Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 20 January 2022

AI in health and medicine
- Pranav Rajpurkar ORCID: orcid.org/0000-0002-8030-3727 1 na1 ,
- Emma Chen 2 na1 ,
- Oishi Banerjee 2 na1 &
- Eric J. Topol ORCID: orcid.org/0000-0002-1478-4729 3
Nature Medicine volume 28 , pages 31–38 ( 2022 ) Cite this article
131k Accesses
563 Citations
619 Altmetric
Metrics details
- Computational biology and bioinformatics
- Medical research
Artificial intelligence (AI) is poised to broadly reshape medicine, potentially improving the experiences of both clinicians and patients. We discuss key findings from a 2-year weekly effort to track and share key developments in medical AI. We cover prospective studies and advances in medical image analysis, which have reduced the gap between research and deployment. We also address several promising avenues for novel medical AI research, including non-image data sources, unconventional problem formulations and human–AI collaboration. Finally, we consider serious technical and ethical challenges in issues spanning from data scarcity to racial bias. As these challenges are addressed, AI’s potential may be realized, making healthcare more accurate, efficient and accessible for patients worldwide.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
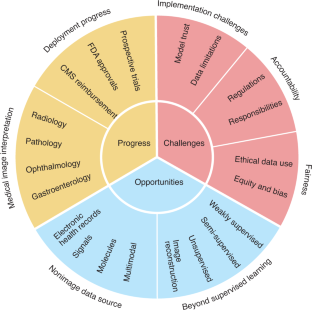
Similar content being viewed by others
Foundation models for generalist medical artificial intelligence
Michael Moor, Oishi Banerjee, … Pranav Rajpurkar

Guiding principles for the responsible development of artificial intelligence tools for healthcare
Kimberly Badal, Carmen M. Lee & Laura J. Esserman
A short guide for medical professionals in the era of artificial intelligence
Bertalan Meskó & Marton Görög
Gulshan, V. et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. J. Am. Med. Assoc. 316 , 2402–2410 (2016).
Article Google Scholar
Esteva, A. et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 542 , 115–118 (2017).
Article CAS PubMed PubMed Central Google Scholar
Rajpurkar, P. et al. Deep learning for chest radiograph diagnosis: a retrospective comparison of the CheXNeXt algorithm to practicing radiologists. PLoS Med. 15 , e1002686 (2018).
Article PubMed PubMed Central Google Scholar
Hannun, A. Y. et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 25 , 65–69 (2019).
Wiens, J. et al. Do no harm: a roadmap for responsible machine learning for health care. Nat. Med. 25 , 1337–1340 (2019).
Article CAS PubMed Google Scholar
Kanagasingam, Y. et al. Evaluation of artificial intelligence-based grading of diabetic retinopathy in primary care. JAMA Netw. Open 1 , e182665 (2018).
Beede, E. et al. A human-centered evaluation of a deep learning system deployed in clinics for the detection of diabetic retinopathy. in Proceedings of the 2020 CHI Conference on Human Factors in Computing Systems 1–12 (Association for Computing Machinery, 2020); https://dl.acm.org/doi/abs/10.1145/3313831.3376718
Kiani, A. et al. Impact of a deep learning assistant on the histopathologic classification of liver cancer. NPJ Digit. Med. 3 , 23 (2020).
Lin, H. et al. Diagnostic efficacy and therapeutic decision-making capacity of an artificial intelligence platform for childhood cataracts in eye clinics: a multicentre randomized controlled trial. EClinicalMedicine 9 , 52–59 (2019).
Gong, D. et al. Detection of colorectal adenomas with a real-time computer-aided system (ENDOANGEL): a randomised controlled study. Lancet Gastroenterol. Hepatol. 5 , 352–361 (2020).
Article PubMed Google Scholar
Wang, P. et al. Effect of a deep-learning computer-aided detection system on adenoma detection during colonoscopy (CADe-DB trial): a double-blind randomised study. Lancet Gastroenterol. Hepatol. 5 , 343–351 (2020).
Hollon, T. C. et al. Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks. Nat. Med. 26 , 52–58 (2020).
Phillips, M. et al. Assessment of accuracy of an artificial intelligence algorithm to detect melanoma in images of skin lesions. JAMA Netw. Open 2 , e1913436 (2019).
Nimri, R. et al. Insulin dose optimization using an automated artificial intelligence-based decision support system in youths with type 1 diabetes. Nat. Med. 26 , 1380–1384 (2020).
Wijnberge, M. et al. Effect of a machine learning-derived early warning system for intraoperative hypotension vs. standard care on depth and duration of intraoperative hypotension during elective noncardiac surgery. J. Am. Med. Assoc. 323 , 1052–1060 (2020).
Wismüller, A. & Stockmaster, L. A prospective randomized clinical trial for measuring radiology study reporting time on Artificial Intelligence-based detection of intracranial hemorrhage in emergent care head CT. in Medical Imaging 2020: Biomedical Applications in Molecular, Structural, and Functional Imaging vol. 11317, 113170M (International Society for Optics and Photonics, 2020).
Liu, X. et al. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Br. Med. J. 370 , m3164 (2020).
Rivera, S. C. et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Nat. Med. 26 , 1351–1363 (2020).
Centers for Medicare & Medicaid Services. Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Final Policy Changes and Fiscal Year 2021 Rates; Quality Reporting and Medicare and Medicaid Promoting Interoperability Programs Requirements for Eligible Hospitals and Critical Access Hospitals. Fed. Regist. 85 , 58432–59107 (2020).
