Skip to Content
- Conquer Cancer
- ASCO Journals
- f Cancer.net on Facebook
- t Cancer.net on Twitter
- q Cancer.net on YouTube
- g Cancer.net on Google

Types of Cancer
- Navigating Cancer Care
- Coping With Cancer
- Research and Advocacy
- Survivorship
Brain Tumor: Introduction
ON THIS PAGE: You will find some basic information about this disease and the parts of the body it may affect. This is the first page of Cancer.Net’s Guide to Brain Tumors. Use the menu to see other pages. Think of that menu as a roadmap for this entire guide.
The possibility of being diagnosed with a brain tumor can be a shocking and life-changing event. If your doctor suspects a brain tumor, it is important to seek out doctors who specialize in diagnosing and treating brain tumors. The brain is a complex and vital organ, and treatment often causes life-long changes. Research about brain tumor treatment is ongoing, so it is important to get updated medical information about treatment options about the specific type of brain tumor and to get specialists' opinions about your treatment plan.
About the brain and central nervous system
The brain and spinal column make up the central nervous system (CNS), where all vital functions are controlled. These functions include thought, speech, and body movements. This means that when a tumor grows in the CNS, it can affect a person's thought processes or the way they talk or move.
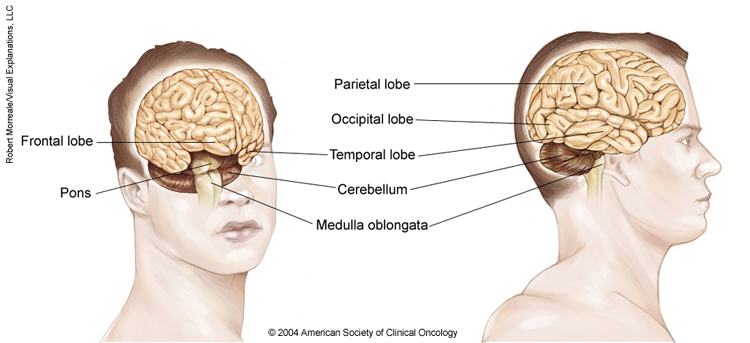
Anatomy of the brain
The brain is made up of 3 main parts: the cerebrum, the cerebellum, and the brain stem. The meninges, which surround the brain, are also considered part of the brain.
The cerebrum. This is the largest part of the brain. It contains 2 cerebral hemispheres on either side of the brain that each control the opposite side of the body. It is divided into 4 lobes where specific functions occur:
The frontal lobe controls reasoning, emotions, problem-solving, expressive speech, and movement.
The parietal lobe controls the sensations of touch, such as pressure, pain, and temperature. It also controls parts of speech, visual-spatial orientation, and calculation.
The temporal lobe controls memory, special senses such as hearing, and the ability to understand spoken or written words.
The occipital lobe controls vision.
The cerebellum. The cerebellum is located at the back part of the brain below the cerebrum. It is responsible for coordination and balance, and it controls functions on the same side of the body.
The brain stem. This is the portion of the brain that connects to the spinal cord and the cerebellum. It controls involuntary functions essential for life, such as the beating of the heart and breathing. Messages for the functions controlled by the cerebrum and cerebellum travel through the brain stem to the body.
The meninges. These are the membranes that surround and protect the brain and spinal cord. There are 3 meningeal layers, called the dura mater, arachnoid, and pia mater. The cerebrospinal fluid (CSF) is made near the center of the brain, in the lateral ventricles. CSF circulates around the brain and spinal cord between the arachnoid and pia layers.
About primary brain tumors
A primary brain tumor is a tumor that starts in the brain. A primary brain tumor is often described as either "low grade" or "high grade." A low-grade tumor generally grows slowly, but it can turn into a high-grade tumor. A high-grade tumor is more likely to grow faster.
In adults, a secondary brain tumor, also called a brain metastasis, is much more common than a primary brain tumor.
About secondary brain tumors
A secondary brain tumor, or brain metastasis, is a cancerous tumor that started in another part of the body, such as the breast, lung, or colon, and then spread to the brain. A secondary brain tumor may also be called metastatic cancer. For instance, lung cancer that has spread to the brain may be called metastatic lung cancer.
If cancer spreads to the meninges and CSF, it is called leptomeningeal metastases or neoplastic meningitis. This condition occurs more commonly in people with leukemia, lymphoma, melanoma, breast cancer, or lung cancer.
The rest of this guide mainly covers primary brain tumors in adults, while providing some information on brain metastases. To learn more detailed information about cancer that started elsewhere in the body and spread to the brain, read about that specific type of cancer . Learn about brain tumors in children in a different guide on this same website.
Types of primary brain tumors in adults
There are many types of primary brain tumors. Some cannot be assigned an exact type because the tumor’s location makes it too difficult to remove for full testing.
Descriptions of more common brain tumor types in adults are described below, divided into glioma and non-glioma tumor types.
As a group, gliomas are one of the most common types of brain tumors. While the exact origin of gliomas is still unknown, they are thought to grow from glial cells or glial precursor cells. A glial cell is a type of supportive cell in the brain. The main types of supportive cells in the brain include astrocytes, oligodendrocytes, and ependymal cells. Gliomas may be considered astrocytoma, oligodendroglioma, or ependymoma. Gliomas are assigned a grade, which is an indication of how aggressive a tumor is likely to be. A higher grade is usually more aggressive and more likely to grow quickly. However, current research is helping doctors move toward using tumor genetics to better classify gliomas. This is discussed elsewhere in this guide. Unlike most tumors that start outside of the brain and CNS, most primary brain tumors like glioma are not assigned a "stage." For tumors that do not begin in the brain, a higher cancer "stage" number usually describes whether the primary tumor has spread to other parts of the body, and this information influences which treatments are selected. Primary brain tumors, like gliomas, only rarely spread outside of the brain. Thus, they do not need to be staged to help the clinical team decide on the appropriate treatments.
Currently, the types of gliomas include:
Astrocytoma. Astrocytoma is the most common type of glioma. Astrocytoma cells look like glial cells called astrocytes that are found in the cerebrum or cerebellum. Historically, there have been 4 grades of astrocytoma, which are described below.
Grade 1 or pilocytic astrocytoma is a slow-growing tumor that is most often benign and rarely spreads into nearby tissue. Benign means the tumor can grow but does not spread to other parts of the body.
Grade 2 astrocytoma is a slow-growing malignant tumor that can often spread into nearby tissue and can become a higher grade. Malignant means it is cancerous and can spread to other parts of the body.
Grade 3 or anaplastic astrocytoma is a malignant tumor that can quickly grow and spread to nearby tissues.
Grade 4 or glioblastoma is a very aggressive form of astrocytoma.
A new international classification system for primary brain tumors was unveiled by the World Health Organization (WHO) in 2021. This system divides astrocytomas and other types of brain tumors into many subgroups depending largely on their genetic makeup and the presence or absence of certain important changes in the tumor's specific genes. The treatment team will use information from an analysis of each tumor sample to precisely classify each tumor using the new guidelines. (Learn more about biomarker testing of the tumor in the Diagnosis section.) Key changes for the most common types of astrocytomas include:
Adult diffuse astrocytomas have now been grouped based on whether there is a mutation in the isocitrate dehydrogenase ( IDH ) gene. There are 2 groups: 1 ) astrocytoma, IDH mutant, and 2 ) glioblastoma, IDH wild-type. "Wild-type" means that the gene is found in its natural, unmutated form.
Astrocytoma, IDH mutant, can be a grade 2, grade 3, or grade 4 tumor, based on whether there are other genetic and tumor features, including a high rate of cell division (called the mitotic index) and alterations in the CDKN2A/B genes. These were previously called IDH -mutant or secondary glioblastomas.
Glioblastoma is now only used to describe IDH wild-type tumors that also have 1 or more of the following features: loss of chromosome 10, gain of chromosome 7, TERT promoter mutation, and increased number of copies of the EGFR gene. Unlike the previous classification system, glioblastomas are do not also have to show signs of cell death and excessive growth of blood vessels.
Oligodendroglioma. Oligodendroglioma is a tumor whose cells look like glial cells called oligodendrocytes. These cells are responsible for making myelin. Myelin surrounds the nerves and is rich in protein and fatty substances called lipids. Under the 2021 WHO guidelines, these tumors must have an IDH mutation and contain a chromosome 1p and 19q codeletion. They are categorized as either grade 2 oligodendroglioma, which is considered low grade, or grade 3 oligodendroglioma, which is considered a high-grade tumor with anaplastic features.
Ependymoma. Ependymoma commonly begins in the passageways in the brain where CSF is made and stored. In adults, they occur more often in the spine and can also be of the myxopapillary subtype. Learn about ependymoma in children .
Diffuse midline glioma. A diffuse midline glioma begins in the glial cells in the brain stem, spinal cord, and other midline structures within the CNS. Learn about brain stem glioma in children .
Non-glioma tumors
Non-glioma tumors are tumors that arise from cells in the brain that are not glial cells. Types of non-glioma tumors include:
Meningioma. Meningioma is the most common primary brain tumor. It begins in the meninges and is most often noncancerous. Meningioma can cause serious symptoms if it grows and presses on the brain or spinal cord or grows into the brain tissue. Learn more about meningioma .
Pineal gland and pituitary gland tumors. These are tumors that start in the pineal gland and pituitary gland .
Primary CNS lymphoma . This is a form of lymphoma . Lymphoma is a cancer that begins in the lymphatic system. Primary CNS lymphoma starts in the brain and can spread to the spinal fluid and eyes.
Medulloblastoma. Medulloblastoma is thought to start from a specific type of cell in the cerebellum. These cells are called cerebellar granule progenitor cells. It is most common in children and is usually cancerous, often spreading throughout the CNS. Learn about medulloblastoma in children .
Craniopharyngioma. Craniopharyngioma is a benign tumor that begins near the pituitary gland located near the base of the brain. These tumors are uncommon. Learn about craniopharyngioma in children .
Schwannoma. Schwannoma is a rare tumor that begins in the nerve sheath, or the lining of the nerves. It may often occur in the vestibular nerve, which is a nerve in the inner ear that helps control balance. It is typically noncancerous.
For a complete list of all types of brain tumors and how often they are diagnosed, the website of the Central Brain Tumor Registry of the United States offers this information in its reports. (Please note that this link takes you to another organization's website.)
Looking for More of an Introduction?
If you would like more of an introduction, explore these related items. Please note that these links will take you to other sections on Cancer.Net:
Cancer.Net En Español: Read about brain tumors in Spanish. Infórmase sobre tumor cerebral en español.
Find a Doctor. Search for a specialist in your local area using this free database of doctors from the American Society of Clinical Oncology (ASCO).
Cancer Terms. Learn what medical phrases and terms used in cancer care and treatment mean.
The next section in this guide is Medical Illustrations . It offers drawings of body parts often affected by a brain tumor. Use the menu to choose a different section to read in this guide.
Brain Tumor Guide
Cancer.Net Guide Brain Tumor
- Introduction
- Medical Illustrations
- Risk Factors
- Symptoms and Signs
- Grades and Prognostic Factors
- Types of Treatment
- About Clinical Trials
- Latest Research
- Coping with Treatment
- Follow-Up Care
- Questions to Ask the Health Care Team
- Additional Resources
View All Pages
Timely. Trusted. Compassionate.
Comprehensive information for people with cancer, families, and caregivers, from the American Society of Clinical Oncology (ASCO), the voice of the world's oncology professionals.
Find a Cancer Doctor
- Patient Care & Health Information
- Diseases & Conditions
- Brain tumor
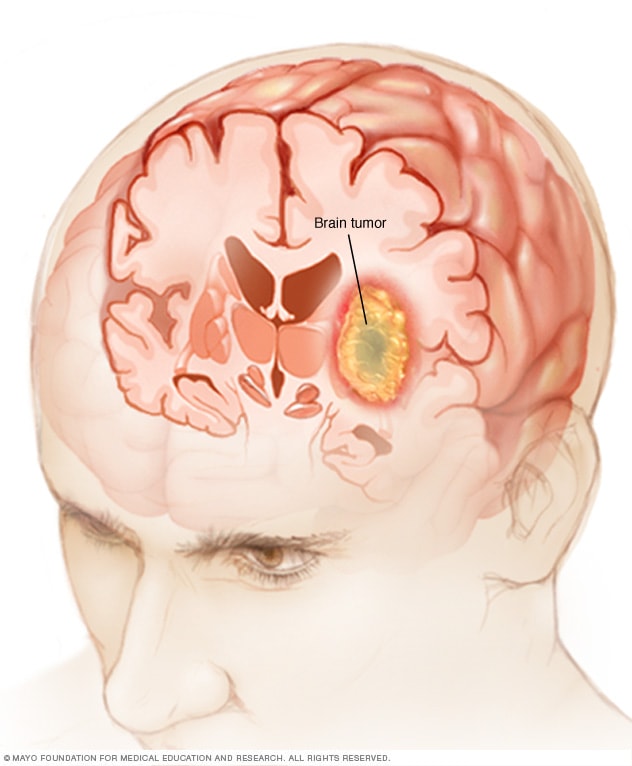
A brain tumor can form in the brain cells (as shown), or it can begin elsewhere and spread to the brain. As the tumor grows, it creates pressure on and changes the function of surrounding brain tissue, which causes signs and symptoms such as headaches, nausea and balance problems.
A brain tumor is a growth of cells in the brain or near it. Brain tumors can happen in the brain tissue. Brain tumors also can happen near the brain tissue. Nearby locations include nerves, the pituitary gland, the pineal gland, and the membranes that cover the surface of the brain.
Brain tumors can begin in the brain. These are called primary brain tumors. Sometimes, cancer spreads to the brain from other parts of the body. These tumors are secondary brain tumors, also called metastatic brain tumors.
Many different types of primary brain tumors exist. Some brain tumors aren't cancerous. These are called noncancerous brain tumors or benign brain tumors. Noncancerous brain tumors may grow over time and press on the brain tissue. Other brain tumors are brain cancers, also called malignant brain tumors. Brain cancers may grow quickly. The cancer cells can invade and destroy the brain tissue.
Brain tumors range in size from very small to very large. Some brain tumors are found when they are very small because they cause symptoms that you notice right away. Other brain tumors grow very large before they're found. Some parts of the brain are less active than others. If a brain tumor starts in a part of the brain that's less active, it might not cause symptoms right away. The brain tumor size could become quite large before the tumor is detected.
Brain tumor treatment options depend on the type of brain tumor you have, as well as its size and location. Common treatments include surgery and radiation therapy.
There are many types of brain tumors. The type of brain tumor is based on the kind of cells that make up the tumor. Special lab tests on the tumor cells can give information about the cells. Your health care team uses this information to figure out the type of brain tumor.
Some types of brain tumors usually aren't cancerous. These are called noncancerous brain tumors or benign brain tumors. Some types of brain tumors usually are cancerous. These types are called brain cancers or malignant brain tumors. Some brain tumor types can be benign or malignant.
Benign brain tumors tend to be slow-growing brain tumors. Malignant brain tumors tend to be fast-growing brain tumors.
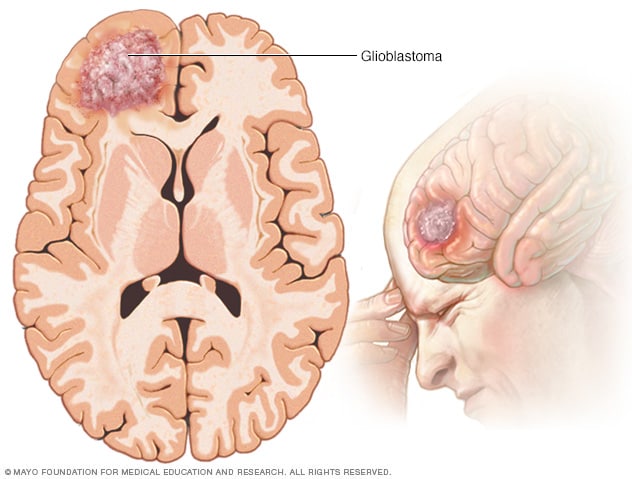
Glioblastoma
Glioblastoma is a type of cancer that starts in cells called astrocytes that support nerve cells. It can form in the brain or spinal cord.
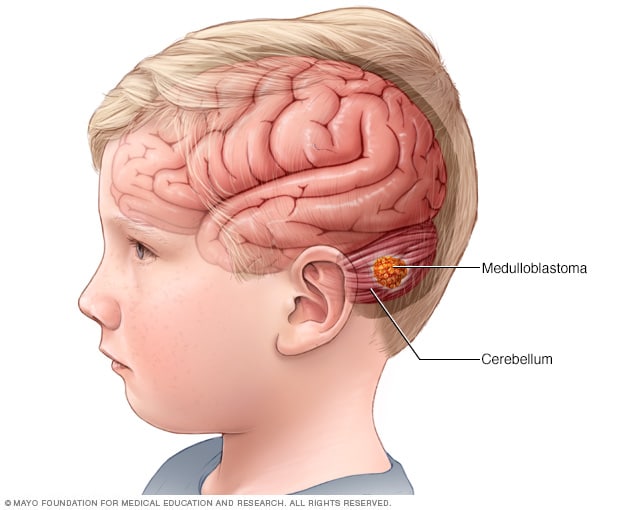
Medulloblastoma
Medulloblastoma is a type of brain cancer that starts in the part of the brain called the cerebellum. Medulloblastoma is the most common type of cancerous brain tumor in children.
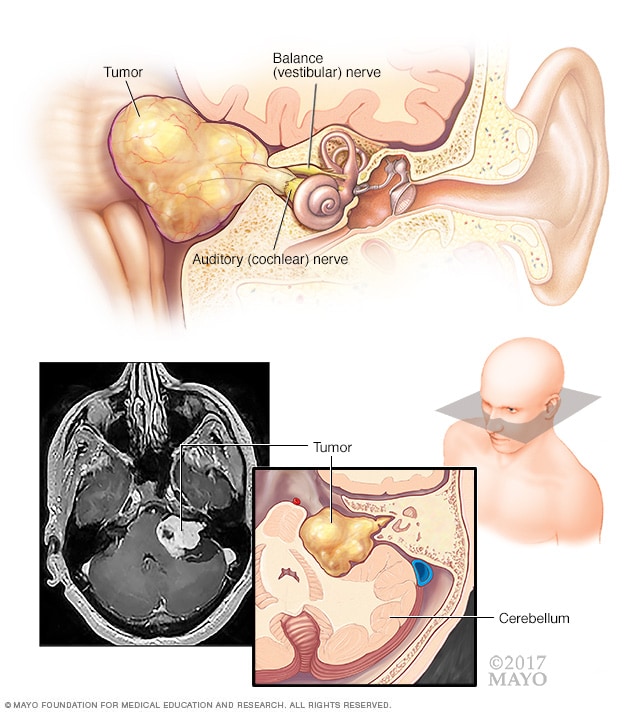
Acoustic neuroma (vestibular schwannoma)
An acoustic neuroma (vestibular schwannoma) is a benign tumor that develops on the balance and hearing nerves leading from the inner ear to the brain. These nerves are twined together to form the vestibulocochlear nerve (eighth cranial nerve). The pressure on the nerve from the tumor may cause hearing loss and imbalance.
Types of brain tumors include:
- Gliomas and related brain tumors. Gliomas are growths of cells that look like glial cells. The glial cells surround and support nerve cells in the brain tissue. Types of gliomas and related brain tumors include astrocytoma , glioblastoma , oligodendroglioma and ependymoma . Gliomas can be benign, but most are malignant. Glioblastoma is the most common type of malignant brain tumor.
- Choroid plexus tumors. Choroid plexus tumors start in cells that make the fluid that surrounds the brain and spinal cord. This fluid is called cerebrospinal fluid. Choroid plexus tumors are located in the fluid-filled cavities in the brain, called the ventricles. Choroid plexus tumors can be benign or malignant. Choroid plexus carcinoma is the malignant form of this type of brain tumor. It's more common in children.
- Embryonal tumors. Embryonal tumors begin in cells that are left over from fetal development. The cells, called embryonal cells, stay in the brain after birth. Embryonal tumors are malignant brain tumors that happen most often in babies and young children. The most common type of embryonal tumor is medulloblastoma . It's usually located in the lower back part of the brain, called the cerebellum.
- Germ cell tumors. Germ cell tumors start in reproductive cells, called germ cells, that go on to become the sperm and egg cells. Germ cells are mostly in the ovaries and testicles. But sometimes they're in other parts of the body, including the brain. When germ cell tumors happen in the brain, they're often located near the pineal gland or the pituitary gland. Germ cell tumors are mostly benign. They're more common in children.
- Pineal tumors. Pineal tumors start in and around the brain's pineal gland. The pineal gland is located in the center of the brain. It makes a hormone called melatonin that helps with sleep. Pineal tumors can be benign or malignant. Pineoblastoma is a malignant type of pineal tumor that's most common in children.
- Meningiomas. Meningiomas are brain tumors that start in the membranes around the brain and spinal cord. Meningiomas are usually benign, but sometimes they can be malignant. Meningiomas are the most common type of benign brain tumor.
- Nerve tumors. Nerve tumors are growths that happen in and around nerves. The most common type that happens in the head is acoustic neuroma , also called schwannoma. This benign tumor is located on the main nerve that connects the inner ear to the brain.
- Pituitary tumors. Brain tumors can begin in and around the pituitary gland. This small gland is located near the base of the brain. Most tumors that happen in and around the pituitary gland are benign. Pituitary tumors happen in the pituitary gland itself. Craniopharyngioma is a type of brain tumor that happens near the pituitary gland.
- Other brain tumors. Many other types of rare tumors can happen in and around the brain. Tumors can start in the muscles, blood vessels and connective tissue around the brain. Tumors can form in the bones of the skull. Malignant brain tumors can start from the germ-fighting immune system cells in the brain. This type of brain cancer is called primary central nervous system lymphoma.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
The signs and symptoms of a brain tumor depend on the brain tumor's size and location. Symptoms also might depend on how fast the brain tumor is growing, which is also called the tumor grade.
General signs and symptoms caused by brain tumors may include:
- Headache or pressure in the head that is worse in the morning.
- Headaches that happen more often and seem more severe.
- Headaches that are sometimes described as tension headaches or migraines.
- Nausea or vomiting.
- Eye problems, such as blurry vision, seeing double or losing sight on the sides of your vision.
- Losing feeling or movement in an arm or a leg.
- Trouble with balance.
- Speech problems.
- Feeling very tired.
- Confusion in everyday matters.
- Memory problems.
- Having trouble following simple commands.
- Personality or behavior changes.
- Seizures, especially if there is no history of seizures.
- Hearing problems.
- Dizziness or a sense that the world is spinning, also called vertigo.
- Feeling very hungry and gaining weight.
Brain tumors that aren't cancerous tend to cause symptoms that develop slowly. Noncancerous brain tumors also are called benign brain tumors. They might cause subtle symptoms that you don't notice at first. The symptoms might get worse over months or years.
Cancerous brain tumors cause symptoms that get worse quickly. Cancerous brain tumors also are called brain cancers or malignant brain tumors. They cause symptoms that come on suddenly. They get worse in a matter of days or weeks.
Brain tumor headaches
Headaches are the most common symptom of brain tumors. Headaches happen in about half of people with brain tumors. Headaches can happen if a growing brain tumor presses on healthy cells around it. Or a brain tumor can cause swelling in the brain that increases pressure in the head and leads to a headache.
Headache pain caused by brain tumors is often worse when you wake up in the morning. But it can happen at any time. Some people have headaches that wake them from sleep. Brain tumor headaches tend to cause pain that's worse when coughing or straining. People with brain tumors most often report that the headache feels like a tension headache. Some people say the headache feels like a migraine.
Brain tumors in the back of the head might cause a headache with neck pain. If the brain tumor happens in the front of the head, the headache might feel like eye pain or sinus pain.
Brain tumor symptoms by location
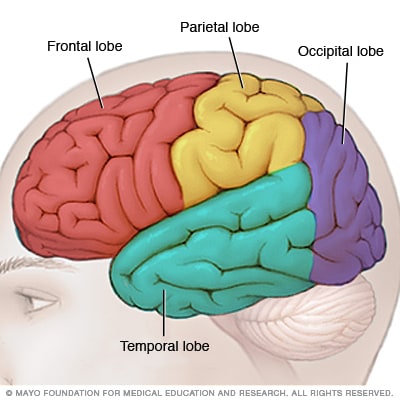
Brain lobes
Each side of your brain contains four lobes. The frontal lobe is important for cognitive functions and control of voluntary movement or activity. The parietal lobe processes information about temperature, taste, touch and movement, while the occipital lobe is primarily responsible for vision. The temporal lobe processes memories, integrating them with sensations of taste, sound, sight and touch.
The main part of the brain is called the cerebrum. Brain tumors in different parts of the cerebrum might cause different symptoms.
- Brain tumors in the front of the brain. The frontal lobes are in the front of the brain. They control thinking and movement. Frontal lobe brain tumors might cause balance problems and trouble walking. There might be personality changes, such as forgetfulness and lack of interest in usual activities. Sometimes family members notice that the person with the brain tumor seems different.
- Brain tumors in the middle of the brain. The parietal lobes are in the upper middle part of the brain. They help process information about touch, taste, smell, vision and hearing. Parietal lobe brain tumors can cause problems related to the senses. Examples include vision problems and hearing problems.
- Brain tumors in the back of the brain. The occipital lobes are in the back of the brain. They control vision. Occipital lobe brain tumors can cause vision loss.
- Brain tumors in the lower part of the brain. The temporal lobes are on the sides of the brain. They process memories and senses. Temporal lobe brain tumors can cause memory problems. They might cause someone to see, taste or smell something that isn't there. Sometimes the taste or smell is unpleasant or unusual.
When to see a doctor
Make an appointment with your health care provider if you have persistent signs and symptoms that worry you.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get the latest brain tumor advice from Mayo Clinic delivered in your inbox.
Sign up for free and receive the latest on brain tumor treatment, diagnosis and surgery.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
You will receive the first brain tumor email in your inbox shortly, which will include information on treatment, diagnosis, surgery and how brain cancer teams at Mayo Clinic approach personalized care.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Brain tumors that begin in the brain
Brain tumors that start as a growth of cells in the brain are called primary brain tumors. They might start right in the brain or in the tissue nearby. Nearby tissue might include the membranes that cover the brain, called meninges. Brain tumors also can happen in nerves, the pituitary gland and the pineal gland.
Brain tumors happen when cells in or near the brain get changes in their DNA. A cell's DNA holds the instructions that tell the cell what to do. The changes tell the cells to grow quickly and continue living when healthy cells would die as part of their natural life cycle. This makes a lot of extra cells in the brain. The cells can form a growth called a tumor.
It's not clear what causes the DNA changes that lead to brain tumors. For many people with brain tumors, the cause is never known. Sometimes parents pass DNA changes to their children. The changes can increase the risk of having a brain tumor. These hereditary brain tumors are rare. If you have a family history of brain tumors, talk about it with your health care provider. You might consider meeting with a health care provider trained in genetics to understand whether your family history increases your risk of having a brain tumor.
When brain tumors happen in children, they're likely to be primary brain tumors. In adults, brain tumors are more likely to be cancer that started somewhere else and spread to the brain.
Cancer that spreads to the brain
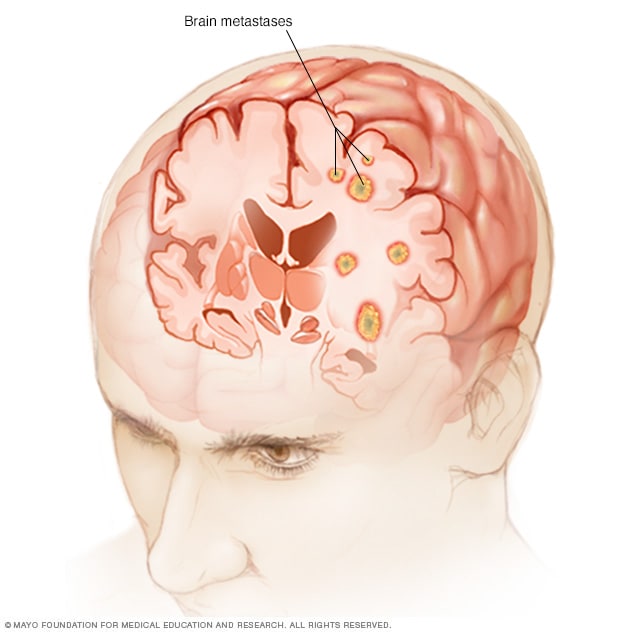
Brain metastases
Brain metastases happen when cancer begins elsewhere in the body and spreads (metastasizes) to the brain.
Secondary brain tumors happen when cancer starts somewhere else and spreads to the brain. When cancer spreads, it's called metastatic cancer.
Any cancer can spread to the brain, but common types include:
- Breast cancer.
- Colon cancer.
- Kidney cancer.
- Lung cancer.
It's not clear why some cancers spread to the brain and others are more likely to spread to other places.
Secondary brain tumors most often happen in people who have a history of cancer. Rarely, a brain tumor may be the first sign of cancer that began somewhere else in the body.
In adults, secondary brain tumors are far more common than are primary brain tumors.
Risk factors
In most people with primary brain tumors, the cause isn't clear. But doctors have identified some factors that may raise the risk.
Risk factors include:
- Age. Brain tumors can happen at any age, but they happen most often in older adults. Some brain tumors mostly affect adults. Some brain tumors happen most often in children.
- Race. Anyone can get a brain tumor. But some types of brain tumors are more common in people of certain races. For example, gliomas are more common in white people. Meningiomas are more common in Black people.
Exposure to radiation. People who have been exposed to a strong type of radiation have an increased risk of brain tumor. This strong radiation is called ionizing radiation. The radiation is strong enough to cause DNA changes in the body's cells. The DNA changes can lead to tumors and cancers. Examples of ionizing radiation include radiation therapy used to treat cancer and radiation exposure caused by atomic bombs.
Low-level radiation from everyday objects isn't linked to brain tumors. Low levels of radiation include the energy that comes from cellphones and radio waves. There is no convincing evidence that using cellphones causes brain tumors. But more studies are happening to make sure.
- Inherited syndromes that increase the risk of brain tumor. Some DNA changes that increase the risk of brain tumor run in families. Examples include the DNA changes that cause neurofibromatosis 1 and 2, tuberous sclerosis, Lynch syndrome, Li-Fraumeni syndrome, Von Hippel-Lindau disease, familial adenomatous polyposis, Cowden syndrome, and Gorlin syndrome.
There's no way to prevent brain tumors. If you get a brain tumor, you didn't do anything to cause it.
People with an increased risk of brain tumor might consider screening tests. Screening isn't brain tumor prevention. But screening might help find a brain tumor when it's small and treatment is more likely to be successful.
If you have a family history of brain tumor or inherited syndromes that increase the risk of brain tumor, talk about it with your health care provider. You might consider meeting with a genetic counselor or other health care provider trained in genetics. This person can help you understand your risk and ways to manage it. For example, you might consider brain tumor screening tests. Testing might include an imaging test or a neurological exam to test your vision, hearing, balance, coordination and reflexes.
Brain tumor care at Mayo Clinic
- Niederhuber JE, et al., eds. Cancer of the central nervous system. In: Abeloff's Clinical Oncology. 6th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Sept. 27, 2022.
- Adult central nervous system tumors treatment (PDQ) — Patient version. National Cancer Institute. https://www.cancer.gov/types/brain/patient/adult-brain-treatment-pdq. Accessed Sept. 27, 2022.
- Brain tumor. Cancer.Net. https://www.cancer.net/cancer-types/brain-tumor/view-all. Accessed Nov. 1, 2022.
- Louis DN, et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncology. 2021; doi:10.1093/neuonc/noab106.
- Chheda MG, et al. Uncommon brain tumors. https://www.uptodate.com/contents/search. Accessed Nov. 10, 2022.
- Childhood medulloblastoma and other central nervous system embryonal tumors treatment (PDQ) — Patient version. National Cancer Institute. https://www.cancer.gov/types/brain/patient/child-cns-embryonal-treatment-pdq. Accessed Nov. 15, 2022.
- Childhood central nervous system germ cell tumors treatment (PDQ) — Patient version. National Cancer Institute. https://www.cancer.gov/types/brain/patient/child-cns-germ-cell-treatment-pdq. Accessed Nov. 15, 2022.
- Ostrom QT, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro-Oncology. 2022; doi:10.1093/neuonc/noac202.
- Winn HR, ed. Youmans and Winn Neurological Surgery. 8th ed. Elsevier; 2023. https://www.clinicalkey.com. Accessed Sept. 27, 2022.
- Wong ET, et al. Overview of the clinical features and diagnosis of brain tumors in adults. https://www.uptodate.com/contents/search. Accessed Sept. 27, 2022.
- Edlow JA, et al. Medical and nonstroke neurological causes of acute, continuous vestibular symptoms. Neurology Clinics. 2015; doi:10.1016/j.ncl.2015.04.002.
- Cellphones and cancer risk. National Cancer Institute. https://www.cancer.gov/about-cancer/causes-prevention/risk/radiation/cell-phones-fact-sheet. Accessed Oct. 21, 2022.
- Central nervous system cancers. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1425. Oct. 28, 2022.
- Stereotactic radiosurgery (SRS) and stereotactic body radiotherapy (SBRT). RadiologyInfo.org. https://www.radiologyinfo.org/en/info/stereotactic. Nov. 4, 2022.
- Distress management. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1431. Accessed Sept. 27, 2022.
- Muthupillai R, et al. Magnetic resonance elastography. Nature Medicine. 1996; doi:10.1038/nm0596-601.
- Murphy MC, et al. MR elastography of the brain and its application in neurological diseases. NeuroImage. 2019l doi:10.1016/j.neuroimage.2017.10.008.
- Warner KJ. Allscripts EPSi. Mayo Clinic. Jan. 7, 2021.
- Member institutions. Alliance for Clinical Trials in Oncology. https://www.allianceforclinicaltrialsinoncology.org/main/public/standard.xhtml?path=/Public/Institutions. Accessed Nov. 30, 2022.
- Genetic/familial high-risk assessment: Breast, ovarian and pancreatic. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1503. Accessed Nov. 30, 2022.
- Lynch syndrome management. AskMayoExpert. 2021.
- Brain tumor FAQs
- Living with Brain Tumors
- Long Term Brain Cancer Survivor
- Punk Guitarist Survives Brain Tumor
- What is a brain tumor? A Mayo Clinic expert explains
Associated Procedures
- Ablation therapy
- Acupuncture
- Brain stereotactic radiosurgery
- Chemotherapy
- Needle biopsy
- Positron emission tomography scan
- Radiation therapy
- Stereotactic radiosurgery
News from Mayo Clinic
- Mayo Clinic Minute: Learn about meningioma and glioblastoma brain tumors Aug. 07, 2023, 02:00 p.m. CDT
- Against the odds Sept. 29, 2022, 11:00 a.m. CDT
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Phoenix/Scottsdale, Arizona, and Mayo Clinic in Jacksonville, Florida, have been ranked among the best Neurology & Neurosurgery hospitals in the nation for 2023-2024 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Let’s celebrate our doctors!
Join us in celebrating and honoring Mayo Clinic physicians on March 30th for National Doctor’s Day.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 20 December 2019
Focusing on brain tumours and brain metastasis
Nature Reviews Cancer volume 20 , page 1 ( 2020 ) Cite this article
11k Accesses
11 Citations
34 Altmetric
Metrics details
This Focus issue highlights current research into the unique biology of brain tumours and brain metastasis and how this research might improve therapy of these often devastating diseases.
Survival for many types of malignant primary brain tumours has not improved much in the past 10 years, despite the introduction of some new treatments and despite our improved understanding of the biological bases of brain tumour development 1 , 2 . In addition, most malignant brain lesions are actually secondary brain tumours (brain metastases), and it is estimated that brain metastases will develop in up to 30% of adults who have a malignant primary tumour at another site 2 , 3 . Furthermore, brain tumours are the most common type of solid tumour in children and are the leading cause of cancer-related deaths in this population 1 , 4 . These statistics all indicate that better treatments for brain tumours and brain metastasis are a pressing need. We have therefore put together this Focus issue to highlight the diverse research in this field and the unique challenges posed by brain tumours and brain metastases.
Brain tumours are a heterogeneous group of diseases, but one important common feature is that they are subject to the unique biology of the brain and its microenvironment. The brain contains many cell types that are distinct from those found elsewhere in the body, making it challenging to extrapolate findings from cancers arising in other organs to those arising in the brain. Furthermore, the anatomy of the brain presents challenges for treating both brain tumours and brain metastases.
A prime example of both the unique biology and the anatomical challenges of treating brain tumours is the blood–brain barrier (BBB), the neurovascular unit that maintains brain homeostasis and acts as a ‘gatekeeper’, controlling the crossing of molecules and cells from the blood into the brain. Although the BBB is often disrupted in brain tumours, effective delivery of anticancer therapeutics through this blood–tumour barrier remains a challenge, as addressed by Arvanitis et al. 5 .
One aspect of the brain microenvironment that might not be as unique as initially presumed is the immune environment. The immune cell types of the brain differ from those in other organs, but, as discussed by Sampson et al. 6 , it is now becoming clear that this organ is not as ‘immune privileged’ as once thought, leading to hope that brain tumours and metastases might be successfully targeted with immunotherapies.
despite the challenges presented by brain tumours, progress is being made on many different fronts against these often devastating diseases
Gliomas account for ~80% of malignant brain tumours, and the highest grade glioma, glioblastoma, is one of the most lethal cancers in adults 3 . Interestingly, genomic sequencing efforts more than 10 years ago jump-started the field of glioma metabolism with their finding of recurrent mutations in the genes encoding the tricarboxylic acid cycle enzymes IDH1 and IDH2, but the role of metabolism in glioma pathogenesis goes beyond IDH, as discussed by Bi et al. 7 .
Medulloblastoma is one of the most common paediatric brain tumours 4 . Our understanding of this disease was advanced substantially by genomic studies reported in 2012. Since then, as discussed by Hovestadt et al. 8 , more genomic studies, as well as epigenomic, transcriptomic and proteomic profiling efforts, have provided new insights into medulloblastoma biology that will hopefully lead to improved diagnosis and therapy.
The prevalence of brain metastases in adults raises the question of what those working on primary brain tumours can learn from research on brain metastasis, and vice versa. This, and other important questions on brain metastasis, is pondered in a Viewpoint article written by four leading experts in this field 9 .
What has emerged from this collection of articles is that despite the challenges presented by brain tumours, progress is being made on many different fronts against these often devastating diseases, which will hopefully lead to improvements in survival in the next 10 years, if not sooner.
Jones, D. T. W. et al. Molecular characteristics and therapeutic vulnerabilities across paediatric solid tumours. Nat. Rev. Cancer 19 , 420–438 (2019).
Article CAS Google Scholar
Lapointe, S. et al. Primary brain tumours in adults. Lancet 392 , 432–446 (2018).
Article Google Scholar
Weller, M. et al. Glioma. Nat. Rev. Dis. Primers 1 , 15017 (2015).
Liu, K. W. et al. Molecular mechanisms and therapeutic targets in pediatric brain tumors. Sci. Signal. 10 , eaaf7593 (2017).
Arvanitis, C. D. et al. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer https://doi.org/10.1038/s41568-019-0205-x (2019).
Article PubMed Google Scholar
Sampson, J. H. et al. Brain immunology and immunotherapy in brain tumours. Nat. Rev. Cancer https://doi.org/10.1038/s41568-019-0224-7 (2019).
Bi, J. et al. Altered cellular metabolism in gliomas — an emerging landscape of actionable co-dependency targets. Nat. Rev. Cancer https://doi.org/10.1038/s41568-019-0226-5 (2019).
Hovestadt, V. et al. Medulloblastomics revisited: biological and clinical insights from thousands of patients. Nat. Rev. Cancer https://doi.org/10.1038/s41568-019-0223-8 (2019).
Boire, A. et al. Brain metastasis. Nat. Rev. Cancer https://doi.org/10.1038/s41568-019-0220-y (2019).
Download references
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Focusing on brain tumours and brain metastasis. Nat Rev Cancer 20 , 1 (2020). https://doi.org/10.1038/s41568-019-0232-7
Download citation
Published : 20 December 2019
Issue Date : January 2020
DOI : https://doi.org/10.1038/s41568-019-0232-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Ultra-efficient mcf-7 cell ablation and chemotherapy-integrated electrothermal therapy with dox–ws2–peg–m13 nanostructures.
- Fitya S. Mozar
- Maria P. Meivita
- Desmond K. Loke
Discover Materials (2024)
Biology of breast cancer brain metastases and novel therapies targeting the blood brain barrier: an updated review
- Hongfang Zhao
- Luxuan Wang
Medical Oncology (2023)
Effect of COVID-19 on patient access to health services for noncommunicable diseases in Latin America: a perspective from patient advocacy organizations
- Meredith H. Kruse
- Alessandra Durstine
- Dabney P. Evans
International Journal for Equity in Health (2022)
Impact of COVID-19 crisis on medical care of patients with metastasized uro-oncologic disease under systemic cancer therapy: a multicenter study in German university hospitals
- Julian P. Struck
- Maike Schnoor
- Axel S. Merseburger
World Journal of Urology (2022)
Integrating deep learning and unbiased automated high-content screening to identify complex disease signatures in human fibroblasts
- Lauren Schiff
- Bianca Migliori
- Bjarki Johannesson
Nature Communications (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
NOTICE MyAANS, password-protected resources, and purchases are currently experiencing issues and are unavailable. We are working to get this fixed as soon as possible.

