

Interesting Chemistry Topics for Seminar Presentation

Chemistry Topics for Seminar Presentation
- COVID-19: Why Chemistry Matters
- COVID‐19 in Chemical Science Perspective
- The Chemistry of the COVID-19 Antigen Test
- The Chemical Reactions
- Surface Chemistry: Adsorption and Absorption (PPT)
- Metals, Nonmetals, and Metalloids
- Acid Rain: Acid Deposition
- Advances in ion chromatography
- Air Analysis
- Aliphatic Compounds
- Analytical technique
- BioMolecular Chemistry
- Biomolecule
- Boron Chemistry and Applications to Cancer Treatment
- Boron-Pnictogen Multiple Bonds: Organometallic Alkenes and Alkynes
- Breathe Analysis
- Carbohydrates
- Carbon Capture and Storage
- Carbon Dating
- Catalytic Converters
- CFCs and the Environment
- Charge Transport across the Metal-Molecule Interface
- Chemical Sensor Technologies for Chemical Analysis and Materials Characterization
- Chemistry at the origin of life
- Chemical Elements in the Human Body
- Chemistry of Coffee
- Chemical Reactions in Biological Systems
- Chemical Reactions in the Metabolism
- Chemistry of Diamonds
- Chemistry of Paints
- Chemistry of Warfare
- Chiral Compounds
- Chiral molecules
- Chlorine trifluoride
- Clean Water Supply
- Cleaning Up: Formulation Chemistry
- Coffee: Friend or Foe
- Counterfeit Medicines
- Curare and the case of Mario Jascalevich
- Desalinating Water for future supply
- Desalination
- Detecting Chemicals in the Universe (Astrochemistry)
- Detecting Drug Abuse in Sport
- Drugs in Drinking Water
- Electrochemical fluorination
- Emulsion – Suspension Technology
- Environmental Impact of pH Balance
- Forensic analysis of explosives
- Extraterrestrial molecules
- Fingerprint Detection
- Functional Group
- Graphene- Wonder Material
- Greenhouses Gases
- Hybrid organic/inorganic nanocomposites
- Hybridization
- Hydrocarbons
- Hydrogen Fuel Cells ( Article )
- Hydrogen Fuel Storage
- Hydrogen production from coal gasification
- Insect Repellants
- Isolating Fluorine Gas
- Lanthanide porphyrin complexes with sandwich structures
- Lead in the Environment
- Mass Spectrometry
- Medicinal Applications of Iodine
- Medicinal Properties of Radium
- Mesoporous materials: Synthesis, structure, and properties
- Modern Fibres for future clothing
- Molecular Design of In-Situ Phosphatizing Coatings for Aerospace Primers
- Mohr's Salt
- Molecule-based magnetic materials and high-spin molecules
- Nanograin Magnetoresistive Manganite Coatings
- Nanomedicines
- Nanoparticle Plasmons
- Nanosilver in the environment
- Nanotechnology: molecular-scale machines.
- New advances in crystal engineering
- New advances in supramolecular chemistry
- New biomaterial for scar healing
- New Chemistry of Superelectrophiles
- A new generation of antimalarials
- New materials for lightweight aircraft
- Nitric oxide as a neurotransmitter:
- Nitrous OxideParticle Size Analysis
- Novel surface analytical techniques
- Ocean Acidification
- Organic Chemistry
- Ozone: Protector or Polluter
- Paracetamol
- People on the Periodic Table
- Photocatalysts for Self-Cleaning Surfaces
- Photodynamic Therapy
- Photoreduction of Metal Ions
- Photoresist chemistry for X-ray lithography
- Phyto Chemistry
- Physical chemistry of surface phenomena
- Polymorphism in the Pharmaceutical Industry
- Preparing and Characterising Nanoparticles
- Prevention of iron corrosion by amine-quinone polymers
- Quasicrystals
- Seaweed extracts to improve battery performance
- Seeing structure: atomic-scale molecular imaging
- Smart Clothing
- Smart Glass
- Solid Oxide fuel cells
- Stereochemical nonrigidity in organometallic chemistry
- Structural characterization of carbon nanomaterials
- Surfactant, Colloids, and Interfaces
- Synthesis of Ammonia
- Synthesis of Carbon Nanotubes (CNTs) on porous Si
- Synthetic Chemistry
- Thallium Poisoning
- The Artificial Leaf
- The chemistry of early life
- The chemistry of smell
- The Discovery of DNA
- The Science of Cocktails
- Thin-film modeling of catalysts
- Use of the Hadamard transform in chemistry
- Uses of Electrochemical Cells
- Violating the Octet Rule: the Chemistry of Hypervalent Bonds
- Water from hydrofracking
- Water-Based Polymers
- Methamphetamine Synthesis
- Human digestion process
- Hemoglobin and Oxygen Transportation
- Pollutants Chemistry
- VSEPR Theory
- Explosives: TNT and nitroglycerine
- Why Alchemists Seeks Philosopher’s Stone
- Chemists Who Are Nobel Prize Awardees
- How Chemical Weapons Become Main Threat for War
- How to Choose Quality Water
- Making a Kid Interested in Chemistry
- Hair Biochemistry and Its Process
- Effects of Lack of Chemical Elements in a Human Body
- Safety Precautions for Chemical Products
- Like on Facebook
- Follow on Twitter
- Follow on Slideshare
- Follow on Pinterest
- Subscribe on Youtube
Trending Seminar Topics
- 100+ Seminar Topics for Youth, Teenagers, College Students Young people are on a never-ending quest for transcendence, which drives them to want to improve the environment, countries, communities,...
- 30+ Technical Seminar Topics for Presentation: Latest Tech Trends Technology is rapidly evolving today, allowing for faster change and progress and accelerating the rate of change. However, it is not just t...
- 100 PowerPoint Presentation Topics in Hindi (Download PPT) विद्यार्थियों के लिए प्रेजेंटेशन का महत्व प्रेजेंटेशन (presentation) देना शैक्षणिक पाठ्यक्रम का एक महत्वपूर्ण व्यावहारिक पाठ्यक्रम है, ...
- 100+ Interesting Biology Presentation Topics with PPT Biology Topics for Presentation & Research Biology is a topic that every school student studies and university student who does major in...
- 100 Interesting Fun Topics for Presentations Fun Topics for Presentations We have prepared for you a fantastic collection of fun topics for presentation with relevant links to the artic...
Recent Seminar Topics
Seminar topics.
- 💻 Seminar Topics for CSE Computer Science Engineering
- ⚙️ Seminar Topics for Mechanical Engineering ME
- 📡 Seminar Topics for ECE Electronics and Communication
- ⚡️ Seminar Topics for Electrical Engineering EEE
- 👷🏻 Seminar Topics for Civil Engineering
- 🏭 Seminar Topics for Production Engineering
- 💡 Physics Seminar Topics
- 🌎 Seminar Topics for Environment
- ⚗️ Chemistry Seminar Topics
- 📈 Business Seminar Topics
- 👦🏻 Seminar Topics for Youth
Investigatory Projects Topics
- 👨🏻🔬 Chemistry Investigatory Projects Topics
- 📧 Contact Us For Seminar Topics
- 👉🏼Follow us in Slideshare
Presentation Topics
- 🌍 Environment Related Presentation Topics
- ⚗️ Inorganic Chemistry Presentation Topics
- 👨🏻🎓 General Presentation Topics
- 🦚 Hindi Presentation Topics
- 🪐 Physics Presentation Topics
- 🧪 Chemistry: Interesting Presentation Topics
- 🌿 Biology Presentation Topics
- 🧬 Organic Chemistry Presentation Topics
Speech Topics and Ideas
- 🦁 Informative and Persuasive Speech Topics on Animals
- 🚗 Informative and Persuasive Speech Topics on Automotives
- 💡 Ideas to Choose Right Informative Speech
- 👩🏻🎓 Informative Speech Topics For College Students
- 🔬 Informative Speech Topics on Science and Technology

Science Presentation for Class 9
Chapter 1: matter in our surroundings, chapter 2: is matter around us pure, chapter 3: atoms and molecules, chapter 4: structure of the atom, chapter 5: the fundamental unit of life, chapter 6: tissues, chapter 7.1: animal kingdom diversities, chapter 7.1: plant kingdom diversities, chapter 8: motion, chapter 9: force and laws of motion, chapter 10: gravitation, chapter 11: work and energy, chapter 12: sound, chapter 13: why do we fall ill, chapter 14: natural resources, chapter 15: improvement in food resources.
Disclaimer: All contents are collected from various sources and updated at this platform to help teachers and students. If content owner (Original Creator) have any objection, Please mail us to [email protected] with ID Proof, content will be removed/credited. Thanks and Regards
Notes Station: To Read Click on Title
Lesson plan for english class 8 chapter wise, कक्षा 6 पाठ योजना संस्कृत पाठ 1 class 6 sanskrit lesson plan chapter 1, split up of syllabus class 6 sanskrit, split up of syllabus class 6 hindi, book cart: to purchase click on title.

