
Our top 5 cancer research breakthroughs of 2022
15th December 2022
2022 has likely been your first full year back to ‘normal’ since the COVID-19 pandemic, and that’s no different for cancer researchers. Back into full swing in the lab, Worldwide Cancer Research scientists have been busy making exciting new discoveries about cancer. Here are the top five cancer research breakthroughs made by our scientists in 2022.
1. Stopping the spread of breast cancer
Our scientists in Italy discovered a previously unknown way that breast cancer cells survive treatment . They found that breast cancer cells hiding in places like the lungs seem to rely on specific antioxidants to survive there. This could be a new way to wipe out breast cancer cells that have escaped treatment.
Click here to read more about this incredible discovery, or find out what other ground-breaking work we're funding .
Our hope is that these findings can be translated into a real drug treatment that can kill “sleeping” cancer cells before they awake into full-blown metastases.
2. Stool samples can reveal pancreatic cancer sooner
Our researchers in Spain, led by Dr Núria Malats, have found a new way to spot if someone is at higher risk of pancreatic cancer, and even diagnose patients at an early stage of the disease . Specific microorganisms in a stool sample could signal that there is a problem in a rapid, non-invasive, and affordable way.
Read the full story here , or find out what else our scientists have discovered about pancreatic cancer this year.

This new breakthrough builds on the growing evidence that the microbiome is linked to the development of cancer. Early detection and diagnosis are just as important an approach to starting new cancer cures as developing treatments.
Become a Curestarter today and join us in supporting pioneering cancer research breakthroughs like these in 2023.
3. targeting cancer’s energy supply.
Our scientists in Germany discovered they could prevent head and neck cancer spreading by stopping it getting the extra energy it needs to do so. If they prevented a change happening in the RNA of mitochondria (the powerhouse of the cell), the cancer didn’t spread as much.
Click here to learn more about this breakthrough, or learn how your donations are used to start cancer cures .

The funding from Worldwide Cancer Research helped us to get a step closer to succeeding in fighting cancer metastasis, the leading cause of cancer death.
4. Making radiotherapy work for more patients
Our researchers in Spain made a breakthrough that could help treat people with cancer that has spread to the brain . In the future, a blood test could reveal if patients will respond to radiotherapy or if the cancer will resist it, then a drug called a RAGE inhibitor could make radiotherapy work better for those that would resist it.
Learn more about this important discovery , or read Anne and Cathrin's personal story.

We are very excited about the findings of this study and specifically the drug we have found. We really hope that what we have discovered will lead to a new way to personalise the use of radiotherapy that maximises the benefits for each patient.
5. Engineering immune cells to hunt down cancer
Our researchers in Italy have made a breakthrough that could lead to better, more effective immunotherapy options for cancer patients . They discovered how to engineer a specific type of immune cell to target and kill cancer cells, then boost its cancer-killing ability using a drug delivered with nanotechnology.
Find out the full story , or see how we've contributed to the discovery of new immunotherapies .

We hope we have laid the foundations for an innovative approach of adoptive cell therapy of cancer, hopefully more efficient than the current ones.

Breakthroughs of the future: 2023!
And finally, we were delighted to commit to funding £5.3 million to 25 new research projects that will start in 2023! It is incredibly important to continue supporting ground-breaking discovery cancer research if we hope to end the suffering caused by cancer.
Breakthroughs like these are vital if we hope to end the suffering caused by cancer. Unfortunately, funding for discovery research has dropped by ~25% in recent years.
Become a Curestarter today and you can help start new cancer cures.

Join our monthly newsletter
Keep up to date with all our latest news, events, groundbreaking research discoveries and much more!
You're now a Curestarter!
Our newsletter usually drops towards the end of each month
Thanks for subscribing
Explore CRI’s 2023 Cancer Research Impact
Cancer Research Institute Media Room

Cellular Cancer Immunotherapy Development Evolves, Expands with New Technologies and Targets
Latest analysis from the Cancer Research Institute of the global landscape of cellular immunotherapies, including R&D trends and real-world usage, highlights key challenges including effective solid tumor targeting, manufacturing complexities, and commercial access to approved therapies
The Cancer Research Institute (CRI), a nonprofit organization dedicated to the discovery and development of powerful immunotherapies for all types of cancer, announced today the publication of its newest analysis of the global landscape of cellular immunotherapies, including R&D trends and real-world usage data. The report, published today in Nature Reviews Drug Discovery , highlights trends in cellular immunotherapy for cancer including top modalities, targets, clinical development, and data from patients receiving CAR-T therapies in clinical practice. This report is an update to CRI’s prior cellular immunotherapy landscape analysis published in July 2021.
In this analysis, author Samik Upadhaya, PhD, assistant director of scientific affairs and member of the Anna-Maria Kellen Clinical Accelerator team at CRI, and colleagues provide an update on the overall status of the cellular cancer immunotherapy landscape as well as observations on key changes within the field including clinical practice. Findings include:
- As of April 15, 2022, there were 2,756 active cell therapy agents in the global immuno-oncology pipeline, an increase of 36% over the 2021 landscape analysis that identified 2,031 such agents, but also a modest deceleration compared to 43% growth in the prior year
- CAR-T therapeutics continue to dominate the cell therapy pipeline with growth of 24% since 2021
- Development continues for non-T cell therapies including NK cell, dendritic cell, stem cell, and other myeloid-derived cell therapies, with the greatest growth in NK cell therapy, up 55% over the prior year
- Clinical usage of cell therapy for cancer treatment is not keeping pace with regulatory approvals, with clinicians citing cost, travel, and supply limitations as key barriers to patient access
This latest report from the Cancer Research Institute, titled, “Landscape of cancer cell therapies: trends and real-world data,” was generated in collaboration with IQVIA, a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry, which provided the authors with access to IQVIA’s proprietary clinical trials database. The report is part of a suite of CRI-owned immuno-oncology landscape analyses that includes reports on cell therapy drug development and the broader IO landscape including clinical development of checkpoint inhibitors, cancer vaccines, and oncolytic viruses in addition to bispecific antibodies and other immunomodulators.
To access an interactive dashboard of the Cancer Research Institute’s cancer cell immunotherapy report, visit the CRI website at cancerresearch.org/cell-therapy .
About the Cancer Research Institute The Cancer Research Institute (CRI), established in 1953, is a highly rated U.S. nonprofit organization dedicated exclusively to saving more lives by fueling the discovery and development of powerful immunotherapies for all cancers. Guided by a world-renowned Scientific Advisory Council that includes four Nobel laureates and 27 members of the National Academy of Sciences, CRI has invested $474 million in support of research conducted by immunologists and tumor immunologists at the world’s leading medical centers and universities and has contributed to many of the key scientific advances that demonstrate the potential for immunotherapy to change the face of cancer treatment. To learn more, go to cancerresearch.org .
About the Anna-Maria Kellen Clinical Accelerator CRI’s clinical program, the Anna-Maria Kellen Clinical Accelerator is a unique academic-nonprofit-industry collaboration model that serves an as “incubator” that delivers multicenter clinical trials of promising new immunotherapy combinations. CRI’s venture philanthropy fund supports clinical trials within the program, which fosters a collaborative environment that enables scientists to advance their most ambitious research ideas by accelerating studies that one group or company could not do alone. To learn more, go to cancerresearch.org/clinical-accelerator .
Let's spread the word about Immunotherapy! Click to share this page with your community.
This website uses tracking technologies, such as cookies, to provide a better user experience. If you continue to use this site, then you acknowledge our use of tracking technologies. For additional information, review our Privacy Policy .
- Skip to main content
- Keyboard shortcuts for audio player
This experimental drug could change the field of cancer research

