Essay on Coronavirus Vaccine
500+ words essay on coronavirus vaccine.
The Coronavirus has infected millions of people so far all over the world. In addition to that, millions of people have lost their lives to it. Ever since the outbreak, researchers all over the world have been working constantly to develop vaccines that will work effectively against the virus. We will take a look at the Coronavirus vaccine that is present today. Vaccines have the ability to save people’s lives. Developing the vaccine for Coronavirus was a huge step to end the pandemic.


Working of Coronavirus Vaccine
As Coronavirus caused a lot of confusion and fear amongst people, it is natural people were not aware of how the vaccine works. To begin with, a vaccine will work by mimicking an infectious agent.
The agent can be viruses, bacteria or any other microorganisms. They carry the potential of causing disease. When it mimics that, our immune system learns how to respond against it rapidly and efficiently.
As per the traditional methods, vaccines have managed to do this as they introduce a weakened form of an infectious agent. It enables our immune system to basically build its memory.
As a result, our immune system can then identify it quickly and fight against it before it gets the chance to harm us or make us ill. Similarly, some of the coronavirus vaccines have been made like that.
On the other hand, there are other coronavirus vaccines that researchers have developed by making use of new approaches. We refer to them as messenger RNA or mRNA vaccines.
Over here, they do not introduce antigens in our bodies. Instead, mRNA vaccines give the genetic code our body needs to enable our immune system for producing the antigen itself.
For several years, researchers have been studying mRNA vaccine technology. Thus, they do not contain any live virus and also do not interfere with the human DNA .
Get the huge list of more than 500 Essay Topics and Ideas
Safety of Coronavirus Vaccine
While the vaccines are being developed at a fast pace, they also require rigorous testing. The tests are done in clinical trials to ensure that they meet the benchmarks for the safety and efficiency of international standards.
When they meet the standards, then only can they get the go-ahead from WHO and national regulatory agencies. UNICEF has said that it will attain and supply only those vaccines that meet the WHO guidelines and have met the regulatory approval.
As of now, the vaccine doses are limited in number. Thus, the healthcare workers, first responders, people over the age of 75 and residents of long-term care facilities will receive the first doses.
After that, everyone will be able to get it once more of them are available. To get the vaccine, a person may require to pay a fee. However, some government institutions are providing it free of cost.
In order to get the vaccine, one must check with their local and state health departments on a regular basis. When they get the chance, they must get the dose right away.
The Coronavirus outbreak has challenged the whole world. Constantly, the experts and authorities are working to develop the vaccines. Therefore, we can also do our bit and adopt preventive measures to limit the spread of this disease. The major goal is to get the vaccine to everyone so that we can go on and about with our normal lives.
FAQ on Essay on Coronavirus Vaccine
Question 1: What are some common side effects of the Coronavirus vaccine?
Answer 1: The most common side effect includes a sore arm, fever , headache, and fatigue. However, not to worry, side effects are good in this case. They indicate that your vaccine is starting to work as it triggers your immune system.
Question 2: When do Coronavirus vaccine side effects kick in?
Answer 2: Usually, most of the side effects start to kick in within the first 3 days after you get your vaccine. Moreover, they will last up to 1 to 2 days only.
Customize your course in 30 seconds
Which class are you in.

- Travelling Essay
- Picnic Essay
- Our Country Essay
- My Parents Essay
- Essay on Favourite Personality
- Essay on Memorable Day of My Life
- Essay on Knowledge is Power
- Essay on Gurpurab
- Essay on My Favourite Season
- Essay on Types of Sports
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

Persuasive Essay Guide
Persuasive Essay About Covid19
How to Write a Persuasive Essay About Covid19 | Examples & Tips
11 min read

People also read
A Comprehensive Guide to Writing an Effective Persuasive Essay
200+ Persuasive Essay Topics to Help You Out
Learn How to Create a Persuasive Essay Outline
30+ Free Persuasive Essay Examples To Get You Started
Read Excellent Examples of Persuasive Essay About Gun Control
Crafting a Convincing Persuasive Essay About Abortion
Learn to Write Persuasive Essay About Business With Examples and Tips
Check Out 12 Persuasive Essay About Online Education Examples
Persuasive Essay About Smoking - Making a Powerful Argument with Examples
Are you looking to write a persuasive essay about the Covid-19 pandemic?
Writing a compelling and informative essay about this global crisis can be challenging. It requires researching the latest information, understanding the facts, and presenting your argument persuasively.
But don’t worry! with some guidance from experts, you’ll be able to write an effective and persuasive essay about Covid-19.
In this blog post, we’ll outline the basics of writing a persuasive essay . We’ll provide clear examples, helpful tips, and essential information for crafting your own persuasive piece on Covid-19.
Read on to get started on your essay.
- 1. Steps to Write a Persuasive Essay About Covid-19
- 2. Examples of Persuasive Essay About Covid19
- 3. Examples of Persuasive Essay About Covid-19 Vaccine
- 4. Examples of Persuasive Essay About Covid-19 Integration
- 5. Examples of Argumentative Essay About Covid 19
- 6. Examples of Persuasive Speeches About Covid-19
- 7. Tips to Write a Persuasive Essay About Covid-19
- 8. Common Topics for a Persuasive Essay on COVID-19
Steps to Write a Persuasive Essay About Covid-19
Here are the steps to help you write a persuasive essay on this topic, along with an example essay:
Step 1: Choose a Specific Thesis Statement
Your thesis statement should clearly state your position on a specific aspect of COVID-19. It should be debatable and clear. For example:
Step 2: Research and Gather Information
Collect reliable and up-to-date information from reputable sources to support your thesis statement. This may include statistics, expert opinions, and scientific studies. For instance:
- COVID-19 vaccination effectiveness data
- Information on vaccine mandates in different countries
- Expert statements from health organizations like the WHO or CDC
Step 3: Outline Your Essay
Create a clear and organized outline to structure your essay. A persuasive essay typically follows this structure:
- Introduction
- Background Information
- Body Paragraphs (with supporting evidence)
- Counterarguments (addressing opposing views)
Step 4: Write the Introduction
In the introduction, grab your reader's attention and present your thesis statement. For example:
Step 5: Provide Background Information
Offer context and background information to help your readers understand the issue better. For instance:
Step 6: Develop Body Paragraphs
Each body paragraph should present a single point or piece of evidence that supports your thesis statement. Use clear topic sentences, evidence, and analysis. Here's an example:
Step 7: Address Counterarguments
Acknowledge opposing viewpoints and refute them with strong counterarguments. This demonstrates that you've considered different perspectives. For example:
Step 8: Write the Conclusion
Summarize your main points and restate your thesis statement in the conclusion. End with a strong call to action or thought-provoking statement. For instance:
Step 9: Revise and Proofread
Edit your essay for clarity, coherence, grammar, and spelling errors. Ensure that your argument flows logically.
Step 10: Cite Your Sources
Include proper citations and a bibliography page to give credit to your sources.
Remember to adjust your approach and arguments based on your target audience and the specific angle you want to take in your persuasive essay about COVID-19.

Paper Due? Why Suffer? That's our Job!
Examples of Persuasive Essay About Covid19
When writing a persuasive essay about the Covid-19 pandemic, it’s important to consider how you want to present your argument. To help you get started, here are some example essays for you to read:
Check out some more PDF examples below:
Persuasive Essay About Covid-19 Pandemic
Sample Of Persuasive Essay About Covid-19
Persuasive Essay About Covid-19 In The Philippines - Example
If you're in search of a compelling persuasive essay on business, don't miss out on our “ persuasive essay about business ” blog!
Examples of Persuasive Essay About Covid-19 Vaccine
Covid19 vaccines are one of the ways to prevent the spread of Covid-19, but they have been a source of controversy. Different sides argue about the benefits or dangers of the new vaccines. Whatever your point of view is, writing a persuasive essay about it is a good way of organizing your thoughts and persuading others.
A persuasive essay about the Covid-19 vaccine could consider the benefits of getting vaccinated as well as the potential side effects.
Below are some examples of persuasive essays on getting vaccinated for Covid-19.
Covid19 Vaccine Persuasive Essay
Persuasive Essay on Covid Vaccines
Interested in thought-provoking discussions on abortion? Read our persuasive essay about abortion blog to eplore arguments!
Examples of Persuasive Essay About Covid-19 Integration
Covid19 has drastically changed the way people interact in schools, markets, and workplaces. In short, it has affected all aspects of life. However, people have started to learn to live with Covid19.
Writing a persuasive essay about it shouldn't be stressful. Read the sample essay below to get idea for your own essay about Covid19 integration.
Persuasive Essay About Working From Home During Covid19
Searching for the topic of Online Education? Our persuasive essay about online education is a must-read.
Examples of Argumentative Essay About Covid 19
Covid-19 has been an ever-evolving issue, with new developments and discoveries being made on a daily basis.
Writing an argumentative essay about such an issue is both interesting and challenging. It allows you to evaluate different aspects of the pandemic, as well as consider potential solutions.
Here are some examples of argumentative essays on Covid19.
Argumentative Essay About Covid19 Sample
Argumentative Essay About Covid19 With Introduction Body and Conclusion
Looking for a persuasive take on the topic of smoking? You'll find it all related arguments in out Persuasive Essay About Smoking blog!
Examples of Persuasive Speeches About Covid-19
Do you need to prepare a speech about Covid19 and need examples? We have them for you!
Persuasive speeches about Covid-19 can provide the audience with valuable insights on how to best handle the pandemic. They can be used to advocate for specific changes in policies or simply raise awareness about the virus.
Check out some examples of persuasive speeches on Covid-19:
Persuasive Speech About Covid-19 Example
Persuasive Speech About Vaccine For Covid-19
You can also read persuasive essay examples on other topics to master your persuasive techniques!
Tips to Write a Persuasive Essay About Covid-19
Writing a persuasive essay about COVID-19 requires a thoughtful approach to present your arguments effectively.
Here are some tips to help you craft a compelling persuasive essay on this topic:
Choose a Specific Angle
Start by narrowing down your focus. COVID-19 is a broad topic, so selecting a specific aspect or issue related to it will make your essay more persuasive and manageable. For example, you could focus on vaccination, public health measures, the economic impact, or misinformation.
Provide Credible Sources
Support your arguments with credible sources such as scientific studies, government reports, and reputable news outlets. Reliable sources enhance the credibility of your essay.
Use Persuasive Language
Employ persuasive techniques, such as ethos (establishing credibility), pathos (appealing to emotions), and logos (using logic and evidence). Use vivid examples and anecdotes to make your points relatable.
Organize Your Essay
Structure your essay involves creating a persuasive essay outline and establishing a logical flow from one point to the next. Each paragraph should focus on a single point, and transitions between paragraphs should be smooth and logical.
Emphasize Benefits
Highlight the benefits of your proposed actions or viewpoints. Explain how your suggestions can improve public health, safety, or well-being. Make it clear why your audience should support your position.
Use Visuals -H3
Incorporate graphs, charts, and statistics when applicable. Visual aids can reinforce your arguments and make complex data more accessible to your readers.
Call to Action
End your essay with a strong call to action. Encourage your readers to take a specific step or consider your viewpoint. Make it clear what you want them to do or think after reading your essay.
Revise and Edit
Proofread your essay for grammar, spelling, and clarity. Make sure your arguments are well-structured and that your writing flows smoothly.
Seek Feedback
Have someone else read your essay to get feedback. They may offer valuable insights and help you identify areas where your persuasive techniques can be improved.
Tough Essay Due? Hire Tough Writers!
Common Topics for a Persuasive Essay on COVID-19
Here are some persuasive essay topics on COVID-19:
- The Importance of Vaccination Mandates for COVID-19 Control
- Balancing Public Health and Personal Freedom During a Pandemic
- The Economic Impact of Lockdowns vs. Public Health Benefits
- The Role of Misinformation in Fueling Vaccine Hesitancy
- Remote Learning vs. In-Person Education: What's Best for Students?
- The Ethics of Vaccine Distribution: Prioritizing Vulnerable Populations
- The Mental Health Crisis Amidst the COVID-19 Pandemic
- The Long-Term Effects of COVID-19 on Healthcare Systems
- Global Cooperation vs. Vaccine Nationalism in Fighting the Pandemic
- The Future of Telemedicine: Expanding Healthcare Access Post-COVID-19
In search of more inspiring topics for your next persuasive essay? Our persuasive essay topics blog has plenty of ideas!
To sum it up,
You have read good sample essays and got some helpful tips. You now have the tools you needed to write a persuasive essay about Covid-19. So don't let the doubts stop you, start writing!
If you need professional writing help, don't worry! We've got that for you as well.
MyPerfectWords.com is a professional essay writing service that can help you craft an excellent persuasive essay on Covid-19. Our experienced essay writer will create a well-structured, insightful paper in no time!
So don't hesitate and get in touch with our persuasive essay writing service today!
Frequently Asked Questions
Are there any ethical considerations when writing a persuasive essay about covid-19.
Yes, there are ethical considerations when writing a persuasive essay about COVID-19. It's essential to ensure the information is accurate, not contribute to misinformation, and be sensitive to the pandemic's impact on individuals and communities. Additionally, respecting diverse viewpoints and emphasizing public health benefits can promote ethical communication.
What impact does COVID-19 have on society?
The impact of COVID-19 on society is far-reaching. It has led to job and economic losses, an increase in stress and mental health disorders, and changes in education systems. It has also had a negative effect on social interactions, as people have been asked to limit their contact with others.
Write Essay Within 60 Seconds!

Caleb S. has been providing writing services for over five years and has a Masters degree from Oxford University. He is an expert in his craft and takes great pride in helping students achieve their academic goals. Caleb is a dedicated professional who always puts his clients first.

Paper Due? Why Suffer? That’s our Job!
Keep reading


- High contrast
- Press Centre
Search UNICEF
What you need to know about covid-19 vaccines, answers to the most common questions about coronavirus vaccines..

- Available in:
Vaccines save millions of lives each year. The development of safe and effective COVID-19 vaccines are a crucial step in helping us get back to doing more of the things we enjoy with the people we love.
We’ve gathered the latest expert information to answer some of the most common questions about COVID-19 vaccines. Keep checking back as we will update this article as more information becomes available.
What are the benefits of getting vaccinated?
Vaccines save millions of lives each year and a COVID-19 vaccine could save yours. The COVID-19 vaccines are safe and effective, providing strong protection against serious illness and death. WHO reports that unvaccinated people have at least 10 times higher risk of death from COVID-19 than someone who has been vaccinated.
It is important to be vaccinated as soon as it’s your turn, even if you already had COVID-19. Getting vaccinated is a safer way for you to develop immunity from COVID-19 than getting infected.
The COVID-19 vaccines are highly effective, but no vaccine provides 100 per cent protection. Some people will still get ill from COVID-19 after vaccination or pass the virus onto someone else.
Therefore, it is important to continue practicing safety precautions to protect yourself and others, including avoiding crowded spaces, physical distancing, hand washing and wearing a mask.
Who should be vaccinated first?
Each country must identify priority populations, which WHO recommends are frontline health workers (to protect health systems) and those at highest risk of death due to COVID-19, such as older adults and people with certain medical conditions. Other essential workers, such as teachers and social workers, should then be prioritized, followed by additional groups as more vaccine doses become available.
The risk of severe illness from COVID-19 is very low amongst healthy children and adolescents, so unless they are part of a group at higher risk of severe COVID-19, it is less urgent to vaccinate them than these priority groups.
Children and adolescents who are at higher risk of developing severe illness from COVID-19, such as those with underlying illnesses, should be prioritized for COVID-19 vaccines.
When shouldn’t you be vaccinated against COVID-19?
If you have any questions about whether you should receive a COVID-19 vaccine, speak to your healthcare provider. At present, people with the following health conditions should not receive a COVID-19 vaccine to avoid any possible adverse effects:
- If you have a history of severe allergic reactions to any ingredients of a COVID-19 vaccine.
- If you are currently sick or experiencing symptoms of COVID-19 (although you can get vaccinated once you have recovered and your doctor has approved).
Should I get vaccinated if I already had COVID-19?
Yes, you should get vaccinated even if you’ve previously had COVID-19. While people who recover from COVID-19 may develop natural immunity to the virus, it is still not certain how long that immunity lasts or how well it protects you against COVID-19 reinfection. Vaccines offer more reliable protection, especially against severe illness and death. Vaccination policies after COVID-19 infection vary by country. Check with your health care provider on the recommendation where you live.