The site navigation utilizes arrow, enter, escape, and space bar key commands. Left and right arrows move across top level links and expand / close menus in sub levels. Up and Down arrows will open main level menus and toggle through sub tier links. Enter and space open menus and escape closes them as well. Tab will move on to the next part of the site rather than go through menu items.
Brain Tumors
Types of brain tumors.
A brain tumor, known as an intracranial tumor, is an abnormal mass of tissue in which cells grow and multiply uncontrollably, seemingly unchecked by the mechanisms that control normal cells. More than 150 different brain tumors have been documented, but the two main groups of brain tumors are termed primary and metastatic .
Primary brain tumors include tumors that originate from the tissues of the brain or the brain's immediate surroundings. Primary tumors are categorized as glial (composed of glial cells ) or non-glial (developed on or in the structures of the brain, including nerves, blood vessels and glands) and benign or malignant .
Metastatic brain tumors include tumors that arise elsewhere in the body (such as the breast or lungs) and migrate to the brain, usually through the bloodstream. Metastatic tumors are considered cancer and are malignant.
Metastatic tumors to the brain affect nearly one in four patients with cancer, or an estimated 150,000 people a year. Up to 40 percent of people with lung cancer will develop metastatic brain tumors. In the past, the outcome for patients diagnosed with these tumors was very poor, with typical survival rates of just several weeks. More sophisticated diagnostic tools, in addition to innovative surgical and radiation approaches, have helped survival rates expand up to years; and also allowed for an improved quality of life for patients following diagnosis.
Types of Benign Brain Tumors
- Chordomas are benign, slow-growing tumors that are most prevalent in people ages 50 to 60. Their most common locations are the base of the skull and the lower portion of the spine. Although these tumors are benign, they may invade the adjacent bone and put pressure on nearby neural tissue. These are rare tumors, contributing to only 0.2 percent of all primary brain tumors.
- Craniopharyngiomas typically are benign, but are difficult tumors to remove because of their location near critical structures deep in the brain. They usually arise from a portion of the pituitary gland (the structure that regulates many hormones in the body), so nearly all patients will require some hormone replacement therapy .
- Gangliocytomas , gangliomas and anaplastic gangliogliomas are rare tumors that include neoplastic nerve cells that are relatively well-differentiated, occurring primarily in young adults.
- Glomus jugulare tumors most frequently are benign and typically are located just under the skull base, at the top of the jugular vein. They are the most common form of glomus tumor . However, glomus tumors, in general, contribute to only 0.6 percent of neoplasms of the head and neck.
- Meningiomas are the most common benign intracranial tumors, comprising 10 to 15 percent of all brain neoplasms, although a very small percentage are malignant. These tumors originate from the meninges , the membrane-like structures that surround the brain and spinal cord.
- Pineocytomas are generally benign lesions that arise from the pineal cells , occurring predominantly in adults. They are most often well-defined, noninvasive, homogeneous and slow-growing.
- Pituitary adenomas are the most common intracranial tumors after gliomas, meningiomas and schwannomas. The large majority of pituitary adenomas are benign and fairly slow-growing. Even malignant pituitary tumors rarely spread to other parts of the body. Adenomas are by far the most common disease affecting the pituitary. They commonly affect people in their 30s or 40s, although they are diagnosed in children, as well. Most of these tumors can be treated successfully.
- Schwannomas are common benign brain tumors in adults. They arise along nerves, comprised of cells that normally provide the "electrical insulation" for the nerve cells. Schwannomas often displace the remainder of the normal nerve instead of invading it. Acoustic neuromas are the most common schwannoma, arising from the eighth cranial nerve, or vestibularcochlear nerve , which travels from the brain to the ear. Although these tumors are benign, they can cause serious complications and even death if they grow and exert pressure on nerves and eventually on the brain. Other locations include the spine and, more rarely, along nerves that go to the limbs.
Types of Malignant Brain Tumors
Gliomas are the most prevalent type of adult brain tumor, accounting for 78 percent of malignant brain tumors. They arise from the supporting cells of the brain, called the glia . These cells are subdivided into astrocytes , ependymal cells and oligodendroglial cells (or oligos). Glial tumors include the following:
- Astrocytomas are the most common glioma, accounting for about half of all primary brain and spinal cord tumors. Astrocytomas develop from star-shaped glial cells called astrocytes, part of the supportive tissue of the brain. They may occur in many parts of the brain, but most commonly in the cerebrum. People of all ages can develop astrocytomas, but they are more prevalent in adults — particularly middle-aged men. Astrocytomas in the base of the brain are more prevalent in children or younger people and account for the majority of children's brain tumors. In children, most of these tumors are considered low-grade, while in adults, most are high-grade.
- Ependymomas are derived from a neoplastic transformation of the ependymal cells lining the ventricular system and account for two to three percent of all brain tumors. Most are well-defined, but some are not.
- Glioblastoma multiforme (GBM) is the most invasive type of glial tumor. These tumors tend to grow rapidly, spread to other tissue and have a poor prognosis. They may be composed of several different kinds of cells, such as astrocytes and oligodendrocytes. GBM is more common in people ages 50 to 70 and are more prevalent in men than women.
- Medulloblastomas usually arise in the cerebellum , most frequently in children. They are high-grade tumors, but they are usually responsive to radiation and chemotherapy .
- Oligodendrogliomas are derived from the cells that make myelin , which is the insulation for the wiring of the brain.
Other Types of Brain Tumors
- Hemangioblastomas are slow-growing tumors, commonly located in the cerebellum. They originate from blood vessels, can be large in size and often are accompanied by a cyst. These tumors are most common in people ages 40 to 60 and are more prevalent in men than women.
- Rhabdoid tumors are rare, highly aggressive tumors that tend to spread throughout the central nervous system. They often appear in multiple sites in the body, especially in the kidneys. They are more prevalent in young children, but also can occur in adults.
Pediatric Brain Tumors
Brain tumors in children typically come from different tissues than those affecting adults. Treatments that are fairly well-tolerated by the adult brain (such as radiation therapy) may prevent normal development of a child's brain, especially in children younger than age five.
According to the Pediatric Brain Tumor Foundation , approximately 4,200 children are diagnosed with a brain tumor in the U.S. Seventy-two percent of children diagnosed with a brain tumor are younger than age 15. Most of these brain tumors grow in the posterior fossa (or back) of the brain. Children often present with hydrocephalus (fluid build up in the brain) or the face or body not working properly.
Some types of brain tumors are more common in children than in adults. The most common types of pediatric tumors are medulloblastomas, low-grade astrocytomas (pilocytic) , ependymomas, craniopharyngiomas and brainstem gliomas .
The World Health Organization (WHO) has developed a grading system to indicate a tumor's malignancy or benignity based on its histological features under a microscope.
- Most malignant
- Rapid growth, aggressive
- Widely infiltrative
- Rapid recurrence
- Necrosis prone
World Health Organization (WHO) Brain Tumor Grades
Incidence in adults.
The National Cancer Institute estimates that 22,910 adults (12,630 men and 10,280 women) will be diagnosed with brain and other nervous system tumors in 2012. It also estimates that in 2012, 13,700 of these diagnoses will result in death.
Between 2005 and 2009, the median age for death from cancer of the brain and other areas of the nervous system was age 64.
Brain Tumor Causes
Brain tumors are thought to arise when certain genes on the chromosomes of a cell are damaged and no longer function properly. These genes normally regulate the rate at which the cell divides (if it divides at all) and repair genes that fix defects of other genes, as well as genes that should cause the cell to self-destruct if the damage is beyond repair. In some cases, an individual may be born with partial defects in one or more of these genes. Environmental factors may then lead to further damage. In other cases, the environmental injury to the genes may be the only cause. It is not known why some people in an "environment" develop brain tumors, while others do not.
Once a cell is dividing rapidly and internal mechanisms to check its growth are damaged, the cell can eventually grow into a tumor. Another line of defense may be the body's immune system, which optimally would detect the abnormal cell and kill it. Tumors may produce substances that block the immune system from recognizing the abnormal tumor cells and eventually overpower all internal and external deterrents to its growth.
A rapidly growing tumor may need more oxygen and nutrients than can be provided by the local blood supply intended for normal tissue. Tumors can produce substances called angiogenesis factors that promote the growth of blood vessels. The new vessels that grow increase the supply of nutrients to the tumor, and, eventually, the tumor becomes dependent on these new vessels. Research is being done in this area, but more extensive research is necessary to translate this knowledge into potential therapies.
Symptoms vary depending on the location of the brain tumor, but the following may accompany different types of brain tumors:
- Headaches that may be more severe in the morning or awaken the patient at night
- Seizures or convulsions
- Difficulty thinking, speaking or articulating
- Personality changes
- Weakness or paralysis in one part or one side of the body
- Loss of balance or dizziness
- Vision changes
- Hearing changes
- Facial numbness or tingling
- Nausea or vomiting , swallowing difficulties
- Confusion and disorientation
Sophisticated imaging techniques can pinpoint brain tumors. Diagnostic tools include computed tomography (CT or CAT scan) and magnetic resonance imaging (MRI) . Other MRI sequences can help the surgeon plan the resection of the tumor based on the location of the normal nerve pathways of the brain. Intraoperative MRI also is used during surgery to guide tissue biopsies and tumor removal. Magnetic resonance spectroscopy (MRS) is used to examine the tumor's chemical profile and determine the nature of the lesions seen on the MRI. Positron emission tomography (PET scan) can help detect recurring brain tumors.
Sometimes the only way to make a definitive diagnosis of a brain tumor is through a biopsy. The neurosurgeon performs the biopsy and the pathologist makes the final diagnosis, determining whether the tumor appears benign or malignant, and grading it accordingly.
Brain Tumor Treatment
Brain tumors (whether primary or metastatic, benign or malignant) usually are treated with surgery, radiation, and/or chemotherapy — alone or in various combinations. While it is true that radiation and chemotherapy are used more often for malignant, residual or recurrent tumors, decisions as to what treatment to use are made on a case-by-case basis and depend on a number of factors. There are risks and side effects associated with each type of therapy.
It is generally accepted that complete or nearly complete surgical removal of a brain tumor is beneficial for a patient. The neurosurgeon's challenge is to remove as much tumor as possible, without injuring brain tissue important to the patient's neurological function (such as the ability to speak, walk, etc.). Traditionally, neurosurgeons open the skull through a craniotomy to insure they can access the tumor and remove as much of it as possible. A drain (EVD) may be left in the brain fluid cavities at the time of surgery to drain the normal brain fluid as the brain recovers from the surgery.
Another procedure that is commonly performed, sometimes before a craniotomy, is called a stereotactic biopsy . This smaller operation allows doctors to obtain tissue in order to make an accurate diagnosis. Usually, a frame is attached to the patient's head, a scan is obtained, and then the patient is taken to the operating area, where a small hole is drilled in the skull to allow access to the abnormal area. Based on the location of the lesion, some hospitals may do this same procedure without the use of a frame. A small sample is obtained for examination under the microscope.
In the early 1990s, computerized devices called surgical navigation systems were introduced. These systems assisted the neurosurgeon with guidance, localization and orientation for tumors. This information reduced the risks and improved the extent of tumor removal. In many cases, surgical navigation systems allowed previously inoperable tumors to be excised with acceptable risks. Some of these systems also can be used for biopsies without having to attach a frame to the skull. One limitation of these systems is that they utilize a scan (CT or MRI) obtained prior to surgery to guide the neurosurgeon. Thus, they cannot account for movements of the brain that may occur intraoperatively. Investigators are developing techniques using ultrasound and performing surgery in MRI scanners to help update the navigation system data during surgery.
Intraoperative language mapping is considered by some as a critically important technique for patients with tumors affecting language function, such as large, dominant-hemisphere gliomas. This procedure involves operating on a conscious patient and mapping the anatomy of their language function during the operation. The doctor then decides which portions of the tumor are safe to resect. Recent studies have determined that cortical language mapping may be used as a safe and efficient adjunct to optimize glioma resection while preserving essential language sites.
Ventriculoperitoneal shunting may be required for some patients with brain tumors. Everyone has cerebrospinal fluid (CSF) within the brain and spine that is slowly circulating all the time. If this flow becomes blocked, the sacs that contain the fluid (the ventricles) can become enlarged, creating increased pressure within the head, resulting in a condition called hydrocephalus. If left untreated, hydrocephalus can cause brain damage and even death. The neurosurgeon may decide to use a shunt to divert the spinal fluid away from the brain and, therefore, reduce the pressure. The body cavity in which the CSF is diverted usually is the peritoneal cavity (the area surrounding the abdominal organs). The shunt usually is permanent. If it becomes blocked, the symptoms are similar to that of the original condition of hydrocephalus and may include headaches, vomiting, visual problems and/or confusion or lethargy, among others. Another method that may be used to control obstruction of the brain fluid pathways is called an Endoscopic Third Ventriculostomy. This helps the brain fluid be diverted around the obstruction without the need for a shunt.
Radiation Therapy
Radiation therapy uses high-energy X-rays to kill cancer cells and abnormal brain cells and to shrink tumors. Radiation therapy may be an option if the tumor cannot be treated effectively through surgery.
- Standard External Beam Radiotherapy uses a variety of radiation beams to create a conformal coverage of the tumor while limiting the dose to surrounding normal structures. The risk of long-term radiation injury with modern delivery methods is very low. Newer techniques of delivery aside from 3-dimensional conformal radiotherapy (3DCRT) include intensity-modulated radiotherapy (IMRT) .
- Proton Beam Treatment employs a specific type of radiation in which protons, a form of radioactivity, are directed specifically to the tumor. The advantage is that less tissue surrounding the tumor incurs damage.
- Stereotactic Radiosurgery (such as Gamma Knife , Novalis and Cyberknife ) is a technique that focuses the radiation with many different beams on the target tissue. This treatment tends to incur less damage to tissues adjacent to the tumor. Currently, there is no data to suggest one delivery system is superior to another in terms of clinical outcome, and each has its advantages and disadvantages.
Chemotherapy
Chemotherapy generally is considered to be effective for specific pediatric tumors, lymphomas and some oligodendrogliomas. While it has been proven that chemotherapy improves overall survival in patients with the most malignant primary brain tumors, it does so in only in about 20 percent of all patients, and physicians cannot readily predict which patients will benefit before treatment. As such, some physicians choose not to use chemotherapy because of the potential side effects ( lung scarring , suppression of the immune system, nausea, etc.).
Chemotherapy works by inflicting cell damage that is better repaired by normal tissue than tumor tissue. Resistance to chemotherapy might involve survival of tumor tissue that cannot respond to the drug, or the inability of the drug to pass from the bloodstream into the brain. A special barrier exists between the bloodstream and the brain tissue called the blood-brain barrier . Some investigators have tried to improve the effect of chemotherapy by disrupting this barrier or by injecting the drug into the tumor or brain. The goal of another class of drugs is not to kill the tumor cells but, rather, to block further tumor growth. In some cases, growth modifiers (such as breast cancer treatment drug Tamoxifen ) have been used to attempt to stop the growth of tumors resistant to other treatments.
In 1996, the U.S. Food and Drug Administration approved the use of chemotherapy-impregnated wafers, which can be applied by the neurosurgeon at the time of surgery. The wafers slowly secrete the drug into the tumor, and the patient receives chemotherapy with the systemic side effects of treatment.
Laser Interstitial Thermal Therapy (LITT)
Laser Thermal Ablation is a newer technique that some centers are using to treat smaller tumors particularly in areas that may be more difficult to reach using previous open surgery procedures. This involves placing a tiny catheter within the lesion, possibly completing a biopsy, then using laser to thermally ablate the lesion. This technique is only more recently used in brain tumor treatments, therefore the long term efficacy has not been established.
Investigational Therapies
Many types of new therapies currently are being studied, especially on tumors for which the prognosis is generally poor through existing conventional therapies. It is unknown whether these therapies will work. Such therapies are given according to a protocol and include various forms of immunotherapy, therapy using targeted toxins, anti-angiogenesis therapy, gene therapy and differentiation therapy. Combinations of treatments also may be able to improve the outlook for patients, while lowering the adverse side effects.
The AANS does not endorse any treatments, procedures, products or physicians referenced in these patient fact sheets. This information is provided as an educational service and is not intended to serve as medical advice. Anyone seeking specific neurosurgical advice or assistance should consult his or her neurosurgeon, or locate one in your area through the AANS’ Find a Board-certified Neurosurgeon”online tool.
Read the Stories of a Patients Diagnosed with Brain Tumors
Support nref.
Make a difference in one minute. Text "Neurosurgery" to 41444 and follow the prompts to donate easily from your mobile device.

Support the NREF When You Shop
Register with iGive.com or AmazonSmile and designate the NREF as your charity.

How Does Brain Cancer Affect Everyday Life?

