An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Huntington disease: a single-gene degenerative disorder of the striatum
Enfermedad de huntington: un trastorno degenerativo monogénico del estriado, la maladie de huntington : une affection dégénérative monogénique du striatum, peggy c nopoulos , md.
- Author information
- Article notes
- Copyright and License information
E-mail: [email protected]
Issue date 2016 Mar.
This is an open-access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by-nc-nd/3.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Huntington disease (HD) is an autosomal dominant, neurodegenerative disorder with a primary etiology of striatal pathology. The Huntingtin gene (HTT) has a unique feature of a DNA trinucleotide (triplet) repeat, with repeat length ranging from 10 to 35 in the normal population. Repeat lengths between 36 and 39 cause HD at reduced penetrance (some will get the disease, others won't) and when expanded to 40 or more repeats (mHTT), causes HD at full penetrance (every person with this length or beyond will definitely develop the disease). The symptoms of HD may be motor, cognitive, and psychiatric, and are consistent with the pathophysiology of frontostriatal circuitry malfunction. Expressed ubiquitously and throughout the entire life cycle (development through adulthood), mHTT causes initial dysfunction and eventual death of a specific cell population within the striatum. Although all areas of the brain are eventually affected, the primary pathology of the disease is regionally specific. As a single-gene disorder, HD has the distinction of having the potential of treatment that is aimed directly at the known pathogenic mechanism by gene silencing, providing hope for neuroprotection and ultimately, prevention.
Keywords: Huntington disease , neurodevelopment , striatum
La Enfermedad de Huntington (EH) es un trastorno neurodegenerativo autosómico dominante con una etiología primaria de la patología estriatal. El gen de la proteína huntingtina (HTT) tiene una característica distintiva cual es la repetición del trinucleótido de DNA (triplete), con una longitud de repetición que va de 10 a 35 veces en la población normal. Las longitudes de repetición entre 36 y 39 veces provocan una EH de pene-tración reducida (algunos adquirirán la enfermedad y otros no) y cuando se expanden a 40 o más repeticiones (mHTT), se provoca la EH de penetración completa (cada persona con esta longitud o más desarrollará definitivamente la enfermedad). Los síntomas de la EH pueden ser motores, cognitivos y psiquiátricos y son consistentes con la fisiopatología de un mal funcionamiento del circuito fronto-estriatal. El mHTT provoca una disfunción inicial y la eventual muerte de una población celular específica dentro del estriado, al expresarse de manera ubicua y durante toda la vida (con un desarrollo en la adultez). Aunque todas las áreas del cerebro están eventualmente afectadas, la patología primaria de la enfermedad ocurre en una región específica. Como un trastorno monogénico, la EH se distingue por tener una terapéutica potencial dirigida directamente al mecanismo patogénico conocido como el silenciamiento génico, dando esperanzas de neuroprotección y finalmente de prevención.
La maladie de Huntington (MH) est une affection neu-rodégénérative autosomique dominante dont l'étiologie primaire est une maladie du striatum. Le gène de la protéine Huntingtine (HTT) se présente sous la forme particulière d'une répétition d'un trinucléotide de l'ADN (triplet), la longueur de la répétition variant de 10 à 35 fois dans la population normale. Des longueurs répétées entre 36 et 39 fois provoquent une MH à pénétrance réduite (certains auront la maladie, d'autres non) et des répétitions de 40 fois et plus (HTTm) entraînent une MH à pénétrance complète (chaque personne porteuse de cette longueur et au-delà développera la maladie de façon certaine). Les symptômes de la MH peuvent être moteurs, cognitifs et psychiatriques et concordent avec la physiopathologie d'une dysfonction du circuit striato-frontal. Exprimé de façon ubiquitaire et tout au long d'une vie entière (il se développe pendant toute la vie adulte), le gène HTTm est responsable de la dysfonction initiale et de la mort finale d'une population cellulaire spécifique dans le striatum. Toutes les zones cérébrales sont finalement touchées mais la pathologie primaire de la maladie est spécifique localement. En tant qu'affection monogénique, la MH a la particularité de pouvoir bénéficier d'un traitement dirigé directement contre le mécanisme pathogène connu par silençage génique, ce qui est porteur d'espoir pour la neuroprotection et enfin pour la prévention.
Introduction
Huntington disease (HD) is an autosomal dominant, neurodegenerative disorder with a primary etiology of corticostriatal pathology. HD is caused by a DNA trinucleotide (triplet) repeat expansion of equal to or greater than 40 CAG repeats within the gene Huntingtin (HTT, OMIM 613004). Repeat numbers vary from 6 to 35 in the general population. When there are less than 27 repeats, there is no manifestation of HD, and the gene is stable upon transmission. Repeat lengths in the range of 27 to 35 also are not associated with development of HD; however, there is a possibility of expansion upon transmission, giving rise to the phenomenon of genetic anticipation. Expansion upon transmission is more likely at longer repeat lengths within this range, and is also more likely to happen during male transmission. 1 CAG repeats in the range of 36 to 39 are of incomplete penetrance with variable disease manifestation.
