- Open supplemental data
- Reference Manager
- Simple TEXT file

People also looked at
Review article, xylitol: bioproduction and applications-a review.

- 1 Department of Biotechnology, School of Bioengineering, College of Engineering and Technology, Faculty of Engineering and Technology, SRM Institute of Science and Technology, Chengalpattu, India
- 2 School of Water, Energy and Environment, Cranfield University, Cranfield, United Kingdom
Xylitol, a natural compound classified as a sugar alcohol, is found diversely in fruits and vegetables in small quantities. Commercial production of xylitol has expanded due to its health benefits and wide applications as an alternative sweetener in food and pharmaceutical products. Production of xylitol on large scale is industrially being achieved by the chemical method. However, the biotechnological method offers the possibilities of lowered cost and energy compared to the chemical methods. It involves the conversion of xylose to xylitol by microbes or enzymes which is environmentally safe. This review highlights the prospects of the biotechnological method of xylitol production. Various microorganisms that have been used to produce xylitol, the bioprocess parameters, and genetic modifications to increase xylitol yield have been reviewed. In addition, the applications, benefits, and safety concerns to health have been discussed.
Introduction
Xylitol is one of the naturally occurring pentitols (five-carbon sugar alcohol) with a molecular formula of C 5 H 12 O 5. It is a white crystalline sugar, commercially used as an artificial sweetener in the food and pharmaceutical industries. Xylitol has a lesser calorific value (2.4 cal/g) compared to sucrose (4.0 cal/g) but has a relative sweetness almost equal to sucrose ( Chen et al., 2010 ; Tiefenbacher, 2017 ). The xylitol metabolism is independent of insulin; hence it has been used as a safe sucrose substitute for patients with diabetes. In the human gastrointestinal tract, 50–75% ingested xylitol is not absorbed ( Rehman et al., 2013 ). Owing to its anti-cariogenic properties, it has been used in the production of chewing gums and toothpaste. Many studies show the role of xylitol to reduce the incidence of respiratory and middle ear infections. Xylitol increases the absorption of calcium thereby helps in combating osteoporosis ( Mussatto, 2012 ).
In 1891, a German and French chemist concurrently discovered xylitol ( Rehman et al., 2016 ). Several years later, xylitol crystals were purified successfully, and it was used widely as an alternative to sugar during World War II, to meet the severe shortage faced during the war ( Rehman et al., 2013 ). Xylitol was recognized as a potent sweetener after the discovery of its property of not elevating blood sugar levels. Countries like Germany, Japan, and Italy started to use xylitol as a part of the diabetic diet. Later in the 1970s, the dental benefits of xylitol came into light after the “Turku Sugar Studies” ( Janakiram et al., 2017 ). Since 1975, various xylitol substituted products were introduced globally.
Xylitol is naturally present in little quantity in diverse vegetables and fruits. Some of the fruits containing xylitol are strawberry, raspberry, banana, yellow plums, and vegetables containing xylitol include cauliflower, spinach, carrot, onion, white mushroom, eggplant, lettuce, and pumpkin. It is also found in hardwood trees like birch and beechwood and in husks and stalks of the plants ( Chen et al., 2010 ). Humans and animals produce small quantities of xylitol as well, during the metabolism of glucose. On average, an adult human produces 5 to 15 g of xylitol per day ( Rehman et al., 2016 ). Since the amount of xylitol present in these natural sources is low, its extraction from these sources is inefficient. Currently, the chemical method of xylitol production is being used commercially to meet the xylitol demand. In this chemical process, xylose extracted from the lignocellulosic biomass undergoes catalytic hydrogenation to produce xylitol. Alternatively, to reduce the high production costs, biotechnological methods of xylitol production from lignocellulosic biomass can be employed ( Mathew et al., 2018 ). There is an increasing interest in commercializing biotechnological methods for the sustainable production of xylitol.
In this review, the production of xylitol has been discussed, with the main focus on the biotechnological process. Although different microorganisms and enzymes have been studied for xylitol production, there is an utmost need for exploring engineered strains for enhanced xylitol production. Hence this review focuses on the microbial and enzymatic production of xylitol and highlights the metabolic engineering of microorganisms as well. Furthermore, the applications of xylitol in the food and pharmaceutical industries, its health benefits, and safety concerns have been summarized.
Chemical And Biological Methods For Xylitol Production
Industrial production of xylitol is carried out by a chemical hydrogenation process where Raney nickel is used as a catalyst to convert xylose from hemicellulosic hydrolysate to xylitol. It involves five steps as follows: (1) Hydrolysis of the lignocellulosic biomass by acid to get monomeric sugars; (2) Treatment of hydrolysate to purify xylose; (3) Catalytic hydrogenation (usually carried out at a temperature of 353–413 K); (4) Xylitol purification; (5) Xylitol crystallization ( Arcaño et al., 2020 ). By employing the catalytic hydrogenation method, about 50–60% of xylan from the hydrolysate can be converted to xylitol ( Naidu et al., 2018 ). Other catalytic systems with Ruthenium, a noble metal, have also been proven to be effective in the hydrogenation of xylose to xylitol ( Baudel et al., 2005 ; Yadav et al., 2012 ; Mishra et al., 2013 ). Recently, a catalytic formulation where noble metals have been replaced by non-noble metals like cobalt supported on silica is a better alternative catalyst ( Audemar et al., 2020 ). However, the dependence of the chemical mode of synthesis seems to be non-sustainable and costly which prompted for biological routes of conversion of sugars to xylitol.
The biotechnological conversion of xylose to xylitol is carried out by microorganisms that produce enzymes for xylose metabolism. The substrate, xylose, is obtained from the hemicellulose-rich fraction of lignocellulosic biomass such as wood, agricultural wastes, or aquatic weeds ( Sindhu et al., 2017 ; Espinoza-Acosta, 2020 ). Yeast is the predominant microorganism that can utilize xylose and ferment it to xylitol. Certain bacteria and filamentous fungi are also known to ferment xylose. In addition to microbial fermentation, enzymes have been used to produce xylitol as well. Furthermore, genetically engineered strains are being developed to improve xylitol yield to meet the industrial requirements ( Xu et al., 2019 ).
The chemical method of xylitol production involves high pressure and temperature. It requires high energy and it is labor-intensive as well ( Chen, 2015 ). Though the raw material for xylitol production is available throughout the world, the industrial expansion is limited to a few countries in Europe, Asia, and the United States due to the expensive production process ( Arcaño et al., 2020 ). In the quest for an alternative method for producing xylitol, biotechnological methods have attracted the interest of researchers, which could address the above-mentioned limitations of the chemical method. Xylose bioconversion requires a simple fermentative approach and the overall energy consumption is relatively less ( Chen, 2015 ). Microbial fermentation can be carried out under milder pressure and temperature compared to chemical methods. In the bioconversion process, organic waste can be extensively utilized and their environmental burden is reduced. Xylitol obtained through bioconversion can be used safely in food products as it does not have the risk of the presence of metal catalyst debris. The biotechnological process is safer and environmentally less polluting. Some inhibitors or impurities formed during hydrolysis of the biomass are either utilized or degraded partially by the microorganisms thus facilitating easier purification of the produced xylitol ( Hernández-Pérez et al., 2019 ).
Consequently, the biotechnological method of producing xylitol has an added advantage in reducing production and purification costs and has the potential to replace chemical methods in terms of efficiency and sustainability. This is one of the main reasons behind the implementation of bioconversion methods of xylitol production by the leading manufacturers such as Thomson Biotech (China) and ZuChem (US) ( Ravella et al., 2012 ). Figure 1 gives an overview of xylitol production strategy and its applications.
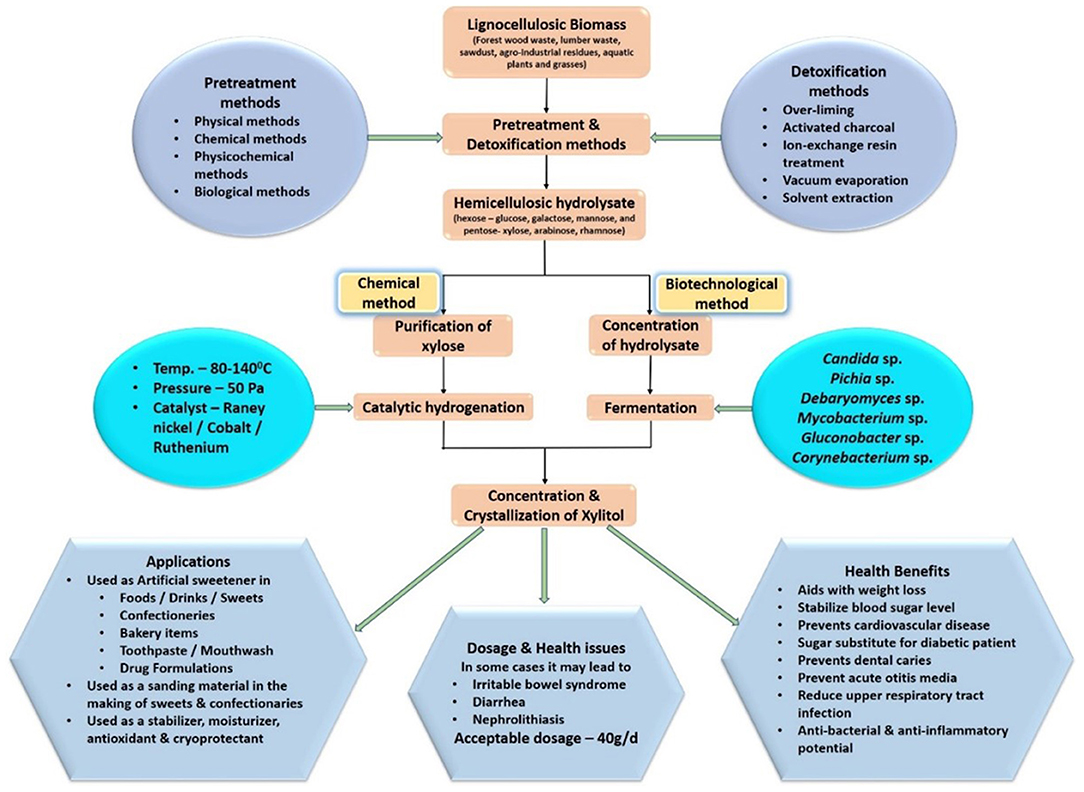
Figure 1 . Overview of Xylitol production and its applications.
Bioproduction Of Xylitol
Xylitol production from lignocellulosic biomass.
Unlike the chemical method of xylitol production, the biotechnological production of xylitol does not solely depend on purified xylose as a substrate. Bioconversion of xylitol can be done effectively by using various lignocellulosic biomass rich in hemicellulosic fraction as a carbon source ( Chandel et al., 2018 ). The major portion of lignocellulosic biomass comprises cellulose, hemicellulose, and lignin while a small amount of pectin, ash, protein, and extractives are also present ( Kumar et al., 2009 ). The cellulose bound to hemicellulose forms a matrix with lignin. Thus, formed lignocellulosic material functions as a protective complex for plants. Based on the species and age, different plant materials have a difference in the compositions of these constituents. Hemicellulose, which commonly makes up 25–35% is the second most abundant polymer of the lignocellulosic biomass, after cellulose. In some plants like water hyacinth, around 38–43% is made up of hemicellulose ( Patel et al., 1993 ; Varanasi et al., 2018 ). Hemicellulose is a branched-chain heteropolymer made up of different sugars based on which it is classified as xylans (xylose and arabinose), mannans (mannose, galactose, glucose), and xyloglucans (xylose and glucose) ( Naidu et al., 2018 ). Among these, xylan, which is comprised of β-D-xylopyranosyl units, makes up most of the hemicellulose fraction.
Lignocellulosic biomass that are rich in xylan content are widely used for xylitol production. Hemicellulose of hardwood (such as birch and oak) is found to have more xylose than softwood ( Winkelhausen and Kuzmanova, 1998 ). In recent decades, wastes generated from agriculture or forestry are being employed to get value-added products such as xylitol. Sugarcane bagasse, corn stover, fruit pomaces, rice, and wheat straw, and sawdust are commonly investigated for xylitol bioproduction ( de Albuquerque et al., 2014 ). The lignocellulosic biomass selection for industrial usage should be based on the presence of high xylan content, availability, and proximity to the industry.
Pretreatment and Hydrolysis
The hemicellulosic fraction of the lignocellulosic biomass must be hydrolyzed to recover the pentose sugar, xylose, which is the main substrate for xylitol production. Compared to cellulose, it is easier to hydrolyze hemicellulose owing to its branched structure ( Rafiqul and Sakinah, 2013 ). The yield of xylitol is influenced by the presence of by-products of hydrolysis and by the contamination of other fractions of the lignocellulose ( Mussatto and Teixeira, 2010 ; Nair and Zhao, 2010 ). Pretreatment processes are included for efficient hydrolysis so that more xylose is accessible to the fermenting microorganisms and enzymes to produce a high xylitol yield. Pretreatment methods can be classified as (1) Physical methods—Microwave and Ultrasound methods; (2) Chemical methods—Dilute acid, ionic solvents, deep eutectic solvents, etc; (3) Physicochemical methods—Steam explosion, ammonia-based pretreatment; (4) Biological methods involving microorganisms like fungi ( Kumar and Sharma, 2017 ). Table 1 compares different pretreatment methods with the respective xylitol yield.
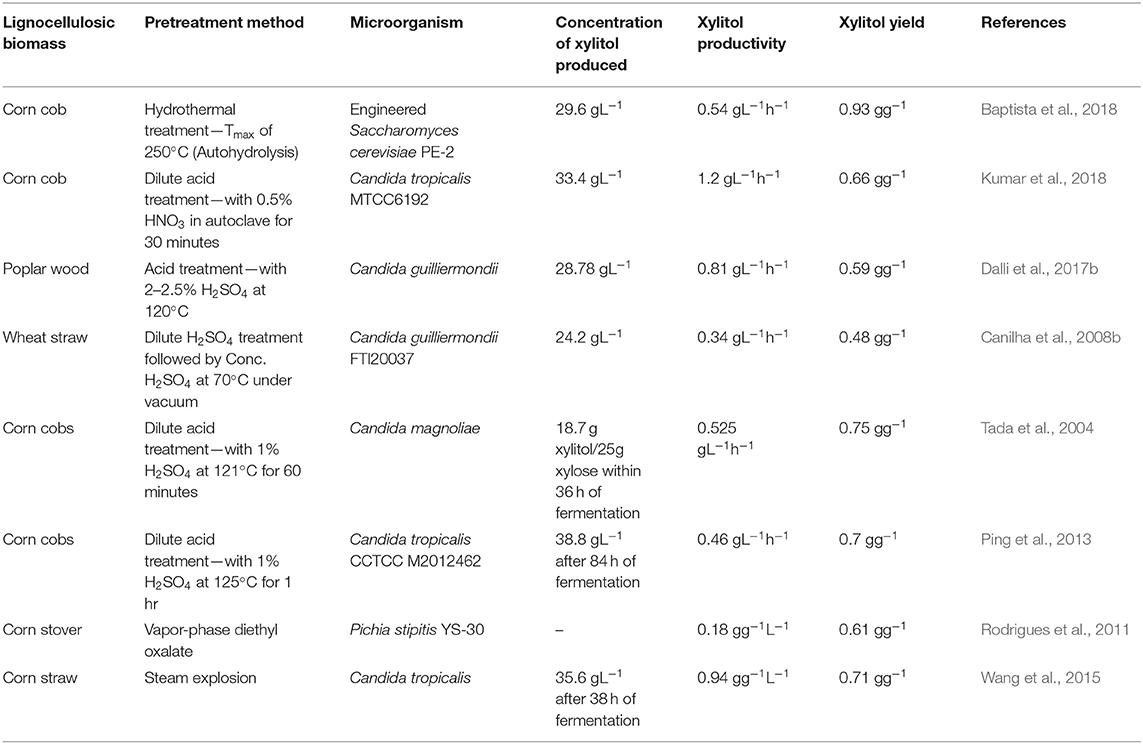
Table 1 . Comparison of different pretreatment methods with the xylitol yield.
Industrially, acid hydrolysis, particularly dilute-acid hydrolysis, is the most commonly employed method owing to its efficiency in hydrolyzing hemicellulose at a fast rate at less cost ( Martin et al., 2013 ). Moraes et al. (2020) have obtained 99% extraction of xylose by pretreating the biomass with 1% sulphuric acid at 120°C. The use of nitric acid hydrolysis has been found to improve xylose extraction ( Dalli et al., 2017b ; Manaf et al., 2018 ; Shah et al., 2020 ). Acid pretreatment releases monomeric sugars from the hemicellulose thereby eliminating the need for a further hydrolysis step. Nevertheless, acid hydrolysis has the drawback of producing inhibitory by-products and it is corrosive to the reactor vessels as well. Furthermore, it hydrolyzes cellulose along with hemicellulose. Enzymatic hydrolysis, on the other hand, can produce xylose specifically and minimize the formation of by-products. In a study by Mardawati et al. (2018) comparing acid and enzymatic hydrolysis methods, it was observed that the specific growth rate of microbes and the xylitol yield were higher in the hydrolysate obtained by enzymes.
The hemicellulosic fraction can be solubilized by an eco-friendly method called auto-hydrolysis. This method, also known as the hydrothermal pretreatment method, uses just water to convert hemicellulose into oligomers from which monomeric sugars can be recovered using further enzymatic hydrolysis ( Mardawati et al., 2020 ). Here, the biomass is treated at high pressure and temperature of around 200°C due to which the acetyl groups in the hemicellulose get hydrolyzed to release acids that break bonds between the carbohydrates and lignin. Baptista et al. (2018 , 2020) have used the auto-hydrolysis method to pretreat corncob and have reported the highest titer of xylitol production using recombinant Saccharomyces cerevisiae .
Solvents such as ionic liquids and deep eutectic solvents (DES) are grabbing researchers' attention in recent years to pretreat hydrolysates ( Behera et al., 2014 ; Liu et al., 2019 ). Although the physiochemical properties of these two solvents are alike, it has been observed that DESs are more advantageous owing to the cost and biocompatible nature. Ai et al. (2020) have obtained xylose and glucose yield of around 85%, employing DES-mediated extrusion for pretreating sorghum bagasse. The choice of the pretreatment method is purely based on the application. Combining two pretreatment methods can also be advantageous in obtaining a better yield of monomeric sugars ( Kumar and Sharma, 2017 ).
Inhibitors and Detoxification
Upon pretreatment and hydrolysis of biomass, apart from releasing monomeric sugars, many inhibitory compounds are produced. These compounds are derived from lignin or generated from the degradation of sugars, and they inhibit the fermentative microorganisms and enzymes. Furan compounds like furfural and hydroxymethylfurfural, phenolic compounds like vanillin and syringaldehyde, and acids such as formic acid, acetic acid, and levulinic acid are some of the common inhibitors formed in the hydrolysate of lignocellulosic biomass ( Valdes et al., 2020 ; Bianchini et al., 2021 ). In the case of acid hydrolysis, inhibitors in the form of heavy metals can be produced due to corrosion of the equipment ( Rehman et al., 2015 ). The type of inhibitors produced, and their concentration depends on the source of biomass and the treatment conditions used. The presence of phenolic compounds can alter the membrane integrity of the microorganisms. Damage to the DNA structure and enzyme activity can occur due to furans ( Rao et al., 2016 ). The acids formed, affect the metabolism of xylose in yeast ( Rafiqul et al., 2015a ). The effect of inhibition depends on the concentration of the compounds, the fermentation conditions, and the ability of microorganisms to tolerate the inhibitory compound ( Hernández-Pérez et al., 2019 ).
To obtain a better yield of xylitol from the xylose present in the hydrolysate, it is necessary to detoxify the hydrolysate and remove or inactivate the inhibitory compounds before fermentation. Neutralization, activated charcoal treatment, overliming, solvent extraction, ion-exchange resin treatment, and microbial detoxification are some of the commonly used detoxification methods. ( Soares et al., 2016 ; Gupta et al., 2017 ; Kumar et al., 2018 ; Agarwal and Singh, 2019 ; Llano et al., 2021 ). Kumar et al. (2019) have reported the efficient removal of acetic acid and salts in the hydrolysate by employing a detoxification method involving activated charcoal followed by membrane filtration. However, the loss of sugar is a limitation of these methods. To minimize sugar loss while removing inhibitors, Dalli et al. (2017a) combined vacuum evaporation with solvent extraction and achieved 97 and 87.5% of furfural and acetic acid removal respectively.
Besides the research on the efficient and cost-effective detoxification methods, some researchers use adapted strains or recombinant strains of yeast to avoid a detoxification step ( Santana et al., 2018 ). It has been reported in a study that a genetically engineered strain of Candida tropicalis has produced 0.98 gg −1 of xylitol from non-detoxified hydrolysate ( Hong et al., 2016 ). The tolerance capacity of the microbial species used, the composition of the hydrolysate, the concentration, and the type of inhibitors present to decide the necessity for a detoxification step before fermentation. Also, a techno-economic analysis considering the cost of the detoxification method and the yield of xylitol should be taken into account.
Xylitol Production From Glucose
Naturally, xylose is found in abundance in the hemicellulosic fraction of lignocellulosic biomass. Yet, there is a drawback in the industrial production of xylitol from xylose due to the lack of an economically viable availability of pure xylose ( Cheng et al., 2014 ). Addressing this limitation, glucose might be used as a better and cheaper alternative for large-scale xylitol production. Early studies on the production of xylitol from glucose involved three steps. In the first step, D-glucose is converted to D-arabitol by Debaryomyces hansenii . Then D-arabitol is oxidized to D-xylulose by Acetobacter suboxydans . Finally, D-xylulose is converted to xylitol by C. guilliermondii var. soya . ( Onishi and Suzuki, 1969 ). However, the yield of xylitol obtained is low, which limits the application of this method in large-scale production.
To convert glucose directly to xylitol, studies on the construction of genetically engineered microorganisms are being done. Povelainen and Miasnikov (2007) have expressed the xylitol-phosphate dehydrogenase gene in a strain of Bacillus subtilis that resulted in the production of xylitol from D-glucose with a yield of 23%. Cheng et al. (2014) have genetically engineered a Pichia pastoris strain with the xylose dehydrogenase gene and D-arabitol dehydrogenase gene from different microorganisms. This engineered strain converted glucose into xylitol in a single fermentation with a yield of 0.078 g xylitol/g glucose. Further, the authors concluded that the yield of D-arabitol could be improved if glucose is made to enter the pentose phosphate pathway and the cell NADPH demand is altered. The major issues to be addressed in the single fermentation process of xylitol production from D-glucose are (1) the yield and productivity of D-arabitol by genetically engineered strains is low and (2) the production of by-products such as other polyols have to be reduced as it may increase the osmotic stress of the cells ( Qi et al., 2014 ). Thus, more studies on the screening of strains producing a better yield of D- arabitol and optimization of the conditions of fermentation are required to make the production of xylitol from D-glucose industrially feasible.
Xylitol Production by Microorganisms
The biotechnological process of xylitol production is centered on the metabolism of xylose by microorganisms that can naturally utilize pentose sugars as a carbon source. Xylitol is produced by these microorganisms as an intermediate in the metabolic pathway of xylose ( Narisetty et al., 2022 ). The first step in the metabolism of D-xylose occurring in yeast and fungi involves xylose reductase which reduces D-xylose to xylitol ( Verduyn et al., 1985 ). The xylitol thus produced is secreted out of the cell or otherwise, it is oxidized by xylitol dehydrogenase to produce D-xylulose ( Rizzi et al., 1989 ; Yablochkova et al., 2003 ). The secretion or oxidation of xylitol depends on the availability of cofactors. Xylose reductase requires NADPH or NADH and xylose dehydrogenase requires NADP or NAD for their activities. D-xylulose further gets phosphorylated by the action of xylulokinase and gets integrated with the non-oxidative route of the pentose phosphate pathway ( Ravella et al., 2012 ) ( Figure 2 ). In the process of xylitol bioconversion, continuous availability of NADPH is required so that there is limited oxidation of xylitol to xylulose. Numerous research works have been done to screen microbial strains that can efficiently produce xylitol ( Hernández-Pérez et al., 2019 ; Narisetty et al., 2022 ).
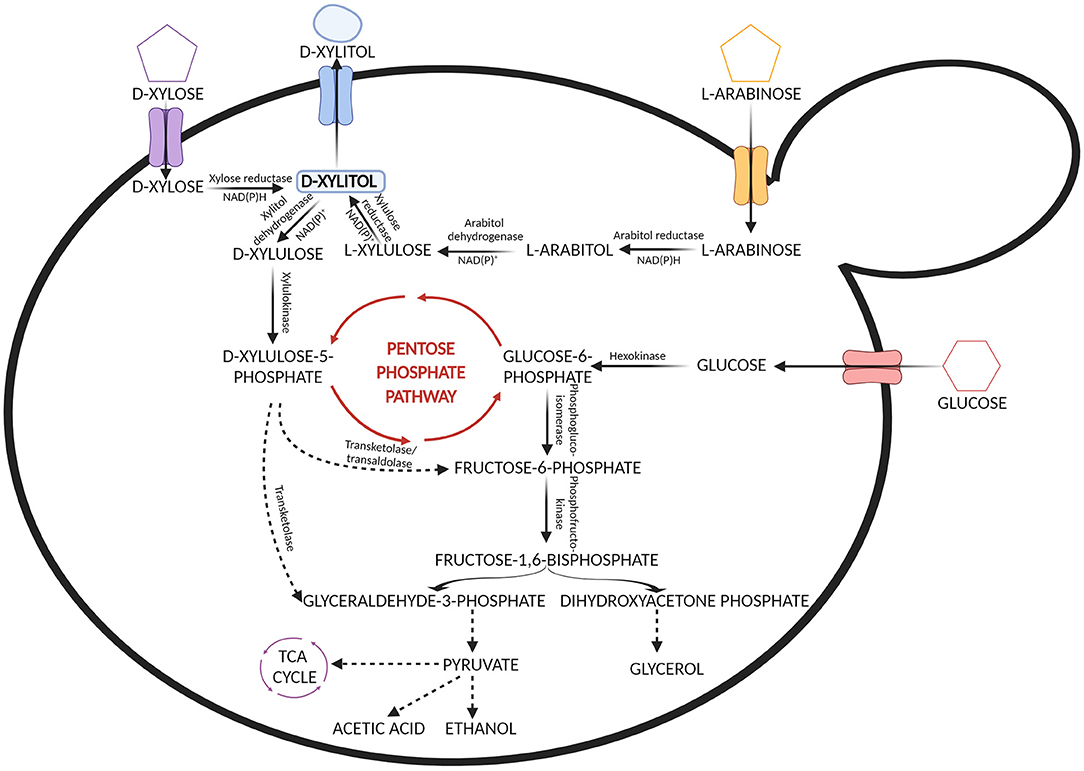
Figure 2 . Metabolic pathways involved in the assimilation of xylose, arabinose and glucose from the hemicellulosic hydrolysate by yeast.
In contrast, bacteria contain the enzyme xylose isomerase that can directly convert D-xylose into D-xylulose which further enters the pentose phosphate pathway after phosphorylation. However, there are some exceptions. Some of the earlier studies done in the 1970s have shown that a few bacterial strains belonging to Corynebacterium and Enterobacter species contain the enzyme xylose reductase and have the ability to accumulate xylitol ( Yoshitake et al., 1973a , b ). In a study by Izumori and Tuzaki (1988) , Mycobacterium smegmatis was found to produce xylitol. Several bacterial cultures belonging to the species Cellulomonas, Corynebacterium , and Serratia were screened for xylitol production by Rangaswamy and Agblevor (2002) and it was observed that among those, Corynebacterium produced maximum xylitol yield. Recently it has been found that Pseudomonas putida can produce xylitol with a volumetric productivity of 0.98 g L −1 h −1 which is higher than other bacterial strains ( Lugani and Sooch, 2020 ). Filamentous fungi such as Penicillium chrysogenum and Petromyces albertensis were first studied for xylitol production ( Dahiya, 1991 ). In 2003, Sampaio et al. screened Aspergillus niger and 10 different strains of Penicillium and it was seen that Penicillium crustosum had the highest yield. However, bacteria and filamentous fungi do not favor xylitol production as much as yeast does. Table 2 shows xylitol bioproduction by different microorganisms.
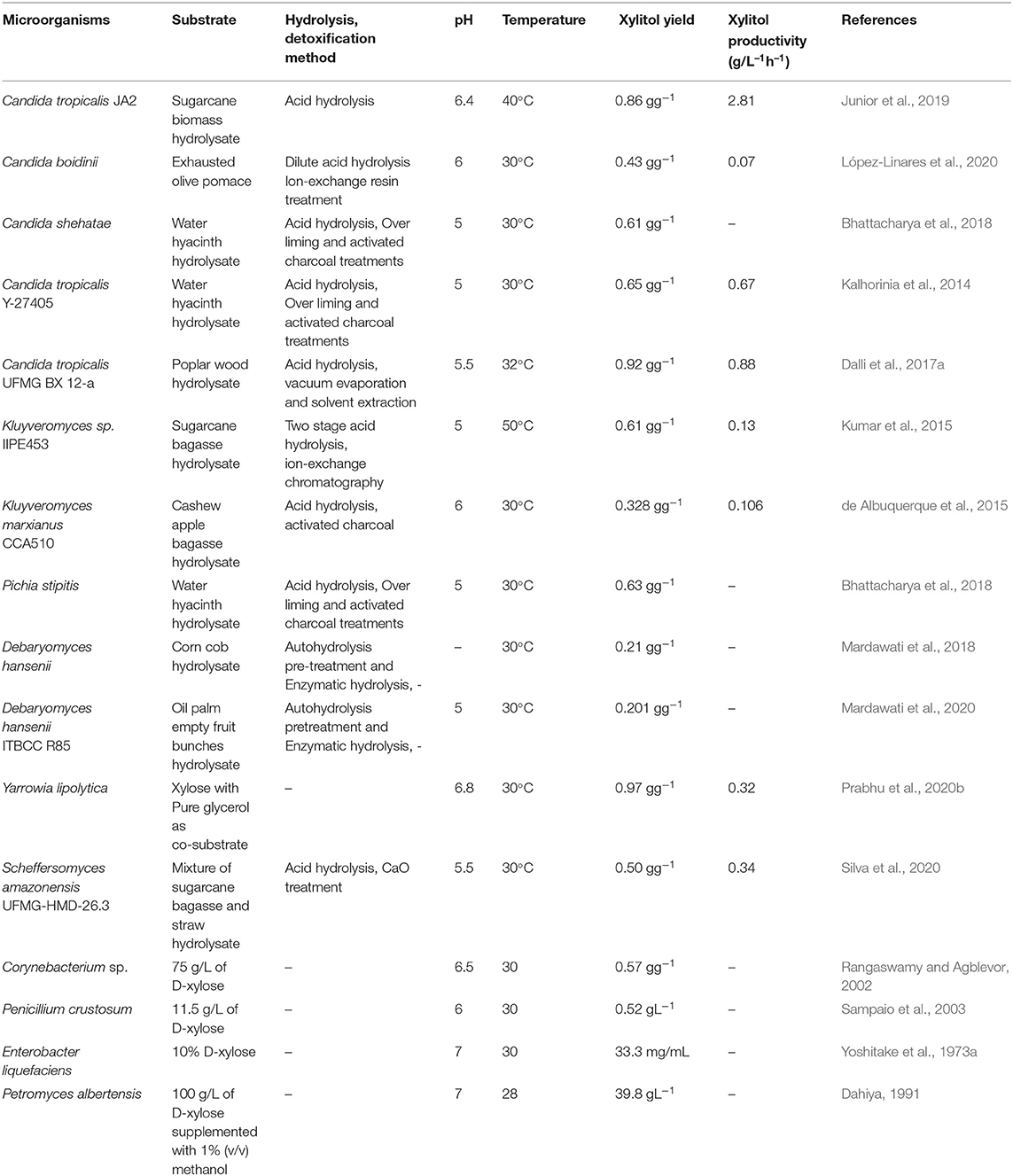
Table 2 . Xylitol bioproduction by different strains of microorganisms.
