Loading metrics
Open Access
Peer-reviewed
Research Article

Midwifery continuity of care: A scoping review of where, how, by whom and for whom?
Roles Data curation, Formal analysis, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing
Affiliations Maternal, Child, and Adolescent Health Program, Burnet Institute, Melbourne, Victoria, Australia, Mater Research, University of Queensland, Brisbane, Queensland, Australia
Roles Data curation, Formal analysis, Validation, Writing – review & editing
Affiliations Maternal, Child, and Adolescent Health Program, Burnet Institute, Melbourne, Victoria, Australia, Melbourne Medical School, University of Melbourne, Melbourne, Victoria, Australia
Roles Conceptualization, Funding acquisition, Methodology, Supervision, Validation, Writing – review & editing
Affiliation Department of Maternal, Newborn, Child, and Adolescent Health, World Health Organisation, Geneva, Switzerland
Roles Conceptualization, Funding acquisition, Methodology, Writing – review & editing
Roles Formal analysis, Methodology, Validation, Writing – review & editing
Affiliation Department of Women and Children’s Health, Kings College London, London, United Kingdom
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing
* E-mail: [email protected]
Affiliation Maternal, Child, and Adolescent Health Program, Burnet Institute, Melbourne, Victoria, Australia
- Billie F. Bradford,
- Alyce N. Wilson,
- Anayda Portela,
- Fran McConville,
- Cristina Fernandez Turienzo,
- Caroline S. E. Homer

- Published: October 5, 2022
- https://doi.org/10.1371/journal.pgph.0000935
- Peer Review
- Reader Comments
Systems of care that provide midwifery care and services through a continuity of care model have positive health outcomes for women and newborns. We conducted a scoping review to understand the global implementation of these models, asking the questions: where, how, by whom and for whom are midwifery continuity of care models implemented? Using a scoping review framework, we searched electronic and grey literature databases for reports in any language between January 2012 and January 2022, which described current and recent trials, implementation or scaling-up of midwifery continuity of care studies or initiatives in high-, middle- and low-income countries. After screening, 175 reports were included, the majority (157, 90%) from high-income countries (HICs) and fewer (18, 10%) from low- to middle-income countries (LMICs). There were 163 unique studies including eight (4.9%) randomised or quasi-randomised trials, 58 (38.5%) qualitative, 53 (32.7%) quantitative (cohort, cross sectional, descriptive, observational), 31 (19.0%) survey studies, and three (1.9%) health economics analyses. There were 10 practice-based accounts that did not include research. Midwives led almost all continuity of care models. In HICs, the most dominant model was where small groups of midwives provided care for designated women, across the antenatal, childbirth and postnatal care continuum. This was mostly known as caseload midwifery or midwifery group practice. There was more diversity of models in low- to middle-income countries. Of the 175 initiatives described, 31 (18%) were implemented for women, newborns and families from priority or vulnerable communities. With the exception of New Zealand, no countries have managed to scale-up continuity of midwifery care at a national level. Further implementation studies are needed to support countries planning to transition to midwifery continuity of care models in all countries to determine optimal model types and strategies to achieve sustainable scale-up at a national level.
Citation: Bradford BF, Wilson AN, Portela A, McConville F, Fernandez Turienzo C, Homer CSE (2022) Midwifery continuity of care: A scoping review of where, how, by whom and for whom? PLOS Glob Public Health 2(10): e0000935. https://doi.org/10.1371/journal.pgph.0000935
Editor: Ahmed Waqas, University of Liverpool, UNITED KINGDOM
Received: June 3, 2022; Accepted: September 5, 2022; Published: October 5, 2022
Copyright: © 2022 Bradford et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data are in the Supporting information files.
Funding: This review was commissioned by the World Health Organization Department of Maternal, Newborn, Child and Adolescent Health and Ageing and funded through a grant received from Merck Sharp and Dohme Corp (MSD). CFT is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration (ARC) South London, a NIHR Global Health Research Group (NIHR133232) and a NIHR Development and Skills Award (NIHR301603). CSEH is supported by an Australian National Health and Medical Research Council Fellowship (APP1137745). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Continuity of care is a concept rooted in primary care involving the care of individuals (rather than populations) over time by the same care provider. It encompasses relational continuity, informational continuity and management continuity [ 1 ]. In the primary care setting, continuity of care has been shown to reduce mortality and hospitalisations, and increase patient satisfaction [ 2 ]. Continuity of care also has an important place in chronic care settings, such as palliative care [ 3 ].
In the maternal and newborn care setting, midwife-led continuity of care refers to a model whereby care is provided by the same midwife, or small team of midwives, during pregnancy, labour and birth, and the postnatal periods with referral to specialist care as needed [ 4 ]. Midwife-led also refers to a model of care which is provided there is a distinct occupational group of midwives [ 5 ] and the person is fully qualified, regulated and deployed only as a midwife. This contrasts to systems in many countries (most countries in Africa for and South East Asia for example) where nurse-midwives are rotated to either nursing or midwifery duties. Midwife-led continuity models in a small number of HICs have been associated with lower rates of preterm birth (24% reduction), and lower fetal loss before and after 24 weeks and neonatal deaths (16%) less likely to lose their babies overall (combined reduction in fetal loss and neonatal death) for women at low and mixed risk of complications compared to other models of care. In addition, women are less likely to experience interventions and more likely to report positive experiences of care [ 4 ]. A Cochrane review of reviews of interventions during pregnancy to prevent preterm birth also found that these models had clear benefit in reducing preterm birth and perinatal death [ 6 ]. Women prefer the personalised experience provided by such models, leading to trust between midwife and woman and empowerment of both women and midwives [ 7 ].
Models of care that provide continuity across the childbearing continuum are complex interventions, and the pathway of influence that produces these positive outcomes is unclear. A number of plausible hypotheses require further investigation. For example, it could be that midwives provide a mechanism that enables effective and equitable care to be provided by better coordination, navigation and referral; and/or that relational continuity and advocacy engenders trust and confidence between women and midwives, resulting in women feeling safer, less stressed and more respected [ 4 ]. Access to organisational infrastructure, innovative partnerships, and robust community networks has been found crucial to overcome barriers, address women’s, newborns’ and parents’ needs and ensure quality of care [ 8 ].
Inequity is a key driver of adverse perinatal outcome, both between and within countries. Some observational studies of midwife-led continuity of care models in socially and economically disadvantaged populations in high-income countries (HIC) have reported significant reductions in pre-term birth and caesarean sections in diverse cohorts of women in the United Kingdom [ 9 – 12 ]. In Australia, a study of maternity care during significant floods in Queensland showed that midwife-led continuity of care mitigated the social and emotional impacts of the floods [ 13 ]. Another Australian study showed reduced preterm births amongst Australian Aboriginal and Torres Strait Islander women who received midwife-led continuity of care [ 14 ]. These studies suggest that women who typically experience a greater burden of adverse perinatal outcome, may derive greater benefit from continuity of care. However, understanding how continuity per se may mitigate inequities in maternal and newborn health remains a research priority.
Despite evidence supporting midwife-led continuity of care and guidelines from the World Health Organization which recommend midwife-led continuity-of-care models for pregnant women in settings with well-functioning midwifery programmes [ 15 – 17 ] only a small proportion of women internationally have access to such care. The current evidence suggests that access to midwife-led continuity of care models is largely confined to a small number of HICs notably Australia, Canada, New Zealand and the United Kingdom [ 17 ] where a distinct occupational group of midwives has been a central part of the health systems for decades. Barriers to implementation of midwifery-led continuity of care exist across all country income levels and include a lack of local health system financing, shortage of personnel including administrative and other support staff [ 4 ]. It is not clear to what extent midwife-led continuity of care has been implemented in low- to middle-income countries (LMIC). Many LMICs have a model of predominantly nurse-midwives who are deployed to both nursing and midwifery duties, often preventing midwife-led continuity of care models. Advancing understanding around which countries have implemented continuity of care models for maternal and newborn health, how, for whom, and in what context, is crucial for successful implementation, scale-up and sustainability.
The overall aim of this review was to understand the global implementation of midwifery continuity of care, asking the questions: Where, how, by whom and for whom are midwifery continuity of care initiatives implemented?
Materials and methods
A scoping review was undertaken guided by the approach described by Arksey and O’Malley [ 18 ] and further defined by Levac and colleagues [ 19 ]. The following five steps were followed: i) identifying the research question; ii) identifying the relevant literature; iii) study selection, iv) charting the data; and v) collating, summarising and reporting the results.
We used the broad definition of midwifery from The Lancet Series on Midwifery as our starting point, that is, “skilled, knowledgeable, and compassionate care for childbearing women, newborn infants and families across the continuum from pre-pregnancy, pregnancy, birth, postpartum and the early weeks of life [ 20 ]. Midwifery continuity of care was defined as care delivered by the same known care provider or care provider team across two or more parts in the care continuum–antenatal, intrapartum, postnatal and neonatal periods. In some settings, continuity of care may be provided by cadre other than midwives, for example, nurses or physicians. Thus, eligible papers could include care providers that were midwives and non-midwives, such as, nurses, community health workers and physicians. We excluded reports on care primarily by traditional birth attendants (TBA).
Identifying the relevant literature—Search strategy and selection criteria
In order to develop the search strategy, a preliminary search of PubMed and Google scholar using the terms ‘midwifery or midwife-led continuity of care’ were used to locate key systematic and scoping reviews on the topic and identify relevant search terms for the systematic search strategy (see S1 Text for the search strategy). We then searched the following electronic databases: MEDLINE, CENTRAL, CINAHL, PsychINFO and Web of Science. A subject librarian reviewed search terms, keywords and strategies. In addition, we searched PubMed, Google Scholar, PROSPERO, Scopus and Dimensions and the WHO International Clinical Trials Registry platform. We conducted the search on the 20 February 2022 and included publications (peer reviewed studies and reports) in the past 10 years.
A key area of interest was implementation of continuity of midwifery care in LMICs but we recognised that reports of implementation may be published in formats other than peer reviewed publications. Eligible papers therefore included implementation studies or reports of implementation of midwifery continuity of care in the grey literature. We sourced grey literature through online searches on the websites of relevant professional groups, United Nations agencies and non-government organisations (NGO). We circulated a call for relevant materials through online list servs (email groups) and through midwifery contacts. The International Confederation of Midwives assisted by emailing all member associations asking for any relevant reports.
Eligible reports could report on midwifery continuity of care efforts in HICs and LMICs. Reports from implementation efforts by government programmes, private providers, professional organisations, NGOs and universities and research studies of any design were eligible for inclusion. Protocols that reported studies that were underway, but not concluded, were also eligible. Opinion pieces, editorials and other materials, which included details of midwifery continuity of care initiatives, were also eligible. Publications in any language were eligible. The search was limited to reports published in the last ten years (January 2012 to January 2022) to ensure the information was contemporary and therefore of greatest relevance to policy makers.
Reports identified through both peer-reviewed and grey literature databases were hand-searched for other potentially relevant studies. These included reference lists of relevant systematic reviews, and published conference abstracts, as well as any reports forwarded to authors in response to a call for notification of new or ongoing initiatives from key global stakeholder organisations, such as the International Confederation of Midwives.
Reports were excluded they if reported on midwifery continuity of care in general but did not report on a continuity of care practice initiative. We excluded systematic and literature reviews although their reference lists were searched for relevant primary studies.
Study selection
All reports identified through database searching were imported into Endnote referencing programme (Endnote 20, Clarivate Analytics, Philadelphia), and duplicates removed. Remaining citations (n = 5789) were uploaded into systematic review software Covidence (Covidence 2022, Veritas Health Innovations, Melbourne). Two authors independently conducted initial title and abstract screening and undertook full-text review. A third author screened a random selection of 10% of studies and discrepancies were discussed and resolved.
Charting the data
The following information was extracted for all included reports: country, income-level (as defined by the World Bank [ 21 ], study design (if applicable), setting (urban/rural and community-based or facility-based), novel or scaled-up initiative, model of care, level of continuity (antenatal and intrapartum, antenatal and postnatal, intrapartum and postnatal) and cadre of care providers, (e.g. mix of providers involved). We also collected information on the inclusion of priority population groups–these are groups of people who are persistently disadvantaged by existing systems of power with demographic features known to be associated with adverse perinatal outcomes, such as ethnic minorities, urban and remote women, socially disadvantaged, and Indigenous women. We have described these specific groups as priority rather than vulnerable populations [ 22 ]. Reporting of the scoping review findings follows the PRISMA-ScR (Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews) format (see S1 Checklist ) and reference ( Fig 1 ) [ 23 ]. Appraisal of study quality or meta-analysis was not undertaken.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pgph.0000935.g001
In total, 6595 references were identified from electronic peer-reviewed databases, 821 duplicate records were removed prior to uploading to Covidence, a further 634 duplicates were removed by automation. Of the 5136 remaining references, 4728 did not meet the inclusion criteria. A further 256 references were excluded at the full-text stage as either: they did not describe continuity of care according to our pre-determined criteria (167); did not include primary source data (42); were duplicates (41); or did not have insufficient detail regarding the model of care (6). One hundred and fifty-two (152) peer-reviewed publications were eligible based on inclusion and exclusion criteria. A further 23 reports were identified following the grey literature search, bringing the total to 175 ( Fig 1 ). Details are listed in S1 Table .
Of the 175 individual reports, 152 (86.8%) were peer reviewed publications, 18 (10.3%) were conference abstracts, and the remaining five (3%) were published or unpublished reports. Reports primarily reported on birth outcomes (n = 54, 31%), women’s (including some partners’) views and experiences (n = 47, 27%) and midwives (including doctors) views and experiences (n = 33, 19%). There were 18 reporting on the model of care more broadly, including implementation challenges (n = 18, 10%), and 14 (7%) that were focussed on the experience of midwifery students providing continuity of care as part of their education. The majority of these student-focused reports were from Australia. Fewer reports focussed on the experience of midwifery managers (n = 3, 2%), while four were cost analyses (2%).
There were 163 unique studies including eight (4.9%) randomised or quasi randomised trials, 58 (38.5%) qualitative, 53 (32.7%) quantitative (cohort, cross sectional, descriptive, observational), 31 (19.0%) survey studies, and three (1.9%) health economics analyses. There were 10 practice-based accounts that did not include research.
‘The where’: Country and setting
Of the 175 included reports, the majority (n = 157, 90%) were from HICs and 18 (10%) from LMICs ( Table 1 ). Most were from Australia, (n = 71, 41%), followed by the United Kingdom (England, Scotland, Wales) (24, 14%), Sweden (13, 8%), Canada (8, 5%), Denmark (6, 4%), New Zealand (7, 4%), Japan (5, 3%), with less than five reports described initiatives conducted in Belgium, Finland, Germany, Greece, Ireland, Netherlands, Norway, Singapore, Switzerland and the United States of America (USA). In the LMICs, three from Palestine, three from China, two each from Bangladesh and Indonesia, and one from each of the remaining countries.
https://doi.org/10.1371/journal.pgph.0000935.t001
Overall, most midwifery continuity of care models were based in urban areas (n = 126, 72%). In HIC, three-quarters of services (n = 118, 75%) were urban-based, whereas in LMICs just under half (n = 8, 44%) were urban-based. Hospital or facility-based services were most common across all income levels (n = 124, 72% overall).
‘The how’: Describing the way continuity of care is provided
There were a number of different terms used to define the model of care, and the level of continuity provided across the continuum of care varied with no single term used. Overall, the most common terms were caseload midwifery (n = 63, 36%), midwifery-led continuity (n = 60, 34%), or team/midwifery group practice (n = 40, 23%). Most described services designed so that the same providers provided care across the continuum–antenatal, intrapartum and postnatal (n = 159, 91%). There were eight which described continuity only across the antenatal and postpartum periods [ 24 – 31 ] (excluding labour and birth), and five reported [ 32 – 36 ] (including 3 unique examples) where the continuity was provided only across antenatal and intrapartum periods without postpartum care.
In HICs, the most dominant approach is where small groups of midwives provide care for designated women, known as caseload midwifery or midwifery group practice in countries like Australia [ 37 ], the United Kingdom [ 9 ], Denmark [ 38 ], Sweden [ 39 ], and Singapore [ 40 ] where the number of midwives is usually two to four. In other countries, for example, Japan [ 41 ] and Switzerland [ 42 ], the approach is also called team midwifery and the number of midwives is five or more.
The continuity of care services were located as part of the usual hospital [ 37 , 43 ], in an alongside birth centre [ 44 – 46 ] or in a free-standing birth centre [ 47 ]. Some midwife-led continuity of care services were offered through homebirth practices, either as part of the hospital system [ 48 , 49 ] or as a private service [ 50 ]. Most services were based in urban areas but there were some examples from rural areas in Australia [ 51 – 53 ], Sweden [ 54 – 56 ] and Scotland [ 57 ] ( Table 2 ).
https://doi.org/10.1371/journal.pgph.0000935.t002
Although midwife-led continuity of care was available in a number of countries, mostly high-income with a cadre of midwives, in select facilities and locations, it was generally not scaled-up nationally. The exception being New Zealand, where the Lead Maternity Carer model is national allowing to midwives provide continuity of care to all women regardless of risk, in either caseloading or small community-based group practices, under a national funding arrangement and with medical or other collaboration when required [ 58 ].
In LMICs there was greater diversity in structure of arrangements for provision of midwifery continuity of care models. Models of care included a lead midwife delivering care across the continuum [ 59 , 60 ], midwives on-call for women during labour who they had previously seen for antenatal care [ 61 ], and a midwifery continuity of care team that ran in parallel with an obstetric team [ 62 ]. An initiative in Ethiopia involved the same midwife providing antenatal, intrapartum and postnatal care to the same women [ 63 , 64 ]. An initiative in Kenya involved midwifery care across the childbearing continuum, embedded within a family planning and HIV care service [ 65 ]. One initiative in China [ 66 ] facilitated continuity of care for women wishing to have a vaginal birth, where efforts were made for women to see the same midwife for intrapartum and postnatal care. In the Palestinian initiative [ 67 – 69 ], midwives were allocated geographical areas to provide antenatal and postnatal care for between 50–100 women. One initiative in Bangladesh [ 70 ] and another in Iran [ 71 ] involved teams of midwives providing care in a private midwifery clinic associated with two local hospitals.
Similar to HICs, continuity of care services were located as part of the usual hospital services (eg in Pakistan [ 59 , 72 ], China [ 61 ], Ethiopia [ 63 , 64 ], Palestine [ 67 – 69 ] or in community health centres or maternity clinics, for example in Bangladesh [ 60 , 70 ], Kenya [ 65 ] and Afghanistan [ 73 ]. Most services were based in urban or semi-urban areas but there were some examples from rural areas, for example, Palestine [ 67 – 69 ], Bangladesh [ 70 ], Afghanistan [ 73 ] and Indonesia [ 74 ].
Reports from China [ 62 ], Ethiopia [ 63 ], Iran [ 71 ], Kenya [ 65 ] and Pakistan [ 59 ] provided some degree of continuity of care across all antenatal, intrapartum and postnatal periods. Two reports, one from China [ 61 ] and the one from Kenya [ 65 ] provided care across antenatal and intrapartum. A study in China [ 66 ] involved the provision of midwife-led care at antenatal, intrapartum and postnatal time points, but continuity of care with the same/a known provider was only guaranteed at intrapartum and postnatal time points. S1 Table provides more details on each of the initiatives and S2 Table gives additional detail on models of care from LMICs.
The ‘by whom’: Providers of midwifery continuity of care
Midwives were the dominant provider of continuity of care across all settings. Services were mostly midwife-led with some reports including other cadre as well. Integration with existing services including systems for referral to obstetric services when needed was usual.
In HIC, almost all models of continuity of midwifery care involved care provided by midwives and/or midwifery students. A small number included midwives and other cadre. For example, programs with midwives and Aboriginal Health Workers (Indigenous health providers) [ 14 , 75 – 78 ]; collaborations with general practitioners, obstetricians or a social worker [ 44 , 79 – 81 ]. Just two examples did not include midwives; a model in Finland where continuity of care is provided by a nurse who takes care of the family from the pregnancy until the child reaches school age [ 26 , 82 ], and an example in Ireland [ 83 ], where continuity of care was provided by a privately practising obstetrician.
All except two of the continuity of care initiatives in LMICs were midwife-led. The initiative from Ghana [ 84 ] was provided by midwives, nurses and doctors while the one in Kenya [ 65 ] was provided by community based midwives who may have nursing or midwifery qualifications and other health professional with obstetric skills who reside in the community.
There were 16 reports which described midwifery students providing continuity of care, most of these were from Australia, Norway and Indonesia [ 74 , 85 – 101 ]. Midwifery students were placed with women, providing continuity of care to a defined number of women over their education program, as a way to engage them in this model of care [ 91 , 94 ].
The ‘for whom’: Priority groups for continuity of care initiatives
Of the 175 initiatives, 44 (25.5%) of these were implemented for women and newborns with risk of adverse outcomes ( Table 3 ). These included women from Indigenous communities, refugee and migrant populations, young mothers, women living in rural and remote areas, women who experience socioeconomic disadvantage, women with a history of substance abuse, chronic illness, and ethnic minority groups. The majority were from Australia [ 23 ], United Kingdom [ 9 ] and Canada [ 3 ]. There were four examples from LMICs, these were designed primarily for rural and remote communities in Palestine [ 67 – 69 ], Bangladesh [ 70 ], Afghanistan [ 73 ] and Kenya [ 84 ].
https://doi.org/10.1371/journal.pgph.0000935.t003
This scoping reviewed aimed to map where, how, by whom, and for whom are midwifery continuity of care models are being implemented globally. The majority of models identified were in HICs, largely in Australia and the United Kingdom. Notably, all countries where five or more continuity of midwifery care initiatives were identified in the last 10 years are high-income and provide free public healthcare to their citizens and have a distinct cadre of midwives which makes this possible (Australia, Canada, Denmark, Japan, New Zealand, Sweden, and United Kingdom). Only 18 initiatives were identified in LMICs.
There is a growing body of literature demonstrating beneficial effects of midwifery continuity of care [ 4 , 8 ]. Midwifery continuity of care is a complex, multi-faceted intervention and teasing out which elements impart benefit to recipients of care is difficult. We found that almost all papers included in this review, involved continuity of care initiatives led by midwives or midwifery students (with midwife supervision). This was despite casting the net wide to identify continuity of care initiatives provided by any health provider across two or parts of the maternal and newborn care continuum.
Reviews of continuity of care in maternal and newborn care have focused on midwife-led continuity of care compared with other models of care such as doctor-led and shared care models [ 4 , 102 ]. However, a previous integrative review of midwife-led care in LMICs, found that just over half of studies included in the review included only midwives, with other cadres of health professionals including nurses, nurse-midwives, doctors, traditional birth attendants and family planning workers [ 103 ]. Whilst there is scope for other non-midwife health providers to provide continuity of care, such as family physicians [ 104 ]and community health workers [ 105 ], which may particularly be of value in LMICs countries where there is a shortage of midwives [ 106 ], there are few studies or reports available about these continuity of care models and their benefits. Although other cadre are not precluded from providing continuity of care, this review has shown that in the global literature, continuity of care across the maternal and newborn continuum is reported to be almost exclusively provided by midwives and is a significant area of quality improvement and research interest for midwives.
An encouraging finding from this review was the significant proportion of initiatives in HICs which focussed on women and newborns with vulnerabilities related to social and economic determinants of health (23.2%). The evidence that such initiatives are feasible for a diverse range of priority groups across many countries could demonstrate recognition of the benefits of continuity of care in improving outcomes for those with greater social and economic barriers to good health outcomes. This has implications for future research in that previous studies exploring childbirth outcomes from midwifery continuity of care frequently involve low-risk women [ 4 ], or women who had self-selected to be part of a midwife-led care project and thus are more likely to experience a positive outcome. This review has revealed that initiatives in a range of settings involve groups acknowledged to be at increased risk of adverse outcome. Larger scale and robust studies of midwifery continuity of care initiatives involving populations who experience social and economic disadvantage, and/or are at increased obstetric risk are both feasible and desirable.
Implications for policy, practice, research
This review has revealed that most studies, or reports, on midwifery continuity of care describe models led by midwives within HICs. Despite the benefits of midwife-led continuity of care, none of these countries has managed to scale-up this approach to being the standard of care at a national level, other than New Zealand. This highlights the organisational challenges of widespread implementation and the importance of system-level reform to enable countries to transition to this model of care and to scale-up. This reform means having adequate funding, support to enable midwives to be educated, and regulated, to work to their full scope of practice including flexibility and autonomy, self-managed time, team space, telephone access, and being able to work safely in the community and having access to transport and referral services [ 8 , 107 ].
Fewer than 10% of initiatives included in this review were from LMICs and only one was a clinical trial. The greatest burden of maternal and newborn deaths and stillbirths exists in LMICs. In HICs, midwife-led continuity of care has potential to reduce preventable maternal and newborn mortality and morbidity and stillbirths, however system-level reform and ensuring an enabling environment is still key [ 44 ]. The lack of midwifery continuity of care initiatives in LMICs, highlights the need for greater investment to ensure well-functioning midwifery systems can be developed with monitoring, evaluation and research to understand the effect of different models and associated benefits and/or challenges in different contexts. Operational research that identifies the barriers, facilitators and blockages to implementing models of midwifery continuity of care is needed, including in settings where there are shortages of midwives. In order to facilitate transition to, and scale-up of, midwifery continuity of care in LMICs, key considerations include strengthening midwifery education and regulation and ensuring the presence of an enabling environment [ 66 , 73 ].
Future systematic and scoping review studies would be enhanced by clear reporting of midwifery continuity model type, implementation details (including on midwife competence, scope of practice, deployment) and degree of continuity achieved within published studies and reports. Establishment of a classification system for this purpose would also enhance implementation efforts. One example of a classification system in a country which has an identifiable cadre of midwives is the Maternity Care Classification System (MaCCS) which was developed to classify, record and report data about maternity models of care in Australia [ 108 , 109 ]. The MaCCS includes a series attributes including the target groups, profession of provider, the caseload size, the extent of planned continuity of care and the location of care to come up with 11 major model categories (see S3 Table for details) [ 110 ]. This classification system is now being included in all routine data systems in Australia so that, in the future, outcomes by model of care will be reported. While this is developed for one high-income country, an adaptation for global utility could be useful.
Measuring the extent to which continuity of care is achieved is the second key area. The health insurance industry in the USA has developed measures to assess patterns of visits to providers and therefore, the level of continuity of care [ 111 ]. The measures include the Bice-Boxerman Continuity of Care Index (measures the degree of coordination required between different providers during an episode), the Herfindahl Index (the degree of coordination required between different providers during an episode), the Usual Provider of Care (the concentration of care with a primary provider) and Sequential Continuity of Care Index (the number of handoffs of information required between providers). The Usual Provider of Care index has also been used to assess continuity of care in general practice in the UK, that is, to assess the proportion of a patient’s contacts that was with their most regularly seen doctor [ 112 ]. For example, if a patient had 10 general practitioner contacts, including six with the same doctor, then their usual provider of care index score would be 0.6. With the exception of one study, none of the papers in this review had applied such indexes. This is an important consideration for the future.
Strengths and limitations
This review provides a summary of midwifery continuity of care efforts globally. As countries look to strengthen midwifery and quality of care for women and newborns during pregnancy, childbirth and postnatal periods, understanding implementation in all resource settings is important. In this review the broad criteria for inclusion allowed for identifying the maximum number of implementation efforts in LMICs to be identified. Despite the efforts to reach out, and although no language filters were applied, search terms were in English thus we may have missed some ongoing efforts. We also did not measure, or account for, the skills and competencies in the different cadres providing care, or if they are always deployed as midwives or provide details about the profile/qualifications of the healthcare providers, the way the midwifery system function, if any affiliation to healthcare centres, support systems, health costs and coverage or safety outcome indicators as these were reported differently or not at all across the papers. Finally, in this review we were not able to reliably determine the extent to which women receiving care were able to see the same individual care provider. Relational continuity is a key element of continuity of care, and possible mechanism for beneficial effects, which requires repeat contact over time between individual care providers and recipients of care.
Conclusions
This review mapped midwifery continuity of care initiatives globally. The majority of initiatives identified were in HICs, with fewer identified in LMICs. Almost all initiatives identified in LMICs were led by midwives (some of whom worked in a model in which they were also deployed as nurses), despite our efforts to identify models led by other skilled health professionals. Almost no countries have managed to scale-up midwifery continuity of care to being the standard of care at a national level. This highlights the organisational challenges of widespread implementation and the importance of system-level reform to enable these models of care to scale-up. Nevertheless, examples of successful implementation of midwifery continuity of care in low-resource settings reported show that advances in this area are possible.
A number of initiatives identified in HICs focused on women and newborns at risk of adverse outcomes, demonstrating the value of midwifery continuity of care in populations who experience social and economic disadvantage and vulnerabilities. There is a need for further research on midwifery continuity of care models in LMICs, and strategies to facilitate transition to, and scale-up of, midwifery continuity of care initiatives globally.
Supporting information
S1 text. search strategy..
https://doi.org/10.1371/journal.pgph.0000935.s001
S1 Table. All included items.
https://doi.org/10.1371/journal.pgph.0000935.s002
S2 Table. Additional details from low- and middle-income countries.
https://doi.org/10.1371/journal.pgph.0000935.s003
S3 Table. Major model categories in MaCCS.
https://doi.org/10.1371/journal.pgph.0000935.s004
S1 Checklist. Preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews.
https://doi.org/10.1371/journal.pgph.0000935.s005
Acknowledgments
Thank you to Rana Islamiah Zahroh, PhD student and researcher at the University of Melbourne in Australia for assistance mapping the data. Thanks also to Rosemary Rowe, Subject Librarian at Faculty of Health, Victoria University of Wellington in New Zealand and to Allisyn Moran and Joao Paolo Souza (WHO) for useful feedback and advice.
