An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Saudi J Biol Sci
- v.22(2); 2015 Mar

Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation
Salinity is one of the most brutal environmental factors limiting the productivity of crop plants because most of the crop plants are sensitive to salinity caused by high concentrations of salts in the soil, and the area of land affected by it is increasing day by day. For all important crops, average yields are only a fraction – somewhere between 20% and 50% of record yields; these losses are mostly due to drought and high soil salinity, environmental conditions which will worsen in many regions because of global climate change. A wide range of adaptations and mitigation strategies are required to cope with such impacts. Efficient resource management and crop/livestock improvement for evolving better breeds can help to overcome salinity stress. However, such strategies being long drawn and cost intensive, there is a need to develop simple and low cost biological methods for salinity stress management, which can be used on short term basis. Microorganisms could play a significant role in this respect, if we exploit their unique properties such as tolerance to saline conditions, genetic diversity, synthesis of compatible solutes, production of plant growth promoting hormones, bio-control potential, and their interaction with crop plants.
1. Introduction
The beginning of 21st century is marked by global scarcity of water resources, environmental pollution and increased salinization of soil and water. Increasing human population and reduction in land available for cultivation are two threats for agricultural sustainability ( Shahbaz and Ashraf, 2013 ). Various environmental stresses viz . high winds, extreme temperatures, soil salinity, drought and flood have affected the production and cultivation of agricultural crops, among these soil salinity is one of the most devastating environmental stresses, which causes major reductions in cultivated land area, crop productivity and quality ( Yamaguchi and Blumwald, 2005; Shahbaz and Ashraf, 2013 ). A saline soil is generally defined as one in which the electrical conductivity (EC) of the saturation extract (EC e ) in the root zone exceeds 4 dS m −1 (approximately 40 mM NaCl) at 25 °C and has an exchangeable sodium of 15%. The yield of most crop plants is reduced at this EC e , though many crops exhibit yield reduction at lower EC e s ( Munns, 2005; Jamil et al., 2011 ). It has been estimated that worldwide 20% of total cultivated and 33% of irrigated agricultural lands are afflicted by high salinity. Furthermore, the salinized areas are increasing at a rate of 10% annually for various reasons, including low precipitation, high surface evaporation, weathering of native rocks, irrigation with saline water, and poor cultural practices. It has been estimated that more than 50% of the arable land would be salinized by the year 2050 ( Jamil et al., 2011 ).
Water and soil management practices have facilitated agricultural production on soil marginalized by salinity but an additional gain from these approaches seems problematic ( Zahir et al., 2008 ). Impacted soils are a major limiting production factor worldwide for every major crop ( Bacilio et al., 2004; Shannon and Grieve, 1999 ). A significant increase (an estimated 50%) in grain yields of major crop plants such as rice ( Oryza sativa L.), wheat ( Triticum aestivum L.) and maize ( Zea mays L.) is required to fulfill the food supply requirements for the projected population by 2050 ( Godfray et al., 2010 ). The urgency of feeding the world’s growing population while combating soil pollution, salinization, and desertification has given plant and soil productivity research vital importance. Under such circumstances, it requires suitable biotechnology not only to improve crop productivity but also to improve soil health through interactions of plant roots and soil microorganisms ( Lugtenberg et al., 2002 ).
Salt stressed soils are known to suppress the growth of plants ( Paul, 2012 ). Plants in their natural environment are colonized both by endocellular and intracellular microorganisms ( Gray and Smith, 2005 ). Rhizosphere microorganisms, particularly beneficial bacteria and fungi, can improve plant performance under stress environments and, consequently, enhance yield both directly and indirectly ( Dimkpa et al., 2009 ). Some plant growth-promoting rhizobacteria (PGPR) may exert a direct stimulation on plant growth and development by providing plants with fixed nitrogen, phytohormones, iron that has been sequestered by bacterial siderophores, and soluble phosphate ( Hayat et al., 2010 ). Others do this indirectly by protecting the plant against soil-borne diseases, most of which are caused by pathogenic fungi ( Lutgtenberg and Kamilova, 2009 ). The problem of soil salinization is a scourge for agricultural productivity worldwide. Crops grown on saline soils suffer on an account of high osmotic stress, nutritional disorders and toxicities, poor soil physical conditions and reduced crop productivity. The present review focuses on the enhancement of productivity under stressed conditions and increased resistance of plants against salinity stress by application of plant growth promoting microorganisms.
2. Problem of soil salinization
Soil salinity is an enormous problem for agriculture under irrigation. In the hot and dry regions of the world the soils are frequently saline with low agricultural potential. In these areas most crops are grown under irrigation, and to exacerbate the problem, inadequate irrigation management leads to secondary salinization that affects 20% of irrigated land worldwide ( Glick et al., 2007 ). Irrigated agriculture is a major human activity, which often leads to secondary salinization of land and water resources in arid and semi-arid conditions. Salts in the soil occur as ions (electrically charged forms of atoms or compounds). Ions are released from weathering minerals in the soil. They may also be applied through irrigation water or as fertilizers, or sometimes migrate upward in the soil from shallow groundwater. When precipitation is insufficient to leach ions from the soil profile, salts accumulate in the soil resulting soil salinity ( Blaylock et al., 1994 ). All soils contain some water-soluble salts. Plants absorb essential nutrients in the form of soluble salts, but excessive accumulation strongly suppresses the plant growth. During the last century, physical, chemical and/or biological land degradation processes have resulted in serious consequences to global natural resources (e.g. compaction, inorganic/organic contamination, and diminished microbial activity/diversity). The area under the affected soils continues to increase each year due to introduction of irrigation in new areas ( Patel et al., 2011 ).
Salinization is recognized as the main threats to environmental resources and human health in many countries, affecting almost 1 billion ha worldwide/globally representing about 7% of earth’s continental extent, approximately 10 times the size of a country like Venezuela or 20 times the size of France ( Metternicht and Zinck, 2003; Yensen, 2008 ). It has been estimated that an approximate area of 7 million hectares of land is covered by saline soil in India ( Patel et al., 2011 ). Most of which occurs in indogangetic plane that covers the states of Punjab, Haryana, U.P. Bihar and some parts of Rajasthan. Arid tracts of Gujarat and Rajasthan and semi-arid tracts of Gujarat, Madhya Pradesh, Maharashtra, Karnataka and Andhra Pradesh are also largely affected by saline lands.
3. Impact of salinity on plants
Agricultural crops exhibit a spectrum of responses under salt stress. Salinity not only decreases the agricultural production of most crops, but also, effects soil physicochemical properties, and ecological balance of the area. The impacts of salinity include—low agricultural productivity, low economic returns and soil erosions, ( Hu and Schmidhalter, 2002 ). Salinity effects are the results of complex interactions among morphological, physiological, and biochemical processes including seed germination, plant growth, and water and nutrient uptake ( Akbarimoghaddam et al., 2011; Singh and Chatrath, 2001 ). Salinity affects almost all aspects of plant development including: germination, vegetative growth and reproductive development. Soil salinity imposes ion toxicity, osmotic stress, nutrient (N, Ca, K, P, Fe, Zn) deficiency and oxidative stress on plants, and thus limits water uptake from soil. Soil salinity significantly reduces plant phosphorus (P) uptake because phosphate ions precipitate with Ca ions ( Bano and Fatima, 2009 ). Some elements, such as sodium, chlorine, and boron, have specific toxic effects on plants. Excessive accumulation of sodium in cell walls can rapidly lead to osmotic stress and cell death ( Munns, 2002 ). Plants sensitive to these elements may be affected at relatively low salt concentrations if the soil contains enough of the toxic element. Because many salts are also plant nutrients, high salt levels in the soil can upset the nutrient balance in the plant or interfere with the uptake of some nutrients ( Blaylock et al., 1994 ). Salinity also affects photosynthesis mainly through a reduction in leaf area, chlorophyll content and stomatal conductance, and to a lesser extent through a decrease in photosystem II efficiency ( Netondo et al., 2004 ). Salinity adversely affects reproductive development by inhabiting microsporogenesis and stamen filament elongation, enhancing programed cell death in some tissue types, ovule abortion and senescence of fertilized embryos. The saline growth medium causes many adverse effects on plant growth, due to a low osmotic potential of soil solution (osmotic stress), specific ion effects (salt stress), nutritional imbalances, or a combination of these factors ( Ashraf, 2004 ). All these factors cause adverse effects on plant growth and development at physiological and biochemical levels ( Munns and James, 2003 ), and at the molecular level ( Tester and Davenport, 2003 ).
In order to assess the tolerance of plants to salinity stress, growth or survival of the plant is measured because it integrates the up- or down-regulation of many physiological mechanisms occurring within the plant. Osmotic balance is essential for plants growing in saline medium. Failure of this balance results in loss of turgidity, cell dehydration and ultimately, death of cells. On the other hand, adverse effects of salinity on plant growth may also result from impairment of the supply of photosynthetic assimilates or hormones to the growing tissues ( Ashraf, 2004 ). Ion toxicity is the result of replacement of K + by Na + in biochemical reactions, and Na + and Cl − induced conformational changes in proteins. For several enzymes, K + acts as cofactor and cannot be substituted by Na + . High K + concentration is also required for binding tRNA to ribosomes and thus protein synthesis ( Zhu, 2002 ). Ion toxicity and osmotic stress cause metabolic imbalance, which in turn leads to oxidative stress ( Chinnusamy et al., 2006 ). The adverse effects of salinity on plant development are more profound during the reproductive phase. Wheat plants stressed at 100–175 mM NaCl showed a significant reduction in spikelets per spike, delayed spike emergence and reduced fertility, which results in poor grain yields. However, Na + and Cl − concentrations in the shoot apex of these wheat plants were below 50 and 30 mM, respectively, which is too low to limit metabolic reactions ( Munns and Rawson, 1999 ). Hence, the adverse effects of salinity may be attributed to the salt-stress effect on the cell cycle and differentiation. Salinity arrests the cell cycle transiently by reducing the expression and activity of cyclins and cyclin-dependent kinases that results in fewer cells in the meristem, thus limiting growth. The activity of cyclin-dependent kinase is diminished also by post-translational inhibition during salt stress. Recent reports also show that salinity adversely affects plant growth and development, hindering seed germination, seedling growth, enzyme activity ( Seckin et al., 2009 ), DNA, RNA, protein synthesis and mitosis ( Tabur and Demir, 2010; Javid et al., 2011 ).
4. Amelioration of salinity
Salinization can be restricted by leaching of salt from root zone, changed farm management practices and use of salt tolerant plants. Irrigated agriculture can be sustained by better irrigation practices such as adoption of partial root zone drying methodology, and drip or micro-jet irrigation to optimize use of water. The spread of dry land salinity can be contained by reducing the amount of water passing beyond the roots. This can be done by re-introducing deep rooted perennial plants that continue to grow and use water during the seasons that do not support annual crop plants. This may restore the balance between rainfall and water use, thus preventing rising water tables and the movement of salt to the soil surface ( Manchanda and Garg, 2008 ). Farming systems can change to incorporate perennials in rotation with annual crops (phase farming), in mixed plantings (alley farming, intercropping), or in site-specific plantings (precision farming) ( Munns et al., 2002 ). Although the use of these approaches to sustainable management can ameliorate yield reduction under salinity stress, implementation is often limited because of cost and availability of good water quality or water resource. Evolving efficient, low cost, easily adaptable methods for the abiotic stress management is a major challenge. Worldwide, extensive research is being carried out, to develop strategies to cope with abiotic stresses, through development of salt and drought tolerant varieties, shifting the crop calendars, resource management practices etc. ( Venkateswarlu and Shanker, 2009 ) as shown in Fig. 1 .

Different approaches for improvement of salt tolerance in agricultural crops.
5. Use of salt tolerant crops and transgenics
Using the salt-tolerant crops is one of the most important strategies to solve the problem of salinity. Tolerance will be required for the “de-watering” species, but also for the annual crops to follow, as salt will be left in the soil when the water table is lowered. Salt tolerance in crops will also allow the more effective use of poor quality irrigation water. To increase the plant salt-tolerance, there is a need for understanding the mechanisms of salt limitation on plant growth and the mechanism of salt tolerance at the whole-plant, organelle, and molecular levels. Under saline conditions, there is a change in the pattern of gene expression, and both qualitative and quantitative changes in protein synthesis. Although it is generally agreed that salt stress brings about quantitative changes in protein synthesis, there is some controversy as to whether salinity activates specialized genes that are involved in salt stress. Salt tolerance does not appear to be conferred by unique gene(s) ( Manchanda and Garg, 2008 ). When a plant is subjected to abiotic stress, a number of genes are turned on, resulting in increased levels of several metabolites and proteins, some of which may be responsible for conferring a certain degree of protection to these stresses ( Bhatnagar-Mathur et al., 2008 ). Efforts to improve crop performance by transgenic approach under environmental stresses have not been that fruitful because the fundamental mechanisms of stress tolerance in plants remain to be completely understood.
Development of salt-tolerant crops has been a major objective of plant breeding programs for decades in order to maintain crop productivity in semiarid and saline lands. Although several salt-tolerant varieties have been released, the overall progress of traditional breeding has been slow and has not been successful as only few major determinant genetic traits of salt tolerance have been identified ( Schubert et al., 2009; Dodd and Perez-Alfocea, 2012 ). 25 years ago Epstein et al. (1980) described the technical and biological constraints to solving the problem of salinity. Although there has been some success with technical solutions to the problem, the biological solutions have been more difficult to develop because a pre-requisite for the development of salt tolerant crops is the identification of key genetic determinants of stress tolerance. The existence of salt-tolerant plants (halophytes) and differences in salt tolerance between genotypes within salt-sensitive plant species (glycophytes) indicates that there is a genetic basis to salt response ( Yamaguchi and Blumwald, 2005 ). Although a lot of approaches have been done for development of salt tolerant plants by transgenics complete success is not achieved yet. The assessment of salt tolerance in transgenic experiments has been mostly carried out using a limited number of seedlings or mature plants in laboratory experiments. In most of the cases, the experiments were carried out in greenhouse conditions where the plants were not exposed to those conditions that prevail in high-salinity soils (e.g. alkaline soil pH, high diurnal temperatures, low humidity, and presence of other sodic salts and elevated concentrations of selenium and/or boron). The salt tolerance of the plants in the field needs to be evaluated and, more importantly, salt tolerance needs to be evaluated as a function of yield. The evaluation of field performance under salt stress is difficult because of the variability of salt levels in field conditions ( Richards, 1983 ) and the potential for interactions with other environmental factors, including soil fertility, temperature, light intensity and water loss due to transpiration. Evaluating tolerance is also made more complex because of variation in sensitivity to salt during the life cycle. For example, in rice, grain yield is much more affected by salinity than in vegetative growth ( Khatun and Flowers, 1995 ). In tomato, the ability of the plants to germinate under conditions of high salinity is not always correlated with the ability of the plant to grow under salt stress because both are controlled by different mechanisms ( Foolad and Lin, 1997 ), although some genotypes might display similar tolerance at germination and during vegetative growth ( Foolad and Chen, 1999 ). Therefore, the assessment of stress tolerance in the laboratory often has little correlation to tolerance in the field. Although there have been many successes in developing stress-tolerant transgenics in model plants such as tobacco, Arabidopsis or rice ( Grover et al., 2003 ), there is an urgent need to test these successes in other crops. There are several technical and financial challenges associated with transforming many of the crop plants, particularly the monocots. First, transformation of any monocot other than rice is still not routine and to develop a series of independent homozygous lines is costly, both in terms of money and time. Second, the stress tolerance screens will need to include a field component because many of the stress tolerance assays used by basic researchers involve using nutrient-rich media (which in some cases include sucrose). This type of screen is unlikely to have a relationship to field performance. Third, because saline soils are often complex and can include NaCl, CaCl 2 , CaSO 4 , Na 2 SO 4 , high boron concentrations and alkaline pH, plants that show particular promise will eventually have to be tested in all these environments ( Joseph and Jini, 2010 ).
6. Microbes: abiotic stress alleviation tool in crops
Several strategies have been developed in order to decrease the toxic effects caused by high salinity on plant growth, including plant genetic engineering ( Wang et al., 2003 ), and recently the use of plant growth-promoting bacteria (PGPB) ( Dimkpa et al., 2009 ). The role of microorganisms in plant growth promotion, nutrient management and disease control is well known and well established. These beneficial microorganisms colonize the rhizosphere/endorhizosphere of plants and promote growth of the plants through various direct and indirect mechanisms ( Nia et al., 2012; Ramadoss et al., 2013 ). Previous studies suggest that utilization of PGPB has become a promising alternative to alleviate plant stress caused by salinity ( Yao et al., 2010 ) and the role of microbes in the management of biotic and abiotic stresses is gaining importance. The subject of PGPR elicited tolerance to abiotic stresses has been reviewed recently ( Dodd and Perez-Alfocea, 2012; Yang et al., 2009 ).
The term Induced Systemic Tolerance (IST) has been proposed for PGPR-induced physical and chemical changes that result in enhanced tolerance to abiotic stress. PGPR facilitate plant growth indirectly by reducing plant pathogens, or directly by facilitating the nutrient uptake through phytohormone production (e.g. auxin, cytokinin and gibberellins), by enzymatic lowering of plant ethylene levels and/or by production of siderophores ( Kohler et al., 2006 ). It has been demonstrated that inoculations with AM (arbuscular mycorrhizal) fungi improves plant growth under salt stress ( Cho et al., 2006 ). Kohler et al., 2006 demonstrated the beneficial effect of PGPR Pseudomonas mendocina strains on stabilization of soil aggregate. The three PGPR isolates P. alcaligenes PsA15, Bacillus polymyxa BcP26 and Mycobacterium phlei MbP18 were able to tolerate high temperatures and salt concentrations and thus confer on them potential competitive advantage to survive in arid and saline soils such as calcisol ( Egamberdiyeva, 2007 ). Kohler et al., 2009 investigated the influence of inoculation with a PGPR, P. mendocina , alone or in combination with an AM fungus, Glomus intraradices or G. mosseae on growth and nutrient uptake and other physiological activities of Lactuca sativa affected by salt stress. The plants inoculated with P. mendocina had significantly greater shoot biomass than the controls and it is suggested that inoculation with selected PGPR could be an effective tool for alleviating salinity stress in salt sensitive plants. Bacteria isolated from different stressed habitats possess stress tolerance capacity along with the plant growth-promoting traits and therefore are potential candidates for seed bacterization. When inoculated with these isolates, plants show enhanced root and shoot length, biomass, and biochemical levels such as chlorophyll, carotenoids, and protein ( Tiwari et al., 2011 ). Investigations on interaction of PGPR with other microbes and their effect on the physiological response of crop plants under different soil salinity regimes are still in incipient stage. Inoculations with selected PGPR and other microbes could serve as the potential tool for alleviating salinity stress in salt sensitive crops. Therefore, an extensive investigation is needed in this area, and the use of PGPR and other symbiotic microorganisms, can be useful in developing strategies to facilitate sustainable agriculture in saline soils.
7. Alleviation of abiotic stress in plants by rhizospheric bacteria
Besides developing mechanisms for stress tolerance, microorganisms can also impart some degree of tolerance to plants towards abiotic stresses like drought, chilling injury, salinity , metal toxicity and high temperature. In the last decade, bacteria belonging to different genera including Rhizobium , Bacillus , Pseudomonas , Pantoea , Paenibacillus , Burkholderia , Achromobacter , Azospirillum , Microbacterium , Methylobacterium , Variovorax , Enterobacter etc. have been reported to provide tolerance to host plants under different abiotic stress environments ( Grover et al., 2011 ). Use of these microorganisms per se can alleviate stresses in agriculture thus opening a new and emerging application of microorganisms. Microbial elicited stress tolerance in plants may be due to a variety of mechanisms proposed from time to time based on studies done. Production of indole acetic acid, gibberellins and some unknown determinants by PGPR, results in increased root length, root surface area and number of root tips, leading to an enhanced uptake of nutrients thereby improving plant health under stress conditions ( Egamberdieva and Kucharova, 2009 ). Plant growth promoting bacteria have been found to improve growth of tomato, pepper, canola, bean and lettuce under saline conditions ( Barassi et al., 2006; Yildirim and Taylor, 2005 ).
Some PGPR strains produce cytokinin and antioxidants, which result in abscisic acid (ABA) accumulation and degradation of reactive oxygen species. High activities of antioxidant enzymes are linked with oxidative stress tolerance ( Stajner et al., 1997 ). Another PGPR strain, Achromobacter piechaudii ARV8 which produced 1-aminocyclopropane-1-carboxylate (ACC) deaminase, conferred IST against drought and salt in pepper and tomato ( Mayak et al., 2004 ). Many aspects of plant life are regulated by ethylene levels and the biosynthesis of ethylene is subjected to tight regulation, involving transcriptional and post-transcriptional factors regulated by environmental cues, including biotic and abiotic stresses ( Hardoim et al., 2008 ). Under stress conditions, the plant hormone ethylene endogenously regulates plant homoeostasis and results in reduced root and shoot growth. In the presence of ACC deaminase producing bacteria, plant ACC is sequestered and degraded by bacterial cells to supply nitrogen and energy. Furthermore, by removing ACC, the bacteria reduce the deleterious effect of ethylene, ameliorating stress and promoting plant growth ( Glick, 2007 ). The complex and dynamic interactions among microorganisms, roots, soil and water in the rhizosphere induce changes in physicochemical and structural properties of the soil ( Haynes and Swift, 1990 ). Microbial polysaccharides can bind soil particles to form microaggregates and macroaggregates. Plant roots and fungal hyphae fit in the pores between microaggregates and thus stabilize macroaggregates. Plants treated with Exo-poly saccharides (EPS) producing bacteria display increased resistance to water and salinity stress due to improved soil structure ( Sandhya et al., 2009 ). EPS can also bind to cations including Na + thus making it unavailable to plants under saline conditions. Chen et al., 2007 correlated proline accumulation with drought and salt tolerance in plants. Introduction of proBA genes derived from B. subtilis into A. thaliana resulted in production of higher levels of free proline resulting in increased tolerance to osmotic stress in the transgenic plants. Increased production of proline along with decreased electrolyte leakage, maintenance of relative water content of leaves and selective uptake of K ions resulted in salt tolerance in Zea mays coinoculated with Rhizobium and Pseudomonas ( Bano and Fatima, 2009 ). Rhizobacteria inhabiting the sites exposed to frequent stress conditions, are likely to be more adaptive or tolerant and may serve as better plant growth promoters under stressful conditions. Moreover Yao et al., 2010 reported that inoculation with P. putida Rs 198 promoted cotton growth and germination under conditions of salt stress. Tank and Saraf (2010) showed that PGPRs which are able to solubilize phosphate, produce phytohormones and siderophores in salt condition promote growth of tomato plants under 2% NaCl stress.
In a study carried out by Naz et al., 2009 , it was shown that strains isolated from Khewra salt range of Pakistan exhibited their tolerance when tested on saline media simulated by rhizosphere soil filtrate. Noteworthy, the isolates produced ABA in a concentration much higher than that of previous reports. Furthermore production of proline, shoot/root length, and dry weight was also higher in soybean plants inoculated with these isolates under induced salt stress. Likewise Upadhyay et al., 2011 studied the impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions and reported that co-inoculation with B. subtilis and Arthrobacter sp. could alleviate the adverse effects of soil salinity on wheat growth with an increase in dry biomass, total soluble sugars and proline content. Jha et al., 2011 reported that P. pseudoalcaligenes , an endophytic bacterium in combination with a rhizospheric B. pumilus in paddy was able to protect the plant from abiotic stress by induction of osmoprotectant and antioxidant proteins than by the rhizospheric or endophytic bacteria alone at early stages of growth. Plants inoculated with endophytic bacterium P. pseudoalcaligenes showed a significantly higher concentration of glycine betaine-like quaternary compounds and higher shoot biomass at lower salinity levels. While at higher salinity levels, a mixture of both P. pseudoalcaligenes and B. pumilus showed better response against the adverse effects of salinity. Nia et al., 2012 studied the effect of inoculation of Azospirillum strains isolated from saline or non-saline soil on yield and yield components of wheat in salinity and they observed that inoculation with the two isolates increased salinity tolerance of wheat plants; the saline-adapted isolate significantly increased shoot dry weight and grain yield under severe water salinity. The component of grain yield most affected by inoculation was grains per plant. Plants inoculated with saline-adapted Azospirillum strains had higher N concentrations at all water salinity levels.
Sadeghi et al., 2012 studied the plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions and reported increases in growth and development of wheat plant. They observed significant increases in germination rate, percentage and uniformity, shoot length and dry weight compared to the control. Applying the bacterial inocula increased the concentration of N, P, Fe and Mn in wheat shoots grown in normal and saline soil and thus concluded that Streptomyces isolate has potential to be utilized as biofertilizers in saline soils. More recently Ramadoss et al., 2013 studied the effect of five plant growth promoting halotolerant bacteria on wheat growth and found that inoculation of those halotolerant bacterial strains to ameliorate salt stress (80, 160 and 320 mM) in wheat seedlings produced an increase in root length of 71.7% in comparison with uninoculated positive controls. In particular, Hallobacillus sp . and B. halodenitrificans showed more than 90% increase in root elongation and 17.4% increase in dry weight when compared to uninoculated wheat seedlings at 320 mM NaCl stress indicating a significant reduction of the deleterious effects of NaCl. These results indicate that halotolerant bacteria isolated from saline environments have potential to enhance plant growth under saline stress through direct or indirect mechanisms and would be most appropriate as bioinoculants under such conditions. The isolation of indigenous microorganisms from the stress affected soils and screening on the basis of their stress tolerance and PGP traits may be useful in the rapid selection of efficient strains that could be used as bioinoculants for stressed crops. Some of the advances and researches carried out in evaluating role of rhizobacteria as salinity stress remediators have been summarized in Table 1 .
Role of plant growth promoting bacteria in salinity stress alleviation in plants.
8. Conclusion
An ideal sustainable agricultural system is one which maintains and improves human health, benefits producers and consumers both economically and spiritually, protects the environment, and produces enough food for an increasing world population. One of the most important constraints to agricultural production in world is abiotic stress conditions prevailing in the environment. Plant-associated microorganisms can play an important role in conferring resistance to abiotic stresses. These organisms could include rhizoplane, rhizosphere and endophytic bacteria and symbiotic fungi and operate through a variety of mechanisms like triggering osmotic response, providing growth hormones and nutrients, acting as biocontrol agents and induction of novel genes in plants. The development of stress tolerant crop varieties through genetic engineering and plant breeding is essential but a long drawn and expensive process, whereas microbial inoculation to alleviate stresses in plants could be a more cost effective environmental friendly option which could be available in a shorter time frame. Taking the current leads available, concerted future research is needed in this area, particularly on field evaluation and application of potential organisms as biofertilizers in stressed soil.
Peer review under responsibility of King Saud University.
- Ahmad M., Zahir Zahir A., Naeem Asghar H., Asghar M. Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol. 2011; 57 (7):578–589. [ PubMed ] [ Google Scholar ]
- Ahmad M., Zahir Z.A., Asghar H.N., Arshad M. The combined application of rhizobial strains and plant growth promoting rhizobacteria improves growth and productivity of mung bean (Vigna radiata L.) under salt-stressed conditions. Ann. Microbiol. 2012; 62 :1321–1330. [ Google Scholar ]
- Ahmad M., Zahir Zahir A., Nazli Farheen, Akram Fareeha, Muhammad Arshad Khalid M. Effectiveness of halo-tolerant, auxin producing pseudomonas and Rhizobium strains to improve osmotic stress tolerance in mung bean (Vigna radiata L.) Braz. J. Microbiol. 2013; 44 (4):1341–1348. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Akbarimoghaddam H., Galavi M., Ghanbari A., Panjehkeh N. Salinity effects on seed germination and seedling growth of bread wheat cultivars. Trakia J. Sci. 2011; 9 (1):43–50. [ Google Scholar ]
- Ashraf M. Some important physiological selection criteria for salt tolerance in plants. Flora. 2004; 199 :361–376. [ Google Scholar ]
- Bacilio M., Rodriguez H., Moreno M., Hernandez Juan-Pablo, Bashan Y. Mitigation of salt stress in wheat seedlings by a gfp-tagged Azospirillum lipoferum . Biol. Fertility Soils. 2004; 40 :188–193. [ Google Scholar ]
- Bano A., Fatima M. Salt tolerance in Zea mays (L.) following inoculation with Rhizobium and Pseudomonas . Biol. Fertility Soils. 2009; 45 :405–413. [ Google Scholar ]
- Barassi C.A., Ayrault G., Creus C.M., Sueldo R.J., Sobero M.T. Seed inoculation with Azospirillum mitigates NaCl effects on lettuce. Sci. Horticulturae (Amsterdam) 2006; 109 :8–14. [ Google Scholar ]
- Bhatnagar-Mathur P., Vadez V., Sharma K.K. Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep. 2008; 27 :411–424. [ PubMed ] [ Google Scholar ]
- Blaylock, A.D., 1994. Soil salinity, salt tolerance and growth potential of horticultural and landscape plants. Co-operative Extension Service, University of Wyoming, Department of Plant, Soil and Insect Sciences, College of Agriculture, Laramie, Wyoming.