Yeast is widely studied due to the high xylitol productivity and assimilation of xylose. The most common yeast that produces high xylitol yield includes Candida and Debaryomyces ( Guo et al., 2006 ; Sampaio et al., 2008 ). In a recent study by Carneiro and Almeida (2019) involving 44 isolates, it was found that Meyerozyma species were the best utilizers of xylose and Wickerhamomyces anomalus produced high xylitol yield. Candida strains such as C. tropicalis and C. silvanorum are the most promising xylitol producers as they have been found to have the highest xylose reductase activity ( Yablochkova et al., 2003 ). López-Linares et al. (2018) compared the production of xylitol by Debaryomyces hansenii and Candida guilliermondii and observed that C. guilliermondii showed high tolerance to inhibitors like furans and acids thus possessing the advantage of not requiring additional detoxification steps. Similarly, a strain of C.tropicalis was found to produce xylitol with high tolerance to acetic acid in the hydrolysate ( Junior et al., 2019 ). Generally, the yield of xylitol is less if the hydrolysate is not detoxified. However, Prabhu et al. (2020a) obtained a high yield of xylitol using Pichia fermentans without detoxifying the hydrolysate. Oleaginous yeast such as Yarrowia lipolytica has also studied in the xylitol bioproduction ( Prabhu et al., 2020b ). Furthermore, xylitol production has been done by genetic manipulation of microorganisms such as Saccharomyces cerevisae and Escherichia coli which has been discussed in section Metabolic engineering for enhanced xylitol production .
Xylitol Production by the Enzymatic Method
Until now microorganisms are commonly used for most studies on xylitol production. However, limitations in product purity and stability and lower enzyme to substrate ratio have paved the way for research on xylose bioconversion through enzymatic method instead of using whole cells, to meet the demands on a large scale. In the microbial production of xylitol, xylose is required for cell growth and cell maintenance. Since the enzymatic method does not require such tedious process, 100% of xylose can be converted to xylitol ( de Freitas Branco et al., 2011 ). The key enzyme acquiring industrial importance in converting xylose to xylitol is xylose reductase (XR) ( Lugani et al., 2021 ). Kitpreechavanich et al. (1984) reported that 90% of xylose was enzymatically converted into xylitol by xylose reductase from Candida pelliculosa coupled with a methanogen oxidoreductase system for NADPH recycling. Other NADPH recycling systems including formate dehydrogenase and glucose dehydrogenase have also been studied ( Xu et al., 2019 ). Yeast like Candida guilliermondii, Candida tenuis, Candida tropicalis which are the best xylitol producers are used to obtain XR enzyme ( Häcker et al., 1999 ; Tomotani et al., 2009 ; Kim et al., 2019 ). Recently, hemicellulosic hydrolysates have been used for the enzymatic bioconversion process and high xylitol yield has been obtained ( Rafiqul and Sakinah, 2015 ; Rafiqul et al., 2015a , 2021 ).
In addition to the advantage of the high yield of xylitol, the energy, water requirement, and time of incubation are comparatively lesser in the enzymatic bioconversion method ( Rafiqul and Sakinah, 2013 ). However, the enzymatic bioconversion faces two main constraints which are (1) the high cost involved in the preparation of xylose reductase enzyme; (2) the requirement of a constant supply of NADPH. The development of stable XR and an effective system for cofactor regeneration are needed for improving enzymatic xylitol production ( de Freitas Branco et al., 2012 ).
Fermentation Strategies in Xylitol Production
Fermentative parameters in xylitol production.
The pH suitable for yeast growth is generally acidic. Characterization of xylose reductase from a Candida tropicalis strain showed that the enzyme was active at a pH of 5 to 7 ( Rafiqul et al., 2015b ). The optimum pH for the production of xylitol by Candida strains has been reported to be 5 and 5.5 ( Rudrangi and West, 2020 ; West, 2021 ). The production of xylitol by yeast generally employs a temperature of 30°C. However, depending on the species used, the optimum temperature range of xylitol producing yeast varies between 30 and 37°C. In a study employing Candida tropicalis for the production of xylitol, it was observed that there was a consumption of about 90% xylose at a temperature range of 29 and 34°C and there was a decrease in xylose consumption below 28°C and above 35°C ( Tamburini et al., 2015 ). Sampaio et al. (2006a) investigated the effect of temperature and pH on xylitol production by a Debaryomyces hansenii strain and concluded that the optimum temperature and pH range was 30–35°C and 4–8 respectively. Nevertheless, some research employs microorganisms tolerant to higher temperatures. Zhang et al. (2014) have used a recombinant strain of Kluyveromyces marxianus that produced high productivity of 1.49 g L −1 h −1 xylitol from 100 g L −1 xylose at a temperature of 42°C. Temperature influences the microbial growth rate and the production of xylitol as the regulation of transport proteins involved in xylose sequestration and the enzyme activity depend on the temperature ( Tamburini et al., 2015 ).
For obtaining a high yield of xylitol, the initial concentration of xylose must be high since xylose is necessary to induce the enzymes involved in xylitol production ( Winkelhausen and Kuzmanova, 1998 ). To increase the initial concentration of xylose, hemicellulosic hydrolysates undergo a concentration step which will also possibly increase the inhibitor concentration present in the hydrolysate ( Hernández-Pérez et al., 2019 ). Moreover, during batch fermentation, there might be substrate inhibition due to high initial substrate concentration. Thus, the initial xylose concentration has to be optimized such that there is a high xylitol yield and the performance of the microorganisms is not affected. It should be noted that the optimum initial xylose concentration depends on the type of hydrolysate and yeast strain used ( Mussatto and Roberto, 2008 ). Apart from using a detoxification step, increasing the inoculum concentration could also help in reducing the effects of the inhibitory compounds in the hydrolysate ( Felipe et al., 1997 ). To enhance the production of xylitol, co-substrates such as glucose and glycerol can be supplemented ( Kogje and Ghosalkar, 2017 ; Ariyan and Uthandi, 2019 ). One of the key elements in the metabolism of D-xylose in yeast is oxygen. El-Baz et al. (2011) have reported that reduced aeration increases the xylitol production rate. In another study, it was reported that Pichia guilliermondii favored the production of xylitol at a very low volumetric oxygen transfer coefficient (k L a) of 0.075/h ( Zou et al., 2010 ). Low k L a value favors xylitol production as an increase in k L a value will direct the metabolism toward cell growth rather than xylitol production ( Martínez et al., 2000 ).
Operation Modes
Batch fermentation in Erlenmeyer flasks or bioreactors is most commonly used for xylitol production ( Mussatto and Roberto, 2003 ; López-Linares et al., 2018 ; Silva et al., 2020 ). A study by Shah et al. (2019) showed that batch fermentation of kenaf hydrolysate by a recombinant Escherichia coli gave a xylitol yield of 0.38 gg −1 . Fed-batch fermentations can give better xylitol productivity as the concentration of substrate can be maintained constant. Li et al. (2012) carried out a two-stage fed-batch fermentation using Candida tropicalis and have reported that the xylitol productivity was 65.57% more than batch fermentation. Su et al. (2015) have reported the highest productivity of xylitol using E. coli by employing fed-batch fermentation. Recently, Ramirez and Escoto (2021) have implemented a genetic algorithm study to enhance xylose fermentation by a fed-batch process. A study by Martinez et al. (2003) describing the xylitol production by continuous fermentation of sugarcane hydrolysate by C. guilliermondii resulted in productivity of 0.68 gL −1 h −1 . Salgado et al. (2012) have employed a technique where two continuous stirred tank reactors were connected for the production of lactic acid and xylitol and this setup gave xylitol productivity of 0.218 gL −1 h −1 .
To increase the fermentation rate and reuse the cells, immobilization of cells is being preferred. Immobilization can be done by entrapment in beads or gels using carriers like sodium alginate, polyvinyl alcohol polyacrylamide agarose gel, κ-carrageenan, and gelatin ( Yewale et al., 2016 ). Abd Rahman et al. (2020) have implemented immobilization of recombinant E. coli cells on multi-walled carbon nanotubes to enhance xylitol production. Recently, carbon fiber treated with Fenton reagent has been found to improve immobilization efficiency which resulted in improved xylitol production ( Wang et al., 2021 ). Production of xylitol using immobilized cells can be carried out using different modes of operations such as batch, continuous, and fed-batch fermentation ( Pérez-Bibbins et al., 2015 ).
Recovery of Xylitol
The viability of the bioproduction of xylitol depends on the downstream processing and recovery of xylitol from the fermentation broth. The nature of the product such as its size, the fermentation broth's concentration, and the presence of impurities like sugars, other sugar alcohols, media constituents, and inhibitory compounds in the broth decide the type of recovery process to be used ( Martínez et al., 2015 ). The most common method employed for xylitol purification is crystallization. However, the broth must be clarified before the crystallization step to produce pure crystals. Clarification of the fermented media is usually done by using ion exchange resins, activated charcoal, liquid-liquid extraction, and membrane separation ( Sampaio et al., 2006b ; Canilha et al., 2008a ; Misra et al., 2011 ). Faneer et al. (2017) have enhanced the purity of xylitol to 92% using polyethersulfone nanofiltration membrane which was efficient in removing other impurities like arabinose as well. After concentrating the broth using ion exchange resins, a two-stage crystallization process was performed by Martínez et al., by which up to 94% pure xylitol crystals were obtained ( Martínez et al., 2007 ). In recent years, there is a search for greener methods for downstream processing that will be eco-friendly and cost-efficient as well. Faneer et al. (2018) have employed a pressure filtration membrane to obtain a high concentration of xylitol. Junior and Rocha (2021) have developed a purification process for xylitol using protic ionic liquids which gave a crystal yield of 70% with 85% purity.
Metabolic Engineering For Enhanced Xylitol Production
Many wild and engineered strains are explored for increased xylitol production. Some of the wild potent microorganisms with inbuilt xylitol producing capacity are Candida sp., Pichia sp., Debaryomyces sp., Mycobacterium sp., Gluconobacter sp., and Corynebacterium sp. ( Guo et al., 2006 ). To enhance xylitol production, genetic engineering techniques such as the introduction of genes coding for the key enzymes, disruption of hindering genes, or overexpression of existing gene is adopted ( Johan et al., 1991 ; Handumrongkul et al., 1998 ). Table 3 shows some of the important engineered microorganisms with enhanced xylitol production. Key enzymes for xylitol production such as xylose reductase and xylose isomerase are isolated from potent fungi and are incorporated into microorganisms using genetic engineering techniques.
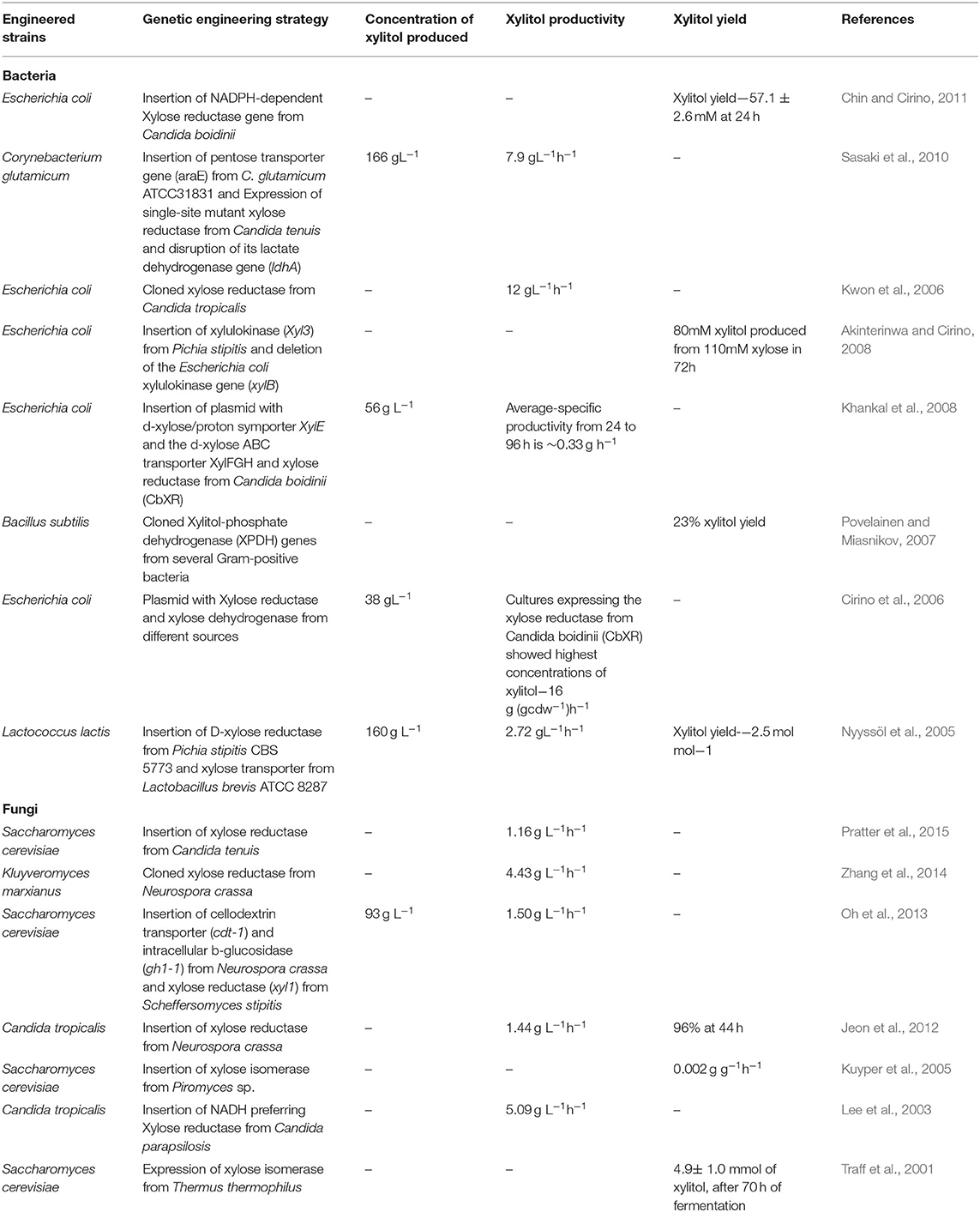
Table 3 . Engineered microorganisms for enhanced xylitol production.
Guirimand et al. (2019) showed that engineered Saccharomyces cerevisiae with cell surface display of Aspergillus aculeatus β-glucosidase ( bgl1 ), Trichoderma reesei endoglucanase II ( egl2 ), Aspergillus oryzae β-xylosidase A ( xyl A ) and cytosolic Scheffersomyces stipitis xylose reductase ( Xyl1 ) results in enhanced xylitol production with 44% conversion rate (xylitol yield-3.7 gL −1 ) after 96 hours. Su et al. (2015) improved xylitol production in E. coli by blocking xylose catabolism and xylitol phosphorylation. Xylose catabolism is blocked by deleting xylulose kinase ( xylB ) and xylose isomerase ( xylA ) genes and phosphorylation of the xylitol produced is blocked by disrupting fructose phosphotransferase system ( ptsF ) resulting in increased production of xylitol (xylitol yield of 172.4 gL −1 is achieved after 110 h of batch fermentation).
Kim et al. (2015) engineered E. coli for increased xylitol production by introducing the following changes in the gene structure: (1) Deletion of arabinose transcriptional regulator ( araC ); (2) Intergenic mutation in xylose isomerase ( xylA ) and Orotate phosphoribosyl transferase ( pyrE ); (3) Missense mutation in Arabinose-proton symporter ( araE ) and Putative undecaprenyl-diphosphatase ( ybjG ). The resulting mutant strain E. coli GX20 showed rapid conversion of xylose to xylitol even in the presence of glucose in the medium. Zha et al. (2013) engineered Saccharomyces cerevisiae with xylose reductase ( xyl1 ) from Scheffersomyces stipitis and β-glucosidase ( gh1-1 ) and cellodextrin transporter ( cdt-1 ) from Neurospora crassa resulting in increased xylitol production by 85.7% when co-fermented with cellobiose and xylose compared to co-fermentation with glucose and xylose. Oh et al. (2013) engineered Saccharomyces cerevisiae with xylose reductase ( xyl1 ) from Scheffersomyces (Pichia) stipitis and β-glucosidase ( gh1-1 ) and cellodextrin transporter ( cdt-1 ) from Neurospora crassa , increased overall xylitol productivity by 40% when co-fermented with cellobiose and xylose.
Market Trends And Future Prospects
One of the largest commercially produced sugar alcohols is xylitol. In the 1970s, a Finnish company commercialized xylitol production ( Honkala et al., 1999 ). DuPont, an American-based company is a highly noted world's largest producer of xylitol from renewable resources such as hardwoods and maize as the feedstocks and commercialize xylitol under the trade name xivia ( Ravella et al., 2012 ). Xylitol is considered to be one of the major value-added chemicals that can be produced from biomass ( Werpy and Petersen, 2004 ). By 2020, the market value of xylitol was 921 million USD globally and it is projected to become 1.37 billion USD in 5 years [ International Market Analysis Research Consulting Group (IMARC), 2020 ]. Xylitol has been certified to be safe for consumption by the US Food and Drug Association ( Food Drug Administration, 2006 ). Nearly 70% of xylitol production is used in the production of confectionery items and chewing gums ( Ahuja et al., 2020 ). In the bioproduction of xylitol, techno-economic analysis and assessment of environmental impact must be done at every step of the production process. This will ensure that the biomass is valorized sustainably with minimal waste production. To achieve a circular bio-based economy, every component of biomass has to be utilized to produce a wide range of products like fuels, chemicals, and energy. Integrating xylitol production with other products makes the bioconversion process industrially feasible ( Morales-Rodriguez et al., 2016 ; Medina et al., 2018 ).
For the success of a biorefinery, it is crucial to understand the environmental impact through a life cycle assessment. Hafyan et al. (2019) have studied the environmental impact, hazard potential, and techno-economic aspects to select the optimum capacity for producing xylitol. Dasgupta et al. (2021) have estimated the material and energy balance and carbon dioxide emission levels in the bioproduction of xylitol using corncob. It has been reported that the energy requirement and greenhouse gas emission can be reduced by employing heat integration between processes.
Commercial Aspects Of Xylitol In Industrial Scale
Currently, more than 35 nations have endorsed the utilization of xylitol in food sources, drugs, and oral wellbeing items, chiefly in chewing gums, toothpaste, syrups, and candy preparations ( Barathikannan and Agastian, 2016 ). Routine xylitol utilization might be characterized as everyday utilization of 5–7 g of xylitol no less than three times each day. The suggested portion for dental caries avoidance is 6–10 g/d but it varies from one individual to another. According to many studies, the maximum dosage of xylitol for children is 45 g/d and for adults is 100 g/d and the acceptable dosage is 40 g/d ( Nayak et al., 2014 ).
Food and Confectionery
Long-term use of conventional white sugar in food and food products leads to many complications such as diabetes, inflammatory diseases, gingivitis, obesity, cardiovascular problems, metabolic syndrome, and dental caries. People suffering from obesity, find it too difficult to lose weight. Weight gain is directly linked to an increase in cholesterol level in the blood and lipid storage and cause many cardiovascular diseases. Replacing white sugar with xylitol helps in stabilizing blood sugar levels and decreasing overall lipid storage ( Islam and Indrajit, 2012 ). It contributes to weight reduction and indirectly prevents the onset of cardiovascular disease. The diet of nursing women and pregnant ladies with gestational diabetes can be replaced by food items containing xylitol to prevent the impact of diabetes ( Yamagata et al., 1969 ). When consumed, xylitol is digested into carbon dioxide and water, requiring no insulin for metabolism and having no effect on blood glucose levels. Compared to glucose only 20–30% of consumed xylitol is absorbed in the upper gastrointestinal tract and then it is non-actively transported through the intestinal tract.
Using xylitol as a sweetness enhancer in food preparation improves certain properties of food such as taste, color, longevity, and texture ( Benahmed et al., 2020 ). Xylitol is extensively used in the manufacturing of chewing gums, chocolate, hard candies, wafer fillings, chocolate, pastilles, pectin jellies, ice cream fillings, and other sweets. Xylitol possesses some of the properties such as remineralization, moisture retention, non-fermentability, microbial stability, high solubility, and prebiotic effect ( Mäkinen, 2000 ). Xylitol provides flexibility, a pleasing and cooling effect to the confectioneries. In the manufacturing of xylitol-based chewing gums, the cooling effect is promoted by the endothermic property (34.8 cal/g) of xylitol. As it does not undergo milliard reaction, xylitol will not char on heating and because of this property, it gives unique taste and color to the food items. It is used to sweeten the flour which is used for making bread, rusks, and cakes. Crystalline xylitol is used as a sanding material in the preparation of sweets and confectioneries. It is also used in the process of protein extraction as a stabilizing agent as it prevents the denaturation of protein. This property of xylitol is used to increase the shelf life of food products and so xylitol is highly recognized as a food preservative. In the food industry, xylitol is used as a stabilizer, moisturizer, antioxidant, and cryoprotectant.
Pharmaceutical Applications
Xylitol is markedly used in the manufacturing of toothpaste and mouth rinses for people with gum problems and sensitive gums due to its anti-cariogenic and tooth rehardening property ( Janket et al., 2019 ). Streptococcus mutans and Helicobacter pylori are the oral pathogenic bacteria causing tooth decay, xerostomia, plaque formation, gingival inflammation, and erosion of teeth. These bacteria metabolize sugar residues present in the mouth and feed on them. Xylitol cannot be metabolized by these bacteria and so brushing teeth with toothpaste containing xylitol prevents dental caries. Due to the endothermic property of xylitol, toothpaste and mouthwashes give a cooling and refreshing effect. Xylitol sweetened night medicines after brushing are highly recommended for children ( Feigal et al., 1981 ). Xylitol shows well-defined anti-bacterial properties and inhibits the growth of microorganisms. It also prevents the attachment of these microorganisms to the teeth surface and reduces their corrosive activity by reducing acid production potential ( Nayak et al., 2014 ).
Streptococcus pneumoniae and Haemophilus influenzae are the common otopathogens causing ear infections such as acute otitis media. Xylitol with its anti-bacterial and anti-inflammatory potential reduces upper respiratory tract and middle ear infections by preventing the growth of the bacteria. Chewing gums and syrup containing xylitol have been displayed to ensure from the middle ear infection in children ( Uhari et al., 1996 ; Vernacchio et al., 2014 ). Xylitol is used in the manufacturing of capsules and is added in oral drug preparations such as syrups, tonics, and vitamin formulations to increase palatability.
Some reports showed that the consumption of xylitol can reduce constipation, and for some people, it induces some side effects such as irritable bowel syndrome, diarrhea, and nephrolithiasis. Many cases have reported the toxic effect of xylitol on dogs. However, according to the animal poison control center of American society, xylitol is considered safe for other mammals like humans and cats, except for dogs [ Peterson, 2013 ; American Association of Poison Control Centers (AAPCC), 2021 ].
Xylitol is a commercially successful, high market value artificial sweetener, produced by the biological action of microorganisms on the pentose sugar xylose. Low calorific value, insulin-independent metabolism, anti-cariogenic property, remineralization property of xylitol is highly exploited in food and pharmaceutical industries. Commercially many food and candy items, confectionery, pharmaceutical products are produced by replacing sucrose with xylitol. Due to the high demand for xylitol, the global production rate had been increased tremendously. The chemical method of xylitol production is cost-ineffective and not environmentally safe and the enzymatic method of production is time-consuming. The production of xylose-rich hemicellulosic hydrolysate is another snag in the large-scale production of xylitol. These difficulties can be overcome by the microbial method of xylitol production. Various genetic engineering strategies to modulate key enzymes such as xylose reductase, xylose isomerase, xylulokinase for enhanced xylitol yield and optimizing fermentative parameters based on kinetics studies, modeling and simulation need to be employed for large scale production of xylitol. Recent advances in integrated biorefinery approach, biomass conversion innovations, and metabolic engineering techniques would disclose new fortuity for economical and eco-friendly production of xylitol to meet the growing demand.
Author Contributions
DU and RK has equally contributed in drafting the manuscript with relevant information. VK involved in critical inputs in framing the manuscript outline. SJ is responsible for coordinating the team, framing the outline, correction, and final drafting of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abd Rahman, N. H., Jahim, J. M., Munaim, M. S., Rahman, R. A., Fuzi, S. F., and Illias, R. M. (2020). Immobilization of recombinant Escherichia coli on ulti-walled carbon nanotubes for xylitol production. Enzyme Microb. Technol . 135:109495. doi: 10.1016/j.enzmictec.2019.109495
PubMed Abstract | CrossRef Full Text | Google Scholar
Agarwal, B., and Singh, L. K. (2019). “Sugar and Sugar Alcohols: Xylitol,” in High Value Fermentation Products: Human Health , ed. S. Saran, V. Babu, A. Chuabey (Wiley online library), 285–307.
PubMed Abstract | Google Scholar
Ahuja, V., Macho, M., Ewe, D., Singh, M., Saha, S., and Saurav, K. (2020). Biological and pharmacological potential of xylitol: a molecular insight of unique metabolism. Foods . 9,1592. doi: 10.3390/foods9111592
Ai, B., Li, W., Woomer, J., Li, M., Pu, Y., Sheng, Z., et al. (2020). Natural deep eutectic solvent mediated extrusion for continuous high-solid pretreatment of lignocellulosic biomass. Green Chem . 22, 6372–6383. doi: 10.1039/D0GC01560A
CrossRef Full Text | Google Scholar
Akinterinwa, O., and Cirino, P. C. (2008). Heterologous expression of D-xylulokinase from Pichia stipitis enables high levels of xylitol production by engineered Escherichia coli growing on xylose. Metab. Eng . 11, 48–55. doi: 10.1016/j.ymben.2008.07.006
American Association of Poison Control Centers (AAPCC) (2021). Available online at: https://www.aapcc.org/ (accessed November 20, 2021).
Arcaño, Y. D., García, O. D., Mandelli, D., Carvalho, W. A., and Pontes, L. A. (2020). Xylitol: A review on the progress and challenges of its production by chemical route. Catal. Today. 344, 2–14. doi: 10.1016/j.cattod.2018.07.060
Ariyan, M., and Uthandi, S (2019). Xylitol Production by Xylose Reductase over producing Recombinant Escherichia coli M15. Madras Agric. J . 106, 247. doi: 10.29321/MAJ.2019.000247
Audemar, M., Ramdani, W., Junhui, T., Raluca Ifrim, A., Ungureanu, A., Jérôme, F., et al. (2020). Selective hydrogenation of xylose to xylitol over Co/SiO2 catalysts. ChemCatChem. 12, 1973-1978. doi: 10.1002/cctc.201901981
Baptista, S. L., Carvalho, L. C., Romaní, A., and Domingues, L. (2020). Development of a sustainable bioprocess based on green technologies for xylitol production from corn cob. Ind. Crops. Prod. 156:112867. doi: 10.1016/j.indcrop.2020.112867
Baptista, S. L., Cunha, J. T., Romaní, A., and Domingues, L. (2018). Xylitol production from lignocellulosic whole slurry corn cob by engineered industrial Saccharomyces cerevisiae PE-2. Bioresour. Technol . 267, 481–491. doi: 10.1016/j.biortech.2018.07.068
Barathikannan, K., and Agastian, P. (2016). “Xylitol: Production, optimization and industrial application.” Int. J. Curr. Microbiol. Appl. Sci . 5, 324–339. doi: 10.20546/ijcmas.2016.509.036
Baudel, H. M., Abreu, C. D., and Zaror, C. Z. (2005). Xylitol production via catalytic hydrogenation of sugarcane bagasse dissolving pulp liquid effluents over Ru/C catalyst. J. Chem. Technol. Biotechnol . 80, 230–233. doi: 10.1002/jctb.1155
Behera, S., Arora, R., Nandhagopal, N., and Kumar, S. (2014). Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 36, 91–106. doi: 10.1016/j.rser.2014.04.047
Benahmed, A. G., Gasmi, A., Arshad, M., Shanaida, M., Lysiuk, R., Peana, M., et al. (2020). Health benefits of xylitol. Appl. Microbiol. Biotechnol . 104, 7225–7237. doi: 10.1007/s00253-020-10708-7
Bhattacharya, A., Ganguly, A., Sadhukhan, A. K., and Chatterjee, P. K. (2018). Investigations on the effect of driving parameters for xylitol production from water hyacinth biomass. Indian J. Biotechnol . 17, 272–283. doi: 10.7904/2068-4738-VII(14)-13
Bianchini, I. D., Sene, L., da Cunha, M. A., and Felipe, M. D. (2021). Short-term Adaptation Strategy Improved Xylitol Production by Candida guilliermondii on Sugarcane Bagasse Hemicellulosic Hydrolysate. Bioenergy Res . 17, 1–3. doi: 10.1007/s12155-021-10324-x
Canilha, L., Carvalho, W., Felipe, M. D. G. A., de Almeida e Silva, J. B. (2008b). Xylitol production from wheat straw hemicellulosic hydrolysate: hydrolysate detoxification and carbon source used for inoculum preparation. Braz. J. Microbiol . 39, 333–336. doi: 10.1590/S1517-83822008000200025
Canilha, L., Carvalho, W., Giulietti, M., Felipe, M. D. G. A., de Almeida e Silva, J. B. (2008a). Clarification of a wheat straw-derived medium with ion-exchange resins for xylitol crystallization. J. Chem. Technol. Biotechnol . 83, 715–721. doi: 10.1002/jctb.1861
Carneiro, C. V., and Almeida, J. R. (2019). Xylitol production: identification and comparison of new producing yeasts. Microorganisms . 7, 484. doi: 10.3390/microorganisms7110484
Chandel, A. K., Antunes, F. A., Terán-Hilares, R., Cota, J., Ellil,ä, S., Silveira, M. H., et al. (2018). “Bioconversion of hemicellulose into ethanol and value-added products: commercialization, trends, and future opportunities,” in Advances in sugarcane biorefinery , eds. A. K. Chandel and M. H. Silveira (Elsevier). 97–134.