Living with brain cancer is a complex journey that can significantly influence various aspects of everyday life. No diagnosis or situation is the same, and because of this, what a patient’s day-to-day life looks like can vary greatly. Let’s dive into the most common ways brain cancer can impact individuals, emphasizing the challenges and adjustments that come with this diagnosis.
Contrary to popular belief, many individuals dealing with a brain tumor diagnosis can still maintain a good quality of life. Personalized supportive care and treatments, clear communication and collaboration with a great healthcare team, and a solid framework of emotional support from loved ones are key to living with this disease.
Navigating Emotional & Psychological Impact
Receiving a brain cancer diagnosis is an emotionally overwhelming experience. Individuals may face a range of emotions, from fear to sadness. Counseling and support groups become invaluable resources in helping patients and their loved ones navigate this emotional turmoil.
Everyone feels a different range of emotions in their own unique way when dealing with a diagnosis. However, here are the most common emotions and phases patients go through when hearing the shocking news.
- Shock: It is entirely normal to feel a profound sense of shock upon receiving a brain cancer diagnosis. The sudden and unexpected nature of such news can be overwhelming, leaving individuals grappling with disbelief and a sense of unreality.
- Denial: Denial often follows shock as a defense mechanism. It's a way for the mind to protect itself from the full impact of the diagnosis. Individuals may find it challenging to accept the reality of having brain cancer, leading to a temporary state of denial.
- Anger: The onset of anger is a common emotional response to a brain cancer diagnosis. Individuals may feel a sense of injustice, questioning why this is happening to them. Anger can serve as a natural outlet for the frustration and fear associated with the situation.
- Guilt: It's not uncommon for feelings of guilt to surface, particularly when individuals begin to question past choices or behaviors, even if unrelated to the diagnosis. It's essential to acknowledge that these feelings are a natural part of the emotional turmoil that accompanies a challenging diagnosis.
- Anxiety/Depression: The weight of a brain cancer diagnosis can trigger intense anxiety and periods of depression. Uncertainty about the future, the impact on loved ones, and the challenges of treatment can contribute to a range of complex emotions that may manifest as anxiety or depression.
- Acceptance: Acceptance is not an immediate response but rather a gradual process. It involves coming to terms with the diagnosis and understanding that it is part of one's life. Achieving acceptance doesn't negate the difficulty of the situation but allows individuals to move forward with a clearer mindset.
It's crucial to validate these emotions as completely normal reactions to the complexities of a brain cancer diagnosis. Each individual's journey is unique, and acknowledging and understanding these emotions is a vital step toward emotional well-being. Seeking support from healthcare professionals, loved ones, or support groups can provide invaluable assistance in navigating and processing these intense feelings.
Tips to Help Improve Mindset
Coping with the emotional stress of a brain cancer diagnosis requires a multifaceted approach that prioritizes mental well-being. Here are some practical tips to help individuals navigate and improve their mindset during this challenging journey:
- Mindful Meditation: Engage in mindful meditation as a tool to center and calm the mind. Mindfulness practices, such as focused breathing or guided meditation, can bring a sense of tranquility amidst the emotional turmoil associated with a brain cancer diagnosis.
- Journaling: Expressing thoughts and emotions through journaling can be therapeutic. Take time to jot down your feelings, fears, and hopes. Reflecting on your journey allows for a deeper understanding of your emotions and promotes a sense of self-awareness.
- Open Communication: Don't hesitate to openly communicate with your healthcare team, loved ones, and friends. Share your thoughts, concerns, and aspirations. Transparent communication fosters understanding, and having a supportive network can alleviate feelings of isolation.
- Reach Out to Peers: Connect with others who share a similar diagnosis. Joining support groups or reaching out to individuals who have faced similar challenges can provide a sense of camaraderie and shared experiences. Learning from others' coping strategies can be empowering.
- Creative Outlets: Engage in creative outlets as a means of self-expression. Whether it's art, music, or writing, channeling emotions into creative endeavors can offer a therapeutic release. Embrace activities that resonate with your interests and provide an avenue for self-discovery.
- Mind-Body Practices: Explore mind-body practices such as yoga or tai chi. These activities not only contribute to physical well-being but also promote a harmonious connection between the mind and body. Gentle movements and focused breathing can be particularly soothing.
- Professional Counseling: Consider seeking professional counseling or therapy to navigate the emotional complexities of a brain cancer diagnosis. Mental health professionals can provide guidance, coping strategies, and a safe space to explore emotions.
- Connecting with Nature: Spend time in nature to foster a sense of calm and connection. Whether it's a walk in the park, sitting by the water, or simply enjoying the outdoors, nature has a profound impact on mental well-being.
- Interaction with Service Dogs or Animals: The companionship of service dogs or animals can bring comfort and joy. Their presence provides a source of emotional support and can alleviate stress. Consider spending time with therapy animals or, if suitable, having a pet as part of your support system.
- Set Realistic Goals: Establish realistic and achievable goals for yourself. Breaking down larger objectives into smaller, manageable tasks provides a sense of accomplishment and helps maintain a positive mindset throughout the journey.
- Embrace Positivity: Surround yourself with positivity. Seek out inspirational stories, motivational quotes, or uplifting activities. Creating a positive environment can significantly impact your mindset and foster resilience in the face of adversity.
Relationships and Communication
Maintaining healthy relationships while dealing with brain cancer can be challenging. Open and honest communication is crucial. Expressing feelings and concerns can foster understanding among family and friends, creating a supportive environment for the individual battling this condition.
Building a Support System
Caregivers play a crucial role in the journey of someone with brain cancer. Their support is invaluable, extending beyond the realms of physical care to emotional and practical assistance. Caregiver support networks provide resources and guidance for those in this essential role. Look for support groups you can join as well to expand your support system.
Advocacy and Awareness
By sharing personal stories and experiences, individuals contribute to the broader conversation, dispelling myths and fostering understanding. Participating in awareness campaigns helps create a supportive community and encourages ongoing research into effective treatments. This is optional but it can help you connect with people who are going through similar challenges as you.
Understanding Your Brain Cancer Diagnosis
When faced with a brain cancer diagnosis, it's paramount to recognize the vast differences among brain tumor types. The location, size, and type of the tumor significantly influence your situation. Some tumors may be benign (non-cancerous), while others are malignant (cancerous).
Each diagnosis comes with its own unique set of challenges, requiring tailored approaches to treatment and care. Ask questions and make sure you fully understand your diagnosis to be better prepared and informed throughout the whole process.
Treatment Options
Navigating the array of treatment options is a crucial aspect of understanding your brain cancer diagnosis. From surgery and chemotherapy to radiation therapy and experimental treatments, the approach depends on the specific characteristics of your tumor.
A healthcare team experienced in treating brain tumors will work closely with you to determine the most effective and personalized treatment plan. Head to our blog, “ What is the Best Treatment for Brain Cancer: Unraveling the Options ” to learn more about potential treatment options.
Managing Brain Tumor Symptoms
The impact of a brain cancer diagnosis extends beyond medical treatments to managing symptoms. Symptoms can vary widely, from headaches and cognitive changes to motor skill impairment.
Understanding how to address and cope with these symptoms is integral to enhancing your quality of life. A comprehensive approach may involve medications, rehabilitation, and lifestyle adjustments tailored to your unique situation.
Lifestyle Adjustments
Individuals with brain cancer may need to make significant lifestyle adjustments. This could include changes in diet, exercise routines, and sleep patterns to support overall well-being. Occupational and physical therapy may also play a crucial role in adapting to the physical limitations imposed by the illness.
Diet & Nutrition
It is advisable to try and incorporate a healthy and nutritious diet to help fuel your body with the nutrients it needs to stay strong. Work with your doctors and nutritionists to craft a plan that fits your needs.
Oftentimes, patients may experience nausea, constipation, diarrhea, and vomiting while going through certain brain cancer treatments. Here are some tips to help minimize symptoms like nausea and vomiting:
- Nutrient-Rich Diet: Focus on incorporating a variety of fruits, vegetables, whole grains, and lean proteins. Nutrient-dense foods provide essential vitamins and minerals, supporting your body's resilience during the challenges of brain cancer.
- Hydration: Adequate water intake helps manage potential side effects of treatments and supports overall health. Consider consulting with a nutritionist to tailor your fluid intake to your individual needs.
- Dietary Restrictions: In some cases, dietary restrictions may be necessary. Certain medications or treatments may impact your appetite or require specific dietary adjustments. Working closely with healthcare professionals and nutritionists ensures a diet plan that aligns with your unique circumstances.
- Smaller Meals: Eat smaller meals more frequently; this can help with digestion and it may be easier to take in smaller portions of food when your stomach is feeling sick.
- Simple Foods: Try incorporating plain foods such as toast, applesauce, crackers, or yogurt to help reduce nausea.
- Avoid Greasy Foods: Try your best to avoid any fried or overly greasy foods, especially if you are experiencing constipation.
- Caloric Food: You may find yourself losing your appetite often, when you do eat, try choosing foods that are higher in calories to give your body more energy.
Exercise
Incorporating exercise into the routine of individuals with a brain cancer diagnosis holds significant benefits for both physical and mental well-being. Before starting any exercise regimen, consult with your healthcare team.
They can provide insights into safe and suitable activities, considering your overall health and the specific implications of your brain cancer diagnosis. Open communication ensures a collaborative approach to integrating exercise into your lifestyle. Try beginning with low impact workouts like swimming or gentle walks; aim for activities that enhance mobility and strength.
Regular exercise contributes to mental well-being. It can help alleviate stress, reduce anxiety, and improve mood. Engaging in activities such as walking, yoga, or gentle stretching supports physical health and enhances the overall quality of life.
Cognitive Side Effects
Cognitive changes are common in individuals with brain cancer. These changes can affect memory, concentration, and problem-solving skills. Cognitive rehabilitation programs may be needed and are designed to address these challenges, offering strategies to enhance cognitive function and improve daily functioning.
Continuing Needed Brain Cancer Treatments
Receiving a brain cancer diagnosis initiates a journey that often involves ongoing treatments, requiring individuals to integrate medical interventions into their daily lives. Understanding and adapting to this new normal is crucial for effectively managing the disease. Here's what to consider:
- Frequent Medical Visits : Continuing brain cancer treatments often entails frequent visits to healthcare professionals for tests, assessments, and adjustments to the treatment plan. Embracing these regular check-ups is essential for monitoring the progress of the treatment and ensuring that it aligns with your evolving needs.
- Adapting to Treatment Schedules: Incorporating treatments into your life means adjusting to specific schedules. Whether it's chemotherapy sessions, radiation therapy, or other interventions, finding a routine that accommodates these treatments becomes integral. Collaborating with your healthcare team to establish a schedule that aligns with your daily life is essential for seamless integration.
- Dealing with Side Effects: Brain cancer treatments may bring about side effects that impact your daily life. From fatigue and nausea to changes in cognitive function, acknowledging and addressing these side effects is crucial. Open communication with your healthcare team allows for proactive management, ensuring that side effects are minimized as much as possible.
- Quality of Life Considerations: Balancing the need for continued treatments with maintaining a reasonable quality of life is a delicate task. It involves making decisions about the intensity and duration of treatments based on your preferences and overall well-being. Open discussions with your healthcare team about your goals and priorities help shape a treatment plan that aligns with your values.
Returning to Work or School
Returning to work or school after a brain cancer diagnosis is a deeply personal decision, and there is no one-size-fits-all approach. It's essential to acknowledge that individuals may have varying perspectives on how employment or academic pursuits align with their recovery. Here are key considerations and tips for those contemplating a return:
- Personal Choices and Recovery: Recognize that there is no definitive "right" answer when it comes to returning to work or school. Some may find the routine and structure beneficial, while others may feel that dedicating more time to recovery or spending quality moments with loved ones is a priority. Trusting your instincts and understanding your unique situation is paramount.
- Open Communication: If contemplating a return to work or school, open communication is key. Discuss your intentions and concerns with your healthcare team, employer, or educators. Establishing transparent communication lines ensures that everyone is on the same page, allowing for necessary accommodations and support.
- Flexible Schedules: Consider negotiating flexible work hours or part-time arrangements. Flexibility in your schedule can provide the balance needed for both work or school commitments and your health. Many employers and educational institutions are open to accommodating individual needs, fostering an environment conducive to overall well-being.
- Emotional and Practical Support: Seek support from loved ones, colleagues, or classmates who can provide understanding and encouragement. Practical assistance, such as having a designated workspace or access to resources that ease your transition, contributes to a smoother reintegration.
- Prioritize Self-Care: Regardless of your decision to return to work or school, prioritizing self-care is non-negotiable. Ensure you have adequate rest, manage stress, and incorporate activities that promote your mental and physical well-being. Balancing responsibilities with self-care is essential for sustained recovery and overall life satisfaction.
- Set Realistic Goals: If returning to work or school, set realistic goals that align with your current capabilities. Understand that adjustments may be necessary and progress may occur gradually. Establishing achievable milestones ensures a sense of accomplishment without overwhelming yourself.
- School Accommodations: Get in touch with your school’s disability services center and see what flexibility they can offer you to make school more manageable. For instance, you can get an extension on the deadline for class work and exams, as well as more time off to meet all your scheduled appointments. Some schools even offer note-taking services and more.

Brain Cancer Doctors Near You
If you or a loved one is facing the complexities of a brain cancer diagnosis, the Preston Robert Tisch Brain Tumor Center is one of the best brain cancer centers in the world. As a world-renowned facility, we are committed to providing exceptional care, cutting-edge treatments, and unwavering support throughout your journey.
Our all-star team comprises dedicated brain cancer doctors , including pediatric brain cancer doctors , recognized globally for their expertise and commitment to advancing treatment options.
We offer a holistic approach to diagnosis and treatment, ensuring every aspect of your unique situation is addressed with the utmost precision and care. Benefit from the latest advancements in brain cancer treatments, personalized to your specific diagnosis, and cutting-edge clinical trials . Our commitment to innovation positions us at the forefront of medical breakthroughs.
At the Preston Robert Tisch Brain Tumor Center, we understand the emotional challenges that come with a brain cancer diagnosis. Our compassionate team is here to provide the support and guidance you need throughout your journey.
Don't let a brain cancer diagnosis define your future. Take the next step toward hope, healing, and exceptional care. Contact our brain cancer center located in Durham, NC, today to schedule a consultation with our renowned team of brain cancer doctors. Your journey to recovery begins here.
Related Readings:
- Best Clinic for Brain Tumor Treatment
- How to Care for Someone with Brain Cancer
- Follow-up Care & Rehabilitation: What Happens After Brain Cancer Treatment?
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

A Survey of Brain Tumor Segmentation and Classification Algorithms
Erena siyoum biratu.
1 College of Electrical and Mechanical Engineering, Addis Ababa Science and Technology University, Addis Ababa 120611, Ethiopia; moc.liamg@muoyisanari (E.S.B.); moc.liamg@amrigeyat (T.G.D.)
Friedhelm Schwenker
2 Institute of Neural Information Processing, Ulm University, 89081 Ulm, Germany
Yehualashet Megersa Ayano
3 Ethiopian Artificial Intelligence Center, Addis Ababa 40782, Ethiopia; moc.liamg@nevuelauhey
Taye Girma Debelee
Associated data.
Not applicable.
A brain Magnetic resonance imaging (MRI) scan of a single individual consists of several slices across the 3D anatomical view. Therefore, manual segmentation of brain tumors from magnetic resonance (MR) images is a challenging and time-consuming task. In addition, an automated brain tumor classification from an MRI scan is non-invasive so that it avoids biopsy and make the diagnosis process safer. Since the beginning of this millennia and late nineties, the effort of the research community to come-up with automatic brain tumor segmentation and classification method has been tremendous. As a result, there are ample literature on the area focusing on segmentation using region growing, traditional machine learning and deep learning methods. Similarly, a number of tasks have been performed in the area of brain tumor classification into their respective histological type, and an impressive performance results have been obtained. Considering state of-the-art methods and their performance, the purpose of this paper is to provide a comprehensive survey of three, recently proposed, major brain tumor segmentation and classification model techniques, namely, region growing, shallow machine learning and deep learning. The established works included in this survey also covers technical aspects such as the strengths and weaknesses of different approaches, pre- and post-processing techniques, feature extraction, datasets, and models’ performance evaluation metrics.
1. Introduction
Machine learning has been applied in different sectors, the majority of the studies indicate that it was applied in agriculture [ 1 ], and health sectors [ 2 , 3 ] for disease detection, prediction, and classifications. In health sectors the most researched areas are breast cancer segmentation and classification [ 4 , 5 , 6 , 7 ], brain tumor detection and segmentation [ 8 ], and lung and colon cancer segmentation and classification [ 3 ].
The gold standard in brain tumor diagnosis is biopsy which includes resection and pathological examination using various cellular (histologic) examination techniques. However, the diagnosis using biopsy is invasive that may result in bleeding and even injury that results in functional loss [ 9 ]. As a result, non-invasive brain tumor diagnosis using magnetic resonance imaging is the mainstay of modern neuroimaging that enables physician to characterize structural, cellular, metabolic, and functional properties of brain tumor [ 9 , 10 ].
In a conventional structural MRI scan, a healthy brain contains white mater (WM), gray matter (GM), cerebrospinal fluid (CSF) [ 11 ]. The main variation of these tissues in a structural MRI scan depends on their water content. The white matter (WM), which is 70% water, is a myelinated axon that connects the cerebral cortex with other brain regions. Furthermore, it carries information between neurons and connects the right and left hemispheres of the brain. The gray matter, which is 80% water, contains neuronal and glial cells that control brain activity, and the basal nuclei which are located deep within the white matter. Whereas, the cerebrospinal fluid is almost 100% water, and fills the space between the infoldings of the brain, between the brain and skull, and between the ventricular system in the brain[ 11 , 12 ].
Clinically, due to the variability in size, locality, rate of growth, and pathology, it is difficult to understand the manifestation of a brain tumor. However, a brain tumor is an abnormal mass of tissue, in which some cells grow and multiply uncontrollably. This uncontrollable growth takes up space within the skull and interferes with normal brain activity and damages the brain cells. The damage may be caused through increasing pressure in the brain, by shifting the brain or pushing against the skull, and by invading nerves and healthy brain tissues [ 13 , 14 ]. Different criteria can be used to classify brain tumor. A layered based tumor classification schema that has been proposed by WHO provides a detailed classification techniques that is more pertinent to radiological use. In this schema the hierarchy from top to bottom four layers, that are, final integrated diagnosis, histologic classification, WHO grade, molecular information [ 15 ]. However, brain tumors can be more generally grouped into primary and secondary (metastatic) tumors depending on their place of origin [ 16 ]. Primary brain tumors originates in the brain itself and are named for the cell types from which they originated. These primary tumors can be benign (non-cancerous) and malignant (cancerous). Benign tumors grow slowly and do not spread elsewhere or invade the surrounding tissues. However, they can put pressure on the brain and compromise its function. On the contrary, the malignant tumors grow rapidly and spread to surrounding tissues. On the other hand, secondary brain tumors originate from another part of the body. These tumors mainly occur due to cancer cells from somewhere else in the patient’s body that spread to the brain. The most common causes of secondary brain tumors are lung cancer, breast cancer, melanoma, kidney cancer, bladder cancer, certain sarcomas, and testicular and germ cell tumors [ 13 , 16 , 17 ]. Each of these tumors has unique clinical, radiographic, and biological characteristics [ 13 ].
In MRI scanning, brain examination can be normal or abnormal. The normal brain tissues in MRI are characterized by gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) tissues. Apart from the normal tissues listed earlier the tumorous brain scan often contains core tumor, necrosis, and edema. Necrosis is a dead cell located inside a core tumor, while edema is located near active tumor borders. Edema is a swelling that exists due to trapped fluids around a tumor. It can be vasogenic in non-infiltrative extra-axial tumors, such as meningioma, or it can be infiltrative that invades WM tracts of a brain in tumors, such as glioma [ 10 , 18 ]. Furthermore, these tissues often have indistinguishable intensity features in structural MRI sequences, such as T1-w, T2-w, FLAIR. For instance, the difficulty in differentiating between the core tumor and associated inflammation was discussed [ 19 ]. In addition to that, Alves et al. [ 19 ] demonstrated the difficulty in differentiating tumors using signal intensities alone. They demonstrated using a case where two patients were diagnosed with two different brain tumor types due to both tumors have similar intensity features and both are surrounded by extensive edema.
1.1. Brain Tumor Imaging Modalities
There are a variety of imaging techniques used to study brain tumors, such as magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET), and single-photon emission computed tomography (SPECT) imaging. However, CT and MR imaging are the most widely used techniques, because of their widespread availability and their ability to produce high-resolution images of normal anatomic structures and pathologies [ 20 ].
1.1.1. Magnetic Resource Imaging
Magnetic resonance imaging (MRI) of a brain generates several 3-dimensional image data that comprise the three anatomical views of a brain (axial, sagittal, and coronal) at different depths of a brain. Depending on the strength of the magnetic field and the sampling protocols, the image quality, slice thickness, and inter-slice gap vary [ 21 , 22 ]. During MR imaging, a patient lay in a strong magnetic field, almost 10,000 times stronger than the earth’s magnetic field, that forces the protons in the water molecule of the body to align in either a parallel (low energy) or anti-parallel (high energy) orientation with the magnetic field. Then, a radiofrequency pulse is introduced that forces the spinning protons to move out of the equilibrium state. When a radiofrequency pulse pauses, the protons return to an equilibrium state and produce a sinusoidal signal at a frequency dependent on the local magnetic field. Finally, a radio antenna within the scanner detects the sinusoidal signal and creates the image [ 22 , 23 ]. The amount of signal produced by specific tissue types is determined by their number of mobile hydrogen protons, the speed at which they are moving, the time needed for the protons within the tissue to return to their original state of magnetization (T1), and the time required for the protons perturbed into coherent oscillation by the radiofrequency pulse to lose their coherence (T2) relaxation times. As T1 (spin-lattice, also known as longitudinal relaxation) and T2 (spin-spin, also known as traversal relaxation) times are time-dependent, the timing of the radio frequency pulse and the reading of the radiated RF energy change the appearance of the image. In addition, the repetition time (TR) describes the time between successive applications of RF pulse sequences, and the echo time (TE) tells the delay before the RF energy radiated by the tissue in question is measured. The variation of T1 and T2 relaxation times between tissues gives image contrast on T1- and T2-weighted (T1-w and T2-w) images. The T1-w sequence is characterized by short TR and short TE while the T2-w sequence is characterized by long TR and short TE. Tissues with shorter T1 (for example, white matter) appear brighter when compared to tissues with a longer T1 (for example, gray matter) in magnetic resonance images. The other intermediate sequence that adopts long TR from T2-w and short TE from T1-w is a proton density-weighted (PD-w). In PD-w, the number of protons per unit volume in tissues is the main factor in determining the formation of image [ 23 , 24 ].
In the current neuroimaging techniques different MRI brain scan procedures can be performed, these include, the conventional structural MRI, functional MRI, diffusion-weighted imaging (DWI), and diffusion tensor imaging (DTI) [ 10 ]. In structural MRI procedure which mainly differentiates healthy and abnormal brain tissues based on their water molecule content is the most commonly employed standard imaging technique. This procedure helps to visualize healthy brain tissues and to map gross brain anatomy, tumoral vascularity, calcification, and radiation-induced micro hemorrhage [ 10 , 11 ]. The structural sequences include T1-w, T2-w, FLAIR, and contrast-enhanced T1-w [ 10 ]. The functional MRI (fMRI) on the other hand is used to capture the neural activity inside a brain through the ratio of oxygenated to the deoxygenated level of blood in the neighboring vasculature while performing a cognitive or motor task. The fMRI is used to localize eloquent cortex and differentiate between tumor grades [ 10 ]. The DWI captures the random motion of water molecules in a brain and it is used to characterize a tumor through identification of its cellularity and hypoxia, peritumoral edema, the integrity of WM tracts, and to differentiate between posterior fossa tumors [ 10 , 25 ]. Whereas, diffusion tensor imaging (DTI) is used to analyze the 3D diffusion direction, also known as diffusion tensor, of the water molecule. The DTI helps to determine local effects of the tumor on white matter tract integrity including tract displacement, the existence of vasogenic edema, tumor infiltration, and tract destruction [ 26 ].
1.1.2. Computed Tomography Imaging
A computed tomography (CT) scan was used in neuroimaging to help understand the functional and structural status of clinically significant signs of diseases. However, it provides less information than an MRI in brain tumor diagnosis. For instance, CT is inferior to MRI in the characterization of soft tissues like a brain and its use of ionizing radiation. However, a computed tomography (CT) scan can provide more detailed images of the bone structures near a brain tumor, such as the skull or spine. A CT scan may also be used to diagnose a brain tumor if the patient has implants like a pacemaker and when an MRI is not available. Currently, a CT is commonly used in the diagnosis of diseases like acute hemorrhage Parkinson’s, head trauma, and in determining age [ 27 , 28 ]. Therefore, in this survey work, brain tumor segmentation and classification techniques that use the brain scan image of MRI are only explored.
The remaining part of the paper is organized as follows, Section 2 illustrates related works to this survey work and shows their strengths and limitations. In Section 3 , the literature search strategy, including the chronological span, journal databases, the keywords used for search, and the inclusion and exclusion criteria, is presented. In Section 4 , the commonly used model performance metrics in evaluating the performance of brain tumor segmentation and classification algorithms are highlighted. In Section 5 , different region growing, conventional shallow supervised machine learning, and deep learning-based brain tumor segmentation techniques are discussed. Furthermore, the reported performances are presented. The techniques used in conventional machine learning-based brain tumor classification and their classification performance are elaborated in Section 6 . In addition, different deep learning models based brain tumor classification techniques with their reported performance are presented. Finally, the paper presents a discussion on Section 7 and a conclusion in Section 8 .
2. Related Works
The quest to find a better autonomous brain tumor segmentation and classification technique that can aid physicians in brain tumor diagnosis have been an active research area. As a result, several survey works have been completed to foster the research in the field and recap techniques used in brain tumor segmentation and classification. In Table 1 , only some of the recent pieces of literature that are related to our survey work are listed. Furthermore, their strengths and limitations are clearly discussed.
Survey literature on brain tumor segmentation and classification techniques.
Our work is tailored to provide a comprehensive survey of recently proposed different brain tumor segmentation and classification techniques, including region growing, shallow machine learning, and deep learning. The established work in this survey also covers technical aspects, such as the strengths and weaknesses of different approaches, together with their performance.
In this survey work, peer reviewed research papers from 2015 to 2021 that were published on Scopus and Web of Science indexed journals are surveyed to investigate the region growing, deep learning based brain tumor segmentation techniques, and machine learning and deep learning based brain tumor classification techniques. The databases that are extensively searched for this survey work were: (1) IEEE Xplore Digital Library, (2) Science Direct, (3) PubMed, (4) Google Scholar, and (5) MDPI. The search criterion includes (“Brain Tumor”) AND (“Region Growing”) AND (“Segmentation”) AND (“Deep Learning”) AND ("Machine Learning") AND ("Classification"). The methodology used for selecting literature is clearly shown in Algorithm algorithm1. In addition, the paper inclusion criteria (IC) and exclusion criteria (EC) is indicated on Table 2 .
Inclusion and exclusion criteria for paper selection.
4. Performance Measuring Metrics
Evaluating the segmentation and classification performance of a machine learning algorithm is an essential part of a research project. A machine learning model may give a satisfying result when evaluated using a metric, for instance, accuracy score but may give poor results when evaluated against other metrics such as precision or any other metric. Therefore, most of the time various evaluation metrics are applied to measure and compare the model performance.
In a segmentation task, true positive (TP) represents a pixel that is correctly predicted to belong to the given class according to the ground truth, whereas a true negative (TN) represents a pixel that is correctly identified as not belonging to the given class. On the other hand, a false positive (FP) is an outcome where the model incorrectly predicts a pixel not belonging to a given class. A false negative (FN) is an outcome where the model incorrectly predicts the pixel belonging to a given class. Similarly, for tumor classification task, TP represents a tumor class that is correctly predicted to belong to the given class according to the ground truth whereas a TN represents a tumor class that is correctly identified as not belonging to the given class. By the same token, false positive (FP) is an outcome where the model incorrectly predicts a tumor class not belonging to a given class. A false negative (FN) is an outcome where the model incorrectly predicts the class belonging to a given class. Therefore, keeping different performance metrics used in brain tumor segmentation and classification literature are listed as follows.
Accuracy (ACC) measures the ability of a model in correctly identifying all class or pixels, no matter if it is positive or negative.
Sensitivity (SEN) indicates the frequency of correctly predicted positive samples/pixels among all real positive/samples. It measures the models ability in identifying positive samples/pixels.
Specificity (SPE) is the proportion of actual negatives, which was predicted as the negative (or true negative). It tells the percentage of classes/pixels could not correctly identified.
Recall (RE) describes the completeness of the machine learning model’s positive predictions relative to the ground truth. It tells the percentage of classes/pixels annotated in our ground truth, are also included in model’s prediction.
Precision (PR) also known as positive predictive value (PPV) describes how often the model predicting correct class/pixel. It tells the the correct proportion of models predicted positives.
F1-Score is the most popular metric that combines both precision and recall. It represents harmonic mean of the two.
Intersection over union (IoU) also known as Jaccard index (JI) measures the percent overlap between the annotated ground truth mask and the model’s prediction output.
Dice similarity coefficient (DSC) measures the spatial overlap between the ground truth tumor region and the model segmented region. A zero DSC value indicates no spatial overlap between the ground truth tumor region and model annotated result whereas a value indicates a indicating complete overlap between the two.
Area under the curve (AUC) measure of the ability of a classifier to distinguish between classes and is used as a summary of the receiver characteristics curve and it is an area under true positive rate vs. false positive rate.
Similarity index (SI) refers to the similarity between the expert annotated ground truth and the model’s segmentation. It describes the similar identity between the input image and the detected tumor region.
5. Brain Tumor Segmentation Methods
Brain tumor imaging using techniques, such as MRI and CT, generate a significantly large number of images. Brain MRI scan of a single individual consists of several slices across the 3D anatomical view. Therefore, manual segmentation of brain tumors from magnetic resonance (MR) images is a challenging and time-consuming task. In addition, the artifacts introduced in the imaging process results in low-quality images that make the interpretation difficult. As a result, the manual brain MRI segment is susceptible for inter and intra observable variability. To alleviate these challenges and help radiologist, different automatic brain tumor segmentation techniques have been proposed in literature.
On these literature, authors have proposed an automated system for brain tumor segmentation techniques that provides objective, reproducible segmentation that are close to the manual results. These automated brain tumor segmentation can help to alleviate the difficulties associated with manually analyzing brain tumors. This will speed-up the brain image analysis process, improve diagnosis outcome, and make easy the follow-up of the disease through evaluating tumor progression [ 34 ].
In this section, among the proposed brain tumor segmentation techniques in the literature; region growing, machine learning, and deep learning based techniques will be surveyed to identify the experimental dataset, pre-processing, feature extraction, segmentation algorithm, and the reported performance.
5.1. Region-Based and Shallow Unsupervised Machine Learning Approach
One of the most commonly used segmentation techniques in automated image processing applications is region-based segmentation. Regions in an image are a group of connected pixels that satisfy certain homogeneity criteria, such as pixel intensity values, shape, and texture [ 35 ]. In a region-based segmentation the image is partitioned into dissimilar regions so that the desired region is located precisely [ 36 ]. The region-based segmentation takes into account the pixel values, such as gray level difference and variance, and spatial proximity of pixels, such as Euclidean distance and region compactness in grouping pixels together. In brain tumor segmentation, region growing, and clustering algorithms are the most commonly used region based segmentation technique.
Clustering-based segmentation is one of the powerful region based segmentation techniques where an image is partitioned into a number of disjoint groups. In clustering based segmentation pixels with high similarity categorized in a given region whereas dissimilar pixels categorized into different regions [ 37 ]. Clustering techniques, which are an unsupervised learning method, have been widely investigated in medical image segmentation. However, in this survey work some of the most popular clustering methods, such as k-means and its varieties [ 38 , 39 , 40 , 41 , 42 , 43 , 44 ], fuzzy c-means [ 38 , 39 , 41 , 45 ], subtractive clustering (SC), and hybrid techniques [ 46 , 47 , 48 ].
K-means clustering is an unsupervised machine learning algorithm and it is commonly used to segment a region of interest from the remaining part of an image. K-means has been extensively tested in brain tumor segmentation and has shown acceptable accuracy [ 48 ]. The minimal computational requirement [ 37 , 48 ], simplicity to implement on large dataset [ 49 ], adaptation to new examples, and guaranteed convergence are some of the advantages that makes K-means popular segmentation algorithm. However, k-means suffers with incomplete delineation of the tumor region [ 49 ], selection of the initial centroid is not optimum [ 37 , 43 ], and it is sensitive to outliers [ 48 , 50 ]. Due to these limitations a number of solutions have been proposed, including, evenly spreading the initial cluster centers (k-means++), hybridizing k-means with other clustering techniques [ 49 ], adaptively initializing cluster centers, such as adaptive k-means [ 43 ], modified adaptive k-means (MAKM), and histogram based k-means.
Fuzzy c-means works by assigning membership values to each of the pixels in an image corresponding to the centers of the clusters depending on a certain similarity criteria [ 51 ]. In fuzzy c-means (FCM) clustering objects can belong to more than one cluster based on its degree of membership. Therefore, in such a type of soft clustering technique, image pixels can occupy multiple clusters. As a result, compared to hard-clustering techniques such as k-means, FCM performs better on relatively noise free images. However, in medical images such as brain MRI that can be easily affected by unknown noises, the FCM performance is severely affected [ 52 ]. A number of researches have been performed to improve the limitation of FCM [ 53 , 54 , 55 , 56 ].
In region growing brain tumor segmentation, tissues including tumorous regions are partitioned based on certain similarity criterion, such as homogeneity, texture, sharpness, and gray levels. The technique starts by selecting an initial seed based on predefined methods. Then, the neighboring pixels are added progressively to the seed pixel [ 57 ]. The region growing based segmentation can properly segment regions with similar properties and spatially separated regions. However, it is sensitive to noise and influenced by the similarity criterion [ 57 ]. Therefore, it may end up with disconnected regions and results in a hole in the segmented region. Furthermore, finding a good initial seed is not an easy task [ 57 ]. Region growing and conventional unsupervised machine learning based brain tumor segmentation techniques proposed in literature are summarized in Table 3 . The table indicates the brain MRI dataset used in the experiment, the centroid initialization techniques, the objective function, and the segmentation performance.
Region growing and shallow unsupervised machine learning based brain tumor segmentation.
1 Darwinian Particle Swarm Optimization, 2 Discrete Wavelet Transform.
5.2. Supervised Shallow Machine Learning Based Approach
Supervised machine learning-based brain tumor segmentation approaches transformed the image segmentation problem into a tumorous pixel classification problem. The input vector for these supervised learning models consisted of different extracted features, and the output is a vector of desired classes for segmentation. In brain tumor segmentation, where tumor regions are often scattered all over the image, pixel classification rather than classical segmentation methods are often preferable [ 65 ]. Therefore, the traditional supervised machine learning algorithms have been used in the segmentation of a brain tumor from a head MRI scan [ 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 ].
In this section, as shown in Table 4 , most relevant literature on brain tumor segmentation using traditional machine learning algorithms, such as support vector machine (SVM), artificial neural network (ANN), random forest (RF) are surveyed to identify data used, the pre-processing, feature extraction techniques, the classifier model, and whether or not post-processing is implemented.
Summary of a shallow machine learning based segmentation.
1 Simple Linear Iterative Clustering, 2 Concatenated and Connected Random Forest, 3 Multiscale Patch Driven Active Contour, 4 Random Decision Forest.
5.3. Deep Learning-Based Approach
Deep learning methodologies produce automatic features that avoid or minimize the need for handcrafted features. In the deep learning-based brain tumor segmentation approach, the general strategy is to pass an image through the pipeline of deep learning building blocks and input image segmentation is performed depending on the deep features. In literature, there are a variety of deep learning techniques proposed for segmenting brain tumors. Some of such blocks contain deep convolutional neural networks (DCNNs), convolutional neural network (CNN), recurrent neural networks (RNNs), long short-term memory (LSTM), deep neural networks (DNNs), deep autoencoders (AEs), and generative adversarial networks (GANs). In this section, literature in terms of these building blocks, the dataset used, and the reported performance are presented as shown in Table 5 .
Summary of deep learning based brain tumor segmentation techniques.
1 Heterogeneous CNN + Conditional Random Fields-Recurrent Regression based Neural Network, 2 Deep Residual Dilate Network with Middle Supervision, 3 Fully Convolutional Neural Network, 4 OCcipito Module, 5 Atrous-Convolution Feature Pyramid.
6. Brain Tumor Classification Methods
Based on the WHO’s classification of central nervous system (CNS) tumors, there are more than 150 types of CNS tumors that are mainly categorized into primary and metastatic (secondary) tumors [ 99 ]. The primary tumors originate from the brain or the immediate surrounding tissues. Whereas, metastatic tumors arise from other body parts and migrate to the brain through the bloodstream. Metastatic tumors are considered cancerous or malignant, while primary tumors can be benign or malignant.
A biopsy is the existing gold standard procedure in brain tumor classification. However, it usually requires definitive brain surgery to take a sample [ 100 , 101 ]. On the other hand, an automated brain tumor classification from an MRI is non-invasive so that it avoids tumor sample taking procedure and it is safer. In addition, the machine learning-based brain tumor classification from an MRI scan can improve the diagnosis and treatment planning [ 101 ]. As a result, an automatic brain tumor classification from MRI images using machine or deep learning techniques is an active research area, and promising results have been achieved [ 100 , 102 , 103 , 104 , 105 , 106 ].
6.1. Conventional Machine Learning Based Approach
Machine learning is a paradigm where a machine is given a task where its performance improves with experience. Machine learning techniques are commonly grouped into three major types: supervised, unsupervised, and reinforcement learning [ 107 ]. Supervised learning is based on training a data sample from the data source with correct classification already assigned by domain experts, whereas, in unsupervised learning, the algorithm finds hidden patterns from the unlabeled data. On the other hand, reinforcement learning is carried out by making a sequence of decisions using reward signals. Therefore, the algorithm learns through receiving either rewards or penalties for the actions it performs [ 107 ]. Machine learning has been used in the classification of brain tumors from MRI images, and promising classification performance has been reported [ 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 ].
The traditional machine learning-based brain tumor classification techniques often consist of preprocessing, segmentation, feature extraction, and classification stages.
6.1.1. Pre-processing
Brain MRI scans are significantly affected by different types of noises, including salt and pepper, Gaussian, Rician, and speckle noise [ 116 , 117 , 118 ]. These noises impose challenges in machine learning-based applications [ 117 , 119 ]. Therefore, obtaining high-quality image denoising is one of the important tasks in the pre-processing stage. Each method used in MRI denoising has its advantages and disadvantages. Several methods have been developed for reducing noises based on statistical property and frequency spectrum distribution [ 119 ]. In addition to denoising, tasks such as removing tags, smoothing the foreground region, intensity inhomogeneity correction, maintaining relevant edges, resizing, cropping, and skull stripping are part of pre-processing [ 110 , 111 , 112 ].
6.1.2. Region of Interest (ROI) Detection
In an MRI brain scan, the segmentation task labels each voxel in an MRI image to specify its tissue type and anatomical structure [ 119 ]. The objective of ROI detection in tumor classification is to locate the tumor region from an MRI scan, improve the visualization, and allow quantitative measurements of image structures in the feature extraction stage [ 108 , 112 ]. Brain tumor segmentation can be performed in three different ways, namely, manual segmentation, semi-automatic segmentation, and fully automatic segmentation [ 119 ]. The autonomous brain segmentation techniques have been briefly discussed in Section 5 .
6.1.3. Feature Extraction
The feature extraction techniques are mathematical models based on various image properties. The different types of features include texture, brightness, contrast, shape, Gabor transforms, gray-level co-occurrence matrix (GLCM), and wavelet-based features [ 115 , 120 ], histogram of local binary patterns (LBP) [ 121 ]. On the other hand, recently, deep features that are obtained from deep neural networks such as CNN have been used as input to SVM classifier to classify brain tumors [ 122 ]. In brain tumor classification, it is customary to fuse several features from different extraction models to improve the discrimination power of the machine learning model [ 123 ]. Furthermore, feature selection is applied for dimensionality reduction.
6.1.4. Classification
Different classification techniques have been proposed by many authors for identifying tumor types from brain images. Different authors have classified tumor into a variety of ways, for instance meningioma, glioma, and pituitary [ 109 , 121 , 122 , 124 , 125 ]; astrocytoma, glioblastoma, and oligodendrogliamo [ 112 ]; glioma tumor grades (I–IV) [ 113 ]; benign and malignant stages(I–IV) [ 126 , 127 , 128 , 129 ]; diffuse midline glioma, medulloblastoma, pilocytic astrocytoma, and ependymoma [ 102 ]; multifocal, multicentric, and gliomatosis [ 130 ]; ependymoma and pilocytic astrocytoma [ 120 ].
In brain tumor classification, the most commonly used classifiers are neural network [ 108 , 109 , 110 , 111 , 131 ], support vector machines (SVM) [ 108 , 115 , 124 , 127 , 128 , 129 , 130 , 132 , 133 ], K-nearest neighbor (KNN) [ 112 , 121 , 130 , 134 ], Adaboost [ 126 ], and hybrid models [ 113 , 135 , 136 ]. The neural network was implemented using different architectures, such as feedforward neural network [ 110 , 125 ], multilayer perceptron neural network [ 109 , 137 ], and probabilistic neural network (PNN) [ 111 , 131 ]. Support vector machine (SVM) was commonly implemented using three kernels, linear, homogeneous polynomial, and Gaussian radial basis function (RBF) [ 108 , 115 ]. In the KNNclassifier, the testing feature vector is classified by finding the k-nearest training neighbor, that is, the classifier does not use any model to match and is only based on memory. However, KNN uses different measurements such as euclidean distance, city block, cosine, and correlation to find the nearest distance between the testing and training class feature vectors [ 134 ].
A summary of recent shallow machine learning-based brain tumor classification techniques is given on Table 6 .
Summary of conventional ML based brain tumor classification techniques.
1 Principal Component Analysis, 2 Particle Swarm Optimization, 3 Regularized Extreme Learning Machine.
6.2. Deep Learning Approach
Even though promising progress has been made in classifying brain tumors into their respective types from an MRI brain scan using shallow supervised machine learning algorithms, there are still challenges in classifying brain tumors from an MRI scan. These challenges are mainly due to the ROI detection, and extracting descriptive information using traditionally handcrafted feature extraction techniques is not efficient [ 122 ]. This inefficiency mainly arises due to the complex structure of brain anatomy and the high-density nature of the brain.
Unlike shallow machine learning algorithms, deep learning is based on learning data representations and hierarchical feature learning. In deep learning-based brain tumor classification, the deep learning models discover the descriptive information that optimally represents different brain tumors. This nature of deep learning transforms the brain tumor classification from handcrafted feature-driven into data-driven problem [ 103 ]. Among the deep learning models, a convolutional neural network (CNN) is widely used in brain tumor classification tasks, and a substantial result has been achieved[ 100 ].
In the reviewed literature, there are differences in the techniques used for the classification of brain tumors. The difference encompasses: (i) the dataset used for classification including tumor types, (ii) the implemented pre-processing and data augmentation techniques, (iii) whether or not the ROI segmentation was used as a prior step in the classification, (iv) whether a pre-trained or custom-designed deep learning model is used.
For instance, Badža and Barjaktarović[ 100 ] used publicly available contrast-enhanced T1-weighted brain tumor MRI scans [ 138 ]. The dataset contains meningioma, glioma, and pituitary brain tumor types scanned along with the three anatomical views, i.e., axial, sagittal, and coronal. The images were preprocessed using techniques, such as normalization and resizing. In addition, images in the dataset are augmented with 90 o rotation and vertical flipping to increase the training dataset. Furthermore, they used a custom-designed CNN model trained with Adam optimizer with a mini-batch size of 16 and tested with 10—fold cross-validation. The weights of the convolution layers are initialized using a Glorot initializer. The model performance was measure using sensitivity, specificity, accuracy, precision, recall, and F1-score. The sensitivity for meningioma, glioma, and pituitary is 89.8%, 96.2%, and 98.4%, respectively. The specificity of the model for meningioma, glioma, and pituitary is 90.2%, 95.5%, and 97.7%, respectively. Furthermore, the models’ overall accuracy, average precision, average recall, and F1-score are 95.4%, 94.81%, 95.07%, and 94.94%, respectively. The summary of this and other literature is presented on Table 7 .
Summary of deep learning based brain tumor classification techniques.
1 Convolutional Neural Network based on Complex Networks, 2 Confidence Interval, 3 Automated Machine Learning, 4 Matthew’s Correlation Coefficient, 5 Visual Geometry Group, 6 Deep Dense Inception Residual Network, 7 High Grade Glioma, 8 Low Grade Glioma.
7. Discussion
This paper presented a thorough survey of techniques used in brain tumor segmentation and classification. The survey encompasses several traditional machine learning and deep learning-based methods with their quantitative performance. The conventional image segmentation techniques, that is, region growing and unsupervised machine learning used in brain tumor segmentation are presented in Table 3 . The region growing with all other conventional image processing segmentation techniques is the earliest approach applied in brain tumor segmentation [ 161 ]. It is mainly affected by noises, poor image quality, and initial seed point. To overcome these challenges, an automatic seed point selection by optimization techniques and artificial intelligence-based seed point selection has been proposed [ 162 ]. In addition, it has a limitation in segmenting tumors that appear scattered across the brain. In the second generation segmentation techniques which are based on shallow unsupervised machine learning, such as fuzzy c-means and k-means grouping of pixels into more than one class has been achieved. However, these methods are also highly sensitive to noise. Therefore, through incorporating additional information and adaptively selecting the centroid, the segmentation performance of medical images can be improved [ 6 ]. In addition, the inherent ambiguous boundaries between normal tissues and brain tumors pose a significant challenge for conventional and clustering segmentation techniques. Therefore, to address this challenge, pixel-level classification-based segmentation techniques using traditional supervised machine learning have been proposed [ 70 ]. These methods are often accompanied by feature engineering, where the tumor descriptive pieces of information are extracted to train the model. Furthermore, the supervised machine learning segmentation output is further improved through post-processing [ 71 , 76 ].
Nowadays, conventional image processing and shallow machine learning-based brain tumor segmentation techniques are becoming obsolete due to the advent of deep learning-based techniques. The deep learning-based approach performs an end-to-end tumor segmentation by passing an MRI image through the pipeline of its building blocks. These models often extract tumor descriptive information automatically and avoid the need for handcrafted features. However, the need for a large dataset to train the models and the difficulty in interpreting the models hinders their usage in medical fields [ 163 ]. In terms of segmentation performance, it is evident from Table 4 and Table 5 that the deep learning-based and supervised shallow machine learning-based with post-processing has comparable performances. Asummary of the number of brain tumor segmentation techniques surveyed in this is given on Figure 1 .

