Cornell Chronicle
- Architecture & Design
- Arts & Humanities
- Business, Economics & Entrepreneurship
- Computing & Information Sciences
- Energy, Environment & Sustainability
- Food & Agriculture
- Global Reach
- Health, Nutrition & Medicine
- Law, Government & Public Policy
- Life Sciences & Veterinary Medicine
- Physical Sciences & Engineering
- Social & Behavioral Sciences
- Coronavirus
- News & Events
- Public Engagement
- New York City
- Photos of the Day
- Big Red Sports
- Freedom of Expression
- Student Life
- University Statements
- Around Cornell
- All Stories
- In the News
- Expert Quotes
- Cornellians

Large-scale study reveals new genetic details of diabetes
By wynne parry weill cornell medicine.
In experiments of unprecedented scale, investigators at Weill Cornell Medicine and the National Institutes of Health have revealed new aspects of the complex genetics behind Type 2 diabetes. Through these discoveries, and by providing a template for future studies, this research furthers efforts to better understand and ultimately treat this common metabolic disease.
Previous studies have generally examined the influence of individual genes. In research described Oct. 18 in Cell Metabolism, senior co-author Shuibing Chen , the Kilts Family Professor of Surgery at Weill Cornell Medicine, working alongside senior co-author Dr. Francis Collins , a senior investigator at the Center for Precision Health Research within the National Human Genome Research Institute of the U.S. National Institutes of Health, took a more comprehensive approach. Together, they looked at the contribution of 20 genes in a single effort.
“It’s very difficult to believe all these diabetes-related genes act independently of each other,” Chen said. By using a combination of technologies, the team examined the effects of shutting each down. By comparing the consequences for cell behavior and genetics, she said, “we found some common themes.”
As with other types of diabetes, Type 2 diabetes occurs when sugar levels in the blood are too high. In Type 2 diabetes, this happens in part because specialized cells in the pancreas, known as β-cells, don’t produce enough insulin, a hormone that tells cells to take sugar out of the blood for use as an energy source. Over time, high levels of blood sugar damage tissues and cause other problems, such as heart and kidney disease. According to the United States Centers for Disease Control and Prevention, nearly 9% of adults in the United States have been diagnosed with Type 2 diabetes.
Both genetic and environmental factors, such as obesity and chronic stress, can increase risk for it. Yet evaluating the role of the genetic contributors alone is a massive project. So far, researchers have identified more than 290 locations within the genome where changes to DNA can raise the likelihood of developing the disease. Some of these locations fall within known genes, but most are found in regions that regulate the expression of nearby genes.
For the new research, the team focused on 20 genes clearly identified as contributors. They began their investigation by using the gene editing system CRISPR-Cas9 to shut down these genes, one at a time, within 20 sets of identical stem cells.
These stem cells had the potential to generate any kind of mature cell, but the researchers coaxed them into becoming insulin-producing β-cells. They then examined the effects of losing each gene on five traits related to insulin production and the health of β-cells. They also documented the accompanying changes in gene expression and the accessibility of DNA for expression.
To make sense of the massive amount of data they collected, the team developed their own computational models to analyze it, leading to several discoveries: By comparing the effects of all 20 mutations on β-cells, they identified four additional genes, each representing a newly discovered pathway that contributes to insulin production. They also found that, of the original 20 genes, only one, called HNF4A, contributed to all five traits, apparently by acting as a master controller that regulates the activity of other genes. In one specific example, they explained how a small variation, located in a space between genes, contributes to the risk of diabetes by interfering with HNF4A’s ability to regulate nearby genes.
Ultimately, this study and others like it hold the promise of benefiting patients, Collins said. “We need to understand all the genetic and environmental factors involved so we can do a better job of preventing diabetes, and to develop new ideas about how to effectively treat it.”
Collins and Chen note that their approach may have relevance beyond diabetes, to other common diseases, such as Alzheimer’s, Parkinson’s and Crohn’s disease, that involve many genetic factors.
The work reported in this newsroom story was supported in part by the United States’ National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases and the American Diabetes Association.
Many Weill Cornell Medicine physicians and scientists maintain relationships and collaborate with external organizations to foster scientific innovation and provide expert guidance. The institution makes these disclosures public to ensure transparency. For this information, see the profile for Shuibing Chen .
Wynne Parry is a freelance writer for Weill Cornell Medicine.
Media Contact
Krystle lopez.
Get Cornell news delivered right to your inbox.
You might also like

Gallery Heading
These New Developments Could Make Living With Type 2 Diabetes More Manageable
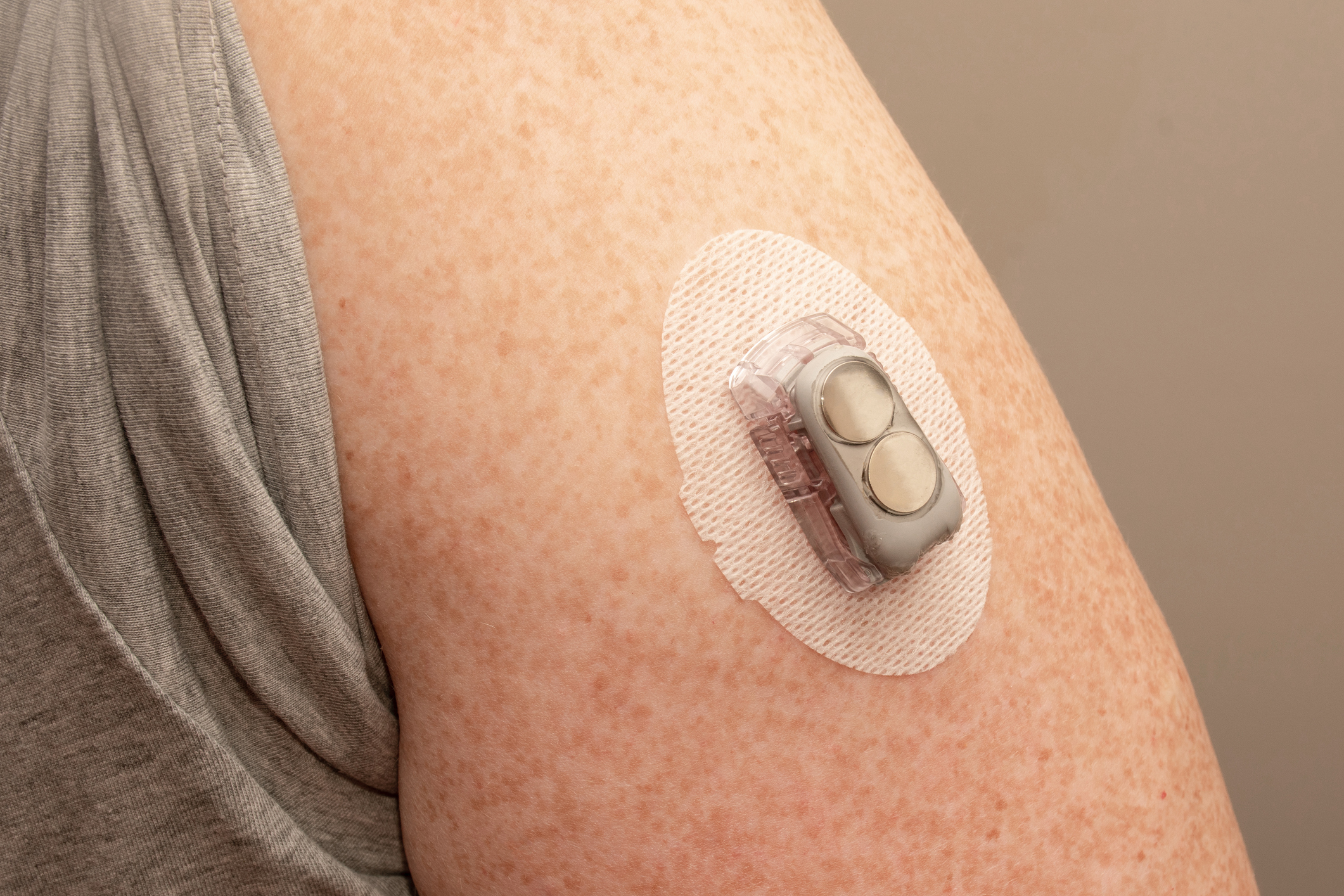
E xperts often talk about the “burden” of a disease or illness. The word acts as a tidy container for all the unpleasantness people with that condition may experience—from their symptoms, to the cost of their care, to the restrictions imposed on their lifestyle, to the health complications that may arise. For people with Type 2 diabetes , this burden can be high.
Routine management of Type 2 diabetes often involves major changes to one’s diet and physical activity . And for many, especially those taking insulin to manage their blood sugar, the disease can necessitate daily blood-glucose monitoring, a process that entails pricking a finger to draw blood and then dabbing that blood onto a glucose monitor’s test strip. Doing this several times a week—month after month—can present overlapping challenges. According to a 2013 survey in the journal Diabetes Spectrum, people find finger-prick glucose monitoring to be painful, and the results can be confusing or unhelpful.
“Patients don’t want to prick their fingers, and they come in all the time and say, ‘I’m tired of this,’” says Dr. Francisco Pasquel, a diabetes specialist and associate professor of medicine at Emory University School of Medicine in Atlanta.
But relief is on the way. Continuous glucose monitors, or CGMs, are small devices-—often about the size of a quarter-—that use a small under-the-skin needle to continuously monitor blood-glucose levels. This information can be transmitted—in some cases wirelessly and automatically—to a smartphone app or other device. “You can look at glucose levels for a single point in time, but you can also look at trends in values over time,” says Dr. Roy Beck, medical director of the nonprofit Jaeb Center for Health Research in Tampa. Beck’s work has found that continuous glucose monitoring may provide a number of benefits for people with Type 2 diabetes.
These monitors are just one of several new advancements in Type 2 diabetes care and management. From connected technologies to new drug treatments, medical science is making steady and sometimes life-changing progress in the treatment of this condition. Here, experts describe some of the latest and greatest developments.
Continuous glucose monitors
People with Type 1 diabetes typically have to check their blood-sugar levels on a daily basis, or even multiple times each day. Because testing is such a big part of managing that disease, the research on continuous glucose monitors started with these patients. That work has shown that CGMs provide multiple benefits, including reduced hemoglobin A1C (HbA1c) levels, which is an important measure of healthy blood glucose. Continuous glucose monitors are now being studied in people with Type 2 diabetes, and research points to multiple benefits.
For a study published in 2021 in the Journal of the American Medical Association, Beck and his colleagues compared continuous glucose monitoring to standard finger-prick tests among people with Type 2 diabetes who were using insulin. They found that continuous monitoring was associated with a significantly greater drop in HbA1c. They also found that continuous monitoring helped people avoid risky and severe drops in blood sugar (a.k.a. hypoglycemia). “It’s pretty clear that there’s a benefit for people with Type 2 diabetes who are using insulin,” he says.
More than 90% of people with diabetes have Type 2 diabetes, and Beck says that roughly 30% of these people are using insulin. In other words, there are many people with Type 2 diabetes who stand to benefit from continuous glucose monitoring. However, use of these monitors is still mostly confined to people with Type 1 diabetes. “Use is slowly increasing in Type 2 patients, but I think it’s still too low considering this is a non-pharmacological approach”—something many people prefer because it avoids the side effects of medications—“that can help people,” he says.
Even for people with Type 2 diabetes who are not taking insulin, Beck says that continuous glucose monitoring could be helpful. “There’s a need for more studies to prove it, but it makes sense that it would likely have benefits,” he says. For example, monitoring blood sugar in real time could help people make diet or lifestyle changes that reduce their risks for long-term health complications. “Normally, blood glucose following a meal shouldn’t go above 140 [mg/dL],” he says. But based on factors like diet, meal timing, and exercise habits, someone with Type 2 diabetes may experience post-meal blood-sugar spikes that surpass 200 or even 300 mg/dL. These spikes could cause few symptoms or short-term consequences, Beck says, but over time they can contribute to the development of common diabetes-related complications such as kidney failure, heart disease, or diabetic retinopathy (an eye condition that can cause blurry vision or blindness). “The first time people use these continuous monitors, it can be a real eye-opener,” he adds. “I think they could be most helpful for self-management, and Type 2 diabetes is a disease where self-management through diet and exercise can make a huge difference.”
Other experts second this. “Patients using these devices can receive a graph of their glucose values over time, which helps them understand the effects of nutrition on glucose control, or how they could modify their exercise to make improvements,” says Dr. Ilias Spanakis, an associate professor of medicine in the division of endocrinology, diabetes, and nutrition at the University of Maryland School of Medicine.
For patients who are reliant on insulin to manage their blood glucose, combining continuous glucose monitors with insulin pumps—devices that automatically inject insulin as needed—could also lead to major improvements. “Smart algorithms that connect the two can automatically adjust glucose based on glucose values,” Spanakis says. This is already possible, and it’s likely to become much more commonplace, he adds.
For many people with diabetes, continuous glucose monitoring could provide a safer and simpler path forward.
Read More: The Link Between Type 2 Diabetes and Psychiatric Disorders
Bariatric surgery for Type 2 diabetes
Historically, bariatric (weight-loss) surgery has been used primarily to help people manage severe obesity, which the U.S. Centers for Disease Control and Prevention defines as a BMI of 40 or higher. Many people who are severely obese also have diabetes, and research has found that these surgical procedures can help reduce the burden of Type 2 diabetes or even send it into remission.
A 2018 study from researchers at the University of Oklahoma found that Roux-en-Y gastric bypass surgery, a common bariatric procedure, vastly outperformed typical medical management techniques—such as diet changes, doctor’s visits, and prescription drugs—among people with Type 2 diabetes. Surgery led to diabetes remission in roughly 28% of patients, compared with a remission rate of just 4% among the non-surgery group, according to the study results. More research has found that bariatric surgery may effectively send Type 2 diabetes into remission.
“Surgery does not just lead to weight loss, but also to an improvement in glycemic control, which happens even before the weight loss occurs,” says Emory’s Pasquel, who has published work on the benefits of bariatric surgery for people with Type 2 diabetes. Exactly how the surgery does this isn’t well understood, he says. However, bariatric surgery affects appetite, food intake, caloric absorption, and multiple neuroendocrine pathways—all of which could contribute to its beneficial actions for people with Type 2 diabetes.
In the future, Pasquel says these procedures are likely to become more commonplace even for people with Type 2 diabetes who are not severely obese.
More from TIME
New pharmaceutical drugs.
There are a lot of different diabetes drugs on the market, each with its own risks and benefits. But experts say two types are emerging as potential “game changers” when it comes to Type 2 diabetes treatment.
Glucagon-like peptide 1 (GLP-1) is a hormone released in the gut during digestion—one that plays a role in blood-sugar homeostasis. A class of drugs known as GLP-1 receptor agonists can interact with GLP-1 receptors in ways that lower appetite, slow digestion, and provide other benefits for people with Type 2 diabetes. These GLP-1 drugs aren’t new. But Pasquel says the latest versions are different in that they work on two different receptors, not one. “Recent evidence shows that activating both receptors has a remarkable impact on weight loss and glycemic control,” he says. Especially for people with Type 2 diabetes who are at high risk for heart or arterial disease, he says that these new drugs seem to be a big upgrade over previous medications.
A second category of drug has also emerged as a standout in the treatment of Type 2 diabetes. Known as sodium-glucose cotransporter-2 (SGLT-2) inhibitors, these drugs help the kidneys remove sugar from a person’s blood. Not only does this improve blood-sugar control in people with Type 2 diabetes, but it also helps protect them from heart failure and kidney disease—two common and serious complications. Pasquel says these drugs are so effective that they’re now being used in people with heart failure or kidney disease who do not have Type 2 diabetes.
Read More: The Truth About Fasting and Type 2 Diabetes
Emerging ways to think about weight loss
Experts have long understood that weight loss can help people reduce their Type 2 diabetes symptoms and risks . This recognition has led to research on a number of weight-loss diets . More research is needed, but some of the latest studies suggest that fasting plans—in particular, intermittent fasting—may be particularly beneficial for people with Type 2 diabetes.
Intermittent fasting involves cutting out calorie-containing foods and drinks for an extended period of time—anywhere from 12 hours to two days depending on the approach a person chooses. A 2019 research review in the journal Nutrients found that intermittent fasting promotes weight loss, increases insulin sensitivity, and reduces insulin levels in the blood. All of this is helpful for people with Type 2 diabetes. “Essentially, fasting is doing what we prescribe diabetes medications to do, which is to improve insulin sensitivity,” says Benjamin Horne, director of cardiovascular and genetic epidemiology at Intermountain Healthcare in Utah.
It’s not yet clear which form of intermittent fasting is best. But Horne says that time-restricted eating—a type of fasting that involves squeezing all the day’s calories into single six- or eight-hour feeding windows—is leading the pack, largely because patients are able to stick with it.
There are more new advancements in Type 2 diabetes care. The interventions described here—from continuous glucose monitors to novel drugs—are some of the most promising, but they have company. It’s safe to say that, looking ahead, more people with Type 2 diabetes will be able to effectively manage or mitigate their symptoms.
More Must-Reads From TIME
- Jane Fonda Champions Climate Action for Every Generation
- Biden’s Campaign Is In Trouble. Will the Turnaround Plan Work?
- Why We're Spending So Much Money Now
- The Financial Influencers Women Actually Want to Listen To
- Breaker Sunny Choi Is Heading to Paris
- Why TV Can’t Stop Making Silly Shows About Lady Journalists
- The Case for Wearing Shoes in the House
- Want Weekly Recs on What to Watch, Read, and More? Sign Up for Worth Your Time
Contact us at [email protected]
You May Also Like
Type 2 Diabetes Research At-a-Glance
The ADA is committed to continuing progress in the fight against type 2 diabetes by funding research, including support for potential new treatments, a better understating of genetic factors, addressing disparities, and more. For specific examples of projects currently funded by the ADA, see below.
Greg J. Morton, PhD
University of Washington
Project: Neurocircuits regulating glucose homeostasis
“The health consequences of diabetes can be devastating, and new treatments and therapies are needed. My research career has focused on understanding how blood sugar levels are regulated and what contributes to the development of diabetes. This research will provide insights into the role of the brain in the control of blood sugar levels and has potential to facilitate the development of novel approaches to diabetes treatment.”
The problem: Type 2 diabetes (T2D) is among the most pressing and costly medical challenges confronting modern society. Even with currently available therapies, the control and management of blood sugar levels remains a challenge in T2D patients and can thereby increase the risk of diabetes-related complications. Continued progress with newer, better therapies is needed to help people with T2D.
The project: Humans have special cells, called brown fat cells, which generate heat to maintain optimal body temperature. Dr. Morton has found that these cells use large amounts of glucose to drive this heat production, thus serving as a potential way to lower blood sugar, a key goal for any diabetes treatment. Dr. Morton is working to understand what role the brain plays in turning these brown fat cells on and off.
The potential outcome: This work has the potential to fundamentally advance our understanding of how the brain regulates blood sugar levels and to identify novel targets for the treatment of T2D.
Tracey Lynn McLaughlin, MD
Stanford University
Project: Role of altered nutrient transit and incretin hormones in glucose lowering after Roux-en-Y gastric bypass surgery
“This award is very important to me personally not only because the enteroinsular axis (gut-insulin-glucose metabolism) is a new kid on the block that requires rigorous physiologic studies in humans to better understand how it contributes to glucose metabolism, but also because the subjects who develop severe hypoglycemia after gastric bypass are largely ignored in society and there is no treatment for this devastating and very dangerous condition.”
The problem: Roux-en-Y gastric bypass (RYGB) surgery is the single-most effective treatment for type 2 diabetes, with persistent remission in 85% of cases. However, the underlying ways by which the surgery improves glucose control is not yet understood, limiting the ability to potentially mimic the surgery in a non-invasive way. Furthermore, a minority of RYGB patients develop severe, disabling, and life-threatening low-blood sugar, for which there is no current treatment.
The project: Utilizing a unique and rigorous human experimental model, the proposed research will attempt to gain a better understanding on how RYGB surgery improves glucose control. Dr. McLaughlin will also test a hypothesis which she believes could play an important role in the persistent low-blood sugar that is observed in some patients post-surgery.
The potential outcome: This research has the potential to identify novel molecules that could represent targets for new antidiabetic therapies. It is also an important step to identifying people at risk for low-blood sugar following RYGB and to develop postsurgical treatment strategies.
Rebekah J. Walker, PhD
Medical College of Wisconsin
Project: Lowering the impact of food insecurity in African Americans with type 2 diabetes
“I became interested in diabetes research during my doctoral training, and since that time have become passionate about addressing social determinants of health and health disparities, specifically in individuals with diabetes. Living in one of the most racially segregated cities in the nation, the burden to address the needs of individuals at particularly high risk of poor outcomes has become important to me both personally and professionally.”
The problem: Food insecurity is defined as the inability to or limitation in accessing nutritionally adequate food and may be one way to address increased diabetes risk in high-risk populations. Food insecure individuals with diabetes have worse diabetes outcomes and have more difficulty following a healthy diet compared to those who are not food insecure.
The project: Dr. Walker’s study will gather information to improve and then will test an intervention to improve blood sugar control, dietary intake, self-care management, and quality of life in food insecure African Americans with diabetes. The intervention will include weekly culturally appropriate food boxes mailed to the participants and telephone-delivered diabetes education and skills training. It will be one of the first studies focused on the unique needs of food insecure African American populations with diabetes using culturally tailored strategies.
The potential outcome: This study has the potential to guide and improve policies impacting low-income minorities with diabetes. In addition, Dr. Walker’s study will help determine if food supplementation is important in improving diabetes outcomes beyond diabetes education alone.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pharmacological Reviews

New Aspects of Diabetes Research and Therapeutic Development
Both type 1 and type 2 diabetes mellitus are advancing at exponential rates, placing significant burdens on health care networks worldwide. Although traditional pharmacologic therapies such as insulin and oral antidiabetic stalwarts like metformin and the sulfonylureas continue to be used, newer drugs are now on the market targeting novel blood glucose–lowering pathways. Furthermore, exciting new developments in the understanding of beta cell and islet biology are driving the potential for treatments targeting incretin action, islet transplantation with new methods for immunologic protection, and the generation of functional beta cells from stem cells. Here we discuss the mechanistic details underlying past, present, and future diabetes therapies and evaluate their potential to treat and possibly reverse type 1 and 2 diabetes in humans.
Significance Statement
Diabetes mellitus has reached epidemic proportions in the developed and developing world alike. As the last several years have seen many new developments in the field, a new and up to date review of these advances and their careful evaluation will help both clinical and research diabetologists to better understand where the field is currently heading.
I. Introduction
Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020 ). Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing pancreatic beta cells. The much more prevalent T2D arises in conjunction with peripheral tissue insulin resistance and beta cell failure and is estimated to increase to 21%–33% of the US population by the year 2050 (Boyle et al., 2010 ). To combat this growing health threat and its cardiac, renal, and neurologic comorbidities, new and more effective diabetes drugs and treatments are essential. As the last several years have seen many new developments in the field of diabetes pharmacology and therapy, we determined that a new and up to date review of these advances was in order. Our aim is to provide a careful evaluation of both old and new therapies ( Fig. 1 ) in a manner that we hope will be of interest to both clinical and bench diabetologists. Instead of the usual encyclopedic approach to this topic, we provide here a targeted and selective consideration of the underlying issues, promising new treatments, and a re-examination of more traditional approaches. Thus, we do not discuss less frequently used diabetes agents, such as alpha-glucosidase inhibitors; these were discussed in other recent reviews (Hedrington and Davis, 2019 ; Lebovitz, 2019 ).