Benjamens, S., Dhunnoo, P. & Meskó, B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit. Med. 3 , 118 (2020).
Wu, N. et al. Deep neural networks improve radiologists’ performance in breast cancer screening. IEEE Trans. Med. Imaging 39 , 1184–1194 (2020).
McKinney, S. M. et al. International evaluation of an AI system for breast cancer screening. Nature 577 , 89–94 (2020).
Ghorbani, A. et al. Deep learning interpretation of echocardiograms. NPJ Digit. Med. 3 , 10 (2020).
Ouyang, D. et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature 580 , 252–256 (2020).
Ardila, D. et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 25 , 954–961 (2019).
Huynh, E. et al. Artificial intelligence in radiation oncology. Nat. Rev. Clin. Oncol. 17 , 771–781 (2020).
Huang, P. et al. Prediction of lung cancer risk at follow-up screening with low-dose CT: a training and validation study of a deep learning method. Lancet Digit. Health 1 , e353–e362 (2019).
Kather, J. N. et al. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat. Med. 25 , 1054–1056 (2019).
Jackson, H. W. et al. The single-cell pathology landscape of breast cancer. Nature 578 , 615–620 (2020).
Campanella, G. et al. Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat. Med. 25 , 1301–1309 (2019).
Fu, Y. et al. Pan-cancer computational histopathology reveals mutations, tumor composition and prognosis. Nat. Cancer 1 , 800–810 (2020).
Courtiol, P. et al. Deep learning-based classification of mesothelioma improves prediction of patient outcome. Nat. Med. 25 , 1519–1525 (2019).
Bera, K., Schalper, K. A., Rimm, D. L., Velcheti, V. & Madabhushi, A. Artificial intelligence in digital pathology: new tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 16 , 703–715 (2019).
Zhou, D. et al. Diagnostic evaluation of a deep learning model for optical diagnosis of colorectal cancer. Nat. Commun. 11 , 2961 (2020).
Zhao, S. et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: a systematic review and meta-analysis. Gastroenterology 156 , 1661–1674 (2019).
Freedman, D. et al. Detecting deficient coverage in colonoscopies. IEEE Trans. Med. Imaging 39 , 3451–3462 (2020).
Liu, H. et al. Development and validation of a deep learning system to detect glaucomatous optic neuropathy using fundus photographs. JAMA Ophthalmol. 137 , 1353–1360 (2019).
Milea, D. et al. Artificial intelligence to detect papilledema from ocular fundus photographs. N. Engl. J. Med. 382 , 1687–1695 (2020).
Wolf, R. M., Channa, R., Abramoff, M. D. & Lehmann, H. P. Cost-effectiveness of autonomous point-of-care diabetic retinopathy screening for pediatric patients with diabetes. JAMA Ophthalmol. 138 , 1063–1069 (2020).
Xie, Y. et al. Artificial intelligence for teleophthalmology-based diabetic retinopathy screening in a national programme: an economic analysis modelling study. Lancet Digit. Health 2 , e240–e249 (2020).
Arcadu, F. et al. Deep learning algorithm predicts diabetic retinopathy progression in individual patients. NPJ Digit. Med. 2 , 92 (2019).
Senior, A. W. et al. Improved protein structure prediction using potentials from deep learning. Nature 577 , 706–710 (2020).
Alley, E. C., Khimulya, G., Biswas, S., AlQuraishi, M. & Church, G. M. Unified rational protein engineering with sequence-based deep representation learning. Nat. Methods 16 , 1315–1322 (2019).
Gainza, P. et al. Deciphering interaction fingerprints from protein molecular surfaces using geometric deep learning. Nat. Methods 17 , 184–192 (2020).
Greener, J.G. et al. Deep learning extends de novo protein modelling coverage of genomes using iteratively predicted structural constraints. Nat. Commun. 10 , 3977 (2019).
Chabon, J. J. et al. Integrating genomic features for non-invasive early lung cancer detection. Nature 580 , 245–251 (2020).
Luo, H. et al. Circulating tumor DNA methylation profiles enable early diagnosis, prognosis prediction, and screening for colorectal cancer. Sci. Transl. Med. 12 , eaax7533 (2020).
Cristiano, S. et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature 570 , 385–389 (2019).
Gussow, A. B. et al. Machine-learning approach expands the repertoire of anti-CRISPR protein families. Nat. Commun. 11 , 3784 (2020).
Wang, D. et al. Optimized CRISPR guide RNA design for two high-fidelity Cas9 variants by deep learning. Nat. Commun. 10 , 4284 (2019).
Bhattacharyya, R. P. et al. Simultaneous detection of genotype and phenotype enables rapid and accurate antibiotic susceptibility determination. Nat. Med. 25 , 1858–1864 (2019).
Stokes, J. M. et al. A deep learning approach to antibiotic discovery. Cell 181 , 475–483 (2020).
Zhavoronkov, A. et al. Deep learning enables rapid identification of potent DDR1 kinase inhibitors. Nat. Biotechnol. 37 , 1038–1040 (2019).
Lee, J. et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics 36 , 1234–1240 (2020).
CAS PubMed Google Scholar
Zhu, Y., Li, L., Lu, H., Zhou, A. & Qin, X. Extracting drug-drug interactions from texts with BioBERT and multiple entity-aware attentions. J. Biomed. Inform. 106 , 103451 (2020).
Smit, A. et al. CheXbert: Combining automatic labelers and expert annotations for accurate radiology report labeling using BERT. in Proceedings of the 2020 Conference on Empirical Methods in Natural Language Processing 1500–1519 (2020).
