AACR Annual Meeting News: Read the latest session previews and recaps from the official news website.
Select "Patients / Caregivers / Public" or "Researchers / Professionals" to filter your results. To further refine your search, toggle appropriate sections on or off.

Cancer Research Catalyst The Official Blog of the American Association for Cancer Research

Home > Cancer Research Catalyst > Experts Forecast Cancer Research and Treatment Advances in 2022
Experts Forecast Cancer Research and Treatment Advances in 2022
The year 2021 defied our expectations in a variety of ways.
The delta and omicron COVID-19 variants imposed unprecedented challenges on the health care system and threatened our hopes of an end to the pandemic, but widespread vaccine distribution provided protection, preventing an estimated 36 million cases and 1 million deaths in the United States. As omicron called into question the efficacy of existing vaccines, tests, and treatments, the U.S. Food and Drug Administration (FDA) provided new options, in the form of emergency use authorizations for the first two oral COVID-19 drugs, nirmatrelvir/ritonavir (Paxlovid) and molnupiravir (Lagevrio).
Aside from the pandemic, supply chain delays and worker shortages sparked frustration, but the national unemployment rate gradually fell to its lowest percentage since February 2020. Through a year of harsh weather conditions ranging from ice storms to wildfires to hurricanes and tornadoes, the United States doubled down on initiatives to battle climate change .
In spite of the year’s setbacks, the field of cancer research also made progress. The FDA approved 16 new oncology drugs —including two to treat genetic conditions that cause high rates of tumor formation—as well as two cancer detection agents that help physicians better identify certain tumors during imaging or surgery. We celebrated the 50th anniversary of the National Cancer Act , saw marked progress in many areas of cancer research , and helped provide cancer patients with reliable information about their COVID-19 risks and vaccine efficacy .
As in previous years , we have asked a panel of experts to reflect on the progress made in 2021 and forecast their predictions for cancer research in the year 2022. We spoke with AACR President-Elect Lisa Coussens, PhD, FAACR , about basic research; AACR board member and co-editor-in-chief of Cancer Discovery Luis Diaz Jr., MD , about precision immunotherapy; co-editor-in-chief of Cancer Prevention Research Michael Pollak, MD , and deputy editor of Cancer Prevention Research Avrum Spira, MD , about cancer prevention; and AACR board member and former Annual Meeting Program Chair John Carpten, PhD, FAACR , about cancer disparities.
Priorities for Basic Research in 2022
“There isn’t a drug on the market that doesn’t have its origins in a basic science discovery,” said Lisa Coussens, PhD, FAACR , chair of the department of Cell Development and Cancer Biology at Oregon Health and Science University, when asked about the ways that laboratory science has shaped the landscape of cancer care. “We can’t lose sight of the importance of basic research at any step in the pipeline toward advancing cancer medicine and improving outcomes for our patients.”
Basic science—fundamental research about the way cells and molecules function and interact—spans applications from protein chemistry to cell genomics to animal models. Such discoveries help researchers determine, for example, which proteins can be targeted with drugs to fight a disease, or which biomarkers might help determine a patient’s prognosis or course of treatment.
An important priority for improving our knowledge of cancer cell biology, Coussens explained, is to better understand how cells shift between different states, especially in response to a disease or therapy.
“We need to understand nuances between different tissue states within our body, and how they respond to changes in their environment,” Coussens said, noting that this is true in healthy organs as well as in evolving tumors, where single cell types typically steer disease processes but are dependent on cues from the multiple cell types surrounding them.
“Understanding those nuances will lead to bigger discoveries about how to target cell state changes so we can return cells back to normal control mechanisms,” she continued.
Tumor cells are not the only cells that might change their patterns of gene expression and metabolism during the course of cancer progression and treatment, however. Other cells that surround and interact with the tumor, such as fibroblasts and immune cells, play a vital role in determining how the tumor behaves.

“A full understanding of tumor ecosystems includes the neoplastic cells—the ‘bad guys’ with mutations—as well as the normal host cells that are recruited or co-opted to help tumor cells survive and disseminate,” Coussens said.
Emerging classes of therapies, such as immune checkpoint inhibitors, leverage elements of the tumor microenvironment to kill cancer cells. In order to develop more drugs targeting these cancer support systems, researchers need to learn more about how tumors interact with their surroundings.
“I think the next years will bring a major focus on understanding communication networks between all the different types of cells in tumor ecosystems,” Coussens said, adding that a basic understanding of cell communications could produce benefits beyond the scope of cancer. “Basic discoveries about tumor ecosystems can have far-reaching impacts on autoimmune diseases, chronic inflammatory diseases, and how individuals respond to therapies that are designed to treat Alzheimer’s, for example,” she explained.
Coussens believes that many of these discoveries will be driven by the expanded use of technology and data science. Since the turn of the century, rapid advances in genomics, proteomics, and metabolomics have created an abundance of biological data from patients, animal models, and cell lines. Designing computational programs capable of integrating these data and determining how to analyze them in meaningful ways has been a constant source of innovation over the past 20 years.
Coussens emphasized that continued progress in this area could significantly shape basic research in the coming years.
“The biggest impact we’re seeing right now is with the emergence of technology development and computational data sciences,” Coussens said. “I think the greatest advances we will see over the next several years will be emerging out of team science embracing technology, data science, and biology.”
As technological advances spur more integration between different disciplines, Coussens predicts that collaboration will become more crucial than ever.
“Science has changed—we no longer do science in isolation,” she said. “The best science today, I think, comes out of multidisciplinary team science. I’m a biologist, but I now need to be able to communicate with data scientists, epidemiologists, and chemists.”
Coussens expressed that young investigators entering the field should consider this new paradigm when planning their training. “The more you can round out your education in a multidisciplinary way, the better. You need to be able to communicate your science with people who don’t necessarily speak your field’s language.”
Part of her advice hinged on trainees finding strong mentors who can help guide them toward these opportunities, especially as they recover from lost time and funding resulting from the COVID-19 pandemic. “Invest your time and energy in identifying mentors who care about who you are and the trajectory of your career. Find mentors who you will grow to respect and love,” she said.
Overall, Coussens was optimistic about the state of basic research moving forward.
“The basic science discoveries we’re going to see in the next five years will reshape the medical landscape for years to come,” she said.
PRIORITIES FOR Precision Immunotherapy IN 2022
The art of deciding which cancer therapies to give a patient, based on their individual tumor characteristics, has evolved over the past several decades, according to Luis Diaz Jr., MD , head of Solid Tumor Oncology at Memorial Sloan Kettering Cancer Center and a member of the National Cancer Advisory Board. Such decisions were first made based on protein markers expressed by the tumor, then by genetic changes in the tumor’s DNA. Now, Diaz said, a precise understanding of tumor characteristics can predict which patients may benefit most from immunotherapy.
“One example has been PD-L1 overexpression, either on the tumors themselves or on the surrounding cells,” Diaz said. “Another is mismatch repair deficiency, which seems to prime cells to become very sensitive to immunotherapy.”
This is just one of the ways that the fields of precision medicine and immunotherapy have grown to complement each other in recent years. As Diaz noted, antibodies targeting PD-1 or PD-L1 have become an effective therapy for patients whose tumors express these immunosuppressive markers.
The treatment of patients with CAR T cells—immune cells which are harvested from a patient’s body, engineered to target tumors, and returned to the patient’s bloodstream—represents an even more patient-specific approach to immunotherapy.
But these therapies are not appropriate for all cancer types, and many patients who receive these therapies eventually relapse, creating a need for the expansion of immunotherapy types and indications.
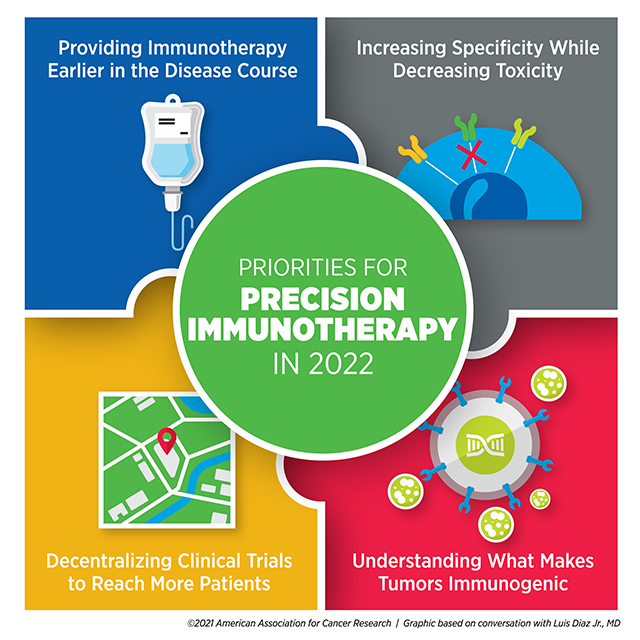
Diaz believes researchers can improve the efficacy of immunotherapy by offering it earlier in a patient’s course of treatment.
“In many cases, we’re testing new therapies on patients for whom all standard therapies have already failed,” he said. “As we move forward, we need to begin to treat earlier in the diagnosis.”
Diaz emphasized that treating advanced cancer poses far more challenges than intervening in early-stage disease or preventing tumor formation altogether. “If we can begin to bring targeted therapy and immunotherapy into the prevention space, I think we’ll see a profound impact,” he said.
A different approach to improving immunotherapy efficiency is to reach more patients by making cell-based immunotherapies, such as CAR T, effective against a broader range of tumor types, including solid tumors.
To overcome these hurdles, Diaz said, “The priority needs to be in maximizing specificity and minimizing toxicity.”
Solid tumors, Diaz explained, are often heterogeneous. An immune response against a single target may kill some of the tumor, but cancer cells that don’t express the target may continue to grow and evade the immune system. Researchers have designed CAR T cells that target multiple tumor cell markers, but more targets also increase the likelihood of harmful side effects.
“It’s a mathematical problem we can’t solve very easily,” Diaz said. “We need some clever new ideas.”
Boosting the number of people who receive immunotherapy also involves addressing accessibility issues, especially for patients in rural or underresourced communities. Diaz speculated that the increase in remote care options resulting from the COVID-19 pandemic might provide a blueprint for the decentralization of clinical trials, paving the way for large cancer centers to collaborate with community hubs.
He emphasized that one way to promote decentralization is to encourage more clinical trial ownership from clinicians rather than pharmaceutical companies. “I’d like to see our investigators becoming the initiators of more trials to be run at large cancer centers and elsewhere,” Diaz said.
He noted that clinical trial decentralization will pose some challenges, such as standardizing procedures and supplies and ensuring that quality does not suffer. However, he was optimistic that it would eventually improve care. “I think it will make clinical development move faster than it ever has before,” he said.
Targeting new populations and tumor types with immunotherapy, however, will only benefit patients whose tumors mount an immune response. Some tumors—deemed immunologically “cold”—expertly evade the immune system, and the mechanisms underlying that process are complex.
“We need a better understanding of what makes tumors immunogenic so we can harness that knowledge to make cancers more immunogenic,” Diaz said.
He noted that research into the interface between immune cells and cancer cells has done a great job of producing the therapies on the market today, but that advancing precision immunotherapy will require those efforts to continue.
“As exciting as everything is that we’re doing, we need to do so much more,” Diaz said. “What’s popular right now is probably only the tip of the iceberg.”
Priorities for Cancer Prevention in 2022
“The most transformative impact we could have on cancer care would be to prevent cancer from happening in the first place,” said Avrum Spira, MD , a professor of medicine, pathology and laboratory medicine, and bioinformatics at the Boston University School of Medicine and global head of the Lung Cancer Initiative at Johnson & Johnson.
Spira and his colleagues study how physicians can better detect early-stage lung cancer or signs of precancerous changes in the lungs. He also studies how to intervene in these early stages to prevent disease progression.
“Researchers have found molecular alterations in late-stage cancer and used that information to develop new targeted therapies and immunotherapies that are transforming the treatment of advanced-stage disease,” Spira said. “It’s absolutely critical to move that fundamental molecular understanding to early-stage and even premalignant disease.”
Understanding what drives benign cells into a tumorigenic state is an important component of this process, Spira emphasized. Drawing on the success of large-scale programs such as The Cancer Genome Atlas , the Human Cell Atlas , and the Human Tumor Atlas Network , dedicated to fully characterizing the blueprints of the human body, researchers have embarked on the development of a Pre-cancer Atlas .
“Within the Human Tumor Atlas Network, researchers are forming large coalitions for multiple different cancer types to develop a temporal and spatial atlas of the cellular and molecular changes associated with the transition of a premalignant lesion to a fully-blown invasive cancer,” Spira said. “I think, in 2022, we’re going to see a proliferation of those types of studies, generating a vast amount of cellular and molecular data from premalignant tissue across many cancer types.”
Spira believes such an atlas will benefit patients in two key ways: the development of biomarkers that can help predict which precancerous lesions will advance to cancer, and the identification of drug targets to stop the progression.
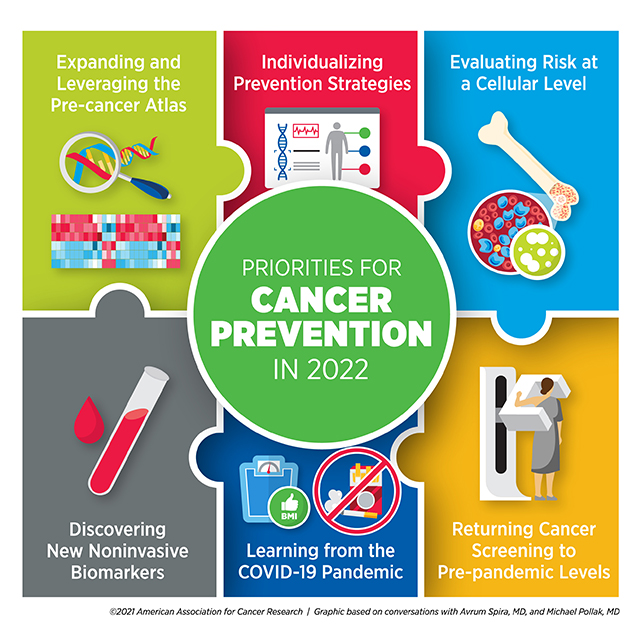
“For most cancer types, we don’t know what those early events are, and therefore, we have no effective way to intercept the disease process,” he said. “I think in 2022, we will begin to understand these events and gain unprecedented insight into targeted approaches aimed at intercepting premalignancy.”
Spira elaborated more on the need for biomarkers, which may not only identify patients at an elevated cancer risk but may also determine which patients with abnormal imaging results may need a biopsy. The most effective biomarkers, he stressed, would be the ones detectable via noninvasive tests.
“I’m excited about the future of blood-based tests looking at nucleic acids,” Spira said. “The technologies are evolving very rapidly to the point where they can now detect very small amounts of DNA or epigenetic changes that are circulating in the blood, and they can screen people across multiple cancer types.”
While blood-based liquid biopsies have attracted a great deal of attention in recent years, Spira also drew attention to other emerging noninvasive tests with the potential to have a significant impact on early cancer detection, such as urine markers of urologic cancer, stool markers of colon cancer, and nasal brushings to assess lung cancer risk.
Spira hopes these noninvasive tests can be integrated with each other and with imaging results to give the best possible assessment of a patient’s risk. “That’s a complicated space, but I think this convergent approach is one that will advance significantly in 2022,” he said.
Even noninvasive tests, however, can only benefit patients who are able to access them. Spira pointed out a few ways the field adapted during the COVID-19 pandemic that could continue to be leveraged moving forward.
“We need to find ways to get screening to patients as opposed to them having to come to the hospital,” Spira said. He highlighted advances such as remote clinical trial management, as well as mobile CT and radiology units, set up in large vans or trucks that can drive to various neighborhoods to perform screening. Used during COVID-19 to promote social distancing and minimize virus exposure, such units could be used in the future to help people catch up on screenings missed during the pandemic, especially in areas with poor health care access.
Spira also noted that the pandemic placed a spotlight on behavioral risk factors that increased COVID-19 susceptibility and the risk for severe disease, such as smoking, alcohol consumption, obesity, and physical inactivity. He pointed out that, often, these same behaviors contribute to cancer risk.
“This has become a teachable moment,” Spira said. “I think we can encourage the public to alter some cancer-causing behaviors that are also related to virus susceptibility.”
Michael Pollak, MD , a professor of oncology and medicine at McGill University in Montreal, Canada, who studies cancer prevention through the lens of reducing risk, also emphasized addressing lifestyle behaviors that affect multiple health conditions.
“An important trend for 2022 may be the concept of healthy lifestyle behaviors integrated across diseases,” Pollak said. “We have to recognize that some of the activities and lifestyles and approaches to cancer risk just contribute to overall good health.”
While many behavioral factors are known to broadly increase risk of several cancers, Pollak noted that risks vary in unique ways among different individuals.
“Oncologists are used to personalization of treatments,” he said. “We try to find out what treatment would be particularly useful for one patient as compared to their neighbor. In prevention, we may discover an analogy to that customization.”
He explained that an individual assessment of risk may make the message of behavioral intervention more personal. “If you hear your doctor saying that, in your particular case, the way your body is put together, your weight especially increases your risk for cancer, it may help motivate some people.”
Pollak believes risk assessment can be further personalized beyond the level of the individual, down to the level of discrete cell types. “We’re used to thinking of a person’s cancer risk as if the person was homogeneous, but carcinogenesis takes place at the cellular level,” he said. “We need to know what’s going on differently in the different cells that might determine risk on a per-cell basis.”
Pollak mentioned the Pre-cancer Atlas as an important vehicle for realizing this goal. “With the Pre-cancer Atlas, we’ll learn more about the cellular composition and subcellular features that lead to carcinogenesis,” he said, noting that such a granular understanding of tumor formation could pave the way for improved therapies.
“We really won’t be able to prevent every cancer, but even if we confine our goals to preventing the subset of cancers that are preventable, that’s estimated to be about half of all cancers,” Pollak concluded. “Even acknowledging the limitations, the potential gains are absolutely enormous.”
Priorities for Cancer Disparities in 2022
The past two years have presented health care challenges beyond COVID-19, encompassing financial and access-related struggles that affected many facets of medicine, including cancer care. Many individuals have had to delay routine cancer screenings, alter the course of treatment, or miss follow-up appointments as a result of the pandemic.
Such problems were more pronounced in some communities than others.
“The pandemic has definitely impacted our opportunities to move forward toward eliminating disparities in all areas of cancer research,” said John Carpten, PhD, FAACR , chair of the department of Translational Genomics at the University of Southern California Keck School of Medicine and chair of the National Cancer Advisory Board. “As we consider gaps in cancer screening and cancer diagnosis, many challenges were further exacerbated in underrepresented minority communities during the pandemic.”
Carpten also pointed out the disproportionate challenges minority cancer researchers faced during COVID-19. “Many underrepresented minority investigators, who may have already had challenges in terms of access to funding, were also impacted severely by the pandemic,” he said. “This is especially true for early-stage investigators and postdoctoral fellows who were unable to be in their laboratories to perform research.”
Although the issue of lost time and funding due to the pandemic may be difficult to solve, Carpten believes that other initiatives to support underrepresented minority researchers—especially trainees and early-career investigators—will positively influence health disparities research in 2022.
Carpten specifically listed diversifying the biomedical workforce as a key priority for tackling health disparities. “Increasing underrepresented minority faculty members will increase the number of mentors who will then be able to train more underrepresented minorities and fellows,” he said.