Phenomenology
HD is a rare disease with a prevalence of approximately 10 to 12 individuals per 100 000 of European ancestry. 2 The number of repeats in HTT is inversely associated with disease onset such that the greater the number, the earlier the onset. 3 Onset of disease is defined as manifestation of significant motor or neurologic symptoms and occurs on average around the age of 40. Although the number of repeats in HTT accounts for roughly 50% to 70% of the variance in age of onset, 4 there remain other influential factors yet to be defined; these are likely to be environmental elements or modifying gene factors. Although repeat length does not account for all of the variance in age of onset, the strong relationship allows for some generalizations. ( Figure 1 ). displays the general relationship between age of onset and CAG repeat number.
Figure 1. In Huntington disease, the length of CAG repeat is inversely related to age of onset. This relationship is shown with approximations of age of onset based on repeat length. JHD, juvenile Huntington disease.

Those with later onset are more likely to have fewer repeats, sometimes in the intermediate range. Classic adult onset between the ages of 30 and 50 are associated with repeat lengths between 40 and 49. Repeat lengths larger than 50 are typically associated with onset between 20 and 30 years of age. When disease onset occurs prior to the age of 21, it is referred to as juvenile HD (JHD), which constitutes about 5% of all HD cases. 5 Within JHD, repeat lengths greater than 60 are associated with age of onset between 10 and 20, and the very highest repeat numbers — over 80 — can manifest in childhood onset, where the diagnosis is made before the age of 10. The earliest reported diagnosis was in an 18-month-old with a repeat length over 200 . 6
The course of disease in HD is relatively long compared with other neurodegenerative disorders, lasting on average 15 years from diagnosis to death. Although the CAG repeat number is highly correlated with age of onset, it does not appear to have much impact on length of disease, suggesting that once the disease process begins, other factors determine course of disease rather than the length of the mutation. Stages of HD can be categorized based on the patient's functional capacity, which is expressed as a standardized scale. 7 The Shoulson-Fahn staging system ranges from stage 1 through stage 5 with the earliest stage having full functional capacity (early in the disease) and stage 5, severely limited functional capacity. Patients in stage 5 typically need total care and are most often in a nursing home.
Sine the discovery of the gene in 1993, it is possible to test persons who are at risk for HD (due to autosomal dominance, each child of a parent with HD has a 50% chance of inheriting the mutation). In terms of clinical aspects, at the age of 18 has been persons at risk can undergo presymptomatic testing to find out if they do indeed carry the mutant gene. This is a choice that relatively few people at risk (roughly 5% to 10%) choose to make, and it requires a multi visit protocol with genetic counselors, neurologists, and often a psychiatrist or psychologist.
Clinical symptoms
The clinical symptoms of HD are classically defined as occurring in three domains: motor, cognitive, and psychiatric. The motor symptoms are progressive and—early in the disease—are mostly hyperkinetic with involuntary movements of chorea. These movements generally begin distally and are of small degree, then become more axial and are of greater amplitude. Movements are often incorporated into natural voluntary movements and, thus, early on may appear as simple restlessness. Although the early stages of motor symptoms are hyperkinetic, in later stages of the disease, motor symptoms tend to be hypokinetic with bradykinesia and dystonia. 8 Other classic symptoms are motor impersistence and abnormal eye movements. Late in the disease, dysphagia becomes a symptom with high morbid impact, as aspiration is a common occurrence, and pneumonia is a common cause of death.
Cognitive dysfunction occurs in the vast majority of patients. In early stages, this can be somewhat limited to executive function, with difficulties in decision making, organization, planning, and multitasking. Eventually, these symptoms progress, and a more global picture of cognitive deficits emerges with an ultimate diagnosis of dementia. In general, the dementia of HD is considered to be “subcortical,” highlighting the involvement of the corticostriatal pathways. Key differences between HD dementia and a classic cortical dementia is that, in memory tasks, patients with HD can recall items better if cued, suggesting that it involves an inefficient search of memory rather than a deficient memory per se. In general, memory loss occurs later in HD, and problems that are typical of cortical dementias, such as aphasia and apraxia, are not as common in HD. Importantly, the cognitive deficits in HD may precede the motor symptoms by many years. It is not uncommon in a clinical setting to have a formal clinical diagnosis of HD made for the first time based on motor abnormality though the cognitive deficits have already reached the level of dementia.
