Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article

Green synthesis and characterization of copper nanoparticles for investigating their effect on germination and growth of wheat
Contributed equally to this work with: Humaira Kausar, Ansar Mehmood
Roles Conceptualization, Investigation
Affiliation Department of Botany, University of Poonch Rawalakot, Azad Kashmir, Pakistan
Roles Conceptualization, Supervision, Writing – original draft, Writing – review & editing
* E-mail: [email protected] , [email protected]
Roles Formal analysis
¶ ‡ RTK, KSA, SH, FN, MSI, MN and TSU also contributed equally to this work.
Affiliation Department of Botany, the University of Azad Jammu and Kashmir (UAJK), Muzaffarabad, Pakistan
Roles Methodology, Writing – review & editing
Roles Validation
Roles Software
Current address: Institute of Crop Science (340 h), University of Hohenheim, Stuttgart, Germany
Affiliation Department of Agronomy, MNS University of Agriculture Multan, Punjab, Pakistan
Affiliation Department of Botany, University of Gujrat, Punjab, Pakistan
Roles Resources
Affiliation Department of Botany, University of Kotli, Azad Jammu and Kashmir, Pakistan
- Humaira Kausar,
- Ansar Mehmood,
- Rizwan Taj Khan,
- Khawaja Shafique Ahmad,
- Sajjad Hussain,
- Fahim Nawaz,
- Muhammad Sajjad Iqbal,
- Muhammad Nasir,
- Tariq Saif Ullah

- Published: June 21, 2022
- https://doi.org/10.1371/journal.pone.0269987
- Reader Comments
Today, different types of nanoparticles (NPs) are being synthesized and used for medical and agricultural applications. In this study, copper nanoparticles (CuNPs) were synthesized using the aqueous extract of mint ( Mentha longifolia L.). For the characterization of CuNPs, UV-visible spectroscopy, scanning electron microscopy, X-ray diffraction, and Fourier transform infrared spectrometry were used. The UV-Visible absorption peak at 558 nm confirmed the formation of CuNPs. The XRD pattern confirmed the phase-centered crystalline nature of CuNPs. FTIR analysis showed the O-H, Cu-H and C-C bonds, indicating the active role of these functional groups as reducing agents of Cu ions to CuNPS. The synthesized NPs were found to have an almost spherical shape with an average size of 23 nm. When applied to wheat, a condition dependent effect of CuNPs was found. Variety 18-Elite Line 1, Elite Line 3, and 18-Elite Line 6 showed maximum germination and growth rate at 50 mg CuNPs/L, while variety 18-Elite Line 5 showed that increase at 25 mg CuNPs/L. Beyond these concentrations, the seed germination and growth of wheat declined. In conclusion, the application of CuNPs showed a beneficial effect in improving the growth of wheat at a certain concentration.
Citation: Kausar H, Mehmood A, Khan RT, Ahmad KS, Hussain S, Nawaz F, et al. (2022) Green synthesis and characterization of copper nanoparticles for investigating their effect on germination and growth of wheat. PLoS ONE 17(6): e0269987. https://doi.org/10.1371/journal.pone.0269987
Editor: Mohammad Shahid, Aligarh Muslim University, INDIA
Received: February 18, 2022; Accepted: June 1, 2022; Published: June 21, 2022
Copyright: © 2022 Kausar et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting information files.
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Research in nanotechnology has evolved rapidly over the past few decades. Nanotechnology is a branch of science that deals with the atomic and molecular analysis of material objects [ 1 ]. Nanoparticles (NPs) are classified as particles up to 100 nm in size and consisting of chemical and physical processes, including unique properties [ 2 ]. They have a very significant role in various applications because of their unique properties such as optical, electrical, catalytic, electromagnetic, and mechanical, which enable them to be used in various sectors such as medical diagnostics, therapy, electronics, clothing, and agriculture [ 3 ]. Nano research is having an impact on a variety of fields, including the environment and catalysts [ 4 – 7 ]. Metal NPs production has been given great attention in the last few years, particularly to control and discover their possible applications and specific properties. The increased production of metal NPs has also increased their discharge into our environment, and the effect of their particular physical and chemical properties on the ecosystem is becoming a key concern [ 8 ]. NPs can have both negative and positive effects on the growth and development of a plant, and their influence on plants depends on the size, shape, and properties of both plant species and NPs [ 9 ].
Copper (Cu), an element in block D of the periodic table, is a microelement essential for plant improvement and growth. It is a cofactor of superoxide phenol oxidases, ascorbate oxidase, a part of regulatory proteins, and is involved in the electron transport chain during photosynthesis and respiration [ 10 , 11 ]. Cu in the form of nanomaterial gains exceptional properties like its small size and high surface area, which give it chemical reactivity, physical resistance, magnetism, and optical effects [ 12 ]. Due to these unique properties, copper nanoparticles (CuNPs) are used for a wide variety of applications, including bioactive coatings, air and liquid filtration, sensors, ceramics, films, skin products, lubricant oils, inks, wood protection, and textiles. Recent estimates suggest that the global production of CuNPs, or Cu-based NPs, has come to 200 tons/year [ 13 ]. Their extensive engineering and use have led them to enter the environment and interact with agriculture. They can cause either positive or negative effects following contact with plants, depending upon the concentration of the NPs and the type of plant. It is therefore the need of the hour to investigate the effect of CuNPs on crops, as there are only a few studies available that show the influence of CuNPs on crop plants.
CuNPs can be engineered by way of specific routes like physical, chemical, and biological techniques [ 14 – 16 ]. The main problems with the physical and chemical methods are the time-consuming nature of them and their usage of exclusive and toxic chemicals [ 17 ]. The chemical methods are eco-incompatible, costly, and also have a low yield. In contrast to physical and chemical methods, biological methods (green synthesis) are more advantageous in the sense of being eco-friendly, cost-effective, and high yielding [ 18 ]. Green synthesis is largely motivated by environmental concerns, with the goal of developing a green pathway for NPs synthesis that is also contamination-free [ 19 ].
Green synthesis involves the use of algae [ 20 ], sea cucumbers [ 6 ], marine animals [ 21 ], plants [ 16 , 22 , 23 ], and microorganisms among many others [ 24 , 25 ]. Biological techniques make it easier to reduce dissolved metal ions to a zero-valence state and produce the corresponding nanoparticles because of their inherent capabilities. NPs synthesis with the aid of using plants is beneficial over the use of microorganisms because it gets rid of the complicated procedure of keeping cellular cultures and can also be well scaled up [ 26 ]. Keeping in mind the importance and benefits of green synthesis, CuNPs were synthesized in this study by using a plant extract and their effect was evaluated on the growth and development of wheat ( T . aestivum ), an essential and widely consumed staple food. In Pakistan, wheat crops provide a living for 80 percent of farmers. It is also a very significant single commodity in Pakistan’s rural areas as a source of earnings [ 27 ]. Although some studies have reported the effect of CuNPs on wheat, they have used either very small concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 ppm) of CuNPs [ 28 ] or chemically engineered CuNPs (50 nm) of large size [ 29 ]. In contrast to these, we used biologically synthesized CuNPs of 23 nm in size at a concentration of 0, 25, 50, and 100 mg/L.
Therefore, the first aim of this study is the green synthesis and characterization of CuNPs. In this study, an aqueous extract of Mentha longifolia will be used for the reduction of Cu ions to CuNPs at room temperature. The CuNPs will be characterized by UV-visible spectroscopy, scanning electron microscopy, X-ray diffraction, and Fourier transform infrared spectrometry. The second aim is the investigation of the effect of biosynthesized CuNPs on germination, growth, and biochemical attributes of the widely consumed staple food wheat.
Material and methods
Synthesis and characterization of cunps.
A biological method, i.e., the use of an aqueous extract of M . longifolia was adopted in this study for the synthesis of CuNPs. The plant material was collected from the Rawalakot region of Azad Jammu and Kashmir, Pakistan. The plant was identified with the help of the flora of Pakistan and other available literature, and a voucher specimen was submitted to the herbarium, Department of Botany, University of Poonch Rawalakot. To prepare the aqueous extract, the whole plant was dried in the shade, ground to powder, and 25 g of powder was dissolved in 400 mL of distilled water, then kept at room temperature for 24 hours. After that, it was filtered through Whatman filter paper no. 42 and the filtrate was used for the synthesis of CuNPs. The CuNPs synthesis was attained by treating 80 mL of 1 mM CuSO 4 at room temperature with 20 mL of plant extract, held in the dark, and color changes were observed. When the brown color developed (after 24 hrs), the reacting mixture was analyzed for UV-vis spectroscopy in the range of wavelengths between 300 to 800 nm using Perkin-Elmer lambda 750 spectrophotometers, indicating the formation of CuNPs. After initial indication, the reacting solution was centrifuged for 15 min at 10,000 rpm, the supernatant was discarded, the pellet was re-dispersed in distilled water and the centrifugation cycle was repeated at the same pace and duration. The final round of re-dispersion and centrifugation was accomplished with acetone to obtain cleaned and purified CuNPs. The morphology, phase, and capped functional groups of CuNPs were determined through scanning electron microscopy (MIRA 3 Tescan), x-ray diffraction analysis, and Fourier transform infrared spectrometry (Perkin Elmer Spectrum 100 FTIR).
Application of CuNPs on wheat using Petri plate assay
Seed source, treatments, and germination..
The seeds of four wheat varieties like 18-Elite Line 1, 18-Elite Line 3, 18-Elite Line 5, and 18-Elite Line 6 have been obtained from the Department of Plant Breeding Genetics, University of Poonch Rawalakot. The seeds were sterilized for 1 minute with 75 percent ethanol and 15 minutes with 2.5 percent calcium hypochlorite after being soaked in 0.6 percent HNO 3 for 10 minutes to end seed dormancy for successful germination [ 30 ]. The experiment was conducted in a completely randomized design (CRD) with 5 replicates. The four concentrations of CuNPs (0, 25, 50, and 100 mg/L) were applied in two ways, i.e., seed treatment and foliar spray. For seed treatment, the seeds were soaked in an aqueous solution of CuNPs for 3 h, and then 10 seeds of each variety were evenly placed in each Petri plate (labeled) already containing wet blotting paper. All the Petri plates were placed at room temperature and allowed to germinate with a daily supply of 5 mL of each concentration as a foliar spray. Samples were collected after 10 days of treatment and examined the effects of CuNPs on germination, growth, and biochemical attributes.
Germination indices measurements.
The germinated seeds were counted in each treatment for the measurement of germination percentage (GP). The GP was determined as GP = GN/SN × 100, where GN and SN are the total germinated seeds and tested seeds, respectively. The germination index (GI) was measured as GI = number of seeds germinated seeds/days of the first count + number of germinated seeds/days of the final count.
Growth parameters measurements.
To estimate growth, the primary root and shoot length of 10 days of seedlings were measured. For this, 5 plants were selected randomly from each treatment, the length was measured in cm, and the average was calculated. Similarly, the fresh weight of seedlings was measured in grams with the help of an analytical balance.
Biochemical contents measurements.
For the extraction of chlorophyll and carotenoid pigments, fresh leaves weighing 0.1 g were homogenized in 10 mL of 80% acetone. The homogenate was then centrifuged at 15000 rpm for 10 min, the pellet was discarded, and the absorbance of the supernatant was recorded at 666, 653, and 470 nm on the Shimadzu UV-1601 spectrophotometer. The formulae developed by [ 31 ] were used for the calculation of chlorophyll and carotenoid: Chla (mg/g) = [(12.25 x A663.2)—(2.79 x A646.8)] × ml acetone / mg leaf tissue, Chlb (mg/g) = [(21.50 x A646.8)—(5.10 x A663.2)] × ml acetone / mg leaf tissue. Total Chl = Chla + Chlb and Carotenoids (mg/g) = (1000 A470–1.8Chla– 85. 02 Chlb) /198. Total phenolic content was measured by Folin–Ciocalteu’s phenol reagent assay [ 32 ]. A 0.2 mL of plant extract mixed with 5 mL of 10-fold diluted Folin–Ciocalteu’s phenol reagent was kept for 4 minutes and then it was aided with 4 mL of sodium carbonate (7.5 percent w/v). The whole mixture was diluted up to a volume of 15 mL with distilled water and mixed well. The reaction was permitted to stand for 90 min, and the absorption of each sample was reported at 760 nm using a spectrophotometer Shimadzu UV-1601. The total phenols were measured using the calibration curve for gallic acid (GA) and presented as the equivalent of mg GA / g FW. To extract total soluble sugars, 50 mg of fresh leaves were crushed thoroughly in 5 mL of 80% ethanol and centrifuged for 10 minutes at 3000 rpm. From the supernatant, 0.1 mL was taken in a test tube and kept in a water bath for the evaporation of ethanol. After the ethanol had completely evaporated, 4 mL of 0.2 percent enthrone reagent was added to the test tube, heated in boiling water for 10 minutes, and then allowed to cool at room temperature. The absorption of each sample was reported at 760 nm using a spectrophotometer Shimadzu UV-1601. Total soluble sugars were measured using the calibration curve for glucose and presented as the equivalent of mg Gu/g FW.
Statistical analysis
The experiment was conducted in CRD using three replicates and analysis of variance (ANOVA) was performed in statistix 8.1. The statistical difference between means was evaluated, considering p < 0.05 as a significance level.
Results and discussion
Synthesis, morphology, size, and structure of cunps.
The CuNPs were synthesized from the aqueous extract of M . longifolia . The addition of aqueous extract to the aqueous solution of CuSO 4 resulted in the development of a color change from pale yellow to dark brown over the course of 24 h, the first indication of the formation of CuNPs in the solution ( Fig 1 ). This color change is developed due to the excitation of surface plasmon resonance (SPR) [ 33 ]. Previous research has also suggested that brown color can be established in the reaction mixture of plant extract and CuSO 4 [ 34 ]. This color change was further accomplished by taking a UV-vis spectrum of a brown-colored solution in the range of 400 to 800 nm ( Fig 1 ). The spectrophotometric study revealed an absorption band at 558 nm, a result of interaction between the free electrons presents on the surface of metal NPs and light of specific wavelengths. This absorption band indicates the formation of CuNPs in the colloidal solution because it is a known fact that CuNPs show characteristic absorption peaks in the range of 200–800 nm [ 35 ]. Moreover, it has been analyzed that a single SPR band represents round-shaped nanoparticles, whereas two or more SPR bands correspond to the anisotropy of nanoparticles [ 36 ]. In our study, CuNPs in the solution showed a single SPR band, which reveals the round shape of CuNPs. The morphology and size of the prepared CuNPs are shown in the images of SEM ( Fig 2a ) and their mean size distribution histogram ( Fig 2b ). The CuNPs exhibited a spherical shape, were distributed irregularly, and had an average size of 23 nm. Fig 2c represents the energy dispersive x-ray (EDX) of the powder and shows the presence of Cu in the material. The peaks belonging to elemental Cu, C, and O were clearly detected, and there were no extra peaks, demonstrating the purity of the synthesized NPs and correlating with the XRD analysis. It’s also possible that the existence of C and O is due to bioactive molecules that have been capped. Furthermore, only a few copper atoms on the surface of the NPs are likely to have been oxidized, yielding modest quantities of CuO and Cu 2 O. The XRD pattern of CuNPs is shown in Fig 3 , confirms the face-centered crystalline nature, as all the peaks match Cu with FCC symmetry and are consistent with JCPDS No: 04–0836, which reveals the crystalline nature of CuNPs [ 37 ]. With the help of FTIR ( Fig 3 ), the functional groups of all the possible biocompounds involved in the reduction and capping of CuNPs are identified. The FTIR spectrum showed peaks at 3754.36, 2054.64, 1937.67, 1587.34, 1266.30, and 1043.06 cm -1 . The peak at 3754.36 cm -1 corresponds to O-H stretching frequency of hydroxyl groups of polyphenols. The peaks at 2054.64 and 1937.67`cm -1 correspond to Cu-H (metal-hydrogen) bonds. The peaks at 1587.34, 1266.30, and 1043.06 cm -1 could be due to C-C stretching vibrations of an aromatic compound, C-O stretching of carboxylic acid, and ester bonds of phenolic compounds. These results suggest an interaction between the functional groups present in plant extract and Cu ions that results in bio-reduction, the formation, and stabilization of CuNPs [ 4 , 6 , 38 ]. CuNPs are thought to be formed from copper salts using plant extracts in three steps: production of copper ions, reduction of the ions, and lastly, oxidation of the reduced ions [ 16 , 20 ].
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
a copper sulfate solution. b plant extract. c mixture of copper sulfate solution and plant extract at zero-time. d change in color of solution after 24 h of time lap. e UV-visible spectrograph of CuNPs.
https://doi.org/10.1371/journal.pone.0269987.g001
(a) FESEM micrograph of CuNPs, showing spherical shape of CuNPs; (b) Size distribution histogram of CuNPs, showing average size of 23 nm. c EDX spectrum of CuNPs.
https://doi.org/10.1371/journal.pone.0269987.g002
(a) XRD pattern of CuNPs, predicting crystalline nature of CuNPs; (b) FTIR spectrum of CuNPs, confirming functional groups from plant extract capping the CuNPs.
https://doi.org/10.1371/journal.pone.0269987.g003
Effect of CuNPs on seed germination of wheat
Seed germination is the beginning of a physiological process of a plant that needs water imbibition and is directly linked to the yield of the plant. In this study, seed germination and germination index of the tested wheat varieties exhibited an almost similar trend, except for the variety 18-Elite line-5. Fig 4 shows the GP and GI of wheat varieties after the 10 th day of treatment with different concentrations of an aqueous solution of CuNPs. When exposed to 50 mg CuNPs/L, GP and GI increased considerably in varieties 18-Elite line-1, 18-Elite line-3, and 18-Elite line-6, while the variety 18-Elite line-5 showed increased GP and GI under 25 mg CuNPs/L. After 50 mg CuNPs/L exposure, the GP increased to approximately 67, 31, 33, and 11 percent in 18-Elite line-1, 18-Elite line-3, 18-Elite line-5, and 18-Elite line-6, respectively, of the GP observed in control. However, in 18-Elite line-5, a higher increase was found at 25 mg CuNPs/L (100%) as compared to control. A similar trend was found for GI, it increased to approximately 76, 37, 41, and 17 percent in 18-Elite line-1, 18-Elite line-3, 18-Elite line-5, and 18-Elite line-6, respectively, of the GI, recorded in control after the application of 50 mg CuNPs/L. More than double the increase in GI was apparent in 18-Elite line-5 after treatment with 25 mg CuNPs/L. When varieties were exposed to 100 mg CuNPs/L, no statistical difference was found in GP compared to control, except in variety 18-Elite line-6, which showed a reduced GP and GI as compared to control. Similar results were reported by [ 39 ], where lettuce seeds showed better germination when treated with CuNPs. It is found that NPs tend to come into contact with seed coats, penetrate the seeds, and improve seed germination and seedling growth of the plants [ 40 ]. They found that CuNPs penetrated the cell wall and formed new pores, so water absorption was improved, and as a result, favorable seed germination was started. Another study demonstrated that high seed germination rates might be due to the photo-generation of active oxygen like hydroxide anions and superoxide, which causes re-activation of aged seeds. In the nanocomposite, activated carbon provides moisture and a large surface area for seed germination [ 41 ].
Different letters in the same sub-group of columns indicate significant difference at p < 0.05 level.
https://doi.org/10.1371/journal.pone.0269987.g004
Effect of CuNPs on seedling growth of wheat
RL, SL, and FW were measured for all the tested varieties after 10 days of seedling ( Fig 5 ) to investigate the effect of CuNPs on seedling growth of wheat. All the concentrations of CuNPs posed a positive effect on the RL of all wheat varieties, but the most prominent increase was found at 50 mg CuNPs/L in 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6 and 25 mg CuNPs/L in 18-Elite line-5. When treated with 50 mg CuNPs/L, the RL increased by 50, 153, and 33% in 18-Elite line-1, 18-Elite line-3, and 18-Elite line-6, respectively, and by 134 percent in 18-Elite line-5 when treated with 25 mg CuNPs/L. When compared to the control, the SL in 18-Elite line-1, 18-Elite line-3, and 18-Elite line-6 increased by 19, 20, and 37 percent, respectively, under 50 mg CuNPs/L, and by 54 percent in 18-Elite line-5 under 25 mg CuNPs/L. The FW also displayed the same results as for RL and SL, where a CuNPs concentration of 50 mg/L exhibited the most prominent effect on the FW of three wheat varieties. The maximum increase of 75, 95, and 35 in FW was observed for 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6, respectively, treated with 50 mg CuNPs/L, while a maximum increase of 18% in FW was observed for 18-Elite line-5, treated with 25 mg CuNPs/L.
https://doi.org/10.1371/journal.pone.0269987.g005
After 10 days of exposure, the CuNPs treatments modified the RL, SL, and FW of wheat. RL, SL, and FW were significantly increased, especially for varieties exposed to 50 mg CuNPs/L. These changes in the root and shoot system might be due to hormonal imbalances, like auxin and cytokinin imbalances [ 42 ]. Our findings are supported by the study [ 33 ], where CuNPs, at low concentration, increased the fresh weight of wheat. Moreover, we have observed that at 100 mg/L treatment of CuNPs, the seedling growth is significantly reduced compared to the treatment of 50 mg CuNPs/L, suggesting that a high dose of CuNPs can be lethal to plants. At high concentrations, CuNPs may release cupric ions that change the physiological processes of the plant. These modifications increase the capacity of particles that cross the cell membrane of a plant cell [ 3 ]. Previous studies also supported this fact that a higher concentration of CuNPs produces toxic effects, e.g., in chickpea and soybean, CuONPs increased root and shoot growth at 100 ppm, but beyond this concentration (200 ppm), the root and shoot growth was found to decrease [ 43 ]. Other studies also suggest the same result, e.g., CuNPs reduced the seedling length by 65 and 75% in Phaseolus radiatus and Triticum aestivum , respectively [ 44 ]. In a similar way, CuONPs decreased the RL in Cucurbita pepo when applied at a 1000 mg/L concentration [ 45 ].
Effect of CuNPs on photosynthetic pigments of wheat seedling
The effect of CuNPs on photosynthetic pigments of wheat varieties was measured in terms of total chlorophyll and carotenoid ( Fig 6 ). Chlorophylls are located in the chloroplast where they play a crucial role in the photosynthesis system, which is highly related to plant productivity and biomass [ 46 ]. As a result, the total content of Chl and carotenoid in wheat varieties was measured in all treatments in the current study. The total chl content started increasing upon exposure to CuNPs and a higher level was found at 50 mg CuNPs/L in 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6, while in 18-Elite line-5, 25 mg CuNPs/L evidenced stimulation of chl up to 48% as compared with no application of CuNPs (control). Carotenoid content in test plants also followed the same trend as total Chl. A maximum increase of 61, 40, and 42% in carotenoid content was found in 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6, respectively, at 50 mg CuNPs/L. Some authors also noticed changes in the content of photosynthetic pigments as a result of nanoparticle application. For example, a concentration-dependent effect of CuNPs on chlorophyll and carotenoid pigments was studied by [ 47 ], where CuNPs increased the chlorophyll and carotenoid content. Similarly, Vigna radiata has shown increased chlorophyll and carotenoid content under the treatment of CuNPs [ 48 ]. We also observed a significant inhibitory effect at higher concentrations (100 mg CuNPs/L). This could be attributed directly to oxidative stress or the interaction of RuBP carboxylase, due to copper interaction with SH groups. Additionally, the Chl content decrease might also be due to the lowered shoot biomass upon contact with higher concentrations of CuONPs or to the membrane damage as a result of excess lipid peroxidation of chloroplast membranes under oxidative stress [ 49 ].
https://doi.org/10.1371/journal.pone.0269987.g006
Effect of CuNPs on phenolic and sugar content of wheat seedling
Treatment with CuNPs significantly influenced the content of phenol and soluble sugar in the seedlings of wheat ( Fig 7 ). A maximum increase in total phenols was found at 100 mg CuNPs/L. It has been identified that plants induce the production of phenolic compounds in response to NPs [ 50 ]. In some other studies, an increase in phenolic content was also observed under a high concentration of nanoparticles. For example, fruits of Jalapeno pepper showed an increase in the total phenols under the application of CuNPs + Chitosan-PVA [ 51 ]. Similarly, the application of zinc nano fertilizer also increased the total phenols in pomegranate fruits [ 52 ]. This increase in phenolic content at higher concentrations may be related to the plant’s response to stress, as we observed 100 mg CuNPs/L caused toxic effects on wheat. Phenols are antioxidant compounds that trigger the synthesis of a series of secondary metabolites from the shikimic acid pathway or through phenylpropanoids under conditions of abiotic stress [ 53 ]. Therefore, the observed response may be related to ROS formation due to CuNPs. However, unlike the phenolic content, the soluble sugar content was found to be higher at 50 mg CuNPs/L treatment, as was the case with all other factors investigated. Overall, we found a positive effect of CuNPs on the growth and development of wheat. These results were supported by previous studies that showed Cu plays an important role in plant growth and development, and plant productivity [ 54 ].
https://doi.org/10.1371/journal.pone.0269987.g007
Conclusions
This study used an eco-friendly and appropriate method for the synthesis of CuNPs using Mentha longifolia extract . There were no chemical reagents or surfactant templates required, allowing green bioprocesses for a range of biomedical applications. During the application of CuNPs on wheat, we found in our study that with an increase in the concentration of CuNPs, the seed germination and growth of wheat plants were also increased. However, after a certain concentration (50 mg CuNPs/L), the seed germination and seedling growth were found to decrease. Overall results showed that application of CuNPs influences the seed germination and seedling growth of wheat at different concentrations. The higher germination and growth were found at 50 mg CuNPs/L treatment for 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6 wheat varieties and at 25 mg CuNPs/L for 18-Elite line-5. Beyond this treatment, germination and growth were inhibited. Effective germination and growth at a certain optimum concentration and decreased germination and growth beyond this concentration may be attributed to the varieties of wheat and the uptake and accumulation of CuNPs by the roots, because the germination and growth were dependent on the exposure concentration of CuNPs. In particular, the contact of plants with NPs and the impact of such contact on plant growth could spur new research focus on nanobiotechnology. Further studies are needed to understand the mechanism of accumulation and uptake of CuNPs in plants and the way they act during seed germination and growth.
Supporting information
https://doi.org/10.1371/journal.pone.0269987.s001
Acknowledgments
We are thankful to Department of Plant Breeding and Genetics, University of Poonch Rawalakot for providing seeds of wheat varieties. We are also thankful to IST Islamabad for providing scanning electron microscopy and X-ray diffraction facilities.
- View Article
- PubMed/NCBI
- Google Scholar
- 26. Kim BS, Song JY. Biological synthesis of metal nanoparticles. In: Hou CT, Shaw JF, editors. Biocatalysis and Agricultural Biotechnology. CRC Press, Boca Raton; 2009. pp. 399–407.
- 49. Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Clarendon Press, Oxford; 1989.

Current Nanomaterials
Editor-in-Chief: Manoj Gupta Department of Mechanical Engineering National University of Singapore (NUS) Singapore
ISSN (Print): 2405-4615 ISSN (Online): 2405-4623
Green Synthesis of Copper Nanoparticles by Using Plant Extracts and their Biomedical Applications – An Extensive Review
- Department of Physics, Vidyasagar University, Paschim Medinipur-721102, West Bengal, India
- Department of Physics, Narajole Raj College, Paschim Medinipur-721211, West Bengal, India
Volume 8, Issue 2, 2023
Published on: 18 July, 2022
Page: [110 - 125] Pages: 16
DOI: 10.2174/2405461507666220516092814

In recent years, the green synthesis of different metal nanoparticles has become a substantial technique for the synthesis of different essential nanoparticles and their potential applications in technological, industrial along with biomedical fields. Among the several essential nanoparticles, copper nanoparticles (CuNPs) have attracted enormous attention for their wide range of applications like the production of gas sensors, solar cells, high-temperature superconductors as well as drug delivery materials and catalysis owing to its distinctive optical, electrical, dielectric, imaging and catalytic, etc. properties. Herein, in this review, our aim is to find out the recent progress of synthesis, as well as different optical and structural characterizations of green, synthesized CuNPs along with their broadspectrum biomedical applications, mainly antibacterial, antifungal, antiviral and anticancer as well as the future perspective of research trends in the green synthesis of CuNPs. CuNPs have been synthesized by different researchers using three methods, namely, physical, chemical, and biological. In this review, the eco-friendly, efficient and low cost different established biological/green synthesis methods of CuNPs using different plant extracts like leaves, flowers, fruits, seeds, latex, etc., as capping and reducing agents have been briefly discussed, along with reaction conditions together with their optical as well as structural analysis. Effects of different parameters on the green synthesis of CuNPs like the presence of phytochemicals and confirmation of phytochemicals, temperature, pH, etc., are elucidated. Studies of the antibacterial activity of biomolecules capped CuNPs by different researchers against both Gram-positive and Gram-negative bacterial strains along with minimum inhibitory concentration (MIC) values have been summarized. Furthermore, antifungal and antiviral effects of green synthesized CuNPs studied by different researchers are mentioned with minimum inhibitory concentration (MIC) values. The anticancer activity of green synthesized CuNPs against different cancer cells studied by different researchers is summarized with correlation sizes of CuNPs on anticancer activity. The review also focuses on in vivo applications of green synthesized CuNPs along with clinical trails. Furthermore, an emphasis is given to the effectiveness of CuNPs in combating COVID-19.
Keywords: Green synthesis , copper nanoparticles , plant extracts , MNPs , phytochemicals , biomedical applications.
Graphical Abstract

Title: Green Synthesis of Copper Nanoparticles by Using Plant Extracts and their Biomedical Applications – An Extensive Review
Volume: 8 Issue: 2
Author(s): Soumen Rakshit, Paresh Chandra Jana and Tapanendu Kamilya*
Abstract: In recent years, the green synthesis of different metal nanoparticles has become a substantial technique for the synthesis of different essential nanoparticles and their potential applications in technological, industrial along with biomedical fields. Among the several essential nanoparticles, copper nanoparticles (CuNPs) have attracted enormous attention for their wide range of applications like the production of gas sensors, solar cells, high-temperature superconductors as well as drug delivery materials and catalysis owing to its distinctive optical, electrical, dielectric, imaging and catalytic, etc. properties. Herein, in this review, our aim is to find out the recent progress of synthesis, as well as different optical and structural characterizations of green, synthesized CuNPs along with their broadspectrum biomedical applications, mainly antibacterial, antifungal, antiviral and anticancer as well as the future perspective of research trends in the green synthesis of CuNPs. CuNPs have been synthesized by different researchers using three methods, namely, physical, chemical, and biological. In this review, the eco-friendly, efficient and low cost different established biological/green synthesis methods of CuNPs using different plant extracts like leaves, flowers, fruits, seeds, latex, etc., as capping and reducing agents have been briefly discussed, along with reaction conditions together with their optical as well as structural analysis. Effects of different parameters on the green synthesis of CuNPs like the presence of phytochemicals and confirmation of phytochemicals, temperature, pH, etc., are elucidated. Studies of the antibacterial activity of biomolecules capped CuNPs by different researchers against both Gram-positive and Gram-negative bacterial strains along with minimum inhibitory concentration (MIC) values have been summarized. Furthermore, antifungal and antiviral effects of green synthesized CuNPs studied by different researchers are mentioned with minimum inhibitory concentration (MIC) values. The anticancer activity of green synthesized CuNPs against different cancer cells studied by different researchers is summarized with correlation sizes of CuNPs on anticancer activity. The review also focuses on in vivo applications of green synthesized CuNPs along with clinical trails. Furthermore, an emphasis is given to the effectiveness of CuNPs in combating COVID-19.
Export Options
About this article.
Cite this article as:
Rakshit Soumen, Jana Chandra Paresh and Kamilya Tapanendu*, Green Synthesis of Copper Nanoparticles by Using Plant Extracts and their Biomedical Applications – An Extensive Review, Current Nanomaterials 2023; 8 (2) . https://dx.doi.org/10.2174/2405461507666220516092814
| | 2405-4615 |
| Bentham Science Publisher | 2405-4623 |
Call for Papers in Thematic Issues
Recent advances in nanomaterials: modeling, simulation, machining and characterization.
This special cover the vast domain of the advanced modeling and simulation of various nanomaterials, and its processing and structures governed by the laws of mechanics. The emphasis is on advanced and innovative modeling approaches and numerical strategies. The main objective is to describe the actual physics of large systems ... read more
“Recent Advances in Design, Characterizations, Applications and Future Prospective of Smart Nanomaterials”
Scope of the Thematic Issue: Smart materials are the family of materials (hydrogels, nitinol, electroactive polymer, graphene structures, carbon-metal nanohybrids …many more) which represent their significant electrical, catalytic, thermal, optical, mechanical and magnetic properties. There are various numbers of categorized smart nanomaterials materials reported so far such as thermoresponsive, piezoelectric, ... read more
Related Journals
Current Catalysis