Sarker, A., Gonzalez-Hernandez, G., Ruan, Y. & Perrone, J. Machine learning and natural language processing for geolocation-centric monitoring and characterization of opioid-related social media chatter. JAMA Netw. Open 2 , e1914672 (2019).
Claassen, J. et al. Detection of brain activation in unresponsive patients with acute brain injury. N. Engl. J. Med. 380 , 2497–2505 (2019).
Porumb, M., Stranges, S., Pescapè, A. & Pecchia, L. Precision medicine and artificial intelligence: a pilot study on deep learning for hypoglycemic events detection based on ECG. Sci. Rep. 10 , 170 (2020).
Attia, Z. I. et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 394 , 861–867 (2019).
Chan, J., Raju, S., Nandakumar, R., Bly, R. & Gollakota, S. Detecting middle ear fluid using smartphones. Sci. Transl. Med. 11 , eaav1102 (2019).
Willett, F. R., Avansino, D. T., Hochberg, L. R., Henderson, J. M. & Shenoy, K. V. High-performance brain-to-text communication via handwriting. Nature 593 , 249–254 (2021).
Green, E. M. et al. Machine learning detection of obstructive hypertrophic cardiomyopathy using a wearable biosensor. NPJ Digit. Med. 2 , 57 (2019).
Thorsen-Meyer, H.-C. et al. Dynamic and explainable machine learning prediction of mortality in patients in the intensive care unit: a retrospective study of high-frequency data in electronic patient records. Lancet Digit. Health 2 , e179–e191 (2020).
Porter, P. et al. A prospective multicentre study testing the diagnostic accuracy of an automated cough sound centred analytic system for the identification of common respiratory disorders in children. Respir. Res. 20 , 81 (2019).
Tomašev, N. et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature 572 , 116–119 (2019).
Kehl, K. L. et al. Assessment of deep natural language processing in ascertaining oncologic outcomes from radiology reports. JAMA Oncol. 5 , 1421–1429 (2019).
Huang, S.-C., Pareek, A., Seyyedi, S., Banerjee, I. & Lungren, M. P. Fusion of medical imaging and electronic health records using deep learning: a systematic review and implementation guidelines. NPJ Digit. Med. 3 , 136 (2020).
Wang, C. et al. Quantitating the epigenetic transformation contributing to cholesterol homeostasis using Gaussian process. Nat. Commun. 10 , 5052 (2019).
Li, Y. et al. Inferring multimodal latent topics from electronic health records. Nat. Commun. 11 , 2536 (2020).
Tshitoyan, V. et al. Unsupervised word embeddings capture latent knowledge from materials science literature. Nature 571 , 95–98 (2019).
Li, X. et al. Deep learning enables accurate clustering with batch effect removal in single-cell RNA-seq analysis. Nat. Commun. 11 , 2338 (2020).
Amodio, M. et al. Exploring single-cell data with deep multitasking neural networks. Nat. Methods 16 , 1139–1145 (2019).
Urteaga, I., McKillop, M. & Elhadad, N. Learning endometriosis phenotypes from patient-generated data. NPJ Digit. Med. 3 , 88 (2020).
Brbić, M. et al. MARS: discovering novel cell types across heterogeneous single-cell experiments. Nat. Methods 17 , 1200–1206 (2020).
Seymour, C. W. et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. J. Am. Med. Assoc. 321 , 2003–2017 (2019).
Article CAS Google Scholar
Fries, J. A. et al. Weakly supervised classification of aortic valve malformations using unlabeled cardiac MRI sequences. Nat. Commun. 10 , 3111 (2019).
Jin, L. et al. Deep learning enables structured illumination microscopy with low light levels and enhanced speed. Nat. Commun. 11 , 1934 (2020).
Vishnevskiy, V. et al. Deep variational network for rapid 4D flow MRI reconstruction. Nat. Mach. Intell. 2 , 228–235 (2020).
Masutani, E. M., Bahrami, N. & Hsiao, A. Deep learning single-frame and multiframe super-resolution for cardiac MRI. Radiology 295 , 552–561 (2020).
Rana, A. et al. Use of deep learning to develop and analyze computational hematoxylin and eosin staining of prostate core biopsy images for tumor diagnosis. JAMA Netw. Open 3 , e205111 (2020).
Liu, X. et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit. Health 1 , e271–e297 (2019).
Chen, P.-H. C. et al. An augmented reality microscope with real-time artificial intelligence integration for cancer diagnosis. Nat. Med. 25 , 1453–1457 (2019).
Patel, B. N. et al. Human–machine partnership with artificial intelligence for chest radiograph diagnosis. NPJ Digit. Med. 2 , 111 (2019).
Sim, Y. et al. Deep convolutional neural network–based software improves radiologist detection of malignant lung nodules on chest radiographs. Radiology 294 , 199–209 (2020).
Park, A. et al. Deep learning–assisted diagnosis of cerebral aneurysms using the HeadXNet model. JAMA Netw. Open 2 , e195600 (2019).
Steiner, D. F. et al. Impact of deep learning assistance on the histopathologic review of lymph nodes for metastatic breast cancer. Am. J. Surg. Pathol. 42 , 1636–1646 (2018).
Jain, A. et al. Development and assessment of an artificial intelligence-based tool for skin condition diagnosis by primary care physicians and nurse practitioners in teledermatology practices. JAMA Netw. Open 4 , e217249 (2021).
Seah, J. C. Y. et al. Effect of a comprehensive deep-learning model on the accuracy of chest x-ray interpretation by radiologists: a retrospective, multireader multicase study. Lancet Digit. Health 3 , e496–e506 (2021).