Psychiatric symptoms associated with HD can span a variety of domains; however, the most common symptoms are consistent with frontal lobe dysfunction, in line with the known pathophysiology of the disease. 9 Initially, these symptoms are in the domain of frontal disinhibition, with symptoms of poor attention, irritability, impulsivity, and poor mood regulation. Early in the disease course, family members often conceptualize such symptoms as a personality change. The irritability associated with HD can be severe, and results in outbursts of anger and aggression, with prevalence recently reported as being as high as between 22% and 66% of patients. 10 Later in the disease, the symptoms often take on more of a frontal abulic constellation of symptoms, with prominent apathy or loss of initiative, creativity, and curiosity. This is accompanied by pervasive emotional blandness. Family members often interpret these symptoms as depression, and objectively, the patient certainly appears more withdrawn, uninterested, and noninteractive. However, subjectively, patients will deny any feelings of sadness or hopelessness and typically describe their mood as fine or good. These frontal lobe symptoms (disinhibition and abulia) of HD are a likely manifestation of the frontostriatal pathology of degeneration. Apathy is, in general, the most common feature of the disease, occurring at the highest prevalence and is progressive, 11 tracking along with other progressive features such as motor symptoms and cognitive decline.
Depression is a feature that is commonly reported to be associated with HD. 12 However, what is not clear is whether depressive symptoms are a manifestation of the disease process with direct connections to the neural underpinnings of pathology. Importantly, the course of depressive symptoms is opposite what one would expect if these symptoms were core features that were directly linked to the pathophysiology of the degenerative process. That is, depression is most common at early points in the illness—the first period is right around the time of diagnosis, and the second is during stage 2 when some impairment begins to hamper function. 13 However, from this point on, depressive symptoms appear to decline in prevalence. Parallel with this time course is risk of suicide, which is highest near the time of diagnosis and which then drops off and diminishes after that. 14 Given the course of a degenerative disease, any associated symptom that is present early in the course of disease but lessens with time suggests that it is not the underlying pathology leading to depressive symptoms. It may well be that the increase in depressive symptoms occurring early in the course of disease may be due to an appropriate psychological reaction to the stressors of dealing with either the knowledge or the emergence of a fatal brain disease. Despite the etiology of depressive symptoms in HD, the treatment of these symptoms and the need for careful screening of suicide risk remains a vital part of the care of this patient population.
A prominent feature of HD is lack of awareness or lack of insight into the nature or severity of symptoms that the patient is experiencing. This can include lack of awareness of any feature of the disease, including all three domains of motor, cognitive, or behavioral symptoms. 16 , 15 This feature makes it important to consider family members as helpful (sometime crucial) sources of information who provide objective appraisals of the patient's symptoms and level of function, and they should be involved in the patient's health care assessments and decision making.
The gene HTT is but one in a class of genes in which a CAG repeat (triplet coding for glutamine, represented as Q) is present, which when expanded causes brain disease. These can be classified as the polyQ (meaning repeating glutamine) diseases and include spinocerebellar ataxia (of which there are several types), dentatorubropallidoluysian atrophy (DRPLA) and spinal and bulbar muscular atrophy (SBMA). All polyQ diseases share the features of being autosomal dominant and causing disease when the number of CAG repeats crosses a certain threshold. Beyond polyQ diseases, there are several other diseases that are caused by triplet repeat, and these include fragile X syndrome (CGG repeat), Friedreich ataxia (GAA repeat), and myotonic dystrophy (CTG repeat).
Although a lot of work has been done on the study of the expanded or mutant form of HTT (mHTT), there has been relatively little work on understanding the normal gene and its function. 17 Some of this is due to the large size of the huntingtin protein (making isolation difficult), the fact that it is located everywhere in the body (ubiquitous expression), and that, within the cell, it interacts with more than 200 partners/proteins. 18 What is known is that like other homopeptide-repeatcontaining proteins, huntingtin functions by creating m ul ti protein complex form at ions. The actual function is not clear and may vary, but reports support functions in transcriptional regulation, nucleocytoplasmic shuttling, synaptic function, and antiapoptic activity. 19
It is also known that HTT is a highly conserved gene. However, the CAG repeats in the gene are not conserved. Work by Tatari et al 20 demonstrated that phylogenetic comparison of HTT homologs reveal the appearance of repeats first in deuteros tomes, and the repeats then increase — the more evolved the species, the greater the number of repeats, with humans having the highest number. For this and a number of reasons, it has been postulated thaXHTT, and possibly other genes like it, may have an important role in the evolution of the brain from primate to human. 21
In terms of mHTT and the disease process, the conundrum is that, despite ubiquitous expression throughout the entire body and from conception through adulthood, the primary site of pathology is specific to the striatum. Even more specific is the type of neuron that is most vulnerable. Striatal GABAergic spiny projection neurons (SPNs) come in two types, as follows: indirect pathway SPNs (iSPNs), which suppress inappropriate movements, and direct pathway SPNs (dSPNs), which promote appropriate movements. It is the iSPNs that are affected first and are consistent with the manifestation of chorea as the presenting motor abnormality. Later in the disease, dSPNs are affected, leading to the hypokinetic motor symptoms of later-stage disease.