Current Materials Science
Journal of Photocatalysis

Current Mechanics and Advanced Materials

Current Applied Materials
Related Books

Thin Film Nanomaterials: Synthesis, Properties and Innovative Energy Applications

Advanced Materials and Nano Systems: Theory and Experiment (Part 3)

Plant Mediated Synthesis of Metal Nanoparticles

Nanoelectronic Devices and Applications

Advanced Materials for Emerging Applications (Innovations, Improvements, Inclusion and Impact)

Metal Matrix Composites: A Modern Approach to Manufacturing

Bioderived Materials: Harnessing Nature for Advanced Biochemical Handiwork

Nanoscale Field Effect Transistors: Emerging Applications

Biocarbon Polymer Composites

Manufacturing and Processing of Advanced Materials

- About Journal
- Editorial Board
- Journal Insight
- Current Issue
- Volumes /Issues
- Author Guidelines
- Graphical Abstracts
- Fabricating and Stating False Information
- Research Misconduct
- Post Publication Discussions and Corrections
- Publishing Ethics and Rectitude
- Increase Visibility of Your Article
- Archiving Policies
- Peer Review Workflow
- Order Your Article Before Print
- Promote Your Article
- Manuscript Transfer Facility
- Editorial Policies
- Allegations from Whistleblowers
- Forthcoming Thematic Issues
- Guest Editor Guidelines
- Editorial Management
- Ethical Guidelines for New Editors
- Reviewer Guidelines
- Abstract Ahead of Print 0
- Article(s) in Press 31
- Free Online Copy
- Most Cited Articles
- Most Accessed Articles
- Highlighted Article
- Most Popular Articles
- Editor's Choice
- Thematic Issues
- Open Access Articles
- Open Access Funding
- Library Recommendation
- Trial Requests
- Advertise With Us
- Meet the Executive Guest Editor(s)
- Brand Ambassador
- Author's Comment & Reviews
- New Journals 2023
- New Journals 2024
- Alert Subscription
Related Articles
Restricted access panel.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Green Synthesis of Copper Nanoparticles and its Characterization

2021, Journal of Scientific Research
80 DOI: 10.37398/JSR.2021.650111 Abstract: The green nanotechnology is generating interest in researchers for the synthesis of nanoparticle in a simple, cost effective, less toxic and ecofriendly manner. The present study reports the biosynthesis of copper nanoparticle using the leaf extract of Ocimum sanctum. The color change in the Ocimum leaf extract when copper sulphate solution is added indicates the presence of copper nanoparticle. The effect of temperature and time of incubation on the biosynthesis of Cu NP were noted. The characterization of the biosynthesized copper nanoparticle was done by UV Vis spectrophotometer, Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD).
Related Papers
International journal of pharmaceutical investigation
Avinash A S H O K Survase
World Journal of Biology Pharmacy and Health Sciences
Mukundraj Rathod
International Journal of Plant & Soil Science
sudeshna baruah
This research aimed at exploring eco-friendly green synthesis of CuNPs using different plant species used for research purpose were Nyctanthes arbor-tristris (Night jasmine), Gardenia jasminoides (Cape jasmine), Tabernaemontana divartica (Crape jasmine), Cascabela thevetia (Yellow oleander), Clerodendrum inerme (Glory bower), Hibiscus rosa-sinensis (China rose) and Allamanda cathartica (Allamanda) for synthesizing CuNPs. Out of seven ornamental plant species CuNPs were synthesized from three species viz. Night jasmine, Yellow oleander, Allamanda which were confirmed through UV-VIS spectrophotometer in wavelength 250-450 nm. The SPR peak was recorded at 301.00 nm, 300.50 nm and 300.00 nm for Allamanda, Yellow oleander and Night jasmine respectively that confirmed the formation of CuNPs. FTIR analysis of CuNPs showed different functional groups such as O−H, N−H, S−H, O=C=O, C≡C, C=O, N−O, C−H, M−O for Allamanda, O−H, N≡N, N−H, C−Cl for Yellow oleander and O−H, C−N, −C≡C−, =C−H, N−O fo...
TJPRC Publication
The plants Asparagus adscendens, Bacopa monnieri, Ocimum bacilicum, and Withania somnifera were used and compared for their extracellular synthesis of metallic copper nanoparticles (CuNPs). Stable Cu nanoparticles were formed by treating aqueous solution of CuSO4•5H2O with the plant leaf extracts as reducing agent. By treatment of 1mM aqueous solutions of CuSO4•5H2O with leaf extract stable CuNPs were formed; the change in color of solution confirm the formation of stable nanoparticles. UV-Visible study revealed qualitative formation of CuNPs and characteristic absorption peak in Asparagus adscendens, Bacopa monnieri and Ocimum bacilicum leaf extract at the range of 500-700nm but in Withania somnifera leaf extract absorption peak of CuNPs is shifted at the range of 500-800nm. These biosynthesized CuNPs were characterized with the help of Fourier transform infrared spectroscopy (FTIR), and Transmission Electron Microscopy (TEM). The involvement of primary and secondary metabolites or possible reducing agent confirmed by FTIR analysis. TEM confirms the formation and the crystalline nature of Copper nanomaterial. This method can be used as effective and environmental friendly technique for the synthesis of Copper nanoparticles using leaf extract of different plants. The antibacterial potentials of the CuNPs were studied and these are shown good antimicrobial activity against Gram positive and Gram negative bacteria.
Physicochemical Problems of Mineral Processing
Zygmunt Sadowski
Plant extract obtained from green tea was used for the synthesis of nanoparticles under anaerobic and aerobic conditions at various ratios of the copper solution to the extract used. The smallest nanoparticles were obtained at a ratio of 1:10. The nanoparticles showed the maximum negative value of the zeta potential around pH 6. An increase in the temperature of reaction caused a decrease in the negative zeta potential value. Synthesis under nitrogen atmosphere favours the formation of smaller copper nanoparticles.
Materials Chemistry and Physics
Iftikhar Hussain Shah
Interventions in Pediatric Dentistry Open Access Journal
Zahid Qureshi
Present approach for synthesizing copper nanoparticles using plant extract is important plant based bio-resource which eliminates the use of synthetic reducing and capping agents. These biocompatible nanoparticles were nontoxic. The plant extracts of Azadiracta indica, Lantana camera, Calotropis procera and Tridax procumbens was successfully used for Synthesis of copper nanoparticles. The synthesized copper nanoparticles from plant extracts showed the signatory colour then optical absorbance was recorded by UV Visible spectrophotometer in 24 and 48 hrs. It clearly showed that the Lantana camera expressed the highest absorbance value compared to other three plants and demonstrated that the smaller particle sizes of synthesized copper nanoparticles and Lantana camera and Tridax procumbens showed maximum zone of inhibition having diameter 10mm while the neem showed the 5mm and the Calotropis procera shows the 6mm zone against E.coli.
G.DAYANA JEYALEELA
Recently, development of reliable experimental protocols for synthesis of metal nanoparticles with desired morphologies and sizes has become a major focus of researchers. Green synthesis of metal nanoparticles using plant extracts emerged as a nontoxic and eco-friendly method for synthesis of metal nanoparticles. In this study biosynthesis of stable copper nanoparticles were done using aqueous leaf extract of Vitex negundo leaf from 3mm copper sulphate solution. Synthesized nanoparticles were characterized under UV-Vis spectroscopy at the range of 400 nm to 800 nm at varying intervals of time. The peak at 254nm revealed the presence of CuNPs. It was observed that the Vitex negundo leaf extract can reduce copper ions into copper nanoparticles within 10 to 15 min of reaction time. These biosynthesized Cu nanoparticles were characterized with the help of X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and SEM techniques. GC-MS analysis revealed that the leaf extract of Vitex negundo.L,contains steroids, saponins, tannins, phenols, triterpenoids, flavonoids, glycosides, and glycerides. These bioactive principles are found to be responsible for bioreduction during the synthesis of spherical copper nanoparticles. The preparation of nano-structured copper particles using Vitex negundo.L leaf extract thus provides an environmentally friendly option, as compared to currently available chemical and physical methods.
Current Science
Vasudeo Kulkarni
Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
RELATED PAPERS
Journal of the Turkish Chemical Society, Section A: Chemistry
hayrunnisa nadaroglu
Oriental Journal Of Chemistry
Dr.C.Alosious Gonsago
TRIDHA Scholars
Alagunambi Ramasubbu
Nanoscale research letters
Muhammad Imran Din
humaira rashied
Editor iajps
American Journal of Biomedical Science & Research
Farrukh Jaleel
gopal shende
Wali Muhammad
RSC Advances
Annadurai Gurusamy
Journal of Agriculture
Yılmaz KOÇAK
Dr. Faiz Rabbani , Reena Rasheed
Balka Chitwas
Journal of chemical and pharmaceutical research
Muhamed haneefa M
IJAR Indexing
A.Antony Lawrence
Dr Antony L A W R E N C E Andrews
Endocrine, metabolic & immune disorders
Monika Vats
International Journal of Scientific Research in Science and Technology
International Journal of Scientific Research in Science and Technology IJSRST
Sharmila Pradhan
Environmental Chemistry Letters
Nav Raten Panwar
Solid State Phenomena
Asian Journal of Pharmaceutical and Clinical Research
Arushi Saloki
Akintelu Sunday Adewale
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Nanoscience
- Nanostructures
- Nanoparticles
- Metal Nanoparticles
- Copper Nanoparticles
Green synthesis of Copper Nanoparticles and Investigation of its Antimicrobial Properties
- This person is not on ResearchGate, or hasn't claimed this research yet.

- Florida State University
- Nigeria Sugar Institute, Ilorin

- Nnamdi Azikiwe University, Awka
Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Anupama Senthilkumar
- Razia Muthuswamy

- Fatimetou Mohamed N'dah

- E. Ech-chihbi
- J MATER SCI-MATER EL

- A U Onuigbo

- Fariba Garkani Nejad

- Bee Luan Khoo
- INT J ELECTROCHEM SC
- Arefeh Mohammadnavaz
- Yunkang Liu

- AFR J AGR RES

- J. M. Gichumbi
- Nkem F Obianagha

- Anurekha Jain

- Samson O. Famuyiwa

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 01 November 2023
Green synthesis, characterization, antibacterial, and antifungal activity of copper oxide nanoparticles derived from Morinda citrifolia leaf extract
- Manogar Priya 1 na1 ,
- Raja Venkatesan 2 ,
- Simon Deepa 1 ,
- Siva Sankar Sana 2 na1 ,
- Soundhar Arumugam 3 ,
- Abdulnasser M. Karami 4 ,
- Alexandre A. Vetcher 5 &
- Seong-Cheol Kim 2
Scientific Reports volume 13 , Article number: 18838 ( 2023 ) Cite this article
7769 Accesses
12 Citations
29 Altmetric
Metrics details
- Materials science
- Nanoscience and technology
The green methodologies of nanoparticles with plant extracts have received an increase of interest. Copper oxide nanoparticles (CuO NPs) have been utilized in a many of applications in the last few decades. The current study presents the synthesis of CuO NPs with aqueous extract of Morinda citrifolia as a stabilizing agent. The leaf extract of Morinda citrifolia was mixed with a solution of copper sulphate (CuSO 4 ·5H 2 O) and sodium hydroxide as a catalyst. UV–visible spectroscopy, FTIR, XRD, SEM, TEM, and EDAX analysis were performed to study the synthesized CuO NPs. Particle size distribution of the synthesized CuO NPs have been measured with dynamic light scattering. The CuO NPs synthesized were highly stable, sphere-like, and have size of particles from 20 to 50 nm. Furthermore, as-formed CuO NPs shown strong antibacterial activity against the Gram-positive bacteria ( Bacillus subtilis, and Staphylococcus aureus ), and Gram-negative bacteria ( Escherichia coli ). CuO NPs revealed a similar trend was analysed for antifungal activity. The zone of inhibition for the fungi evaluated for Aspergillus flavus (13.0 ± 1.1), Aspergillus niger (14.3 ± 0.7), and Penicillium frequentans (16.8 ± 1.4). According to the results of this investigation, green synthesized CuO NPs with Morinda citrifolia leaf extract may be used in biomedicine as a replacement agent for biological applications.
Similar content being viewed by others
Biosynthesis of copper nanoparticles using Alstonia scholaris leaves and its antimicrobial studies
Green synthesis of copper oxide nanoparticles and its efficiency in degradation of rifampicin antibiotic

Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential
Introduction.
Nanotechnology is growing as an essential area with enormous potential for many applications due to the distinctive characteristics of nanoparticles (NPs) 1 . In comparison with their bulk substitutes, these nanoscale materials have enhanced optical, magnetic, catalytic, and electrical capacities 2 , 3 . As a result, there is more interest in producing sustainable and effective methods for synthesizing nanoparticles. Traditional methods of synthesizing nanoparticles often involve the use of hazardous chemicals, high temperatures, and energy-intensive processes, leading to environmental concerns and potential toxicity. To address these issues, green synthesis has gained considerable attention as a promising alternative. Green synthesis, also known as environmentally friendly or sustainable synthesis, involves the use of natural resources, biomolecules, or environmentally benign materials to fabricate nanoparticles 4 , 5 , 6 . It offers several advantages over conventional methods, including reduced energy consumption, minimal use of toxic chemicals, biodegradability, and the potential for large-scale production 7 , 8 .
Metal oxide nanoparticles attract the attention of researchers due to the connect bulk and atomic structures. NPs have unique physicochemical characteristics which include significant reactivity, huge surface area, pore size, and particles shape 9 . Introduction to novel nanoparticles might put immunological in nature and inflammation responses to the challenge 10 . The most rapid adopters of nanotechnology are the areas of information and communication (such as electrical and optoelectronic sectors), food technology, energy technology, and medical products (including a number of pharmaceuticals and drug delivery systems, diagnostics, and medical technology). Toxicity arising from nanomaterials might present new problems. These situations may involve nanoparticle which have been introduced into the environment or which were given to individuals via nanotechnology products. Nanoparticles are can be synthesized by physical, chemical, biological, and hybrid procedures 11 , 12 , 13 . Toxic materials render the production of physical and chemical nanoparticles more difficult. Effective eco-friendly biogenetic methods of production have become more common due to their ease of use and flexibility 14 , 15 . Before, nanoparticles that needed to be produced via chemical and physical methods. Nanoparticles and nanotechnology deal with small materials. Nanoparticles were extensively studied in recent years due to their many potential uses in chemistry, drug delivery, biomedical, and other areas 16 , 17 , 18 , 19 .
As a result of their biocidal characteristics, copper nanoparticles are now attractive wounds treatment. With its cheap price and excellent physical and chemical attributes, copper NPs can be utilized in process bandage. The method for the production of nanomaterials is dependent on their small dimensions and high surface-to-volume ratio 20 . Metal and metal oxide nanoparticles have been employed in a wide range of applications. Several distinctive methods for adjusting shape and size includes metal vapour co-deposition, electrochemical reduction, gas phase evaporation, thermal decomposition, radiolytic reduction, and chemical reduction 21 , 22 , 23 , 24 . Nanosized particles can be produced with chemical and physical methods like micro-emulsion are immersed. For instance, flame-based aerosol techniques, Sono chemical hydrothermal techniques, solid-state techniques, and the system for producing nanoparticles. Nanoparticles cannot be used in healthcare due to there are generated with toxic materials. Clean, biocompatible, nontoxic, and sustainable nanoparticle processing is thus advantageous 25 , 26 . This field is currently concentrating on “green” chemistry and bio-processors.
Plants are used in “green synthesis” for the production of metal nanoparticles. Green synthesis in biotechnology and nanotechnology has an opportunity for advantages for the economy and the environment 27 . Green chemistry synthesizes in an environmentally friendly and efficient method. Nanoparticles have been proposed to be synthesized in plants, algae, bacteria, yeast, and fungi 28 . The nanoparticles of copper can be produced from plant extracts using eco-friendly, low-cost, and biocompatible reducing agents 29 , 30 . Copper oxide nanoparticles development is enhanced with ascorbic acid in Morinda citrifolia leaf extract. In addition to their distinctive characteristics, such as a large surface area, catalytic activity, and antibacterial capabilities, CuO NPs have attracted interest in many other fields. Bioengineered CuO NPs are those that undergo CuO nanoparticle synthesis or modification with biological processes like bacteria, fungi, or plant extracts. The significance of using bioengineered CuO NPs lies in their potential to provide more sustainable, efficient, and biocompatible solutions across various fields, from healthcare and environmental protection to materials science and energy.
In this work, we developed an efficient method to synthesize CuO NPs and studied the crystalline nature, chemical composition, and interactions between NPs and the reducing agent. Morinda citrifolia leaf extract was used as a stabilizing agent in the green synthesis of CuO NPs. The copper oxide nanoparticles with functional components, structure, and particle size were studied with UV–vis, FTIR, XRD, SEM, TEM, and DLS analysis. Furthermore, the antibacterial effects of the CuO NPs were investigated by Gram-positive bacteria ( Bacillus subtilis, and Staphylococcus aureus ), and Gram-negative bacteria ( Escherichia coli ) with the agar diffusion method.
Materials and methods
Copper sulphate (CuSO 4 ·5H 2 O) was purchased from Sigma-Aldrich (98%). Hydrochloric acid (HCl) (35%), sodium hydroxide (98%) was used to monitoring the pH, were received from Merck. The leaves of Morinda citrifolia have been collected in Chennai, Tamil Nadu. The dissolution of 2.5 g of CuSO 4 ·5H 2 O in 100 mL deionized water yielded a 1 × 10 –2 M stock solution of copper sulphate. Bacterial and fungal cultures were grown in the medium, including Bacillus subtilis , Staphylococcus aureus , Escherichia coli , Aspergillus flavus , Aspergillus niger , and Penicillium frequentans . All of the chemical and solvents utilized were of analytical grade.
Preparation of Morinda citrifolia leaf extract
The Morinda citrifolia leaf extract can be seen in Fig. 1 A. Morinda citrifolia , a plant of the Rubiaceae family, had its leaves collected from a garden in Chennai. We weighed and cleaned Morinda citrifolia leaves several times with tap water and deionized water after collecting to get rid of any extra dust or contaminants. After that, a slice the leaf in small pieces, add 100 mL of distilled water, and immerse the mixture in a water bath heated to 60 °C for 1 h. The green extract can be processed in a burette and used as a reducing or capping agent. The extract was kept at 4 °C for further studies.
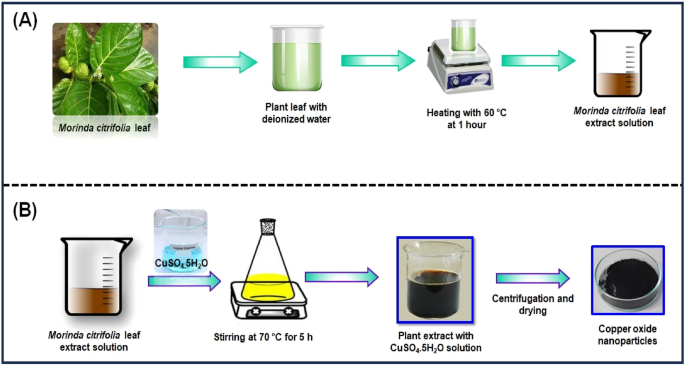
( A ) Schematic representation of eco-friendly synthesis of copper oxide nanoparticles using Morinda citrifolia leaf extract; ( B ) Schematic diagram of CuO NPs from leaf extract of Morinda citrifolia.
Synthesis of CuO NPs from Morinda citrifolia leaf extract
Figure 1 B shows the synthesis of CuO NPs from Morinda citrifolia leaf extract solution. 2.5 g of CuSO 4 ·5H 2 O was dissolved in 100 mL of Deionized water (DI) to initiate the green synthesis process for CuO NPs. After, 50 mL of Morinda citrifolia extract solution to 100 mL of 1 × 10 –2 M CuSO 4 ·5H 2 O solution, the pH was kept at 7.0 with NaOH. The solution then underwent to a reflux at a magnetic stirrer. The colour of the solution changed as it was stirring with a from pale-green to a deep-brown while maintaining for 5 h at 70 °C. After centrifuging the solution for 24 h, it was filtered. The solid precipitate was washed three times with deionised water, followed by an 100% ethanol wash for CuO NPs separation, dried at 60 °C for 4 h, and kept at 4 °C for further application.
The following equations explain the synthesis mechanism for CuO NPs;
Characterization of synthesized CuO NPs
The UV–Visible spectrum of effectively obtained CuO NPs was collected with an ( Oceian optics JAZ, USA ) spectrophotometer. The UV spectrum of copper oxide nanoparticle synthesis in colloidal solution was observed at wavelengths ranging from 200 to 800 nm. The FTIR spectrometer ( Perkin Elmer, Spectrum-2, USA ) with KBr pellet was used for collecting functional group data in the region of 4000–400 cm −1 . The FTIR spectrum of obtained CuO NPs was examined. Different modes of vibration in the CuO NPs have been identified and assigned to evaluate the presence of different functional groups that aid the extract of the Morinda citrifolia plant. XRD measurement of the CuO NPs, where only 5.0 ml of the extract was added, was done on a Shimadzu XRD-6000 diffractometer operating at a voltage of 40 kV and current of 20 mA with Cu-Kα radiation (λ = 1.54 Å). The XRD spectrum has been examined and acquired with scanning range values of 20° and 80°.SEM study of the surface morphology of CuO NPs was performed ( CARL ZEISS, Jena, Germany ). The inner morphology of the CuO nanoparticles was studied with Morinda citrifolia extract, and images were captured using a TEM ( JEOL, JEM-2100, Japan ). For descriptive purposes, a 5.0 ml of the materials were sonicated in ethanol, and a drop of it was cast in a copper grid with a 300-mesh carbon layer by layer for magnetic measurements. The particle size distribution (PSD) of the synthesized CuO NPs have been measured with the Dynamic Light Scattering (DLS) measurements instrument's standard operating procedure.
Antibacterial and antifungal studies
Methodology.
Bacillus subtilis (MTCC6133), Escherichia coli (MTCC6133), and Staphylococcus aureus (MTCC96) were collected from the Microbial Type Culture Collection and Gene Bank (MTCC), Institute of Microbial Technology, Chandigarh, India. Standard cultures of bacteria have been sub-cultured into newly prepared nutrient agar and incubated at 37 °C for 24 h for the production of fresh cultures of bacteria. Marina Labs Research and Development offers fungal cultures of Aspergillus flavus (MLAC1101), Aspergillus niger (MLAC1201), and Penicillium frequentans (MLAC 2101). The fungi were sub-cultured for 72 h to produce the sporulation process and the developing spore were examined for antifungal activity.
Assay for antibacterial activity by well diffusion
The zone of inhabitation method was employed to evaluate the antibacterial activity of the offered materials 31 , 32 , 33 . Mueller–Hinton agar plates were applied to test the samples. The agar plate was streaked with the different cultures (bacterial strains). Then, using a sterile cork-borer, 5 mm diameter wells were cut into the agar medium. For 20 min, the plates are allowed to dry to remove all remaining moisture. The compounds of 15 µL, 20 µL, and 25 µL were administered into each well. As a positive control, a well containing 15 µL of streptomycin antibiotic was used. The plates were incubated at 37 °C. The tests were performed in duplicates. Every plate was evaluated for zones of inhibition 24 h after incubation. The diameter of the inhibitory zone was calculated in millimetres (mm).
Assay for antifungal activity by well diffusion
For testing the antibacterial activity of the offered sample, the agar well diffusion method was employed. Sabouraud’s Dextrose agar plates were employed for testing the specimens. The agar plate’s surface was streaked with the different cultures (fungal strains). The agar medium was then cut into 5 mm diameter wells with a sterile cork-borer. For 20 min, the plates are allowed to dry to remove additional moisture. Compounds of 15 µL, 20 µL, and 25 µL were dispensed into each well, with 5.0 mg of Fluconazole serve as a positive control. At 37 °C, the plates were incubated. The tests have been carried out in duplicate. After 24 h of incubation, each plate was examined for zones of inhibition. The zone of inhibition was recorded as the diameter of inhibition zone in mm.
Leaves collection permission
The Morinda citrifolia leaves have been obtained from Chennai, Tamil Nadu in India, and all of the national guidelines, legislation and/or protocols have adhered appropriately. Morinda citrifolia is a flora species found predominantly in India. In Tamil Nadu, this species is a very common tree seen in road sides and in every gardens. Hence, the usage of this plant needs no permission and licensing.
Ethical approval
We comply with relevant guidelines and legislation regarding the sample collection in the present study. The plant leaves ( Morinda citrifolia ), in the present study is not endangered. In 2023, leaves of the Morinda citrifolia plant were collected in Chennai, Tamil Nadu, India. There are no plant material samples for the current study.
Consent to participate
All person named as author in this manuscript have participated in the planning, design and performance of the research and in the interpretation of the result.
Result and discussion
The change in the colour of the reaction solution suggests the synthesis of CuO NPs by the reduction of CuSO 4 ·5H 2 O during treatment with extracts of Morinda citrifolia leaf. The change in color of the reaction solution after 2 h reveals the synthesis of CuO NPs. The result indicates that the Cu-Extract 2+ ions in the reaction mixture have changed to copper oxide with nanometric size. In the synthesis of CuO NPs, different types of plant extracts are used as reducing and stabilizing agents. The resultant nanoparticles have no surface instead of encased in a medium or gel, and their catalytic and other characteristics can be restricted, while particle stabilized and microgel stabilized nanoparticles characteristics may be altered by modifying the temperature and pH. Table 1 presents the green synthesis of CuO NPs with different plants.
UV–Visible spectroscopy
The CuO NPs were investigated with UV–Visible spectroscopy to identify the optical band gap. A distinctive peak was found at 256 nm, which might be assigned to surface plasmon resonance (SPR), and it was revealed. The SPR at 256 nm indicates the synthesis of CuO NPs. SPR occurred as a result of an oscillation of surface electron of nanoparticles, so this result agreed with earlier research 34 . In accordance with Mie's theory, the quantity of SPR bands is mainly determined by the shape of nanoparticles that are produced. The spherical form of the nanoparticle is mostly because of a single SPR band. With equation, the band gap energy similar to the wavelength of peak absorption was calculated. The band gap energy can be calculated with the formulas below.
where h is the plank constant, C is the velocity of light, Eg is the energy gap, and g is the measured absorption wavelength.
The synthesized Cu nanoparticle’s strongest and most sharp absorption peak appears at 256 nm, and it shows the blue shift absorption observed in Fig. 2 A. The calculated band gap energy from the UV–visible absorption spectrum is 1.006 eV 56 , 57 . The decrease in particle size has been triggered with a shift in absorption towards smaller wavelengths.
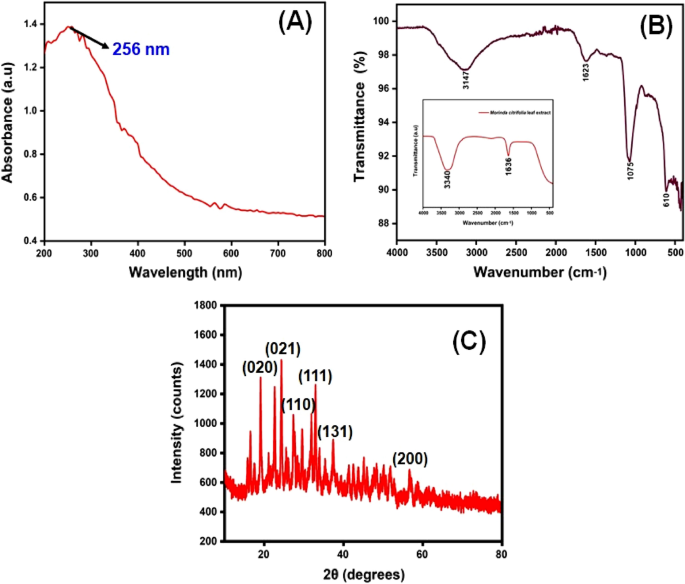
( A ) UV–visible spectrophotometer results of synthesized CuO NPs; ( B ) FTIR spectra and ( C ) XRD pattern.
FTIR spectral analysis
The FTIR spectrum of ecofriendly obtained plants extracts and CuO NPs were studied. The spectra were collected between 4000 and 400 cm −1 . A type of vibrations in the CuO materials have been determined and assigned to identify the existence of different functional groups that aid with the chemical reduction. The FTIR spectra of the plant extract Morinda citrifolia are shown in (Fig. 2 B inset), consistent with the earlier research 42 . The absorption bands at 3340 cm −1 correspond to the absorption band of –OH functional group. The significant peaks at 1636 cm −1 suggest the existence of a functional group denoted as –NO 2 in the plant extract The Morinda citrifolia leaf plant served in the reduction of copper ions as well as the capping of CuO. Figure 2 B presents the results of the research study performed on the peaks. For O–H stretching of water and –C=O stretching of aldehydes and ketones, the major peaks are observed at 3147 cm −1 and 1623 cm −1 , respectively 58 , 59 . The stretching vibration peak for the C=H and H–C–H functional groups is at 495 cm −1 , and the stretching vibration peak for the C=H and H–C–H functional groups is at 2923 cm −1 , confirmation the presence of synthesized CuO nanoparticles in the materials 60 .
XRD analysis
XRD measurements revealed the crystalline characteristics of the obtained copper nanoparticles. The XRD spectrum of the synthesized copper nanoparticles is presented in Fig. 2 C. The CuO NPs exhibited crystalline XRD peaks at 2θ values of 19.03°, 24.36°, 27.39°, 32.99°, 37.55°, and 56.74° which correspond to the planes of crystals of (020), (021), (110), (111), (131) and (200), respectively. The plane alignments of the synthesized CuO NPs were in excellent accordance with the standard CuO nanoparticles obtained for the International Centre of Diffraction Data Card (JCPDS No.: 00-041-0254). The XRD pattern suggested that the synthesized CuO nanoparticles are polycrystalline in characteristic and resembled the monoclinic tenorite phase of the CuO structure. Lattice parameters are α = 4.79 Å, the intensities and positions of peaks are in moral promise with the stated standards 61 , 62 . Additionally, the well- distinct and sharp CuO images detected from XRD patterns approves the moral crystalline nature of the green synthesized CuO NPs. Comparable results were also stated in earlier like works 63 , 64 . Strong orientation and broad diffraction bands in the XRD spectrum can be attributed to the nano dimensional conditions of the obtained nanoparticles. In addition, the XRD pattern indicated the newly synthesised nanoparticles are nanocrystalline. The average crystallite size of CuO nanoparticles was calculated using a Debye–Scherrer formula (Eq. 5 ).
where D is the average diameter of the nanoparticles, K is the Sherrer constant, λ is the wavelength of x-ray diffraction (015,406 nm), β is the full width at half maximum, and θ is Bragg angle (degree).
The average crystalline size of synthesized CuO nanoparticles has been estimated to be in the range of 25–30 nm using the Debye Scherrer formula, and the crystal structure of synthesised CuO nanoparticles has been shown to be face-cantered cubic structure.
SEM analysis
The scanning electron microscope (SEM) confirmed the size and structure of the nanoparticles that were synthesized. The images from SEM suggest that the green synthesized CuO NPs have a major distribution and spherical shapes 65 , and have an average size of 29.2 nm. As predicted, agglomerations decreased as the size of particles increased, due to size of particles increased gain size linear. When the agglomeration of particles can be attributed to an effort to decrease surface free energy, SEM images of CuO nanoparticles are showed in Fig. 3 A–C. The surface alternatives are clearly shown, paying special attention to the fact that nanoparticles were synthesized.
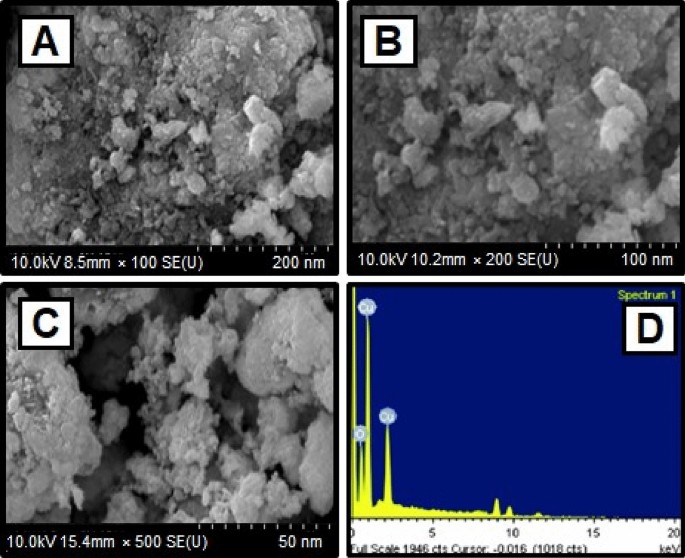
( A )–( C ) SEM image of CuO NPs synthesized using CuSO 4 ·5H 2 O and Morinda citrifolia leaf extract ( D ) EDX analysis of CuO NPs.
The elemental composition of CuO NPs produced with green synthesis method have been identified using a EDAX device. The elemental composition of CuO nanoparticles can be seen in Fig. 3 D. The elements are copper (65%), oxygen (23%), and carbon (12%) shown the Table 2 . The high concentration of copper metal in the advanced levels indicates the synthesis of CuO NPs via a green methodology.
TEM analysis
The TEM images of synthesized CuO nanoparticles are shown in Fig. 4 A–C. TEM was employed to study the particle size and surface morphology of Morinda citrifolia -mediated CuO NPs, and the results suggested that the CuO were polydisperse and cylindrical in structure. The SAED pattern confirmed the crystal structure of CuO NPs. SAED patterns suggest that CuO NPs have distinctive lattice fringes which are similar with the normal CuO structures and have excellent crystalline quality. Padmavathi et. al., observed that produced CuO NPs are surface elements and can serve as a successful reducing agent of CuO ions to CuO NPs in Morinda citrifolia extract 66 . Sodium hydroxide as a catalyst agent, inhibiting CuO NPs aggregation. The TEM results of CuO NPs were fully consistent with the XRD pattern of obtained CuO NPs. This study was aided by the results of Fardood et al., which noticed the FCC structure of CuO NPs using the TEM and SAED patterns of CuO NPs synthesized from Morinda citrifolia leaf extract 67 . The corresponding SAED pattern (inset in Fig. 4 C) indicates that the copper particles given among the CuO NPs are highly crystalline and have the predicted alignment. The Cu, O, and C elements are seen in the element mapping images of the synthesized CuO NPs (Fig. 4 D–F). The presence of nanoparticles in the material is evident as Cu, O, and C are confirmed with synthesized CuO NPs.
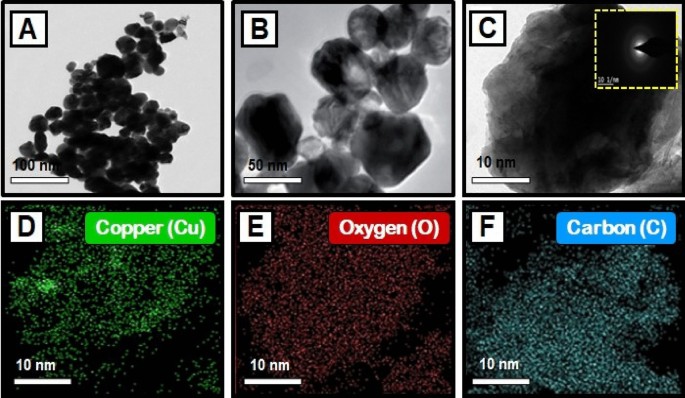
( A – C ) TEM images of copper oxide nanoparticles, and SAED image of CuO NPs [insets Fig. ( C )]; Elemental mapping analysis of CuO NPs from Morinda citrifolia leaf extract, ( D ) Copper, ( E ) Oxygen, and ( F ) Carbon elements.
The particle size distribution of CuO NPs
This method is utilized for synthesizing particles with colloidal structure. The particle size distribution (PSD) for colloids produced at different concentrations of CuSO 4 ·5H 2 O and constant Morinda citrifolia content, as measured with the dynamic light scattering (DLS) method, is shown in Fig. 5 . For the three samples, the types of distribution and average diameters changed. The sample prepared with 1 × 10 –2 M CuSO 4 ·5H 2 O is the most monodisperse and has an average diameter of about 100.0 nm, however the samples obtained with 15, 20 and 25 µL, despite having average dimensions of 49.1 nm, 37.0 nm and 29.2 nm, respectively, have more polydispersity suggested that 15 µL given the best performance. The results reported in previous articles 68 , 69 , comparing Fig. 5 A–C indicates the concentration of the copper sulphate greatly impacts the size distribution of the nanoparticles. Aside from the formation of smaller particles, it was expected that a lower CuSO 4 .5H 2 O concentration would result in a narrower size distribution since the ratio of Morinda citrifolia :Cu 2+ ions would be greater in this case. However, the DLS data shown a trend toward the reverse site. As the concentration of CuSO 4 ·5H 2 O decreased, the size distribution expanded. The result can be addressed if we understand that the average diameter measured with DLS results from nanoparticles surrounded by Morinda citrifolia rather than “naked” CuO NPs. In addition Morinda citrifolia molecules may attach to the surface of particles at lower concentrations of copper sulphate due to the higher Morinda citrifolia :Cu 2+ ions ratio 70 , 71 . Morinda citrifolia molecules may form more than one layer on the nanoparticles. The outermost layer can absorb water, producing tumescence of the composite nanoparticles and, as a result, increasing particle sizes.
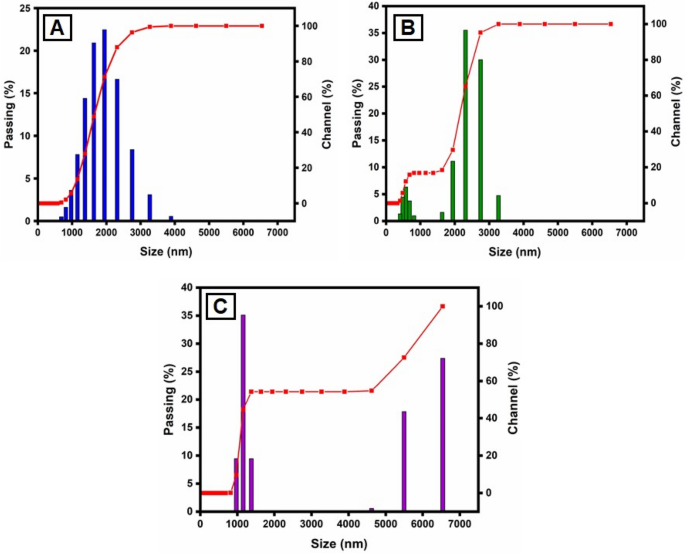
Particle size distribution (PSD) of the synthesized CuO NPs by the DLS methods, for varying CuSO 4 ·5H 2 O concentration: ( A ) 15 µL; ( B ) 20 µL and ( C ) 25 µL.
Antibacterial activity
The disk diffusion method was used to study the antibacterial activity of CuO NPs against gram-positive and gram-negative pathogenic bacteria such as B. subtilis, S. aureus , and E. coli (Fig. 6 a). In laboratories, nutritional broth has been commonly utilized for sustaining live pathogens of bacteria (as subcultures with 0.5 Mc turbidity) cultivated overnight at 37 °C 72 .
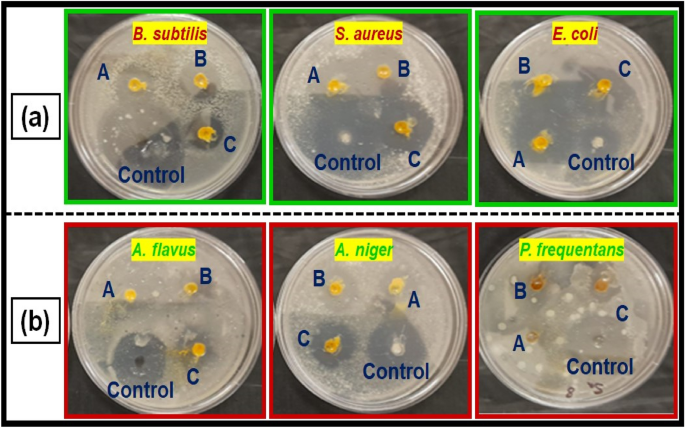
( a ) Antibacterial activity, and ( b ) antifungal activity of copper oxide nanoparticles from Morinda citrifolia leaf extracts; (A) 15 µL, (B) 20 µL and (C) 25 µL; and control of CuO NPs.
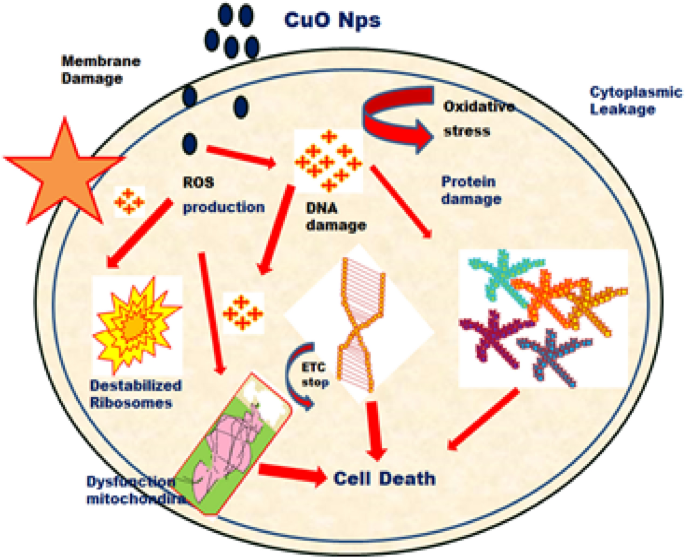
The fresh bacterial culture was swiped evenly on sterilized Petri dishes with nutrient agar. On the clean disks, synthesized CuO NPs (15, 20 and 25 µL) and an aqueous ( Morinda citrifolia ) leaf extract (25 µL) was poured. As a positive control, 25 mL of chloramphenicol disks were maintained, and all plates were incubated overnight at 37 °C for 24–48 h to identify the development of bacterial inhibition zone surrounding the surface of the disk. The results revealed that the CuO NPs has showed antibacterial activity against the bacteria, Bacillus subtilis. It has recorded 13.0 mm zone of inhibition at the concentration of 25 µl . However, there was no zone recorded for the bacteria, Escherichia coli. The compound showed less activity against the bacteria, Staphylococcus aureus. The zone of inhibition recorded for the bacteria, Bacillus subtilis (13.6 ± 1.1), Staphylococcus aureus (13.2 ± 0.2), and Escherichia coli (13.1 ± 1.2) respectively. The antibacterial activity mechanism of green synthesized CuO NPs is shown in Fig. 7 . The antibacterial activity mechanism of copper oxide nanoparticles is dependent on the size, structure, and concentration of copper oxide. The three major ways that antibacterial activity follows are as follows. (1) Degeneration of the cell wall and membrane, (2) Infiltration and cellular disruption, and (3) Oxidation stress 73 , 74 , 75 . The antibacterial activity recorded against each individual bacteria for the CuO nanoparticles is presented in Table 3 .
Schematic representation of green synthesis of copper oxide nanoparticles using Morinda citrifolia leaf extract.
Antifungal activity
Figure 6 b shows a similar pattern for CuO nanoparticle’s antifungal activity. The zone of inhibition recorded for the fungi, Aspergillus flavus (13.1 ± 1.1), Aspergillus niger (14.7 ± 0.7), and Penicillium frequentans (16.2 ± 1.4) respectively. However antifungal activity for the fungus, A . niger was similar to that of control ( Flucanazole ) 76 , 77 . The antifungal activity recorded for the CuO NPs against each individual fungal species is presented in Table 3 .
Conclusions
The copper oxide nanoparticles were synthesized with an eco-friendly methodology obtained from plant extracts such as Morinda citrifolia . The size, shape, elemental composition, and structure of the synthesized CuO NPs were characterized by UV–visible spectroscopy, FTIR, XRD, SEM, TEM and DLS. Within the process of synthesis, the UV–visible absorption spectrum reveals a blue shift as the percentage of plant extract in the resultant mixture rises. XRD patterns suggest that the crystallites of the CuO NPs that developed have a centre cubic structure. The SEM image of the synthesized CuO NPs suggests that these particles exhibit a spherical structure with an average size of the NPs was 29.2 nm. In addition, structural and size studies reveal that CuO NPs synthesized by Morinda citrifolia have a high surface-to-volume ratio. In the EDAX spectrum, the elemental percentage of copper in the CuO NPs was found to be highly uniform. However, the results of bacterial activity showed that the CuO NPs acted well. The synthesized CuO NPs has antibacterial activity against Bacillus subtilis , Escherichia coli , and Staphylococcus aureus . According to the results, CuO NPs is more effective than the other two against Bacillus subtilis . CuO NPs has been proven to be efficient against three distinct types of fungi: Aspergillus flavus , Aspergillus niger , and Penicillium frequentans . Copper oxide nanoparticles has been shown to be most effective against all three kinds of fungi based on the data: Aspergillus flavus , Aspergillus niger , and Penicillium frequentans . As a result, the data show that the antifungal activity of the green synthesised CuO NPs has a higher than its antibacterial activity. This study suggests that the synthesized CuO NPs could be employed in the biomedical, fuel cell, battery and food storage industries. However, more study should be done on minimize the toxicity of CuO NPs though maintaining and improving their biological efficiency in order to promote the biomedical uses of CuO NPs.
Data availability
All data generated or analysed during this study are included in this published article.
Khan, I., Saeed, K. & Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 12 (7), 908–931. https://doi.org/10.1016/j.arabjc.2017.05.011 (2019).
Article CAS Google Scholar
Baig, N., Kammakakam, I. & Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2 (6), 1821–1871. https://doi.org/10.1039/D0MA00807A (2021).
Article Google Scholar
Chandrakala, V., Aruna, V. & Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emerg. Mater. 5 , 1593–1615. https://doi.org/10.1007/s42247-021-00335-x (2022).
Noah, N. M. & Ndangili, P. M. Green synthesis of nanomaterials from sustainable materials for biosensors and drug delivery. Sens. Int. 3 , 100166. https://doi.org/10.1016/j.sintl.2022.100166 (2022).
Aswathi, V. P., Meera, S., Maria, C. G. A. & Nidhin, M. Green synthesis of nanoparticles from biodegradable waste extracts and their applications: A critical review. Nanotechnol. Environ. Eng. 8 , 377–397. https://doi.org/10.1007/s41204-022-00276-8 (2023).
Madani, M. et al. Green synthesis of nanoparticles for varied applications: Green renewable resources and energy-efficient synthetic routes. Nanotechnol. Rev. 11 (1), 731–759. https://doi.org/10.1515/ntrev-2022-0034 (2022).
Harish, V. et al. Review on nanoparticles and nanostructured materials: Bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food applications. Nanomaterials 12 (3), 457. https://doi.org/10.3390/nano12030457 (2022).
Article CAS PubMed PubMed Central Google Scholar
Szczyglewska, P., Feliczak-Guzik, A. & Nowak, I. Nanotechnology-general aspects: A chemical reduction approach to the synthesis of nanoparticles. Molecules 28 (13), 4932. https://doi.org/10.3390/molecules28134932 (2023).
Chavali, M. S. & Nikolova, M. P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 1 , 607. https://doi.org/10.1007/s42452-019-0592-3 (2019).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20 , 101–124. https://doi.org/10.1038/s41573-020-0090-8 (2021).
Article CAS PubMed Google Scholar
Ying, S. et al. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 26 , 102336. https://doi.org/10.1016/j.eti.2022.102336 (2022).
Vijayaram, S. et al. Applications of green synthesized metal nanoparticles—a review. Biol. Trace Elem. Res. https://doi.org/10.1007/s12011-023-03645-9 (2023).
Article PubMed PubMed Central Google Scholar
Sharma, N. K. et al. Green route synthesis and characterization techniques of silver nanoparticles and their biological adeptness. ACS Omega 7 (31), 27004–27020. https://doi.org/10.1021/acsomega.2c01400 (2022).
Sharma, D., Kanchi, S. & Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 12 (8), 3576–3600. https://doi.org/10.1016/j.arabjc.2015.11.002 (2019).
Gudikandula, K., Vadapally, P. & Charya, M. A. S. Biogenic synthesis of silver nanoparticles from white rot fungi: Their characterization and antibacterial studies. OpenNano. 2 , 64–78. https://doi.org/10.1016/j.onano.2017.07.002 (2017).
Haleem, A., Javaid, M., Singh, R. P., Rab, S. & Suman, R. Applications of nanotechnology in medical field: A brief review. J. Glob. Health. 7 (2), 70–77. https://doi.org/10.1016/j.glohj.2023.02.008 (2023).
Ray, S. S. & Bandyopadhyay, J. Nanotechnology-enabled biomedical engineering: Current trends, future scopes, and perspectives. Nanotechnol. Rev. 10 (1), 728–743. https://doi.org/10.1515/ntrev-2021-0052 (2021).
McNamara, K. & Tofail, S. A. M. Nanoparticles in biomedical applications. Adv. Phys.-X 2 (1), 54–88. https://doi.org/10.1080/23746149.2016.1254570 (2017).
Patra, J. K. et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 16 , 71 (2018).
Joudeh, N. & Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 20 , 262. https://doi.org/10.1186/s12951-022-01477-8 (2022).
Theerthagiri, J. et al. Fundamentals and comprehensive insights on pulsed laser synthesis of advanced materials for diverse photo-and electrocatalytic applications. Light Sci. Appl. 11 , 250. https://doi.org/10.1038/s41377-022-00904-7 (2022).
Article ADS CAS PubMed PubMed Central Google Scholar
Thanh, N. T. K., Maclean, N. & Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 114 (15), 7610–7630. https://doi.org/10.1021/cr400544s (2014).
Hui, S. et al. Three-dimensional cathodes for electrochemical reduction of CO 2 : From macro- to nano-engineering. Nanomaterials 10 (9), 1884. https://doi.org/10.3390/nano10091884 (2020).
Ahmed, S. F. et al. Green approaches in synthesising nanomaterials for environmental nanobioremediation: Technological advancements, applications, benefits and challenges. Environ. Res. 204 , 111967. https://doi.org/10.1016/j.envres.2021.111967 (2022).
Nath, D. & Banerjee, P. Green nanotechnology—A new hope for medical biology. Environ. Toxicol. Phar. 36 (3), 997–1014. https://doi.org/10.1016/j.etap.2013.09.002 (2013).
Venkatesan, R. et al. Biodegradable composites from poly(butylene adipate- co -terephthalate) with carbon nanoparticles: Preparation, characterization and performances. Environ. Res. 235 , 116634. https://doi.org/10.1016/j.envres.2023.116634 (2023).
Singh, J. et al. Green synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 16 , 84. https://doi.org/10.1186/s12951-018-0408-4 (2018).
Pandit, C. et al. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ. Sci. 34 (3), 101869. https://doi.org/10.1016/j.jksus.2022.101869 (2022).
Murugappan, G. & Sreeram, K. J. Nano-biocatalyst: Bi-functionalization of protease and amylase on copper oxide nanoparticles. Colloids Surf. B. 197 , 111386. https://doi.org/10.1016/j.colsurfb.2020.111386 (2021).
Manzoor, M. A. et al. Environmental sustainable: Biogenic copper oxide nanoparticles as nano-pesticides for investigating bioactivities against phytopathogens. Environ. Res. 231 (1), 119451. https://doi.org/10.1016/j.envres.2023.115941 (2023).
Venkatesan, R., Rajeswari, N. & Tamilselvi, A. Antimicrobial, mechanical, barrier, and thermal properties of bio-based poly (butylene adipate- co -terephthalate) (PBAT)/Ag 2 O nanocomposite films for packaging application. Polym. Adv. Technol. 29 (1), 61–68. https://doi.org/10.1002/pat.4089 (2018).
Ngamsurach, P. & Praipipat, P. Antibacterial activities against Staphylococcus aureus and Escherichia coli of extracted Piper betle leaf materials by disc diffusion assay and batch experiments. RSC Adv. 12 (40), 26435–26454. https://doi.org/10.1039/D2RA04611C (2022).
Venkatesan, R., Zhang, Y. & Chen, G. Preparation of poly(butylene adipate-co-terephthalate)/ZnSnO 3 composites with enhanced antimicrobial activity. Compos. Commun. 22 , 100469. https://doi.org/10.1016/j.coco.2020.100469 (2020).
Alao, I. I., Oyekunle, I. P., Iwuozor, K. O. & Emenike, E. C. Green synthesis of copper nanoparticles and investigation of its antimicrobial properties. Adv. J. Chem. B. 4 (1), 39–52. https://doi.org/10.22034/ajcb.2022.323779.1106 (2022).
Yallappa, S. et al. Microwave assisted rapid synthesis and biological evaluation of stable copper nanoparticles using T. arjuna bark extract. Spectrochim. Acta A 110 , 108–115. https://doi.org/10.1016/j.saa.2013.03.005 (2013).
Article ADS CAS Google Scholar
Manimaran, K. et al. Eco-friendly approaches of mycosynthesized copper oxide nanoparticles (CuONPs) using Pleurotus citrinopileatus mushroom extracts and their biological applications. Environ. Res. 232 , 116319. https://doi.org/10.1016/j.envres.2023.116319 (2023).
Ismail, M. I. M. Green synthesis and characterizations of copper nanoparticles. Mater. Chem. Phys. 240 , 122283. https://doi.org/10.1016/j.matchemphys.2019.122283 (2020).
Mohamed, E. A. Green synthesis of copper & copper oxide nanoparticles using the extract of seedless dates . Heliyon 6 (1), e03123. https://doi.org/10.1016/j.heliyon.2019.e03123 (2020).
Naika, H. R. et al. Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J. Taibah Univ. Sci. 9 (1), 7–12. https://doi.org/10.1016/j.jtusci.2014.04.006 (2015).
Sivaraj, R., Rahman, P. K. S. M., Rajiv, P., Narendhran, S. & Venckatesh, R. Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim. Acta A 129 , 255–258. https://doi.org/10.1016/j.saa.2014.03.027 (2014).
Abbas, A. H. & Fairouz, N. Y. Characterization, biosynthesis of copper nanoparticles using ginger roots extract and investigation of its antibacterial activity. Mater. Today Proc. 61 (3), 908–913. https://doi.org/10.1016/j.matpr.2021.09.551 (2022).
Dayana, K. S., Mani, R. J. & Durai, S. C. V. Morinda citrifolia leaf extract mediated green synthesis of copper oxide nanoparticles and it’s potential and antibacterial studies. Rasayan J. Chem. 14 (2), 897–904. https://doi.org/10.31788/RJC.2021.1426264 (2021).
Sharma, M., Sharma, S. K., Mathur, M. & Choudhary, M. K. A novel approach towards eco-friendly green synthesis of copper nanoparticles from Bunium persicum and their biomedical applications. Int. J. Health Sci. 6 (5), 3099–3119. https://doi.org/10.53730/ijhs.v6nS5.9155 (2022).
Aheri, H. R., Han, S. H., Vikhe, A. S. & Kuchekar, S. R. Green synthesis of copper nanoparticles using Syzygium Cumin leaf extract, characterization and antimicrobial activity. Chem. Sci. Trans. 8 (1), 1–6. https://doi.org/10.7598/cst2019.1552 (2019).
Wu, S., Rajeshkumar, S., Madasamy, M. & Mahendran, V. Green synthesis of copper nanoparticles using Cissus vitiginea and its antioxidant and antibacterial activity against urinary tract infection pathogens. Artif. Cells Nanomed. Biotechn. 48 (1), 1153–1158. https://doi.org/10.1080/21691401.2020.1817053 (2020).
Suárez-Cerda, J. et al. A green synthesis of copper nanoparticles using native cyclodextrins as stabilizing agents. J. Saudi Chem. Soc. 21 (3), 341–348. https://doi.org/10.1016/j.jscs.2016.10.005 (2017).
Hassanien, R., Husein, D. Z. & Al-Hakkani, M. F. Biosynthesis of copper nanoparticles using aqueous Tilia extract: Antimicrobial and anticancer activities. Heliyon. 4 (12), e01077. https://doi.org/10.1016/j.heliyon.2018.e01077 (2018).
Mali, S. C., Dhaka, A., Githala, C. K. & Trivedi, R. Green synthesis of copper nanoparticles using Celastrus paniculatus . Willd leaf extract and their photocatalytic and antifungal properties. Biotechnol. Rep. 27 , e00518. https://doi.org/10.1016/j.btre.2020.e00518 (2020).
Tahir, A. et al. Green synthesis, characterization and antibacterial, antifungal, larvicidal and anti-termite activities of copper nanoparticles derived from Grewia asiatica L. Bull. Natl. Res. Cent. 46 , 188. https://doi.org/10.1186/s42269-022-00877-y (2022).
Heydari, R., Koudehi, M. F. & Pourmortazavi, S. M. Antibacterial activity of Fe 3 O 4 /Cu nanocomposite: Green synthesis using Carum carvi L. seeds aqueous extract. ChemistrySelect 17 (2), 531–535. https://doi.org/10.1002/slct.201803431 (2019).
Salas, Z. H. et al. Green synthesis of copper nanoparticles and their formulation into face masks: An antibacterial study. Polym. Compos. 44 (2), 907–916. https://doi.org/10.1002/pc.27142 (2023).
Thandapani, G. et al. Green synthesis of copper oxide nanoparticles using Spinacia oleracea leaf extract and evaluation of biological applications: Antioxidant, antibacterial, larvicidal and biosafety assay. Mater. Today Commun. 34 , 105248. https://doi.org/10.1016/j.mtcomm.2022.105248 (2023).
Kalaiyan, G., Prabu, K. M., Suresh, S. & Suresh, N. Green synthesis of CuO nanostructures with bactericidal activities using Simarouba glauca leaf extract. Chem. Phys. Lett. 761 , 138062. https://doi.org/10.1016/j.cplett.2020.138062 (2020).
Lafmejani, Z. N., Jafari, A. A., Moradi, P. & Moghadam, A. L. Impact of foliar application of copper sulphate and copper nanoparticles on some morpho-physiological traits and essential oil composition of peppermint ( Mentha piperita L.). Herba Pol. 64 (2), 13–24. https://doi.org/10.2478/hepo-2018-0006 (2018).
Benassai, E. et al. Green and cost-effective synthesis of copper nanoparticles by extracts of non-edible and waste plant materials from Vaccinium species: Characterization and antimicrobial activity. Mater. Sci. Eng. C 119 , 111453. https://doi.org/10.1016/j.msec.2020.111453 (2021).
Ramasubbu, K. et al. Green synthesis of copper oxide nanoparticles using sesbania grandiflora leaf extract and their evaluation of anti-diabetic, cytotoxic, anti-microbial, and anti-inflammatory properties in an in-vitro approach. Fermentation 9 (4), 332. https://doi.org/10.3390/fermentation9040332 (2023).
Majeed, S., Shukhairi, A. N. B. & Danish, M. Green Approach for the synthesis of copper oxide nanoparticles and its antibacterial effect against methicillin-resistant staphylococcus aureus (MRSA). J. Pure Appl. Microbiol. 16 (1), 708–716. https://doi.org/10.22207/JPAM.16.1.74 (2022).
Amin, F. et al. Green synthesis of copper oxide nanoparticles using Aerva javanica leaf extract and their characterization and investigation of in vitro antimicrobial potential and cytotoxic activities. Evid. Based Complement. Altern. Med. , https://doi.org/10.1155/2021/5589703 (2021).
Raul, P. K. et al. CuO nanorods: A potential and efficient adsorbent in water purification. RSC Adv. 4 , 40580–40587. https://doi.org/10.1039/C4RA04619F (2014).
Ethiraj, A. S. & Kang, D. J. Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res. Lett. 7 , 70. https://doi.org/10.1186/1556-276X-7-70 (2012).
Arockiasamy, J. S. K. & Irudayaraj, J. Natural dye sensitized CuO nanorods for luminescence applications. Ceram. Int. 42 (5), 6198–6205. https://doi.org/10.1016/j.ceramint.2015.12.180 (2016).
Kamble, S. P. & Mote, V. D. Structural, optical and magnetic properties of Co doped CuO nano-particles by sol-gel auto combustion technique. Solid State Sci. 95 , 105936. https://doi.org/10.1016/j.solidstatesciences.2019.105936 (2019).
Kannan, K., Radhika, D., Nesaraj, A. S., Sadasivuni, K. K. & Krishna, L. S. Facile synthesis of NiO-CYSO nanocomposite for photocatalytic and antibacterial applications. Inorg. Chem. Commun. 122 , 108307. https://doi.org/10.1016/j.inoche.2020.108307 (2020).
Karthik, K., Dhanuskodi, S., Gobinath, C., Prabukumar, S. & Sivaramakrishnan, S. Dielectric and antibacterial studies of microwave assisted calcium hydroxide nanoparticles. J. Mater. Sci. Mater. Electron. 28 , 16509–16518. https://doi.org/10.1007/s10854-017-7563-5 (2017).
Kannan, K. et al. Facile fabrication of novel ceria-based nanocomposite (CYO-CSO) via co-precipitation: Electrochemical, photocatalytic and antibacterial performance. J. Mol. Struct. 1256 , 132519. https://doi.org/10.1016/j.molstruc.2022.132519 (2022).
Padmavathi, A. R. et al. Copper oxide nanoparticles as an effective anti-biofilm agent against a copper tolerant marine bacterium, Staphylococcus lentus . Biofouling 35 (9), 1007–1025. https://doi.org/10.1080/08927014.2019.1687689 (2019).
Fardood, S. T., Ramazani, A., Asiabi, P. A. & Joo, S. W. A novel green synthesis of copper oxide nanoparticles using a Henna extract powder. J. Struct. Chem. 59 , 1737–1743. https://doi.org/10.1134/S0022476618070302 (2018).
Yugandhar, P., Vasavi, T., Uma Maheswari Devi, P. & Savithramma, N. Bioinspired green synthesis of copper oxide nanoparticles from Syzygium alternifolium (Wt.) Walp: Characterization and evaluation of its synergistic antimicrobial and anticancer activity. Appl. Nanosci. 7 , 417–427. https://doi.org/10.1007/s13204-017-0584-9 (2017).
Dagher, S., Haik, Y., Ayesh, A. I. & Tit, N. Synthesis and optical properties of colloidal CuO nanoparticles. J. Lumin. 151 , 149–154. https://doi.org/10.1016/j.jlumin.2014.02.015 (2014).
Sondors, R. et al. Size distribution, mechanical and electrical properties of CuO nanowires grown by modified thermal oxidation methods. Nanomaterials 10 , 1051. https://doi.org/10.3390/nano10061051 (2020).
Santhoshkumar, J., Agarwal, H., Menon, S., Rajeshkumar, S. & Venkat Kumar, S. A biological synthesis of copper nanoparticles and its potential applications. In Green Synthesis, Characterization and Applications of Nanoparticles (ed. Shukala, A.K.; Iravani, S.) 2019, 199–221. https://doi.org/10.1016/B978-0-08-102579-6.00009-5 .
Kannan, K., Radhika, D., Gnanasangeetha, D., Krishna, L. S. & Gurushankar, K. Y 3+ and Sm 3+ co-doped mixed metal oxide nanocomposite: Structural, electrochemical, photocatalytic, and antibacterial properties. Appl. Surf. Sci. Adv. 4 , 100085. https://doi.org/10.1016/j.apsadv.2021.100085 (2021).
Naz, S., Gul, A., Zia, M. & Javed, R. Synthesis, biomedical applications, and toxicity of CuO nanoparticles. Appl. Microbiol. Biotechnol. 107 , 1039–1061. https://doi.org/10.1007/s00253-023-12364-z (2023).
Shkodenko, L., Kassirov, I. & Koshel, E. Metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations. Microorganisms 8 , 1545. https://doi.org/10.3390/microorganisms8101545 (2020).
Slavin, Y. N., Asnis, J., Häfeli, U. O. & Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 15 , 65. https://doi.org/10.1186/s12951-017-0308-z (2017).
Chinnaiah, K., Kannan, K., Krishnamoorthy, R. & Gurushankar, K. Datura metal L. leaf extract mediated sodium alginate polymer membrane for supercapacitor and food packaging applications. Int. J. Biol. Macromol. 242 , 125112. https://doi.org/10.1016/j.ijbiomac.2023.125112 (2023).
Rangayasami, A., Kannan, K., Joshi, S. & Subban, M. Bioengineered silver nanoparticles using Elytraria acaulis (L.F.) Lindau leaf extract and its biological applications. Biocatal. Agric. Biotech. 27 , 101690. https://doi.org/10.1016/j.bcab.2020.101690 (2020).
Download references
Acknowledgements
The authors, R.V. and S.-C. Kim, would like to thank their gratitude to the National Research Foundation of Korea (NRF), which was funded supported a by the Ministry of Education (2020R1I1A3052258). This paper has also been supported by the RUDN University Strategic Academic Leadership Program (recipient A.V.). This work was funded by the Researchers Supporting Project Number (RSPD2023R764), King Saud University, Riyadh, Saudi Arabia.
Author information
These authors contributed equally: Manogar Priya and Siva Sankar Sana.
Authors and Affiliations
Department of Chemistry, School of Basic Sciences, Vels Institute of Science, Technology and Advanced Studies, Chennai, Tamil Nadu, 600117, India
Manogar Priya & Simon Deepa
School of Chemical Engineering, Yeungnam University, Gyeongsan, 38541, Republic of Korea
Raja Venkatesan, Siva Sankar Sana & Seong-Cheol Kim
Department of Mechanical Engineering, Indian Institute of Technology Guwahati, Guwahati, Assam, 781039, India
Soundhar Arumugam
Department of Chemistry, College of Science, King Saud University, 11451, Riyadh, Saudi Arabia
Abdulnasser M. Karami
Institute of Biochemical Technology and Nanotechnology, Peoples’ Friendship, University of Russia (RUDN), 6 Miklukho‐Maklaya St., Moscow, Russia, 117198
Alexandre A. Vetcher
You can also search for this author in PubMed Google Scholar
Contributions
M.P.: Formal analysis, Writing—original draft; R.V.: Investigation, Conceptualization, Writing—original draft; S.D.: Data curation; S.S.S.: Formal analysis, Writing—original draft; S.A.: Investigation; A.M.K.: Formal analysis; A.A.V.: Formal analysis, Data curation; S.-C.K.: Supervision, Project administration, Funding acquisition, Writing—review & editing. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Manogar Priya , Raja Venkatesan or Seong-Cheol Kim .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Priya, M., Venkatesan, R., Deepa, S. et al. Green synthesis, characterization, antibacterial, and antifungal activity of copper oxide nanoparticles derived from Morinda citrifolia leaf extract. Sci Rep 13 , 18838 (2023). https://doi.org/10.1038/s41598-023-46002-5
Download citation
Received : 08 September 2023
Accepted : 26 October 2023
Published : 01 November 2023
DOI : https://doi.org/10.1038/s41598-023-46002-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Nir light-activated nanocomposites combat biofilm formation and enhance antibacterial efficacy for improved wound healing.
- Irfan Ullah
- Shahin Shah Khan
Communications Chemistry (2024)
- Ahmad Nasir Labaran
- Zakariyya Uba Zango
- Osamah A. Aldaghri
Scientific Reports (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Green synthesis and characterization of copper nanoparticles for investigating their effect on germination and growth of wheat
Humaira kausar.
1 Department of Botany, University of Poonch Rawalakot, Azad Kashmir, Pakistan
Ansar Mehmood
Rizwan taj khan.
2 Department of Botany, the University of Azad Jammu and Kashmir (UAJK), Muzaffarabad, Pakistan
Khawaja Shafique Ahmad
Sajjad hussain, fahim nawaz.
3 Department of Agronomy, MNS University of Agriculture Multan, Punjab, Pakistan