- Chang P., Gerhardt K.E., Huang Xiao-Dong, Yu Xiao-Ming, Glick B.R., Gerwing P.D., Greenberg B.M. Plant growth-promoting bacteria facilitate the growth of barley and oats in salt-impacted soil: implications for phytoremediation of saline soils. Int. J. Phytorem. 2014; 16 (11):1133–1147. [ PubMed ] [ Google Scholar ]
- Chen M., Wei H., Cao J., Liu R., Wang Y., Zheng C. Expression of Bacillus subtilis proAB genes and reduction of feedback inhibition of proline synthesis increases proline production and confers osmotolerance in transgenic Arabdopsis . J. Biochem. Mol. Biol. 2007; 40 (3):396–403. [ PubMed ] [ Google Scholar ]
- Chinnusamy V., Zhu J., Zhu Jian-Kang. Gene regulation during cold acclimation in plants. Physiol. Plant. 2006; 126 (1):52–61. [ Google Scholar ]
- Cho K., Toler H., Lee J., Owenley B., Stutz J.C., Moore J.L., Auge R.M. Mycorrhizal symbiosis and response of sorghum plants to combined drought and salinity stresses. J. Plant Physiol. 2006; 163 :517–528. [ PubMed ] [ Google Scholar ]
- Dimkpa C., Weinand T., Ash F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009; 32 :1682–1694. [ PubMed ] [ Google Scholar ]
- Dodd I.C., Perez-Alfocea F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012; 63 (9):3415–3428. [ PubMed ] [ Google Scholar ]
- Egamberdieva D., Kucharova Z. Selection for root colonizing bacteria stimulating wheat growth in saline soils. Biol. Fertility Soil. 2009; 45 :563–571. [ Google Scholar ]
- Egamberdiyeva D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl. Soil Ecol. 2007; 36 :184–189. [ Google Scholar ]
- Epstein E.J.D., Norlyn D.W., Rush R.W., Kinsbury D.B., Kelly G.A., Cunningham, Wrona A.F. Saline culture of crops: a genetic approach. Science. 1980; 210 :399–404. [ PubMed ] [ Google Scholar ]
- Foolad M.R., Chen F.Q. RFLP mapping of QTLs conferring salt tolerance during the vegetative stage in tomato. Theor. Appl. Genet. 1999; 99 :235–243. [ Google Scholar ]
- Foolad M.R., Lin G.Y. Absence of a genetic relationship between salt tolerance during seed germination and vegetative growth in tomato. Plant Breed. 1997; 116 :363–367. [ Google Scholar ]
- Glick B.R. Promotion of plant growth by bacterial ACC deaminase. Crit. Rev. Plant Sci. 2007; 26 :227–242. [ Google Scholar ]
- Glick B.R., Cheng Z., Czarny J., Duan J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant Pathol. 2007; 119 :329–339. [ Google Scholar ]
- Godfray H.C.J., Beddington J.R., Crute I.R., Haddad L., Lawrence D., Muir J.F., Pretty J., Robinson S., Thomas S.M., Toulmin C. Food security: the challenge of feeding 9 billion people. Science. 2010; 327 :812–818. [ PubMed ] [ Google Scholar ]
- Gray E.J., Smith D.L. Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signalling processes. Soil Biol. Biochem. 2005; 37 :395–412. [ Google Scholar ]
- Grover A., Aggarwal P.K., Kapoor A., Katiyar-Agarwal S., Agarwal M., Chandramouli A. Addressing abiotic stresses in agriculture through transgenic technology. Curr. Sci. 2003; 84 :355–367. [ Google Scholar ]
- Grover M., Ali Sk.Z., Sandhya V., Rasul A., Venkateswarlu B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011; 27 :1231–1240. [ Google Scholar ]
- Hamdia M.A., Shaddad M.A.K., Doaa M.M. Mechanism of salt tolerance and interactive effect of Azospirillum bransilense inoculation on maize cultivars grown under salt stress conditions. Plant Growth Regul. 2004; 44 :165–174. [ Google Scholar ]
- Hardoim P.R., van Overbeek S.V., van Elsas J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008; 16 :463–471. [ PubMed ] [ Google Scholar ]
- Hayat R., Ali S., Amara U., Khalid R., Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann. Microbiol. 2010; 60 :579–598. [ Google Scholar ]
- Haynes R.J., Swift R.S. Stability of soil aggregates in relation to organic constituents and soil water content. J. Soil Sci. 1990; 41 :73–83. [ Google Scholar ]
- Hu Y., Schmidhalter U. Limitation of salt stress to plant growth. In: Hock B., Elstner C.F., editors. Plant Toxicology. Marcel Dekker Inc.; New York: 2002. pp. 91–224. [ Google Scholar ]
- Jamil A., Riaz S., Ashraf M., Foolad M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011; 30 (5):435–458. [ Google Scholar ]
- Javid M.G., Sorooshzadeh A., Moradi F., Sanavy Seyed A.M.M., Allahdadi I. The role of phytohormones in alleviating salt stress in crop plants. AJCS. 2011; 5 (6):726–734. [ Google Scholar ]
- Jha Y., Subramanian R.B. PGPR regulate caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol. Mol. Biol. Plants. 2014; 20 (2):201–207. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jha Y., Subramanian R.B., Patel S. Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol. Plant. 2011; 33 :797–802. [ Google Scholar ]
- Joseph B., Jini D. Salinity induced programmed cell death in plants: challenges and opportunities for salt tolerant plants. J. Plant Sci . 2010:1–15. [ Google Scholar ]
- Khatun S., Flowers T.J. Effects of salinity on seed set in rice. Plant Cell Environ. 1995; 18 :61–67. [ Google Scholar ]
- Kohler J., Caravaca F., Carrasco L., Roldan A. Contribution of Pseudomonas mendocina and Glomus intraradices to aggregates stabilization and promotion of biological properties in rhizosphere soil of lettuce plants under field conditions. Soil Use Manage. 2006; 22 :298–304. [ Google Scholar ]
- Kohler J., Hernandez J.A., Caravaca F., Roldan A. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ. Exp. Bot. 2009; 65 :245–252. [ Google Scholar ]
- Lugtenberg B., Chin-A-Woeng T., Bloemberg G. Microbe-plant interactions: principles and mechanisms. Antonie Van Leeuwenhoek. 2002; 81 :373–383. [ PubMed ] [ Google Scholar ]
- Lutgtenberg B., Kamilova F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009; 63 :541–556. [ PubMed ] [ Google Scholar ]
- Manchanda G., Garg N. Salinity and its effects on the functional biology of legumes. Acta Physiol. Plant. 2008; 30 :595–618. [ Google Scholar ]
- Mayak S., Tirosh T., Glick B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004; 42 :565–572. [ PubMed ] [ Google Scholar ]
- Metternicht G.I., Zinck J.A. Remote sensing of soil salinity: potentials and constraints. Remote Sens. Environ. 2003; 85 :1–20. [ Google Scholar ]
- Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002; 25 :239–250. [ PubMed ] [ Google Scholar ]
- Munns R. Genes and salt tolerance: bringing them together. New Phytol. 2005; 167 :645–663. [ PubMed ] [ Google Scholar ]
- Munns R., James R.A. Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant Soil. 2003; 253 :201–218. [ Google Scholar ]
- Munns R., Rawson H.M. Effect of salinity on salt accumulation and reproductive development in the apical meristem of wheat and barley. Aust. J. Plant Physiol. 1999; 26 :459–464. [ Google Scholar ]
- Munns R., Husain S., Rivelli A.R., James R.A., Condon A.G., Lindsay M.P., Lagudah E.S., Schachtman D.P., Hare R.A. Avenues for increasing salt tolerance of crops, and the role of physiologically based selection traits. Plant Soil. 2002; 247 (1):93–105. [ Google Scholar ]
- Nadeem S.M., Zahir Z.A., Naveed M., Arshad M. Preliminary investigation on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC-deaminase activity. Can. J. Microbiol. 2007; 53 :1141–1149. [ PubMed ] [ Google Scholar ]
- Nadeem S.M., Zaheer Z.A., Naveed M., Nawaz S. Mitigation of salinity-induced negative impact on the growth and yield of wheat by plant growth-promoting rhizobacteria in naturally saline conditions. Ann. Microbiol. 2013; 63 (1):225–232. [ Google Scholar ]
- Naz I., Bano A., Tamoor-ul-Hassan. Isolation of phytohormones producing plant growth promoting rhizobacteria from weeds growing in Khewra salt range, Pakistan and their implication in providing salt tolerance to Glycine max L. Afr. J. Biotechnol. 2009; 8 (21):5762–5766. [ Google Scholar ]
- Netondo G.W., Onyango J.C., Beck E. Sorghum and salinity: II. Gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Sci. 2004; 44 :806–811. [ Google Scholar ]
- Nia S.H., Zarea M.J., Rejali F., Varma A. Yield and yield components of wheat as affected by salinity and inoculation with Azospirillum strains from saline or non-saline soil. J. Saudi Soc. Agric. Sci. 2012; 11 :113–121. [ Google Scholar ]
- Palaniyandi S.A., Damodharan K., Yang S.H., Suh J.W. Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’ tomato plants. J. Appl. Microbiol. 2014; 117 :766–773. [ PubMed ] [ Google Scholar ]
- Patel B.B., Patel Bharat.B., Dave R.S. Studies on infiltration of saline–alkali soils of several parts of Mehsana and Patan districts of north Gujarat. J. Appl. Technol. Environ. Sanitation. 2011; 1 (1):87–92. [ Google Scholar ]
- Paul D. Osmotic stress adaptations in rhizobacteria. J. Basic Microbiol. 2012; 52 :1–10. [ PubMed ] [ Google Scholar ]
- Ramadoss D., Lakkineni V.K., Bose P., Ali S., Annapurna K. Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. Springer Plus. 2013; 2 (6):1–7. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Richards R.A. Should selection for yield in saline regions be made on saline or non-saline soils. Euphytica. 1983; 32 :431–438. [ Google Scholar ]
- Sadeghi A., Karimi E., Dahaji P.A., Javid M.G., Dalvand Y., Askari H. Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions. World J. Microbiol. Biotechnol. 2012; 28 :1503–1509. [ PubMed ] [ Google Scholar ]
- Sandhya V., Ali Sk.Z., Grover M., Reddy G., Venkateswarlu B. Alleviation of drought stress effects in sunflower seedlings by exopolysaccharides producing Pseudomonas putida strain P45. Biol. Fertility Soil. 2009; 46 :17–26. [ Google Scholar ]
- Saravanakumar D., Samiyappan R. ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut ( Arachis hypogea ) plants. J. Appl. Microbiol. 2007; 102 :1283–1292. [ PubMed ] [ Google Scholar ]
- Schubert S., Neubert A., Schierholt A., Sumer A., Zorb C. Development of salt-resistant maize hybrids: the combination of physiological strategies using conventional breeding methods. Plant Sci. 2009; 177 :196–202. [ Google Scholar ]
- Seckin B., Sekmen A.H., Turkan I. An enhancing effect of exogenous mannitol on the antioxidant enzyme activities in roots of wheat under salt stress. J. Plant Growth Regul. 2009; 28 :12–20. [ Google Scholar ]
- Shahbaz M., Ashraf M. Improving salinity tolerance in cereals. Crit. Rev. Plant Sci. 2013; 32 :237–249. [ Google Scholar ]
- Shannon M.C., Grieve C.M. Tolerance of vegetable crops to salinity. Sci. Horticulturae. 1999; 78 :5–38. [ Google Scholar ]
- Shukla P.S., Agarwal P.K., Jha B. Improved Salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria. J. Plant Growth Regul. 2012; 31 :195–206. [ Google Scholar ]
- Singh K.N., Chatrath R. Salinity tolerance. In: Reynolds M.P., Monasterio J.I.O., McNab A., editors. Application of Physiology in Wheat Breeding. CIMMYT; Mexico, DF: 2001. pp. 101–110. [ Google Scholar ]
- Stajner D., Kevresan S., Gasic O., Mimica-Dukic N., Zongli H. Nitrogen and Azotobacter chroococcum enhance oxidative stress tolerance in sugar beet. Biol. Plantarum. 1997; 39 (3):441–445. [ Google Scholar ]
- Tabur S., Demir K. Role of some growth regulators on cytogenetic activity of barley under salt stress. Plant Growth Regul. 2010; 60 :99–104. [ Google Scholar ]
- Tank N., Saraf M. Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J. Plant Interact . 2010; 5 :51–58. [ Google Scholar ]
- Tester M., Davenport R. Na + tolerance and Na + transport in higher plants. Ann. Bot. 2003; 91 :503–507. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tiwari S., Singh P., Tiwari R., Meena K.K., Yandigeri M., Singh D.P., Arora D.K. Salt-tolerant rhizobacteria-mediated induced tolerance in wheat ( Triticum aestivum ) and chemical diversity in rhizosphere enhance plant growth. Biol. Fertility Soils. 2011; 47 :907–916. [ Google Scholar ]
- Upadhyay S.K., Singh J.S., Saxena A.K., Singh D.P. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol . 2011; 14 :605–611. [ PubMed ] [ Google Scholar ]
- Venkateswarlu B., Shanker A.K. Climate change and agriculture: adaptation and mitigation strategies. Indian J. Agron. 2009; 54 :226–230. [ Google Scholar ]
- Wang W., Vinocur B., Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003; 218 :1–14. [ PubMed ] [ Google Scholar ]
- Wu Z., Yue H., Lu J., Li C. Characterization of rhizobacterial strain Rs-2 with ACC deaminase activity and its performance in promoting cotton growth under salinity stress. World J. Microbiol. Biotechnol. 2012; 28 :2383–2393. [ PubMed ] [ Google Scholar ]
- Yamaguchi T., Blumwald E. Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci. 2005; 10 (12):615–620. [ PubMed ] [ Google Scholar ]
- Yang J., Kloepper J.W., Ryu C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009; 14 :1–4. [ PubMed ] [ Google Scholar ]
- Yao L., Wu Z., Zheng Y., Kaleem I., Li C. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur. J. Soil Biol. 2010; 46 :49–54. [ Google Scholar ]
- Yensen N.P. Halophyte uses for the twenty-first century. In: Khan M.A., Weber D.J., editors. Ecophysiology of High Salinity Tolerant Plants. Springer; Dordrecht: 2008. pp. 367–396. [ Google Scholar ]
- Yildirim E., Taylor A.G. Effect of biological treatments on growth of bean plans under salt stress. Ann. Rep. Bean Improvement Cooperative. 2005; 48 :176–177. [ Google Scholar ]
- Zahir Z.A., Munir A., Asghar H.N., Arshad M., Shaharoona B. Effectiveness of rhizobacteria containing ACC-deaminase for growth promotion of peas ( Pisum sativum ) under drought conditions. J. Microbiol. Biotechnol. 2008; 18 (5):958–963. [ PubMed ] [ Google Scholar ]
- Zhang H., Kim M.S., Sun Y., Dowd S.E., Shi H., Pare P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant Microbe Interact. 2008; 21 :731–744. [ PubMed ] [ Google Scholar ]
- Zhu J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Bol. 2002; 53 :247–273. [ PMC free article ] [ PubMed ] [ Google Scholar ]
ORIGINAL RESEARCH article
Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (oenanthe javanica) cultivars.

- 1 The State Key Laboratory of Freshwater Ecology and Biotechnology, The Key Laboratory of Aquatic Biodiversity and Conservation of Chinese Academy of Sciences, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China
- 2 University of Chinese Academy of Sciences, Beijing, China
- 3 Institute of Vegetables, Wuhan Academy of Agricultural Sciences, Wuhan, China
Salt stress is an important environmental limiting factor. Water dropwort ( Oenanthe javanica ) is an important vegetable in East Asia; however, its phenotypic and physiological response is poorly explored. For this purpose, 48 cultivars of water dropwort were grown hydroponically and treated with 0, 50, 100, and 200 mm NaCl for 14 days. Than their phenotypic responses were evaluated, afterward, physiological studies were carried out in selected sensitive and tolerant cultivars. In the present study, the potential tolerant (V11E0022) and sensitive (V11E0135) cultivars were selected by screening 48 cultivars based on their phenotype under four different levels of salt concentrations (0, 50, 100, and 200 mm). The results depicted that plant height, number of branches and leaves were less effected in V11E0022, and most severe reduction was observed in V11E0135 in comparison with others. Than the changes in biomass, ion contents, accumulation of reactive oxygen species, and activities of antioxidant enzymes and non-enzymatic antioxidants were determined in the leaves and roots of the selected cultivars. The potential tolerant cultivar (V11E0022) showed less reduction of water content and demonstrated low levels of Na + uptake, malondialdehyde, and hydrogen peroxide (H 2 O 2 ) in both leaves and roots. Moreover, the tolerant cultivar (V11E0022) showed high antioxidant activities of ascorbate peroxidase (APX), superoxide dismutase, peroxidase, catalase (CAT), reduced glutathione (GSH), and high accumulation of proline and soluble sugars compared to the sensitive cultivar (V11E0135). These results suggest the potential tolerance of V11E0022 cultivar against salt stress with low detrimental effects and a good antioxidant defense system. The observations also suggest good antioxidant capacity of water dropwort against salt stress. The findings of the present study also suggest that the number of branches and leaves, GSH, proline, soluble sugars, APX, and CAT could serve as the efficient markers for understanding the defense mechanisms of water dropwort under the conditions of salt stress.
Introduction
Salinity is one of the major abiotic stresses that has been significantly affecting the plant growth and yield ( Gharsallah et al., 2016 ). The continuous increase in salinity in arable land due to poor cultivation practices and climate change have devastating global effects, and it is estimated that about 50% of arable land will be lost by the middle of the 21st century ( Islam et al., 2019 ). To date, about 1,125 million hectares of agricultural lands have already been seriously affected by salinity, thus it is considered a serious threat to agriculture ( Islam et al., 2019 ; Sanower-Hossain, 2019 ). In China, a total of 36.7 million hectares of land has been greatly affected by salinity, of which 12.3 million hectares is agricultural land ( Li-ping et al., 2015 ).
A high level of salt results ionic imbalance and osmotic stress in plants which causes severe effects on morphology, biomass, and biochemical processes of the plants, and ultimately result in plant damages ( Zhang et al., 2013 ; Rahneshan et al., 2018 ). Soil salinity enhances the Na + and Cl − contents in plants which then increases the ratio of Na + /K + , which ultimately affects the regular ionic activities in plants ( Singh et al., 2014 ). Several plants have developed different strategies to overcome these challenges. Among these, the first one is the maintenance of homeostasis by the osmotic adjustment that carries out the excessive Na + ions to the vacuole, and the second is the synthesis of osmolyte to cope with this situation ( Queirós et al., 2009 ; Silva et al., 2015 ; Rahneshan et al., 2018 ). A high K + /Na + ratio also plays a vital role in maintaining membrane potential as well as osmotic and turgor pressures. It also helps in enzyme activation and tropisms ( Rahneshan et al., 2018 ). Plants produce osmolytes, such as proline and soluble sugars protect the plant cells against the adverse effects of salt stress. These help in osmotic adjustment, and their higher production can increase the salinity tolerance ( Rahneshan et al., 2018 ). Similarly, antioxidant molecules including glutathione (GSH) and proteins have the role in control of concentration of reactive oxygen species (ROS), which ultimately help in salinity tolerance. Proteins can also help in osmotic adjustment under salt stress ( Zhang et al., 2013 ; Hasanuzzaman et al., 2018 ).
Salt stress also leads to increasing the level of ROS which results in oxidative stress, which in turn affects the plants both at cellular and metabolic levels ( Ali et al., 2017 ; Sahin et al., 2018 ). The plants overcome the oxidative damage through activation of antioxidants through enzymatic and non-enzymatic mechanisms. The enzymatic component includes superoxide dismutase (SOD; EC 1.15.1.1), peroxidase (POD; EC 1.11.1.7), catalase (CAT; EC 1.11.1.6), and ascorbate peroxidase (APX; EC 1.11.1.1; Shaheen et al., 2013 ; Shafeiee and Ehsanzadeh, 2019 ; Soares et al., 2019 ; Sarker and Oba, 2020b ). Moreover, the ROS, such as superoxide radicals ( O 2 − ), hydrogen peroxide (H 2 O 2 ), and small amounts of transition metals, also increases the concentration of OH − . Therefore, plants carry out detoxification to avoid the oxidative damage where these antioxidant enzymes play an important role. A study reported that the antioxidant enzymes positively correlate with the plant tolerance in drought and salt stress ( Wang et al., 2009 ). Moreover, the higher antioxidant activities can help improving death in plants ( Khan et al., 2017 ).
Oenanthe javanica (Blume) DC (also known as water dropwort) is an aquatic perennial herb belonging to the family Apiaceae. It is mainly cultivated in East Asian countries, such as China, Japan, Korea, Thailand, Malaysia, and Australia ( Jeon et al., 2007 ; Lee and Kim, 2009 ; Lu and Li, 2019 ). Water dropwort contains high contents of minerals and vitamins, and demonstrates medicinal properties ( Jiang et al., 2015 ; Lu and Li, 2019 ; Kumar et al., 2020 , 2021 ). It has been traditionally used as a vegetable in China. Various researchers have suggested that persicarin, isorhamnetin, and hyperoside are the three important compounds present in O. javanica , which possess the pharmacological activities for curing various ailments ( Jiang et al., 2015 ; Chan et al., 2017 ; Lu and Li, 2019 ). Therefore, all these properties make the water dropwort a popular edible plant in China. The previous studies reported that O. javanica is sensitive to drought and salt stress, and these are the key limiting factors for its growth and production ( Jiang et al., 2015 ; Kumar et al., 2020 ). There is only limited information available related to the salt tolerance mechanism of water dropwort concerning the regulation of free radicals quenching pathway with the antioxidative defense.
The present study is designed to access phenotypic responses of different water dropwort cultivars under salt stress and to select salt-tolerant and sensitive cultivars based on phenotype among them. Secondly, it aims to study some physiological parameters including enzymatic and the non-enzymatic antioxidant defense system, chlorophyll content, and ionic homeostasis regarding the salt tolerance in selected tolerant and sensitive cultivars of water dropwort. For these objectives, various parameters, such as plant growth, fresh and dry biomass, relative water content (RWC), chlorophyll content, Na + and K + content, production rate of ROS, osmolytes and antioxidant molecules concentration, and activities of antioxidant enzymes, were studied.
Materials and Methods
Plant culture and salt treatment.
Seeds of 48 cultivars of Oenanthe javanica were kept in wet sand for 1 month and then shifted to the wet filter paper and placed in the growth chamber (12/12 h) at 25°C. After germination for 7–10 days, seeds were transferred to Hoagland nutrient solution ( Hoagland and Arnon, 1950 ) and grown for 44 days in greenhouse condition at 20–25°C for 16 h photoperiod. The composition of media was 3.59 mm Ca(NO 3 ) 2 , 8.7 mm KNO 3 , 0.713 mm N₂H₄O₃, 1.516 mm MgSO 4 , 1.314 mm KH 2 PO 4 , 62.5 μm FeSO 4 , 44.6 μm EDTA, 48.5 μm H 3 BO 3 , 13.2 μm MnSO 4 , 1.36 μm ZnSO 4 , 0.501 μm CuSO 4 , and 2.55 μm (NH 4 ) 2 MoO 4 . Initially, the plants were grown hydroponically for 30 days than these plants were treated with 0 (control), 50, 100, and 200 mm NaCl for 14 days. Afterward, these treated plants were used for further analysis. All experiments were conducted in biological triplicates.
Morphological Parameters and Chlorophyll Content
After harvesting, morphological parameters, such as plant height, stem length, root length, and number of branches and leaves, were measured. The total chlorophyll content was determined using the SPAD-502Plus (Konica Minolta, Japan). The fresh and dry biomass of selected cultivars was also measured. The shoots biomass and roots biomass were determined after washing with distilled water and drying them gently on a paper towel. The dry weight (DW) was determined after drying for 72 h at 70°C.
Determination of RWC
Relative water content (RWC) of leaves was measured according to the method described by Sarker and Oba (2018) and Kumar et al. (2020) . After determining the fresh weight (FW), leaves were immersed in distilled water in a closed Petri dish for 4 h, and the turgor weight (TW) of each leaf was noted. Thereafter, the leaf samples were placed in a pre-heated oven at 70°C for 24 h to obtain dry weight (DW). Afterward, RWC was calculated using the following formula:
Determination of Na + and K + Contents
For determination of Na + and K + contents, approximately 100 mg of dried leaves and roots was digested with 6 ml nitric acid in a microwave digestion system (Multiwave 3000, Anton Paar, Austria) for 90 min. The digested samples were diluted up to 10 ml with ultra-deionized water. The ions concentrations were determined by using the inductively coupled plasma-atomic emission spectroscopy ICP-OES (Optima8000, PerkinElmer, United States; Colomer-Winter et al., 2018 ).
Determination of Photosynthetic Pigments
For determination of chlorophyll and carotenoid concentrations, approximately 100 mg of fresh leaves was homogenized with 80% acetone and centrifuged at 7,000 × g for 10 min. The supernatant was collected, and the absorbance (A) was measured at 663 nm for chlorophyll a , 646 nm for chlorophyll b , and 470 nm for carotenoid using an ELISA plate reader (i3× molecular devices, United States; Sarker and Oba, 2020a ; Kumar et al., 2021 ). The concentration of chlorophyll and carotenoids was calculated as follows:
Determination of Lipid Peroxidation
For determination of malondialdehyde (MDA), approximately 50 mg of fresh leaves and roots was homogenized with 450 μl phosphate buffer saline (PBS; pH 7.4, 0.1 M) with a glass homogenizer. The samples were then centrifuged three times at 4,000 × g for 15 s with intervals of 30 s. Afterward, the homogenate was centrifuged at 3500 × g for 10 min. After centrifugation, the supernatant was used for the MDA analysis with a commercially available test kit (A003-1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Finally, absorbance was measured at 530 nm ( Dai et al., 2018 ).
Assays for Hydrogen Peroxide, Proteins, GSH, and Antioxidant Enzymes
For determination of H 2 O 2 , GSH, and antioxidant enzymes, approximately 200 mg of fresh leaves was homogenized with 1.8 ml of PBS (pH 7.4, 0.1 M) with a glass homogenizer and then centrifuged at 3,500 × g for 12 min. The supernatant was used for determination of total protein, H 2 O 2 , GSH contents, and antioxidant enzymes activities including APX, SOD, POD, and CAT with commercially available test kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China; Zhang et al., 2015 ; Hussain et al., 2016 ; Dai et al., 2018 ; Yang et al., 2018 ).
The Coomassie brilliant blue method was used for determining the total protein content with a commercially available total protein assay kit (A045-2; Nanjing Jiancheng Bioengineering Institute, China), and the absorbance was measured at 595 nm. H 2 O 2 forms a complex with molybdate whose absorbance was measured at 405 nm. The GSH content was determined with a glutathione assay kit (A006-1; Nanjing Jiancheng Bioengineering Institute, China) according to the DTNB [5,5,-dithiobis (2-nitrobenzoic acid)] method. The absorbance was measured at 420 nm, and GSH content was expressed as mg g −1 protein ( Zhang et al., 2015 ; Hussain et al., 2016 ; Dai et al., 2018 ; Yang et al., 2018 ).
