Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Open access
- Published: 10 March 2021

The chromosome-level genome of dragon fruit reveals whole-genome duplication and chromosomal co-localization of betacyanin biosynthetic genes
- Jinfang Zheng 1 ,
- Lyndel W. Meinhardt 2 ,
- Ricardo Goenaga 3 ,
- Dapeng Zhang 2 &
- Yanbin Yin ORCID: orcid.org/0000-0001-7667-881X 1
Horticulture Research volume 8 , Article number: 63 ( 2021 ) Cite this article
8807 Accesses
23 Citations
22 Altmetric
Metrics details
- Comparative genomics
- Genome duplication
- Genome evolution
Dragon fruits are tropical fruits economically important for agricultural industries. As members of the family of Cactaceae , they have evolved to adapt to the arid environment. Here we report the draft genome of Hylocereus undatus , commercially known as the white-fleshed dragon fruit. The chromosomal level genome assembly contains 11 longest scaffolds corresponding to the 11 chromosomes of H. undatus . Genome annotation of H. undatus found ~29,000 protein-coding genes, similar to Carnegiea gigantea (saguaro). Whole-genome duplication (WGD) analysis revealed a WGD event in the last common ancestor of Cactaceae followed by extensive genome rearrangements. The divergence time between H. undatus and C. gigantea was estimated to be 9.18 MYA. Functional enrichment analysis of orthologous gene clusters (OGCs) in six Cactaceae plants found significantly enriched OGCs in drought resistance. Fruit flavor-related functions were overrepresented in OGCs that are significantly expanded in H. undatus . The H. undatus draft genome also enabled the discovery of carbohydrate and plant cell wall-related functional enrichment in dragon fruits treated with trypsin for a longer storage time. Lastly, genes of the betacyanin (a red-violet pigment and antioxidant with a very high concentration in dragon fruits) biosynthetic pathway were found to be co-localized on a 12 Mb region of one chromosome. The consequence may be a higher efficiency of betacyanin biosynthesis, which will need experimental validation in the future. The H. undatus draft genome will be a great resource to study various cactus plants.
Similar content being viewed by others
The genome sequence of star fruit (Averrhoa carambola)
Shasha Wu, Wei Sun, … Zhong-Jian Liu

Chromosome-scale genome assembly provides insights into the evolution and flavor synthesis of passion fruit (Passiflora edulis Sims)
Zhiqiang Xia, Dongmei Huang, … Shun Song

