Cannabis (Marijuana) Research Report Is marijuana safe and effective as medicine?
The potential medicinal properties of marijuana and its components have been the subject of research and heated debate for decades. THC itself has proven medical benefits in particular formulations. The U.S. Food and Drug Administration (FDA) has approved THC-based medications, dronabinol (Marinol ® ) and nabilone (Cesamet ® ), prescribed in pill form for the treatment of nausea in patients undergoing cancer chemotherapy and to stimulate appetite in patients with wasting syndrome due to AIDS.
In addition, several other marijuana-based medications have been approved or are undergoing clinical trials. Nabiximols (Sativex ® ), a mouth spray that is currently available in the United Kingdom, Canada, and several European countries for treating the spasticity and neuropathic pain that may accompany multiple sclerosis, combines THC with another chemical found in marijuana called cannabidiol (CBD).
The FDA also approved a CBD-based liquid medication called Epidiolex ® for the treatment of two forms of severe childhood epilepsy, Dravet syndrome and Lennox-Gastaut syndrome. It’s being delivered to patients in a reliable dosage form and through a reproducible route of delivery to ensure that patients derive the anticipated benefits. CBD does not have the rewarding properties of THC.
Researchers generally consider medications like these, which use purified chemicals derived from or based on those in the marijuana plant, to be more promising therapeutically than use of the whole marijuana plant or its crude extracts. Development of drugs from botanicals such as the marijuana plant poses numerous challenges. Botanicals may contain hundreds of unknown, active chemicals, and it can be difficult to develop a product with accurate and consistent doses of these chemicals. Use of marijuana as medicine also poses other problems such as the adverse health effects of smoking and THC-induced cognitive impairment. Nevertheless, a growing number of states have legalized dispensing of marijuana or its extracts to people with a range of medical conditions.
An additional concern with "medical marijuana" is that little is known about the long-term impact of its use by people with health- and/or age-related vulnerabilities—such as older adults or people with cancer, AIDS, cardiovascular disease, multiple sclerosis, or other neurodegenerative diseases. Further research will be needed to determine whether people whose health has been compromised by disease or its treatment (e.g., chemotherapy) are at greater risk for adverse health outcomes from marijuana use.

Medical Marijuana Laws and Prescription Opioid Use Outcomes
A 2019 analysis, also funded by NIDA, re-examined this relationship using data through 2017. Similar to the findings reported previously, this research team found that opioid overdose mortality rates between 1999-2010 in states allowing medical marijuana use were 21% lower than expected. When the analysis was extended through 2017, however, they found that the trend reversed, such that states with medical cannabis laws experienced an overdose death rate 22.7% higher than expected. 79 The investigators uncovered no evidence that either broader cannabis laws (those allowing recreational use) or more restrictive laws (those only permitting the use of marijuana with low tetrahydrocannabinol concentrations) were associated with changes in opioid overdose mortality rates.
These data, therefore, do not support the interpretation that access to cannabis reduces opioid overdose. Indeed, the authors note that neither study provides evidence of a causal relationship between marijuana access and opioid overdose deaths. Rather, they suggest that the associations are likely due to factors the researchers did not measure, and they caution against drawing conclusions on an individual level from ecological (population-level) data. Research is still needed on the potential medical benefits of cannabis or cannabinoids.
ORIGINAL RESEARCH article
Cannabis for medical use: analysis of recent clinical trials in view of current legislation.

- Department of Drug Science and Technology, University of Turin, Turin, Italy
Cannabis has long been regarded as a recreational substance in the Western world. The recent marketing authorization of some medicinal products of industrial origin and the introduction onto the market of inflorescences for medical use mean that medical doctors can now prescribe Cannabis -based medicines in those countries which allow it. Nevertheless, there is still considerable controversy on this topic in the scientific community. In particular, this controversy concerns: the plant species to be used; the pathologies that can be treated and consequently the efficacy and safety of use; the routes of administration; the methods of preparation; the type and dosage of cannabinoids to be used; and, the active molecules of interest. As such, although medical Cannabis has been historically used, the results of currently completed and internationally published studies are inconclusive and often discordant. In light of these considerations, the aim of this work is to analyse the current legislation in countries that allow the use of medical Cannabis , in relation to the impact that this legislation has had on clinical trials. First of all, a literature search has been performed (PubMed and SciFinder) on clinical trials which involved the administration of Cannabis for medical use over the last 3 years. Of the numerous studies extrapolated from the literature, only about 43 reported data on clinical trials on medical Cannabis , with these mainly being performed in Australia, Brazil, Canada, Denmark, Germany, Israel, Netherlands, Switzerland, the United Kingdom and the United States of America. Once the reference countries were identified, an evaluation of the legislation in relation to Cannabis for medical use in each was carried out via the consultation of the pertinent scientific literature, but also of official government documentation and that of local regulatory authorities. This analysis provided us with an overview of the different legislation in these countries and, consequently, allowed us to analyse, with greater awareness, the results of the clinical trials published in the last 3 years in order to obtain general interest indications in the prosecution of scientific research in this area.
1 Introduction
Cannabis was widely used in the past for its curative properties. The earliest records of its medicinal use date back to China where Cannabis has been cultivated for millennia for use as a fiber, food, and medicine. Over time, it spread to the whole of Asia, the Middle East, and Africa. In the West, the plant started to attract scientific interest only in the 20th century. However, in the last century, the cultivation, sale, and use of Cannabis was made illegal in the majority of countries ( Lafaye, et al., 2017 ; Pisanti and Bifulco, 2019 ; Romano and Hazekamp, 2019 ; Arias, et al., 2021 ).
In the last few decades, there has been revived support for its decriminalisation, and legalisation for medical uses thanks to new and scientifically founded indications of its potential therapeutic value. This is partly due to the support gained in the media, and to the high expectations for its efficacy, even though these hopes, for many diseases, are not sufficiently supported by scientific research ( Hill, 2015 ; Whiting, et al., 2015 ).
The phytocomplex of Cannabis plants is made up of more than 500 molecules, of which about a hundred belong to the Cannabinoid chemical class. Among these molecules, even small variations in molecular structure can produce significantly different effects. The molecules of greatest interest to pharmacologists are the decarboxylated forms of 9-tetrahydracannabinol (THC) and cannabidiol since these are easily absorbed in the intestine ( Grotenhermen, 2003 ; Gould, 2015 ; Baratta, et al., 2019 ; Baratta, et al., 2021 ).
Recently, Cannabis based industrial medicines have been approved for sale, and medical use inflorescences have been made available. This has given medical doctors, in those countries which allow it, the option to prescribe Cannabis -based products. At present, the most widely available products are: Marinol ® (AbbVie Inc) and Syndros ® (Benuvia Therapeutics) which contain dronabinol, an isomer of delta-9-tetrahydrocannabinol; Cesamet ® based on nabilone (Meda Pharmaceuticals Inc.), another synthetic cannabinoid; Sativex ® (GW Pharma Ltd.), based on an ethanol extraction of Cannabis sativa ; and Epidiolex ® 1 (Greenwich Biosciences), which contains CBD ( Casiraghi, et al., 2018 ).
A variety of pharmaceutical-grade inflorescence products are also available on the market. Usually, the label only indicates the concentrations of THC and CBD. This is a critical point as the phytocomplex of medical Cannabis contains many active molecules which contribute to the “Entourage effect,” a hypothesis postulating a positive synergic action between cannabinoids and terpenes ( Stella, et al., 2021 ; Baratta, et al., 2022 ).
Given the increasing availability of the above products, many countries have introduced specific legislation, regulations, and guidelines regarding the use of medical use Cannabis in the treatment of various pathologies. Nevertheless, debate continues around this subject within the scientific community. The main points of contention are the correct plant varieties to be used, the pathologies to be treated, and, consequently, the efficacy and safety of their use. There are no universally shared indications on the optimum administration route, the preparation methodology, the definitive types of cannabinoids and dosages to recommend, or even the identity of the active molecule of interest. This controversy stems in large part from the findings of the clinical trials conducted till now. Although the number of studies and publications is growing rapidly, for many diseases the results are often contradictory or inconclusive. All too often, these trials were performed on a non-homogeneous population, and utilising diverse plant material, extraction methods, dosages, pharmaceutical forms, and administration routes. Moreover, the trials were often conducted without a control group ( Stella, et al., 2021 ).
In light of all these considerations, the objective of this work is to analyse the current legislation and regulations in a number of countries where medical use Cannabis is permitted in order to evaluate any relationship of these on the design of clinical trials carried out there.
2 Materials and Methods
We carried out a literature search (PubMed and SciFinder) for clinical trials with medical Cannabis published in the last 3 years (2019/01/01–2021/12/15). We excluded literature reviews, non-clinical trials, and articles about non-medical use Cannabis . We also considered published articles about clinical trial protocols to be carried out. The key search terms used were clinical trials, medical Cannabis , and medical use.
After the publications had been selected, the countries of origin were identified in order to perform an evaluation of the current regulations in each regarding medical Cannabis . The scientific literature, and relevant official publications from government and local authorities were consulted for this analysis.
Finally, the characteristics and the results of the clinical studies were analysed to evaluate any possible link to the state legislation where the studies had been carried out.
Of the 400 matches from the literature search, only 10% (43) of the publications reported data from trials or clinical protocols regarding medical Cannabis . The relevant trials were carried out in: Australia, Brazil, Canada, Denmark, Germany, Israel, Netherlands, Switzerland, the United Kingdom, and the United States of America. Given their geographical distribution, these countries can be considered of interest despite the small number of studies available.
For each of the countries in question, the current legislation on medical Cannabis was analysed, and some specific features are reported such as: prescription procedure, indicated pathologies for medical Cannabis , products available for sale, dispensation forms, authorisation to grow Cannabis for medical use, and reimbursement procedure.
3.1 Current Legislation
3.1.1 australia.
Although there are some regulatory differences among the federal states regarding the importation of products, and the qualification required to write a prescription, medical Cannabis may be prescribed after receiving authorisation from the Therapeutic Goods Administration, through the Special Access Scheme for an individual patient, or through the Authorized Prescriber Scheme for a group of patients with the same condition. Products of industrial origin are exempt from these schemes as approval for sale has already been granted (Sativex ® and Epidiolex ® ).
As well as Sativex ® and Epidiolex ® , indicated for the treatment of spasticity in multiple sclerosis and paediatric epilepsy, herbal- Cannabis based products may also be prescribed. The most common conditions are spasticity in multiple sclerosis, nausea or vomiting caused by anti-tumoral chemotherapy, pain or anxiety in patients with terminal diseases, and refractory child epilepsy. The physician may in any case write a prescription for pathologies other than those indicated.
Pharmacies are authorised to dispense medical Cannabis -based products.
The cost of the therapy is not subsidised by the government.
Alcohol and Drug Foundation, 2021 ; Australian Capital Territory Government, 2021 ; Australian Government, 2017a ; Australian Government, 2017b ; Australian Government, 2018 ; Australian Government, 2020 ; Australian Government, 2021 ; Australian Institute of Health and Welfare, 2019 ; Castle, et al., 2019 ; Centre for Medicinal Cannabis Research and Innovation, 2021 ; Health Direct, 2019 ; Mersiades, et al., 2019 ; The Health Products Regulatory Authority, 2017 ; The Office of Drug Control, 2021 )
3.1.2 Brazil
Various products of industrial origin are available such as Epidiolex ® and Sativex ® , and the importation of Cannabis -derived products is generally authorised. However, the importation of the raw plant or parts of the plant is not permitted. Products with a concentration of THC greater than 0.2% may only be prescribed when no alternative therapy is available, and the patient has reached the irreversible or terminal stage of their disease. Prescription is under the responsibility of the prescribing medical doctor. The medication may be taken either orally or by inhalation.
The cost of the treatment is generally high and is completely at the patient’s expense.
The dispensation may take place in a pharmacy, where Cannabis may not be processed, however.
( Crippa, et al., 2018 ; Marketrealist, 2019 ; Ministério da Saúde, 2019 ; Reuters, 2019 ; Brazilian Government, 2021 )
3.1.3 Canada
The situation in Canada is quite different, medical Cannabis (with the exception of approved industrial products) is not considered as a medicine; hence, it is not dispensed in pharmacies. Medical doctors or nurses may prescribe it for individual patients. The patient can then acquire it from a licensed vendor; grow a quantity sufficient for personal use in residence after registering with the Ministry for Health; nominate a grower in their place (a grower can only cultivate for two people); or acquire it from a provincial or area level licensed retailer. The patient is allowed to prepare Cannabis -based products, but the use of organic solvents such as butane, benzene, methyl-chloride, or chlorinated hydrocarbons is forbidden.
Regarding industrial products, Sativex ® is available for sale; it is indicated for the treatment of spasticity in multiple sclerosis. Other recommended uses include additional pain relief for neuropathic pain in adult patients with multiple sclerosis, and additional pain relief for patients with late-stage cancer who experience moderate to serious pain when already undergoing palliative care with the highest tolerable dosages of opioids. Nabilone is approved for treatment of serious nausea and vomiting associated with chemotherapy, while dronabinol is approved for the treatment of AIDS-related anorexia, and for serious nausea and vomiting associated with chemotherapy. Dronabinol was withdrawn for the Canadian market by the producer in February 2012, but not for health risks.
Generally, Cannabis may be used for any symptom without demonstrating the inefficacy of the previous therapies.
The approved industrial products may be reimbursed by health insurance companies, while all the others are non-reimbursable.
( Fischer, et al., 2015 ; Ablin, et al., 2016 ; Health Canada, 2016 ; The Health Products Regulatory Authority, 2017 ; Abuhasira, et al., 2018 ; Conseil fédéral, 2018 ; Government of Canada, 2019 ; Health Canada, 2022 )
3.1.4 Denmark
All medical doctors are authorised to prescribe Cannabis -based products as part of a 4 years pilot project launched in January 2018. As part of this project, a medical doctor may prescribe medicines that are not approved for distribution or sale in Denmark. However, the medical doctor must take full responsibility for the products they prescribe and must determine the proper dosage for each patient. Medical doctors may refer to the guidelines laid out by the Danish Medicines Agency. The imported plant products available for prescription may vary in content, but they must comply with strict standards and regulations governing the cultivation of the plant species, and the production and standardisation of the Cannabis -based product.
Herbal Cannabis is available by prescription only in pharmacies, which may also prepare magistral preparations.
Regarding industrial products, neurologists may prescribe Sativex ® to treat spasticity from multiple sclerosis. In general, medical doctors may prescribe imported Cannabis -derived medicines that have not been approved for sale in Denmark, such as Marinol ® and Cesamet ® on compassionate grounds, but only if the request is approved by the Danish Medicines Agency.
In general, the Danish Medicines Agency indicates that medical Cannabis be considered as a therapy only for the following conditions: painful spasticity in multiple sclerosis, painful spasticity caused by spinal cord damage, chemotherapy-induced nausea, and neuropathic pain. As part of the pilot project, Cannabis may, however, be prescribed to any patient even outside of the guidelines. The use of Cannabis is not recommended for patients under 18 years of age.
The prices of the prescribed products within the pilot project are set freely by the manufacturers. It is possible to obtain a reimbursement as of 01/01/2019 (retroactive for 2018). Patients in the terminal stages of a disease are fully reimbursed, while patients with other illnesses receive a 50% reimbursement, up to annual maximum of 10,000 Danish Krone. The reimbursement is automatically deducted at the time of the purchase in a pharmacy.
For prescriptions that are not part of the pilot project, the medical doctor may request a reimbursement for an individual patient from the Danish Medicines Agency. It will consider the request for those patients with pathologies where Cannabis -based treatment appears to be effective, and for those whom all other treatments with approved medicines have been used without effect.
( The Health Products Regulatory Authority, 2017 ; Abuhasira, et al., 2018 ; Krcevski-Skvarc, et al., 2018 ; Danish Medicines Agency, 2020 ; Gustavsen, et al., 2021 )
3.1.5 Germany
Medical doctors may prescribe medical Cannabis using a specific “narcotics” prescription form. The prescription may be for any condition that has no standard treatment, or the standard treatment cannot be used owing to reactions, or based on the patient’s specific condition. Among the industrial products available is Sativex ® , which is indicated for spasticity in refractory multiple sclerosis. In addition, it is possible to prescribe dronabinol without particular restrictions regarding its indicated use. Nabilone is approved for nausea and vomiting associated with chemotherapy and unresponsive to conventional therapies. Finally, Epidiolex ® and many types of Cannabis inflorescences may also be prescribed. Magisterial preparations may be prescribed, and pharmacies may dispense extracts of Cannabis and inflorescences.
In the past, Cannabis could also be theoretically grown in residence by private individuals if conventional therapies had been inefficacious, no other alternative treatments were available, and/or to reduce the cost of therapy. Actually, this possibility has never been really applied. Since 2019, however, a system of checks on the production and supply of Cannabis has been introduced by the government.
The patients may request a reimbursement from health insurance companies. For this purpose the prescribing medical doctor has the task of certifying the seriousness of the disease, that the standard therapies have been ineffective, or cannot be used due to the patient’s specific condition, or that there is a reasonable likelihood that medical Cannabis will be effective for that subject.
( Grotenhermen and Müller-Vahl, 2012 ; Ablin, et al., 2016 ; The Health Products Regulatory Authority, 2017 ; Abuhasira, et al., 2018 ; Conseil fédéral, 2018 ; Federal Institute for Drugs and Medical Devices, 2018 ; Krcevski-Skvarc, et al., 2018 ; Rasche, et al., 2019 ; Federal Institute for Drugs and Medical Devices, 2022a ; Federal Institute for Drugs and Medical Devices, 2022b ; Federal Institute for Drugs and Medical Devices, 2022c ; Federal Institute for Drugs and Medical Devices, 2022d ; German Institute for Medical Cannabis , 2022 )
3.1.6 Israel
In Israel, patients with a prescription may use a licensed pharmacy to obtain medical Cannabis . There is a list of conditions for which Cannabis may be used, but the medical doctor may also prescribe it for other pathologies: in any case, it may only be used when other therapies have proved ineffective. The list includes neuropathic pain, serious cachexia in AIDS patients, spasticity from multiple sclerosis, pain associated with Parkinson’s disease, Tourette’s syndrome, treatment of metastatic cancer or chemotherapy-induced symptoms, inflammatory intestinal diseases and post-traumatic stress disorders.
In general, the products available are Cannabis inflorescences, Sativex ® and Epidiolex ® . The number of medical Cannabis patients among the Israeli population is one of the highest in the world (on February 2022 about 100,000 Israelis -about 1% of the population-were allowed to consume medical Cannabis ).
Sativex ® is recommended for spasticity from multiple sclerosis unresponsive to other treatments, or as an additional analgesic therapy in adult patients with advanced stage cancer with moderate to severe pain despite being administered the highest tolerable dosage of opioids; Epidiolex ® is used to treat convulsions in Dravet syndrome, and Lennox-Gastaut syndrome.
As for herbal Cannabis , a government-run programme produces and distributes this product. Medical Cannabis is supplied in two forms: as an oil extract for oral administration or sub-lingual deposition, and as the inflorescence which may be smoked or inhaled with vaporisers. The cost of the therapy is reimbursed in part by some private and state health insurance schemes.
( abcNEWS, 2022 ; Ablin, et al., 2016 ; Abuhasira, et al., 2018 ; Krcevski-Skvarc, et al., 2018 ; State of Israel - Minister of Health, 2017 ; State of Israel - Minister of Health, 2022 ; The Health Products Regulatory Authority, 2017 )
3.1.7 Netherlands
In Netherlands, all medical doctors may prescribe medical Cannabis . The pharmacies may also produce extracts using the plant material produced by the Office of Medical Cannabis . These are usually oil extracts to be taken orally or deposited under the tongue. Some types of inflorescences are available for this purpose: the concentration of the active molecules and granulation properties may vary. The inflorescences may also be taken in the decoction form or inhaled through vaporisers.
Sativex ® is approved for the treatment of spasticity from multiple sclerosis refractory to conventional therapies.
Cannabis is indicated for the treatment of pain (multiple sclerosis, or spinal cord injuries), chronic pain, nausea and vomiting (in chemotherapy or radiotherapy, HIV therapies, adverse reactions to hepatitis C medication), palliative care for cancer or AIDS (to increase appetite and alleviate pain, nausea and weight loss), Tourette’s syndrome, and refractory glaucoma, epilepsy and epileptic syndromes (even in children). In addition, its use is indicated in the reduction in symptomology of the following pathologies: Crohn’s disease, ulcerative colitis, itching, migraine, rheumatic conditions, ADHD, post-traumatic stress disorders, agitation in Alzheimer’s disease and cerebral trauma. Medical doctors are in any case authorised to prescribe these therapies for other conditions if they consider it fit. Cannabis -based products must, however, be considered only in cases where authorised medicines have inefficacious or provoked unacceptable adverse reactions.
As concerns the available herbal Cannabis species, Bediol ® (THC 6.3%; CBD 8%) is usually recommended as the first-choice therapy to alleviate pain or as an anti-inflammatory therapy. Bedrocan ® (THC 22%; CBD <1.0%), Bedica ® (THC 14%; CBD <1.0%) and Bedrobinol ® (THC 13.5%; CBD <1.0%) are considered more effective for the treatment of symptoms such as appetite loss, weight loss, nausea, vomiting, anorexia, cachexia, emesis, Tourette’s syndrome, and glaucoma. Bedrolite ® (THC <1.0%; CBD 7.5%) is employed for certain forms of epilepsy.
The healthcare system does not reimburse the cost of Cannabis -based medicines. In some cases, the patient may be able to claim from private insurance schemes.
( The Health Products Regulatory Authority, 2017 ; Abuhasira, et al., 2018 ; Conseil fédéral, 2018 ; Krcevski-Skvarc, et al., 2018 ; Bedrocan, 2021 ; Office of Medicinal Cannabis, 2022 )
3.1.8 Switzerland
The prescription and use of Cannabis -based magistral preparations is authorised for spasticity (multiple sclerosis), chronic pain, appetite loss in AIDS, and nausea, pain, and appetite loss from cancer.
The magistral preparations are prepared in a pharmacy.
Medical doctors may prescribe Cannabis -based medicines only after receiving authorisation from the Federal office of the Public Health System.
The cost of the therapy is not reimbursed systematically, but on a case-by-case basis.
As well as the inflorescence, it is possible to use dronabinol and Epidiolex ® . Sativex ® is also authorised for use and available for treatment of spasticity from multiple sclerosis.
( Abuhasira, et al., 2018 ; Krcevski-Skvarc, et al., 2018 ; Swiss Confederation, Federal Office of Public Health, 2020 ; Swiss Confederation, Federal Office of Public Health, 2021a ; Swiss Confederation, Federal Office of Public Health, 2021b ; Swiss Confederation, Federal Office of Public Health, 2021c )
3.1.9 United Kingdom
In the United Kingdom, medical Cannabis is generally prescribed to adults and children with rare and serious forms of epilepsy, adults suffering from nausea or vomiting from chemotherapy, and adults with muscular stiffness or spasms from multiple sclerosis. This therapy is considered only in cases in which no alternative treatment is available, or other treatments have been inefficacious. The available products are Epidiolex ® , prescribed to patients with Lennox-Gastaut syndrome or Dravet syndrome; nabilone, which is authorised for nausea and vomiting associated with chemotherapy; dronabinol is also available, but it has no marketing authorization; and Sativex ® , which is prescribed for muscular spasms in multiple sclerosis unresponsive to other treatments (even though it is discouraged by NICE in that it is not cost-effective).
The medical Cannabis therapy cannot be obtained from a general practitioner but must be prescribed by a hospital specialist registered with the General Medical Council. The medical doctor may collect data on adverse reactions, which can also be signalled directly by the patient through a yellow card system.