Pharmacologic targeting of numerous organ systems for the treatment of diabetes. Treatment of diabetes involves targeting of various organ systems, including the kidney by SGLT2 inhibitors; the liver, gut, and adipose tissue by metformin; and direct actions upon the pancreatic beta cell. Beta cell compounds aim to increase secretion or mass and/or to protect from autoimmunity destruction. Ultimately, insulin therapy remains the final line of diabetes treatment with new technologies under development to more tightly regulate blood glucose levels similar to healthy beta cells. hESC, human embryonic stem cell.
II. Diabetes Therapies
A. metformin.
Metformin is a biguanide originally based on the natural product galegine, which was extracted from the French lilac (Bailey, 1992 ; Rojas and Gomes, 2013 ; Witters, 2001 ). A closely related biguanide, phenformin, was also used initially for its hypoglycemic actions. Based on its successful track record as a safe, effective, and inexpensive oral medication, metformin has become the most widely prescribed oral agent in the world in treating T2D (Rojas and Gomes, 2013 ; He and Wondisford, 2015 ; Witters, 2001 ), whereas phenformin has been largely bypassed due to its unacceptably high association with lactic acidosis (Misbin, 2004 ). Unlike sulfonylureas, metformin lowers blood glucose without provoking hypoglycemia and improves insulin sensitivity (Bailey, 1992 ). Despite these well known beneficial metabolic actions, metformin’s mechanism of action and even its main target organ remain controversial. In fact, metformin has multiple mechanisms of action at the organ as well as the cellular level, which has hindered our understanding of its most important molecular effects on glucose metabolism (Witters, 2001 ). Adding to this, a specific receptor for metformin has never been identified. Metformin has actions on several tissues, although the primary foci of most studies have been the liver, skeletal muscle, and the intestine (Foretz et al., 2014 ; Rena et al., 2017 ). Metformin and phenformin clearly suppress hepatic glucose production and gluconeogenesis, and they improve insulin sensitivity in the liver and elsewhere (Bailey, 1992 ). The hepatic actions of metformin have been the most exhaustively studied to date, and there is little doubt that these actions are of some importance. However, several of the studies remain highly controversial, and there are still open questions.
One of the first reported specific molecular targets of metformin was mitochondrial complex I of the electron transport chain. Inhibition of this complex results in reduced oxidative phosphorylation and consequently decreased hepatic ATP production (El-Mir et al., 2008 ; Evans et al., 2005 ; Owen et al., 2000 ). As is the case in many other studies of metformin, however, high concentrations of the drug were found to be necessary to depress metabolism at this site (El-Mir et al., 2000 ; He and Wondisford, 2015 ; Owen et al., 2000 ). Also controversial is whether metformin works by activating 5′ AMP-activated protein kinase (AMPK), a molecular energy sensor that is known to be a major metabolic sensor in cells, or if not AMPK directly, then one of its upstream regulators such as liver kinase B2 (Zhou et al., 2001 ). Although metformin was shown to activate AMPK in several excellent studies, other studies directly contradicted the AMPK hypothesis. Most dramatic were studies showing that metformin’s actions to suppress hepatic gluconeogenesis persisted despite genetic deletion of the AMPK’s catalytic domain (Foretz et al., 2010 ). More recent studies identified additional or alternative targets, such as cAMP signaling in the liver (Miller et al., 2013 ) or glycogen synthase kinase-3 (Link, 2003 ). Other work showed that the phosphorylation of acetyl-CoA carboxylase and acetyl-CoA carboxylase 2 are involved in regulating lipid homeostasis and improving insulin sensitivity after exposure to metformin (Fullerton et al., 2013 ).
Although there are strong data to support each of these pathways, it is not entirely clear which signaling pathway(s) is most essential to the actions of metformin in hepatocytes. Metformin clearly inhibits complex I and concomitantly decreases ATP and increases AMP. The latter results in AMPK activation, reduced fatty acid synthesis, and improved insulin receptor activation, and increased AMP has been shown to inhibit adenylate cyclase to reduce cAMP and thus protein kinase A activation. Downstream, this reduces the expression of phosphoenolpyruvate carboxykinase and glucose 6-phosphatase via decreased cAMP response element-binding protein, the cAMP-sensitive transcription factor. Decreased PKA also promotes ATP-dependent 6-phosphofructokinase, liver type activity via fructose 2,6-bisphosphate and reduces gluconeogenesis, as fructose-bisphosphatase 1 is inhibited by fructose 2,6-bisphosphate, along with other mechanisms (Rena et al., 2017 ; Pernicova and Korbonits, 2014 ).
More recent work has shown that metformin at pharmacological rather than suprapharmacological doses increases mitochondrial respiration and complex 1 activity and also increases mitochondrial fission, now thought to be critical for maintaining proper mitochondrial density in hepatocytes and other cells. This improvement in respiratory activity occurs via AMPK activation (Wang et al., 2019 ).
Although the liver has historically been the major suspected site of metformin action, recent studies have suggested that the gut instead of the liver is a major target, a concept supported by the increased efficacy of extended-release formulations of metformin that reside for a longer duration in the gut after their administration (Buse et al., 2016 ). An older, but in our view an important observation, is that the intravenous administration of metformin has little or no effect on blood glucose, whereas, in contrast, orally administered metformin is much more effective (Bonora et al., 1984 ). Recent imaging studies using labeled glucose have shown directly that metformin stimulates glucose uptake by the gut in patients with T2D to reduce plasma glucose concentrations (Koffert et al., 2017 ; Massollo et al., 2013 ). Additionally, it is possible that metformin may exert its effect in the gut by inducing intestinal glucagon-like peptide-1 (GLP-1) release (Mulherin et al., 2011 ; Preiss et al., 2017) to potentiate beta cell insulin secretion and by stimulating the central nervous system (CNS) to exert control over both blood glucose and liver function. Indeed, CNS effects produced by metformin have been proposed to occur via the local release of GLP-1 to activate intestinal nerve endings of ascending nerve pathways that are involved in CNS glucose regulation (Duca et al., 2015 ). Lastly, several papers have now implicated that metformin may act by altering the gut microbiome, suggesting that changes in gut flora may be critical for metformin’s actions (McCreight et al., 2016 ; Wu et al., 2017 ; Devaraj et al., 2016 ). A new study proposed that activation of the intestinal farnesoid X receptor may be the means by which microbiota alter hyperglycemia (Sun et al., 2018 ). However, these studies will require more mechanistic detail and confirmation before they can be fully accepted by the field. In addition to the action of metformin on gut flora, the production of imidazole propionate by gut microbes in turn has been shown to interfere with metformin action through a p38-dependent mechanism and AMPK inhibition. Levels of imidazole propionate are especially higher in patients with T2D who are treated with metformin (Koh et al., 2020 ).
In summary, the combined contribution of these various effects of metformin on multiple cellular targets residing in many tissues may be key to the benefits of metformin treatment on lowering blood glucose in patients with type 2 diabetes (Foretz et al., 2019 ). In contrast, exciting new work showing metformin leads to weight loss by increasing circulating levels of the peptide hormone growth differentiation factor 15 and activation of brainstem glial cell-derived neurotropic factor family receptor alpha like receptors to reduce food intake and energy expenditure works independently of metformin’s glucose-lowering effect (Coll et al., 2020 ).
B. Sulfonylureas and Beta Cell Burnout
The class of compounds known as sulfonylureas includes one of the oldest oral antidiabetic drugs in the pharmacopoeia: tolbutamide. Tolbutamide is a “first generation” oral sulfonylurea secretagogue whose clinical usefulness is due to its prompt stimulation of insulin release from pancreatic beta cells. “Second generation” sulfonylureas include drugs such as glyburide, gliclazide, and glipizide. Sulfonylureas act by binding to a high affinity sulfonylurea binding site, the sulfonylurea receptor 1 subunit of the K(ATP) channel, which closes the channel. These drugs mimic the physiologic effects of glucose, which closes the K(ATP) channel by raising cytosolic ATP/ADP. This in turn provokes beta cell depolarization, resulting in increased Ca 2+ influx into the beta cell (Ozanne et al., 1995 ; Ashcroft and Rorsman, 1989 ; Nichols, 2006 ). Importantly, sulfonylureas, and all drugs that directly increase insulin secretion, are associated with hypoglycemia, which can be severe, and which limits their widespread use in the clinic (Yu et al., 2018 ). Meglitinides are another class of oral insulin secretagogues that, like the sulfonylureas, bind to sulfonylurea receptor 1 and inhibit K(ATP) channel activity (although at a different site of action). The rapid kinetics of the meglitinides enable them to effectively blunt the postprandial glycemic excursions that are a hallmark (along with elevated fasting glucose) of T2D (Rosenstock et al., 2004). However, the need for their frequent dosing (e.g., administration before each meal) has limited their appeal to patients.
The efficacy of sulfonylureas is known to decrease over time, leading to failure of the class for effective long-term treatment of T2D (Harrower, 1991 ). More broadly, it is now widely accepted that the number of functional beta cells in humans declines during the progression of T2D. Thus, one would expect that due to this decline, all manner of oral agents intended to target the beta cell and increase its cell function (and especially insulin secretion) will fail over time (RISE Consortium, 2019 ), a process referred to as “beta cell failure” (Prentki and Nolan, 2006 ). Currently, treatments that can expand beta cell mass or improve beta cell function or survival over time are not yet available for use in the clinic. As a result, treatments that may be able to help patients cope with beta cell burnout such as islet cell transplantation, insulin pumps, or stem cell therapy are alternatives that will be discussed below.
C. Ca 2+ Channel Blockers and Type 1 Diabetes
Strategies to treat and prevent T1D have historically focused on ameliorating the toxic consequences of immune dysregulation resulting in autoimmune destruction of pancreatic beta cells. More recently, a concerted focus on alleviating the intrinsic beta cell defects (Sims et al., 2020 ; Soleimanpour and Stoffers, 2013 ) that also contribute to T1D pathogenesis have been gaining traction at both the bench and the bedside. Several recent preclinical studies suggest that Ca 2+ -induced metabolic overload induces beta cell failure (Osipovich et al., 2020 ; Stancill et al., 2017 ; Xu et al., 2012 ), with the potential that excitotoxicity contributes to beta cell demise in both T1D and T2D, similar to the well known connection between excitotoxicity and, concomitantly, increased Ca 2+ loading of the cells and neuronal dysfunction. Indeed, the use of the phenylalkylamine Ca 2+ channel blocker verapamil has been successful in ameliorating beta cell dysfunction in preclinical models of both T1D and T2D (Stancill et al., 2017 ; Xu et al., 2012 ). Verapamil is a well known blocker of L-type Ca 2+ channels, and, in normally activated beta cells, it limits Ca 2+ entry into the beta cell (Ohnishi and Endo, 1981 ; Vasseur et al., 1987 ). This would be expected to, in turn, alter the expression of many Ca 2+ influx–dependent beta cell genes (Stancill et al., 2017 ), and the evidence to date suggests it is likely that verapamil preserves beta cell function in diabetes models by repressing thioredoxin-interacting protein (TXNIP) expression and thus protecting the beta cell. This is somewhat surprising given the physiologic role of Ca 2+ is to acutely trigger insulin secretion; this process would be expected to be inhibited by L-type Ca 2+ channel blockers (Ashcroft and Rorsman, 1989 ; Satin et al., 1995 ).
Hyperglycemia is a well known inducer of TXNIP expression, and a lack of TXNIP has been shown to protect against beta cell apoptosis after inflammatory stress (Chen et al., 2008a ; Shalev et al., 2002 ; Chen et al., 2008b ). Excitingly, the use of verapamil in patients with recent-onset T1D improved beta cell function and improved glycemic control for up to 12 months after the initiation of therapy, suggesting there is indeed promise for targeting calcium and TXNIP activation in T1D. Use of verapamil for a repurposed indication in the preservation of beta cell function in T1D is attractive due its well known safety profile as well as its cardiac benefits (Chen et al., 2009 ). Although the long-term efficacy of verapamil to maintain beta cell function in vivo is unclear, a recently described TXNIP inhibitor may also show promise in suppressing the hyperglucagonemia that also contributes to glucose intolerance in T2D (Thielen et al., 2020 ). As there is a clear need for increased Ca 2+ influx into the beta cell to trigger and maintain glucose-dependent insulin secretion (Ashcroft and Rorsman, 1990 ; Satin et al., 1995 ), it remains to be seen how well regulated insulin secretion is preserved in the presence of L-type Ca 2+ channel blockers like verapamil in the system. One might speculate that reducing but not fully eliminating beta cell Ca 2+ influx might reduce TXNIP levels while preserving enough influx to maintain glucose-stimulated insulin release. Alternatively, these two phenomena may operate on entirely different time scales. At present, these issues clearly will require further investigation.
D. GLP-1 and the Incretins
Studies dating back to the 1960s revealed that administering glucose in equal amounts via the peripheral circulation versus the gastrointestinal tract led to dramatically different amounts of glucose-induced insulin secretion (Elrick et al., 1964 ; McIntyre et al., 1964 ; Perley and Kipnis, 1967 ). Gastrointestinal glucose administration greatly increased insulin secretion versus intravenous glucose, and this came to be known as the “incretin effect” (Nauck et al., 1986a ; Nauck et al., 1986b ). Subsequent work showed that release of the gut hormone GLP-1 mediated this effect such that food ingestion induced intestinal cell hormone secretion. GLP-1 so released would then circulate to the pancreas via the blood to prime beta cells to secrete more insulin when glucose became elevated because these hormones stimulated beta cell cAMP formation (Drucker et al., 1987 ). The discovery that a natural peptide corresponding to GLP-1 could be found in the saliva of the Gila monster, a desert lizard, hastened progress in the field, and ample in vitro studies subsequently confirmed that GLP-1 potentiated insulin secretion in a glucose-dependent manner. GLP-1 has little or no significant action on insulin secretion in the absence of elevated glucose (such as might typically correspond to the postprandial case or during fasting), thus minimizing the likelihood of hypoglycemia provoked by GLP-1 in treated patients (Kreymann et al., 1987 ). Although not completely understood, the glucose dependence of GLP-1 likely reflects the requirement for adenine nucleotides to close glucose-inhibited K(ATP) channels and thus subsequently activate Ca 2+ influx–dependent insulin exocytosis. Besides potentiating GSIS at the level of the beta cell, glucagon-like peptide-1 receptor (GLP-1R) agonists also decrease glucagon secretion from pancreatic islet alpha cells, reduce gastric emptying, and may also increase beta cell proliferation, among other cellular actions (reviewed in Drucker, 2018 ; Muller et al., 2019).
Intense interest in the incretins by basic scientists, clinicians, and the pharma community led to the rapid development of new drugs for treating primarily T2D. These drugs include a range of GLP-1R agonists and inhibitors of the incretin hormone degrading enzyme dipeptidyl peptidase 4 (DPP4), whose targeting increases the half-lives of GLP-1 and gastric inhibitory polypeptide (GIP) and thereby increases protein hormone levels in plasma. GLP-1R agonists have been associated with not only a lowering of plasma glucose but also weight loss, decreased appetite, reduced risk of cardiovascular events, and other favorable outcomes (Gerstein et al., 2019; Hernandez et al., 2018; Husain et al., 2019; Marso et al., 2016a; Marso et al., 2016b ; Buse et al., 2004). Regarding their untoward actions, although hypoglycemia is not a major concern, there have been reports of pancreatitis and pancreatic cancer from use of GLP-1R agonists. However, a recent meta-analysis covering four large-scale clinical trials and over 33,000 participants noted no significantly increased risk for pancreatitis/pancreatic cancer in patients using GLP-1R agonists (Bethel et al., 2018).
Ongoing and future developments in the use of proglucagon-derived peptides such as GLP-1 and glucagon include the use of combined GLP-1/GIP, glucagon/GLP-1, and agents targeting all three peptides in combination (reviewed in Alexiadou and Tan, 2020 ). Although short-term infusions of GLP-1 with GIP failed to yield metabolic benefits beyond those seen with GLP-1 alone (Bergmann et al., 2019 ), several GLP-1/GIP dual agonists are currently in development and have shown promising metabolic results in clinical trials (Frias et al., 2017 ; Frias et al., 2020 ; Frias et al., 2018 ). At the level of the pancreatic islet, beneficial effects of dual GLP-1/GIP agonists may be related to imbalanced and biased preferences of these agonists for the gastric inhibitory polypeptide receptor over the GLP-1R (Willard et al., 2020 ) and possibly were not simply to dual hormone agonism in parallel. Dual glucagon/GLP-1 agonist therapy has also been shown to have promising metabolic effects in humans (Ambery et al., 2018 ; Tillner et al., 2019 ). Oxyntomodulin is a natural dual glucagon/GLP-1 receptor agonist and proglucagon cleavage product that is also secreted from intestinal enteroendocrine cells, which has beneficial effects on insulin secretion, appetite regulation, and body weight in both humans and rodents (Cohen et al., 2003 ; Dakin et al., 2001 ; Dakin et al., 2002 ; Shankar et al., 2018 ; Wynne et al., 2005 ). Interestingly, alpha cell crosstalk to beta cells through the combined effects of glucagon and GLP-1 is necessary to obtain optimal glycemic control, suggesting a potential pathway for therapeutic dual glucagon/GLP-1 agonism within the islets of patients with T2D (Capozzi et al., 2019a ; Capozzi et al., 2019b ). Although the early results appear promising, more studies will be necessary to better understand the mechanistic and clinical impacts of these multiagonist agents.
E. DPP4 Inhibitors
Inhibition of DPP4, the incretin hormone degrading enzyme, is one of the most common T2D treatments to increase GLP-1 and GIP plasma hormone levels. These DPP4 inhibitors or “gliptins” are generally used in conjunction with other T2D drugs such as metformin or sulfonylureas to obtain the positive benefits discussed above (Lambeir et al., 2008 ). DPP4 is a primarily membrane-bound peptidase belonging to the serine peptidase/prolyl oligopeptidase gene family, which cleaves a large number of substrates in addition to the incretin hormones (Makrilakis, 2019 ). DPP4 inhibitors provide glucose-lowering benefits while being generally well tolerated, and the variety of available drugs (including sitagliptin, saxagliptin, vildagliptin, alogliptin, and linagliptin) with slightly different dosing frequency, half-life, and mode of excretion/metabolism allows for use in multiple patient populations (Makrilakis, 2019 ). This includes the elderly and individuals with renal or hepatic insufficiency (Makrilakis, 2019 ).
Although hypoglycemia is not a concern for DPP4 inhibitor use, other considerations should be made. DPP4 inhibitors tend to be more expensive than metformin or other second-line oral drugs in addition to having more modest glycemic effects than GLP-1R agonists (Munir and Lamos, 2017 ). Finally, meta-analysis of randomized and observational studies concluded that heart failure in patients with T2D was not associated with use of DPP4 inhibitors; however, this study was limited by the short follow-up and lack of high-quality data (Li et al., 2016 ). Thus, the US Food and Drug Administration (FDA) did recommend assessing risk of heart failure hospitalization in patients with pre-existing cardiovascular disease, prior heart failure, and chronic kidney disease when using saxagliptin and alogliptin (Munir and Lamos, 2017 ).
F. Sodium Glucose Cotransporter 2 Inhibitors
A recent development in the field of T2D drugs are sodium glucose cotransporter 2 (SGLT2) inhibitors, which have an interesting and very different mechanism of action. Within the proximal tubule of the nephron, SGLT2 transports ingested glucose into the lumen of the proximal tubule between the epithelial layers, thereby reclaiming glucose by this reabsorption process (reviewed in Vallon, 2015 ). SGLT2 inhibitors target this transporter and increase glucose in the tubular fluid and ultimately increase it in the urine. In patients with diabetes, SGLT2 inhibition results in a lowering of plasma glucose with urine glucose content rising substantially (Adachi et al., 2000 ; Vallon, 2015 ). These drugs, although they are relatively new, have become an area of great interest for not only patients with T2D (Grempler et al., 2012 ; Imamura et al., 2012 ; Meng et al., 2008 ; Nomura et al., 2010 ) but also for patients with T1D (Luippold et al., 2012 ; Mudaliar et al., 2012 ). Part of their appeal also rests on reports that their use can lead to a statistically significant decline in cardiac events that are known to occur secondarily to diabetes, possibly independently of plasma glucose regulation (reviewed in Kurosaki and Ogasawara, 2013 ). Although the long-term consequences of their clinical use cannot yet be determined, raising the glucose content of the urogenital tract leads to an increased risk of urinary tract infections and other related infections in some patients (Kurosaki and Ogasawara, 2013 ).
Another recent concern about the use of SGLT2 inhibitors has been the development of normoglycemic diabetic ketoacidosis (DKA). Despite the efficacy of SGLT2 inhibitors, observations of hyperglucagonemia in patients with euglycemic DKA has led to a number of recent studies focused on SGLT2 actions on pancreatic islets. Initial studies of isolated human islets treated with small interfering RNA directed against SGLT2 and/or SGLT2 inhibitors demonstrated increased glucagon release. These studies were complemented by the finding of elevations in glucagon release in mice that were administered SGLT2 inhibitors in vivo (Bonner et al., 2015 ). Insights into the possible mechanistic links between SGLT2 inhibition, DKA frequency, and glucagon secretion in humans may relate to the observation of heterogeneity in SGLT2 expression, as SGLT2 expression appears to have a high frequency of interdonor and intradonor variability (Saponaro et al., 2020 ). More recently, both insulin and GLP-1 have been demonstrated to modulate SGLT2-dependent glucagon release through effects on somatostatin release from delta cells (Vergari et al., 2019 ; Saponaro et al., 2019 ), suggesting potentially complex paracrine effects that may affect the efficacy of these compounds.
On the other hand, several recent studies question that the development of euglycemic DKA after SGLT2 inhibitor therapy may be through alpha cell–dependent mechanisms. Three recent studies found no effect of SGLT2 inhibitors to promote glucagon secretion in mouse and/or rat models and could not detect SGLT2 expression in human alpha cells (Chae et al., 2020 ; Kuhre et al., 2019 ; Suga et al., 2019 ). A fourth study demonstrated only a brief transient effect of SGLT2 inhibition to raise circulating glucagon concentrations in immunodeficient mice transplanted with human islets, which returned to baseline levels after longer exposures to SGLT2 inhibitors (Dai et al., 2020 ). Furthermore, SGLT2 protein levels were again undetectable in human islets (Dai et al., 2020 ). These results could suggest alternative islet-independent mechanisms by which patients develop DKA, including alterations in ketone generation and/or clearance, which underscore the additional need for further studies both in molecular models and at the bedside. Nevertheless, SGLT2 inhibitors continue to hold promise as a valuable therapy for T2D, especially in the large segment of patients who also have superimposed cardiovascular risk (McMurray et al., 2019; Wiviott et al., 2019; Zinman et al., 2015).
G. Thiazolidinediones
Once among the most commonly used oral agents in the armamentarium to treat T2D, thiazolidinediones (TZDs) were clinically popular in their utilization to act specifically as insulin sensitizers. TZDs improve peripheral insulin sensitivity through their action as peroxisome proliferator-activated receptor (PPAR) γ agonists, but their clinical use fell sharply after studies suggested a connection between cardiovascular toxicity with rosiglitazone and bladder cancer risk with pioglitazone (Lebovitz, 2019 ). Importantly, an FDA panel eventually removed restrictions related to cardiovascular risk with rosiglitazone in 2013 (Hiatt et al., 2013 ). Similarly, concerns regarding use of bladder cancer risk with pioglitazone were later abated after a series of large clinical studies found that pioglitazone did not increase bladder cancer (Lewis et al., 2015 ; Schwartz et al., 2015 ). However, usage of TZDs had already substantially decreased and has not since recovered.
Although concerns regarding edema, congestive heart failure, and fractures persist with TZD use, there have been several studies suggesting that TZDs protect beta cell function. In the ADOPT study, use of rosiglitazone monotherapy in patients newly diagnosed with T2D led to improved glycemic control compared with metformin or sulfonylureas (Kahn et al., 2006). Later analyses revealed that TZD-treated subjects had a slower deterioration of beta cell function than metformin- or sulfonylurea-treated subjects (Kahn et al., 2011). Furthermore, pioglitazone use improved beta cell function in the prevention of T2D in the ACT NOW study (Defronzo et al., 2013; Kahn et al., 2011). Mechanistically, it is unclear if TZDs lead to beneficial beta cell function through direct effects or through indirect effects of reduced beta cell demand due to enhanced peripheral insulin sensitivity. Indeed, a beta cell–specific knockout of PPAR γ did not impair glucose homeostasis, nor did it impair the antidiabetic effects of TZD use in mice (Rosen et al., 2003 ). However, other reports demonstrated PPAR-responsive elements within the promoters of both glucose transporter 2 and glucokinase that enhance beta cell glucose sensing and function, which could explain beta cell–specific benefits for TZDs (Kim et al., 2002 ; Kim et al., 2000 ). Furthermore, TZDs have been shown to improve beta cell function by upregulating cholesterol transport (Brunham et al., 2007 ; Sturek et al., 2010 ). Additionally, use of TZDs in the nonobese diabetic (NOD) mouse model of T1D augmented the beta cell unfolded protein response and prevented beta cell death, suggesting potential benefits for TZDs in both T1D and T2D (Evans-Molina et al., 2009 ; Maganti et al., 2016 ). With a now refined knowledge of demographics in which to avoid TZD treatment due to adverse effects, together with genetic approaches to identify candidates more likely to respond effectively to TZD therapy (Hu et al., 2019 ; Soccio et al., 2015 ), it remains to be seen if TZD therapy will return to more prominent use in the treatment of diabetes.
H. Insulin and Beyond: The Use of “Smart” Insulin and Closed Loop Systems in Diabetes Treatment
Due to recombinant DNA technology, numerous insulin analogs are now available in various forms ranging from fast acting crystalline insulin to insulin glargine; all of these analogs exhibit equally effective insulin receptor binding. Most are generated by altering amino acids in the B26–B30 region of the molecule (Kurtzhals et al., 2000 ). The American Diabetes Association delineates these insulins by their 1) onset or time before insulin reaches the blood stream, 2) peak time or duration of maximum blood glucose–lowering efficacy, and 3) the duration of blood glucose–lowering time. Insulin administration is independent of the residuum of surviving and/or functioning beta cells in the patient and remains the principal pharmacological treatment of both T1D and T2D. The availability of multiple types of delivery methods, i.e., insulin pens, syringes, pumps, and inhalants, provides clinicians with a solid and varied tool kit with which to treat diabetes. The downsides, however, are that 1) hypoglycemia is a constant threat, 2) proper insulin doses are not trivial to calculate, 3) compliance can vary especially in children and young adults, and 4) there can be side effects of a variety of types. Nonetheless, insulin therapy remains a mainstay treatment of diabetes.
To eliminate the downsides of insulin therapy, research in the past several decades has worked toward generating glucose-sensitive or “smart” insulin molecules. These molecules change insulin bioavailability and become active only upon high blood glucose using glucose-binding proteins such as concanavalin A, glucose oxidase to alter pH sensitivity, and phenylboronic acid (PBA), which forms reversible ester linkages with diol-containing molecules including glucose itself (reviewed in Rege et al., 2017 ). Indeed, promising recent studies included various PBA moieties covalently bonded to an acylated insulin analog (insulin detemir, which contains myristic acid coupled to Lys B29 ). The detemir allows for binding to serum albumin to prolong insulin’s half-life in the circulation, and PBA provided reversible glucose binding (Chou et al., 2015 ). The most promising of the PBA-modified conjugates showed higher potency and responsiveness in lowering blood glucose levels compared with native insulin in diabetic mouse models and decreased hypoglycemia in healthy mice, although the molecular mechanisms have not yet been determined (Chou et al., 2015 ).
An additional active area of research includes structurally defining the interaction between insulin and the insulin receptor ectodomain. Importantly, a major conformational change was discovered that may be exploited to impair insulin receptor binding under hypoglycemic conditions (Menting et al., 2013 ; Rege et al., 2017 ). Challenges in the design, testing, and execution of glucose-responsive insulins may be overcome by the adaptation of novel modeling approaches (Yang et al., 2020 ), which may allow for more rapid screening of candidate compounds.
Technologies have also progressed in the field of artificial pancreas design and development. Currently two “closed loop” systems are now available: Minimed 670G from Medtronic and Control-IQ from Tandem Diabetes Care. Both systems use a continuous glucose monitor, insulin pump, and computer algorithm to predict correct insulin doses and administer them in real time. Such algorithm systems also take into account insulin potency, the rate of blood glucose increase, and the patient’s heart rate and temperature to adjust insulin delivery levels during exercise and after a meal. In addition, so-called “artificial pancreas” systems have also been clinically tested, which use both insulin and glucagon and as such result in fewer reports of hypoglycemic episodes (El-Khatib et al., 2017 ). These types of systems will continue to become more popular as the development of room temperature–stable glucagon analogs continue, such as GVOKE by Xeris Pharmaceuticals (currently available in an injectable syringe) and Baqsimi, a nasally administered glucagon from Eli Lilly.
I. Present and Future Therapies: Beta Cell Transplantation, Replication, and Immune Protection
1. islet transplantation.
The idea to use pancreatic allo/xenografts to treat diabetes remarkably dates back to the late 1800s (Minkowski, 1892 ; Pybus, 1924 ; Williams, 1894 ). Before proceeding to the discovery of insulin (together with Best, MacLeod, and Collip), Frederick Banting also postulated the potential for transplantation of pancreatic tissue emulsions to treat diabetes in dog models in a notebook entry in 1921 (Bliss, 1982 ). Decades later, Paul Lacy, David Scharp, and colleagues successfully isolated intact functional pancreatic islets and transplanted them into rodent models (Kemp et al., 1973 ). These studies led to the initial proof of concept studies for humans, with the first successful islet transplant in a patient with T1D occurring in 1977 (Sutherland et al., 1978 ). A rapid expansion of islet transplantation, inspired by these original studies led to key observations of successfully prolonged islet engraftment by the “Edmonton protocol” whereby corticosteroid-sparing immunosuppression was applied, and islets from at least two allogeneic donors were used to achieve insulin independence (Shapiro et al., 2000 ). More recent work has focused on improving upon the efficiency and long-term engraftment of allogeneic transplants leading to more prolonged graft function (to the 5-year mark) and successful transplantation from a single islet donor (Hering et al., 2016; Hering et al., 2005 ; Rickels et al., 2013 ). Critical to these efforts to improve the success rate was the recognition that the earlier generation of immunosuppressive agents to counter tissue rejection was toxic to islets (Delaunay et al., 1997 ; Paty et al., 2002 ; Soleimanpour et al., 2010 ) and that more appropriate and less toxic agents were needed (Hirshberg et al., 2003 ; Soleimanpour et al., 2012 ).
Certainly, islet transplantation as a therapeutic approach for patients with T1D has been scrutinized due to several challenges, including (but not limited to) the lack of available donor supply to contend with demand, limited long-term functional efficacy of islet allografts, the potential for re-emergence of autoimmune islet destruction and/or metabolic overload-induced islet failure, and significant adverse effects of prolonged immunosuppression (Harlan, 2016 ). Furthermore, although islet transplantation is not currently available for individuals with T2D, simultaneous pancreas-kidney transplantation in T2D had similar favorable outcomes to simultaneous pancreas-kidney transplantation in T1D; therefore, islet-kidney transplantation may eventually be a feasible option to treat T2D, as patients will already be on immunosuppressors (Sampaio et al., 2011 ; Westerman et al., 1983 ). An additional significant obstacle is the tremendous expense associated with islet transplantation therapy. Indeed, the maintenance, operation, and utilization of an FDA-approved and Good Manufacturing Practice–compliant islet laboratory can lead to operating costs at nearly $150,000 per islet transplant, which is not cost effective for the vast majority of patients with T1D (Naftanel and Harlan, 2004 ; Wallner et al., 2016 ). At present, the focus has been to obtain FDA approval for islet allo-transplantation as a therapy for T1D to allow for insurance compensation (Hering et al., 2016; Rickels and Robertson, 2019 ). In the interim, the islet biology, stem cell, immunology, and bioengineering communities have continued the development of cell-based therapies for T1D by other approaches to overcome the challenges identified during the islet transplantation boom of the 1990s and 2000s.
2. Pharmacologic Induction of Beta Cell Replication
Besides transplantation, progress in islet cell biology and especially in developmental biology of beta cells over several decades raised the additional possibility that beta cell mass reduction in diabetes might be countered by increasing beta cell number through mitogenic means. A key method to expand pancreatic beta cell mass is through the enhancement of beta cell replication. Although the study of pancreatic beta cell replication has been an area of intense focus in the beta cell biology field for several decades, only recently has this seemed truly feasible. Seminal studies identified that human beta cells are essentially postmitotic, with a rapid phase of growth occurring in the prenatal period that dramatically tapers off shortly thereafter (Gregg et al., 2012 ; Meier et al., 2008 ). The plasticity of rodent beta cells is considerably higher than that of human beta cells (Dai et al., 2016 ), which has led to a renewed focus on validation of pharmacologic agents to enhance rodent beta cell replication using isolated and/or engrafted human islets (Bernal-Mizrachi et al., 2014 ; Kulkarni et al., 2012 ; Stewart et al., 2015 ). Indeed, a large percentage of agents that were successful when applied to rodent systems were largely unsuccessful at inducing replication in human beta cells (Bernal-Mizrachi et al., 2014 ; Kulkarni et al., 2012 ; Stewart et al., 2015 ). However, several recent studies have begun to make significant progress on successfully pushing human beta cells to replicate.
Several groups have reported successful human beta cell proliferation, both in vitro and in vivo, in response to inhibitors of the dual specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A). These inhibitors include harmine, INDY, GNF4877, 5-iodotubericidin, leucettine-42, TG003, AZ191, CC-401, and more specific, recently developed DYRK1A inhibitors (Ackeifi et al., 2020 ). Although DYRK1A is conclusively established as the important mediator of human beta cell proliferation, comprehensively determining other cellular targets and if additional gene inhibition amplifies the proliferative response is still in process. New evidence from Wang and Stewart shows dual specificity tyrosine phosphorylation-regulated kinase 1B to be an additional mitogenic target and also describes variability in the range of activated kinases within cells and/or levels of inhibition for the many DYRK1A inhibitors listed above (Ackeifi et al., 2020 ). Interestingly, opposite to these human studies, earlier mouse studies from the Scharfmann group demonstrated that Dyrk1a haploinsufficiency leads to decreased proliferation and loss of beta cell mass (Rachdi et al., 2014b ). In addition, overexpression of Dyrk1a in mice led to beta cell mass expansion with increased glucose tolerance (Rachdi et al., 2014a ).
Although important differences in beta cell proliferative capacity have been shown between human and rodent species, there are also significant differences in the mitogenic capacity of beta cells from juvenile, adult, and pregnant individuals. This demonstrates that proliferative stimuli appear to act within the complex islet, pancreas, and whole-body environments unique to each time point. For example, the administration of the hormones platelet-derived growth factor alpha or GLP-1 result in enhanced proliferation in juvenile human beta cells yet are ineffective in adult human beta cells (Chen et al., 2011 ; Dai et al., 2017 ). This has been shown to be due to a loss of platelet-derived growth factor alpha receptor expression as beta cells age but appears to be unrelated to GLP-1 receptor expression levels (Chen et al., 2011 ). Indeed, the GLP-1 receptor is highly expressed in adult beta cells, and GLP-1 secretion increases insulin secretion, as detailed previously; however, the induction of proliferative factors such as nuclear factor of activated T cells, cytoplasmic 1; forkhead box protein 1; and cyclin A1 is only seen in juvenile islets (Dai et al., 2017 ). Human studies using cadaveric pancreata from pregnant donors also showed increased beta cell mass, yet lactogenic hormones from the pituitary or placenta (prolactin, placental lactogen, or growth hormone) are unable to stimulate proliferation in human beta cells despite their ability to produce robust proliferation in mouse beta cells (reviewed in Baeyens et al., 2016 ). Experiments overexpressing mouse versus human signal transducer and activator of transcription 5, the final signaling factor inducing beta cell adaptation, in human beta cells allows for prolactin-mediated proliferation revealing fundamental differences in prolactin pathway competency in human (Chen et al., 2015 ). Overcoming the barrier of recapitulating human pregnancy’s effect on beta cells through isolating placental cells or blood serum during pregnancy may result in the discovery of a factor(s) that facilitates the increase in beta cell mass observed during human pregnancy.
Mechanisms that stimulate beta cell proliferation have also been discovered from studying genetic mutations that result in insulinomas, spontaneous insulin-producing beta cell adenomas. The most common hereditary mutation occurs in the multiple endocrine neoplasia type 1 (MEN1) gene. Indeed, administration of a MEN1 inhibitor in addition to a GLP-1 agonist (which cannot induce proliferation alone) is able to increase beta cell proliferation in isolated human islets through synergistic activation of KRAS proto-oncogene, GTPase downstream signals (Chamberlain et al., 2014 ). Interestingly, MEN1 mutations are uncommon in sporadic insulinomas, yet assaying genomic and epigenetic changes in a large cohort of non-MEN1 insulinomas found alterations in trithorax and polycomb chromatin modifying genes that were functionally related to MEN1 (Wang et al., 2017 ). Stewart and colleagues hypothesized that changes in histone 3 lysine 27 and histone 3 lysine 4 methylation status led to increased enhancer of zeste homolog 2 and lysine demethylase 6A, decreased cyclin-dependent kinase inhibitor 1C, and thereby increased beta cell proliferation, among other phenotypes. They also proposed that these findings help to explain why increased proliferation always occurs despite broad heterogeneity of mutations found between individual insulinomas (Wang et al., 2017 ).
Although factors that induce proliferation are continuing to be discovered, there are drawbacks that still limit their clinical application. Harmine and other DYRK1A inhibitors are not beta cell specific, nor have all their cellular targets been determined (Ackeifi et al., 2020 ). Targeting other pathways to induce human beta cell proliferation such as modulation of prostaglandin E2 receptors (i.e., inhibition of prostaglandin E receptor 3 alone or in combination with prostaglandin E receptor 4 activation) showed promising increases in proliferative rate yet suffers from the same lack of specificity (Carboneau et al., 2017 ). Induction of proliferation may also come at the expense of glucose sensing as in insulinomas, which have an increased expression of “disallowed genes” and alterations in glucose transporter and hexokinase expression (Wang et al., 2017 ). A further untoward consequence that must be avoided is the production of cancerous cells through unchecked proliferation. Finally, increasing beta cell mass through low rates of proliferation may increase the pool of functional insulin-secreting cells in T2D, but without additional measures, these beta cells will still ultimately be targeted for immune cell destruction in T1D.
3. Beta Cell Stress Relieving Therapies
Metabolic, inflammatory, and endoplasmic reticulum (ER) stress contribute to beta cell dysfunction and failure in both T1D and T2D. Although reduction of metabolic overload of beta cells by early exogenous insulin therapy or insulin sensitizers can temporarily reduce loss of beta cell mass/function early in diabetes, a focus on relieving ER and inflammatory stress is also of interest to preserve beta cell health.
ER stress is a well known contributor to beta cell demise both in T1D and T2D (Laybutt et al., 2007 ; Marchetti et al., 2007 ; Marhfour et al., 2012 ; Tersey et al., 2012 ) and a target of interest in the prevention of beta cell loss in both diseases. Preclinical studies suggest that the use of chemical chaperones, including 4-phenylbutyric acid and tauroursodeoxycholic acid (TUDCA), to alleviate ER stress improves beta cell function and insulin sensitivity in mouse models of T2D (Cnop et al., 2017 ; Ozcan et al., 2006 ). Furthermore, TUDCA has been shown to preserve beta cell mass and reduce ER stress in mouse models of T1D (Engin et al., 2013 ). Interestingly, TUDCA has shown promise at improving insulin action in obese nondiabetic human subjects, yet beta cell function and insulin secretion were not assessed (Kars et al., 2010 ). A clinical trial regarding the use of TUDCA for humans with new-onset T1D is also ongoing ( {"type":"clinical-trial","attrs":{"text":"NCT02218619","term_id":"NCT02218619"}} NCT02218619 ). However, a note of caution regarding use of ER chaperones is that they may prevent low level ER stress necessary to potentiate beta cell replication during states of increased insulin demand (Sharma et al., 2015 ), suggesting that the broad use of ER chaperone therapies should be carefully considered.
The blockade of inflammatory stress has long been an area of interest for treatments of both T1D and T2D (Donath et al., 2019 ; Eguchi and Nagai, 2017 ). Indeed, use of nonsteroidal anti-inflammatory drugs (NSAIDs), which block cyclooxygenase, have been observed to improve metabolic control in patients with diabetes since the turn of the 20th century (Williamson, 1901 ). Salicylates have been shown to improve insulin secretion and beta cell function in both obese human subjects and those with T2D (Fernandez-Real et al., 2008; Giugliano et al., 1985 ). However, another NSAID, salsalate, has not been shown to improve beta cell function while improving other metabolic outcomes (Kim et al., 2014 ; Penesova et al., 2015 ), possibly suggesting distinct mechanisms of action for anti-inflammatory compounds. The regular use of NSAIDs to enhance metabolic outcomes is also often limited to the tolerability of long-term use of these agents due to adverse effects. Recently, golilumab, a monoclonal antibody against the proinflammatory cytokine tumor necrosis factor alpha, was demonstrated to improve beta cell function in new-onset T1D, suggesting that targeting the underlying inflammatory milieu may have benefits to preserve beta cell mass and function in T1D (Quattrin et al., 2020). Taken together, both new and old approaches to target beta cell stressors still remain of long-term interest to improve beta cell viability and function in both T1D and T2D.
3. New Players to Induce Islet Immune Protection
Countless researchers have expended intense industry to determine T1D disease etiology and treatments focused on immunotherapy and tolerogenic methods. Multiple, highly comprehensive reviews are available describing these efforts (Goudy and Tisch, 2005 ; Rewers and Gottlieb, 2009 ; Stojanovic et al., 2017 ). Here we will focus on the protection of beta cells through programmed cell death protein-1 ligand (PD-L1) overexpression, major histocompatibility complex class I, A, B, C (HLA-A,B,C) mutated human embryonic stem cell–derived beta cells, and islet encapsulation methods.
Cancer immunotherapies that block immune checkpoints are beneficial for treating advanced stage cancers, yet induction of autoimmune diseases, including T1D, remains a potential side effect (Stamatouli et al., 2018 ; Perdigoto et al., 2019 ). A subset of these drugs target either the programmed cell death-1 protein on the surface of activated T lymphocytes or its receptor PD-L1 (Stamatouli et al., 2018 ; Perdigoto et al., 2019 ). PD-L1 expression was found in insulin-positive beta cells from T1D but not insulin-negative islets or nondiabetic islets, leading to the hypothesis that PD-L1 is upregulated in an attempt to drive immune cell attenuation (Osum et al., 2018 ; Colli et al., 2018 ). Adenoviral overexpression of PD-L1 specifically in beta cells rescued hyperglycemia in the NOD mouse model of T1D, but these animals eventually succumbed to diabetes by the study’s termination (El Khatib et al., 2015 ). A more promising report from Ben Nasr et al. ( 2017 ) demonstrated that pharmacologically or genetically induced overexpression of PD-L1 in hematopoietic stem and progenitor cells inhibited beta cell autoimmunity in the NOD mouse as well as in vitro using human hematopoietic stem and progenitor cells from patients with T1D.
As mentioned above, islet transplantation to treat T1D is limited by islet availability, cost, and the requirement for continuous immunosuppression. Islet cells generated by differentiating embryonic or induced pluripotent stem (iPS) cells could circumvent these limitations. Ideally, iPS-derived beta cells could be manipulated to eliminate the expression of polymorphic HLA-A,B,C molecules, which were found to be upregulated in T1D beta cells (Bottazzo et al., 1985 ; Richardson et al., 2016 ). These molecules allow peptide presentation to CD8+ T cells or cytotoxic T lymphocytes and may lead to beta cell removal. Interestingly, remaining insulin-positive cells in T1D donor pancreas are not HLA-A,B,C positive (Nejentsev et al., 2007; Rodriguez-Calvo et al., 2015 ). However, current differentiation protocols are still limited in their ability to produce fully glucose-responsive beta cells without transplantation into animal models to induce mature characteristics. Additionally, use of iPS-derived beta cells will still lead to concerns regarding DNA mutagenesis resulting from the methods used to obtain pluripotency or teratoma formation from cells that have escaped differentiation.
Encapsulation devices would protect islets or stem cells from immune cell infiltration while allowing for the proper exchange of nutrients and hormones. Macroencapsulation uses removable devices that would help assuage fears surrounding mutation or tumor formation; indeed, the first human trial using encapsulated hESC-derived beta cells will be completed in January 2021 ( {"type":"clinical-trial","attrs":{"text":"NCT02239354","term_id":"NCT02239354"}} NCT02239354 ). Macroencapsulation of islets prior to transplantation using various alginate-based hydrogels has historically been impeded by a strong in vivo foreign body immune response (Desai and Shea, 2017 ; Doloff et al., 2017 ; Pueyo et al., 1993 ). More recently, chemically modified forms of alginate that avoid macrophage recognition and fibrous deposition have been successfully used in rodents and for up to 6 months in nonhuman primates (Vegas et al., 2016 ). Indeed, Bochenek et al. ( 2018 ) successfully transplanted alginate protected islets for 4 months without immunosuppression in the bursa omentalis of nonhuman primates demonstrating the feasibility for this approach to be extended to humans. It remains to be seen if these devices will be successful for long-term use, perhaps decades, in patients with diabetes.
III. Summary
Although existing drug therapies using classic oral antidiabetic drugs like sulfonylureas and metformin or injected insulin remain mainstays of diabetes treatment, newer drugs based on incretin hormone actions or SGLT2 inhibitors have increased the pharmacological armamentarium available to diabetologists ( Fig. 1 ). However, the explosion of progress in beta cell biology has identified potential avenues that can increase beta cell mass in sophisticated ways by employing stem cell differentiation or enhancement of beta cell proliferation. Taken together, there should be optimism that the increased incidence of both T1D and T2D is being matched by the creativity and hard work of the diabetes research community.
Abbreviations
Authorship contributions.
Wrote and contributed to the writing of the manuscript: Satin, Soleimanpour, Walker
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) [Grant R01-DK46409] (to L.S.S.), [Grant R01-DK108921] (to S.A.S.), and [Grant P30-DK020572 pilot and feasibility grant] (to S.A.S.), the Juvenile Diabetes Research Foundation (JDRF) [Grant CDA-2016-189] (to L.S.S. and S.A.S.), [Grant SRA-2018-539] (to S.A.S.), and [Grant COE-2019-861] (to S.A.S.), and the US Department of Veterans Affairs [Grant I01 BX004444] (to S.A.S.). The JDRF Career Development Award to S.A.S. is partly supported by the Danish Diabetes Academy and the Novo Nordisk Foundation.
https://doi.org/10.1124/pharmrev.120.000160
- Ackeifi C, Swartz E, Kumar K, Liu H, Chalada S, Karakose E, Scott DK, Garcia-Ocaña A, Sanchez R, DeVita RJ, et al. (2020) Pharmacologic and genetic approaches define human pancreatic β cell mitogenic targets of DYRK1A inhibitors . JCI Insight 5 :e132594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Adachi T, Yasuda K, Okamoto Y, Shihara N, Oku A, Ueta K, Kitamura K, Saito A, Iwakura I, Yamada Y, et al. (2000) T-1095, a renal Na+-glucose transporter inhibitor, improves hyperglycemia in streptozotocin-induced diabetic rats . Metabolism 49 :990–995. [ PubMed ] [ Google Scholar ]
- Alexiadou K, Tan TM (2020) Gastrointestinal peptides as therapeutic targets to mitigate obesity and metabolic syndrome . Curr Diab Rep 20 :26. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L, Tsai LF, Robertson D, Jain M, Petrone M, et al. (2018) MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study . Lancet 391 :2607–2618. [ PubMed ] [ Google Scholar ]
- Ashcroft FM, Rorsman P (1989) Electrophysiology of the pancreatic beta-cell . Prog Biophys Mol Biol 54 :87–143. [ PubMed ] [ Google Scholar ]
- Ashcroft FM, Rorsman P (1990) ATP-sensitive K+ channels: a link between B-cell metabolism and insulin secretion . Biochem Soc Trans 18 :109–111. [ PubMed ] [ Google Scholar ]
- Baeyens L, Hindi S, Sorenson RL, German MS (2016) β-Cell adaptation in pregnancy . Diabetes Obes Metab 18 ( Suppl 1 ):63–70. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bailey CJ (1992) Biguanides and NIDDM . Diabetes Care 15 :755–772. [ PubMed ] [ Google Scholar ]
- Ben Nasr M, Tezza S, D’Addio F, Mameli C, Usuelli V, Maestroni A, Corradi D, Belletti S, Albarello L, Becchi G, et al. (2017) PD-L1 genetic overexpression or pharmacological restoration in hematopoietic stem and progenitor cells reverses autoimmune diabetes . Sci Transl Med 9 :eaam7543. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bergmann NC, Lund A, Gasbjerg LS, Meessen ECE, Andersen MM, Bergmann S, Hartmann B, Holst JJ, Jessen L, Christensen MB, et al. (2019) Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: a randomised, crossover study . Diabetologia 62 :665–675. [ PubMed ] [ Google Scholar ]
- Bernal-Mizrachi E, Kulkarni RN, Scott DK, Mauvais-Jarvis F, Stewart AF, Garcia-Ocaña A (2014) Human β-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map . Diabetes 63 :819–831. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, Pagidipati NJ, Chan JC, Gustavson SM, Iqbal N, et al.; EXSCEL Study Group (2018) Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis . Lancet Diabetes Endocrinol 6 :105–113. [ PubMed ] [ Google Scholar ]
- Bliss M (1982) Banting’s, Best’s, and Collip’s accounts of the discovery of insulin . Bull Hist Med 56 :554–568. [ PubMed ] [ Google Scholar ]
- Bochenek MA, Veiseh O, Vegas AJ, McGarrigle JJ, Qi M, Marchese E, Omami M, Doloff JC, Mendoza-Elias J, Nourmohammadzadeh M, et al. (2018) Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques . Nat Biomed Eng 2 :810–821. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thévenet J, Beaucamps C, Delalleau N, Popescu I, Malaisse WJ, et al. (2015) Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion . Nat Med 21 :512–517. [ PubMed ] [ Google Scholar ]
- Bonora E, Cigolini M, Bosello O, Zancanaro C, Capretti L, Zavaroni I, Coscelli C, Butturini U (1984) Lack of effect of intravenous metformin on plasma concentrations of glucose, insulin, C-peptide, glucagon and growth hormone in non-diabetic subjects . Curr Med Res Opin 9 :47–51. [ PubMed ] [ Google Scholar ]
- Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR (1985) In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis . N Engl J Med 313 :353–360. [ PubMed ] [ Google Scholar ]
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF (2010) Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence . Popul Health Metr 8 :29. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodrigues B, Johnson JD, Parks JS, et al. (2007) Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment . Nat Med 13 :340–347. [ PubMed ] [ Google Scholar ]
- Buse JB, DeFronzo RA, Rosenstock J, Kim T, Burns C, Skare S, Baron A, Fineman M (2016) The primary glucose-lowering effect of metformin resides in the gut, not the circulation: results from short-term pharmacokinetic and 12-week dose-ranging studies . Diabetes Care 39 :198–205. [ PubMed ] [ Google Scholar ]
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD; Exenatide-113 Clinical Study Group (2004) Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes . Diabetes Care 27 :2628–2635. [ PubMed ] [ Google Scholar ]
- Capozzi ME, Svendsen B, Encisco SE, Lewandowski SL, Martin MD, Lin H, Jaffe JL, Coch RW, Haldeman JM, MacDonald PE, et al. (2019a) β Cell tone is defined by proglucagon peptides through cAMP signaling . JCI Insight 4 :e126742. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Capozzi ME, Wait JB, Koech J, Gordon AN, Coch RW, Svendsen B, Finan B, D’Alessio DA, Campbell JE (2019b) Glucagon lowers glycemia when β-cells are active . JCI Insight 5 :e129954. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carboneau BA, Allan JA, Townsend SE, Kimple ME, Breyer RM, Gannon M (2017) Opposing effects of prostaglandin E 2 receptors EP3 and EP4 on mouse and human β-cell survival and proliferation . Mol Metab 6 :548–559. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chae H, Augustin R, Gatineau E, Mayoux E, Bensellam M, Antoine N, Khattab F, Lai BK, Brusa D, Stierstorfer B, et al. (2020) SGLT2 is not expressed in pancreatic α- and β-cells, and its inhibition does not directly affect glucagon and insulin secretion in rodents and humans . Mol Metab 42 :101071. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chamberlain CE, Scheel DW, McGlynn K, Kim H, Miyatsuka T, Wang J, Nguyen V, Zhao S, Mavropoulos A, Abraham AG, et al. (2014) Menin determines K-RAS proliferative outputs in endocrine cells . J Clin Invest 124 :4093–4101. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen H, Gu X, Liu Y, Wang J, Wirt SE, Bottino R, Schorle H, Sage J, Kim SK (2011) PDGF signalling controls age-dependent proliferation in pancreatic β-cells . Nature 478 :349–355. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen H, Kleinberger JW, Takane KK, Salim F, Fiaschi-Taesch N, Pappas K, Parsons R, Jiang J, Zhang Y, Liu H, et al. (2015) Augmented Stat5 signaling bypasses multiple impediments to lactogen-mediated proliferation in human β-cells . Diabetes 64 :3784–3797. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen J, Cha-Molstad H, Szabo A, Shalev A (2009) Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein . Am J Physiol Endocrinol Metab 296 :E1133–E1139. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen J, Hui ST, Couto FM, Mungrue IN, Davis DB, Attie AD, Lusis AJ, Davis RA, Shalev A (2008a) Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes . FASEB J 22 :3581–3594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A (2008b) Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis . Diabetes 57 :938–944. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chou DH, Webber MJ, Tang BC, Lin AB, Thapa LS, Deng D, Truong JV, Cortinas AB, Langer R, Anderson DG (2015) Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates . Proc Natl Acad Sci USA 112 :2401–2406. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cnop M, Toivonen S, Igoillo-Esteve M, Salpea P (2017) Endoplasmic reticulum stress and eIF2α phosphorylation: the Achilles heel of pancreatic β cells . Mol Metab 6 :1024–1039. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, Frost GS, Ghatei MA, Bloom SR (2003) Oxyntomodulin suppresses appetite and reduces food intake in humans . J Clin Endocrinol Metab 88 :4696–4701. [ PubMed ] [ Google Scholar ]
- Coll AP, Chen M, Taskar P, Rimmington D, Patel S, Tadross JA, Cimino I, Yang M, Welsh P, Virtue S, et al. (2020) GDF15 mediates the effects of metformin on body weight and energy balance . Nature 578 :444–448. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Colli ML, Hill JLE, Marroquí L, Chaffey J, Dos Santos RS, Leete P, Coomans de Brachène A, Paula FMM, Op de Beeck A, Castela A, et al. (2018) PDL1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-α and-γ via IRF1 induction . EBioMedicine 36 :367–375. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- RISE Consortium (2019) Lack of durable improvements in β-cell function following withdrawal of pharmacological interventions in adults with impaired glucose tolerance or recently diagnosed type 2 diabetes . Diabetes Care 42 :1742–1751. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dai C, Hang Y, Shostak A, Poffenberger G, Hart N, Prasad N, Phillips N, Levy SE, Greiner DL, Shultz LD, et al. (2017) Age-dependent human β cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling . J Clin Invest 127 :3835–3844. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dai C, Kayton NS, Shostak A, Poffenberger G, Cyphert HA, Aramandla R, Thompson C, Papagiannis IG, Emfinger C, Shiota M, et al. (2016) Stress-impaired transcription factor expression and insulin secretion in transplanted human islets . J Clin Invest 126 :1857–1870. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dai C, Walker JT, Shostak A, Bouchi Y, Poffenberger G, Hart NJ, Jacobson DA, Calcutt MW, Bottino R, Greiner DL, et al. (2020) Dapagliflozin does not directly affect human α or β cells . Endocrinology 161 :bqaa080. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM, Ghatei MA, Bloom SR (2001) Oxyntomodulin inhibits food intake in the rat . Endocrinology 142 :4244–4250. [ PubMed ] [ Google Scholar ]
- Dakin CL, Small CJ, Park AJ, Seth A, Ghatei MA, Bloom SR (2002) Repeated ICV administration of oxyntomodulin causes a greater reduction in body weight gain than in pair-fed rats . Am J Physiol Endocrinol Metab 283 :E1173–E1177. [ PubMed ] [ Google Scholar ]
- Defronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Gastaldelli A, Henry RR, Kitabchi AE, et al.; ACT NOW Study (2013) Prevention of diabetes with pioglitazone in ACT NOW: physiologic correlates . Diabetes 62 :3920–3926. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Delaunay F, Khan A, Cintra A, Davani B, Ling ZC, Andersson A, Ostenson CG, Gustafsson J, Efendic S, Okret S (1997) Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids . J Clin Invest 100 :2094–2098. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Desai T, Shea LD (2017) Advances in islet encapsulation technologies . Nat Rev Drug Discov 16 :338–350. [ PubMed ] [ Google Scholar ]
- Devaraj S, Venkatachalam A, Chen X (2016) Metformin and the gut microbiome in diabetes . Clin Chem 62 :1554–1555. [ PubMed ] [ Google Scholar ]