Combined genomic, transcriptomic, and metabolomic analyses provide insights into chayote (Sechium edule) evolution and fruit development
Anzhen Fu, Qing Wang, … Jinhua Zuo
Introduction
Dragon fruits (pitahaya or pitaya) are a group of Hylocereus species in the Cactaceae family. The genus Hylocereus was classified into 19 species based on morphological characteristics, and all of these species are indigenous to tropical America 1 . Among them, Hylocereus undatus (commonly known as the white-fleshed pitaya) is the most commonly cultivated species as a fruit crop 2 . The precise origin of H. undatus is not clear, but it is generally considered a native to Southern Mexico and Central America 3 . Today, this species has been commercially grown throughout tropical and subtropical regions of the world, particularly in Southeast Asia and Southern Mexico 4 , 5 .
In recent years, dragon fruits (including H. undatus and the red-fleshed H. polyrhizus ) have been accepted as important fruit crops for their rich nutrient content 6 , 7 , including high amounts of antioxidants (e.g., betacyanin and other phenolics), dietary fibers, prebiotics, vitamins, and minerals (e.g., calcium and potassium). These nutrients have numerous benefits to human health, which may also explain the increased consumption of dragon fruit for health purposes 6 , 7 .
In Asia, dragon fruits are the fifth most popular tropical fruits, and the global production of H. undatus has been increasing. In Vietnam alone, there are 40,000 hectares (Ha) of land dedicated for dragon fruit production, which is worth ~$895 million annually 8 . In addition to being consumed as a fruit, H. undatus is also known as an ornamental plant owing to its exotic appearance and night flowering 7 . Also, its fruit rind or peel has a high content of betacyanins (one type of betalains), and therefore H. undatus has been used to produce coloring dyes and additives in the food and cosmetic industries 6 , 7 .
In addition to their significance in agricultural industries, as members of the family of Cactaceae dragon fruits are also important for the study of cactus plants in general. For example, species of Cactaceae are well known to be highly drought resistant. To adapt to an arid environment, dragon fruits and other cacti have developed fascinating structures, including succulent stems, acicular leaves, and watery fruits. In fact, the family Cactaceae arose ~28.8 (median with a range 15–45) millions of years ago (Mya) 9 , 10 , when the Earth underwent a drop in atmospheric CO 2 concentration and a global expansion of aridity. As a result, plants in this family have gone through rapid genome evolution (e.g., whole-genome duplication and gene family expansion/contraction 11 , 12 ) and species diversification, as well as drastic changes at the phenotypic level 13 , 14 , which leads to a conflict between molecular phylogeny and classification based on morphological characters 15 , 16 . Recently, five cacti genomes have been sequenced to study homoplasy among different cacti and its impact on molecular phylogenies 17 . Obviously, more genomes sequenced in Cactaceae will certainly further improve our understanding of the cacti evolution and adaptation to dry and hot climates.
Due to their economic importance, an increasing number of research papers have been published in recent years to study dragon fruits from the food science perspective 18 , 19 , 20 , 21 , 22 and using RNA sequencing (RNA-seq) technology. For the latter, RNA-seq of Hylocereus species has been used to study abiotic stress response 23 , 24 , 25 , betalain synthetic pathway 26 , 27 , disease-resistant genes 28 , and antioxidant resistance during storage 29 , 30 . Due to the lack of a reference genome, all of these studies are based on mapping RNA-seq reads to de novo assembled transcripts instead of mapping to a reference genome.
In this study, we provide a high-quality genome assembly of H. undatus at the chromosome level. This highly continuous draft genome has allowed us to (i) study the whole-genome duplication of dragon fruits, (ii) date the divergence of H. undatus from other cacti, (iii) identify gene ontology functional groups that distinguish cacti from non-cacti plants, and distinguish H. undatus from other cacti, (iv) better understand the genomic adaptation of H. undatus to drought resistance, (v) study differentially expressed genes in trypsin-treated dragon fruit during storage by mapping RNA-seq reads to the H. undatus draft genome, and lastly, (vi) identify key enzymes that are involved in the synthesis of betacyanin, a red-violet pigment and antioxidant with a very high concentration in dragon fruits.
The draft genome of H. undatus contains 11 long scaffolds at the chromosomal level
The genome of the H. undatus cultivar “David Bowie” (from Puerto Rico, Fig. 1A ) was sequenced using a combination of 10X chromium sequencing, Chicago and Hi-C chromatin proximity ligation from Dovetail Genomics (see “Methods“ for details). The initial 10X raw read assembly had a contig N50 = 31 kb, and scaffold N50 = 769 kb. This assembly was significantly improved with Chicago and Hi-C data for scaffolding using the HiRise pipeline, which produced a much-improved assembly with a scaffold N50 = 109.7 Mb and assembly size = 1.33 Gb. Previous studies have indicated that the chromosome number of H. undatus is 2 n = 22 31 , which has been further confirmed by flow cytometry 32 , 33 , 34 . Furthermore, the genome size of H. undatus was estimated to be 1.44 Gb 33 with DNA 2C content = 3.63, using Arabidopsis as a reference (DNA 2C content = 0.32) 35 . Therefore, it is estimated that over 92.4% (1.33/1.44) of the H. undatus genome was assembled into 33,691 scaffolds. Interestingly, 88.7% of the assembled genome were present in the 11 longest scaffolds (Fig. 1B ), corresponding to the 11 chromosomes reported in dragon fruit 32 . This suggests that our H. undatus genome assembly is close to the chromosomal level.
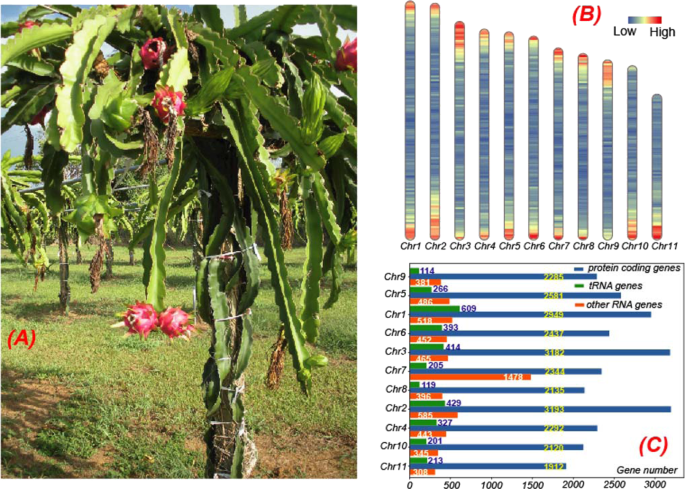
Protein-coding genes, noncoding RNA gene, and tRNA genes resided in these scaffolds account for 87.8%, 72.6%, and 58.0% of all these genes, respectively. A A photo of the whole plant of Hylocereus undatus cultivar “David Bowie” from the USDA-ARS Tropical Agriculture Research Station in Mayaquez, Puerto Rico. B Protein-coding gene density of dragon fruit in the 11 longest scaffolds/pseudochromosomes with a window size 100,000 bp, which is plotted by Rldeogram 111 . C Distribution of protein-coding genes (blue), noncoding RNA genes (including rRNAs, orange), and tRNA genes (green) on the 11 longest scaffolds. The Chr7 (Scaffold 33675) has the most (1478) noncoding RNAs, including 1125 5S rRNAs. The mapping of scaffolds and pseudochromosomes is as follows: Chr1:Scaffold 33678, Chr2:Scaffold 19641, Chr3:Scaffold 33676, Chr4:Scaffold 10417, Chr5:Scaffold 33679, Chr6:Scaffold 33677, Chr7:Scaffold 33675, Chr8:Scaffold 33673, Chr9:Scaffold 33680, Chr10:Scaffold 3410, Chr11:Scaffold 2055
The completeness of the draft genome was further evaluated by BUSCO (Benchmarking Universal Single-Copy Orthologs) 36 . Of the 2121 single-copy orthologous genes in the BUSCO eudicotyledons_odb10 database, 1972 (93.0%) were identified in our draft genome (Supplementary Table S1 ). The distribution of genes (see below) in assembled scaffolds showed that almost all of these genes resided in the 11 longest scaffolds (Fig. 1C ). A total of 89.1% RNA-seq reads, obtained from NCBI (SRR3234546 37 , sampled from the shoot of H. polyrhizus cultivar Zihonglong), were mapped to the draft genome. All these evaluations suggested high completeness and high continuity of the dragon fruit draft genome at the chromosome level.
Genome annotation of H. undatus found ~29,000 protein-coding genes close to the number in Carnegiea gigantea (saguaro)
Repeat regions in the genome were annotated using a combination of ab initio and homology search methods (see “Methods”). As the result, 58.89% of the genome were identified as repeat regions (Supplementary Table S2 ). Long terminal repeat (LTR) elements were the largest category of classified/annotated repeats (22.47% of the genome). Most of the repeat regions (28.58%) were unclassified (Supplementary Table S2 ). For noncoding gene prediction, in total, 5637 tRNAs, 2364 rRNAs, and 5698 other noncoding RNAs were predicted in the dragon fruit draft genome (Fig. 1C ).
For protein-coding gene prediction, MAKER 38 was run with three iterations using RNA-seq and protein homology data as supporting evidence (see “Methods”). In total, 28,992 genes (length > 50 aa) were predicted (Table 1 ). Of the 28,992 proteins, 21,655 (74.7%) were annotated by eggNOG 39 , of which 10,093 were assigned to GO terms and 9920 were assigned to KEGG pathways.
In order to conduct a comparative genomic analysis, MAKER was also run to predict protein-coding genes in draft genomes of Stenocereus thurberi, Lophocereus schottii, Pachycereus pringlei , and Pereskia humboldtii , which were previously sequenced in ref. 17 . The same previous paper also sequenced Carnegiea gigantea with a better genome assembly and the author has kindly provided us the predicted protein sequences, so MAKER prediction was not needed for this species. The statistics of protein-coding genes in the six cactus species (including H. undatus ) are provided in Table 1 . Notably, for L. schottii and S. thurberi , MAKER predicted 94,756 and 93,917 protein-coding genes, respectively. These numbers were three to four times larger than those of other cactus species, implicating possible whole-genome duplications (WGDs) in these two species (see more details below).
Whole-genome duplication analysis predicts a WGD event in the last common ancestor of Cactaceae followed by extensive genome rearrangements in H. undatus
The large variation in the gene contents of the six cactus species (Table 1 ) demanded a whole-genome duplication (WGD) analysis. The paralogous and orthologous genes were identified by using a reciprocal best hit (RBH) approach with BlastP. The synonymous substitution rates (Ks) and the rate of transversions on fourfold degenerate synonymous sites (4dTv) of global paralogous genes within each species were calculated to infer potential WGD events. Similarly, the Ks and 4dTv rates of global orthologous genes between different species were also calculated. In addition to the six genomes of the family Cactaceae , we have also collected five sequenced genomes representing other families within the order of Caryophyllales : Beta vulgaris (Bv) 40 and Spinacia oleracea (So) 41 of Chenopodiaceae , Aldrovanda vesiculosa (Av) of Droseraceae 42 , Simmondsia chinensis (Sc) of Simmondsiaceae 43 , and Fagopyrum tataricum (Ft) of Polygonaceae 44 .
Using 507 single-copy genes identified in the 12 genomes (six Cactaceae + five other Caryophyllales + Arabidopsis thaliana ), a phylogeny (Fig. 2A ) was built to depict the phylogenetic relationship among these species. According to this phylogeny genomes of Cactaceae are closer to Chenopodiaceae , then to Simmondsiaceae , and lastly to Droseraceae and Polygonaceae . This is also supported by the 4dTv (Fig. 2B ) and the Ks (Supplementary Fig. S1A ) distributions of orthologous genes between Hund and the five Caryophyllales genomes.
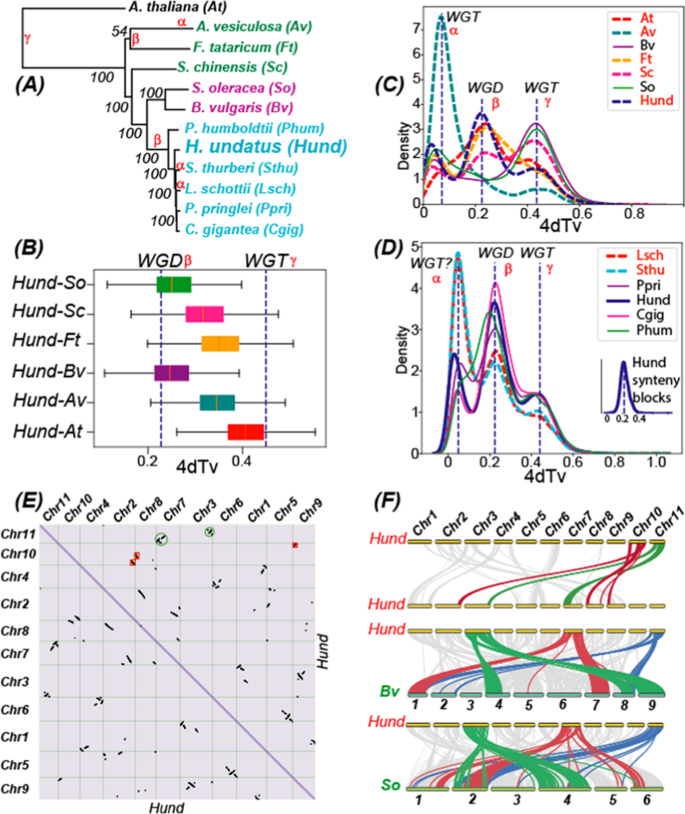
The full names and acronyms of the 11 plants are: Stenocereus thurberi (Sthu), Lophocereus schottii (Lsch), Carnegiea gigantea (Cgig), Pachycereus pringlei (Ppri), and Pereskia humboldtii (Phum) of Cactaceae, Beta vulgaris (Bv) 40 , and Spinacia oleracea (So) 41 of Chenopodiaceae, Aldrovanda vesiculosa (Av) of Droseraceae 42 , Simmondsia chinensis (Sc) of Simmondsiaceae 43 , and Fagopyrum tataricum (Ft) of Polygonaceae 44 , Arabidopsis thaliana . A A phylogeny is built with single-copy orthologous genes (identified by OrthoFinder) in the 12 genomes (concatenated alignment with MAFFT and tree built with RAxML). The WGD events characterized in ( C ) and ( D ) are indicated beside the branches. B Orthologous genes between Hund and other species were identified using the reciprocal best hit (RBH) approach, and fourfold degenerate synonymous sites (4dTv) values were calculated and plotted as boxplots. The dashed lines correspond to the WGD events characterized in ( C ). C Intragenome 4dTv distribution of paralogous genes (identified by RBH approach) in Hund and six other genomes. D Intragenome 4dTv distribution of paralogous genes (identified by RBH approach) in the six Cactaceae genomes. The inset figure shows the 4dTv distribution of only paralogous genes present in the synteny blocks (identified by MCScanX) of Hund. E Whole-genome self-alignment and syntenic blocks within the 11 largest Hund scaffolds/pseudochromosomes are presented as a dot plot. Strings of dots (paralogous genes) that correspond to duplicated regions in Chr11 (scaffold 2055) and Chr10 (scaffold 3410) are highlighted using the same circles or boxes in the same color. F A linear representation of the synteny analysis results is shown for intragenome Hund-Hund, as well as inter-genome Hund-Bv and Hund-So genome pairs. Synteny blocks of selected chromosomes are colored in red, green, and blue as examples. The chromosome sizes are drawn proportional to the actual genome size within each species but not between different species (total genome size: Hund (1.33 Gb), Bv (544 Mb), and So (969 Mb))
The intragenome 4dTv distributions (Fig. 2C ) of paralogous genes reveal three peaks in Hund. This is also true for two other Caryophyllales genomes (Ft and Sc) and the distant At. In contrast, Bv and So have two peaks. However, all the 12 genomes have the last peak (γ), which corresponds to the ancient whole-genome triplication (WGT) shared by all eudicot plants. The middle peak (β) is found in all species except for Bv and So, while its exact position differs slightly among different species. While this β peak is the largest peak in Hund, At, and Ft, it is very small and almost neglectable in Av. Remarkably, Av also distinguishes itself from other species with a very large first peak (α), which has been reported to be a very recent WGT event unique in Av 42 . In all other species, this α peak is much smaller and has very small 4dTv values, suggesting it might not represent a WGD but rather a smaller scale recent duplications such as tandem or dispersed duplications. The reason is that more recent WGD tends to result in larger peaks (e.g., the WGT in Av creates the largest α peak), as more recently duplicated genes have not degenerated as much as more anciently duplicated genes. We also provided the Ks distributions of the paralogous genes in Supplementary Fig. S1A , where the separation of the α and β peaks is not very evident in most species.
Further 4dTv analysis of the other five Cactaceae genomes (Fig. 2D ), despite their low N50 assemblies, found that they all share the β peak and the γ peak with Hund. The positions of the two peaks are also fairly consistent in these six genomes. In contrast, the α peak differs significantly: it is the largest in Lsch and Sthu, very small in Phum (almost neglectable), and medium in Ppri, Hund, and Cgig. Considering that the gene numbers in Lsch and Sthu are at least three times larger than the other four genomes (Table 1 ), one can speculate that this α peak might correspond to a more recent WGT event that had only happened in Lsch and Sthu. However, this speculation needs a more vigorous study once the genome assemblies of the two species are much-improved. The current very fragmented assemblies of two genomes hinder the verification of the putative WGT in these genomes via a syntenic block analysis.
According to Fig. 2B , the β peak (first dashed line) has a lower 4dTv value than the Hund-Bv and Hund-So 4dTv values (box plot), suggesting that the WGD event happened after the divergence of Cactaceae from other Caryophyllales families. Similarly, the γ peak (second dashed line) has a higher 4dTv value than the Hund-At values (box plot), confirming that the ancient WGT event happened before the emergence of the common ancestor of Hund and At, i.e., at the base of all dicot plants. To approximately estimate the absolute dates of the three WGD events, we used the formula: T = Ks/2γ , where γ = 1.5 × 10 −8 is the rate of synonymous substitutions per site per year for dicots following 45 . From the Ks distributions of the paralogous genes in the six Cactaceae (Supplementary Fig. S2B ), we estimated that the α peak (WGT in Lsch and Sthu) corresponds to 6.0 MYA, the β peak (WGD in the last common ancestor of Cactaceae ) corresponds to 25.7 MYA, and the γ peak (the ancient core dicot WGT) corresponds to 135.0 MYA.
With the chromosome-level assembly of Hund, we have also performed a whole-genome self-alignment (Fig. 2E ), an intragenome syntenic block analysis (Fig. 2F ), as well as an inter-genome syntenic block analysis (Fig. 2F ) of Hund vs. the previously published chromosome-level assemblies of Bv 40 and So 41 . These results show that dragon fruit had experienced a WGD event followed by extensive genome rearrangements (e.g., chromosome breakage and reorganization) (Fig. 2E ). For example, Chr11 (Scaffold 2055) shares large syntenic gene blocks with Chr8 (Scaffold 33675) and Chr3 (Scaffold 33676), while Chr10 (Scaffold 3410) shares large syntenic gene blocks with three chromosomes: Chr2, Chr8, and Chr9. It also appears that the genomic synteny is fairly conserved between Cactaceae and Chenopodiaceae genomes according to the inter-genome Hund-Bv and Hund-So synteny alignments (Fig. 2F ). The inter-genome alignments of Hund-Bv and Hund-So were also made (Supplementary Fig. S8A, B ). These alignments show that there is a clear two-to-one relationship between Hund and the other two genomes with respect to the aligned syntenic blocks, which supports the lack of the β WGD peak in Bv and So (Fig. 2C ).
Notably, Bv and So are among the earliest sequenced Caryophyllales genomes and both have high-quality chromosome-level assemblies. The chromosome-level assembly of Bv has been achieved by genetic and physical mapping as well as classic BAC/Fosmid clones and long-read Sanger/454 sequencing 40 . The chromosome-level assembly of So has been supported by BioNano optical mapping data and long-range mate-pair sequencing libraries 41 . Therefore, the inter-genome synteny alignments shown in Fig. 2F also suggest that our chromosome-level assembly of Hund genome is of high quality. In contrast, we were not able to perform the intragenome and inter-genome syntenic block analyses for the other five cacti because their genome assemblies have much lower continuity (low N50 and too many contigs) (Table 1 ).
To study which of the three 4dTv peaks in Fig. 2D correspond to the Hund intragenome syntenic blocks plotted in Fig. 2 E, F , we have extracted the paralogous genes located in the syntenic blocks only and plotted their 4dTv distribution. The inset plot of Fig. 2F clearly shows that these Hund syntenic blocks correspond to the β peak in Fig. 2D . This again supports that the small α peak in Hund is not derived from a WGD; otherwise, the 4dTv distribution of Hund syntenic blocks will have a peak corresponding to the α peak. Furthermore, the Ks distributions of the six Cactaceae genomes (Supplementary Fig. S2B ) also reveal that the α peak is only evident in Hund, Lsch, and Sthu, and it is much smaller than the β peak in Hund. Overall, all the evidence suggests that unlike Lsch and Sthu, Hund does not have a very recent WGD after its divergence from other Cactaceae species.
To further study this small α peak of Hund in Fig. 2D , we have extracted 2,250 Hund genes with 4dTv < = 0.116 (the entire α peak) and performed Pfam (Supplementary Table S3 ) and GO (Supplementary Table S4 ) annotation on them. Interestingly, 632 (28%) of these 2250 genes are located within 10 genes from each other, indicating that these genes were derived from tandem duplications. The Pfam annotation showed that the most abundant protein families include plant transposases, protein kinases, plant disease resistance proteins (leucine-rich repeat), cytochrome P450, and 2OG-Fe(II) oxygenases (Supplementary Table S3 ). All these protein families might have been selected in Hund to have tandem gene duplications as a genomic adaptation to the hot and dry environments.
The last common ancestor of H. undatus and C. gigantea is estimated to appear 9.18 MYA
To examine the evolutionary relationship within the six cactus plants and between cactus plants and other plants, we have included ten additional plant genomes in our analyses. These include three C3 plants ( Oryza sativa 46 , Arabidopsis thaliana 47 , and Cannabis sativa 48 ), three C4 plants ( Zea mays 49 , Sorghum bicolor 50 , and Setaria italica 51 ), and four CAM (crassulacean acid metabolism) plants ( Phalaenopsis equestris 52 , Ananas comosus 53 , Kalanchoe fedtschenkoi 54 , and Sedum album 55 ). Using OrthoFinder 56 , 645,270 proteins of these 16 plant genomes were clustered into 31,276 orthologous gene clusters (OGCs) with each cluster containing more than two proteins. A phylogenetic tree was built using 130 single-copy genes to represent the species tree (Fig. 3 ). The topology of the grass family agrees with the previous analysis 57 , and the topology of the cactus family is also in line with the cactus phylogeny described in ref. 17 , suggesting the high quality of this species tree.
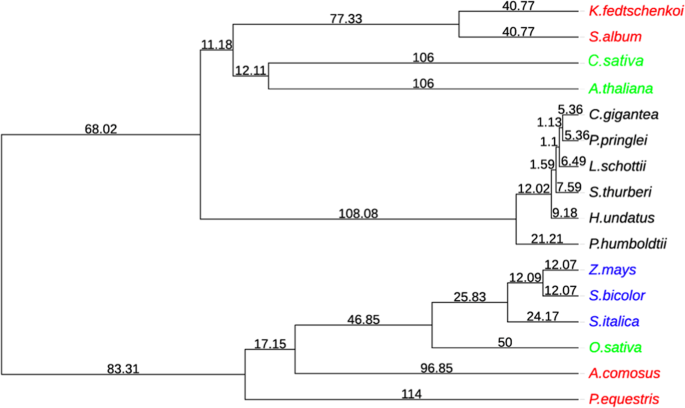
CAM plants are colored in red (noncactus family) and black (cactus family). C3 and C4 are colored in green and blue, respectively. The maximum-likelihood tree was built with 130 single-copy genes using RAxML. The divergence time is estimated by r8s. The full names of the 16 plants are: three C3 plants ( Oryza sativa 46 , Arabidopsis thaliana 47 , and Cannabis sativa 48 ), three C4 plants ( Zea mays 49 , Sorghum bicolor 50 , and Setaria italica 51 ), four CAM plants ( Phalaenopsis equestris 52 , Ananas comosus 53 , Kalanchoe fedtschenkoi 54 , and Sedum album 55 ), and six cactus plants ( Hylocereus undatus , Stenocereus thurberi 17 , Lophocereus schottii 17 , Carnegiea gigantea 17 , Pachycereus pringlei 17 , and Pereskia humboldtii 17 )
With the species tree, r8s 58 was employed to estimate the divergence time of different cactus plants. Three divergence times from the TimeTree database 59 were used as references: (i) 50 MYA (million years ago) between O. sativa and S. bicolor , (ii) 106 MYA between C. sativa and A. thaliana , and (iii) 114 MYA between A. comosus and P. equestris . Shown in Fig. 3 , the divergence times of cactus family species were estimated to be: (i) 5.36 MYA between C. gigantea and P. pringlei , (ii) 6.49 MYA between C. gigantea and L. schottii , (iii) 7.59 MYA beween C. gigantea and S. thurberi , (iv) 9.18 MYA between C. gigantea and H. undatus , and (v) 21.12 MYA between C. gigantea and P. humboldtii . As expected, the estimated time of P. humboldtii diverging from other cacti (21.12 MYA) was more recent than the estimations made for the cactus crown clade in ref. 17 (26.88 MYA) and in ref. 60 (30–30.5 MYA).
Functional enrichment analysis of orthologous gene clusters (OGCs) in cactus plants finds significantly enriched OGCs in drought resistance
Of these 31,276 OGCs, 30,678 contain proteins from more than two species. We have performed gene ontology (GO) enrichment analysis between OGCs of different groups of plants following the method in our previous paper 61 . The goal was to investigate, comparing to the shared OGCs (as control), what GO functional terms are significantly overrepresented in the cactus-specific OGCs. These enriched GO terms may highlight what biological functions are critical for cacti to adapt to their unique living environments. Briefly, the 30,678 OGCs were separated into three groups according to what species are present in the OGCs (Fig. 4A ). A cactus-specific OGC was defined as a cluster containing proteins from at least two cactus species but not from any noncactus species. A shared OGC contained at least one cactus and one noncactus species. Then for each GO term, a binomial test P value was calculated to measure the statistical enrichment of this GO term in cactus-specific OGCs, by considering the count of cactus-specific OGCs and the count of shared OGCs assigned to this GO.
