- Download PDF
- Share X Facebook Email LinkedIn
- Permissions

Young-Onset Dementia—New Insights for an Underappreciated Problem
- 1 Department of Neurology, Mayo Clinic, Rochester, Minnesota
- Original Investigation Global Prevalence of Young-Onset Dementia Stevie Hendriks, MSc; Kirsten Peetoom, PhD; Christian Bakker, PhD; Wiesje M. van der Flier, PhD; Janne M. Papma, PhD; Raymond Koopmans, PhD; Frans R. J. Verhey, MD, PhD; Marjolein de Vugt, PhD; Sebastian Köhler, PhD; Young-Onset Dementia Epidemiology Study Group; Adrienne Withall, PhD; Juliette L. Parlevliet, MD, PhD; Özgül Uysal-Bozkir, PhD; Roger C. Gibson, PhD; Susanne M. Neita, PhD; Thomas Rune Nielsen, PhD; Lise C. Salem, PhD; Jenny Nyberg, PhD; Marcos Antonio Lopes, PhD; Jacqueline C. Dominguez, PhD; Ma Fe De Guzman, PhD; Alexander Egeberg, MD, PhD; Kylie Radford, PhD; Tony Broe, PhD; Mythily Subramaniam, PhD; Edimansyah Abdin, PhD; Amalia C. Bruni, PhD; Raffaele Di Lorenzo, PhD; Kate Smith, PhD; Leon Flicker, PhD; Merel O. Mol, MSc; Maria Basta, PhD; Doris Yu, PhD; Golden Masika, PhD; Maria S. Petersen, PhD; Luis Ruano, MD, PhD JAMA Neurology
At one time in the not-too-distant past, young-onset dementia (YOD) was considered a disease, whereas later-onset dementia (LOD) was considered the inevitable consequence of aging. We now reject such a formulation, but within that misguided dichotomy is a recognition of the dramatic differences in prevalence and incidence of YOD compared with LOD. In this issue of JAMA Neurology , Hendriks et al 1 quantitate dementia prevalence in persons aged 30 to 64 years through a meta-analysis of 74 individual studies.
Read More About
Knopman DS. Young-Onset Dementia—New Insights for an Underappreciated Problem. JAMA Neurol. 2021;78(9):1055–1056. doi:10.1001/jamaneurol.2021.1760
Manage citations:
© 2024
Artificial Intelligence Resource Center
Neurology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Young-Onset Dementia More Prevalent Than Previously Estimated
- Nick Zagorski
Search for more papers by this author
A meta-analysis of 95 studies from around the world suggests that nearly 4 million adults globally may experience dementia before age 65.
Though considered a disease of the elderly, dementia can strike younger adults as well (Alois Alzheimer’s first patient was a woman who began experiencing memory loss and delusions in her 40s). Yet attention to patients with rare young-onset dementia—typically characterized as dementia before age 65—often pales in comparison to that given to the estimated 45 million adults living with late-onset dementia.
A large meta-analysis conducted by Sebastian Köhler, Ph.D., an associate professor of psychiatry and neuropsychology at Maastricht University in the Netherlands, and colleagues suggests young-onset dementia may be more common than previously estimated. Making use of data from 95 individual studies encompassing 2.7 million adults in more than 30 countries, the researchers calculated a global prevalence rate of 119.0 cases of young-onset dementia per 100,000 people. This figure is more than double that of previous estimates and equates to about 200,000 cases of young-onset dementia in the United States and nearly 4 million globally.
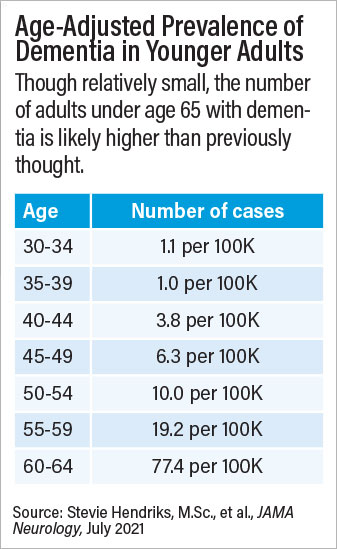
“Although this is higher than previously thought, it is probably an underestimation owing to lack of high-quality data,” senior author Köhler and colleagues wrote in JAMA Neurology . They noted that data on adults under age 50 were sparse, as were data from low- and middle- income countries.
The authors also excluded studies that focused on at-risk population groups like patients with HIV, noted Brian Draper, M.D., a conjoint professor of psychiatry at the University of New South Wales in Australia, who specializes in young-onset dementia. “The bulk of dementia research is related to diseases like Alzheimer’s or frontotemporal dementia, but these secondary dementias that arise from other disorders that can impact the brain should not be discounted,” he said. Draper has done a lot of work with alcohol-related dementia, which overlaps with Wernicke-Korsakoff syndrome, a condition in which chronic alcohol use leads to vitamin B deficiency and subsequent neurodegeneration.
Draper told Psychiatric News that the reduced attention to secondary dementias is not entirely from scientists or physicians. “There has been pushback from patients and advocacy groups for conditions like HIV to avoid associating these disorders with dementia due to stigma,” he said. As a result, he said, places like alcohol treatment centers do not often provide dementia screening, which contributes to patients slipping through the cracks.
“From a personal management and clinical care perspective, it’s imperative to diagnose young-onset dementia as soon as possible,” Draper continued. “By and large, people who develop dementia earlier in life have a faster rate of cognitive decline, yet they live longer with the disease”—a factor that may be due to younger patients having fewer comorbidities at the time of dementia diagnosis.
In an editorial that accompanied Köhler’s JAMA Neurology article, David Knopman, M.D., a professor of neurology at the Mayo Clinic, wrote: “Young-onset dementia is a particularly disheartening diagnosis because it affects individuals in their prime years, in the midst of their careers, and while raising families,” he wrote. “Most dementia care is geared for older patients, and as a consequence, services are rarely available to address the needs of someone diagnosed with dementia in their 50s who has dependent children at home and a spouse who must continue working. Understanding the prevalence and incidence of [young-onset dementia] is a first step in addressing this challenge.”
In comments to Psychiatric News , Knopman said that the results of the meta-analysis offered an important perspective on the rates of dementia over time and the relative risks in older versus younger adults. Overall, the study suggests that about 3% of all dementia cases in the United States are in adults under age 65. However, most of these cases were in adults aged 55 to 64.
“Among the really young, where the burden would be greatest, dementia is exceedingly rare,” Knopman said. In the age range of 30 to 34, for example, Köhler’s meta-analysis calculated a prevalence of 1 case of dementia per 100,000 people. “At that level, routine screening is out of the question,” he said. “So, on a practical level, how can physicians identify a problem they might encounter once a decade?”
It’s a pertinent question for psychiatrists, Draper said, since work by him and others has shown that chronic, treatment-resistant depression can be an early symptom of young-onset dementia. “If you have a middle-aged patient with treatment-resistant depression who begins complaining of memory problems, you should entertain [underlying] dementia as a possibility,” he said.
Draper noted that Alzheimer’s disease—which is the most common cause of dementia in older adults—is less dominant in young-onset cases. More commonly, younger patients may experience Disorders like frontotemporal dementia and Huntington’s disease are more common. This is why psychiatrists need to look at more than just cognition in younger people, as behavioral (personality changes) and/or physical symptoms (such as gait disturbances) are most likely to emerge first, he said.
“There is no specific pattern that can help diagnose patients at an individual level,” Knopman said. “But if physicians keep an open mind that dementia exists before 65, that can help awareness.”
Firsthand clinical experience is also valuable, Knopman continued. “Once you have seen one patient with dementia, it helps make future diagnoses much easier. If more clinicians could do a geriatric rotation, that would help diagnosis tremendously.”
The meta-analysis was supported by the Gieskes-Strijbis Foundation, Alzheimer Netherlands, and the Dutch Young-Onset Dementia Knowledge Centre. ■
“Global Prevalence of Young-Onset Dementia: A Systematic Review and Meta-analysis” is posted here .