The activity of APX was determined with the APX assay kit (A123-1-1; Nanjing Jiancheng Bioengineering Institute, China). APX catalyzed the oxidation of ascorbate at 290 nm and expressed as U mg −1 FW. One unit activity of APX is the amount of enzyme, which oxidizes 1 μmol ascorbate per min in 1 mg fresh sample ( Nakano and Asada, 1981 ). The activity of SOD was determined with SOD assay kit (A001-1; Nanjing Jiancheng Bioengineering Institute, China) and was presented as U mg −1 FW. One unit of SOD activity is the amount of extract that gives 50% inhibition in reducing xanthine monitored at 550 nm ( McCord and Fridovich, 1969 ). The activity of POD was measured by using a POD assay kit (A084-3-1; Nanjing Jiancheng Bioengineering Institute, China) on the basis of guaiacol oxidation at 470 nm by H 2 O 2 and expressed as U mg −1 . The change in absorbance at 470 nm was recorded every 20 s ( Chance and Maehly, 1955 ). One unit of POD activity is the amount of enzyme, which causes the decomposition of 1 μg substrate per minute in 1 mg fresh sample at 37°C. Similarly, the activity of CAT was measured with a CAT assay kit (A007-1; Nanjing Jiancheng Bioengineering Institute, China) and was presented as U mg −1 FW. One unit of CAT activity is the amount of enzyme which causes the decomposition of 1 μmol H 2 O 2 per minute in 1 mg fresh sample at 37°C ( Beers and Sizer, 1952 ).
Determination of Concentrations of Proline and Soluble Sugars
Approximately 100 mg of fresh leaves and roots was homogenized for determination of proline content following the manufacturer’s instructions (A107-1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China), and the absorbance was measured at 520 nm. For the analysis of soluble sugars, approximately 0.1 g of fresh samples was homogenized in 1 ml ddH 2 O with a glass homogenizer. The tubes were boiled at 95°C for 10 min and cooled with tap water. After cooling, the homogenate was centrifuged at 4,000 × g for 10 min. Thereafter, the supernatant was diluted with ddH 2 O at 1:9. The diluted extracts were used for determination of soluble sugar content using a commercially available test kit (A145-1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Finally, the absorbance was measured at 620 nm and the soluble sugar concentration expressed and the results expressed in the fresh weight (FW) basis ( Dai et al., 2018 ; Kumar et al., 2021 ).
Statistical Analysis
All experiments were performed in triplicates, and SPSS 25.0 statistical program (IBM Crop. Armonk, NY, United States) was used for statistical analysis. Tukey tests were performed for determining the significant differences ( p ≤ 0.05) among treatments. GraphPad Prism 7 (San Diego, California, United States) was used for figures, and significant differences were indicated by different letters. All data are presented as mean ± standard error (SE).
Growth and Biomass of Water Dropwort
The growth properties of 48 cultivars were strongly influenced by the salt stress. The phenotypic parameters of water dropwort, such as plant height, stem length, root length, number of branches, and number of leaves in all treatments, were significantly lower than the control ( p < 0.05; Supplementary Table S1 ). The plant growth showed an inverse relation with the different level of salt stress imposed. Moreover, a high reduction in the plant height, root length, stem length, and number of branches, and leaves was observed in all cultivars at 200 NaCl ( Supplementary Table S1 ). Beside the plant height, the drastic effects of salinity were found in the number of branches and leaves of all cultivars. Furthermore, an increase in salt concentration caused a decline in the number of branches and leaves. Based on the phenotypic results, we identified V11E0022 as the potential tolerant cultivar, whereas the V11E0135 as the most sensitive cultivar among the 48 cultivars under consideration in the present study ( Supplementary Table S1 ).
The growth parameters of selected tolerant and sensitive cultivars were greatly affected by the salt stress ( Figure 1 ). A gradual decrease in the plant height, and root and stem length of both cultivars were observed under all treatments (50, 100, and 200 mm) in comparison with the control, and a maximum reduction was detected at 200 mm NaCl ( Table 1 ). The plant height of V11E0135 was decreased by 28.5 and 31.4% by exposure of 100 and 200 mm NaCl, respectively, while it decreases only 16.5 and 22.7% in V11E0022 under 100 and 200 mm NaCl, respectively. Similarly, the number of branches and leaves of both cultivars were significantly reduced under different levels of NaCl compared to the control ( p < 0.05). The number of branches in V11E0135 was reduced by 68.5 and 76.7% by exposure of 100 and 200 mm NaCl, respectively, whereas the reduction in V11E0022 was only 33.7 and 37.5% under 100 and 200 mm NaCl, respectively. Similarly, the number of leaves of V11E0135 decreased by 74.5 and 84.7% under 100 and 200 mm NaCl, respectively. However, V11E0022 showed only 31.3 and 34.9% reduction in number of leaves under 100 and 200 mm NaCl, respectively. The salt stress also significantly reduced the fresh and dry weight of the shoot and root ( p < 0.05), and a maximum reduction was observed under 200 mm NaCl in V11E0135 when compared with the control ( Table 2 ). The shoot fresh weight of V11E0135 was decreased by 70% at 100 mm and 80.3% at 200 mm NaCl. On the other hand, V11E0022 showed 40.7 and 45.3% decrease in shoot fresh weight under 100 and 200 mm NaCl, respectively. Furthermore, root fresh weight of V11E0135 decreased by 61.9 and 71.63% under 100 and 200 mm NaCl, respectively. In contrast, V11E0022 showed reduction of 47.5 and 51.1% at 100 and 200 mm NaCl, respectively. Overall, the shoot and root (fresh and dry) weight of the V11E0135 cultivar was reduced more than that of V11E0022. Overall, V11E0135 showed drastic effects for different growth parameters compared to the V11E0022 cultivar ( Table 1 ).

Figure 1 . Effect of salt stress on the tolerant and sensitive cultivars of water dropwort. (A) potential tolerant cultivar (V11E0022) and (B) sensitive cultivar (V11E0135).
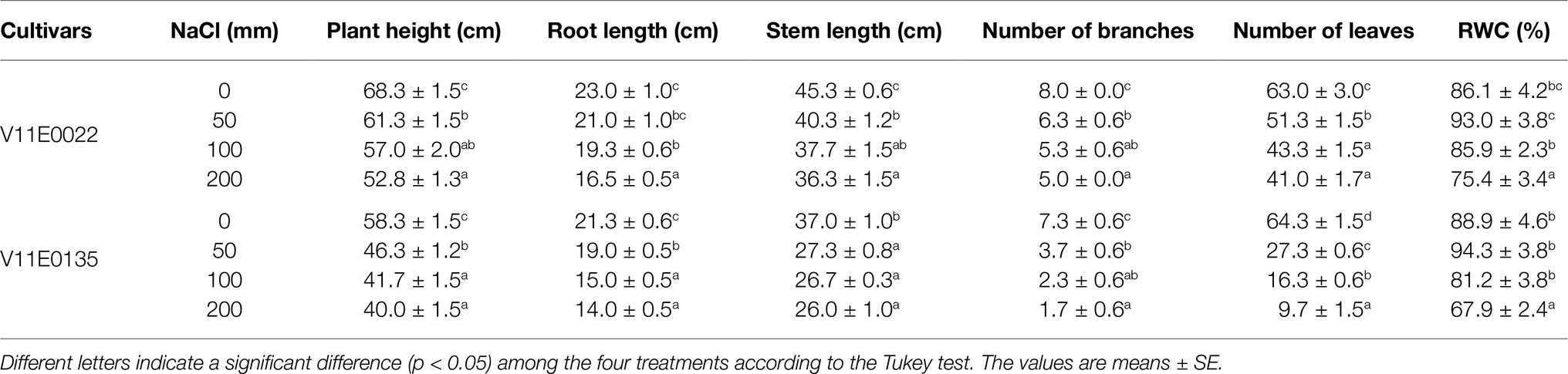
Table 1 . Effect of salt stress on morphological parameters and relative water content (RWC) of two cultivars of water dropwort.
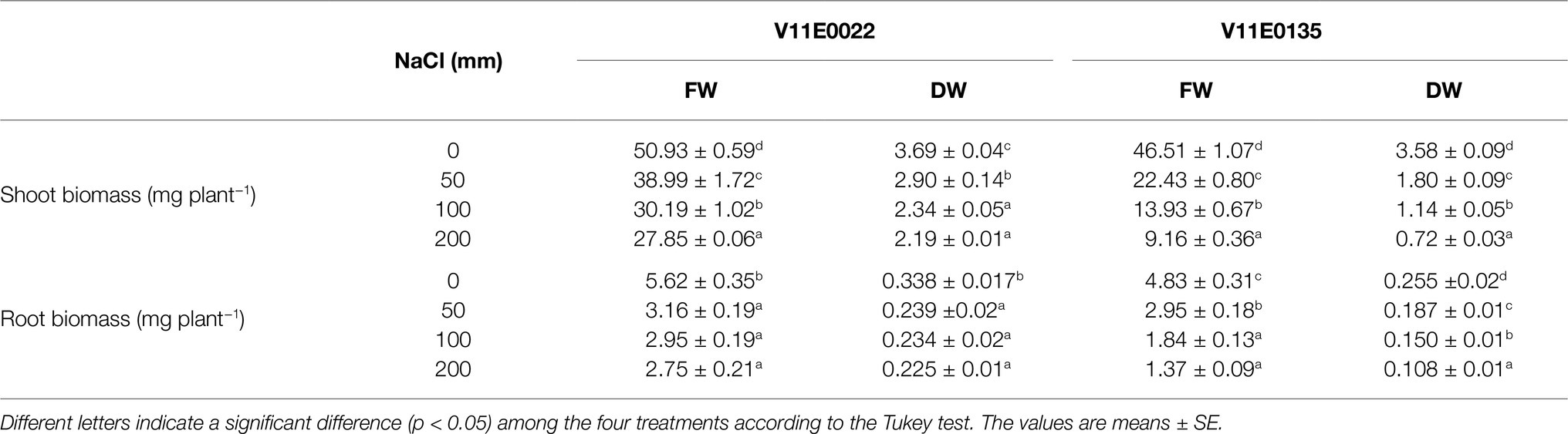
Table 2 . Effect of salt stress on fresh (FW) and dry weight (DW) of the shoot and root of two water dropwort cultivars.
Relative Water Content
Similarly, RWC decreased in both cultivars with the increase of NaCl compared to the control, except for 50 mm, where RWC was increased to 7.99 and 6.06% in V11E0022 and V11E0135, respectively ( Table 1 ). Moreover, a relatively higher reduction of RWC was observed in V11E0135 compared to the V11E0022.
Na + and K + Concentrations
The salt stress significantly enhanced the Na + content in the roots and leaves of both cultivars under all treatments (50, 100, and 200; p < 0.05). Furthermore, the leaves and roots of V11E0135 showed high uptake of Na + ion than its counterpart, and the highest Na + content was detected under 200 mm NaCl ( Table 3 ). Similarly, the K + content in the roots and leaves of both cultivars decreased with increasing NaCl concentration, and the lowest level of K + uptake was observed in V11E0135 at 200 mm NaCl. Moreover, a negative relationship was found between the salt stress and K + /Na + ratio in both cultivars ( Table 3 ).
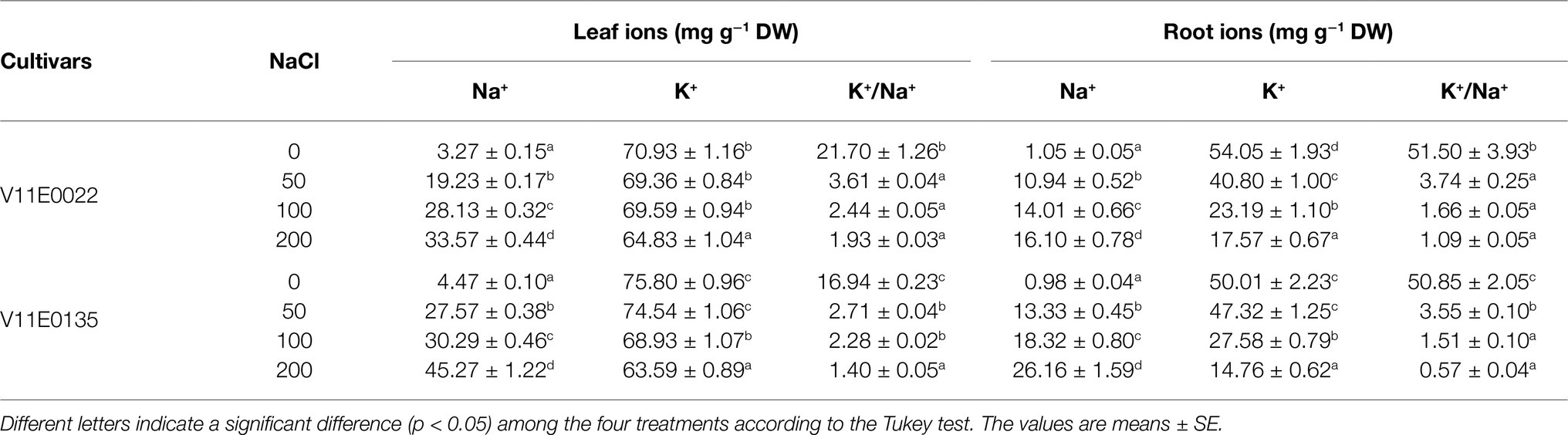
Table 3 . Changes in leaf and root ionic contents of two water dropwort cultivars under salt stress.
Photosynthetic Pigments
A zigzag trend of chlorophyll content was found in the leaves under different salt concentrations of all 48 cultivars ( Supplementary Table S1 ). Interestingly, the chlorophyll content was increased in many cultivars of water dropwort. Similarly, the concentration of photosynthetic pigments, including chlorophyll a (chl a ) and chlorophyll b (chl b ) as well as total chlorophyll (chl a + b ) and carotenoids (Car), was higher in the salt-treated plants compared to the non-treated plants of both selected sensitive and tolerant cultivars ( p < 0.05; Figures 2A – D ). Specifically compared to the control, the concentration of chl ( a + b ) and chl b was higher in both cultivars, and maximum concentration was present at 200 mm NaCl treatment. Although higher than in the control situation, a comparable concentration of chl a and Car was present in all treatments.
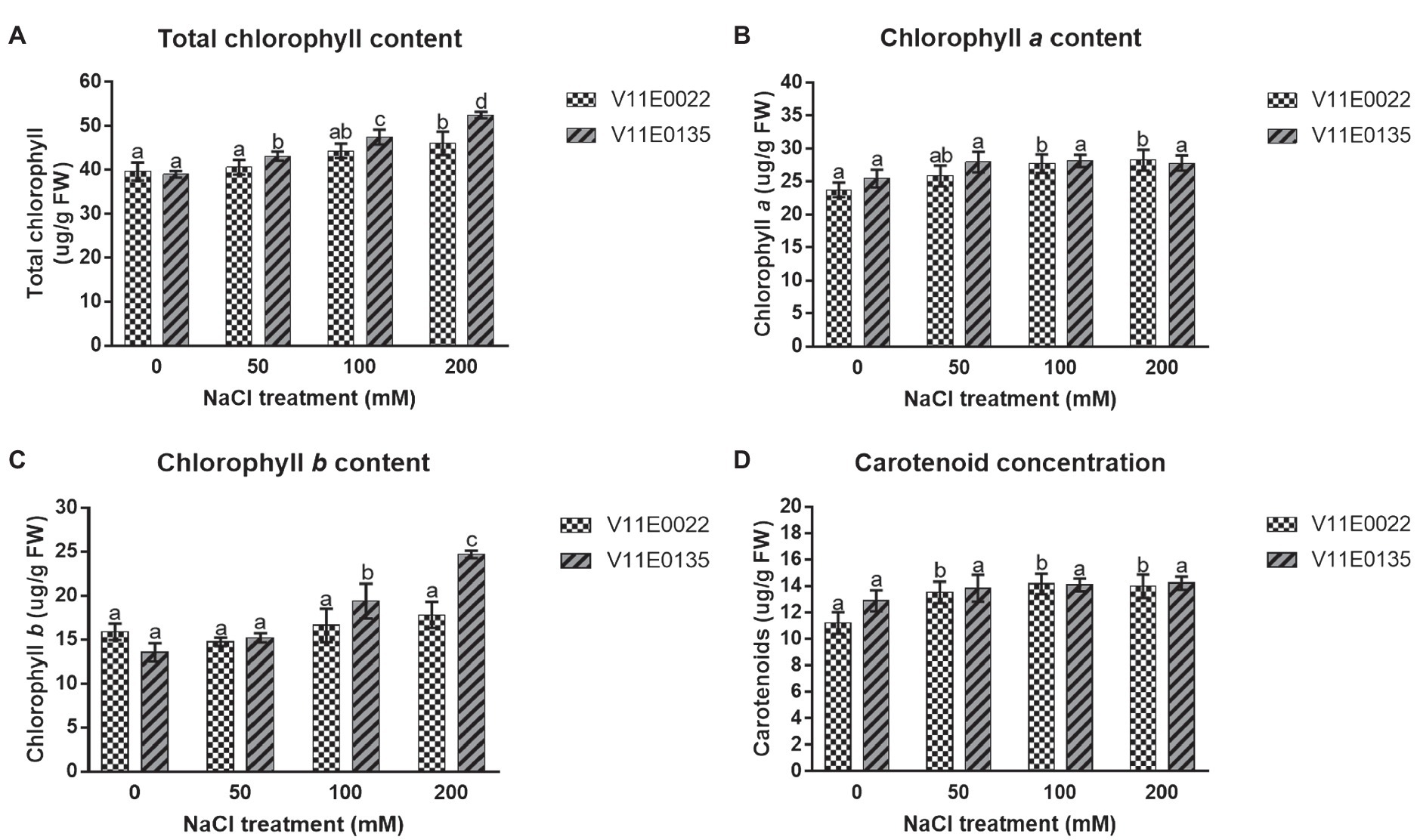
Figure 2 . Changes in the photosynthetic pigments under salt stress in leaves of two water dropwort cultivars. (A) Total chlorophyll content, (B) chlorophyll a content, (C) chlorophyll b content and (D) carotenoid concentration in the leaves of water dropwort. Means followed by different letters indicate a significant difference ( p < 0.05) among the four treatments according to the Tukey test. Error bars show mean ± SE.
Lipid Peroxidation and H 2 O 2 Content
The salt stress significantly induced lipid peroxidation in terms of MDA content in both leaves and roots of water dropwort cultivars ( p < 0.05). Moreover, high MDA content was present in V11E0135 compared to the V11E0022. Compared to the control, the MDA content was increased maximally up to 100 mm in leaves of V11E0022 and V11E0135 ( Figure 3A ), whereas in the roots of both cultivars were found significantly higher under all salt treatments compared to the control ( p < 0.05; Figure 3B ).
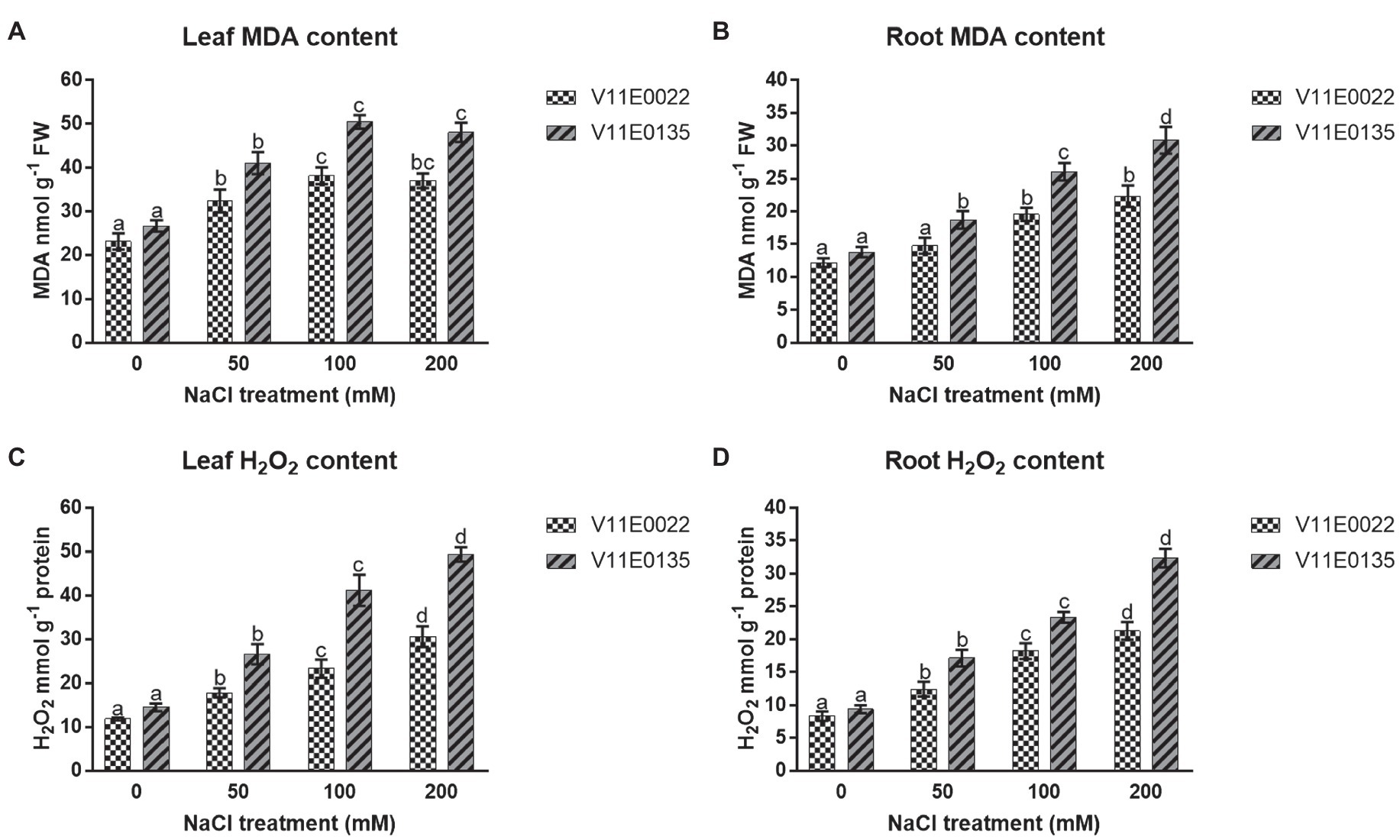
Figure 3 . Changes in the lipid peroxidation and ROS in fresh leaves and roots of two water dropwort cultivars under salt stress. (A) MDA content in the leaves, (B) MDA content in the roots, (C) H2O2 content in the leaves and (D) H2O2 content in the roots of water dropwort. Means followed by different letters indicate a significant difference ( p < 0.05) among the four treatments according to the Tukey test. Error bars show mean ± SE.
The H 2 O 2 production rate was significantly increased in leaves and roots of both cultivars as compared to the control ( p < 0.05). Moreover, a significantly higher content of H 2 O 2 was present in V11E0135 compared to the V11E0022 ( p < 0.05). Compared to untreated plants, maximum H 2 O 2 content was present at 200 mm NaCl concentration in leaves and roots of both cultivars ( Figures 3C , D ).
Osmolytes and Antioxidant Molecules
The proline concentration was found higher in V11E0022 compared to V11E0135. The proline content increases significantly in the leaves and roots of V11E0022 in all NaCl treatments compared to the control ( p < 0.05). The V11E0135 showed a gradual rise in content of proline in leaves and roots up to 100 mm NaCl. Thereafter, a significant decline was observed at 200 mm NaCl ( p < 0.05; Figures 4A , B ). The concentration of soluble sugars was found higher in V11E0022 compared to the V11E0135. In leaves and roots of both cultivars, the concentration of soluble sugar was found to be significantly increasing up to 100 mm NaCl concentration compared to the control ( p < 0.05; Figures 4C , D ).
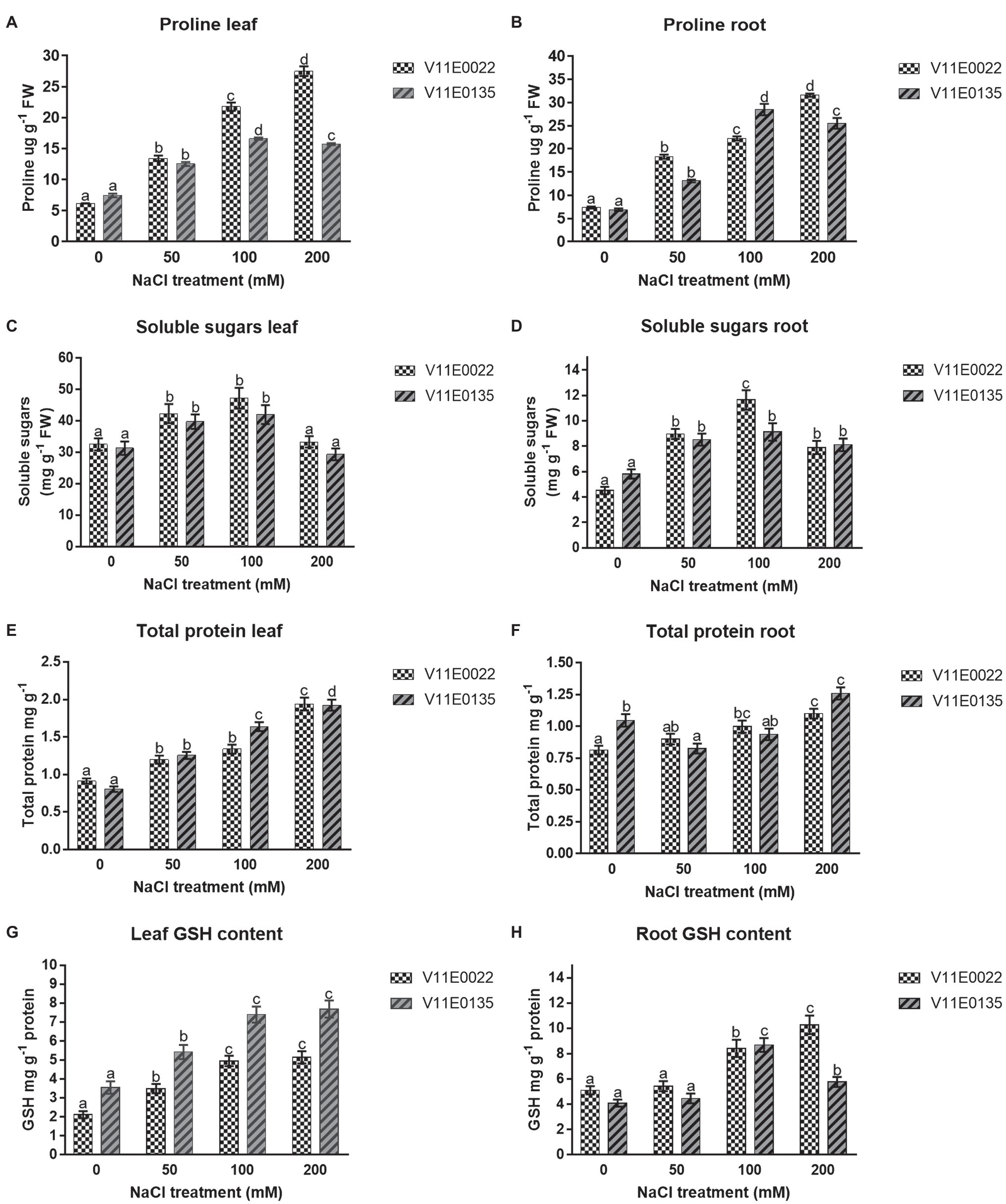
Figure 4 . Changes in the content of osmolytes and non-enzymatic antioxidant compounds in fresh leaves and roots of two water dropwort cultivars under salt stress. (A) Proline content in the leaves, (B) proline content in the roots, (C) soluble sugars content in the leaves, (D) soluble sugars content in the roots, (E) total protein content in the leaves, (F) total protein content in the roots, (G) reduced glutathione (GSH) content in the leaves and (H) GSH content in the roots of water dropwort. Means followed by different letters indicate a significant difference ( p < 0.05) among the four treatments according to the Tukey test. Error bars show mean ± SE.
The results showed that the protein content was increased with the increasing of NaCl concentration in both selected cultivars. A significant difference was observed in protein concentration with the increasing salt concentration in leaves and roots of V11E0022 as compared to the control ( p < 0.05; Figures 4E , F ). In contrast, the leaves of V11E0135 showed a significant increase in all treatments ( p < 0.05; Figure 4E ). However, protein content in roots of V11E0135 was significantly decreased by 21.09% at 50 mm in comparison with its respective control ( p < 0.05), and thereafter increased at 100 and 200 mm NaCl concentrations ( Figure 4F ).
