World First: Stem Cells Reverse Type 1 Diabetes in Clinical Trial
Most people with type 1 diabetes cannot reverse their autoimmune disease or put it into spontaneous remission – all they can do is manage their blood sugar levels with insulin, a healthy diet, and regular exercise.
Now, the successful treatment of the condition in a young woman in China has brought the possibility of a universal cure tantalizingly close.
In June 2023, doctors injected the equivalent of roughly 1.5 million insulin-producing cells into the patient's abdominal muscles. The community of cells was carefully reprogrammed from her own stem cells .
Two and a half months later, the woman's lifelong dependence on injected insulin came to an end – completely reversing her long-term, hard-to-control diabetes.
More than four months after the transplantation, her body was producing enough insulin on its own to keep her in a safe blood glucose range for more than 98 percent of the day.
If this one patient can keep producing insulin naturally in the coming years, she could one day be declared 'cured' – the first successful case of its kind in the scientific literature.
"That's remarkable," diabetes researcher Daisuke Yabe, who was not involved in the research, told Nature reporter Smriti Mallapaty.
"If this is applicable to other patients, it's going to be wonderful."
Researchers in China are already planning to open up their trial to new patients.
Type 1 diabetes occurs when the body's immune system attacks clusters of cells in the pancreas that produce insulin, called islets.
Transplanting islets or an entire pancreas from a donor to a patient with type 1 diabetes can prove curative in some selective cases, but it is an extreme and potentially dangerous option, and there are too few donors to make it accessible for the millions of those with this autoimmune disease worldwide .
For over two decades now , scientists have tried to coax cells from fully-developed adult tissue types back into a blank state known as an induced pluripotent stem cell , and in turn, transform them into insulin-producing cells.
Figuring out how to do that with accuracy, however, is tricky work. The final product doesn't always match real pancreatic islets.
Researchers in China have taken a new route that they say allows them greater control. Instead of introducing proteins to the stem cells, which trigger certain gene expressions, reprogramming the adult tissues into the blank state, the team has engineered induced pluripotent stem cells using small molecules .
After testing the technique in mice and non-human primates with success and safety, the team of researchers got their approval for a human clinical trial . The trial is ongoing, and three people are currently enrolled.
One of those patients is a 26-year-old woman, who was diagnosed with type 1 diabetes at age 14, and her results from one year in the trial have now been published.
Previously, this patient had already had a pancreas transplant for severe hypoglycemia, but the organ had to be removed due to "severe thrombotic complications".
So far, her stem cell transplant seems to be working better, alongside her immunosuppressive drugs.
The findings join several other recent clinical trials that suggest further studies on stem cell transplantations are warranted.
In a trial in the US , for instance, a dozen patients with type 1 diabetes were injected with islets, created from donated stem cells. The group was also treated with immunosuppressants. All 12 participants began producing insulin naturally when glucose entered their bloodstream, according to preliminary results.
"Overall," write the researchers in China, "the findings support further clinical studies in this direction and mark a step forward in achieving the potential of personalized cell therapy… to treat disease."
The study was published in Cell .

Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

Seem like peanut allergies were once rare and now everyone has them?

3 million Americans have dental implants — but procedure wasn’t always ‘routine’
Getting to the bottom of long COVID

“When my son was diagnosed [with Type 1], I knew nothing about diabetes. I changed my research focus, thinking, as any parent would, ‘What am I going to do about this?’” says Douglas Melton.
Kris Snibbe/Harvard Staff Photographer
Breakthrough within reach for diabetes scientist and patients nearest to his heart
Harvard Correspondent
100 years after discovery of insulin, replacement therapy represents ‘a new kind of medicine,’ says Stem Cell Institute co-director Douglas Melton, whose children inspired his research
When Vertex Pharmaceuticals announced last month that its investigational stem-cell-derived replacement therapy was, in conjunction with immunosuppressive therapy, helping the first patient in a Phase 1/2 clinical trial robustly reproduce his or her own fully differentiated pancreatic islet cells, the cells that produce insulin, the news was hailed as a potential breakthrough for the treatment of Type 1 diabetes. For Harvard Stem Cell Institute Co-Director and Xander University Professor Douglas Melton, whose lab pioneered the science behind the therapy, the trial marked the most recent turning point in a decades-long effort to understand and treat the disease. In a conversation with the Gazette, Melton discussed the science behind the advance, the challenges ahead, and the personal side of his research. The interview was edited for clarity and length.
Douglas Melton
GAZETTE: What is the significance of the Vertex trial?
MELTON: The first major change in the treatment of Type 1 diabetes was probably the discovery of insulin in 1920. Now it’s 100 years later and if this works, it’s going to change the medical treatment for people with diabetes. Instead of injecting insulin, patients will get cells that will be their own insulin factories. It’s a new kind of medicine.
GAZETTE: Would you walk us through the approach?
MELTON: Nearly two decades ago we had the idea that we could use embryonic stem cells to make functional pancreatic islets for diabetics. When we first started, we had to try to figure out how the islets in a person’s pancreas replenished. Blood, for example, is replenished routinely by a blood stem cell. So, if you go give blood at a blood drive, your body makes more blood. But we showed in mice that that is not true for the pancreatic islets. Once they’re removed or killed, the adult body has no capacity to make new ones.
So the first important “a-ha” moment was to demonstrate that there was no capacity in an adult to make new islets. That moved us to another source of new material: stem cells. The next important thing, after we overcame the political issues surrounding the use of embryonic stem cells, was to ask: Can we direct the differentiation of stem cells and make them become beta cells? That problem took much longer than I expected — I told my wife it would take five years, but it took closer to 15. The project benefited enormously from undergraduates, graduate students, and postdocs. None of them were here for 15 years of course, but they all worked on different steps.
GAZETTE: What role did the Harvard Stem Cell Institute play?
MELTON: This work absolutely could not have been done using conventional support from the National Institutes of Health. First of all, NIH grants came with severe restrictions and secondly, a long-term project like this doesn’t easily map to the initial grant support they give for a one- to three-year project. I am forever grateful and feel fortunate to have been at a private institution where philanthropy, through the HSCI, wasn’t just helpful, it made all the difference.
I am exceptionally grateful as well to former Harvard President Larry Summers and Steve Hyman, director of the Stanley Center for Psychiatric Research at the Broad Institute, who supported the creation of the HSCI, which was formed specifically with the idea to explore the potential of pluripotency stem cells for discovering questions about how development works, how cells are made in our body, and hopefully for finding new treatments or cures for disease. This may be one of the first examples where it’s come to fruition. At the time, the use of embryonic stem cells was quite controversial, and Steve and Larry said that this was precisely the kind of science they wanted to support.
GAZETTE: You were fundamental in starting the Department of Stem Cell and Regenerative Biology. Can you tell us about that?
MELTON: David Scadden and I helped start the department, which lives in two Schools: Harvard Medical School and the Faculty of Arts and Science. This speaks to the unusual formation and intention of the department. I’ve talked a lot about diabetes and islets, but think about all the other tissues and diseases that people suffer from. There are faculty and students in the department working on the heart, nerves, muscle, brain, and other tissues — on all aspects of how the development of a cell and a tissue affects who we are and the course of disease. The department is an exciting one because it’s exploring experimental questions such as: How do you regenerate a limb? The department was founded with the idea that not only should you ask and answer questions about nature, but that one can do so with the intention that the results lead to new treatments for disease. It is a kind of applied biology department.
GAZETTE: This pancreatic islet work was patented by Harvard and then licensed to your biotech company, Semma, which was acquired by Vertex. Can you explain how this reflects your personal connection to the research?
MELTON: Semma is named for my two children, Sam and Emma. Both are now adults, and both have Type 1 diabetes. My son was 6 months old when he was diagnosed. And that’s when I changed my research plan. And my daughter, who’s four years older than my son, became diabetic about 10 years later, when she was 14.
When my son was diagnosed, I knew nothing about diabetes and had been working on how frogs develop. I changed my research focus, thinking, as any parent would, “What am I going to do about this?” Again, I come back to the flexibility of Harvard. Nobody said, “Why are you changing your research plan?”
GAZETTE: What’s next?
MELTON: The stem-cell-derived replacement therapy cells that have been put into this first patient were provided with a class of drugs called immunosuppressants, which depress the patient’s immune system. They have to do this because these cells were not taken from that patient, and so they are not recognized as “self.” Without immunosuppressants, they would be rejected. We want to find a way to make cells by genetic engineering that are not recognized as foreign.
I think this is a solvable problem. Why? When a woman has a baby, that baby has two sets of genes. It has genes from the egg, from the mother, which would be recognized as “self,” but it also has genes from the father, which would be “non-self.” Why does the mother’s body not reject the fetus? If we can figure that out, it will help inform our thinking about what genes to change in our stem cell-derived islets so that they could go into any person. This would be relevant not just to diabetes, but to any cells you wanted to transplant for liver or even heart transplants. It could mean no longer having to worry about immunosuppression.
Share this article
You might like.
Surgeon, professor Marty Makary examines damage wrought when medicine closes ranks around inaccurate dogma

Surgeon recounts changes in field over 40-year career — from titanium screws to bone regeneration — as he accepts Goldhaber Award
A reservoir of virus in the body may explain why some people experience long COVID symptoms
Drug-free nasal spray blocks, neutralizes viruses, bacteria
In preclinical studies, spray offered nearly 100% protection from respiratory infections by COVID-19, influenza, viruses, and pneumonia-causing bacteria
Falls put older adults at increased risk of Alzheimer’s
Researchers found dementia more frequently diagnosed within one year of a fall, compared to other types of injuries
A blueprint for better conversations
After months of listening and learning, open inquiry co-chairs detail working group’s recommendations
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
October 14, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
written by researcher(s)
Stem cell therapy reverses type 1 diabetes in world first
by Stéphane Berneau, The Conversation

A groundbreaking discovery has recently brought hope to millions of people living with type 1 diabetes around the world. In a world first , scientists have successfully used stem cell therapy to reverse type 1 diabetes in a woman.
This achievement is being hailed as a major medical breakthrough , as it offers a potential cure for a disease that, until now, could only be managed but not cured.
Type 1 diabetes is a serious condition that usually starts in childhood or early adulthood. In people with the condition, the body's immune system mistakenly attacks the cells in the pancreas that produce insulin.
Insulin is a hormone that helps regulate blood sugar levels. Without it, blood sugar can spike to dangerously high levels. Over time, this can lead to severe health complications, such as heart disease , nerve damage , kidney failure and blindness.
People with type 1 diabetes need to take insulin injections or use insulin pumps every day to manage their blood sugar levels. Despite these treatments, managing the disease can be difficult, and patients often face lifelong difficulties. That's why this new stem cell therapy has generated so much excitement—it could offer a real solution.
The average human body is composed of about 37.2 trillion cells , which is 300 times the number of stars in our galaxy. All our adult cells come from a single cell, called the fertilized egg (or zygote), which during our development will divide and differentiate into specialized cells and adult stem cells . The zygote is the initial stem cell capable of generating a new person.
Adult stem cells are special cells in the body that can turn into a limited number of cell types. Scientists have been studying stem cells for years and trying to re-program specialized cells into stem cells, hoping to use them to treat various diseases.
One of the most exciting aspects of stem cells is that they can replace damaged or missing cells in the body. At the University of Central Lancashire, my research team is using induced-pluripotent brain stem cells which were reprogrammed from skin cells of patients with Alzheimer's disease. We aim to learn more about the degenerative brain disease and its development in a petri dish without further invasive techniques.
In the case of type 1 diabetes, scientists wondered if stem cells could be used to replace the insulin-producing cells that the body had destroyed. It is extremely difficult to get stem cells to behave like the specific insulin-producing cells needed in the pancreas.
In a recent case study, scientists at Peking University in Beijing took cells from a patient and modified them in the lab to become insulin-producing cells. These newly developed cells were then implanted into the same patient with type 1 diabetes.
Remarkably, the cells began producing insulin on their own, allowing the patients to regulate their blood sugar levels after two and a half months without requiring daily insulin injections.
This is why the therapy is being referred to as a potential "cure" for type 1 diabetes. While it's still early days, the results are incredibly promising, and the therapy could become widely available in the near future if further large trials are successful.
Hurdles still to overcome
One issue is the body's immune system, which could attack the newly transplanted cells as part of diabetes type 1 conditions. Scientists are working on ways to prevent this and ensure that the transplanted cells are behaving over several years similarly compared to the initial phase in a petri dish.
Making the therapy accessible to more people will be another big challenge. If approved, stem cell treatments are expensive and complicated, so researchers are looking for ways to make the process more scalable while using the patient's own cells to prevent rejection of the transplanted cells.
Despite these hurdles, the recent discovery has created a wave of hope and optimism for patients suffering from type 1 diabetes. Stem cell therapy is showing us that it might be possible to truly cure diseases that have long been considered only manageable and incurable.
Explore further
Feedback to editors

In depth analysis explains why preschoolers are less likely to develop severe COVID-19
24 minutes ago

Fearful memories of others seen in mouse brain—study pinpoints where different types of memories begin
2 hours ago

Countries that choose to do so can reduce premature death by half, researchers say
13 hours ago

Clinical trials confirm zolbetuximab's efficacy in gastric cancer
16 hours ago

Lynch syndrome: New patient database of genetic condition aims to prevent cancer

Gene therapy that converts omega-6 to omega-3 fatty acids in the body could combat effects of childhood obesity
Enhancing MRI with AI to improve diagnosis of brain disorders

Controlled trial shows hearing and vision health support vital in dementia care

New behavioral signature could help quantify the value that people attribute to specific interpersonal relationships
17 hours ago

Both low and high folate levels during pregnancy linked to increased heart defects in babies
18 hours ago
Related Stories

Patient with type 1 diabetes functionally cured using stem cell injections
Sep 30, 2024

Stem cell therapy could be breakthrough against type 1 diabetes
Jun 25, 2024

Bacterial infections could be trigger for type 1 diabetes, new research suggests
Sep 18, 2024
New process produces purer, safer pancreas stem cells for potential transplant
Nov 6, 2023

Boosting function and survival of stem cell-derived pancreatic cells by genetic engineering
Mar 3, 2022
Examining the protein that protects insulin-producing cells
Mar 21, 2024
Recommended for you

Evidence builds for near infrared light treatment in traumatic brain injury
Oct 11, 2024

Magnetically regulated gene therapy tech offers precise brain-circuit control
Oct 10, 2024

What a tiny, 3D gut can tell us about gastrointestinal disorders

Researchers identify signaling mechanism that damages cells in diabetic kidney disease

Gene-edited cells could halt multiple sclerosis progression
Oct 9, 2024

Stitches with internally produced electric charge found to speed up wound healing in rats
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Stem cell therapy reverses type 1 diabetes in world first
Lecturer in Physiology and Pharmacology, School of Pharmacy and Biomedical Sciences, University of Central Lancashire
Disclosure statement
Stéphane Berneau does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
University of Central Lancashire provides funding as a member of The Conversation UK.
View all partners
A groundbreaking discovery has recently brought hope to millions of people living with type 1 diabetes around the world. In a world first , scientists have successfully used stem cell therapy to reverse type 1 diabetes in a woman.
This achievement is being hailed as a major medical breakthrough , as it offers a potential cure for a disease that, until now, could only be managed but not cured.
Type 1 diabetes is a serious condition that usually starts in childhood or early adulthood. In people with the condition, the body’s immune system mistakenly attacks the cells in the pancreas that produce insulin.
Insulin is a hormone that helps regulate blood sugar levels. Without it, blood sugar can spike to dangerously high levels. Over time, this can lead to severe health complications, such as heart disease, nerve damage, kidney failure and blindness.
People with type 1 diabetes need to take insulin injections or use insulin pumps every day to manage their blood sugar levels. Despite these treatments, managing the disease can be difficult, and patients often face lifelong difficulties. That’s why this new stem cell therapy has generated so much excitement — it could offer a real solution.
The average human body is composed of about 37.2 trillion cells , which is 300 times the number of stars in our galaxy. All our adult cells come from a single cell, called the fertilised egg (or zygote) which during our development will divide and differentiate into specialised cells and adult stem cells. The zygote is the initial stem cell capable of generating a new person.
Adults stem cells are special cells in the body that can turn into a limited number of cell types. Scientists have been studying stem cells for years and trying to re-program specialised cells into stem cells, hoping to use them to treat various diseases.
One of the most exciting aspects of stem cells is that they can replace damaged or missing cells in the body. At the University of Central Lancashire, my research team is using induced-pluripotent brain stem cells which were reprogrammed from skin cells of patients with Alzheimer’s disease. We aim to learn more about the degenerative brain disease and its development in a petri dish without further invasive techniques.
In the case of type 1 diabetes, scientists wondered if stem cells could be used to replace the insulin-producing cells that the body had destroyed. It is extremely difficult to get stem cells to behave like the specific insulin-producing cells needed in the pancreas.
In a recent case study, scientists at Peking University in Beijing took cells from a patient and modified them in the lab to become insulin-producing cells. These newly developed cells were then implanted into the same patient with type 1 diabetes.
Remarkably, the cells began producing insulin on their own, allowing the patients to regulate their blood sugar levels after two and a half months without requiring daily insulin injections.
This is why the therapy is being referred to as a potential “cure” for type 1 diabetes. While it’s still early days, the results are incredibly promising, and the therapy could become widely available in the near future if further large trials are successful.
Hurdles still to overcome
One issue is the body’s immune system, which could attack the newly transplanted cells as part of diabetes type 1 conditions. Scientists are working on ways to prevent this and ensure that the transplanted cells are behaving over several years similarly compared to the initial phase in a petri dish.
Making the therapy accessible to more people will be another big challenge. If approved, stem cell treatments are expensive and complicated, so researchers are looking for ways to make the process more scalable while using the patient’s own cells to prevent rejection of the transplanted cells.
Despite these hurdles, the recent discovery has created a wave of hope and optimism for patients suffering from type 1 diabetes. Stem cell therapy is showing us that it might be possible to truly cure diseases that have long been considered only manageable and incurable.
Correction: The sentence that read: ‘In a recent trial, scientists at Peking University in Beijing took cells from a donor and modified them in the lab to become insulin-producing cells. These newly developed cells were then implanted into patients with type 1 diabetes.’ Has been corrected to read: ‘In a recent case study, scientists at Peking University in Beijing took cells from a patient and modified them in the lab to become insulin-producing cells. These newly developed cells were then implanted into the same patient with type 1 diabetes.’
- Autoimmune diseases
- Stem cell therapy
- Type 1 diabetes
- Give me perspective

Resource Development Lead
Integrated management of invasive pampas grass for enhanced land rehabilitation.

Economics Editor

Deputy Vice-Chancellor (Indigenous Strategy and Services)

Chief People & Culture Officer
- Overview & Types
- Symptoms & Diagnosis
- Understanding Blood Sugar
- Diet & Exercise
- Better Living
- Complications
- Related Conditions
- Symptoms & Causes
- Diagnosis & Tests
- Prevention & Treatment
- Living & Managing
- Complications & Related Diseases
- Treating & Managing
- Complications & Related Conditions
- Gestational Diabetes
- Appointment Prep
- View Full Guide
Stem Cell Therapy Implant Shows Promise For Type 1 Diabetes
Dec. 11, 2023 – An experimental device containing millions of stem cells significantly reduced the need for insulin shots among people with type 1 diabetes , according to a new study – a treatment researchers say may someday provide a cure for the chronic, life-altering condition.
Researchers from the University of British Columbia and Vancouver Coastal Health used tiny implants filled with lab-grown pancreatic cells known as VC-02.
The study, published in the journal Nature Biotechnology , involved 10 people who at the start of the study could not produce insulin naturally. After 6 months with the implant, three of them showed significant improvement. Their bodies spent more time within the normal blood sugar range, reducing their need for external insulin.
“The hope is to get these cells strong enough to help stop requiring insulin injections all together,” said David Thompson, MD, principal investigator at the Vancouver trial site and clinical director of the Vancouver General Hospital Diabetes Centre. “I believe this is going to turn into a cure as soon as 2024.”
Type 1 diabetes is a condition in which the immune system destroys insulin-making cells in the pancreas, known as beta cells. Insulin is a hormone that regulates sugar in the blood. The condition – sometimes called juvenile diabetes – is most commonly diagnosed between the ages of 4 and 6 and in early puberty.
In the United States, people who are non-Hispanic and White are most likely to have type 1 diabetes, and it affects men and women at about the same rates. Having a close family member with the illness increases risk. About 1.24 million people in the United States live with type 1 diabetes; that number is expected to reach 5 million by 2050.
With type 1 diabetes, it's as if the body's insulin factory has shut down. People who have the disease need to take insulin from the start.
This differs from type 2 diabetes , in which the body doesn't use insulin properly. It can be managed with lifestyle changes, medications, and sometimes external insulin shots.
Until a century ago – when insulin was discovered – diabetes was a death sentence . A 14-year-old boy who lay dying from diabetes in a Toronto hospital was the first person to receive the new treatment in 1922. Within 24 hours, his high blood glucose levels dropped to near-normal levels.
“Insulin therapy for people with type 1 diabetes is better than it has ever been, but it's still not a cure,” Thompson said. “This is probably the first wave of a new era of medicine using cell therapy.”
The trial tested an experimental cell therapy developed by biotechnology company ViaCyte.
Thompson and his colleagues used devices implanted just beneath the skin, about the size of a small bandage. Unlike a glucose monitor – which is also inserted beneath the skin but only estimates blood glucose levels – the stem cell device delivers a steady supply of insulin to the body.
The trial builds off of a 2021 study that showed this approach could help the human body produce insulin. The latest study increased the number of devices for each person and improved the design to help the lab-grown cells survive.
All the people in the study started out with no insulin production and had surgery at sites in Vancouver, Belgium, and the U.S. to get up to 10 device implants each. After 6 months, three of them showed clear signs of insulin production that stayed steady throughout the yearlong study. One person in the study had showed notable improvement, spending more time in the target blood sugar range and reducing their need for extra daily insulin by 44%.
“Each device is like a miniature insulin-producing factory,” said co-author Timothy Kieffer, PhD, a professor with the departments of surgery and cellular and physiological sciences at the University of British Columbia, and past chief scientific officer of ViaCyte. The cells are “packaged into the device to essentially re-create the blood sugar-regulating functions of a healthy pancreas.”
A cure for type 1 diabetes would also mean preventing several other health complications related to the illness: blindness, kidney problems, limb loss, and even life-threatening blood sugar drops during sleep. Diabetes also significantly heightens the chances of having a heart attack or stroke.
The trial has two big limitations, said Robert Gabbay, MD, chief scientific and medical officer of the American Diabetes Association, who was not involved in the trial. Not only is it small, but the technology failed to normalize blood glucose levels, which is the goal.
But it shows promise, he said. Cell replacement therapies have previously faced a major barrier: The immune system attacks the implanted cells, requiring potentially harmful immunosuppressive drugs.
“This is particularly problematic for people with type I diabetes since the initial cause of type 1 is an autoimmune destruction of beta cells,” Gabbay said. “Placing beta cells sequestered from the immune system has been something that a number of investigative teams have worked on. This early study shows some proof of concept.”