Muhammad Sajjad Iqbal
4 Department of Botany, University of Gujrat, Punjab, Pakistan
Muhammad Nasir
5 Department of Botany, University of Kotli, Azad Jammu and Kashmir, Pakistan
Tariq Saif Ullah
Associated data.
All relevant data are within the paper and its Supporting information files.
Today, different types of nanoparticles (NPs) are being synthesized and used for medical and agricultural applications. In this study, copper nanoparticles (CuNPs) were synthesized using the aqueous extract of mint ( Mentha longifolia L.). For the characterization of CuNPs, UV-visible spectroscopy, scanning electron microscopy, X-ray diffraction, and Fourier transform infrared spectrometry were used. The UV-Visible absorption peak at 558 nm confirmed the formation of CuNPs. The XRD pattern confirmed the phase-centered crystalline nature of CuNPs. FTIR analysis showed the O-H, Cu-H and C-C bonds, indicating the active role of these functional groups as reducing agents of Cu ions to CuNPS. The synthesized NPs were found to have an almost spherical shape with an average size of 23 nm. When applied to wheat, a condition dependent effect of CuNPs was found. Variety 18-Elite Line 1, Elite Line 3, and 18-Elite Line 6 showed maximum germination and growth rate at 50 mg CuNPs/L, while variety 18-Elite Line 5 showed that increase at 25 mg CuNPs/L. Beyond these concentrations, the seed germination and growth of wheat declined. In conclusion, the application of CuNPs showed a beneficial effect in improving the growth of wheat at a certain concentration.
Introduction
Research in nanotechnology has evolved rapidly over the past few decades. Nanotechnology is a branch of science that deals with the atomic and molecular analysis of material objects [ 1 ]. Nanoparticles (NPs) are classified as particles up to 100 nm in size and consisting of chemical and physical processes, including unique properties [ 2 ]. They have a very significant role in various applications because of their unique properties such as optical, electrical, catalytic, electromagnetic, and mechanical, which enable them to be used in various sectors such as medical diagnostics, therapy, electronics, clothing, and agriculture [ 3 ]. Nano research is having an impact on a variety of fields, including the environment and catalysts [ 4 – 7 ]. Metal NPs production has been given great attention in the last few years, particularly to control and discover their possible applications and specific properties. The increased production of metal NPs has also increased their discharge into our environment, and the effect of their particular physical and chemical properties on the ecosystem is becoming a key concern [ 8 ]. NPs can have both negative and positive effects on the growth and development of a plant, and their influence on plants depends on the size, shape, and properties of both plant species and NPs [ 9 ].
Copper (Cu), an element in block D of the periodic table, is a microelement essential for plant improvement and growth. It is a cofactor of superoxide phenol oxidases, ascorbate oxidase, a part of regulatory proteins, and is involved in the electron transport chain during photosynthesis and respiration [ 10 , 11 ]. Cu in the form of nanomaterial gains exceptional properties like its small size and high surface area, which give it chemical reactivity, physical resistance, magnetism, and optical effects [ 12 ]. Due to these unique properties, copper nanoparticles (CuNPs) are used for a wide variety of applications, including bioactive coatings, air and liquid filtration, sensors, ceramics, films, skin products, lubricant oils, inks, wood protection, and textiles. Recent estimates suggest that the global production of CuNPs, or Cu-based NPs, has come to 200 tons/year [ 13 ]. Their extensive engineering and use have led them to enter the environment and interact with agriculture. They can cause either positive or negative effects following contact with plants, depending upon the concentration of the NPs and the type of plant. It is therefore the need of the hour to investigate the effect of CuNPs on crops, as there are only a few studies available that show the influence of CuNPs on crop plants.
CuNPs can be engineered by way of specific routes like physical, chemical, and biological techniques [ 14 – 16 ]. The main problems with the physical and chemical methods are the time-consuming nature of them and their usage of exclusive and toxic chemicals [ 17 ]. The chemical methods are eco-incompatible, costly, and also have a low yield. In contrast to physical and chemical methods, biological methods (green synthesis) are more advantageous in the sense of being eco-friendly, cost-effective, and high yielding [ 18 ]. Green synthesis is largely motivated by environmental concerns, with the goal of developing a green pathway for NPs synthesis that is also contamination-free [ 19 ].
Green synthesis involves the use of algae [ 20 ], sea cucumbers [ 6 ], marine animals [ 21 ], plants [ 16 , 22 , 23 ], and microorganisms among many others [ 24 , 25 ]. Biological techniques make it easier to reduce dissolved metal ions to a zero-valence state and produce the corresponding nanoparticles because of their inherent capabilities. NPs synthesis with the aid of using plants is beneficial over the use of microorganisms because it gets rid of the complicated procedure of keeping cellular cultures and can also be well scaled up [ 26 ]. Keeping in mind the importance and benefits of green synthesis, CuNPs were synthesized in this study by using a plant extract and their effect was evaluated on the growth and development of wheat ( T . aestivum ), an essential and widely consumed staple food. In Pakistan, wheat crops provide a living for 80 percent of farmers. It is also a very significant single commodity in Pakistan’s rural areas as a source of earnings [ 27 ]. Although some studies have reported the effect of CuNPs on wheat, they have used either very small concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 ppm) of CuNPs [ 28 ] or chemically engineered CuNPs (50 nm) of large size [ 29 ]. In contrast to these, we used biologically synthesized CuNPs of 23 nm in size at a concentration of 0, 25, 50, and 100 mg/L.
Therefore, the first aim of this study is the green synthesis and characterization of CuNPs. In this study, an aqueous extract of Mentha longifolia will be used for the reduction of Cu ions to CuNPs at room temperature. The CuNPs will be characterized by UV-visible spectroscopy, scanning electron microscopy, X-ray diffraction, and Fourier transform infrared spectrometry. The second aim is the investigation of the effect of biosynthesized CuNPs on germination, growth, and biochemical attributes of the widely consumed staple food wheat.
Material and methods
Synthesis and characterization of cunps.
A biological method, i.e., the use of an aqueous extract of M . longifolia was adopted in this study for the synthesis of CuNPs. The plant material was collected from the Rawalakot region of Azad Jammu and Kashmir, Pakistan. The plant was identified with the help of the flora of Pakistan and other available literature, and a voucher specimen was submitted to the herbarium, Department of Botany, University of Poonch Rawalakot. To prepare the aqueous extract, the whole plant was dried in the shade, ground to powder, and 25 g of powder was dissolved in 400 mL of distilled water, then kept at room temperature for 24 hours. After that, it was filtered through Whatman filter paper no. 42 and the filtrate was used for the synthesis of CuNPs. The CuNPs synthesis was attained by treating 80 mL of 1 mM CuSO 4 at room temperature with 20 mL of plant extract, held in the dark, and color changes were observed. When the brown color developed (after 24 hrs), the reacting mixture was analyzed for UV-vis spectroscopy in the range of wavelengths between 300 to 800 nm using Perkin-Elmer lambda 750 spectrophotometers, indicating the formation of CuNPs. After initial indication, the reacting solution was centrifuged for 15 min at 10,000 rpm, the supernatant was discarded, the pellet was re-dispersed in distilled water and the centrifugation cycle was repeated at the same pace and duration. The final round of re-dispersion and centrifugation was accomplished with acetone to obtain cleaned and purified CuNPs. The morphology, phase, and capped functional groups of CuNPs were determined through scanning electron microscopy (MIRA 3 Tescan), x-ray diffraction analysis, and Fourier transform infrared spectrometry (Perkin Elmer Spectrum 100 FTIR).
Application of CuNPs on wheat using Petri plate assay
Seed source, treatments, and germination.
The seeds of four wheat varieties like 18-Elite Line 1, 18-Elite Line 3, 18-Elite Line 5, and 18-Elite Line 6 have been obtained from the Department of Plant Breeding Genetics, University of Poonch Rawalakot. The seeds were sterilized for 1 minute with 75 percent ethanol and 15 minutes with 2.5 percent calcium hypochlorite after being soaked in 0.6 percent HNO 3 for 10 minutes to end seed dormancy for successful germination [ 30 ]. The experiment was conducted in a completely randomized design (CRD) with 5 replicates. The four concentrations of CuNPs (0, 25, 50, and 100 mg/L) were applied in two ways, i.e., seed treatment and foliar spray. For seed treatment, the seeds were soaked in an aqueous solution of CuNPs for 3 h, and then 10 seeds of each variety were evenly placed in each Petri plate (labeled) already containing wet blotting paper. All the Petri plates were placed at room temperature and allowed to germinate with a daily supply of 5 mL of each concentration as a foliar spray. Samples were collected after 10 days of treatment and examined the effects of CuNPs on germination, growth, and biochemical attributes.
Germination indices measurements
The germinated seeds were counted in each treatment for the measurement of germination percentage (GP). The GP was determined as GP = GN/SN × 100, where GN and SN are the total germinated seeds and tested seeds, respectively. The germination index (GI) was measured as GI = number of seeds germinated seeds/days of the first count + number of germinated seeds/days of the final count.
Growth parameters measurements
To estimate growth, the primary root and shoot length of 10 days of seedlings were measured. For this, 5 plants were selected randomly from each treatment, the length was measured in cm, and the average was calculated. Similarly, the fresh weight of seedlings was measured in grams with the help of an analytical balance.
Biochemical contents measurements
For the extraction of chlorophyll and carotenoid pigments, fresh leaves weighing 0.1 g were homogenized in 10 mL of 80% acetone. The homogenate was then centrifuged at 15000 rpm for 10 min, the pellet was discarded, and the absorbance of the supernatant was recorded at 666, 653, and 470 nm on the Shimadzu UV-1601 spectrophotometer. The formulae developed by [ 31 ] were used for the calculation of chlorophyll and carotenoid: Chla (mg/g) = [(12.25 x A663.2)—(2.79 x A646.8)] × ml acetone / mg leaf tissue, Chlb (mg/g) = [(21.50 x A646.8)—(5.10 x A663.2)] × ml acetone / mg leaf tissue. Total Chl = Chla + Chlb and Carotenoids (mg/g) = (1000 A470–1.8Chla– 85. 02 Chlb) /198. Total phenolic content was measured by Folin–Ciocalteu’s phenol reagent assay [ 32 ]. A 0.2 mL of plant extract mixed with 5 mL of 10-fold diluted Folin–Ciocalteu’s phenol reagent was kept for 4 minutes and then it was aided with 4 mL of sodium carbonate (7.5 percent w/v). The whole mixture was diluted up to a volume of 15 mL with distilled water and mixed well. The reaction was permitted to stand for 90 min, and the absorption of each sample was reported at 760 nm using a spectrophotometer Shimadzu UV-1601. The total phenols were measured using the calibration curve for gallic acid (GA) and presented as the equivalent of mg GA / g FW. To extract total soluble sugars, 50 mg of fresh leaves were crushed thoroughly in 5 mL of 80% ethanol and centrifuged for 10 minutes at 3000 rpm. From the supernatant, 0.1 mL was taken in a test tube and kept in a water bath for the evaporation of ethanol. After the ethanol had completely evaporated, 4 mL of 0.2 percent enthrone reagent was added to the test tube, heated in boiling water for 10 minutes, and then allowed to cool at room temperature. The absorption of each sample was reported at 760 nm using a spectrophotometer Shimadzu UV-1601. Total soluble sugars were measured using the calibration curve for glucose and presented as the equivalent of mg Gu/g FW.
Statistical analysis
The experiment was conducted in CRD using three replicates and analysis of variance (ANOVA) was performed in statistix 8.1. The statistical difference between means was evaluated, considering p < 0.05 as a significance level.
Results and discussion
Synthesis, morphology, size, and structure of cunps.
The CuNPs were synthesized from the aqueous extract of M . longifolia . The addition of aqueous extract to the aqueous solution of CuSO 4 resulted in the development of a color change from pale yellow to dark brown over the course of 24 h, the first indication of the formation of CuNPs in the solution ( Fig 1 ). This color change is developed due to the excitation of surface plasmon resonance (SPR) [ 33 ]. Previous research has also suggested that brown color can be established in the reaction mixture of plant extract and CuSO 4 [ 34 ]. This color change was further accomplished by taking a UV-vis spectrum of a brown-colored solution in the range of 400 to 800 nm ( Fig 1 ). The spectrophotometric study revealed an absorption band at 558 nm, a result of interaction between the free electrons presents on the surface of metal NPs and light of specific wavelengths. This absorption band indicates the formation of CuNPs in the colloidal solution because it is a known fact that CuNPs show characteristic absorption peaks in the range of 200–800 nm [ 35 ]. Moreover, it has been analyzed that a single SPR band represents round-shaped nanoparticles, whereas two or more SPR bands correspond to the anisotropy of nanoparticles [ 36 ]. In our study, CuNPs in the solution showed a single SPR band, which reveals the round shape of CuNPs. The morphology and size of the prepared CuNPs are shown in the images of SEM ( Fig 2a ) and their mean size distribution histogram ( Fig 2b ). The CuNPs exhibited a spherical shape, were distributed irregularly, and had an average size of 23 nm. Fig 2c represents the energy dispersive x-ray (EDX) of the powder and shows the presence of Cu in the material. The peaks belonging to elemental Cu, C, and O were clearly detected, and there were no extra peaks, demonstrating the purity of the synthesized NPs and correlating with the XRD analysis. It’s also possible that the existence of C and O is due to bioactive molecules that have been capped. Furthermore, only a few copper atoms on the surface of the NPs are likely to have been oxidized, yielding modest quantities of CuO and Cu 2 O. The XRD pattern of CuNPs is shown in Fig 3 , confirms the face-centered crystalline nature, as all the peaks match Cu with FCC symmetry and are consistent with JCPDS No: 04–0836, which reveals the crystalline nature of CuNPs [ 37 ]. With the help of FTIR ( Fig 3 ), the functional groups of all the possible biocompounds involved in the reduction and capping of CuNPs are identified. The FTIR spectrum showed peaks at 3754.36, 2054.64, 1937.67, 1587.34, 1266.30, and 1043.06 cm -1 . The peak at 3754.36 cm -1 corresponds to O-H stretching frequency of hydroxyl groups of polyphenols. The peaks at 2054.64 and 1937.67`cm -1 correspond to Cu-H (metal-hydrogen) bonds. The peaks at 1587.34, 1266.30, and 1043.06 cm -1 could be due to C-C stretching vibrations of an aromatic compound, C-O stretching of carboxylic acid, and ester bonds of phenolic compounds. These results suggest an interaction between the functional groups present in plant extract and Cu ions that results in bio-reduction, the formation, and stabilization of CuNPs [ 4 , 6 , 38 ]. CuNPs are thought to be formed from copper salts using plant extracts in three steps: production of copper ions, reduction of the ions, and lastly, oxidation of the reduced ions [ 16 , 20 ].

a copper sulfate solution. b plant extract. c mixture of copper sulfate solution and plant extract at zero-time. d change in color of solution after 24 h of time lap. e UV-visible spectrograph of CuNPs.

(a) FESEM micrograph of CuNPs, showing spherical shape of CuNPs; (b) Size distribution histogram of CuNPs, showing average size of 23 nm. c EDX spectrum of CuNPs.

(a) XRD pattern of CuNPs, predicting crystalline nature of CuNPs; (b) FTIR spectrum of CuNPs, confirming functional groups from plant extract capping the CuNPs.
Effect of CuNPs on seed germination of wheat
Seed germination is the beginning of a physiological process of a plant that needs water imbibition and is directly linked to the yield of the plant. In this study, seed germination and germination index of the tested wheat varieties exhibited an almost similar trend, except for the variety 18-Elite line-5. Fig 4 shows the GP and GI of wheat varieties after the 10 th day of treatment with different concentrations of an aqueous solution of CuNPs. When exposed to 50 mg CuNPs/L, GP and GI increased considerably in varieties 18-Elite line-1, 18-Elite line-3, and 18-Elite line-6, while the variety 18-Elite line-5 showed increased GP and GI under 25 mg CuNPs/L. After 50 mg CuNPs/L exposure, the GP increased to approximately 67, 31, 33, and 11 percent in 18-Elite line-1, 18-Elite line-3, 18-Elite line-5, and 18-Elite line-6, respectively, of the GP observed in control. However, in 18-Elite line-5, a higher increase was found at 25 mg CuNPs/L (100%) as compared to control. A similar trend was found for GI, it increased to approximately 76, 37, 41, and 17 percent in 18-Elite line-1, 18-Elite line-3, 18-Elite line-5, and 18-Elite line-6, respectively, of the GI, recorded in control after the application of 50 mg CuNPs/L. More than double the increase in GI was apparent in 18-Elite line-5 after treatment with 25 mg CuNPs/L. When varieties were exposed to 100 mg CuNPs/L, no statistical difference was found in GP compared to control, except in variety 18-Elite line-6, which showed a reduced GP and GI as compared to control. Similar results were reported by [ 39 ], where lettuce seeds showed better germination when treated with CuNPs. It is found that NPs tend to come into contact with seed coats, penetrate the seeds, and improve seed germination and seedling growth of the plants [ 40 ]. They found that CuNPs penetrated the cell wall and formed new pores, so water absorption was improved, and as a result, favorable seed germination was started. Another study demonstrated that high seed germination rates might be due to the photo-generation of active oxygen like hydroxide anions and superoxide, which causes re-activation of aged seeds. In the nanocomposite, activated carbon provides moisture and a large surface area for seed germination [ 41 ].

Different letters in the same sub-group of columns indicate significant difference at p < 0.05 level.
Effect of CuNPs on seedling growth of wheat
RL, SL, and FW were measured for all the tested varieties after 10 days of seedling ( Fig 5 ) to investigate the effect of CuNPs on seedling growth of wheat. All the concentrations of CuNPs posed a positive effect on the RL of all wheat varieties, but the most prominent increase was found at 50 mg CuNPs/L in 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6 and 25 mg CuNPs/L in 18-Elite line-5. When treated with 50 mg CuNPs/L, the RL increased by 50, 153, and 33% in 18-Elite line-1, 18-Elite line-3, and 18-Elite line-6, respectively, and by 134 percent in 18-Elite line-5 when treated with 25 mg CuNPs/L. When compared to the control, the SL in 18-Elite line-1, 18-Elite line-3, and 18-Elite line-6 increased by 19, 20, and 37 percent, respectively, under 50 mg CuNPs/L, and by 54 percent in 18-Elite line-5 under 25 mg CuNPs/L. The FW also displayed the same results as for RL and SL, where a CuNPs concentration of 50 mg/L exhibited the most prominent effect on the FW of three wheat varieties. The maximum increase of 75, 95, and 35 in FW was observed for 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6, respectively, treated with 50 mg CuNPs/L, while a maximum increase of 18% in FW was observed for 18-Elite line-5, treated with 25 mg CuNPs/L.

After 10 days of exposure, the CuNPs treatments modified the RL, SL, and FW of wheat. RL, SL, and FW were significantly increased, especially for varieties exposed to 50 mg CuNPs/L. These changes in the root and shoot system might be due to hormonal imbalances, like auxin and cytokinin imbalances [ 42 ]. Our findings are supported by the study [ 33 ], where CuNPs, at low concentration, increased the fresh weight of wheat. Moreover, we have observed that at 100 mg/L treatment of CuNPs, the seedling growth is significantly reduced compared to the treatment of 50 mg CuNPs/L, suggesting that a high dose of CuNPs can be lethal to plants. At high concentrations, CuNPs may release cupric ions that change the physiological processes of the plant. These modifications increase the capacity of particles that cross the cell membrane of a plant cell [ 3 ]. Previous studies also supported this fact that a higher concentration of CuNPs produces toxic effects, e.g., in chickpea and soybean, CuONPs increased root and shoot growth at 100 ppm, but beyond this concentration (200 ppm), the root and shoot growth was found to decrease [ 43 ]. Other studies also suggest the same result, e.g., CuNPs reduced the seedling length by 65 and 75% in Phaseolus radiatus and Triticum aestivum , respectively [ 44 ]. In a similar way, CuONPs decreased the RL in Cucurbita pepo when applied at a 1000 mg/L concentration [ 45 ].
Effect of CuNPs on photosynthetic pigments of wheat seedling
The effect of CuNPs on photosynthetic pigments of wheat varieties was measured in terms of total chlorophyll and carotenoid ( Fig 6 ). Chlorophylls are located in the chloroplast where they play a crucial role in the photosynthesis system, which is highly related to plant productivity and biomass [ 46 ]. As a result, the total content of Chl and carotenoid in wheat varieties was measured in all treatments in the current study. The total chl content started increasing upon exposure to CuNPs and a higher level was found at 50 mg CuNPs/L in 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6, while in 18-Elite line-5, 25 mg CuNPs/L evidenced stimulation of chl up to 48% as compared with no application of CuNPs (control). Carotenoid content in test plants also followed the same trend as total Chl. A maximum increase of 61, 40, and 42% in carotenoid content was found in 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6, respectively, at 50 mg CuNPs/L. Some authors also noticed changes in the content of photosynthetic pigments as a result of nanoparticle application. For example, a concentration-dependent effect of CuNPs on chlorophyll and carotenoid pigments was studied by [ 47 ], where CuNPs increased the chlorophyll and carotenoid content. Similarly, Vigna radiata has shown increased chlorophyll and carotenoid content under the treatment of CuNPs [ 48 ]. We also observed a significant inhibitory effect at higher concentrations (100 mg CuNPs/L). This could be attributed directly to oxidative stress or the interaction of RuBP carboxylase, due to copper interaction with SH groups. Additionally, the Chl content decrease might also be due to the lowered shoot biomass upon contact with higher concentrations of CuONPs or to the membrane damage as a result of excess lipid peroxidation of chloroplast membranes under oxidative stress [ 49 ].

Effect of CuNPs on phenolic and sugar content of wheat seedling
Treatment with CuNPs significantly influenced the content of phenol and soluble sugar in the seedlings of wheat ( Fig 7 ). A maximum increase in total phenols was found at 100 mg CuNPs/L. It has been identified that plants induce the production of phenolic compounds in response to NPs [ 50 ]. In some other studies, an increase in phenolic content was also observed under a high concentration of nanoparticles. For example, fruits of Jalapeno pepper showed an increase in the total phenols under the application of CuNPs + Chitosan-PVA [ 51 ]. Similarly, the application of zinc nano fertilizer also increased the total phenols in pomegranate fruits [ 52 ]. This increase in phenolic content at higher concentrations may be related to the plant’s response to stress, as we observed 100 mg CuNPs/L caused toxic effects on wheat. Phenols are antioxidant compounds that trigger the synthesis of a series of secondary metabolites from the shikimic acid pathway or through phenylpropanoids under conditions of abiotic stress [ 53 ]. Therefore, the observed response may be related to ROS formation due to CuNPs. However, unlike the phenolic content, the soluble sugar content was found to be higher at 50 mg CuNPs/L treatment, as was the case with all other factors investigated. Overall, we found a positive effect of CuNPs on the growth and development of wheat. These results were supported by previous studies that showed Cu plays an important role in plant growth and development, and plant productivity [ 54 ].

Conclusions
This study used an eco-friendly and appropriate method for the synthesis of CuNPs using Mentha longifolia extract . There were no chemical reagents or surfactant templates required, allowing green bioprocesses for a range of biomedical applications. During the application of CuNPs on wheat, we found in our study that with an increase in the concentration of CuNPs, the seed germination and growth of wheat plants were also increased. However, after a certain concentration (50 mg CuNPs/L), the seed germination and seedling growth were found to decrease. Overall results showed that application of CuNPs influences the seed germination and seedling growth of wheat at different concentrations. The higher germination and growth were found at 50 mg CuNPs/L treatment for 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6 wheat varieties and at 25 mg CuNPs/L for 18-Elite line-5. Beyond this treatment, germination and growth were inhibited. Effective germination and growth at a certain optimum concentration and decreased germination and growth beyond this concentration may be attributed to the varieties of wheat and the uptake and accumulation of CuNPs by the roots, because the germination and growth were dependent on the exposure concentration of CuNPs. In particular, the contact of plants with NPs and the impact of such contact on plant growth could spur new research focus on nanobiotechnology. Further studies are needed to understand the mechanism of accumulation and uptake of CuNPs in plants and the way they act during seed germination and growth.
Supporting information
Acknowledgments.
We are thankful to Department of Plant Breeding and Genetics, University of Poonch Rawalakot for providing seeds of wheat varieties. We are also thankful to IST Islamabad for providing scanning electron microscopy and X-ray diffraction facilities.
Funding Statement
The author(s) received no specific funding for this work.
Data Availability
Spectroscopic investigations of green synthesized zinc oxide nanoparticles (ZnO NPs): antioxidant and antibacterial activity
- Open access
- Published: 24 July 2024
- Volume 6 , article number 399 , ( 2024 )
Cite this article
You have full access to this open access article