He mentioned the National Institutes of Health (NIH) FIRST program , a funding opportunity provided to institutions to promote the hiring of early-career investigator cohorts from diverse backgrounds in support of their career development. Providing a supportive environment and sufficient resources to these investigators, Carpten said, can make significant strides toward ensuring a successful career trajectory in academic research.
“We believe that this is going to be a huge component in the growth of underrepresented minorities in the area of biomedical research, specifically cancer research,” he said.
Encouraging diversity of researchers, however, is only one step where meaningful interventions can occur. Another is the broader inclusion of diverse patients and samples in cancer research, especially of patients recruited into clinical trials.
“We need to understand the broader impact of new therapies for all people, preferably prior to approvals, to ensure that we have the most accurate picture relative to effectiveness and toxicity profiles across representative groups of patients,” Carpten said.
Diversity in preclinical studies, including patient-derived samples, genetic data, and model systems, is also key to understanding the biological basis of cancer health disparities.
“Whether it’s understanding the influence of genetic factors on cancer risk or understanding how collections of mutations that occur in cancer cells differ across individuals from different groups, it will be very important for us to continue increasing representation of the reagents, models, and data that we use,” Carpten said.
“Ensuring that we understand how biological changes impact cancer initiation, progression, and growth across an array of models will provide additional information so that we can really capture the full complexity of cancer,” he added.
Carpten also encourages working to address the cultural, social, and access-related issues underlying cancer health disparities by striving harder to engage with the community.
“We need to advance our relationships with various stakeholders, especially in terms of community engagement, outreach, and involvement,” Carpten said. “If we don’t build better relationships with the community, get their feedback, understand their issues, and work together to address them, I think we’ll continue to have challenges.”
As observed during the pandemic , improving community engagement can help health care providers build trust with their patients, bring care to broader geographic areas, and better understand the needs of the populations disparities researchers are working to serve.
“I really look forward to working with my colleagues in academia, industry, and the government, but most importantly, with our colleagues in the community,” Carpten concluded. “Their voice really needs to be heard and will be key in achieving cancer health equity.”
- About This Blog
- Blog Policies
- Tips for Contributors

Arming the Body to Fight Ovarian Cancer

Examining Cancer Health Disparities in the LGBTQ Community
AACR Annual Meeting 2019: Preclinical Steps Toward a Cancer Preventive Vaccine for Lynch Syndrome
Cancel reply
Your email address will not be published. Required fields are marked *
Join the Discussion (max: 750 characters)...
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Thank you so much for sharing such useful information over here with us. This is really a great blog have you written. I really enjoyed reading your article. I will be looking forward to reading your next post. https://atulkulkarnidotblog.wordpress.com/ https://atulkulkarnidotblog.blogspot.com/ https://atulblog123.tumblr.com/
I was distraught when I found out that my grandmother was diagnosed with cancer. I appreciate that this post emphasized that it is important to get the right kind of treatment, to target the cells precisely. I think I will advise my mother to seek the right kind of treatment by speaking with professional oncologists.
This is very informative blog about Cancer research forecast, I will definitely share this content with others.
- Experts Forecast Cancer Research and Treatment...
- Executive Summary
- Experts Forecast 2024, Part 1: Advances in...
What Are Cancer Research Studies?
What is cancer research and why is it important.

Research is the key to progress against cancer and is a complex process involving professionals from many fields. It is also thanks to the participation of people with cancer, cancer survivors, and healthy volunteers that any breakthroughs go on to improve treatment and care for those who need it.
Cancer research studies may lead to discoveries such as new drugs to treat cancer, new therapies to make symptoms less severe, or lifestyle changes to reduce the chances of getting cancer.
Cancer research may also address big picture questions like why cancer is more prevalent in certain populations or how doctors can make existing cancer detection tools more effective in health care settings.
These discoveries can help people with cancer and their caregivers live fuller lives.
Who should join cancer research studies?
When you choose to participate in a research study, you become a partner in scientific discovery. Your generous contribution can make a world of difference for people like you.
As scientists continue to conduct cancer research, anyone can consider joining a research study. The best research includes everyone, and everyone includes you.
Your unique experience with cancer is incredibly valuable and may help current and future generations lead healthier lives.
When more people of all different races, ethnicities, ages, genders, abilities, and backgrounds participate, more people benefit.
It is important for scientists to capture the full genetic diversity of human populations so that the lessons learned are applicable to everyone.
What are the types of cancer research studies?
See below for definitions on the four major types of research and their subtypes:
- basic research
- quality of life/supportive care
- natural history
- longitudinal
- population-based
- epidemiological research
- translational research
Basic Research
Basic cancer research studies explore the very laws of nature. Scientists learn how cancer cells grow and divide, for example, by growing and testing bacteria , viruses , fungi , animal cells, and human cells in a lab. Scientists also study, for example, the genes that make up tumors in mice and rats in the lab. These experiments help build the foundation for further discovery.

Why Participate in a Clinical Trial?
Get information on how to evaluate a clinical trial and what questions to ask.
Clinical Research
Clinical research involves the study of cancer in people. These cancer research studies are further broken down into two types: clinical trials and observational studies .
- Treatment trials test how safe and useful a new treatment or way of using existing treatments is for people with cancer. Test treatments may include drugs, approaches to surgery or radiation therapy , or combinations of treatments.
- Prevention trials are for people who do not have cancer but are at a high risk for developing cancer or for cancer coming back. Prevention clinical trials target lifestyle changes (doing something) or focus on certain nutrients or medicines (adding something).
- Screening trials test how effective screening tests are for healthy people. The goal of these trials is to discover screening tools or methods that reduce deaths from cancer by finding it earlier.
- Quality-of-life/supportive care tests aim to help people with cancer, as well as their family and loved ones, cope with side effects like pain, nutrition problems, nausea and vomiting , sleeping problems, and depression . These trials may involve drugs or activities like therapy and exercising.

Find Observation Studies >
View a studies that are looking for people now.
- Natural history studies look at certain conditions in people with cancer or people who are at a high risk of developing cancer. Researchers often collect information about a person and their family medical history , as well as blood, saliva, and tumor samples. For example, a biomarker test may be used to get a genetic profile of a person’s cancer tissue. This may reveal how certain tumors change over the course of treatment .
- Longitudinal studies gather data on people or groups of people over time, often to see the result of a habit, treatment, or change. For example, two groups of people may be identified as those who smoke and those who do not. These two groups are compared over time to see whether one group is more likely to develop cancer than the other group.
- Population-based studies explore the causes of cancer, cancer trends, and factors that affect cancer care in specific populations. For example, a population-based study may explore the causes of a high cancer rate in a regional Native American population.
Epidemiological Research
Epidemiological research is the study of the patterns, causes, and effects of cancer in a group of people of a certain background. This research encompasses both observational population-based studies but also includes clinical epidemiological studies where the relationship between a population’s risk factors and treatments are tested.
Translational Research
Translational research is when cancer research moves across research disciplines, from basic lab research into clinical settings, and from clinical settings into everyday care. In turn, findings from clinical studies and population-based studies can inform basic cancer research. For example, data from the genetic profile of a tumor during an observational study may help scientists develop a clinical trial to test which drugs to prescribe to cancer patients with specific tumor genes.

Monica Bertagnolli, Director, NIH; former director, NCI; cancer survivor
Participation in Cancer Research Matters
I am so happy to have the opportunity to acknowledge the courage and generosity of an estimated 494,018 women who agreed to participate in randomized clinical trials with results reported between 1971 and 2018.
Their contributions showed that mammography can detect cancer at an early stage, that mastectomies and axillary lymph node dissections are not always necessary, that chemotherapy can benefit some people with early estrogen receptor–positive, progesterone receptor–positive, HER2-negative breast cancer but is not needed for all, and that hormonal therapy can prevent disease recurrence.
For just the key studies that produced these results, it took the strength and commitment of almost 500,000 women. I am the direct beneficiary of their contributions, and I am profoundly grateful.
The true number of brave souls contributing to this reduction in breast cancer mortality over the past 30 years? Many millions. These are our heroes.
— From NCI Director’s Remarks by then-NCI Director Monica M. Bertagnolli, M.D., at the American Society of Clinical Oncology Annual Meeting, June 3, 2023
Advertisement
The Supportive Care Needs of Cancer Patients: a Systematic Review
- Open access
- Published: 25 January 2021
- Volume 36 , pages 899–908, ( 2021 )
Cite this article
You have full access to this open access article
- Madeleine Evans Webb 1 ,
- Elizabeth Murray ORCID: orcid.org/0000-0002-8932-3695 2 ,
- Zane William Younger 2 ,
- Henry Goodfellow 2 &
- Jamie Ross 2
8266 Accesses
33 Citations
4 Altmetric
Explore all metrics
Cancer, and the complex nature of treatment, has a profound impact on lives of patients and their families. Subsequently, cancer patients have a wide range of needs. This study aims to identify and synthesise cancer patients’ views about areas where they need support throughout their care. A systematic search of the literature from PsycInfo, Embase and Medline databases was conducted, and a narrative. Synthesis of results was carried out using the Corbin & Strauss “3 lines of work” framework. For each line of work, a group of key common needs were identified. For illness-work, the key needs idenitified were; understanding their illness and treatment options, knowing what to expect, communication with healthcare professionals, and staying well. In regards to everyday work, patients wanted to maintain a sense of normalcy and look after their loved ones. For biographical work, patients commonly struggled with the emotion impact of illness and a lack of control over their lives. Spiritual, sexual and financial problems were less universal. For some types of support, demographic factors influenced the level of need reported. While all patients are unique, there are a clear set of issues that are common to a majority of cancer journeys. To improve care, these needs should be prioritised by healthcare practitioners.
Similar content being viewed by others