Most recent evidence points to the pathophysiology of mHTT being the impairment of cortical pyramidal neurons to provide the striatum with needed brain derived neurotrophic factor (BDNF). 22 , 23 This has been said to lead to the ”withering“ of the striatal cells. Although eventually all regions and tissues of the brain are affected, the disease process is clearly selective and specific to the striatum.
Another major theme in the research on etiology of HD is the disturbance of cell metabolism. Biochemical studies have shown disrupted metabolic processes of post-mortem HD brains 24 and lymphoblast cell lines of HD patients. 25 Impaired energetics in HD were not limited to neuronal tissues and were also found in HD adult skeletal muscles, 26 - 28 implying an integral role for huntingtin in mitochondrial energy metabolism. This disturbance in metabolism has been linked clinically to the symptoms of lower weight and body mass index (BMI) in patients with HD. This has been shown to be independent of the amount of chorea that is present, and in later stages of the disease, it can lead to cachexia. More importantly, low BMI is present in preHD subjects and points to an energy imbalance that may be primary to the disease process. 29 , 30
The role of abnormal development
In general, HD is classically conceptualized as a neurodegenerative disease of the striatum. However, multiple lines of evidence support the theory that abnormal brain development may play an important role in the etiology of HD, as well as other degenerative brain disorders. Abnormal development and degeneration are not mutually exclusive. There is no doubt that HD is a neurodegenerative disorder. However, persuasive work in molecular biology has supported the theory of neurodevelopmental mechanisms of degeneration. This theory suggests that neurodegenerative diseases such as Alzheimer disease and HD may represent a novel class of developmental disorders in which subsets of neural populations are vulnerable because of abnormal development, and exist in a mutant steady state before succumbing to environmental stressors or toxins that normally would not promote cell death. 31

Huntingtin and development
Normal HTT is necessary for brain development; embryos of HTT knock-out mice have major abnormalities in central nervous system development and die shortly after birth. 32 It is also known that HTT is expressed in the brain throughout development 33 , 34 and plays a vital role in neuronal survival and stability. 35 Therefore, given HTTs key role in development, a partial loss of function may manifest in abnormal neural development. Although the classic theory of HD etiology is that mutant HTT (mHTT) results in a gain-of-function toxicity that results in neural damage, there is also compelling evidence that in addition to this mechanism, loss of function of normal HTT may also be an important mechanism in the disease. 17 , 36
More recent work by Nguyen et al 37 has shown that HTT is essential for the program of neural induction, progressive specification of neural progenitor cell types, and the subsequent elaboration of neural lineage species. Moreover, mHTT was shown to cause impairments in multiple stages of striatal development, supporting the notion that the selective vulnerability of striatal neurons may have a developmental etiology. 37
One of the benefits of the HTT gene discovery was the opportunity it afforded to study presymptomatic HD subjects, also known as preHD. This allows the measurement of brain structure and function in people who are years from the onset of disease. One large multisite study with more than 1300 at-risk adults enrolled has shown that preHD subjects exhibit abnormalities in brain structure, cognition, behavior, and motor function long before (up to 20 years) a clinical diagnosis is made. 9 , 38 - 46 Multiple other studies, both large and small, have also found abnormalities in preHD adults decades before onset. 47 - 51 Some have suggested that these changes are due to early degeneration. 52 - 54 However, an alternate explanation is that these subtle symptoms are manifestations of abnormal brain development and are present throughout life.
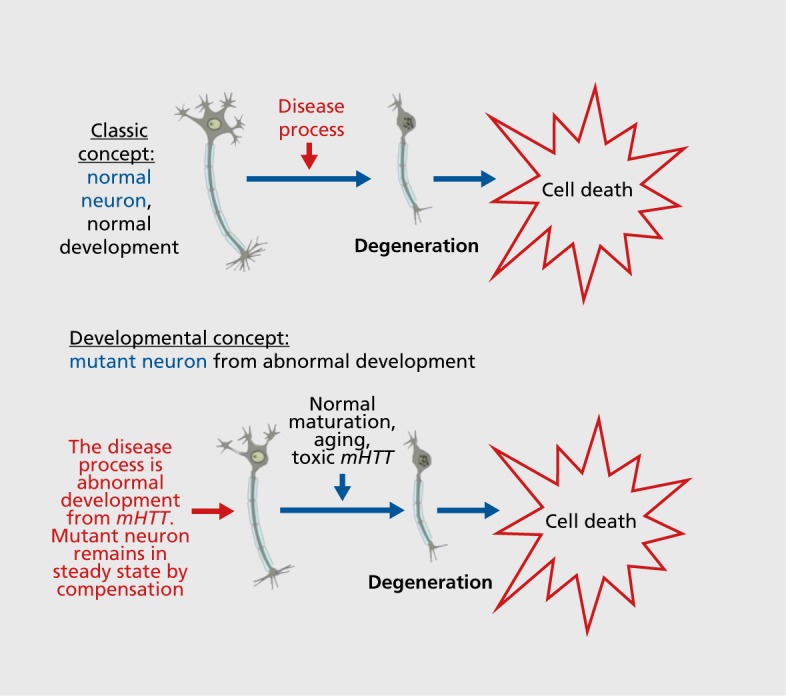
If it is true that HD has a vital portion of its pathophysiology based in abnormal development, a conceptual shift in our understanding of this disease (and other degenerative disorders) will be in order. The current etio logic dogma of HD is that the disease process lies within the toxic effects of mutant huntingtin (encoded by mHTT), which accumulates in the cell, is toxic, and causes degeneration. An alternative theory is that HTTs vital role in development is compromised in the mHTT form, leading to abnormal development, which is, in and of itself, part of the disease process. This mutant neuronal circuit is initially able to compensate and remain relatively functional, though with subtle abnormalities (mutant steady state). Later, maturation and aging processes (and toxic effects of mutant huntingtin) eventually tip the faulty circuit toward degeneration. A schematic of the model is shown in ( Figure 2 ).
