

A Case Study on Endometriosis
Endometriosis is a chronic reproduction condition that still remains a mystery to the medical community. This paper starts off by providing the background information on what endometriosis is, the etiology, and risk factors associated with the condition. Following the introduction is a case study on a 20 year old female who currently suffers from the condition herself. Based on Patient X’s life, the end of this paper focuses on the prognosis she has as far as living with the disease goes, and things she can change in her lifestyle to improve her symptoms.
qnk322f06z_version1_Endometriosis_Case_Study.docx
size: 24.6 KB | mime_type: application/vnd.openxmlformats-officedocument.wordprocessingml.document | date: 2015-04-16
- V1 published November 16, 2020
Collections
This resource is currently not in any collection.
Work History
Version 1 published.
November 16, 2020 23:39 by Scholarsphere 4 Migration
- Open access
- Published: 26 January 2022
Challenges of and possible solutions for living with endometriosis: a qualitative study
- Gabriella Márki 1 , 2 ,
- Dorottya Vásárhelyi 2 ,
- Adrien Rigó 2 ,
- Zsuzsa Kaló 2 ,
- Nándor Ács 3 &
- Attila Bokor 3
BMC Women's Health volume 22 , Article number: 20 ( 2022 ) Cite this article
10k Accesses
14 Citations
29 Altmetric
Metrics details
Endometriosis as a chronic gynecological disease has several negative effects on women’s life, thereby placing a huge burden on the patients and the health system. The negative impact of living with endometriosis (impaired quality of life, diverse medical experiences) is detailed in the literature, however, we know less about patients’ self-management, social support, the meaning of life with a chronic disease, and the needs of patients. To implement a proper multidisciplinary approach in practice, we need to have a comprehensive view of the complexity of endometriosis patients’ life and disease history.
Four focus group discussions were conducted between October 2014 and November 2015 by a team consisting of medical and psychological specialists. 21 women (age: 31.57; SD = 4.45) with surgical and histological confirmation of endometriosis were included in the study. Discussions were audiotaped and transcribed verbatim, and a 62,051-word corpus was analyzed using content analysis.
Four main themes emerged from the analysis: (1) the impact of endometriosis on quality of life, (2) medical experiences, (3) complementary and alternative treatments, and (4) different coping strategies in disease management. All themes were interrelated and highly affected by a lack of information and uncertainty caused by endometriosis. A supporting doctor-patient relationship, active coping, and social support were identified as advantages over difficulties. Finding the positive meaning of life after accepting endometriosis increased the possibility of posttraumatic growth. Furthermore, women’s needs were identified at all levels of the ecological approach to health promotion.
Conclusions
Our results highlight the need for multidisciplinary healthcare programs and interventions to find solutions to the difficulties of women with endometriosis. To achieve this goal, a collaboration of professionals, psychologists, and support organizations is needed in the near future.
Peer Review reports
Introduction
Endometriosis is a chronic inflammatory disease that is defined as the presence of endometrium-like tissues outside the uterus causing pain symptoms (dysmenorrhea, dyspareunia, chronic pelvic pain) and infertility [ 1 ]. This gynecological disease affects approximately 2–10% of the reproductive-aged and 50% of infertile women, and women with endometriosis have increased risk of obstetric outcome [ 2 ]. Because symptoms are not specific, the diagnostic delay is almost 8–10 years [ 3 ].
Endometriosis has a negative impact on health-related quality of life (HRQoL) [ 4 ]. Quantitative studies identified deterioration in physical wellbeing [ 5 ], psychological functioning [ 6 , 7 ], daily life activities and work productivity [ 8 , 9 ], social participation [ 10 ], quality of sexual life [ 11 ], and an increase in financial burden [ 12 ]. Decreased HRQoL has a negative feedback effect on endometriosis progression [ 13 , 14 ]. Furthermore, it is already known that pain is a major cause of these physical, psychosocial, emotional, and work-related difficulties among patients [ 15 , 16 , 17 ].
Previously published qualitative data demonstrated the negative impact of endometriosis on HRQoL and medical experiences but offered fewer findings of self-management, social support, femininity, the meaning of life with a chronic disease, and future directions and needs of patients. Therefore, this study aims to expand knowledge of (i) the difficulties women have when living with endometriosis and (ii) their opportunities and mechanisms for coping with the negative impact of the disease. We assume that by exploring these main areas we can help to develop health promotion strategies to reduce further negative effects on women's lives.
Study design, procedure, and data collection
This qualitative study is part of a comprehensive study on the psychosocial aspects of endometriosis conducted by Eötvös Loránd University in conjunction with Semmelweis University, Budapest, Hungary. Participants with surgical and histological confirmation of endometriosis from our previous study [ 18 ] were invited via e-mail to participate in exploratory focus group discussions, where participation was voluntary. The invitation explained the nature and details of the study. Four focus group discussions were conducted in the department room of the Institute of Psychology, Eötvös Loránd University between October 2014 and November 2015. Within the postpositivist qualitative paradigm [ 19 ] we followed the phenomenological approach to inquiry. Focus groups allow data to be collected through a group, where participants express their opinion more naturally and influence one another, thus it is more likely that new issues will be raised than in a one-to-one interview. Furthermore, focus groups allow the perceptions, emotions, and concerns of participants to be explored [ 20 , 21 ].
Focus group participants were asked to articulate their concerns and experiences on two topics: (i) living with endometriosis, (ii) disease-management, and experiences of medical or other treatments. All conversations were guided by the first author and trained assistant, who took field notes. Focus groups were audiotaped with their permission and transcribed verbatim by the assistant. Overall, there were 462 min of recording which were then transcribed into a 62,051-word corpus for the analysis.
The verbatim transcripts included typical or relevant non-verbal expressions (laughing, long pauses) that were confirmed by the assistant who made observations during focus group sessions. The basic element of analysis was the word. After checking the transcript (rereading the text while listening to the voice recording), the text was analyzed line by line using content analysis [ 22 , 23 ] in ATLAS.ti by two independent coders [ 24 ]. They discussed and compared collected codes from the data and after reaching consensus code groups, defined categories and created themes. Final themes and categories were checked against codes [ 22 ].
Sample characteristics
The study involved 21 patients diagnosed with endometriosis with a mean age of 31.57 (SD = 4.45). Participants (> 18 years) represented a homogenous group in terms of socio-economic background and ethnicity. On average, participants saw more than three gynecologists (range 1–15) and one alternative healer (range 0–4) before being diagnosed with endometriosis. The diagnostic delay was 2.05 years (SD = 3.32; range 0–12). Most participants (66.7%) had endometriosis-related symptoms by the time of the study. In all our participants, peritoneal endometriosis was observed (21/21, 100%), ovarian endometriosis was found in 11 patients (11/21, 52.3%) while deep infiltrating endometriosis affecting the rectum and/or the rectovaginal space was present in 6 cases (6/21, 28.6%). None of our patients had extrapelvic or abdominal wall endometriosis. Most patients of the whole sample used medical hormonal treatment at the time of the conversation. From the whole cohort, 18 (18/21, 85.7%) women received combined oral contraceptive therapy on a continuous regimen. We have observed no difference between the types of oral contraceptives since they were all combined pills containing dienogest and ethinylestradiol. Most of our participants (16/21) struggled to get pregnant, only one of them had a successful clinical pregnancy and delivery at the time of the conversation, further 23.8% of participants were undergoing in vitro fertilization (IVF). None of study participants had a comorbid psychiatric disorder.
Thematic analysis findings
Four main themes emerged from the analysis: (a) impact of endometriosis on quality of life, (b) medical experiences, (c) complementary and alternative treatments, (d) different coping strategies in disease-management (Table 1 ). Notably, that all themes were highly affected by a lack of information and uncertainty related to endometriosis, while all the emerged themes showed a dynamic connection between them and present the patients’ circular pathways (Fig. 1 ).
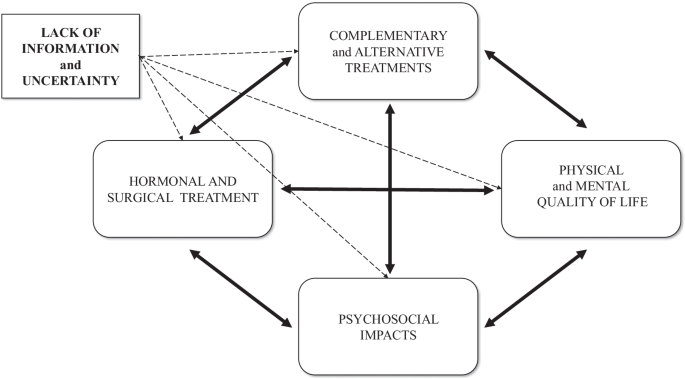
A model of the dynamic relationship of endometriosis-related themes and negative impacts
Theme 1: Impact of endometriosis on quality of life
Physical impacts.
Most women mentioned chronic pelvic pain, dysmenorrhea, and dyspareunia as leading symptoms of their endometriosis. Most of them reported that pain killers or special body positions did not significantly relieve pain.
It was never-ending, so I lived like this every day. I kneeled on the ground. I moved back and forth because it was not good any other way and yet I still held tightly onto my hair because I was in so much pain.
Psychological impacts
Besides the physical burden, participants also reported psychological consequences of endometriosis, namely anxiety, stress, and helplessness , and sometimes these were more confusing and annoying than the physical symptoms. One participant described how depressing it was to realize that she had lost 10 years of her life living in permanent pain without receiving the correct diagnosis and treatment. Feelings of loss and shame were also highlighted by participants. Uncertainty about the possible recurrence of the disease has been identified as a further stress factor in women who wanted to take an active role in their disease management. The negative emotional state negatively shaped their way of thinking, and subsumed their everyday lives.
You can’t do anything about the physical side anymore, but the psychological aspects, they leave their mark on you. I gave in to endometriosis, in fact, my whole life revolved around it. It made me bitter, and I realized after a while that I couldn’t think about anything else.
The psychosocial effects of endometriosis
This category includes common and cumulative effects of physical and psychological impacts. Endometriosis-related uncertainty had several negative impacts on women’s life. Families and friendships were affected by a lack of adequate information and a feeling of helplessness.
Friendships were ruined at that time. There was not one aspect of my life that was not affected by endometriosis. Within the family, you can release the stress that you cannot release anywhere else. It was common for me to cry during family dinners. Even friends who were supportive did not always understand what I was going through.
Intimate relationships were negatively affected by uncertainties. Participants mentioned that explaining the disease and giving reassurance to their husbands was difficult. Women agreed that a supportive partner can be the biggest source of help and support, but not every relationship was able to handle the burden of endometriosis.
It [endometriosis] cost me my marriage… At that time, we had already started in vitro fertilization. The first one ended up in colonic obstruction and I got a stoma for three months. Before the next round of IVF started my ex-husband said it was over for him.
Some women experienced sexual problems and the inconvenience of sharing their experiences of dyspareunia due to the normalizing reaction of society and health care providers. The non-sharable experiences led in two cases to sexual aversion, when “ sex was equal to pain”.
Besides dyspareunia and sexual dysfunctions, the most burden for most women were fertility problems. Participants stated that they pursued one of two options: some women insisted on childbearing and did not give up even after the defeats and inconveniences of IVF, because they thought it was worth the sacrifice; others re-evaluated pregnancy and went on to consider other options for motherhood.
We try and we hope, and I don’t know. I have learned a big lesson from this—that it would be nice to have a baby, but what if I can never have my own baby—because it could happen. Now I can say it out loud: it is okay, I can adopt a child or choose other options.
Female identity was negatively affected by infertility, sexual problems, and impersonal medical examinations. Repression and negative attitudes towards femininity have been mentioned as possible causes of their disease.
If you do not experience your femininity, it will come back to haunt you at some point.
Endometriosis and its treatment had a significant impact on participation in education and employment . Women mentioned sick-leave and semester deferral due to dysmenorrhea, as well as sleeping problems and surgery. The impact on employment usually depended on the boss and the flexibility of the workplace.
It can cause a lot of tension, finding where the line is between asking your boss to let you leave and be patient, or feeling that you are risking your job and tomorrow maybe you don’t have to go to work anymore.
The cost of gynecological consultations, medications, surgery, healthy nutrition, and further treatments caused a financial burden and required a considerable amount of time and energy .
The costs associated with endometriosis are so high; my family has an emergency budget just for this.
Theme 2: Medical experiences
Diagnostic delay.
Participants usually experienced that health professionals normalized symptoms of dysmenorrhea. It was not only normalization but physicians’ lack of adequate knowledge relating to endometriosis that caused misdiagnosis and diagnostic delay.
I went from doctor to doctor for seven years and I knew something was wrong because I could not conceive, so we were looking for the reason behind it. But a lot of doctors did not recognize the disease and that was the biggest problem.
Treatment of endometriosis
The option of pharmacological treatments was divisive among participants; most of them were concerned about side effects. Participants reported being fearful before surgery and stated that they were concerned about reproductive organs and intestinal involvement or getting stoma. Fear and uncertainty were pronounced concerning recovery and lack of information right after surgery. Participants reported that having a child was usually expressly recommended by gynecologists as a potential treatment option . These women often experienced medical and social pressure to have a baby, even if they did not feel ready to become mothers.
A woman can find herself in this trap. Although the gynecologist means well, saying you must have a child as soon as possible is such a burden on the woman. It is unbearable and impossible to process.
Infertility was a sensitive topic in every discussion. Participants who underwent assisted reproductive technology treatment described it as impersonal, physically, and mentally stressful, for men as well. Furthermore, the possible recurrence of endometriosis proved to be one of the biggest uncertainty factors, and it placed a huge burden on women.
Doctor-patient relationship
Most women agreed that having a good, reliable gynecologist specialized in endometriosis is one of the most essential factors in managing endometriosis. Many participants had negative experiences with doctors who were negligent or had insufficient professional knowledge of endometriosis, which increased diagnostic delay by several years. Women highlighted that healthcare professionals’ uncertainty led to mistrust, increased fear, and despondency, and caused them to go ‘doctor-shopping’ because they could not accept their doctor’s negligent attitude towards their symptoms or recommended treatment options. All the women agreed that physicians who reassured and informed them properly as a specialized professional in endometriosis engendered the most trust.
It was an odd experience, that even doctors can’t tell me what is wrong with me and what will make me feel better. So, you have to go until you find someone you can at least trust.
Theme 3: Complementary and alternative treatments
This theme includes women’s motivation towards all kinds of complementary and alternative treatments which may supplement or substitute medical treatments.
Lifestyle changes as treatment
Despite a lack of scientific evidence and findings of the positive effects of lifestyle change , women wanted to achieve better physical health and HRQoL and long-term recovery.
You have to be very conscious and responsible and need an incredible amount of time to develop this routine. I was exhausted and I wanted nothing more than to go to bed, but I knew if I did not prep my lunch for the next day, then I wasn’t going to have a [healthy] meal.
These women were given a great deal of contradictory information about their potential endometriosis diet . Those following a strict diet said it was like being a prisoner and they suffered because of the financial cost. When the diet was ineffective or too strict, women gave in and started to follow the needs of their bodies and developed a unique, personalized diet.
When I accidentally ate something, which was forbidden in the diet I would hate myself. Now I listen to my body, the things it likes or does not like.
Although it is difficult to find enough time and mobilize resources, all women agreed that physical activity is an essential part of managing the disease. Women were doing various sports (yoga, running, cycling, Zumba, swimming, intimate muscle training, and Pilates) regularly, but the efficacy of these sports was not specified during discussions.
Due to the unknown etiology of the disease, participants stated that they had thought about the psychosomatic, stress-related origin of endometriosis. Many women sought psychological help by using cognitive methods, schema therapy, EMDR (Eye Movement Desensitization and Reprocessing), stress management, autogenic training, meditation, and hypnosis to alleviate their symptoms.
I went to a psychologist, and I opened up about this stuff [dyspareunia]. She pointed out things that I could not see myself, and for some reason, I believe that if I defeat this misery the endometriosis will disappear, too.
Naturopathy and other methods
A wide range of naturopathic medicine (acupuncture, reflexology, Chinese medicine, Ayurveda, kinesiology, herbs) was mentioned in focus group discussions. When women find no answer in western medicine, they seek help through alternative treatments.
Then I decided to start taking a path which I normally would not take, as the path that I am currently on is not working.
Participants were not able to agree about the impact of the aforementioned methods, because each woman had a different view of those effects.
Theme 4: Different coping strategies in disease-management
Obtaining information.
One of the most important aspects of disease-management was obtaining reliable information. Women were motivated to access as much information as possible, however, it was the area with the most obstruction. Contradictory information increased the feeling of uncertainty (see Fig. 2 ). Insufficient information from health care professionals also increased uncertainty . Only some women felt that they were properly informed by their gynecologists, and many of them found that they had to drag the information out of their doctors. After the diagnosis, some participants were sent home to read about endometriosis on the internet .
When you are sent home to look into it on the internet, it’s like being thrown into the sea in order to teach you how to swim.
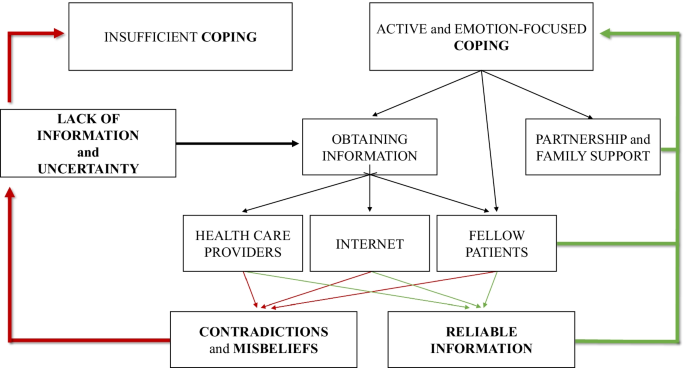
The model of sufficient and insufficient ways of coping with endometriosis
All women experienced that the internet is full of contradictions and misconceptions . Furthermore, destructive opinions, negative experiences, and rumors from fellow patients on blogs and online forums often confused them. Some women learned the most about endometriosis from fellow patients in the waiting room, where they were able to exchange experiences and inform one another. However, they also drew attention to the distress they had experienced.
We [fellow patients] can understand each other’s problems because we are in the same boat, but if I get no positive feedback and I can’t say anything to her that she needs, it’s not good.
Active control and emotion-focused coping
Women described a wide range of active and emotion-focused coping mechanisms they need to be able to use flexibly. In addition to obtaining information, most women avoided passivity and took control, assuming an active role and self-care in managing endometriosis. Some women stated that since changing their lifestyle, they have been able to live a full life. Others mentioned the importance of listening to the signs and needs of their bodies. Many participants coped with the difficulties and uncertainty of endometriosis by having a positive attitude , trying to find the positive aspects, and trying to remain optimistic.
Endometriosis taught me to take care of myself, and try to heal myself, to listen to my body and my inner voice, to look for methods that might help, and to find those which really help.
Social support
All women agreed on the importance of social support and support from their partners . “I cannot tell you how much it helps when he [male partner] stands by you.” They were able to cope with living with endometriosis, operations, and treatment thanks to the personal support of relatives and friends . Several participants mentioned the important role played by endometriosis community members, who can give support by sharing intimate experiences of endometriosis so that women do not have to face their problems alone.
It is always nice to get support from others who have experienced similar things and similar problems, so it feels good to talk about it. You are not alone.
Positive meaning of life after accepting endometriosis
Women described the difficulties, uncertainties, and lack of information surrounding endometriosis as pervasive features of their lives. Nevertheless, despite many difficulties and problems, women described a positive impact ( peace, patience, openness, personality development, and gratitude ) on their life after accepting their condition.
A whole new world has opened up before me. I am not saying that it is good to have endometriosis, but I have completely changed because of it. I would not be the same person if I had not gone through this. I improved as a person, and the journey is not over yet. I would not be as open towards people, I would not have these kinds of relationships, my family and my relationship would not be the same. I have a sense of purpose.
Possible responses to “do patients know what they need?”
Focus group discussions allowed women with endometriosis to demonstrate their desire to take an active role in the management of their disease and to express their needs and options for alleviating the difficulties and deficiencies. These suggestions allow us to understand the real needs of women with endometriosis and design a proper health promotion program.
Giving proper information from reliable sources could be one of the best ways of reducing uncertainty and increasing HRQoL. Participants highlighted the need for information about surgical results right after the postoperative wake-up, which would reduce postoperative stress, anxiety, and uncertainty.
Women suggested that diagnostic delay, the risk of misdiagnosis, and the normalization of dysmenorrhea could be reduced through more extensive training and by improving the specialist knowledge of a broader range of health care professionals and medical students in all related medical areas in relation to the recognition of endometriosis.
Almost every woman agreed that clinical or health psychologists are needed in hospitals to help cope with diagnosis and surgery and to process disease-management.
All women agreed on the importance of raising awareness of endometriosis by involving male partners, friends, and colleagues. Educating and informing men about endometriosis would have long-term advantages, as men could provide effective help and support to women with endometriosis. Women highlighted the fact that it could also be very stressful for men to be involved, and that because of many uncertainty factors there should be educational and supportive groups for men as well.
To prevent more severe conditions, participants agreed that awareness of and education about endometriosis is necessary from menarche. Preventive and educational programs relating to endometriosis in schools would help ensure the early diagnosis of future patients.