Sacha Pfeiffer

Jonaki Mehta

The new treatment is categorized as immunotherapy. skaman306/Getty Images hide caption
The new treatment is categorized as immunotherapy.
A tiny group of people with rectal cancer just experienced something of a scientific miracle: their cancer simply vanished after an experimental treatment.
In a very small trial done by doctors at New York's Memorial Sloan Kettering Cancer Center, patients took a drug called dostarlimab for six months. The trial resulted in every single one of their tumors disappearing. The trial group included just 18 people, and there's still more to be learned about how the treatment worked. But some scientists say these kinds of results have never been seen in the history of cancer research.
Dr. Hanna Sanoff of the University of North Carolina's Lineberger Comprehensive Cancer Center joined NPR's All Things Considered to outline how this drug works and what it could mean for the future of cancer research. Although she was not involved with the study, Dr. Sanoff has written about the results.
This interview has been lightly edited
On her first reaction to the results: I mean, I am incredibly optimistic. Like you said in the introduction, we have never seen anything work in 100 percent of people in cancer medicine.
On how the drug works to treat cancer: This drug is one of a class of drugs called immune checkpoint inhibitors. These are immunotherapy medicines that work not by directly attacking the cancer itself, but actually getting a person's immune system to essentially do the work. These are drugs that have been around in melanoma and other cancers for quite a while, but really have not been part of the routine care of colorectal cancers until fairly recently.
On the kinds of side effects patients experienced: Very, very few in this study - in fact, surprisingly few. Most people had no severe adverse effects at all.
On how this study could be seen as 'practice-changing': Our hope would be that for this subgroup of people - which is only about five percent to 10 percent of people who have rectal cancer - if they can go on and just get six months of immunotherapy and not have any of the rest of this - I don't even know the word to use. Paradigm shift is often used, but this really absolutely is paradigm-shifting.
On why the idea of being able to skip surgery for cancer treatment is so revolutionary: In rectal cancer, this is part of the conversation we have with someone when they're diagnosed. We are very hopeful for being able to cure you, but unfortunately, we know our treatments are going to leave you with consequences that may, in fact, be life-changing. I have had patients who, after their rectal cancer, have barely left the house for years - and in a couple of cases, even decades - because of the consequences of incontinence and the shame that's associated with this.
On next steps for the drug: What I'd really like us to do is get a bigger trial where this drug is used in a much more diverse setting to understand what the real, true response rate is going to be. It's not going to end up being 100 percent. I hope I bite my tongue on that in the future, but I can't imagine it will be 100 percent. And so when we see what the true response rate is, that's when I think we can really do this all the time.
This piece was reported by Sacha Pfeiffer, produced by Jonaki Mehta and edited by Kathryn Fox. It was adapted for the web by Manuela Lopez Restrepo.
- cancer treatment
- History, Facts & Figures
- YSM Dean & Deputy Deans
- YSM Administration
- Department Chairs
- YSM Executive Group
- YSM Board of Permanent Officers
- FAC Documents
- Current FAC Members
- Appointments & Promotions Committees
- Ad Hoc Committees and Working Groups
- Chair Searches
- Leadership Searches
- Organization Charts
- Faculty Demographic Data
- Professionalism Reporting Data
- 2022 Diversity Engagement Survey
- State of the School Archive
- Faculty Climate Survey: YSM Results
- Strategic Planning
- Mission Statement & Process
- Beyond Sterling Hall
- COVID-19 Series Workshops
- Previous Workshops
- Departments & Centers
- Find People
- Biomedical Data Science
- Health Equity
- Inflammation
- Neuroscience
- Global Health
- Diabetes and Metabolism
- Policies & Procedures
- Media Relations
- A to Z YSM Lab Websites
- A-Z Faculty List
- A-Z Staff List
- A to Z Abbreviations
- Dept. Diversity Vice Chairs & Champions
- Dean’s Advisory Council on Lesbian, Gay, Bisexual, Transgender, Queer and Intersex Affairs Website
- Minority Organization for Retention and Expansion Website
- Office for Women in Medicine and Science
- Committee on the Status of Women in Medicine Website
- Director of Scientist Diversity and Inclusion
- Diversity Supplements
- Frequently Asked Questions
- Recruitment
- By Department & Program
- News & Events
- Executive Committee
- Aperture: Women in Medicine
- Self-Reflection
- Portraits of Strength
- Mindful: Mental Health Through Art
- Event Photo Galleries
- Additional Support
- MD-PhD Program
- PA Online Program
- Joint MD Programs
- How to Apply
- Advanced Health Sciences Research
- Clinical Informatics & Data Science
- Clinical Investigation
- Medical Education
- Visiting Student Programs
- Special Programs & Student Opportunities
- Residency & Fellowship Programs
- Center for Med Ed
- Organizational Chart
- Leadership & Staff
- Committee Procedural Info (Login Required)
- Faculty Affairs Department Teams
- Recent Appointments & Promotions
- Academic Clinician Track
- Clinician Educator-Scholar Track
- Clinican-Scientist Track
- Investigator Track
- Traditional Track
- Research Ranks
- Instructor/Lecturer
- Social Work Ranks
- Voluntary Ranks
- Adjunct Ranks
- Other Appt Types
- Appointments
- Reappointments
- Transfer of Track
- Term Extensions
- Timeline for A&P Processes
- Interfolio Faculty Search
- Interfolio A&P Processes
- Yale CV Part 1 (CV1)
- Yale CV Part 2 (CV2)
- Samples of Scholarship
- Teaching Evaluations
- Letters of Evaluation
- Dept A&P Narrative
- A&P Voting
- Faculty Affairs Staff Pages
- OAPD Faculty Workshops
- Leadership & Development Seminars
- List of Faculty Mentors
- Incoming Faculty Orientation
- Faculty Onboarding
- Past YSM Award Recipients
- Past PA Award Recipients
- Past YM Award Recipients
- International Award Recipients
- Nominations Calendar
- OAPD Newsletter
- Fostering a Shared Vision of Professionalism
- Academic Integrity
- Addressing Professionalism Concerns
- Consultation Support for Chairs & Section Chiefs
- Policies & Codes of Conduct
- First Fridays
- Fund for Physician-Scientist Mentorship
- Grant Library
- Grant Writing Course
- Mock Study Section
- Research Paper Writing
- Establishing a Thriving Research Program
- Funding Opportunities
- Join Our Voluntary Faculty
- Child Mental Health: Fostering Wellness in Children
- Faculty Resources
- Research by Keyword
- Research by Department
- Research by Global Location
- Translational Research
- Research Cores & Services
- Program for the Promotion of Interdisciplinary Team Science (POINTS)
- CEnR Steering Committee
- Experiential Learning Subcommittee
- Goals & Objectives
- Issues List
- Print Magazine PDFs
- Print Newsletter PDFs
- YSM Events Newsletter
- Social Media
- Patient Care
INFORMATION FOR
- Residents & Fellows
- Researchers
New Research and Treatment Advances From Yale Cancer Center to be Presented at the World’s Largest Cancer Research Conference
2024 asco annual meeting.
Nearly 50 presentations by researchers and clinicians from Yale Cancer Center (YCC) at Yale School of Medicine will be among the more than 5,000 abstracts available during the annual meeting of the American Society of Clinical Oncology (ASCO) May 31 to June 4 in Chicago, Ill.
This year's meeting, themed “The Art and Science of Cancer Care: From Comfort to Cure” will include over 200 sessions. The 48 YCC presentations will include phase II trial results for a cancer vaccine in combination with an immunotherapy drug, a new tool to predict the risk of recurrence of hormone receptor-positive, HER2-negative breast cancer, and the factors affecting sexual function of young women with breast cancer.
YCC experts will deliver 10 oral presentations and 38 poster presentations. They include:
Poster Session
A randomized phase 2 trial of the IO102-IO103 (IDO and PD-L1) cancer vaccine plus pembrolizumab as neoadjuvant/adjuvant treatment of patients with solid tumors
June 1; 9:00AM CDT
Presenter and Senior Author: Barbara Burtness, MD
Safety and time to response of [177Lu]Lu-DOTATATE in patients with newly diagnosed advanced grade 2 and grade 3, well-differentiated gastroenteropancreatic neuroendocrine tumors: Sub-analysis of the phase 3 randomized NETTER-2 study
June 1; 1:30PM CDT
Presenter: Pamela Kunz, MD
Oral Abstract
A phase 1 dose expansion study of a first-in-class KAT6 inhibitor (PF-07248144) in patients with advanced or metastatic ER+ HER2− breast cancer
June 1; 4:24PM CDT
Presenter: Pat LoRusso, DO
ARC-9: A randomized study to evaluate etrumadenant based treatment combinations in previously treated metastatic colorectal cancer (mCRC)
June 2; 10:24AM CDT
Presenter and Senior Author: Michael Cecchini, MD
ARV-766, a proteolysis targeting chimera (PROTAC) androgen receptor (AR) degrader, in metastatic castration-resistant prostate cancer (mCRPC): Initial results of a phase 1/2 study
June 3; 1:27PM CDT
Presenter: Daniel Petrylak, MD
Factors associated with sexual function and sexual satisfaction in young women with breast cancer
June 3; 1:30PM CDT
Presenter: Ana Ferrigno Guajardo, MD
Updated results from COAST, a phase 2 study of durvalumab (D) ± oleclumab (O) or monalizumab (M) in patients (pts) with stage III unresectable non-small cell lung cancer (uNSCLC)
Presenter and Senior Author: Roy Herbst, MD, PhD
Development and validation of RSClin N+ tool for hormone receptor-positive (HR+), HER2-negative (HER2-), node-positive breast cancer
June 3; 5:24PM CDT
Presenter: Lajos Pusztai, MD
Click here to see the full list of YCC presentations.
Yale Cancer Center combines a tradition of innovative cancer treatment and quality care for our patients. A National Cancer Institute (NCI) designated comprehensive cancer center since 1974, Yale Cancer Center is one of only 56 such centers in the nation and the only one in Connecticut. Yale Cancer Center members include national and internationally renowned scientists and physicians at Yale School of Medicine and Smilow Cancer Hospital. This partnership enables the Center to provide the best approaches for prevention, detection, diagnosis, and treatment for cancer.
Media Contact:
Michael Masciadrelli [email protected]
Featured in this article
- Patricia LoRusso, DO Amy and Joseph Perella Professor of Medicine (Medical Oncology); Chief, Experimental Therapeutics; Associate Cancer Center Director, Experimental Therapeutics
- Roy S. Herbst, MD, PhD Ensign Professor of Medicine (Medical Oncology) and Professor of Pharmacology; Deputy Director, Yale Cancer Center; Chief of Medical Oncology, Yale Cancer Center and Smilow Cancer Hospital; Assistant Dean for Translational Research, Yale School of Medicine; Director, Center for Thoracic Cancers, Yale Cancer Center and Smilow Cancer Hospital; Program Director, Master of Health Science - Clinical Investigation Track (MHS-CI)
- Barbara Burtness, MD Anthony N. Brady Professor of Medicine (Medical Oncology); Chief Translational Research Officer, Yale Cancer Center; Chief, Head and Neck Cancers/Sarcoma; Co-Leader, Developmental Therapeutics, Yale Cancer Center; Associate Cancer Center Director for Translational Research, Yale Cancer Center
- Daniel P. Petrylak, MD Professor of Medicine (Medical Oncology) and of Urology; Chief, Genitourinary Oncology
- Michael Cecchini, MD Assistant Professor of Medicine (Medical Oncology); Co-Director, Colorectal Program in the Center for Gastrointestinal Cancers; Medical Oncology Section Lead for National Accreditation Program for Rectal Cancer, Internal Medicine
- Pamela L. Kunz, MD Associate Professor of Internal Medicine (Medical Oncology); Director, Center for Gastrointestinal Cancers at Smilow Cancer Hospital and Yale Cancer Center; Chief, GI Medical Oncology
- Lajos Pusztai, MD, DPhil Professor of Medicine (Medical Oncology); Co-Leader, Genetics, Genomics and Epigenetics, Yale Cancer Center
- Ana Ferrigno Guajardo, MD Hospital Resident
Cancer News
Top headlines, latest headlines.
- New Tech: Tailoring Cancer Treatment
- Melanoma in Darker Skin Tones
- Production of Stomach Acids
- Control of Stem Cell Differentiation
- 3D Pathology With Help of AI
- AI, Tissues, Drug Discovery and Diagnostics
- New Target for Potential Leukemia Therapy
- Strengthening CAR-T Therapy: Solid Tumors
- AI Predicts Tumor-Killing Cells
- New Cancer Treatments
Earlier Headlines
Tuesday, may 7, 2024.
- US Geographic Region Results in Vastly Different Anal Cancer Risk for People With HIV
- Intermittent Fasting Protects Against Liver Inflammation and Liver Cancer
- Study Sheds Light on Cancer Cell 'tug-of-War'
- Researchers Use Foundation Models to Discover New Cancer Imaging Biomarkers
Monday, May 6, 2024
- Past and Guides Future Efforts to Reduce Cancer Disparities
- Simulated Chemistry: New AI Platform Designs Tomorrow's Cancer Drugs
- Expanding a Lymph Node, Boosting a Vaccine
Friday, May 3, 2024
- Newly Discovered Mechanism of T-Cell Control Can Interfere With Cancer Immunotherapies
- Pan-Cancer Analysis Uncovers a New Class of Promising CAR T--Cell Immunotherapy Targets
- New Immunosuppressive Mechanism Found in Brain Cancer
Thursday, May 2, 2024
- Scientists Track 'doubling' In Origin of Cancer Cells
- Gene Signatures from Tissue-Resident T Cells as a Predictive Tool for Melanoma Patients
- Cancer Patients Gain Important Benefits from Genome-Matched Treatments
- Medical School Scientist Creates Therapy to Kill Hypervirulent Bacteria
Wednesday, May 1, 2024
- Therapy to Kill Hypervirulent Bacteria Developed
- Research Breakthrough on Birth Defect Affecting Brain Size
- Unraveling the Roles of Non-Coding DNA Explains Childhood Cancer's Resistance to Chemotherapy
- Biomarkers in Blood to Predict Liver Cancer
- New mRNA Cancer Vaccine Triggers Fierce Immune Response to Fight Malignant Brain Tumor
- Clogged Arteries Worsened by Cells That Behave Like Cancer Cells
- One-Two Punch Treatment Delivers Blood Cancer Knockout
Tuesday, April 30, 2024
- Difference Found in Pancreatic Cancer Cells, Offering New Hope for Immunotherapy Effectiveness
- New and Improved Way to Grow the Cells That Give Rise to the Kidney's Filtration System
- Regulating Cholesterol Levels Might Be the Key to Improving Cancer Treatment
- Scientists Find Cancer-Like Features in Atherosclerosis, Spurring Opportunity for New Treatment Approaches
Monday, April 29, 2024
- Kaposi Sarcoma Discovery Could Facilitate Drug Development
- The Aspirin Conundrum: Navigating Negative Results, Age, Aging Dynamics and Equity
- Blood Samples Enhance B-Cell Lymphoma Diagnostics and Prognosis
Friday, April 26, 2024
- Breast Cancer Rates Rising Among Canadian Women in Their 20s, 30s and 40s
- Component of Keto Diet Plus Immunotherapy May Reduce Prostate Cancer
Thursday, April 25, 2024
- Vitamin D Alters Mouse Gut Bacteria to Give Better Cancer Immunity
- Physical Activity in Nature Helps Prevent Several Diseases, Including Depression and Type 2 Diabetes
- Circadian Rhythms Can Influence Drugs' Effectiveness
Wednesday, April 24, 2024
- Tumor Cells Evade the Immune System Early On: Newly Discovered Mechanism Could Significantly Improve Cancer Immunotherapies
- Mini-Colons Revolutionize Colorectal Cancer Research
- Artificial Intelligence Can Develop Treatments to Prevent 'superbugs'
- Scientists Identify and Show How to Target a Key Tumor Defense Against Immune Attack
- CAR T Cell Therapy Targeting HER2 Antigen Shows Promise Against Advanced Sarcoma in Phase I Trial
- Discovering Cancers of Epigenetic Origin Without DNA Mutation
Tuesday, April 23, 2024
- Researching Cancer by Studying Lipids Cell by Cell
- Genetics Predict Type 2 Diabetes Risk and Disparities in Childhood Cancer Survivors
- New Study Uncovers Lasting Financial Hardship Associated With Cancer Diagnosis for Working-Age Adults in the U.S.
- Liver Cancer: Molecular Signaling Pathway of Tumor Development Decoded
Monday, April 22, 2024
- Breakthrough Rice Bran Nanoparticles Show Promise as Affordable and Targeted Anticancer Agent
- Genetically Engineering a Treatment for Incurable Brain Tumors
Friday, April 19, 2024
- Researchers Develop a New Way to Safely Boost Immune Cells to Fight Cancer
- Study Opens New Avenue for Immunotherapy Drug Development
Thursday, April 18, 2024
- Mutations in Noncoding DNA Become Functional in Some Cancer-Driving Genes
- AI Tool Predicts Responses to Cancer Therapy Using Information from Each Cell of the Tumor
- Siblings With Unique Genetic Change Help Scientists Progress Drug Search for Type 1 Diabetes
- New Urine-Based Test Detects High-Grade Prostate Cancer, Helping Men Avoid Unnecessary Biopsies
Wednesday, April 17, 2024
- Researchers Uncover Human DNA Repair by Nuclear Metamorphosis
Tuesday, April 16, 2024
- Researchers Discover Urine-Based Test to Detect Head and Neck Cancer
- Nanoparticle Delivery of FZD4 to Lung Endothelial Cells Inhibits Lung Cancer Progression and Metastases
- New Insights Could Unlock Immunotherapy for Rare, Deadly Eye Cancer
Monday, April 15, 2024
- Next-Generation Treatments Hitch a Ride Into Cancer Cells
- Epilepsy Drug Prevents Brain Tumors in Mice With NF1
- New Study Sheds Light on the Mechanisms Underlying the Development of Malignant Pediatric Brain Tumors
Friday, April 12, 2024
- Melanomas Resist Drugs by 'breaking' Genes
- Scientists Uncover a Missing Link Between Poor Diet and Higher Cancer Risk
Thursday, April 11, 2024
- Researchers Identify New Genetic Risk Factors for Persistent HPV Infections
- Colorless, Odorless Gas Likely Linked to Alarming Rise in Non-Smoking Lung Cancer
- Scientists Uncover Key Resistance Mechanism to Wnt Inhibitors in Pancreatic and Colorectal Cancers
- Study Lays the Basis for New Knowledge on Gastrointestinal Diseases
Wednesday, April 10, 2024
- Researchers Identify Protein That Controls CAR T Cell Longevity
- New Insight Into Combating Drug-Resistant Prostate Cancer
- A Promising Target for New RNA Therapeutics Now Accessible
- A New Screening Protocol Can Detect Aggressive Prostate Cancers More Selectively
- AI-Assisted Breast-Cancer Screening May Reduce Unnecessary Testing
Tuesday, April 9, 2024
- Targeting RAS Proteins May Prevent Relapse in Acute Myeloid Leukemia
- Bacteria in Cancer Unmasked
- Periostin Shows Promise to Help Fight a Common Form of Esophageal Cancer
- Research Could Unlock More Precise Prognoses and Targeted Treatments for Children With Cancer
- Immune Key to Chronic Viral Infections Discovered
Monday, April 8, 2024
- The Surprising Connection Between Male Infertility and Family Cancer Risk
- Targeting Vulnerability in B-Cell Development Leads to Novel Drug Combination for Leukemia
- Opening a New Front Against Pancreatic Cancer
Thursday, April 4, 2024
- Less Extensive Breast Cancer Surgery Results in Fewer Swollen Arms
- Microbial Signature of Colorectal Cancer-Associated Mutations Identified in New Study
- Study Finds Less Invasive, Safer Option for Removing Benign Pancreatic Tumors
Tuesday, April 2, 2024
- Investigators Develop Novel Treatment for T-Cell Leukemias and Lymphomas
Monday, April 1, 2024
- New Antibiotic Class Effective Against Multidrug-Resistant Bacteria
- Exposure to Common Environmental Carcinogens Linked to Decreased Lifespan Happiness
Thursday, March 28, 2024
- Genomic Research May Help Explain Cancer Resistance in Tasmanian Devils
- Cell Division Quality Control 'stopwatch' Uncovered
- 'Exhausted' Immune Cells in Healthy Women Could Be Target for Breast Cancer Prevention
Wednesday, March 27, 2024
- A Combination of Approved Drugs Enhances the Delivery of Anti-Bacterial Medications to Treat Tuberculosis
- Combining Epigenetic Cancer Medications May Have Benefit for Colorectal Cancers and Other Tumor Types
- Researchers Turn Back the Clock on Cancer Cells to Offer New Treatment Paradigm
- New Technique for Predicting Protein Dynamics May Prove Big Breakthrough for Drug Discovery
- Researchers Create Biocompatible Nanoparticles to Enhance Systemic Delivery of Cancer Immunotherapy
- Researchers Discover a Mechanism That Could Improve Platinum-Based Cancer Therapy
- Accelerating CAR T Cell Therapy: Lipid Nanoparticles Speed Up Manufacturing
- Beating by Overheating: New Strategy to Combat Cancer
Tuesday, March 26, 2024
- Genetically Engineered Dendritic Cells Enhance the Power of Immunotherapy Against Lung Cancer
Monday, March 25, 2024
- Cancer Therapies Show Promise in Combating Tuberculosis
- Promising Drug Combination for Multiple Myeloma Treatment
Thursday, March 21, 2024
- An Immunotherapy to Overcome Resistant Leukemia
Wednesday, March 20, 2024
- Bacteria Subtype Linked to Growth in Up to 50% of Human Colorectal Cancers
- In the Fight Against Breast Cancer, Researchers Identify Malignancy Hibernation as the Next Battleground
- LATEST NEWS
- Health & Medicine
- Diseases & Conditions
- Alzheimer's Research
- Amyotrophic Lateral Sclerosis
- Attention Deficit Disorder
- Back and Neck Pain
- Birth Defects
- Bladder Disorders
- Blood Clots
- COVID and SARS
- Cervical Cancer
- Bladder Cancer
- Multiple Myeloma
- Pancreatic Cancer
- Brain Tumor
- Colon Cancer
- Breast Cancer
- Ovarian Cancer
- Lung Cancer
- Mesothelioma
- Skin Cancer
- Prostate Cancer
- Cerebral Palsy
- Chikungunya
- Chronic Fatigue Syndrome
- Cold and Flu
- Crohn's Disease
- Cystic Fibrosis
- Dengue Fever
- Down Syndrome
- Eating Disorder Research
- Encephalitis
- Epilepsy Research
- Erectile Dysfunction
- Fibromyalgia
- Gastrointestinal Problems
- HIV and AIDS
- Headache Research
- Hearing Loss
- Heart Health
- Cholesterol
- Stroke Prevention
- Heart Disease
- Hormone Disorders
- Hypertension
- Infectious Diseases
- Insomnia Research
- Irritable Bowel Syndrome
- Kidney Disease
- Liver Disease
- Lung Disease
- Lyme Disease
- Mental Health Research
- Multiple Sclerosis Research
- Mumps, Measles, Rubella
- Muscular Dystrophy
- Osteoporosis
- Parkinson's Research
- Prostate Health
- Restless Leg Syndrome
- Sickle Cell Anemia
- Sleep Disorder Research
- Thyroid Disease
- Triglycerides
- Tuberculosis
- Medical Topics
- Accident and Trauma
- Alternative Medicine
- Birth Control
- Bone and Spine
- Chronic Illness
- Controlled Substances
- Dietary Supplements and Minerals
- Epigenetics
- Food Additives
- Foodborne Illness
- Foot Health
- Gene Therapy
- Health Policy
- Human Biology
- Immune System
- Joint Health
- Medical Imaging
- Nervous System
- Pain Control
- Personalized Medicine
- Pharmacology
- Psychology Research
- Wounds and Healing
- PHYSICAL/TECH
- ENVIRONMENT
- SOCIETY & EDUCATION
- Fastest Rate of CO2 Rise Over Last 50,000 Years
- Like Dad and Like Mum...all in One Plant
- What Makes a Memory? Did Your Brain Work Hard?
- Plant Virus Treatment for Metastatic Cancers
- Controlling Shape-Shifting Soft Robots
- Brain Flexibility for a Complex World
- ONe Nova to Rule Them All
- AI Systems Are Skilled at Manipulating Humans
- Planet Glows With Molten Lava
- A Fragment of Human Brain, Mapped
Trending Topics
Strange & offbeat.
MIT Technology Review
- Newsletters
Cancer vaccines are having a renaissance
After years of lackluster results, cancer vaccines seem poised for success. Finally.
- Cassandra Willyard archive page
This article first appeared in The Checkup, MIT Technology Review’s weekly biotech newsletter. To receive it in your inbox every Thursday, and read articles like this first, sign up here .
Last week, Moderna and Merck launched a large clinical trial in the UK of a promising new cancer therapy: a personalized vaccine that targets a specific set of mutations found in each individual’s tumor. This study is enrolling patients with melanoma. But the companies have also launched a phase III trial for lung cancer. And earlier this month BioNTech and Genentech announced that a personalized vaccine they developed in collaboration shows promise in pancreatic cancer, which has a notoriously poor survival rate.
Drug developers have been working for decades on vaccines to help the body’s immune system fight cancer, without much success. But promising results in the past year suggest that the strategy may be reaching a turning point. Will these therapies finally live up to their promise?
This week in The Checkup, let’s talk cancer vaccines. (And, you guessed it, mRNA.)
Long before companies leveraged mRNA to fight covid, they were developing mRNA vaccines to combat cancer. BioNTech delivered its first mRNA vaccines to people with treatment-resistant melanoma nearly a decade ago. But when the pandemic hit, development of mRNA vaccines jumped into warp drive. Now dozens of trials are underway to test whether these shots can transform cancer the way they did covid.
Recent news has some experts cautiously optimistic. In December, Merck and Moderna announced results from an earlier trial that included 150 people with melanoma who had undergone surgery to have their cancer removed. Doctors administered nine doses of the vaccine over about six months, as well as what’s known as an immune checkpoint inhibitor. After three years of follow-up, the combination had cut the risk of recurrence or death by almost half compared with the checkpoint inhibitor alone.
The new results reported by BioNTech and Genentech, from a small trial of 16 patients with pancreatic cancer, are equally exciting. After surgery to remove the cancer, the participants received immunotherapy, followed by the cancer vaccine and a standard chemotherapy regimen. Half of them responded to the vaccine, and three years after treatment, six of those people still had not had a recurrence of their cancer. The other two had relapsed. Of the eight participants who did not respond to the vaccine, seven had relapsed. Some of these patients might not have responded because they lacked a spleen, which plays an important role in the immune system. The organ was removed as part of their cancer treatment.
The hope is that the strategy will work in many different kinds of cancer. In addition to pancreatic cancer, BioNTech’s personalized vaccine is being tested in colorectal cancer, melanoma, and metastatic cancers.
The purpose of a cancer vaccine is to train the immune system to better recognize malignant cells, so it can destroy them. The immune system has the capacity to clear cancer cells if it can find them. But tumors are slippery. They can hide in plain sight and employ all sorts of tricks to evade our immune defenses. And cancer cells often look like the body’s own cells because, well, they are the body’s own cells.
There are differences between cancer cells and healthy cells, however. Cancer cells acquire mutations that help them grow and survive, and some of those mutations give rise to proteins that stud the surface of the cell—so-called neoantigens.
Personalized cancer vaccines like the ones Moderna and BioNTech are developing are tailored to each patient’s particular cancer. The researchers collect a piece of the patient’s tumor and a sample of healthy cells. They sequence these two samples and compare them in order to identify mutations that are specific to the tumor. Those mutations are then fed into an AI algorithm that selects those most likely to elicit an immune response. Together these neoantigens form a kind of police sketch of the tumor, a rough picture that helps the immune system recognize cancerous cells.
“A lot of immunotherapies stimulate the immune response in a nonspecific way—that is, not directly against the cancer,” said Patrick Ott, director of the Center for Personal Cancer Vaccines at the Dana-Farber Cancer Institute, in a 2022 interview . “Personalized cancer vaccines can direct the immune response to exactly where it needs to be.”
How many neoantigens do you need to create that sketch? “We don’t really know what the magical number is,” says Michelle Brown, vice president of individualized neoantigen therapy at Moderna. Moderna’s vaccine has 34. “It comes down to what we could fit on the mRNA strand, and it gives us multiple shots to ensure that the immune system is stimulated in the right way,” she says. BioNTech is using 20.
The neoantigens are put on an mRNA strand and injected into the patient. From there, they are taken up by cells and translated into proteins, and those proteins are expressed on the cell’s surface, raising an immune response
mRNA isn’t the only way to teach the immune system to recognize neoantigens. Researchers are also delivering neoantigens as DNA, as peptides, or via immune cells or viral vectors. And many companies are working on “off the shelf” cancer vaccines that aren’t personalized, which would save time and expense. Out of about 400 ongoing clinical trials assessing cancer vaccines last fall, roughly 50 included personalized vaccines.
There’s no guarantee any of these strategies will pan out. Even if they do, success in one type of cancer doesn’t automatically mean success against all. Plenty of cancer therapies have shown enormous promise initially, only to fail when they’re moved into large clinical trials.
But the burst of renewed interest and activity around cancer vaccines is encouraging. And personalized vaccines might have a shot at succeeding where others have failed. The strategy makes sense for “a lot of different tumor types and a lot of different settings,” Brown says. “With this technology, we really have a lot of aspirations.”
Now read the rest of The Checkup
Read more from mit technology review’s archive.
mRNA vaccines transformed the pandemic. But they can do so much more. In this feature from 2023, Jessica Hamzelou covered the myriad other uses of these shots , including fighting cancer.
This article from 2020 covers some of the background on BioNTech’s efforts to develop personalized cancer vaccines. Adam Piore had the story .
Years before the pandemic, Emily Mullin wrote about early efforts to develop personalized cancer vaccines—the promise and the pitfalls.
From around the web
Yes, there’s bird flu in the nation’s milk supply. About one in five samples had evidence of the H5N1 virus. But new testing by the FDA suggests that the virus is unable to replicate. Pasteurization works! ( NYT )
Studies in which volunteers are deliberately infected with covid—so-called challenge trials—have been floated as a way to test drugs and vaccines, and even to learn more about the virus. But it turns out it’s tougher to infect people than you might think. ( Nature )
When should women get their first mammogram to screen for breast cancer? It’s a matter of hot debate. In 2009, an expert panel raised the age from 40 to 50. This week they lowered it to 40 again in response to rising cancer rates among younger women. Women with an average risk of breast cancer should get screened every two years, the panel says. ( NYT )
Wastewater surveillance helped us track covid. Why not H5N1? A team of researchers from New York argues it might be our best tool for monitoring the spread of this virus. ( Stat )
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets.
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
- Antonio Regalado archive page
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
- Jessica Hamzelou archive page
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from mit technology review.
Discover special offers, top stories, upcoming events, and more.
Thank you for submitting your email!
It looks like something went wrong.
We’re having trouble saving your preferences. Try refreshing this page and updating them one more time. If you continue to get this message, reach out to us at [email protected] with a list of newsletters you’d like to receive.
New on NCI’s Websites for April 2024
April 12, 2024 , by Daryl McGrath
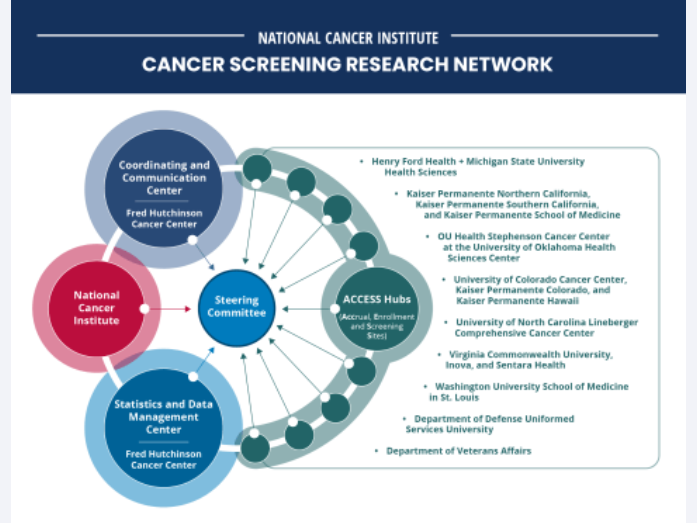
The Cancer Screening Research Network will conduct rigorous, multicenter cancer screening trials.
NCI’s collection of cancer information products is constantly growing, so periodically we provide updates on new and updated content of interest to the cancer community.
NCI Launches Research Network to Evaluate Emerging Cancer Screening Technologies
The Cancer Screening Research Network (CSRN) will support studies that investigate new ways to identify cancers earlier , when they may be easier to treat. In late 2024, CSRN plans to launch the Vanguard Study on Multi-Cancer Detection, a pilot study to inform the design of a larger trial of multi-cancer detection tests. Multi-cancer detection tests measure biomarkers, such as pieces of DNA, that cancer cells release into the blood and can potentially be used to detect many kinds of cancer.
For Childhood Cancer Survivors, Inherited Genetic Factors Influence Risk of Cancers Later in Life
Common inherited genetic factors that predict cancer risk in the general population may also predict elevated risk of new cancers among childhood cancer survivors , according to a study led by researchers at NCI.
NCI Launches Virtual Clinical Trials Office
NCI has launched the Virtual Clinical Trials Office , a centralized team of support staff—including research nurses, clinical research associates, and clinical data specialists—who will work remotely to assist NCI-Designated Comprehensive Cancer Centers and community practices with their clinical trials activities.
Meet the NCI Director Video
During a live social media event that took place February 22, NCI Director Kimryn Rathmell, M.D., Ph.D., introduced herself and answered live questions about the future of cancer research.
President’s Cancer Panel Issues Progress Report on the National Cancer Plan
On February 28, the President’s Cancer Panel released its I nitial Assessment of the National Cancer Plan . This report examines the National Cancer Plan as a road map for the National Cancer Program and offers recommendations in five priority areas to accelerate progress toward the plan’s goals.
Small Business Innovation Research Program
NCI’s Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs support small businesses across the United States to develop innovative cancer technologies. The SBIR Development Center has a new website that features the center’s SBIR Innovation Lab podcast , highlights success stories , and showcases companies chosen to pitch for funding at investor events.
Progress in Basic Cancer Research
NCI’s Division of Cancer Biology (DCB) features recent progress in advancing basic cancer research . A new infographic shows progress made in fiscal year 2023, highlights of recent research, and examples of DCB-sponsored research intended to help achieve the goals of the National Cancer Plan.
Center for Cancer Research Updates
NCI’s Center for Cancer Research (CCR) has added and updated several web pages. CCR’s training page for postdoctoral researchers features a new video explaining the benefits of postdoc training with NCI tenure-track investigators.
CCR also added a new chromosome biology research section to its website. The section includes a new web page for the NCI Center of Excellence in Chromosome Biology , information about specific researchers working on chromosome biology, and related fellowships and jobs in CCR.
CCR also has released its annual Milestones publication. The 10 stories highlighted in this year’s publication illustrate the breadth, creativity, and impact of CCR research.
Why We Love Diverse Data
NCI’s Center for Biomedical Informatics and Information Technology celebrated “Love Data Week!” (February 12–16) with a blog post featuring six scientists talking about why they love diverse data .
Inside Cancer Careers Podcast
NCI’s Center for Cancer Training has launched the second season of the Inside Cancer Careers podcast . The February 1 episode was a “fireside chat” with NCI Director Dr. Kimryn Rathmell , who shared her career journey and the importance of supporting the next generation of cancer researchers. The February 15 episode featured Ophira Ginsburg, M.D. , senior scientific officer and senior advisor for clinical research in NCI’s Center for Global Health, who gave her thoughts on the importance of women's leadership and representation in cancer care.
World Cancer Day 2024 Blog
NCI’s Center for Global Health (CGH) marked World Cancer Day with a blog post by CGH Director Satish Gopal, M.D., M.P.H. Dr. Gopal highlighted important accomplishments and activities in 2023, including CGH’s ongoing NCI Global Cancer Research and Control Seminar Series , NCI support and participation in the November 2023 African Organization for Research and Training in Cancer (AORTIC) meeting in Senegal, initiation of cancer health disparities minilabs to develop transdisciplinary approaches to advance scientific progress globally, and leadership and launch of the Lancet Commission on Women, Cancer, and Power .
NCI Office of Cancer Survivorship – New Resources
The NCI’s Office of Cancer Survivorship (OCS) has revamped its Cancer Survivor and Caregiver Stories page . Along with adding caregiver stories, the page also enables readers to filter stories—such as by cancer type and specific populations—to select those that will be most meaningful to them. Also new is a video library that includes videos sharing the words of advocates on survivorship-related topics and an FAQ with OCS Director Emily Tonorezos, M.D.
HINTS Brief 53 on Telehealth Use Among US Adults
The Health Information National Trends Survey (HINTS) Management Team has released HINTS Brief 53, “Patterns and Predictors of Telehealth Use among US Adults in 2022 .” This brief discusses the impact of the COVID-19 pandemic on the expansion of telehealth; the prevalence of, and factors associated with, telehealth use; and more.

