Advances in Brain and Spinal Cord Tumor Research
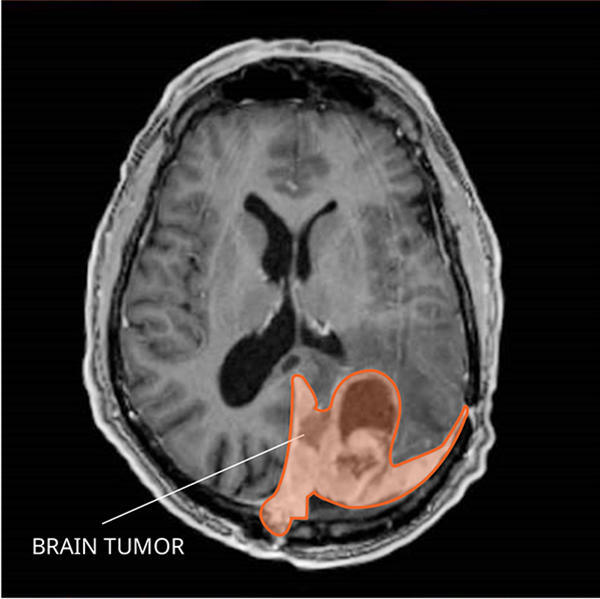
MRI of a meningioma in the brain.
NCI-funded researchers are working to improve our understanding of how to treat tumors that arise in the brain or the spinal cord (together known as the central nervous system , or CNS). Such tumors can be either benign (not cancer) or malignant (cancer). But the tissues of the nervous system are so important and so vulnerable that even some benign tumors may need urgent treatment.
Tumors that begin in the brain or spinal cord account for less than 2% of all cancers diagnosed each year in the United States. And there are over 130 different types. This diversity and the rarity of some types pose unique challenges to developing new treatments.
Often, tumors found in the brain have started somewhere else in the body and then spread to the brain. These are called metastatic brain tumors (or brain metastases ). The research highlighted on this page addresses primary brain tumors (tumors that start in the tissue of the brain), not metastatic brain tumors. It also includes research into primary spinal cord tumors, which are tumors that start in the spine.
The latest research on this page includes clinical advances that may soon translate into improved care and research findings from recent studies.

Diagnosis of Brain and Spinal Cord Tumors
Many types of brain and spinal cord tumors look similar when the cells are examined under the microscope. Even with trained pathologists examining tissue samples, up to 10% of people with a brain or spinal cord tumor receive the wrong diagnosis at first. This can potentially affect outcomes, because tumors that look similar at the cellular level may require very different treatments.
NCI-funded researchers are studying ways to improve the diagnosis of brain and spinal cord tumors. For example:
- A type of blood test called a liquid biopsy was able to distinguish between several different types of brain tumors in adult patients. This test checks for chemical changes in tumor DNA that had been shed into the bloodstream. Researchers hope that such tests could not just improve the accuracy of diagnoses but someday be used to diagnose brain tumors without the need for invasive surgery to get biopsy samples .
- A different liquid biopsy test was able to detect a specific genetic alteration in children with a rare type of brain tumor . Such tests could eventually help select patients for studies of new targeted therapies.
- Another study found that a blood test that detects genomic changes in DNA shed from a brain tumor called medulloblastoma could identify children who still had evidence of cancer after treatment. Such children are at high risk of relapse and may benefit from more aggressive therapy upfront.
- Scientists are also testing the use of artificial intelligence , or AI, for the analysis of images to speed the diagnosis of brain tumors during surgery .
If you have received a diagnosis of a brain and spinal cord tumor and are seeking a second opinion , the NCI-CONNECT program offers free consultations, as well as advice for patients’ cancer care teams at home. The program also runs clinical studies and trials focused on the treatment of rare adult brain and spinal cord tumors and on improving quality of life, symptom control, and management of side effects.
New Treatments for Brain and Spinal Cord Tumors in Adults
The mainstays of treatment for most types of brain and spinal cord tumors are surgery and radiation therapy. For some types of tumors in the CNS, chemotherapy is also used.
Because these treatments can damage normal cells as well as tumor cells in the brain and spinal cord, they may come with serious side effects. These include memory and thinking problems, mood changes, and difficulty walking. In addition to their toxicity , these treatments are not always effective, especially for aggressive types of brain cancer, such as glioblastoma . This cancer, the most common type of brain tumor, usually comes back (recurs) rapidly after treatment.
Researchers are searching for ways to improve the treatment of brain and spinal cord tumors, including the following three strategies:
Targeted therapy for brain and spinal cord tumors
Precision medicine (also called personalized medicine) uses information about a person’s own genes or proteins to prevent, diagnose, or treat disease. Over the last decade, scientists have discovered that tumors arising from the same type of cell can be driven by many different gene changes (also known as gene mutations). Such genetic studies hold the promise of more personalized treatments.
- In a large clinical trial, treatment with the targeted drug vorasidenib slowed the growth of tumors in some people with low-grade gliomas that had mutations in the IDH 1 or IDH 2 genes, postponing the need for additional therapies.
- For tumors with mutations in IDH , treatment with a type of drug called a PARP inhibitor may make them more sensitive to chemotherapy. A clinical trial is testing this strategy in people with a type of brain tumor called a glioma that has recurred after initial treatment.
- Other treatments for IDH - mutated tumors are targeting changes in cancer metabolism .
- Some rare types of brain tumors may depend on specific gene changes to fuel their growth. That makes such changes good targets for treatment. For example, a recent study found that targeting a gene change called BRAF V600 E in a rare type of brain tumor called papillary craniopharyngioma let many people delay invasive surgery or radiation therapy for years .
- Researchers are also beginning to use powerful computers to look through enormous databases of genetic mutations and combinations of genes, called gene fusions, found in brain tumors. Doing so may help them pinpoint which ones the cancer cells rely on to survive. These mutations and fusions could then potentially be targeted with new or existing drugs.
- Other studies are trying to target proteins found widely in CNS tumors. For example, NCI researchers are testing a drug that blocks dopamine receptors in recurrent brain and spine tumors . Dopamine receptors are found in many of these tumor types, including glioblastoma , lower grade gliomas, and medulloblastoma .
Scientists are also trying to understand other biological factors that influence brain tumor development and its response to treatment. For example, studies have found that women are less likely than men to be diagnosed with glioblastoma and their tumors tend to respond better to standard treatments. Such work may uncover further avenues for treatment personalization.
Personalizing treatment of brain and spinal cord tumors makes it challenging to test new drugs, because clinical trials will be limited to fewer patients with already rare cancers. Examples of NCI-led initiatives to overcome this challenge include:
- The NCI-led Brain Tumor Trials Collaborative (BTTC) and NCI-CONNECT clinical trial network are established at 33 cancer centers across the county to make it easier for people with rare brain and spinal cord tumors to participate in clinical trials closer to their homes.
- NCI researchers are leading efforts to reduce other barriers to clinical trial participation for people with brain or spinal cord tumors. These include allowing older adults or people who have previously been treated for other types of cancer to participate. Promoting the use of telehealth and collection of blood tests at local hospitals may also reduce the need for people to travel to participate in a research study.
Improving the response to radiation
Radiation therapy is already personalized to some extent because the amount and shape of the tissue that gets treated is tailored to each tumor’s size and location. However, the dose (or amount) of radiation used is usually the same for everyone with a specific tumor type.
- Researchers want to find ways to figure out whether a tumor’s response to radiation can be predicted before treatment. That would make it possible for people with tumors that are unlikely to shrink after standard doses of radiation to instead join clinical trials that are testing strategies like higher radiation doses. Scientists are studying whether machine learning , also called artificial intelligence or AI, can use data from MRI scans of brain tumors to predict radiation response.
- Scientists are also trying to develop substances called radiation sensitizers , which increase the damage radiation does to tumor cells but not normal cells. Dozens of small clinical trials across the country are studying radiation sensitizers in glioblastoma. For example, a trial led by NCI researchers is looking at whether the drug selinexor (Xpovio), when combined with chemotherapy and radiation , can improve survival.
Immunotherapy
Immunotherapy uses substances to stimulate or suppress the immune system to help the body fight cancer. In some blood cancers and solid tumors, immunotherapy drugs have provided huge gains in survival for some people. But to date, immunotherapy has not worked well for brain tumors. Issues may include:
- The blood–brain barrier, which is a network of blood vessels and tissue that helps protect the brain. This barrier also prevents some drugs and types of immune cells from reaching tumors.
- A recent NCI-funded study found that corticosteroids may reduce the effectiveness of immunotherapy for brain cancer by suppressing the body’s immune response.
- Another study from NCI researchers found that steroids used to treat brain cancer may affect the development of T cells , which are key immune cells for fighting cancer.
However, some people with CNS tumors given immunotherapy in clinical trials have had their tumors shrink or disappear. Researchers want to know if these responses could be predicted, both to spare people unnecessary treatment and to develop new strategies to make resistant CNS tumors respond to immunotherapies.
- NCI researchers are running a clinical trial testing a combination of two immunotherapy drugs in people with newly diagnosed brain tumors. As part of the study, they’re testing whether there are changes in the types and level of activity of immune cells in the blood both before and during treatment. Measuring these changes might help predict who will benefit from immunotherapy and who won’t.
- Another study led by NCI researchers is testing whether the immunotherapy drug nivolumab (Opdivo) can shrink or control the growth or spread of specific types of recurrent rare brain or spine tumors . The trial is also testing the changes that nivolumab induces in immune cells in the blood during treatment, and whether the drug can improve the symptoms of people with these tumors.
- Other studies are examining whether people whose brain tumors have certain mutations, such as those in the IDH gene , are more likely to have their tumors shrink from immunotherapy.
Survivorship and Quality of Life for People with Brain or Spinal Cord Tumors
Because both CNS tumors and their treatments can be debilitating, researchers are looking for new ways to improve quality of life for people with these tumors.
- This may include using people’s quality of life to measure the benefit of new drugs or treatment combinations. For example, in a recent NCI-led trial of a drug combination to treat a rare CNS tumor called ependymoma , most people’s tumors didn’t shrink. However, because they reported a reduction in symptoms from their tumor , the combination is now included in some professional recommendations for treatment of this cancer.
- NCI scientists are looking at whether genomic factors can predict the likelihood that a patient will experience side effects from certain treatments. They’re also performing experiments in the lab to understand the biological processes linked with these side effects. Results from these studies could potentially influence treatment decisions.
- For people with many cancer types, simply monitoring symptoms has been found to improve not just quality of life but survival . An NCI study is tracking symptoms and the well-being of people with brain and spinal cord tumors over time. People in that study can also take part in research to improve sleep , to reduce distress, anxiety , and depression, such as using virtual reality to reduce stress.
Treatment of Brain and Spinal Cord Tumors in Children
Tumors of the brain and spinal cord in children are relatively rare . But about 4,000 children and adolescents nationwide receive a diagnosis of a brain or spinal cord tumor every year, making CNS tumors the second most common cancer type in this age group after leukemia.
Treatment has improved for young patients with these tumors over the last several decades. Although some CNS tumors can’t be cured, almost three-quarters of children and adolescents treated for a brain or spinal cord tumor will be alive 5 years after diagnosis.
However, effective treatments can harm children’s developing nervous systems. Current research in childhood brain and spinal cord tumors focuses on understanding the underlying causes of these cancers, developing new treatments, and reducing the toxic effects of effective therapies. For example,
- One study found that some children with medulloblastoma , a type of brain cancer, can safely get less radiation therapy without reducing their long-term survival. The effectiveness of this approach depended on the genetic alterations found in children’s tumors. A follow-up study is looking more closely at reducing the intensity of treatment in children with medulloblastoma caused by changes in a gene called WNT .
- A 2022 clinical trial found that, for children whose tumors have a BRAF mutation called V600, a combination of two targeted drugs was safer and better than standard chemotherapy at shrinking these tumors and keeping them from growing again. Approved by the FDA in 2023, these drugs, dabrafenib (Tafinlar) and trametinib (Mekinist) , can be given orally as a liquid, making treatment easier for children as well.
- Most low-grade gliomas have BRAF gene changes called rearrangements or fusions. These happen when pieces of the gene get switched around or stuck to pieces of other genes. In 2024, a drug called tovorafenib (Ojemda) received FDA approval for treating low-grade gliomas that have these or other BRAF changes and that have returned after initial treatment . An ongoing study is now comparing tovorafenib against chemotherapy as part of initial treatment for children with low-grade glioma.
- A targeted drug called selumetinib (Koselugo) is approved for treating nerve tumors in children with a rare condition called neurofibromatosis type 1 (NF1) . A small study found that it could also shrink a type of brain tumor called low-grade glioma in some children with NF1 whose tumors have certain BRAF changes. NCI researchers have launched a clinical trial of the drug in children with and without NF1 who have low-grade glioma with these BRAF changes.
- A rare type of brain tumor called diffuse midline glioma, which occurs more commonly in children than adults, currently has no cure. An NCI-funded clinical trial is testing CAR T cells, a type of immunotherapy, that target cells with a mutation found in some of these tumors. The treatment has been found to shrink tumors and reduce neurologic symptoms caused by the tumor in some children.
- Other studies are using information about mutations in children’s brain tumors to test new treatments in those who may benefit the most. One such study, the Pediatric MATCH study is testing new targeted therapies in children with solid tumors—including those in the brain or spinal cord—that have not responded to standard treatments. In the study, children are assigned to an experimental treatment based on the genetic changes found in their tumors rather than on their type of cancer or cancer site.
Additional clinical trials for children with CNS tumors are being performed by the NCI-sponsored Children’s Oncology Group and Pediatric Brain Tumor Consortium .
NCI-Supported Research Programs
Many NCI-funded researchers working at the National Institutes of Health (NIH) campus, as well as across the United States and throughout the world, are seeking ways to address tumors of the brain and spinal cord more effectively. Some research is basic, exploring questions such as the biological underpinnings of cancer. And some is more clinical, seeking to translate this basic information into improving patient outcomes. The programs listed below are a small sampling of NCI’s research efforts in brain and other CNS tumors.
- The NCI-CONNECT Rare Brain and Spine Tumor Network is part of the Rare Tumor Patient Engagement Network, an initiative supported by the Cancer Moonshot℠ . The network aims to advance the understanding of rare adult CNS cancers by establishing and fostering patient–advocacy–provider partnerships and networks to improve approaches to care and treatment. Consultations are free of charge for people seeking a second opinion or expertise on a rare CNS tumor type. Patients also receive support for travel expenses, food, lodging and outpatient treatment.
- The Brain Tumor Trials Collaborative network includes over 30 institutions with expertise in neuro-oncology from across the United States. The network’s mission is to develop and carry out clinical trials in a collaborative environment to advance treatments for patients with brain and spine tumors. NCI serves as the lead institution, providing administrative support, a clinical database, and oversight for the network.
- NCI’s Brain Cancer Specialized Programs of Research Excellence (SPOREs) promote collaborative, interdisciplinary research. SPORE grants involve both basic and clinical/applied scientists working together. They support the efficient movement of basic scientific findings into clinical settings, as well as studies to determine the biological basis for observations made in individuals with cancer or in populations at risk for cancer.
- NCI's National Clinical Trials Network (NCTN) is a collection of organizations and clinicians that coordinate and support cancer clinical trials at more than 3,000 sites across the United States and Canada. NCTN has a variety of trials testing treatments for brain and other CNS tumors .
- NCI has also formed partnerships with the pharmaceutical industry, academic institutions, and individual investigators for the early clinical evaluation of innovative cancer therapies. The Experimental Therapeutics Clinical Trials Network (ETCTN) was created to evaluate these therapies using a coordinated, collaborative approach to early-phase clinical trials. The ETCTN is currently running early-stage trials in brain and other CNS tumors .
- NCI’s Division of Cancer Epidemiology and Genetics conducts studies on people with brain and other CNS tumors to learn about genetic and other risk factors for these diseases .
NCI’s Office of Cancer Survivorship , part of the Division of Cancer Control and Population Sciences, supports many research projects that study survivorship issues in people who have been treated for brain tumors.
Clinical Trials
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Our clinical trials search form can be used to find trials to treat glioblastoma , glioma , medulloblastoma , and other types of brain and spinal cord tumors.
Brain and Spinal Cord Tumor Research Results
The following are some of our latest news articles on brain and spinal cord tumor research.
- Experimental mRNA Vaccine Hints at Potential Against Glioblastoma
- Tovorafenib Approved for Some Children with Low-Grade Glioma
- Genetic Signature May Help Tailor Treatment for Meningioma
- Engaging People with Low-Grade Glioma in Cancer Research
- Targeted Drug Combo May Change Care for Rare Brain Tumor Craniopharyngioma
- Vorasidenib Treatment Shows Promise for Some Low-Grade Gliomas
View the full list of brain cancer research results and study updates .

Brain Cancer
Brain Cancer research articles are listed. Brain cancer news covers topics such as diagnosis, brain tumors, chemotherapy, gamma knife technology, brain cancer treatments, glioblastomas, stem cell research, neurosurgery, medicine, genetics, neurology, and other brain research.

Cancer Genes Crucial for Nervous System Development
Cancer Drug May Halt Parkinson’s Spread
New Insights into Pediatric Brain Tumors

New Gene Discovery Could Halt Metastatic Cancer Spread

Artificial Lymph Node Shows Promise in Cancer Treatment
New Hope for Treating Brain Cancer with Ultrasound
Glioblastoma Drug Resistance Pathway Identified
AI Tool Speeds Up Brain Tumor Classification
Breakthrough in Immunotherapy for Brain Cancer
Nanoparticles Penetrate Blood-Brain Barrier to Treat Cancer
Novel mRNA Vaccine Shows Promise Against Deadly Brain Cancer
Vitamin D Boosts Gut Bacteria for Cancer Immunity
Misleading Information on Depression Hampers Understanding

Sleep-Deprived Memories Restored by Common Medications
Supranormal Hearing Achieved by Boosting Ear Synapses