Top doctors in ,
Find more top doctors on, related links.
- Diabetes Health Center Reference
- Diabetes Health Center Slideshows
- Diabetes Health Center Quizzes
- Diabetes Blogs
- Diabetes Health Center Videos
- Diabetes Health Center Medications
- Find an Endocrinologist
- Book: Take Control of Your Diabetes Risk
- General Diabetes
- Type 1 Diabetes
- Type 2 Diabetes
- Prediabetes
- Blood Sugar Control
- Diabetic Neuropathy
- Eye Problems Assessment
- Heart Disease
- Metabolic Syndrome
- Diabetes Overview
- Diabetes Symptoms
- Diabetes Causes
- Diabetes Diagnosis
- Diabetes Treatment
- Open access
- Published: 08 July 2020
Current progress in stem cell therapy for type 1 diabetes mellitus
- Shuai Chen 1 ,
- Kechen Du 1 &
- Chunlin Zou ORCID: orcid.org/0000-0002-3308-5544 1
Stem Cell Research & Therapy volume 11 , Article number: 275 ( 2020 ) Cite this article
64k Accesses
78 Citations
28 Altmetric
Metrics details
Type 1 diabetes mellitus (T1DM) is the most common chronic autoimmune disease in young patients and is characterized by the loss of pancreatic β cells; as a result, the body becomes insulin deficient and hyperglycemic. Administration or injection of exogenous insulin cannot mimic the endogenous insulin secreted by a healthy pancreas. Pancreas and islet transplantation have emerged as promising treatments for reconstructing the normal regulation of blood glucose in T1DM patients. However, a critical shortage of pancreases and islets derived from human organ donors, complications associated with transplantations, high cost, and limited procedural availability remain bottlenecks in the widespread application of these strategies. Attempts have been directed to accommodate the increasing population of patients with T1DM. Stem cell therapy holds great potential for curing patients with T1DM. With the advent of research on stem cell therapy for various diseases, breakthroughs in stem cell-based therapy for T1DM have been reported. However, many unsolved issues need to be addressed before stem cell therapy will be clinically feasible for diabetic patients. In this review, we discuss the current research advances in strategies to obtain insulin-producing cells (IPCs) from different precursor cells and in stem cell-based therapies for diabetes.
Introduction
Diabetes mellitus (DM) is a group of chronic metabolic disorders characterized by hyperglycemia due to insufficient secretion of insulin or insulin resistance. DM is mainly divided into four categories: type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), gestational diabetes, and monogenic diabetes. Patients with T1DM need daily insulin injections because of the absolute insufficiency of endogenous insulin caused by autoimmune destruction of pancreatic β cells. Thus, type 1 diabetes is also known as insulin-dependent DM. Patients with type 2 diabetes may need exogenous insulin injections when oral medications cannot properly control the blood glucose levels. Diabetes without proper treatment can cause many complications. Acute complications include hypoglycemia, diabetic ketoacidosis, or hyperosmolar nonketotic coma (HHNC). Long-term complications include cardiovascular disease, diabetic nephropathy, and diabetic retinopathy [ 1 ]. Although hyperglycemia can be ameliorated by drugs or exogenous insulin administration, these treatments cannot provide physiological regulation of blood glucose. Therefore, the ideal treatment for diabetes should restore both insulin production and insulin secretion regulation by glucose in patients (Fig. 1 ).
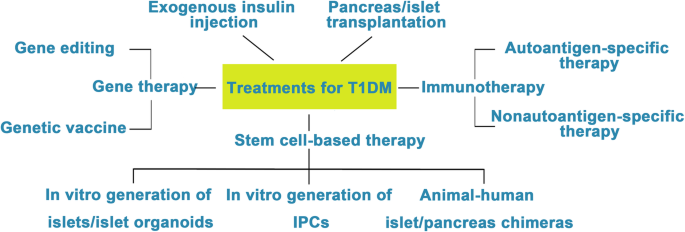
Attempts to cure T1DM. The discovery of insulin has enhanced the life span of T1DM patients, and successes in islet/pancreas transplantation have provided direct evidence for the feasibility of reestablishing β cells in vivo to treat T1DM. However, the restriction of a pancreas shortage has driven scientists to generate IPCs, and even whole pancreas, in vitro from hESCs, iPSCs, and adult stem cells. Studies focusing on the immune mechanism of T/B cell destruction in T1DM have made breakthroughs. Gene therapy has shown great promise as a potential therapeutic to treat T1DM, although its safety still needs to be confirmed in humans
Clinical pancreas or islet transplantation has been considered a feasible treatment option for T1DM patients with poor glycemic control. Dr. Richard Lillehei performed the first pancreas transplantation in 1966 [ 2 ]. Up until 2015, more than 50,000 patients (> 29,000 in the USA and > 19,000 elsewhere) worldwide had received pancreas transplantations according to the International Pancreas Transplant Registry (IPTR) [ 3 ]. Islet cell transplantation was first performed in 1974. However, efforts toward routine islet cell transplantation as a means for reversing type 1 diabetes have been hampered by limited islet availability and immune rejection. In 2000, Shapiro et al. reported that seven consecutive patients with type 1 diabetes attained sustained insulin independence after treatment with glucocorticoid-free immunosuppression combined with the infusion of adequate islet mass. Moreover, tight glycemic control and correction of glycated hemoglobin levels were observed in all seven patients. This treatment became known as the Edmonton protocol [ 4 ]. Over the past two decades, continuous improvements in islet isolation and immunosuppression have increased the efficiency of pancreatic islet transplant, and approximately 60% of patients with T1DM have achieved insulin independence 5 years after islet transplantation [ 3 , 5 , 6 , 7 , 8 ].
However, the worldwide shortage of pancreas donors in clinical islet transplantation remains a major challenge. Intensive studies have been conducted for the generation of IPCs or islet organoids in vitro since human pluripotent stem cells (hPSCs) have been anticipated for application in regenerative medicine. The sources for the generation of IPCs or islet organoids in vitro mainly include hPSCs (human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs)), adult stem cells, and differentiated cells from mature tissues that can be transdifferentiated into IPCs. Current strategies for generating IPCs are mainly based on approaches that mimic normal pancreas development. The obtained IPCs are supposed to express specific biological markers of normal β cells that identify a terminal differentiation status, such as MAFA (a basic leucine zipper transcription factor expressed in mature β cells and absent in pancreatic progenitors and other cell types), NEUROD1 (downstream factor of NGN3 expressed in most pancreatic endocrine cells, including β cells), and PDX1/NKX 6.1 (restricted coexpression in β cells), as well as key functional features of adult β cells, including glucose-stimulated insulin secretion (GSIS) and C-peptide secretion [ 9 , 10 , 11 , 12 , 13 , 14 ]. In addition, after implantation into DM patients or immunodeficient diabetic animals, these in vitro-generated IPCs or islet organoids should respond to changing blood glucose and produce sufficient insulin and finally reverse hyperglycemia.
In the last two decades, many protocols have been successfully designed for the generation of IPCs or islet organoids in vitro. In this review, we summarized the research progress in the generation of IPCs and islet organoids from hPSCs and adult stem cells and the new technological advances in stem cell-based therapy for T1DM.
Generating IPCs from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs)
ESCs are pluripotent cells isolated from the inner cell mass of a blastocyst, the early mammalian embryo that implants into the uterus. ESCs show the characteristics of infinite proliferative capacity and self-renewal and are able to differentiate into multiple types of adult cells in vitro [ 15 ]. iPSCs, which are reprogrammed from somatic cells, hold a similar capacity to proliferate and differentiate like ESCs. Hence, hPSCs provide a promising platform to produce in vitro insulin-secreting cells. Ethical issues in the applications of ESCs are still controversial due to their origins. In contrast, iPSCs are derived from adult somatic cells that have been reprogrammed back into an embryonic-like pluripotent state using Yamanaka factors [ 16 , 17 ]. During the last two decades, numerous methods to generate IPCs from hPSCs have been reported [ 9 , 10 , 11 , 12 , 18 , 19 , 20 , 21 , 22 ].
Ordinarily, the schemes for the generation of functional IPCs from hPSCs were based on imitating the in vivo development of the embryonic pancreas (Fig. 2 ). The pivotal stages of embryonic pancreas development include the development of the definitive endoderm (DE), primitive gut tube (PGT), pancreatic progenitor (PP), endocrine progenitor (EP), and hormone-expressing endocrine cells. By adding diverse cytokines (e.g., epidermal growth factor, bFGF) and signaling modulators (e.g., bone morphogenetic proteins, γ-secretase inhibitors) to each stage to activate or inhibit specific signaling pathways (e.g., Notch, Wnt) involved in the generation of adult β cells, the hPSC cell fate is manipulated into the β cell phenotype [ 18 , 20 , 23 ].
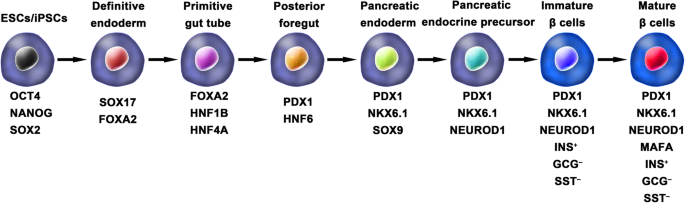
Generation of insulin-producing β cells from hPSCs. Schematic illustration of the differentiation protocol for generating insulin-producing β cells from hPSCs by mimicking the in vivo development of the embryonic pancreas. The key molecules of all key developmental stages of pancreatic islet β cells are illustrated
D’Amour et al. set up the first stepwise protocol to produce endocrine hormone-expressing cells that were able to synthesize and release multiple hormones from hESCs. However, at the final stage, the average percentage of insulin-positive cells in differentiated hES cell cultures was only 7.3%. Furthermore, these polyhormonal cells failed to respond to a high-glucose stimulus [ 18 ]. It is known that the fetal pancreas also possesses these characteristics, and previous studies demonstrated that fetal human pancreatic tissues could develop functionally after transplantation into animals [ 24 , 25 , 26 , 27 ]. Thus, the authors chose to determine whether these immature β cells derived from hESCs could mature into functional β cells under an in vivo environment. They generated pancreatic endoderm cells (similar to fetal 6- to 9-week pancreatic tissue) using an optimized protocol and then transplanted them into immunodeficient mice. The pancreatic endoderm cells successfully differentiated and matured into β-like cells in response to both fasting-induced hypoglycemia and glucose challenge and maintained normal glucose homeostasis for 3 months [ 28 ].
Similarly, the generation of IPCs from iPSCs is based on consecutive regulation of specific signaling pathways involved in pancreas development. Tateishi et al. first demonstrated that skin fibroblast-derived iPSCs were capable of producing islet-like clusters (ILCs) in vitro by mimicking the in vivo development of the pancreas. However, under high glucose stimulation (40 mM), the amount of C-peptide secreted by iPSC-derived ILCs and ESC-derived ILCs was only 0.3 ng/μg DNA and 0.15 ng/μg DNA, respectively [ 29 ].
Although the above studies have confirmed that hESCs and hiPSCs have the potential to differentiate into IPCs, this differentiation is done only cautiously owing to the low differentiation efficiency of protocols and the polyhormonal features of these β-like cells.
One of the breakthroughs comes from Rezania et al. in 2014, and the authors reported a more detailed protocol and generated mature and functional IPCs from hPSCs that were comparable to human β cells. The differentiation protocol was divided into 7 sequential stages, including definitive endoderm (stage 1), primitive gut hub (stage 2), posterior foregut (stage 3), pancreatic endoderm (stage 4), pancreatic endocrine precursors (stage 5), immature β cells (stage 6), and maturing β cells (stage 7). The obtained cells expressed key markers of mature β cells, such as MAFA, PDX1/NKX6.1, and INS, and showed functional similarities to human islets after transplantation in vivo. These β-like cells rapidly reversed hyperglycemia in STZ-diabetic mice by secreting C-peptide and insulin [ 20 ]. Nevertheless, the S7 (stage 7) cells were not equivalent to mature human β cells. S7 cells exhibited a very small and blunt response to high glucose stimulation, which differs from that of mature islet β cells. Moreover, a scalable suspension-based culture system developed by Paliuca et al. showed the possibility of generating large-scale stem cell-derived β cells (SC-β) [ 9 ]. Expression of NGN3 marks the initiation of endocrine differentiation. Previous studies have confirmed that inhibition of the Notch signaling pathway using γ secretase inhibitors or BMP inhibitors is essential for the induction of NGN3, followed by the addition of fibroblast growth factor 10 and keratinocyte growth factor (KGF), resulting in the robust generation of PDX1 + pancreatic progenitors and an increase in insulin expression in hPSC-derived progeny [ 9 , 20 ]. However, Russ et al. demonstrated that the use of BMP inhibitors promoted the precocious induction of endocrine differentiation in PDX1 + pancreatic progenitors and that omitting addition at pancreatic specification could successfully reduce the formation of polyhormonal cells. Subsequent exposure to retinoic acid and epidermal growth factors (EGF)/KGF cocktail efficiently induced the formation of PDX1 + /NKX6.1 + progenitor cells that differentiated into IPCs in vitro [ 10 ]. Recently, Yabe et al. reported that the addition of the selective glycogen synthase-kinase-3 β (GSK-3β) inhibitor (a substitute for Wnt3a; regarded as a key molecule for definitive endodermal induction from hPSCs) during definitive endodermal induction significantly decreased the death rate of endodermal cells [ 12 , 18 , 30 ]; further, spheroid formation of postendocrine progenitor cells rather than monolayer formation was crucial for generating IPCs from hiPSCs, which may be explained by the unique architecture of adult islets.
Among the above studies, the obtained cell population contains an average of 45% β cells, and the phenotypes of the remaining cells were unclarified. Identification of cell types that formed during differentiation is particularly important to improve the differentiated proportion of β cells. In a recent study, single-cell RNA sequencing in hPSCs undergoing in vitro β cell differentiation mapped a comprehensive description of cell production during stem-to-β cell differentiation [ 31 ]. Four distinct cell populations were isolated and identified from stem cell-derived islets, including SC-β cells, α-like polyhormonal cells, nonendocrine cells, and stem cell-derived enterochromaffin (SC-EC) cells. An in vitro study confirmed that α-like polyhormonal cells were transient toward SC-α cells and that nonendocrine cells were capable of generating exocrine cells (pancreatic acinar, mesenchymal and ductal cells). Additionally, CD49a was characterized as a surface marker of SC-β cells but not of adult islet β cells. Furthermore, SC-β cells could be purified up to 80% from SC islets using a scalable reaggregation method and magnetic sorting.
As patient-derived hiPSCs have been shown to provide tremendous advantages for studying the pathogenesis and pathophysiology of disease in vitro, studies on producing iPSCs from diabetic patients have generated great interest. Patient-specific iPSCs can overcome current obstacles in stem cell therapy, such as immune rejection and immune mismatch, and provide a platform to establish a personalized disease model to investigate pathogenic mechanisms and seek therapeutic methods for the disease. Maehr et al. successfully generated hiPSCs from skin fibroblasts of patients with T1DM (T1DM-specific iPSCs, DiPSCs). These DiPSCs resembled ESCs in the global gene expression profile and were capable of differentiating into pancreatic cell lineages, paving the path of generating T1DM SC-β cells and making autologous stem cell-derived pancreatic progeny transplantation for T1DM possible [ 32 ]. In 2015, Millman et al. confirmed that SC-β cells derived from DiPSCs functionally resembled adult islet β cells both in vivo and in vitro. GSIS tests showed that under high glucose stimulation (20 mM incubation for 30 min), T1DM and nondiabetic (ND) SC-β cells secreted 2.0 ± 0.4 and 1.9 ± 0.3 mIU of human insulin per 10 3 cells, respectively, and both of these cells functioned similarly to adult primary islets in a previous study. After transplantation into ND immunodeficient mice, the engraft function was evaluated by serum human insulin before and 30 min after an injection of glucose. At the early time point (2 weeks after transplantation), most engrafts responded to glucose and released more insulin after glucose injection, and the ratio of insulin secretion after glucose stimulation averaged 1.4 and 1.5 for T1DM and ND SC-β cells, respectively. The effects of these engrafts on insulin secretion were observed for several months. Of note, compared to the early time point, after 12–16 weeks, the human insulin content increased approximately 1.5 times after glucose stimulation [ 33 ]. It should be acknowledged that diversities exist among T1DM patients, and a larger number of specific stem cell lines from T1DM need to be developed for future clinical use. Although DiPSCs are an alternative source for cell replacement therapy for diabetes, some T1DM-specific stem cell lines have shown low efficiency in generating PDX1 + pancreatic progenitors [ 34 ]. Evaluated by flow cytometry, the number of IPCs derived from ND iPSCs (25–50.5%) was comparable to that of the β cells found in human primary islets, whereas the number of IPCs differentiated from T1DM iPSC lines was much lower (15.9%) [ 35 , 36 ]. Upon a strict differentiation protocol, pancreatic progenitors derived from T1DM iPSCs showed lower expression of PDX1 than ND iPSCs at a specific differentiation stage. Epigenetic changes resulting from dysmetabolism in T1DM might be responsible for the poor yield of β cells from T1DM iPSCs. Transient demethylation treatment of DE cells rescued the expression of PDX1 by inhibiting methyl group deposition on the cytosine residues of DNA and led to the differentiation of DE cells into IPCs [ 36 ]. The effect of demethylation on IPC differentiation has been shown to promote pancreatic progenitor induction rather than DE induction [ 37 ].
Generating pancreatic progenitors from ESCs and iPSCs
Pancreatic progenitors that coexpress specific markers indispensable for inducing a β-cell fate are a crucial cell state of differentiating hPSCs into β cells in vitro. Pancreatic and duodenal homeobox 1 (PDX1) transcription factor and NK6 homeobox transcription factor-related locus 1 (NKX6.1) have been considered to be the regulatory factors of differentiating DE into pancreatic progenitors [ 38 ]. Notably, high coexpression of PDX1 and NKX6.1 in pancreatic progenitors is essential for the efficient generation of mature and functional β cells [ 39 , 40 ].
Of note, the efficiency and safety of pancreatic progenitors that coexpress PDX1 and NKX6.1 for T1DM treatment are currently being evaluated in clinical trials by ViaCyte Company. Thus, elevating the production of hPSC-derived β cells, optimizing the in vitro differentiation protocols in multiple aspects, and generating a high population of PDX1 + /NKX6.1 + pancreatic progenitors are needed to accelerate the clinical trial. Multiple studies have been carried out to determine the appropriate cocktail of cytokines to mimic in vivo development [ 41 , 42 , 43 ]. Recently, Nostro et al. demonstrated that the combination of EGF and nicotinamide induced a higher production of NKX6.1 + pancreatic progenitors in adherent culture [ 44 ]. Importantly, the authors focused on the temporal window of foregut differentiation into the pancreatic endoderm and confirmed that the size of the NKX6.1 + population decreased with extended duration. Although previous studies have shown that the maintenance of cellular aggregation during the differentiation process could significantly elevate the efficiency of pancreatic progenitors [ 10 , 45 , 46 ], the impact of culture condition changes that affect the physical environment of cells on pancreatic progenitor differentiation is still less studied. Memon et al. showed that the generation of PDX1 + /NKX6.1 + pancreatic progenitors could be dramatically induced after dissociating and replating pancreatic endodermal cells at half density in monolayer culture [ 47 ]. Intriguingly, a novel NKX6.1 + /PDX1 − cell population that holds the potential to generate functional β cells was discovered, and the cell type was confirmed to be a new type of pancreatic progenitor cell by the same team [ 48 ].
Another important issue that needs to be resolved before hPSC-derived pancreatic progenitors can be used in the clinic is how the recipient’s in vivo environment affects the maturation and differentiation of these undifferentiated cells. Although many studies have highlighted the importance of the in vivo environment in promoting islet cell differentiation, the system mechanism regulating the response of the transplanted cells to the in vivo environment has not been well studied [ 9 , 20 , 21 ]. Most recently, Legøy et al. confirmed that short-term exposure of encapsulated pancreatic progenitors to an in vivo environment was beneficial for cell fate determination, as revealed by increased islet proteome characteristics [ 49 ]. These effects could be partially mediated by the levels of hepatocyte nuclear factor 1-α (HNF1A) and hepatocyte nuclear factor 4-α (HNF4A) in recipients.
Generating islet organoids/islets from ESCs and iPSCs
The pancreatic islet of Langerhans is comprised of α, β, δ, ε, and pancreatic polypeptide cells [ 46 , 50 ]. Many studies have highlighted the importance of reciprocal coordination and complementary interactions of different types of islet cells for glucose hemostasis [ 51 , 52 , 53 , 54 ]. Thus, it may be beneficial for producing whole islets or islet organoids rather than differentiating cells into a specific type.
Organoids are defined as 3D cultures maintained in vitro that can be generated from adult tissues or hPSCs and recapitulate the in vivo morphologies, cellular architecture and organ-specific functionality of the original tissue. Kim et al. developed islet-like organoids from hPSCs that showed a glucose response in vitro and in vivo [ 55 ]. Endocrine cells (ECs) were generated from hPSCs using a multistep protocol and expressed pancreatic hormones. Notably, dissociated ECs spontaneously formed islet-like spheroids, referred to as endocrine cell clusters (ECCs), under optimal 3D culture conditions in 24 h. The diameter of the ECCs was approximately 50–150 μm and contained 5 × 10 4 cells. ECCs consisted of several types of islet endocrine cells, apart from α cells, indicating that ECCs derived from hPSCs are partially similar to human adult islets. After high glucose stimulation (27.5 mM) for 1 h, ECCs showed increases in both insulin and C-peptide secretion, from 1.01 ± 0.22% up to 2.6 ± 0.21% and from 159.6 ± 20.01 pmol/L up to 336.3 ± 29.21 pmol/L, respectively. Additionally, ECCs exhibited intracellular Ca 2+ oscillation under a high glucose stimulus. Furthermore, a major breakthrough was that after ECCs were implanted into STZ-induced diabetic mice, normoglycemia was rapidly achieved within 3 days. In previous studies, transplanted hPSC-derived ECs took a long period (over 40 days) to normalize the glucose level in diabetic mice [ 9 , 10 , 20 , 28 ]. Therefore, this study suggested that it was promising to generate functional islet-like organoids from hPSCs and provided an alternative cell source for treating diabetes. Soon after that, based on a biomimetic 3D scaffold, islet organoids were successfully generated from hESCs [ 56 ]. The organoids contained all types of pancreatic cells (α, β, δ, and pancreatic polypeptide cells), specific markers of mature β cells as well as insulin secretory granules, which were characterized by a round electron-dense crystalline core surrounded by a distinctive large, clear halo. Insulin granules have been reported as an indication of mature β cells and a key participant in glucose homeostasis [ 36 , 57 ]. Generally, insulin granules in adult β cells were differentiated according to the shape and density of the core. Through transmission electron microscopy, insulin granules generally possess a characteristic “halo,” which is a product of glutaraldehyde fixation that does not exist in other endocrine granules. Many studies have reported remarkable insulin granules during the differentiation of hPSCs into IPCs [ 9 , 20 ]. Glucose loading experiments demonstrated that islet organoids exhibited a sharp increase in insulin secretion under high glucose conditions. Under the same glucose stimulation conditions (exposure from 5.5 mM to 25 mM), the 3D-induced cells had an insulin content that increased by seven-fold, whereas the 2D-induced cells had an insulin content that increased by 3.7-fold. These results suggested that 3D-induced IPCs are more sensitive to glucose stimulation due to their elevated maturity.
Fundamental studies of islet development during embryogenesis will promote optimization of protocols for differentiating hPSCs into 3D islet clusters or islet organoids. The traditional model of islet development is based on epithelial-mesenchymal transition (EMT) during the differentiation of pancreatic progenitors. However, this hypothesis was recently challenged by a study in which the dynamic changes in transcripts involved in islet formation were mapped [ 46 ]. Sharon et al. reported that along with EP differentiation, they maintained intact cell-to-cell adhesion and formed bud-like islet precursors (defined as peninsula-like structures) rather than undergoing EMT. Further in vitro generation of SC-β cells showed that the maintenance of cell adhesion could efficiently induce hESCs into peninsula-like structures. Importantly, these peninsula-like clusters could generate INS + and GCG + monohormonal cells after transplantation into SCID mice. This study provides a new framework for understanding islet embryogenesis and offers novel ideas to optimize the current protocols for the differentiation of SC-β cells.
Generating interspecific pancreatic chimeras from pancreatic stem cells (PSCs)
Interspecific chimeras, defined as organisms with cells originating from at least two different species, are able to produce organs completely consisting of donor-origin cells. Thus, human-animal chimeras have great potential for providing immune-compatible patient-specific human organs for transplantation.
In 2010, Kobayashi et al. successfully generated a functional rat pancreas in PDX1 −/− (pancreatogenesis knockout) mice via interspecies blastocyst complementation [ 58 ]. The rat iPSC-derived pancreas (rat M pancreas) in PDX1 −/− mice showed both exocrine and endocrine characteristics and expressed several pancreatic enzymes and hormones. In addition, outcomes from glucose tolerance testing (GTT) in adulthood indicated that endogenous insulin secretion was increased under high blood glucose, and glucose homeostasis was preserved. Recently, the same group reported the reverse experiment; mouse PSCs were injected into PDX1 −/− rat blastocysts to generate a pancreas (mouse R pancreas) the size of a rat pancreas with pancreatic cells primarily originating from mouse PSCs [ 59 ]. Most importantly, the isolated islets from the mouse R pancreas were subsequently injected into STZ-induced diabetic mice, and functional glucose-induced insulin secretion was successfully established in recipients for over 1 year. These data strongly supported the hypothesis that donor PSC-derived organs could be generated in a xenogeneic environment and provided the theoretical possibility of applying donor PSC-derived islets generated by animal-human interspecific blastocyst complementation in clinical trials. It is worth noting that rat M pancreases were the size of a rat pancreas, rather than the size of a mouse pancreas or an intermediate size, whereas mouse R pancreases were the size of a mouse pancreas. Thus, to adapt interspecific blastocyst complementation for patients, it seems necessary to generate organs in animals that are closer to humans in both size and evolutionary distance, such as sheep, pigs, and nonhuman primates (NHPs). Exogenic pancreases have been generated in vivo in transgenic cloned pigs by blastocyst complementation [ 60 ]. In this study, donor morula blastomeres derived from female cloned embryos were injected into the morula of male pancreatogenesis-disabled fetuses, and morphologically and functionally normal donor-derived pancreases were formed in adult chimeric pigs. Furthermore, PDX1 −/− sheep generated using CRISPR/Cas9 have been reported and can potentially serve as a host for interspecies organ generation [ 61 ]. However, blastocyst complementation has failed to generate chimeras in NHPs [ 62 ].
Differentiation of adult stem cells into IPCs
The search for adult pancreatic stem cells.
The adult pancreas consists of two unique parts: the exocrine pancreas and the endocrine pancreas, with unique morphology and function, respectively. The pancreas arises from two separate primordia along the dorsal and ventral surfaces of the posterior foregut. Lineage-tracing studies have demonstrated that all of the mature pancreatic cells were developed from PDX1 + /PTF1A + progenitor cells [ 63 , 64 ]. However, if there are detectable pancreatic stem cells in adult animal and human pancreases, how these cells participate in the regeneration of β cells is still under debate. The hypothesis was initially supported by histological observation of neogenesis occurring in adult rodent pancreatic ducts after pancreatic duct ligation (PDL) [ 65 ]. However, genetic lineage-tracing studies indicated that there was no contribution to endocrine regeneration during the adult life or after injury, and the major mechanism was enhanced replication by only preexisting β cells [ 63 , 66 , 67 ]. In 2007, supporting evidence comes from a study by Xu et al., in which NGN3 + (the earliest islet cell-specific transcription factor) endocrine precursors appeared in the ductal lining after PDL in mice and gave rise to all types of islet cells, including glucose-responsive β cells [ 68 ]. Additionally, increased proliferation and ectopic NGN3 + pancreatic progenitors were reported in experiments of α-to-β-cell reprogramming [ 69 , 70 ]. In conclusion, whether adult pancreatic stem cells exist in adulthood is unclear. Recent events in single-cell RNA sequencing are promising for mapping dynamic gene expression changes during the adult lifespan or after injury in animal and human pancreases, for constructing differentiation trajectories of pancreas/islet cells and for illustrating the mechanisms involved in β cell regeneration.
Pancreatic duct-derived stem cells
Theoretically, pancreatic duct epithelial cells possess a promising capacity for β cell generation because both originate from the same embryonic precursor [ 46 , 71 ]. Budding of β cells or new islets generated from ductal epithelium occurs during pancreatic regeneration in adults and has been reported [ 72 , 73 ]. Since then, studies have been designed to reprogram pancreatic ductal cells into β cells. Ramiya et al. isolated pancreatic ductal epithelial cells from prediabetic adult nonobese diabetic (NOD) mice, cultured them in vitro, and ensued the formation of ILCs that contained α, β, and δ cells. Subsequently, the blood glucose level of diabetic NOD mice was decreased from 400 to 180–220 mg/dl in 7 days [ 74 ]. Moreover, Bonner-Weir et al. demonstrated that the pancreatic ductal epithelium could expand and further differentiate into functional islet tissues in a Matrigel-based 3D culture system in vitro [ 75 ]. Further studies demonstrated that CK19 + nonendocrine pancreatic epithelial cells (NEPECs) can be differentiated into β cells in vitro [ 76 ].
Over the past two decades, attempts have been directed toward optimizing the protocols for generating IPCs from pancreas duct-derived stem cells. Since CA19-9 and CD133 were identified as specific membrane proteins of pancreas duct-derived stem cells, it became easier to purify these cells from the adult human pancreas [ 77 , 78 ]. It has been demonstrated that diverse growth factors (e.g., bFGF, EGF, and KGF) benefit the proliferation and differentiation of human pancreatic duct-derived stem cells [ 74 , 79 ]. Generally, epithelial cells show limited mitotic activity in vitro. Corritore et al. developed a differentiation protocol in which isolated human pancreatic duct cells from the pancreas were forced to undergo EMT to achieve a phenotypic change and allow them to extensively proliferate. After proliferation of these cells in vitro, pancreatic duct-derived cells differentiated into IPCs with a large array of specific marker expression and insulin secretion [ 78 ]. More recently, Zhang et al. reported that diabetic mice continuously administered gastrin and EGFs had accelerated transdifferentiation of SOX9 + duct cells into IPCs and consequently maintained blood glucose homeostasis [ 80 ].
Nestin-positive mesenchymal stem cells from islets