Rajpurkar, P. et al. CheXaid: deep learning assistance for physician diagnosis of tuberculosis using chest x-rays in patients with HIV. NPJ Digit. Med. 3 , 115 (2020).
Kim, H.-E. et al. Changes in cancer detection and false-positive recall in mammography using artificial intelligence: a retrospective, multireader study. Lancet Digit. Health 2 , e138–e148 (2020).
Tschandl, P. et al. Human–computer collaboration for skin cancer recognition. Nat. Med. 26 , 1229–1234 (2020).
van der Laak, J., Litjens, G. & Ciompi, F. Deep learning in histopathology: the path to the clinic. Nat. Med. 27 , 775–784 (2021).
Willemink, M. J. et al. Preparing medical imaging data for machine learning. Radiology 295 , 4–15 (2020).
Irvin, J. et al. CheXpert: a large chest radiograph dataset with uncertainty labels and expert comparison. in Proceedings of the AAAI Conference on Artificial Intelligence vol. 33, 590–597 (2019).
Kelly, C. J., Karthikesalingam, A., Suleyman, M., Corrado, G. & King, D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 17 , 195 (2019).
DeGrave, A. J., Janizek, J. D. & Lee, S.-I. AI for radiographic COVID-19 detection selects shortcuts over signal. Nat. Mach. Intell. 3 , 610–619 (2021).
Cutillo, C. M. et al. Machine intelligence in healthcare: perspectives on trustworthiness, explainability, usability, and transparency. NPJ Digit. Med. 3 , 47 (2020).
Sendak, M. P., Gao, M., Brajer, N. & Balu, S. Presenting machine learning model information to clinical end users with model facts labels. NPJ Digit. Med. 3 , 41 (2020).
Saporta, A. et al. Deep learning saliency maps do not accurately highlight diagnostically relevant regions for medical image interpretation. Preprint at medRxiv https://doi.org/10.1101/2021.02.28.21252634 (2021).
Ehsan, U. et al . The who in explainable AI: how AI background shapes perceptions of AI explanations. Preprint at https://arxiv.org/abs/2107.13509 (2021).
Reyes, M. et al. On the interpretability of artificial intelligence in radiology: Challenges and opportunities. Radio. Artif. Intell. 2 , e190043 (2020).
Liu, C. et al . On the replicability and reproducibility of deep learning in software engineering. Preprint at https://arxiv.org/abs/2006.14244 (2020).
Beam, A. L., Manrai, A. K. & Ghassemi, M. Challenges to the reproducibility of machine learning models in health care. J. Am. Med. Assoc. 323 , 305–306 (2020).
Gerke, S., Babic, B., Evgeniou, T. & Cohen, I. G. The need for a system view to regulate artificial intelligence/machine learning-based software as medical device. NPJ Digit. Med. 3 , 53 (2020).
Lee, C. S. & Lee, A. Y. Clinical applications of continual learning machine learning. Lancet Digit. Health 2 , e279–e281 (2020).
Food and Drug Administration. Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD): Discussion Paper and Request for Feedback (FDA, 2019).
Morley, J. et al. The debate on the ethics of AI in health care: a reconstruction and critical review. SSRN http://dx.doi.org/10.2139/ssrn.3486518 (2019.
Price, W. N., Gerke, S. & Cohen, I. G. Potential liability for physicians using artificial intelligence. J. Am. Med. Assoc. 322 , 1765–1766 (2019).
Larson, D. B., Magnus, D. C., Lungren, M. P., Shah, N. H. & Langlotz, C. P. Ethics of using and sharing clinical imaging data for artificial intelligence: a proposed framework. Radiology 295 , 675–682 (2020).
Kaissis, G. A., Makowski, M. R., Rückert, D. & Braren, R. F. Secure, privacy-preserving and federated machine learning in medical imaging. Nat. Mach. Intell. 2 , 305–311 (2020).
Larrazabal, A. J., Nieto, N., Peterson, V., Milone, D. H. & Ferrante, E. Gender imbalance in medical imaging datasets produces biased classifiers for computer-aided diagnosis. Proc. Natl Acad. Sci. USA 117 , 12592–12594 (2020).
Vyas, D. A., Eisenstein, L. G. & Jones, D. S. Hidden in plain sight: reconsidering the use of race correction in clinical algorithms. N. Engl. J. Med. 383 , 874–882 (2020).
Obermeyer, Z., Powers, B., Vogeli, C. & Mullainathan, S. Dissecting racial bias in an algorithm used to manage the health of populations. Science 366 , 447–453 (2019).
Cirillo, D. et al. Sex and gender differences and biases in artificial intelligence for biomedicine and healthcare. NPJ Digit. Med. 3 , 81 (2020).
Download references
Acknowledgements
We thank A. Tamkin and N. Phillips for their feedback. E.J.T. receives funding support from US National Institutes of Health grant UL1TR002550.
Author information
These authors contributed equally: Pranav Rajpurkar, Emma Chen, Oishi Banerjee.
Authors and Affiliations
Department of Biomedical Informatics, Harvard University, Cambridge, MA, USA
Pranav Rajpurkar
Department of Computer Science, Stanford University, Stanford, CA, USA
Emma Chen & Oishi Banerjee
Scripps Translational Science Institute, San Diego, CA, USA
Eric J. Topol
You can also search for this author in PubMed Google Scholar
Contributions
P.R. and E.J.T. conceptualized this Review. E.C., O.B. and P.R. were responsible for the design and synthesis of this Review. All authors contributed to writing and editing the manuscript.