Digital Devices Hinder Kids’ Emotional Regulation Development
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
A noninvasive treatment for “chemo brain”
Press contact :, media download.
*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."
Previous image Next image
Patients undergoing chemotherapy often experience cognitive effects such as memory impairment and difficulty concentrating — a condition commonly known as “chemo brain.”
MIT researchers have now shown that a noninvasive treatment that stimulates gamma frequency brain waves may hold promise for treating chemo brain. In a study of mice, they found that daily exposure to light and sound with a frequency of 40 hertz protected brain cells from chemotherapy-induced damage. The treatment also helped to prevent memory loss and impairment of other cognitive functions.
This treatment, which was originally developed as a way to treat Alzheimer’s disease, appears to have widespread effects that could help with a variety of neurological disorders, the researchers say.
“The treatment can reduce DNA damage, reduce inflammation, and increase the number of oligodendrocytes, which are the cells that produce myelin surrounding the axons,” says Li-Huei Tsai, director of MIT’s Picower Institute for Learning and Memory and the Picower Professor in the MIT Department of Brain and Cognitive Sciences. “We also found that this treatment improved learning and memory, and enhanced executive function in the animals.”
Tsai is the senior author of the new study, which appears today in Science Translational Medicine . The paper’s lead author is TaeHyun Kim, an MIT postdoc.
Protective brain waves
Several years ago, Tsai and her colleagues began exploring the use of light flickering at 40 hertz (cycles per second) as a way to improve the cognitive symptoms of Alzheimer’s disease. Previous work had suggested that Alzheimer’s patients have impaired gamma oscillations — brain waves that range from 25 to 80 hertz (cycles per second) and are believed to contribute to brain functions such as attention, perception, and memory.
Tsai’s studies in mice have found that exposure to light flickering at 40 hertz or sounds with a pitch of 40 hertz can stimulate gamma waves in the brain, which has many protective effects, including preventing the formation of amyloid beta plaques. Using light and sound together provides even more significant protection. The treatment also appears promising in humans: Phase 1 clinical trials in people with early-stage Alzheimer’s disease have found the treatment is safe and does offer some neurological and behavioral benefits.
In the new study, the researchers set out to see whether this treatment could also counteract the cognitive effects of chemotherapy treatment. Research has shown that these drugs can induce inflammation in the brain, as well as other detrimental effects such as loss of white matter — the networks of nerve fibers that help different parts of the brain communicate with each other. Chemotherapy drugs also promote loss of myelin, the protective fatty coating that allows neurons to propagate electrical signals. Many of these effects are also seen in the brains of people with Alzheimer’s.
“Chemo brain caught our attention because it is extremely common, and there is quite a lot of research on what the brain is like following chemotherapy treatment,” Tsai says. “From our previous work, we know that this gamma sensory stimulation has anti-inflammatory effects, so we decided to use the chemo brain model to test whether sensory gamma stimulation can be beneficial.”
As an experimental model, the researchers used mice that were given cisplatin, a chemotherapy drug often used to treat testicular, ovarian, and other cancers. The mice were given cisplatin for five days, then taken off of it for five days, then on again for five days. One group received chemotherapy only, while another group was also given 40-hertz light and sound therapy every day.
After three weeks, mice that received cisplatin but not gamma therapy showed many of the expected effects of chemotherapy: brain volume shrinkage, DNA damage, demyelination, and inflammation. These mice also had reduced populations of oligodendrocytes, the brain cells responsible for producing myelin.
However, mice that received gamma therapy along with cisplatin treatment showed significant reductions in all of those symptoms. The gamma therapy also had beneficial effects on behavior: Mice that received the therapy performed much better on tests designed to measure memory and executive function.
“A fundamental mechanism”
Using single-cell RNA sequencing, the researchers analyzed the gene expression changes that occurred in mice that received the gamma treatment. They found that in those mice, inflammation-linked genes and genes that trigger cell death were suppressed, especially in oligodendrocytes, the cells responsible for producing myelin.
In mice that received gamma treatment along with cisplatin, some of the beneficial effects could still be seen up to four months later. However, the gamma treatment was much less effective if it was started three months after the chemotherapy ended.
The researchers also showed that the gamma treatment improved the signs of chemo brain in mice that received a different chemotherapy drug, methotrexate, which is used to treat breast, lung, and other types of cancer.
“I think this is a very fundamental mechanism to improve myelination and to promote the integrity of oligodendrocytes. It seems that it’s not specific to the agent that induces demyelination, be it chemotherapy or another source of demyelination,” Tsai says.
Because of its widespread effects, Tsai’s lab is also testing gamma treatment in mouse models of other neurological diseases, including Parkinson’s disease and multiple sclerosis. Cognito Therapeutics, a company founded by Tsai and MIT Professor Edward Boyden, has finished a phase 2 trial of gamma therapy in Alzheimer’s patients, and plans to begin a phase 3 trial this year.
“My lab’s major focus now, in terms of clinical application, is Alzheimer’s; but hopefully we can test this approach for a few other indications, too,” Tsai says.
The research was funded by the JPB Foundation, the Ko Hahn Seed Fund, and the National Institutes of Health.
Share this news article on:
Press mentions, new scientist.
MIT scientists have found that a potential treatment for Alzheimer’s disease involving flickering lights and low-pitched sound could also help prevent cognitive problems after cancer treatment, reports Clare Wilson for New Scientist . The treatment is aimed at stimulating 40 Hz brainwaves, which are linked to memory processing. The results suggest targeting such “brainwaves may result in broader benefits for the brain, including increasing the activity of immune cells and, most recently, boosting its drainage system, which could help clear a toxic protein called beta-amyloid.”
Previous item Next item
Related Links
- Li-Huei Tsai
- Picower Institute
- Department of Brain and Cognitive Sciences
Related Topics
- Brain and cognitive sciences
- Pharmaceuticals
Related Articles
Small studies of 40-hertz sensory stimulation confirm safety, suggest Alzheimer’s benefits

Why visual stimulation may work against Alzheimer’s
![brain cancer research articles “…[I]f humans behave similarly to mice in response to this treatment, I would say the potential is just enormous, because it’s so noninvasive, and it’s so accessible,” says Li-Huei Tsai, the Picower Professor of Neuroscience, when describing a new treatment for Alzheimer’s disease.](https://news.mit.edu/sites/default/files/styles/news_article__archive/public/images/201612/MIT-li-huei-tsai.jpg?itok=jrC2K2AI)
Unique visual stimulation may be new treatment for Alzheimer’s
More mit news.

The tenured engineers of 2024
Read full story →
Detachable cardiac pacing lead may improve safety for cardiac patients

“Rollerama” roller rink opens in Kendall Square

A prosthesis driven by the nervous system helps people with amputation walk naturally

Scientists observe record-setting electron mobility in a new crystal film

Faces of MIT: Anthony Hallee-Farrell '13
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram

- Health A to Z
- Alternative Medicine
- Blood Disorders
- Bone and Joint
- Cardiovascular Diseases
- Child Health
- Coronavirus
- Dental Care
- Digestive System
- Disabilities
- Drug Center
- Ear, Nose, and Throat
- Environmental Health
- Exercise And Fitness
- First Aid and Emergencies
- General Health
- Health Technology
- Hearing Loss
- Hypertension
- Infectious Disease
- LGBTQ Health
- Liver Health
- Men's Health
- Mental Health
- Pain Management
- Public Health
- Pulmonology
- Senior Health
- Sexual Health
- Skin Health
- Women's Health
- News For Medical Professionals
- Our Products
- Consumer News
- Physician's Briefing
- HealthDay TV
- Wellness Library
- HealthDay Living
- Conference Coverage
- Custom Products
- Health Writing
- Health Editing
- Video Production
- Medical Review
Why Are Brain Tumors More Deadly for Kids in Poorer Neighborhoods?

Key Takeaways
Children with inoperable brain tumors die sooner if they live in a poorer neighborhood
Kids from wealthy neighborhoods survived more than twice as long as those from poor areas
Less education also had an impact on a child’s treatment
MONDAY, June 24, 2024 (HealthDay News) -- U.S. children with inoperable brain tumors appear to die sooner and find it harder to get care if they live in poorer neighborhoods, a new study finds.
Children from higher-income areas had more than double the average survival time than kids from poorer neighborhoods -- 480 days versus 235 days, depending if a census tract had an average household income higher or lower than $50,000.
Kids in wealthier neighborhoods were also able to travel significantly longer distances to get the best medical care, 1,550 miles versus 1,114 miles for children in lower-income areas, researchers reported recently in the Journal of Neurosurgery .
“Children with these inoperable gliomas require access to specialized centers of care and clinical trials in the hopes of extending their lives, yet we are seeing that socioeconomic factors are linked to worsening survival,” said lead researcher Dr. John Lee , an incoming neurosurgery resident at University of Michigan Health.
“It’s critical that we understand the reasons for this disparity, so that we can ensure all patients have opportunities for life-prolonging care,” Lee added in a university news release.
The study tracked nearly 100 children being treated for two types of incurable brain tumors, diffuse midline glioma (DMG) and diffuse intrinsic pontine glioma (DIPG), between 2000 and 2022.
Education levels also made a difference in a child’s treatment, researchers found.
Kids in higher-educated areas were able to travel an average 2,964 miles for cancer care, versus 478 miles for children in neighborhoods with the least education.
Families with fewer financial resources might be less capable of travel to specialized cancer centers where they can receive second opinions, different treatment options and enrollment in clinical trials , Lee said.
“Additionally, patients whose families have lower levels of education may have less knowledge about the signs and symptoms of a condition, early screening programs and treatment options,” said senior researcher Dr. Karin Muraszko , a professor of neurosurgery at University of Michigan Medical School.
“However, our research uncovered disparities in survival despite no significant differences in rates of clinical trial enrollment or time until initiation of treatment between patients from different census tracts,” Muraszko added. “Future studies should aim to understand the exact mechanism through which this gap is created.”
More information
The National Cancer Institute has more on childhood glioma .
SOURCE: University of Michigan, news release, June 20, 2024
What This Means For You
Children with brain cancers might face tougher odds if they’re from poorer or less-educated parts of the country.
Related Stories


Optimal targeted therapy for multiple cancers based on contrastive Notch signaling networks
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- For correspondence: [email protected]
- Info/History
- Preview PDF
Over decades, cancer understanding has advanced significantly at molecular and cellular levels, leading to various therapies based on intra-/inter-cellular networks. Despite this, cancer still remains a leading cause of death globally. The primary driver of cancer mortality is metastasis, responsible for about 90% of cancer deaths, due to unclear pathophysiological mechanisms that complicate treatment development. The Notch signaling pathway, a crucial intercellular network in many cancers, has been extensively studied and therapies targeting the Notch pathway also have been well-studied based on inhibiting various stages of Notch activation. On the other hand, Notch signaling's role varies between cancers; for instance, in non-small cell lung cancer, Notch1 and Notch2 have opposing effects compared to their roles in embryonal brain tumors. In this study, we assumed a scenario of multiple cancers with contrasting Notch signaling pathways and explored optimal targeted therapies for reducing cancer cells by developing two mathematical models with contrasting Notch signaling pathways. The proposed therapies were compared with existing ones, and strategies were investigated to reduce cancer cell numbers for different stage of cancer. We found that that multiple cancers with contrasting Notch networks can be controlled by a common targeted signal network. Combination therapy enhancing Notch production may be most effective in early-stage cancer, while cleavage therapies may be more effective in late-stage cancer. Our study also suggests that optimal treatment should consider the cancer stage, with careful selection and ordering of medication therapies.
Competing Interest Statement
The authors have declared no competing interest.
https://github.com/TWakamoto/Notch-signaling.git
View the discussion thread.
Thank you for your interest in spreading the word about bioRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Cancer Biology
- Animal Behavior and Cognition (5417)
- Biochemistry (12222)
- Bioengineering (9149)
- Bioinformatics (30190)
- Biophysics (15492)
- Cancer Biology (12605)
- Cell Biology (18098)
- Clinical Trials (138)
- Developmental Biology (9761)
- Ecology (14631)
- Epidemiology (2067)
- Evolutionary Biology (18787)
- Genetics (12557)
- Genomics (17237)
- Immunology (12326)
- Microbiology (29064)
- Molecular Biology (12068)
- Neuroscience (63300)
- Paleontology (464)
- Pathology (1940)
- Pharmacology and Toxicology (3373)
- Physiology (5196)
- Plant Biology (10818)
- Scientific Communication and Education (1710)
- Synthetic Biology (3007)
- Systems Biology (7547)
- Zoology (1693)
Scientific breakthroughs: 2024 emerging trends to watch

December 28, 2023
Across disciplines and industries, scientific discoveries happen every day, so how can you stay ahead of emerging trends in a thriving landscape? At CAS, we have a unique view of recent scientific breakthroughs, the historical discoveries they were built upon, and the expertise to navigate the opportunities ahead. In 2023, we identified the top scientific breakthroughs , and 2024 has even more to offer. New trends to watch include the accelerated expansion of green chemistry, the clinical validation of CRISPR, the rise of biomaterials, and the renewed progress in treating the undruggable, from cancer to neurodegenerative diseases. To hear what the experts from Lawrence Liverpool National Lab and Oak Ridge National Lab are saying on this topic, join us for a free webinar on January 25 from 10:00 to 11:30 a.m. EDT for a panel discussion on the trends to watch in 2024.
The ascension of AI in R&D

While the future of AI has always been forward-looking, the AI revolution in chemistry and drug discovery has yet to be fully realized. While there have been some high-profile set-backs , several breakthroughs should be watched closely as the field continues to evolve. Generative AI is making an impact in drug discovery , machine learning is being used more in environmental research , and large language models like ChatGPT are being tested in healthcare applications and clinical settings.
Many scientists are keeping an eye on AlphaFold, DeepMind’s protein structure prediction software that revolutionized how proteins are understood. DeepMind and Isomorphic Labs have recently announced how their latest model shows improved accuracy, can generate predictions for almost all molecules in the Protein Data Bank, and expand coverage to ligands, nucleic acids, and posttranslational modifications . Therapeutic antibody discovery driven by AI is also gaining popularity , and platforms such as the RubrYc Therapeutics antibody discovery engine will help advance research in this area.
Though many look at AI development with excitement, concerns over accurate and accessible training data , fairness and bias , lack of regulatory oversight , impact on academia, scholarly research and publishing , hallucinations in large language models , and even concerns over infodemic threats to public health are being discussed. However, continuous improvement is inevitable with AI, so expect to see many new developments and innovations throughout 2024.
‘Greener’ green chemistry

Green chemistry is a rapidly evolving field that is constantly seeking innovative ways to minimize the environmental impact of chemical processes. Here are several emerging trends that are seeing significant breakthroughs:
- Improving green chemistry predictions/outcomes : One of the biggest challenges in green chemistry is predicting the environmental impact of new chemicals and processes. Researchers are developing new computational tools and models that can help predict these impacts with greater accuracy. This will allow chemists to design safer and more environmentally friendly chemicals.
- Reducing plastics: More than 350 million tons of plastic waste is generated every year. Across the landscape of manufacturers, suppliers, and retailers, reducing the use of single-use plastics and microplastics is critical. New value-driven approaches by innovators like MiTerro that reuse industrial by-products and biomass waste for eco-friendly and cheaper plastic replacements will soon be industry expectations. Lowering costs and plastic footprints will be important throughout the entire supply chain.
- Alternative battery chemistry: In the battery and energy storage space, finding alternatives to scarce " endangered elements" like lithium and cobalt will be critical. While essential components of many batteries, they are becoming scarce and expensive. New investments in lithium iron phosphate (LFP) batteries that do not use nickel and cobalt have expanded , with 45% of the EV market share being projected for LFP in 2029. Continued research is projected for more development in alternative materials like sodium, iron, and magnesium, which are more abundant, less expensive, and more sustainable.
- More sustainable catalysts : Catalysts speed up a chemical reaction or decrease the energy required without getting consumed. Noble metals are excellent catalysts; however, they are expensive and their mining causes environmental damage. Even non-noble metal catalysts can also be toxic due to contamination and challenges with their disposal. Sustainable catalysts are made of earth-abundant elements that are also non-toxic in nature. In recent years, there has been a growing focus on developing sustainable catalysts that are more environmentally friendly and less reliant on precious metals. New developments with catalysts, their roles, and environmental impact will drive meaningful progress in reducing carbon footprints.
- Recycling lithium-ion batteries: Lithium-ion recycling has seen increased investments with more than 800 patents already published in 2023. The use of solid electrolytes or liquid nonflammable electrolytes may improve the safety and durability of LIBs and reduce their material use. Finally, a method to manufacture electrodes without solvent s could reduce the use of deprecated solvents such as N-methylpyrrolidinone, which require recycling and careful handling to prevent emissions.
Rise of biomaterials

New materials for biomedical applications could revolutionize many healthcare segments in 2024. One example is bioelectronic materials, which form interfaces between electronic devices and the human body, such as the brain-computer interface system being developed by Neuralink. This system, which uses a network of biocompatible electrodes implanted directly in the brain, was given FDA approval to begin human trials in 2023.
- Bioelectronic materials: are often hybrids or composites, incorporating nanoscale materials, highly engineered conductive polymers, and bioresorbable substances. Recently developed devices can be implanted, used temporarily, and then safely reabsorbed by the body without the need for removal. This has been demonstrated by a fully bioresorbable, combined sensor-wireless power receiver made from zinc and the biodegradable polymer, poly(lactic acid).
- Natural biomaterials: that are biocompatible and naturally derived (such as chitosan, cellulose nanomaterials, and silk) are used to make advanced multifunctional biomaterials in 2023. For example, they designed an injectable hydrogel brain implant for treating Parkinson’s disease, which is based on reversible crosslinks formed between chitosan, tannic acid, and gold nanoparticles.
- Bioinks : are used for 3D printing of organs and transplant development which could revolutionize patient care. Currently, these models are used for studying organ architecture like 3D-printed heart models for cardiac disorders and 3D-printed lung models to test the efficacy of drugs. Specialized bioinks enhance the quality, efficacy, and versatility of 3D-printed organs, structures, and outcomes. Finally, new approaches like volumetric additive manufacturing (VAM) of pristine silk- based bioinks are unlocking new frontiers of innovation for 3D printing.
To the moon and beyond

The global Artemis program is a NASA-led international space exploration program that aims to land the first woman and the first person of color on the Moon by 2025 as part of the long-term goal of establishing a sustainable human presence on the Moon. Additionally, the NASA mission called Europa Clipper, scheduled for a 2024 launch, will orbit around Jupiter and fly by Europa , one of Jupiter’s moons, to study the presence of water and its habitability. China’s mission, Chang’e 6 , plans to bring samples from the moon back to Earth for further studies. The Martian Moons Exploration (MMX) mission by Japan’s JAXA plans to bring back samples from Phobos, one of the Mars moons. Boeing is also expected to do a test flight of its reusable space capsule Starliner , which can take people to low-earth orbit.
The R&D impact of Artemis extends to more fields than just aerospace engineering, though:
- Robotics: Robots will play a critical role in the Artemis program, performing many tasks, such as collecting samples, building infrastructure, and conducting scientific research. This will drive the development of new robotic technologies, including autonomous systems and dexterous manipulators.
- Space medicine: The Artemis program will require the development of new technologies to protect astronauts from the hazards of space travel, such as radiation exposure and microgravity. This will include scientific discoveries in medical diagnostics, therapeutics, and countermeasures.
- Earth science: The Artemis program will provide a unique opportunity to study the Moon and its environment. This will lead to new insights into the Earth's history, geology, and climate.
- Materials science: The extreme space environment will require new materials that are lightweight, durable, and radiation resistant. This will have applications in many industries, including aerospace, construction, and energy.
- Information technology: The Artemis program will generate a massive amount of data, which will need to be processed, analyzed, and shared in real time. This will drive the development of new IT technologies, such as cloud computing, artificial intelligence, and machine learning.
The CRISPR pay-off

After years of research, setbacks, and minimal progress, the first formal evidence of CRISPR as a therapeutic platform technology in the clinic was realized. Intellia Therapeutics received FDA clearance to initiate a pivotal phase 3 trial of a new drug for the treatment of hATTR, and using the same Cas9 mRNA, got a new medicine treating a different disease, angioedema. This was achieved by only changing 20 nucleotides of the guide RNA, suggesting that CRISPR can be used as a therapeutic platform technology in the clinic.
The second great moment for CRISPR drug development technology came when Vertex and CRISPR Therapeutics announced the authorization of the first CRISPR/Cas9 gene-edited therapy, CASGEVY™, by the United Kingdom MHRA, for the treatment of sickle cell disease and transfusion-dependent beta-thalassemia. This was the first approval of a CRISPR-based therapy for human use and is a landmark moment in realizing the potential of CRISPR to improve human health.
In addition to its remarkable genome editing capability, the CRISPR-Cas system has proven to be effective in many applications, including early cancer diagnosis . CRISPR-based genome and transcriptome engineering and CRISPR-Cas12a and CRISPR-Cas13a appear to have the necessary characteristics to be robust detection tools for cancer therapy and diagnostics. CRISPR-Cas-based biosensing system gives rise to a new era for precise diagnoses of early-stage cancers.
MIT engineers have also designed a new nanoparticle DNA-encoded nanosensor for urinary biomarkers that could enable early cancer diagnoses with a simple urine test. The sensors, which can detect cancerous proteins, could also distinguish the type of tumor or how it responds to treatment.