- Open access
- Published: 02 January 2022
What do health professionals need to know about young onset dementia? An international Delphi consensus study
- Leah Couzner 1 ,
- Sally Day 1 ,
- Brian Draper 2 ,
- Adrienne Withall 3 ,
- Kate E. Laver 4 ,
- Claire Eccleston 5 ,
- Kate-Ellen Elliott 5 ,
- Fran McInerney 5 &
- Monica Cations 1 , 4
BMC Health Services Research volume 22 , Article number: 14 ( 2022 ) Cite this article
3128 Accesses
7 Citations
7 Altmetric
Metrics details
People with young onset dementia (YOD) have unique needs and experiences, requiring care and support that is timely, appropriate and accessible. This relies on health professionals possessing sufficient knowledge about YOD. This study aims to establish a consensus among YOD experts about the information that is essential for health professionals to know about YOD.
An international Delphi study was conducted using an online survey platform with a panel of experts ( n = 19) on YOD. In round 1 the panel individually responded to open-ended questions about key facts that are essential for health professionals to understand about YOD. In rounds 2 and 3, the panel individually rated the collated responses in terms of their importance in addition to selected items from the Dementia Knowledge Assessment Scale. The consensus level reached for each statement was calculated using the median, interquartile range and percentage of panel members who rated the statement at the highest level of importance.
The panel of experts were mostly current or retired clinicians (57%, n = 16). Their roles included neurologist, psychiatrist and neuropsychiatrist, psychologist, neuropsychologist and geropsychologist, physician, social worker and nurse practitioner. The remaining respondents had backgrounds in academia, advocacy, or other areas such as law, administration, homecare or were unemployed. The panel reached a high to very high consensus on 42 (72%) statements that they considered to be important for health professionals to know when providing care and services to people with YOD and their support persons. Importantly the panel agreed that health professionals should be aware that people with YOD require age-appropriate care programs and accommodation options that take a whole-family approach. In terms of identifying YOD, the panel agreed that it was important for health professionals to know that YOD is aetiologically diverse, distinct from a mental illness, and has a combination of genetic and non-genetic contributing factors. The panel highlighted the importance of health professionals understanding the need for specialised, multidisciplinary services both in terms of diagnosing YOD and in providing ongoing support. The panel also agreed that health professionals be aware of the importance of psychosocial support and non-pharmacological interventions to manage neuropsychiatric symptoms.
Conclusions
The expert panel identified information that they deem essential for health professionals to know about YOD. There was agreement across all thematic categories, indicating the importance of broad professional knowledge related to YOD identification, diagnosis, treatment, and ongoing care. The findings of this study are not only applicable to the delivery of support and care services for people with YOD and their support persons, but also to inform the design of educational resources for health professionals who are not experts in YOD.
Peer Review reports
Young onset dementia (YOD), in which dementia symptoms develop prior to 65 years of age, accounts for up to 8% of all dementia diagnoses [ 1 ]. Young onset dementia is associated with significant challenges for both the individual and their support persons, which may differ to those experienced in late onset dementia (LOD) [ 2 ]. At the time of diagnosis people with YOD are often employed, and they and their supporters may be forced to leave the workforce. This presents financial ramifications [ 2 , 3 ]. They may be balancing caring responsibilities for young children and/or ageing parents and experience a shift in identity from that of a provider to a recipient of support [ 2 , 4 ]. People with YOD are also often physically healthy and active yet may experience a loss of empowerment and independence as their symptoms progress [ 4 ]. Other psychological impacts include shock or embarrassment about being diagnosed with a condition commonly associated with older adults, loss of purpose, and relationship strain [ 4 , 5 , 6 ]. Supporters of people with YOD report greater difficulty managing dementia-related behavioural disturbances than those providing support for someone with LOD [ 7 ]. Other effects reported by support persons include stress, depression, frustration, grief, guilt, loneliness, fear of the future, and social isolation [ 6 , 8 ].
People with YOD have unique needs and experiences, and as such require care to be provided by health professionals with sufficient knowledge and skills. A lack of awareness among health professionals about YOD may contribute to the average 4.7 year diagnosis delay from the onset of symptoms, with misdiagnosis being one reason [ 9 , 10 ]. However recent work by O’Malley et al. has identified, using expert consensus, key elements in the diagnostic workup of YOD to aide decision making for clinicians [ 11 ]. After diagnosis, the use of formal support services in the community may delay the need for permanent residential care and can provide respite and access to peer support [ 3 ]. However, services providing dementia-related support are often designed for older adults. These services are known to lack acceptability for people diagnosed with YOD. Reasons for this include a lack of age-appropriate services, poor service accessibility (e.g. lack of transport, held during work time, lack of child care), inadequate security for physically agile participants, affordability issues, a lack of continuity of care and inadequate information provision about YOD and the support available [ 3 , 12 , 13 ].
It is therefore important that health professionals possess adequate knowledge and skills about YOD presentation, identification, diagnosis, treatment, and care to provide services and information that are timely, appropriate, and accessible. A preliminary step to addressing this is to determine the information that health professionals require to provide this care. This study aims to establish a consensus among those with YOD expertise about the key information that is important for health professionals to know and understand about YOD. This data can then be utilised to inform the upskilling of YOD professionals and in the development of tools to track their knowledge.
The Delphi technique is a multistage method used to obtain a consensus among a panel of experts on a particular topic [ 14 ]. The process involves the iterative distribution of a series of questionnaires asking the participant to rank a list of items or statements in order of importance. When completing the second and subsequent questionnaires, participants are provided with the results of the previous questionnaire and are encouraged to reconsider their individual responses. The process continues until a consensus has been reached, or no further changes are being made [ 15 , 16 ]. The number of iterations or “rounds” varies, but three rounds can offer a balance between rigour and participant burden [ 15 ]. The questionnaires are completed anonymously and via mail or email, minimising the risk of individual participants influencing the rest of the group [ 17 ].
In this Delphi study, three feedback rounds were conducted using an online secure survey platform. The three phases in this study were: (a) identifying key information about young onset dementia, (b) rating agreed knowledge statements, and (c) confirming group consensus via item ratings. The procedure for this study was modelled on a previous study conducted by members of our research team that identified consensus opinion about key information about dementia more generally [ 18 ].
Participants
Participants selected for a Delphi study directly influence the quality of the data generated [ 15 , 19 ]. It is vital that participants possess in-depth knowledge or experience about the topic being explored. The ideal panel size has not been established, but 10 to 30 participants is recommended in the literature [ 19 , 20 ]. In this study, experts on young onset dementia were identified through networks of the research team in the areas of clinical care, psychology, research and education, advocacy and lived experience. Participants were required to have either lived experience or professional experience regarding YOD, be able to read and write in English and provide informed consent. These experts were invited to participate via an email invitation from the study team. Passive snowballing recruitment was also used meaning the experts were encouraged to forward the email invitation to others they considered experts in the area of young onset dementia who would be willing to participate in the study. The research was reviewed and approved by the Flinders University Social and Behavioural Research Ethics Committee (8331).
Experts were contacted via email from the study team throughout the project, and two follow up emails were sent for the second and third rounds. If the experts did not respond or participate in that round they were considered to have withdrawn from the study. All study participants were anonymous to each other and were identifiable to the study team only through their email address (to enable contact of participating experts at each round of the Delphi). Data collection was undertaken using an online survey platform, Qualtrics.
Round one (June 2020): gathering information
In the first round, the panel of experts were presented with the following open-ended questions:
What key facts are essential to understanding young onset dementia? Participants were provided with five concept areas to consider: (a) causes and characteristics, (b) symptoms and progression, (c) assessment and diagnosis, (d) prevention and treatment, and (e) care.
What key facts about young onset dementia are different to late onset dementia and the same as late onset dementia?
What key facts about young onset dementia are frequently misunderstood by health professionals?
These questions were modelled on those used in the development of the Dementia Knowledge Assessment Scale (DKAS) and in consultation with the research team. The questions were selected to identify information that the experts considered important for health professionals with differing levels of knowledge and experience in YOD to understand. The responses were then independently reviewed by two researchers on the project team who collated them to produce a list of statements that reflected the range of information provided. Where possible, the experts’ own words were used to maintain authenticity and reduce researcher bias. This process resulted in a list of 48 statements representing the information that the experts deemed to be essential in understanding YOD. Statements from the Dementia Knowledge Assessment Scale [ 18 ] were also included to build on existing work.
Round two (August 2020): rating knowledge statements
In the second round, the statements identified in round 1 were presented to the panel of experts. They were asked to rate each statement in terms of how essential it was for knowledge of YOD among health professionals from 1 (not important at all) to 5 (very important), or N/A, indicating that they perceived that the statement was not applicable to YOD. This rating scale was selected in accordance with that used in the development of the Dementia Knowledge Assessment Scale [ 21 ]. The responses were then analysed by two researchers on the project team to calculate the level of consensus achieved for each statement.
Round three (November 2020): obtaining consensus
In round 3, participants were presented with the same list of statements, accompanied by each statement’s median rating (group score) and consensus level from the previous round. Participants were not informed which statements had been deemed not applicable as this was collected for analytical purposes only. Participants were asked to review this new information, and again rate each statement on the same scale of 1 to 5. The responses were then analysed to ascertain the level of consensus reached by the participants for each statement. This allowed for comparisons to be made between the results of rounds 2 and 3, where little change would indicate a stability of the consensus levels.
Measurement and analysis
When using the Delphi technique, consensus is typically considered to have been reached when a certain percentage of the responses fall within a pre-determined range, for example 70% of participants rating 3 or higher on a four-point Likert scale [ 15 ]. The statistics commonly used in Delphi studies are measures of central tendency (mean, median and mode) and dispersion (standard deviation and interquartile range (IQR)). This allows for the combined group response to be presented, reflecting the responses of every panel member [ 15 , 22 ].
This study utilised the scoring systems reported by Annear and colleagues and van der Steen and colleagues [ 21 , 23 ] to build on existing work and allow for comparisons between the YOD information identified as essential in this study and that identified as essential to dementia more broadly in previous work. The scoring system is based on the median score and IQR for each statement, and the percentage of participants who scored the statement as either important or very important (the two highest levels). Full consensus was defined as a median score of 5, an IQR of 0, and 100% of participants rating the statement with the highest possible score of 5. Very high consensus was considered to be a median score of 5, an IQR of 0 and ≥ 80% scoring a 4 or 5. High consensus was defined as a median score of 5, an IQR ≤1 and ≥ 80% scoring a 4 or 5. Moderate consensus was considered to be a median score of 4–5, an IQR ≤2 and ≥ 60% scoring a 4 or 5. No consensus (low agreement) was defined as a median score of 4–5 and either IQR ≤2 or ≥ 60% scoring a 4 or 5. Statements with median scores between 2 and 4 were deemed to demonstrate no consensus (no agreement) [ 21 , 23 ].
Forty-six experts on YOD were identified via professional networks and invited to participate in the Delphi study. Of those, twenty-eight individuals completed the first round (61% response rate). Sixteen of the twenty-eight participants remained in the study until completion (57% completion rate), with one additional participant completing round 3 only. Most round 1 participants were from Australia ( n = 15, 54%), followed by Canada, the United Kingdom, the Netherlands, and Norway. Fifty-seven percent ( n = 16) of respondents in round 1 identified themselves as being a current or retired clinician, or having dual clinical and academic roles. The clinical roles included neurologist, psychiatrist and neuropsychiatrist, psychologist, neuropsychologist and geropsychologist, physician, social worker and nurse practitioner. The remaining respondents had backgrounds in academia, advocacy, or other areas such as law, administration, homecare or were unemployed. The characteristics of the participants are summarised in Table 1 .
Round 1: gathering information
Twenty-eight participants responded to the open-ended questions presented in round 1. Their responses were independently reviewed by two researchers and collated into a list of 48 statements summarising the information provided (Table 2 ). These statements were then compared to those in theDKAS [ 18 ]. The DKAS was selected as it is a validated assessment of dementia knowledge based upon dementia information identified as essential using expert consensus, although not YOD-specific. Ten DKAS statements were identified as not corresponding to any of the statements based on the participant responses in this study. To utilise and build on the existing knowledge, 10 statements closely based on statements from the DKAS were added to the original 48 statements. The statements were grouped into six categories: characteristics, causes and prevention, symptoms, diagnosis, treatment, and care.
Round 2: rating knowledge statements
In round 2, the participants were provided with the 58 statements developed in round 1 and asked to rate the importance of each statement on a scale from 1 (not important at all) to 5 (very important), or alternatively identify the statement as being not applicable to YOD. Nineteen participants provided responses in round 2. No statements achieved full consensus, however 16 statements (28%) achieved very high consensus. Very high consensus items most commonly related to post-diagnosis care for people with YOD ( n = 6, 38%).
Round 3: obtaining consensus
In round 3, participants were asked to rate the same statements for a second time. They were also provided with the median rankings and consensus levels from round two. Seventeen participants completed round 3 of the study, with consensus ratings displayed in Table 3 . As with round 2, no statements achieved full consensus, however all statements met some level of agreement. In total, 28% ( n = 16) of statements achieved very high consensus, 46% ( n = 26) reached high consensus, and 24% ( n = 14) reached moderate consensus. Three percent ( n = 2) of statements failed to achieve a consensus, reaching only low levels of agreement.
The statement that “young people with dementia require age-appropriate care programs and accommodation options” ranked highest, with 94% of participants rating the statement with the highest possible score of 5. At least one statement from each thematic category met very high consensus, except for “causes and prevention”. Of the statements that achieved very high consensus, the most prevalent themes were treatment ( n = 5, 31%) and care ( n = 5, 31%). Nineteen percent ( n = 3) related to the diagnosis of YOD, 13% ( n = 2) focused on the characteristics of YOD and 6% ( n = 1) referred to the symptoms of YOD.
Eight statements were identified by participants as not being applicable to YOD, most commonly “there are medications that can slow down the progression of some types of young onset dementia” ( n = 4). Two participants considered the statement “neuropsychiatric (i.e., behavioural and psychological) symptoms are more common in young people with dementia than older people” not relevant to YOD.
The additional 10 statements that were included from the DKAS [ 18 ] all reached consensus, with 10% (n = 1) reaching very high consensus, 40% (n = 4) reaching high consensus and 50% ( n = 5) reaching moderate consensus. Although these statements were not identified spontaneously by the experts, they were nonetheless deemed as important information for health professionals to know about YOD.
The panel of experts in this study reached high to very high consensus on 42 statements (out of 58) that they considered to be important for health professionals to know when providing care and services to people with YOD and their families. There was agreement across all thematic categories, indicating the importance of broad professional knowledge related to YOD identification, diagnosis, treatment, and ongoing care. These data can be used to inform efforts to upskill YOD professionals and track their knowledge and skills over time.
Identification and aetiology
Young onset dementia is an umbrella term that refers to a broad and heterogeneous range of illnesses, and in many cases occurs secondarily to another condition (for example, Huntington’s disease or alcohol use disorders) [ 24 ]. Common symptoms are not limited to cognitive impairment; depression, language impairments, and changes in behaviour or personality are regularly reported particularly by those with frontotemporal dementias [ 25 ]. Physical symptoms can include seizures, peripheral neuropathy, visual impairments, ataxia, skin lesions, headaches and visual impairment [ 26 ]. It is important for health professionals to be aware of the symptoms and stages of YOD as the range of clinical presentations and overlap of symptoms across YOD subtypes can delay the receipt of a diagnosis and often result in a dismissal of symptoms, or misdiagnosis [ 4 , 27 ].
The diversity in presentation, cause, and course of YOD was evident in many of the consensus statements, including that YOD is aetiologically diverse, refers to the emergence of dementia symptoms prior to the age of 65, is distinct from a mental illness, and is not a normal part of the ageing process. Consensus was also reached for facts that are commonly mistaken about YOD, including that most cases of YOD are not directly inherited (i.e. autosomal-dominant) and have a combination of genetic and non-genetic contributing factors. However when cases of directly-inherited occur, they tend to be associated with a younger age of onset [ 28 ]. It is notable that we did not reach consensus on the statement “Alzheimer’s disease is the most common form of young onset dementia”, despite this being supported by extensive research literature [ 29 ]. This may reflect a view among our experts that the diversity in causes of YOD is more important for health professionals to know, especially as Alzheimer’s disease does not predominate in this population to the same extent as in late life.
The panel also highlighted the importance of a healthy lifestyle for reducing the risk of the most common forms of young onset dementia [ 21 ], similar to a previous Delphi study that concluded that this knowledge is essential for dementia more broadly. Several non-genetic risk factors have been identified for YOD, including low participation in cognitive leisure activities, low educational attainment, stroke, transient ischemic attack and very heavy alcohol use [ 30 , 31 ]. Other potentially modifiable risk factors for dementia include physical inactivity, smoking, hypertension, obesity, diabetes, depression and low social contact [ 32 , 33 ]. Awareness of these risk factors can enable health professionals to encourage lifestyle modifications that may positively ameliorate the development and clinical course of YOD.
Delayed diagnosis and misdiagnosis of YOD are very common, with delays often related to the presence of depression or mild cognitive impairment [ 9 ]. Timely and accurate diagnosis is difficult as rarer forms of dementia and non-amnestic presentations are more common at younger ages than among older people [ 34 ]. As such, it is recommended that the diagnostic process for YOD include a formal cognitive assessment, full medical history including family history, risk assessment, physical examination including neurological examination, assessment of psychiatric, psychological and behavioural symptoms, functional assessment, neuroimaging and, where appropriate, amyloid imaging and genetic biomarkers [ 11 , 26 ]. This was acknowledged by our expert panel, who agreed that it was important for health professionals to know that a comprehensive, specialist, multi-disciplinary assessment is required, and identified the benefit of neuropsychological testing and the need to rule out reversible causes of impairment prior to diagnosing YOD. Knowledge about the need to conduct a comprehensive assessment will not only aid in making a timely, accurate diagnosis, but allow for the identification of co-existing symptoms and the initiation of symptom management and support services [ 35 ]. Additionally, it will provide an opportunity for accurate prognosis and future planning.
Treatment and care
The expert panel echoed published recommendations [ 3 , 13 ] that people with YOD require tailored, specialised, multidisciplinary services to support them following diagnosis. Research recommends the provision of support that addresses the physical, mental and social needs of people with YOD [ 32 ]. Programs that reduce social isolation and provide meaningful activities and continued engagement in skills or activities performed prior to diagnosis have been well received by people experiencing YOD and their supporters [ 3 , 4 , 12 , 36 ]. Psychosocial interventions are also recommended to manage neuropsychiatric symptoms, rather than using psychotropic medications which may be ineffective and associated with adverse effects [ 32 ]. These concepts were all agreed as essential knowledge by our panel of experts.