Which COVID-19 vaccine is best for me?
All WHO-approved vaccines have been shown to be highly effective at protecting you against severe illness and death from COVID-19. The best vaccine to get is the one most readily available to you.
You can find a list of those approved vaccines on WHO’s site .
Remember, if your vaccination involves two doses, it’s important to receive both to have the maximum protection.
How do COVID-19 vaccines work?
Vaccines work by mimicking an infectious agent – viruses, bacteria or other microorganisms that can cause a disease. This ‘teaches’ our immune system to rapidly and effectively respond against it.
Traditionally, vaccines have done this by introducing a weakened form of an infectious agent that allows our immune system to build a memory of it. This way, our immune system can quickly recognize and fight it before it makes us ill. That’s how some of the COVID-19 vaccines have been designed.
Other COVID-19 vaccines have been developed using new approaches, which are called messenger RNA, or mRNA, vaccines. Instead of introducing antigens (a substance that causes your immune system to produce antibodies), mRNA vaccines give our body the genetic code it needs to allow our immune system to produce the antigen itself. mRNA vaccine technology has been studied for several decades. They contain no live virus and do not interfere with human DNA.
For more information on how vaccines work, please visit WHO .
Are COVID-19 vaccines safe?
Yes, COVID-19 vaccines have been safely used to vaccinate billions of people. The COVID-19 vaccines were developed as rapidly as possible, but they had to go through rigorous testing in clinical trials to prove that they meet internationally agreed benchmarks for safety and effectiveness. Only if they meet these standards can a vaccine receive validation from WHO and national regulatory agencies.
UNICEF only procures and supplies COVID-19 vaccines that meet WHO’s established safety and efficacy criteria and that have received the required regulatory approval.
How were COVID-19 vaccines developed so quickly?
Scientists were able to develop safe effective vaccines in a relatively short amount of time due to a combination of factors that allowed them to scale up research and production without compromising safety:
- Because of the global pandemic, there was a larger sample size to study and tens of thousands of volunteers stepped forward
- Advancements in technology (like mRNA vaccines) that were years in the making
- Governments and other bodies came together to remove the obstacle of funding research and development
- Manufacturing of the vaccines occurred in parallel to the clinical trials to speed up production
Though they were developed quickly, all COVID-19 vaccines approved for use by the WHO are safe and effective.
What are the side effects of COVID-19 vaccines?
Vaccines are designed to give you immunity without the dangers of getting the disease. Not everyone does, but it’s common to experience some mild-to-moderate side effects that go away within a few days on their own.
Some of the mild-to-moderate side effects you may experience after vaccination include:
- Arm soreness at the injection site
- Muscle or joint aches
You can manage any side effects with rest, staying hydrated and taking medication to manage pain and fever, if needed.
If any symptoms continue for more than a few days then contact your healthcare provider for advice. More serious side effects are extremely rare, but if you experience a more severe reaction, then contact your healthcare provider immediately.
>> Read: What you need to know before, during and after receiving a COVID-19 vaccine
How do I find out more about a particular COVID-19 vaccine?
You can find out more about COVID-19 vaccines on WHO’s website .
Can I stop taking precautions after being vaccinated?
Keep taking precautions to protect yourself, family and friends if there is still COVID-19 in your area, even after getting vaccinated. The COVID-19 vaccines are highly effective against serious illness and death, but no vaccine is 100% effective.
The vaccines offer less protection against infection from the Omicron variant, which is now the dominant variant globally, but remain highly effective in preventing hospitalization, severe disease, and death. In addition to vaccination, it remains important to continue practicing safety precautions to protect yourself and others. These precautions include avoiding crowded spaces, physical distancing, hand washing, and wearing a mask (as per local policies).
Can I still get COVID-19 after I have been vaccinated? What are ‘breakthrough cases’?
A number of vaccinated people may get infected with COVID-19, which is called a breakthrough infection. In such cases, people are much more likely to only have milder symptoms. Vaccine protection against serious illness and death remains strong.
With more infectious virus variants such as Omicron, there have been more breakthrough infections. That’s why it's recommended to continue taking precautions such as avoiding crowded spaces, wearing a mask and washing your hands regularly, even if you are vaccinated.
And remember, it’s important to receive all of the recommended doses of vaccines to have the maximum protection.
How long does protection from COVID-19 vaccines last?
According to WHO, the effectiveness of COVID-19 vaccines wanes around 4-6 months after the primary series of vaccination has been completed. Taking a booster to strengthen your protection against serious disease is recommended if it is available to you.
Do the COVID-19 vaccines protect against variants?
The WHO-approved COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
However, the vaccines offer less protection against infection from Omicron, which is the dominant variant globally. That's why it's important to get vaccinated and continue measures to reduce the spread of the virus – which helps to reduce the chances for the virus to mutate – including physical distancing, mask wearing, good ventilation, regular handwashing and seeking care early if you have symptoms.
Do I need to get a booster shot?
Booster doses play an important role in protecting against severe disease, hospitalization and death.
WHO recommends that you take all COVID-19 vaccine doses recommended to you by your health authority as soon as it is your turn, including a booster dose if recommended.
Booster shots should be given first to high priority groups. Data shows that a booster shot plays a significant role in boosting waning immunity and protecting against severe disease from highly transmissible variants like Omicron.
If available, an additional second booster shot is also recommended for some groups of people, 4-6 months after the first booster. That includes older people, those who have weakened immune systems, pregnant women and healthcare workers.
Check with your local health authorities for guidance and the availability of booster shots where you live.
What do we know about the bivalent COVID-19 booster doses that have been developed to target Omicron?
Bivalent COVID-19 booster shots have now been developed with both the original strain of the coronavirus and a strain of Omicron. These have been designed to better match the Omicron subvariants that have proven to be particularly transmissible. Lab studies have shown that these doses help you to mount a higher antibody response against Omicron. Both Moderna and Pfizer have developed these bivalent vaccines, and some countries have now approved their use.
Check with your local health authorities for information about the availability of these doses and who can get them where you live. And it’s important to note that the original COVID-19 vaccines continue to work very well and provide strong protection against severe illness from Omicron.
Can I receive different types of COVID-19 vaccines?
Yes, however, policies on mixing vaccines vary by country. Some countries have used different vaccines for the primary vaccine series and the booster. Check with your local health authorities for guidance where you live and speak with your healthcare provider if you have any questions on what is best for you.
I’m pregnant. Can I get vaccinated against COVID-19?
Yes, you can get vaccinated if you are pregnant. COVID-19 during pregnancy puts you at higher risk of becoming severely ill and of giving birth prematurely.
Many people around the world have been vaccinated against COVID-19 while pregnant or breastfeeding. No safety concerns have been identified for them or their babies. Getting vaccinated while pregnant helps to protect your baby. For more information about receiving a COVID-19 vaccination while pregnant, speak to your healthcare provider.
>> Read: Navigating pregnancy during the COVID-19 pandemic
I’m breastfeeding. Should I get vaccinated against COVID-19?
Yes, if you are breastfeeding you should take the vaccine as soon as it is available to you. It is very safe and there is no risk to the mother or baby. None of the current COVID-19 vaccines have live virus in them, so there is no risk of you transmitting COVID-19 to your baby through your breastmilk from the vaccine. In fact, the antibodies that you have after vaccination may go through the breast milk and help protect your baby. >> Read: Breastfeeding safely during the COVID-19 pandemic
Can COVID-19 vaccines affect fertility?
No, you may have seen false claims on social media, but there is no evidence that any vaccine, including COVID-19 vaccines, can affect fertility in women or men. You should get vaccinated if you are currently trying to become pregnant.

Could a COVID-19 vaccine disrupt my menstrual cycle?
Some people have reported experiencing a disruption to their menstrual cycle after getting vaccinated against COVID-19. Although data is still limited, research is ongoing into the impact of vaccines on menstrual cycles.
Speak to your healthcare provider if you have concerns or questions about your periods.
Should my child or teen get a COVID-19 vaccine?
An increasing number of vaccines have been approved for use in children. They’ve been made available after examining the data on the safety and efficacy of these vaccines, and millions of children have been safely vaccinated around the world. Some COVID-19 vaccines have been approved for children from the age of 6 months old. Check with your local health authorities on what vaccines are authorized and available for children and adolescents where you live.
Children and adolescents tend to have milder disease compared to adults, so unless they are part of a group at higher risk of severe COVID-19, it is less urgent to vaccinate them than older people, those with chronic health conditions and health workers.
Remind your children of the importance of us all taking precautions to protect each other, such as avoiding crowded spaces, physical distancing, hand washing and wearing a mask.
It is critical that children continue to receive the recommended childhood vaccines.
How do I talk to my kids about COVID-19 vaccines?
News about COVID-19 vaccines is flooding our daily lives and it is only natural that curious young minds will have questions – lots of them. Read our explainer article for help explaining what can be a complicated topic in simple and reassuring terms.
It’s important to note that from the millions of children that have so far been vaccinated against COVID-19 globally, we know that side effects are very rare. Just like adults, children and adolescents might experience mild symptoms after receiving a dose, such as a slight fever and body aches. But these symptoms typically last for just a day or two. The authorized vaccines for adolescents and children are very safe.
My friend or family member is against COVID-19 vaccines. How do I talk to them?
The development of safe and effective COVID-19 vaccines is a huge step forward in our global effort to end the pandemic. This is exciting news, but there are still some people who are skeptical or hesitant about COVID-19 vaccines. Chances are you know a person who falls into this category.
We spoke to Dr. Saad Omer, Director at the Yale Institute for Global Health, to get his tips on how to navigate these challenging conversations. >> Read the interview
How can I protect my family until we are all vaccinated?
Safe and effective vaccines are a game changer, but even once vaccinated we need to continue taking precautions for the time being to protect ourselves and others. Variants like Omicron have proven that although COVID-19 vaccines are very effective at preventing severe disease, they’re not enough to stop the spread of the virus alone. The most important thing you can do is reduce your risk of exposure to the virus. To protect yourself and your loved ones, make sure to:
- Wear a mask where physical distancing from others is not possible.
- Keep a physical distance from others in public places.
- Avoid poorly ventilated or crowded spaces.
- Open windows to improve ventilation indoors.
- Try and focus on outdoor activities if possible.
- Wash your hands regularly with soap and water or an alcohol-based hand rub.
If you or a family member has a fever, cough or difficulty breathing, seek medical care early and avoid mixing with other children and adults.
Can COVID-19 vaccines affect your DNA?
No, none of the COVID-19 vaccines affect or interact with your DNA in any way. Messenger RNA, or mRNA, vaccines teach the cells how to make a protein that triggers an immune response inside the body. This response produces antibodies which keep you protected against the virus. mRNA is different from DNA and only stays inside the cell for about 72 hours before degrading. However, it never enters the nucleus of the cell, where DNA is kept.
Do the COVID-19 vaccines contain any animal products in them?
No, none of the WHO-approved COVID-19 vaccines contain animal products.
I’ve seen inaccurate information online about COVID-19 vaccines. What should I do?
Sadly, there is a lot of inaccurate information online about the COVID-19 virus and vaccines. A lot of what we’re experiencing is new to all of us, so there may be some occasions where information is shared, in a non-malicious way, that turns out to be inaccurate.
Misinformation in a health crisis can spread paranoia, fear and stigmatization. It can also result in people being left unprotected or more vulnerable to the virus. Get verified facts and advice from trusted sources like your local health authority, the UN, UNICEF, WHO.
If you see content online that you believe to be false or misleading, you can help stop it spreading by reporting it to the social media platform.
What is COVAX?
COVAX is a global effort committed to the development, production and equitable distribution of vaccines around the world. No country will be safe from COVID-19 until all countries are protected.
There are 190 countries and territories engaged in the COVAX Facility, which account for over 90 per cent of the world’s population. Working with CEPI, GAVI, WHO and other partners, UNICEF is leading efforts to procure and supply COVID-19 vaccines on behalf of COVAX.
Learn more about COVAX .
This article was last updated on 25 October 2022. It will continue to be updated to reflect the latest information.
Related topics
More to explore, covid-19 response.
Resources and information about UNICEF’s response to the COVID-19 pandemic
How to talk to your children about COVID-19 vaccines
Tips for navigating the conversation
How to talk to friends and family about vaccines
Tips for handling tough conversations with your loved ones
COVAX information centre
UNICEF and partners led the largest vaccine procurement and supply operation in history
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 May 2021
Public attitudes toward COVID-19 vaccination: The role of vaccine attributes, incentives, and misinformation
- Sarah Kreps 1 ,
- Nabarun Dasgupta 2 ,
- John S. Brownstein 3 , 4 ,
- Yulin Hswen 5 &
- Douglas L. Kriner ORCID: orcid.org/0000-0002-9353-2334 1
npj Vaccines volume 6 , Article number: 73 ( 2021 ) Cite this article
19k Accesses
72 Citations
43 Altmetric
Metrics details
- Health care
- Translational research
While efficacious vaccines have been developed to inoculate against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; also known as COVID-19), public vaccine hesitancy could still undermine efforts to combat the pandemic. Employing a survey of 1096 adult Americans recruited via the Lucid platform, we examined the relationships between vaccine attributes, proposed policy interventions such as financial incentives, and misinformation on public vaccination preferences. Higher degrees of vaccine efficacy significantly increased individuals’ willingness to receive a COVID-19 vaccine, while a high incidence of minor side effects, a co-pay, and Emergency Use Authorization to fast-track the vaccine decreased willingness. The vaccine manufacturer had no influence on public willingness to vaccinate. We also found no evidence that belief in misinformation about COVID-19 treatments was positively associated with vaccine hesitancy. The findings have implications for public health strategies intending to increase levels of community vaccination.
Similar content being viewed by others

Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA
Sahil Loomba, Alexandre de Figueiredo, … Heidi J. Larson
Vaccine hesitancy and monetary incentives
Ganesh Iyer, Vivek Nandur & David Soberman
Providing normative information increases intentions to accept a COVID-19 vaccine
Alex Moehring, Avinash Collis, … Dean Eckles
Introduction
In less than a year, an array of vaccines was developed to bring an end to the SARS-CoV-2 pandemic. As impressive as the speed of development was the efficacy of vaccines such as Moderna and Pfizer, which are over 90%. Despite the growing availability and efficacy, however, vaccine hesitancy remains a potential impediment to widespread community uptake. While previous surveys indicate that overall levels of vaccine acceptance may be around 70% in the United States 1 , the case of Israel may offer a cautionary tale about self-reported preferences and vaccination in practice. Prospective studies 2 of vaccine acceptance in Israel showed that about 75% of the Israeli population would vaccinate, but Israel’s initial vaccination surge stalled around 42%. The government, which then augmented its vaccination efforts with incentive programs, attributed unexpected resistance to online misinformation 3 .
Research on vaccine hesitancy in the context of viruses such as influenza and measles, mumps, and rubella, suggests that misinformation surrounding vaccines is prevalent 4 , 5 . Emerging research on COVID-19 vaccine preferences, however, points to vaccine attributes as dominant determinants of attitudes toward vaccination. Higher efficacy is associated with greater likelihood of vaccinating 6 , 7 , whereas an FDA Emergency Use Authorization 6 or politicized approval timing 8 is associated with more hesitancy. Whether COVID-19 misinformation contributes to vaccine preferences or whether these attributes or policy interventions such as incentives play a larger role has not been studied. Further, while previous research has focused on a set of attributes that was relevant at one particular point in time, the evidence and context about the available vaccines has continued to shift in ways that could shape public willingness to accept the vaccine. For example, governments, employers, and economists have begun to think about or even devise ways to incentivize monetarily COVID-19 vaccine uptake, but researchers have not yet studied whether paying people to receive the COVID-19 vaccine would actually affect likely behavior. As supply problems wane and hesitancy becomes a limiting factor, understanding whether financial incentives can overcome hesitancy becomes a crucial question for public health. Further, as new vaccines such as Johnson and Johnson are authorized, knowing whether the vaccine manufacturer name elicits or deters interest in individuals is also important, as are the corresponding efficacy rates of different vaccines and the extent to which those affect vaccine preferences. The purpose of this study is to examine how information about vaccine attributes such as efficacy rates, the incidence of side effects, the nature of the governmental approval process, identity of the manufacturers, and policy interventions, including economic incentives, affect intention to vaccinate, and to examine the association between belief in an important category of misinformation—false claims concerning COVID-19 treatments—and willingness to vaccinate.