- Doloff JC, Veiseh O, Vegas AJ, Tam HH, Farah S, Ma M, Li J, Bader A, Chiu A, Sadraei A, et al. (2017) Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates . Nat Mater 16 :671–680. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Donath MY, Dinarello CA, Mandrup-Poulsen T (2019) Targeting innate immune mediators in type 1 and type 2 diabetes . Nat Rev Immunol 19 :734–746. [ PubMed ] [ Google Scholar ]
- Drucker DJ (2018) Mechanisms of action and therapeutic application of glucagon-like peptide-1 . Cell Metab 27 :740–756. [ PubMed ] [ Google Scholar ]
- Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF (1987) Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line . Proc Natl Acad Sci USA 84 :3434–3438. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Duca FA, Côté CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK (2015) Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats . Nat Med 21 :506–511. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Eguchi K, Nagai R (2017) Islet inflammation in type 2 diabetes and physiology . J Clin Invest 127 :14–23. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- El Khatib MM, Sakuma T, Tonne JM, Mohamed MS, Holditch SJ, Lu B, Kudva YC, Ikeda Y (2015) β-Cell-targeted blockage of PD1 and CTLA4 pathways prevents development of autoimmune diabetes and acute allogeneic islets rejection . Gene Ther 22 :430–438. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- El-Khatib FH, Balliro C, Hillard MA, Magyar KL, Ekhlaspour L, Sinha M, Mondesir D, Esmaeili A, Hartigan C, Thompson MJ, et al. (2017) Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial . Lancet 389 :369–380. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- El-Mir MY, Detaille D, R-Villanueva G, Delgado-Esteban M, Guigas B, Attia S, Fontaine E, Almeida A, Leverve X (2008) Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons . J Mol Neurosci 34 :77–87. [ PubMed ] [ Google Scholar ]
- El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I . J Biol Chem 275 :223–228. [ PubMed ] [ Google Scholar ]
- Elrick H, Stimmler L, Hlad CJ Jr, Arai Y (1964) Plasma Insulin Response to Oral and Intravenous Glucose Administration . J Clin Endocrinol Metab 24 :1076–1082. [ PubMed ] [ Google Scholar ]
- Engin F, Yermalovich A, Nguyen T, Hummasti S, Fu W, Eizirik DL, Mathis D, Hotamisligil GS (2013) Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes [published correction appears in Sci Transl Med (2013) 5 :214er11] . Sci Transl Med 5 :211ra156. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD (2005) Metformin and reduced risk of cancer in diabetic patients . BMJ 330 :1304–1305. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Evans-Molina C, Robbins RD, Kono T, Tersey SA, Vestermark GL, Nunemaker CS, Garmey JC, Deering TG, Keller SR, Maier B, et al. (2009) Peroxisome proliferator-activated receptor gamma activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure . Mol Cell Biol 29 :2053–2067. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fernández-Real JM, López-Bermejo A, Ropero AB, Piquer S, Nadal A, Bassols J, Casamitjana R, Gomis R, Arnaiz E, Pérez I, et al. (2008) Salicylates increase insulin secretion in healthy obese subjects . J Clin Endocrinol Metab 93 :2523–2530. [ PubMed ] [ Google Scholar ]
- Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B (2014) Metformin: from mechanisms of action to therapies . Cell Metab 20 :953–966. [ PubMed ] [ Google Scholar ]
- Foretz M, Guigas B, Viollet B (2019) Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus . Nat Rev Endocrinol 15 :569–589. [ PubMed ] [ Google Scholar ]
- Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state . J Clin Invest 120 :2355–2369. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Frias JPBastyr EJ 3rd, Vignati L, Tschöp MH, Schmitt C, Owen K, Christensen RHDiMarchi RD (2017) The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes . Cell Metab 26 :343–352.e2. [ PubMed ] [ Google Scholar ]
- Frias JP, Nauck MA, Van J, Benson C, Bray R, Cui X, Milicevic Z, Urva S, Haupt A, Robins DA (2020) Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens . Diabetes Obes Metab 22 :938–946. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, Urva S, Gimeno RE, Milicevic Z, Robins D, et al. (2018) Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial . Lancet 392 :2180–2193. [ PubMed ] [ Google Scholar ]
- Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O’Neill HM, Ford RJ, Palanivel R, O’Brien M, et al. (2013) Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin . Nat Med 19 :1649–1654. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, et al.; REWIND Investigators (2019) Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial . Lancet 394 :121–130. [ PubMed ] [ Google Scholar ]
- Giugliano D, Ceriello A, Saccomanno F, Quatraro A, Paolisso G, D’Onofrio F (1985) Effects of salicylate, tolbutamide, and prostaglandin E2 on insulin responses to glucose in noninsulin-dependent diabetes mellitus . J Clin Endocrinol Metab 61 :160–166. [ PubMed ] [ Google Scholar ]
- Goudy KS, Tisch R (2005) Immunotherapy for the prevention and treatment of type 1 diabetes . Int Rev Immunol 24 :307–326. [ PubMed ] [ Google Scholar ]
- Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA, Rhodes CJ (2012) Formation of a human β-cell population within pancreatic islets is set early in life . J Clin Endocrinol Metab 97 :3197–3206. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, Bakker RA, Mark M, Klein T, Eickelmann P (2012) Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors . Diabetes Obes Metab 14 :83–90. [ PubMed ] [ Google Scholar ]
- Harlan DM (2016) Islet transplantation for hypoglycemia unawareness/severe hypoglycemia: caveat emptor . Diabetes Care 39 :1072–1074. [ PubMed ] [ Google Scholar ]
- Harrower AD (1991) Efficacy of gliclazide in comparison with other sulphonylureas in the treatment of NIDDM . Diabetes Res Clin Pract 14 ( Suppl 2 ):S65–S67. [ PubMed ] [ Google Scholar ]
- He L, Wondisford FE (2015) Metformin action: concentrations matter . Cell Metab 21 :159–162. [ PubMed ] [ Google Scholar ]
- Hedrington MS, Davis SN (2019) Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes . Expert Opin Pharmacother 20 :2229–2235. [ PubMed ] [ Google Scholar ]
- Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, et al.; Clinical Islet Transplantation Consortium (2016) Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia . Diabetes Care 39 :1230–1240. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J, et al. (2005) Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes . JAMA 293 :830–835. [ PubMed ] [ Google Scholar ]
- Hernandez AFGreen JBJanmohamed SD’Agostino RB Sr , Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MCet al.; Harmony Outcomes committees and investigators (2018) Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial . Lancet 392 :1519–1529. [ PubMed ] [ Google Scholar ]
- Hiatt WR, Kaul S, Smith RJ (2013) The cardiovascular safety of diabetes drugs--insights from the rosiglitazone experience . N Engl J Med 369 :1285–1287. [ PubMed ] [ Google Scholar ]
- Hirshberg B, Preston EH, Xu H, Tal MG, Neeman Z, Bunnell D, Soleimanpour S, Hale DA, Kirk AD, Harlan DM (2003) Rabbit antithymocyte globulin induction and sirolimus monotherapy supports prolonged islet allograft function in a nonhuman primate islet transplantation model . Transplantation 76 :55–60. [ PubMed ] [ Google Scholar ]
- Hu W, Jiang C, Guan D, Dierickx P, Zhang R, Moscati A, Nadkarni GN, Steger DJ, Loos RJF, Hu C, et al. (2019) Patient adipose stem cell-derived adipocytes reveal genetic variation that predicts antidiabetic drug response . Cell Stem Cell 24 :299–308.e6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, et al.; PIONEER 6 Investigators (2019) Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes . N Engl J Med 381 :841–851. [ PubMed ] [ Google Scholar ]
- Imamura M, Nakanishi K, Suzuki T, Ikegai K, Shiraki R, Ogiyama T, Murakami T, Kurosaki E, Noda A, Kobayashi Y, et al. (2012) Discovery of Ipragliflozin (ASP1941): a novel C-glucoside with benzothiophene structure as a potent and selective sodium glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes mellitus . Bioorg Med Chem 20 :3263–3279. [ PubMed ] [ Google Scholar ]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, et al.; ADOPT Study Group (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy . N Engl J Med 355 :2427–2443. [ PubMed ] [ Google Scholar ]
- Kahn SE, Lachin JM, Zinman B, Haffner SM, Aftring RP, Paul G, Kravitz BG, Herman WH, Viberti G, Holman RR; ADOPT Study Group (2011) Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT . Diabetes 60 :1552–1560. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, et al. (2010) Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women . Diabetes 59 :1899–1905. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kemp CB, Knight MJ, Scharp DW, Lacy PE, Ballinger WF (1973) Transplantation of isolated pancreatic islets into the portal vein of diabetic rats . Nature 244 :447. [ PubMed ] [ Google Scholar ]
- Kim HI, Cha JY, Kim SY, Kim JW, Roh KJ, Seong JK, Lee NT, Choi KY, Kim KS, Ahn YH (2002) Peroxisomal proliferator-activated receptor-gamma upregulates glucokinase gene expression in beta-cells . Diabetes 51 :676–685. [ PubMed ] [ Google Scholar ]
- Kim HI, Kim JW, Kim SH, Cha JY, Kim KS, Ahn YH (2000) Identification and functional characterization of the peroxisomal proliferator response element in rat GLUT2 promoter . Diabetes 49 :1517–1524. [ PubMed ] [ Google Scholar ]
- Kim SH, Liu A, Ariel D, Abbasi F, Lamendola C, Grove K, Tomasso V, Ochoa H, Reaven G (2014) Effect of salsalate on insulin action, secretion, and clearance in nondiabetic, insulin-resistant individuals: a randomized, placebo-controlled study . Diabetes Care 37 :1944–1950. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Koffert JP, Mikkola K, Virtanen KA, Andersson AD, Faxius L, Hällsten K, Heglind M, Guiducci L, Pham T, Silvola JMU, et al. (2017) Metformin treatment significantly enhances intestinal glucose uptake in patients with type 2 diabetes: Results from a randomized clinical trial . Diabetes Res Clin Pract 131 :208–216. [ PubMed ] [ Google Scholar ]
- Koh A, Mannerås-Holm L, Yunn NO, Nilsson PM, Ryu SH, Molinaro A, Perkins R, Smith JG, Bäckhed F (2020) Microbial imidazole propionate affects responses to metformin through p38γ-dependent inhibitory AMPK phosphorylation . Cell Metab 32 :643–653.e4. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kreymann B, Williams G, Ghatei MA, Bloom SR (1987) Glucagon-like peptide-1 7-36: a physiological incretin in man . Lancet 2 :1300–1304. [ PubMed ] [ Google Scholar ]
- Kuhre RE, Ghiasi SM, Adriaenssens AE, Wewer Albrechtsen NJ, Andersen DB, Aivazidis A, Chen L, Mandrup-Poulsen T, Ørskov C, Gribble FM, et al. (2019) No direct effect of SGLT2 activity on glucagon secretion . Diabetologia 62 :1011–1023. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF (2012) Human β-cell proliferation and intracellular signaling: driving in the dark without a road map . Diabetes 61 :2205–2213. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kurosaki E, Ogasawara H (2013) Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data . Pharmacol Ther 139 :51–59. [ PubMed ] [ Google Scholar ]
- Kurtzhals P, Schäffer L, Sørensen A, Kristensen C, Jonassen I, Schmid C, Trüb T (2000) Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use . Diabetes 49 :999–1005. [ PubMed ] [ Google Scholar ]
- Lambeir AM, Scharpé S, De Meester I (2008) DPP4 inhibitors for diabetes--what next? Biochem Pharmacol 76 :1637–1643. [ PubMed ] [ Google Scholar ]
- Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ (2007) Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes . Diabetologia 50 :752–763. [ PubMed ] [ Google Scholar ]
- Lebovitz HE (2019) Thiazolidinediones: the forgotten diabetes medications . Curr Diab Rep 19 :151. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lewis JD, Habel LA, Quesenberry CP, Strom BL, Peng T, Hedderson MM, Ehrlich SF, Mamtani R, Bilker W, Vaughn DJ, et al. (2015) Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes . JAMA 314 :265–277. [ PubMed ] [ Google Scholar ]
- Li L, Li S, Deng K, Liu J, Vandvik PO, Zhao P, Zhang L, Shen J, Bala MM, Sohani ZN, et al. (2016) Dipeptidyl peptidase-4 inhibitors and risk of heart failure in type 2 diabetes: systematic review and meta-analysis of randomised and observational studies . BMJ 352 :i610. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Link JT (2003) Pharmacological regulation of hepatic glucose production . Curr Opin Investig Drugs 4 :421–429. [ PubMed ] [ Google Scholar ]
- Luippold G, Klein T, Mark M, Grempler R (2012) Empagliflozin, a novel potent and selective SGLT-2 inhibitor, improves glycaemic control alone and in combination with insulin in streptozotocin-induced diabetic rats, a model of type 1 diabetes mellitus . Diabetes Obes Metab 14 :601–607. [ PubMed ] [ Google Scholar ]
- Maganti AV, Tersey SA, Syed F, Nelson JB, Colvin SC, Maier B, Mirmira RG (2016) Peroxisome proliferator-activated receptor-γ activation augments the β-cell unfolded protein response and rescues early glycemic deterioration and β cell death in non-obese diabetic mice . J Biol Chem 291 :22524–22533. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Makrilakis K (2019) The role of DPP-4 inhibitors in the treatment algorithm of type 2 diabetes mellitus: when to select, what to expect . Int J Environ Res Public Health 16 :2720. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M (2007) The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients . Diabetologia 50 :2486–2494. [ PubMed ] [ Google Scholar ]
- Marhfour I, Lopez XM, Lefkaditis D, Salmon I, Allagnat F, Richardson SJ, Morgan NG, Eizirik DL (2012) Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes . Diabetologia 55 :2417–2420. [ PubMed ] [ Google Scholar ]
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al.; SUSTAIN-6 Investigators (2016a) Semaglutide and cardiovascular outcomes in patients with type 2 diabetes . N Engl J Med 375 :1834–1844. [ PubMed ] [ Google Scholar ]
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al.; LEADER Steering Committee; LEADER Trial Investigators (2016b) Liraglutide and cardiovascular outcomes in type 2 diabetes . N Engl J Med 375 :311–322. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Massollo M, Marini C, Brignone M, Emionite L, Salani B, Riondato M, Capitanio S, Fiz F, Democrito A, Amaro A, et al. (2013) Metformin temporal and localized effects on gut glucose metabolism assessed using 18F-FDG PET in mice . J Nucl Med 54 :259–266. [ PubMed ] [ Google Scholar ]
- McCreight LJ, Bailey CJ, Pearson ER (2016) Metformin and the gastrointestinal tract . Diabetologia 59 :426–435. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McIntyre N, Holdsworth CD, Turner DS (1964) New interpretation of oral glucose tolerance . Lancet 2 :20–21. [ PubMed ] [ Google Scholar ]
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al.; DAPA-HF Trial Committees and Investigators (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction . N Engl J Med 381 :1995–2008. [ PubMed ] [ Google Scholar ]
- Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC (2008) Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans . Diabetes 57 :1584–1594. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Meng W, Ellsworth BA, Nirschl AA, McCann PJ, Patel M, Girotra RN, Wu G, Sher PM, Morrison EP, Biller SA, et al. (2008) Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes . J Med Chem 51 :1145–1149. [ PubMed ] [ Google Scholar ]
- Menting JG, Whittaker J, Margetts MB, Whittaker LJ, Kong GK, Smith BJ, Watson CJ, Záková L, Kletvíková E, Jiráček J, et al. (2013) How insulin engages its primary binding site on the insulin receptor . Nature 493 :241–245. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ (2013) Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP . Nature 494 :256–260. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Minkowski O (1892) Weitere Mitteilungen über den Diabetes mellitus nach Extirpation des Pankreas . Berliner Klinische Wochenschrift 29 :90–93. [ Google Scholar ]
- Misbin RI (2004) The phantom of lactic acidosis due to metformin in patients with diabetes . Diabetes Care 27 :1791–1793. [ PubMed ] [ Google Scholar ]
- Mudaliar S, Armstrong DA, Mavian AA, O’Connor-Semmes R, Mydlow PK, Ye J, Hussey EK, Nunez DJ, Henry RR, Dobbins RL (2012) Remogliflozin etabonate, a selective inhibitor of the sodium-glucose transporter 2, improves serum glucose profiles in type 1 diabetes . Diabetes Care 35 :2198–2200. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mulherin AJ, Oh AH, Kim H, Grieco A, Lauffer LM, Brubaker PL (2011) Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell . Endocrinology 152 :4610–4619. [ PubMed ] [ Google Scholar ]
- Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, et al. (2019) Glucagon-like peptide 1 (GLP-1) . Mol Metab 30 :72–130. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Munir KM, Lamos EM (2017) Diabetes type 2 management: what are the differences between DPP-4 inhibitors and how do you choose? Expert Opin Pharmacother 18 :839–841. [ PubMed ] [ Google Scholar ]
- Naftanel MA, Harlan DM (2004) Pancreatic islet transplantation . PLoS Med 1 :e58. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nauck M, Stöckmann F, Ebert R, Creutzfeldt W (1986a) Reduced incretin effect in type 2 (non-insulin-dependent) diabetes . Diabetologia 29 :46–52. [ PubMed ] [ Google Scholar ]
- Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W (1986b) Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses . J Clin Endocrinol Metab 63 :492–498. [ PubMed ] [ Google Scholar ]
- Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, Reynolds P, Hardy M, King E, Masters J, et al.; Wellcome Trust Case Control Consortium (2007) Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A . Nature 450 :887–892. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nichols CG (2006) KATP channels as molecular sensors of cellular metabolism . Nature 440 :470–476. [ PubMed ] [ Google Scholar ]
- Nomura S, Sakamaki S, Hongu M, Kawanishi E, Koga Y, Sakamoto T, Yamamoto Y, Ueta K, Kimata H, Nakayama K, et al. (2010) Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus . J Med Chem 53 :6355–6360. [ PubMed ] [ Google Scholar ]
- Ohnishi ST, Endo M, editors. (1981) The Mechanism of Gated Calcium Transport Across Biological Membranes , Academic Press, New York. [ Google Scholar ]
- Osipovich AB, Stancill JS, Cartailler JP, Dudek KD, Magnuson MA (2020) Excitotoxicity and overnutrition additively impair metabolic function and identity of pancreatic β-cells . Diabetes 69 :1476–1491. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Osum KC, Burrack AL, Martinov T, Sahli NL, Mitchell JS, Tucker CG, Pauken KE, Papas K, Appakalai B, Spanier JA, et al. (2018) Interferon-gamma drives programmed death-ligand 1 expression on islet β cells to limit T cell function during autoimmune diabetes . Sci Rep 8 :8295. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Owen MR, Doran E, Halestrap AP (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain . Biochem J 348 :607–614. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ozanne SE, Guest PC, Hutton JC, Hales CN (1995) Intracellular localization and molecular heterogeneity of the sulphonylurea receptor in insulin-secreting cells . Diabetologia 38 :277–282. [ PubMed ] [ Google Scholar ]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes . Science 313 :1137–1140. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Paty BW, Harmon JS, Marsh CL, Robertson RP (2002) Inhibitory effects of immunosuppressive drugs on insulin secretion from HIT-T15 cells and Wistar rat islets . Transplantation 73 :353–357. [ PubMed ] [ Google Scholar ]
- Penesova A, Koska J, Ortega E, Bunt JC, Bogardus C, de Courten B (2015) Salsalate has no effect on insulin secretion but decreases insulin clearance: a randomized, placebo-controlled trial in subjects without diabetes . Diabetes Obes Metab 17 :608–612. [ PubMed ] [ Google Scholar ]
- Perdigoto AL, Quandt Z, Anderson M, Herold KC (2019) Checkpoint inhibitor-induced insulin-dependent diabetes: an emerging syndrome . Lancet Diabetes Endocrinol 7 :421–423. [ PubMed ] [ Google Scholar ]
- Perley MJ, Kipnis DM (1967) Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects . J Clin Invest 46 :1954–1962. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pernicova I, Korbonits M (2014) Metformin--mode of action and clinical implications for diabetes and cancer . Nat Rev Endocrinol 10 :143–156. [ PubMed ] [ Google Scholar ]
- Preiss D, Dawed A, Welsh P, Heggie A, Jones AG, Dekker J, Koivula R, Hansen TH, Stewart C, Holman RR, et al.; DIRECT consortium group (2017) Sustained influence of metformin therapy on circulating glucagon-like peptide-1 levels in individuals with and without type 2 diabetes . Diabetes Obes Metab 19 :356–363. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Prentki M, Nolan CJ (2006) Islet beta cell failure in type 2 diabetes . J Clin Invest 116 :1802–1812. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pueyo ME, Darquy S, Capron F, Reach G (1993) In vitro activation of human macrophages by alginate-polylysine microcapsules . J Biomater Sci Polym Ed 5 :197–203. [ PubMed ] [ Google Scholar ]
- Pybus F (1924) Notes on suprarenal and pancreatic grafting . Lancet 204 :550–551. [ Google Scholar ]
- Quattrin T, Haller MJ, Steck AK, Felner EI, Li Y, Xia Y, Leu JH, Zoka R, Hedrick JA, Rigby MR, et al.; T1GER Study Investigators (2020) Golimumab and Beta-Cell Function in Youth with New-Onset Type 1 Diabetes . N Engl J Med 383 :2007–2017. [ PubMed ] [ Google Scholar ]
- Rachdi L, Kariyawasam D, Aïello V, Herault Y, Janel N, Delabar JM, Polak M, Scharfmann R (2014a) Dyrk1A induces pancreatic β cell mass expansion and improves glucose tolerance . Cell Cycle 13 :2221–2229. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rachdi L, Kariyawasam D, Guez F, Aïello V, Arbonés ML, Janel N, Delabar JM, Polak M, Scharfmann R (2014b) Dyrk1a haploinsufficiency induces diabetes in mice through decreased pancreatic beta cell mass . Diabetologia 57 :960–969. [ PubMed ] [ Google Scholar ]
- Rege NK, Phillips NFB, Weiss MA (2017) Development of glucose-responsive ‘smart’ insulin systems . Curr Opin Endocrinol Diabetes Obes 24 :267–278. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rena G, Hardie DG, Pearson ER (2017) The mechanisms of action of metformin . Diabetologia 60 :1577–1585. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rewers M, Gottlieb P (2009) Immunotherapy for the prevention and treatment of type 1 diabetes: human trials and a look into the future . Diabetes Care 32 :1769–1782. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Richardson SJ, Rodriguez-Calvo T, Gerling IC, Mathews CE, Kaddis JS, Russell MA, Zeissler M, Leete P, Krogvold L, Dahl-Jørgensen K, et al. (2016) Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes . Diabetologia 59 :2448–2458. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rickels MR, Liu C, Shlansky-Goldberg RD, Soleimanpour SA, Vivek K, Kamoun M, Min Z, Markmann E, Palangian M, Dalton-Bakes C, et al. (2013) Improvement in β-cell secretory capacity after human islet transplantation according to the c7 protocol . Diabetes 62 :2890–2897. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rickels MR, Robertson RP (2019) Pancreatic islet transplantation in humans: recent progress and future directions . Endocr Rev 40 :631–668. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rodriguez-Calvo T, Suwandi JS, Amirian N, Zapardiel-Gonzalo J, Anquetil F, Sabouri S, von Herrath MG (2015) Heterogeneity and lobularity of pancreatic pathology in type 1 diabetes during the prediabetic phase . J Histochem Cytochem 63 :626–636. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rojas LB, Gomes MB (2013) Metformin: an old but still the best treatment for type 2 diabetes . Diabetol Metab Syndr 5 :6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu CH, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, et al. (2003) Targeted elimination of peroxisome proliferator-activated receptor gamma in beta cells leads to abnormalities in islet mass without compromising glucose homeostasis . Mol Cell Biol 23 :7222–7229. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosenstock J, Hassman DR, Madder RD, Brazinsky SA, Farrell J, Khutoryansky N, Hale PM; Repaglinide Versus Nateglinide Comparison Study Group (2004) Repaglinide versus nateglinide monotherapy: a randomized, multicenter study . Diabetes Care 27 :1265–1270. [ PubMed ] [ Google Scholar ]
- Sampaio MS, Kuo HT, Bunnapradist S (2011) Outcomes of simultaneous pancreas-kidney transplantation in type 2 diabetic recipients . Clin J Am Soc Nephrol 6 :1198–1206. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Saponaro C, Gmyr V, Thévenet J, Moerman E, Delalleau N, Pasquetti G, Coddeville A, Quenon A, Daoudi M, Hubert T, et al. (2019) The GLP1R agonist liraglutide reduces hyperglucagonemia induced by the SGLT2 inhibitor dapagliflozin via somatostatin release . Cell Rep 28 :1447–1454.e4. [ PubMed ] [ Google Scholar ]
- Saponaro C, Mühlemann M, Acosta-Montalvo A, Piron A, Gmyr V, Delalleau N, Moerman E, Thévenet J, Pasquetti G, Coddeville A, et al. (2020) Interindividual heterogeneity of SGLT2 expression and function in human pancreatic islets . Diabetes 69 :902–914. [ PubMed ] [ Google Scholar ]
- Satin LS, Tavalin SJ, Kinard TA, Teague J (1995) Contribution of L- and non-L-type calcium channels to voltage-gated calcium current and glucose-dependent insulin secretion in HIT-T15 cells . Endocrinology 136 :4589–4601. [ PubMed ] [ Google Scholar ]
- Schwartz AV, Chen H, Ambrosius WT, Sood A, Josse RG, Bonds DE, Schnall AM, Vittinghoff E, Bauer DC, Banerji MA, et al. (2015) Effects of TZD use and discontinuation on fracture rates in ACCORD Bone Study . J Clin Endocrinol Metab 100 :4059–4066. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shalev A, Pise-Masison CA, Radonovich M, Hoffmann SC, Hirshberg B, Brady JN, Harlan DM (2002) Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway . Endocrinology 143 :3695–3698. [ PubMed ] [ Google Scholar ]
- Shankar SS, Shankar RR, Mixson LA, Miller DL, Pramanik B, O’Dowd AK, Williams DM, Frederick CB, Beals CR, Stoch SA, et al. (2018) Native oxyntomodulin has significant glucoregulatory effects independent of weight loss in obese humans with and without type 2 diabetes . Diabetes 67 :1105–1112. [ PubMed ] [ Google Scholar ]
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV (2000) Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen . N Engl J Med 343 :230–238. [ PubMed ] [ Google Scholar ]
- Sharma RB, O’Donnell AC, Stamateris RE, Ha B, McCloskey KM, Reynolds PR, Arvan P, Alonso LC (2015) Insulin demand regulates β cell number via the unfolded protein response . J Clin Invest 125 :3831–3846. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sims EK, Mirmira RG, Evans-Molina C (2020) The role of beta-cell dysfunction in early type 1 diabetes . Curr Opin Endocrinol Diabetes Obes 27 :215–224. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soccio RE, Chen ER, Rajapurkar SR, Safabakhsh P, Marinis JM, Dispirito JR, Emmett MJ, Briggs ER, Fang B, Everett LJ, et al. (2015) Genetic variation determines PPARγ function and anti-diabetic drug response in vivo . Cell 162 :33–44. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soleimanpour SA, Crutchlow MF, Ferrari AM, Raum JC, Groff DN, Rankin MM, Liu C, De León DD, Naji A, Kushner JA, et al. (2010) Calcineurin signaling regulates human islet beta-cell survival . J Biol Chem 285 :40050–40059. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soleimanpour SA, Hirshberg B, Bunnell DJ, Sumner AE, Ader M, Remaley AT, Rother KI, Rickels MR, Harlan DM (2012) Metabolic function of a suboptimal transplanted islet mass in nonhuman primates on rapamycin monotherapy . Cell Transplant 21 :1297–1304. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Soleimanpour SA, Stoffers DA (2013) The pancreatic β cell and type 1 diabetes: innocent bystander or active participant? Trends Endocrinol Metab 24 :324–331. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, Gettinger S, Sznol M, Young A, Rushakoff R, et al. (2018) Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors . Diabetes 67 :1471–1480. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stancill JS, Cartailler JP, Clayton HW, O’Connor JT, Dickerson MT, Dadi PK, Osipovich AB, Jacobson DA, Magnuson MA (2017) Chronic β-cell depolarization impairs β-cell identity by disrupting a network of Ca 2+ -regulated genes . Diabetes 66 :2175–2187. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stewart AF, Hussain MA, García-Ocaña A, Vasavada RC, Bhushan A, Bernal-Mizrachi E, Kulkarni RN (2015) Human β-cell proliferation and intracellular signaling: part 3 . Diabetes 64 :1872–1885. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stojanovic I, Dimitrijevic M, Vives-Pi M, Mansilla MJ, Pujol-Autonell I, Rodríguez-Fernandez S, Palova-Jelínkova L, Funda DP, Gruden-Movsesijan A, Sofronic-Milosavljevic L, et al. (2017) Cell-based tolerogenic therapy, experience from animal models of multiple sclerosis, type 1 diabetes and rheumatoid arthritis . Curr Pharm Des 23 :2623–2643. [ PubMed ] [ Google Scholar ]
- Sturek JM, Castle JD, Trace AP, Page LC, Castle AM, Evans-Molina C, Parks JS, Mirmira RG, Hedrick CC (2010) An intracellular role for ABCG1-mediated cholesterol transport in the regulated secretory pathway of mouse pancreatic beta cells . J Clin Invest 120 :2575–2589. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Suga T, Kikuchi O, Kobayashi M, Matsui S, Yokota-Hashimoto H, Wada E, Kohno D, Sasaki T, Takeuchi K, Kakizaki S, et al. (2019) SGLT1 in pancreatic α cells regulates glucagon secretion in mice, possibly explaining the distinct effects of SGLT2 inhibitors on plasma glucagon levels . Mol Metab 19 :1–12. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sun L, Xie C, Wang G, Wu Y, Wu Q, Wang X, Liu J, Deng Y, Xia J, Chen B, et al. (2018) Gut microbiota and intestinal FXR mediate the clinical benefits of metformin . Nat Med 24 :1919–1929. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sutherland DE, Matas AJ, Najarian JS (1978) Pancreatic islet cell transplantation . Surg Clin North Am 58 :365–382. [ PubMed ] [ Google Scholar ]
- Tersey SA, Nishiki Y, Templin AT, Cabrera SM, Stull ND, Colvin SC, Evans-Molina C, Rickus JL, Maier B, Mirmira RG (2012) Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model . Diabetes 61 :818–827. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thielen LA, Chen J, Jing G, Moukha-Chafiq O, Xu G, Jo S, Grayson TB, Lu B, Li P, Augelli-Szafran CE, et al. (2020) Identification of an anti-diabetic, orally available small molecule that regulates TXNIP expression and glucagon action . Cell Metab 32 :353–365.e8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tillner J, Posch MG, Wagner F, Teichert L, Hijazi Y, Einig C, Keil S, Haack T, Wagner M, Bossart M, et al. (2019) A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: Results of randomized, placebo-controlled first-in-human and first-in-patient trials . Diabetes Obes Metab 21 :120–128. [ PubMed ] [ Google Scholar ]
- Vallon V (2015) The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus . Annu Rev Med 66 :255–270. [ PubMed ] [ Google Scholar ]
- Vasseur M, Debuyser A, Joffre M (1987) Sensitivity of pancreatic beta cell to calcium channel blockers. An electrophysiologic study of verapamil and nifedipine . Fundam Clin Pharmacol 1 :95–113. [ PubMed ] [ Google Scholar ]
- Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, et al. (2016) Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates . Nat Biotechnol 34 :345–352. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Vergari E, Knudsen JG, Ramracheya R, Salehi A, Zhang Q, Adam J, Asterholm IW, Benrick A, Briant LJB, Chibalina MV, et al. (2019) Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion . Nat Commun 10 :139. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wallner K, Shapiro AM, Senior PA, McCabe C (2016) Cost effectiveness and value of information analyses of islet cell transplantation in the management of ‘unstable’ type 1 diabetes mellitus . BMC Endocr Disord 16 :17. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang H, Bender A, Wang P, Karakose E, Inabnet WB, Libutti SK, Arnold A, Lambertini L, Stang M, Chen H, et al. (2017) Insights into beta cell regeneration for diabetes via integration of molecular landscapes in human insulinomas . Nat Commun 8 :767. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang Y, An H, Liu T, Qin C, Sesaki H, Guo S, Radovick S, Hussain M, Maheshwari A, Wondisford FE, O’Rourke B, He L (2019) Metformin improves mitochondrial respiratory activity through activation of AMPK . Cell Rep 29 :1511–1523.e5. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Westerman J, Wirtz KW, Berkhout T, van Deenen LL, Radhakrishnan R, Khorana HG (1983) Identification of the lipid-binding site of phosphatidylcholine-transfer protein with phosphatidylcholine analogs containing photoactivable carbene precursors . Eur J Biochem 132 :441–449. [ PubMed ] [ Google Scholar ]
- World Health Organization (2020) World Health Organization Diabetes Fact Sheet . [ Google Scholar ]
- Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, Capozzi ME, van der Velden WJ, Stutsman C, Cardona GR, et al. (2020) Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist . JCI Insight 5 :e140532. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Williams P (1894) Notes on diabetes treated with extract and by grafts of sheep’s pancreas . BMJ 2 :1303–1304. [ Google Scholar ]
- Williamson RT (1901) On the treatment of glycosuria and diabetes mellitus with sodium salicylate . BMJ 1 :760–762. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Witters LA (2001) The blooming of the French lilac . J Clin Invest 108 :1105–1107. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al.; DECLARE–TIMI 58 Investigators (2019) Dapagliflozin and cardiovascular outcomes in type 2 diabetes . N Engl J Med 380 :347–357. [ PubMed ] [ Google Scholar ]
- Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, et al. (2017) Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug . Nat Med 23 :850–858. [ PubMed ] [ Google Scholar ]
- Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA, et al. (2005) Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial . Diabetes 54 :2390–2395. [ PubMed ] [ Google Scholar ]
- Xu G, Chen J, Jing G, Shalev A (2012) Preventing β-cell loss and diabetes with calcium channel blockers . Diabetes 61 :848–856. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yang JF, Gong X, Bakh NA, Carr K, Phillips NFB, Ismail-Beigi F, Weiss MA, Strano MS (2020) Connecting rodent and human pharmacokinetic models for the design and translation of glucose-responsive insulin . Diabetes 69 :1815–1826. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yu O, Azoulay L, Yin H, Filion KB, Suissa S (2018) Sulfonylureas as initial treatment for type 2 diabetes and the risk of severe hypoglycemia . Am J Med 131 :317.e11–317.e22. [ PubMed ] [ Google Scholar ]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action . J Clin Invest 108 :1167–1174. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al.; EMPA-REG OUTCOME Investigators (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes . N Engl J Med 373 :2117–2128. [ PubMed ] [ Google Scholar ]
Research Summaries
Keep up with the latest diabetes and diabetes-related studies with these brief overviews. Each summary provides main points, methods, and findings and includes a link to the article.
Diabetes Management and Education
Reaching treatment goals could help people living with type 2 diabetes increase their life expectancy by 3 years or in some cases by as much as 10 years. Read the summary .
Adults who receive diabetes education are more likely to follow recommended preventive care practices that lead to better diabetes management. Read the summary .
In 2017, the total cost of diabetes complications was over $37 billion among Medicare beneficiaries 65 or older with type 2 diabetes. Read the summary .
Kids and teens can get both type 1 and type 2 diabetes. New research shows how diabetes rates in young people may rise by 2060. Read the summary .
New USPSTF and ADA guidelines lower the age for prediabetes and type 2 diabetes screening to 35. This study examined if testing practices aligned with guidelines and which populations were less likely to receive testing. Read the summary .
The SEARCH for Diabetes in Youth study reports trends in young people who are being diagnosed with type 1 and type 2 diabetes. Read the summary .
Recent guidelines recommend newer types of diabetes medications, and most Americans living with type 2 diabetes are eligible. Read the summary .
Chronic Kidney Disease
End-stage kidney disease—kidney failure that requires dialysis or a kidney transplant—can lead to disability and early death, is expensive to treat, and cases are on the rise. Read the summary .
- Reports and Publications
- Research Projects
- US Diabetes Surveillance System
To receive updates about diabetes topics, enter your email address:
- Diabetes Home
- State, Local, and National Partner Diabetes Programs
- National Diabetes Prevention Program
- Native Diabetes Wellness Program
- Chronic Kidney Disease
- Vision Health Initiative
- Heart Disease and Stroke
- Overweight & Obesity
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Patient Care & Health Information
- Diseases & Conditions
- Type 2 diabetes
Type 2 diabetes is usually diagnosed using the glycated hemoglobin (A1C) test. This blood test indicates your average blood sugar level for the past two to three months. Results are interpreted as follows:
- Below 5.7% is normal.
- 5.7% to 6.4% is diagnosed as prediabetes.
- 6.5% or higher on two separate tests indicates diabetes.
If the A1C test isn't available, or if you have certain conditions that interfere with an A1C test, your health care provider may use the following tests to diagnose diabetes:
Random blood sugar test. Blood sugar values are expressed in milligrams of sugar per deciliter ( mg/dL ) or millimoles of sugar per liter ( mmol/L ) of blood. Regardless of when you last ate, a level of 200 mg/dL (11.1 mmol/L ) or higher suggests diabetes, especially if you also have symptoms of diabetes, such as frequent urination and extreme thirst.
Fasting blood sugar test. A blood sample is taken after you haven't eaten overnight. Results are interpreted as follows:
- Less than 100 mg/dL (5.6 mmol/L ) is considered healthy.
- 100 to 125 mg/dL (5.6 to 6.9 mmol/L ) is diagnosed as prediabetes.
- 126 mg/dL (7 mmol/L ) or higher on two separate tests is diagnosed as diabetes.
Oral glucose tolerance test. This test is less commonly used than the others, except during pregnancy. You'll need to not eat for a certain amount of time and then drink a sugary liquid at your health care provider's office. Blood sugar levels then are tested periodically for two hours. Results are interpreted as follows:
- Less than 140 mg/dL (7.8 mmol/L ) after two hours is considered healthy.
- 140 to 199 mg/dL (7.8 mmol/L and 11.0 mmol/L ) is diagnosed as prediabetes.
- 200 mg/dL (11.1 mmol/L ) or higher after two hours suggests diabetes.
Screening. The American Diabetes Association recommends routine screening with diagnostic tests for type 2 diabetes in all adults age 35 or older and in the following groups:
- People younger than 35 who are overweight or obese and have one or more risk factors associated with diabetes.
- Women who have had gestational diabetes.
- People who have been diagnosed with prediabetes.
- Children who are overweight or obese and who have a family history of type 2 diabetes or other risk factors.
After a diagnosis
If you're diagnosed with diabetes, your health care provider may do other tests to distinguish between type 1 and type 2 diabetes because the two conditions often require different treatments.
Your health care provider will test A1C levels at least two times a year and when there are any changes in treatment. Target A1C goals vary depending on age and other factors. For most people, the American Diabetes Association recommends an A1C level below 7%.
You also receive tests to screen for complications of diabetes and other medical conditions.
More Information
- Glucose tolerance test
Management of type 2 diabetes includes:
- Healthy eating.
- Regular exercise.
- Weight loss.
- Possibly, diabetes medication or insulin therapy.
- Blood sugar monitoring.
These steps make it more likely that blood sugar will stay in a healthy range. And they may help to delay or prevent complications.
Healthy eating
There's no specific diabetes diet. However, it's important to center your diet around:
- A regular schedule for meals and healthy snacks.
- Smaller portion sizes.
- More high-fiber foods, such as fruits, nonstarchy vegetables and whole grains.
- Fewer refined grains, starchy vegetables and sweets.
- Modest servings of low-fat dairy, low-fat meats and fish.
- Healthy cooking oils, such as olive oil or canola oil.
- Fewer calories.
Your health care provider may recommend seeing a registered dietitian, who can help you:
- Identify healthy food choices.
- Plan well-balanced, nutritional meals.
- Develop new habits and address barriers to changing habits.
- Monitor carbohydrate intake to keep your blood sugar levels more stable.
Physical activity
Exercise is important for losing weight or maintaining a healthy weight. It also helps with managing blood sugar. Talk to your health care provider before starting or changing your exercise program to ensure that activities are safe for you.
- Aerobic exercise. Choose an aerobic exercise that you enjoy, such as walking, swimming, biking or running. Adults should aim for 30 minutes or more of moderate aerobic exercise on most days of the week, or at least 150 minutes a week.
- Resistance exercise. Resistance exercise increases your strength, balance and ability to perform activities of daily living more easily. Resistance training includes weightlifting, yoga and calisthenics. Adults living with type 2 diabetes should aim for 2 to 3 sessions of resistance exercise each week.
- Limit inactivity. Breaking up long periods of inactivity, such as sitting at the computer, can help control blood sugar levels. Take a few minutes to stand, walk around or do some light activity every 30 minutes.
Weight loss
Weight loss results in better control of blood sugar levels, cholesterol, triglycerides and blood pressure. If you're overweight, you may begin to see improvements in these factors after losing as little as 5% of your body weight. However, the more weight you lose, the greater the benefit to your health. In some cases, losing up to 15% of body weight may be recommended.
Your health care provider or dietitian can help you set appropriate weight-loss goals and encourage lifestyle changes to help you achieve them.
Monitoring your blood sugar
Your health care provider will advise you on how often to check your blood sugar level to make sure you remain within your target range. You may, for example, need to check it once a day and before or after exercise. If you take insulin, you may need to check your blood sugar multiple times a day.
Monitoring is usually done with a small, at-home device called a blood glucose meter, which measures the amount of sugar in a drop of blood. Keep a record of your measurements to share with your health care team.
Continuous glucose monitoring is an electronic system that records glucose levels every few minutes from a sensor placed under the skin. Information can be transmitted to a mobile device such as a phone, and the system can send alerts when levels are too high or too low.
Diabetes medications
If you can't maintain your target blood sugar level with diet and exercise, your health care provider may prescribe diabetes medications that help lower glucose levels, or your provider may suggest insulin therapy. Medicines for type 2 diabetes include the following.
Metformin (Fortamet, Glumetza, others) is generally the first medicine prescribed for type 2 diabetes. It works mainly by lowering glucose production in the liver and improving the body's sensitivity to insulin so it uses insulin more effectively.
Some people experience B-12 deficiency and may need to take supplements. Other possible side effects, which may improve over time, include:
- Abdominal pain.
Sulfonylureas help the body secrete more insulin. Examples include glyburide (DiaBeta, Glynase), glipizide (Glucotrol XL) and glimepiride (Amaryl). Possible side effects include:
- Low blood sugar.
- Weight gain.
Glinides stimulate the pancreas to secrete more insulin. They're faster acting than sulfonylureas. But their effect in the body is shorter. Examples include repaglinide and nateglinide. Possible side effects include:
Thiazolidinediones make the body's tissues more sensitive to insulin. An example of this medicine is pioglitazone (Actos). Possible side effects include:
- Risk of congestive heart failure.
- Risk of bladder cancer (pioglitazone).
- Risk of bone fractures.
DPP-4 inhibitors help reduce blood sugar levels but tend to have a very modest effect. Examples include sitagliptin (Januvia), saxagliptin (Onglyza) and linagliptin (Tradjenta). Possible side effects include:
- Risk of pancreatitis.
- Joint pain.
GLP-1 receptor agonists are injectable medications that slow digestion and help lower blood sugar levels. Their use is often associated with weight loss, and some may reduce the risk of heart attack and stroke. Examples include exenatide (Byetta, Bydureon Bcise), liraglutide (Saxenda, Victoza) and semaglutide (Rybelsus, Ozempic, Wegovy). Possible side effects include:
SGLT2 inhibitors affect the blood-filtering functions in the kidneys by blocking the return of glucose to the bloodstream. As a result, glucose is removed in the urine. These medicines may reduce the risk of heart attack and stroke in people with a high risk of those conditions. Examples include canagliflozin (Invokana), dapagliflozin (Farxiga) and empagliflozin (Jardiance). Possible side effects include:
- Vaginal yeast infections.
- Urinary tract infections.
- Low blood pressure.
- High cholesterol.
- Risk of gangrene.
- Risk of bone fractures (canagliflozin).
- Risk of amputation (canagliflozin).
Other medicines your health care provider might prescribe in addition to diabetes medications include blood pressure and cholesterol-lowering medicines, as well as low-dose aspirin, to help prevent heart and blood vessel disease.
Insulin therapy
Some people who have type 2 diabetes need insulin therapy. In the past, insulin therapy was used as a last resort, but today it may be prescribed sooner if blood sugar targets aren't met with lifestyle changes and other medicines.
Different types of insulin vary on how quickly they begin to work and how long they have an effect. Long-acting insulin, for example, is designed to work overnight or throughout the day to keep blood sugar levels stable. Short-acting insulin generally is used at mealtime.
Your health care provider will determine what type of insulin is right for you and when you should take it. Your insulin type, dosage and schedule may change depending on how stable your blood sugar levels are. Most types of insulin are taken by injection.
Side effects of insulin include the risk of low blood sugar — a condition called hypoglycemia — diabetic ketoacidosis and high triglycerides.
Weight-loss surgery
Weight-loss surgery changes the shape and function of the digestive system. This surgery may help you lose weight and manage type 2 diabetes and other conditions related to obesity. There are several surgical procedures. All of them help people lose weight by limiting how much food they can eat. Some procedures also limit the amount of nutrients the body can absorb.
Weight-loss surgery is only one part of an overall treatment plan. Treatment also includes diet and nutritional supplement guidelines, exercise and mental health care.
Generally, weight-loss surgery may be an option for adults living with type 2 diabetes who have a body mass index (BMI) of 35 or higher. BMI is a formula that uses weight and height to estimate body fat. Depending on the severity of diabetes or the presence of other medical conditions, surgery may be an option for someone with a BMI lower than 35.
Weight-loss surgery requires a lifelong commitment to lifestyle changes. Long-term side effects may include nutritional deficiencies and osteoporosis.
People living with type 2 diabetes often need to change their treatment plan during pregnancy and follow a diet that controls carbohydrates. Many people need insulin therapy during pregnancy. They also may need to stop other treatments, such as blood pressure medicines.
There is an increased risk during pregnancy of developing a condition that affects the eyes called diabetic retinopathy. In some cases, this condition may get worse during pregnancy. If you are pregnant, visit an ophthalmologist during each trimester of your pregnancy and one year after you give birth. Or as often as your health care provider suggests.
Signs of trouble
Regularly monitoring your blood sugar levels is important to avoid severe complications. Also, be aware of symptoms that may suggest irregular blood sugar levels and the need for immediate care:
High blood sugar. This condition also is called hyperglycemia. Eating certain foods or too much food, being sick, or not taking medications at the right time can cause high blood sugar. Symptoms include:
- Frequent urination.
- Increased thirst.
- Blurred vision.
Hyperglycemic hyperosmolar nonketotic syndrome (HHNS). This life-threatening condition includes a blood sugar reading higher than 600 mg/dL (33.3 mmol/L ). HHNS may be more likely if you have an infection, are not taking medicines as prescribed, or take certain steroids or drugs that cause frequent urination. Symptoms include:
- Extreme thirst.
- Drowsiness.
- Dark urine.
Diabetic ketoacidosis. Diabetic ketoacidosis occurs when a lack of insulin results in the body breaking down fat for fuel rather than sugar. This results in a buildup of acids called ketones in the bloodstream. Triggers of diabetic ketoacidosis include certain illnesses, pregnancy, trauma and medicines — including the diabetes medicines called SGLT2 inhibitors.
The toxicity of the acids made by diabetic ketoacidosis can be life-threatening. In addition to the symptoms of hyperglycemia, such as frequent urination and increased thirst, ketoacidosis may cause:
- Shortness of breath.
- Fruity-smelling breath.
Low blood sugar. If your blood sugar level drops below your target range, it's known as low blood sugar. This condition also is called hypoglycemia. Your blood sugar level can drop for many reasons, including skipping a meal, unintentionally taking more medication than usual or being more physically active than usual. Symptoms include:
- Irritability.
- Heart palpitations.
- Slurred speech.
If you have symptoms of low blood sugar, drink or eat something that will quickly raise your blood sugar level. Examples include fruit juice, glucose tablets, hard candy or another source of sugar. Retest your blood in 15 minutes. If levels are not at your target, eat or drink another source of sugar. Eat a meal after your blood sugar level returns to normal.
If you lose consciousness, you need to be given an emergency injection of glucagon, a hormone that stimulates the release of sugar into the blood.
- Medications for type 2 diabetes
- GLP-1 agonists: Diabetes drugs and weight loss
- Bariatric surgery
- Endoscopic sleeve gastroplasty
- Gastric bypass (Roux-en-Y)
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Lifestyle and home remedies
Careful management of type 2 diabetes can reduce the risk of serious — even life-threatening — complications. Consider these tips:
- Commit to managing your diabetes. Learn all you can about type 2 diabetes. Make healthy eating and physical activity part of your daily routine.
- Work with your team. Establish a relationship with a certified diabetes education specialist, and ask your diabetes treatment team for help when you need it.
- Identify yourself. Wear a necklace or bracelet that says you are living with diabetes, especially if you take insulin or other blood sugar-lowering medicine.
- Schedule a yearly physical exam and regular eye exams. Your diabetes checkups aren't meant to replace regular physicals or routine eye exams.
- Keep your vaccinations up to date. High blood sugar can weaken your immune system. Get a flu shot every year. Your health care provider also may recommend the pneumonia vaccine. The Centers for Disease Control and Prevention (CDC) also recommends the hepatitis B vaccination if you haven't previously received this vaccine and you're 19 to 59 years old. Talk to your health care provider about other vaccinations you may need.
- Take care of your teeth. Diabetes may leave you prone to more-serious gum infections. Brush and floss your teeth regularly and schedule recommended dental exams. Contact your dentist right away if your gums bleed or look red or swollen.
- Pay attention to your feet. Wash your feet daily in lukewarm water, dry them gently, especially between the toes, and moisturize them with lotion. Check your feet every day for blisters, cuts, sores, redness and swelling. Contact your health care provider if you have a sore or other foot problem that isn't healing.
- Keep your blood pressure and cholesterol under control. Eating healthy foods and exercising regularly can go a long way toward controlling high blood pressure and cholesterol. Take medication as prescribed.
- If you smoke or use other types of tobacco, ask your health care provider to help you quit. Smoking increases your risk of diabetes complications. Talk to your health care provider about ways to stop using tobacco.
- Use alcohol sparingly. Depending on the type of drink, alcohol may lower or raise blood sugar levels. If you choose to drink alcohol, only do so with a meal. The recommendation is no more than one drink daily for women and no more than two drinks daily for men. Check your blood sugar frequently after drinking alcohol.
- Make healthy sleep a priority. Many people with type 2 diabetes have sleep problems. And not getting enough sleep may make it harder to keep blood sugar levels in a healthy range. If you have trouble sleeping, talk to your health care provider about treatment options.
- Caffeine: Does it affect blood sugar?
Alternative medicine
Many alternative medicine treatments claim to help people living with diabetes. According to the National Center for Complementary and Integrative Health, studies haven't provided enough evidence to recommend any alternative therapies for blood sugar management. Research has shown the following results about popular supplements for type 2 diabetes:
- Chromium supplements have been shown to have few or no benefits. Large doses can result in kidney damage, muscle problems and skin reactions.
- Magnesium supplements have shown benefits for blood sugar control in some but not all studies. Side effects include diarrhea and cramping. Very large doses — more than 5,000 mg a day — can be fatal.
- Cinnamon, in some studies, has lowered fasting glucose levels but not A1C levels. Therefore, there's no evidence of overall improved glucose management.
Talk to your health care provider before starting a dietary supplement or natural remedy. Do not replace your prescribed diabetes medicines with alternative medicines.
Coping and support
Type 2 diabetes is a serious disease, and following your diabetes treatment plan takes commitment. To effectively manage diabetes, you may need a good support network.
Anxiety and depression are common in people living with diabetes. Talking to a counselor or therapist may help you cope with the lifestyle changes and stress that come with a type 2 diabetes diagnosis.
Support groups can be good sources of diabetes education, emotional support and helpful information, such as how to find local resources or where to find carbohydrate counts for a favorite restaurant. If you're interested, your health care provider may be able to recommend a group in your area.
You can visit the American Diabetes Association website to check out local activities and support groups for people living with type 2 diabetes. The American Diabetes Association also offers online information and online forums where you can chat with others who are living with diabetes. You also can call the organization at 800-DIABETES ( 800-342-2383 ).
Preparing for your appointment
At your annual wellness visit, your health care provider can screen for diabetes and monitor and treat conditions that increase your risk of diabetes, such as high blood pressure, high cholesterol or a high BMI .
If you are seeing your health care provider because of symptoms that may be related to diabetes, you can prepare for your appointment by being ready to answer the following questions:
- When did your symptoms begin?
- Does anything improve the symptoms or worsen the symptoms?
- What medicines do you take regularly, including dietary supplements and herbal remedies?
- What are your typical daily meals? Do you eat between meals or before bedtime?
- How much alcohol do you drink?
- How much daily exercise do you get?
- Is there a history of diabetes in your family?
If you are diagnosed with diabetes, your health care provider may begin a treatment plan. Or you may be referred to a doctor who specializes in hormonal disorders, called an endocrinologist. Your care team also may include the following specialists:
- Certified diabetes education specialist.
- Foot doctor, also called a podiatrist.
- Doctor who specializes in eye care, called an ophthalmologist.
Talk to your health care provider about referrals to other specialists who may be providing care.
Questions for ongoing appointments
Before any appointment with a member of your treatment team, make sure you know whether there are any restrictions, such as not eating or drinking before taking a test. Questions that you should regularly talk about with your health care provider or other members of the team include:
- How often do I need to monitor my blood sugar, and what is my target range?
- What changes in my diet would help me better manage my blood sugar?
- What is the right dosage for prescribed medications?
- When do I take the medications? Do I take them with food?
- How does management of diabetes affect treatment for other conditions? How can I better coordinate treatments or care?
- When do I need to make a follow-up appointment?
- Under what conditions should I call you or seek emergency care?
- Are there brochures or online sources you recommend?
- Are there resources available if I'm having trouble paying for diabetes supplies?
What to expect from your doctor
Your health care provider is likely to ask you questions at your appointments. Those questions may include:
- Do you understand your treatment plan and feel confident you can follow it?
- How are you coping with diabetes?
- Have you had any low blood sugar?
- Do you know what to do if your blood sugar is too low or too high?
- What's a typical day's diet like?
- Are you exercising? If so, what type of exercise? How often?
- Do you sit for long periods of time?
- What challenges are you experiencing in managing your diabetes?
- Professional Practice Committee: Standards of Medical Care in Diabetes — 2020. Diabetes Care. 2020; doi:10.2337/dc20-Sppc.
- Diabetes mellitus. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetes-mellitus-dm. Accessed Dec. 7, 2020.
- Melmed S, et al. Williams Textbook of Endocrinology. 14th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Dec. 3, 2020.
- Diabetes overview. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/all-content. Accessed Dec. 4, 2020.
- AskMayoExpert. Type 2 diabetes. Mayo Clinic; 2018.
- Feldman M, et al., eds. Surgical and endoscopic treatment of obesity. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 11th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed Oct. 20, 2020.
- Hypersmolar hyperglycemic state (HHS). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/hyperosmolar-hyperglycemic-state-hhs. Accessed Dec. 11, 2020.
- Diabetic ketoacidosis (DKA). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetic-ketoacidosis-dka. Accessed Dec. 11, 2020.
- Hypoglycemia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/hypoglycemia. Accessed Dec. 11, 2020.
- 6 things to know about diabetes and dietary supplements. National Center for Complementary and Integrative Health. https://www.nccih.nih.gov/health/tips/things-to-know-about-type-diabetes-and-dietary-supplements. Accessed Dec. 11, 2020.
- Type 2 diabetes and dietary supplements: What the science says. National Center for Complementary and Integrative Health. https://www.nccih.nih.gov/health/providers/digest/type-2-diabetes-and-dietary-supplements-science. Accessed Dec. 11, 2020.
- Preventing diabetes problems. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/all-content. Accessed Dec. 3, 2020.
- Schillie S, et al. Prevention of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recommendations and Reports. 2018; doi:10.15585/mmwr.rr6701a1.
- Diabetes prevention: 5 tips for taking control
- Hyperinsulinemia: Is it diabetes?
Associated Procedures
News from mayo clinic.
- Mayo study uses electronic health record data to assess metformin failure risk, optimize care Feb. 10, 2023, 02:30 p.m. CDT
- Mayo Clinic Minute: Strategies to break the heart disease and diabetes link Nov. 28, 2022, 05:15 p.m. CDT
- Mayo Clinic Q and A: Diabetes risk in Hispanic people Oct. 20, 2022, 12:15 p.m. CDT
- The importance of diagnosing, treating diabetes in the Hispanic population in the US Sept. 28, 2022, 04:00 p.m. CDT
- Mayo Clinic Minute: Managing Type 2 diabetes Sept. 28, 2022, 02:30 p.m. CDT
Products & Services
- A Book: The Essential Diabetes Book
- A Book: The Mayo Clinic Diabetes Diet
- Assortment of Health Products from Mayo Clinic Store
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Type 2 diabetes articles within Scientific Reports
Article 13 March 2024 | Open Access
KSRP improves pancreatic beta cell function and survival
- Leticia Barssotti
- , Gabriela Moreira Soares
- & Helena Cristina Lima Barbosa
Article 08 March 2024 | Open Access
The clinical relevance of a polygenic risk score for type 2 diabetes mellitus in the Korean population
- Na Yeon Kim
- , Haekyung Lee
- & Seunggeun Lee
Article 06 March 2024 | Open Access
Impact of diabetes distress on glycemic control and diabetic complications in type 2 diabetes mellitus
- Hye-Sun Park
- , Yongin Cho
- & So Hun Kim
Article 26 February 2024 | Open Access
Hepatitis C virus antibody seropositivity is associated with albuminuria but not peripheral artery disease in patients with type 2 diabetes
- Yu-Cheng Cheng
- , Teng-Yu Lee
- & I-Te Lee
Article 18 February 2024 | Open Access
Inverse association of serum albumin levels with diabetic retinopathy in type 2 diabetic patients: a cross-sectional study
- , Wenqing Hao
- & Nailong Yang
Article 15 February 2024 | Open Access
Diabetic peripheral neuropathy among adult type 2 diabetes patients in Adama, Ethiopia: health facility-based study
- Yohannes Mekuria Negussie
- & Nardos Tilahun Bekele
Article 01 February 2024 | Open Access
Amyloid deposition and small vessel disease are associated with cognitive function in older adults with type 2 diabetes
- Orit H. Lesman-Segev
- , Sapir Golan Shekhtman
- & Michal Schnaider Beeri
Article 30 January 2024 | Open Access