Survivorship concerns among individuals diagnosed with metastatic cancer: Findings from the Cancer Experience Registry
Rachelle S. Brick, Lisa Gallicchio, … Melissa F. Miller
Spiritual care interventions in nursing: an integrative literature review
Mojtaba Ghorbani, Eesa Mohammadi, … Monir Ramezani
Network analysis used to investigate the interplay among somatic and psychological symptoms in patients with cancer and cancer survivors: a scoping review
G. Elise Doppenberg-Smit, Femke Lamers, … Joost Dekker
Avoid common mistakes on your manuscript.
Introduction
Over 42 million people worldwide are currently living with cancer [ 1 ]. A cancer diagnosis often results in biographical disruption [ 2 ] and distress [ 3 ], sometimes lasting years post-treatment [ 4 , 5 ]. As survival rates continue to increase, more individuals will have to live with the long-term implications of cancer. It is therefore important that the support offered to cancer patients improves to meet this growing demand.
Cancer care pathways are often spread across multiple facilities and delivered by healthcare practitioners (HCP), which make it challenging for a patient’s wider support needs to be met. This has an impact on patient wellbeing [ 6 ] and survival outcomes [ 7 , 8 ]. Many studies focus on the needs of specific patient groups, defined by diagnosis, treatment or demographics, but there is no broad consensus on how common or dissimilar patients’ supportive care needs are across types of cancer and populations. The aim of this study was to synthesise existing data on the support needs of cancer patients across populations. Identifying the common underlying needs of cancer patients, as well as needs that are specific to a patient’s diagnosis or background, will help HCPs provide comprehensive support more efficiently.
Eligibility Criteria
Inclusion criteria: Any patient undergoing treatment for any form of cancer. Patients in remission or recovery were eligible only if they had not been in remission for longer than 5 years, a key milestone in cancer survivorship [ 9 ].
Intervention and Comparator
Patients that had received any form of treatment, be it curative or palliative, could be included. As this review was not assessing the effectiveness of an intervention program, there was no appropriate comparator or control group.
The primary outcome was the identification of any supportive care needs, categorised into emotional, informational, spiritual, social or “other”. Needs could be specifically identified, or could be inferred from reported distress, e.g. patients reporting high levels of loneliness would be categorised as having an emotional need.
Inclusion criteria: Any study design which included collection of primary data, quantitative or qualitative, was eligible for inclusion.
Exclusion criteria: Papers which did not include new primary data (e.g. reviews, meta-analyses, editorials), had not been peer reviewed or were not available in English.
The search strategy was the keywords: [emotional need] or [spiritual need] or [social need] or [emotional need] AND [Neoplasm(s)] either appearing in the title, abstract, subject heading, keyword heading, protocol supplementary concept, rare disease supplementary concept or as a unique identifier.
The search was carried out on PsycInfo, Embase and Medline databases, on 24 April 2018. This selection was based on a review of which databases have the highest recall rate, while also needing to produce a manageable number of results [ 10 ].
Reference lists of included papers were searched for potentially eligible studies.
Study Selection
Titles and abstracts were screened against the inclusion/exclusion criteria, and 10% of papers were also screened by a second author. For any paper that could not be confidently excluded, the full paper was read to determine whether it should be included. There was 100% agreement between the screeners about which papers should be excluded.
Data Extraction and Management
Data were extracted into an extraction form, which was piloted and refined. Data extracted from each paper were as follows: title, year of publication, country of study setting, study design, population studied, methods of data collection and analysis and results. The needs identified in each paper were classified as informational, emotional, spiritual, social or other. For quantitative data, scores or rankings for each need were recorded, along with whether needs differed between sub-groups. For qualitative data, overarching themes, subthemes and illustrative quotes were extracted.
Data Synthesis
Data were analysed using a narrative synthesis method [ 11 ]; this allowed for the synthesis of qualitative and quantitative data and analysis of whether medical or demographic factors shaped patient needs [ 11 , 12 ].
The first step was to group the needs identified in the papers into the categories specified in the primary literature. Seven categories of need were identified in the included papers: emotional, sexual, spiritual, social, financial, daily living, nutritional and informational. The second step was to map these categories onto the Corbin & Strauss “Three lines of work” model of chronic disease management. The model identifies three types of work associated with managing a long-term condition: illness-related work, everyday life work and biographical work [ 13 ]. Within each group, the relative importance and prevalence of all the needs identified in the primary literature were compared to identify which were the most common and urgent.
Our goal was to clarify the commonality of the experience of “cancer”, irrespective of the type of cancer, thus providing an overview of the common and important support needs faced by people with cancer, and hence an understanding of where supportive care is most needed. In instances where there was conflicting evidence in the primary literature on the importance of a specific need, clinical and demographic differences between study populations were reviewed in order to understand the potential reasons for this conflict.
The Corbin & Strauss model was chosen because the categories of need identified in the primary literature clearly corresponded to the types of work in the model (Fig. 2 ). Using the model as a framework to synthesise the data allowed us to compare the relative importance of needs from different categories that fell under the same type of work. The simplicity of the model meant it could be consistently applied to needs that were identified and categorised using a number of different methodologies.
In total, 2535 papers were identified, and 540 duplicates were removed. After screening against the criteria, 1829 papers were removed, and the remaining 80 papers were read in full (Fig. 1 ). Forty-six papers were found to be eligible for inclusion in this review.
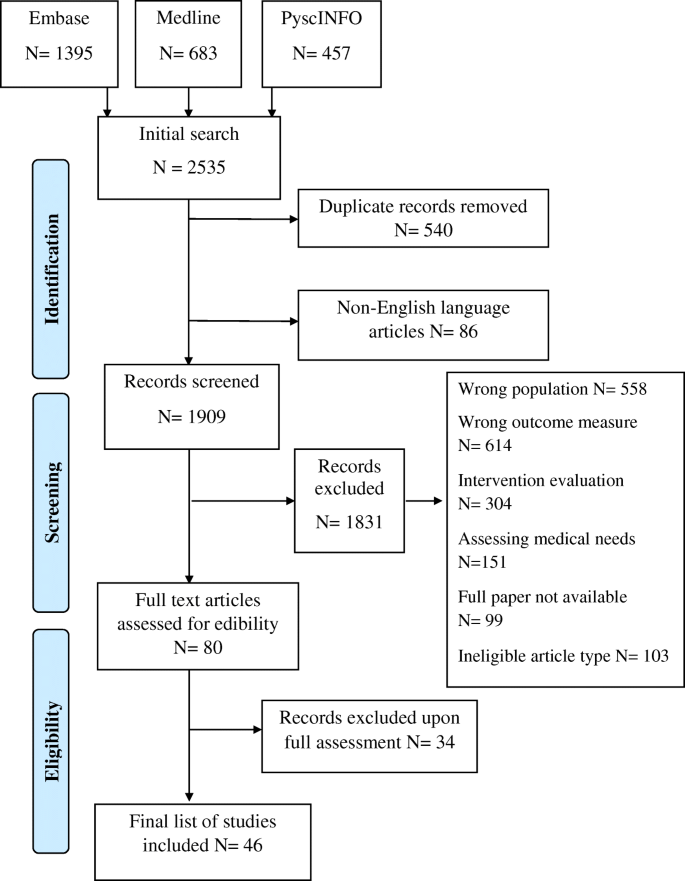
PRISMA flow diagram of the paper identification process
Study Characteristics
Of the 46 studies, 34 were quantitative, 10 were qualitative and two were mixed methods. Study population sizes ranged from 7 to 1059 participants. Fifteen papers focused on patients with a specific type of cancer, with breast and colorectal cancer being the most common. Three studies looked at patients from specific ethnic backgrounds. Eight papers focused on patients receiving a specific form of care/treatment. Three papers focused on children or young adults. Three papers looked at adults within specific age groups. Eleven studies only included patients at a certain stage of cancer or time since diagnosis. Thirty-nine studies took place in high-income countries, 6 were from middle income countries and 1 took place in a low-income country.
Needs of Cancer Patients
Thirty-two papers mentioned informational needs, 31 mentioned emotional needs, 24 mentioned spiritual needs and 19 mentioned social needs. Thirty-five papers mentioned needs in at least one of these other categories: nutritional, sexual, daily living or financial.
The resulting needs identified were grouped according to the different forms of chronic disease “work” defined by the Corbin & Strauss framework (Fig. 2 ).
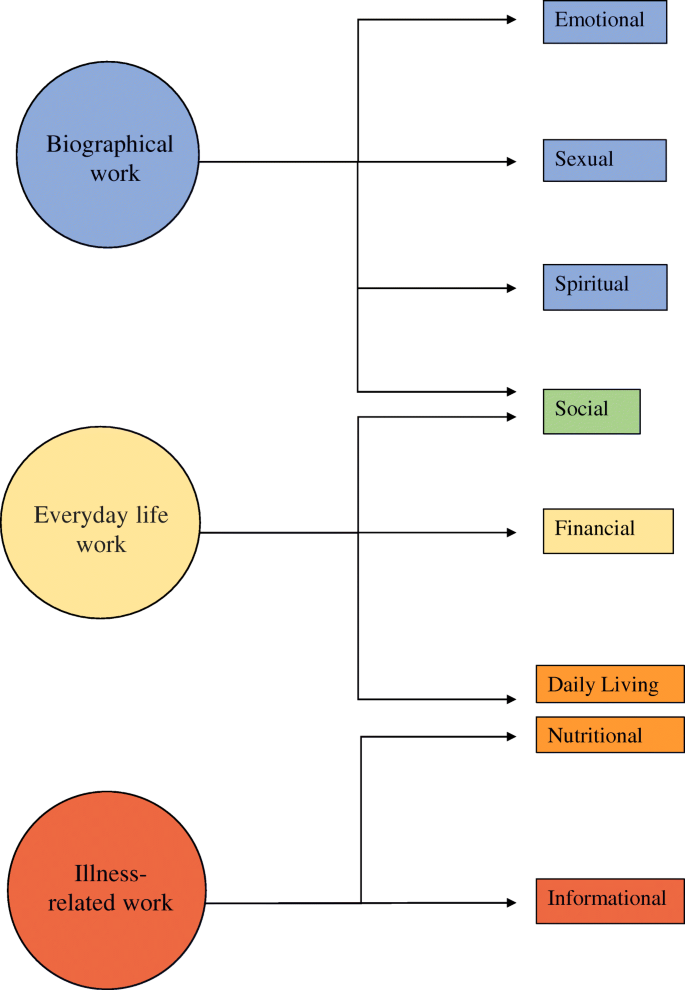
Illustration of how the different domains of need identified fit into Corbin and Strauss’ 3 lines of work model of managing chronic illness
Illness-Related Work
Illness-related work, defined by Corbin & Strauss, is “the tasks of controlling symptoms; monitoring, preventing crises; carrying out regimens and managing limitations of activity” [ 13 ]. The central goal of illness-related work for patients is to understand their illness and treatment, and subsequently the need for information is consistently reported as a high priority [ 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 ]. The only paper that did not find a high level of informational need specifically measured unmet need [ 30 ].
Most frequently patients wanted to know what treatment they were receiving and how it worked [ 20 , 26 , 27 , 29 , 31 , 32 , 33 , 34 , 35 , 36 ], why that treatment had been selected, its effectiveness and its pros and cons [ 14 , 20 , 21 , 22 , 23 , 24 , 26 , 35 ]. Patients also frequently searched for more specific information about their diagnosis and prognosis [ 15 , 20 , 21 , 22 , 23 , 24 , 25 , 29 , 31 , 33 , 34 ].
Patients wanted to know what to expect from their illness and treatment [ 15 , 16 , 31 , 33 , 34 , 35 , 37 , 38 ] (Box 1). This included knowing about the chance of a relapse [ 26 ], the length of their hospital stay [ 32 ] and when their life would return to “normal” [ 26 , 31 , 33 , 39 ]. One paper reported that being given “vague” answers by HCPs frustrated patients [ 38 ].
In regard to treatment, patients most often wanted to know what the possible side effects were [ 16 , 18 , 21 , 22 , 25 , 27 , 31 , 33 , 37 , 38 , 40 , 41 ] and how they could manage or relieve them [ 14 , 17 , 19 , 22 , 23 , 24 , 26 , 31 , 33 , 35 , 42 , 43 ]. The importance of this information may depend on the stage of the patient’s treatment, as patients receiving follow-up or palliative care placed less importance on symptom management [ 25 , 27 ].
Wanting to minimise the impact of side effects speaks to a commonly reported desire among patients to be as healthy as possible [ 14 , 15 , 18 , 19 , 22 , 23 , 24 , 26 , 27 , 28 , 29 , 33 , 38 , 39 , 40 ]. This aim is also seen in the nutritional needs of patients [ 16 , 20 , 33 , 40 , 41 , 44 ]. Rather than receiving generic information about healthy diets, patients wanted more specific advice around foods that could aid recovery or minimise side effects [ 16 , 40 , 41 ]. Nutritional needs had an outsized importance in studies involving Native American patients and colorectal cancer patients [ 16 , 40 ]. For colorectal cancer patients, nutritional needs are likely higher as their cancer directly affects their digestive system. Within the Native American population, there was a strong interest in information about traditional foods, possibly due to culturally specific reasons [ 40 ].
Generally, patients wanted their test results as soon as possible [ 21 , 22 , 24 , 27 , 33 , 43 ] and wanted the meaning of the results explained to them [ 21 , 22 , 26 , 34 , 43 ]. The importance of this information to patients could be due to a desire to have some say in the treatment they are given [ 18 , 33 , 34 , 45 ], although the level of interest in alternative treatments varied significantly [ 14 , 18 , 24 , 40 , 44 ] (Box 2). The only study where information about tests was less important involved newly diagnosed patients [ 18 ].
The final area of illness-related work highlighted by this review was communication. Patients wanted to be able to communicate with their HCPs [ 18 , 27 , 34 , 40 ] but often felt unsure of when or who to direct questions to [ 24 , 26 , 35 , 36 , 38 ]. Having a single HCP who they could talk to about all aspects of treatment was a high priority [ 19 , 21 , 22 , 23 , 28 , 43 ]. Less important was the need to talk to a professional counsellor [ 25 , 27 , 36 , 43 ].
Although a general need for information was consistent across all included studies, not all patients wanted a high volume of information. A significant minority of patients only wanted to know essential information or did not want to receive bad news [ 29 , 31 , 46 ]. Age may play a role in this dynamic, as multiple papers reported older people wanted less information [ 14 , 15 , 20 , 22 , 25 , 26 , 31 , 44 ], while only a couple found no relationship [ 28 , 34 ]. Timing could also be a factor, as some patients felt the amount of information received when diagnosis was overwhelming and preferred receiving information as it became relevant [ 34 , 37 , 39 , 41 ].
Everyday Life Work
This area of need encapsulates “the daily round of tasks that helps keep a household going”, which includes the practical tasks involved in managing an illness, along with trying to maintain the structure of life pre-diagnosis [ 13 ]. The most frequently reported social needs were about patients’ concern for their family [ 17 , 20 , 21 , 26 , 39 , 46 , 47 , 48 ]. The importance of maintaining relationships with their partner, children or friends were all mentioned [ 15 , 29 , 37 , 42 , 45 , 47 ], although notably not among patients with incurable cancer [ 27 ]. There was no consensus on whether patients wanted to discuss their cancer with loved ones; some papers found this to be highly important, others did not [ 20 , 31 , 36 , 42 , 49 ]. While there were no clear demographic or medical factors connected to this variation, Kent (2013) reported that patients whose existing relationships had been heavily affected by their diagnosis were more likely to want to talk about cancer [ 49 ].
Patients wanted to live a life they consider “normal”, reflected by the importance placed on daily living needs. The most common difficulties patients faced were coping with a lack of energy [ 17 , 19 , 21 , 27 , 28 , 30 , 36 , 38 , 43 ] and wanting to do the things they used to do [ 19 , 21 , 26 , 28 , 31 , 39 ] (Box 3). Patients placed a high value on socialising and leisure time [ 15 , 26 , 32 , 45 ] and reported a fear of being isolated or abandoned [ 16 , 18 , 20 ]. The importance of maintaining a job was influenced by age, with younger patients being more interested in how cancer will affect their career and their employment rights [ 15 , 18 , 20 , 26 , 29 , 33 , 39 , 42 ].
The final practical need identified was financial, though the level of need was highly dependent on location. Patient populations with greater access to healthcare placed lower importance on financial needs [ 25 , 27 , 29 , 30 , 33 ] (Box 4). The needs in these groups related to wanting financial stability and informational support [ 45 , 50 ], with low levels of interest in economic aid [ 34 ]. Patients in countries with more limited access to healthcare reported higher levels of financial stress and reliance on family for monetary support [ 32 , 46 ]. This was true in all US-based studies, apart from one in which the mean income of participants was high [ 18 , 20 , 24 , 28 ]. For these populations, financial concerns included managing bills [ 18 , 24 ], bankruptcy assistance [ 18 ], paying for care [ 20 , 32 , 46 ] and homelessness [ 46 ]. A few financial needs were common across healthcare systems, being able to maintain a basic standard of living [ 27 , 30 , 45 , 46 ] and helping understanding financial systems and resources [ 18 , 25 , 26 , 34 ], though again the level of importance varied.
Biographical Work
Biographical work is defined as “the work involved in defining and maintaining an identity” [ 13 ]. This involves coming to terms with and contextualising a diagnosis within a persons’ identity [ 42 , 45 ]. Patients wanted to be treated as individuals [ 16 , 19 , 21 , 22 , 34 ], be reassured [ 19 , 34 ], have their feelings acknowledged [ 19 , 22 ], be respected [ 34 , 45 ] and have their dignity preserved [ 47 ] (Box 5).
Biographical work includes dealing with the emotional impact of cancer. Feelings of despair or depression were common [ 19 , 21 , 23 , 28 , 30 , 42 , 51 , 52 ], as well as distress and anxiety [ 16 , 21 , 28 , 30 , 35 , 38 , 43 ]. Patients also reported a range of fears including cancer itself [ 17 , 21 , 23 , 31 , 43 , 51 ], their treatment [ 35 , 37 ], dying [ 17 , 19 , 42 , 52 ] and pain [ 27 ]. Physical changes also negatively affected patients’ sense of self [ 26 , 27 , 29 , 30 , 38 , 42 , 45 , 46 , 47 ]. Consequently, the need for relaxation and stress management was high [ 23 , 24 , 33 , 48 , 52 ].
Patients struggled to deal with the uncertainty [ 15 , 17 , 19 , 28 , 30 , 42 , 43 , 46 , 51 ] and expressed a desire for more control [ 17 , 19 , 20 , 21 , 27 , 28 , 30 , 42 , 43 , 53 ]. To cope, patients placed a lot of importance on receiving support from loved ones [ 14 , 18 , 32 , 35 , 36 , 42 , 51 ]. However, this directly conflicted with their fear of being a burden and a perceived pressure to “stay strong” [ 20 , 27 , 37 , 45 , 46 , 51 , 53 ] (Box 6). Other patients were identified as a source of support for some [ 18 , 34 , 37 , 49 , 52 ], but others, especially those who were receiving follow-up or palliative care, were less interested in talking to other patients [ 25 , 35 , 45 , 47 ]. This aligns with a reported need among terminal cancer patients to discuss things other than illness [ 54 ].
Sexuality is another part of identity that can be impacted by cancer. Patients wanted to know how cancer would impact their sex drive, sexuality [ 14 , 17 , 20 , 26 , 29 ] and their intimate relationships [ 14 , 17 , 30 , 31 ] but often felt uncomfortable discussing these needs with their HCPs [ 14 , 18 , 20 , 26 ] (Box 7). When ranked alongside other needs, sexuality was reported to be of lesser importance to most patients [ 17 , 19 , 21 , 22 , 35 , 42 , 43 ], apart from prostate cancer patients, who reported the impact on their sex drive and sexual activity as some of the most significant changes they faced [ 14 , 30 , 38 ] (Box 7). Higher sexuality-related needs were also identified in patients with colorectal and breast cancer, although not at the same level [ 22 , 30 ]. Of the papers that looked, five out of the six studies found a relationship between age and importance of sexual identity, with younger patients having a greater need for information on sex [ 17 , 22 , 26 , 31 ] and individuals over 40 wanting more guidance on fertility [ 18 ]. One study involving younger patients did report limited interest in sexuality; however, the majority of patients were under 18 and therefore were less likely to be sexually active [ 42 ].
Much like with sexual identity, patients’ spiritual needs were not highly important when ranked alongside other domains [ 23 , 24 , 27 , 28 , 33 , 34 , 40 , 45 ], but papers that focused solely on spirituality reported widespread need [ 47 , 48 , 52 , 54 , 55 , 56 , 57 , 58 ]. There was no consensus on the importance of accessing religious resources, some papers reported a strong need for religious support [ 23 , 32 , 45 , 55 , 56 , 59 ], but more papers reported low levels of interest [ 24 , 28 , 33 , 48 , 51 , 52 , 54 , 57 , 60 ]. In line with this, the most commonly reported spiritual needs were not explicitly religious. This included maintaining a sense of calm [ 45 , 46 , 47 , 48 , 52 , 53 , 55 , 56 , 58 , 60 ], staying positive or hopeful [ 23 , 24 , 32 , 45 , 47 , 48 , 57 , 58 , 59 ] and being able to appreciate or find meaning in life [ 32 , 45 , 47 , 48 , 55 , 56 , 57 , 59 , 60 ]. Generally, there was little reported interest in discussing death or dying [ 23 , 24 , 27 , 42 , 45 , 48 , 52 , 60 ] or making sense of why this happened [ 34 , 55 , 56 , 57 ]. Much like the importance of family relationships in everyday work, being with loved ones was important for patients’ spiritual wellbeing [ 47 , 51 , 53 , 54 , 55 , 56 , 57 , 58 , 60 ]. However, some patients reported that being part of a religious community gave them similar support [ 46 , 51 , 53 , 55 , 60 ].
The most commonly reported religious need for patients was to pray or be prayed for [ 32 , 46 , 48 , 55 , 56 , 57 , 59 ]. The fact that prayer was also important for non-religious participants suggests that it may be seen as a spiritual practice for some patients. A small number of papers reported that having a relationship with God was important to patients [ 15 , 48 , 56 , 57 , 59 ], with some patients viewing God as a saviour from illness [ 40 , 46 , 59 ], while others felt that God caused their illness as punishment or as a test of faith [ 46 , 47 , 51 ] (Box 8).
Cultural factors may also influence spiritual needs. The afterlife was found to be an important concern for some patients [ 52 , 53 , 55 ], but not if their culture had little belief in the concept [ 47 ]. In the same way, having a legacy was a key need in one paper due to the importance of continuity after death in that culture [ 53 ].
This is the first review to synthesise data about cancer patients’ supportive needs across all populations and cancer types. There was remarkable consistency in the needs identified, and these were well explained by the Corbin & Strauss model of managing a chronic condition [ 13 ]. Almost all studies confirmed patients’ need for high-quality, comprehensible and timely information about their illness, treatments and how best to manage their symptoms. Such information was necessary for patients to undertake illness-related, everyday living and biographical work. In addition, patients needed support in dealing with emotional issues, including existential uncertainty, changing relationships with friends and family and practical support with everyday tasks.
Previous Literature
This review confirms the findings of previous reviews focused on specific types of need or specific populations. The most common needs identified as illness-related work in this study correspond to key informational needs highlighted in previous reviews [ 61 , 62 ]. The spiritual needs discussed have also been found to be key in improving psycho-spiritual wellbeing in other research [ 63 ]. While our review did not assess the ability of current care models to meet these needs, it is noteworthy that the key needs we identified have been found to frequently go unmet in other research [ 64 , 65 ].
Strengths and Limitations
The main strength of this study is its inclusive nature, looking across all populations and all types of cancer. This, combined with the theoretical underpinning and use of the Corbin & Strauss model, provides reassurance about the overall transferability of these findings to other clinical populations.
The main limitation pertains to the scope of the primary literature, with most of the studies coming from high-income countries, and only 7 papers from low- or middle-income countries. While the nature of patients’ financial needs were clearly dependent on country, setting may also influence other needs in less direct ways, limiting how universal the findings are. Additionally, the majority of studies used opportunistic sampling so may not accurately capture the needs of the general cancer population. Most included studies were not longitudinal and therefore could not analyse how patients’ concerns changed over time. Finally, potentially relevant demographic information was not always collected. For example, only one of the papers that examined sexuality collected information about sexual orientation, and only a couple of studies that measured financial need recorded socioeconomic status.
Conclusions
This review highlights a number of underlying issues that affect cancer patients. These findings are consistent with the previous literature and fit well with multiple chronic illness frameworks, which suggests that they are robust enough to inform best practice. The most common needs identified support the argument for empowering people with cancer through a patient-centred form of care.
Priorities for practice should be to ensure patients understand their illness and what they can expect throughout their treatment pathway. Supportive care should work to enable patients to live a life they recognise as “normal” and help them maintain their closest relationships. HCPs should ensure that patients always feel that they are being treated as individuals and know who to go to when they have questions. These key needs should be addressed as a first step to provide a strong basis of care before providing more individualised support.
Further research should focus on how to ensure these needs are addressed effectively. Evaluation of supportive care interventions should remain focused on the experiences of patients to allow them to have a voice in their care. Additional research on when different needs arise over the disease progression would help ensure that resources are provided only when needed.
McGuire S (2016) World Cancer Report 2014. World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Oxford University Press, Geneva, Switzerland
Google Scholar
Park CL, Zlateva I, Blank TO (2009) Self-identity after cancer: “survivor”, “victim”, “patient”, and “person with cancer”. J Gen Intern Med 24(2):430–435
Article PubMed Central Google Scholar
Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S (2001) The prevalence of psychological distress by cancer site. Psycho-oncology. 10(1):19–28
