GenomeWeb News) – The Food and Drug Administration has released a guidance document that lays out and clarifies the rules for how in vitro diagnostic products for research use only (RUO) and investigational use only (IUO) may be used, labeled, or marketed.
FDA created the guidance on RUOs and IUOs, which has been in development for several years , because it is concerned that unapproved or uncleared IVDs are being used for clinical diagnostic use, even though their performance characteristics and manufacturing controls have not met the agency's clinical standards.
The agency allows an investigational device exemption (IDE) for medical devices that enables them to be used in research without receiving premarket approval or 510(k) clearance, but a lack of clarity in the exemption has created a loophole that makes it possible for RUOs and IOUs to seep into clinical use.
The worry is that healthcare providers could be misled about the approved applications for RUO and IUO tests, and patients could be harmed. To avoid such harms, FDA wants this guidance to inform IVD makers on how to comply and ensure that devices are being used as they were intended.
The new FDA document covers the appropriate and inappropriate uses for IVDs, as well as requirements for their labeling, manufacturing, and marketing.
To qualify as an IDE, devices must be non-invasive, must not be used as a diagnostic procedure without confirmation of the diagnosis by another medically established diagnostic, and must not require an invasive sampling procedure that presents a risk.
The guidance states that RUOs must be labeled, "For Research Use Only. Not for use in Diagnostic Procedures." IUOs must be labeled, "For Investigational Use Only. The performance characteristics of this product have not been established.''
Product labeling alone may not keep IVDs from being marketed for clinical uses, so the guidance provides details regarding how the RUOs and IUOs may be marketed, and the kinds of promotional statements that would conflict with the devices' RUO and IUO status.
Filed under
New products posted to genomeweb: speedx, vizgen, dovetail genomics, meridian bioscience, more, people in the news at stanford, illumina, bio-rad, novacyt, scale biosciences, one biosciences, more, exome sequencing leads to diagnosis for 12 percent of infertile men in estonian study, european commission approves illumina's plan for divesting grail, oncocyte, bio-rad partner to globally launch transplant test, consortium hopes to drive adoption of next-gen sequencing for infectious disease diagnostics premium, multi-cancer screening testmakers share updates at aacr as field grapples with limitations premium.
- Blog | The CURRENT
Healthcare and Life Sciences Taking Research Use Only Products from the Research Lab to the IVD Market
Dick Rubin has over 30 years of experience in the lab instrument industry, an educational background in chemistry and a certificate in FDA regulatory affairs. As a Senior Manager of Engineering Solutions at Plexus, Dick works with Plexus engineering staff to draft development solutions and supports active development projects to ensure Plexus’ commitment to flawless execution.
Q: What trends are you noticing in the market right now?
A: Scientific innovation across the life sciences industry is driving the rapid expansion of the instrumentation market with products that provide better results, faster. A pull for more point-of-care and home-use technology is also driving innovation. In particular, we’re seeing more companies looking to move instruments from Research Use Only (RUO) applications to pursue in vitro diagnostic (IVD) applications with the same instrument.
This is a big undertaking, which is why companies find value in working with Plexus. Commercializing an RUO solution for the IVD market comes with challenges, such as greater regulatory demands and finding the optimum balance between high quality and cost-effectiveness. Realizing such products requires a unique, technical skill set and strong understanding of the regulatory environment.
Q: How do you help companies make the leap from the lab to the clinical market?
A: With expertise across the product lifecycle, from initial design to manufacturing to aftermarket services, Plexus can partner with the customer’s clinical team to address any documentation gaps before categorizing the assay, or instrument, as an IVD device capable of clinical applications.
Our team brings deep familiarity with the complex regulatory environment that life sciences products need to navigate for market clearance. We have decades of experience developing healthcare and life sciences products ranging from Class I to Class III, and continually invest in our regulatory and compliance expertise to ensure the safety, dependability and quality of the products we support.
Q: Can you describe an instance where you helped a customer bring an RUO instrument to market?
A: We recently helped a customer transition an RUO instrument to IVD application for COVID-19 in the near term, and other diagnostic applications afterward. This involved critical analysis and partial redesigning of a system that was originally built for lab use to obtain IVD certification. It may sound simple, but one thing that’s frequently missed in RUO product development is a rigorous quality system for documentation of initial design inputs, and the subsequent crosscheck to ensure design outputs meet the input objectives.
For this application especially, time was precious. We leveraged as much of the system’s existing components as possible in the redesign to save time. Using a systems engineering approach, we brought our full depth of experience to the table to get this project to market quickly. We also worked to ensure the customer met all regulatory requirements, including EMC, electrical safety and IEC 62304.
Related Articles:
Aftermarket services for life sciences: finding the right partner for products in the market, life science commercialization: getting complex instruments to market faster, a valuable framework to evaluate maturity of technology for life sciences.
Read our research on: Gun Policy | International Conflict | Election 2024
Regions & Countries
9 facts about americans and marijuana.

The use and possession of marijuana is illegal under U.S. federal law, but about three-quarters of states have legalized the drug for medical or recreational purposes. The changing legal landscape has coincided with a decades-long rise in public support for legalization, which a majority of Americans now favor.
Here are nine facts about Americans’ views of and experiences with marijuana, based on Pew Research Center surveys and other sources.
As more states legalize marijuana, Pew Research Center looked at Americans’ opinions on legalization and how these views have changed over time.
Data comes from surveys by the Center, Gallup , and the 2022 National Survey on Drug Use and Health from the U.S. Substance Abuse and Mental Health Services Administration. Information about the jurisdictions where marijuana is legal at the state level comes from the National Organization for the Reform of Marijuana Laws .
More information about the Center surveys cited in the analysis, including the questions asked and their methodologies, can be found at the links in the text.
Around nine-in-ten Americans say marijuana should be legal for medical or recreational use, according to a January 2024 Pew Research Center survey . An overwhelming majority of U.S. adults (88%) say either that marijuana should be legal for medical use only (32%) or that it should be legal for medical and recreational use (57%). Just 11% say the drug should not be legal in any form. These views have held relatively steady over the past five years.