Updated: Catchment Areas of NCI-Designated Cancer Centers
NCI’s Division of Cancer Control and Population Sciences updated its page on the Catchment Areas of NCI-Designated Cancer Centers , including designation status and catchment area updates for several cancer enters.
Co-Use of Tobacco with Alcohol and Cannabis
The NCI’s Behavioral Research Program has launched a new web page on the co-use of tobacco with alcohol and cannabis . This page, created by program directors in the Health Behaviors Research Branch and the Tobacco Control Research Branch, discusses the importance of addressing co-use of tobacco with alcohol and tobacco with cannabis as targets for cancer prevention and control.
Smokefree.gov Launches SmokefreeNATIVE
NCI’s Smokefree.gov initiative has partnered with the Indian Health Service to launch a new, free text messaging resource to help American Indian and Alaska Native adults and adolescents quit smoking commercial tobacco. People who smoke and are ready to set a date to quit can enroll online or by texting NATIVE to 47848.
Participate in Cancer Research
NCI has enhanced its information about participating in cancer research studies, including how to join clinical trials or observational studies and how to donate medical data and biological samples. The new Participate in Cancer Research section of NCI’s website includes information about cancer research studies , a clinical trials search tool , personal stories from people who have participated in studies, and more.
Lymphedema, the buildup of lymph fluid in tissues causing swelling, affects people’s ability to do certain activities and their quality of life. NCI has revised and consolidated its expert-reviewed content about ways to prevent and treat lymphedema .
The ALCHEMIST Lung Cancer Trials
The ALCHEMIST clinical trials are a group of randomized clinical trials for people with early-stage non-small cell lung cancer whose tumors have been completely removed by surgery. People entering the ALCHEMIST study will have their tumor tissue genetically sequenced to determine if one of the drugs being studied targets a specific biomarker, including certain genetic mutations, found in their tumors. This revised and streamlined page describes the different trials and which are currently recruiting participants.
Updated Fact Sheets
Tumor Markers : This updated fact sheet defines tumor markers and describes how they can be used to help diagnose and treat cancer. This revised and streamlined page also includes a list of tumor marker tests in common use .
Computed Tomography (CT) Scans and Cancer : This updated page explains what CT scans are, how they're used for cancer screening, diagnosis, and treatment, and what people can expect during the procedure.
New and Updated Patient Information Summaries
Skin Cancer Prevention : Skin cancer prevention includes avoiding risk factors like ultraviolet radiation that comes from the sun, sun lamps, and tanning beds. This updated expert-reviewed summary discusses the risks and possible protective factors for skin cancer.
Childhood Colorectal Cancer Treatment : Colorectal cancer is defined as a cancer that starts anywhere along the colon or rectum. Learn about risk factors, symptoms, and tests to diagnose colorectal cancer in children and how it is treated.
Depression : Depression is a treatable medical problem that can affect adults and children with cancer. This updated page describes symptoms of depression, risk factors, diagnosis, and treatment for adults and children with cancer.
Childhood Brain Tumors: NCI’s pages on DIPG (diffuse intrinsic pontine glioma) , childhood ependymoma , and childhood glioma (including astrocytoma) have a new look. The pages cover causes and risk factors, symptoms, screening, diagnosis, prognosis, stages, and treatment.
Spirituality and Cancer Care : Religion and spirituality in cancer care are very personal matters that can affect treatment decisions and the ability to cope. This updated page explains ways a person’s health care team can support their spiritual and religious well-being and how this can lead to improved health and quality of life.
Oral Complications of Cancer Therapies : Mouth and throat problems are common problems of cancer treatment. This updated page explains ways to prevent and manage problems like dry mouth, taste changes, pain, and infection.
Acute Myeloid Leukemia Treatment : Acute myeloid leukemia (AML) is a cancer that causes the rapid growth of abnormal white blood cells. These abnormal cells can replace healthy blood cells, leading to symptoms such as bleeding, anemia, and infection. This recently updated page includes information about the causes, symptoms, diagnosis, and treatment options for AML.
New Health Professional Summary: Hospice
Health care professionals and health care systems face many challenges when caring for patients at the end of life. This new page offers expert-reviewed information about many aspects of hospice care .
Chemotherapy and You : Support for People with Cancer
Chemotherapy and You is a booklet for people who are about to receive or are now receiving chemotherapy for cancer. It includes facts about chemotherapy and its side effects and highlights ways people can care for themselves before, during, and after treatment.
Infographic: Chances of Developing Breast Cancer by Age 70 to 80
Women who have harmful changes in certain genes have a higher risk of some cancers. This updated infographic helps explain the risk of breast cancer for women with harmful BRCA1 and BRCA2 mutations.
Featured Posts
March 27, 2024, by Edward Winstead
March 21, 2024, by Elia Ben-Ari
March 5, 2024, by Carmen Phillips
- Biology of Cancer
- Cancer Risk
- Childhood Cancer
- Clinical Trial Results
- Disparities
- FDA Approvals
- Global Health
- Leadership & Expert Views
- Screening & Early Detection
- Survivorship & Supportive Care
- February (6)
- January (6)
- December (7)
- November (6)
- October (7)
- September (7)
- February (7)
- November (7)
- October (5)
- September (6)
- November (4)
- September (9)
- February (5)
- October (8)
- January (7)
- December (6)
- September (8)
- February (9)
- December (9)
- November (9)
- October (9)
- September (11)
- February (11)
- January (10)
Preventable Premature Deaths from the Five Leading Causes of Death in Nonmetropolitan and Metropolitan Counties, United States, 2010–2022
Surveillance Summaries / May 2, 2024 / 73(2);1–11
Macarena C. García, DrPH 1 ; Lauren M. Rossen, PhD 2 ; Kevin Matthews, PhD 3 ; Gery Guy, PhD 4 ; Katrina F. Trivers, PhD 5 ; Cheryll C. Thomas, MSPH 5 ; Linda Schieb, MSPH 5 ; Michael F. Iademarco, MD 1 ( View author affiliations )
Introduction
Limitations, future directions, acknowledgments.
- Article PDF
- Rural reinvestment: A path forward to addressing geographic health inequities
Problem/Condition: A 2019 report quantified the higher percentage of potentially excess (preventable) deaths in U.S. nonmetropolitan areas compared with metropolitan areas during 2010–2017. In that report, CDC compared national, regional, and state estimates of preventable premature deaths from the five leading causes of death in nonmetropolitan and metropolitan counties during 2010–2017. This report provides estimates of preventable premature deaths for additional years (2010–2022).
Period Covered: 2010–2022.
Description of System: Mortality data for U.S. residents from the National Vital Statistics System were used to calculate preventable premature deaths from the five leading causes of death among persons aged <80 years. CDC’s National Center for Health Statistics urban-rural classification scheme for counties was used to categorize the deaths according to the urban-rural county classification level of the decedent’s county of residence (1: large central metropolitan [most urban], 2: large fringe metropolitan, 3: medium metropolitan, 4: small metropolitan, 5: micropolitan, and 6: noncore [most rural]). Preventable premature deaths were defined as deaths among persons aged <80 years that exceeded the number expected if the death rates for each cause in all states were equivalent to those in the benchmark states (i.e., the three states with the lowest rates). Preventable premature deaths were calculated separately for the six urban-rural county categories nationally, the 10 U.S. Department of Health and Human Services public health regions, and the 50 states and the District of Columbia.
Results: During 2010–2022, the percentage of preventable premature deaths among persons aged <80 years in the United States increased for unintentional injury (e.g., unintentional poisoning including drug overdose, unintentional motor vehicle traffic crash, unintentional drowning, and unintentional fall) and stroke, decreased for cancer and chronic lower respiratory disease (CLRD), and remained stable for heart disease. The percentages of preventable premature deaths from the five leading causes of death were higher in rural counties in all years during 2010–2022. When assessed by the six urban-rural county classifications, percentages of preventable premature deaths in the most rural counties (noncore) were consistently higher than in the most urban counties (large central metropolitan and fringe metropolitan) for the five leading causes of death during the study period.
During 2010–2022, preventable premature deaths from heart disease increased most in noncore (+9.5%) and micropolitan counties (+9.1%) and decreased most in large central metropolitan counties (−10.2%). Preventable premature deaths from cancer decreased in all county categories, with the largest decreases in large central metropolitan and large fringe metropolitan counties (−100.0%; benchmark achieved in both county categories in 2019). In all county categories, preventable premature deaths from unintentional injury increased, with the largest increases occurring in large central metropolitan (+147.5%) and large fringe metropolitan (+97.5%) counties. Preventable premature deaths from CLRD decreased most in large central metropolitan counties where the benchmark was achieved in 2019 and increased slightly in noncore counties (+0.8%). In all county categories, preventable premature deaths from stroke decreased from 2010 to 2013, remained constant from 2013 to 2019, and then increased in 2020 at the start of the COVID-19 pandemic. Percentages of preventable premature deaths varied across states by urban-rural county classification during 2010–2022.
Interpretation: During 2010–2022, nonmetropolitan counties had higher percentages of preventable premature deaths from the five leading causes of death than did metropolitan counties nationwide, across public health regions, and in most states. The gap between the most rural and most urban counties for preventable premature deaths increased during 2010–2022 for four causes of death (cancer, heart disease, CLRD, and stroke) and decreased for unintentional injury. Urban and suburban counties (large central metropolitan, large fringe metropolitan, medium metropolitan, and small metropolitan) experienced increases in preventable premature deaths from unintentional injury during 2010–2022, leading to a narrower gap between the already high (approximately 69% in 2022) percentage of preventable premature deaths in noncore and micropolitan counties. Sharp increases in preventable premature deaths from unintentional injury, heart disease, and stroke were observed in 2020, whereas preventable premature deaths from CLRD and cancer continued to decline. CLRD deaths decreased during 2017–2020 but increased in 2022. An increase in the percentage of preventable premature deaths for multiple leading causes of death was observed in 2020 and was likely associated with COVID-19–related conditions that contributed to increased mortality from heart disease and stroke.
Public Health Action: Routine tracking of preventable premature deaths based on urban-rural county classification might enable public health departments to identify and monitor geographic disparities in health outcomes. These disparities might be related to different levels of access to health care, social determinants of health, and other risk factors. Identifying areas with a high prevalence of potentially preventable mortality might be informative for interventions.