Number of brain tumor segmentation methods.
Aside from segmentation of brain tumor region from head MRI scan, classification of tumor into their respective histological type has great importance in diagnosis and treatment planning which actually requires biopsy procedure in today’s medical practice [ 158 ]. Several methods which encompass shallow machine learning and deep learning have been proposed for brain tumor classification. The conventional shallow machine learning algorithms often consist of preprocessing, ROI detection, and feature extraction. However, due to the inherent noise sensitivity of MRI image acquisition, variations in the shape, size, location, and contrast of tumor tissue cells, extracting descriptive information is a challenging task. Therefore, nowadays, deep learning techniques are becoming the state-of-the-art approach to classify different types of brain tumors, such as astrocytoma, glioma, meningioma, and pituitary. Several brain tumor classifications have been discussed in this survey, and a summary of the number of brain tumor classification techniques surveyed in this paper are given on Figure 2 .

Number of brain tumor classification methods.
Several brain tumor datasets that are collected by researchers datasets and those that are available on repositories were used in the training and testing of brain tumor classification models. The publicly available dataset provided by J. Cheng et al. [ 138 ], which contains meningioma, glioma, and pituitary tumor in T1-WC MRI-images is one of the most commonly used datasets in the training and testing classifier models. Using this dataset, Gumaei, A. et al. [ 125 ] has achieved a classification accuracy of 94.23% using a regularized extreme learning machine, while the Kokkalla, S. et al. [ 153 ] have reported a classification accuracy of 99.69% using custom modified deep-dense inception residual network (DDIRNet). These results indicate that the deep learning-based model outweighs the shallow machine learning-based techniques for this particular dataset.
Challenges in Automatic Brain Tumor Segmentation and Classification
The development of autonomous brain tumor segmentation and classification models using MRI images is still a challenging task. The challenges are due to several constraints including the effect of different types of noises embedded in the brain MRI images [ 116 , 117 , 118 ], motion and metal artifacts during image acquisition [ 164 ], low-resolution MRI images [ 165 ], and lack of deep learning models interpretability and transparency [ 166 , 167 ].
One of the most common challenges in machine learning-based brain tumor segmentation and classification is the noisiness of an MRI image. Therefore, noise estimation and denoising MRI images is a crucial pre-processing task for improving the accuracy of brain tumor segmentation and classification models. Therefore, several techniques have been proposed for denoising MRI images, such as modified iterative grouping median filter [ 118 ], Wiener filter and wavelet transform [ 168 ], non-local means [ 169 ], and deep learning-based approaches [ 170 , 171 ]. However, a robust denoising technique for MRI images is still challenging and the pursuit to obtain an efficient denoising technique has been an active research area [ 170 ]. Similarly, motion, metal, and other artifacts are also a source of challenge to the robustness of machine learning-based brain tumor segmentation and classification. Recently, deep learning-based solutions for minimizing the effects of these artifacts have been proposed [ 164 , 172 ]. MRI provides a high fidelity brain scan image compared to other imaging techniques. However, post-acquisition image processing techniques, including deep learning-based methods have been used to increase the resolution of MR images so that the efficiency of autonomous brain tumor segmentation and classification models improved[ 165 , 173 ]. The other major challenge is the lack of deep models’ interpretability, and often they are perceived as black-box. As a result, attaining any evidence regarding the process they perform is difficult. However, the transparency and interpretability of deep learning techniques are crucial for the complete integration into medical diagnosis [ 166 ].
8. Conclusions
Automating the brain tumor segmentation and classification task has tremendous benefits in improving the diagnosis, treatment planning, and follow-up of patients. Through applying various techniques, including conventional image processing, shallow machine learning, and deep learning techniques, undeniable progress have been achieved in automating brain tumor segmentation and classification tasks. However, building a fully autonomous system that can be used on clinical floors is still a challenging task.
Compared to region-growing and shallow machine learning algorithms, automating the brain tumor segmentation and classification using deep learning techniques have huge benefits. This is mainly due to the powerful feature learning ability of deep learning techniques. In addition, as can be shown in Figure 1 and Figure 2 , deep learning-based brain tumor segmentation and classification techniques are becoming the most active research area. In this paper, a comprehensive survey on region growing, shallow machine learning, and deep learning-based brain tumor segmentation and classification methods are presented. These methods are structurally categorized and summarized to give an insight to the reader of the dataset used, pre-processing, feature extraction, segmentation, classification, post-processing, and the reported model performances in the literature. Furthermore, the pros and cons of the methods and the model evaluation metrics have been discussed.
Author Contributions
Conceptualization, E.S.B.; Methodology, E.S.B.; Validation, Y.M.A., F.S., T.G.D.; Writing—original draft preparation, E.S.B.; Writing—review and editing, E.S.B., Y.M.A., F.S., T.G.D. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Skip to main content
- Keyboard shortcuts for audio player
Health Care
After years in conflict zones, a war reporter reckons with a deadly cancer diagnosis.

Terry Gross

Rod Nordland looks at the Istanbul old city from Galata Tower on Nov. 20, 2016. Nordland was diagnosed with glioblastoma, a terminal brain cancer, in 2019. Yasin Akgul/AFP via Getty Images hide caption
Rod Nordland looks at the Istanbul old city from Galata Tower on Nov. 20, 2016. Nordland was diagnosed with glioblastoma, a terminal brain cancer, in 2019.
As a war correspondent for The New York Times , Newsweek and the Philadelphia Inquirer , Rod Nordland faced death many times over. He's felt bullets whizzing by his head in Cambodia, and once escaped a hotel room in Sarajevo moments before a mortar attack reduced his bed to rubble.
But in 2019, Nordland confronted a different type of danger when he was diagnosed with glioblastoma , the most lethal form of brain tumor.
The median life expectancy for someone with glioblastoma is about 14 months . Less than 7% of people survive five years. Nordland says his time as a war corresponded helped prepare him for his cancer diagnosis.

Shots - Health News
John mccain was diagnosed with a glioblastoma, among the deadliest of cancers.
"One of the most important things I learned as a war correspondent was ... to stay calm and not lose control of your emotions," he says. "And I think that's been a really good lesson for dealing with cancer, too."
Rod Nordland on Afghanistan's Real-Life Romeo And Juliet
Afghanistan's real-life romeo and juliet.
Optimistic by nature, Nordland acknowledges that he's already beaten the odds by living with glioblastoma for as long as he has. He's actively engaged in treatment, but he also recognizes that there is no cure for his type of cancer.
"I had to face the reality that my death was within a fairly short timespan, highly probable," he says. "That had never been the case before. And I think it made me a better person for that."
Nordland writes about facing mortality from war and cancer in his new memoir, Waiting for the Monsoon.
Interview highlights

Waiting for the Monsoon , by Rod Nordland Harper Collins hide caption
Waiting for the Monsoon , by Rod Nordland
On his current treatments for glioblastoma
I'm doing a low-dose of chemo, and I'm also wearing a device on my head called an Optune. It's a series of ceramic arrays that are kind of glued to my head after I shave it. And then they they emit electronic beams that are thought to fight tumors. ... So every three days or so I have to shave my head bald and then reapply the arrays. And I have to make sure that the Optune machine is close to me. So it often means having somebody else carry it for me if I move it around or put it in a backpack or in the back of my wheelchair. So that's a bit annoying and certainly restricts my movement a lot.
On the side effects of the treatments
I do use a wheelchair when I go out to appointments, to doctors appointments, just for safety's sake. Because while I can walk with a cane sometimes without a cane, I'm very prone to falls and tripping because ... when the doctor cut the tumor out, he also cut some nerves that provided sensation to my left side. So I have no sensation on my left, which causes a lot of mobility problems. It gives you what they call poor proprioception, which is a fancy word, meaning your brain's knowledge of where your body is in space. And if your brain doesn't know where your body parts are, you're obviously very prone to falls, which, in my case, are bad for my head [and] can be fatal.
On being a war correspondent
When I began working as a war correspondent, I was still 20-something and still in many ways an adolescent. Like a lot of young people, I really didn't believe in my own mortality. And I think that's true of a lot of people who do that kind of work, because otherwise, who would do it? Who would jump out of an airplane into a parachute if they didn't have some belief in their own immortality? So I lost that arrogance very profoundly when I was on a front line against my own rules in Cambodia, on the outskirts of a refugee camp where there was a nasty little internecine war going on between factions that ran the camp and lived off of the proceeds of the food and supplies they could steal. ... I was standing shoulder to shoulder with one of these militiamen, and there were bullets whizzing over our heads. ... And we just stood there like idiots. And one of those bullets hit the guy next to me and blew his brains out, quite literally.
... After that, I started doing it really differently. That taught me that I was, in fact, mortal, which is an important lesson that all young men should learn as soon as possible. And after that, I never went to the front lines anymore.
On the meaning of life
I asked everybody I met what the meaning of life was. I even asked Alexa. The answer was, to quote Eleanor Roosevelt, that "the purpose of life is to live life to the fullest and to enjoy everything about it." That's somewhat of a lame answer. But at one time I asked that question of a nurse and she turned it around on me and said, "What do you think the meaning of life is?" So I said, "Well, I'm sorry, I'm going to have to punt on that. But I think the meaning of life is, as Raymond Carver said, 'to feel yourself beloved on this earth.'" And that was my answer then. And it's my answer in the book too.
Sam Briger and Susan Nyakundi produced and edited this interview for broadcast. Bridget Bentz, Seth Kelley and Carmel Wroth adapted it for the web.
A survey on brain tumor image analysis
- Review Article
- Published: 13 September 2023
- Volume 62 , pages 1–45, ( 2024 )
Cite this article
- Kashfia Sailunaz 1 ,
- Sleiman Alhajj 2 ,
- Tansel Özyer 3 ,
- Jon Rokne 1 &
- Reda Alhajj 1 , 4 , 5
998 Accesses
Explore all metrics
Medical imaging, also known as radiology, is the field of medicine in which medical professionals recreate various images of parts of the body for diagnostic or treatment purposes. Medical imaging procedures include non-invasive tests that allow doctors to diagnose injuries and diseases without being intrusive TechTarget (n.d.). A number of tools and techniques are used to automate the analysis of medical images acquired with various image processing methods. The brain is one of the largest and most complex organs of the human body and anomaly detection from brain images (i.e., MRI, CT, PET, etc.) is one of the major research areas of medical image analysis. Image processing methods such as filtering and thresholding models, geometry models, graph models, region-based analysis, connected component analysis, machine learning (ML) models, the recent deep learning (DL) models, and various hybrid models are used in brain image analysis. Brain tumors are one of the most common brain diseases with a high mortality rate, and it is difficult to analyze from brain images for the versatility of the shape, location, size, texture, and other characteristics. In this paper, a comprehensive review on brain tumor image analysis is presented with basic ideas of brain tumor, brain imaging, brain image analysis tasks, brain image analysis models, brain tumor image features, performance metrics used for evaluating the models, and some available datasets on brain tumor/medical images. Some challenges of brain tumor analysis are also discussed including suggestions for future research directions. The graphical abstract summarizes the contributions of this paper.
Graphical abstract
Similar content being viewed by others
Brain tumor detection and classification using machine learning: a comprehensive survey
Javaria Amin, Muhammad Sharif, … Ramesh Sundar Nayak
Machine learning and deep learning approach for medical image analysis: diagnosis to detection
Meghavi Rana & Megha Bhushan
Convolutional neural networks: an overview and application in radiology
Rikiya Yamashita, Mizuho Nishio, … Kaori Togashi
Avoid common mistakes on your manuscript.
1 Introduction
Medical imaging is the approach of imaging and visualizing organs or tissues and their functionalities for both clinical and physiological analysis. It does not only reveal the interior of a body and structures of internal organs/tissues, but it also is used for diagnosis and treatment of diseases [ 58 ]. Medical image analysis is the research area that processes the medical images (which are represented in a pixelated, digital form) to extract meaningful information about organs and possible abnormalities or lesions with a non-invasive approach. It analyzes medical images acquired using magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET), ultrasound, etc., to identify the possible abnormalities based on image properties (i.e., intensity, shape, texture, contour) of different body parts or organs. Image segmentation might be performed on the images where image segmentation is defined as is the process of partitioning a digital image into multiple image segments, also known as image regions or image objects (sets of pixels) [ 76 ].
The brain is a central part of the human nervous system and brain image analysis for neurological disorder detection is a major research topic of medical image analysis. This analysis has been used to detect and assess neurological disorders like brain tumor/cancer, Alzheimer disease, and Parkinson disease by researchers from both medical and technology backgrounds. Brain and central nervous system cancers that often start as brain tumors cause over 200,000 deaths every year [ 19 ].
A tumor is defined as an abnormal growth of tissues and they constitute the second leading cause of death. They are formed by uncontrolled cell divisions creating abnormal shapes containing excessive cells that do not follow their normal life cycle of cell genesis, cell growth, and cell death. Tumors are usually named after the organ or body part of their origin; and therefore, the tumors which originate from any brain or skull component like brain nerves, brain tissues, brain membranes, skull bones, etc., are called brain tumors and they have a 70% mortality rate [ 3 , 55 ].
More than 150 types of brain tumors exist based on the specific location of the tumor and they can be either benign (i.e., chordomas, gangliocytomas, meningiomas, etc.) or malignant (i.e., ependymomas, medulloblastomas, ependymomas, etc.) [ 34 , 55 ]. Tumors do not have any active cells and are uniform in structure and are said to be benign tumors or non-cancerous tumors. Tumors containing active cells with non-uniform shapes are called malignant or cancerous. Based on the origin, brain tumors are primary if they originated in the brain or secondary/metastatic if they originated in any other organ and later spread to the brain [ 46 ], while primary tumors that may or may not be malignant secondary tumors are almost always malignant. In many cases, it might be possible to remove a tumor by surgery, but the tumor might recur after the surgery.
Brain tumors can be categorized into four grades where grades I and II are “low-grade” tumors that are slowly growing and mostly benign, whereas grades III and IV are “high-grade” tumors that are mostly malignant and recurring. Grade I tumors do not infiltrate other organs, grow slowly, can be cured with surgeries, and have a high long-term survival rate. Grade II tumors are more severe. This class of tumors may recur after surgery, they might infiltrate other organs, and they grow a bit faster than grade I tumors. Grade III tumors are malignant, they tend to infiltrate other organs and they have a high frequency of recurrence, and grade IV tumors are the most severe ones.
Brain tumor analyses from brain images have been studied by researchers to capture different healthy brain tissues (i.e., white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF)) and tumor tissues. Different tumor tissues are shown in Fig. 1 in different colors. The tumor tissues can be detected as (i) necrotic or permanently dead tissues, (ii) enhancing or active tumor tissues containing active cells that can reproduce and infiltrate, and (iii) edema or swollen tissues that create brain swelling due to plasma leakage and can increase pressure inside the skull.
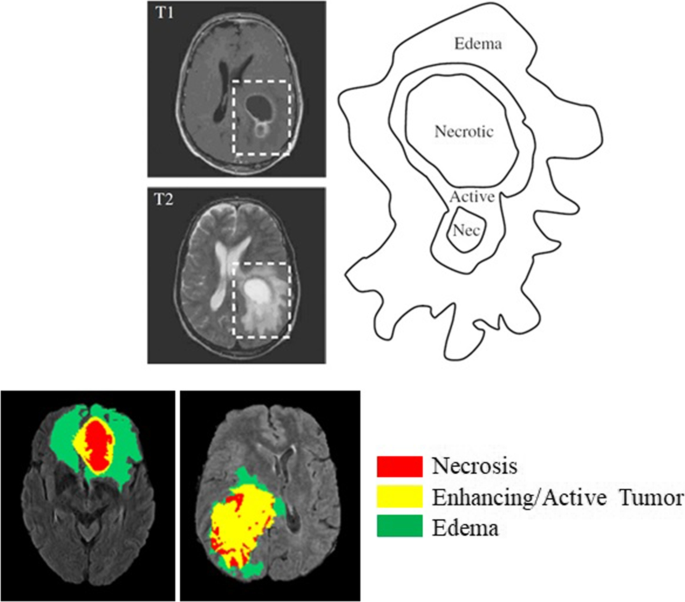
Brain tumor tissues [ 17 , 23 ]
Medical imaging for brain abnormality analysis uses various types of imaging techniques and each imaging method enhances some specific features of the organ. For example, ultrasound [ 63 ] uses soundwaves to capture images of brain, cerebrospinal fluid, and blood flow in the brain; CT uses X-rays to detect swelling, bleeding, or structural changes in the brain; PET uses radioactive tracers to identify the spots with defects; and MRI uses radiowaves to capture the brain, different tissues, functionalities, etc. [ 64 , 67 ]. Among the various types of brain scans, MRI images are the most popular for brain tumor studies and they have been used in most of the recent methods. MRI’s offer low radiation exposure together with high-contrast images. They can detect nervous abnormalities, blood flow, and cryptic vascular diseases and provides clearer images with multiple modalities. It is a non-invasive imaging technique that scans the body to generate detailed cross-sectional images of body parts. MRIs use a magnetic field to align the protons of the brain cell water molecules and a radio signal at a specific frequency is used to disrupt them once they are all aligned [ 36 ]. When the radio signal is stopped, the protons try to realign and while doing so emit signals which are captured by detectors.
Different anatomical planes for brain imaging can be viewed using MRI scans and each plane contains some specific information. These planes are considered as imaginary planes or surfaces passing through the anatomical position of the body or any specific part of the body. Generally, three major anatomical planes are used for brain imaging: (i) axial or horizontal, (ii) coronal or frontal, and (iii) sagittal or longitudinal. Figure 2 shows how the views from the different planes depict cross-sections of the brain as well as the brain MRIs corresponding to the planes [ 12 , 38 ]. Other than the view planes, MRI imaging can also vary based on image sequences. Various types of radio frequencies can be used in MRI imaging with different response times and echo times of the pulse signals that are used to produce multiple types of image sequences (i.e., T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), etc.) [ 39 ], and each of these image sequences stores some novel characteristics. Each type of image sequence highlights certain parts of the brain or the skull, and can be used for analyzing different parts of the brain. Table 1 shows the repetition times (i.e., time duration between two consecutive pulse sequences) and echo times (i.e., time duration between radio frequency remittance and echo signal reception) for some of the different MRI sequences, and Fig. 3 shows sample MRIs for these sequences [ 55 ].
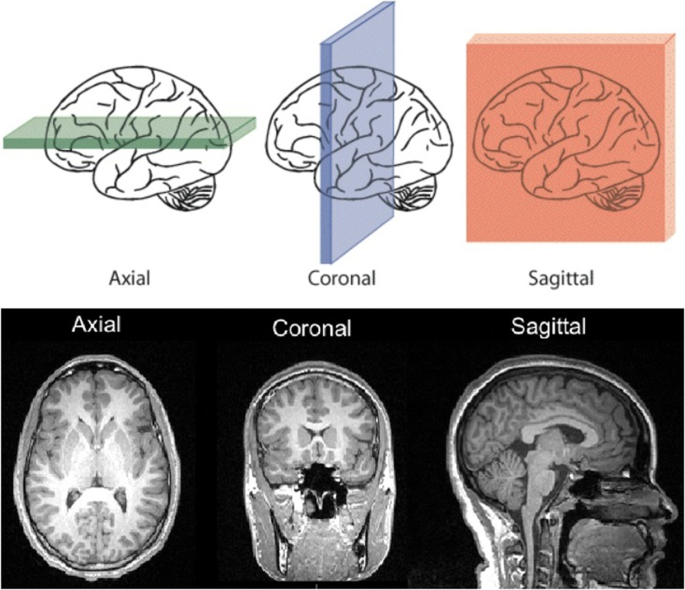
Axial, coronal, and sagittal views [ 12 , 38 ]
T1, T2, and FLAIR MRIs [ 55 ]
Brain tumor analysis from medical images is a popular research field and researchers have been trying to contribute for years towards a perfect automated system for brain tumor detection, segmentation, tumor type classification, tumor tissue identification, and extraction of other tumor properties. Various thresholding, filtering, morphological operations, region growing, connected component detection, supervised and unsupervised machine learning, heuristics, recent deep learning methods, and hybrid models have been deployed to solve these tasks in an efficient manner. Most systems are only tested on limited amounts of clinical and synthetic data. Within this limited scope of data tested, some systems have achieved high performance scores. In theory, lots of the existing systems performed well on individual tasks, but very few integrated all tasks together to create a completely automated brain tumor detection and analysis system. Analyzing the existing research works to compare their contributions, performances, and limitations can help to design a complete system by incorporating the models in a proper way to achieve the highest accuracy. It can also help to find the challenges of the tasks and the possible solutions.
Due to the complexity of the problem, most recent approaches of brain tumor analysis use DL for brain tumor image analysis. Some recent works also focused on metaheuristics algorithms like particle swarm optimization (PSO), various hybrid methods with PSO, cuckoo search, firefly algorithm, chicken-based optimization, artificial bee colony optimization, bacteria foraging optimization, etc. These methods are able to achieve around 90% accuracy as mentioned in recent approaches [ 55 ]. Other than these, supervised and unsupervised machine learning methods like support vector machine (SVM), random forest (RF), K-means clustering, K-nearest neighbor (KNN) algorithm, fuzzy C-means clustering (FCM), and hybrids of these methods are also popular in the literature. Various convolutional neural networks (CNN), fully convolutional networks, cascaded CNNs, deep neural networks (DNN), etc., are the most popular methods among all due to their high accuracy and other performance scores [ 15 , 30 , 46 , 48 , 55 , 56 , 57 ].
Although DL models have been providing better performances than the conventional machine learning methods, researchers are still trying other approaches such as various hybrid, ensemble, and novel methods with different existing conventional approaches by focusing on new perspectives and analyses. Various skull stripping algorithms [ 16 , 43 ], thresholding methods [ 14 , 22 ], region-growing algorithms, level set algorithms [ 25 , 27 ], contour models [ 4 , 24 , 51 ], wavelet transform models, principal component analysis, and morphological models [ 52 ] have been applied for brain tumor analyses that were able to achieve compatible performances as the DL models [ 46 , 55 ]. Some researchers also tried to view the MRI tumor detection problem from a geometric perspective and have been working on various fractal and computational geometry approaches for automatic brain tumor detection, tumor segmentation and reconstruction, tumor area calculation, tumor volume computation, etc. For example, Delaunay triangulation was used for brain tumor reconstruction [ 9 ], whereas convex hull was used for brain tumor segmentation [ 51 ], tumor volume calculation [ 49 ], and artifact removal [ 45 ].
There are hundreds of brain tumor publications over the years. Most of the papers are on brain tumor analysis from different types of inputs using various methods and only a few provide literature reviews on brain tumor researches. In this survey, the publications on brain tumor analysis from medical images were included by collecting publications from “Google Scholar” [ 65 ] and “PubMed” [ 66 ]. The search keywords were the following terms and their combinations—“brain tumor,” “medical images,” “brain MRI,” “brain tumor detection,” “brain tumor segmentation,” “brain tumor classification,” “AI for brain tumor analysis,” “brain tumor analysis with MRI survey,” “deep learning in brain MRI,” “machine learning in brain MRI.” The publication timeline was from January 2015 to June 2021. Only a few publications from 2013 and 2014 were added based on their mentions in the collected publications and their relevance to the search topics. At the first phase, 32 survey papers, 90 ML- and DL-based papers, 20 dataset papers, and 33 papers on the basics of brain tumor analysis (in total 175 papers) were collected. Although the publications from 2015 to mid-2021 based on those search terms were collected at the first phase, the duplicates were removed, and they were filtered based on the relevance and more recent publication dates in the second phase. Another criterion in the second phase to shortlist the collected publications was the uniqueness of the task or the methods or both. After going through the abstracts and publication dates of all papers collected, they were sorted based on these three criteria and 110 publications (i.e., 20 survey papers, 55 ML and DL papers, 15 dataset papers, and 20 brain tumor analysis papers) were listed for further filtering. At the final phase, after going through all 110 publications one by one in detail, 68 were finalized to be summarized in this literature review. Among the final 68 papers, 12 publications were on brain tumor surveys, 34 were on ML and DL methods, 12 were on 12 different datasets, and 10 were on the basics of brain tumor image analysis. The criteria for the final selection for the survey papers were the latest publication dates, the tasks discussed (i.e., analysis, detection, segmentation, classification, skull stripping, feature analysis, etc.), and the discussed approaches (i.e., ML, DL, conventional, etc.). For the ML- and DL-based papers, the criteria were the type of the task (i.e., detection, segmentation, classification, etc.), the uniqueness of the method or part of the method, the novelty of the used features, and the most recent publication dates. The dataset papers were chosen completely based on the uniqueness of the dataset. The basic brain tumor analysis–based papers were selected based on the recent publication dates, coverage of the discussions on brain tumor, brain medical images, brain tumor analysis tasks, brain tumor image analysis evaluations, brain tumor image features, brain tumor image analysis methods, etc. Figure 4 shows the phases of the publication filtering.
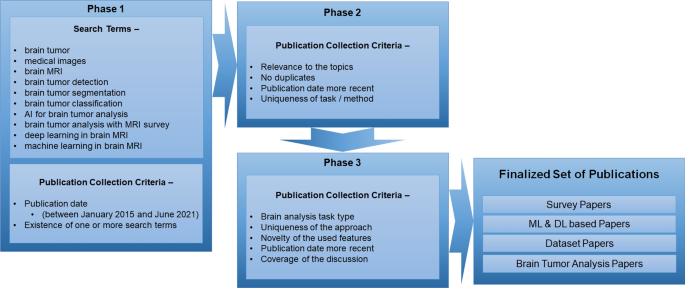
Publication searching criteria and process
Various researchers have reviewed brain medical image analysis and different tasks like brain tumor detection, classification, and segmentation, etc., for the existing researches on brain tumor images [ 1 , 3 , 11 , 21 , 46 ]. Although these reviews contain the contributions of researchers on this topic, most of them have focused on either a specific analysis task (i.e., detection, segmentation, classification, skull stripping, etc.), or specific types of approaches (i.e., conventional, ML, DL, etc.). The contributions of the existing survey papers are discussed in Sect. 4 summarizing the recent existing brain tumor surveys provided within the scope of this paper by generating a more complete literature review. Proposing and implementing automated systems with ML, DL models for each of the brain tumor image analysis task achieve some advancement towards helping the healthcare professionals in detecting the brain tumor and its severity more accurately. The tumor detection task can separate healthy brains from tumorous brains and the accuracy of the detection can save lives of the patients. The tumor segmentation task segments the tumor region from the rest of the brain to specify the exact location, area, and other tumor properties. This allows the healthcare professional to focus on the details of the tumor region only; hence, the accuracy of the tumor segmentation is a key point to treat patients with brain tumors. The accurate segmentation can also specify the severity of the tumor based on the segmented tumor properties. The tumor classification task can also help with assessing the severity of the tumor by classifying different tumor parts and grading the tumor based on its properties. As all of these brain tumor analysis tasks are directly related to help the healthcare professional in saving the lives of their patients and provide them more accurate diagnoses, applying different ML and DL models to improve the detection, segmentation, and classification performances is very crucial. Hence, comprehensive reviews on each of these tasks to understand the current state of the research, the performances of the current systems, and their advantages and limitations can help other researchers to get a clear idea about the existing researches and the scopes of improvements and contributions for better automated systems. The goal of this survey paper is to provide a review on the basics of the brain tumor image analysis and the existing researches with their contributions and our observations on them. The existing recent survey papers are summarized to represent the researches on the various tasks of brain tumor analysis, their features, available datasets, etc. The brain tumor detection, segmentation, and classification researches are added to sum up the approaches (i.e., novel and others) used with types of features extracted from the images for the tasks, the performance scores of the implementations, their contributions to the research field, and our observations to their works to guide the future researchers on all major key points of future research possibilities.
The objective of this paper is to generate a complete review of existing approaches on brain tumor analysis, including the methods used in various recent works with their performance, contributions, and some observations. It includes existing recent reviews on medical image and brain image analysis, novel approaches used with various models, features used for brain image analysis, datasets that are available for research, common measurements used for performance evaluation, etc. After analyzing the relevant works, the paper also discusses the current challenges for these tasks and scopes out the requirements for a completely automated brain tumor analysis system.
The rest of this paper is outlined as follows. Brain tumor analysis tasks are summarized in Sect. 2. Section 3 includes various brain tumor analysis features and models. Sections 4, 5, 6, 7, and 8 include summaries of some existing works on brain tumor analysis—surveys, conventional methods, machine learning methods, deep learning methods, and hybrid methods, respectively. Section 9 lists some benchmark datasets for brain tumor analysis. Section 10 includes the performance metrics used in brain tumor analysis. Section 11 discusses the scope of research and challenges of brain tumor analysis and Sect. 12 concludes the paper.
2 Brain tumor analysis
As shown in Fig. 5 , brain tumor analysis tasks can be divided into three main categories: (i) brain tumor/non-tumor detection, (ii) brain tumor segmentation, and (iii) brain tumor classification. All these tasks have been approached by researchers using different methods. The AI-based systems use various types of brain medical images as inputs. The tumor detection task analyzes the image and detects if there is a tumor present in the image or not. The output of the tumor detection task is the decision (i.e., tumor or non-tumor/healthy). On the other hand, the tumor segmentation task is to determine the exact tumor region in the image and segmenting or extracting that region from the image. Finally, the tumor classification task can incorporate processes like grading the tumor based on the severity (i.e., benign/malignant or grade I–IV) or specifying different tissues in the tumor (i.e., core tumor, edema, enhancing tumor, non-enhancing tumor, etc.).
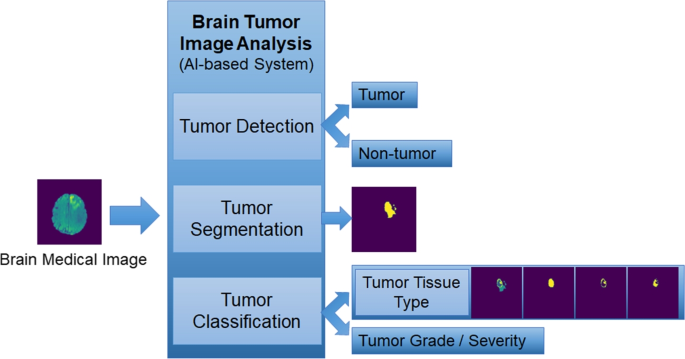
AI-based system for brain tumor analysis tasks
Brain tumor detection is a tumor/non-tumor classification task that detects if a brain MRI is from a healthy brain or has a tumor/abnormality. Various machine learning models, deep learning methods, and hybrid models have been used to classify MRIs into tumor and non-tumor classes [ 20 ]. Tumor/non-tumor classification is based on datasets containing both healthy brain MRIs and tumor brain MRIs with labels to train supervised/unsupervised/deep learning models by extracting distinguishing features of healthy tissues and tumor tissues. Figure 6 shows some examples of brain medical images with healthy brains and brains containing tumors [ 40 ]. Similarly, the brain tumor classification task is to classify or detect the different types of tumor tissues shown in Fig. 1 .
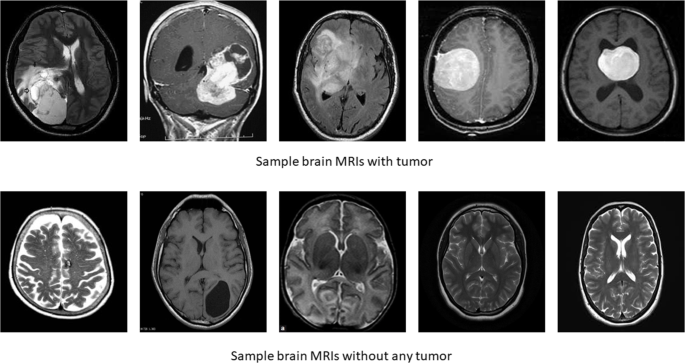
Sample tumor and non-tumor brain MRIs used for brain tumor/non-tumor detection task [ 40 ]
In some works, tumor classification is also used to detect tumor type (i.e., benign/malignant or specific type of tumors) [ 34 ]. But these two tasks are normally not experimented separately, instead they are combined with the brain tumor segmentation task. For brain MRI analysis, the images have been used for deep feature extractions (i.e., automatically extracted features by DL models) in most of the recent works, but a huge amount of research works also have been focusing on other approaches by extracting different intensity features (i.e., mean, variance, skewness, kurtosis, etc.), shape features (i.e., area, circularity, volume, etc.), and texture features (i.e., correlation, contrast, energy, entropy, local binary pattern, etc.) from the MRIs to either incorporate them with the images or to use only the extracted features as system inputs achieving efficient detection and segmentation results [ 46 ].
Brain tumor image segmentation task is an image segmentation problem which is the process of segmenting images to extract a specific part (i.e., the region of interest, denoted ROI) based on certain conditions specific to the problem. Figure 7 shows few samples on original MRI, the ground truth tumor in the original mask (marked as red), and the predicted segmentation results of the tumor (marked as red). Brain tumor can contain different types of tissues as core tumor tissues, edema tissues, enhancing tumor tissues, non-enhancing tumor tissues, etc. Figure 8 shows a sample MRI and the different types of tissue the tumor contains. In recent years, image segmentation became very popular to medical researchers for analyzing medical images like MRI, CT scans, etc. Image segmentation approaches help in identifying different areas or groups of pixels containing ROI organs, or lesions inside the organs, hence forming the research field for medical image segmentation [ 21 ]. Brain tumor image segmentation from non-invasive brain scans (mostly MRIs) has been explored by researchers from various fields to generate efficient and accurate automated brain tumor segmentation systems that can segment tumor, and classify the tumor type and severity so that abnormalities can be detected and treated in a timely manner [ 55 ].