Oswaal Science Question bank - 9

Oswaal English Lang. & Lit. Question bank - 9

Oswaal Social Science Question bank - 9

Oswaal Mathematics Question bank - 9

MTG Foundation Course NTSE-NVS-NSO - 9

Golden Science Refresher for Class - 9

All in One Mathematics for Class - 9

All in One English Lang. & Lit. for Class - 9

All in One Social Science for Class - 9

All in One Hindi Course A for Class - 9

Beehive English Textbook Class - 9

NCERT - Mathematics Solutions - Class 9

Social Sc. Combo Pack NCERT Textbook - 9

Full Marks Hindi Course A Workbook Class - 9

Hindi Sparsh Bhaag 1 - NCERT Class - 9

Mathematics Textbook NCERT Class 9

NCERT Textbook Science Class 9

Full Marks CBSE Science Workbook - Class 9

Full Marks So. Science Workbook - Class 9

NCERT -Set of 8 Books - Class 9

Chapter wise Worksheets for CBSE All Subjects - 9

Full Marks Mathematics Practice Book Class 9

Full Marks English Workbook Class - 9

CBSE All In One Science Class 9

Mathematics by R D Sharma - Class 9
Amazon Affiliate Disclaimer: cbsecontent.com is a part of Amazon Services LLC Associates Program, an affiliate advertising program designed to provide a means for sites to earn advertising fees by advertising and linking to Amazon.in. As an amazon associates we earn from qualifying purchases.
Please show me the notes of Physics ( Chapter Motion ) Class 9
naturally like your web site however you need to take a look at the spelling on several of your posts. A number of them are rife with spelling problems and I find it very bothersome to tell the truth on the other hand I will surely come again again.
I appreciate you sharing this blog post. Thanks Again. Cool.
How can I download presentation
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Unit 2: Matter in Our Surroundings
Matter and particles that make up matter..
- Characteristics of particles of matter (Opens a modal)
- Characteristics of particles of matter Get 4 of 5 questions to level up!
States of matter
- States of matter (Opens a modal)
- Effect of temp and pressure on state change (Opens a modal)
- Evaporation and its cooling effect (Opens a modal)
- The states of matter. Get 3 of 4 questions to level up!
- Evaporation and its cooling effects Get 5 of 7 questions to level up!
Talk to our experts
1800-120-456-456
- Structure of the Atom Class 9 Notes CBSE Science Chapter 4 (Free PDF Download)
- Revision Notes

CBSE Class 9 Science Chapter 4 - Structure of The Atom Revision Notes - Free PDF Download
The smallest unit of any matter which retains all the chemical properties of an element is called an atom. Molecules are formed by combining atoms which then interconnect and form solids, liquids, or gases. From the structure of an atom of Class 9 notes, you will know that an atom has two regions, a nucleus that forms the centre of the atom and contains neutrons and protons, and there is an outer region of an atom which keeps the electron in its orbit around the nucleus. Neutrons have a negative charge equal to the positive charge of a proton.
Vedantu is a platform that provides free CBSE Solutions (NCERT) and other study materials for students. You can also download Class 9 Maths and Class 9 Science NCERT Solutions to help you to revise the complete syllabus and score more marks in your examinations.
Important Topics Covered under the Chapter
Following are the important topics that are covered in Class 9 Science Chapter 4 - Structure of the Atom:
Sub-Atomic Particles of an Atom and Their Discovery
Atomic Model by Thomson
Electronic Arrangement in an Atom
Atomic Model by Rutherford
Bohr’s Model of Atom
Atomic Number
Mass number, download cbse class 9 science revision notes 2024-25 pdf.
Also, check CBSE Class 9 Science revision notes for All chapters:

Access Class 9 Science Chapter 4 – Structure of Atoms Notes in 30 Minutes
Summary of structure of atoms.
Atoms are the basic units of matter and the defining structure of elements.
It consists of three basic particles, i.e., protons, electrons, and neutrons, that build the structure of an atom.
Protons are positively charged particles and were founded by E. Goldstein.
Electrons are negatively charged particles and were founded by J.J. Thomson.
Neutrons have no charge and were founded by Chadwick.
The nucleus is in the centre of the atom and contains protons and neutrons.
The outer region of the atom which holds electrons in orbit around the nucleus is known as shell/energy level/orbits.
These shells are further divided into subshells.
The electrons present in the outermost shell of an atom are known as the valence electrons.
The electronic configuration of an element is the representation of the arrangement of electrons distributed among the orbital shells and subshells. The valence electrons are the determining factor for the unique chemistry of the element.
The atomic number of an element is the same as the number of protons in the nucleus of its atom. As atoms are electrically neutral, an atom contains as many electrons as it has protons. The atomic number is denoted by Z.
The mass number of an atom is equal to the number of nucleons in its nucleus. Nucleons are the collective term for protons and neutrons. The mass number is denoted by A.
In the notation of an atom, the atomic number is written as a subscript on the left of the element symbol and the mass number is written as a superscript on the left of the element symbol.
Isotopes can be defined as elements that possess the same number of protons and electrons, but a different number of neutrons.
For example, Protium, deuterium, and tritium are the isotopes of hydrogen. They each have one single proton Z=1 and a single electron but differ in the number of their neutrons. Hydrogen has no neutrons, deuterium has one, and tritium has two neutrons.
Isobar is an element that differs in chemical property but has the same physical property which means isobars are those elements that have a different atomic number but the same mass number.
For example, Calcium and chlorine are isobars since both have a mass number of 40 but calcium has an atomic number of 20 and chlorine has an atomic number of 17.
Isotones are elements that have the same number of neutrons but different atomic numbers.
For example, Chlorine with atomic number 37 and potassium with atomic number 39 is isotones because both chlorine and potassium have the same number of neutrons i.e., 20.
Chapter 4 Science Class 9 Notes: Atomic Number and Mass Number
Chapter 4 Science Class 9 notes tell us that the total number of protons present in an element is called the atomic number while the mass number is both the number of protons plus the number of neutrons.
According to Class 9 Chapter 4 Science notes, an atomic number is the number of the chemical elements present in the periodic system so that the elements are organised in order of the increasing number of protons present in the nucleus. Also, the total number of protons that are equal to the electrons in a neutral atom is called the atomic number. For example, iron has 26 protons in its nucleus; hence, the atomic number of the matter is 26. An equal number of electrons and protons are presented in a neutral atom.
According to Class 9th Science Chapter 4 notes, the total number of protons and neutrons is combined together and forms an element’s mass number. The contribution of the mass from the electron is disdained and then calculated as the mass number. Hence, the approximation of the mass is used to calculate the number of neutrons that an element has by subtracting the total number of protons from the mass number. Both neutrons and protons weigh about 1 atomic mass unit or amu. Isotopes of this same element will have the same number of atoms but a different mass.
Class 9 Science Chapter 4 Notes: Details of Isotopes
Notes of Chapter 4 Science Class 9 also give us a brief description of isotopes. It is the variant of a specific chemical element that differs in its number of neutrons and also in the number of nucleons. The isotopes have the same number of protons and a different number of neutrons. Thus, they have the same atomic number but a different mass number. All elements are a mixture of isotopes. Each of them is a pure substance. They have the same chemical properties but different physical properties. If there is an absence of isotope in an element, then its mass would be the same as the total number of neutrons and protons. Chapter 4 Science Class 9 notes also tell us that if there is a presence of isotope in any element, then the percentage of all the isotope forms should be known and then the average mass should be calculated.
Science Class 9 Chapter 4 Notes: Details of Isobars
The nuclides of various chemical elements are called isobars. They have the same number of nucleons which means that they have the same mass number. But they differ in atomic number. Alfred Walter Stewart suggested the name “isobars”. It has been derived from the Greek word isos which means “equal” and bars, which means “weight”.
Class 9 Science Notes Chapter 4: A Summary of The Structure of An Atom
An atom is made from the nucleus which is the centre of the atom and carries protons and neutrons and an outer region that holds the electron in its orbit around the nucleus.
All electrons have a negative charge which is equal to the positive charge of a proton.
Neutrons are known as uncharged particles that are found in the nucleus.
Benefits of Studying Vedantu’s Revision Notes on CBSE Class 9 Science Chapter 4 - Structure of the Atom
The advantages of studying the CBSE Revision Notes on Class 9 Science Chapter 4 - Structure of the Atom, by Vedantu, are many. However, the following are the most noteworthy:
Provides quick, clear summaries of key concepts.
Simplifies complex topics for better understanding.
Efficient tool for last-minute exam prep.
Enhances retention of crucial information.
Supports effective exam preparation with key points and tips.
Saves time by consolidating information.
Prioritizes important topics and questions.
Offers practical examples for real-world connections.
Boosts student confidence for exams.
Why Choose Vedantu?
In the case of online learning, Vedantu has been a pioneer. The answers provided in the solutions prepared by Vedantu are contributions of skilful experts. Written in easy language, the solutions and notes are understood by the students most easily. Vedantu also prepares for mock tests for students to check their progress. The PDF versions of the solutions are available for free download.
The Revision Notes on Class 9 Science Chapter 4 - Structure of the Atom will help students to have a clear understanding of the concepts covered in this chapter. Students are advised to refer to these notes to secure good marks in the exams. To access other chapters' revision notes and important study materials for Class 9 students, explore Vedantu’s website.