General characteristics of study population
Table 1 presents sample demographics, which largely reflect those of the US population as a whole. Of the 1335 US adults recruited for the study, a convenience sample of 1100 participants consented to begin the survey, and 1096 completed the full questionnaire. The sample was 51% female; 75% white; and had a median age of 43 with an interquartile range of 31–58. Comparisons of the sample demographics to those of other prominent social science surveys and U.S. Census figures are shown in Supplementary Table 1 .
Vaccination preferences
Each subject was asked to evaluate a series of seven hypothetical vaccines. For each hypothetical vaccine, our conjoint experiment randomly assigned values of five different vaccine attributes—efficacy, the incidence of minor side effects, government approval process, manufacturer, and cost/financial inducement. Descriptions of each attribute and the specific levels used in the experiment are summarized in Table 2 . After seeing the profile of each vaccine, the subject was asked whether she would choose to receive the vaccine described, or whether she would choose not to be vaccinated. Finally, subjects were asked to indicate how likely they would be to take the vaccine on a seven-point likert scale.
Across all choice sets, in 4419 cases (58%) subjects said they would choose the vaccine described in the profile rather than not being vaccinated. As shown in Fig. 1 , several characteristics of the vaccine significantly influenced willingness to vaccinate.
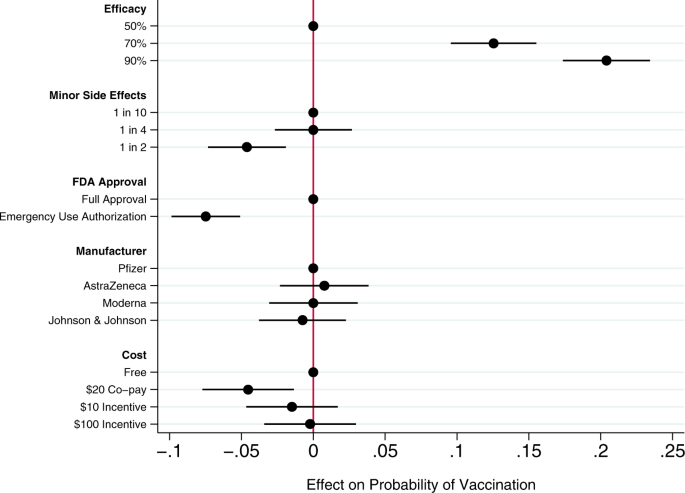
Circles present the estimated effect of each attribute level on the probability of a subject accepting vaccination from the attribute’s baseline level. Horizontal lines through points indicate 95% confidence intervals. Points without error bars denote the baseline value for each attribute. The average marginal component effects (AMCEs) are the regression coefficients reported in model 1 of Table 3 .
Efficacy had the largest effect on individual vaccine preferences. An efficacy rate of 90% increased uptake by about 20% relative to the baseline at 50% efficacy. Even a high incidence of minor side effects (1 in 2) had only a modest negative effect (about 5%) on willingness to vaccinate. Whether the vaccine went through full FDA approval or received an Emergency Use Authorization (EUA), an authority that allows the Food and Drug Administration mechanisms to accelerate the availability and use of treatments or medicines during medical emergencies 9 , significantly influenced willingness to vaccinate. An EUA decreased the likelihood of vaccination by 7% compared to a full FDA authorization; such a decline would translate into about 23 million Americans. While a $20 co-pay reduced the likelihood of vaccination relative to a no-cost baseline, financial incentives did not increase willingness to vaccinate. Lastly, the manufacturer had no effect on vaccination attitudes, despite the public pause of the AstraZeneca trial and prominence of Johnson & Johnson as a household name (our experiment was fielded before the pause in the administration of the Johnson & Johnson shot in the United States).
Model 2 of Table 3 presents an expanded model specification to investigate the association between misinformation and willingness to vaccinate. The primary additional independent variable of interest is a misinformation index that captures the extent to which each subject believes or rejects eight claims (five false; three true) about COVID-19 treatments. Additional analyses using alternate operationalizations of the misinformation index yield substantively similar results (Supplementary Table 4 ). This model also includes a number of demographic control variables, including indicators for political partisanship, gender, educational attainment, age, and race/ethnicity, all of which are also associated with belief in misinformation about the vaccine (Supplementary Table 2 ). Finally, the model also controls for subjects’ health insurance status, past experience vaccinating against seasonal influenza, attitudes toward the pharmaceutical industry, and beliefs about vaccine safety generally.
Greater levels of belief in misinformation about COVID-19 treatments were not associated with greater vaccine hesitancy. Instead, the relevant coefficient is positive and statistically significant, indicating that, all else being equal, individuals who scored higher on our index of misinformation about COVID-19 treatments were more willing to vaccinate than those who were less susceptible to believing false claims.
Strong beliefs that vaccines are safe generally was positively associated with willingness to accept a COVID-19 vaccine, as were past histories of frequent influenza vaccination and favorable attitudes toward the pharmaceutical industry. Women and older subjects were significantly less likely to report willingness to vaccinate than men and younger subjects, all else equal. Education was positively associated with willingness to vaccinate.
This research offers a comprehensive examination of attitudes toward COVID-19 vaccination, particularly the role of vaccine attributes, potential policy interventions, and misinformation. Several previous studies have analyzed the effects of vaccine characteristics on willingness to vaccinate, but the modal approach is to gauge willingness to accept a generic COVID-19 vaccine 10 , 11 . Large volumes of research show, however, that vaccine preferences hinge on specific vaccine attributes. Recent research considering the influence of attributes such as efficacy, side effects, and country of origin take a step toward understanding how properties affect individuals’ intentions to vaccinate 6 , 7 , 8 , 12 , 13 , but evidence about the attributes of actual vaccines, debates about how to promote vaccination within the population, and questions about the influence of misinformation have moved quickly 14 .
Our conjoint experiment therefore examined the influence of five vaccine attributes on vaccination willingness. The first category of attributes involved aspects of the vaccine itself. Since efficacy is one of the most common determinants of vaccine acceptance, we considered different levels of efficacy, 50%, 70%, and 90%, levels that are common in the literature 7 , 15 . Evidence from Phase III trials suggests that even the 90% efficacy level in our design, which is well above the 50% threshold from the FDA Guidance for minimal effectiveness for Emergency Use Authorization 16 , has been exceeded by both Pfizer’s and Moderna’s vaccines 17 , 18 . The 70% efficacy threshold is closer to the initial reports of the efficacy of the Johnson & Johnson vaccine, whose efficacy varied across regions 19 . Our analysis suggests that efficacy levels associated with recent mRNA vaccine trials increases public vaccine uptake by 20% over a baseline of a vaccine with 50% efficacy. A 70% efficacy rate increases public willingness to vaccinate by 13% over a baseline vaccine with 50% efficacy.
An additional set of epidemiological attributes consisted of the frequency of minor side effects. While severe side effects were plausible going into early clinical trials, evidence clearly suggests that minor side effects are more common, ranging from 10% to 100% of people vaccinated depending on the number of doses and the dose group (25–250 mcg) 20 . Since the 100 mcg dose was supported in Phase III trials 21 , we include the highest adverse event probability—approximating 60% as 1 in 2—and 1 in 10 as the lowest likelihood, approximating the number of people who experienced mild arthralgia 20 . Our findings suggest that a the prevalence of minor side effects associated with recent trials (i.e. a 1 in 2 chance), intention to vaccinate decreased by about 5% versus a 1 in 10 chance of minor side effects baseline. However, at a 25% rate of minor side effects, respondents did not indicate any lower likelihood of vaccination compared to the 10% baseline. Public communications about how to reduce well-known side effects, such as pain at the injection site, could contribute to improved acceptance of the vaccine, as it is unlikely that development of vaccine-related minor side effects will change.
We then considered the effect of EUA versus full FDA approval. The influenza H1N1 virus brought the process of EUA into public discourse 22 , and the COVID-19 virus has again raised the debate about whether and how to use EUA. Compared to recent studies also employing conjoint experimental designs that showed just a 3% decline in support conditional on EUA 6 , we found decreases in support of more than twice that with an EUA compared to full FDA approval. Statements made by the Trump administration promising an intensely rapid roll-out or isolated adverse events from vaccination in the UK may have exacerbated concerns about EUA versus full approval 8 , 23 , 24 , 25 . This negative effect is even greater among some subsets of the population. As shown in additional analyses reported in the Supplementary Information (Supplementary Fig. 5 ), the negative effects are greatest among those who believe vaccines are generally safe. Among those who believe vaccines generally are extremely safe, the EUA decreased willingness to vaccinate by 11%, all else equal. This suggests that outreach campaigns seeking to assure those troubled by the authorization process used for currently available vaccines should target their efforts on those who are generally predisposed to believe vaccines are safe.
Next, we compared receptiveness as a function of the manufacturer: Moderna, Pfizer, Johnson and Johnson, and AstraZeneca, all firms at advanced stages of vaccine development. Vaccine manufacturers in the US have not yet attempted to use trade names to differentiate their vaccines, instead relying on the association with manufacturer reputation. In other countries, vaccine brand names have been more intentionally publicized, such as Bharat Biotech’s Covaxin in India and Gamaleya Research Institute of Epidemiology and Microbiology Sputnik V in Russia. We found that manufacturer names had no impact on willingness to vaccinate. As with hepatitis and H. influenzae vaccines 26 , 27 , interchangeability has been an active topic of debate with coronavirus mRNA vaccines which require a second shot for full immunity. Our research suggests that at least as far as public receptiveness goes, interchangeability would not introduce concerns. We found no significant differences in vaccination uptake across any of the manufacturer treatments. Future research should investigate if a manufacturer preference develops as new evidence about efficacy and side effects becomes available, particularly depending on whether future booster shots, if needed, are deemed interchangeable with the initial vaccination.
Taking up the question of how cost and financial incentives shape behavior, we looked at paying and being paid to vaccinate. While existing research suggests that individuals are often willing to pay for vaccines 28 , 29 , some economists have proposed that the government pay individuals up to $1,000 to take the COVID-19 vaccine 30 . However, because a cost of $300 billion to vaccinate the population may be prohibitive, we posed a more modest $100 incentive. We also compared this with a $10 incentive, which previous studies suggest is sufficient for actions that do not require individuals to change behavior on a sustained basis 31 . While having to pay a $20 co-pay for the vaccine did deter individuals, the additional economic incentives had no positive effect although they did not discourage vaccination 32 . Consistent with past research 31 , 33 , further analysis shows that the negative effect of the $20 co-pay was concentrated among low-income earners (Supplementary Fig. 7 ). Financial incentives failed to increase vaccination willingness across income levels.
Our study also yields important insights into the relationship between one prominent category of COVID-19 misinformation and vaccination preferences. We find that susceptibility to misinformation about COVID-19 treatments—based on whether individuals can distinguish between factual and false information about efforts to combat COVID-19—is considerable. A quarter of subjects scored no higher on our misinformation index than random guessing or uniform abstention/unsure responses (for the full distribution, see Supplementary Fig. 2 ). However, subjects who scored higher on our misinformation index did not exhibit greater vaccination hesitancy. These subjects actually were more likely to believe in vaccine safety more generally and to accept a COVID-19 vaccine, all else being equal. These results run counter to recent findings of public opinion in France where greater conspiracy beliefs were negatively correlated with willingness to vaccinate against COVID-19 34 and in Korea where greater misinformation exposure and belief were negatively correlated with taking preventative actions 35 . Nevertheless, the results are robust to alternate operationalizations of belief in misinformation (i.e., constructing the index only using false claims, or measuring misinformation beliefs as the number of false claims believed: see Supplementary Table 4 ).
We recommend further study to understand the observed positive relationship between beliefs in COVID-19 misinformation about fake treatments and willingness to receive the COVID-19 vaccine. To be clear, we do not posit a causal relationship between the two. Rather, we suspect that belief in misinformation may be correlated with an omitted factor related to concerns about contracting COVID-19. For example, those who believe COVID-19 misinformation may have a higher perception of risk of COVID-19, and therefore be more willing to take a vaccine, all else equal 36 . Additional analyses reported in the Supplementary Information (Supplementary Fig. 6 ) show that the negative effect of an EUA on willingness to vaccinate was concentrated among those who scored low on the misinformation index. An EUA had little effect on the vaccination preferences of subjects most susceptible to misinformation. This pattern is consistent with the possibility that these subjects were more concerned with the disease and therefore more likely to vaccinate, regardless of the process through which the vaccine was brought to market.
We also observe that skepticism toward vaccines in general does not correlate perfectly with skepticism toward the COVID-19 vaccine. Therefore, it is important not to conflate people who are wary of the COVID-19 vaccine and those who are anti-vaccination, as even medically informed individuals may be hesitant because of the speed at which the COVID-19 vaccine was developed. For example, older people are more likely to believe vaccines are safe but less willing to receive the COVID-19 vaccine in our survey, perhaps following the high rates of vaccine skepticism among medical staff expressing concerns regarding the safety of a rapidly-developed vaccine 2 . This inverse relationship between age and willingness to vaccinate is also surprising. Most opinion surveys find older adults are more likely to vaccinate than younger adults 37 . However, most of these survey questions ask about willingness to take a generic vaccine. Two prior studies, both recruiting subjects from the Lucid platform and employing conjoint experiments to examine the effects of vaccine attributes on public willingness to vaccinate, also find greater vaccine hesitancy among older Americans 6 , 7 . Future research could explore whether these divergent results are a product of the characteristics of the sample or of the methodological design in which subjects have much more information about the vaccines when indicating their vaccination preferences.
An important limitation of our study is that it necessarily offers a snapshot in time, specifically prior to both the election and vaccine roll-out. We recommend further study to understand more how vaccine perceptions evolve both in terms of the perceived political ownership of the vaccine—now that President Biden is in office—and as evidence has emerged from the millions of people who have been vaccinated. Similarly, researchers should consider analyzing vaccine preferences in the context of online vaccine controversies that have been framed in terms of patient autonomy and right to refuse 38 , 39 . Vaccination mandates may evoke feelings of powerlessness, which may be exacerbated by misinformation about the vaccines themselves. Further, researchers should more fully consider how individual attributes such as political ideology and race intersect with vaccine preferences. Our study registered increased vaccine hesitancy among Blacks, but did not find that skepticism was directly related to misinformation. Perceptions and realities of race-based maltreatment could also be moderating factors worth exploring in future analyses 40 , 41 .
Overall, we found that the most important factor influencing vaccine preferences is vaccine efficacy, consistent with a number of previous studies about attitudes toward a range of vaccines 6 , 42 , 43 . Other attributes offer potential cautionary flags and opportunities for public outreach. The prospect of a 50% likelihood of mild side effects, consistent with the evidence about current COVID-19 vaccines being employed, dampens likelihood of uptake. Public health officials should reinforce the relatively mild nature of the side effects—pain at the injection site and fatigue being the most common 44 —and especially the temporary nature of these effects to assuage public concerns. Additionally, in considering policy interventions, public health authorities should recognize that a $20 co-pay will likely discourage uptake while financial incentives are unlikely to have a significant positive effect. Lastly, belief in misinformation about COVID-19 does not appear to be a strong predictor of vaccine hesitancy; belief in misinformation and willingness to vaccinate were positively correlated in our data. Future research should explore the possibility that exposure to and belief in misinformation is correlated with other factors associated with vaccine preferences.
Survey sample and procedures
This study was approved by the Cornell Institutional Review Board for Human Participant Research (protocol ID 2004009569). We conducted the study on October 29–30, 2020, prior to vaccine approval, which means we captured sentiments prospectively rather than based on information emerging from an ongoing vaccination campaign. We recruited a sample of 1096 adult Americans via the Lucid platform, which uses quota sampling to produce samples matched to the demographics of the U.S. population on age, gender, ethnicity, and geographic region. Research has shown that experimental effects observed in Lucid samples largely mirror those found using probability-based samples 45 . Supplementary Table 1 presents the demographics of our sample and comparisons to both the U.S. Census American Community Survey and the demographics of prominent social science surveys.
After providing informed consent on the first screen of the online survey, participants turned to a choice-based conjoint experiment that varied five attributes of the COVID-19 vaccine. Conjoint analyses are often used in marketing to research how different aspects of a product or service affect consumer choice. We build on public health studies that have analyzed the influence of vaccine characteristics on uptake within the population 42 , 46 .