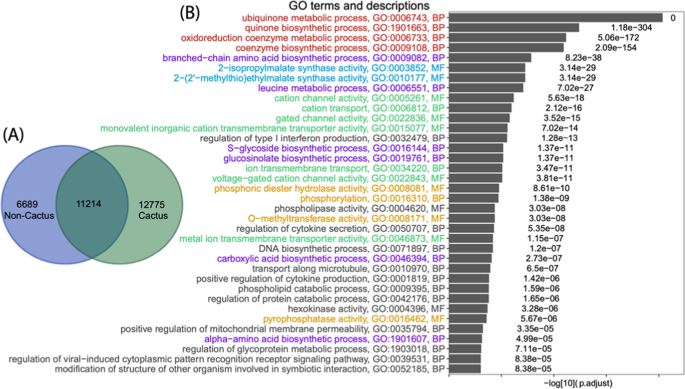
A 30,457 OGCs are clustered into 12,755 cactus-specific OGCs, 6689 noncactus-specific OGCs, and 11,214 shared OGCs. B GO enrichment analysis was conducted with 12,775 cactus-specific OGCs as foreground, and 11,214 shared OGCs as background. The x axis shows the log10 of the adjusted P values, and the y axis shows the GO terms (only molecular function (MP) and biological process (BP) are shown) with adjusted P value < 0.01. Groups of GO terms are colored in green (group I), red (group II), purple (group III), blue (group IV), and orange (group V). See main text for the five groups of GO terms
Comparing to noncactus plants, cactus plants can survive in arid environments with high light, hot temperature extremes, and little water. We anticipated that the GO enrichment analysis could reveal functions that can help explain these traits unique in cactus plants. Indeed, among the list of significantly enriched GO terms shown in Fig. 4B , 23 (63.8%) can be classified into five groups of functions that are related to cactus adapting to the dry and hot environments.
1. Group I (green) contains GO terms related to ion-channel functions. This is expected as they are highly related to osmotic stress and controlling movements of the stoma. For example, during the daytime, cactus plants (and all CAM species) close their stoma to reduce water transpiration.
2. Group II (red) is antioxidant defense-related. To survive in an arid environment, cactus plants must possess the drought response mechanism 62 , particularly the antioxidant defense system. The constant drought stress will lead to the accumulation of the oxidizing substance, such as O 2 , H 2 O 2 , O 2 -, and OH, which will damage the cells and even cause the death 62 . Hence, antioxidant defense system-related GO terms are enriched in cactus plant genomes.
3. Group III (purple) is related to biosynthetic metabolism, particularly amino acid biosynthesis. Evidence has been shown in model plant organisms that drought stress can induce alterations in almost all primary metabolisms 63 including carbohydrates (e.g., glycosides), amino acids (e.g., branched-chain amino acids 64 ), and lipids, as well as secondary metabolisms (e.g., glucosinolate 65 ). Hence, these enriched GO terms can be associated with the drought resistance response of cactus plants as well.
4. Group IV (blue) contains GO:0003852 (2-isopropylmalate synthase activity) and GO:0010177 (2-(2’-methylthio) ethylmalate synthase activity), which are related to the CAM pathway. Therefore, these enriched functions should contribute to the CAM photosynthesis in cactus plants to conserve water and adapt to arid environments.
5. Group V (orange) is highly related to phosphoryl and methyl metabolism. O -methyltransferase is related to the biosynthesis of flavonoid, one of the most abundant classes of plant secondary metabolites 66 . All the other GO terms in this group are related to phosphorylation of proteins, carbohydrates, and lipids, which are critical responses to abiotic stresses including heat and drought 67 .
Similar enrichment analysis was also conducted on KEGG pathways (Supplementary Fig. S2 ). The enriched KEGG terms were classified into two groups: one contains pathways involved in environmental information processing and signal transduction, and the other contains pathways for the metabolism of various molecules (carbohydrates, proteins, lipids, glucosinolate, 2-oxocarboxylic acid, terpenoids, and polyketides). The first KEGG-term group corresponds to enriched GO group I and V, while the second group corresponds to enriched GO group II, III, and IV. Overall, the KEGG enrichment result is consistent with the GO enrichment result.
Fruit flavor-related GO terms are found to be enriched in OGCs significantly expanded in H. undatus
In addition to the GO enrichment analysis performed on cactus-specific OGCs, we have also taken advantage of the species tree reconstructed from the 130 single-copy OGCs to identify OGCs significantly expanded along with specific nodes in the tree (Fig. 5A and Supplementary Fig. S3 ). The significantly contracted and expanded OGCs on different nodes in the tree were determined by CAFE 68 . Particularly, we have focused on the 67 OGCs that are significantly expanded in only dragon fruit (Fig. 5A ).
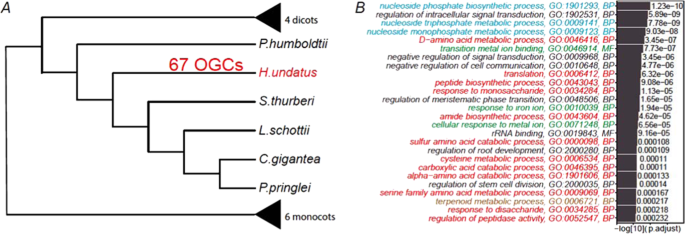
A The 67 significantly expanded OGCs were identified using CAFE with the species tree and OGCs generated by OrthoFinder as input. The complete version of the tree and OGCs on each node can be found in Supplementary Fig. S3 . B Significantly enriched GO terms in the 67 significantly expanded OGCs in H. undatus . The full names of the 16 plants are provided in the legend of Fig. 3
The 67 OGCs were used as the foreground for statistical analysis. For background, we have selected conserved OGCs that contain at least one species from each of the three major clades of the species tree: cactus clade (six species), other dicot clade (four species), and monocot clade (six species). Then, for each GO term in the foreground, the P value of the binomial test was calculated to measure the significance of the GO enrichment.
Most enriched GO terms in these 67 OGCs were related to metabolisms of saccharide, amino acid, and carboxylic acid (colored in red in Fig. 5B ) (gene and GO list in Supplementary Table S5 ). Sugar and acid metabolism processes have been shown to be related to the fruit flavor 69 , 70 , 71 , 72 . Therefore, OGCs significantly expanded in H. undatus have likely contributed to the dragon fruit maturity and ripening or its unique nutrition and flavor. In addition, evidence has also been shown that soluble sugars 73 and other primary metabolites 63 confer tolerance to drought stress. Other enriched GO terms include metal ion response functions (green in Fig. 5B ), nucleoside and ribonucleotide metabolic processes (blue in Fig. 5B ), terpenoid metabolic processes (brown in Fig. 5B ), which are all related to drought resistance and fruit flavor as well 74 , 75 .
Plant cell wall-related functions are enriched in differentially expressed genes (DEGs) in trypsin-treated dragon fruit during storage
Recent work has revealed that antioxidant functions were enriched in the differentially expressed genes (DEGs) between dragon fruit peels treated with and without trypsin 30 . The analysis was based on the de novo assembled transcripts without a reference genome. Hence, in this study, we mapped the RNA-seq reads to the dragon fruit draft genome for a more accurate reference-based DEG analysis. Briefly, the trypsin-treated (SRR8327215, H. undatus cultivar Viet 1 peel) and control (SRR8327214, H. undatus cultivar Viet 1 peel) clean reads were aligned to the H. undatus draft genome. After a reference-based transcript assembly, transcripts corresponding to 18,808 MAKER predicted genes (Supplementary Table S6 ) were analyzed by DESeq2 76 for DEGs. As the result, 1065 significantly upregulated genes ( P .adjusted < 0.05, log2FoldChange > 1) and 1279 significantly downregulated genes ( P .adjusted < 0.05, log2FoldChange < −1) were identified and subject to GO enrichment analysis with all the 18,808 expressed genes as the background.
As shown in Fig. 6A , the enriched GO terms in upregulated DEGs were classified into groups and colored differently. Group I (red) contained five terpenoid metabolic GO terms, which are antioxidants-related 77 , 78 and consistent with the previous DEGs analysis based on de novo assembled transcripts 30 , 79 . Very interestingly, ten GO terms of group II (blue in Fig. 6A ) contained various carbohydrate and plant cell wall-related processes, particularly those regulating functions. This was not found in ref. 30 but was expected as trypsin treatment may indirectly affect the cell wall integrity and carbohydrate metabolisms by directly acting on cell wall proteins. In addition, there were also other GO terms that respond to various biotic and abiotic stresses, such as regulation of phytoalexin metabolic process, ubiquinone metabolic process, phosphatase activity, and photosynthesis-related processes. The downregulated DEGs were also enriched with eight carbohydrate and plant cell wall-related processes (particularly those biosynthetic functions, blue in Fig. 6B ), which is also the largest GO group. The other GO groups were related to ion transporting activities, which was found previously 30 . Overall, the genome reference-based DEG analysis revealed many more enriched GO terms than the previous DEG analysis based on de novo assembled transcripts.
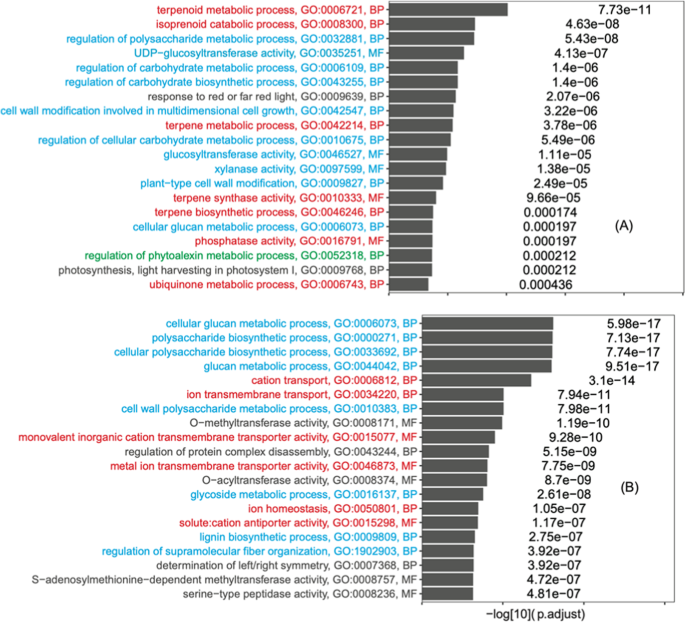
A Upregulated DEGs with trypsin treatment: superoxide scavenging activity-related GO terms are colored in red; saccharide-related GO terms are colored in blue; Phytoalexin is colored in green. B Downregulated DEGs with trypsin treatment: ion-related GO terms are colored in red; saccharide-related GO terms are colored in blue
Most betalain biosynthetic genes are co-localized in a single H. undatus chromosome
Betalains are red-violet (betacyanin) and yellow (betaxanthin) pigments uniquely found in the order Caryophyllales . The betacyanin synthesis pathway is illustrated in Fig. 7A , where the enzymes and their orthologs found in H. undatus genome are indicated. Betaxanthin is made from betalamic acid without enzymes by spontaneously connecting with amino acids, which is not shown in Fig. 7A . Two key enzyme families, L‐DOPA 4,5‐dioxygenase (DODA) and cytochrome P450 enzyme CYP76AD, in the betalain synthesis pathway, had been phylogenetically studied in Caryophyllales 80 . As a result, two DODA subfamilies (DODA-α and DODA-β) and three CYP76AD subfamilies (CYP76AD-α, CYP76AD-β, and CYP76AD-γ) were characterized. Only DODA-α, CYP76AD-α, and CYP76AD-β have been shown to be involved in the betalain synthesis pathway. Two glucosyltransferases (cyclo‐DOPA 5‐ O ‐glucosyltransferase [cDOPA5GT] and betanidin glucosyl‐transferase [5GT/6GT]) of glycosyltransferase family 1 (GT1), which are involved in structural modifications of betalains, were also more recently investigated 81 .
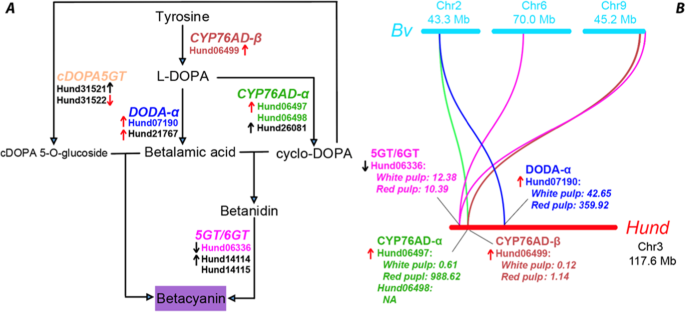
In ( A ), the enzyme subfamilies (larger font) and the H. undatus orthologous genes (smaller font) are shown. The up arrows show increased gene expressions from white pulp to red pulp development stages; the down arrows mean decreased expressions. The red arrows indicate significant expression changes (adjusted P value <0.05 and expression log2 (fold change) >1 or <−1), while black arrows do not. The colored genes are genes that are co-localized in Chr3 of H. undatus . In ( B ), the colors of genes and curved lines (depicting orthologous gene relationships) match the colors of genes in ( A ). The TPM (transcripts per kilobase million reads) values are also indicated for these co-localized genes on Chr3. The detailed expression values can be found in Supplementary Table S8
A homology search of the three enzyme families (DODA, CYP76AD, and GT1) followed by detailed phylogenetic analyses found that all of them have orthologs in the H. undatus genome (Fig. 7A and Supplementary Table S7 ). Specifically, Hund06497, Hund06498, and Hund26081 are the orthologs of CYP76AD-α, Hund06499 is the ortholog of CYP76AD-β, Hund27099, and Hund27100 are the orthologs of CYP76AD-γ (Supplementary Fig. S4 ). In the dragon fruit genome, CYP76AD genes seem to have many recently duplicated copies; these genes have a low Ks <0.3 (see above) and are located adjacently in the genome, suggesting a significant tandem duplication of CYP76AD in the dragon fruit genome. Similarly, for DODA enzymes, Hund07190 and Hund21767 are the orthologs of DODA-α; Hund21766 is the ortholog of DODA-β (Supplementary Fig. S5 ). Note that Hund21767 is located next to Hund21766 in the genome and shares 48.3% sequence identity. Lastly, Hund06336 is the ortholog of betanidin 5GT, Hund14114 and Hund14115 are the orthologs of betanidin 6GT, and Hund31521 and Hund31522 are the orthologs of cDOPA5GT (Supplementary Fig. S6 ).
Interestingly, Hund06336 (5GT), Hund06497 (CYP76AD-α), Hund06498 (CYP76AD-α), Hund06499 (CYP76AD-β), and Hund07190 (DODA-α) are all located in a ~12 Mb region of the Chr3 (Scaffold 33676) (total length 117.6 Mb) (Fig. 7B ). Therefore, all the major genes in the betacyanin biosynthetic pathway except for cDOPA5GT are co-localized in one single chromosome of H. undatus . All these genes were also found in the closely related H. polyrhizus (Supplementary Table S7 ), which currently only have transcriptomes. As a comparison, in Beta vulgaris , these genes are located on three different chromosomes (Fig. 7B ), although two key genes of them (CYP76AD-α and DODA-α) have been known to be adjacent to each other in one gene cluster in B. vulgaris and representative genomes of Amaranthaceae family 82 . We also attempted to locate these genes in the C. gigantea genome. However, as it is very fragmented compared to H. undatus and B. vulgaris , all these C. gigantea genes are found on different short contigs and thus it is not possible to determine if they are also clustered in C. gigantea genome.
A recent study has generated RNA-seq data in H. polyrhizus aiming to identify differentially expressed genes in different stages of dragon fruit pulp development (white pulp vs. red pulp) 27 . We have mapped ~12 Gb reads of this dataset to our H. undatus genome and found that 93% of the reads were mapped. Interestingly, 9 out of the 11 betacyanin biosynthetic genes in Fig. 7A have expression changes, among them, four have significantly increased expression changes ( P value < 0.05 and log2 (fold change) > 1) from white pulp to red pulp development. Particularly, Hund06497 (CYP76AD-α) is the third most upregulated gene with a log2(fold change) = 10.2 among all Hund genes, followed by Hund07190 (DODA-α) with a log2 (fold change) = 2.6 (Fig. 7B and Supplementary Table S8 ).
Interestingly, crassulacean acid metabolism (CAM) genes are also differentially expressed in dragon fruit development. The CAM pathway genes were identified by using a list of query proteins (11 families) from Kalanchoe fedtschenkoi 54 for a BlastP search in H. undatus followed by detailed phylogenetic analyses. As expected, all the main enzymes in the CAM pathway were found in H. undatus . Interestingly, among 38 H. undatus genes of the 11 enzyme families (Supplementary Table S9 ), 34 genes are differentially expressed ( P value < 0.05) from white pulp to red pulp in dragon fruit development 27 . Ten of these genes are significantly upregulated (log2 (fold change) >1) and nine are significantly downregulated (log2 (fold change) < −1). The increased gene expression is especially evident for enzymes active in daytime. What this implicates will need further in-depth studies in the future.
Dragon fruits contain many species of the genus Hylocereus and are increasingly important for food and agricultural industries. In this study, we have sequenced the H. undatus draft genome, the first genome of the Hylocereus genus. This genome is also the sixth sequenced genome of the Cactaceae family. Compared to the previously published five Cactaceae genomes ( S. thurberi (Sthu), L. schottii (Lsch), C. gigantea (Cgig), P. pringlei (Ppri) , and P. humboldtii (Phum) ), the H. undatus genome has the longest scaffold N50 (109 M vs 61.5 k of C. gigantea , the best assembled in ref. 17 , i.e., >1700 times longer) and the largest genome completeness (92.4% vs. 75.3% of C. gigantea ).
The chromosome-level assembly of H. undatus genome is achieved by using the Dovetail Genomics HiRise™ scaffolding software with long-range Chicago libraries and Hi-C libraries sequencing. This pipeline has been widely used by a number of large plant and animal genome-sequencing projects 83 , 84 , 85 , 86 . Evaluation of its performance by resequencing reference genomes of model organisms such as humans has demonstrated that it is an accurate and inexpensive approach to building long-range sequence scaffolds at the chromosome-level even 86 , 87 , 88 . The density report in the Hi-C scaffolding run on our H. undatus genome shows that the Hi-C data is in agreement with the placement and orientation of the input scaffolds in the final chromosome-level assembly (Supplementary Fig. S7 ). In addition, the inter-genome synteny alignments of H. undatus against four other chromosome-level genomes of Caryophyllales (Fig. 2F and Supplementary Fig. S8 ) also suggest that our H. undatus genome assembly is in a high quality.
With this high-quality draft genome, comparative analysis against other cacti genomes revealed a number of interesting findings as described in “Results”. These findings have led to new understandings ranging from whole-genome duplication, species divergence time, to significantly enriched GO functions in Cactaceae and in H. undatus , as well as betacyanin biosynthetic genes co-localized in a 12 Mb region of one single chromosome.
Particularly, the highly continuous chromosome-level assembly of H. undatus genome has allowed us to detect and confidently verify the WGD in H. undatus . This WGD event (Fig. 2 ) happened most likely in the last common ancestor of all cactus plants (β node in Fig. 2A ). This is because the 4dTv β peak is clearly shared by all the six studied cacti. In addition, it is obvious that S. thurberi and L. schottii have had an extra recent WGT event (α peak in Fig. 2D ). This observation is also consistent with the peculiarly higher numbers of protein-coding genes predicted from S. thurberi and L. schottii draft genomes (Table 1 ).
Similarly, it is the chromosome-level H. undatus genome that made it possible to locate the betacyanin pathway genes within a 12-Mb genomic region of the Chr3 (Fig. 7B ). In contrast, although orthologous genes were also identified in C. gigantea and H. polyrhizus , it is unknown if these genes are also co-localized in other cactus genomes. The future improvement of these fragmented draft genome to a chromosome-level assembly will help to answer this question. It is interesting to note that although CYP76AD-α and DODA-α genes are adjacent in Chr2 of the well-assembled B. vulgaris genome, other core genes in the pathway are located on two other chromosomes (Chr6 and Chr9) (Fig. 7B ). The chromosome-level co-localization of betacyanin biosynthetic genes in H. undatus may implicate a more efficient biosynthesis of betacyanin. The experimental verification of this hypothesis in the future is very necessary, given that betacyanin is one type of highly active and abundant antioxidants found in many cactus plants.
Lastly, it is widely accepted that reference-based RNA-seq assembly and expression quantification are preferred whenever a reference genome is available 89 . This is because de novo transcript assembly tends to have various issues (e.g., more fragmented and redundant contigs, missing low-abundance transcripts, mis-assembled transcripts), which may lead to inaccurate or incomplete results in downstream differential expression analysis. Indeed, by using the available H. undatus draft genome as the reference, we have identified antioxidant-related GO terms enriched in differentially expressed genes (DEGs) in trypsin-treated dragon fruits, which confirmed the results in refs. 30 , 79 . Furthermore, we were also able to identify carbohydrate and plant cell wall-related biological processes to be also highly enriched in DEGs.
In summary, the H. undatus draft genome will be a significant contribution to the dragon fruit research community. With a chromosome-level assembly and high genome completeness, it will also be a great reference for the study of various cactus plants. As dragon fruits are increasingly consumed as an important tropical fruit, various dragon fruit cultivars have been developed all over the world. This H. undatus draft genome will be a great resource to develop genomic tools such as SNPs and other molecular markers for genetic characterization of various dragon fruit cultivars.
Materials and methods
The detailed methods can be found in the Supplementary Method file. Here, we only briefly describe the materials and methods used in this study.
Plant material, 10X library prep, and sequencing
Stem (cladode) samples of H. undatus cultivar “David Bowie” (Fig. 1A ) were collected from the USDA-ARS Tropical Agriculture Research Station in Mayaquez, Puerto Rico. Whole-genome sequencing libraries were prepared using Chromium Genome Library & Gel Bead Kit v.2 (10X Genomics, cat. no. 120258) and sequenced on a NovaSeq6000 sequencer (Illumina, San Diego, CA) with paired-end 150 bp reads.
Chicago library preparation and sequencing
Two Chicago libraries were prepared as described previously 88 . The libraries were sequenced on an Illumina HiSeq X. The number and length of read pairs produced for each library were: 145 million, 2 × 150 bp for library 1; 181 million, 2 × 150 bp for library 2. Together, these Chicago library reads provided 235.75× physical coverage of the genome (1–100 kb pairs).
Dovetail Hi-C library preparation and sequencing
Two Dovetail Hi-C libraries were prepared in a similar manner as described previously 88 . The libraries were sequenced on an Illumina HiSeq X. The number and length of read pairs produced for each library were: 204 million, 2 × 150 bp for library 1; 177 million, 2 × 150 bp for library 2. Together, these Dovetail Hi-C library reads provided 6,171.67× physical coverage of the genome (10–10,000 kb pairs).
Scaffolding the assembly with HiRise
The input de novo assembly, shotgun reads, Chicago library reads, and Dovetail Hi-C library reads were used as input data for HiRise, a software pipeline designed specifically for using proximity ligation data to scaffold genome assemblies 90 . An iterative analysis was conducted.
Repeat and noncoding RNA annotation
RepeatModeler 91 and RepeatMasker 92 were employed to annotate repetitive elements in the draft dragon fruit genome and other cactus genomes. tRNA-scan2 93 was used to identify tRNA genes. Infernal package 94 and Rfam 95 were employed to identify noncoding RNA genes.
Protein-coding gene prediction
MAKER 38 was employed to predict protein-coding genes by combining ab initio and homology-based approaches. Three rounds of MAKER runs considered transcriptome and protein evidence as well as ab initio gene prediction. The result of the third round of MAKER run was used as the final protein-coding gene model. In addition to H. undatus , MAKER gene predictions were also performed on four of the five cactus draft genomes sequenced by 17 except for C. gigantea following the same procedure as described above for H. undatus . The redundant proteins from MAKER predictions were removed using seqkit 96 , so were proteins < 50 aa.
Orthologous gene clusters and phylogenetic analyses
Proteins of a total of 16 sequenced plant genomes were selected to define orthologous gene clusters (OGCs) and for phylogenetic analyses. These genomes include three C3 plants, three C4 plants, and ten CAM plants. Six of the ten CAM plants are cacti, and their proteins were obtained by the processes described above. Some genomes have proteins from alternative splicing, and such genomes were processed to only keep the longest isoform protein of each gene.
Proteins of the 16 genomes were combined as input to OrthoFinder 56 . All of the single-copy orthologs were aligned with MUSCLE 97 . The alignments of single-copy OGCs were concatenated into one super alignment, which was further processed by Gblocks 98 . A phylogenetic tree was built using RAxML 99 to represent the species tree with 100 times of bootstrap and the evolutionary model -m PROTGAMMAJTT. The divergence time of the 16 plants was estimated by r8s 58 with three calibrations. The input tree to r8s was the tree built by RAxML. All the OGCs generated by OrthoFinder were analyzed by CAFE 68 to identify significantly expanded and contracted OGCs in different nodes of the species tree.