Article CAS PubMed Google Scholar
Ferrell BR, Dow KH, Leigh S, Ly J, Gulasekaram P (1995) Quality of life in long-term cancer survivors. In: Oncology nursing forum
Stein KD, Syrjala KL, Andrykowski MA (2008) Physical and psychological long-term and late effects of cancer. Cancer. 112(S11):2577–2592
Article PubMed Google Scholar
Li HW, Lopez V, Chung OJ, Ho KY, Chiu SY (2013) The impact of cancer on the physical, psychological and social well-being of childhood cancer survivors. Eur J Oncol Nurs 17(2):214–219
Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I (2006) Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol 24(7):1105–1111
Brown KW, Levy AR, Rosberger Z, Edgar L (2003) Psychological distress and cancer survival: a follow-up 10 years after diagnosis. Psychosom Med 65(4):636–643
Welch HG, Schwartz LM, Woloshin S (2000) Are increasing 5-year survival rates evidence of success against cancer? Jama. 283(22):2975–2978
Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH (2017) Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Systematic reviews 6(1):245
Article PubMed PubMed Central Google Scholar
Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M et al (2006) Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme Version 1:b92
Barnett-Page E, Thomas J (2009) Methods for the synthesis of qualitative research: a critical review. BMC Med Res Methodol 9(1):59
Corbin JM, Strauss A. Unending work and care: managing chronic illness at home: Jossey-Bass; 1988
Boberg EW, Gustafson DH, Hawkins RP, Offord KP, Koch C, Wen K-Y, Kreutz K, Salner A (2003) Assessing the unmet information, support and care delivery needs of men with prostate cancer. Patient Educ Couns 49(3):233–242
Derdiarian AK (1986) Informational needs of recently diagnosed cancer patients. Nurs Res 35(5):276–281
Beaver K, Latif S, Williamson S, Procter D, Sheridan J, Heath J, Susnerwala S, Luker K (2010) An exploratory study of the follow-up care needs of patients treated for colorectal cancer. J Clin Nurs 19(23–24):3291–3300
Dubey C, De Maria J, Hoeppli C, Betticher D, Eicher M (2015) Resilience and unmet supportive care needs in patients with cancer during early treatment: a descriptive study. Eur J Oncol Nurs 19(5):582–588
Goldfarb M, Casillas J (2013) Unmet information and support needs in newly diagnosed thyroid cancer: comparison of adolescent/young adults (AYA) and older patients. J Cancer Surviv 8(3):394–401
Article Google Scholar
Hasegawa T, Goto N, Matsumoto N, Sasaki Y, Ishiguro T, Kuzuya N, Sugiyama Y (2016) Prevalence of unmet needs and correlated factors in advanced-stage cancer patients receiving rehabilitation. Support Care Cancer 24(11):4761–4767
Hawkins NA, Pollack LA, Leadbetter S, Steele WR, Carroll J, Dolan JG, Ryan EP, Ryan JL, Morrow GR (2008) Informational needs of patients and perceived adequacy of information available before and after treatment of cancer. J Psychosoc Oncol 26(2):1–16
Korner A, Garland R, Czajkowska Z, Coroiu A, Khanna M (2016) Supportive care needs and distress in patients with non-melanoma skin cancer: nothing to worry about? Eur J Oncol Nurs 20:150–155
Li WW, Lam WW, Au AH, Ye M, Law WL, Poon J et al (2013) Interpreting differences in patterns of supportive care needs between patients with breast cancer and patients with colorectal cancer. Psycho-Oncol 22(4):792–798
Article CAS Google Scholar
Moadel AB, Morgan C, Dutcher J (2007) Psychosocial needs assessment among an underserved, ethnically diverse cancer patient population. Cancer. 109(Suppl2):446–454
Moody K, Mannix MM, Furnari N, Fischer J, Kim M, Moadel A (2011) Psychosocial needs of ethnic minority, inner-city, pediatric cancer patients. Support Care Cancer 19(9):1403–1410
Ng CHR, Verkooijen HM, Ooi LL, Koh WP (2011) Unmet psychosocial needs among breast, colorectal and gynaecological cancer patients at the National Cancer Centre Singapore (NCCS). Support Care Cancer 20(5):1049–1056
O'Connor G, Coates V, O'Neill S (2010) Exploring the information needs of patients with cancer of the rectum. Eur J Oncol Nurs 14(4):271–277
Rainbird K, Perkins J, Sanson-Fisher R, Rolfe I, Anseline P (2009) The needs of patients with advanced, incurable cancer. Br J Cancer 101(5):759–764
Article CAS PubMed PubMed Central Google Scholar
Sanders SL, Bantum EO, Owen JE, Thornton AA, Stanton AL (2010) Supportive care needs in patients with lung cancer. Psycho-Oncology. 19(5):480–489
Whelan TJ, Mohide EA, Willan AR, Arnold A, Tew M, Sellick S, Gafni A, Levine MN (1997) The supportive care needs of newly diagnosed cancer patients attending a regional cancer center. Cancer. 80(8):1518–1524
White K, D'Abrew N, Katris P, O'Connor M, Emery L (2012) Mapping the psychosocial and practical support needs of cancer patients in Western Australia. Eur J Cancer Care. 21(1):107–116
Giacalone A, Blandino M, Talamini R, Bortolus R, Spazzapan S, Valentini M, Tirelli U (2007) What elderly cancer patients want to know? Differences among elderly and young patients. Psycho-Oncology. 16(4):365–370
Masika GM, Wettergren L, Kohi TW, von Essen L (2012) Health-related quality of life and needs of care and support of adult Tanzanians with cancer: a mixed-methods study. Health and Quality of Life Outcomes 10 (no pagination):(133)
Neumann M, Wirtz M, Ernstmann N, Ommen O, Langler A, Edelhauser F et al (2011) Identifying and predicting subgroups of information needs among cancer patients: an initial study using latent class analysis. Support Care Cancer 19(8):1197–1209
Tamburini M, Gangeri L, Brunelli C, Boeri P, Borreani C, Bosisio M et al (2003) Cancer patients’ needs during hospitalisation: a quantitative and qualitative study. BMC Cancer 3 (no pagination):(12)
van Weert JC, Bolle S, van Dulmen S, Jansen J (2013) Older cancer patients’ information and communication needs: what they want is what they get? Patient Educ Couns 92(3):388–397
Wong RK, Franssen E, Szumacher E, Connolly R, Evans M, Page B, Chow E, Hayter C, Harth T, Andersson L, Pope J, Danjoux C (2002) What do patients living with advanced cancer and their carers want to know? - a needs assessment. Support Care Cancer 10(5):408–415
Beaver K, Williamson S, Briggs J (2016) Exploring patient experiences of neo-adjuvant chemotherapy for breast cancer. Eur J Oncology Nurs 20:77–86
Grimsbo GH, Finset A, Ruland CM (2011) Left hanging in the air: experiences of living with cancer as expressed through e-mail communications with oncology nurses. Cancer Nurs 34(2):107–116
Heidari H, Mardani-Hamooleh M (2016) Cancer patients’ informational needs: qualitative content analysis. J Cancer Educ 31(4):715–720
Doorenbos AZ, Eaton LH, Haozous E, Towle C, Revels L, Buchwald D (2010) Satisfaction with telehealth for cancer support groups in rural American Indian and Alaska native communities. Clin J Oncol Nurs 14(6):765–770
James-Martin G, Koczwara B, Smith E, Miller M (2014) Information needs of cancer patients and survivors regarding diet, exercise and weight management: a qualitative study. Eur J Cancer Care 23(3):340–348
Decker C, Phillips CR, Haase JE (2004) Information needs of adolescents with cancer. J Pediatr Oncol Nurs 21(6):327–334
Beesley VL, Janda M, Goldstein D, Gooden H, Merrett ND, O'Connell DL, Rowlands IJ, Wyld D, Neale RE (2016) A tsunami of unmet needs: pancreatic and ampullary cancer patients’ supportive care needs and use of community and allied health services. Psycho-Oncology. 25(2):150–157
Choi KH, Park JH, Park SM (2011) Cancer patients’ informational needs on health promotion and related factors: a multi-institutional, cross-sectional study in Korea. Support Care Cancer 19(10):1495–1504
Buzgova R, Hajnova E, Sikorova L, Jarosova D (2014) Association between unmet needs and quality of life in hospitalised cancer patients no longer receiving anti-cancer treatment. Eur J Cancer Care. 23(5):685–694
Elsner F, Schmidt J, Rajagopal MR, Radbruch L, Pestinger M (2012) Psychosocial and spiritual problems of terminally ill patients in Kerala, India. Future Oncol 8(9):1183–1191
Hsiao SM, Gau ML, Ingleton C, Ryan T, Shih FJ (2011) An exploration of spiritual needs of Taiwanese patients with advanced cancer during the therapeutic processes. J Clin Nurs 20(7–8):950–959
Sharma RK, Astrow AB, Texeira K, Sulmasy DP (2012) The spiritual needs assessment for patients (SNAP): development and validation of a comprehensive instrument to assess unmet spiritual needs. J Pain Symptom Manag 44(1):44–51
Kent EE, Smith AW, Keegan TH, Lynch CF, Wu X-C, Hamilton AS et al (2013) Talking about cancer and meeting peer survivors: social information needs of adolescents and young adults diagnosed with cancer. J Adolesc Young Adult Oncol 2(2):44–52
O'Connor G, Coates V, O'Neill S (2010) Exploring the information needs of patients with cancer of the rectum. Eur J Oncol Nurs 14(4):271–277
Murray SA, Kendall M, Boyd K, Worth A, Benton T (2004) Exploring the spiritual needs of people dying of lung cancer or heart failure: a prospective qualitative interview study of patients and their carers. Palliat Med 18(1):39–45
Astrow AB, Wexler A, Texeira K, He MK, Sulmasy DP (2007) Is failure to meet spiritual needs associated with cancer patients’ perceptions of quality of care and their satisfaction with care? J Clin Oncol 25(36):5753–5757
Shih FJ, Lin HR, Gau ML, Chen CH, Hsiao SM, Shih SN, Sheu SJ (2009) Spiritual needs of Taiwan’s older patients with terminal cancer. Oncol Nurs Forum 36(1):E31–EE8
Hampton DM, Hollis DE, Lloyd DA, Taylor J, McMillan SC (2007) Spiritual needs of persons with advanced cancer. Am J Hospice Palliat Med 24(1):42–48
Dedeli O, Yildiz E, Yuksel S (2015) Assessing the spiritual needs and practices of oncology patients in Turkey. Holist Nurs Pract 29(2):103–113
Ghahramanian A, Markani AK, Davoodi A, Bahrami A (2016) Spiritual needs of patients with cancer referred to Alinasab and Shahid Ghazi Tabatabaie Hospitals of Tabriz, Iran. Asian Pacific journal of cancer prevention: APJCP 17(7):3105–3109
PubMed Google Scholar
Taylor EJ (2006) Prevalence and associated factors of spiritual needs among patients with cancer and family caregivers. Oncol Nurs Forum 33(4):729–735
Vilalta A, Valls J, Porta J, Vinas J (2014) Evaluation of spiritual needs of patients with advanced cancer in a palliative care unit. J Palliat Med 17(5):592–600
Taylor EJ (2003) Spiritual needs of patients with cancer and family caregivers. Cancer Nurs 26(4):260–266
Hocker A, Krull A, Koch U, Mehnert A (2014) Exploring spiritual needs and their associated factors in an urban sample of early and advanced cancer patients. European Journal of Cancer Care. 23(6):786–794
Rutten LJF, Arora NK, Bakos AD, Aziz N, Rowland J (2005) Information needs and sources of information among cancer patients: a systematic review of research (1980–2003). Patient Educ Couns 57(3):250–261
Fletcher C, Flight I, Chapman J, Fennell K, Wilson C (2017) The information needs of adult cancer survivors across the cancer continuum: a scoping review. Patient Educ Couns 100(3):383–410
Lin HR, Bauer-Wu SM (2003) Psycho-spiritual well-being in patients with advanced cancer: an integrative review of the literature. J Adv Nurs 44(1):69–80
Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ (2009) What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer 17(8):1117–1128
Sanson-Fisher R, Girgis A, Boyes A, Bonevski B, Burton L, Cook P, Supportive Care Review Group (2000) The unmet supportive care needs of patients with cancer. Cancer. 88(1):226–237
Download references
Author information
Authors and affiliations.
UCL Research Department of Epidemiology & Public Health, 1-19 Torrington Place, London, WC1E 6BT, UK
Madeleine Evans Webb
Department of Primary Care and Population Health, Upper 3rd Floor, Royal Free Hospital, Rowland Hill Street, London, NW3 2PF, UK
Elizabeth Murray, Zane William Younger, Henry Goodfellow & Jamie Ross
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Elizabeth Murray .
Ethics declarations
Conflict of interest.
This work was part-funded by the MacMillan Cancer Support Research Grant number 6488115. Elizabeth Murray receives funding from the NIHR School for Primary Care Research and the NIHR Collaboration for Leadership in Applied Health Research and Care North Thames. Henry Goodfellow is funded through an NIHR Academic Clinical Fellowship. Jamie Ross is funded by an NIHR School for Primary Care Research fellowship.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
(DOCX 58 kb)
(DOCX 118 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Evans Webb, M., Murray, E., Younger, Z.W. et al. The Supportive Care Needs of Cancer Patients: a Systematic Review. J Canc Educ 36 , 899–908 (2021). https://doi.org/10.1007/s13187-020-01941-9
Download citation
Accepted : 06 December 2020
Published : 25 January 2021
Issue Date : October 2021
DOI : https://doi.org/10.1007/s13187-020-01941-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Supportive care
- Patient needs
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Psychol
Depression and Anxiety in Patients With Cancer: A Cross-Sectional Study
Abdallah y. naser.
1 Faculty of Pharmacy, Isra University, Amman, Jordan
Anas Nawfal Hameed
Nour mustafa.
2 Department of Pharmacy, King Hussein Cancer Center, Amman, Jordan
Hassan Alwafi
3 Department of Pharmacology and Toxicology, College of Medicine, Umm Al-Qura University, Mecca, Saudi Arabia
Eman Zmaily Dahmash
Hamad s. alyami.
4 Department of Pharmaceutics, College of Pharmacy, Najran University, Najran, Saudi Arabia
Haya Khalil
Associated data.
The original contributions presented in the study are included in the article/ Supplementary Material , further inquiries can be directed to the corresponding author/s.
Depression and anxiety persist in cancer patients, creating an additional burden during treatment and making it more challenging in terms of management and control. Studies on the prevalence of depression and anxiety among cancer patients in the Middle East are limited and include many limitations such as their small sample sizes and restriction to a specific type of cancer in specific clinical settings. This study aimed to describe the prevalence and risk factors of depression and anxiety among cancer patients in the inpatient and outpatient settings.
Materials and Methods
A total of 1,011 patients (399 inpatients and 612 outpatients) formed the study sample. Patients’ psychological status was assessed using the Hospital Anxiety and Depression Scale (HADS), the Patient Health Questionnaire (PHQ-9), and the Generalized Anxiety Disorder 7-item (GAD-7) scale. The prevalence rate of depressive and anxious symptomatology was estimated by dividing the number of patients who exceeded the borderline score: 10 or more for each subscale of the HADS scale, 15 or more for the GAD-7 scale, and 15 or more in the PHQ-9 by the total number of the patients. Risk factors were identified using logistic regression.
The prevalence of depressive and anxious symptomatology among all patients was 23.4% and 19.1–19.9%, respectively. Depressive symptomatology was more prevalent across patients who were hospitalized (37.1%) compared with patients in the outpatient setting (14.5%) ( p < 0.001). Similarly, anxious symptomatology was more prevalent in the inpatient setting ( p < 0.001). In the inpatient setting, depressive symptomatology was more prevalent among patients with bladder cancer, while severe anxious symptomatology was more prevalent across patients with lung cancer. In the outpatient setting, depressive and anxious symptomatology was more prevalent among breast and prostate cancer patients, respectively. Despite that, around 42.7% and 24.8% of the patients, respectively, reported that they feel anxious and depressed, and only 15.5% of them were using medications to manage their conditions.
Our study findings demonstrated a higher prevalence of depressive and anxious symptomatology in the inpatient setting and advanced disease stages. In addition, the underutilization of antidepressant therapy was observed. There is a need to consider mental disorders as part of the treatment protocol for cancer patients. Enhanced clinical monitoring and treatment of depression and anxiety of cancer patients are required.
Introduction
In cancer patients, psychological problems such as depression and anxiety persist and can cause an additional burden during their treatment, making it more challenging in terms of its management and control ( Walker et al., 2013 ; Ahmed, 2019 ), compliance during the treatment course ( Ahmed, 2019 ), duration of hospital stay ( Koenig et al., 1992 ; McDermott et al., 2018 ), and, ultimately, survival rate ( Spiegel et al., 1989 ; Spiegel and Li, 2007 ). Previous studies have reported that the prevalence of depressive disorders among cancer patients is two to three times higher than those of the general population ( Massie, 2004 ; Pirl, 2004 ). Previous studies that evaluated psychological distress among cancer patients have reported various heterogeneous prevalence rates that differed according to clinical settings (outpatient clinics, hospital settings, and palliative care), stage of the disease (newly diagnosed, recurrence, survivorship, or advanced stages), and phase of treatment ( Mitchell et al., 2011 ; Krebber et al., 2014 ; Caruso et al., 2017 ), which ranged between 5.0 and 49.0% ( Walker et al., 2013 ). A previous meta-analysis that explored the prevalence of depression among cancer patients and included 211 studies (representing more than 82,000) reported a different prevalence rate of depression—one that differed by the type of instrument, type of cancer, and treatment phase ( Krebber et al., 2014 ). In addition, the prevalence rate of depressive disorders among cancer patients differed by cancer site and ranged from 5.6% for patients with genitourinary cancer to 13.1% for patients with lung cancer ( Walker et al., 2014 ). Furthermore, depression is more common among patients with severe illness and advanced stages of malignancy ( Kaasa et al., 1993 ; Delgado-Guay et al., 2009 ). A previous critical review that included 11 previous systematic reviews and meta-analyses, which aimed to identify risk factors of depression among cancer patients, has reported a wide range of factors that play a key role in developing depression besides the biological factors (the type of cancer, stage of the disease, and treatment-related factors) ( Caruso et al., 2017 ). This includes individual factors (family history, personal psychiatric history, and personality traits) and interpersonal and social factors (a history of stressful life events, loneliness, social isolation, low-socioeconomic status, and lack of social support) ( Caruso et al., 2017 ). Assessment of psychological distress among cancer patients is important in order to recognize patients who need help and further assessment and, therefore, subsequent healthcare intervention. This increasingly highlights the fact that depression is a substantial problem in cancer patients.
In 2018, a total of 10,898 new cancer cases were diagnosed in Jordan within a population of 9,903,798. An age-standardized incident rate was 157.8 per 100,000, while the age-standardized mortality rate was 89.7 per 100,000. The top five most prevalent cancers were breast cancer, lung cancer, colorectal cancer, bladder cancer, and leukemia ( World Health Organization, 2018 ). Even though depression is a significant complication of cancer and its occurrence is higher than in the general population ( Centre for Disease Control and Prevention, 2019 ), it is often neglected. Studies on the prevalence of depression and anxiety among cancer patients in the Middle East are limited, with many limitations such as small sample size and being restricted to a specific type of cancer in specific clinical settings ( Al Ahwal et al., 2014 ; Abou Kassm et al., 2018 ; Ahmadi Gharaei et al., 2019 ). The primary aim of this study is to describe the prevalence and risk factors of depressive and anxious symptomatology in cancer patients in the inpatient and outpatient settings. Additionally, we aimed to explore the pattern of use of antidepressants among the study participants. This will enable us to identify cancer patients who are at higher risk of depression and anxiety. Mental and medical support can be directed to cancer patients who are at higher risk in order to control their psychological problems and improve their clinical outcome. Early identification is critical in the management of depressive symptoms and plays an important role in improving their adherence to the therapy and the overall control of the disease.
Study Design
This was a cross-sectional study conducted at the King Hussein Cancer Center (KHCC) in Amman, Jordan, between October 2019 and February 2020. According to the last available statistics in 2015, KHCC provides medical care to around 60.0% of all cancer patients in Jordan ( Abdel-Razeq et al., 2015 ). KHCC is the main hospital and the single specialized tertiary hospital in Jordan and provides comprehensive clinical management and cancer care to adults and pediatric patients from Jordan and the surrounding region.
Sampling Strategy
Data were collected from the inpatient and outpatient settings using a convenience sampling technique. Cancer patients who have any type of cancer from any stage and who are willing to participate in the study formed the study population. The inclusion criteria were (a) patients aged 18 years and above with a confirmed cancer diagnosis and (b) patients who had no apparent cognitive deficit. Patients were excluded if they were (a) below 18 years of age and (b) unable to participate in this study due to physical or emotional distress. This is due to the difficulties in detecting depression in cancer patients with emotional distress because of patients’ reluctance to discuss their emotional well-being ( Krebber et al., 2014 ). Eligible patients were identified and assessed by a clinical pharmacist (NM). Recruitment of patients was conducted by two pharmacists (AH and NM). For patients who agreed to participate, the study’s aim and objectives were explained thoroughly. Information sheets were provided to the patients for further clarification about the study. In addition, patients were informed that their agreement to participate in the study is considered as written consent. Patients’ clinical data were obtained from their medical charts in collaboration with a clinical pharmacist at the cancer center.
Sample Size
The target sample size was estimated based on the WHO recommendations for the minimal sample size needed for a prevalence study ( Lwanga and Lemeshow, 1991 ). Using a confidence interval of 95%, a standard deviation of the prevalence rate of 0.5, a margin of error of 5%, we determined that the required sample size was 385 patients.
Depression and Anxiety Assessment Scales
Previously validated assessment scales, the Patient Health Questionnaire (PHQ)-9, Generalized Anxiety Disorder 7-item (GAD-7) scale, and Hospital Anxiety and Depression Scale (HADS) were used to assess depressive and anxious symptomatology among the study participants. These screening instruments were frequently used and validated as brief screening tools among cancer patients for depressive and anxious symptomatology ( Härter et al., 2006 ; Sawaya et al., 2016 ; Terkawi et al., 2017 ). These assessment scales provide a symptomatological assessment based on predefined cut-off points.
Different assessment scales were used to fit the inpatient and outpatient settings, as recommended by previous literature. The HADS and GAD-7 instruments were previously validated to be used for hospitalized patients (secondary care settings) in multiple previous studies, including studies on patients with cancer ( Zigmond and Snaith, 1983 ; Bjelland et al., 2002 ; Hartung et al., 2017 ; Esser et al., 2018 ). In contrast, the PHQ-9 and GAD-7 instruments were recommended for patients in primary care settings ( Hinz et al., 2016 ; Esser et al., 2018 ; Levis et al., 2019 ). The PHQ-9 scale is a nine-question instrument given to patients in a primary-care setting to screen for the presence and severity of depressive symptomatology ( Hartung et al., 2017 ). This instrument was used to assess depressive symptomatology among cancer patients in the outpatient setting, and the GAD-7 instrument was used to screen for anxiety among them ( Esser et al., 2018 ). In the inpatient setting, the HADS instrument was used, which is a 14-question instrument given to patients in a secondary-care setting to screen for the presence and severity of depressive and anxious symptomatology ( Hartung et al., 2017 ).
The use of a pre-existing scale has the advantage of using a validated and tested instrument, which increases the reliability of its measure. The PHQ-9 and the GAD-7 instruments ask the patients about the degree of applicability of each item (question), using a 4-point Likert scale. Patients’ responses ranged from 0 to 3, where 0 means “Not at all” and 3 means “Nearly every day.” The HADS instrument is a 14-question questionnaire that asks the patients about the degree of applicability of each item (question), using a 4-point Likert scale. Patients’ response ranges from 0 to 3, where 0 means “Often” and 3 means “Very seldom” or from “Not at all” to “Most of the time.”