Conjoint experiment
We first designed a choice-based conjoint experiment that allowed us to evaluate the relative influence of a range of vaccine attributes on respondents’ vaccine preferences. We examined five attributes summarized in Table 2 . Past research has shown that the first two attributes, efficacy and the incidence of side effects, are significant drivers of public preferences on a range of vaccines 47 , 48 , 49 , including COVID-19 6 , 7 , 13 , 50 . In this study, we increased the expected incidence of minor side effects from previous research 6 to reflect emerging evidence from Phase III trials. The third attribute, whether the vaccine received full FDA approval or an EUA, examines whether the speed of the approval process affects public vaccination preferences 6 . The fourth attribute, the manufacturer of the vaccine, allows us to examine whether the highly public pause in the AstraZeneca trial following an adverse event, and the significant differences in brand familiarity between smaller and less broadly known companies like Moderna and household name Johnson & Johnson affects public willingness to vaccinate. The fifth attribute examines the influence of a policy tool—offsetting the costs of vaccination or even incentivizing it financially—on public willingness to vaccinate.
Attribute levels and attribute order were randomly assigned across participants. A sample choice set is presented in Supplementary Fig. 1 . After viewing each profile individually, subjects were asked: “If you had to choose, would you choose to get this vaccine, or would you choose not to be vaccinated?” Subjects then made a binary choice, responding either that they “would choose to get this vaccine” or that they “would choose not to be vaccinated.” This is the dependent variable for the regression analyses in Table 3 . After making a binary choice to take the vaccine or not be vaccinated, we also asked subjects “how likely or unlikely would you be to get the vaccine described above?” Subjects indicated their vaccination preference on a seven-point scale ranging from “extremely likely” to “extremely unlikely.” Additional analyses using this ordinal dependent variable reported in Supplementary Table 3 yield substantively similar results to those presented in Table 3 .
To determine the effect of each attribute-level on willingness to vaccinate, we followed Hainmueller, Hopkins, and Yamamoto and employed an ordinary least squares (OLS) regression with standard errors clustered on respondent to estimate the average marginal component effects (AMCEs) for each attribute 51 . The AMCE represents the average difference in a subject choosing a vaccine when comparing two different attribute values—for example, 50% efficacy vs. 90% efficacy—averaged across all possible combinations of the other vaccine attribute values. The AMCEs are nonparametrically identified under a modest set of assumptions, many of which (such as randomization of attribute levels) are guaranteed by design. Model 1 in Table 3 estimates the AMCEs for each attribute. These AMCEs are illustrated in Fig. 1 .
Analyzing additional correlates of vaccine acceptance
To explore the association between respondents’ embrace of misinformation about COVID-19 treatments and vaccination willingness, the survey included an additional question battery. To measure the extent of belief in COVID-19 misinformation, we constructed a list of both accurate and inaccurate headlines about the coronavirus. We focused on treatments, relying on the World Health Organization’s list of myths, such as “Hand dryers are effective in killing the new coronavirus” and true headlines such as “Avoiding shaking hands can help limit the spread of the new coronavirus 52 .” Complete wording for each claim is provided in Supplementary Appendix 1 . Individuals read three true headlines and five myths, and then responded whether they believed each headline was true or false, or whether they were unsure. We coded responses to each headline so that an incorrect accuracy assessment yielded a 1; a correct accuracy assessment a -1; and a response of unsure was coded as 0. From this, we created an additive index of belief in misinformation that ranged from -8 to 8. The distribution of the misinformation index is presented in Supplementary Fig. 2 . A possible limitation of this measure is that because the survey was conducted online, some individuals could have searched for the answers to the questions before responding. However, the median misinformation index score for subjects in the top quartile in terms of time spent taking the survey was identical to the median for all other respondents. This may suggest that systematic searching for correct answers is unlikely.
To ensure that any association observed between belief in misinformation and willingness to vaccinate is not an artifact of how we operationalized susceptibility to misinformation, we also constructed two alternate measures of belief in misinformation. These measures are described in detail in the Supplementary Information (see Supplementary Figs. 3 and 4 ). Additional regression analyses using these alternate measures of misinformation beliefs yield substantively similar results (see Supplementary Table 4 ). Additional analyses examining whether belief in misinformation moderates the effect of efficacy and an FDA EUA on vaccine acceptance are presented in Supplementary Fig. 6 .
Finally, model 2 of Table 3 includes a range of additional control variables. Following past research, it includes a number of demographic variables, including indicator variables identifying subjects who identify as Democrats or Republicans; an indicator variable identifying females; a continuous variable measuring age (alternate analyses employing a categorical variable yield substantively similar results); an eight-point measure of educational attainment; and indicator variables identifying subjects who self-identify as Black or Latinx. Following previous research 6 , the model also controlled for three additional factors often associated with willingness to vaccinate: an indicator variable identifying whether each subject had health insurance; a variable measuring past frequency of influenza vaccination on a four-point scale ranging from “never” to “every year”; beliefs about the general safety of vaccines measured on a four-point scale ranging from “not at all safe” to “extremely safe”; and a measure of attitudes toward the pharmaceutical industry ranging from “very positive” to “very negative.”
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data and statistical code to reproduce the tables and figures in the manuscript and Supplementary Information are published at the Harvard Dataverse via this link: 10.7910/DVN/ZYU6CO.
Hamel, L., Kirzinger, A., Munana, C. & Brodie, M. KFF COVID-19 Vaccine Monitor: December 2020 | KFF (2020).
Dror, A. A. et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 35 , 775–779 (2020).
Article CAS PubMed Google Scholar
Scharf, I. & Ben Zion, I. As Vaccinations Lag, Israel Combats Online Misinformation. AP2 (21AD).
Nyhan, B. & Reifler, J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 33 , 459–464 (2015).
Article PubMed Google Scholar
Hussain, A., Ali, S., Ahmed, M. & Hussain, S. The anti-vaccination movement: a regression in modern medicine. Cureus 10 , e2919 (2018).
PubMed PubMed Central Google Scholar
Kreps, S. et al. Factors Associated With US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw. open 3 , (2020).
Motta, M. Can a COVID-19 vaccine live up to Americans’ expectations? A conjoint analysis of how vaccine characteristics influence vaccination intentions. Soc. Sci. Med . 113642, https://doi.org/10.1016/j.socscimed.2020.113642 (2021).
Bokemper, S. E., Huber, G. A., Gerber, A. S., James, E. K. & Omer, S. B. Timing of COVID-19 vaccine approval and endorsement by public figures. Vaccine , https://doi.org/10.1016/j.vaccine.2020.12.048 (2021).
Quinn, S. C., Jamison, A. M. & Freimuth, V. Communicating effectively about emergency use authorization and vaccines in the COVID-19 pandemic. Am. J. Public Health e1–e4 (2020).
Malik, A. A., McFadden, S. A. M., Elharake, J. & Omer, S. B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine 26 , 100495 (2020).
Article PubMed PubMed Central Google Scholar
Fisher, K. A. et al. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann. Intern. Med. 173 , 964–973 (2020).
Lazarus, J. V. et al . A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med . 1–4 (2020).
Schwarzinger, M., Watson, V., Arwidson, P., Alla, F. & Luchini, S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Heal ., https://doi.org/10.1016/s2468-2667(21)00012-8 (2021).
Wood, S. & Schulman, K. Beyond politics—promoting Covid-19 vaccination in the United States. N. Engl. J. Med . NEJMms2033790, https://doi.org/10.1056/NEJMms2033790 (2021).
Marshall, H. S., Chen, G., Clarke, M. & Ratcliffe, J. Adolescent, parent and societal preferences and willingness to pay for meningococcal B vaccine: a discrete choice experiment. Vaccine 34 , 671–677 (2016).
Administration, F. and D. Development and Licensure of Vaccines to Prevent COVID-19: Guidance for Industry . (2020).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383 , 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med ., https://doi.org/10.1056/nejmoa2035389 (2020).
Johnson & Johnson. Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial. (2020). Available at: https://www.jnj.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial . (Accessed 28 Feb 2021).
Jackson, L. A. et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 383 , 1920–1931 (2020).
Anderson, E. J. et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 383 , 2427–2438 (2020).
Quinn, S. C., Kumar, S., Freimuth, V. S., Kidwell, K. & Musa, D. Public willingness to take a vaccine or drug under emergency use authorization during the 2009 H1N1 pandemic. Biosecurity Bioterrorism 7 , 275–290 (2009).
Holden Thorp, H. A dangerous rush for vaccines. Science 369 , 885 (2020).
Limaye, R. J., Sauer, M. & Truelove, S. A. Politicizing public health: the powder keg of rushing COVID-19 vaccines. Hum. Vaccines Immunother ., https://doi.org/10.1080/21645515.2020.1846400 (2020).
Feuer, W. Trump Says ‘No President’s Ever Pushed’ the FDA Like Jim, Vaccine Coming ‘Very Shortly’. CNBC (2020).
Soysal, A., Gokçe, I., Pehlivan, T. & Bakir, M. Interchangeability of a hepatitis A vaccine second dose: Avaxim 80 following a first dose of Vaqta 25 or Havrix 720 in children in Turkey. Eur. J. Pediatr. 166 , 533–539 (2007).
Greenberg, D. P. & Feldman, S. Vaccine interchangeability. Clin. Pediatrics 42 , 93–99 (2003).
Article Google Scholar
Bishai, D., Brice, R., Girod, I., Saleh, A. & Ehreth, J. Conjoint analysis of French and German parents’ willingness to pay for meningococcal vaccine. PharmacoEconomics 25 , 143–154 (2007).
Cameron, M. P., Newman, P. A., Roungprakhon, S. & Scarpa, R. The marginal willingness-to-pay for attributes of a hypothetical HIV vaccine. Vaccine 31 , 3712–3717 (2013).
Litan, R. Want herd immunity? Pay people to take the vaccine. Brookings (2020).
Kane, R. L., Johnson, P. E., Town, R. J. & Butler, M. A structured review of the effect of economic incentives on consumers’ preventive behavior. Am. J. Preventive Med. 27 , 327–352 (2004).
Volpp, K. G., Loewenstein, G. & Buttenheim, A. M. Behaviorally informed strategies for a National COVID-19 vaccine promotion program. JAMA - J. Am. Med. Assoc. 325 , 125–126 (2020).
Google Scholar
Nowalk, M. P. et al. Improving influenza vaccination rates in the workplace. A randomized trial. Am. J. Prev. Med. 38 , 237–246 (2010).
Bertin, P., Nera, K. & Delouvée, S. Conspiracy beliefs, rejection of vaccination, and support for hydroxychloroquine: a conceptual replication-extension in the COVID-19 pandemic context. Front. Psychol. 11 , 2471 (2020).
Lee, J. J. et al. Associations between COVID-19 misinformation exposure and belief with COVID-19 knowledge and preventive behaviors: cross-sectional online study. J. Med. Internet Res. 22 , e22205 (2020).
Reyna, V. F. Risk perception and communication in vaccination decisions: a fuzzy-trace theory approach. Vaccine 30 , 3790–3797 (2012).
Lin, C., Tu, P. & Beitsch, L. M. Confidence and receptivity for covid‐19 vaccines: a rapid systematic review. Vaccines 9 , 1–32 (2021).
Broniatowski, D. A. et al. Facebook pages, the ‘Disneyland’ measles outbreak, and promotion of vaccine refusal as a civil right, 2009–2019. Am. J. Public Health 110 , S312–S318 (2020).
Moon, K., Riege, A., Gourdon-Kanhukamwe, A. & Vallée-Tourangeau, G. The Moderating Effect of Autonomy on Promotional Health Messages Encouraging Flu Vaccination Uptake Among Healthcare Professionals . (PsyArXiv, 2020), https://doi.org/10.31234/OSF.IO/AJV4Q .
Ferdinand, K. C., Nedunchezhian, S. & Reddy, T. K. The COVID-19 and influenza “Twindemic”: barriers to influenza vaccination and potential acceptance of SARS-CoV2 vaccination in African Americans. J. Natl. Med. Assoc. 112 , 681–687 (2020).
PubMed Google Scholar
Jaiswal, J., LoSchiavo, C. & Perlman, D. C. Disinformation, misinformation and inequality-driven mistrust in the time of COVID-19: lessons unlearned from AIDS denialism. AIDS Behav. 24 , 2776–2780 (2020).
Determann, D. et al. Acceptance of vaccinations in pandemic outbreaks: a discrete choice experiment. PLoS ONE 9 , e102505 (2014).
Simpson, C. R., Ritchie, L. D., Robertson, C., Sheikh, A. & McMenamin, J. Effectiveness of H1N1 vaccine for the prevention of pandemic influenza in Scotland, UK: A retrospective observational cohort study. Lancet Infect. Dis. 12 , 696–702 (2012).
Remmel, A. COVID vaccines and safety: what the research says. Nature 590 , 538–540 (2021).
Coppock, A. & McClellan, O. A. Validating the demographic, political, psychological, and experimental results obtained from a new source of online survey respondents. Res. Polit. 6 , 1–14 (2019).
de Bekker-Grob, E. W. et al. The impact of vaccination and patient characteristics on influenza vaccination uptake of elderly people: A discrete choice experiment. Vaccine 36 , 1467–1476 (2018).
Determann, D. et al. Public preferences for vaccination programmes during pandemics caused by pathogens transmitted through respiratory droplets–A discrete choice experiment in four European countries, 2013. Eurosurveillance 21 , 1–13 (2016).
de Bekker-Grob, E. W. et al. Girls’ preferences for HPV vaccination: A discrete choice experiment. Vaccine 28 , 6692–6697 (2010).
Guo, N., Zhang, G., Zhu, D., Wang, J. & Shi, L. The effects of convenience and quality on the demand for vaccination: results from a discrete choice experiment. Vaccine 35 , 2848–2854 (2017).
Kaplan, R. M. & Milstein, A. Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proc. Natl. Acad. Sci. USA 118 , 2021726118 (2021).
Hainmueller, J., Hopkins, D. J. & Yamamoto, T. Causal inference in conjoint analysis: Understanding multidimensional choices via stated preference experiments. Polit. Anal. 22 , 1–30 (2014).
Organization, W. H. Coronavirus disease (COVID-19) advice for the public: Mythbusters. (2020). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters . (Accessed 14 Jan 2021).
Download references
Acknowledgements
S.K. and D.K. would like to thank the Cornell Atkinson Center for Sustainability for financial support.
Author information
Authors and affiliations.
Department of Government, Cornell University, Ithaca, NY, USA
Sarah Kreps & Douglas L. Kriner
Injury Prevention Research Center, University of North Carolina, Chapel Hill, NC, USA
Nabarun Dasgupta
Department of Pediatrics, Harvard Medical School, Boston, MA, USA
John S. Brownstein
Computational Epidemiology Lab, Boston Children’s Hospital, Boston, MA, USA
Epidemiology and Biostatistics, University of California, San Francisco, CA, USA
Yulin Hswen
You can also search for this author in PubMed Google Scholar
Contributions
S.K. and D.K. designed the experiment/survey instrument and conducted the statistical analysis. S.K., N.D., J.B., Y.H., and D.K. all contributed to the conceptual design of the research and to the writing of the paper.
Corresponding author
Correspondence to Douglas L. Kriner .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kreps, S., Dasgupta, N., Brownstein, J.S. et al. Public attitudes toward COVID-19 vaccination: The role of vaccine attributes, incentives, and misinformation. npj Vaccines 6 , 73 (2021). https://doi.org/10.1038/s41541-021-00335-2
Download citation
Received : 25 January 2021
Accepted : 06 April 2021
Published : 14 May 2021
DOI : https://doi.org/10.1038/s41541-021-00335-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Progress with covid vaccine development and implementation.