FAQs on Structure of the Atom Class 9 Notes CBSE Science Chapter 4 (Free PDF Download)
1. What is an atomic number? Give one word for the following:
Positively charged atom
A group of atoms carrying a charge
The total number of protons present in the nucleus of an atom is called an atomic number. For Example- atomic numbers of carbon, oxygen, magnesium, and neon are 6, 12, 8, and 10 respectively.
Positively charged atom - Cation
A group of atoms carrying a charge – Polyatomic ion.
2. Why are the chemical properties of all isotopes the same? Name the isotopes used in the treatment of cancer and goitre?
Isotopes have the same atomic number, hence the number of the valence electron in them remains the same and this valence electron takes part in the chemical reactions. Thus, isotopes have the same chemical properties.
Goitre – an isotope of iodine
Cancer – an isotope of cobalt
3. What is Bohr's structure of atom Chapter 4 of Class 9 Science?
Bohr's model was presented in the year 1913 by Niels Bohr and Ernest Rutherford. Bohr's model of atoms states that the whole mass of an atom is concentrated as the heavily positively charged nucleus. The atoms consist of electrons that revolve around the nucleus. The electrons revolve around a circular path known as orbits which are also known as energy levels. Every energy level has different amounts of energy. This change in energy takes place when one electron jumps from one energy to another.
4. What is an atom?
Atoms are elements that help create matter. They are the tiniest element available that cannot be further divided. It is the smallest unit of matter that consists of some sort of chemical element. Atoms don't exist independently, instead, they form molecules and ions. These molecules and ions combine together in a huge number which later forms into matter that we can feel, see, and touch. Examples of atoms include Hydrogen, Sodium, Oxygen, and many more.
5. What are the important concepts in Chapter 4 of Class 9 Science?
Class 9 Science Chapter 4 is titled Structure of the atom. This is an important chapter in Class 9 science as it will help you understand the basics of the chapter for your future studies. Some of the important concepts in this chapter include the structure of an atom, charged particles in matter, mass number, isobars, isotopes, atomic number, valency, distribution of electrons in different orbits, neutrons, Rutherford model of an atom, Thomson's model of an atom, and even Bohr's model of an atom.
6. Where can I find notes for Chapter 4 of Class 9 Science?
Vedantu provides easy Revision notes for Class 9 Science Chapter 4. These notes are explained in detail for you to understand the chapter better, free of cost. There are important concepts and topics that are covered in the notes provided. The notes are also available on the Vedantu Mobile app. This chapter has a good weightage from the examination point of view. Therefore, it is important for you to understand this chapter.
7. Briefly explain the Cathode Ray Experiment.
The Cathode Ray Experiment refers to the discovery of electrons which was discovered by J. J. Thomson. He was able to discover this by using the cathode-ray tube. A cathode ray tube refers to a vacuum-sealed tube with an anode and a cathode on one end. This created a beam of electrons that were travelled to the other side of the tube. The experiment stated that an atom is an indivisible and simple particle that has at least one electron.
STUDY MATERIALS FOR CLASS 9

25,000+ students realised their study abroad dream with us. Take the first step today
Here’s your new year gift, one app for all your, study abroad needs, start your journey, track your progress, grow with the community and so much more.

Verification Code
An OTP has been sent to your registered mobile no. Please verify

Thanks for your comment !
Our team will review it before it's shown to our readers.

Class 9 Atoms And Molecules Study Notes
- Updated on
- Jul 31, 2021
The matter that surrounds us is made up of tiny particles. The class 9 syllabus for science entails a very interesting chapter that enlightens us with the actual phenomenon behind the composition of different substances. In addition to that, you will learn about Dalton’s atomic theory, atomic and molecular masses, ions, mole concept and many other interesting topics. Here are the complete study notes for Class 9 Atoms and Molecules to make this chapter easy to study and remember for you!
This Blog Includes:
Laws of chemical combination, dalton’s atomic theory, atomic size, atomic mass, symbols for elements, rules for writing chemical formulae, mole concept, class 9 atoms and molecules: important questions, class 9 atoms and molecules ppt.
The chapter begins with a thorough explanation of the two laws of chemical combination. Great minds like Kannad (600 BC), Democritus, and Leucippus (400 BC), as well as Antoine Lavoisier and John Dalton, made some observations, based upon which these laws have been devised. After a detailed and logical analysis, they all came to some conclusions regarding the formation of compounds and laid down the below-mentioned laws-
Law of Conservation of Mass According to this law, for a general chemical reaction, there is no loss or gain in the total mass of substances. It means that, in a chemical reaction, there is no change in the total mass as the mass of the product is always equal to the mass of the reactant.
Law of Constant or Definite Proportion This law explains that all pure elements of the same compound contain the same elements in the same proportion of weight. In simpler words, every element combines with another in a definite or constant ratio, i.e., depending on their atomic masses.
Check Out Study Notes for Class 9: Work, Energy and Power
The famous chemist, John Dalton, carried out a random experiment which later on became the famous Atomic Theory. He analysed it critically and then presented his insightful observations in the form of postulates of atomic theory. Dalton’s postulates regarding the atomic theory as per class 9 atoms and molecules are:
- Atoms make up all the matter in this world
- Atoms of a given element have exactly the same chemical properties and mass
- Atoms are indivisible particles which can neither be created nor destroyed
- Atoms of different elements have varied chemical properties and mass
- Atoms combine in a ratio of small natural numbers to form compounds.
- A given compound will always have determined kinds and number of atoms
Now we will explore the most vital topic of this chapter. The smallest and the tiniest unit that can be a part of a chemical reaction is known as an Atom. Atoms tend to have some general properties which they showcase, let us have a look at them-
Atoms are invisible to the naked eye. Their shape is spherical and is not visible by an ordinary microscope. The diameter of an atom is about 1 × 10 -10 m. Even on the tip of a pencil, there are thousands, if not millions of atoms.
Every element has a set atomic mass. This was a direct postulate from Dalton’s Theory on atoms. Determining the mass of such a tiny particle was a daunting task. Hence, this is where the concept of Relative Atomic Mass comes in.
What is Motion in a Straight Line ?
As the discovery of elements went on for years and years, every element was given a particular symbol either based on its initials or on the name of the scientist who discovered it. Tabulated below are some important elements from the class 9 atoms and molecules chapter.
The very next important topic of this chapter is of molecules. A molecule is the smallest particle of matter (element or compound) which can exist in a free state. Like atoms, molecules of any given substance are all similar. Consequently, molecules of different substances are different.
Atomicity is the number of atoms contained in a molecule of a substance (element or compound). Based on atomicity, the types of molecules are:
- Monoatomic Molecules
- Diatomic Molecules
- Triatomic Molecules
- Tetratomic Molecules
- Polyatomic Molecules
What is the Difference Between Acid and Base ?
The class 9 atoms and molecules chapter include another integral topic called Ions. The compounds formed by charged particles are called ions. Ions carrying positive charges are called Cations, and the ones with negative charges are called Anions. For Example: NaCl is an ionic compound. That means that it is made up of Sodium ion (Na + ) and Chlorine ion (Cl – ).
Writing a chemical formula has an intricate science that runs behind the process. Till now, you must have learnt them without knowing the actual reason for their placement. But in the class 9 atoms and molecules, you will also get to know that the chemical formula denotes the ratio of elements used to form the given compound. The correct formula can be determined if we know the respective valencies of the given elements.
Before we go on to exploring the rules, the combining power of an element or ion as per which it can react is called Valency. The higher the valency, the more the number of atoms or ions it can accumulate. Mentioned below are some common valencies:
The formula of a compound is given by writing, side by side, the symbols of the constituent elements. The symbol of more a metallic element is written first in the formula, for example, HCl, NaCl, CaCO 3 , etc. Subscripts indicate the number of atoms in each of the constituent elements present. For example, in H 2 O, there are 2 Hydrogen and 1 Oxygen atom. Furthermore, the simple binary compounds are made by crisscrossing the valencies present in the molecules of a compound.
One of the most important concepts of class 9 atoms and molecules is the explanation of the mole concept. A mole of atoms is a collection of atoms whose total mass is the number of grams equal to the atomic mass. A mole (mol) is defined as the amount of substance that contains as many atoms, molecules, ions, electrons, etc., as there are atoms in exactly 12 g of carbon 12 ( 12 C). A mole of a substance represents 6.022 × 10 23 particles of the substance.
Number of Moles = (mass of the given element in grams) ÷ (gram-atomic mass of the element)
Download the PDF for Class 9 Atoms and Molecules.
Some conceptual questions to clarify any doubts about atoms and molecules class 9 are:
- What is the mass of 5 mol of Ammonia?
- Explain ‘relative atomic mass.’
- Write the correct chemical formula of (i) Sodium Carbonate (ii) Magnesium Oxide.
- Define atomicity? What is the atomicity of phosphorus and nitrogen?
- Explain the difference between cation and anion?
- What is polyatomic ion? Give one example.
- What is meant by valency. What is the valency for magnesium and copper?
- Explain the types of ion with examples.
- What is formula unit of mass? Explain how is it different from molecular mass?
- What is Dalton’s Atomic Theory?
These were some essential points for class 9 atoms and molecules. Get in touch with our experts at Leverage Edu as they will help you have a clear picture of your upcoming future.
Team Leverage Edu
Leave a Reply Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Contact no. *

Leaving already?
8 Universities with higher ROI than IITs and IIMs
Grab this one-time opportunity to download this ebook
Connect With Us
25,000+ students realised their study abroad dream with us. take the first step today..

