Clinical Research Associate (CRA) Beginners
Online Course • CGX Training

About the CPD course
The CPD Accredited CRA Online Interactive Version of the course is conducted 2 days a week. Running at 3 hours each day over the course of 3 weeks. Around 21 training hours in total.; for persons wishing to advance their career in the Clinical Research Industry. The course is specifically designed for those who wish to become a Clinical Research Associate (CRA). Delegates will gain a thorough and detailed understanding of life as a CRA. The attendees will adopt the role as a CRA and engage in conducting mock clinical trials. The course is highly interactive and utilises teaching methods and hands on practical activities based on real life scenarios.
CPD Provider
CGX Training
- View Profile
Send an enquiry
By submitting this form, you consent to CPD sending you email regarding your application.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Thank you for your enquiry. Your message will be sent to the relevant CPD provider to contact you directly. We hope this helps.
Are you using the My CPD Portal? Log in here.
You may also be interested in:
Clinical Trial Administrator (CTA) Beginners
Communicate effectively, present fearlessly, write expressively.

Want to learn more?
More cpd courses by cgx training, online course, training course, get industry-related content straight to your inbox.
Thank you for subscribing
By signing up to our site you are agreeing to our privacy policy
Use the form below to submit an enquiry to
To get in touch please call us on +44 (0)208 840 4383 or email us via [email protected]
Thank you for your message, a member of our team will be in touch shortly
"All the world is my school and all humanity is my teacher." George Whitman
"Education is the key to unlocking the world, a passport to freedom." Oprah Winfrey
"Education is not the filling of a pail, but the lighting of a fire." W.B. Yeats
"A man, though wise, should never be ashamed of learning more, and must unbend his mind." Sophocles
"Education is not preparation; education is life itself." John Dewey
"It does not matter how slowly you go as long as you do not stop." Confucius
"The beautiful thing about learning is nobody can take it away from you." B.B. King
"It’s taken me all my life to learn what not to play." Dizzy Gillespie
"Self-education is, I firmly believe, the only kind of education there is." Isaac Asimov
"Learn everything you can, anytime you can, from anyone you can. There will always come a time when you will be grateful you did." Sarah Caldwell
"Learning is a treasure that will follow its owner everywhere." Chinese Proverb
"In learning you will teach, and in teaching you will learn." Phil Collins
"You do not learn to walk by following rules. You learn by doing, and falling over." Richard Branson
"Gold has a price, but learning is priceless." Chinese Proverb
"Live as if you were to die tomorrow. Learn as if you were to live forever." Mahatma Gandhi
"Tell me and I forget. Teach me and I remember. Involve me and I learn." Benjamin Franklin
"The noblest pleasure is the joy of understanding." Leonardo da Vinci
"Wisdom comes not from age, but from education and learning." Anton Chekhov
"I had six honest men. They taught me all I knew. Their names were: Where, What, When, Why, How and Who." Rudyard Kipling
"The roots of education are bitter, but the fruit is sweet." Aristotle
"We are born not to be perfect, but to learn and reflect from imperfections" Princess Ramirez
"Learning is an experience. Everything else is just information." Albert Einstein
"Mistakes are great, the more I make the smarter I get." R. Buckminster Fuller
"Living is easy with eyes closed, misunderstanding all you see." John Lennon and Paul McCartney
"Every time man makes a new experiment he always learns more. He cannot learn less." R. Buckminster Fuller
"Science is organised knowledge. Wisdom is organised life." Will Durant
"The capacity to learn is a gift; the ability to learn is a skill; the willingness to learn is a choice." Brian Herbert
"Knowledge speaks, but wisdom listens." Jimi Hendrix
"It is the art of an educated mind to be able to entertain a thought without accepting it." Aristotle
"Learning never exhausts the mind." Leonardo da Vinci
"I am always doing that which I cannot do, in order that I may learn how to do it." Pablo Picasso
"Commit yourself to lifelong learning. The most valuable asset you'll ever have is your mind and what you put into it." Brian Tracey
"Education is a progressive discovery of our own ignorance." Will Durant
"You cannot open a book without learning something." Confucius
"I am learning all the time. The tombstone will be my diploma." Eartha Kitt
"Teaching is only demonstrating that it is possible. Learning is making it possible for yourself." Paulo Coelho
"For the best return on your money, pour your purse into your head." Benjamin Franklin
"You don't understand anything until you learn it more than one way." Marvin Minsky
"Education is not the learning of facts, but the training of the mind to think." Albert Einstein
"Anyone who stops learning is old, whether at twenty or eighty. Anyone who keeps learning stays young." Henry Ford
"Education is the movement from darkness to light." Allan Bloom
"Much to learn, you still have." Yoda
"Light up the darkness." Bob Marley
"Education's responsibility is to replace an empty mind with an open one." Malcolm Forbes
"We are what we repeatedly do. Excellence, therefore, is not an act, but a habit." Aristotle
"Education is the kindling of a flame, not the filling of a vessel." Socrates
"I was obliged to be industrious. Whoever is equally industrious will succeed equally well." Johann Sebastian Bach
"The mind is not a vessel to be filled, but a fire to be ignited." Plutarch
"You can never be overdressed or overeducated." Oscar Wilde
"I am still learning." Michelangelo, age 87
"Real learning comes about when the competitive spirit has ceased." Jiddu Krishnamurti
This is a courtesy notification to let you know we have recently updated our privacy policy.
Clinical Research MRes/PG Cert

The Florence Nightingale Faculty of Nursing, Midwifery and Palliative Care at King's is a world-leading centre for applied and allied healthcare, clinical research and education. Our focus is on training the healthcare leaders of the future, with a strong commitment to providing post-qualification programmes of the highest quality. The Clinical Research MRes and PGCert is a multi-disciplinary course aimed at practitioners who wish to develop their clinical or academic research careers. The course seeks to enhance the skills and knowledge needed for supporting, delivering and integrating research into clinical practice, as well as fostering evidence-based practice. Practitioners with the appropriate innovation and critical-thinking skills may be supported to undertake further study at MPhil/PhD level with the aim of developing a future clinical academic career.
Key benefits
- You will be studying at the no.1 Nursing Faculty in the UK and 2nd in the world (2023 QS World University Rankings by subject).
- Located in the heart of London, across four of King’s Thames-side campuses (Waterloo, Strand, St Thomas’ and Guy’s) and the Denmark Hill Campus in South London.
- Lectures delivered by experienced multi- disciplinary researchers on contemporary issues in the conduct and utilisation of health, clinical and social care research.
- Support will be given to circulate work relating to your studies, predominantly through publication in high-quality peer-reviewed journals, with the opportunity to develop your research into a PhD Fellowship application.
- Course essentials
- Entry requirements
- Teaching & structure
Employability
Graduates from this course go on to develop a research or a clinical-academic career or move into a senior leadership role.
- Fees & Funding
Application closing date guidance
- How to apply
- Register your interest
- Chat to a student
- Order a prospectus
Related departments
- Florence Nightingale Faculty of Nursing, Midwifery & Palliative Care
- Department of Adult Nursing

Open days and events
Chat with current students and King's staff to find out about the courses we offer, life at King's and ask any questions you may have.

Postgraduate Nursing Scholarships
King's works in partnership with The Burdett Trust for Nursing and University of Edinburgh to educate nurse leaders of the future.
Explore more

Accommodation
Discover your accommodation options and explore our residences.

Connect with a King’s Advisor
Want to know more about studying at King's? We're here to help.

Learning in London
King's is right in the heart of the capital.
clinical research training delivered by industry experts
Ditch the internet searching and start your career in clinical research — get trained to a gold standard by experienced professionals established in the field. Our courses simulate real-world scenarios, gives you insight into the documents you’ll use on the job, and guides your CV and interview preparation — so you’ll be equipped to handle any challenge with confidence.
“I was only able to get the job I have now because of the CRA course with CGX. I’m working with a great company that I’m hoping to stay with for the foreseeable future. I can’t recommend CGX enough.”
R. Batta — CRA Beginners Course
Worked with and trusted by

Get the practical experience you need to skillfully handle your role in the clinical trial world like a pro. Cut out all the trial and error and start your career with an industry-recognised advantage.
MEET YOUR EXPERT TRAINERS

With a BSc in Chemistry, PhD in Organic Chemistry and a Postdoctoral Research Fellowship at the Institute of Biotechnology at Cambridge University, Dr Lia Hunter's career has spanned 30 years in clinical research. Founder and Director of both CGX Training and Clinnovate Ltd, Dr Hunter has worked for and with world-renowned pharmaceutical organisations, including Pfizer, Biogen, Takeda and AbbVie.

Donna-Marie Donalds’ career has spanned more than 20 years. Starting as a CRA for the Thrombosis Research Institute in 2001, she has since worked with the likes of Johnson & Johnson, Covance and Sanofi-Aventis. She is currently the Managing Director of QC Monitoring Solutions while freelancing as a clinical research consultant. Donna-Marie is also Head of Training and the innovative course creator at CGX Training.
“The CTA course and tutors were excellent. I got a very good insight into clinical trials and the roles of a CTA. Thank you CGX, you helped me gain my first job in the industry.”
J.Barbot, Clinical Trial Assistant
Ace that interview with the guidance of trainers who have done the hiring
Who we work with.
“We recruited CGX for a project delivered during STEM Week. The speaker's style was engaging and provoked questions about informed consent, ethics, and the clinical trial drug process. Students had many questions and this may open up a potential career for some of them.”
“I had doubts in myself, but I was reassured I have the transferable skills to transition into the clinical trials sector. It builds up your confidence when someone in the industry sees the potential in you and says you can do it! If I could work as a marketer, then I could do this too.”
“This course was what I have been searching for for some time! It was really hands-on with lots of activities and scenarios. I had no idea of the number of options available in this industry until now. I will definitely be pursuing a career in clinical research.”
WANT TO SEE OUR COURSES?
You're only a few steps away from expanding your knowledge and opportunities with our tailored courses led by our industry experts.
Worried that investing in yourself will cost you an arm and a leg?
Investing in your career doesn't have to break the bank! CGX Training was founded to help drive up the standards in the clinical research industry. We want every trainee to reach their full potential. You'll leave here:
We want to help you invest in your clinical training! Spread the cost with three budget-friendly payments or receive a 10% discount when you pay all in one go. Receive 20% off any communication skills course when you purchase a CTA, CRA or CPM course.
We can help you define your career path and future
Be trained by our highly qualified clinical trial professionals practising within the industry. Your expert trainers have more than 40 years of experience managing clinical trials between them. They will guide you through the responsibilities of your role, ensure your CV is optimised and get you interview-ready.
Save days, weeks and months of trial and error and invest in your career today. Get equipped with the practical skills to effortlessly and confidently manage onsite challenges and progress in your career.
want to see where one of our trainees is now?
Latest blog posts.

6 Ethical Principles You Need to Consider in Clinical Research
The 6 ethical principles that are paramount to the success of clinical trials and how we can train practitioners to the highest standards

Black History Month USA: The Contributions of People of Colour to Clinical Research and Science
Discover some of the great contributions made to science by Black people and how we can tackle diversity and inclusion in clinical research today.