Google Scholar
Chen, H. (2015). “Lignocellulose biorefinery product engineering,” in Lignocellulose Biorefinery Engineering , ed. H. Chan (Cambridge; Woodhead Publishing Limited), 125–165.
Chen, X., Jiang, Z. H., Chen, S., and Qin, W. (2010). Microbial and bioconversion production of D-xylitol and its detection and application. Int. J. Biol. Sci . 6, 834–844. doi: 10.7150/ijbs.6.834
Cheng, H., Lv, J., Wang, H., Wang, B., Li, Z., and Deng, Z. (2014). Genetically engineered Pichia pastoris yeast for conversion of glucose to xylitol by a single-fermentation process. Appl. Microbiol. Biotechnol . 98, 3539–3552. doi: 10.1007/s00253-013-5501-x
Chin, J. W., and Cirino, P. C. (2011). Improved NADPH supply for xylitol production by engineered Escherichia coli with glycolytic mutations. Biotechnol. Prog . 27, 333–341. doi: 10.1002/btpr.559
Cirino, P. C., Chin, J. W., and Ingram, L. O. (2006). Engineering Escherichia coli for xylitol production from glucose-xylose mixtures. Biotechnol. Bioeng . 95, 1167–1176. doi: 10.1002/bit.21082
Dahiya, J. S. (1991). Xylitol production by Petromyces albertensis grown on medium containing D-xylose. Can. J. Microbiol . 37, 14–18. doi: 10.1139/m91-003
Dalli, S. S., Da Silva, S. S., Uprety, B. K., and Rakshit, S. K. (2017a). Enhanced production of xylitol from poplar wood hydrolysates through a sustainable process using immobilized new strain Candida tropicalis UFMG BX 12-a. Appl. Biochem. Biotechnol . 182, 1053–1064. doi: 10.1007/s12010-016-2381-4
Dalli, S. S., Patel, M., and Rakshit, S. K. (2017b). Development and evaluation of poplar hemicellulose prehydrolysate upstream processes for the enhanced fermentative production of xylitol. Biomass. Bioenergy . 105, 402–410. doi: 10.1016/j.biombioe.2017.08.001
Dasgupta, D., Sidana, A., Ghosh, P., Sharma, T., Singh, J., Prabhune, A., et al. (2021). Energy and life cycle impact assessment for xylitol production from corncob. J. Clean. Prod . 278, 123217. doi: 10.1016/j.jclepro.2020.123217
de Albuquerque, T. L., da Silva Jr, I. J., de Macedo, G. R., and Rocha, M. V. (2014). Biotechnological production of xylitol from lignocellulosic wastes: a review. Process Biochem . 49, 1779–1789. doi: 10.1016/j.procbio.2014.07.010
de Albuquerque, T. L., Gomes, S. D., Marques, J. E. Jr„ da Silva, I. J. Jr., and Rocha, M. V. (2015). Xylitol production from cashew apple bagasse by Kluyveromyces marxianus CCA510. Catal. Today . 255, 33–40. doi: 10.1016/j.cattod.2014.10.054
de Freitas Branco, R., Chandel, A. K., and da Silva, S. S. (2012). “Enzymatic production of xylitol: current status and future perspectives,” in D-Xylitol , eds. S. S. Da Silva and A. K. Chandel (Berlin: Springer), 193–204.
de Freitas Branco, R., dos Santos, J. C., and da Silva, S. S. (2011). A novel use for sugarcane bagasse hemicellulosic fraction: xylitol enzymatic production. Biomass. Bioenergy . 35, 3241–3246. doi: 10.1016/j.biombioe.2011.02.014
El-Baz, A. F., Shetaia, Y. M., and Elkhouli, R. R. (2011). Xylitol production by Candida tropicalis under different statistically optimized growth conditions. Afr. J. Biotechnol . 10, 15353–15363. doi: 10.5897/AJB10.1575
Espinoza-Acosta, J. L. (2020). Biotechnological production of xylitol from agricultural waste. Biotecnia . 22, 126–134. doi: 10.18633/biotecnia.v22i1.1160
Faneer, K. A., Rohani, R., and Mohammad, A. W. (2018). Influence of pluronic addition on polyethersulfone membrane for xylitol recovery from biomass fermentation solution. J. Clean. Prod . 171, 995–1005. doi: 10.1016/j.jclepro.2017.10.075
Faneer, K. A., Rohani, R., Mohammad, A. W., and Ba-Abbad, M. M. (2017). Evaluation of the operating parameters for the separation of xylitol from a mixed sugar solution by using a polyethersulfone nanofiltration membrane. Korean J. Chem. Eng . 34, 2944–2957. doi: 10.1007/s11814-017-0186-y
Feigal, R. J., Jensen, M, E., and Mensing, C. A. (1981). Dental caries potential of liquid medications. Pediatr . 68, 416–419. doi: 10.1542/peds.68.3.416
Felipe, M. G., Vitolo, M., Mancilha, I. M., and Silva, S. S. (1997). Environmental parameters affecting xylitol production from sugar cane bagasse hemicellulosic hydrolyzate by Candida guilliermondii . J. Ind. Microbiol. Biotechnol . 18, 251–254. doi: 10.1038/sj.jim.2900374
Food Drug Administration (2006). Food Additive Status List . Available online at: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list , (accessed October 10, 2021).
Guirimand, G., Inokuma, K., Bamba, T., Matsuda, M., Morita, K., Sasaki, K., et al. (2019). Cell-surface display technology and metabolic engineering of Saccharomyces cerevisiae for enhancing xylitol production from woody biomass. Green Chem . 21, 1795–1808. doi: 10.1039/C8GC03864C
Guo, C., Zhao, C., He, P., Lu, D., Shen, A., and Jiang, N. (2006). Screening and characterization of yeasts for xylitol production. J. Appl. Microbiol . 101, 1096–1104. doi: 10.1111/j.1365-2672.2006.02994.x
Gupta, R., Gautam, S., Shukla, R., and Kuhad, R. C. (2017). Study of charcoal detoxification of acid hydrolysate from corncob and its fermentation to xylitol. J. Environ. Chem. Eng . 5, 4573–4582. doi: 10.1016/j.jece.2017.07.073
Häcker, B., Habenicht, A., Kiess, M., and Mattes, R. (1999). Xylose utilisation: cloning and characterisation of the xylose reductase from Candida tenuis . Biol. Chem . 380, 1395–1403. doi: 10.1515/BC.1999.179
Hafyan, R., Bhullar, L., Putra, Z., Bilad, M. R., Wirzal, M. D., and Nordin, N. A. (2019). “Sustainability assessment of xylitol production from empty fruit bunch,” in MATEC Web of Conferences , (EDP Sciences) p. 06018.
Handumrongkul, C., Ma, D. P., and Silva, J. L. (1998). Cloning and expression of Candida guilliermondii xylose reductase gene (xyl1) in Pichia pastoris . Appl. Microbiol. Biotechnol . 49, 399–404. doi: 10.1007/s002530051189
Hernández-Pérez, A. F., de Arruda, P. V., Sene, L., da Silva, S. S., Kumar Chandel, A., and de Almeida Felipe, M. D. (2019). Xylitol bioproduction: state-of-the-art, industrial paradigm shift, and opportunities for integrated biorefineries. Crit. Rev. Biotechnol . 39, 924–943. doi: 10.1080/07388551.2019.1640658
Hong, E., Kim, J., Rhie, S., Ha, S. J., Kim, J., and Ryu, Y. (2016). Optimization of dilute sulfuric acid pretreatment of corn stover for enhanced xylose recovery and xylitol production. Biotechnol. Bioprocess Eng . 21, 612–619. doi: 10.1007/s12257-016-0483-z
Honkala, S., Honkala, E., Tynjäl,ä, J., and Kannas, L. (1999). Use of xylitol chewing gum among Finnish schoolchildren. Acta Odontol. Scand . 57, 306–309. doi: 10.1080/000163599428526
International Market Analysis Research Consulting Group (IMARC) (2020). Xylitol Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2021-2026 . Available online at: https://www.imarcgroup.com/xylitol-market (accessed October 16, 2021).
Islam, M. S., and Indrajit, M. (2012). Effects of xylitol on blood glucose, glucose tolerance, serum insulin and lipid profile in a type 2 diabetes model of rats. Ann. Nutr. Metab . 61, 57–64. doi: 10.1159/000338440
Izumori, K., and Tuzaki, K. (1988). Production of xylitol from D-xylulose by Mycobacterium smegmatis . J. Ferment. Technol . 66, 33–36. doi: 10.1016/0385-6380(88)90126-4
Janakiram, C., Kumar, C. D., and Joseph, J. (2017). Xylitol in preventing dental caries: A systematic review and meta-analyses. J. Nat. Sci. Biol. Med . 8, 16–21. doi: 10.4103/0976-9668.198344
Janket, S. J., Jaspreet, B., Isaac, P., Leland, K. A., and Jukka, H. M. (2019). Oral and systemic effects of xylitol consumption. Caries Res . 53, 491–501. doi: 10.1159/000499194
Jeon, W. Y., Yoon, B. H., Ko, B. S., Shim, W., Y., and Kim, J. H. (2012). Xylitol production is increased by expression of codon-optimized Neurospora crassa xylose reductase gene in Candida tropicalis . Bioprocess Biosyst. Eng . 35, 191–198. doi: 10.1007/s00449-011-0618-8
Johan, H., Walfridsson, M., Airaksinen, U., Ojamo, H., Hägerdal, B. H., et al. (1991). Xylitol production by recombinant Saccharomyces cerevisiae . Bio/technology 9, 1090–1095. doi: 10.1038/nbt1191-1090
Junior, J. E., and Rocha, M. V. (2021). Development of a purification process via crystallization of xylitol produced for bioprocess using a hemicellulosic hydrolysate from the cashew apple bagasse as feedstock. Bioprocess Biosyst. Eng . 44, 713–725. doi: 10.1007/s00449-020-02480-9
Junior, W. G. M., Pacheco, T. F., Trichez, D., Almeida, J. R., and Gonçalves, S. B. (2019). Xylitol production on sugarcane biomass hydrolysate by newly identified Candida tropicalis JA2 strain. Yeast . 36, 349–361. doi: 10.1002/yea.3394
Kalhorinia, S., Goli, J. K., Yadav, K. S., Naseeruddin, S., and Rao, L. V. (2014). Xylitol production from water hyacinth ( Eichhornia crassipes ) by Candida tropicalis Y-27405. Biosci. Biotechnol. Res. Asia 11, 427–434. doi: 10.13005/bbra/1291
Khankal, R., Chin, J. W., and Cirino, P. C. (2008). Role of xylose transporters in xylitol production from engineered Escherichia coli . J. Biotechnol . 134, 246–252. doi: 10.1016/j.jbiotec.2008.02.003
Kim, S., Lee, J., and Sung, B. H. (2019). Isolation and characterization of the stress-tolerant Candida tropicalis YHJ1 and evaluation of its xylose reductase for xylitol production from acid pre-treatment wastewater. Front. Bioeng. Biotechnol . 7:138. doi: 10.3389/fbioe.2019.00138
Kim, S. M., Choi, B. Y., Ryu, Y. S., Jung, S. H., Park, J. M., Kim, G. H., et al. (2015). Simultaneous utilization of glucose and xylose via novel mechanisms in engineered Escherichia coli . Metab. Eng . 30, 141–148. doi: 10.1016/j.ymben.2015.05.002
Kitpreechavanich, V., Hayashi, M., Nishio, N., and Nagai, S. (1984). Conversion of D-xylose into xylitol by xylose reductase from Candida pelliculosa coupled with the oxidoreductase system of methanogen strain HU. Biotechnol. Lett . 6, 651–656. doi: 10.1007/BF00133831
Kogje, A. B., and Ghosalkar, A. (2017). Xylitol production by genetically modified industrial strain of Saccharomyces cerevisiae using glycerol as co-substrate. J. Ind. Microbiol. Biotechnol . 44, 961–971. doi: 10.1007/s10295-017-1914-3
Kumar, A. K., and Sharma, S. (2017). Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour. Bioprocess. 4, 1–9. doi: 10.1186/s40643-017-0137-9
Kumar, P., Barrett, D. M., Delwiche, M. J., and Stroeve, P. (2009). Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res . 48, 3713–3729. doi: 10.1021/ie801542g
Kumar, S., Dheeran, P., Singh, S. P., Mishra, I. M., and Adhikari, D. K. (2015). Bioprocessing of bagasse hydrolysate for ethanol and xylitol production using thermotolerant yeast. Bioprocess Biosyst. Eng . 38, 39–47. doi: 10.1007/s00449-014-1241-2
Kumar, V., Krishania, M., Sandhu, P. P., Ahluwalia, V., Gnansounou, E., and Sangwan, R. S. (2018). Efficient detoxification of corn cob hydrolysate with ion-exchange resins for enhanced xylitol production by Candida tropicalis MTCC 6192. Bioresour. Technol . 251,416–419. doi: 10.1016/j.biortech.2017.11.039
Kumar, V., Sandhu, P. P., Ahluwalia, V., Mishra, B. B., and Yadav, S. K. (2019). Improved upstream processing for detoxification and recovery of xylitol produced from corncob. Bioresour. Technol . 291, 121931. doi: 10.1016/j.biortech.2019.121931
Kuyper, M., Hartog, M. M. P., Toirkens, M. J., Almering, M. J. H., Winkler, A. A., Dijken, J. P. V., et al. (2005). Metabolic engineering of a xylose-isomerase-expressing Saccharomyces cerevisiae strain for rapid anaerobic xylose fermentation. FEMS Yeast Res . 5, 399–409. doi: 10.1016/j.femsyr.2004.09.010
Kwon, S. G., Park, S. W., and Oh, D. K. (2006). Increase of xylitol productivity by cell-recycle fermentation of Candida tropicalis using submerged membrane bioreactor. J. Biosci. Bioeng . 101, 13–18. doi: 10.1263/jbb.101.13
Lee, J. K., Koo, B. S., and Kim, S. Y. (2003). Cloning and characterization of the xyl1 gene, encoding an NADH-preferring xylose reductase from Candida parapsilosis , and its functional expression in Candida tropicalis . Appl. Environ. Microbiol . 69, 6179–6188. doi: 10.1128/AEM.69.10.6179-6188.2003
Li, M., Meng, X., Diao, E., and Du, F. (2012). Xylitol production by Candida tropicalis from corn cob hemicellulose hydrolysate in a two-stage fed-batch fermentation process. J. Chem. Technol. Biotechnol . 87, 387–392. doi: 10.1002/jctb.2732
Liu, Y., Zheng, J., Xiao, J., He, X., Zhang, K., Yuan, S., et al. (2019). Enhanced enzymatic hydrolysis and lignin extraction of wheat straw by triethylbenzyl ammonium chloride/lactic acid-based deep eutectic solvent pretreatment. ACS omega . 4,19829–19839. doi: 10.1021/acsomega.9b02709
Llano, T., Rueda, C., Dosal, E., Andrés, A., and Coz, A. (2021). Multi-criteria analysis of detoxification alternatives: Techno-economic and socio-environmental assessment. Biomass. Bioenergy . 154, 106274. doi: 10.1016/j.biombioe.2021.106274
López-Linares, J. C., Romero, I., Cara, C., Castro, E., and Mussatto, S. I. (2018). Xylitol production by Debaryomyces hansenii and Candida guilliermondii from rapeseed straw hemicellulosic hydrolysate. Bioresour. Technol . 247, 736–743. doi: 10.1016/j.biortech.2017.09.139
López-Linares, J. C., Ruiz, E., Romero, I., Castro, E., and Manzanares, P. (2020). Xylitol production from exhausted olive pomace by Candida boidinii . Appl. Sci . 10, 6966. doi: 10.3390/app10196966
Lugani, Y., Puri, M., and Sooch, B. S. (2021). Recent insights, applications and prospects of xylose reductase: a futuristic enzyme for xylitol production. Eur. Food Res. Technol . 247, 921–946. doi: 10.1007/s00217-020-03674-x
Lugani, Y., and Sooch, B. S. (2020). Fermentative production of xylitol from a newly isolated xylose reductase producing Pseudomonas putida BSX-46. LWT . 134, 109988. doi: 10.1016/j.lwt.2020.109988
Mäkinen, K. K. (2000). The rocky road of xylitol to its clinical application. J. Dent. Res . 79, 1352–1355. doi: 10.1177/00220345000790060101
Manaf, S. F., Jahim, J. M., Harun, S., and Luthfi, A. A. (2018). Fractionation of oil palm fronds (OPF) hemicellulose using dilute nitric acid for fermentative production of xylitol. Ind. Crops. Prod . 115, 6–15. doi: 10.1016/j.indcrop.2018.01.067
Mardawati, E., Andoyo, R., Syukra, K. A., Kresnowati, M. T., and Bindar, Y. (2018). “Production of xylitol from corn cob hydrolysate through acid and enzymatic hydrolysis by yeast,” in IOP Conference Series: Earth and Environmental Science 1 (Bogor: IOP Publishing). 012019. doi: 10.1088/1755-1315/141/1/012019
Mardawati, E., Maharani, N., Wira, D. W., Harahap, B. M., Yuliana, T., and Sukarminah, E. (2020). xylitol production from oil palm empty fruit bunches (OPEFB) via simultaneous enzymatic hydrolysis and fermentation process. J. Ind. Inf. Technol. Agricult. 2, 25064. doi: 10.24198/jiita.v2i1.25064
Martin, J. F., Sánchez, S., and Cuevas, M. (2013). Evaluation of the effect of the dilute acid hydrolysis on sugars release from olive prunings. Renew. Energy . 51, 382–387 doi: 10.1016/j.renene.2012.10.002
Martínez, E. A., Canettieri, E. V., Bispo, J. A., Giulietti, M., De Almeida e Silva, J. B., and Converti, A. (2015). Strategies for xylitol purification and crystallization: a review. Sep. Sci. Technol . 50, 2087–2098. doi: 10.1080/01496395.2015.1009115
Martínez, E. A., e Silva, J. B., Giulietti, M., and Solenzal, A. I. (2007). Downstream process for xylitol produced from fermented hydrolysate. Enzyme Microb. Technol . 40, 1193–1198. doi: 10.1016/j.enzmictec.2006.09.003
Martinez, E. A., Silva, S. S., e Silva, J. B., Solenzal, A. I., and Felipe, M. G. (2003). The influence of pH and dilution rate on continuous production of xylitol from sugarcane bagasse hemicellulosic hydrolysate by C. guilliermondii . Process Biochem . 38, 1677–1683. doi: 10.1016/S0032-9592(02)00244-3
CrossRef Full Text
Martínez, E. A., Silva, S. S., and Felipe, M. G. (2000). Effect of the oxygen transfer coefficient on xylitol production from sugarcane bagasse hydrolysate by continuous stirred-tank reactor fermentation. Appl. Biochem. Biotechnol . 84, 633–641. doi: 10.1385/ABAB:84-86:1-9:633
Mathew, A. K., Abraham, A., Mallapureddy, K. K., and Sukumaran, R. K. (2018). “Lignocellulosic biorefinery wastes, or resources,” in Waste Biorefinery , eds. T. Bhaskar, A. Pandey, S. V. Mohan, D. J. Lee. (Elsevier), 267–297.
Medina, J. D., Woiciechowski, A. L., Zandona Filho, A., Brar, S. K., Magalhaes Junior, A. I., and Soccol, C. R. (2018). Energetic and economic analysis of ethanol, xylitol and lignin production using oil palm empty fruit bunches from a Brazilian factory. J. Clean. Prod . 195, 44–55. doi: 10.1016/j.jclepro.2018.05.189
Mishra, D. K., Dabbawala, A. A., and Hwang, J. S. (2013). Ruthenium nanoparticles supported on zeolite Y as an efficient catalyst for selective hydrogenation of xylose to xylitol. J. Mol. Catal. A. Chem . 376, 63–70. doi: 10.1016/j.molcata.2013.04.011
Misra, S., Gupta, P., Raghuwanshi, S., Dutt, K., and Saxena, R. K. (2011). Comparative study on different strategies involved for xylitol purification from culture media fermented by Candida tropicalis . Sep. Sci. Technol . 78, 266–273. doi: 10.1016/j.seppur.2011.02.018
Moraes, E. D. J. C., Silva, D. D. V., Dussán, K. J., Tesche, L. Z., de Almeida Silva, J. B., Rai, M., et al. (2020). Xylitol-sweetener production from barley straw: optimization of acid hydrolysis condition with the energy consumption simulation. Waste Biomass Valorization . 11, 1837–1849. doi: 10.1007/s12649-018-0501-9
Morales-Rodriguez, R., Perez-Cisneros, E. S., Jose, A., and Rodriguez-Gomez, D. (2016). Evaluation of biorefinery configurations through a dynamic model-based platform: Integrated operation for bioethanol and xylitol co-production from lignocellulose. Renew. Energy . 89, 135–143. doi: 10.1016/j.renene.2015.12.019
Mussatto, S. I. (2012). “Application of xylitol in food formulations and benefits for health,” in D-Xylitol , eds. S. S. Da Silva and A. K. Chandel (Berlin: Springer), 309–323.
Mussatto, S. I., and Roberto, I. C. (2003). Xylitol production from high xylose concentration: evaluation of the fermentation in bioreactor under different stirring rates. J. Appl. Microbiol . 95, 331–337. doi: 10.1046/j.1365-2672.2003.01990.x
Mussatto, S. I., and Roberto, I. C. (2008). Establishment of the optimum initial xylose concentration and nutritional supplementation of brewer's spent grain hydrolysate for xylitol production by Candida guilliermondii . Process Biochem . 43, 540–546. doi: 10.1016/j.procbio.2008.01.013
Mussatto, S. I., and Teixeira, J. A. (2010). “Lignocellulose as raw material in fermentation processes,” in Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology , ed. Méndez-Vilas, A., (Formatex Research Center), 897–907.
Naidu, D. S., Hlangothi, S. P., and John, M. J. (2018). Bio-based products from xylan: A review. Carbohydr. Polym . 179, 28–41. doi: 10.1016/j.carbpol.2017.09.064
Nair, N. U., and Zhao, H. (2010). Selective reduction of xylose to xylitol from a mixture of hemicellulosic sugars. Metab. Eng . 12, 462–468. doi: 10.1016/j.ymben.2010.04.005
Narisetty, V., Cox, R., Bommareddy, R. R., Agrawal, D., Ahmad, E., Pant, K. K., et al. (2022). Valorisation of xylose to renewable fuels and chemicals, an essential step in augmenting the commercial viability of lignocellulosic biorefineries. Sustain. Energy Fuels. 6, 29–65. doi: 10.1039/D1SE00927C
Nayak, P. A., Nayak, U. A, and Khandelwal, V. (2014). The effect of xylitol on dental caries and oral flora. Clin. Cosmet. Investig. Dent . 6, 89–94. doi: 10.2147/CCIDE.S55761
Nyyssöl,ä, A., Pihlajaniemi, A., Palva, A., Weymarn, N. V, and Leisola, M. (2005). Production of xylitol from D-xylose by recombinant Lactococcus lactis . J. Biotechnol . 118, 55–66. doi: 10.1016/j.jbiotec.2005.03.014
Oh, E. J., Ha, S. J., Kim, S. R., Lee, W. H., Galazka, J. M., Cate, J. H. D., et al. (2013). Enhanced xylitol production through simultaneous co-utilization of cellobiose and xylose by engineered Saccharomyces cerevisiae . Metab. Eng . 15, 226–234. doi: 10.1016/j.ymben.2012.09.003
Onishi, H., and Suzuki, T. (1969). Microbial production of xylitol from glucose. Appl. Microbiol . 18, 1031–1035. doi: 10.1128/am.18.6.1031-1035.1969
Patel, V., Desai, M., and Madamwar, D. (1993). Thermochemical pretreatment of water hyacinth for improved biomethanation. Appl. Biochem. Biotechnol . 42, 67–74. doi: 10.1007/BF02788902
Pérez-Bibbins, B., Torrado-Agrasar, A., Salgado, J. M., Mussatto, S. I., and Domínguez, J. M. (2015). Xylitol production in immobilized cultures: a recent review. Crit. Rev. Biotechnol . 36, 691–704. doi: 10.3109/07388551.2015.1004660
Peterson, M. E. (2013). Xylitol. Topics in Compan. An Med . 28, 18–20. doi: 10.1053/j.tcam.2013.03.008
Ping, Y., Ling, H., Song, G., and Ge, J. (2013). Xylitol production from non-detoxified corncob hemicellulose acid hydrolysate by Candida tropicalis . Biochem Eng J . 75, 86–91. doi: 10.1016/j.bej.2013.03.022
Povelainen, M., and Miasnikov, A. N. (2007). Production of xylitol by metabolically engineered strains of Bacillus subtilis . J. Biotechnol . 128, 24–31. doi: 10.1016/j.jbiotec.2006.09.008
Prabhu, A. A., Bosakornranut, E., Amraoui, Y., Agrawal, D., Coulon, F., Vivekanand, V., et al. (2020a). Enhanced xylitol production using non-detoxified xylose rich pre-hydrolysate from sugarcane bagasse by newly isolated Pichia fermentans . Biotechnol. Biofuels . 13, 1–5. doi: 10.1186/s13068-020-01845-2
Prabhu, A. A., Thomas, D. J., Ledesma-Amaro, R., Leeke, G. A., Medina, A., Verheecke-Vaessen, C., et al. (2020b). Biovalorisation of crude glycerol and xylose into xylitol by oleaginous yeast Yarrowia lipolytica . Microb. Cell Factories . 19, 1–8. doi: 10.1186/s12934-020-01378-1
Pratter, S. M., Eixelsberger, T., and Nidetzky, B. (2015). Systematic strain construction and process development: Xylitol production by Saccharomyces cerevisiae expressing Candida tenuis xylose reductase in wild-type or mutant form. Bioresour. Technol . 198, 732–738. doi: 10.1016/j.biortech.2015.09.046
Qi, X., Wang, X., Lin, J., Zhu, J., Luo, Y., Deng, W., et al. (2014). Microbial and enzymatic process for xylitol production from D-glucose. Curr. Org. Chem . 18, 3131–3135. doi: 10.2174/138527281824150119095755
Rafiqul, I. S., Mimi Sakinah, A. M., and Zularisam, A. W. (2021). Improvement of enzymatic bioxylitol production from sawdust hemicellulose: optimization of parameters. Prep. Biochem. Biotechnol . 2, 1–11. doi: 10.1080/10826068.2021.1897840
Rafiqul, I. S., and Sakinah, A. M. (2013). Processes for the production of xylitol—a review. Food Rev. Int . 29, 127–156. doi: 10.1080/87559129.2012.714434
Rafiqul, I. S., and Sakinah, A. M. (2015). Biochemical properties of xylose reductase prepared from adapted strain of Candida tropicalis . Appl. Biochem. Biotechnol . 175, 387–399. doi: 10.1007/s12010-014-1269-4
Rafiqul, I. S., Sakinah, A. M., and Zularisam, A. W. (2015a). Enzymatic production of bioxylitol from sawdust hydrolysate: screening of process parameters. Appl. Biochem. Biotechnol . 176, 1071–1083. doi: 10.1007/s12010-015-1630-2
Rafiqul, I. S., Sakinah, A. M., and Zularisam, A. W. (2015b). Inhibition by toxic compounds in the hemicellulosic hydrolysates on the activity of xylose reductase from Candida tropicalis . Biotechnol. Lett . 37, 191–196. doi: 10.1007/s10529-014-1672-5
Ramirez, O. S., and Escoto, H. H. (2021). Enhancement of xylitol production by fed-batch policy designed through stochastic search. Chem. Eng. Trans . 86, 1009–1014.
Rangaswamy, S., and Agblevor, F. A. (2002). Screening of facultative anaerobic bacteria utilizing D-xylose for xylitol production. Appl. Microbiol. Biotechnol . 60, 88–93. doi: 10.1007/s00253-002-1067-8
Rao, L. V., Goli, J. K., Gentela, J., and Koti, S. (2016). Bioconversion of lignocellulosic biomass to xylitol: an overview. Bioresour. Technol . 213, 299–310. doi: 10.1016/j.biortech.2016.04.092
Ravella, S. R., Gallagher, J., Fish, S., and Prakasham, R. S. (2012). “Overview on commercial production of xylitol, economic analysis and market trends,” in D-Xylitol , eds. S. S. Da Silva and A. K. Chandel (Berlin, Springer), 291–306.