Most importantly, the panel agreed that health professionals should be aware that young people with dementia require age-appropriate care programs and accommodation options that take a whole-family approach. People with YOD and their support persons have expressed dissatisfaction with services that are designed for older adults. Difficulty relating to older participants, lack of security for physically agile people with YOD, and services being offered during business hours without childcare have been identified as reasons for which services targeted at people with LOD are not appropriate for people with YOD [ 3 ]. Previous research has recommended that care instead be tailored to the individual and also their family and/or support persons [ 4 , 26 ]. Support persons of people with YOD report very high rates of stress and burden [ 6 , 37 ] and children can experience a loss of care from both parents as a result.
Finally, our panel emphasised the significant financial impact of YOD. People with YOD may have to leave the workforce earlier than planned and before they are eligible to receive superannuation or pensions. This may result in their care partner being required to increase their working hours, or they may need to cease work to support them [ 5 , 6 , 26 ]. Research has shown that the impact of this can result in reduced access to services from diagnosis through to placement in residential care [ 3 ]. Holistic care for people with YOD therefore requires that health professionals are aware of these additional impacts on family members and friends.
Strengths and limitations
This international Delphi consensus study provides expert guidance about the knowledge that professionals working with people with YOD, and their families need to have to provide best-practice care. There are nonetheless important limitations to this work. Although many countries were represented, a large proportion of participants lived in Australia. This may introduce a geographical bias, and a more equal distribution of countries may provide a greater range of responses and experiences upon which to draw. Another limitation was the predominance of researchers and clinicians in the sample, particularly by round 3. Greater representation from health professionals providing YOD care in the disability and aged care sectors and individuals with a lived experience of YOD may provide a more thorough exploration of the topic. An established limitation of the Delphi study methodology is that it can be time consuming for participants to undertake. This may have contributed to the reduction in response rate over the course of this study. However, this is an issue common to other published Delphi studies [ 20 , 38 , 39 , 40 ] and the number of participants who completed round 3 within the recommended sample size for this method [ 19 , 20 ].
This international Delphi study has established the key pieces of information that experts consider essential for health professionals to understand about YOD to enable to them to deliver best-practice care. The statements indicate the breadth of knowledge that health professionals should be expected to know and can be used to guide the design and delivery of diagnostic, treatment, and support services for people with YOD and their support persons. Additionally, the statements can be used in the development of training and education materials to improve the awareness and understanding of YOD among health professionals providing care to this varied group of clients.
Availability of data and materials
The datasets used and/or analysed during the current study are available in a de-identified format from the corresponding author on reasonable request.
Prince M, Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina M, et al. World Alzheimer Report 2015: The global impact of dementia: An analysis of prevalence, incidence, cost and trends. 2015 [cited 2021 Mar 24]; Available from: https://www.alzint.org/resource/world-alzheimer-report-2015/
Mayrhofer A, Shora S, Tibbs M-A, Russell S, Littlechild B, Goodman C. Living with young onset dementia: reflections on recent developments, current discourse, and implications for policy and practice. Ageing Society. 2020:1–9.
Cations M, Withall A, Horsfall R, Denham N, White F, Trollor J, et al. Why aren’t people with young onset dementia and their supporters using formal services? Results from the INSPIRED study. PLoS One. 2017;12(7):e0180935.
Article Google Scholar
Sansoni J, Duncan C, Grootemaat P, Capell J, Samsa P, Westera A. Younger onset dementia: a review of the literature to inform service development. Am J Alzheimers Dis Other Demen. 2016;31(8):693–705.
Roach P, Drummond N, Keady J. ‘Nobody would say that it is Alzheimer’s or dementia at this age’: family adjustment following a diagnosis of early-onset dementia. J Aging Stud. 2016;36:26–32.
van Vliet D, de Vugt ME, Bakker C, Koopmans RTCM, Verhey FRJ. Impact of early onset dementia on caregivers: a review. Int J Geriatr Psychiatry. 2010;25(11):1091–100.
Arai A, Matsumoto T, Ikeda M, Arai Y. Do family caregivers perceive more difficulty when they look after patients with early onset dementia compared to those with late onset dementia? Int J Geriatr Psychiatry. 2007;22(12):1255–61.
Kaiser S, Panegyres PK. The psychosocial impact of young onset dementia on spouses. Am J Alzheimers Dis Other Demen. 2007;21(6):398–402.
Draper B, Cations M, White F, Trollor J, Loy C, Brodaty H, et al. Time to diagnosis in young-onset dementia and its determinants: the INSPIRED study. Int J Geriatr Psychiatry. 2016;31(11):1217–24.
van Vliet D, de Vugt ME, Bakker C, Pijnenburg Y. a. L, Vernooij-Dassen MJFJ, Koopmans RTCM, et al. time to diagnosis in young-onset dementia as compared with late-onset dementia. Psychol Med. 2013;43(2):423–32.
O’Malley M, Parkes J, Stamou V, LaFontaine J, Oyebode J, Carter J. International consensus on quality indicators for comprehensive assessment of dementia in young adults using a modified e-Delphi approach. Int J Geriatr Psychiatry. 2020;35(11):1309–21.
Millenaar JK, Bakker C, Koopmans RTCM, Verhey FRJ, Kurz A, de Vugt ME. The care needs and experiences with the use of services of people with young-onset dementia and their caregivers: a systematic review. Int J Geriatr Psychiatry. 2016;31(12):1261–76.
Stamou V, Fontaine JL, Gage H, Jones B, Williams P, O’Malley M, et al. Services for people with young onset dementia: the ‘Angela’ project national UK survey of service use and satisfaction. Int J Geriatr Psychiatry. 2021;36(3):411–22.
Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008–15.
CAS PubMed Google Scholar
Hsu C-C, Sandford B. The Delphi Technique: Making Sense of Consensus. Pract Assess Res Eval. 2007;12(12):Article 10.
Keeney S, Hasson F, McKenna H. Consulting the oracle: ten lessons from using the Delphi technique in nursing research. J Adv Nurs. 2006;53(2):205–12.
Dalkey NC. The Delphi method: an experimental study of group opinion. Rand Corporation; 1969. Available from: https://www.rand.org/pubs/research_memoranda/RM5888.html
Annear MJ, Toye C, Elliott K-EJ, McInerney F, Eccleston C, Robinson A. Dementia knowledge assessment scale (DKAS): confirmatory factor analysis and comparative subscale scores among an international cohort. BMC Geriatr. 2017;17(1):168.
Okoli C, Pawlowski SD. The Delphi method as a research tool: an example, design considerations and applications. Inf Manage. 2004;42(1):15–29.
de Villiers PMR de, de Villiers PJT de, Kent AP. The Delphi technique in health sciences education research. Med Teach 2005;27(7):639–643.
Annear MJ, Toye C, McInerney F, Eccleston C, Tranter B, Elliott K-E, et al. What should we know about dementia in the 21st century? A Delphi consensus study. BMC Geriatr. 2015;15(1):5.
Yousuf M. Using Experts` Opinions Through Delphi Technique. Pract Assess Res Eval. 2019;12(12):Article 4.
van der Steen JT, Radbruch L, Hertogh CMPM, de Boer ME, Hughes JC, Larkin P, et al. White paper defining optimal palliative care in older people with dementia: a Delphi study and recommendations from the European Association for Palliative Care. Palliat Med. 2014;28(3):197–209.
Withall A, Draper B, Seeher K, Brodaty H. The prevalence and causes of younger onset dementia in eastern Sydney. Aust Int Psychogeriatrics. 2014;26(12):1955–65.
Rossor MN, Fox NC, Mummery CJ, Schott JM, Warren JD. The diagnosis of young-onset dementia. Lancet Neurol. 2010;9(8):793–806.
Draper B, Withall A. Young onset dementia. Intern Med J. 2016;46(7):779–86.
Article CAS Google Scholar
Spreadbury JH, Kipps CM. Understanding important issues in young-onset dementia care: the perspective of healthcare professionals. Neurodegenerative Disease Manag. 2018;8(1):37–47.
Jarmolowicz AI, Chen H-Y, Panegyres PK. The patterns of inheritance in early-onset dementia: Alzheimer’s disease and frontotemporal dementia. Am J Alzheimers Dis Other Demen. 2015;30(3):299–306.
Vieira RT, Caixeta L, Machado S, Silva AC, Nardi AE, Arias-Carrión O, et al. Epidemiology of early-onset dementia: a review of the literature. Clin Pract Epidemiol Ment Health. 2013;9:88–95.
Cations M, Draper B, Low L-F, Radford K, Trollor J, Brodaty H, et al. Non-genetic risk factors for degenerative and vascular young onset dementia: results from the INSPIRED and KGOW studies. Rouch I, editor. JAD. 2018;62(4):1747–58.
Cations M, Withall A, Draper B. Modifiable risk factors for young onset dementia. Curr Opin Psychiatry. 2019;32(2):138–43.
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396(10248):413–46.
Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–94.
Koedam ELGE, Lauffer V, van der Vlies AE, van der Flier WM, Scheltens P, Pijnenburg YAL. Early-versus late-onset Alzheimer’s disease: more than age alone. J Alzheimers Dis. 2010;19(4):1401–8.
Masellis M, Sherborn K, Neto PR, Sadovnick DA, Hsiung G-YR, Black SE, et al. Early-onset dementias: diagnostic and etiological considerations. Alzheimers Res Ther. 2013;5(1):S7.
Richardson A, Pedley G, Pelone F, Akhtar F, Chang J, Muleya W, et al. Psychosocial interventions for people with young onset dementia and their carers: a systematic review. Int Psychogeriatr. 2016;28(9):1441–54.
Svanberg E, Spector A, Stott J. The impact of young onset dementia on the family: a literature review. Int Psychogeriatr. 2011;23(3):356–71.
Macfarlane L, Owens G, Cruz BDP. Identifying the features of an exercise addiction: a Delphi study. J Behav Addict. 2016;5(3):474–84.
Wong HS, Curry NS, Davenport RA, Yu L-M, Stanworth SJ. A Delphi study to establish consensus on a definition of major bleeding in adult trauma. Transfusion. 2020;60(12):3028–38.
Murphy F, Doody O, Lyons R, Gallen A, Nolan M, Killeen A, et al. The development of nursing quality care process metrics and indicators for use in older persons care settings: a Delphi-consensus study. J Adv Nurs. 2019;75(12):3471–84.
Download references
Acknowledgements
We would like to acknowledge the support of Ian Gladstone, Michael King, and Sue King in completing this research.
This research was funded by a Dementia Australia Research Foundation project grant. MC is supported by a Hospital Research Foundation Early Career Fellowship and a Medical Research Future Fund and National Health and Medical Research Council Investigator Grant. KEL is supported by an Australian Research Council Discovery Early Career Research Award. KEE is supported by a National Health and Medical Research Council and Australian Research Council Dementia Research Development Fellowship.
Author information
Authors and affiliations.
College of Education, Social Work and Psychology, Flinders University, Adelaide, South Australia, Australia
Leah Couzner, Sally Day & Monica Cations
School of Psychiatry, UNSW Sydney, Sydney, New South Wales, Australia
Brian Draper
School of Public Health and Community Medicine, UNSW Sydney, Sydney, New South Wales, Australia
Adrienne Withall
College of Medicine and Public Health, Flinders University, Adelaide, South Australia, Australia
Kate E. Laver & Monica Cations
University of Tasmania, Hobart, Tasmania, Australia
Claire Eccleston, Kate-Ellen Elliott & Fran McInerney
You can also search for this author in PubMed Google Scholar
Contributions
MC conceptualised and designed the study. SD led the data collection and analysis with input from LC. LC and SD drafted the manuscript which was refined by BD, KEL, CE, FM and MC. All authors approved the final manuscript.
Corresponding author
Correspondence to Monica Cations .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Flinders University Social and Behavioural Research Ethics Committee (reference number 8331). All methods were performed in accordance with the relevant guidelines and regulations. Potential participants were provided with an information sheet explaining the study and what their voluntary participation would involve. Participants were informed in the recruitment email and on the participant information sheet that their completion of the questionnaire would be accepted as their consent to participate.
Consent for publication
Not applicable.
Competing interests
MC has been employed in the past 5 years to assist with data collection for Alzheimer’s disease drug trials funded by Janssen and Merck. All other authors declare no conflicts of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Couzner, L., Day, S., Draper, B. et al. What do health professionals need to know about young onset dementia? An international Delphi consensus study. BMC Health Serv Res 22 , 14 (2022). https://doi.org/10.1186/s12913-021-07411-2
Download citation
Received : 21 June 2021
Accepted : 13 December 2021
Published : 02 January 2022
DOI : https://doi.org/10.1186/s12913-021-07411-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Young onset dementia
- Health professionals
- Delphi study
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
Developing dementia: The existential experience of the quality of life with young-onset dementia - A longitudinal case study
Affiliations.
- 1 Norwegian National Advisory, Vestfold Hospital Trust, Unit on Ageing and Health, Norway; Norwegian Social Research (NOVA), Oslo Metropolitan University, Norway.
- 2 Norwegian National Advisory Unit on Ageing and Health, Vestfold Hospital Trust, Norway; University of South-Eastern Norway, Norway.
- 3 Center for Alzheimer's Disease and Related Disorders, Institute of Psychiatry, Universidade Federal do Rio de Janeiro, Brazil.
- PMID: 30058371
- DOI: 10.1177/1471301218789990
Keywords: coping; early-onset dementias; existential needs; health care; health promotion; subjective experiences; young persons.
Publication types
- Case Reports
- Adaptation, Psychological*
- Age of Onset*
- Dementia / diagnosis*
- Longitudinal Studies
- Middle Aged
- Quality of Life / psychology*
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- Winter 2024 | VOL. 36, NO. 1 CURRENT ISSUE pp.A5-81
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
The Diagnostic Challenge of Young-Onset Dementia Syndromes and Primary Psychiatric Diseases: Results From a Retrospective 20-Year Cross-Sectional Study
- Paraskevi Tsoukra , M.D., M.Sc. ,
- Dennis Velakoulis , F.R.A.N.Z.C.P., M.B.B.S. ,
- Pierre Wibawa , M.B.B.S. ,
- Charles B. Malpas , Ph.D., F.C.C.N. ,
- Mark Walterfang , F.R.A.N.Z.C.P., M.B.B.S. ,
- Andrew Evans , F.R.A.N.Z.C.P., M.B.B.S. ,
- Sarah Farrand , F.R.A.N.Z.C.P., M.B.B.S. ,
- Wendy Kelso , C.C.N., M.A.P.S. ,
- Dhamidhu Eratne , F.R.A.N.Z.C.P., M.B.Ch.B. ,
- Samantha M. Loi , F.R.A.N.Z.C.P., M.B.B.S .
Search for more papers by this author
Distinguishing a dementia syndrome from a primary psychiatric disease in younger patients can be challenging and may lead to diagnostic change over time. The investigators aimed to examine diagnostic stability in a cohort of patients with younger-onset neurocognitive disorders.
A retrospective review of records was conducted for patients who were admitted to an inpatient neuropsychiatry service unit between 2000 and 2019, who were followed up for at least 12 months, and who received a diagnosis of young-onset dementia at any time point. Initial diagnosis included Alzheimer’s disease-type dementia (N=30), frontotemporal dementia (FTD) syndromes (N=44), vascular dementia (N=7), mild cognitive impairment (N=10), primary psychiatric diseases (N=6), and other conditions, such as Lewy body dementia (N=30).
Among 127 patients, 49 (39%) had a change in their initial diagnoses during the follow-up period. Behavioral variant FTD (bvFTD) was the least stable diagnosis, followed by dementia not otherwise specified and mild cognitive impairment. Compared with patients with a stable diagnosis, those who changed exhibited a higher cognitive score at baseline, a longer follow-up period, greater delay to final diagnosis, and no family history of dementia. Patients whose diagnosis changed from a neurodegenerative to a psychiatric diagnosis were more likely to have a long psychiatric history, while those whose diagnosis changed from a psychiatric to a neurodegenerative one had a recent manifestation of psychiatric symptoms.
Conclusions:
Misdiagnosis of younger patients with neurocognitive disorders is not uncommon, especially in cases of bvFTD. Late-onset psychiatric symptoms may be the harbinger to a neurodegenerative disease. Close follow-up and monitoring of these patients are necessary.
Young-onset dementia, a dementia syndrome with onset under the age of 65, accounts for approximately 8% of all people with dementia ( 1 ). The presenting symptoms of young-onset dementia may be atypical and differ from those seen in late-onset dementia ( 2 ). The differential diagnosis of young-onset dementia can be broad ( 3 ) and includes neurological, neurodegenerative, and primary psychiatric disorders. The clinical distinction can be most difficult in patients with this type of dementia and prominent frontal or white matter change, as seen in the behavioral variant of frontotemporal dementia (bvFTD) ( 4 , 5 ), the “frontal variant” of Alzheimer’s disease ( 6 ), white matter disorders, such as leukodystrophies ( 7 , 8 ), or vascular dementias ( 9 , 10 ). Such patients may commonly present with personality, mood, or behavioral changes, including apathy, social withdrawal, mood disturbance, stereotyped/compulsive behaviors, disinhibition, or social inappropriateness.
Distinguishing a primary psychiatric disorder from a frontotemporal syndrome can be challenging ( 11 , 12 ), especially in patients with a long-standing history of schizophrenia or bipolar disorder, given the potential overlap at the level of cognition and/or psychiatric symptomatology ( 13 ). In a proportion of patients with young-onset dementia, the difficulty in diagnosis may lead to diagnostic change over time from a psychiatric to a neurodegenerative diagnosis or vice versa. While some studies ( 13 , 14 ) have described patients with a psychiatric diagnosis subsequently diagnosed with an underlying neurodegenerative condition, few studies have specifically addressed the question of diagnostic stability or change in patients with younger-onset dementia.
In a recent study, Perry et al. ( 15 ) examined diagnostic stability across neurodegenerative diseases in patients seen on at least two occasions in a tertiary memory service, aiming to identify factors that could influence diagnostic change. The investigators reviewed clinical records of patients diagnosed with bvFTD, language variants FTD, Alzheimer’s disease, corticobasal syndrome, and progressive supranuclear palsy. Diagnostic change occurred frequently in patients with bvFTD (32%) and was more common in patients with possible bvFTD (70%) compared with probable bvFTD (15%). In 22% of bvFTD patients, the diagnosis changed to a nondementia diagnosis, such as multiple sclerosis, or a psychiatric diagnosis, such as bipolar disorder.
Krudop et al. ( 16 ) examined patients presenting to a specialized memory clinic who were initially diagnosed as having bvFTD. After a 2-year follow-up period, 49% of these patients underwent a change in diagnosis. Thirty-two percent were reclassified with a primary psychiatric diagnosis, most commonly mood disorders, while 17% received other neurologic diagnoses, most commonly neurodegenerative diagnoses.
Woolley et al. ( 17 ) examined rates and risk factors for prior psychiatric diagnoses of neurodegenerative diseases (including Alzheimer’s, bvFTD, and primary progressive aphasia, among others) and reported that 28% of participants were initially given a psychiatric diagnosis. Of the dementia diagnoses, bvFTD was significantly more often initially misdiagnosed as a primary psychiatric condition, with depression being the most common diagnosis.
While the available studies have shown that diagnostic change is bidirectional (i.e., diagnoses may change from neurodegenerative to psychiatric or vice versa), individual studies have been largely unidirectional in that they have either focused on diagnostic change in patients initially diagnosed with dementia ( 15 , 16 ) or have looked retrospectively at rates of prior psychiatric diagnoses in patients with neurodegenerative diseases ( 17 ). In the present study, we aimed to address this limitation by examining diagnostic stability in both directions by investigating diagnostic change in a cohort of patients with younger-onset neurocognitive disorders referred to a neuropsychiatry service.
Study Design and Participants
An inpatient diagnostic admission to neuropsychiatry between 2000 and 2019
At least one inpatient or outpatient follow-up (≥12 months) assessment with neuropsychiatry
A diagnosis of younger-onset dementia or mild cognitive impairment at any time point between 2000 and 2019
The study was conducted in accordance with the Declaration of Helsinki and approved by the Melbourne Health Ethics Committee.
Demographic and Clinical Variables
Patients’ files were reviewed for the following information: age (at symptom onset and hospital admission), sex, education, alcohol and tobacco consumption, medical comorbidities, presenting symptoms, psychiatric history, and family history.
Diagnostic Information
Both inpatient and outpatient assessments included evaluation by a multidisciplinary team, consisting of a neuropsychiatrist, a neurologist, a neuropsychologist, an occupational therapist, and a social worker. Rating instruments included the Neuropsychiatry Unit Cognitive Assessment Tool (NUCOG) ( 18 ) and the Mini-Mental State Examination ( 19 ) to assess bedside cognition and the Global Assessment of Functioning scale to assess level of function. Investigations included blood and urine tests, structural and functional imaging of the brain (MRI, single-photon emission computed tomography, positron emission tomography [PET]), and cerebrospinal fluid (CSF) analysis. Genetic testing was performed where clinically indicated in the presence of a strong family history. Following all multidisciplinary and multimodal assessments, a consensus diagnosis was made for all patients and recorded in the clinical file. Each patient’s inpatient and outpatient medical records were reviewed to collate diagnoses made through the patient’s time with neuropsychiatry.
All diagnoses (initial and final) were made based on established contemporaneous diagnostic criteria (DSM-IV and DSM-5) ( 20 – 23 ).
Diagnoses were grouped into the following six categories: Alzheimer’s-type dementia, FTD (including bvFTD, semantic and nonfluent variant primary progressive aphasia, and FTD with motor neuron disease [FTD-MND]), vascular dementia (VaD), mild cognitive impairment, primary psychiatric diseases (including major depressive disorder, bipolar affective disorder, and schizophrenia), and other diseases not categorized in any of the previous groups, such as Lewy body dementias (including Parkinson’s disease dementia and dementia with Lewy bodies), progressive supranuclear palsy, corticobasal ganglionic degeneration, and dementia not otherwise specified.