Black History Month USA: Why Is There a Lack of Clinical Trial Uptake within the Black Community?
Uncovering the history of racial health disparities amongst Black communities and how they affect clinical trial uptake today.
- Log in
- Site search
Clinical research associate
Clinical drug research is a competitive but growing field and work experience in a scientific or healthcare environment is crucial for a job as a clinical research associate
As a clinical research associate (CRA), you'll run clinical trials to test drugs for their effectiveness, risks and benefits to ensure that they are safe for the intended use.
You'll work on new and existing drugs and will typically be involved in all stages of the clinical trial, including identifying an investigational site and setting up, initiating, monitoring and closing down the trial.
Clinical trials may be carried out at various stages or phases and include trials on healthy humans, trials on patients with a disease, and studies conducted after the launch of a new drug to monitor safety and side effects.
Responsibilities
Tasks vary depending on your employer and level of experience. However, you'll typically need to:
- develop and write trial protocols (outlining purpose and methodology)
- present trial protocols to a steering committee
- design data collection forms, known as case report forms (CRFs)
- coordinate with the ethics committee, which safeguards the rights, safety and wellbeing of all trial subjects
- manage regulatory authority applications and approvals that oversee the research and marketing of new and existing drugs
- identify and assess the suitability of facilities to use as the clinical trial site
- identify/select an investigator who will be responsible for conducting the trial at the trial site
- liaise with doctors, consultants or investigators on conducting the trial
- set up the trial sites - ensuring each centre has the trial materials, including the trial drug often known as the investigational medicinal product (IMP)
- train the site staff to trial-specific industry standards
- monitor the trial throughout its duration, which involves visiting the trial sites on a regular basis and dealing with and solving any issues
- verify that data entered on to the CRFs is consistent with patient clinical notes, known as source data/document verification (SDV)
- collect completed CRFs from hospitals and general practices
- write visit reports and file and collate trial documentation and reports
- meet with team members to discuss on-going trials, results and any trends or adverse events
- ensure all unused trial supplies are accounted for
- close down trial sites on completion of the trial
- discuss results with a medical statistician, who writes technical trial reports
- archive study documentation and correspondence
- prepare final reports and occasionally manuscripts for publication.
- Starting salaries for CRAs are in the region of £26,000 to £34,000. It's likely these posts will require some experience in a related area.
- As a senior CRA (SCRA), also known as a CRA II, you can earn a salary of around £35,000 to £50,000.
- In some senior roles, as a manager or director, salaries of in excess of £55,000 can be achieved.
Salaries vary from company to company. Additional benefits, such as a car allowance and bonus and pension, are typically offered.
Income figures are intended as a guide only.
Working hours
Working conditions vary between companies, although the hours are usually full time, Monday to Friday. You should expect to work some evenings, although weekend or shift work is uncommon.
Part-time work is possible, as are career breaks. Short term contracts of six to 12 months with a company are common, meaning you may work more like a contractor than a permanent employee.
What to expect
- It's likely your role will be a mixture of desk-based work and site visits. You'll visit trial sites to set up and close down a trial as well as to monitor the trial while it is running which could involve visits every four to six weeks. Some trials may be running abroad which will involve international travel. There may be opportunities for home-working the remainder of the time. In some instances you may find a role that is almost exclusively office-based with the focus being on document review.
- Most UK pharmaceutical companies are located in the south of England. Field-based positions are generally found in key locations throughout the UK. Some jobs, for example in a company laboratory, can be found locally, while others are regionally based.
- Self-employment or freelance work is possible once you've gained significant experience.
- The job can involve a lot of travelling and you may be out of the office three or four days a week, possibly including overnight stays. Some companies operate a system whereby the CRA specialises in a specific disease area and covers the whole of the UK. Others operate their CRAs on a regional basis.
- Initiatives are in place to encourage more women into science-based careers, such as Women in STEM .
Qualifications
To become a clinical research associate (CRA) you need to have a degree in life sciences, medical sciences or nursing.
This could include subjects such as:
- biochemistry
- biomedical science
- microbiology
- molecular biology
- pharmacology or pharmacy
- toxicology.
Entry without a degree or with a HND only is unlikely. You might occasionally be able to enter from the administration side - for example, you could start as a study-site coordinator in the NHS or as a clinical trials administrator/assistant. However, you would need substantial experience and further qualifications to progress to the role of CRA.
A postgraduate qualification is not essential, with many employers only looking for a related undergraduate degree. However, it could give you valuable experience in clinical trials and may be an advantage against competition when applying for jobs. A relevant PhD can also be advantageous in some companies, who may consider it as highly-relevant work experience counting towards gaining promotion to senior positions or moving into protocol development. Check with desired employers to find out what they're looking for.
Search for postgraduate courses in clinical research or clinical trials .
You'll need to have:
- excellent communication, both written and verbal, and interpersonal skills
- the ability to build effective relationships with trial centre staff and colleagues
- the ability to motivate others
- strong customer focus
- an excellent grasp of numeracy and a keen eye for detail
- presentation skills
- the ability to multitask and think on your feet
- project management skills
- a flexible and adaptable approach to work
- organisational, IT and administrative skills - the job involves a lot of documenting and recording information through computerised processes, such as clinical trial management systems and electronic data capture
- an understanding of the importance of good clinical practice (GCP) , which is a legal requirement for all CRAs.
You'll usually need a clean driving licence for travel between trial sites and your office.
Skills in an additional language, particularly any European ones, may also be useful for roles abroad.
Work experience
Relevant experience is crucial for securing a job as a CRA. Without it, you're likely to start work at a lower level, as a clinical data coordinator or clinical trials administrator/assistant, where you won't be involved in initiating or designing the trials. Once you've gained experience, you will then move on to a full CRA position.
A small number of companies may recruit graduates without experience if they have the necessary personal skills, but it's more likely that employers will look for someone who has some actual experience in a related workplace. This can include any work that uses scientific and analytical skills, for example:
- academic or pharmaceutical research
- clinical data work
- clinical laboratory work
- medical sales
- nursing or care work
Another useful way of gaining experience is to complete an industrial placement as part of your undergraduate degree. This can give you the real-world experience in a related area as well as helping you to make contacts that could lead to potential job offers in the future.
Competition for jobs is strong and work experience in a clinically-relevant field will considerably improve your chances.
Find out more about the different kinds of work experience and internships that are available.
Typical employers include pharmaceutical companies, medical device manufacturers, biotech companies and contract research organisations (CROs), which conduct research on behalf of pharmaceutical companies.
A CRO will organise the placement of a CRA on behalf of the sponsor (the pharmaceutical company) and may be involved in planning, organising and conducting the whole study or just part of it. The CRA will report back to the organisation and will feed back to the sponsor.
Large contract organisations are more likely to recruit an inexperienced graduate into a monitoring role and provide the training to progress to the level of a CRA.
Hospital academic departments occasionally employ CRAs in clinical trials units.
Look for job vacancies at:
- BMJ Careers
- emedcareers
- New Scientist Jobs
- Pharmiweb Jobs
Contract research organisations and pharmaceutical companies may advertise vacancies on their own websites.
Specialist recruitment agencies also handle vacancies. These include AL Solutions and RBW Consulting .
Professional development
Training takes place mainly in-house and on the job. The nature of the training can vary from company to company, with some employers providing a structured system.
Some companies will pay for relevant external training courses through organisations such as the Institute of Clinical Research (ICR) . They provide training in areas such as:
- advanced monitoring
- effective project management for clinical trials
- essentials of clinical trial monitoring
- process thinking in clinical trials.
You can also complete the ICR Certificate and Diploma to provide evidence of your clinical research knowledge and skills.
Becoming a member of the ICR can aid career development as it provides networking opportunities, discounted training, specialist interest groups and access to industry news. You can progress through the ICR membership levels but to do so you'll need to undertake a certain amount of continuing professional development (CPD) each year.
If you don't already have a postgraduate qualification, you can take a postgraduate certificate, diploma or Masters in areas such as:
- clinical pharmacology
- clinical pharmacy
- clinical research
- pharmaceutical medicine.
It's also possible to do a PhD. These courses can facilitate professional development and career advancement but you should check this against the career route you want to follow and employers of interest.
Career prospects
Career structures vary from company to company. How quickly you move up the grades depends on a range of factors including motivation, the opportunities available for training and development, ability and previous experience.
Before becoming a CRA, you may begin at a lower level such as a clinical trial administrator or junior CRA. As a CRA (also known as a CRA I), you'll work on pre-trial procedures, setting up and organising clinical trial sites (with some supervision), archiving documents and correspondence.
With the right combination of skills and experience you can move into the role of senior CRA (SCRA), also known as a CRA II. Work will include selecting investigators, coordinating ethics committee and regulatory authority applications, supervising trial supplies and attending investigator meetings.
As you progress further you'll also be responsible for supervising, training and mentoring junior staff, project management of whole trials (possibly on an international scale), protocol development and design of case report forms (CRFs). You could become a clinical team manager, clinical trial manager or clinical project manager depending on where your interests and skills lie. Beyond this are director roles within the same areas.
If you work within a contract research organisation you can build up and widen your experience with a variety of sponsor pharmaceutical companies in different therapeutic areas and in different phases of clinical research. This could allow you to move to a pharmaceutical company.
Self-employment may be possible as CRAs are employed on a freelance basis by certain companies. This should usually only be considered when you have developed experience, contacts and clients.
How would you rate this page?
On a scale where 1 is dislike and 5 is like
- Dislike 1 unhappy-very
- Like 5 happy-very
Thank you for rating the page
- Undergraduate courses
- Postgraduate courses
- Foundation courses
- Apprenticeships
- Part-time and short courses
- Apply undergraduate
- Apply postgraduate
Search for a course
Search by course name, subject, and more
- Undergraduate
- Postgraduate
- (suspended) - Available in Clearing Not available in Clearing location-sign UCAS
Fees and funding
- Tuition fees
- Scholarships
- Funding your studies
- Student finance
- Cost of living support
Why study at Kent
Student life.
- Careers and employability
- Student support and wellbeing
- Our locations
- Placements and internships
- Year abroad
- Student stories
- Schools and colleges
- International
- International students
- Your country
- Applicant FAQs
- International scholarships
- University of Kent International College
- Campus Tours
- Applicant Events
- Postgraduate events
- Maps and directions
- Research strengths
- Research centres
- Research impact
Research institutes
- Durrell Institute of Conservation and Ecology
- Institute of Cyber Security for Society
- Institute of Cultural and Creative Industries
- Institute of Health, Social Care and Wellbeing
Research students
- Graduate and Researcher College
- Research degrees
- Find a supervisor
- How to apply
Popular searches
- Visits and Open Days
- Jobs and vacancies
- Accommodation
- Student guide
- Library and IT
- Partner with us
- Career planning
- Making applications
- After graduation
I want to work in Clinical Research
Introduction.
Life sciences or medical sciences degrees can be put to good use in the competitive but growing field of clinical drug research.
A clinical research associate (CRA) runs clinical trials to test drugs for their effectiveness, risks and benefits to ensure that they are safe to allow on to the market. You'll work on new and existing drugs and will usually be employed by either a pharmaceutical company or a contract research organisation (CRO), which works on behalf of pharmaceutical companies.
The Clinical Research Associate coordinates clinical trials of new drugs. Once these trials are successful, the Regulatory Affairs Officer takes the trial data and uses this to gain government approval for the new medicine. Both jobs are well paid and interesting.
Clinical Research Associate
CRAs work for pharmaceutical companies and Contract Research Organisations which plan, organise and conduct Clinical Trials on behalf of pharmaceutical companies.
CRA's are also called Clinical Trials Administrators (CTAs), Clinical Research Scientists, Clinical Secretaries, Clinical Trials Assistants, Clinical Trials Associates, Data Monitors and Clinical Research Monitors!
In the past few years the percentage of clinical trials carried out in the UK has dropped as companies have increasingly carried out trials in Asia and Eastern Europe where it is cheaper. The cost of doing trials in the UK is one of the highest in Europe, but firms have in the past preferred to carry out trials in the UK because of our strong research base. The huge patient base of the NHS (over 50 million patient records) is also a powerful resource.
Bureaucracy in trial approvals has created longer start up times than in other European countries. This is important as the patent on a drug only runs for a limited number of years and once it expires, anyone can copy the drug. It is especially important for smaller companies to get trails up and running quickly to maintain cash flow from investors. A large trial may involve obtaining contracts with many different primary care trusts each one requiring its own set of paperwork. A UK Clinical Research Network has now been set up to speed up approval of trials and this seems to be working.
ICON plc offer a full range of consulting, clinical development and commercialisation services from a global network of offices in 46 countries. They are a global provider of consulting, and outsourced development and commercialisation services to pharmaceutical, biotechnology, medical device and government and public health organisations. ICON has been recognised as one of the world’s leading Contract Research Organisations through a number of high-profile industry awards.
SEC are specialist recruiters in the life sciences and pharmaceutical sector, delivering recruitment excellence to these industries since 1987 across the UK, Europe and the USA. With a diverse workforce encompassing 27 different languages, SEC is proud to live our values of trust, honesty, integrity and commitment by providing our candidates and clients with a first class service, demonstrated by our long-standing relationships.
Key Supporters
BSI is the business standards company that helps organisations all over the world make excellence a habit. For more than a century we have been challenging mediocrity and complacency to help embed excellence into the way people and products work. That means showing businesses how to improve performance, reduce risk and achieve sustainable growth. As a global leader in helping organisations improve, our clients range from high profile brands to small, local companies in 182 countries worldwide.
CK Group is the UK’s specialist in scientific, clinical and technical recruitment. For more than 25 years CK Group has been forging strong partnerships with the world’s most innovative and successful organisations, from small independent start-ups to globally renowned blue-chip pharmaceutical organisations.
DLRC Regulatory Consultancy
Pathway to market can often be complex but DLRC can simplify your journey. With over 450 years combined experience we provide a full range of services from strategic advice in early development to compilation and management of regulatory submissions throughout lifecycle. Since 2005 our innovative approach and collaborative interactions with agencies have helped secure approvals.
European Pharmaceutical Students' Association
EPSA is an umbrella organisation of all European Pharmaceutical Students' Associations, representing more than 100,000 students in 37 European countries.
Linkfield Life Sciences
Linkfield Life Sciences are a Regulatory Affairs specific recruitment company. We provide permanent, contract and retained services across the industry. Supporting our clients and candidates with knowledge gained over the last 8 years, we aim to build long lasting and productive relationships with all our Life Sciences partners. Contact us on 02071835059.
Medicines and Healthcare Products Regulatory Agency
The Medicines and Healthcare products Regulatory Agency regulates medicines, medical devices and blood components for transfusion in the UK. Recognised globally as an authority in its field, the agency plays a leading role in protecting and improving public health and supports innovation through scientific research and development.
PPD is a leading global contract research organisation providing comprehensive, integrated drug development, laboratory and lifecycle management services. Our clients and partners include pharmaceutical, biotechnology, medical device, academic and government organisations. With offices in 48 countries and more than 21,000 professionals worldwide, PPD applies innovative technologies, therapeutic expertise and a firm commitment to quality to help clients and partners bend the cost and time curve of drug development and optimise value in delivering life-changing therapies to improve health.
PharmaLex is a leading provider of specialized services for the pharmaceutical, biotech and medical device industries.
Roche is the world’s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases and neurology. Our UK headquarters in Welwyn houses both the UK business organisation and a growing global drug development group with over 800 world class professionals translating great science into great medicine for patients.
The Royal Society of Chemistry
The Royal Society of Chemistry is a professional body in the United Kingdom with the goal of "advancing the chemical sciences" and “supporting the chemistry community".
We’re Teva , a global pharmaceutical company specialising in medicines within central nervous system, respiratory, pain and oncology therapy areas. We've been around for about 80 years, which is longer than the NHS! We contribute to improving the lives and health of patients all around the UK. We’re the biggest supplier of medicines to the NHS* and a key partner to NHS organisations where we work together to improve healthcare outcomes for patients through joint working projects.
Find out more
- Prospects Job Profile: Regulatory Affairs Officer
- ABPI Job Profile: Regulatory Affairs Associate
- NewScientist: Getting into Regulatory Affairs
CCRA Certification
Ccra® (certified clinical research associate) is a credential formally recognizing clinical research professionals with experience monitoring and supervising the conduct and progress of clinical trials on behalf of a sponsor., this trusted mark of excellence in clinical research is awarded to clinical researchers who have demonstrated proficiency of specific knowledge and skills by passing the standardized ccra® certification exam..
Apply for Your Exam

Clinical research professional with 3,000 hours of verifiable work experience are eligible to sit for the CCRA ® Exam. Complete eligibility criteria is defined in the Academy’s policy manual .
What qualifies as work experience, work related to human subject research, paid contractual agreement – employer/employee, can be verified by acrp through employer, what is excluded from work experience, any work that is part of a degree track or education program, any experience older than ten years, internships paid or unpaid.