Furthermore, women stated that it is a social responsibility to increase publicity and awareness of endometriosis in society, and a campaign like that for breast cancer would help to educate all social groups.
In our study, we first report a mutual dynamic connection between the main endometriosis-related themes (HRQoL, medical experiences, complementary and alternative treatment, and coping strategies), and show that these areas are negatively influenced by the most prominent themes: uncertainty and lack of information. Exploring the connections between these themes will also help to understand patient pathways, which is essential for planning the long-term management of women with endometriosis.
Identified topics are comparable with previous findings [ 25 , 26 ], where negative impact on HRQoL and medical experience of endometriosis appeared as essential topics. Our results highlight that these themes are not independent of one another (see Figs. 1 , 2 ). Prolonged (pain)symptoms of endometriosis decrease quality of life, and direct women to health care, where patients can face a variety of different experiences. An inadequate doctor-patient relationship affects not only medical experiences and the physical condition of patients but also impairs adherence, compliance, and HRQoL. Ineffective medical attention or treatment affects women’s relationship with healthcare and leads them to use (non)evidence-based alternative treatments. Patients need active, emotion-focused coping strategies which are properly supported by positive medical experiences, reliable information, and effective social support. In their absence, patients may use inadequate coping options, which can have a negative impact on HRQoL. Lifestyle change as a potential coping and disease-management strategy [ 27 ] is an obvious opportunity for women to have control over one aspect of their condition. Nonetheless, the effectiveness of nonmedical treatments in endometriosis has not been sufficiently explored by evidence-based medicine [ 3 ]. Our results highlight the importance of finding a scientific response to women’s questions because failed attempts have a negative impact on prognosis, quality of life, and self-esteem [ 25 ].
Uncertainty and lack of information can have a direct impact on HRQoL, medical experiences, coping, and indirectly, on fertility as well [ 15 ]. The normalization and rejection of symptoms as a general problem impact the doctor-patient relationship before diagnosis and leads to diagnostic delay and eliminates the benefits of early diagnosis [ 3 ].
The lack of information at health care centers causes women to seek self-management strategies [ 15 , 28 , 29 ]. The lack of information causes women to seek self-management strategies. Women try to obtain information from various sources, but they come across a great deal of contradictory information, which needs to be dealt with. Studies identified that becoming assertive and taking control can be a potential coping mechanism before diagnosis and treatment [ 28 ], but there are fewer findings of how women cope with endometriosis and achieve an asymptomatic and fertile life after diagnosis. The women in our study used positive emotion-focused coping strategies to focus on the positive and optimistic aspects of their lives. Besides, problem-focused coping (versus non-adaptive focus on emotions) was found as an adaptive and assertive coping strategy that correlates with lower stress and depressive symptoms. [ 30 ]. On the other hand, catastrophizing is a negative cognitive and emotional coping response to pain [ 31 ] and enhances pain perception as a predictor among women with endometriosis [ 32 ]. Roomaney and Kagee [ 33 ] highlight—in line with our results—that both problem-focused and positive emotion-focused coping strategies can be helpful for women with endometriosis. A third means of coping is based on the help and support provided by personal relationships and endometriosis communities. Strong relationships were characterized by admiration for women’s courage, independence, and inner strength [ 34 , 35 ]. Self-help groups and endometriosis foundations can provide effective support to women from the individual (see reliable information; health promotion programs) [ 36 ] to society (see social awareness and publicity) [ 37 , 38 ].
In addition to negative consequences and needs, there were some interesting findings supporting the results of Facchin et al. [ 22 ] about finding the meaning of life with endometriosis. Women with positive emotion-focused coping strategies and a lower level of stress can accept the disease and find positive meaning in their lives from endometriosis. These results suggest the possibility of posttraumatic growth (PTG) in endometriosis. PTG is defined as the “positive psychological change experienced as a result of the struggle with highly challenging life circumstances” [ 39 ] (e.g. chronic disease as trauma or danger to health). Previous studies on women with chronic disease identified that PTG is negatively associated with age, depression, and stress, while positively associated with time since diagnosis, education, income, social support, mental HRQoL, self-efficacy, self-esteem, and optimism [ 40 , 41 , 42 , 43 , 44 ]. These characteristics show similarities with predictors of mental health quality in endometriosis [ 18 , 45 ], although to the author’s knowledge PTG in endometriosis patients has not been measured yet. The authors suggest that PTG may occur due to multiple health behavior changes which improve active coping and the patient’s sense of control [ 46 , 47 , 48 ]. Therefore, it is recommended that the possibility of PTG be explored in future endometriosis studies.
The authors acknowledge that are some limitations to the current study. Firstly, the study sample was low and consisted of participants with homogeneous demographic and disease characteristics. Secondly, we collected our data retrospectively. We asked women about their experiences about living with endometriosis without making differences in the pre- and post-operative period, because we wanted to collect all the affected areas in their life. Although there can be differences before and after the endometriosis surgery for example in the quality of sexual life [ 49 ]. These differences can be analyzed in further qualitative studies.
Thirdly, as endometriosis is a benign disorder, the primary objective of any treatment should be to alleviate symptoms, control progression, and improve quality of life. Laparoscopic surgery is the most widely accepted surgical approach in cases of peritoneal, ovarian, and deep infiltrating endometriosis (DIE) [ 3 , 50 , 51 ]. Peritoneal disease can be excised or vaporized using different energy sources, while ovarian endometriosis can be managed by cystectomy or ablation. According to the recent data the ovarian cystectomy may lead to the loss ovarian reserve [ 52 ]. The optimal type of colorectal resection in case of bowel DIE, whether conservative (shaving, disc resection) or radical technique (segmental bowel resection) has to be applied is under discussion [ 53 , 54 , 55 , 56 , 57 ]. It has been suggested that the conservative surgical therapy of colorectal DIE is associated with lower morbidity, however the unequivocal evidence supporting this hypothesis is still lacking. The external validity of present data regarding the surgical therapy of endometriosis should be investigated in future multicentric prospective randomized trials on a large cohort of patients. A clear limitation of our study is that we did not assess the impact of different surgical methods on the endometriosis related quality of life in our group of patients.
Further, as a result of the recruitment process predominantly women with active coping strategies and an optimistic attitude applied to take part in the study. Thirdly, the themes that emerged were facilitated by means of predetermined questions, and participants would have continued conversations in three areas. This may cause some limitations to the possible themes and topics of endometriosis discussed (e.g. symptoms, medical and surgical experiences).
Finally, coping strategies and PTG in endometriosis would have been identified by using appropriate questionnaires.
Uncertainty and lack of information about endometriosis as main challenges and difficulties have a significant impact on women’s life. The present findings indicate that cooperation between health care professionals, psychologists, and support organizations will be necessary for the future to provide care and find possible solutions to the needs of women living with endometriosis. Communication must be improved, and psychosocial problems need to be recognized by health care providers to ensure that empathetic care is provided. Having evidence-based answers about the efficiency of alternative and complementary therapies could decrease the uncertainty and lack of information. Furthermore, in order to reduce diagnostic delay, health care providers’ knowledge and society’s awareness of endometriosis should be improved in the near future. Health promotion programs and support groups should be managed to facilitate coping and posttraumatic growth in women with endometriosis. Achieving these recommendations would allow women to live an asymptomatic, fertile, and balanced life with endometriosis.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Infiltrating endometriosis
- Health-related quality of life
In vitro fertilization
Posttraumatic growth
Giudice LC, Kao LC. Endometriosis. The Lancet. 2004;364(9447):1789–99.
Article Google Scholar
Berlanda N, Alio W, Angioni S, Bergamini V, Bonin C, Boracchi P, et al. Impact of endometriosis on obstetric outcome after natural conception: a multicenter Italian study. Arch Gynecol Obstet; 2021.
Dunselman GAJ, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod Oxf Engl. 2014;29(3):400–12.
Article CAS Google Scholar
D’Alterio MN, Saponara S, Agus M, Laganà AS, Noventa M, Loi ES, et al. Medical and surgical interventions to improve the quality of life for endometriosis patients: a systematic review. Gynecol Surg. 2021;18(1):13.
De Graaff AA, D’Hooghe TM, Dunselman GAJ, Dirksen CD, Hummelshoj L, WERF EndoCost Consortium, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod Oxf Engl. 2013; 28(10):2677–85.
Pope CJ, Sharma V, Sharma S, Mazmanian D. A systematic review of the association between psychiatric disturbances and endometriosis. J Obstet Gynaecol Can JOGC J Obstet Gynecol Can JOGC. 2015;37(11):1006–15.
Smorgick N, Marsh CA, As-Sanie S, Smith YR, Quint EH. Prevalence of pain syndromes, mood conditions, and asthma in adolescents and young women with endometriosis. J Pediatr Adolesc Gynecol. 2013;26(3):171–5.
Article PubMed Google Scholar
Fourquet J, Gao X, Zavala D, Orengo JC, Abac S, Ruiz A, et al. Patients’ report on how endometriosis affects health, work, and daily life. Fertil Steril. 2010;93(7):2424–8.
Article PubMed PubMed Central Google Scholar
Nnoaham KE, Hummelshoj L, Webster P, d’Hooghe T, de Cicco NF, de Cicco NC, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
Gilmour JA, Huntington A, Wilson HV. The impact of endometriosis on work and social participation. Int J Nurs Pract. 2008;14(6):443–8.
Barbara G, Facchin F, Buggio L, Somigliana E, Berlanda N, Kustermann A, et al. What is known and unknown about the association between endometriosis and sexual functioning: a systematic review of the literature. Reprod Sci Thousand Oaks Calif. 2017;1:1933719117707054.
Google Scholar
Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod Oxf Engl. 2012;27(5):1292–9.
Laganà AS, La Rosa VL, Rapisarda AMC, Valenti G, Sapia F, Chiofalo B, et al. Anxiety and depression in patients with endometriosis: impact and management challenges. Int J Womens Health. 2017;9:323–30.
Tariverdian N, Theoharides TC, Siedentopf F, Gutiérrez G, Jeschke U, Rabinovich GA, et al. Neuroendocrine-immune disequilibrium and endometriosis: an interdisciplinary approach. Semin Immunopathol. 2007;29(2):193–210.
Culley L, Law C, Hudson N, Denny E, Mitchell H, Baumgarten M, et al. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum Reprod Update. 2013;19(6):625–39.
Facchin F, Barbara G, Saita E, Mosconi P, Roberto A, Fedele L, et al. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynaecol. 2015;36(4):135–41.
Moradi M, Parker M, Sneddon A, Lopez V, Ellwood D. Impact of endometriosis on women’s lives: a qualitative study. BMC Womens Health. 2014;14:123.
Márki G, Bokor A, Rigó J, Rigó A. Physical pain and emotion regulation as the main predictive factors of health-related quality of life in women living with endometriosis. Hum Reprod. 2017;32(7):1432–8.
Ponterotto JG. Qualitative research in counseling psychology: A primer on research paradigms and philosophy of science. J Couns Psychol. 2005;52:126–36.
Krueger RA, Casey MA. Focus groups: A practical guide for applied research. Thousand Oaks: Sage Publications; 2014.
Morgan DL. The focus group guidebook, vol. 1. Thousand Oaks: Sage Publications; 1997.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Joffe H, Yardley L. Content and thematic analysis. In: Research methods for clinical and health psychology. 2004th ed. California: Sage; 2004. p. 56–68.
Creswell JW. Qualitative research design and inquiry: choosing among five approaches. 2nd ed. Thousand Oaks: Sage Publications Inc.; 2007.
Facchin F, Saita E, Barbara G, Dridi D, Vercellini P. “Free butterflies will come out of these deep wounds”: A grounded theory of how endometriosis affects women’s psychological health. J Health Psychol. 2017;11:1359105316688952.
Young K, Fisher J, Kirkman M. Women’s experiences of endometriosis: a systematic review and synthesis of qualitative research. J Fam Plann Reprod Health Care. 2015;41(3):225–34.
Buggio L, Barbara G, Facchin F, Frattaruolo MP, Aimi G, Berlanda N. Self-management and psychological-sexological interventions in patients with endometriosis: strategies, outcomes, and integration into clinical care. Int J Womens Health. 2017;9:281–93.
Article CAS PubMed PubMed Central Google Scholar
Cox H, Henderson L, Andersen N, Cagliarini G, Ski C. Focus group study of endometriosis: struggle, loss and the medical merry-go-round. Int J Nurs Pract. 2003;9(1):2–9.
Cox H, Henderson L, Wood R, Cagliarini G. Learning to take charge: women’s experiences of living with endometriosis. Complement Ther Nurs Midwifery. 2003;9(2):62–8.
Donatti L, Ramos DG, de Andres MP, Passman LJ, Podgaec S. Patients with endometriosis using positive coping strategies have less depression, stress and pelvic pain. Einstein Sao Paulo Braz. 2017;15(1):65–70.
Sullivan MJL, Lynch ME, Clark AJ. Dimensions of catastrophic thinking associated with pain experience and disability in patients with neuropathic pain conditions. Pain. 2005;113(3):310–5.
Martin CE, Johnson E, Wechter ME, Leserman J, Zolnoun DA. Catastrophizing: a predictor of persistent pain among women with endometriosis at 1 year. Hum Reprod Oxf Engl. 2011;26(11):3078–84.
Roomaney R, Kagee A. Coping strategies employed by women with endometriosis in a public health-care setting. J Health Psychol. 2016;21(10):2259–68.
Culley L, Law C, Hudson N, Mitchell H, Denny E, Raine-Fenning N. A qualitative study of the impact of endometriosis on male partners. Hum Reprod Oxf Engl. 2017;15:1–7.
Fernandez I, Reid C, Dziurawiec S. Living with endometriosis: the perspective of male partners. J Psychosom Res. 2006;61(4):433–8.
Shoebotham A, Coulson NS. Therapeutic affordances of online support group use in women with endometriosis. J Med Internet Res. 2016;18(5):e109.
Richard L, Potvin L, Kishchuk N, Prlic H, Green LW. Assessment of the integration of the ecological approach in health promotion programs. Am J Health Promot AJHP. 1996;10(4):318–28.
Article CAS PubMed Google Scholar
Facchin F, Saita E, Barbara G, Dridi D, Vercellini P. Free butterflies will come out of these deep wounds: A grounded theory of how endometriosis affects women’s psychological health. J Health Psychol. 2017;23:538–49.
Tedeschi RG, Calhoun LG. Posttraumatic growth: conceptual foundations and empirical evidence. Psychol Inq. 2004;15(1):1–18.
Barskova T, Oesterreich R. Post-traumatic growth in people living with a serious medical condition and its relations to physical and mental health: a systematic review. Disabil Rehabil. 2009;31(21):1709–33.
Danhauer SC, Case LD, Tedeschi R, Russell G, Vishnevsky T, Triplett K, et al. Predictors of posttraumatic growth in women with breast cancer. Psychooncology. 2013;22(12):2676–83.
Koutrouli N, Anagnostopoulos F, Griva F, Gourounti K, Kolokotroni F, Efstathiou V, et al. Exploring the relationship between posttraumatic growth, cognitive processing, psychological distress, and social constraints in a sample of breast cancer patients. Women Health. 2016;56(6):650–67.
Wang M-L, Liu J-E, Wang H-Y, Chen J, Li Y-Y. Posttraumatic growth and associated socio-demographic and clinical factors in Chinese breast cancer survivors. Eur J Oncol Nurs Off J Eur Oncol Nurs Soc. 2014;18(5):478–83.
Yeung NCY, Lu Q. Perceived stress as a mediator between social support and posttraumatic growth among Chinese American breast cancer survivors. Cancer Nurs. 2016;41:53.
Facchin F, Barbara G, Dridi D, Alberico D, Buggio L, Somigliana E, et al. Mental health in women with endometriosis: searching for predictors of psychological distress. Hum Reprod. 2017;32(9):1855–61.
Fong AJ, McDonough MH, Pila E, Sabiston CM. Posttraumatic growth in breast cancer survivors: the roles of physical activity and social support. J Exerc Mov Sport. 2017;49(1):165.
Hawkes AL, Pakenham KI, Chambers SK, Patrao TA, Courneya KS. Effects of a multiple health behavior change intervention for colorectal cancer survivors on psychosocial outcomes and quality of life: a randomized controlled trial. Ann Behav Med Publ Soc Behav Med. 2014;48(3):359–70.
Love C, Sabiston CM. Exploring the links between physical activity and posttraumatic growth in young adult cancer survivors. Psychooncology. 2011;20(3):278–86.
Di Donato N, Montanari G, Benfenati A, Monti G, Leonardi D, Bertoldo V, et al. Sexual function in women undergoing surgery for deep infiltrating endometriosis: a comparison with healthy women. J Fam Plann Reprod Health Care. 2015;41(4):278–83.
Redwine DB, Sharpe DR. Laparoscopic segmental resection of the sigmoid colon for endometriosis. J Laparoendosc Surg. 1991;1(4):217–20.
Bokor A, Hudelist G, Dobó N, Dauser B, Farella M, Brubel R, et al. Low anterior resection syndrome following different surgical approaches for low rectal endometriosis: a retrospective multicenter study. Acta Obstet Gynecol Scand. 2021;100(5):860–7.
Younis JS, Shapso N, Ben-Sira Y, Nelson SM, Izhaki I. Endometrioma surgery-a systematic review and meta-analysis of the effect on antral follicle count and anti-Müllerian hormone. Am J Obstet Gynecol. 2021; S0002-9378(21)00788-2.
Bokor A, Lukovich P, Csibi N, D’Hooghe T, Lebovic D, Brubel R, et al. Natural orifice specimen extraction during laparoscopic bowel resection for colorectal endometriosis: technique and outcome. J Minim Invasive Gynecol. 2018;25(6):1065–74.
Hudelist G, Aas-Eng MK, Birsan T, Berger F, Sevelda U, Kirchner L, et al. Pain and fertility outcomes of nerve-sparing, full-thickness disk or segmental bowel resection for deep infiltrating endometriosis—a prospective cohort study. Acta Obstet Gynecol Scand. 2018;97(12):1438–46.
Roman H, Bubenheim M, Huet E, Bridoux V, Zacharopoulou C, Daraï E, et al. Conservative surgery versus colorectal resection in deep endometriosis infiltrating the rectum: a randomized trial. Hum Reprod Oxf Engl. 2018;33(1):47–57.
Borghese G, Raimondo D, Esposti ED, Aru AC, Raffone A, Orsini B, et al. Preoperative ureteral stenting in women with deep posterior endometriosis and ureteral involvement: Is it useful? Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2021.
Seracchioli R, Raimondo D, Arena A, Zanello M, Mabrouk M. Clinical use of endovenous indocyanine green during rectosigmoid segmental resection for endometriosis. Fertil Steril. 2018;109(6):1135.
Download references
Acknowledgements
The authors would like to thank Szilvia Kassai of the Institute of Psychology, Eötvös Loránd University, Budapest, Hungary, for her expert and methodological advice in relation to this study, and Boglárka Kristóf, a psychology student at Eötvös Loránd University, Budapest, Hungary, for her help with the pilot analysis.
Open access funding provided by Semmelweis University. There were no external sources of funding for this study. We acknowledge the significant contribution of the author’s workplaces, the Institute of Psychology, Eötvös Loránd University, Budapest 1064, Hungary and Department of Obstetrics and Gynecology, Semmelweis University, Budapest 1085, Hungary.
Author information
Authors and affiliations.
Doctoral School of Psychology, Eötvös Loránd University, Budapest, 1064, Hungary
Gabriella Márki
Institute of Psychology, Eötvös Loránd University, Izabella Street 46, Budapest, 1064, Hungary
Gabriella Márki, Dorottya Vásárhelyi, Adrien Rigó & Zsuzsa Kaló
Department of Obstetrics and Gynecology, Faculty of Medicine, Semmelweis University, Baross Street 27, Budapest, 1088, Hungary
Nándor Ács & Attila Bokor
You can also search for this author in PubMed Google Scholar
Contributions
G.M., A.R., N.Á., and A.B. were involved in designing this study. G.M., A.B., and N.Á. helped recruit participants. G.M. and D.V. corrected the database verbatim. G.M., D.V., Z.K., and A.R. were involved in the analysis and interpretation of data. This manuscript was drafted by G.M., V.D., Z.K., and A.R., and A.B. edited the article. All the authors have approved the final draft.
Corresponding author
Correspondence to Attila Bokor .
Ethics declarations
Ethical approval and consent to participate..
This study was approved by the Regional, Institutional Research and Ethics Committee of Semmelweis University, Budapest, Hungary (registration number TUKEB 60/2014), and the work was conducted in accordance with the tenets of the Declaration of Helsinki. Written informed consent was provided by all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Márki, G., Vásárhelyi, D., Rigó, A. et al. Challenges of and possible solutions for living with endometriosis: a qualitative study. BMC Women's Health 22 , 20 (2022). https://doi.org/10.1186/s12905-022-01603-6
Download citation
Received : 22 September 2021
Accepted : 14 January 2022
Published : 26 January 2022
DOI : https://doi.org/10.1186/s12905-022-01603-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Endometriosis
- Psychosocial impact
- Focus group
BMC Women's Health
ISSN: 1472-6874
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Imaging of Endometriosis: The Role of Ultrasound and Magnetic Resonance
- GLOBAL RADIOLOGY (J FRENCHNER, SECTION EDITOR)
- Open access
- Published: 18 February 2022
- Volume 10 , pages 21–39, ( 2022 )
Cite this article
You have full access to this open access article
- Valentina Testini 1 , 2 ,
- Laura Eusebi 3 ,
- Gianluca Grechi 4 ,
- Francesco Bartelli 3 &
- Giuseppe Guglielmi ORCID: orcid.org/0000-0002-4325-8330 1 , 2 , 5
10k Accesses
Explore all metrics
Endometriosis is a chronic gynecological disease characterized by the growth of functional ectopic endometrial glands and stroma outside the uterus. It causes pelvic pain, dysmenorrhea, dyspareunia, or infertility. Diagnosis requires a combination of clinical history, non-invasive and invasive techniques. The aim of the present review was to evaluate the contribution of imaging techniques, mainly transvaginal sonography and magnetic resonance imaging to diagnose different locations and for the most appropriate treatment planning. Endometriosis requires a multidisciplinary teamwork to manage these patients clinically and surgically.