- View Article
- PubMed/NCBI
- Google Scholar
- 5. World Health Organization. Global strategic directions for nursing and midwifery 2021–2025. Geneva: World Health Organization; 2021.
- 15. World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. Geneva: World Health Organization; 2016.
- 16. World Health Organization. WHO recommendations: intrapartum care for a positive childbirth experience. Geneva: World Health Organization; 2018.
- 17. World Health Organization. WHO recommendations on maternal and newborn care for a positive postnatal experience. Geneva: World Health Organization; 2022.
- 21. World Bank. World Development Indicators 2022. https://databank.worldbank.org/reports.aspx?source=world-development-indicators .
- 24. Cummins A, Griew K, Devonport C, Ebbett W, Catling C, Baird K. Exploring the value and acceptability of an antenatal and postnatal midwifery continuity of care model to women and midwives, using the Quality Maternal Newborn Care Framework. Women and Birth. 2021.
- 31. Health Synergy and Collaborative Business. Logan Community Maternity and Child Health Hubs Cost Analysis. Brisbane: Synergy Health and Business Collaborative; 2021.
- 35. Markesjö G. Women’s experience of pregnancy and childbirth in a continuity of care model. A qualitative study at Huddinge Hospital. Stockholm: Karolinska Institute; 2019.
- 36. Holroyd M. Midwives experiences of working within the project “Min Barnmorska”. Stockholm: Karolinska Institute; 2019.
- 63. Hailemeskel S, Alemu K, Christensson K, Tesfahun E, Lindgren H. Midwife-led continuity of care improved maternal and neonatal health outcomes in north Shoa zone, Amhara regional state, Ethiopia: A quasi-experimental study. Women and Birth. 2021.
- 70. Rahman S. Midwife led Care Centre in a Government Facility: Charikata Union Health and Family Welfare Centre (UH&FWC), Jaintiapur, Sylhet. 2021 Wednesday, Apr 20, 2022.
- 85. Baird K, Hastie C, Stanton P, Gamble J. Learning to be a midwife: Midwifery students’ experiences of an extended placement within a midwifery group practice. Women and Birth. 2021.
- 94. Newton M, Faulks F, Bailey C, Davis J, Vermeulen M, Tremayne A, et al. Continuity of care experiences: A national cross-sectional survey exploring the views and experiences of Australian students and academics. Women and Birth. 2021.
- 99. Tickle N, Gamble J, Creedy D. Clinical outcomes for women who had continuity of care experiences with midwifery students. Women and Birth. 2021.
- 106. UNFPA. State of the World’s Midwifery New York: United Nations Population Fund; 2021.
- 107. World Health Organization. Strengthening quality midwifery education for Universal Health Coverage 2030: framework for action. Geneva: World Health Organization; 2019.
- 108. Australian Institute of Health and Welfare. Maternity Care Classification System: Maternity Model of Care Data Set Specification national pilot report November 2014. Canberra: Australian Institute of Health and Welfare; 2014.
Midwife-led birthing centres in four countries: a case study
- Open access
- Published: 17 October 2023
- Volume 23 , article number 1105 , ( 2023 )
Cite this article
You have full access to this open access article
- Oliva Bazirete 1 , 2 ,
- Kirsty Hughes 2 ,
- Sofia Castro Lopes 3 ,
- Sabera Turkmani 4 ,
- Abu Sayeed Abdullah 5 ,
- Tasleem Ayaz 6 ,
- Sheila E. Clow 7 ,
- Joshua Epuitai 8 ,
- Abdul Halim 5 ,
- Zainab Khawaja 9 ,
- Scovia Nalugo Mbalinda 10 ,
- Karin Minnie 11 ,
- Rose Chalo Nabirye 8 ,
- Razia Naveed 9 ,
- Faith Nawagi 10 ,
- Fazlur Rahman 5 ,
- Saad Ibrahim Rasheed 9 ,
- Hania Rehman 9 ,
- Andrea Nove 2 ,
- Mandy Forrester 12 ,
- Shree Mandke 12 ,
- Sally Pairman 12 &
- Caroline S. E. Homer 4
1957 Accesses
2 Altmetric
Explore all metrics
Midwives are essential providers of primary health care and can play a major role in the provision of health care that can save lives and improve sexual, reproductive, maternal, newborn and adolescent health outcomes. One way for midwives to deliver care is through midwife-led birth centres (MLBCs). Most of the evidence on MLBCs is from high-income countries but the opportunity for impact of MLBCs in low- and middle-income countries (LMICs) could be significant as this is where most maternal and newborn deaths occur. The aim of this study is to explore MLBCs in four low-to-middle income countries, specifically to understand what is needed for a successful MLBC.
A descriptive case study design was employed in 4 sites in each of four countries: Bangladesh, Pakistan, South Africa and Uganda. We used an Appreciative Inquiry approach, informed by a network of care framework. Key informant interviews were conducted with 77 MLBC clients and 33 health service leaders and senior policymakers. Fifteen focus group discussions were used to collect data from 100 midwives and other MLBC staff.
Key enablers to a successful MLBC were: (i) having an effective financing model (ii) providing quality midwifery care that is recognised by the community (iii) having interdisciplinary and interfacility collaboration, coordination and functional referral systems, and (iv) ensuring supportive and enabling leadership and governance at all levels.
The findings of this study have significant implications for improving maternal and neonatal health outcomes, strengthening healthcare systems, and promoting the role of midwives in LMICs. Understanding factors for success can contribute to inform policies and decision making as well as design tailored maternal and newborn health programmes that can more effectively support midwives and respond to population needs. At an international level, it can contribute to shape guidelines and strengthen the midwifery profession in different settings.
Similar content being viewed by others

Reasons for home delivery and use of traditional birth attendants in rural Zambia: a qualitative study
Cephas Sialubanje, Karlijn Massar, … Robert AC Ruiter
Iranian midwives’ lived experiences of providing continuous midwife-led intrapartum care: a qualitative study
Leila Amiri-Farahani, Maryam Gharacheh, … Sally Pezaro
Why do women not deliver in health facilities: a qualitative study of the community perspectives in south central Ethiopia?
Meselech Assegid Roro, Emebet Mahmoud Hassen, … Mesganaw Fantahun Afework
Access to quality sexual, reproductive, maternal, newborn and adolescent health care is essential for countries to achieve universal health coverage (UHC). Midwives are key in this endeavour. Midwives have the potential to impact maternal and newborn health outcomes, if they are adequately educated, deployed and supported in an enabling work environment. Universal access to midwife-delivered interventions could prevent two-thirds of the world’s maternal and newborn deaths and stillbirths [ 1 ].
Midwife-led birthing centres (MLBCs) are an option in many countries [ 2 ]. MLBCs are usually provided at a primary care level, have a dedicated space providing childbirth care where midwives are the lead professionals and have arrangements for consultation and referral to secondary and tertiary-level services if needed. Some MLBCs are close to, or an integral part of, a maternity or general hospital (called “alongside” or “on-site” centres) while others are situated apart from hospitals (called “stand-alone” or “freestanding” centres).
MLBCs that are appropriately integrated into the health system have been found to improve maternal and neonatal outcomes by providing woman-centred, high-quality, cost-effective, and safe care [ 3 , 4 , 5 , 6 , 7 , 8 ]. A recent Cochrane review examined 15 trials with 17,674 women from high-income countries and found that midwife-led continuity of care offers various favourable outcomes for women when compared to other care models [ 9 ].
Most of the evidence on MLBCs is from high-income countries, but the impact of MLBCs in low- and middle-income countries (LMICs) could be significant as this is where the vast majority of maternal and newborn mortality and morbidity occurs [ 10 ]. However, we cannot assume that research findings from HICs can be generalised to other settings. For example, MLBC clients in HICs tend to belong to more wealthy demographics, [ 3 ] whereas in many low- and middle-income countries (LMICs), MLBCs primarily cater to clients from disadvantaged and marginalized communities [ 11 ]. The weak referral systems evident in many LMICs may compromise the safety of women and newborns who use MLBCs, especially if the MLBC is a freestanding one (the most prevalent type in LMICs [ 11 ]), whereas in HICs the evidence indicates better outcomes in freestanding MLBCs [ 3 ]. Further, in low-income nations, MLBC services are predominantly offered by private, not-for-profit entities. Consequently, the accessibility of care for clients from underprivileged and marginalized backgrounds heavily relies on sustained support from these sources [ 11 ].
Our previous work has shown that MLBCs exist in at least 24 LMICs [ 11 ]. Much of the evidence about these MLBCs was collected in a survey and there was limited information in the peer reviewed literature, especially understanding how the MLBCs were successful. The aim of this study therefore, was to explore MLBCs in four low- and middle income countries, specifically to understand what is needed for a successful MLBC.
Study design
A descriptive study with four case study countries was undertaken using an Appreciative Inquiry approach [ 12 ]. Appreciative Inquiry is a participatory technique designed to initiate a positive conversation about experiences, needs, and proposed solutions [ 13 , 14 ]. The study was guided by a networks of care (NOC) framework with four domains: agreement and enabling environment, operational standards, quality/efficiency/responsibility, and learning and adaptation. The domains include access, quality of care, financing, community buy in, referral systems, supply and resources of health services [ 15 ]. The NOC framework guided site selection, data collection approaches and the initial analytic processes. The project established an expert advisory group (EAG) that included experts in MLBCs from high-, middle- and low-income contexts and representatives of the International Confederation of Midwives (ICM), World Health Organization (WHO), United Nations Population Fund (UNFPA), Bill and Melinda Gates Foundation and World Bank. This EAG met three times during the project to provide advice and guidance.
Prior to commencing the study, human research ethics approval was received through the Alfred Hospital HREC in Australia and from a relevant ethics committee within each of the four countries.
Country and site selection
Country selection was informed by a literature review and scoping survey, [ 11 , 16 ] and consultation with ICM and the EAG. The main inclusion criteria were: (a) the country was classed by the World Bank in 2022 as low-, lower-middle, or upper-middle income, (b) there was evidence from the literature and the survey that the country had at least four MLBCs that were either in the public sector or well-integrated within the national health system, (c) good research capacity within the country, and (d) data was available for an economic analysis (to be reported separately). Each country was invited to participate through the national Ministry of Health (MoH) with the ICM member association (MA).
Four study sites were selected in each country based on a desk review of the literature and in consultation with the MoH, the MA, the national research team, the site manager(s) and other relevant stakeholders (Table 1 ). To be counted as an MLBC, a facility had to be a dedicated space providing childbirth care where midwives were the lead professionals providing care. The midwives were those recognised in each country as midwives. The MLBCs may also have other staff including nurses, lay health workers, assistants-in-midwifery, laboratory technician and pharmacy staff.
Study participants, recruitment and data collection
Three groups of informants were purposively selected in each country: health service leaders (defined as midwives, doctors, policymakers, or program planners who, within the preceding two years, had held a leadership role within the maternity care system at a national or sub-national level), MLBC staff (92% of whom were midwives) currently working at one of the selected MLBCs (Table S 1 : **Definitions of a midwife by country participating in this study**), and MLBC clients (women aged 18 or over who had planned to give birth in one of the selected MLBCs within the preceding six months, including those who had been transferred to another facility). Leaders were selected in consultation with the MoH and the ICM MA. Staff and clients were nominated by the MLBC managers.
Leaders, staff and clients were interviewed separately. Staff were interviewed in focus group discussions (FGDs), or individually if the MLBC was operated by a single midwife. All interviews with clients and staff were conducted in the most appropriate language for that informant or site. The leader interviews were conducted in the informant’s preferred language. FGDs were conducted in a private location at or close to the MLBC. The FGDs lasted between 60 and 90 min and the individual interviews between 30 and 60 min. The majority of the interviews (105/125; 84%) were conducted in person, with the others on the phone or online using Zoom or MS Teams. All interviews were audio-recorded and transcribed in the original language before being translated into English for analysis. The researchers recorded field notes during and after gathering data in order to document the nonverbal cues and significant details observed from the respondents. The study was conducted from September 2022- April 2023.
A total of 210 informants participated: 34–66 per country (Table 2 ).
Data collection tools were developed by the research team based on a NOC framework [ 15 ] and guided by the Appreciative Inquiry approach [ 14 ]. We did not provide a definition of “successful” for the study participants. Rather, we asked them to describe and explore what was working well; and explored what was valued by the participants, including their vision of the ‘ideal’ situation. These aspects of the Appreciative Inquiry approach provided evidence for what makes a ‘successful’ MLBC from the perspectives of the three participant types.
The country research team had a virtual orientation training with international consultants on data collection using the Appreciative Inquiry approach and the team in each country consisted of members with expertise in qualitative research methodology, maternal health and health care. There was a team of 20 researchers in the countries who undertook the data collection. Of these, 15 were women, and 5 were men and all were nationals in the individual country.
Data analysis
Data analysis was undertaken concurrently with data collection and was initiated after the completion of the first interviews. The translated transcripts were read by a team of four analysts (all women: OB, KH, SCL and ST) who used NVivo software to help organize the data for further analysis. Data were initially categorized under the 4 overarching themes represented in the NOC framework: 1) agreement and enabling environment, 2) operational standards, 3) quality, efficiency and responsibility, 4) learning and adaptation [ 15 ]. The next step interrogated the initial analysis asking: (i) What are the enablers, i.e. factors that make MLBCs successful (as defined by individual informants)? (ii) What are the main challenges facing MLBCs? (iii) Do these enablers and challenges vary according to MLBC type (freestanding vs alongside/onsite) and sector (private vs public)? We identified the themes from each of these questions by country and by participant group using an inductive approach and then searched for commonalities and differences. These findings were presented back to the country teams who had collected the data to ensure that the interpretation was sound. Revisions were made and further cross-checking and refinement took place. We then combined the findings across the matrix of country and participant group to produce the synthesized results.
The results are presented initially by context and then what is needed for a successful MLBC. The wider analysis also included the key barriers facing MLBCs and differences between private/public and freestanding/alongside MLBCs (Table S 2 : **Barriers facing by MLBCs and key differences between different types of MLBCs**).
The four selected countries have different strengths and challenges in relation to maternal and newborn health indicators as indicated by Table S 3 (Table S3** Key maternal and newborn health statistics for the four countries**).
What is needed for a successful MLBC?
Although the findings varied by country, four universal themes described the enabling factors influencing successful MLBCs as illustrated by Fig. 1 . These were: (1) an effective financing model (2) quality midwifery care that is recognised by the community (3) interdisciplinary and interfacility collaboration, coordination and functional referral systems, and (4) supportive and enabling leadership and governance at all levels.
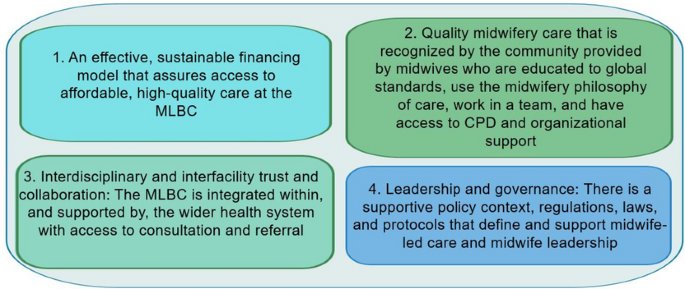
Facilitators of success for midwife-led birthing centres
An effective financing model
A number of different financing models for MLBCs services were identified. These included external funding model (non-governmental organizations (NGOs), donors, faith-based organizations, among others), governmental funding model and a combination of two or more funding models. All four countries had external funding models with funders including UN agencies and NGOs, but few South African MLBCs have external funding. Partnerships with these external funders helped to make childbirth care affordable at MLBCs and provided support through donations, as seen in Bangladesh, Pakistan and Uganda. These partnerships improved service utilisation as seen in Bangladesh:
“Mostly people from the neighbourhood areas come here. They only need to pay for transportation … several NGOs are providing incentives. In that case, a mother is given BDT 500 for a normal delivery. The medication is available here ... It doesn’t cost much. Incentives are supported by UNFPA and [specific programmes], sometimes helps with referrals.” (Staff Bangladesh)
Sometimes, however, the support was limited and staff had to use their own funds:
“NGOs provided us with a painted delivery room … I spent my own money to renovate windows, walls, and doors. They [NGOs] do not understand that we are working without any facilities on a low income.” (Staff Pakistan)
In South Africa, public sector health care included public MLBCs, where pregnant women and children under the age of 6 years are entitled to free health care at the point of care. Government support was also observed in the public health sector in Bangladesh and Pakistan where the government provides infrastructure for MLBCs and the public MLBCs offer free healthcare services. Free services promoted accessibility:
“I will come here again. I like it here; their way of communicating is very good. I didn’t have to pay anything here; everything is done here for free ... Since it is free and the government pays the expenses here, my relatives will come here happily.” (User Bangladesh)
“Mostly our clients are poor and can’t access private services. So they come here… normal delivery is easily done here, and every medicine is free” (Staff Pakistan).
In Uganda, where the selected MLBCs are all private (some for-profit and some not for profit), priority is given to providing health care to the service user, with payment being made later or some initiative to substitute the cost of care, for example, work in exchange. Two staff members explained:
“ … you do not know if she has money or if she doesn’t have money. So you have to first deliver then after she can tell you “I will send the money”.” (Staff Uganda)
“We ask that people either give about five thousand shillings if they can but it is not required or that they can we have some work exchange. People can come and work for a bit and do digging or different things.” (Staff Uganda)
Different financial models according to the context of the country and flexibility were important to ensure that service users have access to necessary health care without facing financial hardship.
Quality midwifery care that is recognised by the community
Successful MLBCs were those recognised by the community as providing quality care in a respectful way. Respect and maintenance of confidentiality for women accessing the MLBC and being treated with dignity and compassion were all important. Midwives in the MLBCs supported physiological labour and birth, catering to the women’s cultural and language needs, establishing strong relationships and ensuring continuity of care. Quality midwifery care included being provided with accurate and unbiased information about their options, being enabled to make informed decisions about their care, and being supported in their choices. Service users explained:
“…someone understands that you are in pain and they comfort you knowing that if she distances herself from you, you can even die. That love that she is there with you at every point, taking care of you and when you say that I have failed here she is there with you. When you need something she is there with you 24/7. She cares about you and wants your baby to be born well, that nothing happens but for the wellbeing of you and your baby.” (User Uganda)
“She [the midwife] never scolded me or anything like that. She treated me with love and respect. She always answered any question I had. She gave me reassurance and told me that I can call for her whenever I need help.” (User Pakistan)
The examples from Bangladesh and South Africa highlight the value that women placed on receiving care that was respectful, supported physiological labour and birth and reduced the risk of unnecessary interventions:
“The service providers helped me by respecting my decision to have a normal delivery. I may need something that tells them what they need to look at carefully. In terms of language, there was no difference between them, whether they were poor or rich. Everyone is treated equally.” (User Bangladesh)
“I wanted proper care for me and my child. I knew early that I wanted to give birth naturally and I knew that my chances for natural birth would be more reduced when going to a doctor [at a hospital] than going to a midwife [at an MLBC].” (User South Africa).
This support of physiology was not at the expense of a healthy baby: “… She [the midwife] cares about you and wants your baby to be born well, that nothing happens but for the wellbeing of you and your baby” (User Uganda).
Midwives in MLBCs ensured that women felt welcomed and supported. The staff and leaders recognised that this built trust and was important:
“Patients trust this [MLBC] as we provide good service, take care of their privacy and their self-respect as well.” (Staff Pakistan)
“First of all, respect for maternity care is encouraging for [MLBCs]. …When mothers come to these places and see that they can open up, get respect, and give birth the way they want, they gain confidence.” (Leader Bangladesh)
In South Africa, the involvement of partners and family members during childbirth was highly valued by some MLBC users. They said that midwives accommodated family members and this partner involvement increased awareness of the MLBC:
“ They [the MLBC] were very accommodating, because initially I thought that I am going to give birth with my husband there and then my two sisters came along and then they were very accommodating and then they also involved them throughout like from the labour process and then also assistance …”(User South Africa)
Cultural and linguistic sensitivity were critical factors in the success of MLBCs. For instance, in Pakistan, service users highlighted that midwives acknowledged and respected the cultural values, beliefs, and practices of the communities they served. The midwives encouraged open communication with the women in their care, which allowed for the sharing of cultural practices and beliefs. Many women from Pakistan felt that the midwives in the MLBC respected their privacy, observed purdah (female seclusion) requirements, and communicated with them in their preferred language:
“We are Baravi speakers. She guides me in Baravi language. She was concerned about my privacy [so] there were no male members in her birth station.” (User Pakistan ).
“She took care of our purdah during check-ups and during childbirth as well.” (User Pakistan ).
Building a good relationship with MLBC users was important to provide quality midwifery services and the evidence to relationship is built on trust between midwives and community and on word of mouth:
“ I think it's the relationship between the client and the midwife and the respectful and kind care that is provided by the midwife. It also depends on the feedback that you receive from other people like your sister or your relatives and a lot of community’s trust is built in that way. So I think the [MLBCs] are affordable, acceptable and provide clean quality care and then there are the sources of comfort and respect ”. (Leader-Pakistan)
“What I found very interesting is that the men/partners become the biggest advocates of midwife-led care or birth centres. They are the ones that then tell their friends, listen you know that this is a good place to give birth.” ( Staff South Africa)
Midwives identified that different aspects of quality of care were key to success, including care across the continuum, and providing remote services. Many MLBCs – especially the freestanding ones—also provided antenatal care. For example:
“ that relationship begins from antenatal … it can begin when we go to the community and we talk about the availability of this facility [the MLBC], so the relationship begins. And then when they come, the way we talk to them, the way when we are examining them, touching them and talking to them as a family, husband and wife … that puts us together.” (Staff Uganda)
“The tasks here are the antenatal care (ANC) check-ups of pregnant mothers, ensuring four visits and quality control of the visits. They also provide remote ANC service through mobile. Many who do not come are called and brought for treatment. Also, the check-ups and various investigations at ANC are done in the hospital itself .” (Staff Bangladesh)
After birth, MLBCs users were also encouraged to attend scheduled postpartum appointments. In some MLBCs, users were provided with cards to contact the MLBC if any complication arises after discharge:
“ They gave us a card while we were going home from here. A contact number was given on that card so that I could contact them if any complications arose. ” (User Bangladesh).
“… she [the midwife] was already planning to come the following day, but she said if anything concerns you in this next 24 hours you can just give me a call you know I can come to the house.” (User South Africa).
Respectful care is a key contributor to success but is still a challenge to achieve, especially when there are midwife shortages. In some settings, clients experienced long wait times, inadequate communication, and lack of timely access to necessary care, especially for the referrals which did not always take place in a timely manner. To address this and ensure success, supportive counselling sessions were organised for midwives at MLBCs in South Africa:
“We used to have a psychologist … because … to be a midwife sometimes can be very draining because they have been long in labour and sometimes you must look after two patients and you don’t have patience all the time because we are also human, you also coming with the baggage so that it came in that the leader must also look at the staff as a person.” (Staff-South Africa)
A key to a successful MLBC was the recognition that the centre provided quality care. Midwives were seen as integral to this success but needed access to ongoing education to ensure they were up-to-date with knowledge and skills. In service training was described as an essential component of ensuring that midwives at MLBCs have the knowledge, skills and attitudes necessary to provide high-quality care. Medical doctors, UN agencies and NGOs provided training that included refresher training, neonatal resuscitation and ultrasound:
“Many NGOs are providing training to fill gaps. NGOs include UNICEF, UNFPA. UNICEF provides us with a wide range of logistical support.” (Leader Bangladesh)
“In three months, basically we get a refresher on different subjects, its normally about the staff need" [referring to Essential Steps in Managing Obstetric Emergencies, Helping Babies Breathe training].” (Staff South Africa)
“We went to Karachi and attended ultrasound training and that was very good. Everyone from here went there one by one from training and that was good. Our knowledge increases from that training.” (Staff Pakistan)
Interdisciplinary and interfacility collaboration, coordination and functional referral systems
Collaboration, coordination and functional referral systems are needed for successful MLBCs. While the MLBC midwives are the lead professionals, collaboration with obstetricians, nurses, and other support staff and working as a wider team were important. “ I would say the common goal that we are having, no mother and child should die. Teamwork is key amongst all of us the midwife fraternity [sic].” (Leader Uganda). This sense of collaboration and teamwork was also expressed in South Africa and Pakistan:
“Yes I want to say what makes my day is everyone getting along and more patients giving birth and it is smooth, happy team work day, everyone is smiling, there is no fighting … It can get very hot and steamy in the labour ward, and … I do find that we all have good relationships with each other under those situations and we can still make good decisions now walking away from that knowing that you did the best with what we had.” (Staff South Africa)
“…We deal with everything together. ….. Our doctors are also very cooperative.” (Staff Pakistan).
Good coordination included flexibility in how the service is organised and enabled continuity of care:
”I liked that idea that even if my midwife was unavailable, there were back up ones that I could meet ahead of time and get to know a little bit about care, yeah just bit more of personal care.” (User South Africa)
Staff also valued being able to organise and coordinate their own work:
“That depends on us [how] we have to divide it [the work] shift wise. Sometimes we divide the burden in the shifts so that not all the burden is on the first shift. So we have to make those policies ourselves. We don’t have any pressure from our admin or head office. This is the work we do on our own with their cooperation and there isn’t any more change needed in that.” (Staff Pakistan)
A well-functioning referral system was critical for successful MLBCs to ensure that, when needed, women received appropriate and timely care at a referral hospital with advanced care. The referral system was facilitated by effective communication and coordination from MLBCs to referral hospitals. Staff and leaders explained:
“So, from up to down, we have free communication, policies and protocols that determine which patient is where, and they are appropriately referred and referred back again.” (Leader South Africa)
“There were identified sites where we would refer the patients and you ring the doctor before you send the patient. So, even if it’s like APH [Antepartum haemorrhage], PPH [Postpartum haemorrhage], now the doctor would say do a,b,c,d as you are coming with this patient and that was really beautiful. So having connections of where to refer, so that by the time the patient reaches there, there is somebody waiting for the patient.” (Staff Uganda)
“I like working here in an [MLBC] which is adjacent to the main labour ward because if there is any complication there are those intrapartum emergencies like shoulder dystocia, PPH, uterine inversion, so you are just close to labour ward of the hospital for further management.” (Staff South Africa)
“What I liked about this clinic is that they are able to quickly see the problem if you have it, then when they see that it is not theirs and they will not be able to help it, they are able to refer you to higher people who will be able to help you.” (User South Africa)
Some sites ensured there was feedback about the transfer or systems where staff keep in touch via phone with service users. For example in Bangladesh, leaders found this feedback important in order to ensure that service users get enough support to maintain health:
“...the patient is then transported by us to another health facility with a midwife. Even after the referral, we keep in touch with the mother every day through her mobile phone.” (Leader Bangladesh).
Supportive and enabling leadership and governance at all levels
Effective leadership and governance systems enabled successful MLBCs. Governance mechanisms in different forms were described and included policy, governmental support and recognition, coordination meetings, support for continues professional development and monitoring and evaluation to ensure the MLBCs function and are effective. Government support to MLBCs was seen as a contributing factor to quality of care and hence reduction in maternal and child mortality rates:
“The government supports this service led by midwives. That is why the government has created separate midwives for maternal and child health and is providing full cooperation. They are providing as much support as needed. The main objective of the government is to reduce maternal and child mortality rates and increase institutional deliveries. It is for these reasons that the government is supporting midwives.” (Staff Bangladesh)
“Well, we get support from the government in the sense that they recognize our practice, and we can transfer patients to [institution names].” (Staff South Africa)
“The district is very supportive. You can’t run this type of, you know, activity without support from the district and the community.” (Staff Uganda)
“[MLBCs] have developed good setups and made a good network but it all depends on how much support they got. It depends on how your seniors, supervisor, NGO or the government held your hand. So that is very important. Once they are backed up, they can do wonders.” (Leader Pakistan)
Coordination meetings and regular supervision involving MLBCs brought together stakeholders to discuss improving the quality and accessibility of MLBC services. Coordination meetings enabled MLBC staff, government officials, healthcare providers, community leaders and other stakeholders to share information and experiences and contribute to effective functioning:
“We have quarterly meetings … to review the performance of each facility. We have also monthly meetings for the in-charges to review our performance and then we also do quarterly supervision and we have tools that guide us that we use to assess the quality-of-care services. Then we also have, they also submit reports to the district and we enter them into our system.” (Leader Uganda)
“There was a monthly perinatal meeting and later there was ESMOE [Essential Steps in Managing Obstetric Emergencies]. So, I suppose I then also got to know how they function and then later on [community obstetrician] kind of really coordinated the [MLBCs] in Cape Town and he set up a meeting called MOUSE [Midwife Obstetric Units Support Executive].” (Leader South Africa)
It was also highlighted by staff in South Africa that availability of guidelines and protocols at the disposal of midwives facilitate in decision-making for transfer. Leaders in Pakistan also had guidelines and frameworks but these were not applied in private MLBCs:
“We do have written guidelines and protocols in labour ward and in the antenatal clinic that we follow especially when it comes to transfers, what to transfer, so we work according to the protocol.” [Staff South Africa]
“In the infrastructure [MLBC], there should be midwifery led policies rather than medical policies. There should be a midwifery led service framework and the policies should be according to it. So that women can get midwifery services rather than medical services.” (Leader Pakistan)
Effective leadership and governance play a crucial role in enabling the timely and accurate reporting of data, ensuring evidence-based services at MLBCs. The establishment of reliable monitoring and evaluation mechanisms at MLBCs is an essential element for their successful operation and impact.:
“Evidence is mainly maintained by register books. We maintain it step by step from entry to discharge ... Thus, evidence-based services are ensured. We have been given a register of maternity services from the government. We fill up the form as given there.” (Leader Bangladesh)
“ In the government centres, the data is present in two ways. Firstly it is present in the paper form where there are separate registers for antenatal patients, delivery and for postnatal. Now in the government centers of Punjab, a new system of registration called EMR has started, which is known as Electronic Medical Reporting. Through it, every patient’s registration is being done through an application.” (Staff Pakistan)
“We report to the district every month. They have a tool that they give us but then we also keep our own private records. So we record with, you know, we have our paper charts that we have on each client and then we have someone who takes those paper charts and puts them into a larger book and then we take that book once a month and we digitize it.” (Staff Uganda)
The need to accelerate progress on maternal and newborn health and wellbeing is widely recognised. Action is required to increase the availability and accessibility of maternal and newborn health care, and also to improve quality of care (including respectful care). Expanding access to MLBCs is one means of achieving these objectives. Prior to this study, most of the evidence about MLBCs came from high-income countries, and the extent to which conclusions based on this evidence were transferable to LMICs was not clear. This study in four LMICs demonstrates that MLBCs are a feasible and acceptable model of care in a wide range of settings. The findings highlight that leadership support, availability of adequate resources, and workforce development as potential factors contributing to the successful implementation of MLBCs in LMICs.