GO and KEGG enrichment analysis
OGCs generated by OrthoFinder were classified into different groups according to what plant species that a CGC contains proteins from. For GO and KEGG annotation, all the proteins within a CGC were BlastP searched against the eggNOG database 39 . The eggNOG hit contains GO term and KEGG term, which were transferred to the query protein. For each CGC, duplicated GO terms or KEGG terms were only counted once for the enrichment analysis. See “Results” for how we have defined foreground and background datasets depending on what questions we wanted to address. The same approach had been used in our previous papers 61 , 100 .
Whole-genome duplication analysis
To examine the WGD in cactus species, wgd 101 and MCScanX 102 were used (unless stated otherwise) to analyze the synteny and calculate the synonymous substitution rate (Ks) and the rate of transversions on fourfold degenerate synonymous sites (4dTv).
RNA-seq data analysis
For the expression analysis of trypsin-treated dragon fruit during storage, the control (SRR8327214) and trypsin-treated (SRR8327215) raw reads were downloaded from the NCBI SRA database. For the expression analysis of betacyanin biosynthetic genes and CAM genes, the white pulp (SRR2924904) and red pulp (SRR3203780) raw reads were downloaded from the NCBI SRA database. The reference genome-based differential expression analysis used HISAT2 103 and StringTie pipeline following 104 . DEseq2 76 was used to calculate the log2 fold change and the adjusted P value of each gene between control and treatment.
Phylogenetic trees of CODA, CYP76AD, cDOPA5GT, and betanidin 5GT/6GT
For CYP76AD, three CYP76AD-α proteins (GenBank accession: HQ656026, HQ656025, and HQ656024) from 105 combined with the other 151 CYP76AD proteins (KR376350–KR376501) from ref. 80 were used as queries to collect homologs from cactus plants.
DODA-α proteins of Beta vulgaris (GenBank accession: HQ656027), Portulaca grandiflora (AJ580598), and Mirabilis jalapa (AB435372) combined with the DODA protein sequences (GenBank accession: KR376141–KR376346) previously studied in ref. 80 were used as queries to collect homologs from cactus plants.
cDOPA5GT and betanidin 5GT/6GT proteins were collected from ref. 106 and ref. 107 , and used as query to collect homologs from cactus plants.
Homologs were filtered with domain e-value < 10 −6 and coverage > = 0.3 (alignment length/HMM length). Filtered full-length protein sequences were aligned by MAFFT 108 , and then the phylogenies were built by FastTree 109 and visualized with iTOL server 110 .
Data availability
The raw DNA sequencing reads and the assembled genome of Hylocereus undatus cultivar “David Bowie” have been submitted to NCBI. The BioProject ID is PRJNA664414, and the BioSample ID is SAMN16213845. The Whole Genome Shotgun accession number is JACYFF000000000.
Bauer, R. A Synopsis of the Tribe Hylocereeae F. Buxb (Hunt, 2003).
Nerd, A., Tel-Zur, N. & Mizrahi, Y. in Cacti: Biology and Uses (ed. Nobel, P. S.) 185–197 (University of California Press, 2002).
Medina, E. D. H. Benzing vascular epiphytes, general biology and related biota Cambridge University Press, Cambridge (1990). J. Tropical Ecol. 8 , 55–56 (1992).
Article Google Scholar
Hoa, T., Clark, C., Waddell, B. & Woolf, A. Postharvest quality of Dragon fruit ( Hylocereus undatus ) following disinfesting hot air treatments. Postharvest Biol. Technol. 41 , 62–69 (2006).
Article CAS Google Scholar
Andrew, P. Invasive Species Compendium (CAB International, 2020).
Le Bellec, F., Vaillant, F. & Imbert, E. Pitahaya (Hylocereus spp.): a new fruit crop, a market with a future. Fruits 61 , 237–250 (2006).
Rachel Nall, M. C. What are the proven benefits of dragon fruit? https://www.medicalnewstoday.com/articles/324655 (2019).
Paull, R. E. & Chen, N. J. Overall dragon fruit production and global marketing. FFTC , http://ap.fftc.agnet.org/ap_db.php (2019).
Arakaki, M. et al. Contemporaneous and recent radiations of the world’s major succulent plant lineages. Proc. Natl Acad. Sci. USA 108 , 8379–8384 (2011).
Article CAS PubMed PubMed Central Google Scholar
Magallón, S., Gómez‐Acevedo, S., Sánchez‐Reyes, L. L. & Hernández‐Hernández, T. A metacalibrated time‐tree documents the early rise of flowering plant phylogenetic diversity. N. Phytologist 207 , 437–453 (2015).
Kohler, M., Reginato, M., Souza-Chies, T. T. & Majure, L. C. Insights into chloroplast genome evolution across Opuntioideae (Cactaceae) reveals robust yet sometimes conflicting phylogenetic topologies. Front Plant Sci. 11 , 729 (2020).
Article PubMed PubMed Central Google Scholar
Wang, N. et al. Evolution of Portulacaceae marked by gene tree conflict and gene family expansion associated with adaptation to harsh environments. Mol. Biol. Evol. 36 , 112–126 (2019).
Article CAS PubMed Google Scholar
Gibson, A. C. & Nobel, P. S. The Cactus Primer (Harvard University Press, 1986).
Hernández‐Hernández, T., Brown, J. W., Schlumpberger, B. O., Eguiarte, L. E. & Magallón, S. Beyond aridification: multiple explanations for the elevated diversification of cacti in the New World Succulent Biome. N. Phytologist 202 , 1382–1397 (2014).
Anderson, E. F. The Cactus Family (Timber Press, 2001).
Demaio, P. H., Barfuss, M. H., Kiesling, R., Till, W. & Chiapella, J. O. Molecular phylogeny of Gymnocalycium (Cactaceae): assessment of alternative infrageneric systems, a new subgenus, and trends in the evolution of the genus. Am. J. Bot. 98 , 1841–1854 (2011).
Article PubMed Google Scholar
Copetti, D. et al. Extensive gene tree discordance and hemiplasy shaped the genomes of North American columnar cacti. Proc. Natl Acad. Sci. USA 114 , 12003–12008 (2017).
Wichienchot, S., Jatupornpipat, M. & Rastall, R. A. Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem. 120 , 850–857 (2010).
Rebecca, O. P. S., Boyce, A. N. & Chandran, S. Pigment identification and antioxidant properties of red dragon fruit ( Hylocereus polyrhizus ). Afr. J. Biotechnol. 9 , 1450–1454 (2010).
Nurliyana, R. d., Syed Zahir, I., Mustapha Suleiman, K., Aisyah, M. & Kamarul Rahim, K. Antioxidant study of pulps and peels of dragon fruits: a comparative study. Int. Food Res. J. 17 , 367–375 (2010).
CAS Google Scholar
Suh, D. H. et al. Metabolite profiling of red and white pitayas ( Hylocereus polyrhizus and Hylocereus undatus ) for comparing betalain biosynthesis and antioxidant activity. J. Agric. Food Chem. 62 , 8764–8771 (2014).
Nurmahani, M., Osman, A., Hamid, A. A., Ghazali, F. M. & Dek, M. P. Antibacterial property of Hylocereus polyrhizus and Hylocereus undatus peel extracts. Int. Food Res. J. 19 , 77 (2012).
Google Scholar
Zhou, J. et al. Proteogenomic analysis of pitaya reveals cold stress-related molecular signature. PeerJ 8 , e8540 (2020).
Nong, Q. et al. RNA-Seq de novo assembly of red pitaya ( Hylocereus polyrhizus ) roots and differential transcriptome analysis in response to salt stress. Tropical Plant Biol. 12 , 55–66 (2019).
Fan, Q. J., Yan, F. X., Qiao, G., Zhang, B. X. & Wen, X. P. Identification of differentially-expressed genes potentially implicated in drought response in pitaya ( Hylocereus undatus ) by suppression subtractive hybridization and cDNA microarray analysis. Gene 533 , 322–331 (2014).
Xi, X. et al. Transcriptome analysis clarified genes involved in betalain biosynthesis in the fruit of red pitayas ( Hylocereus costaricensis ). Molecules 24 , https://doi.org/10.3390/molecules24030445 (2019).
Qingzhu, H. et al. Transcriptomic analysis reveals key genes related to betalain biosynthesis in pulp coloration of Hylocereus polyrhizus . Front Plant Sci. 6 , 1179 (2015).
PubMed Google Scholar
Xu, M. et al. Transcriptomic de novo analysis of pitaya ( Hylocereus polyrhizus ) canker disease caused by Neoscytalidium dimidiatum . BMC Genomics 20 , 10 (2019).
Xiong, R. et al. Transcriptomic analysis of flower induction for long-day pitaya by supplementary lighting in short-day winter season. BMC Genomics 21 , 329 (2020).
Li, X. et al. Transcriptomic analysis reveals key genes related to antioxidant mechanisms of Hylocereus undatus quality improvement by trypsin during storage. Food Funct. 10 , 8116–8128 (2019).
Pinkava, D. J. & McLeod, M. G. Chromosome numbers in some cacti of western North America. Brittonia 23 , 171–176 (1971).
Lichtenzveig, J., Abbo, S., Nerd, A., Tel-Zur, N. & Mizrahi, Y. Cytology and mating systems in the climbing cacti Hylocereus and Selenicereus. Am. J. Bot . 87 , 1058–1065, https://www.ncbi.nlm.nih.gov/pubmed/10898783 (2000).
Tel-Zur, N. et al. Phenotypic and genomic characterization of vine cactus collection (Cactaceae). Genet. Resour. Crop Evolution 58 , 1075–1085 (2011).
Cisneros, A. & Tel-Zur, N. Genomic analysis in three Hylocereus species and their progeny: evidence for introgressive hybridization and gene flow. Euphytica 194 , 109–124 (2013).
Bennett, M. D., Leitch, I. J., Price, H. J. & Johnston, J. S. Comparisons with Caenorhabditis (approximately 100 Mb) and Drosophila (approximately 175 Mb) using flow cytometry show genome size in Arabidopsis to be approximately 157 Mb and thus approximately 25% larger than the Arabidopsis genome initiative estimate of approximately 125 Mb. Ann. Bot. 91 , 547–557 (2003).
Simao, F. A., Waterhouse, R. M., Ioannidis, P., Kriventseva, E. V. & Zdobnov, E. M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31 , 3210–3212 (2015).
Wu, Y. et al. Comparative transcriptome analysis combining SMRT- and Illumina-based RNA-Seq identifies potential candidate genes involved in betalain biosynthesis in pitaya fruit. Int. J. Mol. Sci . 21 , https://doi.org/10.3390/ijms21093288 (2020).
Cantarel, B. L. et al. MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 18 , 188–196 (2008).
Huerta-Cepas, J. et al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 47 , D309–D314 (2019).
Dohm, J. C. et al. The genome of the recently domesticated crop plant sugar beet ( Beta vulgaris ). Nature 505 , 546–549 (2014).
Xu, C. et al. Draft genome of spinach and transcriptome diversity of 120 Spinacia accessions. Nat. Commun. 8 , 15275 (2017).
Palfalvi, G. et al. Genomes of the Venus flytrap and close relatives unveil the roots of plant carnivory. Curr. Biol. 30 , 2312–2320.e5 (2020).
Sturtevant, D. et al. The genome of jojoba ( Simmondsia chinensis ): a taxonomically isolated species that directs wax ester accumulation in its seeds. Sci. Adv. 6 , eaay3240 (2020).
Zhang, L. et al. The Tartary buckwheat genome provides insights into rutin biosynthesis and abiotic stress tolerance. Mol. Plant 10 , 1224–1237 (2017).
Wang, Y. et al. The sacred lotus genome provides insights into the evolution of flowering plants. Plant J. 76 , 557–567 (2013).
Yu, J. et al. A draft sequence of the rice genome ( Oryza sativa L. ssp. indica). Science 296 , 79–92 (2002).
Arabidopsis Genome, I. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana . Nature 408 , 796–815 (2000).
Gao, S. et al. A high-quality reference genome of wild Cannabis sativa . Hortic. Res. 7 , 73 (2020).
Article PubMed PubMed Central CAS Google Scholar
Jiao, Y. et al. Improved maize reference genome with single-molecule technologies. Nature 546 , 524–527 (2017).
Paterson, A. H. et al. The Sorghum bicolor genome and the diversification of grasses. Nature 457 , 551–556 (2009).
Bennetzen, J. L. et al. Reference genome sequence of the model plant Setaria. Nat. Biotechnol. 30 , 555–561 (2012).
Cai, J. et al. The genome sequence of the orchid Phalaenopsis equestris . Nat. Genet. 47 , 65–72 (2015).
Ming, R. et al. The pineapple genome and the evolution of CAM photosynthesis. Nat. Genet. 47 , 1435–1442 (2015).
Yang, X. et al. The Kalanchoe genome provides insights into convergent evolution and building blocks of crassulacean acid metabolism. Nat. Commun. 8 , 1899 (2017).
Wai, C. M. et al. Time of day and network reprogramming during drought induced CAM photosynthesis in Sedum album . PLoS Genet. 15 , e1008209 (2019).
Emms, D. M. & Kelly, S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20 , 238 (2019).
Gaut, B. S. Evolutionary dynamics of grass genomes. New Phytologist 154 , 15–28 (2002).
Sanderson, M. J. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19 , 301–302 (2003).
Kumar, S., Stecher, G., Suleski, M. & Hedges, S. B. TimeTree: a resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 34 , 1812–1819 (2017).
Guerrero, P. C., Majure, L. C., Cornejo-Romero, A. & Hernandez-Hernandez, T. Phylogenetic relationships and evolutionary trends in the cactus family. J. Hered. 110 , 4–21 (2019).
Fitzek, E. et al. Cell wall enzymes in Zygnema circumcarinatum UTEX 1559 respond to osmotic stress in a plant-like fashion. Front. Plant Sci. 10 , 732 (2019).
Fang, Y. & Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol. Life Sci. 72 , 673–689 (2015).
Yang, L. et al. Deciphering drought-induced metabolic responses and regulation in developing maize kernels. Plant Biotechnol. J. https://doi.org/10.1111/pbi.12899 (2018).
Huang, T. & Jander, G. Abscisic acid-regulated protein degradation causes osmotic stress-induced accumulation of branched-chain amino acids in Arabidopsis thaliana . Planta 246 , 737–747 (2017).
Del Carmen Martinez-Ballesta, M., Moreno, D. A. & Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 14 , 11607–11625 (2013).
Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 126 , 485–493 (2001).
Cramer, G. R., Urano, K., Delrot, S., Pezzotti, M. & Shinozaki, K. Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol. 11 , 163 (2011).
De Bie, T., Cristianini, N., Demuth, J. P. & Hahn, M. W. CAFE: a computational tool for the study of gene family evolution. Bioinformatics 22 , 1269–1271 (2006).
Dong, X. et al. De novo assembly of a wild pear ( Pyrus betuleafolia ) genome. Plant Biotechnol. J. 18 , 581–595 (2020).
Sadka, A., Shlizerman, L., Kamara, I. & Blumwald, E. Primary metabolism in citrus fruit as affected by its unique structure. Front. Plant Sci. 10 , https://doi.org/10.3389/fpls.2019.01167 (2019).
Desnoues, E. et al. Profiling sugar metabolism during fruit development in a peach progeny with different fructose-to-glucose ratios. BMC Plant Biol. 14 , 336 (2014).
Li, M., Li, P., Ma, F., Dandekar, A. M. & Cheng, L. Sugar metabolism and accumulation in the fruit of transgenic apple trees with decreased sorbitol synthesis. Hortic. Res. 5 , 60 (2018).
Redillas, M. C. F. R. et al. Accumulation of trehalose increases soluble sugar contents in rice plants conferring tolerance to drought and salt stress. Plant Biotechnol. Rep. 6 , 89–96 (2012).
Oikawa, A. et al. Metabolic profiling of developing pear fruits reveals dynamic variation in primary and secondary metabolites, including plant hormones. PLoS ONE 10 , e0131408 (2015).
Aharoni, A. et al. Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species. Plant Cell 16 , 3110–3131 (2004).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15 , 550 (2014).
Pichersky, E. & Raguso, R. A. Why do plants produce so many terpenoid compounds? N. Phytol. 220 , 692–702 (2018).
Zhang, X. et al. Identification and functional characterization of three new terpene synthase genes involved in chemical defense and abiotic stresses in Santalum album . BMC Plant Biol. 19 , 115 (2019).
Pang, X. et al. Transcriptomic analysis reveals Cu/Zn SODs acting as hub genes of SODs in Hylocereus undatus induced by trypsin during storage. Antioxidants 9 , https://doi.org/10.3390/antiox9020162 (2020).
Brockington, S. F. et al. Lineage-specific gene radiations underlie the evolution of novel betalain pigmentation in Caryophyllales. N. Phytol. 207 , 1170–1180 (2015).
Timoneda, A. et al. The evolution of betalain biosynthesis in Caryophyllales. N. Phytol. 224 , 71–85 (2019).
Sheehan, H. et al. Evolution of l-DOPA 4,5-dioxygenase activity allows for recurrent specialisation to betalain pigmentation in Caryophyllales. N. Phytol. 227 , 914–929 (2020).
Warren, W. C. et al. Sequence diversity analyses of an improved rhesus macaque genome enhance its biomedical utility. Science 370 , https://doi.org/10.1126/science.abc6617 (2020).
Ghosh, A. et al. A high-quality reference genome assembly of the saltwater crocodile, Crocodylus porosus , reveals patterns of selection in Crocodylidae. Genome Biol. Evol. 12 , 3635–3646 (2020).
Jarvis, D. E. et al. The genome of Chenopodium quinoa . Nature 542 , 307–312 (2017).
Dudchenko, O. et al. De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science 356 , 92–95 (2017).
Kadota, M. et al. Multifaceted Hi-C benchmarking: what makes a difference in chromosome-scale genome scaffolding? Gigascience 9 , https://doi.org/10.1093/gigascience/giz158 (2020).
Putnam, N. H. et al. Chromosome-scale shotgun assembly using an in vitro method for long-range linkage. Genome Res. 26 , 342–350 (2016).
Conesa, A. et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 17 , 13 (2016).
Lieberman-Aiden, E. et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326 , 289–293 (2009).
Smit, A. F. A., Hubley, R. & Green, P. RepeatMasker Open-4.0. 2013–2015.
Smit, A., Hubley, R. & Green, P. J. D. D. RepeatMasker Open-4.0. http://www.repeatmasker.org (2018).
Chan, P. P. & Lowe, T. M. tRNAscan-SE: searching for tRNA genes in genomic sequences. Methods Mol. Biol. 1962 , 1–14 (2019).
Nawrocki, E. P. & Eddy, S. R. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics 29 , 2933–2935 (2013).
Kalvari, I. et al. Rfam 13.0: shifting to a genome-centric resource for non-coding RNA families. Nucleic Acids Res. 46 , D335–D342 (2018).
Shen, W., Le, S., Li, Y. & Hu, F. SeqKit: a cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS ONE 11 , e0163962 (2016).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32 , 1792–1797 (2004).
Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17 , 540–552 (2000).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30 , 1312–1313 (2014).
Orton, L. M. et al. Zygnema circumcarinatum UTEX 1559 chloroplast and mitochondrial genomes provide insight into land plant evolution. J. Exp. Bot. 71 , 3361–3373 (2020).
Zwaenepoel, A. & Van de Peer, Y. wgd-simple command line tools for the analysis of ancient whole-genome duplications. Bioinformatics 35 , 2153–2155 (2019).
Wang, Y. et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40 , e49 (2012).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12 , 357–360 (2015).
Pertea, M., Kim, D., Pertea, G. M., Leek, J. T. & Salzberg, S. L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 11 , 1650–1667 (2016).
Hatlestad, G. J. et al. The beet R locus encodes a new cytochrome P450 required for red betalain production. Nat. Genet. 44 , 816–820 (2012).
Sasaki, N. et al. Isolation and characterization of cDNAs encoding an enzyme with glucosyltransferase activity for cyclo-DOPA from four o’clocks and feather cockscombs. Plant Cell Physiol. 46 , 666–670 (2005).
Vogt, T. Substrate specificity and sequence analysis define a polyphyletic origin of betanidin 5- and 6-O-glucosyltransferase from Dorotheanthus bellidiformis . Planta 214 , 492–495 (2002).
Katoh, K., Asimenos, G. & Toh, H. Multiple alignment of DNA sequences with MAFFT. Methods Mol. Biol. 537 , 39–64 (2009).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE 5 , e9490 (2010).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 47 , W256–W259 (2019).
Hao, Z. et al. RIdeogram: drawing SVG graphics to visualize and map genome-wide data on the idiograms. J Comput. Sci. 6 , e251 (2020).
Download references
Acknowledgements
We would like to acknowledge all of our lab members for helpful discussions. We thank Dr. Baolong Liu of Northwest Institute of Plateau Biology of China for providing the assembled UniGene sequences for red- and white-fleshed dragon fruits of their published paper. This work was partially completed utilizing the Holland Computing Center of the University of Nebraska, which receives support from the Nebraska Research Initiative. This work was primarily supported by the United States Department of Agriculture (USDA)/Agricultural Research Service (ARS) award [58-8042-9-089], and partially by National Science Foundation (NSF) CAREER award [DBI-1933521], start-up grant of UNL [2019-YIN] to Y.Y. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Author information
Authors and affiliations.
Nebraska Food for Health Center, Department of Food Science and Technology, University of Nebraska, Lincoln, NE, 68588, USA
Jinfang Zheng & Yanbin Yin
Sustainable Perennial Crops Lab, USDA-ARS, Beltsville, MD, USA
Lyndel W. Meinhardt & Dapeng Zhang
Tropical Agriculture Research Station, USDA-ARS, Puerto Rico, PR, USA
Ricardo Goenaga
You can also search for this author in PubMed Google Scholar
Contributions
Y.Y., D.Z., L.W.M., and R.G. conceived and designed the project. D.Z., L.W.M., and R.G. collected the plant materials and generated the sequencing data. J.Z. performed all the data analysis under the supervision of Y.Y. J.Z. and Y.Y. draft the paper. All authors contributed and approved the final paper.
Corresponding authors
Correspondence to Dapeng Zhang or Yanbin Yin .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Supplementary information
Supplemental methods, supplemental tables, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Zheng, J., Meinhardt, L.W., Goenaga, R. et al. The chromosome-level genome of dragon fruit reveals whole-genome duplication and chromosomal co-localization of betacyanin biosynthetic genes. Hortic Res 8 , 63 (2021). https://doi.org/10.1038/s41438-021-00501-6
Download citation
Received : 01 November 2020
Revised : 19 January 2021
Accepted : 20 January 2021
Published : 10 March 2021
DOI : https://doi.org/10.1038/s41438-021-00501-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Plastome variations reveal the distinct evolutionary scenarios of plastomes in the subfamily cereoideae (cactaceae).
- Jingling Li
- Hongping Deng
BMC Plant Biology (2023)
Trypsin preservation: CsUGT91C1 regulates Trilobatin Biosynthesis in Cucumis sativus during Storage
Plant Growth Regulation (2023)
A chromosome-scale genome sequence of pitaya (Hylocereus undatus) provides novel insights into the genome evolution and regulation of betalain biosynthesis
- Jian-ye Chen
- Fang-fang Xie
- Yong-hua Qin
Horticulture Research (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Phytoconstituents and pharmaco-therapeutic benefits of pitaya: A wonder fruit
Affiliation.
- 1 Shobhaben Pratapbhai Patel School of Pharmacy & Technology Management, SVKM's NMIMS, Mumbai, India.
- PMID: 32378233
- DOI: 10.1111/jfbc.13260
Dragon fruit has caught the attention of many researchers in the last few years because of its vast therapeutic potential. The fruit is enriched with several phytochemical constituents having tremendous pharmacological properties. It is traditionally used as a coloring agent. Some newly explored therapeutic applications include its use as an antioxidant, antimicrobial, antidiabetic, anticancer, and nutraceutical. The phytoconstituents can be extracted from flesh, peel, and seeds of the fruit. The fruit is known to be a rich source of betacyanin, vitamin C, and lycopene. The current review is focused on phytochemical constituents of dragon fruit along with its pharmacological activities. It also sheds light on the safety aspects of the fruit. The review will pave a path for researchers to study this marvel fruit further for societal benefit. Advanced research on dragon fruit will unleash many more therapeutic benefits and can give mechanistic insight about its activities. PRACTICAL APPLICATIONS: Phytoconstituents play a vital role in the treatment of various diseases and for the improvement of human health, in general. Dragon fruit is known to be having antioxidant, anti-microbial, anti-diabetic, anti-cancer applications. The fruit can also be used as a nutraceutical (functional food). To grab all the benefits from this fruit, its phytoconstituents and pharmaco-therapeutic aspect have to be thoroughly studied. This review can be very useful for researchers across different fields like botany, agriculture, pharmacy, etc., to bridge the gap for collaborative work on dragon fruit, which will help in finding solutions for many modern diseases.
Keywords: Hylocereus; anticancer; antimicrobial; dragon fruit; pitaya.
© 2020 Wiley Periodicals LLC.

