Random Assignment in Psychology: Definition & Examples
Julia Simkus
Editor at Simply Psychology
BA (Hons) Psychology, Princeton University
Julia Simkus is a graduate of Princeton University with a Bachelor of Arts in Psychology. She is currently studying for a Master's Degree in Counseling for Mental Health and Wellness in September 2023. Julia's research has been published in peer reviewed journals.
Learn about our Editorial Process
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
In psychology, random assignment refers to the practice of allocating participants to different experimental groups in a study in a completely unbiased way, ensuring each participant has an equal chance of being assigned to any group.
In experimental research, random assignment, or random placement, organizes participants from your sample into different groups using randomization.
Random assignment uses chance procedures to ensure that each participant has an equal opportunity of being assigned to either a control or experimental group.
The control group does not receive the treatment in question, whereas the experimental group does receive the treatment.
When using random assignment, neither the researcher nor the participant can choose the group to which the participant is assigned. This ensures that any differences between and within the groups are not systematic at the onset of the study.
In a study to test the success of a weight-loss program, investigators randomly assigned a pool of participants to one of two groups.
Group A participants participated in the weight-loss program for 10 weeks and took a class where they learned about the benefits of healthy eating and exercise.
Group B participants read a 200-page book that explains the benefits of weight loss. The investigator randomly assigned participants to one of the two groups.
The researchers found that those who participated in the program and took the class were more likely to lose weight than those in the other group that received only the book.

Importance
Random assignment ensures that each group in the experiment is identical before applying the independent variable.
In experiments , researchers will manipulate an independent variable to assess its effect on a dependent variable, while controlling for other variables. Random assignment increases the likelihood that the treatment groups are the same at the onset of a study.
Thus, any changes that result from the independent variable can be assumed to be a result of the treatment of interest. This is particularly important for eliminating sources of bias and strengthening the internal validity of an experiment.
Random assignment is the best method for inferring a causal relationship between a treatment and an outcome.
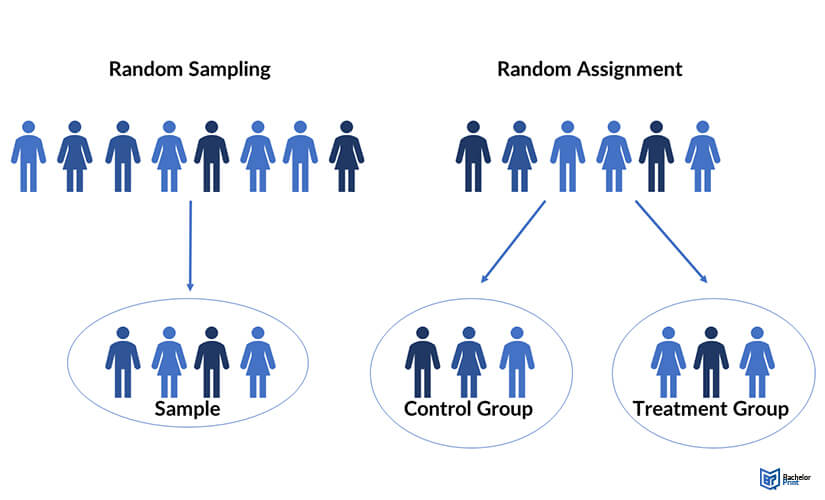
Random Selection vs. Random Assignment
Random selection (also called probability sampling or random sampling) is a way of randomly selecting members of a population to be included in your study.
On the other hand, random assignment is a way of sorting the sample participants into control and treatment groups.
Random selection ensures that everyone in the population has an equal chance of being selected for the study. Once the pool of participants has been chosen, experimenters use random assignment to assign participants into groups.
Random assignment is only used in between-subjects experimental designs, while random selection can be used in a variety of study designs.
Random Assignment vs Random Sampling
Random sampling refers to selecting participants from a population so that each individual has an equal chance of being chosen. This method enhances the representativeness of the sample.
Random assignment, on the other hand, is used in experimental designs once participants are selected. It involves allocating these participants to different experimental groups or conditions randomly.
This helps ensure that any differences in results across groups are due to manipulating the independent variable, not preexisting differences among participants.
When to Use Random Assignment
Random assignment is used in experiments with a between-groups or independent measures design.
In these research designs, researchers will manipulate an independent variable to assess its effect on a dependent variable, while controlling for other variables.
There is usually a control group and one or more experimental groups. Random assignment helps ensure that the groups are comparable at the onset of the study.
How to Use Random Assignment
There are a variety of ways to assign participants into study groups randomly. Here are a handful of popular methods:
- Random Number Generator : Give each member of the sample a unique number; use a computer program to randomly generate a number from the list for each group.
- Lottery : Give each member of the sample a unique number. Place all numbers in a hat or bucket and draw numbers at random for each group.
- Flipping a Coin : Flip a coin for each participant to decide if they will be in the control group or experimental group (this method can only be used when you have just two groups)
- Roll a Die : For each number on the list, roll a dice to decide which of the groups they will be in. For example, assume that rolling 1, 2, or 3 places them in a control group and rolling 3, 4, 5 lands them in an experimental group.
When is Random Assignment not used?
- When it is not ethically permissible: Randomization is only ethical if the researcher has no evidence that one treatment is superior to the other or that one treatment might have harmful side effects.
- When answering non-causal questions : If the researcher is just interested in predicting the probability of an event, the causal relationship between the variables is not important and observational designs would be more suitable than random assignment.
- When studying the effect of variables that cannot be manipulated: Some risk factors cannot be manipulated and so it would not make any sense to study them in a randomized trial. For example, we cannot randomly assign participants into categories based on age, gender, or genetic factors.
Drawbacks of Random Assignment
While randomization assures an unbiased assignment of participants to groups, it does not guarantee the equality of these groups. There could still be extraneous variables that differ between groups or group differences that arise from chance. Additionally, there is still an element of luck with random assignments.
Thus, researchers can not produce perfectly equal groups for each specific study. Differences between the treatment group and control group might still exist, and the results of a randomized trial may sometimes be wrong, but this is absolutely okay.
Scientific evidence is a long and continuous process, and the groups will tend to be equal in the long run when data is aggregated in a meta-analysis.
Additionally, external validity (i.e., the extent to which the researcher can use the results of the study to generalize to the larger population) is compromised with random assignment.
Random assignment is challenging to implement outside of controlled laboratory conditions and might not represent what would happen in the real world at the population level.
Random assignment can also be more costly than simple observational studies, where an investigator is just observing events without intervening with the population.
Randomization also can be time-consuming and challenging, especially when participants refuse to receive the assigned treatment or do not adhere to recommendations.
What is the difference between random sampling and random assignment?
Random sampling refers to randomly selecting a sample of participants from a population. Random assignment refers to randomly assigning participants to treatment groups from the selected sample.
Does random assignment increase internal validity?
Yes, random assignment ensures that there are no systematic differences between the participants in each group, enhancing the study’s internal validity .
Does random assignment reduce sampling error?
Yes, with random assignment, participants have an equal chance of being assigned to either a control group or an experimental group, resulting in a sample that is, in theory, representative of the population.
Random assignment does not completely eliminate sampling error because a sample only approximates the population from which it is drawn. However, random sampling is a way to minimize sampling errors.
When is random assignment not possible?
Random assignment is not possible when the experimenters cannot control the treatment or independent variable.
For example, if you want to compare how men and women perform on a test, you cannot randomly assign subjects to these groups.
Participants are not randomly assigned to different groups in this study, but instead assigned based on their characteristics.
Does random assignment eliminate confounding variables?
Yes, random assignment eliminates the influence of any confounding variables on the treatment because it distributes them at random among the study groups. Randomization invalidates any relationship between a confounding variable and the treatment.
Why is random assignment of participants to treatment conditions in an experiment used?
Random assignment is used to ensure that all groups are comparable at the start of a study. This allows researchers to conclude that the outcomes of the study can be attributed to the intervention at hand and to rule out alternative explanations for study results.
Further Reading
- Bogomolnaia, A., & Moulin, H. (2001). A new solution to the random assignment problem . Journal of Economic theory , 100 (2), 295-328.
- Krause, M. S., & Howard, K. I. (2003). What random assignment does and does not do . Journal of Clinical Psychology , 59 (7), 751-766.
- Skip to secondary menu
- Skip to main content
- Skip to primary sidebar
Statistics By Jim
Making statistics intuitive
Random Assignment in Experiments
By Jim Frost 4 Comments
Random assignment uses chance to assign subjects to the control and treatment groups in an experiment. This process helps ensure that the groups are equivalent at the beginning of the study, which makes it safer to assume the treatments caused any differences between groups that the experimenters observe at the end of the study.

Huh? That might be a big surprise! At this point, you might be wondering about all of those studies that use statistics to assess the effects of different treatments. There’s a critical separation between significance and causality:
- Statistical procedures determine whether an effect is significant.
- Experimental designs determine how confidently you can assume that a treatment causes the effect.
In this post, learn how using random assignment in experiments can help you identify causal relationships.
Correlation, Causation, and Confounding Variables
Random assignment helps you separate causation from correlation and rule out confounding variables. As a critical component of the scientific method , experiments typically set up contrasts between a control group and one or more treatment groups. The idea is to determine whether the effect, which is the difference between a treatment group and the control group, is statistically significant. If the effect is significant, group assignment correlates with different outcomes.
However, as you have no doubt heard, correlation does not necessarily imply causation. In other words, the experimental groups can have different mean outcomes, but the treatment might not be causing those differences even though the differences are statistically significant.
The difficulty in definitively stating that a treatment caused the difference is due to potential confounding variables or confounders. Confounders are alternative explanations for differences between the experimental groups. Confounding variables correlate with both the experimental groups and the outcome variable. In this situation, confounding variables can be the actual cause for the outcome differences rather than the treatments themselves. As you’ll see, if an experiment does not account for confounding variables, they can bias the results and make them untrustworthy.
Related posts : Understanding Correlation in Statistics , Causation versus Correlation , and Hill’s Criteria for Causation .
Example of Confounding in an Experiment

- Control group: Does not consume vitamin supplements
- Treatment group: Regularly consumes vitamin supplements.
Imagine we measure a specific health outcome. After the experiment is complete, we perform a 2-sample t-test to determine whether the mean outcomes for these two groups are different. Assume the test results indicate that the mean health outcome in the treatment group is significantly better than the control group.
Why can’t we assume that the vitamins improved the health outcomes? After all, only the treatment group took the vitamins.
Related post : Confounding Variables in Regression Analysis
Alternative Explanations for Differences in Outcomes
The answer to that question depends on how we assigned the subjects to the experimental groups. If we let the subjects decide which group to join based on their existing vitamin habits, it opens the door to confounding variables. It’s reasonable to assume that people who take vitamins regularly also tend to have other healthy habits. These habits are confounders because they correlate with both vitamin consumption (experimental group) and the health outcome measure.
Random assignment prevents this self sorting of participants and reduces the likelihood that the groups start with systematic differences.
In fact, studies have found that supplement users are more physically active, have healthier diets, have lower blood pressure, and so on compared to those who don’t take supplements. If subjects who already take vitamins regularly join the treatment group voluntarily, they bring these healthy habits disproportionately to the treatment group. Consequently, these habits will be much more prevalent in the treatment group than the control group.
The healthy habits are the confounding variables—the potential alternative explanations for the difference in our study’s health outcome. It’s entirely possible that these systematic differences between groups at the start of the study might cause the difference in the health outcome at the end of the study—and not the vitamin consumption itself!
If our experiment doesn’t account for these confounding variables, we can’t trust the results. While we obtained statistically significant results with the 2-sample t-test for health outcomes, we don’t know for sure whether the vitamins, the systematic difference in habits, or some combination of the two caused the improvements.
Learn why many randomized clinical experiments use a placebo to control for the Placebo Effect .
Experiments Must Account for Confounding Variables
Your experimental design must account for confounding variables to avoid their problems. Scientific studies commonly use the following methods to handle confounders:
- Use control variables to keep them constant throughout an experiment.
- Statistically control for them in an observational study.
- Use random assignment to reduce the likelihood that systematic differences exist between experimental groups when the study begins.
Let’s take a look at how random assignment works in an experimental design.
Random Assignment Can Reduce the Impact of Confounding Variables
Note that random assignment is different than random sampling. Random sampling is a process for obtaining a sample that accurately represents a population .

Random assignment uses a chance process to assign subjects to experimental groups. Using random assignment requires that the experimenters can control the group assignment for all study subjects. For our study, we must be able to assign our participants to either the control group or the supplement group. Clearly, if we don’t have the ability to assign subjects to the groups, we can’t use random assignment!
Additionally, the process must have an equal probability of assigning a subject to any of the groups. For example, in our vitamin supplement study, we can use a coin toss to assign each subject to either the control group or supplement group. For more complex experimental designs, we can use a random number generator or even draw names out of a hat.
Random Assignment Distributes Confounders Equally
The random assignment process distributes confounding properties amongst your experimental groups equally. In other words, randomness helps eliminate systematic differences between groups. For our study, flipping the coin tends to equalize the distribution of subjects with healthier habits between the control and treatment group. Consequently, these two groups should start roughly equal for all confounding variables, including healthy habits!
Random assignment is a simple, elegant solution to a complex problem. For any given study area, there can be a long list of confounding variables that you could worry about. However, using random assignment, you don’t need to know what they are, how to detect them, or even measure them. Instead, use random assignment to equalize them across your experimental groups so they’re not a problem.
Because random assignment helps ensure that the groups are comparable when the experiment begins, you can be more confident that the treatments caused the post-study differences. Random assignment helps increase the internal validity of your study.
Comparing the Vitamin Study With and Without Random Assignment
Let’s compare two scenarios involving our hypothetical vitamin study. We’ll assume that the study obtains statistically significant results in both cases.
Scenario 1: We don’t use random assignment and, unbeknownst to us, subjects with healthier habits disproportionately end up in the supplement treatment group. The experimental groups differ by both healthy habits and vitamin consumption. Consequently, we can’t determine whether it was the habits or vitamins that improved the outcomes.
Scenario 2: We use random assignment and, consequently, the treatment and control groups start with roughly equal levels of healthy habits. The intentional introduction of vitamin supplements in the treatment group is the primary difference between the groups. Consequently, we can more confidently assert that the supplements caused an improvement in health outcomes.
For both scenarios, the statistical results could be identical. However, the methodology behind the second scenario makes a stronger case for a causal relationship between vitamin supplement consumption and health outcomes.
How important is it to use the correct methodology? Well, if the relationship between vitamins and health outcomes is not causal, then consuming vitamins won’t cause your health outcomes to improve regardless of what the study indicates. Instead, it’s probably all the other healthy habits!
Learn more about Randomized Controlled Trials (RCTs) that are the gold standard for identifying causal relationships because they use random assignment.
Drawbacks of Random Assignment
Random assignment helps reduce the chances of systematic differences between the groups at the start of an experiment and, thereby, mitigates the threats of confounding variables and alternative explanations. However, the process does not always equalize all of the confounding variables. Its random nature tends to eliminate systematic differences, but it doesn’t always succeed.
Sometimes random assignment is impossible because the experimenters cannot control the treatment or independent variable. For example, if you want to determine how individuals with and without depression perform on a test, you cannot randomly assign subjects to these groups. The same difficulty occurs when you’re studying differences between genders.
In other cases, there might be ethical issues. For example, in a randomized experiment, the researchers would want to withhold treatment for the control group. However, if the treatments are vaccinations, it might be unethical to withhold the vaccinations.
Other times, random assignment might be possible, but it is very challenging. For example, with vitamin consumption, it’s generally thought that if vitamin supplements cause health improvements, it’s only after very long-term use. It’s hard to enforce random assignment with a strict regimen for usage in one group and non-usage in the other group over the long-run. Or imagine a study about smoking. The researchers would find it difficult to assign subjects to the smoking and non-smoking groups randomly!
Fortunately, if you can’t use random assignment to help reduce the problem of confounding variables, there are different methods available. The other primary approach is to perform an observational study and incorporate the confounders into the statistical model itself. For more information, read my post Observational Studies Explained .
Read About Real Experiments that Used Random Assignment
I’ve written several blog posts about studies that have used random assignment to make causal inferences. Read studies about the following:
- Flu Vaccinations
- COVID-19 Vaccinations
Sullivan L. Random assignment versus random selection . SAGE Glossary of the Social and Behavioral Sciences, SAGE Publications, Inc.; 2009.
Share this:

Reader Interactions
November 13, 2019 at 4:59 am
Hi Jim, I have a question of randomly assigning participants to one of two conditions when it is an ongoing study and you are not sure of how many participants there will be. I am using this random assignment tool for factorial experiments. http://methodologymedia.psu.edu/most/rannumgenerator It asks you for the total number of participants but at this point, I am not sure how many there will be. Thanks for any advice you can give me, Floyd
May 28, 2019 at 11:34 am
Jim, can you comment on the validity of using the following approach when we can’t use random assignments. I’m in education, we have an ACT prep course that we offer. We can’t force students to take it and we can’t keep them from taking it either. But we want to know if it’s working. Let’s say that by senior year all students who are going to take the ACT have taken it. Let’s also say that I’m only including students who have taking it twice (so I can show growth between first and second time taking it). What I’ve done to address confounders is to go back to say 8th or 9th grade (prior to anyone taking the ACT or the ACT prep course) and run an analysis showing the two groups are not significantly different to start with. Is this valid? If the ACT prep students were higher achievers in 8th or 9th grade, I could not assume my prep course is effecting greater growth, but if they were not significantly different in 8th or 9th grade, I can assume the significant difference in ACT growth (from first to second testing) is due to the prep course. Yes or no?
May 26, 2019 at 5:37 pm
Nice post! I think the key to understanding scientific research is to understand randomization. And most people don’t get it.
May 27, 2019 at 9:48 pm
Thank you, Anoop!
I think randomness in an experiment is a funny thing. The issue of confounding factors is a serious problem. You might not even know what they are! But, use random assignment and, voila, the problem usually goes away! If you can’t use random assignment, suddenly you have a whole host of issues to worry about, which I’ll be writing about in more detail in my upcoming post about observational experiments!
Comments and Questions Cancel reply
Random Assignment in Psychology (Definition + 40 Examples)