GSH content was increased in both leaves and roots of V11E0022 with the increasing NaCl concentration, and the highest GSH content was found at 200 mm NaCl concentration ( Figures 4G , H ). The V11E0022 showed higher GSH content than the V11E0135 in roots. Interestingly, the leaves of V11E0135 showed higher content of GSH compared to its counterpart, but its roots showed maximum GSH content at 100 mm NaCl concentration.
Antioxidant Enzymes
APX activity was found higher in V11E0022 compared to the V11E0135. The activity increased significantly with the increasing salt concentration in leaves and roots of V11E0022 compared to its counterpart ( p < 0.05; Figures 5A , B ). In contrast, the APX activity decreased gradually in leaves of V11E0135 with the increasing salt concentration ( Figure 5A ). However, APX activity decreased up to 10.68% at 50 mm NaCl concentration in roots; nevertheless, comparatively higher activity was observed at 100 and 200 mm NaCl concentration ( p > 0.05; Figure 5B ).
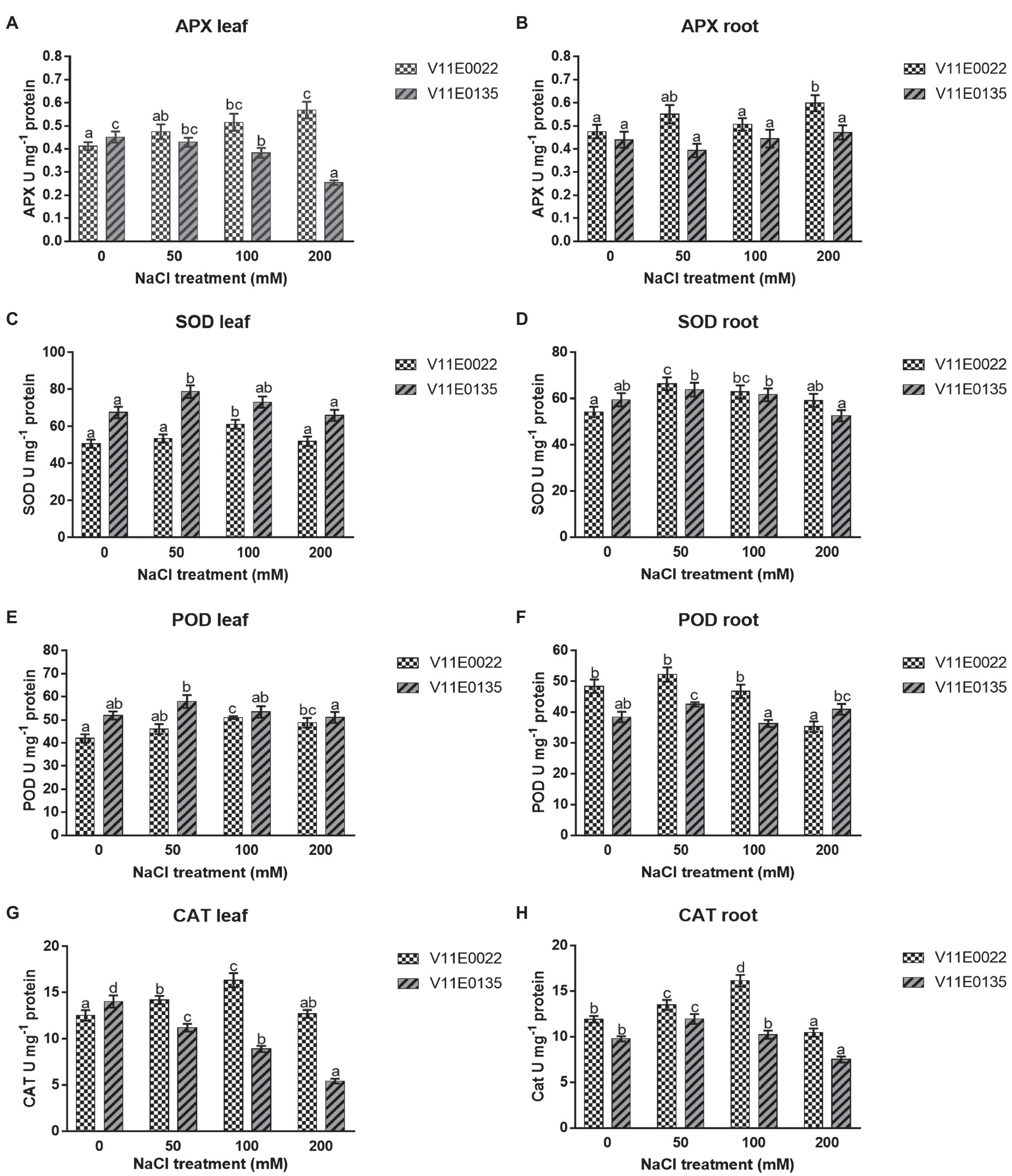
Figure 5 . Changes in activities of antioxidant enzymes in fresh leaves and roots of two water dropwort cultivars under salt stress. (A) APX activity in the leaves, (B) APX activity in the roots, (C) SOD activity in the leaves, (D) SOD activity in the roots, (E) POD activity in the leaves, (F) POD activity in the roots, (G) CAT activity in the leaves and (H) CAT activity in the roots of water dropwort. Means followed by different letters indicate a significant difference ( p < 0.05) among the four treatments according to the Tukey test. Error bars show mean ± SE.
A similar pattern was observed for SOD and POD in both cultivars under different NaCl treatments. Compared to the control, the activities of SOD and POD were increased significantly up to 100 mm in the leaves of V11E0022 ( p < 0.05; Figures 5C , E ), while decreased under 50 mm NaCl in the leaves of V11E0135. Furthermore, SOD and POD activities were decreased after 50 mm NaCl in roots of both cultivars. Interestingly, the POD activity in V11E0135 at 200 mm was 6.53% higher than the control ( Figures 5D , F ). When compared based on the difference in activities with their respective controls, V11E0022 was found comparatively higher than V11E0135 in both antioxidant enzyme.
A significantly higher CAT activity was observed in V11E0022 compared to the V11E0135 ( p < 0.05). The CAT activity increased up to 100 mm NaCl concentration in leaves and roots of V11E0022 ( Figures 5G , H ). In contrast, the CAT activity was decreased significantly with the increasing salt concentration in leaves of V11E0135. In contrast, the CAT activity was found significantly higher in roots at 50 mm and thereafter decreased gradually at 100 and 200 mm NaCl concentrations ( p < 0.05; Figures 5G , H ).
Salinity is a major abiotic stress that significantly affects the plant growth by causing osmotic stress, and inducing ionic and nutrient imbalance. Such imbalances adversely affect different physiological and biochemical mechanisms related to the plant growth and development ( Zhang et al., 2013 ). The present study investigated the phenotypic effects of salt stress on 48 water dropwort cultivars at different NaCl concentration (0–200 mm). The study proposed the tolerant and sensitive cultivars based on their performance against salt stress, and different components of the antioxidant defense system depicted the salt tolerance mechanism in selected sensitive and tolerant cultivars of water dropwort.
The results of the present study show that the plant growth (total height, stem and root lengths, and number of branches and leaves) was decreased significantly with the increasing NaCl concentration in all 48 cultivars of water dropwort, indicating that salt stress suppressed their growth. The growth reduction in V11E0135 was very pronounced in comparison with other cultivars, whereas V11E0022 showed better adaptation as compared to others. Similar studies were previously conducted on different plants also support our findings ( Shaheen et al., 2013 ; Okkaoğlu et al., 2015 ; Menezes et al., 2017 ; Rahneshan et al., 2018 ; Sahin et al., 2018 ). Furthermore, the fresh and dry biomass of shoot and root was significantly decreased in both selected cultivars of water dropwort under all treatments of NaCl, whereas V11E0135 showed more reduction than the V11E0022. Previous studies on different plants showed the reduction of fresh and dry weights of root and shoot under NaCl stress ( Inal et al., 2009 ; Shaheen et al., 2013 ; Kapoor and Pande, 2015 ). According to Meriem et al. (2014) , a higher reduction in biomass was observed in sensitive cultivars than tolerant cultivar of coriander under different NaCl treatments. It is suggested that this decrease in the length and biomass of water dropwort could be due to the negative effect of NaCl treatment. The salinity increases the osmotic stress that inhibits absorption and transport of water. This inhibition leads to hormones-induced sequential reactions, which can reduce the stomatal opening, CO 2 assimilation, and photosynthetic rate ( Odjegba and Chukwunwike, 2012 ; Menezes et al., 2017 ; Sarker and Oba, 2020b ). Another reason for reduction in growth might be the diversion of energy from growth to the homeostasis of salinity stress and a reduction in carbon gains ( Atkin and Macherel, 2009 ; Sarker and Oba, 2020b ).
Based on all phenotypic results of the current study, it is suggested that the decrease in growth and biomass could be due to the adverse effects of salinity on cell division and elongation. Moreover, salinity also causes the nutrient imbalance, overproduction of ROS, and inhibition of enzymatic activities, which significantly affect the cellular components and biological membranes and cause a decrease in biomass production ( Ali et al., 2017 ; Alzahrani et al., 2019 ).
A high concentration of NaCl affects photosynthesis, and the exposure to salt stress for longer time causes a reduction in biosynthesis of chlorophyll protein-lipid complex ( Akbari Ghogdi et al., 2012 ). Different opinions about the salinity effect on the chlorophyll content have been reported, and among these many studies have reported a significantly decreased in chlorophyll content under salt stress ( Çelik and Atak, 2012 ; Meriem et al., 2014 ; Sharif et al., 2017 ). However, the results of higher chlorophyll a , b , and the total chlorophyll content in the present study are in agreement with the studies previously conducted on amaranth ( Amaranthus tricolor ), sugar beet, and cabbage ( Wang and Nii, 2000 ; Jamil et al., 2007 ). These mentioned studies suggest that an increase in the chlorophyll content under salt stress could be due to the increased number of chloroplasts. Similar results on lettuce suggest that the increased chlorophyll content could be due to the accumulation of NaCl in the chloroplast ( Ekinci et al., 2012 ). According to Jamil et al. (2007) , tolerance of photosystem II (PSII) to high salt stress and increased chlorophyll content had played important in salinity tolerance of cabbage and sugar beet; therefore, it might be possible that PSII can play important role in salinity stress tolerance of water dropwort, but it needs further studies. Our results indicate that increased in chlorophyll content under salt stress could be helpful to grow water dropwort in the saline soils. Carotenoid is a type of antioxidant which helps in developing tolerance against salt stress in plants by reducing the free oxygen radicals ( Ali et al., 2017 ). In the current study, the carotenoid concentration was slightly increased under NaCl stress in both selected cultivars compared to the control, where V11E0022 showed relatively high concentration. A previous study also reported an increase in the carotenoids concentration under salt stress ( Çelik and Atak, 2012 ). As an antioxidant, carotenoids help reducing the singlet oxygen for preventing the oxidative damage.
Salt stress induces the concentration of Na + ions in the plant cells. The excess accumulation of Na + in the cytosol is concomitant with salt-induced K + efflux, and the cytosolic K + /Na + ratio decreases dramatically under salinity stress conditions ( Silva et al., 2015 ; Sarker and Oba, 2020b ). This might be linked with the fact that Na + enters the roots passively through voltage-independent or weakly voltage-dependent nonselective cation channels. This could be also linked with other Na + transporters, such as members of the high-affinity K + transporters. Thus, increase in the level of external Na + will sensibly rise the accumulation of Na + in the plants with concomitant decrease in K + uptake ( Odjegba and Chukwunwike, 2012 ; Silva et al., 2015 ; Sarker and Oba, 2020b ). Several authors have suggested that low uptake of Na + and high uptake of K + signifies salinity tolerance in higher plants ( Yassin et al., 2019 ). In the present study, a significant increase in Na + uptake and decrease in the K + uptake was observed in leaves and roots of both cultivars with increasing NaCl concentration, whereas the leaves showed more ionic uptake than the roots. Furthermore, high uptake of Na + was observed in the V11E0135 cultivar as compared to V11E0022. Previous studies on pistachio, paper mulberry, and wheat also showed a higher uptake of Na + in sensitive cultivars, which support the findings of the current study ( Zhang et al., 2013 ; Rahneshan et al., 2018 ; Yassin et al., 2019 ). Roots and shoots of the carrot and amaranth also showed high Na + uptake and decreased K + uptake under salt stress; moreover, the roots showed a considerably low concentration of Na + and K + compared to the shoots ( Inal et al., 2009 ; Menezes et al., 2017 ). In the current study, we found the higher level of Na + in the leaves as compared to the roots, and the reason is that leaves are most porn to Na + than roots because Na + and Cl − accumulate more in shoots than the roots. Roots maintain constant level of NaCl over time and can regulate NaCl levels by export to the shoots or to the soil. Na + is transported to shoots in the rapidly moving transpiration stream in the xylem ( Tester and Davenport, 2003 ). Different studies reported that salinity-tolerant plants either limit the excess salt in the vacuole or compartmentalize essential ions in different plant tissues. This compartmentalization of Na + into the vacuoles or its efflux across the plasma membrane is controlled by the expression and activity of Na + /H + antiporters, V-type H + -ATPase, and H + -PPase, which ultimately increased the K + /Na + ratio ( Türkan and Demiral, 2009 ; Tsujii et al., 2019 ). The results of these parameters in the present study suggest that a low-level uptake of Na + in V11E0022 might be due to these antiporters and membrane transporters, which can help to stand against the salt stress. RWC is considered a useful and reliable parameter to check the salt stress ( Sharif et al., 2017 ; Sarker and Oba, 2020b ). V11E0022 showed the low uptake of Na + and less reduction in K + under salt stress enabled the plant to retain more RWC compared to the V11E0135. Thus, V11E0022 is able to keep a high salt concentration and can absorb more water and consequently has high RWC to adjust osmotic pressure.
Lipid peroxidation is an indicator of oxidative damage caused by salt stress, and the higher concentration of MDA under stress represents the degree of cell membrane damage and it is a common physiological indicator for evaluating plant exposed to biotic or abiotic stress ( Sarker and Oba, 2020b ). In general, the salt-tolerant cultivars exhibit less lipid peroxidation and ROS production (H 2 O 2 ) compared to their sensitive counterparts, which is attributed to efficient protection mechanisms and predominantly high scavenging capacity of the tolerant cultivar ( Yassin et al., 2019 ). The results of the present study showed a significantly higher concentration of MDA and H 2 O 2 in V11E0135 compared to V11E0022 under salt stress, which is in agreement with the previous studies on different plants ( Wang et al., 2009 ; Shafeiee and Ehsanzadeh, 2019 ; Yassin et al., 2019 ; Sarker and Oba, 2020b ). In the current study, a minor decrease in MDA concentration was observed at 200 mm NaCl in the leaves of both cultivars. 200 mm NaCl might induces salt stress-related genes in the leaves of water dropwort ( Kumar et al., 2020 ). Sustained decreases in MDA accumulation might be due to the activation of PSII core proteins and Rubisco ( Morales and Munné-Bosch, 2019 ). A negative correlation between MDA content and electron transport was described by Morales and Munné-Bosch, which implies a feedback of PSII and lower MDA in salt-stressed plants. From the current study, we assume that the higher accumulation of MDA and H 2 O 2 has severely affected the phenotype of V11E0135 as compared to the V11E0022. To cope with this situation, the plants have a defense system in the form of osmolytes, antioxidant molecules, and antioxidant enzymes.
To regulate the osmotic potential, different compatible solute, such as proline, soluble sugars, proteins, and GSH, was accumulated in plants. The higher level of these compounds helps in selecting the tolerant cultivar under stress conditions ( Torabi et al., 2013 ; Sharif et al., 2017 ; Sarker and Oba, 2019 ). Accumulation of proline and soluble sugars under stress conditions protects the cell by maintaining the osmotic strength of cytosol with that of vacuole and external environment. In addition to its osmoprotection role, proline is prominently used against ROS as well as provide protection to enzymes and stabilize their structures ( Rahneshan et al., 2018 ; Alzahrani et al., 2019 ). Previous studies reported that the salinity tolerant cultivars of canola, coriander, and tobacco showed an increment in the proline content and soluble sugars, which is in agreement with the current results ( Çelik and Atak, 2012 ; Meriem et al., 2014 ; Sharif et al., 2017 ). Proline content increased with increasing NaCl stress in both roots and leaves of tolerant cultivar, whereas, in sensitive cultivar it starts to decrease after 100 mm NaCl. The decrease at 200 mm NaCl stress might be due to the low activity of enzymes (P5CS and glutamine dehydrogenase) of the proline biosynthetic pathway in V11E0135 ( Chun et al., 2018 ). Another reason might be proline dehydrogenase (ProDH), which is one of the key enzymes that regulates proline accumulation. Therefore, it might be possible that ProDH genes ( ProDH1 and ProDH2 ) expression has been decreased at higher concentration of NaCl in V11E0135 ( Funck et al., 2010 ; Chun et al., 2018 ).
The reason for the increment of soluble sugars might be the higher enzymatic activities that help in the regulation of cellular structures and functions through the interaction with macromolecules ( Sharif et al., 2017 ; Ibrahimova et al., 2019 ). The tolerant cultivars retain more water due to proline and sugars, and the present study also showed that the higher RWC of V11E0022 is due to the elevated concentrations of proline and soluble sugars, which improves the osmotic adjustment in water dropwort. Different studies revealed that the salt stress reduced the RWC in the plants, and a direct consequence of higher osmolytes in tolerant cultivar is the maintenance of comparatively higher RWC ( Karlidag et al., 2009 ; Alzahrani et al., 2019 ; Shafeiee and Ehsanzadeh, 2019 ).
Furthermore, proteins act as osmotin and their accumulation play a potential role developing tolerance against the salt stress ( Qados, 2011 ; Zhang et al., 2013 ; Sarker et al., 2018 ). Results of the current study showed that the protein content in leaves and roots of both cultivars was increased significantly under salt stress. The current results are in agreement with the previous studies conducted on Vicia faba , Broussonetia papyrifera , and Amaranthus tricolor , which showed an increment in the protein content in both roots and shoots under salt stress ( Qados, 2011 ; Zhang et al., 2013 ; Sarker et al., 2018 ). According to Yan et al. (2018) , the synthesis and accumulation of GSH can improve tolerance against biotic and abiotic stresses. Moreover, GSH helps in ROS scavenging by detoxifying the superoxide and hydroxyl radical ( Ashraf, 2009 ; Hussain et al., 2016 ). In the present study, a higher level of GSH was found in both cultivars of water dropwort under salt stress compared to the control. The roots of V11E0022 showed a higher level of GSH in comparison with its counterparts, it could help in developing salt tolerance. Likewise, studies carried out on wheat and onion showed the positive effect of GSH by improving cell viability under salt stress ( Aly-Salama and Al-Mutawa, 2009 ; Ahanger et al., 2019 ). Surprisingly, the leaves of V11E0135 showed higher GSH than the V11E0022, and this increase in leaves could be due to respiration, which plays a vital role in biosynthesis of GSH. Metabolites, such as glycine, are produced during the respiration that could be used in the biosynthesis of GSH ( Aly-Salama and Al-Mutawa, 2009 ). This higher GSH content is concomitant with a higher respiration rate in V11E0135. All these osmolytes and antioxidants might be responsible for osmotic adjustment as well as the reduction of ROS and oxidative stress, which enhance the tolerance of V11E0022 under salt stress.
Higher activities of antioxidant enzymes (SOD, POD, CAT, and APX) provide tolerance against salt stress by scavenging ROS, and the tolerant plants possess higher enzyme activities than the sensitive counterparts ( Ali et al., 2017 ; Polash et al., 2019 ). In a defense mechanism, the first line of defense is SOD that transforms the superoxides into H 2 O 2 . Thereafter, CAT further converts this H 2 O 2 into H 2 O and oxygen. Likewise, APX converts the H 2 O 2 into H 2 O. In addition to these, POD is also used to scavenge H 2 O 2 from the chloroplast efficiently ( Jalali-e-Emam et al., 2011 ; Polash et al., 2019 ). Similarly, GR converts the glutathione (GSSG) into reduced GSH which regulates the ROS removal ( Elsawy et al., 2018 ; Polash et al., 2019 ). Previous studies reported the higher activities of APX, SOD, POD, and CAT in response to salinity in tomato, cabbage, amaranth, and wheat ( Li, 2009 ; Ali et al., 2017 ; Sahin et al., 2018 ; Sarker and Oba, 2020b ).
Considering biomass and growth as the indicators for salt tolerance, we deduce that V11E0022 is more tolerant than the V11E0135, and this high tolerance could be attributed to better antioxidant enzyme activities viz SOD, POD, CAT, and APX, which reduced the H 2 O 2 and lipid peroxidation level in roots and leaves. SOD and POD of V11E0022 showed higher activity up to 100 mm in the leaves, whereas V11E0135 starts to decrease after 50 mm NaCl. The activity of SOD and POD in roots of both cultivars decreased after 50 mm NaCl, but comparatively higher activities were found in V11E0022. APX activity in the leaves of V11E0022 was increased with increasing NaCl concentration, whereas V11E0135 showed inverse relation with salt stress. Similarly, APX activity in roots was also found significantly higher in V11E0022 in comparison with V11E0135. CAT showed higher activities up to 100 mm in both leaves and roots of V11E0022; however, leaves of V11E0135 showed decrease in CAT activity with increasing NaCl concentration, whereas roots start to decrease after 50 mm NaCl. The observation of augmented antioxidant capacity of water dropwort up to 100 mm NaCl stress. Previous studies also reported the decreased antioxidant capacities after 100 and 150 mm NaCl stress in Vigna unguiculata , Brassica juncea, Oryza sativa , Morus alba , Broussonetia papyrifera and many other plants ( Verma and Mishra, 2005 ; Ahmad et al., 2010 ; Maia et al., 2010 ; Zhang et al., 2013 ; García-Caparrós et al., 2019 ). Moreover, the present study also suggests that the different parts of water dropwort may behave differently against the salt stress, which depend on the type of cellular metabolism of the plant part. The findings of antioxidant capacity also reveal that APX and CAT could be efficient markers for understanding the potential defense mechanisms of water dropwort under NaCl stress conditions compared to other enzymes.
Based on the phenotypic and physiological studies, we found that V11E0022 cultivar is tolerance against salt stress among the 48 cultivars, whereas V11E0135 is the most sensitive. Moreover, the tolerance of water dropwort could be due to the higher content of osmolytes and antioxidants, and better activities of APX, SOD, POD, and CAT, which reduced the level of H 2 O 2 , and MDA in roots and leaves of water dropwort. Comparatively higher K + /Na + ratio and higher concentration of proline and soluble sugars, which acts as osmoregulators helped in retaining higher water content in V11E0022. Based on the antioxidant defense system, it is suggested that this cultivar could efficiently tolerate the salt stress up to 100 mm NaCl. Furthermore, proline, GSH, APX, and CAT could play efficient roles in water dropwort under NaCl stress conditions compared to others and help to understand the salinity tolerance mechanism in water dropwort.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
All authors contributed to the manuscript. Conceptualization and funding acquisition: HH and WK. Data curation, investigation, validation, and writing—original draft: SK. Methodology: SK, XH, QJ, and ZL. Writing—review and editing: SK, GL, and HH. All authors have read and agreed to the published version of the manuscript.
This work was supported by the National Key R&D Program of China (grant number 2020YFD0900305), National Special Vegetable Industry Technology System of China (grant number CARS-24-A-02), and Crop Germplasm Resources Protection Project of the Ministry of Agriculture and Rural Areas (grant number 19200368).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are thankful to the Chinese Scholarship Council. Authors acknowledge all the staff members of the Institute of Vegetables, Wuhan Academy of Agricultural Sciences, Wuhan, Hubei Province, and also Xin Wang and Jun Men from The Analysis and Testing Center of Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China, for providing technical support and help during the experimental work.
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fpls.2021.660409/full#supplementary-material
Ahanger, M. A., Qin, C., Begum, N., Maodong, Q., Dong, X. X., El-Esawi, M., et al. (2019). Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 19:479. doi: 10.1186/s12870-019-2085-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Ahmad, P., Jaleel, C. A., and Sharma, S. (2010). Antioxidant defense system, lipid peroxidation, proline-metabolizing enzymes, and biochemical activities in two Morus alba genotypes subjected to NaCl stress. Russ. J. Plant Physiol. 57, 509–517. doi: 10.1134/S1021443710040084
CrossRef Full Text | Google Scholar
Akbari Ghogdi, E., Izadi-Darbandi, A., and Borzouei, A. (2012). Effects of salinity on some physiological traits in wheat ( Triticum aestivum L.) cultivars. Indian J. Sci. Technol. 5, 1901–1906. doi: 10.17485/ijst/2012/v5i1.23
Ali, Q., Daud, M. K., Zulqurnain, M., and Ali, S. (2017). Seed priming by sodium nitroprusside improves salt tolerance in wheat ( Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol. Biochem. 119, 50–58. doi: 10.1016/j.plaphy.2017.08.010
Aly-Salama, K. H., and Al-Mutawa, M. M. (2009). Glutathione-triggered mitigation in salt-induced alterations in plasmalemma of onion epidermal cells. Int. J. Agric. Biol. 11, 639–642.
Google Scholar
Alzahrani, S. M., Alaraidh, I. A., Migdadi, H., Alghamdi, S., Altaf Khan, M., and Ahmad, P. (2019). Physiological, biochemical, and antioxidant properties of two genotypes of Vicia faba grown under salinity stress. Pak. J. Bot. 51, 786–798. doi: 10.30848/PJB2019-3(3)
Ashraf, M. (2009). Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 27, 84–93. doi: 10.1016/j.biotechadv.2008.09.003
Atkin, O. K., and Macherel, D. (2009). The crucial role of plant mitochondria in orchestrating drought tolerance. Ann. Bot. 103, 581–597. doi: 10.1093/aob/mcn094
Beers, R. F., and Sizer, I. W. (1952). A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195, 133–140. doi: 10.1016/S0021-9258(19)50881-X
Çelik, Ö., and Atak, Ç. (2012). The effect of salt stress on antioxidative enzymes and proline content of two Turkish tobacco varieties. Turk. J. Biol. 36, 339–356. doi: 10.3906/biy-1108-11
Chan, E. W. C., Wong, S. K., and Chan, H. T. (2017). Ulam herbs of Oenanthe javanica and Cosmos caudatus : an overview on their medicinal properties. J. Nat. Remedies 16, 137–147. doi: 10.18311/jnr/2016/8370
Chance, B., and Maehly, A. C. (1955). Assay of catalases and peroxidases. Methods Enzymol. 2, 764–775. doi: 10.1016/S0076-6879(55)02300-8
Chun, S. C., Paramasivan, M., and Chandrasekaran, M. (2018). Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 9:2525. doi: 10.3389/fmicb.2018.02525
Colomer-Winter, C., Flores-Mireles, A. L., Baker, S. P., Frank, K. L., Lynch, A. J. L., Hultgren, S. J., et al. (2018). Manganese acquisition is essential for virulence of Enterococcus faecalis . PLoS Pathog. 14:e1007102. doi: 10.1371/journal.ppat.1007102
Dai, W., Wang, M., Gong, X., and Liu, J. H. (2018). The transcription factor FcWRKY40 of Fortunella crassifolia functions positively in salt tolerance through modulation of ion homeostasis and proline biosynthesis by directly regulating SOS2 and P5CS1 homologs. New Phytol. 219, 972–989. doi: 10.1111/nph.15240
Ekinci, M., Yildirim, E., Dursun, A., and Turan, M. (2012). Mitigation of salt stress in lettuce ( Lactuca sativa L. var. Crispa) by seed and foliar 24-epibrassinolide treatments. HortScience 47, 631–636. doi: 10.21273/HORTSCI.47.5.631
Elsawy, H. I. A., Mekawy, A. M. M., Elhity, M. A., Abdel-dayem, S. M., Abdelaziz, M. N., Assaha, D. V. M., et al. (2018). Differential responses of two Egyptian barley ( Hordeum vulgare L.) cultivars to salt stress. Plant Physiol. Biochem. 127, 425–435. doi: 10.1016/j.plaphy.2018.04.012
Funck, D., Eckard, S., and Müller, G. (2010). Non-redundant functions of two proline dehydrogenase isoforms in Arabidopsis . BMC Plant Biol. 10:70. doi: 10.1186/1471-2229-10-70
García-Caparrós, P., Hasanuzzaman, M., and Lao, M. T. (2019). “Oxidative stress and antioxidant defense in plants under salinity,” in Reactive Oxygen, Nitrogen and Sulfur Species in Plants. eds. M. Hasanuzzaman, V. Fotopoulos, K. Nahar, and M. Fujita (Hoboken: Wiley), 291–309.