Scaling-up the MLBC model of care to reach more women in more countries requires attention to be paid to the enablers identified in this study. High level themes describing enablers are: (i) having an effective financing model (ii) providing quality midwifery care that is recognised by the community (iii) having interdisciplinary and interfacility collaboration, coordination and functional referral systems, and (iv) ensuring supportive and enabling leadership and governance at all levels. With these enablers in place, the potential for MLBCs to contribute to improved maternal and newborn health and wellbeing is enormous. Our findings are discussed in view of evidence from both LMICs and HICs (high-income countries).
There are strong parallels between the findings of this study and recently published research from LMICs about enablers to the implementation of midwife-led care in LMICs, which highlighted the importance of funding, community accessibility and trust, quality of care (including respectful care), leadership and collaboration [ 17 , 18 , 19 , 20 ]. Our findings suggest that financing is crucial for the sustainability and accessibility of midwifery care services. When there is a stable and sufficient funding mechanism in place, MLBCs can maintain their operations, invest in necessary resources, and provide quality care to expectant mothers. Adequate financing can also contribute to improving infrastructure, equipment, and training opportunities for midwives, which, in turn, enhances the overall quality of care. Studies have shown that various financing models can support midwife-led care, including government funding, private insurance coverage, and community support. In a scoping review exploring network of care in implementing MLBCs in LMICs, strategies to make MLBC services affordable were identified: such as establishment of a health insurance scheme and incentivised services or fee waivers for maternity services to increase utilization. Communities utilise MLBC services more when the services were either free or subsidised through an enabling environment that included health insurance, incentives, and donor support [ 16 ].
Our results indicate that community recognition and acceptance of midwifery care are essential for the success of midwife-led birthing centres. When the community values and trusts the expertise of midwives, more women and families will opt for midwifery care during childbirth. This can lead to increased demand for MLBCs, resulting in better outcomes for mothers and babies. Our study also highlights that midwives in the MLBCs support physiological labour and birth, catering to the women’s cultural and language needs, establishing strong relationships and ensuring continuity of care MLBCs. To achieve community recognition, MLBCs need to prioritize providing high-quality, evidence-based care. This includes offering personalized and comprehensive prenatal, intrapartum, and postpartum care to expectant mothers. Additionally, fostering strong communication and relationships between midwives and their clients can contribute to a positive reputation.
Collaboration and coordination among different healthcare professionals and facilities are vital components of a successful MLBC. MLBCs are likely to be successful if they are integrated within the broader health system [ 18 , 21 ]. The results of our study demonstrate that this does not necessarily mean that the MLBCs must be part of the public sector system; they can be private, NGO-run or a combination. Integration can be achieved across different sectors if the MLBCs are part of a well-functioning network of care. In settings with low capacity in the public sector, it is important to pay attention to how such networks can be built and/or strengthened in order to facilitate the expansion of access to high quality MLBCs.
The sites we included were those that provided care during labour and birth. Most of the included sites also provided other maternal and newborn health care services such as antenatal and postnatal care. Although these other elements of care were not the main focus of this study, evidence from other studies indicates that midwife-led continuity of care models providing care across the continuum have numerous benefits over other models of care [ 9 , 22 , 23 , 24 , 25 , 26 ]. Efforts to expand access to high-quality MLBCs should take into consideration the potential benefits of ensuring that care is offered across the full continuum of care where feasible and appropriate.
Our study findings also align with similar studies in HICs [ 27 , 28 , 29 , 30 ] and the frameworks and standards that have been developed based on those studies [ 31 , 32 , 33 ]. For example, the following factors have been identified as important in a range of settings as well as in this study: an enabling policy environment including adequate financial and human resourcing, [ 28 , 29 , 30 , 31 , 32 , 33 ] mainstreaming MLBCs as a core element of the service rather than as an ‘optional extra’, [ 27 , 28 , 30 ] strong and inspirational midwifery leadership, [ 27 , 28 , 30 ] education and training programmes that prepare midwives with the competence and confidence to support physiological birth and make decisions independently, [ 28 , 30 ] interdisciplinary and interfacility collaboration built on relationships of trust and respect [ 28 , 30 ]. Supportive leadership helps create a positive work environment for midwives, encouraging their professional development and job satisfaction. It also ensures that the MLBC operates efficiently, adheres to best practices, and maintains a focus on providing quality care.
The findings of this study have significant implications for improving maternal and neonatal health outcomes, strengthening healthcare systems, and promoting the role of midwives in LMICs. We have identified key factors that enable the successful establishment and operation of MLBCs. Understanding these factors can contribute to inform policies and decision making as well as design tailored maternal and newborn health programmes that can more effectively support midwives and respond to population needs. At an international level, it can contribute to shape guidelines and strengthen the midwifery profession in different settings.
Strengths and limitations
One of the strengths of our study is its approach to triangulation used to obtain credible information on enablers for the establishment and running of MLBCs in LMICs. The use of multiple data collection methods (KIIs and FGDs) with different groups of informants – including women who have used MLBC services—to corroborate findings increased the validity and reliability of the study's findings. The involvement of a team of four data analysts and the data validation from the four country teams was also a strength to ensure rigour in data analysis and increase the credibility of the study. However, there was no systematic attempt to conduct repeat interviews or ask participants to validate the interview transcripts, which may have adversely affected the richness of the data.
Methodological limitations occur often in qualitative study involving multiple sites in multiple countries with different cultural contexts and languages. Differences in the interpretation of questions or cultural norms may affect the quality and consistency of the data collected. To counteract this, each country team of researchers underwent training on the research approach and methods used. The country teams identified experienced translators fluent in the appropriate languages to translate both interview guides and transcripts.
We included MLBCs that had midwives as recognised in their country. We acknowledge that there are a range of midwives including community midwives and nurse-midwives and not all may meet the definition of a midwife according to the ICM [ 34 ]. This is likely in many countries who are transitioning to having midwives educated according to ICM standards, but it will take some countries many years to ensure all their MLBC staff fit this definition. Despite this, these health workers were working in the capacity of midwives providing care within a midwifery model of care and it is very encouraging to see the positive impact they were having in their communities.
We did not systematically include the perspective of other leaders including midwives' associations and regulatory bodies who may have a crucial role in promoting the safety and quality of care in MLBCs. Therefore, future research should explore the perspectives and experiences of midwives' associations and regulatory bodies in supporting MLBCs. This will provide a more comprehensive understanding of the factors that contribute to the success of MLBCs and inform policy and practice in this area.
The aim of this study was to explore MLBCs in four low- and middle-income countries, specifically to understand what is needed for a successful MLBC. We have shown that the key elements of success include having an effective financing model; providing quality midwifery care that is recognised by the community; having interdisciplinary collaboration, coordination and functional referral systems, and ensuring supportive and enabling leadership and governance at all levels. MLBCs are one way to deliver midwifery interventions which have been shown to reduce maternal and newborn deaths and stillbirths. Understanding factors for success can contribute to inform policies and decision making as well as design tailored maternal and newborn health programmes that can more effectively support midwives and respond to population needs. The next step is to scale up MLBCs while collecting evidence on the effectiveness of this model of care on the outcomes for women, babies, midwives and the health system.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due to the risk of compromising participants’ confidentiality, but are available from the corresponding author on reasonable request.
Nove A, Friberg IK, de Bernis L, McConville F, Moran AC, Najjemba M, Ten Hoope-Bender P, Tracy S, Homer CSE. Potential impact of midwives in preventing and reducing maternal and neonatal mortality and stillbirths: a Lives Saved Tool modelling study. Lancet Glob Health. 2021;9(1):e24–32.
Article CAS PubMed Google Scholar
Stevens JR, Alonso C. Commentary: Creating a definition for global midwifery centers. Midwifery. 2020;85:102684.
Article PubMed Google Scholar
Brocklehurst P, Hardy P, Hollowell J, Linsell L, Macfarlane A, McCourt C, Marlow N, Miller A, Newburn M, Petrou S, et al. Perinatal and maternal outcomes by planned place of birth for healthy women with low risk pregnancies: the Birthplace in England national prospective cohort study. BMJ. 2011;343:d7400.
Homer CSE, Cheah SL, Rossiter C, Dahlen HG, Ellwood D, Foureur MJ, Forster DA, McLachlan HL, Oats JJN, Sibbritt D, et al. Maternal and perinatal outcomes by planned place of birth in Australia 2000–2012: a linked population data study. BMJ Open. 2019;9(10):e029192.
Article PubMed PubMed Central Google Scholar
Merz WM, Tascon-Padron L, Puth MT, Heep A, Tietjen SL, Schmid M, Gembruch U. Maternal and neonatal outcome of births planned in alongside midwifery units: a cohort study from a tertiary center in Germany. BMC Pregnancy Childbirth. 2020;20(1):267.
Overgaard C, Moller AM, Fenger-Gron M, Knudsen LB, Sandall J. Freestanding midwifery unit versus obstetric unit: a matched cohort study of outcomes in low-risk women. BMJ Open. 2011;1(2):e000262.
Scarf VL, Rossiter C, Vedam S, Dahlen HG, Ellwood D, Forster D, Foureur MJ, McLachlan H, Oats J, Sibbritt D, et al. Maternal and perinatal outcomes by planned place of birth among women with low-risk pregnancies in high-income countries: A systematic review and meta-analysis. Midwifery. 2018;62:240–55.
Stapleton SR, Osborne C, Illuzzi J. Outcomes of care in birth centers: demonstration of a durable model. J Midwifery Womens Health. 2013;58(1):3–14.
Sandall J, Soltani H, Gates S, Shennan A, Devane D. Midwife-led continuity models versus other models of care for childbearing women. Cochrane Database Syst Rev. 2016;4:CD004667.
PubMed Google Scholar
Betrán AP, Temmerman M, Kingdon C, Mohiddin A, Opiyo N, Torloni MR, Zhang J, Musana O, Wanyonyi SZ, Gülmezoglu AM, et al. Interventions to reduce unnecessary caesarean sections in healthy women and babies. Lancet. 2018;392(10155):1358–68.
Nove A, Bazirete O, Hughes K, Turkmani S, Callander E, Scarf V, Forrester M, Mandke S, Pairman S, Homer CS. Which low- and middle-income countries have midwife-led birthing centres and what are the main characteristics of these centres? A scoping review and scoping survey. Midwifery. 2023;123:103717.
Crowe S, Cresswell K, Robertson A, Huby G, Avery A, Sheikh A. The case study approach. BMC Med Res Methodol. 2011;11:100.
Reed J. Appreciative inquiry. Thousand Oaks: Sage; 2007.
Book Google Scholar
Trajkovski S, Schmied V, Vickers M, Jackson D. Implementing the 4D cycle of appreciative inquiry in health care: a methodological review. J Adv Nurs. 2013;69(6):1224–34.
Carmone AE, Kalaris K, Leydon N, Sirivansanti N, Smith JM, Storey A, Malata A. Developing a common understanding of networks of care through a scoping study. Health Syst Reform. 2020;6(2):e1810921.
Turkmani S, Nove A, Bazirete O, Hughes K, Pairman S, Callander E, Scarf V, Forrester M, Mandke S, Homer CSE. Exploring networks of care in implementing midwife-led birthing centres in low- and middle-income countries: a scoping review. PLOS Global Public Health. 2023;3(5):e0001936.
Sangy MT, Duaso M, Feeley C, Walker S. Barriers and facilitators to the implementation of midwife-led care for childbearing women in low- and middle-income countries: A mixed-methods systematic review. Midwifery. 2023;122:103696.
Pappu NI, Oberg I, Byrskog U, Raha P, Moni R, Akhtar S, Barua P, Das SR, De S, Jyoti HJ, et al. The commitment to a midwifery centre care model in Bangladesh: An interview study with midwives, educators and students. PLoS One. 2023;18(4):e0271867.
Article CAS PubMed PubMed Central Google Scholar
Stevens JR, Sabin LL, Onyango MA, Sarker M, Declercq E. Midwifery centers as enabled environments for midwifery: A quasi experimental design assessing women’s birth experiences in three models of care in Bangladesh, before and during covid. PLoS One. 2022;17(12):e0278336.
Blomgren J, Gabrielsson S, Erlandsson K, Wagoro MC, Namutebi M, Chimala E, Lindgren H. Maternal health leaders' perceptions of barriers to midwife-led care in Ethiopia, Kenya, Malawi, Somalia, and Uganda. Midwifery 2023: https://doi.org/10.1016/j.midw.2023.103734 .
Vedam S, Stoll K, MacDorman M, Declercq E, Cramer R, Cheyney M, Fisher T, Butt E, Yang YT, Powell KH. Mapping integration of midwives across the United States: Impact on access, equity, and outcomes. PLoS One. 2018;13(2):e0192523.
Bogren M, Jha P, Sharma B, Erlandsson K. Contextual factors influencing the implementation of midwifery-led care units in India. Women Birth 2022: https://doi.org/10.1016/j.wombi.2022.1005.1006 .
Hailemeskel S, Alemu K, Christensson K, Tesfahun E, Lindgren H. Midwife-led continuity of care improved maternal and neonatal health outcomes in north Shoa zone, Amhara regional state, Ethiopia: A quasi-experimental study. Women Birth. 2021. https://doi.org/10.1016/j.wombi.2021.08.008
Long Q, Allanson ER, Pontre J, Tuncalp O, Hofmeyr GJ, Gulmezoglu AM. Onsite midwife-led birth units (OMBUs) for care around the time of childbirth: a systematic review. BMJ Glob Health. 2016;1(2):e000096.
Michel-Schuldt M, McFadden A, Renfrew M, Homer C. The provision of midwife-led care in low- and middle-income countries: an integrative review. Midwifery 2020, 84: https://doi.org/10.1016/j.midw.2020.102659 .
Mortensen B, Lieng M, Diep LM, Lukasse M, Atieh K, Fosse E. Improving maternal and neonatal health by a midwife-led continuity model of care - an observational study in one governmental hospital in Palestine. EClinicalMedicine. 2019;10:84–91.
Batinelli L, McCourt C, Bonciani M, Rocca-Ihenacho L. Implementing midwifery units in a European country: Situational analysis of an Italian case study. Midwifery. 2023;116:103534.
Batinelli L, Thaels E, Leister N, McCourt C, Bonciani M, Rocca-Ihenacho L. What are the strategies for implementing primary care models in maternity? A systematic review on midwifery units. BMC Pregnancy Childbirth. 2022;22(1):123.
Kennedy HP, Balaam MC, Dahlen H, Declercq E, de Jonge A, Downe S, Ellwood D, Homer CSE, Sandall J, Vedam S, et al. The role of midwifery and other international insights for maternity care in the United States: An analysis of four countries. Birth. 2020;47(4):332–45.
Walsh D, Spiby H, McCourt C, Grigg C, Coleby D, Bishop S, Scanlon M, Culley L, Wilkinson J, Pacanowski L, et al. Factors influencing the utilisation of free-standing and alongside midwifery units in England: a qualitative research study. BMJ Open. 2020;10(2):e033895.
Midwifery Unit Network. Midwifery unit standards. 2018. https://www.midwiferyunitnetwork.org/wp-content/uploads/PDFs/LY1309BRO-MUNEt-Standards-PRINT-opt.pdf .
Midwifery Unit Network. MUSA Framework. 2023. https://www.musaframework.org/musa-framework .
Yuill C, Keraudren S, Murphy R, Uddin N, Rocca-Ihenacho L. Developing the midwifery Unit Self-Assessment (MUSA) Framework: A mixed methods study in six European midwifery units. Sex Reprod Healthc. 2023;35:100819.
International Confederation of Midwives. International definition of the midwife. The Hague: ICM, 2017. https://www.internationalmidwives.org/assets/files/definitions-files/2018/06/eng-definition_of_the_midwife-2017.pdf .
Download references
Acknowledgements
The authors would like to thank all of the informants who gave up their time to be interviewed, and all members of the project’s expert advisory group who have provided helpful guidance throughout this study.
We also thank Professor Emily Callander and Dr Vanessa Scarf of University of Technology Sydney, Australia, and Mr Martin Boyce of Novametrics Ltd, UK, who provided input into the selection of case study sites and the demographic data.
The study was funded by a grant from the Bill and Melinda Gates Foundation (award number INV—033046). The funding body was not involved in the study design or writing of this manuscript. CSEH and ST were also supported by an Australian National Health and Medical Research Council Investigator Grant (APP 2016379).
Author information
Authors and affiliations.
College of Medicine and Health, Sciences, University of Rwanda, Kigali, Rwanda
Oliva Bazirete
Novametrics Ltd, Duffield, UK
Oliva Bazirete, Kirsty Hughes & Andrea Nove
Independent Consultant, Cape Town, South Africa
Sofia Castro Lopes
Burnet Institute, Melbourne, Australia
Sabera Turkmani & Caroline S. E. Homer
Centre for Injury Prevention and Research, Bangladesh (CIPRB), Dhaka, Bangladesh
Abu Sayeed Abdullah, Abdul Halim & Fazlur Rahman
Independent Consultant, Islamabad, Pakistan
Tasleem Ayaz
University of Cape Town, Rondebosch, South Africa
Sheila E. Clow
Busitema University, Tororo, Uganda
Joshua Epuitai & Rose Chalo Nabirye
Research & Development Solutions, Islamabad, Pakistan
Zainab Khawaja, Razia Naveed, Saad Ibrahim Rasheed & Hania Rehman
Makerere University, Kampala, Uganda
Scovia Nalugo Mbalinda & Faith Nawagi
University of the Western Cape, Cape Town, South Africa
Karin Minnie
International Confederation of Midwives, The Hague, Netherlands
Mandy Forrester, Shree Mandke & Sally Pairman
You can also search for this author in PubMed Google Scholar
Contributions
“CH, AN, SP, MF and SM conceptualized the paper. KH, CH, OB, ST and AN devised the data collection tools and methods in consultation with SA, SC, AH, ZK, SNM, KM, RCN, and SR. SA, SC, AH, ZK, SNM, KM, RCN, and SR adapted and arranged translation of the tools for use in their respective countries, arranged ethics approval, and conducted and/or supervised the data collection, transcription and translation. Under CH’s guidance, OB, KH, SCL and ST conducted the data analysis and contributed to discussions prior to drafting the results section of this paper. CH made an initial draft of the background section. OB made an initial draft of the methods and results sections. AN made an initial draft of the discussion section. All authors provided substantive comments on the initial draft and approved the final version.”
Corresponding author
Correspondence to Oliva Bazirete .
Ethics declarations
Ethics approval and consent to participate.
The study was conducted according to the guidelines of the Declaration of Helsinki and approval was obtained from the following ethics committees:
• Australia: Alfred Hospital Ethics Committee – ref381/22 (on behalf of the Burnet Institute)
• Bangladesh: the Centre for Injury Prevention and Research, Bangladesh (CIPRB) Institutional Review Board – ref CIPRB/ERC/2022/11.
• Pakistan: Research and Development Solutions (RADS) Institutional Review Board – ref IRB00010843
• South Africa: the University of Cape Town (South Africa) Faculty of Health Sciences Human Research Ethics Committee ref HREC 624/2022
• Uganda: Mulago Hospital Research Ethics Committee – ref MHREC-2022–77
In all four countries, informed consent was obtained from study participants in the relevant language before collection of data.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table s1..
**Definition of a midwife by countries included in the study**.
Additional file 2: Table S2.
**Barriers facing by MLBCs and key differences between different types of MLBCs**.
Additional file 3: Table S3.
** Key maternal and newborn health statistics for the four countries**.
Additional file 4:
Supplementary 4. Interview guiding questions.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Bazirete, O., Hughes, K., Lopes, S.C. et al. Midwife-led birthing centres in four countries: a case study. BMC Health Serv Res 23 , 1105 (2023). https://doi.org/10.1186/s12913-023-10125-2
Download citation
Received : 01 June 2023
Accepted : 06 October 2023
Published : 17 October 2023
DOI : https://doi.org/10.1186/s12913-023-10125-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Midwife led birthing centre
- Low and middle income countries
- South Africa
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 14 March 2020
Quality of intrapartum care: direct observations in a low-resource tertiary hospital
- Natasha Housseine ORCID: orcid.org/0000-0002-1849-7815 1 , 2 , 3 ,
- Marieke C. Punt 1 ,
- Ali Gharib Mohamed 4 ,
- Said Mzee Said 4 ,
- Nanna Maaløe 5 ,
- Nicolaas P. A. Zuithoff 2 ,
- Tarek Meguid 3 , 4 ,
- Arie Franx 1 ,
- Diederick E. Grobbee 2 ,
- Joyce L. Browne 2 &
- Marcus J. Rijken 1 , 2
Reproductive Health volume 17 , Article number: 36 ( 2020 ) Cite this article
7712 Accesses
19 Citations
6 Altmetric
Metrics details
The majority of the world’s perinatal deaths occur in low- and middle-income countries. A substantial proportion occurs intrapartum and is avoidable with better care. At a low-resource tertiary hospital, this study assessed the quality of intrapartum care and adherence to locally-tailored clinical guidelines.
A non-participatory, structured, direct observation study was held at Mnazi Mmoja Hospital, Zanzibar, Tanzania, between October and November 2016. Women in active labour were followed and structure, processes of labour care and outcomes of care systematically recorded. Descriptive analyses were performed on the labour observations and compared to local guidelines and supplemented by qualitative findings. A Poisson regression analysis assessed factors affecting foetal heart rate monitoring (FHRM) guidelines adherence.
161 labouring women were observed. The nurse/midwife-to-labouring-women ratio of 1:4, resulted in doctors providing a significant part of intrapartum monitoring. Care during labour and two-thirds of deliveries was provided in a one-room labour ward with shared beds. Screening for privacy and communication of examination findings were done in 50 and 34%, respectively. For the majority, there was delayed recognition of labour progress and insufficient support in second stage of labour. While FHRM was generally performed suboptimally with a median interval of 105 (interquartile range 57–160) minutes, occurrence of an intrapartum risk event (non-reassuring FHR, oxytocin use or poor progress) increased assessment frequency significantly (rate ratio 1.32 (CI 1.09–1.58)).
Conclusions
Neither international nor locally-adapted standards of intrapartum routine care were optimally achieved. This was most likely due to a grossly inadequate capacity of birth attendants; without whom innovative interventions at birth are unlikely to succeed. This calls for international and local stakeholders to address the root causes of unsafe intrafacility care in low-resource settings, including the number of skilled birth attendants required for safe and respectful births.
Peer Review reports
Plain English summary
Every year around the world, 2.1 million babies die in the womb (stillbirths) and another 2.6 million die within 28 days of birth (neonatal deaths). About half of these deaths are associated with problems that occur during birth in resource-poor settings.
In this study, we assessed the quality of labour care at Zanzibar’s tertiary hospital, Tanzania. We directly observed and carefully recorded the care given to 161 women throughout birth and compared our findings to the local clinical guidelines of care at birth.
In this busy hospital, care during birth was provided by young nurses, midwives and doctors with little direct supervision from seniors. Conditions were difficult for both staff and pregnant women. Labour and delivery took place in an open and crowded labour ward. Each midwife took care of four women in labour simultaneously which resulted in insufficient support during birth.
Monitoring was not optimally performed according to the locally-tailored guidelines. For example, the baby’s heart was monitored every 105 min on average instead of the recommended 60 min. Staff, however, increased monitoring when certain problems were detected, such as when the baby’s heart was not beating normally, when progress of labour was slow and when oxytocin was used to increase contraction. Putting up a screen for privacy was done in about half of all vaginal examinations. In most cases, women were not informed of the findings after an examination. Main reasons for birth attendants being unable to follow their clinical guidelines were that they are too few. Thus an investment in sufficient and competent workforce in such labour wards is crucial.
An estimated 300,000 maternal deaths and five million perinatal deaths occur yearly worldwide, with > 98% in low- and middle-income countries (LMICs) [ 1 ]. Although childhood mortality has been reduced significantly, Millennium Development Goal 4 was not met in Sub-Saharan Africa, as neonatal deaths went mostly ignored and now make up 40% of under-5 mortalities [ 1 , 2 ]. Almost half the number of stillbirths and 23% of neonatal deaths in LMICs are intrapartum-related, in contrast to high-income settings [ 3 , 4 ]. Hence, ending intrapartum deaths by improved quality of intra-facility care is pivotal [ 5 ]. Yet, in Sub-Saharan Africa, the increasing numbers of facility-based deliveries, have not resulted in better intrapartum care and progress to improve perinatal health outcomes is slowest [ 6 , 7 , 8 ]. Instead, in many facilities, international standards of intrapartum care have become more difficult to implement in the day-to-day reality. As found in our hospital, after unrealistic international guidelines were adapted to better suit the local resource-limited reality, significant improvements were observed in quality of care, stillbirths were reduced by one-third and the number of neonates with birth asphyxia nearly halved (Box 1 ) [ 9 ].
Quality of intrapartum care has mostly been assessed by retrospective analysis of existing medical records [ 10 ]. However, written records (e.g. partographs) in low-resource settings are often incomplete, missing or inaccurate [ 10 ], and therefore might not reflect the actual care. The gold standard for clinical quality assessment is direct observation that captures the real-life experiences and behaviour of birth attendants; yet, they are rarely used [ 10 , 11 ]. Similarly, few studies have assessed the adequacy of intrapartum foetal monitoring in low-resource settings [ 12 , 13 , 14 , 15 , 16 ]. This study used continuous direct observation to assess the quality of intrapartum care, with a specific focus on foetal monitoring and the structural requirements to delivering intrapartum quality care.
Study design
This was a prospective study consisting of labour observations at the maternity ward of Mnazi Mmoja Hospital (MMH) in Zanzibar, the United Republic of Tanzania, from October to November 2016. The study adhered to a pre-determined protocol (Additional file 1 ) and STROBE standards of reporting [ 17 ]. This manuscript is part of the larger PartoMa Project initiated in 2015 to improve quality of care (Box 1 ) [ 9 ].
About 12,000 annual deliveries are assisted at MMH, and it is the only tertiary hospital on the Zanzibar archipelago [ 12 ]. At the time of the study, the labour unit consisted of an admission room, a one-room labour ward with 19 beds, a three-bed delivery room, two postnatal rooms, and one theatre (Fig. 1 ). For privacy reasons, women were not allowed to have a companion during childbirth in the busy labour ward. Skilled birth attendants (SBAs) comprised 27 nurse-midwives (diploma-level in nursing, except three seniors with university-level degree), 13 resident doctors (general doctors) and six intern doctors (total n = 46) who together provided routine labour care and comprehensive emergency obstetric and newborn care. On average, six to eight nurse-midwives and five doctors were allocated to the maternity unit in the morning shifts and a total of six SBAs were in the remaining shifts (including weekends). These were the main roles of nurse-midwives: assessment on admission; intrapartum and postpartum care including supportive care, routine monitoring, administration of medication, vaginal deliveries, and perineal repair; maintenance of ward hygiene, and delivery sets. The roles of the doctors involved providing labour and postpartum care to high risk women and complicated deliveries and performing obstetric and gynaecological operations. Direct supervision was provided by two senior midwives and two senior (visiting) doctors, only during morning shifts. In addition, there were three obstetricians who could be consulted and called for emergencies (Additional file 2 , cadre definitions). Standards for labour management was according to the peer-reviewed PartoMa Pocket Guide version 1.2 [ 9 ]. where, in collaboration with the SBAs, international guidelines had been tailored to the situation at the hospital, including reductions in information load, ambiguity, and frequency of clinical assessments (Additional file 3 , comparison of FHRM recommendations) [ 9 ]. The stillbirth rate was 39 per 1000 total births, of which 38% occurred during intra-hospital care [ 9 ].
Layout of the maternity unit of Mnazi Mmoja Hospital (MMH) in Zanzibar, the United Republic of Tanzania (2016)
Participants
The first women to reach active phase of labour (≥ 4 cm cervical dilatation), either at admission or already in the ward, were selected for inclusion. Exclusion criteria were absent foetal heart rate (FHR) on admission, elective or emergency caesarean section (CS) decided immediately after admission, and known congenital foetal anomaly incompatible with life. Shifts of inclusion were planned beforehand to ensure representation of morning, evening, and night shifts throughout the week. Labours were observed until delivery or diagnosis of intrauterine foetal death.
Ethical approval
Approval from Mnazi Mmoja Hospital and Zanzibar Medical and Research Ethical Committee the local ethics committee (ZAMREC) was obtained (ZAMREC/0002/May/016). Written informed consent in Swahili was sought from all participating women. The aim of the study and the role of observers were introduced to all staff before commencing the study (not to cause blame or undermine the staff’s devotion to labouring women). In case observers had concerns about the safety of a woman, they could express these concerns to the staff on duty and a senior staff could be called for assistance if needed.