( Department of Health and Social Care, 2018 ; Medicines and healthcare products Regulatory Agency, 2020 ; MS Society, 2021 ; National Health Service, 2021 ; General Medical Council, 2022 ; National Health Service, 2022 ; UK Government, 2022 )
3.1.10 United States of America
There are significant legislative differences among the states concerning Cannabis in the United States. In some states the legislation in force is extremely limiting, in others significantly less restrictive. Therefore, the state laws may not be completely harmonised with federal laws.
Regarding industrial products, the FDA has approved the prescription of dronabinol and nabilone for the treatment of chemotherapy-induced nausea and vomiting. Dronabinol may also be used for the treatment of appetite and weight loss in HIV patients. Epidiolex ® may be prescribed for the treatment of epileptic disorders, Lennox-Gastaut syndrome and Dravet’s syndrome.
Concerning herbal Cannabis , only 36 states have legalised or decriminalised its use. In general, in those states which have authorised the use of medical use Cannabis , there are restrictions on its prescription. Depending to the local laws, therefore, Cannabis may be prescribed for pain, anxiety, epilepsy, glaucoma, appetite and weight loss associated with AIDS, inflammatory intestinal disturbances irritable intestine syndrome, motor disturbances due to Tourette’s syndrome or multiple sclerosis, nausea and vomiting caused by chemotherapy, sleep disorders, posttraumatic stress disorders. Some states allow the addition, at the prescribing medical doctor’s discretion, of pathologies other than those expressly stated.
Generally, medical doctors do not need specific training to prescribe Cannabis , but in many states, it is necessary to register before doing so. In other states, medical doctors must attend a short training course to be able to register. In some states, it is enough that the medical doctor gives advice verbally to take medical Cannabis , or its use may be recommended by a health care professional who is not a medical doctor. On the other hand, in some states, it is necessary that two medical doctors confirm the need for a Cannabis -based treatment for a patient. Depending on the state, Cannabis may be supplied to the patient by licensed dispensaries, or it may be grown at home by the patient or by a caregiver.
Smoking medical Cannabis is prohibited in some states. Similarly even the edible forms are prohibited in some states. Generally, the administration is performed orally or by vaporiser.
Patients are generally registered so that the possession and use of medical Cannabis is not prosecuted.
Abuhasira, et al., 2018 ; Alharbi, 2020 ; Carliner, et al., 2017 ; Choo and Emery, 2017 ; Corroon and Kight, 2018 ; Johnson, et al., 2021 ; Mead, 2017 ; National Conferences of State Legislatures, 2022 ; ProCon, 2022 ; Ryan, et al., 2021 ; The Health Products Regulatory Authority, 2017 )
3.2 Study Protocols and Clinical Trials
There are 43 publications of proposed, or executed, clinical trial protocols in those countries whose legislation has been analysed; eight of these regarded proposed clinical trial protocols.
Hence, 35 publications regarded actual clinical trial data. These were sub-divided into three groups: the first, “positive outcome,” included those studies which demonstrated the efficacy of the preparation administered, or that the actual results were in line with those expected (18). The second group, “negative outcome,” included those studies where the authors reported that the administered product was no more efficacious than the placebo (5). Finally, the third group, “inconclusive outcome,” comprised those studies where the results were not conclusive (12).
The characteristics of the taken into account clinical studies are summarized in Table 1 .
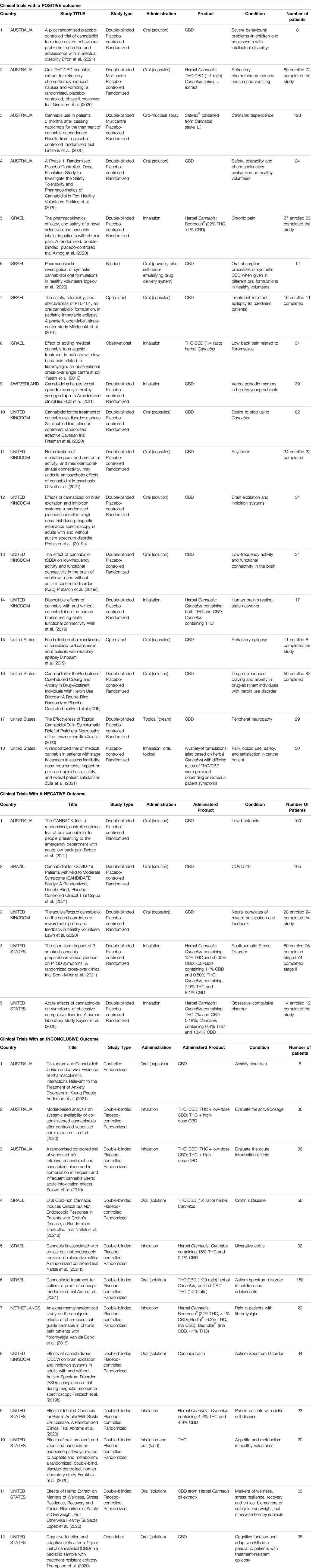
TABLE 1 . Characteristics of the selected clinical trials.
3.2.1 Clinical Trials With a Positive Outcome
Of the 18 studies in this category, 4 were conducted in Australia, 4 in Israel, 1 in Switzerland, 5 in the United Kingdom, and 4 in the United States.
Regarding the study design, 2 were multi-centred, 13 used the double-blind method, 14 had a randomised control design, and 14 used a placebo control group.
The sample size varied greatly, from a minimum of 8 to a maximum of 128 enrolled subjects.
As for the products used in the trials, 12 studies administer CBD, 6 studied herbal Cannabis derivatives.
CBD was administered orally in 10 cases, topically and by inhalation in only one study. The herbal Cannabis derivatives were administered by inhalation in 3 cases, and by the oral route in 2 cases. One study considered products to be administered orally, by inhalation or topically.
In 9 studies, the Cannabis derivatives were administered in addition to a standard therapy.
The most commonly studied conditions were behaviour, cerebral activity, and memory (6), pain (4), addiction or abstinence to drugs (3), epilepsy (2), pharmacokinetic studies, safety, and tolerability (2), and nausea and vomiting (1). Two studies were carried out on a paediatric population.
In general, the studies involving the administration of CBD regarded epilepsy, addiction or abstinence to drugs, behaviour, cerebral activity and memory, peripheral neuropathy, pharmacokinetic studies, and safety and tolerability.
Instead, studies administering herbal Cannabis derivatives focused mainly about pain and then about nausea and vomiting, cerebral activity and Cannabis dependence. In most cases both THC and CBD were administered in different ratios. In some cases, a herbal Cannabis strain was used with a high concentration of THC.
( Almog, et al., 2020 ; Birnbaum, et al., 2019 ; Efron, et al., 2021 ; Freeman, et al., 2020 ; Grimison, et al., 2020 ; Hotz, et al., 2021 ; Hurd, et al., 2019 ; Izgelov, et al., 2020 ; Lintzeris, et al., 2020 ; Mitelpunkt, et al., 2019 ; O'Neill, et al., 2021 ; Perkins, et al., 2020 ; Pretzsch, et al., 2019a ; Pretzsch, et al., 2019c ; Wall, et al., 2019 ; Xu, et al., 2020 ; Yassin, et al., 2019 ; Zylla, et al., 2021 )
3.2.2 Clinical Trials With a Negative Outcome
Five trials had a negative outcome. Two of these were conducted in the United States, 1 in Australia, 1 in Brazil and 1 in the United Kingdom.
All of the trials had a randomised control, used a placebo control group, and a double-blind control. The sample size ranged from 14 to 105 enrolled subjects.
As for the products used, 3 studies administered oral preparations containing CBD. 2 studies were based on the administration of inflorescences by inhalation. 4 studies out of 5 administered the product in addition to a standard therapy.
The conditions studied in these trials with CBD were pain, COVID-19 infection, and the effects on neural correlates of reward anticipation and feedback. Herbal Cannabis , in three different forms and different ratios of THC/CBD), was administered to evaluate its efficacy in the treatment of Obsessive-Compulsive Disorder (OCD) and Post-Traumatic Stress Disorder (PTSD).
None of these studies demonstrated that the administered product was more efficacious than the placebo control.
( Kayser, et al., 2020 ; Lawn, et al., 2020 ; Bebee, et al., 2021 ; Bonn-Miller, et al., 2021 ; Crippa, et al., 2021 )
3.2.3 Clinical Trials With an Inconclusive Outcome
12 studies had an inconclusive outcome: 3 were conducted in Australia, 3 in Israel, 1 in the Netherlands, 1 in the United Kingdom and 4 in the United States.
Regarding study design, 10 included a double-blind system, 11 had a randomised control, and 10 utilised a placebo control group. The sample size ranged from a minimum of 6 subjects to a maximum of 150 individuals. Two of the studies were conducted on paediatric subjects.
Concerning the products used, 2 studies administered CBD alone, one study used THC alone, 1 study administered cannabidivarin, 2 studies administered THC and CBD, both alone and in a mixture, 5 studies administered herbal Cannabis derivatives, and 1 study administered both THC and CBD as well as a herbal Cannabis extract.
CBD and cannabidivarin were administered orally; THC, and the mixtures of THC and CBD were administered by inhalation. THC was also administered orally. The herbal Cannabis derivatives were administered by inhalation in 3 studies, while they were for oral use in 2 studies. 1 study used oral administration of a herbal Cannabis extract or an equivalent mixture of THC and CBD.
Six trials predicted that the administration was additional to standard therapy.
The conditions to be studied for the efficacy of CBD were anxiety and cognitive function in patients suffering from epilepsy. THC and/or CBD were administered to evaluate the active dosage or to study its effects on problems linked to appetite and metabolism, herbal Cannabis derivatives were studied to evaluate their activity in Crohn’s disease, ulcerative colitis, pain, haemolytic anaemia, markers of wellness and clinical biomarkers in obese patients. Trials related to autism were conducted with, as well as cannabidivarin, the administration of a herbal Cannabis extract or an equivalent mixture of THC and CBD.
When herbal Cannabis derivatives were administered, the concentration of THC and CBD, and the ratio of the two varied greatly among the trials. Some used products with a high concentration of THC, while others used products with a high concentration of CBD. In 1 trial, different types of inflorescences were administered to evaluate the most efficacious ratio of THC to CBD concentrations against pain.
( Pretzsch, et al., 2019b ; Solowij, et al., 2019 ; Van de Donk, et al., 2019 ; Abrams, et al., 2020 ; Farokhnia, et al., 2020 ; Liu, et al., 2020 ; Lopez, et al., 2020 ; Thompson, et al., 2020 ; Naftali, et al., 2021a ; Anderson, et al., 2021 ; Aran, et al., 2021 ; Naftali, et al., 2021b )
3.2.4 Study Protocols
There are 8 examples of published protocols that have not yet initiated the clinical trial phase. 4 are in Australia, and 1 each in Denmark, Canada, Germany, and Netherlands. The number of enrolled subjects is between 10 and 180 in total. One study will be carried out among the paediatric population.
Concerning the study design, 3 will be multi-centre studies, 7 use a double-blind system, 8 are randomised, and 7 use a placebo control group.
Regarding the products to be used, 4 protocols will use the oral administration of THC and CBD. The ratio between the components in question varies from study to study. In 2 protocols, the administration of CBD is also foreseen. One protocol foresees the administration of both CBD and a preparation containing a high concentration of THC.
For those studies using THC and CBD mixtures, the pathologies to be studied are, pain, dementia, spasms, and the activation of the immune system in HIV patients. Instead, the CBD alone preparations will be administered for behavioural problems and phobias. The herbal- Cannabis derived product will be administered for chronic tic disorder. The protocol that foresees the administration of both CBD and a preparation with a high concentration of THC will focus on the alleviation of pain.
( Costiniuk, et al., 2019 ; Hendricks, et al., 2019 ; Urbi, et al., 2019 ; Van der Flier, et al., 2019 ; Efron, et al., 2020 ; Hardy, et al., 2020 ; Jakubovski, et al., 2020 ; Timler, et al., 2020 )
4 Discussion
From the analysis of the current legislation in states where clinical trials and proposed protocols on medical Cannabis and derived products have been published in the last 3 years, many significant differences have been found regarding the products available, the indicated pathologies for which it may be prescribed, the production of the raw plant material, as well as its reimbursement and prescription. It was evaluated to consider the studies published in the last 3 years supposing that the researchers have benefited from the latest knowledge on medical Cannabis and to make an overview of the pathologies currently under study.
In particular, regarding industrial products, practically every country, with the exception of the United States, has approved the use of Sativex ® . However, Epidiolex ® , dronabinol.Netherlands, and nabilone are also quite common.
In all the countries, the use of herbal Cannabis is also authorised. The only exception is Brazil, which is certainly the country with the most restrictive legislation. Netherlands is the only country to provide directions for use, which are not binding, but quite strict, regarding the plant strain to be used for a determined pathology based on the concentration of active molecules (THC and CBD). Instead, for the other countries, it must be pointed out that the current legislation provides for the use of inflorescences or herbal Cannabis extracts without providing specific directions concerning the recommended concentration of active molecules to treat a determined condition.
Regarding the pathologies or symptoms associated with the more or less well-defined conditions, the most common are pain, nausea, vomiting, spasticity, and epilepsy followed by spasms, and weight and appetite loss. The less frequently indicated conditions in this case include Tourette’s syndrome, PTSD, and glaucoma. In many countries, additional conditions are considered in more or less detail.
In this regard, it is interesting to note that the country with the greatest number of specifically recommended pathologies not indicated in other countries is the Netherlands: perhaps based on the longstanding use of Cannabis both for medical use and recreational purposes. Although the legislation regarding medical Cannabis is quite comprehensive in all the countries considered, some of them, namely Australia, Canada, Denmark, Germany, Israel, Netherlands, and the United States, also permit the prescription of Cannabis for any therapeutic application at the discretion of the medical doctor. However, in Germany, Netherlands and Israel, this is limited to cases in which other therapies have proved ineffective, excessive adverse reactions to standard treatments have occurred, or valid alternative treatments are not available. Instead, in Australia, Canada, Denmark, and the United States, therapeutic strategies different from those specified are authorised regardless of any prior treatment. The prescription of medical Cannabis for any condition certainly does not conform to the procedures generally in force for other medicinal products, and especially products with a psychoactive effect such as those prepared containing THC.
It is interesting to note that in Canada, and in some states in the United States, the medical inflorescences may be grown directly by the patient, and the treatment may be recommended by a health worker, and not only a medical doctor; in the event that the plant species is not home-grown, it is distributed through a licensed dispensary. In Germany, Israel and Netherlands, herbal Cannabis is grown locally under the supervision of a government agency. This is significant if one considers that, in these three countries, the prescription process is highly deregulated regarding the recommended pathologies to be treated with Cannabis , but the same does not apply to its cultivation.
The normal administration routes are oral or by inhalation. Some countries, such as Israel, authorise smoking Cannabis inflorescences as a route of administration, something that is categorically banned in some states of the United States.
In addition, regarding prescription, it is noteworthy that the United Kingdom is the only country where this must be obtained from a hospital specialist. In some states in the United States, on the other hand, the prescribing medical doctor must be registered to prescribe this therapy and have attended a specific training course. In Australia and Switzerland, medical doctors may write the prescription only after receiving authorisation from a specific agency. Therefore, there is a different focus on the prescription process and hence inhomogeneity in this aspect too. The treatment costs are generally borne by the patient, and no reimbursement is foreseen, unless it is from a private health insurance scheme. This certainly restricts access to this kind of therapy to the more privileged members of society.
Concerning the results of the clinical trials, some interesting observations may be made. In the first place, a greater number of studies have been published in certain countries. These countries are the United States (11) and Australia (9), followed by Israel (7) and the United Kingdom (7). In general, the majority of the studies featured randomisation, the use of a double-blind method, and a placebo control group: these are factors which guarantee the quality of the data gathered. On the other hand, the majority of the studies took place with a small sample size. Moreover, the studies made use of a heterogeneous population: healthy and ill volunteers, adults and children, acute and chronically ill patients, and subjects who had previously used or had never used Cannabis prior to the study. Factors that, being so numerous, make it particularly challenging to draw any conclusive evaluations of the results of these trials, and more in general, the real efficacy of medical Cannabis .
Considering only the studies with a positive outcome, it should be noted that the studied pathologies are coherent with those provided for in current legislation i.e., pain, epilepsy, nausea, and vomiting; on the contrary, psychosis, behavioural problems, memory and cerebral activity represent a novelty. Furthermore, there is a net distinction between the products used based on the different conditions to be treated: the trials on pain, nausea and vomiting with positive outcomes administered herbal Cannabis derivatives in which, in 3 cases out of 4, both THC and CBD are present; the other studies with a positive outcome administered CBD alone. In those trials with a negative outcome, CBD was administered for pain, while herbal Cannabis derivatives were used for conditions such as OCD or PTSD. This consideration supports the use of herbal Cannabis in which both THC and CBD are present for pain, even though it should be stressed that the studies with a positive outcome for this pathology had a maximum of 30 enrolled subjects.
The studies with an inconclusive outcome regarded a variegated list of conditions including anxiety, Crohn’s syndrome, ulcerative colitis, pain, and appetite loss. Many of these are already included in some national regulations although the efficacy of Cannabis in these cases according to the currently available data is not satisfactorily demonstrated.
It is evident that the only pathology present in all three study categories is pain, for which 4 studies had a positive outcome, 1 had a negative outcome, and 1 had an inconclusive outcome.
Among the study protocols to be trialled, pain and spasticity appear again, approved by legislation in most countries and the object of numerous studies, as well as a number of less-investigated conditions such as dementia, phobias, tic disorders and the activation of the immune system in HIV patients.
Based on the research conducted, it is, therefore, possible to stress that in spite of the growing number of recent studies on medical Cannabis , many of which have had a positive outcome while many others have had an inconclusive or negative outcome. The presumed broad spectrum action of Cannabis has led to the initiation of many trials and the preparation of many study protocols for a wide range of pathologies with the enrolment of subjects with diverse characteristics from study to study. This means that there is very little data for each pathology or symptomology.
Another important factor is that the products used are very diverse from each other; consequently, a comparison is extremely difficult to make, especially for the herbal products. All of the trials indicate the precise dosages used in terms of active molecules, but when it comes to inflorescences, or extracts derived from them, the concentration is provided only for the THC and CBD content and not for the other active molecules. Furthermore, the diverse administration routes make a comparison based on pharmacokinetics difficult for the molecules of interest.
Therefore, it is difficult to compare the studies and draw conclusions concerning the efficacy of the protocol for the single pathologies. However, for some, substantial evidence is emerging regarding their efficacy and the suitable products to ensure that. From the analysed data, it is clear that the best pain treatment is herbal Cannabis derivatives containing both THC and CBD, just as the best way to treat epilepsy is to administer CBD.
One interesting point is that for some of the pathologies approved for treatment with medical Cannabis under the current legislation, the data do not paint a definitive picture. This is true for conditions such as anxiety, ulcerative colitis, Crohn’s syndrome, and appetite enhancement.
On the other hand, the current legislation often authorises inflorescences or extracts without indicating the exact concentration of the active molecules. In parallel, many studies use different plant strains or study a small number of subjects, making it difficult to compare and consequently interpret the results. Moreover, in many studies, the Cannabis -based medicines were administered in addition to other treatments making any evaluation of their efficacy it even more complex.
5 Conclusion
Medical Cannabis is often considered as if it were a single active component, but, in fact, there are countless possible variations. Hence, it will be some time before the current list of pathologies that each product may be used for can be updated based on definitive clinical data on the efficacy of the various components. Certainly, the development of standardised industrial products will facilitate the execution of more meaningful trials compared to those that involve the administration of inflorescences or derived extracts prepared using a variety of methods and, thus, highly variable in terms of concentration of the active molecules.
The authors want moreover to put in evidence that, despite legislation authorising the use of medical Cannabis and instituting the national production centre for inflorescences more than 5 years ago, Italy is still among the states where clinical trials have not been conducted. This gap is due to legal restrictions on the approval and conduction of clinical trials in this field, and the difficulty in sourcing the raw plant material, of which there is always a shortage. The result of this is therapies using inflorescences and extracts which have never undergone specific clinical trialling.
In the end, the influence of the media, economic interests, and the demands of associations representing patients affected by these diseases and conditions, for whom Cannabis is a panacea, means that in many countries it is currently possible to use medical Cannabis even though the scientific data do not entirely support the signs of efficacy: certainly this is a special case where the consolidated procedures for the administration of any product in the medical field have been either overlooked or ignored. It is time that the regulatory agencies considered whether this is actually safeguarding the health of patients.
The analysis of the current legislation may not be exhaustive in that it refers only to public texts available online.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
FB and PB performed the conceptualization of the work. FB, IP and LE performed the investigation and took care of the data. FB wrote the manuscript. PB coordinated the project. All authors approved the final version of the study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Dr Tom O Byrne for the linguistic revision of the text.
Abbreviations
ADHD, Attention-Deficit/Hyperactivity Disorder; AIDS, Acquired ImmunoDeficiency Syndrome; CBD, CannaBiDiol; COVID-19, COronaVIrus Disease 2019; FDA, Food and Drug Administration; HIV, Human Immunodeficiency Virus; NICE, National Institute for health and Care Excellence; OCD, Obsessive-Compulsive Disorder; PTSD, Post-Traumatic Stress Disorder; THC, delta-9-TetraHydroCannabinol; United States, United States of America.
1 Epidiolex ® has received approval in the European Union under the tradename Epidyolex ® .
abc NEWS (2022). Israeli Bigwigs Eye Profits from Cannabis Legalization. Available at: https://abcnews.go.com/Health/wireStory/israeli-bigwigs-eye-profits-cannabis-legalization-82617899 (Accessed April 26th, 2022).
Google Scholar
Ablin, J., Ste-Marie, P. A., Schäfer, M., Häuser, W., and Fitzcharles, M. A. (2016). Medical Use of Cannabis Products: Lessons to Be Learned from Israel and Canada. Schmerz 30 (1), 3–13. doi:10.1007/s00482-015-0083-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Abrams, D. I., Couey, P., Dixit, N., Sagi, V., Hagar, W., Vichinsky, E., et al. (2020). Effect of Inhaled Cannabis for Pain in Adults with Sickle Cell Disease: A Randomized Clinical Trial. JAMA Netw. Open 3 (7), e2010874. doi:10.1001/jamanetworkopen.2020.10874
Abuhasira, R., Shbiro, L., and Landschaft, Y. (2018). Medical Use of Cannabis and Cannabinoids Containing Products - Regulations in Europe and North America. Eur. J. Intern Med. 49, 2–6. doi:10.1016/j.ejim.2018.01.001
Alcohol and Drug Foundation (2021). Medicinal Cannabis Products FAQs. Available at: https://adf.org.au/talking-about-drugs/medicinal-cannabis-products/medicinal-cannabis-products-faqs/ (Accessed February 15th, 2022).
Alharbi, Y. N. (2020). Current Legal Status of Medical Marijuana and Cannabidiol in the United States. Epilepsy Behav. 112, 107452. doi:10.1016/j.yebeh.2020.107452
Almog, S., Aharon-Peretz, J., Vulfsons, S., Ogintz, M., Abalia, H., Lupo, T., et al. (2020). The Pharmacokinetics, Efficacy, and Safety of a Novel Selective-Dose Cannabis Inhaler in Patients with Chronic Pain: A Randomized, Double-Blinded, Placebo-Controlled Trial. Eur. J. Pain 24 (8), 1505–1516. doi:10.1002/ejp.1605
Anderson, L. L., Doohan, P. T., Oldfield, L., Kevin, R. C., Arnold, J. C., Berger, M., et al. (2021). Citalopram and Cannabidiol: In Vitro and In Vivo Evidence of Pharmacokinetic Interactions Relevant to the Treatment of Anxiety Disorders in Young People. J. Clin. Psychopharmacol. 41 (5), 525–533. doi:10.1097/JCP.0000000000001427
Aran, A., Harel, M., Cassuto, H., Polyansky, L., Schnapp, A., Wattad, N., et al. (2021). Cannabinoid Treatment for Autism: a Proof-Of-Concept Randomized Trial. Mol. Autism 12 (1), 6. doi:10.1186/s13229-021-00420-2
Arias, S., Leon, M., Jaimes, D., and Bustos, R.-H. (2021). Clinical Evidence of Magistral Preparations Based on Medicinal Cannabis. Pharmaceuticals 14 (2), 78. doi:10.3390/ph14020078
Australian Capital Territory Government (2021). Patient Information for Medicinal Cannabis. Available at: https://www.health.act.gov.au/sites/default/files/2018-09/Medicinal%20Cannabis%20-%20Patient%20Information_0.pdf (Accessed February 15th, 2022).