Have you ever wondered how researchers discover new ways to help people learn, make decisions, or overcome challenges? A hidden hero in this adventure of discovery is a method called random assignment, a cornerstone in psychological research that helps scientists uncover the truths about the human mind and behavior.
Random Assignment is a process used in research where each participant has an equal chance of being placed in any group within the study. This technique is essential in experiments as it helps to eliminate biases, ensuring that the different groups being compared are similar in all important aspects.
By doing so, researchers can be confident that any differences observed are likely due to the variable being tested, rather than other factors.
In this article, we’ll explore the intriguing world of random assignment, diving into its history, principles, real-world examples, and the impact it has had on the field of psychology.
History of Random Assignment
Stepping back in time, we delve into the origins of random assignment, which finds its roots in the early 20th century.
The pioneering mind behind this innovative technique was Sir Ronald A. Fisher , a British statistician and biologist. Fisher introduced the concept of random assignment in the 1920s, aiming to improve the quality and reliability of experimental research .
His contributions laid the groundwork for the method's evolution and its widespread adoption in various fields, particularly in psychology.
Fisher’s groundbreaking work on random assignment was motivated by his desire to control for confounding variables – those pesky factors that could muddy the waters of research findings.
By assigning participants to different groups purely by chance, he realized that the influence of these confounding variables could be minimized, paving the way for more accurate and trustworthy results.
Early Studies Utilizing Random Assignment
Following Fisher's initial development, random assignment started to gain traction in the research community. Early studies adopting this methodology focused on a variety of topics, from agriculture (which was Fisher’s primary field of interest) to medicine and psychology.
The approach allowed researchers to draw stronger conclusions from their experiments, bolstering the development of new theories and practices.
One notable early study utilizing random assignment was conducted in the field of educational psychology. Researchers were keen to understand the impact of different teaching methods on student outcomes.
By randomly assigning students to various instructional approaches, they were able to isolate the effects of the teaching methods, leading to valuable insights and recommendations for educators.
Evolution of the Methodology
As the decades rolled on, random assignment continued to evolve and adapt to the changing landscape of research.
Advances in technology introduced new tools and techniques for implementing randomization, such as computerized random number generators, which offered greater precision and ease of use.
The application of random assignment expanded beyond the confines of the laboratory, finding its way into field studies and large-scale surveys.
Researchers across diverse disciplines embraced the methodology, recognizing its potential to enhance the validity of their findings and contribute to the advancement of knowledge.
From its humble beginnings in the early 20th century to its widespread use today, random assignment has proven to be a cornerstone of scientific inquiry.
Its development and evolution have played a pivotal role in shaping the landscape of psychological research, driving discoveries that have improved lives and deepened our understanding of the human experience.
Principles of Random Assignment
Delving into the heart of random assignment, we uncover the theories and principles that form its foundation.
The method is steeped in the basics of probability theory and statistical inference, ensuring that each participant has an equal chance of being placed in any group, thus fostering fair and unbiased results.
Basic Principles of Random Assignment
Understanding the core principles of random assignment is key to grasping its significance in research. There are three principles: equal probability of selection, reduction of bias, and ensuring representativeness.
The first principle, equal probability of selection , ensures that every participant has an identical chance of being assigned to any group in the study. This randomness is crucial as it mitigates the risk of bias and establishes a level playing field.
The second principle focuses on the reduction of bias . Random assignment acts as a safeguard, ensuring that the groups being compared are alike in all essential aspects before the experiment begins.
This similarity between groups allows researchers to attribute any differences observed in the outcomes directly to the independent variable being studied.
Lastly, ensuring representativeness is a vital principle. When participants are assigned randomly, the resulting groups are more likely to be representative of the larger population.
This characteristic is crucial for the generalizability of the study’s findings, allowing researchers to apply their insights broadly.
Theoretical Foundation
The theoretical foundation of random assignment lies in probability theory and statistical inference .
Probability theory deals with the likelihood of different outcomes, providing a mathematical framework for analyzing random phenomena. In the context of random assignment, it helps in ensuring that each participant has an equal chance of being placed in any group.
Statistical inference, on the other hand, allows researchers to draw conclusions about a population based on a sample of data drawn from that population. It is the mechanism through which the results of a study can be generalized to a broader context.
Random assignment enhances the reliability of statistical inferences by reducing biases and ensuring that the sample is representative.
Differentiating Random Assignment from Random Selection
It’s essential to distinguish between random assignment and random selection, as the two terms, while related, have distinct meanings in the realm of research.
Random assignment refers to how participants are placed into different groups in an experiment, aiming to control for confounding variables and help determine causes.
In contrast, random selection pertains to how individuals are chosen to participate in a study. This method is used to ensure that the sample of participants is representative of the larger population, which is vital for the external validity of the research.
While both methods are rooted in randomness and probability, they serve different purposes in the research process.
Understanding the theories, principles, and distinctions of random assignment illuminates its pivotal role in psychological research.
This method, anchored in probability theory and statistical inference, serves as a beacon of reliability, guiding researchers in their quest for knowledge and ensuring that their findings stand the test of validity and applicability.
Methodology of Random Assignment
Implementing random assignment in a study is a meticulous process that involves several crucial steps.
The initial step is participant selection, where individuals are chosen to partake in the study. This stage is critical to ensure that the pool of participants is diverse and representative of the population the study aims to generalize to.
Once the pool of participants has been established, the actual assignment process begins. In this step, each participant is allocated randomly to one of the groups in the study.
Researchers use various tools, such as random number generators or computerized methods, to ensure that this assignment is genuinely random and free from biases.
Monitoring and adjusting form the final step in the implementation of random assignment. Researchers need to continuously observe the groups to ensure that they remain comparable in all essential aspects throughout the study.
If any significant discrepancies arise, adjustments might be necessary to maintain the study’s integrity and validity.
Tools and Techniques Used
The evolution of technology has introduced a variety of tools and techniques to facilitate random assignment.
Random number generators, both manual and computerized, are commonly used to assign participants to different groups. These generators ensure that each individual has an equal chance of being placed in any group, upholding the principle of equal probability of selection.
In addition to random number generators, researchers often use specialized computer software designed for statistical analysis and experimental design.
These software programs offer advanced features that allow for precise and efficient random assignment, minimizing the risk of human error and enhancing the study’s reliability.
Ethical Considerations
The implementation of random assignment is not devoid of ethical considerations. Informed consent is a fundamental ethical principle that researchers must uphold.
Informed consent means that every participant should be fully informed about the nature of the study, the procedures involved, and any potential risks or benefits, ensuring that they voluntarily agree to participate.
Beyond informed consent, researchers must conduct a thorough risk and benefit analysis. The potential benefits of the study should outweigh any risks or harms to the participants.
Safeguarding the well-being of participants is paramount, and any study employing random assignment must adhere to established ethical guidelines and standards.
Conclusion of Methodology
The methodology of random assignment, while seemingly straightforward, is a multifaceted process that demands precision, fairness, and ethical integrity. From participant selection to assignment and monitoring, each step is crucial to ensure the validity of the study’s findings.
The tools and techniques employed, coupled with a steadfast commitment to ethical principles, underscore the significance of random assignment as a cornerstone of robust psychological research.
Benefits of Random Assignment in Psychological Research
The impact and importance of random assignment in psychological research cannot be overstated. It is fundamental for ensuring the study is accurate, allowing the researchers to determine if their study actually caused the results they saw, and making sure the findings can be applied to the real world.
Facilitating Causal Inferences
When participants are randomly assigned to different groups, researchers can be more confident that the observed effects are due to the independent variable being changed, and not other factors.
This ability to determine the cause is called causal inference .
This confidence allows for the drawing of causal relationships, which are foundational for theory development and application in psychology.
Ensuring Internal Validity
One of the foremost impacts of random assignment is its ability to enhance the internal validity of an experiment.
Internal validity refers to the extent to which a researcher can assert that changes in the dependent variable are solely due to manipulations of the independent variable , and not due to confounding variables.
By ensuring that each participant has an equal chance of being in any condition of the experiment, random assignment helps control for participant characteristics that could otherwise complicate the results.
Enhancing Generalizability
Beyond internal validity, random assignment also plays a crucial role in enhancing the generalizability of research findings.
When done correctly, it ensures that the sample groups are representative of the larger population, so can allow researchers to apply their findings more broadly.
This representative nature is essential for the practical application of research, impacting policy, interventions, and psychological therapies.
Limitations of Random Assignment
Potential for implementation issues.
While the principles of random assignment are robust, the method can face implementation issues.
One of the most common problems is logistical constraints. Some studies, due to their nature or the specific population being studied, find it challenging to implement random assignment effectively.
For instance, in educational settings, logistical issues such as class schedules and school policies might stop the random allocation of students to different teaching methods .
Ethical Dilemmas
Random assignment, while methodologically sound, can also present ethical dilemmas.
In some cases, withholding a potentially beneficial treatment from one of the groups of participants can raise serious ethical questions, especially in medical or clinical research where participants' well-being might be directly affected.
Researchers must navigate these ethical waters carefully, balancing the pursuit of knowledge with the well-being of participants.
Generalizability Concerns
Even when implemented correctly, random assignment does not always guarantee generalizable results.
The types of people in the participant pool, the specific context of the study, and the nature of the variables being studied can all influence the extent to which the findings can be applied to the broader population.
Researchers must be cautious in making broad generalizations from studies, even those employing strict random assignment.
Practical and Real-World Limitations
In the real world, many variables cannot be manipulated for ethical or practical reasons, limiting the applicability of random assignment.
For instance, researchers cannot randomly assign individuals to different levels of intelligence, socioeconomic status, or cultural backgrounds.
This limitation necessitates the use of other research designs, such as correlational or observational studies , when exploring relationships involving such variables.
Response to Critiques
In response to these critiques, people in favor of random assignment argue that the method, despite its limitations, remains one of the most reliable ways to establish cause and effect in experimental research.
They acknowledge the challenges and ethical considerations but emphasize the rigorous frameworks in place to address them.
The ongoing discussion around the limitations and critiques of random assignment contributes to the evolution of the method, making sure it is continuously relevant and applicable in psychological research.
While random assignment is a powerful tool in experimental research, it is not without its critiques and limitations. Implementation issues, ethical dilemmas, generalizability concerns, and real-world limitations can pose significant challenges.
However, the continued discourse and refinement around these issues underline the method's enduring significance in the pursuit of knowledge in psychology.
By being careful with how we do things and doing what's right, random assignment stays a really important part of studying how people act and think.
Real-World Applications and Examples

Random assignment has been employed in many studies across various fields of psychology, leading to significant discoveries and advancements.
Here are some real-world applications and examples illustrating the diversity and impact of this method:
- Medicine and Health Psychology: Randomized Controlled Trials (RCTs) are the gold standard in medical research. In these studies, participants are randomly assigned to either the treatment or control group to test the efficacy of new medications or interventions.
- Educational Psychology: Studies in this field have used random assignment to explore the effects of different teaching methods, classroom environments, and educational technologies on student learning and outcomes.
- Cognitive Psychology: Researchers have employed random assignment to investigate various aspects of human cognition, including memory, attention, and problem-solving, leading to a deeper understanding of how the mind works.
- Social Psychology: Random assignment has been instrumental in studying social phenomena, such as conformity, aggression, and prosocial behavior, shedding light on the intricate dynamics of human interaction.
Let's get into some specific examples. You'll need to know one term though, and that is "control group." A control group is a set of participants in a study who do not receive the treatment or intervention being tested , serving as a baseline to compare with the group that does, in order to assess the effectiveness of the treatment.
- Smoking Cessation Study: Researchers used random assignment to put participants into two groups. One group received a new anti-smoking program, while the other did not. This helped determine if the program was effective in helping people quit smoking.
- Math Tutoring Program: A study on students used random assignment to place them into two groups. One group received additional math tutoring, while the other continued with regular classes, to see if the extra help improved their grades.
- Exercise and Mental Health: Adults were randomly assigned to either an exercise group or a control group to study the impact of physical activity on mental health and mood.
- Diet and Weight Loss: A study randomly assigned participants to different diet plans to compare their effectiveness in promoting weight loss and improving health markers.
- Sleep and Learning: Researchers randomly assigned students to either a sleep extension group or a regular sleep group to study the impact of sleep on learning and memory.
- Classroom Seating Arrangement: Teachers used random assignment to place students in different seating arrangements to examine the effect on focus and academic performance.
- Music and Productivity: Employees were randomly assigned to listen to music or work in silence to investigate the effect of music on workplace productivity.
- Medication for ADHD: Children with ADHD were randomly assigned to receive either medication, behavioral therapy, or a placebo to compare treatment effectiveness.
- Mindfulness Meditation for Stress: Adults were randomly assigned to a mindfulness meditation group or a waitlist control group to study the impact on stress levels.
- Video Games and Aggression: A study randomly assigned participants to play either violent or non-violent video games and then measured their aggression levels.
- Online Learning Platforms: Students were randomly assigned to use different online learning platforms to evaluate their effectiveness in enhancing learning outcomes.
- Hand Sanitizers in Schools: Schools were randomly assigned to use hand sanitizers or not to study the impact on student illness and absenteeism.
- Caffeine and Alertness: Participants were randomly assigned to consume caffeinated or decaffeinated beverages to measure the effects on alertness and cognitive performance.
- Green Spaces and Well-being: Neighborhoods were randomly assigned to receive green space interventions to study the impact on residents’ well-being and community connections.
- Pet Therapy for Hospital Patients: Patients were randomly assigned to receive pet therapy or standard care to assess the impact on recovery and mood.
- Yoga for Chronic Pain: Individuals with chronic pain were randomly assigned to a yoga intervention group or a control group to study the effect on pain levels and quality of life.
- Flu Vaccines Effectiveness: Different groups of people were randomly assigned to receive either the flu vaccine or a placebo to determine the vaccine’s effectiveness.
- Reading Strategies for Dyslexia: Children with dyslexia were randomly assigned to different reading intervention strategies to compare their effectiveness.
- Physical Environment and Creativity: Participants were randomly assigned to different room setups to study the impact of physical environment on creative thinking.
- Laughter Therapy for Depression: Individuals with depression were randomly assigned to laughter therapy sessions or control groups to assess the impact on mood.
- Financial Incentives for Exercise: Participants were randomly assigned to receive financial incentives for exercising to study the impact on physical activity levels.
- Art Therapy for Anxiety: Individuals with anxiety were randomly assigned to art therapy sessions or a waitlist control group to measure the effect on anxiety levels.
- Natural Light in Offices: Employees were randomly assigned to workspaces with natural or artificial light to study the impact on productivity and job satisfaction.
- School Start Times and Academic Performance: Schools were randomly assigned different start times to study the effect on student academic performance and well-being.
- Horticulture Therapy for Seniors: Older adults were randomly assigned to participate in horticulture therapy or traditional activities to study the impact on cognitive function and life satisfaction.
- Hydration and Cognitive Function: Participants were randomly assigned to different hydration levels to measure the impact on cognitive function and alertness.
- Intergenerational Programs: Seniors and young people were randomly assigned to intergenerational programs to study the effects on well-being and cross-generational understanding.
- Therapeutic Horseback Riding for Autism: Children with autism were randomly assigned to therapeutic horseback riding or traditional therapy to study the impact on social communication skills.
- Active Commuting and Health: Employees were randomly assigned to active commuting (cycling, walking) or passive commuting to study the effect on physical health.
- Mindful Eating for Weight Management: Individuals were randomly assigned to mindful eating workshops or control groups to study the impact on weight management and eating habits.
- Noise Levels and Learning: Students were randomly assigned to classrooms with different noise levels to study the effect on learning and concentration.
- Bilingual Education Methods: Schools were randomly assigned different bilingual education methods to compare their effectiveness in language acquisition.
- Outdoor Play and Child Development: Children were randomly assigned to different amounts of outdoor playtime to study the impact on physical and cognitive development.
- Social Media Detox: Participants were randomly assigned to a social media detox or regular usage to study the impact on mental health and well-being.
- Therapeutic Writing for Trauma Survivors: Individuals who experienced trauma were randomly assigned to therapeutic writing sessions or control groups to study the impact on psychological well-being.
- Mentoring Programs for At-risk Youth: At-risk youth were randomly assigned to mentoring programs or control groups to assess the impact on academic achievement and behavior.
- Dance Therapy for Parkinson’s Disease: Individuals with Parkinson’s disease were randomly assigned to dance therapy or traditional exercise to study the effect on motor function and quality of life.
- Aquaponics in Schools: Schools were randomly assigned to implement aquaponics programs to study the impact on student engagement and environmental awareness.
- Virtual Reality for Phobia Treatment: Individuals with phobias were randomly assigned to virtual reality exposure therapy or traditional therapy to compare effectiveness.
- Gardening and Mental Health: Participants were randomly assigned to engage in gardening or other leisure activities to study the impact on mental health and stress reduction.
Each of these studies exemplifies how random assignment is utilized in various fields and settings, shedding light on the multitude of ways it can be applied to glean valuable insights and knowledge.
Real-world Impact of Random Assignment

Random assignment is like a key tool in the world of learning about people's minds and behaviors. It’s super important and helps in many different areas of our everyday lives. It helps make better rules, creates new ways to help people, and is used in lots of different fields.
Health and Medicine
In health and medicine, random assignment has helped doctors and scientists make lots of discoveries. It’s a big part of tests that help create new medicines and treatments.
By putting people into different groups by chance, scientists can really see if a medicine works.
This has led to new ways to help people with all sorts of health problems, like diabetes, heart disease, and mental health issues like depression and anxiety.
Schools and education have also learned a lot from random assignment. Researchers have used it to look at different ways of teaching, what kind of classrooms are best, and how technology can help learning.
This knowledge has helped make better school rules, develop what we learn in school, and find the best ways to teach students of all ages and backgrounds.
Workplace and Organizational Behavior
Random assignment helps us understand how people act at work and what makes a workplace good or bad.
Studies have looked at different kinds of workplaces, how bosses should act, and how teams should be put together. This has helped companies make better rules and create places to work that are helpful and make people happy.
Environmental and Social Changes
Random assignment is also used to see how changes in the community and environment affect people. Studies have looked at community projects, changes to the environment, and social programs to see how they help or hurt people’s well-being.
This has led to better community projects, efforts to protect the environment, and programs to help people in society.
Technology and Human Interaction
In our world where technology is always changing, studies with random assignment help us see how tech like social media, virtual reality, and online stuff affect how we act and feel.
This has helped make better and safer technology and rules about using it so that everyone can benefit.
The effects of random assignment go far and wide, way beyond just a science lab. It helps us understand lots of different things, leads to new and improved ways to do things, and really makes a difference in the world around us.
From making healthcare and schools better to creating positive changes in communities and the environment, the real-world impact of random assignment shows just how important it is in helping us learn and make the world a better place.
So, what have we learned? Random assignment is like a super tool in learning about how people think and act. It's like a detective helping us find clues and solve mysteries in many parts of our lives.
From creating new medicines to helping kids learn better in school, and from making workplaces happier to protecting the environment, it’s got a big job!
This method isn’t just something scientists use in labs; it reaches out and touches our everyday lives. It helps make positive changes and teaches us valuable lessons.
Whether we are talking about technology, health, education, or the environment, random assignment is there, working behind the scenes, making things better and safer for all of us.
In the end, the simple act of putting people into groups by chance helps us make big discoveries and improvements. It’s like throwing a small stone into a pond and watching the ripples spread out far and wide.
Thanks to random assignment, we are always learning, growing, and finding new ways to make our world a happier and healthier place for everyone!
Related posts:
- 19+ Experimental Design Examples (Methods + Types)
- Cluster Sampling vs Stratified Sampling
- 41+ White Collar Job Examples (Salary + Path)
- 47+ Blue Collar Job Examples (Salary + Path)
- McDonaldization of Society (Definition + Examples)
Reference this article:
About The Author
Free Personality Test
Free Memory Test
Free IQ Test
PracticalPie.com is a participant in the Amazon Associates Program. As an Amazon Associate we earn from qualifying purchases.
Follow Us On:
Youtube Facebook Instagram X/Twitter
Psychology Resources
Developmental
Personality
Relationships
Psychologists
Serial Killers
Psychology Tests
Personality Quiz
Memory Test
Depression test
Type A/B Personality Test
© PracticalPsychology. All rights reserved
Privacy Policy | Terms of Use

The Plagiarism Checker Online For Your Academic Work
Start Plagiarism Check
Editing & Proofreading for Your Research Paper
Get it proofread now
Online Printing & Binding with Free Express Delivery
Configure binding now
- Academic essay overview
- The writing process
- Structuring academic essays
- Types of academic essays
- Academic writing overview
- Sentence structure
- Academic writing process
- Improving your academic writing
- Titles and headings
- APA style overview
- APA citation & referencing
- APA structure & sections
- Citation & referencing
- Structure and sections
- APA examples overview
- Commonly used citations
- Other examples
- British English vs. American English
- Chicago style overview
- Chicago citation & referencing
- Chicago structure & sections
- Chicago style examples
- Citing sources overview
- Citation format
- Citation examples
- College essay overview
- Application
- How to write a college essay
- Types of college essays
- Commonly confused words
- Definitions
- Dissertation overview
- Dissertation structure & sections
- Dissertation writing process
- Graduate school overview
- Application & admission
- Study abroad
- Master degree
- Harvard referencing overview
- Language rules overview
- Grammatical rules & structures
- Parts of speech
- Punctuation
- Methodology overview
- Analyzing data
- Experiments
- Observations
- Inductive vs. Deductive
- Qualitative vs. Quantitative
- Types of validity
- Types of reliability
- Sampling methods
- Theories & Concepts
- Types of research studies
- Types of variables
- MLA style overview
- MLA examples
- MLA citation & referencing
- MLA structure & sections
- Plagiarism overview
- Plagiarism checker
- Types of plagiarism
- Printing production overview
- Research bias overview
- Types of research bias
- Example sections
- Types of research papers
- Research process overview
- Problem statement
- Research proposal
- Research topic
- Statistics overview
- Levels of measurment
- Frequency distribution
- Measures of central tendency
- Measures of variability
- Hypothesis testing
- Parameters & test statistics
- Types of distributions
- Correlation
- Effect size
- Hypothesis testing assumptions
- Types of ANOVAs
- Types of chi-square
- Statistical data
- Statistical models
- Spelling mistakes
- Tips overview
- Academic writing tips
- Dissertation tips
- Sources tips
- Working with sources overview
- Evaluating sources
- Finding sources
- Including sources
- Types of sources
Your Step to Success
Plagiarism Check within 10min
Printing & Binding with 3D Live Preview
Random Assignment – A Simple Introduction with Examples
How do you like this article cancel reply.
Save my name, email, and website in this browser for the next time I comment.

Completing a research or thesis paper is more work than most students imagine. For instance, you must conduct experiments before coming up with conclusions. Random assignment, a key methodology in academic research, ensures every participant has an equal chance of being placed in any group within an experiment. In experimental studies, the random assignment of participants is a vital element, which this article will discuss.
Inhaltsverzeichnis
- 1 Random Assignment – In a Nutshell
- 2 Definition: Random assignment
- 3 Importance of random assignment
- 4 Random assignment vs. random sampling
- 5 How to use random assignment
- 6 When random assignment is not used
Random Assignment – In a Nutshell
- Random assignment is where you randomly place research participants into specific groups.
- This method eliminates bias in the results by ensuring that all participants have an equal chance of getting into either group.
- Random assignment is usually used in independent measures or between-group experiment designs.
Definition: Random assignment
Pearson Correlation is a descriptive statistical procedure that describes the measure of linear dependence between two variables. It entails a sample, control group , experimental design , and randomized design. In this statistical procedure, random assignment is used. Random assignment is the random placement of participants into different groups in experimental research.