Publication types
- Antioxidants / therapeutic use
- Betacyanins
- Plant Extracts / therapeutic use
- Antioxidants
- Plant Extracts
A Review on the Scope of Adoption of Underutilized Climate Smart Dragon Fruit ( Hylocereus spp. ) Cultivation
- Review Article / Übersichtsbeitrag
- Published: 18 December 2023
- Volume 66 , pages 297–309, ( 2024 )
Cite this article
- Ashok Yadav ORCID: orcid.org/0000-0001-6787-3883 1 ,
- M. K. Dhakar 2 ,
- A. Arunachalam 1 ,
- Suchisree Jha 3 ,
- Sandeep Garg 1 ,
- Neha Gangwar 1 ,
- A. K. Handa 1 ,
- Badre Alam 1 &
- Darshan Kadam 4
159 Accesses
Explore all metrics
Dragon fruit is an important fruit crop in the Cactaceae family and is known for its high nutraceutical properties, greater monetary returns, low maintenance and stress resistance. Three of its species viz. Hylocereus undatus, H. megalanthus , and H. polyrhizus are extensively grown in the world. Improved agronomic practices have been important for obtaining quality yield. Available literature highlights the novel production techniques such as pruning, training, use of plant hormones, and other horticultural techniques. Nonetheless, there is dearth of complete and comprehensive information about production technology of dragon fruit. This paper reviews all available information related to production technology that would be useful for researchers and farmers.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Analysis of genetic divergence in Psidium cattleyanum Sabine accessions based on morphological fruit descriptors
Paulo César da Silva Santos, Ricardo Gallo, … Diego Silva Batista