Figure 2. Models of degeneration. (Top) The classic concept of the etiology of degeneration, where a normal neuron is attacked by the disease process (effect of the toxic mutant gene) and dies. (Bottom) The etiology based on aberrant development, where the disease process begins with abnormal neuronal development, compensation leads to a mutant steady state, and degeneration occurs due to stress caused by factors such as maturation, aging, or toxic gene effects. mHTT, mutant Huntingtin gene.
Evidence of abnormal brain development in human research includes one large magnetic resonance imaging study showing that pre-HD males had smaller intracranial volumes (ICV) than healthy controls. 40 ICV is a proxy measure of maximum brain growth during development; therefore, lower ICV is indicative of poor general brain growth. Also, a unique study of children at risk for HD has shown that children who are preHD (average age 12) have lower BMI and smaller head circumference values than healthy controls, again supporting the notion that abnormal growth may be a vital part of the pathologic process in HD. 55
Juvenile Huntington disease
Longer expansions of HTT cause early onset of the disease. When this occurs prior to age 21, it is termed JHD. JHD is similar to adult-onset HD in many ways, with the same triad of motor, cognitive, and psychiatric symptoms. However, one distinct difference is the presentation and predominance of motor symptoms: In adult-onset HD, early motor symptoms are chorea/hyperkinetic and later on, hypokinetic. In JHD, the motor presentation is typically hypokinetic with bradykinesia/ stiffness/dystonia and may become hyperkinetic later in the disease. 56 One other accompanying feature that is somewhat unique to JHD is seizures. Most common in childhood-onset JHD (diagnosed before the age of 10), seizures can be severe and difficult to treat. 57 The cognitive changes in JHD are progressive but, in the context of children, may also affect their ability to learn and obtain skills before degeneration of those skills occurs. Of the symptoms of JHD, the psychiatric and behavioral symptoms are far more consistent and prominent. That is, most patients will have behavioral symptoms. In one large study, 70% of JHD subjects presented with behavioral symptoms; during the course of disease, 93% of males and 81% of females experienced psychiatric issues. 58 These symptoms are made up almost exclusively of externalizing behaviors of hyperactivity, impulsivity, poor mood regulation, irritability, and anger outbursts. Although there is a perception that JHD patients may have a more rapid disease course than those with adult onset, the literature has conflicting reports, leaving it a question that has yet to be fully explored. 56
Gene therapy
By far the most exciting and promising advances in research on HD have been made in the work toward treatment with gene therapy. A potential therapeutic for dominant genetic disorders, silencing of mutant genes provides the opportunity for treatment with major impact. In general, the promise of gene therapy could be twofold: (i) restoration of function by returning to health neuronal circuits that are not yet dead, but dysfunctional and (ii) neuroprotection with a lack of disease progression. In fact, the ultimate use of gene therapy would not be in treatment, but in prevention of disease — avoidance of symptoms entirely. The approaches of gene therapy are based on targeting the processes of DNA information being copied into messenger RNA or mRNA (a process called transcription) and on the synthesis of proteins using the information in mRNA (a process called translation). Gene-silencing techniques have three general approaches, as follows: repression of transcription using zinc finger proteins, repression of translation of mHTT by antisense oligonucleotides (ASOs), and blocking protein translation using RNA interference techniques. 59 The large neuroimaging studies done in preHD and early-stage patients have shown that quantitative measures of brain regions such as the striatum are excellent biomarkers of disease progression and will be useful in the context of the upcoming gene therapy studies. 42 , 60 , 61
HD, a single-gene degenerative disorder of the striatum, has seen more than two decades of intense research, spurred by the identification of the gene in 1993. This research has led to a better understanding of the pathoetiology of the disease; however, there is much still to be studied, especially in the context of understanding the role of abnormal development. In addition, very little is known about the normal function of HTT, which is vital to brain development. Despite these areas of uncertainty, much progress has been made, particularly regarding the promise, and now reality, of new methods of treatment and potential prevention in the context of gene therapy approaches.