Nestin is an intermediate filament protein that is specifically expressed in neuronal and muscle precursor cells [ 81 , 82 ]. Recent studies have indicated that nestin-positive (nestin + ) cells resided in pancreatic islets and could differentiate into IPCs and islet-like cell clusters (Fig. 3 ), and now, nestin has been accepted as a critical pancreatic progenitor marker [ 83 , 84 ]. Zulewski et al. first demonstrated the existence of a distinct cell population within islets isolated from the human pancreas that express nestin, termed nestin-positive islet-derived progenitor cells (NIPs). These NIPs displayed features of stem cells and were able to generate cells with either pancreatic exocrine or endocrine phenotypes in vitro. Most importantly, the terminally differentiated cells were capable of secreting pancreatic hormones, such as insulin and glucagon [ 85 ]. Another study performed by the same group reported that NIPs also showed characteristics of bone marrow side population (SP) stem cells due to their coexpression of the ATP-binding cassette transporter ABCG2, which has been previously demonstrated to be a major component of the SP phenotype [ 85 , 86 , 87 ]. This was further supported by a study showing that NIPs isolated from a human fetal pancreas expressed ABCG2 and nestin [ 88 ]. Moreover, CD44, CD90, and CD147, which represent the phenotypes of bone marrow-derived mesenchymal stem cells, were also detected on NIPs. These data strongly indicated that NIPs have a high potential to become an alternative cell source for producing IPCs and islets in vitro. Huang et al. isolated and cultured NIPs from a human fetal pancreas. In this study, NIPs formed islet-like cell clusters (ICCs) in confluent cultures. Moreover, differentiation of ICCs from NIPs results in increased pancreatic islet-specific gene expression, along with a concomitant downregulation of ABCG2 and nestin. Additionally, the transplantation of ICCs reversed hyperglycemia in diabetic NOD-SCID mice [ 89 ].
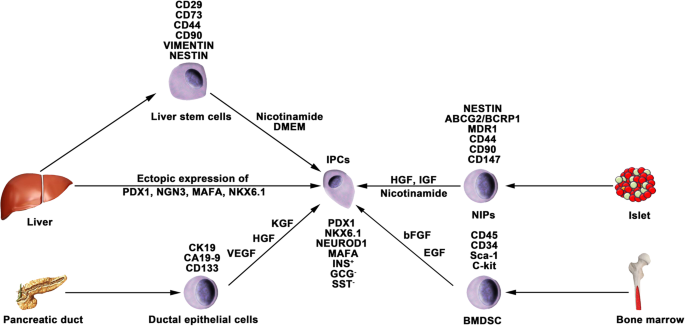
Generation of IPCs from adult stem cells. Adult pancreatic stem cells may be a potential source of IPCs. Functional IPCs have been generated from pancreatic ductal cells and NIPs isolated from adult islets. During embryogenesis, the liver and pancreas arise from common endoderm progenitors. Liver cells can transdifferentiate into IPCs by ectopic expression of pancreatic transcription factors. Additionally, a high pluripotent cell population termed HLSCs can also produce IPCs in vitro. Bone marrow-derived stem cells show the capacity to generate insulin cell clusters
The studies mentioned above about NIPs are based on rodent models. Nonhuman primate models often serve as an important bridge from laboratory research to clinical application; thus, generating pancreatic stem cells/progenitor cells from NHPs has led to great interest. Our previous study indicated that pancreatic progenitor cells existed in the adult pancreases of type 1 diabetic monkeys as well as in the pancreases of normal monkeys. The isolated pancreatic progenitor cells were able to proliferate in vitro and form ICCs in differentiation media. Furthermore, glucose-induced insulin and C-peptide secretion from the ICCs suggested that the ICCs functionally resembled primary islets [ 90 ]. In view of pathogenetic differences between STZ-induced diabetic monkeys and patients with T1DM, it still needs to be clarified whether NIPs also reside in T1DM patients.
Differentiation of bone marrow-derived stem cells (BMDSCs)
Several studies have reported that BMDSCs have the ability to differentiate into IPCs. Tang et al. reported that BMDSCs could spontaneously differentiate and form ICCs when continuously cultured with high glucose concentrations. The ICCs expressed multiple pancreatic lineage genes, including INS, GLUT2, glucose kinase, islet amyloid polypeptide, nestin, PDX-1, and PAX6, with β cell development. Moreover, ICCs could respond to glucose stimulation and release insulin and C-peptide in vitro, and following implantation into diabetic mice, hyperglycemia was reversed [ 91 ]. Since then, numerous studies have demonstrated the generation of IPCs from human and rat bone marrow stem cells (Fig. 3 ). However, the efficacy of BMDSC differentiation is low and highly variable with the current protocols. In particular, the quantity of insulin secreted by these cells was far from that secreted by adult β cells. Gabr and colleagues tested the efficiency of three differentiation protocols using immunolabeling, and the proportion of generated IPCs was modest (≈ 3%) in all protocols [ 92 ]. The expression of pancreatic-associated genes in generated IPCs was quite low compared to the expression in human islets. Optimizing differentiation protocols to upregulate the expression of specific genes by determining optimal molecules and culture conditions is crucial. Extracellular matrix proteins play a vital role in cell differentiation and proliferation. Laminin, one of the pancreatic extracellular matrices, has been confirmed to enhance the expression of insulin and promote the formation of ICCs from BMDSCs, whereas collagen type IV affects the expression of NEUROD1 and GCG [ 93 ]. Generally, differentiation of BMDSCs into IPCs is performed on nonadherent polymer surfaces and hydrogels. A recent study reported that 3D culture of BMDSCs on agar (a hydrogel-forming polysaccharide widely used in biomedical research) for 7 days followed by 2D culture of formed cellular clusters in high glucose media could enhance the production of IPCs from BMDSCs [ 94 ]. IPCs expressed INS genes at a 2215.3 ± 120.8-fold higher level than BMDSCs, whereas this fold change in previous studies was 1.2–2000-fold.
Differentiation of liver cells
The liver and pancreas originate from appendages of the upper primitive foregut endoderm. Later, separation of the liver and pancreas during organogenesis left both tissues with multipotent cells capable of generating both hepatic and pancreatic cell lineages. The common embryonic origin of the liver and pancreas raises the intriguing speculation that it may be possible to convert liver cells to pancreatic ECs (Fig. 3 ). Several studies have demonstrated that adult or fetal liver cells and biliary epithelial cells are capable of reprogramming into IPCs by inducing the expression of endocrine pancreatic-specific transcription factors [ 95 , 96 , 97 , 98 ]. The in vivo data showed that these hepatic cell-derived IPCs could ameliorate hyperglycemia upon implantation into diabetic mice. However, the efficiency of liver-to-pancreas reprogramming is still low, and the obtained IPCs are likely immature β-like cells. In addition, Herrera et al. isolated and characterized a population of human liver stem cells (HLSCs). HLSCs express both mesenchymal stromal cells (MSCs) and immature hepatocyte markers. In addition, HLSCs expressing nestin and vimentin are capable of differentiating into multiple cell lineages, including epithelial, endothelial, osteogenic, and islet-like structure (ILS) cells [ 99 ]. Later, Navarro-Tableros et al. confirmed that HLS-ILS cells expressed β cell transcription factors, such as NKX6.1, NKX6.3, and MAFA, and could respond to glucose loading by releasing C-peptide. Hyperglycemia was rapidly reversed in diabetic SCID mice after implantation [ 100 ]. These data suggest that HLSCs could be a novel potential resource for stem cell-based therapy for diabetes.
Encapsulation technique for stem cell therapy for T1DM
The encapsulation technique is based on a matrix that prevents immune cells, cytokines, and antibodies from reacting to grafts while allowing nutrient, oxygen, and signaling molecule diffusion. An appropriate encapsulation device is especially crucial for T1DM to prevent an autoimmune reaction against transplanted hPSC-derived pancreatic progeny, including allogenic grafts. Criteria to evaluate an encapsulation device should take many variables into consideration, including the biocompatibility, stability and permselectivity of the membrane, interaction with the bloodstream, availability of nutrients and oxygen, among others [ 101 , 102 , 103 ]. Studies have been performed to detect optimal materials to improve these properties and have mainly been developed for pancreatic islet transplantation.
Alginate, a scaffolding polysaccharide produced by brown seaweeds, has been widely employed by virtue of its biocompatibility [ 102 , 104 , 105 ]. Alginates are linear unbranched polymers containing β-(1 → 4)-linked d -mannuronic acid (M) and α-(1 → 4)-linked l -guluronic acid (G) residues and possess eminent gel-forming properties in the presence of polyvalent cations, such as Ca 2+ and Ba 2+ [ 103 , 106 , 107 , 108 ]. Earlier studies have confirmed that compared to nonencapsulated islets, encapsulated islets have significantly improved survival, long-term biocompatibility and function with the use of purified alginate [ 109 , 110 , 111 , 112 ]. Additionally, specific modifications to alginates trigger great interest, as they could circumvent the local immune response after transplantation of an allo- or xenograft. The incorporation of the chemokine CXCL2 with alginate microcapsules prevented allo- or xenoislet transplantation from immune reactions by establishing sustained local immune isolation [ 113 ]. Most recently, the same team confirmed that these modifications on alginates could also efficiently prolong the survival and function of hPSC-derived β cells and achieve long-term immunoprotection in immunocompetent mice with T1DM without systemic immunosuppression [ 114 ]. Of note, CXCL2 enhanced the GSIS activity of β cells, thus making it a crucial biomaterial to study for stem cell-based therapy for T1DM.
ViaCyte, leading the first and only islet cell replacement therapies derived from stem cells for diabetes, is testing for the safety and efficacy of its encapsulation devices PEC-Encap and PEC-Direct in clinical trials. The PEC-Encap is designed to fully contain hPSC-derived pancreatic progenitors in a semipermeable pouch so that vital nutrients and proteins can travel between the cells inside the device and the blood vessels, which grow along the outside of the device. In the case of PEC-Encap, the implanted cells were completely segregated from the recipients’ immune system. Another device called PEC-Direct allowed blood vessels to enter the device and directly interact with the implanted cells. Thus, immune suppression therapy was necessary for patients who received PEC-Direct, which made it suitable only for people with high-risk type 1 diabetes.
Immune modulation in stem cell therapy for T1DM
Human ESC/iPS-derived β cells have been proposed as a potential β cell replacement source for the treatment of T1DM. However, both the alloimmune and autoimmune responses remain a major problem for the wide application of cell replacement therapies for T1DM. Although massive efforts have been made in the progress of encapsulation technology, the engraftment of transplanted hPSC-derived pancreatic progenitors or β cells still faces challenges. The engraftments will certainly be destroyed by the recipient’s immune system if the encapsulation system is eliminated. Certain modulations of these encapsulated cells to circumvent autoimmune attack seem promising. Human leukocyte antigen (HLA) mismatching is the major molecular mechanism of immune rejection in allo- or xenografts [ 115 ]. Studies have proven that elimination of HLA-A genes by zinc-finger nucleases in hematopoietic stem cells could increase donor compatibility [ 116 , 117 ]. Likewise, knocking out the β2-microglobulin (B2M) gene, which abolishes all HLA class I molecules, or deleting HLA-A and HLA-B biallelically, retained one allele of HLA-C to allow the hPSC grafts to avoid T and NK cell attack [ 118 ]. Other protocols for immunosuppressive effects have been reported, such as targeted overexpression of PDL1-CTLA4Ig in β cells, which efficiently prevented the development of T1DM and allo-islet rejection, in turn promoting the survival of β cell mass [ 119 ]. Therefore, immune modulation strategies for hPSCs could be promising to overcome challenges associated with engraft rejection.
Clinical trials in stem cell therapy for T1DM
In the last few years, controlled clinical trials have been carried out to estimate the efficiency and safety of stem cell therapy for T1DM. It has been demonstrated that MSCs can ameliorate or reverse the manifestation of diabetes in animal models of T1DM. In 2014, Carlsson et al. confirmed that MSC treatment could preserve β cell functions in new-onset T1DM patients. Twenty adult patients (aged 18–40 years) with newly diagnosed (< 3 weeks) T1DM were enrolled and randomized to MSC treatment or to the control group and followed by a 1-year follow-up examination [ 120 ]. At the end of the clinical trial, mixed-meal tolerance tests (MMTTs) revealed that both C-peptide peak values and C-peptide significantly decreased in the treatment group. Of note, MSC treatment side effects were not observed during the follow-up examination. During January 2009 and December 2010, 42 patients aged 18–40 years with a history of T1DM for ≥ 2 years and ≤ 16 years were randomized into either the stem cell transplantation (umbilical cord MSCs in combination with autologous bone marrow mononuclear cells) or standard insulin care treatment groups [ 121 ]. A 1-year follow-up examination indicated that the C-peptide increased from 6.6 to 13.6 pmol/mL/180 min in treated patients, whereas it decreased from 8.4 to 7.7 pmol/mL/180 min in control groups; insulin increased from 1477.8 to 2205.5 mmol/mL/180 min in treated patients; and it decreased from 1517.7 to 1431.7 mmol/mL/180 min in control patients. Additionally, HbA 1c and fasting glycemia decreased in the treated groups and increased in the control subjects. Daily insulin requirements in the treated groups also decreased compared to those of the control groups. During the follow-up period, severe hypoglycemic events reported by patients were significantly decreased. Limitations of these studies could be a small sample size and the short follow-up period. Moreover, the treated patients did not achieve complete insulin independence. Even so, these results help to improve clinical trial outcomes in future large-scale trials.
Conclusions and perspectives
Stem cell-based therapy has been considered a promising potential therapeutic method for diabetes treatment, especially for T1DM. As mentioned in this review, major advances in research on the derivation of IPCs from hPSCs have improved our chance of reestablishing glucose-responsive insulin secretion in patients with T1DM. However, the clinical trial results of stem cell therapies for T1DM are still dissatisfactory [ 122 ], and many questions and technical hurdles still need to be solved. The major problems include the following four aspects: (1) how to generate more mature functional β-like cells in vitro from hPSCs; (2) how to improve the differentiation efficiency of IPCs from hPSCs; (3) how to protect implanted IPCs from autoimmune attack; (4) how to generate sufficient numbers of desired cell types for clinical transplantation; and (5) how to establish thorough insulin independence. Despite these obstacles, the application of stem cell-based therapy for T1DM represents the most advanced approach for curing type 1 diabetes.
Availability of data and materials
Not applicable.
Abbreviations
- Type 1 diabetes mellitus
- Insulin-producing cells
Diabetes mellitus
Type 2 diabetes mellitus
Hyperosmolar nonketotic coma
International Pancreas Transplant Registry
Human pluripotent stem cells
Human embryonic stem cells
Human induced pluripotent stem cells
MAF bZIP transcription factor A
Neuronal differentiation 1
Pancreatic and duodenal homeobox 1
NK6 homeobox transcription factor-related locus 1
Glucose-stimulated insulin secretion
Embryonic stem cells
Induced pluripotent stem cells
Definitive endoderm
Primitive gut tube
Pancreatic progenitor
Endocrine progenitor
Basic fibroblast growth factor
Islet-like clusters
Streptozocin
Stem cell-derived β cells
Neurogenin 3
Bone morphogenetic protein
Keratinocyte growth factor
Epidermal growth factors
Glycogen synthase-kinase-3 β
Stem cell-derived enterochromaffin
T1DM-specific iPSCs
Nondiabetic
Hepatocyte nuclear factor 1-α
Hepatocyte nuclear factor 4-α
Endocrine cells
Endocrine cell clusters
Epithelial-mesenchymal transition
Severe combined immunodeficiency
Pancreatic stem cells
Glucose tolerance testing
Nonhuman primates
Pancreas associated transcription factor 1a
Pancreatic duct ligation
Nonobese diabetic
Nonendocrine pancreatic epithelial cells
SRY-box transcription factor 9
Nestin-positive islet-derived progenitor cells
Side population
ATP binding cassette subfamily G member 2
Bone marrow-derived stem cells
Glucose transporter 2
Paired box 6
Human liver stem cells
Mesenchymal stromal cells
Islet-like structure
NK6 homeobox transcription factor-related locus 3
C-X-C motif chemokine ligand 2
Human leukocyte antigen
Major histocompatibility complex, class I, A
Major histocompatibility complex, class I, B
Major histocompatibility complex, class I, C
β2-microglobulin
Natural killer cell
Programmed cell death 1 ligand 1-cytotoxic T-lymphocyte antigen-4
Mixed-meal tolerance tests
Octamer-binding transcription factor-4
Nanog homeobox
SRY-box transcription factor 2
SRY-box transcription factor 17
Forkhead box A2
Hepatocyte nuclear factor 1-β
Hepatocyte nuclear factor 6
Somatostatin
Vascular endothelial growth factor
Hepatocyte growth factor
Insulin-like growth factor
Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–88.
CAS PubMed Google Scholar
Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol. 2013;9(9):555–62.
Lombardo C, et al. Update on pancreatic transplantation on the management of diabetes. Minerva Med. 2017;108(5):405–18.
PubMed Google Scholar
Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8.
Niclauss N, et al. Beta-cell replacement: pancreas and islet cell transplantation. Endocr Dev. 2016;31:146–62.
Dean PG, et al. Pancreas transplantation. BMJ. 2017;357:j1321.
Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017;13(5):268–77.
Rickels MR, Robertson RP. Pancreatic islet transplantation in humans: recent progress and future directions. Endocr Rev. 2019;40(2):631–68.
Pagliuca FW, et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159(2):428–39.
CAS PubMed PubMed Central Google Scholar
Russ HA, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34(13):1759–72.
Mu XP, et al. Enhanced differentiation of human amniotic fluid-derived stem cells into insulin-producing cells in vitro. J Diabetes Investig. 2017;8(1):34–43.
Yabe SG, et al. Efficient generation of functional pancreatic beta-cells from human induced pluripotent stem cells. J Diabetes. 2017;9(2):168–79.
Path G, et al. Stem cells in the treatment of diabetes mellitus - focus on mesenchymal stem cells. Metabolism. 2019;90:1–15.
Tao T, et al. Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab Chip. 2019;19(6):948–58.
Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7.
Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72.
Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20.
D'Amour KA, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392–401.
Shim JH, et al. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50(6):1228–38.
Rezania A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121–33.
Vegas AJ, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med. 2016;22(3):306–11.
Southard SM, Kotipatruni RP, Rust WL. Generation and selection of pluripotent stem cells for robust differentiation to insulin-secreting cells capable of reversing diabetes in rodents. PLoS One. 2018;13(9):e0203126.
PubMed PubMed Central Google Scholar
Zhang D, et al. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19(4):429–38.
Tuch BE. Reversal of diabetes by human fetal pancreas. Optimization of requirements in the hyperglycemic nude mouse. Transplantation. 1991;51(3):557–62.
Beattie GM, Butler C, Hayek A. Morphology and function of cultured human fetal pancreatic cells transplanted into athymic mice: a longitudinal study. Cell Transplant. 1994;3(5):421–5.
Hayek A, Beattie GM. Experimental transplantation of human fetal and adult pancreatic islets. J Clin Endocrinol Metab. 1997;82(8):2471–5.
Castaing M, et al. Ex vivo analysis of acinar and endocrine cell development in the human embryonic pancreas. Dev Dyn. 2005;234(2):339–45.
Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52.
Tateishi K, et al. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283(46):31601–7.
Kunisada Y, et al. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 2012;8(2):274–84.
Veres A, et al. Charting cellular identity during human in vitro beta-cell differentiation. Nature. 2019;569(7756):368–73.
Maehr R, et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A. 2009;106(37):15768–73.
Millman JR, et al. Generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nat Commun. 2016;7:11463.
Chetty S, et al. A simple tool to improve pluripotent stem cell differentiation. Nat Methods. 2013;10(6):553–6.
Brissova M, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53(9):1087–97.
Manzar GS, Kim EM, Zavazava N. Demethylation of induced pluripotent stem cells from type 1 diabetic patients enhances differentiation into functional pancreatic beta cells. J Biol Chem. 2017;292(34):14066–79.
Wang Q, et al. Real-time observation of pancreatic beta cell differentiation from human induced pluripotent stem cells. Am J Transl Res. 2019;11(6):3490–504.
Al-Khawaga S, et al. Pathways governing development of stem cell-derived pancreatic beta cells: lessons from embryogenesis. Biol Rev Camb Philos Soc. 2018;93(1):364–89.
Rezania A, et al. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells. 2013;31(11):2432–42.
Taylor BL, Liu FF, Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Rep. 2013;4(6):1262–75.
Mfopou JK, et al. Noggin, retinoids, and fibroblast growth factor regulate hepatic or pancreatic fate of human embryonic stem cells. Gastroenterology. 2010;138(7):2233–45 2245 e1–14.
Nostro MC, et al. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138(5):861–71.
Elham H, Mahmoud H. The effect of pancreas islet-releasing factors on the direction of embryonic stem cells towards Pdx1 expressing cells. Appl Biochem Biotechnol. 2018;186(2):371–83.
Nostro MC, et al. Efficient generation of NKX6-1+ pancreatic progenitors from multiple human pluripotent stem cell lines. Stem Cell Reports. 2015;4(4):591–604.
Toyoda T, et al. Cell aggregation optimizes the differentiation of human ESCs and iPSCs into pancreatic bud-like progenitor cells. Stem Cell Res. 2015;14(2):185–97.
Sharon N, et al. A peninsular structure coordinates asynchronous differentiation with morphogenesis to generate pancreatic islets. Cell. 2019;176(4):790–804 e13.
Memon B, et al. Enhanced differentiation of human pluripotent stem cells into pancreatic progenitors co-expressing PDX1 and NKX6.1. Stem Cell Res Ther. 2018;9(1):15.
Aigha II, et al. Differentiation of human pluripotent stem cells into two distinct NKX6.1 populations of pancreatic progenitors. Stem Cell Res Ther. 2018;9(1):83.
Legoy TA, et al. In vivo environment swiftly restricts human pancreatic progenitors toward mono-hormonal identity via a HNF1A/HNF4A mechanism. Front Cell Dev Biol. 2020;8:109.
Shahjalal HM, et al. Generation of pancreatic beta cells for treatment of diabetes: advances and challenges. Stem Cell Res Ther. 2018;9(1):355.
Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155–79.
Peiris H, et al. The beta-cell/EC axis: how do islet cells talk to each other? Diabetes. 2014;63(1):3–11.
Johnston NR, et al. Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab. 2016;24(3):389–401.
Liu W, et al. Abnormal regulation of glucagon secretion by human islet alpha cells in the absence of beta cells. EBioMedicine. 2019;50:306–16.
Kim Y, et al. Islet-like organoids derived from human pluripotent stem cells efficiently function in the glucose responsiveness in vitro and in vivo. Sci Rep. 2016;6:35145.
Wang W, Jin S, Ye K. Development of islet organoids from H9 human embryonic stem cells in biomimetic 3D scaffolds. Stem Cells Dev. 2017;26(6):394–404.
Suckale J, Solimena M. The insulin secretory granule as a signaling hub. Trends Endocrinol Metab. 2010;21(10):599–609.
Kobayashi T, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142(5):787–99.
Yamaguchi T, et al. Interspecies organogenesis generates autologous functional islets. Nature. 2017;542(7640):191–6.
Matsunari H, et al. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci U S A. 2013;110(12):4557–62.
Vilarino M, et al. CRISPR/Cas9 microinjection in oocytes disables pancreas development in sheep. Sci Rep. 2017;7(1):17472.
Tachibana M, et al. Generation of chimeric rhesus monkeys. Cell. 2012;148(1–2):285–95.
Pan FC, et al. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development. 2013;140(4):751–64.
Fujitani Y. Transcriptional regulation of pancreas development and beta-cell function [Review]. Endocr J. 2017;64(5):477–86.
Peshavaria M, et al. Regulation of pancreatic beta-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes. 2006;55(12):3289–98.
Dor Y, et al. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–6.
Kopp JL, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138(4):653–65.
Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207.
Al-Hasani K, et al. Adult duct-lining cells can reprogram into beta-like cells able to counter repeated cycles of toxin-induced diabetes. Dev Cell. 2013;26(1):86–100.
Courtney M, et al. The inactivation of Arx in pancreatic alpha-cells triggers their neogenesis and conversion into functional beta-like cells. PLoS Genet. 2013;9(10):e1003934.
Lysy PA, Weir GC, Bonner-Weir S. Making beta cells from adult cells within the pancreas. Curr Diab Rep. 2013;13(5):695–703.
Bonner-Weir S, et al. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans. 2008;36(Pt 3):353–6.
Carpino G, et al. Progenitor cell niches in the human pancreatic duct system and associated pancreatic duct glands: an anatomical and immunophenotyping study. J Anat. 2016;228(3):474–86.
Ramiya VK, et al. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6(3):278–82.
Bonner-Weir S, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A. 2000;97(14):7999–8004.
Hao E, et al. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med. 2006;12(3):310–6.
Lee J, et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife. 2013;2:e00940.
Corritore E, et al. Beta-cell differentiation of human pancreatic duct-derived cells after in vitro expansion. Cell Reprogram. 2014;16(6):456–66.
Hoesli CA, Johnson JD, Piret JM. Purified human pancreatic duct cell culture conditions defined by serum-free high-content growth factor screening. PLoS One. 2012;7(3):e33999.
Zhang M, et al. Growth factors and medium hyperglycemia induce Sox9+ ductal cell differentiation into beta cells in mice with reversal of diabetes. Proc Natl Acad Sci U S A. 2016;113(3):650–5.
Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–95.
Zimmerman L, et al. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12(1):11–24.
Xie L, et al. Characterization of nestin, a selective marker for bone marrow derived mesenchymal stem cells. Stem Cells Int. 2015;2015:762098.
Bernal A, Arranz L. Nestin-expressing progenitor cells: function, identity and therapeutic implications. Cell Mol Life Sci. 2018;75(12):2177–95.
Kim M, et al. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8(1):22–8.
Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99(2):507–12.
Lechner A, et al. Nestin-positive progenitor cells derived from adult human pancreatic islets of Langerhans contain side population (SP) cells defined by expression of the ABCG2 (BCRP1) ATP-binding cassette transporter. Biochem Biophys Res Commun. 2002;293(2):670–4.
Zhang L, et al. Nestin-positive progenitor cells isolated from human fetal pancreas have phenotypic markers identical to mesenchymal stem cells. World J Gastroenterol. 2005;11(19):2906–11.
Huang H, Tang X. Phenotypic determination and characterization of nestin-positive precursors derived from human fetal pancreas. Lab Investig. 2003;83(4):539–47.
Zou C, et al. Isolation and in vitro characterization of pancreatic progenitor cells from the islets of diabetic monkey models. Int J Biochem Cell Biol. 2006;38(5–6):973–84.
Tang DQ, et al. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53(7):1721–32.
Gabr MM, et al. Generation of insulin-producing cells from human bone marrow-derived mesenchymal stem cells: comparison of three differentiation protocols. Biomed Res Int. 2014;2014:832736.
Pokrywczynska M, et al. Transdifferentiation of bone marrow mesenchymal stem cells into the islet-like cells: the role of extracellular matrix proteins. Arch Immunol Ther Exp. 2015;63(5):377–84.
CAS Google Scholar
Daryabor G, Shiri EH, Kamali-Sarvestani E. A simple method for the generation of insulin producing cells from bone marrow mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2019;55(6):462–71.
Sapir T, et al. Cell-replacement therapy for diabetes: generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci U S A. 2005;102(22):7964–9.
Zalzman M, Anker-Kitai L, Efrat S. Differentiation of human liver-derived, insulin-producing cells toward the beta-cell phenotype. Diabetes. 2005;54(9):2568–75.
Meivar-Levy I, Ferber S. Reprogramming of liver cells into insulin-producing cells. Best Pract Res Clin Endocrinol Metab. 2015;29(6):873–82.
Cerda-Esteban N, et al. Stepwise reprogramming of liver cells to a pancreas progenitor state by the transcriptional regulator Tgif2. Nat Commun. 2017;8:14127.
Herrera MB, et al. Isolation and characterization of a stem cell population from adult human liver. Stem Cells. 2006;24(12):2840–50.
Navarro-Tableros V, et al. Islet-like structures generated in vitro from adult human liver stem cells revert hyperglycemia in diabetic SCID mice. Stem Cell Rev Rep. 2019;15(1):93–111.
Opara EC, et al. Design of a bioartificial pancreas(+). J Investig Med. 2010;58(7):831–7.
Kepsutlu B, et al. Design of bioartificial pancreas with functional micro/nano-based encapsulation of islets. Curr Pharm Biotechnol. 2014;15(7):590–608.
Farney AC, Sutherland DE, Opara EC. Evolution of islet transplantation for the last 30 years. Pancreas. 2016;45(1):8–20.
O'Sullivan ES, et al. Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocr Rev. 2011;32(6):827–44.
Memon B, Abdelalim EM. Stem cell therapy for diabetes: beta cells versus pancreatic progenitors. Cells. 2020;9(2):283.
Kizilel S, Garfinkel M, Opara E. The bioartificial pancreas: progress and challenges. Diabetes Technol Ther. 2005;7(6):968–85.
Welman T, et al. Bioengineering for organ transplantation: progress and challenges. Bioengineered. 2015;6(5):257–61.
Hwang PT, et al. Progress and challenges of the bioartificial pancreas. Nano Converg. 2016;3(1):28.
de Vos P, et al. Long-term biocompatibility, chemistry, and function of microencapsulated pancreatic islets. Biomaterials. 2003;24(2):305–12.
Mallett AG, Korbutt GS. Alginate modification improves long-term survival and function of transplanted encapsulated islets. Tissue Eng Part A. 2009;15(6):1301–9.
Souza YE, et al. Islet transplantation in rodents. Do encapsulated islets really work? Arq Gastroenterol. 2011;48(2):146–52.
Rengifo HR, et al. Long-term survival of allograft murine islets coated via covalently stabilized polymers. Adv Healthc Mater. 2014;3(7):1061–70.
Chen T, et al. Alginate encapsulant incorporating CXCL12 supports long-term allo- and xenoislet transplantation without systemic immune suppression. Am J Transplant. 2015;15(3):618–27.
Alagpulinsa DA, et al. Alginate-microencapsulation of human stem cell-derived beta cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression. Am J Transplant. 2019;19(7):1930–40.
Williams RC, et al. The risk of transplant failure with HLA mismatch in first adult kidney allografts 2: living donors, summary, guide. Transplant Direct. 2017;3(5):e152.
Torikai H, et al. Toward eliminating HLA class I expression to generate universal cells from allogeneic donors. Blood. 2013;122(8):1341–9.
Torikai H, et al. Genetic editing of HLA expression in hematopoietic stem cells to broaden their human application. Sci Rep. 2016;6:21757.
Xu H, et al. Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell. 2019;24(4):566–78 e7.
El Khatib MM, et al. Beta-cell-targeted blockage of PD1 and CTLA4 pathways prevents development of autoimmune diabetes and acute allogeneic islets rejection. Gene Ther. 2015;22(5):430–8.
Carlsson PO, et al. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64(2):587–92.
Cai J, et al. Umbilical cord Mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care. 2016;39(1):149–57.
Hwang G, et al. Efficacies of stem cell therapies for functional improvement of the beta cell in patients with diabetes: a systematic review of controlled clinical trials. Int J Stem Cells. 2019;12(2):195–205.
Download references
Acknowledgements
We gratefully acknowledge the funding support from the National Key Research and Development Program of China (2016YFC1305703), the National Natural Science Foundation of China (81670750, 81971191, and 61627807), Guangxi Natural Science Foundation (2014GXNSFDA118030), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.