The study qualitatively described and quantified structural indicators and processes of intrapartum care, as described in the Donabedian model [ 18 ]. It focused on the aspects of intrapartum monitoring and supportive care (Fig. 2 ). In addition, the study determined adherence to the local FHRM guidelines (in terms of frequency and technique) [ 9 ], and the effect of five pre-identified predictors on FHRM frequency: pregnancy risk status (Box 2 ); occurrence of intrapartum risk events (Box 2 ) [ 19 ]; parity (nulliparity and multiparity); SBA’s years of experience with maternity care; and shift of inclusion. Predictors were adopted from the NICE [ 19 ] and local PartoMa guidelines, and from the hypothesis that they would alter the frequency of observations and/or the quality of care. Adherence to FHRM guidelines meant that the number of FHRMs was at least equal to the expected frequency. Expected frequencies of FHRM for low-risk labours, high-risk labours, and non-reassuring/abnormal FHR were set at 60, 30, and 15 min, respectively [ 9 ]. These were based on first stage of labour guidelines, because the start of second stage was often unknown. Other variables recorded consisted of socio-demographic characteristics, risk assessment variables (Box 2 ), cervical dilatation, and FHR on admission (Additional file 4 ).
Variables measured in the study, related to structure and processes of care, by the Donabedian framework
Data sources/measurement
The five observers were not qualified to act as birth attendants. One of the observers was a foreign final year medical student. The other four observers were newly graduated local medical students who had recently completed their studies and awaiting approval to start clinical internships. Therefore, none of the observers were permitted to provide medical care in this setting. Two observers per shift, both located in the labour room, conducted non-participatory direct observations. They recorded all care provided to each woman and performed hourly counts of the structural indicators (Fig. 2 ). When a woman was taken to the delivery room or theatre, one observer followed her until birth. Sociodemographic characteristics, risk assessment variables, assessments on admission and birth outcomes were collected using the women’s records (antenatal card and hospital file). Two data collection forms were created (Additional file 4 ), pilot tested, and used for the systematic recording [ 18 ]; one for processes of care provided per woman as well as cadre of staff who provided the care and birth outcomes, and one for hourly counts of number of birth attendants, labouring women, and functioning FHRM devices. Both forms provided space for free-text description of e.g. supportive care (staff’s and women’s behaviour, presence of SBA during delivery), decision-making, medical treatment, and physical space/environment.
Observer bias was a major concern. Observers could have underreported assessments made by SBAs or could have been involved in providing labour care. To minimise these biases, they were trained to recruit women into the study, make objective structured assessments with minimal intervention in care provision, and record their observations on the forms. They worked in pairs and observed a maximum of eight labouring women at a time. Also, the presence of observers could have altered the behaviour and improved performance of the SBAs (the Hawthorne effect) [ 20 ]. However, this effect was likely to be minimal because the labour ward was extremely busy. Also, observations were made from an adequate distance, rather than by following the SBA.
The a priori sample size was estimated to be 150 labours plus 10% to account for potential hindsight exclusion based on a maximum of five predictors, with the aim to detect a single predictor with an assumed effect size of a risk ratio of 1.3, power of 80% and alpha of 5%.
First, descriptive analyses were conducted on women’s characteristics and number of staff, women, care provision, and FHRM devices. Means were reported with standard deviations (SDs), medians with interquartile ranges (IQRs), and frequencies with percentages. Then, the proportion of observations adhering to local FHRM guidelines, privacy standards and communication practices were calculated using the following formula: number of observations divided by number of expected observations, given the duration of labour. Four intervals were calculated: between two adjacent FHRMs, vaginal examinations, contractions and the last FHR-to-delivery interval. Differences between work shifts (day/evening/nights) were tested using one-way-ANOVA for the number of staff, equipment and labouring women and chi-square test for composite adverse perinatal outcomes (i.e. stillbirths and Apgar score < 7 at 1 min). Furthermore, univariate and multivariate generalized linear models for Poisson distributions were performed with the number of FHRMs for each woman as an outcome to estimate the effect of the above-mentioned pre-selected predictors on local guidelines adherence. In the multivariate analysis, the predictor intrapartum risk event was dichotomised. Results from the analysis were reported as rate ratios (RRs) with 95% confidence intervals and corresponding p -values. For this study, a RR may be interpreted as the increase in the number of FHRMs either compared to a reference category or when a continuous predictor increases by one unit. The set frequency of FHRM was hourly and women with a total pre-delivery observation of less than one hour were excluded from this analysis. The statistical software used was SPSS version 23, and SAS 9.4. Free-text comments were used to supplement quantitative findings on human resources, physical space, equipment, monitoring, and supportive care.
Participants and birth outcomes
The majority of the 161 women observed (95.0%, n = 153) came directly from home, at term (39 (±2.8) weeks), and with a median cervical dilatation of five (IQR: 3–7) cm. Inclusions were spread evenly between morning, evening, and night shifts (34.2%, n = 55; 30.4%, n = 49; and 35.4%, n = 57, respectively). Fifty-one women (31.7%) were classified as high-risk on admission; the most common risk factors being hypertensive disorders (10.5%, n = 17), prematurity (8.1%, n = 13), breech presentation (6.8%, n = 11, including two singletons and one twin pregnancy diagnosed close to delivery), previous uterine scar (5.6%, n = 9) and grand multiparity (5.6%, n = 9). In addition, 38.2% ( n = 42/110) of the remaining labouring women experienced one or more intrapartum risk events. Of the 42 (26%) labours augmented with oxytocin, two thirds ( n = 28) had crossed the action line of the partograph (i.e. poor progress). There were no maternal or neonatal deaths before hospital discharge, but there were four stillbirths (2.4%) and 23 (14.3) babies with Apgar score less than seven at one minute (Table 1 ). There was no statistical significance in composite perinatal outcomes between shifts of delivery ( p = 0.70).
Structure: context in which care was provided
Human resources.
The majority of nurse-midwives and doctors (89%, n = 41/46) had a maximum of five years’ experience in labour care (< 1 year: 41%, n = 19/46; 1–5 years: 48%, n = 22/46; > 5 years: 11%, n = 5/46). Observation at hourly intervals showed an average of nine labouring women and two to three nurse-midwives in the labour and delivery rooms throughout the day (nurse/midwife-to-labouring-women ratio of 1:4). Nurse-midwives performed all admission assessments, conducted 32.6% (134/411) of intrapartum monitoring and the majority of vaginal deliveries (67.1%, n = 94/140) (Table 3 ). The resident doctor on duty also monitored women in labour (38.9% of examinations, n = 160/411) and handled gynaecological and obstetric emergencies such as CS, obstetric haemorrhage and eclampsia. Sharing of information within cadres occurred during handover rounds, and two clinical meetings in the morning shift in which mainly doctors attended. Several SBAs regularly worked at a continuous pace and attended to women; while others showed signs of exhaustion, such as decreased work speed, resting for long periods, sleeping during night shifts, and uncourteous behaviour towards women and colleagues, only offering help when women became too loud or in the presence of a senior. Students and cleaners also shared tasks; they assisted during deliveries, offered psychological support, helped women with food and facilitated communication between women, SBAs and their families who were restricted access to the maternity unit.
Equipment and supplies
Partograph copies were available throughout the study period and were used in at least 87% ( n = 140) of labours; the remaining were either lost after delivery (9.3%, n = 15) or not used at all (3.7%, n = 6). Difficulties encountered with FHRM devices were their scarcity or misplacement (Table 2 ), unavailability of gel for hand-held Dopplers and ultrasound, and non-functioning hand-held Dopplers ( n = 6). Other intermittent shortages included lack of essential medication (e.g. antihypertensive), and supplies for vacuum extraction, CS and normal delivery sets.
Physical space
The single-room labour ward was noisy and crowded, and consisted of women in all stages of labour and post-delivery. The number of women typically exceeded the number of beds, thus women often shared and changed beds. Although specific areas of the room were reserved for high risk women and specific stages of labour, this localisation was not consistently used. Two thirds of vaginal deliveries took place in the shared labour ward (64.3%, n = 90/140), while 32.9% ( n = 46/140) reached the three-bed delivery room that had more privacy (Table 1 ).
Processes of routine care delivery
527 provider-woman contact points were observed during labour and delivery, which included 411 examinations consisting of FHRM ( n = 268), vaginal examinations ( n = 326), and other forms of care ( n = 116) (Table 3 ). Maternal blood pressure and/or temperature were measured at 180 of these time points. The median decision-delivery time interval for the 27 emergency operative deliveries was 60 (IQR: 25–81) minutes.
Caring support
Labouring women were provided intermittent care collectively by the SBAs on duty. They often called for attention, especially during contractions and when they felt the need to push. This prompted examination and diagnosis of second stage of labour in the majority of women who delivered vaginally (95.0%, n = 133/140). In more than a quarter of women (27.9%, n = 39/140), support during second stage was not provided until ≤5 min before delivery; six of whom delivered unattended. Six of the 39 women were admitted with < 4 cm cervical dilatation and also went through the entire first stage of labour unobserved. Other actions associated with caring support, including use of a screen for privacy and communication during and after examinations (FHRM and/or vaginal examination) were conducted in 50.1% ( n = 206/411) and 34.1% ( n = 140/411) respectively. (Table 4 ).
Routine monitoring of labour progress and foetal heart rate
The median duration of labour observation was 290 (IQR:135–570) minutes in which the median frequency of FHRM was one (IQR: 0–3) and of vaginal examination two (IQR:1–3). The median interval between two vaginal examinations in the first stage of labour was 125 (IQR 56–225) minutes. In none of the cases, the strength and frequency of contractions were assessed by 10-min palpation per abdomen.
Two-thirds of FHRM were conducted with a DeLee or Pinard stethoscope (69.4%, n = 186/268; DeLee: n = 117, Pinard: n = 64, both: n = 5). Ultrasound was primarily used in 23.5%, ( n = 63/268) of cases, while hand-held Doppler was rarely used (1.9%, n = 5/268). In the remaining cases, ultrasound, following use of stethoscope, was used to confirm FHR (5.2%, n = 14/268). In 37.9% ( n = 61) of labours, FHRM was only recorded on admission. For labours with more than one FHRM ( n = 254), the median interval between two FHRMs was 105 (IQR 57–160) minutes, with 30% ( n = 76/254) of intervals within 60 min, and 42.5% ( n = 108/254) beyond two hours. The median interval between the last FHRM and delivery was 87 (IQR:41–170) minutes (Table 5 ). Of all FHRMs observed, they were counted with a clock in 35.0% ( n = 94/268), maternal pulse simultaneously palpated in 5.6% ( n = 15/268), and contraction palpated in 16.0% ( n = 43/268) of cases. The locally recommended one-hour frequency of FHRM was adhered to in 3.1% ( n = 5) women throughout labour (Table 4 ). There was no difference in last FHRM to delivery intervals between morning, evening, and night shifts. The presence of intrapartum risk events led to an increase in the number of FHRMs observed, with a rate ratio (RR) of 1.33 (CI 1.11–1.64), when a non-reassuring/abnormal FHR was detected (RR 1.59; CI 1.19–2.13), oxytocin was used (RR 1.25; CI 1.02–1.54, and when labour crossed the action line on the partograph (RR 1.25; CI 1.00–1.56) (Additional file 5 ).
This direct observation study reports on intrapartum care provided to 161 women in a congested tertiary hospital in Sub-Saharan Africa. It shows suboptimal birth attendance and adherence to local and international guidelines on timely care, including surveillance of the woman’s vital signs, FHR, and labour progress. Substantial findings of this study were the discontinuous care. Lack of support and respectful care were reflected by a significant absence of communication, privacy, and support at the time of delivery. Structural challenges observed were high workload compared to staff numbers, an unconducive environment and scarcity of monitoring devices. However, despite this congested and unconducive environment, SBAs showed ability to provide evidence-based triage; they prioritised the monitoring of women who were recognised to have an intrapartum risk event (non-reassuring/abnormal FHR, oxytocin use, and crossing the action line) leading to a significant increase in frequency of FHR assessments.
Lack of attendance and delay in diagnosing second stage of labour in the majority of women was the rationale behind our conservative approach to determine FHRM guidelines adherence. We only compared FHRM to first stage expected frequencies, which were lower than second stage frequency. Although this approach underreports the problem, the findings highlight the challenge of adhering to guidelines in these settings. In other resource-limited settings of Sub-Saharan Africa, randomised control trials on innovative FHRM devices failed to show improvement in perinatal outcomes due to non-adherence to FHRM international guidelines and obstetric response [ 21 , 22 , 23 , 24 ].
In this hospital, international guidelines were adapted to locally-acceptable minimum standards [ 9 ]. Compared to the situation before local guidelines were implemented, 20 months prior, there was sustained multiple improvements in monitoring: including higher FHRM frequency, notably in women with intrapartum risk events, more timely oxytocin use for augmentation, improved care for women with severe hypertensive disorders, and lower intrapartum stillbirth rates [ 9 , 12 ]. An earlier study showed that these guidelines were positively viewed and were used by local staff [ 25 ]. However, they are still far from being adhered to optimally which present an ethical dilemma of whether such guidelines can further reduce frequency of assessments.
Inadequate number of health workers, as reflected in the hourly mid-wife-to-labouring women ratio, remained the major bottleneck to following internationally agreed evidence-based standards of care optimally. For example, with one nurse-midwife attending four women simultaneously, there is insufficient time to palpate contractions in accordance to international recommendations (every 30 min for 10-min duration), not to mention time for any other care or rest. Providing support throughout birth was thus an overarching challenge, notably of women in early labour who were not considered as yet eligible for routine intrapartum care. However, women in general, including in Sub-Saharan Africa, favour continuous birth companionship [ 26 ]. Evidence suggests that it is the most significant intervention during birth associated with positive effects on perinatal outcomes and women’s experience of birth [ 27 ]. As such the lack of support in this study, including in the second stage of labour, meant that women were likely to have had significant distress and negative birth experience. In addition to suboptimal care, this inevitably leads to a workforce with moral distress, burnout, and compassion fatigue hence, even less capable of giving compassionate care [ 28 ]. In order to cope with the high workload, resident doctors performed a significant part of routine intrapartum care that is usually conducted by midwives. However, this may have impaired the ability to provide obstetric care to the large number of high-risk women and further disrupted midwifery-led supportive care. The lack of adequately trained SBAs, in particular nurse-midwives, may have been exacerbated by lack of inter-professional collaboration and supervision of junior staff, as well as by inefficient organisation of space, workforce and tools [ 29 ]. Consequently, care remained severely suboptimal and the stillbirth rate persistently high, emphasising the need to first improve the basic structure of care. Achieving minimal standards of care would require efficient allocation of available resources, organisation, supervision and teamwork as well as an adequate increase in human resources. Moreover, birth companionship is a challenge in open and overcrowded labour wards. Efforts should also be placed on how e.g. relatives and traditional birth attendants may assist the overstrained skilled birth attendants in providing continuous support during labour and delivery.
Strengths, limitations and ethical consideration
We here report the labour observation aspect of a broader work that included an ethnographic study and a locally co-created intervention to improve quality of labour care. Measurement of the quality of labour care is essential for identifying gaps, developing context-tailored interventions and monitoring of quality improvement processes. This unique systematic observation of intrapartum routine care overcomes the shortcomings of records-based studies in assessing quality of care. It allowed evaluation of the interactions between labouring women and their birth attendants, and the structural context care was provided in. The findings are supported by record-based quantitative PartoMa findings and also by mainly qualitative findings that showed similar suboptimal structural challenges to processes of care in numerous areas across Sub-Saharan Africa and other low resource-settings [ 12 , 29 , 30 , 31 ]. However, observations are more resource-consuming than record-based assessments, hold a higher risk of the Hawthorne effect, and may pose ethical dilemmas about the non-participatory nature of the observer and their moral responsibility to participant safety in understaffed settings. Thus, a trade-off of biased results for participant safety may be necessary. As the observers were not qualified birth attendants, they expressed any concerns on patient safety, including imminent delivery, to the staff on duty and in emergency situations, they assisted the staff when requested. Observations show it was common practice for birth attendants to wait until signs of imminent delivery before attending to women in the second stage of labour. Thus a few women delivered unattended, as a result of preventable delays to respond to women’s call for help and not the mere absence of skilled birth attendants from the labour ward.
Limitations included the observer bias and Hawthorne effect described above. Also, the study aimed to measure and identify challenges, rather than detect differences in outcomes for varying quality of care. Hence, it was still unable to determine the effect foetal monitoring has, if any, on birth outcomes [ 16 ]. The results should then lead to adequately-powered studies including clinical auditing in other LMICs to estimate the quality of the intrapartum (foetal) monitoring and linking it to birth outcomes. Moreover, we were not able to determine the effect of staff experience and time of day on the quality of care as women were cared for by several birth attendants across multiple shifts. The admission assessment was retrieved from the patient file and not observed. Numerous findings on admission which included smaller-than expected fundal height, undiagnosed twins, breech presentation, and intrauterine foetal death until close to delivery indicate inadequate risk assessment and suggests admission time as an important point for improvement to explore.
In this reality check of intrapartum care, the quality of basic routine care in a Tanzanian referral hospital remained unacceptable. It was not possible to provide respectful and safe care, and even to optimally follow locally adapted clinical guidelines, which took the local resources into account. This was particularly due to the disproportionate birth-attendant-to-labouring women ratio. Ensuring a safe and positive birth experience requires local stakeholders and international community to urgently address the structural barriers in Sub-Saharan Africa and invest in sufficient numbers of adequately trained and motivated staff for continuous support during labour.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Caesarean section (CS)
Foetal heart rate
Foetal heart rate monitoring
Interquartile range
Low and middle-income countries
Mnazi Mmoja Hospital
National Institute for Health and Care Excellence
Statistical Analysis System
Skilled birth attendant
Standard deviation
Statistical Package for the Social Sciences
Vaginal examination
GBD 2015 Child Mortality Collaborators. Global , regional , national , and selected subnational levels of stillbirths , neonatal , infant , and under-5 mortality , 1980–2015 : a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1725–74. https://doi.org/10.1016/S0140-6736(16)31575-6 .
Article PubMed Central Google Scholar
Lawn JE, Blencowe H, Oza S, et al. Every newborn: Progress, priorities, and potential beyond survival. Lancet. 2014;384(9938):189–205. https://doi.org/10.1016/S0140-6736(14)60496-7 .
Article PubMed Google Scholar
Lawn JE, Blencowe H, Waiswa P, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387(10018):587–603. https://doi.org/10.1016/S0140-6736(15)00837-5 .
Article Google Scholar
Goldenberg RL, McClure EM. Reducing intrapartum stillbirths and intrapartum-related neonatal deaths. Int J Gynaecol Obstet. 2009;107(Suppl):S1–3. https://doi.org/10.1016/j.ijgo.2009.07.014 .
Article PubMed PubMed Central Google Scholar
World Health Organization. Making pregnancy safer : the critical role of the skilled attendant: A joint statement by WHO , ICM and FIGO. World Health Organization. https://www.who.int/maternal_child_adolescent/documents/9241591692/en/ . Published 2004. Accessed June 20, 2019.
Campbell OMR, Calvert C, Testa A, et al. Maternal Health 3 The scale , scope , coverage , and capability of childbirth care. Lancet 2016;388(10056):2193–2208. doi: https://doi.org/10.1016/S0140-6736(16)31528-8 .
Graham WJ, Varghese B. Quality , quality , quality : gaps in the continuum of care A call for coordinated and evidence-based action to protect children outside of family care. Lancet. 2015;379(9811):e5–6. https://doi.org/10.1016/S0140-6736(10)62267-2 .
Bradley S, Mccourt C, Rayment J, Parmar D. Disrespectful intrapartum care during facility-based delivery in sub-Saharan Africa : a qualitative systematic review and thematic synthesis of women ’ s perceptions and experiences. Soc Sci Med. 2016;169:157–70. https://doi.org/10.1016/j.socscimed.2016.09.039 .
Maaløe N, Housseine N, Meguid T, Nielsen BB, Jensen AKG, Khamis RS et al. Effect of locally tailored labour management guidelines on intrahospital stillbirths and birth asphyxia at the referral hospital of Zanzibar: a quasi-experimental pre-post study (The PartoMa study). B J O G. 2018;125(2):235–45. https://doi.org/10.1111/1471-0528.14933 .
Tripathi V. International journal of gynecology and obstetrics a literature review of quantitative indicators to measure the quality of labor and delivery care. Int J Gynecol Obstet. 2016;132(2):139–45. https://doi.org/10.1016/j.ijgo.2015.07.014 .
Hermida J, Nicholas DD, Blumenfeld SN. Comparative validity of three methods for assessment of the quality of primary health care task performed. Int J Qual Heal Care. 1999;11(5):429–33.
Article CAS Google Scholar
Maaløe N, Housseine N, Bygbjerg IC, et al. Stillbirths and quality of care during labour at the low resource referral hospital of Zanzibar : a case-control study. BMC Pregnancy Childbirth. 2016;16(1):1–12. https://doi.org/10.1186/s12884-016-1142-2 .
Ashish KC, Wrammert J, Clark RB, Ewald U, Målqvist M. Inadequate fetal heart rate monitoring and poor use of partogram associated with intrapartum stillbirth: a case-referent study in Nepal. BMC Pregnancy Childbirth. 2016;16(1):233. https://doi.org/10.1186/s12884-016-1034-5 .
Ogwang S, Karyabakabo Z, Rutebemberwa E. Assessment of partogram use during labour in Rujumbura health Sub District, Rukungiri District, Uganda. Afr Health Sci. 2009;9(Suppl 1):S27–34.
PubMed PubMed Central Google Scholar
Nyamtema A, Urassa D, Massawe S, Massawe A, Lindmark G, van Roosmalen J. Partogram use in the Dar es Salaam perinatal care study. Int J Gynecol Obstet. 2008;100(1):37–40. https://doi.org/10.1016/j.ijgo.2007.06.049 .
Kamala BA, Ersdal HL, Dalen I, Abeid MS, Ngarina MM, Perlman JM, et al. Implementation of a novel continuous fetal Doppler (Moyo) improves quality of intrapartum fetal heart rate monitoring in a resource-limited tertiary hospital in Tanzania: An observational study. PLoS ONE. 2018;13(10):e0205698. https://doi.org/10.1371/%20journal.pone.0205698 .
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. PLoS Med. 2007;4(10). https://doi.org/10.18601/01229893.n38.03 .
Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q. 1966;44(3):166–206. https://doi.org/10.1126/science.58.1509.437-a .
National Institute for Health and Clinical Excellence. The use of electronic fetal monitoring: The use and interpretation of cartidotocography in intrapartum foetal surveillance. NICE Clinical Guideline C. National Institute for Clinical Excellence. http://ctgutbildning.se/images/Referenser/NICE-guidelines-FHR-monitoring-2001.pdf . Published 2001. Accessed August 26, 2016.
Leung WC, Lam HSW, Lam KW, To M, Lee CP. Unexpected reduction in the incidence of birth trauma and birth asphyxia related to instrumental deliveries during the study period: was this the Hawthorne effect? BJOG. 2003;110:319–22.
Mdoe PF, Ersdal HL, Mduma E, et al. Randomized controlled trial of continuous Doppler versus intermittent fetoscope fetal heart rate monitoring in a low-resource setting. Int J Gynecol Obstet. 2018. https://doi.org/10.1002/ijgo.12648 .
Mdoe PF, Ersdal HL, Mduma ER, et al. Intermittent fetal heart rate monitoring using a fetoscope or hand held Doppler in rural Tanzania: a randomized controlled trial. BMC Pregnancy Childbirth. 2018;18(1):1–8. https://doi.org/10.1186/s12884-018-1746-9 .
Kamala BA, Kidanto HL, Wangwe PJ, et al. Intrapartum fetal heart rate monitoring using a handheld Doppler versus Pinard stethoscope: a randomized controlled study in Dar Es Salaam. Int J Women's Health. 2018;10:341–8. https://doi.org/10.2147/IJWH.S160675 .
Housseine N, Punt MC, Browne JL, et al. Strategies for intrapartum foetal surveillance in low- and middle-income countries: a systematic review. PLoS One. 2018;13(10):e0206295. https://doi.org/10.1371/journal.pone.0206295 .
Article CAS PubMed PubMed Central Google Scholar
Maaløe N, Housseine N, Van Roosmalen J, et al. Labour management guidelines for a Tanzanian referral hospital : the participatory development process and birth attendants ’ perceptions. BMC Pregnancy Childbirth. 2017;17(175):1–11. https://doi.org/10.1186/s12884-017-1360-2 .
Kabakian-khasholian T, Portela A. Companion of choice at birth : factors affecting implementation. 2017:1–13. https://doi.org/10.1186/s12884-017-1447-9 .
Bohren MA, Hofmeyr GJ, Sakala C, Fukuzawa RK, Cuthbert A. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;(7). https://doi.org/10.1002/14651858.CD003766.pub6 .
Filby A, McConville F, Portela A. What prevents quality midwifery care? A systematic mapping of barriers in low and middle income countries from the provider perspective. PLoS One. 2016;11(5):1–20. https://doi.org/10.1371/journal.pone.0153391 .
Munabi‐Babigumira S, Glenton C, Lewin S, Fretheim A, Nabudere H. Factors that influence the provision of intrapartum and postnatal care by skilled birth attendants in low‐ and middle‐income countries: a qualitative evidence synthesis. Cochrane Database Syst Rev. 2017;(11). https://doi.org/10.1002/14651858.CD011558.pub2 .
Solnes Miltenburg A, Kiritta RF, Bishanga TB, van Roosmalen J, Stekelenburg J. Assessing emergency obstetric and newborn care: can performance indicators capture health system weaknesses? BMC Pregnancy Childbirth. 2017;17(1):1–9. https://doi.org/10.1186/s12884-017-1282-z .
Maaløe N, Housseine N, Meguid T, et al. Effect of locally tailored labour management guidelines on intrahospital stillbirths and birth asphyxia at the referral hospital of Zanzibar: a quasi-experimental pre-post study (the PartoMa study). BJOG An Int J Obstet Gynaecol. 2018;125(2):235–45. https://doi.org/10.1111/1471-0528.14933 .
Download references
Acknowledgements
Our gratitude to Rashid Saleh Khamis and Mbweni Makame Ali for their valuable contribution to data collection.
Global Health scholarship and Dr. Lieve Page-Christiaens at UMC Utrecht; and Mnazi Mmoja Hospital in Zanzibar. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report or the decision to submit for publication. The corresponding author had full access to all the data in the study and all authors had final responsibility for the decision to submit for publication.
Author information
Authors and affiliations.
Division Woman and Baby, University Medical Centre Utrecht, Utrecht, Netherlands
Natasha Housseine, Marieke C. Punt, Arie Franx & Marcus J. Rijken
Julius Global Health, Julius Centre for Health Sciences and Primary Care, University Medical Centre Utrecht, Huispost nr. STR 6.131, P.O. Box 85500, 3508, Utrecht, Netherlands
Natasha Housseine, Nicolaas P. A. Zuithoff, Diederick E. Grobbee, Joyce L. Browne & Marcus J. Rijken
Department of Obstetrics and Gynaecology, Mnazi Mmoja Hospital, Zanzibar, Tanzania
Natasha Housseine & Tarek Meguid
School of Health and Medical Science, State University of Zanzibar (SUZA), Zanzibar, Tanzania
Ali Gharib Mohamed, Said Mzee Said & Tarek Meguid
Global Health Section, Department of Public Health, University of Copenhagen, Copenhagen, Denmark
Nanna Maaløe
You can also search for this author in PubMed Google Scholar
Contributions
NH, MP, JB and MJR conceived and designed the experiment. NH, MP, AGM and SMS carried out data acquisition. NH, MP and NPAZ analysed the data. NH and MP interpreted the results with substantial contribution from JB, TM, NPAZ, AF and MJR. NH drafted the manuscript with substantial contribution from MP, JB, NM, TM, AF and MJR who revised the drafted manuscript on multiple occasions. All authors reviewed, approved and agreed to be accountable for the final version to be published.
Authors’ information
At the time of the study, NH was a clinician at the Obstetrics and Gynaecology Department Mnazi Mmoja Hospital and is a PhD candidate at UMC Utrecht. Her research is based on improving quality of intrapartum care, with a particular focus on foetal monitoring, in low resource setting.
Corresponding author
Correspondence to Natasha Housseine .
Ethics declarations
Ethics approval and consent to participate.
Approval from the local ethics committee (ZAMREC) was obtained (ZAMREC/0002/May/016). Written informed consent in Swahili was sought from all participating women.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
Study protocol
Additional file 2.
Definition of various terms used in the study
Additional file 3.
Comparison of foetal and contraction monitoring recommendations in international, national and local (PartoMa) guidelines
Additional file 4.
Data collection sheets
Additional file 5.
Predictors of the frequency of FHR monitoring
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Housseine, N., Punt, M.C., Mohamed, A.G. et al. Quality of intrapartum care: direct observations in a low-resource tertiary hospital. Reprod Health 17 , 36 (2020). https://doi.org/10.1186/s12978-020-0849-8
Download citation
Received : 25 March 2019
Accepted : 02 January 2020
Published : 14 March 2020
DOI : https://doi.org/10.1186/s12978-020-0849-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Foetal monitoring
- Intermittent auscultation
- Low resource
- Developing countries
Reproductive Health
ISSN: 1742-4755
- General enquiries: [email protected]
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.

- A new version of this title is available

Intrapartum care for healthy women and babies
NICE Clinical Guidelines, No. 190
- Copyright and Permissions
This guideline replaces CG55.
This guideline is partially replaced by NG229.
This guideline is the basis of QS105.
This guideline covers the care of healthy women and their babies, during labour and immediately after the birth. It focuses on women who give birth between 37 and 42 weeks of pregnancy (‘term’). The guideline helps women to make an informed choice about where to have their baby. It also aims to reduce variation in areas of care such as fetal monitoring during labour and management of the third stage of labour.