Diagnostic Outcomes
No diagnostic change
Change from a neurodegenerative diagnosis to another neurodegenerative or neurological condition (e.g., from dementia not otherwise specified to Alzheimer’s disease)
Change from a neurodegenerative diagnosis to a primary psychiatric diagnosis (e.g., from bvFTD to schizophrenia)
Change from a psychiatric diagnosis to a neurodegenerative diagnosis (e.g., from major depressive disorder to bvFTD)
We considered patients whose diagnosis was switched from a neurodegenerative to a psychiatric disorder (or vice versa) as having a “between-category” diagnostic change, while change within the neurodegenerative category was considered a “within-category” change.
Statistical Analysis
Continuous variables are reported as means with standard deviations. Categorical variables are reported as frequencies (%). As a result of our small sample size and after testing for normality, Mann-Whitney U tests were performed to test differences in numerical variables between diagnostically stable and unstable groups. Pearson’s chi-square tests of independence were performed to compare dichotomous variables and determine differences in psychiatric and family history across the categories of change. A two-sided p value <0.05 was considered statistically significant. Statistical analysis was performed with SPSS, version 25 (IBM, Armonk, N.Y.).
Baseline Demographic and Clinical Characteristics
A total of 462 patients were identified as having a dementia syndrome or mild cognitive impairment. Of these, 195 individuals were excluded, either because they were not followed up (N=156) or were followed up for less than 12 months (N=39). We excluded patients whose age at symptom onset was >65 years (N=77). Patients diagnosed with genetically confirmed dementia disorders, such as Huntington’s disease and Niemann-Pick disease type C, at their first inpatient admission were not included in our analysis. A total of 127 patients met inclusion criteria and were included in the analysis ( Figure 1 ).
FIGURE 1. Eligibility criteria for study inclusion among patients admitted to an inpatient neuropsychiatry service unit between 2000 and 2019 and received a diagnosis of young-onset dementia a
a HD=Huntington’s disease; NPC=Niemann-Pick disease type C.
Of the 127 patients who met inclusion criteria, 82 were male (64.6%), the mean age at onset was 50.1 years (SD=8.6), the mean duration of symptoms until initial diagnosis was 3.1 years (SD=2.2), and the mean time of follow-up was 40 months (SD=28.1). A detailed summary of participants’ baseline characteristics is presented in Table 1 .
TABLE 1. Baseline characteristics at first presentation among young-onset dementia patients who met study inclusion criteria
Patients who were not followed up by neuropsychiatry (N=156) included patients who returned to private or public specialist care or were lost to follow-up. Compared with the 127 patients who met inclusion criteria, these patients were older at admission (mean age: 57.6 years versus 51.0 years, p<0.05) and at symptom onset (mean age: 54.4 years versus 47.8 years, p<0.05). The most common diagnoses among patients who were not followed up were Alzheimer’s disease (21.9%) and VaD (12.4%).
Diagnostic Change
The key finding among patients who met inclusion criteria was that there was a change of diagnosis during the study period for 39% of these patients (N=49). bvFTD was the least stable diagnosis, with almost half of patients (47.1%) undergoing a diagnostic change. Dementia not otherwise specified was the second least stable diagnosis, with 16% of patients shifting to another neurodegenerative/neurological or psychiatric condition. Changes in Alzheimer’s and VaD dementia were less common (10.2% and 4.1%, respectively). No patients diagnosed with a language variant FTD (semantic dementia [N=6]), nonfluent primary progressive aphasia (N=3), or FTD-MND (N=1) had a diagnostic change. Male participants had a change more frequently than females (69.4% and 30.6%, respectively). Changes in the 49 patients presenting with unstable diagnoses are summarized in Table 2 .
a AD=Alzheimer’s disease; FTD=frontotemporal dementia; VaD=vascular dementia.
b The patient’s diagnosis changed from behavioral variant FTD to semantic variant FTD.
TABLE 2. Changes between initial and final diagnoses during the follow-up period among patients with young-onset dementia a
For the patients initially diagnosed with bvFTD, five later received “other” diagnoses, including hereditary spastic paraplegia, central nervous system (CNS) vasculitis, Parkinson’s disease dementia, and dementia not otherwise specified, while one patient within the FTD group was rediagnosed as having the semantic variant. Patients presenting with an unclear condition at first admission (categorized in the “other” group) had their diagnosis revised at subsequent visits to more specific diseases, such as multiple system atrophy, Parkinson’s disease dementia, paraneoplastic encephalitis, or mild cognitive impairment. Two patients diagnosed with progressive supranuclear palsy and corticobasal ganglionic degeneration at first admission were switched to bvFTD and dementia with Lewy bodies, respectively.
Comparison of Patients With No Change in Diagnosis With Those With a Diagnostic Change
Compared with patients with a stable diagnosis, patients with a diagnostic change exhibited a higher NUCOG score at baseline (mean=74.9, 95% CI=70.8, 79.1 versus mean=65.4, 95% CI=60.8, 69.9, p<0.05), a longer follow-up period (mean=47.5 months, 95% CI=38.5, 56.5 versus mean=35.3 months, 95% CI=29.7, 41, p<0.05), and a greater delay to final diagnosis (mean=5.7 years, 95% CI=4.7, 6.7 versus mean=3.1 years, 95% CI=2.6, 3.6, p<0.01) and were more likely to have a history of hypertension (30.6% versus 15.4%, χ 2 =4.1, p<0.05). Patients with a family history of dementia were more likely to have a stable diagnosis compared with those who did not report family history (48.1% versus 20.8%, χ 2 =9.3, p<0.01). No difference was detected between the two groups regarding age at onset, education, smoking habits, and alcohol consumption.
Categories of Diagnostic Change
Within-category diagnostic change..
Of the 49 patients whose initial diagnosis was revised, 30 (23.6%) underwent a within-category diagnostic change. For two patients who were initially diagnosed with bvFTD, the change to a diagnosis of Alzheimer’s disease was influenced by CSF and amyloid PET biomarkers. Another participant had the diagnosis revised from bvFTD to hereditary spastic paraplegia after undergoing genetic testing. Genetics led to a change from mild cognitive impairment to Huntington’s disease in one patient. Patients given a diagnosis of dementia not otherwise specified at the time of first admission had a more precise diagnosis of bvFTD, posterior cortical atrophy, and chronic inflammatory encephalopathy when they were subsequently seen at follow-up. Two patients with Alzheimer’s disease developed common vascular copathology, while three cases of mild cognitive impairment were revised to Parkinson’s disease dementia (N=2) and progressive supranuclear palsy (N=1).
For the 49 patients who had a diagnostic change during follow-up, those who reported a negative psychiatric history were more likely to have a within-category change than those who reported a positive psychiatric history (χ 2 =4.8, p<0.05; odds ratio=4.6, 95% CI=1.1, 19.4). No differences were detected in terms of medical comorbidities (hypertension, diabetes mellitus, and dyslipidemia) and alcohol and tobacco consumption.
Between-category diagnostic change.
Thirteen patients (10.2% of the total group) switched from a neurodegenerative to a psychiatric diagnosis (subtype I).
Six patients (4.7% of the total group) switched from a psychiatric to a neurodegenerative diagnosis (subtype II).
Further clinical details of these 19 patients are presented in Table 3 . Of the 13 patients in the first subtype (patients 1–13; Table 3 ), six had schizophrenia, three had bipolar disorder, one had depression, and three had no evidence of a progressive neurodegenerative disorder. Of the six patients with the second subtype (patients 14–19; Table 3 ), half developed an FTD diagnosis.
a AD=Alzheimer’s disease; BPAD=bipolar affective disorder; bvFTD=behavioral variant frontotemporal dementia; Nil=negative history of psychiatric symptoms; NOS=not otherwise specified; NR=not reported; OCD=obsessive-compulsive disorder; STM=short-term memory; VaD=vascular dementia.
TABLE 3. Clinical details of patients with a between-category diagnostic change a
There was no difference between these two subtypes in the age at which participants first exhibited symptoms (subtype I: mean age=47.6 years versus subtype II: mean age=51.6 years, p=0.32). Of the participants in this between-category diagnostic change group, 84.2% (N=16) reported a positive history of psychiatric symptoms (depressive, psychotic). The presence of cardiovascular risk factors (hypertension, diabetes mellitus, dyslipidemia, and smoking) was not associated with a diagnostic change in this group.
Patients in subtype I (i.e., those who changed from a neurodegenerative to a psychiatric diagnosis) were more likely to have a long psychiatric history (>10 years, N=8/13; 61.5%) compared with patients in subtype II (i.e., those who switched from a psychiatric to a neurodegenerative diagnosis), the majority of which had a recent manifestation of psychiatric symptoms (<10 years, N=5/6; 83.3%) (χ 2 =7.2, p<0.05).
This study examined bidirectional diagnostic stability across neurodegenerative and psychiatric diseases in a cohort of patients diagnosed with younger-onset dementia at presentation or subsequent follow-up in a single tertiary specialist clinical service. Several important findings emerged from this investigation. Of 127 patients, 49 (39%) had their initial diagnoses revised during the follow-up period. The observed diagnostic changes included change from neurodegenerative disease to another neurodegenerative or neurological condition (24%), from a neurodegenerative diagnosis to a psychiatric diagnosis (10%), and from a psychiatric diagnosis to a neurodegenerative diagnosis (5%). Alzheimer’s disease-type dementia was the most stable diagnosis over time. Consistent with previous literature, a diagnosis of bvFTD was found to be the most likely diagnosis to change during the follow-up period. For patients with a diagnosis of bvFTD, their diagnosis changed to another neurodegenerative or neurological diagnosis (24%) or to a psychiatric diagnosis (21%). On the other hand, 60% of patients finally diagnosed with bvFTD were given an initial diagnosis of depression, a finding consistent with that of Woolley et al. ( 17 ). Finally, we identified that a higher score on cognitive testing at baseline, a negative family history of dementia, and a positive psychiatric history were associated with a greater likelihood of diagnostic change.
From a clinical and patient perspective, the most significant diagnostic changes were the between-category changes (i.e., when the diagnosis changed from neurodegenerative to psychiatric or vice versa). In 19 patients who presented with a between-category change, the presence and duration of psychiatric symptoms played a significant role in the reconsideration of the patients’ initial diagnosis. Eight of the nineteen patients (42%) had a psychiatric history >10 years at the time of an initial diagnosis of a dementia. In all eight patients, the initial dementia diagnosis was subsequently revised to a psychiatric diagnosis based on lack of progression and, in some cases, improvement of cognitive impairment. Eight of the 19 patients (42%) had a history of psychiatric symptoms <10 years. More than half of these eight patients, who were initially diagnosed with a psychiatric condition (63%), were subsequently diagnosed with a neurodegenerative disease. It was believed that in these individuals, the late-onset psychiatric symptoms reflected a prodromal phase of an underlying neurodegenerative condition.
For the patients with a within-category change (N=30), the bvFTD diagnosis was the most frequent to change to another neurodegenerative or neurological condition (30%, N=9). These final diagnoses had a wide range, including spastic paraplegia, CNS vasculitis, Parkinson’s disease dementia/VaD, Alzheimer’s disease, and semantic dementia, among others. Patients initially diagnosed with dementia not otherwise specified and mild cognitive impairment were given a more specific diagnosis over time, meaning that a longer follow-up period was required in these patients. Genetic testing also influenced diagnostic change and should be taken into consideration in the presence of a strong family history and for further counseling.
The relationship between psychiatric diagnoses and younger-onset neurodegenerative dementia diagnoses has been investigated in several studies. Primary psychiatric diseases, such as major depressive disorder, schizophrenia, and bipolar disorder, are risk factors for the development of dementia later in life ( 24 – 28 ), while other studies have suggested that late-onset depression may represent a prodromal phase of dementia ( 29 – 31 ). Patients with a late-life onset of psychiatric manifestations or patients with a long psychiatric history presenting with cognitive decline should be carefully evaluated and followed up over time, as their symptomatology may signify an early phase of a neurodegenerative disease. In our cohort, patients who changed diagnosis were followed up significantly longer and had a greater delay to final diagnosis than those who remained stable.
Our findings should be considered “red flags,” as clinicians need to be careful and vigilant when evaluating and diagnosing patients with younger-onset dementia syndromes, especially in bvFTD and in treatment-resistant psychiatric symptoms. A validated checklist developed by Ducharme et al. ( 32 ) is a promising clinical tool aimed to improve diagnostic accuracy in these patients. Although clinical diagnostic criteria are available for the commonest disorders (Alzheimer’s disease, FTD, vascular dementia, and Lewy body dementia), they have only moderate sensitivity and specificity. Neurodegenerative diseases progress over time, and disease-modifying therapies are yet to be found. Such a diagnosis has a major impact on an adult’s life, especially in younger patients who are still likely to be active members of society with professional, financial, and caring responsibilities. On the other hand, psychiatric diseases can often be effectively treated, leading to improvement in symptoms and quality of life.
Limitations
The small number of patients included in our analysis and the small sample size of patients with a between-category diagnostic change limit the generalizability of our findings to the wider population of patients with younger-onset dementia or primary psychiatric diseases. A further limitation, related to the clinical basis of this study, is that the follow-up duration and intervals were not the same for all patients. We found that patients with a diagnostic change were followed up over a significantly longer period of time than those without a diagnostic change. The longer follow-up period may have increased the possibility of reconsideration and revision of the diagnosis.
Finally, our tertiary neuropsychiatric service generally sees patients with a complex constellation of cognitive, psychiatric, and neurological symptoms referred by other specialist physicians. We cannot comment on diagnostic change in those patients not referred to our service, but it is likely that patients referred to our service represent a more complex group diagnostically and are more likely to exhibit diagnostic uncertainty and change over time.
CONCLUSIONS
There can be significant overlap of psychiatric, neurological, cognitive, and even neuroimaging changes in younger-onset dementia syndromes and primary psychiatric diseases, causing diagnostic uncertainty and delay. Misdiagnosis is frequent, especially in cases of bvFTD. Because most primary psychiatric diseases occur in adolescence or early adulthood, the manifestation of late-onset (after 40 years old) psychiatric symptoms may be the harbinger to a neurodegenerative disease. On the other hand, a chronic mental illness may predispose to a dementing syndrome. Establishing an accurate diagnosis as early as possible is of paramount importance, because initiation of the appropriate therapeutic intervention may have its greatest potential in the early stages of a disease. Close monitoring and follow-up of these patients are required.
The authors report no financial relationships with commercial interests.
Dr. Tsoukra is the recipient of a Greek Australian Fellowship.
The authors thank the patients, families, and staff at the neuropsychiatry unit at Royal Melbourne Hospital.
1 Draper B, Withall A : Young onset dementia . Intern Med J 2016 ; 46:779–786 Crossref , Medline , Google Scholar
2 Rossor MN, Fox NC, Mummery CJ, et al. : The diagnosis of young-onset dementia . Lancet Neurol 2010 ; 9:793–806 Crossref , Medline , Google Scholar
3 Kuruppu DK, Matthews BR : Young-onset dementia . Semin Neurol 2013 ; 33:365–385 Crossref , Medline , Google Scholar
4 Piguet O, Hornberger M, Mioshi E, et al. : Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management . Lancet Neurol 2011 ; 10:162–172 Crossref , Medline , Google Scholar
5 Lanata SC, Miller BL : The behavioural variant frontotemporal dementia (bvFTD) syndrome in psychiatry . J Neurol Neurosurg Psychiatry 2016 ; 87:501–511 Crossref , Medline , Google Scholar
6 Ossenkoppele R, Pijnenburg YAL, Perry DC, et al. : The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features . Brain 2015 ; 138:2732–2749 Crossref , Medline , Google Scholar
7 Rosebush PI, Garside S, Levinson AJ, et al. : The neuropsychiatry of adult-onset adrenoleukodystrophy . J Neuropsychiatry Clin Neurosci 1999 ; 11:315–327 Link , Google Scholar
8 Kitchin W, Cohen-Cole SA, Mickel SF : Adrenoleukodystrophy: frequency of presentation as a psychiatric disorder . Biol Psychiatry 1987 ; 22:1375–1387 Crossref , Medline , Google Scholar
9 Aharon-Peretz J, Kliot D, Tomer R : Behavioral differences between white matter lacunar dementia and Alzheimer’s disease: a comparison on the neuropsychiatric inventory . Dement Geriatr Cogn Disord 2000 ; 11:294–298 Crossref , Medline , Google Scholar
10 Moretti R, Torre P, Antonello RM, et al. : Frontal lobe dementia and subcortical vascular dementia: a neuropsychological comparison . Psychol Rep 2005 ; 96:141–151 Crossref , Medline , Google Scholar
11 Vismara M, Cirnigliaro G, Piccoli E, et al. : Crossing borders between frontotemporal dementia and psychiatric disorders: an updated overview . J Alzheimers Dis 2020 ; 75:661–673 Crossref , Medline , Google Scholar
12 Ducharme S, Dols A, Laforce R, et al. : Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders . Brain 2020 ; 143:1632–1650 Crossref , Medline , Google Scholar
13 Velakoulis D, Walterfang M, Mocellin R, et al. : Frontotemporal dementia presenting as schizophrenia-like psychosis in young people: clinicopathological series and review of cases . Br J Psychiatry 2009 ; 194:298–305 Crossref , Medline , Google Scholar
14 Snowden JS, Rollinson S, Thompson JC, et al. : Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations . Brain 2012 ; 135:693–708 Crossref , Medline , Google Scholar
15 Perry DC, Datta S, Miller ZA, et al. : Factors that predict diagnostic stability in neurodegenerative dementia . J Neurol 2019 ; 266:1998–2009 Crossref , Medline , Google Scholar
16 Krudop WA, Dols A, Kerssens CJ, et al. : The pitfall of behavioral variant frontotemporal dementia mimics despite multidisciplinary application of the FTDC criteria . J Alzheimers Dis 2017 ; 60:959–975 Crossref , Medline , Google Scholar
17 Woolley JD, Khan BK, Murthy NK, et al. : The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease . J Clin Psychiatry 2011 ; 72:126–133 Crossref , Medline , Google Scholar
18 Walterfang M, Siu R, Velakoulis D : The NUCOG: validity and reliability of a brief cognitive screening tool in neuropsychiatric patients . Aust N Z J Psychiatry 2006 ; 40:995–1002 Crossref , Medline , Google Scholar
19 Folstein MF, Folstein SE, McHugh PR : “Mini-Mental State”: practical method for grading the cognitive state of patients for the clinician . J Psychiatr Res 1975 ; 12:189–198 Crossref , Medline , Google Scholar
20 McKhann GM, Knopman DS, Chertkow H, et al. : The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease . Alzheimers Dement 2011 ; 7:263–269 Crossref , Medline , Google Scholar
21 McKhann G, Drachman D, Folstein M, et al. : Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease . Neurology 1984 ; 34:939–944 Crossref , Medline , Google Scholar
22 Rascovsky K, Hodges JR, Knopman D, et al. : Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia . Brain 2011 ; 134:2456–2477 Crossref , Medline , Google Scholar
23 McKhann GM, Albert MS, Grossman M, et al. : Work Group on Frontotemporal Dementia and Pick’s Disease : Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease . Arch Neurol 2001 ; 58:1803–1809 Crossref , Medline , Google Scholar
24 da Silva J, Gonçalves-Pereira M, Xavier M, et al. : Affective disorders and risk of developing dementia: systematic review . Br J Psychiatry 2013 ; 202:177–186 Crossref , Medline , Google Scholar
25 Dotson VM, Beydoun MA, Zonderman AB : Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment . Neurology 2010 ; 75:27–34 Crossref , Medline , Google Scholar
26 Geerlings MI, den Heijer T, Koudstaal PJ, et al. : History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease . Neurology 2008 ; 70:1258–1264 Crossref , Medline , Google Scholar
27 Ownby RL, Crocco E, Acevedo A, et al. : Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis . Arch Gen Psychiatry 2006 ; 63:530–538 Crossref , Medline , Google Scholar
28 Green RC, Cupples LA, Kurz A, et al. : Depression as a risk factor for Alzheimer disease: the MIRAGE Study . Arch Neurol 2003 ; 60:753–759 Crossref , Medline , Google Scholar
29 Mirza SS, Wolters FJ, Swanson SA, et al. : 10-year trajectories of depressive symptoms and risk of dementia: a population-based study . Lancet Psychiatry 2016 ; 3:628–635 Crossref , Medline , Google Scholar
30 Li G, Wang LY, Shofer JB, et al. : Temporal relationship between depression and dementia: findings from a large community-based 15-year follow-up study . Arch Gen Psychiatry 2011 ; 68:970–977 Crossref , Medline , Google Scholar
31 Brommelhoff JA, Gatz M, Johansson B, et al. : Depression as a risk factor or prodromal feature for dementia? findings in a population-based sample of Swedish twins . Psychol Aging 2009 ; 24:373–384 Crossref , Medline , Google Scholar
32 Ducharme S, Pearl-Dowler L, Gossink F, et al. : The Frontotemporal Dementia versus Primary Psychiatric Disorder (FTD versus PPD) checklist: a bedside clinical tool to identify behavioral variant FTD in patients with late-onset behavioral changes . J Alzheimers Dis 2019 ; 67:113–124 Crossref , Medline , Google Scholar
- Motor Neuron Disease & Frontotemporal Dementia Presenting with a Conversion Disorder Psychiatry Research Case Reports, Vol. 2, No. 1
- Young‐onset dementia diagnosis, management and care: a narrative review 19 February 2023 | Medical Journal of Australia, Vol. 218, No. 4
- Diagnostic instability over time in the late-onset frontal lobe syndrome: When can we say it's FTD? The American Journal of Geriatric Psychiatry, Vol. 114
- Carer burden and psychological distress in young‐onset dementia: An Australian perspective 16 June 2022 | International Journal of Geriatric Psychiatry, Vol. 37, No. 7
- Music and Psychology & Social Connections Program: Protocol for a Novel Intervention for Dyads Affected by Younger-Onset Dementia 15 April 2022 | Brain Sciences, Vol. 12, No. 4
- Recent research advances in young-onset dementia 29 December 2022 | Current Opinion in Psychiatry, Vol. Publish Ahead of Print
- Bias Investigation in Artificial Intelligence Systems for Early Detection of Parkinson’s Disease: A Narrative Review 11 January 2022 | Diagnostics, Vol. 12, No. 1