Author information
Authors and affiliations.
Key Laboratory of Longevity and Ageing-Related Disease of Chinese Ministry of Education, Center for Translational Medicine and School of Preclinical Medicine, Guangxi Medical University, Nanning, 530021, Guangxi, China
Shuai Chen, Kechen Du & Chunlin Zou
You can also search for this author in PubMed Google Scholar
Contributions
CZ designed the concept. SC wrote the manuscript. SC and KD designed the figures. CZ revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Chunlin Zou .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Chen, S., Du, K. & Zou, C. Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell Res Ther 11 , 275 (2020). https://doi.org/10.1186/s13287-020-01793-6
Download citation
Received : 29 January 2020
Revised : 19 June 2020
Accepted : 29 June 2020
Published : 08 July 2020
DOI : https://doi.org/10.1186/s13287-020-01793-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pancreatic islets
- Transplantation
Stem Cell Research & Therapy
ISSN: 1757-6512
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
ViaCyte and CRISPR Introduce New Stem Cell Therapy for Type 1 Diabetes

In a collaboration between ViaCyte and CRISPR Therapeutics, a new clinical trial is investigating a stem cell therapy that may eventually help millions with type 1 diabetes .
Researchers have long sought a cell-based “cure” for type 1 diabetes, and in recent years, this goal has seemed more attainable. Since the condition results from the body’s own immune system destroying the insulin-producing beta cells in the islets of the pancreas, research has focused on finding a way to replace these non-functional cells with new and functional beta cells.
There are now two main methods for generating new and functional beta cells. People can undergo a surgical procedure to transplant healthy insulin-making cells (called beta cells) from a human donor into a person with type 1. Alternatively, researchers have now discovered ways to generate beta cells from stem cells , or cells that have not yet matured into one of the many types of cells in the human body. In 2021, Vertex performed the first successful beta cell transplant in a person with type 1 with functioning beta cells created from stem cells. In that same year, ViaCyte published the first peer-reviewed studies showing that its own stem cell therapy was successful in producing insulin in people with type 1 diabetes.
The drawback to these procedures, however, is that they require recipients to take medicine to suppress their immune system on a daily basis in order to stop the immune system from attacking the implanted cells. While both methods could be considered functional cures for type 1 diabetes, the need for permanent immunosuppressants is a limitation.
However, a new cell therapy developed at ViaCyte , in collaboration with CRISPR Therapeutics , may answer the call.
CRISPR gene-editing technology, often referred to simply as “CRISPR,” is a Nobel Prize-winning technology that allows researchers to alter the genetic code (or DNA) of a cell with extreme precision. CRISPR is one of the most common methods used to create genetically modified organisms, agricultural products, and certain medications.
With current stem cell treatments (like the cell therapy used in Vertex’s current clinical trial), when a person with type 1 receives a beta cell transplant, the implanted cells have their own unique immune “signature” in their genetic code, which is different from that of the recipient. This is why the body’s immune system thinks of the cells as foreign and dangerous, so it will target them for destruction in the same way that a person’s own beta cells are targeted for destruction when they have type 1.
By altering the genetic code of these implanted cells using the CRISPR technology, however, researchers may be able to create beta cells that avoid all recognition by the immune system, creating cells that might evade the attack from the immune system completely.
“This new product could provide a functional cure for type 1 diabetes,” said Dr. Howard Foyt, chief medical officer at ViaCyte. “By implanting these cells in the individual, the hope is that people can throw away their glucometers and insulin syringes, because these cells could provide all the insulin they need.”
According to ViaCyte , pre-clinical studies (which are done in test tubes or in animals, prior to being tested in humans) showed that these beta cell implants were well-tolerated, and had minimal side effects.
This therapy (called PEC-QT ) places genetically modified beta cells (that will hopefully be invisible to the recipient's immune system) into a pouch that is then implanted into the body. Blood vessels should be able to grow and penetrate the pouch coming into direct contact with the cells and giving them all the nutrients and oxygen they need.
“The advantage of having these cells in the pouch is that we can remove them all at once, unlike a typical islet cell transplant or cell infusion,” Foyt said. “So being designed for safety is a big advantage with this procedure. If someone does have an immune reaction, we can remove the cells.”
There are still several questions that need to be answered about these therapies, one of which is how often the pouch needs to be replaced.
“We refer to this as a potential ‘functional’ cure for type 1 diabetes because the device is not permanent; we know it will need to be replaced after a certain time,” Foyt said. “To an extent, because cells directly contact the blood, the pouch is akin to islet cell transplantation. If so, we could potentially see a lifespan of potentially five years, similar to islet transplants, and possibly as long as 10 years, but this question remains unanswered.”
Another therapy (called PEC-Encap ), now in Phase 2 clinical trials , places the modified beta cells into a pouch that blood vessels and immune cells cannot penetrate (a method called encapsulation), but that allows nutrients such as oxygen, glucose and other hormones to pass through the walls of the pouch.
While it will be several years before PEC-QT and other beta cell therapies hopefully become available for people with type 1 diabetes, the beginning of these clinical trials is another step toward the goal of finding a cure for type 1 diabetes.
On Feb. 2, ViaCyte and CRISPR Therapeutics announced that the first participant in the trial had received the PEC-QT implant. This marks the first gene-edited, stem cell-derived pancreatic cells to be implanted in a human that are specifically designed to evade the immune system in treating type 1 diabetes.
All in all, Foyt is optimistic that this therapy could eventually be used by a large portion of the type 1 population, if not people with insulin-requiring type 2 diabetes as well.
“This is the power of stem cells,” he said. “We have the capacity to create a seemingly unlimited supply of gene-edited stem cells to be used in developing this functional cure for type 1 diabetes.”
For more about the latest in the search for type 1 cures, stem cell therapy, or efforts to tackle immunosuppression, check out these articles:
- Hope Stems from Vertex’s New Therapy
- Type 1 Cures: A Sneak Peek into the Lab
- Type 1 Diabetes Research 2021: Science, Hope and Clinical Reality

- Claim credits
- Plan your learning
- Browse journals and articles
- View sessions recordings
- Renew membership
- Submit Your Science!
- Find other members
- Become a member leader
- Get published
- Earn recognition
- Develop your career
- Promote your work
Stem-cell based therapy shows promise in treating high-risk type 1 diabetes
About Endocrine Society
Endocrinologists are at the core of solving the most pressing health problems of our time, from diabetes and obesity to infertility, bone health, and hormone-related cancers. The Endocrine Society is the world’s oldest and largest organization of scientists devoted to hormone research and physicians who care for people with hormone-related conditions.
The Society has more than 18,000 members, including scientists, physicians, educators, nurses, and students in 122 countries. To learn more about the Society and the field of endocrinology, visit our site at www.endocrine.org . Follow us on X (formerly Twitter) at @TheEndoSociety and @EndoMedia .
Colleen Williams Senior Communications Manager, Public Relations Phone: (202)-971-3611 [email protected]
Jenni Glenn Gingery Director, Communications and Media Relations Phone: (202)-971-3655 [email protected]
All News & Advocacy

Endocrine News Podcast

The Endocrine News podcast brings you the latest research and clinical advances from experts in the field, whether you are in your car, office, or out for a run.
Endocrine Society Journals
Our top-ranked peer-reviewed journals are among the first to publish major developments and discovery milestones.
For 100 years, the Endocrine Society has been at the forefront of hormone science and public health. Read about our history and how we continue to serve the endocrine community.