Environmental exposure to lead and cadmium are associated with triglyceride glucose index
- , Eun Young Park
- & Jin-Kyoung Oh
Article 24 January 2024 | Open Access
Identifying top ten predictors of type 2 diabetes through machine learning analysis of UK Biobank data
- , Araz Rawshani
- & Björn Eliasson
Article 05 January 2024 | Open Access
Dapagliflozin ameliorates diabetes-induced spermatogenic dysfunction by modulating the adenosine metabolism along the gut microbiota-testis axis
- , Yalei Cao
- & Jing Hang
Article 02 January 2024 | Open Access
White matter microstructure alterations in type 2 diabetes mellitus and its correlation with cerebral small vessel disease and cognitive performance
- Yangyingqiu Liu
- , Yuhan Jiang
- & Yanwei Miao
Article 03 November 2023 | Open Access
Efficacy and safety of polyethylene glycol loxenatide in type 2 diabetic patients: a systematic review and meta-analysis of randomized controlled trials
- Hazem Mohamed Salamah
- , Ahmed Marey
- & Mohamed Abd-Elgawad
Article 25 October 2023 | Open Access
Diabetes knowledge predicts HbA1c levels of people with type 2 diabetes mellitus in rural China: a ten-month follow-up study
- Xiaoying Wang
- & Weijun Zhang
Article 16 October 2023 | Open Access
The role of high fat diet on serum uric acid level among healthy male first degree relatives of type 2 diabetes mellitus
- Dyah Purnamasari
- , Asri R. M. Umpuan
- & Muhadi Muhadi
Article 27 September 2023 | Open Access
MRPS6 modulates glucose-stimulated insulin secretion in mouse islet cells through mitochondrial unfolded protein response
- Danhong Lin
- , Jingwen Yu
- & Huibiao Quan
Article 20 September 2023 | Open Access
Leptin receptor gene deficiency minimally affects osseointegration in rats
- Martina Jolic
- , Krisztina Ruscsák
- & Anders Palmquist
Article 02 September 2023 | Open Access
Clustering and trajectories of key noncommunicable disease risk factors in Norway: the NCDNOR project
- Knut Eirik Dalene
- , Simon Lergenmuller
- & Inger Ariansen
Article 27 July 2023 | Open Access
A fuzzy based dietary clinical decision support system for patients with multiple chronic conditions (MCCs)
- Leila Marashi-Hosseini
- , Sima Jafarirad
- & Ali Mohammad Hadianfard
Article 07 July 2023 | Open Access
Hyperglycemia is associated with duodenal dysbiosis and altered duodenal microenvironment
- Aarti Darra
- , Vandana Singh
- & Usha Dutta
Article 04 July 2023 | Open Access
Subgroups of adult-onset diabetes: a data-driven cluster analysis in a Ghanaian population
- Ina Danquah
- , Isabel Mank
- & Olov Rolandsson
Article 29 June 2023 | Open Access
Mechanism of TNFα-induced downregulation of salt-inducible kinase 2 in adipocytes
- Magdaléna Vaváková
- , Kaisa Hofwimmer
- & Olga Göransson
Article 19 June 2023 | Open Access
A closer association between blood urea nitrogen and the probability of diabetic retinopathy in patients with shorter type 2 diabetes duration
- Jian-Bo Zhong
- , Yu-Feng Yao
- & Li Cai
Article 11 April 2023 | Open Access
Partial substitution of red or processed meat with plant-based foods and the risk of type 2 diabetes
- Mirkka Maukonen
- , Kennet Harald
- & Satu Männistö
Article 20 February 2023 | Open Access
Socioeconomic inequality in awareness, treatment and control of diabetes among adults in India: Evidence from National Family Health Survey of India (NFHS), 2019–2021
- Suraj Maiti
- , Shamrin Akhtar
- & Sanjay K. Mohanty
Article 02 February 2023 | Open Access
Low thigh muscle strength in relation to myosteatosis in patients with type 2 diabetes mellitus
- Yilong Huang
- & Bo He
Article 28 January 2023 | Open Access
Pre-diabetes is associated with attenuation rather than volume of epicardial adipose tissue on computed tomography
- David Molnar
- , Elias Björnson
- & Göran Bergström
Article 19 January 2023 | Open Access
Fasting plasma glucose level-based formula for estimating starting daily dose in basal-bolus insulin therapy
- Mototsugu Nagao
- , Taro Harada
- & Shinichi Oikawa
Article 12 January 2023 | Open Access
Linagliptin treatment is associated with altered cobalamin (VitB12) homeostasis in mice and humans
- Harald Tammen
- , Martin Kömhoff
- & Thomas Klein
Article 10 January 2023 | Open Access
Identification of stable reference genes in peripheral blood mononuclear cells from type 2 diabetes mellitus patients
- Ankita Hazarika
- , Bajanai Nongkhlaw
- & Arpita Mukhopadhyay
Article 04 January 2023 | Open Access
Efficacy and safety of sitagliptin treatment in older adults with moderately controlled type 2 diabetes: the STREAM study
- , Jun Sasaki
- & M. Yamada
Article 29 December 2022 | Open Access
Genome-wide association study of the response of patients with diabetic macular edema to intravitreal Anti-VEGF injection
- Eun Hee Hong
- , Hoseok Yeom
- & Heeyoon Cho
Article 26 December 2022 | Open Access
GTS-21, a selective alpha7 nicotinic acetylcholine receptor agonist, ameliorates diabetic nephropathy in Lepr db/db mice
- Qinghe Meng
- , Xinghan Tian
- & Robert N. Cooney
Article 10 December 2022 | Open Access
Identifying myoglobin as a mediator of diabetic kidney disease: a machine learning-based cross-sectional study
- , Zhihao Shu
- & Hongwei Lu
Article 03 December 2022 | Open Access
Diabetes medication recommendation system using patient similarity analytics
- Wei Ying Tan
- & Ngiap Chuan Tan
Article 21 November 2022 | Open Access
Machine learning models for prediction of HF and CKD development in early-stage type 2 diabetes patients
- Eiichiro Kanda
- , Atsushi Suzuki
- & Toshitaka Yajima
Article 01 November 2022 | Open Access
BMI variability and incident diabetes mellitus, Tehran Lipid and Glucose Study (TLGS)
- Ladan Mehran
- , Pouria Mousapour
- & Fereidoun Azizi
Article 12 October 2022 | Open Access
Effect of empagliflozin versus linagliptin on body composition in Asian patients with type 2 diabetes treated with premixed insulin
- Yi-Hong Zeng
- , Sung-Chen Liu
- & Jason J. Liu
Article 27 September 2022 | Open Access
Sex differences in frailty of geriatric outpatients with type 2 diabetes mellitus: a multicentre cross-sectional study
- Huan Thanh Nguyen
- , An Huu Nguyen
- & Phuong Thi My Le
Article 13 July 2022 | Open Access
Transgenic overexpression of microRNA-30d in pancreatic beta-cells progressively regulates beta-cell function and identity
- , Jacob Schoenborn
- & Xiaoqing Tang
Article 07 July 2022 | Open Access
Effects of immune-mediated inflammatory diseases on cardiovascular diseases in patients with type 2 diabetes: a nationwide population-based study
- Oh Chan Kwon
- , Kyungdo Han
- & Min-Chan Park
Article 20 June 2022 | Open Access
Development and internal validation of a model to predict type 2 diabetic complications after gestational diabetes
- Ugochinyere Vivian Ukah
- , Robert W. Platt
- & Natalie Dayan
Article 14 June 2022 | Open Access
Glycaemic variability is associated with all-cause mortality in COVID-19 patients with ARDS, a retrospective subcohort study
- Bojan Hartmann
- , Marlo Verket
- & Dirk Müller-Wieland
Article 25 May 2022 | Open Access
Associations of body shape index (ABSI) and hip index with liver, metabolic, and inflammatory biomarkers in the UK Biobank cohort
- Sofia Christakoudi
- , Elio Riboli
- & Konstantinos K. Tsilidis
Article 11 May 2022 | Open Access
High-throughput analysis of ANRIL circRNA isoforms in human pancreatic islets
- Hannah J. MacMillan
- , Yahui Kong
- & Athma A. Pai
Performance assessment across different care settings of a heart failure hospitalisation risk-score for type 2 diabetes using administrative claims
- Alessandro Guazzo
- , Enrico Longato
- & Barbara Di Camillo
Article 04 May 2022 | Open Access
Cordyceps inhibits ceramide biosynthesis and improves insulin resistance and hepatic steatosis
- , Chad Lamar Talbot
- & Bhagirath Chaurasia
Article 28 April 2022 | Open Access
Strength training alters the tissue fatty acids profile and slightly improves the thermogenic pathway in the adipose tissue of obese mice
- Diego Gomes de Melo
- , Chadi Pellegrini Anaruma
- & Leandro Pereira de Moura
Article 27 April 2022 | Open Access
Network analysis reveals dysregulated functional patterns in type II diabetic skin
- , Sudha Ram
- & Bonnie L. Hurwitz
Article 12 April 2022 | Open Access
Laminin matrix regulates beta-cell FGFR5 expression to enhance glucose-stimulated metabolism
- Vidhant Pal
- , Yufeng Wang
- & Jonathan V. Rocheleau
Article 01 April 2022 | Open Access
Metformin is associated with favorable outcomes in patients with COVID-19 and type 2 diabetes mellitus
- , Nishit Patel
- & Mahesh Krishnamurthy
Browse broader subjects
Quick links.
- Explore articles by subject
- Guide to authors
- Editorial policies
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih research matters.
March 26, 2024
Weight-loss surgery yields long-term benefits for type 2 diabetes
At a glance.
- Bariatric surgery helped people with type 2 diabetes better control their blood glucose years later compared to medical and lifestyle interventions.
- The findings support the use of weight-reduction surgery for treating type 2 diabetes in people with obesity.