Clinical research professionals with 1,500 hours of verifiable work experience and a clinical research degree are eligible to site for the CCRA ® Exam.
What qualifies as a clinical research degree, any degree awarded in clinical research from a chea accredited institution, major in clinical research, what does not qualify, any degree not in clinical research (biology, psychology, public health, epidemiology, nursing, doctorate), graduate certificate programs.

The CCRA ® exam consists of 125 multiple choice questions that must be answered within 180 minutes.
The exam is referenced only to the international conference on harmonization (ich) guidelines. no other regulatory framework is tested, including country-specific regulations (i.e, fda or ema)., the following are the only references for which the ccra ® certification exam content can be supported:, ccra ® exam detailed content outline >, guideline for good clinical practice e6 (r2) >, definitions and standards for expedited reporting (e2a) >, general considerations for clinical trials (e8) >, statistical principles for clinical trials (e9) >, clinical trials in pediatric population (e11) >, the declaration of helsinki (doh) >, the global ccra ® exam committee uses psychometrically sound practices to develop certified clinical research associate (ccra) examinations that meet the current test specifications as determined by the most recent job task analysis (jta)..

Review the Detailed Content Outline and make sure your experience and work hours are appropriate, as outlined in the Eligibility tab.
We also strongly encourage you to review the entire acrp certification handbook , which provides full details about every facet of acrp certification..

Create a free ACRP account so you can begin the application process. Follow the on-screen prompts to enter any requested information and documentation.
If you already have an acrp account, please proceed to step three., create account >.

You’re almost there! Please note, applications selected for audit will undergo a formal review by ACRP’s subject matter experts. In most instances, you will receive a status update about your application within 7 business days.
In accordance with the americans with disabilities act, acrp will provide reasonable accommodations for candidates with disabilities. please complete this special accommodations form for submission with your application before proceeding., acrp’s testing partner psi offers in-person testing, as well as on-demand remote testing available 24 hours a day, every day, during the testing windows., watch these videos to learn what to expect from each option before scheduling your exam..

Find Test Centers Near You >
Schedule your in-person exam >.

Schedule Your Remote Exam >
Check system requirements >.

The best way to prepare for the CCRA ® exam is to fully understand the scope of the exam content and its references.
Please be sure to thoroughly review the following:, acrp certification handbook >, remember: the exam is referenced only to the international conference on harmonization guidelines. no other regulatory framework is tested, including country-specific regulations (i.e, fda or ema)..

We also recommend leaning on your community! Thousands of ACRP Certified members have been in your shoes. They are active community members and always willing to share tips and advice for ACRP exam prep.
Visit the acrp community >.

ACRP offers a variety of training and continuing education programs focused on the key ICH guidelines covered in the CCRA ® exam.
Learn more >.

Exam results are shared immediately at the conclusion of your exam, but PSI will send you an email with your full score report within 24 hours.
Your acrp account will reflect your results within 3 weeks of your exam date..


Congratulations! You just passed a major milestone on your professional journey and are now a member of the elite club of ACRP Certified clinical research professionals.
Keep an eye on your email because you will soon receive information from our digital badging partner credly about claiming your digital badge and how you can use it to tout your accomplishment. also learn how to use your new credential by reviewing the certification mark policy ., you have 2 years to keep your certification in good standing by continuing your professional development, and we’ll be right there with you every step of the way. in the meantime, we highly recommend you review all the details about maintenance of certification . don’t leave it to the last minute.

Don’t worry. It happens to the best of us. Give it another try!
Refer to your acrp certification examination results email or the acrp certification handbook for guidance on the next steps in your certification journey., upcoming testing dates, spring 2024 testing february 15 – may 15, 2024, fall 2024 testing july 15 – october 15, 2024, 2024 registration dates and fees, early bird registration acrp members – $435 nonmembers – $485 spring 2024: october 15 – december 31, 2023 fall 2024: may 15 – july 15, regular registration acrp members – $460 nonmembers – $600 spring 2024: january 1 – april 30 fall 2024: july 16 – september 30, join acrp & save, joining acrp helps you save money. more importantly, acrp is where you will find the very best of what you need to design a career path that’s uniquely your own. connections through an engaged community. growth through gold-standard training. and elevation through rigorous certification., explore membership >, exam preparation, congratulations on your decision to earn the most recognized and respected endorsement of clinical research competency — acrp certification. as you start this important journey in your career, we’re here to support you every step of the way..
- Program Overview
The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices, continuous learning, and advancing global health.
The Society of Clinical Research Associates (SOCRA) established the Certification Program for Clinical Research Professionals in order to create an internationally accepted standard of knowledge, education, and experience by which clinical research professionals will be recognized by the clinical research community. Those individuals so recognized may use the "Certified Clinical Research Professional" or "CCRP ® " designation.
Path to Certification
CCRP certification is awarded upon meeting two criteria: a successful written application and a passing CCRP examination score. The benefits of obtaining certification are numerous. It not only validates knowledge, skills, and abilities but also enhances credibility and peer recognition. Career advancement and increased earning potential become tangible outcomes, reflecting a commitment to standards, compliance, and integrity.
Scope and Standards of Practice
The standards upon which this certification program is based have been set forth by SOCRA to promote recognition and continuing excellence in the ethical conduct of clinical trials. It is the goal of SOCRA to encourage members, and assure the competency of certified members, in their knowledge, understanding, and application of the conduct of clinical investigations involving humans in accordance with the ICH Guidelines, the U.S. Code of Federal Regulations, and the ethical principles that guide clinical research. Members are expected to adhere to national, state, local and provincial regulations and to international guidelines published by the International Conference for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and all applicable federal, state and local laws and policies.
Standards of Practice include an understanding of and application of basic concepts of Good Clinical (Research) Practice, including:
- The Nuremberg Code
- The Belmont Report
- The Declaration of Helsinki
- 21 U.S. Code of Federal Regulations – Parts 11, 50, 56, 312, 812
- 45 U.S. Code of Federal Regulations - Part 46
- ICH Harmonised Guideline for Good Clinical Practice E6(R2), and
- ICH Clinical Safety Data Management: Definitions and Standards for Expedited Reporting (E2A)
- 42 CFR Part 11 (ClinicalTrials.gov)
Certification Exam
The SOCRA Certification Examination is offered in two formats: paper and pencil (at SOCRA sponsored sites), and computer based (at Prometric testing centers or through Home Proctoring).
SOCRA Sponsored Sites: Paper and Pencil
- Hosted exams offered in various location throughout the US and Canada.
- Visit the paper and pencil exam schedule for dates and locations.
- A complete application must be received by the deadline date as stated on the examination schedule.
- Score reports mailed to you in 4-6 weeks after exam.
Computer Based Testing: Testing Centers and Remote Proctoring
- Offered at Prometric testing centers throughout the world or through Home Proctoring
- Click here for a list of test centers.
- Allow 2-4 weeks for application processing.
- Once application is approved, schedule exam at a testing center. Exam sessions are available at least 6 weeks in advance.
- Score reports received immediately upon completion of exam.
Candidate Handbook
For more information, please view the Candidate Handbook.
Certification
- CCRP Certification Quick Facts
- Definition of a Clinical Research Professional
- Certification Program Policies
- Removal of CCRP® Credential
- Verify Certification
- Exam Overview
- Candidate Eligibility
- Application and Fee
- Computer Based Testing Exams
- Paper and Pencil Exams
- Refunds, Rescheduling and Retesting
- SOCRA Sponsored Exam Schedule
- Preparing for the Exam
- Preparation Resources
- Examination Results
- Host an Exam at Your Site
- Apply Online
- Exam Schedule SOCRA Sponsored Sites
- Requirements for Maintaining Certification
- Continuing Education Requirements
- Descriptions of Acceptable CE
- CE Recordkeeping Requirements
- Request for SOCRA CE for Courses / Workshops
- Installment Plan Payment
- Renewal of Certification
- Recertification Audit
- Recertification Learning Module
- Accreditation
Summary of Certification Activities
11,145 CCRPs (as of 12/31/2022)
- 1,391 candidates took CCRP exam
- 73% passed CCRP exam
- 2,649 CCRPs recertified
- 946 candidates took CCRP exam
- 65% passed CCRP exam
- 2,783 CCRPs recertified
- 2,060 candidates took CCRP exam
- 70% passed CCRP exam
- 3,801 CCRPs recertified
- 1,980 candidates took CCRP exam
- 71% passed CCRP exam
- 3,188 CCRPs recertified
- 104 exam sites hosted
- 2,175 candidates took CCRP exam
- 2,491 CCRPs recertified
- 91 exam sites hosted
- 2,141 candidates took CCRP exam
- 2,421CCRPs recertified
Internet Explorer is no longer supported by Microsoft. To browse the NIHR site please use a modern, secure browser like Google Chrome, Mozilla Firefox, or Microsoft Edge.

Clinical Research Practitioners

Clinical Research Practitioners (CRPs) work to deliver safe, ethical and high quality clinical research care. They make up around 25% of the research workforce.
What do CRPs do?
CRPs are an essential part of the research delivery team in various clinical and non clinical settings. CRPs:
- work autonomously
- are adaptable team members
- specialise in consent, data collection, and other study protocol activities
- are key project managers liaising with research investigators and research delivery teams
The UK Professional Standards Authority (PSA) recognises CRPs as an occupational group in health and care. The PSA approved adding CRPs to the Academy for Healthcare Science (AHCS) Accredited Register in 2020. The AHCS Accredited Register for CRPs opened in March 2021.
CRP Directory
If you’re starting out on a training pathway as a CRP, you should join the CRP Directory. You’ll be part of a growing community helping to shape the professional identity of CRPs.
You are eligible to join the CRP Directory if you:
work in a research delivery role involving direct contact with patients and activities in health and social care research settings
- are not currently registered with a healthcare profession
- are applying to join the AHCS Accredited Register for CRPs
- are already professionally registered as a CRP
Find out more:
CRP Directory on the AHCS website
Search for registered CRPs
Search the Clinical Research Practitioner Directory on the Academy for Healthcare Science (AHCS) website to find accredited CRPs.
Join the AHCS Accredited Register for CRPs
You can apply to join the AHCS Accredited Register for CRPs if you:
- meet the AHCS Standards of Proficiency for CRPs and
- have the required education, training and experience
Joining the register will help you to:
- demonstrate your proficiency in delivery of research
- define the expectations and accountability relating to your practice
- develop professionally
Find out more About the CRP Accredited Register on the Academy for Healthcare Science (AHCS) website
Career development and training
NIHR programmes to help you further your CRP career:
HEE-NIHR Integrated Clinical and Practitioner Academic Programme
Become a Research Delivery Leader: Clinician Researcher Credentials Framework
Support for CRPs and their managers
Find out more about CRPs and get help:
NIHR Clinical Research Practitioners' Community website
NIHR contact: [email protected]
AHCS contact: [email protected]
Clinical Professionals
Life science recruitment, training and development.
Clinical Professionals offer the pharmaceutical industry a leading package of training and development solutions for a range of specialist technical life sciences subjects as well as soft skills development programmes. Our programmes are run on a regular basis in our in-house training facility or bespoke courses are delivered on our customer sites.
Clinical Professionals also recently launched their Life Science Graduate Training Academy after noticing a lack of opportunity for graduates to join the life science industry and progress while receiving training. The Training Academy consists of a modular training course, followed by a placement in a “first to industry” CTA (Clinical Trial Administrator) job.
Experienced Candidates’ Training and Development Courses.
Our team offer specialist training in the following: Clinical Research Regulatory Affairs Pharmacovigilance
Example training courses include: Introduction to pharmaceutical industry GCP Introduction GCP Update Introduction to CRA role Introduction to Lead CRA role Co-monitoring Advanced Monitoring GMP for IMP Statistics for non statisticians Clinical Trial Designs Preparing for Regulatory Body Inspections CRED: Life Cycle Management – Variations CRED: Introduction to eCTD
Clinical Professionals offer an innovative Clinical Research Associate (CRA) recruitment and training programme in five European countries (Germany, UK, Netherlands, France and Switzerland).
Clinical Professionals offer clients an intensive three day theoretical training and selection course ‘Breakthrough’. This includes two follow-up training days. These are designed and delivered by Clinical Professionals training staff. Ongoing support and mentoring is provided throughout the contract.
Theoretical knowledge is provided on a modular basis by qualified clinical research personnel; the client partner company provides the opportunity to put the learning into practice on the job.
The core elements included: 1. Overview of Medicinal Product Research and Development 2. Good Clinical Practice (ICH GCP) 3. Clinical Trial Development 4. Clinical Trial Management 5. Monitoring Obligations and Methods
Further to our technical training and development programmes Clinical Professionals offers pharmaceutical and biotechnology clients soft skills development courses. These programmes offer personal development and management development. We help people to develop the skills they need to be more confident and effective.
Typical examples include: Conflict resolution, assertiveness and negotiation skills Introduction to Effective Team Leadership The Importance of Team working Effective Communication Issue and crisis management Presentation skills Stress management
For further information or to schedule an introductory meeting please contact + 44 (0)118 959 4990.