Australian Government, Department of Health (2018). Access to Medicinal Cannabis Products: Frequently Asked Questions (FAQs). Available at: https://www.tga.gov.au/access-medicinal-cannabis-products-frequently-asked-questions-faqs (Accessed February 15th, 2022).
Australian Government, Department of Health (2017a). Guidance for the Use of Medicinal Cannabis in Australia: Overview. Available at: https://www.tga.gov.au/publication/guidance-use-medicinal-cannabis-australia-overview (Accessed February 15th, 2022).
Australian Government, Department of Health (2017b). Guidance for the Use of Medicinal Cannabis in Australia: Patient Information. Available at: https://www.tga.gov.au/publication/guidance-use-medicinal-cannabis-australia-patient-information (Accessed February 15th, 2022).
Australian Government, Department of Health (2020). Medicinal Cannabis: Information for Consumers. Available at: https://www.tga.gov.au/medicinal-cannabis-information-consumers (Accessed February 15th, 2022).
Australian Government, Department of Health (2021). Medicinal Cannabis: Information for Health Professionals. Available at: https://www.tga.gov.au/medicinal-cannabis-information-health-professionals (Accessed February 15th, 2022).
Australian Institute of Health and Welfare (2019). National Drug Strategy Household Survey. Available at: https://www.aihw.gov.au/getmedia/108d1761-b523-492b-81cc-a09db6740e85/aihw-phe-270-Chapter6-Medicinal-cannabis.pdf.aspx#:∼:text=Household%20Survey%202019-,Emerging%20topic%3A%20Medicinal%20cannabis,and%20leaves%20of%20the%20plant (Accessed February 15th, 2022).
Baratta, F., Peira, E., Maza, C., Gallarate, M., and Brusa, P. (2022). Cannabis -Based Oral Emulsion for Medical Purposes to Meet the Needs of Patients: Formulation, Quality and Stability. Pharmaceutics 14, 513. doi:10.3390/pharmaceutics14030513
Baratta, F., Simiele, M., Pignata, I., Ravetto Enri, L., Torta, R., De Luca, A., et al. (2019). Development of Standard Operating Protocols for the Optimization of Cannabis -Based Formulations for Medical Purposes. Front. Pharmacol. 10, 701. doi:10.3389/fphar.2019.00701
Baratta, F., Simiele, M., Pignata, I., Ravetto Enri, L., D’Avolio, A., Torta, R., et al. (2021). Cannabis -Based Oral Formulations for Medical Purposes: Preparation, Quality and Stability. Pharmaceuticals 14 (2), 171. doi:10.3390/ph14020171
Bebee, B., Taylor, D. M., Bourke, E., Pollack, K., Foster, L., Ching, M., et al. (2021). The CANBACK Trial: a Randomised, Controlled Clinical Trial of Oral Cannabidiol for People Presenting to the Emergency Department with Acute Low Back Pain. Med. J. Aust. 214 (8), 370–375. doi:10.5694/mja2.51014
Bedrocan (2021). Products. Available at: https://bedrocan.com/it/prodotti/ (Accessed February 15th, 2022).
Birnbaum, A. K., Karanam, A., Marino, S. E., Barkley, C. M., Remmel, R. P., Roslawski, M., et al. (2019). Food Effect on Pharmacokinetics of Cannabidiol Oral Capsules in Adult Patients with Refractory Epilepsy. Epilepsia 60 (8), 1586–1592. doi:10.1111/epi.16093
Bonn-Miller, M. O., Sisley, S., Riggs, P., Yazar-Klosinski, B., Wang, J. B., Loflin, M. J. E., et al. (2021). The Short-Term Impact of 3 Smoked Cannabis Preparations versus Placebo on PTSD Symptoms: A Randomized Cross-Over Clinical Trial. PloS one 16 (3), e0246990. doi:10.1371/journal.pone.0246990
Brazilian Government (2021). Autorização Sanitária de Produtos de Cannabis. Available at: https://www.gov.br/anvisa/pt-br/assuntos/educacaoepesquisa/webinar/medicamentos/arquivos/perguntas-e-respostas-autorizacao-sanitaria-de-produtos-de-cannabis.pdf (Accessed February 15th, 2022).
Carliner, H., Brown, Q. L., Sarvet, A. L., and Hasin, D. S. (2017). Cannabis Use, Attitudes, and Legal Status in the U.S.: A Review. Prev. Med. 104, 13–23. doi:10.1016/j.ypmed.2017.07.008
Casiraghi, A., Roda, G., Casagni, E., Cristina, C., Musazzi, U. M., Franzè, S., et al. (2018). Extraction Method and Analysis of Cannabinoids in Cannabis Olive Oil Preparations. Planta Med. 84 (4), 242–249. doi:10.1055/s-0043-123074
Castle, D. J., Strauss, N., Norman, A., and Bonomo, Y. (2019). Medical Marijuana: The Australian Experience. Mo Med. 116 (4), 270–273.
PubMed Abstract | Google Scholar
Centre for Medicinal Cannabis Research and Innovation (2021). Legal Access to Cannabis Based Medicines. Available at: https://www.medicinalcannabis.nsw.gov.au/__data/assets/pdf_file/0020/2864/Legal-Access-To-Cannabis-Based-Medicines-Fact-Sheet-Medical-Practitioners-FINAL.pdf (Accessed February 15th, 2022).
Choo, E. K., and Emery, S. L. (2017). Clearing the Haze: the Complexities and Challenges of Research on State Marijuana Laws. Ann. N. Y. Acad. Sci. 1394 (1), 55–73. doi:10.1111/nyas.13093
Conseil fédéral (2018). Traiter les personnes gravement malades avec du cannabis. Rapport du Conseil fédéral en réponse à la motion Kessler (14.4164). Available at: https://www.aramis.admin.ch/Default?DocumentID=49994&Load=true (Accessed February 15th, 2022).
Corroon, J., and Kight, R. (2018). Regulatory Status of Cannabidiol in the United States: A Perspective. Cannabis Cannabinoid Res. 3 (1), 190–194. doi:10.1089/can.2018.0030
Costiniuk, C. T., Saneei, Z., Routy, J. P., Margolese, S., Mandarino, E., Singer, J., et al. (2019). Oral Cannabinoids in People Living with HIV on Effective Antiretroviral Therapy: CTN PT028-Study Protocol for a Pilot Randomised Trial to Assess Safety, Tolerability and Effect on Immune Activation. BMJ open 9 (1), e024793. doi:10.1136/bmjopen-2018-024793
Crippa, J. A., Guimarães, F. S., Campos, A. C., and Zuardi, A. W. (2018). Translational Investigation of the Therapeutic Potential of Cannabidiol (CBD): Toward a New Age. Front. Immunol. 9, 2009. doi:10.3389/fimmu.2018.02009
Crippa, J. A. S., Pacheco, J. C., Zuardi, A. W., Guimarães, F. S., Campos, A. C., Osório, F. d. L., et al. (2021). Cannabidiol for COVID-19 Patients with Mild to Moderate Symptoms (CANDIDATE Study): A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Cannabis Cannabinoid Res. Adv. online Publ. [Epub ahead of print] doi:10.1089/can.2021.0093
CrossRef Full Text | Google Scholar
Danish Medicines Agency (2020). Cannabis-containing Products. Available at: https://laegemiddelstyrelsen.dk/en/special/medicinal-cannabis/ (Accessed February 15th, 2022).
Department of Health and Social Care (2018). Cannabis-based Products for Medicinal Use. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/753444/letter-with-guidance-on-cannabis-based-products-for-medicinal-use.pdf (Accessed February 15th, 2022).
Efron, D., Freeman, J. L., Cranswick, N., Payne, J. M., Mulraney, M., Prakash, C., et al. (2021). A Pilot Randomised Placebo-Controlled Trial of Cannabidiol to Reduce Severe Behavioural Problems in Children and Adolescents with Intellectual Disability. Br. J. Clin. Pharmacol. 87 (2), 436–446. doi:10.1111/bcp.14399
Efron, D., Taylor, K., Payne, J. M., Freeman, J. L., Cranswick, N., Mulraney, M., et al. (2020). Does Cannabidiol Reduce Severe Behavioural Problems in Children with Intellectual Disability? Study Protocol for a Pilot Single-Site Phase I/II Randomised Placebo Controlled Trial. BMJ open 10 (3), e034362. doi:10.1136/bmjopen-2019-034362
Farokhnia, M., McDiarmid, G. R., Newmeyer, M. N., Munjal, V., Abulseoud, O. A., Huestis, M. A., et al. (2020). Effects of Oral, Smoked, and Vaporized Cannabis on Endocrine Pathways Related to Appetite and Metabolism: a Randomized, Double-Blind, Placebo-Controlled, Human Laboratory Study. Transl. Psychiatry 10 (1), 71. doi:10.1038/s41398-020-0756-3
Federal Institute for Drugs and Medical Devices (2018). Annual Report 2017/18. Available at: https://www.bfarm.de/SharedDocs/Downloads/EN/BfArM/Publikationen/AnnualReport2017-18.pdf?blob=publicationFile (Accessed February 15th, 2022).
Federal Institute for Drugs and Medical Devices (2022a). BfArM Starts Selling Cannabis for Medical Purposes to Pharmacies. Available at: https://www.bfarm.de/SharedDocs/Pressemitteilungen/DE/2021/pm6-2021.html?nn=471310 (Accessed February 15th, 2022).
Federal Institute for Drugs and Medical Devices (2022b). Cannabis Agency. Available at: https://www.bfarm.de/DE/Bundesopiumstelle/Cannabis-als-Medizin/Cannabisagentur/_artikel.html (Accessed February 15th, 2022).
Federal Institute for Drugs and Medical Devices (2022c). Instructions for Pharmacists. Available at: https://www.bfarm.de/DE/Bundesopiumstelle/Cannabis-als-Medizin/Hinweise-fuer-Apotheker/_node.html (Accessed February 15th, 2022).
Federal Institute for Drugs and Medical Devices (2022d). Notes for Medical Doctors. Available at: https://www.bfarm.de/DE/Bundesopiumstelle/Cannabis-als-Medizin/Hinweise-fuer-Aerzte/_node.html (Accessed February 15th, 2022).
Fischer, B., Kuganesan, S., and Room, R. (2015). Medical Marijuana Programs: Implications for Cannabis Control Policy-Oobservations from Canada. Int. J. Drug Policy 26 (1), 15–19. doi:10.1016/j.drugpo.2014.09.007
Freeman, T. P., Hindocha, C., Baio, G., Shaban, N. D. C., Thomas, E. M., Astbury, D., et al. (2020). Cannabidiol for the Treatment of Cannabis Use Disorder: a Phase 2a, Double-Blind, Placebo-Controlled, Randomised, Adaptive Bayesian Trial. Lancet Psychiatry 7 (10), 865–874. doi:10.1016/S2215-0366(20)30290-X
General Medical Council (2022). Information for Doctors on Cannabis-Based Products for Medicinal Use. Available at: https://www.gmc-uk.org/ethical-guidance/learning-materials/information-for-doctors-on-cannabis-based-products-for-medicinal-use (Accessed February 15th, 2022).
German Institute for Medical Cannabis (2022). Mmedicinal Cannabis. Available at: https://difmc.de/medizinalcannabis/ (Accessed February 15th, 2022).
Gould, J. (2015). The Cannabis Crop. Nature 525 (7570), S2–S3. doi:10.1038/525S2a
Government of Canada (2019). Cannabis for Medical Purposes under the Cannabis Act: Information and Improvements. Available at: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/medical-use-cannabis.html#_Access_to_cannabis (Accessed February 15th, 2022).
Grimison, P., Mersiades, A., Kirby, A., Lintzeris, N., Morton, R., Haber, P., et al. (2020). Oral THC:CBD Cannabis Extract for Refractory Chemotherapy-Induced Nausea and Vomiting: a Randomised, Placebo-Controlled, Phase II Crossover Trial. Ann. Oncol. 31 (11), 1553–1560. doi:10.1016/j.annonc.2020.07.020
Grotenhermen, F., and Müller-Vahl, K. (2012). The Therapeutic Potential of Cannabis and Cannabinoids. Dtsch. Arztebl Int. 109 (29-30), 495–501. doi:10.3238/arztebl.2012.0495
Grotenhermen, F. (2003). Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 42 (4), 327–360. doi:10.2165/00003088-200342040-00003
Hardy, J., Haywood, A., Gogna, G., Martin, J., Yates, P., Greer, R., et al. (2020). Oral Medicinal Cannabinoids to Relieve Symptom Burden in the Palliative Care of Patients with Advanced Cancer: a Double-Blind, Placebo-Controlled, Randomised Clinical Trial of Efficacy and Safety of 1:1 Delta-9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD). Trials 21 (1), 611. doi:10.1186/s13063-020-04541-6
Health Canada (2016). Consumer Information—Cannabis. Available at: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/marihuana/info/cons-eng.pdf (Accessed February 15th, 2022).
Health Canada (2022). Marijuana Laws. Available at: https://medicalmarijuana.ca/patients/marijuana-laws/ (Accessed February 15th, 2022).
Health Direct (2019). Medicinal Cannabis. Available at: https://www.healthdirect.gov.au/medicinal-cannabis (Accessed February 15th, 2022).
Hendricks, O., Andersen, T. E., Christiansen, A. A., Primdahl, J., Hauge, E. M., Ellingsen, T., et al. (2019). Efficacy and Safety of Cannabidiol Followed by an Open Label Add-On of Tetrahydrocannabinol for the Treatment of Chronic Pain in Patients with Rheumatoid Arthritis or Ankylosing Spondylitis: Protocol for a Multicentre, Randomised, Placebo-Controlled Study. BMJ open 9 (6), e028197. doi:10.1136/bmjopen-2018-028197
Hill, K. P. (2015). Medical Marijuana for Treatment of Chronic Pain and Other Medical and Psychiatric Problems: A Clinical Review. JAMA 313 (24), 2474–2483. doi:10.1001/jama.2015.6199
Hotz, J., Fehlmann, B., Papassotiropoulos, A., de Quervain, D. J., and Schicktanz, N. S. (2021). Cannabidiol Enhances Verbal Episodic Memory in Healthy Young Participants: A Randomized Clinical Trial. J. Psychiatr. Res. 143, 327–333. doi:10.1016/j.jpsychires.2021.09.007
Hurd, Y. L., Spriggs, S., Alishayev, J., Winkel, G., Gurgov, K., Kudrich, C., et al. (2019). Cannabidiol for the Reduction of Cue-Induced Craving and Anxiety in Drug-Abstinent Individuals with Heroin Use Disorder: A Double-Blind Randomized Placebo-Controlled Trial. Am. J. Psychiatry 176 (11), 911–922. doi:10.1176/appi.ajp.2019.18101191
Izgelov, D., Davidson, E., Barasch, D., Regev, A., Domb, A. J., and Hoffman, A. (2020). Pharmacokinetic Investigation of Synthetic Cannabidiol Oral Formulations in Healthy Volunteers. Eur. J. Pharm. Biopharm. 154, 108–115. doi:10.1016/j.ejpb.2020.06.021
Jakubovski, E., Pisarenko, A., Fremer, C., Haas, M., May, M., Schumacher, C., et al. (2020). The CANNA-TICS Study Protocol: A Randomized Multi-Center Double-Blind Placebo Controlled Trial to Demonstrate the Efficacy and Safety of Nabiximols in the Treatment of Adults with Chronic Tic Disorders. Front. Psychiatry 11, 575826. doi:10.3389/fpsyt.2020.575826
Johnson, J. K., Johnson, R. M., Hodgkin, D., Jones, A. A., Kritikos, A., Doonan, S. M., et al. (2021). Medical Marijuana Laws (MMLs) and Dispensary Provisions Not Associated with Higher Odds of Adolescent Marijuana or Heavy Marijuana Use: A 46 State Analysis, 1991-2015. Subst. Abus 42 (4), 471–475. doi:10.1080/08897077.2021.1900986
Kayser, R. R., Haney, M., Raskin, M., Arout, C., and Simpson, H. B. (2020). Acute Effects of Cannabinoids on Symptoms of Obsessive-Compulsive Disorder: A Human Laboratory Study. Depress Anxiety 37 (8), 801–811. doi:10.1002/da.23032
Krcevski-Skvarc, N., Wells, C., and Häuser, W. (2018). Availability and Approval of Cannabis-Based Medicines for Chronic Pain Management and Palliative/supportive Care in Europe: A Survey of the Status in the Chapters of the European Pain Federation. Eur. J. Pain 22 (3), 440–454. doi:10.1002/ejp.1147
Lafaye, G., Karila, L., Blecha, L., and Benyamina, A. (2017). Cannabis, Cannabinoids, and Health. Dialogues Clin. Neurosci. 19 (3), 309–316. doi:10.31887/DCNS.2017.19.3/glafaye
Lawn, W., Hill, J., Hindocha, C., Yim, J., Yamamori, Y., Jones, G., et al. (2020). The Acute Effects of Cannabidiol on the Neural Correlates of Reward Anticipation and Feedback in Healthy Volunteers. J. Psychopharmacol. 34 (9), 969–980. doi:10.1177/0269881120944148
Lintzeris, N., Mills, L., Dunlop, A., Copeland, J., Mcgregor, I., Bruno, R., et al. (2020). Amp; Agonist Replacement for Cannabis Dependence Study Group. (Arc-D)Cannabis Use in Patients 3 Months after Ceasing Nabiximols for the Treatment of Cannabis Dependence: Results from a Placebo-Controlled Randomised Trial. Drug alcohol dependence 215, 108220. doi:10.1016/j.drugalcdep.2020.108220
Liu, Z., Galettis, P., Broyd, S. J., van Hell, H., Greenwood, L. M., de Krey, P., et al. (2020). Model-based Analysis on Systemic Availability of Co-administered Cannabinoids after Controlled Vaporised Administration. Intern Med. J. 50 (7), 846–853. doi:10.1111/imj.14415
Lopez, H. L., Cesareo, K. R., Raub, B., Kedia, A. W., Sandrock, J. E., Kerksick, C. M., et al. (2020). Effects of Hemp Extract on Markers of Wellness, Stress Resilience, Recovery and Clinical Biomarkers of Safety in Overweight, but Otherwise Healthy Subjects. J. Diet. Suppl. 17 (5), 561–586. doi:10.1080/19390211.2020.1765941
Marketrealist (2019). Medical Cannabis in Brazil Is Coming. Available at: https://marketrealist.com/2019/12/medical-cannabis-in-brazil-is-coming/ (Accessed February 15th, 2022).
Mead, A. (2017). The Legal Status of Cannabis (Marijuana) and Cannabidiol (CBD) under U.S. Law. Epilepsy Behav. 70 (Pt B), 288–291. doi:10.1016/j.yebeh.2016.11.021
Medicines and healthcare products Regulatory Agency (2020). The Supply, Manufacture, Importation and Distribution of Unlicensed Cannabis-Based Products for Medicinal Use in Humans ‘specials’. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/869284/Cannabis_Guidance__unlicensed_CBPMs__updated_2020.pdf (Accessed February 15th, 2022).
Mersiades, A. J., Stockler, M. R., Olver, I. N., and Grimison, P. (2019). Medicinal Cannabis for Chemotherapy-Induced Nausea and Vomiting: Prescribing with Limited Evidence. Med. J. Aust. 210 (1), 11. doi:10.5694/mja17.01099
Ministério da Saúde (2019). Resolução da Diretoria Colegiada n 327. Available at: https://www.in.gov.br/en/web/dou/-/resolucao-da-diretoria-colegiada-rdc-n-327-de-9-de-dezembro-de-2019-232669072 (Accessed February 15th, 2022).
Mitelpunkt, A., Kramer, U., Hausman Kedem, M., Zilbershot Fink, E., Orbach, R., Chernuha, V., et al. (2019). The Safety, Tolerability, and Effectiveness of PTL-101, an Oral Cannabidiol Formulation, in Pediatric Intractable Epilepsy: A Phase II, Open-Label, Single-Center Study. Epilepsy Behav. 98 (Pt A), 233–237. doi:10.1016/j.yebeh.2019.07.007
MS Society (2021). Sativex (Nabiximols). Available at: https://www.mssociety.org.uk/about-ms/treatments-and-therapies/cannabis/sativex (Accessed February 15th, 2022).
Naftali, T., Bar-Lev Schleider, L., Scklerovsky Benjaminov, F., Konikoff, F. M., Matalon, S. T., and Ringel, Y. (2021b). Cannabis Is Associated with Clinical but Not Endoscopic Remission in Ulcerative Colitis: A Randomized Controlled Trial. PloS one 16 (2), e0246871. doi:10.1371/journal.pone.0246871
Naftali, T., Bar-Lev Schleider, L., Almog, S., Meiri, D., and Konikoff, F. M. (2021a). Oral CBD-Rich Cannabis Induces Clinical but Not Endoscopic Response in Patients with Crohn's Disease, a Randomised Controlled Trial. J. Crohn's colitis 15 (11), 1799–1806. doi:10.1093/ecco-jcc/jjab069
National Conferences of State Legislatures (2022). State Medical Cannabis Laws. Available at: https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx (Accessed February 15th, 2022).
National Health Service (2022). Cannabis-based Products for Medicinal Use: Frequently Asked Questions. Available at: https://www.england.nhs.uk/medicines-2/support-for-prescribers/cannabis-based-products-for-medicinal-use/cannabis-based-products-for-medicinal-use-frequently-asked-questions/ (Accessed February 15th, 2022).
National Health Service (2021). Medical Cannabis (And Cannabis Oils). Available at: https://www.nhs.uk/conditions/medical-cannabis/ (Accessed February 15th, 2022).
O'Neill, A., Wilson, R., Blest-Hopley, G., Annibale, L., Colizzi, M., Brammer, M., et al. (2021). Normalization of Mediotemporal and Prefrontal Activity, and Mediotemporal-Striatal Connectivity, May Underlie Antipsychotic Effects of Cannabidiol in Psychosis. Psychol. Med. 51 (4), 596–606. doi:10.1017/S0033291719003519
Office of Medicinal Cannabis (2022). Products and Services. Available at: https://english.cannabisbureau.nl/ (Accessed February 15th, 2022).
Perkins, D., Butler, J., Ong, K., Nguyen, T. H., Cox, S., Francis, B., et al. (2020). A Phase 1, Randomised, Placebo-Controlled, Dose Escalation Study to Investigate the Safety, Tolerability and Pharmacokinetics of Cannabidiol in Fed Healthy Volunteers. Eur. J. Drug Metab. Pharmacokinet. 45 (5), 575–586. doi:10.1007/s13318-020-00624-6
Pisanti, S., and Bifulco, M. (2019). Medical Cannabis: A Plurimillennial History of an Evergreen. J. Cell Physiol. 234 (6), 8342–8351. doi:10.1002/jcp.27725
Pretzsch, C. M., Freyberg, J., Voinescu, B., Lythgoe, D., Horder, J., Mendez, M. A., et al. (2019a). Effects of Cannabidiol on Brain Excitation and Inhibition Systems; a Randomised Placebo-Controlled Single Dose Trial during Magnetic Resonance Spectroscopy in Adults with and without Autism Spectrum Disorder. Neuropsychopharmacol. official Publ. Am. Coll. Neuropsychopharmacol. 44 (8), 1398–1405. doi:10.1038/s41386-019-0333-8
Pretzsch, C. M., Voinescu, B., Lythgoe, D., Horder, J., Mendez, M. A., Wichers, R., et al. (2019b). Effects of Cannabidivarin (CBDV) on Brain Excitation and Inhibition Systems in Adults with and without Autism Spectrum Disorder (ASD): a Single Dose Trial during Magnetic Resonance Spectroscopy. Transl. Psychiatry 9 (1), 313. doi:10.1038/s41398-019-0654-8
Pretzsch, C. M., Voinescu, B., Mendez, M. A., Wichers, R., Ajram, L., Ivin, G., et al. (2019c). The Effect of Cannabidiol (CBD) on Low-Frequency Activity and Functional Connectivity in the Brain of Adults with and without Autism Spectrum Disorder (ASD). J. Psychopharmacol. 33 (9), 1141–1148. doi:10.1177/0269881119858306
ProCon (2022). Legal Medical Marijuana States and DC. Available at: https://medicalmarijuana.procon.org/legal-medical-marijuana-states-and-dc/ (Accessed February 15th, 2022).