Gharsallah, C., Fakhfakh, H., Grubb, D., and Gorsane, F. (2016). Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants 8:plw055. doi: 10.1093/aobpla/plw055
Hasanuzzaman, M., Oku, H., Nahar, K., Bhuyan, M. H. M. B., Al, J., Baluska, F., et al. (2018). Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol. Rep. 12, 77–92. doi: 10.1007/s11816-018-0480-0
Hoagland, D. R., and Arnon, D. I. (1950). The Water-Culture Method for Growing Plants Without Soil. 2nd Edn . Vol . 347. California: Agricultural Experiment Station, 1–32.
Hussain, S., Khan, F., Hussain, H. A., and Nie, L. (2016). Physiological and biochemical mechanisms of seed priming-induced chilling tolerance in rice cultivars. Front. Plant Sci. 7:116. doi: 10.3389/fpls.2016.00116
Ibrahimova, U. F., Mammadov, A. C., and Feyziyev, Y. M. (2019). The effect of NaCl on some physiological and biochemical parameters in Triticum aestivum L. genotypes. Plant Physiol. Rep. 24, 370–375. doi: 10.1007/s40502-019-00461-z
Inal, A., Gunes, A., Pilbeam, D. J., Kadloglu, Y. K., and Eraslan, F. (2009). Concentrations of essential and nonessential elements in shoots and storage roots of carrot grown in NaCl and Na 2 SO 4 salinity. X-Ray Spectrom. 38, 45–51. doi: 10.1002/xrs.1104
Islam, F., Wang, J., Farooq, M. A., Yang, C., Jan, M., Mwamba, T. M., et al. (2019). “Rice responses and tolerance to salt stress,” in Advances in Rice Research for Abiotic Stress Tolerance. eds. M. Hasanuzzaman, M. Fujita, K. Nahar, and J. Biswas (Cambridge: Woodhead Publishing), 791–819.
Jalali-e-Emam, S. M. S., Alizadeh, B., Zaefizadeh, M., Zakarya, R. A., and Khayatnezhad, M. (2011). Superoxide dismutase (SOD) activity in NaCl stress in salt-sensitive and salt-tolerance genotypes of colza ( Brassica napus L.). Middle-East J. Sci. Res. 7, 7–11.
Jamil, M., Rehman, S., and Rha, E. S. (2007). Salinity effect on plant growth, PSII photochemistry and chlorophyll content in sugar beet ( Beta vulgaris L.) and cabbage ( Brassica oleracea capitata L.). Pak. J. Bot. 39, 753–760.
Jeon, H. R., Abd El-Aty, M., Cho, S. K., Choi, J., Kim, K., Park, R., et al. (2007). Multiresidue analysis of four pesticide residues in water dropwort ( Oenanthe javanica ) via pressurized liquid extraction, supercritical fluid extraction, and liquid–liquid extraction and gas chromatographic determination. J. Sep. Sci. 30, 1953–1963. doi: 10.1002/jssc.200600548
Jiang, Q., Wang, F., Tan, H. W., Li, M. Y., Xu, Z. S., Tan, G. F., et al. (2015). De novo transcriptome assembly, gene annotation, marker development, and miRNA potential target genes validation under abiotic stresses in Oenanthe javanica . Mol. Gen. Genomics 290, 671–683. doi: 10.1007/s00438-014-0953-y
Kapoor, N., and Pande, V. (2015). Effect of salt stress on growth parameters, moisture content, relative water content and photosynthetic pigments of fenugreek variety RMt-1. J. Plant Sci. 10, 210–221. doi: 10.3923/jps.2015.210.221
Karlidag, H., Yildirim, E., and Turan, M. (2009). Salicylic acid ameliorates the adverse effect of salt stress on strawberry. Sci. Agric. 66, 180–187. doi: 10.1590/S0103-90162009000200006
Khan, A., Anwar, Y., Hasan, M., Iqbal, A., Ali, M., Alharby, H., et al. (2017). Attenuation of drought stress in Brassica seedlings with exogenous application of Ca 2+ and H 2 O 2 . Plan. Theory 6:20. doi: 10.3390/plants6020020
Kumar, S., Li, G., Huang, X., Ji, Q., Zhou, K., Hou, H., et al. (2021). Phenotypic, nutritional, and antioxidant characterization of blanched Oenanthe javanica for preferable cultivar. Front. Plant Sci. 12:639639. doi: 10.3389/fpls.2021.639639
Kumar, S., Li, G., Yang, J., Huang, X., Ji, Q., Zhou, K., et al. (2020). Investigation of an antioxidative system for salinity tolerance in Oenanthe javanica . Antioxidants 9:940. doi: 10.3390/antiox9100940
Lee, J. H., and Kim, H. R. (2009). Influence of pretreatments on the dehydration characteristics during vacuum drying of water dropwort ( Oenanthe javanica DC.). J. Food Process. Preserv. 34, 397–413. doi: 10.1111/j.1745-4549.2008.00319.x
Li-ping, L., Xiao-hua, L., Hong-bo, S., Zhao-Pu, L., Ya, T., Quan-suo, Z., et al. (2015). Ameliorants improve saline-alkaline soils on a large scale in northern Jiangsu Province, China. Ecol. Eng. 81, 328–334. doi: 10.1016/j.ecoleng.2015.04.032
Li, Y. (2009). Physiological responses of tomato seedlings ( Lycopersicon esculentum ) to salt stress. Mod. Appl. Sci. 3, 171–176. doi: 10.5539/mas.v3n3p171
Lu, C., and Li, X. (2019). A review of Oenanthe javanica (Blume) DC. as traditional medicinal plant and its therapeutic potential. Evidence-Based Complement. Altern. Med. 2019, 1–17. doi: 10.1155/2019/6495819
Maia, J. M., de Macedo, C. E. C., Voigt, E. L., Freitas, J. B. S., and Silveira, J. A. G. (2010). Antioxidative enzymatic protection in leaves of two contrasting cowpea cultivars under salinity. Biol. Plant. 54, 159–163. doi: 10.1007/s10535-010-0026-y
McCord, J. M., and Fridovich, I. (1969). Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244, 6049–6055. doi: 10.1016/S0021-9258(18)63504-5
Menezes, R. V., Azevedo Neto, A. D. D., Ribeiro, M. D. O., and Cova, A. M. W. (2017). Growth and contents of organic and inorganic solutes in amaranth under salt stress. Pesqui. Agropecuária Trop. 47, 22–30. doi: 10.1590/1983-40632016v4742580
Meriem, B. F., Kaouther, Z., Chérif, H., Tijani, M., André, B., Meriem, B. F., et al. (2014). Effect of priming on growth, biochemical parameters and mineral composition of different cultivars of coriander ( Coriandrum sativum L.) under salt stress. J. Stress Physiol. Biochem. 10, 84–109.
Morales, M., and Munné-Bosch, S. (2019). Malondialdehyde: facts and artifacts. Plant Physiol. 180, 1246–1250. doi: 10.1104/pp.19.00405
Nakano, Y., and Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880. doi: 10.1093/oxfordjournals.pcp.a076232
Odjegba, V. J., and Chukwunwike, I. C. (2012). Physiological responses of Amaranthus hybridus L. under salinity stress. Indian J. Innov. Dev. 1, 742–748.
Okkaoğlu, H., Sönmez, Ç., Şimşek, A. Ö., and Bayram, E. (2015). Effect of salt stress on some agronomical characteristics and essential oil content of coriander ( Coriandrum sativum L.) cultivars. J. Appl. Biol. Sci. 9, 21–24.
Polash, M. A. S., Sakil, M. A., and Hossain, M. A. (2019). Plants responses and their physiological and biochemical defense mechanisms against salinity: a review. Trop. Plant Res. 6, 250–274. doi: 10.22271/tpr.2019.v6.i2.35
Qados, A. M. S. A. (2011). Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). J. Saudi Soc. Agric. Sci. 10, 7–15. doi: 10.1016/j.jssas.2010.06.002
Queirós, F., Fontes, N., Silva, P., Almeida, D., Maeshima, M., Gerós, H., et al. (2009). Activity of tonoplast proton pumps and Na + /H + exchange in potato cell cultures is modulated by salt. J. Exp. Bot. 60, 1363–1374. doi: 10.1093/jxb/erp011
Rahneshan, Z., Nasibi, F., and Moghadam, A. A. (2018). Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio ( Pistacia vera L.) rootstocks. J. Plant Interact. 13, 73–82. doi: 10.1080/17429145.2018.1424355
Sahin, U., Ekinci, M., Ors, S., Turan, M., Yildiz, S., and Yildirim, E. (2018). Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage ( Brassica oleracea var. capitata ). Sci. Hortic. 240, 196–204. doi: 10.1016/j.scienta.2018.06.016
Sanower-Hossain, M. (2019). Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci. 1, 1–3.
Sarker, U., Islam, M. T., and Oba, S. (2018). Salinity stress accelerates nutrients, dietary fiber, minerals, phytochemicals and antioxidant activity in Amaranthus tricolor leaves. PLoS One 13:e0206388. doi: 10.1371/journal.pone.0206388
Sarker, U., and Oba, S. (2018). Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor . Appl. Biochem. Biotechnol. 186, 999–1016. doi: 10.1007/s12010-018-2784-5
Sarker, U., and Oba, S. (2019). Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J. Sci. Food Agric. 99, 2275–2284. doi: 10.1002/jsfa.9423
Sarker, U., and Oba, S. (2020a). Nutritional and bioactive constituents and scavenging capacity of radicals in Amaranthus hypochondriacus . Sci. Rep. 10:19962. doi: 10.1038/s41598-020-71714-3
Sarker, U., and Oba, S. (2020b). The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. 11:559876. doi: 10.3389/fpls.2020.559876
Shafeiee, M., and Ehsanzadeh, P. (2019). Physiological and biochemical mechanisms of salinity tolerance in several fennel genotypes: existence of clearly-expressed genotypic variations. Ind. Crop. Prod. 132, 311–318. doi: 10.1016/j.indcrop.2019.02.042
Shaheen, S., Naseer, S., Ashraf, M., and Akram, N. A. (2013). Salt stress affects water relations, photosynthesis, and oxidative defense mechanisms in Solanum melongena L. J. Plant Interact. 8, 85–96. doi: 10.1080/17429145.2012.718376
Sharif, P., Seyedsalehi, M., Paladino, O., Damme, P., Van Sillanpa, M., and Sharifi, A. A. (2017). Effect of drought and salinity stresses on morphological and physiological characteristics of canola. Int. J. Environ. Sci. Technol. 15, 1859–1866. doi: 10.1007/s13762-017-1508-7
Silva, E. N., Silveira, J. A. G., Rodrigues, C. R. F., and Viégas, R. A. (2015). Physiological adjustment to salt stress in Jatropha curcas is associated with accumulation of salt ions, transport and selectivity of K + , osmotic adjustment and K + /Na + homeostasis. Plant Biol. 17, 1023–1029. doi: 10.1111/plb.12337
Singh, M., Kumar, J., Singh, V. P., and Prasad, S. M. (2014). Plant tolerance mechanism against salt stress: the nutrient management approach. Biochem. Pharmacol. 3:e165. doi: 10.4172/2167-0501.1000e165
Soares, C., Carvalho, M. E. A., Azevedo, R. A., and Fidalgo, F. (2019). Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 161, 4–25. doi: 10.1016/j.envexpbot.2018.12.009
Tester, M., and Davenport, R. (2003). Na + tolerance and Na + transport in higher plants. Ann. Bot. 91, 503–527. doi: 10.1093/aob/mcg058
Torabi, M., Halim, R. A., Mokhtarzadeh, A., and Miri, Y. (2013). “Physiological and biochemical responses of plants in saline environment,” in Crop Biology and Agriculture in Harsh Environments. ed. R. Roychowdhury (Chisinau: Lap Lambert Academic Publishing), 47–80.
Tsujii, M., Kera, K., Hamamoto, S., Kuromori, T., Shikanai, T., and Uozumi, N. (2019). Evidence for potassium transport activity of Arabidopsis KEA1-KEA6. Sci. Rep. 9:10040. doi: 10.1038/s41598-019-46463-7
Türkan, I., and Demiral, T. (2009). Recent developments in understanding salinity tolerance. Environ. Exp. Bot. 67, 2–9. doi: 10.1016/j.envexpbot.2009.05.008
Verma, S., and Mishra, S. N. (2005). Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J. Plant Physiol. 162, 669–677. doi: 10.1016/j.jplph.2004.08.008
Wang, W., Kim, Y., Lee, H., Kim, K., Deng, X., and Kwak, S. (2009). Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 47, 570–577. doi: 10.1016/j.plaphy.2009.02.009
Wang, Y., and Nii, N. (2000). Changes in chlorophyll, ribulose bisphosphate carboxylase-oxygenase, glycine betaine content, photosynthesis and transpiration in Amaranthus tricolor leaves during salt stress. J. Hortic. Sci. Biotechnol. 75, 623–627. doi: 10.1080/14620316.2000.11511297
Yan, Z., Ming, D., Jin-xia, C. U. I., Xian-jun, C., Ze-lin, W. E. N., Jian-wei, Z., et al. (2018). Exogenous GSH protects tomatoes against salt stress by modulating photosystem II efficiency, absorbed light allocation and H 2 O 2 scavenging system in chloroplasts. J. Integr. Agric. 17, 2257–2272. doi: 10.1016/S2095-3119(18)62068-4
Yang, J., Li, G., Bishopp, A., Heenatigala, P. P. M., Hu, S., Chen, Y., et al. (2018). A comparison of growth on mercuric chloride for three Lemnaceae species reveals differences in growth dynamics that effect their suitability for use in either monitoring or remediating ecosystems contaminated with mercury. Front. Chem. 6:112. doi: 10.3389/fchem.2018.00112
Yassin, M., El Sabagh, A., Mekawy, A. M., Islam, M., Hossain, A., Barutcular, C., et al. (2019). Comparative performance of two bread wheat ( Triticum aestivum L.) genotypes under salinity stress. Appl. Ecol. Environ. Res. 17, 5029–5041. doi: 10.15666/aeer/1702_50295041
Zhang, J., Zhao, X. X., Wang, X., and Lu, W. X. (2015). Effects of cadmium stress on the growth and physiological property of Oenanthe javanica . Plant Physiol. J. 51, 1969–1974. doi: 10.13592/j.cnki.ppj.2015.0457
Zhang, M., Fang, Y., Ji, Y., Jiang, Z., and Wang, L. (2013). Effects of salt stress on ion content, antioxidant enzymes and protein profile in different tissues of Broussonetia papyrifera . S. Afr. J. Bot. 85, 1–9. doi: 10.1016/j.sajb.2012.11.005
Keywords: antioxidants, reactive oxygen species, ions, growth, NaCl, water dropwort
Citation: Kumar S, Li G, Yang J, Huang X, Ji Q, Liu Z, Ke W and Hou H (2021) Effect of Salt Stress on Growth, Physiological Parameters, and Ionic Concentration of Water Dropwort ( Oenanthe javanica ) Cultivars. Front. Plant Sci . 12:660409. doi: 10.3389/fpls.2021.660409
Received: 29 January 2021; Accepted: 21 May 2021; Published: 21 June 2021.
Reviewed by:
Copyright © 2021 Kumar, Li, Yang, Huang, Ji, Liu, Ke and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weidong Ke, [email protected] ; Hongwei Hou, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 18 November 2021

Global predictions of primary soil salinization under changing climate in the 21st century
- Amirhossein Hassani ORCID: orcid.org/0000-0002-6470-0490 1 , 2 ,
- Adisa Azapagic ORCID: orcid.org/0000-0003-2380-918X 1 &
- Nima Shokri ORCID: orcid.org/0000-0001-6799-4888 3
Nature Communications volume 12 , Article number: 6663 ( 2021 ) Cite this article
27k Accesses
182 Citations
30 Altmetric
Metrics details
- Biogeochemistry
- Climate change
- Environmental chemistry
Soil salinization has become one of the major environmental and socioeconomic issues globally and this is expected to be exacerbated further with projected climatic change. Determining how climate change influences the dynamics of naturally-occurring soil salinization has scarcely been addressed due to highly complex processes influencing salinization. This paper sets out to address this long-standing challenge by developing data-driven models capable of predicting primary (naturally-occurring) soil salinity and its variations in the world’s drylands up to the year 2100 under changing climate. Analysis of the future predictions made here identifies the dryland areas of South America, southern and western Australia, Mexico, southwest United States, and South Africa as the salinization hotspots. Conversely, we project a decrease in the soil salinity of the drylands in the northwest United States, the Horn of Africa, Eastern Europe, Turkmenistan, and west Kazakhstan in response to climate change over the same period.
Similar content being viewed by others
New water accounting reveals why the Colorado River no longer reaches the sea
Brian D. Richter, Gambhir Lamsal, … John C. Schmidt
Climate velocities and species tracking in global mountain regions
Wei-Ping Chan, Jonathan Lenoir, … Sheng-Feng Shen

Historical impacts of grazing on carbon stocks and climate mitigation opportunities
Shuai Ren, César Terrer, … Dan Liu
Introduction
The Soil Science Society of America 1 defines saline soil as a non-sodic soil containing sufficient amount of soluble salt which could adversely influence most crop plants. Conventionally, electrical conductivity of a saturated soil paste extract (EC e ) has been used as a measure of the soil salinity 2 . Soil salinization is a land degradation process that results in excessive accumulation of soluble salts in the soil 3 , 4 . In naturally occurring or primary soil salinization, the predominant origins of soluble salts are rainfall (wet deposition of oceanic salts), aeolian processes (dry deposition of oceanic salts), and physical or chemical weathering of parent rock materials 5 , 6 . Transport of the accumulated salts from saline geological depositions by streamflow or shallow underground waters is an additional source of primary salinization 7 . In anthropogenic or secondary soil salinization, however, the main sources of salinization are human interventions, such as irrigation with brackish or saline water, rising water tables due to poor land and water management, surface or subsurface sea water intrusion into coastal aquifers as a result of rising sea levels or over-exploitation of the fresh underground waters, and overuse of fertilizers 5 , 7 , 8 .
Excessive accumulation of the soluble salts in the root zone may go beyond the salt tolerance of plants, affecting adversely the growth rate of the plants 9 . A soil with salinity of EC e ≥ 2 dS m −1 (at 25 °C) is traditionally considered as a saline soil 10 ; however, depending on the plant type, climatic conditions, and soil-water balance properties, the salt tolerance of sensitive crops and plants can be different 11 . Salinity stress deteriorates the plants’ transpiring leaves which is known as specific ion effects 12 or directly reduces the plant water uptake from the rooting zone, resulting in osmotic stress on the plant 13 , 14 . Soil salinity also imposes nutritious imbalances in plants 6 . Soil salinity between 2 and 4 dS m −1 can negatively impact the yields of sensitive plants and at salinity levels higher than 8 dS m −1 , the growth of most of crops and plants shows a severe decrease in response to excessive soil salinity 1 , 15 . Vegetation loss in turn reduces the soil stability and exposes the soil to wind or water erosion 16 . In addition to deleterious effects on vegetation, excessive soil salinity decreases the biological functioning of the soil micro-organisms to a level that disturbs the soil nitrogen cycle, respiration, and organic matter input 17 , 18 . Reduced environmental health due to aeolian dispersion of saline dust originated from the saline soils 16 , 19 , land abandonment and desertification 20 , 21 , worsening of economic welfare, and human migration are other detrimental consequences of excessive soil salinity 6 , 19 .
Accurate and reliable data on spatial distribution of salt-affected soils are important to develop action plans for management of soil, water, and vegetation and will contribute toward data-driven policy making 22 , 23 , 24 . These data have also implications for tuning large-scale agro-ecological models 25 and planning sustainable reclamation practices 26 . With varying levels of accuracy and spatial coverage, from the local 27 , 28 , 29 to the global scale 30 , 31 , 32 , 33 , defining the spatial distribution and location of salt-affected soils has been under focus of various studies. According to the global-scale studies, salt-affected soils lie across all climate zones and continents with an estimated global area of ~8.31–11.73 Mkm 2 , depending on the methods used for estimation of area of the salt-affected soils. Nevertheless, the general consensus is that the saline and salt-affected soils (including sodic soils) are particularly found in drylands where the excess of evaporation over water input to the soil accumulates salts in the upper soil layer 3 , 34 , 35 .
Drylands, including hyper-arid, arid, semi-arid, and dry sub-humid lands, are characterized by a multi-annual Aridity Index (AI) of less than 0.65 mm mm −1 , computed as the ratio of total precipitation to potential evapotranspiration 36 , 37 . Drylands occupy a total of ~45% of the Earth’s surface 38 , 39 . With the advance of proximal/remote sensors and digital soil mapping techniques, there is a rising interest in spatio-temporal mapping and monitoring of the soil salinity 40 , 41 , 42 . Due to the temporal and vertical variability in salinity levels of the salt-affected soils 5 , 42 , updated predictions on long-term variations of soil salinity can provide a clearer understanding of the dynamics of the terrestrial carbon sink 43 , climate change impacts 44 , and alterations in the land, vegetation, and water resources 45 . Even though the above-mentioned purely spatial or spatio-temporal studies have substantially advanced our understanding of the current status of the salt-affected soils and processes involved in salinization, predictions of the future extent and dynamics of soil salinization at the global scale are still missing, partly due to the complex processes and many parameters influencing soil salinization at the global scale. This makes the future prediction of soil salinization in the face of future climate uncertainties a grand challenge, which is precisely one of the key objectives of the present investigation.
The projected hydrological consequences of climate change may result in physical, biological, biochemical, and chemical degradation of the soils 46 . As one of the major threats to soil stability, fertility, and biodiversity, it is expected that the soil salinity will be a significant and growing concern in a warmer world 47 , 48 . To formulate appropriate plans for sustainable management of soil, water, and vegetation, reliable predictions on the probable occurrence and expansion or shrinkage of the salt-affected soils in response to the threat of climate change are crucial. Compared to other dynamic soil properties, such as P, N, and organic matter content, prediction of soil-salinity responses to climate variability on a global scale has received much less attention 49 . The available literature on the effect of climate change as a source of soil salinization is mainly descriptive and quantitative predictions of the future status of salt-affected soils on the basis of current trends are rare. The IPCC report 50 predicts that climate change will likely impact all the primary mechanisms for soil salinization, including soluble salts accumulation due to a change in hydrological balance, sea salt intrusion, and wind-born salt deposition. An increase in the rate of evapotranspiration and alteration in precipitation patterns, particularly in arid and semi-arid areas, is expected to reduce the soil leaching efficiency and consequently, increase the salt concentrations in top-soil horizons 51 , 52 , 53 . Expansion of irrigated areas and the higher demand for water use under rising global temperatures, in combination with poor drainage/irrigation practices, are expected to result in the spread of secondary salinization 54 . Land use modifications and occurrence of more extreme climate events, such as prolonged droughts followed by severe floods, have the potential to release and redistribute large amount of salts from the geological substrates with high concentration of salts and may put new areas at risk of soil salinization 55 . In addition, rising sea levels and unsustainable extraction of freshwater resources from coastal aquifers can worsen the issue of sea water-induced soil salinization in coastal regions 53 , 56 .
A few studies investigated some aspects of the relationship between projected climate change and soil salinization. Szabolcs 51 was among the first who estimated that the salt-affected areas in North Mediterranean regions will be doubled by 2050 in response to 1 °C increase in the average annual temperature. Similarly, National Land and Water Resources Audit 45 estimated that Australia’s drylands at risk of soil salinity imposed by dryland management actions may expand to 170,000 km 2 in 2050, relative to approximately 57,000 km 2 in 2000. Schofield et al. 57 developed a set of soil salinization indicators including low relief, high two-way annual moisture flux, and local flow deficit in large catchments to identify the current and future (2079–2099) locations with salinization potential across the globe and concluded that areas at risk of soil salinity are expanding. Although these studies provide an understanding of the salinization potential and limitations of the methods used for projecting the soil salinity, they are not based on up-to-date datasets and they mainly highlight the areas at risk; no quantitative and spatially explicit predictions are provided. Other studies on predicting impacts of climate change on soil salinization are mainly focused on predicting secondary salinization processes imposed by unsustainable irrigation practices 58 , 59 , 60 or sea water intrusion 61 , 62 , 63 at local scales. Thus, there is a need for a quantitative global-scale analysis, characterizing the geographical distribution and projecting the long-term variations in soil salinity in the face of future climate fluctuations and uncertainties, which motivated the present investigation.
This study is among the initial attempts for addressing the need for a quantitative tool capable of predicting long-term primary soil salinity on a global scale with a high spatial and temporal resolution. These models and the results will be of interest to local authorities, land managers, and policy makers, helping to plan mitigation of and adaptation to soil salinization. In particular, we performed comprehensive data-driven modelling and analyses to reveal how the projected or hypothesized variations in the key drivers may influence primary soil salinity on the global scale, in both mid- (2031–2060) and long-term (2071–2100) futures. We only focus on soil salinity in the top-soil horizon (0–1 m), quantified by the concentration of soluble salts which is expressed by the extent of EC e . Other aspects of salt-affected soils, such as sodicity (which is traditionally measured by the soil exchangeable sodium percentage) or alkalinity, are not within the scope of this analysis. The potential soil salinity caused by sea level rise, saline groundwater, or irrigation is also excluded from the study. Note that modelling the salinity intrusion in coastal areas in response to sea rise needs a relatively precise estimation of the future groundwater extraction from the coastal aquifer. Similarly, projected data of groundwater level and salinity change (either natural or anthropogenic) are needed for predicting the groundwater-induced soil salinity, which is not currently available. As mentioned in Yeo 54 , it is difficult to generate a clear prediction of the impacts of climate change on the extent of salinization caused by irrigation as this requires reliable estimations of irrigation expansion and the quality of irrigation water in future. Therefore, this study can be deemed as projection of the primary soil salinization under future climate uncertainty.
Several numerical methods have been developed to simulate the soil salinization by considering different modes of mass transfer mechanisms transporting solute in unsaturated soil (such as Corwin et al. 64 , Schoups et al. 65 ); however, the application of these models remains limited to small-scale simulations where the detailed soil characteristics data are available. Moreover, employing analytical approaches, such as the stochastic model of soil salinity 66 , 67 or the developed frameworks for mechanistic modelling of the climate, vegetation, and soil salinity interactions 68 , 69 , 70 , would be applicable for projecting soil salinity only if the initial soil salinity or required calibration parameters for tuning were available; currently, such data are not available, particularly on a global scale. As a result of these practical limitations, we utilize Machine Learning (ML) algorithms as an alternative approach to predict the future of primary soil salinization on a global scale.
Recent studies demonstrated the great potential of ML algorithms in digital soil mapping and predicting spatio-temporal properties of the soil 71 . In the present study, we used supervised ML algorithms for projecting the long-term (up to year 2100) variations in soil salinity. In summary, the methodology included exposure of a known set of input data (predictors) and a set of known responses (soil salinity profiles) to ML models to develop trained models based on the relations between the two sets. The trained models were later applied to a new set of known input data (with unknown responses) to generate predictions for the response (see Methods).
Dryland areas are generally known as the regions with the highest vulnerability to hydro-climatic consequences of climate change 7 . For this reason, the majority of our measured input soil-profiles data were sampled from the dryland areas of the world. We made predictions of soil EC e only for the dryland areas with an AI ≤ 0.65 37 as extrapolation of the ML results to other areas is a matter of uncertainty 72 . The rest of this paper discusses the significance of the predictors and global variation in primary soil salinization at the grid-cell level, followed by the country-level analysis. Changes in the total area of drylands with an EC e ≥ 2 dS m −1 (and EC e ≥ 4 dS m −1 ) at the country and continental levels are also presented. Finally, methods and their limitations are discussed.
Predictors’ significance and their relation to the predicted soil salinity
Supplementary Table 1 shows the estimates of the predictor importance for the trained models based on the output of the GCMs used for spatio-temporal prediction of the EC e (see Methods for details of predictors and trained models). The percentage values reported in Supplementary Table 1 indicate the relative importance of each predictor in the final trained model in each input dataset. Among the 14 applied predictors, the long-term (5-year average) annual precipitation frequency is relatively the most influential soil predictors with an overall importance of 14% for all 16 best-fitted models. WRB soil classes and daily evapotranspiration are, respectively, the second and the third influential environmental predictors in estimation of the soil EC e with the overall importance of 13.07% and 9.26%, respectively.