Corresponding author
Correspondence to Eric J. Topol .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Medicine thanks Despina Kontos and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Karen O’Leary was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Rajpurkar, P., Chen, E., Banerjee, O. et al. AI in health and medicine. Nat Med 28 , 31–38 (2022). https://doi.org/10.1038/s41591-021-01614-0
Download citation
Received : 23 July 2021
Accepted : 05 November 2021
Published : 20 January 2022
Issue Date : January 2022
DOI : https://doi.org/10.1038/s41591-021-01614-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Development and validation of a machine learning model to predict time to renal replacement therapy in patients with chronic kidney disease.
- Takeshi Nakata
- Hirotaka Shibata
BMC Nephrology (2024)
Individualized estimation of arterial carbon dioxide partial pressure using machine learning in children receiving mechanical ventilation
- Bongjin Lee
- June Dong Park
BMC Pediatrics (2024)
“That’s just Future Medicine” - a qualitative study on users’ experiences of symptom checker apps
- Regina Müller
- Malte Klemmt
- Robert Ranisch
BMC Medical Ethics (2024)
Machine learning in physical activity, sedentary, and sleep behavior research
- Vahid Farrahi
- Mehrdad Rostami
Journal of Activity, Sedentary and Sleep Behaviors (2024)
Physician–machine partnerships boost diagnostic accuracy, but bias persists
Nature Medicine (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Future Healthc J
- v.8(2); 2021 Jul

Artificial intelligence in healthcare: transforming the practice of medicine
Junaid bajwa.
A Microsoft Research, Cambridge, UK
Usman Munir
B Microsoft Research, Cambridge, UK
Aditya Nori
C Microsoft Research, Cambridge, UK

Bryan Williams
D University College London, London, UK and director, NIHR UCLH Biomedical Research Centre, London, UK
Artificial intelligence (AI) is a powerful and disruptive area of computer science, with the potential to fundamentally transform the practice of medicine and the delivery of healthcare. In this review article, we outline recent breakthroughs in the application of AI in healthcare, describe a roadmap to building effective, reliable and safe AI systems, and discuss the possible future direction of AI augmented healthcare systems.
Introduction
Healthcare systems around the world face significant challenges in achieving the ‘quadruple aim’ for healthcare: improve population health, improve the patient's experience of care, enhance caregiver experience and reduce the rising cost of care. 1–3 Ageing populations, growing burden of chronic diseases and rising costs of healthcare globally are challenging governments, payers, regulators and providers to innovate and transform models of healthcare delivery. Moreover, against a backdrop now catalysed by the global pandemic, healthcare systems find themselves challenged to ‘perform’ (deliver effective, high-quality care) and ‘transform’ care at scale by leveraging real-world data driven insights directly into patient care. The pandemic has also highlighted the shortages in healthcare workforce and inequities in the access to care, previously articulated by The King's Fund and the World Health Organization (Box (Box1 1 ). 4,5
Workforce challenges in the next decade
The application of technology and artificial intelligence (AI) in healthcare has the potential to address some of these supply-and-demand challenges. The increasing availability of multi-modal data (genomics, economic, demographic, clinical and phenotypic) coupled with technology innovations in mobile, internet of things (IoT), computing power and data security herald a moment of convergence between healthcare and technology to fundamentally transform models of healthcare delivery through AI-augmented healthcare systems.
In particular, cloud computing is enabling the transition of effective and safe AI systems into mainstream healthcare delivery. Cloud computing is providing the computing capacity for the analysis of considerably large amounts of data, at higher speeds and lower costs compared with historic ‘on premises’ infrastructure of healthcare organisations. Indeed, we observe that many technology providers are increasingly seeking to partner with healthcare organisations to drive AI-driven medical innovation enabled by cloud computing and technology-related transformation (Box (Box2 2 ). 6–8
Quotes from technology leaders
Here, we summarise recent breakthroughs in the application of AI in healthcare, describe a roadmap to building effective AI systems and discuss the possible future direction of AI augmented healthcare systems.
What is artificial intelligence?
Simply put, AI refers to the science and engineering of making intelligent machines, through algorithms or a set of rules, which the machine follows to mimic human cognitive functions, such as learning and problem solving. 9 AI systems have the potential to anticipate problems or deal with issues as they come up and, as such, operate in an intentional, intelligent and adaptive manner. 10 AI's strength is in its ability to learn and recognise patterns and relationships from large multidimensional and multimodal datasets; for example, AI systems could translate a patient's entire medical record into a single number that represents a likely diagnosis. 11,12 Moreover, AI systems are dynamic and autonomous, learning and adapting as more data become available. 13
AI is not one ubiquitous, universal technology, rather, it represents several subfields (such as machine learning and deep learning) that, individually or in combination, add intelligence to applications. Machine learning (ML) refers to the study of algorithms that allow computer programs to automatically improve through experience. 14 ML itself may be categorised as ‘supervised’, ‘unsupervised’ and ‘reinforcement learning’ (RL), and there is ongoing research in various sub-fields including ‘semi-supervised’, ‘self-supervised’ and ‘multi-instance’ ML.
- Supervised learning leverages labelled data (annotated information); for example, using labelled X-ray images of known tumours to detect tumours in new images. 15
- ‘Unsupervised learning’ attempts to extract information from data without labels; for example, categorising groups of patients with similar symptoms to identify a common cause. 16
- In RL, computational agents learn by trial and error, or by expert demonstration. The algorithm learns by developing a strategy to maximise rewards. Of note, major breakthroughs in AI in recent years have been based on RL.