Diabetes affects more than 38 million people nationwide. It occurs when levels of blood sugar, or glucose, are too high. Over time, excess blood glucose can lead to serious health problems, such as heart disease, stroke, nerve damage, and eye disease.
Some people with type 2 diabetes—the most common type—keep blood glucose in check by making lifestyle changes, including diet and exercise. Medications can also help to control blood glucose. Clinical trials over the past few decades have found that bariatric surgery, or weight-control surgery, can also help control type 2 diabetes. But it had been unclear which of these interventions might have better long-term outcomes.
To learn more, NIH-supported researchers at four institutions drew on data collected from four previous clinical trials conducted between May 2007 and August 2013. These trials were single-center studies comparing the effectiveness of bariatric surgeries to medical and lifestyle interventions. The surgeries included sleeve gastrectomy, Roux-en-Y gastric bypass, and adjustable gastric banding. The medical and lifestyle interventions included nutrition counseling, self-monitoring of glucose, and medication to treat diabetes. By pooling data from the four clinical trials, the researchers had a larger, more diverse data set to analyze. Follow-up data was collected 7 to 12 years after the start of the original trials, through July 2022.
In total, 262 study participants agreed to long-term follow-up. All were between ages 18 and 65. Each had overweight or obesity, as measured by body mass index (BMI). Nearly 70% of participants were women, 31% were Black, and 67% were white. More than half (166) were randomized to receive bariatric surgery. The remaining 96 received diabetes medications plus lifestyle interventions known to be effective for weight loss. Results appeared in the Journal of the American Medical Association on February 27, 2024.
The researchers found that, seven years after the original intervention, 54% of those in the surgery group had an A1c measurement less than 7%. A1c is a blood test that measures a person’s average blood sugar levels over the previous two or three months. In contrast, only 27% of those in the medical/lifestyle group had similar A1c values.
In addition, 18% of those in the surgery group no longer had signs or symptoms of diabetes by year seven, compared to 6% in the medical/lifestyle group. The surgery group also had an average weight loss of 20%, compared to 8% in the other group. The differences between groups remained significant at 12 years.
No differences in major side effects were detected. The surgery group did have a higher number of fractures, anemia, low iron, and gastrointestinal events. These might have been due to greater weight loss and associated nutritional deficiencies. Sleeve gastrectomy and Roux-en-Y gastric bypass were both better than adjustable gastric banding at reducing A1c levels.
The surgeries appeared to be beneficial even among those with lower BMI scores, between 27 and 34 at study enrollment. That BMI range includes overweight and low-range obesity. Such people had typically been excluded from receiving bariatric surgery for diabetes. But this finding aligns with other recent data that support the use of surgery for some people with a BMI less than 35.
“These results show that people with overweight or obesity and type 2 diabetes can make long-term improvements in their health and change the trajectory of their diabetes through surgery,” says Dr. Jean Lawrence of NIH’s National Institute of Diabetes and Digestive and Kidney Diseases.
Related Links
- Intermittent Fasting for Weight Loss in People With Type 2 Diabetes
- Popular Diabetes Drugs Compared in Large Trial
- Diabetes Control Worsened Over the Past Decade
- Weight-loss (Metabolic & Bariatric) Surgery
- Type 2 Diabetes
- Calculate Your Body Mass Index
References: Long-Term Outcomes of Medical Management vs Bariatric Surgery in Type 2 Diabetes. Courcoulas AP, Patti ME, Hu B, Arterburn DE, Simonson DC, Gourash WF, Jakicic JM, Vernon AH, Beck GJ, Schauer PR, Kashyap SR, Aminian A, Cummings DE, Kirwan JP. JAMA . 2024 Feb 27;331(8):654-664. doi: 10.1001/jama.2024.0318. PMID: 38411644.
Funding: NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Connect with Us
- More Social Media from NIH