Scientist hopes to cure Type 1 diabetes by disguising stem cells
Dr. Hannah Pizzato, a scientist at the U of A Health Sciences Center for Advanced Molecular and Immunological Therapies, is working on a stem cell-based therapy for Type 1 diabetes.
Research at the Center for Advanced Molecular and Immunological Therapies could lead to stem cell-based therapies that can bypass the body’s immune system and target diseases.
Photo courtesy of Hannah Pizzato
People have been waiting a long time for a cure for Type 1 diabetes. Hannah Pizzato, PhD , is one of them.

Hannah Pizzato, PhD, was diagnosed with Type 1 diabetes when she was 4 years old. This photo was taken on her first family vacation shortly after the diagnosis.
“My whole life I was told a cure is coming and that I won’t have to deal with this very long, but then a cure never came,” said Pizzato, a principal scientist at the University of Arizona Health Sciences Center for Advanced Molecular and Immunological Therapies .
Pizzato was diagnosed with Type 1 diabetes when she was 4 years old. She was too young to understand it is an autoimmune disease. She didn’t know her immune system was attacking cells in her pancreas that are responsible for producing insulin, a hormone that regulates blood sugar. But from an early age, she began to question why people become sick.
“I was always interested in the fundamentals of how the body works and adapts over time to keep us alive,” she said. “I was intrigued by how biology influences human development.”
It isn’t a surprise her educational and research trajectory focused on the immune system and Type 1 diabetes. She received her doctorate from Washington University in St. Louis, where she worked under the mentorship of Deepta Bhattacharya, PhD , who recently was named the inaugural executive director of CAMI.
In 2017, Pizzato followed Bhattacharya to Tucson, where she finished her thesis and became a postdoctoral fellow in his lab at the U of A College of Medicine—Tucson Department of Immunobiology .
In Bhattacharya’s lab, Pizzato worked to overcome immune barriers to stem cell transplantation. Today, as one of the first scientists working at CAMI, Pizzato is building upon that research with the goal of one day using a stem cell therapy to finally cure Type 1 diabetes.
Building toward a cure
Pizzato’s interest in the immune system brought her to the forefront of a field that is pushing the boundaries of modern medicine: stem cell therapy. Stem cells are unique in that they are self-replicating and unspecialized, meaning they can turn into many different types of cells in the body, including blood, muscle and brain cells.

Pizzato received her bachelor’s degree from Purdue University and her doctorate from Washington University in St. Louis. She is one of the first principal scientists at the Center for Advanced Molecular and Immunological Therapies.
Photo by Noelle Haro-Gomez, U of A Health Sciences Office of Communications
Since the first successful bone marrow transplant in 1956, scientists all over the world have been examining stem cells and their potential to fight diseases, regenerate damaged tissues and develop personalized treatments for patients. Today, hematopoietic stem cells found in bone marrow are used to treat blood cancers such as leukemia, lymphoma, multiple myeloma and more.
One of the fastest-growing areas of stem cell research utilizes pluripotent stem cells, which are cells that can turn into any cell type in the body.
“We are researching pluripotent stem cells because they can be differentiated into other kinds of cells that perform specialized functions,” Pizzato said. “In theory, these stem cells could be transformed into anything a patient might need. For example, our goal is to mature stem cells into insulin-producing beta cells and transplant them into someone with Type 1 diabetes. We could replace the beta cells they lost and reverse the disease.”
The biggest challenge to stem cell therapies comes from the immune system, the body’s natural protector against invaders. This complex network of cells, tissues and organs fights off infections, diseases and, unfortunately, regenerative treatments including stem cells.
“In the same way that the body’s immune system will reject a kidney transplant, the body will reject stem cells without the assistance of immunosuppressive drugs,” Pizzato explained. “But lifelong immunosuppression increases your risk of infections and can even allow cancer cells to form and proliferate unchecked.”
Bhattacharya and Pizzato have spent years trying to find a solution to this problem. Now, they may be on the verge of a breakthrough.
“We think modifying stem cells in a way that hides them from the immune system can avoid the need for immunosuppression and potentially make for a really effective therapy,” Pizzato said.
Engineering a disguise for stem cells
Pizzato was the first author on a paper published in Stem Cell Reports earlier this year. In the study, the research team genetically engineered stem cells to evade detection by various parts of the immune system, including T cells.
T cells, a type of white blood cell, are key drivers of immune rejection. They are constantly scanning for anything that doesn’t belong in the body, such as viruses and bacteria. T cells even perform security checks on other cells by checking for human leukocyte antigen, a protein found on the surface of most cells. If that protein isn’t a match to the body’s specific human leukocyte antigen, the T cell is triggered to kill that cell.

Deepta Bhattacharya, PhD, the inaugural executive director of CAMI, mentored Pizzato through her doctorate program and fostered her interest in stem cell research.
“The human leukocyte antigen on transplanted cells is a dead giveaway to T cells that they are not part of the body,” she said. “To get around this problem, we used a genetic engineering tool to cut out the gene that encodes for that protein. This acts as a camouflage to get our modified stem cells around any investigating T cells.”
The lack of human leukocyte antigen solved the T cell problem, but it didn’t address two other parts of the immune system: natural killer cells and complement deposition. Natural killer cells, like T cells, are always on the lookout to destroy damaged or diseased cells. The immune system also relies on a process called complement deposition, where certain proteins bind to the surface of invaders to help the immune system recognize threats.
Pizzato said that after removing human leukocyte antigen, the team added several other proteins, some of which sent inhibitory signals to hinder the natural killer cells and others preventing complement deposition.
“It’s like we took the stem cell’s glasses off, and then added a hat, fake moustache and coat to prevent recognition,” Pizzato said.
The modified stem cells were tested in mice with fully functioning immune systems, where they successfully dodged rejection by the immune system and persisted.
“These findings are very encouraging and give us confidence we are ready to move forward,” Pizzato said.
Moving forward at CAMI
In July, Pizzato moved to Phoenix to set up the first CAMI lab on the second floor of the Biomedical Sciences Partnership Building.
Some of the first experiments Pizzato has planned include narrowing down the number of modifications needed to successfully disguise stem cells from healthy immune systems. Eventually, she and Bhattacharya hope to test the engineered stem cells in nonobese diabetic mouse models of Type 1 diabetes and translate their mouse work into human findings.
“This will be an especially important step and a big test for our modified stem cells,” Pizzato said. “Type 1 diabetes is an autoimmune disease, so the immune system is already primed to be a hostile environment. The first big step was making stem cells that are invisible to the immune system, but invisible, unspecialized stem cells floating around the body don’t really help anyone. We want to turn those invisible stem cells into beta cells and make sure they can help regulate blood sugar.”
For Pizzato, CAMI is helping bridge the gap between her research and a potential product. It might also help a lifelong dream come true.
Hannah Pizzato, PhD Principal Scientist, Center for Advanced Molecular and Immunological Therapies, U of A Health Sciences
Deepta Bhattacharya, PhD Inaugural Executive Director, Center for Advanced Molecular and Immunological Therapies, U of A Health Sciences Professor, Department of Immunobiology, U of A College of Medicine – Tucson Professor, Department of Surgery, U of A College of Medicine – Tucson Member, U of A Cancer Center Member, BIO5 Institute
Related Stories
Researchers genetically modify stem cells to evade immunological rejection
Leading the charge to discover answers in immunology
TEDx Talk tackles the question, can vaccines fight cancer?
Brian Brennan U of A Health Sciences Office of Communications 520-621-3510, [email protected]
- Utility Menu
GA4 tracking code

Diabetes Program
Our goal is to cure diabetes. to do that, we have successfully developed ways to create new insulin-producing beta cells. this critical advance has enabled the first beta cell replacement clinical trial for type 1 diabetes. we are now focused on devising strategies to effectively protect beta cells from attack by the immune system., what we are investigating.
Our researchers have discovered how to reprogram adult and embryonic stem cells into new beta cells. Now, we are exploring how these beta cells can be effectively transplanted into patients, without being rejected. This will require protecting transplanted cells against immune attack, but also determining the best way to transplant them to retain their full function. HSCI researchers are also investigating:
- How and why the immune attack on beta cells in type 1 diabetes begins.
- How beta cell function is affected.
- How the immune attack can be prevented or stopped.
- How beta cells could be replaced or regenerated.
This work dovetails with the goal of many HSCI researchers, who are seeking to identify “universal donor” cells that can be transplanted without triggering an immune reaction.
What we have achieved so far
Researchers in the HSCI Diabetes Program have:
- Coaxed human embryonic and adult stem cells into becoming pancreatic beta cells.
- Mapped out the full set of proteins at play in mouse and human pancreatic islets.
- Pinpointed the genes involved in partitioning parts of the embryo into organs.
- Clarified how genes are controlling the late stages of beta cell development.
- Identified genes that may help protect beta cells from the immune system attack.
The BAIRT collaboration
We are central players in the Boston Autologous Islet Replacement Program ( BAIRT ), a unique collaboration that seeks to accelerate a cure for diabetes. HSCI, Brigham and Women’s Hospital, the Joslin Diabetes Center, and the Dana-Farber Cancer Institute are working together in BAIRT to generate clinical-grade, pluripotent stem cells that are suitable for use in patients. This work will enable us to show that beta cells derived from patients themselves can be a safe and effective therapy in regulating blood sugar. BAIRT aims to advance key concepts including:
- Preparing clinical-grade patient-derived beta cells.
- Identifying the optimal site of implantation in the body.
- Testing the best delivery method.
The JDRF New England Center of Excellence
We are also integral members of the JDRF New England Center of Excellence . This cross-institutional collaboration involves diabetes experts from HSCI, Joslin Diabetes Center, UMass Chan Medical School, Dana-Farber Cancer Institute, and the Jackson Laboratory. The group is working towards genetic engineering of beta cells created from pluripotent stem cells to enable their transplantation without the need for immunosuppression. The goal of the JDRF New England Center of Excellence is to deliver a genome modification strategy that will protect beta cells against both autoimmune destruction and transplant rejection.
Program Leader

Douglas A. Melton, Ph.D.
Get the latest hsci research news, related news, improving stem cell-derived pancreatic cells with genetic engineering, harvard university licenses technology to alkem, aiming to treat ischemic injury and vascular diseases, hsci receives grant from the crown prince of abu dhabi to help advance type 1 diabetes research.
- Blood Program
- Cancer Program
- Cardiovascular Program
- Kidney Program
- Nervous System Diseases Program
- Musculoskeletal Program
- Skin Program
- Aging & Fibrosis
- Stem Cells as Tools
- Early-Stage Research
- For Patients
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Current progress in stem cell therapy for type 1 diabetes mellitus
Affiliations.
- 1 Key Laboratory of Longevity and Ageing-Related Disease of Chinese Ministry of Education, Center for Translational Medicine and School of Preclinical Medicine, Guangxi Medical University, Nanning, 530021, Guangxi, China.
- 2 Key Laboratory of Longevity and Ageing-Related Disease of Chinese Ministry of Education, Center for Translational Medicine and School of Preclinical Medicine, Guangxi Medical University, Nanning, 530021, Guangxi, China. [email protected].
- PMID: 32641151
- PMCID: PMC7346484
- DOI: 10.1186/s13287-020-01793-6
Type 1 diabetes mellitus (T1DM) is the most common chronic autoimmune disease in young patients and is characterized by the loss of pancreatic β cells; as a result, the body becomes insulin deficient and hyperglycemic. Administration or injection of exogenous insulin cannot mimic the endogenous insulin secreted by a healthy pancreas. Pancreas and islet transplantation have emerged as promising treatments for reconstructing the normal regulation of blood glucose in T1DM patients. However, a critical shortage of pancreases and islets derived from human organ donors, complications associated with transplantations, high cost, and limited procedural availability remain bottlenecks in the widespread application of these strategies. Attempts have been directed to accommodate the increasing population of patients with T1DM. Stem cell therapy holds great potential for curing patients with T1DM. With the advent of research on stem cell therapy for various diseases, breakthroughs in stem cell-based therapy for T1DM have been reported. However, many unsolved issues need to be addressed before stem cell therapy will be clinically feasible for diabetic patients. In this review, we discuss the current research advances in strategies to obtain insulin-producing cells (IPCs) from different precursor cells and in stem cell-based therapies for diabetes.
Keywords: Insulin-producing cells; Pancreatic islets; Stem cells; Transplantation; Type 1 diabetes mellitus.
PubMed Disclaimer
Conflict of interest statement
The authors declare that they have no competing interests.
Attempts to cure T1DM. The…
Attempts to cure T1DM. The discovery of insulin has enhanced the life span…
Generation of insulin-producing β cells…
Generation of insulin-producing β cells from hPSCs. Schematic illustration of the differentiation protocol…
Generation of IPCs from adult…
Generation of IPCs from adult stem cells. Adult pancreatic stem cells may be…
Similar articles
- Stem Cell Therapy for the Management of Type 1 Diabetes: Advances and Perspectives. Goyal P, Malviya R. Goyal P, et al. Endocr Metab Immune Disord Drug Targets. 2024;24(5):549-561. doi: 10.2174/0118715303256582230919093535. Endocr Metab Immune Disord Drug Targets. 2024. PMID: 37861029 Review.
- In Vivo Differentiation of Stem Cell-derived Human Pancreatic Progenitors to Treat Type 1 Diabetes. Maloy MH, Ferrer MA, Parashurama N. Maloy MH, et al. Stem Cell Rev Rep. 2020 Dec;16(6):1139-1155. doi: 10.1007/s12015-020-10018-5. Stem Cell Rev Rep. 2020. PMID: 32844324 Review.
- Stem cells therapy for diabetes: from past to future. Li Y, He C, Liu R, Xiao Z, Sun B. Li Y, et al. Cytotherapy. 2023 Nov;25(11):1125-1138. doi: 10.1016/j.jcyt.2023.04.012. Epub 2023 May 29. Cytotherapy. 2023. PMID: 37256240 Review.
- How to make insulin-producing pancreatic β cells for diabetes treatment. Lu J, Xia Q, Zhou Q. Lu J, et al. Sci China Life Sci. 2017 Mar;60(3):239-248. doi: 10.1007/s11427-016-0211-3. Epub 2016 Oct 27. Sci China Life Sci. 2017. PMID: 27796637 Review.
- Human Induced Pluripotent Stem Cells in the Curative Treatment of Diabetes and Potential Impediments Ahead. Dadheech N, James Shapiro AM. Dadheech N, et al. Adv Exp Med Biol. 2019;1144:25-35. doi: 10.1007/5584_2018_305. Adv Exp Med Biol. 2019. PMID: 30569414 Review.
- Association and causality between diabetes and activin A: a two-sample Mendelian randomization study. Wang M. Wang M. Front Endocrinol (Lausanne). 2024 Aug 29;15:1414585. doi: 10.3389/fendo.2024.1414585. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39280004 Free PMC article.
- Dental pulp stem cells regenerate neural tissue in degenerative disorders and stroke rehabilitation: A scope systematic review. Rahnama Sisakht A, Tavasouli Z, Negahi A, Hosseini SA, Satarzadeh M. Rahnama Sisakht A, et al. Heliyon. 2024 Jul 25;10(15):e35080. doi: 10.1016/j.heliyon.2024.e35080. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39166055 Free PMC article.
- Targeting epigenetic alterations in cancer stem cells. F V, V D P, C M, M LI, C D, G P, D C, A T, M G, S DF, M T, V V, G S. F V, et al. Front Mol Med. 2022 Sep 20;2:1011882. doi: 10.3389/fmmed.2022.1011882. eCollection 2022. Front Mol Med. 2022. PMID: 39086963 Free PMC article. Review.
- Exploring the causal effect of omega-3 polyunsaturated fatty acid levels on the risk of type 1 diabetes: a Mendelian randomization study. Abolo L, Ssenkaali J, Mulumba O, Awe OI. Abolo L, et al. Front Genet. 2024 Jul 8;15:1353081. doi: 10.3389/fgene.2024.1353081. eCollection 2024. Front Genet. 2024. PMID: 39040994 Free PMC article.
- The efficiency of stem cell differentiation into functional beta cells for treating insulin-requiring diabetes: Recent advances and current challenges. Luo Y, Yu P, Liu J. Luo Y, et al. Endocrine. 2024 Oct;86(1):1-14. doi: 10.1007/s12020-024-03855-8. Epub 2024 May 10. Endocrine. 2024. PMID: 38730069 Review.
- Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. - PubMed
- Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol. 2013;9(9):555–562. - PubMed
- Lombardo C, et al. Update on pancreatic transplantation on the management of diabetes. Minerva Med. 2017;108(5):405–418. - PubMed
- Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. - PubMed
- Niclauss N, et al. Beta-cell replacement: pancreas and islet cell transplantation. Endocr Dev. 2016;31:146–162. - PubMed
Publication types
- Search in MeSH
Related information
- PubChem Compound (MeSH Keyword)
LinkOut - more resources
Full text sources.
- BioMed Central
- Europe PubMed Central
- PubMed Central
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Talk to us about diabetes
0345 123 2399
customer support
Behind the headlines: Stem cell therapy breakthrough for type 1
Scientists in the United States have announced early results from a pioneering trial testing a new stem cell treatment designed to replace insulin-producing beta cells in people with type 1 diabetes. In a small clinical trial, which is still underway, the first person to be given a transplant of new beta cells made from stem cells could reduce the amount of insulin they were injecting by around 90%.
The results have created a buzz, but what does this research mean for you and for the future of type 1 treatments? Here we take a deeper dive into research to replace lost beta cells and this latest news.

Making strides with stem cells
The stem cell therapy research is being led by a biotech company called Vertex . They’re running an early-stage clinical trial in the US testing a new approach to replacing the beta cells that are destroyed by the immune system in type 1 diabetes.
Transplants of cells taken from donor pancreas already exist, called islet transplants . But islet transplants rely on donated pancreas cells, which are in really short supply. So not many people can be given an islet transplant.
To solve the supply issue, scientists have turned to stem cells. Most cells in our body have one particular job to do, but stem cells are different. They can be coaxed into becoming different types of cells, including beta cells.
Researchers at Vertex are testing how well beta cells made in the lab from stem cells work. Their treatment, called VX-880, involves injecting these cells into a vein that sits near the pancreas. Once inside the body, the scientists hope the cells will start making the right amount insulin to keep blood sugar levels in a healthy range, just like real beta cells do.
The Vertex researchers are early on in their initial trial and so far have looked at the effects of VX-880 in just one person. Before the study, the individual – who has lived with type 1 diabetes for 40 years – was taking an average of 34 units of insulin per day. Three months after the transplant they were making their own supply of insulin and had reduced the amount of insulin they were injecting to just three units a day. That's a drop of 91%. Their HbA1c also dropped from 8.6% to 7.2%.
Safety and side effects
Being able to make new beta cells in the lab that can help people with type 1 to make their own insulin again is hugely exciting. We’re fast approaching exactly 100 years since insulin was discovered , on 10th November 1921, and these results are a moment to celebrate and offer up real hope for the day when can put insulin injections and infusions into retirement. But there a few things to keep in mind.
These are early findings from just one person and there’s much more work to do before we can be confident that the treatment is safe and effective. The researchers plan to test 15 more people in this trial to assess the treatment’s safety and work out the right dose to use. Then larger trials, involving hundreds of people, will be needed to test how effective stem cell therapy is at helping to manage blood sugar levels and how long the benefits last for.
The first participant to receive the VX-880 treatment didn’t have any unexpected side effects, but the immune system in people with type 1 diabetes wants to find and destroy beta cells. Because of this, people on the VX-880 trial need to take immunosuppression drugs that blunt the immune system to prevent it from attacking the transplanted cells. Immunosuppression drugs come with risk of serious side effects, like raising the risk of infections and a small but increased risk of certain cancers.
This means there’s a careful balance of risks versus benefits to consider for treatments like VX-880. The researchers are only testing it in people with type 1 diabetes who have regular severe hypos and can’t detect the symptoms of low blood sugars . This can be extremely dangerous. People can lose consciousness and, in extreme cases, go into a coma. For this group of people, the risks of hypos outweigh the risks of immunosuppression.
The quest to keep cells safe
For stem cell therapy to offer hope for everyone with living with type 1 diabetes, we need treatments other than immunosuppression drugs to protect transplanted cells from the immune system. That’s why we’re funding research to develop and test treatments called immunotherapies . Unlike immunosuppression, which affects the whole immune system, immunotherapies work to retrain the specific parts of the immune system responsible for attacking the pancreas in type 1 diabetes. This means they don’t have the same side effects.
Find out more about how immunotherapies work and how we’re driving forward research to make them a reality sooner.
Another possible way to protect beta cells from the immune system’s attack is to transplant them inside a protective barrier. This is called beta cell encapsulation. Barriers are being tested that allow new beta cells to sense blood sugar levels and let in important nutrients they need to survive, while at the same time shielding them from attacking immune cells.
We also know that beta cells scientists make from stem cells in the lab aren’t perfect yet, and don’t work as well as ‘real’ beta cells. But our researchers, like Dr Natasha Hill and Dr Ildem Akerman , are on the case and figuring out how to improve the way we grow beta cells in the lab, so that they’ll make more insulin and respond better to blood sugar levels.
What about type 2 diabetes?
Stem cell therapies at the moment are being tested in people with type 1 diabetes and frequent hypos and hypo unawareness , for whom this pioneering treatment could be life-saving. But as research advances, we hope stem cells-turned-beta cells could also be used to boost beta cell supply in people with type 2 diabetes and help them to make enough of their own insulin to perfectly control blood sugar levels.
Can I take part?
Vertex is only recruiting people to take part in their study in the US and Canada. But if you’re interested in getting involved in research, you can search for studies near you on our take part in research page.
Share this Page
© The British Diabetic Association operating as Diabetes UK, a charity registered in England and Wales (no. 215199) and in Scotland (no. SC039136). A company limited by guarantee registered in England and Wales with (no.00339181) and registered office at Wells Lawrence House, 126 Back Church Lane London E1 1FH