- 1. Zuhlke C., Riess O., Bockel B., Lange H., Thies U. Mitotic stability and meiotic variability of the (CAG)n repeat in the Huntington disease gene. Hum Mol Genet. 1993;2(12):2063–2067. doi: 10.1093/hmg/2.12.2063. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Evans SJ., Douglas I., Rawlins MD., Wexler NS., Tabrizi SJ., Smeeth L. Prevalence of adult Huntington's disease in the UK based on diagnoses recorded in general practice records. J Neurol Neurosurg Psychiatry. 2013;84(10):1156–1160. doi: 10.1136/jnnp-2012-304636. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Lee JM., Ramos EM., Lee JH., et al CAG repeat expansion in Huntington disease determines age at onset in a fully dominant fashion. Neurology. 2012;78(10):690–695. doi: 10.1212/WNL.0b013e318249f683. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Langbehn DR., Brinkman RR., Falush D., Paulsen JS., Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clin Genet. 2004;65(4):261–211. doi: 10.1111/j.1399-0004.2004.00241.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Quarrell O., O'Donovan KL., Bandmann O., Strong M. The prevalence of juvenile Huntington's disease: a review of the literature and meta-analysis. PLoSCurr. 2012;4:e4f8606b8742ef8603. doi: 10.1371/4f8606b742ef3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Nicolas G., Devys D., Goldenberg A., et al Juvenile Huntington disease in an 18-month-old boy revealed by global developmental delay and reduced cerebeIlar volume . Am J Med Gen et A. 2011;155 A(4):815–818. doi: 10.1002/ajmg.a.33911. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Shoulson I., Fahn S. Huntington's disease: clinical care and evaluation. Neurology. 1979;29:1–3. doi: 10.1212/wnl.29.1.1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Novak MJ., Tabrizi SJ. Huntington's disease. BMJ. 2010;340:c3109. doi: 10.1136/bmj.c3109. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Duff K., Paulsen JS., Beglinger LJ., et al “Frontal” behaviors before the diagnosis of Huntington's disease and their relationship to markers of disease progression: evidence of early lack of awareness. J Neuropsychiatry Clin Neurosci. 2010;22(2):196–207. doi: 10.1176/appi.neuropsych.22.2.196. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Fisher CA., Sewell K., Brown A., Churchyard A. Aggression in Huntington's disease: a systematic review of rates of aggression and treatment methods. J Huntingtons Dis. 2014;3(4):319–332. doi: 10.3233/JHD-140127. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. van Duijn E., Craufurd D., Hubers AA., et al Neuropsychiatry symptoms in a European Huntington's disease cohort (REGISTRY). J Neurol Neurosurg Psychiatry. 2014;85(12):1411–1418. doi: 10.1136/jnnp-2013-307343. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Ghosh R., Tabrizi SJ. Clinical aspects of Huntington's disease. Curr Top Behav Neurosci. 2015;22:3–31. doi: 10.1007/7854_2013_238. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Paulsen JS., Nehl C., Hoth KF., et al Depression and stages of Huntington's disease. J Neuropsychiatry Clin Neurosci. 2005;17(4):496–502. doi: 10.1176/jnp.17.4.496. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Paulsen JS., Hoth KF., Nehl C., Stierman L. Critical periods of suicide risk in Huntington's disease. Am J Psychiatry. 2005;162(4):725–731. doi: 10.1176/appi.ajp.162.4.725. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Hoth KF., Paulsen JS., Moser DJ., Tranel D., Clark LA., Bechara A. Patients with Huntington's disease have impaired awareness of cognitive, emotional, and functional abilities. J Clin Exp Neuropsychol. 2007;29(4):365–376. doi: 10.1080/13803390600718958. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. McCusker EA., Gunn DG., Epping EA., et al Unawareness of motor phenoconversion in Huntington disease. Neurology. 2013;81(13):1141–1147. doi: 10.1212/WNL.0b013e3182a55f05. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Cattaneo E., Zuccato C., Tartari M. Normal huntingtin function: an alternative approach to Huntington's disease. Nat Rev Neurosci. 2005;6(12):919–930. doi: 10.1038/nrn1806. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Harjes P., Wanker EE. The hunt for huntingtin function: interaction partners tell many different stories. Trends Biochem Sci. 2003;28(8):425–433. doi: 10.1016/S0968-0004(03)00168-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. De Souza RA., Leavitt BR. Neurobiology of Huntington's disease. Curr Top Behav Neurosci. 2015;22:81–100. doi: 10.1007/7854_2014_353. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Tartari M., Gissi C., Lo Sardo V., et al Phylogenetic comparison of huntingtin homologues reveals the appearance of a primitive polyQ in sea urchin. Mol Biol Evol. 2008;25(2):330–338. doi: 10.1093/molbev/msm258. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Nithianantharajah J., Hannan AJ. Dynamic mutations as digital genetic modulators of brain development, function and dysfunction. Bioessays. 