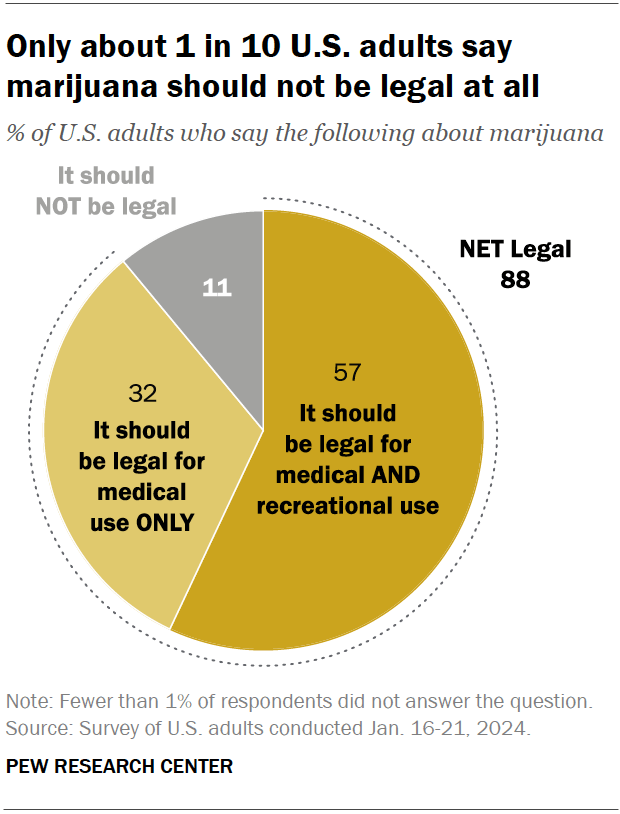
Views on marijuana legalization differ widely by age, political party, and race and ethnicity, the January survey shows.

While small shares across demographic groups say marijuana should not be legal at all, those least likely to favor it for both medical and recreational use include:
- Older adults: 31% of adults ages 75 and older support marijuana legalization for medical and recreational purposes, compared with half of those ages 65 to 74, the next youngest age category. By contrast, 71% of adults under 30 support legalization for both uses.
- Republicans and GOP-leaning independents: 42% of Republicans favor legalizing marijuana for both uses, compared with 72% of Democrats and Democratic leaners. Ideological differences exist as well: Within both parties, those who are more conservative are less likely to support legalization.
- Hispanic and Asian Americans: 45% in each group support legalizing the drug for medical and recreational use. Larger shares of Black (65%) and White (59%) adults hold this view.
Support for marijuana legalization has increased dramatically over the last two decades. In addition to asking specifically about medical and recreational use of the drug, both the Center and Gallup have asked Americans about legalizing marijuana use in a general way. Gallup asked this question most recently, in 2023. That year, 70% of adults expressed support for legalization, more than double the share who said they favored it in 2000.
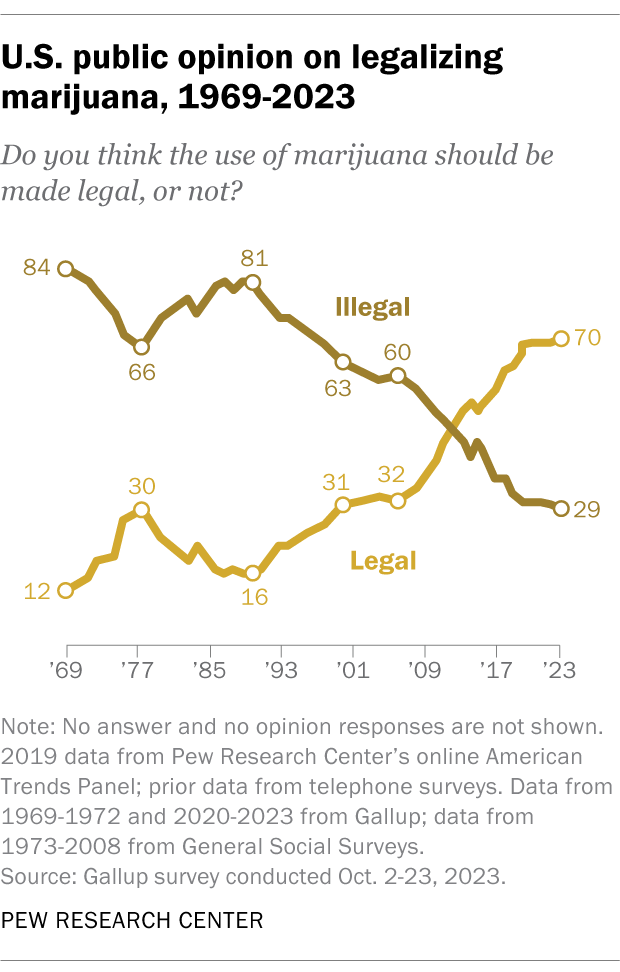
Half of U.S. adults (50.3%) say they have ever used marijuana, according to the 2022 National Survey on Drug Use and Health . That is a smaller share than the 84.1% who say they have ever consumed alcohol and the 64.8% who have ever used tobacco products or vaped nicotine.
While many Americans say they have used marijuana in their lifetime, far fewer are current users, according to the same survey. In 2022, 23.0% of adults said they had used the drug in the past year, while 15.9% said they had used it in the past month.
While many Americans say legalizing recreational marijuana has economic and criminal justice benefits, views on these and other impacts vary, the Center’s January survey shows.
- Economic benefits: About half of adults (52%) say that legalizing recreational marijuana is good for local economies, while 17% say it is bad. Another 29% say it has no impact.
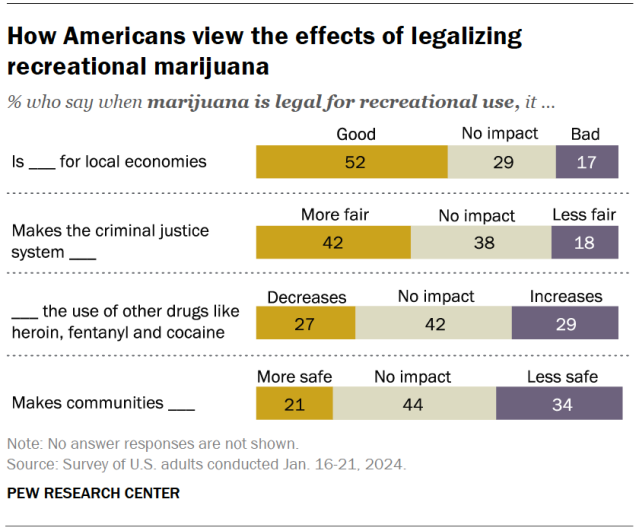
- Criminal justice system fairness: 42% of Americans say legalizing marijuana for recreational use makes the criminal justice system fairer, compared with 18% who say it makes the system less fair. About four-in-ten (38%) say it has no impact.
- Use of other drugs: 27% say this policy decreases the use of other drugs like heroin, fentanyl and cocaine, and 29% say it increases it. But the largest share (42%) say it has no effect on other drug use.
- Community safety: 21% say recreational legalization makes communities safer and 34% say it makes them less safe. Another 44% say it doesn’t impact safety.
Democrats and adults under 50 are more likely than Republicans and those in older age groups to say legalizing marijuana has positive impacts in each of these areas.