The Royal College of Obstetricians and Gynaecologists has produced guidance on COVID-19 and intrapartum care for all midwifery and obstetric services.
Who is it for?
- Healthcare professionals
- Commissioners and providers
- Healthy women who have had a straightforward pregnancy and give birth between 37 and 42 weeks of pregnancy
- Recommendations
People have the right to be involved in discussions and make informed decisions about their care, as described in NICE’s information on making decisions about your care .
Making decisions using NICE guidelines explains how we use words to show the strength (or certainty) of our recommendations, and has information about prescribing medicines (including off-label use), professional guidelines, standards and laws (including on consent and mental capacity), and safeguarding.
1.1. Place of birth
Choosing planned place of birth, women at low risk of complications.
Explain to both multiparous and nulliparous women who are at low risk of complications that giving birth is generally very safe for both the woman and her baby. [2014]
- Advise low-risk multiparous women that planning to give birth at home or in a midwifery-led unit (freestanding or alongside) is particularly suitable for them because the rate of interventions is lower and the outcome for the baby is no different compared with an obstetric unit.
- Advise low-risk nulliparous women that planning to give birth in a midwifery-led unit (freestanding or alongside) is particularly suitable for them because the rate of interventions is lower and the outcome for the baby is no different compared with an obstetric unit. Explain that if they plan birth at home there is a small increase in the risk of an adverse outcome for the baby. [2014]
- planning birth at home or in a freestanding midwifery unit is associated with a higher rate of spontaneous vaginal birth than planning birth in an alongside midwifery unit, and these 3 settings are associated with higher rates of spontaneous vaginal birth than planning birth in an obstetric unit
- planning birth in an obstetric unit is associated with a higher rate of interventions, such as instrumental vaginal birth, caesarean section and episiotomy, compared with planning birth in other settings
- there are no differences in outcomes for the baby associated with planning birth in any setting. [2014]
Rates of spontaneous vaginal birth, transfer to an obstetric unit and obstetric interventions for each planned place of birth: low-risk multiparous women (sources: Birthplace 2011; Blix et al. 2012).
Outcomes for the baby for each planned place of birth: low-risk multiparous women (source: Birthplace 2011).
- there are no differences in outcomes for the baby associated with planning birth in an alongside midwifery unit, a freestanding midwifery unit or an obstetric unit
- planning birth at home is associated with an overall small increase (about 4 more per 1,000 births) in the risk of a baby having a serious medical problem compared with planning birth in other settings. [2014]
Rates of spontaneous vaginal birth, transfer to an obstetric unit and obstetric interventions for each planned place of birth: low-risk nulliparous women (sources: Birthplace 2011; Blix et al. 2012).
Outcomes for the baby for each planned place of birth: low-risk nulliparous women (source: Birthplace 2011).
Ensure that all healthcare professionals involved in the care of pregnant women are familiar with the types and frequencies of serious medical problems that can affect babies (see appendix A ), in order to be able to provide this information to women if they request it. [2014]
Commissioners and providers (this can also include networks of providers) should ensure that all 4 birth settings are available to all women (in the local area or in a neighbouring area). [2014]
the likelihood of being cared for in labour by a familiar midwife
the likelihood of receiving one-to-one care throughout labour (not necessarily being cared for by the same midwife for the whole of labour).
- Access to medical staff (obstetric, anaesthetic and neonatal).
- Access to pain relief, including birthing pools, Entonox, other drugs and regional analgesia.
- The likelihood of being transferred to an obstetric unit (if this is not the woman’s chosen place of birth), the reasons why this might happen and the time it may take. Refer to table 5 if no local data are available. [2014]
Primary reasons for transfer to an obstetric unit (source: Birthplace 2011).
If further discussion is wanted by either the midwife or the woman about the choice of planned place of birth, arrange this with a consultant midwife or supervisor of midwives, and/or a consultant obstetrician if there are obstetric issues. [2014]
When discussing the woman’s choice of place of birth with her, do not disclose personal views or judgements about her choices. [2014]
Medical conditions and other factors that may affect planned place of birth
- Table 6 and table 7 show medical conditions or situations in which there is increased risk for the woman or baby during or shortly after labour, where care in an obstetric unit would be expected to reduce this risk.
- The factors listed in table 8 and table 9 are not reasons in themselves for advising birth within an obstetric unit, but indicate that further consideration of birth setting may be required.
- Discuss these risks and the additional care that can be provided in the obstetric unit with the woman so that she can make an informed choice about planned place of birth. [2007, amended 2014]
Medical conditions indicating increased risk suggesting planned birth at an obstetric unit.
Other factors indicating increased risk suggesting planned birth at an obstetric unit.
Medical conditions indicating individual assessment when planning place of birth.
Other factors indicating individual assessment when planning place of birth.
Women’s experience in all birth settings
For all women giving birth in all birth settings, follow the principles in the NICE guideline on patient experience in adult NHS services . [2014]
Providers, senior staff and all healthcare professionals should ensure that in all birth settings there is a culture of respect for each woman as an individual undergoing a significant and emotionally intense life experience, so that the woman is in control, is listened to and is cared for with compassion, and that appropriate informed consent is sought. [2014]
Senior staff should demonstrate, through their own words and behaviour, appropriate ways of relating to and talking about women and their birth companion(s), and of talking about birth and the choices to be made when giving birth. [2014]
One-to-one care in all birth settings
- provide a model of care that supports one-to-one care in labour for all women and
- benchmark services and identify overstaffing or understaffing by using workforce planning models and/or woman-to-midwife ratios. [2014]
Service organisation and clinical governance
Ensure that all women giving birth have timely access to an obstetric unit if they need transfer of care for medical reasons or because they request regional analgesia. [2014]
- robust protocols in place for transfer of care between settings (see also the section on general principles for transfer of care )
when crossing provider boundaries
if the nearest obstetric or neonatal unit is closed to admissions or the local midwifery-led unit is full. [2014]
Commissioners and providers (this can also include networks of providers) should ensure that there are multidisciplinary clinical governance structures in place to enable the oversight of all birth settings. These structures should include, as a minimum, midwifery (including a supervisor of midwives), obstetric, anaesthetic and neonatal expertise, and adequately supported user representation. [2014]
1.2. Care throughout labour
Communication.
Treat all women in labour with respect. Ensure that the woman is in control of and involved in what is happening to her, and recognise that the way in which care is given is key to this. To facilitate this, establish a rapport with the woman, ask her about her wants and expectations for labour, and be aware of the importance of tone and demeanour, and of the actual words used. Use this information to support and guide her through her labour. [2007]
- Greet the woman with a smile and a personal welcome, establish her language needs, introduce yourself and explain your role in her care.
- Maintain a calm and confident approach so that your demeanour reassures the woman that all is going well.
- Knock and wait before entering the woman’s room, respecting it as her personal space, and ask others to do the same.
- Ask how the woman is feeling and whether there is anything in particular she is worried about.
- If the woman has a written birth plan, read and discuss it with her.
- Assess the woman’s knowledge of strategies for coping with pain and provide balanced information to find out which available approaches are acceptable to her.
- Encourage the woman to adapt the environment to meet her individual needs.
- Ask her permission before all procedures and observations, focusing on the woman rather than the technology or the documentation.
- Show the woman and her birth companion(s) how to summon help and reassure her that she may do so whenever and as often as she needs to. When leaving the room, let her know when you will return.
- Involve the woman in any handover of care to another professional, either when additional expertise has been brought in or at the end of a shift. [2007]
Mobilisation
Encourage and help the woman to move and adopt whatever positions she finds most comfortable throughout labour. [2007]
Encourage the woman to have support from birth companion(s) of her choice. [2007]
Hygiene measures
Tap water may be used if cleansing is required before vaginal examination. [2007]
Routine hygiene measures taken by staff caring for women in labour, including standard hand hygiene and single-use non-sterile gloves, are appropriate to reduce cross-contamination between women, babies and healthcare professionals. [2007]
Selection of protective equipment must be based on an assessment of the risk of transmission of microorganisms to the woman, and the risk of contamination of the healthcare worker’s clothing and skin by women’s blood, body fluids, secretions or excretions. This is in accordance with the following health and safety legislation (current at the time NICE’s guideline on healthcare-associated infections was published [March 2012]): Health and Safety at Work Act 1974 , Management of Health and Safety at Work Regulations 1999 , Health and Safety Regulations 2002 , Control of Substances Hazardous to Health Regulations 2002 , Personal Protective Equipment Regulations 2002 and Health and Social Care Act 2008 ). [2007, amended 2014]
This recommendation is adapted from NICE’s guideline on healthcare-associated infections .
1.3. Latent first stage of labour
Definitions of the latent and established first stages of labour.
there are painful contractions and
there is some cervical change, including cervical effacement and dilatation up to 4 cm.
there are regular painful contractions and
there is progressive cervical dilatation from 4 cm. [2007]
Education and early assessment
- what to expect in the latent first stage of labour
- how to work with any pain they experience
- how to contact their midwifery care team and what to do in an emergency. [2014]
- how to differentiate between Braxton Hicks contractions and active labour contractions
- the expected frequency of contractions and how long they last
- recognition of amniotic fluid (‘waters breaking’)
- description of normal vaginal loss. [2014]
Consider an early assessment of labour by telephone triage provided by a dedicated triage midwife for all women. [2014]
- at home (regardless of planned place of birth) or
- in an assessment facility in her planned place of birth (midwifery-led unit or obstetric unit), comprising one-to-one midwifery care for at least 1 hour. [2014]
- ask the woman how she is, and about her wishes, expectations and any concerns she has
- ask the woman about the baby’s movements, including any changes
- give information about what the woman can expect in the latent first stage of labour and how to work with any pain she experiences
- give information about what to expect when she accesses care
- agree a plan of care with the woman, including guidance about who she should contact next and when
- provide guidance and support to the woman’s birth companion(s). [2014]
The triage midwife should document the guidance that she gives to the woman. [2014]
- recognise that a woman may experience painful contractions without cervical change, and although she is described as not being in labour, she may well think of herself as being ‘in labour’ by her own definition
- offer her individualised support, and analgesia if needed
- encourage her to remain at or return home, unless doing so leads to a significant risk that she could give birth without a midwife present or become distressed. [2014]
Pain relief
Advise the woman and her birth companion(s) that breathing exercises, immersion in water and massage may reduce pain during the latent first stage of labour. (See also the recommendation on timing of regional analgesia .) [2014]
Do not offer or advise aromatherapy, yoga or acupressure for pain relief during the latent first stage of labour. If a woman wants to use any of these techniques, respect her wishes. [2014]
1.4. Initial assessment
When performing an initial assessment of a woman in labour, listen to her story and take into account her preferences and her emotional and psychological needs. [2014]
Review the antenatal notes (including all antenatal screening results) and discuss these with the woman.
Ask her about the length, strength and frequency of her contractions.
Ask her about any pain she is experiencing and discuss her options for pain relief.
Record her pulse, blood pressure and temperature, and carry out urinalysis.
Record if she has had any vaginal loss.
Ask the woman about the baby’s movements in the last 24 hours.
Palpate the woman’s abdomen to determine the fundal height, the baby’s lie, presentation, position, engagement of the presenting part, and frequency and duration of contractions.
- Auscultate the fetal heart rate for a minimum of 1 minute immediately after a contraction. Palpate the woman’s pulse to differentiate between the heartbeats of the woman and the baby. In addition (see also recommendation 1.4.5 ):
- If there is uncertainty about whether the woman is in established labour, a vaginal examination may be helpful after a period of assessment, but is not always necessary.
- If the woman appears to be in established labour, offer a vaginal examination. [2014]
pulse over 120 beats/minute on 2 occasions 30 minutes apart
a single reading of either raised diastolic blood pressure of 110 mmHg or more or raised systolic blood pressure of 160 mmHg or more
either raised diastolic blood pressure of 90 mmHg or more or raised systolic blood pressure of 140 mmHg or more on 2 consecutive readings taken 30 minutes apart
a reading of 2+ of protein on urinalysis and a single reading of either raised diastolic blood pressure (90 mmHg or more) or raised systolic blood pressure (140 mmHg or more)
temperature of 38°C or above on a single reading, or 37.5°C or above on 2 consecutive readings 1 hour apart
any vaginal blood loss other than a show
rupture of membranes more than 24 hours before the onset of established labour (see the section on babies born to women with prelabour rupture of the membranes at term )
the presence of significant meconium (see the section on presence of meconium )
pain reported by the woman that differs from the pain normally associated with contractions
any risk factors recorded in the woman’s notes that indicate the need for obstetric led care.
any abnormal presentation, including cord presentation
transverse or oblique lie
high (4/5 to 5/5 palpable) or free-floating head in a nulliparous woman
suspected fetal growth restriction or macrosomia
suspected anhydramnios or polyhydramnios
fetal heart rate below 110 or above 160 beats/minute
a deceleration in fetal heart rate heard on intermittent auscultation
reduced fetal movements in the last 24 hours reported by the woman.
If none of these are observed, continue with midwifery-led care unless the woman requests transfer (see also the section on measuring fetal heart rate as part of initial assessment ). [2014]
If any of the factors in recommendation 1.4.3 are observed but birth is imminent, assess whether birth in the current location is preferable to transferring the woman to an obstetric unit and discuss this with the coordinating midwife. [2014]
- be sure that the examination is necessary and will add important information to the decision-making process
- recognise that a vaginal examination can be very distressing for a woman, especially if she is already in pain, highly anxious and in an unfamiliar environment
- explain the reason for the examination and what will be involved
- ensure the woman’s informed consent, privacy, dignity and comfort
- explain sensitively the findings of the examination and any impact on the birth plan to the woman and her birth companion(s). [2014]

Measuring fetal heart rate as part of initial assessment
- Use either a Pinard stethoscope or doppler ultrasound.
- Carry out auscultation immediately after a contraction for at least 1 minute and record it as a single rate.
- Record accelerations and decelerations if heard.
- Palpate the maternal pulse to differentiate between the maternal and fetal heartbeats. [2017]
Be aware that for women at low risk of complications there is insufficient evidence about whether cardiotocography as part of the initial assessment either improves outcomes or results in harm for women and their babies, compared with intermittent auscultation alone. [2017]
- discuss the risks, benefits and limitations of cardiotocography with her, and support her in her choice
- explain that, if she is in a setting where cardiotocography is not available, she will need to be transferred to obstetric-led care. [2017]
Offer continuous cardiotocography if any of the risk factors listed in recommendation 1.4.3 in the section on initial assessment are identified on initial assessment, and explain to the woman why this is being offered. (See also the NICE guideline on fetal monitoring in labour for further guidance on fetal monitoring.) [2017]
Offer cardiotocography if intermittent auscultation indicates possible fetal heart rate abnormalities, and explain to the woman why this is being offered. If the trace is normal (see the section on the use of cardiotocography for monitoring during labour in the NICE guideline on fetal monitoring in labour ) after 20 minutes, return to intermittent auscultation unless the woman asks to stay on continuous cardiotocography. [2017]
If fetal death is suspected despite the presence of an apparently recorded fetal heart rate, offer real-time ultrasound assessment to check fetal viability. [2017]
1.5. Ongoing assessment
temperature of 38°C or above on a single reading, or 37.5°C or above on 2 consecutive occasions 1 hour apart
confirmed delay in the first or second stage of labour
request by the woman for additional pain relief using regional analgesia
obstetric emergency – including antepartum haemorrhage, cord prolapse, postpartum haemorrhage, maternal seizure or collapse, or a need for advanced neonatal resuscitation
retained placenta
third-degree or fourth-degree tear or other complicated perineal trauma that needs suturing.
a deceleration in fetal heart rate heard on intermittent auscultation.
If none of these are observed, continue with midwifery-led care unless the woman requests transfer (see also recommendation 1.4.6 in the section on measuring fetal heart rate as part of initial assessment ). [2014]
Presence of meconium
As part of ongoing assessment, document the presence or absence of significant meconium. This is defined as dark green or black amniotic fluid that is thick or tenacious, or any meconium-stained amniotic fluid containing lumps of meconium. [2014]
- healthcare professionals trained in fetal blood sampling are available during labour and
- healthcare professionals trained in advanced neonatal life support are readily available for the birth. [2014]
If significant meconium is present, transfer the woman to obstetric-led care provided that it is safe to do so and the birth is unlikely to occur before transfer is completed. Follow the general principles for transfer of care described in section 1.6 . [2014]
1.6. General principles for transfer of care
Transfer of care refers to the transfer between midwifery-led care and obstetric-led care. This may or may not involve transport from one location to another. Women who are receiving midwifery-led care in an obstetric unit can have their care transferred to obstetric-led care without being moved.
Base any decisions about transfer of care on clinical findings, and discuss the options with the woman and her birth companion(s). [2014]
- talk with the woman and her birth companion(s) about the reasons for this and what they can expect, including the time needed for transfer
- address any concerns she has and try to allay her anxiety
- ensure that her wishes are respected and her informed consent is obtained. [2014]
When arranging transfer of care, the midwife attending the labour should contact the ambulance service (if appropriate) and the coordinating midwife in the obstetric unit. The coordinating midwife should then alert the relevant healthcare professionals (obstetric, anaesthetic and neonatal). [2014]
- Before transfer, the woman is dressed, wrapped in a blanket or otherwise covered in a way that she feels is comfortable and appropriate.
- The woman is made to feel as comfortable as possible before and during transfer.
- Any ambulance staff or other personnel involved are aware that some positions may make the woman uncomfortable or afraid and could affect her labour, so she should be encouraged to choose how to move and what position to adopt if possible, in accordance with ambulance service protocols.
- Communication and companionship are maintained. Explain the arrangements for transfer to the woman and her birth companion(s). A midwife who has been involved in her care up to that point should travel with her and carry out a handover of care that involves the woman.
- Arrangements are in place to enable the woman’s birth companion(s) to travel with her in the ambulance if that is what she wants. If this is not possible or not wanted, check that the birth companion(s) have or can arrange their own transport. [2014]
If a woman is transferred to an obstetric unit after the birth (see the section on care of the woman after birth ), ensure that her baby goes with her. [2014]
1.7. Care in established labour
Support in labour.
Provide a woman in established labour with supportive one-to-one care. [2007]
Do not leave a woman in established labour on her own except for short periods or at the woman’s request. [2007]
For guidance on ensuring continuity of care, see recommendation 1.4.1 in the NICE guideline on patient experience in adult NHS services . [2016]
Controlling gastric acidity
Do not offer either H 2 -receptor antagonists or antacids routinely to low-risk women. [2007]
Either H 2 -receptor antagonists or antacids should be considered for women who receive opioids or who have or develop risk factors that make a general anaesthetic more likely. [2007]
Inform the woman that she may drink during established labour and that isotonic drinks may be more beneficial than water. [2007]
Inform the woman that she may eat a light diet in established labour unless she has received opioids or she develops risk factors that make a general anaesthetic more likely. [2007]
1.8. Pain relief in labour: non-regional
Attitudes to pain and pain relief in childbirth.
Healthcare professionals should think about how their own values and beliefs inform their attitude to coping with pain in labour and ensure their care supports the woman’s choice. [2007]
Pain-relieving strategies
If a woman chooses to use breathing and relaxation techniques in labour, support her in this choice. [2007]
If a woman chooses to use massage techniques in labour that have been taught to birth companions, support her in this choice. [2007]
Offer the woman the opportunity to labour in water for pain relief. [2007]
For women labouring in water, monitor the temperature of the woman and the water hourly to ensure that the woman is comfortable and not becoming pyrexial. The temperature of the water should not be above 37.5°C. [2007]
Keep baths and birthing pools clean using a protocol agreed with the microbiology department and, in the case of birthing pools, in accordance with the manufacturer’s guidelines. [2007]
Do not use injected water papules. [2007]
Do not offer acupuncture, acupressure or hypnosis, but do not prevent women who wish to use these techniques from doing so. [2007]
Support the playing of music of the woman’s choice in labour. [2007]
Non-pharmacological analgesia
Do not offer transcutaneous electrical nerve stimulation (TENS) to women in established labour. [2007]
Inhalational analgesia
Ensure that Entonox (a 50:50 mixture of oxygen and nitrous oxide) is available in all birth settings as it may reduce pain in labour, but inform the woman that it may make her feel nauseous and light-headed. [2007]
Intravenous and intramuscular opioids
Ensure that pethidine, diamorphine or other opioids are available in all birth settings. Inform the woman that these will provide limited pain relief during labour and may have significant side effects for both her (drowsiness, nausea and vomiting) and her baby (short-term respiratory depression and drowsiness which may last several days). [2007]
Inform the woman that pethidine, diamorphine or other opioids may interfere with breastfeeding. [2007]
If an intravenous or intramuscular opioid is used, also administer an antiemetic. [2007]
Women should not enter water (a birthing pool or bath) within 2 hours of opioid administration or if they feel drowsy. [2007]
1.9. Pain relief in labour: regional analgesia
Information about regional analgesia.
If a woman is contemplating regional analgesia, talk with her about the risks and benefits and the implications for her labour, including the arrangements and time involved for transfer of care to an obstetric unit if she is at home or in a midwifery unit (follow the general principles for transfer of care ). [2007, amended 2014]
- It is available only in obstetric units.
- It provides more effective pain relief than opioids.
- It is not associated with long-term backache.
- It is not associated with a longer first stage of labour or an increased chance of a caesarean birth.
- It is associated with a longer second stage of labour and an increased chance of vaginal instrumental birth.
- It will be accompanied by a more intensive level of monitoring and intravenous access, and so mobility may be reduced. [2007, amended 2014]
Timing of regional analgesia
If a woman in labour asks for regional analgesia, comply with her request. This includes women in severe pain in the latent first stage of labour. [2007]
Care and observations for women with regional analgesia
Always secure intravenous access before starting regional analgesia. [2007]
Preloading and maintenance fluid infusion need not be administered routinely before establishing low-dose epidural analgesia and combined spinal–epidural analgesia. [2007]
- During establishment of regional analgesia or after further boluses (10 ml or more of low-dose solutions), measure blood pressure every 5 minutes for 15 minutes.
- If the woman is not pain-free 30 minutes after each administration of local anaesthetic/opioid solution, recall the anaesthetist.
- Assess the level of the sensory block hourly. [2007]
Encourage women with regional analgesia to move and adopt whatever upright positions they find comfortable throughout labour. [2007]
Once established, continue regional analgesia until after completion of the third stage of labour and any necessary perineal repair. [2007]
Upon confirmation of full cervical dilatation in a woman with regional analgesia, unless the woman has an urge to push or the baby’s head is visible, pushing should be delayed for at least 1 hour and longer if the woman wishes, after which actively encourage her to push during contractions. [2007]
After diagnosis of full dilatation in a woman with regional analgesia, agree a plan with the woman in order to ensure that birth will have occurred within 4 hours regardless of parity. [2007]
Do not routinely use oxytocin in the second stage of labour for women with regional analgesia. [2007]
Perform continuous cardiotocography for at least 30 minutes during establishment of regional analgesia and after administration of each further bolus of 10 ml or more. [2007, amended 2014]
Establishing and maintaining regional analgesia
Use either epidural or combined spinal–epidural analgesia for establishing regional analgesia in labour. [2007]
If rapid analgesia is required, use combined spinal–epidural analgesia. [2007]
Establish combined spinal–epidural analgesia with bupivacaine and fentanyl. [2007]
Establish epidural analgesia with a low-concentration local anaesthetic and opioid solution with, for example, 10 to 15 ml of 0.0625 to 0.1% bupivacaine with 1 to 2 micrograms per ml fentanyl. The initial dose of local anaesthetic plus opioid is essentially a test dose, so administer cautiously to ensure that inadvertent intrathecal injection has not occurred. [2007]
Use low-concentration local anaesthetic and opioid solutions (0.0625 to 0.1% bupivacaine or equivalent combined with 2.0 micrograms per ml fentanyl) for maintaining epidural analgesia in labour. [2007]
Do not use high concentrations of local anaesthetic solutions (0.25% or above of bupivacaine or equivalent) routinely for either establishing or maintaining epidural analgesia. [2007]
Either patient-controlled epidural analgesia or intermittent bolus given by healthcare professionals are the preferred modes of administration for maintenance of epidural analgesia. [2007]
1.10. Monitoring during labour
The recommendations in this section have been withdrawn. For advice on monitoring during labour, see the NICE guideline on fetal monitoring in labour .
1.11. Prelabour rupture of membranes at term
Do not carry out a speculum examination if it is certain that the membranes have ruptured. [2007]
If it is uncertain whether prelabour rupture of the membranes has occurred, offer the woman a speculum examination to determine whether the membranes have ruptured. Avoid digital vaginal examination in the absence of contractions. [2007]
- the risk of serious neonatal infection is 1%, rather than 0.5% for women with intact membranes
- 60% of women with prelabour rupture of the membranes will go into labour within 24 hours
- induction of labour (see NICE’s guideline on inducing labour ) is appropriate approximately 24 hours after rupture of the membranes. [2007]
- do not offer lower vaginal swabs and measurement of maternal C-reactive protein
- to detect any infection that may be developing, advise the woman to record her temperature every 4 hours during waking hours and to report immediately any change in the colour or smell of her vaginal loss
- inform the woman that bathing or showering is not associated with an increase in infection, but that having sexual intercourse may be. [2007]
Assess fetal movement and heart rate at initial contact and then every 24 hours after rupture of the membranes while the woman is not in labour, and advise the woman to report immediately any decrease in fetal movements. [2007]
If labour has not started 24 hours after rupture of the membranes, advise the woman to give birth where there is access to neonatal services and to stay in hospital for at least 12 hours after the birth. [2007]
1.12. First stage of labour
See the recommendation on definitions of the latent and established first stages of labour .
Do not offer or advise clinical intervention if labour is progressing normally and the woman and baby are well. [2007]
In all stages of labour, women who have left the normal care pathway because of the development of complications can return to it if/when the complication is resolved. [2007]
Duration of the first stage
- first labours last on average 8 hours and are unlikely to last over 18 hours
- second and subsequent labours last on average 5 hours and are unlikely to last over 12 hours. [2007]
Observations during the established first stage
Do not routinely use verbal assessment using a numerical pain score. [2007]
Use a pictorial record of labour (partogram) once labour is established. [2007]
Where the partogram includes an action line, use the World Health Organization (WHO) recommendation of a 4-hour action line (the WHO partograph in management of labour, published in 1994 as part of the Maternal Health and Safe Motherhood Programme. Lancet 343: 1399 to 404). [2007]
See also the WHO Multicountry Survey on Maternal and Newborn Health .
- half-hourly documentation of frequency of contractions
- hourly pulse
- 4-hourly temperature and blood pressure
- frequency of passing urine
- offer a vaginal examination (see recommendation 1.4.5 in the section on initial assessment ) 4-hourly or if there is concern about progress or in response to the woman’s wishes (after abdominal palpation and assessment of vaginal loss). [2007] If any of the indications for transfer are met (see the recommendation on ongoing assessment ), transfer the woman to obstetric-led care. Follow the general principles for transfer of care . [2014]
Give ongoing consideration to the woman’s emotional and psychological needs, including her desire for pain relief. [2007]
Encourage the woman to communicate her need for analgesia at any point during labour. [2007]
Possible routine interventions in the first stage
Do not routinely offer the package known as active management of labour (one-to-one continuous support; strict definition of established labour; early routine amniotomy; routine 2-hourly vaginal examination; oxytocin if labour becomes slow). [2007]
In normally progressing labour, do not perform amniotomy routinely. [2007]
Do not use combined early amniotomy with use of oxytocin routinely. [2007]
Delay in the first stage
- cervical dilatation and rate of change
- uterine contractions
- station and position of presenting part
- the woman’s emotional state
- referral to the appropriate healthcare professional. Offer the woman support, hydration, and appropriate and effective pain relief. [2007]
- cervical dilatation of less than 2 cm in 4 hours for first labours
- cervical dilatation of less than 2 cm in 4 hours or a slowing in the progress of labour for second or subsequent labours
- descent and rotation of the baby’s head
- changes in the strength, duration and frequency of uterine contractions. [2007] If delay is diagnosed, transfer the woman to obstetric-led care. Follow the general principles for transfer of care . [2014]
If delay in the established first stage of labour is suspected, amniotomy should be considered for all women with intact membranes, after explanation of the procedure and advice that it will shorten her labour by about an hour and may increase the strength and pain of her contractions. [2007]
Whether or not a woman has agreed to an amniotomy, advise all women with suspected delay in the established first stage of labour to have a vaginal examination 2 hours later, and diagnose delay if progress is less than 1 cm. [2007]
For women with intact membranes in whom delay in the established first stage of labour is confirmed, advise the woman to have an amniotomy, and to have a repeat vaginal examination 2 hours later whether her membranes are ruptured or intact. [2007]
- transfer the woman to obstetric-led care for an obstetric review and a decision about management options, including the use of oxytocin (follow the general principles for transfer of care ) [2014]
- explain to her that using oxytocin after spontaneous or artificial rupture of the membranes will bring forward the time of birth but will not influence the mode of birth or other outcomes. [2007]
For a multiparous woman with confirmed delay in the established first stage of labour, an obstetrician should perform a full assessment, including abdominal palpation and vaginal examination, before a decision is made about using oxytocin. [2007]
Offer all women with delay in the established first stage of labour support and effective pain relief. [2007]
Inform the woman that oxytocin will increase the frequency and strength of her contractions and that its use will mean that her baby should be monitored continuously. Offer the woman an epidural before oxytocin is started. [2007]
If oxytocin is used, ensure that the time between increments of the dose is no more frequent than every 30 minutes. Increase oxytocin until there are 4 to 5 contractions in 10 minutes. (See also recommendation 1.12.14 and recommendation 1.3.8 in the NICE guideline on fetal monitoring in labour .) [2007]
- If cervical dilatation has increased by less than 2 cm after 4 hours of oxytocin, further obstetric review is required to assess the need for caesarean section.