Premature deaths, all-cause mortality, and poor health outcomes are greater among residents of rural counties than of urban counties in the United States ( 1 ). In 2021, the all-cause age-adjusted death rate in the United States was 841.6 per 100,000 population. The gap in all-cause mortality between rural (nonmetropolitan) and urban (metropolitan) areas of the United States continues to widen. In 1999, the death rate in rural areas was 7% higher than in urban areas; by 2019, it was 20% higher ( 2 ). Describing premature mortality rates from the five leading causes of death (cancer, unintentional injury [e.g., unintentional poisoning including drug overdose, unintentional motor vehicle traffic crash, unintentional drowning, and unintentional fall], heart disease, stroke, and chronic lower respiratory disease [CLRD]) and related rural disparities might help guide public health messaging and interventions.
The risk for premature death is associated with modifiable factors that vary by disease ( 3 ). Four of the five leading risk factors for premature death are more prevalent in rural areas of the United States: using tobacco, obesity, physical inactivity, and drinking alcohol or drinking in excess ( 4 , 5 ). Extensive literature on social determinants of health has established the importance of community context in shaping all aspects of health ( 6 ). Structural factors (e.g., lower socioeconomic status, limited access to health care professionals, and limited job opportunities) increase the risk for premature death among rural residents ( 7 ).
Multiple factors influence the rural-urban gap in preventable premature deaths. Because each of the five leading causes of death is age related, these conditions are more prevalent in rural areas of the United States where residents typically are older than their urban counterparts. Working-age adults might leave rural areas to seek better economic opportunities elsewhere ( 8 ), and older persons might be more likely to retire in rural areas ( 9 ). However, the population’s age structure alone does not explain the disparity in mortality. Instead, differences in social circumstances, socioeconomic characteristics, health-related behaviors, and access to health care services affect mortality and potentially contribute to approximately half of all preventable premature deaths ( 10 ). County-level disparities in all-cause premature deaths by rurality, race, and ethnicity have been documented ( 11 ). Data on cause-specific preventable premature deaths from the leading causes of death by rurality, sex, race, and ethnicity are limited, and direct comparisons accounted for by these factors will be reported in subsequent analyses.
Rural public health needs and sociodemographic characteristics of rural populations are changing ( 12 ). Although the proportion of the U.S. population that lives in rural areas is gradually declining, any rural population growth can be attributed to in-migration, which might require sensitivity to cultural differences ( 13 , 14 ). With gradual declines in population, the wealth and tax bases of rural counties also are decreasing, resulting in reduced funding for social and health services ( 15 ).
In this analysis, mortality data were used to estimate the number and percentage of deaths from each of the five leading causes of death that could have been prevented if all states had similarly low death rates. Disparities in premature mortality from the five leading causes of death in rural areas in the United States during 2010–2022 also were estimated. The results of this analysis are intended to serve as a critical resource for policymakers, public health officials, and researchers striving to understand and address the root causes of preventable premature deaths.
This analysis used mortality data for U.S. residents from the National Vital Statistics System ( https://www.cdc.gov/nchs/index.htm ) to calculate preventable premature deaths by urban-rural county classification from the five leading causes of death during 2010–2022 (heart disease, cancer, unintentional injury, CLRD, and stroke). Deaths from COVID-19 were excluded to maintain consistency and facilitate the assessment of trends over time. Data for 2022 are provisional counts from January through June and were annualized for comparability with previous years.
The number of preventable premature deaths for a specific cause (also described as potentially preventable premature or excess deaths) is equal to the difference in the number of observed deaths among persons aged <80 years and the number of deaths expected if the mortality rate in all states were equivalent to the average rate of the three states with the lowest mortality. Rates in the three states define the benchmarks. The benchmark for each cause of death is derived from a unique set of three states.
Rural and urban categories were identified using the National Center for Health Statistics 2013 urban-rural classification scheme for counties ( 16 ). County of residence of the decedent was used to determine urban-rural county classification. The categories are 1: large central metropolitan (most urban), 2: large fringe metropolitan, 3: medium metropolitan, 4: small metropolitan, 5: micropolitan, and 6: noncore (most rural).
Preventable premature deaths were calculated individually for the two nonmetropolitan categories (micropolitan and noncore) and the four metropolitan categories (large central metropolitan, large fringe metropolitan, medium metropolitan, and small metropolitan) as well as for the broader categories of metropolitan and nonmetropolitan. Analyses were restricted to deaths with an underlying cause of death from the five leading causes of death based on the International Classification of Diseases, 10th Revision (ICD-10): heart disease (I00–I09, I11, I13, and I20–I51), cancer (C00–C97), unintentional injury (V01–X59 and Y85–Y86), CLRD (J40–J47), and stroke (I60–I69). The analysis of preventable premature deaths during 2010–2022 was restricted to persons aged <80 years at the time of death. The age restriction is consistent with the average life expectancy for the U.S. population in 2010, which was approximately 79 years ( 17 ).
Age-specific mortality rates for each of the five leading causes of death were used to derive the number of preventable premature deaths using methods described elsewhere ( 18 ). Age groupings varied by cause of death. (Most were 10-year age groups; however, the size of the youngest age group ranged from 0 to 9 years for unintentional injury to 0 to 49 years for CLRD and cerebrovascular disease because deaths from those causes are rare among younger persons.) For each age group and cause of death, the death rates of the three states with the lowest rates during 2008–2010 (benchmark states) were averaged to produce benchmark rates ( 18 ) ( https://stacks.cdc.gov/view/cdc/42342 ). These benchmarks were chosen to represent the lowest death rates achievable by states at the beginning of the study period and did not vary by year to allow for the examination of trends over time. Although using time-varying benchmarks would better account for potential improvements over time in the benchmark rates, time-varying benchmarks also would make temporal and geographic comparisons more difficult. The same benchmarks were applied to both nonmetropolitan and metropolitan counties, and benchmarks were not adjusted for other characteristics that might affect death rates (e.g., race, ethnicity, socioeconomic status, and urbanicity). Deaths attributed to COVID-19 from 2020 through June 2022 were excluded from this study.
The numbers of preventable premature deaths for each cause of death were assumed to follow a Poisson distribution. SEs were calculated using standard formulas that incorporated the variance around both the observed and the expected counts ( 18 ), and pairwise z-tests were performed to determine whether the differences during 2010–2022 were statistically significant (p<0.05). All differences during 2010–2022 are statistically significant unless otherwise noted. The percentage of preventable premature deaths was calculated by dividing the number of preventable premature deaths by the total observed number of premature deaths.
The percentage of preventable premature deaths from cancer decreased from 2010 through June 2022 (from 21% to 0.3%) ( Figure 1 ). Regardless of urban-rural classification, all county categories experienced decreases ( Figure 2 ). However, the decreases in urban counties were larger than those in rural counties, which widened the rural-urban disparities in preventable premature deaths from cancer (Figure 2) ( Table ). The percentage of preventable premature deaths from cancer in noncore counties in 2022 (18.1%) was similar to the percentage in large central metropolitan counties in 2010 (17.9%).
The percentage of preventable premature deaths from heart disease decreased from 2010 through 2019 (from 33.5% to 28.8%), followed by a steep increase to 33.6% from 2020 through June 2022 (Figure 1). Increases from 2020 through June 2022 occurred across all rural-urban categories except for large central metropolitan counties, which experienced a decrease from 32.9% in 2020 to 30.1% in 2021 (Figure 2) (Table). Rural counties had the highest percentage of preventable premature deaths from heart disease in 2022 (45.8% in micropolitan counties and 49.4% in noncore counties) (Figure 2) (Table). Most states experienced an increase in preventable early deaths from heart disease and stroke (96% and 88% of states, respectively) from 2019 through June 2022 (Supplementary Table, https://stacks.cdc.gov/view/cdc/147842 ).
The percentage of preventable premature deaths from unintentional injury increased from 2010 to 2019 (from 38.8% to 53.8%), followed by a steep increase from 2019 to 2021 and a slight decrease through June 2022 to 63.5% (Figure 1). Increases in preventable premature deaths from unintentional injury during 2010–2022 were statistically significant for all metropolitan categories except micropolitan. Rural percentages were higher than in urban areas, but the gap narrowed (Figure 2). The percentages increased in all states except Wyoming, but the increase varied widely at the state level (Supplementary Table, https://stacks.cdc.gov/view/cdc/147842 ).
The percentage of preventable premature deaths from CLRD decreased from 2010 through 2022 (from 38.6% to 25.5%) (Figure 1). The percentage of preventable premature deaths varied widely when stratified by rural-urban county category, but all county categories except for noncore counties experienced decreases. Rural-urban disparities widened when large central metropolitan percentages decreased from 23.4% in 2010 to 0% in 2022, whereas the rural percentages hovered between 50.7% and 54.8% in 2022 (Figure 2) (Table).
The percentage of preventable premature deaths from stroke decreased slightly from 2010 through 2019 (32.4% to 26.4%), followed by an increase to 33.9% through June 2022 (Figure 1). Each rural-urban category experienced steep increases from 2019 to June 2022, except for noncore counties that experienced a slight decrease from 2021 to June 2022; rural counties had the highest percentages from January to June 2022 (42.0% in micropolitan counties and 40.9% in noncore counties) (Figure 2) (Table). The highest percentages of preventable premature deaths from stroke in 2022 were in southern states (Supplementary Table, https://stacks.cdc.gov/view/cdc/147842 ).
Rural residents, particularly those in noncore counties, experienced high percentages of preventable premature deaths during the study period. The rural-urban disparities in premature deaths varied by cause of death. However, disparities were not limited to place of residence. Disparities in all-cause premature deaths also were associated with other demographic factors (e.g., sex, race, and ethnicity) ( 11 ). For example, the highest rates of premature deaths were observed in rural counties where a majority of the population was Black, African American, American Indian, or Alaska Native ( 11 ). To address disparities in preventable premature deaths across rural and urban counties, data on disparities in cause-specific premature deaths from the five leading causes by rural-urban county category, race, and ethnicity are needed to inform interventions and health care policies for specific racial and ethnic groups. A follow-up of this analysis stratified by race and ethnicity will be published in subsequent reports, further contributing evidence to guide existing and new programs and policies.
Overall, the decrease in preventable premature deaths from cancer was substantial and was greatest in urban counties where access to preventive services, treatment, survivor care, and specialty care is much higher than in rural counties ( 19 ). Large central metropolitan and fringe metropolitan areas achieved the benchmark rates in 2019. This is consistent with overall declines in cancer mortality, which decreased 27% between 2001 and 2020 ( 20 ). The decrease in preventable premature deaths likely reflects multiple factors. Increases in recommended screening for the leading causes of deaths from cancer (e.g., lung, colon, cervical, and female breast) have led to earlier detection, when treatment is more effective, and prevention by detecting cellular changes before they turn into cancer, as in the case of colorectal cancer ( 21 ). Increases in vaccination rates for cancer-causing viruses and decreases in prevalence of risk factors (e.g., combustible tobacco use) also have driven cancer mortality downward ( 22 ). Access to these cancer prevention and early detection strategies was increased with the expansion of Medicaid ( 23 ). New cancer treatments and therapies, specifically for lung cancer and melanoma, also have led to longer survival for those with a cancer diagnosis ( 24 ). CDC conducted a demonstration project on how to best provide care for persons living in rural areas who had cancer diagnosed ( 25 ). Although cancer is categorized as a single disease group in this analysis, each cancer site has different risk factors, has varying treatment methods, and can manifest itself in different ways among groups by sex, age, race, and ethnicity. Preventable premature death might vary depending on the cancer site and might not have decreased for cancers with increasing prevalence of risk factors (e.g., obesity), no recommended screening modalities, or therapies that have not changed. Lung cancer, the leading cause of cancer mortality, accounted for 23% of all cancer deaths in 2020 ( 20 ). Geographic differences in combustible tobacco use and use of lung cancer screening likely partially drive differences in lung cancer mortality. Access to lung cancer screening facilities is more limited in rural counties than in urban counties ( 26 ). Despite overall reductions in preventable premature deaths from cancer, premature deaths surpass the national average in micropolitan and noncore counties, highlighting the need in rural areas to reduce cancer-related premature deaths. Because more urban areas surpassed the 2010 benchmarks for cancer death rates in 2019, future updates to the cancer-specific benchmarks using more recent years of data might better reflect the lowest achievable death rates.
Unintentional Injury
The worsening and expanding drug overdose epidemic, increases in motor vehicle traffic fatalities, and falls drive the growth in preventable premature deaths from unintentional injury ( 27 ). Narrowing rural-urban disparities in the percentage of preventable premature deaths from unintentional injury were driven by worsening rates of preventable mortality in more urban areas, with the percentage more than doubling in large central metropolitan areas over the study period. For drug overdoses, access to medications for opioid use disorder continues to be more limited in rural counties, as evidenced by low buprenorphine dispensing rates and reduced treatment capacity ( 28 ). For motor vehicle traffic crashes, rural residents have an increased risk for death and are less likely than urban residents to wear seat belts ( 29 ). Evidence-based interventions reduce rural-urban disparities in seat belt use and motor vehicle death rates ( 30 ). Many fall risk factors are modifiable, implying that many falls can be prevented ( 31 ).
Heart Disease and Stroke
Disparities in preventable premature deaths from heart disease and stroke between rural and urban areas existed across the study period. These gaps increased from 2019 to June 2022, except in large central metropolitan counties where a decrease of three percentage points was observed from 2020 to 2021. Increases in preventable premature deaths from heart disease and stroke in 2020 and 2021 were likely associated with COVID-19–related conditions that contributed to risk-associated increased mortality from heart disease and stroke ( 32 ). Increases in systolic and diastolic blood pressure, a leading risk factor for heart disease and stroke, were observed among all age groups when comparing 2020 with 2019 ( 33 ). Inequities in control of hypertension (i.e., systolic blood pressure values of ≥130 mm Hg, diastolic blood pressure of >80 mm Hg, or both) were observed during the COVID-19 pandemic and are related to insufficient health care access, medication adherence, and monitoring ( 34 ). Patients might have delayed or avoided seeking emergency care when experiencing a life-threatening event during the height of the COVID-19 pandemic ( 35 ). Emergency department visits for heart attack and stroke decreased by 20% during the weeks after the declaration of COVID-19 as a national emergency on March 13, 2020, and hospital admissions for heart attack and stroke decreased during the pandemic ( 35 ). In addition, COVID-19 was associated with an increased risk for stroke and heart disease ( 36 , 37 ).
Chronic Lower Respiratory Disease
Despite the overall decrease during 2010–2020 (because of decreases observed in larger urban areas), the percentage of preventable premature deaths from CLRD was relatively stable in medium and small urban counties and rural counties during 2010–2015. During 2010–2022, the sharpest decline in preventable premature death from CLRD in urban areas occurred from 2019 through 2021 and could be the result of deaths from COVID-19 that otherwise would have been attributable to CLRD. Persons with CLRD (e.g., chronic obstructive pulmonary disease) are at increased risk for death from COVID-19 ( 38 ).
The findings in this report are subject to at least six limitations. First, applying benchmarks (e.g., the three states with the lowest rates) to all urban-rural county categories facilitates comparisons but might not represent the lowest death rates achievable by certain subgroups. For example, large metropolitan areas met the benchmarks for cancer in 2019 and, therefore, had negative estimates of preventable premature deaths during 2019–2022. In these instances, negative estimates were truncated at zero to indicate that the 2010 benchmarks had been achieved. Using urban-rural–specific benchmarks would likely result in larger numbers of preventable premature deaths in certain categories and larger rural-urban disparities in certain cases. However, using benchmarks specific to state, age, cause, and subgroup (e.g., urban-rural, sex, race, and ethnicity) also could lead to less stable estimates because of smaller numbers of deaths when stratifying by multiple demographic and geographic dimensions. Second, the differences cannot be attributed solely to population size and geographic location because risk factors do not occur randomly in populations and are related to well-known social, demographic, environmental, economic, and geographic attributes of the neighborhoods in which persons live and work ( 39 ). Third, estimates of preventable premature deaths using historical benchmarks (e.g., 2008–2010) might not reflect improvements in mortality that could have occurred in a later year. For example, the 2010 benchmarks for cancer are higher than if benchmarks were based on 2022 data because of decreases in cancer deaths during 2010–2022. Fourth, the numbers of preventable premature deaths by cause are not necessarily independent, and the numbers of potentially excess deaths from the five causes cannot be combined to generate a total. For example, the number of preventable premature deaths from cancer might be lower because persons with cancer died from another cause (e.g., heart disease). Fifth, deaths from certain causes might have increased partly because of COVID-19 and the pandemic. Specifically, misclassification of deaths from COVID-19 that were attributed to other causes (because of lack of testing or reporting on the death certificate) could have contributed to a greater number of deaths across other causes and by rural and urban areas. In addition, certain causes of death might have increased because of indirect pandemic-related effects (e.g., reduced access to emergency care or life-saving treatments). Finally, data for 2022 were based on provisional data from the first half of the year, and results might differ when the final data for the full year are available.
The findings in this report demonstrate the value of analyzing preventable premature deaths according to the six National Center for Health Statistics 2013 urban-rural county classifications. Reporting trends in preventable premature deaths over a 12-year period highlights differences over time and might aid in understanding underlying structural, environmental, and social risk factors. Because of increasing percentages of preventable premature deaths in recent years for specific causes of death and certain demographic groups, these data might augment traditional rate comparisons and help guide focused public health interventions. Comparing the findings in this report with data from tools such as the CDC Interactive Atlas of Heart Disease and Stroke ( https://www.cdc.gov/dhdsp/maps/atlas/index.htm ) might help identify social determinants of health, health care infrastructure, and public policies that could be related to increases or decreases in preventable premature deaths in specific nonmetropolitan areas. Detailed community-based evaluations might clarify how various risk factors and social determinants of health relate to premature mortality and related rural-urban disparities. In addition, other methods for developing benchmark rates might be helpful, including using benchmarks based on the nonmetropolitan areas with the lowest death rates, or updating benchmarks based on more recent data, especially for causes of death (e.g., cancer) that have decreased substantially over time. In addition, more detailed analyses by race, ethnicity, and age, along with examining preventable deaths among persons aged >80 years, and preventable premature deaths from other causes (especially causes that are more prevalent in rural counties) might be informative.
Defining preventable premature deaths within the context of the five leading causes of death does not capture the full spectrum of preventable mortality. The degree to which these deaths could be prevented is related to various factors, including distinct prevention strategies, varying risk factors, and the availability of effective interventions, all of which vary by cause. Not all premature deaths are equally preventable among the leading causes or even within each specific leading cause category, as exemplified by certain types of cancer ( 40 ).
Routine tracking of preventable premature deaths by urban-rural county classification might facilitate the identification of areas with high prevalence of preventable premature mortality along with related geographic and urban-rural disparities in health outcomes. These disparities might be related to different levels of access to health care, social determinants of health, and other risk factors. Findings might help guide more focused interventions to reduce premature death from the five leading causes of death and reduce disparities by rural-urban residence and geographic region.
Health care professionals, medical examiners, coroners, vital registrars, and vital statistics offices across the United States.
Corresponding author: Macarena C. García, Office of Science and Medicine, Office of the Assistant Secretary of Health, U.S. Department of Health and Human Services, Washington, DC. Telephone: 678-770-6220; Email: [email protected] .
1 Office of Science and Medicine, Office of the Assistant Secretary of Health, U.S. Department of Health and Human Services, Washington, DC; 2 National Center for Health Statistics, CDC, Hyattsville, Maryland; 3 National Center for State, Tribal, Local, and Territorial Public Health Infrastructure and Workforce, CDC, Atlanta, Georgia; 4 National Center for Injury Prevention and Control, CDC, Atlanta, Georgia; 5 National Center for Chronic Disease Prevention and Health Promotion, CDC, Atlanta, Georgia
Conflicts of Interest
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
- James CV, Moonesinghe R, Wilson-Frederick SM, Hall JE, Penman-Aguilar A, Bouye K. Racial/ethnic health disparities among rural adults—United States, 2012–2015. MMWR Surveill Summ 2017;66(No. SS-23):1–9. https://doi.org/10.15585/mmwr.ss6623a1 PMID:29145359
- Curtin SC, Spencer MR. Trends in death rates in urban and rural areas: United States, 1999–2019. NCHS data brief no. 417, September 2021. Hyattsville, MD: US Department of Health and Human Services, CDC, National Center for Health Statistics; 2021. https://www.cdc.gov/nchs/data/databriefs/db417.pdf
- Committee on Population; Division of Behavioral and Social Sciences and Education; Board on Health Care Services; National Research Council; Institute of Medicine. Measuring the risks and causes of premature death: summary of workshops. Washington, DC: The National Academies Press; 2015.
- Matthews KA, Croft JB, Liu Y, et al. Health-related behaviors by urban-rural county classification—United States, 2013. MMWR Surveill Summ 2017;66(No. SS-5):1–8. https://doi.org/10.15585/mmwr.ss6605a1 PMID:28151923
- Yoon PW, Bastian B, Anderson RN, Collins JL, Jaffe HW; CDC. Potentially preventable deaths from the five leading causes of death—United States, 2008–2010. MMWR Morb Mortal Wkly Rep 2014;63:369–74. PMID:24785982
- Office of the Assistant Secretary of Health, Office of Disease Prevention and Health Promotion. Healthy People 2030. Social determinants of health [Internet]. Washington, DC: US Department of Health and Human Services, Office of the Secretary, Office of the Assistant Secretary of Health, Office of Disease Prevention and Health Promotion. https://health.gov/healthypeople/priority-areas/social-determinants-health
- McMaughan DJ, Oloruntoba O, Smith ML. Socioeconomic status and access to healthcare: interrelated drivers for healthy aging. Front Public Health 2020;8:231. https://doi.org/10.3389/fpubh.2020.00231 PMID:32626678
- Jensen L, Monnat SM, Green JJ, Hunter LM, Sliwinski MJ. Rural population health and aging: toward a multilevel and multidimensional research agenda for the 2020s. Am J Public Health 2020;110:1328–31. https://doi.org/10.2105/AJPH.2020.305782 PMID:32673118
- Hayward MD, Majmundar MK, eds. Future directions for the demography of aging: proceedings of a workshop. Washington, DC: The National Academies Press, 2018.
- Committee on Population; Division of Behavioral and Social Sciences and Education; Board on Health Care Services; National Research Council; Institute of Medicine. Measuring the risks and causes of premature death: summary of workshops, Washington, DC: The National Academies Press; 2015.
- Henning-Smith C, Hernandez A, Ramirez M, et al. Dying too soon: county-level disparities in premature death by rurality, race, and ethnicity. Minneapolis, MN: University of Minnesota Rural Health Research Center; 2019. 1552267547UMNpolicybriefPrematureDeath.pdf
- Gilbert PA, Laroche HH, Wallace RB, Parker EA, Curry SJ. Extending work on rural health disparities: a commentary on Matthews and colleagues’ report. J Rural Health 2018;34:119–21. https://doi.org/10.1111/jrh.12241 PMID:28397970
- Lichter DT, Johnson KM. A demographic lifeline? Immigration and Hispanic population growth in rural America. Popul Res Policy Rev 2020;39:785–803. https://doi.org/10.1007/s11113-020-09605-8
- Khullar D, Chokshi DA. Challenges for immigrant health in the USA—the road to crisis. Lancet 2019;393:2168–74. https://doi.org/10.1016/S0140-6736(19)30035-2 PMID:30981536
- Leider JP, Meit M, McCullough JM, et al. The state of rural public health: enduring needs in a new decade. Am J Public Health 2020;110:1283–90. https://doi.org/10.2105/AJPH.2020.305728 PMID:32673103
- Ingram DD, Franco SJ. 2013 NCHS urban-rural classification scheme for counties [Internet]. Hyattsville, MD: US Department of Health and Human Services, CDC, National Center for Health Statistics; 2014. https://www.cdc.gov/nchs/data_access/urban_rural.htm
- Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep 2013;61:1–117. PMID:24979972
- García MC, Rossen LM, Bastian B, et al. Potentially excess deaths from the five leading causes of death in metropolitan and nonmetropolitan counties—United States, 2010–2017. MMWR Surveill Summ 2019;68(No. SS-10):1–11. https://doi.org/10.15585/mmwr.ss6810a1 PMID:31697657
- Hung P, Shi K, Probst JC, et al. Trends in cancer treatment service availability across critical access hospitals and prospective payment system hospitals. Med Care 2022;60:196–205. https://doi.org/10.1097/MLR.0000000000001635 PMID:34432764
- CDC. An update on cancer deaths in the United States. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://stacks.cdc.gov/view/cdc/119728
- Zheng S, Schrijvers JJA, Greuter MJW, Kats-Ugurlu G, Lu W, de Bock GH. Effectiveness of colorectal cancer (CRC) screening on all-cause and CRC-specific mortality reduction: a systematic review and meta-analysis. Cancers (Basel) 2023;15:1948. https://doi.org/10.3390/cancers15071948 PMID:37046609
- Williams PA, Zaidi SK, Sengupta R. AACR cancer progress report 2023: advancing the frontiers of cancer science and medicine. Clin Cancer Res 2023;29:3850–1. https://doi.org/10.1158/1078-0432.CCR-23-2591 PMID:37702621
- Barnes JM, Johnson KJ, Adjei Boakye E, et al. Early Medicaid expansion and cancer mortality. J Natl Cancer Inst 2021;113:1714–22. https://doi.org/10.1093/jnci/djab135 PMID:34259321
- Cronin KA, Scott S, Firth AU, et al. Annual report to the nation on the status of cancer, part 1: national cancer statistics. Cancer 2022;128:4251–84. https://doi.org/10.1002/cncr.34479 PMID:36301149
- CDC. Using project ECHO and patient navigation to improve the health and wellness of cancer survivors in rural communities. Atlanta, GA: US Department of Health and Human Services, CDC; 2023. https://www.cdc.gov/cancer/ncccp/success-stories/echo-navigation.htm
- Sahar L, Douangchai Wills VL, Liu KKA, et al. Geographic access to lung cancer screening among eligible adults living in rural and urban environments in the United States. Cancer 2022;128:1584–94. https://doi.org/10.1002/cncr.33996 PMID:35167123
- CDC. WISQARS fatal injury data visualization. Atlanta, GA: US Department of Health and Human Services, CDC; 2020. https://wisqars.cdc.gov/data/lcd/home
- Roehler DR, Guy GP Jr, Jones CM. Buprenorphine prescription dispensing rates and characteristics following federal changes in prescribing policy, 2017–2018: a cross-sectional study. Drug Alcohol Depend 2020;213:108083. https://doi.org/10.1016/j.drugalcdep.2020.108083 PMID:32505044
- National Highway Traffic Safety Administration. US Census Bureau American Community Survey. Fatality Analysis Reporting System (FARS), 2016–2020. Washington, DC: National Highway Traffic Safety Administration. https://www-fars.nhtsa.dot.gov/Main/index.aspx
- Shaw KM, West B, Kendi S, Zonfrillo MR, Sauber-Schatz E. Urban and rural child deaths from motor vehicle crashes: United States, 2015–2019. J Pediatr 2022;250:93–9. https://doi.org/10.1016/j.jpeds.2022.07.001 PMID:35809653
- Stevens JA, Lee R. The potential to reduce falls and avert costs by clinically managing fall risk. Am J Prev Med 2018;55:290–7. https://doi.org/10.1016/j.amepre.2018.04.035 PMID:30122212
- Sidney S, Lee C, Liu J, Khan SS, Lloyd-Jones DM, Rana JS. Age-adjusted mortality rates and age and risk-associated contributions to change in heart disease and stroke mortality, 2011–2019 and 2019–2020. JAMA Netw Open 2022;5:e223872. https://doi.org/10.1001/jamanetworkopen.2022.3872 PMID:35319764
- Laffin LJ, Kaufman HW, Chen Z, et al. Rise in blood pressure observed among US adults during the COVID-19 pandemic. Circulation 2022;145:235–7. https://doi.org/10.1161/CIRCULATIONAHA.121.057075 PMID:34865499
- Bress AP, Cohen JB, Anstey DE, et al. Inequities in hypertension control in the United States exposed and exacerbated by COVID-19 and the role of home blood pressure and virtual health care during and after the COVID-19 pandemic. J Am Heart Assoc 2021;10:e020997. https://doi.org/10.1161/JAHA.121.020997 PMID:34006116
- Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions—United States, January–May 2020. Am J Transplant 2020;20:2612–7. https://doi.org/10.1111/ajt.16239 PMID:32862556
- Yang Q, Tong X, George MG, Chang A, Merritt RK. COVID-19 and risk of acute ischemic stroke among Medicare beneficiaries aged 65 years or older: self-controlled case series study. Neurology 2022;98:e778–89. https://doi.org/10.1212/WNL.0000000000013184 PMID:35115387
- Italia L, Tomasoni D, Bisegna S, et al. COVID-19 and heart failure: from epidemiology during the pandemic to myocardial injury, myocarditis, and heart failure sequelae. Front Cardiovasc Med 2021;8:713560. https://doi.org/10.3389/fcvm.2021.713560 PMID:34447795
- Schultze A, Walker AJ, MacKenna B, et al.; OpenSAFELY Collaborative. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med 2020;8:1106–20. https://doi.org/10.1016/S2213-2600(20)30415-X PMID:32979987
- Frieden TR. Foreword. In: CDC health disparities and inequalities report—United States, 2013. MMWR Suppl 2013;62:(No. Suppl-3):1–2. PMID:24264482
- Kilic H, Arguder E, Karalezli A, et al. Effect of chronic lung diseases on mortality of prevariant COVID-19 pneumonia patients. Front Med (Lausanne) 2022;9:957598. https://doi.org/10.3389/fmed.2022.957598 PMID:36314036
FIGURE 1 . Percentages of preventable premature deaths* among persons aged <80 years from the five leading causes of death, by year — National Vital Statistics System, United States, 2010–2022 †
* Preventable premature deaths are defined as deaths among persons aged <80 years in excess of the number that would be expected if the death rates for each cause in all states were equivalent to those in the benchmark states (i.e., the three states with the lowest rates).
† Data for 2022 are provisional counts from January through June and were annualized for comparability with previous years.
FIGURE 2 . Percentages of preventable premature deaths* among persons aged <80 years from the five leading causes of death, by rural-urban county classification and year — National Vital Statistics System, United States, 2010–2022 †
* Preventable premature deaths are defined as deaths among persons aged <80 years in excess of the number that would be expected if the death rates for each cause in all states were equivalent to those in the benchmark states (i.e., the three states with the lowest rates). Estimates of potentially excess deaths that were negative were set to zero. These negative excess estimates occurred in cases where deaths were fewer than expected (i.e., mortality was lower than benchmark rates). † Data for 2022 are provisional counts from January through June and were annualized for comparability with previous years. § Includes unintentional poisoning (e.g., drug overdose), unintentional motor vehicle traffic crash, unintentional drowning, and unintentional fall ( https://www.cdc.gov/injury/wisqars/animated-leading-causes.html ).
Suggested citation for this article: García MC, Rossen LM, Matthews K, et al. Preventable Premature Deaths from the Five Leading Causes of Death in Nonmetropolitan and Metropolitan Counties, United States, 2010–2022. MMWR Surveill Summ 2024;73(No. SS-2):1–11. DOI: http://dx.doi.org/10.15585/mmwr.ss7302a1 .
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services. Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services. References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed in MMWR were current as of the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version ( https://www.cdc.gov/mmwr ) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