Most Americans support easing penalties for people with marijuana convictions, an October 2021 Center survey found . Two-thirds of adults say they favor releasing people from prison who are being held for marijuana-related offenses only, including 41% who strongly favor this. And 61% support removing or expunging marijuana-related offenses from people’s criminal records.
Younger adults, Democrats and Black Americans are especially likely to support these changes. For instance, 74% of Black adults favor releasing people from prison who are being held only for marijuana-related offenses, and just as many favor removing or expunging marijuana-related offenses from criminal records.
Twenty-four states and the District of Columbia have legalized small amounts of marijuana for both medical and recreational use as of March 2024, according to the National Organization for the Reform of Marijuana Laws (NORML), an advocacy group that tracks state-level legislation on the issue. Another 14 states have legalized the drug for medical use only.
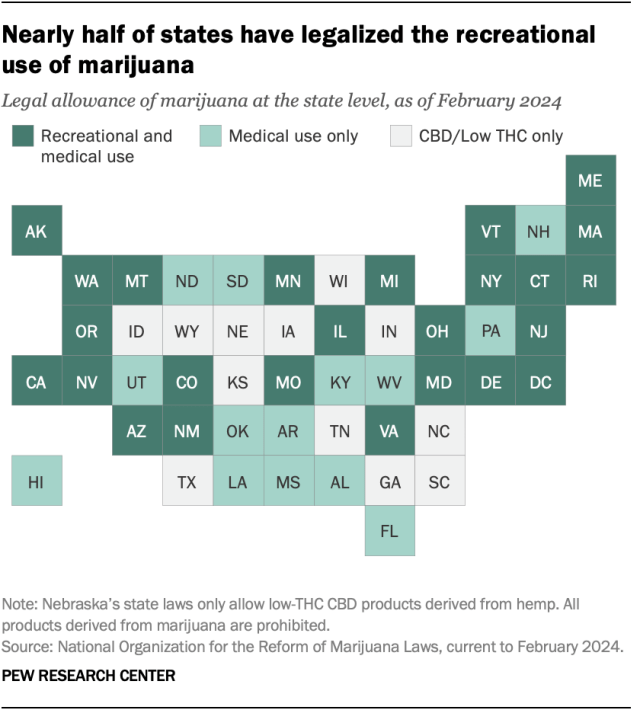
Of the remaining 12 states, all allow limited access to products such as CBD oil that contain little to no THC – the main psychoactive substance in cannabis. And 26 states overall have at least partially decriminalized recreational marijuana use , as has the District of Columbia.
In addition to 24 states and D.C., the U.S. Virgin Islands , Guam and the Northern Mariana Islands have legalized marijuana for medical and recreational use.
More than half of Americans (54%) live in a state where both recreational and medical marijuana are legal, and 74% live in a state where it’s legal either for both purposes or medical use only, according to a February Center analysis of data from the Census Bureau and other outside sources. This analysis looked at state-level legislation in all 50 states and the District of Columbia.
In 2012, Colorado and Washington became the first states to pass legislation legalizing recreational marijuana.
About eight-in-ten Americans (79%) live in a county with at least one cannabis dispensary, according to the February analysis. There are nearly 15,000 marijuana dispensaries nationwide, and 76% are in states (including D.C.) where recreational use is legal. Another 23% are in medical marijuana-only states, and 1% are in states that have made legal allowances for low-percentage THC or CBD-only products.
The states with the largest number of dispensaries include California, Oklahoma, Florida, Colorado and Michigan.
Note: This is an update of a post originally published April 26, 2021, and updated April 13, 2023.

Sign up for our weekly newsletter
Fresh data delivered Saturday mornings
Americans overwhelmingly say marijuana should be legal for medical or recreational use
Religious americans are less likely to endorse legal marijuana for recreational use, four-in-ten u.s. drug arrests in 2018 were for marijuana offenses – mostly possession, two-thirds of americans support marijuana legalization, most popular.
About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .

Why Taiwan Was So Prepared for a Powerful Earthquake
Decades of learning from disasters, tightening building codes and increasing public awareness may have helped its people better weather strong quakes.
Search-and-rescue teams recover a body from a leaning building in Hualien, Taiwan. Thanks to improvements in building codes after past earthquakes, many structures withstood Wednesday’s quake. Credit...
Supported by
- Share full article
By Chris Buckley , Meaghan Tobin and Siyi Zhao
Photographs by Lam Yik Fei
Chris Buckley reported from the city of Hualien, Meaghan Tobin from Taipei, in Taiwan.
- April 4, 2024
When the largest earthquake in Taiwan in half a century struck off its east coast, the buildings in the closest city, Hualien, swayed and rocked. As more than 300 aftershocks rocked the island over the next 24 hours to Thursday morning, the buildings shook again and again.
But for the most part, they stood.
Even the two buildings that suffered the most damage remained largely intact, allowing residents to climb to safety out the windows of upper stories. One of them, the rounded, red brick Uranus Building, which leaned precariously after its first floors collapsed, was mostly drawing curious onlookers.
The building is a reminder of how much Taiwan has prepared for disasters like the magnitude-7.4 earthquake that jolted the island on Wednesday. Perhaps because of improvements in building codes, greater public awareness and highly trained search-and-rescue operations — and, likely, a dose of good luck — the casualty figures were relatively low. By Thursday, 10 people had died and more than 1,000 others were injured. Several dozen were missing.
“Similar level earthquakes in other societies have killed far more people,” said Daniel Aldrich , a director of the Global Resilience Institute at Northeastern University. Of Taiwan, he added: “And most of these deaths, it seems, have come from rock slides and boulders, rather than building collapses.”
Across the island, rail traffic had resumed by Thursday, including trains to Hualien. Workers who had been stuck in a rock quarry were lifted out by helicopter. Roads were slowly being repaired. Hundreds of people were stranded at a hotel near a national park because of a blocked road, but they were visited by rescuers and medics.

On Thursday in Hualien city, the area around the Uranus Building was sealed off, while construction workers tried to prevent the leaning structure from toppling completely. First they placed three-legged concrete blocks that resembled giant Lego pieces in front of the building, and then they piled dirt and rocks on top of those blocks with excavators.
“We came to see for ourselves how serious it was, why it has tilted,” said Chang Mei-chu, 66, a retiree who rode a scooter with her husband Lai Yung-chi, 72, to the building on Thursday. Mr. Lai said he was a retired builder who used to install power and water pipes in buildings, and so he knew about building standards. The couple’s apartment, near Hualien’s train station, had not been badly damaged, he said.
“I wasn’t worried about our building, because I know they paid attention to earthquake resistance when building it. I watched them pour the cement to make sure,” Mr. Lai said. “There have been improvements. After each earthquake, they raise the standards some more.”