Ending cancer

The immuno-oncology field has seen tremendous growth in the last few years. Approved products such as cytokines, vaccines, tumor-directed monoclonal antibodies, and immune checkpoint blockers continue to grow in market size. Novel therapies like TAC01-HER2 are currently undergoing clinical trials. This unique therapy uses autologous T cells, which have been genetically engineered to incorporate T cell Antigen Coupler (TAC) receptors that recognize human epidermal growth factor receptor 2 (HER2) presence on tumor cells to remove them. This could be a promising therapy for metastatic, HER2-positive solid tumors.
Another promising strategy aims to use the CAR-T cells against solid tumors in conjunction with a vaccine that boosts immune response. Immune boosting helps the body create more host T cells that can target other tumor antigens that CAR-T cells cannot kill.
Another notable trend is the development of improved and effective personalized therapies. For instance, a recently developed personalized RNA neoantigen vaccine, based on uridine mRNA–lipoplex nanoparticles, was found effective against pancreatic ductal adenocarcinoma (PDAC). Major challenges in immuno-oncology are therapy resistance, lack of predictable biomarkers, and tumor heterogenicity. As a result, devising novel treatment strategies could be a future research focus.
Decarbonizing energy

Multiple well-funded efforts are underway to decarbonize energy production by replacing fossil fuel-based energy sources with sources that generate no (or much less) CO2 in 2024.
One of these efforts is to incorporate large-scale energy storage devices into the existing power grid. These are an important part of enabling the use of renewable sources since they provide additional supply and demand for electricity to complement renewable sources. Several types of grid-scale storage that vary in the amount of energy they can store and how quickly they can discharge it into the grid are under development. Some are physical (flywheels, pumped hydro, and compressed air) and some are chemical (traditional batteries, flow batteries , supercapacitors, and hydrogen ), but all are the subject of active chemistry and materials development research. The U.S. government is encouraging development in this area through tax credits as part of the Inflation Reduction Act and a $7 billion program to establish regional hydrogen hubs.
Meanwhile, nuclear power will continue to be an active R&D area in 2024. In nuclear fission, multiple companies are developing small modular reactors (SMRs) for use in electricity production and chemical manufacturing, including hydrogen. The development of nuclear fusion reactors involves fundamental research in physics and materials science. One major challenge is finding a material that can be used for the wall of the reactor facing the fusion plasma; so far, candidate materials have included high-entropy alloys and even molten metals .
Neurodegenerative diseases

Neurodegenerative diseases are a major public health concern, being a leading cause of death and disability worldwide. While there is currently no cure for any neurodegenerative disease, new scientific discoveries and understandings of these pathways may be the key to helping patient outcomes.
- Alzheimer’s disease: Two immunotherapeutics have received FDA approval to reduce both cognitive and functional decline in individuals living with early Alzheimer's disease. Aducannumab (Aduhelm®) received accelerated approval in 2021 and is the first new treatment approved for Alzheimer’s since 2003 and the first therapy targeting the disease pathophysiology, reducing beta-amyloid plaques in the brains of early Alzheimer’s disease patients. Lecanemab (Leqembi®) received traditional approval in 2023 and is the first drug targeting Alzheimer’s disease pathophysiology to show clinical benefits, reducing the rate of disease progression and slowing cognitive and functional decline in adults with early stages of the disease.
- Parkinson’s disease: New treatment modalities outside of pharmaceuticals and deep brain stimulation are being researched and approved by the FDA for the treatment of Parkinson’s disease symptoms. The non-invasive medical device, Exablate Neuro (approved by the FDA in 2021), uses focused ultrasound on one side of the brain to provide relief from severe symptoms such as tremors, limb rigidity, and dyskinesia. 2023 brought major news for Parkinson’s disease research with the validation of the biomarker alpha-synuclein. Researchers have developed a tool called the α-synuclein seeding amplification assay which detects the biomarker in the spinal fluid of people diagnosed with Parkinson’s disease and individuals who have not shown clinical symptoms.
- Amyotrophic lateral sclerosis (ALS): Two pharmaceuticals have seen FDA approval in the past two years to slow disease progression in individuals with ALS. Relyvrio ® was approved in 2022 and acts by preventing or slowing more neuron cell death in patients with ALS. Tofersen (Qalsody®), an antisense oligonucleotide, was approved in 2023 under the accelerated approval pathway. Tofersen targets RNA produced from mutated superoxide dismutase 1 (SOD1) genes to eliminate toxic SOD1 protein production. Recently published genetic research on how mutations contribute to ALS is ongoing with researchers recently discovering how NEK1 gene mutations lead to ALS. This discovery suggests a possible rational therapeutic approach to stabilizing microtubules in ALS patients.
Gain new perspectives for faster progress directly to your inbox.
Drive industry-leading advancements and accelerate breakthroughs by unlocking your data’s full potential. Contact our CAS Custom Services SM experts to find the digital solution to your information challenges.

The Epistemological Consequences of Artificial Intelligence, Precision Medicine, and Implantable Brain-Computer Interfaces
Article sidebar.
Main Article Content
I argue that this examination and appreciation for the shift to abductive reasoning should be extended to the intersection of neuroscience and novel brain-computer interfaces too. This paper highlights the implications of applying abductive reasoning to personalized implantable neurotechnologies. Then, it explores whether abductive reasoning is sufficient to justify insurance coverage for devices absent widespread clinical trials, which are better applied to one-size-fits-all treatments.
INTRODUCTION
In contrast to the classic model of randomized-control trials, often with a large number of subjects enrolled, precision medicine attempts to optimize therapeutic outcomes by focusing on the individual. [i] A recent publication highlights the strengths and weakness of both traditional evidence-based medicine and precision medicine. [ii] Plus, it outlines a tension in the shift from evidence-based medicine’s inductive reasoning style (the collection of data to postulate general theories) to precision medicine’s abductive reasoning style (the generation of an idea from the limited data available). [iii] The paper’s main example is the application of precision medicine for the treatment of cancer. [iv] I argue that this examination and appreciation for the shift to abductive reasoning should be extended to the intersection of neuroscience and novel brain-computer interfaces too.
As the name suggests, brain-computer interfaces are a significant advancement in neurotechnology that directly connects someone’s brain to external or implanted devices. [v] Among the various kinds of brain-computer interfaces, adaptive deep brain stimulation devices require numerous personalized adjustments to their settings during the implantation and computation stages in order to provide adequate relief to patients with treatment-resistant disorders. What makes these devices unique is how adaptive deep brain stimulation integrates a sensory component to initiate the stimulation. While not commonly at the level of sophistication as self-supervising or generative large language models, [vi] they currently allow for a semi-autonomous form of neuromodulation. This paper highlights the implications of applying abductive reasoning to personalized implantable neurotechnologies. Then, it explores whether abductive reasoning is sufficient to justify insurance coverage for devices absent widespread clinical trials, which are better applied to one-size-fits-all treatments. [vii]
I. The State of Precision Medicine in Oncology and the Epistemological Shift
While a thorough overview of precision medicine for the treatment of cancer is beyond the scope of this article, its practice can be roughly summarized as identifying clinically significant characteristics a patient possesses (e.g., genetic traits) to land on a specialized treatment option that, theoretically, should benefit the patient the most. [viii] However, in such a practice of stratification patients fall into smaller and smaller populations and the quality of evidence that can be applied to anyone outside these decreases in turn. [ix] As inductive logic helps to articulate, the greater the number of patients that respond to a particular therapy the higher the probability of its efficacy. By straying from this logical framework, precision medicine opens the treatment of cancer to more uncertainty about the validity of these approaches to the resulting disease subcategories. [x] Thus, while contemporary medical practices explicitly describe some treatments as “personalized”, they ought not be viewed as inherently better founded than other therapies. [xi]
A relevant contemporary case of precision medicine out of Norway focuses on the care of a patient with cancer between the ventricles of the heart and esophagus, which had failed to respond to the standard regimen of therapies over four years. [xii] In a last-ditch effort, the patient elected to pay out-of-pocket for an experimental immunotherapy (nivolumab) at a private hospital. He experienced marked improvements and a reduction in the size of the tumor. Understandably, the patient tried to pursue further rounds of nivolumab at a public hospital. However, the hospital initially declined to pay for it given the “lack of evidence from randomised clinical trials for this drug relating to this [patient’s] condition.” [xiii] In rebuttal to this claim, the patient countered that he was actually similar to a subpopulation of patients who responded in “open‐label, single arm, phase 2 studies on another immune therapy drug” (pembrolizumab). [xiv] Given this interpretation of the prior studies and the patient’s response, further rounds of nivolumab were approved. Had the patient not had improvements in the tumor’s size following a round of nivolumab, then pembrolizumab’s prior empirical evidence in isolation would have been insufficient, inductively speaking, to justify his continued use of nivolumab. [xv]
The case demonstrates a shift in reasoning from the traditional induction to abduction . The phenomenon of ‘cancer improvement’ is considered causally linked to nivolumab and its underlying physiological mechanisms. [xvi] However, “the weakness of abductions is that there may always be some other better, unknown explanation for an effect. The patient may for example belong to a special subgroup that spontaneously improves, or the change may be a placebo effect. This does not mean, however, that abductive inferences cannot be strong or reasonable, in the sense that they can make a conclusion probable .” [xvii] To demonstrate the limitations of relying on the abductive standard in isolation, commentators have pointed out that side effects in precision medicine are hard to rule out as being related to the initial intervention itself unless trends from a group of patients are taken into consideration. [xviii]
As artificial intelligence (AI) assists the development of precision medicine for oncology, this uncertainty ought to be taken into consideration. The implementation of AI has been crucial to the development of precision medicine by providing a way to combine large patient datasets or a single patient with a large number of unique variables with machine learning to recommend matches based on statistics and probability of success upon which practitioners can base medical recommendations. [xix] The AI is usually not establishing a causal relationship [xx] – it is predicting. So, as AI bleeds into medical devices, like brain-computer interfaces, the same cautions about using abductive reasoning alone should be carried over.
II. Responsive Neurostimulation, AI, and Personalized Medicine
Like precision medicine in cancer treatment, computer-brain interface technology similarly focuses on the individual patient through personalized settings. In order to properly expose the intersection of AI, precision medicine, abductive reasoning, and implantable neurotechnologies, the descriptions of adaptive deep brain stimulation systems need to deepen. [xxi] As a broad summary of adaptive deep brain stimulation, to provide a patient with the therapeutic stimulation, a neural signal, typically referred to as a local field potential, [xxii] must first be detected and then interpreted by the device. The main adaptive deep brain stimulation device with premarket approval, the NeuroPace Responsive Neurostimulation system, is used to treat epilepsy by detecting and storing “programmer-defined phenomena.” [xxiii] Providers can optimize the detection settings of the device to align with the patient’s unique electrographic seizures as well as personalize the reacting stimulation’s parameters. [xxiv] The provider adjusts the technology based on trial and error. One day machine learning algorithms will be able to regularly aid this process in myriad ways, such as by identifying the specific stimulation settings a patient may respond to ahead of time based on their electrophysiological signatures. [xxv] Either way, with AI or programmers, adaptive neurostimulation technologies are individualized and therefore operate in line with precision medicine rather than standard treatments based on large clinical trials.
Contemporary neurostimulation devices are not usually sophisticated enough to be prominent in AI discussions where the topics of neural networks, deep learning, generative models, and self-attention dominate the conversation. However, implantable high-density electrocorticography arrays (a much more sensitive version than adaptive deep brain stimulation systems use) have been used in combination with neural networks to help patients with neurologic deficits from a prior stroke “speak” through a virtual avatar. [xxvi] In some experimental situations, algorithms are optimizing stimulation parameters with increasing levels of independence. [xxvii] An example of neurostimulation that is analogous to the use of nivolumab in Norway surrounds a patient in the United States who was experiencing both treatment-resistant OCD and temporal lobe epilepsy. [xxviii] Given the refractory nature of her epilepsy, implantation of an adaptive deep brain stimulation system was indicated. As a form of experimental therapy, her treatment-resistant OCD was also indicated for the off-label use of an adaptive deep brain stimulation set-up. Another deep brain stimulation lead, other than the one implanted for epilepsy, was placed in the patient’s right nucleus accumbens and ventral pallidum region given the correlation these nuclei had with OCD symptoms in prior research. Following this, the patient underwent “1) ambulatory, patient-initiated magnet-swipe storage of data during moments of obsessive thoughts; (2) lab-based, naturalistic provocation of OCD-related distress (naturalistic provocation task); and (3) lab-based, VR [virtual reality] provocation of OCD-related distress (VR provocation task).” [xxix] Such signals were used to identify when to deliver the therapeutic stimulation in order to counter the OCD symptoms. Thankfully, following the procedure and calibration the patient exhibited marked improvements in their OCD symptoms and recently shared her results publicly. [xxx]
In both cases, there is a similar level of abductive justification for the efficacy of the delivered therapy. In the case study in which the patient was treated with adaptive deep brain stimulation, they at least had their neural activity tested in various settings to determine the optimum parameters for treatment to avoid them being based on guesswork. Additionally, the adaptive deep brain stimulation lead was already placed before the calibration trials were conducted, meaning that the patient had already taken on the bulk of the procedural risk before the efficacy could be determined. Such an efficacy test could have been replicated in the first patient’s cancer treatment, had it been biopsied and tested against the remaining immunotherapies in vitro . Yet, in the case of cancer with few options, one previous dose of a drug that appeared to work on the patient may justify further doses. However, as the Norwegian case presents, corroboration with known responses to a similar drug (from a clinical trial) could be helpful to validate the treatment strategy. (It should be noted that both patients were resigned to these last resort options regardless of the efficacy of treatment.)
There are some elements of inductive logic seen with adaptive deep brain stimulation research in general. For example, abductively the focus could be that patient X’s stimulation parameters are different from patient Y’s and patient Z’s. In contrast, when grouped as subjects who obtained personalized stimulation, patients X, Y, and Z demonstrate an inductive aspect to this approach’s safety and/or efficacy. The OCD case holds plenty of abductive characteristics in line with precision medicine’s approach to treating cancer and as more individuals try the method, there will be additional data. With the gradual integration of AI into brain-computer interfaces in the name of efficacy, this reliance on abduction will continue, if not grow, over time. Moving forward, if a responsive deep brain stimulation treatment is novel and individualized (like the dose of nivolumab) and there is some other suggestion of efficacy (like clinical similarities to other patients in the literature), then it may justify insurance coverage for the investigative intervention, absent other unrelated reasons to deny it.
III. Ethical Implications and Next Steps
While AI’s use in oncology and neurology is not yet as prominent as its use in other fields (e.g., radiology), it appears to be on the horizon for both. [xxxi] AI can be found in both the functioning of the neurotechnologies as well as the implementation of precision medicine. The increasing use of AI may serve to further individualize both oncologic and neurological therapies. Given these implications and the handful of publications cited in this article, it is important to have a nuanced evaluation of how these treatments, which heavily rely on abductive justification, ought to be managed.
The just use an abductive approach may be difficult as AI infused precision medicine is further pursued. At baseline, such technology relies on a level of advanced technology literacy among the general public and could exclude populations who lack access to basic technological infrastructure or know-how from participation. [xxxii] Even among nations with adequate infrastructure, as more patients seek out implantable neurotechnologies, which require robust healthcare resources, the market will favor patient populations that can afford this complex care. [xxxiii]
If patients already have the means to pay for an initial dose/use of a precision medicine product out of pocket, should insurance providers be required to cover subsequent treatments? [xxxiv] That is, if a first dose of a cancer drug or a deep brain stimulator over its initial battery life is successful, patients may feel justified in having the costs of further treatments covered. The Norwegian patient’s experience implies there is a precedent for the idea that some public insurance companies ought to cover successful cancer therapies, however, insurance companies may not all see themselves as obligated to cover neurotechnologies that rely on personalized settings or that are based on precision/abductive research more than on clinical trials.
The fact that the cases outlined above rely on abductive style of reasoning implies that there may not be as strong a justification for coverage by insurance, as they are both experimental and individualized, when compared to the more traditional large clinical trials in which groups have the same or a standardized protocol (settings/doses). If a study is examining the efficacy of a treatment with a large cohort of patients or with different experimental groups/phases, insurance companies may conclude that the resulting symptom improvements are more likely to be coming from the devices themselves. A preference for inductive justification may take priority when ruling in favor of funding someone’s continued use of an implantable neurostimulator. There are further nuances to this discussion surrounding the classifications of these interventions as research versus clinical care that warrant future exploration, since such a distinction is more of a scale [xxxv] than binary and could have significant impacts on the “right-to-try” approach to experimental therapies in the United States. [xxxvi] Namely, given the inherent limitations of conducting large cohort trials for deep brain stimulation interventions on patients with neuropsychiatric disorders, surgically innovative frameworks that blend abductive and inductive methodologies, like with sham stimulation phases, have traditionally been used. [xxxvii] Similarly, for adaptive brain-computer interface systems, if there are no large clinical trials and instead only publications that demonstrate that something similar worked for someone else, then, in addition to the evidence that the first treatment/dose worked for the patient in question, the balance of reasoning would be valid and arguably justify insurance coverage. As precision approaches to neurotechnology become more common, frameworks for evaluating efficacy will be crucial both for insurance coverage and for clinical decision making.
ACKNOWLEDGEMENT
This article was originally written as an assignment for Dr. Francis Shen’s “Bioethics & AI” course at Harvard’s Center for Bioethics. I would like to thank Dr. Shen for his comments as well as my colleagues in the Lázaro-Muñoz Lab for their feedback.
[i] Jonathan Kimmelman and Ian Tannock, “The Paradox of Precision Medicine,” Nature Reviews. Clinical Oncology 15, no. 6 (June 2018): 341–42, https://doi.org/10.1038/s41571-018-0016-0.
[ii] Henrik Vogt and Bjørn Hofmann, “How Precision Medicine Changes Medical Epistemology: A Formative Case from Norway,” Journal of Evaluation in Clinical Practice 28, no. 6 (December 2022): 1205–12, https://doi.org/10.1111/jep.13649.
[iii] David Barrett and Ahtisham Younas, “Induction, Deduction and Abduction,” Evidence-Based Nursing 27, no. 1 (January 1, 2024): 6–7, https://doi.org/10.1136/ebnurs-2023-103873.
[iv] Vogt and Hofmann, “How Precision Medicine Changes Medical Epistemology,” 1208.
[v] Wireko Andrew Awuah et al., “Bridging Minds and Machines: The Recent Advances of Brain-Computer Interfaces in Neurological and Neurosurgical Applications,” World Neurosurgery , May 22, 2024, S1878-8750(24)00867-2, https://doi.org/10.1016/j.wneu.2024.05.104.
[vi] Mark Riedl, “A Very Gentle Introduction to Large Language Models without the Hype,” Medium (blog), May 25, 2023, https://mark-riedl.medium.com/a-very-gentle-introduction-to-large-language-models-without-the-hype-5f67941fa59e.
[vii] David E. Burdette and Barbara E. Swartz, “Chapter 4 - Responsive Neurostimulation,” in Neurostimulation for Epilepsy , ed. Vikram R. Rao (Academic Press, 2023), 97–132, https://doi.org/10.1016/B978-0-323-91702-5.00002-5.
[viii] Kimmelman and Tannock, 2018.
[ix] Kimmelman and Tannock, 2018.
[x] Simon Lohse, “Mapping Uncertainty in Precision Medicine: A Systematic Scoping Review,” Journal of Evaluation in Clinical Practice 29, no. 3 (April 2023): 554–64, https://doi.org/10.1111/jep.13789.
[xi] Kimmelman and Tannock, “The Paradox of Precision Medicine.”
[xii] Vogt and Hofmann, 1206.
[xiii] Vogt and Hofmann, 1206.
[xiv] Vogt and Hofmann, 1206.
[xv] Vogt and Hofmann, 1207.
[xvi] Vogt and Hofmann, 1207.
[xvii] Vogt and Hofmann, 1207.
[xviii] Vogt and Hofmann, 1210.
[xix] Mehar Sahu et al., “Chapter Three - Artificial Intelligence and Machine Learning in Precision Medicine: A Paradigm Shift in Big Data Analysis,” in Progress in Molecular Biology and Translational Science , ed. David B. Teplow, vol. 190, 1 vols., Precision Medicine (Academic Press, 2022), 57–100, https://doi.org/10.1016/bs.pmbts.2022.03.002.
[xx] Stefan Feuerriegel et al., “Causal Machine Learning for Predicting Treatment Outcomes,” Nature Medicine 30, no. 4 (April 2024): 958–68, https://doi.org/10.1038/s41591-024-02902-1.
[xxi] Sunderland Baker et al., “Ethical Considerations in Closed Loop Deep Brain Stimulation,” Deep Brain Stimulation 3 (October 1, 2023): 8–15, https://doi.org/10.1016/j.jdbs.2023.11.001.
[xxii] David Haslacher et al., “AI for Brain-Computer Interfaces,” 2024, 7, https://doi.org/10.1016/bs.dnb.2024.02.003.
[xxiii] Burdette and Swartz, “Chapter 4 - Responsive Neurostimulation,” 103–4; “Premarket Approval (PMA),” https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100026.
[xxiv] Burdette and Swartz, “Chapter 4 - Responsive Neurostimulation,” 104.
[xxv] Burdette and Swartz, 126.
[xxvi] Sean L. Metzger et al., “A High-Performance Neuroprosthesis for Speech Decoding and Avatar Control,” Nature 620, no. 7976 (August 2023): 1037–46, https://doi.org/10.1038/s41586-023-06443-4.
[xxvii] Hao Fang and Yuxiao Yang, “Predictive Neuromodulation of Cingulo-Frontal Neural Dynamics in Major Depressive Disorder Using a Brain-Computer Interface System: A Simulation Study,” Frontiers in Computational Neuroscience 17 (March 6, 2023), https://doi.org/10.3389/fncom.2023.1119685; Mahsa Malekmohammadi et al., “Kinematic Adaptive Deep Brain Stimulation for Resting Tremor in Parkinson’s Disease,” Movement Disorders 31, no. 3 (2016): 426–28, https://doi.org/10.1002/mds.26482.
[xxviii] Young-Hoon Nho et al., “Responsive Deep Brain Stimulation Guided by Ventral Striatal Electrophysiology of Obsession Durably Ameliorates Compulsion,” Neuron 0, no. 0 (October 20, 2023), https://doi.org/10.1016/j.neuron.2023.09.034.
[xxix] Nho et al.
[xxx] Nho et al.; Erik Robinson, “Brain Implant at OHSU Successfully Controls Both Seizures and OCD,” OHSU News, accessed March 3, 2024, https://news.ohsu.edu/2023/10/25/brain-implant-at-ohsu-successfully-controls-both-seizures-and-ocd.
[xxxi] Awuah et al., “Bridging Minds and Machines”; Haslacher et al., “AI for Brain-Computer Interfaces.”
[xxxii] Awuah et al., “Bridging Minds and Machines.”
[xxxiii] Sara Green, Barbara Prainsack, and Maya Sabatello, “The Roots of (in)Equity in Precision Medicine: Gaps in the Discourse,” Personalized Medicine 21, no. 1 (January 2024): 5–9, https://doi.org/10.2217/pme-2023-0097.
[xxxiv] Green, Prainsack, and Sabatello, 7.
[xxxv] Robyn Bluhm and Kirstin Borgerson, “An Epistemic Argument for Research-Practice Integration in Medicine,” The Journal of Medicine and Philosophy: A Forum for Bioethics and Philosophy of Medicine 43, no. 4 (July 9, 2018): 469–84, https://doi.org/10.1093/jmp/jhy009.
[xxxvi] Vijay Mahant, “‘Right-to-Try’ Experimental Drugs: An Overview,” Journal of Translational Medicine 18 (June 23, 2020): 253, https://doi.org/10.1186/s12967-020-02427-4.
[xxxvii] Michael S. Okun et al., “Deep Brain Stimulation in the Internal Capsule and Nucleus Accumbens Region: Responses Observed during Active and Sham Programming,” Journal of Neurology, Neurosurgery & Psychiatry 78, no. 3 (March 1, 2007): 310–14, https://doi.org/10.1136/jnnp.2006.095315.
Ian Stevens
MA Philosophy University of Tasmania in Australia, MS Bioethics Harvard Medical School Center for Bioethics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.30(1); 2023
Recent advances in drug delivery systems for targeting brain tumors
a Department of Translational Medicine Center, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
b The Academy of Medical Science, College of Medical, Zhengzhou University, Zhengzhou, China
c Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu, China
Yuanyuan Sun
d Department of Orthopedics, the First Affiliated Hospital of Henan Polytechnic University (the Second People’s Hospital of Jiaozuo City), Jiaozuo, China
Bingjie Han
Brain tumor accounts for about 1.6% of incidence and 2.5% of mortality of all tumors, and the median survival for brain tumor patients is only about 20 months. The treatment for brain tumor still faces many challenges, such as the blood-brain barrier (BBB), blood-brain tumor barrier (BBTB), the overexpressed efflux pumps, the infiltration, invasion, high heterogeneity of tumor cells, drug resistance and immune escape caused by tumor microenvironment (TME) and cancer stem cells (CSC). This review attempts to clarify the challenges for multi-functional nano drug delivery systems (NDDS) to cross the BBB and target the cancer cells or organelles, and also provides a brief description of the different types of targeted multi-functional NDDS that have shown potential for success in delivering drugs to the brain. Further, this review also summarizes the research progress of multi-functional NDDS in the combination therapy of brain tumors from the following sections, the combination of chemotherapy drugs, chemotherapy-chemodynamic combination therapy, chemotherapy-immunization combination therapy, and chemotherapy-gene combination therapy. We also provide an insight into the recent advances in designing multi-functional NDDS for combination therapy.
1. Introduction
Brain tumor accounts for about 1.6% of incidence and 2.5% of mortality of all tumors respectively according to the latest global cancer data released by the World Health Organization (WHO) in 2020 (Sung et al., 2021 ). While, in China, the morbidity and mortality of brain tumors rank first in the global brain tumors, up to 32% and 26% respectively, and the incidence rate is still rising year by year and younger (Patel et al., 2019 ). Among the brain tumors, glioma, the most common and invasive type of brain tumor, with the characteristics of strong invasion, high recurrence rate and poor prognosis, accounts for 30% of all brain tumors (Reifenberger et al., 2017 ; Lin et al., 2020 ).
Surgery is the treatment of choice for brain tumors, but the invasiveness and fuzzy boundary make it difficult to completely remove the tumor. And, the postoperative recurrence rate is more than 90% (Ganz, 2022 ). Moreover, postoperative chemotherapy and radiotherapy have become the standard therapy for brain tumors. An alkylating agent, temozolomide (TMZ), functions as a first-line chemotherapy drug for brain tumors and delivers the methyl group to purine bases of DNA to cause cell death (Zhang et al., 2012 ). However, the increased dosage due to its short half-life, which leading to a series of side effects, such as thrombocytopenia, neutropenia and lymphopenia. In addition, the tumor cells may become resistant to TMZ due to the dysregulation of signaling pathways, DNA repair, autophagy and other related mechanisms (Yan et al., 2016 ). Apart from TMZ, bevacizumab has been approved by USA FDA for the treatment of brain tumors as a VEGFR inhibitor. However, this anti-angiogenesis therapy has failed to improve the overall survival of patients, and its use remains controversial (Ozdemir-Kaynak et al., 2018 ). Other therapeutic drugs, such as nitrosoureas (carmustine, lomustine), anthracyclines (adriamycin), platinums (cisplatin, carboplatin, oxaliplatin), topoisomerase inhibitors (camptothecin, irinotecan, etoposide), integrin receptor inhibitors (cilengitide), EGFR inhibitors (erlotinib, gefitinib, afatinib), and histone deacetylase inhibitors (vorinostat, panobinostat), are difficult to become specific drugs for the treatment of brain tumors due to the low efficacy and severe toxic and side effects (Aparicio-Blanco et al., 2020 ). New therapies such as gene therapy, angiogenesis inhibition and immunotherapy have shown potential but limited efficacy in the treatment of glioma (Sousa et al., 2019 ; Weenink et al., 2020 ; Chelliah et al., 2021 ; Conniot et al., 2021 ). Therefore, there is an urgent need to develop high-efficiency, low-toxicity and specific drugs for brain tumors.
2. Challenges in developing drugs for brain cancer
Compared with peripheral tumors, the treatment of brain tumors faces many challenges ( Figure 1 ). On the one hand, the physiological barriers (such as blood-brain barrier (BBB), blood-brain tumor barrier (BBTB)) and the over-expressed efflux pumps prevent drugs from entering the central nervous system (CNS) and reaching the tumor site. On the other hand, the inherent characteristics of brain tumors, such as the infiltration, invasion, high heterogeneity, drug resistance and immune escape caused by tumor microenvironment (TME) and cancer stem cells (CSC), further restrict the therapeutic effects, which leading to high failure rate and recurrence rate (Zhao et al., 2020 ). The median survival of brain tumor patients receiving standard therapy is only about 20 months, and the 2- and 5-year survival rates are only 27% and 10%, respectively (Ashby et al., 2016 ).