- If cervical dilatation has increased by 2 cm or more, advise 4-hourly vaginal examinations. [2007]
1.13. Second stage of labour
Definition of the second stage.
the finding of full dilatation of the cervix before or in the absence of involuntary expulsive contractions.
the baby is visible
expulsive contractions with a finding of full dilatation of the cervix or other signs of full dilatation of the cervix
active maternal effort following confirmation of full dilatation of the cervix in the absence of expulsive contractions. [2007]
Observations during the second stage
- half-hourly documentation of the frequency of contractions [2007]
- hourly blood pressure [2007]
- continued 4-hourly temperature [2007]
- frequency of passing urine [2007]
- offer a vaginal examination (see recommendation 1.4.5 in the section on initial assessment ) hourly in the active second stage, or in response to the woman’s wishes (after abdominal palpation and assessment of vaginal loss). [2007] In addition:
- Continue to take the woman’s emotional and psychological needs into account. [2007]
- Assess progress, which should include the woman’s behaviour, the effectiveness of pushing and the baby’s wellbeing, taking into account the baby’s position and station at the onset of the second stage. These factors will assist in deciding the timing of further vaginal examination and any need for transfer to obstetric led care. [2007, amended 2014]
- Perform intermittent auscultation of the fetal heart rate immediately after a contraction for at least 1 minute, at least every 5 minutes. Palpate the woman’s pulse every 15 minutes to differentiate between the two heartbeats. [2007, amended 2014]
- Ongoing consideration should be given to the woman’s position, hydration, coping strategies and pain relief throughout the second stage. [2007]
Duration of the second stage and definition of delay
- birth would be expected to take place within 3 hours of the start of the active second stage in most women
- diagnose delay in the active second stage when it has lasted 2 hours and refer the woman to a healthcare professional trained to undertake an operative vaginal birth if birth is not imminent. [2007]
- birth would be expected to take place within 2 hours of the start of the active second stage in most women
- diagnose delay in the active second stage when it has lasted 1 hour and refer the woman to a healthcare professional trained to undertake an operative vaginal birth if birth is not imminent. [2007]
For a nulliparous woman, suspect delay if progress (in terms of rotation and/or descent of the presenting part) is inadequate after 1 hour of active second stage. Offer vaginal examination and then offer amniotomy if the membranes are intact. [2007, amended 2014]
For a multiparous woman, suspect delay if progress (in terms of rotation and/or descent of the presenting part) is inadequate after 30 minutes of active second stage. Offer vaginal examination and then offer amniotomy if the membranes are intact. [2014]
If full dilatation of the cervix has been confirmed in a woman without regional analgesia, but she does not get an urge to push, carry out further assessment after 1 hour. [2007]
Oxytocin in the second stage
Consideration should be given to the use of oxytocin, with the offer of regional analgesia, for nulliparous women if contractions are inadequate at the onset of the second stage. [2007]
The woman’s position and pushing in the second stage
Discourage the woman from lying supine or semi-supine in the second stage of labour and encourage her to adopt any other position that she finds most comfortable. [2007]
Inform the woman that in the second stage she should be guided by her own urge to push. [2007]
If pushing is ineffective or if requested by the woman, offer strategies to assist birth, such as support, change of position, emptying of the bladder and encouragement. [2007]
Intrapartum interventions to reduce perineal trauma
Do not perform perineal massage in the second stage of labour. [2007]
Either the ‘hands on’ (guarding the perineum and flexing the baby’s head) or the ‘hands poised’ (with hands off the perineum and baby’s head but in readiness) technique can be used to facilitate spontaneous birth. [2007]
Do not offer lidocaine spray to reduce pain in the second stage of labour. [2007]
Do not carry out a routine episiotomy during spontaneous vaginal birth. [2007]
Inform any woman with a history of severe perineal trauma that her risk of repeat severe perineal trauma is not increased in a subsequent birth, compared with women having their first baby. [2007]
Do not offer episiotomy routinely at vaginal birth after previous third- or fourth-degree trauma. [2007]
- current urgency or incontinence symptoms
- the degree of previous trauma
- risk of recurrence
- the success of the repair undertaken
- the psychological effect of the previous trauma
- management of her labour. [2007]
Inform any woman with infibulated genital mutilation of the risks of difficulty with vaginal examination, catheterisation and application of fetal scalp electrodes. Inform her of the risks of delay in the second stage and spontaneous laceration together with the need for an anterior episiotomy and the possible need for defibulation in labour. [2007]
If an episiotomy is performed, the recommended technique is a mediolateral episiotomy originating at the vaginal fourchette and usually directed to the right side. The angle to the vertical axis should be between 45 and 60 degrees at the time of the episiotomy. [2007]
Perform an episiotomy if there is a clinical need, such as instrumental birth or suspected fetal compromise. [2007]
Provide tested effective analgesia before carrying out an episiotomy, except in an emergency because of acute fetal compromise. [2007]
Water birth
Inform women that there is insufficient high-quality evidence to either support or discourage giving birth in water. [2007]
Delay in the second stage
If there is delay in the second stage of labour, or if the woman is excessively distressed, support and sensitive encouragement and the woman’s need for analgesia/anaesthesia are particularly important. [2007]
An obstetrician should assess a woman with confirmed delay in the second stage (after transfer to obstetric-led care, following the general principles for transfer of care ) before contemplating the use of oxytocin. [2014]
After initial obstetric assessment of a woman with delay in the second stage, maintain ongoing obstetric review every 15 to 30 minutes. [2007]
Instrumental birth and delayed second stage
Think about offering instrumental birth if there is concern about the baby’s wellbeing or there is a prolonged second stage. [2007]
Recognise that, on rare occasions, the woman’s need for help in the second stage may be an indication to assist by offering instrumental birth when supportive care has not helped. [2007]
The choice of instrument depends on a balance of clinical circumstance and practitioner experience. [2007]
Because instrumental birth is an operative procedure, advise the woman to have tested effective anaesthesia. [2007]
If a woman declines anaesthesia, offer a pudendal block combined with local anaesthetic to the perineum during instrumental birth. [2007]
If there is concern about fetal compromise, offer either tested effective anaesthesia or, if time does not allow this, a pudendal block combined with local anaesthetic to the perineum during instrumental birth. [2007]
Advise the woman to have a caesarean section if vaginal birth is not possible (see the NICE guideline on caesarean birth ). [2007] .
Expediting birth
- the degree of urgency
- clinical findings on abdominal and vaginal examination
- choice of mode of birth (and whether to use forceps or ventouse if an instrumental birth is indicated)
- anticipated degree of difficulty, including the likelihood of success if instrumental birth is attempted
- any time that may be needed for transfer to obstetric-led care
- the need for additional analgesia or anaesthesia
- the woman’s preferences. [2014]
Talk with the woman and her birth companion(s) about why the birth needs to be expedited and what the options are. [2014]
Inform the team about the degree of urgency. [2014]
Record the time at which the decision to expedite the birth is made. [2014]
1.14. Third stage of labour
Recognise that the time immediately after the birth is when the woman and her birth companion(s) are meeting and getting to know the baby. Ensure that any care or interventions are sensitive to this and minimise separation or disruption of the mother and baby. [2014]
Definition of the third stage
- The third stage of labour is the time from the birth of the baby to the expulsion of the placenta and membranes.
routine use of uterotonic drugs
deferred clamping and cutting of the cord
controlled cord traction after signs of separation of the placenta.
no routine use of uterotonic drugs
no clamping of the cord until pulsation has stopped
delivery of the placenta by maternal effort. [2014]
Prolonged third stage
Diagnose a prolonged third stage of labour if it is not completed within 30 minutes of the birth with active management or within 60 minutes of the birth with physiological management. Follow the recommendations on managing retained placenta . [2014]
Observations in the third stage
- her general physical condition, as shown by her colour, respiration and her own report of how she feels
- vaginal blood loss. [2014]
- transfer her to obstetric-led care (following the general principles for transfer of care )
- carry out frequent observations to assess whether resuscitation is needed. [2014]
Active and physiological management of the third stage
Explain to the woman antenatally about what to expect with each package of care for managing the third stage of labour and the benefits and risks associated with each. [2014]
- shortens the third stage compared with physiological management
- is associated with nausea and vomiting in about 100 in 1,000 women
- is associated with an approximate risk of 13 in 1,000 of a haemorrhage of more than 1 litre
- is associated with an approximate risk of 14 in 1,000 of a blood transfusion. [2014]
- is associated with nausea and vomiting in about 50 in 1,000 women
- is associated with an approximate risk of 29 in 1,000 of a haemorrhage of more than 1 litre
- is associated with an approximate risk of 40 in 1,000 of a blood transfusion. [2014]
Discuss again with the woman at the initial assessment in labour (see the section on initial assessment ) about the different options for managing the third stage and ways of supporting her during delivery of the placenta, and ask if she has any preferences. [2014]
Advise the woman to have active management of the third stage, because it is associated with a lower risk of a postpartum haemorrhage and/or blood transfusion. [2014]
If a woman at low risk of postpartum haemorrhage requests physiological management of the third stage, support her in her choice. [2014]
Document in the records the decision that is agreed with the woman about management of the third stage. [2014]
For active management, administer 10 IU of oxytocin by intramuscular injection with the birth of the anterior shoulder or immediately after the birth of the baby and before the cord is clamped and cut. Use oxytocin as it is associated with fewer side effects than oxytocin plus ergometrine. [2014]
- Do not clamp the cord earlier than 1 minute from the birth of the baby unless there is concern about the integrity of the cord or the baby has a heart rate below 60 beats/minute that is not getting faster.
- Clamp the cord before 5 minutes in order to perform controlled cord traction as part of active management.
- If the woman requests that the cord is clamped and cut later than 5 minutes, support her in her choice. [2014]
After cutting the cord, use controlled cord traction. [2014]
Perform controlled cord traction as part of active management only after administration of oxytocin and signs of separation of the placenta. [2014]
Record the timing of cord clamping in both active and physiological management. [2014]
- haemorrhage
- the placenta is not delivered within 1 hour of the birth of the baby. [2014]
Offer a change from physiological management to active management if the woman wants to shorten the third stage. [2014]
Do not use either umbilical oxytocin infusion or prostaglandin routinely in the third stage of labour. [2014]
Retained placenta
Secure intravenous access if the placenta is retained, and explain to the woman why this is needed. [2014]
Do not use umbilical vein agents if the placenta is retained. [2014]
Do not use intravenous oxytocic agents routinely to deliver a retained placenta. [2014]
Give intravenous oxytocic agents if the placenta is retained and the woman is bleeding excessively. [2014]
- offer a vaginal examination to assess the need to undertake manual removal of the placenta
- explain that this assessment can be painful and advise her to have analgesia. [2014]
If the woman reports inadequate analgesia during the assessment, stop the examination and address this immediately. [2014]
If uterine exploration is necessary and the woman is not already in an obstetric unit, arrange urgent transfer (following the general principles for transfer of care ). [2014]
Do not carry out uterine exploration or manual removal of the placenta without an anaesthetic. [2014]
Postpartum haemorrhage
Risk factors.
previous retained placenta or postpartum haemorrhage
maternal haemoglobin level below 85 g/litre at onset of labour
BMI greater than 35 kg/m 2
grand multiparity (parity 4 or more)
antepartum haemorrhage
overdistention of the uterus (for example, multiple pregnancy, polyhydramnios or macrosomia)
existing uterine abnormalities
low-lying placenta
maternal age of 35 years or older.
prolonged first, second or third stage of labour
oxytocin use
precipitate labour
operative birth or caesarean section. [2007]
If a woman has risk factors for postpartum haemorrhage, highlight these in her notes, and make and discuss with her a care plan covering the third stage of labour. [2007]
- call for help
emptying of the bladder and
uterine massage and
uterotonic drugs and
intravenous fluids and
controlled cord traction if the placenta has not yet been delivered
- continuously assess blood loss and the woman’s condition, and identify the source of the bleeding
- give supplementary oxygen
- arrange for transfer of the woman to obstetric-led care (following the general principles for transfer of care ). [2014]
- oxytocin (10 IU intravenous) or
- ergometrine (0.5 mg intramuscular) or
- combined oxytocin and ergometrine (5 IU/0.5 mg intramuscular). [2014]
oxytocin (intravenous)
ergometrine (intramuscular, or cautiously intravenously)
combined oxytocin and ergometrine (intramuscular)
- misoprostol
- oxytocin infusion
- carboprost (intramuscular). [2014]
- tranexamic acid (intravenous)
- rarely, in the presence of otherwise normal clotting factors, rFactor VIIa, in consultation with a haematologist. [2014]
Allocate a member of the healthcare team to stay with the woman and her birth companion(s), explain what is happening, answer any questions and offer support throughout the emergency situation. [2014]
- perform examination under anaesthetic
- ensure that the uterus is empty and repair any trauma
- consider balloon tamponade before surgical options. [2014]
Be aware that no particular surgical procedure can be recommended over any other for treating postpartum haemorrhage. [2014]
The maternity service and ambulance service should have strategies in place in order to respond quickly and appropriately if a woman has a postpartum haemorrhage in any setting. [2014]
1.15. Care of the newborn baby
Initial assessment of the newborn baby and mother–baby bonding.
Recommendations 1.15.6 , 1.15.8 and 1.15.9 have been adapted from NICE’s guideline on postnatal care ; refer to this guideline for more information on immediate postnatal care (within 2 hours of birth).
Record the Apgar score routinely at 1 and 5 minutes for all births. [2007]
Record the time from birth to the onset of regular respirations. [2014]
- follow the recommendations in the section on neonatal resuscitation and
- take paired cord-blood samples for blood gas analysis, after clamping the cord using 2 clamps. Continue to evaluate and record the baby’s condition until it is improved and stable. [2014]
Do not take paired cord blood samples (for blood gas analysis) routinely. [2014]
Ensure that a second clamp to allow double-clamping of the cord is available in all birth settings. [2014]
Encourage women to have skin-to-skin contact with their babies as soon as possible after the birth. [2007]
In order to keep the baby warm, dry and cover him or her with a warm, dry blanket or towel while maintaining skin-to-skin contact with the woman. [2007]
Avoid separation of a woman and her baby within the first hour of the birth for routine postnatal procedures, for example, weighing, measuring and bathing, unless these measures are requested by the woman, or are necessary for the immediate care of the baby. [2007]
Encourage initiation of breastfeeding as soon as possible after the birth, ideally within 1 hour. [2007]
Record head circumference, body temperature and birth weight soon after the first hour following birth. [2007]
Undertake an initial examination to detect any major physical abnormality and to identify any problems that require referral. [2007]
Ensure that any examination or treatment of the baby is undertaken with the consent of the parents and either in their presence or, if this is not possible, with their knowledge. [2007]
Neonatal resuscitation
In the first minutes after birth, evaluate the condition of the baby – specifically respiration, heart rate and tone – in order to determine whether resuscitation is needed according to nationally accredited guidelines on neonatal resuscitation. [2014]
All relevant healthcare professionals caring for women during birth should attend annually a course in neonatal resuscitation that is consistent with nationally accredited guidelines on neonatal resuscitation. [2014]
- bear in mind that it will be necessary to call for help if the baby needs resuscitation, and plan accordingly
- ensure that there are facilities for resuscitation, and for transferring the baby to another location if necessary
- develop emergency referral pathways for both the woman and the baby, and implement these if necessary. [2014]
If a newborn baby needs basic resuscitation, start with air. [2014]
Minimise separation of the baby and mother, taking into account the clinical circumstances. [2014]
Throughout an emergency situation in which the baby needs resuscitation, allocate a member of the healthcare team to talk with, and offer support to, the woman and any birth companion(s). [2014]
Care of babies in the presence of meconium
- do not suction the baby’s upper airways (nasopharynx and oropharynx) before birth of the shoulders and trunk
- do not suction the baby’s upper airways (nasopharynx and oropharynx) if the baby has normal respiration, heart rate and tone
- do not intubate if the baby has normal respiration, heart rate and tone. [2014]
If there has been significant meconium (see recommendation 1.5.2 in the section on presence of meconium ) and the baby does not have normal respiration, heart rate and tone, follow nationally accredited guidelines on neonatal resuscitation, including early laryngoscopy and suction under direct vision. [2014]
If there has been significant meconium and the baby is healthy, closely observe the baby within a unit with immediate access to a neonatologist. Perform these observations at 1 and 2 hours of age and then 2-hourly until 12 hours of age. [2014]
If there has been non-significant meconium, observe the baby at 1 and 2 hours of age in all birth settings. [2014]
- respiratory rate above 60 per minute
- the presence of grunting
- heart rate below 100 or above 160 beats/minute
- capillary refill time above 3 seconds
- body temperature of 38°C or above, or 37.5°C on 2 occasions 30 minutes apart
- oxygen saturation below 95% (measuring oxygen saturation is optional after non-significant meconium)
- presence of central cyanosis, confirmed by pulse oximetry if available. [2014] Be aware that some pulse oximeters can underestimate or overestimate oxygen saturation levels, especially if the saturation level is borderline. Overestimation has been reported in people with dark skin. See also the NHS England Patient Safety Alert on the risk of harm from inappropriate placement of pulse oximeter probes .
Explain the findings to the woman, and inform her about what to look out for and who to talk to if she has any concerns. [2014]
Babies born to women with prelabour rupture of the membranes at term
- temperature
- respiratory rate
- presence of respiratory grunting
- significant subcostal recession
- presence of nasal flare
- presence of central cyanosis, confirmed by pulse oximetry if available
- skin perfusion assessed by capillary refill
- floppiness, general wellbeing and feeding. If any of these are observed, ask a neonatologist to assess the baby (transfer both the woman and baby if they are at home or in a freestanding midwifery unit, following the general principles for transfer of care ). [2014] Be aware that some pulse oximeters can underestimate or overestimate oxygen saturation levels, especially if the saturation level is borderline. Overestimation has been reported in people with dark skin. See also the NHS England Patient Safety Alert on the risk of harm from inappropriate placement of pulse oximeter probes .
If there are no signs of infection in the woman, do not give antibiotics to either the woman or the baby, even if the membranes have been ruptured for over 24 hours. [2007]
If there is evidence of infection in the woman, prescribe a full course of broad-spectrum intravenous antibiotics. [2007]
Advise women with prelabour rupture of the membranes to inform their healthcare professionals immediately of any concerns they have about their baby’s wellbeing in the first 5 days after birth, particularly in the first 12 hours when the risk of infection is greatest. [2007]
Do not perform blood, cerebrospinal fluid and/or surface culture tests in an asymptomatic baby. [2007]
Refer a baby with any symptom of possible sepsis, or born to a woman who has evidence of chorioamnionitis, to a neonatal care specialist immediately. [2007]
1.16. Care of the woman after birth
Initial assessment.
- Record her temperature, pulse and blood pressure. Transfer the woman (with her baby) to obstetric-led care if any of the relevant indications listed in the recommendation on ongoing assessment are met.
- Uterine contraction and lochia.
- Examine the placenta and membranes: assess their condition, structure, cord vessels and completeness. Transfer the woman (with her baby) to obstetric-led care if the placenta is incomplete.
- Early assessment of the woman’s emotional and psychological condition in response to labour and birth.
- Successful voiding of the bladder. Assess whether to transfer the woman (with her baby) to obstetric-led care after 6 hours if her bladder is palpable and she is unable to pass urine. If transferring the woman to obstetric-led care, follow the general principles for transfer of care . [2014]
Perineal care
- first degree – injury to skin only
- second degree – injury to the perineal muscles but not the anal sphincter
3a – less than 50% of external anal sphincter thickness torn
3b – more than 50% of external anal sphincter thickness torn
3c – internal anal sphincter torn.
- fourth degree – injury to the perineum involving the anal sphincter complex (external and internal anal sphincter) and anal epithelium. [2007]
- explain to the woman what is planned and why
- offer inhalational analgesia
- ensure good lighting
- position the woman so that she is comfortable and so that the genital structures can be seen clearly. [2007]
Perform the initial examination gently and with sensitivity. It may be done in the immediate period after birth. [2007]
If genital trauma is identified after birth, offer further systematic assessment, including a rectal examination. [2007]
- further explanation of what is planned and why
- confirmation by the woman that tested effective local or regional analgesia is in place
- visual assessment of the extent of perineal trauma to include the structures involved, the apex of the injury and assessment of bleeding
- a rectal examination to assess whether there has been any damage to the external or internal anal sphincter if there is any suspicion that the perineal muscles are damaged. [2007]
Ensure that the timing of this systematic assessment does not interfere with mother–baby bonding unless the woman has bleeding that requires urgent attention. [2007]
Assist the woman to adopt a position that allows adequate visual assessment of the degree of trauma and for repair. Only maintain this position for as long as necessary for systematic assessment and repair. If it is not possible to adequately assess the trauma, transfer the woman (with her baby) to obstetric-led care, following the general principles for transfer of care . [2007, amended 2014]
Seek advice from a more experienced midwife or obstetrician if there is uncertainty about the nature or extent of the trauma. Transfer the woman (with her baby) to obstetric-led care (following the general principles for transfer of care ) if the repair needs further surgical or anaesthetic expertise. [2007, amended 2014]
Document the systematic assessment and its results fully, possibly pictorially. [2007]
All relevant healthcare professionals should attend training in perineal/genital assessment and repair, and ensure that they maintain these skills. [2007]
Undertake repair of the perineum as soon as possible to minimise the risk of infection and blood loss. [2007]
- ensure that tested effective analgesia is in place, using infiltration with up to 20 ml of 1% lidocaine or equivalent
- top up the epidural or insert a spinal anaesthetic if necessary. [2007]
If the woman reports inadequate pain relief at any point, address this immediately. [2007]
Advise the woman that in the case of first-degree trauma, the wound should be sutured in order to improve healing, unless the skin edges are well opposed. [2007]
Advise the woman that in the case of second-degree trauma, the muscle should be sutured in order to improve healing. [2007]
If the skin is opposed after suturing of the muscle in second-degree trauma, there is no need to suture it. [2007]
If the skin does require suturing, use a continuous subcuticular technique. [2007]
Undertake perineal repair using a continuous non-locked suturing technique for the vaginal wall and muscle layer. [2007]
Use an absorbable synthetic suture material to suture the perineum. [2007]
Offer rectal non-steroidal anti-inflammatory drugs routinely after perineal repair of first- and second-degree trauma provided these drugs are not contraindicated. [2007]
- Repair perineal trauma using aseptic techniques.
- Check equipment and count swabs and needles before and after the procedure.
- Good lighting is essential to see and identify the structures involved.
- Ensure that difficult trauma is repaired by an experienced practitioner in theatre under regional or general anaesthesia.
- Insert an indwelling catheter for 24 hours to prevent urinary retention.
- Ensure that good anatomical alignment of the wound is achieved and that consideration is given to the cosmetic results.
- Carry out rectal examination after completing the repair to ensure that suture material has not been accidentally inserted through the rectal mucosa.
- After completion of the repair, document an accurate detailed account covering the extent of the trauma, the method of repair and the materials used.
- Give the woman information about the extent of the trauma, pain relief, diet, hygiene and the importance of pelvic-floor exercises (see the NICE guideline on pelvic floor dysfunction ). [2007]
- Putting this guideline into practice
NICE has produced tools and resources to help you put this guideline into practice .
Putting recommendations into practice can take time. How long may vary from guideline to guideline, and depends on how much change in practice or services is needed. Implementing change is most effective when aligned with local priorities.
Changes recommended for clinical practice that can be done quickly – like changes in prescribing practice – should be shared quickly. This is because healthcare professionals should use guidelines to guide their work – as is required by professional regulating bodies such as the General Medical and Nursing and Midwifery Councils.
Changes should be implemented as soon as possible, unless there is a good reason for not doing so (for example, if it would be better value for money if a package of recommendations were all implemented at once).
Different organisations may need different approaches to implementation, depending on their size and function. Sometimes individual practitioners may be able to respond to recommendations to improve their practice more quickly than large organisations.
- Raise awareness through routine communication channels, such as email or newsletters, regular meetings, internal staff briefings and other communications with all relevant partner organisations. Identify things staff can include in their own practice straight away.
- Identify a lead with an interest in the topic to champion the guideline and motivate others to support its use and make service changes, and to find out any significant issues locally.
- Carry out a baseline assessment against the recommendations to find out whether there are gaps in current service provision.
- Think about what data you need to measure improvement and plan how you will collect it. You may want to work with other health and social care organisations and specialist groups to compare current practice with the recommendations. This may also help identify local issues that will slow or prevent implementation.
- Develop an action plan , with the steps needed to put the guideline into practice, and make sure it is ready as soon as possible. Big, complex changes may take longer to implement, but some may be quick and easy to do. An action plan will help in both cases.
- For very big changes include milestones and a business case, which will set out additional costs, savings and possible areas for disinvestment. A small project group could develop the action plan. The group might include the guideline champion, a senior organisational sponsor, staff involved in the associated services, finance and information professionals.
- Implement the action plan with oversight from the lead and the project group. Big projects may also need project management support.
- Review and monitor how well the guideline is being implemented through the project group. Share progress with those involved in making improvements, as well as relevant boards and local partners.
NICE provides a comprehensive programme of support and resources to maximise uptake and use of evidence and guidance. See our into practice pages for more information.
Also see Leng G, Moore V, Abraham S, editors (2014) Achieving high quality care – practical experience from NICE. Chichester: Wiley.
Giving birth is a life-changing event. The care that a woman receives during labour has the potential to affect her – both physically and emotionally, in the short and longer term – and the health of her baby. Good communication, support and compassion from staff, and having her wishes respected, can help her feel in control of what is happening and contribute to making birth a positive experience for the woman and her birth companion(s).
This guideline covers the care of healthy women who go into labour at term (37 +0 to 41 +6 weeks). About 700,000 women give birth in England and Wales each year, of whom about 40% are having their first baby. Most of these women are healthy and have a straightforward pregnancy. Almost 90% of women will give birth to a single baby after 37 weeks of pregnancy, with the baby presenting head first. About two-thirds of women go into labour spontaneously. Therefore, most women giving birth in England and Wales are covered by this guideline.
Since the original guideline was published in 2007, the number of women giving birth in England and Wales each year has risen, the rate of intervention (instrumental births and caesarean section) has increased slightly, and there has been some reconfiguration of services. The decision to update the guideline in 2014 was made based on developments in the NHS and new evidence becoming available that could affect the recommendations from 2007.
It is important that the woman is given information and advice about all available settings when she is deciding where to have her baby, so that she is able to make a fully informed decision. This includes information about outcomes for the different settings. It is also vital to recognise when transfer of care from midwifery-led care to obstetric-led care is indicated because of increased risk to the woman and/or her baby resulting from complications that have developed during labour.
Uncertainty and inconsistency of care has been identified in a number of areas, such as choosing place of birth, care during the latent first stage of labour, fetal assessment and monitoring during labour (particularly cardiotocography compared with intermittent auscultation) and management of the third stage of labour. These and other related topics are addressed in the guideline.
The guideline is intended to cover the care of healthy women with uncomplicated pregnancies entering labour at low risk of developing intrapartum complications. In addition, recommendations are included that address the care of women who start labour as ‘low risk’ but who go on to develop complications. These include the care of women with prelabour rupture of membranes at term, care of the woman and baby when meconium is present, indications for continuous cardiotocography, interpretation of cardiotocograph traces, and management of retained placenta and postpartum haemorrhage. Aspects of intrapartum care for women at risk of developing intrapartum complications are covered by a range of NICE guidelines on specific conditions and a further guideline is planned on the intrapartum care of women at high risk of complications during pregnancy and the intrapartum period.
- Recommendations for research
The guideline committee has made the following recommendations for research.
1. Models of midwifery-led care
In December 2021, we stood down this recommendation for research as continuity of care in maternity services is now a national policy in the UK following on from the National Maternity Review on Better Births and the subsequent Maternity Transformation Programme .
2. Effect of information giving on place of birth
How does the provision of accurate, evidence-based information affect women’s decision-making processes and choice of place of birth?
Why this is important
A report by Coxon et al. (2013) identifies in detail why women make choices about where to give birth and how these choices can be influenced. Influences may include written and verbal information (both online and from midwives and doctors), previous experience, and word-of-mouth advice from friends and family. The Birthplace study concluded that giving birth outside an obstetric unit is the optimal choice for low-risk women. This finding should be used to restructure the way in which information is provided, so that it is presented in a more accurate, less risk-based way in order to support women’s choices. This change should be evaluated in a quantitative observational study and/or qualitative study that records any changes in women’s choice-making about place of birth. Outcomes include understanding why and how women make choices about where to give birth and how this can influence the provision of appropriate and accessible information, a measure of informed decision-making, and fearfulness and absence of fearfulness when choosing place of birth.
3. Long-term consequences of planning birth in different settings
What are the long-term consequences for women and babies of planning birth in different settings?
The long-term consequences of birth experiences and birth outcomes are poorly understood, particularly in relation to place of birth. A large population-based observational study would compare women’s experiences and outcomes in different birth settings (with subgroup analysis by mode of birth) in relation to the wellbeing of the women and their children over different periods of time (for example, 2, 5, 10, 15, 20 and 30 years). A secondary analysis could compare different providers where birth philosophies are different. Outcomes would be compared by accessing medical records and through qualitative interviews. Primary outcomes are long-term physical morbidity, pain after birth, readmission to hospital, infection, psychological morbidity (for example, postnatal depression, bonding, relationship breakdown with partner, fear of giving birth in future) and breastfeeding rates. Secondary outcomes are impact on attachment between mother and child, obesity in children, autoimmune disease, chronic illness, educational achievement and family functioning.
4. Education about the latent first stage of labour
Does enhanced education specifically about the latent first stage of labour increase the number of nulliparous women who wait until they are in established labour before attending the obstetric or midwifery unit (or calling the midwife to a home birth), compared with women who do not receive this education?