The effect of each of the 13 non-categorical predictors (see Methods) on the predicted outcome of the trained models is shown in Supplementary Fig. 1 (Partial Dependency Plots, PDPs). The effect of long-term daily wet and dry deposition rates of sea salts are presented in Supplementary Fig. 2 . Supplementary Fig. 1a, b suggest that shallower depths are not necessarly associated with higher EC e in soil under natural conditions. However, in many previous experimental, analytical and numerical investigations 73 , 74 , 75 , 76 , 77 , higher solute concentrations and solute precipitation close to the evaporation surface were observed when the Peclet number (quantifying the relative importance of chemical diffusion and advection) was greater than the one during saline water evaporation from porous media. It must be noted that under natural environmental conditions (which is the case in our investigation), many parameters influence the complex dynamics of solute transport and deposition in soil, including the vegetation and land cover, rainfall, micro-organisms’ activities, depth of water tables, soil chemical compositions and heterogeneity, human interventions, and land-atmosphere interactions. These parameters, which could not be included in the majority of the previous experiments conducted under well-controlled laboratory conditions or numerical simulations, could induce significant impacts on solute distribution in soil under natural conditions 3 , 29 .
Fine-textured soils (soils with the higher clay content) show higher Water Holding Capacity (WHC, the difference between field capacity and wilting point) and lower saturated hydraulic conductivity. Overall, the predicted EC e values provided by each of the 16 trained models show a reverse relation with the soil clay content and WHC which is in line with previous experimental results 78 and a literature review 78 (Supplementary Fig. 1c, g, h ). Similarly, based on numerical, experimental, and field-scale investigations, Shokri‐Kuehni et al. 79 concluded that soil salinity for coarse‐textured soils is greater than for medium and fine‐textured soils when the water table is shallow and hydraulically connected to the evaporation surface. Our predicted results regarding the effects of soil texture on soil salinity are generally in agreement with the above-mentioned physically based determined trends and behaviour.
Moreover, the analysis of PDPs shows that the effective plant rooting depth influences the predicted EC e approximately up to the depth of 4 m. The PDPs also demonstrate a strong negative correlation between soil salinity and terrain elevation, topographic slope, and precipitation frequency (Supplementary Fig. 1d, e, i ). These correlations can be explained by the prior pedologic knowledge: the lower hillslope and the higher precipitation frequency result in more efficient leaching of the salts accumulated in the root zone 66 , resulting in lower salinity. The relationship between the predicted soil EC e values and other predictors, however, is more complicated and deriving general trends remains a challenge.
Projected soil salinity in drylands up to the year 2100
The trained models based on the output of Global Circulation Models (GCMs) were applied to new input predictor data to estimate the annual soil salinity for each grid-cell (0.5° spatial resolution) of the global soil base map of the drylands between 1904 and 2100 (see Methods for details of GCMs, predictors, and trained models). Figure 1 shows the spatial distribution of the change in primary soil EC e projected by the multi-model ensembles in the mid- (2031–2060) and long-terms (2071–2100), relative to the reference period (1961–1990) at the 0.5° spatial resolution. The RCP 4.5 and RCP 8.5 scenarios (Representative Concentration Pathways which result in a respective radiative forcing of 4.5 and 8.5 W m −2 in year 2100, relative to pre-industrial conditions) are related to CMIP5 (Coupled Model Inter-comparison Project Phase 5 80 ) data project, while the SSP 2-4.5 and SSP 5-8.5 scenarios (projections forced by RCP 4.5 and RCP 8.5 global forcing pathways for the Shared Socio-economic Pathways 2 and 5) refer to CMIP6 (CMIP Phase 6 81 ).
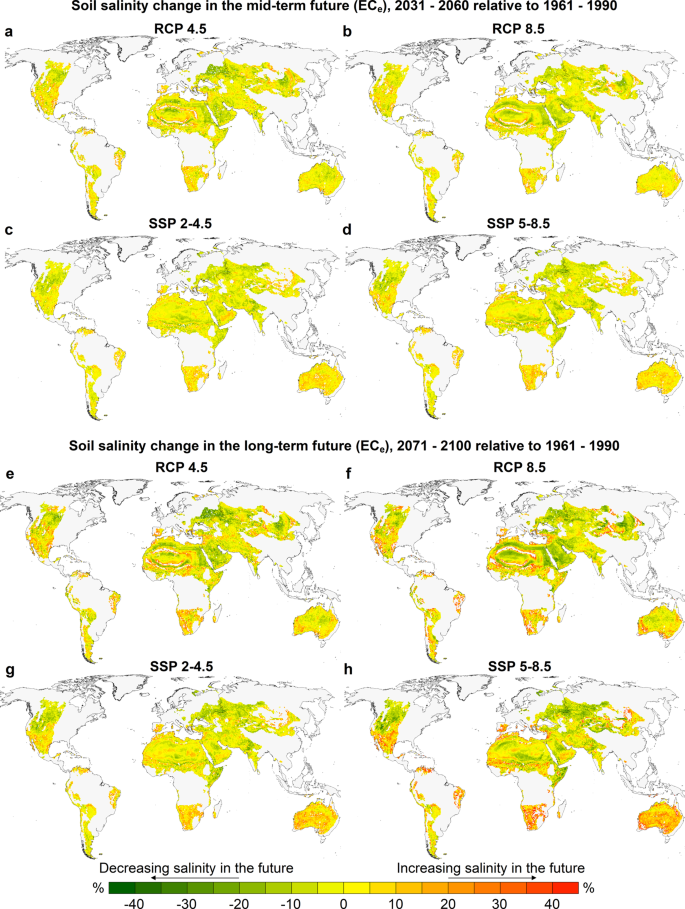
a – d Mid-term prediction of changes in EC e (2031–2060). e – h Long-term prediction of changes in EC e (2071–2100). The average of the predictions to the depth of 1 m were used for calculations of salinity change. At each map cell (pixel) and based on each GCM, we calculated the mean of soil salinity for the reference, mid-, and long-term future periods and then computed the relative change as: (Future mean − Reference mean)/Reference mean; the percentage value of each cell represents the multi-GCM mean of the calculated relative changes presented by the colour map. Positive values indicate an increase in soil salinity while the negative values are indicative of a decreasing trend.
Our results reveal that the sign (positive: indicative of a higher EC e and negative: indicative of a lower EC e ) and intensity of changes in primary soil salinity are geographically highly variable; the variations are more extreme at the end of the 21th century compared to the mid-term future. Generally, the relative changes in soil salinity are more severe for the GHG emission rates which result in higher radiative forcing scenarios (RCP 8.5 and SSP 5-8.5). However, the intensity and spatial distribution of the projected changes based on the CMIP5 models are not necessarily the same as the CMIP6-based models predictions. Although our aim was to include all available projections in the analysis, in the case of discrepancy between CMIP5 and CMIP6 models, the predictions made based on the CMIP6 GCMs should be prioritized as they are more recent, forced by more updated data, and generally of higher spatial resolutions 81 .
According to our long-term predictions based on all multi-model ensembles, the drylands areas of South America, southern Australia, Mexico, south-west United States, and South Africa are generally at the highest risk of increased soil salinity, compared to the reference period. The threat of climate-induced soil salinity is also projected to increase in drylands of Spain, Morocco, and northern Algeria. To a lesser extent, western and southern Sahara and central Indian drylands, in addition to the desert soils of southeast Mongolia and north of China, are estimated to become saltier in response to the projected climate change by 2100 for different GHG concentration trajectories. On the other hand, our results indicate that the extent of soil salinity will remain constant or decrease relative to the reference period in the drylands located across the northwest United States, the Horn of Africa, Eastern Europe, Turkmenistan, and west Kazakhstan.
Additionally, Supplementary Fig. 3 shows the long-term future relative change in the five-year moving averages of daily dry and wet deposition rates of the sea salts (the 1971–2100 mean minus the 1961–1990 mean) projected by the multi-GCM ensemble means, as the two predictors used for training the models. Overall, the CMIP6 models predict a more severe increase or decrease in dry and wet deposition rates; however, all ensemble means are in agreement on an increasing trend in the dry deposition rate of sea salts in coastal regions, particularly in the southern hemisphere. All models also project a decreasing trend in dry deposition rates in north-western United States, west Canada, and central Asian regions; however, for these locations, the projected sign of the change in wet deposition rates is different between the CMIP5 and CMIP6 models. To some extent, the projections of these deposition rate can explain why soil salinity decreases in some regions, e.g. central Asia and Kazakhstan, where there is less certainty on the projected sign of changes in precipitation and evapotranspiration 82 .
Not all of the predictions generated based on the CMIP5 and CMIP6 GCMs used in this study are in agreement on the extent and sign of the soil salinity by the end of the century. Figure 2 , in particular, shows the multi-model ensemble agreements on the sign of the predicted change in soil salinity in the long-term future under different trajectory scenarios of GHG concentration. A cell value close to 100% indicates a complete agreement of the ensemble members on the sign of the salinity change. For the RCP 4.5 ensemble, as an example, an ensemble agreement of 100% of a grid-cell shows that all seven models in the ensemble are predicting an increase or a decrease in soil salinity in the long-term future relative to the reference period (depending on the sign of change). Especially under the SSP 2-4.5 and SSP 5-8.5 scenarios, the multi-GCM certainty of the predictions for a great proportion of the drylands of southern/eastern Australia, South America, and southern Africa indicate the southern hemisphere is at a higher risk of salinity caused by climate change. The projected increase in soil salinity in south-west and southern Australia induced by rising shallow groundwater tables as a result of dryland resource management and activities 45 can exacerbate the climate-induced soil salinization projected here. However, the certainty of the predictions made for drylands located in the Middle East, Russia, and Sahara is seemingly lower than for the other zones. For those dryland regions, the uncertainty is also recognizable through the difference in the predictions made based on the CMIP5 and CMIP6 models in Fig. 1 . For example, the CMIP5 models predict an increase in soil salinity in Russian drylands, while the CMIP6 models show the opposite trend in those regions.
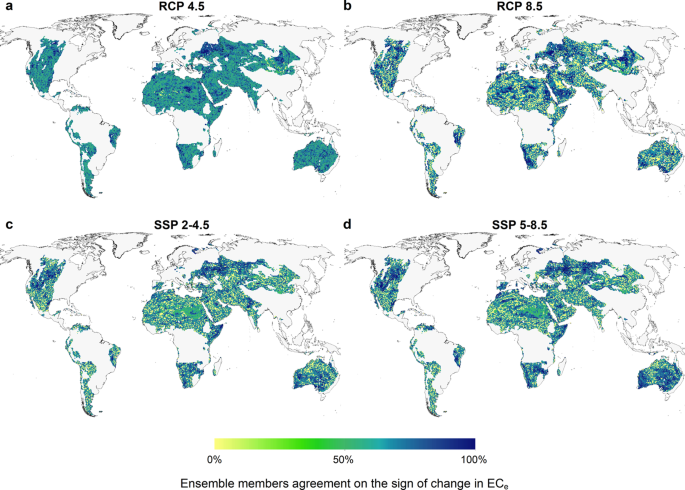
a , b Multi-GCM ensemble agreement of the models adopted from Coupled Model Inter-comparison Project Phase 5 (CMIP5) forced by RCP 4.5 and RCP 8.5 scenarios (Representative Concentration Pathways, which result in a respective radiative forcing of 4.5 and 8.5 W m −2 in year 2100, relative to pre-industrial conditions), respectively. c , d respective multi-GCM ensemble agreement of the models adopted from CMIP6 project under SSP 2-4.5 and SSP 5-8.5 pathways (projections forced by RCP 4.5 and RCP 8.5 global forcing pathways for the Shared Socio-economic Pathways 2 and 5, respectively). 100% shows the full agreement of the models on the sign of change, while zero indicates inconsistency among the models’ predictions.
Country-level projected changes in soil salinity
At the country level, we calculated descriptive statistics for the relative changes in soil salinity estimated at each grid-cell (in the mid- and long-term futures compared to the reference period) based on the multi-model ensemble mean, including grid-cells mean, 95% confidence intervals of the mean, standard error of the mean, and variance (Supplementary Tables 2 – 9 ). We did not calculate these descriptive statistics at the continental level as there was no noticeable difference between the results for various continents due to the high number of grid-cells within each continent.
Although the country-level results mask the majority of the local-scale variabilities of the soil salinity, the provided statistics help to have a better understanding of the countries with the highest risk of salinization. We ranked the countries based on the total number of grid-cells located in each country and calculated all aforementioned statistics only for the 30 countries with the highest number of grid-cells (Supplementary Table 10 shows the top 30 countries and the total estimated area of their drylands).
For the 2071–2100 period relative to 1961–1990 and under RCP 8.5 as the worst case scenario, the countries with the highest relative increase in the soil salinity were Brazil (with a mean grid-cell increase in EC e of 15.1% and the 95% confidence intervals of 13.25–16.95%), Namibia (13.57%; 12.1–15.04%), South Africa (11.2%; 9.41–13%), and Mexico (6.38%; 4.96–7.8%). The increase in soil salinity for Australia was much lower (3.31% and 2.88–3.73%). Under SSP 5-8.5, the countries with the highest relative increase in grid-cell means of soil salinity in the same period were Botswana (24.94%; 22.71–27.16%), South Africa (21.35%; 19.84–22.85%), Namibia (17.69%; 16.14–19.24%), and Brazil (16.21%; 14.77–17.66%). Overall, our calculated statistics suggest that the soil salinity will be increased more extensively by the climate change impacts in the regions spread across the southern latitudes, specifically below −20°.
Change in the total area of salt-affected soils in drylands
Additionally, based on our predictions for soil salinity extent in each grid-cell, we estimated the total area of salt-affected soils up to year 2100. Currently, no unique definition is available for the salt-affected soils. Contingent on the soil classification system, different values of EC e , ranging from 2 dS m −1 to even 30 dS m −1 , are adopted as the minimum threshold of salinity for characterizing the saline soils 35 , 83 , 84 . Accordingly, here we quantified the areal variation of the soils exposed to the threat of primary salinization assuming an EC e equal to 2 dS m −1 as the critical threshold, corresponding to the upper salinity limit tolerable by sensitive crops 11 . The results were computed at the country (Supplementary Table 11 ), continental (Table 1 , Fig. 3 ; Supplementary Fig. 4 ), and global levels (Supplementary Fig. 5 ). Additionally, Supplementary Figs. 6 – 8 and Supplementary Tables 12 , 13 show the projected variation in the total area of naturally occurring salt-affected soils assuming 4 dS m −1 as the critical threshold at the continent and country levels. As before, at the country level, only the top 30 countries with the highest number of the grid-cells were included. This analysis could be an indicator of the spatial expansion of the soil salinity in drylands in response to climate change.
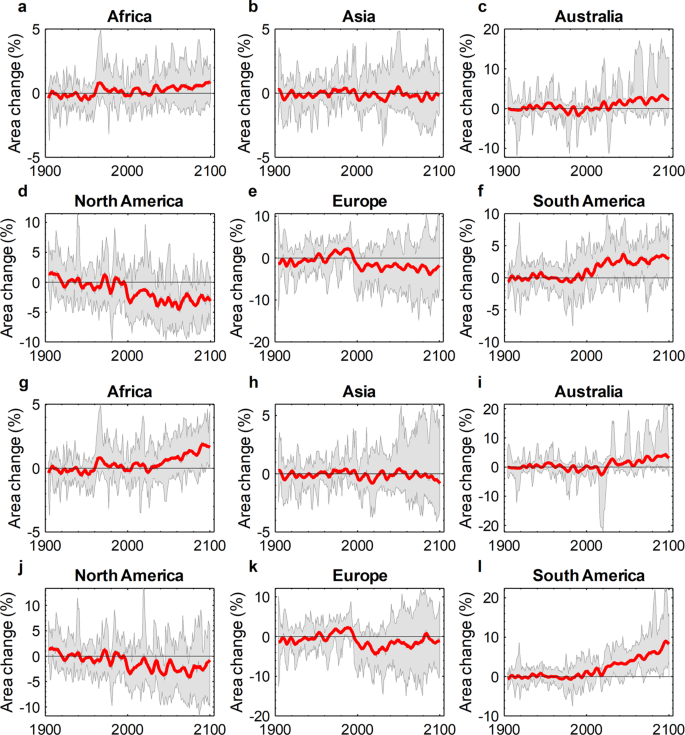
a – f Relative change under SSP 2-4.5 greenhouse gas concentration trajectory. g – l Relative change under SSP 5-8.5 greenhouse gas concentration trajectory. Shaded areas show the minimum and maximum range of the relative changes predicted by multi-model ensemble members. Red lines show the low-pass filtered (5-year running window) of the multi-model ensemble mean of the predicted variations; since all spatio-temporal predictors are five-year moving averages, 1904 is the beginning of the period.
Overall, under emission rates resulting in the radiative forcing of 8.5 W m −2 , all CMIP5 and CMIP6-derived predictions indicate an increasing trend in the total area of dryland soils with an EC e ≥ 2 dS m −1 for Australia and South America and a decreasing trend for Asia and Europe relative to the average of 1904–1999 period. For Australia and South America, we estimate the respective increases of 3.4% and 6.7% in the total area of dryland soils with EC e ≥ 2 dS m −1 between 2071–2100 relative to 1904–1999 period according to the multi-GCM ensemble means under the SSP 5-8.5 scenario. The CMIP5 and CMIP6-derived predictions of the total area of dryland soils with EC e ≥ 2 dS m −1 , however, are not in agreement on the sign and extent of the change for Africa and North America. The multi-model ensemble means under the SSP 5-8.5 scenario predict an increase of 1.5% and a decrease of 2.5% for the total area of dryland soils with a salinity ≥2 dS m −1 located in Africa and North America (between 2071–2100 relative to 1904–1999), respectively. Brazil (with 43%), Mexico (14.5%), and Mongolia (8%) had the highest estimated expansion in the total area of dryland soils with a salinity ≥2 dS m −1 between 2071–2100 relative to 1904–1999 periods under SSP 5-8.5 at the country level. On the opposite side of the continuum, Canada (with −10%), Somalia (−8.5%), and Ethiopia (−5%) had the largest predicted shrinkage of saline soils under SSP 5-8.5 (among the top 30 counties with the highest number of grid-cells in our analysis).
One of the questions that arises from this research is if the projected changes in primary soil salinization can actually occur in the time scales (10–40 years or 50–80 years) used for projections, especially in inland (hyper) arid regions where salt deposition is minimal and weathering very slow. The fast transition in near surface salt-budget has been reported in some studies which evaluated the temporal variations of naturally occurring soil salinity in arid environments using laboratory analysis and remote sensing techniques. Bannari and Al-Ali 85 examined the effect of climate change on spatio-temporal variability of soil salinity during the last 30 years (1987–2017) in the state of Kuwait using Landsat images and 100-geo-referenced soil data; for instance, only between 1987 and 1992, they estimated an increase equivalent to 350% in total area of salt-affected soils compared to the salt-affected area approximated in map of 1987 (433 km 2 ). As another example, Wang et al. 86 investigated the spatio-temporal changes of soil salinity in Kashgar region, north-western China with respective annual precipitation and potential evapotranspiration of 67.5 mm and 2100 mm using multi-temporal Landsat images and saline soil types from 19 field survey sites in the years 2000, 2010, and 2017. They estimated a total of 6.13% decrease (relative to 26,500 km 2 ) in the net area of salt-affected soils between 2000 and 2010, followed by further decrease of 1.75% between 2010 and 2017. Another example is the study by Taghadosi and Hasanlou 87 who monitored the salinity changes in bare soils near the arid district of Bakhtegan Lake in Iran between 2000 and 2016 using multi-temporal Landsat images. Through a comparative analysis, the authors concluded that 92% of these soils have become saltier over the studied period (referring to Fig. 6 in their paper). Although according to the literature, the predicted changes in soil salinity in arid and hyper-arid regions are feasible in the period considered in our study spanning over almost two centuries (1905–2100), physically constrained models are still required to evaluate the feasibility of occurrence of the conclusions obtained from our ML models. To conduct such a physically based analysis on a global scale, one would need detailed soil and environmental data to model precipitation, leaching events as well as in situ salt amount in the root zone on a global scale which is currently not available.
The changes predicted here do not agree with the global-scale predictions of Schofield et al. 57 who estimated that Australia and western North America would be the areas with lower salinization potential in the 2070–2099 period, while they predicted a high potential for salinization in lands across Eastern Europe and Kazakhstan. In addition to the difference between the methodologies used for the projections of soil salinity, this discrepancy is due to various other reasons. For example, unlike the current study, Schofield et al. 57 only used one GCM (HadCM3GGa), developed before 2000, to specify their salinization indicators. Furthermore, they estimated the future potential evapotranspiration as an empirical function of air temperature to calculate AI as an indicator of soil salinity, while we used total evapotranspiration derived from the more physically based GCMs.
The results of ML models are primarily based on the trends they capture from the input data used for training. Therefore, projected changes of EC e in the hotspots of climate-induced soil salinization can be mainly attributed to the variations in spatio-temporal input data projected by GCMs. As mentioned before, precipitation frequency and evapotranspiration were the most influential spatio-temporal predictors for the predictions of the trained models. According to the analytical salt mass balance, higher evapotranspiration rate and precipitation with a lower frequency and intensity accumulate more salts in the root zone 66 . By the end of the century, an ensemble mean decrease in precipitation (under RCP 8.5) of up to 40% was reported by Giorgi et al 88 . for the southern hemisphere, particularly southern and western Australia, Namibia, and Brazil for the June–July–August months, which are also the salinization hotspots according to our results. Similarly, in the northern hemisphere, they predicted a more severe decrease in precipitation for Mexico, West Africa, and Mediterranean coasts for December–January–February. At smaller spatial scales, other studies projected an increase in the number and duration of drought events, higher potential and actual evapotranspiration, decreasing trends in frequency and intensity of precipitation, and in general drier conditions by the mid and end of the century.
Using 34 GCMs under the two different emission scenarios of RCP 4.5 and RCP 8.5, Shi et al. 89 predicted that potential evapotranspiration tends to increase in south-eastern Australia. Likewise, using 22 CMIP5 models, a substantial increase in the number of warm temperature extremes and periods of dryness was projected by Alexander et al. 90 for Australia, one of the predicted salinization hotspots in the current study. Similar trends for Australia were projected by Grose et al. 91 by analysing the available CMIP6 multi-model ensemble. By analysis of 14 GCMs under the RCP 4.5 and RCP 8.5 future scenarios, a substantial decrease in precipitation during the summer (up to 1.5 mm day −1 ) is expected by Colorado‐Ruiz et al. 92 in southern Mexico, also a projected salinization hotspots in the present study. A decrease in the frequency of precipitation during winter and spring in south-western United States is projected by Easterling et al. 93 , as also found in this study to be a hotspot. An increase in the number of consecutive dry days in west Sahara 94 and actual evapotranspiration in arid areas across north-western China 95 under the 1.5 °C and 2.0 °C global warming scenarios reported in the literature is congruent with the findings of the current study.
To conclude, lack of reliable predictive tools and data to assist land managers and policy makers for understanding the land cover dynamics is one of the main obstacles to long-term sustainable land and environment management. In the present study, we used legacy soil-profiles data and a set of purely spatial and spatio-temporal predictors to develop some predictive ML models for projection of the primary soil salinity (represented by electrical conductivity) as one of the major threats to the soil fertility, stability, and biodiversity in world drylands. Our analysis provides long-term gridded (at 0.5° spatial resolution) predictions of primary soil salinity change in drylands globally in response to projected key climatic drivers of soil salinity, which is currently missing in the soil and land management literature. In the face of projected future climatic uncertainties, the developed predictive models and generated data in the present investigation can help with decision-making regarding land and water resources management to recognize the hotspots of soil salinization, devise the necessary action plans, and implement those plans towards sustainable land and water resources management.
Under different GHG concentration trajectories, our predictions suggest that by the late 21th century the drylands areas of South America, southern Australia, Mexico, south-west United States, and South Africa are at the risk of higher soil salinity caused by climate change, compared to the reference period (1961–1990). In addition, increase in climate-induced soil salinity threatens the drylands of Spain, Morocco, and northern Algeria by the end of the century. On the other hand, our results project a decreasing trend in primary soil salinity of the drylands located in the northwest United States, the Horn of Africa, Eastern Europe, Turkmenistan, and west Kazakhstan, relative to the reference period. The reliability of the predictions made here are different: the projected soil salinities for the drylands located in North America and Australia are of the highest level of reliability while the drylands of central Asia, Middles East, and the Great Sahara have the highest uncertainty in predictions for soil salinity. Other zones such as India, South America, and South Africa are in the middle in terms of the reliability of predictions.
In a previous study 33 , we developed tree-based two-part predictive ML models for determining annual surface (referring to top 30 cm of the soil) soil salinity and sodicity (represented by exchangeable sodium percentage) over the past four decades (1980–2018) at ~1 km 2 spatial resolution on a global scale. In the present study, however, we aimed to predict the future dynamics of soil salinization up to the year 2100 under changing climate. In the present investigation, we focused on primary salinization and the trained tree-based ML models were only of regressive models. The next sections explain the details of the workflow for predicting soil salinity (EC e ) including: (1) collection of the measured soil-salinity profiles, (2) collection and processing of salinity predictors, (3) exposing the salinity profiles and predictors data to ML models, training the models, and validation of the trained models, and (4) employing the trained models to project the spatio-temporal variation of the soil EC e up to the year 2100 under different greenhouse gas (GHG) concentration trajectories. Finally, we discuss the accuracy of the trained models for prediction of EC e .
Soil-salinity profiles
We obtained the geo-referenced soil profiles (points) with measured values of EC e from the soil-profile dataset of World Soil Information Service (WoSIS) 96 . The spatial distribution of the profiles data used as an input into the ML models is presented in Fig. 4a . The WoSIS EC e database includes 19,434 soil profiles and each individual profile (with a unique profile ID) may include one or more samples for various depths below the soil surface. The data cover the sampling period from 1950 to 2014. Since the date of sampling was an essential parameter in model training, we removed the EC e profiles without sampling dates. This reduced the total number of EC e samples from 73,517 to 59,649, with the number of samples per year shown in Fig. 4b . In addition, we dropped the soil EC e profiles sampled from the croplands to remove the effects of human interventions from the analysis. As a result, a total 44,708 samples (11,517 profiles) remained in our analysis for model training and accuracy assessment.
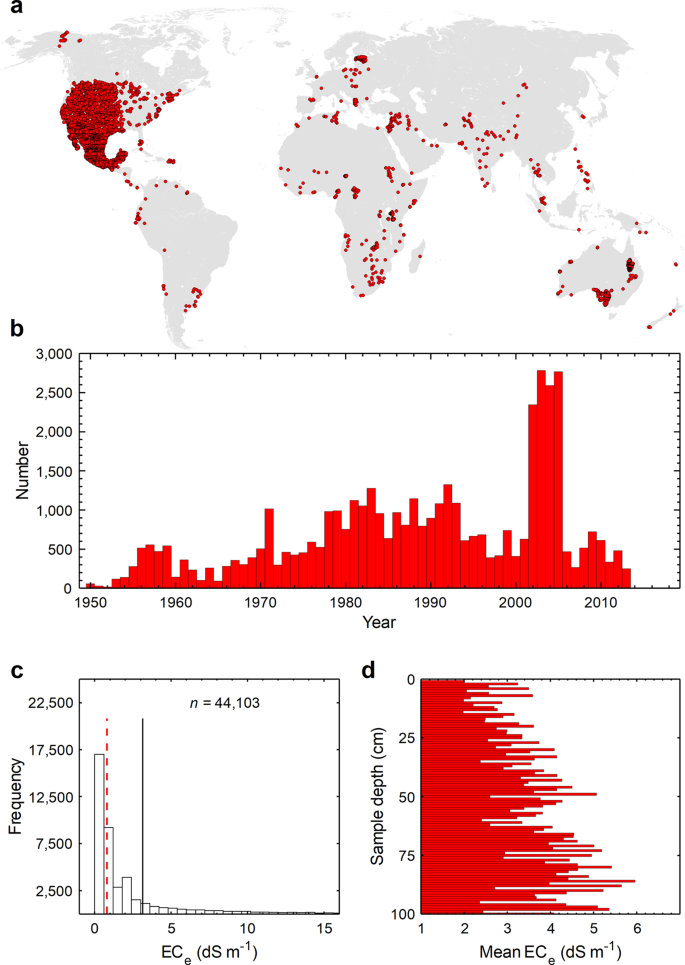
a spatial distribution of the soil salinity profiles used for model training and prediction of the soil salinity. Each profile includes one or more soil samples. b temporal distribution of the samples used for training the predictive models of soil salinity. Each bar shows the number of samples within one year. c frequency distribution of the measured values of EC e . The solid and dashed vertical lines represent the mean and median values, respectively. d average of the measured soil salinity values at 1 cm intervals to the depth of 1 m below the surface.