- Ajay Kumar Tiwari 1 na1 ,
- Saket Jha 2 na1 ,
- Sharad Kumar Tripathi 3 ,
- Rohit Shukla 4 ,
- Ram Raseele Awasthi 5 ,
- Abhishek Kumar Bhardwaj 6 ,
- Abhimanyu Kumar Singh 7 &
- Anupam Dikshit 8
In the recent decade, zinc oxide nanoparticles (ZnO NPs) have been widely explored owing to their versatile properties and prodigious demands in the drug delivery, medical, energy storage, cosmetics, and the healthcare sectors. Therefore, the current work opts for an environmentally benign method to prepare ZnO NPs. The leaf extract of Calendula officinalis L. acts as a reducing agent for the metal ions; therefore, in the current research, ZnO NPs were prepared via green route by using Calendula officinalis leaf extract. Furthermore, the ZnO NPs were analysed with different spectroscopic techniques to confirm the structure and stability of nanomaterials. The prepared ZnO NPs were characterized by XRD, FE-SEM, FT-IR and UV–Vis studies. Also, the antioxidant and antimicrobial properties of the synthesized ZnO NPs were investigated. The XRD result of synthesized ZnO NPs showed the crystalline size 28.23 nm with wurtzite hexagonal structure along with the most intense peak (101). Following preliminary confirmations of the intended ZnO NPs, both big and small agglomerated forms were observed in the FE-SEM, which is often used to determine their exterior assembly. Further, the results of Fourier transform infrared spectroscopy (FT-IR) indicated the formation of pure ZnO NPs with an absorption peak of the Zn–O bond between 4000 cm −1 and 500 cm −1 and no discernible peak in the monitoring range. The UV–Vis spectrum of the green synthesized ZnO NPs were revealed two prominent absorption peaks at 355 nm and 370 nm with energy band gap of 2.986 eV. Using the 1, 1-di phenyl-2-picrylhydrazyl (DPPH) test, the antioxidant activity of the described ZnO NPs was assessed. It demonstrated how, ZnO NPs significantly increased their antioxidant activity by scavenging 1, 1-di phenyl-2-picrylhydrazyl (DPPH) free radicals. It could be seen that synthesis of the naturally occurring plant product ZnO NPs have been acting as an alternate of chemical antioxidant. The antimicrobial analysis was also performed with the help of disk diffusion method where three multi-drug resistant human pathogens namely Staphylococcus aureus, Klebsiella pneumoniae and E.coli were used. The Zone of Inhibition diameter values are 35.2 mm ± 0.9, 23.6 mm ± 0.1 and 13.5 mm ± 0.1, respectively, which showed that the ZnO NPs was highly effective against S. aureus. Thus, the green synthesis method of ZnO NPs using leaf extract of Calendula officinalis is evidence that it is superior and environmentally friendly method for the preparation of ZnO NPs and hence it can be utilized in various nano-medicine approaches.
Article Highlights
Green synthesis (hydrothermal method) of ZnO-NPs using leaf extract of Calendula officinalis L . and its spectroscopic investigation.
Found effective Antioxidant activities of green synthesised ZnO that can be employed as skin care and cosmetics.
Green synthesized ZnO NPs having a significant bactericidal activity observed against bacteria S. aureus , K. pneumonia and E. coli.
Avoid common mistakes on your manuscript.
1 Introduction
Metal oxide nanoparticles (NPs) have opened a new horizon in the fields of drug delivery, gene delivery, nanomedicine, and biosensing in the twenty-first century [ 1 , 2 ]. Nanoparticles are also applicable in environmental protection, sensing, communication, cosmetics, medical industry etc. [ 3 ]. The magnetic and catalytic properties of nanoparticles, along with their improved surface to volume ratio, can be enhanced by some unique modified processes [ 4 , 5 ]. Nanoparticles can be synthesized by using an array of physical, chemical, and biological methods. The hydrothermal processes, physical vapor deposition, microemulsion, precipitation, ultrasonic irradiation, chemical reduction, plasma, and sol–gel methods are the best examples of the above methods [ 6 ]. But these methods required a variety of hazardous chemicals and a huge amount of energy to synthesize the nanoparticles. The above methods also utilize very high temperatures and pressures, which are very harmful to the environment. Accordingly, the product cast gets very high due to the higher order of the equipment used in these methods [ 7 ]. As a result, an environmentally friendly method is required to synthesize the nanoparticles without utilizing any toxic components. Recently, many scholars have taken an interest in preparing the nanoparticles via green route. In medical science, especially in the diagnosis and treatment of several diseases, the biological method via the green route is comparatively more advantageous than the physical and chemical methods. Thus, the biological method via green route has replaced the physical and chemical methods for the synthesis of nanoparticles due to the high energy consumption, low cost, environmental impact, and production of more stable and biocompatible nanoparticles [ 8 , 9 ]. Moreover, this method controls the morphology of nanoparticles, including nanorods, nanospheres, nanoporous structures, and nanowires, crucial for various emerging potentials applications [ 10 ].
Microorganisms [ 11 ], enzymes [ 12 ], fungi [ 13 ], and plant extracts [ 14 ] are used to prepare metal and metal oxide nanoparticles. Nevertheless, it is challenging to develop an effective and environment friendly method to prepare nanoparticles with absolute sizes, shapes, and compositions. Nontoxic and environment friendly nanoparticles are prepared by a variable phyto-chemistry based process using biological entities [ 15 , 16 ]. There are several harmful compounds including radiation, smoke, and pollution; which pose serious effect to human health concern. Antioxidants are being used more frequently in an effort to mitigate the negative effects of these free radicals. Determining the reactive oxygen species (ROS) reaction requires examining the physical and chemical characteristics of nanoparticles, such as their size, surface charge, and chemical makeup. This is important because ROS generation can be control due to the intrinsic properties of nanoparticles. Numerous studies have demonstrated the ability of metal or metal oxide nanoparticles produced with plant extracts to scavenge radicals. These extracts phytochemicals have two functions: they stabilize the nanoparticles and function as antioxidants to combat free radicals [ 17 , 18 ].
Among the various metal oxide nanoparticles, zinc oxide nanoparticles (ZnO NPs) are one of the most cost-effective metal oxide nanoparticles with minimal toxicity and the highest biocompatibility. Nowadays ZnO NPs are used in photo-thermal therapy, antibacterial and anticancer drug distributions, cell imaging, and in bio-sensing by making them a promising tool in the field of biomedical sciences [ 19 , 20 ]. Various methods of synthesis are utilized to improve both the crystal size and their properties for the production of ZnO NPs [ 21 ]. Among them, green synthesis method for the preparation of ZnO NPs are widely accepted due to its non-toxicity, environmental friendliness, and wide range of applications viz. antimicrobial properties, antioxidants, anti-aging, anticancer and so on. In the green synthesis method, secondary metabolites trap the metal ions and act as capping agents that help stabilize the nanoparticles in terms of electrostatic, steric, hydration forces, depletion, and Vander-Waals forces [ 22 , 23 ]. The interactions of ZnO NPs with microbial cells are possible due to the smaller surface area and higher charge density [ 24 , 25 ].
Researchers have used a variety of physico-chemical [ 26 , 27 ] and biological or plant-mediated [ 28 , 29 ] methods to synthesize the ZnO NPs. Green synthesis becomes more preferable technique in recent decades for producing the metallic nanoparticles because of cost effectiveness, lesser toxicity, and additional environmental suitability [ 30 ]. Plant extracts are used by researchers to create ZnO NPs through a green synthesis process. For example, several kind biological extracts including algal seaweeds [ 31 ], Hibiscus sabdariffa [ 32 ] Limonium pruinosum [ 33 ], Laurus nobilis [ 34 ], Rosa indica [ 35 ] Parthenium hysterophorus [ 36 ], Thymus spp. [ 37 ], Solanum nigrum [ 38 ], Lawsonia inermis [ 39 ], Syzygium cumini [ 40 ], and Agathosma betulina [ 41 ]. These natural extracts having stabilizing and reducing agents, play a significant role in the production of ZnO NPs. This chemical free approach of synthesizing nanoparticles is in line with the worldwide movement toward sustainability and is a viable substitute for traditional techniques that mainly rely on hazardous chemicals and energy-intensive procedures.
Calendula officinalis is a flowering plant which belongs to the family of Asteraceae. A wide range of pharmacological effects can be observed in the extract of Calendula officinalis due to which it can be utilized as an antiseptic, stimulant, diaphoretic, antispasmodic, and anti-pyretic agent [ 42 , 43 ]. It has been observed that the leaf extracts of the Calendula officinalis have anti-cancerous, antiviral anti-genotoxic, anti-oxidant, anti-microbial and anti-inflammatory properties [ 44 , 45 ].
The present study is focused on the synthesis of ZnO NPs via the green route by using Calendula officinalis leaf extract. The prepared ZnO NPs are further characterized by XRD, FE-SEM, UV–Vis and FT-IR spectroscopy for the size, shape, optical properties and stability of the ZnO NPs, respectively. The study also emphasizes the application approach of these nanoparticles towards medicinal area due to their effectiveness in antimicrobial and antioxidant properties. The antimicrobial study was done against multi-drug resistant human pathogens. Also an antioxidant activity was analyzed against DPPH free radical scavenging properties.
2 Method and reagents
2.1 chemical products.
Zinc acetate dihydrate (C 4 H 6 O 4 Zn.2H 2 O) (219 g/mol, purity 98.5%), and sodium hydroxide (NaOH) (39.997 g/mol, purity 97%) were supplied by Merck Life Science Pvt. Ltd. Mumbai. Nutrient agar (NA), Mueller Hinton Broth (MHB), Mueller Hinton Agar (MHA) and 2, 2-diphenyl-1-picrylhydrazyl (DPPH) (394.32 g/mol, purity 95%) were procured from Himedia Pvt. Ltd. Mumbai for antibacterial assay. As a solvent deionized water was employed. All the chemicals used in this experiment, are analytical grade.
2.2 Leaves procurement and processing
The fresh Calendula officinalis leaves were collected from the Roxburgh Botanical Garden, University of Allahabad, Prayagraj, Uttar Pradesh, India. The Calendula officinalis plants were identified by Prof. Anupam Dikshit, Department of Botany, University of Allahabad. Leaves were cleaned three times to get rid of the dust and other contaminants, and left in the shade for a week to dry. Leaves were chopped into small pieces to prepare the leaf extract.
2.3 Preparation of the leaf extract
Chopped Calendula officinalis leaves (25 g) were added in 100 ml deionized water and kept on magnetic stirrer for 6 h at 60 0 C for 1200 rpm [ 46 ]. The aqueous solution was filtered using filter paper and kept for 24 h at 4 0 C. The zinc ions which are supportive to form the ZnO NPs, were reduced from the fused aqueous extract and zinc acetate.
2.4 Green synthesis of ZnO NPs
A beaker of Calendula officinalis leaf extract (50 ml) was kept on magnetic stirrer (1200 rpm) at 60 0 C temperature. The aqueous solution of ZnC 4 H 6 O 4 .2H 2 O (0.45 M of 50 ml) was dropped continuously and stirring it for two hours with constant stirring (1200 rpm). The colour of the reaction mixture started converting in to brown precipitate. The resulting precipitate was centrifuged at 6000 rpm for 15 min. It was washed twice with deionized water before spending the entire night at 100 0 C [ 47 ]. Figure 1 shows the systematic procedure of synthesis of ZnO NPs.

Flow diagram of synthesis of ZnO NPs using leaf extract of Calendula officinalis
2.4.1 Mechanism for the green synthesis of ZnO NPs
Phytochemicals in plant extracts can reduce metal precursors to nanoparticles, acting as both reducing and stabilizing agents. Calendula officinalis leaf extract contains numerous phytochemicals including triterpenes, Flavonoids, Phenolic compounds, coumarins, and carotenoids, which play a significant role in the reduction of metal ions. The concentration of these phytochemical reducing agents varies in different plant extracts, significantly influencing the composition of leaf extract in the process of the formation of nanoparticles. Apart from it various factor such as pH, temperature, contact time, metal salt concentration, and the phytochemical profile of the plant extract impact the synthesis, stabilization, and quantity of nanoparticles produced. Jaychandran et al. [ 48 ] suggested that metal ions undergo encapsulation with an organic covering in three steps for stabilization after reduction by plant extracts: 1) Activation phase, involving metal ion reduction and nucleation, 2) Growth phase, ensuring nanoparticle stability, 3) Termination phase, determining the shape of nanoparticles. Metals like copper, silver, gold, titanium, zinc, iron, and nickel form metal oxides through phytochemical activity. Phytochemicals enable metal ions to reach growth and stabilization, ultimately linking metal ions and defining their shape [ 48 ]. The clear cut mechanism of green synthesis of ZnO NPs using calendula officinalis leaf extract, is represented in Fig. 2 .
Mechanism for the synthesis of ZnO NPs using Calendula officinalis leaf extract
2.5 Spectroscopic investigation of ZnO NPs
2.5.1 x-ray powdered diffraction (xrd) analysis.
The X-Ray powder diffraction technique was used to investigate the crystalline structure, size and formation of the compounds. ZnO NPs which were synthesized via the green route using Calendula officinalis leaf extract were analyzed using single crystal X-Ray Diffractometer (Rikagu, Ultima IV Japan) with Cu-Kα radiation (λ = 1.54020 Å). The scanning was done in the region of 2 θ from 20° to 70°.
2.5.2 Field emission-scanning electron microscopy (FE-SEM)
The surface morphology of synthesized ZnO NPs were further analyzed by FE-SEM (JEOL India Pvt. Ltd., Delhi India) for superficial phenotype. A very small amount of nano-powder was kept on the carbon coated copper grid, and then FE-SEM grid was dried with a fine layer of sample and fixed with gold for 5 min.
2.5.3 Fourier transform infra-red (FT-IR) spectroscopy
The Perkin-Elmer Bucking Hamashire, UK FT-IR spectroscopy was used to investigate bond and functional groups existing in the green synthesized ZnO NPs. During the analysis, the band and fictional group were identified by using FT-IR spectra with a resolution of 4.0 cm −1 in order to 4000–450 cm −1 .
2.5.4 Ultraviolet–Visible (UV–Vis) spectroscopy
The optical properties of the green synthesized ZnO NPs were investigated using UV–Vis spectroscopy. The shape and size of the synthesized ZnO NPs, affect the sharpness of the absorption peak. In order to check the optical properties of green synthesized ZnO NPs, 0.05 g of ZnO NPs was dispersed in 4 mL of double ionized water. The absorption spectrum was recorded using an UV–Vis spectrophotometer (Spectra Max, San Jose, CA, USA) in a wavelength range of 350–460 nm.
2.6 Free radical scavenging activity
Brand William conducted a comprehensive investigation into the free radical scavenging activity of ZnO nanoparticles (NPs) synthesized through green methods, with adaptations made to the protocol outlined by a prior study [ 49 ]. The 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical, known for its stable purple color and characteristic absorption peak at 517 nm, served as the substrate for this assessment. Upon exposure to antioxidants present in the medium, the purple hue of DPPH underwent a discernible transition to transparency, indicative of radical scavenging activity. To prepare the solution for the antioxidant assay, 39.44 mg of DPPH was dissolved in 100 ml of methanol, yielding a concentration of 0.14 mM. Similarly, stock solutions of green-synthesized ZnO NPs and standard ascorbic acid were prepared in methanol, followed by appropriate dilutions to achieve the desired working concentrations. Ascorbic acid, employed as the standard, underwent evaluation at concentrations spanning 2–6 μg/ml to establish a calibration curve. In a controlled environment within a darkened laboratory, 140 μl of freshly prepared 1 mM DPPH was combined with 860 μl of test samples. To minimize the influence of ambient light, all test tubes were carefully wrapped in aluminum foil. A comparative analysis was conducted using a control sample comprising 1 ml of DPPH solution mixed with 3 ml of methanol. Data interpretation involved calculating the percent inhibition of DPPH free radicals relative to the control, with further determination of IC50 values derived from the descending order of test sample readings. This meticulous approach enabled a thorough assessment of the antioxidant potential of the green-synthesized ZnO NPs and facilitated valuable insights into their free radical scavenging capabilities. The percentage of scavenging free radical values was calculated by the following formula-[ 50 ]
2.6.1 Antioxidant radical power
The antioxidant radical power of the green synthesized ZnO NPs and Ascorbic acid can be evaluated by the given formula [ 51 ]
2.7 Antibacterial activities
The antibacterial assessment employed the widely recognized disc diffusion method to discern the efficacy of green-synthesized ZnO nanoparticles (NPs) against three distinct bacterial strains: Staphylococcus aureus (Gram positive), Escherichia coli (Gram negative), and Klebsiella pneumoniae (Gram negative). These bacterial strains represent diverse microbial morphologies and pathogenic profiles, allowing for a comprehensive evaluation of the nanoparticles' antibacterial potential across different bacterial types. Prior to testing, the green-synthesized ZnO NPs were meticulously dissolved in dimethyl sulfoxide (DMSO) to facilitate the creation of standardized stock solutions, ensuring consistency and accuracy in subsequent analysis. This step is crucial in maintaining the stability and reproducibility of the experimental conditions, thereby enhancing the reliability of the results obtained. In a controlled laboratory environment, cultures of the respective bacterial strains were inoculated into separate batches of nutrient broth and then subjected to an incubation period of 12 h at 37°C. This incubation duration allows for optimal bacterial growth, ensuring that the cultures reach a logarithmic phase of growth before further experimentation. Following incubation, 100 μL of each bacterial culture was uniformly spread onto sterile Muller-Hinton agar (MHA) plates using sterile swabs. The choice of MHA as the growth medium is deliberate, as it provides a standardized and optimal environment for bacterial growth, enabling accurate assessment of antibacterial activity. Subsequently, discs impregnated with varying concentrations (50, 75, and 100 μg/ml) of green-synthesized ZnO NPs were carefully placed onto the agar plates, with sterile water discs serving as negative controls. This setup allows for the assessment of dose-dependent antibacterial effects, elucidating the relationship between NP concentration and inhibitory activity. The plates were then incubated at 37°C for a period of 24 h to allow for bacterial growth and subsequent assessment of inhibition zones [ 47 ]. The formation of clear zones of inhibition around the NP-loaded discs indicates the antibacterial activity of the synthesized nanoparticles against the respective bacterial strains. To ensure the reliability and robustness of the experimental data, each assay was performed in triplicate, minimizing experimental variability and enhancing the statistical significance of the findings [ 47 ]. The inhibition zone diameters were meticulously measured, and the data were expressed as mean values ± standard deviation, providing a comprehensive understanding of the antibacterial efficacy of the green-synthesized ZnO NPs.
3 Results and discussion
3.1 x-ray diffraction analysis of green synthesized zno nps.
The recorded X-ray diffraction pattern shows the phase and crystallinity of the produced ZnO NPs. The produced ZnO NPs XRD patterns are displayed in Fig. 3 . Lattice planes (hkl) of (100), (002), (101), (110), (200), (112), and (201) could be responsible for the diffraction peaks at 31.6 0 , 34.3 0 , 36.1 0 , 47.4 0 , 56.4 0 , 62.7 0 , 66.2 0 , 67.8 0 , and 69 0 (Table 1 ) for the hexagonal wurtzite ZnO phase (JCPDS-file: 36–1451) [ 52 ]. Furthermore, the complete conversion of the Zn precursor into ZnO NPs is shown by the lack of an impurity peak in the diffraction pattern. Calendula officinalis leaf extracts phenols and flavonoids work as reducing agents and shield the zinc acetate molecule's outermost surface, they promote the development of ZnO NPs. Diffraction peaks that are both conspicuous and narrow indicate that the product's particles have a distinct crystalline structure. The strong peak intensity indicates the high crystalline nature of the produced ZnO NPs. The Debye-Scherer equation is used to further estimate the diameter of the produced ZnO NPs [ 53 ].
where: D = Average crystallite size of the particles, K = Debye-Scherer’s constant (= 0.94), λ = Wavelength of the Cu Kα-radiation (= 0.1540 nm), β = Full width at half maximum (FWHM) in radian, θ = Bragg’s diffraction angle.
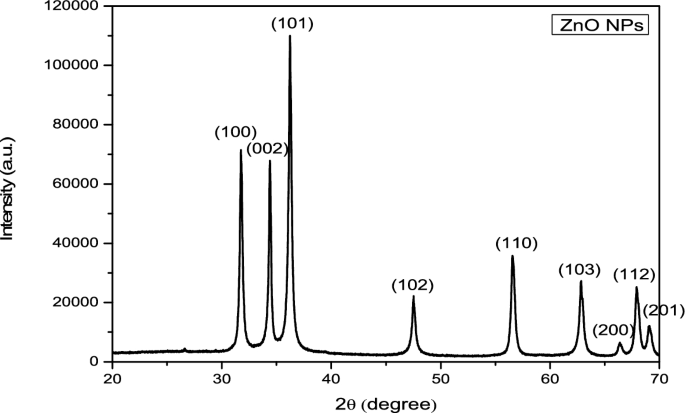
XRD pattern of green synthesized ZnONPs
The XRD analysis proves that the Calendula officinalis leaves are the effective reducing agents for the green synthesis of ZnO NPs.
The FWHM of the most intense peak, which corresponded to the (101) plane located at 36.1°, was used to compute the crystallite diameter of the generated ZnO NPs, which came out to be 28.23 nm. ZnO NPs were found to have a well-crystalline character and a hexagonal wurtzite crystal structure. After calculations, it was discovered that the lattice has dimensions of (a = b) = 3.2535 Å and (c = 5.2151 Å).
The change in crystallographic properties can also be understood with the help of W–H plot between βCosθ versus 4Sinθ. Figure 4 depict W–H plots from mathematical relations-

Williamson-Hall (W–H) Plot for green synthesized ZnO NPs
βCosθ = \(\frac{k\lambda }{D}\) + 4µ s Sinθ (2).
Strain is determined from the slope of straight line. The values of micro-strain have been calculated and found to be 0.00451. The reciprocal of intercept reveals the grain size of the sample. The variations of grain sizes calculated from both Debye-Scherer’s as well as W–H plot are approximately the same.
3.2 FE-SEM analysis of green synthesized ZnO NPs
The generated ZnO NPs FE-SEM images are displayed in Fig. 5 . The surface morphology displays the aggregated particulates. FE-SEM provides the microstructure and surface morphology of samples and XRD provides the sample's crystallite size. From FE-SEM image of ZnO NPs, it is revealed that most of the particles are spherical in shape. Surface morphology also provides evidence that ZnO NPs were formed in their agglomerated state [ 54 ]. The microstructures have varying sizes, as seen by the FE-SEM image (Fig. 5 ).