Resend OTP in

Need help with?
Study abroad.
UK, Canada, US & More
IELTS, GRE, GMAT & More
Scholarship, Loans & Forex
Country Preference
New Zealand
Which English test are you planning to take?
Which academic test are you planning to take.
Not Sure yet
When are you planning to take the exam?
Already booked my exam slot
Within 2 Months
Want to learn about the test
Which Degree do you wish to pursue?
When do you want to start studying abroad.
September 2024
January 2025
What is your budget to study abroad?

How would you describe this article ?
Please rate this article
We would like to hear more.
LAWS OF CHEMICAL COMINATION- PPT (Powerpoint Presentation), Chemistry - Class 9 PDF Download
Top courses for class 9, faqs on laws of chemical comination- ppt (powerpoint presentation), chemistry - class 9, practice quizzes, sample paper, laws of chemical comination- ppt (powerpoint presentation), mock tests for examination, chemistry - class 9, shortcuts and tricks, semester notes, extra questions, important questions, objective type questions, past year papers, video lectures, study material, viva questions, previous year questions with solutions.

LAWS OF CHEMICAL COMINATION- PPT (Powerpoint Presentation), Chemistry Free PDF Download
Importance of laws of chemical comination- ppt (powerpoint presentation), chemistry, laws of chemical comination- ppt (powerpoint presentation), chemistry notes, laws of chemical comination- ppt (powerpoint presentation), chemistry class 9 questions, study laws of chemical comination- ppt (powerpoint presentation), chemistry on the app, welcome back, create your account for free.

Forgot Password
Unattempted tests, change country.
- Bihar Board
SRM University
Pseb 10th result.
- UP Board 10th Result
- UP Board 12th Result
- Punjab Board Result 2024
- MP Board Result 2024
- Rajasthan Board Result 2024
- Karnataka Board Result
- Shiv Khera Special
- Education News
- Web Stories
- Current Affairs
- नए भारत का नया उत्तर प्रदेश
- School & Boards
- College Admission
- Govt Jobs Alert & Prep
- GK & Aptitude
CBSE Class 9 Science Exam 2020: Chapter-wise important topics and questions
You will get here the cbse class 9 science, chapterwise important topics & questions to prepare for class 9 science examination 2020. all the questions have been prepared to cover most of the important topics included in each chapter..

A strategic study plan is a must for effective learning and improving your performance in school. Students must have an authentic, meaningful problem-solving experience that requires the use of various study tips and strategies.
CBSE Class 9 Science Syllabus 2019-2020
Jagranjosh brings you a collection of the Important Topics and Questions for all the chapters of CBSE Class 9 Science. With all these sources in one place, you may find it easy to access the chapters easily and make the most of your study time. These tips will surely provide you with an idea of the important topics from each chapter and help you in building a strong foundation to get better results.
Find below the chapter-wise links to access the Important Topics and Questions for Class 9 Science:
Chapter 1: Matter in Our Surroundings
Chapter 2: Is matter arround us pure
Chapter 3: Atoms and Molecules
Chapter 4: Structure of Atom
Chapter 5: The fundamental unit of life
Chapter 6: Tissues
Chapter 7: Diversity in kiving Organisms
Chapter 8: Motion
Chapter 9: Force and Law of Motion
Chapter 10: Gravitation
Chapter 11: Work and Energy
Chapter 12: Sound
Chapter 13: Why do we fall ill
Chapter 14: Natural Resources
Chapter 15: Improvement in Food Resources
You may also like to read:
NCERT Solutions for CBSE Class 9 Science
Get here latest School , CBSE and Govt Jobs notification in English and Hindi for Sarkari Naukari and Sarkari Result . Download the Jagran Josh Sarkari Naukri App . Check Board Result 2024 for Class 10 and Class 12 like CBSE Board Result , UP Board Result , Bihar Board Result , MP Board Result , Rajasthan Board Result and Other States Boards.
- PSEB 10th Result 2024
- PSEB Class 10th Result 2024
- Punjab Board 10th Result 2024
- pseb.ac.in 10th Result 2024
- Punjab Board Class 10th Result 2024
- ਪੀਐਸਈਬੀ 10ਵੀਂ ਦਾ ਨਤੀਜਾ 2024
- PSEB १०थ रिजल्ट २०२४
- पंजाब बोर्ड १०थ टॉपर्स लिस्ट 2024
- PSEB 10th Topper List 2024
- CBSE Class 9 Tips & Strategies for Science
- CBSE Class 9 Science
- CBSE Class 9 Subjects
- CBSE Class 9 Study Material
- CBSE Class 9 Tips & Strategies
- Tips & Strategies for CBSE Exams
- CBSE Study Material
- CBSE Class 9
Latest Education News
Punjab Board 10th Toppers List 2024: अदिति ने 650 अंक लाकर पंजाब बोर्ड 10वीं की परीक्षा में किया टॉप, जानें कैसा रहा रिजल्ट
SSC GD Result 2024 Live: Constable Scorecard Soon on ssc.gov.in; Check Expected Cut Off
Punjab Board 10th Result 2024: PSEB Matric Result Link at pseb.ac.in
PSEB 10th Result 2024 Analysis: 97.24% Pass Out 2.7 Lakh Students Appeared, Girls Outshine Boys
Optical Illusion IQ Test: Use Your Razor-Sharp Vision To Spot A Flag In This Garden In 8 Seconds!
CBSE Class 11 Geography Syllabus 2024-2025 PDF Download
PSEB Class 10th Result 2024 Declared: Punjab Board Matriculation Results Online on Official Website pseb.ac.in, How to Check Scorecard
दुनिया की सबसे ऊंची राजधानी कौन-सी है, जानें
PSEB 10th Result 2024 Released: Aditi, Alisha and Karmanpreet got 1st, 2nd and 3rd Position, 97.2% Students Passed and Live Link Tomorrow
Punjab Board 10th Result 2024: When, Where, and How to Check PSEB Matric Results Online and via SMS
PSEB 10th Result 2024 Roll Number and Name-wise: Check Punjab Board Matric Marks Online
WBJEE Admit Card 2024 Released: Download Hall Ticket PDF Using Application Number at wbjeeb.nic.in
PSEB 10th Toppers List 2024 Released: Aditi Tops Punjab Board Class 10 Exam, Check Toppers Name, Marks and Percentage Here
Army Agniveer Admit Card 2024 Out At joinindianarmy.nic.in; Check Exam Date, Pattern and other details
PSEB 10th Class Result 2024 Out: ਪੰਜਾਬ ਬੋਰਡ ਦਾ 10ਵੀਂ ਦਾ ਨਤੀਜਾ ਜਾਰੀ, pseb.ac.in 'ਤੇ ਕੱਲ੍ਹ ਤੋਂ ਸਿੱਧਾ ਲਿੰਕ ਹੋਵੇਗਾ ਐਕਟਿਵ
PSEB 10th Result 2024 Out: पंजाब बोर्ड मैट्रिक के नतीजे pseb.ac.in पर, इन स्टेप्स से चेक करें नतीजे
Bihar Panchayati Raj Vibhag Recruitment 2024 for 6570 Accountant IT Assistant, Check Application Dates
NIOS 12th Maths Syllabus 2023-2024: Download Class 12 Maths Syllabus PDF
Punjab Board Result 2024: Check PSEB 10th, 12th Result Link at pseb.ac.in
Calcutta High Court LDC Recruitment 2024: Apply Online For Stenographer And Other Posts, Check Eligibility
- CBSE Notes For Class 9
- Class 9 Science Notes
- Chapter 3: Atoms And Molecules

Atoms And Molecules Class 9 Notes - Chapter 3
Cbse class 9 science notes chapter 3 atoms and molecules.
Atoms and molecules are responsible for forming tiny sand particles, gargantuan black holes and everything in between. The atom is the most fundamental unit of matter, making up everything that we see around us. It is extremely small, measuring in at less than 0.1 to 0.5 nanometers.
For Chapter Summary on Atoms And Molecules, watch the below video