Search our Roles
Latest news.
- Digital vacancies on the rise but Big Pharma still dominating force
- New study finds Clinical Research cut by 87% at peak of pandemic
- First patient dosing commences in GSK 5 in 1 meningitis jab
Like to Talk to a Consultant?
Call or email us directly and find out your next career step: [email protected] or +44 (0)118 9594 990
CRTI Online LLC | Houston, TX 77079 | 1-866-378-8206
Privacy Policy
Student Login
The CRA Training Institute

Training & Onboarding Clinical Research Professionals since 1989
Online cra, crc & cdm clinical research courses .
Receive your Accredited Certificate and be
job-ready in as little as 3-4 Weeks!
CRA Certificate Course
COURSE FEE: $79 9.00
DURATION: 3-4 Weeks Average
No Time Limit
DELIVERY: 100% online
Self-Paced Stu dy
START DATE: Immediately
CRC Certificate Course
COURSE FEE: $74 9.00
DURATION: 2-4 Weeks Average
Self-Paced Study
CDM Certificate Course
COURSE FEE: $6 9 9.00
DURATION: 2-3 Weeks
DELIVERY: 100% o nline
Trusted by CROs & Biotech Companies Globally

Curavit Clinical Research

Accreditation
The cra training institute and its online clinical research courses are accredited by the accreditation council for clinical research education (accre)..
Global Program Code
463-04-112-GPC02
www.accre-accredit.org 1840 W Whittier Blvd #4320 La Habra, CA 90631 USA Phone: 1-(888) 512-6760

Student Testimonials
Got hired as a pharmacovigilance officer for IQVIA working with Moderna on their Phase III mRNA vaccine candidate! Thanks so much for your help.
S. Ray, MD, MSc, CRA - Georgia
Job placement assistance.

Professional Resume preparation

Interview preparation

Connecting with Employers
Our Alumni have either found employment with or have been referred from a wide cross-section of hospitals, clinics, CROs, biotech and pharmaceutical companies across North America and globally.
With the knowledge and job skills gained as a result of your study, you can help to meet the ever-growing demand for trained CRAs, CRCs or CDMs to develop exciting new therapeutics for the 21st century in the biotechnology, pharmaceutical and medical device industries.
Clinical Trials in a Nutshell
.png)
Our Accredited Interactive-Online Clinical Research Courses are designed for persons with or without prior on-site clinical trials monitoring experience, seeking qualifications and/or current practical knowledge to effectively work as a Clinical Research Associate (CRA), Clinical Research Coordinator (CRC), or Clinical Data Manager (CDM) in the monitoring of clinical trials globally.
Online CRA, CRC & CDM Clinical Research Courses