- Richard W. Titball
- David I. Bernstein
- Alan D. T. Barrett
npj Vaccines (2024)
A synthesis of evidence for policy from behavioural science during COVID-19
- Kai Ruggeri
- Friederike Stock
- Robb Willer
Nature (2024)
The rapid progress in COVID vaccine development and implementation
- Nicolas V. J. Fanget
npj Vaccines (2022)
COVID-19 Vaccine Acceptability and Financial Incentives among Unhoused People in Los Angeles County: a Three-Stage Field Survey
- Allison D. Rosen
- Jacqueline Beltran
- Chelsea L. Shover
Journal of Urban Health (2022)
The shot, the message, and the messenger: COVID-19 vaccine acceptance in Latin America
- Pablo Argote
- Elena Barham
- Oscar Pocasangre
npj Vaccines (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Toward trustworthy COVID-19 interventions: Building vaccine trust through community-university partnerships
Roles Conceptualization, Formal analysis, Investigation, Project administration, Writing – original draft
* E-mail: [email protected]
Affiliation Center for Applied Social Research, University of Oklahoma, Norman, Oklahoma, United States of America
Roles Conceptualization, Investigation, Writing – original draft
Affiliations Center for Applied Social Research, University of Oklahoma, Norman, Oklahoma, United States of America, Department of Anthropology, University of Oklahoma, Norman, Oklahoma, United States of America
Roles Funding acquisition, Investigation, Writing – review & editing
Affiliation Department of Biostatistics and Epidemiology, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, United States of America
Roles Investigation, Writing – review & editing
Affiliation Public Health Institute of Oklahoma, Oklahoma City, Oklahoma, United States of America
Roles Writing – review & editing
Affiliations Department of Pediatrics, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, United States of America, Oklahoma Clinical and Translational Science Institute, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, United States of America
¶ Membership of the CATCH-UP Vaccines Team is provided in the Acknowledgments.
- Laura A. Bray,
- Lori L. Jervis,
- Amanda E. Janitz,
- Laura Ross,
- Gloria Tallbull,
- Timothy M. VanWagoner,
- the CATCH-UP Vaccines Team

- Published: March 27, 2024
- https://doi.org/10.1371/journal.pone.0300872
- Reader Comments
Prior research identifies trust as critical to increase vaccine acceptance and uptake. However, few intervention studies have sought to develop or test strategies for bolstering vaccine-related trust. To address this gap, this exploratory study identifies features of COVID-19 vaccine hesitancy interventions that can promote or undermine trust across three interconnected domains: institutional, interpersonal, and product (the vaccine itself). We draw on focus groups (N = 27 participants) with community and university partners involved with hosting COVID-19 testing and vaccine events in underserved Oklahoma communities. Focus groups explored participants’ experiences serving community health needs and elicited feedback on proposed vaccine hesitancy interventions. Proposed interventions included two technology-based strategies (text message reminders and tablet-based testimonials and education) and one dialogue-based strategy (anti-body test interpretation). We find that community partners perceived local universities as trustworthy institutions because of their association with popular sports programs, academic credentials, and proximity, creating opportunities to address vaccine-related distrust through community-university partnerships. The most promising intervention strategies for building interpersonal trust included engaging in one-on-one dialogue and using autonomy enhancing approaches. Finally, interventions that successfully encouraged vaccine trust did so by incorporating personalized health information about individuals’ potential level of protection and susceptibility to the COVID-19 virus. These findings can inform future public health efforts to create trustworthy vaccine hesitancy interventions.
Citation: Bray LA, Jervis LL, Janitz AE, Ross L, Tallbull G, VanWagoner TM, et al. (2024) Toward trustworthy COVID-19 interventions: Building vaccine trust through community-university partnerships. PLoS ONE 19(3): e0300872. https://doi.org/10.1371/journal.pone.0300872
Editor: Nicola Diviani, Swiss Paraplegic Research, SWITZERLAND
Received: December 28, 2023; Accepted: March 6, 2024; Published: March 27, 2024
Copyright: © 2024 Bray et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Data cannot be shared publicly to protect participant confidentiality. Data are available from the Center for Applied Social Research (contact via [email protected] ) for researchers who meet the criteria for access to confidential data.
Funding: Research reported in this RADx® Underserved Populations publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number U54GM104938. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
In 2019, the World Health Organization recognized vaccine hesitancy as a top ten threat to public health [ 1 ]. The consequences of vaccine resistance and refusal have since been realized on a global scale with the COVID-19 pandemic, as increasing numbers of people question the public health recommendations coming from scientific, medical, and governance institutions [ 2 , 3 ]. In response, a large body of scholarship has emerged focused on understanding vaccine hesitancy and testing interventions to increase vaccine uptake. This research consistently identifies (dis)trust—defined as a person’s willingness to defer to others on matters beyond their knowledge and power and in ways that can potentially harm them [ 4 ]—as a key factor within vaccine hesitancy and refusal, including distrust of the vaccine itself, institutions involved in their development and regulation, and providers responsible for administering the vaccines [ 5 – 14 ]. Yet despite the demonstrated importance of multiple forms of trust for increasing vaccine uptake, few intervention studies focus on developing or testing strategies that can rebuild or repair trust as a means to address vaccine hesitancy [for related work, see 15 – 17 ]. As a result, researchers currently know little about what makes an intervention worthy or unworthy of trust to the vaccine hesitant publics. Developing trustworthy interventions is particularly important for underserved and marginalized communities where ongoing experiences of medical mistreatment and neglect contribute to high levels of distrust [ 16 , 18 ].
This exploratory study examines what makes COVID-19 vaccine hesitancy interventions trustworthy. We report focus group findings from community health organizations and university partners on their experience conducting COVID-19 testing and vaccine events, as well as feedback on several proposed vaccine hesitancy interventions. Through qualitative analysis of these data, we explore features of vaccine hesitancy interventions that can promote institutional, interpersonal, and vaccine trust, as well factors that can undermine trust across these three domains.
(Dis)trust and the roots of vaccine hesitancy
Research both preceding and following the 2020–23 COVID-19 pandemic consistently identifies (dis)trust as a key factor within vaccine hesitancy and refusal [ 5 – 14 ]. Vaccine hesitancy refers to “an attitude of ambivalence regarding vaccines” [ 7 ], particularly during the decision-making process [ 19 ], with vaccine refusal focused on the behavior of declining to vaccinate despite availability. The causes of vaccine hesitancy are multi-faceted and complex, embedded within interpersonal and institutional relationships and shifting over time. Because of the highly specialized and complex nature of modern biomedicine and healthcare, vaccine acceptance requires that the public rely on scientific and medical institutions to act in their best interest, illustrating the critical role of trust for achieving public health goals. As Adhikari et al. [ 6 ] explain, “Default asymmetry in information, comprehensibility, and power between the vaccine providers and the vaccine recipients makes the… [recipient] vulnerable as they have to invest some degree of faith in the trusted party.” Survey research consistently finds a strong relationship between trust and COVID-19 vaccine attitudes and behavior, with respondents indicating low levels of trust in traditional media, public health institutions, government, and science less likely to accept or receive vaccines [ 5 , 20 – 22 ].
As a sociological concept, trust refers to the willingness to defer “to others about something beyond our knowledge and power [and] in ways that can potentially hurt us” [ 4 ]. This definition places power differentials and vulnerability at the core of trust relationships. Trust is required because of differential levels of knowledge and power and thus involves a “leap of faith” to overcome knowledge gaps [ 23 ]. As a future oriented concept, trust also invokes risk and uncertainty [ 24 ]. Placing trust in a person or institution means risking negative consequences if that trust is betrayed, making it “rational” to withhold or withdraw trust in some instances [ 25 ]. Finally, as a relational concept, trust is made and remade through lived experience and interactions over time [ 23 ]. This means that events, reasons, or information that allow people to suspend or “bracket off” uncertainties to make a leap of faith may change over time [ 23 ].
Vaccine hesitancy research examines trust across three primary domains: institutional, interpersonal, and in the vaccine itself [ 6 , 26 ]. Although conceptually distinct, these three domains intersect in important ways, with institutional aspects often underlying other forms of distrust. Institutional trust focuses on the parties responsible for developing, testing, distributing, and regulating vaccines including governments, public health agencies, the healthcare system, pharmaceutical industry, and science itself. In the US, trust in institutions declined during the COVID-19 pandemic [ 27 – 29 ] because of conflicting and confusing official communication [ 30 ], the increasing partisan divide over pandemic policies and public health governance [ 31 ], and widespread mis/disinformation [ 32 ].
Institutional trust lays the groundwork for the development of interpersonal and vaccine trust [ 23 ]. Interpersonal trust related to vaccines centers on relationships with representatives of institutions, such as doctors, nurses, and public health officials. Despite reduced trust in recent years, doctors and healthcare professionals remain the most trusted sources of vaccine and health information [ 30 ]. Because healthcare professionals represent the point of contact between the public and health/medical institutions, these interpersonal relationships hold the potential to rebuild institutional trust more broadly. However, trust relations with healthcare professionals do not always increase vaccine acceptance because many doctors and nurses share the vaccine concerns of the public more generally [ 33 ]. Healthcare practitioners also face time constraints that limit their interactions with patients, making it difficult to engage in personalized conversations that can help address vaccine concerns and anxieties [ 33 ]. Additionally, those who are poor, uninsured, or medically underserved frequently do not have a regular provider to develop a trusting relationship [ 34 , 35 ].
In the context of vaccine hesitancy, loss of confidence in scientific institutions and medical providers entails a loss of epistemic trust, or willingness to accept information or knowledge from another party as true and relevant [ 7 ]. This loss of epistemic trust in official sources of information damages vaccine trust , or trust that vaccines are necessary, safe, and effective [ 26 ]. Although vaccine distrust is often attributed to public ignorance (referred to as the “deficit model” in science and health communication), this approach fails to recognize how the vaccine hesitant public understands risk. For example, health officials emphasize vaccine safety based on population-level data and the statistical rarity of severe adverse outcomes. Vaccine hesitant individuals, by contrast, understand vaccine risk in more individualized ways and focus on whether the vaccine is safe for themselves and their children based on personal history and experience [ 8 , 25 , 36 ]. These epistemic mismatches mean that health officials and institutions often fail to effectively address the public’s vaccine concerns and anxieties, focusing instead on the public’s perceived knowledge deficiencies, and thereby deepening distrust.
Scholars emphasize the need for multi-layered interventions to address vaccine related public distrust. Common recommendations include community-engaged approaches, use of trusted messengers, and dialogue-based interventions [ 37 – 40 ]. These approaches make intuitive sense, but more research is needed to understand whether and how they work to increase trust. In this exploratory study, we examine the trustworthiness of vaccine hesitancy interventions to gain insights into how institutions can begin to build or repair trust relations with the public.
Data and methods
Study setting: underserved oklahoma communities.
Oklahoma, a state in the southcentral US, consistently ranks near the bottom of all states for health outcomes and health system performance [ 41 ]. The state has low rates of adult vaccine uptake coupled with high prevalence of chronic health conditions that increase risk for infectious disease-related morbidity and mortality [ 42 – 45 ]. During the early emergency period of the pandemic (Jan 2020 through Jul 2021), Oklahoma had among the highest COVID-19 mortality rates in the country (493 deaths per 100,000 population, compared to 372 nationwide) [ 46 , 47 ]. COVID-19 vaccination uptake has likewise lagged in Oklahoma across the pandemic, reflecting widespread vaccine hesitancy concerns [ 48 ]. As of May 2023, 15.3% of Oklahoma adults had received the updated COVID-19 bivalent booster dose, compared to 20.5% of the population nationally [ 49 ]. Underserved rural populations are particularly susceptible to severe impacts from vaccine-preventable infectious disease. Oklahoma is a highly rural state, with one third of all residents residing outside of metro areas [ 50 ]. Rural Oklahomans are also less vaccinated than their urban counterparts. Less than half (46.7%) of nonmetro residents in the state have completed their initial COVID-19 vaccine series compared to 58.3% in metro counties [ 51 ].
CATCH-UP: Community COVID-19 testing and vaccine events
Data for this study derive from the NIH-funded CATCH-UP project (Community-engaged Approaches to Testing in Community and Healthcare Settings for Underserved Populations, NIH grant number 3U54GM104938-08S1), which provided technical assistance and staffing support for communities to design, organize, and host COVID-19 testing events in rural and underserved areas with limited access to these services. Depending on availability and need, testing services included polymerase chain reaction (PCR), antigen (rapid), and serology (antibody) testing. As vaccines became available, community partners also began arranging for COVID-19 vaccines to be offered at testing events and organizing separate vaccine events.
Community COVID-19 testing events occurred across Oklahoma between December 2020 and April 2023, organized in partnership with the University of Oklahoma Health Sciences Center (OUHSC), the Public Health Institute of Oklahoma (PHIO), and numerous community health organizations. The testing events were made possible because PHIO, an organization founded in 2004, has developed a robust statewide network of health organizations to bridge government, academia, and communities. In 2012, PHIO established the County Health Improvement Organization model and the Oklahoma Primary Health Care Extension System alongside OU Family Medicine. The system was designed to mobilize communities for public and community health action, through community-designed efforts, including academic-community research efforts. To support CATCH-UP events, PHIO provided a total of $75,000 ($2,500 per community health coalition) to purchase supplies and cover startup costs. PHIO adapted a Community Testing Guide from a tribal partner to provide testing site partners with a document to organize their local testing efforts. Community health coalitions also received incentive funds based on the number of events hosted and tests performed. Incentive dollars ranged from $1,250 to $9,500 per event, with a total of $888,000 dispersed. Incentives funds were required to be invested in the community to reduce COVID-19 related morbidity and mortality but had no other restrictions on their use. For example, incentive funds supported after school programming, walking trails and fitness equipment, food interventions for families experiencing COVID-19 isolation, diabetes prevention, and professional development.
Community partners hosted a total of 465 COVID-19 testing and/or vaccine events and performed 8,054 tests (44% antibody, 31% PCR, and 25% rapid). Events occurred in 29 out of Oklahoma’s 77 counties and included African American urban communities in Tulsa and Oklahoma City, rural Tribal communities, and white rural communities throughout the state. In all, 48 total testing site partners or community coalitions took part in organizing testing events. Community partners encompassed a wide variety of organizations, including workplaces, chambers of commerce, Black fraternities and sororities, educational literacy centers, fire departments, federally qualified health centers, and local health departments. Event sites were similarly varied and ranged from churches, schools, community centers, and concert halls to low-income housing complexes, mobile home parks, and parking lots. Many events were organized alongside other services or activities such as social services (e.g., food distribution, back-to-school drives, and dental clinics) or large social events (e.g., Pride Festival, car show, and sports events). In other events, coalitions partnered with local schools, assisted living facilities, and employers to provide services to residents or employees. In coordinating testing and vaccine events, community health partners took on complex and sophisticated roles, operating in many cases as “mini health departments.” This involved emergency preparedness training, managing supply chains, navigating multiple state-mandated reporting and scheduling platforms, learning CDC reporting guidelines, interfacing with the Public Health Lab, coordinating specimen transport, and responding to diverse community needs during outbreaks.
In August 2021, the research team received a supplementary grant award (CATCH-UP Vaccines, NIH grant number 3U54GM104938-09S3), funded through the Rapid Acceleration of Diagnostics–Underserved Populations program (RADx-UP), to design and test interventions to reduce vaccine hesitancy [for the final intervention protocol, see 52 ]. With a primary aim to inform intervention design, we conducted focus groups with community partners and others involved in organizing or running community testing and vaccine events. Below, we follow the “Consolidated criteria for reporting qualitative research” (COREQ) checklist to ensure transparency in our methods and findings [ 53 ].
Recruitment
All community and academic partners involved with organizing and staffing the CATCH-UP testing and vaccine events were eligible and invited to participate in a focus group. LR, the CATCH-UP director at PHIO, recruited participants through program listservs, virtual meetings, and email outreach. LB, LJ, and LR regularly evaluated the representativeness of the sample and LR extended personalized email and phone invitations to individuals from geographic, racial, and organizational groups that were not well represented in the sample. Recruitment ended once all potential participants had been given multiple opportunities to attend and event registration dropped to consistently low levels (recruitment period: 12/01/2021-03/31/2023). As incentive, participants received a $40 Walmart gift card following data collection.
Data collection
Data collection took place either in-person or virtually (through Zoom videoconferencing software), depending on participant preference and COVID-19 safety considerations. Three researchers (LB, LJ, and GT) from the OU Center for Applied Social Research, all experienced in qualitative data collection, organized and facilitated the focus groups/interviews. Two researchers were present at each focus group, one acting as the primary moderator and one taking notes and providing technical support. LB (a sociologist and research scientist) and LJ (a medical anthropologist and professor) moderated most of the focus groups/interviews, with GT (a research scientist) primarily serving in a support role.