Laws of Chemical Combination
Chemical reactions.
- In a chemical reaction, two or more molecules interact to produce new compounds; they are called reactants, whereas the newly formed compounds are called products.
- In a chemical reaction, a chemical change must occur, which is generally observed with physical changes like precipitation, heat production, colour change, etc.
Law of Conservation of Mass
- According to the law of conservation of mass, matter can neither be created nor destroyed in a chemical reaction. It remains conserved.
- The mass of reactants will be equal to the mass of products.
Law of Constant Proportions
- A pure chemical compound containing the same elements combined together in a fixed proportion by mass is given by the law of definite proportions.
- For e.g., If we take water from a river or from an ocean, both have oxygen and hydrogen in the same proportion.
The elements are present in chemical compounds in a predetermined mass ratio. The “Law of Constant Proportions” is this. This “Law of Constant Proportions” is also known as “Proust’s law” or the “law of defined proportions.” For instance, the oxygen and hydrogen content in pure water is always 1:8.
An atom is the defining structure of an element, which cannot be broken by any chemical means.
The atomic symbol has three parts:-
- The symbol X: the usual element symbol
- The atomic number A: equal to the number of protons
- The mass number Z: equal to the total number of protons and neutrons in an element.
Atomic Radius
The distance between an atom’s nucleus and outer electron shell. The atomic radius is calculated by measuring the distance between the nuclei of two identical atoms bonded together. Half this distance is the atomic radius.
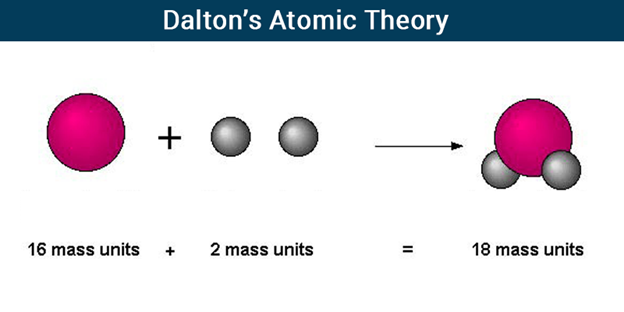
Dalton’s Atomic Theory
According to Dalton’s Atomic Theory, atoms, which are indestructible and indivisible building blocks, make up all substances. Unlike other elements, which have atoms of different sizes and weights, an element’s atoms have all the same size and mass.
Dalton proposed that the concept of atoms could be used to explain the laws of conservation of mass and definite proportions. He proposed that atoms, which he described as “solid, massy, hard, impenetrable, moving particle(s)”, are the smallest, indivisible units of matter.
- The matter is made up of indivisible particles known as atoms.
- The properties of all the atoms of a given element are the same, including mass. This can also be stated as – all the atoms of an element have identical mass and chemical properties; atoms of different elements have different masses and chemical properties.
- Atoms of different elements combine in fixed ratios to form compounds.
- Atoms are neither created nor destroyed. The formation of new products (compounds) results from the rearrangement of existing atoms (reactants) in a chemical reaction.
- The relative number and kinds of atoms are constant in a given compound.
To know more about the Laws of Chemical Combination, visit here .
Atomic Mass
Atomic mass and atomic mass unit.
- Atomic mass is the total of the masses of the electrons, neutrons, and protons in an atom, or in a group of atoms, the average mass.
- The mass of an atomic particle is called the atomic mass.
- This is commonly expressed as per the international agreement in terms of a unified atomic mass unit (AMU).
- It can be best defined as 1/12 of the mass of a carbon-12 atom in its ground state.
To know more about Atomic Mass, visit here .
Molecular mass
Molecular mass of an element is defined as the sum of the masses of the elements present in the molecule.
- Molecular mass is obtained by multiplying the atomic mass of an element by the number of atoms in the molecule and then adding the masses of all the elements in the molecule.
To know more about Molecular mass, visit here .
The smallest identifiable unit into which a pure substance may be divided while retaining its composition and chemical properties is a molecule, which is a collection of two or more atoms.
Molecules of Elements
A molecule is a collection of two or more chemically bound atoms, whether they are from the same element or another.
For example, when two hydrogen (H 2 ) atoms and one oxygen (O 2 ) atom interact, one water molecule is created.
Molecules of Compounds
Salts and molecular compounds are the two categories into which compounds can be divided. Covalent bonds hold the atoms together in molecular molecules. Ionic bonds hold it together in salts. Every compound is composed of one of these two types of bonds.
Actually, a compound is a kind of molecule. The atoms that join together must be distinct from one another for the substance to qualify as a compound. O 2 , for instance, is a molecule, not a compound, due to its atomic connection with another oxygen atom. NaCl, however, is a compound since it is made up of two distinct atoms that are chemically bound together.
Mole Concept
Mole concept & avogadro number.
- In a substance, the amount of entities present. For e.g. atoms, molecules, and ions are defined as a mole. A mole of any substance is 6.022×10 23 molecules.
- The Mole concept is one of the most convenient ways of expressing the amount of reactants and products in the reaction.
The value of Avogadro’s number is approximately 6.022×10 23 . The definition of Avogadro’s number is that it tells us the number of particles in 1 mole (or mol) of a substance. These particles could be electrons or molecules, or atoms.
To know more about Mole Concept, visit here .
A substance is something which has mass and occupies space. The molar mass/molecular weight is actually the sum of the total mass in grams of the atoms present to make up a molecule per mole. The unit of molar mass is grams/mole.
To know more about Molar Mass, visit here .
Atomic Valency
Molecules and atomicity.
A molecule is defined as the smallest unit of a compound that contains the chemical properties of the compound.
- The atomicity of an element is the number of atoms in one molecule of the element.
- For e.g., Hydrogen, nitrogen, oxygen, chlorine, iodine, and bromine all have two atoms in each of their molecules. So, the atomicity of hydrogen, nitrogen, oxygen, chlorine, iodine, and bromine is two each.
Structure of an Atom
- Atom is made of three particles; electron, proton and neutron.
- The centre of the atom is called the nucleus. The nucleus of an atom contains the whole mass of an atom.
- Electrons in an atom are arranged in shells/orbitals.
Valence electrons are those electrons which are present in the outermost orbit of the atom.
- The capacity of an atom to lose, gain or share valence electrons in order to complete its octet determines the valency of the atom.
To know more about Valency, visit here .
Writing Chemical Formulae
- When two or more elements chemically combine in a fixed ratio by mass, the obtained product is known as a compound.
- Compounds are substances consisting of two or more different types of elements in a fixed ratio of its atoms.
- An ion is defined as an atom or molecule which has gained or lost one or more of its valence electrons, giving it a net positive or negative charge.
- A negatively charged particle is called an anion, and a positively charged particle is called a cation.
Ionic Compounds: Chemical Formula
Each constituent element in a chemical formula is identified by its chemical symbol, along with the relative number of atoms that make up each element. These ratios are used in empirical equations to start with a key element and then assign atom counts for the remaining elements in the compound in relation to the key element.
- Ionic compounds are chemical compounds in which ions are held together by specialised bonds called ionic bonds.
- An Ionic compound always contains an equal amount of positive and negative charge.

To know more about Writing Chemical Formulae, visit here .
Explore more questions, concepts and tips on atoms and molecules Class 9 notes by registering at BYJU’S.
Further Reading:-
- Important Questions for Class 9 Science Chapter 3 –Atoms and Molecules
- Maths Notes For Class 9
- CBSE Class 9 Social Science Notes
Frequently Asked Questions on CBSE Class 9 Science Notes Chapter 3 Atoms and Molecules
What is the difference between atoms and molecules.
Atoms refer to the most basic or smallest unit of a chemical element. In comparison, molecules are composed of 2 or more atoms held together.
Who was Dalton?
John Dalton was an English chemist and physicist, and he was best known for his Atomic Theory.
What is a mole?
Mole is a standard unit for measuring the amount of any substance.
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
It is the best website for maths , science
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