Challenges in treatment for brain cancer (Drawn by Figdraw).
2.1. Blood-brain barrier and blood-brain tumor barrier
The BBB consists of brain capillary endothelial cells (BCEC), pericytes, astrocytes, neurons, and basement membranes, and it plays a crucial role in the transport of endogenous and exogenous molecules between the blood and the brain (Zhao et al., 2022 ). The BBB prevents all the macromolecular drugs and over 98% of small molecule drugs to permeate into CNS based on the following mechanisms. (1) Paracellular barrier: The tight junctions between BCEC cells strictly limit the passive diffusion of drugs into CNS, and only lipophilic substances and hydrophilic small molecules are allowed to enter the brain. (2) Transcellular barrier: The endocytosis activity is lower in BCEC cells than that in other brain cells, which significantly limits the transcellular transport of drugs across the BBB (Azarmi et al., 2020 ). (3) Enzyme barrier: The BBB cells have a strong metabolic capacity due to the significant high expression of peptidase, phosphatase, nucleotidase, esterase, and cytochrome P450 enzymes in BCEC, which enhances the ability to degrade drugs (Alexander, 2018 ). (4) Immunologic barrier: The microglia, mastocyte, and macrophages form an immunologic barrier to accelerate the clearance of drugs (Alexander, 2018 ). (5) Efflux proteins: The efflux proteins, such as ATP-binding cassette transporters (P-gp, BCRP, MRPs) and solute carrier transporters, are over-expressed at the BBB that actively pump out the drugs and limit the permeability (Saidijam et al., 2018 ). And it is also one of the main reasons for drug resistance in brain tumors.
When the brain tumor becomes larger than 2 mm 3 , the progression of angiogenesis results in the loss of normal functions and integrity of the BBB, and the BBTB comes into being (Mojarad-Jabali et al., 2021 ). Passive tumor targeting via enhanced permeability and retention (EPR) effect has long been considered as the most effective mechanism for the accumulation of nanoparticles. Whereas, the vascular pore size of brain tumors is much smaller (only 7 ∼ 100 nm) and the EPR is much weaker. Therefore, it is still difficult for drugs to reach brain tumor sites through the EPR effect (Caro et al., 2021 ). Hence, BBTB is considered to be another major obstacle for the drug transport in the treatment of brain tumors, critically restricting the delivery of drugs to tumor tissues.
2.2. Infiltration and invasion
The brain tumor cells show an aggressive characteristic against the surrounding tissues. Even individual brain tumor cell can infiltrate normal tissues and form tumors through the following steps. The brain tumor cells migrate and accumulate at the nearby vessels, and secrete the glioma-derived factors, such as TGF-β2, reactive oxygen species (ROS), and proinflammatory peptides that disrupt the normal contact between endothelial cells and the basement membrane. Subsequently, the factors induce and activate matrix metalloproteinases (MMPs), which further induce the degradation of tight junctions by downregulating claudin proteins. These processes contribute to the degradation of the vascular basement membrane and extracellular matrix, migration of endothelial cells, and the formation of abnormal new blood vessels due to VEGF overexpression (Ishihara et al., 2008 ; Dubois et al., 2014 ; Oishi et al., 2022 ). Therefore, the abnormally rapid proliferation of the vasculature leads to the function loss of tight junctions, i.e. the destruction of the BBB, which conduces to the infiltrative growth of the tumor with blurred tumor margins and metastasis.
2.3. Brain cancer stem cells
The cell subsets in brain tumors show stem cell-like characteristics and express stem cell markers, including CD133, A2B5 and EGFRvIII (Emlet et al., 2014 ; Ishii et al., 2021 ; Smiley et al., 2021 ). The stem cells exhibit the following characteristics. (1) Aggressiveness: Including the highly migratory and invasive, and the resistance to chemoradiotherapy. (2) Similar to normal stem cells or progenitor cells, the CSC can self-renew and differentiate into different types of cancer cell lines in specific tumor tissues. (3) Drug resistance: The multidrug resistance (MDR) of CSC is embodied in repairing DNA damage and excreting harmful substances (Phi et al., 2018 ). In addition, CSC can also enhance the transcription of anti-apoptotic genes and efflux transporters, and the angiogenesis. Although standard therapy kills most tumor cells, stem cells that have invaded the brain parenchyma will eventually lead to disease recurrence due to the invasiveness, resistance, self-renewal, and differentiation (Alcantara Llaguno & Parada, 2021 ). Therefore, eradicating tumor stem cells is an important research field to overcome MDR and improve the efficiency of tumor treatment.
2.4. Immune escape
The BBB prevents the entry of most harmful components, leaving the brain in a relatively safe environment and rarely launching immune attacks. Once the brain cells are attacked by autoimmunity, the consequences are serious. Therefore, the immune system of CNS is usually inhibited. On the other hand, the dogma has been established that the CNS lacks normal running lymphatic and dendritic cells for antigen presentation (D'Agostino et al., 2012 ). In consequence, active immune surveillance in the CNS rarely occurs, which provides a safe environment for tumor growth (Rustenhoven & Kipnis, 2019 ). Immunotherapy has been proved to have therapeutic potential for various solid tumors, including melanoma and non-small cell lung cancer (Waldman et al., 2020 ). However, the current immunotherapy has not been confirmed to significantly improve the survival rate of patients with brain tumors in clinic. It is mainly because the immune components such as antibodies and immune cells cannot enter the CNS through BBB (Desbaillets & Hottinger, 2021 ).
2.5. Tumor microenvironment
The tumor microenvironment includes tumor cells, tumor stem cells, blood vessels, lymphatic, immune cells, fibroblasts, and extracellular matrix, which provides a suitable environment for the growth, division, angiogenesis and metastasis of tumor cells (Petrova et al., 2018 ). And TME protects tumor cells mainly through the following mechanisms. The increased activity of vascular endothelial growth factor leads to the high proliferation of microvessels. Tumor cells interact with secreted cytokines or growth factors to obtain nutrients from abnormal blood vessels, which in turn induce fibroblasts and macrophages to proliferate and invade, resulting in drug resistance. The cross-linking structure of extracellular matrix formed by the fibrous collagen, proteoglycan, stromal cell protein and hyaluronic acid prevents drugs from reaching tumor cells through the microenvironment, thus resisting the drugs treatment. In addition to providing integral structure, extracellular matrix also contributes to the transport of nutrients and oxygen, thereby promoting tumor initiation and progression.
3. Design strategy of brain tumor targeting nano drug delivery system
Nano drug delivery system has unique advantages in drug delivery. The appropriate physicochemical properties including solubility, particle size, potential, and morphology contribute to improving the pharmacokinetics and tissue distribution. What’s more, surface modification may enhance the accumulation of drugs in the target tissue to improve the therapeutic effect. In addition, the NDDS has specific drug release behavior, which increases the concentration of drug in the target site and reduces the concentration of drugs in the non-target site, thereby reducing adverse reactions. Furthermore, the NDDS is easy to realize the combined treatment to achieve synergistic effects. Therefore, NDDS provides an excellent platform for the study of brain tumor-targeted drugs (Yeini et al., 2021 ). The commonly used design strategies are optimizing the physicochemical properties, overcoming the BBB and BBTB, introducing stimulus-responsive functional groups, and targeting organelles, et al.
3.1. Optimizing the physicochemical properties
The size, surface charge, morphology, and surface modification of nanoparticles influence the drug circulation in the blood and accumulation in the brain, which should be taken into consideration when designing NDDS.
Nanocarriers preferentially accumulate in tumor through passive targeting due to the leaky vasculature and defective lymphatic drainage (Subhan et al., 2021 ). Nanostructures smaller than 10 nm are rapidly cleared by the kidney, while larger than 200 nm are easily cleared by the liver and recognized by the reticuloendothelial system (RES) to reduce the circulation time in the blood (Kibria et al., 2013 ; Golombek et al., 2018 ). In addition, the vascular aperture of brain tumors is only 7 ∼ 100 nm, which is much smaller than that of other tumors. Moreover, the EPR effect is also much weaker than that of peripheral tumors. Therefore, the particle size of 10 ∼ 100 nm seems to be more efficient in crossing the BBB and delivering drugs into the brain.
The charge of nanoparticles has an effect on the interaction between nanomaterials and cells (Sanita et al., 2020 ). Compared with neutral nanoparticles, the charged nanoparticles have the following advantages. (1) High stability: Due to the lack of electrostatic interaction, the neutral nanoparticles have low physical stability and can’t inhibit the self-aggregation of nanoparticles. Meanwhile, the charges on the surface prevent nanoparticles from polymerizing and flocculating by zeta electrostatic interaction. (2) High permeability: Nanoparticles can interact with cells through surface electrostatic charges, so as to improve the accumulation of drugs in cells (Smith et al., 2017 ). For example, cationic nanoparticles interact with negatively charged BBB and are transported into the brain (Lombardo et al., 2020 ). However, cationic nanoparticles are easy to be rapidly cleared by RES and the positive surface charge leads to systemic adverse effects.
The morphology of nanoparticles also influences on the distributions. As reported, the cell uptake of rod-shaped particles (larger than 100 nm) is superior to spherical, cylindrical and cubic nanoparticles (Jia et al., 2021 ). However, spherical nanoparticles show the highest absorption when the diameter is less than 100 nm (Qiu et al., 2010 ).
The interaction between nanoparticles and biological microenvironment is an important factor to influence the fate of particles in vivo. This interaction depends not only on the physicochemical properties, but also on the surface modification and biomolecules in the biological environment. Qie et al. prepare the nanoparticles coated with polyethylene glycol (PEG) and CD47 to avoid the phagocytosis by macrophages (Qie et al., 2016 ). Although the PEGylation reduces the capacity of nanoparticles to adsorb a variety of soluble proteins, the immunogenicity is a potential limiting factor that may lead to the increased clearance rate and decreased efficacy of PEGylated nanoparticles upon repeated administration, which is known as accelerated blood clearance (ABC) phenomenon (Mohamed et al., 2019 ).
3.2. Overcoming the BBB and BBTB
Paracellular and transcellular transport are the main routes for substances to cross BBB. The paracellular transport is restricted by the tight junction between endothelial cells, and can only transport micromolecule (such as CO 2 , O 2 , H 2 O and C 2 H 5 OH) across the BBB. Transcellular transport includes passive diffusion and endocytosis (Hersh et al., 2016 ), and the former is only applicable for lipophilic drugs below 500 Da (Grabrucker et al., 2016 ). Therefore, the transport of drugs across BBB mainly depends on endocytosis, including carrier-mediated, receptor-mediated, adsorption-mediated and cell-mediated transport ( Figure 2 ) (Chen & Liu, 2012 ).

Schematic diagram of drug transport across the BBB (Drawn by Figdraw).
3.2.1. Carrier-mediated transport
There are many transporters on the BBB for the transportation of nutrients, such as the glucose transporter 1 (GLUT1), vitamin C transporter 2 (SVCT2), Na + -dependent vitamin transporter (SMVT), L-amino acid transporter 1 (LAT1), monocarboxylic acid transporter 1 (MCT1), et al (Zhao et al., 2014 ; Jiang et al., 2021 ; Zhao et al., 2021 ). The high affinity between these transporters and substrates facilitates the substrate crossing the BBB through carrier-mediated transport. Modification of the NDDS with the substrates or their analogues can promote drugs entry into the brain. As shown in Figure 3 , our group has designed the liposome ligands modified with glucose, vitamin C, biotin, glucose-vitamin C (Glu-Vc), and glucose-biotin (Glu-Bio) to enhance the drug transport through the highly expressed GLUT1, SVCT2, SMVT transporter on the BBB, which significantly improves the ability of drugs to enter the CNS in different degrees (Lei et al., 2011 ; Peng et al., 2018 ; Huang et al., 2020 ).

The structures of liposome ligands Glu-Chol, Vc-Chol, Bio-Chol, Glu-Vc-Chol, and Glu-Bio-Chol.
3.2.2. Receptor-mediated transport
Receptor-mediated transport is the primary way for internalizing large biomolecules and growth factors in the brain, and is the most widely used strategy in brain tumor targeted drug delivery. The low density lipoprotein receptor (LDL-R), apolipoprotein E (ApoE) receptor, epidermal growth factor receptor (EGFR), transferrin receptor (TfR), insulin receptor (IR) and integrin receptor (αvβ3) are the commonly used targets that promote the delivery of drugs into the brain. Therefore, the ligands modification on the NDDS can specifically bind to the receptor on BBB, which effectively increases the drug concentration in the brain. In our previous studies, the RGD-modified liposomes that could be mediated by αvβ3 across the BBB ( Figure 4 ), and the distributions of drugs in brain are improved with 2.44 and 4.72 times than that of naked drug (Fu et al., 2019 ).