Clinical trials: A significant part of cancer care
Share this:.
Editor's note: May is National Cancer Research Month.
By Mayo Clinic staff
A cancer diagnosis is an emotional experience. Learning that you have cancer can create feelings of hopelessness, fear and sadness. This is especially true if your cancer is advanced or available treatments are unable to stop or slow its growth.
"Often, when patients are diagnosed with cancer , they feel hopeless and scared. Clinical trials are one way patients can be proactive. They can make a choice in how their care is going to be," says Matthew Block, M.D., Ph.D. , a Mayo Clinic medical oncologist.
Cancer clinical trials help physician-scientists test new and better ways to control and treat cancer. During a clinical trial, participants receive specific interventions, and researchers determine if those interventions are safe and effective. Interventions studied in clinical trials might be new cancer drugs or new combinations of drugs, new medical procedures, new surgical techniques or devices, new ways to use existing treatments, and lifestyle or behavior changes.
Clinical trials provide access to potential treatments under investigation, giving options to people who otherwise may face limited choices. "Clinical trials open the door to a new hope that maybe we can fight their cancer back and give them a better quality of life," says Geoffrey Johnson, M.D., Ph.D. , a Mayo Clinic radiologist, nuclear medicine specialist and co-chair of the Mayo Clinic Comprehensive Cancer Center Experimental and Novel Therapeutics Disease Group.
You will receive cancer treatment if you participate in a clinical trial. "I think one common misperception about clinical trials is that if you enter a clinical trial, you may not get treatment (receive a placebo). And that's actually very much not true. Most clinical trials are looking at one treatment compared to another treatment," says Judy C. Boughey, M.D. , a Mayo Clinic surgical oncologist, chair of Breast and Melanoma Surgical Oncology at Mayo Clinic in Rochester, Minnesota, and chair of the Mayo Clinic Comprehensive Cancer Center Breast Cancer Disease Group.
"I think one common misperception about clinical trials is that if you enter a clinical trial, you may not get treatment (receive a placebo). And that's actually very much not true. Most clinical trials are looking at one treatment compared to another treatment." Judy C. Boughey, M.D.
Watch this video to hear the experiences of people who have participated in cancer clinical trials and to hear Drs. Block, Johnson and Boughey discuss the importance of clinical trials in cancer care:
Clinical trials are a significant part of cancer care at Mayo Clinic Comprehensive Cancer Center. Cancer care teams work together across specialties to make sure the right clinical trials are available to serve the needs of people with cancer who come to Mayo Clinic.
"We are very particular in how we select the clinical trials that we have available for patients," says Dr. Boughey. "We want to have the best trials available for our patients. Some of the clinical trials are evaluating drugs — we are so excited about those drugs, but we can't prescribe those drugs for patients without having that trial. And so we will actually fight to try to get that trial open here to have it available as an opportunity for our patients."
If you choose to participate in a clinical trial, you will continue to receive cancer care. "For most patients that we evaluate, there's always the standard of care treatment option for those patients. And then, in many situations, there's also a clinical trial that the patient can participate in," says Dr. Boughey.
People who participate in clinical trials help make new and better cancer care available for future patients. The treatments available for cancer patients today exist because of the clinical trial participants of yesterday. "We couldn't advance medicine if it wasn't for people volunteering for trials. And the promise from our side is to say we're not going to put patients on trials or offer trials for them to consider unless we think there's a good chance that they'll get a benefit or that society at large will get a benefit," says Dr. Johnson.
"We couldn't advance medicine if it wasn't for people volunteering for trials. And the promise from our side is to say we're not going to put patients on trials or offer trials for them to consider unless we think there's a good chance that they'll get a benefit or that society at large will get a benefit." Geoffrey Johnson, M.D., Ph.D.
Participating in a clinical trial may give you access to cutting-edge treatment, improve your quality of life and extend your time with loved ones.
"It's definitely worth reaching out to your healthcare provider and asking, 'What clinical trials could I be a potential candidate for?'" says Dr. Boughey. "And remember, you can ask this of your surgical oncologist, your medical oncologist, your radiation oncologist, or any of the physicians you're seeing because there are trials in all disciplines. There are also ongoing trials that require the collection of tissue or the donation of blood. They can also be important in trying to help future generations as we continue to work to end cancer."
Participating in a clinical trial is an important decision with potential risks and benefits. Explore these FAQ about cancer clinical trials, and ask your care team if a clinical trial might be right for you.
Learn more about cancer clinical trials and find a clinical trial at Mayo Clinic.
Join the Cancer Support Group on Mayo Clinic Connect , an online community moderated by Mayo Clinic for patients and caregivers.
Read these articles about people who have participated in clinical trials at Mayo Clinic:
- A silent tumor, precancerous polyps and the power of genetic screening
- Mayo Clinic’s DNA study reveals BRCA1 mutations in 3 sisters, prompts life-changing decisions
Read more articles about Mayo Clinic cancer research made possible by people participating in clinical trials.
Related Posts