25 Useful Presentation Topics for Science
By: Author Shrot Katewa

We are mostly asked questions about Presentation Design. But, sometimes, we do have our patrons reaching out to us to seek help with the “content” that needs to be created even before we begin with the design of the presentation.
So, today we are sharing a few really easy-to-cover super useful presentation topics for Science. This is especially helpful for all those teachers and parents who are looking to increase the curiosity of aspiring students and children.
So, let’s dive right into it –
A Quick Note Before We Begin – if you want to make jaw-dropping presentations, I would recommend using one of these Presentation Designs . The best part is – it is only $16.5 a month, but you get to download and use as many presentation designs as you like! I personally use it from time-to-time, and it makes my task of making beautiful presentations really quick and easy!
1. Big Bang Theory – Origin of Our Universe
As a kid, I was always curious about how we came into existence! How the planet Earth was created? How did it all start? This is a great topic to really generate and at times, even quench the curiosity of your students or children. While it is a great topic for presentation in class, it is also an equally good topic for a dinner conversation with your kids.
2. DNA structure
Our DNA is the very core of our life. If the Big Bang Theory is how the universe came into being, DNA is where our personal journey begins. While the structure of DNA is quite fascinating, the impact it has on our lives and how it affects our characteristics is mind-boggling!
It is another great topic for a Science Presentation. Do keep in mind, use of visual aids will most likely improve comprehension and retention among your audience.
3. Gene Editing & Its Uses
In case you choose to go with the previous topic of DNA, Gene Editing serves as a perfect extension of that topic even though it can be a great topic in itself. Sharing insights on Gene Editing and how it works, can showcase the capacity of human endeavors and its resolve to make things better.
4. Important Discoveries of Science
Okay, so this can really be a fun topic. As a kid, it was always fascinating to know about some of the world’s greatest discoveries and inventions.
Be it Penicillium or the first flight by the Wright Brothers, such topics allow you to take your audience on a journey and relive the times in which these discoveries and inventions were made. The thing that I like the most about this topic is that it doesn’t have to be completed in one session.
In fact, this can be turned into a knowledge series of multiple sessions as the list of discoveries is endless.
5. Aerodynamics
Most kids and students are really fascinated with planes. But, only a few really understand the basic principles of how a plane works. Explaining Aerodynamics can be an interesting topic.
It also allows you to introduce props such as a plane and practical exercises such as creating your own plane and analyzing its aerodynamics. The introduction of visuals for such a topic can greatly enhance the learning experience.
So this is a topic that most of the kids and students would have at least heard of, most might know about it a little. But very few would really understand how gravity truly changed our concepts not just on Earth, but also beyond our Planet in our Solar System.
Gravity alone is responsible for the tectonic shift of mindset that the Earth was the center of our Solar System to the fact that the Sun is the center of our Solar System around which the rest of the planets revolve. That and much more!
Explaining the stories of Galileo who first challenged this assumption and how Newton turned everything we knew upside down (almost literally!)
7. Photosynthesis
Another interesting Science topic for a presentation.
How do non-moving organisms produce and consume food? How Photosynthesis is not just limited to trees but virtually drives all lifeforms on Earth through the transfer of energy.
Also, touching upon the fact how Photosynthesis has led to the revolutionary discovery of Solar cells and how it is potentially going to be powering our future.
8. Artificial Intelligence – Boon or Bane
When it comes to Artificial Intelligence, there is a lot that we can do to engage the curiosity of our kids and students. It is an evolving part of Science as we haven’t fully applied and utilized AI.
One of the reasons this can be a great topic is because it engages your students or kids to really think. You may consider forming 2 teams and allowing an open debate on how AI could be a boon or a bane – a great way to promote cross-learning.
9. Ocean – The Unknown World
Our Ocean is what sets our planet Earth apart from the other planets in our solar planet. It is not only one of the main factors contributing to life on earth, the Ocean holds a world of its own with hidden creatures which have only recently been explored.
There is a lot to cover when it comes to the Ocean. Don’t limit your imagination to just lifeforms as you can even talk about treasures troves contained in the ships that sank!
10. Astronomy
So I have a confession to make. Which is this – Astronomy astonished me as a kid, and it amazes me even now! There have been countless nights that I gazed at the stars in the sky in amazement trying to locate a planet, and falling stars and other man-made satellites in the sky.
This is not just an amazing topic for a presentation, but if you could get hold of a telescope for a practical session, it will make a night to remember for the kids and the students!
11. Light and its effects
This is another topic that can turn into a great practical session!
Presentations can be accompanied by a trip to the physics lab or even using equipment like a prism to take the session experience of your audience to a totally different level! Experiencing the various colors that form light is one thing, but understanding how it impacts almost every single thing in our day-to-day activities makes us admire it.
12. Atoms – Building Blocks of Matter
While there is a whole universe outside of our Planet, there is a completely different world that exists when we go granular inside any matter.
There are literally billions and billions of atoms inside just our human body. Each atom has its own world making it as diverse as you can imagine.
How these atoms interact with each other and what makes an atom can be a really engaging topic to bubble the curiosity of the students or your kids!
13. Sound & Waves
Another super interesting presentation topic for Science for kids and students is to understand how Sound works.
There are several things to cover as part of this ranging from simple waves to frequency and resonance experiments. Sound is not just a good topic for a presentation but also for experiments and physical demos.
14. Technology
Technology as a topic has a lot to cover. As we all know that technology touches each of our lives on a daily basis, students can find this topic relatable quite easily. The canvas for exploration and presentation is quite broad giving you a wide range of technology topics to present from.
15. Human Brain
Many believe that we only use 10% of the capacity of our human brain. We have to date only barely managed to understand how our brain works.
Even the parts that we have gathered an understanding about, we don’t quite fully understand. The human brain has remained a topic of astonishment for scientists for a long time. It is only logical to conclude that if presented effectively, this can be a good presentation topic on science.
16. Evolution
When Charles Darwin presented his Theory of Evolution by Natural Selection in his book “The Origin of Species”, it took the world of science by storm.
How the species have evolved over a period of millions of years is quite interesting. There were quite a few interesting learnings that Darwin had and he shared that as a summary. This is something that has been also covered in the TV series Cosmos by Neil Degrasse Tyson.
I highly recommend giving this TV series a watch to get inspiration for some topics for presentation.
17. Magnetism
The majority of the kids have handled and spent hours in awe playing with a magnet. Many try to understand how a magnet really works! But, only a few are able to really understand the science behind it.
Magnetism can be a really fun topic to give a presentation on. Additionally, this topic also allows enough space to display, experiment, and have fun with real magnet and iron filings to showcase the effect of magnetism.
18. Electricity
Electricity is pretty much everywhere.
Today, if there is no electricity, the region is considered underdeveloped or backward. The discovery and the use of electricity is probably one of the greatest inventions of the 20th century.
It has been single-handedly responsible for industrialization, powering growth, and the development of the human race.
19. Steam Engine
Steam Engine was the first step of the human race towards powered locomotives.
From the discovery of the steam engine to how it was responsible for creating a time standard and time zones along with the stories related to it, can all be very fascinating and take you back in time to relive history!
A perfect presentation topic for science students.
20. Science of Medicine
No list of presentation topics for Science would be complete without mentioning medicine and its benefits.
The discovery of medicines and drugs has been responsible for nearly doubling the average human age. The impact is far-reaching with several pros and cons that constitute an interesting topic for presentation.
21. Periodic Table
Students often find this topic very dull. However, if you can help them understand the beauty and significance of this periodic table, it can be an amazing topic.
To really understand how Mendeleev could predict the existence of various elements even before they were discovered, is mind-boggling!
The periodic table is such a perfect table that explains how the elements are arranged in a well-structured manner in nature. This topic can be turned into a very interesting topic but a bit of effort and some out-of-the-box thinking may be required.
22. Buoyancy
Okay, so we all may have heard the story of Archimedes in a bathtub and how he shouted “Eureka” when he managed to solve the problem that was tasked to him. He did this using the Buoyancy principle.
While this story is something we relate to buoyancy the most, there is a lot more than we can truly learn and apply using this principle. This can be a very helpful topic for a presentation as well as a practical science experiment.
23. Health & Nutrition
Health & Nutrition is a very important aspect of our life. Its importance is often not completely understood by kids and students alike. Presenting about Health & Nutrition can go a long way to benefit the students to maintain a very healthy life!
24. Our Solar System
Our Solar System is a topic that is mostly taught since you join the school.
However, while most of us know about our solar system, there are enough mysteries about it to capture and captivate the attention of your audience. Questions like – why is Pluto not a planet anymore?
Or other questions such as – are we alone in this universe or even topics around the Sun as a star or even the asteroid belt between Mars and Jupiter can all lead to great engaging presentations and discussions.
25. Stem Cell
Stem cell research has become cutting-edge medical research. Thus, it is often a hot topic for discussion but is often not completely understood.
This topic will also provide you an opportunity to engage your audience in a debate that could be centered around the ethics of stem cells and their application.
This is a perfect topic as this allows your students or kids to learn and share their opinion with others.
Science is a vast world. Even though there are several other topics that can be covered, we decided to list topics that are relatively common such that it widely applies to a large set of people. If you have shortlisted your presentation topic and are looking for help to create a visually appealing presentation that captures the attention of your audience, be sure to reach out to us!
Our goal on this blog is to create content that helps YOU create fantastic presentations; especially if you have never been a designer. We’ve started our blog with non-designers in mind, and we have got some amazing content on our site to help YOU design better.
If you have any topics in mind that you would want us to write about, be sure to drop us a comment below. In case you need us to work with you and improve the design of your presentation, write to us on [email protected] . Our team will be happy to help you with your requirements.
Lastly, your contribution can make this world a better place for presentations . All you have to do is simply share this blog in your network and help other fellow non-designers with their designs!
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

49 templates

18 templates

32 templates

42 templates

40 templates

16 templates
Science Subject for High School - 9th Grade: Chemistry
Science subject for high school - 9th grade: chemistry presentation, free google slides theme, powerpoint template, and canva presentation template.
Future scientists always start somewhere, for example, in high school! Let's make your chemistry lessons more dynamic with a slideshow that can help with your lecture. It contains several illustrations of lab objects, a slide frame composed of squares and green backgrounds. Don't worry about the layouts because they're easier to edit than you think. Take a look at the periodic table we've also included! That will save you some time!
Features of this template
- 100% editable and easy to modify
- 35 different slides to impress your audience
- Available in five colors:Blue, Green, Pink, Yellow and Orange
- Contains easy-to-edit graphics such as graphs, maps, tables, timelines and mockups
- Includes 500+ icons and Flaticon’s extension for customizing your slides
- Designed to be used in Google Slides, Canva, and Microsoft PowerPoint
- 16:9 widescreen format suitable for all types of screens
- Includes information about fonts, colors, and credits of the free resources used
How can I use the template?
Am I free to use the templates?
How to attribute?
Combines with:
This template can be combined with this other one to create the perfect presentation:

Attribution required If you are a free user, you must attribute Slidesgo by keeping the slide where the credits appear. How to attribute?
Related posts on our blog.