Studies show that antenatal education about labour and birth in general makes a difference to some birth outcomes, but there is limited evidence focusing on education about the latent first stage of labour specifically. The aim of this study (randomised controlled trial or prospective observational study) would be to compare 2 groups of women experiencing their first labour and birth: a group who receive an education session in late pregnancy covering what to expect in the latent first stage of labour and how to recognise the onset of established labour, and a group who have not received this focused education. Primary outcomes would be mode of birth, satisfaction with the birth experience and the woman’s physical and emotional wellbeing after birth. Secondary outcomes would be use of pharmacological pain relief, use of oxytocin to augment labour, and time from first contact in confirmed established labour to birth.
5. Postpartum haemorrhage
What is the most effective treatment for primary postpartum haemorrhage?
There is uncertainty about the most effective drug treatments and dosage regimes, and about which other treatments should be used, for women who develop a postpartum haemorrhage. The most effective sequencing of interventions is also uncertain. The psychological impact of postpartum haemorrhage for women can be significant, and identifying the approach that minimises this impact is important. Randomised controlled trials comparing different dosage regimes for oxytocin and misoprostol, as well as comparisons with ergometrine and carboprost, are needed. Trials of mechanical measures such as intrauterine balloons or interventional radiology as early second-line treatment (rather than an alternative drug treatment) are also needed. Alternatively, a trial comparing the effectiveness of a complex intervention (for example, an educational component, sequence of interventions, immediate feedback and quality improvements) compared with standard care could be undertaken. Important outcomes include blood and blood product transfusion, need for further intervention, need for hysterectomy and psychological outcomes for the woman.
Appendix A. Adverse outcomes
Adverse outcome: in order to be able to count enough adverse events to be able to say that the results recorded are not just a result of chance, the Birthplace UK (2011) study used a composite definition of ‘adverse outcome’. The definition includes the following outcomes: stillbirth during labour, death of the baby in the first week after birth, neonatal encephalopathy (disordered brain function caused by oxygen deprivation before or during birth), meconium aspiration syndrome, and physical birth injuries (brachial plexus injury and bone fractures). The term ‘serious medical problems’ has been used to describe this composite outcome in the guideline recommendations.
Table A1 Numbers and proportions of the individual components of the composite adverse outcomes measure recorded in the Birthplace UK (2011) study
View in own window
Note: Each of the categories above are mutually exclusive and outcomes listed higher in the table take precedence over outcomes listed lower down. For example, if a baby with neonatal encephalopathy died within 7 days the outcome is classified as an early neonatal death.
Denominator varies because of missing values.
Does not equal 100% because of rounding.
- Finding more information and committee details
To find NICE guidance on related topics, including guidance in development, see the NICE topic page on intrapartum care . For full details of the evidence and the guideline committee’s discussions, see the full guideline . You can also find information about how the guideline was developed , including details of the committee .
NICE has produced tools and resources to help you put this guideline into practice . For general help and advice on putting our guidelines into practice, see resources to help you put NICE guidance into practice .
- Update information
December 2022: We removed section 1.10 on monitoring during labour because these recommendations have been replaced by the NICE guideline on fetal monitoring in labour . We also updated the links in recommendations 1.4.9 , 1.4.10 and 1.12.22 .
February 2017: The evidence has been reviewed for the sections on measuring fetal heart rate as part of the initial assessment and on fetal monitoring during labour. Recommendations for which the evidence was reviewed are labelled [2017] . Recommendations were added or amended, or the committee agreed that no changes were needed to the recommended actions.
Where recommendations end [2007] , [2007, amended 2014] , [2014] or [2016] , the evidence has not been reviewed.
As part of this update, the committee has also removed a research recommendation on intermittent auscultation compared with cardiotocography. Details can be found in addendum 190.1 .
November 2016: A recommendation about team midwifery was deleted, and a new recommendation was added (labelled [2016] ) that cross-refers to information about continuity of care in the NICE guideline on patient experience in NHS services.
Minor changes since publication
October 2022: We added text to indicate that pulse oximetry may be less reliable in people with dark skin. We also added a link to the NHS patient safety alert on the risk of harm from inappropriate placement of pulse oximeter probes. See recommendations 1.15.23 and 1.15.25 .
December 2021: We added a link to NICE’s guideline on pelvic floor dysfunction in recommendation 1.16.22 . We also stood down the recommendation for research on models of midwifery-led care as continuity of care in maternity services is now a national policy in the UK following on from the National Maternity Review on Better Births and the subsequent Maternity Transformation Programme .
Your responsibility : The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals and practitioners are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or the people using their service. It is not mandatory to apply the recommendations, and the guideline does not override the responsibility to make decisions appropriate to the circumstances of the individual, in consultation with them and their families and carers or guardian.
All problems (adverse events) related to a medicine or medical device used for treatment or in a procedure should be reported to the Medicines and Healthcare products Regulatory Agency using the Yellow Card Scheme .
Local commissioners and providers of healthcare have a responsibility to enable the guideline to be applied when individual professionals and people using services wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with complying with those duties.
Commissioners and providers have a responsibility to promote an environmentally sustainable health and care system and should assess and reduce the environmental impact of implementing NICE recommendations wherever possible.
Created: December 3, 2014; Last Update: December 14, 2022.
- Cite this Page Intrapartum care for healthy women and babies. London: National Institute for Health and Care Excellence (NICE); 2022 Dec 14. (NICE Clinical Guidelines, No. 190.)
- PDF version of this title (415K)
In this Page
- Adverse outcomes
Other titles in this collection
- National Institute for Health and Care Excellence: Guidelines
Related NICE guidance and evidence
- Intrapartum Care: Care of Healthy Women and Their Babies During Childbirth
- Addendum to intrapartum care: care for healthy women and babies
- Addendum to Clinical Guideline CG190, Intrapartum care for healthy women and babies
- 2019 surveillance of intrapartum care for healthy women and babies (NICE guideline CG190)
Supplemental NICE documents
- NICE Quality Standard QS105: Intrapartum care (PDF)
Similar articles in PubMed
- Review Intrapartum care [ 2023] Review Intrapartum care . 2023 Sep 29
- Review Postnatal care [ 2021] Review Postnatal care . 2021 Apr 20
- Vaginal delivery of breech presentation. [J Obstet Gynaecol Can. 2009] Vaginal delivery of breech presentation. Kotaska A, Menticoglou S, Gagnon R, MATERNAL FETAL MEDICINE COMMITTEE. J Obstet Gynaecol Can. 2009 Jun; 31(6):557-566.
- Review Antenatal care [ 2021] Review Antenatal care . 2021 Aug 19
- Review Fetal monitoring in labour [ 2022] Review Fetal monitoring in labour . 2022 Dec 14
Recent Activity
- Intrapartum care for healthy women and babies Intrapartum care for healthy women and babies
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
This website is intended for healthcare professionals

- { $refs.search.focus(); })" aria-controls="searchpanel" :aria-expanded="open" class="hidden lg:inline-flex justify-end text-gray-800 hover:text-primary py-2 px-4 lg:px-0 items-center text-base font-medium"> Search
Search menu

Kathleen's journey: improving mental health outcomes for women with bipolar affective disorder
Given the statistics related to women with bipolar affective disorder, promoting interdisciplinary mental health services is critical across the perinatal period (Knight et al, 2022). The specialist...

Public health prevention in maternity programme: supporting the local system
Programme priorities were refreshed in 2021, informed by local healthcare data on the scale of potential benefit and maternity system requirements, among other criteria. Objectives for each new...
The importance of the user voice in clinical decision making: a reflective account
When reflecting on the factors affecting the decision-making process, it is useful to consider decision-making theory. The dual processing theory suggests that thought process can be distinguished as...
A critical analysis of a tripartite clinical decision involving a student, midwife and client
Midwives often make clinical decisions with missing or ambiguous information, requiring skill and a degree of managed risk consideration (NHS, 2022) or trade off, as per Sherif et al's (1965) degrees...

Lack of policy consideration for breastfeeding co-mothers in maternity services
The written narratives from both Jane and Ayesha show mothers-to-be in a great deal of emotional distress. NHS antenatal services for lactation support do not always guarantee support for...

A midwifery team's journey implementing and sustaining continuity of care
Caseloading is one way of providing continuity, either antenatally and postnatally or including intrapartum care. Teams provide a named midwife and continuity of care, leading to a safer and better...
Giant placenta chorioangioma with postpartum bleeding and uterine atony
A 35-year-old patient was undergoing routine prenatal checkup at gestational week 35, with suspicion of preterm delivery as a result of advanced stage labor contractions. The patient had a history of...

SARS-CoV-2 infection complicated by intrahepatic cholestasis of pregnancy
This article presents the case of a 28-year-old South Asian woman in her first pregnancy with no past medical history and no family history of ICP. She provided written consent for the publication of...

The effect of COVID-19 on intrapartum care: a case review from early in the pandemic
A 36-year-old primigravida booked at 11 weeks for antenatal care. She was of East Asian ethnicity, with a BMI of 26. She had no significant past medical or surgical history, however, a first-degree...

Induced abortions in Pakistan: an afflicting challenge needing addressal
Unsafe abortion is one of the major causes of maternal mortality that accounts for approximately 4.7%–13.2% maternal deaths every year (Say et al, 2014). In developing countries, every year, nearly...
SARS-CoV-2: do corticosteroids for fetal lung maturation worsen maternal or fetal outcomes?
The SARS-Cov-2 virus is a virulent pathogen which first emerged in December 2019 in Hubei province of China. This virus is thought to cause severe acute respiratory syndrome and the associated illness...
Cerebral venous sinus thrombosis viewed as a postpartum complication
Cerebral venous sinus thrombosis (CVST) is a rare type of stroke caused by a clot forming in one of the intracranial sinuses and subsequent blockage in blood drainage. Even though venous...
Showing 1 to 12 of 14 results
Why choose British Journal of Midwifery?
BJM supports midwives by sharing expertise and advice to help you build confidence, grow professionally and improve care.
What's included
Evidence-based best practice
Peer-reviewed research
Practical guidance
CPD support
Subscriptions start:
- Open access
- Published: 15 June 2023
Women’s and midwives’ views on the optimum process for informed consent for research in a feasibility study involving an intrapartum intervention: a qualitative study
- Mary Alvarez ORCID: orcid.org/0000-0001-6848-2067 1 , 2 ,
- Emily J. Hotton ORCID: orcid.org/0000-0002-8570-9136 1 , 3 ,
- Sam Harding ORCID: orcid.org/0000-0002-5870-2094 1 , 4 ,
- Jonathan Ives ORCID: orcid.org/0000-0002-5233-5000 2 , 5 ,
- Joanna F. Crofts ORCID: orcid.org/0000-0001-9416-0176 1 &
- Julia Wade ORCID: orcid.org/0000-0001-6486-6477 2
Pilot and Feasibility Studies volume 9 , Article number: 98 ( 2023 ) Cite this article
1139 Accesses
2 Citations
13 Altmetric
Metrics details
Recruitment to intrapartum research is complex. Women are expected to understand unfamiliar terminology and assess potential harm versus benefit to their baby and themselves, often when an urgent intervention is required. Time pressures of intrapartum interventions are a major challenge for recruitment discussions taking place during labour, with research midwives expected to present, discuss and answer questions whilst maintaining equipoise. However, little is known about these interactions. An integrated qualitative study (IQS) was used to investigate information provision for women invited to participate in the Assist II feasibility study investigating the OdonAssist™—a novel device for use in assisted vaginal birth with an aim to generate a framework of good practice for information provision.
Transcripts of in-depth interviews with women participants ( n = 25), with recruiting midwives ( n = 6) and recruitment discussions between midwives and women ( n = 21), accepting or declining participation, were coded and interpreted using thematic analysis and content analysis to investigate what was helpful to women and what could be improved.
Recruiting women to intrapartum research is complicated by factors that impact on women’s understanding and decision-making. Three key themes were derived from the data: (i) a woman-centred recruitment process, (ii) optimising the recruitment discussion and (iii) making a decision for two.
Despite evidence from the literature that women would like information provision and the research discussion to take place in the antenatal period, intrapartum studies still vary in the recruitment processes they offer women. Particularly concerning is that some women are given information for the first time whilst in labour, when they are known to feel particularly vulnerable, and contextual factors may influence decision-making; therefore, we propose a framework for good practice for information provision for research involving interventions initiated in the intrapartum period as a woman centred, and acceptable model of recruitment, which addresses the concerns of women and midwives and facilitates fair inclusion into intrapartum trials.
Trial registration
ISRCTN. This qualitative research was undertaken as part of the ASSIST II Trial (trial registration number: ISRCTN38829082. Prospectively registered on 26/06/2019).
Peer Review reports
Key messages regarding feasibility
Women were willing to have their recruitment discussions audio-recorded and take part in an interview. The inclusion of vignettes elicited less guarded views on the informed consent process.
Recruitment to intrapartum trials should be women-centred and acceptable to women. All efforts should be made to inform women of trial information before the birth admission. Most women believed this should be done via the community midwife with whom they have a trusted relationship.
The findings of the IQS demonstrated that there needed to be alternative approaches and modifications made to the consenting process to facilitate a woman-centred strategy to recruitment, guided by the principles of the framework for good practice (Table 6 ).
Informed consent is a not an event, but a complex process [ 1 ] central to the ethical conduct of research [ 2 ]. Gaining consent for research interventions initiated during the intrapartum period involves added complexities to the norm: not only are women required to assess the potential harm versus benefit to their baby in addition to themselves, but there may also be time pressure for the intervention to be initiated. These complexities add an additional layer of challenge to the usual requirements of consent including understanding the study purpose, the nature of the intervention(s), the right of withdrawal, risks and benefits of participation, how findings will be shared, confidentiality maintained and, in randomised studies, concepts such as equipoise and randomisation. The recruitment discussion, which involves sharing study information and the researcher assessing the woman’s capacity to make a decision to participate, is fundamental to a good quality consent process [ 3 , 4 ].
During recruitment discussions, recruiters face a dual challenge: conveying a clear account of essential research information [ 5 ] and allowing women as much time as they require to be confident with their decision, whilst simultaneously optimising recruitment [ 6 , 7 ]. Ensuring the potential participant has sufficient understanding to give informed consent can be complicated by factors that are a normal feature of the intrapartum period. At this time, women’s capacity to consent can be affected by, for example, lack of sleep, opiate analgesia and pain [ 8 ]. The decision to participate in intrapartum studies is also influenced by contextual factors such as the environment, timing of consent in relation to birth, birthing support, the women’s physical and/or mental state and the accessibility of the study information [ 9 ].
In 1997, the Association for Improvements in Maternity Services (AIMS) highlighted the failure of researchers to adequately inform pregnant women of the risks and benefits of participating in research [ 10 ]. Inadequate information provision, recognition of vulnerability during labour, inappropriate timing of consent and the lack of women’s involvement in research design led to the mantra ‘research should be undertaken with women, not on women’ . However, despite this mantra and accompanying recommendations, there is still evidence that this is not always achieved [ 11 ].
This qualitative research study investigated women’s and midwives’ perspectives on a model of information provision and informed consent for an intervention initiated in the intrapartum period to identify what was helpful to women and what could be improved, with a view to developing a best practice framework.
The Assist II study was a non-randomised feasibility study investigating the clinical impact, safety and acceptability of the OdonAssist™ inflatable device for assisted vaginal birth (previously known as the Odon Device) and is an innovative device for use during assisted vaginal birth (AVB—an intrapartum intervention). The OdonAssist™ comprises of a plastic applicator, fastening band and plastic sleeve that slips over the baby’s head—a circumferential air cuff is then inflated and with maternal effort, traction applied to the air cuff to achieve an assisted birth. The mechanism of action and design has been fully described in detail [ 12 ]. An integrated qualitative study (IQS) embedded within the larger Assist II study, as a sub-study, investigated the model of information provision and informed consent within the Assist II study and is reported here [ 12 ].
The study took place at Southmead Hospital, Bristol—a single tertiary hospital in the southwest of England.
The Assist II study recruitment processes involved distributing the patient information leaflet (PIL) from 20 weeks’ gestation and receiving consent from 28 weeks onwards. Women were approached for a recruitment discussion once in hospital for the birth (spontaneous labour or planned admission). They were offered a PIL, a 10-min study information video and the opportunity to discuss the study with a research midwife. In line with current Royal College of Obstetricians and Gynaecologists (RCOG) guidance for consent to intrapartum research [ 13 ], a recruitment approach was permitted from admission to the maternity unit until full dilatation with regional anaesthesia. Research midwives used the ‘hints and tips’ document derived from the Assist sub-study before they began recruitment consultations. It clarified what terminology should be used and avoided and helped communicate the concept of equipoise [ 14 ].
Qualitative study design
Qualitative methods were used to investigate this recruitment process by triangulating findings from the following data sources: in-depth interviews with women invited to take part in Assist II, interviews with Assist II research midwives and audio-recorded Assist II recruitment discussions. The Assist II study [ 12 ] trialists were investigating a complex intrapartum intervention. It was decided that integrating a qualitative study into the quantitative stud would allow a more complete analysis and add credibility to the results of the (IQS).
Participants
IQS participants included a subset of the women invited to participate in the Assist II study [ 12 ] and all research midwives involved in study recruitment. Women were eligible to participate in the study if they were anticipating a vaginal birth. Inclusion criteria required women to be 18 years of age and over, have a singleton pregnancy, be over 28 weeks gestation at the time of consent, have a good understanding of the English language, not be in prison, may require an assisted vaginal birth, be at least 36 weeks gestation at the time of the assisted birth, have no latex allergy, have a negative virology, have no intra-uterine death, have no foetal osteogenesis imperfecta, have no foetal bleeding disorders, have no intramuscular or intravenous opiates within 6 h of consent and may consent up to 4 cm of cervical dilatation without regional analgesia or up to full dilatation with regional analgesia.
A purposive sampling strategy was used to include women accepting and declining participation in Assist II , with a range of birth experiences (successful and unsuccessful assistance with the OdonAssist™) and a range of characteristics (age, parity and previous birth mode, occupation and ethnicity).
Data collection
Women participating in the IQS were invited to consent to an audio-recording of the Assist II recruitment discussion and an in-depth interview conducted within 2 weeks of the birth. All six midwives approaching participants within the Assist II study [ 12 ] were invited to audio-record all recruitment discussions with potential Assist II participants (with the consent of all involved) and take part in an in-depth interview.
Data collection took place between 3 September 2019 and 4 March 2020. Interviews followed a topic guide informed by existing literature on information provision and consent to interventions in the intrapartum period (Table 1 ) but allowing interviewees to introduce unanticipated issues of relevance to them.
To help elucidate interview participants’ views on the appropriateness of different recruitment strategies, three contrasting vignettes depicting differences in the timing, manner and stage of labour for information provision and approach were used (Table 2 ). The purpose of the vignettes was to provide scenarios where women and midwives could give opinions on the timing of approaches without necessarily referring to their own experiences.
The first two vignettes represented common scenarios around timing of the recruitment approach and information provision within the Assist II study [ 12 ], and the third was constructed to explore the acceptability of an alternative informed consent strategy.
Recruitment discussions and interviews were recorded using a digital voice recorder and were transcribed verbatim, with identifying data removed.
Data analysis
Reflexive thematic analysis [ 14 ] was used to analyse both interview and recruitment discussion data [ 15 ] focussing on participants’ recounted experiences for the former, and the nature and content of the discussion for the latter [ 16 ]. Content analysis including simple quantification of content identified the most frequent patterns within recruitment discussion data. Data were hand coded by MA to allow familiarisation before using NVivo12 software to organise codes and develop themes. A proportion (20%) of the recruitment discussions and interviews were co-coded by JW and discrepancies discussed and resolved. These multiple data sets enabled triangulation of data relating to each woman’s experience (woman’s interview, recruitment discussion, midwife interview) as well as cross-case comparison resulting in a deeper understanding [ 17 ], and an increased confidence in the findings [ 18 ]. Initial themes were discussed between MA, JW, EH, SH and JI and further developed and revised, before a final thematic account was created. Reflexivity was used throughout data analysis and collection as a methodological tool to enhance credibility of the findings [ 19 ].
Twenty-five women and six research midwives were included (Tables 3 and 4 for participant characteristics), and 17 of those women had their Assist II recruitment interviews recorded. Five of those 17 women ultimately declined participation in the Assist II study, and the remaining 12 participated. Six midwives (all those working on the Assist II study) agreed to participate in the interviews. Despite all research midwives agreeing to the audio-recording of recruitment discussions in principle, only three engaged fully with this process, with these three midwives capturing fewer than 10 audio recordings between them.
Three themes were developed from the data: (i) a woman-centred recruitment process, (ii) optimising the recruitment discussion and (iii) making a decision for two. Quotes are identified as originating either from women’s interviews (e.g. IP0162A, with A indicating accepting participation, D indicating declining), midwife interviews (e.g. Midwife 01) or recruitment discussions RD (e.g. RDP0162A indicates data from the recruitment discussion of a patient who accepted participation).
Woman-centred recruitment process
Women’s and midwives’ accounts suggested a range of practices that would keep women and their interests at the centre of the recruitment process, most notably that study information should be freely available to all during pregnancy.
If you don’t inform everybody, how could you possibly know which ones to inform? So, it’s better that the information is wider spread. (IP0162A)
The first time I heard about it was when I was having an antenatal appointment prior to even looking at my birth plan... I read a little bit about it then. I was thinking, oh that’s interesting, I wonder what’s that about. (IP0082A)
Women also suggested community midwives should be the first point of contact with study information and research midwives agreed, aware the rapport women develop with community midwives was hard to replicate during an initial research approach in hospital. Further, whilst there was a clear belief that information should be given throughout pregnancy, many women suggested the third trimester was the optimum timing for receiving information.
It’s worth people giving information leaflets out at [community] midwives’ appointments at like 36 weeks … (IP0302A)
You’d build up trust and rapport with your [community] midwife…but when you meet a new member of staff who’s then giving you information about a study, it’s quite a big ask for them to trust you and agree to take part. (IMidwife01)
It was felt that being signposted to information in advance of labour, through collaboration between community and research midwives, would avoid women receiving information for the first time during birth admission, when there is little time to make a considered judgement on participation. This latter experience was reported by 15 women.
It was sprung on me when I was about to be induced .... I think they should do it beforehand, maybe given time like when they go to their next scan or when they do their birth plan...not spring it when you’re about to go into labour. IP0352A
...having the opportunity to think about things, so perhaps have seen it in the hospital but be able to come back and watch [the patient video] again and have more processing time... I’d have been on a better footing then rather than having all the information straight away and making a decision at the time. (IP0162A)
...if they’ve had the information leaflet at least or they know a bit about the study before they come in... they’ve got that little seed in their head...it’s not such a big deal. They’re ready for it in a way. (IMidwife01)
The third vignette involved ‘Agnes’ (Table 2 ) who was informed of the study prior to labour and initiated discussion with the research midwife in advanced labour. Most women and midwives favoured this scenario, some citing it as ideal.
It sounds like she’d already decided she wanted to do it, she’d already accessed all the information and she actively asked for a midwife to come and speak to her about it. So, yeah, no problem. (IP0234A)
Optimising the recruitment discussion
Finding the opportune time for midwives to make an approach and initiate a discussion was challenging given that many women were already anxious due to being admitted with a pregnancy complication or being in labour. Women, including those given information and approached about the study for the first time during labour, did not overtly criticise the timing of their own approach. However, in apparent contradiction to this stance, they expressed concern about the timing of approach in ‘Libby’s’ vignette, approached during labour (Table 2 ), with comments on vignettes possibly providing less guarded insights into women’s views:
I think you’d have to question, is Libby in a sound state of mind...probably in a significant amount of pain, about to face one of the toughest things in her life that should not be the first time someone hears of the study, let alone is asked to consent for research purposes. (IP0264A)
I think I would have listened, tried to watch the video, tried to read or get someone to read it and then tell me or read it to me but then it would probably have been like, do you know what? Just shush please. [laugh] (Participant IP0308A)
Women reported factors that hindered their ability to make an informed choice during labour including pain, pain relief, tiredness, vulnerability and anxiety, the clinical environment and a lack of privacy. Pain relief was perceived to contribute to diminished capacity and some women relied on their partners to support their decision-making.
.. being approached when I was being induced worked for us... so he was able to kind of like, be there and watch [the patient information video] with me...I was drugged at the time [had been administered codeine]. (IP0086A)
The term ‘vulnerability’ was used by six women. It was used to describe being pregnant, labouring alone, a lack of control and feeling scared.
Well, I think the whole pregnancy thing...it certainly made me feel quite vulnerable because it's not something I'd ever experienced before, and I had no control over... (IP0082A)
I was just so scared, in my head I was terrified that he'd be stillborn...So, I think if someone approached me and started giving me information [prior to hospital] ...I think that would probably scare the life out of me for labour even more than it already was scaring me. (IP0086A)
This highlighted the importance of getting the timing right, but also that women were vulnerable in different ways and at different times, meaning the ‘right timing’ will vary between women and reinforced the need for recruitment to be tailored to individual needs.
The midwives recognised that the hospital environment was not ideal for a research approach and were aware that women were unlikely to expect a first approach for research participation during their admission. The second vignette presented a scenario where the midwife initially offered clinical advice, then introduced the ASSIST II study (Table 2 ). Most women found this acceptable, but midwives raised concerns about this approach in this context, questioning whether it gave priority to research when clinical needs should be addressed first:
I don’t personally think it matters that she was approached by the research midwife before the clinical one. I don’t see the issue with that. (IP0332A)
...the fact that she hadn’t seen a clinical midwife before the research midwife went in, I’d feel a little bit more uneasy about that because that’s not the reason she is here, to take part in a study, the reason she is here is to be induced. (IMidwife06)
Regardless of the timing, however, allowing time to discuss the study with partners in privacy was valued.
She left us with the [Assist II study video] so we could talk to each other and see, because obviously if she was sat there, you’d kind of feel a bit awkward if [partner] didn’t want to do it or something, but yeah, it was quite nice that she left you... you weren’t feeling pressured or anything. (IP0078A)
I’d just like to approach you both about the Assist II study which we are currently running in this hospital, and I believe that [Partner name], you have had a chance to look at the information leaflet. (RCMidwife04)
The midwives reported that providing engaging, accessible and coherent information was, in their experience, important to women. Some modes of information provision, however, were rated more highly than others. The PIL was intended to raise awareness of the study during pregnancy but was criticised by some women and midwives for its appearance, content and length. Midwives noted that decisions to participate were rarely based on its content , and this was supported by some women, who described it as wordy and off-putting:
... it was quite wordy almost put you off wanting to read it because you think, oh like if they were shorter paragraphs or whatever you think, oh I can read this in two minutes, done. (IP0215A)
In contrast, women valued the information video’s visual demonstration of how the OdonAssist™ would be used in practice. Midwives valued the video for the clarity it offered women.
I remember being quite impressed that it felt very much like everything had been thought through so that video gave you all the information you needed. (IP0082A)
So, it was the visual explanation of the bag going over the head and how the baby was then moved down the birth canal and out. I remember seeing that bit... (IP0215A)
Before we launched, I thought it was an additional tool to our chat but now I think it’s an instrumental part...I think probably it’s the seeing the device in operation rather than people talking around it. (IMidwife02)
The recruitment discussion was seen as pivotal in promoting understanding. Women commented positively on the content and manner of information provision, regardless of their decision about participation, with sixteen (thirteen accepters and three decliners) recalling the discussion as being essential for decision-making.
I just bombarded her with questions…So, I’d say her knowledge was quite brilliant, reassuring...it gave me confidence. (IP0332A)
…it is a special time for you, your husband, and the midwife, that relationship, so I think it’s important not to impose too much on that, and the researcher I spoke to didn’t. I think she was very courteous in that way, but yeah, I think if you took up too much of someone’s time on the day, it perhaps takes away a little bit of the special experience and I think that just adds more to the argument about giving the information earlier. (IP0181D)
It is worth noting the final point made by IP0181D, which highlights the risk of a recruitment approach imposing on a special time and supports the idea that an approach prior to labour is preferable.
Combined, women asked 40 different questions during recruitment consultations, (presented in Table 5 ). Most questions focused on the OdonAssist™ and the baby’s ability to breathe during birth, whilst the fewest focused on research follow-up. Six women asked no questions. One research midwife noted that because of the comprehensive nature of the information video, conversations were often brief.
Most women turn round and they’re like ‘oh, I was going to ask you that question actually about the baby breathing, but it was all covered in the video. (IMidwife04)
‘Good’ conversations were defined by midwives as exchanges where the midwives’ believed women were optimally informed, either because of evidence of previous access to study information or because women were able to ask probing questions.
Good conversations, definitely the majority of women have had the information before. (IMidwife06)
The people that I’ve approached, had a conversation with and walked away feeling like yeah that was really, really good were the ones where they asked the most questions...you feel that they’ve got all the information and they’ve made the right decision. (IMidwife02)
The physical state of the women, access to prior information, the timing and location of the discussion, and the length of time given to make a decision about participation were most influential in how positively women perceived the discussion, irrespective of whether they accepted or declined participation. At interview, 2 weeks postpartum, there was no consistency in the elements of participation that women could recall, although most acknowledged their lack of knowledge about study detail. This inability to recall was reported by women regardless of whether they gave consent on Central Delivery Suite or in pain. Those who had only partial understanding at the time of their decision appeared to accept this as sufficient and place their trust in the study team:
To be honest with you...sort of understood it a little bit, what would happen and why I would need it. (IP0308A)
Other women demonstrated a clear understanding of what they consented to:
I consented to, if the right person was available at the time and I needed an assisted delivery that I would have one. So, I consented to going along with it to the point of delivery and then sort of re-evaluating whether I definitely still wanted at that point. (IP0235A)
Most women were given a short time by the research midwives in which to consider participation, but some indicated having ample time to consider, as illustrated by the following excerpt from a recruitment discussion:
So, I’m around for another few hours now and then I'm here again tomorrow. If you decide to sign up prior to that ask the [clinical] midwife to give me a shout and I'll come back down. (RCMidwife02)
This woman was followed up the next day. After all her queries had been answered, she felt comfortable with her decision to participate.