Rasche, T., Emmert, D., Radbruch, L., Conrad, R., and Mücke, M. (2019). Cannabis and Cannabinoids in Palliative Care. Bundesgesundheitsblatt Gesundheitsforsch. Gesundheitsschutz 62 (7), 830–835. doi:10.1007/s00103-019-02967-1
Reuters (2019). Brazil Approves Medical Marijuana Rules, Blocks Cannabis Cultivation. Available at: https://www.reuters.com/article/us-brazil-marijuana/brazil-approves-medical-marijuana-rules-blocks-cannabis-cultivation-idUSKBN1Y726W (Accessed February 15th, 2022).
Romano, L., and Hazekamp, A. (2019). An Overview of Galenic Preparation Methods for Medicinal Cannabis. Cbc 15 (2), 174–195. doi:10.2174/1573407214666180612080412
Ryan, J. E., McCabe, S. E., and Boyd, C. J. (2021). Medicinal Cannabis: Policy, Patients, and Providers. Policy, Polit. Nurs. Pract. 22 (2), 126–133. doi:10.1177/1527154421989609
S, G., Hb, S., K, L., R, T., Bs, R., Ps, S., et al. (2021). Safety and Efficacy of Low-Dose Medical Cannabis Oils in Multiple Sclerosis. Mult. Scler. Relat. Disord. 48, 102708. doi:10.1016/j.msard.2020.102708
Solowij, N., Broyd, S., Greenwood, L. M., van Hell, H., Martelozzo, D., Rueb, K., et al. (2019). A Randomised Controlled Trial of Vaporised Δ9-tetrahydrocannabinol and Cannabidiol Alone and in Combination in Frequent and Infrequent Cannabis Users: Acute Intoxication Effects. Eur. Arch. Psychiatry Clin. Neurosci. 269 (1), 17–35. doi:10.1007/s00406-019-00978-2
State of Israel - Minister of Health (2022). Cannabis for Medical Use and for Research. Available at: https://health.gov.il/English/Topics/cannabis/Pages/default.aspx (Accessed February 15th, 2022).
State of Israel - Minister of Health (2017). The First Course for Doctors to Prescribe Medical Cannabis approval Was Completed. 81 Doctors Will Begin to Authorize the Use of Medical Cannabis. Available at: https://health.gov.il/English/News_and_Events/Spokespersons_Messages/Pages/07092017_2.aspx (Accessed February 15th, 2022).
Stella, B., Baratta, F., Della Pepa, C., Arpicco, S., Gastaldi, D., and Dosio, F. (2021). Cannabinoid Formulations and Delivery Systems: Current and Future Options to Treat Pain. Drugs 81 (13), 1513–1557. doi:10.1007/s40265-021-01579-x
Swiss Confederation, Federal Office of Public Health (2021a). Cannabidiol (CBD) Containing Products - Overview and Implementation Guide. Available at: https://www.swissmedic.ch/swissmedic/it/home/news/mitteilungen/prodotti-contenenti-cbd--cannabidiol---panoramica.html (Accessed February 15th, 2022).
Swiss Confederation, Federal Office of Public Health (2020). Fact Sheet: Hemp-Based Medicines. Available at: https://www.bag.admin.ch/bag/it/home/medizin-und-forschung/heilmittel/med-anwend-cannabis/gesetzesaenderung-cannabisarzneimittel.html (Accessed February 15th, 2022).
Swiss Confederation, Federal Office of Public Health (2021c). Medical Application of Hemp. Available at: https://www.bag.admin.ch/bag/it/home/medizin-und-forschung/heilmittel/med-anwend-cannabis.html (Accessed February 15th, 2022).
Swiss Confederation, Federal Office of Public Health. (2021b). Hemp-based Medicines: Amendment of the Law. Available at: https://www.bag.admin.ch/bag/it/home/medizin-und-forschung/heilmittel/med-anwend-cannabis/gesetzesaenderung-cannabisarzneimittel.html (Accessed February 15th, 2022).
The Health Products Regulatory Authority (2017). Cannabis for Medical Use - A Scientific Review. Available at: https://www.hpra.ie/docs/default-source/publications-forms/newsletters/cannabis-for-medical-use---a-scientific-review.pdf?sfvrsn=7 (Accessed February 15th, 2022).
The Office of Drug Control (2021). Manufacturers and Suppliers of Medicinal Cannabis Products. https://www.odc.gov.au/manufacturers-and-suppliers-medicinal-cannabis-products (Accessed February 15th, 2022).
Thompson, M. D., Martin, R. C., Grayson, L. P., Ampah, S. B., Cutter, G., Szaflarski, J. P., et al. (2020). Cognitive Function and Adaptive Skills after a One-Year Trial of Cannabidiol (CBD) in a Pediatric Sample with Treatment-Resistant Epilepsy. Epilepsy Behav. 111, 107299. doi:10.1016/j.yebeh.2020.107299
Timler, A., Bulsara, C., Bulsara, M., Vickery, A., Smith, J., and Codde, J. (2020). Use of Cannabinoid-Based Medicine Among Older Residential Care Recipients Diagnosed with Dementia: Study Protocol for a Double-Blind Randomised Crossover Trial. Trials 21 (1), 188. doi:10.1186/s13063-020-4085-x
UK Government (2022). Medicinal Cannabis: Information and Resources. Available at: https://www.gov.uk/government/collections/medicinal-cannabis-information-and-resources (Accessed February 15th, 2022).
Urbi, B., Broadley, S., Bedlack, R., Russo, E., and Sabet, A. (2019). Study Protocol for a Randomised, Double-Blind, Placebo-Controlled Study Evaluating the Efficacy of Cannabis-Based Medicine Extract in Slowing the Disease pRogression of Amyotrophic Lateral Sclerosis or Motor Neurone Disease: the EMERALD Trial. BMJ open 9 (11), e029449. doi:10.1136/bmjopen-2019-029449
Van de Donk, T., Niesters, M., Kowal, M. A., Olofsen, E., Dahan, A., and van Velzen, M. (2019). An Experimental Randomized Study on the Analgesic Effects of Pharmaceutical-Grade Cannabis in Chronic Pain Patients with Fibromyalgia. Pain 160 (4), 860–869. doi:10.1097/j.pain.0000000000001464
Van der Flier, F. E., Kwee, C. M. B., Cath, D. C., Batelaan, N. M., Groenink, L., Duits, P., et al. (2019). Cannabidiol Enhancement of Exposure Therapy in Treatment Refractory Patients with Phobias: Study Protocol of a Randomized Controlled Trial. BMC psychiatry 19 (1), 69. doi:10.1186/s12888-019-2022-x
Wall, M. B., Pope, R., Freeman, T. P., Kowalczyk, O. S., Demetriou, L., Mokrysz, C., et al. (2019). Dissociable Effects of Cannabis with and without Cannabidiol on the Human Brain's Resting-State Functional Connectivity. J. Psychopharmacol. 33 (7), 822–830. doi:10.1177/0269881119841568
Whiting, P. F., Wolff, R. F., Deshpande, S., Di Nisio, M., Duffy, S., Hernandez, A. V., et al. (2015). Cannabinoids for Medical Use: A Systematic Review and Meta-Analysis. JAMA 313 (24), 2456–2473. doi:10.1001/jama.2015.6358
Xu, D. H., Cullen, B. D., Tang, M., and Fang, Y. (2020). The Effectiveness of Topical Cannabidiol Oil in Symptomatic Relief of Peripheral Neuropathy of the Lower Extremities. Curr. Pharm. Biotechnol. 21 (5), 390–402. doi:10.2174/1389201020666191202111534
Yassin, M., Oron, A., and Robinson, D. (2019). Effect of Adding Medical Cannabis to Analgesic Treatment in Patients with Low Back Pain Related to Fibromyalgia: an Observational Cross-Over Single Centre Study. Clin. Exp. Rheumatol. 37, 13–20.
Zylla, D. M., Eklund, J., Gilmore, G., Gavenda, A., Guggisberg, J., VazquezBenitez, G., et al. (2021). A Randomized Trial of Medical Cannabis in Patients with Stage IV Cancers to Assess Feasibility, Dose Requirements, Impact on Pain and Opioid Use, Safety, and Overall Patient Satisfaction. Support Care Cancer 29 (12), 7471–7478. doi:10.1007/s00520-021-06301-x
Keywords: medical Cannabis , clinical trials, study protocols, legislation, law
Citation: Baratta F, Pignata I, Ravetto Enri L and Brusa P (2022) Cannabis for Medical Use: Analysis of Recent Clinical Trials in View of Current Legislation. Front. Pharmacol. 13:888903. doi: 10.3389/fphar.2022.888903
Received: 03 March 2022; Accepted: 09 May 2022; Published: 25 May 2022.
Reviewed by:
Copyright © 2022 Baratta, Pignata, Ravetto Enri and Brusa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: F. Baratta, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Introduction
- Conclusions
- Article Information
AE indicates adverse event; RCT, randomized controlled trial; and SR, systematic review.
a These excluded reports were screened as full-text articles/reports.
b The number of included RCTS does not sum because some were included in more than 1 indication category.
Odds indicate 30% or greater improvement in pain with cannabinoid compared with placebo, stratified according to cannabinoid. The square data markers indicate odds ratios (ORs) from primary studies, with sizes reflecting the statistical weight of the study using random-effects meta-analysis. The horizontal lines indicate 95% CIs. The blue diamond data markers represent the subtotal and overall OR and 95% CI. The vertical dashed line shows the summary effect estimate, the dotted shows the line of no effect (OR = 1).
The square data markers indicate mean differences from primary studies, with sizes reflecting the statistical weight of the study using random-effects meta-analysis. The horizontal line indicate, 95% CIs. The blue diamond data markers represent the subtotal and overall weighted mean difference and 95% CI. The vertical dashed line shows the summary effect estimate, the solid vertical line shows the line of no effect (mean difference = 0).
The square data markers indicate odds ratios (ORs) from primary studies, with sizes reflecting the statistical weight of the study using random-effects meta-analysis. The horizontal lines indicate 95% CIs. The blue diamond data markers represent the subtotal and overall OR and 95% CI. The vertical dashed line shows the summary effect estimate, the dotted line shows the line of no effect (OR = 1).
eAppendix 1. Review Protocol
eAppendix 2. Search Strategy
eAppendix 3. Nausea and Vomiting Due to Chemotherapy
eAppendix 4. Appetite Stimulation in HIV/AIDS
eAppendix 5. Chronic Pain
eAppendix 6. Spasticity in MS and Paraplegia
eAppendix 7. Depression
eAppendix 8. Anziety Disorder
eAppendix 9. Sleep Disorder
eAppendix 10. Psychosis
eAppendix 11. Glaucoma
eAppendix 12. Tourette Syndrome
eAppendix 13. Risk of Bias in Each Included Study, Grouped by Indication
eReferences
- Medical Marijuana JAMA Editorial June 23, 2015 This Editorial discusses some of the medical and legal considerations surrounding use of medical marijuana and cannabinoid drugs. Deepak Cyril D'Souza, MBBS, MD; Mohini Ranganathan, MD
- Medical Marijuana for Treatment of Chronic Pain and Other Problems JAMA Clinical Crossroads June 23, 2015 This synopsis of a medicine grand rounds conference uses an example of a patient with chronic low back pain as the basis for discussing the use of medical marijuana for treating chronic pain and other disorders. Kevin P. Hill, MD, MHS
- Incorrect Figure Label JAMA Correction August 4, 2015
- Incorrect Values Reported JAMA Correction August 25, 2015
- Medical Use of Cannabinoids JAMA Comment & Response October 27, 2015 Igor Grant, MD
- Medical Use of Cannabinoids JAMA Comment & Response October 27, 2015 Michael E. Schatman, PhD
- Medical Use of Cannabinoids JAMA Comment & Response October 27, 2015 Penny Whiting, PhD; Robert Wolff, MD
- Incorrect Nonproprietary Drug Name and Approved Use JAMA Correction December 1, 2015
- Incorrect Effect Estimate JAMA Correction April 12, 2016
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Whiting PF , Wolff RF , Deshpande S, et al. Cannabinoids for Medical Use : A Systematic Review and Meta-analysis . JAMA. 2015;313(24):2456–2473. doi:10.1001/jama.2015.6358
Manage citations:
© 2024
- Permissions
Cannabinoids for Medical Use : A Systematic Review and Meta-analysis
- 1 School of Social and Community Medicine, University of Bristol, Bristol, United Kingdom
- 2 The National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care West at University Hospitals, Bristol NHS Foundation Trust, Bristol, United Kingdom
- 3 Kleijnen Systematic Reviews Ltd, Escrick, York, United Kingdom
- 4 Department of Medical, Oral, and Biotechnological Sciences, University “G. D'Annunzio” of Chieti-Pescara, Chieti, Italy
- 5 Department of Vascular Medicine, Academic Medical Center, Amsterdam, the Netherlands
- 6 Medical School, Universidad Peruana de Ciencias Aplicadas (UPC), Lima, Peru
- 7 Health Outcomes and Clinical Epidemiology Section, Department of Quantitative Health Sciences, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio
- 8 Department of Pathology, Radboud University Medical Center, Nijmegen, the Netherlands
- 9 Institut für Epidemiologie und kongenitale Erkrankungen, Cepicon GmbH, Hamburg, Germany
- 10 School for Public Health and Primary Care (CAPHRI), Maastricht University, Maastricht, the Netherlands
- Editorial Medical Marijuana Deepak Cyril D'Souza, MBBS, MD; Mohini Ranganathan, MD JAMA
- Clinical Crossroads Medical Marijuana for Treatment of Chronic Pain and Other Problems Kevin P. Hill, MD, MHS JAMA
- Correction Incorrect Figure Label JAMA
- Correction Incorrect Values Reported JAMA
- Comment & Response Medical Use of Cannabinoids Igor Grant, MD JAMA
- Comment & Response Medical Use of Cannabinoids Michael E. Schatman, PhD JAMA
- Comment & Response Medical Use of Cannabinoids Penny Whiting, PhD; Robert Wolff, MD JAMA
- Correction Incorrect Nonproprietary Drug Name and Approved Use JAMA
- Correction Incorrect Effect Estimate JAMA
Importance Cannabis and cannabinoid drugs are widely used to treat disease or alleviate symptoms, but their efficacy for specific indications is not clear.
Objective To conduct a systematic review of the benefits and adverse events (AEs) of cannabinoids.
Data Sources Twenty-eight databases from inception to April 2015.
Study Selection Randomized clinical trials of cannabinoids for the following indications: nausea and vomiting due to chemotherapy, appetite stimulation in HIV/AIDS, chronic pain, spasticity due to multiple sclerosis or paraplegia, depression, anxiety disorder, sleep disorder, psychosis, glaucoma, or Tourette syndrome.
Data Extraction and Synthesis Study quality was assessed using the Cochrane risk of bias tool. All review stages were conducted independently by 2 reviewers. Where possible, data were pooled using random-effects meta-analysis.
Main Outcomes and Measures Patient-relevant/disease-specific outcomes, activities of daily living, quality of life, global impression of change, and AEs.
Results A total of 79 trials (6462 participants) were included; 4 were judged at low risk of bias. Most trials showed improvement in symptoms associated with cannabinoids but these associations did not reach statistical significance in all trials. Compared with placebo, cannabinoids were associated with a greater average number of patients showing a complete nausea and vomiting response (47% vs 20%; odds ratio [OR], 3.82 [95% CI, 1.55-9.42]; 3 trials), reduction in pain (37% vs 31%; OR, 1.41 [95% CI, 0.99-2.00]; 8 trials), a greater average reduction in numerical rating scale pain assessment (on a 0-10-point scale; weighted mean difference [WMD], −0.46 [95% CI, −0.80 to −0.11]; 6 trials), and average reduction in the Ashworth spasticity scale (WMD, −0.12 [95% CI, −0.24 to 0.01]; 5 trials). There was an increased risk of short-term AEs with cannabinoids, including serious AEs. Common AEs included dizziness, dry mouth, nausea, fatigue, somnolence, euphoria, vomiting, disorientation, drowsiness, confusion, loss of balance, and hallucination.
Conclusions and Relevance There was moderate-quality evidence to support the use of cannabinoids for the treatment of chronic pain and spasticity. There was low-quality evidence suggesting that cannabinoids were associated with improvements in nausea and vomiting due to chemotherapy, weight gain in HIV infection, sleep disorders, and Tourette syndrome. Cannabinoids were associated with an increased risk of short-term AEs.
Cannabis is a generic term used for drugs produced from plants belonging to the genus Cannabis . 1 It is one of the most popular recreational drugs; worldwide, an estimated 178 million people aged 15 to 64 years used cannabis at least once in 2012. 2 Cannabis was included as a controlled drug in the United Nations’ Single Convention on Narcotic Drugs, held in 1961, 3 and its use is illegal in most countries.
Medical cannabis refers to the use of cannabis or cannabinoids as medical therapy to treat disease or alleviate symptoms. Cannabinoids can be administered orally, sublingually,or topically; they can be smoked, inhaled, mixed with food, or made into tea. They can be taken in herbal form, extracted naturally from the plant, gained by isomerisation of cannabidiol, or manufactured synthetically. 4 Prescribed cannabinoids include dronabinol capsules, nabilone capsules, and the oromucosal spray nabiximols. 4 Some countries have legalized medicinal-grade cannabis for chronically ill patients. Canada and the Netherlands have government-run programs in which specialized companies supply quality-controlled herbal cannabis. 5 In the United States, 23 states and Washington, DC (May 2015), have introduced laws to permit the medical use of cannabis 6 ; other countries have similar laws. The aim of this systematic review was to evaluate the evidence for the benefits and adverse events (AEs) of medical cannabinoids across a broad range of indications.
This review followed guidance published by the Centre for Reviews and Dissemination and the Cochrane Collaboration. 7 , 8 We established a protocol for the review (eAppendix 1 in Supplement 1 ).
Randomized clinical trials (RCTs) that compared cannabinoids with usual care, placebo, or no treatment in the following indications were eligible: nausea and vomiting due to chemotherapy, appetite stimulation in HIV/AIDS, chronic pain, spasticity due to multiple sclerosis (MS) or paraplegia, depression, anxiety disorder, sleep disorder, psychosis, intraocular pressure in glaucoma, or Tourette syndrome. These indications were prespecified by the project funders, the Swiss Federal Office of Public Health. If no RCTs were available for a particular indication or outcome (eg, long-term AEs such as cancer, psychosis, depression, or suicide), nonrandomized studies including uncontrolled studies (such as case series) with at least 25 patients were eligible.
Twenty-eight databases and gray literature sources were searched from inception to April 2015 without language restriction (Embase search strategy and details of databases searched available in eAppendix 2 in Supplement 2 ). The search strategy was peer reviewed 9 by a second information specialist. Reference lists of included studies were screened. Search results and full-text articles were independently assessed by 2 reviewers; disagreements were resolved through consensus or referral to a third reviewer.
We extracted data about baseline characteristics and outcomes (patient-relevant and disease-specific outcomes, activities of daily living, quality of life, global impression of change, and specified AEs). For dichotomous data such as number of patients with at least 30% improvement in pain, we calculated the odds ratio (OR) and 95% CI. For categorical data, we extracted details about each category assessed and the numbers of patients with an outcome in each category. Continuous data such as the Ashworth spasticity score 10 were extracted as means and SDs at baseline, follow-up, and the change from baseline and used to calculate mean differences with 95% CIs. Results (mean difference, 95% CIs, and P values) from the between-group statistical analyses reported by the study were also extracted. All relevant sources were used for data extraction including full-text journal articles, abstracts, and clinical trial registry entries. Where available, the journal article was used as the primary publication because it had been peer reviewed.
RCTs were assessed for methodological quality using the Cochrane Risk of Bias tool. 11 If at least one of the domains was rated as high, the trial was considered at high risk of bias. If all domains were judged as low, the trial was considered at low risk of bias. Otherwise, the trial was considered as having unclear risk of bias. Data extraction and risk-of-bias assessment were performed independently by 2 reviewers; disagreements were resolved by a third reviewer.
Clinical heterogeneity was assessed by grouping studies by indication, cannabinoid, and outcome. If there were 2 or more trials within a single grouping, data were pooled using random-effects meta-analysis. 12 For continuous outcomes, we analyzed the mean difference in change from baseline; if this was not reported and could not be calculated from other data, we used the mean difference at follow-up. 13 For dichotomous data, we used the OR. In order to avoid double counting, we selected a single data set from each study to contribute to the analysis. For studies evaluating multiple interventions, we selected the intervention or dose that was most similar to the other interventions being evaluated in the same analysis. Heterogeneity was investigated using forest plots and the I 2 statistic. Where data were considered too heterogeneous to pool or not reported in a format suitable for pooling (eg, data reported as medians), we used a narrative synthesis.
Sensitivity analyses were used to assess the statistical effect of trial design. The primary analysis included only parallel-group trials, results from crossover trials were included in an additional analysis. For the analysis of AEs, data for all conditions were combined. We conducted stratified analyses and meta-regression to investigate whether associations varied according to type of cannabinoid, study design (parallel group vs crossover trial), indication (each of the indication categories included in this report), comparator (active vs placebo), and duration of follow-up (<24 hours, 24 hours-1 week, >1 week-4 weeks, >4 weeks) for the outcome of any AE. Statistical analyses were performed using Stata statistical software (version 10).
GRADE (Grading of Recommendations Assessment, Development and Evaluation) was used to rate the overall quality of the evidence for risk of bias, publication bias, imprecision, inconsistency, indirectness, and magnitude of effect. The GRADE ratings of very low–, low-, moderate-, or high-quality evidence reflect the extent to which we are confident that the effect estimates are correct. 14
The searches identified 23 754 hits (records) of which 505 were considered potentially relevant, based on title and abstract screening, and obtained as full-text studies. A total of 79 studies (6462 participants), available as 151 reports, were included; 3 studies (6 reports) were included in multiple indication categories ( Figure 1 ). Thirty-four studies were parallel-group trials (4436 participants), and 45 were crossover trials (2026 participants). Four studies were available only as an abstract, 15 - 18 a further 3 were available only as abstracts 19 - 21 but with additional details available on trial registries including full results in one, 19 and details of 2 trials (including full trial results) were available only as trial registry entries 22 , 23 ; all other trials were reported in full-length journal articles. Where reported, the proportion of participants who were men ranged from 0% to 100% (median, 50% [57 studies]), and the proportion of white participants ranged from 50% to 99% (median, 78% [18 studies]). Publication dates ranged from 1975 to 2015 (median, 2004 [with one-third of trials published before 1990]). Studies were conducted in a wide range of countries. A variety of cannabinoids were evaluated and compared with various different active comparators or placebos; most active comparators were included in the nausea and vomiting indication ( Table 1 ). eAppendices 3 to 12 in Supplement 1 provide an overview of the included studies and their findings.