At the beginning of each focus group, all parties introduced themselves, and researchers explained the purpose and goals of the study before obtaining oral consent from each participant. Focus groups were audio recorded through Zoom or an internal recorder when data collection occurred virtually and using a digital recorder when in-person. Demographic information was collected from participants via an anonymous survey (online or paper, depending on the data collection format) at the conclusion of each focus group. The response rate for the anonymous demographic survey was 74.1%.
During focus groups, a semi-structured interview guide was used to explore (1) experiences organizing, hosting, and running testing and vaccine events, (2) community response to events, including COVID-19 vaccine beliefs and attitudes, and (3) feedback on three proposed vaccine hesitancy interventions intended for implementation at testing and vaccine events. For intervention feedback, we asked participants about potential community response and likely effectiveness of the interventions, logistical or technological obstacles to implementation, and suggested improvements. Interventions varied slightly across focus groups/interviews as the research team refined the strategies and incorporated feedback from participants.
The CATCH-UP Vaccines Team developed the proposed interventions based on disciplinary expertise, prior research, and experience with the testing and vaccine events. Proposed interventions included: (1) text message reminders, (2) tablet-delivered testimonial and educational content, and (3) one-on-one dialogue focused on antibody test interpretation. Text message reminders would be sent either before or during events to inform attendees about COVID-19 vaccine availability and encourage vaccination. Different wording options were presented for feedback, including “Your COVID-19 vaccine is reserved for you” and “Remember to get your COVID-19 vaccine at the event today. First, second, and booster doses are available.” Tablet-delivered testimonial and educational content was designed to address specific COVID-19 vaccine concerns of community members. Event attendees would first take a short digital survey via tablet to gather information about their vaccine concerns and status. Based on survey responses, attendees would then see tailored testimonial and educational material responding to those concerns. Researchers also presented a sample educational infographic that responded to common vaccine concerns.
One-on-one dialogue encouraged vaccination while delivering antibody test results. The intervention included a semi-structured script for testers to use while delivering test results, as well as a visual showing the difference between individuals with high antibodies and no antibodies (an idea that came out of an early focus group). During focus groups, participants were read a sample script for delivering antibody test results that was meant to help standardize the process across testers. Following antibody results interpretation, testers would engage attendees in one-on-one conversation centered on vaccine questions and concerns.
Between December 8, 2021 and March 10, 2022, we conducted 7 focus groups with 2–5 participants present in each group. Because of scheduling conflicts, 6 additional individual interviews were completed with participants who were unable to attend a scheduled focus group. In total, 27 people participated in a CATCH-UP focus group or interview. Due to the COVID-19 pandemic, all but two focus groups and interviews took place virtually over Zoom. In December 2021, when social distancing requirements had eased due to relatively low COVID-19 case counts, one in-person focus group took place in a rural community center. One interview occurred over the phone because of issues with poor internet connectivity. Focus groups/interviews ranged from 43 minutes to 2 hours (average of 1.2 hours). All data collection occurred in English.
Most participants (82%) represented community partners who organized and hosted testing events, but we also talked to PHIO staff (7.4%) who served administrative and support roles, OUHSC employees who performed serology testing, and nursing staff who assisted with viral testing and vaccines (11.1%, see Table 1 ). Reflecting the gender composition of community partners more broadly, women made up most participants (82%), with the remaining 19% identifying as men. Racially, participants identified as 44.4% white, 11.1% American Indian/Alaska Native, 11.1% Black/African American, and 7.4% more than one race. Seven percent (7.4%) identified as Hispanic.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0300872.t001
Data analysis
Data processing began with verbatim transcription of audio recordings using NVivo auto-transcription services, followed by researcher review and correction of all transcripts for accuracy. We then analyzed the transcripts using NVivo qualitative data analysis software [ 54 ]. LB coded the data with regular discussion and input from LJ and GT on themes and analytic categories. Throughout the data collection and coding process, the core research team (LB, LJ, GT) also met weekly with the larger CATCH-UP Vaccines Team to present and discuss preliminary findings and themes. Because of the small sample size and exploratory nature of the study, we did not enlist a second independent coder but rely on other well established means of ensuring analytic rigor, including regular team discussions, transparency in methods reporting, and substantial use of raw data in the findings [ 55 ].
Data analysis followed the flexible coding method, a strategy that takes advantage of the capabilities of qualitative data analysis software and recognizes that researchers generally combine deductive and inductive approaches in practice [ 56 ]. Flexible coding therefore takes an abductive approach by using previous research to inform data analysis while remaining open to new and unexpected findings [ 57 ]. The process involves three stages: indexing, analytic coding, and validity checking [ 56 ]. First, we indexed the data by coding transcripts into broad thematic categories based on the interview protocol. Index codes included the organization and success of past testing events, community vaccine attitudes and concerns, strategies used to respond to community vaccine concerns, and feedback on the three interventions. Second, we applied analytic codes to indexed extracts, focused on better understanding how participant strategies and proposed interventions worked to either encourage or reduce trust. Analytic coding combined conceptual categories from past research with inductively developed codes by identifying characteristics of strategies that facilitated trust-building across three pre-determined domains (institutional, interpersonal, and product). Fig 1 presents the analytic codes and their conceptual relationship. Third, we checked the theoretical validity of the coding by examining the distribution of analytic codes across cases, exploring alternative explanations, and identifying negative cases.
https://doi.org/10.1371/journal.pone.0300872.g001
The research protocol and materials received expediated approval from the University of Oklahoma Health Sciences Center Institutional Review Board for the Protection of Human Subjects (OUHSC IRB# 13436). We obtained verbal consent from study participants during virtual data collection and written consent during in-person data collection.
Institutional trust through community-university partnerships
Community partners identified government and institutional distrust as underlying much of the vaccine hesitancy in their communities and described widespread beliefs that the vaccine had been developed too quickly with experimental technology, was inadequately tested, inferior to natural immunity, and ineffective at preventing infection. Yet despite low levels of trust in national public health and governance institutions, Oklahoma’s large state universities retained some credibility within communities, providing an entry point for community engagement and trust building through community-university partnerships. Working with community partners further bolstered trustworthiness by ensuring the interventions included shared values and identities.
Community health partners attributed the success of COVID-19 community testing and vaccine events in part to the presence of the University of Oklahoma (OU). Participants saw communities as more willing to engage and receive COVID-19 information coming from OU or other large state universities than from large federal agencies (e.g., CDC) and believed that this trust stemmed from the university’s popular sports programs (primarily football), academic and medical credentials, and general proximity/familiarity. One community partner from a small rural community explained how college sports loyalties contributed to institutional trust:
I think that OU , there’s a trust there and I hate to say it , simply because of the football…Whether it’s OU or OSU [Oklahoma State University] , either one , it just seems to be something that people pay more attention to . (Participant 9, Community Health Partner)
Echoing this sentiment, another community partner suggested that athletic coaches and players from OU or other local teams would be good messengers for health information within their community.
For other participants, the academic and medical credentials of OU made the institution trustworthy to communities. When offering COVID-19 testing services, participants stressed the need to use university branding and OU representatives to establish expertise:
I think that they [community members] want to see somebody dressed professionally in their OU gear and know that they’re speaking with someone who’s bringing that [expert] knowledge to the table . (Participant 4, Community Health Partner) I did feel , even from our first event , it was so important to establish the fact [that the University of] Oklahoma , OU Health is behind this . This is OU Health here . (Participant 18, PHIO staff)
The perceived expertise behind OU—and particularly OU Health—bolstered the legitimacy of events and enabled community partners to bring trustworthy information to communities. The most commonly noted characteristic that set state academic institutions apart from large national or out-of-state institutions with similar credentials was proximity, suggesting that familiarity increased trustworthiness. Participants frequently stressed that when it came to trusted information sources, “the more local, the better” (Participant 16, Community Health Partner). For this reason, the partnership between OU and local organizations helped to further strengthen the trustworthiness of information.
Community partners emphasized the importance of the community-university partnership for delivering trustworthy information, with the collaboration bolstering the credibility of both parties.
I think [information from] an organization like OU…and then if it’s filtered through our coalition , I think it would help to bring the message home . So they would know the information came from an institution that has the data , has the brainpower behind it , and then maybe we can help to deliver the information . (Participant 17, Community Health Partner)
This “filtering” of information through local organizations was important because community members tended to place higher trust in peers or community leaders who shared key identity characteristics or values, for example racial or regional identity or religious beliefs. Participants believed that some community members felt more comfortable discussing concerns with non-expert peers with whom they could more easily relate but also wanted assurances that the information came from credible sources. This local translating of information from experts increased the perceived trustworthiness of information. The community-university partnership was, therefore, able to combine institutional reputation and shared local values to help establish trusting relations with a broad range of community members.
Two main issues emerged that threatened to undermine rather than build institutional trust through community-university partnerships: (1) under resourcing events and (2) introducing value conflicts through the research. Many community members had a baseline level of trust in the community-university partnership that made them willing to attend and engage with event staff. However, early lessons suggested the importance of organizational competency and capacity for maintaining and building on this trust. For example, at times too few OU employees were available to staff events, resulting in long wait times and making attendees impatient and less willing to engage in meaningful conversations about COVID-19 and vaccines. This could reduce the perceived competency and trustworthiness of both the community-university partnership and institution more broadly.
The second threat to institutional trust concerned cultural and value conflicts introduced by the research. At testing events, researchers invited attendees to participate in a standardized COVID-19 survey, a required study component as part of the NIH CATCH-UP parent project (NIH RADx-UP) [ 58 ]. The survey was administered on a tablet and took about 15 minutes and was completed while participants waited for rapid COVID-19 test results. Community partners described multiple complaints related to the survey that indicated a lack of cultural compatibility between the research instrument and communities, particularly in more conservative rural areas:
It’s a trust issue . I personally think that the survey that went along with this really caused more distrust with people because it had so many questions , so many things that really have nothing apparently to do with the COVID itself… I mean , we had a lot of people , with the gender [question] and the different things , [questioning] like , “Why are they asking about this for the COVID [study] ? ” (Participant 22, Community Health Partner)
The gender question refers to a survey item asking about participants’ gender identify that included multiple options outside of the male/female gender binary (e.g., transgender and nonbinary). As alluded to by this exchange, the survey—and the gender question in particular—alienated many community members and made them less likely to engage during events. Community health partners serving rural and conservative areas witnessed multiple attendees become so upset in response to questions related to identity or other topics perceived as irrelevant to COVID-19 that they refused to complete the survey or even walked out. As one tester recalled, “One guy at one of our very first events came back and just dropped the iPad on the table and said, ‘I’m done. This is too woke.’ He said, ‘This is too woke’” (Participant 28, OUHSC Employee). The length of the survey was also a common source of complaints within communities. In many instances, community partners were able to mitigate the impacts, but the negative reactions suggest that the potential of the conflict to damage institutional trust.
Interpersonal trust through dialogue
The testing and vaccine events, organized and ran through community-university health partnerships, provided effective settings for interpersonal dialogue around COVID-19 and vaccines. Both community partners (many of whom did not have medical or healthcare backgrounds) and OU employees reported facilitating effective conversations about vaccines that led attendees to reconsider their decision and, in some cases, vaccinate at the event. While this was best accomplished via one-on-one dialogue, community health partners also believed technology and other educational aids could play a role as well. Participant strategies for facilitating trustworthy vaccine dialogue included using autonomy enhancing language (such as stressing that vaccinating was attendee’s decision), acknowledging their own knowledge limitations on COVID-19 and vaccines, and sharing personal experiences with the vaccine. Barriers to interpersonal trust through dialogue included the time- and labor-intensive nature of the intervention and lack of knowledge and confidence on the part of some community partners to act as peer educators.
During CATCH-UP focus groups, participants gave feedback on three potential interventions that could be implemented at community health events, including two technology-based interventions (text message reminders and an interactive tablet-based education program) and one dialogue-based intervention. The dialogue-based intervention received the most favorable responses, while the technology-based interventions were seen as most helpful in aiding direct, in-person dialogue. Participants’ assessment emerged from their prior experience engaging in one-on-one vaccine conversation during community testing and vaccine events:
[T]hat one-on-one discussion about the person individually [works] because then you can go into , what’s the reason for not getting vaccinated ? Do you have any health concerns ? Really one-on-one works the best for us to get people vaccinated . (Participant 20, Community Health Partner)
This type of direct, personal interaction was seen as the most promising approach to reduce vaccine hesitancy. However, participants emphasized that conversations must be conducted and approached in an appropriate manner to establish or maintain interpersonal trust.
Communication strategies varied based on participants’ level of comfort, experience, and expertise. OU employees responsible for conducting and explaining the antibody tests described breaking down the science about how the immune system responded to the COVID-19 virus and vaccines and answering questions in a straightforward but nonjudgmental way. Most community partners, although lacking the same level of expertise, were comfortable acting as peer educators to address COVID-19 questions and concerns as well. The most common strategy, both for presenting specific information and as a general logic for organizing events, was to educate attendees by providing factual information while emphasizing individual autonomy and choice:
I always , always say , it doesn’t matter to me what personal choice you make . It matters to me if you’re making an educated choice . And then people are usually open to what you have to say . (Participant 1, Nurse) [My approach] was always , regardless of what you decide to do , let’s just have the conversation . Let’s get the education . Let’s figure out what you want to do and then you can make your own choice . I’ve never told anybody what to do . (Participant 3, Community Health Partner)
This approach was seen as vital for breaking down individual resistance and overcoming divisions within the community because of widespread concerns about government overreach and loss of personal freedoms from pandemic restrictions. Within black communities, community partners saw this approach as important because of historical medical abuses (such as the Tuskegee syphilis study) that deprived individuals of autonomy and created widespread distrust in medical institutions. Participants reported that community members were generally receptive to information presented in this way and believed their communities were “still listening” despite the politically polarized and entrenched positions depicted by much of the media.
In addition to the interpersonal trust between the public and event staff, interpersonal trust between community partners, OU employees, and PHIO was crucial for the success of events by increasing their knowledge and confidence as peer educators during events. During CATCH-UP focus groups, community partners reported continuously learning from OU employees and relaying that information to community members.
Participant 6: We would use the time when we didn’t have anybody … [to] carry on conversations [with OU employees] to get our questions answered that people had … Participant 3: [So we could] share that information with other people . Participant 6: So the peer side of that says, “OK, did you get your [test] results? … Peer-to-peer, let’s talk about this…” And the facts that we had weren’t so far out there because they were legit from the group. And so I think the peer interaction is huge because… they’re not afraid to ask us questions once they’ve warmed up … And knowing the answer, that’s the thing you have to know the answer and not just talk like you know it .
This example shows how interpersonal trust with OU employees enabled community partners to act as peer educators, as well as how interpersonal trust operated at both the professional and peer levels to address vaccine hesitancy issues. Similarly, community partners noted PHIO staff as a trusted source of information who they felt comfortable contacting and had strong working relationships. Participants also saw transparency about the limitations of their knowledge as important for maintaining community trust. Citing their sources and referring attendees to professionals with greater expertise provided a means to stay within their comfort level while continuing to serve the community.
A second common strategy for both community partners and OU employees was to share their personal experiences with COVID-19 vaccination to help alleviate community members’ fears and concerns.
One way that we’ve been able to get people to become more accepting of the vaccine at some of these events is giving your own personal experience and being real about it… They usually had a positive reception to that and sometimes changed their minds . (Participant 12, OUHSC Employee) I think that too often we don’t have enough people in the community who look like those who we’re serving , who can come and share their experiences and say , “Hey , you know what , I got the vaccine . ” You know , who can kind of talk to them and let them know , “Hey , it’s OK , I’m alive . I made it . I don’t have an extra arm . ” (Participant 7, Community Health Partner)
Community and university partners described sharing both the positive and negative aspects of their vaccine experiences, including uncomfortable and dayslong vaccine side effects. Community partners also saw acknowledging that they too had been hesitant at one time as an important way to build trust by validating the vaccine hesitancy concerns of community members.