- Young-Onset Dementia
- Neurodegenerative Disorder
- Late-Onset Psychiatric Disorders
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Study Protocol
Social care planning and provision for people with young onset dementia and their families: Protocol for the DYNAMIC study
Contributed equally to this work with: Catherine Quinn, Jan Oyebode
Roles Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations Centre for Applied Dementia Studies, University of Bradford, Bradford, United Kingdom, Wolfson Centre for Applied Health Research, Bradford, United Kingdom
Roles Conceptualization, Methodology, Writing – review & editing
Affiliation Centre for Applied Dementia Studies, University of Bradford, Bradford, United Kingdom
Roles Conceptualization, Funding acquisition, Methodology, Writing – review & editing
Affiliation Social Policy Research Unit, University of York, York, United Kingdom
Roles Conceptualization, Funding acquisition, Writing – review & editing
- Catherine Quinn,
- Helen Young,
- Kate Gridley,
- Vasileios Stamou,
- Clare Mason,
- Jan Oyebode

- Published: February 5, 2024
- https://doi.org/10.1371/journal.pone.0297747
- Peer Review
- Reader Comments
Social care is vital to quality of life for people with young onset dementia and their families. Yet care is hugely variable, frequently lacking and poorly coordinated. We aim to establish current practice in English social care for people with young onset dementia and co-produce evidence-based recommendations and resources for improvement.
Methods and analysis
In Work-Package 1, we will gather qualitative data from 25 people with young onset dementia and/or main supporters residing in England. We will ask them about their experiences of social care (broadly defined, including independent and voluntary sector provision) and suggestions for improvement. In Work-Package 2, we will conduct a short on-line survey with a wide range of staff with a role in adult social care in England. We will find out about current awareness, knowledge and practice and suggestions for improvements. Quantitative and qualitative analysis will provide a picture of current practice. In Work-Package 3, we will use convergence analysis to synthesise the findings from Work-Packages 1 and 2 and present the findings to a stakeholder workshop, to identify feasible priorities for improvement. We will establish what is already known about good practice relating to these key priorities using a scoping review and interviews with professionals. This knowledge will then feed into the co-production of resources and recommendations with key stakeholders to improve social care for people with young onset dementia and their families.
This study seeks to address a gap in our understanding of social care provision for people with young onset dementia and develop recommendations and practical resources for improvements. The findings will help people with young onset dementia and supporters to receive higher quality social care.
Trial registration
Study registration number: ISRCTN10653250 .
Citation: Quinn C, Young H, Gridley K, Stamou V, Mason C, Oyebode J (2024) Social care planning and provision for people with young onset dementia and their families: Protocol for the DYNAMIC study. PLoS ONE 19(2): e0297747. https://doi.org/10.1371/journal.pone.0297747
Editor: Vanessa Carels, PLoS ONE, UNITED STATES
Received: December 12, 2023; Accepted: January 9, 2024; Published: February 5, 2024
Copyright: © 2024 Quinn et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: No datasets were generated or analysed during the current study. All relevant data from this study will be made available upon study completion.
Funding: This study is funded by the National Institute for Health and Care Research (NIHR) Research for Social Care (RfSC) Programme through grant NIHR204266. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Competing interests: The authors have declared that no competing interests exist.
Introduction
It is estimated that 55 million people are currently living with dementia worldwide, a number estimated to increase to 139 million by 2050 [ 1 ]. There are many different types of dementia. Although young onset dementia (dementia with an onset under 65 years of age) is relatively rare, its prevalence in the UK at 70,800 [ 2 ] is higher than other well-known conditions, e.g. over 8 times as many people have young onset dementia than have motor neuron disease [ 3 ]. The condition has great impact on those diagnosed and costs families the equivalent of £10,000 in family care per 3 months [ 4 ]. There is also a wider cost to society as both the person diagnosed and/or their main supporter may be unable to work [ 5 ]. There are also long-term consequences for the well-being, education and mental health of children/young people in the family [ 6 , 7 ]. Formal services currently fund little care or support for people with young onset dementia, unless the person is admitted to residential care [ 4 ].
Social care has an important role in supporting individuals. The purpose of social care planning is to enable people to remain independent, have control over their life, do things they enjoy, know what type of care may help and understand their condition and care needs [ 8 , 9 ]. Social care is vital to the quality of life of people with young onset dementia and their families [ 10 , 11 ] but their social care needs are seldom well addressed [ 4 , 6 , 12 ]. Research in the Angela Project found that almost a third of people living with young onset dementia had no regular appointments with any social or health care professional and fewer than 1 in 3 had a care plan [ 6 ]. Lack of social care has far-reaching consequences, exposing people with young onset dementia and their families to risks, including strained relationships, financial hardship, mental distress and ill-being. Unmet social care needs are significantly associated with distress and psychiatric symptoms [ 13 ]. They may precipitate crises for the person with young onset dementia and their families [ 7 ]. Services may then have to step in to address issues that could have been averted by earlier social care provision. There is a great need to improve social care for people with young onset dementia to address the inequalities experienced by this population.
There is little published research and no published reviews specifically addressing young onset dementia social care. A 2016 review on young onset dementia [ 12 ] found only 10 sources focused on service experiences, concluding that there was: ‘lack of a clear diagnostic pathway, poor provision of information, lack of appropriate referrals to support services, and a high volume of informal care provided’ (p.5). A 2018 systematic review [ 10 ] of service provision for people with young onset dementia located six UK-based studies on social care needs and service provision. The authors concluded these initiatives enabled people with young onset dementia to continue living at home for longer but also that: ‘The evidence on the experience of living with young onset dementia is not matched by research and the innovation needed to mitigate the impact of young onset dementia’ (p.933). A 2022 meta-synthesis [ 8 ] identified 11 publications on views of specific supportive services and found there was great overlap in preferences of those with young onset dementia and their carers. The authors identified the need for further studies that include both people with young onset dementia and their carers and focus specifically on services. Against this backdrop of limited research, the Angela project [ 14 ], identified eight key needs of people with young onset dementia and family members, highlighting a strong social care component [ 15 , 16 ]. The project confirmed that care was hugely variable, frequently lacking and poorly coordinated [ 4 ]. In particular, the person with young onset dementia was often discharged from secondary to primary health care, with little focus on social care [ 4 ]. Due to the predominantly healthcare focus of the Angela project, findings on social care were patchy and ‘fragmented’. Additionally, as the Angela project focused on positive experiences of diagnostic and post-diagnostic services, it did not identify unmet social care needs or shortcomings in services. Given the lack of research in this area, it is essential to establish gaps in social care practice and areas for improvement to inform the development of recommendations and resources to improve social care for people with young onset dementia.
Research [ 4 , 10 – 12 , 15 , 17 ] has established the high level and breadth of social care needs in young onset dementia, the lack of social care for young onset dementia and the deleterious consequences of living with unmet social care needs for the person [ 13 ], main supporter [ 4 , 17 ] and children/young people within the family [ 6 , 7 ]. When developing this study our consultation work with a range of stakeholders indicated that no-one seems to have a clear picture of current social care for young onset dementia. In this project, we aim to address this gap and co-produce evidence-based recommendations.
The DYNAMIC study aims to:
- Gain in-depth understanding of the social care needs, experiences and preferences of those living with young onset dementia and their supporters.
- Establish levels of awareness, knowledge and practice among professionals regarding social care needs, care planning, and provision for people with young onset dementia.
- Co-produce recommendations and resources to improve social care for people with young onset dementia and their supporters.
Materials and methods
In this study we aim to establish current practice in English social care for people with young onset dementia and co-produce evidence-based recommendations and resources to improve social care. This study involves two stages: The first stage (to establish current practice) incorporates Work-packages 1 and 2 and Work-package 3 (to co-produce evidence-based recommendations and resources) will comprise the second stage.
Work-package 1
In this Work-package we will employ qualitative methods to better understand experiences of social care and areas for improvement from the perspectives of people living with young onset dementia and their main supporters. Recruitment for this Work-package commenced on 3/11/2023.
We will recruit 25 ‘cases’, with each case constituting an individual with young onset dementia and/or a main supporter (typically a family member or friend), from England. People with young onset dementia who do not have a main supporter will still be eligible to participate in the study. The inclusion and exclusion criteria are provided in Box 1 .
INCLUSION CRITERIA
For people with dementia:
- Diagnosed with young onset dementia (defined as when the first symptoms of dementia occur before the age of 65 years)
- Living alone or living with others
For main supporters:
- Relatives, friends or neighbours self-identified as involved in supporting a person with young onset dementia.
- May be living within or outside the home environment of the person with dementia.
Staff will be:
- Staff with a role in or awareness of adult/older adult social care planning, provision, management or commissioning
EXCLUSION CRITERIA
People with dementia will be excluded if they are any of the following:
- Diagnosed with dementia caused by HIV, traumatic brain injury, Down’s syndrome, Huntington’s chorea or alcohol-related dementia.
- Lack capacity to consent.
- Unable to communicate.
- Residing outside of England.
Main supporters will be excluded if they are any of the following:
- Not caring for someone with young onset dementia
- Caring for the person in a professional capacity e.g. paid carers.
- Residing outside of England
Staff will be excluded if they:
- Do not have a role in or awareness of adult/older adult social care planning, provision, management or commissioning.
- Work outside of England.
We will raise awareness of the study and recruit potential participants through a variety of media, including face-to-face meetings, with the help of national and local charitable and community-based organisations. Purposive sampling will ensure variation in characteristics that may influence experiences of social care e.g. gender, ethnicity and type of dementia. We will ensure inclusion and representation through consultation with patient and public involvement (PPI) members of the project team about the recruitment strategies. People who express an interest in taking part will be screened to ensure they meet the inclusion criteria.
We will offer a range of ways for people to express interest in taking part including in person (when the researcher visits community and peer support groups), online (by clicking a link or using a QR code), or by directly phoning or emailing the researcher. The researcher will contact those who express an interest to answer any questions about the study and check the individual meets the inclusion criteria. If the person meets these criteria the researcher will send them the information sheet (through email or post) and follow this up with further phone/email contact. Those who are ineligible or do not wish to participate will have their details destroyed. If the person has capacity to give informed consent and decides to take part the researcher will arrange a date and time for the research interview. The interview will take place in person, online or over the phone depending on the preference of the individual. Those with young onset dementia will be given a choice of having someone they trust present to support them. If both a person with young onset dementia and a supporter take part, they will be given a choice to be interviewed jointly or individually. Participants will be contacted with a reminder about the interview through their preferred contact method 1–2 days before the date of the meeting. In-person interviews will be conducted at the home of the interviewee or a mutually agreed suitable location, e.g. University premises.
On the day of the interview the researcher will re-confirm the participant’s capacity to consent and obtain consent either orally or in writing before the interview commences. Interviews will take the form of an extended conversation [ 18 ] guided by a semi-structured topic guide, allowing participants to focus on issues of most importance to them and enabling the researcher to probe for greater depth. Potential topics include experiences of social care; social care needs (met and unmet); contextual factors and nuances of the person’s unique situation, and suggested improvements or changes to social care provision. The interviews will last approximately 30–45 minutes but could be conducted over two occasions at participants’ requests. Interviews will be digitally recorded and transcribed. Participants will be given a voucher as a token of appreciation for their time.
Data will be managed using the Framework approach [ 19 ] and analysed inductively using reflexive thematic analysis [ 20 ]. We will take an ‘experiential’ approach, focusing on the meanings and experiences articulated by participants, underpinned by hermeneutics of empathy [ 20 ]. From this approach, themes are ‘conceptually founded patterns’ [ 21 ] reflexively developed by the research team but grounded in the expressed experiences of participants. After initial familiarisation and summarising, development and refinement of themes will be undertaken using a series of case by theme matrices (frameworks) to enable us to visualise relationships between cases, topics and themes. Involvement of the project team (including PPI members) at key points will bring in multiple perspectives, reducing the influence of any one researcher and ensuring rigour and accountability throughout [ 22 ].
Work-package 2
In this work-package we will employ a quantitative methodology to establish awareness, knowledge and practice among staff regarding social care needs, care planning and provision for people with young onset dementia. This will involve a short on-line survey distributed across England.
Participants will be staff with a role in or awareness of adult social care planning, provision, management or commissioning. Staff could be employed by Local Authorities, the third sector, the healthcare sector, the private sector or integrated care boards. They do not have to have seen people with young onset dementia but need to have a remit that could include people with young onset dementia. The inclusion and exclusion criteria are listed in Box 1 . To ensure recruitment across the whole range of social care professionals, we aim to establish the size and nature of the key population/ subpopulations (e.g., social workers, home carers, social prescribers) and check the distribution of respondents two and four months into recruitment. This will allow us to target later recruitment drives to increase representation from under-represented groups. Socio-demographic questions will enable us to check diversity of recruitment and this will allow focused recruitment campaigns.
The survey will be publicised through social media, key contacts of the research team and existing networks such as the Association of Directors of Adult Social Services. The publicity leaflet will include a link to the online survey. Contact details of the researcher will be provided so that those interested in taking part can find out more information about the study. The information sheet will be accessible through a link in the online survey or posted to those participants who request it. The survey will be available on paper for participants who do not have access to the internet or prefer to complete a paper version.
The survey will be kept concise and primarily consist of closed-ended questions, with some open-ended questions to enable participants to elaborate on their responses. Survey items will be informed by previous research and consultation with PPI members of the project team. Questions will address frequency of contact with people with young onset dementia; practice in the provision of social care in young onset dementia; and perceived areas for improvement in social care. We will also collect participants’ socio-demographic information and geographical location (e.g. rurality and ethnicity). To ensure the appropriateness of the length, wording, and content we will pilot the survey and will also use cognitive interviewing, where the person ‘thinks aloud’, explaining their thoughts as they answer each question [ 23 ], with at least two individuals.
Data analysis
All data will be imported into SPSS for analysis. We will produce descriptive data; for example, about the frequency of contact with people with young onset dementia. Some open-ended data will be imported into NVivo (qualitative data management software) or Excel for analysis. Content analysis [ 24 ] will be used to analyse responses to open-ended questions, and identify common themes, or categories, in participants’ responses; for example, in relation to improvements in social care. Emergent themes will be discussed with the wider research team, including PPI members, to ensure rigour in data analysis and relevance of the findings [ 22 ].
Work-package 3
This Work-package will have several stages. The first stage comprises a synthesis of Work-package 1 and Work-package 2 findings to identify potential areas for improvement in social care provision. Themes from work-packages 1 and 2 will be compared to identify areas of convergence (similarities) and divergence (differences) [ 25 ]; e.g., if few Work-package 1 participants report receiving a social care assessment, this can be compared with Work-package 2 findings on staff reports of conducting social care assessments for people with young onset dementia. A consistent finding (e.g. few received and few delivered) could indicate a gap in provision, whereas an inconsistent finding (e.g. few received but many delivered) could indicate a mismatch of perceptions, implying a need for clearer communications. Suggestions for service change and improvement will be directly compared. These could include aspects such as improving social workers’ awareness of the impact of young onset dementia or development of more meaningful personalised care plans. The synthesised findings of Work packages 1 and 2 will be summarised for the next stage.
The second stage will involve identifying impactful feasible priorities for improvement in social care provision. Priorities will be established through an on-line consensus workshop. A purposive sample of up to 20 diverse stakeholders will be recruited through our existing contacts with organisations and individuals and the suggestions of Steering Group members. People who took part in Work-packages 1 and 2 will also be approached to take part. Stakeholders will include those with young onset dementia and main supporters, local authority and third sector staff. PPI members of the research team and the PPI lead will advise on ways to ensure meaningful involvement.
The online workshop will last approximately 90 minutes. It will begin with a presentation and discussion of the Stage One findings, followed by break-out groups focused on the broad areas of service improvement identified through Work-packages 1 and 2. To address existing issues of inequalities in access to services, one group will focus specifically on diversity. Groups will be tasked with considering the findings relevant to their service improvement area and with suggesting improvements (e.g., tools, guidelines, training interventions) that would be impactful and feasible to introduce. Each group will be facilitated by a member of the research team who will ensure the voices of people living with young onset dementia are included and represented in the process. The workshop will end with feedback from each group and the collation of a set of feasible priorities for improvement in social care [ 25 ]. The workshop notes will be typed up and each group’s comments, priorities and feasibility ratings will be checked against the collated list of feasible priorities from the day. This will result in three areas of focus to be taken forward in the remainder of the work package. A summary of the workshop will be sent to all attendees by email or other preferred means of communication. All will be invited to join a reference group to continue their involvement as the recommendations and resources are developed.
The third stage will comprise a scoping review and interviews with professionals to establish existing knowledge on good practice within the priority areas, which will inform the development of the recommendations and resources on social care provision. A scoping review will be conducted on the key identified priorities for improvement, to explore, map and summarise relevant evidence and inform initial practice recommendations. Studies of all methodologies will be included, including grey literature. Systematic searches will be conducted on relevant databases (e.g., PubMed, Scopus, EBSCO), supplemented by reference-checking and hand-searching of relevant journals. Titles and abstracts retrieved will be reviewed against pre-defined inclusion and exclusion criteria. Full manuscripts of retained articles will then be checked against the criteria. The review process will align with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Extension for Scoping reviews [ 26 , 27 ]. We will appraise the quality of studies using the Mixed Methods Appraisal Tool [ 28 ] and grey literature using the AACODS (Authority, Accuracy, Coverage, Objectivity, Date, Significance) checklist [ 29 ].
Findings from the included studies will be extracted according to their relevance to the identified improvement priorities. Data analysis will involve an initial description of the sample of studies and their quality. The findings will be thematically grouped and presented descriptively. A diagrammatic map of the detailed findings will be developed, encompassing the key areas of improvement and themes/sub-themes related to key aspects of good practice for each. This will serve as a template for the analysis of the subsequent interviews with professionals.
Interviews with professionals will complement the scoping review and gather good practice examples of social care, supplemented, where possible, by anonymised copies of relevant documents (e.g. policy documents, care plan templates, examples of leaflets). The participants will be 10–12 social care practitioners/policy-makers who will be interviewed to provide data of sufficient depth and breadth [ 30 ]. We will use purposive sampling to recruit a diverse sample across sectors, with the focus depending on the particular improvements identified. Interviewees will be identified from survey respondents from Work-package 2 who agreed to be contacted, known centres of good practice, recommendations from the Project Steering group, other networks and social media. All participants will be asked to give informed consent and will also be invited to express interest in continuing involvement in the project, through joining a reference group to develop the resources and recommendations for social care improvement.
A flexible semi-structured interview guide will be developed, with advice and feedback from the PPI members of the research team and the Steering Group. It will be informed by the scoping reviews and focus on the key priorities for improvement in social care. In-depth insights and understanding of relevant examples of good practice will be sought, including participants’ understanding of the key improvement areas and what good practice in these entails (e.g. organisational and contextual factors facilitating the process, delivery format, time, budget). We will also gather suggestions for further improvement and elaborate on key facilitators and barriers. Interviews will be digitally recorded and transcribed for analysis. Data will be analysed using template analysis [ 31 ]. The diagrammatic map resulting from the scoping reviews will provide the initial template. Relevant text excerpts will be labelled according to the template, clustering new codes into pre-existing themes and/or constructing new themes from participants’ accounts. Evolving themes and codes will be discussed within the research team to bring in multiple perspectives to ensure the rigour and relevance of the findings [ 22 ].
Stage four will involve the co-production of resources and recommendations for social care improvement. We will co-produce, with stakeholders, evidence- and practice-based recommendations and resources focused on the three priorities for improvement identified by stakeholders at the consensus workshop [ 32 ]. We will follow the principles of good practice in co-production [ 33 ] including relationship-building, flexibility in arrangements, agreeing clear ground rules, inclusion and reflection. We will purposively recruit 4–6 diverse stakeholders with a particular interest in each of the three improvement priorities to act as reference groups and inform resource development. In line with co-production principles [ 33 ], the process will be tailored to the wishes of the reference groups. A short on-line meeting/email discussion with each reference group will take place at the outset to discuss and agree this. The general process will be one of iteration, with feedback cycles involving direction from reference group members and production by the researchers [ 34 ]. The templates summarising good practice will form the basis for development of tangible resources/recommendations. Initially, the reference group will identify the form of the preferred resource/ recommendation (e.g. on-line guidance, downloadable templates, videos). Once the nature of the resource is agreed a draft will be created. The reference group will then feedback on the draft. If required, this cycle will be repeated until we have generated practice- and evidence-informed recommendations and resources. The academic research team will be joined by a member of staff from DementiaUK, a national charity with a specialist arm focused on young onset dementia, who will bring expertise in the co-production of online, paper, video and other resources for people with young onset dementia, families, and health and social care professionals.
Patient and public involvement
In developing the proposal, we consulted with people directly affected by young onset dementia and providers from social services, voluntary and health sectors. We also worked directly with two people with dementia and two main supporters. Within the project we have a dedicated PPI lead (CM), two PPI members on the Project Management Group and two PPI members on the Project Steering Group. PPI members will be involved in the development of recruitment and data collection materials, information sheets and discussions about the research findings. They will support knowledge exchange, dissemination and impact throughout the project. Additional PPI members will support the co-production work in Work-package 3. They will contribute to the development of project recommendations/ resources, through participation in: a) the stakeholder workshop which will set the priorities for improvement and b) the smaller reference groups which will co-produce the resources/recommendations and associated impact plans.
Data management
All study documentation and data will be stored on a secure University of Bradford shared drive, accessible only to the research team. All interviews will be audio recorded on a password protected device. Recordings will be uploaded to a shared drive which will only be accessible through password protected computers. Following the uploading, the original recording will be deleted from the recording device. The audio files will be transcribed verbatim into a Word document by the researchers or by a University of Bradford approved supplier. Recordings and anonymised transcripts will be stored using a unique identification number for each participant and will be stored separately to participants’ personal details. All quotations used in the study final report, publications and presentations will be pseudonymised to ensure that no identifying details are present (e.g. names of individuals).
All Work-package 2 survey data will be entered into a secure online database. Each participant will be assigned a unique identity code. The data will be anonymised with the exception of the names of services and their location.
Participants may decide to provide personal contact details to allow the research team to make contact about following stages of the study, to hear about the findings of the study or to be informed of future studies. This data will be stored in a separate password-protected electronic file in the secure servers of the University of Bradford which will only be accessible by the research team through password protected computers.
The DYNAMIC study seeks to address an important topic area around social care provision for people with young onset dementia. There are distinctive social care needs associated with young onset dementia; yet due to the rarity of the condition, these needs are not well known to social care practitioners and are rarely met, leading to poorer quality of life and crises in families affected by young onset dementia. In exploring current provision, we seek to develop recommendations and resources to improve social care and support. This may result in improved quality of social care services and may have a positive impact on the quality of life and well-being of people with young onset dementia and their supporters.
A strength of this study lies in the active involvement of people with young onset dementia and their supporters in major aspects of project development and implementation. Members of our PPI group have been involved in the design of the study to ensure it is inclusive of those affected by young onset dementia. They will also be involved in different aspects of study delivery to ensure the relevance of the study and the associated findings. The study will also incorporate multiple perspectives of people with young onset dementia, supporters, and professionals, to better understand current practice in social care provision and how it can be improved. Due to the small scale of the study, we will only include people with young onset dementia who have capacity to consent. However, main supporters of people with advanced dementia will be able to participate, to ensure wide representation. The study recommendations will also be co-produced in collaboration with people with young onset dementia, supporters, and professionals, to increase the relevance and potential applicability of the findings in real-life practice.
The young-onset-dementia-specific recommendations and resources will provide tools which may improve real-life social care provision. The resources/recommendations will raise awareness of younger people’s needs among social care professionals and policy makers and may inform the re-designing of services or social care improvements beyond the few existing pockets of good practice. Our wide dissemination strategy will increase the impact of the study and may also enhance initiatives of service recipients to advocate for higher quality social care in young onset dementia.
Acknowledgments
We would like to acknowledge Elaine Daniels and Julie Hayden who are on the Project Management Group of the study. We would like to thank everyone who is part of the PPI and Project Steering Group. We are grateful for the support from Young Dementia Network and Tessa Gutteridge, Young Dementia Lead, Dementia UK. For the purpose of open access a Creative Commons Attribution (CC BY) licence is applied to any Author Accepted Manuscript version arising from this submission.
- 1. Alzheimer’s Disease International. From Plan to Impact VI: Making every step count. London: Alzheimer’s Disease International; 2023.
- View Article
- Google Scholar
- PubMed/NCBI
- 9. NHS England. Available from: https://www.england.nhs.uk/personalisedcare/social-prescribing/ .
- 14. Young Dementia Network. Available from: https://www.youngdementianetwork.org/research-evidence/the-angela-project/ .
- 19. Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE publications 2003.
- 20. Braun V, Clarke V. Thematic analysis: A practical guide London: Sage Publications 2021.
- 21. Braun V, Clarke V, Hayfield N, Terry G. Thematic Analysis. In: Liamputtong P, editor. Handbook of Research Methods in Health Social Sciences. Singapore: Springer Singapore; 2019. p. 843–60.
- International edition
- Australia edition
- Europe edition
Smartphone app could help detect early-onset dementia cause, study finds
App-based cognitive tests found to be proficient at detecting frontotemporal dementia in those most at risk
A smartphone app could help detect a leading cause of early-onset dementia in people who are at high risk of developing it, data suggests.
Scientists have demonstrated that cognitive tests done via a smartphone app are at least as sensitive at detecting early signs of frontotemporal dementia in people with a genetic predisposition to the condition as medical evaluations performed in clinics.
Frontotemporal dementia is a neurological disorder that often manifests in midlife, where the part of the brain responsible for skills such as the capacity to plan ahead and prioritise tasks, filter distractions and control impulses, shrinks as the disease progresses.
About a third of such cases have a genetic cause, but research into the condition has been hampered by problems with early diagnosis and difficulty tracking how people are responding to treatments that may only be effective during the early stages of disease.
“Most frontotemporal dementia patients are diagnosed relatively late in the disease, because they are young, and their symptoms are mistaken for psychiatric disorders,” said the study’s senior author, Prof Adam Boxer, at the University of California, San Francisco.
Smartphones are already attracting interest as a tool for diagnosing and assessing Alzheimer’s, Parkinson’s and Huntington’s diseases. To investigate their utility in frontotemporal dementia, Boxer and his colleagues collaborated with the US-based software company Datacubed Health to develop an app that could record people’s speech while they engaged with several cognitive tests, including executive functioning assessments.
“We also created tests of walking, balance and slowed movements, as well as different aspects of language,” said Dr Adam Staffaroni, a clinical neuropsychologist at the University of California, San Francisco, and the study’s first author.
They tested the app in 360 adults at high genetic risk of developing frontotemporal dementia, including some who had not developed any obvious symptoms yet.
The research, published in JAMA Network Open , found that the app could accurately detect dementia in such individuals, and might even be more sensitive to the earliest stages of the condition than gold-standard neuropsychological evaluations that are usually performed in clinics.
Although there are no immediate plans to make the app available to the public, Staffaroni said it could help bolster research into the condition.
More than 30 such clinical trials are under way or in the planning stages, including trials of therapies that might help to slow progression of the disease in some gene carriers. “A major barrier has been a lack of outcome measures that can be easily collected and are sensitive to treatment effects at early stages of the disease.”
Frequent in-person assessments are also burdensome for patients, caregivers and clinicians. “We hope that smartphone assessments will facilitate new trials of promising therapies,” Staffaroni said.
“Eventually, the app may be used to monitor treatment effects, replacing many or most in-person visits to clinical trials’ sites.”
- Smartphones
- Mental health
- Medical research
- Neuroscience
Most viewed
Study Reveals 15 Risk Factors for Young-Onset Dementia
Experts explain the surprising findings.