The structures of liposome ligands RGD-Chol and Glu-RGD-Chol.
3.2.3. Adsorption-mediated transport
When the nanoparticles are modified with cationic components, such as protamine, cell-penetrating peptide (CPP), etc., they bind to the anion membrane of brain microvascular endothelial cells to promote endocytosis by the cells. However, the adsorption-mediated transport does not involve the specific binding to the cell membrane, so the cationic nanoparticles can also enter normal tissues and cause inevitable side effects (Meng et al., 2017 ). The CPPs are the most frequently-used ligands for adsorption-mediated transport, which remain electropositive under physiological conditions due to the abundant arginine and lysine residues. What’s more, the interaction with anionic substances on the endothelial cell membrane further enhances the cell uptake (Herve et al., 2008 ). Due to the lack of specific adsorption of CPP on tumor cells, most studies utilize the CPP combined with tumor targeted ligands to prepare the dual- or multiple-targeting NDDS. For example, Sun et al. synthesize the copolymer TfR-T12-PEG-PLGA targeting transferrin receptor and the CPP-modified polymer TATH7-PEG-PLGA (Sun et al., 2022 ). The nano polymer micelles are prepared with the polymers for synchronously delivery of paclitaxel (PTX) and immunomodulator imiquimod. The modification of TfR-T12 peptide can achieve the targeted delivery of chemotherapeutic drugs through the BBB mediated by TfR. The pH-sensitive TATH7 can increase the uptake efficiency for the micelles by tumor cells through adsorption-mediated in pH 5.5 medium than that under pH 7.4 medium. Therefore, the micelles have enhanced the therapeutic effect on brain tumors through chemotherapy and immunotherapy.
3.2.4. Cell-mediated transport
Nanoparticles can also cross the BBB through cell-mediated transport, called ‘Trojan’. In general, leukocytes and stem cells are widely used as carriers for cell-mediated transport to deliver nanoparticles to the target region. These biomimetic delivery systems have unique advantages compared with other delivery systems, including prolonged blood circulation time and biological half-life, low immunogenicity, and enhanced biocompatibility (Charabati et al., 2020 ). In recent years, the application of several leukocytes, such as neutrophils and mononuclear macrophages, has made good progress in brain-targeted drug delivery across the BBB (Pang et al., 2018 ; Wu et al., 2018 ). Wu et al. developed the inflammation-activatable engineered neutrophils by internalizing doxorubicin-loaded magnetic mesoporous silica nanoparticles (ND-MMSNs) (Wu et al., 2018 ). After systemic injection of ND-MMSNs, the nanoparticles migrate along the molecular guidance signals, and accumulate in the inflamed glioma sites. Subsequently, highly activated neutrophils carrying D-MMSNs release neutrophil extracellular traps in the inflammatory region. In the meanwhile the drug-loaded nanoparticles were released and uptaken by infiltrating glioma cells, achieving visualization of diagnosis and treatment of postoperative glioma. Pang et al. prepared M1 macrophage-loaded nanoparticles (M1-NPs) by incubating poly(lactide-co-glycolide) nanoparticles with primary M1 macrophages for glioma therapy (Pang et al., 2018 ). The macrophages that present a strong phagocytic capacity to incorporate the drug-loaded NPs were able to effectively migrate and infiltrate into orthotopic glioma tumor models for DOX release. It was noteworthy that M1-NPs significantly prolonged mice survival with median survival 38.5 days (PBS group, 21 days). What’s more, the M1-NPs also increased caspase-3 protein expression. All the results indicated that DOX@M1-NPs exhibited a significant improvement in anti-tumor activities.
3.2.5. Reducing drug efflux
As mentioned above, the high expressed efflux proteins (such as P-glycoprotein, P-gp) on the BBB are also important factors that affect the entry of drugs into brain tissue. Therefore, reducing the expression of efflux proteins or inhibiting its activity is an important means to increase the drug concentration in the CNS to improve the therapeutic effect and reverse drug resistance. For example, the ‘cocktail’ liposomes co-loading with verapamil and riluzole overcome drug resistance by inhibiting P-gp in brain endothelial cells and astrocytes (Tang et al., 2015 ).
3.2.6. Overcoming the blood-brain tumor barrier
Compared with BBB, BBTB has the higher permeability, so the nanoparticles can cross BBTB through the EPR effect. On the other hand, many receptors are overexpressed on BBTB, such as EGFR, matrix metalloproteinase-2 (MMP-2), TfR, interleukin-13 receptor (IL-13R), etc, are widely used for targeting BBTB. The ideal brain targeted drug delivery systems could not only overcome the barriers of BBB, but also overcome BBTB and selectively target cancer cells, thereby reducing the distribution in normal brain cells. Therefore, it is urgently needed to develop a dual-targeted drug delivery system with BBB-targeting and BBTB-targeting capabilities. The widely used carriers/receptors are highly expressed in the cells and their ligands are shown in Table 1 . Our group have designed a novel dual-targeting ligand modified with glucose and RGD (Glu-RGD-Chol, Figure 4 ). PTX-loaded liposome is prepared with this ligand, which contributes to crossing BBB and targeting glioma (Fu et al., 2019 ). Compared with naked PTX, the brain targeting performance of this dual targeting liposome was increased with 4.41 times.
The transporters/receptors and their ligands targeting blood-brain barrier and brain tumors.
| Target | BBB | Tumor | Ligand | |
| Transporter | GLUT | √ | √ | Glucose, Glucose analogues (2-deoxy-D-glucose), other hexoses (Mannose, Galactose, Glucosamine) |
| GSH transporter | √ | × | GSH | |
| LAT1 | √ | × | Large neutral amino acids (L-tyrosine, L-phenylalanine, L-leucin, L-isoleucine) | |
| MCT | √ | × | Lactate, Pyruvate, Biotin, Sialic acid | |
| Nucleoside transporter | √ | × | Adenosine, Guanosine, Uridine | |
| Choline transporter | √ | × | Choline, Thiamin | |
| Receptor | TfR | √ | √ | Tf, OX26, T7, TfR-lytic hybrid peptide |
| LR | √ | √ | Lf | |
| LDLR | √ | √ | nLDL, Peptide-22 | |
| LRP | √ | √ | Angiopep-2, MTf, ApoE, Peptide-22 | |
| Insulin Receptor | √ | √ | 83-14 murine monoclonal antibody | |
| Integrin αvβ3 | √ | √ | RGD, c(RGDyK), cRGD | |
| EGFR | √ | √ | GE11, EGF, mAb225 | |
| TGN receptor | √ | × | TGN peptide | |
| CD44 | × | √ | HA | |
| IL-13 Receptor | × | √ | IL-13 | |
| FR | × | √ | Folate, Pteroic acid |
3.3. Stimulate-responsive nano drug delivery system
The active-targeting strategy depends on the high expression of specific receptors/transporters in tumors, and the heterogeneous expression may greatly reduce its targeting efficiency (Srinivasarao et al., 2015 ). In addition, most receptors/transporters are also expressed in normal tissues, potentially causing off-target effect. Therefore, the active-targeting strategy based on ligand modification is not enough to achieve efficient delivery of drugs for brain tumors. The ‘stimulate-responsive’ strategy is emerged for drug delivery by introducing sensitive groups. The sensitive groups will undergo physical and chemical changes (such as protonation, deprotonation, fracture or degradation) when the nanoparticles are stimulated at the pathological site, resulting in structural changes in the nanoparticles ( Figure 5 ). These changes help to enhance cellular uptake, release drugs, promote lysosomal escape, turn on imaging signals, and penetrate into tumors (Chen et al., 2017 ). Therefore, the ‘stimulus-response’ strategy can improve the bioavailability of antitumor drugs and the antitumor efficacy.

Schematic diagram of stimulate-responsive nano drug delivery system.
3.3.1. Ph-responsive nano drug delivery system
The decreased pH in extracellular and interstitial is a sign of malignancy, which is due to the excess of metabolites (lactic acid, CO 2 , etc), as well as the increased expression and activity of vacuolar (V-type) H + -ATPase (Helmlinger et al., 2002 ). Compared with normal tissue (pH 7.0 ∼ 7.4), the extracellular pH of the tumor can be reduced to 5.6 ∼ 6.8. In addition, the pH in lysosomes is only about 4.5 ∼ 5.5 (Shi et al., 2020 ).
Generally, pH-sensitive materials work based on the following two mechanisms: the cleavage of acid sensitive chemical bonds and the protonation of materials. Various chemical functional groups, such as (semi) acetals, amides, orthoesters, amines, imines, and hydrazones, can be used as pH-sensitive groups are showed in Table 2 (Shi et al., 2020 ). The structures of commonly used acid sensitive chemical bonds and their degradation products (Shi et al., 2020 ). He et al. prepared the pH-sensitive polymer vesicles (Au-DOX@PO-ANG) to deliver the gold nanoparticles-doxorubicin complex to treat glioma (He et al., 2021 ). The tertiary amide group binds to hydrogen ions in solution and forms hydrogen bonds under acidic conditions, which would disrupt the core-shell structure, thereby reducing the stability of the polymersomes and releasing the drugs.
pH-sensitive chemical bonds and degradation products.
| Type | Acid sensitive chemical bonds | Degradation products |
|---|---|---|
| Vinyl ester | ||
| Amide | ||
| Imine | ||
| Oxime | ||
| Hydrazone | ||
| Acetals | ||
| Orthoester |
3.3.2. Redox-responsive nano drug delivery system
Compared with healthy tissues, the tumor tissue is highly reduced. As the main contributor to the redox state in cells, the concentration of glutathione (γ-Glutamyl-cyste-glycine, GSH) is about 0.5 ∼ 10 mm, which is more than 100 times higher than that in healthy cells (2 ∼ 20 µm) (Zhao et al., 2021 ). Disulfide bond, sulfide, selenide and telluride are commonly used functional groups for designing GSH-sensitive nano carriers. The structures and their products after reacting with GSH are shown in Table 3 . Wen et al. prepare angiopep-2 (AP)-modified redox-responsive nanoparticles to co-deliver siVEGF and PTX for glioma targeted therapy (Wen et al., 2020 ). The disulfide bond was broken by GSH, which allows the cleavage of the nano carrier and release of the drugs. As anticipated, Ap-CSssSA/P/R showed slower release during the whole experiment period with 0 mM GSH, however, PTX and siVEGF releases were significantly enhanced with 10 mM GSH, and the cumulative release was over 90% after 48 h.
The widely used GSH- and ROS-sensitive chemical bonds and broken products.
| Type | Chemical bonds | Degradation products | |
|---|---|---|---|
| GSH | Disulfide | ||
| Thioether | |||
| Selenium | |||
| Telluride | |||
| ROS | Arylboronic esters | ||
| Sulfhydryl | |||
| Diselenide/Ditelluride | |||
| Thioketal | |||
| Thioether | |||
| Peroxalate ester |
Rapid cell proliferation and high metabolic rate lead to a higher level of ROS (100 µm) in the tumor environment, much higher than that in the normal tissues (20 nm) (Xu et al., 2017 ). Table 3 lists the structure of commonly used ROS-responsive groups and their products after reacting with ROS. Zheng et al. design an ROS-responsive siRNA nanomedicine, 3I-NM@siRNA stabilized by ‘triple interactions’ (Zheng et al., 2019 ). 3I-NM@siRNA exhibited an active ROS response and efficient siRNA release upon treatment with H 2 O 2 , whereas 2I-NM@siRNA was very stable and no siRNAs were released. When 3I-NM@siRNA encounters ROS stimulus inside brain tumor, the hydrophobic phenylboronic ester is converted to its hydrophilic counterpart with carboxyl groups, which depletes the hydrophobic stabilization force and subsequently the newly produced carboxyl groups interfere with electrostatic and hydrogen bond interactions. This sequential ‘self-destruct’ process enables effective siRNA release.
3.3.3. Enzyme-responsive nano drug delivery system
The abnormal expression of enzymes has been used as a agonist for drug delivery and tumor targeting (Park et al., 2021 ). Some commonly used enzymes and their substrates are shown in Table 4 (Park et al., 2021 ), among which, MMPs, cathepsin B, hyaluronidase (HAase) and β-glucuronidase are widely used in NDDS. MMPs, members of the proteolytic enzyme family, are overexpressed in many types of tumors and play a key role in degrading extracellular matrix and promoting tumor metastasis (Shahriari et al., 2019 ). Hua et al. design and develop a dual-functional peptide-drug conjugate, SynB3-PVGLIG-PTX (Hua et al., 2021 ). The PTX binds to SynB3 through an MMP-2-sensitive linker (PVGLIG), which helps drug release at the target sites with high MMP-2 expression level. In the presence of MMP-2, SynB3-PVGLIG-PTX could completely disappear, while the percentage of PVG-PTX peaked (100%). In contrast, SynB3-PVGLIG-PTX could not be cleaved without MMP-2, and neither PVG-PTX nor free PTX could be detected.
The commonly used enzymes and their substrates.
| Enzyme | Linker |
|---|---|
| Hyaluronidase | Hyaluronic acid (HA) |
| MMP-9 | GFFLGLDD peptide |
| MMP-2 | GPLGLAG peptide, GPLGVRGK peptide, PVGLIG peptide, GPLGVRGC peptide, VPLGVRTK peptide, PLGVRG peptide, Gelatin |
| Cathepsin B | GFLGKGLFG peptide, GFLG peptide , Val-Cit peptide, |
| FAP-α | DRGETGPAC peptide |
| β-glucuronidase | Glucuronide |
3.4. Organelle targeting
In recent years, the nano materials with subcellular-targeting abilities have attracted much attention in the field of cancer therapy (Jin et al., 2020 ). Based on the molecular mechanism of the drug, targeting subcellular organelles has gradually become an important part of precision medicine (Gu et al., 2021 ). For example, drugs that produce reactive oxygen species are delivered to mitochondria, and therapeutic agents that blind DNA are delivered to the nucleus (Vankayala et al., 2015 ; Chen et al., 2019 ). Transporting drugs into the target organelles can maximize the efficacy of drugs, which is conducive to completely eradicating tumors and preventing tumor recurrence, invasion and metastasis ( Figure 6 ) (Chen et al., 2019 ).

Schematic diagram of organelle targeting nano drug delivery system.
3.4.1. Nucleus targeting
Many anti-tumor drugs widely used in the clinic are toxins related to DNA replication, and the drugs inhibit DNA replication by interacting with DNA or inactivating related enzymes. Therefore, delivering these drugs into the nucleus contributes to improving the efficacy (Vankayala et al., 2015 ). At present, the design of nuclear targeted nano-carriers can be summarized into the following two methods: (1) modifying nano-carriers with nuclear-targeted peptides to promote the enrichment of drugs in the nucleus; (2) preparing the nano-carriers with switchable size (Wei et al., 2021 ).
The first method is to modify the nano-carriers with specific ligands that can activate and internalize nuclear receptors to promote the interaction between carriers and nuclear membranes, so as to promote the entry of nanoparticles into the nucleus (Tanaka et al., 2017 ). The nuclear localization sequence (NLS) is a short peptide that is rich in lysine, arginine or proline, which can transfer the molecules attached to the nucleus through the nuclear pore. The structures of several commonly used nuclear targeting groups are shown in Figure 7 (Lange et al., 2010 ). For example, CB5005, a nuclear targeting peptide, is composed of membrane permeation sequence (CB5005M) and nuclear localization sequence (CB5005N), which can significantly enhance the enrichment of drugs in the cytoplasm and nucleus. In addition, CB5005 can also target intracellular NF-κB and inhibit its activation. Co-administration of CB5005 and doxorubicin (DOX) shows a synergistic effect in anti-glioma (Louzoun-Zada et al., 2019 ).

The structure of several commonly used nucleus targeting groups.
Nuclear pores control the transport process between cytoplasm and nucleoplasm. The diameter of the pores is only about 39 nm, which makes it difficult for nanoparticles to penetrate the nucleoplasm through passive diffusion (Haddad et al., 2020 ). To enhance the penetration capacity of nano drugs into nucleus, Wang et al. develop a pH- and GSH-responsive micelle. The charge reverses in the tumor microenvironment, so as to contribute to the entry into tumor cells through an adsorption-mediated effect. In the presence of GSH, the disulfide bond is broken and the particle size becomes smaller. In addition, surface conjugated dexamethasone can effectively dilate the nuclear pores, which facilitates the free entry of micelles into the cell nucleus (Wang et al., 2017 ).
3.4.2. Mitochondria targeting
As the ‘power room’ of eukaryotic cells, mitochondria are responsible for energy production, electron transmission, calcium metabolism, ROS production and immune regulation (Cho et al., 2020 ). Therefore, the function changes will affect biosynthetic pathways, cell signal transduction, chromatin structure and the activation of apoptosis. Targeted delivery of drugs to mitochondria and regulation of mitochondrial function provide great potential for the treatment of tumors.
The mitochondrial membrane potential (-160 ∼ −180 MV) of malignant cells is higher than that of normal cells, which indicates that it is feasible to selectively target the mitochondria of tumor cells. In order to enter the mitochondria, compounds must pass through the cell membrane and mitochondrial membrane. Fortunately, the both membrane potentials are negative, which allows the cationic compounds to accumulate initially in the cell cytosol and then inside the mitochondria. At present, several molecules targeting mitochondria have been reported ( Figure 8 ), such as mitochondrial penetrating polypeptide (MPP), delocalized lipophilic cations such as triphenylphosphonium (TPP), rhodamine, berberine, guanidine and (E-4-(2-(indole-3-yl) vinyl)-1-methylpyridinium salt (F16), etc (Louzoun-Zada et al., 2019 ).

The structure of molecules targeting mitochondria.
3.4.3. Lysosome targeting
Lysosome, the digestive organs in cells, containing many hydrolases for degrading, repairing and recycling biomolecules, which plays a crucial role in autophagy, secretion, and membrane repair (Yu et al., 2010 ). Nanomaterials are attractive in lysosome targeting because most nano preparations are eventually transported into lysosome via membrane receptor-mediated endocytosis. Therefore, there are few studies on promoting the entry of nanoparticles into the lysosome through ligand modification. On the contrary, there are many studies on lysosome escape, for example, cationic liposomes are more likely to escape from the lysosome through the ‘proton sponge’ effect. While, there are still some nano preparations that functionalized by lysosome-targeting groups to improve the accumulation in lysosome and further improve anticancer efficiency (Wang et al., 2016 ). For instance, mannose 6-phosphate is a promising lysosomal guiding group that can promote the entry into lysosome (Coutinho et al., 2012 ). Delivery vehicles using M6P-decorated nanoparticles have been developed for anti-cancer therapy. Crucianelli et al. developed M6P-decorated liposomes made of a M6P cholesteryl conjugate where a sufficiently rigid aryl-incorporated linker connects the M6P moiety to a steroid structure and ensured exposure of the M6P function to favor tight association with the liposome with the receptor (Crucianelli et al., 2014 ). And the enhanced uptake of M6P-decorated liposomes in cancer cells was confirmed. Furthermore, this group prepared another M6P-decorated liposomes loading with C6-ceramide, which behaves as a detergent and was found to induce lysosomal membrane permeabilization, in order to target lysosomes in cancer cells and induce apoptosis (Minnelli et al., 2018 ).
3.4.4. Endoplasmic reticulum targeting
Endoplasmic reticulum (ER) plays an important role in protein synthesis, folding and post-translational modification. In addition, ER is also involved in lipid biosynthesis, maintaining calcium homeostasis and other physiological functions. At present, the commonly used strategy for ER-targeting is to modify the nanoparticle with an ER-targeting peptide, such as the KKXX and KXKXX search signal, RXR retention/recovery signal, and the KDEL (Lys-Asp-Glu-Leu) retention/recovery signal (Ma et al., 2016 ). In addition, some small molecular groups such as p-toluenesulfonamide, hydrazide can also be used to target ER. Wang et al. design gold nanoparticles (KDEL-AuNPs) modified with KDEL, which can be internalized and accumulated in ER (Wang et al., 2013 ).
3.4.5. Golgi targeting
Golgi is the site for post-translational protein modification, and the structural integrity of golgi is important for certain signaling pathways, especially those related to migration, invasion and angiogenesis (Wang et al., 2015 ; Nishita et al., 2017 ; Yu et al., 2018 ). Therefore, the destruction of golgi structure in tumor cells may be a potential method to destroy multiple signaling pathways and a good strategy for targeted tumor therapy. Gong’s group has developed a golgi-targeted prodrug nanoparticles by combining chondroitin sulfate (CS) with retinoic acid (RA). This nanoparticles accumulate in the golgi of cancer cells and then release RA in the acidic environment. The evaluation in vitro and in vivo further confirms that CS-RA inhibits the expression of many metastasis-related proteins by destroying the golgi structure. After loading with PTX, the CS-RA based nanoformulation (PTX-CS-RA) suppresses tumor growth and metastasis (Li et al., 2019 ).
4. Drug combination strategy based on multifunctional nano drug delivery system
In recent years, combination therapy has received attention for decreasing side effects and increasing efficacy (Zhao et al., 2020 ). At present, the combination therapy in clinical research for brain tumors includes the combination of chemotherapy drugs (temozolomide, paclitaxel, camptothecin, methotrexate, etc.), chemotherapy drugs and small molecule targeted drugs (sildenab + lomustine, bortezomib + temozolomide), t small molecule targeted drugs (erlotinib + vorinolta), small molecule drugs and monoclonal antibodies (bevacizumab + irinotecan), small molecule drugs and nucleic acid drugs (temozolomide + SGT-53), etc (Zhao et al., 2020 ). Although these combination therapies have improved the clinical outcomes to some extent, they are still not ideal due to the complexity of brain tumors. Therefore, there is an urgent need to improve the existing combination strategies to solve these problems, and the combined drug therapy based on multi-functional drug delivery system has made good progress in brain tumors (Khan et al., 2021 ).
Two or more drugs with different anti-tumor mechanisms are commonly selected for combined administration, such as chemotherapy drugs with different mechanisms, chemotherapy drugs + chemotherapy sensitizers, chemotherapy drugs + small molecule targeted drugs, and small molecule drugs and biological drugs (chemotherapy drugs + monoclonal antibodies, chemotherapy drugs + immune agents, chemotherapy drugs + nucleic acid drugs), et al ( Figure 9 ). The combination of drugs with different mechanisms has the following advantages. Firstly, the drugs kill tumor cells against different targets to improve the efficacy. Secondly, the combined administration can reduce the dose, thereby decreasing the toxic and side effects. It can also avoid the drug resistance caused by a single drug. In addition, the administration can improve the killing effect on tumor stem cells and metastatic tumor cells, so as to reduce the recurrence of the disease. What’s more, the proportion and the release sequence of drugs are also important for the synergistic treatment. This section will summarize the application of multifunctional NDDS in the combined treatment of brain tumors, including the combined use of small molecular chemotherapy drugs, chemotherapy-immunotherapy, and chemotherapy-gene therapy.