Dr. Sujay Vora is studying a new approach to glioblastoma treatment that is improving health outcomes and quality of life for elderly people like Richard Casper.

Dr. S. John Weroha discusses new treatments and research that are helping more people survive ovarian cancer.

Hypothesis-driven AI offers an innovative way to use massive datasets to help discover the complex causes of diseases such as cancer and improve treatment strategies.
Select "Patients / Caregivers / Public" or "Researchers / Professionals" to filter your results. To further refine your search, toggle appropriate sections on or off.
Cancer Research Catalyst The Official Blog of the American Association for Cancer Research

Home > Cancer Research Catalyst > AACR Annual Meeting 2024: A Global View on Cancer
AACR Annual Meeting 2024: A Global View on Cancer
With more than 23,200 registrants, the American Association for Cancer Research (AACR) Annual Meeting 2024 not only set a record number of registrants but witnessed the highest international participation to date.
As the world’s largest professional organization dedicated to advancing cancer research, the AACR has members in 141 countries and territories around the world and offers numerous opportunities to help investigators in the international community.
In fact, 62 out of 67 grants at the AACR are open globally including the AACR Scholar-in-Training Awards , the Global Scholar-in-Training Awards (GSITA), Women in Cancer Research (WICR) Scholar Awards , the Beginning Investigator Grant for Catalytic Research (BIG Cat) , and Maximizing Opportunity for New Advancements in Research in Cancer (MONARCA) . Additionally, education and training opportunities include AACR on Campus and several conferences around the world.
Even domestic conferences like the Annual Meeting provide international researchers with the opportunity to exchange knowledge and new ideas with other scientists dedicated to cancer research. This year’s meeting welcomed more than 8,000 international registrants—representing 36% of participants—from 78 countries and multiple territories.
“We are proud to highlight cutting-edge research from groups of scientists from around the world,” said Elizabeth M. Jaffee, MD, FAACR , chair of the AACR Global Affairs Committee. “Global collaboration enables strong scientific contributions to research, and we trust that the Annual Meeting provides a platform for researchers globally, to absorb and share new knowledge on emerging research, therapies, and treatment with colleagues and communities at home.”
The Annual Meeting also offers a platform to shine a light on cancer researchers and journalists through a number of awards with several international attendees among this year’s recipients. We asked a few of these award recipients about their experience attending the meeting and the importance of global collaboration in the conquest of cancer.
Exploring the International Perspective
Hellen Shikanda from Kenya became a journalist because she wanted to be a voice for the voiceless.
“Speaking to people who would not ordinarily get an opportunity to have their stories out there helps in empowering communities that they come from,” Shikanda said. “I have learnt that their stories, if told well, always have an impact on the policies that are enacted after those in leadership positions realize that there has been a gap that urgently needed to be filled.”
One of her stories was honored with the AACR June L. Biedler Award for Cancer Journalism , which was given to five journalists in four categories. Her article, “New Drug Promises Better Treatment for Cancer Patients,” relays a patient’s journey through five countries to get properly diagnosed with colon cancer and ultimately find hope for treatment in a clinical trial.
“I was happy to work on a story that explored clinical trials for a new drug that tackles a specific gene mutation—and the research was done in Africa for the first time,” she explained. “Global collaboration means that there will be funding opportunities for all researchers who can share insights on how they can help defeat cancer.”

Anshika Chauhan, PhD , from India, is the recipient of one such funding opportunity as one of the many international winners of the AACR-WICR Scholar Awards. This award is given to members of WICR, a constituency group focused on women scientists within the AACR that celebrated its 25th anniversary at the meeting , who are scientists-in-training and presenters of meritorious scientific papers at AACR Annual Meetings. She presented her research on increased mitochondrial DNA copy number via EPOR signaling as a potential mechanism for metastatic dissemination in head and neck squamous cell carcinoma.
“The AACR gives women from different backgrounds, different geographical regions, and different phases of their career the platform to work together, learn from each other, and make a difference in fighting cancer,” Chauhan said. “So, it’s important to welcome women from all over the world into cancer research. By working together and sharing our ideas, we can make a big impact in the fight against this disease.”
Seifegebriel Feleke Teshome, from Ethiopia, was one of 15 early-career scientists from nine countries who were offered the chance to attend the meeting and present their work as a 2024 GSITA recipient . His poster focused on profiling the CpG methylation patterns of Epstein-Barr virus DNA in lymphoma patients from Ethiopia.
“During the meeting, numerous scientific researchers and young scientists from different parts of the world gathered to share their findings, achievements, and innovative approaches in cancer research,” he said. “This diverse community of attendees created a vibrant atmosphere conducive to engaging discussions and fruitful interactions.”
Many of these award recipients will share what they learned about the AACR’s global programs and initiatives with their colleagues back home as they work to overcome the worldwide challenge of cancer through scientific research.
- About This Blog
- Blog Policies
- Tips for Contributors

Cultivating a More Diverse Scientific Work Force

Behind the Scenes: Building a Robust Scientific Program at the AACR Annual Meeting

SABCS 2016: World’s Largest Breast Cancer Meeting Draws to a Close
Cancel reply
Your email address will not be published. Required fields are marked *
Join the Discussion (max: 750 characters)...
This site uses Akismet to reduce spam. Learn how your comment data is processed .
- Healthcare Professionals Membership
- What Is Cancer Research
- Various Types of Cancer
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Focus 19 April 2022
The Future of Cancer Research
Nature Medicine presents a special Focus dedicated to the future of cancer research and care. We take stock of the latest exciting developments, as well as the challenges, gaps and inequities that must be resolved as we navigate the cancer research landscape of the next decade.
Call for papers - Nature Medicine is inviting submission of innovative research on the prevention, detection and treatment of cancer. We welcome in particular clinical trials leveraging new technologies and designs to address unmet clinical needs, as well as population-based studies with global impact. Find out more here .

Focus Issue: The Future Of Cancer Research
New treatments and technologies offer exciting prospects for cancer research and care, but their global impact rests on widespread implementation and accessibility.

How to correct the market for children’s cancer drugs
The development of pediatric cancer drugs is vastly underfunded compared with that for adults, but legislation can correct market failures.
- Nancy F. Goodman

Strategies for inclusive grantmaking
Grantmaking organizations play a crucial role in increasing diversity and equity in the biomedical workforce. Collecting demographic data, increasing the diversity of applicants and reducing bias in peer review are valuable strategies to achieve these goals.
- Maryrose Franko
- Sindy Escobar-Alvarez
- Kristen L. Mueller

Cancer treatments should benefit patients: a common-sense revolution in oncology
Many newly approved cancer therapeutics offer limited clinical benefits yet are still prescribed to patients. A common-sense revolution in oncology would prioritize treatments that meaningfully improve survival and quality of life.
- Bishal Gyawali
- Christopher M. Booth

Reimagining patient-centric cancer clinical trials: a multi-stakeholder international coalition
The Bloomberg New Economy International Cancer Coalition brings together academia, industry, government, patient advocacy and policy think tanks to leverage technology and collaboration to improve patient access to clinical trials and to harmonize regulations aiming to accelerate cancer cures and prevention worldwide in the post-pandemic era.
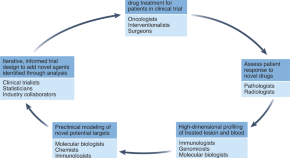
Neoadjuvant clinical trials provide a window of opportunity for cancer drug discovery
Window-of-opportunity trials, during which patients receive short-duration pre-surgical therapies, provide a platform for understanding the therapies’ mechanisms of action, but will require a paradigm shift in trial design, specimen collection and analysis.
- Thomas U. Marron
- Matthew D. Galsky
- Miriam Merad

A golden era of cancer clinical trials
Cancer immunotherapy is generating huge excitement, but the future may lie elsewhere, in antibody–drug conjugates, proteolysis-targeting chimeras, and liquid biopsy for early detection.
- Paul Webster
Theranostics could be big business in precision oncology
Theranostics aim to both diagnose and treat cancer. Although few such drugs are on the market, many are being tested in clinical trials, with early results showing promise.
- Carrie Arnold

Five young investigators tackle big questions in oncology
Early-career researchers share their priorities for cancer research, from early screening and immunotherapy to nanotechnology and counseling.
- Meghie Rodrigues
Reviews and Perspectives
Priorities for cancer research in low- and middle-income countries: a global perspective.
Radical rethinking is needed to address the burning issues in cancer care in low- and middle-income countries. In this Perspective, the authors outline the main challenges and top priorities for cancer research now and into the future.
- C. S. Pramesh
- Rajendra A. Badwe
- Elisabete Weiderpass
Delivering precision oncology to patients with cancer
Precision medicine is reshaping cancer care, but the benefits are not accessible to all patients. This Perspective outlines the major challenges to the implementation of precision oncology and discusses critical steps toward resolving these.
- Joaquin Mateo
- Lotte Steuten
- Emile Voest
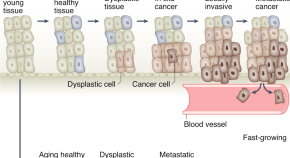
The future of early cancer detection
Technological advances are producing exquisitely sensitive cancer detection tests. This Review discusses who should be tested, and how—and looks to the future of personalized, risk-based cancer screening.
- Rebecca C. Fitzgerald
- Antonis C. Antoniou
- Nitzan Rosenfeld
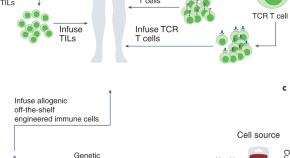
Engineered cellular immunotherapies in cancer and beyond
Oncology is trailblazing the field of engineered cellular therapeutics. This Review discusses the goals of cellular immunotherapy in cancer, key challenges facing the field and strategies to overcome them—paving the way for treatment of other diseases.
- Amanda V. Finck
- Tatiana Blanchard
- Carl H. June
Targeting the gut and tumor microbiota in cancer
There exists tremendous opportunity to target microorganisms in the gut and other niches to help treat or even prevent cancer. This Review outlines how microbial targeting could become a pillar of personalized cancer care over the next 5 to 10 years.
- Elizabeth M. Park
- Manoj Chelvanambi
- Jennifer A. Wargo
New Research
Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial
A randomized trial in treatment-naive patients with metastatic renal cell carcinoma shows that the addition of a live bacterial product to an immunotherapy combination elicits promising clinical benefit in association with an enrichment of bacterial species, circulating cytokines and immune cell populations in responders.
- Nazli Dizman
- Sumanta K. Pal
A probiotic supplement boosts response to cancer immunotherapy
Patients with kidney cancer who took probiotic supplements of Clostridium butyricum had improved response to immunotherapy, according to a randomized phase 1 study.
- Lisa Derosa
- Laurence Zitvogel
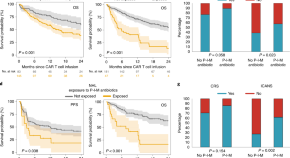
Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy
In an analysis of adult patients with hematologic malignancies who received anti-CD19 chimeric antigen receptor T cell therapy, baseline gut microbiome composition was correlated with clinical response and treatment with broad-spectrum antibiotics in the four weeks prior to infusion was associated with worse survival and increased neurotoxicity.
- Melody Smith
- Marco Ruella
PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial
CAR T cells targeting PSMA and engineered to be resistant to immunosuppressive TGFβ signaling exhibit dose-dependent toxicity and expansion following infusion, with some transient antitumor activity, in patients with metastatic castration-resistant prostate cancer
- Vivek Narayan
- Julie S. Barber-Rotenberg
- Naomi B. Haas
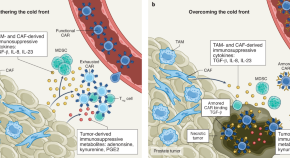
CAR T cells reach clinical milestone in prostate cancer
Armored CAR T cells show early signs of clinical activity in patients with castration-resistant prostate cancer, paving the way for further development and optimization.
- Nicholas P. Tschernia
- Scott M. Norberg
- James L. Gulley
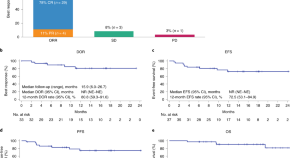
Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial
In a phase 2 trial, first-line treatment with axicabtagene ciloleucel, an autologous CD19-targeting CAR T-cell therapy, exhibited a high complete response rate and a manageable safety profile in adults with high-risk large B-cell lymphoma.
- Sattva S. Neelapu
- Michael Dickinson
- Julio C. Chavez
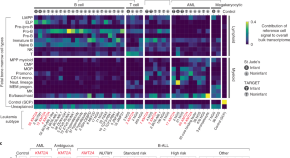
Single-cell transcriptomics reveals a distinct developmental state of KMT2A -rearranged infant B-cell acute lymphoblastic leukemia
Single-cell transcriptomic and phylogenetic analyses reveal new insights into the developmental origin and potential therapeutic targets for a particularly aggressive form of B-cell acute lymphoblastic leukemia in infants.
- Eleonora Khabirova
- Laura Jardine
- Sam Behjati
Stratification of radiosensitive brain metastases based on an actionable S100A9/RAGE resistance mechanism
A comprehensive analysis of models of brain metastasis and multiple cohorts of patient samples identifies a targetable molecular mechanism underlying the resistance to whole-brain radiotherapy that can inform patient selection for personalized radiotherapy.
- Cátia Monteiro
- Lauritz Miarka
- Manuel Valiente
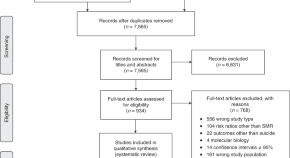
Suicide risk and mortality among patients with cancer
A global analysis across 62 studies, of which 28 were meta-analyzed, reveals an increased risk of death by suicide in patients with cancer and underscores the need for comprehensive psycho-oncological therapy during clinical routine to improve the quality of life of patients.
- Michael Heinrich
- Luisa Hofmann
- Corinna Seliger
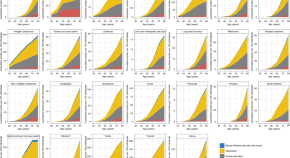
Cumulative burden of psychiatric disorders and self-harm across 26 adult cancers
A large-scale population analysis quantifies the burden of mental illness and self-harm events in patients diagnosed with the most common adult cancers and highlights opportunities for advancing patient care.
- Wai Hoong Chang
- Alvina G. Lai

Shining a light on the psychological burden of cancer
Two studies incorporating data from millions of patients highlight the need for consistent screening and access to supportive care.
- Cristiane Decat Bergerot
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
FIGO 2023 endometrial staging: a leap of faith into the new “prognostic based’ rather than “anatomical based” staging—too fast too furious??
- Open access
- Published: 11 May 2024
- Volume 150 , article number 251 , ( 2024 )
Cite this article
You have full access to this open access article