It was possible to walk for city blocks without seeing clear signs of the powerful earthquake. Many buildings remained intact, some of them old and weather-worn; others modern, multistory concrete-and-glass structures. Shops were open, selling coffee, ice cream and betel nuts. Next to the Uranus Building, a popular night market with food stalls offering fried seafood, dumplings and sweets was up and running by Thursday evening.
Earthquakes are unavoidable in Taiwan, which sits on multiple active faults. Decades of work learning from other disasters, implementing strict building codes and increasing public awareness have gone into helping its people weather frequent strong quakes.
Not far from the Uranus Building, for example, officials had inspected a building with cracked pillars and concluded that it was dangerous to stay in. Residents were given 15 minutes to dash inside and retrieve as many belongings as they could. Some ran out with computers, while others threw bags of clothes out of windows onto the street, which was also still littered with broken glass and cement fragments from the quake.
One of its residents, Chen Ching-ming, a preacher at a church next door, said he thought the building might be torn down. He was able to salvage a TV and some bedding, which now sat on the sidewalk, and was preparing to go back in for more. “I’ll lose a lot of valuable things — a fridge, a microwave, a washing machine,” he said. “All gone.”
Requirements for earthquake resistance have been built into Taiwan’s building codes since 1974. In the decades since, the writers of Taiwan’s building code also applied lessons learned from other major earthquakes around the world, including in Mexico and Los Angeles, to strengthen Taiwan’s code.
After more than 2,400 people were killed and at least 10,000 others injured during the Chi-Chi quake of 1999, thousands of buildings built before the quake were reviewed and reinforced. After another strong quake in 2018 in Hualien, the government ordered a new round of building inspections. Since then, multiple updates to the building code have been released.
“We have retrofitted more than 10,000 school buildings in the last 20 years,” said Chung-Che Chou, the director general of the National Center for Research on Earthquake Engineering in Taipei.
The government had also helped reinforce private apartment buildings over the past six years by adding new steel braces and increasing column and beam sizes, Dr. Chou said. Not far from the buildings that partially collapsed in Hualien, some of the older buildings that had been retrofitted in this way survived Wednesday’s quake, he said.
The result of all this is that even Taiwan’s tallest skyscrapers can withstand regular seismic jolts. The capital city’s most iconic building, Taipei 101, once the tallest building in the world, was engineered to stand through typhoon winds and frequent quakes. Still, some experts say that more needs to be done to either strengthen or demolish structures that don’t meet standards, and such calls have grown louder in the wake of the latest earthquake.
Taiwan has another major reason to protect its infrastructure: It is home to the majority of production for the Taiwan Semiconductor Manufacturing Company, the world’s largest maker of advanced computer chips. The supply chain for electronics from smartphones to cars to fighter jets rests on the output of TSMC’s factories, which make these chips in facilities that cost billions of dollars to build.
The 1999 quake also prompted TSMC to take extra steps to insulate its factories from earthquake damage. The company made major structural adjustments and adopted new technologies like early warning systems. When another large quake struck the southern city of Kaohsiung in February 2016, TSMC’s two nearby factories survived without structural damage.
Taiwan has made strides in its response to disasters, experts say. In the first 24 hours after the quake, rescuers freed hundreds of people who were trapped in cars in between rockfalls on the highway and stranded on mountain ledges in rock quarries.
“After years of hard work on capacity building, the overall performance of the island has improved significantly,” said Bruce Wong, an emergency management consultant in Hong Kong. Taiwan’s rescue teams have come to specialize in complex efforts, he said, and it has also been able to tap the skills of trained volunteers.

Taiwan’s resilience also stems from a strong civil society that is involved in public preparedness for disasters.
Ou Chi-hu, a member of a group of Taiwanese military veterans, was helping distribute water and other supplies at a school that was serving as a shelter for displaced residents in Hualien. He said that people had learned from the 1999 earthquake how to be more prepared.
“They know to shelter in a corner of the room or somewhere else safer,” he said. Many residents also keep a bag of essentials next to their beds, and own fire extinguishers, he added.
Around him, a dozen or so other charities and groups were offering residents food, money, counseling and childcare. The Tzu Chi Foundation, a large Taiwanese Buddhist charity, provided tents for families to use inside the school hall so they could have more privacy. Huang Yu-chi, a disaster relief manager with the foundation, said nonprofits had learned from earlier disasters.
“Now we’re more systematic and have a better idea of disaster prevention,” Mr. Huang said.
Mike Ives contributed reporting from Seoul.
Chris Buckley , the chief China correspondent for The Times, reports on China and Taiwan from Taipei, focused on politics, social change and security and military issues. More about Chris Buckley
Meaghan Tobin is a technology correspondent for The Times based in Taipei, covering business and tech stories in Asia with a focus on China. More about Meaghan Tobin
Siyi Zhao is a reporter and researcher who covers news in mainland China for The Times in Seoul. More about Siyi Zhao
Advertisement
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Diagnostics (Basel)
- PMC10529212

IVDR: Analysis of the Social, Economic, and Practical Consequences of the Application of an Ordinance of the In Vitro Diagnostic Ordinance in Switzerland
Alix t. coste.
1 Institute of Microbiology, University of Lausanne & University Hospital Center, 1011 Lausanne, Switzerland; [email protected]
Adrian Egli
2 Institute of Medical Microbiology, University of Zurich, 8006 Zurich, Switzerland; hc.hzu.mmi@ilgea (A.E.); hc.hzu.mmi@nednibza (A.Z.)
Jacques Schrenzel
3 Bacteriology Laboratory, Service of Laboratory Medicine, Department of Diagnostic, Geneva University Hospitals, 1211 Geneva, Switzerland; [email protected]
Beatrice Nickel
4 Swiss Tropical and Public Health Institute, 4123 Allschwil, Switzerland; [email protected]
5 University of Basel, 4002 Basel, Switzerland
Andrea Zbinden
Reto lienhard.