- Deep learning (DL) is a class of algorithms that learns by using a large, many-layered collection of connected processes and exposing these processors to a vast set of examples. DL has emerged as the predominant method in AI today driving improvements in areas such as image and speech recognition. 17,18
How to build effective and trusted AI-augmented healthcare systems?
Despite more than a decade of significant focus, the use and adoption of AI in clinical practice remains limited, with many AI products for healthcare still at the design and develop stage. 19–22 While there are different ways to build AI systems for healthcare, far too often there are attempts to force square pegs into round holes ie find healthcare problems to apply AI solutions to without due consideration to local context (such as clinical workflows, user needs, trust, safety and ethical implications).
We hold the view that AI amplifies and augments, rather than replaces, human intelligence. Hence, when building AI systems in healthcare, it is key to not replace the important elements of the human interaction in medicine but to focus it, and improve the efficiency and effectiveness of that interaction. Moreover, AI innovations in healthcare will come through an in-depth, human-centred understanding of the complexity of patient journeys and care pathways.
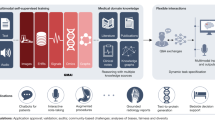
In Fig Fig1, 1 , we describe a problem-driven, human-centred approach, adapted from frameworks by Wiens et al , Care and Sendak to building effective and reliable AI-augmented healthcare systems. 23–25

Multi-step, iterative approach to build effective and reliable AI-augmented systems in healthcare.
Design and develop
The first stage is to design and develop AI solutions for the right problems using a human-centred AI and experimentation approach and engaging appropriate stakeholders, especially the healthcare users themselves.
Stakeholder engagement and co-creation
Build a multidisciplinary team including computer and social scientists, operational and research leadership, and clinical stakeholders (physician, caregivers and patients) and subject experts (eg for biomedical scientists) that would include authorisers, motivators, financiers, conveners, connectors, implementers and champions. 26 A multi-stakeholder team brings the technical, strategic, operational expertise to define problems, goals, success metrics and intermediate milestones.
Human-centred AI
A human-centred AI approach combines an ethnographic understanding of health systems, with AI. Through user-designed research, first understand the key problems (we suggest using a qualitative study design to understand ‘what is the problem’, ‘why is it a problem’, ‘to whom does it matter’, ‘why has it not been addressed before’ and ‘why is it not getting attention’) including the needs, constraints and workflows in healthcare organisations, and the facilitators and barriers to the integration of AI within the clinical context. After defining key problems, the next step is to identify which problems are appropriate for AI to solve, whether there is availability of applicable datasets to build and later evaluate AI. By contextualising algorithms in an existing workflow, AI systems would operate within existing norms and practices to ensure adoption, providing appropriate solutions to existing problems for the end user.
Experimentation
The focus should be on piloting of new stepwise experiments to build AI tools, using tight feedback loops from stakeholders to facilitate rapid experiential learning and incremental changes. 27 The experiments would allow the trying out of new ideas simultaneously, exploring to see which one works, learn what works and what doesn't, and why. 28 Experimentation and feedback will help to elucidate the purpose and intended uses for the AI system: the likely end users and the potential harm and ethical implications of AI system to them (for instance, data privacy, security, equity and safety).
Evaluate and validate
Next, we must iteratively evaluate and validate the predictions made by the AI tool to test how well it is functioning. This is critical, and evaluation is based on three dimensions: statistical validity, clinical utility and economic utility.
- Statistical validity is understanding the performance of AI on metrics of accuracy, reliability, robustness, stability and calibration. High model performance on retrospective, in silico settings is not sufficient to demonstrate clinical utility or impact.
- To determine clinical utility, evaluate the algorithm in a real-time environment on a hold-out and temporal validation set (eg longitudinal and external geographic datasets) to demonstrate clinical effectiveness and generalisability. 25
- Economic utility quantifies the net benefit relative to the cost from the investment in the AI system.
Scale and diffuse
Many AI systems are initially designed to solve a problem at one healthcare system based on the patient population specific to that location and context. Scale up of AI systems requires special attention to deployment modalities, model updates, the regulatory system, variation between systems and reimbursement environment.
Monitor and maintain
Even after an AI system has been deployed clinically, it must be continually monitored and maintained to monitor for risks and adverse events using effective post-market surveillance. Healthcare organisations, regulatory bodies and AI developers should cooperate to collate and analyse the relevant datasets for AI performance, clinical and safety-related risks, and adverse events. 29
What are the current and future use cases of AI in healthcare?
AI can enable healthcare systems to achieve their ‘quadruple aim’ by democratising and standardising a future of connected and AI augmented care, precision diagnostics, precision therapeutics and, ultimately, precision medicine (Table (Table1 1 ). 30 Research in the application of AI healthcare continues to accelerate rapidly, with potential use cases being demonstrated across the healthcare sector (both physical and mental health) including drug discovery, virtual clinical consultation, disease diagnosis, prognosis, medication management and health monitoring.
Widescale adoption and application of artificial intelligence in healthcare
Timings are illustrative to widescale adoption of the proposed innovation taking into account challenges / regulatory environment / use at scale.
We describe a non-exhaustive suite of AI applications in healthcare in the near term, medium term and longer term, for the potential capabilities of AI to augment, automate and transform medicine.
AI today (and in the near future)
Currently, AI systems are not reasoning engines ie cannot reason the same way as human physicians, who can draw upon ‘common sense’ or ‘clinical intuition and experience’. 12 Instead, AI resembles a signal translator, translating patterns from datasets. AI systems today are beginning to be adopted by healthcare organisations to automate time consuming, high volume repetitive tasks. Moreover, there is considerable progress in demonstrating the use of AI in precision diagnostics (eg diabetic retinopathy and radiotherapy planning).