Similar content being viewed by others
Magnetic resonance imaging for deep infiltrating endometriosis: current concepts, imaging technique and key findings
Filomenamila Lorusso, Marco Scioscia, … Arnaldo Scardapane

- Endometriosis
Endometriosis: clinical features, MR imaging findings and pathologic correlation
Pietro Valerio Foti, Renato Farina, … Giovanni Carlo Ettorre
Avoid common mistakes on your manuscript.
Introduction
Endometriosis is a common gynecological inflammatory condition that is defined as functional ectopic endometrial glands and stroma outside the uterus. This disease affects women of reproductive age, with a prevalence of approximately 10% [ 1 ]. Patients can be asymptomatic or present with chronic pelvic pain and/or infertility.
The phenotypes of endometriotic lesions can be divided into three groups: pelvic endometriosis, ovarian endometriomas (OMA) and deep infiltrating endometriosis (DIE) [ 2 ]. In particular, pelvic endometriosis is defined as the presence of any endometrial tissue within the pelvic cavity, including the peritoneum, within any of the pelvic organs and inside the pouch of Douglas (POD). Ovarian endometriosis, an endometrioma, is defined as an ovarian cyst, of different size, lined by endometrial tissue. DIE is defined as endometriotic tissue that penetrates the retroperitoneal space for a distance of 5 mm or more and may be present in multiple locations, involving anterior or posterior pelvic compartments, or both [ 3 ]. Posterior DIE, a multifocal disease that may affect a variety of anatomical sites, represents the most common type of DIE [ 4 ]. The most typical sites of DIE include uterosacral ligaments (USL), rectovaginal septum (RVS), vaginal wall, POD and bowel, predominantly below the rectosigmoid junction. Anterior DIE corresponds to disease involving the anterior pouch or bladder and is much less common. DIE is a source of pain and infertility [ 5 ].
A frequently association with endometriosis is represented by adenomyosis, a disease characterized by infiltration of endometrial tissue into the myometrium [ 6 ].
Etiopathogenesis
Although the pathogenesis of endometriosis has not been fully elucidated, it is commonly thought that endometriosis occurs when endometrial tissue contained within menstrual fluid flows retrogradely through the fallopian tubes and implants at an ectopic site within the pelvic cavity [ 7 ]. In this process, menses transports viable endometrial fragments through the fallopian tubes to the peritoneal cavity, where they are able to implant, develop and sometimes invade other tissues of the pelvis [ 8 ]. In favor of this hypothesis is that all known factors that increase menstrual flow are also risk factors for endometriosis, including early age at menarche, heavy and long periods as well as short menstrual cycles [ 9 ]. The anatomical distribution of endometriotic lesions can also be explained by the hypothesis of retrograde menstruation as endometriotic lesions tend to have an asymmetrical distribution, which could be explained by the effect of gravity on menstrual flow, the abdominopelvic anatomy and the peritoneal clockwise flow of menses [ 10 ]. However, this theory does not explain the fact that although retrograde menstruation is seen in up to 90% of women, only 10% of women develop endometriosis [ 3 ]. Moreover retrograde menstruation does not explain the mechanism of endometrial tissue grafting onto the peritoneum. It is therefore evident that a variety of environmental, immunological and hormonal factors contribute to the onset of endometriosis, with mechanisms not yet known [ 11 ].
Genetic factors play an important role in the genesis of endometriosis, with an up to six times greater risk of developing the disease for first degree relatives of patients with endometriosis [ 12 ]. Despite this clear inheritance, the identification of the genetic factors that drive the disease is still incomplete.
The first step in diagnosing DE is to establish the patient's clinical history with particular emphasis on symptoms (dysmenorrhea, dyspareunia, dysuria, dyschezia, and chronic pelvic pain) as well as, age, height, weight, ethnic origin, gravidity, parity, previous surgery for endometriosis, family history of endometriosis, previous non-surgical treatment for endometriosis, and infertility. No symptom is specific to endometriosis [ 13 ].
However, several authors have underlined the poor relationship between symptoms exhibited by patients and the severity of the lesions rendering clinical diagnosis difficult. Moreover, it is thought that up to 50% of women could have asymptomatic endometriosis [ 14 , 15 ].
The second step is based on physical examination including a systematic analysis of the posterior vaginal fornix with a speculum to look for retraction and dark nodules. Digital examinations should be performed of the vagina to assess the characteristics of the uterus and adnexa, of the vesicouterine pouch to detect bladder invasion, and of the retrocervical area to detect infiltration of the torus uterinus, uterosacral ligaments (USLs), pouch of Douglas (POD), vagina, and rectovaginal septum (RVS) [ 13 ]. Rectal digital examination can help in assessing the involvement of the rectum, parametrium and visceral pelvic fascia. In the particular setting of DE, few data are available to evaluate the accuracy of physical examination. One retrospective study found that routine clinical examination detected DE in only 36% of 140 women with DE, and the authors suggest the accuracy of physical examination improves during menstruation [ 16 ]. To detect rectosigmoid and retrocervical DE without differentiating between the different specific DE locations, Abrao et al. reported that digital vaginal examination had a sensitivity of 72% and 68%, a specificity of 54% and 46%, respectively [ 17 ].
Laboratory tests are limited in the diagnosis of endometriosis (CA-125 has a detection rate of only 54% in patients with severe endometriosis 5 and is neither sensitive nor specific for the diagnosis) [ 18 ].
The gold standard for diagnosis of endometriosis is based on laparoscopy or surgery with histological verification of endometrial glands and/or stroma.
Imaging is needed to diagnose endometriosis and to plan treatment. The techniques used are transvaginal ultrasound (TVUS) and magnetic resonance imaging (MRI), the latter should be considered as a second-line technique after ultrasound [ 1 ].
Treatment of endometriosis is complicated and involves conservative approaches combined with medical therapies or surgery. Imaging is crucial to guide the type of treatment. The American Society for Reproductive Medicine Practice Committee states that “endometriosis should be viewed as a chronic disease that requires a lifelong management plan with the goal of maximizing the use of medical treatment and avoiding repeat surgical procedures” [ 18 ]. Current treatment is essentially surgical, medical, or a combination of both approaches. To this end, many patients are stratified for medical treatment with or without surgical treatment based on symptom severity or imaging results and desire to have children, with medical therapy typically including non-steroidal anti-inflammatory drugs, oral contraceptives, androgens, progestogens and gonadotropin-releasing hormone (GnRH) and/or surgery [ 19 ].
Laparoscopy is an effective surgical approach with the goal of excision of visible endometriosis in a hemostatic fashion. Radical surgery is reserved for those patients with severe symptoms where there is no desired fertility potential and especially when other forms of treatment have failed. Total abdominal hysterectomy and bilateral salpingo-oophorectomy are performed along with resection of any endometriotic lesions as completely as possible [ 4 ].
Transvaginal ultrasound (TVUS) is typically the initial imaging evaluation performed in patients with pelvic pain and infertility or when there is clinical suspicion for endometriosis. This examination is widely available with low relative cost and the sensitivity and specificity for detection of ovarian endometriomas and lesions in the rectal wall are high [ 18 ].
All patients should be examined systematically and carefully using an endocavitary sonography, with a microconvex array probe inserted transvaginally or transrectally. Both techniques are optimal approaches for examining uterus (including the different uterine zones: cervix, endometrium, junctional zone, and myometrium), adnexa, paracolpium, parametrium, vesicocervical, vesicovaginal, and rectovaginal spaces as well as urinary bladder, ureters, and rectum [ 20 ].
Limitations of sonographic technique include anterior compartment detection of endometriosis (bladder and vesicouterine pouch detection) and detection within the middle compartment (torus uterinus and round ligaments) [ 18 ].
The pelvic localization of endometriosis can be described according to three compartments (central, anterior, and posterior) according to functional and clinical relevance [ 21 ]. The anterior compartment includes the insertion site of the ureters, the bladder, the vesicouterine pouch, and the vesicovaginal pouch. The middle compartment contains the uterine body, fallopian tube, and uterine ligaments. The posterior compartment contains the uterosacral ligaments, rectovaginal septum, anterior rectal wall, and sigmoid colon [ 21 ].
Recently, the IDEA (International Deep Endometriosis Analysis group) published a consensus report 18 on the appropriate terms, definitions, and measurements that may be used to describe the sonographic features of the different phenotypes of endometriosis. A standardized pelvic TVS approach is proposed by this IDEA report consisting in four step pelvic evaluation [ 22 ]:
Evaluation of uterus and ovaries:
Evaluation of uterus: 2D–3D sonographic signs of adenomyosis
Evaluation of the adnexa: presence or absence of endometrioma or tubal pathology
Evaluation of TVS organ mobility (adhesions): adnexa and uterine mobility site-specific tenderness
Pouch of Douglas (POD) assessment using real-time ultrasound-based “sliding sign”
Assessment for DIE nodules in anterior lateral and posterior compartments

Anterior Compartment
The anterior compartment is comprised of the urinary bladder, the vesicouterine pouch, round ligaments and ureters. Involvement of the urinary tract occurs in approximately 1–2% of patients with endometriosis and involves bladder in 85% of these cases [ 23 ]. Ureteric involvement is found in 4% of patients with rectovaginal endometriosis [ 24 ]. The prevalence of round ligament endometriosis is estimated between 4.3% and 13.8% [ 25 ].
Bladder endometriosis is considered only in case of infiltration of the bladder wall and not in case of adhesions or superficial peritoneal implants on the bladder serosa.
Before TVS scan, patients are asked not to empty completely the bladder, because the slightly filled bladder permits to better evaluate the structure of the walls.
On ultrasound, bladder endometriosis appears as hypoechoic lesion, either containing cystic lesions or not, with regular/irregular margins of the bladder wall, bulging toward the lumen, involving the serosa, muscularis (most common), or (sub)mucosa of the bladder [ 26 ].
In the assessment of the bladder DIE localization, the bladder wall can be divided into three zones: the trigonal zone and vesical base; the vesical dome (which lies superior to the trigone and is intra-abdominal) and the anterior retroperitoneal bladder. Most frequently bladder endometriosis is located in the vesical dome on the posterior bladder wall close to the vesicouterine pouch [ 27 ].
Bladder adhesions of the vesicouterine pouch are evaluated by the presence or absence of the “sliding sign” between the uterus and the bladder [ 28 ].
During examination, from a longitudinal section through the cervix and moving the probe toward the lateral pelvic wall, it is possible to assess the distal part of the ureter adjacent to the bladder trigone, in order to evaluate the presence of stenosis and subsequent cephalad dilatation of the pelvic ureters. This finding can suggest direct invasion or compression of the ureter by endometriotic nodules, ovarian endometriomas, or adhesions [ 28 ].
The prevalence of ureteral endometriosis ranges from 0.01 to 1% of all patients with the disease and most often affect the distal segment of the ureter [ 29 ]. There are two types of endometriosis involvement of ureters: (1) extrinsic that represents 75–80% of the cases and is defined as the presence of endometrial tissue in the outer adventitia of the ureter that occurs as a nodule encasing the ureter by extension from pelvic foci; (2) intrinsic that represents 20–25% of cases and is defined as the presence of endometrial tissue in the mucosal and/or muscular layer of the ureter. Imaging signs are nodule or mass occurring in the ureter along its course, dilatation of the pelvic ureteral tract, or ureteropelvic hydronephrosis superior to the suspected lesion [ 22 ]. Pelvic ureteral dilation can be easily seen by TVS as a tubular anechoic image, very similar to a blood vessel but with negative color/power Doppler signs. An extrinsic compression, also without ureteral dilatation, is suspected in cases where a DIE lesion is located close to the ureter. The observation of a possible ureteral involvement requires transabdominal ultrasound to evaluate the renal pelvis. In all women with DIE, a transabdominal scan of the kidney to search for ureteral stenosis is necessary because the prevalence of endometriotic lesions in the urinary tract may be underestimated and women with DIE involving the ureter may be asymptomatic [ 30 ].
A review of the literature for bladder endometriosis reveals a reported mean US sensitivity of 55% and specificity of 93.5% [ 31 ].
For vesicouterine pouch endometriosis, two series reported US sensitivities and specificities of 16.7% and 33% and 99% and 100% [ 32 ]. These discrepant results could be partly explained by selection bias of inclusion among studies.
Central Compartment
The central compartment includes uterus and adnexa.
Adenomyosis is characterized by the migration and proliferation of endometrial glands and stroma from the basal layer of endometrium into the myometrium. It is associated with smooth muscle hyperplasia leading to an ultrasound image of ill-defined lesions within the myometrium [ 22 ].
Recently, the ultrasound features have been systematically described by the international Morphological Uterus Sonographic Assessment (MUSA) group [ 33 ].
According to the MUSA consensus, adenomyosis should be described as localized and diffuse.
Adenomyosis is classified as diffuse, if the total involvement of myometrium exceeds 50% of the corpus uteri (when the findings are present in only one part of the myometrium on one or more sites within the uterine wall), and localized (or focal) when less than 50% of myometrium is involved (one or more lesions) [ 33 ]. An adenomyoma is defined as a focal consolidation of endometrial glands and or endometrial stroma located within the myometrium with additional compensatory hypertrophy of the surrounding myometrium [ 27 ]. In rare cases it may present as a large cyst (adenomyotic cyst or cystic adenomyoma, with largest diameter 2 mm and echogenic rim) [ 34 ].
To evaluate adenomyosis, the size of the lesions should be measured (in particular the largest diameter of each focal lesion or the myometrial wall thickness in cases of a diffuse lesion), the involvement of the uterine layers and of the extent of the disease, based on the estimated volume of the uterine corpus affected by adenomyosis (mild < 25%, moderate 25–50%, and severe > 50%) [ 27 , 35 ].
According to several studies, there are different features associated with adenomyosis visible on 2D transvaginal sonographic [ 33 ].
On ultrasound, an adenomyotic uterus appears with a globular shape, enlarged dimensions, and uterine wall asymmetry. The myometrium typically appears inhomogeneous on gray-scale, characterized by the presence of an indistinctly defined area with either decreased or increased echogenicity with myometrial hypoechoic linear striations [ 36 ]. Round anechoic areas of 1-mm to 7-mm diameter, named myometrial cysts, also could be present within the myometrium [ 36 ]. In cases of focal adenomyosis, the adenomyotic lesion appears as a heterogeneous and hypoechogenic area within the myometrium, usually with anechoic lacunae or cysts with ill-defined contours and fan-shaped shadowing. These hypoechogenic areas reflect muscular hypertrophy of the myometrial tissue [ 27 ]. Irregularities of the endometrial-myometrial junctional zone is another common ultrasound marker in the diagnosis of adenomyosis [ 27 ]. This endomyometrial interface is normally visualized as hypoechoic tissue layer seen beyond the endometrial basal layer. In women with adenomyosis, the diffuse or focal hyperplasia and hypertrophy of myocytes determine whether diffuse or focal thickening of this zone is seen [ 37 ].
A characteristic sign is the “question mark sign” defined when the corpus uterus was flexed backward, the fundus of uteri was facing the posterior pelvic compartment, and the cervix was directed frontally toward the urinary bladder [ 38 ]. Investigators found 93% specificity and 75% sensitivity of this sign in detecting adenomyosis [ 22 ].
Power Doppler can be used to distinguish myometrial cysts from blood vessels and discriminate between leiomyomas and focal adenomyosis. Uterine leiomyomas manifest a circular flow along the myoma pseudocapsule, while localized adenomyosis and adenomyomas are characterized by diffusely spread vessels inside the lesions [ 39 ]. 2D ultrasound can yield equivocal result in the case of focal adenomyosis especially if there are coexistent fibroids. A meta-analysis of 14 trials and 1985 participants reported the sensitivity and specificity of ultrasound in the diagnosis of adenomyosis to be as high as 82.5 and 84.6%, respectively, values in line with MRI values [ 40 ].
The use of 3D vaginal ultrasound for the diagnosis of adenomyotic pathology allows a more complete evaluation in the sagittal, transverse and coronal planes, evaluating the ultrasound signs on the acquired 3D volume of the uterus [ 41 ].
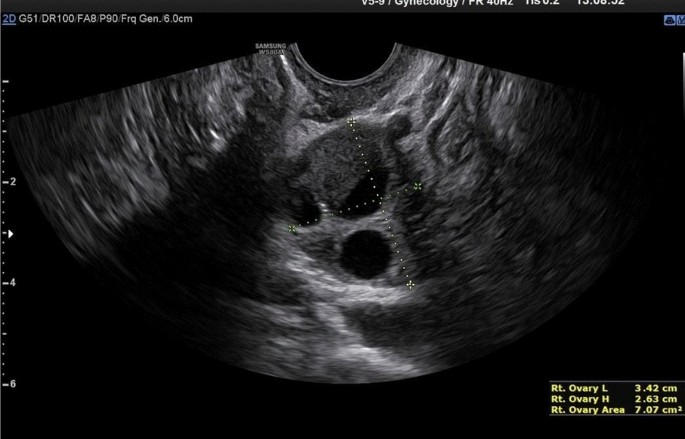
Ovarian endometriomas occur when ectopic endometrial tissue in the ovary hemorrhages, forms a hematoma, enveloped by ovarian parenchyma (Fig. 1 ).
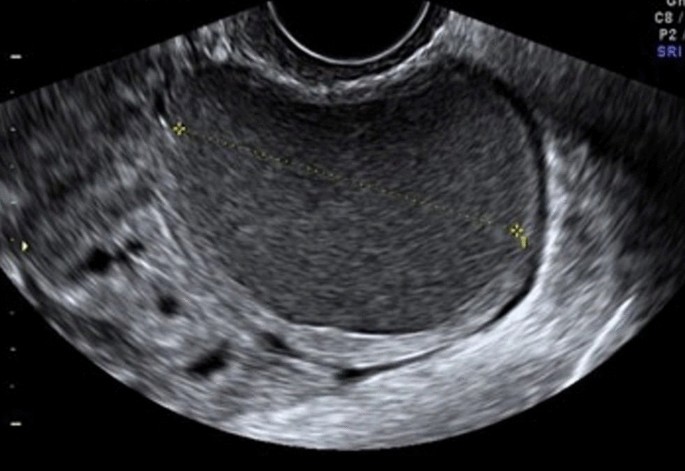
Endometriotic cyst, with regular parenchyma at the periphery, called “crescent sign” characteristic of benign lesions. Absent intralesional vascularization
An ovarian endometrioma has different imaging appearances on US, with the classic appearance being a cyst unilocular or multilocular (less than five locules) with homogeneous low-level echogenicity (ground glass echogenicity) of the cyst fluid, with increased posterior through transmission and no vascularization on color Doppler [ 22 ] (Fig. 2 ).
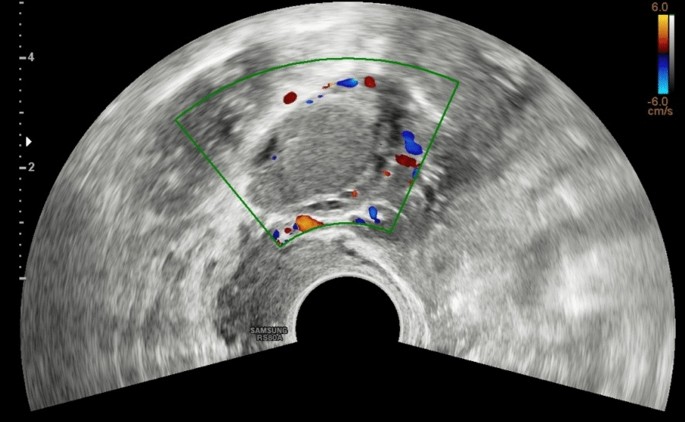
Color Doppler of multiple endometriomas of both ovaries, which appear enlarged and sharpened (ovarian kissing)
Another feature is the presence of peripheral echogenic foci (thought to reflect cholesterol deposits) seen in up to 36% of endometriomas. Endometriomas tend to be multilocular and bilateral (up to 50%) [ 42 ] (Fig. 3 ).
Endometrioma with evidence of the characteristic double fluid–fluid level. Inside the formation, hyperechoic spots can be highlighted, a symptom of hemosiderin accumulation
However, endometriomas may have a variable appearance because of the range of appearance of the internal blood products within them, which can cause fluid–fluid levels, echogenic regions, and papillary projections. In these cases, additional evaluation with MR imaging may be warranted to better evaluate and to exclude malignancy [ 43 ]. There is evidence that ovarian endometriomas originate from ovulatory events and it is likely that the number of endometriomas may increase with age, and multiple endometriomas in the same ovary may assume a multilocular morphology [ 44 ]. Guerriero and colleagues reported that ultrasound appearance of endometriomas differed between premenopausal and postmenopausal patients [ 44 ]. The endometriomas in the postmenopausal patients were less often unilocular cysts and less likely to exhibit ground glass echogenicity [ 27 ].