Combination strategy for brain tumors.
4.1. The combination of small molecular chemical drugs
4.1.1. the combination of chemotherapy drugs.
The chemotherapy drugs have a limit in specificity targeting the tissue, which may lead to the obvious toxic and side effects, and MDR. Therefore, the combination of two or more chemotherapeutic drugs with different mechanisms is the most commonly used strategy (Shrestha et al., 2021 ).
Zhang et al. designed a dual-targeting ligand modified with lactoferrin (Lf) and RGD peptide to prepare lipid nanoparticles (L/R-T/V-NLC) loading with TMZ and vincristine (VCR). The RGD peptide can recognize the α v β 3 receptor overexpressed on neurovascular endothelial cells and the Lf can recognize transferrin receptor (TFR) on the brain tumors to facilitate the nanoparticles crossing the BBB and then targeting tumor cells. The results show that L/R-T/V-NLCs inhibited the tumor growth better than that treated with single-ligand-modified NLCs, single-drug-loaded NLCs, and drug solutions (Zhang et al., 2018 ). Xu et al. prepare the nanoparticles with the cationic micellar core loading curcumin and the anion shell loading DOX to target both cancer cells and stem cells (Xu et al., 2018 ). And an obvious inhibition of tumor growth was observed after treatment with the nanoparticles. The rat survival after treatment with this nanoparticles (64.5 days) was significantly prolonged in comparison to the control group (31.4 days), the combinational Cur/DOX solution group (36.3 days), the DOX-VPDP group (33.5 days) and the Cur-VPDP group (38.7 days). Liu et al. develop the HA-grafted micelles encapsulating lauroyl-gemcitabine and honokiol. The micelles penetrate into the tumor sphere mediated by the CD44 receptor, and enhances the cytotoxicity to glioma cells (Liu et al., 2018 ). In vivo, drug-loaded HA-M significantly increased the survival rate of mice bearing orthotopic xenograft GBM compared with the negative control (1.85-fold).
4.1.2. The combination of chemotherapy drugs and sensitizer
In addition to the combined use of chemotherapeutic drugs, the combination therapy with chemotherapy/radiotherapy and sensitizer can also improve the therapeutic effect on brain tumor and prevent drug resistance. Hua et al. develop the novel angiopep-2-lipid-poly-(metronidazoles) n (ALP-(MIs) n ) hypoxic radiosensitizer-polyprodrug nanoparticles to enhance the radiosensitizing effect on gliomas (Hua et al., 2018 ). The nanoparticles are aggregated in glioma through specifically binding with low-density lipoprotein receptor-related protein-1 (LRP-1) highly expressed on the surface of brain microvascular endothelial cells and glioma cells. The hydrophobic P-(MIs) n core encapsulating DOX is converted into hydrophilic amino groups under low oxygen conditions to mimic the oxygen-increased sensitization and provoke the release of DOX. The activity evaluation shows that ALP-(MIs) n /DOX can effectively accumulate in the glioma after systemic administration in vivo, which shows a significant radiosensitizing effect for glioma treatment. The median survival time for mice treated with LP-(MIs) 25 +RT (61 days) and ALP-(MIs) 48 +RT (63 days) were longer than those of PBS, PBS + RT and AL-PLGA + RT, suggesting that ALP-(MIs) 25 and ALP-(MIs) 48 improved the efficacy of the radiotherapy. Similarly, Lam et al. develop transferrin-functionalized nanoparticles (TF-NP) to co-deliver temozolomide and chemotherapy sensitizer JQ1 (a bromine domain inhibitor), which can effectively enhance chemotherapy-induced DNA damage and cytotoxicity (Lam et al., 2018 ). Treatment of tumor-bearing mice with TF-NP loaded with TMZ and the JQ1 leads to increased DNA damage and apoptosis that correlates with a 1.5- to 2-fold decrease in tumor burden and corresponding increase in survival compared to equivalent free-drug dosing.
In addition to combining with sensitizers, introducing photosensitizers and thermosensitive agents can also improve the effect. Lu et al. developed a disulfide bond-conjugated prodrug polymer consisting of camptothecin (CPT) and polyethylene glycol (PEG) with further modification of iRGD peptide. The polymer could self-assemble into nanosized polymeric micelles and load with photosensitizer IR780 for combination therapy. Interestingly, conjugation of iRGD on the surface of micelles obviously enhances the ability to cross the BBB and target glioma cells, which displays a better tumor killing ability (Lu et al., 2020 ). CPT-S-S-PEG-iRGD@IR780 micelles combined chemotherapy with photodynamic therapy (PDT) showed the longest median survival time (49 days), while PBS, CPT, CPC@IR780 micelles and CPD@IR780 micelles treatments achieved the median survival times of 29, 31, 31 and 38 days, respectively. In our previous study, we prepared the biomimetic nanoparticles (ICG/PTX@RGE-EV) co-loading indocyanine green (ICG) and PTX by modifying Neuropilin-1 targeting peptide (RGE) on the extracellular vesicles (EV) membrane. ICG/PTX@RGE-EV shows good photothermal properties and promotion of PTX release from EVs, when stimulated by 808-nm laser light. Then, they target U251 cells, with activation of the Caspase-3 pathway and elevated apoptosis, which increases the median survival of glioma mice (Wang et al., 2021 ). What’s more, the significantly reduced tumor volume was observed in mice following targeting combined therapy (ICG/PTX@RGE-EV + NIR) compared with mice treated with chemotherapy, (PTX@RGE-EV), hyperthermia (ICG@RGEEV + NIR), or non-targeted chemotherapy-hyperthermia (ICG/PTX@EV + NIR).
4.1.3. The combination of other small molecule chemical drugs
Antitumor drugs achieve the treatment of tumors through a variety of mechanisms. In addition to directly inducing tumor cell apoptosis, they can also inhibit angiogenesis, regulate tumor autophagy, reshape the tumor microenvironment, and regulate tumor related signal pathways. Lakkadwala et al. develop a dual-functional liposome, which is modified with Tf to target the brain endothelial cells and glioblastoma cells. The liposome is also decorated with CPP (Pen) to promote the transport of DOX and erlotinib across the BBB to glioblastoma tumor (Lakkadwala et al., 2019 ). The biodistribution of Tf-Pen liposomes demonstrated 12- and 3.3- fold increase in DOX and erlotinib accumulation in mice brain, respectively compared to free drugs. In addition, Tf-Pen liposomes showed excellent antitumor efficacy by regressing ∼90% of tumor in mice brain with significant increase in the median survival time (36 days) along with no toxicity.
Glioblastoma (GBM) treatment is undermined by the suppressive tumor immune microenvironment (TIME). Zheng et al. develop a liposome modified with α7 nicotinicacetylcholine eceptors (nAChRs)-binding peptide D CDX to achieve a ‘three birds-one-stone’ delivery strategy, namely, targeting the glioma vessel endothelium, glioma cells, and tumor-associated macrophages that all overexpressing α7 nAChRs (Zheng et al., 2020 ). This multifunctional liposome co-encapsulates honokiol and disulfiram/copper complex to remodel the tumor metabolism and TIME through the mammalian target of rapamycin. The median survival time of the orthotopic cancer mice in the CDX-LIPO group was 27 days, which was significantly longer than that of the mice treated with PBS (9 days), free drug injections (17 days), free-drug combo (21 days), and LIPO (21 days).
Biotinylated PAMAM G3 dendrimers with BBB penetrating ability are used to load anticancer agent cyclooxygenase-2 inhibitor celecoxib and peroxisome proliferator-activated receptor γ agonist Fmoc-L-Leucine, which have a synergistic effect on glioma (Uram et al., 2019 ). Huang et al. develop acid-sensitive CaCO 3 /TPGS nanoparticles (ICG-PDA-TPZ NPs), modified with RGD peptide (Huang et al., 2019 ). The nanoparticles encapsulate near-infrared photosensitizer ICG, photothermal conversion agent polydopamine (PDA), and tirapazamine (TPZ), which have the synergistic treatment for brain tumors through chemo-photodynamic and photothermal therapy. In addition, the multifunctional NDDS used for the co-delivery of cobstatin-A4 (anti-angiogenesis agent) and DOX, PTX and melittin, PTX and artemether has also made good progress in the treatment of glioma (Gao et al., 2014 ; Li et al., 2014 ; Wang et al., 2019 ).
4.2. The combination of chemotherapy and chemodynamic therapy
The ROS, such as hydrogen peroxide, hydroxyl radical, superoxide anion radical and singlet oxygen, etc, are widely present in mammalian cells. When the content exceeds the tolerance value of cells, it will induce cell necrosis and apoptosis (Trachootham et al., 2009 ). Chemodynamic therapy (CDT), first proposed by Shi and coworkers in 2016, is an emerging cancer therapeutic method (Zhang et al., 2016 ). It uses various transition metal ions, such as Fe 2+ , Mn 2+ and Cu + , to catalyze H 2 O 2 decomposition in the cancer region. It has emerged as an efficient strategy for cancer treatment utilizing Fenton or Fenton-like reactions to destroy cancer cells by converting endogenous H 2 O 2 into highly toxic reactive oxygen species (Tang et al., 2019 ). However, the in vivo therapeutic outcomes are highly dependent on the endogenous H 2 O 2 amount, which is the power source for Fenton-like reactions (Ren et al., 2020 ).
In recent years multifunctional nano-delivery systems have made good progress in combination of chemotherapy and chemodynamic therapy for brain tumors. It has been found that a combination of nanoparticles can efficiently cross the blood-brain barrier and precisely target glioblastoma to inhibit cancer cells through chemotherapy and chemodynamic therapy, achieving excellent anti-cancer efficacy (Pan et al., 2022 ). There is currently research into the development of theranostic nanodrug (iRPPA@TMZ/MnO) where the presence of iRGD provides the nanodrug with a high ability to cross the BBB and penetrate the tumor tissue (Tan et al., 2020 ). Upon accumulation in glioma, the nanodrug responds to the tumor microenvironment with the simultaneous release of TMZ, Mn 2+ and O 2 . The released TMZ and Mn 2+ provide significant benefits for glioma growth inhibition through the synergistic anti-cancer effects of chemo-chemodynamic therapy. In addition, the generated O 2 in situ reduces tumor hypoxia and enhances the therapeutic effect of chemotherapy/chemotherapy kinetics on glioma. And the in vivo anti-GBM efficacy results suggested that CuFeSe2-LOD@Lipo-CM + NIR group had a remarkable tumor inhibition rate of 84.9% which was significantly higher than those of CuFeSe2-LOD@Lipo-CM group (54.8%) and CuFeSe2-LOD@Lipo + NIR group (61.2%).
4.3. The combination of chemotherapy and immunization therapy
The immune system is one of the key components of TME and plays an important role in the occurrence and development of tumors. However, due to the ‘immune privilege’ of CNS, the current immunotherapy has not been clinically proven to significantly improve the survival rate of brain tumor patients. In recent years, studies have found a small number of immune cells (including T cells) in the choroid plexus matrix, cerebrospinal fluid, subarachnoid space and perivascular space, proving that there is indeed active surveillance in the CNS (Ratnam et al., 2019 ). This indicates that immunotherapy is important in the treatment of brain tumors.
4.3.1. Combination chemotherapy with immune checkpoint inhibitors
In the immune system, immune checkpoint inhibitors are responsible for negatively regulating the activation of T lymphocytes, thereby limiting the over activation of the immune system and maintaining immune homeostasis. However, the tumors prefer to escape the clearance of the immune system by utilizing the immune checkpoints. The checkpoints, such as the over-expressed cytotoxic T lymphocyte associated antigen 4 (CTLA-4) and programmed death ligand-1 (PD-L1), hinder the recognition of tumor cells by T cells. Blocking immune checkpoints is the most effective approach in immunotherapy. The inhibition of CTLA-4 on T cells can be alleviated by using CTLA-4 molecular inhibitors or CTLA-4 monoclonal antibodies. Similarly, using PD-1 or its ligand PD-L1 to selectively block the binding between tumor cells and T cells can also promote T cells to recognize and eliminate cancer cells (Topalian et al., 2012 ). Although many clinical trials using immune checkpoint blocking therapy to fight against glioblastoma, the results are unsatisfactory due to the poor permeability across the BBB into the tumor. Therefore, the combination therapy based on multifunctional NDDS plays an important role in immune checkpoint blocking therapy.
Indoleamine 2,3-dioxygenase (IDO) is an immune checkpoint receptor produced by tumor cells, macrophages, and dendritic cells (DCs) within draining lymph nodes and the tumor microenvironment. 1-methyltryptophan (1MT), an IDO-specific competitive inhibitor, not only activates effector CD8 + T cells and inhibits immunosuppressive regulatory CD4 + T cells, but also activates DCs to increase antigen presentation. Kuang et al. discover iRGD-modified nanoparticles to simultaneously deliver DOX and 1MT into glioma. The nanoparticles show the capability of penetrating through BBB into the tumor, and significantly improve the accumulation of drugs in brain tumors with minimal side effects (Kuang et al., 2018 ). Meng et al. propose a combination therapy targeting BBB regulation and microenvironment amelioration (Meng et al., 2021 ). Firstly, biomimetic nanovesicles are designed to achieve targeted regulation using biomimetic technology with favorable biocompatibility and long circulation. They encapsulate an appropriate ratio of perfluorocarbon (PF) and the A 2A R agonist, 5′-(Nethylcarboxamido)adenosine (NECA), in the red-blood-cell membrane to form BBB-regulating nanovesicles. Ultrasound is performed to gasify these PFs to break the nanostructures. Subsequently, the NECA activate A 2A R to induce effects on endothelial cells, which transiently increases BBB permeability. TMZ is encapsulated in manganese dioxide that attached outside with an acid-responsive material, poly(ethylene glycol)-poly(β-amino ester) to improve its stability in circulation. Manganese dioxide could react with overexpressed H 2 O 2 to produce oxygen to improve the hypoxic microenvironment. Following, the encapsulated TMZ is released. Combined with radiotherapy, more PD-L1 antibodies enter glioblastoma tissues and release the immune brake to initiate tumor-specific immune responses, so as to achieve enhanced therapeutic efficiencies of chemoradiation and immune therapy.
4.3.2. Targeting tumor-associated immune cells
Tumor-associated macrophages (TAM) are the main constituents of the tumor microenvironment. These cells are usually derived from monocyte precursors of the CNS with an M2-like phenotype, which is unfavorable for the immune system to detect and kill tumor cells (Vidyarthi et al., 2019 ). Transforming macrophage phenotype from anti-inflammatory M2 (TAM2, immunosuppression) to pro-inflammatory M1 (TAM1, anti-tumor) phenotype not only relieves immunosuppression and triggers cytotoxic T-cell immunity, but also enhances the chemotherapy efficacy improves the prognosis of patients, and prolongs the survival time. Zhao et al. prepare the albumin nanoparticles modified with dual ligands, a TfR-binding peptide T12 and mannose (Zhao et al., 2018 ). The nanoparticles can efficiently pass through the BBB mediated by TfR and albumin-binding receptor SPARC that were overexpressed in both the BBB and glioma cells, thus achieving biomimetic delivery to glioma. The group given the T12/Man-BSA NPs displayed the longest survival time, with a median survival time of 42 days, compared to 32 days for the Man-BSA NP group and 28 days for the BSA NP group. Through the co-delivery of disulfiram/copper complex and regorafenib, the system efficiently inhibits the glioma cell proliferation and successfully ‘re-educated’ the protumor TAM2 toward antitumor TAM1.
4.4. The combination of chemotherapy and gene therapy
Within the last decade, researchers have paid close attention to the gene therapy, which is considered to be one of the most promising methods to treat cancer. Nucleic acid-based drugs, such as siRNA, miRNA, mRNA, DNA and CRISPR/Cas9, are a new class of highly specific anticancer drugs, which play the anti-tumor role in the cytoplasm or nucleus of cancer cells (Peng et al., 2020 ).
At present, fifteen nucleic acid drugs developed all over the world, including ten antisense nucleic acid (ASOs) drugs, four small interfering RNA (siRNA) drugs, and one nucleic acid aptamer. In addition, there are two other mRNA drugs approved as COVID-19 vaccines. However, there are no nucleic acid drugs approved for brain tumors. The application of nucleic acid drugs on brain tumors is a new field, and the combination with other drugs is also under preclinical research.
VEGF is a key regulator of tumor angiogenesis, and RNAi interference therapy can down-regulate the expression of VEGF through siRNA. Wen et al. develop angiopep-2 (AP)-modified redox-responsive nanoparticles (Ap-CSssSA/P/R) to co-deliver siVEGF and PTX (Wen et al., 2020 ). In vitro and in vivo Ap-CSssSA/P/R complexes showed an excellent silencing effect of VEGF gene, and complexes via LRP1-mediated targeting delivery exerted a higher neovascularization inhibition, compared to naked PTX-loaded nanoparticles. An angiopep-2 (A2)-modified cationic lipidpoly (lactic-co-glycolic acid) (PLGA) nanoparticle (A2-N) is developed by Ye et al. that can release gefitinib (Ge) and GOLPH3 siRNA (siGOLPH3) upon entering glioma cells, thus serving as a combinatorial anti-tumor therapy (Ye et al., 2019 ). The released siGOLPH3 effectively silences GOLPH3 mRNA expression and further promotes EGFR and pEGFR degradation, and Ge also markedly inhibits EGFR signaling. The median survival time of mice treated with A2-N/Ge/siGOLPH3 was 45 days, longer than that of the other groups. These researches confirm the feasibility of combined anti-angiogenesis and pro-apoptotic therapy for brain tumors.
Cancer stem cells are a subset of tumor cells with high self-renewal and stem cell properties. Traditional therapeutic drugs can target and eliminate tumor cells, while they lack effectiveness on tumor stem cells and are prone to recurrence. Developing new therapeutic strategies that selectively target tumor stem cells to improve efficacy has become a research hotspot in recent years (Bhargav et al., 2020 ). Sun et al. prepare the cationic liposomes (DP-CLPs) loaded with survivin siRNA and paclitaxel (DP-CLPs-PTX-siRNA) modified with a low-density lipoprotein receptor-related protein and an RNA aptamer bound CD133 (Sun et al., 2018 ). The liposome displays durable ability to target glioma cells and brain microvascular endothelial cells (BCECs) and to deliver drugs (PTX/siRNA) to CD133 + glioma stem cells, which exhibits great potential for targeted imaging and therapy of brain glioma stem cells. The tumor size at 19 days of the nude mice in situ implanted by CD133 + +DP-CLPs-PTX-survivin siRNA were much more significantly decreased compared with the group of CD133 + +PTX, CD133 + +CLPs-PTX-survivin siRNA and the control.
5. Conclusions
Brain tumor is one of the most complex and lethal tumors. The challenges in treatment mainly include the BBB, BBTB, the heterogeneity and invasiveness of brain tumor cells, immune escape, tumor stem cells and tumor microenvironment. To overcome the above obstacles, it is urgently to design the multi-functional NDDS for the delivery of drugs. Compared with the single drug administration, the combination therapy can synergistically enhance the efficacy, further reduce toxic and side effects and decrease the recurrence rate. The multifunctional NDDS that target brain tumors usually has the following characteristics: the suitable physicochemical property to improve the stability in blood circulation; the ligand modification to facilitate the penetration across the BBB and further enter into tumor cells; the stimulation-response groups to promote the delivery and controlled release of drugs in cells; the modification that targeting organelles to contribute to the precision delivery to the target sites. This paper also summarizes the research progress of multifunctional NDDS in the combination therapy of brain tumors from the following sections, the combination of chemotherapy drugs, chemotherapy-chemodynamic combination therapy, chemotherapy-immunization combination therapy, and chemotherapy-gene combination therapy.
In addition to the design strategies mentioned above, the following points should be considered when designing multifunctional NDDS for combination therapy: (1) the potential neurotoxicity of nano preparations, (2) the leakage of drugs during transportation, (3) the shielding effect of the protein corona, (4) the off-target effect. Therefore, when designing brain tumor targeting NDDS, it is necessary to reasonably select the target and combined drugs, rationally design the delivery system according to the molecular mechanism, develop new nontoxic or low-toxic materials, promote the delivery and release of drugs in brain, improve the production process, pay attention to personalized administration and to achieve precise treatment. With the continuous understanding of the physiological structure of brain tumors, the discovery of new targets, the development of anti-tumor drugs, the progress of materials science and nanotechnology, and the maturity of emerging therapies such as immunotherapy, gene therapy, cell therapy, etc., the combination therapy based on multifunctional NDDS will gradually move from theory to practice in the treatment of brain tumors.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 81903448 & 22007085), the Henan Medical Science and Technology Joint Building Program (No. LHGJ20220417 & LHGJ20210925), the Henan Key Project of Research and Development Plan (Science and Technology) (No. 222102310091), the Fundamental Research Funds for the Universities of Henan Province (No. NSFRF210319), and the Postdoctoral Science Foundation of China (No. 2020M682366).
Disclosure statement
The authors declare no competing financial interest.
- Khan M, Sherwani S, Khan S, et al. (2021). Insights into multifunctional nanoparticle-based drug delivery systems for glioblastoma treatment . Molecules 26 :2262. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Alcantara Llaguno S, Parada LF. (2021). Cancer stem cells in gliomas: evolving concepts and therapeutic implications . Curr Opin Neurol 34 :868–74. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Alexander JJ. (2018). Blood-brain barrier (BBB) and the complement landscape . Mol Immunol 102 :26–31. [ PubMed ] [ Google Scholar ]
- Aparicio-Blanco J, Sanz-Arriazu L, Lorenzoni R, et al. (2020). Glioblastoma chemotherapeutic agents used in the clinical setting and in clinical trials: nanomedicine approaches to improve their efficacy . Int J Pharm 581 :119283. [ PubMed ] [ Google Scholar ]
- Ashby LS, Smith KA, Stea B. (2016). Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review . World J Surg Oncol 14 :225. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Azarmi M, Maleki H, Nikkam N, et al. (2020). Transcellular brain drug delivery: a review on recent advancements . Int J Pharm 586 :119582. [ PubMed ] [ Google Scholar ]
- Bhargav AG, Mondal SK, Garcia CA, et al. (2020). Nanomedicine revisited: next generation therapies for brain cancer . Adv Therap 3 :2000118. [ Google Scholar ]
- Caro C, Avasthi A, Paez-Munoz JM, et al. (2021). Passive targeting of high-grade gliomas via the EPR effect: a closed path for metallic nanoparticles? Biomater Sci 9 :7984–95. [ PubMed ] [ Google Scholar ]
- Charabati M, Rabanel J, Ramassamy C, et al. (2020). Overcoming the brain barriers: from immune cells to nanoparticles . Trends Pharmacol Sci 41 :42–54. [ PubMed ] [ Google Scholar ]
- Chelliah SS, Paul EAL, Kamarudin MNA, et al. (2021). Challenges and perspectives of standard therapy and drug development in high-grade gliomas . Molecules 26 :1169. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen B, Dai W, He B, et al. (2017). Current multistage drug delivery systems based on the tumor microenvironment . Theranostics 7 :538–58. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chen WH, Luo GF, Zhang XZ. (2019). Recent advances in subcellular targeted cancer therapy based on functional materials . Adv Mater 31 :1802725. [ PubMed ] [ Google Scholar ]
- Chen Y, Liu LH. (2012). Modern methods for delivery of drugs across the blood-brain barrier . Adv Drug Deliv Rev 64 :640–65. [ PubMed ] [ Google Scholar ]
- Cho H, Cho Y, Shim M, et al. (2020). Mitochondria-targeted drug delivery in cancers . Biochim Biophys Acta Mol Basis Dis 1866 :165808. [ PubMed ] [ Google Scholar ]
- Conniot J, Talebian S, Simoes S, et al. (2021). Revisiting gene delivery to the brain: silencing and editing . Biomater Sci 9 :1065–87. [ PubMed ] [ Google Scholar ]
- Coutinho MF, Prata MJ, Alves S. (2012). Mannose-6-phosphate pathway: a review on its role in lysosomal function and dysfunction . Mol Genet Metab 105 :542–50. [ PubMed ] [ Google Scholar ]
- Crucianelli E, Bruni P, Frontini A, et al. (2014). Liposomes containing mannose-6-phosphate-cholesteryl conjugates for lysosome-specific delivery . RSC Adv 4 :58204–7. [ Google Scholar ]
- D'Agostino PM, Gottfried-Blackmore A, Anandasabapathy N, et al. (2012). Brain dendritic cells: ciology and pathology . Acta Neuropathol 124 :599–614. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Desbaillets N, Hottinger AF. (2021). Immunotherapy in glioblastoma: a clinical perspective . Cancers 13 :3721. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dubois LG, Campanati L, Righy C, et al. (2014). Gliomas and the vascular fragility of the blood brain barrier . Front Cell Nurosci 8 :418. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Emlet DR, Gupta P, Holgado-Madruga M, et al. (2014). Targeting a glioblastoma cancer stem-cell population defined by EGF receptor variant III . Cancer Res 74 :1238–49. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fu QY, Zhao Y, Yang ZZ, et al. (2019). Liposomes actively recognizing the glucose transporter GLUT1 and integrin alphav beta3 for dual-targeting of glioma . Arch Pharm Chem Life Sci 352 :1800219. [ PubMed ] [ Google Scholar ]