We may earn commission from links on this page, but we only recommend products we back. Why Trust Us?
- New research reveals 15 risk factors for young-onset dementia.
- The study’s findings challenge the notion that genetics are the sole cause of the condition.
- Experts explain how this may apply to your risk for early-onset dementia.
A study published in JAMA Neurology followed more than 350,000 participants across the United Kingdom from the UK Biobank. Participants were under 65 years old and did not have a dementia diagnosis upon initial assessment. Researchers evaluated participants for an array of risk factors ranging from genetic predispositions to lifestyle and environmental influences.
Some of the risk factors associated with a higher risk of young-onset dementia named in the study include:
- Lower formal education
- Lower socioeconomic status
- Genetic variation
- Lower handgrip strength
- Alcohol use disorder
- Social isolation
- Vitamin D deficiency
- Hearing impairment
- Heart disease
These findings challenge the idea that genetics are the sole cause of a young dementia diagnosis, and may help future prevention strategies.
Young-onset dementia happens before the age of 65, with late-onset dementia occurring after, says Dale Bredesen, M.D. , neuroscience researcher and singleton chair in neurology at the Pacific Neuroscience Institute.
Young-onset dementia is relatively rare compared to a diagnosis later in life—however, instances of young-onset dementia are occurring at a rate higher than ever observed before, says Patrick Porter, Ph.D., neuroscience expert and founder of BrainTap . “This form of dementia can be particularly challenging because it affects individuals during their prime working years and can have substantial emotional, social, and financial impacts,” adds Porter.
According to Porter, the biggest risk factors for dementia in general include:
- Genetic factors
- Lifestyle factors, including smoking, excessive alcohol consumption, poor diet, and lack of physical exercise
- Medical conditions such as cardiovascular diseases, diabetes, obesity, depression, and head injuries
- Education and cognitive engagement
“The point of this study, given that dementia is a leading cause of death in the UK (as in the US and elsewhere), is to identify and address changeable risk factors, says Dr. Bredesen. “Most of those identified in this study are indeed modifiable, and thus there is hope that dementia incidence and ultimately prevalence will be reduced as a result of this study.”
As far as reducing your risk for dementia , Kavita Desai, Pharm. D., women’s health specialist, and founder of Revivele , recommends reducing daily stress, cutting back on alcohol, staying socially engaged, and prioritizing sleep quality.
It’s crucial to understand that while adopting certain strategies can mitigate risk, they do not provide absolute prevention against dementia, says Porter. “Individual differences, including genetic factors, play a significant role in one’s health and are not entirely within our control. However, embracing healthy habits can offer a broad spectrum of benefits for overall health and well-being.”
Madeleine, Prevention ’s assistant editor, has a history with health writing from her experience as an editorial assistant at WebMD, and from her personal research at university. She graduated from the University of Michigan with a degree in biopsychology, cognition, and neuroscience—and she helps strategize for success across Prevention ’s social media platforms.
Brain Health

What Is Menopause Brain?