Sample tumor area segmentation task outputs
Sample MRI and different types of tumor tissues
Most of the existing research focus on brain tumor image segmentation task since correctly segmenting the region of tumor from the rest of the image is crucial for diagnosis and it is also the most complex task among the three tasks depicted in Fig. 1 . It should also be noted that few researchers have worked on more than one of these tasks due to the unavailability of appropriate datasets and due to the complexity of each task. Research on developing a complete and accurate system able to solve all of the tasks without any human intervention is, therefore, one of the major future scopes of the brain tumor image analysis problem.
3 Features and methods
Brain image analysis needs to be able to perform feature extraction from brain MRIs. Various models and algorithms can then use these features to generate outputs for the brain analysis task. Different types of features and models have been used for brain image analysis in the literature and they are summarized below.
3.1 Features
The features collected from brain MRIs can be divided into four main classes as noted by [ 46 ]. These classes are (i) intensity-based features, (ii) texture-based features, (iii) shape-based features, and (iv) deep learning–based features and they are diagrammed in Fig. 9 .
Brain tumor analysis feature types
3.1.1 Intensity-based features
Intensity features are based on the first order histogram which has four statistical features: mean, variance, skewness, and kurtosis. The values for the features of an image are calculated using the grayscale pixel values of the image. Let, \({G}_{i}\) be the pixel value of a pixel i of the greyscale image. If the total number of pixels is N , then the mean value represents the center of distribution of the first-order histogram of the image. Variance measures how dispersed the pixel values are, skewness provides the degree of asymmetry of the histogram, and kurtosis measures the peakedness or the flatness of the histogram. Equations 1 , 2 , 3 , and 4 can be used for calculating the four features.
3.1.2 Texture-based features
An image texture is a set of metrics calculated in image processing designed to quantify the perceived texture of an image. Image texture gives us information about the spatial arrangement of color or intensities in an image or selected region of an image [ 77 ]. Specifically, gray-level co-occurrence matrix (GLCM), autocorrelation, correlation, homogeneity, energy, entropy, local binary pattern, dissimilarity, contrast, cluster shade, and prominence are the mostly used texture-based features for brain image analysis.
3.1.3 Shape-based features
Shape-based features characterize the shape of objects. Some of these features are area, volume, perimeter, convex area, polygon shape, circularity/irregularity of the object, etc. They can be extracted as shape-based features from a brain MRI.
3.1.4 Deep learning-based features
Deep learning features are the simple and complex features collected automatically by the DL models at every level of the neural network. At each layer of the network, new features are found and feature maps are created. For example, the first layer may extract the edges, the second layer may extract the corners, the third layer may extract the shape of the objects, and so on. Every layer extracts some more detailed feature information of the input image and these features are used to create feature maps/vectors and passed through the network. The features are concatenated (based on the network architecture) and used for brain tumor detection, segmentation, and classification.
3.2 Methods
Various types of methods and models are used for brain tumor image analysis. They can be categorized into four classes: (i) conventional methods, (ii) machine learning models, (iii) deep learning models, and (iv) hybrid methods [ 46 , 55 ]. Figure 10 shows the commonly used methods for brain image analysis.
Types of brain tumor analysis method
Conventional image analysis methods were used in brain tumor image analysis for a long period before the ML and DL models/algorithms were introduced. They are still being used in the pre-processing and post-processing steps in recent researches when incorporating algorithms to enhance image and/or pixel features. Various thresholding models are applied on images to define a threshold for the pixel scores and then removing the pixels below the threshold. This process helps with removing noise from images and enhancing the ROI pixels. Figure 11 shows some sample outputs of few popular thresholding methods applied on brain MRIs. The region-based models use the connected pixels to find the regions from the images and apply contouring algorithms to outline the ROIs, and Fig. 12 shows an example of region extraction using connected component analysis and contouring of the region. Basic morphological operations are applied to reduce or expand the boundaries of objects in an image. Figure 13 shows four basic morphological operations [ 71 ] on brain MRIs and all of these operations are based on the neighbor pixels to decide whether to remove some pixels from boundary of the object (i.e., erosion), or to add pixels (i.e., dilation), or to use a combination of both like opening (i.e., erosion followed by dilation) or closing (i.e., dilation followed by erosion) to represent the ROI more appropriately. Although geometry-based algorithms are not commonly used in brain tumor analysis for lack of appropriate geometric properties of organs, some non-Euclidean geometry models are rarely used in brain tumor image detection or segmentation. The popular conventional methods include basic thresholding (i.e., binary, Otsu, Yen, Adaptive, Manual thresholding, etc.) and filtering models (i.e., anisotropic filtering), region-based models (i.e., region growing, connected component extraction, contouring, etc.), geometry-based models (i.e., graph-based algorithms, fractal geometry, computational geometry, etc.), and morphology-based models (i.e., erosion, dilation, opening, closing, etc.).
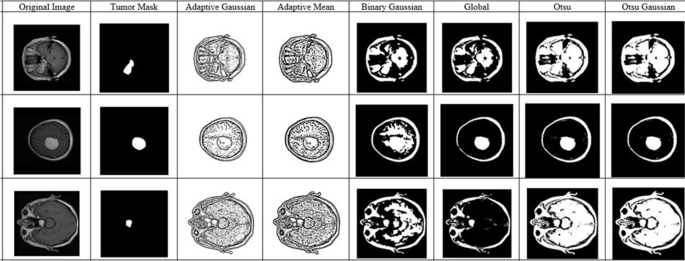
Examples of various thresholding methods
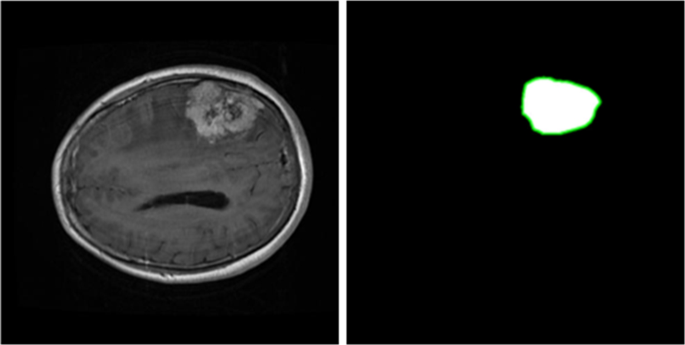
Example of ROI extraction with region-based algorithms
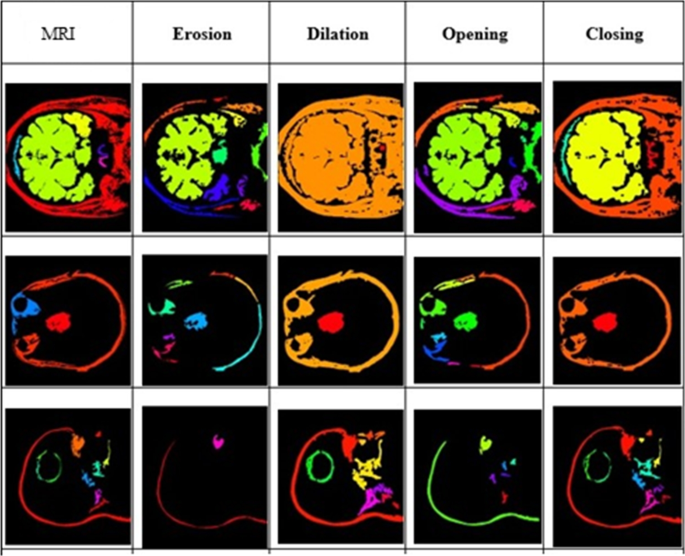
Examples of basic morphological operations
Another category of research is based on AI algorithms that are in general used to imitate human intelligence with computer-based systems [ 75 ] and medical data analyses have been using AI models to develop automated systems to assist healthcare professionals. Machine learning (ML) is a branch of artificial intelligence (AI) and computer science which focuses on the use of data and algorithms to imitate the way that humans learn, gradually improving its accuracy [ 72 , 79 , 78 ]. The machine learns from the data provided and improves the prediction accuracy using various methods when supplied with further data. ML algorithms can be supervised, unsupervised, semi-supervised, and reinforcement learning based on the data and training process. Supervised ML algorithms use data containing labels/ground truths to learn mapping input data into output data. Unsupervised ML algorithms on the other hand use unlabeled data and find patterns/structures from the data to learn. Regression, SVM, DT are some examples of supervised ML algorithms and classification algorithms, KNN are examples of unsupervised algorithms and clustering algorithms. Semi-supervised ML algorithms combine both labeled and unlabeled data to develop the classifier outputs. Reinforcement ML algorithms learn from the observation of the environment and the feedbacks on the actions from the environment. Examples of the most commonly used machine learning methods for brain tumor analysis are SVM, RF, K-Means clustering, and FCM clustering.
Most of the recent works mainly focus on deep learning models. Deep learning is a subset of ML that uses a hierarchical structure of artificial neural networks (ANN) with multiple hidden layers to extract high-level features from inputs [ 73 ]. Different types of DL algorithms are applied with variations in network structures like CNN, DNN, RNN, etc. [ 74 ]. DNN is a neural network with multiple hidden layers that creates a sequence of fully connected layers for deep learning. At each layer of DNN, more complex features are computed by transforming the input data or the data from previous layers and the DNN learns to generate outputs by the data encoding and decoding process within the hidden layers. Figure 14 shows a sample DNN with input layer, hidden layers, and output layer. CNN mirrors how human intelligence recognizes patterns from images. CNN models use convolution layers for feature map extraction from images, pooling layers for dimension pruning, and fully connected layers for connecting each neuron of one layer with all neurons of other layers. Each layer contributes to the feature extraction and computes more complex features incorporating the feature maps of previous layers. The efficiency of CNN in automatically extracting deep features and training with those features for more appropriate classification and segmentation makes CNN and its variants a prominent choice in medical image analysis. Figure 15 shows a sample CNN structure with the basic CNN components. Different variants of CNNs can have different structures with sequences of multiple convolution and pooling layers, with additional batch normalization, dropout, and different hyperparameter settings required for the image analysis task. As DL models achieve significantly high performance for brain image analysis, hence CNN models and their variations like VGG, AlexNet, GoogleNet, ResNet, attention-based CNNs, etc. [ 69 ], and variants of fully convolutional networks like U-Nets (popular for biomedical image segmentation) [ 70 ], and DNNs [ 76 ] have been very popular for brain medical image analysis tasks. Other than these methods, various hybrid models containing multiple methods from these three (i.e., conventional, ML, and DL), or a combination of these with heuristics, genetic algorithms, statistical models, and other novel architectures are also popular and they have shown to achieve high accuracy comparable to DL models. Some of the recent approaches from all these categories are discussed in the next five sections.
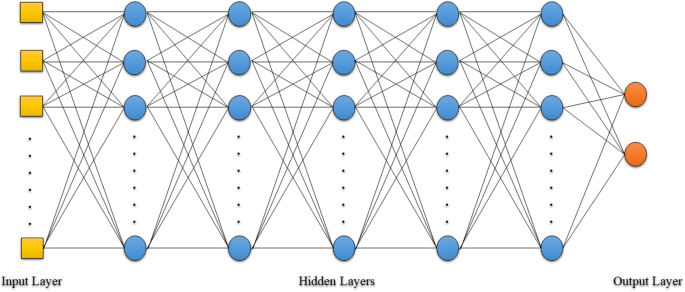
Sample DNN structure
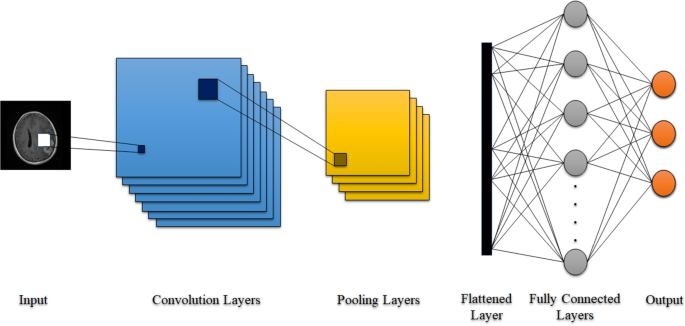
Sample CNN structure
4 Survey/review papers
A significant amount of research has been produced on medical image processing and brain tumor analysis since researchers have been working in the area for decades. Some researchers have also reviewed and summarized the existing works from different perspectives and for different timelines. Medical image analyses with various semi-supervised machine learning, multi-instance learning, and transfer learning for different organs have been discussed in detail in [ 11 ] with references to relevant systems. Some survey papers like [ 21 ] have discussed more recent methods based on DL models for medical image analysis, including their challenges and solutions.
Tiwari et al. [ 55 ] reviewed the recent methods used for brain tumor segmentation and classification of brain MRIs in 62 research papers from 2014 to 2019. They included details of brain MRIs, various types of MRI imaging techniques, their outcomes, differences, advantages of MRIs to extract the gray matter (GM), white matter (WM), and cerebrospinal fluid spaces (CSF) individually. They also discussed all the superclasses and subclasses of brain tumor classifications, detail classification of gliomas, and tumor grades based on their severity and location in the brain. The image segmentation methods used up to now including thresholding-based (i.e., region growing), supervised (i.e., ANN, SVM, RF, KNN), and unsupervised (i.e., clustering and active contour models) machine learning were also discussed as they were used for brain tumor segmentation. Both supervised (i.e., DT, NB, bayesian, SVM, LDA, perceptron, KNN) and unsupervised (i.e., hierarchical clustering, FCM, k-means, SOM) tumor classification methods were listed with references. The techniques, performance measurements, true-positive and false-positive rates, and the datasets were summarized in a tabular format for all the 62 reviewed articles with their textual summary. A list of 19 datasets used in previous brain tumor segmentation studies with their references and frequencies of usage also provided a clear idea about the benchmark datasets and their availability as well as showing that the datasets BRATS 2013 and BRATS 2015 were being used in most recent works. The authors also included some statistical results on the country of origin of the reviewed articles, publishers, citations, publication years, used datasets, used methodologies, etc. The comparisons between the used methods showed the dominance of CNN and PSO in recent years, whereas SVM, FCM, and k-means had the second rank among the used methods from 2014 to 2019. Different performance metrics used in brain tumor analysis were explained with proper equations and their network visualization maps of the terms and their links were helpful to get a general idea about the terms used in similar articles. Their conclusion on various deep learning and PSO methods being the current and future focus on this research field has been validated with our review on the recent works on brain tumor segmentation and classification. The review paper was quite thorough on the recent research works and correctly mentioned the contributions of all the 62 articles. The tabular summaries and statistical analysis on various topics were helpful for quick comparisons between different methods and the datasets, publications, performance metrics, etc. Summaries for some papers were very brief due to their similarities to some other discussed works and the methods used in various papers were mentioned with their names. The benchmark datasets were listed with their names and references. More details on the datasets, such as the number of items, type of data, public availability, and ground truth availability, could help for understanding the datasets better when choosing an appropriate one for further related research.
Saman et al. [ 46 ] published a survey on brain tumor segmentation and various feature extraction techniques discussing MRI systems, the technology behind MRI processing, and MRI sequences with explanation and examples. Different grades of brain tumor, their locations, symptoms, and their treatment methods were listed in the survey. The paper reviewed works on brain tumor detection from the last ten years with a main focus on works from the last three years. The authors discussed pre-processing of the MRIs like skull stripping, bias field correction, noise removal, object detection, and segmentation using existing tools and algorithms. The existing research works on brain tumor segmentation were summarized as manual, intensity-based (i.e., threshold-based, region-based, classification-based, clustering-based), atlas-based, and deformable model-based (i.e., level set and active contour models), and hybrid and deep learning methods (i.e., CNN, cascaded CNN, FCN, DCNN, etc.). The advantages and disadvantages of some of the papers from these methods were expressed in tabular format. The authors also analyzed and reviewed recent works on feature extraction methods for brain tumor analysis. Different types of features, including texture-based (i.e., autocorrelation, correlation, contrast, cluster prominence, shade, dissimilarity, energy, entropy, homogeneity, and local binary pattern), intensity-based (i.e., mean, variance, skewness, kurtosis, and pixel orientation), and shape-based (i.e., area, perimeter, circularity, irregularity, and shape index) were defined and described with their corresponding equations. Deep learning–based automated feature extraction techniques were also discussed. The recent focus on various CNNs and U-Nets with their variations was mentioned by the authors as well. The detailed analysis on the features of brain tumor segmentation mentioned in the paper was helpful to understand and compare the features for feature extraction and processing of similar systems. Mentioning and referring to more than 130 research works from 10 years was impressive and useful in order to analyze the popular methods and their performances in brain tumor research. Although a huge number of relevant articles were listed in the references, most of them were not properly discussed or summarized which made it difficult to generate any comparison-based decisions without actually going through the cited papers individually.
Another review on brain tumor segmentation was presented in [ 57 ]. The authors covered recent developments in brain tumor segmentation by summarizing 19 recent articles with their methods, datasets, data dimensions, MRI modalities, execution time, detected abnormality types, and the supervision status (i.e., automatic, semi-automatic, etc.). They also added a comparative analysis of their performance metrics (i.e., Dice score, precision, sensitivity) from two benchmark datasets—BRATS 2013 and BRATS 2015—guiding future research towards the most promising approaches. Conventional methods such as thresholding and region growing were defined and explained with mentions of some existing morphological methods applied in relevant research works. Supervised methods (like KNN, SVM, RF, and ANN), unsupervised methods (like k-means and FCM), active contour models, and hybrid methods combining the aforementioned ones were explained with brief summaries of more than 100 existing methods. The authors concluded their work with the recommendation that the application of conditional random field (CRF) with FCNN, DeepMedic, or ensemble systems was superior from the performance comparison presented in the paper. The authors presented a detailed summary on brain tumor segmentation research works from recent years. Most of the works among the more than 100 articles they included were focused on the last 10 years, and they were discussed with brief summaries of their contributions in separate sections based on the methods used. The different methods existing in the literature were defined and described properly with their advantages and disadvantages. Although a large number of papers were covered in this survey, similar to the previous survey, the contributions of most works were not mentioned properly with their outcomes or performance metrics. The comparison of performances was done on few selected works to include as many methods as possible in the comparison, but comparing few works was not sufficient to make any generalized decisions on their scope and possible future research directions.
Abd-Ellah et al. [ 1 ] reviewed brain tumor MRI image segmentation from a machine learning and deep learning perspective. Machine learning techniques for image denoising, reconstruction, registration, and skull stripping were mentioned as preliminaries to brain tumor analysis. A generic computer-aided diagnosis (CAD) system was analyzed with a framework containing data collection, pre-processing with masking, thresholding, filtering, etc., segmentation with various techniques, texture, intensity and other feature extraction, feature selection with genetic or sequential algorithms, feature reduction with principal component analysis, classification with machine learning and deep learning, performance evaluation, and final diagnosis. Brain MRIs, MRI types, and a discussion of 14 popular datasets, namely, AANLIB, ADNI1, Allen brain atlas, BrainWeb, Brain-development, BRATS 2012 to 2017, Cjdata, Rider, IBSR with their sources, modalities, number of images, image types, number of patients, and source link, were included by the authors. Traditional machine learning methods and deep learning methods for tumor detection, segmentation, and classification were then discussed with relevant references. CNN, cascaded correlation artificial neural network (CCANN), feedforward backpropagation neural network (FFBPNN), backpropagation neural network (BPNN), probabilistic neural network (PNN), fuzzy Hopfield neural network (FHNN), and many other DL models were discussed in detail. Thirty-two machine learning–based papers with their feature extraction methods, detection methods, datasets, performance metrics (i.e., sensitivity, specificity, and accuracy), and limitations were summarized for tumor detection. Similarly, 41 papers on tumor segmentation with their methods, datasets, performance metrics (i.e., dice score, accuracy, sensitivity, and specificity), and limitations were discussed. The comparison showed that DL performed better than other methods for all these approaches. A discussion of 15 tumor classification–based papers was also added with their features, classification methods, datasets, performance metrics, and limitations. The authors discussed the scope of brain tumor methods based on the limitations extracted from existing works and mentioned the small amount of work on the detection of tumors as well as the limited number of datasets for the process. The advantages of DL models over traditional machine learning methods were also mentioned with the necessity of providing a more precise approach to specify the location, area, and performance measures for the tumor. The authors concluded the paper with the observation that more complete systems combining detection, segmentation, classification, and precision analysis of brain tumor are needed in this research area. The review on brain tumor analysis provided by the authors covered all major issues of brain tumor studies. They discussed all basic components of the analysis in detail with a general framework structure and mentioned the specific works of existing approaches with their limitations and scope. Although the individual summaries of the papers were very brief, the authors were able to point out all the major contributions and challenges of the field and the possible future works towards a combined system.
A very recent survey on brain tumor segmentation with deep learning was presented in [ 30 ]. The authors discussed image segmentation and various brain tumor segmentation techniques such as manual, semi-automatic, and automatic methods, and then focused on automatic methods that used deep learning. To introduce the deep learning process, the authors started with a detailed explanation of neural networks. CNNs and each building block of CNN (i.e., convolutional layer, pooling layer, non-linearity layer, fully connected layer, optimization, loss function, parameter initialization, hyperparameter training, and regularization) were defined and explained with proper references to some conventional and novel methods for each of them representing the original methods and their various optimizations. Similarly, the structures of deep CNN structures like single/dual pathway, cascading, and U-Nets were included with their definitions and descriptions with various approaches to optimize the results for a network. The paper included a detailed discussion on the various steps and techniques for brain tumor segmentation starting from pre-processing (i.e., bias field correction, normalization, etc.) to post-processing (i.e., CRF, MRF, connected component analysis, morphological operations, etc.) with class imbalance solution (i.e., loss functions, hard negative mining, two-phased training, hierarchical segmentation, etc.), data augmentation (i.e., data transformation, artificial data generation, etc.), datasets (i.e., Decathlon, BRATS 2012 to 2019, etc.), and performance evaluation metrics (i.e., Hausdorff measure, Dice score, sensitivity, specificity, etc.), and various software and frameworks (i.e., Theano, Pylearn2, Pytorch, Kears, Tensorflow, Caffe, etc.) were used for brain tumor segmentation. An overview of recent deep learning methods with their input type, pre-processing methods, optimizer, activation and loss function, and regularization was added for 26 methods based on U-Net architectures, and for 13 works on dual-pathway architecture, for 8 works on single-pathway architecture, for 7 works on ensemble architecture, and for 13 works on cascaded architectures. The performance metrics of the papers that used BRATS 2017, BRATS 2018, and BRATS 2019 datasets were compared and discussed with the conclusion that U-Net with good set of parameters performs better than other deep learning methods for brain tumor segmentation. This survey on the recent deep learning methods for brain tumor segmentation was quite unique in its representation of the works. Instead of summarizing the referred methods individually, the authors created a complete introductory analysis and workflow for brain tumor segmentation and referred parts of the recent works in each component of the introduction and workflow. The approach helped to better understand the whole process while learning the existing methods used for each part of the process. The discussion and comparisons of the methods helped to realize the best available options for similar works up to recently. Keeping track of the complete processes used in each of the recent works was a bit inconvenient as they were distributed throughout the workflow.
Another deep learning–based brain tumor image analysis survey that was recently published [ 3 ] reviewed 427 research works published from 2018 until February 2020 in the Science Direct database, the IEEEXplore Library, and Scopus by following a systematic review protocol. After scanning titles and abstracts and checking the relevance of all the 427 works, 92 papers were read thoroughly and summarized for the survey. The authors focused on two types of methods—brain tumor segmentation and brain tumor classification. They started by mentioning the large-scale use of SVM, then random forest, and the evolution to deep learning (DL). They included an overview of the datasets used and the DL method. Performance measurements were included in tabular and summary paragraph formats to show how DL methods were able to achieve more than 90% accuracy and Dice score comparison to machine learning (ML) techniques with around 70% Dice scores. The BRATS datasets and around 10 other frequently used datasets (i.e., TCIA, REMBRANDT, etc.) were also mentioned. Different methods applied for brain tumor segmentation research with references were also discussed briefly. Data augmentation with image transformation or synthetic data generation, CNN architecture with convolutional layer, fully connected layer, pooling layer, activation function, and evaluation metrics used in recent DL methods were described. Popular CNN structures like GoogleNet, AlexNet, VGG, ResNet, U-Net, etc., and their implementation in brain tumor analysis were explained, and the application of transfer learning for those structures was mentioned. The advantages of the DL methods for higher accuracy and consistency were explained and the challenges when using smaller datasets and corresponding ground truth were mentioned in the conclusion. The survey paper was organized in a very efficient manner to get an overview on the different DL methods for both segmentation and classification used in recent years, their performances, datasets, and some discussion on the general brain tumor analysis framework and steps. The paper covered all recent research directions and challenges.
Some brain image analysis reviews discussed only parts of the complete brain image processing such as skull stripping and mentioned the existing works and datasets [ 16 , 43 ]. The methods were sometimes used either as a pre- or post-processing for brain tumor analysis or overlapping with brain tumor studies, and some of those datasets were used in brain tumor analysis methods. Table 2 contains summaries of these survey papers.
5 Conventional methods
Although machine learning and deep learning methods are more popular now, the methods for brain tumor segmentation originally started with thresholding and morphological operations. With the rise of machine learning and deep learning, more researchers focused on the recent approaches, but some thresholding, filtering, and morphology based methods are still being developed individually or in hybrid approaches with machine learning or deep learning and they show promising outcomes. A novel region-based level set method on topological changes of curve evolution using superpixel fuzzy clustering and Lattice Boltzmann Method (LBM) was proposed very recently [ 27 ]. A watershed transform (WT) algorithm with multiscale morphological gradient reconstruction (MMGR) to handle over-segmentation was used for segmentation. Dilation, erosion, opening, and closing were applied for morphological reconstruction. Then the En-FCM method was implemented for the superpixels and the results were used to generate a two-region level set. Finally, the contours were produced after minimizing the energy of the level set function using LBM to produce the final segmentation. The proposed method was tested on both synthetic and BRATS2017 data and the segmentation results were compared to 6 other recent segmentation methods to show that the proposed method outperformed other methods by at least 3% to at most 20% for the Dice score, and it had better sensitivity and specificity. The proposed method was computationally efficient and took less than 4 s for each image, whereas the similar methods like LINC, LIC, GINC, and VLS required at least 20 s to more than 100 s to process each image. The inclusion of superpixels and LBM helped to reduce the running time significantly. The method was also robust to noise, heterogeneity, and initial contour. The proposed method was computationally at least 5 times faster than the existing approaches, but the method was used with only a single modality that was applicable for whole tumor and it was unable to specify the core or other tissues. It was mentioned that the proposed method was applied to both synthetic and BRATS2017 datasets, but there was no proper description about the synthetic data. Only 40 2D slices from FLAIR modality of the BRATS2017 dataset were used for the experimentation without proper justification. Although the proposed system was computationally efficient and performed better than other recent approaches, the results were from experiments on a very limited dataset and hence not enough to establish the performance of the method as a comparable method for brain tumor segmentation.
Islam et al. [ 24 ] proposed a novel parametric active contour model (PACM) that reduced the processing time of brain MRIs by extracting three initial points for initial contouring of a snake model. The proposed model also provided the optimal set of parameters for PACM model by trial and error and calculated the area of the detected tumor region by measuring the pixels within the extracted tumor region. After reshaping the input MRIs, the proposed method applied a median filter on them to reduce noise while keeping the contrast variation information. An initial contour interpolation method was then used to find three initial points to form a triangle and then to draw a circle through the points so that the tumor was inside the circle. Then, the PACM was applied to draw the tumor contour starting from the circumscribed circle with 3 initial points and found the optimal parameter set for the PACM with 180 iterations using step size = 1, scaling factor = 0.5, elasticity controller = 0.38, rigidity controller = 0.21, weighting factor for intensity-based potential = 0.32, weighting factor for edge-based potential = 0.38, and termination potential weighting factor = 0.7. The sample output images showed that the proposed method was able to correctly contour the tumor region in the MRIs. As the intensities of the pixels in brain MRIs are very similar, threshold-based, contrast-based, or edge-based methods had issues trying to find the tumor region correctly. The proposed snake method using only three initial points for contouring seemed a simple but efficient solution for brain tumor segmentation. The details of the used dataset and performance metric measurements were not available so that the accuracy of the method or a comparison with other existing similar approaches was not possible. It was mentioned that the dataset was collected from available resources without any detail on the number of data instances, ground truth, or the images themselves. All of the sample images included in the paper had a very visible and high-intensity tumor region which is not the case for all brain MRIs. So, it was not clear if the method would work on other brain MRIs where the tumor region is not clearly visible. For these reasons, any generalization of the performance of the method was difficult.
A region-based fuzzy active contour model was proposed recently in [ 4 ] for brain tumor segmentation. The MRIs were divided by Otsu thresholding [ 79 ] into three non-overlapping areas (i.e., tumor, dark region and background, and the rest of the brain region), and an energy-based membership degree calculation was done on the pixels to separate them into two parts. After repeating the process until the total energy became stable, the contours for the regions were then generated. After normalizing the MRIs, anisotropic filtering was applied for the segmentation algorithm to get the texture features, and then the SLIC algorithm was executed to create clusters of superpixels. The three-region division with Otsu thresholding was then used and repeated as mentioned earlier to generate the final tumor boundary. The novel approach was experimented on with both synthetic data and the BRATS2013 and BRATS2019 datasets. The performance metrics comparisons between the proposed method and nine other baseline methods for the BRATS dataset, four other baseline methods for the synthetic dataset, and two methods for both datasets showed that the model outperformed most of them and were compatible with the remaining ones. The idea of clustering based on the energy of the pixels to create clusters of tumor/light pixels, dark pixels, and other pixels was an impressive idea which helped to increase the region contouring efficiency. But to generalize the result decisions, some more experiments on the proposed approach were needed with MRIs having overlapping regions and very similar intensities or energy for most pixels to check if the clustering algorithm was able to identify them correctly and was able to draw tumor boundary contour properly for overlapping or vaguely distinct areas.
A model using ROI extraction, region growing and morphological operations (i.e., dilation and erosion), FCM, and Gaussian filters was proposed in [ 52 ] for brain tumor segmentation, area, and centroid detection. The model applied FCM on the MRIs to get clusters with tumor pixels and non-tumor pixels for extracting and removing the non-tumor regions. Then, the tumor regions were labeled and after thresholding of the MRIs, two morphological operations were used on them. Dilation was used to identify region growth, and erosion was applied to detect seed points. Sequential application of the morphological outputs was then used to capture the complete tumor region edge for tumor segmentation. Performance scores on specificity and sensitivity of 10 patient MRIs were compared to show the efficiency of the system. The idea of applying a hybrid model by combining morphological operations, clustering, and filtering for tumor segmentation was promising. But the lack of detailed explanation of every step of the methodology, the experimental setups, the comparison of the method with other baseline methods, and the absence of a comprehensive dataset details made it difficult to assess the efficiency of the model and to re-implement the model for testing the system on a benchmark dataset. A novel thresholding method with similar idea was proposed in [ 22 ] for brain tumor segmentation. Basic morphological operations and pixel subtraction were used for pre-processing. Then, a new thresholding based on the uniquely valued pixels was applied and median filtering was used for refining the output. The TCIA dataset was used and achieved 96% accuracy which outperformed the standard Otsu thresholding–based segmentation by at least 8%.
Brain tumor image analysis and brain tumor segmentation methods were also explored from a geometric point of view where in most cases “fractal geometry (FD)” was used [ 18 ]. Fractal geometry is a non-Euclidean geometry that is defined recursively using self-similarity. As the elements in nature and animal/human body do not follow proper Euclidean geometrical shapes or rules, fractal geometry introduces self-similarly fine structures that use magnification to create infinitely complex objects. Various fractal geometric features and algorithms were implemented for brain tumor image segmentation by various researchers. Iftekharuddin et al. [ 45 ] extracted fractal features for pediatric brain tumor segmentation and classification. The piece-wise triangular prism surface area (PTPSA) algorithm was used for fractal feature extraction, and a novel fractional Brownian motion framework was used for fractal wavelet feature extraction from 204 T1, T2, and FLAIR MRI images. The fusion of those two features with intensity values was very effective for automatic tumor detection from single and multimodal MRIs where the tumor was clearly visible. Although the model worked better than existing fractal feature–based systems, it was unable to properly distinguish tumors from the other parts of the brain when the tumor was not the most visible area (i.e., the only region containing highest intensity pixels) in the image. Multiple fractal algorithms (i.e., PTPSA, piece-wise modified box counting (PMBC), and blanket) were used for brain tumor segmentation from MRI images as described in [ 25 , 56 ]. After dividing each MRI image into multiple pieces, the algorithms used the pixel intensities of each piece to detect the tumor. Different intensity threshold values were used for fractal dimension (FD) and cumulative histograms. The PMBC algorithm worked better for tumor detection than the other methods described in [ 25 ]. In [ 56 ], 80 MRI and CT images were tested for statistical validation of using fractal dimension in brain tumor detection. The comparison of average tumor FD and non-tumor FD in 80 images, negative and positive FD difference of half images for PMBC, PTPSA, and blanket algorithms showed the effectiveness of FD as an important attribute for tumor detection. The experiments showed how FD values differed for the tumor area and the non-tumor area so that the tumor could be detected and distinguished properly from the other parts of the brain.
A few researchers worked on brain tumor MRI image analysis using some well-known computational geometry algorithms. Delaunay triangulation was used for reconstruction of a brain tumor in 3D using 2D parallel cross-sectional MRI segmented slices [ 9 ]. Sobel operators and morphological operations were used to find the boundaries and pre-processing before applying Delaunay triangulations between points of two planes. The 3D model was constructed with those connected 2D slices with a stacking algorithm. The research used a novel idea for 3D tumor image reconstruction using geometry that improved the segmentation quality. But the authors did not include any details of the datasets used or any proper experimental results to show the performance of the algorithm. Although the work contributed to tumor location, size, and volume calculation, the decision that their “proposed method” was better than other segmentations still needed to be proved by proper benchmark datasets and experimentation. “Convex hull,” another major topic from computational geometry, was used for brain tumor segmentation by Shivhare et al. [ 51 ]. Although the convex hull was only generated to be the input for an active contour model of brain tumor segmentation, it was one of the few approaches where a computational geometry approach was used for brain tumor image analysis. After pre-processing the MRI images from the BRATS2015 dataset, the key points were extracted from high-energy regions of the image to draw the convex hull for the tumor. The convex hull was then used to draw the exact tumor using an active contour model and to decide on the tumor core, complete tumor, and enhanced tumor. The proposed method produced outputs with 81 to 92% DCC, and performed better than state-of-the-art image processing methods. The convex hull was also used for a part of the process in [ 49 ] for convex area generation. A spectral clustering-based brain tumor segmentation method was used for tumor detection using the similarity graph on the MRI image. The connected component labeling algorithm was applied on the processed image to find all connected regions in the image, and define the largest region as the tumor. Then the tumor volume calculation was done by measuring the convex area generated from the convex hull of the tumor.
Compared to the ML and DL methods, the performances of conventional methods for brain tumor analysis tasks are generally inferior. But as shown in this section, the combination of multiple operations can provide comparable performances in some cases. Although applying only the conventional models is not popular in recent researches, they are still part of pre-processing and post-processing phases in almost every brain tumor analysis task. Different types of thresholding and filtering (i.e., Otsu, Sobel), watershed superpixel model, region growing algorithms, contouring algorithms, fractal geometry algorithms, few computational geometry algorithms (i.e., convex hull, Delaunay triangulation, etc.), and basic morphological operations are still used to enhance the input image properties, separate the ROIs from the background and from other parts of the image, creating boundaries of the tumors, locating the ROIs in image, removing noise from both input and output images, and computing geometric properties of the tumor and other similar tasks. The major advantage of the conventional methods is the simplicity of the application of the algorithms and that they work well with simple 2D images with distinguishable pixel properties between the tumor region and the rest of the image. The limitation of the conventional methods is the lack of features to handle complex multidimensional medical images where the differences between the tumor region and the other regions are subtle. The conventional methods can still be recommended for use for brain tumor analysis methods but the preferred option will be applying a hybrid of these models as part of pre-processing and post-processing while DL models (or efficient ML models) are used for main training modules.
6 Machine learning methods
Both supervised and unsupervised machine learning and their hybrid methods have been used for brain tumor detection/classification and tumor region segmentation tasks. Although most recent works focused on deep learning methods due to their high performance scores, the basic machine learning methods and their variations are still compatible with the deep neural network performance.
Reddy et al. [ 42 ] proposed a method using modified region growing and adaptive SVM for brain tumor prediction and segmentation. They summarized the proposed methods, advantages, and limitations of 18 related works from 2001 to 2019 covering different methods like SVM (with different variations), fuzzy clustering, kernel clustering, thresholding, and CNN for different medical image analysis (but mostly on brain tumor segmentation and classification). The pre-processing started with denoising the images and applying median filter after RGB to HSV conversion and V-image selection. The skull-stripped versions of the pre-processed images were then used for a modified region growing algorithm with gridding and seed point selection for ROI detection. To overcome the shading and over-segmentation, the proposed algorithm used two defined threshold values for intensity and orientation to create a guided region growing system. The segmented images were then used for 22 GLCM feature extraction from each direction of the four orientation angles as 0, 45, 90, and 135 making a total of 88 features for each input image. Then a grasshopper optimization algorithm (GOA) was applied for important feature set selection that was fed to a SVM classifier used for tumor classification. The BRATS 2015 dataset was used in the experiments and the performance of the proposed method was evaluated by peak signal-to-noise ratio (PSNR), mean-square error (MSE), and structural similarity index (SSIM) in order to compare them with existing k-means, hybrid k-means, and FCM methods. The results showed that the proposed approach either performed very similarly to the comparison algorithms or outperformed them by at least a small percentage. The accuracy, execution time, specificity, sensitivity, and other measurements outperformed other methods, however with more than 98% accuracy in minimum time. The tumor prediction approach with GOA-SVM was also measured against PSO-SVM and SVM for 24 low-grade glioma (LGG) and high-grade glioma (HGG) tumor images to prove that their method outperformed the other two by at least 8% higher accuracy by achieving more than 95% prediction accuracy. The authors outlined their proposed method step by step from pre-processing to experiments. They mentioned and discussed the results in detail with complete analysis of every aspect of the results, but they were not very descriptive at some parts of the methodology section. Rather than including the general theoretical definitions and equations of the steps, a more detailed and well-explained methodology mentioning the relevant values or components for their method could better clarify the proposed approach. Some ambiguity and abstractedness in pre-processing (i.e., skull removal), seed-point selection (i.e., histogram analysis), thresholds and other parameters of the algorithm, etc., throughout the complete methodology made it difficult to understand or duplicate their method.
Another ML-based tumor/non-tumor detection and segmentation model was proposed recently [ 54 ]. The input images were normalized and a median filter was used for pre-processing. Then, feature extraction was applied using a histogram of oriented gradients (HoG) after decomposing the images with DWT. Four texture-based features such as contrast, energy, correlation, and homogeneity were measured for each image. The images were classified as normal or tumorous with random forest, SVM, and decision tree classifiers. A basic principal component analysis (PCA) was used for image segmentation. Although the sensitivity value was higher for SVM, the random forest model achieved better performance for all metrics and achieved the accuracy of 98.37%. The proposed model outperformed few existing similar models; however, the unique contributions of the proposed approach and the segmentation results were not clearly mentioned.
A combination of supervised and unsupervised machine learning methods was proposed recently by [ 19 ]. Pre-processing of the images from the BRATS 2015 and 2019 datasets was done by histogram normalization. Gradient-based features and Gabor wavelet features were then extracted from the normalized images. An atlas-based feature enhancement process was applied that used the local average and standard deviation of pixel intensities. The values were applied to create atlases for training and test sets to enhance the features for an ensemble learning classifier. The ensemble learning framework consisted of 125 decision trees for accommodating the feature vectors of the training data. The dataset was randomly divided into six groups and five of them were used as training data and the rest as test data. Randomly selected feature vectors from 100 to 1000 K were used to train the decision trees with different depths varying from 25 to 45 based on the data entropy. The segmentation outputs from the decision trees were then post-processed with a random forest model to separate positive and negative pixels and finally a region growing algorithm was applied to the positive outputs to generate the final segmentation. The proposed method was tested against some existing CNN, deep CNN, RF, GA, FCNN, and hybrid approaches. The DCC of the proposed method varied from 0.84 to 0.88 that was similar to the deep CNN and it was able to outperform all other methods by at least a small percent. The proposed method combined multiple supervised and unsupervised machine learning creating an ensemble method which showed that the correct combination of machine learning methods was able to achieve similar and in cases better results than the CNN frameworks. As most recent works are mostly focusing on deep learning, a novel idea like this combining multiple machine learning methods to create an ensemble method that was able to generate efficient outputs similar to deep learning opened up the possibilities of a new research direction with the expectation of a ML-based method outperforming all recent DL methods.
Pitchai et al. [ 39 ] proposed a method combining machine learning and fuzzy k-means clustering for brain tumor classification and segmentation from MRIs where the machine learning was used for tumor/non-tumor classification and clustering was used for tumor segmentation. The MRIs were pre-processed to remove noise by a Wiener filter that limits the mean square error while smoothing the noise in the images. Then, sixteen GLCM features were extracted from the pre-processed images, and the most optimized set of features was selected with a genetic algorithm called Crow Search Optimization Algorithm (CSOA). A simple three-layer artificial neural network with a backpropagation layer was used to classify the images as normal images or abnormal tumor images based on the optimized feature set as input to the ANN. The weights of the ANN were adjusted by the mean squared error (MSE) at the training phase. The images classified as tumor images were then used as inputs to a fuzzy k-means algorithm executed for 12 iterations for tumor region segmentation. Various experiments on BRATS 2015 and BRATS 2017 datasets were performed by varying the neuron numbers of the ANN, the iteration numbers of the clustering, and over-segmentation and under-segmentation scores to choose the best parameter set for the proposed method. The performance metrics showed that the approach outperformed 2D ConvNet, FCNN, CNN, and KNN by at least 3% and achieved 94% accuracy. As CNN-based approaches generally outperformed the conventional machine learning methods, the proposed hybrid method outperforming basic CNN-based methods generated a new direction for future studies on brain tumor analysis combining basic machine learning algorithms with genetic algorithm optimizations to achieve better results without a high computation cost. Although the authors explained the process in detail, more information about the features and the optimized set of features could be helpful for future work.
Another hybrid k-means clustering and ANN-based method was proposed in [ 8 ] using k-means, ANN, and texture features for brain tumor segmentation using data from the BRATS 2015 dataset. The method classified a tumor as benign or malignant and then segmented the tumor region. For the classification, after applying the k-means clustering, the non-region of interest (ROI) parts were removed from consideration based on the clustering results. Then the texture features were calculated for the images so they could be applied to a basic ANN structure for tumor type classification. Another similar structure was used for the segmentation where after filtering out the non-ROI parts, each clustered object was used to extract features to train another simple 3-layered ANN for tumor region segmentation. The proposed segmentation achieved almost 87% accuracy whereas the basic SVM-based segmentation had around 67% accuracy. The proposed hybrid machine learning method outperformed the basic supervised methods by almost 10%. The idea of combining k-means clustering as a pre-processing for ANN helped in improving the segmentation performance, but the paper did not share proper details of the parameters of two structures combining the clustering and ANN, the features, performance metrics, etc., making the process a little bit difficult to follow.
Angulakshmi et al. [ 5 ] proposed a novel simple linear iterative clustering (SLIC) based approach for brain tumor segmentation using Walsh–Hadamard transform (WHT) [ 80 ] texture features of MRIs from the BRATS 2015 dataset. At the pre-processing step, non-local means (NLM) filtering was used to denoise the data and then N4ITK was applied for bias correction of only T1c images. After dividing each MRI into blocks, diagonal kernels of the WHT were used on each block to generate texture features and finally they were used to create the saliency texture map for the image that was used as input for the SLIC algorithm. The algorithm generated superpixels from the texture saliency maps and spectral clustering was used to segment the tumors. Then the member pixels of the superpixels were recovered to generate the final segmentation output. At the post-processing step, the regions other than the connected components were removed and a polygon fill algorithm was used to refine the segmented tumor. The proposed approach was compared to three other spectral clustering segmentation methods and outperformed all by 12 to 17% with DCC scores varying from 0.69 to 0.89. The method was also compared to k-means, Gaussian mixture model (GMM), and FCM clustering models and outperformed them all by 10 to 16%. The novel approach proposed by the authors using WHT and SLIC definitely improved the performance of the clustering and transformation methods and achieved results compatible with deep learning models providing an alternative way for efficient brain tumor segmentation with texture features and spectral clustering.
A brain tumor detection and segmentation framework containing ML with heuristics and thresholding was proposed by Devanathan et al. [ 14 ]. The MRIs were pre-processed by bilateral filtering, contrast enhancement, and skull-stripping. Then the pre-processed images were used for tumor segmentation with an artificial bee colony–based multi-level thresholding. After segmentation, the GLCM features were extracted and used by a SVM for classification into normal and abnormal MRI classes. Experiments using the Kaggle brain tumor dataset showed 97.56% accuracy, 97.9% sensitivity, and 97.91% specificity. A comparison with other ML models showed that the proposed model outperformed most of them and was comparable to the best model. Another ML model with heuristic for brain tumor type classification (benign or malignant) was proposed recently in [ 7 ]. After removing the noise from the MICCAI dataset, the tumor areas were segmented with a weighted Fuzzy Clustering Algorithm (FCA). Then features were extracted from the segmented images with LBP, GLCM, Gabor filter, discrete Fourier transform, and other first and higher order transforms. The features were used for a deep auto encoder (DAE) that applied a Barnacle Mating Optimization Algorithm (BMOA) and finally the random forest classifier classified the images into benign or malignant tumor classes. The F-measures were around 0.9 and were at least 20% higher than the basic ML algorithms. The proposed method combining multiple approaches with random forest outperformed the existing models. But the sequential steps of the methodology were not very clearly explained. The experimental results section did not provide the performance scores directly as specific values, which made it difficult to understand the results. Other than the mention of MICCAI, the dataset specifications and details were also missing.
A very recent ML-based approach for brain tumor segmentation was proposed in [ 28 ]. Each image was divided into 3 × 3 blocks to extract GLCM features following a pre-processing of the images with a median filter. An adaptive KNN (AKNN) classifier used the GLCM features to detect the tumor/non-tumor images. The abnormal images were then used for tumor segmentation. An optimal possibilistic FCM (OP-FCM) was used to segment the tumors, and a Binary Competitive Swarm Optimizer (BCSO) was used to optimize the centroids of the clusters. The experiments on BRATS and an internet dataset showed 99.9% accuracy and almost similar sensitivity and specificity as 97 and 98. The Matthews correlation coefficient (MCC) and F1-score of the proposed approach were compared to similar models and showed around 10% improvement. The proposed ML-based method clearly outperformed other models by a huge margin and performed better than DL-based methods for brain tumor segmentation. More details on the dataset and the specifications for the results could justify the performance in a better way.
ML models are popular mostly for brain tumor detection and classification tasks. These models are highly dependent on the data used to train the models, and similar to any other medical image datasets, brain tumor datasets generally have a limited amount of images (i.e., at most few hundreds). Some ML algorithms are used for tumor segmentation or part of the segmentation process. Although all types of ML algorithms have been used for brain tumor analysis, SVM and RF supervised classifications have been proved to be the most efficient for the tasks. On the other hand, K-means, KNN, FCM, and GMM clustering have been the most popular ones for the unsupervised ML models. Although, the clustering models performed well, ANN models and their variations performed better for the segmentation tasks. The ML models definitely have significantly better performance in brain tumor analysis tasks compared to the conventional models. The ML models can extract more specific and implicit features of the image pixels and can apply more accurate classification models by training automated systems on various types of data. ML can also be used to apply weights on different parts and parameters based on their contributions to the output and their tuning can improve the image analysis greatly. There are also some limitations to the ML models. The computational complexity of ML models is slightly higher than the conventional models, but the trained models can be saved to reduce the complexity. As noted the performance of the ML models depends on the training data, the limited amount of training data can lead to overfitting of the models. The limited amount of labeled data also creates issues with supervised ML models where not only labeled datasets are needed, but also the distribution of labels/classes affects the performance of the ML model. Hence, data augmentation methods are popular in medical image–based ML models to increase the amount of labeled data by applying various modifications to existing data and adding them to the datasets. The SVM, RF, K-means, KNN, FCM, GMM, and ANN variations can be recommended as preferred algorithms for ML-based brain tumor analysis. The supervised models can be more appropriate for the detection and classification tasks, whereas the clusterings can be more accurate for segmentation. The ANN models can be applied for all brain tumor image analysis tasks to achieve high performance.
7 Deep learning methods
Deep learning methods are the current focus of brain tumor analysis methods for their higher accuracy scores. Various frameworks of DL algorithms have been used in recent works by researchers all over the world. According to the review papers, analyzing the different deep learning methods showed that U-Net is currently one of the best methods for brain tumor analysis as well as other medical image analysis approaches. The network was proposed by Ronneberger et al. [ 44 ] by combining a contracting and expanding path containing convolutional layers that used strong data augmentation methods and was capable of producing more accurate outputs in a very short amount of time. The network consisted of five contraction layers with convolution blocks followed by max pooling layers and their corresponding expansion layers with convolution blocks and upsampling layers. The contraction path started with the dataset images and their augmented versions as inputs and at each layer then worked with the output of the previous layer while downsampling the images. On the other hand, the expansion layers used the outputs from the previous layer and concatenated that with the corresponding contraction layer features while upsampling at each layer to reach to the original size at the final layer. The contraction and expansion paths helped extracting both global and local features, hence improving the outputs. The results generated by U-Net for three different segmentations achieved the least amount of errors compared to the other approaches with at least more than 10% accuracy. The authors discussed the proposed U-Net structure and the data augmentation process in detail to clearly explain each step of the framework and its implementation. The advantages of U-Net over existing CNNs were properly mentioned with logical explanations. The experimental results showed the efficiency of the system, but the paper lacked the details of the datasets, data structures, and their implications in U-Net structure. More detailed discussions of the results and U-Net performance comparisons would help to clarify the contributions of the paper.
Since the performance of U-Net was significantly better and the algorithm was faster than other existing CNN structures, U-Net became one of the mostly used architecture for medical image analysis and different variations of U-Nets have been used by researchers since the U-Net framework was published. One recent variation of U-Net for brain tumor segmentation was the path aggregation U-Net [ 29 ] with a path aggregation encoder, an enhanced decoder, and an efficient feature pyramid (EFP). The encoder was used to shorten the distance between the output layers and the features and to extract deep features from the dataset by concatenating the outputs of the corresponding contraction layers whereas the decoder added extra convolutional layers and combined different filter sizes to extract the features more accurately. Then an efficient pyramid structure was used to combine all the path aggregation outputs from every layer after some upsampling to make the inputs consistent and used a final softmax layer to generate the segmentation output. The BRATS2017 and BRATS2018 datasets were used for experimentation and the results were compared to the VGG, DUNet, and FCNN to show that the proposed framework outperformed the baseline methods by at least 1% at Dice score, sensitivity, and specificity. Also, the proposed network was able to segment the scattered points of a tumor that were not detected by the baseline methods. The proposed framework was built on the idea of the U-Net structure and was an enhanced version of the basic U-Net. The structure helped to incorporate more specific features and improved the performance of tumor segmentation a little bit. Correct segmentation of scattered points of a tumor was definitely a major improvement over the existing approaches and this was possible because of the efficient feature extraction with the path aggregation structure. But it also increased the execution time due to the network size being at least three times bigger than the original U-Net and the performance was not significantly higher when incorporating the extra parts for small datasets.
Another recent brain tumor–based U-Net variation was the HTTU-Net proposed by Aboelenein et al. [ 2 ]. The proposed model included a hybrid network containing two tracks of U-Nets with slightly different structures. One U-Net track had the original U-Net structure with five layers and 3 × 3 filters with the only change being in the activation function. Rather than the ReLU function, the HTTU-Net used leaky ReLU for the complete structure. The other U-Net track had four layers and 5 × 5 filters. Both tracks received the same patches as inputs from the images of BRATS2018 dataset. The networks used a hybrid loss function combining the focal loss and generalized Dice score. The outputs of both tracks were concatenated and a final softmax layer was applied to get the segmented tumors. The performance metrics of the HTTU-Net were compared to the original U-Net and individual tracks of HTTU-Nets to show that the Dice score of HTTU-Net varied from 0.75 to 0.86, whereas the other ones varied between 0.69 to 0.85. Incorporating different U-Net structures was helpful for extracting features from different-sized tumors and combining the results and applying leaky ReLU and a hybrid loss function helped to segment the tumors more accurately. But the HTTU-Net had a similar disadvantage as the path aggregation U-Net. The performance improvement was mostly around 1 to 2% on average with a higher execution time for the network structure.
A triple intersecting U-Net (TIU-Net) was proposed in [ 61 ] and tested on BRATS2015 and BrainWeb datasets to show that the approach outperformed existing U-Nets, FCNNs, DeepMedic, and other similar deep learning structures. The TIU-Net contained binary class segmentation U-Net (BU-Net) and multi-class segmentation U-Net (MU-Net), where the MU-Net used the outputs of the BU-Net and fused the features of BU-Net with its own features to enhance the feature set using a novel polarized cross-entropy loss function. The edge detection maps generated from MU-Net were used to refine the tumor boundaries for the final segmented output. Slices containing more than 2% glioma of the image region were selected for 2D TIU-Nets and 3D patches of dimensions 32 × 96 × 96 were selected for 3D TIU-Net. The normalized images were then used for the models and the performance metrics were calculated to compare with attention-based TIU-Net, without edge TIU-Net, and 13 other 2D and 7 other 3D deep learning methods showing that the proposed approach outperformed them by at least 1%. By incorporating three intersecting U-Nets, and using the outputs of one to guide the others with a novel feature sharing architecture, the proposed method was able to outperform the existing DL methods. Compared to the other U-Net variations with hybrids of two U-Nets, the triple intersecting U-Net was more effective but at the same time the added deep neural network frameworks also increased the computational cost while it only gained a small improvement in the performance.
Recently, Sharif et al. [ 50 ] proposed an active deep learning–based feature selection method for brain tumor segmentation in order to increase the performance efficiency of the CNN-based segmentation of multimodal brain MRIs. They proposed a novel method named saliency-based deep learning (SbDL) for tumor detection and used a pre-trained deep model Inception V3 for tumor classification. The approach was tested on the BRATS2013, BRATS2015, BRATS2017, and BRATS2018 datasets and achieved more than 80% Dice score for detection with more than 92% average accuracy for classification. The authors proposed a pixel increase along limit (PIaL) process for contrast stretching combining clustering based on pixel intensity and a histogram-based contrast enhancement that increased the visibility of the tumor region. For tumor segmentation, the saliency-based method with segmentation-based fractal texture analysis (SFTA) and local binary pattern (LBP) texture features on Alexnet deep CNN pre-trained model was used. Extracted features were mapped on the image with deep CNN and k-means clustering to extract the ROI with a boundary being generated around it. Another pre-trained deep model Inception V3 with 316 layers was used for tumor modality classification. The weighted dominant rotated LBP (DRLBP) features were used on rotated images based on the texture of the pixels; they were combined and concatenated with the deep CNN feature set to create the feature vector for modality classification. The comparison between the proposed method and few existing texture features and contour model–based methods showed that the proposed system was comparable in their performances and in some cases outperformed recent methods and achieved accuracy from 92 to 98%. The proposed feature selection method not only increased classification accuracy but also decreased classification execution time. The proposed feature selection method of combining deep CNN-based features extracted from the MRIs and the texture-based features was an important contribution towards the tumor MRI feature processing. It contributed to the efficiency and accuracy of the classifier, and then decreased the classification execution time. Although the feature-based contribution was effective, the system used some pre-trained existing DL models for detection and classification. The models were tuned for their experiments, but as they were pre-trained and used on BRATS datasets (most BRATS datasets have very similar format and structure), generalizing the results for making decisions on brain tumor feature selection could be problematic. Also, as it was not clear if the proposed method could work for any features of MRIs since using a different set of features extracted from the data could lead to different outcomes.
Sun et al. [ 52 ] recently proposed a novel 3D fully convolutional network for brain tumor segmentation, inspired by U-Net models, which achieved 0.77 to 0.90 DCC for BRATS2018 and 0.76 to 0.89 DCC for BRATS2019 datasets respectively. The model used a multi-pathway feature extraction method to correctly extract features from multi-modal data. The authors discussed the challenges of segmenting the whole tumor, enhancing tumor and tumor core including the complexity of extracting well-defined boundaries, MRI noise, feature reduction during the pooling operation and over-fitting CNN in detail, and explained how the proposed model solved those issues. After pre-processing the input images by cropping them to 144 × 192 × 192 dimensions to remove the black region, randomly selecting 9 consecutive slices from the cropped versions and normalizing them by z -scores, the pre-processed images were used for the proposed model. The model used a FCN with 5D arrays at each layer containing batch size, channel, and spatial dimensions and was built of a FCN structure containing convolutional layers, max pooling layers, batch normalizations, dilated convolutional layer, transposed convolutional layer, and activation function. Four different pathways with different structures, filters, and strides were implemented to extract the feature maps individually and the four feature maps were then fused followed by a transposed convolutional layer to make the output sizes consistent. A ReLU activation function and a categorical cross-entropy loss function were used for the network. The novel method included few unique ideas like cropping the images, taking random sequential slices, applying the dilation convolutional layer, and using multiple pathways for feature extraction that clearly improved the performance of a basic FCN model. The segmentation performance metrics showed that the proposed model was completely compatible to and in some cases better than the existing similar FCN models. Although the authors mentioned various advantages of their model over the existing ones, applying batch normalization in each layer was not a novel idea since it was already used in multiple CNN and U-Net structures.
Another similar work on cross-modality deep feature learning method was proposed in [ 60 ]. The novel idea of using a cross-modality feature transition system and a cross-modality feature fusion system for medical images (specifically for brain tumor) was introduced and tested on BRATS2017 and BRATS2018 datasets and the proposed model achieved an average DCC of 0.84. The performance metrics comparison between the proposed method and some other similar methods showed the compatibility of the proposed network. The cross-modality feature transition method used a generative adversarial learning strategy to extract informative patterns from each modality of the MRIs. A U-Net structure was used for the feature transition model with leaky ReLU activation function, 4 × 4 × 4 filter size for 16, 32, 64, and 128 filters, stride 2, whereas another similar structure with different kernel sizes was used for feature fusions. The DCC and Hausdorff95 scores were calculated for the segmentation output to compare their results with state-of-the-art ensemble and single prediction models and showed that the proposed model was better than most of the state-of-the-art models. The proposed model introduced a novel feature transition and fusion process that was able to overcome the limitation of small datasets and was able to extract important features and patterns without human annotation. The model used U-Net structures for both feature transition and fusion that was very similar to few existing models mentioned earlier where multiple U-Net structures were used for feature extraction. A brief description of the differences between the proposed system and the other similar multiple U-Net systems is needed to clearly identify the uniqueness of the approach.
Chen et al. [ 10 ] proposed a computationally faster novel approach called deep convolutional symmetric neural network (DCSNN) using symmetric masks in few layers to improve the performance of basic DCNN-based segmentation. The normalized versions of the original images, and their left-flipped versions and right-flipped versions were used as inputs to the DCSNN system and the similarity between these images was used as an extra location-based feature for the segmentation model. The framework was built on ResBlocks [ 22 ] and left–right similarity masks and applied to the BRATS2015 dataset. Seven convolutional layers used different filters, strides, parameters, focal loss function with stochastic gradient descent (SGD), and Adam optimizer. The performance of the proposed approach was compared with two other symmetric models—the LRSM2 and the Siamese-based methods—to show that the proposed method outperformed them by at least 2% of DCC score. The comparison with four state-of-the-art DCNN methods showed at least 1% improvement in segmentation DCC scores. The proposed method not only outperformed the existing methods in segmentation accuracy, but it was also able to process each patient image within 10 s whereas the other methods took at least 1–2 min for the same task. Using the similarity feature of the flipped images to create a symmetric network was a unique idea that performed better than some other ideas. At the same time, the augmented dataset lacked variations as none of the other data augmentation methods like rotating, cropping, random selections, etc., was applied.
A deep-LSTM based tumor/non-tumor detection model was proposed in [ 6 ]. The framework pre-processed the input MRIs with bias-field correction and Gaussian filtering. Then, a 4-layer LSTM model was used where the first layer used the pre-processed images as inputs and the consecutive layers used the previous layer outputs as their input. They also tested the model with different numbers of hidden units in each LSTM layer and found that the optimal numbers were 200, 225, 200, and 225 for layers 1, 2, 3, and 4, respectively. Their experiments on multiple BRATS datasets, the ISLES 2015 dataset, and a collected patient data showed 92 to 100% accuracy in tumor/non-tumor detection. Zhang et al. [ 59 ] proposed a novel architecture with a ResU-Net brain tumor segmentation model by adding a series of attention gates. The system added residual blocks in a U-Net architecture and the attention gates were included in the skip connections. The model was able to collect the salient features and the semantic information extracted were able to segment not only large tumors but also smaller tumors more accurately. Their experiments on BRATS 2017–2019 datasets showed DCC scores between 0.70 and 0.88 which outperformed basic U-Net and ResU-Net architectures.
An efficient encoder-decoder framework for brain tumor segmentation was proposed in [ 62 ]. The authors proposed a structure similar to U-Net but with the additional novel idea of shuffle blocks to shuffle the channel data in the contraction path. They also proposed a hybrid loss function by combining the cross-entropy and Dice loss to improve the training performance. They applied a thresholding-based post-processing and used the ERV-Net on a BRATS 2018 dataset. The model achieved DCC scores of 91.21, 81.81, and 86.62 for whole tumor, enhancing tumor, and tumor core, respectively. Some researchers also applied DL models on images from multiple organs. Moeskops et al. [ 37 ] worked on 3 different datasets—brain MRIs (OASIS), breast MRIs, and cardiac CTAs for segmenting various tissues from brain, breast, and heart images. A simple CNN was trained with all possible combinations of tasks (i.e., 7 models) and generated the segmentation outputs. The output DCC score varied from 0.7 to 0.9 which was impressive when considering the simple network structure and 3 different organ images.
DL models performed generally better than most other models for image analysis in general. The same statement can also be applied to medical image analysis. The ML models extract patterns in data as features and train on them. The DL model tries to mimic the complex human brain by using deep neural networks with multiple hidden layers between the input and output layers. The complex hidden layers in DL can extract obvious and implicit features from the image such as the pixels, the context, and the feature maps that then are passed to the next hidden layers to accumulate more intense features. The structure of the DL models allows the training to be much more extensive compared to ML models. The DL models work well for the tumor detection task, but their accuracy for tumor segmentation and tumor tissue classification can definitely outdo other models. Due to the extraction of more specific, detailed, implicit, and microscopic features from the image, DL models can segment the exact tumor region more accurately and can distinguish the subtle differences among various tissues. The hyperparameters of the DL models like learning rate, optimizer, activation function, dropout, kernel size, epochs, etc., are used to improve the performance of the models. The DL models are efficient at automatic feature generation from input images and they perform well with unstructured data. The self-learning ability of the DL models outperforms the conventional and ML models by a huge margin. The ability of processing huge amount of data efficiently makes DL model appropriate for real-time automated system implementation for medical centers to help healthcare professionals. The complexity of the DL models can also increase the computation cost. The aforementioned limited amount of available brain tumor image datasets is considered as a more severe limitation for DL models compared to ML models as the DL models work better with huge amount of training data due to their complex structure. Data augmentation with noise addition, color modifications, intensity modification, rotation, translation, scaling, cropping, flipping, etc., can help in solving the limited training data issue. Different variations of CNNs, VGGs, LSTMs, U-Nets, and attention-based models are popular in recent brain tumor detection, segmentation, and classification researches. As U-Net was originally proposed for medical image analysis and shows better performance in brain tumor and other medical image analysis tasks, variations of U-Nets can be recommended as a major candidate for DL-based models. More recent LSTM and attention-based models can also be applied as preferred DL models for brain tumor image analysis.
8 Hybrid methods
Many of the existing papers used conventional methods as pre-processing or post-processing steps, and some models used a combination of multiple brain analysis methods which means that a number of research works can be classified as hybrid methods. Some of the papers which worked on multiple tasks like detection and segmentation or segmentation and classification, etc., can be included in this category. Some of the papers already mentioned in earlier sections can also be classified as hybrid methods—conventional and ML [ 4 , 8 , 27 , 52 , 49 ], conventional, ML and heuristics [ 14 , 42 ], ML and heuristics [ 39 ], conventional, ML and transform models [ 5 ], conventional, DL and heuristics [ 50 ], conventional, ML, DL, and heuristics [ 7 ], etc.
The hybrid methods also included the models that worked on multiple organs, brain image analysis, and other relevant issues. Han et al. [ 20 ] proposed a model for synthetic brain MRI data generation and data augmentation to solve the issues with limited data availability. They designed a progressive growing generative adversarial network (PGGAN) for training the system for synthetic data generation. ResNet-50 was used for testing the system and provided more than 91% accuracy for the proposed system. A simple ML- and DL-based tumor segmentation and classification method was proposed in [ 34 ]. A FCM model was used for MRI segmentation to segment each image into 5 sections. A discrete wavelet transform (DWT) was applied on the segmented images to extract 1024 features with a 3-level decomposition by Haar wavelet. Principal component analysis (PCA) was used to select the best features. A simple DNN with 7 hidden layers was then used for tumor type classification. The AANLIB dataset was used for experimentation. The system outperformed KNN, LDA, and SMO methods with more than 96% accuracy, 0.97 recall, precision and F-measure, and 0.984 ROC.
Khan et al. [ 26 ] proposed a hybrid model with ML and DL where the k-means algorithm was used to segment the ROI and then VGG19 was used for tumor stage detection (i.e., benign/malignant) and experiments on BRATS 2015 dataset showed 90.03% accuracy. The system was also tested after augmenting with some synthetic data and achieved 94.06% accuracy. The model performance was therefore comparable to other existing approaches. Another ML-DL hybrid system was recently proposed by Raja et al. [ 41 ]. Bayesian Fuzzy Clustering (BFC) was used for extracting the ROIs. Then, three types of features (i.e., information-theoretic measures, wavelet packet Tsallis entropy (WPTE), and scattering transform (ST)) were extracted and used by a deep auto encoder (DAE) for tumor type classification. The BRATS 2015 dataset was used for the experiments and achieved 98.5% accuracy which was higher than 17 existing approaches containing various conventional, ML, DL, and hybrid methods. Although the theoretical details of the methodology were clear, some more specification of how the segmentation was done in the experiments should have been provided to clarify the process completely.
A hybrid method for brain tumor detection and segmentation on MRI and PET of brain was proposed in [ 37 ]. It combined conventional, ML, DL, and heuristic methods. Each input image was decomposed by discrete wavelet transformation (DWT) and they were fused with a modality-coefficient–based fusion. Then a final weighted fusion was introduced in the paper as a novel fusion method. Then 22 GLCM features were extracted from each image to increase the classifier efficiency. An Optimal DNN (ODNN) was used for tumor/non-tumor classification with the GLCM features, and a Spider Monkey Optimization (SMO) algorithm was used for its weight optimization. Finally, the detected abnormal images were segmented to extract the tumor region with a weighted K-means algorithm. Experiments on images collected from the internet showed sensitivity 1.00, specificity 0.89, accuracy 0.93, PPV 0.86, NPV 1.00, FPR 0.11, FNR 0.00, and FDR 0.14, and the performance outperformed basic DNN by around 30%. The proposed model was also compared with 3 other models from recent approaches and outperformed them by 1–3%. The idea to combine multiple methods and to propose new fusion rules to increase the classification and segmentation was impressive. But the lack of dataset information and the absence of experiments on benchmark datasets made it difficult to generalize their results.
A hybrid of DL and heuristics for tumor detection and segmentation is proposed in [ 13 ]. After pre-processing with bi-lateral filtering, the GLCM features were extracted. An adaptive fuzzy deep neural network (AFDNN) with frog leap optimization (FLO) was used to detect the normal and abnormal images. The abnormal images were then used for segmentation with adaptive flying squirrel (AFS) algorithm. Finally, the segmentations were refined with anisotropic diffusion filter (ADF). BRATS 2012, BRATS 2015, BRATS 2016, and BRATS 2018 datasets were tested and achieved accuracy of 99.2 to 99.6%. The proposed model was either comparable or outperformed 5 other hybrid models.
Tables 3 , 4 , 5 , 6 , 7 , 8 , and 9 show the summaries of the papers of conventional, ML, DL, and hybrid methods discussed above.
One of the major challenges of brain tumor or any medical image analysis is data availability. Even though some brain tumor image datasets are available, there are few reliable resources that provide brain tumor data with correct annotations and labeling. Most of these datasets are small and mostly include only few hundreds of images. Table 10 shows some resources that provide multiple medical datasets including brain datasets.
Tables 11 , 12 , and 13 show some commonly used datasets of brain images. More detail on the datasets as their type, modality, tumor type, plane, ground truth availability with labels (GT), and URLs are provided in the tables for the datasets—BRATS 2012, BRATS 2013, BRATS 2014, BRATS 2015, BRATS 2016, BRATS 2017, BRATS 2018, BRATS 2019, BRATS 2020, BRATS 2021, IBSR, IBSR2, BrainWeb, Figshare/CjData, Kaggle brain tumor dataset, Decathlon, BITE, AANLIB/Whole brain atlas, RIDER, REMBRANDT, and Brain-development. Some of these datasets include only healthy brain MRIs, some include only tumorous MRIs, and some include both. The tumorous MRI datasets also sometimes include labels for different types of tumors. Some datasets like ISLES 2015, NAMIC, ABIDE, OASIS, and ADNI include MRIs for other neurological disorders as stroke, autism, schizophrenia, and Alzheimer’s.
As MRI is the most popular brain imaging method in recent years, most of the datasets mentioned here are MRI datasets featuring various characteristics and labels. Only few datasets have additional data with gene information [ 47 ], ultrasound images [ 33 ], PET images [ 32 ], and other signal-based information [ 31 ] and they are included in this review to accommodate the results of a thorough search on brain image datasets. Some of the datasets mentioned in the tables provide synthetic or simulated data, some of them provide patient data, and some datasets combine both types. Few of these datasets are subsets of others. Only few of the datasets are labeled properly with valid annotations. For example, the BRATS dataset [ 68 ] is one of the major benchmark datasets for brain tumor detection and segmentation and has been updated every year with more data and concrete labeling with consistent image features providing a core source for reliable brain medical images with different modalities and ground truth. There are a few other datasets that are mentioned in some existing works but are not mentioned here. Most of those datasets are obsolete now and some are relocated to other resources or not publicly available anymore.
10 Performance metrics
The major performance metrics used for the tumor/non-tumor detection are accuracy, loss, precision, recall, specificity, and F 1 -score. The accuracy of the model represents the percentage of correctly classified data whereas loss represents the distance between the prediction and the ground truth. Hausdorff distance (HD), Dice coefficient (DCC), Jaccard coefficient (JCC), precision, recall, and specificity are the common performance metrics for segmentation. They are used to compare the actual tumor and the segmentation of detected tumor region [ 53 ], and they work with the objects of two binary images: the reference image (i.e., the ground truth) and the resultant image (i.e., the segmented image). A sample confusion matrix is shown in Fig. 16 .
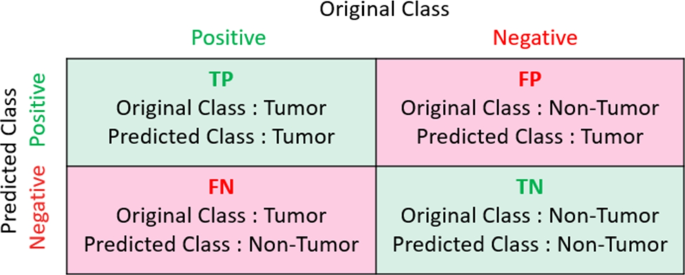
Sample confusion matrix for tumor detection
Precision is the measure to provide the fraction of the retrieved parts that are relevant. Recall represents the fraction of relevant parts from the retrieved set. Precision (i.e., positive predictive value) and recall (i.e., true positive rate) can also be expressed with true positive (TP), true negative (TN), false positive (FP), and false negative (FN) as given in Eqs. 6 and 7 . Specificity (i.e., true negative rate) shows the fraction of correctly returned negative parts. It can be measured by Eq. 8 . \({F}_{1}\) -score can be calculated by Eq. 9 ; it represents the harmonic mean of precision and recall.
According to the confusion matrix,
For HD, DCC (also known as the overlap index), and JCC calculation, let A be the result image and B be the reference image. Figure 17 shows a sample brain MRI, the tumor mask (i.e., ground truth or reference image) or image B, and the predicted tumor (i.e., result image) or image A. HD refers to the distances between the set of points and is represented with Eq. 10 . DCC measures the similarity between image A and image B using Eq. 12 . JCC gives a more proper distance metric with Eq. 14 by achieving triangle inequality. Both DCC and JCC can also be calculated by the confusion matrix values as given in Eqs. 13 and 15 .