2007;29(6):525–535. doi: 10.1002/bies.20589. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Zuccato C., Cattaneo E. Role of brain-derived neurotrophic factor in Huntington's disease. Prog Neurobiol. 2007;81(5-6):294–330. doi: 10.1016/j.pneurobio.2007.01.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Plotkin JL., Surmeier DJ. Corticostriatal synaptic adaptations in Huntington's disease. Curr Opin Neurobiol. 2015;33:53–62. doi: 10.1016/j.conb.2015.01.020. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Browne SE., Bowling AC., Macgarvey U., et al Oxidative damage and metabolic dysfunction in Huntington's disease: selective vulnerability of the basal ganglia. Ann Neurol. 1997;41(5):646–653. doi: 10.1002/ana.410410514. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Seong IS., Ivanova E., Lee JM., et al HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Hum Mol Genet. 2005;14(19):2871–2880. doi: 10.1093/hmg/ddi319. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Ciammola A., Sassone J., Sciacco M., et al Low anaerobic threshold and increased skeletal muscle lactate production in subjects with Huntington's disease. Mov Disord. 2011;26(1):130–137. doi: 10.1002/mds.23258. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Koroshetz WJ., Jenkins BG., Rosen BR., Beal MR. Energy metabolism defects in Huntington's disease and effects of coenzyme Q10. Ann Neurol. 1997;41(2):160–165. doi: 10.1002/ana.410410206. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Lodi R., Schapira AH., Manners D., et aI AbnormaI in vivo ske letaI muscle energy metabolism in Huntington's disease and dentatorubropallidoluysian atrophy. Ann Neurol. 2000;48(1):72–76. [ PubMed ] [ Google Scholar ]
- 29. Djousse L., Knowlton B., Cupples L., Marder K., Shoulson I., Myers R. Weight loss in early stage of Huntington's disease. Neurology. 2002;59(9):1325–1330. doi: 10.1212/01.wnl.0000031791.10922.cf. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Mochel F., Charles P., Seguin F., et al Early energy deficit in Huntington disease: identification of a plasma biomarker traceable during disease progression. PloS One. 2007;2(7):e647–e647. doi: 10.1371/journal.pone.0000647. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Mehler MF., Gokhan S. Mechanisms underlying neural cell death in neurodegenerative diseases: alterations of a developmentally-mediated cellular rheostat. Trends Neurosci. 2000;23(12):599–605. doi: 10.1016/s0166-2236(00)01705-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Nasir J., Floresco SB., O'Kusky JR., et al Targeted disruption of the Huntington's disease gene results in embryonic lethality and behavioral and morphological changes in heterozygotes. Cell. 1995;81(5):811–823. doi: 10.1016/0092-8674(95)90542-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Bhide PG., Day M., Sapp E., et al Expression of normal and mutant huntingtin in the developing brain. J Neurosc. 1996;16(17):5523–5535. doi: 10.1523/JNEUROSCI.16-17-05523.1996. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Zeitlin S., Liu JP., Chapman DL., Papaioannou VE., Efstratiadis A. Increased apoptosis and early embryonic lethality in mice nullizygous for the Huntington's disease gene homologue. Nat Genet. 1995;11(2):155–163. doi: 10.1038/ng1095-155. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Rigamonti D., Bauer JH., De-Fraja C., et al Wild-type huntingtin protects from apoptosis upstream of caspase-3. J Neurosci. 2000;20(10):3705–3713. doi: 10.1523/JNEUROSCI.20-10-03705.2000. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Cattaneo E., Rigamonti D., Goffredo D., Zuccato C., Squitieri F., Sipione S. Loss of normal huntingtin function: new developments in Huntington's disease research. Trends Neurosci. 2001;24(3):182–188. doi: 10.1016/s0166-2236(00)01721-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Nguyen GD., Gokhan S., Molero AE., Mehler MF. Selective roles of normal and mutant huntingtin in neural induction and early neurogenesis. PLoS One. 2013;8(5):e64368. doi: 10.1371/journal.pone.0064368. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Nopoulos P., Magnotta VA., Mikos A., Paulson H., Andreasen NC., Paulsen JS. Morphology of the cerebral cortex in preclinical Huntington's disease. Am J Psychiatry. 2007;164(9):1428–1434. doi: 10.1176/appi.ajp.2007.06081266. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Nopoulos PC., Aylward EH., Ross CA., et al Cerebral cortex structure in prodromal Huntington disease. Neurobiol Dis. 2010;40(3):544–554. doi: 10.1016/j.nbd.2010.07.014. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Nopoulos PC., Aylward EH., Ross CA., et al Smaller intracranial volume in prodromal Huntington's disease: evidence for abnormaI neurodevelopment. Brain. 2010;134(Pt 1):137–142. doi: 10.1093/brain/awq280. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Paulsen JS., Magnotta VA., Mikos AE., et al Brain structure in preclinical Huntington's disease. Biol Psychiatry. 