Importance of random assignment
Random assessment is essential for strengthening the internal validity of experimental research. Internal validity helps make a casual relationship’s conclusions reliable and trustworthy.
In experimental research, researchers isolate independent variables and manipulate them as they assess the impact while managing other variables. To achieve this, an independent variable for diverse member groups is vital. This experimental design is called an independent or between-group design.
Example: Different levels of independent variables
- In a medical study, you can research the impact of nutrient supplements on the immune (nutrient supplements = independent variable, immune = dependent variable)
Three independent participant levels are applicable here:
- Control group (given 0 dosages of iron supplements)
- The experimental group (low dosage)
- The second experimental group (high dosage)
This assignment technique in experiments ensures no bias in the treatment sets at the beginning of the trials. Therefore, if you do not use this technique, you won’t be able to exclude any alternate clarifications for your findings.
In the research experiment above, you can recruit participants randomly by handing out flyers at public spaces like gyms, cafés, and community centers. Then:
- Place the group from cafés in the control group
- Community center group in the low prescription trial group
- Gym group in the high-prescription group
Even with random participant assignment, other extraneous variables may still create bias in experiment results. However, these variations are usually low, hence should not hinder your research. Therefore, using random placement in experiments is highly necessary, especially where it is ethically required or makes sense for your research subject.
Random assignment vs. random sampling
Simple random sampling is a method of choosing the participants for a study. On the other hand, the random assignment involves sorting the participants selected through random sampling. Another difference between random sampling and random assignment is that the former is used in several types of studies, while the latter is only applied in between-subject experimental designs.
Your study researches the impact of technology on productivity in a specific company.
In such a case, you have contact with the entire staff. So, you can assign each employee a quantity and apply a random number generator to pick a specific sample.
For instance, from 500 employees, you can pick 200. So, the full sample is 200.
Random sampling enhances external validity, as it guarantees that the study sample is unbiased, and that an entire population is represented. This way, you can conclude that the results of your studies can be accredited to the autonomous variable.
After determining the full sample, you can break it down into two groups using random assignment. In this case, the groups are:
- The control group (does get access to technology)
- The experimental group (gets access to technology)
Using random assignment assures you that any differences in the productivity results for each group are not biased and will help the company make a decision.
How to use random assignment
Firstly, give each participant a unique number as an identifier. Then, use a specific tool to simplify assigning the participants to the sample groups. Some tools you can use are:
| Computer programs to generate numbers from the list of participants | |
| Place the numbers in a container and draw them randomly for each group | |
| If you have two sets or groups only, you can toss a coin to determine which one will be the regulated or trial group | |
| If you have three groups, you can roll a dice to determine which participant joins each group. |
Random member assignment is a prevailing technique for placing participants in specific groups because each person has a fair opportunity of being put in either group.
Random assignment in block experimental designs
In complex experimental designs , you must group your participants into blocks before using the random assignment technique.
You can create participant blocks depending on demographic variables, working hours, or scores. However, the blocks imply that you will require a bigger sample to attain high statistical power.
After grouping the participants in blocks, you can use random assignments inside each block to allocate the members to a specific treatment condition. Doing this will help you examine if quality impacts the result of the treatment.
Depending on their unique characteristics, you can also use blocking in experimental matched designs before matching the participants in each block. Then, you can randomly allot each partaker to one of the treatments in the research and examine the results.
When random assignment is not used
As powerful a tool as it is, random assignment does not apply in all situations. Like the following:
Comparing different groups
When the purpose of your study is to assess the differences between the participants, random member assignment may not work.
If you want to compare teens and the elderly with and without specific health conditions, you must ensure that the participants have specific characteristics. Therefore, you cannot pick them randomly.
In such a study, the medical condition (quality of interest) is the independent variable, and the participants are grouped based on their ages (different levels). Also, all partakers are tried similarly to ensure they have the medical condition, and their outcomes are tested per group level.
No ethical justifiability
Another situation where you cannot use random assignment is if it is ethically not permitted.
If your study involves unhealthy or dangerous behaviors or subjects, such as drug use. Instead of assigning random partakers to sets, you can conduct quasi-experimental research.
When using a quasi-experimental design , you examine the conclusions of pre-existing groups you have no control over, such as existing drug users. While you cannot randomly assign them to groups, you can use variables like their age, years of drug use, or socioeconomic status to group the participants.
What is the definition of random assignment?
It is an experimental research technique that involves randomly placing participants from your samples into different groups. It ensures that every sample member has the same opportunity of being in whichever group (control or experimental group).
When is random assignment applicable?
You can use this placement technique in experiments featuring an independent measures design. It helps ensure that all your sample groups are comparable.
What is the importance of random assignment?
It can help you enhance your study’s validity . This technique also helps ensure that every sample has an equal opportunity of being assigned to a control or trial group.
When should you NOT use random assignment
You should not use this technique if your study focuses on group comparisons or if it is not legally ethical.
Because of the positive experience, I recommend this printing service not just...
We use cookies on our website. Some of them are essential, while others help us to improve this website and your experience.
- External Media
Individual Privacy Preferences
Cookie Details Privacy Policy Imprint
Here you will find an overview of all cookies used. You can give your consent to whole categories or display further information and select certain cookies.
Accept all Save
Essential cookies enable basic functions and are necessary for the proper function of the website.
Show Cookie Information Hide Cookie Information
| Name | |
|---|---|
| Anbieter | Eigentümer dieser Website, |
| Zweck | Speichert die Einstellungen der Besucher, die in der Cookie Box von Borlabs Cookie ausgewählt wurden. |
| Cookie Name | borlabs-cookie |
| Cookie Laufzeit | 1 Jahr |
| Name | |
|---|---|
| Anbieter | Bachelorprint |
| Zweck | Erkennt das Herkunftsland und leitet zur entsprechenden Sprachversion um. |
| Datenschutzerklärung | |
| Host(s) | ip-api.com |
| Cookie Name | georedirect |
| Cookie Laufzeit | 1 Jahr |
Statistics cookies collect information anonymously. This information helps us to understand how our visitors use our website.
| Akzeptieren | |
|---|---|
| Name | |
| Anbieter | Google Ireland Limited, Gordon House, Barrow Street, Dublin 4, Ireland |
| Zweck | Cookie von Google zur Steuerung der erweiterten Script- und Ereignisbehandlung. |
| Datenschutzerklärung | |
| Cookie Name | _ga,_gat,_gid |
| Cookie Laufzeit | 2 Jahre |
Content from video platforms and social media platforms is blocked by default. If External Media cookies are accepted, access to those contents no longer requires manual consent.
| Akzeptieren | |
|---|---|
| Name | |
| Anbieter | Meta Platforms Ireland Limited, 4 Grand Canal Square, Dublin 2, Ireland |
| Zweck | Wird verwendet, um Facebook-Inhalte zu entsperren. |
| Datenschutzerklärung | |
| Host(s) | .facebook.com |
| Akzeptieren | |
|---|---|
| Name | |
| Anbieter | Google Ireland Limited, Gordon House, Barrow Street, Dublin 4, Ireland |
| Zweck | Wird zum Entsperren von Google Maps-Inhalten verwendet. |
| Datenschutzerklärung | |
| Host(s) | .google.com |
| Cookie Name | NID |
| Cookie Laufzeit | 6 Monate |
| Akzeptieren | |
|---|---|
| Name | |
| Anbieter | Meta Platforms Ireland Limited, 4 Grand Canal Square, Dublin 2, Ireland |
| Zweck | Wird verwendet, um Instagram-Inhalte zu entsperren. |
| Datenschutzerklärung | |
| Host(s) | .instagram.com |
| Cookie Name | pigeon_state |
| Cookie Laufzeit | Sitzung |
| Akzeptieren | |
|---|---|
| Name | |
| Anbieter | Openstreetmap Foundation, St John’s Innovation Centre, Cowley Road, Cambridge CB4 0WS, United Kingdom |
| Zweck | Wird verwendet, um OpenStreetMap-Inhalte zu entsperren. |
| Datenschutzerklärung | |
| Host(s) | .openstreetmap.org |
| Cookie Name | _osm_location, _osm_session, _osm_totp_token, _osm_welcome, _pk_id., _pk_ref., _pk_ses., qos_token |
| Cookie Laufzeit | 1-10 Jahre |
| Akzeptieren | |
|---|---|
| Name | |
| Anbieter | Twitter International Company, One Cumberland Place, Fenian Street, Dublin 2, D02 AX07, Ireland |
| Zweck | Wird verwendet, um Twitter-Inhalte zu entsperren. |
| Datenschutzerklärung | |
| Host(s) | .twimg.com, .twitter.com |
| Cookie Name | __widgetsettings, local_storage_support_test |
| Cookie Laufzeit | Unbegrenzt |
| Akzeptieren | |
|---|---|
| Name | |
| Anbieter | Vimeo Inc., 555 West 18th Street, New York, New York 10011, USA |
| Zweck | Wird verwendet, um Vimeo-Inhalte zu entsperren. |
| Datenschutzerklärung | |
| Host(s) | player.vimeo.com |
| Cookie Name | vuid |
| Cookie Laufzeit | 2 Jahre |
| Akzeptieren | |
|---|---|
| Name | |
| Anbieter | Google Ireland Limited, Gordon House, Barrow Street, Dublin 4, Ireland |
| Zweck | Wird verwendet, um YouTube-Inhalte zu entsperren. |
| Datenschutzerklärung | |
| Host(s) | google.com |
| Cookie Name | NID |
| Cookie Laufzeit | 6 Monate |
Privacy Policy Imprint
10 Things You Need to Know About Randomization
This guide will help you design and execute different types of randomization in your experiments. We focus on the big ideas and provide examples and tools that you can use in R. For why to do randomization see this methods guide .
1 Some ways are better than others
There are many ways to randomize. The simplest is to flip a coin each time you want to determine whether a given subject gets treatment or not. This ensures that each subject has a .5 probability of receiving the treatment and a .5 probability of not receiving it. Done this way, whether one subject receives the treatment in no way affects whether the next subject receives the treatment, every subject has an equal chance of getting the treatment, and the treatment will be independent from all confounding factors — at least in expectation.
This is not a bad approach but it has shortcomings. First, using this method, you cannot know in advance how many units will be in treatment and how many in control. If you want to know this, you need some way to do selections so that the different draws are not statistically independent from each other (like drawing names from a hat). Second, you may want to assert control over the exact share of units assigned to treatment and control. That’s hard to do with a coin. Third, you might want to be able to replicate your randomization to show that there was no funny business. That’s hard to do with coins and hats. Finally, as we show below, there are all sorts of ways to do randomization to improve power and ensure balance in various ways that are very hard to achieve using coins and hats.
Fortunately though, flexible replicable randomization is very easy to do with freely available software. The following simple R code can, for example, be used to generate a random assignment, specifying the number of units to be treated. Here, N (100) is the number of units you have and m (34) is the number you want to treat. The “seed” makes it possible to replicate the same draw each time you run the code (or you can change the seed for a different draw). 1

2 Block randomization: You can ensure that treatment and control groups are balanced
It is possible, when randomizing, to specify the balance of particular factors you care about between treatment and control groups, even though it is not possible to specify which particular units are selected for either group.
For example, you can specify that treatment and control groups contain equal ratios of men to women. In doing so, you avoid any randomization that might produce a distinctly male treatment group and a distinctly female control group, or vice-versa.
Why is this desirable? Not because our estimate of the average treatment effect would otherwise be biased, but because it could be really noisy. Suppose that a random assignment happened to generate a very male treatment group and a very female control group. We would observe a correlation between gender and treatment status. If we were to estimate a treatment effect, that treatment effect would still be unbiased because gender did not cause treatment status. However, it would be more difficult to reject the null hypothesis that it was not our treatment but gender that was producing the effect. In short, the imbalance produces a noisy estimate, which makes it more difficult for us to be confident in our estimates.
Block (sometimes called stratified) randomization helps us to rig our experiment so that our treatment and control groups are balanced along important dimensions but are still randomly assigned. Essentially, this type of randomization design constructs multiple mini-experiments: for example, it might take women and randomly assign half to treatment and half to control, and then it would assign half of men to treatment and half to control. This guarantees a gender balance when treatment and control groups are pooled.
Another advantage of block randomization is that it ensures that we will be able to estimate treatment effects for subgroups of interest. For example, imagine that we are interested in estimating the effect of the treatment among women. If we do not block on gender, we may, by chance, end up with a random assignment that puts only few women into the treatment group. Our estimate of the treatment effect among women would then be very noisy. However, if we assign treatment separately among women and among men, we can ensure that we will have enough women in, respectively, the treatment and control group to obtain a precise estimate among this subgroup.
The blockTools package is a useful package for conducting block randomization. Let’s start by generating a fake data set for 60 subjects, 36 of whom are male and 24 of whom are female.
Suppose we would like to block on gender. Based on our data, blockTools will generate the smallest possible blocks, each a grouping of two units with the same gender, one of which will be assigned to treatment, and one to control.
You can check the mean of the variable on which you blocked for treatment and control to see that treatment and control groups are in fact perfectly balanced on gender.
3 Factorial designs: You can randomize multiple treatments at the same time without using up power
Suppose there are multiple components of a treatment that you want to test. For example, you may want to evaluate the impact of a microfinance program. Two specific treatments might be lending money to women and providing them with training. A factorial design looks at all possible combinations of these treatments: (1) Loans, (2) Training, (3) Loans + Training, and (4) Control. Subjects are then randomly assigned to one of these four conditions.

Factorial designs are especially useful when evaluating interventions that include a package of treatments. As in the example above, many development interventions come with several arms, and it is sometimes difficult to tell which arms are producing the observed effect. A factorial design separates out these different treatments and also allows us to see the interaction between them.
The following code shows you how to randomize for a factorial design.
4 You can randomize whole clusters together (but the bigger your clusters, the weaker tends to be your power)
Sometimes it is impossible to randomize at the level of the individual. For example, a radio appeal to get individuals to a polling station must inherently be broadcast to a whole media market; it is impossible to broadcast just to some individuals but not others. Whether it is by necessity or by choice, sometimes you will randomize clusters instead of individuals.
The disadvantage of cluster randomization is that it reduces your power, since the number of randomly assigned units now reflects the number of clusters and not simply your total number of subjects. If your sample consisted of two clusters of 1,000 individuals each, the functional number of units might be closer to 2, not 2,000. For this reason, it is preferable to make clusters as small as possible.
The degree to which clustering reduces your power depends on the extent to which units in the same cluster resemble each other. It is desirable to have heterogeneity within your clusters so that they are as representative as possible of your broader population. If the individuals within clusters are very similar to each other, they may have similar potential outcomes, which means that groups of individuals with similar potential outcomes will all be assigned to treatment or control together. If a cluster has particularly high or low potential outcomes, this assignment procedure will increase the overall correlation between potential outcomes and treatment assignment. As a result, your estimates become more variable. In brief, if your clusters are more representative of the broader population, your estimates of the average treatment effect will be more precise. See our guide on cluster random assignment for more details.
A frequently asked question is how cluster randomization differs from block randomization. Block randomization is conducted in order to achieve balance based on pre-treatment covariates. For example, an education intervention might block randomize on the previous year’s test scores in order to track the progress of both low- and high-performing students. Cluster randomization is when multiple units are treated as a group–they all receive treatment or control status together. For example, the same education intervention might randomize at the level of the classroom, so the classrooms constitute the clusters. It is possible to block and cluster randomize simultaneously. In our example, you might calculate the average test score for each classroom and block randomize based on the classroom’s average score.
The following graphic demonstrates what your data might look like in the cases of block, cluster, and block + cluster randomization, relative to a simple case of randomization with no blocking or clustering. The example imagines that we conduct an experiment in four schools, where each school comprises four classrooms with four students in each classroom. The top left panel represents simple random assignment. Note that the number of students assigned to treatment (tiles shaded in blue) varies across schools. For example, in school 1, 9 students are assigned to treatment but in school 4, the treatment group comprises only 6 students. The top right panel represents a random assignment procedure that uses schools as blocks. This procedure ensures that exactly eight students are assigned to treatment in each school. The bottom left panel shows cluster random assignment. The idea is that the four students in each quadrant of a school are in the same classroom, and we assign entire classrooms to treatment. Note again that, because there is no blocking involved, the number of classrooms assigned to treatment varies across schools. For example, school 1 assigns three classrooms to treatment while school 3 assigns all classrooms to control. Finally, the bottom right panel corresponds to assignment that is both blocked by school and clustered by classroom. Blocking ensures that exactly two classrooms per school are assigned to treatment.