Four (5%) trials were judged at low risk of bias, 55 (70%) were judged at high risk of bias, and 20 (25%) at unclear risk of bias (eAppendix 13 in Supplement 2 ). The major potential source of bias in the trials was incomplete outcome data. More than 50% of trials reported substantial withdrawals and did not adequately account for this in the analysis. Selective outcome reporting was a potential risk of bias in 16% of trials. These studies did not report data for all outcomes specified in the trial register, protocol, or methods section or changed the primary outcome from that which was prespecified. Most studies reported being double-blinded but only 57% reported that appropriate methods had been used for participant blinding and only 24% reported that outcome assessors had been appropriately blinded.
Full results from included studies are presented in eAppendices 3-12 in Supplement 2 ; pooled results and GRADE ratings are presented in Table 2 .
Nausea and vomiting due to chemotherapy was assessed in 28 studies (37 reports; 1772 participants). 15 , 16 , 24 - 58 Fourteen studies assessed nabilone and there were 3 for dronabinol, 1 for nabiximols, 4 for levonantradol, and 6 for THC. Two studies also included a combination therapy group of dronabinol with ondansetron or prochlorperazine. Eight studies included a placebo control, 3 of these also included an active comparator, and 20 studies included only an active comparator. The most common active comparators were prochlorperazine (15 studies), chlorpromazine (2 studies) and domperidone (2 studies). Other comparators (alizapride, hydroxyzine, metoclopramide and ondansetron) were evaluated in single studies ( Table 1 ). Of all 28 studies, risk of bias was high for 23 or unclear for 5. All studies suggested a greater benefit of cannabinoids compared with both active comparators and placebo, but these did not reach statistical significance in all studies. The average number of patients showing a complete nausea and vomiting response was greater with cannabinoids (dronabinol or nabiximols) than placebo (OR, 3.82 [95% CI, 1.55-9.42]; 3 trials). There was no evidence of heterogeneity for this analysis ( I 2 = 0%) and results were similar for both dronabinol and nabiximols.
Appetite stimulation in HIV/AIDS was assessed in 4 studies (4 reports; 255 participants). 59 - 62 All studies assessed dronabinol, 3 compared with placebo (1 of which also assessed marijuana), and 1 compared with megastrol acetate. All studies were at high risk of bias. There was some evidence that dronabinol is associated with an increase in weight when compared with placebo. More limited evidence suggested that it may also be associated with increased appetite, greater percentage of body fat, reduced nausea, and improved functional status. However, these outcomes were mostly assessed in single studies and associations failed to reach statistical significance. The trial that evaluated marijuana and dronabinol found significantly greater weight gain with both forms of cannabinoid when compared with placebo. 59 The active comparison trial found that megastrol acetate was associated with greater weight gain than dronabinol and that combining dronabinol with megastrol acetate did not lead to additional weight gain. 60
Chronic pain was assessed in 28 studies (63 reports; 2454 participants). 19 , 20 , 22 , 23 , 63 - 120 Thirteen studies evaluated nabiximols, 4 were for smoked THC, 5 for nabilone, 3 for THC oromucosal spray, 2 dronabinol, 1 vaporized cannabis (included 2 doses), 1 for ajuvenic acid capsules, and 1 for oral THC. One trial compared nabilone with amitriptyline 64 ; all other studies were placebo controlled. One of these studies evaluated nabilone as an adjunctive treatment to gabapentin. 121 The conditions causing the chronic pain varied between studies and included neuropathic pain (central, peripheral, or not specified; 12 studies), 3 for cancer pain, 3 for diabetic peripheral neuropathy, 2 for fibromyalgia, 2 for HIV-associated sensory neuropathy, and 1 study for each of the following indications: refractory pain due to MS or other neurological conditions, for rheumatoid arthritis, for noncancer pain (nociceptive and neuropathic), central pain (not specified further), musculoskeletal problems, and chemotherapy-induced pain.
Two studies were at low risk of bias, 9 at unclear risk, and 17 at high risk of bias. Studies generally suggested improvements in pain measures associated with cannabinoids but these did not reach statistical significance in most individual studies.
The average number of patients who reported a reduction in pain of at least 30% was greater with cannabinoids than with placebo (OR, 1.41 [95% CI, 0.99-2.00]; 8 trials; Figure 2 ). One trial assessed smoked THC 77 and reported the greatest beneficial effect (OR, 3.43 [95% CI, 1.03-11.48]), and 7 trials assessed nabiximols ( Figure 2 ). Pain conditions evaluated in these trials were neuropathic pain (OR, 1.38 [95% CI, 0.93-2.03]; 6 trials) and cancer pain (OR, 1.41 [95% CI, 0.99-2.00]; 2 trials), with no clear differences between pain conditions. Nabiximols was also associated with a greater average reduction in the Numerical Rating Scale (NRS; 0-10 scale) assessment of pain (weighted mean difference [WMD], −0.46 [95% CI, −0.80 to −0.11]; 6 trials), brief pain inventory-short form, severity composite index (WMD, −0.17 [95% CI, −0.50 to 0.16]; 3 trials), neuropathic pain scale (WMD, −3.89 [95% CI, −7.32 to −0.47]; 5 trials), and the proportion of patients reporting improvement on a global impression of change score (OR, 2.08 [95% CI, 1.21 to 3.59]; 6 trials) compared with placebo. There was some evidence to support this based on continuous data but this was not consistent across trials. There was no difference in average quality-of-life scores as measured by the EQ-5D health status index (WMD, −0.01 [95% CI, −0.05 to 0.02]; 3 trials) between nabiximols and placebo. Two of the studies included in the meta-analysis for the NRS (0-10 scale) assessed patients with cancer pain, all other studies assessed patients with neuropathic pain. There were no clear differences based on cause of pain in the meta-analysis of NRS. Sensitivity analyses that included crossover trials showed results consistent with those based on parallel-group trials alone.
Fourteen studies (33 reports; 2280 participants) assessed spasticity due to MS or paraplegia. 17 , 19 , 65 , 87 , 91 , 122 - 149 Eleven studies (2138 participants) included patients with MS and 3 included patients with paraplegia (142 participants) caused by spinal cord injury. Six studies assessed nabiximols, 3 for dronabinol, 1 for nabilone, 4 for THC/CBD (2 of these also assessed dronabinol), and 1 each for ECP002A and smoked THC. All studies included a placebo control group; none included an active comparator. Two studies were at low risk of bias, 5 were at unclear risk of bias, and 7 were at high risk of bias. Studies generally suggested that cannabinoids were associated with improvements in spasticity, but this failed to reach statistical significance in most studies. There were no clear differences based on type of cannabinoid. Only studies in MS patients reported sufficient data to allow summary estimates to be generated. Cannabinoids (nabiximols, dronabinol, and THC/CBD) were associated with a greater average improvement on the Ashworth scale for spasticity compared with placebo, although this did not reach statistical significance (WMD, −0.12 [95% CI, −0.24 to 0.01]; 5 trials; Figure 3 ). Cannabinoids (nabilone and nabiximols) were also associated with a greater average improvement in spasticity assessed using numerical rating scales (mean difference, −0.76 [95% CI, −1.38 to −0.14]; 3 trials). There was no evidence of a difference in association according to type of cannabinoid for either analysis. Other measures of spasticity also suggested a greater benefit of cannabinoid but did not reach statistical significance ( Table 2 ). The average number of patients who reported an improvement on a global impression of change score was also greater with nabiximols than placebo (OR, 1.44 [95% CI, 1.07 to 1.94]; 3 trials); this was supported by a further crossover trial of dronabinol and oral THC/CBD that provided continuous data for this outcome. 132 Sensitivity analyses that included crossover trials showed results consistent with those based on parallel group trials alone.
No studies evaluating cannabinoids for the treatment of depression fulfilled inclusion criteria. Five studies included for other indications reported depression as an outcome measure; 4 evaluated chronic pain and 1 evaluated spasticity in MS patients. 67 , 73 , 75 , 80 , 129 One trial assessed dronabinol (2 doses), 3 assessed nabiximols, and 1 assessed nabilone. Two studies were rated as having unclear risk of bias and 3 as having high risk of bias. Three studies suggested no difference between cannabinoids (dronabinol and nabiximols) and placebo in depression outcomes. One parallel-group trial that compared different doses of nabiximols with placebo reported a negative effect of nabiximols for the highest dose (11-14 sprays per day) compared with placebo (mean difference from baseline, 2.50 [95% CI, 0.38 to 4.62]) but no difference between placebo and the 2 lower doses. 67
One small parallel-group trial, judged at high risk of bias, evaluated patients with generalized social anxiety disorder. 150 The trial reported that cannabidiol was associated with a greater improvement on the anxiety factor of a visual analogue mood scale (mean difference from baseline, −16.52; P value = .01)compared with placebo during a simulated public speaking test. Additional data about anxiety outcomes provided by 4 studies (1 parallel group) in patients with chronic pain also suggested a greater benefit of cannabinoids (dronabinol, nabilone, and nabiximols) than placebo but these studies were not restricted to patients with anxiety disorders. 73 - 75 , 80
Two studies (5 reports; 54 participants) evaluated cannabinoids (nabilone) specifically for the treatment of sleep problems. One was a parallel-group trial judged at high risk of bias. This reported a a greater benefit of nabilone compared with placebo on the sleep apnea/hypopnea index (mean difference from baseline, −19.64; P value = .02). The other was a crossover trial judged at low risk of bias in patients with fibromyalgia and compared nabilone with amitriptyline. This suggested that nabilone was associated with improvements in insomnia (mean difference from baseline, −3.25 [95% CI, −5.26 to −1.24]) and with greater sleep restfulness (mean difference from baseline, 0.48 [95% CI, 0.01 to 0.95]). Nineteen placebo-controlled studies included for other indications (chronic pain and MS) also evaluated sleep as an outcome. 22 , 23 , 65 , 67 - 69 , 75 , 76 , 79 - 81 , 87 , 88 , 123 - 125 , 129 - 131 Thirteen studies assessed nabiximols, 1 for nabilone, 1 for dronabinol, 2 for THC/CBD capsules, and two assessed smoked THC (one at various doses). Two of the studies that assessed nabiximols also assessed oral THC and the trial of dronabinol also assessed oral THC/CBD. There was some evidence that cannabinoids may improve sleep in these patient groups. Cannabinoids (mainly nabiximols) were associated with a greater average improvement in sleep quality (WMD, −0.58 [95% CI, −0.87 to −0.29]; 8 trials) and sleep disturbance (WMD, −0.26 [95% CI, −0.52 to 0.00]; 3 trials). One trial assessed THC/CBD, all others assessed nabiximols, results were similar for both cannabinoids.
Psychosis was assessed in 2 studies (9 reports; 71 participants) judged at high risk of bias, which evaluated cannabidiol compared with amisulpride or placebo. 21 , 151 - 158 The trials found no difference in mental health outcomes between treatment groups.
One very small crossover trial (6 participants) 159 judged at unclear risk of bias compared tetrahydrocannabinol (THC; 5 mg), cannabidiol (20 mg), cannabidiol (40 mg) oromucosal spray, and placebo. This trial found no difference between placebo and cannabinoids on measures of intraocular pressure in patients with glaucoma.
Two small placebo-controlled studies (4 reports; 36 participants) 160 - 163 suggested that THC capsules may be associated with a significant improvement in tic severity in patients with Tourette syndrome.
Data about AEs were reported in 62 studies (127 reports). Meta-regression and stratified analysis showed no evidence for a difference in the association of cannabinoids with the incidence of “any AE” based on type of cannabinoid, study design, indication, comparator, or duration of follow-up 15 , 16 , 18 , 22 - 26 , 28 - 31 , 33 - 38 , 41 , 42 , 44 - 47 , 51 , 57 , 58 , 60 , 62 , 64 - 69 , 72 - 85 , 87 , 88 , 123 - 127 , 129 - 131 , 159 , 160 , 162 ; further analyses were conducted for all studies combined. Figure 4 shows the results of the meta-analyses for the number of participants experiencing any AE compared when compared with controls, stratified according to cannabinoid. Cannabinoids were associated with a much greater risk of any AE, serious AE, withdrawals due to AE, and a number of specific AEs ( Table 3 ). No studies evaluating the long-term AEs of cannabinoids were identified, even when searches were extended to lower levels of evidence.
We conducted an extensive systematic review of the benefits and AEs associated with medical cannabinoids across a broad range of conditions. We included 79 RCTs (6462 participants), the majority of which evaluated nausea and vomiting due to chemotherapy or chronic pain and spasticity due to MS and paraplegia. Other patient categories were evaluated in fewer than 5 studies.
Most studies suggested that cannabinoids were associated with improvements in symptoms, but these associations did not reach statistical significance in all studies. Based on the GRADE approach, there was moderate-quality evidence to suggest that cannabinoids may be beneficial for the treatment of chronic neuropathic or cancer pain (smoked THC and nabiximols) and spasticity due to MS (nabiximols, nabilone, THC/CBD capsules, and dronabinol). There was low-quality evidence suggesting that cannabinoids were associated with improvements in nausea and vomiting due to chemotherapy (dronabinol and nabiximols), weight gain in HIV (dronabinol), sleep disorders (nabilone, nabiximols), and Tourette syndrome (THC capsules); and very low-quality evidence for an improvement in anxiety as assessed by a public speaking test (cannabidiol). There was low-quality evidence for no effect on psychosis (cannabidiol) and very low-level evidence for no effect on depression (nabiximols). There was an increased risk of short-term AEs with cannabinoid use, including serious AEs. Common AEs included asthenia, balance problems, confusion, dizziness, disorientation, diarrhea, euphoria, drowsiness, dry mouth, fatigue, hallucination, nausea, somnolence, and vomiting. There was no clear evidence for a difference in association (either beneficial or harmful) based on type of cannabinoids or mode of administration. Only 2 studies evaluated cannabis. 59 , 77 There was no evidence that the effects of cannabis differed from other cannabinoids.
This review followed recommendations for rigorous systematic reviews. 7 , 8 In order to identify as many relevant studies as possible and reduce the risk of publication bias, a highly sensitive search strategy was used and an extensive range of resources were searched including electronic databases, guidelines, and systematic reviews. Both published and unpublished trials were eligible for inclusion. There were no date or language restrictions. In order to minimize bias and errors, the main Embase strategies were peer reviewed by a second independent information specialist 165 and all stages of the review process were performed independently by 2 reviewers. We used the Cochrane risk of bias tool 11 to assess the included RCTs. This highlighted a number of methodological weaknesses in the included trials including failure to appropriately handle withdrawals, selective outcome reporting, and inadequate description of methods of randomization, allocation concealment, and blinding. An additional limitation of many included studies was their very small sample sizes. This was particularly the case for the trial of glaucoma (N = 6), Tourette syndrome (average N = 18), sleep disorder (average N = 27), and anxiety disorder (N = 24), which means these studies may have lacked the power to detect differences between treatment groups.
The synthesis combined a narrative discussion of individual study results with meta-analysis (for studies in which suitable data were available), supplemented by interpretation (following guidance of the GRADE Working Group). 14 The data analysis was complicated by a number of issues. The included studies used a large variety of measures to evaluate outcomes, and even very similar outcomes were often assessed using different measures. Furthermore, a wide range of time points were reported in the included trials, which limited the applicability of the findings of these studies. Multiple different cannabinoids were evaluated in the included studies. We stratified analyses based on type of cannabinoid to investigate whether there were differences in associations based on type of cannabinoid. The majority of the studies were 2-group trials with a placebo control group; however, some studies included active comparisons and multiple groups comparing more than 1 form of cannabinoid, different doses of cannabinoids, or active and placebo comparator groups. This necessitated selecting a single result from each trial to contribute to the meta-analysis to avoid double counting of studies. Where possible, we selected the result for the treatment or dose most similar to the other studies contributing to that meta-analysis and for placebo-controlled comparisons rather than active comparisons. For the short-term AE analysis, we selected the highest-reported cannabinoids dose because we hypothesized that this would be most likely to be associated with AEs—additionally, this analysis would present a worst-case scenario. Studies evaluated various forms of cannabis administered via various routes (oral capsules, smoked, vaporized, oromucosal spray, intramuscular injection) and active comparators differed across trials. These differences in form, combined with the variety of outcome measures and the broad indication groupings considered by this review, resulted in a very heterogeneous set of included studies, which meant that meta-analysis was not always possible or appropriate. Many studies reported insufficient information to allow meta-analysis (eg, reporting only P values for group differences) or no information on the analysis performed. A further difficulty with the continuous data were that even for the same outcomes, some studies reported results as difference between groups at follow-up and others reported results for difference in change from baseline. As advised by the Cochrane Handbook for Systematic Reviews of Interventions , we combined both types of data when estimating summary mean differences. 7 A potential problem with RCTs using crossover designs is the possible unblinding due to strong treatment or AEs. Additionally, studies of this design were rarely analyzed appropriately and none reported the required data accounting for their crossover design to permit appropriate inclusion in meta-analyses. 166 Primary analyses were therefore based on parallel-group studies, with crossover trials included as sensitivity analyses.
Our search identified a number of existing reviews that assessed the use of medical cannabinoids for MS, 167 - 170 nausea and vomiting due to chemotherapy, 171 - 175 pain, 176 - 191 psychosis, 192 - 194 and Tourette syndrome. 195 , 196 Almost all previous reviews focused on single indications and all but one (which evaluated cannabinoids in 4 trials in patients with pain due to rheumatoid arthritis) 188 did not use the GRADE approach to rating the quality of the evidence. As far as we are aware, our review is the first comprehensive review to evaluate the safety and efficacy of cannabinoids across a broad range of indications. A key strength of review was that it allowed us to conduct pooled analysis for the AEs associated with medicinal cannabinoids, adding considerable power to this analysis.
Further large, robust, RCTs are needed to confirm the effects of cannabinoids, particularly on weight gain in patients with HIV/AIDS, depression, sleep disorders, anxiety disorders, psychosis, glaucoma, and Tourette syndrome are required. Further studies evaluating cannabis itself are also required because there is very little evidence on the effects and AEs of cannabis. Future trials should adhere to the CONSORT (Consolidated Standards of Reporting Trials) reporting standards 197 and ensure that appropriate methods are used for randomization, allocation concealment, patient and outcome assessor blinding, handling of withdrawals, and avoiding selective outcome reporting. Future studies should assess patient-relevant outcomes (including disease-specific end points, quality of life, and AEs) using standardized outcome measures at similar time points to ensure inclusion in future meta-analyses.
There was moderate-quality evidence to support the use of cannabinoids for the treatment of chronic pain and spasticity. There was low-quality evidence suggesting that cannabinoids were associated with improvements in nausea and vomiting due to chemotherapy, weight gain in HIV, sleep disorders, and Tourette syndrome. Cannabinoids were associated with an increased risk of short-term AEs.
Corresponding Author: Penny Whiting, PhD, NIHR CLAHRC West, University Hospitals Bristol NHS Foundation Trust, Ninth Floor, Whitefriars, Lewins Mead, Bristol BS1 2NT, United Kingdom ( [email protected] ).
Author Contributions: Dr Whiting had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Whiting, Wolff, Misso, Kleijnen.
Acquisition, analysis, or interpretation of data: Whiting, Wolff, Deshpande, Di Nisio, Duffy, Hernandez, Keurentjes, Lang, Misso, Ryder, Schmidlkofer, Westwood.
Drafting of the manuscript: Whiting, Keurentjes, Ryder.
Critical revision of the manuscript for important intellectual content: Whiting, Wolff, Deshpande, Di Nisio, Duffy, Hernandez, Keurentjes, Lang, Misso, Ryder, Schmidlkofer, Westwood, Kleijnen.
Statistical analysis: Whiting, Wolff, Di Nisio, Hernandez, Keurentjes, Schmidlkofer.
Obtained funding: Kleijnen.
Administrative, technical, or material support: Deshpande, Lang, Ryder.
Study supervision: Whiting, Kleijnen.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and declare support from the Swiss Federal Office of Public Health (FOPH) for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. Dr Whiting reports that part of her time on this review was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care West at University Hospitals Bristol NHS (National Health Service) Foundation Trust. No additional disclosures were reported.
Funding/Support: This funded by the Swiss Federal Office of Public Health (FOPH) under grant agreement 14.001443/204.0001/-1257.
Role of the Funder/Sponsor: The FOPH had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The decision to submit the article for publication was a condition of the funding and was made before any results were available.
Additional Author Contributions: Dr Whiting drafted the article, produced tables and figures and performed the analysis. Drs Whiting, Wolff, and Kleijnen and Ms Misso and Mr Duffy drafted the protocol. Mr Duffy and Ms Misso conducted the literature searches. Drs Whiting, Wolff, and Lang screened searched results and selected full-text studies for inclusion. Drs Whiting, Wolff, Lang, Westwood, Keurentjes, Di Nisio, Hernandez, and Messrs Deshpande and Ryder, and Ms Schmidlkofer performed data extraction and risk-of-bias assessment. Dr Wolff performed the GRADE assessments. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclaimer: The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health.
Additional Contributions: We would like to thank Julie Harker (MRes, Kleijnen Systematic Reviews at the time of this project) for help with inclusion screening and data extraction and Gillian Worthy (MSc, Kleijnen Systematic Reviews) for advice on data analysis. Neither of these individuals received additional compensation in association with their work on this article.
Correction: This article was corrected online June 26, 2015, for incorrect axis labeling in Figure 4; on July 13, 2015, for a corrected average reduction to the Ashworth spasticity scale (as reported in the Abstract); on November 5, 2015, for an incorrect nonproprietary name and approved use for a drug in Table 1; and on April 12, 2016, for an incorrect effect estimate.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Understanding the evidence for medical cannabis and cannabis-based medicines for the treatment of chronic non-cancer pain
Affiliations.
- 1 National Drug and Alcohol Research Centre (NDARC), Faculty of Medicine, UNSW Sydney, 22-32 King Street, Randwick, NSW, 2031, Australia. [email protected].
- 2 National Drug and Alcohol Research Centre (NDARC), Faculty of Medicine, UNSW Sydney, 22-32 King Street, Randwick, NSW, 2031, Australia.
- 3 Monash Addiction Research Centre, Eastern Health Clinical School, Faculty of Medicine Nursing and Health Sciences, Monash University, Level 2, 5 Arnold Street, Box Hill, VIC, 3128, Australia.
- PMID: 30635715
- DOI: 10.1007/s00406-018-0960-9
The use of medical cannabis and cannabis-based medicines has received increasing interest in recent years; with a corresponding surge in the number of studies and reviews conducted in the field. Despite this growth in evidence, the findings and conclusions of these studies have been inconsistent. In this paper, we outline the current evidence for medical cannabis and cannabis-based medicines in the treatment and management of chronic non-cancer pain. We discuss limitations of the current evidence, including limitations of randomised control trials in the field, limits on generalisability of previous findings and common issues such as problems with measurements of dose and type of cannabinoids. We discuss future directions for medicinal cannabinoid research, including addressing limitations in trial design; developing frameworks to monitor for use disorder and other unintended outcomes; and considering endpoints other than 30% or 50% reductions in pain severity.
Keywords: Cannabis; Cannabis-based medicines; Chronic pain; Medical cannabis.
Publication types
- Cannabinoid Receptor Modulators / pharmacology*
- Cannabinoids / pharmacology*
- Chronic Pain / drug therapy*
- Medical Marijuana / pharmacology*
- Cannabinoid Receptor Modulators
- Cannabinoids
- Medical Marijuana
Grants and funding
- 1119992/National Health and Medical Research Council
- 1104600/National Health and Medical Research Council
- 113243/National Health and Medical Research Council
Journal of Cannabis Research
Aims and scope.
The Journal of Cannabis Research is an international, fully open access, peer-reviewed journal covering all topics pertaining to cannabis , including original research, perspectives, commentaries and protocols. Our goal is to provide an accessible outlet for expert interdisciplinary discourse on cannabis research.