The primary differential diagnosis of an endometrioma is a hemorrhagic cyst. On US, a hemorrhagic cyst classically has internal reticular strands with retractile clot [ 18 ]. However, these features may not be seen, and instead, homogeneous low-level echoes mimicking that of an endometrioma may be present. Hemorrhagic cysts are unlikely to have the peripheral echogenic foci occasionally seen in endometriomas, and they are less likely to be bilateral or multifocal. Sonographic follow-up demonstrating resolution at 6–12 weeks is diagnostic of a hemorrhagic cyst [ 18 ].
Another differential diagnosis of an endometrioma is an ovarian epithelial neoplasm, which may contain low-level internal homogeneous echoes similar to an endometrioma. This imaging appearance was seen in up to 6% of ovarian serous cystadenomas in the study by Patel et al. [ 42 ] and in up to 20% of mucinous cystadenomas in the study by Van Holsbeke [ 45 ]. To better evaluate for the presence of malignancy (cystadenocarcinomas) in these cases, careful interrogation of the cyst should be performed to assess for internal solid components, such as papillary projections, mural nodules, and thickened septations.
Doppler helps avoid classifying malignancies as endometriomas, especially when evaluating a papillary projection. Generally, these different ultrasound criteria proposed have a sensitivity ranging from 62 to 73%, a specificity of 94–98% [ 46 ].
Masses in postmenopausal women whose cystic contents have a ground glass appearance have a high risk of malignancy. Borderline tumors and carcinomas arising from endometrioid cysts show a vascularized solid component on ultrasound examination [ 47 ].
The uterine tubes can be involved with endometriosis either with adhesions occluding the tube up to 6%) or by DIE foci affecting the tubal walls (up to 26% of the time) [ 18 ].
In case of endometriosis of the tube, we can observe a dilated tube with thick walls and incomplete septa with a fluid dense content similar to an endometrioma (hematosalpinx) [ 48 ]. A “cog-wheel” appearance of the longitudinal folds can be seen when the tube is imaged in cross-section. The presence of a hematosalpinx may be the only sign on imaging of endometriosis in the pelvis [ 49 ].
In case of occlusion of the tube due to adhesion or DIE that involved the distal part and the fimbriae, a hydrosalpinx is seen with the typical “beads-on-a-string” sign, defined as hyperechoic mural nodules measuring approximately 2–3 mm as seen on the cross-section of the fluid-filled distended structure [ 48 ].
The differential diagnosis of a hematosalpinx includes pelvic inflammatory disease (PID) or fallopian tube malignancies. Pyosalpinx of PID can be differentiated clinically by the presence of extreme tenderness on examination as well as the clinical signs of infection (fever, white count). On imaging, hyperemia surrounding the fallopian tube with fatty proliferation/edema in the adjacent fat suggests a pyosalpinx. Fallopian tube carcinoma presents sonographically with solid, vascular internal nodules within the fallopian tube and tends to occur in an older demographic group [ 43 ].
Posterior Compartment
Recently, the ultrasound features of the deep infiltrating endometriosis nodules have been systematically defined by the International Deep Endometriosis Analysis group [ 34 ]. The most common sites of the posterior compartment are posterior vaginal fornix/rectovaginal septum, uterosacral ligaments, anterior rectum/anterior rectosigmoid junction, and sigmoid colon [ 10 ] (Fig. 4 ).
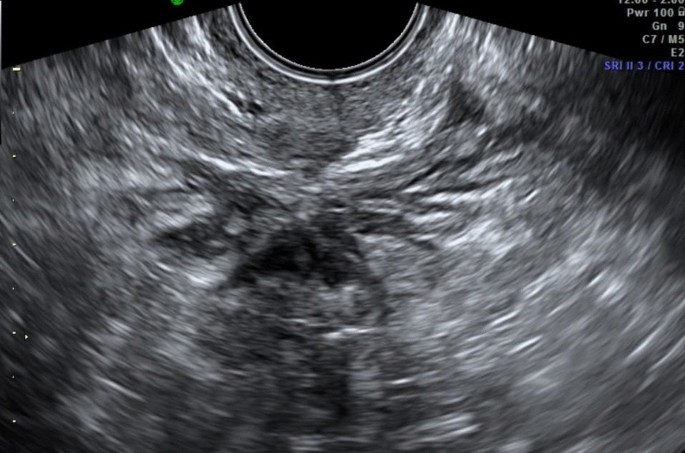
Hypoechoic nodule of the rectosigmoid portion that obliterates the Douglas. At this level, the “sliding sign” can be seen, the sign of the sliding structures on each other which does not occur in the case of endometriosis
Deep endometriosis on sonography is subtle and presents as hypoechoic nodular or infiltrating regions. Occasionally, the infiltrative regions of DIE may have internal hyperechoic foci or complex internal cysts [ 50 ]. The differential diagnosis for DIE includes peritoneal implants; in these cases, to help differential diagnosis, an additional evaluation with MR is recommended [ 18 ]. Three-dimensional (3D) TVS has been also proposed in the evaluation of posterior locations of DIE without intestinal involvement, improving the diagnostic accuracy of 2D ultrasonography [ 51 ].
In the cases of DIE, it is necessary to describe of the anatomical localizations, the size and number of DIE nodules, the depth of infiltration of the nodules, and the degree of stenosis of the bowel lumen which is important to plan the surgical procedures [ 22 ].
Uterosacral Ligaments
The uterosacral ligaments (USL) are usually not visible on ultrasound. The uterosacral ligaments affected by deep infiltrating endometriosis can be seen in the longitudinal view of the uterus at the insertion on the posterior lateral cervix wall, as hypoechoic tissue, with regular/irregular margins within the peritoneal fat surrounding the uterosacral ligaments [ 13 ]. On the transverse cervical section, these hypoechoic nodules appear on the posterior lateral part of the cervix and interrupt the hyperechoic external cervical fascia. Sometimes, the uterosacral ligaments appear thickened and hyperechoic, probably as the morphologic expression of fibrosis, due to the chronic process of inflammation [ 27 ].
USLs lesions may be isolated or may be part of a larger nodule extending into the vagina or into other surrounding structures. In some cases, the DIE lesion involving the USL is located at the torus uterinus as a central thickening of the retrocervical area between USLs [ 52 ]. Two recent meta-analyses of USL endometriosis have reported pooled sensitivities and specificities of 53–64% and 93–97%, respectively [ 53 ].
In case of endometriotic lesions involving the uterosacral ligaments, special attention must be paid to the parametrium. Parametria are examined lateral to the uterine cervix first on the sagittal planes moving the probe from the lateral sites where the parametrium is attached to the cervix, to the uterine vessels bifurcation, to the lateral pelvic wall, and then on the transverse planes moving the probe from the uterine isthmus to the external cervical os. The parametrial involvement is seen as an infiltrating hypoechogenic irregular tissue, and it can be medially delimitated from the cervical vascular plexuses using color or power Doppler [ 22 ].
Rectovaginal Septum
Involvement of the rectovaginal septum should be suspected when an endometriotic nodule, which appears as hypoechoic solid nodule with smooth or irregular contours, that replaces the normal hyperechoic aspect of this layer between the vagina and the rectum, seen in the rectovaginal space below the horizontal plane passing through the lower border of the posterior lip of the cervix (under the peritoneum) [ 34 ].
Isolated rectovaginal septum nodule is rare, and it is usually an extension of posterior vaginal wall, anterior rectal wall, or both posterior vaginal wall and anterior rectal wall involvement. Hourglass-shaped or diabolo-like nodules can occur when endometriosis lesions from the posterior vaginal fornix extend to the anterior rectal wall [ 54 ].
DIE of the RVS may extend into the rectum and/or in the posterior vaginal fornix [ 22 ].
Major discrepancies exist between the pooled sensitivities and specificities provided by meta-analyses reporting values from 49% to 88% and 98% to 100%, respectively [ 34 ].
Vaginal endometriosis is diagnosed when the posterior or a lateral vaginal fornix shows a nodular wall thickened (> 5 mm) (mean normal vaginal thickness ranges from 3 to 5 mm), with or without round cystic anechoic areas, that does not get thinner with probe compression [ 13 ].
The nodule may be hypoechoic, homogeneous, or inhomogeneous with or without cystic areas and there may also be some vascularization at power Doppler. More frequently, the lesions are localized in the posterior vaginal fornix [ 22 ].
The insertion of saline solution in the vagina (sonovaginography) could improve the visualization of these lesions [ 55 ].
In a meta-analysis including ten studies, the pooled sensitivity and specificity of TVS was 57% and 99%, respectively [ 56 ]. Among the various TVS techniques, SVG provided the highest sensitivity and specificity reaching 91% and 89%, respectively [ 56 ].
The endometriosis affecting the bowel can appear as a thickening of the muscularis propria or as a hypoechoic nodule penetrating the intestinal wall with blurred margins, with or without hypoechoic or hyperechoic foci, usually associated with retraction and adhesion (the so-called Indian headdress sign), and few vessels at power Doppler evaluation [ 57 ].
The rectum and the rectosigmoid segment is the most frequent site of bowel involvement accounting for 70–88% of cases of bowel involvement with endometriosis, followed by the sigmoid colon, rectum, ileum, appendix, and cecum [ 10 ]. Intestinal nodules located below the peritoneum of the POD (or the level of the insertion of the USLs on the cervix in case the cul-de-sac is obliterated) are considered low rectal lesions, while the ones above this level are considered upper rectal or the rectosigmoid junction lesions. This virtual line should delineate the plane under the peritoneum of the POD and correspond laterally to the parametria and medially to the RVS. The lowest limit of the nodule on the bowel wall should be determined, because the lower rectal lesions are more difficult to remove surgically by shaving or segmental resection and have higher complication rate [ 22 ].
Endometriotic nodules of the rectum can be evaluated if necessary also by transrectal examination as well with the same transvaginal convex probe. This has the advantage of visualizing better the vagina, the rectovaginal septum (RVS), and the low rectal walls. Moreover, during the transrectal or transvaginal examination, a fluid contrast medium can be inserted in the vagina to visualize better the RVS (sonovaginography) [ 55 ]. It has been reported that adding water contrast in the rectum during transvaginal ultrasonography (RWC-TVS) improves the diagnosis of rectal infiltration in women with rectovaginal endometriosis. RWC-TVS is performed by injecting saline solution into the rectal lumen under ultrasonographic control through a catheter [ 58 ].
During the evaluation of posterior compartment, a negative “sliding sign” between the rectosigmoid and uterus could indicate an obliteration of the pouch of Douglas (POD), frequently associated with severe DE. Using this new technique, Reid et al. found sensitivity, specificity of 83.3%, 97.1% [ 59 ].
Multifocal lesions are defined as the presence of deep lesions within 2-cm area of the main lesions or multiple endometriotic lesions affecting the same intestinal segment. Multicentric lesions are defined as a satellite deep nodule found more than 2 cm from the main lesions or endometriotic lesions affecting several digestive segments [ 60 ].
The use of volume acquisition with 3D TVS permits a more accurate measurement and evaluation of the DIE lesion in different planes.
The pooled sensitivity and specificity of TVS for rectosigmoid endometriosis are reported as 90% and 96%, respectively, with similar results being provided by RES [ 56 ]. Guerriero et al., in a one-paired study, suggested that 2D-TVS was more sensitive but less specific than 3D TVS [ 61 ].
MR imaging of the pelvis is frequently performed for the detection of endometriosis, either as the second-line imaging examination (after US) for the detection/confirmation of endometriosis, in particular in deep infiltrating endometriosis, or as the initial examination in a patient for whom there is a high clinical suspicion for endometriosis and not ultrasound confirm [ 1 ].
MR imaging can be performed with either a 1.5-T or 3-T magnet, using a high-resolution phased-array surface coil for improved resolution. There is no consensus regarding whether to perform the examination around the timing of the patient’s menstrual cycle [ 13 ].
Patients are positioned supine on the scanner, with abdominal strapping after phased coil array placement. Fasting before the examination for 4 h is typically recommended in order to empty the upper gastrointestinal tract; the use of antiperistaltic agents is recommended to reduce motion artifacts caused by intestinal peristalsis. However, the type of agent (oral agents, nonoral agents), dose, and route (intramuscular, subcutaneous, or IV) is debated [ 18 ].
Patient preparation required a moderately filled bladder; this is required to change the angle of uterine anteversion, leading to better detection of implants in the anterior compartment. Moreover, a moderately filled bladder displaces the bowel superiorly, by reducing the artifacts from bowel motion [ 62 ].
For the evaluation of deep endometriosis it was suggested to introduce intra-vaginal aqueous gel to distend the vaginal cavity and better explore the vaginal fornices and the retrocervical area [ 62 ].
In the presence of symptoms that may be related to rectal involvement, gel may also be useful to distend the rectal\sigmoid bowel wall.
The typical imaging protocol [ 21 , 63 ] includes three T2 turbo-spin-echo (TSE)—weighted sequences (T2W) in different slice orientations (sagittal, coronal, and axial planes), followed by three T1-weighted (T1W) sequences in an identical imaging plane (TR 500 ms, TE 14 ms) without fat suppression and fat-suppressed T1W before and after intravenous injection of contrast media because the fat suppression is useful for the detection of subtle foci of hemorrhage, which may be obscured on non–fat-saturated images [ 64 ].
Use of contrast-enhanced imaging is primarily required to identify solid enhancing nodules within endometriotic cysts when malignant transformation is suspected.
Dixon technique or conventional in- and out-of-phase T1-weighted images are useful for the differentiation of fat-containing lesions, such as dermoid cysts from endometriomas, both of which have high signal on non–fat-saturated T1-weighted images [ 18 ].
No recommendation can be achieved for the use of DWI and SWI sequences.
Half-Fourier acquisition single shot turbo-spin-echo (HASTE) is recommended for the evaluation of uterine peristalsis because it enables multiphase and multislice image acquisition producing kinematic images for the evaluation of pelvic adhesions [ 1 ]. During the peri-ovulatory phase, uterine peristalsis is significantly reduced in subjects with endometriosis when compared to normal controls that may be due to increased, sustained contractions in endometriosis patients [ 65 ].
MRI Evaluation
At MR imaging the signal intensity of endometriotic lesions is a function of the quantity and age of the hemorrhage on the one hand and the proportion of endometrial cells and stroma on the other [ 66 ].
The lesions have a micronodular or microcystic appearance; however, cysts do not enlarge except in the ovary. Only pigmented lesions can be detected at non-contrast-enhanced MR imaging because of the presence of hemorrhage [ 67 ]. At MR imaging these small implants manifest as multiple round (cystic or nodular) lesions homogeneously hyperintense on fat-suppressed T1-weighted images, due to old hemorrhagic content, regardless of their signal intensity on T2-weighted images [ 67 ]. Involvement of peritoneal reflections over the cul-de-sac and the uterus may also manifest on contrast-enhanced fat-saturated T1-weighted images as diffuse peritoneal enhancement secondary to the inflammatory reaction induced by endometrial implants. Over time a fibrotic reaction may occur, thus leading to adhesions formation between pelvic structures [ 67 ].
T2W sequences are used for the evaluation of fibrotic lesions, notably those that involve the pelvic ligaments, retrocervical space, or prevesical recess [ 21 ].
They appear as speculated hypointense peritoneal strands arranged in confluent angles. Posterior displacement of uterus and ovaries, angulation of rectosigmoid colon and bowel loops, elevation of the posterior vaginal fornix, loculated fluid collections, and a hydrosalpinx may be indirect signs of adhesions [ 68 ].
At MR imaging both fibrous tissue and smooth muscle show intermediate signal intensity on T1-weighted images and low-signal intensity on T2-weighted images. Therefore, on T2-weighted images, solid endometriotic lesions appear as hypointense nodular structures with irregular or stellate margins due to fibrous tissue and smooth muscle proliferation. In certain cases, deep endometriotic lesions may also appear as irregular and hypointense soft-tissue nodular thickening on T2-weighted sequences, as it occurs when the disease involves the USL or the vaginal or rectal wall [ 66 ].
In some cases the glandular component can be predominant, compared to the fibrous tissue and in this case the MRI appearance will show high signal intensity of T2W images; in this case the use of contrast material can be useful because this will show enhancement, thus distinguishing it from intramural hemorrhage or necrosis [ 63 ]. Usually, endometrial glands without hemorrhage are not detectable on fat-suppressed T1-weighted images; so deep lesions may show homogeneous intermediate signal intensity on T1-weighted images. When red cell extravasation outside the glandular ducts into the surrounding stroma occurs, these small hemorrhages become visible as small hyperintense spots on fat-saturated T1-weighted images. After the intravenous administration of gadolinium, lesion enhancement may occur due to inflammatory reaction, glandular and fibrous tissue.
Owing to the possibility to perform a complete assessment of all pelvic compartments at one time, MRI represents the best imaging technique for preoperative staging of endometriosis [ 66 ].
Endometriotic lesions may affect the urinary tract in up to 20% of cases.
Bladder lesions appear as small masses of round or lobulated hypertrophic tissue covered by normal mucosa [ 67 ].
Bladder involvement is often multifocal, the trigone and the dome being the most frequently affected sites [ 23 ]. According to the degree of wall infiltration, bladder involvement may be classified as extrinsic or intrinsic. In extrinsic involvement, the most common form, implants are confined to the serosal surface or the surrounding connective tissue; in intrinsic involvement lesions infiltrate the muscular layer manifesting as mural masses [ 66 ].
At MR imaging bladder endometriosis may manifest as localized or diffuse wall thickening and signal intensity abnormalities [ 63 ]. The appearance is of low-signal intensity on T2-weighted and intermediate signal intensity on T1-weighted images, with or without spots of high signal intensity on T1-weighted images, representing hemorrhagic content. Sometimes, hyperintense foci on T2W images corresponding to the dilated endometrial glands may be found [ 66 ]. Implants minimally enhance after injection of a gadolinium-based contrast material [ 64 ].
The maximum lesion diameter varies between 1 and 5 cm. MRI reaches sensitivity up to 88%, specificity up to 99% and diagnostic accuracy of about 98% for the diagnosis of bladder endometriosis [ 23 ]. The differential diagnosis of bladder endometriosis includes urachal remnant, epithelial tumors (bladder carcinoma) and mesenchymal tumors (angiomas, leiomyoma) [ 23 ].
Urethral endometriosis is uncommon; direct implantation of endometrial tissues during procedures is hypothesized to be the etiologic mechanism [ 69 ]. In some cases, endometriosis may be mistaken for a urethral diverticulum and therefore a precise diagnosis is essential to facilitate optimal management. Usually, urethral involvement is observed as a contiguous extension from the bladder and the MRI findings are the same as those described for bladder endometriosis [ 64 ].
Ureteral endometriosis may be defined as any situation where endometriosis or surrounding associated fibrosis causes compression or distortion of the normal ureteral anatomy, even when hydroureteronephrosis is not yet present [ 70 ]. Ureteral endometriosis is quite uncommon, most often unilateral, with a left predisposition; bilateral involvement is present in approximately 10–20% of cases [ 71 ]. Ureteral involvement is often associated with an ipsilateral endometrioma or with a rectovaginal nodule larger than 3 cm. The distal ureter, 3–4 cm above the vesicoureteral junction, is the most common ureteral segment involved [ 71 ].
The symptoms can vary according to the type of nodule infiltration: indeed endometrial tissue can directly infiltrate the muscularis propria, lamina propria, or ureteral lumen causing symptoms that may be related to the pelvic endometriosis itself (dysmenorrhea, dyspareunia) or secondary to urinary tract involvement (flank pain, obstruction, and in some cases decline of renal function) [ 64 ]. Also in the ureteral endometriosis there are two major pathological types: extrinsic and intrinsic. Extrinsic endometriosis is the most common form (80%) of ureteral involvement and it represents endometrial glandular and stromal tissue within the submucosa and adventitia of the ureter [ 21 ]. In addition, scar tissue or fibrosis without true endometriotic invasion of the ureter may also be classified as extrinsic disease. In contrast, intrinsic endometriosis (about 20%) involves the uroepithelial and muscular layer. At MRI, ureteral endometriosis usually appears as hypointense solid nodules on T2W images with spiculated margins, that envelop the ureter, causing dilatation of the ureter upstream [ 72 ]. Extrinsic disease may be hypothesized when the interface of fat between the nodule and ureter is no longer visible. MR urographic techniques can be used to obtain three-dimensional reconstructed images from coronal volumetric excretory phase T1W data. The differential diagnosis of ureteral endometriosis includes ureteral invasion by cervical cancer [ 64 ].
Vesicouterine Pouch
The vesicouterine pouch or anterior cul-de-sac is a common site of endometriotic involvement [ 21 ]. These lesions are associated with anteflexion of the uterus and obliteration of the anterior cul-de-sac due to extensive adhesions between the peritoneum of the bladder fold and the uterus. At MR imaging deep endometriotic implants involving the anterior uterine serosa demonstrate infiltrative pattern with indistinct margins and show hypointense nodules on T2-weighted images, with small cystic areas that typically adhere to the anterior uterine surface, forming an obtuse angle with the vesical wall [ 72 ].