Mediterranean diet may protect against diabetes more than realized, research shows
T he Mediterranean diet has already been shown to help protect the aging brain and may significantly lower risk of heart disease . A new study has now found a much stronger link than previously realized between the Mediterranean diet — which is filled with whole grains, fish, fruits and olive oil — and a reduced risk of Type 2 diabetes.
Previous research looking at the impact of a Mediterranean diet on diabetes have shown mixed results, possibly because the studies were based on participants remembering and self-reporting the type of food they ate.
For the new research, published Thursday in PLOS Medicine, British scientists used blood samples to develop a biomarker scoring system. They first ran a small trial, called the Medley trial, with 128 adults ages 65 and older who were randomized to consume either the Mediterranean diet or continue eating as they usually did for a period of six months.
A comparison of blood samples from the two groups turned up a host of biomarkers of fatty acids and car o tenoids , the substances that give color to vegetables such as pumpkins, carrots and tomatoes.
For the second part of the research, scientists from the University of Cambridge, in the U.K., analyzed data — including blood samples and self-reported diet information — from more than 340,000 middle-aged participants in a long-running European study . Over about 10 years, 9,453 had developed Type 2 diabetes.
Then the researchers compared biomarker scores from the 9,453 to 12,749 randomly selected participants without diabetes in the large study.
They found a nearly 30% reduction in diabetes risk using the biomarker data, compared to 10% reduction from the self-reported data. That means previous studies probably underestimated the impact of the Mediterranean diet.
The findings strengthen the case for recommending the Mediterranean diet for the prevention of Type 2 diabetes, said senior author Dr. Nita Forouhi, a professor of population health and nutrition and program leader in nutritional epidemiology at the University of Cambridge.
“The 20% of participants with the highest values of the biomarker score had a 62% lower risk of new-onset type 2 diabetes relative to the 20% with the lowest biomarker score values,” Forouhi said in an email.
What is a Mediterranean diet?
For someone consuming an average of 2,000 calories per day, researchers defined it as, at least:
- 2 to 3 tablespoons of olive oil.
- Five servings of vegetables (75 g each).
- Two to three servings of fruits (150 g each).
- Five servings of cereal products (between 30-120 g each depending on the specific product).
And a daily maximum of:
- One medium potato.
- One serving of milk (250 mL).
- Two glasses of red wine (150 mL each).
Weekly recommendations included:
- Six servings of Greek low-fat yogurt (170 g each).
- One to three servings of poultry (100 g each).
- At least five servings of nuts (35 g each).
- At least three servings of legumes (75 g each).
- At least three servings of fish (150 g each).
And a maximum of:
- Four servings of cheese (40 g each).
- One serving of red meat (100 g).
Dr. Peter Goulden, chief of the division of endocrinology, diabetes and bone diseases at Mount Sinai Morningside and Mount Sinai West, called the new biomarker scoring an exciting development.
“If you compare the biomarker score with self-reports, the effect is three times larger,” said Goulden, who is also an associate professor at the Icahn School of Medicine at Mount Sinai. “So that’s very powerful.”
Further studies will be needed to corroborate the findings and determine if they would apply to a wider population, he said.
A downside is the biomarkers don't determine whether the benefits are from fruit and vegetable consumption or some other aspect of the diet, said Linda Van Horn, a clinical nutrition epidemiologist at the Feinberg School of Medicine, Northwestern University in Chicago.
Still, this approach “is more objective, so I would say it’s a step in the right direction,” she said.
The importance of the findings is underscored by the rising rates of diabetes around the world, said Dr. Anna Beth Bradley, an assistant professor of medicine, diabetes, endocrinology and metabolism at the Vanderbilt University School of Medicine.
“If you look at the prevalence of diabetes globally, you see that in Europe, the incidence is around 6% to 7%, while in the U.S., it’s around 11%, which suggests that the standard American diet probably has contributed to the rise in diabetes,” Bradley said. “That terrible American diet is leaking over to other countries with diabetes rising in pretty much every country.”
This article was originally published on NBCNews.com