We have emailed you a PDF version of the article you requested.
Can't find the email?
Please check your spam or junk folder
You can also add [email protected] to your safe senders list to ensure you never miss a message from us.
Type 1 Diabetic No Longer Needs Insulin After First-Of-Its-Kind Stem Cell Treatment
Complete the form below and we will email you a PDF version
Cancel and go back
IFLScience needs the contact information you provide to us to contact you about our products and services. You may unsubscribe from these communications at any time.
For information on how to unsubscribe, as well as our privacy practices and commitment to protecting your privacy, check out our Privacy Policy
Complete the form below to listen to the audio version of this article
Advertisement
Subscribe today for our Weekly Newsletter in your inbox!
It’s still a bit too early to call this a “cure”, but several clinical trials are returning exciting results.
Laura Simmons
Editor and Staff Writer
Laura is an editor and staff writer at IFLScience. She obtained her Master's in Experimental Neuroscience from Imperial College London.
Book View full profile
Book Read IFLScience Editorial Policy
Maddy Chapman
Editor & Writer
Maddy is an editor and writer at IFLScience, with a degree in biochemistry from the University of York.
DOWNLOAD PDF VERSION

Type 1 diabetics must closely monitor their blood sugar levels to ensure they're supplementing with the right amount of insulin.
Image credit: superbeststock/Shutterstock.com
A 25-year-old woman with type 1 diabetes has been able to stop taking insulin after a groundbreaking stem cell treatment. This is the first human trial of the procedure, and while it’s too soon yet to say that the woman is “cured”, it certainly demonstrates that the approach merits a closer look.
In most people with type 1 diabetes, the immune system – which is meant to protect us from invasion by pathogens – turns on the body’s own tissues, destroying cells in the pancreas that produce insulin. Without the right level of insulin, blood sugar cannot be properly controlled. Over time, this can cause damage to many organs and tissues, and there’s also the risk of the acute, life-threatening condition diabetic ketoacidosis .
That’s why people with type 1 diabetes must take insulin themselves, calibrating the dose carefully depending on the food they eat and their level of activity. It has been described it as a “ full time, 24/7 job ”.
Modern technologies like continuous glucose monitors, insulin pumps, and artificial pancreases have revolutionized the management of this lifelong condition, but these are not always accessible to people, and the cost of insulin itself can be prohibitive.
But if we want to talk about a cure, we need to find a way to replace the body’s own ability to produce insulin as and when it needs to. That hope is now being tentatively turned to reality thanks to a handful of pioneering stem cell treatment trials.
A recent report details the case of a woman from Tianjin, China who was diagnosed with type 1 diabetes in her early teens. The patient, who wished to remain anonymous, became the first type 1 patient in the world to receive a transplant of stem cells extracted from her own body, with the aim of replacing specialized pancreatic cells called islet cells.
The woman’s medical history was somewhat complicated; she had previously undergone two liver transplant operations as well as a pancreas transplant, which had subsequently failed. Because of the liver transplants, she was on immunosuppressant medication.
In 2020, medics harvested the stem cells and reprogrammed them using a comparatively new chemical process . They were left with induced pluripotent stem cells (iPSCs), which have the potential to develop into any human tissue you can think of. In this case, the researchers pushed them down the path to develop into clusters of islet cells.
After testing the cells for safety in animal models, the woman’s transplant surgery finally went ahead in 2023. It only took around 30 minutes and involved the injection of 1.5 million cells into her abdominal muscles, where they could be easily monitored by scans.
Just two-and-a-half months later, the woman’s body was producing enough insulin that she no longer needed to inject herself with top ups. The report details the clinical follow-up at a year post-transplant, at which time the woman was still managing without supplementary insulin and was not seeing dangerous peaks and troughs in her blood sugar levels.
Diabetes researcher Daisuke Yabe from Kyoto University, who was not involved in the study, described the results as “remarkable” to Nature News , adding, “If this is applicable to other patients, it’s going to be wonderful.”
This case is the first to use a patient’s own stem cells, but similarly positive results have been seen in small trials using embryonic stem cells. The Washington Post recently reported on the case of Amanda Smith, a 35-year-old nurse from Canada, who has also stopped taking insulin. Smith was part of a trial of 12 patients , of whom 11 have been able to dramatically reduce or stop their insulin altogether.
One remaining question centers around how long these effects might last. The underlying autoimmune reaction that causes the damage to the pancreas in the first place could come back and start attacking the donor cells. The case of the woman from Tianjin is interesting here, in that she was already taking immunosuppressants; further trials will explore whether this kind of treatment is needed in general.
For the estimated 8.4 million people with type 1 diabetes worldwide, taking insulin is a non-negotiable. The hope with these stem cell treatments is that instead of this daily regime, we might one day be able to tackle the problem at the source.
For Smith, the trial has been transformative, as she told the Washington Post: “You didn’t realize how much of your life it took up – until it’s taking up none, now.”
The case report is published in the journal Cell .
ARTICLE POSTED IN
stem cells,
Type 1 Diabetes,
autoimmune condition
More Health and Medicine Stories
link to article

Quarter Of US Adults Believe They Have ADHD – But Only 13 Percent Have Told A Doctor

Astigmatism: Here’s What To Know About This Common Eye Condition

Deadly Bacteria Could Thrive In Floods Left By Hurricane Milton, Florida Residents Warned

Fusing Jellies, Bad Robot Jokes, And Elephants Evolve Before Our Eyes

How Many Times Could Earth Fit Inside The Sun?

Glowing Crystals, Radioactive Storms, And A “Google Maps” For The Brain
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- 17 June 2021
Towards a stem-cell therapy for diabetes
- Anna Melidoni 0
Senior Editor, BMC Endocrine Disorders
You can also search for this author in PubMed Google Scholar
You have full access to this article via your institution.
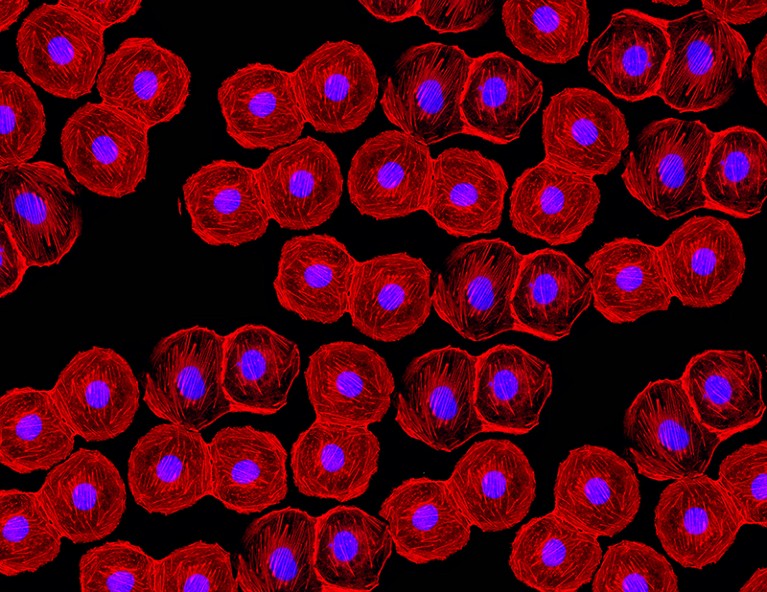
Credit: Edgloris Marys / Alamy Stock Photo
The isolation of the first human embryonic stem cell (hESC) lines in 1998 opened the possibility of stem cell therapies for a variety of conditions. Type 1 diabetes (T1D) is particularly suited to this approach, as transplantation of the insulin-producing pancreatic β-cells could provide long-lasting therapy or even a cure. By the early 2000s, the stem cell differentiation field was benefitting from embryo studies in model organisms that had identified the role of various signalling pathways in controlling pancreatic cell lineage specification.
In 2005, D’Amour et al. reported the production of hESC differentiation cultures containing >80% definitive endoderm cells: the embryonic progenitors of pancreatic cells. Differentiation occurred in 2D culture, using a low serum medium containing activin A, a protein that is essential for endoderm specification in the embryo.
Following this advance, in 2006, D’Amour et al. published a key paper reporting the generation of endocrine pancreatic cells, capable of secreting insulin, glucagon, somatostastin, pancreatic polypeptide and ghrelin from differentiating hESCs. Their differentiation protocol included five stages, during which hESCs went through a series of intermediate cellular states: definitive endoderm, primitive gut tube, posterior foregut, pancreatic endoderm and endocrine precursors and hormone-expressing endocrine cells. Specific growth factors were supplied at each step, in an effort to mimic embryonic pancreas development. Indeed, characterization of mRNA and proteins in the different cell states showed that they resembled the equivalent embryonic endodermal progenitors.
At stage five of this study, staining by zinc-chelating agent dithizone was used to identify the pancreatic endocrine cells. Their insulin content was similar to that of primary adult human islets and the de novo synthesis of insulin was confirmed by mRNA, C-peptide and pro-insulin protein measurement. However, compared with adult islets, the C-peptide content of hESC-derived cells was inferior, indicating a reduced efficiency in processing pro-insulin. C-peptide secretion was responsive to multiple secretory stimuli, albeit only minimally responsive to glucose.
Subsequently, in 2008, the same group (Kroon et al.) succeeded in generating glucose-responsive endocrine cells in vivo, following transplantation of hESC-derived stage-four pancreatic endoderm in mice. These cells expressed key β-cell transcription factors, showed efficient processing of pro-insulin and contained mature secretory granules. Importantly, the hESC-derived endocrine cells protected the mice against hyperglycaemia.
In 2014, Rezania et al. described a seven-stage hESC differentiation protocol that generated glucose-responsive insulin-producing β-cells that reversed diabetes in mice upon transplantation. In the same year, Pagliuca et al. reported the in vitro large-scale generation of glucose-responsive, insulin-expressing β-cells derived from human pluripotent stem cells (hPSCs), by sequentially modulating key signalling pathways in a 3D culture system. These hPSC-derived β-cells were very similar to human β-cells in terms of gene expression and ultrastructure and also ameliorated hyperglycaemia in diabetic mice upon transplantation.
In 2015, Russ et al. reported a simplified suspension-based, directed differentiation protocol to generate glucose-responsive insulin-producing human β-cells from hPSCs in vitro and in vivo. The resulting cells reduced blood levels of glucose in diabetic mice in a matter of weeks, as opposed to months in previous studies.
In 2019, Nair et al. reported the generation in vitro of mature β-cells from hESCs, by allowing the immature β-like cells to form islet-like clusters, thus recapitulating endocrine cell clustering in vivo. Mature β-cells showed enhanced metabolic and structural maturation of mitochondria and hyperglycaemia was reduced within days of transplantation in diabetic mice.
In 2020, Hogrebe et al. discovered that the state of actin polymerization influences pancreatic differentiation in vitro. Thus, timed actin depolymerization during differentiation led to the generation of β-cells from hPSCs that rapidly reversed diabetes in mice and maintained normoglycaemia for 9 months.
Fifteen years after the first insulin-secreting cells were generated by differentiating hESCs in vitro, we still do not have a β-cell replacement therapy for T1D. However, thanks to the studies mentioned here, and many others, we are much closer to this goal. Apart from further optimizing the efficiency and scalability of mature β-cell production, important issues, such as shielding the transplanted stem-cell-derived β-cells from immune rejection and ensuring their purity, need to be resolved before a stem-cell-based therapy becomes a reality.
Further reading
D’Amour, K. A. et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23 , 1534–1541 (2005).
Kroon, E. et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26 , 443–452 (2008).
Rezania, A. et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 32 , 1121–1133 (2014).
Pagliuca, F. W. et al. Generation of functional human pancreatic β cells in vitro. Cell 159 , 428–439 (2014).
Russ, H. A. et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 34 , 1759–1772 (2015).
Nair, G. G. et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat. Cell Biol. 21 , 263–274 (2019).
Hogrebe, N. J. et al. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat. Biotechnol. 38 , 460–470 (2020).
Related Articles
Sponsor content: Diabetes: Following the science in the search for a cure
Sponsor content: Artificial intelligence: The next frontier in diabetes therapy
Sponsor content: Driving change to defeat diabetes and other serious chronic diseases
Faculty Positions at SUSTech Department of Biomedical Engineering
We seek outstanding applicants for full-time tenure-track/tenured faculty positions. Positions are available for both junior and senior-level.
Shenzhen, Guangdong, China
Southern University of Science and Technology (Biomedical Engineering)
Faculty Positions in Bioscience and Biomedical Engineering (BSBE) Thrust, Systems Hub, HKUST (GZ)
Tenure-track and tenured faculty positions at all ranks (Assistant Professor/Associate Professor/Professor)
Guangzhou, Guangdong, China
The Hong Kong University of Science and Technology (Guangzhou)
Publisher - Chemistry
London (Greater) (GB)
In-house Senior Editor
Scientist for epigenetic basis of pediatric brain disease - jamy peng lab.
Memphis, Tennessee (US)
St. Jude Children's Research Hospital (St. Jude)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
By providing an email address. I agree to the Terms of Use and acknowledge that I have read the Privacy Policy .
Stem cells show promise in reversing type 1 diabetes
In June 2023, Peking University researchers successfully reversed type 1 diabetes in a patient, opening the possibility of a universal cure.
ScienceAlert reports these doctors injected roughly 1.5 million insulin-producing cells into a woman’s abdominal muscles.
READ: How to manage PCOS with exercise
Two and a half months later, her lifelong dependence on injected insulin ended. As a result, the treatment reversed her long-term health condition.
How does the type 1 diabetes trial work?
❗Could it really be possible that one day, diabetes patients can finally be free of needles?! 🌟 #PKUResearch team led by Deng Hongkui, with their collaborators, has successfully used chemically induced pluripotent stem cells to create insulin-producing islets, leading to the… pic.twitter.com/g9SYgGnIpe — Peking University (@PKU1898) October 2, 2024
The treatment uses molecules to revert fully-developed adult tissues into a blank slate called induced pluripotent stem cells.
Then, these stem cells turn into insulin-producing cells that do not trigger adverse reactions in patients.
In contrast, conventional treatments involve transplanting insulin-producing cell clusters called islets or, in some cases, an entire pancreas.
However, it is a highly dangerous option with a lack of donors, compounded by a lack of donors, which prevents millions from treating their type 1 diabetes worldwide.
Scientists have tried turning cells into pluripotent stem cells for two decades, but the results varied.
The PKU researchers claim their method allows them greater control over the outcomes. This led to their testing on a 26-year-old woman who had been suffering from type 1 diabetes since age 14.
As highlighted earlier, her dependence on injected insulin ended two and a half months after the cell transplantation.
Later, her body produced enough insulin by itself to maintain a safe blood sugar range of over 98 percent daily.
If she continues to produce insulin in the coming years, healthcare professionals could declare that she’s cured. As a result, these findings could become the world’s first in the scientific community.
ScienceAlert says these findings coincide with other clinical trials that suggest stem cell transplantations have promising possibilities. Consequently, the Peking University researchers stated:
Subscribe to our daily newsletter
“Overall, the findings support further clinical studies in this direction and mark a step forward in achieving the potential of personalized cell therapy… to treat disease.”
Subscribe to our newsletter!
Disclaimer: Comments do not represent the views of INQUIRER.net. We reserve the right to exclude comments which are inconsistent with our editorial standards. FULL DISCLAIMER
© copyright 1997-2024 inquirer.net | all rights reserved.
This is an information message
We use cookies to enhance your experience. By continuing, you agree to our use of cookies. Learn more here.

Stem Cell-Derived Islets Functionally Cure Patient With Type 1 Diabetes
Key takeaways.
- Stem-cell-derived islets achieved insulin independence and stable glycemic control in a T1D patient, showing promising results in a first-in-human study.
- Challenges in stem cell therapy for T1D include immunoisolation and genetic manipulation to prevent immune response complications.
- The FDA approved donislecel, an allogeneic pancreatic islet cellular therapy, for T1D patients with severe hypoglycemia.
- The FORWARD trial data supports stem-cell-derived islets' potential to reduce or eliminate insulin use in T1D patients.
The analysis assessed the feasibility of autologous transplantation of chemically-induced pluripotent stem cell-derived islets for type 1 diabetes.
Investigators have released a preliminary analysis of a first-in-human study showing that stem-cell-derived islets functionally cured a patient’s type 1 diabetes (T1D), according to the analysis published in Cell . The analysis included 1 year of data, which assessed the feasibility of autologous transplantation of chemically induced pluripotent stem cell-derived islets. The islets were injected beneath the abdominal anterior rectus sheath, according to the study authors. 1

Image Credit: Nikish Hiraman/peopleimages.com - stock.adobe.com

The patient had sustained insulin independence 75 days post-transplant, with a time-in-target glycemic range increase from 43.18% at baseline to 96.2% at 4 months after transplantation. Combined with a decrease in glycated hemoglobin reaching approximately 5%, the patient presented with stable glycemic control. 1
In a review published in Stem Cell Research and Therapy in January 2024, the authors discussed the current state of stem cell therapy for T1D as well as challenges with the therapy. Of note, investigators have developed protocols to differentiate pluripotent stem cells into pancreatic progenitors or fully differentiated β-cells, which have shown control of chemically induced diabetes. One challenge with these cells is the need for an immunoisolation device and/or immunosuppressive as needed, according to the authors of the review. 2
Additionally, there have been a growing number of studies focused on genetic manipulation to produce immune evasive cells, but the authors note the evidence needs to show that the genetic manipulation does not lead to any unforeseen complications. For mesenchymal stem/stromal cells (MSC), investigators note that these can form insulin-producing cells, which show that transplantation of allogenic insulin-producing cells from MSCs are associated with muted allogenic response without interfering with functionality. 2
Furthermore, exosome derived from naïve MSCs have also been shown to have varying degrees of success in rodent models, including a reduction in insulin resistance, promotion of autophagy, and increase in T regulatory populations. 2
In June 2023, the FDA approved the first allogeneic pancreatic islet cellular therapy, donislecel (Lantidra; CellTrans), comprised of deceased donor pancreatic cells. It is approved for individuals with T1D who are unable to approach the target glycated hemoglobin due to repeated episodes of severe hypoglycemia, despite intensive diabetes management and education. The primary mechanism of action is the secretion of insulin by infused allogenic islet β-cells, which can sometimes produce enough insulin that the patient no longer needs to take insulin to control their blood sugar. 3
Furthering the potential of stem cells for the treatment of T1D, new data from the phase 1/2 FORWARD clinical study were presented at the American Diabetes Association 84th Scientific Session in Orlando, Florida, in June 2024. The results showed the VX-880 reduced or eliminated the need for insulin use for patients with T1D, further showing that stem-cell-derived islets can restore physiological islet function and improve glycemic control. 4
1. Wang S, Du Y, Zhang B, et al. Transplantation of chemically induced pluripotent stem-cell-derived islets under abdominal anterior rectus sheath in a type 1 diabetes patient. Cell . Published online September 20, 2024. doi:10.1016/j.cell.2024.09.004
2. ghoneim ma, gabr mm, el-halawani sm, refaie af. current status of stem cell therapy for type 1 diabetes: a critique and a prospective consideration. stem cell res ther . 2024;15(1):23. published 2024 jan 29. doi:10.1186/s13287-024-03636-0, 3. fda approves first cellular therapy to treat patients with type 1 diabetes. news release. fda. june 28, 2023. accessed october 8, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-first-cellular-therapy-treat-patients-type-1-diabetes, 4. expanded forward trial demonstrates continued potential for stem cell-derived islet cell therapy to eliminate need for insulin for people with t1d. news release. american diabetes association. june 21, 2024. accessed october 8, 2024. https://diabetes.org/newsroom/press-releases/expanded-forward-trial-demonstrates-continued-potential-stem-cell-derived.

Pharmacy Times Launches Behind The Script Campaign to Highlight Pharmacists

Public Health Matters: The Difference Between Misinformation and Disinformation, and How Information Can Inform Evidence-Based Decisions

Celebrating Pharmacy Technician Day: Investing in the Future of Health Care
Public Health Matters: Healthcare Advocate Summit Emphasizes Advocates Have a Voice in Creating Solutions
Outsourcing Facilities Association Sues FDA Over Sudden Removal of Tirzepatide From Drug Shortage List