Rehman, S., Murtaza, M. A., and Mushtaq, Z. (2016). “Xylitol as sweetener,” in Sweeteners, Reference Series in Phytochemistry , eds J. M. Merillon and K. Ramawat (Cham: Springer), 1–21. doi: 10.1007/978-3-319-26478-3_30-1
Rehman, S. U., Mushtaq, Z., Zahoor, T., Jamil, A., and Murtaza, M. A. (2013). Xylitol: A review on bioproduction, application, health benefits, and related safety issues. Crit. Rev. Food Sci. Nutr . 55, 1514–1528. doi: 10.1080/10408398.2012.702288
Rehman, S. U., Mushtaq, Z., Zahoor, T., Jamil, A., and Murtaza, M. A. (2015). Xylitol: a review on bioproduction, application, health benefits, and related safety issues. Crit. Rev. Food Sci. Nutr . 55, 1514–1528. doi: 10.1080/1408398.2012.702288
Rizzi, M., Harwart, K., Erlemann, P., Bui-Thanh, N. A., and Dellweg, H. (1989). Purification and properties of the NAD+-xylitol-dehydrogenase from the yeast Pichia stipitis . J. Ferment. Bioeng . 67, 20–24. doi: 10.1016/0922-338X(89)90080-9
Rodrigues, R. C. L. B.Kenealy, W. R., et al. (2011). Xylitol production from DEO hydrolysate of corn stover by Pichia stipitis YS-30. J Ind Microbiol Biotechnol . 38, 1649–1655. doi: 10.1007/s10295-011-0953-4
Rudrangi, S. S., and West, T. P. (2020). Effect of pH on xylitol production by Candida species from a prairie cordgrass hydrolysate. Z. Naturforsch. C . 75, 489–493. doi: 10.1515/znc-2020-0140
Salgado, J. M., Rodríguez, N., Cortés, S., and Domínguez, J. M. (2012). Coupling two sizes of CSTR-type bioreactors for sequential lactic acid and xylitol production from hemicellulosic hydrolysates of vineshoot trimmings. N. Biotechnol . 29, 421–427. doi: 10.1016/j.nbt.2011.07.003
Sampaio, F. C., Chaves-Alves, V. M., Converti, A., Passos, F. M., and Coelho, J. L. (2008). Influence of cultivation conditions on xylose-to-xylitol bioconversion by a new isolate of Debaryomyces hansenii . Bioresour. Technol . 99, 502–508. doi: 10.1016/j.biortech.2007.01.017
Sampaio, F. C., De Moraes, C. A., De Faveri, D., Perego, P., Converti, A., and Passos, F. M. (2006a). Influence of temperature and pH on xylitol production from xylose by Debaryomyces hansenii UFV-170. Process Biochem . 41, 675–681. doi: 10.1016/j.procbio.2005.08.019
Sampaio, F. C., Passos, F. M., Passos, F. J., De Faveri, D., Perego, P., and Converti, A. (2006b). Xylitol crystallization from culture media fermented by yeasts. Chem. Eng. Process.: Process Intensif . 45, 1041–1046 doi: 10.1016/j.cep.2006.03.012
Sampaio, F. C., Silveira, W. B., Chaves-Alves, V. M., Passos, F. M., and Coelho, J. L. (2003). Screening of filamentous fungi for production of xylitol from D-xylose. Brazilian J. Microbiol . 34, 325–328. doi: 10.1590/S1517-83822003000400007
Santana, N. B., Dias, J. C., Rezende, R. P., Franco, M., Oliveira, L. K., and Souza, L. O. (2018). Production of xylitol and bio-detoxification of cocoa pod husk hemicellulose hydrolysate by Candida boidinii XM02G. PLoS ONE . 13, e0195206 doi: 10.1371/journal.pone.0195206
Sasaki, M., Jojima, T., Inui, M., and Yukawa, H. (2010). Xylitol production by recombinant Corynebacterium glutamicum under oxygen deprivation. Appl. Microbiol. Biotechnol . 86, 1057–1066. doi: 10.1007/s00253-009-2372-2
Shah, S. S., Luthfi, A. A., Jahim, J. M., Harun, S., and Low, K. O. (2020). An improvement in fermentability of acid-hydrolysed hemicellulose from kenaf stem for xylitol production. Int. J. Food Eng . 9, 4080. doi: 10.1515/ijfe-2019-0230
Shah, S. S., Luthfi, A. A., Low, K. O., Harun, S., Manaf, S. F., Illias, R. M., et al. (2019). Preparation of kenaf stem hemicellulosic hydrolysate and its fermentability in microbial production of xylitol by Escherichia coli BL21. Sci. Rep . 9, 1–3. doi: 10.1038/s41598-019-40807-z
Silva, D. D., Dussán, K. J., Idarraga, A., Grangeiro, L., Silva, S. S., Cardona, C. A., et al. (2020). Production and purification of xylitol by Scheffersomyces amazonenses via sugarcane hemicellulosic hydrolysate. Biofuel. Bioprod. Biorefin . 14, 344–356. doi: 10.1002/bbb.2085
Sindhu, R., Binod, P., Pandey, A., Madhavan, A., Alphonsa, J. A., Vivek, N., et al. (2017). Water hyacinth a potential source for value addition: an overview. Bioresour. technol . 230, 152–162. doi: 10.1016/j.biortech.2017.01.035
Soares, L. C., Chandel, A. K., Pagnocca, F. C., Gaikwad, S. C., Rai, M., and da Silva, S. S. (2016). Screening of yeasts for selection of potential strains and their utilization for in situ microbial detoxification (ISMD) of sugarcane bagasse hemicellulosic hydrolysate. Indian J. Microbiol . 56, 172–181. doi: 10.1007/s12088-016-0573-9
Su, B., Wu, M., Zhang, Z., Lin, J., and Yang, L. (2015). Efficient production of xylitol from hemicellulosic hydrolysate using engineered Escherichia coli . Metab. Eng . 31, 112–122. doi: 10.1016/j.ymben.2015.07.003
Tada, K., Horiuchi, J. I., Kanno, T., and Kobayashi, M. (2004). Microbial xylitol production from corn cobs using Candida magnoliae . J. Biosci. Bioeng . 98, 228–230. doi: 10.1016/S1389-1723(04)00273-7
Tamburini, E., Costa, S., Marchetti, M. G., and Pedrini, P. (2015). Optimized production of xylitol from xylose using a hyper-acidophilic Candida tropicalis . Biomolecules . 5, 1979–1989. doi: 10.3390/biom5031979
Tiefenbacher, F, . (ed.). (2017). “Technology of Main Ingredients—Sweeteners and Lipids,” in The Technology of Wafers and Waffles: Operational Aspects , (Academic Press), 123–225. doi: 10.1016/B978-0-12-809438-9.00003-X
Tomotani, E. J., Arruda, P. V., Vitolo, M., and Felipe, M. D. (2009). Obtaining partial purified xylose reductase from Candida guilliermondii . Braz. J. Microbiol . 40, 631–635. doi: 10.1590/S1517-83822009000300027
Traff, K. L., Cordero, R. R. O., Zyl, W., H, V., and Hagerdal, B. H. (2001). Deletion of the GRE3 aldose reductase gene and its influence on xylose metabolism in recombinant strains of Saccharomyces cerevisiae expressing the xylA and XKS1 genes. Appl. Environ. Microbiol . 67, 5668–5674. doi: 10.1128/AEM.67.12.5668-5674.2001
Uhari, M., Kontiokari, T., Koskela, M., and Niemela, M. (1996). Xylitol chewing gum in prevention of acute otitis media: double blind randomised trial. BMJ . 313, 1180–1183. doi: 10.1136/bmj.313.7066.1180
Valdes, G., Mendonça, R. T., and Aggelis, G. (2020). Lignocellulosic biomass as a substrate for oleaginous microorganisms: a review. Appl. Sci . 10, 7698. doi: 10.3390/app10217698
Varanasi, J. L., Kumari, S., and Das, D. (2018). Improvement of energy recovery from water hyacinth by using integrated system. Int. J. Hydrog. Energy 43, 1303–1318. doi: 10.1016/j.ijhydene.2017.11.110
Verduyn, C., Van Kleef, R., Frank, J., Schreuder, H., Van Dijken, J. P., and Scheffers, W. A. (1985). Properties of the NAD (P) H-dependent xylose reductase from the xylose-fermenting yeast Pichia stipitis . Biochem. J . 226, 669–677 doi: 10.1042/bj2260669
Vernacchio, L., Corwin, M. J., Vezina, R. M., Pelton, S. I., Feldman, H. A., Beasley, T. C., et al. (2014). Xylitol syrup for the prevention of acute otitis media. Pediatr . 133, 289–295. doi: 10.1542/peds.2013-2373
Wang, L., Shen, Y., Zhang, Y., Wei, Q., Liang, Y., Tian, H., et al. (2021). A novel surface treatment of carbon fiber with Fenton reagent oxidization for improved cells immobilization and xylitol fermentation. Microporous. Mesoporous. Mater . 325, 111318. doi: 10.1016/j.micromeso.2021.111318
Wang, W., Ling, H., and Zhao, H. (2015). Steam explosion pretreatment of corn straw on xylose recovery and xylitol production using hydrolysate without detoxification. Process Biochem . 50, 1623–1628. doi: 10.1016/j.procbio.2015.06.001
Werpy, T., and Petersen, G. (2004). Top value added chemicals from biomass: volume I–results of screening for potential candidates from sugars and synthesis gas. National Renewable Energy Lab . p. 76. doi: 10.2172/15008859
West, T. P. (2021). Xylitol Production by Candida Species from Hydrolysates of Agricultural Residues and Grasses. Ferment . 7, 243. doi: 10.3390/fermentation7040243
Winkelhausen, E., and Kuzmanova, S. (1998). Microbial conversion of D-xylose to xylitol. J. Ferment. Bioeng . 86, 1–4. doi: 10.1016/S0922-338X(98)80026-3
Xu, Y., Chi, P., Bilal, M., and Cheng, H. (2019). Biosynthetic strategies to produce xylitol: an economical venture. Appl. Microbiol. Biotechnol . 103, 5143–5160. doi: 10.1007/s00253-019-09881-1
Yablochkova, E. N., Bolotnikova, O. I., Mikhailova, N. P., Nemova, N. N., and Ginak, A. I. (2003). The activity of xylose reductase and xylitol dehydrogenase in yeasts. Microbiology . 72, 414–417. doi: 10.1023/A:1025032404238
Yadav, M., Mishra, D. K., and Hwang, J. S. (2012). Catalytic hydrogenation of xylose to xylitol using ruthenium catalyst on NiO modified TiO2 support. Appl. Catal. A: Gen . 425, 110–116. doi: 10.1016/j.apcata.2012.03.007
Yamagata, S., Goto, Y., Ohneda, A., Anzai, M., Kawashima, S., Kikuchi, J., et al. (1969). “Clinical Application of Xylitol in Diabetes”, in International Symposium on Metabolism, Physiology, and Clinical Use of Pentoses and Pentitols , (Berlin, Heidelberg: Springer), 316–325.
Yewale, T., Panchwagh, S., Rajagopalan, S., Dhamole, P. B., and Jain, R. (2016). Enhanced xylitol production using immobilized Candida tropicalis with non-detoxified corn cob hemicellulosic hydrolysate. Biotech . 6, 75. doi: 10.1007/s13205-016-0388-8
Yoshitake, J., Ishizaki, H., Shimamura, M., and Imai, T. (1973a). Xylitol production by an Enterobacter species. Agr Biol Chem . 37, 2261–2267 doi: 10.1080/00021369.1973.10861002
Yoshitake, J., Shimamura, M., and Imai, T. (1973b). Xylitol production by a Corynebacterium species. Agr Biol Chem . 37, 2251–2259. doi: 10.1080/00021369.1973.10861001
Zha, J., Li, B. Z., Shen, M. H., Hu, M. L., Song, H., and Yuan, Y. J. (2013). Optimization of CDT-1 and XYL1 expression for balanced co-production of ethanol and xylitol from cellobiose and xylose by engineered Saccharomyces cerevisiae . PLoS One . 8:e68317. doi: 10.1371/journal.pone.0068317
Zhang, J., Zhang, B., Wang, D., Gao, X., and Hong, J. (2014). Xylitol production at high temperature by engineered Kluyveromyces marxianus . Bioresour. Technol . 152, 192–201. doi: 10.1016/j.biortech.2013.10.109
Zou, Y. Z., Qi, K., Chen, X., Miao, X. L., and Zhong, J. J. (2010). Favorable effect of very low initial KLa value on xylitol production from xylose by a self-isolated strain of Pichia guilliermondii . J. Biosci. Bioeng . 109, 149–152. doi: 10.1016/j.jbiosc.2009.07.013
Keywords: bioproduction, hemicellulose valorization, lignocellulosic biomass, xylitol, xylose
Citation: Umai D, Kayalvizhi R, Kumar V and Jacob S (2022) Xylitol: Bioproduction and Applications-A Review. Front. Sustain. 3:826190. doi: 10.3389/frsus.2022.826190
Received: 30 November 2021; Accepted: 17 January 2022; Published: 15 February 2022.
Reviewed by:
Copyright © 2022 Umai, Kayalvizhi, Kumar and Jacob. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel Jacob, samueljb@srmist.edu.in
Oral and Systemic Effects of Xylitol Consumption
Affiliations.
- 1 Translational Oral Medicine, Forsyth Institute, Cambridge, Massachusetts, USA.
- 2 Boston University School of Public Health, Boston, Massachusetts, USA, [email protected].
- 3 Research Externship,Edwin O. Smith High School, Storrs, Connecticut, USA.
- 4 Department of Public Health, University of Massachusetts, Lowell, Massachusetts, USA.
- 5 Department of Oral and Maxillofacial Diseases, Helsinki University Hospital and University of Helsinki, Helsinki, Finland.
- PMID: 31060040
- DOI: 10.1159/000499194
Recent results of randomized trials testing the efficacy of xylitol in caries prevention have been conflicting. This narrative review reveals the sources of discrepancy. The following databases were searched for the terms "xylitol" or "artificial sweeteners" restricted to the English language: PubMed, Web of Science, Evidenced-Based Medicine, Scopus, and the Cochrane database. In a separate search, the terms "dental caries" or "cariogenicity" or "glucosyltransferase" or "low glycemic" or "low insulinemic" or "dysbiosis" or "gut microbiome" were used and then combined. In section I, findings regarding the role of xylitol in dental caries prevention, the appropriateness of research methods, and the causes for potential biases are summarized. In section II, the systemic effects of xylitol on gut microbiota as well as low-glycemic/insulinogenic systemic effects are evaluated and summarized. The substitution of a carbonyl group with an alcohol radical in xylitol hinders its absorption and slowly releases sugar into the bloodstream. This quality of xylitol is beneficial for diabetic patients to maintain a constant glucose level. Although this quality of xylitol has been proven in in vitro and animal studies, it has yet to be proven in humans. Paradoxically, recent animal studies reported hyperglycemia and intestinal dysbiosis with artificial sweetener consumption. Upon careful inspection of evidence, it was revealed that these reports may be due to misinterpretation of original references or flaws in study methodology. Any systemic benefits of xylitol intake must be weighed in consideration with the well-established adverse gastrointestinal consequences. The contribution of xylitol to gut dysbiosis that may affect systemic immunity warrants further research.
Keywords: Antiacidogenicity; Anticariogenicity; Glucosyltransferase; Gut dysbiosis; Gut microbiome; Low-glycemic effects.
© 2019 S. Karger AG, Basel.
Publication types
- Dental Caries / prevention & control*
- Gastrointestinal Microbiome
- Non-Nutritive Sweeteners / administration & dosage*
- Non-Nutritive Sweeteners / therapeutic use
- Xylitol / administration & dosage*
- Xylitol / therapeutic use
- Non-Nutritive Sweeteners
Advertisement
Effects of xylitol chewing gum and candies on the accumulation of dental plaque: a systematic review
- Open access
- Published: 22 October 2021
- Volume 26 , pages 119–129, ( 2022 )
Cite this article
You have full access to this open access article
- Eva Söderling ORCID: orcid.org/0000-0001-7565-703X 1 &
- Kaisu Pienihäkkinen 1
6915 Accesses
12 Citations
12 Altmetric
Explore all metrics
A systematic review of published data was conducted with the aim of assessing the effects of xylitol consumption on the amount of dental plaque.
Materials and methods
Electronic and hand searches were performed to find clinical studies concerning the effects of xylitol chewing gum or candies on dental plaque. Prospective randomized controlled clinical trials published between 1971 and 2020 conducted in healthy subjects were included in the review.
The initial search identified 424 xylitol articles. After applying inclusion and exclusion criteria, altogether 14 articles (16 studies) were reviewed. The review identified 12 of the total of 14 xylitol chewing gum studies as having fair or high quality. In 13 of the 14 chewing gum studies, xylitol gum decreased plaque accumulation. In six studies, xylitol gum chewing decreased plaque compared to sorbitol gum, and in three studies compared to gum base/no gum. In three fair-quality studies conducted with xylitol candies, plaque accumulation did not change.
Conclusions
Habitual xylitol gum chewing appears to show plaque-reducing effects that differ from those of sorbitol gum. This suggests specific effects for xylitol on plaque accumulation. Xylitol candies appear not to decrease plaque. The heterogeneity of the studies warrants further research.
Clinical relevance
Habitual xylitol gum chewing is likely to decrease plaque.
Similar content being viewed by others
The effect of xylitol chewing gums and candies on caries occurrence in children: a systematic review with special reference to caries level at study baseline
K. Pienihäkkinen, A. Hietala-Lenkkeri, … E. Söderling
Effects of sugar-free polyol chewing gums on gingival inflammation: a systematic review
Eva Söderling, Kaisu Pienihäkkinen & Ulvi Kahraman Gursoy

Should dentists recommend sugar-free chewing gum to help prevent decay?
Duncan Parker-Groves
Avoid common mistakes on your manuscript.
Introduction
Dental caries is a lifestyle-related disease, poor oral hygiene and poor dietary habits playing a key role. Caries is initially reversible and can be halted by removing enough of dental biofilm, i.e. dental plaque [ 1 ]. Dental biofilm is also a risk factor of periodontal disease. Gingival inflammation in response to dental plaque accumulation is considered a key factor for the onset of periodontitis [ 2 ]. Several intrinsic and extrinsic factors, such as saliva and frequent carbohydrate consumption, influence plaque accumulation [ 3 ]. These “disease drivers” are also crucial for symbiosis/dysbiosis of the oral microbiota. In recent years, research has focused on ways to increase resistance of the microbiota to dysbiosis [ 4 , 5 ]. However, plaque accumulation by itself increases the risk of dental disease.
Xylitol is a naturally occurring five-carbon polyol sweetener that appears to have specific, beneficial effects on oral health but also other health benefits [ 6 ]. Habitual consumption of xylitol is suggested to reduce caries occurrence [ 7 ]. Systematic reviews on the caries-preventive effect of xylitol have nevertheless resulted in varying outcomes [ 7 – 9 ]. The “chewing effect” is considered by some authors to explain the caries-preventive effects of xylitol chewing gum [ 10 , 11 ]. However, xylitol administered with pastilles [ 12 ], syrup [ 13 ] and wipes [ 14 ] has also reduced caries. There is good evidence that habitual xylitol consumption decreases counts of caries-associated mutans streptococci [ 15 ]. Xylitol appears to act as an oral prebiotic decreasing mutans streptococci without affecting the overall microbiota, and may thus increase the resistance of the microbiota to dysbiosis [ 15 ].
Habitual xylitol consumption has been associated with a decrease in the amount of dental plaque [ 16 ], though studies that do not support this result have also been published [ 11 ]. Habitual, long-term xylitol consumers were reported to have low levels of dental plaque compared to non-consumers of xylitol [ 17 ]. It has been suggested that the plaque of xylitol users is less adhesive due to a decrease in counts of plaque mutans streptococci and/or reduced amounts of extracellular polysaccharides in the plaque [ 18 ]. Also, the so-called xylitol-resistant mutans streptococci which were suggested to be easily shed to saliva have been connected with a decrease in the amount of plaque [ 18 , 19 ].
Most of the xylitol studies showing a decrease in the amount of plaque have been conducted with chewing gum [ 16 , 18 ]. Also, other sugar-free polyol gums may reduce plaque accumulation [ 20 ]. There is evidence that regular use of sugar-free chewing gum, in conjunction with normal oral hygiene, provides a small, but significant reduction in plaque [ 20 , 21 ]. Sugar-free gum is recommended by several organizations, for example the American Dental Association ( www.ada.org ). However, to our knowledge, only one systematic review concerning sugar-free polyol gums and plaque accumulation has been published [ 20 ].
With this systematic review, we wanted to answer the defined research questions: (1) can the consumption of xylitol chewing gum or candies/lozenges/pastilles reduce the accumulation of dental plaque, (2) are the effects specific for xylitol? To achieve this, we described and evaluated the literature published during 1971–2020 in relation to the effect of xylitol chewing gums and candies on the amount of dental plaque in healthy children and adults.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA: www.prisma-statement.org ) was used as a guideline in the present systematic review. The review was submitted for registration in PROSPERO on November 11, 2020 before the data collection started.
Information sources for data extraction
A systematic review to identify all the relevant studies published was conducted from three databases: PubMed, Embase and the Cochrane Library. Grey literature was searched on www.clinicaltrials.gov . The searches were conducted on November 11, 2020 and updated on December 31, 2020.
Literature screening strategies
The following terms were used in the search for studies:
('xylitol'/exp OR xylitol*) AND ('tooth plaque'/exp OR tooth NEXT/1 plaque* OR dental NEXT/1 plaque*)—Embase
(xylitol* OR "Xylitol"[Mesh]) AND (dental plaque* OR "dental plaque"[Mesh] OR tooth plaque*)—PubMed
(xylitol*) AND (dental NEXT plaque* OR tooth NEXT plaque*)—Cochrane Library
Study inclusion and exclusion criteria
Prospective randomized controlled clinical trials (RCT) conducted in healthy subjects were included in the review. Only healthy subjects were included in the evaluation since if health claims are made on functional foods such as xylitol, the evaluated studies should be conducted in subjects who do not have problems with their general health. The aim of the included trials was to study the effects of xylitol on the amount of dental plaque. Chewing gums or candies (including lozenges/pastilles) were the vehicles included in the review. Plaque was either the primary or secondary outcome measure in the evaluated studies. The included studies compared baseline or no treatment values with values obtained in the same subjects after the intervention period. The comparison/control (product) was a polyol gum or candy, chewing gum base or no product. In order to meet the inclusion criteria, xylitol had to be the polyol with a concentration of 50% or more in the tested product. The comparison/control product could not contain xylitol.
Exclusion criteria used when evaluating abstracts: in vitro studies; animal studies; studies in subjects undergoing orthodontic treatment; studies in mentally retarded or disabled subjects; studies in children, elderly subjects or geriatric patients living in institutions; studies not related to oral health; reviews, abstracts, comments or study protocols; the polyol vehicles were oral rinses, toothpastes, oral sprays, pacifiers, milk or wipes; dental plaque was not an outcome of the study; other plaque reducers than xylitol were studied; no control group; mother-to-child transmission studies; the study was not available in English.
Exclusion criteria used when evaluating full-text articles: in five studies, baseline values were not available [ 22 – 26 ]; three studies were not properly controlled or the control did not fulfill the inclusion criteria [ 27 – 29 ]; two studies were cross-sectional [ 30 , 31 ]; in one study, the test product contained less than 50% of all polyols [ 32 ]; one study was not randomized [ 33 ]; in one study, plaque was analyzed only at baseline [ 34 ]; and in one study, there was no information on the daily dose of xylitol [ 35 ].
Data extraction and assessment of methodological quality and risk of bias
The articles that fulfilled the inclusion criteria were selected for full-text review and data extraction. The following data were collected: author and year of publication, study site, number and age of participants, study design, intervention and controls, oral hygiene instructions, assessment method, and main results.
The risk of bias of the selected articles was assessed using the Cochrane Collaboration tool for assessing risk of bias in randomized trials [ 36 ]. Two authors (ES, KP) independently evaluated the included abstracts and full-length articles and, based on mutual agreement, eliminated discrepancies between each individual assessment. A third evaluator (VL) evaluated the articles in which the first author of this review was an author.
The studies were appraised according to the following aspects: random sequence generation, allocation concealment, blinding, completeness of outcome data, selective reporting and funding bias. Each aspect was classified as having either low, high or unclear risk of bias. The bias was estimated to be unclear, for example, if the study was randomized but details on randomization were not given. Also, when information not found in the paper was submitted by the authors, the bias was classified as unclear. The overall level of risk for each study was classified as low (all quality items were met: high quality), unclear (unclear risk of bias for one or more domain: fair quality), or high (high risk of bias for one or more domain: low quality) [ 15 , 36 , 37 ].
Study selection
In the search for xylitol articles, a total of 802 titles were screened for relevance: (336 PubMed, 348 Embase, 118 Cochrane). Removing the duplicates left 424 titles to be evaluated. Based on the information of the abstract, 396 articles were removed. When full-text articles were assessed for eligibility, 14 articles were removed leaving 14 articles to be reviewed (Fig. 1 ). One of the articles consisted of three substudies, bringing the total number of evaluated studies to 16.
Study characteristics
All studies included in the review were prospective, randomized, controlled studies published between 1971 and 2020 [ 38 – 51 ]. In the 16 studies included in the review, all participants were classified as healthy by the authors of the studies. All studies reported the age of the participants (age range 5–60 years or older), sample size (ranging from 14 to 485), and study duration (from 6 days to 3 years). The delivery modalities included chewing gums or candies (lozenges/pastilles). In four of the studies, the subjects were children (< 18 years); in 12 studies, the participants were adults (Table 1 ).
In the majority of the studies, the primary outcome measure was the amount of plaque (Table 1 ). In one study, the primary outcome measure was the acidogenicity of plaque [ 49 ], and in another, caries occurrence [ 48 ]. In one of the studies, the stimulated saliva flow rate was the primary outcome measure [ 47 ], in one, bleeding on marginal probing [ 50 ], and in one, pro-inflammatory cytokines [ 51 ].
Quality assessment of the selected studies
Figure 2 summarizes the risks of bias in the evaluated studies. The risk-of-bias assessment revealed that two studies had a low risk of bias [ 46 , 47 ], two studies [ 49 , 50 ] were scored as having a high risk of bias, and the rest (12 studies) had an unclear risk of bias.
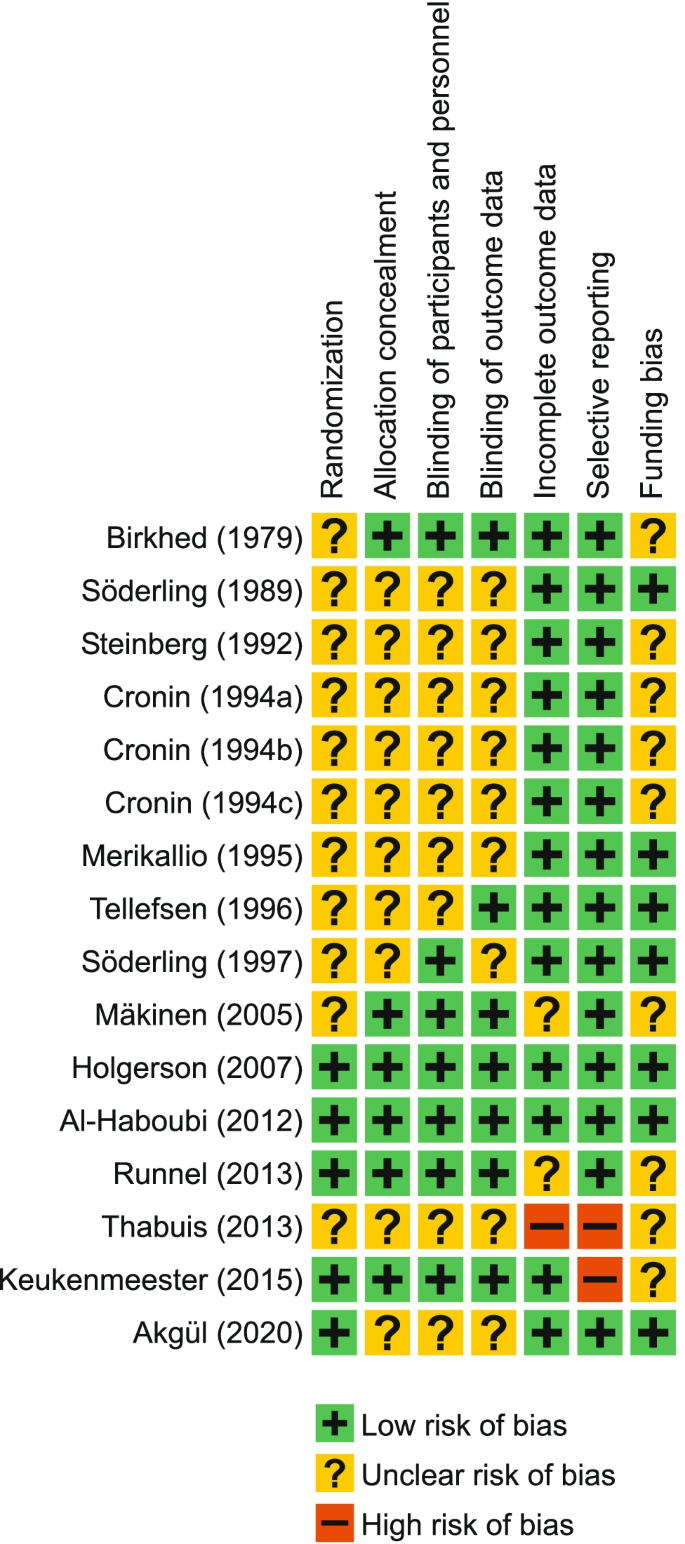
Risk of bias summary
Since the present review includes studies from the 1970s, 1980s and 1990s, it is evident that details concerning for example randomization or blinding were lacking. In fact, only in four studies were the participants randomized on an individual basis using computer-generated randomization [ 46 , 47 , 50 , 51 ]. In the study by Mäkinen et al. [ 45 ], the randomization was done according to kindergarten, which is a practical way to perform chewing gum studies, but may result in some bias.
The amount of plaque was the primary outcome of the present review, and thus it was important that the plaque collection or estimation of the plaque index were performed blinded. This important point was addressed only in a few studies; most studies merely included the statement that they were performed blinded, without providing much detail. However, problems with allocation concealment and blinding are inevitable when the control group does not chew gum or consume candies [ 42 , 47 ].
The availability of individual baseline values increased the probability of finding true intervention-related changes in the amount of plaque in the evaluated studies. Also, differences in changes in the amount of plaque between intervention and comparison groups could be detected. In most studies, the baseline plaque values were comparable with the post-intervention values; however, in two studies, this point remained unclear [ 40 , 43 ]. The crossover designs and controls of these studies should nevertheless compensate for the possible bias in the incomplete outcome data, thus presenting an unclear risk of bias [ 40 , 43 ]. In the study by Runnel et al. [ 48 ], an unclear risk of bias in relation to incomplete outcome data was based on not taking the high number of dropouts into consideration.
Not including in the abstract a report of significant plaque reductions detected in the study led to a high risk of bias in relation to selective reporting in the study by Keukenmeester et al. [ 50 ]. In the study by Mäkinen et al. [ 45 ], results for the sorbitol control group were shown, but not for the no-gum control group leading to an unclear risk of bias in relation to incomplete outcome data. In the Thabuis et al. [ 49 ] study, the results for the gum-base control were not reported leading to a high risk of bias both in relation to incomplete outcome data and selective reporting.
In six of the studies, the tested xylitol and control products had been obtained as gifts from various companies without other apparent funding [ 39 , 42 , 44 , 46 , 47 , 51 ]. Seven studies appeared partly or fully industry-funded resulting in and unclear risk of bias [ 38 , 40 , 43 , 45 , 48 – 50 ]. Also, the three separate clinical studies by Cronin et al. [ 41 a, b, c] had an unclear risk of funding bias.
Influence of xylitol chewing gum on the amount of plaque
Thirteen of the total of 14 chewing gum studies found a significant decrease in the amount of plaque in association with xylitol gum chewing [ 39 – 41 a, b, c, 43 – 47 , 49 – 51 ]. The finding was similar in children [ 45 , 46 , 49 ] and adults [ 39 , 40 , 43 , 44 , 47 , 50 , 51 ]. Eleven of the studies were short-term studies lasting 6–30 days and three were long-term studies lasting 1.5–6 months.