The ovaries are the most common site of endometriosis (20–40% of cases) [ 72 ]. They may be affected in two ways: (i) the endometriomas or chocolate cysts that are associated with ovarian enlargement, and which are caused by repeated episodes of hemorrhage; and (ii) small nodular superficial implants which may cause paraovarian fibrous scarring and adhesions [ 64 ] (Fig. 5 A–C).
An Example of multiple endometriomas of both ovaries, which appear enlarged and sharpened (ovarian kissing): A sagittal T1w fat sat imaging; B , C axial and sagittal T2w imaging
Peritoneal implants confined to the ovarian surface are often underdiagnosed at imaging due to their small size (< 5 mm) [ 66 ].
Endometriomas are frequently multilocular and bilateral (50% of cases). In MRI an endometrioma appears as a homogeneously hyperintense mass on T1W MR images; on T2W MR images, it appears as a low-signal intensity mass with areas of high signal intensity [ 67 ] (Fig. 6 A–F). Endometriomas have a wall with a variable appearance (from thin to thick and fibrotic) and they usually contain dark/brown semi-solid material that represents the degenerated blood products (the so-called ‘‘chocolate cyst’’) [ 67 ] (Fig. 7 A–E). Because the endometriomas contain blood products of different ages and concentrations, they may show a variable appearance, in fact as free water in the cyst is resorbed, the iron concentration increases along with the viscosity of the contents of the cyst: this condition determines the ‘‘shading effect’’. Shading is present when a cyst, hyperintense on a T1W image, shows a gradient from hypointense to hyperintense on a T2W image. Shading can range from faint, dependent layering to complete signal void, according to the concentration of blood products [ 64 ]. It reflects the chronic nature of endometriomas and is the result of cyclic bleeding occurring over time. Old blood products contain high iron and protein concentrations which determine a decrease in T2 relaxation time. Therefore, on T2-weighted images endometriomas will show a gradual loss of signal within the lesion with low-signal intensity till complete signal void in the declivous portion.
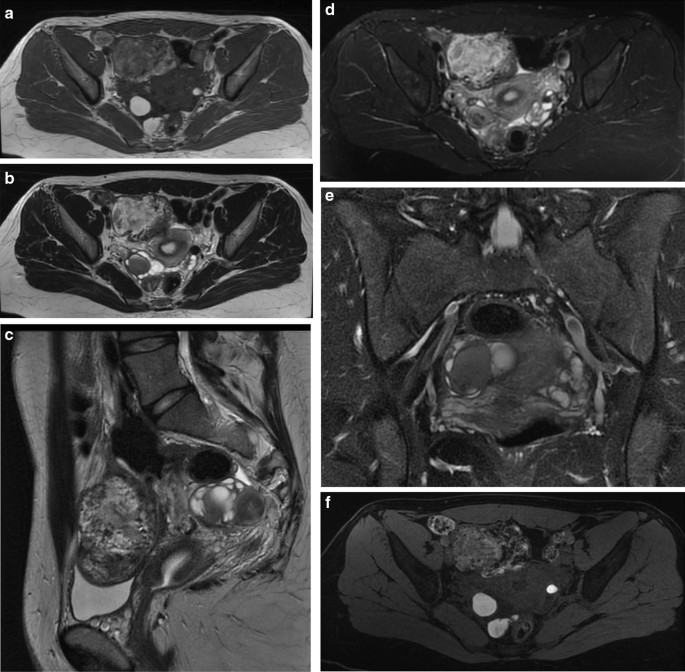
An example of ovarian endometriosis: A T1w imaging; B , C axial and sagittal T2w imaging; D , E axial and coronal T2 fat sat imaging; F T1w imaging after contrast administration. Hyperintensity in T1w is typical of a recent bleeding
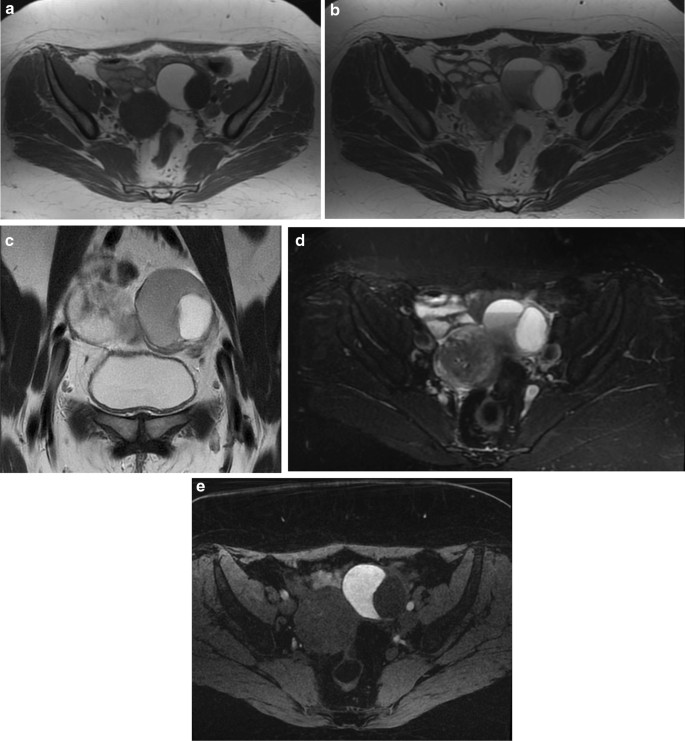
An example of endometrioma: A T1w imaging; B , C axial and coronal T2w imaging; D axial T2 fat sat imaging; E axial T1w imaging after contrast administration. It’s visible a double fluid–fluid level, indicating different bleedings
The most specific pathologic feature of endometrioma is the thick fibrous capsule containing a cluster of hemosiderin-laden macrophages due to repeated hemorrhage [ 66 ].
In certain cases, the ovaries may be joined together behind the uterus in the pouch of Douglas due to adhesion formation between the adjacent peritoneal surfaces, a sign described at US as B kissing ovaries ^ and suggestive of severe pelvic endometriosis [ 73 ].
Togashi et al. found that an extremely sensitive sign for the presence of an endometrioma was the presence of a cyst hyperintense on T1W images and the presence of shading on T2W images [ 74 ]. Another criterion is to observe multiple hyperintense cysts on T1W images (and T1W fat-suppressed images) regardless of their signal intensity on T2W images (Fig. 8 A–F).
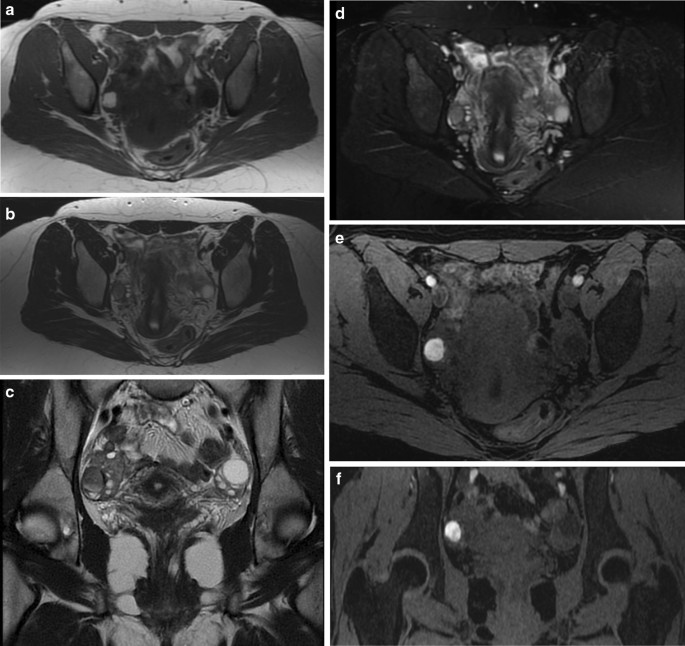
An example of endometriotic cyst: A axial T1w imaging; B , C axial and coronal T2w imaging; D axial T2 fat sat imaging; E , F axial and coronal T1w imaging after contrast administration
The differential diagnosis of endometriomas includes lesions with high signal intensity on T1-weighted images: dermoids, mucinous cystic neoplasms, and hemorrhagic masses. Fat-saturated T1-weighted sequences are helpful to rule out a fat-containing lesion (such as dermoids) and to confirm the presence of blood [ 75 ]. Mucinous lesions may show hyperintensity on T1-weighted images, but signal intensity is lower than that of blood.
It is possible to recognize and differentiate dermoids from endometriomas by the presence of chemical shift artifact and signal drop-out on the fat suppression image. Thus, on a T1-weighted frequency selective fat saturation sequence, the mature cystic teratoma will be low in signal, whereas an endometrioma will have high signal [ 64 ].
Hence, the most challenging differential diagnosis is with other hemorrhagic masses. To differentiate endometriomas from functional hemorrhagic cysts is important in order to prevent unnecessary surgical interventions. Functional hemorrhagic cysts (i.e., hemorrhagic follicular cysts and hemorrhagic corpus luteum cysts) are usually unilocular and unilateral, do not display shading on T2-weighted images, and mostly disappear on follow-up examinations (generally in 4–6 weeks), while endometriomas are usually multilocular and bilateral [ 68 ].
The role of DWI sequences in differentiating endometriomas from functional hemorrhagic ovarian cysts is still debated. Balaban et al. found significantly lower ADC values in endometriomas compared with functional hemorrhagic ovarian cysts in all b values [ 76 ]. Large lesions with wall nodularity, thick septations and enhancing solid components may be suggestive of malignancy.
Fallopian Tubes
Endometriotic involvement of the fallopian tubes is strongly associated with infertility. Serosal or subserosal implants involves the peritoneal surface of the fallopian tubes, where repeated hemorrhages lead to fibrosis and retraction of the tube with hydrosalpinx. Intraluminal implants determine cyclic hemorrhage thus causing hematosalpinx [ 66 ].
Moreover, an association has been described between the endometriosis in the fallopian tubes and a predisposition to endometrial malignancies such as clear cell carcinoma and endometrioid carcinoma [ 67 ].
At MR imaging hematosalpinx appears as a tortuous enlarged tubular adnexal structure filled with hemorrhagic fluid. Endoluminal content shows high signal intensity on fat- suppressed T1-weighted images and intermediate signal intensity, (40% of distended tubes in endometriosis have hyperintense contents) with or without internal fluid–fluid level, on T2-weighted images [ 77 ]. According to Siegelman the presence of T1-weighted hyperintensity within a dilated fallopian tube is suggestive of endometriosis [ 78 ].
In addition, it is atypical to see T2 shading within the lumen of the distended fallopian tube even when there is high signal on T1-weighted images [ 78 ]. T2 shading is not seen because of the fact that the endometriotic implants are mostly along the surface of the tube and not within the lumen of the tube, such that the chronic bleeding within the implants leads to adhesions along the tubal surface, but not within the lumen. The differential diagnosis of a hematosalpinx on MR imaging includes PID or fallopian tube malignancies. Pyosalpinx of PID can be differentiated by the clinical signs of infection (fever, white count) [ 18 ].
On MR imaging, hyperemia surrounding the fallopian tube with stranding in the adjacent fat would suggest a pyosalpinx. Fallopian tube carcinoma demonstrates solid, enhancing internal nodules within the fallopian tube and tends to occur in an older demographic group [ 43 ].
Uterus and Vagina
The most common localization of ectopic endometrial tissue within the uterus is the adenomyosis. Whereas it is important to remember that uterine involvement by endometriosis is usually subserosal, sometimes it is possible to find nodules of endometriosis in the serosal surface of the uterus [ 64 ].
Vaginal endometriosis is usually associated with implants in other pelvic locations, mostly retrocervical and rectal lesions; seldom isolated involvement of the vagina may occur. The upper one-third of the vagina and the posterior fornix are the most commonly affected sites. Generally, the vaginal wall implants show a thickened or nodular appearance [ 21 ], but may also have a polypoid structure. At MR imaging vaginal endometriotic implants show low-signal intensity on T2-weighted images. They often have a multiloculated internal appearance because of the presence of cystic areas [ 66 ]. These locules can show hyperintense content on T1-weighted images due to subacute blood products. Polypoid variant may have a T2 hypointense rim corresponding to surrounding fibrous tissue associated with endometriosis [ 79 ]. Rectovaginal fistulation represents a complication of vaginal endometriosis. Differential diagnosis includes epithelial neoplasms arising from the uterine cervix or vaginal wall [ 66 ].
Uterine Ligaments
At MRI the round ligaments can be identified as thin structures with hypointense fibrous signal on T1- and T2-weighted images, extending from the uterine horns to the pelvic side- wall, passing anteriorly to the external iliac vessels. They have an intra- and an extra-pelvic portion, the latter being the distal part of the ligament in the canal of Nuck [ 80 ]. When involved by endometriosis, round ligaments appear thickened (more than 1 cm), nodular, shortened and irregular. Usually endometriotic implants are a mixture of fibrous tissue and hemorrhage. Fibrous tissue shows hypointense signal on T1- and T2-weighted images; small hemorrhagic foci displays hyperintense signal on fat-suppressed T1-weighted images [ 66 ]. The presence of free fluid around the intra-pelvic portion of the round ligaments may represent an indirect sign of endometriosis [ 80 ].
Endometriosis of the broad ligaments usually manifests as thickening and nodularity of these peritoneal folds extending between the uterus and the lateral walls of the pelvis. These nodules are visible as hypo-intensity signal in T2 sequences and after administration of contrast material it is possible to observe a diffuse peritoneal enhancement secondary to the inflammatory reaction incited by microscopic endometrial implants on contrast-enhanced fat-saturated MRI [ 64 ].
Uterosacral ligaments (USL) are the most frequent location of deep endometriosis. Bilateral USL involvement is often associated with other posterior deep endometriotic locations, mostly the rectosigmoid colon [ 81 ]. At MR imaging normal USL are depicted as thin, regular, semicircular hypointense cords that originate from the lateral aspect of the uterine cervix and the vaginal vault and course dorsocranially toward the sacrum [ 21 ]. USL endometriosis is depicted as nodularity within the ligament or as unilateral or bilateral hypointense thickening of the ligament, with regular or irregular margins [ 21 ]. The proximal medial portion of the USL is most commonly affected by endometriosis.
According to Bazot et al. thin-section oblique axial T2-weighted sequences (3 mm thick, perpendicular to the long axis of the cervix) can improve the capability of conventional MRI to assess USL endometriosis [ 81 ].
Saba et al. suggested that the diagnosis of endometriosis of the USL is simple when ligaments are involved together with the torus uterinus, whereas, when there is only a thickening or an asymmetric nodular irregularity the involvement of the USL can be difficult [ 64 ].
In a recently published meta-analysis the sensitivity and specificity of MRI for the diagnosis of endometriosis of USL were 85% and 80%, respectively [ 82 ].
Retrocervical Area
The retrocervical area is a virtual extraperitoneal space behind the cervix, located above the rectovaginal septum [ 21 ]. It is a common site of deep pelvic endometriosis. Retrocervical implants are often associated with USL involvement and with the retroversion of the uterus [ 83 ].
Deep endometriotic lesions of the retrocervical area frequently appear as ill-defined infiltrative tissue, hypointense on T2-weighted images, extending from the posterior uterine serosa to the retrocervical region [ 21 ]. Nevertheless, some lesions may contain abundant glandular component and little fibrotic reaction, thus showing high signal intensity on T1-weighted images and variable signal intensity on T2-weighted images [ 21 , 63 ]. The solid glandular component enhances after intravenous administration of contrast material [ 63 ].
Del Frate et al. identified a condition they called ‘‘hourglass-shaped’’ lesions that are found in 25% of cases and are due to posterior extension of a posterior forniceal lesion toward the anterior rectal muscularis. These lesions are usually larger than 3 cm, with a greater risk of extension to the rectal wall [ 63 ].
In a recently published meta-analysis the sensitivity and specificity of MRI for the diagnosis of endometriosis of the pouch of Douglas were 89% and 94%, respectively [ 82 ].
The differential diagnosis of retrocervical lesions includes peritoneal metastases from intraperitoneal malignancies (i.e., gastrointestinal and ovarian neoplasms). Peritoneal metastases usually show intermediate to high signal intensity on T2-weighted images and, as the primary cancer site, high signal intensity on DWI; moreover, ascites and a tumor mass into the abdominal cavity may be identified [ 84 ]. On the other hand, solid endometriosis shows low-signal intensity on T2-weighted images [ 66 ].
Rectovaginal Pouch
The rectovaginal pouch is the anatomical region located between the posterior vaginal wall and the anterior rectal wall. It extends from the deepest part of the pouch of Douglas to the top of the perineal body [ 66 ]. The inferior two thirds of this space constitute the rectovaginal septum, a thin membranous partition usually filled with fat [ 21 ]. Usually rectovaginal implants represent extensions from retrocervical or posterior vaginal lesions.
In MR I , nodules of endometriosis affecting the rectovaginal pouch usually appear as hypointense nodules on both T1W and T2W MRI images with signal intensity close to that of pelvic muscle [ 85 ]. Sometimes, foci of endometriosis may also have an abundant glandular component and discrete fibrotic reaction. In such cases, the endometriotic foci are hyperintense nodules on T1W and fat-saturated T1W MRI images, irrespective of their appearance on T2W MRI images. Moreover, the solid glandular component shows variable enhancement after the intravenous administration of contrast material [ 64 ].
In normal conditions the MRI depicts the rectovaginal septum as a hyperintense signal area in T1W and T2W images whereas nodules of endometriosis affecting the rectovaginal septum usually appear as hypointense nodules on both T1W and T2W MRI images [ 64 ].
In a recently published meta-analysis the sensitivity and specificity of MRI for the diagnosis of rectovaginal septum endometriosis were 82% and 77%, respectively [ 82 ].
Rectosigmoid Colon
Among the bowel segments the rectosigmoid is the most commonly involved by endometriosis (65.7%), followed by vermiform appendix, terminal ileum, cecum and descending colon, in order of frequency [ 86 ]. Rectosigmoid endometriosis is often associated with other pelvic locations and with a second intestinal lesion in 55% of cases [ 72 ].
The rectosigmoid endometriosis may cause adhesions, bowel strictures, or intestinal obstruction may result from the inflammatory response to cyclic hemorrhage. The implants are usually serosal but can sometimes involve the subserosal layers and cause thickening and fibrosis of the muscularis propria. Usually, an intact overlying mucosa is present, since the implanted tissue only rarely invades through to the mucosa [ 21 ].
Typically, endometriotic lesions infiltrating the anterior rectal wall have a characteristic fan-shaped configuration (or a pyramidal shape, with the base adhering to the rectal wall and the apex oriented anteriorly toward the retrocervical region). The core of the lesion shows isointense signal compared to muscle on T2-weighted and T1-weighted sequences and at histopathology corresponds to thickening and distortion of the muscularis propria and smooth muscle hyperplasia [ 66 ]. The overlying layer, hyperintense on T2-weighted images, at the luminal side of the bowel wall corresponds to (sub)mucosal thickening, as a consequence of non-specific inflammation with or without infiltration of endometriosis [ 86 ]. When the longitudinal extent of the parietal lesion along the bowel wall is short, a pattern of intraluminal endophytic growth, called mushroom cap, may be observed [ 87 ].
When nodules of endometriosis are localized in the retroperitoneal section frequent concomitant findings are the adherences that identify fibrotic tissue originating from the nodules of endometriosis and involves the closest organs. Sometimes the adherences determine traction of the affected organs [ 88 ]. This is an important finding because it can be considered an indirect sign of endometriosis.
In a recently published meta-analysis the sensitivity and specificity of MRI for the diagnosis of rectosigmoid colon endometriosis were 83% and 88%, respectively [ 82 ]. MR imaging is useful to predict infiltration of the muscular layer of the bowel with a sensitivity of 100% and specificity of 75%. On the other hand, it is of limited value in diagnosing (sub)mucosal infiltration, as (sub)mucosal thickening may be caused by edema without infiltration of endometriosis. Nevertheless, extensive irregularities of the (sub)mucosal layer may raise suspicion of (sub)mucosal involvement [ 86 ]. Differential diagnosis includes rectal cancer and metastatic implants to the bowel [ 66 ].
Conclusions
Endometriosis is a chronic condition that affects women during the reproductive lifespan. Diagnosis of endometriosis is complex as it must take into account non-pathognomonic clinical symptoms and non-specific laboratory tests. The physical examination and above all the different imaging techniques, in particular US and MRI, constitute the gold standard for diagnosis. The role of the radiologist is fundamental both in the diagnosis of endometriosis, especially in the deep sites that cannot be seen with ultrasound, and in planning the type of therapeutic approach. A multidisciplinary study is essential for the management of these patients from both a clinical and surgical point of view.
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
Bazot M, Bharwani N, Huchon C, et al. European society of urogenital radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur Radiol. 2017;27(7):2765–75. https://doi.org/10.1007/s00330-016-4673-z .
Article CAS PubMed Google Scholar
Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68:585–96.
CAS PubMed Google Scholar
Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2(2):CD009591. https://doi.org/10.1002/14651858.CD009591.pub2 .
Article PubMed Google Scholar
Kinkel K, Frei KA, Balleyguier C, Chapron C. Diagnosis of endometriosis with imaging: a review. Eur Radiol. 2006;16(2):285–98. https://doi.org/10.1007/s00330-005-2882-y .