5 You can randomize in a way that makes it easier to see if there are spillovers
When designing your experiment, think critically about whether “spillovers” pose a threat to your ability to identify the causal effect of your treatment. Spillovers arise if one units outcome is affected by the treatment status of another unit. This can be tricky if units have the ability to interact with each other: one member of a village may learn of another villager’s receipt of a cash grant and may change their behavior accordingly.
One way to make spillovers more evident is to use double randomization. You would first randomly assign some clusters to treatment and others to control, and within clusters, you would assign some individuals to treatment and others to control. Comparing control individuals in your treatment cluster to individuals in your control cluster will enable you to assess the role of spillovers in your experiment.
6 Different units can be assigned to treatment with different probabilities
Sometimes people think that “random” means that two events are equally likely, but in fact, random assignment is “random” so long as the probability of assignment to treatment is strictly between 0 and 1. If a subject has a 0 or a 100 percent chance of being assigned to treatment, that subject should be excluded from your experimental analysis because there is no randomization occurring. However, all subjects with a probability of assignment to treatment strictly between 0 and 1 may be included, even if their probabilities differ, so long as their probabilities are known.
Why might you want to assign different probabilities of assignment to treatment? Suppose you are working with an implementing partner to randomize the allocation of election observers in order to measure the effect on electoral fraud. Your implementing partner can afford to send only a few election observers to a rural part of the country. You could address this constraint by blocking on geographic area and assigning a higher probability of assignment to treatment to more proximate villages to which it is less costly to travel. So long as the probability of assignment to treatment for more accessible villages is less than 1, the probability of assignment to treatment for less accessible villages is greater than zero, and these probabilities are known, it is possible to estimate the effect of the treatment.
When subjects have differing probabilities of assignment to treatment, however, you can no longer simply merge all subjects in the analysis of your data. If you do, then treatment assignment will be correlated with background characteristics on which you blocked. There are two ways of handling this.
The first way is to estimate the average treatment effect block by block and then to average the treatment effects, each weighted by the size of the block relative to the entire sample.
The second way is inverse probability weighting (IPW). In IPW, weights are defined as the 1/p for treated units and 1/(1-p) for control units, where p refers to the probability of assignment to treatment. This method allows you to run a weighted regression of your outcome on treatment assignment.
7 Restricted randomization: If you don’t like what you get you can start over
Sometimes you might want to make sure that randomization does not produce particular types of pattern (for example, too many people who know each other all being in treatment). But the patterns you care about might be too hard to set up in advance. What you can do is take a random draw and then check whether the draw meets the criteria you care about. If it doesn’t, then draw again. Be warned, though, that if you do this, you create a couple of complications: (1) each unit will not necessarily be assigned to treatment with the same probability, (2) units may not be independently assigned to treatment. You need to take into account both of these facts in your analysis. You can do so by generating inverse probability weights as we did in point 6. Here, you will need to use the same restricted randomization code that you used to assign treatment to figure out how likely it is that each subject is assigned to treatment under these restrictions. You simply run the code a large number of times and calculate the proportion of times that a given unit is assigned to treatment across all repetitions. Next, you use the distribution of possible treatment assignments to implement randomization inference. These analyses are complex so proceed with caution.
8 Write randomization code that lets you simulate many possible randomizations
A benefit of using R code to randomize is that you can perform thousands of possible randomizations in seconds. Why is this beneficial?
- It can be useful as a way to check whether your randomization code worked. For example, if one or more subjects in your experiment never received treatment over 10,000 possible random assignments, then you would suspect a flaw in your randomization code.
- You can use re-randomization to calculate the exact probability of assignment to treatment for each individual in your experiment. This is especially helpful if your randomization code is more complex. Perhaps you employ both block and cluster randomization or a restricted randomization procedure, resulting probabilities of assignment to treatment that vary greatly across individuals in a large experiment. These probabilities would be difficult to calculate by hand, but an easy solution is to run your original randomization code many times and generate a variable representing each individual’s proportion of times they were assigned to treatment: this represents his or her individual probability of assignment to treatment. The inverse of this variable can then be used in a weighted regression when estimating the average treatment effect.
- Simulating possible randomizations is a design-based approach to calculating statistical significance. This approach, called randomization inference, generates an exact \(p\) -value by calculating possible average treatment effects that would be observed under hypothetical random assignments if in fact the treatment had no effect. The \(p\) -value is then the proportion of the estimated treatment effects that is at least as large in magnitude as the one that your experiment observed. Randomization inference avoids making distributional assumptions and instead uses the distribution of data observed in your experiment. This approach is preferable to standard calculations of statistical significance when the sampling distribution is not normal – a problem that is more likely to arise when your experimental sample is small and when your outcomes do not follow a normal distribution. For more information on randomization inference, including sample code, visit the 10 Things to Know About Randomization Inference Methods Guide.
9 You can randomize as you go along
In many experiments, you may not know the entirety of your sample at the beginning of the experiment; some subjects may join over time. This presents a complication when we want to use a simple blocking algorithm because the addition of subjects to our pool may change the composition of our blocks and therefore their probabilities of assignment to treatment.
To maintain the ability to block and therefore the ability to assert control over the balance between treatment and control groups, you can use covariates to calculate a new subject’s similarity to other previously assigned subjects and assign the new subject to the treatment condition with fewer similar units. 2 3
10 Randomization can sometimes be an ethical way of assigning a treatment, but sometimes it isn’t
Randomization is the key ingredient for isolating the causal effect of a treatment from a research design perspective, but it is also important to consider the ethical implications of randomization as well. When we think about the long-term effects of an experiment, randomization enables us to test which programs are most effective so that resources can be directed to programs that make the most difference in the lives of future populations. In the short term, randomizing access to a program (as opposed to distributing based on arbitrary characteristics) can be a particularly ethical way of distributing scarce goods that cannot be extended to everyone.
However, sometimes, it is the neediest populations that need to be served by an intervention in an experiment. A randomized design that treats equal numbers of low-income and high-income participants with loans is letting resources flow to less rather than more needy individuals. If we believe there are beneficial effects of the loan, then this raises concerns about the ethics of allocating resources away from the neediest. 4 One would need a strong case for social benefits of the research and would also seek designs that provide benefits ultimately to control groups.
A wait list randomization design is one way of treating an entire subject pool while enabling the researcher to test the effectiveness of the treatment experimentally. In this design, the program could roll out the intervention in phases and randomly assign the units to the phase in which they will be treated. For example, if a program wanted to treat 90 villages in total, it could treat 30 villages each year, and measure outcomes at the end of each year. If you wanted to compare outcomes in treatment and control villages, you would compare the 30 treated villages to the 60 yet-untreated villages at the end of the first year. At the end of the second year, you could compare the 30 villages that were treated in the previous year to the 30 villages that are yet-untreated. Essentially, this creates two experiments, identical but for the year’s time that separates them. In the table below, you can see that in the first year, we could compare the dark blue treatment group to the two light blue control groups. In the second year, we could compare the dark red treatment group to the light red treatment group, but we would want to avoid pooling the two treatment groups because one has been treated for longer than the other. You can see that after the third year, no more comparisons may be made because all units have been treated.
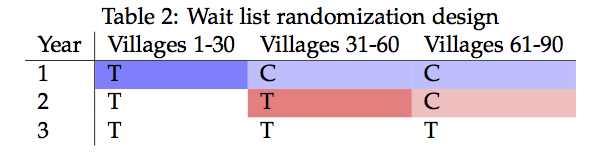
The only requirement is that a subject’s assignment to treatment in a particular phase is randomly assigned and unrelated to their potential outcomes of interest. A design in which more eager participants received treatment earlier would violate this assumption and would not yield an unbiased estimate of the treatment effect, as unobserved factors that predispose them to seeking out treatment may be influencing their schedule of potential outcomes. The wait list design is an example of a creative randomization design that could address ethical concerns about limiting the distribution of a valuable treatment.
Ethics are often highly intertwined with randomized designs, especially in social science and medical research. As a researcher, you should carefully consider the possible implications of randomizing any given treatment. You will also need to solicit approval for your research design from your research institution’s Institutional Review Board (IRB).
11 References
Random number generators are actually pseudo-random because they generate a vector of random numbers based on a small set of initial values, known as a seed state. Random number generators operate this way in order to improve computational speed. However, the series of random numbers generated is as random as you need to it to be for the purposes of random assignment because it is wholly unrelated to the potential outcomes of your subjects. ↩︎
For more, see Moore and Moore ( 2013 ) . ↩︎
For a more detalied walkthrough on the randomization procedures available in the R package randomizr, see here . ↩︎
But if we are certain about the loan’s effects, then it’s also unclear why we are running an experiment to test it. In medical research, randomized controlled trials often stop if it becomes clear early on that a drug is undoubtedly curing life-threatening diseases, and therefore withholding it from control subjects is dangerous. (Similarly, a trial would also stop if it were clear early on that a drug is undoubtedly causing negative and harmful effects.) ↩︎
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Best Family Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
The Definition of Random Assignment According to Psychology
Materio / Getty Images
Random assignment refers to the use of chance procedures in psychology experiments to ensure that each participant has the same opportunity to be assigned to any given group in a study to eliminate any potential bias in the experiment at the outset. Participants are randomly assigned to different groups, such as the treatment group versus the control group. In clinical research, randomized clinical trials are known as the gold standard for meaningful results.
Simple random assignment techniques might involve tactics such as flipping a coin, drawing names out of a hat, rolling dice, or assigning random numbers to a list of participants. It is important to note that random assignment differs from random selection .
While random selection refers to how participants are randomly chosen from a target population as representatives of that population, random assignment refers to how those chosen participants are then assigned to experimental groups.
Random Assignment In Research
To determine if changes in one variable will cause changes in another variable, psychologists must perform an experiment. Random assignment is a critical part of the experimental design that helps ensure the reliability of the study outcomes.
Researchers often begin by forming a testable hypothesis predicting that one variable of interest will have some predictable impact on another variable.
The variable that the experimenters will manipulate in the experiment is known as the independent variable , while the variable that they will then measure for different outcomes is known as the dependent variable. While there are different ways to look at relationships between variables, an experiment is the best way to get a clear idea if there is a cause-and-effect relationship between two or more variables.
Once researchers have formulated a hypothesis, conducted background research, and chosen an experimental design, it is time to find participants for their experiment. How exactly do researchers decide who will be part of an experiment? As mentioned previously, this is often accomplished through something known as random selection.
Random Selection
In order to generalize the results of an experiment to a larger group, it is important to choose a sample that is representative of the qualities found in that population. For example, if the total population is 60% female and 40% male, then the sample should reflect those same percentages.
Choosing a representative sample is often accomplished by randomly picking people from the population to be participants in a study. Random selection means that everyone in the group stands an equal chance of being chosen to minimize any bias. Once a pool of participants has been selected, it is time to assign them to groups.
By randomly assigning the participants into groups, the experimenters can be fairly sure that each group will have the same characteristics before the independent variable is applied.
Participants might be randomly assigned to the control group , which does not receive the treatment in question. The control group may receive a placebo or receive the standard treatment. Participants may also be randomly assigned to the experimental group , which receives the treatment of interest. In larger studies, there can be multiple treatment groups for comparison.
There are simple methods of random assignment, like rolling the die. However, there are more complex techniques that involve random number generators to remove any human error.
There can also be random assignment to groups with pre-established rules or parameters. For example, if you want to have an equal number of men and women in each of your study groups, you might separate your sample into two groups (by sex) before randomly assigning each of those groups into the treatment group and control group.
Random assignment is essential because it increases the likelihood that the groups are the same at the outset. With all characteristics being equal between groups, other than the application of the independent variable, any differences found between group outcomes can be more confidently attributed to the effect of the intervention.
Example of Random Assignment
Imagine that a researcher is interested in learning whether or not drinking caffeinated beverages prior to an exam will improve test performance. After randomly selecting a pool of participants, each person is randomly assigned to either the control group or the experimental group.
The participants in the control group consume a placebo drink prior to the exam that does not contain any caffeine. Those in the experimental group, on the other hand, consume a caffeinated beverage before taking the test.
Participants in both groups then take the test, and the researcher compares the results to determine if the caffeinated beverage had any impact on test performance.
A Word From Verywell
Random assignment plays an important role in the psychology research process. Not only does this process help eliminate possible sources of bias, but it also makes it easier to generalize the results of a tested sample of participants to a larger population.
Random assignment helps ensure that members of each group in the experiment are the same, which means that the groups are also likely more representative of what is present in the larger population of interest. Through the use of this technique, psychology researchers are able to study complex phenomena and contribute to our understanding of the human mind and behavior.
Lin Y, Zhu M, Su Z. The pursuit of balance: An overview of covariate-adaptive randomization techniques in clinical trials . Contemp Clin Trials. 2015;45(Pt A):21-25. doi:10.1016/j.cct.2015.07.011
Sullivan L. Random assignment versus random selection . In: The SAGE Glossary of the Social and Behavioral Sciences. SAGE Publications, Inc.; 2009. doi:10.4135/9781412972024.n2108
Alferes VR. Methods of Randomization in Experimental Design . SAGE Publications, Inc.; 2012. doi:10.4135/9781452270012
Nestor PG, Schutt RK. Research Methods in Psychology: Investigating Human Behavior. (2nd Ed.). SAGE Publications, Inc.; 2015.
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
Statistical Thinking: A Simulation Approach to Modeling Uncertainty (UM STAT 216 edition)
3.6 causation and random assignment.
Medical researchers may be interested in showing that a drug helps improve people’s health (the cause of improvement is the drug), while educational researchers may be interested in showing a curricular innovation improves students’ learning (the curricular innovation causes improved learning).
To attribute a causal relationship, there are three criteria a researcher needs to establish:
- Association of the Cause and Effect: There needs to be a association between the cause and effect.
- Timing: The cause needs to happen BEFORE the effect.
- No Plausible Alternative Explanations: ALL other possible explanations for the effect need to be ruled out.
Please read more about each of these criteria at the Web Center for Social Research Methods .
The third criterion can be quite difficult to meet. To rule out ALL other possible explanations for the effect, we want to compare the world with the cause applied to the world without the cause. In practice, we do this by comparing two different groups: a “treatment” group that gets the cause applied to them, and a “control” group that does not. To rule out alternative explanations, the groups need to be “identical” with respect to every possible characteristic (aside from the treatment) that could explain differences. This way the only characteristic that will be different is that the treatment group gets the treatment and the control group doesn’t. If there are differences in the outcome, then it must be attributable to the treatment, because the other possible explanations are ruled out.
So, the key is to make the control and treatment groups “identical” when you are forming them. One thing that makes this task (slightly) easier is that they don’t have to be exactly identical, only probabilistically equivalent . This means, for example, that if you were matching groups on age that you don’t need the two groups to have identical age distributions; they would only need to have roughly the same AVERAGE age. Here roughly means “the average ages should be the same within what we expect because of sampling error.”
Now we just need to create the groups so that they have, on average, the same characteristics … for EVERY POSSIBLE CHARCTERISTIC that could explain differences in the outcome.
It turns out that creating probabilistically equivalent groups is a really difficult problem. One method that works pretty well for doing this is to randomly assign participants to the groups. This works best when you have large sample sizes, but even with small sample sizes random assignment has the advantage of at least removing the systematic bias between the two groups (any differences are due to chance and will probably even out between the groups). As Wikipedia’s page on random assignment points out,
Random assignment of participants helps to ensure that any differences between and within the groups are not systematic at the outset of the experiment. Thus, any differences between groups recorded at the end of the experiment can be more confidently attributed to the experimental procedures or treatment. … Random assignment does not guarantee that the groups are matched or equivalent. The groups may still differ on some preexisting attribute due to chance. The use of random assignment cannot eliminate this possibility, but it greatly reduces it.
We use the term internal validity to describe the degree to which cause-and-effect inferences are accurate and meaningful. Causal attribution is the goal for many researchers. Thus, by using random assignment we have a pretty high degree of evidence for internal validity; we have a much higher belief in causal inferences. Much like evidence used in a court of law, it is useful to think about validity evidence on a continuum. For example, a visualization of the internal validity evidence for a study that employed random assignment in the design might be:

The degree of internal validity evidence is high (in the upper-third). How high depends on other factors such as sample size.
To learn more about random assignment, you can read the following:
- The research report, Random Assignment Evaluation Studies: A Guide for Out-of-School Time Program Practitioners
3.6.1 Example: Does sleep deprivation cause an decrease in performance?
Let’s consider the criteria with respect to the sleep deprivation study we explored in class.
3.6.1.1 Association of cause and effect
First, we ask, Is there an association between the cause and the effect? In the sleep deprivation study, we would ask, “Is sleep deprivation associated with an decrease in performance?”
This is what a hypothesis test helps us answer! If the result is statistically significant , then we have an association between the cause and the effect. If the result is not statistically significant, then there is not sufficient evidence for an association between cause and effect.
In the case of the sleep deprivation experiment, the result was statistically significant, so we can say that sleep deprivation is associated with a decrease in performance.
3.6.1.2 Timing
Second, we ask, Did the cause come before the effect? In the sleep deprivation study, the answer is yes. The participants were sleep deprived before their performance was tested. It may seem like this is a silly question to ask, but as the link above describes, it is not always so clear to establish the timing. Thus, it is important to consider this question any time we are interested in establishing causality.
3.6.1.3 No plausible alternative explanations
Finally, we ask Are there any plausible alternative explanations for the observed effect? In the sleep deprivation study, we would ask, “Are there plausible alternative explanations for the observed difference between the groups, other than sleep deprivation?” Because this is a question about plausibility, human judgment comes into play. Researchers must make an argument about why there are no plausible alternatives. As described above, a strong study design can help to strengthen the argument.
At first, it may seem like there are a lot of plausible alternative explanations for the difference in performance. There are a lot of things that might affect someone’s performance on a visual task! Sleep deprivation is just one of them! For example, artists may be more adept at visual discrimination than other people. This is an example of a potential confounding variable. A confounding variable is a variable that might affect the results, other than the causal variable that we are interested in.
Here’s the thing though. We are not interested in figuring out why any particular person got the score that they did. Instead, we are interested in determining why one group was different from another group. In the sleep deprivation study, the participants were randomly assigned. This means that the there is no systematic difference between the groups, with respect to any confounding variables. Yes—artistic experience is a possible confounding variable, and it may be the reason why two people score differently. BUT: There is no systematic difference between the groups with respect to artistic experience, and so artistic experience is not a plausible explanation as to why the groups would be different. The same can be said for any possible confounding variable. Because the groups were randomly assigned, it is not plausible to say that the groups are different with respect to any confounding variable. Random assignment helps us rule out plausible alternatives.
3.6.1.4 Making a causal claim
Now, let’s see about make a causal claim for the sleep deprivation study:
- Association: There is a statistically significant result, so the cause is associated with the effect
- Timing: The participants were sleep deprived before their performance was measured, so the cause came before the effect
- Plausible alternative explanations: The participants were randomly assigned, so the groups are not systematically different on any confounding variable. The only systematic difference between the groups was sleep deprivation. Thus, there are no plausible alternative explanations for the difference between the groups, other than sleep deprivation
Thus, the internal validity evidence for this study is high, and we can make a causal claim. For the participants in this study, we can say that sleep deprivation caused a decrease in performance.
Key points: Causation and internal validity
To make a cause-and-effect inference, you need to consider three criteria:
- Association of the Cause and Effect: There needs to be a association between the cause and effect. This can be established by a hypothesis test.
Random assignment removes any systematic differences between the groups (other than the treatment), and thus helps to rule out plausible alternative explanations.
Internal validity describes the degree to which cause-and-effect inferences are accurate and meaningful.
Confounding variables are variables that might affect the results, other than the causal variable that we are interested in.
Probabilistic equivalence means that there is not a systematic difference between groups. The groups are the same on average.
How can we make "equivalent" experimental groups?
| Elements of Research | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
An official website of the United States government The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site. The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
 A roadmap to using randomization in clinical trialsVance w. berger. 1 National Institutes of Health, Bethesda, MD USA Louis Joseph Bour2 Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany Kerstine Carter3 Boehringer-Ingelheim Pharmaceuticals Inc, Ridgefield, CT USA Jonathan J. Chipman4 Population Health Sciences, University of Utah School of Medicine, Salt Lake City UT, USA 5 Cancer Biostatistics, University of Utah Huntsman Cancer Institute, Salt Lake City UT, USA Colin C. Everett6 Clinical Trials Research Unit, University of Leeds, Leeds, UK Nicole Heussen7 RWTH Aachen University, Aachen, Germany 8 Medical School, Sigmund Freud University, Vienna, Austria Catherine Hewitt9 York Trials Unit, Department of Health Sciences, University of York, York, UK Ralf-Dieter HilgersYuqun abigail luo. 10 Food and Drug Administration, Silver Spring, MD USA Jone Renteria11 Open University of Catalonia (UOC) and the University of Barcelona (UB), Barcelona, Spain 12 Department of Human Development and Quantitative Methodology, University of Maryland, College Park, MD USA Yevgen Ryeznik13 BioPharma Early Biometrics & Statistical Innovations, Data Science & AI, R&D BioPharmaceuticals, AstraZeneca, Gothenburg, Sweden Oleksandr Sverdlov14 Early Development Analytics, Novartis Pharmaceuticals Corporation, NJ East Hanover, USA Diane Uschner15 Biostatistics Center & Department of Biostatistics and Bioinformatics, George Washington University, DC Washington, USA Associated DataAll results reported in this paper are based either on theoretical considerations or simulation evidence. The computer code (using R and Julia programming languages) is fully documented and is available upon reasonable request. Randomization is the foundation of any clinical trial involving treatment comparison. It helps mitigate selection bias, promotes similarity of treatment groups with respect to important known and unknown confounders, and contributes to the validity of statistical tests. Various restricted randomization procedures with different probabilistic structures and different statistical properties are available. The goal of this paper is to present a systematic roadmap for the choice and application of a restricted randomization procedure in a clinical trial. We survey available restricted randomization procedures for sequential allocation of subjects in a randomized, comparative, parallel group clinical trial with equal (1:1) allocation. We explore statistical properties of these procedures, including balance/randomness tradeoff, type I error rate and power. We perform head-to-head comparisons of different procedures through simulation under various experimental scenarios, including cases when common model assumptions are violated. We also provide some real-life clinical trial examples to illustrate the thinking process for selecting a randomization procedure for implementation in practice. Restricted randomization procedures targeting 1:1 allocation vary in the degree of balance/randomness they induce, and more importantly, they vary in terms of validity and efficiency of statistical inference when common model assumptions are violated (e.g. when outcomes are affected by a linear time trend; measurement error distribution is misspecified; or selection bias is introduced in the experiment). Some procedures are more robust than others. Covariate-adjusted analysis may be essential to ensure validity of the results. Special considerations are required when selecting a randomization procedure for a clinical trial with very small sample size. ConclusionsThe choice of randomization design, data analytic technique (parametric or nonparametric), and analysis strategy (randomization-based or population model-based) are all very important considerations. Randomization-based tests are robust and valid alternatives to likelihood-based tests and should be considered more frequently by clinical investigators. Supplementary InformationThe online version contains supplementary material available at 10.1186/s12874-021-01303-z. Various research designs can be used to acquire scientific medical evidence. The randomized controlled trial (RCT) has been recognized as the most credible research design for investigations of the clinical effectiveness of new medical interventions [ 1 , 2 ]. Evidence from RCTs is widely used as a basis for submissions of regulatory dossiers in request of marketing authorization for new drugs, biologics, and medical devices. Three important methodological pillars of the modern RCT include blinding (masking), randomization, and the use of control group [ 3 ]. While RCTs provide the highest standard of clinical evidence, they are laborious and costly, in terms of both time and material resources. There are alternative designs, such as observational studies with either a cohort or case–control design, and studies using real world evidence (RWE). When properly designed and implemented, observational studies can sometimes produce similar estimates of treatment effects to those found in RCTs, and furthermore, such studies may be viable alternatives to RCTs in many settings where RCTs are not feasible and/or not ethical. In the era of big data, the sources of clinically relevant data are increasingly rich and include electronic health records, data collected from wearable devices, health claims data, etc. Big data creates vast opportunities for development and implementation of novel frameworks for comparative effectiveness research [ 4 ], and RWE studies nowadays can be implemented rapidly and relatively easily. But how credible are the results from such studies? In 1980, D. P. Byar issued warnings and highlighted potential methodological problems with comparison of treatment effects using observational databases [ 5 ]. Many of these issues still persist and actually become paramount during the ongoing COVID-19 pandemic when global scientific efforts are made to find safe and efficacious vaccines and treatments as soon as possible. While some challenges pertinent to RWE studies are related to the choice of proper research methodology, some additional challenges arise from increasing requirements of health authorities and editorial boards of medical journals for the investigators to present evidence of transparency and reproducibility of their conducted clinical research. Recently, two top medical journals, the New England Journal of Medicine and the Lancet, retracted two COVID-19 studies that relied on observational registry data [ 6 , 7 ]. The retractions were made at the request of the authors who were unable to ensure reproducibility of the results [ 8 ]. Undoubtedly, such cases are harmful in many ways. The already approved drugs may be wrongly labeled as “toxic” or “inefficacious”, and the reputation of the drug developers could be blemished or destroyed. Therefore, the highest standards for design, conduct, analysis, and reporting of clinical research studies are now needed more than ever. When treatment effects are modest, yet still clinically meaningful, a double-blind, randomized, controlled clinical trial design helps detect these differences while adjusting for possible confounders and adequately controlling the chances of both false positive and false negative findings. Randomization in clinical trials has been an important area of methodological research in biostatistics since the pioneering work of A. Bradford Hill in the 1940’s and the first published randomized trial comparing streptomycin with a non-treatment control [ 9 ]. Statisticians around the world have worked intensively to elaborate the value, properties, and refinement of randomization procedures with an incredible record of publication [ 10 ]. In particular, a recent EU-funded project ( www.IDeAl.rwth-aachen.de ) on innovative design and analysis of small population trials has “randomization” as one work package. In 2020, a group of trial statisticians around the world from different sectors formed a subgroup of the Drug Information Association (DIA) Innovative Designs Scientific Working Group (IDSWG) to raise awareness of the full potential of randomization to improve trial quality, validity and rigor ( https://randomization-working-group.rwth-aachen.de/ ). The aims of the current paper are three-fold. First, we describe major recent methodological advances in randomization, including different restricted randomization designs that have superior statistical properties compared to some widely used procedures such as permuted block designs. Second, we discuss different types of experimental biases in clinical trials and explain how a carefully chosen randomization design can mitigate risks of these biases. Third, we provide a systematic roadmap for evaluating different restricted randomization procedures and selecting an “optimal” one for a particular trial. We also showcase application of these ideas through several real life RCT examples. The target audience for this paper would be clinical investigators and biostatisticians who are tasked with the design, conduct, analysis, and interpretation of clinical trial results, as well as regulatory and scientific/medical journal reviewers. Recognizing the breadth of the concept of randomization, in this paper we focus on a randomized, comparative, parallel group clinical trial design with equal (1:1) allocation, which is typically implemented using some restricted randomization procedure, possibly stratified by some important baseline prognostic factor(s) and/or study center. Some of our findings and recommendations are generalizable to more complex clinical trial settings. We shall highlight these generalizations and outline additional important considerations that fall outside the scope of the current paper. The paper is organized as follows. The “ Methods ” section provides some general background on the methodology of randomization in clinical trials, describes existing restricted randomization procedures, and discusses some important criteria for comparison of these procedures in practice. In the “ Results ” section, we present our findings from four simulation studies that illustrate the thinking process when evaluating different randomization design options at the study planning stage. The “ Conclusions ” section summarizes the key findings and important considerations on restricted randomization procedures, and it also highlights some extensions and further topics on randomization in clinical trials. What is randomization and what are its virtues in clinical trials?Randomization is an essential component of an experimental design in general and clinical trials in particular. Its history goes back to R. A. Fisher and his classic book “The Design of Experiments” [ 11 ]. Implementation of randomization in clinical trials is due to A. Bradford Hill who designed the first randomized clinical trial evaluating the use of streptomycin in treating tuberculosis in 1946 [ 9 , 12 , 13 ]. Reference [ 14 ] provides a good summary of the rationale and justification for the use of randomization in clinical trials. The randomized controlled trial (RCT) has been referred to as “the worst possible design (except for all the rest)” [ 15 ], indicating that the benefits of randomization should be evaluated in comparison to what we are left with if we do not randomize. Observational studies suffer from a wide variety of biases that may not be adequately addressed even using state-of-the-art statistical modeling techniques. The RCT in the medical field has several features that distinguish it from experimental designs in other fields, such as agricultural experiments. In the RCT, the experimental units are humans, and in the medical field often diagnosed with a potentially fatal disease. These subjects are sequentially enrolled for participation in the study at selected study centers, which have relevant expertise for conducting clinical research. Many contemporary clinical trials are run globally, at multiple research institutions. The recruitment period may span several months or even years, depending on a therapeutic indication and the target patient population. Patients who meet study eligibility criteria must sign the informed consent, after which they are enrolled into the study and, for example, randomized to either experimental treatment E or the control treatment C according to the randomization sequence. In this setup, the choice of the randomization design must be made judiciously, to protect the study from experimental biases and ensure validity of clinical trial results. The first virtue of randomization is that, in combination with allocation concealment and masking, it helps mitigate selection bias due to an investigator’s potential to selectively enroll patients into the study [ 16 ]. A non-randomized, systematic design such as a sequence of alternating treatment assignments has a major fallacy: an investigator, knowing an upcoming treatment assignment in a sequence, may enroll a patient who, in their opinion, would be best suited for this treatment. Consequently, one of the groups may contain a greater number of “sicker” patients and the estimated treatment effect may be biased. Systematic covariate imbalances may increase the probability of false positive findings and undermine the integrity of the trial. While randomization alleviates the fallacy of a systematic design, it does not fully eliminate the possibility of selection bias (unless we consider complete randomization for which each treatment assignment is determined by a flip of a coin, which is rarely, if ever used in practice [ 17 ]). Commonly, RCTs employ restricted randomization procedures which sequentially balance treatment assignments while maintaining allocation randomness. A popular choice is the permuted block design that controls imbalance by making treatment assignments at random in blocks. To minimize potential for selection bias, one should avoid overly restrictive randomization schemes such as permuted block design with small block sizes, as this is very similar to alternating treatment sequence. The second virtue of randomization is its tendency to promote similarity of treatment groups with respect to important known, but even more importantly, unknown confounders. If treatment assignments are made at random, then by the law of large numbers, the average values of patient characteristics should be approximately equal in the experimental and the control groups, and any observed treatment difference should be attributed to the treatment effects, not the effects of the study participants [ 18 ]. However, one can never rule out the possibility that the observed treatment difference is due to chance, e.g. as a result of random imbalance in some patient characteristics [ 19 ]. Despite that random covariate imbalances can occur in clinical trials of any size, such imbalances do not compromise the validity of statistical inference, provided that proper statistical techniques are applied in the data analysis. Several misconceptions on the role of randomization and balance in clinical trials were documented and discussed by Senn [ 20 ]. One common misunderstanding is that balance of prognostic covariates is necessary for valid inference. In fact, different randomization designs induce different extent of balance in the distributions of covariates, and for a given trial there is always a possibility of observing baseline group differences. A legitimate approach is to pre-specify in the protocol the clinically important covariates to be adjusted for in the primary analysis, apply a randomization design (possibly accounting for selected covariates using pre-stratification or some other approach), and perform a pre-planned covariate-adjusted analysis (such as analysis of covariance for a continuous primary outcome), verifying the model assumptions and conducting additional supportive/sensitivity analyses, as appropriate. Importantly, the pre-specified prognostic covariates should always be accounted for in the analysis, regardless whether their baseline differences are present or not [ 20 ]. It should be noted that some randomization designs (such as covariate-adaptive randomization procedures) can achieve very tight balance of covariate distributions between treatment groups [ 21 ]. While we address randomization within pre-specified stratifications, we do not address more complex covariate- and response-adaptive randomization in this paper. Finally, randomization plays an important role in statistical analysis of the clinical trial. The most common approach to inference following the RCT is the invoked population model [ 10 ]. With this approach, one posits that there is an infinite target population of patients with the disease, from which n eligible subjects are sampled in an unbiased manner for the study and are randomized to the treatment groups. Within each group, the responses are assumed to be independent and identically distributed (i.i.d.), and inference on the treatment effect is performed using some standard statistical methodology, e.g. a two sample t-test for normal outcome data. The added value of randomization is that it makes the assumption of i.i.d. errors more feasible compared to a non-randomized study because it introduces a real element of chance in the allocation of patients. An alternative approach is the randomization model , in which the implemented randomization itself forms the basis for statistical inference [ 10 ]. Under the null hypothesis of the equality of treatment effects, individual outcomes (which are regarded as not influenced by random variation, i.e. are considered as fixed) are not affected by treatment. Treatment assignments are permuted in all possible ways consistent with the randomization procedure actually used in the trial. The randomization-based p- value is the sum of null probabilities of the treatment assignment permutations in the reference set that yield the test statistic values greater than or equal to the experimental value. A randomization-based test can be a useful supportive analysis, free of assumptions of parametric tests and protective against spurious significant results that may be caused by temporal trends [ 14 , 22 ]. It is important to note that Bayesian inference has also become a common statistical analysis in RCTs [ 23 ]. Although the inferential framework relies upon subjective probabilities, a study analyzed through a Bayesian framework still relies upon randomization for the other aforementioned virtues [ 24 ]. Hence, the randomization considerations discussed herein have broad application. What types of randomization methodologies are available?Randomization is not a single methodology, but a very broad class of design techniques for the RCT [ 10 ]. In this paper, we consider only randomization designs for sequential enrollment clinical trials with equal (1:1) allocation in which randomization is not adapted for covariates and/or responses. The simplest procedure for an RCT is complete randomization design (CRD) for which each subject’s treatment is determined by a flip of a fair coin [ 25 ]. CRD provides no potential for selection bias (e.g. based on prediction of future assignments) but it can result, with non-negligible probability, in deviations from the 1:1 allocation ratio and covariate imbalances, especially in small samples. This may lead to loss of statistical efficiency (decrease in power) compared to the balanced design. In practice, some restrictions on randomization are made to achieve balanced allocation. Such randomization designs are referred to as restricted randomization procedures [ 26 , 27 ]. Suppose we plan to randomize an even number of subjects n sequentially between treatments E and C. Two basic designs that equalize the final treatment numbers are the random allocation rule (Rand) and the truncated binomial design (TBD), which were discussed in the 1957 paper by Blackwell and Hodges [ 28 ]. For Rand, any sequence of exactly n / 2 E’s and n / 2 C’s is equally likely. For TBD, treatment assignments are made with probability 0.5 until one of the treatments receives its quota of n / 2 subjects; thereafter all remaining assignments are made deterministically to the opposite treatment. A common feature of both Rand and TBD is that they aim at the final balance, whereas at intermediate steps it is still possible to have substantial imbalances, especially if n is large. A long run of a single treatment in a sequence may be problematic if there is a time drift in some important covariate, which can lead to chronological bias [ 29 ]. To mitigate this risk, one can further restrict randomization so that treatment assignments are balanced over time. One common approach is the permuted block design (PBD) [ 30 ], for which random treatment assignments are made in blocks of size 2 b ( b is some small positive integer), with exactly b allocations to each of the treatments E and C. The PBD is perhaps the oldest (it can be traced back to A. Bradford Hill’s 1951 paper [ 12 ]) and the most widely used randomization method in clinical trials. Often its choice in practice is justified by simplicity of implementation and the fact that it is referenced in the authoritative ICH E9 guideline on statistical principles for clinical trials [ 31 ]. One major challenge with PBD is the choice of the block size. If b = 1 , then every pair of allocations is balanced, but every even allocation is deterministic. Larger block sizes increase allocation randomness. The use of variable block sizes has been suggested [ 31 ]; however, PBDs with variable block sizes are also quite predictable [ 32 ]. Another problematic feature of the PBD is that it forces periodic return to perfect balance, which may be unnecessary from the statistical efficiency perspective and may increase the risk of prediction of upcoming allocations. More recent and better alternatives to the PBD are the maximum tolerated imbalance (MTI) procedures [ 33 – 41 ]. These procedures provide stronger encryption of the randomization sequence (i.e. make it more difficult to predict future treatment allocations in the sequence even knowing the current sizes of the treatment groups) while controlling treatment imbalance at a pre-defined threshold throughout the experiment. A general MTI procedure specifies a certain boundary for treatment imbalance, say b > 0 , that cannot be exceeded. If, at a given allocation step the absolute value of imbalance is equal to b , then one next allocation is deterministically forced toward balance. This is in contrast to PBD which, after reaching the target quota of allocations for either treatment within a block, forces all subsequent allocations to achieve perfect balance at the end of the block. Some notable MTI procedures are the big stick design (BSD) proposed by Soares and Wu in 1983 [ 37 ], the maximal procedure proposed by Berger, Ivanova and Knoll in 2003 [ 35 ], the block urn design (BUD) proposed by Zhao and Weng in 2011 [ 40 ], just to name a few. These designs control treatment imbalance within pre-specified limits and are more immune to selection bias than the PBD [ 42 , 43 ]. Another important class of restricted randomization procedures is biased coin designs (BCDs). Starting with the seminal 1971 paper of Efron [ 44 ], BCDs have been a hot research topic in biostatistics for 50 years. Efron’s BCD is very simple: at any allocation step, if treatment numbers are balanced, the next assignment is made with probability 0.5; otherwise, the underrepresented treatment is assigned with probability p , where 0.5 < p ≤ 1 is a fixed and pre-specified parameter that determines the tradeoff between balance and randomness. Note that p = 1 corresponds to PBD with block size 2. If we set p < 1 (e.g. p = 2 / 3 ), then the procedure has no deterministic assignments and treatment allocation will be concentrated around 1:1 with high probability [ 44 ]. Several extensions of Efron’s BCD providing better tradeoff between treatment balance and allocation randomness have been proposed [ 45 – 49 ]; for example, a class of adjustable biased coin designs introduced by Baldi Antognini and Giovagnoli in 2004 [ 49 ] unifies many BCDs in a single framework. A comprehensive simulation study comparing different BCDs has been published by Atkinson in 2014 [ 50 ]. Finally, urn models provide a useful mechanism for RCT designs [ 51 ]. Urn models apply some probabilistic rules to sequentially add/remove balls (representing different treatments) in the urn, to balance treatment assignments while maintaining the randomized nature of the experiment [ 39 , 40 , 52 – 55 ]. A randomized urn design for balancing treatment assignments was proposed by Wei in 1977 [ 52 ]. More novel urn designs, such as the drop-the-loser urn design developed by Ivanova in 2003 [ 55 ] have reduced variability and can attain the target treatment allocation more efficiently. Many urn designs involve parameters that can be fine-tuned to obtain randomization procedures with desirable balance/randomness tradeoff [ 56 ]. What are the attributes of a good randomization procedure?A “good” randomization procedure is one that helps successfully achieve the study objective(s). Kalish and Begg [ 57 ] state that the major objective of a comparative clinical trial is to provide a precise and valid comparison. To achieve this, the trial design should be such that it: 1) prevents bias; 2) ensures an efficient treatment comparison; and 3) is simple to implement to minimize operational errors. Table Table1 1 elaborates on these considerations, focusing on restricted randomization procedures for 1:1 randomized trials. Considerations for the choice of a restricted randomization procedure