The greatest barriers to addressing vaccine hesitancy through interpersonal dialogue was resource limitations and comfort level of community partners to act as peer educators. The time- and labor-intensive nature of personalized conversations made them difficult to implement on a consistent basis when events became busy or were short staffed as described above. These conditions did not allow for the type of trustworthy interpersonal dialogue needed to address vaccine hesitancy. Additionally, several focus group participants, particularly those without healthcare backgrounds, explained that they did not feel comfortable answering COVID-19 or vaccine related questions:
I have no answers for anyone , I want to know too , you know…I can’t give answers that I don’t know…because that’s not healthy for any of us . (Participant 9, Community Health Partner) Honestly , I didn’t address [vaccine questions] too much because I didn’t feel confident enough in my knowledge to really fully answer those questions . (Participant 5, Community Health Partner)
Participants suggested regular training and educational opportunities to increase community partners’ COVID-19 knowledge and build skills for navigating difficult conversations, as well as ensuring community partners presented consistent information across the state.
In addition to a lack of confidence in their own COVID-19 and vaccine knowledge, several focus group participants expressed vaccine hesitancy concerns, in some cases leading to vaccine refusal. Expressed reasons for vaccine hesitancy mirrored those of the community more generally and included concerns about long-term health impacts, government overreach, and lack of transparency from pharmaceutical and medical institutions. Notably, vaccine hesitant community partners nonetheless supported and helped to facilitate COVID-19 testing and vaccine events as a means of educating communities—an activity largely seen as neutral and without an ulterior agenda—and because of the financial incentive that their organizations received for hosting events.
Vaccine trust through individualized health information
By developing institutional and interpersonal trust, community events ultimately sought to increase trust in the COVID-19 vaccine. The dialogue-based intervention proposed to focus groups was built around antibody testing and emerged from earlier events where this service proved unexpectedly popular. Antibody tests used a blood draw (finger prick) to measure the presence or absence of two types of COVID-19 antibodies, IgG and IgM. At testing events, OU employees explained test results to attendees and, time permitting, facilitated a conversation about vaccines. These interactions simplified aspects of immunity for a lay audience and focused on encouraging vaccination rather than providing medical advice. The proposed intervention added a semi-structured script to help standardize the process. Based on experience, participants saw this intervention as the most effective strategy to increase vaccine trust because of its ability to deliver individualized health information in a concrete way and to facilitate vaccine conversations. Specifically, antibody information helped to address questions about personal vulnerability and vaccine effectiveness, both of which represent key factors in vaccine hesitancy [ 59 , 60 ]. However, antibody testing had the potential to backfire and reduce vaccine trust when community members interpreted results as showing that the vaccines were ineffective or unnecessary. The ability of the tests to deliver new information also varied by individuals and across the pandemic.
For community partners, the antibody testing represented a compelling pathway to transition into providing vaccine services to communities and addressing vaccine hesitancy. Organizers did not initially conceive of antibody testing as a core part of testing events but responded to their popularity and community demand. As one PHIO staff member explained, community partners immediately recognized the opportunity to encourage vaccine acceptance through antibody testing, leading to greater efforts to incorporate COVID-19 vaccine services at events:
[I]t was once [community organizers] saw that popularity of antibody testing that they really started thinking about vaccines and trying to get vaccines to their events because they felt like they had a clear communication message . (Participant 8, PHIO Staff)
Unlike other more generic forms of education or viral testing, community partners saw the antibody testing as delivering a clear message about individuals’ potential level of protection and susceptibility to the virus that, in some instances, led them to rethink their vaccine decision. The same PHIO staff member quoted above went on to explain the line of communication:
A lot of people… would come [to events] and say , “Well , I had [COVID-19] , I’m sure I had it . So I have immunity . ” And you’d be like , “Well… . let’s test it . " If they were negative [for antibodies] , they were suddenly questioning , “What can I do ? What should I do ? Like , I’m surprised” in a way that they didn’t do if it was a PCR or rapid test … It was a clear message of “I’m susceptible to this disease that I maybe thought I wasn’t susceptible to at this time . ” (Participant 8, PHIO Staff)
At a time when many scientific questions remained about the level and duration of protection acquired through different COVID-19 vaccines, as well as disease exposure, the antibody testing was able to provide real-time information that increased people’s confidence in making vaccine decisions.
Several community partners described their own experience with antibody testing and how it informed their vaccination decision:
I was not vaccinated . I had COVID in January last year . My doctor said I should be good to go , I should have antibodies or whatever . I thought I was completely safe . The [community coalition]… did an event at the church up here and I went [and tested] and I had no antibodies . And that scared me , you know . And it made me start thinking… maybe I better go get vaccinated . And that’s what the antibody testing caused me to do , go get vaccinated . If I hadn’t done the antibody testing , I don’t know if I would be vaccinated today . (Participant 9, Community Health Partner)
In this case, the participant described how their fear about being unprotected outweighed other concerns about vaccine safety. The antibody test also helped many attendees who had received an initial vaccine series but were undecided about whether or when to get additional shots. One community partner, for example, recalled: “We had a person in our office get tested and she’s on immunosuppressants and she realized that she needed the booster before that was a recommendation” (Participant 16, Community Health Partner). Attendees making decisions about boosters generally expressed less vaccine hesitancy and distrust than their unvaccinated peers. Nonetheless, the antibody testing helped to cut through confusion and conflicting information that could potentially diminish vaccine trust.
In addition to delivering individualized health information, the antibody testing proved appealing for its ability to make the COVID-19 vaccine’s effect on the body and immune system visible and concrete. People were able to “see” the vaccines’ potential for protection at work through the appearance of the bright lines indicating the presence of antibodies in their blood. Community partners discovered that this visibility and concreteness could be enhanced through comparison with other’s (anonymous) test results. One community partner explained, “It was absolutely a turnaround point for us when we could show a comparison. You know, ‘This is somebody who was vaccinated last year. This is somebody who was vaccinated a month ago’” (Participant 6, Community Health Partner). Event staff were then able to explain how vaccines “wore off” over time and the need to update vaccine protection.
The antibody testing provided an important opening for dialogue as vaccine hesitant community members began to question their vaccine beliefs and decisions. However, some community partners reported instances where the antibody testing increased vaccine distrust, as explained by one organizer: “And now the mistrust is higher because… we had people who had been vaccinated and four weeks later got a serology test and didn’t have any antibodies in their system” (Participant 20, Community Health Partner). Test results also had little impact on vaccine trust when they confirmed prior assumptions about protection levels and therefore failed to offer any new information. For example, tests showing that community members had some level of protection from a previous exposure risked reinforcing pre-existing ideas that vaccines are unnecessary and make people less receptive to engaging in further vaccine conversations. In cases such these, the presence of an OUHSC staff member who could explain the science behind such results using lay language was crucial. These examples underscore the importance of dialogue with trusted messengers to accompany antibody testing or other services to avoid unintentionally reinforcing vaccine distrust.
The COVID-19 pandemic revealed widespread public distrust of key societal institutions—including the government, media, and healthcare systems—that fueled vaccine hesitancy and refusal. Trust is a crucial element for increasing vaccine confidence and uptake, yet building trust in a highly polarized context and across demographic differences remains a significant challenge that will require long-term institutional commitment and change. This study reports findings from a community-engaged study centered on COVID-19 testing and vaccine events in underserved Oklahoma communities. We focus on what makes institutions, interpersonal interactions, and vaccines trustworthy. Through focus groups and interviews with community partners, we identify several strategies that successfully facilitated vaccine-related trust. As an exploratory study based on a relatively small sample, this research has inherent limitations regarding generalizability. Future research, some of which is currently in progress by our research team [ 52 ], should test interventions that incorporate “trustworthy” characteristics identified in this study to determine their effectiveness at both increasing trust and vaccine confidence. The extent to which our findings apply to distrust and hesitancy surrounding other types of vaccines is also unknown. Nonetheless, our findings offer important lessons for designing and implementing effective vaccine hesitancy interventions.
First, our findings indicate that success of the community-university partnership, including effectively implementing vaccine hesitancy strategies, depended on both institutional and interpersonal trust between partners. The institutional arrangement behind the partnership played a large role in facilitating this trust. PHIO has developed statewide organizational infrastructure and long-term relationships with communities. As a result, the community coalitions hosting and organizing events had pre-existing relationships and experience partnering with OU on health projects. This working relationship provided a foundation of trust between the community and university partners. On an interpersonal level, study personnel spent countless hours working alongside community partners during events developing personal and trusting relationships. These relationships were critical for providing community partners with the knowledge and confidence to act as peer educators.
Second, the cultural resonance of interventions set the stage for trust building. Community partners indicated that community members placed a relatively high level of trust in the university. This trust stemmed in part from its academic and medical credentials, but also from familiarity and the popularity of sports teams. Some saw the university’s association with football and sports as helping to drive attendance at events, as well as increasing epistemic trust. Although popular throughout the country, in the US South, football plays a particularly important role in creating community bonds and collective identity [ 61 ]. While some study personnel found the degree of affinity with the University of Oklahoma at odds with previous perceptions that rural Oklahomans viewed the university with suspicion, participants repeatedly reassured us that this was not the case. Our findings suggest that public health campaigns can productively leverage the social and cultural importance of sports to build vaccine trust, for example, by recruiting athletes and coaches to participate in public health campaigns. While sports loyalty alone is unlikely to automatically translate into epistemic trust and survey research indicates low levels of trust in athletes and sports personalities as sources of health information [ 62 ], we suggest that it can establish common ground and an entry point for further dialogue and trust building, especially in contexts with high levels of sports enthusiasm and identification with teams. Conversely, cultural misalignment of study components eroded institutional trust.
Cultural misalignment emerged primarily from a survey administered at testing events as required by the NIH RADx-UP parent project. Because the survey was standardized across data collection sites nationwide, the CATCH-UP team had limited control over survey content but did successfully advocate for some small changes in the wording of demographic questions to reduce resistance in Oklahoma communities, while also remaining inclusive of diverse identities. Community partners’ ability to manage cultural conflicts also proved important. Community health partners described successfully using jokes (e.g., “the survey clearly was not written by Oklahomans”) and explaining how the survey research would benefit their communities to ameliorate negative reactions. Additionally, our findings suggest that navigating cultural tensions can be productive when done in the context of pre-existing trust. Participants from one rural health coalition, for example, reported positive experiences providing testing at a Pride event and serving a demographic who they described having little previous interaction.
Third, antibody testing proved capable of increasing vaccine trust through its ability to deliver individualized health information in a visible and concrete way. The concreteness of the test contrasted with the invisibility of the virus and offered evidence of vaccine effectiveness that resonated more strongly than other types of available information. However, the resonance and popularity of the antibody testing can itself be seen as a reflection of institutional distrust, as well as neoliberal policies that shift the responsibility for health from society to individuals [ 63 ]. This individualized responsibility encourages people to “do their own research” (consisting primarily of searching the internet) and make decisions based on personal experience rather than turning to expert knowledge or institutions. In allowing people to “see” vaccine effectiveness for themselves, the antibody testing provided a more direct form of evidence, presumably bypassing the need for institutional trust. As seen in the examples described above where antibody testing led to reduced trust, this strategy can easily backfire if not embedded within broader efforts to build institutional and interpersonal trust.
Lastly, we emphasize the importance of mutual (bi-directional) trust and long-term commitments for repairing distrustful relations. Most scholarship focuses on public distrust of institutions, yet researchers and institutions must also be willing to extend trust to communities. Notably, antibody testing remained popular despite official recommendations against its use for assessing immunity against COVID-19 [ 64 , 65 ]. Although our goal was to facilitate vaccine conversations and testers encouraged vaccination regardless of the results, community members may have still interpreted and used their results in ways that conflicted with the prevailing scientific understanding. Despite these limitations from a strictly scientific perspective, we chose to trust community health partners’ insights on how their communities could best be reached.
Conclusions
The ability to leverage community partnerships was made possible by years of outreach and collaboration prior to the pandemic. If not for these earlier efforts, it would have been difficult to forge and build these bonds amid a worldwide pandemic. This demonstrates the need to proactively develop and maintain community-university partnerships before a crisis ensues. Other innovations arising from this study, e.g., the unexpected effectiveness of antibody testing as a means of concretizing the virus and providing information in a manner that community members could comprehend, demonstrates the importance of identifying ways to better engage in science communication with communities. Also crucial to these efforts is the ability to determine and appropriately utilize institutional authority in ways that benefit communities, which requires understanding what does and doesn’t resonate locally. These findings–the strength of expert-community collaborations, the importance of developing community relevant science communication, and the ability to effectively understand and deploy institutional authority–provide a roadmap for an improved response in future pandemics and community crises.
Acknowledgments
The CATCH-UP Vaccines Team includes Janis Campbell, 1 Mark Doescher, 2 Judith James, 3 April Lopez, 1 Jordan Neil, 2 Paul Spicer, 4 Conce Uribe-Frias, 1 and Mary L. Williams. 1 Lead author: Laura Bray, [email protected]
1 Department of Biostatistics & Epidemiology, University of Oklahoma Health Sciences, Oklahoma City, Oklahoma, United States of America
2 Family and Preventative Medicine, University of Oklahoma Health Sciences, Oklahoma City, Oklahoma, United States of America
3 Oklahoma Clinical and Translational Science Institute, University of Oklahoma Health Sciences, Oklahoma City, Oklahoma, United States of America
4 Department of Anthropology, University of Oklahoma, Norman, Oklahoma, United States of America
- 1. WHO. The Thirteenth General Programme of Work, 2019–2023. World Health Organization [Internet]. 2019 [cited 2023 Nov 25]. Available from: https://www.who.int/publications/i/item/thirteenth-general-programme-of-work-2019-2023 .
- View Article
- Google Scholar
- PubMed/NCBI
- 34. NACHC. Closing the Primary Care Gap: How Community Health Center can Address the Nation’s Primary Care Crisis. National Association of Community Health Centers [Internet]. 2023 [cited 2023 Oct 17]. Available from: https://www.nachc.org/wp-content/uploads/2023/06/Closing-the-Primary-Care-Gap_Full-Report_2023_digital-final.pdf .
- 36. Fairhead J, Leach M. Vaccine anxieties: Global science, child health and society. London, UK: Earthscan; 2007.
- 42. CDC. People at Higher Risk of Flu Complications. Centers for Disease Control and Prevention [Internet]. 2022 Sep 8 [cited 2023 Nov 10]. Available from: https://www.cdc.gov/flu/highrisk/index.htm .
- 43. KFF. Adult Flu Vaccination Rates by Age, 2020–2021. Kaiser Family Foundation [Internet]. n.d. [cited 2023 Aug 2]. Available from: https://www.kff.org/other/state-indicator/adult-flu-vaccination-rates-by-age/ .
- 47. United Health Foundation. America’s Health Rankings: 2022 Annual Report. United Health Foundation [Internet]. 2022 [cited 2023 Aug 28]. Available from: https://www.americashealthrankings.org/learn/reports/2022-annual-report/state-rankings .
- 50. RHI Hub. Rural Data Explorer. Rural Health Information Hub [Internet]. n.d. [cited 2023 Nov 10]. Available from: https://www.ruralhealthinfo.org/data-explorer?id=179&state=OK .
- 51. OSU Center for Rural Health. Rural Oklahoma COVID-19 Dashboard. OSU Center for Rural Health [Internet]. 2022 [cited 2023 Nov 12]. Available from: https://experience.arcgis.com/experience/52adb920af37454fb76e6c19578d77e1 .
- 54. Lumivero. NVivo (Version 13, 2020 R1). 2020.
- 61. Bain-Selbo E. Game day and God: Football, faith, and politics in the American South. Macon, GA: Mercer University Press; 2009.
- 63. Brown BJ, Baker S. Responsible citizens: Individuals, health, and policy under neoliberalism. London, UK: Anthem Press; 2012.
- 64. FDA. Antibody testing is not currently recommended to assess immunity after COVID-19 vaccination: FDA safety communication. US Food and Drug Administration [Internet]. 2021 Nov 30 [cited 2023 Nov 10]. Available from: https://www.fda.gov/medical-devices/safety-communications/antibody-testing-not-currently-recommended-assess-immunity-after-covid-19-vaccination-fda-safety .
- Skip to main content
- Accessibility help
Information
We use cookies to collect anonymous data to help us improve your site browsing experience.
Click 'Accept all cookies' to agree to all cookies that collect anonymous data. To only allow the cookies that make the site work, click 'Use essential cookies only.' Visit 'Set cookie preferences' to control specific cookies.
Your cookie preferences have been saved. You can change your cookie settings at any time.
Coronavirus (COVID-19) and society: what matters to people in Scotland?
Findings from an open free text survey taken to understand in greater detail how the pandemic has changed Scotland.
- This research has captured the diversity and complexity of people’s experiences.