In six of the studies, xylitol gum chewing decreased plaque accumulation compared to the sorbitol control gum [ 39 , 43 , 45 ]. This suggests specific effects for xylitol on plaque accumulation. In the 2-week American study, xylitol and xylitol-sorbitol chewing gum decreased the fresh weight of plaque by 24–29%, while an increase in plaque was seen in the sorbitol gum group [ 39 ]. The three 2-week studies by Cronin et al. [ 41 a, b, c] compared, among other things, the effects of various daily doses of xylitol on fresh weights of plaque in association with consumption of xylitol/sorbitol and sorbitol gum. In the first study [ 41 ]a], plaque regrowth was reduced by 29% in the xylitol/sorbitol gum group, by 23–32% (consumption 2 pieces 3 day or higher) in the second study [ 41 b] and by 32–38% in the third study [ 41 c]. In the sorbitol control groups, the plaque regrowth reductions were small, 8–10% [ 41 a, b, c]. In the 6-month study by Mäkinen et al. [ 45 ], xylitol gum chewing four times a day, 5 min at a time, decreased the mean plaque index of 5-year-old children by 8% while no change was seen in the sorbitol control group. The chewing gum study by Tellefsen et al. [ 43 ] compared plaque accumulation using a plaque index after 6 days of no oral hygiene combined with xylitol or sorbitol gum chewing, 3 × 20 min a day, xylitol gum reducing plaque formation more than the sorbitol gum.
In four studies, both the xylitol gum and the control polyol gums showed similar decreases in plaque accumulation [ 40 , 46 , 49 , 50 ]. In all of these studies, the gum chewing recommendation was 3–5 times a day, 10 min at a time. In the study by Steinberg et al. [ 40 ], 1.5 months of chewing xylitol or sorbitol gum decreased the mean plaque index by 15% in the xylitol group, and by 12% in the sorbitol control group, while in the no-gum group, no decrease was detected. In the 4-week, Swedish study, the plaque index measured from six buccal surfaces decreased both in the xylitol and sorbitol gum group [ 46 ]. In two low-quality studies, the xylitol gum and the controls, maltitol gum [ 49 ] or maltitol gum and gum base [ 50 ], decreased the plaque index. In the 30-day study by Thabuis et al. [ 49 ], a plaque index decrease of 43% was reported both in the xylitol and maltitol groups. In the Keukenmeester et al. [ 50 ] 3-week study, a decrease in the plaque index of 7–11% was found in the brushed upper jaw in the xylitol and maltitol groups. However, in the absence of brushing, no differences were detected in any group in plaque accumulation in the lower jaw.
Gum base was used as a control in four xylitol chewing gum studies [ 44 , 49 – 51 ], but results were reported only in three studies [ 44 , 49 , 51 ]. In two studies, it was the only control [ 44 , 51 ]. In a Finnish study, xylitol and xylitol-sorbitol gums decreased the fresh weight of plaque by 32–34% while the gum base comparison did not [ 44 ]. In the study, the gums were recommended to be chewed 3–5 times a day, 3 min at a time. In the recent study by Akgül et al. [ 51 ], xylitol gum chewing decreased plaque accumulation by 46% while a small reduction of 9% was seen in the gum base group. The recommended chewing time was 3 times a day, 10 min at a time. In the Keukenmeester et al. study [ 50 ], similar small decreases in the amount of plaque were observed in the xylitol and gum base groups. In the high-quality Al-Haboubi et al. [ 47 ] study, the comparison of xylitol gum was no gum. The low mean plaque scores of elderly people further decreased in association with xylitol gum chewing for 6 months, twice a day, for 15 min at a time. The plaque index did not change in the no-gum control group.
The only chewing gum study not demonstrating a plaque decrease for xylitol gum was a 2-week study comparing the effects of xylitol gum and army-made pastilles with no gum [ 42 ]. The very high plaque amounts suggest that the recruits omitted oral hygiene totally in field conditions.
Influence of xylitol candies/pastilles on the amount of plaque
In three studies, the consumption of xylitol candies/lozenges/pastilles showed no effect on plaque accumulation [ 38 , 42 , 48 ]. In the study by Birkhed et al. [ 38 ], the subjects consumed xylitol, sorbitol, maltitol and Lycasin lozenges for 3 months. The mean fresh weights of plaque did not decrease in any of the groups. In the 3-year study by Runnel et al. [ 48 ], the effects of xylitol, erythritol and sorbitol candies on plaque accumulation were compared. Erythritol candies appeared to decrease plaque, while no effect was seen for either xylitol or sorbitol candies.
Adverse effects
Possible adverse effects connected with the use of the test and control products were recorded and reported in five of the 16 studies [ 41 c, 45 , 47 , 49 , 50 . One subject discontinued the study based on feeling nauseous due to gum chewing [ 41 c], no other adverse effects were reported in the five studies.
The main finding of this review is that habitual xylitol chewing gum consumption reduces the amount of plaque. The result appeared to be similar in short- and long-term studies, and in children and adults. In all but one study [ 47 ], the daily doses of xylitol were high enough, approximately 5 g or higher, to achieve “xylitol effects” demonstrated, for example, in association with decreases in counts of mutans streptococci [ 15 , 52 ]. In six studies, xylitol gum chewing decreased plaque compared to sorbitol gum suggesting specific effects for xylitol on plaque accumulation [ 39 , 41 a, b, c, 43 , 45 ].
It has been suggested that chewing gum has no relevant tooth-cleaning effects [ 10 ]; however, a small, but significant plaque-reducing effect has been shown for sugarfree chewing gums in several studies [ 20 ]. Polyols like sorbitol and maltitol are used commonly as controls in chewing gum studies. They are considered microbiologically rather inert but they are sweet and thus add to the saliva secretion-enhancing effect of gum base chewing. Gum base is often a problematic control in the studies, since without the polyol sweetener, the gum may be harder to chew and the taste is not as agreeable as in the polyol-containing gums. In three of the evaluated studies, results for a control gum base were reported [ 44 , 49 , 51 ]. In our evaluation, gum base chewing showed no [ 44 ] or small 7–9% decreases [ 50 , 51 ] in the mean plaque indices. Thus, the plaque-reducing effects of polyol chewing gums may not be attributed to chewing per se.
Our results suggest that chewing time may be a confounding factor in chewing gum studies even though all evaluated studies did not support this idea. In six studies with short chewing time recommendations [ 44 , 45 ] or no recommendations [ 39 , 41 a, b, c], the xylitol gum decreased plaque while sorbitol gum/gum base did not, suggesting that xylitol gum exerts specific plaque-reducing effects. Xylitol dissolves from a chewing gum with a high concentration peak in the saliva at 1 min, the bulk of the xylitol (and sweetness) being dissolved at 3 min of gum chewing [ 53 ]. Thus, if no chewing time recommendations are given, the tasteless gum is most probably discarded after a short chewing period. The short chewing time resulting in high xylitol levels in the plaque may be important for the mechanism of action of xylitol. Extended gum chewing may actually decrease the beneficial effects of xylitol by stimulating the salivary flow for a long time and thus promoting oral clearance of xylitol. This may be crucial for the effects of xylitol chewing gum on plaque. In fact, the longer chewing recommendations, 3–5 × 10 min, were in three studies associated with similar small plaque reductions for xylitol, sorbitol and maltitol gums [ 40 , 46 , 50 ]. This result is in accordance with earlier studies with sorbitol gum [ 40 , 41 , 43 , 44 , 45 , 54 , 55 , 56 ]. In these studies, the sorbitol gum was chewed several times a day, 20–30 min at a time, and the plaque decreases were significant but rather small. Since the chewing time may influence the outcome of chewing gum studies, it could be an interesting research aspect of future chewing gum studies.
The three xylitol studies with candies/lozenges/pastilles did not find effects for xylitol on the amount plaque [ 38 , 42 , 48 ]. In the study by Birkhed et al. [ 38 ], the subjects showed high amounts of plaque compared to similar studies with 2 days of no oral hygiene [ 39 , 44 ], which may be a confounding factor. Also, the intervention lasted only 4 days and the daily dose of xylitol was rather low, 4 g, which may have affected the outcome of the study. In the Runnel et al. [ 48 ] study, the authors postulated that treatment during the span of the study was relatively mild: test products were only consumed three times a day with the last consumption around 2 pm, and the products were consumed during weekdays, and not during the school vacation. Studies which did not fulfill the inclusion criteria of the present review have, however, demonstrated plaque decreases for xylitol candies/pastilles. Significant plaque reductions have been observed in association with consumption of xylitol candy and pastilles in disabled Finnish [ 57 , 58 ] and Kuwaitian subjects [ 59 ].
Xylitol may influence plaque accumulation through several mechanisms. Xylitol consumption has reduced the acid production potential of plaque [ 60 ], thus not favouring acidogenic and aciduric microorganisms like mutans streptococci. There is good evidence suggesting that habitual xylitol consumption reduces mutans streptococci counts in plaque [ 15 ], which could result in less adhesive plaque. In our evaluation, in one fair-quality study, associations between the magnitudes of the decreases in the fresh weights of plaque vs. levels of both mutans streptococci and xylitol-resistant mutans streptococci were analyzed, but no associations were detected [ 44 ]. It has also been suggested that a xylitol-induced decrease in the extracellular polysaccharides could reduce plaque [ 61 ]. In one study, no connection was detected between decreases in the plaque fresh weights and the polysaccharide contents of plaque [ 44 ]. However, in the low-quality study of Thabuis et al. [ 49 ], insoluble glucans of plaque were reported to decrease in the xylitol and maltitol gum groups but not in the no-gum or gum-base groups. Clearly, there is a need for more research on this topic.
We included in the present evaluation studies with individual baseline values available. The baseline values clearly increased the transparency of the studies and were usually associated with relevant statistical testing. Also, studies with relatively low numbers of subjects could give relevant results concerning changes in the amount of plaque. If the methods were valid, even old studies could be considered to be of at least fair quality. In addition to the study design, also other methodological aspects are of importance, especially in plaque studies. Eight of the present studies estimated the amount of plaque with gravimetric methods [ 38 , 39 , 41 a, b, c, 42 , 44 , 48 ] and seven used plaque indices. The plaque indices used were Quigley-Hein or its modification [ 40 , 43 , 45 , 49 , 50 ], Silness-Löe [ 47 , 51 ] or a simplified oral index (OHI-S; 46). In most of these studies, plaque indices were presented as means of plaque scores [ 40 , 43 , 45 , 47 , 49 – 51 ]. Since plaque is not accumulated evenly in the mouth and the index values are categorical in nature, the means of the scores may not reflect properly plaque accumulation. Also, plaque indices do not take into account, for example, the thickness of plaque. In two studies, no oral hygiene was practiced for 6 days [ 43 ] and 3 weeks [ 50 ], but this was poorly reflected in the mean scores of plaque. As for the Holgerson et al. [ 46 ] study, as the authors themselves state, the simplified oral debris index may not be an adequate way to quantify plaque. Gravimetric methods should be more accurate compared to plaque indices, especially when mean scores are calculated and/or only a few index teeth were used for estimating the plaque index. This idea is supported by a study by Birkhed et al. [ 25 ] which compared plaque indices and gravimetric methods in an intervention study.
Also, the recommendations concerning no oral hygiene before the plaque collections should be of importance. The study subjects will not omit oral hygiene if no instructions are given, and the effects of the interventions may be difficult to detect if there is very little plaque. Our results support this idea. In subjects adhering to 2–2.5 days of no oral hygiene before the plaque collections, clinically relevant changes in the amount of plaque, approximately 20–40%, were detected for xylitol gum chewing [ 39 , 41 a, b, c, 44 , 49 ]. If no recommendations were given or oral hygiene was omitted only in the morning of the plaque collection date, the changes in the amount of plaque were, as a rule, small [ 40 , 45 , 50 ]. In two studies, the subjects refrained from all oral hygiene measures during the study for 6 days or 3 weeks [ 43 , 50 ]. In these studies, xylitol gum chewing reduced plaque accumulation compared to sorbitol gum [ 43 ] or had no effect on it [ 50 ]. In the 2-week study conducted in the army, no effects of xylitol gum chewing or consumption of xylitol pastilles were detected. Based on the very high amounts of plaque, the recruits apparently did not follow the 2-day no-oral-hygiene recommendation but omitted toothbrushing totally during the study [ 42 ]. It is clear that xylitol gum is no substitute for toothbrushing.
Adverse effects were registered in 5 out of the 16 reviewed studies [ 41 , 45 , 47 , 49 , 50 ], and reported only for one subject who discontinued the study based on feeling nauseous when chewing gum [ 41 ]. In three studies, one of high and two of low quality [ 46 , 49 , 50 ], the chewing gums were chewed 3–5 times a day, and the recommended chewing times were rather long, 10 min at a time. In two studies, the recommended chewing times were even longer, 15/20 min [ 43 , 47 ]. Thus, it may be expected that the subjects would experience flatulence or have problems with temporomandibular joint dysfunction, especially if they were older [ 47 ], but this was not the case. Also, the polyol sweetener could have been associated with adverse effects. Xylitol, sorbitol and maltitol belong to FODMAP (Fermentable Oligo-, Di-, Mono-saccharides And Polyols) substances which may not suit persons with a tendency for digestive disorders. No such adverse effects connected with the polyol sweeteners were reported in the evaluated studies. In fact, complaints about digestive discomfort in xylitol studies are rare [ 15 , 62 ].
Effects of xylitol and other polyols on plaque accumulation have received little interest after 2000. Only seven studies published between 2005 and 2020, two of them of low quality, fulfilled our inclusion criteria. Recently, Indian research groups have published results on the effects of xylitol on the amount of plaque [ 35 , 63 ]. These papers did not meet the inclusion criteria of the present review. Xylitol could, however, especially in chewing gums, be an important adjunct to routine oral hygiene methods such as toothbrushing [ 64 ]. A reduction in dental plaque formation would benefit subjects of all ages and health conditions. Improving oral hygiene is an important issue for example in elderly people, who would benefit from an agreeable way to decrease plaque formation. In the high-quality study by Al-Haboubi et al. [ 47 ], older people living in the community chewed xylitol gum for 6 months and showed improvement in plaque indices, gingival scores and also self-perceived oral health [ 47 ]. Most studies published on the effects of xylitol on plaque accumulation report results on caries-associated variables like levels of mutans streptococci and/or the acid production potential of plaque [ 38 , 39 , 45 , 46 , 48 , 49 ]. Only a few studies, however, have been published on the effects of xylitol on the composition of the microbiota reflecting the resistance of the microbiota to dysbiosis [ 15 ]. There is also a demand for studies on the impact of polyol products on gingival health. Of the evaluated papers, in addition to the study by Al-Haboubi et al. [ 47 ], only three studies dealt with the effects of xylitol gum chewing on gingival health [ 40 , 50 , 51 ].
To our knowledge, our systematic review is the first review to deal with the effects of xylitol consumption on plaque accumulation. Seven of the present xylitol chewing gum articles were conducted in adults, and thus could have been evaluated in the Keukenmeester et al. [ 20 ] systematic review on the effects of sugar-free gum on plaque. However, only the old study by Steinberg et al. [ 40 ] is included in both our and the Keukenmeester et al. [ 20 ] review. The review evaluated three xylitol gum studies from the 1970s; these studies did not fulfill our inclusion criteria. Two of them had no baseline values [ 22 , 23 ] making proper interpretation of the results difficult and in one, the amount of plaque was not an outcome measure [ 65 ].
We found 16 studies that met the inclusion criteria of the review. Surprisingly, considering that most of the studies were rather old, 14 showed a high or fair quality. A meta-analysis or high-detailed scoring might have improved the review [ 66 ]. The studies were, however, very heterogeneous with respect to subjects, methods and study designs making a meta-analysis difficult to perform and interpret. Regardless, it is a strength of the review that the included studies compared baseline or no treatment values with values obtained after the intervention period decreasing a possible risk of bias. The review also takes into consideration methodological issues, which are often overlooked.
The present review identified 12 of the altogether 14 xylitol chewing gum studies with either high or fair quality. Based on their results, it is likely that habitual use of xylitol chewing gum decreases plaque in children and adults both in short-term and long-term consumption. The plaque-reducing effects of xylitol gum also appear to differ from those of sorbitol gum/gum base. The recommended chewing time may be a confounding factor in chewing gum studies. Based on three fair quality studies, xylitol consumption of candies/lozenges/pastilles appears not to decrease plaque accumulation. Effects of xylitol consumption on plaque accumulation and its clinical impact clearly need further, well-controlled studies.
Selvitz RH, Ismail AI, Pitts NB (2007) Dental caries. Lancet 369:51–59
Article Google Scholar
Murakami S, Mealey BL, Mariotti A (2018) Dental plaque-induced gingival conditions. J Periodontol 89(Suppl 1):S17–S27
PubMed Google Scholar
Nyvad B, Takahashi N (2020) Integrated hypothesis of dental caries and periodontal disease. J Oral Microbiol 12:1710953
Article PubMed PubMed Central Google Scholar
Lamont RJ, Koo H, Hajishengallis G (2018) The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol 16:745–759
Rosier BT, Marsh PD, Mira A (2018) Resilience of the oral microbiota: mechanisms that prevent dysbiosis. J Dent Res 97:371–380
Article PubMed Google Scholar
Salli K, Lehtinen MJ, Tiihonen K et al (2019) Xylitol’s health benefits beyond dental health: a comprehensive review. Nutrients 11:1813
Article PubMed Central Google Scholar
Desphande A, Jadad AR (2008) The impact of polyol-containing chewing gums on dental caries: a systematic review of original randomized controlled trials and observational studies. J Am Dent Assoc 139:1602–1614
Riley P, Moore D, Ahmed F et al (2015) Xylitol-containing products for preventing dental caries in children and adults. Cochrane Database Syst Rev 26(3):CD010743
Newton JT, Awojobi O, Nasseripour M et al (2020) A systematic review and meta-analysis of the role of sugar-free chewing gum in dental caries. JDR Clin Trans Res 5:214–223
Imfeld T (1999) Chewing gum — facts and fiction: a review of gum chewing and oral health. Crit Rev Oral Biol Med 10:405–419
van Loveren C (2004) Sugar alcohols: what is the evidence for caries-preventive and caries-therapeutic effects? Caries Res 38:286–293
Alanen P, Isokangas P, Gutmann K (2000) Xylitol candies in caries prevention: results of a field study in Estonian children. Community Dent Oral Epidemiol 28:218–224
Milgrom P, Ly KA, Tut OK et al (2009) Xylitol pediatric topical oral syrup to prevent dental caries: a double-blind randomized clinical trial efficacy. Arch Pediatr Adolesc Med 163:601–607
Zhan L, Cheng J, Chang P et al (2012) Effects of xylitol wipes on cariogenic bacteria and caries in young children. J Dent Res 91:85S-90S
Söderling E, Pienihäkkinen K (2020) Effects of xylitol and erythritol consumption on mutans streptococci and the oral microbiota: a systematic review. Acta Odontol Scand 78:599–608
Maguire A, Rugg-Gunn AJ (2003) Xylitol and caries prevention — is it a magic bullet? Br Dent J 194:429–436
Söderling E, Isokangas P, Tenovuo J et al (1991) Long-term xylitol consumption and mutans streptococci in plaque and saliva. Caries Res 25:153–157
Söderling EM (2009) Xylitol, mutans streptococci, and dental plaque. Adv Dent Res 21:74–78
Trahan L, Söderling E, Dréan MF et al (1992) Effect of xylitol consumption on the plaque-saliva distribution of mutans streptococci and the occurrence and long-term survival of xylitol-resistant strains. J Dent Res 71:1785–1791
Keukenmeester RS, Slot DE, Putt MS et al (2013) The effect of sugar-free chewing gum on plaque and clinical parameters of gingival inflammation: a systematic review. Int J Dent Hygiene 11:2–14
Dodds MW (2012) The oral health benefits of chewing gum. J Ir Dent Assoc 58:253–261
Mouton C, Scheinin A, Mäkinen KK (1975) Effect of a xylitol chewing gum on plaque quantity and quality. Acta Odontol Scand 33:251–257
Plüss EM (1978) Effect on plaque growth of xylitol- and sucrose-containing chewing gums. J Clin Periodontol 5:35–40
Rateitschak-Plüss EM, Guggenheim B (1982) Effects of a carbohydrate-free diet and sugar substitutes on dental plaque accumulation. J Clin Periodontol 9:239–251
Birkhed D, Edwardsson S, Wikesjö U et al (1983) Effect of 4 days consumption of chewing gum containing sorbitol or a mixture of sorbitol and xylitol on dental plaque and saliva. Caries Res 17:76–88
Topitsoglou V, Birkhed D, Larsson L-Å et al (1983) Effect of chewing gums containing xylitol, sorbitol or a mixture of xylitol and sorbitol on plaque formation, pH changes and acid production in human dental plaque. Caries Res 17:369–378
Scheinin A, Mäkinen KK (1971) The effect of various sugars on the formation and chemical composition of dental plaque. Int Dent J 21:302–321
Grenby TH, Bashaarat AH, Gey KF (1982) A clinical trial to compare the effects of xylitol and sucrose chewing-gums on dental plaque growth. Br Dent J 152:339–343
Scheie AAa, Fejerskov O, Danielsen B, (1998) The effects of xylitol-containing chewing gums on dental plaque and acidogenic potential. J Dent Res 77:1647–1552
Google Scholar
Mäkinen KK, Söderling E, Hämäläinen M et al (1985) Effect of long-term use of xylitol on dental plaque. Proc Finn Dent Soc 81:28–35
Mäkinen KK, Chen C-Y, Mäkinen P-L et al (1996) Properties of whole saliva and dental plaque in relation to 40-month consumption of chewing gums containing xylitol, sorbitol or sucrose. Caries Res 30:180–188
Fure S, Lingström P, Birkhed D (1998) Effect of three moths’ frequent use of sugar-free chewing gum with and without urea on calculus formation. J Dent Res 77:1630–1637
Harjola U, Liesmaa H (1978) Effect of polyol and sucrose candies on plaque, gingivitis and lactobacillus index scores. Acta Odontol Scand 36:237–242
Hashiba T, Takeuchi K, Shimazaki Y et al (2015) Chewing xylitol gum improves self-rated and objective indicators of oral health status under conditions interrupting regular oral hygiene. Tohuku J Exp Med 235:39–46
Saheer PA, Parmar P, Majid SA et al (2019) Effect of sugar-free chewing gum on plaque and gingivitis among 14–15-year-old school children: a randomized controlled trial. Indian J Dent Res 30.61–66
Higgins JP, Altman DG, Gøzsche PC, et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 343:d5928
Wang Y, Li J, Sun W, et al (2017) Effect of non-fluoride agents on the prevention of dental caries in primary dentition: a systematic review. PLoS ONE 12:e0182221
Birkhed D, Edwardsson S, Ahldén M-L et al (1979) Effects of 3 months frequent consumption of hydrogenated starch hydrolysate (Lycasin®), maltitol, sorbitol and xylitol on human dental plaque. Acta Odontol Scand 37:103–115
Söderling E, Mäkinen KK, Chen C-Y et al (1989) Effect of sorbitol, xylitol, and xylitol/sorbitol chewing gums on dental plaque. Caries Res 23:378–384
Steinberg LM, Odusola F, Mandel ID (1992) Remineralizing, antiplaque and antigingivitis effects of xylitol and sorbitol sweetened chewing gum. Clin Prev Dent 14:31–34
Cronin M, Gordon J, Reardon R et al (1994) Three clinical trials comparing xylitol- and sorbitol-containing chewing gums for their effect on supragingival plaque accumulation. J Clin Dent 5:106–109
Merikallio T, Söderling E (1995) Xylitol as a plaque-control agent in military conditions. Mil Med 160:256–258
Tellefsen G, Larsen G, Kaligithi R et al (1996) J Periodontol 67:181–183
Söderling E, Trahan L, Tammiala-Salonen T et al (1997) Effects of xylitol, xylitol-sorbitol, and placebo chewing gums on the plaque of habitual xylitol consumers. Eur J Oral Sci 105:170–177
Mäkinen KK, Isotupa KP, Mäkinen P-L et al (2005) Six-month polyol chewing-gum programme in kindergarten-age children: a feasibility study focusing on mutans streptococci and dental plaque. Int Dent J 55:81–88
Holgerson PL, Sjöström I, Stecksén-Blicks C et al (2007) Dental plaque formation and salivary mutans streptococci in schoolchildren after use of xylitol-containing chewing gum. Int J Paediatr Dent 17:79–85
Al-Haboubi M, Zoitopoulos L, Beighton D et al (2012) The potential benefits of sugar-free chewing gum on the oral health and quality of life of older people living in the community: a randomized controlled trial. Commun Dent Oral Epidemiol 40:415–424
Runnel R, Mäkinen KK, Honkala S et al (2013) Effect of three-year consumption of erythritol, xylitol and sorbitol candies on various plaque and salivary caries-related variables. J Dent 41:1236–1244
Thabuis C, Cheng CY, Wang X et al (2013) Effects of maltitol and xylitol chewing-gums on parameters involved in dental caries development. Eur J Paediatr Dent 14:303–308
Keukenmeester RS, Slot DE, Rosema NAM et al (2015) Effects of sugar-free chewing gum sweetened with xylitol or maltitol on the development of gingivitis and plaque: a randomized clinical trial. Int J Dent Hygiene 12:238–244
Agkül Ö, Ak AT, Zorlu S, Özdas DÖ, Uslu M, Cayirgan D (2020) Effects of short-term xylitol chewing gum on pro-inflammatory cytokines and Streptococcus mutans : a randomised, placebo-controlled trial. Int J Clin Pract 74e:13623
Milgrom P, Ly KA, Roberts MC et al (2006) Mutans streptococci dose response to xylitol chewing gum. J Dent Res 85:177–181
Lif Holgerson P, Stecksén-Blicks C, Sjöström I (2006) Xylitol concentration in saliva and dental plaque after use of various xylitol-containing products. Caries Res 40:393–397
Addy M, Perriam E, Sterry A (1982) Effects of sugared and sugar-free chewing gum on the accumulation of plaque and debris on the teeth. J Clin Periodontol 9:346–354
Hoerman KC, Gasior EJ, Zibell SE et al (1990) Effect of gum chewing on plaque accumulation. J Clin Dent 2:17–21
Hanham A, Addy M (2001) The effect of chewing sugar-free gum on plaque regrowth at smooth and occlusal surfaces. J Clin Periodontol 28:255–257
Pakkala U, Liesmaa H, Mäkinen KK (1981) The use of xylitol in the control of oral hygiene in mentally retarded children. Proc Finn Dent Soc 77:271–277
Mäkinen KK, Isotupa KP, Kivilompolo T et al (2001) Comparison of erythritol and xylitol in the control of dental plaque and mutans streptococci. Caries Res 35:129–135
Shyama M, Honkala E, Honkala S et al (2006) Effect of xylitol candies on plaque and gingival indices in physically disabled school pupils. J Clin Dent 17:17–21
Splieth CH, Alkilzy M, Schmitt J et al (2009) Effect of xylitol and sorbitol on plaque acidogenesis. Quintessence Int 40:279–285
Mäkinen KK, Söderling E, Hurttia H et al (1985) Biochemical, microbiologic, and clinical comparisons between two dentifrices that contain different mixtures of sugar alcohols. JADA 111:745–750
Mäkinen KK (2016) Gastrointestinal disturbances associated with the consumption of sugar alcohols with special consideration of xylitol: scientific review and instructions for dentists and other health-care professionals. In J Dent 2016:5967907
Oza S, Patel K, Bhosale S et al (2018) To determine the effect of chewing gum containing xylitol and sorbitol on mutans streptococci and lactobacilli count in saliva, plaque, and gingival health and to compare the efficacy of chewing gums. J Int Soc Prev Community Dent 8:354–360
Fontana M (2016) Enhancing fluoride: clinical human studies of alternatives or boosters for caries management. Caries Res 50(suppl 1):22–37
Mouton C, Scheinin A, Mäkinen KK (1975) Effect on plaque of a xylitol-containing chewing gum: a clinical and biochemical study. Acta Odontol Scand 33:33–40
Downs SH, Black N (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and nonrandomised studies of health care interventions. J Epidemiol Community Health 52:377–384
Download references
Acknowledgements
The authors wish to thank docent Vuokko Loimaranta (VL) for evaluating the papers in which the first author of this review was an author. Thank you to emeritus professor Kauko Mäkinen, emeritus professor Dowen Birkhed, Dr. Dagmar Slot and Dr. Özer Akgül for providing details on the studies.
Open access funding provided by University of Turku (UTU) including Turku University Central Hospital.
Author information
Authors and affiliations.
Institute of Dentistry, University of Turku, Lemminkäisenkatu 2, 20520, Turku, Finland
Eva Söderling & Kaisu Pienihäkkinen
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Eva Söderling .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions

About this article
Söderling, E., Pienihäkkinen, K. Effects of xylitol chewing gum and candies on the accumulation of dental plaque: a systematic review. Clin Oral Invest 26 , 119–129 (2022). https://doi.org/10.1007/s00784-021-04225-8
Download citation
Received : 05 May 2021
Accepted : 12 October 2021
Published : 22 October 2021
Issue Date : January 2022
DOI : https://doi.org/10.1007/s00784-021-04225-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Chewing gum
- Dental plaque
- Dental biofilm
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 26 April 2003
Xylitol and caries prevention — is it a magic bullet?
- A Maguire 1 &
- A J Rugg-Gunn 2
British Dental Journal volume 194 , pages 429–436 ( 2003 ) Cite this article
14k Accesses
85 Citations
51 Altmetric
Metrics details
Xylitol is one of a number of non-sugar sweeteners approved for use in foods and other items, in many countries.
It is well-established that xylitol is non-cariogenic.
Xylitol in chewing gum is anti-cariogenic as are other polyols in chewing gum.
Xylitol exhibits dental health benefits which are superior to other polyols in all areas where polyols have been shown to have an effect.
The inhibition of mother/child transmission of cariogenic oral flora leading to reduced caries development in young children is caries-preventive.
Xylitol's specific effects on oral flora and especially on certain strains of mutans streptococci add to its caries-preventive profile and give it a unique role in preventive strategies for dental health.