Global land cover data provided by Earth-Observation Satellites before 1997 were scarce. Accordingly, we divided the profiles into two categories based on the date of sampling: before 1997 and after 1997. For the period before 1997, we identified the profiles located in croplands using the Global Land Cover Characteristics Database, Version 2.0 at ~1 km resolution 97 . Due to a lack of historical land cover data, we assumed that the land cover/land use did not change considerably before the 1980s. For profiles sampled after January 1997, however, we identified the samples/profiles located in croplands using land cover maps for years 2000, 2006, 2014, and 2018 with similar International Geosphere-Biosphere Programme (IGBP) land cover legend adopted from the MODIS Data Collection (MCD12Q1 and MCD12C1) 98 . We selected the IGBP land cover legend as it was available in both datasets. Each profile sampling date was attributed to the layer with the nearest year of acquisition. The MODIS land cover layers were first re-projected to the World Geodetic System (WGS 1984) spatial coordinates at 0.004° (~500 m) using the nearest neighbour method.
We used two types of predictor to train the models for predicting EC e as the target variable: purely spatial and spatio-temporal. Purely spatial predictors included the land and soil attributes, which were relatively constant during the period of the analysis, while spatio-temporal predictors were the large-scale hydro-climatic variables derived from the output of selective GCMs. In total, 14 predictors were used, of which nine purely spatial and the rest spatio-temporal. The pre-processing details, projection, extent, and resolution of the predictors’ layers are summarized in Table 2 . These predictors were primarily selected to represent the main factors affecting the salt balance in the root zone in non-irrigated soils 66 . In addition, we included in our model training additional soil formation factors, including topography and parent material (weathered rock or deposit from which the soil is formed) 96 , 99 .
The purely spatial predictors comprised:
soil classes based on the World Reference Base (WRB) classification 72 , 100 ;
soil texture represented by the percentage of clay content, obtained from the ISRIC global gridded soil information at ~250 m spatial resolution 72 ;
soil wilting point in mm 101 ;
soil field capacity in mm 101 ;
effective plant rooting depth in m 102 ;
topographic slope in degrees; and
terrain elevation in m.
Slope and terrain elevation layers were derived from the World Elevation Terrain data adopted from ArcGIS Living Atlas of the World 103 and were re-projected to the WGS 1984 coordinates system at 0.002° (~250 m) spatial resolution using the cubic convolution method. We filled the missing grid-cells (or cells with no data values) in purely spatial predictor layers with an average from the cells surrounding the missing grid-cell. We used a circle with a radius of four cells from the neighbouring cells to calculate the average and fill the data gap. All purely spatial predictors were assumed to be vertically constant. Raster processing was conducted in ArcGIS 10.7 104 . Then, we obtained the values of grid-cells of purely spatial predictors at the locations of EC e profiles (Fig. 4a ) to later train predictive models of soil salinity (see “model training for prediction of soil salinity”). The upper and lower depths of the measured EC e samples derived from the original WoSIS database were the additional purely spatial predictors used for model training; these were introduced to account for the effect of depth on soil salinization processes.
The spatio-temporal predictors considered here were precipitation intensity, precipitation frequency, daily evapotranspiration, and sea salts wet and dry deposition rates (Table 2 ). To make predictions for future periods, we needed the projected values of the predictors. Therefore, we derived the values of spatio-temporal predictors from the outputs of the GCMs under different GHG concentration trajectories.
For training the models, we used the GCMs available in both CMIP5 and CMIP6 data projects to consider the uncertainty in GCMs predictions and to cover all available projections for dry and wet sea salt deposition rates. Additionally, this gave us the opportunity to analyse the differences between the CMIP5 and CMIP6 model outputs in terms of the derived predictors’ values and their effects on the projected soil salinity. The historical outputs of GCMs, including precipitation, evapotranspiration, and dry and wet deposition rates of sea salts, were used for training the predictive ML models (CMIP5: 1900–2005; CMIP6: 1900–2014). The projected outputs of GCMs for the same parameters were used to make future predictions of soil salinity (CMIP5: 2006–2100, CMIP6: 2015–2100). For the CMIP5 models, predictors were calculated based on the future projections forced by the RCP 4.5 and RCP 8.5 scenarios. Likewise, for the GCMs models of CMIP6, predictors were computed using future projections forced by RCP 4.5 and RCP 8.5 global forcing pathways for the Shared Socio-economic Pathways (SSP) 2 and 5, respectively. These medium (4.5) and high (8.5) radiative forcing pathways were chosen because they, respectively, represent the most plausible (or stabilization) and worst case scenarios of emissions by the end of the 21th century.
Since the total number of wet days and the total annual precipitation values were calculated from the daily precipitation fluxes, the GCMs with precipitation data at daily resolution were required. Additionally, not all of the available GCMs in the CMIP5 and CMIP6 projects had the dry and wet deposition rates of the sea salts. Accordingly, our analysis was narrowed down to a total of 16 GCMs outputs under different GHG concentration trajectories from both CMIP5 and CMIP6 projects. For the GCMs with different ensemble members (MIROC5 and CESM2-WACCM-gn, in particular), we computed an ensemble mean to avoid a bias in the results of final multi-GCM ensembles toward the GCMs with the higher number of participating ensemble members. In total, data of eight GCMs (seven with projections under RCP 4.5 and six GCMs with projections under RCP 8.5) and eight GCMs (with projections under both SSP 2-4.5 and SSP 5-8.5) were downloaded from the CMIP5 and CMIP6 data 105 , respectively. Details on the final chosen GCMs, their spatial resolution, and their used ensemble members are presented in Table 3 .
The original longitude values of netCDF files were set in the range −90° and 90°, referenced to the Greenwich Prime Meridian, to be in the same spatial extent as the purely spatial predictors. Then, using the bilinear interpolation method, all were interpolated to 0.5° × 0.5° WGS 1984 longitude-latitude regular grid to be able to generate multi-GCM ensemble from the outputs of our predictive models. Calculation of the spatio-temporal predictors and processing of the original netCDF files were conducted in the Climate Data Operators 106 environment. The prepared netCDF data based on the outputs of GCMs were then converted to multi-band rasters, after which we obtained the values of spatio-temporal predictors at locations of EC e profiles. These values combined with the values of purely spatial predictors were used to train the predictive models of soil salinity. It was not practical to use these spatio-temporal predictors at daily or monthly temporal resolutions because of strong intra/inter-annual fluctuations in these predictors 66 . Therefore, we used a 5-year moving average instead (as a smoother input) to better capture the effect of intra/inter-annual trends in these predictors on soil salinity variations. Finally, the 5-year moving averages of the spatio-temporal predictors were attributed to each observation of EC e according to the year of sampling.
Model training for prediction of soil salinity
The measured values of EC e (target or response variable) and the values of each of the 14 predictors (each represented by one column of data), attributed to the measured values of EC e , were then imported to MATLAB for model training and validation. For each GCM, a separate matrix of data was prepared, with a total of 16 matrices. The WRB soil classes (as the only categorical predictor) were represented by a vector of positive integers that contained values assigned to different soil classes. The other 13 predictors were non-categorical represented by a set of real numbers. In spite of employing the method explained earlier for estimation of the missing cells in predictors’ layers, the values of some purely spatial predictors were still missing in the final imported matrices. Therefore, the corresponding EC e values (each represented by a row of data) were eliminated and not used for model training. As a result, 1.28% of the sample rows were excluded from the analysis.
We applied MATLAB Statistics and ML toolbox (MATLAB, R2019b) for building and validating the predictive models of EC e . Here, we used an ensemble of regression trees for training and projecting the soil salinity based on the predictor datasets obtained from each of the 16 GCMs shown in Table 3 . We chose tree-based models due to their relatively higher accuracy and computational speed compared to other ML algorithms 33 , 107 . Additionally, tree-based predictive models are highly flexible in mapping non-linear relations between the known predictors and known responses 108 , 109 and are robust in handling outliers and collinearity concerns in environmental modelling 110 , 111 . The MATLAB built-in “fitrenemble” function was applied for training the regression ensembles.
The model hyperparameters, or parameters that should be set before launching the training process of a ML algorithm, were tuned using MATLAB automatic hyperparameter optimizer. These comprised ensemble aggregation method, number of learning cycles, learn rate, minimum leaf size, maximum number of splits, and number of variables to sample 107 . By varying the hyperparameters, the optimizer attempts to find a combination of their values which minimizes the “log (1 + cross-validation loss)”. Holdout cross-validation method (with 25% of data being held out) was used for optimization and the cross-validation loss was quantified using mean squared error. The optimizer used the Bayesian optimization algorithm with the “expected-improvement-per-second-plus” acquisition function. The maximum number of objective function evaluations was 100 since there was no notable decrease in the value of the observed minimum objective function after 100 evaluations. We repartitioned the cross-validation at every iteration and assumed the weight of all observation rows to be equal to one. We applied the log-transform to address the issue of right skewness in frequency distribution of the target variable; however, the log-transformation and back-transform of the predicted responses had a negligible impact on the accuracy of the trained modes.
The Bayesian optimization algorithm could return different results since its chosen acquisition function depends on the runtime of the objective function; the optimizer avoids the regions with extremely high runtimes. According to the non-reproducibility of the tuned set of hyperparameters, the model training and hyperparameter tuning jobs on each of 16 datasets were repeated 30 times (480 models in total). The maximum number of learning cycles was limited to 500 to keep the runtime for each training task below 10 min. High runtime and computational costs did not allow us to repeat the trainings more than 30 times. We accelerated the model training process by running the computations on a machine with 48 cores using the MATLAB Parallel Computing Toolbox. The goodness-of-fit of the trained models was evaluated by 10-fold cross-validation R 2 (the extent of variation explained by the model 112 ), root mean squared error (RMSE), mean absolute error (MAE), and Nash-Sutcliffe model efficiency coefficient (NSE 113 ). Then we used the bias corrected and accelerated percentile method to calculate the 95% confidence intervals of the mean for each validation metric based on 1,000 bootstrap samples (with replacement) derived from the results of the 30 runs performed for each of the 16 datasets. Among the 30 trained models for each input training set, the one with the lowest RMSE was selected; we chose RMSE as it is more sensitive to large errors 114 . In total, 16 models remained in our analysis for soil salinity projections.
Model implementation and soil salinity projection
We converted the world drylands layer delineated by the United Nations Environment Programme World Conservation Monitoring Centre 37 to a raster layer at 0.5° spatial resolution for generation of a global soil base map of the drylands. From that layer, we constrained our analysis to areas with an AI ≤ 0.65 and masked out the grid-cells (pixels) with an AI > 0.65 to keep only the drylands in our analysis 37 . The remained raster had 24,045 grid-cells and we used it as the global soil base map of the drylands.
Similar to input training profiles data, we extracted the values of purely spatial and spatio-temporal predictors to the location of the base map grid-cells and then a 5-year moving average from the values of spatio-temporal predictors was computed. We applied the best chosen trained models to these new locations (cells) and the corresponding values of the predictors. As mentioned before, the degree of soil salinity and solute concentration change along the soil depth. Usage of the upper and lower depths of the samples as predictors in the model training enabled us to make predictions of EC e at different depths below the soil surface. In this regard, the trained models can be considered as four-dimensional predictive models of soil salinity that make predictions for different longitudes, latitudes, depths, and times. For each pixel and each year, we predicted the values of soil salinity at five depths: 0, 10, 30, 60, and 100 cm. We used the trapezoidal rule to compute an average of the EC e (dS m −1 ) to the depth of 1 m as follows: 72
where EC e is the predicted salinity at the corresponding depth. The outlier that is more than three scaled Median Absolute Deviations (MAD) away from the median of all predictions of a year were removed by the MATLAB “isoutlier” built-in function; this was the most robust method for removing outliers according to the user guide (see MATLAB “isoutlier” documentation for further details). In total, for each grid-cell of the global soil base map of the drylands, 197 predictions of EC e were made in the period between 1904 and 2100 (one prediction for each year); since all spatio-temporal predictors are five-year moving averages, 1904 is the beginning of the period.
To compare the future state of the drylands soil salinity against the past conditions, we considered three time periods in our analysis: reference period (1961–1990), mid-term future (2031–2060), and long-term future (2071–2100). We used 30-year periods and 1961–1990 as the reference period based on the recommendations of the World Meteorological Organization for evaluations of the long-term changes in climatic variables 115 . Soil salinity predictions for years in the future periods were averaged and compered to the average of the predictions for years in the reference period.
We calculated the area of each grid-cell of the global soil base map of the drylands in the WGS 1984 spatial coordinates using the computer code presented in the Supplementary Information. We estimated the total annual area of salt-affected soils between 1904 and 2100 and then computed the annual percentage change in the area of those soils by dividing the total area at each year by the average area of salt-affected soils over the period. We assumed an average of 95 years would be enough to remove the potential noise introduced by the spatio-temporal predictors. We used global administrative areas dataset 116 to estimate the total area of salt-affected soils at the national and continental levels. Numerical values representing the countries and continents were attributed to each cell of the base soil map.
Accuracy assessment of the trained models
The results of hyperparameter tuning and the 10-fold cross-validation accuracy metrics of the best-fitted models are summarized in Supplementary Table 14 . Supplementary Table 15 also presents the calculated lower and upper limits of 95% confidence intervals of the 10-fold cross-validation accuracy metrics, calculated for the trained models. For all 16 models, the MATLAB ensemble aggregation method of “LSBoost” was superior in fitting the models, compared to the “Bagged” method.
For the best-fitted models, the lowest R 2 was 71.72% (with the 95% confidence intervals of 67.62–69.89%) related to the GISS-E2-R model, while the highest R 2 between the measured and predicted values of EC e was 73.95% (67.34–70.32%), calculated for the CNRM-ESM2-1 model (see Table 3 for the details of GCMs). For all 16 models, the average calculated 10-fold cross-validation R 2 was 72.79%. Likewise, GISS-E2-R and CNRM-ESM2-1 were the models with the highest and lowest calculated values of RMSE, respectively. The average of 10-fold cross-validation RMSE for all 16 best-fitted models was 3.6, ranging from 3.52 (3.78–3.93) to 3.67 (3.76–3.95). This represents a normalized RMSE equal to ~6% (normalized to the observed range of the EC e values).
To understand better how well the best-fitted models predict the response values, the relation between the measured (values sampled from the soil profiles) and predicted values of EC e is visualized in Fig. 5 via bin scatter plots. Taking a conservative approach, Fig. 5 shows only the validation plots for the six (out of the 16 best-fitted) models with the worst performance (i.e. with highest RMSE values). Predictions of the models are fairly concentrated around the y = x line, suggesting a good agreement of the modelled values with measured data. The accuracy of predictions increases with EC e values, with a tendency for over-estimations for EC e ≤ 1 dS m −1 . Overall, the relatively high R 2 (>70%) values indicate a satisfactory model fitting, particularly as such values are not common in digital soil mapping 117 .
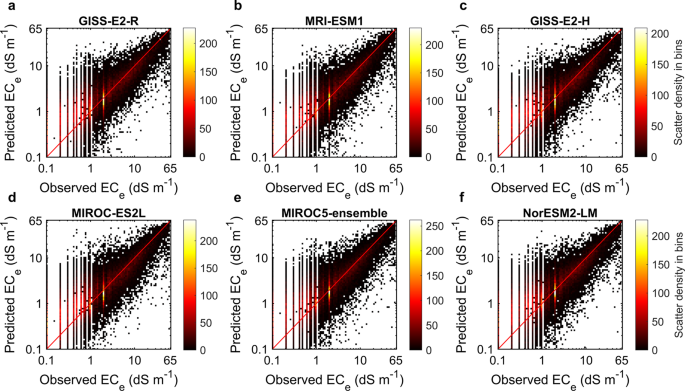
The RMSE decreases from a – f . The colour maps show the scatter density in each bin. The red lines represent the y = x line.
Additionally, we evaluated the accuracy of the vertical prediction of the 16 best-fitted models, i.e. the prediction accuracy at various depths from the soil surface. To do so, we categorized the measured and predicted (by 10-fold cross-validation) values of EC e into six bins of 0–20, 20–40, 40–60, 60–80, 80–100, and 100–200 cm based on an average from the lower and upper depths of the samples (each bin included its left edge); the bins edges were chosen so that the number of samples available for each bin stayed roughly equal and the deeper depths were not considered due to lack of data. The calculated R 2 values for each bin and each of the 16 models are reported in Supplementary Table 16 . The averages of the 16 models R 2 values for the shallowest to deepest soil layers (bins) were 63.59%, 72.99% 77.39%, 77.31%, 79.59%, and 72.51%, respectively. These accuracies are in line with the reported R 2 values of Taghizadeh-Mehrjardi et al. 28 who developed separate regression tree-based models to predict soil salinity (78% for 0–15 cm soil layer). However, their analysis was purely spatial and was only focused on the saline soils located in a local area in central Iran (72,000 ha), while the current analysis projects the spatio-temporal variability in soil salinity on the global scale. We did not observe a decrease in predictive accuracy of the digital soil models at the higher depths reported in other studies, such as Malone et al. 117 and Minasny et al. 118 .
In addition to global accuracy assessment of the trained models, we evaluated the predictive power of the best-fitted models at the country and continental levels (Fig. 6a, b ). We grouped the measured sample values of EC e according to the continent or the country where the samples were acquired and compared the mean of each group with the mean of the 10-fold cross-validated predictions for each group. Only 87 countries had measured input profiles data of EC e required for our analysis. At the country level, the R 2 between the mean of predictions of the 16 models and the mean of measured values of EC e was 80.41% while at the continental level, this value was 99.64%. The reason for such a high accuracy at the continental level is the high number of data points within each continent, which makes the predicted and estimated averages close to each other.
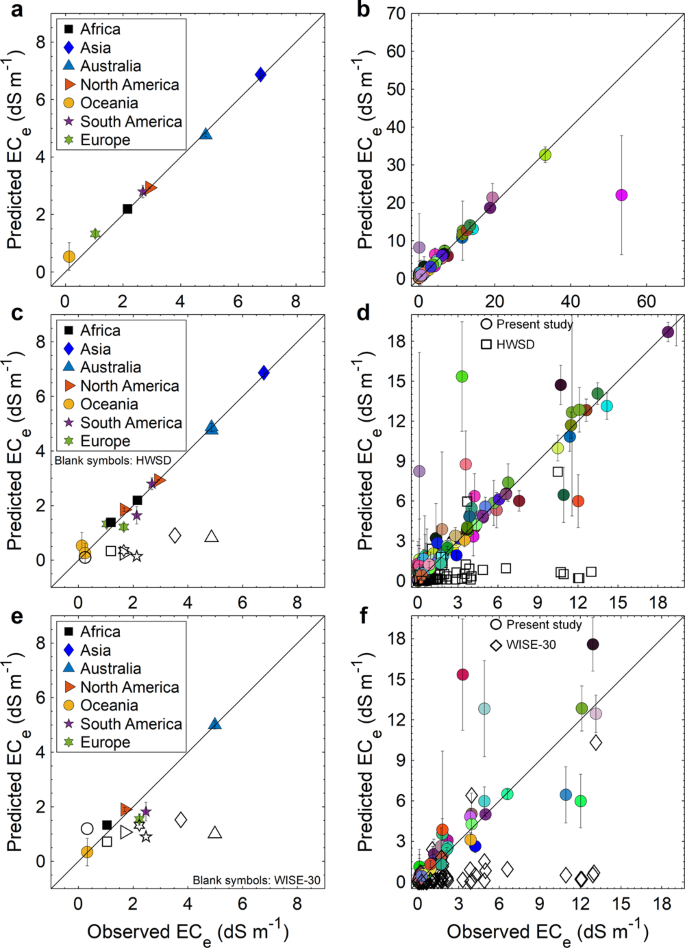
a , b Average predicted values versus average measured values at the continental and country levels (87 countries), respectively. c , d Average of the surface (0–30 cm) salinity (EC e ) values predicted by the present study and Harmonised World Soil Database (HWSD) versus the average of measured surface salinity at the continental and country levels (74 countries), respectively. e , f Average of the surface (0–20 cm) salinity predicted by the present study and WISE-30 (World Inventory of Soil Emission Potentials derived soil properties) dataset versus the average of measured surface salinity at the continental and country levels (71 countries), respectively. The error bars represent the minimum and maximum of average values calculated for the 29 models used in the study.
Similarly, we compared the predictions of our models with other available gridded datasets on soil EC e , including HWSD (Harmonised World Soil Database 10 ) and WISE (World Inventory of Soil Emission Potentials) which derived soil properties on a 30 × 30 arc-seconds global grid (WISE-30; ref. 119 ), at the country and continental levels. Since these two datasets provide data for different soil layers (HWSD: two layers at 0–30 cm and 30–70 cm; WISE-30: seven layers, with five fixed depth intervals of 20 cm up to the depth of 100 cm and two 50 cm depth intervals between 100 and 200 cm), we only focused on surface measurements. For comparison with HWSD, any soil sample with the upper sample depth of 0 cm and a lower sample depth ≤30 was chosen as the surface measurement (a total of 8,995 samples) while for WISE-30, any EC e sample with the lower sample depth of 20 cm was chosen as the surface measurement (a total of 7,535 samples).
At the location of each particular surface measurement, we predicted the soil salinity for 0–20 or 0–30 cm (depending on the target dataset for comparison) soil layers using the purely spatial and spatio-temporal values of predictors corresponding to the year of sampling of that particular surface measurement. Then we grouped the predictions and surface measurements based on the country and continent of sampling. At the country level, the R 2 between the mean of our models predictions and the mean of surface measured values (0–30 cm) of EC e for 74 countries was 68.55%, while this value for HWSD was 13.6%. At the continental level, these values were 91.48% and 74.98%, respectively (Fig. 6c, d ). Compared to the WISE-30 predictions, the R 2 between the mean of our models predictions and the mean of surface measured values (0–20 cm) of EC e was 69.33% and 87.99% at the country (71 countries) and continental levels, respectively whereas the WISE-30 values were 17.22% and 5.53% (Fig. 6e, f ). Although HWSD and WISE-30 datasets are purely spatial (they do not include information on the temporal variability of the soil salinity) and comparison is carried out with the same data used to train the ML models (as currently there are no other independent soil salinity datasets), comparing the predictions made by the models developed here against the predictions of those datasets can provide a better quantitative understanding of the improved predictive performance of our models.
Model limitations, uncertainties, and perspectives for future research
ML models are one of the solutions suggested for time series projection challenges 120 . However, unlike the analytical models, ML models do not enable consideration of the mechanistic insights in the predictive algorithms of soil properties 72 . As mentioned earlier, no harmonized dataset is currently available quantifying the concentration of the soluble salts in salt-affected soils and, to a great extent, quantification of the severity of soil salinity in the field is limited to EC e measurements. Provision of such dataset can be a baseline for developing more mechanistic and physically constrained approaches in projections of soil salinity. Although very challenging, partly due to the lack of the required environmental and soil data, development of root zone salt-budget models for projecting large-scale soil salinity driven by groundwater table, irrigation practices, and sea level rise is an important area for future research.
Captured trends and projections in this study depend on the input data used for training the models. Inconsistency in accuracy and methods applied by different laboratories for measuring soil properties can negatively impact the trends captured by the trained models. As we go towards the past, the number of available samples and their accuracy decreases (Fig. 4b ); this in turn may influence the validation procedures applied to the predictions made by ML models 72 . It may also generate predictions biased towards the recent periods when more data samples are available. Additionally, more care should be given to application of the predictions made here at locations underrepresented by input data for training the ML models. In the current study, the majority of soil profiles used for training were sampled from North America and Australia due to a greater data availability. Thus, there is a possibility that the results are biased towards the soil and hydro-climatic conditions of these two continents. One solution to address this issue can be to develop more regional ML models; yet, this is challenging in the locations with the low number of sample data. Decrease in the number of available input data reduces the efficiency of the model training, resulting in less accurate and unsatisfactory validation outcomes. More updated and geographically scattered profile data are required in future studies to address the issue of inconsistency in the legacy soil-profile data. Although our analysis is an estimation of a relative change (relative to the reference period) in primary soil salinity and biases in GCMs outputs are not significant, application of reanalysis data for the reference historical period may address the biases issue in GCMs.
More importantly, the extent of uncertainty in the predictors used for training the models is not spatially constant. All the predictors used here are large-scale estimations of other models, which inherently include some degrees of uncertainty. Particularly, purely spatial predictors including the wilting point, field capacity, and effective plant rooting depth, are less certain in large deserts where observations are scarce for tuning and validation of the models. One way to address this issue is to provide spatially explicit maps of uncertainty for the predictions of the ML algorithms. However, this needs spatially explicit uncertainty maps of the predictors or their probability distributions. In the case of our study, such data were not available for the predictors. Additionally, ML algorithms are highly computationally demanding and estimation of the outputs uncertainty ranges by methods such as Monte Carlo simulations was not feasible by our computational resources (assuming hypothetical distributions of uncertainty in the predictors and input profiles data). Thus, we did not quantify the posterior distribution and uncertainty of the predictions and instead we estimated the global accuracy of the projected results via the 10-fold cross-validation method. A less computationally intensive framework is needed in the future for provision of the spatially explicit estimations of uncertainties in outputs of the ML models. Furthermore, comparison of our predictions accuracy with HWSD and WISE-30 datasets was based on the data used here for ML training and more independent datasets of soil salinity are required to benchmark our models performance against previous datasets/models of soil salinity.
The number of GCMs with projected wet and dry sea salt deposition rates (which are also necessary for mechanistic approaches) were rather limited in both CMIP5 and CMIP6 data projects. More ensemble members could improve the certainty of the projected soil salinity. Furthermore, the spatial resolution of our salinity projections was relatively coarse (0.5°); although the purely spatial predictors were of the adequate resolution, there was no point in prediction of the soil salinity values at finer resolutions since the spatio-temporal resolution of the GCMs grids was roughly between 1 and 3°. Such issues might be addressed with improvement of the spatial resolution of GCMs and the number of GCMs with sea salt aerosols projections in upcoming years.
Data availability
Data generated in this study including input data for training the predictive models, objects of the predictive models, annual predictions made by the models for each location, and spatially explicit maps quantifying the change in predicted soil salinity in the mid- (2031–2060) and long-term futures (2071–2100), relative to the reference period (1961–1990) have been deposited in the “figshare” database, freely available at https://doi.org/10.6084/m9.figshare.14548947 .
Code availability
Computer codes required for regeneration of the main results presented in this paper can be found in Supplementary Information appendix (computer codes section).
Soil Science Glossary Terms Committee. Glossary of soil science terms (Soil Science Society of America, ASA-CSSA-SSSA, 2008).
Burt, R. Soil Survey Investigations Report, no. 45, version 2.0 . (Natural Resources Conservation Service, 2011).
Abrol, I., Yadav, J. S. P. & Massoud, F. Salt-affected soils and their management (Food & Agriculture Org., 1988).
Bleam, W. F. Soil and Environmental Chemistry (Academic Press, 2016).
Zaman, M., Shahid, S. A. & Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation using Nuclear and Related Techniques (Springer, 2018).
Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 57 , 1017–1023 (2006).
Article CAS PubMed Google Scholar
Daliakopoulos, I. et al. The threat of soil salinity: a European scale review. Sci. Total Environ. 573 , 727–739 (2016).
Article ADS CAS PubMed Google Scholar
Pannell, D. J. & Ewing, M. A. Managing secondary dryland salinity: options and challenges. Agric. Water Manag. 80 , 41–56 (2006).
Article Google Scholar
Ayub, M. A. et al. In Plant Life Under Changing Environment , 47–76 (Elsevier, 2020).
Fao/Iiasa/Isric/Isscas/Jrc. Harmonized world soil database (version 1.2) . (FAO, 2012).
Maas, E. V. & Grattan, S. Crop yields as affected by salinity. Agric. Drain. 38 , 55–108 (1999).