Improving Schizophrenia Outcomes: The Role of Long-Acting Injectables and Medication Adherence
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cold Spring Harb Perspect Biol
- v.13(1); 2021 Jan
A Stem Cell Approach to Cure Type 1 Diabetes
Treatment of type 1 diabetes with insulin injection is expensive, complicated, and insufficient. While cadaveric islet transplantations coupled with immunosuppressants can cure diabetes, the scarcity of acceptable islets is problematic. Developmental research on pancreas formation has informed in vitro differentiation of human pluripotent stem cells into functional islets. Although generating β cells from stem cells offers a potential cure for type 1 diabetes, several challenges remain, including protecting the cells from the immune system.
THE PROBLEM OF DIABETES
Diabetes is a metabolic disease that results from damage to β cells, which are endocrine cells in the pancreas that produce and secrete insulin ( Ashcroft and Rorsman 2012 ). In type 1 diabetes (T1D), an autoimmune destruction of β cells results in a lifelong dependence on exogenous insulin ( Katsarou et al. 2017 ). In type 2 diabetes, which is linked with obesity, β cells become dysfunctional and fail to supply sufficient insulin ( DeFronzo et al. 2015 ). In T1D, invasion and autoimmune attack by both CD4 + and CD8 + T cells specifically destroys β cells and results in almost complete elimination of β-cell mass. The cause for this autoimmune attack in T1D may involve genetic and environmental factors, but it is not clear whether it is the immune system that loses the ability to distinguish between self and nonself, or whether β cells are defective in a manner that makes them a target for clearance.
The destruction or dysfunction of β cells results in high blood sugar levels because β cells secrete insulin and possibly other gene products that are essential for proper glucose homeostasis. β cells are highly sensitive to glucose, constantly monitoring blood glucose levels and secreting exacting amounts of insulin in response to increasing glucose concentrations. Without sufficient insulin secretion, diabetic patients are not able to metabolize glucose normally and control blood glucose levels. T1D is a chronic condition, usually diagnosed in children and young adults, in which insulin replacement is required for survival. There is no known way to prevent or cure T1D. Untreated T1D is a life-threatening issue due to ketoacidosis that can result in cerebral edema and coma, and children are at a higher risk than adults ( Cryer 2013 ). The current T1D treatment protocol combines intensive attention to diet coupled with exogenous insulin administration, either using multiple daily injections or by insulin pumps. In addition, there have been advances in the development of modified insulin analogs, which are fast and long acting, and can provide benefit ( Pathak et al. 2019 ).
Despite diligent and intensive attention to blood glucose control with exogenous insulin, T1D patients are prone to severe episodes of hypoglycemia, insulin resistance, mild obesity, and other serious medical complications.
A SHORT HISTORY OF TREATING DIABETES
Diabetes (to flow or pass through in ancient Greek) mellitus is named after symptoms of the disease—frequent urination with a high content of glucose in the urine. Diabetes was lethal in ancient times; life expectancy of children with diabetes mellitus was short and the prognosis for adult-onset diabetes was very poor. In the 18th century, diabetes was treated with a meat-heavy diet (low in carbohydrates) and, during the early twentieth century before insulin was discovered, physicians Allen and Joslin recommended fasting and calorie-restricted diets. All diabetics were advised to reduce sugar and starch intake from their diet, and those who were obese were advised to lose weight. This resulted in some improvement of T1D symptoms, decreased coma, and delayed death among children.
The first understanding of a role for the pancreas in diabetes, in the late 19th century, is attributed to Joseph von Mering and Oskar Minkowski, who found that dogs whose pancreas was removed developed symptoms of diabetes. Insulin was discovered in 1921, when Banting and Best demonstrated a reversal of induced diabetes in dogs by injection of pancreatic islets extract (discovered in 1869 by Langerhans) of healthy dogs. Banting and Best were also able to purify insulin from bovine pancreases, which led to the first effective diabetes treatment in 1922 ( Banting et al. 1922 ). Animal insulin derived from cows and pigs was the first type of insulin to be administered to humans to control diabetes. In 1923, Eli Lilly and Company, with the University of Toronto, began to produce commercial animal insulin, supplying treatment for thousands of patients in Canada and the United States. Banting and Best made the patent available without charge and did not try to control commercial production. Rapidly, insulin treatment for diabetes became affordable and accessible around the world. Insulin produced from large animals was the only available insulin until the 1980s when the first recombinant human insulin was prepared and mass-produced in bacteria ( Quianzon and Cheikh 2012 ).
While lifesaving, insulin treatment is complicated and does not prevent long-term complications that result from chronic increase in blood glucose levels, including damage to the microvasculature causing retinopathy, nephropathy, and neuropathy, as well as macrovascular disease. Furthermore, treatment with excessive insulin injections can cause hypoglycemia, which is still a common and dangerous complication of the disease. In short, insulin injection is onerous, complicated, and it is not a cure.
Cell-based therapy with cadaveric islet transplantation can provide what is essentially a cure for diabetes ( Scharp et al. 1990 ; Socci et al. 1991 ). In 2000, Shapiro et al. reported a year of follow-up in seven subjects with transplanted islets prepared from pancreases from deceased donors into their liver, a treatment known as the Edmonton protocol. Using this approach, years of complete insulin independence have been achieved in patients with T1D ( Shapiro et al. 2003 , 2006 ; Bellin et al. 2008 ; Berney et al. 2009 ; Langer 2010 ).
While islet transplantation can provide a very significant improvement, there is a severely limited availability and quality of donor islets, and the need for chronic immunosuppression, which increases the risk of infections and malignancies, is an undesirable requirement. At present, a small proportion of T1D patients, those who suffer from repeating or severe hypoglycemia events, are eligible for cadaveric islet transplants.
UNDERSTANDING β-CELL DEVELOPMENT
Extensive developmental research over the last decades has provided a deep understanding of the mechanisms that coordinate pancreas and β-cell development ( Gittes 2009 ; Larsen and Grapin-Botton 2017 ; Sharon et al. 2019a ). The pancreas consists of two compartments: an exocrine compartment that participates in digestion of macronutrients through the production and release of digestive enzymes to the intestine and the endocrine compartment. The pancreatic endocrine cells (mainly glucagon-secreting α cells, β cells, somatostatin-secreting δ cells, and pancreatic polypeptide-secreting cells) form spherical clusters, the islets of Langerhans, within the larger exocrine tissue ( Jennings et al. 2013 , 2015 ).
As in many major organs, including the liver, lung, and the intestines, the development of the pancreas initiates from patterning and differentiation of the definitive endoderm ( Wells and Melton 1999 ). In the embryo, specification of the pancreatic domain is mediated by a combination of signals from the mesoderm, including transforming growth factor β (TGF-β), retinoic acid (RA), and fibroblast growth factor (FGF) ( Henry et al. 1996 ; Apelqvist et al. 1997 , 1999 ; Kim et al. 1997a , b , 2000 ; Kim and Melton 1998 ; Hart et al. 2003 ; Rankin et al. 2018 ). Pancreas specification becomes evident with the expression of pancreatic duodenal homeobox 1 (PDX1) in ventral and dorsal domains within the gut endoderm ( Jonsson et al. 1994 , 1995 ; Offield et al. 1996 ). Epithelial budding of the foregut endoderm generates two pancreatic buds (dorsal and ventral buds) that later fuse to form the pancreas. The ventral and dorsal pancreatic buds are marked by the transcription factors SOX9, PDX1, and GATA4, which are required for pancreatic growth ( Fukuda et al. 2008 ; Wandzioch and Zaret 2009 ; Carrasco et al. 2012 ). The fused ventral and dorsal buds form a multilayered epithelium consisting of multipotent pancreatic progenitor cells, which give rise to ductal, endocrine, and exocrine cell lineages. Lineage-tracing studies reveal that PDX1-expressing cells are pancreatic progenitor cells and PDX1-null mice display pancreatic agenesis ( Jonsson et al. 1994 ). Similarly, human congenital pancreatic agenesis is caused by homozygous deletion of IPF1, the human ortholog of PDX1 ( Stoffers et al. 1997 ). The early pancreas undergoes a large expansion through proliferation of the progenitor cells. By receiving appropriate niche signals from their microenvironment, the progenitors differentiate into endocrine cell types and leave the epithelial cords while maintaining cell-to-cell contacts, thus creating a cohesive bud-like structure ( Pan and Wright 2011 ; Sharon et al. 2019a ). Endocrine differentiation from epithelial cords is first marked by expression of the bHLH transcription factor Neurogenin3 (NGN3) ( Gradwohl et al. 2000 ; Gu et al. 2002 , 2003 ; Gouzi et al. 2011 ). Upon transient increase in the expression levels of NGN3, these progenitors stop proliferating and differentiate into endocrine cells. The expression of NGN3 is required for the commitment of progenitor cells to an endocrine cell fate as NGN3-null mice are completely lacking both intestinal and pancreatic endocrine lineages. The mechanisms by which NGN3 + cells give rise to the different endocrine lineages are not completely understood; however, lineage-tracing experiments and conditional gene-ablation studies has revealed that many transcription factors, including PAX4, HNF4a, FOXA2, NKX6.1, and MAFA, have important roles in β-cell formation, differentiation, and function ( Sosa-Pineda et al. 1997 ; Gupta et al. 2005 ; Henseleit et al. 2005 ; Zhang et al. 2005 ; Gao et al. 2008 ; Taylor et al. 2013 ; Röder et al. 2016 ; Villamayor et al. 2018 ; Lee et al. 2019 ).
Importantly, endocrine progenitors or pancreatic adult stem cells do not exist in the mature pancreas. Therefore, β-cell maintenance and regeneration depend, perhaps entirely, on β-cell replication ( Dor et al. 2004 ). However, baseline and compensatory β-cell replication decline sharply with age, in both rodents and humans, as a mechanism for improving the function of differentiated β cells while sacrificing the ability to mount a regenerative response ( Helman et al. 2016 ; Puri et al. 2018 ). Consequently, if β cells are destroyed, there is no significant replacement or regeneration, unlike other tissues.
STEM CELL THERAPY FOR DIABETES
The discovery of pluripotent embryonic stem cells (ESCs) and engineered induced pluripotent stem cells (iPSCs) that have a potential to differentiate, in vitro, into any cell type, created a new promising strategy for β-cell replacement therapy ( Takahashi and Yamanaka 2006 ; Okita et al. 2007 ; Takahashi et al. 2007 ; Wernig et al. 2007 ; Latres et al. 2019 ).
Studies over the past decade have clearly demonstrated that the most effective way to generate specific cell types from iPSCs in vitro is to recapitulate embryonic development ( Murry and Keller 2008 ). This approach has been successful and revealed that many of the signaling pathways and transcription factors regulate embryonic development of the pancreas. However, because of their high potency, directing stem cells to generate a specific differentiated cell type is difficult to control, and years of research were needed to succeed in directing human stem cells toward a functional β-like cell fate.
Induction of endoderm from pluripotent cells, which is the first step of this process, had been studied in frog and fish and murine models and identified molecular signals that induce differentiation into endoderm, including the TGF-β superfamily members ( Henry et al. 1996 ; Wells and Melton 2000 ; Brennan et al. 2001 ; Tremblay 2010 ; Nostro and Keller 2012 ). Another TGF-β family member, Activin A, can signal via similar downstream pathways as Nodal but is easier to produce as a recombinant protein ( Chen et al. 2013 ), enabling the first protocol for generating definitive endoderm in vitro from human pluripotent stem cells ( D'Amour et al. 2005 ). This was followed by differentiation of ESCs into progenitors that could further differentiate into mature pancreatic cells after transplantation into immune-deficient mice ( Kroon et al. 2008 ). To advance regenerative medicine for diabetes with stem cells, a major challenge in the field was to make functional human β cells in vitro. These required discoveries using different inducing factors and genetic markers for the in vitro differentiation of stem cells and was achieved in 2014 by development of a protocol that produced stem cell–derived (SC) β cells that secreted insulin in response to successive glucose challenges in vitro ( Pagliuca et al. 2014 ). The fact that these β-like cells performed glucose-responsive insulin secretion in response to multiple glucose stimulations, like primary human islets, showed that it is possible to make physiologically functional cells entirely in vitro in the absence of signals from other mesenchymal cells and a natural blood supply. This opened up the possibility of producing islets for further physiological studies and drug studies in vitro as well as transplantation to directly treat the disease.
Subsequent modification of differentiation protocols has enhanced the quality of β-like cells generated in vitro from polyhormonal, glucose-unresponsive cells to a high percentage of monohormonal, insulin-expressing β-like cells ( Rezania et al. 2014 ; Nostro et al. 2015 ; Russ et al. 2015 ; Nair et al. 2019 ; Rosado-Olivieri et al. 2019 ; Sharon et al. 2019b ; Velazco-Cruz et al. 2019 ; Veres et al. 2019 ). The common underlying principle for the different protocols is that millions of human pluripotent stem cells grown in 3D clusters are differentiated in a stepwise manner by exposing them to various growth factors and small molecules to activate or inhibit embryonic signaling pathways, such as Nodal, WNT, RA, FGF, bone morphogenetic protein (BMP), and Notch. For most protocols, it takes between five and seven stages that last 20–30 days in total to transform human stem cells into β-like cells. The terminally differentiated cells maintain their identity for long periods in culture and can rescue diabetes after transplantation in animal models.
The first three stages of differentiation generate a nearly homogenous (about 90%) population of pancreatic progenitors that express the master transcription factor PDX1. Thereafter, distinct populations are identified by staining for C-peptide (processed insulin fragment), the pan-endocrine marker CHGA, and the β-cell transcription factor NKX6.1. Single-cell analysis of the different populations in SC islets identifies three types of endocrine cells: SC β cells that express β-cell markers including INS and NKX6.1, α-like cells that express GCG and ARX, and nonpancreatic endocrine cells that mostly resemble intestinal endocrine cells. There are also remaining nonendocrine progenitor cells in the final SC islets clusters. In addition to β-like cells, these protocols also generate α-like cells that may contribute to proper islet function after transplantation. The undesired cells such as nonendocrine progenitors can be reduced by different reaggregation methods ( Nair et al. 2019 ; Veres et al. 2019 ).
The progress that has been made in generation of an unlimited supply of functional, insulin-secreting cells from human ESCs and iPSCs holds the most promise for transplantation therapy to treat T1D. Moreover, the ability to generate functional β cells in large quantities makes a novel tool for studying mechanisms of human β-cell formation and maturation and would establish a platform for modeling disease in vitro and for screening drugs and molecules that could impact proliferation and/or function of β cells and to identify new targets for diabetes therapy ( Huch and Koo 2015 ; Dutta et al. 2017 ; Bakhti et al. 2019 ).
REMAINING CHALLENGES
Improvements in the efficiency of generating functional SC β cells depend on understanding the extracellular signals that control cell fate choices during embryonic and postnatal development. There is still a lack of complete understanding of the cues required to fully control ESC differentiation toward all the endocrine cells found in islets. A typical SC islet cluster contains 40%–50% of the desired endocrine cells, while the rest of the cluster contains progenitor cells that fail to reach terminal differentiation and other undesired nonpancreatic endocrine cells. Methods for β- and α-cell enrichment, using FACS and magnetic beads sorting, are currently used to reduce the numbers of the undesired cells within the final SC islet clusters. But the overall efficiency of β-like cell production is greatly reduced during the sorting process because of high cell loss ( Veres et al. 2019 ).
It will be important to control the ratio between β and non-β endocrine cells in the clusters to best mimic human islets function. Differentiation protocols are directed toward the generation of β-like cells and generate fewer α- and δ-like cells. It is an open question whether sufficient insulin secretion by β-like cells will be achieved by a pure population or whether the presence of other endocrine cells will be beneficial in achieving optimal β-cell function.
SC β cells made in vitro are highly similar to mature human β cells but lack expression of the maturation markers UCN3, MAFA, and SIX3, and glucose-stimulated insulin secretion capacity does not meet the magnitude of that found in fully mature human β cells ( Blum et al. 2012 ; Hrvatin et al. 2014 ; Arda et al. 2016 ). The inability to achieve the same functionality in vitro reflects the lack of knowledge of the factors that normally regulate β-cell maturation during neonatal development in vivo. For example, the drastic nutritional and metabolic changes that might affect the β-cell niche are not completely known and not recapitulated into SC β-cell differentiation protocols. In agreement with the effects of in vivo environment on SC β-cell maturation, in vivo maturation markers are expressed after transplantation. Functionally, β-cell maturation is characterized by secreting insulin in a glucose-dependent manner; in general, mature β cells secrete similar levels of insulin in response to glucose and to potassium (that induces membrane polarization and maximal insulin secretion), while in fetal β cells and SC β cells glucose induces much less than maximal insulin secretion. In addition, SC β cells secrete insulin in response to amino acids that are normally insufficient to induce insulin secretion in mature β cells. These functional differences may mirror the role of fetal β cells in mediating embryonic growth, which requires continuous insulin secretion, as opposed to mature β cells that secrete insulin only after meals. Recent work highlights the differences between fetal β cells to mature β cells in terms of glucose metabolism, mitochondrial activity, and nutrient sensing. It was recently shown that endocrine cell clustering or expression of mitochondrial activity regulators induces metabolic maturation by driving mitochondrial oxidative respiration, a process central to insulin secretion in mature β cells ( Yoshihara et al. 2016 ). These findings are encouraging, as generating cells that function more robustly could reduce the number of transplanted cells in diabetic patients and/or reverse diabetes more quickly.
It may be that the most significant challenge remaining for transplanting SC β cells to T1D patients is protecting the cells from immune rejection. Physical protection, by encapsulating the SC β cells in alginate microspheres or macrodevices, has shown promise ( Sneddon et al. 2018 ). Macroencapsulation devices have been used with SC progenitor cells to demonstrate blood glucose control in immunocompromised mice ( Motté et al. 2014 ; Robert et al. 2018 ).
Modified alginate spheres containing SC β cells have been transplanted into diabetic rodents, without immunosuppressants, and achieved long-term control of blood glucose levels with human insulin ( Bruin et al. 2013 ; Agulnick et al. 2015 ; Vegas et al. 2016 ; Chang et al. 2017 ; An et al. 2018 ; Alagpulinsa et al. 2019 ).
One of the challenges with physical protection by alginates or macrodevices is providing an adequate nutrient and oxygen supply without direct contact with blood oxygen. In all, encapsulation is a promising approach that could provide long-term glucose control with SC β cells.
A complementary approach is to use engineering strategies to provide immunity protection. Suggested gene modifications in the transplanted cells that could be used to induce immune tolerance include tolerogenic cytokines and immunomodulatory proteins such as HLA-G, PD-L1, and CTLA-4 as well as reducing HLA expression ( El Khatib et al. 2015 ; Figueiredo and Blasczyk 2015 ; Li et al. 2015 ; Gornalusse et al. 2017 ). In this manner, expression may be able to increase survival also after allogeneic transplantation and could be used as universal donor β cells that would match all patients, independent of the genetic background and HLA type of the recipient. These modifications could enhance tolerance to β-cell antigens without affecting the patient's immune system by applying immunosuppressive drugs. One could also take the complementary approach of trying to blunt the T-cell-specific attack on β cells, perhaps by modulating Treg cells ( Bluestone and Tang 2018 ).
Importantly, approaches to induce immune protection to SC cells has the potential concern of creating rogue cells that cannot be eliminated by the immune system. These contaminating or rogue cells might proliferate and cause harm. Using inducible suicide genes may provide protection against this happenstance ( Liang et al. 2018 ). Transplanting cells within a protective device in an accessible location where they can be readily removed will reduce this risk.
Tremendous progress has been made in the last decades toward understanding how β cells are formed during normal development and how to generate functional β-like cells through stem-cell differentiation. Yet additional challenges remain for this field. The immune system problem needs to be addressed, whether through systemic immunomodulation or by using immunoprotective devices. Moreover, clinical trials should be executed to address safety and efficiency questions including optimal implant size and whether other endocrine cells may be required for the best metabolic control. The clinical need for an unlimited source of β-like cells will require methods to generate homogenous cell populations on a very large scale by moving toward industrial production. In summary, 100 years after the discovery of insulin, we have the possibility of more breakthroughs and a meaningful cure for insulin-dependent diabetics.
Editors: Cristina Lo Celso, Kristy Red-Horse, and Fiona M. Watt
Additional Perspectives on Stem Cells: From Biological Principles to Regenerative Medicine available at www.cshperspectives.org
- Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, Kelly JR, Haakmeester C, Srijemac R, Wilson AZ, et al. 2015. Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo . Stem Cells Transl Med 4 : 1214–1222. 10.5966/sctm.2015-0079 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Alagpulinsa DA, Cao JJL, Driscoll RK, Sirbulescu RF, Penson MFE, Sremac M, Engquist EN, Brauns TA, Markmann JF, Melton DA, et al. 2019. Alginate-microencapsulation of human stem cell-derived β cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression . Am J Transplant 19 : 1930–1940. [ PubMed ] [ Google Scholar ]