Clinical Research Associate: A Full Guide on Becoming A CRA

Clinical Research Associate
A complete guide on how to become a clinical research associate.
Over 1.9 million students receive a bachelors of science every year. While a few go on to PhD, Masters, and Medical programs; many are ready to start clinical research certification online to start a career in the frontiers of medical research and patient care.
As a new student applying to the science job market, you may only find internships or recognize that even entry-level science jobs requires 1-2 years of experience. More so, you may realize many of these jobs require intense labor in the lab or just did not meet your expectations for your science degree.
This is why a career as a CRA should be considered with clinical research coordinator training. We train over 500 students each month in clinical research coordinator training and clinical research associate training (depending on prior background).
For those who have always wanted a career in medicine or have a gap year before medical school; Clinical Research Training is the next step to getting a head start in your career.
Because the position is unlike actually working in the lab and more of a management role; you get 1-on-1 connections with physicians and medical staff that can lead to a better application for medical school and other medical careers later on.
Best of all; many of these positions accept remote staff (and some allow you to travel 45-75% with full expenses including travel, accommodation, meals, and other per-dime expenses covered).
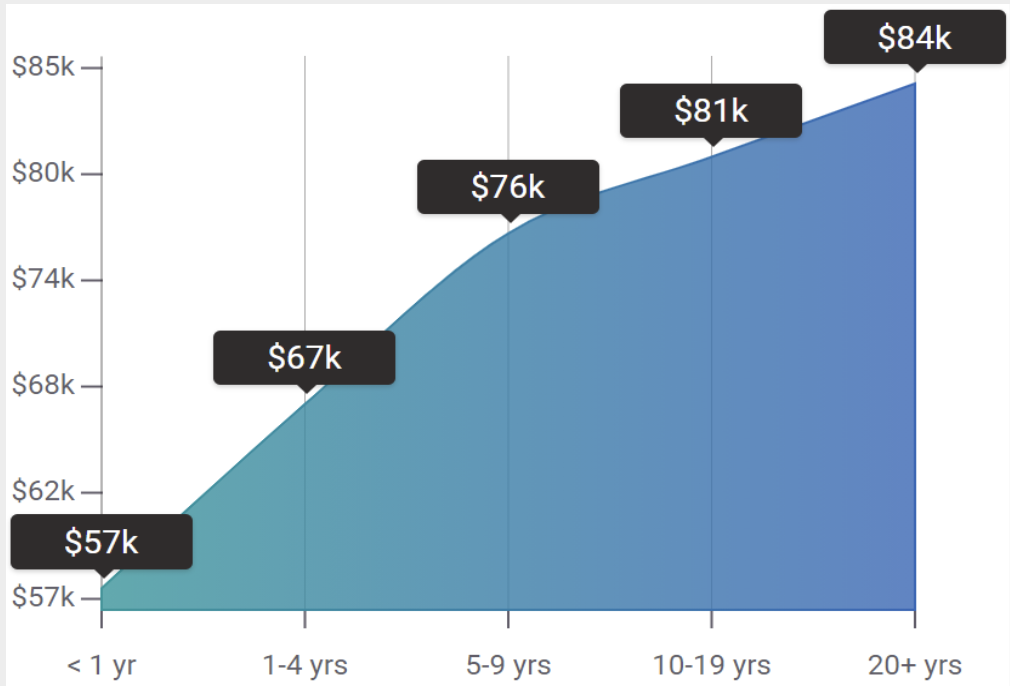
Clinical Research Training can help you save money while also increasing your salary. CRA’s with our level of training can expect to make between $6,500-$12,000 a month with an estimated promotion rate of 33% a year: an amount that is uncommon in other science-degree careers.
CCRPS is one of the only major US-based ACCRE, ACCME, ANCC, ACPE, and Transcelerate Biopharma accredited CRA certification courses that accepts students with no prior background for certification. T
his is because our course is thorough and created by Senior CRAs who have been in the field for long enough to understand what you need to know to begin working and applying. The course can be completed in as little as 7 days with dedicated full-day study time.
Clinical Research Associate Certification Qualifications
Foreign Doctors Welcome: A Clinical Research Associate or Coordinator plays a vital role in directing and supervising clinical trials conducted by physicians, nurses, and other science professionals. This career path is particularly attractive to many foreign doctors with completed medical degrees (MBBS) who can utilize their expertise in the US healthcare system by pursuing a CRA career instead of taking the USMLE or repeating residency training.
Distinct Skillset: Unlike the traditional medical field you may be familiar with after years of schooling, Clinical Research Associate training provides a distinct and valuable skillset.
Most Extensive Online Course: Our program goes beyond basic introductions, offering a comprehensive curriculum with over 110 modules – the most extensive Clinical Research Associate course available online. This in-depth training ensures you're well-prepared to secure a coveted CRA position.
Superior Coursework: Securing a CRA role is a strategic career move compared to the limitations of many traditional medical positions. While generic courses abound, we've observed that graduates often struggle due to a lack of substantive content. Our Clinical Research Associate course addresses this gap by providing Senior Clinical Research Associate-level training through 110 intensive modules grounded in the latest scientific principles.
Diverse Career Opportunities: This high-demand science-based medical field offers diverse opportunities:
Work in the Private Sector: Pursue a CRA career with renowned pharmaceutical companies like Pfizer.
Academic Opportunities: Work in the academic sphere at medical schools.
Unmatched Flexibility and Knowledge: In addition to our exceptional course content, we boast the largest number of clinical research courses available online, providing you with unmatched flexibility and knowledge.
Why Take A CRA Certification Course
The role of the clinical research associate is to ensure that medical devices, new treatments and new drugs are approved for patients' use.
This field is taken as a certificate program course in many schools. For example, you may find associate degree programs. These programs can be completed in two years and can be offered through both the online and the hybrid formats. Hybrid formats combine both online and on-campus courses together.
If you opt for a fully online program, you can still get an immersive education. Different platforms like emails and discussion boards are used to ensure and promote interaction between the students as well as the lecturers.
Online learning platforms are used to upload the syllabus, course materials, lectures and assignments. Some online programs include field work as part of their requirements, in order for students to gain first hand experience working with clinical trials and patients. Depending on the school, they may have a list of approved clinical research institutes and other facilities. Otherwise, you will have to find a facility for yourself and get the school's approval.
These certificate programs are generally designed for professionals that are already in the medical fields (like medical assistants or nurses) and are interested in moving to the field of clinical research.
They may therefore ask for a copy of your CV or resumé or they may ask for a letter from your employers to verify that you have the needed medical experience. Some programs may require just an undergraduate degree in a medical science or life science related field.
Clinical research associates are trained to assist clinical researchers and investigators in the coordination, administration and management of clinical trials.
During this training, different courses will be taught revolving around subjects like safety procedures, subject recruitment, regulatory requirements, drug development, accountability, trial management, medical terminology etc.
The importance of the role of the clinical research associate means that companies that conduct clinical trials are usually very selective. The need to comply with strict regulations often inform their decision when making a choice of their clinical research associate. It is therefore very difficult to get a job as a clinical research associate without previous experience in clinical trials.
Many companies require around at least two years experience in clinical monitoring as a clinical project assistant or clinical trial administrator before considering applicants for this important role.
In applying for the post of a clinical research associate , ensure that you read the job description and indicate or highlights the relevant experience on your curriculum vitae. Your cover letter should be specific to the company you're applying to.
Do not use a one-for-all cover letter. Personalize your cover letter to each company and highlight the skills that fit the specific requirements of the role. Not all companies advertise their vacancies, so you can try to find out about other unadvertised vacancies, you might increase your chances.
Further certification can enhance your resume such as the ACCRE accredited CRA program which contains 110 learning modules for Clinical Research Associate Training and Placement
The Best CRA Certification Course For Entry-Levels
There is a huge shortage of well-trained CRAs, but many companies are reluctant to hire untrained entry-level clinical monitors because of patient and trial safety. Because of this, even the beginner entry-level jobs require certification or training.
Our program is considered one of the top clinical research graduate programs online. Most courses provide very light training that may look good because of the company names, but alone is not sufficient to pass the interview rounds a company conducts.
Because our modules are prepared help even Senior Clinical Research Associates, we find more of our students with no background quickly passing their interview rounds.
CCRPS Course covers double to triple the amount of course content than other courses. While many courses are simply 5-20 simple interactive modules, our course covers 140 dense modules in thorough detail.
After each session, students can ask their questions privately with the course instructor, all of whom have 15+ years of CRA experience.
Currently, 82% of our students are hired within the first month of taking the course. Students with limited background or those looking to gain extra experience are offered a remote internship of up to 6 months during the time they are interviewing.
This advantage allows many students with limited experience to get hired with a higher paying job than previously offered.
While a majority of our students are physicians, a majority of the CRA workforce are Science Grads and Nurses. nonetheless, we train all students at a Senior CRA level regardless of background because clinical research monitoring is vastly different from any lab or science course you may have taken.
Clinical research associates are given the protocol of a study including all medical protocol that must be followed but because they do not diagnose or treat. Medical knowledge is supplemental but not sufficient in this career path.
This is the main reason why our Clinical Research Training includes all possible scenarios you may face at the protocol and guideline level in your future company.
How To Get Experience For Clinical Research Associate Jobs
CCRPS, like other educational institutes, is only associated with educating and certifying clinical research professionals so we do not provide job placement. We want to make sure you apply with your best foot forward. Below are links we readily refer to graduates who are looking for job support. Having a great CV and cover letter are essential to applying for jobs. Recruiters are paid by the company which hires you and thus are free for searching employees. Be realistic but also be driven. Make sure you get continue reaching out until you get a true rejection from any job you apply to as they may never have seen your application if you received no response.
Clinical Research Job Advising: Kunal at ClinicalTrialPodcast
Free Resume Review: TopCV TopCV provides a free review and feedback for your current resume.
Resume Distribution: ResumeRabbit Resume rabbit distributes your resume to 60 job posting sites.
Clinical Research Recruiters: I-Recruit I-Recruit distributes your resume to clinical research recruiters.
Clinical Research Job Bulletin: Indeed Indeed usually provides the most uptodate job bulletin for clinical research jobs
Always use a cover letter specific for the company and job when applying if you are not using a recruiter.
The ICH-GCP in Clinical Research
Regardless of the type of clinical research or function of an IP being tested, it is important that clinical research should meet two critical criteria:
The clinical research process should respect the rights, freedom and dignity of tested patients (human participants).
Data from the clinical research process should be accurately collected, safely stored, rigorously scrutinized and correctly interpreted.
One way to ensure that these requirements are met is to follow a set of internationally recognized and accepted standards for clinical research.
Most countries across the world today follow ICH-GCP, that is, International Committee for Harmonization of Good Clinical Practice guidelines in conducting clinical research on human participants7.
The ICH-GCP outlines procedures and precautions that are essential in order to protect the safety and wellbeing of human research participants during clinical research, and to ensure the integrity of data from clinical research studies.
In the USA, clinical studies are required to comply with the FDA Guidance for Good Clinical Practice, outlined in a document titled ‘E6(R2) Good Clinical Practice: Integrated Addendum to E6(R1)’8.
In the USA, clinical studies are required to comply with the FDA Guidance for Good Clinical Practice, outlined in a document titled ‘E6(R2) Good Clinical Practice: Integrated Addendum to E6(R1)’8.z
Qualifications and Qualities of a CRA
According to the International Accrediting Organization for Clinical Research (IAOCR), candidates for CRA positions usually hold either a biological science degree, or one in medicine or nursing10.
The New Scientist recommends that aspiring CRAs should possess a good working knowledge of one or more of the following subjects – anatomy, biology, biochemistry, chemistry, immunology, microbiology, pharmacology, physiology or toxicology11.
In addition to a background in medical or life sciences, a CRA is required to have a good grasp of data management, including Electronic Data Capture (EDC), data analytics and reporting12.
Sketching the CRA work profile, the authors Diane St. Germain and Marjorie Good state that CRAs are the ones who scrutinize clinical study data most closely from start to finish—as a result, they are often the first to notice critical patterns and interesting trends, and to report these to the research team as well as to the CRO13.
Equally if not more importantly, a CRA must possess a high level of emotional and interpersonal savvy. This is a crucial area, since a CRA’s success hin ges upon his/her ability to elicit the best from team members, in terms of both performance and probity.
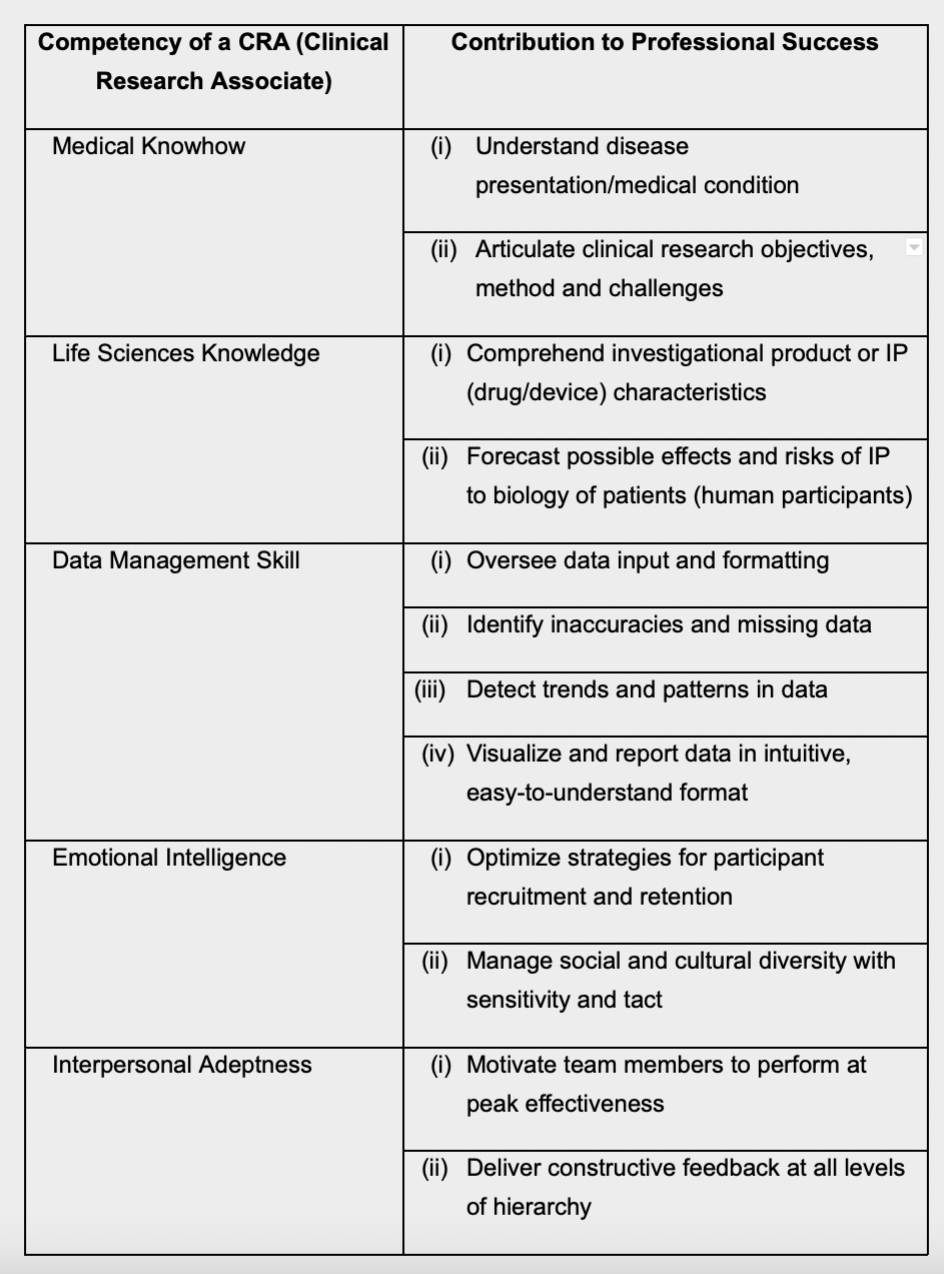
Core Competency Framework for CRAs
To illustrate, the ACRP’s ‘Core Competency
Framework for Clinical Study Monitoring’
requires that a CRA should be able to identify
and correct compliance violations at a study
site. The CRA must not only bring such
violations to the attention of site staff, s/he
must induce them to take corrective action,
as well as reporting the matter and even
escalating it, where necessary14.
The table below summarizes the ideal
competencies of a CRA, and provides
insights on how each ability contributes to
the CRA’s performance.
CRA Career Path
In the past, CRA positions were often filled by individuals with medical or nursing backgrounds, with little thought given to their lack of research training15. As awareness grew about the importance of research experience for a CRA, employers began preferring those with years of experience in clinical research settings, such as Clinical Trials Assistants (CTAs) and Clinical Research Coordinators (CRCs)16.
However, in recent years, the focus has shifted once again from a tenure-based mindset to a skills-based evaluation17. In part, this change has been brought about by the growth in professional courses and training programs in the field.
For instance, many leading US Universities today offer master’s programs in clinical research18. In addition, there are some widely recognized certification programs for clinical research associates, such as those offered by the ACRP19 and the Society of Clinical Research Associates (SOCRA) 20.
Note: You must already be working as a CRA to qualify for the ACRP and SOCRA certification programs.
A Toe in the Door: CRA Certification for a Non-CRA
By this point, you might be wondering, “I have no research experience… I’ve never worked as a Clinical Trials Assistant (CTA) or a s a Clinical Research Coordinator (CRC). Nor do I have a degree in Clinical Research. Can I still become a CRA?”
The simple answer is, yes, you can.
You might be a life sciences graduate looking for a lucrative career in the pharmaceutical or biotechnology sectors. Or, you’re excited by a career in research, but unsure whether the drudgery of a Ph.D. is your thing.
Maybe you’re just looking for a job that represents a great option for someone with your combo of science background plus detail-orientedness.
Whichever of these descriptions best applies to you, a career as a Clinical Research Associate could be exactly right for you.
With the right training, you can be recruited directly to a Clinical Research Associate position, even without a background in clinical research.
So, what kind of training will help me break through the ‘experience’ barrier and land a job as a CRA?
As you’ve already gathered from the table, the skill-set required to be a successful CRA is pretty extensive.
Aside from an in-depth knowledge of scientific and medical concepts and principles, a CRA must have a sound grasp of medical research regulatory requirements, a penchant for being thorough and systematic, as well as a knack for coordinating and managing people with diverse skills, roles and backgrounds.
To our knowledge, CCRPC’s ‘Advanced Clinical Research Associate Certification’ (ACRAC) is one of a kind: The ACRAC is the only multi-accredited* certification program in the US that offers the kind of exhaustive as well as intensive training that equips candidates from a non-clinical background with the abilities and competencies that make a good CRA.
Best of all? The ACRAC is open to fresh graduates holding a B.S. degree in any of the life sciences, with no requirement for prior exposure or experience in clinical research.
*The ACRAC program offered by CCRPC is accredited to ACCRE (Accreditation Council for Clinical Research & Education), ACCME (Accreditation Council for Continuing Medical Education), ACPE (Accreditation Council for Pharmacy Education), ANCC (A merican Nurses Credentialing Center), as well as Transcelerate Biopharma.

Training to be a CRA through CCRPS ACRAC
The ACRAC program includes over 100 course modules that cover all the important knowledge domains and skill-sets required by a CRA.
Designed for a total study time of approximately 250 hours, this training program can be completed at your own pace, or, for those able to dedicate the whole day to study, in as little as two to three weeks.
Starting with a broad overview of clinical research jargon and terminology, the course walks students through the principles of Good Clinical Practice, familiarizing you with the relevant sections of the ICH-GCP and the FDA’s E6(R2).
The program places particular emphasis on ethical practices in research with vulnerable populations.
Students going through the ACRAC are trained in all major aspects of designing a Clinical Trial Protocol in keeping with the Code of Federal Regulations (CFR).
They additionally learn the steps involved in the IRB/IEC approvals process and how to prepare required documents.
Finally, students become aware of the importance of pharmacovigilance and the regulatory process for new drug testing.
A major chunk of the ACRAC certification centers around equipping the CRA for day-to-day responsibilities, such as different types of site visits – preliminary (Site Qualification), preparatory (Site Initiation) and progress monitoring visits (Routine Monitoring).
Crucially, the ACRAC covers essential documentation such as the Case Report Form and Trial Master File, as well as electronic data capture (EDC) and remote monitoring systems.
A vital component of the training program involves empowering students to tackle challenging situations.
For a CRA, these include identifying protocol deviations and violations, and recognizing as well as reporting research fraud and ethical misconduct.
In addition to its comprehensive coverage, the ACRAC certification offers the great advantage of including 17.5 CME credits – that is, course credits that count towards ‘Continuing Medical Education’.
These credits can be used by individuals desiring to further their education and/or careers in healthcare-related fields, including medicine, nursing, pharmacy and research.