Cannabis and cancer: unveiling the potential of a green ally in breast, colorectal, and prostate cancer
Authors: Husam A. ALSalamat, Sara Feras Abuarab, Hazem Mohamed Salamah, Anas Hasan Ishqair, Mohammad Fuad Dwikat, Anas Zakarya Nourelden, Aseel N. Qandil, Yasmeen Barakat and Muna Barakat
Envisaging challenges for the emerging medicinal Cannabis sector in Lesotho
Authors: Regina M. Thetsane
Cannabidiol’s cytotoxicity in pancreatic cancer is induced via an upregulation of ceramide synthase 1 and ER stress
Authors: Nagina Mangal, Vikash Reebye, Nagy Habib and Mikael H. Sodergren
An emerging trend in Novel Psychoactive Substances (NPSs): designer THC
Authors: Cristian Caprari, Elena Ferri, Maria Angela Vandelli, Cinzia Citti and Giuseppe Cannazza
Investigating sex differences and age of onset in emotion regulation, executive functioning, and cannabis use in adolescents and young adults
Authors: Natasha E. Wade, Kelly E. Courtney, Alexander L. Wallace, Laura Hatz and Joanna Jacobus
Most recent articles RSS
View all articles
Processing and extraction methods of medicinal cannabis: a narrative review
Authors: Masoumeh Pourseyed Lazarjani, Owen Young, Lidya Kebede and Ali Seyfoddin
Delta-8-THC: Delta-9-THC’s nicer younger sibling?
Authors: Jessica S. Kruger and Daniel J. Kruger
Cannabis consumption is associated with lower COVID-19 severity among hospitalized patients: a retrospective cohort analysis
Authors: Carolyn M. Shover, Peter Yan, Nicholas J. Jackson, Russell G. Buhr, Jennifer A. Fulcher, Donald P. Tashkin and Igor Barjaktarevic
Δ9-Tetrahydrocannabivarin (THCV): a commentary on potential therapeutic benefit for the management of obesity and diabetes
Authors: Amos Abioye, Oladapo Ayodele, Aleksandra Marinkovic, Risha Patidar, Adeola Akinwekomi and Adekunle Sanyaolu
Total and differential white blood cell count in cannabis users: results from the cross-sectional National Health and Nutrition Examination Survey, 2005–2016
Authors: Omayma Alshaarawy
Most accessed articles RSS
Editor-in-Chief
We are recruiting!
Journal of Cannabis Research is recruiting Associate Editors. As the growth of the journal continues, we are looking to expand our editorial team. If you have experience in any form of cannabis research, we would like to hear from you. Please follow the link below to find out more about the role and apply.
Webinar Series: Institute of Cannabis Research
Register for the Webinar Series hosted by the Institute of Cannabis Research and watch a comprehensive archive of cutting edge science presented by experts across the spectrum of cannabis research. The Cannabis Research Series hosted in collaboration with the Lambert Center at Thomas Jefferson University Cannabis Plant Science and Cultivation , hosted in collaboration with the Volcani Institute in Israel.
The Two Sides of Hemp
Do you have an idea for a thematic series? Let us know!

About the Institute
The Journal of Cannabis Research is the official publication of the Institute of Cannabis Research (ICR), which was established in June 2016 through an innovative partnership between Colorado State University Pueblo, the state of Colorado, and Pueblo County.
The ICR is the first US multi-disciplinary cannabis research center at a regional, comprehensive institution. The primary goal of the ICR is to conduct or fund research related to cannabis and publicly disseminate the results of the research, which it does so in partnership with the Journal. It also advises other Colorado-based higher education institutions on the development of a cannabis-related curriculum and supports the translation of discoveries into innovative applications that improve lives.
Find out more about the the Institute via the link below, as well as the Colorado state University–Pueblo website and Institute's research funding outcomes .

Affiliated with

An official publication of the Institute of Cannabis Research
- Editorial Board
- Instructions for Authors
- Instructions for Editors
- Sign up for article alerts and news from this journal
Annual Journal Metrics
2022 Citation Impact 3.7 - 2-year Impact Factor 3.4 - 5-year Impact Factor 0.741 - SNIP (Source Normalized Impact per Paper)
2023 Speed 21 days submission to first editorial decision for all manuscripts (Median) 211 days submission to accept (Median)
2023 Usage 596,678 downloads 1,834 Altmetric mentions
- More about our metrics
ISSN: 2522-5782
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- News & Events
- Public Health Focus
FDA and Cannabis: Research and Drug Approval Process
On this page:, fda supports sound scientific research.
- Cannabis Study Drugs Controlled Under Schedule I of the CSA
- Cannabis Study Drugs Containing Hemp
Additional Resources
The FDA understands that there is increasing interest in the potential utility of cannabis for a variety of medical conditions, as well as research on the potential adverse health effects from use of cannabis.
To date, the FDA has not approved a marketing application for cannabis for the treatment of any disease or condition. The agency has, however, approved one cannabis-derived drug product: Epidiolex (cannabidiol), and three synthetic cannabis-related drug products: Marinol (dronabinol), Syndros (dronabinol), and Cesamet (nabilone). These approved drug products are only available with a prescription from a licensed healthcare provider. Importantly, the FDA has not approved any other cannabis, cannabis-derived, or cannabidiol (CBD) products currently available on the market.

- Cannabis sativa L. is a plant that contains over 80 different naturally occurring compounds called “cannabinoids”
- Cannabidiol (CBD)
- Tetrahydrocannabinol (THC)
- Plants are grown to produce varying concentrations of cannabinoids – THC or CBD
- These plant variations are called cultivars
Cannabis-derived compounds
- Compounds occurring naturally in the plant – like CBD and THC
- These compounds are extracted directly from the plant
- Can be used to manufacture drug products
- Example: highly-purified CBD extracted from the plant
Cannabis-related compounds
- These synthetic compounds are created in a laboratory
- Can be used to manufacture drug products
- Some synthetic compounds may also occur naturally in the plant and some may not
- Examples: synthetically-derived dronabinol (also naturally occurring) and nabilone (not naturally occurring)
FDA has approved Epidiolex, which contains a purified form of the drug substance cannabidiol (CBD) for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients 2 years of age and older. That means FDA has concluded that this particular drug product is safe and effective for its intended use.
The agency also has approved Marinol and Syndros for therapeutic uses in the United States, including for nausea associated with cancer chemotherapy and for the treatment of anorexia associated with weight loss in AIDS patients. Marinol and Syndros include the active ingredient dronabinol, a synthetic delta-9- tetrahydrocannabinol (THC) which is considered the psychoactive intoxicating component of cannabis (i.e., the component responsible for the “high” people may experience from using cannabis). Another FDA-approved drug, Cesamet, contains the active ingredient nabilone, which has a chemical structure similar to THC and is synthetically derived. Cesamet, like dronabinol-containing products, is indicated for nausea associated with cancer chemotherapy.
FDA is aware that unapproved cannabis and/or unapproved cannabis-derived products are being used to treat a number of medical conditions including, AIDS wasting, epilepsy, neuropathic pain, spasticity associated with multiple sclerosis, and cancer and chemotherapy-induced nausea. Caregivers and patients can be confident that FDA-approved drugs have been carefully evaluated for safety, efficacy, and quality, and are monitored by the FDA once they are on the market. However, the use of unapproved cannabis and cannabis-derived products can have unpredictable and unintended consequences, including serious safety risks. Also, there has been no FDA review of data from rigorous clinical trials to support that these unapproved products are safe and efficacious for the various therapeutic uses for which they are being used.
FDA understands the need to develop therapies for patients with unmet medical needs, and does everything it can to facilitate this process. FDA has programs such as Fast Track, Breakthrough Therapy, Accelerated Approval and Priority Review that are designed to facilitate the development of and expedite the approval of drug products. In addition, the FDA’s expanded access (sometimes called “compassionate use”) statutory and regulatory provisions are designed to facilitate the availability of investigational products to patients with serious diseases or conditions when there is no comparable or satisfactory alternative therapy available, either because the patients have exhausted treatment with or are intolerant of approved therapies, or when the patients are not eligible for an ongoing clinical trial. Through these programs and the drug approval process, FDA supports sound, scientifically-based research into the medicinal uses of drug products containing cannabis or cannabis-derived compounds and will continue to work with companies interested in bringing safe, effective, and quality products to market.
↑ Back to top
The FDA has an important role to play in supporting scientific research into the medical uses of cannabis and its constituents in scientifically valid investigations as part of the agency’s drug review and approval process. As a part of this role, the FDA supports those in the medical research community who intend to study cannabis by:
- Providing information on the process needed to conduct clinical research using cannabis.
- Providing information on the specific requirements needed to develop a human drug that is derived from a plant such as cannabis. In December 2016, the FDA updated its Guidance for Industry: Botanical Drug Development , which provides sponsors with guidance on submitting investigational new drug (IND) applications for botanical drug products. The FDA also has issued “ Cannabis and Cannabis-Derived Compounds: Quality Considerations for Clinical Research, Draft Guidance for Industry .”
- Providing specific support for investigators interested in conducting clinical research using cannabis and its constituents as a part of the IND or investigational new animal drug (INAD) process through meetings and regular interactions throughout the drug development process.
- Providing general support to investigators to help them understand and follow the procedures to conduct clinical research through the FDA Center for Drug Evaluation and Research (CDER) Small Business and Industry Assistance group .
To conduct clinical research that can lead to an approved new drug, including research using materials from plants such as cannabis, researchers need to work with the FDA and submit an IND application to CDER. The IND application process gives researchers a path to follow that includes regular interactions with the FDA to support efficient drug development while protecting the patients who are enrolled in the trials. An IND includes protocols describing proposed studies, the qualifications of the investigators who will conduct the clinical studies, and assurances of informed consent and protection of the rights, safety, and welfare of the human subjects. The FDA reviews the IND to ensure that the proposed studies, generally referred to as “clinical trials,” do not place human subjects at an unreasonable risk of harm. The FDA also requires obtaining the informed consent of trial subjects and human subject protection in the conduct of the clinical trials. For research intending to develop an animal drug product, researchers would establish an INAD file with the Center for Veterinary Medicine (CVM) to conduct their research, rather than an IND with CDER.
FDA is committed to encouraging the development of cannabis-related drug products, including CBD. Those interested in cannabis-derived and cannabis-related drug development are encouraged to contact the relevant CDER review division and CDER’s Botanical Review Team (BRT) to answer questions related to their specific drug development program. The BRT serves as an expert resource on botanical issues and has developed the Botanical Drug Development Guidance for Industry to assist those pursuing drug development in this area. FDA encourages researchers to request a Pre-Investigational New Drug application (PIND) meeting to discuss questions related to the development of a specific cannabis-derived and cannabis-related drug product.
Please note that certain cultivars and parts of the Cannabis sativa L. plant are controlled under the Controlled Substances Act (CSA) since 1970 under the drug class "Marihuana" (commonly referred to as "marijuana") [21 U.S.C. 802(16)]. "Marihuana" is listed in Schedule I of the CSA due to its high potential for abuse, which is attributable in large part to the psychoactive intoxicating effects of THC, and the absence of a currently accepted medical use in the United States. From 1970 until December of 2018, the definition of “marihuana” included all types of Cannabis Sativa L. , regardless of THC content. However, in December 2018, the Agriculture Improvement Act of 2018 (also known as the Farm Bill) removed hemp, a type of cannabis that is very low in THC (cannabis or cannabis derivatives containing no more than 0.3% THC on a dry weight basis), from controls under the CSA. This change in the law may result in a more streamlined process for researchers to study cannabis and its derivatives, including CBD, that fall under the definition of hemp, a result which could speed the development of new drugs containing hemp.
Conducting clinical research using cannabis-derived substances that are considered controlled substances under the CSA often involves interactions with several federal agencies. For example:
- Protocols to conduct research with controlled substances listed in Schedule I are required to be conducted under a site-specific DEA investigator registration. For more information, see 21 CFR 1301.18 .
- National Institute on Drug Abuse (NIDA) Drug Supply Program provides research-grade marijuana for scientific study. Through registration issued by DEA, NIDA is responsible for overseeing the cultivation of marijuana for medical research and has contracted with the University of Mississippi to grow marijuana for research at a secure facility. Marijuana of varying potencies and compositions along with marijuana-derived compounds are available. DEA also may allow additional growers to register with the DEA to produce and distribute marijuana for research purposes. DEA that, as the result of a recent amendment to federal law, certain forms of cannabis no longer require DEA registration to grow or manufacture.
- Researchers work with the FDA and submit an IND or INAD application to the appropriate CDER divisions or other center offices depending on the therapeutic indication or population. If the research is intended to support the approval of an animal drug product, an INAD file should be established with CVM. Based on the results obtained in studies conducted at the IND or INAD stage, sponsors may submit a marketing application for formal approval of the drug.
Cannabis Study Drugs Controlled Under Schedule I of the CSA (greater than 0.3% THC on a dry weight basis)
Sponsor obtains pre-IND number through CDER review division to request a pre-IND meeting. For new animal drug research, a sponsor may engage with CVM to establish an INAD file. A pre-IND meeting with CDER is optional, and an opportunity to obtain FDA guidance on sponsor research plans and required content for an IND submission .
The sponsor contacts NIDA or another DEA-registered source of cannabis and/or cannabis-derived substances to obtain information on the specific cultivars available, so that all necessary chemistry, manufacturing, and controls (CMC) and botanical raw material (BRM) information can be included in the IND. Importation of products controlled under the CSA are subject to DEA authorization.
The sponsor may contact DEA to discuss Schedule I drug research plans that may require DEA inspection for an investigator and study site Schedule I license.
Step 4: If the selected BRM or drug substance manufacturer holds a Drug Master File (DMF) , the sponsor must obtain a Letter of Authorization (LOA) to reference CMC and BRM information. Alternatively, an IND submission would need to contain all necessary CMC data characterizing their study drug and ensuring it is safe for use in humans.
The sponsor sends a copy of the IND and clinical protocol, including a LOA (if applicable), to FDA.
FDA reviews the submitted IND. The sponsor must wait 30 calendar days following IND submission before initiating any clinical trials, unless FDA notifies the sponsor that the trials may proceed sooner. During this time, FDA has an opportunity to review the submission for safety to assure that research subjects will not be subjected to unreasonable risk.
If the IND is authorized by FDA as “safe to proceed” the sponsor may then submit their clinical protocol registration application, including referenced IND number, to DEA to obtain the protocol registration. Once this is received, the sponsor contacts NIDA or another DEA-registered source to obtain the cannabis and/or cannabis-derived substances and they can then begin the study.
For nonclinical research, including research conducted under an INAD file submitted established with CVM, there is no requirement of prior authorization of the protocol by FDA before the investigators may proceed with a protocol registration application submitted to DEA. For these nonclinical protocols, investigators may immediately pursue investigator and study site licensure, and protocol registration with DEA, so they may then obtain their Schedule I cannabis-derived study drug from supplier.
Cannabis Study Drugs Containing Hemp (no more than 0.3% THC on a dry weight basis)
Sponsor provides all applicable chemistry, manufacturing, and controls (CMC) and botanical raw material (BRM) information in the IND for review by FDA, including hemp cultivars.
If the selected hemp manufacturer holds a Drug Master File (DMF) , the sponsor must obtain a Letter of Authorization (LOA) to reference CMC and BRM information. Alternatively, an IND submission would need to contain all necessary CMC data characterizing their study drug and ensuring it is safe for use in humans.
FDA’s Role in the Drug Approval Process
The FDA’s role in the regulation of drugs, including cannabis and cannabis-derived products, also includes review of applications to market drugs to determine whether proposed drug products are safe and effective for their intended indications. The FDA’s drug approval process requires that clinical trials be designed and conducted in a way that provides the agency with the necessary scientific data upon which the FDA can make its approval decisions. Without this review, the FDA cannot determine whether a drug product is safe and effective. It also cannot ensure that a drug product meets appropriate quality standards. For certain drugs that have not been approved by the FDA, the lack of FDA approval and oversight means the safety, effectiveness, and quality of the drug – including how potent it is, how pure it is, and whether the labeling is accurate or false – may vary considerably.
- Product-Specific Guidance for Generic Drug Development: Draft Guidance on Cannabidiol Oral Solution (PDF - 42KB)
- Cannabis Clinical Research: Drug Master Files (DMFs) & Quality Considerations Webinar
- Cannabis and Cannabis-Derived Compounds: Quality Considerations for Clinical Research, Draft Guidance for Industry
- FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD): Questions and Answers
- Development & Approval Process (Drugs)
- From an Idea to the Marketplace: The Journey of an Animal Drug through the Approval Process
- FDA Center for Drug Evaluation and Research Small Business and Industry Assistance group
- CVM Small Business Assistance
- National Institutes of Health (NIH): Guidance on Procedures for Provision of Marijuana for Medical Research
- National Institute on Drug Abuse's (NIDA) Role in Providing Marijuana for Research
- Drug Enforcement Administration - Registration of Manufacturers, Distributors, and Dispensers of Controlled Substances
- International Narcotics Control Board: Single Convention on Narcotic Drugs (1961)
- National Institute on Drug Abuse (NIDA): Ordering Guidelines for Marijuana and Marijuana Cigarettes
This website uses cookies.
By clicking the "Accept" button or continuing to browse our site, you agree to first-party and session-only cookies being stored on your device to enhance site navigation and analyze site performance and traffic. For more information on our use of cookies, please see our Privacy Policy .
- Journal of Economic Literature
The Public Health Effects of Legalizing Marijuana
- D. Mark Anderson
- Daniel I. Rees
- Article Information
Additional Materials
- Author Disclosure Statement(s) (158.63 KB)
JEL Classification
- R41 Transportation: Demand, Supply, and Congestion; Travel Time; Safety and Accidents; Transportation Noise
Marijuana and Cannabinoids: Health, Research and Regulatory Considerations (Position Paper)
Executive summary.
Marijuana and related substance misuse are complex issues impacting family medicine, patient health, and public health. The American Academy of Family Physicians (AAFP) believes family physicians are essential in addressing all forms of inappropriate substance use. The AAFP urges its members to be involved in the diagnosis, treatment, and prevention of substance use, as well as secondary diseases impacted or caused by use. The World Health Organization (WHO) reports approximately 2.5% of the global population uses cannabis annually, making it the most commonly used drug worldwide. 1 Simultaneously, the AAFP acknowledges preliminary evidence indicates marijuana and cannabinoids may have potential therapeutic benefits, while also recognizing subsequent negative public health and health outcomes associated with cannabis use. 2
During the 20 th century, law enforcement and public policy activities have undermined opportunities for scientific exploration. Barriers to facilitating both clinical and public health research regarding marijuana is detrimental to treating patients and the health of the public. The lack of regulation poses a danger to public health and impedes meaningful, patient-centered research to exploring both therapeutic and negative impacts of marijuana and cannabinoids.
Relevant AAFP Policy
Marijuana Possession for Personal Use The American Academy of Family Physicians (AAFP) opposes the recreational use of marijuana. However, the AAFP supports decriminalization of possession of marijuana for personal use. The AAFP recognizes the benefits of intervention and treatment for the recreational use of marijuana, in lieu of incarceration, for all individuals, including youth. 3
The AAFP also recognizes that several states have passed laws approving limited recreational use and/or possession of marijuana. Therefore, the AAFP advocates for further research into the overall safety and health effects of recreational use, as well as the effects of those laws on patient and societal health. 4
It should be noted that cannabis and marijuana are not interchangeable terms. In this position paper, cannabis is an overarching term used to refer to the plant Cannabis sativa . Substances derived from the cannabis plant include marijuana, hemp, and cannabinoids.
Call to Action Family physicians have a vested interest in policies that advance and protect the health of their patients and the public. The regulatory environment surrounding cannabis, medical and recreational marijuana, and cannabidiol (CBD) is rapidly changing, along with the retail environment. This shift has not been accompanied by robust scientific research regarding the health effects of cannabis, both therapeutic or detrimental. The AAFP recognizes the need for substantial clinical, public health, and policy evidence and research regarding cannabis, marijuana, cannabinoids, and CBD to inform evidence-based practice and the impact on public health.
- The AAFP promotes a society which is free of substance misuse, including alcohol and drugs. 3
- The AAFP recognizes there is support for the medical use of marijuana and cannabinoids, but advocates that usage be based on high-quality, evidence-based public health, policy, and patient-centered research, including the impact on vulnerable populations. 3
- The AAFP advocates for further studies into the use of medical marijuana and related compounds. This process should also ensure appropriate funding allocated for this research.
- The AAFP calls for decreased regulatory barriers to facilitate clinical and public health cannabis research, including reclassifying cannabis from a Schedule I controlled substance. 3
- The AAFP advocates for further research into the overall safety and health effects of recreational use, as well as the impact of legal recreational marijuana use laws on patient and societal health. 4
- The AAFP advocates for robust regulation regarding labeling and child-proof packaging of all marijuana and cannabinoid products.
- The AAFP opposes the recreational use and legalization of marijuana, but supports decriminalization of marijuana for personal use. The AAFP recognizes the benefits associated with intervention and treatment, in lieu of incarceration. 4
- The AAFP advocates for regulation regarding marketing claims, labeling, and advertising of all marijuana and cannabinoid products.
- The AAFP supports requirements testing current marijuana and cannabinoid products for safety, dosing, and product consistency.
In the Exam Room
- The AAFP urges its members to be involved in the diagnosis, treatment, and prevention of substance use, as well as the secondary diseases impacted by use.
- The AAFP calls for family physicians to discuss the health consequences of marijuana and cannabis use, as well as prevention strategies to prevent use and unintended consequences of marijuana exposure in at-risk populations.