Sample brain MRI, image A , and image B
The DCC and JCC scores can also be calculated from one another as given in Eq. 16 .
11 Challenges
There are a number of challenges with brain tumor detection, segmentation, and classification like any other medical image analysis problem. A small amount of available datasets, datasets with unavailable ground truths, noisy data, inconsistent data, randomness of abnormalities, possible variations of abnormalities, shape and area variations of human organs, uniqueness of human organs and abnormalities, etc., are some examples of the challenges. These issues complicate the possibility to provide one set of rules or conditions for all data of the same organ that creates the requirement for complex systems. To efficiently and effectively analyze medical data for producing correct outputs, a complex system needs to define multidimensional constraints and apply several types of features to cover all characteristics.
11.1 Possible variations
Randomness of abnormalities in brain images is one of the major challenges of brain tumor analysis from brain MRIs. Possible variations of abnormalities, shape and area variations of human organs, uniqueness of human organs, and abnormalities are some of the common limitations of medical data analysis in general. For brain MRIs, tumors can vary in size, shape, location, area, volume, etc. The large amounts of possible varieties are the origins of the challenging task of applying any general pixel characteristics based methods for brain tumor segmentation. Tumors can also vary in pixel values with variable intensity and textures. For example, in some MRIs, tumors are the highest intensity regions, and in some others they are not. Intensity similarities or differences between pixels due to the MRI technique can effect any generalized assumptions about the intensity of the tumor region or any intensity characteristic of the image (i.e., the tumor region is always the highest intensity region and is one of the possible generalizations). The tumor and healthy area distortion or lesion can also create incorrect area segmentation for unclear borders.
11.2 Dataset availability
There are multiple ethical constraints when creating medical datasets since medical data is confidential. The medical image datasets have another major issue when labeling the ROI. For brain tumor analysis, only the brain is not enough. The correct annotation or segmentation of brain tumors as ground truth is also needed for accurate output. But the ground truth annotation is mostly done manually by medical professionals which requires both human and other resources. Validation of the ground truth is another issue. The segmentation annotation for each image needs correct labeling by multiple medical professionals and the final annotation needs to be approved by them. The data collection, annotation, and validation require time and human resources for generating standard image datasets.
Another issue with data availability are the lack of datasets containing both healthy and tumorous images and absence of datasets with multiple types of brain tumors. Most of the existing datasets work with gliomas or very few other types of tumors. But there are more than 150 types of brain tumors. Tumors can also have different levels of severity to indicate if they are benign or malignant, grades I, II, III, or IV. Lack of brain tumor datasets with various types, severity, and grade annotation is another major challenge for a completely automated brain tumor analysis system.
11.3 Dataset issues
There are also issues with the existing datasets. Most of the datasets only contain a few hundreds of images which are not sufficient for a deep learning process. The same dataset can also contain different modality images or images generated with different imaging techniques. Different modalities of MRIs highlight different regions of the brain. As most of the brain tumor image datasets have different modality data with different MRI sequences, it is difficult to follow one particular method or set of parameters that is applicable for tumor segmentation from any type of MRIs. Some datasets have other inconsistencies related to image dimensions, image type, and other properties that affect the correct analysis of those images. Lots of datasets also have noisy images that disrupt a correct image analysis.
11.4 Complete system
Although lots of research works are based on the individual brain tumor analysis tasks, the lack of a complete frameworks that can analyze a brain MRI and apply all tasks to produce a comprehensive output is still a major challenge. An automated system to detect if there is any abnormality in the brain MRI, and then to segment the tumor region correctly, classify different tumor tissues, identify the tumor severity (i.e., benign/malignant), grade (i.e., I, II, III, IV), and specific tumor type (i.e., name of the tumor) is still not available.
The possible scopes and future research problems can include (i) creating consistent and sufficient datasets with correct annotations and labels, (ii) designing hybrid frameworks for a completely automated system for all the three brain tumor analysis tasks without human intervention, (iii) applying real-time patient data to these systems that can be observed by medical professionals to evaluate the actual (i.e., not theoretical) performance of the frameworks/models, (iv) developing automated systems to analyze brain MRIs and other patient data to generate comprehensive reports to assist the healthcare professionals, and (v) designing interactive recommendation systems to provide suggestions to healthcare professionals about the brain medical image data and to receive feedbacks from the professionals for fine-tuning the system performance. Although developing automated systems for more accurate and efficient medical data analysis is crucial, valid, complete, and detailed datasets are equally essential for training, testing, and validating those automated systems. Hence, healthcare professionals and researchers need to focus on contributing to the reduction of limitations regarding the datasets and the development of real-time automated data processing and analysis systems simultaneously.
12 Conclusion
Medical image analysis with segmentation and classification is a well-known research area that has been explored using manual, semi-automatic, and automatic approaches to increase the precision of the medical decisions to help both patients and healthcare workers. Brain tumor analysis is one of the research directions within this area. It used thresholding, supervised machine learning, unsupervised machine learning, semi-supervised machine learning, deep learning, and hybrid methods, and it was able to achieve high accuracy on a limited number of available datasets. Although the existing systems performed well, there are still lots of challenges to medical image analysis methods. The main objective of this paper is to explore the possibilities of different approaches to brain tumor image detection, segmentation, and classification. The goal is to identify all the challenges and scopes properly to provide some future directions for the researchers to address them with multiple approaches from different perspectives to achieve better results for medical image analysis.
This paper contains a comprehensive review on brain tumor medical image analysis covering all major aspects of brain tumor image analysis. Basic brain tumor and brain medical image-related terms, and their definitions, examples, and applications of brain tumor image analysis are introduced with mentions of AI models. All major brain tumor analysis tasks (i.e., detection, segmentation, classification) are discussed with appropriate examples. Image feature analysis and AI models with conventional, ML, DL, and hybrid algorithms used in existing research works are discussed in detail. The paper includes the summaries on relevant researches with all necessary information like tasks, features, datasets, performance, and contributions in both text and tabular formats for ease of comparisons between methods as needed. This review paper does not just summarize the existing researches but also adds observations (both positive and negative) on the papers like their uniqueness and limitations to provide a complete narrative to future researchers. The datasets and performance metrics used in recent and previous researches are also mentioned with their sources, image properties, data labels, abnormality types, metrics definitions and equations, etc., to help future researchers to check and select appropriate datasets and performance metrics for their research. The review and observations on the existing research also provided the directions for current challenges and future scopes in this research domain. Although this paper provides a complete summary on the major components of brain tumor image analysis researches, there are a few limitations which are intended to be resolved in future. This survey mainly focuses on the AI-based researches on different brain tumor analysis tasks, but more analysis can be done on each step of the image processing and analysis to figure out the scopes of better novel approaches. A more detailed analysis on each step (i.e., feature extraction, pre-processing, post-processing, decision generation, etc.) can lead to specifying the current limitations and scopes of improvements in each, contributing towards a novel and more accurate complete automated system for brain tumor image analysis.
Abbreviations
Anisotropic diffusion filter
Artificial intelligence
Adaptive k-nearest neighbor
Artificial neural network
Binary competitive swarm optimizer
Bayesian fuzzy clustering
Barnacle Mating Optimization Algorithm
Backpropagation neural network
Computer-aided diagnosis
Cascaded correlation artificial neural network
Convolutional neural network
Conditional random field
Crow Search Optimization Algorithm
Cerebrospinal fluid
Computed tomography
Deep auto encoder
Dice coefficient
- Deep learning
Decision tree
Discrete wavelet transform
Efficient feature pyramid
Fuzzy Clustering Algorithm
Fuzzy C-means
Fully convolutional neural network
Feedforward backpropagation neural network
Fuzzy Hopfield neural network
Fluid-attenuated inversion recovery
Frog leap optimization
False negative
False negative rate
False positive
False positive rate
Genetic algorithm
Gray-level co-occurrence matrix
Gray matter
Gaussian mixture model
Grasshopper Optimization Algorithm
Hausdorff distance
High-grade glioma
Histogram of oriented gradients
Jaccard coefficient
K-Nearest neighbor
Lattice Boltzmann method
Local binary pattern
Linear discriminant analysis
Low-grade glioma
Logistic regression
Long short-term memory
Matthews correlation coefficient
- Machine learning
Multiscale morphological gradient reconstruction
Markov random field
Magnetic resonance imaging
Mean-square error
Non-local means
Optimal possibilistic fuzzy C-mean
Parametric active contour model
Principal component analysis
Positron emission tomography
Piece-wise modified box counting
Probabilistic neural network
Positive predictive value
Peak signal-to-noise ratio
Particle Swarm Optimization
Piece-wise triangular prism surface area
Random forest
Recurrent neural network
Region of interest
Segmentation-based fractal texture analysis
Stochastic gradient descent
Simple linear iterative clustering
Social mimic optimization
Structural similarity index
Scattering transform
Support vector machine
The Cancer Imaging Archive
Transfer learning
True negative
True negative rate
True positive
True positive rate
Visual geometry group
Walsh Hadamard transform
White matter
Wavelet packet Tsallis entropy
Watershed transform
Abd-Ellah MK, Awad AI, Khalaf AA, Hamed HF (2019) A review on brain tumor diagnosis from MRI images: practical implications and key achievements and lessons learned. Magn Reson Imaging 61:300–318
Article PubMed Google Scholar
Aboelenein NM, Songhao P, Koubaa A, Noor A, Afifi A (2020) Httu-Net: hybrid two track U-Net for automatic brain tumor segmentation. IEEE Access 8:101406–101415
Article Google Scholar
Al-Galal SAY, Alshaikhli IFT, Abdulrazzaq MM (2021) MRI brain tumor medical images analysis using deep learning techniques: a systematic review. Health Technol 11(7):1–16
Alipour N, Hasanzadeh RP (2021) Superpixel-based brain tumor segmentation in MR images using an extended local fuzzy active contour model. Multimed Tools Appl 80(6):8835–8859
Angulakshmi M, Priya GL (2019) Walsh Hadamard transform for simple linear iterative clustering (SLIC) superpixel based spectral clustering of multimodal MRI brain tumor segmentation. Irbm 40(5):253–262
Amin J, Sharif M, Raza M, Saba T, Sial R, Shad SA (2020) Brain tumor detection: a long short-term memory (LSTM)-based learning model. Neural Comput Appl 32(20):15965–15973
Anantharajan S, Gunasekaran S (2021) Automated brain tumor detection and classification using weighted fuzzy clustering algorithm and deep auto encoder with barnacle mating algorithm and random forest classifier techniques. Int J Imaging Syst Technol 31(4):970–1988
Arunkumar N, Mohammed MA, Ghani MKA, Ibrahim DA, Abdulhay E, Ramirez-Gonzalez G, Albuquerque VHCD (2019) K-means clustering and neural network for object detecting and identifying abnormality of brain tumor. Soft Comput 23(19):9083–9096
Bharathi AS, Manimegalai D (2015) 3d digital reconstruction of brain tumor from MRI scans using Delaunay triangulation and patches. ARPN J Eng Appl Sci 10:9227–9232
Google Scholar
Chen H, Qin Z, Ding Y, Tian L, Qin Z (2020) Brain tumor segmentation with deep convolutional symmetric neural network. Neurocomputing 392:305–313. Accessed May 2023
Cheplygina V, de Bruijne M, Pluim JP (2019) Not-so-supervised: a survey of semi-supervised and multi-instance and transfer learning in medical image analysis. Med Image Anal 54:280–296
Clare S (1998) Magnetic resonance imaging of brain function. URL https://users.fmrib.ox.ac.uk/stuart/thesis/chapter-3/section3-2.html . Accessed May 2023
Deb D, Roy S (2021) Brain tumor detection based on hybrid deep neural network in MRI by adaptive squirrel search optimization. Multimed Tools Appl 80(2):2621–2645
Devanathan B, Venkatachalapathy K (2020) An optimal multilevel thresholding based segmentation and classification model for brain tumor diagnosis, 2020 4th International Conference on Electronics, Communication and Aerospace Technology (ICECA), Coimbatore, India, pp. 1133–1138. https://doi.org/10.1109/ICECA49313.2020.9297571
Díaz-Pernas FJ, Martínez-Zarzuela M, Antón-Rodríguez M, González-Ortega D (2021) A deep learning approach for brain tumor classification and segmentation using a multiscale convolutional neural network. Healthcare Multidiscip Digit Publ Inst 9(2):153
Fatima A, Shahid AR, Raza B, Madni TM, Janjua UI (2020) State-of-the-art traditional to the machine-and deep-learning-based skull stripping techniques, models and algorithms. J Digit Imaging 33(6):1443–1464
Article PubMed PubMed Central Google Scholar
Gordillo N, Montseny E, Sobrevilla P (2013) State of the art survey on MRI brain tumor segmentation. Magn Reson Imaging 31(8):1426–1438
Graham RL, Yao FF (1983) Finding the convex hull of a simple polygon. J Algorithms Elsevier 4(4):324–331
Győrfi Á, Szilágyi L, Kovács L (2021) A fully automatic procedure for brain tumor segmentation from multi-spectral MRI records using ensemble learning and atlas-based data enhancement. Appl Sci 11(2):564
Han C, Rundo L, Araki R, Furukawa Y, Mauri G, Nakayama H, Hayashi H (2020) Infinite brain MR images: PGGAN-Based Data Augmentation for Tumor Detection. In Smart Innovation, Systems and Technologies (pp. 291–303). (Smart Innovation, Systems and Technologies; Vol. 151). Springer Science and Business Media Deutschland GmbH. https://doi.org/10.1007/978-981-13-8950-4_27
Hesamian MH, Jia W, He X, Kennedy P (2019) Deep learning techniques for medical image segmentation: achievements and challenges. J Digit Imaging 32(4):582–596
Ilhan U, Ilhan A (2017) Brain tumor segmentation based on a new threshold approach. Procedia Comput Sci 120:580–587
Intel AI developer program. Brain tumor segmentation using fully convolutional tiramisu deep learning architecture. URL https://software.intel.com/content/www/xl/es/develop/articles/brats-2017-glioma-segmentation-using-fully-convolutional-neural-networks.html . Accessed May 2023
Islam MM, Kashem MA (2021) Parametric active contour model-based tumor area segmentation from brain MRI images using minimum initial points. Iran J Comput Sci 4;125–132. https://doi.org/10.1007/s42044-020-00078-8
Khalil HA, Darwish S, Ibrahim YM, Hassan OF (2020) 3D-MRI brain tumor detection model using modified version of level set segmentation based on dragonfly algorithm. Symmetry 12(8):1256
Khan AR, Khan S, Harouni M, Abbasi R, Iqbal S, Mehmood Z (2021) Brain tumor segmentation using k-means clustering and deep learning with synthetic data augmentation for classification. Microsc Res Tech 84(7):1389–1399. Accessed May 2023
Khosravanian A, Rahmanimanesh M, Keshavarzi P, Mozaffari S (2021) Fast level set method for glioma brain tumor segmentation based on superpixel fuzzy clustering and lattice Boltzmann method. Comput Methods Programs Biomed 198:105809. Accessed May 2023
Kumar DM, Satyanarayana D, Prasad MG (2021) MRI brain tumor detection using optimal possibilistic fuzzy c-means clustering algorithm and adaptive k-nearest neighbor classifier. J Ambient Intell Humaniz Comput 12(2):2867–2880
Lin F, Wu Q, Liu J, Wang D, Kong X (2021) Path aggregation U-Net model for brain tumor segmentation. Multimed Tools Appl 80:22951–22964
Magadza T, Viriri S (2021) Deep learning for brain tumor segmentation: a survey of state-of-the-art. J Imaging 7(2):19
Maier O, Menze BH, Gablentz JVD, Häni L, Heinrich MP, Liebr M, Winzeck S, Basit A, Bentley P, Chen L, Christiaens D (2017) Isles 2015-a public evaluation benchmark for ischemic stroke lesion segmentation from multispectral mri. Med Image Anal 35:250–269. Accessed May 2023
Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL (2007) Open access series of imaging studies (OASIS): cross-sectional MRI data in young and middle aged and nondemented and demented older adults. J Cogn Neurosci 19(9):1498–1507
Mercier L, Maestro RFD, Petrecca K, Araujo D, Haegelen C, Collins DL (2012) Online database of clinical MR and ultrasound images of brain tumors. Med Phys 39(6(Part1)):3253–3261
Mohsen H, El-Dahshan EA, El-Horbaty EM, Salem AM (2018) Classification using deep learning neural networks for brain tumors. Fut Comput Inf J 3(1):68–71
Nazir M, Shakil S, Khurshid K (2021) Role of deep learning in brain tumor detection and classification (2015 to 2020): a review. Comput Med Imaging Graphics 91:101940
Plewes DB, Kucharczyk W (2012) Physics of MRI: a primer. Magn Reson Imaging 35(5):1038–1054
Preethi S, Aishwarya P (2021) An efficient wavelet-based image fusion for brain tumor detection and segmentation over PET and MRI image. Multimed Tools Appl 80(10):14789–14806
Cambridge University Press. Chapter three - cognitive neuroscience methods to study the adolescent brain. URL https://www.cambridge.org/core/books/neuroscience-of-adolescence/cognitive-neuroscience-methods-to-study-the-adolescent-brain/FC6F05A89E2A35EBE37E686BA7BE489D . Accessed May 2023
Preston DC (1997) Magnetic resonance imaging (MRI) of the brain and spine: basics. URL https://case.edu/med/neurology/NR/MRI%20Basics.htm . Accessed May 2023
Rai S, Chowdhury S, Sarkar S, Chowdhury K, Singh KP (2019) A hybrid approach to brain tumor detection from MRI images using computer vision. J Innov Comput Sci Eng 8(2):8–12
Raja PS, Rani AV (2020) Brain tumor classification using a hybrid deep autoencoder with Bayesian fuzzy clustering-based segmentation approach. Biocybernetics Biomed Eng 40(1):440–453
Reddy AS, Reddy PC (2021) MRI brain tumor segmentation and prediction using modified region growing and adaptive SVM. Soft Comput 25(5):4135–4148
Rehman HZU, Hwang H, Lee S (2020) Conventional and deep learning methods for skull stripping in brain MRI. Appl Sci 10(5):1773
Ronneberger O, Fischer P, Brox T (2015) U-Net: convolutional networks for biomedical image segmentation. International Conference on Medical Image Computing and Computer-Assisted Intervention and Springer and Cham , 234–241
Roy S, Nag S, Maitra IK, Bandyopadhyay SK (2013) Artefact removal and skull elimination from MRI of brain image. Int J Sci Eng Res 4(6):163–170
Saman S, Narayanan SJ (2019) Survey on brain tumor segmentation and feature extraction of MR images. Int J Multimed Inf Retr 8(2):79–99
Scarpace L, Flanders A, Jain R, Mikkelsen T, Andrews DW (2015) Data from REMBRANDT. Cancer Imaging Arch. Accessed May 2023
Sharif M, Amin J, Raza M, Yasmin M, Satapathy SC (2020) An integrated design of particle swarm optimization (PSO) with fusion of features for detection of brain tumor. Pattern Recogn Lett 129:150–157. Accessed May 2023
Shally HR, Chitharanjan K (2013) Tumor volume calculation of brain from MRI slices. Int J Comput Sci Eng Technol (IJCSET) 4(8):1126–1132. Accessed May 2023
Sharif MI, Li JP, Khan MA, Saleem MA (2020) Active deep neural network features selection for segmentation and recognition of brain tumors using MRI images. Pattern Recogn Lett 129:181–189
Shivhare SN, Kumar N, Singh N (2019) A hybrid of active contour model and convex hull for automated brain tumor segmentation in multimodal MRI. Multimed Tools Appl 78(24):34207–34229
Sun J, Peng Y, Guo Y, Li D (2021) Segmentation of the multimodal brain tumor image used the multi-pathway architecture method based on 3d fcn. Neurocomputing 423:34–45
Taha AA, Hanbury A (2015) Metrics for evaluating 3d medical image segmentation: analysis and selection and tool. BMC Med Imaging 15(1):1–28
Thayumanavan M, Ramasamy A (2021) An efficient approach for brain tumor detection and segmentation in MR brain images using random forest classifier. Concurr Eng 29(3):266–274
Tiwari A, Srivastava S, Pant M (2020) Brain tumor segmentation and classification from magnetic resonance images: review of selected methods from 2014 to 2019. Pattern Recogn Lett 131:244–260
Verma H, Verma D, Tiwari PK (2020) A population based hybrid FCM-PSO algorithm for clustering analysis and segmentation of brain image. Expert Syst Appl 167:114121
Wadhwa A, Bhardwaj A, Verma VS (2019) A review on brain tumor segmentation of MRI images. Magn Reson Imaging 61:247–259
Wikipedia. Medical imaging. URL https://en.wikipedia.org/wiki/Medical-imaging . Accessed May 2023
Zhang J, Jiang Z, Dong J, Hou Y, Liu B (2020) Attention gate resu-net for automatic MRI brain tumor segmentation. IEEE Access 8:58533–58545
Zhang D, Huang G, Zhang Q, Han J, Han J, Yu Y (2021) Cross-modality deep feature learning for brain tumor segmentation. Pattern Recogn 110:107562
Zhang J, Zeng J, Qin P, Zhao L (2021) Brain tumor segmentation of multi-modality MR images via triple intersecting U-Nets. Neurocomputing 421:195–209. Accessed May 2023
Zhou X, Li X, Hu K, Zhang Y, Chen Z, Gao X (2021) Erv-Net: an efficient 3d residual neural network for brain tumor segmentation. Expert Syst Appl 170:114566
Radiological Society of North America, Inc. (RSNA). Cranial Ultrasound. URL https://www.radiologyinfo.org/en/info/ultrasound-cranial . Accessed May 2023
Conversations in Science at Indiana University. Neuroimaging: three important brain imaging techniques. URL https://blogs.iu.edu/sciu/2022/02/05/three-brain-imaging-techniques/ . Accessed May 2023
Google. Google Scholar. URL https://scholar.google.com/ . Accessed May 2023
NCBI. PubMed. URL https://pubmed.ncbi.nlm.nih.gov/ . Accessed May 2023
Psych Central. Types of brain imaging techniques. URL https://psychcentral.com/lib/types-of-brain-imaging-techniquestypes . Accessed May 2023
Synapse. BraTS continuous evaluation. URL https://www.synapse.org/!Synapse:syn27046444/wiki/616571 . Accessed May 2023
Bhatt D, Patel C, Talsania H, Patel J, Vaghela R, Pandya S, Modi K, Ghayvat H (2021) CNN variants for computer vision: history, architecture, application, challenges and future scope. Electronics 10(20):2470
Siddique N, Paheding S, Elkin CP, Devabhaktuni V (2021) U-Net and its variants for medical image segmentation: a review of theory and applications. IEEE Access 9:82031–82057
Smith SW (1997) The scientist and engineer’s guide to digital signal processing (Vol 14). San Diego: California Technical Pub. Accessed May 2023
Ayodele TO (2010) Types of machine learning algorithms. New Adv Mach Learn 3:19–48
Shrestha A, Mahmood A (2019) Review of deep learning algorithms and architectures. IEEE Access 7:53040–53065
Guo Y, Liu Y, Oerlemans A, Lao S, Wu S, Lew MS (2016) Deep learning for visual understanding: a review. Neurocomputing 187:27–48
Hamet P, Tremblay J (2017) Artificial intelligence in medicine. Metabolism 69:S36–S40
Article CAS Google Scholar
Wikipedia. Image segmentation. URL https://en.wikipedia.org/wiki/Image−segmentation . Accessed May 2023
Shapiro LG, Stockman GC (2001) Computer vision. Upper Saddle River: Prentice-Hall . Accessed May 2023
IBM. What is machine learning?. URL https://www.ibm.com/topics/machine-learning . Accessed May 2023
Wikipedia. Otsu’s method. URL https://en.wikipedia.org/wiki/Otsuśmethod . Accessed May 2023
Wikipedia. Fast Walsh-Hadamard transform. URL https://en.wikipedia.org/wiki/Fast−Walsh%E2%80%93Hadamard_transform . Accessed May 2023
Download references
Author information
Authors and affiliations.
Department of Computer Science, University of Calgary, Alberta, Canada
Kashfia Sailunaz, Jon Rokne & Reda Alhajj
International School of Medicine, Istanbul Medipol University, Istanbul, Turkey
Sleiman Alhajj
Department of Computer Engineering, Ankara Medipol University, Ankara, Turkey
Tansel Özyer
Department of Computer Engineering, Istanbul Medipol University, Istanbul, Turkey
Reda Alhajj
Department of Health Informatics, University of Southern Denmark, Odense, Denmark
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Reda Alhajj .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Sailunaz, K., Alhajj, S., Özyer, T. et al. A survey on brain tumor image analysis. Med Biol Eng Comput 62 , 1–45 (2024). https://doi.org/10.1007/s11517-023-02873-4
Download citation
Received : 17 November 2022
Accepted : 20 June 2023
Published : 13 September 2023
Issue Date : January 2024
DOI : https://doi.org/10.1007/s11517-023-02873-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Brain tumor
- Medical image analysis
- Tumor features
- Tumor detection
- Tumor segmentation
- Find a journal
- Publish with us
- Track your research
- Share full article
Advertisement
Supported by
Guest Essay
‘If You See a Fox and I’ve Died, It Will Be Me’