Clinical Research Associate Training
Get ahead in clinical research with advanced accredited online CRA certification for $450. Demo our on-demand course below.
Clinical Research Associate Certification
Advanced clinical research associate certification (acrac).
Chapter 1: Introduction
This chapter orients you to the concept of Continuing Medical Education (CME) and outlines how the CCRPS CRA program contents meets AMA requirements for CME. Given that, across the US, physician practitioners are required to complete between 20 and 50 hours of CME credits yearly, the ACCME-accredited CCRPS CRA course can be used not only to build knowledge and skills in the field of clinical trial management, but also to further a successful medical career. Additionally, the introductory chapter introduces you to the clinical terminology and abbreviations commonly encountered in clinical research, for example, Investigational Product (IP), Good Clinical Practice (GCP), Institutional Review Board (IRB) and so on.
Chapter 2: Roles and Relationships in Clinical Trials
The unit presents the foundational background to beginning and building a career as a clinical research associate (CRA). As you know, a CRA plays a critical role in setting up as well as monitoring the clinical trials process for an investigational product or IP – a medical drug or device under development. In this unit, you will learn how a CRA interacts with other stakeholders, including the Clinical Research Organization (CRO) or Sponsor of the clinical trials, the Principal Investigator (PI) as well as other research site staff, the trials monitoring team including the Clinical Research Coordinator (CRC),other CRAs and the Data Safety Monitoring Board (DSMB), as well as the research ethics committee (Institutional Review Board or IRB).
Chapter 3: Sponsor and Investigator Roles
In this unit, you will gain insight into the ICH-GCP guidelines, particularly addendum E6, sections 2 through 5, which outline procedures and precautions essential for protecting the safety and wellbeing of human research participants during clinical research. These include guidelines for obtaining informed consent from human subjects, maintenance of trial records, reporting of compliance, safety and research progress, as well as procedures for suspension or termination of the trials process. The chapter familiarizes you with the critical importance of monitoring for Adverse Events (AEs), including types of AEs and regulations for documentation and reporting.
Chapter 4: Clinical Trial Design
In this chapter, you will acquire insight into the different phases of the clinical trials process, from the pre-clinical phase through Phases 0 to 4 of clinical testing. The unit will familiarize you with important concepts of clinical trials, such as the structure and goals of each phase of clinical trials, approaches to dosing, toxicology of pharmaceutical products, in vitro and in vivo testing, dose escalation and so on. Finally, the chapter reviews the FDA’s drug approval process.
Chapter 5: ICH-GCP – Overview
The chapter dives deep into GCP, including a review of the history of medical research leading up to the ICH-GCP. The unit covers all four QSEM categories of the guidelines for ensuring Quality, Safety and Efficacy of the IP, as well as Multidisciplinary guidelines (mainly pertaining to documentation and electronic data safety standards). In addition, the chapter includes an overview of MedDRA software that provides a standardized system of terminology and notation for documenting clinical research, as well as principles of budgeting for clinical trials.
Chapter 6: Ethical Research in Vulnerable Populations
The unit provides a detailed walk-through of the regulations and compliance requirements for conducting clinical trials with human subjects who meet the definition of a ‘vulnerable population’, including pregnant women and fetuses, children, mentally incapacitated individuals (those with cognitive functioning impaired by neurolopsychological conditions or chronic substance abuse), as well as prisoners. You will acquire familiarity with the challenges of research in such populations, including the requirement for parental consent, fair but not excessive incentive, justifiable deception or incomplete disclosure, coercive practices and so forth.
Chapter 7: Adverse Events
Through this module, you will gain a bird’s eye view of the protocol for documenting, reporting and responding to AEs or adverse events during the clinical trials process. The unit covers concepts such as expectedness, severity and seriousness of AEs, Adverse Drug Reactions (ADRs) as a sub-category of AEs, Investigational New Drug or IND reports, causality analysis for AEs and so on. In addition, the chapter reviews the responsibilities of both research sponsors as well as IRBs in sharing AE information with subjects.
Chapter 8: Clinical Trial Protocol
The chapter provides an in-depth tutorial on the structure and elements of a CTP or clinical trial protocol, as well as guidelines on writing a CTP. Important concepts reviewed include study Risk Benefit Analysis (RBA), study sample statistics (sample size, statistical power, plan for data analysis), risk management and study administration. Additionally, the module covers concepts central to study sample selection, addressing inclusion and exclusion criteria, especially safety and ethics considerations in sampling.
Chapter 9: Protocol Deviations and Violations
Through this unit, you will gain familiarity with the many potential causes of protocol deviations and violations, learning to distinguish between minor (deviations) and major departures or violations of protocol. Content provides understanding of the most commonly occurring violations, including both minor (off-schedule subject assessments, subjects’ use of prohibited drugs, and so on) as well as major violations (failure to obtain informed consent, failure to report AEs and so forth). Further, the chapter reviews principles for reporting protocol deviations, IRB approval for planned deviations and related concepts.
Chapter 10: IRB and DSMB
This chapter briefly reviews the history of IRBs and examines the principles guiding IRB decision-making. In addition, the unit discusses recent developments in compliance, including sIRB (single IRB) and SmartIRB for institutions that are part of the CTSA (Clinical and Translational Science Awards). The bulk of this module dives into the categories of IRB review, including full board and expedited review, examining criteria for review exemption such as educational or purely behavioral research, as well as studies collecting identifiable data, surveys and interviews.
Chapter 11: Review Questions
The module provides a self-assessment tool by including questions that review the content covered in previous chapters. The set of 71 questions examines all aspects of ICH-GCP previously discussed.
Chapter 12: Site Monitoring Visits
In this module, an overview is provided of the different types of site monitoring visits, including site selection or qualification visit, study initiation visit, routine or progress monitoring visit, as well as study termination or close-out visit. Important concepts discussed include pre-qualification preparations and site feasibility assessment as well as study monitoring criteria (data omission, incorrect entries, inaccurate calculations, documentation of corrections and so on). For each type of site monitoring visit, the chapter reviews relevant documentation.
Chapter 13: Site Qualification Visit (SQV)
The chapter gives an in-depth understanding of the stages and steps involved in selecting a study site. Elements reviewed within the module include the process of investigator selection and criteria for site evaluation (the four P’s: Patient, Protocol, Performance, Profit). Importantly, the module reviews the most common errors in feasibility assessment, including overestimation of sample availability at site, selection of site staff with low motivation, poor-performing sites owing to high competition for personnel and resources (for example, owing to multiple studies running on a single site), and so on.
Chapter 14: Site Initiation Visit (SIV)
The module dives into the details of an SIV or site initiation visit. You will review the procedure for pre-SIV preparation, including filing for IRB and other necessary approvals, permits and licenses. Additionally, the chapter examines elements of the SIV agenda, mainly orientation and training of site staff, creation of important study-related documents such as the Trial Master File (TMF) and post-SIV filing of compliance documents such as FDA form 1572 and Financial Disclosure Form (FDF) for relevant site personnel.
Chapter 15: Routine Monitoring Visit (RMV)
In this unit, the elements of a routine or periodic monitoring visit are discussed in detail. You will become familiar with the agenda of an RMV, which prioritizes receiving updates on AEs from site staff (incidence, documentation, seriousness and so on), as well as oversight of the overall progress of trials. The chapter covers different approaches to site monitoring, contrasting traditional (full-scale) monitoring with risk-based monitoring (RBM), as well as comparing on-site monitoring with remote monitoring. A crucial concept addressed by the unit is Source Data Verification (SDV), which is central to obtaining meaningful, high-quality data from clinical trials.
Chapter 16: Site Close-Out Visit (SCOV)
The module gives you a comprehensive overview of the protocol and procedures involved in terminating or closing out a trial site. Aspects covered in the chapter include pre-SCOV preparations such as IRB notification and schedule coordination among site staff (PI, other investigators, medical staff) and monitoring team (CRC, CRAs and so on), agenda for an SCOV – drug inventory management, database verification and lockdown, subject intimation and completion of all subject-related documents, staff-related documentation as well as other administrative tasks including close-out report compilation.
Chapter 17: Tools for Monitoring Visits
This unit outlines a host of tips and tools that can help a CRA in successfully tackling the complex process of monitoring clinical trials. The chapter lists numerous physical accessories you can use for effective monitoring, including scheduling and calculation aids, ready reckoners for drug information and medical terminology, as well as document templates to speed up the process of obtaining trial updates while also serving as checklists for the site visit agenda. Additionally, the unit highlights helpful strategies that a CRA can use to ensure that site visits go smoothly, from travel advice to team-building suggestions.
Chapter 18: Audit and Inspections
The module deals with one of the most crucial and often most feared aspects of a CRA’s career – audits and inspections by the CRO (sponsor), FDA or other regulatory authority. Starting from the basic distinction between an audit and an inspection, the chapter covers in detail the protocols for both audits and inspections. Crucially, the chapter will enable you to grasp the difference between a routine audit/ inspection and a ‘for-cause’ audit/ inspection. Further, it lays out the sequence of an FDA inspection in full (including a detailed walk-through of the FDA BIMO or Biomedical Research Monitoring Program inspection), and provides important guidelines on the do’s and dont’s for CRAs during an audit/ inspection, such as the critical ‘3 to 5 minute rule’. You will acquire familiarity with important audit and inspection-related documents such as FDA Form 482 (Notice of Inspection) and Form 483 (Notice of Observation) as well as the Establishment Inspection Report (EIR) prepared by the auditor/ inspector. Finally, you will gain insight into the classes of observations provided in an EIR, including NAI (no action indicated), VAI (voluntary action indicated) and OAI (official action indicated)—the last is commonly termed an ‘FDA warning letter’.
Chapter 19: Review Questions
The unit contains a self-assessment tool comprising 65 questions that review the content covered in previous chapters, as well as a 15-item quiz. Questions and quiz examine all aspects of clinical trial quality monitoring, including monitoring visits, tools as well as audits and inspections.
Chapter 20: SDV and Informed Consent
In this chapter, the ICH-GCP section 4.8 guidelines on obtaining informed consent from subjects are discussed in detail, highlighting the need for using non-technical language, transparent delineation of risks, consent without undue influence, obtaining consent (and assent) from minors and their Legally Acceptable Representatives (LARs), as well as consent from non-English speakers and sedated subjects. The chapter additionally covers important aspects of Source Data Verification (SDV) with respect to electronic as well as paper-based medical records, and highlights the central goal of SDV, which is to conform to ICH-GCP requirements that subject trial data (as recorded in Case Report Forms or CRFs) must correspond to source data (previous medical records).
Chapter 21: Case Report Form
The module provides an in-depth tutorial on the structure and elements of a Case Report Form or CRF, including the different forms for PI verification, subject enrollment, eligibility and randomization, medical history, physical examination and laboratory data, compliance, adverse events and so on. In addition, the chapter outlines important data notation rules, such as the use of accepted acronyms (‘ND’ for missing data and ‘UNK’ for unknown information, MM-DD-YY format, time-stamp data and so forth), as well as guidelines for the design of CRFs (such as consistency of notation, avoidance of data fields that can be computed and of duplicate data fields and so on).
Chapter 22: Quality Control and Safety
Within this unit, you will learn the central concepts of Quality Control (QC) in the context of clinical trials, including definitions of QC and its relationship with the complementary process of Quality Assurance (QA), the use of Key Performance Indicators (KPIs) in QC, need for a Corrective and Preventive Action (CAPA) plan and so on. Additionally, the module examines the QA process, focusing on the central role of RBM or risk-based monitoring in present-day QA as well as providing guidelines on Quality Metrics (QMs) for evaluating the trials process. The chapter also reviews ICH-GCP guidelines on subject safety, underlining risk-benefit assessment, stoppage rules (for instance, in case of SAEs) and reporting responsibilities. Finally, it introduces the FDA’s Human Research Protection Program (HRPP) as a platform that provides training and support for personnel involved in clinical trials.
Chapter 23: Technology in Trials
In this chapter, an in-depth tutorial is provided of the systems used in modern clinical trials for Electronic Data Capture (EDC) and database management. Systems such as Interactive Response Technologies (IRTs) including IVRS and IWRS (Interactive Voice and Web Response Systems, respectively) as well as RTSM systems for Randomization and Trial Supply Management are examined. The unit reviews the benefits of standardized data management and data sharing, approaches to database management and the concept of an Independent Data Monitoring Committee (IDMC). Critical elements of data integrity, such as proper anonymisation and coding, completeness of data, data safety precautions and logging of site visits and other progress reports are highlighted. The unit further examines the essential features of a good Clinical Data Management (CDM) system that complies with FDA CFR Title 21 and HIPAA regulations, such as setting access privileges, tracking changes and updates, data security and locking, flagging and reconciliation of AEs and so forth. Finally, the chapter looks at CTMSs (Clinical Trial Management Systems) in depth, covering the aspects that allow management of day-to-day trials in multi-site studies.
Chapter 24: Modernized Monitoring (Remote, Risk-based, Centralized)
This chapter offers a detailed walk-through of modern, remote monitoring of clinical trials, which evolved into a full-fledged system in response to the COVID-19 pandemic. Important concepts discussed include the critical site initiation process, Electronic Source Data Verification (ESDV) and FDA regulatory guidance for remote monitoring of clinical trials. In this module, you will learn how FDA’s ALCOA (Attributable, Legible, Contemporaneous, Original and Accurate) criteria for data quality have been adapted to remote monitoring. Further, the unit discusses how HIPAA compliance in remote monitoring is achieved by using limited data sets (wherein sensitive individual information is concealed through anonymous subject codes) regulated by data use agreements. Finally, the unit examines how risk-based monitoring approaches have allowed centralized monitoring to evolve into a cost-effective and safe method for clinical trial monitoring.
Chapter 25: Pharmacovigilance and Regulatory Affairs
Through this unit, you will gain insight into the process and rationale behind pharmacovigilance (PV) and its central role in the clinical trials process. The chapter reviews the statistics on AEs, distinguishes between Type A and Type B AEs, and profiles seriousness of ADRs or Adverse Drug Reactions as well as the iGuard Drug Risk Rating System. Importantly, the unit covers ADR causality assessment in detail, including both severity and probability assessment. An important element of PV addressed in this module is the Individual Case Safety Report (ICSR), its structure, content and role in trial monitoring. Other concepts discussed include types of PV inspections (routine vs. ‘for cause’), PSURs or Periodic Safety Update Reports and study criteria for instituting DSMBs (Data Safety Management Boards). Finally, the module also reviews the domain of Regulatory Affairs (RA) as a function of PV, outlining roles and responsibilities of RA personnel as well as the importance of RA in streamlining the process of drug development by ensuring compliance throughout manufacturing, clinical trials, marketing and advertising.
Chapter 26: Investigational Product
In this chapter, an in-depth review is provided of the protocol for receiving, storing and dispensing the IP or investigational product. At every stage, guidelines lay down strategies for ensuring verifiability, accountability and safety of both study subjects and staff. Thus, IP handling precautions include the need for logging date of manufacture, temperature throughout transit, as well as batch number and individual unit numbers (such as bottle or tube identifiers) carefully and accurately, as well as recording shipping details and filing shipping receipts. Additionally, the unit addresses the need for IP dispensing precautions, such as limiting dispensation to authorized personnel only, as well as maintaining individual subject IP logs.