Several recent publications have focused discussion on the value of xylitol in caries prevention. Some reviewers have concluded that xylitol has a unique active role in caries prevention, while other reviewers have been more cautious saying that the case is not yet proven. Chewing xylitol gum is certainly effective at preventing caries development compared with chewing sugared gum or not chewing any gum. Xylitol gum appears to be more effective than sorbitol gum or combinations of xylitol and sorbitol. One recent trial suggested that the effectiveness of eating a xylitol candy could be similar to that of chewing xylitol gum: this is valuable as it would remove the necessity of disposing of spent gum; it has also been suggested that xylitol has a positive action in addition to the favourable effect of chewing. A further recent publication reported substantial reductions in caries development in children whose mothers had chewed xylitol gum. The main explanation appears to be that xylitol changed the plaque flora of the mothers so that transmission of cariogenic oral micro-organisms from mother to child was reduced. Further developments in this field are awaited, but at present we may conclude that xylitol exhibits dental health benefits which are superior to other polyols in all areas where polyols have been shown to have an effect. In addition, xylitol's specific effects on oral flora and especially on certain strains of mutans streptococci add to its caries-preventive profile and give it a unique role in preventive strategies for dental health.
You have full access to this article via your institution.
Similar content being viewed by others

Should dentists recommend sugar-free chewing gum to help prevent decay?
Duncan Parker-Groves

The oral health of secondary school pupils: baseline data from the Brushing RemInder 4 Good oral HealTh (BRIGHT) trial
Zoe Marshman, Caroline Fairhurst, … Nicola Innes
Effects of a mouthwash containing Lespedeza cuneata extract on risk of dental caries: a randomized, placebo-controlled clinical trial
Yu-Rin Kim & Seoul-Hee Nam
Thirty years ago, there was no information on the effect of xylitol on dental caries. Since then, some 270 articles have been published describing clinical studies and investigations into possible mechanisms for xylitol's seemingly remarkable efficacy. The first of these studies was the Turku sugar studies conducted between 1972 and 1974 1 at a time when caries experience was very high in northern Europe, and in Finland which is a major xylitol producer. Xylitol production is now over 10,000 tonnes per year, mainly going to confectionery manufacturers and the pharmaceutical and oral hygiene industries.
While there is no doubt that xylitol is non-cariogenic and the cariostatic effect of xylitol chewing gum is well accepted, the existence of an active anti-caries role of xylitol per se remains controversial. More recently, remarkable results have emerged from a trial where development of dental caries was much lower in children whose mothers had chewed xylitol gum when their children were young (beginning at three months of age) during the so-called 'discrete window of infectivity' from mother to child (between 19 –31 months of age), compared with control children. 2 , 3 , 4
For many years, many thousands of Finnish children have participated in caries preventive programmes which involved chewing xylitol gum in school. 5 , 6 Disposal of spent gum has been seen as a draw-back for such community programmes by public health authorities in many countries and it was of considerable interest that, in a recently published trial, the caries preventive effectiveness of chewing xylitol sweets was observed to be similar to that of xylitol chewing gum. 7
Because of these recent events, it was thought to be useful to summarise the evidence concerning 'is xylitol a magic bullet?'
Xylitol is one of a number of non-sugar sweeteners permitted for use in foods. 8 It is found naturally in some foods but it is mass-produced principally from sustainable xylan-rich hardwood sources such as birch and beech wood — a process first reported over a hundred years ago. Chemically, it is a pentitol which is a five-carbon polyol. In this, it differs from other common polyols such as sorbitol and mannitol, which contain a six-carbon ring. It was approved for use in foods in the UK in 1983 ( Table 1 ) as one of several non-sugar sweeteners. 8 The Department of Health COMA report on 'Dietary sugars and human disease' gave encouragement to their use. 9 Presently in the UK, consumption of xylitol is about 1,000 tonnes per year, principally in chewing gums, confectionery, toothpaste and medicines.
Many clinical trials have shown that chewing sugarless gum leads to substantial caries prevention, with xylitol-containing gums being particularly effective. Chewing sugarless gum increases saliva flow considerably and thus fast flowing saliva with its high pH and high concentration of calcium and phosphate aids remineralisation of dental enamel and resists caries development. It has also been observed in such trials with xylitol-containing gums that the bacterial flora of plaque changes with the more cariogenic bacteria becoming less frequent. Some research has suggested that xylitol has a unique, positive role in preventing dental caries, 10 , 11 while other research workers refute xylitol's unique action suggesting that the caries-preventive effects of xylitol chewing gum can be explained adequately by the favourable action of chewing gum alone. 12 One of the main purposes of this review is to summarize the evidence upon which these arguments rest. Other purposes of this review are to describe progress in the so-called 'mother and child' study 4 and to compare the effectiveness in caries prevention of xylitol with other bulk non-sugar sweeteners. The intense non-sugar sweeteners listed in Table 1 will not be discussed.
Terms used to describe xylitol's action.
When considering the literature, it is important that the terminology used to describe xylitol's effects is accurate and consistent. Acidogenicity and fermentability are essentially terms used to describe findings from in vitro experiments and in vivo studies other than clinical trials, whereas cariogenicity, non-cariogenic and anti-cariogenic are clinical terms. It is clear from the literature that some authors have interpreted a number of definitions relating to these cariological and bacteriological terms in slightly different ways, which have led to some difficulties interpreting the findings of some studies. The terms 'cariostatic', 'anti-cariogenic' and 'anti-caries' have all been used when discussing dental therapeutic claims of xylitol, as have 'active' and 'passive' effects. For the purposes of this review, the properties of non-fermentability and non-cariogenicity will be classed as passive effects while active caries-preventive (or caries-inhibitory) effects will include the terms bacteriostatic and cariostatic. Only a reversal in the caries process, that is the remineralisation of a carious lesion, will be described as a therapeutic or anti-cariogenic effect.
Evidence from clinical trials
Evidence of the effect of xylitol and other sweeteners on dental caries comes from many different types of study — laboratory incubation experiments, in vivo plaque pH and enamel slab caries experiments, and animal experiments. The best form of evidence, though, is from clinical trials — particularly randomised clinical trials (RCTs) where subjects are randomly allocated to treatment groups and they and their assessors do not know the group identity of the subject. Often it is not possible for a subject to be unaware of the treatment he or she is receiving and allocation to groups sometimes has to be done on a school or community basis rather than on an individual basis.
Clinical trials involving xylitol and other polyols can be divided into three main designs: total substitution of normal dietary sugars for xylitol, partial substitution, and supplementation of normal dietary sugars with xylitol or other polyols. A few of the clinical trials using partial substitution or supplementation have involved confectionery but most have studied the effects of chewing gums containing xylitol and/or sorbitol. A few trials which have looked at supplementation with xylitol have involved toothpastes or mouthrinses.
Total substitution studies
The Turku sugar studies are one of the milestones of dental caries research. 1 The main study tested the effect of almost total substitution of normal dietary sugars with xylitol on development of dental caries over two years in adults. This long-term clinical trial was a great organisational feat since it involved the special manufacture and distribution of over a hundred food products. The daily consumption of xylitol was about 50 g per day. While the sucrose and fructose groups developed caries during the two-year study, little caries developed in the xylitol group. There have been some criticisms of the study, especially regarding its design, since allocation to the various test groups was based mainly on personal preference, and the nature of the study excluded the possibility of it being 'blind'. 12 In addition, groups varied with respect to their sweetener intake and possibly their dental health awareness.
The caries data for buccal surfaces 13 and approximal 14 surfaces of teeth of subjects in the Turku study were analysed blind in greater detail. The buccal surfaces were photographed seven months after the beginning and at the end of the two-year trial. While the area of white spots increased over the seventeen months in the sucrose group, the area decreased in the xylitol group: the author concluded that xylitol consumption caused remineralisation of incipient white spot lesions on buccal surfaces. A quantitative measure of the mean size of lesions seen on bitewing radiographs was made using a digitised planimetric technique. The mean lesion size increased in the sucrose group but there was no increase in the mean size of lesion in the xylitol group.
Partial substitution studies and confectionery supplementation studies
Since the Turku sugar substitution study, a number of clinical field trials have been conducted on daily use of xylitol products as part of the usual sugar-containing diet — either partial substitution or supplementation. 1 , 7 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 Field trials differ from clinical trials in that they may have no particular control group; the study may not be blind or there may be no special selection or supervision of participants. Even with these intrinsic weaknesses, field trials are important as they allow the effectiveness and acceptability of preventive agents or methods previously shown in a clinical trial to be effective, to be evaluated in a particular setting. In two of these field trials, xylitol was given as several items of confectionery 17 , 21 while in other studies, xylitol was given in chewing gum only 20 , 23 , 27 and these studies will be considered later.
Both of the confectionery studies lasted three years. The 6–11-year-old Hungarian children consuming xylitol confectionery developed 45% less caries than control children who consumed usual sugar confectionery. Partial substitution of xylitol for dietary sugars had been intended in this trial but analysis revealed that the pattern of consumption of xylitol was largely additive; the frequency of sucrose consumption had not decreased. 16 The study therefore demonstrated the cariostatic effect of xylitol through its use as a supplement. The field trial of Kandelman 21 recorded 37% less caries in 6–12-year-old Polynesian children who consumed up to 20 g xylitol confectionery per day compared with a control group who ate sugar confectionery. Both field studies were plagued by significant numbers of drop-outs among subjects; approximately 30% in the Hungary study and 37% in the Polynesian study and the studies were not blind. However, comparisons of the caries prevalence of participants and drop-outs in this latter study demonstrated that, within each age group, there were only small differences in baseline mean caries values and that the participants appeared to be a representative sample of the entire population.
Chewing gum supplementation studies
There has been considerable growth in the use of sugarless chewing gums — about 85% of gum sold in the UK is now sugar-free. The benefits of sugarless gum have been investigated in a number of clinical trials. In some of these, the control group had chewed sugared gum, thus testing the substitution of polyols for sugar. In other trials, the control group did not chew any gum, testing the beneficial effect of chewing sugarless gum — its non- or anti-cariogenic properties. 36 , 37 Follow-on studies from these clinical trials of chewing gum have also been important in establishing the mechanism for the efficacy of sugarless gums.
The one year Turku chewing gum study assessed the effect on caries development of low doses of xylitol compared with the use of sugared gum, and showed a cumulative caries increment of +292 tooth surfaces in the group chewing sugared gum compared with a negative caries increment of –104 tooth surfaces in the group chewing a mean of 45 xylitol gums (each containing 15 g xylitol) per day. 38
A further two-year study was designed to determine whether the daily use of xylitol gum increased the efficacy of routine caries preventive measures in 11–15-year-old school children in Finland — a country with low baseline caries levels. After two years, this blind study showed a mean reduction in caries in the children chewing xylitol gum of 44% compared with the control group who did not chew any gum. 18 The caries preventive effectiveness was observed three 22 and five years 26 after discontinuation of the use of xylitol — the greatest long-term preventive effect being seen on second permanent molars which erupted during the xylitol gum trial. 39 Scheie and Fejerskov, 12 in their review of this trial, point out that an important factor to be considered in the interpretation of the results was the impact that chewing xylitol gum had on decreasing the intake of conventional solid sweets during the trial and they also suggest that participation in the trial may have raised oral health awareness during the subsequent five years. However, Isokangas 18 stated that 'the frequency of consumption of sweets was not, however, significantly affected by the use of xylitol gums.'
The results of a similarly-designed (although not blind) field study in Montreal showed that children who chewed xylitol gum had significantly lower net progression of caries than the control group children after 24 months, and a significant number of reversals of carious lesions were seen in the test group suggesting that remineralisation had occurred. 19
A series of double blind clinical studies carried out in Belize approximately 10 years ago were the first to provide direct comparisons between xylitol and sorbitol gums. 27 , 28 , 30 , 31 Before this study, trials of xylitol-containing gum had given superior results to trials of sorbitol-containing gums, but they had not been compared in the same trial. The trials investigated caries-preventive effects in primary teeth of younger children and permanent teeth of older children. The study on older children included nine groups; testing, among other things, the effectiveness of chewing xylitol gum compared with chewing no gum and chewing a sugared gum. Compared with the no gum control group, the relative risk of caries development for each of the groups was: sugared gum 120 (ie an increase in risk); xylitol pellet five times per day 027 (ie a decreased risk of caries); xylitol pellet three times a day 041; xylitol stick five times a day 044; xylitol stick three times a day 048 The findings that the pellet gums with a harder texture were more effective than sticks and that chewing five times per day was better than three times a day, appear to confirm a dose response and/or suggest that factors related to stimulating salivary secretion are important in the sugar-free chewing gum effect. However, the more rapid release of xylitol from the coating of a pellet form may be a significant factor. In the youngest children with primary teeth the use of all polyol gums resulted in a significant decrease of the caries onset rate (p<005) with no significant difference in the caries onset risk between xylitol stick gum and sorbitol stick gum. 30 The largest caries risk reduction compared with no gum was found in the group receiving xylitol pellet gum (relative risk 035) and the sorbitol pellet gum (relative risk 044).
The long-term effects of chewing sugar-free gum were demonstrated by Hujoel et al , 34 five years after the two year Belize chewing gum programme ended. In this blind follow-up study, xylitol gum had reduced the caries risk by 59% and sorbitol gum by 35%, compared with a no gum group. In view of the long-term caries risk reduction of 93% found after 1–2 years of gum chewing compared with no-gum, the authors concluded that the optimum time for introducing gum for caries prevention should be at least one year before permanent teeth start to erupt.
Xylitol candies versus chewing gum study
A recent clinical study in Estonia tested the effect of dietary supplementation of two types of xylitol candies and xylitol gum on dental caries occurrence compared with a control group who received no supplements. 7 The subjects of the study were children aged ten years who were given two pieces of gum or two candies three times a day on school days. A double blind design was possible between the use of the two candies, but not between candy and gum use. The gum was chewed for ten minutes and then collected for disposal while the candies were consumed in the usual way, the authors reporting that 'it took approximately the same time for the candies to disappear from the mouth.' The results showed that, for each cluster of schools, the caries increment was 35%–60% higher in the control group who received no supplements than in the xylitol groups. Furthermore, there was no difference between the two groups consuming xylitol candies and xylitol chewing gum.
Mother and child study
This innovative clinical study initially investigated the effect of a mother's habitual xylitol consumption on transmission of mutans streptococci to her child. 2 The 106 mothers randomly allocated to the first study group chewed xylitol gum at least two to three times a day, starting three months after the birth of their child. There were two other study groups: in one, thirty mothers received chlorhexidine varnish six, twelve and eighteen months after delivery; in the other, fluoride varnish was used at the same intervals. The children received no intervention. Follow-up studies have looked at the occurrence of dental decay in children as well as their plaque and salivary colonisation with mutans streptococci at three and six years of age. 3 , 4 There was a 74% reduction in dmft seen in the 5-year-olds whose mothers had used the xylitol around the period described as 'the discrete window of infectivity' 40 compared with children whose mothers had used chlorhexidine. There was a 71% reduction in dmft seen in the 5-year-olds whose mothers had used the xylitol compared with children whose mothers had used fluoride varnish. In all three groups, children in whom Streptococcus mutans had not been detected at two years of age showed lower caries experience at all annual examinations than children who had been colonised with mutans streptococci.
Specific caries-preventive actions of xylitol
The caries-preventive effect of total substitution of dietary sugars by xylitol could be explained by the exclusion of fermentable sugars from the diet. But the impressive caries-preventive effect of partial substitution or supplementation by xylitol requires other explanations: the caries-preventive effect seems to be greater than could be expected from simple substitution, and the result was intense research into the properties of xylitol. Proposed mechanisms are listed in Table 2 .
Xylitol is not fermented by dental plaque. 41 , 42 , 43 There is ample evidence that the oral flora does not adapt to metabolise xylitol when tested over prolonged periods in humans. 44 , 45 Any ability of a few organisms to ferment xylitol is negated by the inaction of other more numerous plaque organisms so that no fall in plaque pH occurs on exposure to this polyol. 46 , 47
The use of xylitol has been shown to lead to a reduction in the proportion of mutans streptococci in plaque. 46 , 48 This is most probably due in part to both non-specific and specific effects of xylitol ( Table 3 ). The non-specific effect is a result of non-fermentability not encouraging bacterial growth. 48 , 49 In addition, there appears to be a number of effects specific to xylitol. First, a selective effect on mutans streptococci resulting in the development of mutant xylitol-resistant strains which may be less virulent in the oral environment. 45 , 50 , 51 Second, the concentrations of ammonia and basic amino acids increase when plaque is exposed to xylitol, resulting in neutralisation of plaque acids. 52 , 53 , 54 Third, in-vitro studies have shown some strains of oral streptococci take up xylitol and convert it to xylitol-5-phosphate resulting in the development of intra-cellular vacuoles and degraded cell membranes in mutans and sobrinus streptococci, and through this mechanism xylitol is acting in a bacteriostatic way. 55 , 56 , 57 , 58 , 59 Lastly, some streptococcal strains take up xylitol which participates in what is termed 'the futile metabolic cycle'. 59 , 60 , 61 , 62 In this cycle, xylitol is taken into the cell, phosphorylated to xylitol-5-phosphate, and is then split by sugar-phosphate phosphatases and the resulting xylitol is expelled from the cell. The clinical relevance of this process has not yet been established, but it is more likely to benefit oral health than damage it.
Much evidence from well controlled clinical studies indicates that xylitol decreases the growth of plaque compared with sugars and other polyols. 1 , 15 , 25 , 46 , 49 , 63 , 64 These studies include the Turku sugar studies and trials of partial substitution and supplementation. There is also good evidence that the ability of plaque to produce acids by metabolism of sugars is reduced by xylitol. 46 , 47 This seems to be adequately explained by a selective decrease in mutans streptococci in plaque exposed to xylitol and possibly by a decrease in plaque quantity.
Of the above intra-plaque mechanisms of xylitol in caries prevention, only the conversion of xylitol to xylitol-5-phosphate, with its subsequent accumulation, as well as the induction of less virulent strains of cariogenic bacteria, can be considered to be truly bacteriostatic. These mechanisms have been emphasised but further research is needed to assess their clinical importance. Although not truly bacteriostatic, other mechanisms listed earlier ( Table 2 ) will assist caries prevention.
As well as these specific effects, xylitol consumption by the mother at the critical period of mother-child transmission of oral flora can reduce the transmission and colonisation of mutans streptococci to her child on a long-term basis, as described previously. 2 , 4 Other evidence has shown that xylitol leads to a reduction in the quantity of plaque, possibly by interfering with mechanisms of adhesion between plaque organisms and the tooth's surface. 65 , 66 , 67 , 68 This is the most likely explanation for the reduced colonisation of mutans streptococci in the mouths of these children.
As far as the evidence regarding salivary flow is concerned, flow rate increases during and immediately after chewing, and a sweet taste increases flow rate even further. 69 , 70 There is no evidence, though, that xylitol is better than any other sweetener in this respect. While chewing results in substantial immediate increases in salivary flow, there have been several investigations into whether chewing gum results in increased capacity for salivary flow long term. The majority of these latter studies have found no increase in the capacity to produce saliva after chewing sweetened gum over varying lengths of time, in people with normal salivary flow; certainly, there was no indication that xylitol had any specific effect. 46 , 47 , 69 , 70 , 71
Pre-cavitation (white spot) carious lesions were observed to remineralise (heal) during clinical studies of xylitol, as mentioned above. This led to speculation that xylitol had a specific action on enamel, and a number of studies have investigated the effect of xylitol and other sweeteners on tooth structure and the potential for remineralisation; laboratory-based studies have included in vitro experiments and rat caries studies. Remineralisation occurred in nearly all experiments where non-sugar sweeteners were used during the 'healing' phase but there was no clear indication that xylitol had any greater effect than other non-sugar sweeteners when evaluated in short-term studies. 72 , 73 , 74 , 75 , 76
Remineralisation is likely to be adequately explained by the increased flow of saliva, rich in calcium and phosphate, and by the shorter time that plaque pH is low and has the potential to cause demineralisation. Any specific anti-caries action of xylitol is therefore likely to be due to its effect on plaque and plaque organisms.
Xylitol compared with other non-sugar bulk sweeteners
All the bulk sweeteners listed in Table 1 have been investigated and shown to be non-cariogenic or to have very low cariogenic potential. The amount and type of evidence varies greatly between sweeteners, with xylitol and sorbitol being the most thoroughly investigated.
Studying the fermentability of sweeteners by plaque and plaque organisms is the simplest type of investigation: these can be carried out entirely in vitro or in the mouth where they are known as plaque pH experiments. One study reported xylitol fermentation by plaque bacteria: 77 however, these bacteria represent only a small proportion of plaque organisms and, in mixed cultures, they were outgrown or their acid production masked by the activities of other micro-organisms. In contrast, sorbitol, manitol, lactitol, maltitol, hydrogenated glucose syrup and isomalt are all fermented slowly by plaque organisms but the rates are very much slower than that for sucrose or fructose. 41 , 42 , 78 , 79
Reduction in plaque quantity on using xylitol appears to be a reflection not only of the non-fermentability of xylitol, ensuring its non-availability metabolically as an energy source for oral bacteria, but also its ability to change the adhesive and cohesive properties of plaque leading to decreased plaque quantity. A number of chewing gum studies, in particular, have investigated changes in plaque quantity after use of xylitol, sorbitol or xylitol-sorbitol mixtures. Most of these studies show that while plaque quantity reduces with xylitol, there is little change in the plaque quantity after the use of sorbitol; with the xylitol-sorbitol mixtures reducing the plaque quantity compared with sorbitol but not as much as with xylitol only. 25 , 32 , 46 , 80 , 81 , 82 The clinical significance of these changes, however, has been questioned. If one accepts that reduced adhesion of plaque organisms was the major explanation for the fairly large dental effect of xylitol in the mother and child study, inclusion of a sorbitol group into any further study would be helpful. Xylitol would appear to have a unique effect in reducing adhesion and it could be expected that other polyols might not show this clinical effect.
Reports of the first clinical trials of sorbitol chewing gums appeared thirty-five years ago. 36 Caries increments were much less with their use compared with sugared gums. Since then, many clinical trials and field studies have indicated the dental benefit of chewing gum and other confectionery made with sorbitol, 84 hydrogenated glucose syrup, 85 as well as xylitol. 36 The majority of studies tested xylitol and results indicate that dental effects seemed to be greater with xylitol, but it was not until the Belize study 27 , 30 that sorbitol and xylitol were compared 'head to head'. The results showed clearly that xylitol in gum was superior to sorbitol, and that mixtures of xylitol and sorbitol were not as good as xylitol but were better than sorbitol alone. The results of the study in older children 27 ( Table 4 ) recorded that, compared with the no gum control group, the relative risk for xylitol pellet gum used five times daily was 027 (ie a decreased risk of caries) and for sorbitol pellet gum used five times daily 074 These equate to 73% and 26% reduction in caries development respectively. Two different ratios of xylitol to sorbitol were also investigated: the 3:2 xylitol:sorbitol gum gave an odds ratio of 056, while the 1:3 xylitol:sorbitol gum gave an odds ratio of 049 The xylitol:sorbitol mixtures were more effective in reducing caries risk than sorbitol alone, but were less effective than xylitol alone. In the study of younger children with primary teeth 30 all polyol gums resulted in a significant decrease of the caries onset rate (p<005); the difference in the caries onset risk between xylitol stick gum and sorbitol stick gum was not statistically significant. The largest caries risk reduction compared with no gum was found in those children receiving xylitol pellet gum and the sorbitol pellet gum (relative risks 035 and 044 respectively).
In summary, xylitol exhibits dental health benefits which are superior to other polyols in all areas where polyols are shown to have an effect. In addition, xylitol's specific effects on oral flora and especially on certain strains of mutans streptococci add to its caries-preventive profile and give it a potentially unique role in caries prevention.
Other issues related to xylitol use
While some of the intense sweeteners are cheaper than sugar, for any given level of sweetness, all the bulk sweeteners are more expensive than sugar. This means that while sugar-free soft drinks should be no more expensive than sugared drinks, sugar-free confectionery could be more expensive than its sugar-containing counterparts. While sorbitol is about twice as expensive as sucrose, xylitol is about six times the price of sucrose. 86 , 87
The flavour profile of bulk sweeteners is generally considered to be good and combinations of sweeteners are often used to produce the best sweet taste. In addition, a cool sensation is experienced when eating polyols due to the unusual property of a negative heat of dissolution.
Perhaps the biggest potential disadvantage of polyols is their liability to cause osmotic diarrhoea if eaten in large amounts. For xylitol, little discomfort is experienced with intakes of about 20 g per day, although threshold levels will be lower for children. It should be remembered that adults in the Turku sugar studies consumed about 50 g of xylitol per day for two years: only one of the 52 subjects withdrew from the study because of intestinal discomfort. 1 In Switzerland and Finland, countries with high levels of consumption of polyols by children, intestinal discomfort does not appear to be a problem.
Is xylitol a unique magic bullet?
In one of the earliest reviews, Bär 88 concluded that 'xylitol may be regarded as the best of all nutritive sugar substitutes with respect to caries prevention.' He drew attention to human studies which showed 'massive reductions in caries following consumption of relatively small amounts of xylitol' but stated that consensus on anti-cariogenic status of xylitol had not been reached. Soderling and Scheinin 89 also commented that 'partial substitution of dietary sucrose by low doses of xylitol was associated with pronounced caries reduction' and that the favourable action of xylitol was likely to be multi-factorial, but did not conclude that xylitol was anti-cariogenic. Makinen 90 reviewed the large amount of evidence on this topic and concluded 'all adequately supervised clinical caries studies have yielded essentially identical results providing evidence of the cariostatic and even anti-cariogenic effect of xylitol.' ( Table 5 )
Imfeld 91 reviewed the clinical caries studies of polyalcohols and concluded that sorbitol, mannitol, xylitol, maltitol, lactitol, hydrogenated glucose syrup and isomalt have all been proven to be non-cariogenic or of extremely low cariogenicity in rat caries experiments and/or human clinical studies. He stated that claims of possible active effects of xylitol due to its bacteriostatic and/or cariostatic properties 'have not yet been substantiated in clinical trials.' Following publication of further studies, Trahan 92 concluded that the reduction in dental caries associated with xylitol consumption could be attributed mainly to xylitol not being significantly metabolised by the oral microflora, and other mechanisms, mostly saliva and plaque related. More recently, Levine, 93 in a briefing paper on xylitol, described xylitol as exhibiting both passive and active anti-caries properties.
Scheie and Fejerskov 12 agreed that all clinical studies concerning the effect of xylitol on caries development consented to its non-cariogenicity. However, they felt that claims that xylitol possessed anti-caries or therapeutic effects and was superior to other polyols were still to be confirmed 'by well designed and conducted studies from independent research groups.' Recognition of the need for independent research is an important recurring issue in the xylitol debate. This appears to have arisen since much of the research into xylitol has been carried out by one group of researchers led by Dr K. K. Makinen. In contrast to the conclusions of Scheie and Fejerskov mentioned above, Makinen, in an editorial published concurrently in the same journal 10 stated that 'there is enough scientific evidence to argue that there indeed exists a pentitol-specific or a xylitol-specific caries-preventive effect that is different from that exerted by hexitols such as sorbitol.'
Very recently in the United States, Hayes 94 reviewed the evidence for the effect of non cariogenic sweeteners on the prevention of dental caries, particularly in relation to criteria for causality — consistency, strength, association, biologic plausibility, temporal sequence and dose response relationship. She concluded that 'Given that several of the criteria for causality are met, it is concluded that xylitol can significantly decrease the incidence of dental caries.'
The dramatic effects of consuming small amounts of xylitol referred to by Bär 88 above, were observed in chewing gum studies, and one of the difficulties has been to distinguish between the caries-preventive effects of salivary stimulation due to chewing gum, and xylitol. One pointer is that xylitol gum was more effective than sorbitol gum in the Belize trial. 27 , 28 , 29 , 30 , 31 Another approach has been to compare the effect of chewing xylitol gum with chewing an unsweetened gum base. Two such studies have been undertaken — one short-term plaque study in habitual xylitol consumers showed a xylitol-specific effect 51 and the other study — a 3 year community intervention trial 35 — did not, although this study did have some problems in its design which may have been reflected in the results. Of some relevance is the Estonian xylitol trial, 7 which compared the dental effects of xylitol in candy and chewing gum form. Although sucking the candy stimulated salivary flow, the results suggested that the xylitol was active in caries prevention, as well as the form of the vehicle used (ie chewing gum or sucking candy). The favourable properties of xylitol within plaque ( Table 2 ) are likely to explain xylitol's superior caries-preventive effectiveness. Those most likely to be of clinical relevance are: xylitol's non-fermentability by plaque micro-organisms, selective reduction of mutans streptococci in plaque and selection within plaque of xylitol-resistant mutans streptococci which appear to have reduced adherence and therefore reduced transference. The remarkable result of the mother and child study has been explained by the reduced transmission of plaque micro-organisms from mother to child. Only one chewing gum group (using xylitol gum) was included in this trial: it is hoped that this mother and child trial will be replicated and, if so, a clearer idea of the clinical importance of reduced transference would emerge if a sorbitol gum group were to be included in this trial.
In summary, from the available evidence it can be concluded that:
xylitol is non-cariogenic;
xylitol in chewing gum is anti-cariogenic as are other polyols in chewing gum;
the inhibition of mother/child transmission of cariogenic oral flora leading to reduced caries development in young children is caries preventive; and
the dental properties of xylitol are superior to other polyols so far investigated — this is likely to be due to a combination of several specific effects of xylitol as well as the general effects of polyols in sucrose substitution and saliva stimulation.
Xylitol exhibits dental health benefits which are superior to other polyols in all areas where polyols have been shown to have an effect. In addition, xylitol's specific effects on oral flora and especially on certain strains of mutans streptococci add to its caries-preventive profile and give it a unique role in preventive strategies for dental health.
Scheinin A, Makinen KK . Turku Sugar Studies I-XXI. Acta Odontol Scand 1975; 33 : 1– 349.
Article Google Scholar
Soderling E, Isokangas P, Pienihakkinen K, Tenovuo J . Influence of maternal xylitol consumption on acquisition of mutans streptococci by infants. J Dent Res 2000; 79 : 882– 887.
Article PubMed Google Scholar
Isokangas P, Soderling E, Pienihakkinen K, Alanen P . Occurrence of dental decay in children after maternal consumption of xylitol chewing gum, a follow-up from 0 to 5 years of age. J Dent Res 2000; 79 : 1885– 1889.
Soderling E, Isokangas P, Pienihakkinen K, Tenovuo J, Alanen P . Influence of maternal xylitol consumption on mother-child transmission of mutans streptococci : 6 year follow-up. Caries Res 2001; 35 : 173– 177.
Nordblad A, Suominen-Taipale L, Murtomaa H, Vartiainen E, Koskela K . Smart Habit Xylitol campaign, a new approach in oral health promotion. Community Dent Health 1995; 12 : 230– 234.