By Sarah Wildman
Ms. Wildman is a staff writer and editor in Opinion.
A block from my house at the edge of Washington, there is a winding park with a road running through it. One Sunday recently, walking my regular loop along the trail, I heard leaves rustling on the wooded hill above me. I often see deer here; this time it was a bright young fox.
She paused. We stood there for a moment, she and I, aware. I wanted desperately for her to come closer, to stay in her orbit a moment longer. I lingered long after she left.
Sometime in my daughter Orli’s last months of life, she told me, lightly, “If you see a fox and I’ve died, it will be me.” I had never seen a fox in my neighborhood. Over the past several months, I have seen maybe a half dozen, here and elsewhere. Each time, I try to quell my desire to shout out, to ask the animal to stay, to call it by her name. It feels crazy; it feels sane.
I had never believed in signs. Now I notice when an interview runs exactly 1 hour and 13 minutes or when the hour is exactly 1:13. Orli was born on Jan. 13. It means nothing; it means something. A double rainbow stretched over a farm in Maine represents more than beauty.
March 17 will be one year since Orli died in our house, in her room, in my arms; March 20 a year since her burial. (In a quirk of this year’s Jewish calendar, the date of her yahrzeit, or memorial date, is some weeks farther on.)
A year is a strange and terrible marker of time, simultaneously endless and instant. A year of loss is a new form of permanence: This is the life we lead. It will not change. A year furthers us on the long march toward our altered future. In the life of a child, a year is transformative. Her peers have molted in the year from 14 to 15. They no longer attend the same school; they have begun new sports, met new friends, moved forward, moved on.
There is an immutability to a year of grief, a sense of solidity to the loss, a movement from the surreality of her absence into a hardened space. It’s not as though I believe she might return, but in the year between her death and now, I remain connected to her presence. My partner, Ian, has spent part of this year adding tattoos to his arms, each an ode to Orli, permanent signifiers of permanent loss. My younger daughter, Hana, has written through her grief; she notes, often, the lack of insight her peers have into the depth of losing a sister. Meanwhile, I wonder if I should keep every item of clothing I can picture Orli in, I wonder what she would say about each movie I see, each book I read. I yearn for her commentary.
On Orli’s birthday, one of her long-distance friends wrote to me, “Whether you consent or not, I bring Orli along in every escapade,” in good decisions, in hidden poor ones. She understood the essence of being human is to be mischievous, of both choosing well and of making bad decisions. I never craved a perfect child, just a living one.
The day before our first birthday without Orli, Hana, Ian and I — walking from separate directions — came upon a fox idling on a street corner, as though waiting for us.
Most of this year I have worked to center memories of Orli’s better moments, the joy she infused in each minute she got to live. One month after her first brain tumor surgery, when she’d rebounded better than any of us could have hoped, we met old friends from Spain for dinner. As we ate, a sudden, drenching storm came up. Orli got up and ran into the warm rain with our friends’ children, dancing, thrilled. It was, she told me, a “bucket list moment.”
She seemed to realize, far earlier than I, she had to lean into each experience, to expand it, to let it fuel her for whatever came next. In her journal she worried she might not see ninth grade. She did not share that with her friends.
Each of us in our rump family has felt an almost visceral physicality of these past few weeks — the slide from her birthday toward this anniversary, the terrible knowledge that we each hold of the last moments of her life, the good minutes we had, the harder hours, the terror of those final days.
In her last week, one doctor cornered me at the hospital to tell me Orli shouldn’t be here anymore. It was not clear if he meant “here, still receiving palliative treatment,” or “here, on earth.” She was fading, I knew. But it felt an awful thing to say — unforgivable, really. I thought of Abraham arguing with God to save the wicked towns . I wanted to ask: But what if I get 15 good minutes with her each hour? Or five? Orli was adamant she did not want to die.
In Judaism a child who is an avel, or mourner, is to stop saying Mourner’s Kaddish for her parent at 11 months as she re-emerges into the community. But because parents who have lost a child have no obligation beyond the first 30 days, this marker holds no meaning. And because those who have lost children are, in many ways, forever seen as mourners, forever noted for their loss, we remain on the margin — in the community but not entirely of it. Once, early in Orli’s illness, on the same path where I saw the fox, I overheard a woman, just slightly still within my earshot, who passed me. “That’s Sarah Wildman, the woman whose daughter …”
I tend to walk alone on this path. Grief of this kind is simultaneously universal and unshareable; loneliness is its inherent point of reference. I cannot conceive that March 18 will be drastically different from March 17.
When 2023 turned to 2024, I thought: It is a terrible thing to buy a calendar for a year Orli will not see. Still, I put up a calendar in her old room, the same feminist calendar she chose each year. As February turned to March, I found the page hard to flip over. Until this point, I have been able to look at the photos in my phone and say: This time last year, we were at this concert, we were at this movie, we had this meal. Now those memories slide farther back. These days Ian often sits in her room, working. He likes to be near her, and so, most nights, in homage to her, I straighten up after him — he is a mess, she craved order. I do it for her, I do it for me.
In early September, not quite six months after Orli died, I interviewed the actor Rob Delaney, who wrote a bracing, visceral book about his young son Henry’s life and death from brain cancer. “You probably at this point regularly — what, every day? — are shocked by the fact that she’s gone. Right?” Mr. Delaney asked me halfway through our call. “For better or for worse — I guess for the survival of the species, it’s for the better — but the acute physical pain will not go away. But it’ll weave itself into your life in a way where threads of Orli will be in the tapestry of your life forever,” he said.
“And in a few years, you’re going to wrap yourself in the tapestry of your life and marvel at the beauty of the threads of Hana and Orli and Ian, and it’ll all be — you will metabolize her life and her death, in a way where you feel a thousand things.” One of those things will always be “disbelief and pain,” he said. “That won’t go away.”
In the first days of March, Hana and I went to speak at Orli’s old school at a Women’s History Month assembly held in her honor. Orli had an “intuitive sense of justice, about doing what’s right in the world, about showing up for her friends and herself,” I told sixth, seventh and eighth graders, aware some of them would have known Orli only as that girl who died.
It was Hana who spoke best. “Orli was like an emotion,” she told the assembled children, all older than she. “I think I will never get over her. It might get less hard, but I will never not be sad.”
It wasn’t until that night, in bed, that I wept. The teachers still knew her as she was, I realized. I craved their memories.
“How are you?” each of them asked, as people often do. “Aquí estoy,” I said, as I have come to say. I’m here.
Sarah Wildman is a staff editor and writer in Opinion. She is the author of “Paper Love: Searching for the Girl My Grandfather Left Behind.”
The Times is committed to publishing a diversity of letters to the editor. We’d like to hear what you think about this or any of our articles. Here are some tips . And here’s our email: [email protected] .
Follow the New York Times Opinion section on Facebook , Instagram , TikTok , WhatsApp , X and Threads .