- People’s experiences of the pandemic and their ability to stay safe has been impacted by a range of factors, including: their geographical environment, their financial situation, profession, their living situation and if they have any physical or mental health conditions.
- Even though the direct level of threat from COVID-19 has reduced (for some people), there is still concern about the longer term harm and disruption that COVID-19 has caused to people and communities, and worry about the threat of future waves of infection.
- This report captures a number of specific suggestions for support. For example, support for key workers, creating safer public environments, wide-scale financial support, greater awareness around the experiences of those who are at higher risk to COVID-19 and putting in place robust processes for learning and reflection on the impact of the pandemic.
- Public engagement in this open and unfiltered format is an essential part of making sense of people’s attitudes and behaviours within the context of their life.
Email: [email protected]
There is a problem
Thanks for your feedback
Your feedback helps us to improve this website. Do not give any personal information because we cannot reply to you directly.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

Editor in Chief's Introduction to Essays on the Impact of COVID-19 on Work and Workers
On March 11, 2020, the World Health Organization declared that COVID-19 was a global pandemic, indicating significant global spread of an infectious disease ( World Health Organization, 2020 ). At that point, there were 118,000 confirmed cases of the coronavirus in 110 countries. China had been the first country with a widespread outbreak in January, and South Korea, Iran and Italy following in February with their own outbreaks. Soon, the virus was in all continents and over 177 countries, and as of this writing, the United States has the highest number of confirmed cases and, sadly, the most deaths. The virus was extremely contagious and led to death in the most vulnerable, particularly those older than 60 and those with underlying conditions. The most critical cases led to an overwhelming number being admitted into the intensive care units of hospitals, leading to a concern that the virus would overwhelm local health care systems. Today, in early May 2020, there have been nearly 250,000 deaths worldwide, with over 3,500,000 confirmed cases ( Hopkins, 2020 ). The human toll is staggering, and experts are predicting a second wave in summer or fall.
As the deaths rose from the virus that had no known treatment or vaccine countries shut their borders, banned travel to other countries and began to issue orders for their citizens to stay at home, with no gatherings of more than 10 individuals. Schools and universities closed their physical locations and moved education online. Sporting events were canceled, airlines cut flights, tourism evaporated, restaurants, movie theaters and bars closed, theater productions canceled, manufacturing facilities, services, and retail stores closed. In some businesses and industries, employees have been able to work remotely from home, but in others, workers have been laid off, furloughed, or had their hours cut. The International Labor Organization (ILO) estimates that there was a 4.5% reduction in hours in the first quarter of 2020, and 10.5% reduction is expected in the second quarter ( ILO, 2020a ). The latter is equivalent to 305 million jobs ( ILO, 2020a ).
Globally, over 430 million enterprises are at risk of disruption, with about half of those in the wholesale and retail trades ( ILO, 2020a ). Much focus in the press has been on the impact in Europe and North America, but the effect on developing countries is even more critical. An example of the latter is the Bangladeshi ready-made-garment sector ( Leitheiser et al., 2020 ), a global industry that depends on a supply chain of raw material from a few countries and produces those garments for retail stores throughout North America and Europe. But, in January 2020, raw material from China was delayed by the shutdown in China, creating delays and work stoppages in Bangladesh. By the time Bangladeshi factories had the material to make garments, in March, retailers in Europe and North American began to cancel orders or put them on hold, canceling or delaying payment. Factories shut down and workers were laid off without pay. Nearly a million people lost their jobs. Overall, since February 2020, the factories in Bangladesh have lost nearly 3 billion dollars in revenue. And, the retail stores that would have sold the garments have also closed. This demonstrates the ripple effect of the disruption of one industry that affects multiple countries and sets of workers, because consider that, in turn, there will be less raw material needed from China, and fewer workers needed there. One need only multiply this example by hundreds to consider the global impact of COVID-19 across the world of work.
The ILO (2020b) notes that it is difficult to collect employment statistics from different countries, so a total global unemployment rate is unavailable at this time. However, they predict significant increase in unemployment, and the number of individuals filing for unemployment benefits in the United States may be an indicator of the magnitude of those unemployed. In the United States, over 30 million filed for unemployment between March 11 and April 30 ( Bureau of Labor Statistics, 2020 ), effectively this is an unemployment rate of 18%. By contrast, in February 2020, the US unemployment rate was 3.5% ( Bureau of Labor Statistics, 2020 ).
Clearly, COVID-19 has had an enormous disruption on work and workers, most critically for those who have lost their employment. But, even for those continuing to work, there have been disruptions in where people work, with whom they work, what they do, and how much they earn. And, as of this writing, it is also a time of great uncertainty, as countries are slowly trying to ease restrictions to allow people to go back to work--- in a “new normal”, without the ability to predict if they can prevent further infectious “spikes”. The anxieties about not knowing what is coming, when it will end, or what work will entail led us to develop this set of essays about future research on COVID-19 and its impact on work and workers.
These essays began with an idea by Associate Editor Jos Akkermans, who noted to me that the global pandemic was creating a set of career shocks for workers. He suggested writing an essay for the Journal . The Journal of Vocational Behavior has not traditionally published essays, but these are such unusual times, and COVID-19 is so relevant to our collective research on work that I thought it was a good idea. I issued an invitation to the Associate Editors to submit a brief (3000 word) essay on the implications of COVID-19 on work and/or workers with an emphasis on research in the area. At the same time, a group of international scholars was coming together to consider the effects of COVID-19 on unemployment in several countries, and I invited that group to contribute an essay, as well ( Blustein et al., 2020 ).
The following are a set of nine thoughtful set of papers on how the COVID-19 could (and perhaps will) affect vocational behavior; they all provide suggestions for future research. Akkermans, Richardson, and Kraimer (2020) explore how the pandemic may be a career shock for many, but also how that may not necessarily be a negative experience. Blustein et al. (2020) focus on global unemployment, also acknowledging the privileged status they have as professors studying these phenomena. Cho examines the effect of the pandemic on micro-boundaries (across domains) as well as across national (macro) boundaries ( Cho, 2020 ). Guan, Deng, and Zhou (2020) drawing from cultural psychology, discuss how cultural orientations shape an individual's response to COVID-19, but also how a national cultural perspective influences collective actions. Kantamneni (2020) emphasized the effects on marginalized populations in the United States, as well as the very real effects of racism for Asians and Asian-Americans in the US. Kramer and Kramer (2020) discuss the impact of the pandemic in the perceptions of various occupations, whether perceptions of “good” and “bad” jobs will change and whether working remotely will permanently change where people will want to work. Restubog, Ocampo, and Wang (2020) also focused on individual's responses to the global crisis, concentrating on emotional regulation as a challenge, with suggestions for better managing the stress surrounding the anxiety of uncertainty. Rudolph and Zacher (2020) cautioned against using a generational lens in research, advocating for a lifespan developmental approach. Spurk and Straub (2020) also review issues related to unemployment, but focus on the impact of COVID-19 specifically on “gig” or flexible work arrangements.
I am grateful for the contributions of these groups of scholars, and proud of their ability to write these. They were able to write constructive essays in a short time frame when they were, themselves, dealing with disruptions at work. Some were home-schooling children, some were worried about an absent partner or a vulnerable loved one, some were struggling with the challenges that Restubog et al. (2020) outlined. I hope the thoughts, suggestions, and recommendations in these essays will help to stimulate productive thought on the effect of COVID-19 on work and workers. And, while, I hope this research spurs to better understand the effects of such shocks on work, I really hope we do not have to cope with such a shock again.
- Akkermans J., Richardson J., Kraimer M. The Covid-19 crisis as a career shock: Implications for careers and vocational behavior. Journal of Vocational Behavior. 2020; 119 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Blustein D.L., Duffy R., Ferreira J.A., Cohen-Scali V., Cinamon R.G., Allan B.A. Unemployment in the time of COVID-19: A research agenda. Journal of Vocational Behavior. 2020; 119 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bureau of Labor Statistics (2020). Labor Force Statistics from the Current Population Survey. Retrieved May 6, 2020 from https://data.bls.gov/cgi-bin/surveymost .
- Cho E. Examining boundaries to understand the impact of COVID-19 on vocational behaviors. Journal of Vocational Behavior. 2020; 119 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Guan Y., Deng H., Zhou X. Understanding the impact of the COVID-19 pandemic on career development: Insights from cultural psychology. Journal of Vocational Behavior. 2020; 119 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Johns Hopkins (2020) Coronavirus Outbreak Mapped: Retrieved May 5, 2020 from https://coronavirus.jhu.edu/map.html .
- International Labor Organization ILO monitor: COVID-19 and the world of work. Third edition updated estimates and analysis. 2020. https://www.ilo.org/wcmsp5/groups/public/@dgreports/@dcomm/documents/briefingnote/wcms_743146.pdf Retrieved May 5, 2020 from:
- International Labor Organization (2020b) COVID-19 impact on the collection of labour market statistics. Retrieved May 6, 2020 from: https://ilostat.ilo.org .
- Kantamneni, N. (2020). The impact of the COVID-19 pandemic on marginalized populations in the United States: A research agenda. Journal of Vocational Behavior, 119 . [ PMC free article ] [ PubMed ]
- Kramer A., Kramer K.Z. The potential impact of the Covid-19 pandemic on occupational status, work from home, and occupational mobility. Journal of Vocational Behavior. 2020; 119 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Leitheiser, E., Hossain, S.N., Shuvro, S., Tasnim, G., Moon, J., Knudsen, J.S., & Rahman, S. (2020). Early impacts of coronavirus on Bangladesh apparel supply chains. https://www.cbs.dk/files/cbs.dk/risc_report_-_impacts_of_coronavirus_on_bangladesh_rmg_1.pdf .
- Restubog S.L.D., Ocampo A.C., Wang L. Taking control amidst the Chaos: Emotion regulation during the COVID-19 pandemic. Journal of Vocational Behavior. 2020; 119 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rudolph C.W., Zacher H. COVID-19 and careers: On the futility of generational explanations. Journal of Vocational Behavior. 2020; 119 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Spurk D., Straub C. Flexible employment relationships and careers in times of the COVID-19 pandemic. Journal of Vocational Behavior. 2020; 119 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- World Health Organization (2020). World Health Organization Coronavirus Update. Retrieved May 5, 2020 from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 .

IMAGES
VIDEO
COMMENTS
500+ Words Essay on Coronavirus Vaccine. The Coronavirus has infected millions of people so far all over the world. In addition to that, millions of people have lost their lives to it. Ever since the outbreak, researchers all over the world have been working constantly to develop vaccines that will work effectively against the virus.
Body Paragraph: One compelling reason for implementing COVID-19 vaccination mandates is the overwhelming evidence of vaccine effectiveness. According to a study published in the New England Journal of Medicine, the Pfizer-BioNTech and Moderna vaccines demonstrated an efficacy of over 90% in preventing symptomatic COVID-19 cases.
Introduction. The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 192 million cases and 4.1 million deaths as of July 22, 2021. 1 This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems. Despite the implementation of stringent public health measures, there ...
For some COVID-19 vaccines, two doses are required . It's important to get the second dose if the vaccine requires two doses. For vaccines that require two doses, the first dose presents antigens - proteins that stimulate the production of antibodies - to the immune system for the first time. Scientists call this priming the immune response.
A novel coronavirus (CoV) named '2019-nCoV' or '2019 novel coronavirus' or 'COVID-19' by the World Health Organization (WHO) is in charge of the current outbreak of pneumonia that began at the beginning of December 2019 near in Wuhan City, Hubei Province, China [1-4]. COVID-19 is a pathogenic virus. From the phylogenetic analysis ...
العربية. 25 October 2022. Vaccines save millions of lives each year. The development of safe and effective COVID-19 vaccines are a crucial step in helping us get back to doing more of the things we enjoy with the people we love. We've gathered the latest expert information to answer some of the most common questions about COVID-19 ...
The Pfizer-BioNTech COVID-19 vaccine has been approved for this age group, and they can now receive it . Until 19 June, 2021, about 21% of the world's population have been vaccinated with at least one dose, however, only 0.8% of people in low-income countries have received at least one dose of a COVID-19 vaccine . Of all the countries, the ...
The 95% effectiveness actually means that people with the vaccine have a 95% lower risk of COVID-19 when compared to a control group. Without the vaccine, we would expect roughly 1% of the ...
An introduction to the CFR Task Force Report on pandemic preparedness and the lessons of COVID-19. ... What Is the World Doing to Create a COVID-19 Vaccine? By Claire Felter Aug 26, 2020.
There is no question that the current vaccines are effective and safe. The risk of severe reaction to a COVID-19 jab, say researchers, is outweighed by the protection it offers against the deadly ...
While efficacious vaccines have been developed to inoculate against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; also known as COVID-19), public vaccine hesitancy could still ...
Background Despite reliable evidence-based research supporting the COVID-19 vaccines, population-wide confidence and trust remain limited. We sought to expand prior knowledge about COVID-19 vaccine perceptions, while determining which population groups are at greatest risk for not getting a vaccine. Methods Study participants in the U.S. (79% female, median age group 46-60 years) were ...
Innovative financing expedited the introduction of the pneumococcal vaccine, enabling it to be launched in 2011, in a world-first, in rich and poor countries simultaneously. Immunisation saves millions of lives every year. We now have vaccines to prevent and control 25 infections, helping people of all ages live longer, healthier lives.
DRAFT Prepared by the SAGE Working Group on COVID-19 Vaccines 22 December 2020 3 . I Epidemiology . Coronavirus disease 2019 (COVID-19) is caused by SARS-CoV-2, a newly emergent coronavirus, that was first recognized in Wuhan, China, in December 2019. Globally, as of 19 December 2020, there
The incidence of COVID-19-associated cardiac injury or myocarditis can be 100 times higher than COVID-19 mRNA-vaccine-related myocarditis [159,160]. Another relevant observation is that cases of myocarditis and pericarditis have been reported mainly for mRNA vaccines [ 161 ]; in Brazil, no cases have been reported related to inactivated virus ...
Ahead of the COVID-19 vaccine rollout, health agencies in many countries issued guidance for phased vaccination campaigns, with health-care workers and residents of nursing homes and long-term ...
Coronavirus disease (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus. Most people infected with the virus will experience mild to moderate respiratory illness and recover without requiring special treatment. However, some will become seriously ill and require medical attention. Older people and those with underlying medical ...
Introduction. In 2019, the World Health Organization recognized vaccine hesitancy as a top ten threat to public health [].The consequences of vaccine resistance and refusal have since been realized on a global scale with the COVID-19 pandemic, as increasing numbers of people question the public health recommendations coming from scientific, medical, and governance institutions [2, 3].
27 June 2022. Directorate. Performance, Delivery and Resilience Directorate. Part of. Coronavirus (COVID-19) in Scotland. ISBN. 9781804356142. Findings from an open free text survey taken to understand in greater detail how the pandemic has changed Scotland. Supporting documents.
Results. Thirteen randomized, blinded, controlled trials, which involved the safety and efficacy of 11 COVID-19 vaccines, were included. In 10 studies, the 28-day seroconversion rate of subjects exceeded 80%. In two 10 000-scale clinical trials, the vaccines were effective in 95% and 70.4% of the subjects, respectively.
Emily Dickinson, 1891. Introduction. This book has documented the unequal impact of the COVID-19 pandemic. It has sought out the causes of the health inequalities that emerged from the pandemic, and located them in the syndemic of social inequality and the novel coronavirus. We have argued that the COVID-19 pandemic is unequal in four ways:
Immune responses to influenza (flu) antigens reflect memory of prior infections or vaccinations, which might influence immunity to new flu antigens. Memory of past antigens has been termed "original antigenic sin" or, more recently, "immune imprinting" and "seniority". We have researched a comparison between the immune response to live flu infections and inactivated flu vaccinations.
Introduction. The novel COVID-19 pandemic started at the Chinese province of Hubei in December 2019 after which the WHO declared it to be a public global health emergency. 1 The COVID-19 virus has a zoonotic source with similarities to other highly infectious zoonotic coronaviruses. COVID-19 infection is highly contagious and continues to spread in both developed and developing countries. 2 It ...
Editor in Chief's Introduction to Essays on the Impact of COVID-19 on Work and Workers. On March 11, 2020, the World Health Organization declared that COVID-19 was a global pandemic, indicating significant global spread of an infectious disease ( World Health Organization, 2020 ). At that point, there were 118,000 confirmed cases of the ...