PubMed Google Scholar
Honkala E, Rimpela A, Karvonen S, Rimpela M . Chewing of xylitol gum--a well adopted practice among Finnish adolescents. Caries Res 1996; 30 : 34– 39.
Alanen P, Isokangas P, Gutmann K . Xylitol candies in caries prevention: results of a field study in Estonian children. Community Dent Oral Epidemiol 2000; 28 : 218– 224.
Department of Health. The sweeteners in foods regulations . London: HMSO; 1983. SI 1983, 1211 as amended by SI 1988, 2122.
Department of Health. Report of panel on dietary sugars . No.37. Dietary sugars and human disease. Committee on Medical Aspects of Food Policy, (COMA). London: HMSO, 1989.
Makinen KK . Xylitol-based caries prevention: is there enough evidence for the existence of a specific xylitol effect? Oral Dis 1998; 4 : 226– 230.
Makinen KK . The rocky road of xylitol to its clinical application. J Dent Res 2000; 79 : 1352– 1355.
Scheie AA, Fejerskov OB . Xylitol in caries prevention: what is the evidence for clinical efficacy? Oral Dis 1998; 4 : 268– 278.
Rekola M . Changes in buccal white spots during 2-year consumption of dietary sucrose or xylitol. Acta Odontol Scand 1986; 44 : 285– 290.
Rekola M . Approximal caries development during 2-year total substitution of dietary sucrose with xylitol. Caries Res 1987; 21 : 87– 94.
Birkhed D, Edwardsson S, Wikesjo U, Ahlden ML, Ainamo J . Effect of 4 days consumption of chewing gum containing sorbitol or a mixture of sorbitol and xylitol on dental plaque and saliva. Caries Res 1983; 17 : 76– 88.
Scheinin A, Banoczy J . Collaborative WHO xylitol field studies in Hungary. An overview. Acta Odontol Scand 1985; 43 : 321– 325.
Scheinin A, Banoczy J, Szoke J, Esztari I, Pienihakkinen K, Scheinin U, et al. Collaborative WHO xylitol field studies in Hungary. I. Three year caries activity in institutionalised children. Acta Odontol Scand 1985; 43 : 327– 347.
Isokangas P . Xylitol chewing gum in caries prevention. A longitudinal study on Finnish school children. Proc Finn Dent Soc 1987; 83 (Suppl 1): 1– 117.
Kandelman D, Gagnon G . Clinical results after 12 months from a study of the incidence and progression of dental caries in relation to consumption of chewing-gum containing xylitol in school preventive programs. J Dent Res 1987; 66 : 1407– 1411.
Isokangas P, Alanen P, Tiekso J, Makinen KK . Xylitol chewing gum in caries prevention: a field study in children. J Am Dent Assoc 1988; 117 : 315– 320.
Kandelman D, Bar A, Hefti A . Collaborative WHO xylitol field study in French Polynesia. I. Baseline prevalence and 32-month caries increment. Caries Res 1988; 22 : 55– 62.
Isokangas P, Tiekso J, Alanen P, Makinen KK . Long term effect of xylitol chewing gum on dental caries. Community Dent Oral Epidemiol 1989; 17 : 200– 203.
Kandelman D, Gagnon G . A 24-month clinical study of the incidence and progression of dental caries in relation to consumption of chewing gum containing xylitol in school preventive programs. J Dent Res 1990; 69 : 1771– 1775.
Petersson LG, Birkhed D, Gleerup A, Johansson M, Jonsson G . Caries-preventive effect of dentifrices containing various types and concentrations of fluorides and sugar alcohols. Caries Res 1991; 25 : 74– 79.
Steinberg LM, Odusola F, Mandel ID . Remineralizing potential, antiplaque and antigingivitis effects of xylitol and sorbitol sweetened chewing gum. Clin Prev Dent 1992; 14 : 31– 34.
Isogangas P, Makinen KK, Tiekso J, Alanen P . Long-term effect of xylitol chewing gum in the prevention of dental caries: a follow-up 5 years after termination of a prevention program. Caries Res 1993; 27 : 495– 498.
Makinen KK, Bennett CA, Hujoel PP, Isokangas PJ, Isotupa KP, Pape HR . Xylitol chewing gums and caries rates: a 40-month cohort study. J Dent Res 1995; 74 : 1904– 1913.
Makinen KK, Makinen PL, Pape HR, Allen P, Bennett CA, Isokangas PJ, et al. Stabilisation of rampant caries: polyol gums and arrest of dentine caries in two long-term cohort studies in young subjects. Int Dent J 1995; 45 (Suppl 1): 93– 107.
Sintes JL, Escalante C, Stewart B, McCool JJ, Garcia L, Volpe AR, et al. Enhanced anticaries efficacy of a 0.243% sodium fluoride/10% xylitol/silica dentifrice: 3-year clinical results. Am J Dent 1995; 8 : 231– 235.
Makinen KK, Hujoel PP, Bennett CA, Isotupa KP, Makinen PL, Allen P . Polyol chewing gums and caries rates in primary dentition: a 24-month cohort study. Caries Res 1996; 30 : 408– 417.
Makinen KK, Makinen PL, Pape HR, Jr.,, Peldyak J, Hujoel P, Isotupa KP, et al. Conclusion and review of the Michigan Xylitol Programme (1986-1995) for the prevention of dental caries. Int Dent J 1996; 46 : 22– 34.
Makinen KK, Chen CY, Makinen PL, Bennett CA, Isokangas PJ, Isotupa KP, et al. Properties of whole saliva and dental plaque in relation to 40-month consumption of chewing gums containing xylitol, sorbitol or sucrose. Caries Res 1996; 30 : 180– 188.
Makinen KK, Olak J, Russak S, Saag M, Seedre T, Vasar R, et al. Polyol-combinant saliva stimulants: a 4-month pilot study in young adults. Acta Odontol Scand 1998; 56 : 90– 94.
Hujoel PP, Makinen KK, Bennett CA, Isotupa KP, Isokangas PJ, Allen P, et al. The optimum time to initiate habitual xylitol gum-chewing for obtaining long-term caries prevention. J Dent Res 1999; 78 : 797– 803.
Machiulskiene V, Nyvad B, Baelum V . Caries preventive effect of sugar-substituted chewing gum. Community Dent Oral Epidemiol 2001; 29 : 278– 288.
Edgar WM, Geddes DA . Chewing gum and dental health – a review. Br Dent J 1990; 168 : 173– 177.
Edgar WM . Sugar substitutes, chewing gum and dental caries - a review. Br Dent J 1998; 184 : 29– 32.
Scheinin A, Makinen KK, Tammisalo E, Rekola M . Turku sugar studies XVIII. Incidence of dental caries in relation to 1-year consumption of xylitol chewing gum. Acta Odontol Scand 1975; 33 : 269– 278.
Virtanen JI, Bloigu RS, Larmas MA . Timing of first restorations before, during, and after a preventive xylitol trial. Acta Odontol Scand 1996; 54 : 211– 216.
Caulfield PW, Cutter GR, Dasanayake AP . Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res 1993; 72 : 37– 45.
Edwardsson S, Birkhed D, Mejare B . Acid production from Lycasin, maltitol, sorbitol and xylitol by oral streptococci and lactobacilli. Acta Odontol Scand 1977; 35 : 257– 263.
Hayes ML, Roberts KR . The breakdown of glucose, xylitol and other sugar alcohols by human dental plaque bacteria. Arch Oral Biol 1978; 23 : 445– 451.
Drucker D, Verran J . Comparative effects of the substance-sweeteners glucose, sorbitol, sucrose, xylitol and trichlorosucrose on lowering of pH by two oral Streptococcus mutans strains in vitro . Arch Oral Biol 1980; 24 : 965– 970.
Knuuttila ML, Makinen K . Effect of xylitol on the growth and metabolism of Streptococcus mutans . Caries Res 1975; 9 : 177– 189.
Google Scholar
Trahan L, Soderling E, Drean MF, Chevrier MC, Isokangas P . Effect of xylitol consumption on the plaque-saliva distribution of mutans streptococci and the occurrence and long-term survival of xylitol-resistant strains J Dent Res 1992; 71 : 1785– 1791. [published erratum appears in J Dent Res 1993 Jan; 72 : 87– 88].
Soderling E, Makinen KK, Chen CY, Pape HR, Loesche W, Makinen PL . Effect of sorbitol, xylitol, and xylitol/sorbitol chewing gums on dental plaque. Caries Res 1989; 23 : 378– 384.
Aguirre-Zero O, Zero DT, Proskin HM . Effect of chewing xylitol chewing gum on salivary flow rate and the acidogenic potential of dental plaque. Caries Res 1993; 27 : 55– 59.
Gehring F, Makinen KK, Larmas M, Scheinin A . Turku sugar studies X. Occurrence of polysaccharide-forming streptococci and ability of the mixed plaque microbiota to ferment various carbohydrates. Acta Odontol Scand 1976; 34 : 329– 343.
Gehring F . Formation of acids by cariogenically important streptococci from sugars and sugar alcohols with special reference to isomaltitol and isomaltulose. Z Ernahrungswiss Suppl 1973; 15 : 16– 27.
Beckers HJ . Influence of xylitol on growth, establishment, and cariogenicity of Streptococcus mutans in dental plaque of rats. Caries Res 1988; 22 : 166– 173.
Soderling E, Trahan L, Tammiala-Salonen T, Hakkinen L . Effects of xylitol, xylitol-sorbitol, and placebo chewing gums on the plaque of habitual xylitol consumers. Eur J Oral Sci 1997; 105 : 170– 177.
Makinen KK, Scheinin A . Turku sugar studies. VII. Principal biochemical findings on whole saliva and plaque. Acta Odontol Scand 1975; 34 : 241– 283.
Makinen KK, Soderling E, Hurttia H, Lehtonen OP, Luukkala E . Biochemical, microbiologic, and clinical comparisons between two dentifrices that contain different mixtures of sugar alcohols. J Am Dent Assoc 1985; 111 : 745– 751.
Soderling E, Talonpoika J, Makinen KK . Effect of xylitol-containing carbohydrate mixtures on acid and ammonia production in suspensions of salivary sediment. Scand J Dent Res 1987; 95 : 405– 410.
Assev S, Vegarud G, Rolla G . Growth inhibition of streptococcus mutans strain OMZ176 by xylitol. Acta Pathol Microbiol Scand 1980; 88 : 61– 63.
Tuompio H, Meurman JH, Lounnatmaa K, Linkola J . Effect of xylitol and other carbon sources on the cell wall of Streptococcus mutans . Scand J Dent Res 1983; 91 : 17– 25.
Trahan L, Bareil M, Gauthier L, Vadeboncoeur C . Transport and phosphorylation of xylitol by a fructose phosphotransferase system in Streptococcus mutans . Caries Res 1985; 19 : 53– 63.
Scheie AA, Fejerskov O, Assev S, Rolla G . Ultrastructural changes in Streptococcus sobrinus induced by xylitol, NaF and ZnCl2. Caries Res 1989; 23 : 320– 327.
Pihlanto-Leppala A, Soderling E, Makinen KK . Expulsion mechanism of xylitol- 5-phosphate in Streptococcus mutans . Scand J Dent Res 1990; 98 : 112– 119.
Soderling E, Pihlanto-Leppala A . Uptake and expulsion of 14C-xylitol by xylitol-cultured Streptococcus mutans ATCC 25175 in vitro . Scand J Dent Res 1989; 97 : 511– 519.
Rogers AH, Pilowsky KA, Zilm PS, Gully NJ . Effects of pulsing with xylitol on mixed continuous cultures of oral streptococci. Aust Dent J 1991; 36 : 231– 235.
Trahan L, Neron S, Bareil M . Intracellular xylitol-phosphate hydrolysis and efflux of xylitol in Streptococcus sobrinus . Oral Microbiol Immunol 1991; 6 : 41– 50.
Grenby TH, Bashaarat AH, Gey KF . A clinical trial to compare the effects of xylitol and sucrose chewing-gums on dental plaque growth. Br Dent J 1982; 152 : 339– 343.
Topitsoglou V, Birkhed D, Larsson LA, Frostell G . Effect of chewing gums containing xylitol, sorbitol or a mixture of xylitol and sorbitol on plaque formation, pH changes and acid production in human dental plaque. Caries Res 1983; 17 : 369– 378.
Rekola M . Comparative effects of xylitol- and sucrose-sweetened chew tablets and chewing gums on plaque quantity. Scand J Dent Res 1981; 89 : 393– 399.
Soderling E, Alaraisanen L, Scheinin A, Makinen KK . Effect of xylitol and sorbitol on polysaccharide production by and adhesive properties of Streptococcus mutans . Caries Res 1987; 21 : 109– 116.
Soderling E, Isokangas P, Tenovuo J, Mustakallio S, Makinen KK . Long-term xylitol consumption and mutans streptococci in plaque and saliva. Caries Res 1991; 25 : 153– 157.
Lingstrom P, Lundgren F, Birkhed D, Takazoe I, Frostell G . Effects of frequent mouthrinses with palatinose and xylitol on dental plaque. Eur J Oral Sci 1997; 105 : 162– 169.
Soderling E, Rekola M, Makinen KK, Scheinin A . Turku Sugar Studies XXI; xylitol-, sorbitol-, fructose-, and sucrose-induced physico-chemical changes in saliva. Acta Odontol Scand 1975; 33 (Suppl 70): 337– 343.
Mouton C . The efficacy of gum chewing and xylitol to reduce oral glucose clearance time. J Can Dent Assoc 1983; 9 : 655– 660.
Makinen KK, Soderling E, Isokangas P, Tenovuo J, Tiekso J . Oral biochemical status and depression of Streptococcus mutans in children during 24- to 36-month use of xylitol chewing gum. Caries Res 1989; 23 : 261– 267.
Leach SA, Green RM . Effect of xylitol-supplemented diets on the progression and regression of fissure caries in the albino rat. Caries Res 1980; 14 : 16– 23.
Leach SA, Green RM . Reversal of fissure caries in the albino rat by sweetening agents. Caries Res 1981; 15 : 508– 511.
Havenaar R, Huis in'tVeldJH, de StoppelaarJD, Dirks OB . Anti-cariogenic and remineralizing properties of xylitol in combination with sucrose in rats inoculated with Streptococcus mutans . Caries Res 1984; 18 : 269– 277.
Bowen WH, Pearson SK . The effects of sucralose, xylitol, and sorbitol on remineralization of caries lesions in rats. J Dent Res 1992; 71 : 1166– 168.
Scheinin A, Soderling E, Scheinin U, Glass RL, Kallio ML . Xylitol-induced changes of enamel microhardness paralleled by microradiographic observations. Acta Odontol Scand 1993; 51 : 241– 246.
Gallagher IH, Fussell SJ . Acidogenic fermentation of pentose alcohols by human dental plaque microorganisms. Arch Oral Biol 1979; 24 : 673– 679.
Ziesenitz SC, Siebert G . The metabolism and utilization of polyols and other bulk sweeteners compared with sugar. In : Grenby T H (ed) Developments in sweeteners - 3. London: Elsevier Applied Science; 1987. p. 109– 149.
Grenby TH, Phillips A, Mistry M . Studies of the dental properties of lactitol compared with five other bulk sweeteners in vitro . Caries Res 1989; 23 : 315– 319.
Cornick DER, Bowen WH . The effect of sorbitol on the microbiology of the dental plaque in monkeys. Arch Oral Biol 1972; 17 : 1637– 1648.
Cronin M, Gordon J, Reardon R, Balbo F . Three clinical trials comparing xylitol- and sorbitol-containing chewing gums for their effect on supragingival plaque accumulation. J Clin Dent 1994; 5 : 106– 109.
Isotupa KP, Gunn S, Chen CY, Lopatin D, Makinen KK . Effect of polyol gums on dental plaque in orthodontic patients. Am J Orthod Dentofacial Orthop 1995; 107 : 497– 504.
Scheie AA, Fejerskov O, Danielsen B . The effects of xylitol-containing chewing gums on dental plaque and acidogenic potential. J Dent Res 1998; 77 : 1547– 1552.
Banoczy J, Hadas E, Esztari I, Fozy I, Szanto S, Felsovalyi A, et al. 3-year experience with clinical experiments on sorbitol used at the Fot children's town. Fogorv Sz 1980; 73 : 321– 329.
Frostell G, Blomlof L, Blomqvist T, Dahl GM, Edward S, et al. Substitution of sucrose by Lycasin in candy. 'The Roslagen Study'. Acta Odontol Scand 1974; 32 : 235.
Sugden K, Jolliffe IG . Development and marketing of non-sugar medicines. In : A.J.Rugg-Gunn (Ed) Sugarless - towards the Year 2000 . Cambridge: Royal Society of Chemistry; 1994. p. 172– 80.
Zumbe A, Lee A, Storey DM . Manufacture and marketing of non-sugar chocolate. In : A.J.Rugg-Gunn (Ed). Sugarless - Towards the Year 2000 . Cambridge: Royal Society of Chemistry; 1994. p. 147– 71.
Bär A . Caries prevention with xylitol. A review of the scientific evidence. World Rev Nutr Diet 1988; 55 : 183– 209.
Soderling E, Scheinin A . Perspectives on xylitol-induced oral effects. Proc Finn Dent Soc 1991; 87 : 217– 229.
Makinen K . Dietary prevention of dental caries by xylitol - clinical effectiveness and safety. J Appl Nutr 1992; 44 : 16– 28.
Imfeld T . Clinical caries studies with polyalcohols. A literature review. Schweiz Monatsschr Zahnmed 1994; 104 : 941– 945.
Trahan L . Xylitol: a review of its action on mutans streptococci and dental plaque – its clinical significance. Int Dent J 1995; 45 (1 Suppl 1): 77– 92.
Levine RS . Briefing paper: xylitol, caries and plaque. Br Dent J 1998; 185 : 520.
Hayes C . The effect of non-cariogenic sweeteners on the prevention of dental caries: A review of the evidence. J Dent Educ 2001; 65 : 1106– 1109.
Download references
Acknowledgements
The work on which this paper is based was supported by Danisco (UK)
Author information
Authors and affiliations.
Clinical Lecturer, School of Dental Sciences, Newcastle University,
Professor Emeritus, School of Dental Sciences, Newcastle University,
A J Rugg-Gunn
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to A Maguire .
Additional information
Refereed paper
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Maguire, A., Rugg-Gunn, A. Xylitol and caries prevention — is it a magic bullet?. Br Dent J 194 , 429–436 (2003). https://doi.org/10.1038/sj.bdj.4810022
Download citation
Received : 13 March 2002
Accepted : 05 November 2002
Published : 26 April 2003
Issue Date : 26 April 2003
DOI : https://doi.org/10.1038/sj.bdj.4810022
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Coupled production of fatty acid alkyl esters as biodiesel and fermentative xylitol from indian palm (elaeis guineensis jacq.) kernal oil in a biorefinery loom.
- Jayacumar Sanjana
- S. P. Jeevan Kumar
- Samuel Jacob
Waste and Biomass Valorization (2024)
Biotransformation of lignocellulosic biomass to xylitol: an overview
- Vasundhara Jain
- Sanjoy Ghosh
Biomass Conversion and Biorefinery (2023)
Effects of xylitol chewing gum and candies on the accumulation of dental plaque: a systematic review
- Eva Söderling
- Kaisu Pienihäkkinen
Clinical Oral Investigations (2022)
Xylitol and erythritol inhibit real-time biofilm formation of Streptococcus mutans
- Vuokko Loimaranta
- Danuta Mazurel
BMC Microbiology (2020)
Health benefits of xylitol
- Asma Gasmi Benahmed
- Geir Bjørklund
Applied Microbiology and Biotechnology (2020)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Help | Advanced Search
Computer Science > Computation and Language
Title: realm: reference resolution as language modeling.
Abstract: Reference resolution is an important problem, one that is essential to understand and successfully handle context of different kinds. This context includes both previous turns and context that pertains to non-conversational entities, such as entities on the user's screen or those running in the background. While LLMs have been shown to be extremely powerful for a variety of tasks, their use in reference resolution, particularly for non-conversational entities, remains underutilized. This paper demonstrates how LLMs can be used to create an extremely effective system to resolve references of various types, by showing how reference resolution can be converted into a language modeling problem, despite involving forms of entities like those on screen that are not traditionally conducive to being reduced to a text-only modality. We demonstrate large improvements over an existing system with similar functionality across different types of references, with our smallest model obtaining absolute gains of over 5% for on-screen references. We also benchmark against GPT-3.5 and GPT-4, with our smallest model achieving performance comparable to that of GPT-4, and our larger models substantially outperforming it.
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .

- Vehicle Technologies Office
- About the Vehicle Technologies Office
- Technology Areas
- Technology Integration
- Reports and Publications
Office: Vehicle Technologies Office FOA number: DE-FOA-0003248 Link to apply: Apply on EERE Exchange FOA Amount: $45,800,000
Today, the Department of Energy (DOE) announced $45.8 million in new funding for projects that will advance research, development, demonstration, and deployment (RDD&D) critical to achieving net-zero greenhouse gas emissions in the transportation sector. The funding will drive innovation in equitable clean transportation and is aligned with strategies detailed in the U.S. National Blueprint for Transportation Decarbonization .
The funding is through DOE’s Office of Energy Efficiency and Renewable Energy (EERE). Topic areas in the Vehicle Technologies Office (VTO) Fiscal Year (FY) 2024 R&D funding opportunity include:
- Next-generation phosphate-based cathodes.
- Advancing the state of the art for sodium-ion batteries.
- Developing concepts for decreasing greenhouse gas emissions from off-road vehicles such as construction, agriculture, mining, and forestry vehicles.
- Developing and deploying vehicle-to-everything technologies that can lead to meaningful savings at the vehicle and transportation system level.
- Developing high-performance, domestically produced electrical steels (E-steels) for use in electrified powertrains.
- Addressing critical cybersecurity needs for smart and secure electric vehicle charging.
As part of the Biden-Harris Administration’s commitment to ensuring the benefits of a clean transportation system are shared equally, the funding seeks the participation of underserved communities and underrepresented groups. Applicants are required to describe how diversity, equity, and inclusion objectives will be incorporated into their project.
VTO provides a series of funding opportunity announcement (FOA) information session videos , which help applicants understand VTO’s FOA process and requirements. The recently released, Session 3: Tips for a Strong FOA Application, includes best practices for incorporating Diversity, Equity, Inclusion, and Accessibility in a project.
Learn more about this and other funding opportunities on VTO’s funding webpage .
Topic Areas
Topic Area 1: Next-Generation Phosphate-Based Cathodes
This topic area targets the development of phosphate-based cathode materials that surpass the performance of state-of-the-art lithium iron phosphate (LFP) cathode materials, which are currently gaining traction as an alternative low-cost solution. The primary objective of this area of interest is to develop high energy density battery cells containing phosphate-based cathodes at the material and cell level.
Topic Area 2: Na-ion Battery Seedling Projects for Electric Vehicle Applications
While shifting to alternative cathode materials like LFP can alleviate the impact of nickel and cobalt, the impact of lithium has not been adequately addressed. One alternative to lithium is sodium (Na). While there is much promise for Na-ion chemistries, key issues still limit their adoption. This objective of this topic area is to advance the state of the art for Na-ion batteries by solving key challenges for the cathode, anode, or electrolyte through the development of 1 Ah full cells utilizing cell chemistries that are significant advancements over current industry state-of-the-art Na-ion technology.
Topic Area 3: Low-GHG Concepts for Off-Road Vehicles
The objective of this topic area is to develop and validate technology concepts capable of significantly decreasing greenhouse gas emissions, energy use, harmful criteria emissions, and total cost of ownership across the entire off-road vehicle sector, including construction, agriculture, mining, forestry, ports, warehouses, etc. Concepts must demonstrate they can meet the unique requirements for off-road vehicles and gain customer acceptance.
Topic Area 4: Saving Energy with Connectivity
Research has shown that vehicle-to-everything (V2X) communications can lead to meaningful energy savings at the vehicle and transportation system level by integrating interoperable vehicle-to-vehicle (V2V), vehicle-to-infrastructure (V2I), and vehicle-to-pedestrian (V2P) communications. The objective of this topic area is to develop and deploy V2X technologies with a focus on the efficiency and convenience of the mobility ecosystem, while reducing transportation’s environmental impacts. Examples could include but are not limited to eco-driving along connected corridors, transit or freight priority, integrated corridor management, or passenger or freight trip-chaining optimization.
Topic Area 5: Domestically Produced Electrical Steels (E-Steels)
The US transportation sector is in a technology revolution where light-duty vehicles are rapidly transitioning from internal combustion engines to electrified powertrains. Although most of the vehicles are produced in the US, many of the powertrain components rely on imports and foreign supply chains. Of particular interest are traction motors and their components. The objective of this topic are is to develop E-Steels meeting properties including frequency, thickness, ductility, cost, and manufacturability.
Topic Area 6: Cybersecurity for Smart and Secure Electric Vehicle Charging
This topic area is addressing critical cybersecurity needs to address through two subtopics:
- Subtopic 6.a: Enabling Wide-scale, Cybersecure EV/EVSE Aggregation for Grid Services : To support the integration of electric vehicles (EVs) and their charging requirements with the electric grid, both government and the private sector have made significant investments in the development of smart charge management (SCM) systems and technologies for EV charging infrastructure. The objective of this subtopic area is to research, develop, and demonstrate systems, technologies, and tools necessary for the cybersecure aggregation of EVs and charging infrastructure to provide widescale, cybersecure grid services.
- Subtopic 6.b: Tools to Assess EV/EVSE/Charging System Cybersecurity Posture and Compliance with Standards and Protocols for Communications, Controls, and Monitoring : Testing and evaluation of Electric Vehicle Supply Equipment (EVSE) by DOE national laboratories has clearly indicated a lack of compliance by many vendors with certified and/or regulated EV charging standards and protocols. In addition to creating cybersecurity vulnerabilities, this non-compliance greatly inhibits interoperability, supplier-managed SCM, and right-to-repair. The objective of this subtopic is to research, develop, and validate a suite of tools and associated procedures to comprehensively assess EV/EVSE/charging system compliance with relevant standards and protocols and cybersecurity posture.
Additional Information
- Download the full funding opportunity on the EERE Exchange website.
- For FOA-specific support, contact [email protected] .
- Sign up for the Office of Energy Efficiency and Renewable Energy (EERE) funding email list to get notified of new EERE funding opportunities. Also sign up for VTO’s newsletter to stay current with the latest news.
- Watch the VTO Funding Opportunity Announcement information series webinars.
Talking politics with strangers isn't as awful as you'd expect, research suggests
Many of us avoid discussing politics with someone who holds an opposing viewpoint, assuming the exchange will turn nasty or awkward. But having those conversations is far more gratifying than we expect, a new research paper suggests.
Across a series of experiments involving hundreds of U.S. adults, a team of scientists found that individuals underestimate the social connection they can make with a stranger who disagrees with them. The findings are published in Psychological Science, a journal of the Association for Psychological Science.
These low expectations may help to explain why people think those on the opposite side of the political spectrum have more extreme views than they actually do, behavioral scientists Kristina A. Wald (University of Pennsylvania), Michael Kardas (Oklahoma State University), and Nicholas Epley (University of Chicago) wrote in an article about their research.
"Mistakenly fearing a negative interaction may create misplaced partisan divides," they wrote, "not only keeping people from connecting with each other but also keeping people from learning about each other and from each other."
The experimenters found evidence, through experiments conducted online and in person, that people prefer to avoid hot-button issues, especially with people who disagree with them. People also tend to advise their friends and relatives to avoid such conversations.
But Wald, Kardas, and Epley believed people would find discussing their political differences to be a more positive experience than expected, at least partly because people fail to appreciate the extent to which conversations are informative and draw people closer together.
To test their theory, they asked nearly 200 participants in one experiment for their opinions on divisive political and religious topics, such as abortion and climate change. The researchers then divided the participants into pairs and assigned them to discuss one of these topics. Some participants were told in advance whether their partners agreed with them or not, but others entered the discussions unaware of their partners' views.
All the participants reported how positively or negatively they expected the conversation to be, then engaged in the discussion while being video recorded. Afterward, the participants rated their sentiments about the dialogue. Research assistants also viewed the videos of the conversations and evaluated them across several dimensions.
As predicted, the participants underestimated how positive their conversation experience would be, but this tendency was largest when they disagreed with their partner. Participants in this disagreement condition also underestimated the similarities in their opinions. Coders who watched the videos of these conversations confirmed that participants tended to stay on topic, and that the conversations were consistently positive whether the participants agreed or disagreed.
In another experiment, the researchers tested their hypothesis that people underestimate how the process of conversation itself -- actual back-and-forth dialogue -- connects people. To do so, they randomly assigned participants to discuss a divisive topic they agreed or disagreed on, but they also randomly assigned participants to either have a conversation about the topic in a dialogue format or to simply learn of their partners' beliefs on the topic in a monologue format. In the monologue format, each person separately recorded themselves talking about their opinion and then watched the other person's recording.
Overall, the participants underestimated how positive their interactions would be, especially when they disagreed with their partner, the researchers noted. But this tendency was especially strong when people actually had a conversation with their partner rather than simply learning of their beliefs in a monologue. The social forces in conversation that draw people together through back-and-forth dialogue are not only powerful, but they appear to be even more powerful than people expect.
The researchers cautioned that their experiments involved participants talking with strangers; the experiments did not reveal how disagreements unfold among family and friends. Still, they said their findings illustrate the benefits of talking and listening to others rather than typing and broadcasting in debates on social media.
Our reluctance to discuss our differences denies us some positive social interactions, the authors concluded.
"Misunderstanding the outcomes of a conversation," they wrote, "could lead people to avoid discussing disagreements more often, creating a misplaced barrier to learning, social connection, free inquiry, and free expression."
- Relationships
- Racial Issues
- Social Psychology
- STEM Education
- Political Science
- Surveillance
- Poverty and Learning
- Social movement
- Intellectual giftedness
- Social science
- Social psychology
- Double blind
- Social cognition
- Social inclusion
Story Source:
Materials provided by Association for Psychological Science . Note: Content may be edited for style and length.
Journal Reference :
- Kristina A. Wald, Michael Kardas, Nicholas Epley. Misplaced Divides? Discussing Political Disagreement With Strangers Can Be Unexpectedly Positive . Psychological Science , 2024; DOI: 10.1177/09567976241230005
Cite This Page :
Explore More
- High Carbon Impact of Tourism at Yellowstone
- Extreme Starburst Galaxy
- Asthma: Disease May Be Stoppable
- Stellar Collisions and Zombie-Like Survivors
- Tiny Robot Swarms Inspired by Herd Mentality
- How the Brain Regulates Emotions
- Evolution in Action? Nitrogen-Fixing Organelles
- Plastic-Free Vegan Leather That Dyes Itself
- Early Mesozoic Animals: High Growth Rates
- Virus to Save Amphibians from Deadly Fungus?