Postharvest Management of Fruits and Vegetables Storage

Vegetable Seed Production
Data availability statement.
All the data available in the manuscript are based on experience as well as on research publications. Websites are properly cited along with references and links.
Ahmad H, Mirana AS, Mahbuba S, Tareq SM, Uddin JAFM (2016) Performance of IBA concentrations for rooting of Dragon fruit ( Hylocereus undatus ) stem cuttings. Int J Acad Res Bus Soc Sci 4:231–234
Google Scholar
Anderson EF (2001) The cactus family. Timber Press, Pentland, p 101 ISBN 978-0-88192-498‑5.
Bárcenas P (1994) Efecto de tres substratos en ele enrizamiento y desarrollo de pitahaya ( Hylocereus undatus ). Proc Interamer Soc Trop Hort 38:120–121
Barthlott W, Hunt DR (1993) Cactaceae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants Vol II Flowering plants. Dicotyledons. Springer, Berlin
Charoensiri R, Kongkachuichai R, Suknicom S, Sungpuag P (2009) Beta-carotene, lycopene, and alpha-tocopherol contents of selected Thai fruits. Food Chem 113(1):202–207
Article CAS Google Scholar
Cheah LK, Eid AM, Aziz A, Ariffin FD, Elmahjoubi A, Elmarzugi NA (2016) Phytochemical properties and health benefits of Hylocereus undatus . Nanomed Nanotechnol 1:1–10
Crane J, Balerdi C (2004) Dragon fruit. Institute of Food and Agricultural Sciences, University of Florida. IFAS Extension, Gainsville, 32611
Duncan RE, Carrillo D, Peña JE (2021) Pitaya (dragon fruit) ( Hylocereus undatus ) pests and beneficial insects: ENY-2050/IN1292, 12/2020. EDIS 2021(1):13–13
Article Google Scholar
FAMA (Federal Agricultural Marketing Authority) (2007) http://www.fama.gov.my/utama
FCEC (Flora of China Editorial Committee) (2015) Flora of China. Missouri Botanical Garden, Harvard University Herbaria, St. Louis, Missouri, Cambridge
Fernandes DR, Moreira RA, Rabelo JM, Oliveira JD (2018) Improvement of production and fruit quality of pitayas with potassium fertilization. Acta Sci Agron 40:
Flores VE, Engleman EM (1976) Apuntes sobre anatomía y morfología de las semillas de cactáceas. I. Desarrollo y estructura. Rev Biol Trop 24:199–227
Fumuro M (2011) Effects of the character of cuttings and the type of auxin on rooting ability in dragon fruit. In Com. Proc Int Plant Prop Soc 61:270–274
Gunasena HPM, Pushpakumara DKNG, Kariyawasam M (2007) Dragon fruit— Hylocereus undatus (Haw.) Britton and Rose. In: Gunasena HPM, Singh VP (eds) Underutilized fruit trees in Sri Lanka, by Pushpakumara DKNG. World Agroforestry Centre, South Asia Office, New Delhi, pp 110–142
Guo LW, Wu YX, Ho HH, Su YY, Mao ZC, He PF, He YQ (2014) First report of dragon fruit ( Hylocereus undatus ) anthracnose caused by Colletotrichum truncatum in China. J Phytopathol 162:272–275
Harivaindaram KV, Rebecca OPS, Chandran S (2008) Study of optimal temperature, pH and stability of dragon fruit ( Hylocereus polyrhizus ) peel for use as potential natural colorant. Pak J Biol Sci 11(18):2259–2263
IPGRI. International Plant Genetic Resources Institute (2006) GIS software to explore and conserve genetic resources. http://www.ipgri.cgiar.org/regions/americas/programmes/gissoftware.htm
Ismail SNS, Mahiddin NAK, Praveena SM (2018) The used of dragon fruit peels as eco-friendly wastewater coagulants. Asian J Agric Biol 6:66–71
Jaafar RA, Rahman, ARBA. Mahmod NZC, Vasudevan R (2009) Proximate analysis of dragon fruit ( Hylecereus polyhizus ). Am J Appl Sci 6:1341–1346
Jeronimo MC, Orsine JVC, Borges KK, Novaes MRCG (2015) Chemical and physical-chemical properties, antipitaya [ Hylocereus undatus (Haw.) Britton & Rose] grown in Brazil. J Drug Metab Toxicol 6:1–6
Jiang YL, Lin TS, Lee CL, Yen CR, Yang WJ (2011) Phenology, canopy composition, and fruit quality of yellow pitaya in tropical Taiwan. HortScience 46:1497–1502
Karunakaran G, Arivalagan M (2019) Dragon Fruit-A New Introduction Crop with Promising Market. Indian Hortic 63:8–11
Karunakaran G, Arivalagan M, Sriram S (2019) Dragon Fruit Country Report From India. In: Y2019 FFTC and VAAS-SOFRI joint workshop “Dragon Fruit Network: Marketing and the Whole Value Chain” and Steering Committee Meeting international workshop on Dragon Fruit Network (DFNet): Marketing and the Whole Value Chain, held at Vietnam during 9–11 September 2019
Khaimov A, Mizrahi Y (2006) Effects of day-length, radiation, flower thinning and growth regulators on flowering of the vine cacti Hylocereus undatus and Selenicereus megalanthus . J Hortic Sci Biotechnol 81:465–470
Khalili RM, Norhayati AH, Rokiah MY, Asmah R, Nasir MM, Muskinah MS (2006) Proximate composition and selected mineral determination in organically grown red pitaya ( Hylocereus sp.). J Trop Agric 34(2):269
Korotkova N, Borsch T, Arias S (2017) A phylogenetic framework for the Hylocereeae (Cactaceae) and implications for the circumscription of the genera. Phytotaxa 327:1–46
Kugler F, Stintzing FC, Carle R (2007) Evaluation of the antioxidant capacity of betalainic fruits and vegetables. J Appl Bot Food Qual 81(1):69–76
CAS Google Scholar
Kumar KM, Hanumanthappa M, Marimuthu S. Meenambigai C (2018) A review on enhancing the fertilizers use efficiency to minimize environmental impacts. Int J Chem Stud 6(3):2167–2174
Le Bellec F (2004) Pollinisation et fécondation d’ Hylocereus undatus et d’ H. Costaricensis à l’île de la Réunion. Fruits 59:411–422
Le Bellec F, Judith RC (2002) La pitaya ( Hylocereus spp.) En culture de diversification à l’île de la Réunion: stratégie d’accompagnement de son développement. Fruits 57:219–230
Le Bellec F, Vaillant F, Imbert E (2006) Pitaya ( Hylocereus spp.): a new crop, a market with a future. Fruits 61:237–250
Lichtenzveig J, Abbo S, Nerd A, Tel-Zur N (2000) Cytology and mating systems in the climbing cacti Hylocereus and Selenicereus . Am J Bot 87:1058–1065
Article CAS PubMed Google Scholar
Lim HK, Tan CP, Karim R, Ariffin AA, Bakar J (2010) Chemical composition and DSC thermal properties of two species of Hylocereus cacti seed oil: Hylocereus undatus and Hylocereus polyrhizus . Food Chem 119:1326–1331
Lum MS, Norazira MA (2011) Effects of hot water, submergence time and storage duration on quality of dragon fruit. J Agric Sci 3:146–152
Luo H, Cai Y, Peng Z et al (2014) Chemical composition and in vitro evaluation of the cytotoxic and antioxidant activities of supercritical carbon dioxide extracts of pitaya (dragon fruit) peel. Chem Cent J. https://doi.org/10.1186/1752-153X-8-1
Article PubMed PubMed Central Google Scholar
Martini MY, Ridzwan AH, Mahmud TMM, Omar SSR, Zainudin M (2008) Growth, yield and fruit quality of red dragon fruit ( Hylocereus polyrhizus ) as affected by plant support system and intercropping with long bean ( Vigna sinensis ). J Food Agric Environ 6:305–311
Masyahit M, Sijam K, Awang Y, Ghazali M (2009) First report on bacterial soft rot disease on dragon fruit ( Hylocereus spp.) caused by Enterobacter cloacae in peninsular Malaysia. Int J Agric Biol 11:659–666
Mizrahi Y, Mouyal J, Nerd A, Sitrit Y (2004) Metaxenia in the Vine Cacti Hylocereus polyrhizus and Selenicereus spp. Ann Bot 93:469–472
Muas Irwan, Mansyah E, Yuliati S, Hendr (2019) Dragon fruit production and marketing in Indonesia: standard quality in the global and regional levels, p 550
Nerd A, Gutman F, Mizrahi Y (1999) Ripening and post-harvest behavior of fruits of two Hylocereus species (Cactaceae). Postharvest Biol Technol: 39–45
Nor S, Razifah MR, Mamat AS, Adzemi MA (2014) Application of gibberellic acid (GA 3 ) in stem cutting of dragon fruit ( Hylocereus polyrhizus ): effects on fruit quality and yield at harvest. J Biol Agric Healthcare 4:51–55
Nyffeler R, Eggli U (2010) A farewell to dated ideas and concepts: Molecular phylogenetics and a revised suprageneric classification of the family Cactaceae. Schumannia 6:109–149
Ortiz-Hernández YD, Livera MM, Colinas LMT, Carrillo SA (1999) Water stress and CO 2 exchange rate of pitahaya ( Hylocereus undatus ). Agrociencia 33:397–405
Pagliaccia D, Vidalakis G, Douhan GW, Lobo R, Tanizaki G (2015) Genetic characterization of pitahaya accessions based on amplified fragment length polymorphism analysis. HortScience 50:332–336
Paull RE, Duarte O (2012) Tropical fruits: crop production science in horticulture 24. CABI, p 325
Book Google Scholar
Perween T (2017) Thesis entitled “Studies on the effect of nutrient application in vegetative and reproductive phenology of dragon fruit”, pp 29–44 (submitted to the Bidhan Chandra Krishi Viswavidyalaya, Mohanpur, West Bengal, India)
Pushpakumara DKNG, Gunasena HPM, Karyawasam M (2005) Flowering and fruiting phenology, pollination vectors and breeding system of dragon fruit ( Hylocereus spp.). Sri Lankan J Agric Sci 42:81–91
Raveh EA, Nerd Mizrahi Y (1997) Responses of two hemiepiphytic fruit-crop cacti to different degrees of shade. Sci Hortic 53:115–122
Ruzainah AJ, Ahmad Ridhwan AR, Nor Zaini CM, Vasudevan R (2009) Proximate analysis of dragon fruit ( Hylecereus polyhizus ). Am J Appl Sci 6(7):1341–1346
Saradhuldhat P, Kaewsongsang K, Suvittawat K (2009) Induced Off-Season flowering by supplemented fluorescent light in dragon fruit. J Int Soc Southeast Asian Agric Sci 15:236–237
Seran TH, Thiresh A (2015) Root and shoot growth of dragon fruit stem cutting as influenced by indole butyric acid. Agric Biol J 1:27–30
Sheng WKW, Sundarasekar J, Sathasivam K, Subramaniam S (2016) Effects of plant growth regulators on seed germination and callus induction of Hylocereus costaricensis . PAK J BOT 48:977–982
Shetty AA, Rana MK, Preetham SP (2012) Cactus: a medicinal food. J Food Sci Technol 49:530–536
Article PubMed Google Scholar
Siddiqua A, Thippesha D, Shivakumar BS, Adivappar N, Ganapathi M (2018) Effect of growth regulators on rooting and shooting of stem cuttings in dragon fruit. J Pharmacogn Phytochem 7:1595–1598
Stintzing FC, Schieber A, Carle R (2002) Betacyanins in fruits from red-purple pitaya, Hylocereus polyrhizus (Weber) Britton & Rose. Food Chem 77(1):101–106
Sushmitha HS, Sathyamurthy B (2018) In silico drug designing studies on dengue virus envelope protein. World J Pharm Pharm Sci 6(9):138–143
Then KH, Faiz OM, Norshafiqah K (2020) The flowering pattern and fruit production of red Pitaya (H. polyrhizus ) under Malaysian growing condition. Int J Agric Innov Res 8:2319–1473
Thomson PH (2002) Pitahaya ( Hylocereus species ) A Promising New Fruit Crop for Southern California (Second edition). Bonsall Publications (California) pp 46
Tran D, Yen C (2014) Morphological characteristics and pollination requirement in red Pitaya ( Hylocereus spp .) Int J Biol Biomol Agric Food Biotechnol Eng 8:274–278
Tran DH, Yen CR, Chen YKH (2015) Flowering response of a red pitaya germplasm collection to lighting addition. Int J Biol Biomol Agric Food Biotechnol Eng 9:175–179
Tripathi PC, Karunakaran G, Sankar V, Senthil Kumar R (2014) Dragon fruit—Nutritive and Ruminative fruit. Technical Bulletin 11/2014. ICAR-IIHR, Central Horticultural Experiment Station, Chettalli Kodagu (PP10)
Uyen DTK, Van Hoa N (2018) Identification of Colletotrichum trucatum causing anthracnose disease on dragon fruit and the efficacy of some biological tools on the mycelial growth of the fungus and disease control management, p 155
Vaillant F, Perez A, Davila I, Dornier M, Reynes M (2005) Colorant and antioxidant properties of red pitahaya ( Hylocereus sp.). Fruits 60:1–7
Valiente-Banuet A, Verdu M (2007) Facilitation can increase the phylogenetic diversity of plant communities. Ecol Lett 10(11):1029–1036
Van To L, Ngu N, Duc ND, Huong HTT (2000) Dragon fruit quality and storage life: effect of harvesting time, use of plant growth regulators and modified atmosphere packaging. Int Symp Trop Subtrop Fruits 575:611–621
Verma RS, Lata R, Ram RB, Verma SS, Prakash S (2019) Effect of organic, inorganic and bio-fertilizers on vegetative characters of dragon fruit ( Hylocereus undatus L.). Plant Pharma Innov J 28:726–728
Wakchaure GC, Kumar S, Meena KK, Rane J, Pathak H (2021) Dragon fruit cultivation in India: scope, constraints and policy issues. Tech Bull 27:47
Wu LC, Hsu HW, Chen YC, Chiu CC, Lin YI, Ho JA (2006) Antioxidant and antiproliferative activities of red pitaya. Food Chem 95:319–327
Wu SH, Sun HT, Teng YC, Rejmanek M, Chaw SM, Yang TYA, Hsieh CF (2010) Patterns of plant invasions in China: Taxonomic, biogeographic, climatic approaches and anthropogenic effects. Biol Invasions 12:2179–2206
Download references
Acknowledgements
We thank the Director of the ICAR CAFRI and the Director of the ICAR RCER for guiding us to write this review.
Author information
Authors and affiliations.
Agroforestry System Research, ICAR-Central Agroforestry Research Institute, Jhansi, Uttar Pradesh, India, 284003
Ashok Yadav, A. Arunachalam, Sandeep Garg, Neha Gangwar, A. K. Handa & Badre Alam
ICAR - Research Complex for Eastern Region, Research Centre, Ranchi, Jharkhand, India, 834010
M. K. Dhakar
Indofil Industries Limited, Thane, Maharashtra, India, 400607
Suchisree Jha
ICAR – Indian Institute of Soil & Water Conservation Research Centre,, Datia, Madhya Pradesh, India, 475661
Darshan Kadam
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ashok Yadav .
Ethics declarations
Conflict of interest.
A. Yadav, M.K. Dhakar, A. Arunachalam, S. Jha, S. Garg, N. Gangwar, A.K. Handa, B. Alam and D. Kadam declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Yadav, A., Dhakar, M.K., Arunachalam, A. et al. A Review on the Scope of Adoption of Underutilized Climate Smart Dragon Fruit ( Hylocereus spp. ) Cultivation. Applied Fruit Science 66 , 297–309 (2024). https://doi.org/10.1007/s10341-023-01006-3
Download citation
Received : 23 May 2023
Accepted : 27 November 2023
Published : 18 December 2023
Issue Date : February 2024
DOI : https://doi.org/10.1007/s10341-023-01006-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Dragon fruit
- Horticulture
- Production technology
- Post-harvest operation
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Antioxidative and Anti-Inflammatory Phytochemicals and Related Stable Paramagnetic Species in Different Parts of Dragon Fruit
Chalermpong saenjum.
1 Cluster of Excellence on Biodiversity-Based Economic and Society (B.BES-CMU), Chiang Mai University, Chiang Mai 50200, Thailand; [email protected]
2 Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand
Thanawat Pattananandecha
Kouichi nakagawa.
3 Division of Regional Innovation, Graduate School of Health Sciences, Hirosaki University, 66-1 Hon-Cho, Hirosaki 036-8564, Japan
Associated Data
The original contributions generated for this study are included in the article; the data presented in this study are available on request from the corresponding author.
In this study, we investigated the antioxidant and anti-inflammatory phytochemicals and paramagnetic species in dragon fruit using high-performance liquid chromatography (HPLC) and electron paramagnetic resonance (EPR). HPLC analysis demonstrated that dragon fruit is enriched with bioactive phytochemicals, with significant variations between each part of the fruit. Anthocyanins namely, cyanidin 3-glucoside, delphinidin 3-glucoside, and pelargonidin 3-glucoside were detected in the dragon fruit peel and fresh red pulp. Epicatechin gallate, epigallocatechin, caffeine, and gallic acid were found in the dragon fruit seed. Additionally, 25–100 mg × L −1 of dragon fruit pulp and peel extracts containing enrichment of cyanidin 3-glucoside were found to inhibit the production of reactive oxygen species (ROS), reactive nitrogen species (RNS), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) in cell-based studies without exerted cytotoxicity. EPR primarily detected two paramagnetic species in the red samples. These two different radical species were assigned as stable radicals and Mn 2+ (paramagnetic species) based on the g-values and hyperfine components. In addition, the broad EPR line width of the white peel can be correlated to a unique moiety in dragon fruit. Our EPR and HPLC results provide new insight regarding the phytochemicals and related stable intermediates found in various parts of dragon fruit. Thus, we suggest here that there is the potential to use dragon fruit peel, which contains anthocyanins, as a natural active pharmaceutical ingredient.
1. Introduction
Dragon fruit ( Hylocereus sp.), commonly known as pitaya or pitahaya, is a member of the cactus family Cactaceae [ 1 ]. It is native to the tropical forest region of Mexico and Central South America [ 2 ]. The peel of the fruit is usually red, with white ( Hylocereus undatus ) and red ( H. polyrhizus , H. costaricensis ) flesh. The fruit is a rich source of nutrients and minerals, including vitamins B 1 , B 2 , B 3 , and C, protein, fat, carbohydrate, crude fiber, thiamin, trigonelline, niacin, alanine, arginine, leucine/isoleucine, glutamate, glutamine, shikimate, rutin, gamma-aminobutyric acid (GABA), valine, choline, pyridoxine, kobalamin, glucose, sucrose, fructose, inositol, betacyanins, phosphorus, iron, formic acid, malic acid, citric acid, fumaric acid, succinic acid, azelaic acid, ascorbic acid, aspartic acid, betalamic acid, betanin, oleic acid, fatty acids, catechins, quercetin derivatives, caffeic acid, 2’- O -glucosyl-betanin, hylocerenin, 2’- O -apiosyl-betanin, phytoalbumin, 2’- O -apiosyl-phyllocactin, and 2’-(5’- O -E-feruloylapiosyl) betanin [ 3 , 4 , 5 , 6 ]. Fiber and vitamins promote the proper functioning of the gastrointestinal system, prevent colorectal cancer and diabetes, remove or increase the secretion of toxic substances such as heavy metals, and control cholesterol levels and blood pressure [ 7 , 8 ]. Betacyanins and betaxanthins, water-soluble pigments in the peel and pulp of dragon fruit [ 9 , 10 ], are used as natural food colorants in the food industry [ 11 ]. The seed of dragon fruit also contains catechin, epicatechin, rutin, quercetin, and myricetin [ 12 ]. Anthocyanins can be found in the skin of H. undatus , including cyanidin 3- O -glucoside, cyanidin 3,5- O -glucoside, and pelargonidin 3,5- O -glucoside [ 13 ]. However, much remains unknown about the paramagnetic species (Mn 2+ , antioxidative organic radicals, etc.) present in dragon fruits and their antioxidant and anti-inflammatory activities.
Electron paramagnetic resonance (EPR) spectroscopy is a very sensitive technique that can non-destructively detect free radicals [ 14 ]. The magnetic-field position and relative intensities of the EPR lines occur between the energy levels of electrons with unpaired spins. Transitions occur between energy levels, which give rise to lines in the spectrum. The EPR spectrum appears either as a series of multiple overlapped lines or as an asymmetric line shape, depending on the paramagnetic species within the sample [ 15 ]. Numerous plants contain various antioxidants to reduce the damage caused by reactive oxygen species (ROS) and reactive nitrogen species (RNS). The antioxidative processes result in the production of antioxidant intermediates. Various antioxidants contain OH groups and conjugated double bonds. The delocalization of unpaired spin forms stable intermediates which are detected by EPR. Additionally, high-performance liquid chromatography (HPLC) is used to separate, identify, and quantitate various antioxidants in plants. However, there are no reports describing the distribution of antioxidative phytochemicals and related intermediates in dragon fruits.
In this investigation, the paramagnetic species in each part of the dragon fruit were examined using EPR and antioxidative phytochemicals were analyzed HPLC. Additionally, the antioxidative and anti-inflammatory activities were also investigated in a cell-based study. The EPR technique measured two types of paramagnetic species in dragon fruits, which displayed antioxidative effects. The EPR line widths and phytochemical activities of various parts of the dragon fruit were discussed.
2. Results and Discussion
2.1. tpc, tfc, and tac content.
Polyphenolic compounds have been reported to be commonly found in both edible and inedible plants [ 16 ]. Flavonoid compounds are usually found in peels, seeds, and stems, while non-flavonoid compounds are located in pulps [ 17 ]. All parts of the dragon fruits have color, except the white pulp ( Figure 1 ). As listed in Table 1 , phenolic and flavonoid compounds were detected in all dragon fruit samples. Both red and white pulp seed were found to have significantly greater phenolic and flavonoid contents than the other parts ( p < 0.05), which is similar to the findings of the study of Nguyen et al. [ 18 ], who reported on the total phenolic compounds in the seed and pulp of red and white dragon fruits. Moreover, Nurliyana et al. [ 19 ] also reported that the peels of both red dragon fruit ( H. polyrhizus ) and white dragon fruit ( H. undatus ) contained higher phenolic compounds than the pulps. The flavonoid content in red and white dragon fruits was reported to be higher in the peel when compared to the pulp [ 20 ]. Interestingly, the extracts from fresh white pulp showed very low levels of antioxidant phytochemicals. In addition, anthocyanin was found in fresh red pulp, red peel, and white peel. Anthocyanins are plant pigments that are responsible for the color of the pulp and the peel of dragon fruits. Malvidin, cyanidin, and delphinidin are anthocyanin compounds found in the peel of the red dragon fruit ( H. polyrhizus ) using liquid chromatography-mass spectrometry (LC-MS) assay [ 21 ].