- Karthik Chandra Bassetty 1 ,
- Dimpy Begum 1 ,
- Debabrata Barmon 1 ,
- Upasana Baruah 1 ,
- Sakshi Gupta 1 ,
- Mahendra Kumar 1 ,
- Jyotiman Nath 1 ,
- Duncan Khanikar 1 ,
- Mouchumee Bhattacharyya 1 &
- P. S. Roy 1
30 Accesses
Explore all metrics
In 2023 FIGO revised the endometrial cancer staging system after 13 years. There is a lacuna of data regarding the performance and practicality of the revised 2023 FIGO staging schema for endometrial cancer from Low Middle-Income Countries (LMIC).
To estimate the shift of stage and adjuvant management of endometrial cancer based on the FIGO 2023 system compared to the FIGO 2009 system and assess the predictive potential of the FIGO 2023 system.
Material and methods
A retrospective study was conducted from 1st January 2017 to 31st December 2022. All patients with endometrial cancer were staged according to the FIGO 2023 and FIGO 2009 staging system. Follow-up of patients was done to determine recurrence.
A total of 152 patients were included. Aggressive histology was seen in 66 (45%) patients. Eighteen (11%) had subserosal involvement. Substantial LVSI was noted in 23 (15%) of patients. Twenty-four (47%) patients of FIGO 2009 Stage IA and 26 patients (63%) of FIGO 2009 Stage IB were upstaged. Eleven (50%) patients of FIGO 2009 Stage IIIA were down staged to IA3. Overall 23 patients (15%) had a shift of stage. Fifteen out of 152 patients (15%) would have had a possible risk stratification change which would imply 23 patients (15%) would have needed a more radical treatment. Molecular classification was done in 32 patients; however, only 2 patients could afford POLE testing. Kaplan–Meier curves showed significant PFS differences in FIGO 2009 Stage IB and Stage IIIA when restaged according to the FIGO 2023 system.
The FIGO 2023 endometrial staging is a more robust prognosticator; however, the practicality of molecular classification in LMICs is still a distant dream.
Avoid common mistakes on your manuscript.
Introduction
Staging systems are the backbone of any cancer treatment. They are meant to be valid, reliable and practical (Odicino et al. 2008 ). Stage is a strong predictor of patient prognosis. The main bodies for cancer staging include the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) (Brierley. 2006 ). Concerning gynaecological cancer, The International Federation of Gynaecology and Obstetrics (FIGO) takes centre stage and a collaborative arrangement between the three staging systems ensures synchrony with changes in FIGO staging manifesting in the AJCC and UICC versions (Greene and Sobin 2009 ).
The earlier anatomy-based staging was the norm earlier, however, over the years staging has included histological prognostic factors, biomarkers and molecular data as evident in breast, head-neck and prostate cancers (Odicino et al. 2008 ; Amin et al. 2017 ). Keeping with this trend a radical shift in endometrial cancer (EC) staging was formulated by FIGO in June 2023 (Berek 2023 ). The changes in the FIGO 2023 endometrial staging system compared to the FIGO 2009 endometrial staging system are described in Table 1 . It has created quite a stir in the oncology fraternity on its inception. Keeping this background in mind, we devised a retrospective study to study the impact of the FIGO 2023 endometrial staging system compared to its predecessor FIGO 2009 endometrial staging system.
Primary objective
To determine the percentage change in stage and risk stratification of patients with endometrial cancer from the FIGO 2009 endometrial staging system to the FIGO 2023 endometrial staging system.
Secondary objective
To determine the possible impact of the stage shift on the management of patients and assess the prognostic impact of the FIGO 2023 endometrial staging system.
Materials and methods
Study design.
All patients who were diagnosed with EC and initially underwent surgery at the Dr. Bhubaneswar Borooah Cancer Institute (BBCI), Guwahati, Assam, India between 1st January 2017 and 31st December 2022 were included in the study. All patients underwent surgical staging which included a total hysterectomy and bilateral salpingo-oophorectomy. Lymph node dissection was conducted in all patients except in patients with Stage IA—Grades I and II endometrioid endometrial cancer with a tumor size of less than 2 cm. Patients who received neoadjuvant chemotherapy, had recurrent endometrial cancer, operated outside BBCI, had multiple cancers, had no available formalin fixed paraffin embedded samples and whose tissue samples were inadequate were excluded. We had incorporated immunohistochemistry testing for MMR proteins (mismatch repair proteins MLH 1, MSH 2, MSH 6 and PMS 2) and p53 from 1st January 2023. POLE testing is not done in our institute due to the cost factor and depending on the affordability of the patient it was outsourced. The patients were divided into four groups—POLEmut, MMR-D, p53abn and NSMP. In the event of a patient testing positive for multiple IHCs the algorithm of ProMisE study was followed (Kommoss et al. 2018 ). Patients who were treated before molecular testing were classified without the molecular testing. All the histopathology reports were reviewed by two expert oncopathologists and were reported as per the 2020 World Health Organization tumor classification criteria which included the histopathological type, extent of LVSI and size of lymph node metastasis. The histopathological slides were reviewed where a discrepancy or doubt occurred regarding the findings. Demographic and clinical data were obtained retrospectively from the electronic medical registry (EMR). In this study the patients who were previously stratified based on FIGO 2009 endometrial staging system were restratified based on FIGO 2023 endometrial staging system. Upstaging was described as the reclassification to a higher group and downstaging was the reclassification to the lower group in the FIGO 2023 staging system compared to the FIGO 2009 staging system.
Operational definitions as per the FIGO 2023 endometrial staging system (Berek 2023 )
Non-aggressive histological types are composed of low-grade (Grades 1 and 2) endometrioid cancers, while aggressive histological types are composed of high-grade endometrioid cancer (Grade 3), serous carcinoma, clear cell carcinoma, mixed carcinoma, undifferentiated variant, carcinosarcoma and mesonephric-like and gastrointestinal type mucinous carcinomas.
Lymphovascular space invasion (LVSI):
Involvement of ≥ 5 vessels is considered as substantial LVSI and less than 5 vessels is considered as focal LVSI.
Involvement of sub-serosa:
As per the ISGYP recommendations (Singh 2019 ) uterine serosa involvement is defined as a tumour reaching submesothelial fibro connective tissue or the mesothelial layer, regardless of whether tumour cells may or may not be present on the serosal surface of the uterus.
Adnexal involvement:
The 2023 FIGO staging for endometrial carcinoma assigns the category of Stage IA3 when the following criteria are met in a low-grade endometrioid endometrial cancer:
No more than superficial myometrium invasion is present (< 50%).
The absence of substantial LVSI.
The absence of additional metastases.
The ovarian tumour is unilateral, limited to the ovary, without capsule invasion/breach (equivalent to pT1a).
The cases not fulfilling these criteria were interpreted as extensive spread of the endometrial carcinoma to the ovary (Stage IIIA1).
All patients underwent risk stratification as per standard guidelines existing (Concin et al. 2021 ; Colombo et al. 2016 ; Endometrical Cancer 2024 ) and received appropriate adjuvant radiotherapy and chemotherapy. We also studied the possible change in risk stratification when patients were stratified as per the ESMO 2022 risk stratification system (Endometrical Cancer 2024 ) using the FIGO 2023 staging system.
Statistical analysis
Demographic and clinical data were expressed as the number of patients and percentage (%). Progression-free survival (PFS) was calculated from the date of completion of treatment to the date of recurrence or last contact. The Kaplan–Meier method was used to analyze the PFS and the log-rank test was used to evaluate the between-group differences in PFS. The cutoff p value for statistical significance was < 0.05. SPSS version 29.0 was used for statistical analysis.
A total of 152 patients were included in the study. The median age of the patients was 52 years (range 23–84 years). The majority of patients (144, 95%) underwent open surgery whereas eight patients (5%) underwent minimally invasive surgery. Table 2 shows the histo-pathological data of all 152 patients. The majority (57%) had non-aggressive histology. Substantial LVSI was noted in 15% of cases. Out of 28 women who had adnexal involvement with low-grade endometrioid carcinoma only eleven patients fulfilled the criteria to be reclassified as Stage IA3 as shown in Fig. 1 . Metastasis to the pelvic nodes was seen in 13% of patients whereas para-aortic nodal metastasis was documented in 3% of cases.
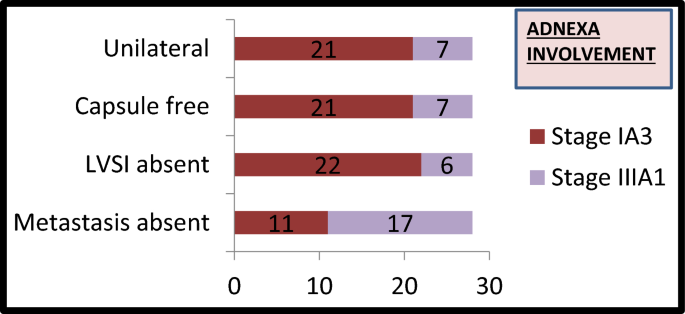
Bar diagram depicting the 28 women with adnexal involvement with characteristics about unilaterality, capsule involvement, LVSI and presence of metastasis
Figure 2 shows the Sankey diagram depicting the change in staging of patients as per the FIGO 2009 and FIGO 2023 endometrial staging systems. Due to reclassification number of patients in Stage I decreased from 92 to 38. There was a sharp increase in Stage II patients (39) in the 2023 system and a decline in Stage III with a dramatic decrease in number of patients with Stage IIIA from 22 to 5 patients. Overall 35 out of 152 (23%) patients had a change in the stage.
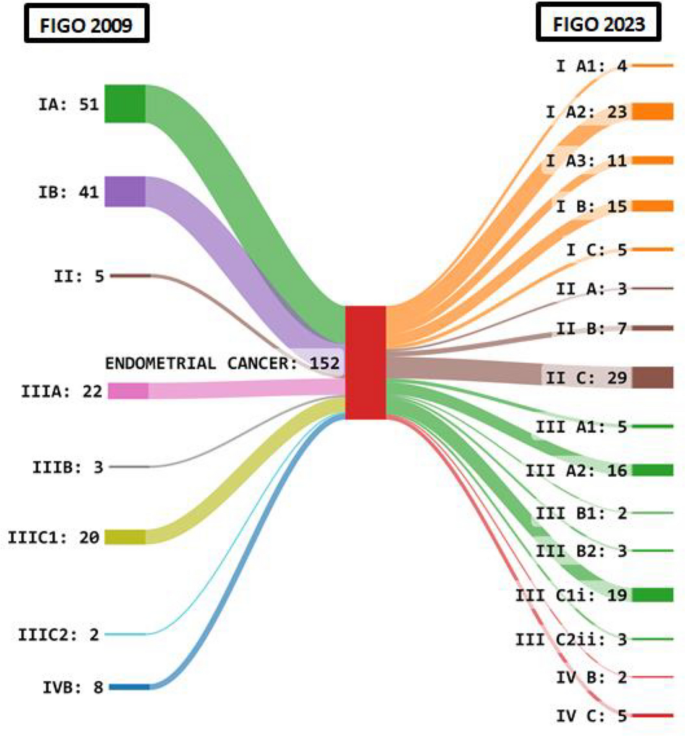
Sankey diagram showing the difference in staging of patients as per the FIGO 2009 and FIGO 2023 endometrial staging systems
Table 3 shows the stage shift concerning the individual sub-stages of FIGO 2009 and FIGO 2023 endometrial staging systems. It was seen that 24 out of 51 patients (47%) were staged in Stage IA whereas 26 out of 41 patients (67%) in Stage IB were upstaged. In Stage IIIA 11 out of 22 patients (50%) were down staged to IA3 whereas in Stage IVB 5 out of 8 patients (62.5%) were upstaged.
Figure 3 a shows the possible shift in the risk stratification category and Fig. 3 b shows the possible change in adjuvant treatment that would have resulted by the application of the ESMO 2022 risk scoring and FIGO 2023 staging system. It was observed that 15 out of 152 patients (10%) would have had a change in the risk stratification score as shown in Fig. 3 a. In Fig. 3 b as shown 7 out of 152 patients (5%) would have been undertreated whereas 23 out of 152 patients (15%) would have been overtreated.
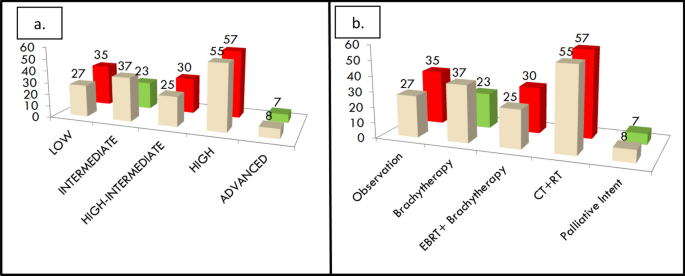
a The possible change in risk stratification between FIGO 2009 and FIGO 2023 staging systems. b The possible change in adjuvant treatment that would have been needed by patients when restaged as per FIGO 2023 staging system. The cream shade refers to the FIGO 2009 system and the green and red refer to the FIGO 2023 system wherein green depicts a decrease in numbers and red depicts the increase in numbers
Table 4 shows the risk stratification shift between FIGO 2009 and FIGO 2023 staging systems using the ESMO 2022 stratification system. It is observed that 16 out of 37 patients (43%) had increased risk stratification in the FIGO 2009 intermediate risk strata group whereas 10 out of 55 patients (18%) had decreased risk stratification in FIGO 2009 high-risk strata group.
When we analyzed the molecular profiling of 32 patients in the 2023 as shown in Fig. 4 a and b it was observed only two patients could afford POLEmut testing and both of the reports were negative for POLE mutation. It was observed that 3 out of 32 patients (9%) would have had their risk stratification strata changed as seen with the high-risk strata group increasing from 9 to 12 cases. We treated those patients with sequential chemotherapy and radiation therapy as dictated by the high-risk group management guidelines.
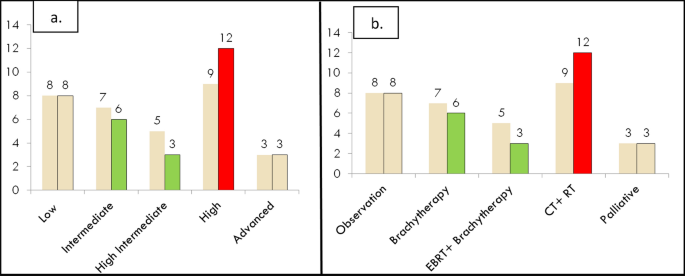
a The impact of molecular profiling on risk stratification. b The change in adjuvant treatment based on the risk strata
When patients were followed up for a median duration of 45 months, restaging of FIGO 2009 Stage IB and Stage IIIA as per the new FIGO 2023 staging system using the Kaplan–Meier analysis was found to be statistically significant for PFS (p 0.01) as shown in Fig. 5 . When we compared the other groups the values were not statistically significant.
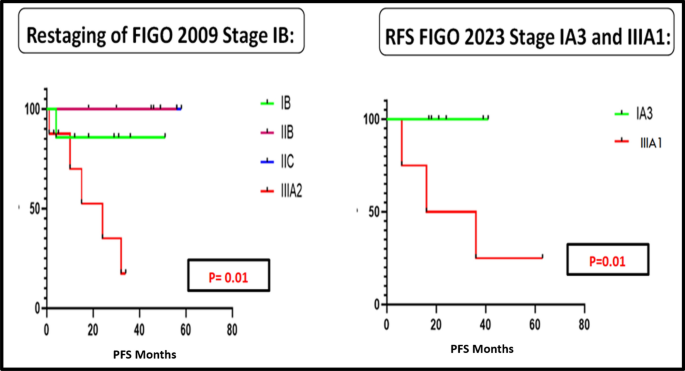
The PFS curves for the FIGO 2009 endometrial staging system when reclassified as per the FIGO 2023 staging system for Stage IB and IIIA
Figure 5 depicts the PFS curves for the FIGO 2009 endometrial staging system when reclassified as per the FIGO 2023 staging system for Stages IB and IIIA.
FIGO presented their revised staging of endometrial cancer nearly 14 years after their last update in 2009. This staging was different from its predecessor in that it moved from an anatomy-based staging into a prognosis-based staging system and was based on new molecular stratification. This created quite a furore among the medical fraternity concerning several factors (McCluggage 2023 ). In the subsequent sections, we will analyze the results of our study with respect to the above factors.
In a study done by Kobayashi et al. they observed a stage migration in 23.4% of the patients which included a larger shift in Stage I to Stage II as per the FIGO 2023 system (Kobayashi-Kato et al. 2023 ). Schwameis et al. showed 27.6% of patients had a stage shift of which 23.6% were upstaged (Schwameis et al. 2023 ). We observed that 23% of the patients had a stage shift in our study with the majority of the shifts occurring to Stage II as per FIGO 2023 endometrial system.
Molecular classification is not widely available in many parts of the world, especially in Low Middle-Income Countries (LMICs) and POLEmut testing has no defined surrogate IHC marker like MMRd or p53 groups (McCluggage 2023 ). This adds to the extra pinch of the patient’s wallet in an already economically backward community. This was evident in our study where only two patients could afford the POLE testing out of 32 patients. This also created an issue while analyzing the other patients as we could not rule out the multiple classifiers which account for 10–15% of the population. It is preferred to keep the molecular classification out of the staging system until a day comes when medical resources are equally available all around the globe. This is not to say that molecular classification is not prognostic (Gilks 2013 ) but it would be prudent to avoid it in staging and reserve it for the prognostic scoring systems. It would be helpful if a collaborative laboratory system is developed in LMIC countries which can reduce the costs involved as the tests would be done in larger numbers making it cheaper for the general population.
The FIGO 2023 staging system being a “heavy-weight” pathology-based system leads to a phenomenon called “stage migration” where the patient with the same disease has a high probability of being accorded a different stage when reviewed at an advanced diagnostic center due to the availability of molecular testing, determination of depth of sub-serosa involvement (Gilks 2013 ) and use of certain confusing histological parameters like absence of myometrium invasion and ≤ 50% myometrium invasion. It is well known that the depth of myometrium invasion has poor interobserver variation due to irregular endometrial/myometrium interface (Singh 2019 ; Gilks 2013 ). These factors lead to difficulty and confusion while assigning the stage of the patient as it keeps changing during the review of the histopathological report. This was evident in our study as we analyzed all the cases of endometrial cancer and whenever in doubt especially concerning subserosal involvement the opinion of two senior oncopathologists was taken. Not all centres can afford to have this luxury. Moreover, there is no definite consensus in terms of absolute distance from serosa to what constitutes as subserosal involvement. This could be a point that would need further clarification in the subsequent updates of the FIGO staging system.
As per the FIGO 2023 staging system the 5-year mortality in the IA3 group was lower when compared to the IIIA1 group (Berek 2023 ; McCluggage 2023 ). This was also shown in a study done by Matsuo et al. (Matsuo 2017 ). We were not able to study the overall survival due to the shorter follow-up duration in our study. However, we observed the PFS was significantly different for the IA3 Stage compared to IIIA1 Stage. We observed a significant difference in PFS when restaging of FIGO 2009 Stage IB was done. Matsuo et al. demonstrated the prognostic significance of the size of lymph nodal metastatic focus and the nature of peritoneal metastases (Matsuo 2017 ). With adoption of newer technologies such as sentinel node mapping and ultrastaging, micro metastasis are detected which are missed on routine histopathological analysis (Bogani et al. 2019 ).
We analyzed the possible impact on adjuvant risk stratification according to the new FIGO 2023 staging system based on the latest ESMO risk stratification system which had not been studied prior. It revealed that seven out of eleven (63%) patients who would have needed adjuvant chemotherapy and radiotherapy but had received lesser form of adjuvant therapy and later developed distant recurrences. We postulate that had this group of patients received more radical treatment we could have prevented these recurrences.
The role of molecular analysis has been exemplified in the latest therapeutic trials of the GARNET study and KEYNOTE -775 study which highlighted the beneficial effect of dostarlimab and a combination of pembrolizumab plus lenvtinib, respectively, in the treatment of endometrial cancer (Oaknin et al. 2022 ; Makker 2022 ).
However, keeping the above-discussed limitations of costly molecular profiling in mind it is time we explore an upcoming technology of radiomics (Bogani et al. 2022a ). Radiomics is a novel technology that uses a large number of quantitative features from radiological images from ultrasound, contrast-enhanced computed tomography(CECT) and magnetic resonance imaging(MRI) using data characterization algorithms (Rizzo et al. 2018 ; Jong et al. 2019 ). Correlation of this information with clinical data and molecular profiling can open up a new frontier in the management of endometrial cancer which will be cost-effective and widely available in the long run if proven to be an effective prognostic indicator (Bogani et al. 2020 ; Leone Roberti Maggiore et al. 2019 ). Proper use of radio genomics knowledge can reduce the health expenditure on upcoming molecular testing of new molecular targets such as PI3K/Akt/mTOR pathway which are well beyond the reach of the common man (Bogani et al. 2022b ).
The merits of this study include the application of the present ESGO 2022 risk stratification system to analyze the impact of the new staging system on adjuvant treatment. An accurate picture of the fallacies of the staging system performance in LMICs was also discussed.
There are important limitations in this study. The retrospective nature of the study combined with the smaller sample size and limited follow-up meant we could not analyze the impact on overall survival. Our data reflect the availability of resources in this part of the world and need not be applicable in other developed areas.
There are both advantages and drawbacks of the FIGO 2023 endometrial staging system. While it serves as a radical shift to the prognostic implication of the staging system it still has grey areas which can be resolved through proper appraisal at the global community level to ensure its acceptability to the wider scientific community. Hence we recommend universal adoption of the FIGO 2023 endometrial staging system as it is prognostic in nature and it should be followed by validation studies of the system from various parts of the globe.
Data availability
No datasets were generated or analysed during the current study
Amin et al (2017) The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging - PubMed [Internet]. [cited 2023 Dec 17]. Available from: https://pubmed.ncbi.nlm.nih.gov/28094848/
Berek (2023) IGO staging of endometrial cancer. Int J Gynecol Obstet Wiley Online Library [Internet]. [cited 2023 Dec 17]. Available from: https://obgyn.onlinelibrary.wiley.com/doi/ https://doi.org/10.1002/ijgo.14923
Bogani G, Mariani A, Paolini B, Ditto A, Raspagliesi F (2019) Low-volume disease in endometrial cancer: the role of micrometastasis and isolated tumor cells. Gynecol Oncol 153(3):670–675
Article PubMed Google Scholar
Bogani G, Cappuccio S, Casarin J, Narasimhulu DMM, Cilby WA, Glaser GE et al (2020) Role of adjuvant therapy in stage IIIC2 endometrial cancer. Int J Gynecol Cancer 30(8):1169–1176
Bogani G, Chiappa V, Lopez S, Salvatore C, Interlenghi M, D’Oria O et al (2022a) Radiomics and Molecular Classification in Endometrial Cancer (The ROME Study): a Step Forward to a Simplified Precision Medicine. Healthcare (basel) 10(12):2464
Bogani G, Ray-Coquard I, Concin N, Ngoi NYL, Morice P, Enomoto T et al (2022b) Clear cell carcinoma of the endometrium. Gynecol Oncol 164(3):658–666
Article CAS PubMed Google Scholar
Brierley (2006) The evolving TNM cancer staging system: an essential component of cancer care - PubMed [Internet]. [cited 2023 Dec 17]. Available from: https://pubmed.ncbi.nlm.nih.gov/16415456/
Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al (2016) ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol 27(1):16–41
Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S et al (2021) ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 31(1):12–39
de Jong EEC, Sanders KJC, Deist TM, van Elmpt W, Jochems A, van Timmeren JE et al (2019) Can radiomics help to predict skeletal muscle response to chemotherapy in stage IV non-small cell lung cancer? Eur J Cancer 120:107–113
Endometrial Cancer: ESMO Clinical Practice Guidelines [Internet]. [cited 2024 Feb 15]. Available from: https://www.esmo.org/guidelines/guidelines-by-topic/esmo-clinical-practice-guidelines-gynaecological-cancers/clinical-practice-guideline-endometrial-cancer
Gilks (2013) Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma - PubMed [Internet]. [cited 2023 Dec 17]. Available from: https://pubmed.ncbi.nlm.nih.gov/23629444/
Greene FL, Sobin LH (2009) Aworldwide approach to the TNM staging system: collaborative efforts of the AJCC and UICC - PubMed [Internet]. [cited 2023 Dec 17]. Available from: https://pubmed.ncbi.nlm.nih.gov/19170124/
Kobayashi-Kato (2023) Utility of the revised FIGO2023 staging with molecular classification in endometrial cancer - PubMed [Internet]. [cited 2023 Dec 17]. Available from: https://pubmed.ncbi.nlm.nih.gov/37748269/
Kommoss S, McConechy MK, Kommoss F, Leung S, Bunz A, Magrill J et al (2018) Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol 29(5):1180–1188
Leone Roberti Maggiore U, Martinelli F, Dondi G, Bogani G, Chiappa V, Evangelista MT, et al. Efficacy and fertility outcomes of levonorgestrel-releasing intra-uterine system treatment for patients with atypical complex hyperplasia or endometrial cancer: a retrospective study. J Gynecol Oncol. 2019;30(4):e57
Makker (2022) Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer - PubMed [Internet]. [cited 2024 Mar 28]. Available from: https://pubmed.ncbi.nlm.nih.gov/35045221/
Matsuo (2017) Impact of adjuvant therapy on recurrence patterns in stage I uterine carcinosarcoma - PubMed [Internet]. [cited 2023 Dec 17]. Available from: https://pubmed.ncbi.nlm.nih.gov/28215838/
McCluggage (2023) FIGO 2023 endometrial cancer staging: too much, too soon? Int J Gynecol Cancer [Internet]. [cited 2023 Dec 17]. Available from: https://ijgc.bmj.com/content/early/2023/11/07/ijgc-2023-004981
Oaknin A, Gilbert L, Tinker AV, Brown J, Mathews C, Press J et al (2022) Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET—a phase I, single-arm study. J Immunother Cancer 10(1):e003777
Article PubMed PubMed Central Google Scholar
Odicino F, Pecorelli S, Zigliani L, Creasman WT (2008) History of the FIGO cancer staging system. Int J Gynaecol Obstet 101(2):205–210
Rizzo S, Botta F, Raimondi S, Origgi D, Buscarino V, Colarieti A et al (2018) Radiomics of high-grade serous ovarian cancer: association between quantitative CT features, residual tumour and disease progression within 12 months. Eur Radiol 28(11):4849–4859
Schwameis et al (2023) Verification of the prognostic precision of the new 2023 FIGO staging system in endometrial cancer patients - An international pooled analysis of three ESGO accredited centres - PubMed [Internet]. [cited 2023 Dec 17]. Available from: https://pubmed.ncbi.nlm.nih.gov/37748967/
Singh (2019) Pathologic prognostic factors in endometrial carcinoma (Other Than Tumor Type and Grade) - PubMed [Internet]. [cited 2023 Dec 17]. Available from: https://pubmed.ncbi.nlm.nih.gov/30550486/
Download references
The authors received no financial support for the research, authorship and/or publication of this article.
Author information
Authors and affiliations.
Dr. Bhubaneswar Borooah Cancer Institute, Guwahati, Assam, India
Karthik Chandra Bassetty, Dimpy Begum, Debabrata Barmon, Upasana Baruah, Sakshi Gupta, Mahendra Kumar, Jyotiman Nath, Duncan Khanikar, Mouchumee Bhattacharyya & P. S. Roy
You can also search for this author in PubMed Google Scholar
Contributions
A.B wrote the main manuscript C..D.E.F.G.H.I.J-reviewed the manuscript
Corresponding author
Correspondence to Dimpy Begum .
Ethics declarations
Conflict of interest.
The authors declare there is no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Bassetty, K.C., Begum, D., Barmon, D. et al. FIGO 2023 endometrial staging: a leap of faith into the new “prognostic based’ rather than “anatomical based” staging—too fast too furious??. J Cancer Res Clin Oncol 150 , 251 (2024). https://doi.org/10.1007/s00432-024-05739-w
Download citation
Received : 09 March 2024
Accepted : 02 April 2024
Published : 11 May 2024
DOI : https://doi.org/10.1007/s00432-024-05739-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Endometrial cancer
- Stage shift
- Find a journal
- Publish with us
- Track your research

IMAGES
COMMENTS
1964-1981: Emerging Leadership in the Cancer Community; 1982-1999: A New Era of Expansion; 2000-Present: At the Forefront of Cancer Science in the 21st Century ... we have asked a panel of experts to reflect on the progress made in 2021 and forecast their predictions for cancer research in the year 2022.
In February 2022, researchers tried 130 compounds on cells grown from Sander's cancer — essentially trying everything at their disposal to see what might work. One option looked promising.
Focus Issue: The Future Of Cancer Research. Nature Medicine 28 , 601 ( 2022) Cite this article. New treatments and technologies offer exciting prospects for cancer research and care, but their ...
Back into full swing in the lab, Worldwide Cancer Research scientists have been busy making exciting new discoveries about cancer. Here are the top five cancer research breakthroughs made by our scientists in 2022. 1. Stopping the spread of breast cancer. Our scientists in Italy discovered a previously unknown way that breast cancer cells ...
Doctors at Memorial Sloan Kettering Cancer Center (MSK) pioneered advances in many types of cancer treatment for patients in 2022. Clinical trials reported results of cutting-edge treatments, including new types of immunotherapy, radiation therapy, and experimental targeted drugs.In many cases, these trials resulted in new standards of care that have the potential to benefit people with cancer ...
She is also President of the American Association for Cancer Research (AACR) for 2022-2023. ... In 2022, several new ADCs were developed and tested in clinical trials, with promising results. ...
In the United States alone, it is predicted that 1,918,030 new cases of cancer will be diagnosed in 2022 and that 609,360 people will die from the disease (ref. 2; see Fig. 1 ). These estimates do not account for the consequences of COVID-19, which has proven to have an adverse impact across the spectrum of cancer research and patient care ...
Scientists at MSK and Weill Cornell Cancer Center identified a previously uncharacterized subtype of hormone-resistant prostate cancer that accounts for about 30% of all cases. The finding, reported in Science on May 27, 2022, could pave the way for targeted therapies for people with this subtype of prostate cancer.
A total of 1.9 million new cancer cases and 609,360 deaths from cancer are expected to occur in the US in 2022, which is about 1,670 deaths a day. ... Cancer research that expands knowledge and advances treatment options; ... deaths from lung cancer in 2022 are expected to be caused from smoking cigarettes.
Latest analysis from the Cancer Research Institute of the global cell therapy landscape shows new technologies and targets advancing, but barriers to patient access are slowing progress. ... As of April 15, 2022, there were 2,756 active cell therapy agents in the global immuno-oncology pipeline, an increase of 36% over the 2021 landscape ...
April 19, 2024 , by Sharon Reynolds. NCI-funded researchers have pinpointed a single type of the bacterium F. nucleatum that appears to fuel the development and growth of colorectal cancer. In mice, the bacterium, Fna C2, appeared to cause more adenomas to form in the large intestine and it was often found in human tumor samples.
The concentration of PD-L1 in cancer cells can be higher than 90%, making it a highly targetable protein. The drug has been used successfully to treat melanoma and other skin cancers, as well as breast, lung, endometrial, kidney, esophageal and many other types of cancer, driving once advanced, even deadly, diseases into remission.
skaman306/Getty Images. A tiny group of people with rectal cancer just experienced something of a scientific miracle: their cancer simply vanished after an experimental treatment. In a very small ...
The Facts & Figures annual report provides: Estimated numbers of new cancer cases and deaths in 2022 (In 2022, there will be an estimated 1.9 million new cancer cases diagnosed and 609,360 cancer deaths in the United States.) Current cancer incidence, mortality, and survival statistics. Information on cancer symptoms, risk factors, early ...
NCI study advances personalized immunotherapy for metastatic breast cancer. February 1, 2022. News releases from the National Cancer Institute for the year 2022.
This article presents global cancer statistics by world region for the year 2022 based on updated estimates from the International Agency for Research on Cancer (IARC). There were close to 20 million new cases of cancer in the year 2022 (including nonmelanoma skin cancers [NMSCs]) alongside 9.7 million deaths from cancer (including NMSC).
For cancer therapy, 2022 continued to look bright. Elie Dolgin's news analysis of the regulatory approvals and setbacks of the past 12 months reveals a thriving cancer drug pipeline that brought ...
Clinical Trials 2022. Clinical Cancer Research ( CCR) publishes innovative clinical trials and translational cancer research studies that bridge the laboratory and the clinic. The Journal is especially interested in clinical trials evaluating new treatments, accompanied by research on pharmacology, and molecular alterations or biomarkers that ...
Studies highlight research on treatment toxicity, disparities in insurance, gaps in metastatic care, new therapies and more. December 14, 2022 • By Diane Mapes / Fred Hutch News Service. Fred Hutch breast cancer oncologists and researchers Drs. Jennifer Specht (second from right) and Hannah Linden (far right) chat with other scientists during ...
Nearly 50 presentations by researchers and clinicians from Yale Cancer Center (YCC) at Yale School of Medicine will be among the more than 5,000 abstracts available during the annual meeting of the American Society of Clinical Oncology (ASCO) May 31 to June 4 in Chicago, Ill. . This year's meeting, themed "The Art and Science of Cancer Care: From Comfort to Cure" will include over 200 ...
Melanoma in Darker Skin Tones. May 10, 2024 — Melanoma, an aggressive form of skin cancer that accounts for 75% of all skin-cancer-related deaths, is often detected later in people with darker ...
The new results reported by BioNTech and Genentech, from a small trial of 16 patients with pancreatic cancer, are equally exciting. After surgery to remove the cancer, the participants received ...
April 12, 2024 , by Daryl McGrath. The Cancer Screening Research Network will conduct rigorous, multicenter cancer screening trials. Credit: NCI. NCI's collection of cancer information products is constantly growing, so periodically we provide updates on new and updated content of interest to the cancer community.
Through the first eight months of 2022, the US Food and Drug Administration (FDA) gave the go-ahead to just four new anti-cancer treatments. But then the pace of marketing authorizations picked up ...
For example, the 2010 benchmarks for cancer are higher than if benchmarks were based on 2022 data because of decreases in cancer deaths during 2010-2022. Fourth, the numbers of preventable premature deaths by cause are not necessarily independent, and the numbers of potentially excess deaths from the five causes cannot be combined to generate ...
Cancer clinical trials help physician-scientists test new and better ways to control and treat cancer. During a clinical trial, participants receive specific interventions, and researchers determine if those interventions are safe and effective. Interventions studied in clinical trials might be new cancer drugs or new combinations of drugs, new ...
With more than 23,200 registrants, the American Association for Cancer Research (AACR) Annual Meeting 2024 not only set a record number of registrants but witnessed the highest international participation to date. As the world's largest professional organization dedicated to advancing cancer research, the AACR has members in 141 countries and territories around the world and offers numerous ...
New treatments and technologies offer exciting prospects for cancer research and care, but their global impact rests on widespread implementation and accessibility. Editorial 19 Apr 2022 Nature ...
A retrospective study was conducted from 1st January 2017 to 31st December 2022. All patients with endometrial cancer were staged according to the FIGO 2023 and FIGO 2009 staging system. Follow-up of patients was done to determine recurrence. A total of 152 patients were included. Aggressive histology was seen in 66 (45%) patients.