6 ADMed Microbiologie Laboratory, 2300 La Chaux-de-Fonds, Switzerland; [email protected]
Alexis Dumoulin
7 Department of Infectious Diseases, Institut Central des Hôpitaux, Hôpital du Valais, 1950 Sion, Switzerland; [email protected]
Martin Risch
8 Dr Risch Medical Laboratory, Wuhrstrasse 14, 9490 Vaduz, Switzerland; [email protected]
Gilbert Greub
9 Infectious Disease Service, University of Lausanne & University Hospital Center, 1011 Lausanne, Switzerland
IVDR regulation represents a major challenge for diagnostic microbiology laboratories. IVDR complicates a broad range of aspects and poses a risk given the high diversity of pathogens (including rare but highly virulent microbes) and the large variety of samples submitted for analysis. The regular emergence of new pathogens (including Echovirus E-11, Adenovirus 41, Monkeypox virus, Alongshan virus, and Enterovirus D68, as recent examples in Europe in the post SARS-CoV-2 era) is another factor that makes IVDR regulation risky, because its detrimental effect on production of in-house tests will negatively impact knowledge and expertise in the development of new diagnostic tests. Moreover, such regulations negatively impact the availability of diagnostic tests, especially for neglected pathogens, and has a detrimental effect on the overall costs of the tests. The increased regulatory burden of IVDR may thereby pose an important risk for public health. Taken together, it will have a negative impact on the financial balance of diagnostic microbiology laboratories (especially small ones). The already-high standards of quality management of all ISO-accredited and Swissmedic-authorized laboratories render IVDR law of little value, at least in Switzerland, while tremendously increasing the regulatory burden and associated costs. Eventually, patients will need to pay for diagnostic assays outside of the framework of their insurance in order to obtain a proper diagnostic assessment, which may result in social inequity. Thus, based on the risk assessment outlined above, the coordinated commission for clinical microbiology proposes adjusting the IvDO ordinance by (i) introducing an obligation to be ISO 15189 accredited and (ii) not implementing the IvDO 2028 milestone.
1. Introduction
On 5 April 2017, the European Parliament approved the new Regulation on in Vitro Diagnostic Medical Devices (IVDR Regulation (EU) 2017/746) [ 1 ], which replaces the previous regulation known as the IVDD directive. The new regulation aims to introduce stricter conformity assessment procedures and performance studies to ensure the safety and quality of in vitro medical devices (IVDs). The Swiss Federal Council, to harmonize with the European Union (EU), implemented these changes by adopting the new Ordinance on in Vitro Diagnostic Medical Devices (IvDO RS 812.219) [ 2 ] on 4 May 2022 in Switzerland (CH) (see Table 1 for correspondence of terms between EU and CH). These new regulations came into force on 26 May 2022, both in Switzerland and in the EU. This new regulation will lead to significant changes and adaptations for medical laboratories. In this article, we will discuss the difficulties for diagnostic laboratories and the associated risks for Switzerland to comply with this new regulation, with a particular focus on microbiology laboratories. We would like (i) to highlight the impact on the routine diagnostic activity and on the quality of the work, (ii) to question the relevance of continuing with the following steps as planned by this regulation, and (iii) highlight the associated risk for the diagnostic capacities and public health in Switzerland.
Correspondence of terms and entities between the European Union (EU) and Switzerland (CH).
* IvDV: die Verordnung über In-vitro-Diagnostika, in German, and ODiv: ordonnance sur les dispositifs médicaux de diagnostics in vitro, in French. ** In french: Dispositifs médicaux de diagnostic in vitro (DIV); in german: IVD (In-vitro-Diagnostika).
2. What IVDR and IvDO Imply
The new European law codifies the placing on the market of commercial IVDs produced by industrial manufacturers, and in-house IVDs developed by diagnostic laboratories or larger health institutions themselves, as designated in the IVDR ( Table 2 ). Note that for all devices for general laboratory use, the processing and operating instructions in a given laboratory are not subject to this new regulation [ 3 ].
Examples of in vitro diagnostics devices affected by the IVD regulations (IvDO/IVDR).
See [ 4 , 5 ] for further details and explanations.
As far as industrial manufacturers are concerned, the number of definitions of an IVD has increased to 74, and the text defines more precisely the different types of diagnostic procedures. The notified bodies (Swissmedic in Switzerland) would therefore be entitled to examine a previously unregulated product for CE marking. In addition, the IVDR introduced a new classification system considering the possible impact on the patient. This replaces the previous categories of IVDs. It divides IVDs into four categories, Class A, Class B, Class C, and Class D, depending on the purpose of the device and the risks involved for patients ( Table 3 ). De facto , CE markings obtained under the previous regulation are no longer valid. Each product must be re-certified under the new regulation within different schedules according to the IVD class. Therefore, if the IVD was certified before the 26 May 2022 in accordance with the former legislation, notification must now occur before 26 May 2025 for Class D, 26 May 2026 for Class C, and 26 May 2027 for Class A and B, respectively ( Figure 1 ). To alleviate the effects of a lack of update to the MRA (mutual recognition of conformity assessments) with the EU, the Swiss Federal Council adopted mitigating measures. Therefore, notification in Switzerland for IVDs from EU manufacturers is not necessary if an authorized representative person is domiciled in Switzerland (article 44 paragraph 1 IvDO). In addition, Article 2 of the IVDR specifies that post-market surveillance reports should be updated each year.

Notification and registration timelines. * Not necessary in Switzerland if already done in EU and authorized representative person domiciled in Switzerland.
IVD classification definitions (IVDR2017/746 Annex VIII) and examples in microbiology.
Table adapted from: Guidance on Classification Rules for in Vitro Diagnostic Medical Devices under Regulation (EU) 2017/746 [ 6 ].
Concerning in-house IVDs, also known as laboratory-developed tests (LDTs), their definition, use, and obligation of notification are defined in article 5.5 of the IVDR and articles 9 and 10 of the IvDO. Any test is considered to be LDT when developed and validated according to the EN ISO 15189 norm in a health institution, or any CE-marked commercial test used off label, meaning not precisely following the manufacturer’s recommendations. Thus, for example, the use of CE-IVD tests on other sample types and/or another population from the one(s) listed in the certification are considered off label. Similarly, any modification of the interpretation thresholds or of the manufacturer’s instruction for use defines the test as off label (see Table 2 ). All LDTs or off-label tests will be submitted to the IVDR/IvDO from 26 May 2024 with different notification deadlines according to the IVD class, i.e., 1 July 2024 for class D, 1 January 2025 for classes B and C, and 1 July 2025 for class A, according to article 90 paragraph 3 of the IvDO ( Figure 1 ). In addition, as of 26 May 2028, to continue using their LDTs, healthcare institutions will have to demonstrate that there is no CE-IVD test with equivalent performance (article 82, paragraph 4 IvDO) or to justify the application of the LDT ( Figure 1 ). In addition, this will have to be demonstrated regularly. This concerns tests that are assumed to be equivalent, such as the use of a home-made database versus a manufacturer’s database on a MALDI-ToF. In no case is this required if the technique used is technically superior to another, typically in cases such as the sequencing of strains versus identification using various biochemical tests.
3. Difficulties Encountered in Clinical Microbiology Laboratories
This extremely restrictive law will thus have a profound impact on diagnostic laboratories. In microbiology, this regulation may lead to an overall loss of quality and to a decrease in the portfolio of analyses offered to clinicians, in complete opposition to the objectives of the law ( Table 4 ).
Major potential risks for microbiology laboratories due to IVDR.
Concerning manufactured IVDs, even before the implementation of the IVDR/IvDO, the availability of diagnostic tests for pathogens that are infrequent or difficult to culture ( Coxiella , Bartonella , Brucella , Rickettsia , Chlamydia psittaci , dengue virus, Trypanosoma cruzi, (chagas disease), and many other neglected diseases, was very limited. In addition, some companies were already beginning to discontinue tests in 2022 due to the potential regulatory burden and associated costs, prompting users to urgently evaluate the few remaining tests on the market. In particular, not every company will have a representative in Switzerland. In addition, in the short term, small companies not able to cope with the cost and workload linked to IVDR will have to stop their activity. We will rapidly run into a massive selection of companies with narrow options, which will lead in the medium term to a massive reduction in industrial innovation due to a lack of incentive for smaller start-up companies.
For example, the main manufacturer of the immunofluorescence tests for the detection of Coxiella as well as Bartonella stopped production in 2021, leaving only two other commercial alternatives for the Swiss market. The same holds true for the Wright test used for the detection of brucellosis, which is still considered the gold standard. Thus, the current law, despite the adjustments already made to mitigate its risk, and the postponements of its implementation (REGULATION (EU) 2022/112), entails an increased risk of test shortages, in particular concerning infrequent or difficult-to-diagnose pathogens.
This law adds a critical layer of difficulty for Swiss diagnostic laboratories. Currently, laboratories are already struggling with the massive cost pressure due to the FOPH-induced reduction of 10% and the current explosion in the cost of reagents around the globe; validating cheap in-house assays or switching to more expensive commercial solutions would add additional cost pressure. This additional layer of IVDR regulation is unlikely to improve quality, since the vast majority of microbiology laboratories are ISO 15189 accredited and consequently perform adequate quality control and have implemented a global quality management system. In addition, all microbiology laboratories need to work according to the epidemiology law (Lep—Loi sur les épidémies), which stipulate the necessity of having Swissmedic authorization to practice; the latter is provided for a five-year period. Both aspects are regularly controlled and supervised by both authorities.
Concerning LDTs, microbiology is a field that must remain on constant diagnostic surveillance in order to be able to react quickly in case of (i) the emergence of new pathogens, such as SARS-CoV-2 virus [ 7 ] or highly resistant Candida auris [ 8 ], (ii) the emergence of new variants of already-known pathogens that could escape the commonly used tests, such as the Swedish variant of Chlamydia trachomatis [ 9 ], or a Neisseria meningitidis variant [ 10 ], or (iii) the new epidemiological spread of organisms previously restricted to a geographical area, such as Zikavirus, the Monkeypox virus, and others [ 11 , 12 , 13 ]. Additionally, when a novel emergent agent is discovered (e.g., the Alongshan virus [ 14 , 15 ]), it is necessary to rapidly establish diagnostic tools for assessing its pathogenic role and dissemination. Thus, the current knowledge and flexibility in implementing new diagnostic tests in Swiss diagnostic laboratories is pivotal.
In addition, tests are often validated on patients and samples reflecting the more frequent clinical presentations of the disease, but this list cannot be exhaustive. Laboratories are often confronted with the need to use those tests on different patient populations or various sample types. For example, the GeneXpert ® Xpert MTB/RIF Ultra test was developed to detect Mycobacterium tuberculosis in sputum, but not in biopsies. Similarly, the TPHA and RPR serologic tests for diagnosing syphilis were developed to assess antibodies in serum, plasma but not in the cerebrospinal fluid (CSF) (which is of paramount importance when neurosyphilis is suspected), and many more examples are common in daily practice.
In all such situations, diagnostic laboratories, and academic laboratories in particular, are on the front line in addressing the urgent and evolving demand for specific, specialized, or new diagnostic assays. Therefore, these labs need to be able to regularly explore and evaluate new tests or off-label usages of commercial tests and to maintain their expertise in that activity. In academic centers, the case selection of patients with rare infectious diseases or who are critically ill is more pronounced. If tests must be sent to a foreign country due to a lack of IVDR/IvDO approval within Switzerland, this may cause significant time delay and present a substantial risk for the patient and public health. The IVDR/IvDO may affect this and other activities due to its substantial administrative workload. In addition, if the law is not changed by 2028, laboratories will have to regularly prove the superiority of their LDTs with respect to manufactured IVDs, generating additional costs and workload due to this extra monitoring activity. Currently, post-market evaluation is guaranteed by regular internal controls and external quality assessments.
4. Foreseen Impact of the Law
The consequences of implementing IVDR/IvDO in microbiology diagnostic laboratories are numerous and potentially severe.
Taken together, the decreased diversity of commercial tests and the strong pressure against LDTs will increase the demand for the remaining commercially available tests, which could lead to increased prices (due to monopoly), company dependence, and logistical challenges, as well as a shortage of reagents for all laboratories. Such a shortage, and now with no redundancy, is likely to have a dramatic effect on patients, and may translate into a drop in diagnostic quality, or even result in a lack of diagnostic capacity in the country. As about 70% to 80% of medical decisions are based on in vitro laboratory diagnostic tests, reduced test availability or even shortages thereof will dramatically impact patient care and potentially lead to clinical/legal consequences.
The workload and the charge of laboratories will strongly increase with the need (i) to assess all tests developed in house, despite existing clearance by Swissmedic certification and ISO accreditation, and compare them to CE-marked IVDs, (ii) to validate newly implemented CE tests, (iii) to re-assess all tests now considered off label, e.g., due to using different materials, and (iv) to provide notification of all such measures to Swissmedic, the Swiss notified body.
It can be easily imagined that small laboratories will not survive the additional costs of reagents and controls, not to mention the heavy IVDR-related workload, prompting them to reduce their test portfolio, at the risk of becoming less attractive and competitive in retaining their clients. This will result in overall economic damage with unemployment. A heavy transfer of diagnostic tests will take place towards large centers, e.g., academic centers, which will also face increased costs, higher workloads, and a shortage of clinically useful, but less frequently used assays. Lower quality can be expected due to the non-execution of some assays, delays in reporting results due to shipping to foreign centers, increased administration, and, again, additional costs.
The work of microbiological experts (FAMH) will be hampered. Indeed, with in-house tests, all test details are available, such as the amplification curve of a real-time or quantitative PCR (qPCR) assay, which is not the case for many commercial tests. Usually, laboratory experts know the exact performances of LDTs (sensitivity, specificity, positive and negative predictive value), and their limits (type of samples, patients, potential cross reactions, etc.). In contrast, commercial tests are often black boxes that do not disclose any details, e.g., the composition of the assay, which negatively affects the interpretation and medical validation of the results.