FE-SEM micrographs of green synthesized ZnO NPs
The histogram of the FE-SEM image is formed by IMAGE-J software. The histogram gives statistics about the distribution of pixel values in the whole image. Histogram of FE-SEM images is made by IMAGE-J. The software program Image J (version 1.46r) was used for FE-SEM image analysis. The observed standard deviations and mean deviations of ZnO NPs using Image-J software were found to decrease with increasing the magnification from × 200 to × 1100 (Fig. 6 ). The standard deviation (51.287) revealed that pixels spread out around the mean of the crystallite of ZnO NPs. The mean deviation (127.148) was measured as the closest alternative to the standard deviation.
Histogram of SEM images by IMAGE-J gives the information of microstructure particle distribution in the pixel values
Thus, the instability of the FE-SEM micrograph was expressed as the significance of the standard deviation. The observed irregular-shaped nanoparticles in FE-SEM images indicated that green synthesized nanoparticles using leaf extract contained a large volume-to-surface ratio. The histogram of the FE-SEM image showed that the ZnO NPs at different magnifications had a uniform particle size distribution on the surface of the cluster.
3.3 FT-IR analysis of green synthesized ZnO NPs
Figure 7 shows the FT-IR spectrum of the green synthesized ZnO NPs. From the spectrum it is clear that the compositional and functional groups are present in the green synthesized ZnO NPs. The spectroscopic grade KBr was utilized in the form of a pallet to record the FT-IR spectrum in the wave number range of 4000–500 cm −1 in the different reflectance modes. FT-IR spectrum has shown the numerous bands at various absorption peaks (Table 2 , Fig. 7 ) which are similar to the ones earlier reported [ 55 , 56 ]. To be more precise, an extensive band at 3401 cm –1 is assigned to the O–H stretching mode of the hydroxyl group present in the green synthesized ZnO NPs. The peak of 3080 cm –1 is due to the C-H stretching vibration of alkenes functional groups. The peaks detected in spectrum (Fig. 7 ) at 2345 cm –1 assign the C = C alkane/C = N stretching.
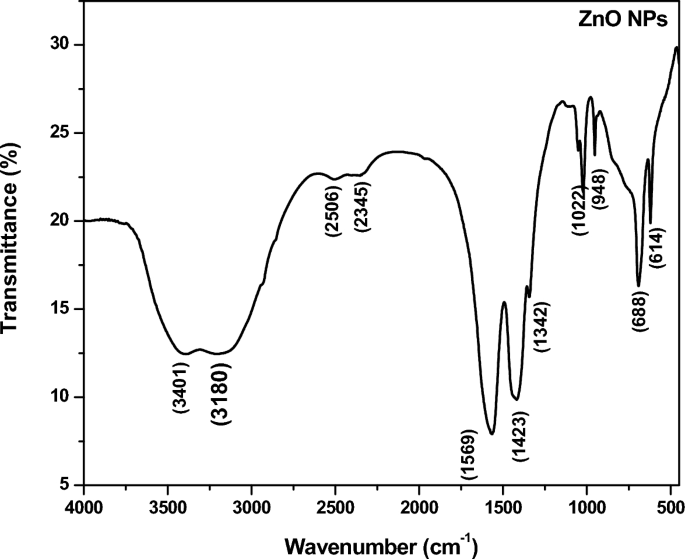
FT-IR spectra of green synthesized ZnO NPs
However, the peak at the 1569 cm –1 is assigned to the (N–H) stretching of the amine functional group and 1423 cm –1 is assigned to the (C-O), C-F, alkyl halide strong groups present in the green synthesized ZnO NPs. Probably 1022 cm –1 , 948 cm –1 , 773 cm –1 , 688 cm –1 is assigned the C-N stretching amine functional group which are present synthesized ZnO NPs [ 57 ]. The FT-IR spectrum confirms the formation of chemical bonding due to functional groups present in the green synthesized ZnO NPs.
3.4 UV–Visible analysis of green synthesized ZnO NPs
The leaves extract not only acts as a reducing agent but also a stabilizing agent. The zinc ions are reduced in the zinc oxide solution by the secondary metabolite of the plants. This was studied by taking the UV-Vis spectrum in between 350 nm and 460 nm. Figure 8 showed the absorption spectrum of green synthesized ZnO NPs. Two strong absorption peaks were visualized at 355 nm and 370 nm which confirmed the formation of ZnO at nanoscale [ 58 ]. For the formation of ZnO NPs, the absorbance peak was found in the previous report in the range of 300–360 nm [ 59 ]. The smaller size particles were also visualized at FE-SEM image (Fig. 5 ) that also supported the above statement and bump around 370 nm. This was because of more interaction of Zn ions with secondary metabolites. Bioactive constituents of plant extract interacted with the Zn ions and acted as stabilizing as well as abbreviating agents in the reduction of the metal size [ 60 , 61 ]. The band gap energy was evaluated by equation-
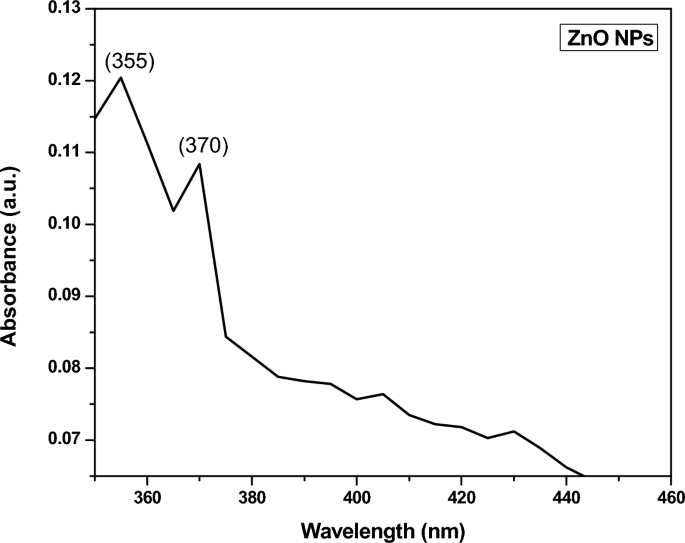
UV–Visible Spectrum of green synthesized ZnO NPs
The optical band gap energy of green synthesized ZnO NPs were observed 2.986 eV (Fig. 9 ) which was comparable to the previously reported one [ 62 , 63 ]. As ZnO is a direct wide band-gap (2.986 eV) semiconductor material at a room temperature, it has gained widespread research interest for a variety of applications such as, optical and opto-electronic devices because of its intriguing features including non-toxicity, non-expensive, large electron mobility and various novel morphological features.
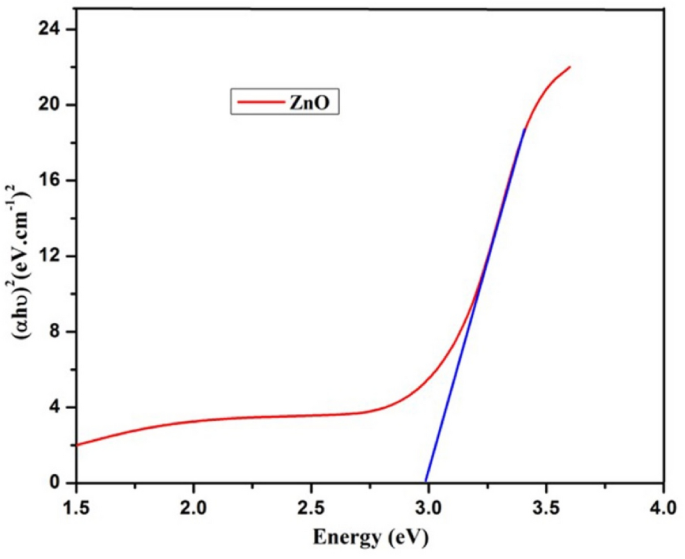
Optical band gap of green synthesized ZnO NPs
3.5 Free radical scavenging activity:
The evaluation of antioxidant activity entailed assessing the DPPH-mediated free radical scavenging capability of ZnO NPs. DPPH, a stable nitrogen-centered free radical, readily undergoes electron or proton transfer, converting into a stable diamagnetic free radical. Consequently, the purple color of the DPPH solution diminishes in proportion to the electrons received from antioxidant agents like ZnO NPs, resulting in the formation of 1, 1-diphenyl-2-picryl-hydrazine with a light yellowish colour. The remarkable antioxidant prowess of green-synthesized ZnO NPs arises from their ability to transfer electrons from oxygen to the electron density of nitrogen within DPPH, effectively neutralizing its unpaired electron. This electron transfer process engenders an electronegative force, which is particularly pronounced in ZnO NPs and hinges upon the structural configuration and oxygen moiety.A pivotal aspect of green-synthesized NPs lies in the electrostatic interaction between negatively charged bioactive compounds sourced from plant extracts and positively charged metal ions within the NPs. This interaction enhances the antioxidant activity manifold compared to chemically synthesized counterparts. In this context, the IC 50 value, derived from the mediated antioxidant assay, stands at 6600 µl/ml. Discrepancies in the antioxidant activity of ZnO NPs originating from different sources can be attributed to variations in size and surface characteristics due to the synthesis process and the botanical origins. Previous studies have underscored that smaller-sized ZnO NPs exhibit the highest percentage of radical scavenging activity, a trend paralleled in gold nanoparticles as well. This emphasizes the nuanced interplay between nanoparticles properties and antioxidant efficacy, thereby highlighting the significance of synthesis methodologies and precursor materials in dictating the antioxidant potential of nanomaterials. Over all, the utilization of nano-sized ZnO particles represents a strategic approach to bolstering antioxidant activity through synergistic interactions, enhanced reactivity, and size-dependent effects. In recent study green synthesized dual doped Co–Cu from plant Tinospora cordifolia exhibited a remarkable antioxidant activity against DPPH, HO and NO free radicals [ 64 ]. In another study Cu-doped hematite nanoparticles was created through green synthesis from the plant Azadirachta indica leaves extract used to examine the antioxidant activities and doped hematite nanoparticles contains high amount of free radical scavenging activity against DPPH free radicals [ 65 ]. In some advanced study iron oxide nanoparticles (Fe 2 O 3 -NPs) were synthesized by chemical and green precipitation methods had been tested against photo-catalytic and antioxidant activities was significantly high of green synthesized nanoparticles of Azadirachta indica [ 66 ].The green synthesized nanoparticles from A. indica used to create hematite nanoparticles with varied Co/Cu dopant concentrations. Methyl orange and Methylene blue industrial wastes can be degraded by using Co/Cu doped hematite nanoparticles. It also possessed remarkable photo-catalytic and antioxidant activities [ 67 ]. Incorporating these nanoparticles into antioxidant formulations holds immense potential for developing novel therapeutics and functional materials aimed at combating oxidative stress-related diseases and promoting overall health and well-being [ 68 ].
3.6 Antioxidant reducing power
The antioxidant values also evaluated in terms of ARP values showed 50% scavenging concentration of both the ascorbic acid and ZnO NPs (Table 3 Fig. 10 ). Approximately the values of 50% scavenging of free radical are equal to ascorbic acid which showed that the ZnO NPs are also potent scavengers of antioxidants [ 69 ]
Free radical scavenging activity of ZnO NPs and Ascorbic acid against DPPH
3.7 Antibacterial activity
The antibacterial activities were performed using the disc diffusion method. The disc diffusion method has several limitations such as: 1. It provides primary confirmation of the antibacterial activity but fails to provide accurate cytotoxicity. 2. It is not able to provide the exact minimum inhibitory concentration (MIC) of targeted nanoparticles. 3. It is also not able to depict mechanistic cytotoxicity against the pathogens. Thus our study is only focused to confirm the antibacterial property of ZnO NPs. Apart from these limitations this method have positive approach such as simple, effective and convenient method to check the antibacterial property of a test sample. It also provide effective concentration or lethal concentration (not accurately) of a test sample [ 70 ]. The different concentrations of green synthesized ZnO NPs were tested against the pathogenic bacteria, i.e., S. aureus (Gram + ve) E. coli (Gram -ve), and K. pneumoniae (Gram -ve). The green synthesized ZnO NPs were observed against these bacteria, as indicated in Table 4 and Fig. 10 . For the standard drug, amoxicillin was taken for reference and for the comparison of the activity of ZnO NPs. Double-distilled water was used as a negative control. ZnO NPs showed maximum inhibition against Staphylococcus aureus (35.2 mm ± 0.9) and minimum inhibition against Klebsiella pneumoniae (23.6 mm ± 0.1) and E. coli (13.5 mm ± 0.1). The efficacy of inhibition percentage is directly proportional to concentration, which means increasing concentration will increase the zone of the diameter [ 71 ]. The results reveal that the diameter of the inhibitory zone increases with increasing nanoparticles concentration for all tested strains [ 71 ]. It is also observed that the green synthesized ZnO NPs showed better inhibition against Gram + ve in comparison to gram-ve. This may be, the size of nanoparticles was less than 22 nm. which increase its cell membrane penetration property and cause severe cell toxicity (antibacterial property). It is reported that, in metal oxide nanoparticles such as ZnO NPs, the size of nanoparticles is inversely proportional to effective antibacterial property which means lesser the size of nanoparticles couses more antibacterial property [ 72 ] This might be because the cell wall of gram-negative bacteria is more complex than that of gram-positive bacteria [ 2 ]. Here, the presence of ZnO NPs leads to damage the cell wall of S. aureus (Fig. 11 ). This effect can be explained by the strong direct interactions with the bacterial membrane surface and antibacterial agents like ZnO NPs. For the ZnO NPs to penetrate the bacteria’s cells, the bacteria’s membrane has small pores [ 71 ]. ZnO NPs cause cell harm by encouraging microorganisms to emit reactive oxygen species once they have broken through a membrane [ 71 ]. The antibacterial efficacy can be explained based on literature by free radical formations like singlet oxygen, H 2 O 2 , hydroxyl radicals, and Zn 2+ ions which are released from the surface of ZnO NPs and damage the cell wall, they damage DNA, and create pores in the membrane where bacteria’s can’t survive [ 72 ]. UV and visible rays can activate ZnO and form electron–hole pairs (e-/h +). It has also been reported that the presence of both these findings demonstrate that ZnO NPs are hazardous to the tested bacterial strains [ 73 , 74 ]. This means that they have great potential as an antibacterial agent in commercial and medical settings.
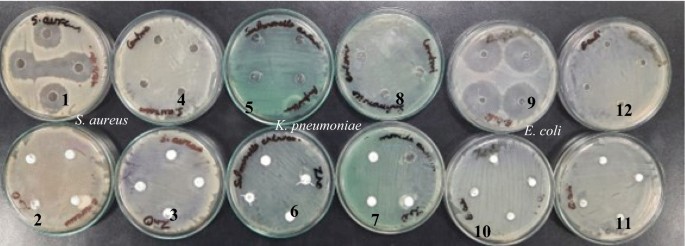
Bacterial cultures showing the inhibition zones of green synthesized ZnO NPs. against bacteria S. aureus (1–3) , K. pneumonia (5–7) and E. coli (9–11) with control (4, 8, 12)
Green synthesized ZnO NPs using Calendula officinalis is found more effective than the previous studies. Hence green synthesized ZnO NPs can be utilized effectively in various biomedical applications including skin care, ointment, and cosmetics. The present study is focused on antioxidant and antibacterial analysis of the green synthesized ZnO NPs. The antibacterial assay done against S. aureus, K. pneumonia, and E. coli pathogenic bacteria which is supported by different researcher (Table 5 ).
Several methods have been introduced to synthesize ZnO NPs, but recently, biological methods have been advanced over physico-chemical approaches due to their lower energy consumption, cost-effectiveness, and eco-friendliness. The present work enlightened the ZnO NPs using an aqueous greenery extract of Calendula officinalis. The secondary metabolites of plant extracts provide stabilizing agents for the synthesis. The optical prosperities, stability, structural characteristics, and morphology of the green synthesized ZnO NPs were analyzed. The FE-SEM micrograph showed that most of the synthesized ZnO NPs have a spherical in shape. The crystalline sizes of the synthesized ZnO NPs were evaluated using Debye-Scherer formula along the most intense peaks (101) and found to be 28.23 nm. FT-IR studies clearly showed the oxide of zinc and bioactive photochemical present in the synthesized nanomaterials which indicate the possibility of formation of ZnO NPs pore shell. The optical band gap energy was evaluated 2.986 eV which can be employed in various optical applications. Furthermore, ZnO NPs were analyzed as an antioxidant against DPPH and antibacterial properties against several multidrug resistance pathogens. The antioxidant reducing power (ARP) value of green-synthesized ZnO NPs was found to be less than that of ascorbic acid. However, despite having a lower ARP value, the experimental results indicate that green-synthesized ZnO NPs exhibit more antioxidant properties than the standard drugs. The antimicrobial activity of green-synthesized ZnO NPs against both Gram-positive ( S. aureus ) and Gram-negative bacteria ( K. pneumoniae and E. coli ) was positive. The above studies suggested that synthesized ZnO NPs can be employed in various fields including pharmaceuticals, cosmeceuticals, environmental industries, and biomedical applications. This study would be a promising avenue for further research and development.
Data availability
The experimental data and the results that support the findings of this study are available.
Ananthalakshmi R, Rajarathinam SR, Sadiq AM. Antioxidant activity of ZnO Nanoparticles synthesized using Luffa acutangula peel extract. Res J Pharm Technol. 2019;12(4):1569–72. https://doi.org/10.5958/0974-360X.2019.00260.9 .
Article Google Scholar
Bhardwaj AK, Sundaram S, Yadav KK, Srivastav AL. An overview of silver nano-particles as promising materials for water disinfection. Environ Technol Innov. 2021;23: 101721. https://doi.org/10.1016/j.eti.2021.101721 .
Ijaz I, Gilani E, Nazir A, Bukhari A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem Lett Rev. 2020;13(3):223–45. https://doi.org/10.1080/17518253.2020.1802517 .
Tiwari AK, Jha S, Singh AK, Mishra SK, Pathak AK, Ojha RP, Dikshit A. Innovative investigation of zinc oxide nanoparticles used in dentistry. Crystals. 2022;12(8):1063. https://doi.org/10.3390/cryst12081063 .
Ramos AP, Cruz MA, Tovani CB, Ciancaglini P. Biomedical applications of nanotechnology. Biophys Rev. 2017;9(2):79–89. https://doi.org/10.1007/s12551-016-0246-2 .
Singh MP, Bhardwaj AK, Bharati K, Singh RP, Chaurasia SK, Kumar S, Vikram K. Biogenic and non-biogenic waste utilization in the synthesis of 2D materials (graphene, h-BN, g-C2N) and their applications. Front Nanotechnol. 2021;3:685427. https://doi.org/10.3389/fnano.2021.685427 .
Bhardwaj AK, Naraian R. Cyanobacteria as biochemical energy source for the synthesis of inorganic nanoparticles, mechanism and potential applications: a review. Biotech. 2021;11(10):445. https://doi.org/10.1007/s13205-021-02992-5 .
Mittal R, Sharma A, Bhardwaj AK, Bhateria R, Bansal S, Kashyap R, Bhukal S. Removal of chromium (VI) using spirulina assisted synthesized mesoporous iron oxide nanoparticles. Inorg Chem Commun. 2023. https://doi.org/10.1016/j.inoche.2023.110881 .
Debroy A, Joshi S, Yadav M, George N. Green synthesis of nanoparticles from bio-waste for potential applications: Current trends, challenges, and prospects. Bio-Based Mater Waste Energ Generation Res Manage. 2023. https://doi.org/10.1016/B978-0-323-91149-8.00009-0 .
Thakur N, Thakur N, Kumar K, Arya V, Kumar A. Encapsulation of Tinospora cordifolia plant in Ni doped TiO2 nanoparticles for the degradation of malachite green dye. Nanofabrication. 2023. https://doi.org/10.37819/nanofab.8.305 .
Jha AK, Prasad K, Kulkarni AR. Synthesis of TiO2 nanoparticles using microorganisms. Colloids Surfaces B Biointerfaces. 2009;71(2):226–9. https://doi.org/10.1016/j.colsurfb.2009.02.007 .
Yaraki MT, Zahed Nasab S, Zare I, Dahri M, Moein Sadeghi M, Koohi M, Tan YN. Biomimetic metallic nanostructures for biomedical applications, catalysis, and beyond. Indus Eng Chem Res. 2022;61(22):7547–93. https://doi.org/10.1021/acs.iecr.2c00285 .
Jain M, Kapadia R, Jadeja RN, Thounaojam MC, Devkar RV and Mishra SH. Traditional uses, phytochemistry and pharmacology of Tecomellaundulata–A review. Asian Pacific Journal of Tropical Biomedicine. 2012; 2 Issue: 3. S1918-S1923. https://doi.org/10.1016/S2221-1691(12)60521-8
Umaralikhan L, Jaffar MJM. Green synthesis of ZnO and Mg doped ZnO nanoparticles, and its optical properties. J Mater Sci Mater Electron. 2017;28:7677–85. https://doi.org/10.1007/s10854-017-6461-1 .
Ijaz M, Zafar M, Islam A, et al. A review on antibacterial properties of biologically synthesized zinc oxide nanostructures. J Inorg Organomet Polym. 2020;30:2815–26. https://doi.org/10.1007/s10904-020-01603-9 .
Khanuja M, Uma Varma A. Synthesis and Characterization of Pure and Doped ZnO Nanostructures for Antimicrobial Applications: Effect of Dopant Concentration with Their Mechanism of Action. In: Ghorbanpour, M., Manika, K., Varma, A. (eds) Nanoscience and Plant–Soil Systems. Soil Biology, vol 48. Springer, Cham. 2017; https://doi.org/10.1007/978-3-319-46835-8_6
Thakur N, Kumar A, Thakur N. Tinospora cordifolia and polyvinylpyrrolidone encapsulated dual doped Ni-Cu TiO2 emerging nanocatalyst for the removal of organic dyes from wastewater and its free radical assay activity. Hybrid Adv. 2023;4: 100086. https://doi.org/10.1016/j.hybadv.2023.100086 .
Kumar P, Thakur N, Tapwal A, Kumar S. Enhancing the adsorption capacity of green/chemical synthesized hematite nanoparticles by copper doping: removal of toxic Congo red dye and antioxidant activity. Appl Nanosci. 2023;13(9):6591–604. https://doi.org/10.1007/s13204-023-02943-x .
Jiang J, Pi J, Cai J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg Chem Appl. 2018;2018:1–18. https://doi.org/10.1155/2018/1062562 .
El-Fallal AA, Elfayoumy RA, El-Zahed MM. Antibacterial activity of biosynthesized zinc oxide nanoparticles using Kombucha extract. SN Appl Sci. 2023;5:332. https://doi.org/10.1007/s42452-023-05546-x .
Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Mohamad D. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-micro Lett. 2015;7:219–42. https://doi.org/10.1007/s40820-015-0040-x .
Ajitha B. Reddy, Y.A.K. Reddy, P.S. Jeon, H.J. and Ahn C.W.(2016). Role of capping agents in controlling silver nanoparticles size, antibacterial activity and potential application as optical hydrogen peroxide sensor. RSC advances. 6(42): 36171–36179. https://doi.org/10.1039/C6RA03766F
Tari O, Aronne A, Addonizio ML, Daliento S, Fanelli E, Pernice P. Sol–gel synthesis of ZnO transparent and conductive films: a critical approach. Solar Energ Mater Solar Cells. 2012;105:179–86. https://doi.org/10.1016/j.solmat.2012.06.016 .
Cao W, Zhang Y, Wang X, Li Q, Xiao Y, Li P, Xing X. Novel resin-based dental material with anti-biofilm activity and improved mechanical property by incorporating hydrophilic cationic copolymer functionalized nano-diamond. J Mater Sci Mater Med. 2018;29(10):1–13. https://doi.org/10.1007/s10856-018-6172-z .
Bala N, Saha S, Chakraborty M, Maiti M, Das S, Basu R, Nandy P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015;7(5):4993–5003. https://doi.org/10.1039/C4RA12784F .
Verma R, Dwivedi GK, Singh JP, Singh AP, Tamta N, Kumar A. Characterization of synthesized zinc oxide nanoparticles and their effect on growth, productivity and zinc use efficiency of wheat and field pea in the Indian Himalayan foothills. Curr Sci. 2023. https://doi.org/10.18520/cs/v124/i11/1319-1328 .
Mishra SK, Srivastava RK, Prakash SG. ZnO nanoparticles: Structural, optical and photoconductivity characteristics. J Alloys Compounds. 2012;539:1–6. https://doi.org/10.1016/j.jallcom.2012.06.024 .
Jha AK, Prasad K, Prasad K, Kulkarni AR. Plant system: nature’s nanofactory. Colloids Surf B. 2009;73(2):219–23. https://doi.org/10.1016/j.colsurfb.2009.05.018 .
Pantidos N, Horsfall LE. Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. J Nanomed Nanotechnol. 2014;5(5):1. https://doi.org/10.4172/2157-7439.1000233 .
Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31(2):346–56. https://doi.org/10.1016/j.biotechadv.2013.01.003 .
Sangeetha G, Rajeshwari S, Venckatesh R. Green synthesis of zinc oxide nanoparticles by aloe barbadensis miller leaf extract: structure and optical properties. Mater Res Bull. 2011;46(12):2560–6. https://doi.org/10.1016/j.materresbull.2011.07.046 .
Chemingui H, Moulahi A, Missaoui T, Al-Marri AH, Hafiane A. A novel green preparation of zinc oxide nanoparticles with Hibiscus sabdariffa L.: photocatalytic performance, evaluation of antioxidant and antibacterial activity. Environ Technol. 2024;45(5):926–44. https://doi.org/10.1080/09593330.2022.2130108 .
Naiel B, Fawzy M, Halmy MWA, Mahmoud AED. Green synthesis of zinc oxide nanoparticles using Sea Lavender (Limonium pruinosum L. Chaz) extract: characterization, evaluation of anti-skin cancer, antimicrobial and antioxidant potentials. Sci Rep. 2022;12(1):20370. https://doi.org/10.1038/s41598-022-24805-2 .
Chemingui H, Missaoui T, Mzali JC, Yildiz T, Konyar M, Smiri M, Yatmaz HC. Facile green synthesis of zinc oxide nanoparticles (ZnO NPs): antibacterial and photocatalytic activities. Mater Res Express. 2019;6(10):10504. https://doi.org/10.1088/2053-1591/ab3cd6 .
Yang Z, Xie C. Zn2+ release from zinc and zinc oxide particles in simulated uterine solution. Colloids Surf B Biointerfaces. 2006;47(2):140–5. https://doi.org/10.1016/j.colsurfb.2005.12.007 .
Datta A, Patra C, Bharadwaj H, Kaur S, Dimri N, Khajuria R. Green synthesis of zinc oxide nanoparticles using parthenium hysterophorus leaf extract and evaluation of their antibacterial properties. J Biotechnol Biomater. 2017;7(3):271–6. https://doi.org/10.4172/2155-952X.100027 .
Karam ST, Abdulrahman AF. Green synthesis and characterization of ZnO nanoparticles by using thyme plant leaf extract. Photonics. 2022. https://doi.org/10.3390/photonics9080594 .
Ramesh M, Anbuvannan M, Viruthagiri GJSAPAM. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim Acta Part A Mol Biomol Spectr. 2015;136:864–70. https://doi.org/10.1016/j.saa.2014.09.105 .
Upadhyaya H, Shome S, Sarma R, Tewari S, Bhattacharya MK, Panda SK. Green synthesis, characterization and antibacterial activity of ZnO nanoparticles. Am J Plant Sci. 2018;9(6):1279–91. https://doi.org/10.4236/ajps.2018.96094 .
Sadiq H, Sher F, Sehar S, Lima EC, Zhang S, Iqbal HM, Nuhanović M. Green synthesis of ZnO nanoparticles from SyzygiumCumini leaves extract with robust photocatalysis applications. J Mol Liquids. 2021;335:116567. https://doi.org/10.1016/j.molliq.2021.116567 .
Thema FT, Manikandan E, Dhlamini MS, Maaza MJML. Green synthesis of ZnO nanoparticles via Agathosmabetulina natural extract. Mater Lett. 2015;161:124–7. https://doi.org/10.1016/j.matlet.2015.08.052 .
Butnariu M, Coradini CZ. Evaluation of biologically active compounds from Calendula officinalis flowers using spectrophotometry. Chem Central J. 2012;6:1–7. https://doi.org/10.1186/1752-153X-6-35 .
Perveen R, Shujaat S, Qureshi Z, Nawaz S, Khan MI, Iqbal M. Green versus sol-gel synthesis of ZnO nanoparticles and antimicrobial activity evaluation against panel of pathogens. J Mater Res Technol. 2020;9(4):7817–27. https://doi.org/10.1016/j.jmrt.2020.05.004 .
Wood M. The book of herbal wisdom: using plants as medicines. North AtlantiBooks. 2017. https://www.google.co.in/books/edition/The_Book_of_Herbal_Wisdom/Czw-DwAAQBAJ?hl=en&gbpv=0
Medina EJ, Angel LG, Paco L, Algarra I, Collado A, Garrido F. A new extract of the plant Calendula officinalis produces a dual in vitro effect. Cytotoxic anti-tumor activity and lymphocyte activation. BMC Cancer. 2006;6:1–4. https://doi.org/10.1186/1471-2407-6-119 .
Shahen MZ, Mahmud S, Rony MH, Sohana SN, Imran MAS, Al Maruf MA, Alam MS. Effect of antibiotic susceptibility and inhibitory activity for the control of growth and survival of microorganisms of extracts of Calendula officinalis. Eur J Med Health Sci. 2019;1(1):1–9.
Google Scholar
Tiwari AK, Jha S, Agrawal R, Mishra SK, Pathak AK, Singh AK, Awasthi RR. Anti-bacterial efficacy of phyto-synthesized zinc oxide nanoparticles using Murraya paniculata l. leaf extract. Sciences. 2022;13(4):864–71.
Jayachandran A, Aswathy TR, Nair AS. Green synthesis and characterization of zinc oxide nanoparticles using Cayratiapedata leaf extract. Biochem Biophys Rep. 2021;26: 100995. https://doi.org/10.1016/j.bbrep.2021.100995 .
Kumar MP, Suresh D, Nagabhushana H, Sharma SC. Beta vulgaris aided green synthesis of ZnO nanoparticles and their luminescence, photocatalytic and antioxidant properties. Eur Phys J Plus. 2015;130(6):109. https://doi.org/10.1140/epjp/i2015-15109-2 .
Tripathi SK, Jha S, Dikshit A, Kumar R. Phytochemical and antioxidant assay of Ecliptaalba (L.) leaf extract. Int J Pharm Sci Res. 2021;12(4):2288–95.
Benmehdi H, Behilil A, Memmou F, Amrouche A. Free radical scavenging activity, kinetic behaviour and phytochemical constituents of Aristolochiaclematitis L. roots. Arab J Chem. 2017;10:S1402–8. https://doi.org/10.1016/j.arabjc.2013.04.015 .
Mydeen SS, Kumar RR, Kottaisamy M, Vasantha VS. Biosynthesis of ZnO nanoparticles through extract from Prosopis juliflora plant leaf: Antibacterial activities and a new approach by rust-induced photocatalysis. J Saudi Chem Soc. 2020;24(5):393–406. https://doi.org/10.1016/j.jscs.2020.03.003 .
Muniz FTL, Miranda MR, Morilla dos Santos C, Sasaki JM. The Scherrer equation and the dynamical theory of X-ray diffraction. Acta Crystallographica Sec A Foundation Adv. 2016;72(3):385–90. https://doi.org/10.1107/S205327331600365X .
Article MathSciNet Google Scholar
Jha S, Singh R, Tiwari AK, Tripathi SK, Pandey A, Dikshit A. Microbial and physic-chemical analysis of palatable water samples from different localities of Prayagraj district. Biochem Cell Arch. 2021;21(2):5149–59.
Peng X, Palma S, Fisher NS, Wong SS. Effect of morphology of ZnO nanostructures on their toxicity to marine algae. Aquat Toxicol. 2011;102(3–4):186–96. https://doi.org/10.1016/j.aquatox.2011.01.014 .
Rad SS, Sani AM, Mohseni S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.). Microb Pathog. 2019;131:239–45. https://doi.org/10.1016/j.micpath.2019.04.022 .
Narendra Kumar HK, Chandra Mohana N, Nuthan BR, Ramesha KP, Rakshith D, Geetha N, Satish S. Phyto-mediated synthesis of zinc oxide nanoparticles using aqueous plant extract of Ocimum americanum and evaluation of its bioactivity. SN Appl Sci. 2019;1:1–9. https://doi.org/10.1007/s42452-019-0671-5 .
Sasani Ghamsari M, Alamdari S, Han W, Park HH. Impact of nanostructured thin ZnO film in ultraviolet protection. Int J Nanomed. 2017. https://doi.org/10.2147/IJN.S118637 .
Babayevska N, Przysiecka Ł, Iatsunskyi I, Nowaczyk G, Jarek M, Janiszewska E, Jurga S. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci Rep. 2022;12(1):8148. https://doi.org/10.1038/s41598-022-12134-3 .
Nayagam V, Gabriel M, Palanisamy K. Green synthesis of silver nanoparticles mediated by Coccinia grandis and Phyllanthus emblica: a comparative comprehension. Appl Nanosci. 2018;8:205–19. https://doi.org/10.1007/s13204-018-0739-3 .
Pai S, Sridevi H, Varadavenkatesan T, Vinayagam R, Selvaraj R. Photocatalytic zinc oxide nanoparticles synthesis using Peltophorumpterocarpum leaf extract and their characterization. Optik. 2019;185:248–55. https://doi.org/10.1016/j.ijleo.2019.03.101 .
Tan ST, Chen BJ, Sun XW, Fan WJ, Kwok HS, Zhang XH, Chua SJ. Blueshift of optical band gap in ZnO thin films grown by metal-organic chemicalvapor deposition. J Appl Phys. 2005. https://doi.org/10.1016/j.ijleo.2019.03.101 .
Thakur N, Thakur N. Removal of organic dyes and free radical assay by encapsulating polyvinylpyrrolidone and Tinospora Cordifolia in dual (Co–Cu) doped TiO2 nanoparticles. Environ Pollut. 2023;335: 122229. https://doi.org/10.1016/j.envpol.2023.122229 .
Kumar P, Thakur N, Tapwal A, et al. Enhancing the adsorption capacity of green/chemical synthesized hematite nanoparticles by copper doping: removal of toxic Congo red dye and antioxidant activity. Appl Nanosci. 2023;13:6591–604. https://doi.org/10.1007/s13204-023-02943-x .
Kumar P, Kumar S, Thakur N. Azadirachta indica and polyvinylpyrrolidone encapsulated Fe2O3 nanoparticles to enhance the photocatalytic and antioxidant activity. Inorg Chem Commun. 2023;155:111084. https://doi.org/10.1016/j.inoche.2023.111084 .
Kumar P, Kumar S, Tapwal A, et al. Chemical/green synthesized cobalt/copper-doped α-Fe2O3 nanoparticles: potential for environmental remediation. J Mater Res. 2024;39:836–49. https://doi.org/10.1557/s43578-023-01274-5 .
Debanath MK, Karmakar S. Study of blueshift of optical band gap in zinc oxide (ZnO) nanoparticles prepared by low-temperature wet chemical method. Mater Lett. 2013;111:116–9. https://doi.org/10.1016/j.matlet.2013.08.069 .
Yakimovich NO, Ezhevskii AA, Guseinov DV, Smirnova LA, Gracheva TA, Klychkov KS. Antioxidant properties of gold nanoparticles studied by ESR spectroscopy. Russian Chem Bullet. 2008;57:520–3. https://doi.org/10.1007/s11172-008-0080-1 .
Stan M, Popa A, Toloman D, Silipas TD, Vodnar DC. Antibacterial and antioxidant activities of ZnO nanoparticles synthesized using extracts of Allium sativum, Rosmarinus officinalis and Ocimumbasilicum. Acta Metallurgica Sinica. 2016;29:228–36. https://doi.org/10.1007/s40195-016-0380-7 .
Hossain TJ. Methods for screening and evaluation of antimicrobial activity: a review of protocols, advantages and limitations. Adv Limitations (July 17, 2023).
MuthuKathija M, Badhusha MSM, Rama V. Green synthesis of zinc oxide nanoparticles using Pisonia Alba leaf extract and its antibacterial activity. Appl Surf Sci Adv. 2023;15: 100400. https://doi.org/10.1016/j.apsadv.2023.100400 .
Gudkov SV, Burmistrov DE, Serov DA, Rebezov MB, Semenova AA, Lisitsyn AB. A mini review of antibacterial properties of ZnO nanoparticles. Front Phys. 2021;9: 641481.
Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Mohamad D. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-micro Lett. 2015;7:219–42.
Steffy K, Shanthi G, Maroky AS, Selvakumar S. Synthesis and characterization of ZnOphytonanocomposite using Strychnos nux-vomica L. (Loganiaceae) and antimicrobial activity against multidrug-resistant bacterial strains from diabetic foot ulcer. J Adv Res. 2018;9:69–77. https://doi.org/10.1016/j.jare.2017.11.001 .
Mahendra C, Chandra MN, Murali M, Abhilash MR, Singh SB, Satish S, Sudarshana MS. Phyto-fabricated ZnO nanoparticles from Canthiumdicoccum (L.) for antimicrobial, anti-tuberculosis and antioxidant activity. Process Biochem. 2020;89:220–6. https://doi.org/10.1016/j.procbio.2019.10.020 .
Download references
Acknowledgements
Authors are feeling gratitude to Prof. Ravindra Dhar, Department of Material Science, University of Allahabad for XRD; Dr. Subodh Kumar, Birbal Sahni Institute of Palaeosciences, University of Lucknow, Lucknow for FE-SEM; Dr. Vivek Bhadoria, Ewing Christian College, University of Allahabad for FT-IR. Also feeling grateful to the Head, Department of Botany, University of Allahabad for providing lab facility.
Author information
Ajay Kumar Tiwari and Saket Jha have contributed equally to this work.
Authors and Affiliations
Department of Physics, Nehru Gram Bharati (Deemed to be University), Prayagraj, 221505, U.P., India
Ajay Kumar Tiwari
Department of Surgery, University of Illinois at Chicago (UIC), Chicago, Illinois, 60612, USA
Department of Life Sciences, Arni University, Kangra, H.P., 176401, India
Sharad Kumar Tripathi
Centre of Science and Society, Under IIDS, University of Allahabad, Prayagraj, 211002, U.P., India
Rohit Shukla
Faculty of Engineering and Technology, Khwaja Moinuddin Chishti Language University, Lucknow, 226013, U.P., India
Ram Raseele Awasthi
Department of Environmental Science, Amity School of Life Sciences, Amity University Madhya Pradesh, Gwalior, 474 005, M.P., India
Abhishek Kumar Bhardwaj
Department of Physics, Shyama Prasad Mukherjee Govt. Degree College, University of Allahabad, Prayagraj, 211013, U.P., India
Abhimanyu Kumar Singh
Biological Product Laboratory, Department of Botany, University of Allahabad, Prayagraj, 211002, U.P., India
Anupam Dikshit
You can also search for this author in PubMed Google Scholar
Contributions
The study’s conception and design were contributed by all authors. Material preparation, data collection and writing were conducted by Ajay Kumar Tiwari, Saket Jha, and Rohit Shukla. The spectroscopic investigations were performed by Ajay Kaumr Tiwari, and Ram Raseele Awasthi. The antioxidant and antimicrobial activities were performed by Sharad Kumar Tripathi, Abhishek Kumar Bhardwaj, and Saket Jha. The initial draft of the manuscript was composed by Ajay Kumar Tiwari and Saket Jha. Review, Editing, and Supervision of the manuscript was composed by Abhimanyu Kumar Singh, and Anupam Dikshit. Authors have contributed feedback and approved for the final manuscript.
Corresponding authors
Correspondence to Ajay Kumar Tiwari or Abhishek Kumar Bhardwaj .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publications
Competing interests.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. There is no conflict of interest in this manuscript. The authors declare that they have no known competing financial.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Tiwari, A.K., Jha, S., Tripathi, S.K. et al. Spectroscopic investigations of green synthesized zinc oxide nanoparticles (ZnO NPs): antioxidant and antibacterial activity. Discov Appl Sci 6 , 399 (2024). https://doi.org/10.1007/s42452-024-06049-z
Download citation
Received : 11 November 2023
Accepted : 20 June 2024
Published : 24 July 2024
DOI : https://doi.org/10.1007/s42452-024-06049-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Multi-drug resistant
- Antioxidant assay
- Antimicrobial assay
Advertisement
- Find a journal
- Publish with us
- Track your research
- DOI: 10.1007/s10971-024-06490-x
- Corpus ID: 271321420
Synthesis and comparison of different metal-doped ZnO nanoparticles for catalytic degradation and adsorptive removal of direct orange: kinetics, isotherms, and thermodynamics
- Anam Batool , Ruba Munir , +1 author S. Noreen
- Published in Journal of Sol-Gel Science… 20 July 2024
- Chemistry, Environmental Science, Materials Science
54 References
Actas pink-2b dye removal in biochar nanocomposites augmented vertical flow constructed wetland (vf-cws), solar-driven removal of selected organics with binary zno based nanomaterials from aquatic environment: chemometric and toxicological assessments on wheat, evaluation of a ph- and time-dependent model for the sorption of heavy metal cations by poultry litter-derived biochar., improved removal of methyl orange dye by adsorption using modified clay: combined experimental study using surface response methodology, removal of reactive blue 49 (rb) onto untreated peanut shell (ups) waste—kinetic study, modeling, mechanism, and regeneration of the adsorbent, photocatalytic degradation of methylene blue dye from wastewater by using doped zinc oxide nanoparticles, enhancing cu2+ ion removal: an innovative approach utilizing modified frankincense gum combined with multiwalled carbon tubes and iron oxide nanoparticles as adsorbent, zeolite supported cds/tio2/ceo2 composite: synthesis, characterization and photocatalytic activity for methylene blue dye degradation, adsorptive dephenolization of aqueous solutions using thermally modified corn cob: mechanisms, point of zero charge, and isosteric heat studies, green metal oxides coated biochar nanocomposites preparation and its utilization in vertical flow constructed wetlands for reactive dye removal: performance and kinetics studies., related papers.
Showing 1 through 3 of 0 Related Papers
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Synthesis and characterization of sio 2 nanoparticles for application as nanoadsorbent to clean wastewater.