RCMidwife02: ‘Would you like some time to think about it you wanted to take part in the study?’
RCP0235A: ‘No. I think we’re happy to participate in it’.
Making a decision for two
One issue above all others influenced decision making: women were making a decision for two. Any other motivation for participation came with the caveat that there must be no risk to their baby.
...as long as it definitely doesn’t hurt little one that you know, I’m happy to help out with the research. That was my only main priority. (IP0308A)
Midwives suggested that some women were taking some control over their baby’s birth by participating. One midwife recalled a woman, traumatised by her first child’s birth, initially rejecting participation before thinking again after her husband made the point that participating gave her another option during labour.
...she said, ‘oh no, I’m really anxious, this is just too much now’...then she came sobbing back into the office....‘my husband just made the really good point of it gives me another option before having to have another caesarean section’. (IMidwife05)
Women also compared the OdonAssist™ to other methods used for AVB. After engaging with the study information most women believed the OdonAssist™ to be a better option, with the absence of hard surfaces on the device leading women to believe the OdonAssist™ was gentler for both themselves and their baby—which suggests an incomplete understanding of equipoise. Twenty women perceived the OdonAssist™ to be a preferable alternative to forceps and participated with the aim of avoiding a forceps-assisted birth.
...I think anyone who’s either had it or nearly had to use forceps, it’s always been like, ‘oh no, they had to use forceps. (IP0264A)
It just looked a bit better and a little bit safer, for me, ‘because it went over the head...I don’t know, just looked a bit safer for me than the forceps. (IP0081A)
Women declining participation in Assist II gave a range of reasons (including fear of something going wrong, concern for the baby’s wellbeing, resistance from family members). When comparing women who declined with those women who accepted participation, none of the decliners had study information prior to the discussion. Most women who declined participation reported doing so because of late information, explaining they would have considered participation with prior information. Decliners also included proportionally more women with English as a second language who did not have translated information available to them (three of five decliners compared to two in twenty of those accepting).
Framework for good practice
Key principles derived from the above findings are summarised in Table 6 . These principles are proposed as a potential framework for good practice for information provision for research involving interventions initiated in the intra-partum period.
This study explored the experiences of women and midwives who were invited to participate in, or recruiting into, the Assist II study—an intrapartum research study in which a recruitment approach could take place between 28 weeks’ gestation and full dilatation [ 12 ]. The study identified three key themes that capture features of recruitment practice that are important to women and midwives: a woman-centred recruitment process, optimising the recruitment discussion and making a decision for two. One implication for future research is that the information provision and recruitment process within Assist II was largely acceptable to women, as recruitment targets were met more rapidly than anticipated, and women’s views about processes were predominantly positive. Furthermore, as a result of this, IQS modifications for improvement were identified and implemented, to improve the recruitment experience. For example, 4 months into the 16-month recruitment period, informed by the developing themes of this study, the recruitment process in Assist II was changed so that only potential participants who had prior information about the study could be approached in labour. The three themes described above form the basis for a proposed framework for good practice (Table 6 ), which aims to place women at the centre of the recruitment process using the following principles: that every woman be provided with the opportunity to participate in research, the promotion of women’s autonomy, acknowledging vulnerability, avoiding professional gatekeeping and supporting the understanding of research information.
There is no current guidance from professional bodies stipulating that women who consent to interventions initiated in the intrapartum period should have information before labour. Current recommendation from the RCOG is that study information should be given based on the likelihood of an intrapartum complication occurring. For example, if the likelihood of the complication is greater than 1:10, women should be given only brief study information in pregnancy; if the complication occurs, women should be given full study information and informed consent given ‘at the time of the emergency’ [ 13 ]. This guidance is based on 21-year-old research that sought to establish whether women in pain understood the risks associated with the siting of an epidural and is intended to prevent psychological trauma caused by information about events unlikely to happen [ 20 , 21 ].
We argue that a research discussion is not comparable to counselling for an epidural. An epidural is a clinical procedure carried out for the benefit of the labouring woman, and the threshold for consent is likely to be lower than that for research. Treatment and research are different, and the consent process required is different. The RCOG guidance aims to spare women the full details of intrapartum research in pregnancy, because of the assumed psychological trauma it may cause [ 13 ]. Findings from our study suggest this stance may result in exclusion of potential participants from research and so may be viewed as inappropriately paternalistic and disempowering for women. All women in our study, including those who would have chosen not to access the information due to concerns about it increasing their anxiety, were clear that they believed access to this information should be given ahead of the intrapartum period. This position is in line with that taken by AIMS [ 10 ] and is consistent the concept of women-centred care as it gives them control.
Woman-centred care, which simply means care that is individualised and developed in consultation with the affected woman, has long been recognised as a quality marker in maternity services, where the woman’s needs, as defined by the woman herself, are prioritised, and she is given choice, control and continuity of care [ 22 ]. This model promotes shared decision-making with the intention of satisfying the woman’s individual wishes and clinical needs. We argue that this model will have implications for research practice and should be extended to the research environment where the needs of women take precedence over the quantitative requirements of the study.
Our findings also confirm previous research and have practical implications for information provision. The third trimester was described by most as optimum timing for information provision, when women turned their attention to labour [ 23 , 24 , 25 ]. A prior awareness of the study, described as ‘a seed in their head’, was reported to be conducive to accepting a research midwife’s later approach. Midwives’ felt that prior awareness of the study is an essential ingredient of a ‘good’ recruitment discussion, because women with prior awareness were more engaged and asked more questions [ 24 , 26 , 27 ]. Two women preferred not to have the information prior to admission to hospital as they believed it would have increased their anxieties around labour and birth; however, they accepted that information should be available to women, to engage with as they chose. The women who reported being most satisfied with their decision were the women who were given extended periods to decide, sometimes overnight.
A practical and ethical implication and one of the key factors favouring information provision prior to the intra-partum period was the difficulty reported by women, and observed by midwives, in absorbing information and making participation decisions when not in the ‘right frame of mind’. Of the women recruited in labour, none could recall the information provision or discussion, and of note was that the presence of epidural anaesthesia did not make for a better ‘state of mind’. Rather, pain was replaced by exhaustion and difficulty staying awake. In principle, labouring women cannot be considered to lack capacity to make their own decision simply because they are labouring—but both women and midwives in our study felt that decision-making capacity can be impaired during labour.
Similar findings have been reported elsewhere. The ‘Got-it’ study [ 23 ] required an intrapartum consent in the event of a retained placenta (the clinical incidence of a retained placenta being approximately 2% of vaginal births). Both researchers and women considered the study information (both written and verbal), given at the time of the emergency, as straightforward and the giving and receiving of consent as uncomplicated. However, qualitative interviews with women and researchers generated conflicting views on whether valid consent had been obtained. The researchers felt they had provided clear, succinct information about participation, whereas women felt that they did not fully absorb the information, particularly the risks of participation, because of their inability to engage with researchers after labour and delivery. They did not feel they gave informed consent and would have preferred information antenatally.
This finding was consistent with the views of acutely unwell women in the QUOTE study, which reported their experiences of recruitment to the Magpie trial [ 25 ]. The trial investigated the use of prophylactic anticonvulsants for women with severe pre-eclampsia. A key finding was that women suggested that they should have been given information in the antenatal period, even if there was only a small chance of them being able to participate. Such information sharing had been rejected by the QUOTE researchers in the design phase, as they felt a duty of care to not distress the 98% of women who would be ineligible to participate [ 25 ].
Women articulated a sense of vulnerability, which they associated with being pregnant, labouring alone, a lack of control or feeling scared. Research midwives recognised this vulnerability and sought to protect vulnerable women from research activities. However, this translated into gatekeeping, choosing which women were approached and which were not. There is an argument that midwives should not act on these protection motives, but hand ownership of the decision to women entirely, and let them decide when and if they receive a research approach [ 26 , 27 ]. Not offering an approach removes a women’s right to self-determination which is ethically hard to defend as it insults women’s autonomy and most women want information [ 28 ]. However, if we accept the potential for vulnerability and loss of capacity to make that decision during labour, it may well be absolutely appropriate (both ethically and legally) for the research midwife to act as gatekeeper and make a decision in the best interests of the labouring women. However, this dilemma of whether to approach or not could be resolved entirely by ensuring that information is given, and a decision about in-principal participation made, prior to labour.
The study video was valued by all women and the OdonAssist™ demonstration stood out as the section most well-recalled, and the most valued feature. The demonstration enabled women to visualise the OdonAssist™ in use, which appeared to play an important and often decisive role in their decision-making. Midwives welcomed the video as it pre-empted many of the women’s questions and anxieties, suggesting that video can be very useful for a specific purpose [ 29 ] and may enhance the quality of consent and be of particular benefit in studies like Assist II [ 12 ] where demonstration of a procedure is needed.
Women, and most midwives, agreed with other intrapartum research study participants that the PIL was less useful than other modes of information provision [ 30 , 31 ]. It could be that the necessity of including research governance text in the pamphlet made it unnecessarily long and negatively impacted on presentation and accessibility. However, it may simply be the case that requiring women to read and understand written information at a time of heightened anxiety and vulnerability, in the context of labour, is simply too demanding. If women were given time to engage with the PIL outside of the birth admission, and not in a state of heightened anxiety, they might be able to read, absorb and discuss it, and might have felt differently about it. Either way, what this suggests is the most appropriate of the mode of information provision is likely to be context and person dependent, and the imperative for potential research participants to consume a written PIL, regardless of context, is misplaced. Most women reported the recruitment discussion was pivotal in their decision-making in terms of helping them understand what was involved [ 9 , 30 ] and more important than any other mode of information provision [ 24 ]. Women were impressed with the research midwives’ knowledge, professionalism and kindness. They emphasised the importance of the research midwives’ interpersonal skills, factors widely reported as influencing recruitment to trials [ 9 , 25 , 32 , 33 , 34 , 35 ].
The original model of information provision for Assist II had envisaged community midwives providing information in the third trimester, but many women were presenting to the research team on admission to hospital without prior knowledge of the study. To promote Assist II, we placed the study on Facebook and Twitter, used the Trust website to host up-to-date research study information and placed standing banners with QR codes in the maternity unit, and a PIL (with a QR code) was sent to all women with their 20-week scan appointment. The new recruitment strategy, implemented after presenting interim findings form this study to the Assist II team, involved approaching women attending the Maternity Assessment Unit in person, and giving women the opportunity to view the study information video at home, followed by a short telephone discussion with a research midwife. Research midwives reported that women valued this new approach. An important practical implication from this study is that going forward future research requiring consent during the intrapartum period will require research information to be disseminated to the community midwives without a further burden of work, i.e. a slot in an antenatal class or a short video on current studies that is periodically updated and sent to women via the hospital app.
Training is important in studies where midwives are the primary recruiters. There is currently no prerequisite training (outside of good clinical practice) when midwives move into the role of the research midwife. The Assist II study [ 12 ] training consisted of a ‘Hints and Tips’ document derived from the findings of the Assist sub-study [ 36 ]. The document was an example of a discussion structure that research midwives could personalise [ 1 ] and served as an ‘aide memoire’. The women commented favourably on the midwives’ depth of knowledge, reporting this positively influenced their decision making—suggesting that well trained and knowledgeable research midwives are likely to have a beneficial impact on women’s experience.
A limitation of the IQS was that the data comes from a single study and the findings cannot simplistically be generalised across all intrapartum studies—although many of the findings are consistent with existing literature, suggesting that they are not entirely specific to this study setting. Time and resources were limited, so care was taken in the selection of participants and in the prioritisation of the questions asked in the interviews, in order to maximise the richness of the data [ 37 ]. The majority of women had accepted participation in Assist II study, and those declining may have had different experiences which were not adequately captured. Participants were mostly White European and minority groups may have had different views about information provision. During the IQS, it became clear that audio recordings for some women approached were not being captured due to midwives’ reluctance to record these data, and insights from the recruitment consultation data were limited by the relatively small number of recordings collected. However, this study captured a range of views and experiences of women and midwives and was strengthened by triangulation of data from the audio recorded recruitment discussions, and interviews with both women and midwives. Questions emerging from this study, which would benefit from further research, include what capacity do midwives have to deliver the required information at the preferred time point?; what factors might increase participation in (trials like) Assist II?; and what factors might discourage participation by women for whom English is not a first language?
Understanding the views of women and midwives on the optimum process for informed consent for research that involves procedures initiated in the intrapartum period is crucial if the informed consent process is to be optimised. Currently, limited data guides evidence-based practice [ 27 ] and there is no clarity, and questionable guidance, on the timing of information provision and the conditions that need to be met before a woman is asked to decide on participation and provide consent. We propose a framework of good practice for moving the consent process to a more woman centred approach, in which recruitment and information provision begins earlier in the pregnancy.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Assisted vaginal birth
Integrated qualitative study
Patient information leaflet
Association for Improvements in the Maternity Services
Royal College of Obstetricians and Gynaecologists
Allmark P, Mason S. Improving the quality of consent to randomised controlled trials by using continuous consent and clinician training in the consent process. J Med Ethics. 2006;32(8):439–43. https://doi.org/10.1136/jme.2005.013722 .
Article CAS PubMed PubMed Central Google Scholar
Gupta U. Informed consent in clinical research: revisiting few concepts and areas. Perspect Clin Res. 2013;4(1):26–32. https://doi.org/10.4103/2229-3485.106373 .
Article PubMed PubMed Central Google Scholar
Flory J, Emmanuel E. Interventions to improve research participants’ understanding in informed consent for research. A systematic review. JAMA. 2004;292(13):1593–601. https://doi.org/10.1001/jama.292.13.1593 .
Article CAS PubMed Google Scholar
Nishimura A, Carey J, Erwin P, Tilburt J, Murad M, McCormick J. Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomized control trials. Med Ethics. 2013;14:28. https://doi.org/10.1186/1472-6939-14-28 .
Article Google Scholar
Wade J, Donovan J, Athene Lane J, Neal D, Hamdy F. It’s not just what you say, it’s also how you say it: opening the ‘black box’ of informed consent appointments in randomised controlled trials. Soc Sci Med. 2009;68(11):2018–28. https://doi.org/10.1016/j.socscimed.20009.02.023 .
Article PubMed Google Scholar
Health Research Authority. Applying a proportionate approach to the process of seeking consent. HRA Policy and public affairs directorate. 2019. Available from: https://s3.eu-west-2amazonaws.com/www.hra.nhs.uk/media/documents/applying-proportionate-approach-process-seeking-consent_R3gbJKn.pdf [cited 26 Nov 2019].
European Medicines Agency. Guideline for good clinical practice E6 (R2) Addendum Step 5 version. 2016:5–69. Avalable from: https://www.ema.europa.eu/en/documents/scientific-guidelines/ich-e-6-r2-guideline/ich-e-6-r2-guideline-good-clinical-practice-step-5_5en.pdf [cited 10 Oct 2019].
Vernon G, Alfirevic Z, Weeks A. Issues of informed consent for intrapartum trials: a suggested pathway from the experience of the Release trial. Trials. 2006;7:13. https://doi.org/10.1186/1745-6215-6215-7-13 .
Tooher RL, Middleton PF, Crowther CA. A thematic analysis of factors influencing recruitment to maternal and perinatal trials. BMC Pregnancy Childbirth. 2008;8:36. https://doi.org/10.1186/1471-2393-8-36 .
Association for improvements in Maternity Services. A Charter for Ethical Research in Maternity Care. AIMS. 1997. Available from: https://www.aims.org.uk/assets/media/5/charter-for-ethical-research.pdf [cited 17 Sep 2019].
Dhumale H, Goudar S. Ethical issues related to consent for intrapartum trials. Reprod Health. 2017;14(Suppl 3):166. https://doi.org/10.1186/s12978-017-0426-y .
Hotton EJ, Alvarez M, Lenguerrand E, Wade J, Blencowe N, Draycott T, et al. The Odon Device for assisted vaginal birth: a feasibility study to investigate safety and efficacy -The ASSIST II study. Pilot Feasibility Stud. 2021;72:7. https://doi.org/10.1186/s40814-021-00814-2 .
Royal College of Obstetricians and Gynaecologists. Clinical governance advice No. 6a. London: RCOG. 2016. Available from: https://www.rcog.org.uk/en/guidelines-reserach-services/guidelines/clinical-governance-advice-6a/ [25 Oct 2019].
Braun V, Clarke V. Successful qualitative research. 1st ed. London: SAGE; 2013.
Google Scholar
Glaser BG, Strauss AL. The discovery of grounded theory. 1st ed. Chicago: Aldine; 1967.
Elo S, Kaariainen M, Kanste O, Polkki T, Utriainen K, Kyngas H. Qualitative content analysis: a focus on trustworthiness. SAGE Open. 2014:1–10. https://doi.org/10.1177/2158244014522633 .
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. https://doi.org/10.1191/1478088706qp063oa .
Tracy S, Hinrichs M. Qualitative quality: eight “big-tent” criteria for excellent qualitative research. Qual Inq. 2010;16(10):837–51. https://doi.org/10.1177/1077800410383121 .
Darawsheh W. Reflexivity in research: promoting rigour, reliability and validity in qualitative research. Int J Ther Rehabil. 2014;21:560–8. https://doi.org/10.12968/ijtr.2014.21.12.560 . [cited 17 Sep 2019].
Jackson A, Henry R, Avery N, Van Den Kerkhof E, Milne B. Informed consent for labour epidurals: what labouring women want to know. Can J Anaesth. 2000;47(11):1068–73. https://doi.org/10.1007/BF03027957 .
Affleck P, Waisel D, Cusick J, Van de Car T. Recall of risks following labor epidural analgesia. J Clin Anesth. 1998;10(2):141–4. https://doi.org/10.1016/s0952-8180(97)00258-4 .
Fontein-Kuipers Y, de Groot R, van Staa A. Woman-centred care 2.0: bringing the concept into focus. Eur J Midwifery. 2018;2:5. https://doi.org/10.18332/ejm/91492 .
Lawton J, Snowdon C, Morrow S, Norman JE, Denison FC, Hallowell. Recruiting and consenting into a peripartum trial in an emergency setting: a qualitative study of the experiences and views of women and healthcare professionals. Trials. 2016;17:195. https://doi.org/10.1186/s13063-016-1323-3 .
Baker L, Lavender T, Tintello D. Factors that influence women’s decisions about whether to participate in research: an exploratory study. Birth. 2005;32(1):60–6. https://doi.org/10.1111/j.0730-7659.2005.00346.x .
Smyth RMD, Jacoby A, Elbourne D. Deciding to join a perinatal randonised controlled trial: experiences and views of pregnant women enroled in the Magpie Trial. Midwifery. 2012;28(4):E478–85. https://doi.org/10.1016/j.midw.2011.08.006 .
Stuart J, Barnes J, Spiby H, Elbourne D. Understanding barriers to involving community midwives in identifying research participants; experience of the first steps randomised controlled trial. Midwifery. 2015;31(8):779–86. https://doi.org/10.1016/j.midw.2015.04.011 .
Hanrahan V, Gillies K, Biesty L. Recruiters perspectives of recruiting women during pregnancy and childbirth to clinical trials: a qualitative evidence synthesis. PLoS one. 2020;15(6):e0234783. https://doi.org/10.1371/journal.pone.0234783 .
Snowdon C, Elbourne D, Forsey M, Alfirevic Z. Information -hungry and disempowered: a qualitative study of women and their partners’ experiences of severe postpartum haemorrhage. Midwifery. 2012;28(6):791–9. https://doi.org/10.1016/j.midw.2011.12.012 .
Taylor HA, Washington D, Wang NY, Patel H, Ford D, Kass NE, Ali J. Randomized comparison of two interventions to enhance understanding during the informed consent process for research. Clin Trials. 2021;18:466–76. https://doi.org/10.1177/17407745211009529 .
Kenyon S, Dixon-Woods M, Jackson C, Pitchforth E, Windridge K. Participating in a trial in a critical situation: a qualitative study in pregnancy. Qual Saf Health Care. 2006;15(2):98–101. https://doi.org/10.1136/qshc.2005.015636 .
Blehar M, Spong C, Grady C, Goldkind S, Sahin L, Clayton J. Enrolling pregnant women: issues in clinical research. Womens Health Issues. 2013;23(1):e39–45. https://doi.org/10.1016/j.whi.2012.10.003 .
Chhoa CY, Sawyer A, Ayers S, Pushpa-Rajah A, Duley L. Clinicians’ views and experiences of offering two alternative consent pathways for participation in a preterm intrapartum trial: a qualitative study. Trials. 2017;18(1):196. https://doi.org/10.1186/s13063-017-1940-5 .
Houghton G, Kingdon C, Dower M, Shakur-Still H, Alfirevic Z. What women think about consent to research at the time of an obstetric emergency: a qualitative study of the views of a cohort of World Maternal Antifibrinolytic Trial participants. BJOG. 2018;125(13):1744–53. https://doi.org/10.1111/1471-0528.15333 .
Lawton J, Kirkham J, White S, Rankin D, Cooper C, Heller S. Uncovering the emotional aspects of working on a clinical trial: a qualitative study of the experiences an views of staff involved in a type 1 diabetes trial. Trials. 2015;16:3. https://doi.org/10.1186/1745-6215-16-3 .
Meshaka R, Jeffares S, Sadrudin F, Huisman N, Saravanan P. Why do pregnant women participate in research? A patient participation investigation using Q-methodology. Health Expect. 2017;20(2):188–97. https://doi.org/10.1111/hex.12446 .
Alvarez ML, Hotton EJ, Harding S, Crofts JF, Wade J. Investigation of informed consent procedures initiated in the intrapartum period. Br J Midwifery. 2020;28(4):251–8. https://doi.org/10.12968/bjom.2020.28.4.251 .
O’Cathain A, Hoddinott P, Lewin S, Thomas KJ, Young B, Adamson J, Jansen YJEM, Mills N, Moore G, Donovan JL. Maximising the impact of qualitative research in feasibility studies for randomised controlled trials: guidance for researchers. Pilot Feasibility Stud. 2015;1:32. https://doi.org/10.1186/s40814-015-0026-y .
Download references
Acknowledgements
The research team are grateful to the women and midwives who agreed to participate in this study and for their contribution to the development of the informed consent process.
This work was supported by the Bill & Melinda Gates Foundation—INV-010180. The Gates foundation was not involved in the design of the study and collection, analysis and interpretation of data.
Author information
Authors and affiliations.
Department of Women’s and Children’s Health, Southmead Hospital, North Bristol NHS Trust, BS10 5NB, Bristol, UK
Mary Alvarez, Emily J. Hotton, Sam Harding & Joanna F. Crofts
Bristol Medical School, Bristol University, BS8 2PS, Bristol, UK
Mary Alvarez, Jonathan Ives & Julia Wade
Translational Health Sciences, Bristol University, Bristol, UK
Emily J. Hotton
Research and Innovation, Learning and Research Building, Southmead Hospital, North Bristol NHS Trust, BS10 5NB, Bristol, UK
Sam Harding
Centre for Ethics in Medicine, Bristol University, Bristol, UK
Jonathan Ives
You can also search for this author in PubMed Google Scholar
Contributions
MA, EJH and JW contributed to the study design. MA carried out the data collection and analysis and wrote the initial draft of the manuscript. JW, JI, SH and EH contributed to the data analysis. All authors reviewed and approved the final manuscript.
Corresponding author
Correspondence to Mary Alvarez .
Ethics declarations
Ethics approval and consent to participate.
All qualitative aspects of the Assist II study 12 were approved by the South Central, Berkshire Research Ethics Committee, UK, on 7 June 2019 (ref: 19/SC/0226). A certificate of non-objection was received from the Medicines and Healthcare products Regulatory Agency on 12 June 2019 and final approval from the HRA was granted on 19 June 2019.
Consent for publication
Not applicable. All identities redacted. No images or videos used.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Alvarez, M., Hotton, E.J., Harding, S. et al. Women’s and midwives’ views on the optimum process for informed consent for research in a feasibility study involving an intrapartum intervention: a qualitative study. Pilot Feasibility Stud 9 , 98 (2023). https://doi.org/10.1186/s40814-023-01330-1
Download citation
Received : 19 December 2022
Accepted : 26 May 2023
Published : 15 June 2023
DOI : https://doi.org/10.1186/s40814-023-01330-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Informed consent
- Intrapartum intervention
- Qualitative research
- Research ethics
Pilot and Feasibility Studies
ISSN: 2055-5784
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Background. Quality of care is a focus area for improvement to reduce avoidable mortality and morbidity in mothers and newborn babies. According to the WHO Quality of Care Framework for maternal and newborn health, evidence-based practice is a key quality of care component [].Two entities underpin the implementation of evidence-based care; firstly, is the provision of 'recommended ...
Background We aim to assess competencies (knowledge, skills and attitudes) of midwifery care providers as well as their experiences and perceptions of in-service training in the four study countries; Benin, Malawi, Tanzania and Uganda as part of the Action Leveraging Evidence to Reduce perinatal mortality and morbidity in sub-Saharan Africa project (ALERT). While today more women in low- and ...
This guideline includes 56 evidence-based recommendations on intrapartum care - 26 new recommendations adopted by the Guideline Development Group (GDG) at the 2017 meetings, and 30 existing recommendations relevant to intrapartum care that were integrated from previously published WHO guidelines. Sections 3.1-3.6 outline the narrative summaries and the corresponding recommendations ...
Evidence-based quality care is essential for reducing sub-Saharan Africa's high burden of maternal and newborn mortality and morbidity. Provision of quality care results from interaction between several components of the health system including competent midwifery care providers and the working environment. We assessed midwifery care providers' ability to provide quality intrapartum and ...
Two reports, one from China and the one from Kenya provided care across antenatal and intrapartum. A study in China involved the provision of midwife-led care at antenatal, intrapartum and ... Flora K, Blandthorn J. A midwifery case management model for women with complex substance use in pregnancy. Women and Birth. 2018;31:S8-S9. View Article
Denis Walsh. This book is an attempt to bring together experts in their respective fields to place in one volume, for the first time, a comprehensive examination of normal birth practice. A glance through the Contents pages will reveal the variety of perspectives included here.
Continuity of care 4. Midwife-led continuity-of-care models, in which a known midwife or small group of known midwives supports a woman throughout the antenatal, intrapartum and postnatal continuum, are recommended for pregnant women in settings with well functioning midwifery programmes. a Context-specific recommendation First stage of labour
Clinical practice guidelines (hereafter referred to as guidelines) can be defined as "systematically developed statements to assist practitioners' decisions about appropriate health care for specific clinical circumstances". 30 Guidelines are considered to decrease the gap between research and current practice and, therefore, to reduce ...
Studies in Cambodia revealed that there was a gap between practices and evidence-based guidelines, although substantial technical support in obstetric and midwifery training courses had been provided for two decades [16,17,18,19,20]. One possible reason behind this gap is a lack of evidence-based knowledge in maternity care [16, 20]. However ...
The current study found that obstetric care providers' knowledge was strongly associated with the evidence-based intrapartum practice, where obstetric care providers who had good knowledge were nearly two times more likely to adhere to evidence-based intrapartum practice than those who had poor knowledge [AOR = 2.1; 95% CI (1.30-3.38)].
Background Midwives are essential providers of primary health care and can play a major role in the provision of health care that can save lives and improve sexual, reproductive, maternal, newborn and adolescent health outcomes. One way for midwives to deliver care is through midwife-led birth centres (MLBCs). Most of the evidence on MLBCs is from high-income countries but the opportunity for ...
The objective of the study was to explore and describe midwives' perceptions about the birth environment that support the implementation of the best practice during intrapartum care at a public hospital. ... Intrapartum care is a shared encounter and once we start to see ourselves as two different teams, then we are not doing good to the ...
The majority of the world's perinatal deaths occur in low- and middle-income countries. A substantial proportion occurs intrapartum and is avoidable with better care. At a low-resource tertiary hospital, this study assessed the quality of intrapartum care and adherence to locally-tailored clinical guidelines. A non-participatory, structured, direct observation study was held at Mnazi Mmoja ...
This guideline covers the care of healthy women and their babies, during labour and immediately after the birth. It focuses on women who give birth between 37 and 42 weeks of pregnancy ('term'). The guideline helps women to make an informed choice about where to have their baby. It also aims to reduce variation in areas of care such as ...
The effect of COVID-19 on intrapartum care: a case review from early in the pandemic. A 36-year-old primigravida booked at 11 weeks for antenatal care. She was of East Asian ethnicity, with a BMI of 26. She had no significant past medical or surgical history, however, a first-degree...
OBJECTIVES OF MIDWIFERY CASE STUDY ANTENATALLY To prepare the mother physically, emotionally, psychologically for labour, lactation and care of the new born. To ensure healthy and mature infant is born at the end of the pregnancy. Asses the progress of pregnancy to detect any risk and act upon it very fast for it is correction. To prepare the family psychologically for the awaiting of the new ...
Background Recruitment to intrapartum research is complex. Women are expected to understand unfamiliar terminology and assess potential harm versus benefit to their baby and themselves, often when an urgent intervention is required. Time pressures of intrapartum interventions are a major challenge for recruitment discussions taking place during labour, with research midwives expected to ...
OBJECTIVES OF MIDWIFERY CASE STUDY ANTENATALLY To prepare the mother physically, emotionally, psychologically for labour, lactation and care of the new born. To ensure healthy and mature infant is born at the end of the pregnancy. Asses the progress of pregnancy to detect any risk and act upon it very fast for it's correction. To prepare the family psychologically for the awaiting of the new ...