Chapron C, Fauconnier A, Vieira M, Barakat H, Dousset B, Pansini V, et al. Anatomical distribution of deeply infiltrating endometriosis: surgical implications and proposition for a classification. Hum Reprod. 2003;18:157–61.
PubMed Google Scholar
Pham M, Sommer C, Wessig C, et al. Magnetic resonance neurography for the diagnosis of extrapelvic sciatic endometriosis. Fertil Steril. 2010;94(1):351.e11-351.e14. https://doi.org/10.1016/j.fertnstert.2009.12.046 .
Article Google Scholar
Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:442–69.
Google Scholar
Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15(11):666–82. https://doi.org/10.1038/s41574-019-0245-z .
Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–75.
Chapron C, Chopin N, Borghese B, et al. Deeply infiltrating endometriosis: pathogenetic implications of the anatomical distribution. Hum Reprod. 2006;21(7):1839–45. https://doi.org/10.1093/humrep/del079 .
BuckLouis GM, et al. Bisphenol A and phthalates and endometriosis: the endometriosis: natural history, diagnosis and outcomes study. Fertil Steril. 2013;100:162-169.e1-2.
CAS Google Scholar
Stefansson H, et al. Genetic factors contribute to the risk of developing endometriosis. Hum Reprod. 2002;17:555–9.
•• Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. 2017;108(6):886–94. https://doi.org/10.1016/j.fertnstert.2017.10.026 For the explanation of the imaging features of the different localization of endometriosis .
Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11:595–606.
Ballard K, Lane H, Hudelist G, Banerjee S, Wright J. Can specific pain symptoms help in the diagnosis of endometriosis? A cohort study of women with chronic pelvic pain. Fertil Steril. 2009;94:20–7.
Koninckx PR, Meuleman C, Oosterlynck D, Cornillie FJ. Diagnosis of deep endometriosis by clinical examination during menstruation and plasma CA-125 concentration. Fertil Steril. 1996;65:280–7.
Abrao MS, Goncalves MO, Dias JA Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22:3092–7.
•• Hindman N, VanBuren W. Imaging spectrum of endometriosis (endometriomas to deep infiltrative endometriosis). Radiol Clin North Am. 2020;58(2):275–89. https://doi.org/10.1016/j.rcl.2019.11.001 For the explanation of the imaging features of the different localization of endometriosis .
Schenken RS. Delayed diagnosis of endometriosis. Fertil Steril. 2006;86(5):1305–6 ( Discussion: 1317 ).
Bean E, Naftalin J, Jurkovic D. How to assess the ureters during pelvic ultrasound. Ultrasound Obstet Gynecol. 2019;53(6):729–33.
Coutinho A, Bittencourt LK, Pires CE, et al. MR imaging in deep pelvic endometriosis: a pictorial essay. Radiographics. 2011;31:549–67.
Exacoustos C, Zupi E, Piccione E. Ultrasound imaging for ovarian and deep infiltrating endometriosis. Semin Reprod Med. 2017;35(1):5–24. https://doi.org/10.1055/s-0036-1597127 .
Maccagnano C, Pellucchi F, Rocchini L, et al. Diagnosis and treatment of bladder endometriosis: state of the art. Urol Int. 2012;89:249–58.
Pateman K, Holland TK, Knez J, et al. Should a detailed ultrasound examination of the complete urinary tract be routinely performed in women with suspected pelvic endometriosis? Hum Reprod. 2015;30:2802–7.
Crispi CP, de Souza CA, Oliveira MA, Dibi RP, Cardeman L, Sato H, et al. Endometriosis of the round ligament of the uterus. J Minim Invasive Gynecol. 2012;19:46–51.
Hudelist G, Ballard K, English J, et al. Transvaginal sonography vs. clinical examination in the preoperative diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol. 2011;37:480–7.
• Moro F, Leombroni M, Testa AC. Ultrasound Imaging in Endometriosis. Obstet Gynecol Clin North Am. 2019;46(4):643–59. https://doi.org/10.1016/j.ogc.2019.07.004 . For the description of the role of US and MRI in the diagnosis of endometriosis
Carfagna P, De Cicco NC, De Cicco NA, et al. Role of transvaginal ultrasound in evaluation of ureteral involvement in deep infiltrating endometriosis. Ultrasound Obstet Gynecol. 2018;51:550–5.
Maccagnano C, Pellucchi F, Rocchini L, et al. Ureteral endometriosis: proposal for a diagnostic and therapeutic algorithm with a review of the literature. Urol Int. 2013;91(1):1–9.
Knabben L, Imboden S, Fellmann B, Nirgianakis K, Kuhn A, Mueller MD. Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril. 2015;103(1):147–52.
Noventa M, Saccardi C, Litta P, Vitagliano A, D’Antona D, Abdulrahim B, et al. Ultrasound techniques in the diagnosis of deep pelvic endometriosis: algorithm based on a systematic review and meta-analysis. Fertil Steril. 2015;104:366-83.e2.
Holland TK, Cutner A, Saridogan E, Mavrelos D, Pateman K, Jurkovic D. Ultrasound mapping of pelvic endometriosis: does the location and number of lesions affect the diagnostic accuracy? A multicentre diagnostic accuracy study. BMC Womens Health. 2013;13:43.
PubMed PubMed Central Google Scholar
Van den Bosch T, Dueholm M, Leone FP, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46:284–98.
Guerriero S, Condous G, Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318–32.
Van den Bosch T, de Bruijn AM, de Leeuw RA, et al. A sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet Gynecol. 2019;53(5):576–82.
Reinhold C, Atri M, Mehio A, et al. Diffuse uterine adenomyosis: morphologic criteria and diagnostic accuracy of endovaginal sonography. Radiology. 1995;197:609–14.
Kepkep K, Tuncay YA, Goynumer G, et al. Transvaginal sonography in the diagnosis of adenomyosis: which findings are most accurate? Ultrasound Obstet Gynecol. 2007;30:341–5.
Di Donato N, Bertoldo V, Montanari G, Zannoni L, Caprara G, Seracchioli R. Question mark form of uterus: a simple sonographic sign associated with the presence of adenomyosis. Ultrasound Obstet Gynecol. 2015;46(1):126–7.
Dueholm M. Transvaginal ultrasound for diagnosis of adenomyosis: a review. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):569–82.
Meredith SM, Sanchez-Ramos L, Kaunitz AM. Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and metaanalysis. Am J Obstet Gynecol. 2009;201(1):107.e1-107.e6.
Luciano DE, Exacoustos C, Albrecht L, et al. Three-dimensional ultrasound in diagnosis of adenomyosis: histologic correlation with ultrasound targeted biopsies of the uterus. J Minim Invasive Gynecol. 2013;20(6):803–10.
Patel MD, Feldstein VA, Chen DC, et al. Endometriomas: diagnostic performance of US. Radiology. 1999;210(3):739–45.
Jones LP, Morgan MA, Chauhan A. The sonographic spectrum of pelvic endometriosis: pearls, pitfalls, and mimics. Ultrasound Q. 2019;35(4):355–75.
Guerriero S, Van Calster B, Somigliana E, et al. Age-related differences in the sonographic characteristics of endometriomas. Hum Reprod. 2016;31:1723–31.
Van Holsbeke C, Zhang J, Van Belle V, et al. Acoustic streaming cannot discriminate reliably between endometriomas and other types of adnexal lesion: a multicenter study of 633 adnexal masses. Ultrasound Obstet Gynecol. 2010;35(3):349–53.
Van Holsbeke C, Van Calster B, Bourne T, et al. External validation of diagnostic models to estimate the riskof malignancy in adnexal masses. Clin Cancer Res. 2012;18(3):815–25.
Mascilini F, Moruzzi C, Giansiracusa C, et al. Imaging in gynecological disease. 10: Clinical and ultrasound characteristics of decidualized endometriomas surgically removed during pregnancy. Ultrasound Obstet Gynecol. 2014;44(3):354–60.
Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol. 1998;12(1):56–66.
Rezvani M, Shaaban AM. Fallopian tube disease in the nonpregnant patient. Radiographics. 2011;31(2):527–48.
Chamié LP, Ribeiro DMFR, Tiferes DA, Macedo Neto AC, Serafini PC. Atypical sites of deeply infiltrative endometriosis: clinical characteristics and imaging findings. Radiographics. 2018;38(1):309–28. https://doi.org/10.1148/rg.2018170093 .
Guerriero S, Alcázar JL, Ajossa S, Pilloni M, Melis GB. Three-dimensional sonographic characteristics of deep endometriosis. J Ultrasound Med. 2009;28(8):1061–6.
Exacoustos C, Manganaro L, Zupi E. Imaging for the evaluation of endometriosis and adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2014;28(5):655–81.
Guerriero S, Ajossa S, Minguez JA, Jurado M, Mais V, Melis GB, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46:534–45.
Squifflet J, Feger C, Donnez J. Diagnosis and imaging of adenomyotic disease of the retroperitoneal space. Gynecol Obstet Invest. 2002;54:43–51.
Reid S, Lu C, Hardy N, et al. Office gel sonovaginography for the prediction of posterior deep infiltrating endometriosis: a multicenter prospective observational study. Ultrasound Obstet Gynecol. 2014;44(6):710–8.
Nisenblat V, Prentice L, Bossuyt PM, Farquhar C, Hull ML, Johnson N. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev. 2016. https://doi.org/10.1002/14651858.CD012281 .
Article PubMed PubMed Central Google Scholar
Guerriero S, Ajossa S, Gerada M, et al. “Tenderness-guided” transvaginal ultrasonography: a new method for the detection of deep endometriosis in patients with chronic pelvic pain. Fertil Steril. 2007;88:1293–7.
Valenzano Menada M, Remorgida V, Abbamonte LH, Nicoletti A, Ragni N, Ferrero S. Does transvaginal ultrasonography combined with water-contrast in the rectum aid in the diagnosis of rectovaginal endometriosis infiltrating the bowel? Hum Reprod. 2008;23(5):1069–75.
Reid S, Lu C, Casikar I, Reid G, Abbott J, Cario G, et al. Prediction of pouch of Douglas obliteration in women with suspected endometriosis using a new real-time dynamic transvaginal ultrasound technique: the sliding sign. Ultrasound Obstet Gynecol. 2013;41:685–91.
Rossi L, Palazzo L, Yazbeck C, et al. Can rectal endoscopic sonography be used to predict infiltration depth in patients with deep infiltrating endometriosis of the rectum? Ultrasound Obstet Gynecol. 2014;43(3):322–7.
Guerriero S, Saba L, Ajossa S, Peddes C, Angiolucci M, Perniciano M, et al. Three-dimensional ultrasonography in the diagnosis of deep endometriosis. Hum Reprod. 2014;29:1189–98.
Guerriero S, Spiga S, Ajossa S, et al. Role of imaging in the management of endometriosis. Minerva Ginecol. 2013;65:143–66.
Del Frate C, Girometti R, Pittino M, et al. Deep retroperitoneal pelvic endometriosis: MR imaging appearance with laparoscopic correlation. Radiographics. 2006;26:1705–18.
Saba L, Sulcis R, Melis GB, et al. Endometriosis: the role of magnetic resonance imaging. Acta Radiol. 2015;56(3):355–67. https://doi.org/10.1177/0284185114526086 .
Nakai A, Togashi K, Kosaka K, et al. Uterine peristalsis: comparison of transvaginal ultrasound and two different sequences of cine MR imaging. J Magn Reson Imaging. 2004;20(3):463–9.
• Foti PV, Farina R, Palmucci S, et al. Endometriosis: clinical features, MR imaging findings and pathologic correlation. Insights Imaging. 2018;9(2):149–72. https://doi.org/10.1007/s13244-017-0591-0 . For the description of the role of US and MRI in the diagnosis of endometriosis
Bis KG, Vrachliotis TG, Agrawal R, Shetty AN, Maximovich A, Hricak H. Pelvic endometriosis: MR imaging spectrum with laparoscopic correlation and diagnostic pitfalls. Radiographics. 1997;17(3):639–55.
Woodward PJ, Sohaey R, Mezzetti TP Jr. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001;21(1):193–216.
Liang CC, Tsai CC, Chen TC, et al. Management of perineal endometriosis. Int J Gynaecol Obstet. 1996;53:261–5.
Donnez J, Nisolle M, Squifflet J. Ureteral endometriosis: a complication of rectovaginal endometriotic (adenomyotic) nodules. Fertil Steril. 2002;77:32–7.
Seracchioli R, Raimondo D, Di Donato N, et al. Histological evaluation of ureteral involvement in women with deep infiltrating endometriosis: analysis of a large series. Hum Reprod. 2015;30(4):833–9. https://doi.org/10.1093/humrep/deu360 .
Chamié LP, Blasbalg R, Pereira RM, Warmbrand G, Serafini PC. Findings of pelvic endometriosis at transvaginal US, MR imaging, and laparoscopy. Radiographics. 2011;31(4):E77–100. https://doi.org/10.1148/rg.314105193 .
Ghezzi F, Raio L, Cromi A, et al. “kissing ovaries”: a sonographic sign of moderate to severe endometriosis. Fertil Steril. 2005;83(1):143–7.
Togashi K, Nishimura K, Kimura I. Endometrial cysts: diagnosis with MR imaging. Radiology. 1991;180:73–8.
Onbas O, Kantarci M, Alper F, et al. Nodular endometriosis: dynamic MR imaging. Abdom Imaging. 2007;32:451–6.
Balaban M, Idilman IS, Toprak H, Unal O, Ipek A, Kocakoc E. The utility of diffusion-weighted magnetic resonance imaging in differentiation of endometriomas from hemorrhagic ovarian cysts. Clin Imaging. 2015;39(5):830–3. https://doi.org/10.1016/j.clinimag.2015.05.003 .
Foti PV, Ognibene N, Spadola S, et al. Non-neoplastic diseases of the fallopian tube: MR imaging with emphasis on diffusion-weighted imaging. Insights Imaging. 2016;7(3):311–27. https://doi.org/10.1007/s13244-016-0484-7 .
Siegelman ES, Oliver ER. MR imaging of endometriosis: ten imaging pearls. Radiographics. 2012;32(6):1675–91. https://doi.org/10.1148/rg.326125518 .
Tham WP, Busmanis I, Tan WC, Kwek JW. Polypoid endometriosis of post vaginal fornix: utility of MRI imaging of pelvis with diffusion weighted imaging for diagnosis. Med J Malaysia. 2016;71(3):144–6.
Gui B, Valentini AL, Ninivaggi V, Marino M, Iacobucci M, Bonomo L. Deep pelvic endometriosis: don’t forget round ligaments. Review of anatomy, clinical characteristics, and MR imaging features. Abdom Imaging. 2014;39(3):622–32. https://doi.org/10.1007/s00261-014-0091-3 .
Bazot M, Gasner A, Ballester M, Daraï E. Value of thin-section oblique axial T2-weighted magnetic resonance images to assess uterosacral ligament endometriosis. Hum Reprod. 2011;26(2):346–53. https://doi.org/10.1093/humrep/deq336 .
Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291(3):611–21. https://doi.org/10.1007/s00404-014-3470-7 .
Chamié LP, Blasbalg R, Gonçalves MO, Carvalho FM, Abrão MS, de Oliveira IS. Accuracy of magnetic resonance imaging for diagnosis and preoperative assessment of deeply infiltrating endometriosis. Int J Gynaecol Obstet. 2009;106(3):198–201. https://doi.org/10.1016/j.ijgo.2009.04.013 .
Namimoto T, Awai K, Nakaura T, Yanaga Y, Hirai T, Yamashita Y. Role of diffusion-weighted imaging in the diagnosis of gynecological diseases. Eur Radiol. 2009;19(3):745–60. https://doi.org/10.1007/s00330-008-1185-5 .
Caramella T, Novellas S, Fournol M, et al. Deep pelvic endometriosis: MR imaging features. J Radiol. 2008;89:473–9.
Busard MP, van der Houwen LE, Bleeker MC, et al. Deep infiltrating endometriosis of the bowel: MR imaging as a method to predict muscular invasion. Abdom Imaging. 2012;37(4):549–57. https://doi.org/10.1007/s00261-011-9790-1 .
Yoon JH, Choi D, Jang KT, et al. Deep rectosigmoid endometriosis: B mushroom cap ^ sign on T2-weighted MR imaging. Abdom Imaging. 2010;35(6):726–31.
Balleyguier C, Chapron C, Dubuisson JB, et al. Comparison of magnetic resonance imaging and transvaginal ultrasonography in diagnosing bladder endometriosis. J Am Assoc Gynecol Laparosc. 2002. https://doi.org/10.1016/s1074-3804(05)60099-0 .
Download references
The authors received no financial sponsors or other funding for this research.
Author information
Authors and affiliations.
Radiology Unit, ‘‘Dimiccoli’’ Hospital, Viale Ippocrate 15, 70051, Barletta, Italy
Valentina Testini & Giuseppe Guglielmi
Department of Clinical and Experimental Medicine, Foggia University School of Medicine, Viale L. Pinto 1, 71121, Foggia, Italy
Radiology Unit, ‘‘Carlo Urbani’’ Hospital, Jesi, Italy
Laura Eusebi & Francesco Bartelli
Obstetrics and Gynecology Unit, ‘‘Carlo Urbani’’ Hospital, Jesi, Italy
Gianluca Grechi
Radiology Unit, Hospital ‘‘Casa Sollievo Della Sofferenza’’, Viale Cappuccini 2, 71013, San Giovanni Rotondo, Foggia, Italy
Giuseppe Guglielmi
You can also search for this author in PubMed Google Scholar
Contributions
All authors participated in the writing of the paper. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Giuseppe Guglielmi .
Ethics declarations
Conflict of interests.
The authors declare that they have no conflict of interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Global Radiology .
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Testini, V., Eusebi, L., Grechi, G. et al. Imaging of Endometriosis: The Role of Ultrasound and Magnetic Resonance. Curr Radiol Rep 10 , 21–39 (2022). https://doi.org/10.1007/s40134-022-00393-x
Download citation
Accepted : 07 February 2022
Published : 18 February 2022
Issue Date : March 2022
DOI : https://doi.org/10.1007/s40134-022-00393-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Deep endometriosis
- Endometrioma
- Transvaginal ultrasonography
Advertisement
- Find a journal
- Publish with us
- Track your research
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State

Patient Case Study
, mrs. m.d. is a 30 year old caucasian female who was referred to the gynecology clinic by her pcp after complaining of pain in her pelvic area along with increased bleeding during her menstrual cycle. she states that she has experienced this pain for quite some time, but recently it seems to have been worsening with each cycle. she also states that she is noticing pain with sexual intercourse., vital signs during office visit, temperature: 98.8 f, respiratory rate: 18, blood pressure: 110/75, physical examination: no abdominal or pelvic masses noted on palpation. physical examination shows no abnormalities., past medical and surgical history, tonsillectomy and adenoidectomy, age 8 years, asthma, age 10 years, age of menarche, age 9 years , dysmenorrhea, age 16 years, problem conceiving, age 27 years, pertinent family history, mother alive at 62 years of age with a history of, endometriosis post cesarean-section at age 35 years, father alive and healthy at age 65 years, sister alive with similar symptoms at age 27, pertinent social history, has worked full time as a teacher for 8 years, has been married to her husband for 7 years and has had trouble conceiving for the past 5 years, has only been sexually active with current spouse, and spouse reports no other sexual partners as well..
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Advanced Search
- Journal List
- Int J Mol Sci

Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature)
Beata smolarz.
1 Laboratory of Cancer Genetics, Department of Pathology, Polish Mother’s Memorial Hospital Research Institute, Rzgowska 281/289, 93-338 Lodz, Poland; lp.pw@zciwonamor-annah
Krzysztof Szyłło
2 Department of Operative Gynaecology and Oncological Gynaecology, Polish Mother’s Memorial Hospital Research Institute, Rzgowska 281/289, 93-338 Lodz, Poland; lp.2o@ollyzsk
Hanna Romanowicz
Associated data.
Not applicable.
Endometriosis is a “mysterious” disease and its exact cause has not yet been established. Among the etiological factors, congenital, environmental, epigenetic, autoimmune and allergic factors are listed. It is believed that the primary mechanism of the formation of endometriosis foci is retrograde menstruation, i.e., the passage of menstrual blood through the fallopian tubes into the peritoneal cavity and implantation of exfoliated endometrial cells. However, since this mechanism is also observed in healthy women, other factors must also be involved in the formation of endometriosis foci. Endometriosis is in many women the cause of infertility, chronic pain and the deterioration of the quality of life. It also represents a significant financial burden on health systems. The article presents a review of the literature on endometriosis—a disease affecting women throughout the world.
1. Endometriosis—History
Endometriosis is defined as the presence of the endometrium outside the uterine cavity accompanied by chronic inflammation. This disease was first described by Daniel Shroen in 1690 in the work “ Disputatio Inauguralis Medica de Ulceribus Ulceri ”. The symptoms of this disease were presented by Arthur Duff in 1769 [ 1 ].
The first appearances in the literature regarding the pathogenesis of endometriosis appeared in the second half of the nineteenth century. This condition was described by Karl von Rokitansky in 1860, who defined it as the presence of an active endometrium outside the uterine cavity [ 2 ].
In 1882, Von Recklinghausen suggested the name adenomyoma and by the end of the nineteenth century, several more authors had described this disease.
In 1908, a monograph was published by T.S. Cullen on adenomyosis [ 3 ].
Cullen was the first to describe the two main symptoms of adenomyosis: prolonged menstrual bleeding and severe pain. He believed that endometrial tissue came from the remains of Müller’s ducts [ 3 , 4 ].
In 1870, the German anatomist, physiologist and pathologist Heinrich Wilhelm Waldeyer was the first to put forward the theory of metaplasia.
One of the outstanding doctors of the nineteenth century, Iwanhofen in 1898 was the author of the thesis that endometrial tissue arises from metaplasia of the peritoneal epithelium.
According to Meyer’s research from 1903, metaplasia would promote the inclusion of the epithelium into the lining, under the influence of hormonal and inflammatory factors.
In Poland, Leonard Lorentowicz was the first to describe endometriosis in Polish Gynecology in 1937 in an article entitled: “On the pathogenesis of intrauterine adenomatosis in the peritoneal cavity (endometriosis peritonealis). Contribution to the etiology of abdominal pregnancy” [ 4 ].
Until the 1920s, endometriosis was considered a benefactory hyperplasia disease occurring under various names: cystadenoma, cystic fibrosis, adenomyoma.
In 1927, J.A. Sampson was the first to introduce the term “endometriosis” into medical nomenclature. According to the researcher, the cause of the disease is “retrogradea menstruation” or retrograde transport of menstrual blood with the consequent implantation of exfoliated endometrial mucosa cells within the peritoneal cavity [ 5 , 6 ].
Starting from the first theory of J.A. Sampson, as the years passed and research continued, new theories were formed that attempted to explain the phenomenon of endometriosis. Despite the passage of such a long period of time and much scientific research, Sampson’s theory is still dominant among other hypotheses regarding the etiopathogenesis of endometriosis. It has still not been possible to fully explain why the retrograde transport of menstrual blood, which occurs in nearly 90% of women of childbearing age, only leads to the survival of endometrial tissue outside the uterine cavity in a minority of women [ 7 ].
All the theories were sorted out by K. Schweppe in 1984 into three main groups:
- - First group—includes theories of endometrial transplantation by retrograde menstrual blood as well as mechanical transplantation (e.g., during surgery), and transport by lymphatic and blood vessels.
- - Second group—these are theories of the development of endometriosis in situ; that is, from local tissue from the remains of cells or tissues of the fetal embryo or the genitourinary system. The research of Waldeyer, Russell, Reclinghausen, Gebhard and Meyer and Novak contributed to the above theories [ 4 ].
- - Third group—includes Levander’s 1941 inductive theory. According to her, exfoliated endometrial cells enter the peritoneal cavity and stimulate its epithelium to transform into endometrial tissue [ 7 , 8 , 9 ].
Table 1 presents the main theories of endometriosis emerging over years of research on this disease [ 10 , 11 ].
Theories of the formation of endometriosis—a historical outline.
It is worth emphasizing that despite many years of research on endometriosis that tried to explain its etiology and pathogenesis, it still remains an enigmatic disease.
2. Endometriosis—Epidemiology
Endometriosis is characterized by the presence of active foci of the endometrium (glandular cells and stroma) or endometrial tissue (endometrioides; Gr. eides-similar) occurring outside its cavity, that is, in the muscular layer of the uterus, other genitals and their surroundings, and even in places distant from the genital organs of the body [ 12 ].
Endometrial foci outside the uterine cavity may appear, for example, in the peritoneal cavity, ovaries, bladder or ureters [ 13 ]. The ectopic endometrium is functionally similar to the eutopic endometrium.
Endometriosis is a benign, estrogen-dependent, gynecological disease; however, due to the accompanying ailments and chronic nature, it is a very important medical, social and economic problem.
Infertility is a relatively common symptom in patients with endometriosis. Up to 30 to 50% of women with endometriosis may experience infertility [ 14 ]. Endometriosis can influence fertility in several ways: distorted anatomy of the pelvis, adhesions, scarred fallopian tubes, inflammation of the pelvic structures, altered immune system functioning, changes in the hormonal environment of the eggs, impaired implantation of a pregnancy, and altered egg quality. Often, this infertility remains unexplained due to a delay in diagnosis, causing significant levels of stress [ 15 ].
Endometriosis is a common gynecological disease in Poland and in the world [ 16 ]. This disease affects from 10–15% of women of reproductive age and 35–50% of women with pelvic pain and/or infertility. However, it should be noted that there are also cases of patients with endometriosis after menopause, and it also happens in adolescent women [ 17 ].
The vast majority of cases of endometriosis occur in women between menarche and menopause. The peak of the disease falls in the period between 25 and 45 years of age [ 18 ].
Literature data indicate that endometriosis is found in 0.1–53% of women operated on laparoscopically or by laparotomy, of which 12–32% are women after diagnostic laparoscopy due to pelvic pain delays and 10–60% of the patient after diagnostic laparoscopy due to disability [ 19 , 20 , 21 ].
Endometriosis in 7% of women is associated with their genetic predisposition in the family. This disease was found in 2% of women undergoing tubal ligation and 17% of women after surgery to remove the ovaries [ 22 , 23 ].
World literature also reports the occurrence of foci of endometriosis in fetuses [ 24 ].
There have been isolated cases of endometriosis in men around the world who have been treated with hormones for prostate cancer [ 25 ].
The risk of developing endometriosis is the lowest in black women, the highest in Asian women. Caucasian women have a higher risk of getting sick than black women [ 26 ].
Endometriosis is a problem of enormous importance not only from the medical and social angles but also from an economic point of view. The annual costs of endometriosis treatment in Europe range from €0.8 billion to €12.5 billion depending on the country and are comparable to other chronic diseases such as diabetes [ 27 ].
Endometriosis has a significant negative impact on aspects of social life, family, and sexual, educational and professional life [ 27 , 28 , 29 ]. Pain and the associated dysfunction of the body worsen the quality of life and reduce professional productivity. In cases where there is no clear cause or medication, the disease can be chronic and recurrent. Due to its impact on sexuality and fertility, it can have a negative impact on partner relationships.
3. Endometriosis—Symptomatology
Endometriosis-related symptoms can affect a woman’s overall health and mental and social well-being. It causes a significant deterioration in the quality of life [ 30 , 31 ]. In 66% of women with endometriosis, the first symptoms of the disease appear before the age of 20 [ 32 ]. Symptoms of endometriosis include: gradually increasing acute premenstrual pain, pelvic pain, pain in the sacral region of the spine, dysmenorrhea, painful ovulation, pain during intercourse, pain when defecating, pain when urinating, pain radiating to the back, abundant irregular menstruation, blood in the stool, diarrhea or constipation, infertility and chronic fatigue [ 18 , 33 ].
Patients may also experience uncharacteristic accompanying symptoms such as subfebrile conditions, nausea, dizziness and headaches, symptoms of depression, anxiety, hypoglycemia, rectal bleeding, hematuria during menstruation or susceptibility to infections and allergies.
The pain associated with endometriosis most often takes the form of painful menstruation. It precedes the appearance of bleeding; over time it intensifies and its location concerns the lower abdomen and deeper pelvic areas. Pain can radiate to the sacral region. The pain can extend beyond the bleeding period and also be present throughout the menstrual cycle. There is a hypothesis that the intensification of menstruation soreness is associated with the involvement of the Douglas sinus and the formation of adhesions in it [ 34 ].
Sometimes very advanced endometriosis may not cause any symptoms, and, paradoxically, small foci within the peritoneum can cause great pain. Intraperitoneal adhesions or overgrowth of the fallopian gouges are the most common causes of the problem with the treatment of endometriosis. Sometimes foci of endometriosis produce antibodies to the eutopic endometrium, which can induce poor embryo implantation or spontaneous abortions. Increased and profuse menstruation is one of the symptoms of endometriosis, e.g., in adenomyosis (so-called internal endometriosis) [ 34 ].
Adenomyosis is defined as the occurrence of ectopic foci of the endometrium outside the uterine cavity. Their effect is lower abdominal pain and abnormal menstrual bleeding. Due to its significant similarity to endometriosis, adenomyosis has so far been classified as endometriosis genitalis interna, in which endometrial foci are located within the muscle membrane of the uterus. In recent years, the distinctiveness of this disease entity has been proven, indicating differences in symptomatology, pathogenesis and treatment [ 35 ].
The cause of adenomyosis is still unknown. This disease usually resolves after menopause. The only effective treatment for adenomyosis remains surgery.
The range of diagnosis of adenomyosis varies between 5% and 70% of patients. Adenomyosis is more common in multiparous women than in nulliparous women [ 36 ].
The average age of diagnosis of adenomyosis is between 40 and 50 years of age. However, this disease can occur in young women as well as after menopause. The pathogenesis of adenomyosis remains a mystery.
In addition to typical ailments such as menstrual pain, pain during intercourse or pain in the lower abdomen, there are also problems in partner relationships and symptoms of depressed mood [ 37 ].
The time from the appearance of the first symptoms of the disease to the diagnosis is up to 8 to 10 years [ 38 ].
Despite such frequent occurrence of this disease, the mechanism of its formation remains unexplained, and a good marker of this disease has still not been discovered [ 39 , 40 ].
Deep infiltrating endometriosis (DIE) is defined as the presence of ectopic endometrial tissue infiltration under the peritoneum, pelvic structure, and organ walls, including the uterosacral ligaments, rectosigmoid colon, vagina, rectovaginal septum, bladder, ureter, and lateral parametrium (LP). DIE may cause pelvic pain and thus negatively affect the function of different structures. Studies indicate that women with DIE may have dysfunctions of the pelvic floor muscles (PFMs) and lower limb muscles (LLMs). Pain was associated with PFM hypertonia and difficulty in PFM relaxation [ 41 ].
The presence of lateral parametrial endometriosis (LPE) can be considered a reflection of a more severe disease, ureteral stenosis and dilatation, and voiding dysfunctions, mainly because of the involvement of the inferior hypogastric plexus. Patients with LPE reported more frequent constipation and voiding symptoms [ 42 ]. Associations exist between LPE and straining to void, the feeling of incomplete emptying, intermittency, and abnormal residual urine and bladder outlet obstruction. LPE might stimulate sympathetic fibers of the pelvic plexus, promoting an increase in urethral sphincter tone and thus leading to different degrees of outlet obstruction. These findings emphasize the importance of obtaining a focused history and objective evaluations of urinary and rectal function in patients presenting with clinical or instrumental findings suggestive of DIE [ 42 ].
4. Risk Factors for Endometriosis
Risk Factors for Endometriosis Include:
- Early menarche—epidemiological studies analyzing the cycle of women with endometriosis have shown that the early first cycle (before the age of 11) is associated with the risk of endometriosis [ 43 , 44 , 45 , 46 ],
- Shorter than 27-day genital cycles, genital defects, including hymen overgrowth or narrowing of the cervical canal [ 47 ]. The risk of endometriosis is increased in women with short cycles, i.e., lasting less than 27 days, but is unrelated to the number of bleeding days and the volume of menstruation [ 48 ],
- Small number of births,
- Caucasian race,
- Age 25–29,
- Daily consumption of alcohol in the amount of at least 10 g per day,
- Endometriosis is more often diagnosed in infertile women who are active smokers and whose body mass index (BMI) is normal or low [ 49 ].
Interestingly, the latest data indicate that there is generally no association between BMI and the incidence of endometriosis, but there has been a significant increase in the incidence of endometriosis in obese women compared to women with normal body weight. Obesity is also a risk factor for severe dysmenorrhea [ 50 ].
Diet plays a very important role in preventing the development of endometriosis. The consumption of green vegetables and fresh fruits is considered to be the most beneficial [ 51 ]. They contain antioxidants, which play an important role in the proper functioning of the immune system and the removal of free radicals. It is worth noting that the fiber contained in vegetables interacts in the control of the intestinal bacterial flora and affects hormonal balance.
Red meat exerts an antagonistic effect on the development of endometriosis compared to vegetables and fruits. It is characterized by a high content of dioxins, hormones and fat, increasing the concentration of estrogens [ 51 ].
In the latest research by the team of Yamamoto et al., an attempt was made to determine whether higher consumption of red meat, poultry, fish and seafood is associated with the risk of laparoscopically confirmed endometriosis [ 52 ]. The study group consisted of 81,908 women, and the observations covered the years from 1991 to 2013. Diet was assessed using a properly prepared nutrition questionnaire administered every 4 years. It was shown that respondents who reported eating > 2 servings/day of red meat had a 56% higher risk of endometriosis compared to those consuming ≤ 1 serving/week. Women who were classified in the category that consumed the most red meat were more likely to have endometriosis. No association with poultry, fish, shellfish and egg consumption and the risk of endometriosis was demonstrated [ 52 ].
A very important factor in the prevention of primary endometriosis is the maintenance of an appropriate lifestyle, in which a significant part should be occupied by rest, movement and physical activity [ 51 ].
5. Theories of the Formation of Endometriosis
So far, the pathomechanisms of the formation of endometriosis have not been definitively explained. The theory of Samson (“retrograde menstruation”) is more widespread. It says that the foci of endometriosis arise as a result of the displacement of menstrual blood into the peritoneal cavity through the fallopian tubes [ 5 , 6 , 53 ].
Literature data indicate that in 80% of women with open fallopian tubes there is a retrograde outflow of menstrual blood, while endometriosis occurs only in some. This suggests the presence of other factors determining the survival of endometrial cells in the peritoneal cavity and their implantation [ 54 ].
Implantation in the peritoneum is explained by a local disorder of the mechanisms that prevent adhesion. The consequence is an increased production of cytokine macrophages, including tumor necrosis factor α (TNF-α) and interleukin [ 55 ].
Immune phenomena are important for explaining the possible pathomechanisms of endometriosis foci, as studies indicate altered humoral and cellular immunity [ 56 ].
There are literature data on the statistically higher occurrence of certain immune diseases together with endometriosis, for example, rheumatoid arthritis or hypothyroidism [ 57 ]. Another way of creating endometriosis foci was presented by Mayer’s theory, which says that peritoneal cells are transformed into Muller-type cells under the influence of hormones [ 58 ]. This theory is based on the assumption of the existence of cells capable of differentiating in the endometrium and those cells being precursors of the mesodermal epithelium of the ovary and the pelvic peritoneum. This theory is particularly useful in explaining the existence of endometriosis in different regions of the body where there is a mesothelium, e.g., pleural cavity [ 59 , 60 ].
The last theory is, in the light of two previous theories, that endogenous biochemical and immunological factors responsible for the non-functioning of endometrial factors are not being used for endometrial systems [ 61 ].
Endometriosis is also considered a chronic inflammatory process associated with immune processes [ 62 ]. Disorders of the immune system accompany virtually every stage of endometriosis development [ 63 ]. Macrophages are known to participate in recognizing foreign cells and presenting them to T cells. An increased number of activated macrophages with a reduced ability to phagocytose in the peritoneal cavity is characteristic of women with endometriosis.
They secrete pro-inflammatory cytokines, such as IL-6, TNF-α, IL-1β and IL-8, in increased amounts. Peritoneal macrophages in women with endometriosis have increased mRNA expression of the cyclooxygenase-2 (COX-2), which resulted in increased prostaglandin secretion [ 64 ]. Increased release of pro-inflammatory cytokines and reduced production of anti-inflammatory factors from stromal, epithelial, smooth or immune cells contribute to the initiation, development and progression of endometriosis [ 65 ] ( Table 2 ).
Cytokines associated with the development of endometriosis.
Another important factor in the pathogenesis of endometriosis is the disturbed balance between type 1 (Th1) and type 2 (Th2) helper lymphocytes. Activated T cells differentiate into Th1 lymphocytes and Th2 lymphocytes. The main function of Th1 is the production of cytokines and the promotion of cell-type responses, whereas Th2 secretes cytokines involved in the differentiation of B lymphocytes, suppression of cell-type responses, as well as the severity of humoral type responses. According to literature data, Th2 lymphocytes gain an advantage in women with endometriosis [ 79 ].
In women with endometriosis, reduced Natural Killer (NK) cell activity is found. This is the main group of cells of the immune system responsible for the phenomenon of natural cytotoxicity. Impaired function of NK cells reduces their ability to cleanse the peritoneal cavity of endometrial elements after retrograde outflow of menstrual blood [ 80 ].
The formation of new vessels is a necessary condition for the development of the ectopic endometrium, especially in the peritoneal microenvironment. Neo-angiogenesis is accompanied by the formation of nerves, which may explain the pain in patients [ 81 ].
Vascular endothelial growth factor (VEGF) is responsible for the formation and growth of new vessels. In women with endometriosis, elevated concentrations of VEGF in peritoneal fluid and its correlation with the stages of the disease were found [ 82 ].
At the moment, scientists agree that many factors are responsible for the formation of endometriosis, and in particular genetic, immunological and environmental factors [ 83 ].
It has been confirmed that genetic conditions may be factors influencing the development of this disease. Currently, a group of genes that can enable the formation of endometriosis is listed. These include the cytochrome P450 gene, estrogen, progesterone and androgen receptor, as well as the p53 gene [ 83 ].
6. Types of Endometriosis
There are several types of endometriosis:
- Ovarian endometriosis—occurs in the form of superficial lesions and as endometrial cysts,
- Peritoneal—can occur in various forms: white raids on the peritoneum, peritoneal defects, red, brown, black-blue and black foci, colorless bright vesicles and focal dilated blood vessels and petechiae,
- Deep infiltrating endometriosis—DIE,
- Endometriosis of other locations.
The three most typical types of endometriosis are peritoneal endometriosis, ovarian cysts (chocolate cysts) and nodules of deeply infiltrating endometriosis in the gut or vaginal–rectal septum [ 84 ].
Peritoneal endometriosis can occur in the form of intraperitoneal and sub-peritoneal. Foci of endometriosis within the peritoneum are found in 15–50% of all women diagnosed with endometriosis [ 85 ]. The best diagnostic method for detecting these changes is to recognize them during laparoscopic surgery [ 86 ].
Ovarian endometriosis occurs in 2–10% of women of reproductive age and 50% of patients treated for infertility [ 87 ]. Ovarian endometriosis is one of the most common localizations of this condition.
Deep infiltrating endometriosis is characterized by the fact that endometrioid changes can reach deep into the extraperitoneal space and occupy various pelvic organs such as the bladder, ureters, large intestine, sacro-uterine ligaments or the vagina. The pathomechanism of DIE is not clearly defined.
7. Classifications of Endometriosis
In order to assess the location and severity of the disease, many divisions and classifications have been proposed.
According to Sampson [ 88 ], we will divide it into:
- - Internal endometriosis affecting the uterine muscle
- - External endometriosis occurring outside the uterine muscle
Divisions of endometriosis by location were developed according to the classification of Martius and Kistner [ 89 , 90 ] ( Table 3 ).
Types of divisions of endometriosis by location.
Based on the histological classification according to Brosens (1993), we can distinguish types of endometriosis [ 91 ]:
- Mucosal type (occurs in endometrial cysts of the ovary),
- - early, active, glandular or follicular lesions,
- - advanced, black, wrinkled changes,
- - white fibrotic lesions,
- Glandular type (the main element is fibrous-muscular tissue and concerns deeply infiltrating endometriosis).
Among the many divisions of endometriosis according to the extent and severity of lesions, the division developed by the American Fertility Association (AFS) is the most commonly used. The American Society of Reproductive Medicine distinguishes four stages of endometriosis, where stage I and II are fairly mild types, and stages III and IV are advanced disease [ 92 , 93 ]. Currently, there is a division corrected by AFS in 1985.
The classification developed by the American Society of Reproductive Medicine (ASRM), based on the results of laparoscopy or laparotomy, it is the most common system used in clinical practice. The most important goal of classifying and determining the severity of endometriosis is to propose an effective treatment plan for it [ 94 ].
The ENZIAN scale in deeply infiltrating endometriosis is a descriptive scale, considering both the existence of the lesion and the depth of the invasion. In the ENZIAN classification, the location of foci was assigned to separate anatomical compartments [ 95 ].
- - Compartment A—foci located in the vagina and the rectovaginal septum,
- - Compartment B—foci located in the sacro-uterine ligaments up to the pelvic walls,
- - Compartment C—foci located in the sigmoid colon and rectum.
This classification also describes the foci of the ectopic endometrium depending on the place of their occurrence as:
- FA—adenomyosis,
- FB—urinary bladder endometriosis,
- FU—ureter endometriosis,
- FI—endometriosis of the bowel wall above the sigmoid colon,
- FO—infiltration of other anatomical structures, e.g., abdominal integuments.
Endometriosis occurring outside the pelvis is a rare phenomenon. However, literature data provide information on respiratory endometriosis or pericardial endometriosis or endometriosis in a scar after surgery with laparotomy access [ 96 , 97 ].
8. Diagnostics
Histopathological examination clearly allows for the diagnosis of endometriosis. However, a good medical history, gynaecological examination with specula, two-handed examination, additional diagnostic tests using imaging techniques, laparoscopy and biochemical tests are helpful in the initial diagnosis of the disease.
The basic examination in the diagnosis of endometriosis is an ultrasound examination [ 98 ]. Ultrasound examination (ultrasonography, USG) is helpful in the diagnosis of endometrial cysts of the ovary and of congenital defects of the reproductive organs favoring the retrograde outflow of menstrual blood into the peritoneal cavity [ 99 ]. In the case of endometriosis infiltrating the urinary bladder or the large intestine, it is justified to perform cystoscopy, colonorectoscopy and transrectal ultrasound examination [ 99 ].