Study: Fiber Supplement May Improve Brain Function

What a Brain Expert Wants You to Know

Wendy Williams Diagnosed With Dementia and Aphasia

Forgetful Much? How to Remember Things Better

These 5 Habits Help Prevent Dementia

How to Age-Proof Your Whole Body

These Specific Exercises Boost Memory

Study Finds Wasabi May Boost Memory

Hot Flashes May Indicate Higher Alzheimer’s Risk

Study: Eating Cheese May Lower Dementia Risk

15 health and lifestyle risk factors for young-onset dementia revealed in new study
Researchers have identified 15 lifestyle and health risk factors for a rare type of dementia that affects people younger than 65.
Some 370,000 people develop young-onset dementia around the world each year, and now researchers from the University of Exeter in England and Maastricht University in the Netherlands say targeting these health and lifestyle traits may help prevent, identify, and treat the neurodegenerative condition.
Alcohol abuse, vitamin D deficiency, stroke, hearing impairment, heart disease, and high concentrations of C-reactive proteins, which signify inflammation in the body, were determined to be some of the major contributors to young-onset dementia after researchers observed 356,000 people in the UK over several years.
Their findings were published Tuesday in JAMA Neurology .
Social isolation and depression were also listed as major factors, along with less formal education and lower socioeconomic status.
“In addition to physical factors, mental health also plays an important role, including avoiding chronic stress, loneliness and depression,” researcher Sebastian Köhler said in a statement .
Lead study author Dr. Stevie Hendriks, a postdoctoral researcher in psychiatry and neuropsychology at Maastricht University, said the cause of young-onset dementia “is often assumed to be genetic, but for many people we don’t actually know exactly what the cause is.”
She noted that most stricken with the condition “still have a job, children, and a busy life.”
Hendriks gave CNN some recommendations to break some of these risky lifestyle habits.
“Be curious: learn new things, spend time on a hobby, stay engaged and socially active by visiting friends and families or going to social gatherings,” Hendriks said.
“Exercise regularly: keep moving, all levels of exercise work, from walking to vigorous exercise, find something that works for you,” she continued.
Fellow researcher Dr. Janice Ranson is optimistic that this research could “herald a new era in interventions to reduce new cases of this condition.”
Experts around the world have warned for years that COVID-19 has had detrimental psychological effects.
Declared the “loneliness epidemic” of modern times, current research finds extended isolation to be as bad for one’s health as smoking 15 cigarettes.
Young-onset dementia can be difficult to spot, as the tell-tale symptom of memory loss does not commonly present itself at first, according to the UK-based Alzheimer’s Society .
Instead, be on the lookout for problems with balance, movement, and coordination.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- IOS Press Open Library

Extremely Early-Onset Frontotemporal Dementia: A Case Report and Literature Review
a Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China
Haitian Nan
Deming jiang, pedro rosa-neto.
b McGill Centre for Studies in Aging, Alzheimer’s Disease Research Unit, Montreal, Canada
Yueshan Piao
c Department of Neuropathology, Xuanwu Hospital, Capital Medical University, Beijing, China
Associated Data
Background:.
In most cases, the onset of frontotemporal dementia (FTD) occurs between the ages of 45 and 65 years. However, some patients experience an extremely early disease onset.
To investigate the clinical, genetic, and pathological features of extremely early-onset FTD.
We conducted a comprehensive clinical, genetic, and neuropathological analysis of a 25-year-old patient experiencing the onset of behavioral variant frontotemporal dementia (bvFTD). In addition, we conducted a literature review and summarized the clinical, genetic, and pathological features of patients with FTD with onset age≤25 years.
The patient was diagnosed with bvFTD; however, there was no family history of FTD, no positive genetic test results and no deposition of TDP43, tau, ubiquitin, and synuclein in the brain. Literature screening identified 18 patients with onset age ≤25 years with FTD. The youngest patient was 14 years of age. Most patients (8/14) had a positive family history. The most common clinical phenotype was the behavioral variant (12/14). Genetic results were reported for 11 patients; the most common pathogenic gene was MAPT (10/12), with four cases of G389 R, two cases of P301 S, one case of G335 S, one case of G335A, one case of G335 V, and one case of L315 R. Pathological results were reported for 13 patients; the most common pathological subtype was tau (8/13).
Conclusion:
FTD can start at an extremely early age. The most common phenotype of extremely early onset FTD was the behavioral variant, the most common pathogenic gene was MAPT, and the most common neuropathological type was tau.
INTRODUCTION
Frontotemporal dementia (FTD) is a neurodegenerative disease with behavioral and language variants [ 1 ]. One clinical feature of FTD is an early onset; most patients experience disease onset between the ages of 45 and 65 years [ 2, 3 ]. However, some cases of extremely early onset have been reported in which the initiation of neurodegeneration occurred in patients in their 20 s to 30 s. The youngest onset age reported thus far is 14 years [ 4 ], although this is relatively rare in clinical practice.
The most challenging subtype in young patients is the behavioral variant (bvFTD). Patients with bvFTD present with early personality changes and inappropriate or disruptive behaviors that influence their normal work and daily life, thus causing heavier management burdens for both caregivers and society [ 1, 5 ]. In addition, bvFTD can be easily misdiagnosed in young patients thus delaying timely genetic tests and effective treatment; the rate of misdiagnosis is 70% [ 6 ]. The potential hazards and diagnostic challenges encountered during the stages of initial presentation highlight the importance of recognizing young patients with FTD as early as possible.
In this study, we conducted a comprehensive clinical, neuroimaging, neuropsychological, genetic, and neuropathological analysis of a 25-year-old patient experiencing the onset of sporadic bvFTD with four years of follow-up. Furthermore, we conducted a literature review of patients with FTD with an onset age of 25 years or younger to summarize their clinical subtype, along with relevant genetic and pathological features.
Clinical and neuropsychological workup
The patient was evaluated at Xuanwu hospital in Beijing, China. We collated a range of clinical data relating to initial symptoms, disease progression, family history, and other medical history. A comprehensive neurological physical examination was conducted by a professional neurologist. Longitudinal follow-up was conducted every two years to track disease progression.
A neuropsychologist administered a standardized battery of tests every two years. The neuropsychological test battery consisted of assessments that measured cognitive function in the domains of memory, executive function, language, and behavior. Global cognitive screening measures included the Mini-Mental State Examination (MMSE) [ 7 ], the Montreal Cognitive Assessment (MoCA) [ 8 ], and the Frontotemporal Lobar Degeneration (FTLD)-Clinical Dementia Rating Scale [ 9 ]. Executive function was measured using the Trail Making Test [ 10 ]. Visuo-spatial function was assessed using the Rey–Osterrieth Complex Figure Test [ 11 ]. Word list memory was evaluated using Rey’s Auditory-Verbal Learning Test [ 12 ]. Language was measured using the Boston Naming Test [ 13 ]. The severity of behavioral abnormality was assessed using the Frontal Behavior Inventory (FBI) [ 14 ] and the Neuropsychiatric Inventory (NPI) [ 15 ]. The ability to perform daily living activities was assessed using the Activities of Daily Living Scale (ADL) [ 16 ].
This study was conducted according to the Declaration of Helsinki. The clinical protocols were approved by the ethics committee and local institutional review board of Xuanwu Hospital, Capital Medical University, China. The study was conducted in accordance with relevant guidelines and regulations for the use of human subjects in research. Written informed consent was obtained from the participant and his guardians before the start of the study.
Neuroimaging analysis
Magnetic resonance imaging (MRI) and positron emission tomography (PET) images were acquired on a hybrid 3.0 T TOF PET/MRI scanner (SIGNA PET/MR, GE Healthcare, WI, USA) at Xuanwu Hospital. MRI and PET data were acquired simultaneously using a vendor-supplied 19-channel head and neck union coil. Images of amyloid PET (AV45), Tau PET (PI2620), and 18 F fluoro-deoxy-glucose ( 18 F-FDG)-PET were acquired. PET data were reconstructed using an ordered subset expectation maximization algorithm with weighted attenuation. Images were smoothened using an 8-mm Gaussian kernel with scatter correction and evaluated before conducting an analysis of patient motion and adequacy of statistical counts. Standardized uptake value ratios were calculated using the cerebellar gray matter reference region to normalize mean activity from acquired intervals.
We conducted a two-sample t-test to compare structural MRI and FDG-PET images between the patient and 22 healthy men matched for age and educational level (mean age = 26 years, range = 24-30 years; mean education = 15 years, range = 12-19 years); these tests applied a False Discovery Rate (FDR) corrected p < 0.05 and cluster size > 200. Details relating to the imaging processing steps, analyses and software are given in the Supplementary Material . Patients were diagnosed as AV45-positive or PI2620-positive based on both visual interpretations of elevated binding in the neocortex and semi-quantitative assessment.
Genetic analysis
We extracted genomic DNA from fresh peripheral blood leukocytes taken from the patient and his parents and used an Agilent SureSelect Human All Exon V6 Kit (Agilent Technologies, Santa Clara, CA, USA) to generate a sequencing library for whole-exome sequencing. The prepared libraries were sequenced using the HiSeq-2000 platform (Illumina, San Diego, CA, USA). The sequenced reads were then aligned to the human genome (GRCh37/hg19). Reads were then aligned to the targeted regions and collated for single nucleotide polymorphism calling and subsequent analysis using Burrows-Wheeler Aligner software. Our final analysis included 42 genes ( GRN, C9orf72, MAPT, CHMP2B, VCP, TARDBP, SQSTM1, FUS, UBQLN2, OPTN, TREM2, CHCHD10, TBK1, CYLD, TIA1, CCNF, hnRNPA1, hnRNPA2B1, TMEM106B, RAB38, CSF1 R, MATR3, TUBA4A, CFAP410, KIF5A, DCTN1, C21ORF2, ITM2B, PSEN1, PSEN2, APP, AARS2, PLP1, PSAP, PRKAR1B, MOBP, BTNL2, HLA-DRA, CTSC, APOE, TOMM40, ARHGAP35, SERPINA1, GFRA2, UNC13A, SORT1, CIAO1, PRNP, SIGMAR1, GBA, NOTCH3, TRPM7, ABCC1, ABCA7, APBB2, ATP13A2, SPG21, DMT1, VPS2B, ALS17, EIF4G1, SCN8A, COQ2, TSC1, TSC2, HCFC1, ITPR3, PLA2G6 , and SLC9A6 ) that were associated with FTD and other neurodegenerative diseases. Repeat primed polymerase chain reaction was performed to obtain a qualitative estimation of the presence of C9orf72-expanded repeats.
Neuropathological analysis
A biopsy test was conducted in the third year from disease onset. The tissues were extracted from the deep white matter and the borderline between the gray matter and the white matter of the right frontal lobe using robotic stereotactic assistance (ROSA) brain biopsy. Brian tissue samples were fixed with 10% formalin and embedded in paraffin. For immunostaining, deparaffinized sections were incubated with 1% H 2 O 2 in methanol for 10 min to eliminate endogenous peroxidase activity in the tissue. Sections were then pretreated by autoclaving for 10 min in 10 mM sodium citrate buffer (pH 6.0) at 120°C. After washing three times with 0.01 M phosphate-buffered saline (pH 7.4), the sections were processed with the polymer horseradish peroxidase detection system (Polink-1 HRP Broad Spectrum DAB Detection Kit, Golden Bridge International, Mukilteo, WA, USA). The antibodies employed for immunohistochemistry were glial fibrillary acidic protein (GFAP, OriGene USA; monoclonal, clone UMAB129, 1 : 200), neuronal nuclear protein (NeuN, Chemicon, USA; monoclonal, 1 : 4000), Olig-2 (Millipore, USA; Polyclonal, AB9610, 1 : 250), ubiquitin (Abcam, UK; monoclonal, ab140601, 1 : 250), AT8 (hyperphosphorylated tau, Ser202 and Thr205; Thermo, USA; monoclonal, Clone: AT8, 1 : 4000), TDP-43 (Proteintech, USA; monoclonal, Clone 6H6E12, 1 : 10000) and synuclein (Clone 3D5,1 : 10000, a gift from Professor S. Yu) [ 17 ].
Literature review
To preliminary describe the clinical, genetic, and pathological features of young patients with FTD with an age of onset similar to the present case, two of the investigators (MC and LL) performed a literature review using PubMed and Embase databases, from inception to September 2021. We included all patients who had been diagnosed with FTD with an onset of≤25-years-of-age. The first author’s name and year of publication, as well as each patient’s age of onset, sex, clinical, neuroimaging, genetics, and pathological results, were extracted from the literature. Two neurologists (MC and LL) independently made a clinical classification of the phenotype involved in each case according to the clinical and neuroimaging features and by referring to the diagnostic criteria for bvFTD [ 1 ], non-fluent variant primary progressive aphasia (nfvPPA) [ 18 ] and semantic variant PPA (svPPA) [ 18 ]. It should be noted that behavioral, language, and motor syndromes overlapped in some patients. The definition of “family history” used in the literature review was 1) a definite report of a family history and 2) some of the relatives presented with dementia, motor dysfunction or other neurodegenerative disease.
Clinical course
The patient was a 30-year-old male with 14 years of education, who used to be a worker in a company selling mineral water. At the age of 25 years, the patient’s family and friends observed marked personality changes; previously, the patient was outgoing but became introverted over time. He stopped working for the mineral water company, became socially withdrawn and lost sympathy with a reduced response to the feelings of his parents and friends; notably, he was indifferent to his brother’s wedding. He exhibited apathy and self-neglect and did not care about personal hygiene. He was obsessed with computer games and had hyperorality presenting with the increased consumption of excessive snacks and alcohol. Sometimes he was restless and quarreled with his family. Depression was initially suspected but the patient was unresponsive to anti-depressive drugs.
Within one year, his condition worsened. He was still restless, apathetic with a loss of sympathy. Behavioral disinhibition was observed; this manifested as impulsive and socially inappropriate behavior (stealing the possessions of others and scratching cars belonging to others). Hallucination and delusion were identified as the patient reported seeing birds in his room; he also thought he had been discarded by his family. However, the hallucination and delusion were moderate and only occurred on two occasions. He was diagnosed with schizophrenia in another hospital nine months after onset but did not respond to antipsychotic drugs.
Approximately one year after onset, he was admitted to our hospital. Physical examination revealed an abnormal cognitive status with memory and executive dysfunction; this is described in the neuropsychological section in Table 1 . The patient had normal cranial signs, and normal limb motor, reflex, sensory and cerebellar function; there were no signs of meningeal irritation. He denied a history of drug use. There was no family history of dementia, motor dysfunction or any other neurodegenerative disease. His parents are still alive and cognitively intact; no other relatives died from neurodegenerative disease. MRI revealed frontal and temporal lobe atrophy and FDG-PET revealed severe hypometabolism in the frontal and temporal lobes. Thus, we considered probable behavior variant frontotemporal dementia as the first diagnosis according to the 2011 diagnostic criteria [ 1 ]. To exclude the early onset of Alzheimer’s disease, we conducted amyloid and tau PET and found no deposition of pathological protein. The neuroimaging results of Aβ and tau PET are shown in the neuroimaging section of Fig. 2 . Metabolic disease including methylmalonic aciduria was also taken into consideration; however, no abnormality was found in the hematuria organic acid screening and genetic examination.
Spatial coordinates and peak values of brain areas showing significant differences in gray matter volume and metabolism of this patient

Neuroimages of the patient. Axial, sagittal, and coronal images of (A) structural MRI, (B) FDG-PET, (C) AV45-PET, and (D) PI2620 PET. (E) Atrophy and (F) Hypometabolism patterns in the patient in comparison with healthy controls. Detailed radiological Montreal Neurosciences Institution (MNI) coordinates of the clusters are shown in Table 1 .
Two years after onset, the patient experienced severe memory and cognitive decline. He was still restless, unstable, and easily lost his temper. Apathy and loss of sympathy were severe in that he did not care about his parents and declined to meet any of his relatives and friends. Some social disinhibition behaviors were observed including talking and physical contact with strangers. Oral exploration was observed in that he always took something inedible, such as papers, to his mouth. Cognitive function declined severely, including some of the long-term memory, executive function, and judgement. Compared with the first follow-up, no more changes were found in the two-year follow-up physical examination.
At the third telephone follow-up, he became mute, bed-ridden, incontinent of urine and feces, and lost the ability to take care of himself. The telephone follow-up was conducted without physical examination. The clinical course is summarized in Fig. 1 . At the time of writing the patient is still alive.

Clinical course of the patient. Symptoms began at 25 years of age and the patient was first admitted to our hospital at 26 years of age. A second and third follow-up were conducted at 28 and 30 years of age, respectively. The main clinical presentations were listed at each time point.
Neuroimaging results
Structural MRI, FDG-PET, amyloid PET (AV45), and tau PET (PI2620) were conducted one year after disease onset. Marked frontotemporal atrophy and hypometabolism were observed on raw MRI images ( Fig. 2A ) and FDG-PET images ( Fig. 2B ). Negative results were found on the AV45-PET ( Fig. 2C ) and PI2620-PET ( Fig. 2D ). The patient showed predominant volume loss and hypometabolism in the frontal, temporal, anterior cingulate, and subcortical brain regions when compared with healthy controls ( Fig. 2E ). The detailed spatial coordinates and peak values of brain areas showing significant differences in gray matter volume and metabolism in the patient are shown in Table 2 . Atrophy and hypometabolism overlapped several brain regions including the left (inferior temporal, middle temporal, middle temporal pole, superior temporal pole, superior orbital frontal, inferior orbital frontal, medial orbital frontal, rectus, anterior cingulate, fusiform, hippocampus, para-hippocampus, thalamus, caudate, and putamen) and right brain regions (superior orbital frontal, medial orbital frontal, rectus, anterior cingulate, middle temporal pole, and caudate). Due to agitation and uncooperative behavior, we were unable to perform follow-up PET/MRI.
Neuropsychological tests of the patient with extremely early-onset FTD
MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; TMT-A, Trail Making Test-A; TMT-B, Trail Making Test-B; RAVLT, Rey’s Auditory-Verbal Learning Test; ROCFT, Rey–Osterrieth Complex Figure Test; BNT, Boston Naming test; FBI, Frontal Behavior Inventory; NPI, neuropsychiatric Inventory; ADL, Activity of Daily Living; FTLD-CDR, Frontotemporal Lobar Degeneration Clinical Dementia Rating Scale.
Neuropsychological tests
Neuropsychological test results are shown in Table 2 . At the first admission, the patient could cooperate with some neuropsychological tests. In terms of general mental status, the MMSE score was 16/30 (23/24 for individuals with 7 or more years of education) and the MoCA score was 11/30 (normal range > 24). In terms of executive function, the Trail Making Test-A complete time was 60 s with no error lines (normal range < 78 s); the Trail Making Test-B was not completed. In terms of memory, Rey’s Auditory-Verbal Learning Test total score was 11 (abnormal range≤20); no words were repeated in the recall and recognition phase. With regards to visual and spatial function, the Rey–Osterrieth Complex Figure Test score was 7/36 (normal range > 29). In terms of language skills, the Boston Naming Test score was 13/30 (normal range > 22); sentence-making (0/1) and the reading test were 0/1 and 1/1 in the MMSE, respectively. Sentence repetition (0/1) and word fluency were 0/1 and 1/1 in the MoCA, respectively. In terms of disease severity, the FTLD-Clinical Dementia Rating Scale sum of box score was 12/32 (abnormal > 0). At the second follow-up, the patient’s cognitive function declined; he was restless, irritable and did not cooperate well during the neuropsychological tests. At the third follow-up, the patient’s condition had become more severe; he was bedridden, mute and was losing the ability to communicate with the physicians.
Cases of young onset frontotemporal dementia (25-years-of-age or younger) in the literature
*Two papers did not provide detailed information on the clinical symptoms of the patients and could not be classified to a clinical subtype. M, Male; F, female; NA, not available; FTD, frontotemporal dementia; FTDP-17, frontotemporal dementia with Parkinsonism-17; PLS, primary lateral sclerosis; FTLD, frontotemporal lobe degeneration; bvFTD, behavior variant frontotemporal dementia; nfvPPA, non-fluent variant primary progressive aphasia; svPPA, semantic variant primary progressive aphasia; MAPT microtubule-associated protein tau.
The NPI, FBI, and ADL were conducted. At the first admission, the patient had an NPI score of 69; in addition, the patient showed severe behavioral symptoms, including agitation, depression, apathy, disinhibition, irritability, aberrant motor behavior, and appetite changes. His FBI score was 35 and negative symptoms were scored at 19, including apathy, aspontaneity, indifference, inflexibility, disorganization, inattention, personal neglect, and loss of insight. Positive symptoms were scored at 16, including irritability, poor judgment, inappropriate behavior, impulsivity, restlessness, aggression, and utilization behavior. The ADL score was 25 at admission. At the second follow-up, the patient’s NPI score was 78, the FBI total score was 38, the FBI disinhibition subscale score was 20, the FBI apathy subscale score was 18, and the ADL score was 40. In the third follow-up, the patient became bedridden and mute, thus we only conducted the ADL test; the score was 80.
Genetic tests
Genetic tests of the patient and his parents for hexanucleotide mutation in the chromosome 9 open reading frame 72 gene and whole-exome sequencing for mutations in known genes associated with FTD and other neurodegenerative diseases were negative. No abnormality was found in the genetic results related to metabolic disease.
Neuropathological tests
There was mild neuronal loss and shrinkage accompanied by gliosis in the deep cortical layers of the frontal lobe ( Fig. 3 ). The subcortical white matter looked a little sparse, but no inclusion bodies were detected by immunohistochemical staining with ubiquitin, phosphorylated tau protein (AT8), DNA-binding protein of 43 kDa (TDP-43), and synuclein.