2006;59(1):57–63. doi: 10.1016/j.biopsych.2005.06.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Paulsen JS., Nopoulos PC., Aylward E., et al Striatal and white matter predictors of estimated diagnosis for Huntington disease. Brain Res Bull. 2010;82(3-4):201–207. doi: 10.1016/j.brainresbull.2010.04.003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Duff K., Paulsen JS., Beglinger LJ., Langbehn DR., Stout JC. Psychiatric symptoms in Huntington's disease before diagnosis: the predict-HD study. Biol Psychiatry. 2007;62(12):1341–1346. doi: 10.1016/j.biopsych.2006.11.034. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Aylward EH., Sparks BF., Field KM., et al Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63(1):66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Beglinger LJ., Paulsen JS., Watson DB., et al Obsessive and compulsive symptoms in prediagnosed Huntington's disease. J Clin Psychiatry. 2008;69(11):1758–1765. doi: 10.4088/jcp.v69n1111. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. BigIan KM., Ross CA., Langbehn DR., et al PREDICT-HD Investigators of the Huntington Study Group. Motor abnormalities in premanifest persons with Huntington's disease: The PREDICT-HD study. Mov Disord. 2009;24(12):1763–1772. doi: 10.1002/mds.22601. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Unschuld PG., Edden RA., Carass A., et al Brain metabolite alterations and cognitive dysfunction in early Huntington's disease. Mov Disord. 2012;27(7):895–902. doi: 10.1002/mds.25010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Reynolds NC Jr., Prost RW., Mark LP. Heterogeneity in 1H-MRS profiles of presymptomatic and early manifest Huntington's disease. Brain Res. 2005;1031(1):82–89. doi: 10.1016/j.brainres.2004.10.030. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Hobbs NZ., Barnes J., Frost C., et al Onset and progression of pathologic atrophy in Huntington disease: a longitudinal MR imaging study. AJNR Am J Neuroradiol. 2010;31(6):1036–1041. doi: 10.3174/ajnr.A2018. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Tabrizi SJ., Langbehn DR., Leavitt BR., et al TRACK-HD Investigators. Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8(9):791–801. doi: 10.1016/S1474-4422(09)70170-X. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. van den Bogaard SJ., Dumas EM., Teeuwisse WM., et al Exploratory 7-Tesla magnetic resonance spectroscopy in Huntington's disease provides in vivo evidence for impaired energy metabolism. J Neurol. 2011;258(12):2230–2239. doi: 10.1007/s00415-011-6099-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Aylward EH., Li Q., Stine OC., et al Longitudinal change in basal ganglia volume in patients with Huntington's disease. Neurology. 1997;48(2):394–399. doi: 10.1212/wnl.48.2.394. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Aylward EH., Sparks BF., Feild KM., et al Onset and rate of striatal atrophy in preclinical Huntington's disease. Neurology. 2004;63(1):66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Paulsen JS., Zhao H., Stout JC., et al Clinical markers of early disease in persons near onset of Huntington's disease. Neurology. 2001;57(4):658–662. doi: 10.1212/wnl.57.4.658. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Lee JK., Mathews K., Schlaggar B., et al Measures of growth in children at risk for Huntington disease. Neurology. 2012;79(7):668–674. doi: 10.1212/WNL.0b013e3182648b65. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. QuarrelI OW., Nance MA., Nopoulos P., Paulsen JS., Smith JA., Squitieri F. Managing juvenile Huntington's disease. Neurodegener Dis Manag. doi:10.2217/nmt.13.18. 2013;3(3) doi: 10.2217/nmt.13.18. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Nance MA. Genetic testing of children at risk for Huntington's disease. US Huntington Disease Genetic Testing Group. Neurology. 1997;49(4):1048–1053. doi: 10.1212/wnl.49.4.1048. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Siesling S., Vegter-van der Vlis M., Roos RA. Juvenile Huntington disease in the Netherlands. Pediatr Neurol. 1997;17(1):37–43. doi: 10.1016/s0887-8994(97)00069-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Shannon KM., Fraint A. Therapeutic advances in Huntington's disease. Mov Disord. 201;30(11):1539–1546. doi: 10.1002/mds.26331. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Andre R., Scahill Rl., Haider S., Tabrizi SJ. Biomarker development for Huntington's disease. Drug Discov Today. 2014;19(7):972–979. doi: 10.1016/j.drudis.2014.03.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Paulsen JS., Long JD., Johnson HJ., et al PREDICT-HD Investigators and Coordinators of the Huntington Study Group. Clinical and biomarker changes in premanifest Huntington disease show trial feasibility a decade of the PREDICT-HD study. Front Aging Neurosci. 2014;6:78. doi: 10.3389/fnagi.2014.00078. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (3.2 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO