Website maintenance is scheduled for Saturday, August 17, and Sunday, August 18. Short disruptions may occur during these days.

AARON SAGUIL, MD, MPH, EDWIN A. FARNELL, IV, MD, AND TENEISHA S. JORDAN, MD
Am Fam Physician. 2022;106(2):173-183
Author disclosure: No relevant financial relationships.
Multiple sclerosis (MS) is a demyelinating disorder of the central nervous system and the most common cause of nontraumatic neurologic disability in young adults. Types of MS include relapsing-remitting (most common), secondary progressive, and primary progressive. Clinically isolated syndrome and radiologically isolated syndrome are additional categories for patients with findings concerning for MS who do not yet meet the diagnostic criteria for the disease. Symptoms of MS depend on the areas of neuronal involvement. Common symptoms include sensory disturbances, motor weakness, impaired gait, incoordination, optic neuritis, and Lhermitte sign. A patient history, neurologic examination, and application of the 2017 McDonald Criteria are needed to diagnose MS accurately. Patients with MS should be treated by a multidisciplinary team that may include physical and occupational therapists, speech and language therapists, mental health professionals, pharmacists, dietitians, neurologists, and family physicians. Steroids are the mainstay of treatment for the initial presentation of MS and relapses. Patients who do not adequately respond to steroids may benefit from plasmapheresis. Patients with MS who smoke tobacco should be strongly encouraged to quit. Disease-modifying therapy has been shown to slow disease progression and disability; options include injectable agents, infusions, and oral medications targeting different sites in the inflammatory pathway. Symptom-based care is important to address the bowel and bladder dysfunction, depression, fatigue, movement disorders, and pain that often complicate MS.
Multiple sclerosis (MS) is a demyelinating disorder of the central nervous system and the most common cause of nontraumatic neurologic disability in young adults. 1 Prevalence differs by latitude, with higher rates among those living further from the equator. The prevalence of MS is 40 per 100,000 people in Lubbock, Tex., compared with 191 per 100,000 people in Olmstead County, Minn. 2 An estimated 1 million people in the United States live with MS. 1 Risk factors include smoking and a history of infectious mononucleosis. Women are twice as likely as men to have MS, and there is a modest genetic influence. 3 , 4
| Clinical practice guideline | ||
| , | Cochrane review and a separate systematic review and meta-analysis of good-quality clinical trials | |
| , | Cohort study and cross-sectional study | |
| , | Clinical practice guidelines supported by randomized controlled trials and systematic review and meta-analyses | |
| Clinical practice guideline |
A woman with MS diagnosed at 35 years of age has an average life expectancy of seven to eight years less than that of the general population. Because MS has a relatively high prevalence and patients have a long life span after diagnosis, many family physicians care for patients with the disease. 5

Pathophysiology
Types of MS include relapsing-remitting (RRMS; most common), secondary progressive, and primary progressive ( Table 1 6 – 13 ) . There are also classifications for people with first episodes concerning for MS who do not meet the diagnostic criteria for MS (clinically isolated syndrome) and those with incidental radiologic findings concerning for MS in the absence of clinical symptoms (radiologically isolated syndrome). 13
| Clinically isolated syndrome | First episode of symptoms characteristic of MS, with acute or subacute onset and lasting at least 24 hours; does not yet meet diagnostic criteria for MS; 80% of patients with clinically isolated syndrome and abnormal MRI findings progress to MS within 20 years compared with 20% of those with normal MRI findings |
| Radiologically isolated syndrome | Radiography shows evidence of inflammatory demyelination without clinical manifestations (i.e., incidental findings on radiography performed for other purposes); 30% to 40% of patients with radiologically isolated syndrome later meet criteria for clinically isolated syndrome or MS |
| Relapsing-remitting MS | Episodes of acute neurologic dysfunction (relapses) followed by partial or complete improvement, with a stable clinical course between relapses; 85% of MS cases |
| Secondary progressive MS | Progressive worsening of neurologic function following initial relapsing-remitting disease; acute exacerbations may occur during progressive phase; develops in 50% of patients with relapsing-remitting MS |
| Primary progressive MS | Progressive worsening of neurologic function from onset of symptoms; acute exacerbations may also occur; 15% of MS cases |
MS is characterized by focal areas of inflammation, demyelination, gliosis (proliferation and activation of glial cells), and degeneration (axonal loss) secondary to immune-mediated attacks. 10 There is debate about whether the inflammation leading to MS is initiated within or outside the central nervous system; however, T cells, B cells, macrophages (including central nervous system microglia), astrocytes, inflammatory mediators, and blood-brain barrier permeability are all involved in a response that is associated with myelin sheath destruction, axonal injury, and clinical symptoms. 4 , 10 , 14 – 16 In RRMS, clinical lesions may resolve through mechanisms such as axonal changes, neuroplasticity, and remyelination. 13 Progressive forms of MS are associated with cumulative axonal loss and increasing neurologic deficits. 10
Clinical Presentation
Symptoms and signs of MS depend on the areas of neuronal involvement 17 ( Table 2 1 , 18 – 22 ) . Common presenting symptoms include sensory disturbances, motor weakness, impaired gait, incoordination, optic neuritis (unilateral vision loss with pain worsened by extraocular movements), and Lhermitte sign (an electric shock–like sensation down the spine on neck flexion). 18 – 20 Other symptoms include urinary, bowel, and sexual dysfunction.
| Cognitive dysfunction (e.g., learning, memory, processing speed) Decreased sensation (e.g., vibration, position, pain) Depressed mood Dysarthria Fatigue Focal sensory disturbances (e.g., numbness, tingling) Focal weakness Hearing loss or tinnitus Heat sensitivity | Lhermitte sign (an electric shock–like sensation down the spine on neck flexion) Motor disturbances (e.g., ataxia, imbalance, incoordination, tremor, weakness) Nystagmus Pain Sexual dysfunction (e.g., erectile dysfunction; problems with arousal, lubrication, pain, orgasm) Urinary or bowel disturbances Vertigo Visual disturbances (e.g., blurring, diplopia, optic neuritis) and defects |
In RRMS, relapse symptoms evolve over days before partially or fully resolving, and patients are typically stable between acute exacerbations. Some symptoms, such as fatigue, can be persistent. 20 , 23
Multiple diseases may mimic MS clinically and radiologically ( Table 3 ) . 13 , 18 , 23 , 24 The differential diagnosis includes genetic, infectious, inflammatory, metabolic, and neoplastic processes. Psychiatric diseases, ingestions, and nutritional deficiencies may also be mistaken for MS. 13 , 18 , 23 , 24 Table 4 lists tests that may help differentiate MS from other diseases. 18
| Central and peripheral nervous system disease | |
| Degenerative diseases | Amyotrophic lateral sclerosis, Huntington disease |
| Demyelinating disorders | Acute inflammatory demyelinating polyneuropathy (Guillain-Barré syndrome), chronic inflammatory demyelinating polyneuropathy, neuromyelitis optica, paraneoplastic syndromes |
| Structural lesions | Arnold-Chiari malformation, arteriovenous malformation, compressive spinal cord lesions, neoplasm |
| Vascular lesions | Cerebrovascular accident, CADASIL, hypertensive disease, migraine, vasculitis |
| Endocrine disorders | Hypothyroidism |
| Genetic disorders | Leukodystrophy, mitochondrial disease |
| Infections | HIV infection, Lyme disease, neurosyphilis, progressive multifocal leukoencephalopathy |
| Inflammatory and infiltrative disorders | Behçet syndrome, granulomatosis with polyangiitis, sarcoidosis, systemic lupus erythematosus, Sjögren syndrome, Susac syndrome |
| Medications and illicit substances | Alcohol, anticholinergic drugs, cocaine, etanercept (Enbrel), infliximab (Remicade), isoniazid, methanol, phenytoin (Dilantin) |
| Nutritional | Manganese toxicity, vitamin B deficiency |
| Psychiatric disease | Anxiety disorders, conversion disorder, somatization |
| Antinuclear antibody titers titers Complete blood count Erythrocyte sedimentation rate Rapid plasma reagin Thyroid-stimulating hormone level Vitamin B level | Systemic lupus erythematosus, rheumatologic disease Lyme disease Infection, inflammation, neoplasm Infection, inflammation Syphilis Hypothyroidism Vitamin B deficiency | Angiotensin-converting enzyme level Autoantibody assays (e.g., antineutrophil cytoplasmic, anticardiolipin, antiphospholipid, Sjögren [anti–SS-A and anti–SS-B] antibodies) HIV screening Human T-lymphotropic virus I screening Very long-chain fatty acid levels | Sarcoidosis Behçet syndrome, Sjögren syndrome, systemic lupus erythematosus, vasculitis HIV infection T-cell leukemia Adrenoleukodystrophy |
A patient history, neurologic examination, and application of the 2017 McDonald Criteria are needed to accurately diagnose MS ( Table 5 ) . 25 Diagnosis relies on the acute exacerbations of MS being disseminated in space and time ( Figure 1 18 ) . In cases where only part of the diagnostic criteria are met, magnetic resonance imaging (MRI) of the brain and spine may be used to confirm the presence of lesions consistent with MS ( Figure 2 , Figure 3 , and Figure 4 ) . 18 Cerebrospinal fluid assays demonstrating oligoclonal bands may also aid in meeting diagnostic criteria. 25
| ≥ 2 clinical attacks | ≥ 2 | None |
| ≥ 2 clinical attacks | 1 (as well as clear-cut historical evidence of a previous attack involving a lesion in a distinct anatomical location) | None |
| ≥ 2 clinical attacks | 1 | Dissemination in space demonstrated by an additional clinical attack implicating a different CNS site or by MRI |
| 1 clinical attack | ≥ 2 | Dissemination in time demonstrated by an additional clinical attack or by MRI OR demonstration of CSF-specific oligoclonal bands |
| 1 clinical attack | 1 | Dissemination in space demonstrated by an additional clinical attack implicating a different CNS site or by MRI AND Dissemination in time demonstrated by an additional clinical attack or by MRI OR demonstration of CSF-specific oligoclonal bands |
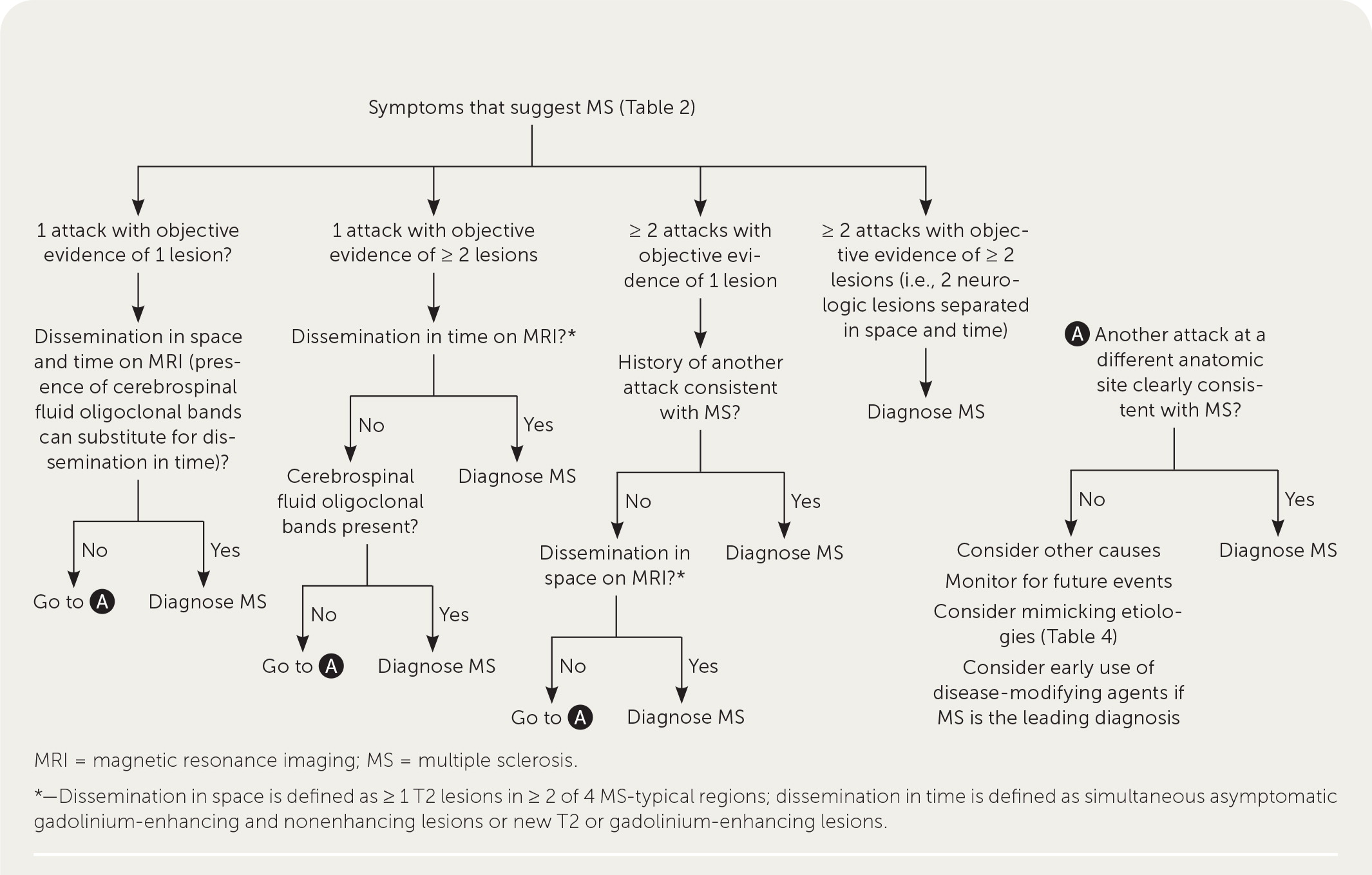
The diagnosis should be questioned if the patient has a family history of neurologic disorders other than MS, an abrupt or transient (less than 24 hours) presentation, progressive ataxia, cognitive dysfunction, other organ involvement, or nonspecific neurologic symptoms that are difficult to localize. 13 , 20 , 26
Patients with MS should be treated by a multidisciplinary team that may include physical and occupational therapists, speech and language therapists, mental health professionals, pharmacists, dietitians, neurologists, and family physicians. 27
INITIAL PRESENTATION AND ACUTE RELAPSES
Steroids are the mainstay of treatment for the initial presentation of MS and MS relapses. A Cochrane review and another systematic review and meta-analysis found no difference in effectiveness between intravenous and oral steroids for relapse recovery or MRI activity. 28 , 29 A higher dosage of steroids, such as 1,000 mg per day of methylprednisolone (intravenously or orally) for three days, is recommended. 30 , 31 Patients who do not have an adequate response to treatment with steroids may benefit from plasmapheresis. 30 , 32 A randomized controlled trial involving six plasmapheresis treatments in patients unresponsive to steroids found higher rates of complete recovery at one month than in those treated with placebo. 33
SMOKING CESSATION
Patients with MS who smoke tobacco should be strongly encouraged to quit. A cohort study found that each smoke-free year was associated with a decrease in disability progression. 34 A cross-sectional study found that each additional year of smoking accelerated the development of secondary progressive MS by 4.7% (95% CI, 2.3 to 7.2). 35
DISEASE-MODIFYING THERAPY
In patients with active MS, long-term disease-modifying therapy should be initiated to decrease new clinical attacks and radiographic lesions and delay disability progression. 36 , 37 There is disagreement about whether to use disease-modifying therapy in patients with clinically isolated syndrome. 36 – 38
Interferon beta-1b (Betaseron, Extavia) was the first disease-modifying therapy approved for use in 1993. Since then, multiple injectable agents, infusions, and oral medications such as monoclonal antibodies and other immunomodulatory medications targeting multiple steps in the MS inflammatory pathway have been approved by the U.S. Food and Drug Administration ( Table 6 ) . 13 , 37 – 39
| Alemtuzumab (Lemtrada) | 12 mg per day for five days, IV; 12 months later, 12 mg once per day for three days, IV | Infusion reaction, increased risk of infection, thyroid problems, blood clots, immune thrombocytopenia, kidney problems | — (only available at specialty pharmacy) |
| Cladribine (Mavenclad) | 1.75 mg per kg twice yearly, orally | Increased risk of infection, headache, tuberculosis, malignancy, PML | — (only available at specialty pharmacy) |
| Dimethyl fumarate | 240 mg twice per day, orally | Flushing, gastrointestinal symptoms, PML | $130 (—) |
| Diroximel fumarate (Vumerity) | 231 mg twice per day, orally | Flushing, gastrointestinal symptoms, PML | — (only available at specialty pharmacy) |
| Fingolimod (Gilenya) | 0.5 mg once per day, orally | Arrhythmia, hepatic dysfunction, increased risk of infection, PML | — ($10,000) |
| Glatiramer (Copaxone, Glatopa) | 20 mg per mL once per day, subcutaneously 40 mg per mL three times per week, subcutaneously | Injection site reactions | 20 mg: $4,700 ($26,600, $4,700) 40 mg: $6,000 ($22,000, $5,000) |
| Interferon beta-1a (Avonex, Rebif) | 30 mcg once per week, intramuscularly 22 mcg or 44 mcg three times per week, subcutaneously | Influenza-like symptoms, injection site reactions, rare liver toxicity | 30 mcg: — ($7,200) 22 mcg or 44 mcg: — ($35,000) |
| Interferon beta-1b (Betaseron, Extavia) | 0.25 mg once every other day, subcutaneously | Influenza-like symptoms, injection site reactions, rare liver toxicity | — ($125,300, $6,500) |
| Mitoxantrone | 12 mg per m every three months, IV | Heart failure, increased risk of infection, leukemia | Only available at specialty pharmacy (—) |
| Monomethyl fumarate (Bafiertam) | 190 mg twice per day, orally | Flushing, gastrointestinal symptoms, PML | — (only available at specialty pharmacy) |
| Natalizumab (Tysabri) | 300 mg every four weeks, IV | Dizziness, nausea, rash, increased risk of infection, PML | — (only available at specialty pharmacy |
| Ocrelizumab (Ocrevus) | 600 mg every six months, IV | Infusion reactions, herpes, increased risk of malignancy | — (only available at specialty pharmacy) |
| Ofatumumab (Kesimpta) | 20 mg at weeks 0, 1, and 2, then 20 mg per month starting at week 4, subcutaneously | Liver injury, PML, increased risk of infections | — (only available at specialty pharmacy) |
| Ozanimod (Zeposia) | 0.92 mg once per day, orally | Arrhythmia, increased risk of infection, hepatic dysfunction, PML | — (only available at specialty pharmacy) |
| Peginterferon beta-1a (Plegridy) | 125 mcg every two weeks, subcutaneously | Influenza-like symptoms, injection site reactions, rare liver toxicity | — (only available at specialty pharmacy) |
| Ponesimod (Ponvory) | 20 mg once per day, orally | Arrhythmia, increased risk of infection, hepatic dysfunction, PML | — ($8,300) |
| Siponimod (Mayzent) | 2 mg once per day, orally | Arrhythmia, increased risk of infection, hepatic dysfunction, PML | — ($8,900) |
| Teriflunomide (Aubagio) | 7 mg or 14 mg once per day, orally | Nausea, diarrhea, rash, teratogenic | — (only available at specialty pharmacy) |
The choice of initial disease-modifying therapy is dependent on patient preference, disease activity, potential adverse effects, and specialist input. All approved agents help prevent disease progression, with a relative risk of progression from 0.47 for mitoxantrone to 0.87 for interferon beta-1a (Avonex, Rebif). 40 For patients with less active disease, agents with a lower risk of adverse effects (e.g., cardiac arrhythmia, increased risk of malignancy, progressive multifocal leukoencephalopathy) are preferred at the cost of effectiveness. For patients with more active disease, effectiveness may be considered more important than avoiding adverse effects. Shared decision-making conversations should consider the availability of the medication options, route and frequency of administration, patient preferences regarding effectiveness vs. adverse effects, and the patient's ability to tolerate and comply with monitoring regimens. 36 , 37
For patients who have newly diagnosed RRMS with minimal symptoms and MRI burden of disease, an appropriate regimen may include a moderately effective agent such as interferon or glatiramer (Copaxone, Glatopa) to control disease activity while minimizing adverse effects. In patients with newly diagnosed, rapidly evolving RRMS, a highly effective agent such as alemtuzumab (Lemtrada), cladribine (Mavenclad), natalizumab (Tysabri), or ocrelizumab (Ocrevus) may be considered. A greater risk of debilitating adverse effects is weighed against a greater chance of controlling disease activity in this strategy. 38 Ocrelizumab is the only disease-modifying therapy currently approved by the U.S. Food and Drug Administration for primary progressive MS. 39
Medications should be continued for at least six months to allow time for benefits to occur. If the disease is not controlled by initial therapy, the patient should be offered a more effective medication, recognizing the increased potential for adverse effects. 37 , 38 It is appropriate to consider switching medications if adverse effects develop. 37
Once started, disease-modifying therapy is generally continued for the patient's lifetime; however, guidelines allow for exceptions. Discontinuation can be considered for patients with secondar y progressive MS who have a higher level of disability, are nonambulatory, and have not had a relapse in two years. Discontinuation can also be considered before conception for patients who want to become pregnant and have well-controlled MS. 37 , 38 During pregnancy, patients tend to have a lower risk of flare-ups, with overall better-controlled disease. 41
In addition to disease-modifying therapy, preliminary research suggests that hematopoietic stem cell transplantation may be a more effective alternative in preventing relapses and disability accumulation. 42
SYMPTOM-BASED CARE
In addition to treatment directed at acute relapses and disease progression, patients with MS require a comprehensive program that addresses overall wellness, symptom management, and comorbid mental health and physical conditions ( Table 7 ) . 13 , 22 , 38 , 43 – 85 A multidisciplinary approach is most effective for many symptoms. Physical activity has good evidence for improving walking ability (increased distance on six-minute walking test, faster times on 10-minute walking test), balance (as measured by the Berg Balance Scale), and depression (decreased scores on depression scales). 43 – 45 Pharmacotherapy used for symptoms associated with MS is often off-label and supported by low-quality evidence. A notable exception is dalfampridine extended-release (Ampyra), which has been approved by the U.S. Food and Drug Administration to improve walking in patients with MS. 86 Pain is treated with analgesics, neuromodulators, hydrotherapy, and sometimes cannabinoids. 49 , 82 , 84
| Bladder dysfunction | Detrusor spasm: imipramine, muscarinics, detrusor muscle onabotulinumtoxinA (Botox) injections Nocturia: intranasal desmopressin Outlet disorder: alpha adrenergic blockers, cannabinoids | Detrusor spasm: avoidance of spicy or acidic foods, caffeine, and alcohol; bladder training; sacral neuromodulation Outlet disorder: catheterization |
| Bowel dysfunction | Constipation: bisacodyl (Dulcolax), docusate sodium (Colace), enemas, lubiprostone (Amitiza), magnesium oxide, polyethylene glycol (Miralax), psyllium (Metamucil) | Abdominal massage, biofeedback, bowel timing (planning toileting times), electrostimulation of abdominal muscles, transanal irrigation |
| Cognitive impairment | Donepezil (Aricept) Amantadine, ginkgo, and rivastigmine (Exelon) were found to have no clear benefit | Neuropsychological rehabilitation, occupational therapy |
| Depression and emotional lability | Bupropion (Wellbutrin), duloxetine (Cymbalta), escitalopram (Lexapro), fluoxetine (Prozac), sertraline (Zoloft), venlafaxine | Cognitive behavior therapy, multidisciplinary rehabilitation, physical activity |
| Fatigue | Amantadine, dextroamphetamine, methylphenidate (Ritalin), modafinil (Provigil), selective serotonin reuptake inhibitors (fluoxetine) | Aerobic exercise; avoidance of heat, overexertion, and stress; cognitive behavior therapy; mindfulness training |
| Movement disorders | Ataxia: baclofen (Lioresal), cannabinoids, dantrolene (Dantrium), threonine, tizanidine (Zanaflex) Impaired ambulation: dalfampridine extended-release (Ampyra) Tremor: onabotulinumtoxinA for focal tremors, beta blockers, diazepam (Valium), isoniazid | Ataxia: deep brain stimulation, vestibular rehabilitation Impaired ambulation: behavior change therapy, physiotherapy, supervised resistance training programs |
| Pain | Neuropathic pain First-line: amitriptyline, duloxetine, gabapentin (Neurontin), nortriptyline (Pamelor), pregabalin (Lyrica) Second-line: capsaicin cream, venlafaxine Trigeminal neuralgia First-line: carbamazepine (Tegretol), oxcarbazepine (Trileptal) Second-line: baclofen, gabapentin, lamotrigine (Lamictal), pregabalin Musculoskeletal pain: analgesics, baclofen | Hydrotherapy, physiotherapy, surgical procedures for trigeminal neuralgia |
| Sexual dysfunction | Female: duloxetine Male First-line: phosphodiesterase-5 inhibitors Second-line: intercavernous vasodilators | Female: clitoral vibratory stimulation, vaginal lubrication Male: penile prostheses, vacuum appliances |
| Spasticity | Benzodiazepines, cannabinoids, dantrolene, gabapentin, intrathecal or oral baclofen, onabotulinumtoxinA, tizanidine | Electromagnetic therapy, physiotherapy, structured exercise program, transcranial magnetic stimulation, transcutaneous electrical nerve stimulation, whole body vibration |
| Vision problems (oscillopsia) | First-line: gabapentin Second-line: memantine (Namenda) | Vestibular rehabilitation |
More than one-half of patients with untreated RRMS transition to secondary progressive disease. 36 Greater disability and brain atrophy at the time of diagnosis, male sex, and older age are risk factors for progression to more significant functional limitations. 13 Disease-modifying therapy has been shown to alter the course of MS, decreasing the rate at which disability progresses, and is also associated with a lower likelihood of transitioning to progressive disease. 37 , 87
Many governments, nonprofit organizations, and websites provide information and support for individuals and families affected by MS ( eTable A ) .
| Multiple Sclerosis Association of America | |
| Multiple Sclerosis Foundation | |
| Multiple Sclerosis Society of Canada | |
| National Institute of Neurological Disorders and Stroke | |
| National Multiple Sclerosis Society | |
| Patients Like Me |
This article updates previous articles on this topic by Saguil, et al. , 18 and Calabresi . 88
Data Sources: PubMed, the Cochrane Database of Systematic Reviews, Essential Evidence Plus, the National Institute for Health and Care Excellence (UK), and the European Committee for Treatment and Research in Multiple Sclerosis were searched for relevant articles and clinical practice guidelines. Key words included multiple sclerosis, demyelinating disorders, disease-modifying treatment, and others as directed by the search. Search dates: August 2021 and May 2022.
Editor's Note: Dr. Saguil is a contributing editor for AFP .
The views expressed in this article are those of the authors and do not reflect the official policy of the U.S. Army or the Uniformed Services University of the Health Sciences.
Hauser SL, Cree BAC. Treatment of multiple sclerosis: a review. Am J Med. 2020;133(12):1380-1390.e2.
Howard J, Trevick S, Younger DS. Epidemiology of multiple sclerosis. Neurol Clin. 2016;34(4):919-939.
Belbasis L, Bellou V, Evangelou E, et al. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14(3):263-273.
Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180.
Palmer AJ, van der Mei I, Taylor BV, et al. Modelling the impact of multiple sclerosis on life expectancy, quality-adjusted life years and total lifetime costs: evidence from Australia. Mult Scler. 2020;26(4):411-420.
Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8(11):647-656.
Antel J, Antel S, Caramanos Z, et al. Primary progressive multiple sclerosis: part of the MS disease spectrum or separate disease entity?. Acta Neuropathol. 2012;123(5):627-638.
Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol. 2012;11(2):157-169.
Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545-558.
Lublin FD, Reingold SC National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology. 1996;46(4):907-911.
Koch-Henriksen N, Magyari M. Apparent changes in the epidemiology and severity of multiple sclerosis. Nat Rev Neurol. 2021;17(11):676-688.
Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet. 2018;391(10130):1622-1636.
Kutzelnigg A, Lassmann H. Pathology of multiple sclerosis and related inflammatory demyelinating diseases. Handb Clin Neurol. 2014;122:15-58.
Bø L, Vedeler CA, Nyland HI, et al. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62(7):723-732.
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15(2):180-188.
Ledesma J, Puttagunta PP, Torabi S, et al. Presenting symptoms and disease severity in multiple sclerosis patients. Neurol Int. 2021;13(1):18-24.
Saguil A, Kane S, Farnell E. Multiple sclerosis: a primary care perspective. Am Fam Physician. 2014;90(9):644-652.
Colombo B, Martinelli Boneschi F, Rossi P, et al. MRI and motor evoked potential findings in nondisabled multiple sclerosis patients with and without symptoms of fatigue. J Neurol. 2000;247(7):506-509.
Brownlee WJ, Hardy TA, Fazekas F, et al. Diagnosis of multiple sclerosis: progress and challenges. Lancet. 2017;389(10076):1336-1346.
Nazari F, Shaygannejad V, Mohammadi Sichani M, et al. Sexual dysfunction in women with multiple sclerosis: prevalence and impact on quality of life. BMC Urol. 2020;20(1):15.
Amato MP, Langdon D, Montalban X, et al. Treatment of cognitive impairment in multiple sclerosis: position paper. J Neurol. 2013;260(6):1452-1468.
Gelfand JM. Multiple sclerosis: diagnosis, differential diagnosis, and clinical presentation. Handb Clin Neurol. 2014;122:269-290.
Ömerhoca S, Akkaş SY, İçen NK. Multiple sclerosis: diagnosis and differential diagnosis. Noro Psikiyatr Ars. 2018;55(suppl 1):S1-S9.
Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
Toledano M, Weinshenker BG, Solomon AJ. A clinical approach to the differential diagnosis of multiple sclerosis. Curr Neurol Neurosci Rep. 2015;15(8):57.
Kraft AK, Berger K. Quality of care for patients with multiple sclerosis—a review of existing quality indicators. Front Neurol. 2021;12:708723.
Burton JM, O'Connor PW, Hohol M, et al. Oral versus intravenous steroids for treatment of relapses in multiple sclerosis. Cochrane Database Syst Rev. 2012(12):CD006921.
Lattanzi S, Cagnetti C, Danni M, et al. Oral and intravenous steroids for multiple sclerosis relapse: a systematic review and meta-analysis. J Neurol. 2017;264(8):1697-1704.
Le Page E, Veillard D, Laplaud DA, et al.; COPOUSEP investigators; West Network for Excellence in Neuroscience. Oral versus intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): a randomized, controlled, double-blind, non-inferiority trial [published correction appears in Lancet . 2016; 387(10016): 340]. Lancet. 2015;386(9997):974-981.
Smets I, Van Deun L, Bohyn C, et al.; Belgian Study Group for Multiple Sclerosis. Corticosteroids in the management of acute multiple sclerosis exacerbations. Acta Neurol Belg. 2017;117(3):623-633.
Cortese I, Chaudhry V, So YT, et al. Evidence-based guideline update: plasmapheresis in neurologic disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;76(3):294-300.
Brochet B, Deloire M, Germain C, et al. Double-blind, randomized controlled trial of therapeutic plasma exchanges vs. sham exchanges in moderate-to-severe relapses of multiple sclerosis. J Clin Apher. 2020;35(4):281-289.
Tanasescu R, Constantinescu CS, Tench CR, et al. Smoking cessation and the reduction of disability progression in multiple sclerosis: a cohort study. Nicotine Tob Res. 2018;20(5):589-595.
Ramanujam R, Hedström AK, Manouchehrinia A, et al. Effect of smoking cessation on multiple sclerosis prognosis. JAMA Neurol. 2015;72(10):1117-1123.
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis [published correction appears in Mult Scler . 2020; 26(4): 517]. Mult Scler. 2018;24(2):96-120.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology [published correction appears in Neurology . 2019; 92(2): 112]. Neurology. 2018;90(17):777-788.
National Health Service England. Treatment algorithm for multiple sclerosis disease-modifying therapies. Updated March 8, 2019. Accessed November 23, 2021. https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2019/03/Treatment-Algorithm-for-Multiple-Sclerosis-Disease-Modifying-Therapies-08-03-2019-1.pdf
U.S. Food and Drug Administration. Drugs@FDA: FDA-approved drugs. Accessed November 23, 2021. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm
Li H, Hu F, Zhang Y, et al. Comparative efficacy and acceptability of disease-modifying therapies in patients with relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. J Neurol. 2020;267(12):3489-3498.
Vukusic S, Michel L, Leguy S, et al. Pregnancy with multiple sclerosis. Rev Neurol (Paris). 2021;177(3):180-194.
Burt RK, Balabanov R, Burman J, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs. continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA. 2019;321(2):165-174.
National Institute for Health and Care Excellence. Multiple sclerosis in adults: management. Updated November 11, 2019. Accessed November 30, 2021. https://www.nice.org.uk/guidance/cg186/chapter/Recommendations#ms-symptom-management-and-rehabilitation
Haselkorn JK, Hughes C, Rae-Grant A, et al. Summary of comprehensive systematic review: rehabilitation in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2015;85(21):1896-1903.
Selph SS, Skelly AC, Wasson N, et al. Physical activity and the health of wheelchair users: a systematic review in multiple sclerosis, cerebral palsy, and spinal cord injury. Arch Phys Med Rehabil. 2021;102(12):2464-2481.e33.
Frohman TC, Castro W, Shah A, et al. Symptomatic therapy in multiple sclerosis. Ther Adv Neurol Disord. 2011;4(2):83-98.
Samkoff LM, Goodman AD. Symptomatic management in multiple sclerosis. Neurol Clin. 2011;29(2):449-463.
Filli L, Zörner B, Kapitza S, et al. Monitoring long-term efficacy of fampridine in gait-impaired patients with multiple sclerosis. Neurology. 2017;88(9):832-841.
Koppel BS, Brust JC, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563.
Herring MP, Puetz TW, O'Connor PJ, et al. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(2):101-111.
Rietberg MB, Brooks D, Uitdehaag BM, et al. Exercise therapy for multiple sclerosis. Cochrane Database Syst Rev. 2005(1):CD003980.
Nicholas RS, Friede T, Hollis S, et al. Anticholinergics for urinary symptoms in multiple sclerosis. Cochrane Database Syst Rev. 2009(1):CD004193.
Rosti-Otajärvi EM, Hämäläinen PI. Neuropsychological rehabilitation for multiple sclerosis. Cochrane Database Syst Rev. 2014(2):CD009131.
Coggrave M, Norton C, Cody JD. Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane Database Syst Rev. 2014(1):CD002115.
He D, Zhang Y, Dong S, et al. Pharmacological treatment for memory disorder in multiple sclerosis. Cochrane Database Syst Rev. 2013(12):CD008876.
Xiao Y, Wang J, Luo H. Sildenafil citrate for erectile dysfunction in patients with multiple sclerosis. Cochrane Database Syst Rev. 2012(4):CD009427.
Khan F, Turner-Stokes L, Ng L, et al. Multidisciplinary rehabilitation for adults with multiple sclerosis. Cochrane Database Syst Rev. 2007(2):CD006036.
Khan F, Ng L, Turner-Stokes L. Effectiveness of vocational rehabilitation intervention on the return to work and employment of persons with multiple sclerosis. Cochrane Database Syst Rev. 2009(1):CD007256.
Koch MW, Glazenborg A, Uyttenboogaart M, et al. Pharmacologic treatment of depression in multiple sclerosis. Cochrane Database Syst Rev. 2011(2):CD007295.
Mills RJ, Yap L, Young CA. Treatment for ataxia in multiple sclerosis. Cochrane Database Syst Rev. 2007(1):CD005029.
Shakespeare DT, Boggild M, Young C. Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst Rev. 2001(4):CD001332.
Thomas PW, Thomas S, Hillier C, et al. Psychological interventions for multiple sclerosis. Cochrane Database Syst Rev. 2006(1):CD004431.
Silveira SL, Huynh T, Kidwell A, et al. Behavior change techniques in physical activity interventions for multiple sclerosis. Arch Phys Med Rehabil. 2021;102(9):1788-1800.
Molhemi F, Monjezi S, Mehravar M, et al. Effects of virtual reality vs. conventional balance training on balance and falls in people with multiple sclerosis: a randomized controlled trial. Arch Phys Med Rehabil. 2021;102(2):290-299.
Kim Y, Mehta T, Lai B, et al. Immediate and sustained effects of interventions for changing physical activity in people with multiple sclerosis: meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2020;101(8):1414-1436.
Lincoln NB, Bradshaw LE, Constantinescu CS, et al. Group cognitive rehabilitation to reduce the psychological impact of multiple sclerosis on quality of life: the CRAMMS RCT. Health Technol Assess. 2020;24(4):1-182.
Khan F, Amatya B. Rehabilitation in multiple sclerosis: a systematic review of systematic reviews. Arch Phys Med Rehabil. 2017;98(2):353-367.
Andreu-Caravaca L, Ramos-Campo DJ, Chung LH, et al. Dosage and effectiveness of aerobic training on cardiorespiratory fitness, functional capacity, balance, and fatigue in people with multiple sclerosis: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2021;102(9):1826-1839.
Tramontano M, Russo V, Spitoni GF, et al. Efficacy of vestibular rehabilitation in patients with neurologic disorders: a systematic review. Arch Phys Med Rehabil. 2021;102(7):1379-1389.
Abou L, Alluri A, Fliflet A, et al. Effectiveness of physical therapy interventions in reducing fear of falling among individuals with neurologic diseases: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2021;102(1):132-154.
Minden SL, Feinstein A, Kalb RC, et al.; Guideline Development Subcommittee of the American Academy of Neurology. Evidence-based guideline: assessment and management of psychiatric disorders in individuals with MS. Neurology. 2014;82(2):174-181.
Latimer-Cheung AE, Pilutti LA, Hicks AL, et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil. 2013;94(9):1800-1828.e3.
Amatya B, Khan F, La Mantia L, et al. Non pharmacological interventions for spasticity in multiple sclerosis. Cochrane Database Syst Rev. 2013(2):CD009974.
Phé V, Chartier-Kastler E, Panicker JN. Management of neurogenic bladder in patients with multiple sclerosis. Nat Rev Urol. 2016;13(5):275-288.
Van Der Walt A, Sung S, Spelman T, et al. A double-blind, randomized, controlled study of botulinum toxin type A in MS-related tremor. Neurology. 2012;79(1):92-99.
Oliveria SF, Rodriguez RL, Bowers D, et al. Safety and efficacy of dual-lead thalamic deep brain stimulation for patients with treatment-refractory multiple sclerosis tremor: a single-centre, randomised, single-blind, pilot trial. Lancet Neurol. 2017;16(9):691-700.
Motl RW, Sandroff BM, Kwakkel G, et al. Exercise in patients with multiple sclerosis. Lancet Neurol. 2017;16(10):848-856.
Hempel S, Graham GD, Fu N, et al. A systematic review of the effects of modifiable risk factor interventions on the progression of multiple sclerosis. Mult Scler. 2017;23(4):513-524.
Ploughman M. A new era of multiple sclerosis rehabilitation: lessons from stroke. Lancet Neurol. 2017;16(10):768-769.
Boesen F, Nørgaard M, Trénel P, et al. Longer term effectiveness of inpatient multidisciplinary rehabilitation on health-related quality of life in MS patients: a pragmatic randomized controlled trial – The Danish MS Hospitals Rehabilitation Study. Mult Scler. 2018;24(3):340-349.
Abo Youssef N, Schneider MP, Mordasini L, et al. Cannabinoids for treating neurogenic lower urinary tract dysfunction in patients with multiple sclerosis: a systematic review and meta-analysis. BJU Int. 2017;119(4):515-521.
Thompson AJ, Toosy AT, Ciccarelli O. Pharmacological management of symptoms in multiple sclerosis: current approaches and future directions. Lancet Neurol. 2010;9(12):1182-1199.
Goverover Y, Chiaravalloti ND, O'Brien AR, et al. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: an updated review of the literature from 2007 to 2016. Arch Phys Med Rehabil. 2018;99(2):390-407.
Castro-Sánchez AM, Matarán-Peñarrocha GA, Lara-Palomo I, et al. Hydrotherapy for the treatment of pain in people with multiple sclerosis: a randomized controlled trial. Evid Based Complement Alternat Med. 2012;2012:473963.
Yadav V, Bever C, Bowen J, et al. Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2014;82(12):1083-1092.
Zhang E, Tian X, Li R, et al. Dalfampridine in the treatment of multiple sclerosis: a meta-analysis of randomised controlled trials. Orphanet J Rare Dis. 2021;16(1):87.
Iaffaldano P, Lucisano G, Patti F, et al.; Italian MS Register. Transition to secondary progression in relapsing-onset multiple sclerosis: definitions and risk factors. Mult Scler. 2021;27(3):430-438.
Calabresi PA. Diagnosis and management of multiple sclerosis. Am Fam Physician. 2004;70(10):1935-1944.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2022 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.

- myCME Login
- Monthly CME eNewsletter
Live Events
Text-based cme, journal cme, self-study cme, multiple sclerosis, carrie m. hersh, do, msc, robert j. fox, md.
Published: April 2018 Expire: April 2021
Introduction
Definition and disease course, pathophysiology, signs and symptoms, treatment strategies, considerations in special populations.
Multiple Sclerosis (MS) is a chronic inflammatory, demyelinating, and neurodegenerative disorder of the central nervous system (CNS) that affects the white and grey matter of the brain, spinal cord, and optic nerve. MS is one of the most common causes of non-traumatic disability among young and middle-aged adults. Direct MS-related healthcare costs are estimated to be more than $10 billion annually in the United States. 1 As symptoms of MS are extremely variable and often quite subtle, diagnosis and management have been greatly enhanced by the use of magnetic resonance imaging (MRI). Therapies that target inflammation and slow progression of disease are available; therefore, early diagnosis and treatment are important in limiting the impact of this potentially devastating disease. Complementary approaches such as symptom management and healthy lifestyle practices also have an important role in MS care.
There are several different forms of MS. Since these classifications were based upon clinical characteristics, they are empiric and do not reflect specific biologic pathophysiology. Nonetheless, they provide an organized framework for diagnosis and long-term management. Approximately 85% of patients present with a relapsing-remitting MS (RRMS) disease course at onset, where symptoms appear for several days to weeks, after which they usually resolve spontaneously. 2 After tissue damage accumulates over many years and reaches a critical threshold, about two-thirds of the patients transition to secondary progressive MS (SPMS), where pre-existing neurologic deficits gradually worsen over time. Relapses can be seen during the early stages of SPMS, but are uncommon as the disease further progresses. About 10% to 15% of patients have gradually worsening manifestations from the onset without clinical relapses, known as primary progressive MS (PPMS). 3 Patients with PPMS tend to be older, have fewer abnormalities on brain MRI, and generally respond less effectively to standard MS therapies. 4
Back to Top
MS affects approximately 1 million individuals in the US and 2.5 million worldwide. 5,6 Initial symptoms typically occur between 20 and 50 years of age, and women have about 3 times increased likelihood of developing MS compared with men. 1 Although MS is more frequently seen in white than African American and Hispanic populations, the latter groups overall have poorer disease outcomes in that they accumulate disability more quickly, suggesting more destructive tissue injury in these groups. The prevalence of MS varies by location and generally increases the further one travels from the equator in either hemisphere. It remains unclear whether this altered incidence represents an environmental influence (eg, vitamin D deficiency), genetic difference, variable surveillance, or other, as yet unidentified, differences.
Early in the disease course, MS involves recurrent bouts of CNS inflammation that results in damage to both the myelin sheath surrounding axons as well as the axons themselves. Histologic examination reveals foci of severe demyelination, decreased axonal and oligodendrocyte numbers, and glial scarring. The exact cause of inflammation remains unclear, but an autoimmune response directed against CNS antigens is suspected.
In progressive MS, inflammation is a less defining pathological hallmark. Instead, progressive MS is characterized by neurodegeneration of the white and grey matter resulting in brain and spinal cord atrophy on a background of mild-moderate inflammation. 7 Predominant factors driving neurodegeneration include mitochondrial dysfunction due to defective oxidative phosphorylation and nitric oxide production, resulting in a chronic state of virtual hypoxia due to unmet energy demands, 8 and age-dependent iron accumulation in myelin and oligodendrocytes leading to oxidative tissue damage. 9 Further research is needed to understand how these different pathologic subtypes affect prognosis and response to treatments. Currently, brain biopsy is the only method to definitively determine pathologic subtypes, but studies are underway to find blood, cerebrospinal fluid, and MRI biomarkers.
Historically, MS was classified as an inflammatory disease targeting white matter, with diagnostics and therapeutics focused on this mechanism of pathology. However, more recent imaging and histopathological studies suggest that cortical demyelination plays a crucial role in MS pathogenesis and cognitive dysfunction. Cortical demyelination is now recognized in early MS. 10 Although some investigative MRI modalities capture some cortical involvement, including double inversion recovery sequences at 3 tesla and ultra-high field MRI, conventional MRI metrics used in clinical practice do not show these changes well. Likewise, extensive cortical demyelination that is seen in histopathological studies is not clearly demonstrated on any current MRI modality. This pathology/imaging discordance demonstrates that we are still technologically disadvantaged in accurately assessing cortical lesion pathology in the live patient.
In the past, inflammation was thought to involve only demyelination, but pathologic studies have found significant axonal pathology as well. In actively demyelinating MS lesions, an average of more than 11,000 transected axons/mm 3 were observed, while control brain tissue had less than one transected axon/mm. 11 Significant axonal injury is also observed in cortical demyelinating lesions. Clearly, axonal injury is significant in the early stages of disease.

Later in the disease course, gradual progression of disability is observed. However, there is significantly less active inflammation during this period, so clinical progression may arise instead from degenerative changes. Nonetheless, oligodendrocyte progenitor cells capable of remyelinating axons have been observed even in white matter plaques from patients with chronic MS ( Figure 1 ). 12 This observation suggests that the potential for remyelination persists even very late in the disease course, which is an encouraging indicator for possible therapeutic targets at this late stage of disease.

Current concepts of the pathophysiology of MS are illustrated in Figure 2 . 13 In the preclinical phase, patients may develop lesions characteristic of MS visible on MRI before they phenotypically manifest symptoms, known as radiologically isolated syndrome. In a different scenario, patients may develop MS symptoms compatible with inflammatory demyelination without other characteristic lesions on MRI. This phenomenon is called clinically isolated syndrome.
On average, patients with RRMS experience clinical relapses every 1 to 2 years. Serial MRI studies show that lesions develop up to 10 to 20 times more frequently than clinical relapses Thus, although RRMS appears to have clinically active and quiescent periods, inflammatory lesions are developing and evolving almost continuously. A current hypothesis states that overt progression of disability, which marks the transition from RRMS to SPMS, occurs when ongoing irreversible tissue injury exceeds a critical threshold beyond which the nervous system can no longer compensate. It is thought that at this point the disease has primarily transitioned to a neurodegenerative condition with neurologic deterioration independent of ongoing inflammation, although superimposed inflammation can continue to cause additional injury. An important implication of this hypothesis is that the accumulation of irreversible tissue damage limits the potential for anti-inflammatory disease modifying therapies (DMTs) when used in the progressive stage of the disease. To be maximally effective, DMTs should be started early in patients with RRMS before permanent disability develops. Overall, an incomplete understanding of progressive MS pathogenesis has slowed the development of effective therapies and requires further inquiry.
MS can cause a wide variety of neurologic symptoms since it can affect numerous areas of the brain, optic nerve, and spinal cord ( Figure 3 ). Characteristic lesions are located in the periventricular and juxtacortical regions, in addition to the brainstem, cerebellum, spinal cord, and optic nerve. Disease localized to the spinal cord may cause partial or complete transverse myelitis, involving sensory or motor changes involving 1 or both sides of the body. Lhermitte’s phenomenon is a nonspecific symptom whereby flexion of the neck causes an electrical-like shooting sensation that extends into the arms or down the back. It is thought to arise from partially demyelinated tissue, whereby mechanical stimulation leads to axonal activation. Other common symptoms of MS often stemming from spinal cord lesions include bladder and bowel dysfunction. Posterior fossa (eg, brainstem and cerebellum) involvement may present as diplopia, dysphagia, altered sensation or weakness of the face, or ataxia. Inflammation of the optic nerve (optic neuritis) usually presents as blurry vision with painful eye movements, and is often an early clinical manifestation of RRMS.

Of all the lesions in MS, cerebral lesions are the most common but cause the fewest symptoms early in MS. Most cerebral lesions are not located in eloquent regions and so are thus clinically silent and identified only by brain MRI. Very large cerebral lesions may present with weakness or numbness and rarely may cause aphasia or other cortical dysfunction. Cerebral and cortical lesions may also cause subtle symptoms, such as cognitive impairment, fatigue, and affective disorders like depression. Although these symptoms are not uncommon in patients with MS, they are also nonspecific and can be seen in a multitude of disorders.
Symptoms of a clinical relapse typically arise over hours to days, worsen over several weeks, and then gradually subside over several weeks or months. Residual enduring neurologic symptoms are common. The gradual progression of progressive MS can manifest as worsening myelopathy causing asymmetric limb weakness, ataxia, spasticity, and bladder/bowel and sexual dysfunction; impaired mobility; impaired motor dexterity; and cognitive impairment.
There are no pathognomonic clinical, laboratory, or imaging findings in MS. The diagnosis ultimately is a clinical decision based on weighing the factors that support the diagnosis against those that fail to support it or point to the possibility of an alternative diagnosis.
The Schumacher criteria from 1965 capture the essence of the diagnosis of MS: CNS lesions disseminated in space and time and the elimination of alternative diagnoses. 14 These core diagnostic characteristics remain relevant today.
The International Panel on MS Diagnosis criteria, also called the McDonald criteria, are diagnostic criteria for MS that incorporate the clinical characteristics and MRI features. 15 Revisions were made in 2005, 2010, and most recently in 2017 as a reflection of an increased understanding of the natural history of MS and improved MRI techniques.
The latest version of the McDonald criteria (2017) simplifies the diagnostic process and allows earlier diagnosis ( Table 1 ). 16 A diagnosis of clinically definite multiple sclerosis requires fulfillment of dissemination in space and time. Dissemination in space is defined as 1 or more T2-hyperintense lesions in more than 1 characteristic location of MS, which includes the periventricular, juxtacortical, and infrantentorial regions (eg, brainstem and cerebellum), and spinal cord. Cerebrospinal-fluid restricted oligoclonal bands can be used as paraclinical support for an early diagnosis of MS ( see Table 1 ). The 2016 magnetic resonance imaging in multiple sclerosis (MAGNIMS) criteria now include the optic nerve as 1 of the characteristic locations fulfilling an MS diagnosis, 17 though it still remains separate from the McDonald criteria. Dissemination in time is defined as 1) the simultaneous presence of a gadolinium-enhancing (GdE) and non-enhancing lesion at any time on initial MRI or 2) a new T2-hyperintense or GdE lesion on follow-up MRI with reference to a baseline scan, irrespective of the timing of the baseline MRI.
Table 1: Summary of the 2017 McDonald Criteria
| Clinical presentation | Additional findings needed for MS diagnosis |
|---|---|
| ≥ 2 clinical attacks and objective clinical evidence of ≥ 2 lesions | None; however, magnetic resonance imaging (MRI) is typically obtained to both exclude other diagnoses and stage the severity of disease. |
| ≥ 2 clinical attacks and objective clinical evidence of 1 lesion | Dissemination in space (DIS) : an additional clinical attack implicating a different central nervous system site OR by MRI |
| 1 clinical attack and objective clinical evidence of ≥ 2 lesions | Dissemination in time (DIT) : an additional clinical attack OR by MRI cerebrospinal fluid-specific oligoclonal bands |
| 1 clinical attack and objective clinical evidence of 1 lesion | DIS: an additional clinical attack implicating a different CNS cite by MRI DIT: an additional clinical attack by MRI cerebrospinal fluid-specific oligoclonal bands |
a DIS and DIT can be made by clinical features alone or by a combination of clinical and MRI features. b DIS principle requires that there are asymptomatic lesions typical of MS present in 2 or more sites within the central nervous system: periventricular, subcortical, infrantentorial, and spinal cord. c DIT principle requires that 2 attacks separated by more than 30 days have occurred in different parts of the central nervous system. MRI criteria for DIT stipulate either an asymptomatic enhancing T2 lesion along with a non-enhancing T2 lesion on any one scan, or a new T2 or gadolinium-enhancing lesion on a follow-up scan. d In a patient with a typical clinically isolated syndrome and fulfillment of clinical or MRI criteria for DIS with no better explanation for the clinical presentation, demonstration of cerebrospinal fluid-specific oligoclonal bands allows an MS diagnosis to be made (change from the 2010 McDonald Criteria).
Adapted from AJ Thompson, et al. 16
In all cases, the practitioner must rule out better explanations for the clinical presentation other than multiple sclerosis. In the context of the MacDonald criteria, a single episode of demyelination and certain findings on a single MRI can fulfill the diagnostic criteria for MS, even before a second clinical episode or new MRI lesion. The revisions also preserve diagnostic sensitivity and specificity and address their applicability across different populations, allowing for more uniform and widespread use across groups.

In 2013, an international panel of MS experts proposed changes to the classification of MS to more effectively characterize the disease course. 18 One of the changes included the categorization of disease as either manifesting active inflammation (‘active’) or no active inflammation (‘non-active’) based on new clinical relapses or new T2 or GdE MRI lesions or in combination within the past year. Another change was the categorization of disease based on the presence or absence of continued gradual clinical decline (with progression or without progression) ( Figure 4 ). 18 These disease classifications were intended to provide a clearer conceptualization of progressive MS and its differentiation from active inflammation.
Although the diagnosis of MS cannot be based on MRI alone, typical MRI lesions in the periventricular and juxtacortical regions, as well as the brainstem, cerebellum, and spinal cord can raise the suspicion of MS, warranting further diagnostic workup or monitoring. MRI is typically obtained at the time of diagnosis to both exclude other diagnoses and stage the severity of disease. Patients with a typical history of MS without typical MRI findings are highly unusual and should prompt consideration of an alternative diagnosis.
Management of MS requires multiple therapeutic approaches. The current goals of MS management involve the treatment of acute relapses, prevention of new disease activity and disability progression, management of symptoms that affect quality of life, and adherence to a healthy lifestyle.
Acute Relapse
Several studies have found that treatment with corticosteroids can shorten the length of relapses and may even improve long-term outcomes. 19,20 A typical regimen is 500 mg to 1,000 mg of intravenous methylprednisolone with or without a tapering dose of oral prednisone over several weeks. The standard protocol at the Cleveland Clinic is intravenous methylprednisolone 1,000 mg daily for 3 to 5 days, followed by a 12-day prednisone taper (60 mg daily, decreasing by 20 mg every 4 days). Evaluation of a relapse should include a search for precipitating factors such as fever, upper respiratory illness, or bladder infection. For patients who do not respond sufficiently to corticosteroids or who do not tolerate corticosteroids, adrenocorticotropic hormone or plasma exchange can be considered.
Disease-Modifying Therapies for Relapsing MS: Treatment Targets and Therapeutic Strategies
After the acute relapse is treated, consideration should be given to use of DMTs, which primarily target the inflammatory, demyelinating aspects of the disease. A list of DMTs for MS approved by the U.S. Food & Drug Administration (FDA) is presented in Table 2 .
Table 2: Disease-Modifying Therapies (Brand Name) for MS
| Injectable platform therapies | Interferon beta-1a (Avonex, Rebif, Plegridy) Interferon beta-1b (Betaseron, Extavia) Glatiramer acetate (Copaxone, Glatopa) |
|---|
| Injectable therapy/monoclonal antibody therapy | Daclizumab (Zinbryta) |
| Oral therapies | Fingolimod (Gilenya) Teriflunomide (Aubagio) Dimethyl fumarate (Tecfidera) |
| Infusion therapies/monoclonal antibody therapy | Natalizumab (Tysabri) Alemtuzumab (Lemtrada) Ocrelizumab (Ocrevus) |
| Infusion therapy/chemotherapeutic | Mitoxantrone (Novantrone) |
ª Voluntarily withdrawn from the market in March 2018 due to concern about the benefit/risk profile, see https://www.fda.gov/Drugs/DrugSafety/ucm600999.htm
Current therapies target the immune dysfunction in MS and resultant neural tissue damage with the goal of preventing or at least reducing the long-term risk of clinically significant disability. Early treatment is key since it offers the greatest chance of preventing or delaying tissue injury and long-term disability. Although the underlying pathogenesis of MS still remains poorly understood, remarkable progress has been made in the development of drug therapies that inhibit disease activity. Using available options, including the advent of newer more effective drugs, there is the potential to achieve a disease-free status, characterized by the prevention of clinical relapses and disability progression and absence of new lesions on MRI. This widely accepted treat-to-target approach is known as “No Evidence of Disease Activity” (NEDA). Patients who achieve NEDA may have better long-term outcomes, although maintaining NEDA over the course of years or decades is challenging.
Escalation therapy and high-efficacy early therapy (HET) are 2 general management strategies to achieve NEDA in RRMS. In escalation therapy, the patient is initially started on a lower efficacy agent, such as one of the standard injectable DMTs. The rationale for using lower efficacy treatment is that such agents typically have a more desirable safety profile for better long-term safety overall. In the presence of disease activity, the patient is subsequently switched to higher efficacy treatment, often in a step-wise approach (eg, injectable → oral → infusion). HET is an alternative approach in which patients are started on high-efficacy therapy (eg, natalizumab and ocrelizumab) early in the course of their disease, and even as first-line agents. This approach may carry more risk in certain circumstances, especially given that patients will have more long-term exposure to drugs with significant immune-altering effects. However, the rationale behind this treatment strategy is that the benefits of early disease control outweigh the risks and may have longer-term benefit compared with escalation therapy. There are middle-ground approaches, too, that utilize oral therapies such as fingolimod and dimethyl fumarate as first-line treatments. Multicenter, prospective, pragmatic clinical trials are needed to address these important clinical questions.
It is important to note that all current MS therapies are preventative and not restorative. As the disease progresses, response to DMT typically declines. The key to successful treatment of MS is to slow the inflammatory process early in the disease. The therapeutic nihilism of the past should be replaced by aggressive treatment and monitoring, while carefully balancing the potential risks and benefits. Monitoring patients clinically and with surveillance MRI scans during treatment is important to detect non-responders and modify therapy accordingly.
It is likely that the accumulation of irreversible tissue damage limits the potential for benefit from DMT as the disease progresses. However, with better understanding of MS pathogenesis and identification of appropriate outcomes measures for progressive MS in clinical trials, the therapeutic landscape of DMT strategies for this disabling and neurodegenerative disease state is rapidly evolving and holds great promise for the future.
Injectable Platform Therapies
Four first-line injectable therapies, otherwise known as platform agents, are currently available in the US: intramuscular interferon (IFN) beta-1a (Avonex), subcutaneous IFN beta-1a (Rebif; Plegridy), subcutaneous IFN beta-1b (Betaseron, Extavia), and glatiramer acetate (Copaxone, Glatopa) ( Table 3 ). The IFN medications are recombinant products with an amino acid sequence that is identical or nearly identical to that of human IFN beta-1. Glatiramer acetate is a random polypeptide based on the amino acid sequence of a myelin protein. All of these medications appear to modulate the immune response in MS, although glatiramer acetate and IFN beta medications probably work through different mechanisms.
Table 3: Injectable Platform Therapies
| Drug (brand name) | Dose | Adverse Effects | Lab monitoring/risk mitigation |
|---|---|---|---|
| Interferon β-1a (Avonex) | 30 mcg intramuscular once weekly | Injection site reactions Flu-like symptoms Lymphopenia Hepatotoxicity Exacerbation of preexisting thyroid disease | Baseline complete blood count (CBC) and liver function tests (LFTs) Periodic CBC and LFTs every 3 to 6 months Baseline thyroid function assessment, then periodically with clinical symptoms |
| (Rebif) | 44 mcg subcutaneous 3 times weekly | ||
| (Plegridy) | 125 mcg subcutaneous every 2 weeks | ||
| Interferon β-1b (Betaseron)(Extavia) | 0.25 mg subcutaneous every other day | ||
| Glatiramer acetate Copaxone, Glatopa | 20 mg subcutaneous once daily | Injection site reactions Post injection systemic reactions Lipoatrophy | None |
Based on data from Oh J, et al. 21
In randomized, placebo-controlled trials, all of these medications decreased the rate of clinical relapses by about 30%, decreased the severity of the relapses, and had beneficial effects on measures of disease activity on MRI. 22-25 All of the platform medications are reasonably well tolerated, and 15 to 20 years of accumulated data and clinical experience suggest strong long-term safety. The platform therapies are similar in efficacy, and selection is generally based on physician and patient preferences and side-effect profile. Potential adverse effects of the IFN medications include hepatic and hematological toxicities, flu-like side effects, and worsening of headaches, depression, and spasticity. Glatiramer acetate may have the potential for more bothersome injection site reactions, particularly in thin patients. All 4 injectable platform therapies are appropriate first-line therapies in RRMS.
Daclizumab (Zinbryta, 150 mg once monthly subcutaneous injection) was voluntarily withdrawn from the market in March 2018 due to concern about the drug’s benefit/risk profile( https://www.fda.gov/Drugs/DrugSafety/ucm600999.htm ). Approved by the FDA for relapsing forms of MS in May 2016, daclizumab is a humanized monoclonal antibody that binds to and blocks the high-affinity interleukin-2 receptor alpha chain, which inhibits T-cell activation and proliferation. Its clinical benefit is also thought to result from the expansion of a subset of regulatory natural killer cells. 26
- Liver function tests: baseline before initiations, monthly prior to next dose, and monthly for 6 months after discontinuation
- Purified protein derivative skin or blood tuberculosis test: before initiation
- Hepatitis serology: before initiation
- Pregnancy test: before initiation
- Cutaneous reactions: after initiation.
Randomized controlled trials (RCTs) of daclizumab found that it reduces the annualized relapse rate (ARR) by 54% compared with placebo 27 and by 45% compared with active comparator intramuscular IFN beta-1a. 28 The number of new or newly enlarging T2-hyperintense lesions was 54% lower with daclizumab than with intramuscular IFN beta-1a over a period of 96 weeks. 28
Overall, the incidence of adverse effects was comparable between daclizumab and comparator treatments in phase 3 clinical trials. 27,28 However, daclizumab is associated with a higher proportion of serious infections. Of these, urinary tract infections, upper respiratory tract infections, pharyngitis, and sinusitis are the most common adverse effects. Most resolve with standard treatments without subsequent complications. There was 1 patient treated with daclizumab who died from complications of a local psoas abscess. Other side effects include cutaneous reactions, 1 case of autoimmune hepatitis, 29 and hepatotoxicity including 1 case of fatal fulminant hepatic failure. 30
Oral Therapies
There are currently 3 oral DMTs approved by the FDA. These therapies include fingolimod (Gilenya), teriflunomide (Aubagio), and dimethyl fumarate (Tecfidera) ( Table 4 ).
Table 4: Oral Therapies
| Drug (brand name) | Dose | Adverse effects | Lab monitoring/risk mitigation |
|---|---|---|---|
| 0.5 mg by mouth daily | |||
| 7 mg or14 mg by mouth daily | |||
| 240 mg by mouth twice daily |
CBC = complete blood count; ECG = electrocardiogram; LFTs = liver function tests.
Fingolimod was approved in September 2010 as the first oral disease therapy for MS. Fingolimod acts by binding to the sphingosine-1-phosphate receptor on lymphocytes, which prevents egress of lymphocytes from lymph nodes. The sequestration of autoreactive lymphocytes prevents their recirculation to the CNS, thus inhibiting one of the primary steps in MS pathogenesis. Fingolimod crosses into the CNS and may have direct effects within the CNS, as well.
Most fingolimod-associated side effects are mild to moderate in severity and include upper respiratory tract infections, headache, diarrhea, and back pain. The most concerning adverse effects include cardiac events (bradycardia and atrioventricular block at treatment initiation), elevated liver enzymes, rare serious infections (eg, herpes virus infections), and macular edema. The development of these serious side effects during clinical trials led to strict FDA recommendations for close monitoring during first dose administration and risk factor mitigation strategies to reduce potential serious complications. These parameters include baseline complete blood count (CBC), liver function tests (LFTs), electrocardiogram, ophthalmological evaluation, and serum varicella virus immunoglobulin G titer prior to fingolimod initiation. First-dose administration is conducted under the supervision of a healthcare provider (either at home or in a medical center) where patients are monitored for 6 hours with hourly vital sign checks and a repeat electrocardiogram after 6 hours. Extended monitoring for a total of 24 hours is needed if bradycardia or QT prolongation is observed, and with other cardiac risk factors. Periodic labs including CBC and LFTs and ophthalmological reassessment are used for continued safety surveillance.
Cases of progressive multifocal leukoencephalopathy (PML) have been reported in association with fingolimod. PML is a serious viral infection of the brain, arising from the ubiquitous John Cunningham virus (JCV), which resides in the kidneys and bone marrow in about half of adults. The estimated rate of PML with fingolimod is about 1:10,000 overall, with a higher rate in those treated for more than 2 years. 33
Teriflunomide
Teriflunomide was the second oral DMT approved by the FDA in September 2012. It is an active metabolite of leflunomide and acts by inhibiting the de novo synthesis of pyrimidine nucleotides through the inhibition of dihydroorotate dehydrogenase. 34 It also inhibits T-lymphocyte activation and cytokine production in addition to cytostatic effects on proliferating B- and T-lymphocytes. 35 Phase 3 trials of teriflunomide showed that it reduces the ARR by 35 compared with placebo. 36,37 However, a phase 3 trial comparing teriflunomide and subcutaneous IFN beta-1a showed them to have relatively similar efficacy. 38
Teriflunomide is relatively safe and generally well-tolerated. There is no increased risk of opportunistic infections, and most of the adverse effects related to the medication are transitory. The most common adverse effects observed in RCTs and in clinical practice are mild to moderate in severity and include nasopharyngitis, gastrointestinal symptoms, decrease in hair density, mildly elevated LFTs, rash, and fatigue. Patients should be screened for tuberculosis before initiating therapy. Risk mitigation strategies include LFTs at baseline and every 6 months while on the medication and a baseline and 6-month CBC. 39
Although teriflunomide was not carcinogenic in mice and rats, it was found to be mutagenic and resulted in embryo lethality in rats. Thus, it has a pregnancy category X designation. In this context, pregnancy must be excluded in all women of childbearing potential prior to treatment, and effective contraceptive methods must be employed for both women and men. An accelerated removal process is available for patients who become pregnant or desire to become pregnant (or father a child) while taking teriflunomide.
Dimethyl Fumarate
The most frequent adverse effects associated with DMF are gastrointestinal symptoms, including stomach pain, nausea, vomiting, and diarrhea. Gastrointestinal symptoms are generally more prominent during the first several weeks of treatment and usually improve significantly thereafter. Taking DMF with food and slower initial dose titration may offset potential gastrointestinal side effects. Transient skin flushing is also observed intermittently. Concomitant use of low-dose aspirin substantially reduces associated skin flushing.
Lymphopenia is a possible side effect without associated increased risk of serious infections. Cases of PML have been reported in association with dimethyl fumarate. The estimated rate of PML with dimethyl fumarate is less than 1:15,000 overall, with a higher rate in those with sustained lymphopenia (ie, 6 months). In this context, baseline and periodic CBC monitoring every 6 months is recommended surveillance measures while on DMF.
Infusion Therapies
There are currently 3 infusion DMTs approved by the FDA to reduce disease activity in relapsing forms of MS and are considered highly effective therapies. These DMTs include natalizumab (Tysabri), alemtuzumab (Lemtrada,), and ocrelizumab (Ocrevus) ( Table 5 ). Ocrelizumab is also the first DMT for treating patient with PPMS approved by the FDA.
Table 5: Common Symptoms in MS and Potential Treatments
| Natalizumab (Tysabri) | 300 mg intravenous every 4 week | . | |
| 12 mg intravenous daily for 5 consecutive 12 mg intravenous daily for 3 consecutive days at month 12 from initial course | | ||
| 300 mg intravenous at week 0 and week 2, then 600 mg intravenous every 24 weeks | |
Ab = antibody; bid = twice daily; CBC = complete blood count; CMP = complete metabolic profile; CSP = cerebrospinal fluid; HIV = human immunodeficiency virus; JCV = John Cunningham Virus; LFTs = liver function tests; MRI = magnetic resonance imaging; PCR = polymerase chain reaction; PO = oral; SCr = serum creatinine; TB = tuberculosis; TSH = thyroid stimulating hormone; VZV = varicella zoster.
Based on data from Oh J, et al. 21 and Ontaneda D, et al. 44
Natalizumab
Natalizumab, approved by the FDA in November 2004, is a monoclonal antibody targeting the cellular adhesion molecule very late antigen-4. By blocking very late antigen-4, fewer inflammatory cells enter the brain and thereby blunt CNS inflammation typical of MS. Clinical trials of natalizumab showed that it reduces clinical relapses by 67% and new brain lesions by 83% in pivotal RCTs. 45,46 Thus natalizumab is considered one of the most clinically effective DMTs for relapsing MS to date.
Natalizumab is relatively well-tolerated with mild headache, fatigue, anxiety, menstrual irregularities, peripheral edema, and routine infections (eg, upper respiratory infection and pharyngitis) occasionally observed. Infusion-related hypersensitivity reactions (eg, hives and pruritus) occur in 2% to 4% of patients and are thought to represent immune-mediated hypersensitivity reactions. 47 Anaphylactic reactions are very rarely observed, but when observed, are typically during the second infusion. Patients who demonstrate a serious infusion reaction should discontinue natalizumab immediately and not be retreated.
The most concerning serious adverse effect of natalizumab is PML, which occurs at an overall incidence of 2.1 per 1,000 population. 48-50 Three identified risk factors that substantially alter an individual’s risk of PML include duration of natalizumab treatment, prior history of immunosuppressive therapy, and serum anti-JCV antibody status. Patients with natalizumab treatment exceeding 60 months, prior use of immunosuppressant drugs, and positive serum anti-JCV antibody testing, carry the highest estimated risk for PML at 1:119 persons. Patients who are negative for JCV antibody have a low risk of PML, estimated at 1:14,285 persons. 51
Because of PML, natalizumab was withdrawn from clinical use in February 2005 but received a second FDA approval in June 2006. Due to the serious potential for PML, natalizumab is generally reserved for patients with worrisome baseline disease activity or negative prognosticators or both, or patients who respond sub-optimally or do not tolerate other MS therapies. However, the growing experience of PML risk stratification suggests that in subjects with persistently negative JCV serology, natalizumab can be considered as first-line therapy. Like with all medications, discussions with potential natalizumab recipients should review the risks and potential benefits of this medication.
In the context of natalizumab’s risk of PML, careful risk stratification prior to treatment initiation is recommended, which enables more informed clinical decision making. It appears that natalizumab-related PML has a better prognosis than PML in other settings, although fatalities or persistent deficits are common. Accelerated removal of natalizumab from the blood (ie, through plasmapheresis or leukopheresis) likely accelerates immune reconstitution and is recommended in patients with natalizumab-related PML.
Alemtuzumab
Alemtuzumab, approved by the FDA in November 2014 for relapsing MS, is a humanized antibody that targets CD52, a cell surface protein expressed on T-lymphocytes, B-lymphocytes, natural killer cells, monocytes, and dendritic cells. 52 Alemtuzumab induces rapid depletion of circulating T- and B-lymphocytes followed by repopulation that leads to a distinctive lymphocyte profile, including an increased proportion of regulatory T-lymphocytes and memory B- and T-lymphocytes. In contrast to the slow recovery of T-lymphocytes, B-lymphocytes return to baseline levels by 3 months, which may explain the occasional development of secondary humoral autoimmune disorders. 53
RCTs of alemtuzumab showed that it reduces the number of clinical relapses versus active comparator, subcutaneous IFN beta-1a, by about 49% to 55%, in both treatment-naïve 54,55 and treatment-experienced patients. 56 Two of these trials showed reduction in the risk of confirmed worsening of disability, and all 3 showed slowing in progression of cerebral atrophy.
The most common adverse effect is infusion-related reactions (IRRs), which occurs in over 90% of patients treated with alemtuzumab without pre-medications. 54,55 The most common symptoms are headache, rash, and pyrexia; which are mostly mild-moderate in severity. IRRs are mitigated by a pretreatment protocol with intravenous methylprednisolone and symptom management with antihistamine and antipyretics.
The principal adverse effect of alemtuzumab is secondary autoimmune disorders, which is theorized to be related to the distinct lymphocyte repertoire that develops following alemtuzumab exposure. Thyroid disease is the most common, occurring in up to 34% in patients followed over 7 years. 57 Other rare secondary autoimmune adverse effects include immune thrombocytopenic purpura and antiglomerular basement membrane disease that led to renal transplant in a patient outside of clinical trials. 58 In the extension phases of clinical trials through year 6, the peak of thyroid disease occurred during year 3, nephropathy during years 1 to 2, and immune thrombocytopenic purpura throughout the study period. 59,60
The subsequent period of rapid lymphocyte depletion immediately following alemtuzumab exposure is associated with a mild increase in infections. Most of these infections are mild to moderate nasopharyngitis, urinary tract infections, and upper respiratory tract infections, although serious listeria and herpes viral infections can occur. Due to the risk of listeria and herpes viral infections, it is recommended that patients be treated with twice daily trimethoprim/sulfamethoxazole for 2 months and daily oral acyclovir for 1 year following each treatment course of alemtuzumab. 61
Ocrelizumab
Ocrelizumab, approved by the FDA in March 2017, is a humanized anti-CD20 monoclonal antibody that binds to an epitope that overlaps with that of rituximab. It is hypothesized that ocrelizumab has comparable efficacy of B-cell depletion to rituximab. In contrast, ocrelizumab is purported to have fewer side effects than rituximab because it is a more humanized antibody and thus produces greater antibody-dependent cellular toxicity and less complement-dependent cytotoxicity. 62 Pivotal phase 3 trials (OPERA I and OPERA II) demonstrated a significant ARR reduction for ocrelizumab compared with subcutaneous IFN beta-1a by 46% to 47%. 63 In a pooled analysis, there was a 40% risk reduction in time to 12-week confirmed disability worsening and a 40% risk reduction in time to 24-week confirmed disability worsening between ocrelizumab and subcutaneous IFN beta-1a.
The most common adverse effects associated with ocrelizumab are IRRs. They typically are mild in degree and managed by symptomatic therapy or slowing the infusion rate. There are no increased rates of serious adverse effects or serious infections.
Malignancy occurred in 0.5% of patients treated with ocrelizumab compared with 0.2% of subcutaneous IFN beta-1a treated patients in clinical trials. 63 Two cases of breast cancer emerged in patients receiving ocrelizumab in 1 of the pivotal RCTs versus none in patients treated with subcutaneous IFNβ-1a. 63 An additional 2 cases of breast cancer occurred during the open-label extension study, during which all patients received ocrelizumab. Given these small numbers, it remains unclear whether ocrelizumab treatment increases the risk of cancer.
Ocrelizumab may increase the risk of PML, but to date there have been no cases of PML directly attributable to ocrelizumab.
Role of Immunosuppressant Drugs in MS
Cyclophosphamide, methotrexate, azathioprine, and cyclosporine have all been studied in small- to medium-sized trials. The Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and MS Council for Clinical Practice Guidelines have made recommendations regarding these therapies. 64 Methotrexate, azathioprine, and cyclosporine were each found to be possibly effective (type C recommendation) in altering the course of disease, but cyclosporine was found to have an unacceptable risk/benefit ratio. In their review, pulse cyclophosphamide treatment did not alter the course of MS (type B recommendation), although a more recent clinical trial showed reduced relapses and MRI lesions in patients treated with cyclophosphamide. 65 Given that there are more than a dozen therapies approved by the FDA for relapsing MS and relatively weak evidence supporting the efficacy of immunosuppressant therapies, they are infrequently used in the treatment of MS.
Mitoxantrone (Novantrone, 12 mg/m 2 every 3 months; maximum lifetime dose of 140 mg/m 2 ) is a chemotherapy medication with demonstrated efficacy in very active relapsing and progressive MS. 66 Administration is via intravenous infusion every 3 months, although a monthly induction course is sometimes used in patients with very active disease. Infusion side effects include nausea and alopecia. Adverse effects include cardiotoxicity, acute myeloid leukemia, bone marrow suppression, and gonadal dysfunction. Because of the potential for long-term toxicities including cardiac injury and lymphoproliferative disorders, mitoxantrone is now rarely used in treating MS. Cardiac injury can occur years after completing therapy, which warrants surveillance echocardiogram or multigated acquisition scans prior to each infusion and after the medication has been discontinued.
Disease Modifying Therapy for Progressive MS
Treatment of progressive MS is more challenging than relapsing MS. Previously, certain DMTs (eg, subcutaneous IFN beta-1a and mitoxantrone) approved for RRMS were also found in some trials to slow progression of disability in SPMS, though the effect was modest and seen primarily in those subjects with superimposed active inflammation. It is likely worthwhile to use the DMTs in progressive MS if there is evidence of persistent active inflammation (eg, clinical relapses or active lesions on MRI) and side effects are tolerated, but patients should be informed that these therapies have limited efficacy in slowing the gradual progression of disability seen in progressive MS.
An encouraging advancement in the treatment of progressive MS was seen with ocrelizumab in PPMS, where ocrelizumab treatment was associated with a 24% relative risk reduction of progression of disability compared with placebo at 12 weeks. However, this trial enriched patients with active inflammation by limiting enrollment to those under 56 years of age and disease duration less than 10 to 15 years. 67 The benefit of ocrelizumab was markedly diminished in those without GdE lesions at baseline and those aged over 50 years. 68 The efficacy of ocrelizumab in PPMS patients over age 55 is unknown. Accordingly, the benefit of ocrelizumab on the underlying progressive aspects of PPMS appears to be limited.
Positive phase 2 and 3 clinical trial findings for high dose biotin 69,70 and siponimod 71,72 for progressive MS have been reported. These advances are encouraging for the future development of neuroprotective and neurorestorative agents.
Changing Disease Modifying Therapy and Monitoring Treatment
Broadly speaking, it is appropriate to consider changing DMT for 3 reasons: intolerable adverse effects, safety concerns, and breakthrough disease. With respect to adverse effects, every attempt should be made to manage them symptomatically. Of paramount importance is the patient’s tolerance of and adherence to DMT. It is important to address poor adherence, as it can lead to higher relapse rates and disease progression. If the patient remains intolerant of adverse effects, switching to an alternative agent is advisable.
With the emergence of newer therapeutics with various mechanisms of action and risk profiles, it is imperative that safety is monitored over the course of treatment. If at some point during treatment, the perceived benefits of the DMT no longer outweigh potential risks, then switching to a different agent should be considered. For example, in a patient treated with natalizumab who seroconverts to a highly positive JCV antibody titer, an alternative strategy without similar safety concerns, such as ocrelizumab, should be considered.
Despite its frequency in routine clinical practice, there is no consensus on the optimal approach to either defining or managing breakthrough disease. Breakthrough disease is generally defined as continued clinical or radiographic evidence of inflammatory disease activity despite treatment with an established DMT. Continued clinical relapses or new MRI lesions, particularly after 6 months of treatment with an established DMT, typically constitutes breakthrough disease. The severity of relapses, their subsequent recovery, and the number and size of new or active MRI lesions all contribute to defining when patients are considered to have sufficient breakthrough disease activity to merit changing therapies. Continued surveillance of clinical and radiographic measures of disease activity is important throughout treatment. In general, patients are seen clinically every 3 to 12 months, with repeat brain MRI every 6 to 12 months, depending on the patient’s baseline disease status and DMT.
Novel methods for longitudinal assessment of neurological performance and quality of life metrics for patients with MS are available for clinical use. At Cleveland Clinic, the Multiple Sclerosis Performance Test is used prior to each visit with the healthcare provider. 73 Completed by patients using an iPad, it includes neuro-performance testing that objectively measures walking speed, manual dexterity, low contrast visual acuity, and cognitive processing speed. Patients also complete questionnaires that screen for depression and evaluate patients’ impression of his or her overall health. These longitudinal data are used to carefully monitor patient function and may detect changes that would otherwise escape notice during traditional neurological assessments.
Symptomatic Therapies
Besides neurologic disability, MS can produce a variety of other symptoms that can interfere with daily activities. Identification and treatment of these symptoms should be considered throughout the disease course ( Table 6 ). Specific recommendations for management of fatigue and urinary dysfunction have been outlined by the Multiple Sclerosis Council for Clinical Practice Guidelines. Aggressive evaluation and treatment for these and other symptoms of MS can significantly improve quality of life and are an important component of long-term management of patients with MS.
Table 6: Common Symptoms in MS and Potential Treatments
| Symptom | Pharmacotherapy (brand name) | Miscellaneous |
|---|---|---|
| | ||
| | | |
| : : : | ||
| 5 mg to 10 mg per day, maximum of 80 mg per day in 3 to 4 divided doses 2 mg to 4 mg per day, maximum of 36 mg per day in 3 to 4 divided doses 2 mg at bedtime (maximum 30 mg per day in 3 to 4 divided doses) 0.5 mg at bedtime, max 2 mg per day 100 mg to 300 mg per day, maximum of 3,600 mg per day in 3 to 4 divided doses | ||
| Sildenafil(Revatio, Viagra) β |
α The sacral neurostimulation device precludes the ability to undergo MRI studies, limiting its use in patients with MS where monitoring MRIs are often an integral part of patient management.
β Evidence for efficacy for sexual dysfunction in women with MS has been negative.
Healthy Lifestyle and Wellness
In addition to conventional pharmacologic therapy, there is growing interest in the use of lifestyle strategies to support wellness and mitigate disease-related outcomes in MS. This interest is based on a growing appreciation of the role of certain comorbidities and lifestyle factors on disease activity, disability, mortality, and overall quality of life. For example, key observational studies suggest an association between vascular comorbidities (eg, hypertension, hyperlipidemia, and type 2 diabetes) and an increased risk of disability and mortality. 74 While evidence from randomized clinical trials is limited, there is evidence to suggest benefit from vitamin D supplementation, tobacco smoking cessation, routine exercise, and maintenance of emotional well-being as adjunct therapies to DMTs.
MS is a heterogeneous disease with a variable clinical course. Patients can progress rapidly over several years to significant disability or may have a few relapses and then remain clinically stable for many decades. The accumulation of disability in MS is slower than previously thought and varies widely between individuals. Early studies reported a relatively quick progression from disease onset to walking with a cane, with a median time of about 15 years. 75 However, more recent natural history studies reported a longer time to reaching this disability milestone, with a median time from onset to cane of about 30 years. Likewise, in PPMS, early studies reported short median time from disease onset to cane of less than 10 years, whereas more current studies showed that median time is closer to 15 years. 75 The advent of effective immunomodulating therapy for relapsing MS may in part explain a better long-term prognosis, but newer diagnostic criteria may have increased inclusion of MS patients with mild disease as well.
It is difficult to predict which patients will progress and which patients will remain relatively stable over time. Although there are clearly patients in whom the disease remains relatively mild, it is very difficult to predict which patients will eventually follow this course. There are several prognostic factors for unfavorable clinical outcomes. Older age at onset, Black race, Hispanic ethnicity, and initial symptoms involving cerebellar, spinal cord or pyramidal systems, and higher initial clinical activity (eg, high attack frequency and increased disability progression in the first 5 years) are all unfavorable prognostic factors. 76 Smoking and low serum vitamin D levels have also emerged as additional predictors of poor long-term outcome. Prognostic radiologic measures include brain and spinal cord atrophy and number of GdE lesions. MRI measures are also useful tools when evaluating the effect of MS therapies and should be used routinely for DMT monitoring. 76
Pregnancy and MS
Pregnancy does not seem to have a detrimental effect on the overall disease course of MS. In general, DMTs are not recommended during pregnancy, so efficient family planning with the help of the obstetrician can help minimize the amount of time the patient is off DMT. Pregnancy during MS is associated with a decreased incidence of relapses, but there is a rebound in relapse frequency in the postpartum period. 78 Relapses during pregnancy can be treated with short courses of high-dose corticosteroids if needed, though it is preferable to not treat mild relapses since adverse effects to glucocorticoids can be seen. A mid-pregnancy visit with the treating neurologist is recommended for postpartum planning. It is also generally recommended that patients who were previously treated with DMT prior to pregnancy resume treatment immediately postpartum unless they plan to breastfeed. If breastfeeding is pursued, cranial MRI 2 months after delivery for disease surveillance is appropriate. If there is evidence of active disease, the benefits of breastfeeding should be balanced with the need to resume DMT.
Unfortunately, no DMT is proven to be safe during pregnancy or while breastfeeding, and so they are generally not recommended. 79 Most of the available DMTs, including interferon beta, daclizumab, fingolimod, natalizumab, and alemtuzumab are pregnancy category C. Glatiramer acetate is pregnancy category B and is the safest DMT to use in women who need to continue DMT. The potential impact of brief exposures to DMTs (ie, during the first few weeks of pregnancy, before pregnancy is recognized) is relatively unknown, but appears to be minimal. Accordingly, although women who become pregnant while taking DMTs are generally recommended to discontinue DMTs, they can be reassured that the potential impact on their pregnancy is very low. As stated above, teriflunomide is pregnancy category X and should not be used in women of childbearing potential without effective contraception and counseling.
Vaccines and MS
The effect of vaccines on MS has been studied very carefully and there appears to be no adverse effect of vaccines on the course of disease. 80 Vaccines can be given safely in patients with MS and should be administered when clinically indicated, unless patients are on specific medications with an impact on response to vaccination. Inactivated vaccines are generally preferred, including in patients taking DMTs. Live attenuated vaccines are generally not recommended for a person with MS because of their theoretical ability to stimulate MS inflammation, although there is no compelling evidence showing an increased risk in the MS population of live attenuated vaccines at this time.
- Multiple sclerosis (MS) is a chronic inflammatory, demyelinating, and neurodegenerative disorder affecting the brain, optic nerve, and spinal cord.
- Symptoms of MS can involve almost any neurologic function; therefore, accurate diagnosis relies on a combination of clinical history, neurological examination, and paraclinical testing such as magnetic resonance imaging (MRI) and sometimes cerebrospinal fluid analysis.
- The 2017 McDonald Criteria simplify the diagnostic process, while preserving high sensitivity and specificity, and allowing early diagnosis of MS and prompt treatment.
- Many disease modifying therapies (DMTs) are available for relapsing forms of MS. They decrease the clinical episodes of inflammation, new MRI lesions, and slow the progression of disability.
- Available DMTs, including newer, more effective agents, allow for the increasingly accepted treat-to-target approach of “No Evidence of Disease Activity” (NEDA), which is defined as freedom from clinical and radiographic disease.
- The first DMT indicated for primary progressive MS (PPMS), ocrelizumab, suggests a potential role for B-cell therapy for progressive MS in younger patients with more active disease. However, the benefit of ocrelizumab on the underlying progressive aspects of PPMS appears to be limited.
- Despite emerging neurotherapeutics for progressive MS, early diagnosis and treatment are still key in preventing central nervous system inflammation and forestalling progressive disability related to neurodegeneration.
- Symptom management and healthy lifestyle strategies are important complementary approaches for better outcomes and quality of life for patients with MS.
- Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: A summary report and call to action. Ann Neurol 2017; 81:479–484.
- Weinshenker BD. Natural history of multiple sclerosis. Ann Neurol 1994; 36(Suppl):S6–S11.
- Koch M, Kingwell E, Rieckmann P, Tremlett H. The natural history of primary progressive multiple sclerosis. Neurology 2009; 73:1996–2002.
- Cottrell DA, Kremenchutzky M, Rice GPA, et al. The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain 1999; 122:625–639.
- Dilokthornsakul P, Valuck RJ, Nair KV, Corboy JR, Allen RR, Campbell JD. Multiple sclerosis prevalence in the United States commercially insured population. Neurology 2016; 86:1014–1021
- Atlas of MS FAQs. MS International Federation website. https://www.msif.org/about-us/who-we-are-and-what-we-do/advocacy/atlas/atlas-of-ms-faqs/. Updated March 14 2017. Accessed April 2, 2018.
- Lucchinetti CF, Brueck W, Rodriguez M, Lassmann H. Multiple sclerosis: lessons from neuropathology. Semin Neurol 1998; 18:337–349.
- Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 2015; 14:183–193.
- Hametner S, Wimmer I, Haider L, Pfeifenbring S, Brück W, Lassmann H. Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol 2013; 74:848–861.
- Lucchinetti CF, Popescu BFG, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Eng J Med 2011; 365:2188–2197.
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998; 338:278–285.
- Chang A, Tourtellotte WW, Rudick RA, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med 2002; 346:165–173.
- Fox RJ, Cohen JA: Multiple sclerosis: the importance of early recognition and treatment. Cleve Clin J of Med, 2001; 68:157–70.
- Schumacher GA, Beebe G, Kibler RF, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci 1965; 122:552–568.
- McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 2001; 50:121–127.
- Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald Criteria. Lancet Neurol 2018; 17:162–173.
- Filippi M, Rocca MA, Ciccarelli O, et al; on behalf of the MAGNIMS Study Group. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS concensus guidelines. Lancet Neurol 2016; 15:292–303.
- Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 83:278–286.
- Milligan NM, Newcombe R, Compston DA. A double-blind controlled trial of high dose methylprednisolone in patients with multiple sclerosis: 1. Clinical effects. J Neurol Neurosurg Psychiatry 1987; 50:511–516.
- Sellebjerg F, Frederiksen JL, Nielsen PM, Olesen J. Double-blind, randomized, placebo-controlled study of oral, high-dose methylprednisolone in attacks of MS. Neurology 1998; 51:529–534.
- Oh J, Calabresi PA. Disease-modifying therapies in relapsing multiple sclerosis. In: Rae-Grant A, Fox RJ, Bethoux F. Multiple Sclerosis and Related Disorders: Clinical Guide to Diagnosis, Medical Management, and Rehabilitation. New York, NY: Demos Medical ; 2013:104.
- Jacobs LD, Cookfair DL, Rudick RA, et al; The Multiple Sclerosis Collaborative Research Group (MSCRG). Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol 1996; 39:285–294.
- Johnson KP, Brooks BR, Cohen JA, et al; Copolymer 1 Multiple Sclerosis Study Group. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind, placebo-controlled trial. Neurolog y 1995; 45:1268–1276.
- Ebers GC, Rice G, Lesaux J; on behalf of the PRISMS Study Group. Randomized double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet 1998; 352:1498–1504.
- IFNB Multiple Sclerosis Study Group, University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized, controlled trial. Neurology 1995; 45:1277–1285.
- Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2Rα-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A 2006; 103:5941–5946.
- Gold R, Giovannoni G, Selmaj K, et al; for the SELECT study investigators. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet 2013; 381:2167–2175.
- Kappos L, Wiendl H, Selmaj K, et al. Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2015; 373:1418–1428.
- Giovannoni G, Gold R, Selmaj K, et al; for the SELECTION Study Investigators. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECTION): a multicentre, randomised, double-blind extension trial. Lancet Neurol 2014; 13:472–481.
- Restrictions of use of Zinbryta (daclizumab) in view of fatal fulminant liver failure. Biogen Health Products Regulatory Authority website. https://www.hpra.ie/docs/default-source/Safety-Notices/important-safety-information---zinbryta-(daclizumab).pdf?sfvrsn=0). Published July 11, 2017. Accessed March 9, 2018.
- Kappos L, Radue E-W, O'Connor P, et al; for the FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362:387–401.
- Cohen JA, Barkhof F, Comi G, et al; for the TRANSFORMS Study Group. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362:402–415.
- Berger JR. Classifying PML risk with disease modifying therpaties. Mult Scler Relat Disord 2017; 12:59–63.
- Greene S, Watanabe K, Braatz-Trulson J, Lou L. Inhibition of dihydroorotate dehydrogenase by the immunosuppressive agent leflunomide. Biochem Pharmacol 1995; 50:861–867.
- Xu X, Blinder L, Shen J, et al. In vivo mechanism by which leflunomide controls lymphoproliferative and autoimmune disease in MRL/MpJ-lpr/lpr mice. J Immunol 1997; 159:167–174.
- O'Connor P, Wolinsky JS, Confavreux C, et al; for the TEMSO Trial Group. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011; 365:1293–1303.
- Confavreux C, O'Connor P, Comi G, et al; for the TOWER Trial Group. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13:247–256.
- Vermersch P, Czlonkowska A, Grimaldi LME, et al; for the TENERE Trial Group. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler 2014; 20:705–716.
- Aubagio [package insert]. Cambridge, MA: Genzyme Corporation; 2012.
- Linker RA, Lee D-H, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011; 134:678–692.
- Reich K, Thaci D, Mrowietz U, Kamps A, Neureither M, Luger T. Efficacy and safety of fumaric acid esters in the long-term treatment of psoriasis—a retrospective study (FUTURE). J Dtsch Dermatol Ges 2009; 7:603–611.
- Gold R, Kappos L, Arnold DL, et al; for the DEFINE Study Investigators. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367:1098-1107.
- Fox RJ, Miller DH, Phillips JT, et al; for the CONFIRM Study Investigators. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367:1087–1097.
- Ontaneda D, Fox RJ. Emerging therapies for relapsing multiple sclerosis. In: Rae-Grant A, Fox RJ, Bethoux F. Multiple Sclerosis and Related Disorders: Clinical Guide to Diagnosis, Medical Management, and Rehabilitation. New York, NY: Demos Medical; 2013:133.
- Rudick RA, Stuart WH, Calabresi PA, et al; for the SENTINEL Investigators. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006; 354:911–923.
- Polman CH, O'Connor PW, Havrdova E, et al; for the AFFIRM Investigators. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354:899–910.
- Tysabri [package insert]. Cambridge, MA: Biogen Idec Inc.; 2012.
- Sørensen PS, Bertolotto A, Edan G, et al. Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult Scler 2012; 18:143–152.
- Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012; 366:1870–1880.
- Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 2006; 354:924–933.
- Berger JR, Fox RJ. Reassessing the risk of natalizumab-associated PML. J Neurovirol 2016; 22:533–535. doi:10.1007/s13365-016-0427-6.
- Bielekova B, Becker BL. Monoclonal antibodies in MS: mechanisms of action. Neurology 2010; 74(suppl 1):S31–S40.
- Thompson SAJ, Jones JL, Cox AL, Compston DAS, Coles AJ. B-cell reconstitution and BAFF after alemtuzumab (campath-1H) treatment of multiple sclerosis. J Clin Immunol 2010; 30:99–105.
- Coles AJ, Compston DAS, Selmaj KW, et al; CAMMS223 Trial Investigators. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008; 359:1786–1801.
- Cohen JA, Coles AJ, Arnold DL, et al; for the CARE-MS I investigators. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012; 380:1819–1828.
- Coles AJ, Twyman CL, Arnold DL, et al; for the CARE-MS II investigators. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012; 380:1829–1839.
- Lemtrada [package insert]. Cambridge, MA: Genzyme Corporation; 2017.
- Clatworthy MR, Wallin EF, Jayne DR. Anti-glomerular basement membrane disease after alemtuzumab. N Engl J Med 2008; 359:768–769.
- Fox EJ, Alroughani R, Brassat D, et al; on behalf of the CARE-MS II Investigators. Efficacy of alemtuzumab is durable over 6 years with active relapsing-remitting multiple sclerosis and an inadequate response to prior therapy in the absence of continuous treatment (CARE-MS II). Abstract presented at the 32nd Congress of ECTRIMS; September 16, 2016; London, UK. Abstract P1150.
- Singer B, Dunn J, Izquierdo G, et al; on behalf of the CARE-MS II Investigators. Alemtuzumab significantly improves disability in patients with active relapsing-remitting MS and an inadequate response to prior therapy as assessed using a novel, composite measure: confirmed disability improvement-plus: results of CARE-MS II. Abstract presented at the 32nd Congress of ECTRIMS; September 15, 2016; London, UK. Abstract P610.
- Coles A, Robertson N, Al-Araji A, et al; MS Advisory group. Guidance on the prevention of Listeria infection after alemtuzumab treatment of multiple sclerosis. Website: https://www.theabn.org/media/Guidance%20on%20the%20prevention%20of%20Listeria%20infection%20after%20alemtuzumab%20treatment% 20of%20multiple%20sclerosis.pdf. Published May 15, 2017. Accessed March 12, 2018.
- Morschhauser F, Marlton P, Vitolo U, et al. Results of a phase I/II study of ocrelizumab, a fully humanized anti-CD20 mAb, in patients with relapsed/refractory follicular lymphoma. Ann Oncol 2010; 21:1870–1876.
- Hauser SL, Bar-Or A, Comi G, et al; for the OPERA I and OPERA II Clinical Investigators. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376:221–234.
- Goodin DS, Frohman EM, Garmany GP Jr, et al. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology 2002; 58:169–178.
- Smith DR, Weinstock-Guttman B, Cohen JA, et al. A randomized blinded trial of combination therapy with cyclophosphamide in patients with active multiple sclerosis on interferon beta. Mult Scler 2005; 11:573–582.
- Hartung H-P, Gonsette R, König N, et al; Mitoxantrone in Multiple Sclerosis Study Group (MIMS). Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet 2002; 360:2018–2025.
- Montalban X, Hauser SL, Kappos L, et al; for the ORATORIO Clinical Investigators. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017; 376:209–220.
- Ocrelizumab Summary of Product Characteristics. European Medicines Agency website. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004043/WC500241124.pdf. Accessed March 12, 2018.
- Sedel F, Papeix C, Bellanger A, et al. High doses of biotin in progressive multiple sclerosis: a pilot study. Mult Scler Relat Disord 2015; 4:159–169.
- Tourbah A, Lebrun-Frenay C, Edan G, et al; on behalf of the MS-SPI study group. MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: a randomized, double-blind, placebo-controlled study. Mult Scler 2016; 22:1719–1731.
- Selmaj K, Li DKB, Hartung H-P, et al. Siponimod for patients with relapsing-remitting multiple sclerosis (BOLD): an adaptive, dose-ranging, randomised, phase 2 study. Lancet Neurol 2013; 12:756–767.
- Kappos L, Bar-Or A, Cree B, et al. Efficacy and safety of siponimod in secondary progressive multiple sclerosis: results of the placebo controlled, double-blind, phase III EXPAND study. Abstract presented at the 32nd Congress of ECTRIMS; September 16, 2016; London, UK. Abstract 250.
- Rudick RA, Miller D, Bethoux F, et al. The multiple sclerosis performance test (MSPT): an iPad-based disability assessment tool. J Vis Exp 2014; 88:e51318.
- Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbitity is associated with more rapid disability progression in multple sclerosis. Neurology 2010; 74:1041–1047.
- Tremlett H, Zhao Y, Rieckmann P, Hutchinson M. New perspectives in the natural history of multiple sclerosis. Neurology 2010; 74:2004–2015.
- Renoux C. Natural history of multiple sclerosis: long-term prognostic factors. Neurol Clin 2011; 29:293–308.
- Kaunzner UW, Gauthier SA. MRI in the assessment and monitoring of multiple sclerosis: an update on best practice. Ther Adv Neurol Discord 2017; 10:247–261.
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T; Pregnancy in Multiple Sclerosis Group. Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med 1998; 339:285–291.
- Cree BAC. Update on reproductive safety of current and emerging disease-modifying therapies for multiple sclerosis. Mult Scler 2013:19:835–843.
- Confavreux C, Suissa S, Saddier P, Bourdès V, Vukusic S; Vaccines in Multiple Sclerosis Study Group. Vaccinations and the risk of relapse in multiple sclerosis. N Engl JMed 2001; 344:319–326.
- Site Disclaimer
- Privacy Policy
- Editorial Policy
- Editorial Board

- Multiple Sclerosis
- Author: Christopher Luzzio, MD; Chief Editor: Jasvinder Chawla, MD, MBA more...
- Sections Multiple Sclerosis
- Practice Essentials
- Pathophysiology
- Epidemiology
- Patient Education
- Physical Examination
- Clinical Rating Scales
- Criteria for Categorizing MS
- Approach Considerations
- McDonald Criteria for MS Diagnosis
- Blood Studies
- Magnetic Resonance Imaging
- Other Imaging Studies in Multiple Sclerosis
- Evoked Potentials
- Electroencephalography
- Lumbar Puncture
- Emergency Department Management
- Treatment of Acute Relapses
- Immunomodulatory Therapy for Relapsing-Remitting MS
- Treatment of Aggressive MS
- Immunomodulatory Therapy for Progressive MS
- Experimental Agents
- Stem Cell Transplantation
- Treatment of MS in Pregnancy
- Symptom Management
- Rehabilitation
- Surgery for Alleviating Symptoms
- Deterrence and Prevention
- Consultations
- Long-Term Monitoring
- Medication Summary
- Immunomodulators
- Corticosteroids
- Immunosuppressants
- Sphingosine 1-Phosphate Receptor Modulators
- Dopamine Agonists
- Skeletal Muscle Relaxant
- Neuromuscular Blockers, Botulinum Toxins
- Alpha2-Adrenergic Agonists
- Benzodiazepines
- Anticonvulsants, Other
- Anticonvulsants, Hydantoin
- Selective Serotonin/Norepinephrine Reuptake Inhibitors
- Nonsteroidal Anti-Inflammatory Drugs
- Antispasmodic Agents, Urinary
- Acetylcholinesterase Inhibitors, Central
- Antidiarrheals
- Potassium Channel Blockers
- Questions & Answers
- Media Gallery
Multiple sclerosis (MS) is an immune-mediated inflammatory disease that attacks myelinated axons in the central nervous system, destroying the myelin and the axon in variable degrees and producing significant physical disability within 20–25 years in more than 30% of patients. The hallmark of MS is symptomatic episodes that occur months or years apart and affect different anatomic locations. See the image below.
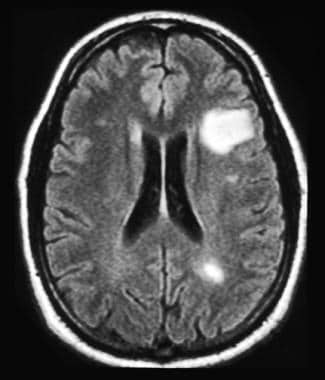
See Multiple Sclerosis , a Critical Images slideshow, for more information on incidence, presentation, and intervention, as well as additional resources.
Also, see the Autoimmune Disorders: Making Sense of Nonspecific Symptoms slideshow to help identify several diseases that can cause a variety of nonspecific symptoms.
Signs and symptoms
Classic MS signs and symptoms are as follows:
Sensory loss (ie, paresthesias): Usually an early complaint
Spinal cord symptoms (motor): Muscle cramping secondary to spasticity
Spinal cord symptoms (autonomic): Bladder, bowel, and sexual dysfunction
Cerebellar symptoms: Charcot triad of dysarthria (scanning speech), nystagmus, and intention tremor
Optic neuritis
Trigeminal neuralgia: Bilateral facial weakness or trigeminal neuralgia
Facial myokymia (irregular twitching of the facial muscles): May also be a presenting symptom
Eye symptoms: Including diplopia on lateral gaze (33% of patients)
Heat intolerance
Constitutional symptoms: Especially fatigue (70% of cases) and dizziness
Pain: Occurs in 30–50% of patients at some point in their illness
Subjective cognitive difficulties: With regard to attention span, concentration, memory, and judgment
Depression: A common symptom
Euphoria: Less common than depression
Bipolar disorder or frank dementia: May be a late finding but is sometimes found at initial diagnosis
Symptoms associated with partial acute transverse myelitis
See Clinical Presentation for more detail.
MS is diagnosed on the basis of clinical findings and supporting evidence from ancillary tests. Tests include the following:
Magnetic resonance imaging: The imaging procedure of choice for confirming MS and monitoring disease progression in the CNS
Evoked potentials: Used to identify subclinical lesions; results are not specific for MS
Lumbar puncture: May be useful if MRI is unavailable or MRI findings are nondiagnostic; CSF is evaluated for oligoclonal bands and intrathecal immunoglobulin G (IgG) production
Classification
MS is divided into the following categories, principally on the basis of clinical criteria, including the frequency of clinical relapses, time to disease progression, and lesion development on MRI: [ 1 , 2 , 3 , 4 ]
Relapsing-remitting MS (RRMS): Approximately 85% of cases
Secondary progressive MS (SPMS)
Primary progressive MS (PPMS)
Progressive-relapsing MS (PRMS)
The following 2 subgroups are sometimes included in RRMS:
Clinically isolated syndrome (CIS): A single episode of neurologic symptoms
Benign MS: MS with almost complete remission between relapses and little if any accumulation of physical disability over time
See Workup for more detail.
Treatment of MS has 2 aspects: immunomodulatory therapy (IMT) for the underlying immune disorder and therapies to relieve or modify symptoms.
Treatment of acute relapses is as follows:
Methylprednisolone (Solu-Medrol) can hasten recovery from an acute exacerbation of MS
Plasma exchange (plasmapheresis) can be used short term for severe attacks if steroids are contraindicated or ineffective [ 5 ]
Dexamethasone is commonly used for acute transverse myelitis and acute disseminated encephalitis
Most of the disease-modifying agents for MS (DMAMS) have been approved for use only in relapsing forms of MS. However, siponimod, ocrelizumab, ozanimod, and cladribine are also approved for active secondary progressive disease. The DMAMS currently approved for use by the US Food and Drug Administration (FDA) include the following:
- Interferons (eg, IFN beta-1a, IFN beta-1b, peginterferon beta-1a) [ 6 , 7 , 8 ]
- Sphingosine 1-phosphate (S1P) receptor modulators (eg, siponimod, fingolimod, ozanimod) [ 9 , 10 , 11 , 12 ]
- Monoclonal antibodies (eg, natalizumab, alemtuzumab, ocrelizumab, ublituximab) [ 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 ]
- Miscellaneous immunomodulators (eg, glatiramer, mitoxantrone, teriflunomide, dimethyl fumarate, cladribine) [ 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 ]
A single-use autoinjector is also available for self-injection of interferon beta-1a (Rebif) in patients with relapsing forms of MS. [ 30 ]
The following agents are used for treatment of aggressive MS:
High-dose cyclophosphamide (Cytoxan) has been used for induction therapy
Mitoxantrone is approved for reducing neurologic disability and/or the frequency of clinical relapses in patients with SPMS, PRMS, or worsening RRMS
Treatment of the symptoms of MS involves both pharmacologic and nonpharmacologic measures. The following symptoms may be amenable to pharmacologic therapy:
Fatigue: Off-label treatments include amantadine, methylphenidate, and fluoxetine
Depression: Selective serotonin reuptake inhibitors are preferred
Spasticity: Baclofen is effective in most cases
Pain: Tricyclic antidepressants are first-line drugs for primary pain
Sexual dysfunction: Oral phosphodiesterase type 5 inhibitors (eg, sildenafil, tadalafil, vardenafil)
Optic neuritis: Intravenous methylprednisolone may speed recovery
See Treatment and Medication for more detail.
Multiple sclerosis (MS) is an immune-mediated inflammatory disease that attacks myelinated axons in the central nervous system (CNS), destroying the myelin and the axon in variable degrees. In most cases, the disease follows a relapsing-remitting pattern, with short-term episodes of neurologic deficits that resolve completely or almost completely. A minority of patients experience steadily progressive neurologic deterioration.
The cause of MS is not known, but it likely involves a combination of genetic susceptibility and a presumed nongenetic trigger (eg, viral infection, low vitamin D levels) that together result in a self-sustaining autoimmune disorder that leads to recurrent immune attacks on the CNS (see Etiology). Geographic variation in the incidence of MS (see Epidemiology) supports the probability that environmental factors are involved in the etiology.
MS is diagnosed on the basis of clinical findings and supporting evidence from ancillary tests, such as magnetic resonance imaging (MRI) of the brain and cerebrospinal fluid examination. (See Workup.) Traditionally, MS could not be diagnosed after only a single symptomatic episode, as diagnosis required the occurrence of repeat clinical attacks suggesting the appearance of lesions separated in time and space; however, recent guidelines allow diagnosis of MS even with a first clinical episode as long as ancillary tests support separation of lesions in time or space.
A common misconception is that any attack of CNS demyelination means a diagnosis of acute MS. When a patient has a first attack of demyelination, the physician should not rush to diagnose MS, because the differential diagnosis includes a number of other diseases. For example, MS must be distinguished from other neuroinflammatory disorders (see DDx.)
Treatment consists of immunomodulatory therapy for the underlying immune disorder and management of symptoms, as well as nonpharmacologic treatments, such as physical and occupational therapy (see Treatment). In the United States, various disease-modifying agents for MS are currently approved for use in relapsing MS.
Multiple sclerosis is an inflammatory, demyelinating disease of the CNS. In pathologic specimens, the demyelinating lesions of MS, called plaques (see the image below), appear as indurated areas—hence the term sclerosis.
Examination of the demyelinating lesions in the spinal cord and brain of patients with MS shows myelin loss, destruction of oligodendrocytes, and reactive astrogliosis, often with relative sparing of the axon cylinder. [ 31 ] In some MS patients, however, the axon is also aggressively destroyed.
The location of lesions in the CNS usually dictates the type of clinical deficit that results. As neural inflammation resolves in MS, some remyelination occurs, but some recovery of function that takes place in a patient could be due to nervous system plasticity. MS is also characterized by perivenular infiltration of lymphocytes and macrophages, as demonstrated in the image below. Infiltration of inflammatory cells occurs in the parenchyma of the brain, brainstem, optic nerves, and spinal cord.
One of the earliest steps in lesion formation is the breakdown of the blood-brain barrier. Enhanced expression of adhesion molecules on the surface of lymphocytes and macrophages seems to underlie the ability of these inflammatory cells to penetrate the blood-brain barrier.
The elevated immunoglobulin G (IgG) level in the cerebrospinal fluid, which can be demonstrated by an oligoclonal band pattern on electrophoresis, suggests an important humoral (ie, B-cell activation) component to MS. In fact, variable degrees of antibody-producing plasma cell infiltration have been demonstrated in MS lesions. The image below provides an overview of demyelination.
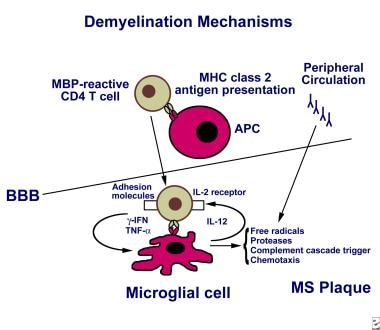
Immune cells in MS
Molecular studies of white matter plaque tissue have shown that interleukin (IL)-12, a potent promoter of inflammation, is expressed at high levels in lesions that form early in MS. B7-1, a molecule required to stimulate lymphocytes to release proinflammatory cytokines, is also expressed at high levels in early MS plaques. [ 31 ] Evidence exists of elevated frequencies of activated myelin-reactive T-cell clones in the circulation of patients with relapsing-remitting MS and higher IL-12 production in immune cells of patients with progressive MS.
Decreased function of T-lymphocytes with a regulatory role (Tregs) has been implicated in MS. [ 32 ] These Tregs are CD4 + CD25 + T cells that can be identified by their expression of a transcription factor known as Foxp3.
Conversely, the cytokine IL-23 has been shown to drive cells to commit to a pathogenic phenotype in autoimmune diseases, including MS. These pathogenic CD4 + T cells act reciprocally to counteract Treg function and can be identified by their high expression of the proinflammatory cytokine IL-17; they are therefore referred to as T H 17 cells. [ 33 ]
Tregs and T H 17 cells are not the only critical immune cells in the pathogenesis of MS. Immune cells such as microglia (resident macrophages of the CNS), dendritic cells, natural killer (NK) cells, and B cells are gaining increased attention by MS researchers. In addition, nonimmune cells (ie, endothelial cells) have also been implicated in mechanisms that lead to CNS inflammation. [ 34 ]
Approximately 55–75% of patients with MS have spinal cord lesions at some point during the course of the disease. Spinal MS is often associated with concomitant brain lesions; however, as many as 20% of patients with spinal lesions do not have intracranial plaques. No strong correlation has been established between the extent of the plaques and the degree of clinical disability.
Spinal MS has a predilection for the cervical spinal cord (67% of cases), with preferential, eccentric involvement of the dorsal and lateral areas of the spinal cord abutting the subarachnoid space around the cord. The gray matter may be involved.
Myelocortical MS
Myelocortical MS (MCMS) is a new subtype of MS identified in 2018. It is marked by demyelination of the spinal cord and cerebral cortex but not of cerebral white matter. Researchers studied the brain and spinal cords from 100 patients with MS who had died between May 1998 and November 2012. Twelve of these individuals (12%) had demyelinated lesions in the spinal cord and cerebral cortex, but not in cerebral white matter. Researchers then compared the demyelinated lesion area in tissue sections of cerebral white matter, spinal cord, and cerebral cortex of individuals with MCMS with those collected from individuals with traditional MS and found that only the typical MS patients had lesions in the cerebral white matter. This suggests that neurodegeneration can be independent of demyelination in MCMS patients. [ 35 ]
Optic neuritis in MS
Approximately 20% of patients with MS present with optic neuritis ( ON ) as a first demyelinating event, and 40% of patients may experience ON during the course of their disease. Sequential episodes of optic nerve involvement and a longitudinally extensive myelopathy suggest a separate disorder, known as neuromyelitis optica [NMO], or Devic disease (see the images below). [ 36 ] Although Devic disease is sometimes categorized as an MS variant, typical MS therapies are ineffective in Devic disease, and most experts consider Devic disease to be separate from MS.
The cause of MS is unknown, but it is likely that multiple factors act in concert to trigger or perpetuate the disease. It has been hypothesized that MS results when an environmental agent or event (eg, viral or bacterial infection, exposure to chemicals, lack of sun exposure) acts in concert with a genetic predisposition to immune dysfunction.
Genetic and molecular factors
The concordance rate for MS among monozygotic twins is only 20–35%, suggesting that genetic factors have only a modest effect. The presence of predisposing non-Mendelian factors (ie, epigenetic modification in 1 twin), along with environmental effects, plays an important role. For first-degree family members (children or siblings) of people affected with MS, the risk of developing the disorder is sevenfold higher than in the general population, but familial excess lifetime risk is only 2.5–5%. [ 37 ]
Different variants of genes normally found in the general population, commonly referred to as polymorphisms, may lead to different gradations of cellular expression of those genes and therefore of the proteins that they encode. With MS susceptibility, it may be that a polymorphism within the promoter region of a gene involved in immune reactivity generates an exaggerated response (eg, elevated expression of a proinflammatory gene) to a given antigen, leading to uncontrolled immune cell proliferation and autoimmunity.
Research on single-nucleotide polymorphisms (SNPs) that confer risk of more severe disease or of developing particular forms of MS will be of great interest to the clinicians treating this complex disorder in the early stages. To date, however, HLA-DRB1 is the only chromosomal locus that has been consistently associated with MS susceptibility. Multiple other polymorphisms that may act in concert to predispose to MS have been described with genome-wide approaches, but their individual contribution to risk is not nearly as high as the risk conferred by the HLA locus. [ 38 ]
Genes that instead of conferring susceptibility to MS confer relative protection against it are also being investigated, and clues are emerging from within the major histocompatibility complex (MHC) region. For example, it has been suggested that the HLA-C*05 allele confers protection against MS. [ 39 ]
Molecular mimicry has been proposed as an etiologic process in MS. The molecular mimicry hypothesis refers to the possibility that T cells in the peripheral blood may become activated to attack a foreign antigen and then erroneously direct their attack toward brain proteins that share similar epitopes.
Viral infection
Another hypothesis is that a virus may infect the immune system, activating self-reactive T cells (myelin reactive) that would otherwise remain quiescent. A virus that infects cells of the immune and nervous systems can possibly be reactivated periodically and thus lead to acute exacerbations in MS.
Epstein-Barr virus (EBV) infection has been found to become periodically reactivated, but a possible causative role in MS has been difficult to prove. Evidence supporting EBV infection as an etiologic factor includes (1) long-term studies showing a higher association with MS in individuals with early presence of serum antibodies against specific EBV antigens and (2) high expression of EBV antigens within MS plaques. [ 40 ]
Evidence that argues against an etiologic role for EBV infection includes the fact that MS is a highly heterogeneous disease; EBV might help trigger some cases but not others, making associations in populations difficult. In addition, it is possible that EBV reactivation is an effect rather than a cause (ie, instead of viral reactivation being the trigger for MS, reactivation might be an epiphenomenon of a dysregulated immune system).
Environmental factors
Geography is clearly an important factor in the etiology of MS. The incidence of the disease is lower in the equatorial regions of the world than in the southernmost and northernmost regions. However, a systematic review by Alonso and Hernán found that this latitude gradient became attenuated after 1980, apparently due to an increased incidence of MS in lower latitudes. [ 41 ]
Apparently, whatever environmental factor is involved must exert its effect in early childhood. If an individual lives in an area with low incidence of MS until age 15 years, that person's risk remains low even if the individual subsequently moves to an area of high incidence.
On the other hand, certain ethnic groups (eg, Eskimos), despite living in areas of higher incidence, do not have a high frequency of MS. Therefore, the exact role played by geography versus genetics is not clear.
Vitamin D levels
Low levels of vitamin D have been proposed as one environmental factor contributing to the development of MS. Vitamin D has a role in regulating immune response, by decreasing production of proinflammatory cytokines and increasing production of anti-inflammatory cytokines; also, high circulating levels of vitamin D appear to be associated with a reduced risk of MS. [ 42 ]
Thus, lower vitamin D levels due to lower sunlight exposure at higher latitudes may be one reason for the geographic variations in MS incidence, and the protective effect of traditional diets high in vitamin D could help explain why certain areas (eg, Norway) have a lower incidence of MS despite having limited sunlight. [ 43 ] This hypothesis would also provide an explanation for the correlation between childhood sun exposure and MS in monozygotic twins discordant for MS. [ 44 ]
Chronic cerebrospinal venous insufficiency
A controversial hypothesis proposes a vascular rather than an immunologic cause for some cases of MS. In 2008, Paolo Zamboni described an association between MS and chronic cerebrospinal venous insufficiency (CCSVI). [ 45 ]
The CCSVI hypothesis posits that stenosis of the main extracranial venous outflow pathways results in compromised drainage and a high rate of cerebral venous reflux. The CCSVI hypothesis has been linked with the potential effects of iron deposition in the brain parenchyma, which some authors suggest is modestly to strongly predictive of disability progression, lesion volume accumulation, and atrophy in some patients with MS. [ 46 , 47 ]
A small, open-label study suggested that internal jugular vein and azygous vein angioplasty had a positive effect on MS symptoms in patients with CCSVI. [ 48 ] A meta-analysis found a positive association between CCSVI and MS, but poor reporting of the success of blinding and marked heterogeneity among studies of CCSVI precluded definitive conclusions. [ 49 ]
Because of the potential danger of such experimental procedures in treating this unproven vascular condition, the US Food and Drug Administration (FDA) has issued a warning. See FDA issues alert on potential dangers of unproven treatment for multiple sclerosis .
Given the paucity of supporting evidence, most MS experts also question the CCSVI hypothesis and do not recommend this therapy. Nevertheless, CCSVI has received widespread attention in the lay press and MS support groups, so physicians should be prepared for inquiries from patients on this highly controversial subject.
Hepatitis B vaccine
Worldwide anecdotal reports suggesting a connection between hepatitis B vaccination and MS prompted the US Centers for Disease Control and Prevention (CDC) to investigate this possibility. The CDC concluded that the weight of the available scientific evidence does not support the suggestion that hepatitis B vaccine causes or worsens MS. [ 50 ]
On the basis of the CDC findings, a National Multiple Sclerosis Society expert panel concluded as follows: “ People with MS should not be denied access to health-preserving and potentially-life saving vaccines because of their MS , and should follow the CDC guidelines for any given vaccine.” [ 51 ]
United States statistics
Prevalence estimates for MS in the United States vary from 58 to 95 per 100,000 population. [ 52 ] According to the National Multiple Sclerosis Society, 400,000 individuals in the United States are affected by MS. [ 53 ] Misdiagnosis is common, however.
As is true of autoimmune diseases in general, MS is more common in women. The female-to-male ratio of MS incidence has increased since the mid-20th century, from an estimated 1.4 in 1955 to 2.3 in 2000. [ 41 ] MS is usually diagnosed in persons aged 15–45 years; however, it can occur in persons of any age. The average age at diagnosis is 29 years in women and 31 years in men.
International statistics
Worldwide, approximately 2.1 million people are affected by MS. The disease is seen in all parts of the world and in all races, but rates vary widely. [ 53 ] In general, the prevalence of MS tends to increase with latitude (eg, lower rates in the tropics, higher rates in northern Europe), but there are many exceptions to this gradient (eg, low rates among Chinese, Japanese, and African blacks; high rates among Sardinians, Parsis, and Palestinians).
The presence of these exceptions implies that racial and ethnic differences affect risk. In addition, a substantial increase in MS incidence has been reported from different regions, suggesting that environmental factors, as well as geographic and genetic ones, play an important role in MS. [ 54 ] (See Etiology.)
Epidemiologic studies indicate an increase in MS prevalence in Latin America. Susceptibility to MS and clinical behavior of the disease varies genetically in Latin America; for example, MS apparently does not occur in Amerindians with Mongoloid genes. [ 55 ]
If left untreated, more than 30% of patients with MS will develop significant physical disability within 20–25 years after onset. Several of the disease-modifying agents used in MS have slowed disability progression within the duration of research trials; whether these effects will be maintained over longer periods is not known.
Less than 5–10% of patients have a clinically milder MS phenotype, in which no significant physical disability accumulates despite the passage of several decades after onset (sometimes in spite of multiple new lesions seen on MRI). Detailed examination of these patients in many instances reveals some degree of cognitive deterioration.
Male patients with primary progressive MS have the worst prognosis, with less favorable response to treatment and rapidly accumulating disability. The higher incidence of spinal cord lesions in primary progressive MS is also a factor in the rapid development of disability.
Life expectancy is shortened only slightly in persons with MS, and the survival rate is linked to disability. Death usually results from secondary complications (50–66%), such as pulmonary or renal causes, but can also be due to primary complications, suicide, and causes unrelated to MS. The Marburg variant of MS is an acute and clinically fulminant form of the disease that can lead to coma or death within days.
Patients should be educated on the purposes of medications, doses, and the management of adverse effects. Patients and caregivers need education on appropriate management of problems related to pain, fatigue, and spasticity, as well as on issues related to bowel, bladder, and sexual function. For patients with advanced disease, caregivers need hands-on training in transfer techniques, as well as in management of skin integrity, bowel programs, and urinary collection devices.
Patients with MS report a high incidence of falling. Contributing factors are similar to those in other populations with neurologic diseases. Patients with MS can benefit from receiving information about preventing falls from their healthcare practitioner. [ 56 ]
To ensure a successful outcome, family members and caregivers should be included in any education provided. Community agencies, such as the state chapters of the National Multiple Sclerosis Society , can provide valuable information concerning community resources, as well as social support and education.
Patients may benefit from referral to comprehensive and professional organizations and Web sites that are dedicated to MS. Among these, the National Multiple Sclerosis Society is highly recommended for information on current hypotheses, ongoing research, general resources, and educational programs. Other highly recommended MS-related Web sites include MultipleSclerosis.com and The Consortium of Multiple Sclerosis Centers .
For patient education information, see the Brain & Nervous System Center .
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol . 2018 Feb. 17 (2):162-173. [QxMD MEDLINE Link] .
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol . 1983 Mar. 13(3):227-31. [QxMD MEDLINE Link] .
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology . 1996 Apr. 46(4):907-11. [QxMD MEDLINE Link] .
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol . 2001 Jul. 50(1):121-7. [QxMD MEDLINE Link] .
Cortese I, Chaudhry V, So YT, Cantor F, Cornblath DR, Rae-Grant A. Evidence-based guideline update: Plasmapheresis in neurologic disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology . 2011 Jan 18. 76(3):294-300. [QxMD MEDLINE Link] . [Full Text] .
Sanford M, Lyseng-Williamson KA. Subcutaneous recombinant interferon-ß-1a (Rebif®): a review of its use in the treatment of relapsing multiple sclerosis. Drugs . 2011 Oct 1. 71(14):1865-91. [QxMD MEDLINE Link] .
Betaseron [package insert]. Montville, NJ: Bayer Healthcare Pharmaceuticals Inc. May 2010.
Calabresi PA, Kieseier BC, Arnold DL, Balcer LJ, Boyko A, Pelletier J, et al. Pegylated interferon ß-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. Lancet Neurol . 2014 Jul. 13(7):657-65. [QxMD MEDLINE Link] .
Gilenya [package insert]. East Hanover, NJ: Novartis. September 2010.
Kappos L, Bar-Or A, Cree BAC, Fox RJ, Giovannoni G, Gold R, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet . 2018 Mar 31. 391 (10127):1263-1273. [QxMD MEDLINE Link] .
Comi G, Kappos L, Selmaj KW, Bar-Or A, Arnold DL, Steinman L, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol . 2019 Nov. 18 (11):1009-1020. [QxMD MEDLINE Link] .
Cohen JA, Comi G, Selmaj KW, Bar-Or A, Arnold DL, Steinman L, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol . 2019 Nov. 18 (11):1021-1033. [QxMD MEDLINE Link] .
Pucci E, Giuliani G, Solari A, et al. Natalizumab for relapsing remitting multiple sclerosis. Cochrane Database Syst Rev . 2011 Oct 5. CD007621. [QxMD MEDLINE Link] .
Tysabri [package insert]. South San Francisco, CA: Biogen Idec Inc. 2011.
Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet . 2012 Nov 24. 380(9856):1819-28. [QxMD MEDLINE Link] .
Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet . 2012 Nov 24. 380(9856):1829-39. [QxMD MEDLINE Link] .
Coles AJ, Fox E, Vladic A, Gazda SK, Brinar V, Selmaj KW, et al. Alemtuzumab more effective than interferon ß-1a at 5-year follow-up of CAMMS223 clinical trial. Neurology . 2012 Apr 3. 78(14):1069-78. [QxMD MEDLINE Link] .
Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med . 2017 Jan 19. 376 (3):221-234. [QxMD MEDLINE Link] .
Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med . 2017 Jan 19. 376 (3):209-220. [QxMD MEDLINE Link] .
Steinman L, Fox E, Hartung HP, Alvarez E, Qian P, Wray S, et al. Ublituximab versus Teriflunomide in Relapsing Multiple Sclerosis. N Engl J Med . 2022 Aug 25. 387 (8):704-714. [QxMD MEDLINE Link] .
Copaxone [package insert] [package insert]. North Wales, PA: Teva Pharmaceuticals USA. February 2009.
Novantrone [package insert]. Rockland, MA: Serono, Inc. May 2012.
Aubagio (teriflunomide) [package insert]. Cambridge, MA: Genentech Corp. September, 2012. Available at [Full Text] .
Jeffrey S. FDA approves third oral agent for MS. March 27, 2013. Medscape Medical News. Available at https://www.medscape.com/viewarticle/781450 . Accessed: April 2, 2013.
US Food and Drug Administration. FDA approves new multiple sclerosis treatment: Tecfidera. March 27, 2013. Available at https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm345528.htm . Accessed: April 2, 2013.
Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med . 2012 Sep 20. 367(12):1098-107. [QxMD MEDLINE Link] . [Full Text] .
Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med . 2012 Sep 20. 367(12):1087-97. [QxMD MEDLINE Link] . [Full Text] .
Leist TP, Comi G, Cree BA, Coyle PK, Freedman MS, Hartung HP, et al. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurol . 2014 Mar. 13 (3):257-67. [QxMD MEDLINE Link] .
Giovannoni G, Soelberg Sorensen P, Cook S, Rammohan K, Rieckmann P, Comi G, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult Scler . 2018 Oct. 24 (12):1594-1604. [QxMD MEDLINE Link] .
Jeffrey S. FDA Approves Interferon Autoinjector for MS. Available at https://www.medscape.com/viewarticle/777065 . Accessed: February 20, 2013.
Windhagen A, Newcombe J, Dangond F, et al. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J Exp Med . 1995 Dec 1. 182(6):1985-96. [QxMD MEDLINE Link] . [Full Text] .
Huan J, Culbertson N, Spencer L, et al. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res . 2005 Jul 1. 81(1):45-52. [QxMD MEDLINE Link] .
Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev . 2008 Jun. 223:87-113. [QxMD MEDLINE Link] . [Full Text] .
Minagar A, Jy W, Jimenez JJ, et al. Elevated plasma endothelial microparticles in multiple sclerosis. Neurology . 2001 May 22. 56(10):1319-24. [QxMD MEDLINE Link] .
Trapp BD, Vignos M, Dudman J, Chang A, Fisher E, Staugaitis SM, et al. Cortical neuronal densities and cerebral white matter demyelination in multiple sclerosis: a retrospective study. Lancet Neurol . 2018 Aug 21. [QxMD MEDLINE Link] .
Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med . 2005 Aug 15. 202(4):473-7. [QxMD MEDLINE Link] . [Full Text] .
Nielsen NM, Westergaard T, Rostgaard K, et al. Familial risk of multiple sclerosis: a nationwide cohort study. Am J Epidemiol . 2005 Oct 15. 162(8):774-8. [QxMD MEDLINE Link] .
Nischwitz S, Muller-Myhsok B, Weber F. Risk conferring genes in multiple sclerosis. FEBS Lett . 2011 Dec 1. 585(23):3789-97. [QxMD MEDLINE Link] .
Yeo TW, De Jager PL, Gregory SG, et al. A second major histocompatibility complex susceptibility locus for multiple sclerosis. Ann Neurol . 2007 Mar. 61(3):228-36. [QxMD MEDLINE Link] . [Full Text] .
Salvetti M, Giovannoni G, Aloisi F. Epstein-Barr virus and multiple sclerosis. Curr Opin Neurol . 2009 Jun. 22(3):201-6. [QxMD MEDLINE Link] .
Kampman MT, Brustad M. Vitamin D: a candidate for the environmental effect in multiple sclerosis - observations from Norway. Neuroepidemiology . 2008. 30(3):140-6. [QxMD MEDLINE Link] .
Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA . 2006 Dec 20. 296(23):2832-8. [QxMD MEDLINE Link] .
Islam T, Gauderman WJ, Cozen W, Mack TM. Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology . 2007 Jul 24. 69(4):381-8. [QxMD MEDLINE Link] .
Zamboni P, Galeotti R, Menegatti E, et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry . 2009 Apr. 80(4):392-9. [QxMD MEDLINE Link] . [Full Text] .
Zivadinov R, Schirda C, Dwyer MG, et al. Chronic cerebrospinal venous insufficiency and iron deposition on susceptibility-weighted imaging in patients with multiple sclerosis: a pilot case-control study. Int Angiol . 2010 Apr. 29(2):158-75. [QxMD MEDLINE Link] .
Study To Evaluate Treating Chronic Cerebrospinal Venous Insufficiency (CCSVI) in Multiple Sclerosis Patients. Available at https://clinicaltrials.gov/ct2/show/NCT01089686 . Accessed: 10/4/2010.
Zamboni P, Galeotti R, Menegatti E, et al. A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency. J Vasc Surg . 2009 Dec. 50(6):1348-58.e1-3. [QxMD MEDLINE Link] .
Laupacis A, Lillie E, Dueck A, et al. Association between chronic cerebrospinal venous insufficiency and multiple sclerosis: a meta-analysis. CMAJ . 2011 Nov 8. 183(16):E1203-12. [QxMD MEDLINE Link] . [Full Text] .
Centers for Disease Control and Prevention. FAQs about Hepatitis B Vaccine (Hep B) and Multiple Sclerosis. [Full Text] .
National Multiple Sclerosis Society. Vaccination. Available at https://www.nationalmssociety.org/living-with-multiple-sclerosis/healthy-living/vaccinations/index.aspx . Accessed: November 17, 2011.
Noonan CW, Williamson DM, Henry JP, et al. The prevalence of multiple sclerosis in 3 US communities. Prev Chronic Dis . 2010 Jan. 7(1):A12. [QxMD MEDLINE Link] . [Full Text] .
National Multiple Sclerosis Society. Who Gets MS?. Available at https://www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-about-ms/who-gets-ms/index.aspx . Accessed: 10/04/2010.
Rosati G. The prevalence of multiple sclerosis in the world: an update. Neurol Sci . 2001 Apr. 22(2):117-39. [QxMD MEDLINE Link] .
Aguirre-Cruz L, Flores-Rivera J, De La Cruz-Aguilera DL, Rangel-Lopez E, Corona T. Multiple sclerosis in Caucasians and Latino Americans. Autoimmunity . 2011 Nov. 44(7):571-5. [QxMD MEDLINE Link] .
Matsuda PN, Shumway-Cook A, Bamer AM, Johnson SL, Amtmann D, Kraft GH. Falls in multiple sclerosis. PM R . 2011 Jul. 3(7):624-32; quiz 632. [QxMD MEDLINE Link] .
Roodhooft JM. Ocular problems in early stages of multiple sclerosis. Bull Soc Belge Ophtalmol . 2009. 65-8. [QxMD MEDLINE Link] .
Braley TJ, Chervin RD. Fatigue in multiple sclerosis: mechanisms, evaluation, and treatment. Sleep . 2010 Aug. 33(8):1061-7. [QxMD MEDLINE Link] .
Optic Neuritis Study Group. The clinical profile of optic neuritis. Experience of the Optic Neuritis Treatment Trial. Optic Neuritis Study Group. Arch Ophthalmol . 1991 Dec. 109(12):1673-8. [QxMD MEDLINE Link] .
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology . 1983 Nov. 33(11):1444-52. [QxMD MEDLINE Link] .
Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol . 2005 Dec. 58(6):840-6. [QxMD MEDLINE Link] .
Lonergan R, Kinsella K, Duggan M, Jordan S, Hutchinson M, Tubridy N. Discontinuing disease-modifying therapy in progressive multiple sclerosis: can we stop what we have started?. Mult Scler . 2009 Dec. 15(12):1528-31. [QxMD MEDLINE Link] .
Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med . 1998 Jan 29. 338(5):278-85. [QxMD MEDLINE Link] .
Prashanth LK, Taly AB, Sinha S, Arunodaya GR, Swamy HS. Wilson's disease: diagnostic errors and clinical implications. J Neurol Neurosurg Psychiatry . 2004 Jun. 75(6):907-9. [QxMD MEDLINE Link] . [Full Text] .
Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain . 1997 Nov. 120 ( Pt 11):2059-69. [QxMD MEDLINE Link] .
Bonhomme GR, Waldman AT, Balcer LJ, et al. Pediatric optic neuritis: brain MRI abnormalities and risk of multiple sclerosis. Neurology . 2009 Mar 10. 72(10):881-5. [QxMD MEDLINE Link] .
Filippi M. Enhanced magnetic resonance imaging in multiple sclerosis. Mult Scler . 2000 Oct. 6(5):320-6. [QxMD MEDLINE Link] .
Filippi M, Bozzali M, Horsfield MA, et al. A conventional and magnetization transfer MRI study of the cervical cord in patients with MS. Neurology . 2000 Jan 11. 54(1):207-13. [QxMD MEDLINE Link] .
Filippi M, Yousry TA, Alkadhi H, Stehling M, Horsfield MA, Voltz R. Spinal cord MRI in multiple sclerosis with multicoil arrays: a comparison between fast spin echo and fast FLAIR. J Neurol Neurosurg Psychiatry . 1996 Dec. 61(6):632-5. [QxMD MEDLINE Link] . [Full Text] .
Grossman RI, Barkhof F, Filippi M. Assessment of spinal cord damage in MS using MRI. J Neurol Sci . 2000 Jan 15. 172 Suppl 1:S36-9. [QxMD MEDLINE Link] .
Neema M, Goldberg-Zimring D, Guss ZD, et al. 3 T MRI relaxometry detects T2 prolongation in the cerebral normal-appearing white matter in multiple sclerosis. Neuroimage . 2009 Jul 1. 46(3):633-41. [QxMD MEDLINE Link] . [Full Text] .
Poonawalla AH, Hou P, Nelson FA, Wolinsky JS, Narayana PA. Cervical Spinal Cord Lesions in Multiple Sclerosis: T1-weighted Inversion-Recovery MR Imaging with Phase-Sensitive Reconstruction. Radiology . 2008 Jan. 246(1):258-264. [QxMD MEDLINE Link] .
Stankiewicz JM, Glanz BI, Healy BC, et al. Brain MRI lesion load at 1.5T and 3T versus clinical status in multiple sclerosis. J Neuroimaging . 2011 Apr. 21(2):e50-6. [QxMD MEDLINE Link] . [Full Text] .
Vaneckova M, Seidl Z, Krasensky J, et al. Patients' stratification and correlation of brain magnetic resonance imaging parameters with disability progression in multiple sclerosis. Eur Neurol . 2009. 61(5):278-84. [QxMD MEDLINE Link] .
Wattjes MP, Barkhof F. High field MRI in the diagnosis of multiple sclerosis: high field-high yield?. Neuroradiology . 2009 May. 51(5):279-92. [QxMD MEDLINE Link] .
[Guideline] Traboulsee, A. et al. Revised Recommendations of the CMSC Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-up of Multiple Sclerosis. Consortim of Multiple Sclerosis Centers. Available at https://c.ymcdn.com/sites/www.mscare.org/resource/collection/9C5F19B9-3489-48B0-A54B-623A1ECEE07B/MRIprotocol2015.pdf . Accessed: August 13, 2015.
Agosta F, Absinta M, Sormani MP, et al. In vivo assessment of cervical cord damage in MS patients: a longitudinal diffusion tensor MRI study. Brain . 2007 Aug. 130:2211-9. [QxMD MEDLINE Link] .
Fazekas F, Offenbacher H, Fuchs S, et al. Criteria for an increased specificity of MRI interpretation in elderly subjects with suspected multiple sclerosis. Neurology . 1988 Dec. 38(12):1822-5. [QxMD MEDLINE Link] .
Zivadinov R, Tavazzi E, Bergsland N, Hagemeier J, Lin F, Dwyer MG, et al. Brain Iron at Quantitative MRI Is Associated with Disability in Multiple Sclerosis. Radiology . 2018 Jul 17. 180136. [QxMD MEDLINE Link] .
Colorado RA, Shukla K, Zhou Y, Wolinsky JS, Narayana PA. Multi-task functional MRI in multiple sclerosis patients without clinical disability. Neuroimage . 2012 Jan 2. 59(1):573-81. [QxMD MEDLINE Link] . [Full Text] .
Wang J, Xiao Y, Luo M, Zhang X, Luo H. Statins for multiple sclerosis. Cochrane Database Syst Rev . 2010 Dec 8. CD008386. [QxMD MEDLINE Link] .
Arnold DL, Matthews PM, Francis G, Antel J. Proton magnetic resonance spectroscopy of human brain in vivo in the evaluation of multiple sclerosis: assessment of the load of disease. Magn Reson Med . 1990 Apr. 14(1):154-9. [QxMD MEDLINE Link] .
Henning A, Schar M, Kollias SS, Boesiger P, Dydak U. Quantitative magnetic resonance spectroscopy in the entire human cervical spinal cord and beyond at 3T. Magn Reson Med . 2008 Jun. 59(6):1250-8. [QxMD MEDLINE Link] .
Marliani AF, Clementi V, Albini-Riccioli L, Agati R, Leonardi M. Quantitative proton magnetic resonance spectroscopy of the human cervical spinal cord at 3 Tesla. Magn Reson Med . 2007 Jan. 57(1):160-3. [QxMD MEDLINE Link] .
Berg D, Maurer M, Warmuth-Metz M, Rieckmann P, Becker G. The correlation between ventricular diameter measured by transcranial sonography and clinical disability and cognitive dysfunction in patients with multiple sclerosis. Arch Neurol . 2000 Sep. 57(9):1289-92. [QxMD MEDLINE Link] .
Walter U, Wagner S, Horowski S, Benecke R, Zettl UK. Transcranial brain sonography findings predict disease progression in multiple sclerosis. Neurology . 2009 Sep 29. 73(13):1010-7. [QxMD MEDLINE Link] .
Vazquez-Marrufo M, Gonzalez-Rosa JJ, Vaquero E, et al. Quantitative electroencephalography reveals different physiological profiles between benign and remitting-relapsing multiple sclerosis patients. BMC Neurol . 2008 Nov 24. 8:44. [QxMD MEDLINE Link] . [Full Text] .
Jeffrey S. TOPIC: Teriflunomide Delays Clinically Definite MS. Medscape Medical News. Available at https://www.medscape.com/viewarticle/803177 . Accessed: May 8, 2013.
Rodriguez M, Karnes WE, Bartleson JD, Pineda AA. Plasmapheresis in acute episodes of fulminant CNS inflammatory demyelination. Neurology . 1993 Jun. 43(6):1100-4. [QxMD MEDLINE Link] .
Spelman T, Mekhael L, Burke T, Butzkueven H, Hodgkinson S, Havrdova E, et al. Risk of early relapse following the switch from injectables to oral agents for multiple sclerosis. Eur J Neurol . 2016 Jan 19. [QxMD MEDLINE Link] .
Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology . 1993 Apr. 43(4):655-61. [QxMD MEDLINE Link] .
Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol . 1996 Mar. 39(3):285-94. [QxMD MEDLINE Link] .
Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet . 1998 Nov 7. 352(9139):1498-504. [QxMD MEDLINE Link] .
Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon beta-1a treatment regimens in MS: The EVIDENCE Trial. Neurology . 2002 Nov 26. 59(10):1496-506. [QxMD MEDLINE Link] .
Schwid SR, Panitch HS. Full results of the Evidence of Interferon Dose-Response-European North American Comparative Efficacy (EVIDENCE) study: a multicenter, randomized, assessor-blinded comparison of low-dose weekly versus high-dose, high-frequency interferon beta-1a for relapsing multiple sclerosis. Clin Ther . 2007 Sep. 29(9):2031-48. [QxMD MEDLINE Link] .
Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology . 1995 Jul. 45(7):1268-76. [QxMD MEDLINE Link] .
Johnson KP, Brooks BR, Ford CC, et al. Sustained clinical benefits of glatiramer acetate in relapsing multiple sclerosis patients observed for 6 years. Copolymer 1 Multiple Sclerosis Study Group. Mult Scler . 2000 Aug. 6(4):255-66. [QxMD MEDLINE Link] .
Khan O, Rieckmann P, Boyko A, Selmaj K, Zivadinov R. Three times weekly glatiramer acetate in relapsing-remitting multiple sclerosis. Ann Neurol . 2013 Jun. 73(6):705-13. [QxMD MEDLINE Link] .
Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med . 2006 Mar 2. 354(9):899-910. [QxMD MEDLINE Link] .
Cadavid D, Jurgensen S, Lee S. Impact of natalizumab on ambulatory improvement in secondary progressive and disabled relapsing-remitting multiple sclerosis. PLoS One . 2013. 8(1):e53297. [QxMD MEDLINE Link] . [Full Text] .
Chun J, Brinkmann V. A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya). Discov Med . 2011 Sep. 12(64):213-28. [QxMD MEDLINE Link] .
Hughes S. Shorter washout reduces MS relapse switching off natalizumab. Medscape Medical News . October 7, 2013. [Full Text] .
Hughes S. Shorter Washout Better for Natalizumab-to-Fingolimod Switch. Medscape Medical News. Available at https://www.medscape.com/viewarticle/822567 . Accessed: April 1, 2014.
Cohen M, Maillart E, Tourbah A, De Sèze J, Vukusic S, Brassat D, et al. Switching From Natalizumab to Fingolimod in Multiple Sclerosis: A French Prospective Study. JAMA Neurol . 2014 Feb 24. [QxMD MEDLINE Link] .
Weber MS, Kappos L, Hauser SL, et al. The Patient Impact of 10 Years of Ocrelizumab Treatment in Multiple Sclerosis: Long-Term Data from the Phase III OPERA and ORATORIO Studies. Presented at the 9th Joint European Committee for Treatment and Research in MS - Americas Committee for Treatment and Research in MS Meeting in Milan; October 11-13, 2023. ECTRIMS-ACTRIMS Poster #P302. Available at https://medically.gene.com/global/en/unrestricted/neuroscience/ECTRIMS-2023/ectrims-2023-poster-weber-the-patient-impact-of-10-year.html .
O'Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med . 2011 Oct 6. 365(14):1293-303. [QxMD MEDLINE Link] .
Semedo, D. Aubagio (Teriflunomide) Slows Brain Atrophy in Patients with Relapsing Multiple Sclerosis. Multiple Sclerosis News Today. Available at https://multiplesclerosisnewstoday.com/2015/10/08/aubagio-teriflunomide-slows-brain-atrophy-patients-relapsing-multiple-sclerosis/ . October 8, 2015; Accessed: October 14, 2015.
A study comparing the effectiveness and safety of teriflunomide and interferon beta-1a in patients with relapsing multiple sclerosis (TENERE). 4th Cooperative Meeting of the Consortium of Multiple Sclerosis Centers (CMSC)/Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS). June 2, 2012 (ClinicalTrials.gov identifier: NCT00883337).
A multicenter double-blind parallel-group placebo-controlled study of the efficacy and safety of teriflunomide in patients with relapsing multiple sclerosis who are treated with interferon-beta. (ClinicalTrials.gov identifier: NCT01252355).
Fox EJ, Sullivan HC, Gazda SK, et al. A single-arm, open-label study of alemtuzumab in treatment-refractory patients with multiple sclerosis. Eur J Neurol . 2012 Feb. 19(2):307-11. [QxMD MEDLINE Link] .
Anderson P. Alemtuzumab Benefits Hard-to-Treat MS Patients. Medscape Medical News. Available at https://www.medscape.com/viewarticle/805173 . Accessed: June 12, 2013.
Brauser, D. Ocrelizumab Linked to Improved Visual Outcomes in Relapsing MS. Medscape Medical News. Available at https://www.medscape.com/viewarticle/887722 . October 27, 2017; Accessed: October 27, 2017.
Kesimpta [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation. August 2020. Available at [Full Text] .
Harrison DM, Gladstone DE, Hammond E, et al. Treatment of relapsing-remitting multiple sclerosis with high-dose cyclophosphamide induction followed by glatiramer acetate maintenance. Mult Scler . 2012 Feb. 18(2):202-9. [QxMD MEDLINE Link] .
Rojas JI, Romano M, Ciapponi A, Patrucco L, Cristiano E. Interferon beta for primary progressive multiple sclerosis. Cochrane Database Syst Rev . 2009 Jan 21. CD006643. [QxMD MEDLINE Link] .
Goodkin DE, Rudick RA, VanderBrug Medendorp S, et al. Low-dose (7.5 mg) oral methotrexate reduces the rate of progression in chronic progressive multiple sclerosis. Ann Neurol . 1995 Jan. 37(1):30-40. [QxMD MEDLINE Link] .
Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med . 2010 Feb 4. 362(5):387-401. [QxMD MEDLINE Link] .
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med . 2010 Feb 4. 362(5):402-15. [QxMD MEDLINE Link] .
Khatri B, Barkhof F, Comi G, et al. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol . 2011 Jun. 10(6):520-9. [QxMD MEDLINE Link] .
Killestein J, Rudick RA, Polman CH. Oral treatment for multiple sclerosis. Lancet Neurol . 2011 Nov. 10(11):1026-34. [QxMD MEDLINE Link] .
Multiple Sclerosis Association of America (MSAA). MS Research Update. Available at https://mymsaa.org/PDFs/MSAA_Research_Update_2013.pdf . Accessed: March 27, 2013.
Anderson P. Myelin peptide skin patch safe, reduces MS activity. Medscape Medical News . July 29, 2013. [Full Text] .
Walczak A, Siger M, Ciach A, Szczepanik M, Selmaj K. Transdermal application of myelin peptides in multiple sclerosis treatment. JAMA Neurol . 2013 Jul 1. 1-6. [QxMD MEDLINE Link] .
Muraro PA, Pasquini M, Atkins HL, Bowen JD, Farge D, et al. Long-term Outcomes After Autologous Hematopoietic Stem Cell Transplantation for Multiple Sclerosis. JAMA Neurol . 2017 Feb 20. [QxMD MEDLINE Link] .
Herman AO. "Unprecedented" Findings for Stem Cell Therapy in MS. NEJM Journal Watch. Available at https://www.jwatch.org/fw113985/2018/03/21/unprecedented-findings-stem-cell-therapy-ms . March 21, 2018; Accessed: March 28, 2018.
Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med . 1998 Jul 30. 339(5):285-91. [QxMD MEDLINE Link] .
Tsui A, Lee MA. Multiple sclerosis and pregnancy. Curr Opin Obstet Gynecol . 2011 Dec. 23(6):435-9. [QxMD MEDLINE Link] .
Krupp LB, Christodoulou C, Melville P, et al. Multicenter randomized clinical trial of donepezil for memory impairment in multiple sclerosis. Neurology . 2011 Apr 26. 76(17):1500-7. [QxMD MEDLINE Link] . [Full Text] .
Attarian HP, Brown KM, Duntley SP, Carter JD, Cross AH. The relationship of sleep disturbances and fatigue in multiple sclerosis. Arch Neurol . 2004 Apr. 61(4):525-8. [QxMD MEDLINE Link] .
MacAllister WS, Krupp LB. Multiple sclerosis-related fatigue. Phys Med Rehabil Clin N Am . 2005 May. 16(2):483-502. [QxMD MEDLINE Link] .
Solaro C, Uccelli MM. Management of pain in multiple sclerosis: a pharmacological approach. Nat Rev Neurol . 2011 Aug 16. 7(9):519-27. [QxMD MEDLINE Link] .
Goodman AD, Brown TR, Krupp LB, et al. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet . 2009 Feb 28. 373(9665):732-8. [QxMD MEDLINE Link] .
Ampyra [package insert]. Hawthorne, NY: Acorda Therapeutics, Inc. 2010.
Nicholas RS, Friede T, Hollis S, Young CA. Anticholinergics for urinary symptoms in multiple sclerosis. Cochrane Database Syst Rev . 2009 Jan 21. CD004193. [QxMD MEDLINE Link] .
US Food and Drug Administration. FDA approves Botox to treat specific form of urinary incontinence. August 25, 2011. Available at https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm269509.htm . Accessed: November 28, 2011.
Beck RW, Cleary PA, Anderson MM Jr, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med . 1992 Feb 27. 326(9):581-8. [QxMD MEDLINE Link] .
Myhr KM. Vitamin D treatment in multiple sclerosis. J Neurol Sci . 2009 Nov 15. 286(1-2):104-8. [QxMD MEDLINE Link] .
Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. November 30, 2010. Available at https://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D.aspx . Accessed: December 29, 2011.
Summerday NM, Brown SJ, Allington DR, Rivey MP. Vitamin D and multiple sclerosis: review of a possible association. J Pharm Pract . 2012 Feb. 25(1):75-84. [QxMD MEDLINE Link] .
Jagannath VA, Fedorowicz Z, Asokan GV, Robak EW, Whamond L. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst Rev . 2010 Dec 8. CD008422. [QxMD MEDLINE Link] .
DeStefano F, Verstraeten T, Jackson LA, et al. Vaccinations and risk of central nervous system demyelinating diseases in adults. Arch Neurol . 2003 Apr. 60(4):504-9. [QxMD MEDLINE Link] .
Confavreux C, Suissa S, Saddier P, Bourdès V, Vukusic S. Vaccinations and the risk of relapse in multiple sclerosis. Vaccines in Multiple Sclerosis Study Group. N Engl J Med . 2001 Feb 1. 344(5):319-26. [QxMD MEDLINE Link] .
Farez MF, Correale J. Yellow fever vaccination and increased relapse rate in travelers with multiple sclerosis. Arch Neurol . 2011 Oct. 68(10):1267-71. [QxMD MEDLINE Link] .
Rovira À, Wattjes MP, Tintoré M, Tur C, Yousry TA, Sormani MP, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol . 2015 Aug. 11 (8):471-82. [QxMD MEDLINE Link] .
[Guideline] Filippi M, Rocca A, Arnold DL, Bakshi R, Barkhof F, De Stefano N, et al. Use of Imaging in Multiple Sclerosis. Gilhus NE, Barnes MP, Brainin M. European Handbook of Neurological Management . 2nd ed. Oxford (UK): Wiley-Blackwell; 2011. Vol 1: 35-51.
Wattjes MP, Rovira À, Miller D, Yousry TA, Sormani MP, de Stefano MP, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis--establishing disease prognosis and monitoring patients. Nat Rev Neurol . 2015 Oct. 11 (10):597-606. [QxMD MEDLINE Link] .
[Guideline] Multiple Sclerosis Coalition. The Use of Disease-Modifying Therapies in Multiple Sclerosis: Principles and Current Evidence: A Consensus Paper. The Consortium of Multiple Sclerosis Centers. Available at https://www.mscare.org/?page=dmt . July 2014;
Hughes, S. European MS Treatment Guidelines Released. Medscape Medical News. Available at https://www.medscape.com/viewarticle/887730 . October 27, 2017; Accessed: October 27, 2017.
Jeffrey S. New AAN Guidelines Advocate Early MS Treatment. Medscape Medical News. Available at https://www.medscape.com/viewarticle/895598 . April 23, 2018; Accessed: April 23, 2018.
Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology . 2018 Apr 24. 90 (17):777-788. [QxMD MEDLINE Link] .
Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, et al. Comprehensive systematic review summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology . 2018 Apr 24. 90 (17):789-800. [QxMD MEDLINE Link] .
[Guideline] Farez MF, Correale J, Armstrong MJ, Rae-Grant A, Gloss D, Donley D, et al. Practice guideline update summary: Vaccine-preventable infections and immunization in multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology . 2019 Sep 24. 93 (13):584-594. [QxMD MEDLINE Link] .
Azasan [package insert] [package insert]. Wilmington, NC: Salix pharmaceuticals Inc. August 2011.
Cyclophosphamide [package insert]. Deerfield, IL: Baxter Healthcare Corporation. June 2004.
Brooks M. New AAN guideline on psychiatric disorders in MS. Medscape Medical News . January 3, 2014. [Full Text] .
Hughes S. New Test to Identify PML Risk With Natalizumab in MS. Medscape Medical News. Available at https://www.medscape.com/viewarticle/832504 . Accessed: October 7, 2014.
Jeffrey S. No Cognitive Disadvantage in Pediatric- vs Adult-Onset MS. Medscape Medical News. Available at https://www.medscape.com/viewarticle/831536 . Accessed: September 15, 2014.
Keller DM. Fingolimod Reduces Annual Brain Volume Loss in MS. Medscape Medical News . Jun 6 2014. [Full Text] .
Minden SL, Feinstein A, Kalb RC, Miller D, Mohr DC, Patten SB, et al. Evidence-based guideline: Assessment and management of psychiatric disorders in individuals with MS: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology . 2013 Dec 27. [QxMD MEDLINE Link] .
- The mechanism of demyelination in multiple sclerosis may be activation of myelin-reactive T cells in the periphery, which then express adhesion molecules, allowing their entry through the blood-brain barrier (BBB). T cells are activated following antigen presentation by antigen-presenting cells such as macrophages and microglia, or B cells. Perivascular T cells can secrete proinflammatory cytokines, including interferon gamma and tumor necrosis factor alpha. Antibodies against myelin also may be generated in the periphery or intrathecally. Ongoing inflammation leads to epitope spread and recruitment of other inflammatory cells (ie, bystander activation). The T cell receptor recognizes antigen in the context of human leukocyte antigen molecule presentation and also requires a second event (ie, co-stimulatory signal via the B7-CD28 pathway, not shown) for T cell activation to occur. Activated microglia may release free radicals, nitric oxide, and proteases that may contribute to tissue damage.
- MRI of the head of a 35-year-old man with relapsing-remitting multiple sclerosis. MRI reveals multiple lesions with high T2 signal intensity and one large white matter lesion. These demyelinating lesions may sometimes mimic brain tumors because of the associated edema and inflammation.
- MRI of the head of a 35-year-old man with relapsing-remitting multiple sclerosis. This MRI, performed 3 months after the one in the related image, shows a dramatic decrease in the size of lesions.
- Inflammation in multiple sclerosis. Hematoxylin and eosin (H&E) stain shows perivascular infiltration of inflammatory cells. These infiltrates are composed of activated T cells, B cells, and macrophages.
- Demyelination in multiple sclerosis. Luxol fast blue (LFB)/periodic acid-Schiff (PAS) stain confers an intense blue to myelin. Loss of myelin is demonstrated in this chronic plaque. Note that absence of inflammation may be demonstrated at the edge of chronic lesions.
- Gadolinium-enhanced, T1-weighted image showing enhancement of the left optic nerve (arrow).
- Corresponding axial images of the spinal cord showing enhancing plaque (arrow). The combination of optic neuritis and longitudinally extensive spinal cord lesions constitutes Devic neuromyelitis optica.
- Table 1. 2017 Revised McDonald Criteria for the Diagnosis of Multiple Sclerosis [ 1 ]
|
|
None; clinical evidence will suffice. Additional evidence (eg, brain MRI) desirable, but must be consistent with MS | |
Dissemination in space demonstrated by MRI Await further clinical attack implicating a different site | |
Dissemination in time demonstrated by MRI second clinical attack demonstration of CSF-specific oligoclonal bands | |
Dissemination in space demonstrated by MRI await a second clinical attack implicating a different CNS site
Dissemination in time, demonstrated by MRI or second clinical attack | |
· Insidious neurologic progression suggestive of MS | One year of disease progression and dissemination in space, demonstrated by 2 of the following: |
Notes: An attack is defined as a neurologic disturbance of the kind seen in MS. It can be documented by subjective report or by objective observation, but it must last for at least 24 hours. Pseudoattacks and single paroxysmal episodes must be excluded. To be considered separate attacks, at least 30 days must elapse between onset of one event and onset of another event. | |

Contributor Information and Disclosures
Christopher Luzzio, MD Clinical Assistant Professor, Department of Neurology, University of Wisconsin at Madison School of Medicine and Public Health Christopher Luzzio, MD is a member of the following medical societies: American Academy of Neurology Disclosure: Nothing to disclose.
Fernando Dangond, MD, FAAN Head of US Medical Affairs, Neurodegenerative Diseases, EMD Serono, Inc Fernando Dangond, MD, FAAN is a member of the following medical societies: American Academy of Neurology , American Medical Association Disclosure: Received salary from EMD Serono, Inc. for employment.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference Disclosure: Received salary from Medscape for employment. for: Medscape.
Jasvinder Chawla, MD, MBA Chief of Neurology, Hines Veterans Affairs Hospital; Professor of Neurology, Loyola University Medical Center Jasvinder Chawla, MD, MBA is a member of the following medical societies: American Academy of Neurology , American Association of Neuromuscular and Electrodiagnostic Medicine , American Clinical Neurophysiology Society , American Medical Association Disclosure: Nothing to disclose.
Martin K Childers, DO, PhD Professor, Department of Neurology, Wake Forest University School of Medicine; Professor, Rehabilitation Program, Institute for Regenerative Medicine, Wake Forest Baptist Medical Center
Martin K Childers, DO, PhD is a member of the following medical societies: American Academy of Physical Medicine and Rehabilitation , American Congress of Rehabilitation Medicine , American Osteopathic Association , Christian Medical & Dental Society , and Federation of American Societies for Experimental Biology
Disclosure: Allergan pharma Consulting fee Consulting
Edmond A Hooker II, MD, DrPH, FAAEM Assistant Professor, Department of Emergency Medicine, University of Cincinnati College of Medicine
Edmond A Hooker II, MD, DrPH, FAAEM is a member of the following medical societies: American Academy of Emergency Medicine , American Public Health Association , Society for Academic Emergency Medicine , and Southern Medical Association
Disclosure: Nothing to disclose.
J Stephen Huff, MD Associate Professor of Emergency Medicine and Neurology, Department of Emergency Medicine, University of Virginia School of Medicine
J Stephen Huff, MD is a member of the following medical societies: American Academy of Emergency Medicine , American Academy of Neurology , American College of Emergency Physicians , and Society for Academic Emergency Medicine
Marjorie Lazoff, MD Editor-in-Chief, Medical Computing Review
Marjorie Lazoff, MD is a member of the following medical societies: Alpha Omega Alpha , American College of Emergency Physicians , American Medical Informatics Association , and Society for Academic Emergency Medicine
Consuelo T Lorenzo, MD Physiatrist, Department of Physical Medicine and Rehabilitation, Alegent Health, Immanuel Rehabilitation Center
Consuelo T Lorenzo, MD is a member of the following medical societies: American Academy of Physical Medicine and Rehabilitation
William J Nowack, MD Associate Professor, Epilepsy Center, Department of Neurology, University of Kansas Medical Center
William J Nowack, MD is a member of the following medical societies: American Academy of Neurology , American Clinical Neurophysiology Society , American Epilepsy Society , American Medical Electroencephalographic Association, American Medical Informatics Association , and Biomedical Engineering Society
Richard Salcido, MD Chairman, Erdman Professor of Rehabilitation, Department of Physical Medicine and Rehabilitation, University of Pennsylvania School of Medicine
Richard Salcido, MD is a member of the following medical societies: American Academy of Pain Medicine , American Academy of Physical Medicine and Rehabilitation , American College of Physician Executives , American Medical Association , and American Paraplegia Society
Daniel D Scott, MD, MA Associate Professor, Department of Physical Medicine and Rehabilitation, University of Colorado School of Medicine; Attending Physician, Department of Physical Medicine and Rehabilitation, Denver Veterans Affairs Medical Center, Eastern Colorado Health Care System
Daniel D Scott, MD, MA is a member of the following medical societies: Alpha Omega Alpha , American Academy of Physical Medicine and Rehabilitation , American Association of Neuromuscular and Electrodiagnostic Medicine , American Paraplegia Society , Association of Academic Physiatrists , National Multiple Sclerosis Society , and Physiatric Association of Spine, Sports and Occupational Rehabilitation
Fu-Dong Shi, MD, PhD Director of Neuroimmunology Laboratory, Barrow Neurological Institute, St Joseph's Hospital and Medical Center
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
Florian P Thomas, MD, MA, PhD, Drmed Director, Spinal Cord Injury Unit, St Louis Veterans Affairs Medical Center; Director, National MS Society Multiple Sclerosis Center; Director, Neuropathy Association Center of Excellence, Professor, Department of Neurology and Psychiatry, Associate Professor, Institute for Molecular Virology, and Department of Molecular Microbiology and Immunology, St Louis University School of Medicine
Florian P Thomas, MD, MA, PhD, Drmed is a member of the following medical societies: American Academy of Neurology , American Neurological Association , American Paraplegia Society , Consortium of Multiple Sclerosis Centers , and National Multiple Sclerosis Society
Timothy Vollmer, MD Consulting Staff, Department of Emergency Medicine, Geisinger Medical Center
Sandra F Williamson, MS, ANP-C, CRRN Clinic Coordinator, Department of Rehabilitation Medicine, Denver Veterans Affairs Medical Center
Sandra F Williamson, MS, ANP-C, CRRN is a member of the following medical societies: Phi Beta Kappa , Phi Kappa Phi, and Sigma Theta Tau International
What would you like to print?
- Print this section
- Print the entire contents of
- Print the entire contents of article

- Pediatric Multiple Sclerosis
- Neuro-Ophthalmologic Manifestations of Multiple Sclerosis
- Multiple Sclerosis Spine Imaging
- Brain Imaging in Multiple Sclerosis
- The Patient's Journey Through Multiple Sclerosis
- Biomarker Testing in Multiple Sclerosis
- Migraine a Forerunner of Multiple Sclerosis?
- Frexalimab Promising for Relapsing Multiple Sclerosis
- US FDA Declines to Approve Viatris's Injection for Multiple Sclerosis

- Drug Interaction Checker
- Pill Identifier
- Calculators

CKS is only available in the UK
The NICE Clinical Knowledge Summaries (CKS) site is only available to users in the UK, Crown Dependencies and British Overseas Territories.
CKS content is produced by Clarity Informatics Ltd (trading as Agilio Software | Primary Care). It is available to users outside the UK via subscription from the Agilio | Prodigy website .

If you believe you are seeing this page in error please contact us .
- Patient Care & Health Information
- Diseases & Conditions
- Multiple sclerosis
Neurological exam
A complete neurological exam and medical history are needed to diagnose MS .
- Multiple sclerosis FAQs
Neurologist Oliver Tobin, M.B., B.Ch., B.A.O., Ph.D., answers the most frequently asked questions about multiple sclerosis.
So people who are overweight have a higher chance of developing MS and people who have MS who are overweight tend to have more active disease and a faster onset of progression. The main diet has been shown to be neuroprotective is the Mediterranean diet. This diet is high in fish, vegetables, and nuts, and low in red meat.
So this question comes up a lot because patients who have multiple sclerosis can sometimes get a transient worsening of their symptoms in heat or if they exercise strenuously. The important thing to note is that heat does not cause an MS attack or MS relapse. And so it's not dangerous. You're not doing any permanent damage if this occurs. Exercise is strongly recommended and is protective to the brain and spinal cord.
Scientists do not yet know which stem cells are beneficial in MS, what route to give them or what dose to give them or what frequency. So at the moment, stem cell treatments are not recommended outside of the context of a clinical trial.
Neuromyelitis optica spectrum disorder or NMOSD and MOG-associated disorder can give features similar to multiple sclerosis. These are more common in people of Asian or African-American ethnicity. And your doctor may recommend blood tests to exclude these disorders.
Well, the first drug approved by the FDA for treatment of multiple sclerosis was in 1993. Since then, over 20 drugs have become available for treatment of MS. And the potency of these drugs has increased over time to the point where we can almost completely suppress the inflammatory component of the disease. This would not be possible if patients like you did not enroll in research studies. There are many different types of research studies, not just drug trials, but also observational studies, as all of these enhance our understanding of the disease, hopefully to lead to even better cures for multiple sclerosis.
Well, the most important thing about having a diagnosis of multiple sclerosis is that you are at the center of your medical team. A comprehensive MS center is the best place for management of multiple sclerosis, and this typically includes physicians with expertise in multiple sclerosis, neurologists, but also urologists, physiatrists or physical medicine and rehabilitation providers, psychologists, and many other providers who have specialty interest in multiple sclerosis. Engaging this team around you and your particular needs will improve your outcomes over time.
There are no specific tests for MS . Instead, a diagnosis of multiple sclerosis often relies on ruling out other conditions that might produce similar signs and symptoms, known as a differential diagnosis.
Your doctor is likely to start with a thorough medical history and examination.
Lumbar puncture, also known as a spinal tap
During a lumbar puncture, also known as a spinal tap, you typically lie on your side with your knees drawn up to your chest. Then a needle is inserted into your spinal canal — in your lower back — to collect cerebrospinal fluid for testing.
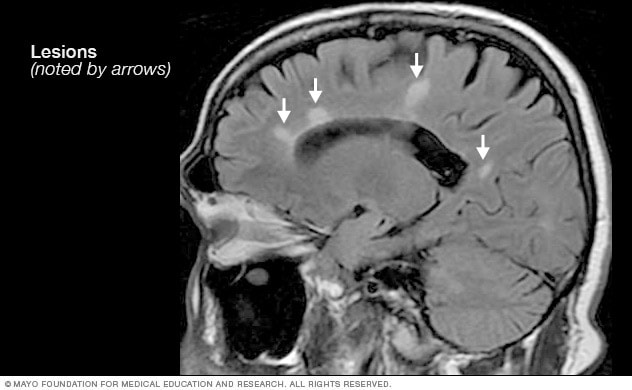
- MRI multiple sclerosis lesions
Brain MRI scan showing white lesions associated with multiple sclerosis.
Your doctor may then recommend:
- Blood tests, to help rule out other diseases with symptoms like MS . Tests to check for specific biomarkers associated with MS are currently under development and may also aid in diagnosing the disease.
- Spinal tap (lumbar puncture), in which a small sample of cerebrospinal fluid is removed from your spinal canal for laboratory analysis. This sample can show abnormalities in antibodies that are associated with MS . A spinal tap can also help rule out infections and other conditions with symptoms like MS . A new antibody test (for kappa free light chains) may be faster and less expensive than previous spinal fluid tests for multiple sclerosis.
- MRI, which can reveal areas of MS (lesions) on your brain, cervical and thoracic spinal cord. You may receive an intravenous injection of a contrast material to highlight lesions that indicate your disease is in an active phase.
- Evoked potential tests that record the electrical signals produced by your nervous system in response to stimuli may be done. An evoked potential test may use visual stimuli or electrical stimuli. In these tests, you watch a moving visual pattern, as short electrical impulses are applied to nerves in your legs or arms. Electrodes measure how quickly the information travels down your nerve pathways.
In most people with relapsing-remitting MS , the diagnosis is straightforward and based on a pattern of symptoms consistent with the disease and confirmed by brain imaging scans, such as an MRI.
Diagnosing MS can be more difficult in people with unusual symptoms or progressive disease. In these cases, further testing with spinal fluid analysis, evoked potentials and additional imaging may be needed.
Brain MRI is often used to help diagnose multiple sclerosis.
- Care at Mayo Clinic
Our caring team of Mayo Clinic experts can help you with your multiple sclerosis-related health concerns Start Here
More Information
Multiple sclerosis care at Mayo Clinic
- Lumbar puncture (spinal tap)
- Explaining multiple sclerosis
There is no cure for multiple sclerosis. Treatment typically focuses on speeding recovery from attacks, reducing new radiographic and clinical relapses, slowing the progression of the disease, and managing MS symptoms. Some people have such mild symptoms that no treatment is necessary.

Multiple sclerosis research laboratory at Mayo Clinic
Treatments for MS attacks
- Corticosteroids, such as oral prednisone and intravenous methylprednisolone, are prescribed to reduce nerve inflammation. Side effects may include insomnia, increased blood pressure, increased blood glucose levels, mood swings and fluid retention.
- Plasma exchange (plasmapheresis). The liquid portion of part of your blood (plasma) is removed and separated from your blood cells. The blood cells are then mixed with a protein solution (albumin) and put back into your body. Plasma exchange may be used if your symptoms are new, severe and haven't responded to steroids.
Treatments to modify progression
There are several disease modifying therapies (DMTs) for relapsing-remitting MS . Some of these DMTs can be of benefit for secondary progressive MS , and one is available for primary progressive MS .
Much of the immune response associated with MS occurs in the early stages of the disease. Aggressive treatment with these medications as early as possible can lower the relapse rate, slow the formation of new lesions, and potentially reduce risk of brain atrophy and disability accumulation.
Many of the disease-modifying therapies used to treat MS carry significant health risks. Selecting the right therapy for you will depend on careful consideration of many factors, including duration and severity of disease, effectiveness of previous MS treatments, other health issues, cost, and child-bearing status.
Treatment options for relapsing-remitting MS include injectable, oral and infusions medications.
Injectable treatments include:
Interferon beta medications. These drugs used to be the most prescribed medications to treat MS . They work by interfering with diseases that attack the body and may decrease inflammation and increase nerve growth. They are injected under the skin or into muscle and can reduce the frequency and severity of relapses.
Side effects of interferons may include flu-like symptoms and injection-site reactions. You'll need blood tests to monitor your liver enzymes because liver damage is a possible side effect of interferon use. People taking interferons may develop neutralizing antibodies that can reduce drug effectiveness.
- Glatiramer acetate (Copaxone, Glatopa). This medication may help block your immune system's attack on myelin and must be injected beneath the skin. Side effects may include skin irritation at the injection site.
- Monoclonal antibodies. Ofatumumab (Kesimpta, Arzerra) targets cells that damage the nervous system. These cells are called B cells. Ofatumumab is given by an injection under the skin and can decrease multiple sclerosis brain lesions and worsening symptoms. Possible side effects are infections, local reactions to the injection and headaches.
Oral treatments include:
- Teriflunomide (Aubagio). This once-daily oral medication can reduce relapse rate. Teriflunomide can cause liver damage, hair loss and other side effects. This drug is associated with birth defects when taken by both men and women. Therefore, use contraception when taking this medication and for up to two years afterward. Couples who wish to become pregnant should talk to their doctor about ways to speed elimination of the drug from the body. This drug requires blood test monitoring on a regular basis.
- Dimethyl fumarate (Tecfidera). This twice-daily oral medication can reduce relapses. Side effects may include flushing, diarrhea, nausea and lowered white blood cell count. This drug requires blood test monitoring on a regular basis.
- Diroximel fumarate (Vumerity). This twice-daily capsule is similar to dimethyl fumarate but typically causes fewer side effects. It's approved for the treatment of relapsing forms of MS .
- Monomethyl fumarate (Bafiertam) was approved by the FDA as a delayed release medicine that has a slow and steady action. Because of its time release, it is hoped that side effects will be decreased. Possible side effects are flushing, liver injury, abdominal pain and infections.
Fingolimod (Gilenya). This once-daily oral medication reduces relapse rate.
You'll need to have your heart rate and blood pressure monitored for six hours after the first dose because your heart rate may be slowed. Other side effects include rare serious infections, headaches, high blood pressure and blurred vision.
- Siponimod (Mayzent). Research shows that this once-daily oral medication can reduce relapse rates and help slow progression of MS . It's also approved for secondary-progressive MS . Possible side effects include viral infections, liver problems and low white blood cell count. Other possible side effects include changes in heart rate, headaches and vision problems. Siponimod is harmful to a developing fetus, so women who may become pregnant should use contraception when taking this medication and for 10 days after stopping the medication. Some might need to have the heart rate and blood pressure monitored for six hours after the first dose. This drug requires blood test monitoring on a regular basis.
- Ozanimod (Zeposia). This oral medication decreases the relapse rate of multiple sclerosis and is given once a day. Possible side effects are an elevated blood pressure, infections and liver inflammation.
- Ponesimod (Ponvory). This oral medication is taken once a day with a gradually increasing dosing schedule. This medicine has a low relapse rate and has demonstrated fewer brain lesions than some other medications used to treat multiple sclerosis. The possible side effects are respiratory tract infections, high blood pressure, liver irritation and electrical problems in the heart that affect heart rate and rhythm.
- Cladribine (Mavenclad). This medication is generally prescribed as a second line treatment for those with relapsing-remitting MS . It was also approved for secondary-progressive MS . It is given in two treatment courses, spread over a two-week period, over the course of two years. Side effects include upper respiratory infections, headaches, tumors, serious infections and reduced levels of white blood cells. People who have active chronic infections or cancer should not take this drug, nor should women who are pregnant or breastfeeding. Men and women should use contraception when taking this medication and for the following six months. You may need monitoring with blood tests while taking cladribine.
Infusion treatments include:
Natalizumab (Tysabri). This is a monoclonal antibody that has been shown to decrease relapse rates and slow down the risk of disability.
This medication is designed to block the movement of potentially damaging immune cells from your bloodstream to your brain and spinal cord. It may be considered a first line treatment for some people with severe MS or as a second line treatment in others.
This medication increases the risk of a potentially serious viral infection of the brain called progressive multifocal leukoencephalopathy (PML) in people who are positive for antibodies to the causative agent of PML JC virus. People who don't have the antibodies have extremely low risk of PML .
Ocrelizumab (Ocrevus). This treatment reduces the relapse rate and the risk of disabling progression in relapsing-remitting multiple sclerosis. It also slows the progression of the primary-progressive form of multiple sclerosis.
This humanized monoclonal antibody medication is the only DMT approved by the FDA to treat both the relapse-remitting and primary-progressive forms of MS . Clinical trials showed that it reduced relapse rate in relapsing disease and slowed worsening of disability in both forms of the disease.
Ocrelizumab is given via an intravenous infusion by a medical professional. Infusion-related side effects may include irritation at the injection site, low blood pressure, a fever and nausea, among others. Some people may not be able to take ocrelizumab, including those with a hepatitis B infection. Ocrelizumab may also increase the risk of infections and some types of cancer, particularly breast cancer.
Alemtuzumab (Campath, Lemtrada). This treatment is a monoclonal antibody that decreases annual relapse rates and demonstrates MRI benefits.
This drug helps reduce relapses of MS by targeting a protein on the surface of immune cells and depleting white blood cells. This effect can limit potential nerve damage caused by the white blood cells. But it also increases the risk of infections and autoimmune disorders, including a high risk of thyroid autoimmune diseases and rare immune mediated kidney disease.
Treatment with alemtuzumab involves five consecutive days of drug infusions followed by another three days of infusions a year later. Infusion reactions are common with alemtuzumab.
The drug is only available from registered providers, and people treated with the drug must be registered in a special drug safety monitoring program. Alemtuzumab is usually recommended for those with aggressive MS or as second line treatment for patients who failed another MS medication.
Recent developments or emerging therapies
Bruton's tyrosine kinase (BTK) inhibitor is an emerging therapy being studied in relapsing-remitting multiple sclerosis and secondary-progressive multiple sclerosis. It works by mostly modulating B cells, which are immune cells in the central nervous system.
Stem cell transplantation destroys the immune system of someone with multiple sclerosis and then replaces it with transplanted healthy stem cells. Researchers are still investigating whether this therapy can decrease inflammation in people with multiple sclerosis and help to "reset" the immune system. Possible side effects are fever and infections.
Researchers are learning more about how existing disease modifying therapies work to lessen relapses and reduce multiple sclerosis-related lesions in the brain. Further studies will determine whether treatment can delay disability caused by the disease.
For primary-progressive MS , ocrelizumab (Ocrevus) is the only FDA-approved disease-modifying therapy (DMT). Those who receive this treatment are slightly less likely to progress than those who are untreated.
For secondary progressive MS , some might consider the use of FDA-approved disease modifying therapies such as ozanimod, siponimod and cladribine, which can potentially slow down disabilities.
Treatments for MS signs and symptoms

Physical therapy can build muscle strength and ease some of the symptoms of MS .
Therapy. A physical or occupational therapist can teach you stretching and strengthening exercises and show you how to use devices to make it easier to perform daily tasks.
Physical therapy along with the use of a mobility aid, when necessary, can also help manage leg weakness and other gait problems often associated with MS .
- Muscle relaxants. You may experience painful or uncontrollable muscle stiffness or spasms, particularly in your legs. Muscle relaxants such as baclofen (Lioresal, Gablofen), tizanidine (Zanaflex) and cyclobenzaprine may help. Onabotulinumtoxin A treatment is another option in those with spasticity.
- Medications to reduce fatigue. Amantadine (Gocovri, Osmolex), modafinil (Provigil) and methylphenidate (Ritalin) have been used to reduce MS -related fatigue. However, a recent study did not find amantadine, modafinil or methylphenidate to be superior to a placebo in improving MS -related fatigue and caused more frequent adverse events. Some drugs used to treat depression, including selective serotonin reuptake inhibitors, may be recommended.
- Medication to increase walking speed. Dalfampridine (Ampyra) may help to slightly increase walking speed in some people. Possible side effects are urinary tract infections, vertigo, insomnia and headaches. People with a history of seizures or kidney dysfunction should not take this medication.
- Other medications. Medications also may be prescribed for depression, pain, sexual dysfunction, insomnia, and bladder or bowel control problems that are associated with MS .
- Acetyl-L-carnitine: Can it relieve MS fatigue?
- Emerging treatments for multiple sclerosis
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Lifestyle and home remedies
To help relieve the signs and symptoms of MS , try to:
- Get plenty of rest. Look at your sleep habits to make sure you're getting the best possible sleep. To make sure you're getting enough sleep, you may need to be evaluated — and possibly treated — for sleep disorders such as obstructive sleep apnea.
- Exercise. If you have mild to moderate MS , regular exercise can help improve your strength, muscle tone, balance and coordination. Swimming or other water exercises are good options if you have intolerance to heat. Other types of mild to moderate exercise recommended for people with MS include walking, stretching, low-impact aerobics, stationary bicycling, yoga and tai chi.
- Cool down. MS symptoms may worsen when the body temperature rises in some people with MS . Avoiding exposure to heat and using devices such as cooling scarves or vests can be helpful.
- Eat a balanced diet. Since there is little evidence to support a particular diet, experts recommend a generally healthy diet. Some research suggests that vitamin D may have potential benefit for people with MS .
- Relieve stress. Stress may trigger or worsen your signs and symptoms. Yoga, tai chi, massage, meditation or deep breathing may help.
- Exercise and multiple sclerosis
- Vitamin D and MS: Any connection?
- Vitamins for MS: Do supplements make a difference?
Alternative medicine
Many people with MS use a variety of alternative or complementary treatments or both to help manage their symptoms, such as fatigue and muscle pain.
Activities such as exercise, meditation, yoga, massage, eating a healthier diet, acupuncture and relaxation techniques may help boost overall mental and physical well-being in patients with MS .
According to guidelines from the American Academy of Neurology, research strongly indicates that oral cannabis extract (OCE) may improve symptoms of muscle spasticity and pain. There is a lack of evidence that cannabis in any other form is effective in managing other MS symptoms.
Daily intake of vitamin D3 of 2,000 to 5,000 international units daily is recommended in those with MS . The connection between vitamin D and MS is supported by the association with exposure to sunlight and the risk of MS .
Coping and support
Living with any chronic illness can be difficult. To manage the stress of living with MS , consider these suggestions:
- Maintain normal daily activities as best you can.
- Stay connected to friends and family.
- Continue to pursue hobbies that you enjoy and are able to do.
- Contact a support group, for yourself or for family members.
- Discuss your feelings and concerns about living with MS with your doctor or a counselor.
Preparing for your appointment
You may be referred to a doctor who specializes in disorders of the brain and nervous system (neurologist).
What you can do
- Write down your symptoms, including any that may seem unrelated to the reason why you scheduled the appointment.
- Make a list of all your medications, vitamins and supplements.
- Bring any clinical notes , scans, laboratory test results or other information from your primary care provider to your neurologist.
- Write down your key medical information, including other conditions.
- Write down key personal information, including any recent changes or stressors in your life.
- Write down questions to ask your doctor.
- Ask a relative or friend to accompany you, to help you remember what the doctor says.
What to expect from your doctor
Your doctor is likely to ask you questions. Being ready to answer them may reserve time to go over points you want to spend more time on. You may be asked:
- When did you begin experiencing symptoms?
- Have your symptoms been continuous or occasional?
- How severe are your symptoms?
- What, if anything, seems to improve your symptoms?
- What, if anything, appears to worsen your symptoms?
- Does anyone in your family have multiple sclerosis?
Questions to ask your doctor
- What's the most likely cause of my symptoms?
- What kinds of tests do I need? Do they require any special preparation?
- Is my condition likely temporary or chronic?
- Will my condition progress?
- What treatments are available?
- I have these other health conditions. How can I best manage them together?
In addition to the questions that you've prepared to ask your doctor, don't hesitate to ask other questions during your appointment.
- What is multiple sclerosis? National Multiple Sclerosis Society. https://www.nationalmssociety.org/What-is-MS. Accessed June 2, 2022.
- Daroff RB, et al. Multiple sclerosis and other inflammatory demyelinating diseases of the central nervous system. In: Bradley's Neurology in Clinical Practice. 7th ed. Philadelphia, Pa.: Elsevier Saunders; 2012. https://www.clinicalkey.com. Accessed June 2, 2022.
- Ferri FF. Multiple sclerosis. In: Ferri's Clinical Advisor 2019. Philadelphia, Pa.: Elsevier; 2019. https://www.clinicalkey.com. Accessed June 2, 2022.
- Olek MJ. Clinical presentation, course, and prognosis of multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Wingerchuk DM (expert opinion). Mayo Clinic, Phoenix/Scottsdale, Ariz. Jan. 21, 2019.
- Ciccarelli O. Multiple sclerosis in 2018: New therapies and biomarkers. The Lancet. 2019; doi: 10.1016/S14744422 (18)30455-1.
- Keegan BM. Therapeutic decision making in a new drug era in multiple sclerosis. Seminars in Neurology. 2013; doi:10.1055/s0033-1345709.
- Goldman L, et al., eds. Multiple sclerosis and demyelinating conditions of the central nervous system. In: Goldman-Cecil Medicine. 25th ed. Philadelphia, Pa.: Saunders Elsevier; 2016. https://www.clinicalkey.com. Accessed Jun. 2, 2022.
- Lotze TE. Pathogenesis, clinical features, and diagnosis of pediatric multiple sclerosis. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Kantarci OH, et al. Novel immunomodulatory approaches for the management of multiple sclerosis. Clinical Pharmacology & Therapeutics. 2014; doi:10.1038/clpt.2013.196.
- Olek MJ. Disease-modifying treatment of relapsing-remitting multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Olek MJ, et al. Treatment of acute exacerbations of multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Wingerchuk DM. Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo Clinic Proceedings. 2014; doi:10.1016/j.mayocp.2013.11.002.
- Pizzorno JE, et al. Multiple sclerosis. In: Textbook of Natural Medicine. 4th ed. St. Louis, Mo.: Churchill Livingstone Elsevier; 2013. https://www.clinicalkey.com. Accessed June 2, 2022.
- Olek MJ, et al. Evaluation and diagnosis of multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Gaetani L, et al. 2017 revisions of McDonald criteria shorten the time to diagnosis of multiple sclerosis in clinically isolated syndromes. Journal of Neurology. 2018;265:2684.
- http://onlinelibrary.wiley.com/doi/10.1002/ana.22366.
- Olek MJ, et al. Pathogenesis and epidemiology of multiple sclerosis.
- Ingram G, et al. Cannabis and multiple sclerosis. Practical Neurology. 2019; doi:10.1136/practneurol-2018-002137.
- Olek MJ, et al. Symptom management of multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Yadav Y, et al. Summary of evidence-based guideline: Complementary and alternative medicine in multiple sclerosis. Neurology. 2014; doi: 10.1212/WNL.0000000000000250.
- Nimmagadda R. Allscripts EPSi. Mayo Clinic. April 22, 2022.
- National MS Society. Network of Pediatric MS Centers. https://www.nationalmssociety.org/What-is-MS/Who-Gets-MS/Pediatric-MS/Care-for-Pediatric-MS. Accessed June 2, 2022.
- Rodriguez M. Plasmapheresis in acute episodes of fulminant CNS inflammatory demyelination. Neurology. 1993; doi:10.1212/wnl.43.6.1100.
- Deb C. CD8+ T cells cause disability and axon loss in a mouse model of multiple sclerosis. PLoS One. 2010; doi:101371/journal.pone.0012478.
- FDA approves new drug to treat multiple sclerosis. U.S. Food & Drug Administration. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm549325.htm. Accessed June 1, 2022.
- Keegan BM (expert opinion). Mayo Clinic, Rochester, Minn. January 15, 2019.
- FDA approves new oral drug to treat multiple sclerosis. U.S. Food and Drug Administration. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm634469.htm. Accessed June 2, 2022.
- Kappos L, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomized, phase 3 study. The Lancet. 2018; doi: 10.1016/S0140-6736(18)30475-6.
- Marin Collazo IV (expert opinion). Mayo Clinic, Rochester, Minn. April 2, 2019.
- AskMayoExpert. Multiple sclerosis. Mayo Clinic; 2020.
- AskMayoExpert. Medication monitoring guidelines. Mayo Clinic; 2020.
- Vumerity. National MS Society. https://www.nationalmssociety.org/Treating-MS/Medications/Vumerity. Accessed March 16, 2020.
- Gianfrancesco M, et al. Obesity during childhood and adolescence increases susceptibility to multiple sclerosis after accounting for established genetic and environmental risk factors. Obesity Research and Clinical Practice. 2014; doi.org/10.1016/j.orcp.2014.01.002.
- Pantavou KG, et al. Season of birth and multiple sclerosis: A systematic review and multivariate meta-analysis. Journal of Neurology. 2020; doi:10.1007/s00415019-09346-5.
- Cifu DX, et al., eds. Multiple sclerosis. In Braddom's Physical Medicine and Rehabilitation. 6th ed. Elsevier; 2021 https://www.clinicalkey.com. Accessed Jun. 2, 2022.
- Langer-Gould AM, et al. Racial and ethnic disparities in multiple sclerosis prevalence. Neurology. 2022; doi:10.1212/WNL.0000000000200151.
- Kasper LH, et al. Immunomodulatory activity of interferon-beta. Annals of Clinical and Translational Neurology. 2014; doi:10.1002/acn3.84.
- Goldschmidt CH, et al. Re-evaluating the use of IFN-B and relapsing multiple sclerosis: Safety, efficacy and place in therapy. Degenerative Neurological and Neuromuscular Disease. 2020; doi:10.2147/DNND.S224912.
- Kieseie BC. The mechanism of action of interferon-B in relapsing multiple sclerosis. Central Nervous System Drugs. 2011; doi:10.1007/s10067-008-0972-3.
- Betaseron. Bayer AG; 1993. www.bayer.com. Accessed Jun. 1, 2022.
- Hauser SL, et al. Ofatumumab versus teriflunomide in multiple sclerosis. The New England Journal of Medicine. 2020; doi:10.1056/NEJMoa1917246.
- Kesimpta. Novartis; 2020. www.novartis.com. Accessed Jun. 1, 2022.
- Marin Collazo V (expert opinion). Mayo Clinic. June 13, 2020.
- Olek MJ. Treatment of progressive multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed Jun. 2, 2022.
- Wingerchuk DM, et al. Disease modifying therapies for relapsing multiple sclerosis. British Medical Journal. 2016; doi:10.1136/bmj.i3518.
- Saadeh RS, et al. CSF kappa free light chains: Cutoff validation for diagnosing multiple sclerosis. Mayo Clinic Proceedings. 2022; doi:10.1016/j.mayocp.2021.09.014.
- Goldschmidt C, et al. Advances in the treatment of multiple sclerosis. Neurologic Clinics. 2021; doi:10.1016/j.ncl.2020.09.002.
- Bafiertam. Banner Life Sciences LLC; 2013. www.bannerls.com. Accessed Jun. 1, 2022.
- Baliertam delayed release capsule. Banner Life Sciences LLC; 2013. www.bannerls.com. Accessed Jun. 1, 2022.
- Oral ponesimod versus teriflunomide in relapsing multiple sclerosis (OPTIMUM). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02425644. Accessed Jun. 2, 2022.
- Ponvory. Janssen Pharmaceuticals; 2021. www.janssen.com. Accessed Jun. 1, 2022.
- Torke S, et al. Inhibition of Bruton's tyrosine kinase as a novel therapeutic approach in multiple sclerosis. Expert Opinion on Investigational Drugs. 2020.
- Nash RA, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for relapsing-remitting multiple sclerosis (HALT-MS): A 3-year interim report. Journal of the American Medical Association Neurology. 2015; doi:10.1001/jamaneurol.2014.3780.
- Reston, et al. Autologous hematopoietic cell transplantation for multiple sclerosis: A systematic review. Multiple Sclerosis. 2011; doi:10,1177/1352458510383609.
- Petrou P, et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain. 2020; doi:10.1093/brain/awaa333.
- Liang J, et al. Allogenic mesenchymal stem cell transplantation in the treatment of multiple sclerosis. Multiple Sclerosis. 2009; doi:10.1177/1352458509104590.
- Wingerchuk DM, et al. Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo Clinic Proceedings. 2014; doi:101016/j.mayocp.2013.11.002.
- Multiple sclerosis information page. National institute of neurological disorders and stroke. https://www.ninds.nih.gov/Disorders/All-Disorders/Multiple-Sclerosis-Information-Page. Accessed Jun. 2, 2022.
- Sadovnick AD. Genetic background of multiple sclerosis. Autoimmunity Reviews. 2012; doi:10.1016/j.autrev.2011.05.007.
- Demyelinating disease: What can you do about it?
- Infographic: Multiple Sclerosis
- Multiple sclerosis: Can it cause seizures?
- Myelin damage and the nervous system
- Physical therapy for multiple sclerosis
- What is multiple sclerosis? An expert explains
Associated Procedures
Products & services.
- A Book: Mayo Clinic Family Health Book
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Phoenix/Scottsdale, Arizona, and Mayo Clinic in Jacksonville, Florida, have been ranked among the best Neurology & Neurosurgery hospitals in the nation for 2024-2025 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Help transform healthcare
Your donation can make a difference in the future of healthcare. Give now to support Mayo Clinic's research.

Multiple Sclerosis
- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE , Medicine , Surgery , Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Table of Contents
Suggest an improvement
- Hidden Post Title
- Hidden Post URL
- Hidden Post ID
- Type of issue * N/A Fix spelling/grammar issue Add or fix a link Add or fix an image Add more detail Improve the quality of the writing Fix a factual error
- Please provide as much detail as possible * You don't need to tell us which article this feedback relates to, as we automatically capture that information for you.
- Your Email (optional) This allows us to get in touch for more details if required.
- Which organ is responsible for pumping blood around the body? * Enter a five letter word in lowercase
- Name This field is for validation purposes and should be left unchanged.
Introduction
Multiple sclerosis (MS) is an autoimmune inflammatory disease of the central nervous system (CNS) which is characterised by demyelination . It is the most common progressive neurological disorder in high-income countries, and in young adults. 1,2
Pathophysiology
MS is an autoimmune inflammatory process in the central nervous system thought to likely be mediated by aberrant T-cell activation . 3
The underlying cause of this process, however, remains unclear. 2-5
It is thought to be a complex relationship between a genetic predisposition and exposure to environmental triggers . 2,4
Classification
It is helpful to think of MS as a continuous spectrum of disease , where the rate of progression and severity worsens over time.
However, for management and research purposes it is simpler to categorise patients into four main groups .
Clinically/radiologically isolated syndrome (CIS/RIS)
Clinically isolated syndrome (CIS) is an otherwise unexplained clinical episode of neurologic dysfunction, and radiologically isolated syndrome (RIS) is evidence of white matter pathology on neuroimaging not attributable to any other pathology in the absence of clinical symptoms. 4
Both syndromes have features suggestive of MS, however, neither CIS nor RIS satisfies the diagnostic criteria that must be met to make a diagnosis of MS (see McDonald’s criteria in investigations section). 3-7
Patients who present with a CIS/RIS are much more likely than the general population to develop MS. Some 34% of patients with RIS develop acute neuropathic symptoms consistent with multiple sclerosis within 5 years. 4
Relapsing-remitting MS (RRMS)
Relapsing-remitting MS (RRMS) is by far the most common form of disease at presentation, encompassing approximately 85% of patients. 3,5,6
RRMS constitutes unpredictable attacks of neurological dysfunction (lasting >24 hours in the absence of fever), followed by relief of symptoms though patients may not return fully to baseline function. 3-7
Secondary progressive MS
Secondary progressive MS initially presents as RRMS, then later declines steadily and progressively without remission. 3-7
Primary progressive MS
Primary progressive MS is a steady, progressive worsening of disease severity from the onset without remission. 3-7
Risk factors
Risk factors for MS include: 1-4
- Family history
- Sex (F>M)
- Age between 25-35
- Other co-existing autoimmune diseases, such as type 1 diabetes mellitus
- Previous EBV infection; infectious mononucleosis / raised anti-EBV nuclear antigen 1 (EBNA1) antibody titres
- Latitude of habitat
- Vitamin D deficiency
Clinical features
While there are no clinical findings that are unique to MS, there is a range of recognised characteristic symptoms (table 1).
The most common symptoms on initial presentation are: 5
- Limb numbness/tingling
- Limb weakness (subacute onset)
- Cerebellar symptoms
Certain phenomena are also considered characteristic of MS: 5
- Uhthoff’s phenomenon : worsening of symptoms on exercise/in warm environments (e.g. in a bath).
- Lhermitte ’ s phenomenon : lightning-shock pain down the spine on flexion of the neck secondary to cervical cord plaque formation.
Table 1. An overview of the clinical features of MS.
|
| Motor: Sensory: Cerebellar: Fatigue Depression/labile mood |
|
| Nystagmus Optic neuritis (pain on movement, visual field defect, loss of colour vision – particularly red) Diplopia – internuclear ophthalmoplegia (INO) |
|
| Dysphagia Slurring/stuttering speech |
|
| Weakness Cramping Spasm/contractures |
|
| Urinary frequency Incontinence Retention |
|
| Constipation/diarrhoea |
Other important areas to cover in the history include:
- Past medical history (such as any history of focal neurologic deficit, or other autoimmune diseases)
- Family history of MS/other autoimmune diseases
- Smoking status
- Impact on activities of daily living
- Driving status
- Falls risk assessment

Clinical examination
MS has the potential to involve multiple different systems. As such, it is important to carry out a thorough neurological examination including a cranial nerve examination and cerebellar examination .
Patients with MS may have a wide range of clinical signs on cranial nerve examination.
Optic nerve (CN II)
Optic neuritis , which is usually monocular, can be the first sign of MS. Fundoscopy may reveal blurring of the optic disc in the acute setting, though often no changes are apparent. A previous episode of optic neuritis is often characterised by disc pallor, which is often far more helpful.
Relative afferent pupillary defect (RAPD) is another manifestation MS which may be revealed by the pendular swinging light test.
The pupil of the healthy eye will constrict as light is shone on it, exhibiting a normal direct light reflex. The contralateral pupil will also constrict as there is a normal consensual light reflex.
When the light is then swung to the affected eye, the previously constricted pupil will dilate as there is no afferent (outgoing) signal being transmitted by the inflamed optic nerve , impairing the direct light reflex .
See the Geeky Medics visual assessment guide for more information on pupillary reflexes.
Oculomotor (CN III), trochlear (CN IV) and abducens (CNVI) nerve
Ophthalmoplegia arises from involvement of the cranial nerve nuclei, resulting in symptoms of a cranial nerve palsy, or involvement of the medial longitudinal fasciculus (MLF) resulting in internuclear ophthalmoplegia (INO).
INO is a conjugate lateral gaze palsy , where there is a failure of adduction of the affected eye during horizontal eye movement (figure 1).
Nystagmus is usually observed with the abduction of the contralateral eye, as it tries to normalise the two discordant images being sent to the brain simultaneously (figure 1).
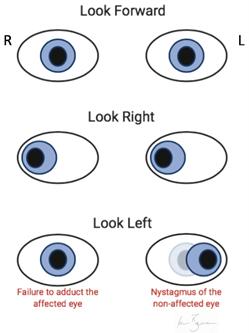
Trigeminal nerve (CN V)
Involvement of CN V nucleus may result in facial paraesthesia , and/or weakness of the muscles of mastication.
Facial nerve (CN VII)
CN VII lesions will result in weakness of facial muscles of expression. This can mimic the signs of an acute stroke. MS is an upper motor neuron lesion, and as such there will be sparing of frontalis controlling expression of the forehead.
Vestibulocochlear nerve (CN VIII)
Loss of balance, and/or sensorineural deafness.
Glossopharyngeal (CN IX), vagus (CN X) and hypoglossal nerve (CN XII)
Loss of motor function to tongue/pharynx resulting in speech and swallowing problems.
Accessory nerve (CN XI)
Loss of motor function to sternocleidomastoid and trapezius resulting in neck weakness and hypertonia.
Cerebellar signs
MS has the potential to affect any part of the CNS. As such, sometimes patients may present with symptoms of cerebellar dysfunction including:
- Nystagmus : slow, large-amplitude nystagmus while eyes are resting at the midline, flickering towards the side of the lesion.
- Intention tremor : a slow, coarse tremor that gets worse on the extension of a limb towards an intended object/target, often associated with dysmetria.
- Scanning dysarthria : sentences or even words are broken up into a number of separate syllables that can be expressed at varying volume. It may appear as though the patient is searching for (or scanning for) the correct next word/sound.
Peripheral nervous system signs
Sensory signs :
- Abnormal sensation
- Romberg’s test positive: this suggests the involvement of the dorsal column of the spinal cord, affecting proprioception.
Motor signs :
- Decreased power
- Hyperreflexia
Differential diagnoses
The presentation of multiple sclerosis can be varied . MS can resemble a broad number of other disorders which should be considered/ruled out ahead of making a diagnosis: 8
- Migraine with aura
- Hypoglycaemia
- Hypothyroidism
- Electrolyte abnormalities (e.g. hyponatraemia)
- Peripheral neuropathy (e.g. B12 deficiency, diabetic neuropathy, radiculopathy, motor neuron disease)
- Space occupying lesion (e.g. glioblastoma/meningioma/lymphoma or cerebral abscess)
- Compression of brainstem or spinal cord (e.g. Chiari malformation, cervical spondylosis, disc herniation)
- Infection (e.g. Lyme disease, syphilis, HIV)
- Inflammatory/autoimmune (e.g. sarcoidosis, systemic lupus erythematosus, CNS vasculitis)
Investigations
Laboratory investigations.
Laboratory investigations are important for ruling out other causes of neurological dysfunction: 8,9
- Full blood count: white cell count for infection.
- C‑reactive protein: a marker of an inflammatory process (e.g. infective/autoimmune).
- Liver function tests: for basic baseline biochemistry and to rule out hepatic pathology associated with MS mimics.
- Urea and electrolytes: to rule out electrolyte disturbance, which might mimic MS.
- Calcium and angiotensin-converting enzyme: to rule out sarcoidosis.
- Thyroid function tests: to rule out hypothyroidism.
- Vitamin B12: to rule out B12 peripheral neuropathy.
- HIV serology: to rule out HIV.
Radiological investigations
The main investigation for MS is MRI of the brain and spinal cord with gadolinium contrast. MS lesions will be apparent as T2-hyperintense white matter plaques (figure 2).
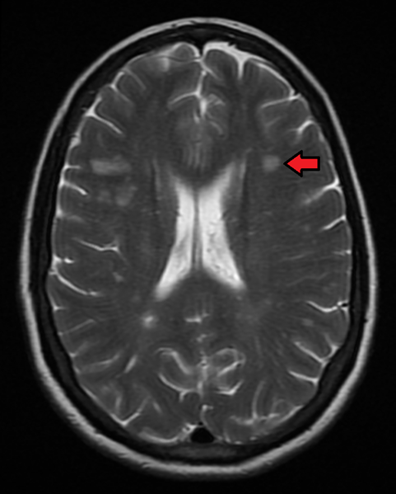
Other investigations
Lumbar puncture for CSF analysis is useful, especially when there is no clear radiological evidence of white matter pathology consistent with MS on MRI (see McDonald’s criteria below).
Typical findings in the CSF of an MS patient include a high protein content and oligoclonal bands of immunoglobulin aggregates on CSF electrophoresis. 9
CSF electrophoresis must be accompanied by serum protein electrophoresis (SPEP) concurrently for results to have any meaning. Oligoclonal bands should not appear on SPEP.
Evoked potentials are also a supportive investigation that historically would have been considered useful in the workup of MS, visual evoked potentials being the most useful. In clinical practice today it is considered solely supportive and not diagnostic in isolation. 10
McDonald diagnostic criteria
The McDonald criteria outline the clinical, radiological and biochemical findings which must be met to make a diagnosis of MS. 6
It is centred around the principle that MS is a disease which demonstrates pathology that is disseminated in time (i.e. events occur at >1 distinct time periods) and disseminated in space (i.e. ≥2 lesions affecting distinctly different locations in the CNS).
Table 2 describes further what dissemination in time and space means in a clinical and radiological context, and table 3 describes how the McDonald criteria apply this information to make a diagnosis of MS.
Table 2a : Description of the clinical findings required to satisfy the McDonald criteria, focussing on the necessity to demonstrate dissemination in time and dissemination in space.
| For example, 2 episodes of left leg weakness that have occurred months apart | For example, a patient presents with optic neuritis and symptoms of wrist drop |
Table 2b : Description of the radiological findings required to satisfy the McDonald criteria, focussing on the necessity to demonstrate dissemination in time and dissemination in space.
| For example, a patient’s scan demonstrates T2-hyperintense paraventricular white matter lesions, and a second scan 4 months later demonstrates a new juxtacortical white matter lesion that was not present previously ≥2 lesions identified simultaneously in areas of the CNS typically associated with MS*, but where only one lesion enhances upon injection of gadolinium contrast** | For example, a patient’s MRI shows evidence of T2-hyperintense white matter lesions in the cerebellum as well as in the spinal cord |
*periventricular, cortical, juxtacortical, infratentorial and the spinal cord **active lesions will enhance with gadolinium, old/inactive lesions will not
Table 3: The McDonald diagnostic criteria for the diagnosis of MS, adapted from Thompson et al., 2018. 6
| Can demonstrate ≥2 neuropathic symptoms that are disseminated in both time and space | None, clinical evidence sufficient MRI desirable to support diagnosis, but must be consistent with MS |
| Can demonstrate recurrence of a single neuropathic symptom (i.e. dissemination in time, but not in space) | Dissemination in space, demonstrated by MRI await further clinical attack implicating different site |
| First/single attack that has resulted in ≥2 neuropathic symptoms (i.e. evidence of dissemination in space but not time) | Dissemination in time, demonstrated by MRI/second clinical attack presence of oligoclonal bands in the CSF in the absence of otherwise atypical CSF findings (biochemical evidence of MS) |
| First attack that has resulted in a solitary neuropathic symptom (i.e. no evidence of dissemination in either space or time) | Dissemination in space, demonstrated by MRI/second clinical attack an involving a different part of the CNS dissemination in time demonstrated by MRI/second clinical attack presence of oligoclonal bands in the CSF in the absence of otherwise atypical CSF findings (biochemical evidence of MS)
|
Management of an acute episode
NICE guidelines state that a relapse/attack can be diagnosed if a patient presents with: 9
- New symptoms or worsening of existing symptoms
- Subacute onset (>24hrs)
- Absence of fever/signs of active infection
Not all relapses require medical intervention. If left alone, some symptoms may remit on their own.
If symptoms are severe, however, or interfere with activities of daily living then you should consider medical intervention. Treatment of an acute attack/relapse requires high dose steroid therapy with methylprednisolone (500mg by mouth for five days, or 1g by mouth for three to five days). 4,9
Patients can be managed in the community by their GP or may require admission to hospital for specialist input, depending on the severity of their symptoms and the impact of symptoms on their activities of daily living. 9
Plasmapheresis is also an option if the exacerbation is refractory to steroids. 4,5
Flares of MS symptoms may also often be caused by something other than an attack, such as an infection . In such an event, treatment of the underlying cause should be the primary concern.
Long-term management
There are currently no curative therapies for MS. Management of the disease focuses on curtailing the demyelination process and managing side-effects of the disease process.
Management of the demyelinating process
Beta-interferon (beta-IFN) and glatiramer acetate are injectable disease-modifying agents regularly used in the management of MS, which have both been shown to be similarly effective in decreasing relapses in RRMS. These medications do not, however, alter disease progression. 4,9,11,12
Additionally, there are a number of approved oral disease-modifying therapies for RRMS, including dimethyl fumarate , fingolimod and cladribine . 11,12
Monoclonal antibody therapies such as alemtuzumab and natalizumab are effective therapies in the treatment of RRMS. These drugs carry more dangerous side effect profiles. Most notably, natalizumab can rarely cause progressive multifocal leukoencephalopathy via reactivation of the JC virus , a very common virus often otherwise found dormant in glial cells. 4,9,11
In addition, alemtuzumab also has had an FDA black box warning for strokes and arterial dissections and thus should be used extremely cautiously. 13,14
With regard to PPMS, ocrelizumab is the only medication to date that has been shown to be effective in slowing the progression of symptoms in patients. 4,15
It is also worth mentioning the emerging role of autologous haematopoietic stem cell transplantation in patients with MS refractory to disease-modifying drug therapy, which has shown great promise to date. 4
The symptoms secondary to the demyelinating process in MS can have marked and severe impacts on a patient’s quality of life. As such, it is paramount that these be treated effectively and in a timely manner. These symptoms are outlined in table 3, along with multidisciplinary management options that should be considered. 4,5,9
Other management strategies
Other important management strategies for MS include:
- Amelioration of modifiable risk factors (e.g. smoking cessation, obesity, vitamin D) 4,5
- Ensuring immunisations are up to date
A multidisciplinary approach is important, other professionals who may be involved include:
- Nurse specialist involvement for assistance in managing the complex needs individuals with MS, their families and carers experience on a day-to-day basis.
- Physiotherapy for assistance and rehabilitation of balance, strength and mobility difficulties.
- Occupational therapy for management around the home and any modifications/aids required.
- Speech and language therapy for the assessment, diagnosis and rehabilitation of speech and/or swallowing difficulties.
- Social care worker for the management of emotional, social and economic need of patients with MS (e.g. social welfare entitlements).
- Psychologist involvement for any mood disturbance. Conversion disorder and depression are common complications of MS.
- GP involvement in the co-ordination of community care.
Complications
MS is a chronic, progressive disease, with a variable clinical course . 3-5
Not much is understood of its aetiology, and as such, there is difficulty identifying reliable biomarkers of disease severity/rate of disability onset. 5
Morbidity 5
The majority of people diagnosed with MS become unemployed within the following 15 years.
50% of patients require some form of mobility aid within 20 years of diagnosis.
Approximately half of patients eventually develop substantial cognitive deficits.
Mortality 5
Patients with multiple sclerosis have a reduction in life expectancy of 7 to 14 years compared with the general population.
At least half die from causes directly related to multiple sclerosis.
Primary progressive disease and older age at onset are associated with shorter survival.
There are numerous recognised complications of MS in addition to the classical motor/sensory symptoms associated with the disease (table 4).
Table 4. An overview of the complications of MS.
|
|
|
|
| Physiotherapy input Exercise therapy |
|
| Physiotherapy Occupational therapy, focusing on mobility aids and home modifications Fampridine can be used to increase walking speed in certain patients |
|
| Physiotherapy Consider pharmacological management with baclofen (an anti-spasmodic medication) and/or botulinum toxin injection of the affected muscle(s) |
|
| Catheterisation (either intermittent or self) Laxatives Anticholinergic medications can be effective in the pharmacological management of trigonal muscle dysfunction in urinary bladder incontinence. |
|
| May need psychiatric/psychological management |
|
| Ophthalmology input Consider gabapentin/memantine in managing oscillopsia |
|
| Pain specialist |
|
| Involvement of dementia specialists Home assistance Occupational therapy input +/- aids |
- Multiple sclerosis (MS) is an autoimmune inflammatory disease of the central nervous system (CNS) which is characterised by demyelination .
- Risk factors include being young, having a family/personal history of autoimmune disease and previous infection with EBV. There are also a number of recognised preventable risk factors such as vitamin D deficiency, the latitude of habitat and smoking.
- The presentation of MS can be quite varied, and may potentially involve quite a number of systems.
- Diagnosis is made using the McDonald criteria , which encompasses clinical and radiological findings consistent with MS, disseminated in time and space .
- Examination of MS patients includes the full examination of the peripheral nervous system, along with a cerebellar exam and a cranial nerve exam.
- The mainstay for management of an acute attack is high-dose steroid therapy to reduce inflammation, along with the management of any specific complications.
- Long term management requires multidisciplinary input. Treatment focuses on the disease itself (beta-IFN/Glatiramer acetate + monoclonal antibodies) along with the long-term management of side effects.
Neurology Registrar
Dr chris jefferies.
- Wallin MT, Culpepper WJ, Nichols E, Bhutta ZA, Gebrehiwot TT, Hay SI, et al. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Published in 2019. Available from: [LINK]
- Belbasis L, Bellou V, Evangelou E, Ioannidis JPA, Tzoulaki I. Environmental risk factors and multiple sclerosis: An umbrella review of systematic reviews and meta-analyses. Published in 2015. Available from: [LINK]
- Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple Sclerosis: Mechanisms and Immunotherapy. Published in 2018. Available from: [LINK]
- Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple Sclerosis. Published in 2018. Available from: [LINK]
- ClinicalKey Clinical Overview; Multiple sclerosis Last updated: 08 November 2019. Available from: [LINK]
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Published in 2018. Available from: [LINK]
- Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Published in 2014. Available from: [LINK]
- Miller DH, Weinshenker BG, Filippi M, Banwell BL, Cohen JA, Freedman MS, et al. Differential diagnosis of suspected multiple sclerosis: a consensus approach. Published in 2008. Available from: [LINK]
- NICE. Recommendations | Multiple sclerosis in adults: management | Guidance. Published in 2014, Last updated: 11 November 2019. Available from: [LINK]
- Walsh P, Kane N, Butler S. The clinical role of evoked potentials. Published in 2005. Available from: [LINK]
- Tramacere I, Del Giovane C, Salanti G, D’amico R, Filippini G. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: A network meta-analysis. Published in 2015.Available from: [LINK]
- Derfuss T, Mehling M, Papadopoulou A, Bar-Or A, Cohen JA, Kappos L. Advances in oral immunomodulating therapies in relapsing multiple sclerosis. Published in 2020. Available from: [LINK]
- McCall B. Alemtuzumab to be restricted pending review, says EMA Reports of stroke in patients having taken alemtuzumab for multiple sclerosis prompt a safety review by the European Medicines Agency. Published in 2019. Available from: [LINK]
- European Medicines Agency Measures to minimise risk of serious side effects of multiple sclerosis medicine Lemtrada. Published in 2020. Available from: [LINK]
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med. Published in 2017. Available from: [LINK]
- Internuclear Ophthalmoplegia . License: [ CC BY-SA ]. Available from: [LINK]
- James Heilman, MD. Multiple Sclerosis . License: [ CC BY-SA ]. Available from: [LINK]

Other pages
- Product Bundles 🎉
- Join the Team 🙌
- Institutional Licence 📚
- OSCE Station Creator Tool 🩺
- Create and Share Flashcards 🗂️
- OSCE Group Chat 💬
- Newsletter 📰
- Advertise With Us
Join the community
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Early Warning Signs of Multiple Sclerosis (MS)
Early warning signs, most common symptoms.
- Males vs. Females
Frequently Asked Questions
While no two people experience multiple sclerosis (MS) the same way, some symptoms tend to crop up earlier in the disease course than others. These symptoms may serve as warning signs of the disease, potentially allowing you or a loved one to receive a diagnosis of MS sooner than later.
In multiple sclerosis, your immune system goes awry and damages the fatty covering ( myelin ) that insulates nerve fibers within your central nervous system (CNS). Your CNS consists of your brain, spinal cord, and the optic nerves of your eyes.
As a result of myelin damage, nerve signals cannot be transmitted rapidly or efficiently between the CNS and the rest of your body. This can lead to various symptoms like blurry vision, pain, abnormal sensations, and muscle weakness, among many others.
This article reviews some of the common early symptoms and signs of MS. It also gives a brief overview of differences of MS between males and females and how MS is diagnosed.
peakSTOCK / Getty Images
Two phenomena—clinically isolated syndrome and optic neuritis—may serve as early warning signs of MS. People who experience one (or both) of these may or may not go on to develop MS.
Clinically Isolated Syndrome
Clinically isolated syndrome (CIS) refers to a person's first-time episode of neurological symptoms caused by inflammation and damaged myelin in the CNS.
As an example, a patient diagnosed with CIS may experience numbness and tingling in their legs. This would be accompanied by magnetic resonance imaging (MRI) findings that reveal damage to the CNS.
CIS is followed by a recovery period where the symptoms improve or completely go away.
Difference Between CIS and MS
The key difference between CIS and MS is that CIS is diagnosed after a person experiences one episode of neurological symptoms. MS can only be diagnosed when a person has experienced more than one episode of neurological symptoms.
Optic Neuritis
Optic neuritis —inflammation of one of your two optic nerves—is a common first presentation of MS. In fact, CIS may be diagnosed from an attack of optic neuritis.
Your optic nerve delivers messages to your brain about what your eye sees. When the myelin covering the optic nerve is damaged, signals related to sight are interrupted.
The common symptoms of optic neuritis include pain with eye movements, blurry or "foggy" vision, and seeing colors less vividly. Vision symptoms usually improve and fully recover within three to five weeks. That said, up to 10% of patients may experience long-term vision problems.
Even though the symptoms of MS vary in type, severity, and duration, there are some that are more common than others. The following is a brief snapshot of such symptoms:
Vision Problems
Besides optic neuritis, other common vision problems seen in MS are:
- Nystagmus is uncontrolled, jerking movement of the eyes, sometimes referred to as "dancing eyes." This symptom is caused by damage to the area of the brainstem that controls eye movements.
- Diplopia (double vision) is uncoordinated eye movements that cause you to see double. This symptom results from damage to the nerves that control your eye muscles.
Muscle Spasms
Muscle spasms are common in MS and are primarily caused by damaged myelin in the nerves that innervate or connect to your muscles. As a result of disrupted nerve signals, your muscles cannot relax properly. This causes muscle stiffness and/or a tightening, cramping, or heavy sensation in the affected muscle(s).
The legs are most commonly affected by spasms, but they can occur anywhere in the body. Muscle spasms also tend to be asymmetric, meaning they are more likely to happen on one side of the body versus both sides.
Nerve fiber damage in MS causes neuropathic pain , which is associated with burning, stabbing, sharp, itching, or squeezing sensations. This type of pain is associated with disability, depression, and fatigue in MS.
Specific types of neuropathic pain that may be early signs of MS include:
- Lhermitte's sign is a sensation of electricity that runs down your spine when you touch your chin to your chest. In MS, it's caused by damage to nerve fibers in your upper spine.
- MS hug is a tightening sensation around the chest and ribs caused by damage to the nerve fibers in your spine.
- Trigeminal neuralgia is an electric-shock-like or stabbing pain in the face or jaw area that is caused by damage to the trigeminal nerve (the fifth cranial nerve ).
Fatigue and Weakness
MS fatigue is often felt both physically and mentally. Described by many as "having the flu," MS fatigue is not eased by sleep and tends to come on suddenly and worsen with heat and humidity.
The overwhelming exhaustion and depletion of energy seen with MS fatigue may arise from the disease itself and/or other factors like medications, sleep disorders, or depression.
Timeline of MS Fatigue
Fatigue can occur at any time during the course of MS, and its development is not necessarily related to the progression of more objective neurological symptoms (e.g., walking problems).
Weakness is also common in MS and may arise from damage to the nerve fibers in the CNS that normally control muscle movements. Lack of activity due to MS-related pain, fatigue, or other symptoms can also contribute to MS weakness.
Bladder and Bowel Problems
Bladder dysfunction is common in MS, affecting the majority of patients at some point in the course of their disease. Urinary symptoms as the first presentation of MS occur in around 3% to 10% of people.
Symptoms and signs of bladder dysfunction in MS vary from mild to severe. They may include:
- Urgency : Feeling like you have to urinate right away
- Hesitancy : Having trouble initiating urination or you cannot maintain a steady stream
- Nocturia : Having to urinate often at night
- Incontinence : Having an involuntary loss of urine control
Recurrent urinary tract infections may also be a sign of bladder dysfunction in MS.
Bowel problems are common in MS, with constipation being the most frequent complaint. Constipation can aggravate other MS symptoms including muscle spasms, pain, bladder dysfunction, and walking problems. It can also contribute to fecal incontinence , which is the loss of control of your bowels.
Depression and Emotional Changes
Depression is associated with constant sadness and a lack of interest in activities you once enjoyed. In MS, depression can occur at any time in the course of the disease, including early or later on.
Depression in MS may stem from a number of different factors, including:
- MS itself : Damage to the areas of the brain that regulate emotion
- Side effects of MS medications : For example, corticosteroids (used to treat MS relapses ) and interferon drugs (used as disease-modifying therapies )
- Stress associated with living with MS : Undergoing a new diagnosis, relapse, or major change in function.
Other common emotional symptoms in MS include grief, anxiety , irritability, and anger. Many of these emotions stem from the unpredictable nature of MS, and the physical and emotional impact the disease has on a person's life.
Presentation in Males vs. Females
Differences exist in MS in males and females. For instance, research has found that females are twice as likely to live with MS as males. Moreover, those diagnosed with primary progressive MS (PPMS) are more likely to be male.
What Is PPMS?
PPMS is characterized by worsening symptoms from the onset of the disease. People with PPMS do not experience relapses or periods of symptom improvement ("remission").
Experts haven't yet teased out fully why these differences between sexes exist. Sex hormones, pregnancy, social factors (delayed care-seeking behavior), and/or differences in genes or environmental exposures may be involved.
How MS Is Diagnosed
The diagnosis of MS is often challenging, considering the symptoms are so variable. In addition, symptoms early on can often be vague or mimic those of other conditions, such as systemic lupus erythematosus (SLE) (an autoimmune disease that can affect many body systems) or vitamin B12 deficiency .
A neurologist —a doctor who specializes in diseases of the nervous system—will use the following tools to confirm a diagnosis of MS:
- Your medical history and neurological exam
- The McDonald criteria (a set of guidelines that focuses on diagnosing MS by showing evidence of damage to the CNS at different dates and to different parts)
- Magnetic resonance imaging (MRI) of the brain and spinal cord (which uses strong magnets to produce detailed images)
- Laboratory tests, mostly to rule out other conditions
- Other tests, including a spinal tap (lumbar puncture) and evoked potential tests (which measure electrical activity of the nerves of the eye)
Even though no two people experience MS in the same way, there are some symptoms, including vision problems and sensory disturbances, that may serve as early warning signs of the disease. Other common symptoms of MS include fatigue, muscle spasms, pain, bladder problems, and constipation.
A Word From Verywell
If you are concerned that you may be experiencing possible symptoms of MS, schedule an appointment with your healthcare provider or a neurologist. Diagnosing and treating MS as early as possible is associated with better long-term outcomes .
Keep in mind that many symptoms of MS overlap with other common medical conditions. Be proactive and get checked out, but try not to worry yourself until you know more information.
Most people are diagnosed with MS between the ages of 20 and 50 years old. That said, MS can develop at any age, and symptoms may predate a diagnosis by years.
Yes. In fact, research suggests MS may have a prodromal ("very early") phase. This phase includes various nonspecific symptoms, like fatigue, depression, pain, and headache. These symptoms may precede an MS diagnosis by several years.
There is no blood test that can diagnose MS. If you or a loved one are being evaluated for MS, your neurologist will use a variety of diagnostic tools, including your medical history, neurological exam, an MRI, and various blood or spinal fluid tests.
MS occurs when your immune system mistakingly attacks myelin, a protective coating on your nerves. These attacks lead to inflammation in the brain and spinal cord. The inflammation shows up as "lesions" or "plaques" on an MRI .
National MS Society. Clinically isolated syndrome .
Kale N. Optic neuritis as an early sign of multiple sclerosis . Eye Brain. 2016;8:195–202. doi:10.2147/EB.S54131
Cavenaghi VB, Dobrianskyj FM, Sciascia do Olival G, Castello Dias Carneiro RP, Tilbery CP. Characterization of the first symptoms of multiple sclerosis in a Brazilian center: cross-sectional study . Sao Paulo Med J. 2 017;135(3):222-225. doi:10.1590/1516-3180.2016.0200270117
National MS Society. Vision problems in multiple sclerosis .
Heitmann H, Biberacher V, Tiemann L et al. Prevalence of neuropathic pain in early multiple sclerosis . Mult Scler. 2016;22(9):1224-30. doi:10.1177/1352458515613643
Tur C. Fatigue management in multiple sclerosis . Curr Treat Options Neurol. 2016;18:26. doi:10.1007/s11940-016-0411-8
Aharony SM, Lam O, Corcos J. Evaluation of lower urinary tract symptoms in multiple sclerosis patients: Review of the literature and current guidelines . Can Urol Assoc J. 2017;11(1-2):61–64. doi:10.5489/cuaj.4058
Walton C, Rechtman L. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition . Mult Scler. 2020 Dec; 26(14): 1816–1821. doi:10.1177/1352458520970841
Eccles A. Delayed diagnosis of multiple sclerosis in males: may account for and dispel common understandings of different MS 'types .' Br J Gen Pract. 2019;69(680):148–149. doi:10.3399/bjgp19X701729
Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges . Lancet . 2017;389(10076):1336-1346. doi:10.1016/S0140-6736(16)30959-X
DiSanto G, Zecca C, MacLachlan S et al. Prodromal symptoms of multiple sclerosis in primary care . Ann Neurol 2018;83(6):1162-1173. doi:10.1002/ana.25247
By Colleen Doherty, MD Dr. Doherty is a board-certified internist and writer living with multiple sclerosis. She is based in Chicago.
| --> | | | | | | | | |
Lectures: Clinical Presentation Introduction Initial Symptoms Ongoing Symptoms Clinical Cases Introduction It is important to note that patients with MS have subjective complaints and objective signs that frequently are not attributable to one specific lesion in the CNS. It is usually possible to distinguish at least two or more separate foci of involvement based on the clinical assessment of the patient. Multiple Sclerosis most often is characterized by episodes of neurological dysfunction followed by periods of stabilization or partial to complete remission of symptoms. These symptoms (relapses or exacerbations) can appear over a few hours or days, can be gradually worsening over a period of a few weeks, or sometimes can present themselves acutely. Depending on a course and a subtype of the disease, these symptoms will either persist or slowly resolve over weeks or months and may even culminate as a complete remissions. A relapsing-remitting pattern is the most common and is characteristic for this disease . Initial Symptoms Certain signs and symptoms are more common in the early stages of the disease. Patients may be complaining of double or blurred vision, numbness, weakness in one or two extremities, instability in walking, tremors and problems with bladder control, heat intolerance. As is well known, sensory exam is the most difficult one to perform reliably and accurately in evaluation of patients with neurologic complaints. However, certain distributions of sensory problems can be suspicious for early MS. Among those are: - ascending numbness starting in the feet; - bilateral hand numbness; - hemiparesthesia; - dysesthesia in one of the above distributions; - generalized heat intolerance Objectively the most common sensory findings in the"numb" areas are dorsal column signs, such as reduction of vibration, proprioception and stereognosis, rather than problems with spinothalamic tract. Usually double vision in MS patients results from a unilateral or bilateral partial of complete internuclear ophthalmoplegia . VI nerve paresis and palsy also have been described as presenting symptoms of MS. III and IV nerves palsy are rather uncommon. Optic Neuritis is a frequent presenting symptom of MS. It is characterized by blurred vision, a change in color perception, visual field defect i.e.,. Central scotoma, and possible headaches and retro-orbital pain precipitated by eye movements. These symptoms may require neuro-ophthalmologic evaluation, MRI imaging and Visual Evoked Potential studies to establish a degree of optic nerve function. Motor weakness often is accompanied by upper motor neuron signs, such as mild spasticity, hyperreflexia, and pathologic signs. The most common initial presentation is paraparesis, but weakness can be also found in just one extremity (monoparesis) or all four extremities (quadriparesis). Ongoing Symptoms and Signs As the disease progresses, the original signs and symptoms may worsen, and the new ones may appear. The most common symptoms and signs include: Motor system: -weakness (variable severity mono- and paraparesis, hemiparesis, quadriparesis) -increased spasticity resulting in spastic gait -pathologic signs (Babinski's, Chaddock's, Hoffmann, Oppenheim's, etc.) -dysarthria Cerebellar signs: -incoordination (dysdiadochokinesia, problems with heel-to-shin test) -slowing of rapid repeating movements -cerebellar ataxia (ataxic gait) -scanning speech -loss of balance Sensory systems: -Lhermitte's sign -dysesthetic pain -paresthesia -numbness -dorsal column signs (i.e.,. severe decrease or loss of vibratory sense and proprioception, positive Romberg's test) Urinary incontinence, incomplete emptying, increased frequency of urination. All of these problems may result in urinary tract infections. Optic disc pallor, atrophy, blurred vision, diplopia, nystagmus, oscillopsia, intranuclear ophthalmoplegia, central scotomas or other visual field defects Cognitive and emotional abnormalities (emotional lability, depression, anxiety) Fatigue Sexual dysfunction At this stage in the disease, uncommon but important problems may include bowel incontinence, difficulty swallowing, seizures, trigeminal neuralgia, dystonia, hearing loss, and facial nerve (Bell's) palsy. All of the above-mentioned symptoms can be precipitated by heat, i.e.,. being in a hot, humid environment, or taking a hot bath. Clinical Cases Case 1 History Case 1 Questions Case 2: History Case 2: Questions Case 3 History Case 3 Questions © John W.Rose, M.D., Maria Houtchens, MSIII, Sharon G. Lynch, M.D.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Multiple sclerosis: diagnosis, differential diagnosis, and clinical presentation
Affiliation.
- 1 Department of Neurology, University of California, San Francisco, USA. Electronic address: [email protected].
- PMID: 24507522
- DOI: 10.1016/B978-0-444-52001-2.00011-X
The diagnosis of multiple sclerosis (MS) is based on demonstrating evidence of inflammatory-demyelinating injury within the central nervous system that is disseminated in both time and space. Diagnosis is made through a combination of the clinical history, neurologic examination, magnetic resonance imaging and the exclusion of other diagnostic possibilities. Other so-called "paraclinical" tests, including the examination of the cerebrospinal fluid, the recording of evoked potentials, urodynamic studies of bladder function, and ocular coherence tomography, may be helpful in establishing the diagnosis for individual patients, but are often unnecessary. Differential diagnosis in MS must be guided by clinical presentation and neurologic localization. While the list of conditions that can mimic MS clinically or radiologically is long, in clinical practice there are few conditions that truly mimic MS on both fronts. A positive test for a putative MS "mimic" does not unto itself exclude the diagnosis of MS. Typical symptoms of MS include discrete episodes ("attacks" or "relapses") of numbness, tingling, weakness, vision loss, gait impairment, incoordination, imbalance, and bladder dysfunction. In between attacks, patients tend to be stable, but may experience fatigue and heat sensitivity. Some MS patients go on to experience, or only experience, an insidious worsening of neurologic function and accumulation of disability ("progression") that is not associated with discrete relapse activity. Progression accounts for most of the long-term disability in MS. Diagnostic criteria for MS have evolved over the past several decades, with each revision impacting the apparent prevalence and prognosis of the disorder - the result has been to encourage earlier diagnosis without compromising accuracy.
Keywords: Diagnostic Criteria; MRI; Prognosis; Progression; Relapse; magnetic resonance imaging; myelitis; optic neuritis.
© 2014 Elsevier B.V. All rights reserved.
PubMed Disclaimer
Similar articles
- Pediatric multiple sclerosis. Bigi S, Banwell B. Bigi S, et al. J Child Neurol. 2012 Nov;27(11):1378-83. doi: 10.1177/0883073812452784. Epub 2012 Aug 21. J Child Neurol. 2012. PMID: 22914372 Review.
- Association between clinical conversion to multiple sclerosis in radiologically isolated syndrome and magnetic resonance imaging, cerebrospinal fluid, and visual evoked potential: follow-up of 70 patients. Lebrun C, Bensa C, Debouverie M, Wiertlevski S, Brassat D, de Seze J, Rumbach L, Pelletier J, Labauge P, Brochet B, Tourbah A, Clavelou P; Club Francophone de la Sclérose en Plaques. Lebrun C, et al. Arch Neurol. 2009 Jul;66(7):841-6. doi: 10.1001/archneurol.2009.119. Arch Neurol. 2009. PMID: 19597085
- Diagnosis and management of multiple sclerosis. Calabresi PA. Calabresi PA. Am Fam Physician. 2004 Nov 15;70(10):1935-44. Am Fam Physician. 2004. PMID: 15571060 Review.
- Differential diagnosis of multiple sclerosis. Fadil H, Kelley RE, Gonzalez-Toledo E. Fadil H, et al. Int Rev Neurobiol. 2007;79:393-422. doi: 10.1016/S0074-7742(07)79018-9. Int Rev Neurobiol. 2007. PMID: 17531852 Review.
- The role of MRI in the diagnosis of multiple sclerosis. Traboulsee AL, Li DK. Traboulsee AL, et al. Adv Neurol. 2006;98:125-46. Adv Neurol. 2006. PMID: 16400831 Review.
- Astrocyte Activation and Drug Target in Pathophysiology of Multiple Sclerosis. Bisht P, Rathore C, Rathee A, Kabra A. Bisht P, et al. Methods Mol Biol. 2024;2761:431-455. doi: 10.1007/978-1-0716-3662-6_30. Methods Mol Biol. 2024. PMID: 38427254 Review.
- A Closed-Loop Falls Monitoring and Prevention App for Multiple Sclerosis Clinical Practice: Human-Centered Design of the Multiple Sclerosis Falls InsightTrack. Block VJ, Koshal K, Wijangco J, Miller N, Sara N, Henderson K, Reihm J, Gopal A, Mohan SD, Gelfand JM, Guo CY, Oommen L, Nylander A, Rowson JA, Brown E, Sanders S, Rankin K, Lyles CR, Sim I, Bove R. Block VJ, et al. JMIR Hum Factors. 2024 Jan 11;11:e49331. doi: 10.2196/49331. JMIR Hum Factors. 2024. PMID: 38206662 Free PMC article.
- Factors associated with the number of months of delaying in multiple sclerosis diagnosis: Comparison of count regression models. Hosseinnataj A, Nikbakht R, Mousavinasab SN, Eskandarieh S, Sahraian MA, Baghbanian SM. Hosseinnataj A, et al. Curr J Neurol. 2023 Apr 4;22(2):65-71. doi: 10.18502/cjn.v22i2.13330. Curr J Neurol. 2023. PMID: 38011390 Free PMC article.
- Neuroprotective effects of gemfibrozil in neurological disorders: Focus on inflammation and molecular mechanisms. Ivraghi MS, Zamanian MY, Gupta R, Achmad H, Alsaab HO, Hjazi A, Romero-Parra RM, Alwaily ER, Hussien BM, Hakimizadeh E. Ivraghi MS, et al. CNS Neurosci Ther. 2024 Mar;30(3):e14473. doi: 10.1111/cns.14473. Epub 2023 Oct 30. CNS Neurosci Ther. 2024. PMID: 37904726 Free PMC article. Review.
- Physical activity assessment among patients with multiple sclerosis in Saudi Arabia. Alkahtani RF, Alhinti MF, AlRashid MH, Alomar AA, Alrumaih SS, Alkanhal AF, AlHarbi AA, AlAbdulSalam AM. Alkahtani RF, et al. Neurosciences (Riyadh). 2023 Oct;28(4):243-249. doi: 10.17712/nsj.2023.4.20230024. Neurosciences (Riyadh). 2023. PMID: 37844952 Free PMC article.
Publication types
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- Elsevier Science
Other Literature Sources
- scite Smart Citations
- Genetic Alliance
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.9(7); 2017 Jul

Psychiatric Symptoms as the First Clinical Presentation of Multiple Sclerosis: A Case Report
Muhammad aadil.
1 Department of Medicine, FMH College of Medicine
Aitzaz Munir
2 Department of Psychiatry, Howard University Hospital
Muhammad Jahanzaib Anwar
3 Department of Internal Medicine, Rush University Medical Center
Hasnain Arshad
4 Department of Internal Medicine, Howard University Hospital
Ibrar Anjum
5 Internal medicine, University of health science Lahore
Aniqa Faraz
6 Department of Internal Medicine, King Edward Medical University Lahore, Pakistan
Multiple sclerosis (MS) is an autoimmune condition which affects the axon myelination in the brain. There can be multiple ways it can present initially, but physical signs and symptoms are the most common ones. We are reporting a case of MS from Pakistan which presented with neuropsychiatric features and was offered psychiatric care, but the patient declined treatment because of stigma related to psychiatric care. Four months later when her condition worsened, further investigation showed it to be a case of MS. The purpose of this case report is that psychiatric features should be considered for differentials of MS.
Introduction
Multiple sclerosis (MS) is a disease caused by an immune-mediated process against central nervous system (CNS) causing neural demyelination and degeneration resulting in interruption of nerve impulses traveling to and from the brain [ 1 ]. This inflammatory process causes progressive loss of neural functions causing sensory loss, spasticity, muscle cramping, pain, optic neuritis, transverse myelitis, trigeminal neuralgia, etc. which are common presentations of MS [ 2 ]. Currently, available therapies for MS are rarely effective to halt disease progression and cause undesirable side effects. It is one of the most common causes of neural disability in young adults, affecting about 2.3 million people worldwide in 2013 [ 3 ]. The current prevalence of this disease in the USA is 90 per 100,000 population [ 1 ]. However, the data is not available to confirm its prevalence rate in Pakistan. Multiple sclerosis can present as relapsing-remitting MS (most common 85% of cases), primary progressive, secondary progressive and progressive-relapsing [ 1 - 2 ]. Peculiar disease course of relapsing-remitting MS has always been the key to its diagnosis in the past. It very rarely presents with a psychiatric symptom as reported by Mahboobi in his case report, where delirium was the first symptom of MS, in 2014-15 [ 4 ].
In the current case, a young woman presented with inappropriate behavior suggestive of bipolar disorder as a presenting symptom of MS which was overlooked for a long time by the healthcare providers until she started showing signs of physical disability.
Case presentation
A 27-year-old Pakistani woman presented in outpatient department with complaints of spells of crying, laughing without any reason and aggressive behavior for three years with few period of being symptom-free. Her general physician considering her symptoms referred her to a psychiatrist. The psychiatrist keeping histrionic personality disorder as the top most differential diagnosis gave her some medication (which she didn’t have any record of) and offered some cognitive therapy. The patient's family stopped taking her to the session due to the stigma of psychiatric treatment. Her condition did not improve. Four months before presentation, she started to develop gradual, progressive and painful weakness of right lower limb. The next three months she could not do her daily routine activities and eventually became bedridden. On examination, she had bilaterally positive Babinski sign, increased tone in the lower extremity, exaggerated knee reflex, and diminished ankle reflex, power in lower limb was ⅖ and decreased sensation as well. In upper limbs, she had a normal tone with inverted biceps reflex and absent brachioradialis reflex, sensations were intact in the upper limb. There was decreased sensation on left side of the face suggesting left trigeminal nerve involvement. Taste sensation was lost on the right side of anterior two-third of the tongue indication right facial damage. Rest of the cranial nerves were normal in function. The fundoscopic examination was normal. A provisional diagnosis of inflammatory demyelinating diseases was made. Magnetic resonance imaging (MRI) of brain and spinal cord without gadolinium contrast were done which showed abnormal signal intensity areas involving multiple bilateral subcortical superficial white matter of frontal, parietal and temporal regions and deep white matter of right parietal and left temporal regions. Lesions were hypointense on T1 weight image (T1W1) and hyperintense on T2W1 and fluid-attenuated inversion recovery (FLAIR) sequence. There was no perilesional edema, mass effect or midline shift. MRI of spine did not show any abnormality. Cerebrospinal fluid (CSF) examination showed positive oligoclonal bands, and hence the patient was labeled as Primary progressive multiple sclerosis (Figure (Figure1 1 ).

This case report highlights that behavioral changes can be the first presenting symptoms of MS. This presentation is very rare. We conducted a brief literature review search using Pubmed, Google Scholar, Scopus which yielded no similar presentation in Pakistan. To our knowledge, this is the first case reported from this region.
Claudia Diaz-Olavarrieta along with her co-authors conducted a study on patients with MS, and the results showed patients developing neuropsychiatric symptoms as the disease evolved. Depression was the most common manifestation in the studied patients (79%). The other psychiatric alterations these patients developed include, agitation 40%, anxiety 37%, irritability 35%, apathy 20%, euphoria 13%, disinhibition 13% and hallucinations 10% [ 5 ]. The pathophysiology of the development of these symptoms remains to be poorly understood. Studies indicate that demyelinating lesions and neural degeneration at the temporal lobe of the brain, which is the language and emotion center, can be the cause of these symptoms [ 6 - 7 ]. Fazzito, et al. reported multiple cases of MS with some psychiatric alterations. In these cases, three patients manifested psychosis as the disease progressed, only one presented with depression and one with changes in behavior as presenting complaints, respectively. She described these alterations being common during the disease course but only 1% of MS cases present with psychiatric symptoms as an initial symptom [ 8 ]. Psychiatric symptoms manifestation is prevalent in established MS, but initial presentation with these symptoms is rare. Studies showed that more than half of the patients with MS develop depression or another psychiatric symptom during the disease, overlooking these symptoms can result in underdiagnosis and complications [ 9 ]. In another case report, published in 2009 by Smith, a female patient presented with erotomanic delusion and complaints of sexual harassment. Her diagnosis of MS was later confirmed by MRI of the brain and lumbar puncture [ 10 ].
In our case, the patient presented with laughing and crying spells along with aggressive behavior. She was diagnosed with a psychiatric condition and was being treated. This resulted in progression of her underlying MS which caused her pain and suffering. When the patient developed signs of physical disability limiting her activities, the physician did further examination which showed signs suggestive of demyelinating disease. As her symptoms started three years ago, she was diagnosed with primary progressive MS. MRI of brain and spinal cord along with CSF examination confirmed the diagnosis of MS.
Conclusions
Formal evaluation for any organic cause should be done if the patient presents with a psychiatric symptom unresponsive to therapy. An underlying cause should be kept in mind as misdiagnosis could result in poor prognosis and increased patient suffering. By diagnosing such patients early and providing them with proper treatment, we can reduce physical disability.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study. Informed consent obtained.
- Open access
- Published: 12 August 2024
Micropapillary breast carcinoma in comparison with invasive duct carcinoma. Does it have an aggressive clinical presentation and an unfavorable prognosis?
- Yasmine Hany Abdel Moamen Elzohery 1 , 5 ,
- Amira H. Radwan 2 , 5 ,
- Sherihan W. Y. Gareer 2 , 5 ,
- Mona M. Mamdouh 3 , 5 ,
- Inas Moaz 4 , 5 ,
- Abdelrahman Mohammad Khalifa 5 ,
- Osama Abdel Mohen 5 ,
- Mohamed Fathy Abdelfattah Abdelrahman Elithy 5 nAff6 &
- Mahmoud Hassaan 5 nAff7
BMC Cancer volume 24 , Article number: 992 ( 2024 ) Cite this article
288 Accesses
Metrics details
Invasive micropapillary carcinoma (IMPC) was first proposed as an entity by Fisher et al. In the 2003 World Health Organization (WHO) guidelines for histologic classification of the breast tumors. IMPC was recognized as a distinct, rare histological subtype of breast cancer.
IMPC is emerging as a surgical and oncological challenge due to its tendency to manifest as a palpable mass, larger in size and higher in grade than IDC with more rate of lymphovascular invasion (LVI) and lymph node (LN) involvement, which changes the surgical and adjuvant management plans to more aggressive, with comparative prognosis still being a point of ongoing debate.
Aim of the study
In this study, we compared the clinicopathological characteristics, survival and surgical management of breast cancer patients having invasive micropapillary carcinoma pathological subtype in comparison to those having invasive duct carcinoma.
This is a comparative study on female patients presented to Baheya center for early detection and treatment of breast cancer, in the period from 2015 to 2022 diagnosed with breast cancer of IMPC subtype in one group compared with another group of invasive duct carcinoma. we analyzed 138 cases of IMPC and 500 cases of IDC.
The incidence of LVI in the IMPC group was 88.3% in comparison to 47.0% in the IDC group (p < 0.001). IMPC had a higher incidence of lymph node involvement than the IDC group (68.8% and 56% respectively). IMPC had a lower rate of breast conserving surgery (26% vs.37.8%) compared with IDC.
The survival analysis indicated that IMPC patients had no significant difference in overall survival compared with IDC patients and no differences were noted in locoregional recurrence rate and distant metastasis rate comparing IMPCs with IDCs.
The results from our PSM analysis suggested that there was no statistically significant difference in prognosis between IMPC and IDC patients after matching them with similar clinical characteristics. However, IMPC was found to be more aggressive, had larger tumor size, greater lymph node metastasis rate and an advanced tumor stage.
Peer Review reports
Introduction
Breast cancer is the most common cancer in women. In the 2012 World Health Organization (WHO) classification of breast cancer. Breast Cancer is classified into up to 21 different histological types depending on cell growth, morphology and architecture patterns [ 1 ]. The invasive carcinoma of no special type (IBC-NST), which is known as invasive ductal carcinoma (IDC), is the most frequently occurring histological type, which constitutes around 75% of invasive breast carcinoma [ 2 ].
Invasive micropapillary carcinoma (IMPC) was first proposed as an entity by Fisher et al. in 1980 [ 3 ] and first described as the term “invasive micropapillary carcinoma” by Siriaunkgul et al. [ 4 ] in 1993.
In the 2003 World Health Organization (WHO) guidelines for histologic classification of the breast tumors [ 5 ]. IMPC was recognized as a distinct, rare histological subtype of breast cancer. While micropapillary histological architecture is present in 2–8% of breast carcinomas, pure micropapillary carcinoma is uncommon and accounts for 0.9–2% of all breast cancers [ 6 ].
IMPC exhibits more distinct morphologic architecture than the IDC, characterized by pseudopapillary and tubuloalveolar arrangements of tumor cell clusters in clear empty sponge-like spaces that resemble extensive lymphatic invasion [ 7 ]. The neoplastic cell exhibits an “inside-out” pattern, known as the reverse polarity pattern [ 2 ].
Most studies demonstrate that the radiological findings of IMPC are irregular-shaped masses with an angular or spiculated margin on ultrasound, mammography and MRI with heterogeneous enhancement and washout kinetics on MRI [ 8 ].
IMPC had tendency to manifest as a palpable mass, larger in size and higher in grade than IDC with more rate of lymphovascular invasion (LVI) and lymph node (LN) involvement, which changes the surgical and adjuvant management plans to more aggressive, with comparative prognosis still being a point of ongoing debate [ 9 ].
In this study, we compared the clinicopathological characteristics, survival and surgical management of breast cancer patients having invasive micropapillary carcinoma pathological subtype in comparison to those having invasive ductal carcinoma.
Patient and method
This is a comparative study on female patients presented to Baheya center for early detection and treatment of breast cancer, in the period from 2015 to 2022 diagnosed with breast cancer of IMPC subtype in one group compared with another group of invasive duct carcinoma.
This retrospective study analyzed 138 cases of IMPC and 500 cases of IDC. Informed consent was obtained from all patients. Ethical approval is obtained from Baheya center for early detection and treatment of breast cancer and National research center ethics committee. Baheya IRB protocol number:202305150022.
The following clinical-pathological features were analyzed for each case: patient age at diagnosis, clinical presentation, laterality, imaging findings, histopathological examination, treatment plan with either primary surgical intervention or other treatment protocol according to tumor stage and biological subtypes.
A breast pathologist evaluated the tumor size, type, grade, lymphovascular invasion, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) receptor and the axillary lymph node involvement.
According to the ASCO/CAP guideline update, 2019: Samples with 1% to 100% of tumor nuclei positive for ER or progesterone receptor (PgR) are interpreted as positive. If ER (not PgR), 1% to 10% of tumor cell nuclei are immunoreactive, the sample are reported as ER Low Positive. There are limited data on the overall benefit of endocrine therapies for patients with low level (1%-10%) ER expression, but they currently suggest possible benefit, so patients are considered eligible for endocrine treatment. A sample is considered negative for ER or PgR if < 1% or 0% of tumor cell nuclei are immunoreactive [ 10 ]. An Allred score between 0 and 8. This scoring system looks at what percentage of cells test positive for hormone receptors, along with how well the receptors show up after staining, called intensity: proportion of cells staining (0, no staining; 1, < 1%; 2, between 1 and 10%; 3, between 11 and 33%; 4, between 34 and 66% and 5, between 67%–100% of the cells staining). Intensity of positive tumor cells (0, none; 1, weak, 2, intermediate; and 3, strong) [ 11 ].
HER2 Test Guideline IHC Recommendations, 2018. IHC 0: as defined by no staining observed or membrane staining that is incomplete and is faint/barely perceptible and within < = 10% of the invasive tumor cells. IHC 1 + : as defined by incomplete membrane staining that is faint/barely perceptible and within > 10% of the invasive tumor cells. IHC 2 + : The revised definition of IHC 2 + (equivocal) is weak to moderate complete membrane staining observed in > 10% of tumor cells. IHC 3 + : based on circumferential membrane staining that is complete, intense in > 10% of tumor cells. [ 12 ].
ASCO–CAP HER2 SISH Test Guideline Recommendations,2018 Twenty nuclei (each containing red (Chr17) and black (HER2) signals) should be enumerated. The final results for the HER2 status are reported based on the ratio formed by dividing the sum of HER2 signals for all 20 nuclei divided by the sum of Chromosome 17 signals for all 20 nuclei. The amplification status is defined as Amplified if the HER2/Chromosome 17 ratio > / = 2.0 and the average Her2 gene copy number is > / = 4.0. It is non-Amplified if the HER2/Chromosome 17 ratio < 2.0 with the Her2 gene copy number is < 4.0. If the HER2/Chr17 ratio is < 2 and the Her2 gene copy number is between 4.0 and 6.0, or, HER2/Chr17 ratio is > / = 2 and the Her2 gene copy number is < 4, or HER2/Chr17 ratio is < 2 and the Her2 gene copy number is > / = 6.0, an additional work should be done. [ 12 ].
Follow-up duration was calculated from the date of diagnosis to the date of the last follow-up. Patients still alive at the last follow-up censored or to the date of occurrence of any event or death.
Disease-free survival was defined as the duration (months) from the initial diagnosis of breast cancer to first any type of recurrence (invasive ipsilateral breast tumor recurrence, local invasive recurrence, regional invasive recurrence, invasive contra lateral breast cancer, distant metastasis.
Overall survival (OS) is defined as the time from diagnosis of breast cancer to death from any cause.
Data were statistically analyzed using an IBM-compatible personal computer with Statistical Package for the Social Sciences (SPSS) version 23. Quantitative data were expressed as mean, standard deviation (SD) and range (minimum–maximum). Qualitative data were expressed as Number (N) and percentage (%), while A P value of < 0.05 was statistically significant. For comparison of unmatched data, chi-square tests were used for categorical variables and t-tests or Mann–Whitney tests for continuous variables.
In this study, we analyzed 138 cases of IMPC which presented to our center in the period from 2015 to 2022.We included a total number of 500 cases of IDC as controls with a ratio of controls to cases 4:1.
Propensity score matching (PSM) is a method for filtrating experimental and control cases of similar characteristics, which are called the matching variables, from existing data to make them comparable in a retrospective analysis. PSM reduce the effect of selection bias. So, the comparison of outcomes between two groups can be fair.
The variables for propensity score matching were selected as follows: age (years), tumour size (cm), nodal status, HR status and HER2 status.
To diminish the effects of baseline differences and potential confounds in clinical characteristics and patients across histology subtypes for outcome differences (disease-free survival and overall survival), PSM method was applied with each micropapillary patient matched to one IDC patient who showed similar baseline characteristics in terms of: menopausal status, comorbidities, multiplicity, histologic grade, tumor size, stage, nodal status, ER /PR status. Differences in prognosis were assessed by Kaplan–Meier analysis.
Most of the patients were postmenopausal, the mean age of patients in IMPC group was 57.36 ± 11.321 years while the mean age of the IDC group was 56.63 ± 9.719 years ( p = 0.45) (Table 1 ).
The most common presentation of IMPC on breast mammography was an irregular shaped mass with a non-circumscribed spiculated margin. while, the most common sonographic finding of IMPC was hypoechoic mass with irregular shapes and spiculated margins. Associated microcalcifications were found in 49 patients (35.5%) of IMPC group. Figs. ( 1 , 2 ): Radiological characteristics of IMPC.
A , B 37-years-old female patient presented with Left breast UOQ extensive fine pleomorphic and amorphous calcifications of segmental distribution, with UOQ multiple indistinct irregular masses. C ultrasound showed left breast UOQ multiple irregular hypoechoic masses with calcific echogenic foci, the largest is seen at 1 o’clock measuring 13 × 15mm. Intraductal echogenic lesions are noted
A , B , C 40-years-old female patient presented with left UOQ extensive pleomorphic microcalcifications of segmental distribution reaching the areola, with multiple well-circumscribed small obscured masses. D , E complementary Ultrasound showed left 2 o’clock multiple ill-defined and well-defined hypoechoic masses (BIRADS 5)
All patients underwent axillary sonography where 77 patients (55.8%) of the IMPC group exhibited pathological lymph nodes and 18 patients (13%) had indeterminate lymph nodes demonstrating preserved hila and associated with either a symmetrical increase of their cortical thickness reaching 3mm or with a focal increase in the cortical thickness.
Multiple lesions were detected in 30% of IMPC patients in comparison to 7% of IDC patients. Intra-ductal extension with nipple involvement was found in 44 patients (31.9%) of the IMPC group (Table 2 ).
MRI was done for 5 cases (3.6%), while CESM was performed for 18 cases (13%) of the IMPC group, the commonest presentation of IMPC in contrast study was irregular shaped enhanced mass in 21 patients and non-mass enhancement was found in 5 patients. Figs. ( 3 , 4 ).
Further imaging modalities. A , B , C 60-years-old female patient had right breast irregular hypoechoic solid mass by ultrasound (BIRADS 5). D , E CESM showed a right breast irregular heterogeneously enhancing solid mass
Role of CESM in diagnosis of IMPC patients. A , B 42-years-old patient presented with a left LIQ irregular spiculated mass with suspicious microcalcifications, other similar lesions were seen anterior and posterior at the same line. C Ultrasound showed a heterogeneously hypoechoic irregular mass with a spiculated outline with multiple similar satellite lesions were seen anterior and posterior to the main lesions
The average tumor size in the IMPC and IDC groups was 3.37 ± 2.04 cm and 2.72 ± 1.39 cm, respectively ( P < 0.001).
The percentage of tumors larger than 5cm, was reported 9.5% in IMPC and 7.4% in IDC.
The pure form of IMPC was the most common type and found in 90 cases (65%) and 47 cases (34%) were mixed type where IDC was the commonest associated type.
There are 6 cases in the IMPC group diagnosed as invasive mucinous carcinoma on biopsy, then in the specimen was mixed invasive micropapillary, IBC-NST and invasive mucinous carcinoma.
On core biopsy, 28 cases were diagnosed as IMPC with focal IDC component, but in corresponding specimens 10 cases were only approved to be mixed invasive micropapillary and invasive duct carcinoma, while others diagnosed as pure invasive micropapillary carcinoma without IDC component.
On the other hand, 48 of our cases were diagnosed as IDC on core biopsy, but in the final specimen examination, 17 of these cases were diagnosed as pure invasive micropapillary carcinoma without invasive ductal component.
The explanation of controversy in proper histologic subtyping of carcinoma on core biopsy and the definite subtype on the corresponding specimen was that the ductal component which only represented in the biopsy is a very minor component of the tumor or the limited sampling, tissue fragmentation and architecture distortion in core biopsy may cause diagnostic pitfalls as regard precise subtyping of the tumor.
The incidence of LVI in the IMPC group was 88.3% in comparison to 47.0% in the IDC group ( p < 0.001).
IMPC had a higher incidence of lymph node involvement than the IDC group (68.8% and 56% respectively) with N3 stage reported in 12.4% of IMPC patients.
IMPC had a higher nuclear grade than the IDC group (25.1% and 15.2% respectively).
The percentage of ER-positive patients was 97.8% in the IMPC group and 87.6% in the IDC group ( p < 0.001), while PR-positive cases were 98.6% in the IMPC group and 88.8% in the IDC group ( p < 0.001). HER2 status was positive in 4.3% of IMPCs and 8% of IDCs ( p = 0.23) (Table 3 ) (Figs. 5 , 6 ).
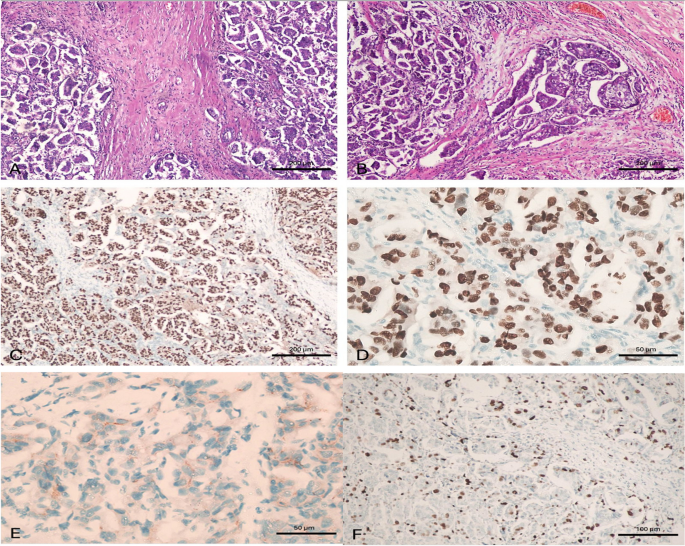
A case of invasive micropapillary carcinoma. A case of invasive micropapillary carcinoma, grade II. A Tissue core biopsy, × 100, B MRM specimen × 100 with Positive metastatic L. nodes 2/15, C ER is positive in > 90% of tumor cells, × 100, D PR is positive in > 90% of tumor cells, × 400, E HER2/neu is negative, × 400 and F) Ki-67 labelling index is high, × 200. This case was considered as luminal type pure invasive micropapillary carcinoma. (100 micron 20__ 50 micron 40)
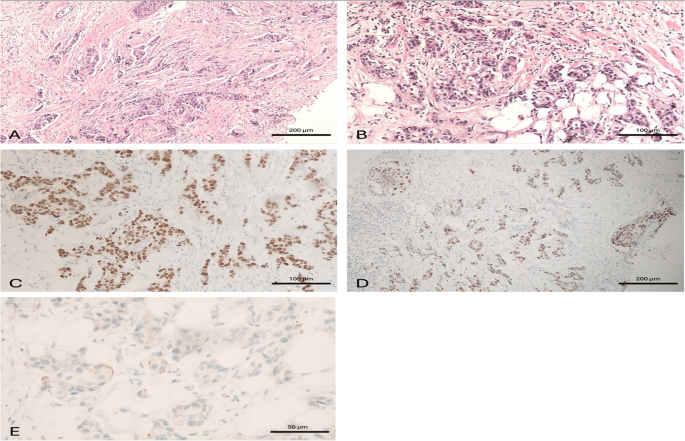
A case of invasive duct carcinoma. A case of invasive duct carcinoma, grade II. A Tissue core biopsy, × 100, B MRM specimen, × 200 with negative L. nodes 0/16, C ER is positive in > 90% of tumor cells, × 200, D PR is positive in > 90% of tumor cells, × 100, E HER2/neu is negative, × 400. This case was considered as luminal type pure invasive duct carcinoma
Regarding definitive surgical management, IMPC had a lower rate of breast conserving surgery (26% vs.37.8%) compared with IDC. While, 49.3% of IMPC patients underwent modified radical mastectomy in comparison to 46% of the IDC patients. Such high incidence of mastectomy was due to the advanced stage at presentation, presence of multiple lesions and presence of intra-ductal extension with nipple involvement.
The incidence of re-surgery in the IMPC group was only in 3 cases, two of them underwent completion mastectomy after the initial conservative breast surgery and axillary clearance. While one patient underwent wider margin excision as positive margin for an invasive residual disease was found.
Two patients in the IMPC group had distant metastasis at the initial diagnosis, they had multiple metastatic lesions and received systemic treatment but one of them underwent palliative mastectomy.
Systemic chemotherapy was administered to 107 patients (77.5%) in the IMPC group and to 207 patients (41%) in the IDC group. Hormonal therapy was administered to all IMPC patients and 76% patients in the IDC group (Table 4 ).
The overall median follow-up duration was 21 months (range 6 – 88 months) with mean follow up duration = 29.8months.
Among the 138 IMPC patients, local recurrence developed in 3 cases, they developed a recurrence at 6,18 and 48 months postoperative. Distant metastasis developed in 5 patients in the form of bone, lung, hepatic and mediastinal lymph node metastasis.
The survival analysis indicated that IMPC patients had no significant difference in overall survival compared with IDC patients and no differences were noted in locoregional recurrence rate comparing IMPCs with IDCs (2.2% and 0.4% respectively). P value for local recurrence = 0.12 (yates corrected chi square).
Distant metastasis rate comparing IMPCs with IDCs was (3.7% and 5.4% respectively). P value for distant metastasis = 0.53 (Table 5 ).
Comparison of OS between IDC and micropapillary cases (Matched by propensity score matching -PSM).
Case Processing Summary
Type | Total N | N of Events | Censored | |
|---|---|---|---|---|
N | Percent | |||
IDC | 125 | 7 | 118 | 94.4% |
Micropapillary | 128 | 3 | 125 | 97.7% |
Overall | 253 | 10 | 243 | 96.0% |
Type | Mean survival time | |||
|---|---|---|---|---|
Estimate | Std. Error | 95% Confidence Interval | ||
Lower Bound | Upper Bound | |||
IDC | 84.596 | 2.314 | 80.061 | 89.131 |
Micropapillary | 57.530 | .844 | 55.876 | 59.185 |
Overall | 85.807 | 1.633 | 82.606 | 89.008 |
Overall Comparisons
Chi-Square | df | Sig. | |
|---|---|---|---|
Log Rank (Mantel-Cox) | .438 | 1 | .508 |
- Test of equality of survival distributions for the different levels type
Disease free survival
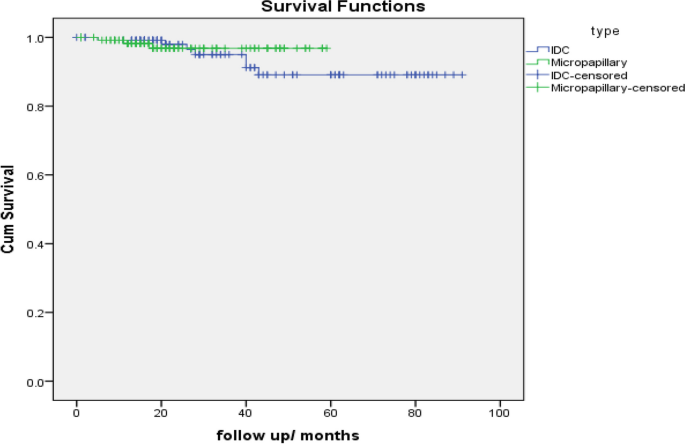
Type | Total N | N of Events | Censored | |
|---|---|---|---|---|
N | Percent | |||
IDC | 124 | 11 | 113 | 91.1% |
Micropapillary | 129 | 5 | 124 | 96.1% |
Overall | 253 | 16 | 237 | 93.7% |
Type | Mean | |||
|---|---|---|---|---|
Estimate | Std. Error | 95% Confidence Interval | ||
Lower Bound | Upper Bound | |||
IDC | 77.324 | 3.019 | 71.407 | 83.242 |
Micropapillary | 56.062 | 1.355 | 53.407 | 58.718 |
Overall | 78.725 | 2.333 | 74.152 | 83.299 |
Chi-Square | df | Sig. | |
|---|---|---|---|
Log Rank (Mantel-Cox) | .380 | 1 | .537 |
- Test of equality of survival distributions for the different levels of type
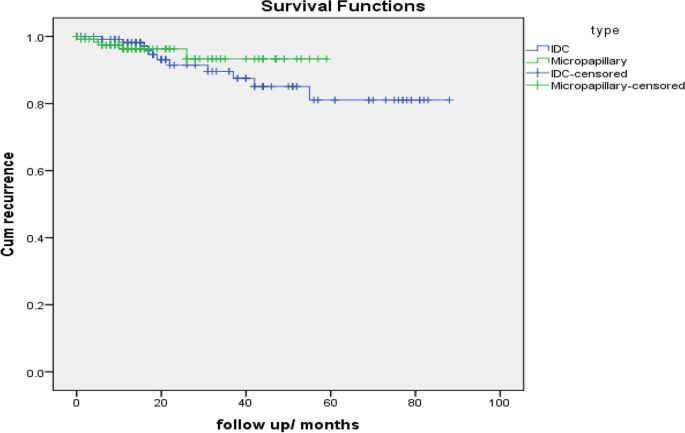
IMPC is a highly invasive type of breast cancer. Hashmi A.A. et al. [ 13 ] found that the incidence of IMPC is very low accounting for 0.76–3.8% of breast carcinomas.
Shi WB et al.; [ 7 ] in a study comparing 188 IMPC cases and 1,289 invasive ductal carcinoma (IDC) cases from China showed that IMPC can occur either alone or mixed with other histological types, such as ductal carcinoma in situ, mucinous carcinoma and IDC. Furthermore, the majority of patients had mixed IMPC.
Fakhry et al. [ 14 ] reported that 64.7% of IMPC patients were pure type. In our study, we found that the pure form of IMPC was the commonest type and presented in 90 patients (65%) and 47 cases (34%) were mixed type which was similar to that reported by Nassar et al. [ 15 ], and Guo et al. [ 16 ] in their studies.
In our study, the commonest finding of IMPC on breast mammography was an irregular shaped mass with a non-circumscribed spiculated margin. While, the commonest sonographic finding of IMPC was hypoechoic mass with irregular shapes and spiculated margins.
These findings were similar to the results demonstrated by Jones et al., [ 17 ] which found that the commonest morphologic finding of IMPC was an irregular high-density lesion (50% of patients) with spiculated margin (42% of patients). However, Günhan-Bilgen et al. [ 18 ] reported that an ovoid or round lesion was found in 53.8% of patients.
Alsharif et al., [ 19 ] reported that the commonest sonographic finding of IMPC was hypoechoic masse (39/41, 95%) with irregular shape (30/41, 73.2%) and angular or spiculated margin (26/41, 63.4%).
In our study, MRI was done for 5 cases (3.6%), while CESM was performed for 18 cases (13%) of the IMPC group, the commonest presentation of IMPC in contrast study was irregular shaped enhanced lesion in 21 cases and non-mass enhancement was presented in 5 cases.
Nangogn et al. [ 20 ] and yoon et al. [ 8 ] recorded that the commonest finding of IMPCs in MRI was spiculated irregular mass with early rapid initial heterogenous enhancement, indicating that the MRI findings correlated with the invasiveness of IMPC.
Fakhry et al. [ 14 ] conducted a study on 68 cases, out of which 17 cases underwent CEM. In all of these cases, the masses showed pathological enhancement, which was either in the form of mass enhancement (12/17 patients, 70.6%) or non-mass enhancement (4/17 patients, 23.5%). The majority of the enhanced masses were irregular in shape (11/12 patients, 91.7%).
All patients underwent axillary sonography and 77 patients (55.8%) of the IMPC group exhibited pathological lymph nodes; this percentage was similar to that recorded by Nangong et al. [ 20 ] which was 54.8% and lower than that recorded by Jones et al. [ 17 ] but higher than that of Günhan et al. [ 18 ] which were 67% and 38% respectively.
Günhan et al. [ 18 ] reported microcalcification in about 66.7% of the cases. In our study, associated microcalcifications were found in 49 patients (35.5%) of the IMPC group. Yun et al. [ 21 ] and Adrada et al. [ 22 ] showed a fine pleomorphic appearance (66.7% and 68%).
Hao et al. [ 23 ] compared the rate of tumors larger than 5cm, reporting 3% in IDC and 4.3% in IMPC. In our study, the rate of tumors larger than 5cm, was reported 7.4% in the IDC patients and 9.5% in the IMPC patients.
Yu et al., et al. [ 24 ] documented in a study comparing 72 cases of IMPC and 144 cases of IDC of the breast that IMPC had a higher nuclear grade than IDC (52.8% vs. 37.5% respectively). In our study, IMPC had a higher nuclear grade than the IDC group (25.1% and 15.2% respectively).
Verras GI et al.; [ 9 ] demonstrated that IMPC was an aggressive breast cancer subtype with a great tendency to lymphovascular invasion and lymph node metastasis. In our study, the incidence of LVI in the IMPC patients was 88.3% in comparison to 47.0% in the IDC patients ( p < 0.001). Tang et al., [ 25 ] also reported that lymphovascular involvement was more common among the IIMPC group than IDC group, with a percentage of 14.7% compared to only 0.1% in the IDC group.
Also, Shi et al. [ 7 ] reported that LVI was detected in 74.5% of cases. Furthermore, the frequency of LVI was found to be greater in IMPC cases when compared to IDC cases. Jones et al., [ 17 ] recorded angiolymphatic invasion in 69% of cases.
Hashmi et al. [ 13 ] reported in his comparative study that nodal involvement was present in 49.5% of IDC patients and N3 stage was only 15.6% in IDC patients compared to 33% in IMPC patients. In our study, the percentage of lymph node involvement of IMPC and IDC patients were 68.8% and 56% respectively with N3 stage reported in 12.4% of IMPC patients.
Guan et al. [ 26 ], Lewis et al., [ 27 ], Pettinato et al., [ 28 ] and De La Cruz et al., [ 29 ] recorded a higher percentage of lymph node metastasis in IMPC patients, reaching 90%, 92.9%,55.2% and 60.9% respectively.
The management of IMPC remains controversial, particularly among breast surgeons. Modified radical mastectomy was the preferred surgical procedure for the majority of IMPC case reports, as found in a study conducted by Yu et al., [ 24 ] where 99% of IMPC cases underwent modified radical mastectomy. Fakhry et al. [ 14 ] reported that 76.5% of the patients underwent modified radical mastectomy. In our study, 49.3% of IMPC patients received modified radical mastectomy.
IMPC patients were also prone to accept BCS rather than mastectomy in the previous series conducted by Lewis GD,et al. [ 27 ] and Vingiani, A. et al. [ 30 ]. However, the precise prognosis value of BCS for patients with IMPC remained unknowable. In our study, IMPC had a lower rate of breast conserving surgery (26% vs.37.8%) compared with IDC.
IMPC was characterized by a high incidence of ER and PR positivity. Our study recorded a high percentage of ER (97.8%) and PR (98.6%) expression. Our findings are similar to those found by Walsh et al., [ 31 ] who reported ER and PR expression of 90% and 70%, respectively. Zekioglu et al. [ 32 ] demonstrated a rate of ER and PR expression of 68% and 61%respectively.
In this study, we reported a relatively lower percentage of HER-2 positivity (4.3%). Also, Nangong et al. [ 20 ] showed HER 2 overexpression in 26.4% of cases.
However, Cui et al. [ 33 ] reported a much higher incidence of HER 2 positivity and Perron et al., [ 34 ] reported that 65% of IMPCs were HER-2 positive.
Chen, A et al. [ 35 ] reported that that the percentage of radiation therapy for IMPC patients was similar to those seen in IDC patients and demonstrates a similar benefit of radiation treatment in both groups. In our study,77.5% patients received radiotherapy in IMPC group in compared to 59.4% patients in IDC group.
Shi et al. [ 7 ] found that patients with IMPC had worse recurrence-free survival (RFS) and overall survival (OS) rates as compared to those with IDC. However, because IMPC is relatively rare, most studies had reported on small sample sizes with limited follow-ups.
Yu et al., [ 24 ] conducted a comparison between IMPC and IDC patients, and the results showed that the IMPC group had a greater tendency for LRR compared to the IDC group ( P = 0.03), but the distant metastasis rate ( P = 0.52) and OS rate ( P = 0.67) of the IMPC showed no statistical differences from the IDC group.
Nevertheless, several recent studies documented that IMPC had better or similar prognosis in comparison to IDC.
Hao et al. [ 23 ] and Vingiani et al. [ 30 ] documented that there was no statistically significant difference in OS and disease-free survival between IMPC patients and IDC patients which was similar to our results. locoregional recurrence rate comparing IMPCs with IDCs was (2.2% and 0.4% respectively). P value for local recurrence = 0.12 (yates corrected chi square). Distant metastasis rate comparing IMPCs with IDCs was (3.7% and 5.4% respectively). P value for distant metastasis = 0.53.
Chen H et al. [ 36 ], compared the overall survival in patient groups with similar nodal involvement and found that IMPC group had better breast cancer–specific survival and overall survival than IDC group.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
Invasive micropapillary carcinoma
Invasive duct carcinoma
Modified radical mastectomy
Conserving breast surgery
Estrogen receptor
Progesterone receptor
Lymphovascular invasion
Contrast enhanced spectral mammography
Overall survival
Lakhani SR. International Agency for Research on Cancer Press and World Health Organization. WHO Classification of Tumours of the Breast. Lyon: International Agency for Research on Cancer Press; 2012.
Wu Y, Zhang N, Yang Q. The prognosis of invasive micropapillary carcinoma compared with invasive ductal carcinoma in the breast: a meta-analysis. BMC Cancer. 2017;17:839.
Article PubMed PubMed Central Google Scholar
Fisher ER, Palekar AS, et al. Pathologic findings from the national surgical adjuvant breast project (protocol no. 4). Vi. Invasive papillary cancer. Am J Clin Pathol. 1980;73:313–22.
Article PubMed CAS Google Scholar
Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol. 1993;6:660–2.
PubMed CAS Google Scholar
Hanby AM, walker C, Tavassoli FA, Devilee P. Pathology and Genetics: Tumours of the Breast and Female Genital Organs. WHO Classification of Tumours series. Breast Cancer Res. Lyon: IARC Press; 2004;4(6):133. https://doi.org/10.1186/bcr788 .
Yang YL, Liu BB, Zhang X, Fu L. Invasive micropapillary carcinoma of the breast: an update. Arch Pathol Lab Med. 2016;140(8):799–805. https://doi.org/10.5858/arpa.2016-0040-RA .
Shi WB, Yang LJ, et al. Clinico-pathological features and prognosis of invasive micropapillary carcinoma compared to invasive ductal carcinoma: a population-based study from china. PLoS ONE. 2014;9:e101390.
Yoon GY, Cha JH, Kim HH, Shin HJ, Chae EY, Choi WJ. Comparison of invasive micropapillary and invasive ductal carcinoma of the breast: a matched cohort study. Acta Radiol. 2019;60(11):1405–13.
Article PubMed Google Scholar
Verras GI, et al. Micropapillary breast carcinoma: from molecular pathogenesis to prognosis. Breast Cancer (Dove Med Press). 2022;12(14):41–61.
Google Scholar
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J Clin Oncol. 2020;38(12):1346–66. https://doi.org/10.1200/JCO.19.02309 .
Fitzgibbons PL, Dillon DA, Alsabeh R, Berman MA, Hayes DF, Hicks DG, Hughes KS, Nofech-Mozes S. Template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. Arch Pathol Lab Med. 2014;138(5):595–601.
Ahn S, Woo JW, Lee K, Park SY. HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. J PatholTransl Med. 2020;54(1):34.
Hashmi AA, et al. Clinicopathologic features of invasive metaplastic and micropapillary breast carcinoma: comparison with invasive ductal carcinoma of breast. BMC Res Notes. 2018;11:1–7.
Article Google Scholar
Fakhry S, et al. Radiological characteristics of invasive micropapillary carcinoma of the breast. Clin Radiol. 2024;79(1):e34–40.
Nassar H, Wallis T, Andea A, et al. Clinicopathologic analysis of invasive micropapillary differentiation in breast carcinoma. Mod Pathol. 2001;14:836e41.
Guo X, Chen L, Lang R, et al. Invasive micropapillary carcinoma of the breast: association of pathologic features with lymph node metastasis. Am J Clin Pathol. 2006;126:740e6.
Jones KN, Guimaraes LS, Reynolds CA, Ghosh K, Degnim AC, Glazebrook KN. Invasive micropapillary carcinoma of the breast: imaging features with clinical and pathologic correlation. AJR Am J Roentgenol. 2013;200:689–95.
Günhan-Bilgen I, et al. Invasive micropapillary carcinoma of the breast: clinical, mammographic, and sonographic findings with histopathologic correlation. AJR Am J Roentgenol. 2002;179:927–31.
Alsharif S, et al. Mammographic, sonographic and MR imaging features of invasive micropapillary breast cancer. Eur J Radiol. 2014;83(8):1375–80.
Nangong J, Cheng Z, Yu L, Zheng X, Ding G. Invasive micropapillary breast carcinoma: a retrospective study on the clinical imaging features and pathologic findings. Front Surg. 2022;23(9):1011773.
Yun SU, Choi BB, Shu KS, et al. Imaging findings of invasive micropapillary carcinoma of the breast. J Breast Cancer. 2012;15:57e64.
Adrada B, Arribas E, Gilcrease M, et al. Invasive micropapillary carcinoma of the breast: mammographic, sonographic, and MRI features. AJR Am J Roentgenol. 2009;193:58e63.
Hao S, Zhao Y, Peng J, et al. Invasive micropapillary carcinoma of the breast had no difference in prognosis compared with invasive ductal carcinoma: a propensity-matched analysis. Sci Rep. 2019;9:1–8.
Yu JI, Choi DH, Huh SJ, et al. Differences in prognostic factors and failure patterns between invasive micropapillary carcinoma and carcinoma with micropapillary component versus invasive ductal carcinoma of the breast: retrospective multicenter case-control study (KROG 13–06). Clin Breast Cancer. 2015;15:353–361.e2.
Tang S-L, Yang J-Q, Du Z-G, et al. Clinicopathologic study of invasive micropapillary carcinoma of the breast. Oncotarget. 2017;8:42455–65.
Guan X, Xu G, Shi A, et al. Comparison of clinicopathological characteristics and prognosis among patients with pure invasive ductal carcinoma, invasive ductal carcinoma coexisted with invasive micropapillary carcinoma, and invasive ductal carcinoma coexisted with ductal carcinoma. Medicine (Baltimore). 2020;99:e23487.
Lewis GD, Xing Y, Haque W, et al. The impact of molecular status on survival outcomes for invasive micropapillary carcinoma of the breast. Breast J. 2019;25:1171e6.
Pettinato G, Pambuccian SE, Di Prisco B, et al. Fine needle aspiration cytology of invasive micropapillary (pseudopapillary) carcinoma of the breast: report of 11 cases with clinicopathologic findings. Acta Cytol. 2002;46:1088e94.
De La Cruz C, et al. Invasive micropapillary carcinoma of the breast: clinicopathological and immunohistochemical study. Pathol Int. 2004;54:90–6.
Vingiani A, et al. The clinical relevance of micropapillary carcinoma of the breast: a case–control study. Histopathology. 2013;63:217–24.
Walsh MM, Bleiweiss IJ. Invasive micropapillary carcinoma of the breast: eighty cases of an underrecognized entity. Hum Pathol. 2001;32:583–9.
Zekioglu O, et al. Invasive micropapillary carcinoma of the breast: high incidence of lymph node metastasis with extranodal extension and its immunohistochemical profile compared with invasive ductal carcinoma. Histopathology. 2004;44:18–23.
Cui ZQ, et al. Clinicopathological features of invasive micropapillary carcinoma of the breast. Oncol Lett. 2015;9:1163–6.
Perron M, Wen HY, Hanna MG, Brogi E, Ross DS. HER2 Immunohistochemistry in invasive micropapillary breast carcinoma: complete assessment of an incomplete pattern. Arch Pathol Lab Med. 2021;145:979–87.
Article PubMed PubMed Central CAS Google Scholar
Chen A, Paulino A, Schwartz M, et al. Population-based comparison of prognostic factors in invasive micropapillary and invasive ductal carcinoma of the breast. Br J Cancer. 2014;111:619–22.
Chen H, Wu K, Wang M, Wang F, Zhang M, Zhang P. Invasive micropapillary carcinoma of the breast has a better long-term survival than invasive ductal carcinoma of the breast in spite of its aggressive clinical presentations: a comparison based on large population database and case–control analysis. Cancer Med. 2017;6:2775–86.
Download references
Acknowledgements
Not applicable.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Mohamed Fathy Abdelfattah Abdelrahman Elithy
Present address: Department of Surgical Oncology, Faculty of Medicine, Al Azhar University, Cairo, Egypt
Mahmoud Hassaan
Present address: Departement of Surgical Oncology, National Cancer Institute, Cairo University, Giza, Egypt
Authors and Affiliations
Department of General Surgery, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Yasmine Hany Abdel Moamen Elzohery
Department of Radiodiagnosis, NCI, Cairo University, Giza, Egypt
Amira H. Radwan & Sherihan W. Y. Gareer
Department of Pathology, National Cancer Institute, Cairo University, Giza, Egypt
Mona M. Mamdouh
Department of Epidemiology and Preventive Medicine, National Liver Institute, Menoufia, Egypt
Baheya Center for Early Detection and Treatment of Breast Cancer, Giza, Egypt
Yasmine Hany Abdel Moamen Elzohery, Amira H. Radwan, Sherihan W. Y. Gareer, Mona M. Mamdouh, Inas Moaz, Abdelrahman Mohammad Khalifa, Osama Abdel Mohen, Mohamed Fathy Abdelfattah Abdelrahman Elithy & Mahmoud Hassaan
You can also search for this author in PubMed Google Scholar
Contributions
Mohamed fathy participated in the sequence alignment and Yasmine hany drafted the manuscript. Mahmoud Hassan participated in the design of the study. Inas Moaz and Abdelrahman Mohammad performed the statistical analysis. Amira H. Radwan and Sherihan WY Gareer conceived the study. Mona M Mamdouh and Osama abdel Mohen participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Yasmine Hany Abdel Moamen Elzohery .
Ethics declarations
Ethics approval and consent to participate.
Available all patients provided informed consent for publication. All patients provided signed written informed consent.
Consent for publication
Ethical approval is obtained from Baheya center for early detection and treatment of breast cancer and National research center ethics committee. Baheya IRB protocol number: 202305150022.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Elzohery, Y.H.A.M., Radwan, A.H., Gareer, S.W.Y. et al. Micropapillary breast carcinoma in comparison with invasive duct carcinoma. Does it have an aggressive clinical presentation and an unfavorable prognosis?. BMC Cancer 24 , 992 (2024). https://doi.org/10.1186/s12885-024-12673-0
Download citation
Received : 05 April 2024
Accepted : 23 July 2024
Published : 12 August 2024
DOI : https://doi.org/10.1186/s12885-024-12673-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
COMMENTS
Key points The diagnosis of multiple sclerosis is a clinical diagnosis supported by investigation findings. There is no single sensitive and specific diagnostic test for multiple sclerosis. The principle of dissemination of lesions in time and space underpins the diagnosis.
One attack. Objective clinical evidence of 1 lesion (clinically isolated syndrome) or. and. One or more T2 lesions in brain, in regions characteristic of MS. Two or more T2 focal lesions in spinal cord. Positive CSF. Multiple sclerosis (MS) is an immune-mediated inflammatory disease that attacks myelinated axons in the central nervous system ...
INTRODUCTION. Multiple sclerosis (MS) is the most common immune-mediated inflammatory demyelinating disease of the central nervous system. The onset and phenotypes of MS will be reviewed here. Other aspects of MS are discussed separately: Pathogenesis and epidemiology of multiple sclerosis. Management of clinically and radiologically isolated ...
Multiple sclerosis (MS) is the most common immune-mediated inflammatory demyelinating disease of the central nervous system. MS is characterized pathologically by multifocal areas of demyelination with loss of oligodendrocytes and astroglial scarring. Axonal injury is also a prominent pathologic feature, especially in the later stages. Certain clinical features are typical of MS, but the ...
Multiple sclerosis (MS) is a potentially disabling disease of the brain and spinal cord (central nervous system). In MS, the immune system attacks the protective sheath (myelin) that covers nerve fibers and causes communication problems between your brain and the rest of your body.
Multiple sclerosis (MS) is a demyelinating disorder of the central nervous system and the most common cause of nontraumatic neurologic disability in young adults. Types of MS include relapsing ...
The first formally defined MS phenotypes, relapsing remitting MS (RRMS), primary progressive MS (PPMS), secondary progressive MS (SPMS), and progressive relapsing MS (PRMS), were proposed in 1996 by the U.S. National Multiple Sclerosis Society (NMSS) Advisory Committee on Clinical Trials in Multiple Sclerosis as a result of increased need for standardized terminology in the field. It was felt ...
In all cases, the practitioner must rule out better explanations for the clinical presentation other than multiple sclerosis. In the context of the MacDonald criteria, a single episode of demyelination and certain findings on a single MRI can fulfill the diagnostic criteria for MS, even before a second clinical episode or new MRI lesion.
Practice Essentials. Multiple sclerosis (MS) is an immune-mediated inflammatory disease that attacks myelinated axons in the central nervous system, destroying the myelin and the axon in variable degrees and producing significant physical disability within 20-25 years in more than 30% of patients. The hallmark of MS is symptomatic episodes ...
Multiple sclerosis (MS) typically presents between 20-50 years of age. About 0.5% of adults with MS first develop symptoms aged 60 years or older — older age at onset is associated with a progressive course. The person may have: A history of previous neurological symptoms. Symptoms that evolve over more than 24 hours, may persist over ...
Multiple sclerosis is a chronic autoimmune disease affecting the central nervous system (CNS) and is characterized by inflammation, demyelination, gliosis, and neuronal loss.[1] This condition manifests with a wide range of neurological symptoms, such as vision impairment, numbness and tingling, focal weakness, bladder and bowel dysfunction, and cognitive impairment.
There is no cure for multiple sclerosis. Treatment typically focuses on speeding recovery from attacks, reducing new radiographic and clinical relapses, slowing the progression of the disease, and managing MS symptoms. Some people have such mild symptoms that no treatment is necessary. Multiple sclerosis research laboratory at Mayo Clinic.
Multiple sclerosis is typically diagnosed based on the presenting signs and symptoms, in combination with supporting medical imaging and laboratory testing. [4] It can be difficult to confirm, especially early on, since the signs and symptoms may be similar to those of other medical problems. [5] [6]
An overview of multiple sclerosis (MS) including aetiology, clinical features (symptoms, signs), investigations, diagnostic criteria and management.
No two people experience multiple sclerosis the same, but there are some common signs and symptoms to note so you can get a diagnosis and treatment.
Multiple sclerosis (MS) is the most common immune-mediated inflammatory demyelinating disease of the central nervous system. The onset and phenotypes of MS will be reviewed here. Other aspects of MS are discussed separately: Pathogenesis and epidemiology of multiple sclerosis.
There are characteristic clinical presentations based on the areas of the central nervous system involved, for example optic nerve, brainstem and spinal cord. The main pattern of MS at onset is relapsing-remitting with clinical attacks of neurological dysfunction lasting at least 24 hours. The differential diagnosis includes other inflammatory ...
MS can also look like this Another potential harbinger of MS is clinically isolated syndrome, or CIS, but this condition is complicated. "CIS is kind of within the spectrum of MS. In fact, sometimes CIS is just the initial presentation of MS. And people with CIS often will go on to develop multiple sclerosis, but not always," Shoemaker explains.
Multiple Sclerosis most often is characterized by episodes of neurological dysfunction followed by periods of stabilization or partial to complete remission of symptoms. These symptoms (relapses or exacerbations) can appear over a few hours or days, can be gradually worsening over a period of a few weeks, or sometimes can present themselves acutely. Depending on a course and a subtype of the ...
The diagnosis of multiple sclerosis (MS) is based on demonstrating evidence of inflammatory-demyelinating injury within the central nervous system that is disseminated in both time and space. Diagnosis is made through a combination of the clinical history, neurologic examination, magnetic resonance imaging and the exclusion of other diagnostic ...
Multiple Sclerosis (MS) is a chronic demyelinating disorder of the central nervous system (CNS) that affects predominately patients aged 20-40 years. The epidemiology of MS is changing worldwide, as is the understanding of its immunopathogenesis and natural history, with new evidence pointing towards a multifactorial etiology involving both environmental and genetic factors (Goodin, 2014 ...
Introduction Multiple sclerosis (MS) is a disease caused by an immune-mediated process against central nervous system (CNS) causing neural demyelination and degeneration resulting in interruption of nerve impulses traveling to and from the brain [ 1 ].
Background Invasive micropapillary carcinoma (IMPC) was first proposed as an entity by Fisher et al. In the 2003 World Health Organization (WHO) guidelines for histologic classification of the breast tumors. IMPC was recognized as a distinct, rare histological subtype of breast cancer. IMPC is emerging as a surgical and oncological challenge due to its tendency to manifest as a palpable mass ...
INTRODUCTION A clinically isolated syndrome (CIS) is a first symptomatic episode compatible with demyelination or multiple sclerosis (MS). In a radiologically isolated syndrome (RIS), an individual presents without overt clinical symptoms but with magnetic resonance imaging (MRI) findings highly suggestive of MS. CIS and RIS can create diagnostic and therapeutic dilemmas, since a substantial ...