Before delving into a detailed discussion, let us introduce some important definitions. Following [ 10 ], a randomization sequence is a random vector δ n = ( δ 1 , ⋯ , δ n ) , where δ i = 1 , if the i th subject is assigned to treatment E or δ i = 0 , if the i th subject is assigned to treatment C. A restricted randomization procedure can be defined by specifying a probabilistic rule for the treatment assignment of the ( i +1)st subject, δ i + 1 , given the past allocations δ i for i ≥ 1 . Let N E i = ∑ j = 1 i δ j and N C i = i - N E i denote the numbers of subjects assigned to treatments E and C, respectively, after i allocation steps. Then D i = N E i - N C ( i ) is treatment imbalance after i allocations. For any i ≥ 1 , D i is a random variable whose probability distribution is determined by the chosen randomization procedure. Balance and randomnessTreatment balance and allocation randomness are two competing requirements in the design of an RCT. Restricted randomization procedures that provide a good tradeoff between these two criteria are desirable in practice. Consider a trial with sample size n . The absolute value of imbalance, D ( i ) ( i = 1 , ⋯ , n ) , provides a measure of deviation from equal allocation after i allocation steps. D ( i ) = 0 indicates that the trial is perfectly balanced. One can also consider Pr ( | D i | = 0 ) , the probability of achieving exact balance after i allocation steps. In particular Pr ( | D n | = 0 ) is the probability that the final treatment numbers are balanced. Two other useful summary measures are the expected imbalance at the i th step, E D ( i ) and the expected value of the maximum imbalance of the entire randomization sequence, E max 1 ≤ i ≤ n D i . Greater forcing of balance implies lack of randomness. A procedure that lacks randomness may be susceptible to selection bias [ 16 ], which is a prominent issue in open-label trials with a single center or with randomization stratified by center, where the investigator knows the sequence of all previous treatment assignments. A classic approach to quantify the degree of susceptibility of a procedure to selection bias is the Blackwell-Hodges model [ 28 ]. Let G i = 1 (or 0), if at the i th allocation step an investigator makes a correct (or incorrect) guess on treatment assignment δ i , given past allocations δ i - 1 . Then the predictability of the design at the i th step is the expected value of G i , i.e. E G i = Pr ( G i = 1 ) . Blackwell and Hodges [ 28 ] considered the expected bias factor , the difference between expected total number of correct guesses of a given sequence of random assignments and the similar quantity obtained from CRD for which treatment assignments are made independently with equal probability: E ( F ) = E ∑ i = 1 n G i - n / 2 . This quantity is zero for CRD, and it is positive for restricted randomization procedures (greater values indicate higher expected bias). Matts and Lachin [ 30 ] suggested taking expected proportion of deterministic assignments in a sequence as another measure of lack of randomness. In the literature, various restricted randomization procedures have been compared in terms of balance and randomness [ 50 , 58 , 59 ]. For instance, Zhao et al. [ 58 ] performed a comprehensive simulation study of 14 restricted randomization procedures with different choices of design parameters, for sample sizes in the range of 10 to 300. The key criteria were the maximum absolute imbalance and the correct guess probability. The authors found that the performance of the designs was within a closed region with the boundaries shaped by Efron’s BCD [ 44 ] and the big stick design [ 37 ], signifying that the latter procedure with a suitably chosen MTI boundary can be superior to other restricted randomization procedures in terms of balance/randomness tradeoff. Similar findings confirming the utility of the big stick design were recently reported by Hilgers et al. [ 60 ]. Validity and efficiencyValidity of a statistical procedure essentially means that the procedure provides correct statistical inference following an RCT. In particular, a chosen statistical test is valid, if it controls the chance of a false positive finding, that is, the pre-specified probability of a type I error of the test is achieved but not exceeded. The strong control of type I error rate is a major prerequisite for any confirmatory RCT. Efficiency means high statistical power for detecting meaningful treatment differences (when they exist), and high accuracy of estimation of treatment effects. Both validity and efficiency are major requirements of any RCT, and both of these aspects are intertwined with treatment balance and allocation randomness. Restricted randomization designs, when properly implemented, provide solid ground for valid and efficient statistical inference. However, a careful consideration of different options can help an investigator to optimize the choice of a randomization procedure for their clinical trial. Let us start with statistical efficiency. Equal (1:1) allocation frequently maximizes power and estimation precision. To illustrate this, suppose the primary outcomes in the two groups are normally distributed with respective means μ E and μ C and common standard deviation σ > 0 . Then the variance of an efficient estimator of the treatment difference μ E - μ C is equal to V = 4 σ 2 n - L n , where L n = D ( n ) 2 n is referred to as loss [ 61 ]. Clearly, V is minimized when L n = 0 , or equivalently, D n = 0 , i.e. the balanced trial. When the primary outcome follows a more complex statistical model, optimal allocation may be unequal across the treatment groups; however, 1:1 allocation is still nearly optimal for binary outcomes [ 62 , 63 ], survival outcomes [ 64 ], and possibly more complex data types [ 65 , 66 ]. Therefore, a randomization design that balances treatment numbers frequently promotes efficiency of the treatment comparison. As regards inferential validity, it is important to distinguish two approaches to statistical inference after the RCT – an invoked population model and a randomization model [ 10 ]. For a given randomization procedure, these two approaches generally produce similar results when the assumption of normal random sampling (and some other assumptions) are satisfied, but the randomization model may be more robust when model assumptions are violated; e.g. when outcomes are affected by a linear time trend [ 67 , 68 ]. Another important issue that may interfere with validity is selection bias. Some authors showed theoretically that PBDs with small block sizes may result in serious inflation of the type I error rate under a selection bias model [ 69 – 71 ]. To mitigate risk of selection bias, one should ideally take preventative measures, such as blinding/masking, allocation concealment, and avoidance of highly restrictive randomization designs. However, for already completed studies with evidence of selection bias [ 72 ], special statistical adjustments are warranted to ensure validity of the results [ 73 – 75 ]. Implementation aspectsWith the current state of information technology, implementation of randomization in RCTs should be straightforward. Validated randomization systems are emerging, and they can handle randomization designs of increasing complexity for clinical trials that are run globally. However, some important points merit consideration. The first point has to do with how a randomization sequence is generated and implemented. One should distinguish between advance and adaptive randomization [ 16 ]. Here, by “adaptive” randomization we mean “in-real-time” randomization, i.e. when a randomization sequence is generated not upfront, but rather sequentially, as eligible subjects enroll into the study. Restricted randomization procedures are “allocation-adaptive”, in the sense that the treatment assignment of an individual subject is adapted to the history of previous treatment assignments. While in practice the majority of trials with restricted and stratified randomization use randomization schedules pre-generated in advance, there are some circumstances under which “in-real-time” randomization schemes may be preferred; for instance, clinical trials with high cost of goods and/or shortage of drug supply [ 76 ]. The advance randomization approach includes the following steps: 1) for the chosen randomization design and sample size n , specify the probability distribution on the reference set by enumerating all feasible randomization sequences of length n and their corresponding probabilities; 2) select a sequence at random from the reference set according to the probability distribution; and 3) implement this sequence in the trial. While enumeration of all possible sequences and their probabilities is feasible and may be useful for trials with small sample sizes, the task becomes computationally prohibitive (and unnecessary) for moderate or large samples. In practice, Monte Carlo simulation can be used to approximate the probability distribution of the reference set of all randomization sequences for a chosen randomization procedure. A limitation of advance randomization is that a sequence of treatment assignments must be generated upfront, and proper security measures (e.g. blinding/masking) must be in place to protect confidentiality of the sequence. With the adaptive or “in-real-time” randomization, a sequence of treatment assignments is generated dynamically as the trial progresses. For many restricted randomization procedures, the randomization rule can be expressed as Pr ( δ i + 1 = 1 ) = F D i , where F · is some non-increasing function of D i for any i ≥ 1 . This is referred to as the Markov property [ 77 ], which makes a procedure easy to implement sequentially. Some restricted randomization procedures, e.g. the maximal procedure [ 35 ], do not have the Markov property. The second point has to do with how the final data analysis is performed. With an invoked population model, the analysis is conditional on the design and the randomization is ignored in the analysis. With a randomization model, the randomization itself forms the basis for statistical inference. Reference [ 14 ] provides a contemporaneous overview of randomization-based inference in clinical trials. Several other papers provide important technical details on randomization-based tests, including justification for control of type I error rate with these tests [ 22 , 78 , 79 ]. In practice, Monte Carlo simulation can be used to estimate randomization-based p- values [ 10 ]. A roadmap for comparison of restricted randomization proceduresThe design of any RCT starts with formulation of the trial objectives and research questions of interest [ 3 , 31 ]. The choice of a randomization procedure is an integral part of the study design. A structured approach for selecting an appropriate randomization procedure for an RCT was proposed by Hilgers et al. [ 60 ]. Here we outline the thinking process one may follow when evaluating different candidate randomization procedures. Our presented roadmap is by no means exhaustive; its main purpose is to illustrate the logic behind some important considerations for finding an “optimal” randomization design for the given trial parameters. Throughout, we shall assume that the study is designed as a randomized, two-arm comparative trial with 1:1 allocation, with a fixed sample size n that is pre-determined based on budgetary and statistical considerations to obtain a definitive assessment of the treatment effect via the pre-defined hypothesis testing. We start with some general considerations which determine the study design:
An important point to consider is calibration of the design parameters. Many restricted randomization procedures involve parameters, such as the block size in the PBD, the coin bias probability in Efron’s BCD, the MTI threshold, etc. By fine-tuning these parameters, one can obtain designs with desirable statistical properties. For instance, references [ 80 , 81 ] provide guidance on how to justify the block size in the PBD to mitigate the risk of selection bias or chronological bias. Reference [ 82 ] provides a formal approach to determine the “optimal” value of the parameter p in Efron’s BCD in both finite and large samples. The calibration of design parameters can be done using Monte Carlo simulations for the given trial setting. Another important consideration is the scope of randomization procedures to be evaluated. As we mentioned already, even one method may represent a broad class of randomization procedures that can provide different levels of balance/randomness tradeoff; e.g. Efron’s BCD covers a wide spectrum of designs, from PBD(2) (if p = 1 ) to CRD (if p = 0.5 ). One may either prefer to focus on finding the “optimal” parameter value for the chosen design, or be more general and include various designs (e.g. MTI procedures, BCDs, urn designs, etc.) in the comparison. This should be done judiciously, on a case-by-case basis, focusing only on the most reasonable procedures. References [ 50 , 58 , 60 ] provide good examples of simulation studies to facilitate comparisons among various restricted randomization procedures for a 1:1 RCT. In parallel with the decision on the scope of randomization procedures to be assessed, one should decide upon the performance criteria against which these designs will be compared. Among others, one might think about the two competing considerations: treatment balance and allocation randomness. For a trial of size n , at each allocation step i = 1 , ⋯ , n one can calculate expected absolute imbalance E D ( i ) and the probability of correct guess Pr ( G i = 1 ) as measures of lack of balance and lack of randomness, respectively. These measures can be either calculated analytically (when formulae are available) or through Monte Carlo simulations. Sometimes it may be useful to look at cumulative measures up to the i th allocation step ( i = 1 , ⋯ , n ); e.g. 1 i ∑ j = 1 i E D ( j ) and 1 i ∑ j = 1 i Pr ( G j = 1 ) . For instance, 1 n ∑ j = 1 n Pr ( G j = 1 ) is the average correct guess probability for a design with sample size n . It is also helpful to visualize the selected criteria. Visualizations can be done in a number of ways; e.g. plots of a criterion vs. allocation step, admissibility plots of two chosen criteria [ 50 , 59 ], etc. Such visualizations can help evaluate design characteristics, both overall and at intermediate allocation steps. They may also provide insights into the behavior of a particular design for different values of the tuning parameter, and/or facilitate a comparison among different types of designs. Another way to compare the merits of different randomization procedures is to study their inferential characteristics such as type I error rate and power under different experimental conditions. Sometimes this can be done analytically, but a more practical approach is to use Monte Carlo simulation. The choice of the modeling and analysis strategy will be context-specific. Here we outline some considerations that may be useful for this purpose:
Overall, there should be a well-thought plan capturing the key questions to be answered, the strategy to address them, the choice of statistical software for simulation and visualization of the results, and other relevant details. In this section we present four examples that illustrate how one may approach evaluation of different randomization design options at the study planning stage. Example 1 is based on a hypothetical 1:1 RCT with n = 50 and a continuous primary outcome, whereas Examples 2, 3, and 4 are based on some real RCTs. Example 1: Which restricted randomization procedures are robust and efficient?Our first example is a hypothetical RCT in which the primary outcome is assumed to be normally distributed with mean μ E for treatment E, mean μ C for treatment C, and common variance σ 2 . A total of n subjects are to be randomized equally between E and C, and a two-sample t-test is planned for data analysis. Let Δ = μ E - μ C denote the true mean treatment difference. We are interested in testing a hypothesis H 0 : Δ = 0 (treatment effects are the same) vs. H 1 : Δ ≠ 0 . The total sample size n to achieve given power at some clinically meaningful treatment difference Δ c while maintaining the chance of a false positive result at level α can be obtained using standard statistical methods [ 83 ]. For instance, if Δ c / σ = 0.95 , then a design with n = 50 subjects (25 per arm) provides approximately 91% power of a two-sample t-test to detect a statistically significant treatment difference using 2-sided α = 5%. We shall consider 12 randomization procedures to sequentially randomize n = 50 subjects in a 1:1 ratio.
These 12 procedures can be grouped into five major types. I) Procedures 1, 2, 3, and 4 achieve exact final balance for a chosen sample size (provided the total sample size is a multiple of the block size). II) Procedures 5 and 6 ensure that at any allocation step the absolute value of imbalance is capped at MTI = 3. III) Procedures 7 and 8 are biased coin designs that sequentially adjust randomization according to imbalance measured as the difference in treatment numbers. IV) Procedures 9, 10, and 11 (GBCD’s with γ = 1, 2, and 5) are adaptive biased coin designs, for which randomization probability is modified according to imbalance measured as the difference in treatment allocation proportions (larger γ implies greater forcing of balance). V) Procedure 12 (CRD) is the most random procedure that achieves balance for large samples. Balance/randomness tradeoffWe first compare the procedures with respect to treatment balance and allocation randomness. To quantify imbalance after i allocations, we consider two measures: expected value of absolute imbalance E D ( i ) , and expected value of loss E ( L i ) = E D ( i ) 2 / i [ 50 , 61 ]. Importantly, for procedures 1, 2, and 3 the final imbalance is always zero, thus E D ( n ) ≡ 0 and E ( L n ) ≡ 0 , but at intermediate steps one may have E D ( i ) > 0 and E L i > 0 , for 1 ≤ i < n . For procedures 5 and 6 with MTI = 3, E L i ≤ 9 / i . For procedures 7 and 8, E L n tends to zero as n → ∞ [ 49 ]. For procedures 9, 10, 11, and 12, as n → ∞ , E L n tends to the positive constants 1/3, 1/5, 1/11, and 1, respectively [ 47 ]. We take the cumulative average loss after n allocations as an aggregate measure of imbalance: I m b n = 1 n ∑ i = 1 n E L i , which takes values in the 0–1 range. To measure lack of randomness, we consider two measures: expected proportion of correct guesses up to the i th step, P C G i = 1 i ∑ j = 1 i Pr ( G j = 1 ) , i = 1 , ⋯ , n , and the forcing index [ 47 , 84 ], F I ( i ) = ∑ j = 1 i E ϕ j - 0.5 i / 4 , where E ϕ j - 0.5 is the expected deviation of the conditional probability of treatment E assignment at the j th allocation step ( ϕ j ) from the unconditional target value of 0.5. Note that P C G i takes values in the range from 0.5 for CRD to 0.75 for PBD(2) assuming i is even, whereas F I ( i ) takes values in the 0–1 range. At the one extreme, we have CRD for which F I ( i ) ≡ 0 because for CRD ϕ i = 0.5 for any i ≥ 1 . At the other extreme, we have PBD(2) for which every odd allocation is made with probability 0.5, and every even allocation is deterministic, i.e. made with probability 0 or 1. For PBD(2), assuming i is even, there are exactly i / 2 pairs of allocations, and so ∑ j = 1 i E ϕ j - 0.5 = 0.5 · i / 2 = i / 4 , which implies that F I ( i ) = 1 for PBD(2). For all other restricted randomization procedures one has 0 < F I ( i ) < 1 . A “good” randomization procedure should have low values of both loss and forcing index. Different randomization procedures can be compared graphically. As a balance/randomness tradeoff metric, one can calculate the quadratic distance to the origin (0,0) for the chosen sample size, e.g. d ( n ) = I m b ( n ) 2 + F I ( n ) 2 (in our example n = 50 ), and the randomization designs can then be ranked such that designs with lower values of d ( n ) are preferable. We ran a simulation study of the 12 randomization procedures for an RCT with n = 50 . Monte Carlo average values of absolute imbalance, loss, I m b i , F I i , and d ( i ) were calculated for each intermediate allocation step ( i = 1 , ⋯ , 50 ), based on 10,000 simulations. Figure 1 is a plot of expected absolute imbalance vs. allocation step. CRD, GBCD(1), and GBCD(2) show increasing patterns. For TBD and Rand, the final imbalance (when n = 50 ) is zero; however, at intermediate steps is can be quite large. For other designs, absolute imbalance is expected to be below 2 at any allocation step up to n = 50 . Note the periodic patterns of PBD(2) and PBD(4); for instance, for PBD(2) imbalance is 0 (or 1) for any even (or odd) allocation.  Simulated expected absolute imbalance vs. allocation step for 12 restricted randomization procedures for n = 50. Note: PBD(2) and PBD(4) have forced periodicity absolute imbalance of 0, which distinguishes them from MTI procedures Figure 2 is a plot of expected proportion of correct guesses vs. allocation step. One can observe that for CRD it is a flat pattern at 0.5; for PBD(2) it fluctuates while reaching the upper limit of 0.75 at even allocation steps; and for ten other designs the values of proportion of correct guesses fall between those of CRD and PBD(2). The TBD has the same behavior up to ~ 40 th allocation step, at which the pattern starts increasing. Rand exhibits an increasing pattern with overall fewer correct guesses compared to other randomization procedures. Interestingly, BSD(3) is uniformly better (less predictable) than ABCD(2), BCD(2/3), and BCDWIT(2/3, 3). For the three GBCD procedures, there is a rapid initial increase followed by gradual decrease in the pattern; this makes good sense, because GBCD procedures force greater balance when the trial is small and become more random (and less prone to correct guessing) as the sample size increases.  Simulated expected proportion of correct guesses vs. allocation step for 12 restricted randomization procedures for n = 50 Table Table2 2 shows the ranking of the 12 designs with respect to the overall performance metric d ( n ) = I m b ( n ) 2 + F I ( n ) 2 for n = 50 . BSD(3), GBCD(2) and GBCD(1) are the top three procedures, whereas PBD(2) and CRD are at the bottom of the list. Ranking of 12 restricted randomization procedures with respect to balance/randomness tradeoff for a trial with n = 50 subjects
Figure 3 is a plot of F I n vs. I m b n for n = 50 . One can see the two extremes: CRD that takes the value (0,1), and PBD(2) with the value (1,0). The other ten designs are closer to (0,0).  Simulated forcing index (x-axis) vs. aggregate expected loss (y-axis) for 12 restricted randomization procedures for n = 50 Figure 4 is a heat map plot of the metric d ( i ) for i = 1 , ⋯ , 50 . BSD(3) seems to provide overall best tradeoff between randomness and balance throughout the study.  Heatmap of the balance/randomness tradeoff d i = I m b ( i ) 2 + F I ( i ) 2 vs. allocation step ( i = 1 , ⋯ , 50 ) for 12 restricted randomization procedures. The procedures are ordered by value of d(50), with smaller values (more red) indicating more optimal performance Inferential characteristics: type I error rate and powerOur next goal is to compare the chosen randomization procedures in terms of validity (control of the type I error rate) and efficiency (power). For this purpose, we assumed the following data generating mechanism: for the i th subject, conditional on the treatment assignment δ i , the outcome Y i is generated according to the model where u i is an unknown term associated with the i th subject and ε i ’s are i.i.d. measurement errors. We shall explore the following four models:
We consider three statistical test procedures:
We consider four scenarios of the true mean difference Δ = μ E - μ C , which correspond to the Null case ( Δ = 0 ), and three choices of Δ > 0 which correspond to Alternative 1 (power ~ 70%), Alternative 2 (power ~ 80%), and Alternative 3 (power ~ 90%). In all cases, n = 50 was used. Figure 5 summarizes the results of a simulation study comparing 12 randomization designs, under 4 models for the outcome (M1, M2, M3, and M4), 4 scenarios for the mean treatment difference (Null, and Alternatives 1, 2, and 3), using 3 statistical tests (T1, T2, and T3). The operating characteristics of interest are the type I error rate under the Null scenario and the power under the Alternative scenarios. Each scenario was simulated 10,000 times, and each randomization-based test was computed using L = 10 , 000 sequences.  Simulated type I error rate and power of 12 restricted randomization procedures. Four models for the data generating mechanism of the primary outcome (M1: Normal random sampling; M2: Linear trend; M3: Errors Cauchy; and M4: Selection bias). Four scenarios for the treatment mean difference (Null; Alternatives 1, 2, and 3). Three statistical tests (T1: two-sample t-test; T2: randomization-based test using mean difference; T3: randomization-based test using ranks) From Fig. 5 , under the normal random sampling model (M1), all considered randomization designs have similar performance: they maintain the type I error rate and have similar power, with all tests. In other words, when population model assumptions are satisfied, any combination of design and analysis should work well and yield reliable and consistent results. Under the “linear trend” model (M2), the designs have differential performance. First of all, under the Null scenario, only Rand and CRD maintain the type I error rate at 5% with all three tests. For TBD, the t-test is anticonservative, with type I error rate ~ 20%, whereas for nine other procedures the t-test is conservative, with type I error rate in the range 0.1–2%. At the same time, for all 12 designs the two randomization-based tests maintain the nominal type I error rate at 5%. These results are consistent with some previous findings in the literature [ 67 , 68 ]. As regards power, it is reduced significantly compared to the normal random sampling scenario. The t-test seems to be most affected and the randomization-based test using ranks is most robust for a majority of the designs. Remarkably, for CRD the power is similar with all three tests. This signifies the usefulness of randomization-based inference in situations when outcome data are subject to a linear time trend, and the importance of applying randomization-based tests at least as supplemental analyses to likelihood-based test procedures. Under the “Cauchy errors” model (M3), all designs perform similarly: the randomization-based tests maintain the type I error rate at 5%, whereas the t-test deflates the type I error to 2%. As regards power, all designs also have similar, consistently degraded performance: the t-test is least powerful, and the randomization-based test using ranks has highest power. Overall, under misspecification of the error distribution a randomization-based test using ranks is most appropriate; yet one should acknowledge that its power is still lower than expected. Under the “selection bias” model (M4), the 12 designs have differential performance. The only procedure that maintained the type I error rate at 5% with all three tests was CRD. For eleven other procedures, inflations of the type I error were observed. In general, the more random the design, the less it was affected by selection bias. For instance, the type I error rate for TBD was ~ 6%; for Rand, BSD(3), and GBCD(1) it was ~ 7.5%; for GBCD(2) and ABCD(2) it was ~ 8–9%; for Efron’s BCD(2/3) it was ~ 12.5%; and the most affected design was PBD(2) for which the type I error rate was ~ 38–40%. These results are consistent with the theory of Blackwell and Hodges [ 28 ] which posits that TBD is least susceptible to selection bias within a class of restricted randomization designs that force exact balance. Finally, under M4, statistical power is inflated by several percentage points compared to the normal random sampling scenario without selection bias. We performed additional simulations to assess the impact of the bias effect ν under selection bias model. The same 12 randomization designs and three statistical tests were evaluated for a trial with n = 50 under the Null scenario ( Δ = 0 ), for ν in the range of 0 (no bias) to 1 (strong bias). Figure S1 in the Supplementary Materials shows that for all designs but CRD, the type I error rate is increasing in ν , with all three tests. The magnitude of the type I error inflation is different across the restricted randomization designs; e.g. for TBD it is minimal, whereas for more restrictive designs it may be large, especially for ν ≥ 0.4 . PBD(2) is particularly vulnerable: for ν in the range 0.4–1, its type I error rate is in the range 27–90% (for the nominal α = 5 %). In summary, our Example 1 includes most of the key ingredients of the roadmap for assessment of competing randomization designs which was described in the “ Methods ” section. For the chosen experimental scenarios, we evaluated CRD and several restricted randomization procedures, some of which belonged to the same class but with different values of the parameter (e.g. GBCD with γ = 1 , 2 , 5 ). We assessed two measures of imbalance, two measures of lack of randomness (predictability), and a metric that quantifies balance/randomness tradeoff. Based on these criteria, we found that BSD(3) provides overall best performance. We also evaluated type I error and power of selected randomization procedures under several treatment response models. We have observed important links between balance, randomness, type I error rate and power. It is beneficial to consider all these criteria simultaneously as they may complement each other in characterizing statistical properties of randomization designs. In particular, we found that a design that lacks randomness, such as PBD with blocks of 2 or 4, may be vulnerable to selection bias and lead to inflations of the type I error. Therefore, these designs should be avoided, especially in open-label studies. As regards statistical power, since all designs in this example targeted 1:1 allocation ratio (which is optimal if the outcomes are normally distributed and have between-group constant variance), they had very similar power of statistical tests in most scenarios except for the one with chronological bias. In the latter case, randomization-based tests were more robust and more powerful than the standard two-sample t-test under the population model assumption. Overall, while Example 1 is based on a hypothetical 1:1 RCT, its true purpose is to showcase the thinking process in the application of our general roadmap. The following three examples are considered in the context of real RCTs. Example 2: How can we reduce predictability of a randomization procedure and lower the risk of selection bias?Selection bias can arise if the investigator can intelligently guess at least part of the randomization sequence yet to be allocated and, on that basis, preferentially and strategically assigns study subjects to treatments. Although it is generally not possible to prove that a particular study has been infected with selection bias, there are examples of published RCTs that do show some evidence to have been affected by it. Suspect trials are, for example, those with strong observed baseline covariate imbalances that consistently favor the active treatment group [ 16 ]. In what follows we describe an example of an RCT where the stratified block randomization procedure used was vulnerable to potential selection biases, and discuss potential alternatives that may reduce this vulnerability. Etanercept was studied in patients aged 4 to 17 years with polyarticular juvenile rheumatoid arthritis [ 85 ]. The trial consisted of two parts. During the first, open-label part of the trial, patients received etanercept twice weekly for up to three months. Responders from this initial part of the trial were then randomized, at a 1:1 ratio, in the second, double-blind, placebo-controlled part of the trial to receive etanercept or placebo for four months or until a flare of the disease occurred. The primary efficacy outcome, the proportion of patients with disease flare, was evaluated in the double-blind part. Among the 51 randomized patients, 21 of the 26 placebo patients (81%) withdrew because of disease flare, compared with 7 of the 25 etanercept patients (28%), yielding a p- value of 0.003. Regulatory review by the Food and Drug Administrative (FDA) identified vulnerability to selection biases in the study design of the double-blind part and potential issues in study conduct. These findings were succinctly summarized in [ 16 ] (pp.51–52). Specifically, randomization was stratified by study center and number of active joints (≤ 2 vs. > 2, referred to as “few” or “many” in what follows), with blocked randomization within each stratum using a block size of two. Furthermore, randomization codes in corresponding “few” and “many” blocks within each study center were mirror images of each other. For example, if the first block within the “few” active joints stratum of a given center is “placebo followed by etanercept”, then the first block within the “many” stratum of the same center would be “etanercept followed by placebo”. While this appears to be an attempt to improve treatment balance in this small trial, unblinding of one treatment assignment may lead to deterministic predictability of three upcoming assignments. While the double-blind nature of the trial alleviated this concern to some extent, it should be noted that all patients did receive etanercept previously in the initial open-label part of the trial. Chances of unblinding may not be ignorable if etanercept and placebo have immediately evident different effects or side effects. The randomized withdrawal design was appropriate in this context to improve statistical power in identifying efficacious treatments, but the specific randomization procedure used in the trial increased vulnerability to selection biases if blinding cannot be completely maintained. FDA review also identified that four patients were randomized from the wrong “few” or “many” strata, in three of which (3/51 = 5.9%) it was foreseeable that the treatment received could have been reversed compared to what the patient would have received if randomized in the correct stratum. There were also some patients randomized out of order. Imbalance in baseline characteristics were observed in age (mean ages of 8.9 years in the etanercept arm vs. that of 12.2 years in the placebo arm) and corticosteroid use at baseline (50% vs. 24%). While the authors [ 85 ] concluded that “The unequal randomization did not affect the study results”, and indeed it was unknown whether the imbalance was a chance occurrence or in part caused by selection biases, the trial could have used better alternative randomization procedures to reduce vulnerability to potential selection bias. To illustrate the latter point, let us compare predictability of two randomization procedures – permuted block design (PBD) and big stick design (BSD) for several values of the maximum tolerated imbalance (MTI). We use BSD here for the illustration purpose because it was found to provide a very good balance/randomness tradeoff based on our simulations in Example 1 . In essence, BSD provides the same level of imbalance control as PBD but with stronger encryption. Table Table3 3 reports two metrics for PBD and BSD: proportion of deterministic assignments within a randomization sequence, and excess correct guess probability. The latter metric is the absolute increase in proportion of correct guesses for a given procedure over CRD that has 50% probability of correct guesses under the “optimal guessing strategy”. 1 Note that for MTI = 1, BSD is equivalent to PBD with blocks of two. However, by increasing MTI, one can substantially decrease predictability. For instance, going from MTI = 1 in the BSD to an MTI of 2 or 3 (two bottom rows), the proportion of deterministic assignments decreases from 50% to 25% and 16.7%, respectively, and excess correct guess probability decreases from 25% to 12.5% and 8.3%, which is a substantial reduction in risk of selection bias. In addition to simplicity and lower predictability for the same level of MTI control, BSD has another important advantage: investigators are not accustomed to it (as they are to the PBD), and therefore it has potential for complete elimination of prediction through thwarting enough early prediction attempts. Predictability of permuted block design (PBD) and big stick design (BSD) for different values of maximum tolerated imbalance (MTI)
Our observations here are also generalizable to other MTI randomization methods, such as the maximal procedure [ 35 ], Chen’s designs [ 38 , 39 ], block urn design [ 40 ], just to name a few. MTI randomization procedures can be also used as building elements for more complex stratified randomization schemes [ 86 ]. Example 3: How can we mitigate risk of chronological bias?Chronological bias may occur if a trial recruitment period is long, and there is a drift in some covariate over time that is subsequently not accounted for in the analysis [ 29 ]. To mitigate risk of chronological bias, treatment assignments should be balanced over time. In this regard, the ICH E9 guideline has the following statement [ 31 ]: “...Although unrestricted randomisation is an acceptable approach, some advantages can generally be gained by randomising subjects in blocks. This helps to increase the comparability of the treatment groups, particularly when subject characteristics may change over time, as a result, for example, of changes in recruitment policy. It also provides a better guarantee that the treatment groups will be of nearly equal size...” While randomization in blocks of two ensures best balance, it is highly predictable. In practice, a sensible tradeoff between balance and randomness is desirable. In the following example, we illustrate the issue of chronological bias in the context of a real RCT. Altman and Royston [ 87 ] gave several examples of clinical studies with hidden time trends. For instance, an RCT to compare azathioprine versus placebo in patients with primary biliary cirrhosis (PBC) with respect to overall survival was an international, double-blind, randomized trial including 248 patients of whom 127 received azathioprine and 121 placebo [ 88 ]. The study had a recruitment period of 7 years. A major prognostic factor for survival was the serum bilirubin level on entry to the trial. Altman and Royston [ 87 ] provided a cusum plot of log bilirubin which showed a strong decreasing trend over time – patients who entered the trial later had, on average, lower bilirubin levels, and therefore better prognosis. Despite that the trial was randomized, there was some evidence of baseline imbalance with respect to serum bilirubin between azathioprine and placebo groups. The analysis using Cox regression adjusted for serum bilirubin showed that the treatment effect of azathioprine was statistically significant ( p = 0.01), with azathioprine reducing the risk of dying to 59% of that observed during the placebo treatment. The azathioprine trial [ 88 ] provides a very good example for illustrating importance of both the choice of a randomization design and a subsequent statistical analysis. We evaluated several randomization designs and analysis strategies under the given time trend through simulation. Since we did not have access to the patient level data from the azathioprine trial, we simulated a dataset of serum bilirubin values from 248 patients that resembled that in the original paper (Fig. 1 in [ 87 ]); see Fig. 6 below.  Cusum plot of baseline log serum bilirubin level of 248 subjects from the azathioprine trial, reproduced from Fig. 1 of Altman and Royston [ 87 ] For the survival outcomes, we use the following data generating mechanism [ 71 , 89 ]: let h i ( t , δ i ) denote the hazard function of the i th patient at time t such that where h c ( t ) is an unspecified baseline hazard, log H R is the true value of the log-transformed hazard ratio, and u i is the log serum bilirubin of the i th patient at study entry. Our main goal is to evaluate the impact of the time trend in bilirubin on the type I error rate and power. We consider seven randomization designs: CRD, Rand, TBD, PBD(2), PBD(4), BSD(3), and GBCD(2). The latter two designs were found to be the top two performing procedures based on our simulation results in Example 1 (cf. Table Table2). 2 ). PBD(4) is the most commonly used procedure in clinical trial practice. Rand and TBD are two designs that ensure exact balance in the final treatment numbers. CRD is the most random design, and PBD(2) is the most balanced design. To evaluate both type I error and power, we consider two values for the true treatment effect: H R = 1 (Null) and H R = 0.6 (Alternative). For data analysis, we use the Cox regression model, either with or without adjustment for serum bilirubin. Furthermore, we assess two approaches to statistical inference: population model-based and randomization-based. For the sake of simplicity, we let h c t ≡ 1 (exponential distribution) and assume no censoring when simulating the data. For each combination of the design, experimental scenario, and data analysis strategy, a trial with 248 patients was simulated 10,000 times. Each randomization-based test was computed using L = 1 , 000 sequences. In each simulation, we used the same time trend in serum bilirubin as described. Through simulation, we estimated the probability of a statistically significant baseline imbalance in serum bilirubin between azathioprine and placebo groups, type I error rate, and power. First, we observed that the designs differ with respect to their potential to achieve baseline covariate balance under the time trend. For instance, probability of a statistically significant group difference on serum bilirubin (two-sided P < 0.05) is ~ 24% for TBD, ~ 10% for CRD, ~ 2% for GBCD(2), ~ 0.9% for Rand, and ~ 0% for BSD(3), PBD(4), and PBD(2). Second, a failure to adjust for serum bilirubin in the analysis can negatively impact statistical inference. Table Table4 4 shows the type I error and power of statistical analyses unadjusted and adjusted for serum bilirubin, using population model-based and randomization-based approaches. Type I error and power of seven randomization designs under a time trend
If we look at the type I error for the population model-based, unadjusted analysis, we can see that only CRD and Rand are valid (maintain the type I error rate at 5%), whereas TBD is anticonservative (~ 15% type I error) and PBD(2), PBD(4), BSD(3), and GBCD(2) are conservative (~ 1–2% type I error). These findings are consistent with the ones for the two-sample t-test described earlier in the current paper, and they agree well with other findings in the literature [ 67 ]. By contrast, population model-based covariate-adjusted analysis is valid for all seven randomization designs. Looking at the type I error for the randomization-based analyses, all designs yield consistent valid results (~ 5% type I error), with or without adjustment for serum bilirubin. As regards statistical power, unadjusted analyses are substantially less powerful then the corresponding covariate-adjusted analysis, for all designs with either population model-based or randomization-based approaches. For the population model-based, unadjusted analysis, the designs have ~ 59–65% power, whereas than the corresponding covariate-adjusted analyses have ~ 97% power. The most striking results are observed with the randomization-based approach: the power of unadjusted analysis is quite different across seven designs: it is ~ 37% for TBD, ~ 60–61% for CRD and Rand, ~ 80–87% for BCD(3), GBCD(2), and PBD(4), and it is ~ 90% for PBD(2). Thus, PBD(2) is the most powerful approach if a time trend is present, statistical analysis strategy is randomization-based, and no adjustment for time trend is made. Furthermore, randomization-based covariate-adjusted analyses have ~ 97% power for all seven designs. Remarkably, the power of covariate-adjusted analysis is identical for population model-based and randomization-based approaches. Overall, this example highlights the importance of covariate-adjusted analysis, which should be straightforward if a covariate affected by a time trend is known (e.g. serum bilirubin in our example). If a covariate is unknown or hidden, then unadjusted analysis following a conventional test may have reduced power and distorted type I error (although the designs such as CRD and Rand do ensure valid statistical inference). Alternatively, randomization-based tests can be applied. The resulting analysis will be valid but may be potentially less powerful. The degree of loss in power following randomization-based test depends on the randomization design: designs that force greater treatment balance over time will be more powerful. In fact, PBD(2) is shown to be most powerful under such circumstances; however, as we have seen in Example 1 and Example 2, a major deficiency of PBD(2) is its vulnerability to selection bias. From Table Table4, 4 , and taking into account the earlier findings in this paper, BSD(3) seems to provide a very good risk mitigation strategy against unknown time trends. Example 4: How do we design an RCT with a very small sample size?In our last example, we illustrate the importance of the careful choice of randomization design and subsequent statistical analysis in a nonstandard RCT with small sample size. Due to confidentiality and because this study is still in conduct, we do not disclose all details here except for that the study is an ongoing phase II RCT in a very rare and devastating autoimmune disease in children. The study includes three periods: an open-label single-arm active treatment for 28 weeks to identify treatment responders (Period 1), a 24-week randomized treatment withdrawal period to primarily assess the efficacy of the active treatment vs. placebo (Period 2), and a 3-year long-term safety, open-label active treatment (Period 3). Because of a challenging indication and the rarity of the disease, the study plans to enroll up to 10 male or female pediatric patients in order to randomize 8 patients (4 per treatment arm) in Period 2 of the study. The primary endpoint for assessing the efficacy of active treatment versus placebo is the proportion of patients with disease flare during the 24-week randomized withdrawal phase. The two groups will be compared using Fisher’s exact test. In case of a successful outcome, evidence of clinical efficacy from this study will be also used as part of a package to support the claim for drug effectiveness. Very small sample sizes are not uncommon in clinical trials of rare diseases [ 90 , 91 ]. Naturally, there are several methodological challenges for this type of study. A major challenge is generalizability of the results from the RCT to a population. In this particular indication, no approved treatment exists, and there is uncertainty on disease epidemiology and the exact number of patients with the disease who would benefit from treatment (patient horizon). Another challenge is the choice of the randomization procedure and the primary statistical analysis. In this study, one can enumerate upfront all 25 possible outcomes: {0, 1, 2, 3, 4} responders on active treatment, and {0, 1, 2, 3, 4} responders on placebo, and create a chart quantifying the level of evidence ( p- value) for each experimental outcome, and the corresponding decision. Before the trial starts, a discussion with the regulatory agency is warranted to agree upon on what level of evidence must be achieved in order to declare the study a “success”. Let us perform a hypothetical planning for the given study. Suppose we go with a standard population-based approach, for which we test the hypothesis H 0 : p E = p C vs. H 0 : p E > p C (where p E and p C stand for the true success rates for the experimental and control group, respectively) using Fisher’s exact test. Table Table5 5 provides 1-sided p- values of all possible experimental outcomes. One could argue that a p- value < 0.1 may be viewed as a convincing level of evidence for this study. There are only 3 possibilities that can lead to this outcome: 3/4 vs. 0/4 successes ( p = 0.0714); 4/4 vs. 0/4 successes ( p = 0.0143); and 4/4 vs. 1/4 successes ( p = 0.0714). For all other outcomes, p ≥ 0.2143, and thus the study would be regarded as a “failure”. All possible outcomes, p- values, and corresponding decisions for an RCT with n = 8 patients (4 per treatment arm) with Fisher’s exact test
a F Declare study a failure, S Declare study a success Now let us consider a randomization-based inference approach. For illustration purposes, we consider four restricted randomization procedures—Rand, TBD, PBD(4), and PBD(2)—that exactly achieve 4:4 allocation. These procedures are legitimate choices because all of them provide exact sample sizes (4 per treatment group), which is essential in this trial. The reference set of either Rand or TBD includes 70 = 8 4 unique sequences though with different probabilities of observing each sequence. For Rand, these sequences are equiprobable, whereas for TBD, some sequences are more likely than others. For PBD( 2 b ), the size of the reference set is 2 b b B , where B = n / 2 b is the number of blocks of length 2 b for a trial of size n (in our example n = 8 ). This results in in a reference set of 2 4 = 16 unique sequences with equal probability of 1/16 for PBD(2), and of 6 2 = 36 unique sequences with equal probability of 1/36 for PBD(4). In practice, the study statistician picks a treatment sequence at random from the reference set according to the chosen design. The details (randomization seed, chosen sequence, etc.) are carefully documented and kept confidential. For the chosen sequence and the observed outcome data, a randomization-based p- value is the sum of probabilities of all sequences in the reference set that yield the result at least as large in favor of the experimental treatment as the one observed. This p- value will depend on the randomization design, the observed randomization sequence and the observed outcomes, and it may also be different from the population-based analysis p- value. To illustrate this, suppose the chosen randomization sequence is CEECECCE (C stands for control and E stands for experimental), and the observed responses are FSSFFFFS (F stands for failure and S stands for success). Thus, we have 3/4 successes on experimental and 0/4 successes on control. Then, the randomization-based p- value is 0.0714 for Rand; 0.0469 for TBD, 0.1250 for PBD(2); 0.0833 for PBD(4); and it is 0.0714 for the population-based analysis. The coincidence of the randomization-based p- value for Rand and the p- value of the population-based analysis is not surprising. Fisher's exact test is a permutation test and in the case of Rand as randomization procedure, the p- value of a permutation test and of a randomization test are always equal. However, despite the numerical equality, we should be mindful of different assumptions (population/randomization model). Likewise, randomization-based p- values can be derived for other combinations of observed randomization sequences and responses. All these details (the chosen randomization design, the analysis strategy, and corresponding decisions) would have to be fully specified upfront (before the trial starts) and agreed upon by both the sponsor and the regulator. This would remove any ambiguity when the trial data become available. As the example shows, the level of evidence in the randomization-based inference approach depends on the chosen randomization procedure and the resulting decisions may be different depending on the specific procedure. For instance, if the level of significance is set to 10% as a criterion for a “successful trial”, then with the observed data (3/4 vs. 0/4), there would be a significant test result for TBD, Rand, PBD(4), but not for PBD(2). Summary and discussionRandomization is the foundation of any RCT involving treatment comparison. Randomization is not a single technique, but a very broad class of statistical methodologies for design and analysis of clinical trials [ 10 ]. In this paper, we focused on the randomized controlled two-arm trial designed with equal allocation, which is the gold standard research design to generate clinical evidence in support of regulatory submissions. Even in this relatively simple case, there are various restricted randomization procedures with different probabilistic structures and different statistical properties, and the choice of a randomization design for any RCT must be made judiciously. For the 1:1 RCT, there is a dual goal of balancing treatment assignments while maintaining allocation randomness. Final balance in treatment totals frequently maximizes statistical power for treatment comparison. It is also important to maintain balance at intermediate steps during the trial, especially in long-term studies, to mitigate potential for chronological bias. At the same time, a procedure should have high degree of randomness so that treatment assignments within the sequence are not easily predictable; otherwise, the procedure may be vulnerable to selection bias, especially in open-label studies. While balance and randomness are competing criteria, it is possible to find restricted randomization procedures that provide a sensible tradeoff between these criteria, e.g. the MTI procedures, of which the big stick design (BSD) [ 37 ] with a suitably chosen MTI limit, such as BSD(3), has very appealing statistical properties. In practice, the choice of a randomization procedure should be made after a systematic evaluation of different candidate procedures under different experimental scenarios for the primary outcome, including cases when model assumptions are violated. In our considered examples we showed that the choice of randomization design, data analytic technique (e.g. parametric or nonparametric model, with or without covariate adjustment), and the decision on whether to include randomization in the analysis (e.g. randomization-based or population model-based analysis) are all very important considerations. Furthermore, these examples highlight the importance of using randomization designs that provide strong encryption of the randomization sequence, importance of covariate adjustment in the analysis, and the value of statistical thinking in nonstandard RCTs with very small sample sizes and small patient horizon. Finally, in this paper we have discussed randomization-based tests as robust and valid alternatives to likelihood-based tests. Randomization-based inference is a useful approach in clinical trials and should be considered by clinical researchers more frequently [ 14 ]. Further topics on randomizationGiven the breadth of the subject of randomization, many important topics have been omitted from the current paper. Here we outline just a few of them. In this paper, we have focused on the 1:1 RCT. However, clinical trials may involve more than two treatment arms. Extensions of equal randomization to the case of multiple treatment arms is relatively straightforward for many restricted randomization procedures [ 10 ]. Some trials with two or more treatment arms use unequal allocation (e.g. 2:1). Randomization procedures with unequal allocation ratios require careful consideration. For instance, an important and desirable feature is the allocation ratio preserving property (ARP). A