1. Introduction
2. materials and methods, 2.1. synthesis of sio 2 nanoparticles, 2.2. x-ray diffraction analysis (xrd), 2.3. uv - vis spectroscopy (uv-vis), 2.4. removal of methylene blue in water, 3.1. ftir spectrum analysis, 3.2. x-ray diffraction of sio 2 nanoparticles, 3.3. uv-vis of sio 2 nanoparticles, 3.4. sem of sio 2 nanoparticles, 3.5. energy-dispersive x-ray (edx) analysis of sio 2 nanoparticles, 3.6. tem of sio 2 nanoparticles, 3.7. methylene blue degradation uv-vis, 3.8. percentage of methylene blue removal, 4. discussion, 5. conclusions, author contributions, data availability statement, acknowledgments, conflicts of interest.
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Kurmus, H.; Milas, J.; Arulrajah, A.; Horpibulsuk, S.; Abdul Kadir, A. Nanoparticles in construction materials and other applications, and implications of nanoparticle use. Materials 2019 , 12 , 3052. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Khajeh, M.; Laurent, S.; Dastafkan, K. Nanoadsorbents: Classification, preparation, and applications (with emphasis on aqueous media). Chem. Rev. 2013 , 113 , 7728–7768. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mahmoudi, M.; Bouras, O.; Hadjersi, T.; Baudu, M.; Aissiou, S. Synthesis of CuO-modified silicon nanowires as a photocatalyst for the degradation of malachite green. React. Kinet. Mech. Catal. 2021 , 134 , 971–987. [ Google Scholar ] [ CrossRef ]
- Akhter, F.; Rao, A.A.; Abbasi, M.N.; Ahmed Wahocho, S.; Mallah, M.A.; Anees-ur-Rehman, H.; Chandio, Z.A. A Comprehensive Review of Synthesis, Applications and Future Prospects for Silica Nanoparticles (SNPs). Silicon 2022 , 14 , 8295–8310. [ Google Scholar ] [ CrossRef ]
- Bhatnagar, A.; Vilar, V.J.P.; Botelho, C.M.; Boaventura, R.A.R. Recent advances in nano-adsorbents for water treatment. Environ. Chem. Lett. 2022 , 20 , 125–145. [ Google Scholar ] [ CrossRef ]
- Jain, K.; Patel, A.S.; Pardhi, V.P.; Flora, S.J.S. Nanotechnology in Wastewater Management: A New Paradigm Towards Wastewater Treatment. Moleculs 2021 , 26 , 1797. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Benhadria, N.; Hachemaoui, M.; Zaoui, F.; Mokhtar, A.; Boukreris, S.; Attar, T.; Belarbi, L.; Boukoussa, B. Catalytic reduction of methylene blue dye by copper oxide nanoparticles. J. Clust. Sci. 2022 , 33 , 249–260. [ Google Scholar ] [ CrossRef ]
- Manasa, R.L.; Mehta, A. Wastewater: Sources of Pollutants and Its Remediation. In Environmental Biotechnology ; Gothandam, K., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World, Vol 45.; Springer: Cham, Switzerland, 2020; Volume 2. [ Google Scholar ] [ CrossRef ]
- Jesús Ruíz-Baltazar, Á.; Reyes-López, S.Y.; de Lourdes Mondragón-Sánchez, M.; Robles-Cortés, A.I.; Pérez, R. Eco-friendly synthesis of Fe 3 O 4 nanoparticles: Evaluation of their catalytic activity in methylene blue degradation by kinetic adsorption models. Results Phys. 2019 , 12 , 989–995. [ Google Scholar ] [ CrossRef ]
- Cheng, K.; Heng, S.; Tieng, S.; David, F.; Dine, S.; Haddad, O.; Colbeau-Justin, C.; Traore, M.; Kanaev, A. Mixed Metal Oxide W-TiO 2 Nanopowder for Environmental Process: Synergy of Adsorption and Photocatalysis. Nanomaterials 2024 , 14 , 765. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- George, A.; Raj, D.M.A.; Venci, X.; Raj, A.D.; Irudayaraj, A.A.; Josephine, R.L.; Sundaram, S.J.; Al-Mohaimeed, A.M.; Al Farraj, D.A.; Chen, T.-W.; et al. Photocatalytic effect of CuO nanoparticles flower-like 3D nanostructures under visible light irradiation with the degradation of methylene blue (MB) dye for environmental application. Environ. Res. 2022 , 203 , 111880. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Modi, S.; Yadav, V.K.; Gacem, A.; Ali, I.H.; Dave, D.; Khan, S.H.; Yadav, K.K.; Rather, S.-u.; Ahn, Y.; Son, C.T.; et al. Recent and emerging trends in remediation of methylene blue dye from wastewater by using zinc oxide nanoparticles. Water 2022 , 14 , 1749. [ Google Scholar ] [ CrossRef ]
- Suvith, V.S.; Philip, D. Catalytic degradation of methylene blue using biosynthesized gold and silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014 , 118 , 526–532. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ceroni, L.; Benazzato, S.; Pressi, S.; Calvillo, L.; Marotta, E.; Menna, E. Enhanced Adsorption of Methylene Blue Dye on Functionalized Multi-Walled Carbon Nanotubes. Nanomaterials 2024 , 14 , 522. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kumari, P.; Alam, M.; Siddiqi, W.A. Usage of nanoparticles as adsorbents for waste water treatment: An emerging trend. Sustain. Mater. Technol. 2019 , 22 , e00128. [ Google Scholar ] [ CrossRef ]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019 , 17 , 145–155. [ Google Scholar ] [ CrossRef ]
- Qi, D.; Lin, C.; Zhao, H.; Liu, H.; Lü, T. Size regulation and prediction of the SiO 2 nanoparticles prepared via Stöber process. J. Dispers. Sci. Technol. 2017 , 38 , 70–74. [ Google Scholar ] [ CrossRef ]
- Zaheer, S.; Shehzad, J.; Chaudhari, S.K.; Hasan, M.; Mustafa, G. Morphological and Biochemical Responses of Vigna radiata L. Seedlings Towards Green Synthesized SiO 2 NPs. Silicon 2023 , 15 , 5925–5936. [ Google Scholar ] [ CrossRef ]
- Rahimzadeh, C.Y.; Barzinjy, A.A.; Mohammed, A.S.; Hamad, S.M. Green synthesis of SiO 2 nanoparticles from Rhus coriaria L. Extract: Comparison with chemically synthesized SiO 2 nanoparticles. PLoS ONE 2022 , 17 , e0268184. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Post, P.; Wurlitzer, L.; Maus-Friedrichs, W.; Weber, A.P. Characterization and applications of nanoparticles modified in-flight with silica or silica-organic coatings. Nanomaterials 2018 , 8 , 530. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sharma, P.; Kherb, J.; Prakash, J.; Kaushal, R. A novel and facile green synthesis of SiO 2 nanoparticles for removal of toxic water pollutants. Appl. Nanosci. 2023 , 13 , 735–747. [ Google Scholar ] [ CrossRef ]
- Abuzeid, H.M.; Julien, C.M.; Zhu, L.; Hashem, A.M. Green Synthesis of Nanoparticles and Their Energy Storage, Environmental, and Biomedical Applications. Crystals 2023 , 13 , 1576. [ Google Scholar ] [ CrossRef ]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of Green Synthesized Metal Nanoparticles—A Review. Biol. Trase Elem. Res. 2024 , 202 , 360–386. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jimenéz-Vivanco, M.R.; García, G.; Carrillo, J.; Morales-Morales, F.; Coyopol, A.; Gracia, M.; Doti, R.; Faubert, J.; Lugo, J.E. Porous Si-SiO 2 UV microcavities to modulate the responsivity of a broadband photodetector. Nanomaterials 2020 , 10 , 222. [ Google Scholar ] [ CrossRef ]
- Ghufran, M.; Huitink, D. Synthesis of nano-size paraffin/silica-based encapsulated phase change materials of high encapsulation ratio via sol–gel method. J. Mater. Sci. 2023 , 58 , 7673–7689. [ Google Scholar ] [ CrossRef ]
- Shi, B.; Xie, L.; Ma, B.; Zhou, Z.; Xu, B.; Qu, L. Preparation and properties of highly transparent SiO 2 aerogels for thermal insulation. Gels 2022 , 8 , 744. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alavi, M.; Hamblin, M.R.; Mozafari, M.R.; Rose Alencar De Menezes, I.; Douglas Melo Coutinho, H. Surface modification of SiO 2 nanoparticles for bacterial decontaminations of blood products. Cell. Mol. Biomed. Rep. 2022 , 2 , 87–97. [ Google Scholar ] [ CrossRef ]
- Sun, J.; Xu, Z.; Li, W.; Shen, X. Effect of nano-SiO 2 on the early hydration of alite-sulphoaluminate cement. Nanomaterials 2017 , 7 , 102. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gomes, B.R.; Lopes, J.L.; Coelho, L.; Ligonzo, M.; Rigoletto, M.; Magnacca, G.; Deganello, F. Development and Upscaling of SiO 2 @TiO 2 Core-Shell Nanoparticles for Methylene Blue Removal. Nanomaterials 2023 , 13 , 2276. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mikhnenko, M.D.; Cherepanova, S.V.; Gerasimov, E.Y.; Pochtar, A.A.; Alekseeva (Bykova), M.V.; Kukushkin, R.G.; Yakovlev, V.A.; Bulavchenko, O.A. Defect Structure of Nanocrystalline NiO Oxide Stabilized by SiO 2 . Inorganics 2023 , 11 , 97. [ Google Scholar ] [ CrossRef ]
- Eissa, D.; Hegab, R.H.; Abou-Shady, A.; Kotp, Y.H. Green synthesis of ZnO, MgO and SiO 2 nanoparticles and its effect on irrigation water, soil properties, and Origanum majorana productivity. Sci. Rep. 2022 , 12 , 5780. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Imoisili, P.E.; Nwanna, E.C.; Jen, T.-C. Facile Preparation and Characterization of Silica Nanoparticles from South Africa Fly Ash Using a Sol–Gel Hydrothermal Method. Processes 2022 , 10 , 2440. [ Google Scholar ] [ CrossRef ]
- Aly, H.F.; Abd-Elhamid, A.I. Photocatalytic degradation of methylene blue dye using silica oxide nanoparticles as a catalyst. Water Environ. Res. 2018 , 90 , 807–817. [ Google Scholar ] [ CrossRef ]
- Nandanwar, R.; Singh, P.; Haque, F.Z. Synthesis and characterization of SiO 2 nanoparticles by sol-gel process and its degradation of methylene blue. Am. Chem. Sci. J. 2015 , 5 , 1–10. [ Google Scholar ] [ CrossRef ]
- Zhu, Z.; Liang, H.; Sun, D.W. Infusing silicone and camellia seed oils into micro-/nanostructures for developing novel anti-icing/frosting surfaces for food freezing applications. ACS Appl. Mater. Interfaces 2023 , 15 , 14874–14883. [ Google Scholar ] [ CrossRef ]
- Jain, A.; Wadhawan, S.; Mehta, S.K. Biogenic synthesis of non-toxic iron oxide NPs via Syzygium aromaticum for the removal of methylene blue. Environ. Nanotechnol. Monit. Manag. 2021 , 16 , 100464. [ Google Scholar ] [ CrossRef ]
- Sharma, P.; Prakash, J.; Kaushal, R. An insight into the green synthesis of SiO 2 nanostructures as a novel adsorbent for removal of toxic water pollutants. Environ. Res. 2022 , 212 , 113328. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Babyszko, A.; Wanag, A.; Kusiak-Nejman, E.; Morawski, A.W. Effect of Calcination Temperature of SiO 2 /TiO 2 Photocatalysts on UV-VIS and VIS Removal Efficiency of Color Contaminants. Catalysts 2023 , 13 , 186. [ Google Scholar ] [ CrossRef ]
- Chen, Q.; Xue, X.; Liu, Y.; Guo, A.; Chen, K.; Yin, J.; Yu, F.; Zhu, H.; Guo, X. Shear-induced fabrication of SiO 2 nano-meshes for efficient uranium capture. J. Hazard. Mater. 2022 , 438 , 129524. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alonso-De la Garza, D.A.; Guzmán, A.M.; Gómez-Rodríguez, C.; Martínez, D.I.; Elizondo, N. Influence of Al 2 O 3 and SiO 2 nanoparticles addition on the microstructure and mechano-physical properties of ceramic tiles. Ceram. Int. 2022 , 48 , 12712–12720. [ Google Scholar ] [ CrossRef ]
- Rong, Z.; Zhao, M.; Wang, Y. Effects of modified nano-SiO 2 particles on properties of high-performance cement-based composites. Materials 2020 , 13 , 646. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dehsheikh, H.G.; Ghasemi-Kahrizsangi, S. The influence of silica nanoparticles addition on the physical, mechanical, thermo-mechanical as well as microstructure of Mag-Dol refractory composites. Ceram. Int. 2017 , 43 , 16780–16786. [ Google Scholar ] [ CrossRef ]
- Wardiyati, S.; Adi, W.A. Synthesis and characterization of microwave absorber SiO 2 by sol-gel methode. In IOP Conference Series: Materials Science and Engineering ; IOP Publishing: Bristol, UK, 2017; Volume 202, p. 012059. [ Google Scholar ] [ CrossRef ]
- El-Feky, H.H.; Behiry, M.S.; Amin, A.S.; Nassar, M.Y. Facile fabrication of nano-sized SiO 2 by an improved sol–gel route: As an adsorbent for enhanced removal of Cd (II) and Pb (II) ions. J. Inorg. Organomet. Polym. Mater. 2022 , 32 , 1129–1141. [ Google Scholar ] [ CrossRef ]
- Hong, Y.; Cha, B.J.; Kim, Y.D.; Seo, H.O. Mesoporous SiO 2 particles combined with Fe oxide nanoparticles as a regenerative methylene blue adsorbent. ACS Omega 2019 , 4 , 9745–9755. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ruchi, N.; Bamne, J.; Singh, N.; Sharma, P.K.; Singh, P.; Umar, A.; Haque, F.Z. Synthesis of titania/silica nanocomposite for enhanced photodegradation of methylene blue and methyl orange dyes under uv and mercury lights. ES Mater. Manuf. 2022 , 16 , 78–88. [ Google Scholar ] [ CrossRef ]
- Eddy, D.R.; Luthfiah, A.; Permana, M.D.; Deawati, Y.; Firdaus, M.L.; Rahayu, I.; Izumi, Y. Rapid Probing of Self-Cleaning Activity on Polyester Coated by Titania–Natural Silica Nanocomposite Using Digital Image-Based Colorimetry. ACS Omega 2023 , 8 , 7858–7867. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Biradar, A.I.; Sarvalkar, P.D.; Teli, S.B.; Pawar, C.A.; Patil, P.S.; Prasad, N.R. Photocatalytic degradation of dyes using one-step synthesized silica nanoparticles. Mater. Today Proc. 2021 , 43 , 2832–2838. [ Google Scholar ] [ CrossRef ]
- Muguruza, A.R.; di Maio, A.; Hodges, N.J.; Blair, J.M.; Pikramenou, Z. Chelating silica nanoparticles for efficient antibiotic delivery and particle imaging in Gram-negative bacteria. Nanoscale Adv. R. Soc. Chem. 2023 , 5 , 2453–2461. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Song, Y.; Zheng, X.; Hu, J.; Ma, S.; Li, K.; Chen, J.; Xu, X.; Lu, X.; Wang, X. Recent advances of cell membrane-coated nanoparticles for therapy of bacterial infection. Front. Microbiol. 2023 , 14 , 3428. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Characteristic | Commercial SiO NPs | Synthesized SiO NPs |
|---|---|---|
| Purity | 99.00% | 98.10% |
| Particle Size | 80 nm | 60 nm |
| Specific surface area | 70 m /g | 90 m /g |
| Morphology | Spherical | Spherical |
| Dispersibility | Good in aqueous and non-aqueous solvents | Good in aqueous solvents |
| Color | White powder | White powder |
| Applications | Used in coatings, electronics, drug delivery, catalysis, and more | Clean wastewater |
| Sample/Weight NPs | 10 mg | 20 mg | 30 mg |
|---|---|---|---|
| COM | 10 min 38% | 10 min 66% | 10 min 77% |
| 30 min 39% | 30 min 70% | 30 min 85% | |
| 60 min 46% | 60 min 72% | 60 min 89% | |
| pH 2 | 10 min 55% | 10 min 48% | 10 min 51% |
| 30 min 45% | 30 min 66% | 30 min 82% | |
| 60 min 67% | 60 min 51% | 60 min 83% | |
| pH 5 | 10 min 34% | 10 min 64% | 10 min 92% |
| 30 min 38% | 30 min 89% | 30 min 95% | |
| 60 min 59% | 60 min 93% | 60 min 97% | |
| pH 7 | 10 min 63% | 10 min 67% | 10 min 85% |
| 30 min 67% | 30 min 81% | 30 min 93% | |
| 60 min 80% | 60 min 97% | 60 min 97% |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Elizondo-Villarreal, N.; Gandara-Martínez, E.; García-Méndez, M.; Gracia-Pinilla, M.; Guzmán-Hernández, A.M.; Castaño, V.M.; Gómez-Rodríguez, C. Synthesis and Characterization of SiO 2 Nanoparticles for Application as Nanoadsorbent to Clean Wastewater. Coatings 2024 , 14 , 919. https://doi.org/10.3390/coatings14070919
Elizondo-Villarreal N, Gandara-Martínez E, García-Méndez M, Gracia-Pinilla M, Guzmán-Hernández AM, Castaño VM, Gómez-Rodríguez C. Synthesis and Characterization of SiO 2 Nanoparticles for Application as Nanoadsorbent to Clean Wastewater. Coatings . 2024; 14(7):919. https://doi.org/10.3390/coatings14070919
Elizondo-Villarreal, Nora, Eleazar Gandara-Martínez, Manuel García-Méndez, Miguel Gracia-Pinilla, Ana María Guzmán-Hernández, Víctor M. Castaño, and Cristian Gómez-Rodríguez. 2024. "Synthesis and Characterization of SiO 2 Nanoparticles for Application as Nanoadsorbent to Clean Wastewater" Coatings 14, no. 7: 919. https://doi.org/10.3390/coatings14070919
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

Oxygen-tolerant, eosin Y mediated synthesis of protein–polymer biohybrids and protein-coated polymer nanoparticles † ‡