- An D, Chiu A, Flanders JA, Song W, Shou D, Lu YC, Grunnet LG, Winkel L, Ingvorsen C, Christophersen NS, et al. 2018. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes . Proc Natl Acad Sci 115 : E263–E272. 10.1073/pnas.1708806115 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Apelqvist A, Ahlgren U, Edlund H. 1997. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas . Curr Biol 7 : 801–804. 10.1016/S0960-9822(06)00340-X [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, de Angelis M H, Lendahl U, Edlund H. 1999. Notch signalling controls pancreatic cell differentiation . Nature 400 : 877–881. 10.1038/23716 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Arda HE, Li L, Tsai J, Torre EA, Rosli Y, Peiris H, Spitale RC, Dai C, Gu X, Qu K, et al. 2016. Age-dependent pancreatic gene regulation reveals mechanisms governing human β cell function . Cell Metab 23 : 909–920. 10.1016/j.cmet.2016.04.002 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ashcroft FM, Rorsman P. 2012. Diabetes mellitus and the β cell: the last ten years . Cell 148 : 1160–1171. 10.1016/j.cell.2012.02.010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bakhti M, Böttcher A, Lickert H. 2019. Modelling the endocrine pancreas in health and disease . Nat Rev Endocrinol 15 : 155–171. 10.1038/s41574-018-0132-z [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. 1922. Pancreatic extracts in the treatment of diabetes mellitus . Can Med Assoc J 12 : 141–146. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bellin MD, Kandaswamy R, Parkey J, Zhang HJ, Liu B, Ihm SH, Ansite JD, Witson J, Bansal-Pakala P, Balamurugan AN, et al. 2008. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes . Am J Transplant 8 : 2463–2470. 10.1111/j.1600-6143.2008.02404.x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Berney T, Ferrari-Lacraz S, Bühler L, Oberholzer J, Marangon N, Philippe J, Villard J, Morel P. 2009. Long-term insulin-independence after allogeneic islet transplantation for type 1 diabetes: over the 10-year mark . Am J Transplant 9 : 419–423. 10.1111/j.1600-6143.2008.02481.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bluestone JA, Tang Q. 2018. T reg cells—the next frontier of cell therapy . Science 362 : 154–155. 10.1126/science.aau2688 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Blum B, Hrvatin S, Schuetz C, Bonal C, Rezania A, Melton DA. 2012. Functional β-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3 . Nat Biotechnol 30 : 261–264. 10.1038/nbt.2141 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, Robertson EJ. 2001. Nodal signalling in the epiblast patterns the early mouse embryo . Nature 411 : 965–969. 10.1038/35082103 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bruin JE, Rezania A, Xu J, Narayan K, Fox JK, O'Neil JJ, Kieffer TJ. 2013. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice . Diabetologia 56 : 1987–1998. 10.1007/s00125-013-2955-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Carrasco M, Delgado I, Soria B, Martín F, Rojas A. 2012. GATA4 and GATA6 control mouse pancreas organogenesis . J Clin Invest 122 : 3504–3515. 10.1172/JCI63240 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chang R, Faleo G, Russ HA, Parent AV, Elledge SK, Bernards DA, Allen JL, Villanueva K, Hebrok M, Tang Q, et al. 2017. Nanoporous immunoprotective device for stem-cell-derived β-cell replacement therapy . ACS Nano 11 : 7747–7757. 10.1021/acsnano.7b01239 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen AE, Borowiak M, Sherwood RI, Kweudjeu A, Melton DA. 2013. Functional evaluation of ES cell-derived endodermal populations reveals differences between Nodal and Activin A–guided differentiation . Development 140 : 675–686. 10.1242/dev.085431 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cryer PE. 2013. Mechanisms of hypoglycemia-associated autonomic failure in diabetes . N Engl J Med 369 : 362–372. 10.1056/NEJMra1215228 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. 2005. Efficient differentiation of human embryonic stem cells to definitive endoderm . Nat Biotechnol 23 : 1534–1541. 10.1038/nbt1163 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, et al. 2015. Type 2 diabetes mellitus . Nat Rev Dis Primers 1 : 15019 10.1038/nrdp.2015.19 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dor Y, Brown J, Martinez OI, Melton DA. 2004. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation . Nature 429 : 41–46. 10.1038/nature02520 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dutta D, Heo I, Clevers H. 2017. Disease modeling in stem cell-derived 3D organoid systems . Trends Mol Med 23 : 393–410. 10.1016/j.molmed.2017.02.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- El Khatib MM, Sakuma T, Tonne JM, Mohamed MS, Holditch SJ, Lu B, Kudva YC, Ikeda Y. 2015. β-Cell-targeted blockage of PD1 and CTLA4 pathways prevents development of autoimmune diabetes and acute allogeneic islets rejection . Gene Ther 22 : 430–438. 10.1038/gt.2015.18 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Figueiredo C, Blasczyk R. 2015. A future with less HLA: potential clinical applications of HLA-universal cells . Tissue Antigens 85 : 443–449. 10.1111/tan.12564 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fukuda A, Kawaguchi Y, Furuyama K, Kodama S, Horiguchi M, Kuhara T, Kawaguchi M, Terao M, Doi R, Wright CV, et al. 2008. Reduction of Ptf1a gene dosage causes pancreatic hypoplasia and diabetes in mice . Diabetes 57 : 2421–2431. 10.2337/db07-1558 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. 2008. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development . Genes Dev 22 : 3435–3448. 10.1101/gad.1752608 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gittes GK. 2009. Developmental biology of the pancreas: a comprehensive review . Dev Biol 326 : 4–35. 10.1016/j.ydbio.2008.10.024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gornalusse GG, Hirata RK, Funk SE, Riolobos L, Lopes VS, Manske G, Prunkard D, Colunga AG, Hanafi LA, Clegg DO, et al. 2017. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells . Nat Biotechnol 35 : 765–772. 10.1038/nbt.3860 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gouzi M, Kim YH, Katsumoto K, Johansson K, Grapin-Botton A. 2011. Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development . Dev Dyn 240 : 589–604. 10.1002/dvdy.22544 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. 2000. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas . Proc Natl Acad Sci 97 : 1607–1611. 10.1073/pnas.97.4.1607 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gu G, Dubauskaite J, Melton DA. 2002. Direct evidence for the pancreatic lineage: NGN3 + cells are islet progenitors and are distinct from duct progenitors . Development 129 : 2447–2457. [ PubMed ] [ Google Scholar ]
- Gu G, Brown JR, Melton DA. 2003. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis . Mech Dev 120 : 35–43. 10.1016/S0925-4773(02)00330-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gupta RK, Vatamaniuk MZ, Lee CS, Flaschen RC, Fulmer JT, Matschinsky FM, Duncan SA, Kaestner KH. 2005. The MODY1 gene HNF-4α regulates selected genes involved in insulin secretion . J Clin Invest 115 : 1006–1015. 10.1172/JCI200522365 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hart A, Papadopoulou S, Edlund H. 2003. Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells . Dev Dyn 228 : 185–193. 10.1002/dvdy.10368 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Helman A, Avrahami D, Klochendler A, Glaser B, Kaestner KH, Ben-Porath I, Dor Y. 2016. Effects of ageing and senescence on pancreatic β-cell function . Diabetes Obes Metab 18 : 58–62. 10.1111/dom.12719 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Henry GL, Brivanlou IH, Kessler DS, Hemmati-Brivanlou A, Melton DA. 1996. TGF-β signals and a pattern in Xenopus laevis endodermal development . Development 122 : 1007–1015. [ PubMed ] [ Google Scholar ]
- Henseleit KD, Nelson SB, Kuhlbrodt K, Hennings JC, Ericson J, Sander M. 2005. NKX6 transcription factor activity is required for α- and β-cell development in the pancreas . Development 132 : 3139–3149. 10.1242/dev.01875 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hrvatin S, O'Donnell CW, Deng F, Millman JR, Pagliuca FW, DiIorio P, Rezania A, Gifford DK, Melton DA. 2014. Differentiated human stem cells resemble fetal, not adult, β cells . Proc Natl Acad Sci 111 : 3038–3043. 10.1073/pnas.1400709111 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Huch M, Koo BK. 2015. Modeling mouse and human development using organoid cultures . Development 142 : 3113–3125. 10.1242/dev.118570 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jennings RE, Berry AA, Kirkwood-Wilson R, Roberts NA, Hearn T, Salisbury RJ, Blaylock J, Piper Hanley K, Hanley NA. 2013. Development of the human pancreas from foregut to endocrine commitment . Diabetes 62 : 3514–3522. 10.2337/db12-1479 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jennings RE, Berry AA, Strutt JP, Gerrard DT, Hanley NA. 2015. Human pancreas development . Development 142 : 3126–3137. 10.1242/dev.120063 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jonsson J, Carlsson L, Edlund T, Edlund H. 1994. Insulin-promoter-factor 1 is required for pancreas development in mice . Nature 371 : 606–609. 10.1038/371606a0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jonsson J, Ahlgren U, Edlund T, Edlund H. 1995. IPF1, a homeodomain protein with a dual function in pancreas development . Int J Dev Biol 39 : 789–798. [ PubMed ] [ Google Scholar ]
- Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, Jacobsen LM, Schatz DA, Lernmark A. 2017. Type 1 diabetes mellitus . Nat Rev Dis Primers 3 : 17016 10.1038/nrdp.2017.16 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim SK, Melton DA. 1998. Pancreas development is promoted by cyclopamine, a hedgehog signaling inhibitor . Proc Natl Acad Sci 95 : 13036–13041. 10.1073/pnas.95.22.13036 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim SK, Hebrok M, Melton DA. 1997a. Notochord to endoderm signaling is required for pancreas development . Development 124 : 4243–4252. [ PubMed ] [ Google Scholar ]
- Kim SK, Hebrok M, Melton DA. 1997b. Pancreas development in the chick embryo . Cold Spring Harb Symp Quant Biol 62 : 377–383. 10.1101/SQB.1997.062.01.045 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim SK, Hebrok M, Li E, Oh SP, Schrewe H, Harmon EB, Lee JS, Melton DA. 2000. Activin receptor patterning of foregut organogenesis . Genes Dev 14 : 1866–1871. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, et al. 2008. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo . Nat Biotechnol 26 : 443–452. [ PubMed ] [ Google Scholar ]
- Langer RM. 2010. Islet transplantation: lessons learned since the Edmonton breakthrough . Transplant Proc 42 : 1421–1424. 10.1016/j.transproceed.2010.04.021 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Larsen HL, Grapin-Botton A. 2017. The molecular and morphogenetic basis of pancreas organogenesis . Semin Cell Dev Biol 66 : 51–68. 10.1016/j.semcdb.2017.01.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Latres E, Finan DA, Greenstein JL, Kowalski A, Kieffer TJ. 2019. Navigating two roads to glucose normalization in diabetes: automated insulin delivery devices and cell therapy . Cell Metab 29 : 545–563. 10.1016/j.cmet.2019.02.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lee K, Cho H, Rickert RW, Li QV, Pulecio J, Leslie CS, Huangfu D. 2019. FOXA2 is required for enhancer priming during pancreatic differentiation . Cell Rep 28 : 382–393.e7. 10.1016/j.celrep.2019.06.034 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li R, Lee J, Kim MS, Liu V, Moulik M, Li H, Yi Q, Xie A, Chen W, Yang L, et al. 2015. PD-L1-driven tolerance protects neurogenin3-induced islet neogenesis to reverse established type 1 diabetes in NOD mice . Diabetes 64 : 529–540. 10.2337/db13-1737 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liang Q, Monetti C, Shutova MV, Neely EJ, Hacibekiroglu S, Yang H, Kim C, Zhang P, Li C, Nagy K, et al. 2018. Linking a cell-division gene and a suicide gene to define and improve cell therapy safety . Nature 563 : 701–704. 10.1038/s41586-018-0733-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Motté E, Szepessy E, Suenens K, Stangé G, Bomans M, Jacobs-Tulleneers-Thevissen D, Ling Z, Kroon E, Pipeleers D; Beta Cell Therapy Consortium EU-FP7. 2014. Composition and function of macroencapsulated human embryonic stem cell-derived implants: comparison with clinical human islet cell grafts . Am J Physiol Endocrinol Metab 307 : E838–E846. 10.1152/ajpendo.00219.2014 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Murry CE, Keller G. 2008. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development . Cell 132 : 661–680. 10.1016/j.cell.2008.02.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nair GG, Liu JS, Russ HA, Tran S, Saxton MS, Chen R, Juang C, Li ML, Nguyen VQ, Giacometti S, et al. 2019. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells . Nat Cell Biol 21 : 263–274. 10.1038/s41556-018-0271-4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nostro MC, Keller G. 2012. Generation of β cells from human pluripotent stem cells: potential for regenerative medicine . Semin Cell Dev Biol 23 : 701–710. 10.1016/j.semcdb.2012.06.010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nostro MC, Sarangi F, Yang C, Holland A, Elefanty AG, Stanley EG, Greiner DL, Keller G. 2015. Efficient generation of NKX6-1 + pancreatic progenitors from multiple human pluripotent stem cell lines . Stem Cell Reports 4 : 591–604. 10.1016/j.stemcr.2015.02.017 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. 1996. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum . Development 122 : 983–995. [ PubMed ] [ Google Scholar ]
- Okita K, Ichisaka T, Yamanaka S. 2007. Generation of germline-competent induced pluripotent stem cells . Nature 448 : 313–317. 10.1038/nature05934 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. 2014. Generation of functional human pancreatic β cells in vitro . Cell 159 : 428–439. 10.1016/j.cell.2014.09.040 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pan FC, Wright C. 2011. Pancreas organogenesis: from bud to plexus to gland . Dev Dyn 240 : 530–565. 10.1002/dvdy.22584 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pathak V, Pathak NM, O'Neill CL, Guduric-Fuchs J, Medina RJ. 2019. Therapies for type 1 diabetes: current scenario and future perspectives . Clin Med Insights Endocrinol Diabetes 12 : 1179551419844521 10.1177/1179551419844521 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Puri S, Roy N, Russ HA, Leonhardt L, French EK, Roy R, Bengtsson H, Scott DK, Stewart AF, Hebrok M. 2018. Replication confers β cell immaturity . Nat Commun 9 : 485 10.1038/s41467-018-02939-0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Quianzon CC, Cheikh I. 2012. History of insulin . J Community Hosp Intern Med Perspect 2 10.3402/jchimp.v2i2.18701 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rankin SA, McCracken KW, Luedeke DM, Han L, Wells JM, Shannon JM, Zorn AM. 2018. Timing is everything: reiterative Wnt, BMP and RA signaling regulate developmental competence during endoderm organogenesis . Dev Biol 434 : 121–132. 10.1016/j.ydbio.2017.11.018 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O'Dwyer S, Quiskamp N, Mojibian M, Albrecht T, et al. 2014. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells . Nat Biotechnol 32 : 1121–1133. 10.1038/nbt.3033 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Robert T, De Mesmaeker I, Stangé GM, Suenens KG, Ling Z, Kroon EJ, Pipeleers DG. 2018. Functional β cell mass from device-encapsulated hESC-derived pancreatic endoderm achieving metabolic control . Stem Cell Reports 10 : 739–750. 10.1016/j.stemcr.2018.01.040 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Röder PV, Wu B, Liu Y, Han W. 2016. Pancreatic regulation of glucose homeostasis . Exp Mol Med 48 : e219 10.1038/emm.2016.6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rosado-Olivieri EA, Anderson K, Kenty JH, Melton DA. 2019. YAP inhibition enhances the differentiation of functional stem cell-derived insulin-producing β cells . Nat Commun 10 : 1464 10.1038/s41467-019-09404-6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Russ HA, Parent AV, Ringler JJ, Hennings TG, Nair GG, Shveygert M, Guo T, Puri S, Haataja L, Cirulli V, et al. 2015. Controlled induction of human pancreatic progenitors produces functional β-like cells in vitro . EMBO J 34 : 1759–1772. 10.15252/embj.201591058 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Scharp DW, Lacy PE, Santiago JV, McCullough CS, Weide LG, Falqui L, Marchetti P, Gingerich RL, Jaffe AS, Cryer PE, et al. 1990. Insulin independence after islet transplantation into type I diabetic patient . Diabetes 39 : 515–518. 10.2337/diab.39.4.515 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shapiro AM, Nanji SA, Lakey JR. 2003. Clinical islet transplant: current and future directions towards tolerance . Immunol Rev 196 : 219–236. 10.1046/j.1600-065X.2003.00085.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, et al. 2006. International trial of the Edmonton protocol for islet transplantation . N Engl J Med 355 : 1318–1330. 10.1056/NEJMoa061267 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharon N, Chawla R, Mueller J, Vanderhooft J, Whitehorn LJ, Rosenthal B, Gürtler M, Estanboulieh RR, Shvartsman D, Gifford DK, et al. 2019a. A peninsular structure coordinates asynchronous differentiation with morphogenesis to generate pancreatic islets . Cell 176 : 790–804.e13. 10.1016/j.cell.2018.12.003 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharon N, Vanderhooft J, Straubhaar J, Mueller J, Chawla R, Zhou Q, Engquist EN, Trapnell C, Gifford DK, Melton DA. 2019b. Wnt signaling separates the progenitor and endocrine compartments during pancreas development . Cell Rep 27 : 2281–2291.e5. 10.1016/j.celrep.2019.04.083 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sneddon JB, Tang Q, Stock P, Bluestone JA, Roy S, Desai T, Hebrok M. 2018. Stem cell therapies for treating diabetes: progress and remaining challenges . Cell Stem Cell 22 : 810–823. 10.1016/j.stem.2018.05.016 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Socci C, Falqui L, Davalli AM, Ricordi C, Braghi S, Bertuzzi F, Maffi P, Secchi A, Gavazzi F, Freschi M, et al. 1991. Fresh human islet transplantation to replace pancreatic endocrine function in type 1 diabetic patients—report of six cases . Acta Diabetol 28 : 151–157. 10.1007/BF00579718 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. 1997. The Pax4 gene is essential for differentiation of insulin-producing β cells in the mammalian pancreas . Nature 386 : 399–402. 10.1038/386399a0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. 1997. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence . Nat Genet 15 : 106–110. 10.1038/ng0197-106 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Takahashi K, Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors . Cell 126 : 663–676. 10.1016/j.cell.2006.07.024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors . Cell 131 : 861–872. 10.1016/j.cell.2007.11.019 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Taylor BL, Liu FF, Sander M. 2013. Nkx6.1 is essential for maintaining the functional state of pancreatic β cells . Cell Rep 4 : 1262–1275. 10.1016/j.celrep.2013.08.010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tremblay KD. 2010. Formation of the murine endoderm: lessons from the mouse, frog, fish, and chick . Prog Mol Biol Transl Sci 96 : 1–34. 10.1016/B978-0-12-381280-3.00001-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, et al. 2016. Long-term glycemic control using polymer-encapsulated human stem cell-derived β cells in immune-competent mice . Nat Med 22 : 306–311. 10.1038/nm.4030 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Velazco-Cruz L, Song J, Maxwell KG, Goedegebuure MM, Augsornworawat P, Hogrebe NJ, Millman JR. 2019. Acquisition of dynamic function in human stem cell-derived β cells . Stem Cell Reports 12 : 351–365. 10.1016/j.stemcr.2018.12.012 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Veres A, Faust AL, Bushnell HL, Engquist EN, Kenty JH, Harb G, Poh YC, Sintov E, Gürtler M, Pagliuca FW, et al. 2019. Charting cellular identity during human in vitro β-cell differentiation . Nature 569 : 368–373. 10.1038/s41586-019-1168-5 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Villamayor L, Rodríguez-Seguel E, Araujo R, Carrasco M, Bru-Tarí E, Mellado-Gil JM, Gauthier BR, Martinelli P, Quesada I, Soria B, et al. 2018. GATA6 controls insulin biosynthesis and secretion in adult β-cells . Diabetes 67 : 448–460. 10.2337/db17-0364 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wandzioch E, Zaret KS. 2009. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors . Science 324 : 1707–1710. 10.1126/science.1174497 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wells JM, Melton DA. 1999. Vertebrate endoderm development . Annu Rev Cell Dev Biol 15 : 393–410. 10.1146/annurev.cellbio.15.1.393 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wells JM, Melton DA. 2000. Early mouse endoderm is patterned by soluble factors from adjacent germ layers . Development 127 : 1563–1572. [ PubMed ] [ Google Scholar ]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. 2007. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state . Nature 448 : 318–324. 10.1038/nature05944 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yoshihara E, Wei Z, Lin CS, Fang S, Ahmadian M, Kida Y, Tseng T, Dai Y, Yu RT, Liddle C, et al. 2016. ERRγ is required for the metabolic maturation of therapeutically functional glucose-responsive β cells . Cell Metab 23 : 622–634. 10.1016/j.cmet.2016.03.005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, et al. 2005. MafA is a key regulator of glucose-stimulated insulin secretion . Mol Cell Biol 25 : 4969–4976. 10.1128/MCB.25.12.4969-4976.2005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
A 25-year-old woman with type 1 diabetes started producing her own insulin less than three months after receiving a transplant of reprogrammed stem cells 1. She is the first person with the ...
With the advent of research on stem cell therapy for various diseases, breakthroughs in stem cell-based therapy for T1DM have been reported. However, many unsolved issues need to be addressed before stem cell therapy will be clinically feasible for diabetic patients. ... Keywords: Type 1 diabetes mellitus, Stem cells, Insulin-producing cells ...
Promising early results show that longstanding Harvard Stem Cell Institute (HSCI) research may have paved the way for a breakthrough treatment of Type 1 diabetes. Utilizing research from the Melton Lab, Vertex Pharmaceuticals has developed VX-880, an investigational stem cell-derived, fully differentiated pancreatic islet cell replacement therapy for people with type 1 diabetes (T1D).
Keywords: type 1 diabetes mellitus, stem cell, β-cell, immunotolerance, ... In recent research, stem cell therapy has demonstrated itself as a rapidly expanding and potentially limitless source of β-cells to arrive at a cure for T1DM by reconstitution of immunotolerance and differentiation into islet β-cell clusters. As the immunosuppression ...
Most people with type 1 diabetes cannot reverse their autoimmune disease or put it into spontaneous remission - all they can do is manage their blood sugar levels with insulin, a healthy diet, and regular exercise. ... Stem Cells Reverse Type 1 Diabetes in Clinical Trial. Health 09 October 2024. ... who was not involved in the research, told ...
Over the past decade, there had been progress in the development of cell therapy for insulin-dependent diabetes. Nevertheless, important hurdles that need to be overcome still remain. Protocols for the differentiation of pluripotent stem cells into pancreatic progenitors or fully differentiated β-cells have been developed. The resulting insulin-producing cells can control chemically induced ...
How stem cells could fix type 1 diabetes. Trials to replace the pancreatic β cells that are destroyed by this autoimmune disease are raising hopes of a cure. Encapsulated stem cell-derived islets ...
Metabolic engineering. Type 1 diabetes. For over two decades pluripotent stem cells have promised a renewable source of β cells to treat patients with type 1 diabetes. Major efforts to optimize ...
Glucose-Dependent Insulin Production and Insulin-Independence in Type 1 Diabetes from Stem Cell-Derived, Fully Differentiated Islet Cells—Updated Data from the VX-880 Clinical Trial ... the world's largest scientific meeting focused on diabetes research, prevention, and care, will be held in San Diego, CA on June 23-26. More than 12,000 ...
For Harvard Stem Cell Institute Co-Director and Xander University Professor Douglas Melton, whose lab pioneered the science behind the therapy, the trial marked the most recent turning point in a decades-long effort to understand and treat the disease. In a conversation with the Gazette, Melton discussed the science behind the advance, the ...
A groundbreaking discovery has recently brought hope to millions of people living with type 1 diabetes around the world. In a world first, scientists have successfully used stem cell therapy to ...
It's rare for medical research to be truly 'groundbreaking', but the case study from China showing that stem cell therapy can reverse type 1 diabetes is worthy of this adjective.
Dec. 11, 2023 - An experimental device containing millions of stem cells significantly reduced the need for insulin shots among people with type 1 diabetes, according to a new study - a ...
To accurately identify clinical trials of stem cell transplantation in DM patients, a search was performed using a combination of medical subject heading (MeSH) terms and text words: "diabetes mellitus, type 1" or "diabetes mellitus, type 2" and "stem cell transplantation" and "therapy" or "therapeutic use." Inclusion and Exclusion Criteria
Type 1 diabetes mellitus (T1DM) is the most common chronic autoimmune disease in young patients and is characterized by the loss of pancreatic β cells; as a result, the body becomes insulin deficient and hyperglycemic. Administration or injection of exogenous insulin cannot mimic the endogenous insulin secreted by a healthy pancreas. Pancreas and islet transplantation have emerged as ...
On Feb. 2, ViaCyte and CRISPR Therapeutics announced that the first participant in the trial had received the PEC-QT implant. This marks the first gene-edited, stem cell-derived pancreatic cells to be implanted in a human that are specifically designed to evade the immune system in treating type 1 diabetes. All in all, Foyt is optimistic that ...
Encapsulated stem cell-derived β cells exert glucose control in patients with type 1 diabetes. Nature Biotechnology , 2023; DOI: 10.1038/s41587-023-02055-5 Cite This Page :
An investigative stem cell-based therapy called PEC-Direct, designed to act as a replacement pancreas, has the potential to provide blood sugar control in patients with high-risk type 1 diabetes, suggests a clinical study presented Saturday, June 11at ENDO 2022, the Endocrine Society's annual meeting in Atlanta, Ga.
For example, our goal is to mature stem cells into insulin-producing beta cells and transplant them into someone with Type 1 diabetes. We could replace the beta cells they lost and reverse the disease." The biggest challenge to stem cell therapies comes from the immune system, the body's natural protector against invaders.
Clinical studies on the treatment of type 1 diabetes with device-encapsulated pancreatic precursor cells derived from human embryonic stem cells found that insulin output was insufficient for ...
This critical advance has enabled the first beta cell replacement clinical trial for type 1 diabetes. We are now focused on devising strategies to effectively protect beta cells from attack by the immune system. What we are investigating Our researchers have discovered how to reprogram adult and embryonic stem cells into new beta cells.
Type 1 diabetes mellitus (T1DM) is the most common chronic autoimmune disease in young patients and is characterized by the loss of pancreatic β cells; as a result, the body becomes insulin deficient and hyperglycemic. ... With the advent of research on stem cell therapy for various diseases, breakthroughs in stem cell-based therapy for T1DM ...
Stem cell therapies at the moment are being tested in people with type 1 diabetes and frequent hypos and hypo unawareness, for whom this pioneering treatment could be life-saving. But as research advances, we hope stem cells-turned-beta cells could also be used to boost beta cell supply in people with type 2 diabetes and help them to make ...
A curated list of completed, active, recruiting, and suspended stem cell-based clinical trials for both T1D and T2D, registered at ClinicalTrials.gov within the last ten years, is presented in Table 1 . The majority of the recently completed and active trials use adult mesenchymal stem cells (MSC) derived from different origins, hematopoietic ...
This review provides a detailed overview of the latest research and clinical applications of HSCT in treating ADs, offering new insights for clinicians aiming to optimize its use for ADs management. ... Immunological Balance Is Associated with Clinical Outcome after Autologous Hematopoietic Stem Cell Transplantation in Type 1 Diabetes. Front ...
A 25-year-old woman with type 1 diabetes has been able to stop taking insulin after a groundbreaking stem cell treatment. This is the first human trial of the procedure, and while it's too soon ...
The isolation of the first human embryonic stem cell (hESC) lines in 1998 opened the possibility of stem cell therapies for a variety of conditions. ... Type 1 diabetes (T1D) is particularly ...
In June 2023, Peking University researchers successfully reversed type 1 diabetes in a patient, opening the possibility of a universal cure. ScienceAlert reports these doctors injected roughly 1.5
Investigators have released a preliminary analysis of a first-in-human study showing that stem-cell-derived islets functionally cured a patient's type 1 diabetes (T1D), according to the analysis published in Cell.The analysis included 1 year of data, which assessed the feasibility of autologous transplantation of chemically induced pluripotent stem cell-derived islets.
Developmental research on pancreas formation has informed in vitro differentiation of human pluripotent stem cells into functional islets. Although generating β cells from stem cells offers a potential cure for type 1 diabetes, several challenges remain, including protecting the cells from the immune system.