- Ganz JC. (2022). Low grade gliomas . Prog Brain Res 268 :271–7. [ PubMed ] [ Google Scholar ]
- Gao HL, Yang Z, Cao SJ, et al. (2014). Tumor cells and neovasculature dual targeting delivery for glioblastoma treatment . Biomaterials 35 :2374–82. [ PubMed ] [ Google Scholar ]
- Golombek SK, May JN, Theek B, et al. (2018). Tumor targeting via EPR: strategies to enhance patient responses . Adv Drug Deliver Rev 130 :17–38. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Grabrucker AM, Ruozi B, Belletti D, et al. (2016). Nanoparticle transport across the blood brain barrier . Tissue Barriers 4 :e1153568. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gu W, Meng F, Haag R, et al. (2021). Actively targeted nanomedicines for precision cancer therapy: concept, construction, challenges and clinical translation . J Control Release 329 :676–95. [ PubMed ] [ Google Scholar ]
- Haddad S, Abanades Lazaro I, et al. (2020). Design of a functionalized metal-organic framework system for enhanced targeted delivery to mitochondria . J Am Chem Soc 142 :6661–74. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- He C, Ding H, Chen J, et al. (2021). Immunogenic cell death induced by chemoradiotherapy of novel pH-sensitive cargo-loaded polymersomes in glioblastoma . Int J Nanomed 16 :7123–35. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Helmlinger G, Sckell A, Dellian M, et al. (2002). Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism . Clin Cancer Res 8 :1284–91. [ PubMed ] [ Google Scholar ]
- Hersh DS, Wadajkar AS, Roberts NB, et al. (2016). Evolving drug delivery strategies to overcome the blood brain barrier . Curr Pharm Des 22 :1177–93. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Herve F, Ghinea N, Scherrmann JM. (2008). CNS delivery via adsorptive transcytosis . Aaps J 10 :455–72. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hua D, Tang L, Wang W, et al. (2021). Improved antiglioblastoma activity and BBB permeability by conjugation of paclitaxel to a cell-penetrative MMP-2-cleavable peptide . Adv Sci (Weinh) 8 :2001960. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hua L, Wang Z, Zhao L, et al. (2018). Hypoxia-responsive lipid-poly-(hypoxic radiosensitized polyprodrug) nanoparticles for glioma chemo- and radiotherapy . Theranostics 8 :5088–105. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Huang M, Pu Y, Peng Y, et al. (2020). Biotin and glucose dual-targeting, ligand-modified liposomes promote breast tumor-specific drug delivery . Bioorg Med Chem Lett 30 :127151. [ PubMed ] [ Google Scholar ]
- Huang X, Wu J, He M, et al. (2019). Combined cancer chemo-photodynamic and photothermal therapy based on ICG/PDA/TPZ-loaded nanoparticles . Mol Pharm 16 :2172–83. [ PubMed ] [ Google Scholar ]
- Ishihara H, Kubota H, Lindberg RLP, et al. (2008). Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor β2 involves matrix metalloproteinases and tight junction proteins . J Neuropathol Exp Neurol 67 :435–48. [ PubMed ] [ Google Scholar ]
- Ishii H, Mimura Y, Zahra MH, et al. (2021). Isolation and characterization of cancer stem cells derived from human glioblastoma . Am J Cancer Res 11 :441–57. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jia W, Wang Y, Liu R, et al. (2021). Shape transformable strategies for drug delivery . Adv Funct Mater 31 :2009765. [ Google Scholar ]
- Jiang B, Zhao Y, Cao J, et al. (2021). Synthesis and preliminary biological evaluation of naproxen-probenecid conjugate for central nervous system (CNS) delivery . Pak J Pharm Sci 34 :2197–203. [ PubMed ] [ Google Scholar ]
- Jin H, Lin X, Gao M, et al. (2020). Peptide-decorated supramolecules for subcellular targeted cancer therapy: recent advances . Front Chem 8 :824. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kibria G, Hatakeyama H, Ohga N, et al. (2013). The effect of liposomal size on the targeted delivery of doxorubicin to integrin alpha v beta 3-expressing tumor endothelial cells . Biomaterials 34 :5617–27. [ PubMed ] [ Google Scholar ]
- Kuang J, Song W, Yin J, et al. (2018). iRGD modified chemo-immunotherapeutic nanoparticles for enhanced immunotherapy against glioblastoma . Adv Funct Mater 28 :1800025. [ Google Scholar ]
- Lakkadwala S, Rodrigues BD, Sun CW, et al. (2019). Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma . J Control Release 307 :247–60. ( [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lam FC, Morton SW, Wyckoff J, et al. (2018). Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles . Nat Commun 9 :1991. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lange A, McLane LM, Mills RE, et al. (2010). Expanding the definition of the classical bipartite nuclear localization signal . Traffic 11 :311–23. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lei F, Fan W, Li XK, et al. (2011). Design, synthesis and preliminary bio-evaluation of glucose–cholesterol derivatives as ligands for brain targeting liposomes . Chinese Chem Lett 22 :831–4. [ Google Scholar ]
- Li H, Zhang P, Luo J, et al. (2019). Chondroitin sulfate-linked prodrug nanoparticles target the golgi apparatus for cancer metastasis treatment . ACS Nano 13 :9386–96. [ PubMed ] [ Google Scholar ]
- Li XY, Zhao Y, Sun MG, et al. (2014). Multifunctional liposomes loaded with paclitaxel and artemether for treatment of invasive brain glioma . Biomaterials 35 :5591–604. [ PubMed ] [ Google Scholar ]
- Lin S, Xu H, Zhang A, et al. (2020). Prognosis analysis and validation of m6A signature and tumor immune microenvironment in glioma . Front Oncol 10 :541401. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Liu X, Li W, Chen T, et al. (2018). Hyaluronic acid-modified micelles encapsulating gem-C12 and HNK for glioblastoma multiforme chemotherapy . Mol Pharm 15 :1203–14. [ PubMed ] [ Google Scholar ]
- Lombardo SM, Schneider M, Türeli AE, et al. (2020). Key for crossing the BBB with nanoparticles: the rational design . Beilstein J Nanotechnol 11 :866–83. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Louzoun-Zada S, Jaber QZ, Fridman M. (2019). Guiding drugs to target-harboring organelles: stretching drug-delivery to a higher level of resolution . Angew Chem Int Ed Engl 58 :15584–94. [ PubMed ] [ Google Scholar ]
- Lu L, Zhao X, Fu T, et al. (2020). An iRGD-conjugated prodrug micelle with blood-brain-barrier penetrability for anti-glioma therapy . Biomaterials 230 :119666. [ PubMed ] [ Google Scholar ]
- Ma XW, Gong NQ, Zhong L, et al. (2016). Future of nanotherapeutics: targeting the cellular sub-organelles . Biomaterials 97 :10–21. [ PubMed ] [ Google Scholar ]
- Meng J, Agrahari V, Youm I. (2017). advances in targeted drug delivery approaches for the central nervous system tumors: the inspiration of nanobiotechnology . J Neuroimmune Pharmacol 12 :84–98. [ PubMed ] [ Google Scholar ]
- Meng L, Wang C, Lu Y, et al. (2021). Targeted regulation of blood-brain barrier for enhanced therapeutic efficiency of hypoxia-modifier nanoparticles and immune checkpoint blockade antibodies for glioblastoma . ACS Appl Mater Interfaces 13 :11657–71. [ PubMed ] [ Google Scholar ]
- Minnelli C, Cianfruglia L, Laudadio E, et al. (2018). Selective induction of apoptosis in MCF7 cancer-cell by targeted liposomes functionalised with mannose-6-phosphate . J Drug Target 26 :242–51. [ PubMed ] [ Google Scholar ]
- Mohamed M, Abu Lila AS, Shimizu T, et al. (2019). PEGylated liposomes: immunological responses . Sci Technol Adv Mater 20 :710–24. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mojarad-Jabali S, Farshbaf M, Walker PR, et al. (2021). An update on actively targeted liposomes in advanced drug delivery to glioma . Int J Pharm 602 :120645. [ PubMed ] [ Google Scholar ]
- Nishita M, Park SY, Nishio T, et al. (2017). Ror2 signaling regulates golgi structure and transport through IFT20 for tumor invasiveness . Sci Rep 7 :1. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Oishi T, Koizumi S, Kurozumi K. (2022). Molecular mechanisms and clinical challenges of glioma invasion . Brain Sci 12 :291. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ozdemir-Kaynak E, Qutub AA, Yesil-Celiktas O. (2018). Advances in glioblastoma multiforme treatment: new models for nanoparticle therapy . Front Physiol 9 :170. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pan Y, Xu C, Deng H, et al. (2022). Localized NIR-II laser mediated chemodynamic therapy of glioblastoma . Nano Today 43 :101435. [ Google Scholar ]
- Pang L, Zhu Y, Qin J, et al. (2018). Primary M1 macrophages as multifunctional carrier combined with PLGA nanoparticle delivering anticancer drug for efficient glioma therapy . Drug Deliv 25 :1922–31. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Park H, Saravanakumar G, Kim J, et al. (2021). Tumor microenvironment sensitive nanocarriers for bioimaging and therapeutics . Adv Healthcare Mater 10 :2000834. [ PubMed ] [ Google Scholar ]
- Patel AP, Fisher JL, Nichols E, et al. (2019). Global, regional, and national burden of brain and other CNS cancer, 1990–2016: a systematic analysis for the global burden of disease study 2016 . Lancet 18 :376–93. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Peng T, Deng Z, He J, et al. (2020). Functional nucleic acids for cancer theranostics . Coordin Chem Rev 403 :213080. [ Google Scholar ]
- Peng Y, Zhao Y, Chen Y, et al. (2018). Dual-targeting for brain-specific liposomes drug delivery system: synthesis and preliminary evaluation . Bioorg Med Chem 26 :4677–86. [ PubMed ] [ Google Scholar ]
- Petrova V, Annicchiarico-Petruzzelli M, Melino G, et al. (2018). The hypoxic tumour microenvironment . Oncogenesis 7 :10. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Phi LTH, Sari IN, Yang YG, et al. (2018). Cancer stem cells (CSCs) in drug resistance and their therapeutic lmplications in cancer treatment . Stem Cells Int 2018 :5416923. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Qie Y, Yuan H, von Roemeling CA, et al. (2016). Surface modification of nanoparticles enables selective evasion of phagocytic clearance by distinct macrophage phenotypes . Sci Rep 6 :26269. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Qiu Y, Liu Y, Wang LM, et al. (2010). Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods . Biomaterials 31 :7606–19. [ PubMed ] [ Google Scholar ]
- Ratnam NM, Gilbert MR, Giles AJ. (2019). Immunotherapy in CNS cancers: the role of immune cell trafficking . Neuro Oncol 21 :37–46. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Reifenberger G, Wirsching HG, Knobbe-Thomsen CB, et al. (2017). Advances in the molecular genetics of gliomas – implications for classification and therapy . Nat Rev Clin Oncol 14 :434–52. [ PubMed ] [ Google Scholar ]
- Ren ZG, Sun SC, Sun RR, et al. (2020). A metal-polyphenol-coordinated nanomedicine for synergistic cascade cancer chemotherapy and chemodynamic therapy . Adv Mater 32 :1906024. [ PubMed ] [ Google Scholar ]
- Rustenhoven J, Kipnis J. (2019). Bypassing the blood-brain barrier . Science 366 :1448–9. [ PubMed ] [ Google Scholar ]
- Saidijam M, Karimi DF, Sohrabi S, et al. (2018). Efflux proteins at the blood-brain barrier: review and bioinformatics analysis . Xenobiotica 48 :506–32. [ PubMed ] [ Google Scholar ]
- Sanita G, Carrese B, Lamberti A. (2020). Nanoparticle surface functionalization: how to improve biocompatibility and cellular internalization . Front Mol Biosci 7 :587012. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shahriari M, Zahiri M, Abnous K, et al. (2019). Enzyme responsive drug delivery systems in cancer treatment . J Control Release 308 :172–89. [ PubMed ] [ Google Scholar ]
- Shi Z, Li Q, Mei L. (2020). pH-sensitive nanoscale materials as robust drug delivery systems for cancer therapy . Chin Chem Lett 31 :1345–56. [ Google Scholar ]
- Shrestha B, Wang L, Brey EM, et al. (2021). Smart nanoparticles for chemo-based combinational therapy . Pharmaceutics 13 :853. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Smiley SB, Yun Y, Ayyagari P, et al. (2021). Development of CD133 targeting multi-drug polymer micellar nanoparticles for glioblastoma – in vitro evaluation in glioblastoma stem cells . Pharm Res 38 :1067–79. [ PubMed ] [ Google Scholar ]
- Smith MC, Crist RM, Clogston JD, et al. (2017). Zeta potential: a case study of cationic, anionic, and neutral liposomes . Anal Bioanal Chem 409 :5779–87. [ PubMed ] [ Google Scholar ]
- Sousa F, Dhaliwal HK, Gattacceca F, et al. (2019). Enhanced anti-angiogenic effects of bevacizumab in glioblastoma treatment upon intranasal administration in polymeric nanoparticles . J Control Release 309 :37–47. [ PubMed ] [ Google Scholar ]
- Srinivasarao M, Galliford CV, Low PS. (2015). Principles in the design of ligand-targeted cancer therapeutics and imaging agents . Nat Rev Drug Discov 14 :203–19. [ PubMed ] [ Google Scholar ]
- Subhan MA, Yalamarty SSK, Filipczak N, et al. (2021). Recent advances in tumor targeting via EPR effect for cancer treatment . JPM 11 :571. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sun P, Wu Z, Xiao Y, et al. (2022). TfR-T12 short peptide and ph sensitive cell transmembrane peptide modified nano-composite micelles for glioma treatment via remodeling tumor microenvironment . Nanomed-Nanotechnol 41 :102516. [ PubMed ] [ Google Scholar ]
- Sun X, Chen Y, Zhao H, et al. (2018). Dual-modified cationic liposomes loaded with paclitaxel and survivin siRNA for targeted imaging and therapy of cancer stem cells in brain glioma . Drug Deliv 25 :1718–27. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sung H, Ferlay J, Siegel RL, et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries . CA Cancer J Clin 71 :209–49. [ PubMed ] [ Google Scholar ]
- Tan J, Duan X, Zhang F, et al. (2020). Theranostic nanomedicine for synergistic chemodynamic therapy and chemotherapy of orthotopic glioma . Adv Sci (Weinh) 7 :2003036. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tanaka N, Aoyama T, Kimura S, et al. (2017). Targeting nuclear receptors for the treatment of fatty liver disease . Pharmacol Ther 179 :142–57. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tang B, Fang GH, Gao Y, et al. (2015). Lipid-albumin nanoassemblies co-loaded with borneol and paclitaxel for intracellular drug delivery to C6 glioma cells with P-gp inhibition and its tumor targeting . Asian J Pharm Sci 10 :363–71. [ Google Scholar ]
- Tang Z, Liu Y, He M, et al. (2019). Chemodynamic therapy: tumour microenvironment-mediated Fenton and Fenton-like reactions . Angew Chem Int Ed Engl 58 :946–56. [ PubMed ] [ Google Scholar ]
- Topalian SL, Hodi FS, Brahmer JR, et al. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer . N Engl J Med 366 :2443–54. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Trachootham D, Alexandre J, Huang P. (2009). Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8 :579–91. [ PubMed ] [ Google Scholar ]
- Uram L, Misiorek M, Pichla M, et al. (2019). The effect of biotinylated PAMAM G3 dendrimers conjugated with COX-2 inhibitor (celecoxib) and PPARgamma agonist (Fmoc-L-Leucine) on human normal fibroblasts, immortalized keratinocytes and glioma cells in vitro . Molecules 24 :3801. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Vankayala R, Kuo CL, Nuthalapati K, et al. (2015). Nucleus-targeting gold nanoclusters for simultaneous in vivo fluorescence imaging, gene delivery, and NIR-light activated photodynamic therapy . Adv Funct Mater 25 :5934–45. [ Google Scholar ]
- Vidyarthi A, Agnihotri T, Khan N, et al. (2019). Predominance of M2 macrophages in gliomas leads to the suppression of local and systemic immunity . Cancer Immunol Immunother 68 :1995–2004. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Waldman AD, Fritz JM, Lenardo MJ. (2020). A guide to cancer immunotherapy: from T cell basic science to clinical practice . Nat Rev Immunol 20 :651–68. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang GK, Norton AS, Pokharel D, et al. (2013). KDEL peptide gold nanoconstructs: promising nanoplatforms for drug delivery . Nanomedicine 9 :366–74. [ PubMed ] [ Google Scholar ]
- Wang H, Wang S, Wang R, et al. (2019). Co-delivery of paclitaxel and melittin by glycopeptide-modified lipodisks for synergistic anti-glioma therapy . Nanoscale 11 :13069–77. [ PubMed ] [ Google Scholar ]
- Wang HB, Li Y, Bai HS, et al. (2017). A cooperative dimensional strategy for enhanced nucleus-targeted delivery of anticancer drugs . Adv Funct Mater 27 :1700339. [ Google Scholar ]
- Wang M, Lv CY, Li SA, et al. (2021). Near infrared light fluorescence imaging-guided biomimetic nanoparticles of extracellular vesicles deliver indocyanine green and paclitaxel for hyperthermia combined with chemotherapy against glioma . J Nanobiotechnol 19 :210. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang R, Ke ZF, Wang F, et al. (2015). GOLPH3 overexpression is closely correlated with poor prognosis in human non-small cell lung cancer and mediates its metastasis through upregulating MMP-2 and MMP-9 . Cell Physiol Biochem 35 :969–82. [ PubMed ] [ Google Scholar ]
- Wang T, Wang D, Yu H, et al. (2016). Intracellularly acid-switchable multifunctional micelles for combinational photo/chemotherapy of the drug-resistant tumor . ACS Nano 10 :3496–508. [ PubMed ] [ Google Scholar ]
- Weenink B, French PJ, Sillevis Smitt PAE, et al. (2020). Immunotherapy in glioblastoma: current shortcomings and future perspectives . Cancers 12 :751. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wei G, Wang Y, Yang G, et al. (2021). Recent progress in nanomedicine for enhanced cancer chemotherapy . Theranostics 11 :6370–92. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wen L, Wen C, Zhang F, et al. (2020). siRNA and chemotherapeutic molecules entrapped into a redox-responsive platform for targeted synergistic combination therapy of glioma . Nanomed 28 :102218. [ PubMed ] [ Google Scholar ]
- Wu M, Zhang H, Tie C, et al. (2018). MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma . Nat Commun 9 :4777. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Xu HL, Fan ZL, ZhuGe DL, et al. (2018). Ratiometric delivery of two therapeutic candidates with inherently dissimilar physicochemical property through pH sensitive core shell nanoparticles targeting the heterogeneous tumor cells of glioma . Drug Deliv 25 :1302–18. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Xu XD, Saw PE, Tao W, et al. (2017). ROS-responsive polyprodrug nanoparticles for triggered drug delivery and effective cancer therapy . Adv Mater 29 :1700141. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yan Y, Xu Z, Dai S, et al. (2016). Targeting autophagy to sensitive glioma to temozolomide treatment . J Exp Clin Cancer Res 35 :23. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ye C, Pan B, Xu H, et al. (2019). Co-delivery of GOLPH3 siRNA and gefitinib by cationic lipid-PLGA nanoparticles improves EGFR-targeted therapy for glioma . J Mol Med (Berl) 97 :1575–88. [ PubMed ] [ Google Scholar ]
- Yeini E, Ofek P, Albeck N, et al. (2021). Targeting glioblastoma: advances in drug delivery and novel therapeutic approaches . Adv Therap 4 :2000124. [ Google Scholar ]
- Yu L, McPhee CK, Zheng L, et al. (2010). Termination of autophagy and reformation of lysosomes regulated by mTOR . Nature 465 :942–6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yu RY, Xing L, Cui PF, et al. (2018). Regulating the golgi apparatus by co-delivery of a COX-2 inhibitor and brefeldin A for suppression of tumor metastasis . Biomater Sci 6 :2144–55. [ PubMed ] [ Google Scholar ]
- Zhang C, Bu W, Ni D, et al. (2016). Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized Fenton reaction . Angew Chem 128 :2141–6. [ PubMed ] [ Google Scholar ]
- Zhang J, Xiao X, Zhu J, et al. (2018). Lactoferrin- and RGD-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers for gliomatosis cerebri combination therapy . Int J Nanomedicine 13 :3039–51. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhang JH, Stevens MFG, Bradshaw TD. (2012). Temozolomide: mechanisms of action, repair and resistance . Curr Mol Pharmacol 5 :102–14. [ PubMed ] [ Google Scholar ]
- Zhao M, van Straten D, Broekman MLD, et al. (2020). Nanocarrier-based drug combination therapy for glioblastoma . Theranostics 10 :1355–72. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhao P, Wang Y, Kang X, et al. (2018). Dual-targeting biomimetic delivery for anti-glioma activity via remodeling the tumor microenvironment and directing macrophage-mediated immunotherapy . Chem Sci 9 :2674–89. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhao Y, Peng Y, Yang Z, et al. (2022). pH-redox responsive cascade-targeted liposomes to intelligently deliver doxorubicin prodrugs and lonidamine for glioma . Eur J Med Chem 235 :114281. [ PubMed ] [ Google Scholar ]
- Zhao Y, Qu B, Wu X, et al. (2014). Design, synthesis and biological evaluation of brain targeting l-ascorbic acid prodrugs of ibuprofen with "lock-in" function . Eur J Med Chem 82 :314–23. [ PubMed ] [ Google Scholar ]
- Zhao Y, Zhao Z, Cui Y, et al. (2021). Redox-responsive glycosylated combretastatin A-4 derivative as novel tubulin polymerization inhibitor for glioma and drug delivery . Drug Dev Res 82 :1063–72. [ PubMed ] [ Google Scholar ]
- Zheng M, Liu Y, Wang Y, et al. (2019). ROS-responsive polymeric sirna nanomedicine stabilized by triple interactions for the robust glioblastoma combinational RNAi therapy . Adv Mater 31 :1903277. [ PubMed ] [ Google Scholar ]
- Zheng Z, Zhang J, Jiang J, et al. (2020). Remodeling tumor immune microenvironment (TIME) for glioma therapy using multi-targeting liposomal codelivery . J Immunother Cancer 8 :e000207. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 26 June 2024
These 3D model brains with cells from several people are first of their kind
- Asher Mullard 0
Asher Mullard is a science journalist based in Ottawa.
You can also search for this author in PubMed Google Scholar
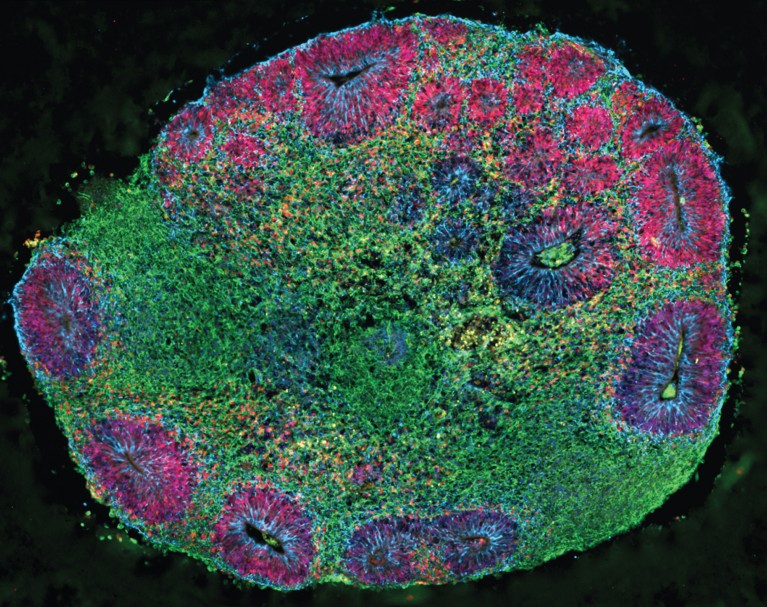
After one month of growth, a brain organoid comprised of cells from multiple human donors is just over one millimetre wide. Credit: N. Antón-Bolaños et al./Nature
For the first time, researchers have grown 3D models of the brain that include a wide variety of cell types from several people 1 . These ‘village in a dish’ organoids could help to reveal why the brain’s response to drugs differs from person to person.
Other teams have made 2D sheets of brain cells sourced from more than one human donor 2 , but this work reports 3D systems that are robust enough for research.
“It’s a really powerful technology, and a powerful approach,” says Tomasz Nowakowski, a biologist at the University of California, San Francisco, who was not involved in the study. Many groups are likely to embrace this method, he adds. “It’s a technical tour de force.”
These chimeric cultures, which the authors call Chimeroids, combine cells from as many as five donors. But future iterations could host cells from hundreds of people. “What if one day we could use Chimeroids as avatars to predict individual responses to new therapeutics before testing these in a trial? I like to imagine that future,” says Paola Arlotta, a stem-cell biologist at Harvard University in Cambridge, Massachusetts, and senior author of the study, which was published today in Nature .
It takes a village
Model systems called organoids mimic the cellular make-up of organs, such as the gut and the lungs. Researchers make them by bathing stem cells from a human donor in a precisely formulated cocktail of chemicals, which encourages the stem cells to mature into all the cell types that are typically present in a given organ. The culture conditions also encourage the cells to gather into a complex 3D shape.
Brain organoids are particularly slow-growing and finicky to use, and researchers have been on the hunt for better ways to make them. One approach has been to combine cells from several donors into a single organoid. Multi-donor clumps of cells might be easier to work with, and would capture a broad diversity of human genetics in a single model. However, because the starting stem cells grow at different paces, fast-growing lines inevitably take over.
Out of many, one
The trick, Arlotta and her colleagues now report, is to first make a set of single-donor organoids. As these mature, the cells in all the organoids take on similar growth rates. By then homogenizing these structures and pooling the cells together, it is possible to grow a composite organoid. The authors’ Chimeroids expanded to about 3–5 millimetres after three months and contain the same cell types that are present in fetal cortical tissue.
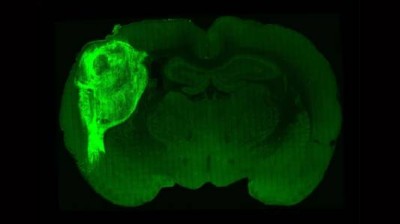
Human brain cells implanted in rats prompt excitement — and concern
“This is a really good advance,” says Robert Vries, chief executive of organoid-research firm HUB Organoids in Utrecht, the Netherlands. The community that studies the central nervous system “really needs more organoid systems”.
Chimeroids should enable researchers to work out whether drugs will have distinct effects on different people. As a test case, the team treated the multi-donor organoids with neurotoxic drugs. Ethanol, which causes fetal alcohol syndrome , reduced the number of cells from just one donor’s cell line. Cells from that donor grew faster when combined with valproic acid, an anti-epileptic drug linked to an increased risk of autism spectrum disorder in children who’d been exposed to it in utero .
Growing pains
But careful follow-on work will be needed to ensure that any effects seen in the chimeric models come from the genetics of a given cell line, rather than from an interaction between closely packed cells, cautions Vries.
Chimeroids are also labour-intensive to grow, adds Nowakowski, who is experimenting with the model in his laboratory. But automated cell-culture systems should ease the workload and make these models viable for more-efficient experiments into diseases of the brain.
doi: https://doi.org/10.1038/d41586-024-02096-z
Read the related News & Views: ‘ Chimeric brain organoids capture human genetic diversity .’
Antón-Bolaños, N. et al. Nature https://doi.org/10.1038/s41586-024-07578-8 (2024).
Article Google Scholar
Wells, M. F. et al. Cell Stem Cell 30 , 312–332 (2023).
Article PubMed Google Scholar
Download references
Reprints and permissions
Related Articles
- Neuroscience