AI in the medium term (the next 5–10 years)
In the medium term, we propose that there will be significant progress in the development of powerful algorithms that are efficient (eg require less data to train), able to use unlabelled data, and can combine disparate structured and unstructured data including imaging, electronic health data, multi-omic, behavioural and pharmacological data. In addition, healthcare organisations and medical practices will evolve from being adopters of AI platforms, to becoming co-innovators with technology partners in the development of novel AI systems for precision therapeutics.
AI in the long term (>10 years)
In the long term, AI systems will become more intelligent , enabling AI healthcare systems achieve a state of precision medicine through AI-augmented healthcare and connected care. Healthcare will shift from the traditional one-size-fits-all form of medicine to a preventative, personalised, data-driven disease management model that achieves improved patient outcomes (improved patient and clinical experiences of care) in a more cost-effective delivery system.
Connected/augmented care
AI could significantly reduce inefficiency in healthcare, improve patient flow and experience, and enhance caregiver experience and patient safety through the care pathway; for example, AI could be applied to the remote monitoring of patients (eg intelligent telehealth through wearables/sensors) to identify and provide timely care of patients at risk of deterioration.
In the long term, we expect that healthcare clinics, hospitals, social care services, patients and caregivers to be all connected to a single, interoperable digital infrastructure using passive sensors in combination with ambient intelligence. 31 Following are two AI applications in connected care.
Virtual assistants and AI chatbots
AI chatbots (such as those used in Babylon ( www.babylonhealth.com ) and Ada ( https://ada.com )) are being used by patients to identify symptoms and recommend further actions in community and primary care settings. AI chatbots can be integrated with wearable devices such as smartwatches to provide insights to both patients and caregivers in improving their behaviour, sleep and general wellness.
Ambient and intelligent care
We also note the emergence of ambient sensing without the need for any peripherals.
- Emerald ( www.emeraldinno.com ): a wireless, touchless sensor and machine learning platform for remote monitoring of sleep, breathing and behaviour, founded by Massachusetts Institute of Technology faculty and researchers.
- Google nest: claiming to monitor sleep (including sleep disturbances like cough) using motion and sound sensors. 32
- A recently published article exploring the ability to use smart speakers to contactlessly monitor heart rhythms. 33
- Automation and ambient clinical intelligence: AI systems leveraging natural language processing (NLP) technology have the potential to automate administrative tasks such as documenting patient visits in electronic health records, optimising clinical workflow and enabling clinicians to focus more time on caring for patients (eg Nuance Dragon Ambient eXperience ( www.nuance.com/healthcare/ambient-clinical-intelligence.html )).
Precision diagnostics
Diagnostic imaging.
The automated classification of medical images is the leading AI application today. A recent review of AI/ML-based medical devices approved in the USA and Europe from 2015–2020 found that more than half (129 (58%) devices in the USA and 126 (53%) devices in Europe) were approved or CE marked for radiological use. 34 Studies have demonstrated AI's ability to meet or exceed the performance of human experts in image-based diagnoses from several medical specialties including pneumonia in radiology (a convolutional neural network trained with labelled frontal chest X-ray images outperformed radiologists in detecting pneumonia), dermatology (a convolutional neural network was trained with clinical images and was found to classify skin lesions accurately), pathology (one study trained AI algorithms with whole-slide pathology images to detect lymph node metastases of breast cancer and compared the results with those of pathologists) and cardiology (a deep learning algorithm diagnosed heart attack with a performance comparable with that of cardiologists). 35–38
We recognise that there are some exemplars in this area in the NHS (eg University of Leeds Virtual Pathology Project and the National Pathology Imaging Co-operative) and expect widescale adoption and scaleup of AI-based diagnostic imaging in the medium term. 39 We provide two use cases of such technologies.
Diabetic retinopathy screening
Key to reducing preventable, diabetes-related vision loss worldwide is screening individuals for detection and the prompt treatment of diabetic retinopathy. However, screening is costly given the substantial number of diabetes patients and limited manpower for eye care worldwide. 40 Research studies on automated AI algorithms for diabetic retinopathy in the USA, Singapore, Thailand and India have demonstrated robust diagnostic performance and cost effectiveness. 41–44 Moreover, Centers for Medicare & Medicaid Services approved Medicare reimbursement for the use of Food and Drug Administration approved AI algorithm ‘IDx-DR’, which demonstrated 87% sensitivity and 90% specificity for detecting more-than-mild diabetic retinopathy. 45
Improving the precision and reducing waiting timings for radiotherapy planning
An important AI application is to assist clinicians for image preparation and planning tasks for radiotherapy cancer treatment. Currently, segmentation of the images is time consuming and laborious task, performed manually by an oncologist using specially designed software to draw contours around the regions of interest. The AI-based InnerEye open-source technology can cut this preparation time for head and neck, and prostate cancer by up to 90%, meaning that waiting times for starting potentially life-saving radiotherapy treatment can be dramatically reduced (Fig (Fig2 2 ). 46,47

Potential applications for the InnerEye deep learning toolkit include quantitative radiology for monitoring tumour progression, planning for surgery and radiotherapy planning. 47
Precision therapeutics
To make progress towards precision therapeutics, we need to considerably improve our understanding of disease. Researchers globally are exploring the cellular and molecular basis of disease, collecting a range of multimodal datasets that can lead to digital and biological biomarkers for diagnosis, severity and progression. Two important future AI applications include immunomics / synthetic biology and drug discovery.