Methods of Analysis
An estimate of prevalence and classification of depression and anxiety.
Prevalence rates of depressive and anxious symptomatology were determined using a cut-off point as recommended by the authors of the PHQ-9, GAD-7, and HADS scale. At the inpatient setting using the HADS instrument, depressive and anxious symptomatology were defined as a total score of (10 or more) at “depression subscale” or “anxiety subscale,” respectively ( Zigmond and Snaith, 1983 ). At the outpatient setting, depressive symptomatology was defined as a total score of 15 and above in the PHQ-9 instrument, indicating a case with moderately severe or severe depression ( Kroenke et al., 2001 ). Anxious symptomatology was defined using the GAD-7 instrument with a total score of 15 and above, indicating a case with severe anxious symptomatology ( Spitzer et al., 2006 ). The higher the score, the more severe the case identified by any scale.
The prevalence rate of depressive symptomatology was estimated by dividing the number of patients who exceeded the borderline score by the total number of patients. The same procedure was followed to calculate the prevalence rate of anxious symptomatology in the inpatient and the outpatient settings.
At The Inpatient Setting
The HADS instrument was used in the inpatient setting, which includes two subscales (anxiety and depression) with seven items for each. Items are scored from 0 to 3, generating a total score ranging from 0 to 21 on each subscale. A total score of 0–7 indicates a normal case, 8–10 a borderline case, and 11–21 an abnormal case of depressive or anxious symptomatology according to the subscale score ( Zigmond and Snaith, 1983 ). In addition, anxious symptomatology was assessed using the GAD-7 scale as a second measure and results were compared with HADS.
At The Outpatient Setting
The PHQ-9 instrument was used in the outpatient setting, which includes nine items. Items are scored from 0 to 3, generating a total score ranging from 0 to 27. A total score of 0–4 indicates minimal depression, 5–9 mild depression, 10–14 moderate depression, 15–19 moderately severe depression, and 20–27 severe depression ( Schwenk et al., 2011 ). The GAD-7 instrument includes seven items. Items are scored from 0 to 3, generating a total score ranging from 0 to 21. A total score of 5–9 indicates mild anxiety, 10–14 moderate anxiety, and 15–21 severe anxiety ( Spitzer et al., 2006 ).
Statistical Analysis
Descriptive statistics were used to describe patients’ demographic characteristics, medication use, and comorbidities. Continuous data were reported as mean ± SD. Categorical data were reported as percentages (frequencies). Logistic regression was used to estimate odds ratios (ORs), with 95% confidence intervals (CIs) for anxious or depressive symptomatology. Logistic regression models were carried out using anxious or depressive symptomatology scores above the cut-off points as highlighted in the section “Materials and Methods.” The cut-off point for the PHQ-9 and GAD-7 scale that was used to identify severe depressive symptomatology and severe anxious symptomatology in the outpatient setting was 15 and above. The cut-off for the HADS scales that was used to identify depressive symptomatology and anxious symptomatology in the inpatient setting was 10 and above (whether for the depression or anxiety subscale). A two-sided p < 0.05 was considered statistically significant. The statistical analyses were carried out using SPSS for Windows (version 25).
Patient Characteristics
Out of 1,041 patients who were approached during the study period, a total of 1,011 patients (response rate of 97.1%) participated in the study: inpatient setting = 399, outpatient setting = 612. Table 1 details the baseline characteristics of the patients in the inpatient and outpatient settings. The mean age of the patients was 54.9 years (±15.2). The majority of patients ( n = 560, 55.4%) were males, married ( n = 833, 82.4%), and unemployed ( n = 471, 46.6%), with an income of 500 JD or below ( n = 737, 72.9%). Around 18.4% ( n = 186) of the patients reported that their cancer was metastatic. More than half ( n = 566, 56.0%) of the patients reported being treated with chemotherapy.
The baseline characteristics of the patients in the inpatient and the outpatient settings.
The most common cancer type in the study was blood cancer ( n = 196, 19.3%), followed by colorectal cancer ( n = 178, 17.6%) and lung cancer ( n = 120, 11.9%). Please refer to Supplementary Table 1 , which shows the distribution of cancer types among study participants.
Use of Antidepressants
Around 42.7% ( n = 432) and 24.8% ( n = 251) of the patients reported that they feel anxious and depressed, respectively, and only 15.5% ( n = 39) of them were using antidepressants. The two most commonly used antidepressant medications were sertraline (29.0%, n = 9) and citalopram (16.1%, n = 5).
The mean age of the patients when they started using antidepressants was 52.2 years (±17.4). Around 51.6% ( n = 16) of these patients were using antidepressants last year, and 67.7% ( n = 21) are using it currently. The majority of the patients (80.6%, n = 25) were using antidepressant medications under medical supervision. The main indication for the use of antidepressants was to alleviate depression for 83.9% ( n = 26), and 35.5% ( n = 11) were using them to alleviate anxiety. Around 92.3% ( n = 36) of the patients reported that they had received instructions on how to use antidepressants. The main source of these instructions was a physician (94.4%, n = 34). The majority of the patients (75.0%, n = 27) believe that these instructions are important ( Table 1 ).
The main reasons patients consider the healthcare professionals’ instructions important include the notion that they increase the safety (27.3%, n = 6) and effectiveness (27.3%, n = 6) of the medication; they decrease side effects and drug interactions (27.3%, n = 6) and increase confidence in therapy (18.2%, n = 4). Nine patients reported that they have questions about antidepressants, and they were mainly considering treatment side effects, mechanisms of action, and treatment time ( Supplementary Table 2 ).
Supplementary Table 3 highlights characteristics of antidepressant use and patient knowledge. More than half of the patients (56.8%, n = 21) reported that they have increased the dose of antidepressant medication without consulting the doctor. Around 40.5% ( n = 15) of the patients reported that they were experiencing side effects from the use of antidepressants. The main three side effects were nausea, dizziness, and insomnia, suffered by 73.3%, 60.0%, and 40.0% of patients, respectively. About 29.7% ( n = 11) and 21.6% ( n = 8) of the patients reported that they think that antidepressant use can cause addiction and tolerance, respectively. More than half of the patients (59.5%, n = 22) reported that antidepressant therapy should gradually be withdrawn at the end of the treatment. When the patients were asked about whether they had stopped using antidepressant without consulting the doctor, 32.4% ( n = 12) reported they had. The two most common reasons for this practice were improved depressive symptoms (50.0%, n = 6) and low tolerance of side effects of the medication (41.7%, n = 5).
Prevalence of Depression and Anxiety
The prevalence of depressive symptomatology among all patients was 23.4% ( n = 237; 89 from the outpatient setting and 148 from the inpatient setting). Depressive symptomatology was more prevalent in the inpatient setting (37.1%; n = 148) compared to the outpatient setting (14.5%; n = 89). The prevalence of anxious symptomatology among all patients was 19.1% ( n = 193) using the HADS for the inpatients or 19.9% ( n = 201), using the GAD-7 for the inpatients. Similarly, anxious symptomatology was more prevalent in the inpatient setting, at 35.6% ( n = 142), using the HADS, or 37.6% ( n = 150) (using the GAD-7). Table 2 below details the prevalence of depressive and anxious symptomatology among the patients stratified by severity.
Prevalence of depression and anxiety among the patients’ stratified by severity.
Table 3 below details the prevalence of depressive and anxious symptomatology stratified by severity and type of cancer in the inpatient and the outpatient setting. In the inpatient setting, depressive symptomatology was more common across patients with bladder cancer, and severe anxious symptomatology was more prevalent among patients with lung cancer. In the outpatient setting, depressive symptomatology was more common among patients with breast cancer, and the highest prevalence of anxious symptomatology was among patients with prostate cancer.
Prevalence of depression and anxiety stratified by type of cancer and severity.
Risk Factors of Depression and Anxiety
In the inpatient setting, logistic regression analysis identified the following groups as being at a higher risk of depressive symptomatology: a) patients with metastatic cancer, OR: 2.62 (95% CI 1.61–4.28) and b) patients at an advanced stage of the disease, stage 3, OR: 5.26 (95% CI 1.05–26.41) and stage 4, OR: 2.73 (95% CI 1.61–4.62). In the outpatient setting, patients with metastatic cancer were the only group that showed a statistically significant increased risk of depressive symptomatology, OR: 3.36 (95% CI 1.33–8.50), compared with others.
Regarding anxious symptomatology, in the inpatient setting the following groups were identified to be at a higher risk using the HADS: a) patients with metastatic cancer, OR: 2.10 (1.29–3.42) and b) patients at stage four of the disease, OR: 2.39 (95% CI 1.42–4.04). On the other hand, patients who are treated with a combination of chemotherapy and surgery showed a lower risk of anxious symptomatology, OR: 0.54 (95% CI 0.33–0.86). On the basis of the GAD-7 scale, the only patient group that showed a higher risk of anxious symptomatology was the group with metastatic cancer, OR: 2.23 (95% CI 1.32–3.74). In the outpatient setting, unemployed patients—OR: 1.89 (95% CI 1.03–3.35)—and patients with metastatic cancer—OR: 2.47 (95% CI 1.15–5.33)—were at higher risk of anxious symptomatology ( Table 4 ).
Logistic regression analysis.
This study aimed to identify the prevalence of depressive and anxious symptomatology among cancer patients and to identify key risk factors using validated assessment tools. Additionally, we explored the pattern of use of antidepressants among the study participants. Several dimensions were investigated in this study, including patients’ characteristics, the prevalence of anxious and depressive symptomatology according to the type of cancer and treatment settings (inpatient versus outpatient). Our findings showed that the prevalence of depressive and anxious symptomatology among cancer patients was 23.4% and 19.1–19.9%, respectively. Increased likelihood of depressive and anxious symptomatology was detected among patients in the inpatient setting (37.1% and 35.6–37.6%, respectively). Screening of frequently prescribed anxiolytics and antidepressants was investigated, revealing that for the most part, SSRIs were prescribed, but as low as 15.5% of depressed and anxious patients received the required treatment ( Waraich et al., 2004 ; Brothers et al., 2011 ; Findley et al., 2012 ; Li et al., 2012 ; Baltenberger et al., 2014 ; Nakash et al., 2014 ; Jassim et al., 2015 ; van den Berg et al., 2015 ; Kanera et al., 2016 ; Lengacher et al., 2016 ; Reich et al., 2017 ; Ahmed, 2019 ).
Our research employed two validated tools (GAD-7 and HADS) to assess the prevalence of anxiety among cancer patients in the inpatient setting, and both of them were reliable and showed a significant correlation (correlation coefficient: 0.812) in terms of the prevalence of anxiety (37.6% versus 35.6% in the inpatient setting). The increased cancer-specific depressive symptomatology was noted across settings (inpatient and outpatient) and was significantly higher in the inpatient settings.
Several factors may impact the development of depression and anxiety among cancer patients, including the cancer type, stage, grade, and treatment option ( Smith, 2015 ). Interestingly, our results are aligned with the findings of several studies, where specific tumor types can lead to depression and anxiety, particularly head and neck, lung, breast, and prostate cancer ( Pitman et al., 2018 ), which, in our research, showed that depression and anxiety are more prevalent in the inpatient setting in patients with head and neck cancer, lung cancer, and bladder cancer, while in the outpatient setting, they were more prevalent among patients diagnosed with prostate and breast cancer.
In addition to the type of cancer, the treatment option impacted the prevalence of anxiety and depression among cancer patients. Cancer treatments that entail chemotherapy may induce depression through specific biological mechanisms. Furthermore, the literature reported that antiemetic medications, steroids, and androgen suppression therapy (for prostate cancer) were reported to induce depression ( Smith, 2015 ; Ismail et al., 2017 ; Nead et al., 2017 ; Niedzwiedz et al., 2019 ). The findings of our study revealed that most cancer patients receive chemotherapy alone (56.0%) or chemotherapy and surgery (34.8%), thus increasing their risk of depression and/or anxiety. The use of chemotherapy-induced nausea and vomiting (CINV) medications, as well as steroids, are a mainstay for the prevention and treatment of CINV ( Rao and Faso, 2012 ) and, consequently, contribute to the high prevalence of anxiety and depression among cancer patients.
Antidepressant Use Patterns
This study also explored the pattern of use of antidepressants among cancer patients stratified by type, where the rate of using antidepressants among patients diagnosed with depression was as low as 15.5%. Low use of antidepressant therapy is an alarming sign, especially for cancer patients who are receiving specialized cancer services ( Waraich et al., 2004 ; Findley et al., 2012 ; Nakash et al., 2014 ). Antidepressants should be introduced as soon as the patient is diagnosed with depression. Use of antidepressants should be individualized according to the patient’s health profile to address his or her symptoms. The choice of antidepressants should be based on the patient’s concurrent medications, history, and symptoms ( Ahmed, 2019 ). Pharmacological therapy is recommended for patients with severe depression, while patients with mild to moderately severe depression are recommended to receive psychotherapy. Several behavioral approaches can be implemented to improve the psychological status of cancer patients, such as cognitive behavioral therapy, mindfulness-based approaches, and self-management strategies. Management of depression and treatment initiation should be under medical supervision, due to the nature of antidepressants and the high probability of drug-drug interaction, adverse effects, and the need for dose adjustment ( Ahmed, 2019 ).
The majority of patients were prescribed selective serotonin reuptake inhibitor (SSRI) antidepressants (sertraline, citalopram, fluoxetine, fluvoxamine, and paroxetine), while tetracyclic antidepressants (mirtazapine) and tricyclic antidepressants (amitriptyline) were prescribed to a lesser extent. The selection of an anxiolytic or antidepressant medication needs to be made under clear guidelines that consider interactions with chemotherapeutic and other concurrently administered medication, as well as side effects, to enable identification of specific contraindications. Sertraline and citalopram are usually recommended for depression and anxiety treatment, as they have the least tendency for interactions and are usually well tolerated ( Chochinov, 2001 ; Smith, 2015 ). Although the use of medications for depression and anxiety among cancer patients in this study was low, the selection of pharmacological agents is in line with the above recommendations. However, a patient-centered approach and a customized treatment plan need to be considered, as studies reported that the SSRIs need to be avoided in elderly patients due to the risk of hyponatremia. Fluoxetine and paroxetine are contraindicated in patients being treated with tamoxifen. Furthermore, mirtazapine should be avoided where white blood cells are compromised and SSRIs are to be avoided where platelets are compromised ( Pitman et al., 2018 ). Finally, since almost 70.0% of the patients reported that they experienced nausea, SSRIs need to be avoided as this will augment chemotherapy-induced nausea and vomiting.
Our findings suggest that severe depressive and anxious symptomatology are substantially more common in patients with cancer in the inpatient than in the outpatient setting. This significant difference could be attributed to the severity of the disease, where hospitalized treatment of cancer patients is mainly employed for the management of acute phases, initial onsets, severe cases, or late stages. A previous study involving more than 5,000 patients has reported that more symptoms of anxiety are associated with cancer within the inpatient setting and in patients in the advanced stages, whereas patients at early stages demonstrated lower anxiety symptoms. Furthermore, disease stage was associated with depression, particularly in men ( Vodermaier et al., 2011 ).
Further analysis using logistic regression was conducted to identify patients at risk of developing depressive and anxious symptomatology. Similar to reported data, the prevalence of anxiety and depression among women is higher than in men ( Smith, 2015 ). As discussed earlier, the advancement of the stage of the disease has an impact on depression and anxiety. Patient vulnerability exacerbates the ability to become depressed and anxious, which is reported to be caused by some socioeconomic factors and disease stages ( Chochinov, 2001 ; Smith, 2015 ). Such results align with our findings about patients with metastasis, advanced disease stages, and lower-income were more vulnerable to developing anxiety and/or depression. Such findings support the need to consider mental disorders as part of the treatment protocol for cancer patients and calls for enhanced clinical monitoring and treatment of depression and anxiety symptoms among cancer patients.
Strengths and Limitations
To the best of our knowledge, this is the first and largest study in the Middle East region to investigate the prevalence of depressive and anxious symptomatology and use of antidepressants among cancer patients without restriction on the type of cancer or clinical settings. Previous studies have focused on a specific type of cancer (breast cancer and colorectal cancer) ( Al Ahwal et al., 2014 ; Abou Kassm et al., 2018 ; Ahmadi Gharaei et al., 2019 ) and had a small sample size (not more than 100 patients) ( Al Ahwal et al., 2014 ; Abou Kassm et al., 2018 ).
We have explored the prevalence of depressive and anxious symptomatology among cancer patients without any restriction on the age, gender, duration of the disease, or treatment phase. Our broad inclusion criteria have enabled us to explore the difference in severity of depressive and anxious symptomatology among different demographic groups and across different stages of the treatment and the course of illness. This study has many strengths that increase its value and reliability: (a) using validated assessment tools for depressive and anxious symptomatology, (b) anxious symptomatology was assessed using two assessment tools (HADS and GAD-7), and both of them showed consistent findings, (c) employing a large sample size, (d) not restricting the inclusion criteria for the specific type of cancer or specific settings (inpatients or outpatients), which increased the generalizability of our findings, and (e) our exclusion criteria minimized the risk of deriving imprecise information (related to the patients’ psychological status) from any suspected physical or emotional distress. On the other hand, this study has limitations: (a) the study design itself, a cross-sectional study design, limited our ability to identify causality between study variables, as it is only capable of showing an association between variables, and (b) the sample size of a few cancer subgroups was small due to a small population in this category nationwide. This might affect our ability to determine the prevalence of depressive and anxious symptomatology among patients with specific types of cancer, especially types for which we have a very low number of patients, such as head and neck cancer; (c) the use of convenience sampling techniques might affect the generalizability of our findings as a prevalent study and may introduce sampling bias (which might not precisely represent the targeted population); (d) the use of different assessment tools to describe the prevalence of depressive symptomatology between the inpatient and the outpatient settings might not provide a fair comparison; (e) there is a lack of non-responder data; and (f) the antidepressant medication information is based on small subsamples making conclusions difficult to sustain. Therefore, our findings should be interpreted carefully.
Our study demonstrated a higher prevalence of both depressive and anxious symptomatology within the inpatient setting and advanced stages of the disease. There is a need for cancer management clinical guidelines to consider early assessment and management of depression and anxiety and to continue to monitor it throughout treatment.
Data Availability Statement
Ethics statement.
Approval for this study was obtained from the Institutional Review Board Committee at King Hussein Cancer Centre in Jordan (No. 19 KHCC 94). For patients who agreed to participate, the study’s aim and objectives were explained thoroughly. Information sheets were provided to the patients for further clarification about the study. In addition, patients were informed that their agreement to participate in the study is considered as a written consent.
Author Contributions
AN contributed to the study design and did the data analysis. AN, AH, NM, and HK conducted the study and collected the data. AN, ED, NM, and HA wrote the first draft of the article. AN, HA, ED, HA, and NM were involved in interpretation of the data. All authors reviewed the manuscript for important intellectual content, provided final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the King Hussein Cancer Center for the support in conducting this research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2021.585534/full#supplementary-material
Supplementary Table 1
Type of cancer among the patient stratified by settings.
Supplementary Table 2
Questions about antidepressants treatment.
Supplementary Table 3
Characteristics of antidepressants utilization and patients’ knowledge about them.
- Abdel-Razeq H., Attiga F., Mansour A. (2015). Cancer care in Jordan. Hematol. Oncol. Stem Cell Ther. 8 64–70. [ PubMed ] [ Google Scholar ]
- Abou Kassm S., Hlais S., Khater C., Chehade I., Haddad R., Chahine J., et al. (2018). Depression and religiosity and their correlates in Lebanese breast cancer patients. Psychooncology 27 99–105. 10.1002/pon.4386 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ahmadi Gharaei H., Dianatinasab M., Kouhestani S. M., Fararouei M., Moameri H., Pakzad R., et al. (2019). Meta-analysis of the prevalence of depression among breast cancer survivors in Iran: an urgent need for community supportive care programs. Epidemiol. Health 41 : e2019030 . 10.4178/epih.e2019030 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ahmed E. (2019). Antidepressants in patients with advanced cancer: when they’re warranted and how to choose therapy. Oncology 33 62–68. [ PubMed ] [ Google Scholar ]
- Al Ahwal M., Al Zaben F., Khalifa D. A., Sehlo M. G., Ahmad R. G., Koenig H. G. (2014). Depression in patients with colorectal cancer in Saudi Arabia. Psychooncology 24 1043–1050. 10.1002/pon.3706 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baltenberger E., Schmitt G., Thomas C. (2014). Treatment of depressive symptoms in patients with cancer. Mental Health Clin. 4 114–117. 10.9740/mhc.n194575 [ CrossRef ] [ Google Scholar ]
- Bjelland I., Dahl A. A., Haug T. T., Neckelmann D. (2002). The validity of the hospital anxiety and depression scale. An updated literature review. J. Psychosom. Res. 52 69–77. [ PubMed ] [ Google Scholar ]