Trending Topics
Strange & offbeat.
The Geography of Capital Allocation in the Euro Area
We assess the pattern of Euro Area financial integration adjusting for the role of “onshore offshore financial centers” (OOFCs) within the Euro Area. The OOFCs of Luxembourg, Ireland, and the Netherlands serve dual roles as both hubs of investment fund intermediation and centers of securities issuance by foreign firms. We provide new estimates of Euro Area countries' bilateral portfolio investments which look through both roles, attributing the wealth held via investment funds to the underlying holders and linking securities issuance to the ultimate parent firms. Our new estimates show that the Euro Area is less financially integrated than it appears, both within the currency union and vis-a-vis the rest of the world. While official data suggests a sharp decline in portfolio home bias for Euro Area countries relative to other developed economies following the introduction of the euro, we demonstrate that this pattern only remains true for bond portfolios, while it is artificially generated by OOFC activities for equity portfolios. Further, using new administrative evidence on the identity of non-Euro Area investors in OOFC funds, we document that the bulk of the positions constituting missing wealth in international financial accounts are now accounted for by United Kingdom counterparts.
We thank the Stanford Impact Labs, the NSF (1653917), the Andrew Carnegie Corporation, the Sloan Foundation, and the Jerome A. Chazen Center for financial support. We thank Luca Fornaro, Galina Hale, Zhengyang Jiang, Niels Johannesen, Philip Lane, Alberto Martin, Gian Maria Milesi-Ferretti, Pablo Ottonello, Diego Perez, Isabel Schnabel, Hyun Song Shin, Paolo Surico, Alexandra Tabova, Silvana Tenreyro, Liliana Varela, Adrien Verdelhan, Frank Warnock, and Gabriel Zucman for helpful comments. We are also grateful to Sergio Florez-Orrego, Bianca Piccirillo, Ziwen Sun, and Serdil Tinda for outstanding research assistance. The ECB has provided access to proprietary data and research support services. The views expressed are those of the authors and do not necessarily reflect those of the ECB. Coppola, Lewis, Maggiori, and Schreger are unpaid consultants of the ECB for the purpose of accessing data for this project, while Beck and Schmitz are employed by the ECB. Our analysis makes use of data that are proprietary to Morningstar and/or its content providers. Neither Morningstar nor its content providers are responsible for any of the views expressed in this article. The views expressed herein are those of the authors and do not necessarily reflect the views of the National Bureau of Economic Research.
Financial support was provided by the Harvard Business School Doctoral Programs Office, Princeton University, and a Harvard University International Economics grant. Markit had the right to review this paper for disclosure of proprietary data and for prejudicial statements regarding Markit or its industry. Schreger: No interests to disclose. Hébert: Hébert’s spouse works in the financial services industry and has business interactions with firms involved in the litigation described in this paper. His spouse has not contributed to or participated in the preparation of this paper, and neither Hébert nor his spouse have a direct or material indirect financial interest in the outcome of the litigation.
MARC RIS BibTeΧ
Download Citation Data
Working Groups
Conferences, more from nber.
In addition to working papers , the NBER disseminates affiliates’ latest findings through a range of free periodicals — the NBER Reporter , the NBER Digest , the Bulletin on Retirement and Disability , the Bulletin on Health , and the Bulletin on Entrepreneurship — as well as online conference reports , video lectures , and interviews .

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Research Findings on Xylitol and the Development of Xylitol Vehicles to Address Public Health Needs
1 Northwest/Alaska Center to Reduce Oral Health Disparities, University of Washington, Seattle
2 Regional Clinical Dental Research Center, University of Washington, Seattle
Xylitol has been demonstrated to be a safe and effective tooth decay preventive agent when used habitually. Nevertheless, its application has been limited by absence of formulations that demand minimal adherence and are acceptable and safe in settings where chewing gum may not be allowed. A substantial literature suggests that a minimum of five to six grams and three exposures per day from chewing gum or candies are needed for a clinical effect. At the same time there is conflicting evidence in the literature from toothpaste studies suggesting that lower-doses and less frequent exposures might be effective. The growing use of xylitol as a sweetener in low amounts in foods and other consumables is, simultaneously, increasing the overall exposure of the public to xylitol and may have additive benefits.
In this paper the authors address the questions: (1) What is the minimum dose and frequency for use of xylitol containing chewing gum for significantly lowering mutans streptococci levels? And (2) can delivery vehicles be produced that are applicable in settings where chewing gum or similar confections might be permitted?
A randomized controlled trial was carried out to determine the dose-response effects of S. mutans in plaque and unstimulated saliva to xylitol gum ( Milgrom et al., 2006 ). Participants (N=132) were randomized into four groups: controls of 9.83 g sorbitol/0.702 g maltitol/day (G1), 3.44 g xylitol/day (G2), 6.88 g xylitol/day (G3), and 10.32 g xylitol/day (G4) in the form of 12 pellets (3 pellets/4 times/day). Plaque was collected in a standardized manner from specific sites but was not weighed. Baseline, 5-week, and 6-month samples of plaque and unstimulated saliva showed decreasing levels of S. mutans across treatment groups of increasing dose. Xylitol at 6.88 g/day and 10.32 g/day reduced S. mutans in plaque at 5 weeks, and in plaque and saliva at 6 months ( Figure 1 ). Results suggested a plateau effect for both plaque and saliva, indicating that exceeding the daily dose of xylitol 10.32 g/day is not likely to increase effectiveness. Alternatively, a dose of 3.44 g/day is not likely to show reductions in S. mutans levels.

Mean log10 CFU mutans streptococci/mL in plaque and unstimulated saliva by xylitol dose at 5 wks and at 6 mos (N=33 in each group). *Significant-difference group compared with placebo (G1) in least-significant-difference multiple comparisons. (Reprinted from Milgrom P, et al. 2006 with permission)
A five week randomized controlled trial was conducted in order to determine the reduction in S. mutans levels in plaque and unstimulated saliva to increasing frequency of xylitol gum use at a fixed daily dose of 10.32 g ( Ly et al., 2006 .) Participants (N=132) received either 10.32 g xylitol/day in the active group or 9.83 g sorbitol/0.7 g maltitol/day in the control group. The 10.32 g dose was used because it clearly would allow testing of the hypothesis even though a smaller dose (e.g. 6.88 g) might also have been possible. The number of pieces of gum did not change, and frequency of chewing (times per day) varied from 2 to 4 times/day within the active group; the control group chewed gum 4 times/day. There were no significant differences in S. mutans level among the groups at baseline. At five weeks, there was a linear reduction in S. mutans in plaque and unstimulated saliva to increasing frequency of xylitol gum use at a constant daily dose of 10.32 g ( Figure 2 ). Although the difference observed for the xylitol two times/day group was consistent with the model, the difference was not statistically significant.

Mutans streptococci counts in plaque and unstimulated saliva at five weeks and best fit linear line. Linear reduction of mutans streptococci levels of xylitol chewing gum use at constant daily dose (10.32 g/day). Linear line equations: plaque -*log mutans streptococci = −.21(Frequency)+5.21; unstimulated saliva-**log mutans streptococci =−.19(frequency)+5.07. Group F0 = Sorbitol Control; F2 = xylitol 2x/d; F3 = xylitol 3x/d; F4 = xylitol 4x/d. N=33 subjects per group. (reprinted from Ly KA, et al., 2006 )
Alternative Vehicles
Study one of a recent experiment compared the potential of pediatric topical syrup to deliver xylitol versus chewing gum. The basic rationale was that if the salivary xylitol concentrations were similar to chewing gum over a similar period, the effect on the oral flora should be the same and a xylitol delivery system for the very young is desirable. Others also have considered syrup or child’s dummy (pacifier) as a delivery vehicle ( Uhari, 1996 , 1998 ; Taipale et al., 2006)
A within-subjects study design was employed to compare the presence and time course of xylitol concentrations in saliva from different delivery methods. Xylitol-containing pellet chewing gum (2.6 g) and 33% xylitol syrup (2.67 g) are presented here ( Riedy et al., 2008 ). Adult subjects (N=15) consumed one product per visit with a 7-day washout period between products. Saliva samples were collected according to a standardized protocol at baseline and at ten regular intervals following exposure. HPLC was used to quantify xylitol concentrations. Mean salivary xylitol concentrations and bimodal time curves were similar for the two delivery methods ( Figure 3 ); the correlation coefficient (r 2 ) between the mean xylitol concentrations at each time point for xylitol pellet chewing gum and the syrup was 0.96. Total AUC for the two products did not differ significantly (pellet gum – 63.0 ng.min/mL, syrup – 59.0 ng.min/mL).

Comparison of salivary xylitol concentrations (ng/mL) after using xylitol-containing gum and syrup (N=15). (Adapted from Riedy CA et al., 2008 )
A randomized control trial of xylitol syrup on early childhood caries has been conducted ( Milgrom et al., 2008 (under peer-review). Children at 9 to 15 months of age were randomized to three conditions in which all were given syrup orally three times per day by their mother/caretaker. The groups were: 3 doses of 2.67 g xylitol each (8 g/day); 2 doses of 4.0 g xylitol per day plus a single dose of a sorbitol placebo (8 g/day xylitol); or a single dose of 2.67 g xylitol plus two sorbitol placebo doses. Results show the pediatric topical syrup was highly effective in preventing early childhood caries in a population with very high rates of disease by 24 months of age.
In the second study of the xylitol salivary level experiment above ( Riedy et al., 2008 ), bear shaped xylitol confections (2.6 g) were compared to xylitol pellet gum (2.6 g) at similar concentration. Another set of subjects (N=15) served as their own control. The study method and saliva sampling were as described for study one, the pellet gum compared to syrup study above. Mean salivary xylitol concentrations and bimodal time curves were similar for the two delivery methods; the correlation coefficient (r 2 ) between the mean xylitol concentrations at each time point for xylitol pellet chewing gum and the gummy bears was 0.99. Total AUC for the two products did not differ significantly (pellet gum – 63.0 ng.min/mL, gummy bears – 55.9 ng.min/mL).
A randomized trial of the same bear shaped confection is now being conducted in which the target is prevention of tooth decay in first permanent molars. About 30 percent of first molars are decayed by first grade. This current study is designed to address the targeted use of xylitol when the first permanent molars are erupting ( Hujoel et al., 1999 ). The study is a two group, 30-month randomized controlled clinical trial designed to assess the use of xylitol gummy bears as snack food during school hours to reduce dental caries among kindergarten children. Nearly all the children have untreated tooth decay in their primary teeth. Three hundred children are being randomized over two years into one of two treatment groups, receiving either six xylitol (1.3 g/piece, 2.6 g/dose—7.8 g/day) or six placebo gummy bears, distributed in the classroom evenly three times a day, for nine months.
The work presented confirms the interpretation of data from clinical studies regarding frequency and dose ( Isokangas, 1987 ; Rekola, 1989 ; Mäkinen et al., 1995 ). One caution is that the effectiveness of the lowest dose in the Milgrom and colleagues study (2006) may have been masked because the subjects had background levels of xylitol exposure, apparently from the diet. The bacterial reductions are a surrogate for reductions in tooth decay but this is permissible because the mechanism of action of xylitol is specifically antibacterial and a number of studies have demonstrated parallel reductions of S. mutans and tooth decay. Thus, the correspondence between the findings in the Milgrom series and the clinical studies already in the literature means that chewing gum can be used as a vehicle in institutional programs. However, there will still be adherence issues related to those who must administer or supervise use. Gum has been shown to be less effective in individual treatment programs because of lack of adherence ( Isotupa et al., 1995 ; Stecksén-Blicks, 2004 ).
A controlled study of complex design of xylitol containing candies and gum was conducted in children about 10 years old ( Alanen et al., 2000 ). This age group was targeted because of the potential to protect erupting second permanent molars. Three xylitol test groups received either candies (xylitol-maltitol or xylitol-polydextrose) or gum at 5 grams per day divided into three doses over several years depending on the group. The results showed 35 to 60 percent reductions in caries incidence in the test groups relative to the controls and no difference between xylitol delivery vehicles. This study is important both because of its result in the same dosage/frequency range as the previous studies and because the trial was intentionally sized to have adequate statistical power even with anticipated attrition.
In contrast there have been at least two studies attempting to demonstrate an effect of lower dosages. A non-randomized trial ( Honkala et al., 2006 ) compared one xylitol candy three times per day (assumed to be 1.9 g total/day; the paper is unclear as to dose) to an untreated control in children and young adults in a school for the disabled. The control group consisted of students whose parents did not consent to the study. Baseline caries scores were fairly high and similar yet the test group showed a significant reduction in caries incidence relative to the untreated controls. This may indeed have been because the test candy, according to the manufacturer’s website, was actually a 1:1 mixture of xylitol and maltitol. Other studies have shown that confections sweetened with maltitol alone reduced S. mutans levels in daily use with children (Ly et al., 2008). Thus, it is likely inaccurate to assert that 1.9 g xylitol per day alone is effective.
Oscarson and colleagues (2006) attempted to prevent caries in preschool children using 0.5 to 1.0 grams of xylitol in lozenges beginning around age 2. This study failed to show any effect largely because the underlying caries rate was extremely low (less than 1 dmfs per child at 4 years old) and the study had not been designed to detect such small, perhaps clinically insignificant, differences in the first place. Neither of these publications gives any rationale for the low dosages.
Xylitol-containing dentifrice
Several studies have evaluated sodium fluoride toothpaste formulations with xylitol. In all they raise questions, in view of the previous data presented, as to how an exposure of as little as 0.1 to 0.2 g per day xylitol (assuming a 1 g dose of toothpaste that is 10% xylitol and given no more than twice per day) could result in significant reductions of S. mutans and dental caries. Unpublished work by Söderling and colleagues has shown that low-dose xylitol decreases the growth of specific mutans strains in culture during the growth phase but this is hardly the same situation as in the mouth. Early short–term study of a xylitol-glycerol dentifrice showed reductions in salivary mutans ( Svanberg & Birkhed, 1991 ).
In a study of 155 university students with high S. mutans levels comparing three fluoridated dentifrices (toothpaste with or without triclosan, or triclosan plus 10% xylitol), only the toothpaste with triclosan and xylitol showed significant reductions in plaque and saliva mutans levels from the placebo at 6 months although the levels dropped in all the groups ( Janneson et al. 2002 ). In this study the students were instructed to use about 1.5 cm of the dentifrice (about 1 g) and to refrain from rinsing. The authors argue that the proprietary toothpaste was formulated to optimize the bioavailability of the xylitol and that the dose used was larger than in other studies (for example, see Twetman & Peterson, 1995 ). No data were presented on how long the xylitol was present in the mouth after the exposures nor were there data on adherence. It is possible that the effects of triclosan and xylitol are synergistic. The time-response effect seen in this study is consistent with the Milgrom and colleagues studies of xylitol-containing chewing gum.
A prospective study of 2,630 Costa Rican children, initially eight to 10 years of age, brushing twice daily with fluoride toothpaste with 10% xylitol or fluoride toothpaste alone reported a 12% reduction in decayed/filled surfaces (DFS) and 11% reduction in decayed/filled buccal and lingual surfaces (DFS-BL) among those children brushing with fluoride toothpaste and xylitol after three years ( Sintes et al., 1995 ). This study should to be interpreted cautiously as there was nearly 40 percent attrition in the subject population and the analysis did not employ intent-to-treat analytical methods. Another 30-month study by the same investigators of 3,394 seven to 12 year old children who used either fluoride toothpaste with and without 10% xylitol showed DFS and DFT increments of 1.30 and 0.69, respectively, for the 10% xylitol group when compared with the fluoride toothpaste only group ( Sintes et al., 2002 ). Again, there were limitations in the study design and synergy between fluoride and xylitol cannot be ruled out. An additional concern is that these toothpastes contained sodium lauryl sulfate as a detergent, which may decrease the effectiveness of the xylitol ( Assev et al., 1997 ).
Low-Dose Non-Intentional Exposure to Xylitol
In the U.S., for example, xylitol is being added in small non-clinical amounts as a sweetener or advertising gimmick to various foods and children’s vitamins. Tables 1 and and2 2 give examples of many of the products containing xylitol in the US. It is possible that frequent lower dose exposure to xylitol is beneficial without the effort to maintain special programs. It is not possible to answer this question from the existing literature; however, two-thirds of the subjects in the Milgrom and colleagues study (2006) had been exposed to low levels of xylitol in their diets ( Roberts et al., 2002 ).
Xylitol Containing Gums and Mints Available in U.S. Markets, Their Xylitol Content, Preventive Potential, and Availability
Xylitol Containing Oral Hygiene, Healthcare, and Diet Products Available in U.S. Markets, and Their Xylitol Content
Conclusions
In spite of the considerable evidence that xylitol is an effective caries preventive and cariostatic agent; an effective delivery system for xylitol, especially for children, demanding minimal adherence yet safe has not been developed. A substantial body of work suggests that a minimum of five to six grams and three exposures per day are needed for a clinical effect. At the same time there is conflicting evidence in the literature from the xylitol toothpaste studies suggesting that lower-doses and less frequent exposures might be effective but the synergistic effects of xylitol and fluoride or triclosan cannot be ruled out. Studies of new vehicles for xylitol such as a xylitol releasing dummy and a pediatric syrup have been conducted.
Acknowledgments
Supported by Grants U54 DE14254 from the NIDCR/NIH, R40MC03622 from the Maternal and Child Health Research Bureau, HRSA, and a Head Start Innovation and Improvement Project Grant No. 90YD0188 from the Office of Head Start, Agency for Children and Families.
- Alanen P, Isokangas P, Gutmann K. Xylitol candies in caries prevention: results of a field study in Estonian children. Community Dent Oral Epidemiol. 2000; 28 (3):218–24. [ PubMed ] [ Google Scholar ]
- Assev S, Wåler SM, Rolla G. Are sodium lauryl sulfate-containing toothpastes suitable vehicles for xylitol? Eur J Oral Sci. 1997; 105 (2):178–82. [ PubMed ] [ Google Scholar ]
- Honkala E, Honkala S, Shyama M, Al-Mutawa SA. Field trial on caries prevention with xylitol candies among disabled school students. Caries Res. 2006; 40 :508–13. [ PubMed ] [ Google Scholar ]
- Hujoel PP, Mäkinen KK, Bennett CA, Isotupa KP, Isokangas PJ, Allen P, Mäkinen P-L. The optimum time to initiate habitual xylitol gum-chewing for obtaining long-term caries prevention. J Dent Res. 1999; 778 (3):797–803. [ PubMed ] [ Google Scholar ]
- Isokangas P. Xylitol chewing gum in caries prevention. A longitudinal study on Finnish school children. Proc Finn Dent Soc. 1987; 83 (Suppl 1):1–117. [ PubMed ] [ Google Scholar ]
- Isotupa KP, Gunn S, Chen CY, Lopatin D, Mäkinen KK. Effect of polyol gums on dental plaque in orthodontic patients. Am J Orthod Dentofacial Orthop. 1995; 107 :497–504. [ PubMed ] [ Google Scholar ]
- Jannesson L, Renvert S, Kjellsdotter P, Gaffar A, Nabi N, Birkhed D. Effect of a triclosan-containing toothpaste supplemented with 10% xylitol on mutans streptococci in saliva and dental plaque. A 6-month clinical study. Caries Res. 2002; 36 :36–39. [ PubMed ] [ Google Scholar ]
- Ly KA, Milgrom P, Roberts MC, Yamaguchi DK, Rothen M, Mueller G. Linear response of mutans streptococci to increasing frequency of xylitol chewing gum use: a randomized controlled trial [ISRCTN43479664] BMC Oral Health. 2006; 6 :6. doi: 10.1186/1472-6831-6-6. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mäkinen KK, Bennett CA, Hujoel PP, Isokangas PJ, Isotupa KP, Pape HR, et al. Xylitol chewing gums and caries rates: A 40-month study. J Dent Res. 1995; 74 :1904–1913. [ PubMed ] [ Google Scholar ]
- Milgrom P, Ly KA, Roberts MC, Rothen M, Mueller G, Yamaguchi DK. Mutans streptococci dose response to xylitol chewing gum. J Dent Res. 2006; 85 :117–181. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Milgrom P, Tut OK, Ly KA, Gancio MJ, Roberts M, Mancl L, Langidrik JR, Briand K. Xylitol topical oral syrup prevents early childhood caries: A RCT. Abstract No. 3331, International Association of Dental Research Annual Meeting; Toronto. 2008. [ Google Scholar ]
- Oscarson P, Lif Holgerson P, Sjöström I, Twetman S, Stecksén-Blicks C. Influence of a low xylitol-dose on mutans streptococci colonisation and caries development in preschool children. Eur Arch Paediatric Dent. 2006; 7 (3):142–7. [ PubMed ] [ Google Scholar ]
- Rekola M. Correlation between caries incidence and frequency of chewing gum sweetened with sucrose or xylitol. Proc Finn Dent Soc. 1989; 85 :21–4. [ PubMed ] [ Google Scholar ]
- Riedy CA, Milgrom P, Ly KA, Rothen M, Mueller G, Hagstrom MK, Tolentino E, Zhou L, Roberts MC. A surrogate method for comparison analysis of salivary concentrations of Xylitol-containing products. BMC Oral Health. 2008 Feb 11;:8–5. doi: 10.1186/1472-6831-8-5. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Roberts MC, Riedy CA, Coldwell SE, Nagahama S, Judge K, Lam M, et al. How xylitol-containing products affect cariogenic bacteria. J Am Dent Assoc. 2002; 133 :435–441. [ PubMed ] [ Google Scholar ]
- Sintes JL, Elias-Boneta A, Stewart B, Volpe AR, Lovett J. Anticaries efficacy of a sodium monofluorophosphate dentifrice containing xylitol in a dicalcium phosphate dihydrate base. A 30-month caries clinical study in Costa Rica. Am J Dent. 2002; 15 :215–219. [ PubMed ] [ Google Scholar ]
- Sintes JL, Escalante C, Stewart B, McCool JJ, Garcia L, Volpe AR, et al. Enhanced anticaries efficacy of a 0.243% sodium fluoride/10% xylitol/silica dentifrice: 3-year clinical results. Am J Dent. 1995; 8 :231–235. [ PubMed ] [ Google Scholar ]
- Stecksén-Blicks C, Holgerson PL, Olsson M, Bylund B, Sjöström I, Sköld-Larsson K, et al. Effect of xylitol on mutans streptococci and lactic acid formation in saliva and plaque from adolescents and young adults with fixed orthodontic appliances. Eur J Oral Sci. 2004; 112 :244–248. [ PubMed ] [ Google Scholar ]
- Svanberg M, Birkhed D. Effect of dentifrices containing either xylitol and glycerol or sorbitol on mutans streptococci in saliva. Caries Res. 1991; 25 :449–53. [ PubMed ] [ Google Scholar ]
- Taipale T, Pienihäkkinen K, Alanen P, Jokela J, Söderling E. Dissolution of xylitol from a food supplement administered with a novel slow-release pacifier: preliminary results. Eur Arch Paediatr Dent. 2007; 8 :123–125. [ PubMed ] [ Google Scholar ]
- Twetman S, Petersson LG. Influence of xylitol in dentifrice on salivary microflora of preschool children at caries risk. Swed Dent J. 1995; 19 :103–8. [ PubMed ] [ Google Scholar ]
- Uhari M, Kontiokari T, Koskela M, Niemelä M. Xylitol chewing gum in prevention of acute otitis media: double blind randomised trial. BMJ. 1996; 313 (7066):1180–1184. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Uhari M, Kontiokari T, Niemelä M. A novel use of xylitol sugar in preventing acute otitis media. Pediatrics. 1998; 102 :879–884. [ PubMed ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
The present paper discusses the research advances focused on integrating several types of catalytic processes in a single container. The mechanism of catalytic hydrogenation of xylose to xylitol is detailed. The domain of the different parameters of the reaction will allow to increase the efficiency of the transformation of the biomass ...
Abstract. Xylitol is a white crystalline, amorphous sugar alcohol and low-calorie sweetener. Xylitol prevents demineralization of teeth and bones, otitis media infection, respiratory tract infections, inflammation and cancer progression. NADPH generated in xylitol metabolism aid in the treatment of glucose-6-phosphate deficiency-associated ...
1. Introduction. Xylitol is a five-carbon sugar alcohol (C 5 H 12 O 5, Figure 1) with a molecular weight of 152.15 g/mol, which is commonly used as a sweetener in sugar-free confectionery.It also naturally occurs in fruits and vegetables (plums, strawberries, cauliflower, and pumpkin []).It is equisweet to sucrose and has a very similar sweetness-time intensity to sucrose.
The present review is devoted to comprehensive analyses of the positive and negative effects of this polyol on human health.Key Points• The health benefits of xylitol are not limited to oral hygiene.•. Xylitol efficiently stimulates the immune system, digestion, lipid and bone metabolism.•. Xylitol helps in glycemic and obesity control ...
The review of the literature has also concluded that the most effective xylitol product in caries prevention was the xylitol (100%), chewed or consumed three to five times per day, after meals with a total dose of 5-10 g/day. Frequencies less than three times a day (less than 3.44g/day) did not show any caries preventive benefit. Doses up to ...
Xylitol, a natural compound classified as a sugar alcohol, is found diversely in fruits and vegetables in small quantities. Commercial production of xylitol has expanded due to its health benefits and wide applications as an alternative sweetener in food and pharmaceutical products. Production of xylitol on large scale is industrially being achieved by the chemical method.
Recent results of randomized trials testing the efficacy of xylitol in caries prevention have been conflicting. This narrative review reveals the sources of discrepancy. The following databases were searched for the terms "xylitol" or "artificial sweeteners" restricted to the English language: PubMe …
The scope of this research aims at merging a new deep eutectic mixture (DES) into a biopolymer-based membrane for a pervaporation application in dehydrating ethanol. Herein, an L-proline:xylitol ...
Xylitol is a white crystalline, amorphous sugar alcohol and low-calorie sweetener. Xylitol prevents demineralization of teeth and bones, otitis media infection, respiratory tract infections, inflammation and cancer progression. NADPH generated in xylitol metabolism aid in the treatment of glucose-6-phosphate deficiency-associated hemolytic anemia. Moreover, it has a negligible effect on blood ...
According to some authors, the prevalence of permanent tooth decay in children aged 6-16 exceeds 70 percent with an intensity of 2,86 index SIC 5,20 ± 0,26. The. -. highest increase in permanent tooth decay is observed in the age groups of 6 -7 years (by 2.91-fold) and 11 -12 years (by 1.37-fold) (Chukhray 2018).
Numerous studies have reported that xylitol has potential beneficial effects in the management and prevention of obesity, diabetes, and other related metabolic disorders (Chukwuma & Islam, 2016 ...
Research showed that the administration of xylitol could induce a rapid release of insulin in plasma. The study has been done in dogs (0.4 g/kg xylitol, intravenously). The prompt rise in plasma insulin was observed as a result, though the procedure or mechanism behind the stimulation of the release of insulin is still ambiguous.
Xylitol metabolism is insulin-independent, and has low energy value (2.5 cal g −1 ) compared with sucrose (4 cal g −1 ) [1]. Therefore, both the food and pharmaceutical industries use xylitol ...
Xylitol is known to be harmful for many microorganisms, and in 2004, research was organized to determine its effect on pneumococci. Xylitol was shown to decrease the growth of streptococcus ...
Of the evaluated papers, in addition to the study by Al-Haboubi et al. , only three studies dealt with the effects of xylitol gum chewing on gingival health [40, 50, 51]. To our knowledge, our systematic review is the first review to deal with the effects of xylitol consumption on plaque accumulation.
Xylitol is a sugar alcohol having the properties that reduce levels of mutans streptococci (MS) in the plaque and saliva. To assess the role of xylitol in preventing dental caries. Systematic review and meta-analysis developed by Cochrane cooperation were adapted. ... In 1967, the National Institute of Dental Research's National Dental Advisory ...
Some research has suggested that xylitol has a unique, ... Levine, 93 in a briefing paper on xylitol, described xylitol as exhibiting both passive and active anti-caries properties.
Xylitol is another emerging prebiotic which is found naturally in many fruits and vegetables like lettuce, cauliflower, yellow plums, strawberry, grapes, banana, meshrooms etc. 25. These ...
Transactions on Machine Learning Research. Yang Xu, Yiheng Xu, Tengchao Lv, Lei Cui, Furu Wei, Guoxin Wang, Yijuan Lu, Dinei Florencio, Cha Zhang, Wanxiang Che, Min Zhang, and Lidong Zhou. 2021.LayoutLMv2: Multi-modal pre-training for visually-rich document understanding. In Proceed-ings of the 59th Annual Meeting of the Association for
ReALM: Reference Resolution As Language Modeling. Reference resolution is an important problem, one that is essential to understand and successfully handle context of different kinds. This context includes both previous turns and context that pertains to non-conversational entities, such as entities on the user's screen or those running in the ...
The effect of xylitol or erythritol intake on the area under the curve for 3-OMG concentration was not significant. Neither the time (pre or post intervention), nor the group (control, xylitol, or erythritol), nor the time-by-group interaction effects were significant (p = 0.829, p = 0.821, and p = 0.572, respectively). Therefore, our results ...
Office: Vehicle Technologies Office FOA number: DE-FOA-0003248 Link to apply: Apply on EERE Exchange FOA Amount: $45,800,000 Today, the Department of Energy (DOE) announced $45.8 million in new funding for projects that will advance research, development, demonstration, and deployment (RDD&D) critical to achieving net-zero greenhouse gas emissions in the transportation sector.
Talking politics with strangers isn't as awful as you'd expect, research suggests. ScienceDaily . Retrieved April 4, 2024 from www.sciencedaily.com / releases / 2024 / 04 / 240403171027.htm
Xylitol, a five-carbon sugar polyol, has been found to be promising in reducing dental caries disease and also reversing the process of early caries. This paper throws light on the role and effects of various forms of xylitol on dental caries and oral hygiene status of an individual. Keywords: xylitol, caries preventive effect, oral flora. Go to:
Working Paper 32275. DOI 10.3386/w32275. Issue Date March 2024. We assess the pattern of Euro Area financial integration adjusting for the role of "onshore offshore financial centers" (OOFCs) within the Euro Area. The OOFCs of Luxembourg, Ireland, and the Netherlands serve dual roles as both hubs of investment fund intermediation and ...
A five week randomized controlled trial was conducted in order to determine the reduction in S. mutans levels in plaque and unstimulated saliva to increasing frequency of xylitol gum use at a fixed daily dose of 10.32 g (Ly et al., 2006.)Participants (N=132) received either 10.32 g xylitol/day in the active group or 9.83 g sorbitol/0.7 g maltitol/day in the control group.