randomization procedure targeting unequal allocation is said to be ARP, if at each allocation step the unconditional probability of a particular treatment assignment is the same as the target allocation proportion for this treatment [ 92 ]. Non-ARP procedures may have fluctuations in the unconditional randomization probability from allocation to allocation, which may be problematic [ 93 ]. Fortunately, some randomization procedures naturally possess the ARP property, and there are approaches to correct for a non-ARP deficiency – these should be considered in the design of RCTs with unequal allocation ratios [ 92 – 94 ]. In many RCTs, investigators may wish to prospectively balance treatment assignments with respect to important prognostic covariates. For a small number of categorical covariates one can use stratified randomization by applying separate MTI randomization procedures within strata [ 86 ]. However, a potential advantage of stratified randomization decreases as the number of stratification variables increases [ 95 ]. In trials where balance over a large number of covariates is sought and the sample size is small or moderate, one can consider covariate-adaptive randomization procedures that achieve balance within covariate margins, such as the minimization procedure [ 96 , 97 ], optimal model-based procedures [ 46 ], or some other covariate-adaptive randomization technique [ 98 ]. To achieve valid and powerful results, covariate-adaptive randomization design must be followed by covariate-adjusted analysis [ 99 ]. Special considerations are required for covariate-adaptive randomization designs with more than two treatment arms and/or unequal allocation ratios [ 100 ]. In some clinical research settings, such as trials for rare and/or life threatening diseases, there is a strong ethical imperative to increase the chance of a trial participant to receive an empirically better treatment. Response-adaptive randomization (RAR) has been increasingly considered in practice, especially in oncology [ 101 , 102 ]. Very extensive methodological research on RAR has been done [ 103 , 104 ]. RAR is increasingly viewed as an important ingredient of complex clinical trials such as umbrella and platform trial designs [ 105 , 106 ]. While RAR, when properly applied, has its merit, the topic has generated a lot of controversial discussions over the years [ 107 – 111 ]. Amid the ongoing COVID-19 pandemic, RCTs evaluating various experimental treatments for critically ill COVID-19 patients do incorporate RAR in their design; see, for example, the I-SPY COVID-19 trial (https://clinicaltrials.gov/ct2/show/ {"type":"clinical-trial","attrs":{"text":"NCT04488081","term_id":"NCT04488081"}} NCT04488081 ). Randomization can also be applied more broadly than in conventional RCT settings where randomization units are individual subjects. For instance, in a cluster randomized trial, not individuals but groups of individuals (clusters) are randomized among one or more interventions or the control [ 112 ]. Observations from individuals within a given cluster cannot be regarded as independent, and special statistical techniques are required to design and analyze cluster-randomized experiments. In some clinical trial designs, randomization is applied within subjects. For instance, the micro-randomized trial (MRT) is a novel design for development of mobile treatment interventions in which randomization is applied to select different treatment options for individual participants over time to optimally support individuals’ health behaviors [ 113 ]. Finally, beyond the scope of the present paper are the regulatory perspectives on randomization and practical implementation aspects, including statistical software and information systems to generate randomization schedules in real time. We hope to cover these topics in subsequent papers. AcknowledgementsThe authors are grateful to Robert A. Beckman for his continuous efforts coordinating Innovative Design Scientific Working Groups, which is also a networking research platform for the Randomization ID SWG. We would also like to thank the editorial board and the two anonymous reviewers for the valuable comments which helped to substantially improve the original version of the manuscript. Authors’ contributionsConception: VWB, KC, NH, RDH, OS. Writing of the main manuscript: OS, with contributions from VWB, KC, JJC, CE, NH, and RDH. Design of simulation studies: OS, YR. Development of code and running simulations: YR. Digitization and preparation of data for Fig. 5 : JR. All authors reviewed the original manuscript and the revised version. The authors read and approved the final manuscript. None. The opinions expressed in this article are those of the authors and may not reflect the opinions of the organizations that they work for. Availability of data and materialsDeclarations. Not applicable. 1 Guess the next allocation as the treatment with fewest allocations in the sequence thus far, or make a random guess if the treatment numbers are equal. Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Contributor InformationRalf-Dieter Hilgers, Email: ed.nehcakku@sreglihr . Oleksandr Sverdlov, Email: [email protected] . for the Randomization Innovative Design Scientific Working Group: Robert A Beckman Have a language expert improve your writingRun a free plagiarism check in 10 minutes, automatically generate references for free.
Random Assignment in Experiments | Introduction & ExamplesPublished on 6 May 2022 by Pritha Bhandari . Revised on 13 February 2023. In experimental research, random assignment is a way of placing participants from your sample into different treatment groups using randomisation. With simple random assignment, every member of the sample has a known or equal chance of being placed in a control group or an experimental group. Studies that use simple random assignment are also called completely randomised designs . Random assignment is a key part of experimental design . It helps you ensure that all groups are comparable at the start of a study: any differences between them are due to random factors. Table of contentsWhy does random assignment matter, random sampling vs random assignment, how do you use random assignment, when is random assignment not used, frequently asked questions about random assignment. Random assignment is an important part of control in experimental research, because it helps strengthen the internal validity of an experiment. In experiments, researchers manipulate an independent variable to assess its effect on a dependent variable, while controlling for other variables. To do so, they often use different levels of an independent variable for different groups of participants. This is called a between-groups or independent measures design. You use three groups of participants that are each given a different level of the independent variable:
Random assignment to helps you make sure that the treatment groups don’t differ in systematic or biased ways at the start of the experiment. If you don’t use random assignment, you may not be able to rule out alternative explanations for your results.
With this type of assignment, it’s hard to tell whether the participant characteristics are the same across all groups at the start of the study. Gym users may tend to engage in more healthy behaviours than people who frequent pubs or community centres, and this would introduce a healthy user bias in your study. Although random assignment helps even out baseline differences between groups, it doesn’t always make them completely equivalent. There may still be extraneous variables that differ between groups, and there will always be some group differences that arise from chance. Most of the time, the random variation between groups is low, and, therefore, it’s acceptable for further analysis. This is especially true when you have a large sample. In general, you should always use random assignment in experiments when it is ethically possible and makes sense for your study topic. Prevent plagiarism, run a free check.Random sampling and random assignment are both important concepts in research, but it’s important to understand the difference between them. Random sampling (also called probability sampling or random selection) is a way of selecting members of a population to be included in your study. In contrast, random assignment is a way of sorting the sample participants into control and experimental groups. While random sampling is used in many types of studies, random assignment is only used in between-subjects experimental designs. Some studies use both random sampling and random assignment, while others use only one or the other. 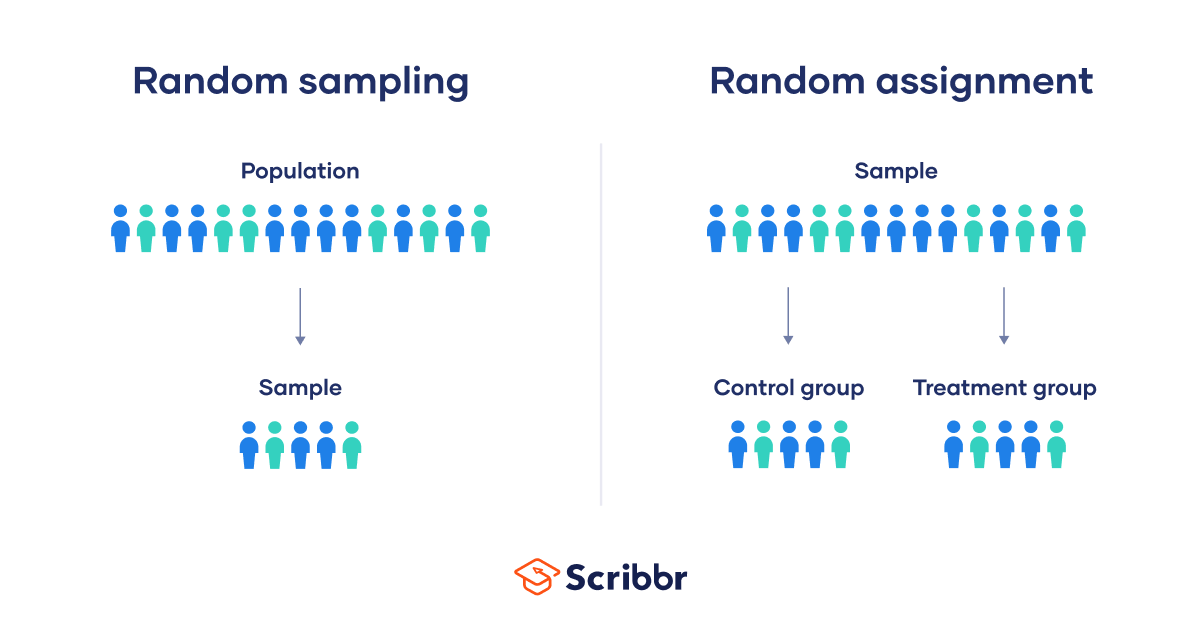 Random sampling enhances the external validity or generalisability of your results, because it helps to ensure that your sample is unbiased and representative of the whole population. This allows you to make stronger statistical inferences . You use a simple random sample to collect data. Because you have access to the whole population (all employees), you can assign all 8,000 employees a number and use a random number generator to select 300 employees. These 300 employees are your full sample. Random assignment enhances the internal validity of the study, because it ensures that there are no systematic differences between the participants in each group. This helps you conclude that the outcomes can be attributed to the independent variable .
You use random assignment to place participants into the control or experimental group. To do so, you take your list of participants and assign each participant a number. Again, you use a random number generator to place each participant in one of the two groups. To use simple random assignment, you start by giving every member of the sample a unique number. Then, you can use computer programs or manual methods to randomly assign each participant to a group.
This type of random assignment is the most powerful method of placing participants in conditions, because each individual has an equal chance of being placed in any one of your treatment groups. Random assignment in block designsIn more complicated experimental designs, random assignment is only used after participants are grouped into blocks based on some characteristic (e.g., test score or demographic variable). These groupings mean that you need a larger sample to achieve high statistical power . For example, a randomised block design involves placing participants into blocks based on a shared characteristic (e.g., college students vs graduates), and then using random assignment within each block to assign participants to every treatment condition. This helps you assess whether the characteristic affects the outcomes of your treatment. In an experimental matched design , you use blocking and then match up individual participants from each block based on specific characteristics. Within each matched pair or group, you randomly assign each participant to one of the conditions in the experiment and compare their outcomes. Sometimes, it’s not relevant or ethical to use simple random assignment, so groups are assigned in a different way. When comparing different groupsSometimes, differences between participants are the main focus of a study, for example, when comparing children and adults or people with and without health conditions. Participants are not randomly assigned to different groups, but instead assigned based on their characteristics. In this type of study, the characteristic of interest (e.g., gender) is an independent variable, and the groups differ based on the different levels (e.g., men, women). All participants are tested the same way, and then their group-level outcomes are compared. When it’s not ethically permissibleWhen studying unhealthy or dangerous behaviours, it’s not possible to use random assignment. For example, if you’re studying heavy drinkers and social drinkers, it’s unethical to randomly assign participants to one of the two groups and ask them to drink large amounts of alcohol for your experiment. When you can’t assign participants to groups, you can also conduct a quasi-experimental study . In a quasi-experiment, you study the outcomes of pre-existing groups who receive treatments that you may not have any control over (e.g., heavy drinkers and social drinkers). These groups aren’t randomly assigned, but may be considered comparable when some other variables (e.g., age or socioeconomic status) are controlled for. In experimental research, random assignment is a way of placing participants from your sample into different groups using randomisation. With this method, every member of the sample has a known or equal chance of being placed in a control group or an experimental group. Random selection, or random sampling , is a way of selecting members of a population for your study’s sample. In contrast, random assignment is a way of sorting the sample into control and experimental groups. Random sampling enhances the external validity or generalisability of your results, while random assignment improves the internal validity of your study. Random assignment is used in experiments with a between-groups or independent measures design. In this research design, there’s usually a control group and one or more experimental groups. Random assignment helps ensure that the groups are comparable. In general, you should always use random assignment in this type of experimental design when it is ethically possible and makes sense for your study topic. To implement random assignment , assign a unique number to every member of your study’s sample . Then, you can use a random number generator or a lottery method to randomly assign each number to a control or experimental group. You can also do so manually, by flipping a coin or rolling a die to randomly assign participants to groups. Cite this Scribbr articleIf you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator. Bhandari, P. (2023, February 13). Random Assignment in Experiments | Introduction & Examples. Scribbr. Retrieved 30 July 2024, from https://www.scribbr.co.uk/research-methods/random-assignment-experiments/ Is this article helpful? Pritha BhandariOther students also liked, a quick guide to experimental design | 5 steps & examples, controlled experiments | methods & examples of control, control groups and treatment groups | uses & examples.  In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation. Have a language expert improve your writingRun a free plagiarism check in 10 minutes, generate accurate citations for free.
Methodology
Quasi-Experimental Design | Definition, Types & ExamplesPublished on July 31, 2020 by Lauren Thomas . Revised on January 22, 2024. Like a true experiment , a quasi-experimental design aims to establish a cause-and-effect relationship between an independent and dependent variable . However, unlike a true experiment, a quasi-experiment does not rely on random assignment . Instead, subjects are assigned to groups based on non-random criteria. Quasi-experimental design is a useful tool in situations where true experiments cannot be used for ethical or practical reasons. 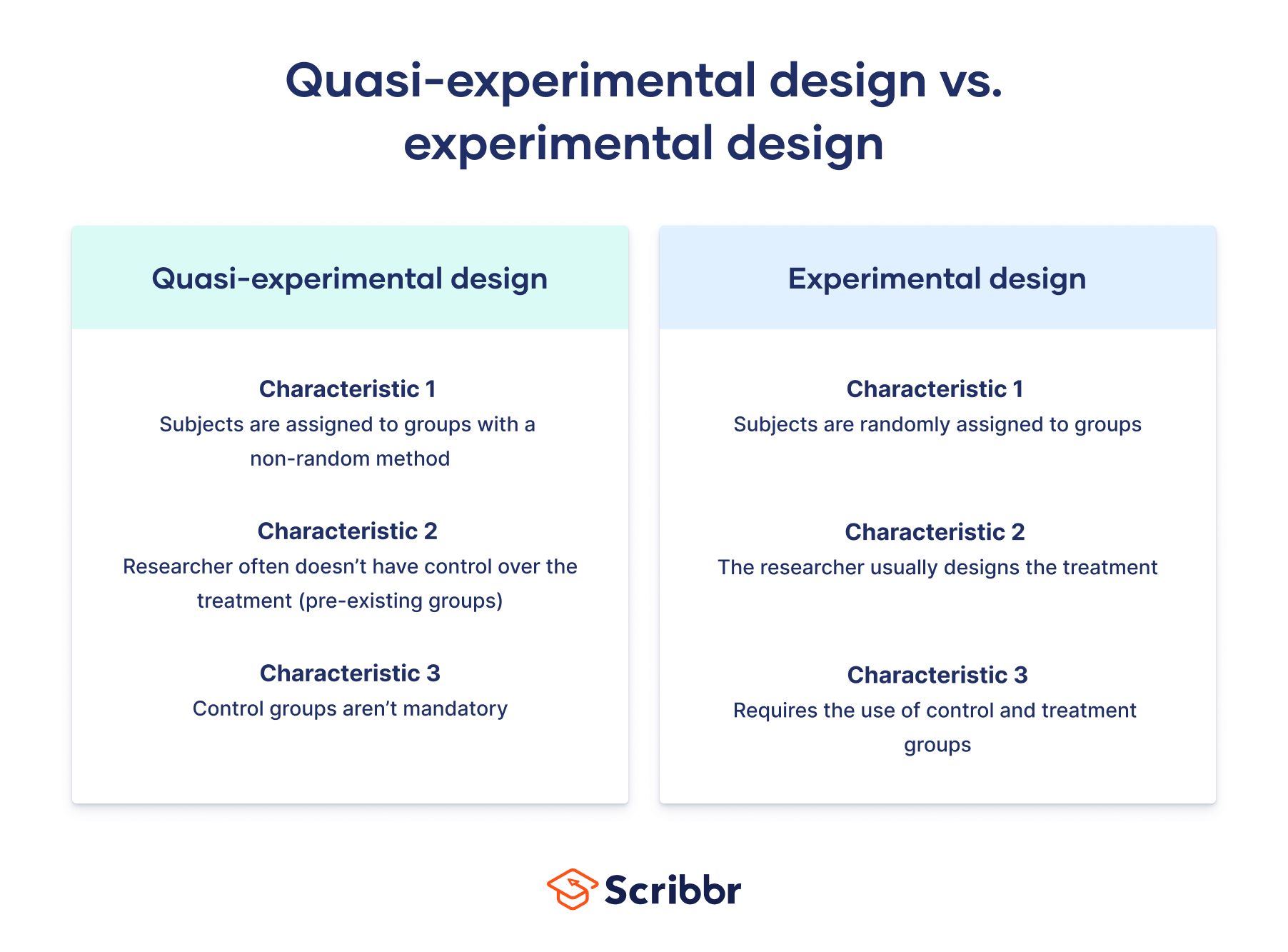 Table of contentsDifferences between quasi-experiments and true experiments, types of quasi-experimental designs, when to use quasi-experimental design, advantages and disadvantages, other interesting articles, frequently asked questions about quasi-experimental designs. There are several common differences between true and quasi-experimental designs.
Example of a true experiment vs a quasi-experimentHowever, for ethical reasons, the directors of the mental health clinic may not give you permission to randomly assign their patients to treatments. In this case, you cannot run a true experiment. Instead, you can use a quasi-experimental design. You can use these pre-existing groups to study the symptom progression of the patients treated with the new therapy versus those receiving the standard course of treatment. Receive feedback on language, structure, and formattingProfessional editors proofread and edit your paper by focusing on:
See an example  Many types of quasi-experimental designs exist. Here we explain three of the most common types: nonequivalent groups design, regression discontinuity, and natural experiments. Nonequivalent groups designIn nonequivalent group design, the researcher chooses existing groups that appear similar, but where only one of the groups experiences the treatment. In a true experiment with random assignment , the control and treatment groups are considered equivalent in every way other than the treatment. But in a quasi-experiment where the groups are not random, they may differ in other ways—they are nonequivalent groups . When using this kind of design, researchers try to account for any confounding variables by controlling for them in their analysis or by choosing groups that are as similar as possible. This is the most common type of quasi-experimental design. Regression discontinuityMany potential treatments that researchers wish to study are designed around an essentially arbitrary cutoff, where those above the threshold receive the treatment and those below it do not. Near this threshold, the differences between the two groups are often so minimal as to be nearly nonexistent. Therefore, researchers can use individuals just below the threshold as a control group and those just above as a treatment group. However, since the exact cutoff score is arbitrary, the students near the threshold—those who just barely pass the exam and those who fail by a very small margin—tend to be very similar, with the small differences in their scores mostly due to random chance. You can therefore conclude that any outcome differences must come from the school they attended. Natural experimentsIn both laboratory and field experiments, researchers normally control which group the subjects are assigned to. In a natural experiment, an external event or situation (“nature”) results in the random or random-like assignment of subjects to the treatment group. Even though some use random assignments, natural experiments are not considered to be true experiments because they are observational in nature. Although the researchers have no control over the independent variable , they can exploit this event after the fact to study the effect of the treatment. However, as they could not afford to cover everyone who they deemed eligible for the program, they instead allocated spots in the program based on a random lottery. Although true experiments have higher internal validity , you might choose to use a quasi-experimental design for ethical or practical reasons. Sometimes it would be unethical to provide or withhold a treatment on a random basis, so a true experiment is not feasible. In this case, a quasi-experiment can allow you to study the same causal relationship without the ethical issues. The Oregon Health Study is a good example. It would be unethical to randomly provide some people with health insurance but purposely prevent others from receiving it solely for the purposes of research. However, since the Oregon government faced financial constraints and decided to provide health insurance via lottery, studying this event after the fact is a much more ethical approach to studying the same problem. True experimental design may be infeasible to implement or simply too expensive, particularly for researchers without access to large funding streams. At other times, too much work is involved in recruiting and properly designing an experimental intervention for an adequate number of subjects to justify a true experiment. In either case, quasi-experimental designs allow you to study the question by taking advantage of data that has previously been paid for or collected by others (often the government). Quasi-experimental designs have various pros and cons compared to other types of studies.
Here's why students love Scribbr's proofreading servicesDiscover proofreading & editing If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
Research bias
A quasi-experiment is a type of research design that attempts to establish a cause-and-effect relationship. The main difference with a true experiment is that the groups are not randomly assigned. In experimental research, random assignment is a way of placing participants from your sample into different groups using randomization. With this method, every member of the sample has a known or equal chance of being placed in a control group or an experimental group. Quasi-experimental design is most useful in situations where it would be unethical or impractical to run a true experiment . Quasi-experiments have lower internal validity than true experiments, but they often have higher external validity as they can use real-world interventions instead of artificial laboratory settings. Cite this Scribbr articleIf you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator. Thomas, L. (2024, January 22). Quasi-Experimental Design | Definition, Types & Examples. Scribbr. Retrieved July 30, 2024, from https://www.scribbr.com/methodology/quasi-experimental-design/ Is this article helpful? Lauren ThomasOther students also liked, guide to experimental design | overview, steps, & examples, random assignment in experiments | introduction & examples, control variables | what are they & why do they matter, what is your plagiarism score.  | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
IMAGES
VIDEO
COMMENTS
Random sampling (also called probability sampling or random selection) is a way of selecting members of a population to be included in your study. In contrast, random assignment is a way of sorting the sample participants into control and experimental groups. While random sampling is used in many types of studies, random assignment is only used ...
Random selection (also called probability sampling or random sampling) is a way of randomly selecting members of a population to be included in your study. On the other hand, random assignment is a way of sorting the sample participants into control and treatment groups. Random selection ensures that everyone in the population has an equal ...
By Jim Frost 4 Comments. Random assignment uses chance to assign subjects to the control and treatment groups in an experiment. This process helps ensure that the groups are equivalent at the beginning of the study, which makes it safer to assume the treatments caused any differences between groups that the experimenters observe at the end of ...
Advances in technology introduced new tools and techniques for implementing randomization, such as computerized random number generators, which offered greater precision and ease of use. The application of random assignment expanded beyond the confines of the laboratory, finding its way into field studies and large-scale surveys.
Random assignment or random placement is an experimental technique for assigning human participants or animal subjects to different groups in an experiment (e.g., a treatment group versus a control group) using randomization, such as by a chance procedure (e.g., flipping a coin) or a random number generator. This ensures that each participant or subject has an equal chance of being placed in ...
Randomization is a statistical process in which a random mechanism is employed to select a sample from a population or assign subjects to different groups. The process is crucial in ensuring the random allocation of experimental units or treatment protocols, thereby minimizing selection bias and enhancing the statistical validity. It facilitates the objective comparison of treatment effects in ...
Example. Your study researches the impact of technology on productivity in a specific company. In such a case, you have contact with the entire staff. So, you can assign each employee a quantity and apply a random number generator to pick a specific sample. For instance, from 500 employees, you can pick 200.
1.1 Describe key concepts, principles, and overarching themes in psychology 2.4 Interpret, design, and conduct basic psychological research ... They must explain how and why researchers use random assignment. ... For teachers who do not have the technology to project words on a screen, they could hold up pieces of paper with a word written in ...
Sometimes people think that "random" means that two events are equally likely, but in fact, random assignment is "random" so long as the probability of assignment to treatment is strictly between 0 and 1. If a subject has a 0 or a 100 percent chance of being assigned to treatment, that subject should be excluded from your experimental ...
Materio / Getty Images. Random assignment refers to the use of chance procedures in psychology experiments to ensure that each participant has the same opportunity to be assigned to any given group in a study to eliminate any potential bias in the experiment at the outset. Participants are randomly assigned to different groups, such as the ...
The use of random assignment cannot eliminate this possibility, but it greatly reduces it. We use the term internal validity to describe the degree to which cause-and-effect inferences are accurate and meaningful. Causal attribution is the goal for many researchers. Thus, by using random assignment we have a pretty high degree of evidence for ...
Pull out 40 slips of paper and assign these subjects to Treatment 1. Then pull out 40 more slips of paper and assign these subjects to Treatment 2. The remaining 40 subjects are assigned to Treatment 3. Describe how you would randomly assign the subjects to the treatments using technology. Assign the students numbers from 1 to 120.
Random assignment is a procedure used in experiments to create multiple study groups that include participants with similar characteristics so that the groups are equivalent at the beginning of the study. The procedure involves assigning individuals to an experimental treatment or program at random, or by chance (like the flip of a coin).
Objective: To review and describe randomization techniques used in clinical trials, including simple, block, stratified, and covariate adaptive techniques. Background: Clinical trials are required to establish treatment efficacy of many athletic training procedures. In the past, we have relied on evidence of questionable scientific merit to aid ...
Random selection, or random sampling, is a way of selecting members of a population for your study's sample. In contrast, random assignment is a way of sorting the sample into control and experimental groups. Random sampling enhances the external validity or generalizability of your results, while random assignment improves the internal ...
Randomization based on a single sequence of random assignments is known as simple randomization. This technique maintains complete randomness of the assignment of a subject to a particular group. The most common and basic method of simple randomization is flipping a coin. For example, with two treatment groups (control versus treatment), the ...
With the adaptive or "in-real-time" randomization, a sequence of treatment assignments is generated dynamically as the trial progresses. For many restricted randomization procedures, the randomization rule can be expressed as Pr ( δ i + 1 = 1) = F D i, where F · is some non-increasing function of D i for any i ≥ 1.
Random sampling (also called probability sampling or random selection) is a way of selecting members of a population to be included in your study. In contrast, random assignment is a way of sorting the sample participants into control and experimental groups. While random sampling is used in many types of studies, random assignment is only used ...
Describe how the random assignment of subjects to treatments might be conducted in the context of this particular experiment. Step 1: Identify the number of participants in the experiment.
Random Assignment In the context of the all causes model, we may state the random assignment assumption as follows: Assumption 1 (Random assignment; RA) Let (Y, W, U) be a random vector with joint distribution characterized by Equation (1). Random assignment assumes W ‹‹ U. (3) In words: the policy W is independent of all other determinants U.
Revised on January 22, 2024. Like a true experiment, a quasi-experimental design aims to establish a cause-and-effect relationship between an independent and dependent variable. However, unlike a true experiment, a quasi-experiment does not rely on random assignment. Instead, subjects are assigned to groups based on non-random criteria.
using carefully constructed comparison groups often shows that the results are different, sometimes much different. For example, a recent random assignment study of dropout prevention programs showed that some types of inter-ventions were effective, but when a matched comparison group design was used instead of a random assignment