Chapter 27: Local and Central Labs
The module profiles the evolution of lab testing in clinical trials, from error-prone localized laboratory testing to centralized testing that allows homogeneity of testing procedures and measurements, thus minimizing errors and improving outcomes. The chapter reviews standards for clinical trial laboratories as per the GLCP (Good Clinical Laboratory Practice) and CLIA norms (Clinical Laboratory Improvement Amendments), as well as providing guidelines for lab audits, including fire safety, protective gear, staff training and so forth.
Chapter 28: Review Questions
The unit contains a self-assessment tool comprising 65 questions that review the content covered in previous chapters, as well as a 15-item quiz. Questions and quiz examine all aspects of trial documentation (SDV, CRF, ICSR), quality control, pharmacovigilance, as well as IP and lab guidelines.
Chapter 29: Regulatory Documents in Clinical Trials
The chapter reviews essential documentation to be created and maintained throughout the course of the clinical trials, including the Trial Master File (TMF), FDA forms 1571, 1572, 3674, 3454/3455 and CFR Title 21 Form 312, besides ethics approval documents such as the IRB-approved protocol, informed consent form, subject education and study advertising materials. You will acquire in-depth familiarity with each of these forms, and learn the importance of maintaining and updating records, for example by incorporating IRB revisions and amendments, periodic renewals of permissions and licenses and copies of submitted reports. In addition, the unit summarizes the need for filing documents outlining study- and site-specific procedures, including SOP (Standard Operating Procedure), MOP (Manual of Procedures), Investigator Brochure (IB), Delegation of Authority Log (DOAL), site staff CVs, SAE notifications, logs of subject screening and enrollment, IP storage (temperature, humidity, etc.) and all relevant study parameters.
Chapter 30: CFR Title 21 Part 11 – Electronic Signatures
This unit gives you an overview of Title 21 of the FDA Code of Federal Regulations (CFR), including Chapter 1 sections on informed consent (Section 50), IRB approval (Section 56) and so on, Series on food (100), pharmaceuticals (200 and 300) and so on, as well as FDA Drug Schedules. The major part of the module focuses on Part 11 which deals with Electronic Records and Electronic Signatures (ERES), laying down the criteria for determining safety and reliability (trustworthiness) of electronic data and signatures.
Chapter 31: New Drug Application
Through this module, you will gain knowledge of the FDA process for evaluating a drug under development, and the role of a CRA in streamlining this process. An important distinction covered here is the difference between an IND (Investigational New Drug) and an NDA (New Drug Application). The chapter discusses in-depth the criteria used in evaluating an IND, including toxicology and pharmacokinetics data, as well as requirements for different drug classes (oncology vs. non-oncology). Additionally, the unit covers FDA requirements for AE reporting, including assessment of seriousness, expectedness and format for expedited reporting of life-threatening SARs, as well as safety reporting requirements for investigators.
Chapter 32: Trial Master File
The unit provides a detailed breakdown of the organization of a TMF or Trial Master File, listing the various binders that should be included within the TMF, as well as their contents. Thus, the TMF should contain binders pertaining to the study protocol and IRB, investigator qualifications, FDA forms and correspondence, FDFs or Financial Disclosure Forms, communications with the CRO, and other relevant trial aspects. A helpful templatic guide to creating a TMF is also provided in this chapter, as well as a self-assessment quiz of 10 items on important sections of a TMF.
Chapter 33: Disclosures and Payments for PI, Site, Patients
In this chapter, FDA guidelines regulating financial disclosure are discussed in-depth, covering the definition of ‘conflict of interest’ and the stipulations of Title 21 Section 54 on disclosure requirements. The unit helpfully contrasts FDA requirements with Canadian and UK/EU policies. You will study real life case examples of conflict of interest, as well as lawsuits pertaining to financial disclosure disputes to help gain a better understanding of the potential problems arising from failure to disclose financial interests in clinical trials. Another important dimension covered in the module is the regulation of payments to PIs and other investigators as well as patient payments, which must comply with CMS (Center for Medicare and Medicaid Services) policy on ‘fair market value’ as well as the Federal ‘Anti-Kickback Statute’. The unit contains guidelines on clinical trial budgeting and subject payments. Finally, the chapter reviews IRB guidelines on advertising to recruit human participants for clinical trials, including stipulations against misleading and coercive language, as well as excessive incentives.
Chapter 34: Patient Recruitment, Retention and Compliance
The unit provides an overview of the process of patient (subject) recruitment in clinical trials, from population research to identify motives for participation, to media support for building up public awareness and interest, to community and physician outreach for referrals and enrollment. Additionally, the chapter identifies common barriers to meeting recruitment goals and outlines strategies for maximizing recruitment, such as relaxing overly stringent criteria, offering reasonable incentives such as travel reimbursement and highlighting benefits of participation. Similarly, the unit covers common causes of patient drop-out as well as strategies for minimizing drop-outs, such as improving patient experience (increased attention and listening to patients, flexible scheduling of visits to suit patients’ convenience and so on). Finally, the unit discusses novel strategies to increase patient retention and improve compliance in clinical trials; these techniques harness technology to yield better outcomes, for example, simplifying form completion through digitized forms with auto-fill features, gamifying elements of compliance reporting, and so forth.
Chapter 35: Misconduct and Fraud
This module discusses the various motives for committing scientific fraud and the fallout of fraudulent practices in clinical trials. A scale for classifying errors in clinical trial data is presented, with ‘honest, isolated mistake’ at one end of the spectrum and ‘deliberate data falsification with malicious intent’ at the other. Types of clinical data that may be falsified, methods used in falsification (fabrication, substitution, omission), as well as scenarios in clinical trials where falsification may be occurring are presented. Through this chapter, you will gain familiarity with the signs to watch out for during the actual clinical trials process.
Chapter 36: Review Questions
The unit contains a self-assessment tool comprising 65 questions that review the content covered in previous chapters, including questions on all aspects of regulatory documents, site documents (TMF and contents), trial budgeting and payments, patient recruitment and scientific fraud.
Chapter 37: Site Visit Templates
This module contains a set of templates that you can use for documenting the details of site monitoring as a CRA, either in their current form, or in a form adapted to the needs of your own study. The templates included in this unit include:
Site Qualification Visit (SQV) – checklist for preparations, questionnaire for assessing the site prior to the actual visit, assessment form and follow-up letter
Site Initiation Visit (SIV) – agenda for visit, confirmation letter to request PI attendance during SIV, report following SIV
Routine Monitoring Visit (RMV) – confirmation letter to request PI attendance, report following RMV, follow-up letter
Site Close-Out Visit (SCOV) – confirmation letter to request PI attendance, agenda for SCOV, report following SCOV, follow-up letter
CRA transition letter – document notifying site PI of appointment of new monitor (yourself as CRA)
Chapter 38: Interviewing and Career
In this unit, you will find suggestions and recommendations for making a positive impact in interviews for CRA positions, as well as tips and strategies for making rapid progress in a clinical research career.
Chapter 39: Final Examination
This module comprises a comprehensive 51-item, self-paced quiz to assess your competency in the skills and knowledge required for a Clinical Research Associate position.
https://www.beroeinc.com/category-intelligence/clinical-research-organizations-market/
https://www.linkedin.com/jobs/search?keywords=Clinical%20Research%20Associate&location=United%20States&geoId=103644278&trk=public_jobs_jobs-search-bar_search-submit&position=1&pageNum=0
https://www.centerwatch.com/articles/24791-demand-for-experienced-clinical-trial-professionals-outpacing-supply-acrp-says
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3317309/
https://www.niaid.nih.gov/research/dmid-investigational-product
https://www.fda.gov/patients/clinical-trials-what-patients-need-know/what-are-different-types-clinical-research
Dixon JR. 1999. The international conference on harmonization good clinical practice guideline. Quality Assurance. 6(2): 65-74. DOI: 10.1080/105294199277860
https://www.fda.gov/files/drugs/published/E6%28R2%29-Good-Clinical-Practice--Integrated-Addendum-to-ICH-E6%28R1%29.pdf
https://www.who.int/groups/research-ethics-review-committee/recommended-format-for-a-research-protocol/
https://iaocr.com/finding-first-clinical-research-job/
https://jobs.newscientist.com/en-au/article/a-career-in-clinical-research/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3326906/
St. Germain DC, Good MJ. 2017. Data management in clinical trials. In: Gallin JI, Ognibene FP, Lee Johnson L, editors. Principles and practice of clinical research. San Diego: Academic Press. p. 531-545. ISBN 978-0-12-849905-4
https://acrpnet.org/wp-content/uploads/dlm_uploads/2017/04/clinical-study-monitoring-competencies.pdf
https://www.clinicalleader.com/doc/starting-a-career-in-clinical-research-things-we-wish-we-knew-0001
https://www.proclinical.com/blogs/2021-9/how-to-get-a-job-as-a-clinical-research-associate-cra
https://acrpnet.org/2018/06/11/5-clinical-research-trends-emerge-at-acrp-2018/
https://www.collegechoice.net/sciences/clinical-research/best-masters-degrees/
https://acrpnet.org/certifications/cra-certification/
https://www.socra.org/certification/program-overview/
Pharmacovigilance: A Complete Guide to Pharmacovigilance and Drug Safety Training
The ultimate guide to clinical research monitoring.
Get extra -20% OFF THE ALL-IN-ONE COURSE with "EXTRA20"
SAVE UP TO -50%
ON YOUR FIRST PURCHASE
Clinical Research Associate Academy
- Level: Intermediate
- Continuing Education Units: up to 500 contact hours, 50 CEU
- Certificates: GCP, VIARES
- Duration: up to 500 contact hours, 50 CEU
THE CRA ACADEMY PROVIDES YOU WITH
Industry required competencies for a Clinical Research Associate role including
- introduction to clinical research
- understanding GCP and general regulatory requirements
- on-site trial monitoring
- remote and centralized monitoring
- monitoring oncology trials
- patient protection and informed consent
- handling of adverse events
- data management basics for clinical trials
A proven process to prepare your job application
- identify your dream job
- understand what are the requirements and how to meet them
- create a clear and concise CV
- prepare your interview
- negotiate your package
You can start your program any time after purchase and define your own pace with our online setup. This is ideal for anyone with a current employment.
ACADEMY CONTENT
Clinical research associate.
- What is clinical research
- How are clinical trials managed
- Who can participate in clinical trials
- What are the key phases and roles in clinical trials
- ICH GCP E6(R2) Certificate
- Investigator Oversight
- Background of medicines development
- Research and discovery stage & product development
- Clinical development
- Regulatory submission, Health Technology Assessment, lifecycle management
- ICH GCP and other applicable regulations
- Applicable international and national regulations
- ICH GCP E6 (R2)
- FDA Regulation
- Quality Assurance: Audits and Inspections
- Elements of a Study Protocol according to ICH GCP
- Trial Design
- Methodologies
- Ethics Committees
- Sponsor / Monitor
- Investigator
- Competent Authority
- Assessing Investigational Sites
- Training and Upgrading Investigational Sites
- Organizing the Initiation Visit
- Setting up the required documentation – Essential Documents
- Planning, Conducting, Documenting, and Reporting Monitoring Visits
- Managing Issues
- Organizing the Close-Out Visit
- Documentation
- Patient Information
- Collecting Patient Consent
- Special Patient Populations
- Types of Adverse Events
- Identifying and Reporting Serious Adverse Events
- Definition of investigational medicinal product (IMP) / study drug
- Provision of the IMP
- Drug Accountability
- Randomization, blinding and un-blinding processes
- Managing Expiry Dates
- Collecting IMP after Site Close-out
- Clinical Research Form (CRFs)
- Process of Data Collection
- Data Collection Systems
- Data validation process
- Query Process
- Data Quality Assurance
Remote & Centralized Monitoring
- Including on-site, remote, and statistical monitoring
- Regulatory guidance to remote and statistical monitoring
- Monitoring concepts in the framework of risk-based monitoring
- From concept to reality: Remote monitoring and centralized statistical monitoring
- Responsibilities
- Tools and resources
- How to handle centralized statistical monitoring
- Monitoring activity and their focus
- Documentation and follow-up
- Team interactions
- What makes a good Remote Monitor
Monitoring Oncology Trials
- Understand the characteristics of cell formation, the major causes of cancer and global impact of the disease
- Examine the impact of Oncology studies and current treatment
- Be able to discuss available treatments and objectives of treating cancer
- Examine various cancer treatments and their adverse reactions
- Explore alternative treatment options
- Identify common cancer drug and cancer combinations
- Thoroughly understand the relationship between risk and benefits of tumor therapies
- Recognize chemotherapy drug types and different principles
- Differentiate between cancer and uncontrolled tumor growths
- Recognize the six major categories of cancer classifications
- Understand components of medical terminology related to cancer
- Be able to discuss Oncology trial schematics and understand the design features.
- Be able to describe the four phases of Oncology studies.
- Understand Endpoint requirements in Oncology
- Recognize common clinical assessments related to oncology trials
- Gain proficiency in reviewing clinical reports
- Identify disease history and common Inclusion/Exclusion criterion
- Define each line of therapy and understand the timing of each line
- Understand dosing design in oncology studies
- Recognize most common Adverse Events and be able to discuss clinical events related to oncology studies
- Recognize common toxicities and understand the DLT process
- Be able to discuss recruitment challenges in Oncology studies
- Describe the screening and enrollment process
- Understand the role of the medical monitor and various key members during screening process
- Clinical Reports and Source
- Distinguish between RECIST 1.0 and RECIST 1.1
- Utilize appropriate oncology disease progression algorithms
- Understand disease progression
- Prepare for challenges with site structure and Delegation of Responsibility at oncology sites
- Describe key site staff and associated roles
- Discuss the key function of site staff
- Learn Sponsor/CRO expectations of Oncology Monitors
- Understand the stress and pressures of monitoring oncology studies
- Learn time saving techniques while monitoring
Application support program
included with one-time payment plan
- Setting clear goals and expectations is the crucial first step when it comes to your job search.
- Understand WHAT are the required skills of your dream job and how to acquire them
- you will address THE most important piece to get your dream job
- As often, it’s more about preparation and practice than any other “miracle tip”. There is no secret – however, there is a good way to prepare correctly for any interview (a method), and this method works well.
- Negotiate and Get What You Truly Deserve.
3 VIARES CERTIFICATES
Upon enrollment you will receive your first certification “Confirmation of Enrollment”. After successful completion of the program and your exams, you get 3 (C linical Research Associate, Remote & Centralized Monitoring, Oncology CRA) personal certificates including:
- course title
- contact hours
- continuing education units (CEU)
- your overall course score
- date of completion
- personal certificate verification code
We also show you how to best share your certificate on LinkedIn and other social media platforms.
Obtaining your certificates can provide several potential benefits, including:
- Professional recognition: A Clinical Research certification demonstrates to employers and colleagues that you have the knowledge and skills necessary to excel in your role as a clinical research professional.
- Competitive edge: In a competitive job market, having a Clinical Research certification can set you apart from other candidates and increase your chances of being hired or promoted.
- Career advancement: With a Clinical Research certification, you may be eligible for higher-paying and more advanced roles in the field.
- Increased knowledge and skills: Pursuing a Clinical Research certification can help you gain a deeper understanding of clinical research methods, regulations, and ethical considerations, which can help you perform your job more effectively.
Overall, obtaining a Clinical Research certification can be a worthwhile investment for those who are committed to their career in clinical research and interested in advancing their knowledge and skills.
Your expert instructors