First published on 10th July 2024
To avoid metal catalysts that are commonly used in conventional approaches for the synthesis of protein–polymer conjugates, an eosin Y/TEMED mediated, photoinduced polymerization of vinyl monomers was optimized. This oxygen tolerant, photoinduced approach allowed the grafting of a series of hydrophobic, hydrophilic and responsive polymers with quantitative protein macroinitiator consumption. CALB bioconjugates were also synthesized and found to retain part of the parent protein activity for extended periods of time. Notably, when BSA was used in the absence of an initiator, protein-coated nanoparticles were shown to form during emulsion polymerization.
Introduction
Recent advances in polymer synthesis have also fostered significant progress in polymer bioconjugate synthesis. In the early synthesis of protein–polymer conjugates, most reports employed grafting-to approaches involving amino acid-specific or random couplings of prefunctionalized polymers to the protein 22–24 or bioaffinity couplings. 25–27 More recently, by capitalizing on the ability to synthesize polymers in rapid, efficient, and precise manners, 28 numerous new methodologies have emerged for the synthesis of protein–polymer conjugates. 2,29–33 These are mostly grafting-from methodologies involving controlled radical polymerization (CRP) approaches such as atom transfer radical polymerization (ATRP), 34–41 Cu(0)-mediated radical polymerization, 42,43 and ring-opening polymerization, 44,45 as well as reversible addition–fragmentation chain transfer (RAFT) 46–48 polymerization approaches and chain growth polymerization techniques.
Very recent reports on the synthesis of protein–polymer conjugates have focused on oxygen tolerant 49–52 and photomediated metal catalyzed approaches. 39,51,53 A significant advantage of photochemical approaches is that they offer temporal and spatial control under mild reaction conditions while oxygen tolerance is fundamental for the development of sustainable applications. 54–57 However, several of these approaches require metal-based catalysts and metal contamination is a limiting factor when aiming at biomedical applications. For this reason, the metal-free organocatalyzed ATRP (O-ATRP) 58–61 mediated synthesis of protein–polymer conjugates recently reported in seminal works by the groups of Sumerlin, 62,63 Matyjaszewski, 64 and Boyer 65 provides a new, powerful tool in the realm of oxygen tolerant bioconjugation. In this respect, the commonly used xanthene electron acceptor derivative eosin Y (EY) has been used as the organocatalyst as it is known to mediate photoinduced polymerization of several families of monomers in conjunction with alkyl halides and amines. 66–68 EY is cheap and commercially available, displays excellent biocompatibility and has therefore been widely used in biological applications. 69,70 Taking these beneficial characteristics into account, Sumerlin and collaborators were the first to employ EY-catalyzed PET-RAFT for the synthesis of polymer–protein conjugates in the presence of a tertiary amine and under visible-light irradiation. 62,63 Following this grafting-from approach, rapid synthesis of water soluble biohybrids over a range of targeted molecular weights became possible. In a more recent contribution, eosin Y acrylate was copolymerized with N -isopropylacrylamide (NIPAM) to afford a polymeric photocatalyst with temperature dependent hydrophilic-to-hydrophobic transition which enabled easy purification of the bioconjugates post polymerization and recovery of the catalyst. 63 Importantly, ascorbic acid (AscA) was shown to efficiently deoxygenate the polymerization solution. Matyjaszewski and collaborators developed green-light-induced dual catalysis ATRP, i.e. , using EY in combination with a copper complex which enabled rapid and well-controlled polymerization in water without the need for deoxygenation. 71,72 Following this approach, hydrophilic acrylate-based protein–polymer hybrids were also synthesized under ambient conditions. 64 Finally, Boyer and collaborators 65 developed a photo-RAFT system allowing the synthesis of protein–polymer conjugates with excellent oxygen tolerance. During this study, the photocatalyst EY was combined with the reactive oxygen species (ROS), generating cocatalysts AscA and triethanolamine (TEOA). Evaluation of the impact of the ROS on model proteins led to the selection of EY/TEOA as the optimal photo-RAFT initiating system for preserving enzymatic activity.
Inspired by these reports, we developed an oxygen tolerant, EY photocatalyzed, grafting-from approach for the synthesis of protein–polymer conjugates in the absence of metal cocatalysts with targeted quantitative macroinitiator consumption. The latter is essential to avoid tedious purification that might render the approach difficult to scale-up for applications. We further reasoned that the strong adsorption of EY on proteins 73 and/or its potential entrapment in the assemblies would enable direct imaging of the bioconjugates via fluorescence, offering in addition one pot synthesis and labeling for studies using advanced microscopy. Following this approach, we present herein the synthesis of hydrophilic, amphiphilic, and responsive bioconjugates under mild blue light irradiation and with quantitative macroinitiator consumption attained in the presence of a tertiary amine cocatalyst. 65 To this end, BSA was selected as a model protein since it offers valuable characteristics to biohybrid systems including a lack of cytotoxicity, high stability, and the ability to evade interactions with blood serum components. 74 Styrene, methacrylates, acrylates, and acrylamides were used as monomers. Expanding the biohybrid scope to enzymes, catalytically active biohybrids were also synthesized using the lipase B from Candida antarctica and were imaged with fluorescence microscopy. Considering that the tertiary amines act as co-initiators, 60,75 the polymerization was studied in depth, revealing tandem formation of polymeric by-products. To valorize these polymers, emulsion polymerization was employed in the presence of native proteins, yielding protein-coated polymer nanoparticles.
Results and discussion
| Synthesis of BSA-poly(styrene) via oxygen tolerant, organocatalyzed ATRP (top scheme). (A) Native PAGE, lanes 1–4 and 7–10: BSA-poly(styrene) lane 1: styrene/BSA-Br, I /EY = 4000/1/0.02, lane 2: styrene/BSA-Br, I /EY = 4000/1/0.2, lane 3: styrene/BSA-Br, I /EY = 4000/1/0.5, lane 4: styrene/BSA-Br, I /EY = 4000/1/1, lane 5: BSA-Br (I ), lane 6: native BSA, lane 7: styrene/BSA-Br, I /EY/TEMED = 4000/1/0.02/0.2, lane 8: styrene/BSA-Br, I /EY/TEMED = 4000/1/0.2/2, lane 9: styrene/BSA-Br, I /EY/TEMED = 4000/1/0.5/5, lane 10: styrene/BSA-Br, I /EY/TEMED = 4000/1/1/10, and lane 11: polystyrene. (B) SEC chromatographs of BSA-Br (I ) and BSA-poly(styrene) synthesized under different conditions. (C) ON/OFF time course experiment using a feed molar ratio of styrene/BSA-Br, I /EY/TEMED = 4000/1/1/10. Left: native PAGE lanes 1–9: samples were withdrawn every 2 minutes of alternating blue LED ON and OFF periods, lane 10: native BSA, and lane 11: BSA-Br (I ). Right: semiquantitative analysis plot of BSA-Br (I ) consumption during the course of the reaction. (D) ON/OFF time course experiment using a feed molar ratio of styrene/BSA-Br, I /EY/TEMED = 4000/1/0.5/5. Left: native PAGE lanes 1–9: samples were withdrawn every 3 minutes of alternating blue LED ON and OFF periods, lane 10: BSA-Br (I ), and lane 11: native BSA. Right: semiquantitative analysis plot of BSA-Br (I ) consumption during the course of the reaction. | ||
| Entry | Styrene/BSA-Br, I /EY | Reaction time (min) | BSA-Br (I ) consumption |
|---|---|---|---|
| 1 | 4000/1/1 | 120 | Near quantitative |
| 2 | 4000/1/0.5 | 120 | Partial |
| 3 | 4000/1/0.2 | 120 | Partial |
| 4 | 4000/1/0.02 | 120 | Low |
| 5 | 4000/1/0 | 120 | No reaction |
| 6 | 4000/1/1 | 120 | No reaction |
| 7 | 4000/0/1 | 120 | n.a. |
| Styrene/BSA-Br, I /EY/AscA | Reaction time (min) | BSA-Br (I ) consumption | |
|---|---|---|---|
| 8 | 4000/1/1/0.5 | 120 | High |
| Styrene/BSA-Br, I /EY/TEMED | Reaction time (min) | BSA-Br (I ) consumption | |
|---|---|---|---|
| Without irradiation. Formation of polystyrene nanoparticles. | |||
| 9 | 4000/1/1/10 | 10–15 | Quantitative |
| 10 | 4000/1/0.5/5 | 30–45 | Quantitative |
| 11 | 4000/1/0.2/2 | 120 | Near quantitative |
| 12 | 4000/1/0.02/0.2 | 240 or 480 | Low |
| 13 | 4000/0/1/10 | 240 | n.a. |
| 14 | 4000/0/0.2/2 | 240 | n.a. |
When control experiments were performed in the absence of a selected reaction component such as the catalyst ( Table 1 , entry 5), the monomer or irradiation ( Table 1 , entry 6 and Fig. S3 ‡ ), no biohybrid formation could be detected while in all cases, the macroinitiator was recovered unaffected. On the other hand, polystyrene nanoparticles were formed when styrene was subjected to emulsion polymerization conditions in the absence of the protein macroinitiator BSA-Br (I o ) ( Table 1 , entry 7 and Fig. 2 ). The produced polystyrene was isolated and characterized with 1 H-NMR spectroscopy (Fig. S5 ‡ ).
| (A) FE-SEM micrographs and (B) TEM micrographs of BSA-poly(styrene) ( , entry 9) observed as two distinct populations of spherical nanoparticles with diameters between 100 and 130 nm and between 20 and 40 nm; (C) FE-SEM micrographs of poly(styrene) ( , entry 13) observed as spherical nanoparticles with diameters between 10 and 40 nm; and (D) FE-SEM micrographs of BSA coated poly(styrene) nanoparticles with diameters between 10 and 50 nm. | ||
Throughout this study, quantitative macroinitiator consumption was targeted as it minimizes the effort required to isolate the biohybrids by rendering only a simple dialysis step necessary. We evaluated grafting in the presence of AscA since it was elegantly employed in recent protocols as a means to deoxygenate the reaction mixtures. 63,65 Under the conditions used herein, grafting of styrene was found to proceed yet without quantitative macroinitiator consumption ( Table 1 , entry 8 and Fig. S3, S7 ‡ ). For this reason, we proceeded to investigate the effect of a tertiary amine sacrificial electron donor since it has been previously shown in EY mediated PET RAFT polymerizations that the stability of the generated amine radical cation enhanced both the efficiency of the reduction of excited-state EY and oxygen tolerance. 80–82 Indeed, when N , N , N ′, N ′-tetramethylethylenediamine (TEMED) was added as a sacrificial electron donor, 48 quantitative macroinitiator consumption could be attained after merely 10 to 15 minutes of blue LED irradiation at a feed molar ratio of styrene/BSA-Br, I o /EY/TEMED = 4000/1/1/10 ( Fig. 1 , Table 1 , entry 9, Fig. S3 and S8 ‡ ). When a reduced EY feed molar ratio was used, quantitative macroinitiator consumption could again be achieved, albeit at increased irradiation times (between 30 minutes and 2 hours depending on the photoredox catalyst loading, Table 1 , entries 10–12, Fig. S8 ‡ ).
Imaging of the products ( Table 1 , entry 9) with FE-SEM revealed two distinct populations of spherical nanoparticles with defined diameters between 100 and 130 nm and between 20 and 40 nm ( Fig. 2A and B ). Poly(styrene) formed in the absence of a macroinitiator appeared as spherical nanoparticles with significantly smaller diameters between 10 and 40 nm ( Fig. 2C , Table 1 , entries 13 and 14, Fig. S5 ‡ ). Hence, the spherical assemblies observed using FE-SEM can be most possibly attributed to hybrid polymer/bioconjugate nanoparticles ( Fig. 2A and B ). The nature of the nanoparticles was further elucidated through the synthesis of BSA-responsive polymer bioconjugates ( vide infra ). Taking into account the amphiphilicity of the bioconjugates and thus the lack of a solvent that would both dissolve them and preserve the conformation of the protein, the free polymer could not be efficiently removed from the product assemblies.
Next, intermittent light exposure was investigated to assess the possibility of activating and deactivating polymerization. Rapid macroinitiator consumption was observed after 2–3 minutes of irradiation, as can be observed in Fig. 1C (styrene/BSA-Br, I o /EY/TEMED = 4000/1/1/10, Table 1 , entry 9 and Fig. S9 ‡ ). This short induction period can be ascribed to the time required to in situ remove the oxygen from the polymerization solution and the time required for the EY radical anion to form and in turn interact with the amine co-initiator to kick-start the polymerization. 83–85 Under these conditions, the polymerization could be activated and deactivated by switching on and off the irradiation source until fully consuming BSA-Br (after 10 minutes of total ON irradiation time); nevertheless, temporal control was poor. We reasoned that the concentrations of EY and the tertiary amine would influence both the induction period and polymerization control, and therefore, to attain better temporal control, we lowered the concentration of the catalyst, i.e. , styrene/BSA-Br, I o /EY/TEMED = 4000/1/0.5/5 ( Table 1 , entry 10 and Fig. 1C ). The reaction could be again triggered or halted by turning the blue LEDs on and off with improved temporal control.
To further exploit this photoinduced methodology, we synthesized BSA-poly(styrene) on a larger scale using the same experimental setup. SEC verified that the macroinitiator consumption was quantitative on a 6 times larger scale without the need for further optimization (Fig. S9 ‡ ).
Monomer scope
| Characterization of amphiphilic, hydrophilic, and responsive protein–polymer conjugates. (A) IR spectra of the bioconjugates. (B) H-NMR spectra acquired for hydrophilic BSA-polymer conjugates. (C) Transmittance vs. time curve at different temperatures showing the rapid response of BSA-poly(NIPAM). (D) Transmittance vs. pH curves of BSA-poly(DPA). Two cycles are shown for the same sample in which the response was induced by changing the pH with the addition of HCl (pH decrease) or NaOH (pH increase). (E) SEM and FE-SEM micrographs of amphiphilic protein–polymer conjugate nanoparticles. | ||
| Entry | Monomer | Monomer/BSA-Br, I /EY | BSA-Br (I ) consumption |
|---|---|---|---|
| Addition of 5% v/v toluene. | |||
| 1 | MA | 4000/1/1/10 | Quantitative |
| 2 | MMA | 4000/1/1/10 | Quantitative |
| 3 | VAc | 8000/1/5/50 | High |
| 4 | VP | 4000/1/0.5/5 | Quantitative |
| 5 | NAM | 4000/1/1/10 | Near quantitative |
| 6 | HEA | 4000/1/1/10 | Quantitative |
| 7 | HEMA | 4000/1/1/10 | Quantitative |
| 8 | NIPAM | 1000/1/1/10 | Quantitative |
| 9 | NIPAM | 1000/1/0.2/10 | Quantitative |
| 10 | NIPAM | 100/1/0.2/10 | Quantitative |
| 11 | DMAEMA | 4000/1/0.2/10 | Quantitative |
| 12 | DPA | 4000/1/0.2/10 | Quantitative |
As seen with styrene, several other monomers used in this study (MMA, DPA and NIPAM vide infra ) were also shown to polymerize in the absence of a macroinitiator (Fig. S6 ‡ ).
Grafting of the less activated monomer vinyl acetate (VAc) proved to be more demanding. In general, ATRP of VAc is considered highly challenging because the homolytic bond dissociation energy of the dormant poly(VAc) chains makes reactivation difficult while at the same time the VAc propagating radical is not stabilized either. 86,87 Indeed, neither addition of an organic cosolvent nor increased catalyst loadings or grafting times could significantly increase biomacroinitiator consumption. The lowest amount of unreacted macroinitiator was detected at a feed molar ratio of VAc/BSA-Br, I o /EY/TEMED = 8000/1/5/10 (Fig. S14 ‡ and Fig. 3E ).
We also sought to graft hydrophilic monomers from BSA-Br (I o ), and for this reason, vinyl pyrrolidone (VP), N -acryloyl morpholine (NAM) and 2-hydroxyethyl acrylate (HEA) were selected since all produce polymers useful in a variety of pharmaceutical and biomedical applications ( Fig. 3 ). 88,89 1 H-NMR spectroscopy provided an additional means to characterize hydrophilic protein–polymer conjugates while dialysis was sufficient to remove both unreacted monomers and the produced polymers from the bioconjugate solution. It should be noted that for hydrophilic monomers, the optimum conditions of emulsion polymerization did not result in macroinitiator consumption which was more difficult to attain. A feed molar ratio of VP/BSA-Br, I o /EY/TEMED = 4000/1/0.5/5 was found to be sufficient to yield BSA-poly(vinyl pyrrolidone) biohybrids ( Fig. 3 and Fig. S15 ‡ ). For the synthesis of BSA-poly( N -acryloyl morpholine), near quantitative macroinitiator consumption was observed after optimization with NAM/BSA-Br/EY/TEMED = 4000/1/1/10 ( Fig. 3 and Fig. S16 ‡ ). At the same molar loading, both 2-hydroxyethyl acrylate (HEA) and 2-hydroxyethyl methacrylate (HEMA) led to the formation of BSA-poly(HEA) ( Fig. 3 and Fig. S187 ‡ ) and BSA-poly(HEMA) ( Fig. 3 and Fig. S18 ‡ ), respectively.
Targeting the synthesis of responsive bioconjugates, N -isopropylacrylamide (NIPAM), 2-(dimethylamino)ethyl methacrylate (DMAEMA) and 2-(diisopropylamino)ethyl methacrylate (DPA) were grafted from the protein macroinitiator BSA-Br ( Fig. 3 ). The conditions identified for full macroinitiator consumption are summarized in Table 2 (Fig. S19–S24 ‡ ). To get insight into the kinetics of this oxygen-tolerant approach, the photoinduced grafting of NIPAM from BSA-Br (I o ) was further studied. In time course experiments performed under the conditions identified to be optimal (NIPAM/BSA-Br, I o /EY/TEMED = 2000/1/0.2/10, Fig. S19 ‡ ), the formation of biohybrids was apparent within the first 5 minutes of irradiation and full macroinitiator consumption could be achieved within 30 minutes. Importantly, when samples of the reaction mixture were withdrawn at fixed time points and studied with 1 H-NMR spectroscopy without purification, full monomer consumption was also seen after 60 minutes (Fig. S19 ‡ ). The lower critical solution temperature (LCST) of BSA-poly(NIPAM) was determined to be between 32.8 and 33 °C at sufficiently dilute concentrations and was found to be reversible ( Fig. 3 and Fig. S20 ‡ ). The spherical assemblies formed at temperatures higher than the LCST were imaged by SEM (Fig. S20 ‡ ). The response of BSA-poly(DPA) was also found to be reversible with the turning point determined to be at pH 5.8 ( Fig. 3 and Fig. S21 ‡ ). 90 Taking advantage of their response, both BSA-poly(NIPAM) and BSA-poly(DPA) could be effectively isolated from independently formed polymer chains, i.e. , by performing dialysis after phase transition while retaining the conditions required for the biopolymer to be hydrophilic (at 20 °C for BSA-poly(NIPAM) and at pH below 5.8 for BSA-poly(DPA)). BSA-poly(DPA) samples were collected before and after dialysis performed at pH 5.5 and analyzed with native PAGE (Fig. S22 † ). The dialysate was collected and the released polymer was isolated and characterized with 1 H-NMR spectroscopy (Fig. S22 ‡ ). Similar trends were observed in the synthesis of BSA-poly(DMAEMA) (Fig. S23 and S24 ‡ ).
Grafting multiple monomers
Protein-coated polymer nanoparticles.
We therefore performed styrene polymerization in the presence of native BSA without adding the ATRP initiator BSA-Br, I o . In PAGE electrophoresis, a band not migrating past the stacking gel front was predominant upon completion of the reaction while native BSA could also be detected (Fig. S28 ‡ ). After the dialysis step, the presence of both BSA and poly(styrene) was confirmed in the product mixture with FT-IR. The nanoparticles were visualized via FE-SEM imaging to be spherical with diameters varying between 10 and 50 nm ( Fig. 2D ). Despite numerous efforts, the protein could not be fully detached from the nanoparticles by simple means that would allow further characterization post polymerization. We therefore proceeded to synthesize responsive poly(DPA) nanoparticles in the presence of native BSA ( Fig. 4 and Fig. S23 ‡ ). To determine the nature of the produced nanoparticles, the product was characterized after synthesis and was then subjected to dialysis against phosphate buffer, pH 5.0, i.e. , below the turning point (5.8, Fig. 3 ). Both the product and the dialysate were characterized. As seen in native PAGE analyses of the samples collected before and after the phase transition, native BSA (pI 4.8–5.0) was liberated, leaving no trace of the band attributed to the nanocarrier (Fig. S22 ‡ ). Our initial assumption was further supported through the detection of poly(DPA) obtained by acquiring a 1 H-NMR spectrum of the dialysate (Fig. S22 ‡ ).
| Proposed pathways to produce BSA-polymer and BSA-coated polymer nanoparticles. | ||
Imaging with fluorescence microscopy
| (A) Left: imaging of BSA coated poly(styrene) with internal reflection fluorescence (TIRF) microscopy. Right: 3D intensity plot of one nanoparticle. (B) Activity of CALB-poly(styrene), polystyrene nanoparticles coated with CALB and native CALB. The graph depicts the slopes of the activity kinetics recorded at 20, 25 and 37 °C. | ||
Protein scope – synthesis of CALB-poly(styrene)
CALB catalyzes the hydrolysis of esters, converting triglycerides into glycerol and fatty acids, while being also one of the most used enzymes in biocatalysis with widespread applications. 94 5-(6)-Carboxyfluorescein diacetate (CFDA) was used to test in vitro the catalytic activity of the CALB-poly(styrene) biohybrids by monitoring the formation of the hydrolysis product carboxyl fluorescein (CF) at 453 nm. The biohybrids were shown to retain part of the catalytic activity of the parent enzyme.
CALB-coated polystyrene nanoparticles
The CALB-coated polystyrene nanoparticles were also found to retain part of the esterase activity of native CALB ( Fig. 5 ). Notably, the coated nanoparticles were proven to be remarkably stable as they retained their activity after storing for one year at 4 °C (Fig. S33, ‡ activity data not shown). To the best of our knowledge, this is the first time that such significant stability of such nanocarriers is being reported.
Experimental
Materials and methods, general polymerization protocol for the oxygen tolerant, ey/temed mediated grafting of monomers from protein macroinitiators, conclusions, author contributions, data availability, conflicts of interest, acknowledgements.
- R. A. Olson, A. B. Korpusik and B. S. Sumerlin, Chem. Sci. , 2020, 11 , 5142 RSC .
- M. S. Messina, K. M. Messina, A. Bhattacharya, H. R. Montgomery and H. D. Maynard, Prog. Polym. Sci. , 2020, 100 , 101186 CrossRef CAS .
- A. J. Russell, S. L. Baker, C. M. Colina, C. A. Figg, J. L. Kaar, K. Matyjaszewski, A. Simakova and B. S. Sumerlin, AIChE J. , 2018, 64 , 3230 CrossRef CAS .
- S. C. Tam, J. Blumenstein and K. Wong, Proc. Natl. Acad. Sci. U. S. A. , 1976, 73 , 2128 CrossRef CAS PubMed .
- A. Abuchowski, T. Van Es, N. C. Palczuk and F. F. Davis, J. Biol. Chem. , 1977, 252 , 3578 CrossRef CAS .
- F. M. Veronese, R. Largajolli, E. Boccù, C. A. Benassi and O. Schiavon, Appl. Biochem. Biotechnol. , 1985, 11 , 141 CrossRef CAS .
- J. M. Harris and R. B. Chess, Nat. Rev. Drug Discovery , 2003, 2 , 214 CrossRef CAS .
- E. M. Pelegri-O'Day, E. W. Lin and H. D. Maynard, J. Am. Chem. Soc. , 2014, 136 , 14323 CrossRef .
- S. N. Alconcel, A. S. Baas and H. D. Maynard, Polym. Chem. , 2011, 2 , 1442 RSC .
- A. M. Ramos-de-la-Peña and O. Aguilar, Int. J. Pept. Res. Ther. , 2019, 26 , 333 CrossRef .
- C. A. Stevens, K. Kaur and H.-A. Klok, Adv. Drug Delivery Rev. , 2021, 174 , 447 CrossRef CAS .
- P. Kiran, A. Khan, S. Neekhra, S. Pallod and R. Srivastava, Front. Med. Technol. , 2021, 3 , 676025 CrossRef PubMed .
- S. Bhattacharjee, W. G. Liu, W. H. Wang, I. Weitzhandler, X. H. Li, Y. Z. Qi, J. Y. Liu, Y. Pang, D. F. Hunt and A. Chilkoti, ChemBioChem , 2015, 16 , 2451 CrossRef CAS PubMed .
- A. S. Hoffman, Adv. Drug Delivery Rev. , 2013, 65 , 10 CrossRef CAS PubMed .
- Z. Liu, C. Dong, X. Wang, H. Wang, W. Li, J. Tan and J. Chang, ACS Appl. Mater. Interfaces , 2014, 6 , 2393 CrossRef CAS .
- F. Duan, W. Jin, T. Zhang, Y. Sun, X. Deng and W. Gao, Adv. Mater. , 2023, 2209765 CrossRef CAS PubMed .
- X. Huang, M. Li, D. Green, D. S. Williams, A. J. Patil and S. Mann, Nat. Commun. , 2013, 4 , 2239 CrossRef PubMed .
- J. M. Hoffman, P. S. Stayton, A. S. Hoffman and J. J. Lai, Bioconjugate Chem. , 2015, 26 , 29–38 CrossRef CAS PubMed .
- K. Velonia, A. E. Rowan and R. J. M. Nolte, J. Am. Chem. Soc. , 2002, 124 , 4224 CrossRef CAS PubMed .
- M. J. Boerakker, J. M. Hannink, P. H. H. Bomans, P. M. Frederik, R. J. M. Nolte, E. M. Meijer and N. A. J. M. Sommerdijk, Angew. Chem., Int. Ed. , 2002, 41 , 4239 CrossRef CAS .
- C. Bao, Y. Yin and Q. Zhang, Biomacromolecules , 2018, 19 , 1539 CrossRef CAS ; C. Y. Bao and Q. Zhang, Eur. Polym. J. , 2019, 112 , 263–272 CrossRef ; C. W. Chiang, X. Liu, J. Sun, J. Guo, L. Tao and W. Gao, Nano Lett. , 2020, 20 , 1383 CrossRef PubMed .
- G. Mantovani, F. Lecolley, L. Tao, D. M. Haddleton, C. Clerx, J. J. L. M. Cornelissen and K. Velonia, J. Am. Chem. Soc. , 2005, 127 , 2966 CrossRef CAS .
- Y. Wang and C. Wu, Biomacromolecules , 2018, 19 , 1804 CrossRef CAS .
- K. Velonia, Polym. Chem. , 2010, 1 , 944 RSC .
- C. A. Lackey, N. Murthy, O. W. Press, D. A. Tirrell, A. S. Hoffman and P. S. Stayton, Bioconjugate Chem. , 1999, 10 , 401 CrossRef CAS PubMed .
- S. Kulkarni, C. Schilli, A. Müller, A. Hoffman and P. Stayton, Bioconjugate Chem. , 2004, 15 , 747 CrossRef CAS PubMed .
- X. Wan, G. Zhang, Z. Ge, R. Narain and S. Liu, Chem. – Asian J. , 2011, 6 , 2835 CrossRef CAS .
- K. Parkatzidis, H. S. Wang, N. P. Truong and A. Anastasaki, Chem , 2020, 7 , 1575 Search PubMed .
- B. Kaupbayeva and A. J. Russell, Prog. Polym. Sci. , 2020, 101 , 101194 CrossRef CAS .
- X. Liu and W. Gao, Angew. Chem., Int. Ed. , 2021, 60 , 2 CrossRef .
- M. Heredero and A. Beloqui, ChemBioChem , 2023, 24 , e202200611 CrossRef CAS .
- M. J. Tamasi, R. A. Patel, C. H. Borca, S. Kosuri, H. Mugnier, R. Upadhya, N. S. Murthy, M. A. Webb and A. J. Gormley, Adv. Mater. , 2022, 34 , 2201809 CrossRef CAS .
- K. L. Heredia, D. Bontempo, T. Ly, J. T. Byers, S. Halstenberg and H. D. Maynard, J. Am. Chem. Soc. , 2005, 127 , 16955 CrossRef CAS PubMed .
- B. Le Droumaguet and K. Velonia, Angew. Chem., Int. Ed. , 2008, 47 , 6263 CrossRef CAS .
- H. Murata, C. S. Cummings, R. R. Koepsel and A. J. Russell, Biomacromolecules , 2014, 15 , 2817 CrossRef CAS PubMed .
- D. Cohen-Karni, M. Kovaliov, T. Ramelot, D. Konkolewicz, S. Graner and S. Averick, Polym. Chem. , 2017, 8 , 3992 RSC .
- C. Bao, J. Chen, D. Li, A. Zhang and Q. Zhang, Polym. Chem. , 2020, 11 , 1386 RSC .
- A. Theodorou, E. Liarou, D. M. Haddleton, I. G. Stavrakaki, P. Skordalidis, R. Whitfield, A. Anastasaki and K. Velonia, Nat. Commun. , 2020, 11 , 1486 CrossRef CAS PubMed .
- A. Theodorou, D. Gounaris, E. Voutyritsa, N. Andrikopoulos, C. I. M. Baltzaki, A. Anastasaki and K. Velonia, Biomacromolecules , 2022, 23 , 4241 CrossRef CAS PubMed .
- E. Voutyritsa, C. Gryparis, A. Theodorou and K. Velonia, Macromol. Rapid Commun. , 2023, 2200976 CrossRef CAS PubMed .
- Q. Zhang, M. Li, C. Zhu, G. Nurumbetov, Z. Li, P. Wilson, K. Kempe and D. M. Haddleton, J. Am. Chem. Soc. , 2015, 137 , 9344 CrossRef CAS .
- Y. Liu, T. K. Nevanen, A. Paananen, K. Kempe, P. Wilson, L. S. Johansson, J. J. Joensuu, M. B. Linder, D. M. Haddleton and R. Milani, ACS Appl. Mater. Interfaces , 2019, 11 , 3599 CrossRef CAS .
- J. Lu, H. Wang, Z. Tian, Y. Hou and H. Lu, J. Am. Chem. Soc. , 2020, 142 , 1217 CrossRef CAS .
- C. Bao, X. Xu, J. Chenvand and Q. Zhang, Polym. Chem. , 2020, 11 , 682 RSC .
- J. Liu, V. Bulmus, D. L. Herlambang, C. Barner-Kowollik, M. H. Stenzel and T. P. Davis, Angew. Chem., Int. Ed. , 2007, 46 , 3099 CrossRef CAS .
- J. Xu, K. Jung, N. A. Corrigan and C. Boyer, Chem. Sci. , 2014, 5 , 3568 RSC .
- M. Kovaliov, M. L. Allegrezza, B. Richter, D. Konkolewicz and S. Averick, Polymer , 2018, 137 , 338 CrossRef CAS .
- A. E. Enciso, L. Fu, A. J. Russell and K. Matyjaszewski, Angew. Chem., Int. Ed. , 2018, 57 , 933 CrossRef CAS PubMed .
- J. Yeow, R. Chapman, A. J. Gormley and C. Boyer, Chem. Soc. Rev. , 2018, 47 , 4357 RSC .
- L. Fu, Z. Wang, S. Lathwal, A. E. Enciso, A. Simakova, S. R. Das, A. J. Russell and K. Matyjaszewski, ACS Macro Lett. , 2018, 7 , 1248 CrossRef CAS PubMed .
- Y. Sun, S. Lathwal, Y. Wang, L. Fu, M. Olszewski, M. Fantin, A. E. Enciso, G. Szczepaniak, S. Das and K. Matyjaszewski, ACS Macro Lett. , 2019, 8 , 603 CrossRef CAS .
- A. Theodorou, P. Mandriotis, A. Anastasaki and K. Velonia, Polym. Chem. , 2021, 12 , 2228 RSC .
- X. Pan, M. Fantin, F. Yuan and K. Matyjaszewski, Chem. Soc. Rev. , 2018, 47 , 5457 RSC .
- A. Anastasaki, V. Nikolaou, Q. Zhang, J. Burns, S. R. Samanta, C. Waldron, A. J. Haddleton, R. McHale, D. Fox, V. Percec, P. Wilson and D. M. Haddleton, J. Am. Chem. Soc. , 2014, 136 , 1141 CrossRef CAS .
- N. D. Dolinski, Z. A. Page, E. H. Discekici, D. Meis, I. H. Lee, G. R. Jones, R. Whitfield, X. Pan, B. G. McCarthy, S. Shanmugam, V. Kottisch, B. P. Fors, C. Boyer, G. M. Miyake, K. Matyjaszewski, D. M. Haddleton, J. R. de Alaniz, A. Anastasaki and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem. , 2019, 57 , 268 CrossRef CAS PubMed .
- S. Dadashi-Silab, I.-H. Lee, A. Anastasaki, F. Lorandi, B. Narupai, N. D. Dolinski, M. L. Allegrezza, M. Fantin, D. Konkolewicz, C. J. Hawker and K. Matyjaszewski, Macromolecules , 2020, 53 , 5280 CrossRef CAS .
- D. A. Corbin and G. M. Miyake, Chem. Rev. , 2022, 122 , 1830 CrossRef CAS PubMed .
- C. Kutahya, F. S. Aykac, G. Yilmaz and Y. Yagci, Polym. Chem. , 2016, 7 , 6094 RSC .
- G. Yilmaz and Y. Yagci, Polym. Chem. , 2018, 9 , 1757 RSC .
- S. de Ávila Gonçalves, P. R. Rodrigues and R. Pioli Vieira, Macromol. Rapid Commun. , 2021, 42 , e2100221 CrossRef PubMed .
- B. S. Tucker, M. L. Coughlin, C. A. Figg and B. S. Sumerlin, ACS Macro Lett. , 2017, 6 , 452 CrossRef CAS PubMed .
- R. A. Olson, J. S. Levi, G. M. Scheutz, J. J. Lessard, C. A. Figg, M. N. Kamat, K. B. Basso and B. S. Sumerlin, Macromolecules , 2021, 54 , 4880 CrossRef CAS .
- K. Kapil, A. M. Jazani, G. Szczepaniak, H. Murata, M. Olszewski and K. Matyjaszewski, Macromolecules , 2023, 56 , 2017 CrossRef CAS PubMed .
- T. Zhang, Z. Wu, G. Ng and C. A. Boyer, Angew. Chem., Int. Ed. , 2023, e202309582 CAS .
- C. Kutahya, F. C. Aykac, G. Yilmaz and Y. Yagci, Polym. Chem. , 2016, 7 , 6094 RSC .
- K. Parkatzidis, N. P. Truong, M. N. Antonopoulou, R. Whitfield, D. Konkolewicz and A. Anastasaki, Polym. Chem. , 2020, 11 , 4968 RSC .
- V. Bellotti, K. Parkatzidis, H. S. Wang, N. D. A. Watuthanthrige, M. Orfano, A. Monguzzi, N. P. Truong, R. Simonutti and A. Anastasaki, Polym. Chem. , 2023, 14 , 253 RSC .
- V. Srivastava and P. P. Singh, RSC Adv. , 2017, 7 , 31377 RSC .
- S. Shanmugam, S. Xu, N. N. M. Adnan and C. Boyer, Macromolecules , 2018, 51 , 779 CrossRef CAS .
- G. Szczepaniak, J. Jeong, K. Kapil, S. Dadashi-Silab, S. S. Yerneni, P. Ratajczyk, S. Lathwal, D. J. Schild, S. R. Das and K. Matyjaszewski, Chem. Sci. , 2022, 13 , 11540 RSC .
- K. Kapil, G. Szczepaniak, M. R. Martinez, H. Murata, A. M. Jazani, J. Jeong, S. R. Das and K. Matyjaszewski, Angew. Chem., Int. Ed. , 2023, e202217658 CAS .
- A. Cvetkovic, A. J. J. Straathof, R. Krishna and L. A. M. van der Wielen, Langmuir , 2005, 21 , 1475 CrossRef CAS PubMed .
- J. Mariam, S. Sivakami and P. M. Dongre, Drug Delivery , 2016, 23 , 2668 CrossRef CAS .
- J.-P. Fouassier, F. Morlet-Savary, J. Lalevée, X. Allonas and C. Ley, Materials , 2010, 3 , 5130 CrossRef CAS PubMed .
- E. Liarou, R. Whitfield, A. Anastasaki, N. G. Engelis, G. R. Jones, K. Velonia and D. M. Haddleton, Angew. Chem., Int. Ed. , 2018, 57 , 8998 CrossRef CAS .
- E. Liarou, A. Anastasaki, R. Whitfield, C. E. Iacono, G. Patias, N. G. Engelis, A. Marathianos, G. R. Jones and D. M. Haddleton, Polym. Chem. , 2019, 10 , 963 RSC .
- E. Liarou, Y. Han, A. M. Sanche, M. Walker and D. M. Haddleton, Chem. Sci. , 2020, 11 , 5257 RSC .
- N. A. Swisher, D. A. Corbin and G. M. Miyake, ACS Macro Lett. , 2021, 10 , 453 CrossRef CAS PubMed .
- Y. Lee, C. Boyer and M. S. Kwon, Chem. Soc. Rev. , 2023, 52 , 3035 RSC .
- B. Nomeir, O. Fabre and K. Ferji, Macromolecules , 2019, 52 , 6898 CrossRef CAS .
- Z. Liang, S. Xu, W. Tian and R. Zhang, Beilstein J. Org. Chem. , 2015, 11 , 425 CrossRef CAS PubMed .
- B. Cornils, W. A. Herrmann, J.-H. Xu and H.-W. Zanthoff, Catalysis from A to Z: a concise encyclopedia , John Wiley and Sons, Weinheim, 2020. DOI: 10.1002/9783527809080 .
- N. El Achi, Y. Bakkour, W. Adhami, J. Molina, M. Penhoat, N. Azaroual, L. Chausset-Boissarie and C. Rolando, Front. Chem. , 2020, 8 , 740 CrossRef CAS .
- D. Bondarev, K. Borská, M. Šoral, D. Moravčíková and J. Mosnáček, Polymer , 2019, 161 , 122 CrossRef CAS .
- F. Lorandi and K. Matyjaszewski, Isr. J. Chem. , 2020, 60 , 108 CrossRef CAS .
- P. G. Falireas, V. Ladmiral, A. Debuigne, C. Detrembleur, R. Poli and B. Ameduri, Macromolecules , 2019, 52 , 1266 CrossRef CAS .
- M. Teodorescu and M. Bercea, Polym.-Plast. Technol. Eng. , 2015, 54 , 923 CrossRef CAS .
- A. Oucif, N. Haddadine, D. Zakia, N. Bouslah, A. Benaboura, K. Beyaz, B. Guedouar and M. S. El-Shall, Polym. Bull. , 2022, 79 , 153 CrossRef .
- L. Papadimitriou, A. Theodorou, M. Papageorgiou, E. Voutyritsa, A. Papagiannaki, K. Velonia and A. Ranella, J. Drug Delivery Sci. Technol. , 2022, 75 , 103591 CrossRef CAS .
- H. D. Pinholt, S. S.-R. Bohr, J. F. Iversen, W. Boomsma and N. S. Hatzakis, Proc. Natl. Acad. Sci. U. S. A. , 2021, 31 , 118 Search PubMed .
- S. S.-R. Bohr, P. M. Lund, A. S. Kallenbach, H. Pinholt, J. Thomsen, L. Iversen, A. Svendsen, S. M. Christensen and N. S. Hatzakis, Sci. Rep. , 2019, 9 , 2654 CrossRef .
- H. Zhao, E. Ibarboure, V. Ibrahimova, Y. Xiao, E. Garanger and S. Lecommandoux, Adv. Sci. , 2021, 8 , 2102508 CrossRef CAS PubMed .
- F. Akram, A. S. Mir, I. u. Haq and A. Roohi, Mol. Biotechnol. , 2022, 65 , 521 Search PubMed .
- M. Skjøt, L. De Maria, R. Chatterjee, A. Svendsen, S. A. Patkar, P. R. Østergaard and J. Brask, ChemBioChem , 2009, 10 , 520 CrossRef .
| † Dedicated to the memory of Prof. R. J. M. Nolte, whose seminal contributions to the field and mentorship shaped our academic journeys. |
| ‡ Electronic supplementary information (ESI) available. See DOI: . Dedicated to the memory of Prof. R. J. M. Nolte, whose seminal contributions to the field and mentorship shaped our academic journeys. |
| § These authors contributed equally to this work. |

IMAGES
VIDEO
COMMENTS
The synthesis of copper nanoparticles is a difficult task for a researcher to find the best valuable points, such as pH, temperature, concentration ratio and time. Time plays an important role in the synthesis of nanoparticles. In some cases, a color change occurs within 30 min, while sometimes it takes up to 48 h.
The green synthesis of copper nanoparticles (CuNPs) using a leaf extract from Jatropha curcas (JC) has been documented in our present research work. The existence of flavonoids, tannins, glycosides, and alkaloids was confirmed by the phytochemical analysis of the plant extract and these chemicals can be used as reducing, stabilizing and capping agents.
2. Green synthesis of nanoparticles. Green synthesis can be defined as the derivation of materials from green or eco-friendly resources by the use of solvent, good reducing agent, and harmless material for stabilization (Citation 37).Additionally, this synthesis route is straightforward, cost-effective, dependable, sustainable, and relatively repeatable, and results in more stable compounds.
The use of biomaterials in the synthesis of nanoparticles is one of the most up-to-date focuses in modern nanotechnologies and nanosciences. More and more research on green methods of producing metal oxide nanoparticles (NP) is taking place, with the goal to overcome the possible dangers of toxic chemicals for a safe and innocuous environment. In this study, we synthesized copper nanoparticles ...
Today, different types of nanoparticles (NPs) are being synthesized and used for medical and agricultural applications. In this study, copper nanoparticles (CuNPs) were synthesized using the aqueous extract of mint (Mentha longifolia L.). For the characterization of CuNPs, UV-visible spectroscopy, scanning electron microscopy, X-ray diffraction, and Fourier transform infrared spectrometry were ...
Top-Down Synthesis breaks intermolecular connections to reduce bulk materials to nanoparticles. Physical or chemical erosion or biological disintegration may do this (Bashir and Liu, 2015).Bottom-Up Synthesis assembles nanostructures atom-by-atom or molecule-by-molecule, frequently by self-assembly (Majumder et al., 2007).Top-down procedures can make nano and microscale materials, whereas ...
Abstract. With the increasing concern over the environmental impact of conventional chemical methods, environmentally friendly processes, commonly known as green chemistry, for the synthesis of nanoparticles have gained growing interest in the field of nanobiotechnology. This review focuses on synthesis of metallic nanoparticles (NPs) based on ...
Volume 65, Issue 1, 2021 Journal of Scientific Research Institute of Science, Banaras Hindu University, Varanasi, India. 10.37398/JSR.2021.650111 DOI: 80 Abstract: synthesized including their density, crystal structure and the The green nanotechnology is generating interest in researchers for the synthesis of nanoparticle in a simple, cost
In recent ages, green nanotechnology has gained attraction in the synthesis of metallic nanoparticles due to their cost-effectiveness, simple preparation steps, and environmentally-friendly. In ...
Green synthesis of copper nanoparticles. The copper nanoparticles were synthesized using the method described by Ullah, N. et al., 2020 with minor modifications show in Fig. 1 [15]. In brief, the copper nanoparticles were created by dissolving 5 g of CuSO4·.5H2O in 50 mL of distilled water. A 250-mL beaker was filled with an equal volume of ...
To summarise for the first time, green synthesis of copper nanoparticles (CuNPs) using Nigella sativa seeds' extract of different concentrations (5%, 6%, 8%, and 10%) were successfully prepared through green methods which showed several benefits, such as easy availability, eco-friendliness, non-toxicity, and economic feasibility. The proposed ...
In conclusion, this study presented the eco-friendly, cost-effective green synthesis of copper nanoparticles using W. somnifera aqueous root extract as a reducing agent in the presence of copper nitrate. Though the preliminary confirmation of the formation of CuNPs was the change in solution color, UV-Vis spectroscopy confirmed its formation ...
Abstract and Figures. Copper and Copper oxide nanoparticles have garnered a lot of attention among the metal oxide nanoparticles, especially because of their many characteristics and applications ...
In recent years, the green synthesis of different metal nanoparticles has become a substantial technique for the synthesis of different essential nanoparticles and their potential applications in technological, industrial along with biomedical fields. Among the several essential nanoparticles, copper nanoparticles (CuNPs) have attracted enormous attention for their wide range of applications ...
Green synthesis of copper nanoparticles was initially confirmed by the position of SPR band at 340 nm in UV-Vis spectra. The particle size of about 20 nm with a spherical shape. XRD spectrum shows crystalline nanostructured copper particles and AFM techniques illustrate the spherical shape of nanoparticles with aggregation. TEM image shows poly ...
The plant extracts of Azadiracta indica, Lantana camera, Calotropis procera and Tridax procumbens was successfully used for Synthesis of copper nanoparticles. The synthesized copper nanoparticles from plant extracts showed the signatory colour then optical absorbance was recorded by UV Visible spectrophotometer in 24 and 48 hrs.
Development of eco-friendly Green synthesis of copper nanoparticles (CuNPs) is an important aspect in the field of nanotechnology and is also inexpensive method for nanoparticles biosynthesis. Recently the utilization of secondary metabolites from plant leaf extract has emerged as a novel technology for the synthesis of various nanoparticles.
Green synthesis of Cu/CuO-NPs by using fungi. Various fungal species have been utilized to synthesize copper oxide and other metal nanoparticles in recent years (127). Fungi, as compared to other microbes, have a lot of potential for nanoparticle production. In comparison to bacteria, fungi tolerate agitation, ow. fl.
green was observed, indicating the fo rmation of. copper nanoparticles, (CuNPs). This colour. change is as a result of interaction between. conduction electrons of metal NPs and incident. photons ...
The green methodologies of nanoparticles with plant extracts have received an increase of interest. Copper oxide nanoparticles (CuO NPs) have been utilized in a many of applications in the last ...
In solar panels, copper is the second-most-valuable metal after silver. We propose an innovative method to recycle copper from waste solar panels and convert it into copper oxide nanoparticles (CuONPs) using a green synthesis method. Synthesizing CuONPs is advantageous due to their large surface area compared to bulk material.
2.1. Plant mediated synthesis of copper oxide nanoparticles. Plant extracts have been widely used for the production of copper oxide nanoparticles [42], [43], [44].Even with the different advantages of the fabrication of copper oxide nanoparticles from bacteria, algae, and fungi, there are some limitations to using these organisms [45], [46].The toxicity of bacteria, isolation of the ...
Facile Synthesis of Sodium Alginate (SA)-Based Quaternary Bio-Nanocomposite (SA@Co-Zn-Ce) for Antioxidant Activity and Photocatalytic Degradation of Reactive Red 24 ... Kim, Y.; Lee, J.; Kim, B.S. Green Synthesis of Copper Oxide Nanoparticles from Waste Solar Panels Using Piper nigrum Fruit Extract and Their Antibacterial Activity. Catalysts ...
Copper oxide nanoparticles (CuO NPs) will accumulate in soil and water due to human and natural activities, eventually finding their way into the human body through direct or indirect pathways. ... This process leads to the inhibition of mitochondrial membrane lipid synthesis, resulting in mitochondrial damage and disruption of intracellular ...
Today, different types of nanoparticles (NPs) are being synthesized and used for medical and agricultural applications. In this study, copper nanoparticles (CuNPs) were synthesized using the aqueous extract of mint (Mentha longifolia L.).For the characterization of CuNPs, UV-visible spectroscopy, scanning electron microscopy, X-ray diffraction, and Fourier transform infrared spectrometry were ...
2.4.1 Mechanism for the green synthesis of ZnO NPs. Phytochemicals in plant extracts can reduce metal precursors to nanoparticles, acting as both reducing and stabilizing agents. Calendula officinalis leaf extract contains numerous phytochemicals including triterpenes, Flavonoids, Phenolic compounds, coumarins, and carotenoids, which play a significant role in the reduction of metal ions.
@article{Batool2024SynthesisAC, title={Synthesis and comparison of different metal-doped ZnO nanoparticles for catalytic degradation and adsorptive removal of direct orange: kinetics, isotherms, and thermodynamics}, author={Anam Batool and Ruba Munir and Nageen Mushtaq and Saima Noreen}, journal={Journal of Sol-Gel Science and Technology}, year ...
Green chemistry was used to synthesize copper nanoparticles (CuNPs), using an aqueous extract of Rosa 'Andeli' double delight fresh petals (RAFE) or Gardenia jasminoides Ellis fresh leaves (GJLE), as stabilizing agents. The effect of the RAFE and GJLE on the CuNPs morphology, size, polydispersity, density of dislocations (δ), and their antibacterial activity against S. aureus and E. coli ...
By way of the sol-gel chemical synthesis method, it is possible to synthesize SiO2 nanoparticles with a defined specific particle size, a surface area, and a defined crystal structure that can be effectively used as a nanoadsorbent to remove various organic dyes. SiO2 nanoparticles were synthesized by the sol-gel method using sodium silicate (Na2SiO3) by a green method without using a ...
Oxygen-tolerant, eosin Y mediated synthesis of protein-polymer biohybrids and protein-coated polymer nanoparticles† ‡. Errika Voutyritsa§ abc, Thomai Lazou§ a, Jonida Bushi§ a, Stavroula Margaritaki a, Myrto Charitaki a, Sune M. Christensen d, Nikos S. Hatzakis bc and Kelly Velonia * a a Department of Materials Science and Engineering, University of Crete, University Campus Voutes ...