Cannabis use, both medically and recreationally, is prevalent throughout history. Extensive evidence indicates cannabis was used by ancient civilizations, dating back more than 5,000 years ago. 1 In the U.S. in the 19th and early 20th centuries, cannabis was frequently used for medicinal purposes, often prescribed by clinicians. 1,5 Cannabis was first listed in the United States Pharmacopoeia in 1851, indicating use as an analgesic, hypnotic, and anticonvulsant agent. 5 After the 1937 Marihuana Tax Act , in 1942, cannabis was removed from the United States Pharmacopoeia . 5
Attitudes and perceived risk of marijuana use have changed with the varying levels of legalization in the U.S. Surveying marijuana use is essential to gauge public health implications of increased access to marijuana, cannabinoid, and cannabis products. According to the 2018 National Institute on Drug Abuse (NIDA) Monitoring the Future Survey (MTF), daily, past month, past year, and lifetime marijuana use among 8 th graders has declined, and remained unchanged in 10 th and 12 th graders, when compared to the 2013 MTF survey. 6 Despite the changing landscape of marijuana regulations nationwide, past year use of marijuana reached and maintained its lowest levels in more than two decades in 2016 among 8 th and 10 th graders. 6 However, marijuana vaping did significantly increase between 2017 and 2018, mirroring trends in youth tobacco use. 6 The NIDA 2017 National Survey on Drug Use and Health indicates nearly 53% of adults between the ages of 18-25 have tried marijuana at some point in their lifetime, 35% have used marijuana within the past year, and 22% within the past month. 7 While the lifetime use remains relatively stable for this cohort, from 2015-2017, past year and past month use increased 2.7% and 2.3%, respectively. 7 Nearly half of adults 26 or older reported using marijuana at some point in their lifetime. 7 Although adults ages 26 and up report the highest percentage of lifetime use, this age group has a significantly lower past year use (12%) and past month use (8%). 7
Forms and Use of Cannabis The cannabis plant, Cannabis sativa , is comprised of both non-psychoactive and psychoactive chemicals called cannabinoids. 5 The cannabinoid commonly known for its psychoactive properties is delta-9-tetrahydrocannabinol (THC). 5 CBD is the most abundant cannabinoid in cannabis, and is considered to be largely non-psychoactive. 5 The biological system responsible for the synthesis and degradation of cannabinoids in mammals is referred to as the endocannabinoid system, which is largely comprised of two g-coupled protein receptors (GPCRs). 8 The GPCRs—CB1 and CB2—are found throughout many bodily tissues. However, CB1 is most concentrated in the neural tissues. 5,8 CB2 receptors are found in the brain, but are mostly found in immune cells, like macrophages, microglia, osteoclasts, and osteoblasts. 5,8
There are many forms of, and products derived from, the Cannabis sativa plant, including hemp, CBD, and marijuana. Cannabis sativa with less than 0.3% THC is considered industrial hemp, and can be used for industrial agriculture cultivation. 9,10 Industrial hemp can be harvested and used for many things, including fibers for textiles, food products, and building materials. 11,12 CBD, the non-psychoactive cannabinoid, is extracted from the flower of industrial hemp. 13 Marijuana and hemp, technically speaking, are the same plant. 13 However, the hemp variety of cannabis contains no more than 0.3% THC, while the marijuana variety contains 5-20% THC. 13
Marijuana and CBD are most commonly used via inhalation, ingestion, and topical absorption. 5 Inhalation can be through combustible mechanisms using dried flowers, including the use of a pipe, rolled joints, blunts, and water pipes (also called bongs). 14 Vaping marijuana and CBD concentrates are an increasingly popular inhalation method. 5,6 Concentrates, the concentrated form of marijuana and CBD, come in various forms, including oil, butter, or a dark sticky substance often referred to as shatter. 15 Concentrates can be both smoked or vaporized, and may also be used as additives or cooking agents for ingestion. 5,15 There are many different ways to ingest cannabinoids. Food products—called edibles—like brownies, gummies, cookies, and candies are common forms of cannabis ingestion, as well as liquid forms like juices, soda, and tea. 5,16 Tinctures are liquid, ultra-concentrated alcohol-based cannabis extracts commonly applied in and absorbed through the mouth. 17 Topical cannabis is applied to, and absorbed through, the skin in a cream or salve form. 18
Routes or methods of administration affect cannabis delivery. When cannabis is smoked or vaporized, onset of effect is within 5-10 minutes with a duration of 2-4 hours. 19 When ingested, effect is within 60-180 minutes with a duration of 6-8 hours. 19 The oromucosal route has an onset of 15-45 minutes and a duration of 6-8 hours. 19 Topical administration of cannabis or cannabinoids has variable onset and duration. 19 The smoked or vaporized method offers the more rapid activity for acute symptoms with the topical preparations offering less systemic effects. 19
Health Effects of Cannabis
Although there is preliminary evidence indicating cannabinoids may have some therapeutic benefit, a large portion of the evidence is very limited for many reasons. These include small sample sizes, lack of control groups, poor study design, and the use of unregulated cannabis products. There are also clear negative health and public health consequences that must be considered, as well as the need for a significant increase in evidence. More research is needed to create a robust evidence base to weigh the potential therapeutic benefits against potential negative impacts on health and public health. Currently, there are three medical formulations of cannabis approved for use in the U.S.; dronabinol, nabilone, and epidiolex. 20 Nabiximols is approved for use in the United Kingdom. 21 Dronabinol is delta-9 THC and ingested as either an oral solution or an oral capsule. 22 Nabilone is an oral capsule containing synthetic THC. 23 Epidiolex is a CBD oral solution. 24 Nabiximols is an oral mucosa spray containing the cannabinoids THC and CBD. 25
In 2015, Whiting, et al, performed a meta-analysis and systematic review of research on the medical use of cannabis. 25 This systematic review served as the basis for many recommendations in 2017 by the National Academy of Science, Engineering, and Health Report on medical marijuana. 5 Dronabinol, nabilone, and nabiximols were included in the studies. However, other cannabis formulations were found in research trials, including CBD, marijuana, and other cannabinoids. 26 Evidence is most substantial for nausea and vomiting associated with chemotherapy, chronic pain treatment, multiple sclerosis spasticity, and intractable seizures associated with Dravet syndrome and Lennox-Gastaut syndrome. 27 There is moderate evidence for the use of cannabinoids for sleep and limited evidence for use in psychiatric conditions, such as post-traumatic stress disorder, depression, anxiety, and psychosis; appetite stimulation and weight gain; and no evidence for cancer treatment. 5
Dronabinol and nabilone were both approved in 1985 for use in treating refractory chemotherapy-induced nausea and vomiting. 5,23 Dronabinol is approved by the Food and Drug Administration (FDA) for appetite stimulation and weight gain, despite limited and often inconclusive evidence that it or other cannabinoids are effective. 22 This drug has traditionally been used in human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) patients to mitigate weight loss and to treat anorexia-cachexia syndrome associated with cancer and anorexia nervosa. 5,22
Cannabinoids have been assessed for chronic pain management. Many forms of chronic pain management were studied, including cancer and chemotherapy-induced pain, fibromyalgia, neuropathic pain, rheumatoid arthritis, non-cancer pain, and musculoskeletal pain. Several studies indicate smoked THC and nabiximols were both associated with pain reduction. 5,25,26 There is limited, mixed evidence regarding the viability of cannabinoids for some forms of chronic pain management. 5 However, limitations exist with these studies, including the variable doses of THC and CBD; unregulated, non-FDA approved products; and conflicting evidence. Studies assessing cannabinoids in treating the spasticity due to multiple sclerosis or paraplegia have mixed results. The cannabinoids nabiximols, dronabinol, and TCH/CBD have all been associated with decreased spasticity. Nabilone and nabiximols were the only drugs with statically-significant decreases. 2,25
In 2018, the FDA approved a cannabidiol oral solution called epidiolex for the treatment of refractory seizures associated with Dravet syndrome and Lennox-Gastaut syndrome. 28 Epidiolex was associated with significant seizure reduction when compared to placebo. 29–31 Dravet syndrome and Lennox-Gastaut syndrome are disorders associated with severe seizures, impaired cognitive skills and development, and uncontrollable muscle contractions. 29–31
Moderate evidence exists for the use of cannabis for sleep. Nabilone and nabiximols have been associated with improvement in sleep from a baseline and sleep restfulness. 2,5,25 Improved sleep was also considered a secondary outcome when evaluating other conditions (chronic pain, multiple sclerosis) with various cannabinoids. 2,5,25
There is limited evidence for the use of cannabis or cannabinoids for the treatment of post-traumatic stress disorder (PTSD), anxiety, depression, or psychosis. Of the limited evidence, nabilone was associated with a decrease in PTSD related nightmares. 5,25 One small study indicated CBD improved public speaking anxiety. 5 There are no studies directly evaluating the effectiveness of cannabis in the treatment of depression. However, some studies measured depression as a secondary outcome, but indicated no difference in depression when compared to placebo. 25 Limited evidence (two studies) have shown no difference in treating psychosis with CBD, amisulpride, or placebo. 25 Evidence indicates individuals who use marijuana are more likely to experience temporary psychosis and chronic mental illness, including schizophrenia. 5,32
There was no evidence or insufficient evidence for the use of cannabis or cannabinoids in the treatment of cancer; neurodegenerative disorders like Huntington’s chorea, Parkinson’s disease, or amyotrophic lateral sclerosis; irritable bowel syndrome; or addiction. 5
Cannabis overdose is rare in adults and adolescents. 33 Children who experience acute intoxication from cannabis generally ingest marijuana or other cannabinoids through experimentation. 33 When compared to adults and adolescents, children are more likely to experience life-threatening symptoms of acute cannabis intoxication, which may include depressed respiration rates, hyperkinesis, or coma. 33 Management consists of supportive care dependent on the manifestation of symptoms. 33 Adults and adolescents may experience increased blood pressure and respiratory rates, red eyes, dry mouth, increased appetite, and slurred speech. 33
Negative health effects are also associated with marijuana and cannabinoid use. Frequent marijuana use has been associated with disorientation. In teens, it has been linked with depression, anxiety, and suicide. 5,32 However, this is not a proven causal relationship. Lung health can also be negatively impacted depending on the delivery mechanism. 34 Smoking marijuana can cause lung tissue scarring and damage blood vessels, further leading to an increased risk of bronchitis, cough, and phlegm production. 34 This generally decreases when users quit. 34
Secondhand smoke is a serious issue associated with marijuana use. However, there is limited evidence on how it impacts heart and lung health. 34 Detectable THC has been found in children who live in the home or have a caretaker who use marijuana, subjecting children to developmental risks of THC exposure. 35 Fetal, youth, and adolescent exposure to THC is associated with negative health effects, including impacting brain development. 34 There is inconsistent, insufficient evidence to determine the long-term effects of marijuana and cannabinoid use while breastfeeding. 36 However, THC has been detected in breast milk for up to six days post-cannabinoid use, and exposure to cannabinoids is known to impact development in children. 37 Evidence also suggests cannabis use during pregnancy may be linked with preterm birth. 38 Cardiovascular health may be impacted by smoked marijuana use. However, the negative health effects are associated with the harmful chemicals in smoke similar to tobacco smoke. 34
Approximately 9% of all individuals who use marijuana develop an addiction, which is variable by age of first use and frequency of use. 34 That number for addiction jumps to 17% for individuals who begin using marijuana as teenagers and 25-50% of those who smoke marijuana daily. 34 Marijuana use does not typically lead to harder drug use, like cocaine and heroin, in most individuals. 39 Further research is needed to evaluate any potential gateway effect. 39
Mental health outcomes associated with marijuana use include an increased risk of anxiety and depression. Marijuana has been linked to schizophrenia, psychoses, and advancing the trajectory of the disease, particularly in individuals with pre-existing genetic indicators. 5,34 Global research also suggests daily use of high-potency marijuana increases risk for psychotic episodes among individuals with no underlying mental health condition. 40 While it is widely accepted that marijuana acutely impairs cognitive function, studies suggest differential outcomes regarding short- versus long-term cognitive impairment. 34
Research Considerations
The regulatory environment surrounding cannabis, marijuana, and cannabinoid research creates barriers detrimental to facilitating meaningful medical, public health, policy, and public safety research. Approval for research expands beyond institutional review boards. Due to the Schedule I classification by the Drug Enforcement Agency (DEA), researchers seeking to investigate health effects associated with cannabis must follow a regimented application process. 41 Applicants must submit an Investigational New Drug (IND) application to the FDA, which will then be reviewed to determine scientific validity and research subjects’ rights and safety. 42 Researchers must also follow the NIDA regulatory procedures for obtaining cannabis for research purposes. 41 Researchers may only use cannabis supplied by the University of Mississippi, the single NIDA-approved source for cannabis research. 41 Requiring research to rely on one source of cannabis limits availability and the variety of products. While the University of Mississippi cultivates different strains of cannabis, it is unable to supply the vast array of strains of cannabis found in the evolving retail environment with varying levels of THC, CBD, and cannabinoid content. 5 Substantial funding and capacity is required for researchers to obtain all regulatory approval and remain in compliance while conducting cannabis-related research. The required processes and procedures present a serious burden, dissuading researchers from pursuing cannabis-related projects. This has led to a lack of empirical evidence regarding a myriad of health-related issues, including potential therapeutic benefits of cannabis, public health impact, health economics, and the short- and long-term health effects from cannabis use.
In order to address the research gaps associated with both beneficial and harmful effects of cannabinoids used in both medical and recreational capacities, the AAFP calls for a comprehensive review of processes and procedures required to obtain approval for cannabis research.
The AAFP encourages the appropriate regulatory bodies, such as the DEA, NIDA, FDA, Department of Health and Human Services (DHHS), National Institutes of Health (NIH), and the Centers for Disease Control and Prevention (CDC), to collaborate with non-governmental stakeholders to determine procedures to decrease the burden of cannabis-related research while maintaining appropriate regulatory safety guards. This should include a reclassification of marijuana from Schedule I to facilitate clinical research. The AAFP calls for increased funding from both public and private sectors to support rigorous scientific research to address gaps in evidence regarding cannabis to protect the health of the public and inform evidence-based practices. 3 Future research should address the impact of cannabis use on vulnerable and at-risk populations.
Regulatory Considerations
While cannabis was federally regulated in 1906 for consumer and safety standards and labeling requirements, the Marihuana Tax Act of 1937 was the first federal regulation to impose a fine or imprisonment for non-medical use and distribution of cannabis. 5 The tax act also regulated production, distribution, and use of cannabis, further requiring anyone dealing with cannabis to register with the federal government. 5 In 1970, the DEA classified marijuana as a Schedule I drug, which is defined as a drug with no current acceptable medical use and a high potential for abuse. 43 Other Schedule 1 drugs include heroin, lysergic acid diethylamide (LSD), 3,4-methylenedioxymethamphetamine (ecstasy), methaqualone, and peyote. 43 Since this class of substances is determined as having no medical usage, they cannot be legally prescribed and thus, there is no medical coverage for them.
Marijuana is illegal under federal law. Penalties cover possession, sale, cultivation, and paraphernalia. However, the Agriculture Improvement Act of 2018 included a U.S. Department of Agriculture (USDA) Hemp Production Program, removing hemp from the Controlled Substances Act. 10,44 As a result, CBD sourced from hemp plants containing no more than 0.3% THC is legal to produce. 10,44 The FDA has approved three medications containing cannabinoids: epidiolex (CBD), dronabinol, and nabilone (synthetic cannabinoids). 5 No other forms of cannabis are currently regulated by the FDA. The AAFP calls upon the FDA to take swift action to regulate CBD and cannabinoid products now legal in order to protect the health of the public.
States have separate marijuana, cannabinoid, and cannabis laws, some of which mirror federal laws, while others may be more harsh, or have decriminalized and even legalized marijuana and cannabis. 45 In 1996, California was the first state to legalize the medical use of marijuana. 46 States have subsequently decriminalized and/or legalized cannabinoids, medical marijuana, and recreational marijuana. 46 As of August 2019, 30 states, along with the District of Columbia, Guam, and Puerto Rico have legalized marijuana in varying forms. 46 Decriminalization laws may include reduction of fines for possession of small amounts of marijuana, reclassification of criminal to civil infractions, excluding the infraction from criminal records and expunging prior offenses and convictions related to marijuana. 47 Thirty-three states, along with the District of Columbia, Guam, Puerto Rico, and the U.S. Virgin Islands have a comprehensive, publicly-available medical marijuana/cannabis program, and 13 of these states have also removed jail time for possessing small amounts of non-medical marijuana. 47 Adult recreational marijuana use is legal in 13 states and the District of Columbia. 47 Vermont and the District of Columbia, however, do not allow the sale of marijuana for recreational purposes. This means it is not a crime to use and possess marijuana recreationally, but commercial sales are not allowed. 47 States have also authorized the sale of products that have low levels of THC, but high levels of CBD. These products are widely available in retail locations, but are highly unregulated. 47 The benefits of CBD touted by the public and retailers are largely anecdotal. The vast majority of these claims are not substantiated by valid research.
Decriminalizing and legalizing marijuana can decrease the number of individuals arrested and subsequently prosecuted for possession and/or use. 48 However, evidence suggests that these practices are not applied equitably. People of color are more likely to be arrested and prosecuted for marijuana possession despite overall decreased arrest rates. 48 Incarceration impacts health. People who are incarcerated have significantly higher rates of disease than those who are not, and are less likely to have access to adequate medical care. 49
The AAFP “opposes the recreational use of marijuana. However, the AAFP supports decriminalization of possession of marijuana for personal use. The AAFP recognizes the benefits of intervention and treatment for the recreational use of marijuana, in lieu of incarceration, for all individuals, including youth.” 4 The AAFP calls for family physicians to advocate to prevent unnecessary incarceration by diverting eligible people from the justice system to substance abuse and/or mental health treatment. 49
There are many public health considerations when regulating cannabis products. Serious public health concerns include impaired driving, youth exposure to advertisements, and accidental poisoning in children. Second to alcohol, marijuana is the most common illicit drug associated with impaired driving and accidents. 34 Marijuana slows reaction time and decision making, substantially increasing risk for traffic accidents. 50 Some states have a zero-tolerance policy, where there is no allowable detectable level of THC while driving, while other states have set five nanograms per milliliter or higher limits of THC, or minimally-detectable amounts of THC. 51
Evidence indicates adolescents who are exposed to medical marijuana advertising are more likely to have positive views of and subsequently use marijuana. 52 Those exposed to medical marijuana advertising were more likely to report past use and expectant future use. 52 These adolescents also reported agreeing with statements like, marijuana helps people relax and get away from their problems. 52 Adolescent exposure to medical marijuana advertising was also associated with self-reporting negative consequences associated with marijuana use, including missing school and concentration issues. 52 The AAFP calls for immediate regulation of advertising of all marijuana and cannabinoid products to decrease youth exposure to aid in preventing initiation and subsequent use of marijuana.
Children are most susceptible to severe effects associated with marijuana poisoning, including decreased coordination, lethargy, sedation, difficulty concentrating, and slurred speech. 53 Exposure may also include serious, potentially life-threatening symptoms like respiratory distress and coma. 33 Unintentional exposures to marijuana in children have increased each year since 2012, likely due to legalization policies across the U.S. and popularity of edibles. 53 Edibles often look exactly like their non-THC counterparts, and come in brightly colored packaging appealing to children, often mimicking candy products. 53 Effective legislation requiring childproof packaging for edible products can help mitigate and prevent unintentional exposure in children. 54 Family physicians should discuss safe storage of all cannabis products with their patients who live with children. 54 Under the Child Abuse Prevention and Treatment Act (CAPTA), physicians are mandated reporters of suspected child abuse and neglect. 55 The 2010 law requires states to enact laws for reporting substance use-exposed infants to child protective services. 55
Family physicians play a key role in addressing marijuana, cannabinoid, and cannabis product use; reducing barriers to research; and advocating for appropriate policy to protect the health of patients and the public.
Family physicians can address the inappropriate use of marijuana, cannabinoid, and cannabis products. Family physicians should discuss safe storage of all cannabis products with patients who live with or serve as primary caregivers for children to prevent unintended exposure. 56 It is important to discuss the developmental and negative impacts of marijuana and cannabis products with individuals who are or can become pregnant, children, and adolescents. Family physicians should also emphasize the serious consequences of impaired driving and marijuana intoxication.
It is essential to decrease barriers to research all forms of marijuana, cannabis, and cannabinoids, including a reclassification of cannabis as a Schedule I drug. High-quality research regarding the impact on patients, public health, society, and health policy are essential to providing patient-centered care and promoting evidence-based public health practices. Immediate regulations for marijuana and cannabinoid products, including CBD, like product safety and consistency safeguards, child-proof packaging, labeling, marketing claims and advertising, and impairment standards are vital for consumer safety and injury prevention. Regulatory measures focused on preventing youth initiation of marijuana and cannabinoid product use must be prioritized to prevent a public health epidemic.
The health benefits associated with intervention and treatment of recreational marijuana and cannabinoid use, in lieu of incarceration, is an important policy consideration.
Utilizing an interdisciplinary, evidence-based approach to addressing both medical and recreational marijuana and cannabis use is essential to promote public health, inform policy, and provide patient-centered care. Family physicians, in partnership with public health and policy professionals, can play an imperative role in addressing the changing landscape of marijuana and cannabis products.
- Bridgeman MB, Abazia DT. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. P T . 2017;42(3):180-188. Accessed August 20, 2019.
- Abrams DI. The therapeutic effects of cannabis and cannabinoids: An update from the National Academies of Sciences, Engineering and Medicine report. Eur J Intern Med . 2018;49:7-11.
- American Academy of Family Physicians. Substance abuse and addiction . Accessed August 20, 2019.
- American Academy of Family Physicians. Marijuana possession for personal use . Accessed August 20, 2019.
- The National Academies of Science, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids . Washington, D.C.: National Academies Press; 2017.
- National Institute on Drug Abuse. DrugFacts: Monitoring the Future Survey: high school and youth trends. www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Accessed August 20, 2019.
- National Institute on Drug Abuse. National Survey of Drug Use and Health . Accessed August 20, 2019.
- Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol . 2008;20(s1):10-14.
- Hilderbrand RL. Hemp & cannabidiol: what is a medicine? Mo Med . 2018;115(4):306-309. Accessed August 20, 2019.
- U.S. Department of Agriculture. Hemp production program . Accessed August 20, 2019.
- Heidi H. Oregon State University launches largest, most comprehensive hemp research center in the nation. Oregon State University. www.today.oregonstate.edu/news/oregon-state-university-launches-largest-most-comprehensive-hemp-research-center-nation. Accessed August 20, 2019.
- Lee MJ. The legalization of hemp. Accessed August 20, 2019.
- Shipman M. Is hemp the same thing as marijuana? NC State University. Accessed August 20, 2019.
- National Institute on Drug Abuse. What is marijuana? Accessed August 20, 2019.
- Drug Policy Alliance. Marijuana concentrates. Accessed August 20, 2019.
- MacCoun RJ, Mello MM. Half-baked — the retail promotion of marijuana edibles. N Engl J Med . 2015;372(11):989-991.
- Peschel W, Peschel, Wieland. Quality control of traditional cannabis tinctures: pattern, markers, and stability. Sci Pharm . 2016;84(3):567-584.
- Rosenblatt S, Tucker J, DeAngelo S. Methods and compositions of cannabis extracts. Accessed August 20, 2019.
- MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med . 2018;49:12-19.
- U.S. Food and Drug Administration. FDA regulation of cannabis and cannabis-derived products: questions and answers . Accessed August 20, 2019.
- National Cancer Institute. NCI Drug Dictionary. Nabiximols. Accessed August 20, 2019.
- U.S. Food and Drug Administration. Syndros (dronabinol) oral solution . Accessed August 20, 2019.
- U.S. Food and Drug Administration. CESAMETTM (nabilone) capsules. Accessed August 20, 2019.
- Greenwich Biosciences. Epidiolex® (cannabidiol). www.epidiolex.com/. Accessed August 20, 2019.
- Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use. A systematic review and meta-analysis. JAMA . 2015;313(24):2456-2473.
- Abuhasira R, Shbiro L, Landschaft Y. Medical use of cannabis and cannabinoids containing products – Regulations in Europe and North America. Eur J Intern Med . 2018;49:2-6.
- Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia . 2014;55(6):791-802.
- U.S. Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. Accessed August 20, 2019.
- Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N Engl J Med . 2018;378(20):1888-1897.
- Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med . 2017;376(21):2011-2020.
- Perucca E. Cannabinoids in the treatment of epilepsy: hard evidence at last? J Epilepsy Res . 2017;7(2):61-76.
- Volkow ND, Swanson JM, Evins AE, et al. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry . 2016;73(3):292-297.
- Wang GS. Cannabis (marijuana): acute intoxication. UpToDate. Accessed August 20, 2019.
- Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med . 2014;370(23):2219-2227.
- Wilson KM, Torok MR, Wei B, et al. Detecting biomarkers of secondhand marijuana smoke in young children. Pediatr Res . 2017;81(4):589-592.
- Reece-Stremtan S, Marinelli KA. ABM Clinical Protocol #21: Guidelines for Breastfeeding and Substance Use or Substance Use Disorder, Revised 2015. Breastfeed Med . 2015;10(3):135-141.
- Bertrand KA, Hanan NJ, Honerkamp-Smith G, Best BM, Chambers CD. Marijuana use by breastfeeding mothers and cannabinoid concentrations in breast milk. Pediatrics . 2018;142(3):e20181076.
- Corsi DJ, Walsh L, Weiss D, et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA . 2019;322(2):145-152.
- Centers for Disease Control and Prevention. Does marijuana use lead to other drug use? Accessed August 20, 2019.
- Di Forti M, Quattrone D, Freeman TP, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. The Lancet Psychiatry . 2019;6(5):427-436.
- U.S. Food and Drug Administration. Marijuana research with human subjects . Accessed August 20, 2019.
- National Institute on Drug Abuse (NIDA). NIDA’s role in providing marijuana for research. www.drugabuse.gov/drugs-abuse/marijuana/nidas-role-in-providing-marijuana-research. Accessed August 20, 2019.
- U.S. Drug Enforcement Administration. Drug scheduling . Accessed August 20, 2019.
- U.S. Food and Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on signing of the Agriculture Improvement Act and the agency's regulation of products containing cannabis and cannabis-derived compounds. Accessed August 20, 2019.
- American Bar Assocation. Conflicting state and federal marijuana laws create ethical complications for lawyers. Accessed August 20, 2019.