Google Scholar
Greenway, H. & Munns, R. Mechanisms of salt tolerance in nonhalophytes. Annu. Rev. plant Physiol. 31 , 149–190 (1980).
Article CAS Google Scholar
Parihar, P., Singh, S., Singh, R., Singh, V. P. & Prasad, S. M. Effect of salinity stress on plants and its tolerance strategies: a review. Environ. Sci. Pollut. Res. 22 , 4056–4075 (2015).
Munns, R. & Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59 , 651–681 (2008).
Hillel, D. Salinity management for sustainable irrigation: integrating science, environment, and economics (The World Bank, 2000).
De la Paix, M. et al. Physicochemical properties of saline soils and aeolian dust. Land Degrad. Dev. 24 , 539–547 (2013).
Singh, K. Microbial and enzyme activities of saline and sodic soils. Land Degrad. Dev. 27 , 706–718 (2016).
Rath, K. M. & Rousk, J. Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: a review. Soil Biol. Biochem. 81 , 108–123 (2015).
Hassani, A., Azapagic, A., D’Odorico, P., Keshmiri, A. & Shokri, N. Desiccation crisis of saline lakes: a new decision-support framework for building resilience to climate change. Sci. Total Environ. 703 , 134718 (2020).
Sentis, I. Soil salinization and land desertification. Soil degradation and desertification in Mediterranean environments , 105–129 (Geoforma Ediciones, 1996).
Perri, S. et al. River basin salinization as a form of aridity. Proc. Natl Acad. Sci. USA 117 , 17635–17642 (2020).
Article CAS PubMed PubMed Central Google Scholar
Oldeman, L. R., Hakkeling, R. & Sombroek, W. G. World map of the status of human-induced soil degradation: an explanatory note (International Soil Reference and Information Centre, 2017).
Omuto, C., Nachtergaele, F. & Rojas, R. V. State of the Art Report on Global and regional Soil Information: Where are we? Where to go? (Food and Agriculture Organization of the United Nations Rome, 2013).
Pannell, D. J. Dryland salinity: economic, scientific, social and policy dimensions. Aust. J. Agric. Resour. Econ. 45 , 517–546 (2001).
Folberth, C. et al. Uncertainty in soil data can outweigh climate impact signals in global crop yield simulations. Nat. Commun. 7 , 1–13 (2016).
Amini, S., Ghadiri, H., Chen, C. & Marschner, P. Salt-affected soils, reclamation, carbon dynamics, and biochar: a review. J. Soils Sediment. 16 , 939–953 (2016).
Paz, A. M. et al. Prediction of soil salinity and sodicity using electromagnetic conductivity imaging. Geoderma 361 , 114086 (2020).
Article ADS CAS Google Scholar
Taghizadeh-Mehrjardi, R., Minasny, B., Sarmadian, F. & Malone, B. Digital mapping of soil salinity in Ardakan region, central Iran. Geoderma 213 , 15–28 (2014).
Scudiero, E., Skaggs, T. H. & Corwin, D. L. Regional scale soil salinity evaluation using Landsat 7, western San Joaquin Valley, California, USA. Geoderma Reg. 2 , 82–90 (2014).
Ghassemi, F., Jakeman, A. J. & Nix, H. A. Salinisation of land and water resources: human causes, extent, management and case studies . (CAB international, 1995).
Szabolcs, I. Salt-affected Soils (CRC Press, Inc., 1989).
FAO ITPS. Status of the world’s soil resources (SWSR)–main report . Food and agriculture organization of the United Nations and intergovernmental technical panel on soils, Rome, Italy (2015).
Hassani, A., Azapagic, A. & Shokri, N. Predicting long-term dynamics of soil salinity and sodicity on a global scale. Proc. Natl Acad. Sci. USA 117 , 33017–33027 (2020).
Article ADS CAS PubMed PubMed Central Google Scholar
Fischer, G. et al. Global agro-ecological zones assessment for agriculture (GAEZ 2008) . (IIASA, 2008).
Richards, L. A. Diagnosis and improvement of saline and alkali soils. Handbook No. 60 . (US Department of Agriculture, 1954).
Middleton, N. & Thomas, D. World atlas of desertification edn. 2. (Arnold, Hodder Headline, PLC, 1997).
UNEP-WCMC, L. A spatial analysis approach to the global delineation of dryland Areas of relevance to the CBD programme of work on dry and sub-humid lands, Dataset based on spatial analysis between WWF terrestrial ecoregions (WWF-US, 2004) and aridity zones (CRU/UEA; UNEPGRID, 1991) . Dataset checked and refined to remove many gaps, overlaps and slivers (July 2014) . https://www.unep-wcmc.org/resources-and-data/a-spatial-analysis-approach-to-the-global-delineation-of-dryland-areas-of-relevance-to-the-cbd-programme-of-work-on-dry-and-subhumid-lands (2007).
Prăvălie, R. Drylands extent and environmental issues. A global approach. Earth Sci. Rev. 161 , 259–278 (2016).
Article ADS Google Scholar
Schimel, D. S. Drylands in the earth system. Science 327 , 418–419 (2010).
Ivushkin, K. et al. Global mapping of soil salinity change. Remote Sens. Environ. 231 , 111260 (2019).
Gorji, T., Sertel, E. & Tanik, A. Monitoring soil salinity via remote sensing technology under data scarce conditions: a case study from Turkey. Ecol. Indic. 74 , 384–391 (2017).
Mulder, V., De Bruin, S., Schaepman, M. E. & Mayr, T. The use of remote sensing in soil and terrain mapping—a review. Geoderma 162 , 1–19 (2011).
Wong, V. N., Greene, R., Dalal, R. C. & Murphy, B. W. Soil carbon dynamics in saline and sodic soils: a review. Soil Use Manag. 26 , 2–11 (2010).
Várallyay, G. In Soil Responses to Climate Change 39–54 (Springer, 1994).
National Land and Water Resources Audit. Australian Dryland Salinity Assessment 2000: extent, impacts, processes, monitoring and management options (Commonwealth of Australia, 2001).
Várallyay, G. The impact of climate change on soils and on their water management. Agron. Res. 8 , 385–396 (2010).
Talat, N. In Climate Change and Soil Interactions 305–329 (Elsevier, 2020).
Tomaz, A., Palma, P., Alvarenga, P. & Gonçalves, M. C. Soil Salinity Risk in A Climate Change Scenario and Its Effect on Crop Yield (Elsevier, 2020).
Corwin, D. L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 72 , 842–862 (2021).
Intergovernmental Panel on Climate Chang. Climate Change 1995: Impacts, Adaptations and Mitigation of Climate Change: Scientific Technical Analyses: Special Report of Working Group II (Cambridge Univ. Press, 1996).
Szabolcs. In Developments in Soil Science , Vol. 20, 61–69 (Elsevier, 1990).
Bates, B. Climate change and water: IPCC technical paper VI (World Health Organization, 2009).
Karmakar, R., Das, I., Dutta, D. & Rakshit, A. Potential effects of climate change on soil properties: a review. Sci. Int. 4 , 51–73 (2016).
Yeo, A. Predicting the interaction between the effects of salinity and climate change on crop plants. Sci. Hortic. 78 , 159–174 (1998).
Van Weert, F., Van der Gun, J. & Reckman, J. Global overview of saline groundwater occurrence and genesis (International Groundwater Resources Assessment Centre, 2009).
Dasgupta, S., Hossain, M. M., Huq, M. & Wheeler, D. Climate change and soil salinity: the case of coastal Bangladesh. Ambio 44 , 815–826 (2015).
Article PubMed PubMed Central Google Scholar
Schofield, R. & Kirkby, M. Application of salinization indicators and initial development of potential global soil salinization scenario under climatic change. Glob. Biogeochem. Cycles 17 , 1078–1091 (2003).
Daliakopoulos, I. N., Pappa, P., Grillakis, M. G., Varouchakis, E. A. & Tsanis, I. K. Modeling soil salinity in greenhouse cultivations under a changing climate with SALTMED: model modification and application in Timpaki, Crete. Soil Sci. 181 , 241–251 (2016).
Martín-Rosales, W. et al. Hydrological implications of desertification in southeastern Spain/Implications hydrologiques de la désertification dans le sud-est de l’Espagne. Hydrol. Sci. J./J. des. Sci. Hydrol. 52 , 1146–1161 (2007).
Zanchi, C. & Cecchi, S. Soil Salinisation in The Grosseto plain (Maremma, Italy): An Environmental and Socio-economic Analysis of The Impact on The Agro-ecosystem (Springer, 2010).
Oude Essink, G., Van Baaren, E. S. & De Louw, P. G. Effects of climate change on coastal groundwater systems: a modeling study in the Netherlands. Water Resour. Res . 46 , W00F04 (2010).
Colombani, N., Mastrocicco, M. & Giambastiani, B. M. S. Predicting salinization trends in a lowland coastal aquifer: Comacchio (Italy). Water Resour. Manag. 29 , 603–618 (2015).
Chen, J. & Mueller, V. Coastal climate change, soil salinity and human migration in Bangladesh. Nat. Clim. Change 8 , 981–985 (2018).
Corwin, D. L., Rhoades, J. D. & Šimůnek, J. Leaching requirement for soil salinity control: steady-state versus transient models. Agric. Water Manag. 90 , 165–180 (2007).
Schoups, G., Hopmans, J. & Tanji, K. Evaluation of model complexity and space–time resolution on the prediction of long‐term soil salinity dynamics, western San Joaquin Valley, California. Hydrol. Process. Int. J. 20 , 2647–2668 (2006).
Suweis, S. et al. Stochastic modeling of soil salinity. Geophys. Res. Lett . 37 , L07404 (2010).
Perri, S., Suweis, S., Entekhabi, D. & Molini, A. Vegetation controls on dryland salinity. Geophys. Res. Lett. 45 , 11,669–11,682 (2018).
Runyan, C. W. & D’Odorico, P. Ecohydrological feedbacks between salt accumulation and vegetation dynamics: Role of vegetation‐groundwater interactions. Water Resour. Res. 46 , W11561 (2010).
Porporato, A. et al. Ecohydrological modeling in agroecosystems: examples and challenges. Water Resour. Res. 51 , 5081–5099 (2015).
Mau, Y. & Porporato, A. A dynamical system approach to soil salinity and sodicity. Adv. Water Resour. 83 , 68–76 (2015).
Padarian, J., Minasny, B. & McBratney, A. B. Machine learning and soil sciences: a review aided by machine learning tools. Soil 6 , 35–52 (2020).
Hengl, T. et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 12 , e0169748 (2017).
Huinink, H., Pel, L. & Michels, M. V. A. How ions distribute in a drying porous medium: a simple model. Phys. Fluids 14 , 1389–1395 (2002).
Article ADS CAS MATH Google Scholar
Guglielmini, L., Gontcharov, A., Aldykiewicz, A. J. Jr & Stone, H. A. Drying of salt solutions in porous materials: intermediate-time dynamics and efflorescence. Phys. Fluids 20 , 077101 (2008).
Article ADS MATH Google Scholar
Shokri, N. Pore-scale dynamics of salt transport and distribution in drying porous media. Phys. Fluids 26 , 012106 (2014).
Shokri‐Kuehni, S. M., Vetter, T., Webb, C. & Shokri, N. New insights into saline water evaporation from porous media: Complex interaction between evaporation rates, precipitation, and surface temperature. Geophys. Res. Lett. 44 , 5504–5510 (2017).
Rad, M. N., Shokri, N., Keshmiri, A. & Withers, P. J. Effects of grain and pore size on salt precipitation during evaporation from porous media. Transp. Porous Media 110 , 281–294 (2015).
Li, X., Chang, S. X. & Salifu, K. F. Soil texture and layering effects on water and salt dynamics in the presence of a water table: a review. Environ. Rev. 22 , 41–50 (2014).
Shokri‐Kuehni, S. M. et al. Water table depth and soil salinization: from pore‐scale processes to field‐scale responses. Water Resour. Res. 56 , e2019WR026707 (2020).
Taylor, K. E., Stouffer, R. J. & Meehl, G. A. An overview of CMIP5 and the experiment design. Bull. Am. Meteorol. Soc. 93 , 485–498 (2012).
Eyring, V. et al. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. Discuss. 9 , 1937–1958 (2016).
Miao, L. et al. Future drought in the dry lands of Asia under the 1.5 and 2.0 °C warming scenarios. Earth’s Future 8 , e2019EF001337 (2020).
Gupta, R. K. et al. in Encyclopedia of Soil Science (ed. Ward Chesworth) 737–738 (Springer, 2008).
Soil Survey Staff. Keys to soil taxonomy (United States Department of Agriculture, Soil Conservation Service, 2010).
Bannari, A. & Al-Ali, Z. M. Assessing climate change impact on soil salinity dynamics between 1987–2017 in arid landscape using Landsat TM, ETM+ and OLI data. Remote Sens. 12 , 2794 (2020).
Wang, B., Dong, X., Wang, Z. & Qin, G. Characterizing spatiotemporal variations of soil salinization and its relationship with eco-hydrological parameters at the Regional Scale in the Kashi Area of Xinjiang, China from 2000 to 2017. Water 13 , 1075 (2021).
Taghadosi, M. M. & Hasanlou, M. Trend analysis of soil salinity in different land cover types using landsat time series data (case study bakhtegan salt lake). International Archives of the Photogrammetry, Remote Sensing & Spatial Information Sciences 42 , Volume XLII-4/W4, 251–257 (2017).
Giorgi, F., Raffaele, F. & Coppola, E. The response of precipitation characteristics to global warming from climate projections. Earth Syst. Dyn. 10 , 73–89 (2019).
Shi, L. et al. Projecting potential evapotranspiration change and quantifying its uncertainty under future climate scenarios: a case study in southeastern Australia. J. Hydrol. 584 , 124756 (2020).
Alexander, L. V. & Arblaster, J. M. Historical and projected trends in temperature and precipitation extremes in Australia in observations and CMIP5. Weather Clim. Extremes 15 , 34–56 (2017).
Grose, M. R. et al. Insights from CMIP6 for Australia’s future climate. Earth’s Future 8 , e2019EF001469 (2020).
Colorado‐Ruiz, G., Cavazos, T., Salinas, J. A., De Grau, P. & Ayala, R. Climate change projections from Coupled Model Intercomparison Project phase 5 multi‐model weighted ensembles for Mexico, the North American monsoon, and the mid‐summer drought region. Int. J. Climatol. 38 , 5699–5716 (2018).
Easterling, D. R. et al. Precipitation change in the United States . In: Climate Science Special Report: A Sustained Assessment Activity of the U.S. Global Change Research Program (eds Wuebbles, D. J. et al.) 301–335 (U.S. Global Change Research Program, Washington, DC, USA, 2017). https://digitalcommons.unl.edu/usdeptcommercepub/586/ .
Klutse, N. A. B. et al. Potential impact of 1.5 C and 2 C global warming on consecutive dry and wet days over West Africa. Environ. Res. Lett. 13 , 055013 (2018).
Ma, X., Zhao, C., Tao, H., Zhu, J. & Kundzewicz, Z. W. Projections of actual evapotranspiration under the 1.5 C and 2.0 C global warming scenarios in sandy areas in northern China. Sci. Total Environ. 645 , 1496–1508 (2018).
Batjes, N. H. et al. WoSIS: providing standardised soil profile data for the world. Earth System Science. Data 9 , 1 (2017).
Belward, A. S., Estes, J. E. & Kline, K. D. The IGBP-DIS global 1-km land-cover data set DISCover: a project overview. Photogrammetric Eng. Remote Sens. 65 , 1013–1020 (1999).
Sulla-Menashe, D. & Friedl, M. A. User guide to collection 6 MODIS land cover (MCD12Q1 and MCD12C1) product , 1–18 (USGS, 2018).
Jenny, H. Factors of soil formation: a system of quantitative pedology (Courier Corporation, 1994).
IUSS Working Group WRB. World reference base for soil resources 2014, update 2015: International soil classification system for naming soils and creating legends for soil maps (FAO, 2015).
Global Soil Data Task Group. Global Gridded Surfaces of Selected Soil Characteristics (IGBP-DIS). Global Gridded Surfaces of Selected Soil Characteristics (International Geosphere-Biosphere Programme—Data and Information System) . https://doi.org/10.3334/ORNLDAAC/569 (2000).
Yang, Y., Donohue, R. J. & McVicar, T. R. Global estimation of effective plant rooting depth: Implications for hydrological modeling. Water Resour. Res. 52 , 8260–8276 (2016).
Esri. World Elevation Terrain data, ArcGIS Living Atlas of the World . www.arcgis.com/home/item.html?id=58a541efc59545e6b7137f961d7de883 (2020).
Desktop, ESRI ArcGIS. Release 10. Redlands, CA: Environmental Systems Research Institute 437 , 438 (2011).
Cinquini, L. et al. The Earth System Grid Federation: an open infrastructure for access to distributed geospatial data. Future Gener. Computer Syst. 36 , 400–417 (2014).
Schulzweida, U. CDO user’s guide Climate data operators, Version 1.9.8 . https://doi.org/10.5281/zenodo.3539275 (2019).
Breiman, L. Random forests. Mach. Learn. 45 , 5–32 (2001).
Article MATH Google Scholar
Kuhn, M. & Johnson, K. Applied Predictive Modeling . Vol. 26 (Springer, 2013).
Hengl, T. et al. Mapping soil properties of Africa at 250 m resolution: random forests significantly improve current predictions. PLoS ONE 10 , e0125814 (2015).
Elith, J. & Leathwick, J. Boosted Regression Trees for ecological modeling. R Documentation . https://cran.r-project.org/web/packages/dismo/vignettes/brt.pdf (2017).
De’Ath, G. Boosted trees for ecological modeling and prediction. Ecology 88 , 243–251 (2007).
Article PubMed Google Scholar
Moriasi, D. N., Gitau, M. W., Pai, N. & Daggupati, P. Hydrologic and water quality models: performance measures and evaluation criteria. Trans. ASABE 58 , 1763–1785 (2015).
Nash, J. E. & Sutcliffe, J. V. River flow forecasting through conceptual models part I—A discussion of principles. J. Hydrol. 10 , 282–290 (1970).
Hyndman, R. J. & Koehler, A. B. Another look at measures of forecast accuracy. Int. J. Forecast. 22 , 679–688 (2006).
World Meteorological Organization. WMO guidelines on the calculation of climate normals (World Meteorological Organization, 2017).
GADM. Database of Global Administrative Areas . https://gadm.org/ (2020).
Malone, B. P., McBratney, A., Minasny, B. & Laslett, G. Mapping continuous depth functions of soil carbon storage and available water capacity. Geoderma 154 , 138–152 (2009).
Minasny, B., McBratney, A. B., Mendonça-Santos, M., Odeh, I. & Guyon, B. Prediction and digital mapping of soil carbon storage in the Lower Namoi Valley. Soil Res. 44 , 233–244 (2006).
Batjes, N. H. World soil property estimates for broad-scale modelling (WISE30sec) . (ISRIC-World Soil Information, 2015).
Ye, L., Gao, L., Marcos-Martinez, R., Mallants, D. & Bryan, B. A. Projecting Australia’s forest cover dynamics and exploring influential factors using deep learning. Environ. Model. Softw. 119 , 407–417 (2019).
Schmidt, G. A. et al. Present-day atmospheric simulations using GISS ModelE: comparison to in situ, satellite, and reanalysis data. J. Clim. 19 , 153–192 (2006).
Watanabe, M. et al. Improved climate simulation by MIROC5: Mean states, variability, and climate sensitivity. J. Clim. 23 , 6312–6335 (2010).
Watanabe, S. et al. MIROC-ESM 2010: Model description and basic results of CMIP5-20c3m experiments. Geosci. Model Dev. 4 , 845 (2011).
Yukimoto, S. et al. A new global climate model of the Meteorological Research Institute: MRI-CGCM3—Model description and basic performance—. J. Meteorol. Soc. Jpn. Ser. II 90 , 23–64 (2012).
Bentsen, M. et al. The Norwegian earth system model, NorESM1-M—Part 1: Description and basic evaluation of the physical climate. Geosci. Model Dev. 6 , 687–720 (2013).
Yukimoto, S. Meteorological research institute earth system model version 1 (MRI-ESM1): model description . (Meteorological Research Institute, 2011).
Danabasoglu, G. et al. The Community Earth System Model version 2 (CESM2). J. Adv. Model. Earth Syst. 12 , e2019MS001916 (2020).
Séférian, R. et al. Evaluation of CNRM Earth System Model, CNRM‐ESM2‐1: role of Earth system processes in present‐day and future climate. J. Adv. Model. Earth Syst. 11 , 4182–4227 (2019).
Dunne, J. et al. The GFDL Earth System Model version 4.1 (GFDL-ESM4. 1): model description and simulation characteristics. J. Adv. Model. Earth Syst. 11 , 3167–3211 (2019).
Volodin, E. et al. INM-CM4-8 model output prepared for CMIP6 PMIP . https://doi.org/10.22033/ESGF/CMIP6.2295 (2019).
Volodin, E. et al. INM INM-CM5-0 model output prepared for CMIP6 CMIP , https://doi.org/10.22033/ESGF/CMIP6.1423 (2019).
Hajima, T. et al. Description of the MIROC‐ES2L Earth system model and evaluation of its climate—Biogeochemical processes and feedbacks. Geosci. Model Dev. Discuss. 2019 , 1–73 (2019).
Yukimoto, S. et al. The Meteorological Research Institute Earth System Model version 2.0, MRI-ESM2. 0: Description and basic evaluation of the physical component. J. Meteorol. Soc. Jpn. Ser. II 97 , 931–965 (2019).
Seland, Ø. et al. The Norwegian Earth System Model, NorESM2–Evaluation of theCMIP6 DECK and historical simulations. Geosci. Model Dev. 13 , 6165–6200 (2020).
Download references
Acknowledgements
We gratefully acknowledge the funding by the Presidential Doctoral Scholarship Award at The University of Manchester, UK Research Councils (grant no. EP/K011820/1) and the Institute of Geo-Hydroinformatics at Hamburg University of Technology.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Department of Chemical Engineering and Analytical Science, The University of Manchester, Sackville Street, Manchester, M13 9PL, UK
Amirhossein Hassani & Adisa Azapagic
NILU - Norwegian Institute for Air Research, PO Box 100, Kjeller, 2027, Norway
Amirhossein Hassani
Institute of Geo-Hydroinformatics, Hamburg University of Technology, Am Schwarzenberg-Campus 3 (E), 21073, Hamburg, Germany
Nima Shokri
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: A.H., A.A. and N.S. Methodology: A.H., A.A. and N.S. Funding acquisition: A.A. and N.S. Supervision: A.A. and N.S. Data acquisition: A.H. Data Analysis and programming: A.H. Investigation: A.H. and N.S. Visualization: A.H. Validation: A.H., A.A. and N.S. Writing—original draft: A.H. Writing—review and editing: A.H., A.A. and N.S.
Corresponding authors
Correspondence to Amirhossein Hassani , Adisa Azapagic or Nima Shokri .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Sun Yean Teh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Hassani, A., Azapagic, A. & Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat Commun 12 , 6663 (2021). https://doi.org/10.1038/s41467-021-26907-3
Download citation
Received : 01 November 2020
Accepted : 28 October 2021
Published : 18 November 2021
DOI : https://doi.org/10.1038/s41467-021-26907-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Enhancing salinity tolerance in cucumber through selenium biofortification and grafting.
- Masoomeh Amerian
- Amir Palangi
- Georgia Ntatsi
BMC Plant Biology (2024)
Salts out, water in
Nature Plants (2024)
A new high-resolution global topographic factor dataset calculated based on SRTM
- Hongming Zhang
- Linlin Yuan
Scientific Data (2024)
Does integrating climate change projection with agriculture policy improve agricultural adaptation and food security? Evidence from Bangladesh
- Shrinidhi Ambinakudige
- Boniface Fosu
Theoretical and Applied Climatology (2024)
Effects of soil type and salinity levels on the performance and bacteriome of the halophyte Atriplex nummularia (old man saltbush)
- Douglas Alfradique Monteiro
- Gordon F. Custer
- Caio Tavora Coelho da Costa Rachid
Plant and Soil (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
The evaluation of field performance under salt stress is difficult because of the variability of salt levels in field conditions (Richards, 1983) and the potential for interactions with other environmental factors, including soil fertility, temperature, light intensity and water loss due to transpiration. Evaluating tolerance is also made more ...
General Aspects of Plant Salt Stress. Soil salinity is one of the most important global problems that negatively affects crop productivity. Salinity impairs plant growth and development via water stress, cytotoxicity due to excessive uptake of ions such as sodium (Na +) and chloride (Cl −), and nutritional imbalance.Additionally, salinity is typically accompanied by oxidative stress due to ...
Salinity inhibits seed germination by either exerting osmotic stress that thwarts water uptake or causes ionic toxicity. These consequences collectively inhibit cell division and expansion, as well as modulates the activity of some key enzymes, thus lastly reduces the seed reserves utilization (El-Hendawy et al., 2019).Thus, it can be said that salinity negatively affected the process of ...
Considering the worldwide salinity crisis, better policies, and allocation of grants and funding for salinity stress research will assist in the sustainable development of salt-tolerant elite ...
Salinity is an important abiotic environmental stress factor threatening agricultural productivity throughout the world. The detrimental effects of salinity stress are observed at cellular, organ and whole plant level at osmotic phase (early/short-term response) and ionic phase (late/long-term response). High salinity exerts its negative impact on major plant processes such as disrupting the ...
To counteract the effects of salt stress, plants have developed various mechanisms, including modulating ion homeostasis, ion compartmentalization and export, and the biosynthesis of osmoprotectants. ... Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a ...
Introduction. The extent of agricultural land that is affected by high salinity is increasing worldwide, due to both natural phenomena and agricultural practices such as irrigation systems (Munns and Tester Citation 2008).Salinity poses two major threats to plant growth: osmotic stress and ionic stress (Flower and Colmer Citation 2008).In addition, it also manifested an oxidative stress.
As shown in A. thaliana, the genes CBF1/2/3 are specifically induced by cold but not drought and high salinity 87,89, whereas CBF4 and DREB2 genes are induced by osmotic stress or high salinity ...
Soil salinization negatively impacts plant development and induces land degradation, thus affecting biodiversity, water quality, crop production, farmers' well-being, and the economic situation in the affected region. Plant germination, growth, and productivity are vital processes impaired by salinity stress; thus, it is considered a serious threat to agriculture. The extent to which a plant ...
Significant advances in the sensory mechanisms to salt stress have been made in plants (Fig. 2).Plants might sense osmotic stress caused by soil salinization; however, the rapid and Na +-specific halotropism (i.e., roots grow away from salt) implies that there might also exist a root-based Na + sensor (Sun et al., 2008; Galvan-Ampudia et al., 2013).
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Salinity stress was caused a ...
Introduction. Salinity is one of the major abiotic stresses that has been significantly affecting the plant growth and yield (Gharsallah et al., 2016).The continuous increase in salinity in arable land due to poor cultivation practices and climate change have devastating global effects, and it is estimated that about 50% of arable land will be lost by the middle of the 21st century (Islam et ...
In on hand, the salt stress results obtained revealed a non-significant decreases in all the studied parameters of Faba bean at the salinity level S3 = 32 dS/m = 8.8 g/l, compared to S0 (control).
Salinity stress deteriorates the plants' transpiring leaves which is known as specific ion effects 12 or directly reduces the plant water uptake from the rooting zone, resulting in osmotic ...
Abstract and Figures. At present more than 20% of all the irrigated land in the world is estimated as affected by salinity and this trend is increasing with the rapid climate changes as well as ...
1. Introduction. Soil salinity, defined as a high concentration of soluble salts in soil, is one of the biggest global challenges that severely affects agricultural productivity and environmental sustainability (Shahid et al., 2018).It has a severe negative effect on the international economy, with an annual global income loss of US$ 27.3 billion (Qadir et al., 2014).
In this paper, we discussed the effect of salinity stress on the wheat crop, possible mechanisms to deal with salinity stress, and management options to improve wheat performance under salinity ...
Different measures were used to describe the fish response to the changes in salinity; cortisol as a measure of acute stress, the plasma ions Na+, Cl-, K+ and Ca2+ as a measure of osmotic stress and the fish ability to regulate the salt-water balance during stress and environmental changes, as well as pH as an indication of the fish metabolic ...
PDF | On Jun 7, 2022, Soumya Das and others published Impact of Salinity Stress on Paddy Production: A Review | Find, read and cite all the research you need on ResearchGate ... The review paper ...