- Brothers B., Yang H.-C., Strunk D. R., Andersen B. L. (2011). Cancer patients with major depressive disorder: testing a biobehavioral/cognitive behavior intervention. J. Consult. Clin. Psychol. 79 253–260. 10.1037/a0022566 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Caruso R., Nanni M. G., Riba M., Sabato S., Mitchell A. J., Croce E., et al. (2017). Depressive spectrum disorders in cancer: prevalence, risk factors and screening for depression: a critical review. Acta Oncol. 56 146–155. 10.1080/0284186x.2016.1266090 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Centre for Disease Control and Prevention (2019). Global Health- Jordan. Available online at: https://www.cdc.gov/globalhealth/countries/jordan/default.htm (accessed May 1, 2020). [ Google Scholar ]
- Chochinov H. M. (2001). Depression in cancer patients. Lancet 2 499–505. [ PubMed ] [ Google Scholar ]
- Delgado-Guay M., Parsons H. A., Li Z., Palmer J. L., Bruera E. (2009). Symptom distress in advanced cancer patients with anxiety and depression in the palliative care setting. Support Care Cancer 17 573–579. 10.1007/s00520-008-0529-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Esser P., Hartung T. J., Friedrich M., Johansen C., Wittchen H.-U., Faller H., et al. (2018). The generalized anxiety disorder screener (GAD-7) and the anxiety module of the hospital and depression scale (HADS-A) as screening tools for generalized anxiety disorder among cancer patients. Psychooncology 27 1509–1516. 10.1002/pon.4681 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Findley P. A., Shen C., Sambamoorthi U. (2012). Depression treatment patterns among elderly with cancer. Depress Res. Treat. 2012 : 676784 . [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Härter M., Woll S., Wunsch A., Bengel J., Reuter K. (2006). Screening for mental disorders in cancer, cardiovascular and musculoskeletal diseases. Comparison of HADS and GHQ-12. Soc Psychiatry Psychiatr. Epidemiol. 41 56–62. 10.1007/s00127-005-0992-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hartung T., Friedrich M., Johansen C., Wittchen H. U., Faller H., Koch U., et al. (2017). The hospital anxiety and depression scale (HADS) and the 9-item patient health questionnaire (PHQ-9) as screening instruments for depression in patients with cancer. Cancer 123 4236–4243. 10.1002/cncr.30846 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hinz A., Mehnert A., Kocalevent R. D., Brähler E., Forkmann T., Singer S., et al. (2016). Assessment of depression severity with the PHQ-9 in cancer patients and in the general population. BMC Psychiatry 16 : 22 . 10.1186/s12888-016-0728-6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ismail M., Lavelle C., Cassidy E. (2017). Steroid-induced mental disorders in cancer patients: a systematic review. Future Oncol. 13 2719–2731. 10.2217/fon-2017-0306 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jassim G. A., Whitford D. L., Hickey A., Carter B. (2015). Psychological interventions for women with non-metastatic breast cancer. Cochrane Database Syst. Rev. 28 : CD008729 . [ PubMed ] [ Google Scholar ]
- Kaasa S., Malt U., Hagen S., Wist E., Moum T., Kvikstad A. (1993). Psychological distress in cancer patients with advanced disease. Radiother. Oncol. 27 193–197. 10.1016/0167-8140(93)90073-h [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kanera I., Willems R. A., Bolman C. A. W., Mesters I., Zambon V., Gijsen B. C. M., et al. (2016). Use and appreciation of a tailored self-management ehealth intervention for early cancer survivors: process evaluation of a randomized controlled trial. J. Med. Internet Res. 18 : e229 . 10.2196/jmir.5975 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Koenig H., Cohen H. J., Blazer D. G., Meador K. G., Westlund R. (1992). A brief depression scale for use in the medically ill. Int. J. Psychiatry Med. 22 183–195. [ PubMed ] [ Google Scholar ]
- Krebber A. M., Buffart L. M., Kleijn G., Riepma I. C., de Bree R., Leemans C. R., et al. (2014). Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology 23 121–130. 10.1002/pon.3409 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kroenke K., Spitzer R., Williams J. (2001). The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern Med. 16 606–613. 10.1046/j.1525-1497.2001.016009606.x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lengacher C., Reich R. R., Paterson C. L., Ramesar S., Park J. Y., Alinat C., et al. (2016). Examination of broad symptom improvement resulting from mindfulness-based stress reduction in breast cancer survivors: a randomized controlled trial. J. Clin. Oncol. 34 2827–2834. 10.1200/jco.2015.65.7874 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Levis B., Benedetti A., Thombs B. D. DEPRESsion Screening Data (Depressd) Collaboration. (2019). Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ 365 1–11. 10.1017/s0033291721000131 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li M., Fitzgerald P., Rodin G. (2012). Evidence-based treatment of depression in patients with cancer. J. Clin. Oncol. 30 1187–1196. 10.1200/jco.2011.39.7372 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lwanga S. K., Lemeshow S. (1991). Sample size Determination in Health Studies: A Practical Manual. Geneva: World Health Organisation, 1–58. [ Google Scholar ]
- Massie M. J. (2004). Prevalence of depression in patients with cancer. J. Natl. Cancer Inst. Monogr. 57–71. [ PubMed ] [ Google Scholar ]
- McDermott C., Bansal A., Ramsey S. D., Lyman G. H., Sullivan S. D. (2018). Depression and health care utilization at end of life among older adults with advanced non-small-cell lung cancer. J. Pain Symptom Manage. 56 699–708. 10.1016/j.jpainsymman.2018.08.004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mitchell A., Chan M., Bhatti H., Halton M., Grassi L., Johansen C., et al. (2011). Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interviewbased studies. Lancet Oncol. 12 160–174. 10.1016/s1470-2045(11)70002-x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nakash O., Levav I., Aguilar-Gaxiola S., Alonso J., Andrade L. H., Angermeyer M. C., et al. (2014). Comorbidity of common mental disorders with cancer and their treatment gap: findings from the world mental health surveys. Psychooncology 23 40–51. 10.1002/pon.3372 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nead K., Sinha S., Yang D. D., Nguyen P. L. (2017). Association of androgen deprivation therapy and depression in the treatment of prostate cancer: a systematic review and meta-analysis. Urologic Oncol. 35 664.e1–664e9. [ PubMed ] [ Google Scholar ]
- Niedzwiedz C. L., Knifton L., Robb K. A., Katikireddi S. V., Smith D. J. (2019). Depression and anxiety among people living with and beyond cancer: a growing clinical and research priority. BMC Cancer 19 : 943 . 10.1186/s12885-019-6181-4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pirl W. F. (2004). Evidence report on the occurrence, assessment, and treatment of depression in cancer patients. J. Natl. Cancer Inst. Monogr. 32–39. 10.1093/jncimonographs/lgh026 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pitman A., Suleman S., Hyde N., Hodgkiss A. (2018). Depression and anxiety in patients with cancer. BMJ 361 : k1415 . [ PubMed ] [ Google Scholar ]
- Rao K., Faso A. (2012). Chemotherapy-induced nausea and vomiting: optimizing prevention and management. Am. Health Drug Benefits 5 232–240. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Reich R., Lengacher C. A., Alinat C. B., Kip K. E., Paterson C., Ramesar S., et al. (2017). Mindfulness-based stress reduction in post-treatment breast cancer patients: immediate and sustained effects across multiple symptom clusters. J. Pain Symptom. Manage. 53 85–95. 10.1016/j.jpainsymman.2016.08.005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sawaya H., Atoui M., Hamadeh A., Zeinoun P., Nahas Z. (2016). Adaptation and initial validation of the Patient Health Questionnaire - 9 (PHQ-9) and the Generalized Anxiety Disorder - 7 Questionnaire (GAD-7) in an Arabic speaking Lebanese psychiatric outpatient sample. Psychiatry Res. 239 245–252. 10.1016/j.psychres.2016.03.030 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schwenk T., Terrell L., Harrison R., Tremper A., Valenstein M., Bostwich J. (2011). UMHS depression guideline. Guidelines for Clinical Care Ambulatory , 1–-15. Available online at: http://www.med.umich.edu/1info/FHP/practiceguides/depress/depress.pdf (accessed August, 2011). [ Google Scholar ]
- Smith H. R. (2015). Depression in cancer patients: pathogenesis, implications and treatment (Review). Oncol. Lett. 9 1509–1514. 10.3892/ol.2015.2944 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Spiegel D., Bloom J. R., Kraemer H. C., Gottheil E. (1989). Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet 2 888–891. 10.1016/s0140-6736(89)91551-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Spiegel D., Li Y. (2007). Effects of supportive-expressive group therapy on survival of patients with metastatic breast cancer: a randomized prospective trial. Cancer 110 1130–1138. 10.1002/cncr.22890 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Spitzer R., Kroenke K., Williams J. B. W., Löwe B. (2006). A brief measure for assessing generalized anxiety disorder. Arch. Intern Med. 166 1092–1097. 10.1001/archinte.166.10.1092 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Terkawi A., Tsang S., AlKahtani G. J., Al-Mousa S. H., Al Musaed S., AlZoraigi U. S., et al. (2017). Development and validation of Arabic version of the hospital anxiety and depression scale. Saudi J. Anaesth. 11 11–18. 10.4103/sja.sja_43_17 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- van den Berg S., Gielissen M. F. M., Custers J. A. E., Van der Graaf W. T. A., Ottevanger P. B., Prins J. B. (2015). BREATH: web-based self-management for psychological adjustment after primary breast cancer–results of a multicenter randomized controlled trial. J. Clin. Oncol. 33 2763–2771. 10.1200/jco.2013.54.9386 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vodermaier A., Linden W., MacKenzie R., Greig D., Marshall C., et al. (2011). Disease stage predicts post-diagnosis anxiety and depression only in some types of cancer. Br. J. Cancer 105 1814–1817. 10.1038/bjc.2011.503 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Walker J., Hansen C. H., Martin P., Sawney A., Thekkumpurath P., Beale C., et al. (2013). Prevalence of depression in adults with cancer: a systematic review. Ann. Oncol. 24 895–900. [ PubMed ] [ Google Scholar ]
- Walker J., Hansen C. H., Martin P., Symeonides S., Ramessur R., Murray G., et al. (2014). Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry 1 343–350. 10.1016/s2215-0366(14)70313-x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Waraich P., Goldner E. M., Somers J. M., Hsu L. (2004). Prevalence and incidence studies of mood disorders: a systematic review of the literature. Can. J. Psychiatry. 49 124–138. 10.1177/070674370404900208 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- World Health Organization (2018). International Agency for Research on Cancer, Globocan 2018- Jordan. Available online at: https://gco.iarc.fr/today/data/factsheets/populations/400-jordan-fact-sheets.pdf (accessed May 1, 2020). [ Google Scholar ]
- Zigmond A. S., Snaith R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatr. Scand. 67 361–370. 10.1111/j.1600-0447.1983.tb09716.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Open access
- Published: 06 April 2024
Real-world patient characteristics and treatment patterns in US patients with advanced non-small cell lung cancer
- Hozefa A. Divan 1 ,
- Marisa A. Bittoni 2 ,
- Ashok Krishna 1 &
- David P. Carbone 2
BMC Cancer volume 24 , Article number: 424 ( 2024 ) Cite this article
487 Accesses
Metrics details
Patients from non-small cell lung cancer (NSCLC) controlled clinical trials do not always reflect real-world heterogeneous patient populations. We designed a study to describe the real-world patient characteristics and treatment patterns of first-line treatment in patients in the US with NSCLC.
This was an observational, retrospective cohort study based on electronic medical records of US adults with locally advanced or metastatic disease in the ConcertAI Patient360 NSCLC database who initiated first-line treatment with anti-programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) therapy between July 2016 and December 2020. The analysis used patient attributes, clinical characteristics, and treatments from each patient’s medical records.
A total of 2175 patients were eligible for analysis. The median age was 68 years, and 26.2% of the patients were ≥75 years old. At treatment initiation, 96.4% and 3.6% of the patients had Stage 4 and Stage 3 (B or C) NSCLC, respectively. The most common histology type was nonsquamous adenocarcinoma (66.4%), and 19.8% had Eastern Cooperative Oncology Group performance status ≥2. Immunosuppressive medications were being used by 17.7% of patients, and 11.0% were immunocompromised. Almost all patients had metastases: 64.6% had 1, 23.2% had 2, and 8.0% had ≥3 metastatic sites. Brain metastases were present in 22.9% of patients. Treatment evolution was observed with first-line standard of care shifting from single-agent immunotherapy in 2016 (90.2%) to combination immunotherapy and chemotherapy in 2020 (60.2%).
Between 2016 and 2020, the first-line treatment paradigm for advanced NSCLC in the US shifted from anti–PD-1/PD-L1 monotherapy to combination chemoimmunotherapy, with increasing biomarker testing. Further research in heterogeneous patient populations to characterize treatment strategies is warranted.
Peer Review reports
Lung cancer was the leading cause of cancer-related deaths worldwide in 2020, and approximately 70% of cases were locally advanced or metastatic disease at diagnosis, of which 80–85% were non-small cell lung cancer (NSCLC) [ 1 , 2 , 3 ].
Clinical trials in recent years have examined a variety of treatment strategies, including monotherapy and combination immunotherapy (IO) compared with chemo-therapy, the previous standard of care for advanced NSCLC [ 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 ]. As a result, the treatment landscape for advanced NSCLC without actionable driver mutations in EGFR or ALK has shifted from chemotherapy to IO with chemotherapy. Immunotherapies target negative immunologic regulators such as cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and the programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathway [ 16 ].
Between 2015 and 2021, IO agents approved by the United States (US) Food and Drug Administration (FDA) as first- or second-line therapy for NSCLC without driver mutations included: the anti–PD-1 antibodies nivolumab, pembrolizumab, and cemiplimab; the anti–PD-L1 antibodies durvalumab and atezolizumab; and the anti–CTLA-4 antibody ipilimumab [ 17 ]. Use of first-line IO to treat advanced NSCLC has increased substantially in the US since the initial approvals in 2016 [ 18 ]. First-line FDA approvals occurred for IO monotherapy with pembrolizumab in October 2016 and for pembrolizumab combination therapy with chemotherapy in May 2017 [ 19 ]. Atezolizumab combination therapy was approved in December 2018 [ 19 ]. Nivolumab + ipilimumab was approved in May 2020, along with atezolizumab monotherapy [ 19 ]. Finally, cemiplimab monotherapy was approved in February 2021 [ 19 ].
Clinical trials are designed to enroll selected patients (ie, those with good performance status, adequate organ function, without certain comorbidities, and who are not immunocompromised), and treatments are administered in highly controlled settings. Therefore, it can be challenging to generalize the findings to the more clinically heterogeneous patient populations seen in practice [ 18 ]. Here we report the findings from a real-world observational study that examined the ConcertAI Patient360 NSCLC database to describe key evidence gaps related to clinical characteristics and treatment patterns in patients in the US who initiated first-line treatment with IO mono-therapy or combination therapy for advanced NSCLC from 2016 to 2020.
Study design and data source
This was a non-interventional, observational, retrospective cohort study of patients with advanced NSCLC who received treatment as documented in the Patient360 NSCLC electronic medical record (EMR) database (ConcertAI, Cambridge, MA). This database sources patient EMRs, including unstructured notes and scans, from multiple oncologic partnerships that are EMR-system agnostic. The database consists of de-identified data from patients treated at various academic (~20%) and community (~80%) oncology centers across the US (~15% in the Northeast, ~25% in the Midwest, ~40% in the South, and ~20% in the West, as defined by US Census Bureau geographic regions).
The overall study period was from July 1, 2015, to March 31, 2021 (Fig. 1 ). The study design included a baseline period, a patient identification period, and a follow-up period. The index date was defined as the date on which the patient initiated first-line anti–PD-1/PD-L1 therapy for locally advanced or metastatic NSCLC. The baseline period spanned from the patient’s earliest NSCLC diagnosis in the database, starting from July 1, 2015, to the index date. If more than one assessment for the same variable of interest was available within this baseline period, the assessment closest to the index date was selected. During the patient identification period (July 1, 2016, to December 31, 2020; 3 months prior to the end of the follow-up period), eligible patients with advanced NSCLC who initiated first-line systemic treatment were identified. The follow-up period began one day post-index date and ended either on the date of death or on March 31, 2021 (end date of the database).
Study period timeline, including baseline period, patient identification period, index date, and follow-up period
The focus of this analysis was patients with advanced NSCLC who were treated with first-line anti–PD-1/PD-L1 therapy. Patients included in the final study cohort for this analysis (Fig. 2 ) had to have (i) a diagnosis of locally advanced, unresectable Stage 3B and 3C, or Stage 4 metastatic NSCLC; (ii) no evidence of candidacy for surgical reconstruction or definitive chemoradiation; (iii) started first-line therapy from July 1, 2016, through December 3, 2020; (iv) age ≥18 years at the time of first-line therapy initiation; (v) absence of a multiple primary cancer diagnosis at the time of initiating first-line therapy; (vi) no evidence of IO treatment in Stage 3 (A, B, or C) NSCLC as part of neoadjuvant or adjuvant treatment; (vii) no evidence of targetable genetic alterations (eg, EGFR , ALK , ROS1 ); and (viii) received first-line treatment with an anti–PD-1/PD-L1 agent for NSCLC.
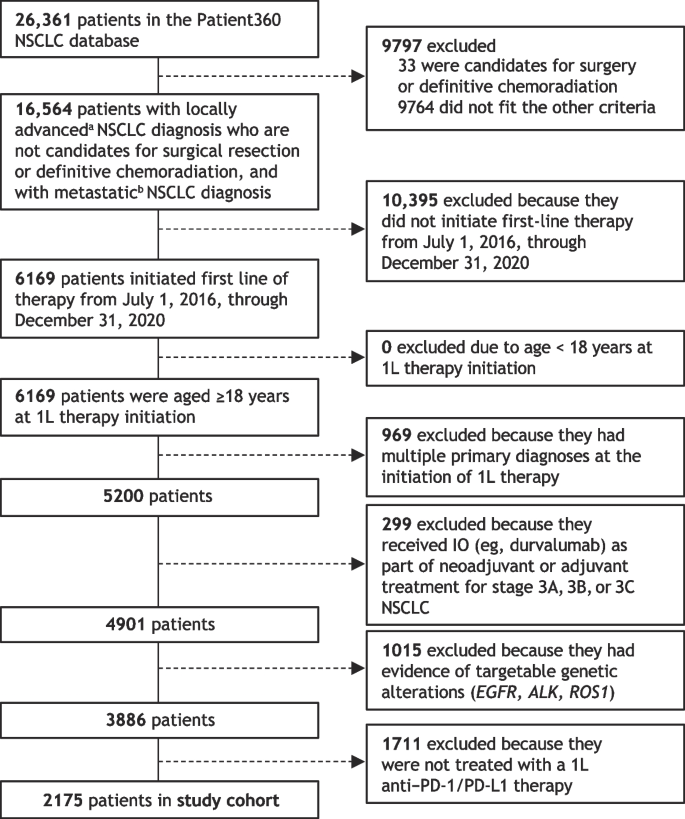
Patient selection and study cohort derived from the Patient360 NSCLC database. 1L, first line; ALK , anaplastic lymphoma kinase; EGFR , epidermal growth factor receptor; IO, immunotherapy; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; ROS1 , ROS proto-oncogene 1, receptor tyrosine kinase. a Stage 3 (3B, 3C) or neoplasm, secondary; b Stage 4, M1 or neoplasm, metastatic
Study objectives
The study objectives were to describe the demographics, clinical characteristics, and drug treatment patterns in patients with advanced NSCLC who were treated with first-line anti–PD-1/PD-L1 therapy. This was done for the overall cohort and by year of initiation of first-line therapy (2016 to 2020) and stratified by subgroups of interest that represent clinically relevant, unmet-needs populations. Additionally, clinical characteristics were stratified by first-line therapy, ie, anti–PD-1/PD-L1 monotherapy or anti–PD-1/PD-L1 therapy combined with platinum-based chemotherapy.
Ethical considerations
The study was performed in accordance with the Declaration of Helsinki and relevant International Council for Harmonisation, Good Clinical Practice, and Good Pharmacoepidemiological Practice guidelines. As no identifiable protected health information was extracted or accessed for the conduct of this study, ethics approval was deemed unnecessary under the Code of Federal Regulations Title 45, Part 46, Section 46.104(d)(4)(ii) (45CFR46.104[d][4][ii]).
Statistical analysis
This was an observational, descriptive cohort study of real-world patients with advanced NSCLC; hence, no hypothesis was tested, and no formal sample size calculation was required. Study measures (ie, patient demographics, disease and clinical characteristics, recorded treatment) were summarized with descriptive statistics and presented as frequencies and percentages. Statistical analyses were conducted using the Palantir Foundry implementation of PySpark.
Cohort disposition
From an initial population of 26,361 curated patients available in the NSCLC database, 3886 patients met all criteria and were started on first-line therapy in the study period, and 2175 of these were treated with an anti–PD-1/PD-L1 agent in the first line during the specified period and were included in the study cohort (Fig. 2 ).
- Patient characteristics
Baseline demographics and clinical characteristics of the patients in the study cohort ( n = 2175) are summarized in Table 1 , overall and by year of first-line therapy initiation. In this eligible population, the median age was 68.0 years (range, 19.0 to 88.0 years), and 53.7% were male. The age group distribution of the cohort remained constant from 2016 to 2020. Most patients were White (76.8%); African Americans comprised the second largest racial group (13.2%). Current or former smokers made up 87.9% of study patients, with the proportion increasing from 83.3% of those who initiated first-line therapy in 2016 to 92.9% of those who initiated first-line therapy in 2020.
Approximately two-thirds of patients (66.4%) had nonsquamous NSCLC with adenocarcinoma histology (Table 1 ). Nearly all patients (96.4%) had Stage 4 disease, and 95.7% had one or more metastatic sites. Bone (29.8%), brain (22.9%), and other lung (19.9%) were the most common metastatic sites at the index date. More patients younger than 65 years (29.3%) had evidence of brain metastases than those aged 65–74 years (21.7%) and ≥75 years (15.1%) (Table 2 ). More than three-quarters (78.9%) of patients had visceral site(s) of metastases at the index date, and 37.6% had nonvisceral site(s) of metastases (Table 1 ). Almost two-thirds of patients (64.7%) with reported Eastern Cooperative Oncology Group performance status (ECOG PS) had a score of 0 or 1, and 19.8% had a score of 2 or higher.
Only one-quarter (24.4%) of patients had recorded evidence of receiving a second line of therapy (Table 1 ). Of those with first-line therapy starting in 2016–2017, only 31.6% received a subsequent, second line of therapy in the study period. Among patients with first-line therapy starting between 2018 and 2020, 22.4% had subsequent treatment with a second line of therapy in the study interval.
Most patients (89.0%) were not immunocompromised, defined as having human immunodeficiency virus (HIV) or taking long-term (≥30 days) immunosuppressive medications, at the index date. Nearly all patients had a negative history of HIV, hepatitis B and C, and autoimmune disease at the index date (99.8%, 100.0%, and 98.5%, respectively). A higher percentage of female patients used immunosuppressive medication at the index date compared with male patients (20.1% versus 15.7%) (data not shown). Use of immunosuppressive medication at the index date decreased each year, with initial use at 25.5% of patients in 2016, down to 13.5% in 2020.
Overall, only 60.1% of study patients had evidence of testing for RET- , BRAF- , or MET -targetable genetic alterations, and 8.2% of all patients had one or more positive test results (Table 1 ). More patients who initiated first-line therapy between 2018 and 2020 had evidence of testing (64.2%) than those who initiated first-line therapy from 2016 to 2017 (45.6%). Almost three-quarters (72.0%) of study patients had a PD-1/PD-L1 expression test recorded, and 45.0% of study patients had a qualitative positive test result recorded. However, only 18.1% of patients with a positive PD-1/PD-L1 test had a result reported numerically. The proportion of patients tested for PD-1/PD-L1 expression increased from 53.9% in 2016 to 79.2% in 2020, and the proportion of study patients who had a positive result increased from 33.3% to 47.2% over the same period.
Treatment patterns