IMAGES
VIDEO
COMMENTS
A brain tumor is an abnormal growth or mass of cells in or around your brain. Together, spinal tumors and brain tumors are called central nervous system (CNS) tumors. Brain tumors can be malignant (cancerous) or benign (noncancerous). Some tumors grow quickly, while others are slow growing.
Essay On Brain Tumor. The brain is a sophisticated, elegant and an elaborate mass of tissue and nerve cells. It seamlessly controls our senses, our personality, helps regulate vital body functions and controls how we move in our surroundings. When abnormal cells grow in the brain to develop a tumor, it can disrupt how we function and will ...
Brain cancer is the leading cause of cancer-related death in people under the age of 39. This cancer cannot be detected without a thorough physical examination and there are no risk factors. Thus, we cannot prevent brain cancer. Once diagnosed, patients undergo brain surgery to remove the tumour bulk. However, because the brain is such a ...
A secondary brain tumor, or brain metastasis, is a cancerous tumor that started in another part of the body, such as the breast, lung, or colon, and then spread to the brain. A secondary brain tumor may also be called metastatic cancer. For instance, lung cancer that has spread to the brain may be called metastatic lung cancer.
The signs and symptoms of a brain tumor depend on the brain tumor's size and location. Symptoms also might depend on how fast the brain tumor is growing, which is also called the tumor grade. General signs and symptoms caused by brain tumors may include: Headache or pressure in the head that is worse in the morning.
Nature Reviews Cancer 20 , 1 ( 2020) Cite this article. This Focus issue highlights current research into the unique biology of brain tumours and brain metastasis and how this research might ...
Neurological Cancer. There are signs that could be the result of a brain tumor. Symptoms include seizures, severe or persistent headaches, increasing irritability, changes in personalities, unusual fatigue or sleeplessness, nausea and vomiting, difficulties with hearing, speech, taste, smell, or bladder. Seen in [Siegel, Mary-Ellen.
Metastatic brain tumors include tumors that arise elsewhere in the body (such as the breast or lungs) and migrate to the brain, usually through the bloodstream. Metastatic tumors are considered cancer and are malignant. Metastatic tumors to the brain affect nearly one in four patients with cancer, or an estimated 150,000 people a year.
Abstract and Figures. Brain tumor is an abnormal growth of mass of cells in (or) around the brain. Brain tumors can be malignant (cancerous) or being non-cancerous. It is the most common malignant ...
Nursing. Undergraduate. Oath of the Horatii 1784, Essay Example. Essay. Identification of Health Related Issues in the Homeless, Essay Example. Essay. Essays.io ️ Brain Tumor, Essay Example from students accepted to Harvard, Stanford, and other elite schools.
Introduction. Primary central nervous system (CNS) tumors begin in the brain or spinal cord. Brain and other CNS tumors had an average annual age-adjusted incidence of 11.20 per 100,000 population in the age 15-39 years and 44.47 per 100,000 population in the age 40+ years (Palmer, 2008; Ostrom et al., 2018). According to World Health Organization Classification of Tumors, the CNS tumors are ...
Brain Tumor Essay. A brain tumor is a collection (or mass) of abnormal cells in the brain. The skull is very rigid and the brain is enclosed, so any growth inside such a restricted space can cause problems. Brain tumors can be cancerous or non-cancerous. When benign (non-cancerous) or malignant (cancerous) tumors grow, they can increase the ...
College Essay On Brain Cancer. Tumors can be classified into three types: 1) benign 2) pre malignant 3) malignant tumor. Benign tumors are those which are incapable of abrupt expanding and affecting the other healthy brain tissues. Premalignant tumor is a pre cancerous stage, if not treated properly it may lead to cancers.
Living with brain cancer is a complex journey that can significantly influence various aspects of everyday life. No diagnosis or situation is the same, and because of this, what a patient's day-to-day life looks like can vary greatly. Let's dive into the most common ways brain cancer can impact individuals, emphasizing the challenges and ...
Additionally, MICCAI workshop papers related to brain tumors have also been included in this review. In summary, the aim of this review is (a) to show the deep learning development in the entire field of brain tumor, (b) the identification of open research challenges for successful deep learning methods for brain tumor tasks, (c) to highlight ...
Background: The present narrative review aims to discuss cognitive-emotional-behavioral symptoms in adults with brain tumors at the time of diagnosis. Methods: The PubMed database was searched considering glioma, pituitary adenoma, and meningioma in adulthood as pathologies, together with cognitive, neuropsychological, or behavioral aspects.
A brain tumor is an abnormal growth of cells within the brain, which can be cancerous or non-cancerous (benign). It is generally caused by abnormal and uncontrolled cell division, normally either in the brain itself (neurons, glial cells (astrocytes, oligodendrocytes, ependymal cells), lymphatic blood vessels), in the cranial nerves (myelin ...
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers. ... Brain tumors are a heterogeneous group of diseases which include either primary or secondary brain tumors. In recent years, significant improvement has been made to identify tumor ...
Essay On Brain Tumors. 1041 Words5 Pages. The brain is the most complex and magnificent organ of the human body. It controls the muscle movements, the secretions of glands,breathing and internal temperature. Every creative thought, feeling, and plan is developed by the brain. The brain acts as the body's control center.
Additionally, MICCAI workshop papers related to brain tumors have also been included in this review. In summary, the aim of this review is (a) to show the deep learning development in the entire field of brain tumor, (b) the identification of open research challenges for successful deep learning methods for brain tumor tasks, (c) to highlight ...
Therefore, manual segmentation of brain tumors from magnetic resonance (MR) images is a challenging and time-consuming task. In addition, an automated brain tumor classification from an MRI scan is non-invasive so that it avoids biopsy and make the diagnosis process safer. ... In this survey work, peer reviewed research papers from 2015 to 2021 ...
Nordland was diagnosed with glioblastoma, a terminal brain cancer, in 2019. As a war correspondent for The New York Times, Newsweek and the Philadelphia Inquirer, Rod Nordland faced death many ...
Malignant brain tumors can occur at all ages [1-2] and are associated with poor prognosis. Surgery forms the mainstay of treatment for brain tumors [2-5]. It is well known that significant tumor decompression is associated with a relatively favourable prognosis [6-10]. An important challenge in brain tumor surgery is the accurate recognition of
A new way to fight an extremely aggressive kind of brain tumor is showing promise in two experiments with a small number of patients. For the experiments, scientists took patients' own immune ...
At the first phase, 32 survey papers, 90 ML- and DL-based papers, 20 dataset papers, and 33 papers on the basics of brain tumor analysis (in total 175 papers) were collected. Although the publications from 2015 to mid-2021 based on those search terms were collected at the first phase, the duplicates were removed, and they were filtered based on ...
We stood there for a moment, she and I, aware. I wanted desperately for her to come closer, to stay in her orbit a moment longer. I lingered long after she left. Sometime in my daughter Orli's ...