From 2028 onwards, the virtual obligation to adopt commercial tests will lead to a drastic reduction in the research and development carried out by diagnostic laboratories and start-up companies, as these will become too cumbersome for less frequent usage in the laboratory. This will result in a reduction in innovation in Switzerland, even in new fields of investigation such as next-generation sequencing (NGS) approaches. Indeed, new applications of next generation sequencing NGS (whole-genome sequencing with genome typing and taxo-genomics, and microbiota, resistome, and virulome analyses [ 16 , 17 , 18 , 19 , 20 ]) have for few years been implemented in routine microbiology laboratories. Given the increasing use of genomics for diagnosis, IVDR regulations may impair this recent development.
This will lead to a considerable loss of skills for all laboratory co-workers. Routine development, with regular research and development (R&D) meetings, is helped by having trained persons to rapidly implement new assays (PCR, serology, antigen and culture-based diagnostics). The value of the FAMH experts may ultimately be called into question, with a foreseeable lack of interest in this discipline resulting in personnel shortages in the medium term.
In the long run, we fear a global impoverishment of our diagnostic capacities in microbiology, a loss of knowledge and competence, and a dramatic impact on the quality of service for patients, which is ironically the absolute opposite of the stated goals of this law. Indeed, the law clearly protects industrial manufacturers from the in-house development of devices in the presence of commercial ones, as laboratories will not invest too much money and time into new devices if the ability to use them in future is not protected. The only big winners will be—in the short term—large manufacturers, who will be able to meet the resulting increases in costs and workload, while being given the opportunity to consolidate their business. The dependencies on these large diagnostic companies will further increase. They will direct their efforts toward tests that are in high demand, with high foreseen incomes, neglecting niche investigations, which are, however, the heart of modern precision quality medicine and are of high importance for patients with rare infectious diseases.
5. How to Face the Problems Raised by the Regulation
As discussed at the ECCMID 2023 meeting in Copenhagen (15 to 18 April 2023), in session SY241, microbiologists wondered whether we should “wait and see” or “do more lobbying towards authorities” with the aim of modifying the IVDR/IvDO.
The Swiss example of an abrupt cut in test reimbursement by 10% by the FOPH serves as a good indicator that the strategy of “wait and see” is not the way to go. Laboratories already knew that PCR was reimbursed at a rate that was too high, but did not actively alert the Swiss authorities. Millions of francs earned by laboratories during the COVID crisis highlighted the large discrepancy between the real cost of high-throughput PCRs (including reagents, automated systems, and manpower costs) and the level of reimbursement. The discrepancy was especially large given the large number of tests executed each day, creating a situation in which performing a few tests per week for rare pathogens (e.g., Coxiella burnetii, Chlamydia psittaci, parasites, exotic viruses such as Monkeypox, West Nile fever virus, Zikavirus, and many more) was not cost-effective, and cross-financing with other more cost-efficient tests was important for running a lab. This sudden cut in test reimbursement had a major impact on the smallest entities (laboratories and hospitals) and laboratoriess specialized in rare diseases, risking a further reduction in test availability in the most remote areas of Switzerland, accelerating imbalances and inequity in our country.
Conversely, increased lobbying toward authorities seems fruitful. Indeed, the lobbying already undertaken at the European level helped much, achieving (i) increased flexibility (more options to interpret the law according in specific situations), (ii) extended timelines for implementation (Regulation (EU) 2022/112), and (iii) abandonment of the sell-off deadline for manufactured products (Regulation (EU) 2023/607). Lobbying at the Swiss level was also successful in obtaining an attenuation measure by the Swiss Federal council (CE-marked IVD do not need to be accredited by Swissmedic, as long as there is a representative of the company domiciled in Switzerland). However, due to the market size of Switzerland, especially for rare pathogens, this is unlikely to happen.
Consequently, the Coordinated Commission of Clinical Microbiology of the Swiss Society of Microbiology considers it very important to make all efforts to lobby against this complexification of the regulations, which will clearly fail to meet its objectives. The authors clearly state that the ISO 15189 accreditation (or equivalent accreditation) norm and its routine application, as well as the Swissmedic certification, provide a legal framework that is strict enough to push all laboratories towards high-quality practices, as already exemplified by other medical laboratory disciplines [ 21 , 22 ].
To avoid further complexification of current regulations, we recommend adjusting the IvDO regulation as follows:
- ISO-15189-accredited laboratories may replace all LDT notifications with submission of their accreditation certificate.
- The IvDO 2028 milestone, which requires the superiority of an LDT against any CE-IVD test to be proven, should not be implemented. Mitigation measures could, for instance, include scientific surveillance and comparison with commercial tests, up to the level of equivalence (but not superiority).
6. Conclusions
The IVDR and the IvDO are two examples of bureaucratic decisions that are disconnected from reality and driven by strong financial lobbies. CE-marked assays such as lateral flow have for a long time been shown to often be insufficiently accurate, with low sensitivity (ranging from 30 to 80%), thus clearly calling into question the real value of CE marking, and more generally the competencies of the so-called competent authorities that deliver CE-marking labels. Notably, one of the few possible positive impacts of this new regulation might be a real improvement in CE-labeling procedures, subsequently resulting in increased quality.
However, there is no doubt that IvDO, if left unchanged, will have detrimental effects on patient care and public health by reducing the portfolio of tests; therefore, Swiss clinical microbiologists have decided—by means of this short article—to officially question this new regulation and its application, while proposing some important modifications. We are deeply convinced that laboratories should continue active R&D programs, active training of young microbiologists, and preserve the level of expertise and knowledge in order to continue to serve the aims of patient care, public health, and adaptation to the emerging biological (rather than administrative) threats.
Acknowledgments
We thank El Wessels (Netherlands) and Nina Nowka (Biel, Switzerland) for fruitful discussions.
Funding Statement
This opinion article received no external funding.
Conflicts of Interest
All authors are lab managers active in Switzerland and are impacted by the IvDO. G Greub has a research agreement with Becton-Dickinson on their BD Kiestra lab automated system and is medical advisor of Resistell, a start-up developing a new device for testing antibiotic susceptibility of bacteria. G Greub is also co-director of JeuPRO, a game company distributing games on microbes. A Egli is a medical advisor of two start-up companies, Sefunda and PhAST, both of which are developing rapid pathogen detection assays. However, no authors have a direct conflict of interest with the present position paper.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.

IMAGES
VIDEO
COMMENTS
Definition in Europe. In Europe, the MEDDEV 2.14/2 guidance document (IVD Guidance: Research Use Only products - A guide for manufacturers and notified bodies) provides clues as to the definition of RUOs. This guidance was written within the framework of the now obsolete Directive 98/79/EC on in vitro diagnostic medical devices (IVDD) and, in the absence of an up-to-date replacement, it can ...