Red and white dragon fruits used in this study ( A ) and anthocyanin extract prepared from the red peel ( B ).
Total phenolic, flavonoid, and anthocyanin contents.
All values are expressed as means ± standard deviation (SD; n = 3). Different letters in each column indicate a significant difference ( p < 0.05). ND = not detectable.
Three sample extracts, including fresh purple pulp, red peel, and white peel were selected for the analysis of anthocyanin by HPLC. Red pulp seed and white pulp seed were also analyzed for catechin and related compounds by HPLC.
2.2. Chromatographic Analysis of Antioxidative Phytochemicals
The HPLC chromatogram identifying anthocyanins in RP compared with mixed anthocyanin standards, including delphinidin 3-glucoside, cyanidin 3-glucoside, peonidin 3-glucoside, pelargonidin 3-glucoside, delphinidin, and cyanidin, is shown in Figure 2 . HPLC chromatograms indicated that cyanidin 3-glucoside, delphinidin 3-glucoside, and pelargonidin 3-glucoside in average concentrations of 12.67 ± 0.63, 0.82 ± 0.17, and 1.76 ± 0.23 mg/ 100 g dried samples, respectively, were found in the reddish parts of the dragon fruit, including fresh red pulp, red peel, and white peel. The results corresponded to those of Fan et al. [ 22 ], who reported that cyanidin 3-glucoside, cyanidin 3-rutinoside, and delphinidin 3- O -beta- d -glucoside were detected in the reddish coloration of dragon fruit. Moreover, cyanidin 3- O -glucoside, cyanidin 3,5- O -glucoside, and pelargonidin 3,5- O -glucoside were also identified in the peel of dragon fruit ( H. undatus ) extracted using 1% trifluoroacetic acid in methanol as an extraction solvent, with an average concentration of 44.3865 ± 1.3125 mg/100 g of the sample [ 13 ]. It has been suggested that extraction methods and physical, chemical, and biological processes affect the content of anthocyanins in dragon fruit parts due to enzymatic and non-enzymatic changes [ 21 , 23 ].

High-performance liquid chromatography (HPLC) chromatograms of mixed anthocyanin standards and red pulp (RP) extract. The peaks indicate ( a ) delphinidin 3-glucoside, ( b ) cyanidin 3-glucoside, ( c ) peonidin 3-glucoside, ( d ) pelargonidin 3-glucoside, ( e ) delphinidin, and ( f ) cyanidin.
Figure 3 shows the HPLC chromatogram of RP-S compared with the mixed catechin and the related compounds. Among the 10 standards used in this HPLC system, gallic acid, epigallocatechin, catechin, caffeine, catechin, epicatechin, and epicatechin gallate were detected in the seeds of dragon fruit. Catechin and epicatechin were detected as the major components of the dragon fruit seeds. These results corresponded with those of Adnan et al. [ 12 ], who reported that catechin, quercetin, epicatechin, myricetin, and epicatechin were detected in red dragon fruit seed, while catechin was the major flavonoid detected, at 3.60 ± 2.33 mg/g dry weight followed by quercetin at 1.31 ± 0.45 mg/g dry weight. A recent study using ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UPLC-QTOF-MS/MS) analysis by Nguyen et al. [ 18 ] reported that isorhamnetin glycoside, rutin, quercetin hexoside, kaempferol glucorhamnoside, isorhamnetin, and galloylglucoside were detected in both red and white dragon fruit peels, while kaempferol glucorhamnoside, kaempferol glucoside, kaempferol 3-glucoside, gallic acid, and ellagic acid were detected in both red and white dragon fruit seeds, but only isorhamnetin glycoside and isorhamnetin were detected in red dragon fruit seed. There were differences in the content of antioxidative phytochemicals that may be affected by several factors, including fruit species, maturity stage of fruits, geographical location, growing and environmental conditions, and methods of cultivation, which can cause variations in the quality of bioactive compounds in fruits [ 24 , 25 ].

HPLC chromatograms of mixed catechin and related compounds and the extract of the seeds of red pulp (RP-S). The peaks indicate ( a ) gallic acid, ( b ) pyrogallol, ( c ) gallocatechin, ( d ) epigallocatechin, ( e ) caffeine, ( f ) catechin, ( g ) epicatechin, ( h ) epigallocatechin gallate, ( i ) gallocatechin gallate, and ( j ) epicatechin gallate.
2.3. Determination of the Inhibitory Effects on Intracellular ROS and RNS Production
The antioxidant and free radical scavenging activities were strongly correlated with the content of total phenolic and total flavonoid compounds because of the mechanism of the hydrogen donation and electron transfer of both phenolic and flavonoid compounds to free radical molecules [ 26 , 27 ]. The inhibitory effects of the extracts of each part of the dragon fruit on nitric oxide production in LPS/IFN-γ-induced RAW 264.7 cells and on intracellular ROS production in PBMC cells are illustrated in Table 2 . The fresh red pulp extract exhibited the highest inhibition of induced RNS and ROS production in cell-based studies. Both tested samples containing anthocyanin and catechin and related compounds exhibited inhibitory effects on intracellular ROS and RNS production. Interestingly, the tested samples containing anthocyanin including fresh red pulp, red peel, and white peel demonstrated a higher inhibition activity for induced RNS and ROS production compared with samples containing catechin and related compounds (red pulp seed and white pulp seed). These results corresponded with the report of Wang et al. [ 28 ] and Kuskoski et al. [ 29 ], who reported that anthocyanins namely, delphinidine and cyanidin 3-glucoside have higher antioxidative properties in comparison with known antioxidants such as catechin and vitamin E derivatives with the trolox equivalence antioxidant capacity (TEAC) 2.45 ± 0.45 and 1.85 ± 0.26 mM, respectively compared to the TEAC of peonidin 3-glucoside, pelargonidin 3-glucoside, and malvidin 3-glucoside in the value of 1.49 ± 0.19, 1.50 ± 0.08, and 1.41 ± 0.07 mM, respectively. The antioxidative property of anthocyanins depends on the number of free hydroxyl groups around the pyrone ring, while the position of the hydroxyl groups and the conjugated double bonds also plays an important role in antioxidant activity. Additionally, 3’,4’ di-OH group in B ring of delphinidine and cyanidine plays a key role in antioxidant and free radical scavenging activities [ 30 ].
The 50% inhibitory concentrations (µg/mL) of extracts for the production of reactive nitrogen species (RNS) and reactive oxygen species (ROS).
All values are expressed as means ± standard deviations (SD; n = 3). Different letters in each column indicate a significant difference ( p < 0.05). ND = not detectable.
2.4. Determination of Anti-Inflammatory Activities
Inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) play a pivotal role in catalyzing the rate-limiting step in prostaglandin biosynthesis and in the mediation of the inflammation process. The inhibitory effects of the selected anthocyanin extracts, including fresh red pulp, red peel, and white peel, on iNOS and COX-2 production in LPS/IFN-γ-induced RAW 264.7 cells are illustrated in Figure 4 and Figure 5 , respectively. The result revealed that the tested samples in the concentrations of 25 to 100 mg × L −1 exhibited an inhibitory effect on both iNOS and COX-2 production in LPS/IFN-γ-induced RAW 264.7 cells in a dose-dependent manner without any cytotoxicity. Our results correspond to those of Jung et al. [ 31 ], who reported that cyanidin-3-glucoside blackberry reduced nitric oxide and prostaglandin E2 production in LPS-stimulated RAW264.7 cells by values of 39.7% and 52.6%, respectively. Additionally, the protein expressions of iNOS and COX-2 in LPS-stimulated RAW264.7 cells were also decreased in cells treated with cyanidin-3-glucoside prepared from blackberry via down-regulated nuclear factor-kappa B (NF-κB) expression and up-regulated I-κB expression in LPS-stimulated macrophages. Moreover, cyanidin-3-glucoside enriched fraction prepared from black rice containing the total anthocyanin and total flavonoid content in the values of 8.1 ± 1.9 mg cyanidin 3-glucoside/g extract and 42.9 ± 2.1 mg catechin/g extract, respectively, also significantly inhibited the LPS-induced production of NO and the expression of iNOS and COX-2 in RAW 264.7 cells via the downregulation of the NF-kB and activator protein 1 (AP-1) signaling pathways [ 32 ]. Furthermore, cyanidin 3-glucoside prevented the tumor necrosis factor-αTNF-α) induced inflammation process in Caco-2 cells through increasing the translocation of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor that may regulate the expression of antioxidant proteins which protect against oxidative damage-induced by inflammatory cytokines, into the nucleus, thus activating antioxidant and detoxifying genes [ 33 ].

The inhibition effect on iNOS production in LPS/IFN-γ-induced RAW 264.7 cells of selected anthocyanin extracts (RP, WP, RPe, and WPe). * Significant differences at the 95% confidence interval compared to control RAW 264.7 cells treated with LPS and IFN-γ.

The inhibition effect on COX-2 production in LPS/IFN-γ-induced RAW 264.7 cells of selected anthocyanin extracts (RP, WP, RPe, and WPe). * Significant differences at the 95% confidence interval compared to control RAW 264.7 cells treated with LPS and IFN-γ.
The overall results indicate that the cyanidin 3-glucoside-enriched extract prepared from the red pulp and peel of dragon fruit exhibited potent antioxidant effects and may suppress inflammatory response through the inhibition of NO and ROS production, and it has been proposed to modulate gene expression.
2.5. EPR of Dragon Fruits
The EPR spectrum of dragon fruit peel, pulp, and one to two whole seeds is shown in Figure 6 . The EPR spectrum here is composed of two kinds of signals; these signals were relatively stable, with no depreciation in the signal for at least a month when the samples were kept at 5 °C. One signal corresponds to Mn 2+ complexes, whereas the other indicates organic radicals.

EPR spectra obtained by a wide-range scan (300 mT) of the red and white peel of dragon fruits. ( A ) shows the EPR spectrum of the red peel. The upper figure shows the expansion of the 300 to 375 mT region. The arrow indicates the magnetic field at ~336 mT. ( B ) shows the EPR spectrum of the white peel. The arrow (~335 mT) indicates organic radicals. The asterisk is not known at this point.
The first signal is characteristic of a Mn 2+ (M I = 5/2) -related sextet in Figure 6 ; the natural abundance of manganese ( 55 Mn isotope) was known to be 100%. The upper inset figure shows the expansion of the 300 to 375 mT region. The arrow indicates the magnetic field at approximately 336 mT. The signal at the arrow has a narrower line width than the other signals. This indicates that the signal is different in different species. Thus, the EPR spectra composed from this sextet were attributed to Mn 2+ peaks. The hyperfine structure of Mn 2+ is at 6.8 to 10.7 mT ( Figure 6 B). The hyperfine values of the sextet are also similar to previously reported values [ 15 ]. The apparent changes in hyperfine couplings from low to high fields were found to be larger in high fields, owing to the correlation time and overlap with other broad features. A similar EPR spectrum was previously reported in apple seeds [ 15 ]. Thus, the observed signatures from these two different radical species were assigned as indicators of stable organic radicals and Mn 2+ complexes. The asterisk features (~260 mT) are not clear at this time ( Figure 6 B). The EPR spectrum of Figure 6 B also overlaps with the organic radical and broad features. The Mn 2+ signals were clearly detected. The broad signal at ~260 mT is not known at this point. It was noted that the EPR intensities of dragon fruits became larger when the samples were submerged in H 2 O and dried out in a refrigerator overnight. The amplitude of the signal may be related to the oxidation and/or antioxidation of the compounds in each sample.
We further examined the EPR signal for the red peel samples. We cut out approximately 1 × 2 × 3 mm 3 red peel (~0.0009 g) and submerged it in distilled water (~40 μL) overnight in a 5 °C refrigerator. We took ~10 μL of the solution using a glass capillary (i.d., 0.9 mm; o.d., 1.2 mm) and measured the solution. We observed the EPR signal from the distilled water extracted solution. The EPR spectrum is presented in Figure 7 . The ΔH pp of the EPR peak is ~1.8 mT. The wider signal indicates various radicals overlapping in the region. In addition, the results suggest that the motional states of various intermediates (radicals) differ between the dry and aqueous sample solutions. Note that the dilution is the major concern for the detection, although the amount of compounds depends on the plant sample.

EPR spectra of red peel-related samples. Upper spectrum is for the dry red peel sample. The bottom one is for the solution of red peel samples submerged in distilled water. The bottom spectrum was accumulated four times.
Figure 8 shows the EPR spectra of various dragon fruits (red and white). The g-value of the EPR signal is approximately 2.004. The EPR spectra of the red dragon fruit peel and pulp are similar; the line widths are in the range 0.59–0.66 mT, as indicated in Table 3 . However, the peel of white dragon fruits has a broader line width (ΔHpp = 1.45 mT) ( Figure 8 B), about twice as broad as that of the red peel. In addition, the EPR intensities of the white peel and pulp are very weak, even taking into account the weights measured. The broad EPR line may be due to the unresolved hyperfine coupling of one radical due to an inhomogeneous environment and/or the overlap of spectra from different organic radicals with different g-values and hyperfine coupling constants. Thus, one can detect the broader line in the sample [ 34 ]. Relatively sharp line widths were obtained for red and white dragon fruit seeds ( Figure 8 E,F). The baselines of the EPR spectra in Figure 8 are not flat due to the overlap with other features, as the organic radicals appear between the third and the fourth Mn 2+ hyperfine couplings.

EPR spectra of various parts of dragon fruits. ( A , B ) show the spectra of the peel of red and white dragon fruits, respectively. ( C , D ) show the spectra of the pulp of red and white dragon fruits, respectively. ( E , F ) show the spectra of the seeds of red and white dragon fruits, respectively. The sample weight measured is indicated at the right side of each spectrum.
EPR peak-to-peak line width of dragon fruits.
All values are expressed as means ± error ( n = 3).
In general, the EPR line width can be influenced by two factors. One is the motion of the radical, and the other is the number of different radicals overlapping. The colored samples (peel and pulp) show very similar line widths, but not the white peel. The border line width of the white peel could have been caused by the overlapping of multiple EPR lines. These multiple signals could be due to different species in the white peel.
Figure 9 shows a bar plot of the relative area of the EPR signal for various dragon fruit parts. The peel and seeds exhibit large signal areas. The analysis of the EPR signal was obtained by taking a double integral of the spectrum. Then, each area was divided by the weight of the samples measured, because the signal intensity is proportional to the amount of the sample measured. The relative area of the seeds is approximately eight to nine times larger than that of the peel. The results suggest that the total radical concentration here is much higher than in other parts of the dragon fruits. Although the peak intensity of the white peel is very weak, the signal area of the peel is close to that of the red peel due to the broad line width. It is also interesting to note that the pulp parts show very weak signals for both.