Of the 2175 study cohort patients, 102 initiated first-line therapy in 2016, 376 in 2017, 586 in 2018, 717 in 2019, and 394 in 2020. First-line treatment patterns are summarized in Fig. 3 .
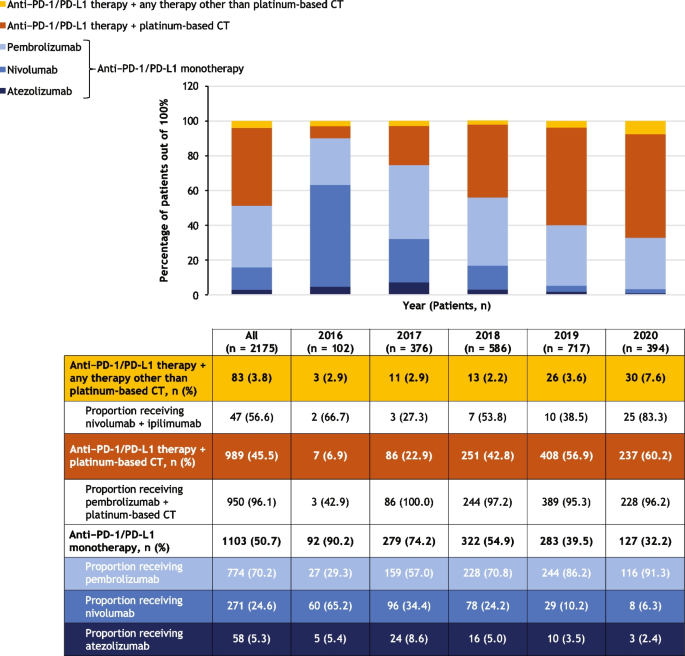
Treatment patterns overall and stratified by year of first-line therapy initiation. The graph shows the numbers of patients who received the treatments indicated in the legend. The table shows the numbers and percentages of patients who received each treatment regimen, along with proportions of patients receiving different types of anti–PD-1/PD-L1 monotherapy, pembrolizumab plus platinum-based chemotherapy, and nivolumab plus ipilimumab, which are shown below the relevant regimen category. During 2020, the COVID-19 pandemic may have impacted routine clinical care. Percentages reported for subcategories are proportions in the respective category, not the whole. CT, chemotherapy; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1
The most common first-line treatment overall was anti–PD-1/PD-L1 monotherapy (in 50.7% of 2175 patients), followed by anti–PD-1/PD-L1 therapy in combination with a platinum-based chemotherapy (45.5%) and anti–PD-1/PD-L1 therapy in combination with any other therapy (3.8%) (Fig. 3 ). The proportion of patients initiating first-line treatment with anti–PD-1/PD-L1 monotherapy decreased from 90.2% of the 102 patients in 2016 to 32.2% of the 394 patients in 2020, while the proportion of patients initiating first-line anti–PD-1/PD-L1 combined with platinum-based chemotherapy increased from 6.9% to 60.2%. PD-1/PD-L1 expression levels influence treatment selection, and PD-1/PD-L1 expression data was limited at the time of the data cutoff.
Most patients who initiated treatment with an anti–PD-1/PD-L1 monotherapy in this study cohort received pembrolizumab (70.2% of the 1103 patients who received monotherapy), followed by nivolumab (24.6%), then atezolizumab (5.3%) (Fig. 3 ). Use of pembrolizumab increased from 29.3% of the 92 patients treated with anti–PD-1/PD-L1 monotherapy in 2016 to 91.3% of the 127 monotherapy-treated patients in 2020, whereas the use of nivolumab declined from 65.2% in 2016 to 6.3% in 2020. Pembrolizumab was consistently the most commonly used anti–PD-1/PD-L1 mono-therapy across patient subgroups including age group, sex, number of metastatic sites, evidence of brain metastasis, ECOG PS, smoking status, and immunocompromised status (Table 3 ). It was the most common anti–PD-1/PD-L1 monotherapy, having been administered to 73.2% of 691 patients with nonsquamous and 61.4% of 267 patients with squamous NSCLC histology.
Pembrolizumab combined with platinum-based chemo-therapy was the most common combination regimen, accounting for 96.1% of the 989 study cohort patients who initiated first-line treatment with anti–PD-1/PD-L1 therapy combined with platinum-based chemotherapy (Fig. 3 ). Of the 83 patients (3.8% of the cohort) who initiated first-line treatment with anti–PD-1/PD-L1 therapy in combination with any other therapy, 56.6% received nivolumab plus ipilimumab.
From 2018 to 2020, the most common first-line treatment among patients younger than 65 years was anti–PD-1/PD-L1 in combination with platinum-based chemotherapy (61.1%); and in those 75 years or older, the most common regimen was anti–PD-1/PD-L1 monotherapy (56.7%) (data not shown). During the same period, 55.3% of patients with nonsquamous NSCLC were treated with anti–PD-1/PD-L1 in combination with platinum-based chemotherapy, and 40.8% received anti–PD-1/PD-L1 monotherapy (data not shown). The proportions of patients with 1, 2, or ≥3 metastatic sites treated with anti–PD-1/PD-L1 in combination with platinum-based chemotherapy were 50.9%, 54.6%, and 64.7%, respectively (data not shown). From 2018 to 2020, anti–PD-1/PD-L1 in combination with platinum-based chemotherapy was also the most commonly used regimen in patients with ECOG PS 0 or 1 (55.5%), current or former smokers (53.6%), and those who were not immunocompromised (53.9%) (data not shown).
This retrospective, real-world cohort study using the ConcertAI Patient360 NSCLC database demonstrated that, from 2016 to 2020, the most common first-line treatment among US patients with locally advanced or metastatic NSCLC who received IO treatment was anti–PD-1/PD-L1 monotherapy, followed by anti–PD-1/PD-L1 agents combined with platinum-based chemotherapy. Nivolumab was the most common monotherapy in 2016, but after the negative CheckMate-026 trial, was overtaken by pembrolizumab in 2017, which remained the most frequently used monotherapy agent until the end of the study period in 2020, corresponding with the positive KEYNOTE-024 trial results [ 15 , 20 ]. A shift in the most commonly used treatments occurred during the study period, from predominantly anti–PD-1/PD-L1 monotherapy in 2016 to combination treatment with anti–PD-1/PD-L1 agents and platinum-based chemotherapy. This was likely driven by US regulatory approval of IO-chemotherapy combination regimens and positive clinical trial results after earlier approvals of IO monotherapies [ 21 , 22 , 23 , 24 , 25 ].
Several patient characteristics in this real-world cohort differed from those in pivotal IO clinical trials [ 5 , 9 , 11 , 12 ]. The median age of 68.0 years was numerically higher than the median of approximately 60–65 years in several clinical trials [ 5 , 9 , 11 , 12 , 26 ], and 26.2% of the patients in this study were 75 years or older. This finding is consistent with another real-world study [ 26 ], which demonstrated that patients receiving IO in the clinic are substantially older than patients studied in the trials that led to these agents’ approvals, as NSCLC trials often recruit fewer elderly patients [ 26 ]. Clinical trials also typically exclude patients with ECOG PS >1, but almost 20% of the patients in this study had ECOG PS ≥2. In our study, ECOG PS deteriorated with age, suggesting that including greater proportions of elderly patients may make clinical trials substantially more generalizable to the real-world setting. Compared with real‑world studies of patients receiving first-line treatment for metastatic NSCLC [ 18 , 27 ], our study included a higher proportion of patients with an ECOG score ≥2 and a higher proportion of patients with brain metastases.
Patients with a history of autoimmune disease and those who are immunocompromised or receiving immuno-suppressive medications are also generally excluded from clinical trials of IO, but these types of patients comprised 1.5%, 11.0%, and 17.7%, respectively, of this real-world cohort and received first-line IO therapy for NSCLC. The fact that 78.9% of this study cohort had visceral metastases and 37.6% had nonvisceral metastases suggests that a substantial proportion of this cohort had dual visceral and nonvisceral metastases.
This study has several strengths and limitations. The database provided valuable real-world data on diagnosis, clinical assessment, and recorded treatments in patient groups not typically enrolled in clinical trials, such as older patients and those with higher ECOG PS, immuno-suppression, and various types and numbers of metastatic sites.
This was a descriptive observational study (no hypothesis testing) and has several limitations. The study may have been subject to confounding if physicians preferentially prescribed certain therapies to patients who were perceived to have worse adverse effects if their underlying disease was more severe or if they had poorer overall health. The study was not designed to evaluate patient outcomes; therefore, no conclusions on prognosis may be drawn. As with all retrospective epidemiological studies, unmeasured confounding and missing data may have an impact on the descriptive estimates presented.
Data entry errors at the points of care could not be detected nor corrected during analysis. Missing data in the form of information not routinely and repeatedly captured may also have impacted the completeness, validity, and reliability of some variables (eg, PD-L1 testing). Potentially interesting data that were unavailable for analysis included the rate of transition to second-line treatment when stratified by first-line therapy and information on local radiotherapy. This study included patients who were treated during the COVID-19 pandemic, which may have impacted routine clinical care in the year 2020. Finally, patients treated at individual sites included in this study may not be representative of all patients with NSCLC across all the sites of care in the US. This study highlights the differences in patient characteristics between real-world populations and clinical trial populations, presenting difficulties in treating patients underrepresented in clinical trial populations. Incorporating more diverse, traditionally excluded patient populations will increase the generalizability of studies and provide the evidence-base required to support decision-making in routine clinical practice.
Conclusions
The initial adoption of anti–PD-1/PD-L1 monotherapy as first-line treatment for advanced NSCLC in the US quickly shifted to combination anti–PD-1/PD-L1 therapy with platinum-based chemotherapy between 2016 and 2020. This real-world study was conducted during these important inflection points for the treatment of advanced NSCLC with anti–PD-1/PD-L1 therapy. This study has emphasized the real-world patient characteristics, how they differ from clinical trial populations, and how these characteristics impact treatment patterns.
In conclusion, we have shown that evidence gaps exist for patients who are older, have ECOG PS ≥2, and are on immunosuppressive medications—patients who make up a substantial proportion of real-world patient populations. To optimize the personalized treatment of advanced NSCLC, further real-world studies will be needed to elucidate the clinical characteristics of patients with advanced NSCLC who are most likely to benefit from an evolving first-line IO treatment landscape.
Availability of data and materials
The data that support the findings of this study are available from ConcertAI but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. The corresponding author may be contacted regarding potential access to the data upon reasonable request and with permission of ConcertAI.
Abbreviations
Anaplastic lymphoma kinase
Proto-oncogene B-Raf
Chemotherapy
Cytotoxic T lymphocyte-associated antigen 4
Eastern Cooperative Oncology Group performance status
Epidermal growth factor receptor
Electronic medical record
Food and Drug Administration
Human immunodeficiency virus
Immunotherapy
Mesenchymal epithelial transition factor
Not otherwise specified
- Non-small cell lung cancer
Programmed cell death protein 1
Programmed cell death ligand 1
RET proto-oncogene
ROS proto-oncogene 1
United States
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660 .
Article CAS PubMed Google Scholar
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94. https://doi.org/10.4065/83.5.584 .
Article PubMed Google Scholar
Jones CM, Brunelli A, Callister ME, Franks KN. Multimodality treatment of advanced non-small cell lung cancer: where are we with the evidence? Curr Surg Rep. 2018;6(2):5. https://doi.org/10.1007/s40137-018-0202-0 .
Article PubMed PubMed Central Google Scholar
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–50. https://doi.org/10.1056/NEJMoa1809697 .
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301. https://doi.org/10.1056/NEJMoa1716948 .
Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397(10274):592–604. https://doi.org/10.1016/S0140-6736(21)00228-2 .
Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab versus docetaxel in previously treated patients with advanced non–small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924–33. https://doi.org/10.1200/jco.2017.74.3062 .
Article CAS PubMed PubMed Central Google Scholar
Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic non-squamous non–small-cell lung cancer. J Clin Oncol. 2020;38(14):1505–17. https://doi.org/10.1200/jco.19.03136 .
Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020–31. https://doi.org/10.1056/NEJMoa1910231 .
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. https://doi.org/10.1016/s0140-6736(18)32409-7 .
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%. J Clin Oncol. 2021;39(21):2339–49. https://doi.org/10.1200/jco.21.00174 .
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. https://doi.org/10.1016/s0140-6736(16)32517-x .
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–46. https://doi.org/10.1016/s0140-6736(16)00587-0 .
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. https://doi.org/10.1056/NEJMoa1507643 .
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33. https://doi.org/10.1056/NEJMoa1606774 .
Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–82. https://doi.org/10.1200/JCO.2014.59.4358 .
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-small cell lung cancer. v1.2022 - December 7, 2021. 2021. https://www.nccn.org/patientresources/patient-resources/guidelines-for-patients . Accessed 25 Jan 2022.
Velcheti V, Hu X, Piperdi B, Burke T. Real-world outcomes of first-line pembrolizumab plus pemetrexed-carboplatin for metastatic nonsquamous NSCLC at US oncology practices. Sci Rep. 2021;11(1):9222. https://doi.org/10.1038/s41598-021-88453-8 .
FDA approval timeline of active immunotherapies. https://www.cancerresearch.org/regulatory-approval-timeline-of-active-immunotherapies . Accessed 20 Apr 2023.
Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–26. https://doi.org/10.1056/NEJMoa1613493 .
FDA approves Genentech’s Tecentriq in combination with Avastin and chemotherapy for the initial treatment of metastatic non-squamous non-small cell lung cancer. 2018. https://www.drugs.com/newdrugs/fda-approves-genentech-s-tecentriq-combination-avastin-chemotherapy-initial-metastatic-non-squamous-4883.html . Accessed 28 Mar 2022.
FDA approves Opdivo (nivolumab) + Yervoy (ipilimumab) as first-line treatment of patients with metastatic non-small cell lung cancer whose tumors express PD-L1≥1%. 2020. https://www.drugs.com/newdrugs/fda-approves-opdivo-nivolumab-yervoy-ipilimumab-first-line-patients-metastatic-non-small-cell-lung-5239.html . Accessed 28 Mar 2022.
FDA approves Genentech’s Tecentriq plus chemotherapy (Abraxane and carboplatin) for the initial treatment of metastatic non-squamous non-small cell lung cancer. 2019. https://www.drugs.com/newdrugs/fda-approves-genentech-s-tecentriq-plus-chemotherapy-abraxane-carboplatin-initial-metastatic-non-5117.html . Accessed 28 Mar 2022.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. https://doi.org/10.1056/NEJMoa1801005 .
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–51. https://doi.org/10.1056/NEJMoa1810865 .
O’Connor JM, Fessele KL, Steiner J, Seidl-Rathkopf K, Carson KR, Nussbaum NC, et al. Speed of adoption of immune checkpoint inhibitors of programmed cell death 1 protein and comparison of patient ages in clinical practice vs pivotal clinical trials. JAMA Oncol. 2018;4(8):e180798. https://doi.org/10.1001/jamaoncol.2018.0798 .
Simeone JC, Nordstrom BL, Patel K, Klein AB. Treatment patterns and overall survival in metastatic non-small-cell lung cancer in a real-world, US setting. Future Oncol. 2019;15(30):3491–502. https://doi.org/10.2217/fon-2019-0348 .
Download references
Acknowledgements
Samantha Santangelo and Micaela Genca provided medical writing support on behalf of inScience Communications (Philadelphia, PA). This work was performed in accordance with current Good Publication Practice guidelines, and was funded by Sanofi, Inc.
This work was supported by Sanofi, which was involved in the study design; data analysis and interpretation; manuscript writing; and the decision to submit to BMC Cancer .
Author information
Authors and affiliations.
Sanofi, Inc., 450 Water Street, Cambridge, MA, 02142, USA
Hozefa A. Divan & Ashok Krishna
James Comprehensive Cancer Center, The Ohio State University, 460 West 10th Avenue, Columbus, OH, 43210, USA
Marisa A. Bittoni & David P. Carbone
You can also search for this author in PubMed Google Scholar
Contributions
H.A.D. provided conceptualization, methodology, data curation, formal analysis, and writing of the manuscript. M.A.B. provided data curation, formal analysis, and writing of the manuscript. A.K. provided conceptualization, methodology, data curation, writing, and supervision of the manuscript. D.P.C. provided conceptualization, methodology, writing, and supervision of the manuscript.
Corresponding author
Correspondence to Ashok Krishna .
Ethics declarations
Ethics approval and consent to participate.
The study was performed in accordance with the Declaration of Helsinki and relevant International Council for Harmonisation, Good Clinical Practice, and Good Pharmacoepidemiological Practice guidelines. As no identifiable protected health information was extracted or accessed for the conduct of this study, ethics approval and informed consent were deemed unnecessary under the Code of Federal Regulations Title 45, Part 46, Section 46.104(d)(4)(ii) (45CFR46.104[d][4][ii]).
Consent for publication
Not applicable.
Competing interests
H.A.D. was an employee of Sanofi at the time of the study and holds stock in the company. M.A.B. received consulting fees from Sanofi. A.K. is an employee of Sanofi and holds stock in the company. D.P.C. has received funding from clinical trial grants from Genentech and Merck Sharp & Dohme to The Ohio State University; received consulting fees from Bristol Myers Squibb (BMS), BMS KK, Boehringer Ingelheim, Curio Science, Genentech/Roche, GI Therapeutics (Intellisphere), GlaxoSmithKline (GSK), Janssen, Mirati, Novartis, Novacure, OncoCyte, OncoHost, Roche China, and Seattle Genetics; received honoraria from AstraZeneca and BMS; participated in Data Safety Monitoring Boards for European Organisation for Research and Treatment of Cancer (EORTC), AbbVie, and Lilly; and participated in advisory boards for Amgen, Arcus Biosciences, AstraZeneca, Cantargia, Daiichi Sankyo, EMD Serono/Merck, Flame Biosciences, Gritstone Oncology, GSK, Lilly, Regeneron, Sanofi, and Seattle Genetics.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Divan, H.A., Bittoni, M.A., Krishna, A. et al. Real-world patient characteristics and treatment patterns in US patients with advanced non-small cell lung cancer. BMC Cancer 24 , 424 (2024). https://doi.org/10.1186/s12885-024-12126-8
Download citation
Received : 17 November 2023
Accepted : 14 March 2024
Published : 06 April 2024
DOI : https://doi.org/10.1186/s12885-024-12126-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- PD-1 inhibitors
- PD-L1 inhibitors
- Real-world evidence
- Observational study
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 10 April 2024
How to supercharge cancer-fighting cells: give them stem-cell skills
- Sara Reardon 0
Sara Reardon is a freelance journalist based in Bozeman, Montana.
You can also search for this author in PubMed Google Scholar
A CAR T cell (orange; artificially coloured) attacks a cancer cell (green). Credit: Eye Of Science/Science Photo Library
Bioengineered immune cells have been shown to attack and even cure cancer , but they tend to get exhausted if the fight goes on for a long time. Now, two separate research teams have found a way to rejuvenate these cells: make them more like stem cells .
Both teams found that the bespoke immune cells called CAR T cells gain new vigour if engineered to have high levels of a particular protein. These boosted CAR T cells have gene activity similar to that of stem cells and a renewed ability to fend off cancer . Both papers were published today in Nature 1 , 2 .
The papers “open a new avenue for engineering therapeutic T cells for cancer patients”, says Tuoqi Wu, an immunologist at the University of Texas Southwestern in Dallas who was not involved in the research.
Reviving exhausted cells
CAR T cells are made from the immune cells called T cells, which are isolated from the blood of person who is going to receive treatment for cancer or another disease. The cells are genetically modified to recognize and attack specific proteins — called chimeric antigen receptors (CARs) — on the surface of disease-causing cells and reinfused into the person being treated.
But keeping the cells active for long enough to eliminate cancer has proved challenging, especially in solid tumours such as those of the breast and lung. (CAR T cells have been more effective in treating leukaemia and other blood cancers.) So scientists are searching for better ways to help CAR T cells to multiply more quickly and last longer in the body.
Cutting-edge CAR-T cancer therapy is now made in India — at one-tenth the cost
With this goal in mind, a team led by immunologist Crystal Mackall at Stanford University in California and cell and gene therapy researcher Evan Weber at the University of Pennsylvania in Philadelphia compared samples of CAR T cells used to treat people with leukaemia 1 . In some of the recipients, the cancer had responded well to treatment; in others, it had not.
The researchers analysed the role of cellular proteins that regulate gene activity and serve as master switches in the T cells. They found a set of 41 genes that were more active in the CAR T cells associated with a good response to treatment than in cells associated with a poor response. All 41 genes seemed to be regulated by a master-switch protein called FOXO1.
The researchers then altered CAR T cells to make them produce more FOXO1 than usual. Gene activity in these cells began to look like that of T memory stem cells, which recognize cancer and respond to it quickly.
The researchers then injected the engineered cells into mice with various types of cancer. Extra FOXO1 made the CAR T cells better at reducing both solid tumours and blood cancers. The stem-cell-like cells shrank a mouse’s tumour more completely and lasted longer in the body than did standard CAR T cells.
Master-switch molecule
A separate team led by immunologists Phillip Darcy, Junyun Lai and Paul Beavis at Peter MacCallum Cancer Centre in Melbourne, Australia, reached the same conclusion with different methods 2 . Their team was examining the effect of IL-15, an immune-signalling molecule that is administered alongside CAR T cells in some clinical trials. IL-15 helps to switch T cells to a stem-like state, but the cells can get stuck there instead of maturing to fight cancer.
The team analysed gene activity in CAR T cells and found that IL-15 turned on genes associated with FOXO1. The researchers engineered CAR T cells to produce extra-high levels of FOXO1 and showed that they became more stem-like, but also reached maturity and fought cancer without becoming exhausted. “It’s the ideal situation,” Darcy says.
Stem-cell and genetic therapies make a healthy marriage
The team also found that extra-high levels of FOXO1 improved the CAR T cells’ metabolism, allowing them to last much longer when infused into mice. “We were surprised by the magnitude of the effect,” says Beavis.
Mackall says she was excited to see that FOXO1 worked the same way in mice and humans. “It means this is pretty fundamental,” she says.
Engineering CAR T cells that overexpress FOXO1 might be fairly simple to test in people with cancer, although Mackall says researchers will need to determine which people and types of cancer are most likely to respond well to rejuvenated cells. Darcy says that his team is already speaking to clinical researchers about testing FOXO1 in CAR T cells — trials that could start within two years.
And Weber points to an ongoing clinical trial in which people with leukaemia are receiving CAR T cells genetically engineered to produce unusually high levels of another master-switch protein called c-Jun, which also helps T cells avoid exhaustion. The trial’s results have not been released yet, but Mackall says she suspects the same system could be applied to FOXO1 and that overexpressing both proteins might make the cells even more powerful.
doi: https://doi.org/10.1038/d41586-024-01043-2
Doan, A. et al. Nature https://doi.org/10.1038/s41586-024-07300-8 (2024).
Article Google Scholar
Chan, J. D. et al. Nature https://doi.org/10.1038/s41586-024-07242-1 (2024).
Download references
Reprints and permissions
Related Articles
- Medical research
ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D
Article 10 APR 24
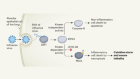
Blocking cell death limits lung damage and inflammation from influenza
News & Views 10 APR 24