- National Conference of State Legislatures. Marijuana laws. www.ncsl.org/bookstore/state-legislatures-magazine/marijuana-deep-dive.aspx. Accessed August 20, 2019.
- National Conference of State Legislatures. Marijuana laws. Accessed August 20, 2019.
- Drug Policy Alliance. From prohibition to progress: a status report on marijuana legalization. Accessed August 20, 2019.
- American Academy of Family Physicians. Incarceration and health: a family medicine perspective (position paper) . Accessed August 20, 2019.
- Centers for Disease Control and Prevention. What you need to know about marijuana use and driving . Accessed August 20, 2019.
- National Conference of State Legislatures. Drugged driving | Marijuana-impaired driving . Accessed August 20, 2019.
- RAND Corporation. Adolescents who view more medical marijuana advertising are more likely to use marijuana, have positive views about the drug . Accessed August 20, 2019.
- Center on Addiction. Childhood poisoning: safeguarding young children from addictive substances. Accessed August 20, 2019.
- Center on Addiction. Toddlers and THC: the number of young children exposed to marijuana is on the rise. Accessed August 20, 2019.
- Child Welfare Information Gateway. Mandatory reporters of child abuse and neglect. Accessed August 20, 2019.
- Children’s Hospital Colorado. Marijuana safety in the home . Accessed August 20, 2019.
(July 2019 BOD)
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Mack A, Joy J. Marijuana as Medicine? The Science Beyond the Controversy. Washington (DC): National Academies Press (US); 2000.

Marijuana as Medicine? The Science Beyond the Controversy.
- Hardcopy Version at National Academies Press
1 INTRODUCTION
There are many reasons for wanting to understand what science has so far revealed—and what remains unknown—about marijuana's medical potential. Can marijuana really help people with AIDS (acquired immune deficiency syndrome), cancer, glaucoma, multiple sclerosis, or any of several other conditions it is purported to relieve? How does marijuana affect the human body? Could the potential benefits of legalizing marijuana for medicinal use possibly outweigh the risk of encouraging drug abuse? All of these questions remain to be answered completely, but over the past two decades scientists have made significant progress in revealing how chemicals in marijuana act on the body. Researchers have also studied how marijuana use affects individuals and society as a whole.
Unfortunately, much of what scientists have learned about the medical use of marijuana has been obscured by highly polarized debate over the drug's legal status. At times advocates for medical marijuana have appeared to be discussing a different drug than their opponents. Consider the following statements:
There are over ten thousand documented studies available that confirm the harmful physical and psychological effects of . . . marijuana.
—from the California Narcotic Officers' Association
Marijuana is NOT a Medicine, Santa Clarita, CA (1996), p. 2.
The cannabis plant (marijuana) . . . [has] therapeutic benefits and could ease the suffering of millions of persons with various illnesses such as AIDS, cancer, glaucoma, multiple sclerosis, spinal cord injuries, seizure disorders, chronic pain, and other maladies.
—from the editor's introduction to Cannabis in Medical Practice, by Mary Lynn Mathre, R.N.
Conflicts regarding the legitimacy of medical marijuana use extend even to the level of state versus federal law. Between 1996 and 1999, voters in eight states (Alaska, Arizona, California, Colorado, Maine, Nevada, Oregon, and Washington) and the District of Columbia * registered their support for the prescription of marijuana by physicians, defying the policies of the federal government and the convictions of many of its leaders.
Prior to the 1998 election, former Presidents Ford, Carter, and Bush released a statement urging voters to reject state medical marijuana initiatives because they circumvented the standard process by which the Food and Drug Administration (FDA) tests medicines for safety and effectiveness. “Compassionate medicine,” these leaders insisted, “must be based on science, not political appeals.” Nevertheless, medical marijuana initiatives proceeded to pass in every state in which they appeared on the ballot.
Both those who advocate and those who oppose the medical use of marijuana claim to have science on their side. Each camp selectively cites research that supports its position, and each occasionally misrepresents study findings. Unfortunately, these skewed interpretations have frequently served as the main source of scientific information on the subject. Until now it has been difficult for people other than scientists to find unbiased answers to questions about the medical use of marijuana—questions that have often drawn conflicting responses from either side of the debate.
But the public controversy over the medical use of marijuana does not reflect scientific controversy. Scientists who study marijuana and its effects on the human body largely agree about the risks posed by its use as well as the potential benefits it may provide. That is what researchers at the Institute of Medicine (IOM) learned when they undertook the study on which this book is based.
The goal of the study, performed at the request of the White House Office of National Drug Control Policy, was to conduct a critical review of all scientific evidence pertaining to the medical use of marijuana and its chemical components. For more than a year, researchers from the IOM—an arm of the National Academy of Sciences, which acts as an independent adviser to the federal government—compiled and assessed a broad range of information on the subject. One of us (Janet E. Joy) coordinated the IOM study. John A. Benson, Jr., dean and professor of medicine emeritus from the Oregon Health Sciences University School of Medicine and Stanley J. Watson, Jr., codirector and research scientist at the University of Michigan's Health Research Institute in Ann Arbor, served as its chief investigators. Nine other medical scientists with expertise concerning the medical use of marijuana served as technical advisers throughout the project.
In the course of its work, the study team examined research on how marijuana exerts its effects in the body and its ability to treat a wide variety of medical conditions. Team members compared the effectiveness of using marijuana versus approved medicines to treat numerous specific disorders. They also evaluated the effects of chronic marijuana use on physical and mental health as well as its possible role as a “gateway” drug to cocaine, heroin, and other illicit drugs.
To gather this information, the researchers analyzed scientific publications, consulted extensively with biomedical and social scientists, and conducted public scientific workshops. They also visited four so-called cannabis buyers' clubs and two HIV-AIDS clinics. Organizations and individuals were encouraged to express their views on the medical use of marijuana at the public workshops as well as via the Internet, by mail, and by telephone. The team's draft report was reviewed and critiqued anonymously by more than a dozen experts, whose comments were addressed in preparing the final version of the document. Entitled Marijuana and Medicine: Assessing the Science Base, the final report was released in March 1999. The report was subsequently published as a clothbound book by the National Academy Press; it can also be viewed on the Press's web site.
At the time of its release, the study received considerable attention from the news media. For example, the next week more than 50 U.S. newspapers carried stories on the study. While many of the articles reflected the balanced nature of the report's findings, most of the headlines—which tend to stick in readers' minds—gave the impression that the IOM had fully endorsed the medical use of marijuana. Scores of editorials followed suit, including several expressing uncritical acceptance of marijuana as a medicine.
In fact, the IOM researchers found little reason to recommend crude marijuana as a medicine, particularly when smoked, but they did conclude that active ingredients in marijuana could be developed into a variety of promising pharmaceuticals. Responding to the report's call for clinical trials on such marijuana-based medications, the National Institutes of Health and the Canadian equivalent of that agency, Health Canada, subsequently announced new policies intended to encourage medical research on marijuana (see Chapter 11 ).
While the IOM report was directed at policymakers, the purpose of this book is to present the main findings of that study for use by anyone who wants unbiased, scientifically sound medical information on marijuana. To adapt the IOM's publication for a general audience, considerable technical detail has been removed and in-depth explanations added of several key studies reviewed in the original report. For studies discussed in detail, references are provided in the form of footnotes. When the results of a group of studies are summarized, readers are referred to the relevant pages of the IOM report for more information and complete references. In a few instances, where more recent survey data became available after the IOM report was published, the most current information is used.
This book is divided into three parts, each of which offers a different perspective on marijuana as medicine. Along with this introduction, Chapter 2 and Chapter 3 lay out the scientific and historical foundation of current knowledge on the potential benefits and dangers of marijuana-based medicines. The second section— Chapter 4 , Chapter 5 , Chapter 6 , Chapter 7 , Chapter 8 through Chapter 9 —focuses on specific diseases, including cancer, AIDS, glaucoma, and a variety of movement and neurological disorders. In each case, the current state of knowledge regarding marijuana's effectiveness in treating symptoms of specific disorders is described and compared with conventional therapies. We explain why some marijuana-related studies that may seem convincing are actually inconclusive and what evidence is needed to support various claims about marijuana's harms or benefits. Finally, although this is primarily a book about science, two chapters in Part III are devoted to related issues: the economic prospects for developing pharmaceuticals from marijuana ( Chapter 10 ) and the complex legal environment surrounding the medical use of marijuana ( Chapter 11 ). Much of the information that is included about the legal status of marijuana did not appear in the IOM report but was added here to place the science of medical marijuana in a broader social context.
In addition to providing a critical and up-to-date summary of scientific knowledge that pertains to the medical use of whole marijuana, chemicals derived from the marijuana plant are also discussed, as well as synthetic compounds that represent “improved” versions of marijuana derivatives. This information can help readers evaluate future research news and participate in the ongoing public discussion of medical marijuana.
At the same time, it is important to recognize that science is but one aspect of the medical marijuana controversy. Ultimately, drug laws must address moral, social, and political concerns as well as science and medicine. Although we present scientific evidence related to the social impact of medical marijuana, the intent is not to prescribe policy but to encourage continued debate based on a firm understanding of scientific knowledge. As you read, please bear this in mind, along with the following caveats:
- Neither this book, nor the IOM study on which it is based, is intended to promote specific social policies. Both were designed to provide an objective scientific analysis of marijuana's current and potential usefulness in treating a variety of symptoms.
- In no way do we wish to suggest that patients should, un der any circumstance, medicate themselves with marijuana, an illegal drug.
- The medical information in this book is not intended to substitute for the advice of a physician or other health care professional.
Now that you know where this book came from and where it's going, we offer a few guideposts to aid your journey through it. Because the following key concepts underlie our discussion of medical marijuana, familiarizing yourself with them will help you make the most of your reading.
Marijuana contains a complex mixture of chemicals. Marijuana leaves or flower tops can be smoked, eaten, or drunk as a tea (see Figure 1.1 ). People who use marijuana in these ways expose themselves to the complex mixture of chemical compounds present in the plant. One of these chemicals, tetrahydrocannabinol (THC), is the main cause of the marijuana “high.” Thus, the effects of marijuana on the body include those of THC, but not all of marijuana's effects are necessarily due to THC alone.
Leaves and flower tops of female marijuana plants. (Photo by André Grossman.)
According to federal law, marijuana belongs to a category of substances that have a high potential for abuse and no accepted medical use. Other drugs in this category include LSD (lysergic acid diethylamide) and heroin. By contrast, doctors can legally prescribe THC, in the form of the medicine Marinol (a brand name for a specific formulation of the generic drug dronabinol), under highly regulated conditions. Dronabinol, the “synthetic” THC in Marinol, is identical in every way to the “natural” THC in marijuana.
The FDA has approved Marinol for the treatment of nausea and vomiting associated with cancer chemotherapy and also to counteract weight loss in AIDS patients. Currently classified with controlled substances such as anabolic steroids, Marinol was moved from a more restrictive category, which included cocaine and morphine, in July 1999.
Some of the medical studies discussed in later chapters deal with the effects of marijuana, while others focus on specific chemicals present in the marijuana plant. This distinction should be kept in mind when considering the results of these studies. The psychoactive chemicals in marijuana are members of a family of molecules known as cannabinoids, derived from the plant's scientific name, Cannabis sativa. Most cannabinoids are closely related to THC. Scientists also refer to chemicals that are not found in marijuana but that resemble THC either in their chemical structure or the way they affect the body as cannabinoids.
Occasionally, we also refer to “marijuana-based medicines. ” These encompass the entire spectrum of potential medications derived from marijuana, from whole-plant remedies to extracts to individual cannabinoids, both natural and synthetic.
Marijuana is not a modern medicine. Although people have used marijuana for centuries to soothe a variety of ills, it cannot be considered a medicine in the same sense as, for example, aspirin. Aspirin's chemical cousin, found in willow bark, was long used as a folk remedy for pain. But unlike marijuana, aspirin has been proven safe and effective through rigorous testing. Aspirin tablets contain a pure measured dose of medicine, so they can be relied on to give consistent and predictable results.
By contrast, two identical-looking marijuana cigarettes could produce quite different effects, even if smoked by the same per son. If one of the cigarettes were made mostly from leaves and the other from flower tops, for instance, they would probably contain different amounts of active chemicals. Growing conditions also affect marijuana's potency, which can vary greatly from region to region and even from season to season in the same place. This variability makes marijuana at best a crude remedy, more akin to herbal supplements such as St. John's wort or ginkgo than to conventional medications.
To date, few herbal supplements have been tested for safety and efficacy in the United States, nor are such products subject to mandatory quality controls. Yet despite these drawbacks, increasing numbers of consumers are using herbal treatments, prompted by their desire for “natural” alternatives to man-made medicines. However, another way to view herbal remedies is to recognize that if they are effective, they contain specific active ingredients. Willow bark contains a pain-relieving compound; marijuana contains cannabinoids such as THC, which lessens nausea. Once identified, chemists can duplicate active compounds in the laboratory. Scientists can also use natural compounds as a basis for creating new medicines. By introducing subtle structural changes in natural molecules, chemists have produced drugs that are more effective and easier to administer and that have fewer side effects than their natural counterparts. So far, a few such analogs or derivatives of cannabinoids are known to exist; others are currently under investigation.
Marijuana used as medicine is not a recreational drug. People who use marijuana solely as a medication do so in order to relieve specific symptoms of AIDS, cancer, multiple sclerosis, and other debilitating conditions. Some do so under the advice or consent of doctors after conventional treatments have failed to help them. In mentioning medical marijuana users, we are referring to people who smoke or eat marijuana exclusively as a treatment for medical symptoms. The fact that many such patients may have prior recreational experience with the drug does not mean that they are using illness as an excuse to get high, although it is possible that some patients might do so. Surveys of marijuana buyers' clubs indicate that most of their members do, in fact, have serious medical conditions.
Medical marijuana users tend to come from different seg ments of the population than recreational users. In the United States recreational marijuana use is most prevalent among 18 to 25 year olds and declines sharply after age 34. By contrast, reports on medical marijuana users indicate that most are over 35, as are typical consumers of herbal medicine and other alternative therapies. Most tend to suffer from chronic illnesses or pain that defy conventional treatments.
Medical marijuana advocates assert that patients usually obtain relief with smaller doses of the drug than would be used recreationally and that they rarely feel high when treating their symptoms with marijuana; however, no objective study has tested this claim. As discussed in detail in Chapter 3 , marijuana and its constituent chemicals can produce both physical and psychological dependence. These risks must be taken into account if marijuana or cannabinoids are to be used as medicines.
Many effective medicines have side effects. The fact that marijuana affects the human body adversely does not preclude its use as a source of useful medicines. Many legitimate drugs—including opiates, chemotherapy agents, and steroids—have side effects ranging from the dangerous to the merely unpleasant. When used carefully, though, the benefits of these medications far outweigh their drawbacks. Patients may also develop tolerance, dependence, and withdrawal—conditions associated with marijuana use—when taking proper doses of several commonly prescribed medications. For example, the correct use of some prescription medicines for pain, anxiety, and even hypertension normally produces tolerance and some physiological dependence.
As researchers learn more about the chemicals present in marijuana and their effects on the body, it may be possible to identify beneficial compounds and separate them from harmful substances in the plant. Finding a rapid way to deliver cannabinoids to the body, other than smoking, could lessen some of marijuana's worst side effects. It may also be possible to reduce the adverse effects of specific cannabinoids through chemical modification, as previously noted.
Marijuana's effects vary with different delivery methods. Traditionally, medicinal marijuana has not been smoked but rather swallowed in the form of an extract or applied to the underside of the tongue in the form of an alcohol-based tincture. Although the lat ter method allows the THC to pass directly into the bloodstream, it is far less efficient than smoking. When swallowed, drugs pass through the stomach, intestine, and liver before entering the bloodstream, so they act slowly. This is especially true of the main active ingredient in marijuana. Because THC is barely soluble in water, the body absorbs only a small fraction of the available drug when it is swallowed.
The same is true of Marinol, which is simply THC in capsule form. Marijuana smoke, on the other hand, efficiently delivers THC into the bloodstream via the lungs. Inhaled THC takes effect quickly, allowing patients to use just enough to relieve their symptoms; it is not so easy to fine-tune the dose of oral medications. For this reason, pharmaceutical firms are investigating the use of smokeless inhalers and nasal sprays to deliver THC and possibly other cannabinoids.
The Colorado vote was later disallowed after a court determined that the petition to place the initiative on the ballot did not have enough valid signatures. Congress has prohibited the counting of actual ballots in the District of Columbia referendum, but exit polls indicated that a majority of voters approved the measure. Nevada voters must reapprove their proposal in the year 2000 before it becomes law.
- Cite this Page Mack A, Joy J. Marijuana as Medicine? The Science Beyond the Controversy. Washington (DC): National Academies Press (US); 2000. 1, INTRODUCTION.
- PDF version of this title (3.6M)
Recent Activity
- INTRODUCTION - Marijuana as Medicine? INTRODUCTION - Marijuana as Medicine?
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
INTRODUCTION. Medicinal cannabis, or medicinal marijuana, is a therapy that has garnered much national attention in recent years. Controversies surrounding legal, ethical, and societal implications associated with use; safe administration, packaging, and dispensing; adverse health consequences and deaths attributed to marijuana intoxication; and therapeutic indications based on limited ...
Background. Interest in medical applications of marijuana (Cannabis sativa) has increased dramatically during the past 20 years.A 1999 report from the National Academies of Sciences, Engineering, and Medicine supported the use of marijuana in medicine, leading to a number of regulatory medical colleges providing recommendations for its prescription to patients [].
The potential medicinal properties of marijuana and its components have been the subject of research and heated debate for decades. THC itself has proven medical benefits in particular formulations. The U.S. Food and Drug Administration (FDA) has approved THC-based medications, dronabinol (Marinol) and nabilone (Cesamet), prescribed in pill form for the treatment of nausea in patients ...
Cannabis sativa has a long history as a medicinal plant, likely dating back more than two millennia (Russo et al., 2007). It was available as a licensed medicine in the United States for about a century before the American Medical Association removed it from the 12th edition of the U.S. Pharmacopeia (IOM, 1999). In 1985, pharmaceutical companies received approval to begin developing Δ9 ...
A new cycle has begun for the use of cannabis derivatives as medicines, this time with more consistency than in the past. Interest in the therapeutic benefits of medical cannabis has grown rapidly in the past 20 years, with patients using cannabinoids to treat a variety of conditions, from chronic pain, cancer and neurological disorders to anxiety and sleep disorders (Schlag et al., 2020 ...
In 2017, the National Academies of Sciences, Engineering, and Medicine released The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research, a report by an expert committee that reviewed the available evidence for 20 indications. 1 As expected, the committee found conclusive evidence that oral ...
In a recent systematic review and meta-analysis of randomized clinical trials of medical cannabis for chronic pain (n = 32 trials with 5174 patients), oral medical cannabis was associated with a 4% increase in the proportion of patients experiencing an improvement of more than 10 points (the minimally clinically important difference) on the ...
Marijuana use, diet, body mass index, and cardiovascular risk factors (from the CARDIA study). Am J Cardiol. 2006; 98:478-484. doi: 10.1016/j.amjcard.2006.03.024 Crossref Medline Google Scholar; 49. Wang GS, Hall K, Vigil D, Banerji S, Monte A, VanDyke M. Marijuana and acute health care contacts in Colorado. Prev Med.
Department of Drug Science and Technology, University of Turin, Turin, Italy; Cannabis has long been regarded as a recreational substance in the Western world. The recent marketing authorization of some medicinal products of industrial origin and the introduction onto the market of inflorescences for medical use mean that medical doctors can now prescribe Cannabis-based medicines in those ...
ABSTRACT: Cannabis, or marijuana, has potential therapeutic and medicinal properties related to multiple compounds, particularly Δ-9-tetrahydrocannabinol and cannabidiol. Over the past 25 years, attitudes toward cannabis have evolved rapidly, with expanding legalization of medical and recreational use at the state level in the United States and
The average number of patients showing a complete nausea and vomiting response was greater with cannabinoids (dronabinol or nabiximols) than placebo (OR, 3.82 [95% CI, 1.55-9.42]; 3 trials). There was no evidence of heterogeneity for this analysis ( I2 = 0%) and results were similar for both dronabinol and nabiximols.
Background Recently, the renewed global interest in cannabis' therapeutic properties has resulted in shifting attitudes and legislative policies worldwide. The aim of this systematic review is to explore the existing literature on medical professionals' and students' attitudes and knowledge regarding medicinal cannabis (MC) to assess any relevant and significant trends. Methods This ...
1 National Drug and Alcohol Research Centre (NDARC), Faculty of Medicine, UNSW Sydney, 22-32 King Street, Randwick, NSW, ... the findings and conclusions of these studies have been inconsistent. In this paper, we outline the current evidence for medical cannabis and cannabis-based medicines in the treatment and management of chronic non-cancer ...
The Journal of Cannabis Research is an international, fully open access, peer-reviewed journal covering all topics pertaining to cannabis, including original research, perspectives, commentaries and protocols. Our goal is to provide an accessible outlet for expert interdisciplinary discourse on cannabis research. Read Aims & Scope.
Medical Cannabis Real World Evidence [ 44 - 46] A Canadian, prospective, non-interventional, observational study led by the University Health Network in Toronto. It aims to explore the benefits of medical cannabis in an observational setting for adults with conditions such as chronic pain, anxiety or depression.
Abstract. The use of cannabis for medical purposes has been a subject for discussion for so many years. Cannabis as a source of medical treatment first came to light in the 19 th century. However, origins of cultivation of marijuana as a medical plant can be traced back to thousands of years. Attempts to completely legalize the use of cannabis ...
A new report from the National Academies of Sciences, Engineering, and Medicine offers a rigorous review of scientific research published since 1999 about what is known about the health impacts of cannabis and cannabis-derived products - such as marijuana and active chemical compounds known as cannabinoids - ranging from their therapeutic effects to their risks for causing certain cancers ...
National Bureau of Economic Research. NBER working papers are circulated for discussion and comment purposes. They have not been ... Thirty-six states have legalized medical marijuana and 14 states have legalized the use of marijuana for recreational purposes. In this paper, we review the literature on the public health ...
Through registration issued by DEA, NIDA is responsible for overseeing the cultivation of marijuana for medical research and has contracted with the University of Mississippi to grow marijuana for ...
Thirty-six states have legalized medical marijuana and 18 states have legalized the use of marijuana for recreational purposes. In this paper, we review the literature on the public health consequences of legalizing marijuana, focusing on studies that have appeared in economics journals as well as leading public policy, public health, and ...
Only recently, cannabis-based medicine was tested more systematically for the treatment of mental disorders. The aim of this systematic review was to analyze its efficacy, tolerability and safety in patients with a diagnosed mental disorder. The literature research identified 4 SRs (of 11 RCTs) and 14 additional RCTs.
The use of cannabis has steadily grown in recent years, and more than 200 million people worldwide used cannabis in 2019 alone. 9 It remains the most widely cultivated and trafficked illicit substance worldwide. 10 In India, according to a nationwide survey, 31 million people (2.8% of the total population) reported using cannabis in 2018, and 0.25% (2.5 million) also showed signs of cannabis ...
In 2015, Whiting, et al, performed a meta-analysis and systematic review of research on the medical use of cannabis. 25 This systematic review served as the basis for many recommendations in 2017 ...
2 has relaxation side effects. This effect would keep the individuals in correctional facilities calm, away from trouble and possible fights. Due to this, correctional officers would be able to maintain law and order in the correctional facilities as medical marijuana would help manage violent inmates with mental disorders. In conclusion, considering the nature and situation of correctional ...
The cannabis plant (marijuana) . . . [has] therapeutic benefits and could ease the suffering of millions of persons with various illnesses such as AIDS, cancer, glaucoma, multiple sclerosis, spinal cord injuries, seizure disorders, chronic pain, and other maladies. —from the editor's introduction to Cannabis in Medical Practice, by Mary Lynn ...