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.

Premium template
Unlock this template and gain unlimited access

Register for free and start editing online

Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Join our Telegram Channel for Free PDF Download
Class 9 Science PPT for Online Teaching
- Last modified on: 3 weeks ago
- Reading Time: 16 Minutes
Are you in search of engaging and comprehensive PowerPoint presentations (PPTs) for teaching CBSE Class 9 Science online? Look no further! Our meticulously crafted PPTs are designed to make your online teaching sessions dynamic and effective. Explore our collection to enhance your teaching experience and captivate your students.
We are providing class 9 Science PPTs. Make your online teaching interesting with our interactive PowerPoint presentations for Science. A must buy for teachers teaching online.

Get Latest Highly Animated PowerPoint presentations for complete Science. These PPTs are very useful for teaching purpose. These Science PPTs based on class 9 syllabus are completely editable. So, if you want to add some slides or want to make some changes in it, then you can easily do. These PPTs covers CBSE level.
Science PPT for Class 9
For more details and in case of any query do connect on WhatsApp (7065827902). We will be happy to help you.
Key Features
- Interactive Presentations : Our PPTs are highly animated and interactive, making learning enjoyable and engaging.
- Complete Syllabus Coverage : Aligned with the CBSE Class 9 Science syllabus, our presentations cover all essential topics thoroughly.
- Editable Slides : Customize the presentations according to your teaching style and requirements effortlessly.
- Suitable for CBSE Curriculum : Our PPTs are tailored to meet the specific requirements of the CBSE curriculum.
Why Teachers Should Buy Our Highly Interactive PPTs for CBSE Class 9 Science?
PowerPoint presentations have become an indispensable tool for teaching Class 9 Science, and for good reason. They offer a wide range of benefits that enhance the learning experience and make complex scientific concepts more accessible to students. Here are some of the key reasons why PowerPoint presentations are important for teaching Class 9 Science:
1. Visual Aid and Engagement: Our PowerPoint presentations for CBSE Class 9 Science use visuals, such as images, diagrams, and charts, to support the content being presented. Visual aids are known to significantly enhance the learning process by helping students better understand abstract concepts and making the learning experience more engaging and memorable.
2. Organized and Structured Content: Our PowerPoint presentations for CBSE Class 9 Science allow teachers to present information in a structured and organized manner. This helps students follow the flow of the lesson, grasp the key points, and retain information more effectively.
3. Multimedia Integration: Science often involves dynamic processes and phenomena that are best understood through multimedia elements like videos and animations. PowerPoint presentations enable teachers to seamlessly integrate multimedia into their lessons, providing students with a more comprehensive understanding of scientific principles.
4. Interactive Learning: Our PowerPoint presentations includes interactive elements, such as quizzes and questions. This interactivity actively engages students in the learning process, encouraging participation and fostering a deeper understanding of the subject matter.
6. Time Management: Using PowerPoint presentations allows teachers to manage time effectively during the class. They can structure the content into logical segments, ensuring that the essential topics are covered within the given class duration.
7. Revision and Review: PowerPoint presentations can be saved and shared with students after the class, allowing them to review and revise the material at their own pace. This serves as a valuable resource for exam preparation and reinforces learning.
8. Teacher-Student Interaction: PowerPoint presentations serve as a visual aid that helps teachers maintain eye contact and interact more effectively with students. This fosters a more conducive learning environment and encourages students to participate in class discussions.
Our PPTs empower both teachers and students to create an enriched educational experience that is both effective and enjoyable.
PowerPoint Presentations (PPTs) for CBSE Class 9 Science
Here is the list of chapters covered in this PowerPoint Presentation Package for CBSE Class 9 Science.
Class 9 Science Chapters PPT
Chapter 1 – Matter in Our Surroundings Class 9 PPT Chapter 2 – Is Matter Around Us Pure Class 9 PPT Chapter 3 – Atoms and Molecules Class 9 PPT Chapter 4 – Structure of The Atom Class 9 PPT Chapter 5 – The Fundamental Unit of Life Class 9 PPT Chapter 6 – Tissues Class 9 PPT Chapter 7 – Diversity in Living Organisms Class 9 PPT Chapter 8 – Motion Class 9 PPT Chapter 9 – Force and Laws of Motion Class 9 PPT Chapter 10 – Gravitation Class 9 PPT Chapter 11 – Work and Energy Class 9 PPT Chapter 12 – Sound Class 9 PPT Chapter 13 – Why Do We Fall Ill Class 9 PPT Chapter 14 – Natural Resources Class 9 PPT Chapter 15 – Improvement in Food Resources Class 9 PPT
*Other Subjects Available for CBSE Class 9: Maths and Social Science
Ready to revolutionize your online teaching? Get in touch with us today to access our comprehensive collection of CBSE Class 9 Science PowerPoint presentations. Elevate your teaching experience and empower your students with interactive and engaging learning materials.
Science PPT for Class 9 Chapters
CBSE Class 9 Science comprises several chapters that cover a wide range of topics in Physics, Chemistry, and Biology. Here are the details of each chapter in CBSE Class 9 Science:
Chapter 1: Matter in Our Surroundings Class 9 PPT
- States of matter: Solid, Liquid, and Gas
- Changes of state: Melting, Evaporation, Boiling, Condensation, Sublimation
- Evaporation causing cooling
Chapter 2: Is Matter Around Us Pure Class 9 PPT
- Mixture and Pure Substances
- Types of Mixtures: Homogeneous and Heterogeneous
- Separation techniques: Filtration, Distillation, Evaporation, Sublimation, Chromatography
Chapter 3: Atoms and Molecules Class 9 PPT
- Law of Conservation of Mass
- Laws of Chemical Combination: Law of Constant Proportions, Law of Multiple Proportions
- Molecules and Ions
- Atomic and Molecular Mass
- Writing Chemical Formulas
Chapter 4: Structure of the Atom Class 9 PPT
- Discovery of Subatomic Particles: Electrons, Protons, and Neutrons
- Atomic Number, Mass Number, and Isotopes
- Bohr’s Model of the Atom
Chapter 5: The Fundamental Unit of Life Class 9 PPT
- Introduction to Cells
- Cell Theory
- Plant Cell and Animal Cell
- Structure and Functions of Cell Organelles
Chapter 6: Tissues Class 9 PPT
- Plant and Animal Tissues
- Meristematic and Permanent Tissues
Chapter 7: Diversity in Living Organisms Class 9 PPT
- Classification of Organisms
- Kingdom Monera, Protista, Fungi, Plantae, and Animalia
- Characteristics and Examples of Each Kingdom
Chapter 8: Motion Class 9 PPT
- Types of Motion: Rectilinear, Circular, and Periodic
- Speed, Velocity, and Acceleration
- Distance-Time and Velocity-Time Graphs
Chapter 9: Force and Laws of Motion Class 9 PPT
- Balanced and Unbalanced Forces
- Newton’s Laws of Motion: First Law, Second Law, Third Law
- Inertia and Momentum
Chapter 10: Gravitation Class 9 PPT
- Universal Law of Gravitation
- Free Fall and Acceleration due to Gravity
- Mass and Weight
Chapter 11: Work and Energy Class 9 PPT
- Work, Energy, and Power
- Types of Energy: Kinetic and Potential Energy
- Conservation of Energy
Chapter 12: Sound Class 9 PPT
- Production and Propagation of Sound
- Characteristics of Sound Waves
- Reflection, Refraction, and Echoes
Chapter 13: Why Do We Fall Ill Class 9 PPT
- Health and Disease
- Acute and Chronic Diseases
- Causes of Diseases and Prevention
Chapter 14: Natural Resources Class 9 PPT
- Types of Resources: Renewable and Non-Renewable
- Conservation of Natural Resources
Chapter 15: Improvement in Food Resources Class 9 PPT
- Plant and Animal Breeding
- Crop Production and Management
- Storage and Quality of Food
Science PPT for Class 9 Contains
Physics PPT Class 9
Chemistry PPT Class 9
Biology PPT Class 9
All Physics, Chemistry and Biology PPT for Class 9 Covered in this package.
Elevate your online teaching experience with our meticulously crafted PowerPoint presentations for CBSE Class 9 Science. Engage your students, simplify complex concepts, and make learning a delightful experience. Don’t miss out on this invaluable resource for effective teaching in the digital age.
Download CBSE Books
Exam Special Series:
- Sample Question Paper for CBSE Class 10 Science (for 2024)
- Sample Question Paper for CBSE Class 10 Maths (for 2024)
- CBSE Most Repeated Questions for Class 10 Science Board Exams
- CBSE Important Diagram Based Questions Class 10 Physics Board Exams
- CBSE Important Numericals Class 10 Physics Board Exams
- CBSE Practical Based Questions for Class 10 Science Board Exams
- CBSE Important “Differentiate Between” Based Questions Class 10 Social Science
- Sample Question Papers for CBSE Class 12 Physics (for 2024)
- Sample Question Papers for CBSE Class 12 Chemistry (for 2024)
- Sample Question Papers for CBSE Class 12 Maths (for 2024)
- Sample Question Papers for CBSE Class 12 Biology (for 2024)
- CBSE Important Diagrams & Graphs Asked in Board Exams Class 12 Physics
- Master Organic Conversions CBSE Class 12 Chemistry Board Exams
- CBSE Important Numericals Class 12 Physics Board Exams
- CBSE Important Definitions Class 12 Physics Board Exams
- CBSE Important Laws & Principles Class 12 Physics Board Exams
- 10 Years CBSE Class 12 Chemistry Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Physics Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Maths Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Biology Previous Year-Wise Solved Papers (2023-2024)
- ICSE Important Numericals Class 10 Physics BOARD Exams (215 Numericals)
- ICSE Important Figure Based Questions Class 10 Physics BOARD Exams (230 Questions)
- ICSE Mole Concept and Stoichiometry Numericals Class 10 Chemistry (65 Numericals)
- ICSE Reasoning Based Questions Class 10 Chemistry BOARD Exams (150 Qs)
- ICSE Important Functions and Locations Based Questions Class 10 Biology
- ICSE Reasoning Based Questions Class 10 Biology BOARD Exams (100 Qs)
✨ Join our Online JEE Test Series for 499/- Only (Web + App) for 1 Year
✨ Join our Online NEET Test Series for 499/- Only for 1 Year
2 thoughts on “ Class 9 Science PPT for Online Teaching ”
How can we buy without seeing ppt
Pl connect on whatsapp for samples.
Leave a Reply Cancel reply
Join our Online Test Series for CBSE, ICSE, JEE, NEET and Other Exams