Research Use Only (RUO) RUO stands for Research Use Only. The RUO label serves as a warning to clinical laboratory professionals that the materials in question are not intended for use with patient diagnostics. ... (IVDR) and do not have any regulatory requirements. Therefore, RUO materials are to be used only for testing with no direct impact ...
The In Vitro Medical Devices Regulation (EU) 2017/746 (IVDR) is a new regulation that will create a robust, transparent, and sustainable regulatory framework that "improves clinical safety and creates fair market access for manufacturers and healthcare professionals"(1). The IVDR "brings EU legislation into line with technical advances, changes in medical science, and progress in law ...
The 21 CFR 809.10 and 21 CFR 864 define four types of IVDs: General Purpose Reagent (GPR), Investigational Use Only (IUO), Analyte Specific Reagent (ASR) and Research Use Only (RUO). This is why in the U.S., RUOs are also called RUO IVDs - In contrast, in EU, only the term RUO prevails. As per the 21 CFR, RUO products are IVD products in the ...
What are Research Use Only (RUO) products? ... However, it is worth noting that the use of in-house assays is regulated in IVDR and is subject to certain conditions. In essence, RUO products provide researchers and physicians with the necessary tools to conduct experiments and studies, contributing to the overall progress in medical research. ...
The second misconception clinical laboratories should be aware of involves material labeled as Research Use Only (RUO). RUO labeling is intended for products that are still under development and are not commercially distributed. A developer would use this labeling to ship product for "investigation relating to product development" as ...
There are four different regulatory levels for IVDs: Research Use Only (RUO) General Laboratory Use (GLU) For Performance Studies Only (PSO) In Vitro Diagnostic Medical Device (IVD) The simplest explanation for these different levels is that each increasing level requires more testing and oversight. Research Use Only products are at the lowest ...
Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only Guidance for Industry and FDA Staff November 2013. Download the Final Guidance Document.
It is important to remind that per Article 1(3) of the IVDR, the following products are not considered IVDs and are henceforth out of scope of this guidance: a) products for general laboratory use or research-use only products, unless such products, in view of their characteristics, are specifically
01. Introduction. This document has been developed as a result of the outcome of initial discussions on "research only products" at the Medical Devices Expert Group (MDEG) meeting of July 2003. It aims to clarify a number of issues raised by Competent Authorities with regard to products labeled as "For Research Use Only" (RUO) and their ...
Research product (Research Use Only/RUO) is medical device and in-vitro medical device product that is in research development stage and has not been approved to be used for clinical purposes; or which is declared RUO by the authorized body in country of origin of the manufacturer. Category 3. 1.
In-house tests will only be allowed to be used, under the IVDR, if there is no equivalent CE-IVD kit on the market or when a target patient-group's specific needs cannot be met at the appropriate level of performance by an equivalent CE-IVD. ... RUO = Research Use Only. These responses, albeit on a voluntary basis, serve to illustrate that CE ...
The combined use of CE-certified IVDs (CE-IVDs), in-house IVDs (IH-IVDs), and research use only (RUO) devices are a cornerstone of diagnostics in pathology departments and crucial for optimal patient care. The IVDR not only regulates the manufacture and placement on the market of industrially manufactured IVDs, but also imposes conditions on ...
MEDDEV 2.14/2 demarcates so-called "Research Use Only" (RUO) products from devices for performance evaluation, from devices manufactured and used only within the same health institution and from further uses in the context of research or IVD design and development. ... Only few IVDR requirements can be fully covered (italic) by compliance ...
The new AVANCE IVDr system, presently for research use only, is a complete, proven and standardized platform for NMR pre-clinical research and screening, as well as for IVD-by-NMR research. It features high sensitivity and information-rich output at 600 MHz proton-NMR frequency, and incorporates advanced hardware, software, automation, spectral ...
Under the IVDD, product s used for Research Use Only (RUO) and Lab-Developed Tests (LDTs) were considered outside the scope of the directive. IVDR however also regulates LDTs. RUO products are still considered outside the scope of the IVDR, provided the product is used for research purposes only and not for the benefit of a specific patient.
IVDR). To the extent necessary to resolve issues of divergent interpretation and of practical application, the Commission may adopt implementing acts, in order to ensure the uniform application of the classification rules, taking into account the relevant scientific opinions of the relevant scientific committees (Article 47 (5) of the IVDR).
Save for later. NEW YORK (GenomeWeb News) - The Food and Drug Administration has released a guidance document that lays out and clarifies the rules for how in vitro diagnostic products for research use only (RUO) and investigational use only (IUO) may be used, labeled, or marketed. FDA created the guidance on RUOs and IUOs, which has been ...
A: We recently helped a customer transition an RUO instrument to IVD application for COVID-19 in the near term, and other diagnostic applications afterward. This involved critical analysis and partial redesigning of a system that was originally built for lab use to obtain IVD certification. It may sound simple, but one thing that's frequently ...
Article 5(5)d,e: Justification of use. Under the IVDR, use of CE-IVDs is the default option. Only when no equivalent CE-IVD is available, or a target patient group's specific needs cannot be met at the appropriate level of performance by an equivalent CE-IVD, use of an IH-IVDs is allowed (Figure (Figure3). 3). This implies that IH-IVDs can be ...
(a) products for general laboratory use or research-use only products, unless such products, in view of their characteristics, are specifically intended by their manufacturer to be used for in vitro diagnostic examination; (b) invasive sampling products or products which are directly applied to the human body for the purpose of obtaining a ...
However, the EU had suffered at the hands of poor device provision and had been hounded to tighten their regulations. Thus, as delineated in Article 5(5) of the IVDR, there is now a more stringent framework for the manufacture and use of in vitro diagnostic LDTs, especially regarding the control of their safety and performance.
The combined use of CE-certified IVDs (CE-IVDs), in-house IVDs (IH-IVDs), and research use only (RUO) devices are a cornerstone of diagnostics in pathology departments and crucial for optimal patient care. The IVDR not only regulates the manufacture and placement on the market of industrially manufactured IVDs, but also imposes conditions on ...
Around nine-in-ten Americans say marijuana should be legal for medical or recreational use, according to a January 2024 Pew Research Center survey.An overwhelming majority of U.S. adults (88%) say either that marijuana should be legal for medical use only (32%) or that it should be legal for medical and recreational use (57%).Just 11% say the drug should not be legal in any form.
Decades of learning from disasters, tightening building codes and increasing public awareness may have helped its people better weather strong quakes.
Given the increasing use of genomics for diagnosis, IVDR regulations may impair this recent development. This will lead to a considerable loss of skills for all laboratory co-workers. Routine development, with regular research and development (R&D) meetings, is helped by having trained persons to rapidly implement new assays (PCR, serology ...