How blockbuster obesity drugs create a full feeling — even before one bite of food
News 27 JUN 24

Chimeric brain organoids capture human genetic diversity
News & Views 26 JUN 24
How the ‘mind’s eye’ calls up visual memories from the brain
News 14 JUN 24
Brain Chimeroids reveal individual susceptibility to neurotoxic triggers
Article 26 JUN 24
Embryo models need consistent ethical oversight
Correspondence 11 JUN 24

Bionic leg moves like a natural limb — without conscious thought
News 01 JUL 24
Osaka University Immunology Frontier Research Center Postdoctoral Researcher
IFReC, Osaka University in Japan offers Advanced Postdoc Positions for Immunology, Cell Biology, Bioinformatics and Bioimaging.
Suita Campus, Osaka University in Osaka, Japan
Immunology Frontier Research Center, Osaka University
PostDoc Researcher, Magnetic Recording Materials Group, National Institute for Materials Science
Starting date would be after January 2025, but it is negotiable.
Tsukuba, Japan (JP)
National Institute for Materials Science
Tenure-Track/Tenured Faculty Positions
Tenure-Track/Tenured Faculty Positions in the fields of energy and resources.
Suzhou, Jiangsu, China
School of Sustainable Energy and Resources at Nanjing University
Postdoctoral Associate- Statistical Genetics
Houston, Texas (US)
Baylor College of Medicine (BCM)
Senior Research Associate (Single Cell/Transcriptomics Senior Bioinformatician)
Metabolic Research Laboratories at the Clinical School, University of Cambridge are recruiting 3 senior bioinformatician specialists to create a dynam
Cambridge, Cambridgeshire (GB)
University of Cambridge
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
An NCI-supported study called OPTIMUM, part of the Cancer Moonshot, was launched to improve the care of people with brain tumors called low-grade glioma in part by bringing them into glioma-related research. Treating craniopharyngioma often requires surgery, radiation therapy, or both.
A paper published alongside the two 2019 brain-cancer papers showed that breast-cancer metastases in the brain could also form synapse-like connections 9. And previous research has linked brain ...
Nature Reviews Cancer 20 , 1 ( 2020) Cite this article. This Focus issue highlights current research into the unique biology of brain tumours and brain metastasis and how this research might ...
Brain cancer. Research on one of the deadliest malignancies is yielding potentially game-changing insights — and treatments. Any type of cancer presents an insidious risk to life. But the ...
The published articles are based on neuro-oncological research and deal with proposing novel effective therapeutic strategies, focusing on different targets and aspects typical of brain tumors: tumor heterogeneity and microenvironment, cancer cell response to new chemotherapeutics and innovative radiotherapy treatments settings (often tested in ...
Brain tumors are among the most lethal tumors. Glioblastoma, the most frequent primary brain tumor in adults, has a median survival time of approximately 15 months after diagnosis or a five-year survival rate of 10%; the recurrence rate is nearly 90%. Unfortunately, this prognosis has not improved for several decades.
1. Introduction. Brain and central nervous system cancer is an important public health issue worldwide, considering the high mortality, economic burden for persons and society, the low survival rate, and the effect on the patients' quality of life [1,2].Based on Global Cancer Observatory (GLOBOCAN) 2020 estimates, brain and central nervous system cancer is a considerable part of the global ...
March 14, 2024 5 min read. A collaborative project to bring the promise of cell therapy to patients with a deadly form of brain cancer has shown dramatic results among the first patients to receive the novel treatment. In a paper published Wednesday in The New England Journal of Medicine, researchers from Mass General Cancer Center shared the ...
Brain Cancer. Brain cancers--both those originating from the central nervous system and those that metastasize to the brain from elsewhere in the body--are highly lethal, heterogeneous, and intractable tumors. Their clinical management is further complicated by the blood-brain barrier, which excludes the majority of conventional therapeutics ...
Learn about the latest research on diagnosis, treatment, and survivorship of brain and spinal cord tumors, funded by the National Cancer Institute. Find out how NCI-supported scientists are using personalized medicine, targeted therapy, and artificial intelligence to improve outcomes for people with these rare cancers.
1 INTRODUCTION. Although primary brain tumours are relatively uncommon, representing only 1.6% of all cancers (Bray et al., 2018), they carry with them significant disability and mortality.Glioblastoma, usually diagnosed in the sixth or seventh decade of life, is the most aggressive glioma in adults (Ostrom et al., 2018), with a median survival of one and a half years (Gilbert et al., 2014 ...
The genomics of brain cancer. A flood of sequencing data is enabling researchers to uncover how tumours differ from each other, and what such variation means for treatment strategies. In 2016, the ...
Read current medical research and news articles on brain tumor surgery and related information. ... May 30, 2024 — Brain cancer is difficult to treat when it starts growing, and a prevalent type ...
Modern radiation techniques such as intensity modulated radiation therapy (IMRT), volumetric modulated arc therapy (VMAT), proton beam therapy, and image-guided radiation therapy (IGRT) are described in Radiation Therapy for Adult Brain and Spinal Cord Tumors. Other new methods of planning and delivering radiation therapy are also being studied.
Brain Cancer research articles are listed. Brain cancer news covers topics such as diagnosis, brain tumors, chemotherapy, gamma knife technology, brain cancer treatments, glioblastomas, stem cell research, neurosurgery, medicine, genetics, neurology, and other brain research.
Introduction. Brain tumours are among the most feared of all forms of cancer. More than two-thirds of adults diagnosed with glioblastoma — the most aggressive type of brain cancer — will die within 2 years of diagnosis 1,2.Brain cancers are also the most common and most lethal of all paediatric solid tumours 3.Furthermore, children with these tumours who survive and enter adulthood will ...
Brain metastases occur in approximately 40% of patients presenting with metastatic cancer and often occur in tumor types driven by RAS-MAPK pathway dysregulation, including melanoma, where response rates are low in intracranial disease (39, 40). To model melanoma brain metastases, we used a luciferase-tagged model system for the study of ...
A noninvasive treatment may help to counter "chemo brain" impairment often seen in chemotherapy patients: Exposure to light and sound with a frequency of 40 hertz protected brain cells from chemotherapy-induced damage in mice, MIT researchers found. Patients undergoing chemotherapy often experience cognitive effects such as memory ...
Challenge 1: redesign research pipeline. Clinical trials have yet to reveal an effective therapy for most brain tumours. This harsh reality stems, in part, from an incomplete understanding of ...
MONDAY, June 24, 2024 (HealthDay News) -- U.S. children with inoperable brain tumors appear to die sooner and find it harder to get care if they live in poorer neighborhoods, a new study finds. Children from higher-income areas had more than double the average survival time than kids from poorer ...
Over decades, cancer understanding has advanced significantly at molecular and cellular levels, leading to various therapies based on intra-/inter-cellular networks. Despite this, cancer still remains a leading cause of death globally. The primary driver of cancer mortality is metastasis, responsible for about 90% of cancer deaths, due to unclear pathophysiological mechanisms that complicate ...
The role of mRNA vaccines in brain cancer is an area of active research and development. Although mRNA vaccines have shown promise in other disease areas, their specific application for brain cancer is still in the early stages. Due to their potency in stimulation of immunity, as well as the precedent of one FDA approved immunotherapy (Provenge ...
The non-invasive medical device, Exablate Neuro (approved by the FDA in 2021), uses focused ultrasound on one side of the brain to provide relief from severe symptoms such as tremors, limb rigidity, and dyskinesia. 2023 brought major news for Parkinson's disease research with the validation of the biomarker alpha-synuclein.
A transcranial 532 nm picosecond laser activates nanoparticles within the tumour, temporarily weakening the blood-brain barrier (BBB) for enhanced drug delivery. The BBB is depicted by endothelial ...
The machine produces particles for use in cancer research, testing and therapies. ... Christian, a medical physicist who is UW-Madison's director of PET physics and co-director of UW's Waisman Center Brain Imaging Laboratory. The new machine's energy capacity will range up to 30 megaelectron volts, compared to the existing cyclotron's ...
The National Brain Tumor Society and the Parker Institute for Cancer Immunotherapy partnered to host a workshop to share recent data, ideas and identify both hurdles and new opportunities for harnessing immunotherapy against pediatric and adult brain tumors. Adoptively transferred cell therapies have recently shown promising early clinical results.
Researchers are unsure why this might be. About 55% of malignant brain tumours occurred in men, compared with 45% in women, between 2008 and 2012 in the United States 1. However, only around 36% ...
ABSTRACT I argue that this examination and appreciation for the shift to abductive reasoning should be extended to the intersection of neuroscience and novel brain-computer interfaces too. This paper highlights the implications of applying abductive reasoning to personalized implantable neurotechnologies. Then, it explores whether abductive reasoning is sufficient to justify insurance coverage ...
1. Introduction. Brain tumor accounts for about 1.6% of incidence and 2.5% of mortality of all tumors respectively according to the latest global cancer data released by the World Health Organization (WHO) in 2020 (Sung et al., 2021 ). While, in China, the morbidity and mortality of brain tumors rank first in the global brain tumors, up to 32% ...
Mini-colon and brain 'organoids' shed light on cancer and other diseases Subjects. Brain; Stem cells; ... Research articles News Opinion ...