Immunomics and synthetic biology
Through the application of AI tools on multimodal datasets in the future, we may be able to better understand the cellular basis of disease and the clustering of diseases and patient populations to provide more targeted preventive strategies, for example, using immunomics to diagnose and better predict care and treatment options. This will be revolutionary for multiple standards of care, with particular impact in the cancer, neurological and rare disease space, personalising the experience of care for the individual.
AI-driven drug discovery
AI will drive significant improvement in clinical trial design and optimisation of drug manufacturing processes, and, in general, any combinatorial optimisation process in healthcare could be replaced by AI. We have already seen the beginnings of this with the recent announcements by DeepMind and AlphaFold, which now sets the stage for better understanding disease processes, predicting protein structures and developing more targeted therapeutics (for both rare and more common diseases; Fig Fig3 3 ). 48,49

An overview of the main neural network model architecture for AlphaFold. 49 MSA = multiple sequence alignment.
Precision medicine
New curative therapies.
Over the past decade, synthetic biology has produced developments like CRISPR gene editing and some personalised cancer therapies. However, the life cycle for developing such advanced therapies is still extremely inefficient and expensive.
In future, with better access to data (genomic, proteomic, glycomic, metabolomic and bioinformatic), AI will allow us to handle far more systematic complexity and, in turn, help us transform the way we understand, discover and affect biology. This will improve the efficiency of the drug discovery process by helping better predict early which agents are more likely to be effective and also better anticipate adverse drug effects, which have often thwarted the further development of otherwise effective drugs at a costly late stage in the development process. This, in turn will democratise access to novel advanced therapies at a lower cost.
AI empowered healthcare professionals
In the longer term, healthcare professionals will leverage AI in augmenting the care they provide, allowing them to provide safer, standardised and more effective care at the top of their licence; for example, clinicians could use an ‘AI digital consult’ to examine ‘digital twin’ models of their patients (a truly ‘digital and biomedical’ version of a patient), allowing them to ‘test’ the effectiveness, safety and experience of an intervention (such as a cancer drug) in the digital environment prior to delivering the intervention to the patient in the real world.
We recognise that there are significant challenges related to the wider adoption and deployment of AI into healthcare systems. These challenges include, but are not limited to, data quality and access, technical infrastructure, organisational capacity, and ethical and responsible practices in addition to aspects related to safety and regulation. Some of these issues have been covered, but others go beyond the scope of this current article.
Conclusion and key recommendations
Advances in AI have the potential to transform many aspects of healthcare, enabling a future that is more personalised, precise, predictive and portable. It is unclear if we will see an incremental adoption of new technologies or radical adoption of these technological innovations, but the impact of such technologies and the digital renaissance they bring requires health systems to consider how best they will adapt to the changing landscape. For the NHS, the application of such technologies truly has the potential to release time for care back to healthcare professionals, enabling them to focus on what matters to their patients and, in the future, leveraging a globally democratised set of data assets comprising the ‘highest levels of human knowledge’ to ‘work at the limits of science’ to deliver a common high standard of care, wherever and whenever it is delivered, and by whoever. 50 Globally, AI could become a key tool for improving health equity around the world.
As much as the last 10 years have been about the roll out of digitisation of health records for the purposes of efficiency (and in some healthcare systems, billing/reimbursement), the next 10 years will be about the insight and value society can gain from these digital assets, and how these can be translated into driving better clinical outcomes with the assistance of AI, and the subsequent creation of novel data assets and tools. It is clear that we are at an turning point as it relates to the convergence of the practice of medicine and the application of technology, and although there are multiple opportunities, there are formidable challenges that need to be overcome as it relates to the real world and the scale of implementation of such innovation. A key to delivering this vision will be an expansion of translational research in the field of healthcare applications of artificial intelligence. Alongside this, we need investment into the upskilling of a healthcare workforce and future leaders that are digitally enabled, and to understand and embrace, rather than being intimidated by, the potential of an AI-augmented healthcare system.
Healthcare leaders should consider (as a minimum) these issues when planning to leverage AI for health:
- processes for ethical and responsible access to data: healthcare data is highly sensitive, inconsistent, siloed and not optimised for the purposes of machine learning development, evaluation, implementation and adoption
- access to domain expertise / prior knowledge to make sense and create some of the rules which need to be applied to the datasets (to generate the necessary insight)
- access to sufficient computing power to generate decisions in real time, which is being transformed exponentially with the advent of cloud computing
- research into implementation: critically, we must consider, explore and research issues which arise when you take the algorithm and put it in the real world, building ‘trusted’ AI algorithms embedded into appropriate workflows.

IMAGES
VIDEO
COMMENTS
The New England Journal of Medicine (NEJM) is a weekly general medical journal that publishes new medical research and review articles, and editorial opinion on a wide variety of topics of ...
Artificial intelligence (AI) is poised to broadly reshape medicine, potentially improving the experiences of both clinicians and patients. We discuss key findings from a 2-year weekly effort to ...
Introduction. Healthcare systems around the world face significant challenges in achieving the ‘quadruple aim’ for healthcare: improve population health, improve the patient's experience of care, enhance caregiver experience and reduce the rising cost of care. 1–3 Ageing populations, growing burden of chronic diseases and rising costs of healthcare globally are challenging governments ...
Donabedian [. 1. ] includes it as an outcome of healthcare services; hence, it is. of utmost importance to evaluate care quality. Several authors argue that satisfaction. and the result in terms ...