- April 2, 2024 | New Insights Into Type 3 Diabetes – Groundbreaking Study Reveals How Diet Influences Alzheimer’s Risk
- April 2, 2024 | Greenland’s Largest Floating Ice Tongue Is Melting
- April 2, 2024 | Don’t Miss: Total Solar Eclipse, Mars and Saturn Rising, Slim Lunar Crescent, Comet 12P
- April 2, 2024 | Rethinking Aging: Could Long Genes Be the Culprits Behind Growing Older?
- April 2, 2024 | Scientists Have Uncovered a Safe Path to Overcoming Food Allergies
New Insights Into Type 3 Diabetes – Groundbreaking Study Reveals How Diet Influences Alzheimer’s Risk
By American Society for Biochemistry and Molecular Biology April 2, 2024

New research conducted on mice has unveiled the molecular link between diabetes and Alzheimer’s disease, suggesting that these conditions, particularly Type 2 diabetes and Alzheimer’s, are closely connected. This connection, often referred to as “Type 3 diabetes” by some researchers, indicates that effective diabetes management or prevention could significantly reduce the risk of Alzheimer’s.
Scientists uncover a connection between the gut and the brain, suggesting that controlling diabetes could help prevent dementia.
New research conducted in mice sheds light on what’s going on at the molecular level that could cause people with diabetes to develop Alzheimer’s disease.
The study adds to a growing body of research on the links between Type 2 diabetes and Alzheimer’s disease, which some scientists have called “Type 3 diabetes.” The findings suggest that it should be possible to reduce the risk of Alzheimer’s by keeping diabetes well controlled or avoiding it in the first place, according to researchers.
Narendra Kumar, an associate professor at Texas A&M University in College Station, led the study.
“We think that diabetes and Alzheimer’s disease are strongly linked,” Kumar said, “and by taking preventative or amelioration measures for diabetes, we can prevent or at least significantly slow down the progression of the symptoms of dementia in Alzheimer’s disease.”
Kumar presented the new research at Discover BMB, the annual meeting of the American Society for Biochemistry and Molecular Biology, which was held March 23–26 in San Antonio.
Growing Concerns and Dietary Influences
Diabetes and Alzheimer’s are two of the fastest-growing health concerns worldwide. Diabetes alters the body’s ability to turn food into energy and affects an estimated 1 in 10 U.S. adults. Alzheimer’s, a form of dementia that causes progressive decline in memory and thinking skills, is among the top 10 leading causes of death in the United States.
Diet is known to influence the development of diabetes as well as the severity of its health impacts. To find out how diet could influence the development of Alzheimer’s in people with diabetes, the researchers traced how a particular protein in the gut influences the brain.
They found that a high-fat diet suppresses the expression of the protein, called Jak3, and that mice without this protein experienced a cascade of inflammation starting with the intestine, moving through the liver and onto the brain. Ultimately, the mice showed signs of Alzheimer’s-like symptoms in the brain, including an overexpressed mouse beta-amyloid and hyperphosphorylated tau, as well as evidence of cognitive impairment.
“Liver being the metabolizer for everything we eat, we think that the path from gut to the brain goes through the liver,” Kumar said.
His lab has been studying functions of Jak3 for a long time, he added, and they now know that the impact of food on the changes in the expression of Jak3 leads to leaky gut. This in turn results in low-grade chronic inflammation, diabetes, decreased ability of the brain to clear its toxic substances, and dementia-like symptoms seen in Alzheimer’s disease.
The good news, according to Kumar, is that it may be possible to stop this inflammatory pathway by eating a healthy diet and getting blood sugar under control as early as possible. In particular, people with prediabetes — which includes an estimated 98 million U.S. adults — could benefit from adopting lifestyle changes to reverse prediabetes, prevent the progression to Type 2 diabetes, and potentially reduce the risk of Alzheimer’s.
Meeting: Discover BMB
Narendra Kumar presented research at the interest group session on inter-organ communication in cellular and immune homeostasis from 12:30 to 2:30 p.m. on Saturday, March 23, in Room 214BC and from 4:30–6:30 p.m. on Tuesday, March 26, in the exhibit hall of the Henry B. González Convention Center (Poster Board No. 315) ( abstract ).
More on SciTechDaily
Superconductor breakthrough: scientists discover an invisible phenomenon.
Quantum Computing Breakthrough: Silicon Encoded Spin Qubits Achieve Universality