Bar plot showing the relative areas of EPR signals as a function of the part of the dragon fruit used. The area of each EPR spectrum was obtained by the double integral. The results are expressed as the mean ± standard error. The letters (R and W) represent red and white, respectively.
The EPR signal area is proportional to the total concentration of radicals in the samples. The seeds have more radicals than the other samples. The seeds have larger amounts of antioxidants, as determined by HPLC. These results are consistent with those of the HPLC. In addition, the seeds are different in color and contain more phenolics than the other parts of the fruit. The results from the EPR and HPLC are consistent, although nondestructive EPR can detect biochemical intermediates in fruits. Stable intermediates, which delocalize unpaired electrons in antioxidants, are present in the seeds.
3. Materials and Methods
3.1. samples preparation.
Two kinds of dragon fruits, red and white pulp, were purchased from the royal project shop, Chiang Mai, Thailand, in June 2020. Samples were cut out from the dragon fruits for EPR measurements and stored in a 5 °C refrigerator to dry them. Each sample was inserted into an EPR tube (o.d., 5.0 mm; i.d., 4.0 mm) for measurements. For other experiments, fresh red pulp (RP), red pulp seed (RP-S), red peel (RPe), fresh white pulp (WP), white peel (WPe), and white pulp seed (WP-S) were freeze-dried (0.07 mbar, −45 °C for 48 h) and cut into small pieces. Then, all the samples were placed in a hydroalcoholic solution at pH 2 to obtain the extracts. The obtained solutions were filtrated, and the solvent was evaporated under reduced pressure and vacuum-dried [ 35 ].
3.2. Total Phenolic Measurement
The total phenolic contents (TPC) of each dragon fruit extract were measured using a slightly modified Folin–Ciocalteu colorimetric assay. All the results are expressed as mg gallic acid equivalent (GAE) per g of dried weight [ 36 ].
3.3. Total Flavonoid Measurement
The total flavonoid contents (TFC) of each dragon fruit extract were analyzed using a modified colorimetric assay, as described by Phromnoi et al. [ 37 ]. Briefly, 150 μL of 5% sodium nitrite was mixed with 2 mL of distilled water. Then, 500 μL of dragon fruit extracts or positive control (quercetin) were added to a mixture solution. Sample mixtures were incubated at room temperature for 5 min and protected from light. Then, 10% aluminum chloride hexahydrate solution was added and the mixture was incubated for 5 min. Finally, 1 mL of 1 M sodium hydroxide was added and the total volume was adjusted to 5 mL using deionized water. The mixture solutions were then incubated for 10 min at room temperature. After incubation, the absorbance was measured at 510 nm and the TFC was expressed as the mg quercetin equivalent (QE) per g of dried weight.
3.4. Total Anthocyanin Measurement
The total anthocyanin content (TAC) was measured using the slightly modified pH differential method of Pengkumsri et al. [ 38 ]. Briefly, 4.0 mL of the buffer solution of pH 1.0 or 4.5 and 1 mL of samples or positive control (cyanidin chloride) were mixed and incubated at room temperature for 20 min with protection from light. After incubation, the absorbance was measured at 510 and 700 nm. TAC was expressed as the mg cyanidin chloride equivalent per g of dried weight.
3.5. Chromatographic Analysis of Anthocyanins
All the samples were analyzed for anthocyanins by reverse-phase HPLC with slight modifications from Pengkumsri et al. [ 38 ] using an Agilent 1200 equipped with a multiwavelength detector (Agilent Technologies Inc., Santa Clara, CA, USA). Symmetry RP18 Column (4.6 mm of diameter × 250 mm of length, 5 µm particle diameter, Waters Co., Ltd., Milford, MA, USA) was used to separate each form of anthocyanin and the detection wavelength was set at 520 nm. Phosphoric acid (3%) in acetonitrile and phosphoric acid (3%) in deionized water was used as the mobile phase at a flow rate of 1.0 mL/min. The linear gradient elution was operated from 0 to 40 min, with acetonitrile ranging from 10% to 20%. All the samples were tested in triplicate.
3.6. Chromatographic Analysis of Catechin and Related Compounds
Catechin and related compounds, including epicatechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, gallocatechin, gallocatechin gallate, gallic acid, and caffeine, were analyzed by reverse-phase HPLC according to the conditions of Saenjum et al. [ 39 ] using an Agilent 1200 equipped with a multiwavelength detector. Briefly, the detection wavelength was set at 210, 278, and 325 nm. The Symmetry Shield RP18 Column (4.6 mm of diameter × 250 mm of length, 5 µm particle diameter, Waters Co., Ltd.) was used to separate each form of catechin and its related compounds. The mobile phase consisted of 10% acetonitrile in 0.1% acetic acid and deionized water at a flow rate of 1.0 mL/min.
3.7. Determination on Inhibition Effect on Intracellular Reactive Oxygen Species (ROS) Production
The inhibitory effect of dragon fruit extracts on intracellular ROS production was determined following the modified method of Banjerdpongchai et al. [ 40 ]. Briefly, peripheral blood mononuclear cells were pretreated with 10–100 mg × L −1 of tested samples for 12 h. Then, 5 mM of hydrogen peroxide (H 2 O 2 ) was added for 30 min to initiate ROS production. DCFH-DA solution was added to each mixture and incubated at 37 °C and 5% CO 2 for 30 min. Fluorescence intensity was measured using a fluorescent microplate reader at an excitation wavelength of 480 nm and an emission wavelength of 525 nm. N -Acetyl cysteine, cyanidin 3-glucoside, and L -ascorbic acid were used as positive controls.
3.8. Determination of the Inhibitory Effect on Intracellular Reactive Nitrogen Species (RNS) Production and Anti-Inflammatory Activities
The inhibition of nitric oxide production was assayed using the improved method of Sirithunyalug et al. [ 41 ] and Phromnoi et al. [ 19 ], with some modifications. Initially, RAW 264.7 cells were pretreated with various concentrations of the tested samples at concentrations of 10–100 mg × L −1 and the positive controls, curcumin and cyanidin 3-glucoside, at concentrations of 2.5–50 mg × L −1 . After a 12 h incubation period, LPS and IFN-γ were added to the media. After 48 h of incubation, the culture medium supernatants were collected to analyze the level of nitric oxide. Griess reagent was used to quantify the nitrile level as an indicator of nitric oxide production; the absorbance was measured at 540 nm. Moreover, cells were lysed to yield cell lysates using the CelLytic TM M Cell Lysis Buffer (Sigma, C2978, Merck KGaA, Darmstadt, Germany) to perform the assay for iNOS and COX-2 using the mouse ELISA kit following the manufacturer’s protocol. The quantity of DNA was measured by the Quant-iT PicoGreen Assay (Invitrogen, {"type":"entrez-protein","attrs":{"text":"P11496","term_id":"461779","term_text":"P11496"}} P11496 , Thermo Fisher Scientific Inc., MA, USA), while that of the protein produced by the HT-29 cells was analyzed using Bradford reagent (Sigma Chemical Co., Ltd., St. Louis, MO, USA).
3.9. EPR Measurements
A modified JEOL RE-3X 9 GHz EPR spectrometer (JEOL Ltd., Tokyo, Japan) was used for various measurements. The system was operated in X-band mode at approximately 9.43 GHz using a 100 kHz modulation frequency. All the EPR spectra were obtained from a single scan. The typical EPR settings were as follows: microwave power, 5 mW; time constant, 0.1 s; sweep time, 4 min; magnetic field modulation, 0.32 mT; and sweep width, 10 mT. The details were described in the previous report [ 42 ].
3.10. Statistical Analysis
IBM SPSS Statistics Baes 22.0 was used for statistical analysis. Data are expressed as mean ± standard deviation (SD). Statistical analysis was determined using one-way analysis of variance. Significant difference at the levels of p < 0.05 was measured in this study.
4. Conclusions
Anthocyanins namely, cyanidin 3-glucoside, delphinidin 3-glucoside, and pelargonidin 3-glucoside were detected in the dragon fruit peel and red pulp. Cyanidin 3-glucoside was analyzed as a major component in the dragon fruit peel and red pulp. Catechin, epicatechin, epicatechin gallate, epigallocatechin, caffeine, and gallic acid were found in the dragon fruit seed. Moreover, cyanidin 3-glucoside enriched extracts prepared from dragon fruit were found to inhibit the production of reactive oxygen species (ROS), reactive nitrogen species (RNS), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) without exerting cytotoxicity in this cell-based study. Additionally, non-destructive EPR detected two different paramagnetic species in dragon fruits. The line broadening of the radical in the white peel could be due to the different radical moieties present. The red peel of dragon fruits contained higher amounts of antioxidants than the white pulp of dragon fruits. The results regarding antioxidative phytochemicals and related intermediates were consistent between the EPR and HPLC measurements. Thus, HPLC and EPR spectroscopy provided useful information regarding antioxidants and related phytochemicals in dragon fruits. EPR is a useful method that can be applied for the evaluation of stable paramagnetic species and related antioxidative intermediates in bioresources or waste biomass samples. In summary, the obtained results demonstrated the potential of cyanidin 3-glucoside-enriched extract prepared from a waste biomass, dragon fruit peel, to be used as a natural active pharmaceutical ingredient (natural API) for nutraceutical and nutricosmetic products.
Acknowledgments
Part of this research was supported by KAKENHI, grant number 18K19890, from the Japan Society for the Promotion of Science (JSPS) (K.N.); the Cluster of Excellence on Biodiversity-based Economics and Society (B.BES-CMU), Chiang Mai University, Chiang Mai, Thailand (C.S.); and a postdoctoral fellowship granted by Chiang Mai University, Chiang Mai, Thailand (T.P.).
Author Contributions
C.S. and K.N. designed the study; C.S. and T.P. collected and extracted the plant materials; C.S., T.P. and K.N. performed the experiments; C.S., T.P. and K.N. wrote, reviewed, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
This research was funded by KAKENHI, grant number 18K19890, from the Japan Society for the Promotion of Science (JSPS).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Sample Availability
Samples of the dragon fruits are available from the authors.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
Dragon fruit has been used as a medicinal food since ancient times by Mayas—using the fruit and the flowers as a hypoglycemic, wound disinfectant and diuretic, for dysentery, tumor dissolution, and as a healing agent. ... Quantitative research type quasi-experiment (pre-test and post-test nonequivalent control group) with 32 students (4 ♂ ...
Dragon fruit or pitaya is an exotic tropical plant that brings multiple benefits to human health thanks to its high nutritional value and bioactive compounds, including powerful natural antioxidants.
Dragon fruit (Hylocereus spp.), also known as Pitaya or Pitahaya belonging to the family Cactaceae, is an most important tropical fruit crop as it is rich in antioxidants (a source of vitamins and ...
Dragon fruit peel is a rich source of antioxidant dietary fibre that can improve the quality and shelf life of meat products. This article presents the results of a study that evaluated the effects of dragon fruit peel powder on the physicochemical, microbiological, and sensory characteristics of chicken nuggets. The article also discusses the potential health benefits of dragon fruit peel as ...
1. Introduction. Dragon fruit, otherwise called pitaya or pitahaya, is an edible fruit of the Hylocereus genus.Hylocereus species are herbaceous perennial climbing cactus mainly distributed in subtropical and tropical regions, and highly tolerant to drought. It is a native of Southern Mexico, Guatemala, and Costa Rica (Mizrahi et al., 1997).The crop has about 20 years of life span and, and ...
Research Method: Two varieties (BAU Dragon fruit 1 and BAU Dragon fruit 2) and four flowering times (May, June, July, and August) were selected for this investigation.
The color of dragon fruit flesh comes from betacyanins and betaxanthins content [].In this research, significant differences were observed for total betacyanins content among the DI and DT, with DI showing doubled amount in comparison to DT (Table 1).These results are in agreement with the literature data [], where high content of betacyanin in the peel of red dragon fruit was shown.
Total phenolics and flavonoids content varied between 25 and 55 mg GAE and 15-35 mg CE per 100 g, respectively. H. polyrhizus have a significantly high quantum of phenolics and antioxidant potential than H. undatus. 100 g fruit contained about 120-200 mg K, 30-45 mg Mg, 20-45 mg Ca, 20-35 mg P, 0.70-1.5 mg Fe, and 0.20-0.40 mg Zn.
Abstract Dragon fruit (DF) ... This review paper provides detailed insights of DF processing into different value-added products, its medicinal benefits, bioactive potential, and the physico-chemical changes occurring in DF during processing such as drying, fermentation, extraction, and so forth. ... 65°C for 30 min, the RFDF juice was treated ...
Dragon fruit (DF) is an exotic awe‐inspiring fruit, newly entered the Indian market and gaining wide popularity in agriculture sector, and among general public due to its mesmerizing color, shape, size, and the esh of the fruit. It. fl. is loaded with small black colored edible seeds enclosed within the esh.
Due to their economic importance, an increasing number of research papers have been published in recent years to study dragon fruits from the food science perspective 18,19,20,21,22 and using RNA ...
H. Polyrhizus (red skinned fruit with red flesh) (Hunt 2006, Hamidah et al. 2017. Dragon fruit is best eaten as fresh in the form of juice, jam or preserves (Perween et al. 2018) or dried fruit or ...
Advanced research on dragon fruit will unleash many more therapeutic benefits and can give mechanistic insight about its activities. PRACTICAL APPLICATIONS: Phytoconstituents play a vital role in the treatment of various diseases and for the improvement of human health, in general. Dragon fruit is known to be having antioxidant, anti-microbial ...
Agricultural crops especially fruit trees are constrained by edaphic stresses in shallow soils with low water retention and poor fertility. Therefore, interventions of shifting to trench planting for better root anchorage and replacing the filling soil were evaluated for 8 years in dragon fruit (Hylocereus undatus) cultivated in Deccan Plateau of peninsular India.
Dragon fruit is an important fruit crop in the Cactaceae family and is known for its high nutraceutical properties, greater monetary returns, low maintenance and stress resistance. Three of its species viz. Hylocereus undatus, H. megalanthus, and H. polyrhizus are extensively grown in the world. Improved agronomic practices have been important for obtaining quality yield. Available literature ...
Dragon fruit belongs to family Cactaceae, is a perennial semi epiphytic vine. It was initially used as ornamental plant and. latter d ue to its health be nefits and market v alue emerged a s a new ...
The dragon fruit, thanks to its nutritional properties, biological activities, and commercial value has become a costeffective product for the Vietnamese economy, particularly in the poorest areas of the Mekong Delta region, and a driving force in the sustainable development of Vietnam under the challenges posed by the global climate change. Dragon fruit or pitaya is an exotic tropical plant ...
In Guizhou, recent research interest in dragon fruit has mainly focused on the molecular mechanisms of plant abiotic stress tolerance (Fan et al., 2014; Li et al., 2019) and betalain metabolism (Wu et al., 2019, 2020; Zhou et al., 2020).There are few reports concerning processing of downstream products of dragon fruit.
Dragon fruit contains 73.9 energy, 1 g protein, 0 g fat, 0.5 g fiber, 23 g carbohydrates, 23 g. sugar, calcium 30 mg. Sodium 10.9 mg, Vitamin-C 1.79 mg. G) Use of fruit. The lotus/dragon fruit can ...
In this study, we investigated the antioxidant and anti-inflammatory phytochemicals and paramagnetic species in dragon fruit using high-performance liquid chromatography (HPLC) and electron paramagnetic resonance (EPR). HPLC analysis demonstrated that dragon fruit is enriched with bioactive phytochemicals, with significant variations between each part of the fruit. Anthocyanins namely ...
The total flavonoid contents (TFC) of each dragon fruit extract were analyzed using a modified colorimetric assay, as described by Phromnoi et al. . Briefly, 150 μL of 5% sodium nitrite was mixed with 2 mL of distilled water. Then, 500 μL of dragon fruit extracts or positive control (quercetin) were added to a mixture solution.
Summary Recovery of bioactive or functional compounds from fruit wastes is a current research trend, ... Search for more papers by this author. Ngoc Lieu Le, Corresponding Author. Ngoc Lieu Le ... the main groups of functional compounds from dragon fruit peels including betalains, phenolics and dietary fibres are comprehensively scrutinised ...
act as natural antioxidants and anti-inflammatory agents (Jiang et al., 2021). Carica papaya, commonly known as papaya, is a large herbaceous plant found in tropical areas. Different parts of the ...