Histopathological findings. Mild neuronal loss was evident in (A) H&E staining and (B) NeuN immunostaining. C) The proliferation of astrocytes was evident in GFAP immunostaining (Scale bar = 400μm). No inclusion bodies were detected by immunohistochemical staining with (D) ubiquitin, (E) AT8, (F) TDP43, and (G) synuclein (Scale bar = 200μm).
Through the literature search, we identified 16 patients with FTD with an age of onset of 25 years or younger. The detailed clinical, genetic, and pathological data of these patients are shown in Table 1 . The youngest patient was 14-years-of-age. The mean age at onset was 21.06±3.70 years (range: 14-25 years). The age of death was reported for 13 patients; the mean age of death was 30.92±4.84 years (range: 24-39 years). Most of the patients with FTD (8/14) had a positive family history. The most common reclassified clinical phenotype was the behavioral variant (12/14); two papers did not report detailed information relating to clinical symptoms. Genetic results were reported for 12 patients; the most common pathogenic gene was MAPT (10/12), with four cases of G389 R, two cases of P301 S, one case of G335 S, one case of G335A, one case of G335 V and one case of L315 R. Pathological results were reported in 13 patients; the most common pathological subtype was tau (8/13). Detailed data extracted from the literature is shown in Supplementary Table 1 .
In this study, we comprehensively investigated the clinical, neuroimaging, genetic, and pathological features of a 25-year-old patient who met the diagnosis of probable bvFTD. We also delineated some unique features of extremely young patients with FTD. The most common clinical subtype was the behavioral variant, most of the reported mutations were on the MAPT gene and the most common pathological subtype was tau pathology. This young-onset patient adds to our knowledge of extremely early-onset FTD and serves as a reminder that FTD should be taken into consideration when young patients present with behavioral deficits.
Our patient met the diagnosis for probable bvFTD with a typical clinical pattern that manifested as disinhibition, apathy, loss of sympathy, and oral exploration. The early-onset patient described herein presented with behavioral and cognitive deficits, which could be differentiated from Alzheimer’s disease, some psychiatric disease (including schizophrenia or depression) and metabolic disease, such as methylmalonic acidemia. The patient was negative for amyloid and tau PET, this helping us to differentiate from AD. Psychiatric disease, including depression or schizophrenia, was considered at first, although obvious brain atrophy and hypometabolism were detected by MRI and FDG-PET. Furthermore, the patient did not respond to anti-depression and anti-psychotic drugs. In addition, metabolic diseases, including methylmalonic aciduria were taken into consideration; however, no abnormality was detected by hematuria organic acid screening and genetic analysis.
Marked fronto-temporal impairment was observed on MRI and FDG-PET, thus supporting the diagnosis of bvFTD that was consistent with the typical patterns of bvFTD atrophy and hypometabolism [ 35 ]. However, we did not find any pathogenic mutations in the patient or his parents, and no family history was reported; therefore, we concluded that the patient was experiencing sporadic bvFTD. No positive inclusion bodies were detected by immunohistochemical staining with ubiquitin, phosphorylated tau protein (AT8), DNA-binding protein of 43 kDa (TDP-43), and synuclein when we conducted a frontal biopsy. However, because of laboratory limitations, we were unable to stain for FTLD-FET, thus limiting our diagnosis to probable bvFTD. The neuropathological results should be interpreted with caution because the pathological brain tissue was acquired through a stereotactic biopsy that was limited to the bilateral frontal tissue. Further post-mortem autopsy needs to be conducted when possible.
Patients with FTD and an age of onset that is 25 years or younger appear to have unique and characteristic features. Most patients had a positive family history (55.6%); this was higher than the 30-50% with typical FTD [ 36 ]. This pedigree investigation represents a significant step forward in terms of identifying the features of young patients with FTD. If a patient has pathogenic mutations, then blood samples from the proband’s biological parents are important to identify whether a patient carries a de novo mutation or whether autosomal dominant inheritance is involved.
Most patients exhibited a behavioral clinical phenotype (77.8%); this is a higher frequency than for typical FTD (in which bvFTD was reported to account for half of such cases) [ 37 ]. The clinical manifestations of these patients are the same as those of typical bvFTD, including apathy and behavior disinhibition; most patients had personality changes as their initial symptoms [ 19, 20, 23-26, 28, 30, 32-34 ]. These might be considered as psychiatric disorders, including depression, schizophrenia, or solvent abuse at the first evaluation and had undergone extensive tests to reach an accurate diagnosis of FTD [ 4, 19, 23–25, 30, 33 ]. The behavioral deficit of these extremely young patients causes heavy social and family burdens and is a huge challenge for patient care and management [ 25 ]. This issue therefore deserves more attention.
All the reported pathogenic mutations were located on the MAPT gene [ 4, 19, 20, 23, 26–28, 30–32 ]; this differs from the genetic distribution of typical FTD in that C9 has been reported as the most common type in Western countries [ 38 ]; MAPT abnormalities are more common in China [ 39 ]. In addition, we also found some specific mutation sites that might contribute to extremely early onset; the most common mutations were MAPT G389 R [ 19, 20, 23, 27 ] and G335A [ 4 ]/S [ 26 ]/V [ 30 ] which are located in exons 13 and 12. We found some discrepancies in the frequency of MAPT mutations between extremely early onset and typical FTD. In cases with typical FTD, the most common MAPT mutations were P301 L (rs63751273; 234 individuals and 59 families), IVS10 + 16C⟶T (rs63751011; 149 individuals and 48 families), R406 W (rs63750424; 67 individuals and 9 families), and N279K (rs63750756; 44 individuals, 17 families) [ 2 ]. However, the mechanisms underlying the more significant contributions of G389 R or G335A/S/V to an early onset need further investigation. Most patients had tau pathology; this differed from the main pathology of typical FTD, in which the most common subtype was reported to be TDP43 [ 40 ]. The pathological distribution discrepancy of high tau proportion might be related to a genetic-pathology MAPT-Tau corresponding relationship [ 41 ].
This study had some limitations that need to be considered. First, this is a case report and literature review; the papers which report positive results from patients with pathogenic mutations or specific pathological results tend to be more easily accepted than those without pathogenic mutations or non-specific pathological results. This might create some bias when summarizing the frequencies in the literature. Second, due to the rarity of this condition, the sample of patients with FTD with a disease onset of 25 years or younger was small. Therefore, the results reported in this study should be considered as exploratory. Third, the pathological tests were not comprehensive as they lacked FTLD-FET due to laboratory limitations, thus limiting the diagnosis to probable bvFTD. Finally, our understanding of the genetics and pathologies of FTD has progressed over the last 20 years, thus, the results reported herein should be interpreted with caution and validated in a larger cohort.
Conclusions
Our analysis showed that FTD can occur at an extremely early age; the youngest patient ever reported was 14 years of age. The most common mutations reported in cases of extremely early onset were involved in the MAPT gene. The most common mutation site was G389 R, and the most common pathological subtype was tau pathology.
Supplementary Material
Acknowledgments.
The authors are grateful to all subjects for their participation in the study.
This work was supported by grants from the National Natural Science Foundation of China [no.81971011].
Authors’ disclosures available online ( https://www.j-alz.com/manuscript-disclosures/22-0679r1 ).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220679 .

IMAGES
COMMENTS
Young-onset dementia (YOD) refers to onset of symptoms under 65 years old. A recent meta-analysis indicated a prevalence of more than 1 in 1000 people, equating to 3.9 million people worldwide living with YOD [1].YOD disorders include early-onset Alzheimer's disease, frontotemporal dementia (FTD) and rarer types of dementia [2], [3].In YOD, the potential for a dominant genetic cause is ...
Young-onset dementia (YOD), arbitrarily described as dementia diagnosed under 65 years, is poorly recognised and often misdiagnosed. 1, 2 This clinical review evaluates the current evidence about best practice in diagnosis to guide thorough assessment of the complex presentations of YOD. Many clinical practice guidelines on the diagnosis and management of dementia exist, but vary in the ...
In the UK, it is estimated that people living with young-onset dementia (YOD) accounts for a small, but significant, number of the total population living with dementia. The diagnosis happens at an unexpected time in one's life, and as a life-limiting condition, there are implications for the whole family, including managing employment, finances and debt, supporting dependent children and ...
Young-onset dementia (YOD) refers to any dementia where symptom onset occurs at less than 65 years of age 1 and is distinct from childhood dementia, which is often diagnosed at 14 years of age or less. 2 YOD accounts for about 5% of all dementias, with an estimated prevalence of 119 per 100 000. 3 In Australia, about 28 000 people are living with YOD, 4 with major associated personal ...
The first is that standard criteria for dementia require that cognitive impairment is sufficiently severe to compromise social and occupational functioning. 1 The second is that memory must be specifically impaired. A consequence of the first challenge is a delay in a specific diagnosis of the cause of the dementia.
This is most commonly referred to as young onset dementia (YOD), and it is estimated that there are 42,325 people living with YOD in the UK, with these figures expected to rise to 50,401 by 2025 (Alzheimer's Society, 2014; ... By using my case study within a research project, I feel that others can benefit from my experience. ...
A total of 42 people participated in the study (14 people with young-onset dementia, 28 family caregivers) (Table 1), which was made up of 11 dyads (person with young-onset dementia and family caregiver), three triads (person with young-onset dementia and two family caregivers), and 11 lone family caregivers. Therefore, this study concerned a ...
At one time in the not-too-distant past, young-onset dementia (YOD) was considered a disease, whereas later-onset dementia (LOD) was considered the inevitable consequence of aging. We now reject such a formulation, but within that misguided dichotomy is a recognition of the dramatic differences in prevalence and incidence of YOD compared with LOD.
A diagnosis of dementia is devastating at any age but diagnosis in younger patients presents a particular challenge. The differential diagnosis is broad as late presentation of metabolic disease is common and the burden of inherited dementia is higher in these patients than in patients with late-onset dementia. The presentation of the common degenerative diseases of late life, such as ...
Overall, the study suggests that about 3% of all dementia cases in the United States are in adults under age 65. However, most of these cases were in adults aged 55 to 64. "Among the really young, where the burden would be greatest, dementia is exceedingly rare," Knopman said. In the age range of 30 to 34, for example, Köhler's meta ...
The full title of the study was: 'Living well with young onset dementia -- Humphrey Booth Resource Centre as a hub of excellence in a system of support'. Competing interests: None declared. Patient consent: Obtained. Ethics approval: Approval for the study was obtained from the Health Research Authority - Preston Research Ethics Committee ...
Background People with young onset dementia (YOD) have unique needs and experiences, requiring care and support that is timely, appropriate and accessible. This relies on health professionals possessing sufficient knowledge about YOD. This study aims to establish a consensus among YOD experts about the information that is essential for health professionals to know about YOD. Methods An ...
Dementia is a major worldwide public health concern in view of the global ageing phenomenon. Dementia usually occurs in old age. However, if the symptoms occur in young patients, the diagnosis can be challenging. Posterior cortical atrophy is a variant of the Alzheimer's disease, which is described as a presenile disease affecting relatively ...
Background: Studies on disease-related obstructions experienced in everyday life of younger people with dementia (YOD ≤ 65 years) and their families are encouraged.Aim: To explore how the family carers experience six predefined topics that influence the everyday life and needs of persons with YOD.Method: A quantitative and a qualitative study including family carers of persons with young ...
Developing dementia: The existential experience of the quality of life with young-onset dementia - A longitudinal case study. ... The existential experience of the quality of life with young-onset dementia - A longitudinal case study Dementia (London). 2020 Apr;19(3):878-893. doi: 10.1177/1471301218789990. Epub 2018 Jul 30. ...
Young-onset dementia, a dementia syndrome with onset under the age of 65, accounts for approximately 8% of all people with dementia ().The presenting symptoms of young-onset dementia may be atypical and differ from those seen in late-onset dementia ().The differential diagnosis of young-onset dementia can be broad and includes neurological, neurodegenerative, and primary psychiatric disorders.
Background Social care is vital to quality of life for people with young onset dementia and their families. Yet care is hugely variable, frequently lacking and poorly coordinated. We aim to establish current practice in English social care for people with young onset dementia and co-produce evidence-based recommendations and resources for improvement. Methods and analysis In Work-Package 1, we ...
A case study comprised of formal interviews, ... Dementia services were ill-informed about how to support persons with young onset dementia, with pre-existing mental health conditions, from an ethnic minority. Education and training to remove barriers to all dementia care services is required for persons with dementia, their families and within ...
Interest in dementia and working life is increasing, framed mainly as an issue for people with young onset dementia, i.e., below the age of 65 years (Evans, Citation 2019; Ikeuchi et al., Citation 2020; Roach, Citation 2017). ... Using a multiple case study approach, our aim was to perform an in-depth exploration of the unfolding experiences of ...
App-based cognitive tests found to be proficient at detecting frontotemporal dementia in those most at risk A smartphone app could help detect a leading cause of early-onset dementia in people who ...
Changes in personality, behavior and language are hallmarks of frontotemporal dementia (FTD), the most common form of dementia in patients under the age of 65, which is associated with degeneration of the frontal and temporal lobes of the brain.Researchers have known that a less common protective variant of a gene called TMEM106B may slow disease progression, and now they have new insight into ...
A total of 42 people participated in the study (14 people with young-onset dementia, 28 family caregivers) , which was made up of 11 dyads (person with young-onset dementia and family caregiver), three triads (person with young-onset dementia and two family caregivers), and 11 lone family caregivers. Therefore, this study concerned a total of ...
Young-onset dementia happens before the age of 65, with late-onset dementia occurring after, says Dale Bredesen, M.D., neuroscience researcher and singleton chair in neurology at the Pacific ...
The large-scale study identified 15 risk factors, which are similar to those for late-onset dementia. For the first time, they indicate that it may be possible to reduce the risk of young-onset ...
Dementia usually develops in people ages 65 years and older. So-called young-onset dementia, occurring in those younger than age 65, is uncommon. Now, a new study published in December 2023 in ...
Risk factors for young-onset dementia. In the new study, researchers investigated the association between 39 potential risk factors and the incidence of young-onset dementia among 356,052 ...
Although dementia care is usually organized for people in the 70s and 80s, the study said there is a growing amount of "young-onset dementia" in the country. In fact, by 2050, the study ...
Researchers have identified 15 lifestyle and health risk factors for a rare type of dementia that affects people younger than 65. Some 370,000 people develop young-onset dementia around the world ...
Frontotemporal dementia (FTD) is a neurodegenerative disease with behavioral and language variants [ 1 ]. One clinical feature of FTD is an early onset; most patients experience disease onset between the ages of 45 and 65 years [ 2, 3 ]. However, some cases of extremely early onset have been reported in which the initiation of neurodegeneration ...
This study revealed that the HRs after crude and adjusted for dementia related to TBI with epilepsy were greater in younger patients. After considering confounding factors in our study, there was a higher proportion of cases in younger older adults with dementia (HR: 3.41; 95% CI: 1.44-8.08) compared with controls.