FOXO1 enhances CAR T cell stemness, metabolic fitness and efficacy
Biological age surges in survivors of childhood cancer
Research Highlight 11 APR 24
FOXO1 is a master regulator of memory programming in CAR T cells
The PARTNER trial of neoadjuvant olaparib in triple-negative breast cancer
Article 08 APR 24
mRNA drug offers hope for treating a devastating childhood disease
News 03 APR 24
Diabetes drug slows development of Parkinson’s disease
Junior Group Leader Position at IMBA - Institute of Molecular Biotechnology
The Institute of Molecular Biotechnology (IMBA) is one of Europe’s leading institutes for basic research in the life sciences. IMBA is located on t...
Austria (AT)
IMBA - Institute of Molecular Biotechnology
Open Rank Faculty, Center for Public Health Genomics
Center for Public Health Genomics & UVA Comprehensive Cancer Center seek 2 tenure-track faculty members in Cancer Precision Medicine/Precision Health.
Charlottesville, Virginia
Center for Public Health Genomics at the University of Virginia
Husbandry Technician I
Memphis, Tennessee
St. Jude Children's Research Hospital (St. Jude)
Lead Researcher – Department of Bone Marrow Transplantation & Cellular Therapy
Researcher in the center for in vivo imaging and therapy.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

UC experts present at national neurology conference

University of Cincinnati researchers will present abstracts at the American Academy of Neurology annual meeting 2024, April 13-18 in Denver, Colorado.
Two-component treatment leads to improvement for patients
Hani Kushlaf, MD. Photo/University of Cincinnati.
Late-onset Pompe disease (LOPD) is a rare, inherited genetic disease caused by the accumulation of glycogen, the body’s stored form of glucose, in muscles and other organs. Left untreated, the muscle weakness it causes can lead to the loss of the ability to walk and breathing impairment.
A research team led by UC’s Hani Kushlaf, MD, looked at the effect of a two-component enzyme replacement therapy (ERT) of drugs cipaglucosidase and miglustat (cipa+mig) compared to a single ERT drug, alglucosidase alfa (alg) and a placebo. UC researchers participated in the Phase 3 trials that led to the Food and Drug Administration approval of both ERT regimens.
“The research question was to look at the magnitude and practical significance of the effect of cip+mig versus alg using patient data from the PROPEL trial on outcomes that included motor function, pulmonary function, muscle strength, biomarkers and patient- and physician-reported quality of life,” said Kushlaf, associate professor and director of Neuromuscular Research and the Neuromuscular Disorders Division in UC’s Department of Neurology & Rehabilitation Medicine in the College of Medicine.
Patients who switched to the dual ERT regimen experienced improvement or stability across the measured outcomes with no worsening of outcomes. Those who remained on the single ERT drug plus placebo experienced worsening or stability across the measured outcomes.
“This analysis highlights the potential of cipaglucosidase+miglustat to become an important treatment option for patients with LOPD, including patients already on enzyme replacement therapy,” Kushlaf said.
This research was sponsored by Amicus Therapeutics, Inc.
Research team learns more about events following immunotherapy treatment
Luca Marsili, MD, PhD. Photo/University of Cincinnati.
Immune checkpoint inhibitors (ICIs), an immunotherapy that activates the body’s immune system to fight cancer cells, has revolutionized cancer treatment. But while boosting anti-tumor immunity, the treatments may cause severe neurological-immune related adverse events.
“These neurological-immune-related adverse events include meningitis, encephalitis, demyelinating diseases, vasculitis, neuropathy, neuromuscular junction disorders and myopathy,” said Luca Marsili, MD, PhD, movement disorder fellow in the Department of Neurology and Rehabilitation Medicine in the University of Cincinnati College of Medicine.
Marsili said the frequency of these adverse events, and the best way to manage them, is still largely unknown.
A team led by Marsili and Alberto Espay, MD, reviewed reported neurological-immune-related adverse events in patients treated with immune checkpoint inhibitors at UC from 2011-2023. They found the adverse events are rare, affecting 28 patients out of 1,677 treated, or 1.66%.
The adverse events were most often associated with melanoma treatment with pembrolizumab, a common immunotherapy treatment.
“The adverse events were most expressed as peripheral neuropathies and encephalitis, manifesting early during treatment within a mean of 2.3 months after ICI initiation,” Marsili said. “Most ICIs, 68%, were discontinued, and in only 10.7% of cases they were restarted without complications.”
Moving forward, the team said further research is needed to determine clinical susceptibility factors and appropriate timing of restarting ICI treatment after discontinuing due to an adverse event. They are also planning to do more detailed demographic and clinical comparisons of the 28 patients identified to have adverse events to see if there are any predictive factors like tumor type, age, sex or ethnicity.
“This study is part of a broader project in collaboration with the University of Udine in Italy and with the Department of Internal Medicine at UC,” Marsili said. “We would like to gather a high number of participants to assess incidence/prevalence of these adverse events and also to raise awareness among neurologists on how to treat/manage them.”
Safe, effective treatment for Parkinson’s
Alberto Espay, MD. Photo/University of Cincinnati.
Alberto Espay, MD, will present findings recently published in the Lancet Neurology journal that found Parkinson’s disease medication delivered through an infusion pump is safe and effective at reducing symptoms for longer periods of time.
Parkinson’s symptoms such as tremors, slowness and stiffness are caused by low levels of dopamine in the body. For decades, doctors have treated Parkinson’s by giving patients levodopa, the inactive substance in the brain that once converted makes dopamine.
“Levodopa is a replacement strategy. We all make levodopa, but Parkinson's patients make less of it,” said Espay, co-principal investigator of the trial, the James J. and Joan A. Gardner Family Center for Parkinson’s Disease Research Endowed Chair in UC’s Department of Neurology and Rehabilitation Medicine and a physician at the UC Gardner Neuroscience Institute.
Levodopa is most commonly administered orally, but this trial tested continuous, 24-hour levodopa delivery through a subcutaneous infusion pump. A total of 381 patients with Parkinson’s disease in 16 countries enrolled in the trial and were randomized to receive levodopa through the infusion pump or through traditional oral medication.
The researchers found levodopa delivered through the infusion pump was safe and led to almost two hours a day (1.72) of additional “on time,” or the time when the medication is working and symptoms are lessened, compared to taking levodopa orally.
“Once approved, this will become an important treatment strategy to consider for patients with Parkinson’s disease experiencing motor fluctuations not adequately controlled with medication,” he said. “Future studies will need to determine the durability of the long-term benefits and whether any safety issues could emerge, as well as how it might compare with deep brain stimulation.”
Impact Lives Here
The University of Cincinnati is leading public urban universities into a new era of innovation and impact. Our faculty, staff and students are saving lives, changing outcomes and bending the future in our city's direction. Next Lives Here.
UC research being presented at AAN includes:
- Kushlaf presenting “ Effect Size Analysis of Cipaglucosidase Alfa Plus Miglustat Versus Alglucosidase Alfa in ERT-experienced Adults with Late-onset Pompe Disease in PROPEL .”
- Marsili and Espay presenting “ Neurological Immune-related Adverse Events of Immune Checkpoint Inhibitors: A Single-center Retrospective Study. ”
- Espay presenting “ Efficacy of ND0612, a 24-hour Subcutaneous Levodopa/Carbidopa Infusion for People with Parkinson’s Disease Experiencing Motor Fluctuations: Subgroup-analyses from a Randomized, Controlled Phase 3 Study .”
- Stacie Demel, DO, PhD, a physician-researcher at the UC Gardner Neuroscience Institute and associate professor of clinical neurology and rehabilitation medicine in UC’s College of Medicine, presenting “ Methylation Patterns Differ Between ICH Cases and Controls .”
- Yang Yu, MD, UC medical resident/fellow, presenting “ Multiple Sclerosis in a Patient with Friedreich's Ataxia .”
- Rhonna Shatz, DO, adjunct associate professor, division director for behavioral neurology, and the Bob and Sandy Heimann Endowed Chair in Research and Education in Alzheimer’s Disease in the UC College of Medicine, presenting “ Identifying a Relationship Between Executive Dysfunction, Poor Sleep Hygiene/Sleep Apnea, and Ventriculomegaly in Cancer-related Cognitive Impairment (CRCI) ”
Featured illustration at top of brain. Photo/iStock/Onurdongel.
- Clinical Research
- Faculty Staff
- College of Medicine
- UC Gardner Neuroscience Institute
- Academic Health Center
- Neurology & Rehabilitation Medicine
Related Stories
Learning more about how cancer affects stroke risk.
October 16, 2023
A collaborative team led by University of Cincinnati, University of North Carolina and Duke University researchers is studying how specific cancers and treatments affect patients' risk of stroke.
UC trial tests tongue exercises to improve swallowing function after stroke
January 9, 2024
A new trial at the University of Cincinnati Gardner Neuroscience Institute, funded by a $660,000 National Institutes of Health (NIH) grant, will test an at-home tongue endurance exercise to improve patients’ swallowing function after a stroke.
Collaborative University of Cincinnati Cancer Center team opens Phase 2 brain tumor trial
March 26, 2024
A multidisciplinary team of University of Cincinnati Cancer Center researchers have opened a Phase 2 clinical trial to test a new combination treatment for glioblastomas, the most deadly form of brain tumors.

IMAGES
COMMENTS
The American Cancer Society (ACS) has helped make possible almost every major cancer breakthrough since 1946. Since then, we've invested more than $5 billion in cancer research, making us the largest nonprofit funder of cancer research in the United States, outside of the federal government. We remain committed to finding more - and better ...
Find research articles on cancer treatment, including news stories, clinical trials, blog posts, and descriptions of active studies. ... A comprehensive analysis of patients with cancer who had exceptional responses to therapy has revealed molecular changes in the patients' tumors that may explain some of the exceptional responses.
NCI joins the cancer community in advancing the goals of the National Cancer Plan as part of its research programs. The ComboMATCH trials are testing targeted-therapy drug combinations for cancer. NCI is the nation's leader in cancer research. Learn about key research areas, initiatives, progress, and research tools, including specimens and data.
2021 Special Section: COVID-19 and Cancer. This year's special section focuses on the impact of the COVID-19 pandemic on people living with cancer, cancer surveillance capabilities, and the overall American health system. This section is intended to inform any person (which may include policy makers, researchers, clinicians, cancer control advocates, patients, and/or caregivers) with interest ...
Your donation to the American Association for Cancer Research helps our more than 58,000 members worldwide drive progress against cancer. The AACR is the first and largest cancer research organization. Our mission is to prevent and cure cancer through research, education, communication, collaboration, funding, and advocacy. Our 58,000 members ...
Population research (also known as epidemiological research) is the study of causes and patterns of occurrence of cancer and evaluation of risk. Population scientists, also known as epidemiologists, study the patterns, causes, and effects of health and diseases in defined groups. Population research is highly collaborative and can span the ...
Thanks to NCI-funded research, patients with cancer have a greater number of therapeutic options than ever before, many of which are more effective and less toxic than earlier options. But more research is needed to ensure the most effective treatment possible, including better ways to use existing therapies in combination, while maintaining ...
Cancer therapies have evolved considerably in recent decades, substantially improving the quality of life and survival of patients with cancer. In this issue, we launch our Series on Cancer ...
Abstract. Cancer research has played a pivotal role in improving patient outcomes. However, despite the significant investment in fundamental cancer research over the past few decades, the ...
The Facts & Figures annual report provides: Estimated numbers of new cancer cases and deaths in 2022 (In 2022, there will be an estimated 1.9 million new cancer cases diagnosed and 609,360 cancer deaths in the United States.) Current cancer incidence, mortality, and survival statistics. Information on cancer symptoms, risk factors, early ...
Among patients with cancers bearing the KRAS G12C mutation who received divarasib at a 400-mg dose, 56% with lung cancer, 36% with colorectal cancer, and 36% with other tumor types had a confirmed ...
Another is the broader inclusion of diverse patients and samples in cancer research, especially of patients recruited into clinical trials. "We need to understand the broader impact of new therapies for all people, preferably prior to approvals, to ensure that we have the most accurate picture relative to effectiveness and toxicity profiles ...
Clinical Research. Clinical research involves the study of cancer in people. These cancer research studies are further broken down into two types: clinical trials and observational studies. Clinical trials are research studies that involve an intervention, which is a treatment or change that may affect the results of cancer.These can lead to new treatments, care, and improved results for ...
Cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, or nearly one in six deaths. The most common cancers are breast, lung, colon and rectum and prostate cancers. Around one-third of deaths from cancer are due to tobacco use, high body mass index, alcohol consumption, low fruit and vegetable intake, and ...
The Biology of Cancer. Cancer is a disease that begins with genetic and epigenetic alterations occurring in specific cells, some of which can spread and migrate to other tissues. 4 Although the biological processes affected in carcinogenesis and the evolution of neoplasms are many and widely different, we will focus on 4 aspects that are particularly relevant in tumor biology: genomic and ...
Cancer, and the complex nature of treatment, has a profound impact on lives of patients and their families. Subsequently, cancer patients have a wide range of needs. This study aims to identify and synthesise cancer patients' views about areas where they need support throughout their care. A systematic search of the literature from PsycInfo, Embase and Medline databases was conducted, and a ...
Patients' quality of life has become a major objective of care in oncology. At the same time, it has become the object of increasing interest by researchers, working with both quantitative and qualitative methods. Progress in oncology has enabled more patients to survive longer, so that cancer is increasingly often a chronic disease that ...
Dedicated to helping people who face cancer. Learn about cancer research, patient services, early detection, treatment and education at cancer.org. ... We've invested more than $5 billion in cancer research since 1946, all to find more - and better - treatments, uncover factors that may cause cancer, ...
There was little variation by gender in whether patients reported a conversation about taking part in research. There was a clear decline in being asked to participate in cancer research for patients aged over 75. Skin and urological cancer patients were less likely to be asked to participate in cancer research. Mc Grath-Lone et al., 2015
Abstract. The novel coronavirus, also known as SARS-Cov-2 or COVID-19 has become a worldwide threat and the major healthcare concern of the year 2020. Cancer research was directly affected by the emerging of this disease. According to some Chinese studies, cancer patients are more vulnerable to COVID-19 complications.
This was a cross-sectional study conducted at the King Hussein Cancer Center (KHCC) in Amman, Jordan, between October 2019 and February 2020. According to the last available statistics in 2015, KHCC provides medical care to around 60.0% of all cancer patients in Jordan ( Abdel-Razeq et al., 2015 ).
RESULTS Distribution of cases and deaths by world region and cancer types. Figure 2 presents the distribution of new cases and deaths according to world region for both sexes combined and for men and women separately. For both sexes combined, there were an estimated 20.0 million new cases worldwide (19.96 million including NMSC and 18.73 million excluding NMSC) and 9.7 million cancer deaths (9 ...
Patients from non-small cell lung cancer (NSCLC) controlled clinical trials do not always reflect real-world heterogeneous patient populations. We designed a study to describe the real-world patient characteristics and treatment patterns of first-line treatment in patients in the US with NSCLC. This was an observational, retrospective cohort study based on electronic medical records of US ...
Patient advocacy in cancer research. Many articles have been written about patient advocacy in cancer research. One of the earliest in 1998 addressing the different types of advocacy as well as several areas of research advocacy [Citation 17].Interestingly, the difference in perception between patients and researchers was highlighted, especially noting that research advocacy was a relatively ...
Cancer Facts & Figures 2023 is an educational companion for Cancer Statistics 2023, a scientific paper published in the American Cancer Society journal, CA: A Cancer Journal for Clinicians. These annual reports provide: Estimated numbers of new cancer cases and deaths in 2023 by cancer site and US state. Current cancer incidence, mortality, and ...
Now, two separate research teams have found a way to rejuvenate these cells: ... The papers "open a new avenue for engineering therapeutic T cells for cancer patients", says Tuoqi Wu, an ...
Almost everyone knows someone with cancer, and they might also know how challenging the diagnosis can be for their loved one's mental health. But...
Opioids are types of medicine used to relieve moderate to severe pain. They are also called opiates or narcotics and are a type of analgesic (painkilling) medicine. Opioids work in the brain and other parts of the body by attaching to pain receptors to block the feeling of pain. Some people with cancer need opioids for cancer-related pain.
Yet research has shown that when physicians show empathy, that can generally lead to better clinical outcomes, at least over the near-term. Now, a new study, published Thursday in JAMA Network ...
"The research question was to look at the magnitude and practical significance of the effect of cip+mig versus alg using patient data from the PROPEL trial on outcomes that included motor function, pulmonary function, muscle strength, biomarkers and patient- and physician-reported quality of life," said Kushlaf, associate professor and ...