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs
Discover more from Gurukul of Excellence
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading

IMAGES
VIDEO
COMMENTS
Class 9 Chemistry Index. This Class 9 Chemistry Index page contains all the topics that fall under each chapter of the class 9 chemistry syllabus as per the NCERT textbook. Students may follow the links on the subtopics to access free study material on the associated concepts (prepared by chemistry subject experts for CBSE students).
Cleaning Up: Formulation Chemistry. Coffee: Friend or Foe. Counterfeit Medicines. Curare and the case of Mario Jascalevich. Desalinating Water for future supply. Desalination. Detecting Chemicals in the Universe (Astrochemistry) Detecting Drug Abuse in Sport. Drugs in Drinking Water.
Next Post →. Science Presentation for Class 9 Science Chapter 1: Matter in Our Surroundings Click Here Click Here Click Here Chapter 2: Is Matter Around Us Pure Click Here Click Here Chapter 3: Atoms and Molecules Click Here Click Here Click Here Chapter 4: Structure of the Atom Click Here Click Here Chapter 5: The fundamental Unit.
Class 9 Chemistry (India) 4 units · 15 skills. Unit 1. Is Matter Around Us Pure? Unit 2. Matter in Our Surroundings. Unit 3. Atoms and Molecules. Unit 4. Structure of the Atom. Course challenge. Test your knowledge of the skills in this course. Start Course challenge. Science; Class 9 Chemistry (India) 1,500 possible mastery points. Mastered.
Best PowerPoint presentation on NCERT class 9 Atoms and Molecules as per CBSE syllabus it covers full chapter with all information. By Raxit Gupta 9C KENDRIYA VIDYALAYA BALLYGUNGE. Read more. Education. 1 of 32. Download Now. Download to read offline. Atoms and molecules class 9 - Download as a PDF or view online for free.
️📚👉 Watch Full Free Course:- https://www.magnetbrains.com ️📚👉 Get Notes Here: https://www.pabbly.com/out/magnet-brains In this playlist, we will cover ...
NehaRohtagi1. PowerPoint Presentation on the topic - 'Atoms And Molecules'. For Class:- 9th Created By - 'Neha Rohtagi'. I hope that you will found this presentation useful and it will help you out for your concept understanding. Thank You! Please give feedbacks and suggestions to get presentations on more interesting topics. Read more. Science.
4 units · 15 skills. Unit 1. Unit 3. Course challenge. Test your knowledge of the skills in this course. Start Course challenge. Science. Class 9 Chemistry (India)
Structure Of The Atom Class 9 CBSE Notes - Chapter 4. Essentially, the structure of an atom comprises protons, neutrons and electrons. These basic components provide the mass and charge of the atoms. The nucleus comprises protons and neutrons, with the electron orbiting around that.
According to Class 9 Chapter 4 Science notes, an atomic number is the number of the chemical elements present in the periodic system so that the elements are organised in order of the increasing number of protons present in the nucleus. Also, the total number of protons that are equal to the electrons in a neutral atom is called the atomic ...
Powerpoint Presentation on the Topic - Structure Of The Atom. Made By - NehaRohtagi1. Science. 1 of 17. Download Now. Download to read offline. Structure Of The Atom - Class 9 - Download as a PDF or view online for free.
A mole (mol) is defined as the amount of substance that contains as many atoms, molecules, ions, electrons, etc., as there are atoms in exactly 12 g of carbon 12 ( 12 C). A mole of a substance represents 6.022 × 10 23 particles of the substance. Number of Moles = (mass of the given element in grams) ÷ (gram-atomic mass of the element ...
The following topics, however are easier and are asked frequently: Kepler's First and Second Laws. Density and Relative Density. Thrust and Pressure. 4. Work, Energy and Power. This chapter is one of the most scoring chapters among others, but even then there are certain topics which are very easy and frequently asked.
Term 2. Unit 1- Matter-Its Nature and Behaviour Chapter 3- Atoms and Molecules. 1. Verification of the law of conservation of mass in a chemical reaction. Please note that the above tables give the Chemistry Syllabus of class 9 for 2021-2022. The core subject Science includes other disciplines -Physics and Biology also.
The "LAWS OF CHEMICAL COMINATION- PPT (Powerpoint Presentation), Chemistry Class 9 Questions" guide is a valuable resource for all aspiring students preparing for the Class 9 exam. It focuses on providing a wide range of practice questions to help students gauge their understanding of the exam topics.
Evaporation: The process of a liquid changing into vapour (or gas) at any temperature below its boiling point is called evaporation. The wet clothes dry due to evaporation of water present in them. Common salt is also recovered from sea-water by the process of evaporation.
NCERT Solutions Class 9 Science Chapter 1 - Free PDF Download. NCERT Solutions for Class 9 Science (Chemistry) Chapter 1 Matter in Our Surroundings is an important study material from the standpoint of your CBSE Class 9 Science examination. Detailed NCERT Solutions for Class 9 Chemistry provided here will help you understand the fundamental concepts taught in the chapter.
Find below the chapter-wise links to access the Important Topics and Questions for Class 9 Science: Chapter 1: Matter in Our Surroundings. Chapter 2: Is matter arround us pure. Chapter 3: Atoms ...
Atoms. An atom is the defining structure of an element, which cannot be broken by any chemical means. The atomic symbol has three parts:-. The symbol X: the usual element symbol. The atomic number A: equal to the number of protons. The mass number Z: equal to the total number of protons and neutrons in an element.
NCERT Solutions for Class 9 Chemistry Chapterwise Free PDF Download. CBSE Class 9 Chemistry Chapter 1 - Matter in our Surroundings. CBSE Class 9 Chemistry Chapter 2 - Is Matter Around Us Pure. CBSE Class 9 Chemistry Chapter 3 - Atoms and Molecules. CBSE Class 9 Chemistry Chapter 4 - Structure of the Atom.
This is a great topic to really generate and at times, even quench the curiosity of your students or children. While it is a great topic for presentation in class, it is also an equally good topic for a dinner conversation with your kids. 2. DNA structure. Our DNA is the very core of our life.
Science Subject for High School - 9th Grade: Chemistry Presentation. Free Google Slides theme, PowerPoint template, and Canva presentation template. Future scientists always start somewhere, for example, in high school! Let's make your chemistry lessons more dynamic with a slideshow that can help with your lecture.
Key Features. Interactive Presentations: Our PPTs are highly animated and interactive, making learning enjoyable and engaging.; Complete Syllabus Coverage: Aligned with the CBSE Class 9 Science syllabus, our presentations cover all essential topics thoroughly.; Editable Slides: Customize the presentations according to your teaching style and requirements effortlessly.