Solar Dynamics Observatory Captures Image of Mid-level Flare

Substance Derived From Licorice May Have Anti-Inflammatory and Anti-Cancer Effects
Unlocking the Secret: How One Protein Can Halt Lung Cancer Spread

Rewiring Children’s Anxious Minds With Cognitive Behavioral Therapy

Meet Selam: The Stellar New Discovery From NASA’s Lucy Mission

NASA, SpaceX Dragon To Deliver 6,200 Pounds of Science Experiments & Crew Supplies to Space Station
1 comment on "new insights into type 3 diabetes – groundbreaking study reveals how diet influences alzheimer’s risk".
In the Philippine islands, the dinosaur’s body is known to be underwater.In the Papua island, by mapping the outer and inner parts of the surface of the said islands, the shape of the hands and feet of the dinosaur that they created can be found under the water using the data of the map of the underwater islands.The computer just needs to lower the water of the ocean to determine the shape of the hands and feet of the dinosaur.In the general images of the islands of the Philippines, Papua, Malaysia, and Indonesia, the story of the consciousness of intelligent people is related to the moon and… if the moon does not plow the earth’s crust, the human skull It wasnot split, and the souls of humans and jinns were not thinking, the moon caused an earthquake and the skull of humans split under the soil, and the soul of the corpse and the body of humans came out more complete, and the humans who buriedtheir dead under the soil, a great soul came out of the brain.And they found a more complete memory of the souls, and humans became thinking, because they can test human corpses and remove the skull, so that a greater memory comes out of the brain, and the efficiency of the soul increases, and the soul cangrow and develop from the oxygen of the air.The work should be done in special coffins and it will work and the efficiency of the human soul will increase
Leave a comment Cancel reply
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
Scientists link certain gut bacteria to lower heart disease risk
Study finds several species of cholesterol-metabolizing bacteria in people with lower cholesterol levels.
Changes in the gut microbiome have been implicated in a range of diseases including type 2 diabetes, obesity, and inflammatory bowel disease. Now, a team of researchers at the Broad Institute of MIT and Harvard along with Massachusetts General Hospital has found that microbes in the gut may affect cardiovascular disease as well. In a study published in Cell , the team has identified specific species of bacteria that consume cholesterol in the gut and may help lower cholesterol and heart disease risk in people.
Members of Ramnik Xavier's lab, Broad's Metabolomics Platform, and collaborators analyzed metabolites and microbial genomes from more than 1,400 participants in the Framingham Heart Study, a decades-long project focused on risk factors for cardiovascular disease. The team discovered that bacteria called Oscillibacter take up and metabolize cholesterol from their surroundings, and that people carrying higher levels of the microbe in their gut had lower levels of cholesterol. They also identified the mechanism the bacteria likely use to break down cholesterol. The results suggest that interventions that manipulate the microbiome in specific ways could one day help decrease cholesterol in people. The findings also lay the groundwork for more targeted investigations of how changes to the microbiome affect health and disease.
"Our research integrates findings from human subjects with experimental validation to ensure we achieve actionable mechanistic insight that will serve as starting points to improve cardiovascular health," said Xavier, who is a core institute member, director of the Immunology Program, and co-director of the Infectious Disease and Microbiome Program at the Broad. He is also a professor at Harvard Medical School and Massachusetts General Hospital.
Postdoctoral researcher Chenhao Li and research scientist Martin Stražar, both in Xavier's lab, were co-first authors on the study.
Cholesterol cues
In the past decade, other researchers have uncovered links between composition of the gut microbiome and elements of cardiovascular disease, such as a person's triglycerides and blood sugar levels after a meal. But scientists haven't been able to target those connections with therapies in part because they lack a complete understanding of metabolic pathways in the gut.
In the new study, the Broad team gained a more complete and detailed picture of the impact of gut microbes on metabolism. They combined shotgun metagenomic sequencing, which profiles all of the microbial DNA in a sample, with metabolomics, which measures the levels of hundreds of known and thousands of unknown metabolites. They used these tools to study stool samples from the Framingham Heart Study.
"The project outcomes underline the importance of high-quality, curated patient data," Stražar said. "That allowed us to note effects that are really subtle and hard to measure and directly follow up on them."
The approach uncovered more than 16,000 associations between microbes and metabolic traits, including one that was particularly strong: People with several species of bacteria from the Oscillibacter genus had lower cholesterol levels than those who lacked the bacteria. The researchers found that species in the Oscillibacter genus were surprisingly abundant in the gut, representing on average 1 in every 100 bacteria.
The researchers then wanted to figure out the biochemical pathway the microbes use to break down cholesterol. To do this, they first needed to grow the organism in the lab. Fortunately, the lab has spent years collecting bacteria from stool samples to create a unique library that also included Oscillibacter .
After successfully growing the bacteria, the team used mass spectrometry to identify the most likely byproducts of cholesterol metabolism in the bacteria. This allowed them to determine the pathways the bacteria uses to lower cholesterol levels. They found that the bacteria converted cholesterol into intermediate products that can then be broken down by other bacteria and excreted from the body. Next, the team used machine-learning models to identify the candidate enzymes responsible for this biochemical conversion, and then detected those enzymes and cholesterol breakdown products specifically in certain Oscillibacter in the lab.
The team found another gut bacterial species, Eubacterium coprostanoligenes, that also contributes to decreased cholesterol levels. This species carries a gene that the scientists had previously shown is involved in cholesterol metabolism. In the new work, the team discovered that Eubacterium might have a synergistic effect with Oscillibacter on cholesterol levels , which suggests that new experiments that study combinations of bacterial species could help shed light on how different microbial communities interact to affect human health.
Microbial messages
The vast majority of genes in the human gut microbiome remains uncharacterized, but the team is confident that their success in pinpointing cholesterol-metabolizing enzymes paves the way for the discovery of other similar metabolic pathways impacted by gut microbes, which could be targeted therapeutically.
"There are many clinical studies trying to do fecal microbiome transfer studies without much understanding of how the microbes interact with each other and the gut," Li said. "Hopefully stepping back by focusing on one particular bug or gene first, we'll get a systematic understanding of gut ecology and come up with better therapeutic strategies like targeting one or a few bugs."
"Because of the large number of genes of unknown function in the gut microbiome, there are gaps in our ability to predict metabolic functions," Li added. "Our work highlights the possibility that additional sterol metabolism pathways may be modified by gut microbes. There are potentially a lot of new discoveries to be made that will bring us closer to a mechanistic understanding of how microbes interact with the host."
Funding: This work was supported by the National Institutes of Health.
- Cholesterol
- Heart Disease
- Gastrointestinal Problems
- Microbes and More
- New Species
- Veterinary Medicine
- Low density lipoprotein
- Physical exercise
- Coronary heart disease
- Huntington's disease
Story Source:
Materials provided by Broad Institute of MIT and Harvard . Original written by Allessandra DiCorato. Note: Content may be edited for style and length.
Journal Reference :
- Chenhao Li, Martin Stražar, Ahmed M.T. Mohamed, Julian A. Pacheco, Rebecca L. Walker, Tina Lebar, Shijie Zhao, Julia Lockart, Andrea Dame, Kumar Thurimella, Sarah Jeanfavre, Eric M. Brown, Qi Yan Ang, Brittany Berdy, Dallis Sergio, Rachele Invernizzi, Antonio Tinoco, Gleb Pishchany, Ramachandran S. Vasan, Emily Balskus, Curtis Huttenhower, Hera Vlamakis, Clary Clish, Stanley Y. Shaw, Damian R. Plichta, Ramnik J. Xavier. Gut microbiome and metabolome profiling in Framingham heart study reveals cholesterol-metabolizing bacteria . Cell , 2024; DOI: 10.1016/j.cell.2024.03.014
Cite This Page :
Explore More
- Australia On Track for Decades-Long Megadroughts
- Speed of Visual Perception Ranges Widely
- 3D Printed Replica of an Adult Human Ear
- Extremely Fast Wound Healing: New Treatment
- Micro-Lisa! Novel Nano-Scale Laser Writing
- Simple Brain-Computer Link: Gaming With Thoughts
- Clinical Reasoning: Chatbot Vs Physicians
- Understanding People Who Can't Visualize
- Illuminating Oxygen's Journey in the Brain
- DNA Study IDs Descendants of George Washington
Trending Topics
Strange & offbeat.

IMAGES
VIDEO
COMMENTS
The new research, published in the journal Nature Communications, offers a potential strategy for developing new therapies that could restore dysfunctional pancreatic beta-cells or, perhaps, even prevent Type 2 diabetes from developing. The new study shows that the beta-cells of Type 2 diabetes patients are deficient in a cell trafficking ...
June 24, 2021 — Across the world, type 2 diabetes is on the rise. A research group has discovered a new gene that may hold the key to preventing and treating lifestyle related diseases such as ...
By Andrew Stewart, MD, as told to Hallie Levine. Type 2 diabetes is one of the world's greatest health problems. It affects about 400 million people globally. If it's uncontrolled, it can lead ...
Type 2 diabetes mellitus, the most frequent subtype of diabetes, is a disease characterized by high levels of blood glucose (hyperglycaemia). It arises from a resistance to and relative deficiency ...
New cause of diabetes discovered, offering potential target for new classes of drugs to treat the disease. ScienceDaily . Retrieved March 29, 2024 from www.sciencedaily.com / releases / 2023 / 12 ...
The FDA approved a new treatment to improve blood sugar control in adults with type 2 diabetes, ... and Obesity in the FDA's Center for Drug Evaluation and Research. Type 2 diabetes, the most ...
A new study found that tiny biodegradable particles improved insulin sensitivity in the liver cells of humans and in diabetic mice, which could offer a promising new treatment for type 2 diabetes.
Large-scale study reveals new genetic details of diabetes. In experiments of unprecedented scale, investigators at Weill Cornell Medicine and the National Institutes of Health have revealed new aspects of the complex genetics behind Type 2 diabetes. Through these discoveries, and by providing a template for future studies, this research ...
The chronic complications of type 2 diabetes are a major cause of mortality and disability worldwide [ 1, 2 ]. Clinical research is the main way to gain knowledge about long-term diabetic complications and reduce the burden of diabetes. This allows for designing effective programs for screening and follow-up and fine-targeted therapeutic ...
A non-coding region on chromosome 9p21 was previously shown to associate with coronary artery disease and type 2 diabetes, and the region has been implicated in regulating neighbouring genes. Here ...
Prolonged benefits of bariatric surgery for patients with Type 2 Diabetes. People with type 2 diabetes who underwent bariatric surgery compared to medical or lifestyle interventions had better ...
February 1, 2022. Source: Scripps Research Institute. Summary: A team of scientists has conducted promising early tests of a new strategy that might one day be used to prevent or treat type 2 ...
Surgery led to diabetes remission in roughly 28% of patients, compared with a remission rate of just 4% among the non-surgery group, according to the study results. More research has found that ...
Amelioration of Both Central and Peripheral Neuropathy in Mouse Models of Type 1 and Type 2 Diabetes by the Neurogenic Molecule NSI-189. Diabetes, 68(11), 2143-2154. Read more. ADA-funded researcher studying link between ageing and type 2 diabetes. One of the most important risk factors for developing type 2 diabetes is age.
The ADA is committed to continuing progress in the fight against type 2 diabetes by funding research, including support for potential new treatments, a better understating of genetic factors, addressing disparities, and more. For specific examples of projects currently funded by the ADA, see below. Greg J. Morton, PhD.
There are many known risk factors for type 2 diabetes, including family members having it, being of African or Asian ancestry, high blood pressure, obesitym and polycystic ovary syndrome (PCOS ...
I. Introduction. Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020).Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing ...
Clinical spectrum associated with Wolfram Syndrome Type 1 and type 2: A review on genotype-phenotype correlations. International Journal of Environmental Research and Public Health , 18 (9 ...
Researchers forecasted the number of kids and teens who will be diagnosed with diabetes from 2017 to 2060 based on two scenarios: Constant incidence: If the rate of new cases stays the same, type 1 diabetes cases would remain about the same. Type 2 diabetes cases would increase about 70%. Total diabetes cases would increase about 12%.
An Additional 12 Million US Adults Become Eligible for Diabetes Screening. New USPSTF and ADA guidelines lower the age for prediabetes and type 2 diabetes screening to 35. This study examined if testing practices aligned with guidelines and which populations were less likely to receive testing. Read the summary.
Develop new habits and address barriers to changing habits. ... Research has shown the following results about popular supplements for type 2 diabetes: Chromium supplements have been shown to have few or no benefits. Large doses can result in kidney damage, muscle problems and skin reactions. ... Type 2 diabetes is a serious disease, and ...
Diabetes and Alzheimer's are two of the fastest-growing health concerns worldwide. Diabetes alters the body's ability to turn food into energy and affects an estimated 1 in 10 U.S. adults.
Read the latest Research articles in Type 2 diabetes from Scientific Reports. ... Diabetic peripheral neuropathy among adult type 2 diabetes patients in Adama, Ethiopia: health facility-based ...
Some people with type 2 diabetes—the most common type—keep blood glucose in check by making lifestyle changes, including diet and exercise. Medications can also help to control blood glucose. Clinical trials over the past few decades have found that bariatric surgery, or weight-control surgery, can also help control type 2 diabetes.
(Carol Yepes/Moment via Getty Images) Eating more dietary fiber may help prevent Type 2 diabetes by promoting beneficial gut bacteria and substances produced during metabolism, according to new research in Hispanic adults. "Consistent evidence suggests diabetes-protective effects of dietary fiber intake, but exactly how that protection occurs remains unclear," said Dr. Zheng Wang, a study co ...
According to a new study published April 19, 2023 in the journal The BMJ, among people diagnosed with type 2 diabetes, those who drink the most sugar-sweetened beverages may be at a 20% higher ...
People with type 2 diabetes who underwent bariatric surgery achieved better long-term blood glucose control compared to people who received medical management plus lifestyle interventions, according to a new study supported by the NIDDK. ... More research is needed to determine if these changes are beneficial or detrimental and what effect they ...
Over about 10 years, 9,453 had developed Type 2 diabetes. Then the researchers compared biomarker scores from the 9,453 to 12,749 randomly selected participants without diabetes in the large study.
New research conducted in mice sheds light on what's going on at the molecular level that could cause people with diabetes to develop Alzheimer's disease. The study adds to a growing body of research on the links between Type 2 diabetes and Alzheimer's disease, which some scientists have called "Type 3 diabetes."
Changes in the gut microbiome have been implicated in a range of diseases including type 2 diabetes, obesity, and inflammatory bowel disease. Now, a team of researchers has found that microbes in ...