BARTEK JAROSZ

Gabi Disselhoff

GAVIN CHAIT

Joanna Wilinska Mackowiak

Andreas Beust

PATRICIA HOLLIS
Hear from our graduates.

The VIARES Clinical Research Academy Program offers a great way to learn the basics of clinical research and monitoring. I am now confident in my knowledge and ready to take the next steps on this path. The VIARES team is always there to guide
you and the experienced tutors are keeping you focused while presenting the informative courses. Thank you VIARES for the opportunity to take part in this exciting program!

The VIARES CRA Talent Program was an opportunity for me to achieve rich and tailored knowledge on Clinical Trials. It offered an opportunity for me to learn considering a career switch from the Investigator operations to monitoring. Being guided
by professionals with extensive first-hand experience made the intensive program easily manageable.
I strongly recommend the VIARES Talent Program and will spread the word to acquaintances.
Customer Reviews
So far my journey here to become a CRA has been so insightful and impactful. I look forward to more exciting topics and lectures as far as the CRA program is concerned.
My only critique is with the navigation. When you finish one module it should have an arrow to go to menu of next module The course content is great and informative
Alot of content relevant to my area of work.
The course structure is very simple to follow and above all the topics are taught by experts in the field.
reviews curated by judge.me

- cancel any time
- TODAY 30% off your first month
- Information about pricing
- Language - English
- Access on desktop, tablet and mobile
- Online Exam & Certificate of Completion
- Contact Hours and CEUs
Courses you may also like

Navigating ICH GCP E6(R3)

Clinical Research Career Accelerator

Application Coaching 3 Session
€ 299,90 Original price was: € 299,90. € 149,90 Current price is: € 149,90.
€ 29,90 Original price was: € 29,90. € 14,90 Current price is: € 14,90. / month for 12 months Pay monthly
Get new job posts and all the news about our VIARES clinical research courses in your inbox!
- All Courses
- Verify Certificate
- Privacy Policy
- Term Of Service
- VIARES Trainer
- Contract Sales Specialist
Our support and sales team is available 24 /7 to answer your queries.
Clinical Research Training courses all prices incl. VAT
Copyright © 2023. All rights reserved.

18 Years experience
Bartek has 18 years experience in clinical research business, mostly spent at largest global Contract Research Organizations. At Quintiles he was responsible for pioneer development of clinical start-up and regional contract departments. He served as executive member of clinical start-up leadership team at INC Research, acting as global head of site contract division. His most recent experience includes support for pharmaceutical organizations in various project specific roles. Bartek enables biopharma to get their sites up and running faster. He is author of several training programs about site contract related matters and successfully delivered to hundreds of individuals worldwide.

38 years in pharmaceutical development
Gabriele is currently the Managing Director of CRQS (Creative Regulatory & Quality Solutions), specializing in Strategic Regulatory Affairs, Quality Management, Organizational Development and Change Management. She has 38 years of experience under her belt in pharmaceutical development, with 29 years in pharmaceutical industry (Merck KGaA, Abbott) and 9 years consulting. She has extensive industry experience in regulatory affairs, clinical research and clinical quality assurance, and a thorough knowledge of document management and electronic submissions.

Data Scientist at Whythawk
Gavin Chait is a Data Scientist at Whythawk, which specializes in integrated open data consulting, and training for open knowledge, freedom of information, and economic development projects. He spent more than a decade in economic and development initiatives in South Africa. He was a commercial director at the Open Knowledge Foundation, and led the implementation of numerous open data projects around the world. Gavin has twenty years of experience in teaching at all academic levels, and is a passionate advocate for knowledge development through open access to data and freedom of information.

in the industry for 21 years
Patricia has been in the clinical research industry for over 20 years. She began her career as a clinical research coordinator at the Medical College of Virginia where she coordinated pharmaceutical and PI initiated trials. She has been a Clinical Research Associate for 16 years and has had the opportunity to serve as a Lead CRA, Clinical Trial Manager, Auditor, Mentor, and Clinical Research Instructor. She has worked in all phases of research and is experienced in numerous therapeutic areas, including Oncology, Endocrinology, CNS, Immunology, Cardiology, Nephrology and Device. Currently, she is a contract CRA through her company, The Hollis Group, LLC, which she owns with her husband and serves as a consultant at local research sites. Patricia completed a Bachelor of Science degree at Virginia Commonwealth University, MBA at Texas Woman’s University and has had specialized oncology training from the Oncology of Nursing Society.
Request Course outline
Explore our pricing plans, 12 payments.
- One year access to your training courses
- Personalized certificate upon completion
- VIARES exam – 2 attempts included
- All online-training course material
- Online access to our learning management system
- One year access to your training courses
- VIARES exam – 2 attempts included
- Including the VIAES CAREER ACCELERATOR
- Plan ends automatically after 12 payments
- No training course handouts for offline learning
- No unlimited access
- All benefits from the montly payment plan
- Life-time access to your purchased course
- Training course handouts included
- Life-time support from the VIARES tutors
- And much more...

IMAGES
VIDEO
COMMENTS
The course is specifically designed for those who wish to become a Clinical Research Associate (CRA). Delegates will gain a thorough and detailed understanding of life as a CRA. The attendees will adopt the role as a CRA and engage in conducting mock clinical trials. The course is highly interactive and utilises teaching methods and hands on ...
COURSE DESCRIPTION. If you are looking to enter the field of clinical research, this CPD-accredited 3-day course is for you. It will provide you with a comprehensive insight into an entry-level Clinical Research Associate (CRA) role. This fundamental "how-to" and "why" course, focuses on current practice. You will learn about the CRA's role and ...
Clinical Research Associate Training UK . Students International. Feb 19. Written By Guest User. United Kingdom Clinical Research Career Guide. Clinical research is a field with a lot of career potential all over the world. Clinical research associates, or CRAs, are one of the most sought-after positions in the field. CRAs monitor labs and ...
The ACRP offers the Certified Clinical Research Associate credential. To earn this certification, you must have one of the following: A bachelor's degree and at least 3,000 hours of experience as a CRA. A current CCRC, CPI or ACRP-CP certification and be able to substitute 1,500 hours of work experience.
The Florence Nightingale Faculty of Nursing, Midwifery and Palliative Care at King's is a world-leading centre for applied and allied healthcare, clinical research and education. Our focus is on training the healthcare leaders of the future, with a strong commitment to providing post-qualification programmes of the highest quality.
1. Complete an undergraduate degree. The type of undergraduate degree necessary to become a CRA mostly depends on the specific role you aspire to within this field. Typically, a bachelor's degree in a relevant scientific discipline, such as biology or chemistry, is necessary.
A clinical Research Associate (CRA) primarily manages the managerial aspects of a number of clinical trials, at every stage of the process. This is fundamentally a "practical" and "hands on" workshop that focuses on current practice to ensure the CRA delegate develops practical experience of the role. Participants will learn about the ...
Ditch the internet searching and start your career in clinical research — get trained to a gold standard by experienced professionals established in the field. +44 (0)208 406 1766 [email protected]. courses. ... We want to help you invest in your clinical training! ...
Training takes place mainly in-house and on the job. The nature of the training can vary from company to company, with some employers providing a structured system. Some companies will pay for relevant external training courses through organisations such as the Institute of Clinical Research (ICR). They provide training in areas such as:
CRAs work for pharmaceutical companies and Contract Research Organisations which plan, organise and conduct Clinical Trials on behalf of pharmaceutical companies. CRA's are also called Clinical Trials Administrators (CTAs), Clinical Research Scientists, Clinical Secretaries, Clinical Trials Assistants, Clinical Trials Associates, Data Monitors ...
What the course involves. There are three components involved for completing the course: Online learning course through NIHR Learn - covering the principles and practice of clinical research, statistics and critical appraisal. *Practical experience can be achieved via participation in the NIHR Associate Principal Investigators scheme if desired.
ACRP Certification is an ideal solution for managers looking to enhance team knowledge, grow leaders, and signify to others that their study teams are among the best of the best. Clinical research professional with 3,000 hours of verifiable work experience are eligible to sit for the CCRA ® Exam. Complete eligibility criteria is defined in the ...
According to the Bureau of Labor Statistics, which classifies Clinical Research Associates as Clinical and Medical Informaticians, the starting salary range in Clinical Research ranges between $60,000 and $65,000 per year. Managerial roles boast a significant range, with Clinical Trial Managers earning $97,000 annually.
The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices, continuous learning ...
Watch on. The Clinical Professionals Group launched their graduate training Academy for UK graduates looking to work in clinical trials research and development in 2014. Within the Academy, Clinical Professionals identifies, fully funds and trains the most talented Life Science University Graduates and deploys them into "first to industry ...
The Directory for Clinical Research Practitioners (CRPs) is a space for CRPs seeking accredited registration. ... are key project managers liaising with research investigators and research delivery teams; The UK Professional Standards Authority (PSA) recognises CRPs as an occupational group in health and care. ... If you're starting out on a ...
A clinical Research Associate (CRA) primarily manages the managerial aspects of a number of clinical trials, at every stage of the process. This is fundamentally a "practical" and "hands on" workshop that focuses on current practice to ensure the CRA delegate develops practical experience of the role. Participants will learn about the ...
CRAs certified through clinical research associate training have up-to-date knowledge of both ICH and FDA regulatory requirements for human subject safety in clinical research. The program is flexible, allowing trainees to fit the training into a busy schedule. There is an emphasis on hands-on training using real-life clinical research examples ...
Call or email us directly and find out your next career step: [email protected] or +44 (0)118 9594 990. Clinical Professionals offers training and development programmes for pharmaceutical professionals working in clinical research, reg affairs and pharmacovigilance.
Global Program Code. 463-04-112-GPC02 www.accre-accredit.org 1840 W Whittier Blvd #4320 La Habra, CA 90631 USA Phone: 1-(888) 512-6760
ISBN 978--12-849905-4. Clinical research associate job requirements Enter the field as a Clinical Research Associate (CRA) with CCRPS's accredited training. Remote roles, $6,500-$12,000 monthly, and 33% annual promotion. 7-day CRA certification for a swift career start.
Here's how to get started as a clinical research associate. 1. Qualify for certification. You can take several paths to becoming a certified CRA in Canada. One path is to earn a high school diploma and clock 3,000 to 3,500 part-time hours of work experience in the field.
Search similar titles. Today's top 368 Clinical Research Associate jobs in United Kingdom. Leverage your professional network, and get hired. New Clinical Research Associate jobs added daily.
12 payments - £ 14.90 £ 7.90. The Clinical Trials Assistant Academy is specifically designed for anyone new to clinical research. With or without a life-science education or work experience. This Clinical Trials Assistant Academy will help you to cross the path from your current job into the clinical research industry.
Example Major-Specific Courses: Fundamentals of research, group counseling Concentrations Available: N/A In-Person Requirements: Yes, includes a 2,000-hour internship and at least six practicum ...