In the case of deeply infiltrating endometriosis, the Rectal Water Contrast Transvaginal Sonography (RWC TVS) is also appropriate. The water contrast allows us to detect foci in the intestinal area and assess their progression ( Figure 1 ).

Intestinal endometriosis (from Department of Operative Gynaecology and Oncological Gynaecology, Polish Mother’s Memorial Hospital Research Institute, Lodz, Poland).
Deeply infiltrating endometriosis is characterized by the infiltration of endometrial cells > 5 mm below the surface of the peritoneum [ 100 ]. Profound lesions mainly affect the posterior pelvic compartment, including the rectovaginal septum, posterior vaginal vault, utero sacral ligaments and anterior rectal wall, causing adhesions and distortions of pelvic anatomy [ 101 , 102 ]. In addition to the classic DIE pain syndrome (characterized by dysmenorrhea, dyspareunia, chronic pelvic pain, dystrophy and dyschesia), profound changes are associated with dysfunction of the pelvic organs and pelvic floor muscles (PFM) [ 103 ]. A series of events or a combination of factors may contribute to the development of non-relaxing PFM in women with chronic pelvic pain, including direct or indirect (neuropathic) pelvic floor muscle injury, pelvic pain symptoms and inflammation. Evaluation of PFM by palpation or electromyography can cause pain, causing pelvic muscle spasm, which can be a confounding factor. Transperineal ultrasound has been shown to be an important, reliable and non-invasive tool for assessing pelvic floor morphometry [ 104 , 105 ]. Women with DIE have a smaller area of the levator hiatal area (LHA), and dynamic maneuvers with three-dimensional (3D) and four-dimensional (4D) transperineal ultrasound suggest they have higher muscle tone and lower strength. [ 106 ] than those without DIE. In a 3D/4D transperineal ultrasound examination, women with ovarian endometriosis and concomitant profound lesions appear to have increased PFM tone and decreased PFM strength compared to women with isolated ovarian endometriosis [ 107 ]. Transperineal ultrasound is a reliable and non-invasive tool for assessing pelvic floor morphometry [ 108 ]. Pelvic floor hypertonic dysfunction is a complex condition, and the specific cause or event that triggers this disorder is often not clearly identified [ 109 ]. A higher tone of PFM is associated with its damage and innervation (i.e., surgery, trauma or endometriosis), musculoskeletal changes (i.e., skeletal asymmetry), chronic pelvic pain syndromes and/or dyspareunia [ 110 , 111 ].
Moreover, this condition can also be attributed to abnormal learned pelvic floor activity or poor activity acquired in adulthood, through constant voluntary urination or defecation, or through sexual abuse [ 112 ]. The relationship between hypertonic disorder PFM and DIE can be explained by two main mechanisms. First, women with ovarian endometriosis alone tend to have less severity of pain symptoms than women with peritoneal endometriosis. In addition, in DIE lesions, increased density of nerve fibers and the phenomenon of perineural and intraneural invasion were clearly demonstrated [ 113 ]. Women affected by DIE showed smaller LHA than women with isolated ovarian endometriosis. Hypertonic dysfunction of PFM may be associated with pain symptoms (myofascial pain) and changes in pelvic organ function in women with DIE. Indeed, the role of PFM is not only to support the pelvic organs, but its contraction and relaxation are necessary for the proper functioning of the intestines and bladder and to enable painless sexual intercourse. While hypotonic pelvic floor disorders, such as pelvic organ prolapse, are often easily identified, symptoms of hypertonic PFM disorders are often not well studied, making these disorders unreported or misdiagnosed as other, better known concomitant conditions [ 109 ]. Because PFM dysfunction can cause pain symptoms and pelvic organ dysfunction potentially resistant to hormonal or surgical endometriosis therapy, transperineal ultrasound may enable a more complete functional assessment in women with DIE, with the goal of achieving tailored therapy, including pelvic floor rehabilitation.
It is also helpful to have a magnetic resonance imaging (MRI) examination, but the ultrasound examination is the basic tool in the diagnosis of this disease.
However, the gold standard in the diagnosis of endometriosis is laparoscopic surgery, with simultaneous confirmation in histopathological examination [ 114 ].
The less frequent areas of endometriosis include the vaginal vault and cervix, which is why examination with a speculum is an obligatory element of the gynaecological examination. Palpation can detect endometriosis in the area of the vaginal-rectal septum and the sacro-uterine ligaments. A characteristic and frequently encountered feature in patients with endometriosis is also uterine retroflexion, often forced by intraperitoneal adhesions.
9. Treatment
Treatment of endometriosis is a big medical problem due to the fact that this disease is difficult to treat and is chronic in nature. Pharmacological, surgical or combination treatment is possible.
Symptoms associated with the presence of foci of endometriosis are the main factor determining the need to start treatment. These symptoms are pain complaints of varying severity and location, and problems with getting pregnant. Women who notice abnormalities in their body report to the doctor, which allows them to apply a selected treatment in advance. A big problem remains patients who, despite the occurrence of pain, report late and therefore the disease, especially DIE, causes the involvement of other organs and the need to perform extensive complicated surgical procedures. Pharmacological, surgical and combination treatments are used to treat endometriosis [ 115 ]. In pharmacological treatment, we distinguish between hormonal and symptomatic treatment. Currently, hormonal treatment is allowed, for three months without histopathological confirmation of the disease. The choice of treatment is made depending on the age of the patient and her procreative desires, the ailments present and the form of endometriosis. Proper diet and lifestyle also play an important role.
9.1. Pharmacological Treatment
Drug treatment is used in women in their reproductive years. The goal of pharmacological treatment is to reduce or eliminate pain, inhibit further development and regression of endometrial foci and restore fertility. Initiation of pharmacological treatment of endometriosis is possible only on the basis of a clinical exam without the need to confirm the existence of the disease in laparoscopic examination (empirical therapy).
Pharmacotherapy may be part of the preparation for surgery, as well as a complementary procedure in the postoperative period. There are certain types of drugs that are used in the treatment of endometriosis ( Table 4 ).
Groups of medicines used in the treatment of endometriosis.
Non-steroidal anti-inflammatory drugs inhibit the synthesis of prostaglandins, contribute to reducing the inflammatory process and resolve pain [ 114 ]. Complex estrogen-progestogen therapy can be used cyclically or continuously.
In hormone therapy of endometriosis, the following groups of drugs are used [ 116 ]:
Danazol—a derivative of the male sex hormone testosterone. It inhibits the secretion of GnRH, causing a decrease in the secretion of LH and FSH by the pituitary gland. Therapy is long-term (6–9 months) and is started on the scond day of the cycle. The drug has many side effects. These include weight gain, fluid retention, breast reduction, oily skin and acne, hot flashes, decreased voice timbre.
Gonadoliberin analogues are substances with a structure similar to GnRH, which “deceive” the hypothalamus, causing a decrease in the production of real GnRH. This results in a decrease in LH and FSH levels and a significant reduction in estrogen levels. Therapy lasts at least 3–6 months. Side effects are caused by low estrogen levels. These include loss of calcium from the bones leading to osteoporosis, vaginal dryness, decreased libido, headaches, insomnia.
Progestogen preparations—contain progesterone derivatives, whose function is to reduce the level of GnRH, and thus the levels of FSH, LH and estrogen. Therapy should be used for at least 6 months. The most common side effects include fluid retention, weight gain, breast tenderness, spotting and irregular bleeding from the genital tract.
Oral contraceptives (estrogen-progestogen preparations)—their action consists in lowering the level of FSH and stabilizing the endometrium, which leads to a reduction in pain.
An intrauterine device releasing levonorgestrel is a progesterone derivative. The effect is the same as progestogen preparations. Recommended especially for women with severe pain.
Aromatase inhibitors—the latest group of drugs used in the treatment of endometriosis. Their action takes advantage of the fact of specific behavior of endometriosis cells. Foci of endometriosis, regardless of the ovaries, produce estrogens that cause an increase in endometrial lesions. Aromatase inhibitors interrupt estrogen production in both endometriosis foci and ovaries, causing a significant reduction in estrogen levels. Side effects are due to low estrogen levels. The most important of these is the significant loss of calcium from the bone causing osteoporosis. Others include loss of libido, vaginal dryness, insomnia [ 116 ].
All recognized methods of pharmacological treatment have similar therapeutic effectiveness. They differ in the scope and speed of the appearance of side effects. There is still an urgent need to develop new types of pharmacological therapies that will guarantee a complete cure of patients without the occurrence of side effects.
The latest drug to be approved by the Food and Drug Administration (FDA) is elagolix, a drug for the treatment of moderate to severe pain associated with endometriosis [ 117 ]. The results of a study in which about 1700 women with moderate or severe pain in endometriosis participated played a part in the above decision. Doses of this drug—150 mg once a day or 200 mg twice a day—significantly reduced the most common types of endometrial pain: pelvic pain and sex-related pain. For a dose of 150 mg, the duration of use of the drug is 24 months; for a dose of 200 mg duration is limited to 6 months, as the drug causes a decrease in bone mineral density. The drug is taken orally [ 117 ].
Elagolix is a non-peptide GnRH (gonadotropin-releasing hormone) antagonist. Elagolix enables dose-dependent reductions in estrogen levels and is effective in relieving moderate to severe endometriosis pain with long-lasting, sustained effects. As a GnRH antagonist, elagolix may also reduce bone mineral density through its estrogen reduction mechanism [ 118 ].
As evidenced by literature data, although there is a risk of bone loss when elagolix is used, the effect of elagolix on the long-term risk of fracture is minimal [ 119 , 120 ].
Results from the final model simulations indicate support for recommendations for the use of elagolix at a dose of 150 mg q.d. for 24 months [ 121 ].
9.2. Surgical Treatment
In the case of surgical treatment, it can be sparing or radical. Sparing treatment applies to adolescent patients and women of childbearing age planning to become pregnant. Radical surgical treatment is carried out in patients who do not plan pregnancy or those who continue to have pain despite the pharmacotherapy used.
Indications for surgical treatment of endometriosis are as follows:
- - pelvic pain,
- - infertility in endometriosis,
- - endometrial ovarian cysts.
Laparoscopy is the recommended surgical technique for the treatment of endometriosis, regardless of its stage [ 122 ]. The best therapeutic effects are achieved as a result of the combination of surgical treatment with pharmacological treatment. Medications are applied both before and after surgery.
Laparoscopy with the assistance of a robot is a viable method of resection of deeply infiltrating endometriosis, especially in the rectal-sigmoid region. In the case of endometriosis, a mini-invasive laparoscopic or laparoscopic approach using a robot is strongly recommended [ 108 ]. The disadvantage of surgery, however, is that when removing DIE lesions, complications that affect the functions of the gastrointestinal, urinary or sexual tract often arise. Complications after DIE surgery include rectal fistula (0.3–2%), intestinal stenosis (2%), and bladder atony (4–6%) [ 123 , 124 , 125 ].
Several studies have compared laparoscopic or robotically assisted methods in the surgical treatment of endometriosis [ 126 , 127 , 128 ].
Laparoscopically assisted robotics are associated with longer duration of relief than laparoscopic surgery, but the results are controversial [ 129 , 130 ].
The results of previous studies on the benefits of robot-assisted laparoscopy compared to conventional laparoscopy are somewhat heterogeneous. However, patients with features of a complex pelvic situation, such as severe endometriosis, increased body mass index, or previous surgeries, may benefit from robotic surgery [ 131 ].
Endometriosis treatment between robot-assisted laparoscopy and conventional laparoscopy was compared in [ 130 ].
This study did not detect any differences in perioperative outcomes of surgery between robotic surgery and laparoscopy.
The results of other smaller studies, mainly retrospective, were heterogeneous. Some of these studies have shown longer surgery times for robotic assisted procedures than for laparoscopy, whereas other studies have shown benefits of robotic surgery [ 132 ].
Laparoscopic rectal nodulectomy with nerve-sparing robots has been shown to be a feasible and safe treatment for isolated sting-rectal DIE [ 133 ].
The latest study suggests that robot-assisted laparoscopy is a possible method of DIE resection [ 134 ].
9.3. Physiotherapy in Endometriosis
Physiotherapy in endometriosis focuses on non-invasive and conservative treatment of pelvic floor disorders in women. It deals with the restoration of the efficiency and function of tissues and organs in the pelvic area, supports the process of surgical treatment, relieves pain, thus improving the quality of life [ 135 ].
In women suffering from endometriosis, pelvic floor structures are very often dysfunctional. Therefore, it is an indication for urogynecological physiotherapy. Inflammation accompanying recurrent endometriosis can cause tissue damage, and thus the formation of scar tissue to heal the affected areas. Unfortunately, a side effect of this process is the formation of adhesions, which, depending on the location, can cause different symptoms. Occurring in the abdominal cavity, they can limit the mobility of internal organs and contribute to the formation of symptoms, i.e., flatulence, constipation, digestive problems, problems with maintaining proper body posture and pain. For such ailments, it is a good idea to use manual therapy and osteopathic techniques to improve the mobility of internal organs and structures of the pelvis and spine. The following types of techniques can be successfully used: mobilization of the lumbosacral spine, needle therapy, pinopressure, visceral therapy (general abdominal maneuvers, therapy of female organs, intestines, liver), if necessary and with the consent of the patient of the technique per vaginum. As a consequence, in combination with the classic treatment of endometriosis and urogynecological physiotherapy, the course of the disease may become much milder and will allow the patient to return to everyday life [ 135 ].
Physical therapy techniques are known to reduce pain and improve quality of life in endometriosis. The overall goal of treatment is to teach the patient to relax the muscles, which in turn helps to break the pain cycle [ 136 ].
Evidence suggests that the symptoms of endometriosis are the result of a local peritoneal inflammatory response triggered by an ectopic endometrial implant that undergoes cyclic bleeding [ 137 ].
Regular exercise, on the other hand, has a protective effect against inflammatory diseases because it induces an increase in systemic levels of cytokines with anti-inflammatory properties [ 138 ].
Regular exercise is thought to promote reduced menstrual flow, ovarian stimulation, and estrogen effects [ 139 ].
Analysis of the available literature data shows that there are no controlled and randomized trials determining whether and to what extent exercise can be beneficial for women with endometriosis. So far, researchers have only speculated on this subject [ 140 ].
Physical exercise has been shown to have a beneficial effect on muscle relaxation in patients suffering from endometriosis, which in turn helps to break their pain cycle [ 141 ].
Progressive muscle relaxation training has been shown to be more effective in reducing pain, anxiety, and depression in women with endometriosis undergoing hormone therapy [ 142 ].
Cardiovascular activity helps endometriosis patients maintain good energy levels. Exercise is one of the most effective strategies for increasing serotonin levels; physical activity and deep breathing exercises can increase the rate of burning serotonin neurons in the brain, which can stimulate the production of mood-enhancing substances. Aerobic exercise, such as walking and swimming, can have a more significant effect on serotonin levels, strengthening the muscles of the whole body and improving overall circulation [ 143 ].
However, there are literature data that draw attention to the possibility of inferring the non-protective effects of exercise in women with endometriosis, which may result from the discomfort felt that prevents physical exercise [ 141 , 144 ].
10. Biomarkers
So far, no specific marker for endometriosis has been identified. The search is underway for a biomarker obtained from serum, plasma or urine, which would allow for a quick and simple diagnosis. In recent years, many substances have been tested that could be potential markers for endometriosis ( Figure 2 ). With peritoneal endometriosis, serum and plasma are not good diagnostic material; attention is paid to the eutopic endometrium. Eutopic endometrial tissue is available by taking the endometrium through a biopsy.

The potential endometriosis biomarkers.
The endometrium comes from the intermediate mesoderm during mesenchymal-epithelial transition (MET) during the formation of the genitourinary system. It is a reversible process, therefore, during the epithelial-mesenchymal transition (EMT, reverse process MET), it is possible to reach the formation of foci of endometriosis [ 145 ]. Foci of endometriosis behave in the same way as eutopic endometrium. There is monthly bleeding within them, which trigger an immune response. This causes the foci of endometriosis to increase [ 146 ].
Pain is a typical symptom of endometriosis, so the conduction pathways were analyzed and the brain-derived neurotrophic factor (BDNF) was found to be elevated in women with endometriosis compared to the control group [ 147 ]. BDNF is a protein that affects sensory neurons. As research shows, BDNF is not useful in detecting peritoneal endometriosis and deeply infiltrating endometriosis [ 148 ].
The marker of endometriosis was sought among growth factors and cytokines, because the development of ectopic implants is accompanied by the processes of invasion, proliferation and angiogenesis. The most promising results were obtained for hepatocyte growth factor (HGF), fibroblasts growth factor (FGF), epithelial growth factor (EGF), vascular endothelial growth factor (VEGF) and interleukin IL-1, IL-6 and IL-8. The most reproducible results were obtained for IL-6. IL-6 is the most characteristic of endometriosis. Its sensitivity is 63% and its specificity is 69% [ 149 ].
YKL-40, a new biomarker of inflammation, is secreted by activated macrophages and neutrophils in various inflamed tissues. Serum concentrations of YKL-40 are elevated in patients with diseases characterized by inflammation. Protein YKL-40 (CHI3L1–chitinase-3-like protein 1, HC-gp-39–human cartilage glycoprotein-39, gp-38k, chondrex) is the subject of scientific research looking for new biomarkers of endometriosis. Literature data suggest that circulating YKL-40 levels may be a new biomarker for diagnosing and monitoring endometriosis. The studies established serum concentrations of YKL-40 in patients with endometriosis compared with healthy people of the same age. It was shown that the level of YKL-40 was significantly higher in patients with endometriosis compared with healthy women [ 150 ].
It is known that in places affected by endometriosis, oxidative stress further intensifies the immune response, as a result of which there may be an exacerbation of the development of endometriosis [ 151 , 152 , 153 ]. Women with endometriosis have increased oxidative stress in the peritoneal cavity. Data indicate that oxidative stress occurs mainly in the peritoneal cavity in women with advanced stage of the disease [ 154 ].
Polak et al. showed that the peritoneal fluid of women with endometriosis is characterized by impaired iron metabolism [ 154 ]. This is most likely related to the increased number of erythrocytes in the peritoneal cavity of women with endometriosis, which leads to a higher haemoglobin concentration in this environment. Impaired iron homeostasis can have a significant effect on the pathophysiology of peritoneal endometriosis through the direct influence of haemoglobin derivatives and/or the creation of a pro-inflammatory and pro-oxidative environment.
Oxidative stress has been shown to activate the transcription factor NF-κB. The NF-kB protein family consists of the proteins RelA (p65), RelB, c-Rel, p50 and p52. The active transcription factor always consists of two subunits and the most common variant of NF-kB is the RelA (p65) and p50 complexes. NF-kB contains a signalling sequence that allows the transport of the active complex to the cell nucleus and binding to a specific DNA consensus. In an inactive form, the NF-kB factor is localized in the cytoplasm of the cell in association with the inhibitor protein IkB. Activation of the transcription factor NF-kB occurs through the interaction with the receptors of the cells of pro-inflammatory cytokines (e.g., TNF-alpha, LPS or IL-1beta), but also such factors as UV rays, antigens or free radicals. Activation of the specific receptor subsequently leads to phosphorylation of the inhibitory IkB protein by IKK kinases followed by proteosomal degradation of IkB. The released complex can be translocated to the cell nucleus [ 142 ]. This pathway of activation of the NF-kB factor is referred to as the canonical pathway. In addition to the canonical NF-kB activation pathway, the action of LPS, CD40 or lymphotoxin (TNFbeta) may lead to the so-called non-canonical NF-kB activation pathway. The transcriptional activity of NF-kB is crucial for cell function and proliferation. The elevated concentrations of TNF-alpha, TNF-beta, LPS, IL-1beta and CD40 in peritoneal fluid in women with endometriosis promote processes such as adhesion, invasion, proliferation and angiogenesis that stimulate the development of the disease [ 155 ].
Unfortunately, despite the passage of years and many studies in which an endometriosis marker was sought, none of the tested substances can be recommended for the diagnosis of endometriosis.
The only markers that have found partial clinical use in the diagnosis of endometriosis are the glycoproteins Ca-125 and Ca-19-9. The sensitivity of Ca-125 is characteristic of the advanced form of the disease, but its specificity is low because it is often elevated in other gynecological diseases [ 156 ]. The concentrations of Ca-125 and Ca-19-9 glycoproteins do not give us definitive answers in the differential diagnostic and therapeutic process, but they help in decision-making and direction of management and therefore remain the recommended complementary tool.
Despite the fact that research on biomarkers of endometriosis are still ongoing, there are still no satisfactory results, which makes it impossible to carry out effective laboratory diagnostics used in the diagnosis and monitoring of the treatment of the disease [ 157 ].
Some hope for the discovery of the endometriosis marker is carried by studies of microRNAs circulating in the blood [ 158 ]. MicroRNAs are small ribonucleic acid molecules about 22 nucleotides long that regulate gene expression by influencing the translation process. MicroRNAs show stability in tissues and can be easily detected in patients’ serum using quantitative methods such as qPCR. Determination of a single microRNA can help distinguish a healthy person from a sick person. However, the greatest prognostic force is the determination of several microRNAs, the expression of which varies in a given disease.
In studies of microRNAs in endometriosis, it has been shown that a certain group of genes is regulated with the participation of short, relatively stable fragments of RNA. MicroRNAs from the let family are among the dominant in endometrial cells.
In the paper of Sahin et al., Let-7b microRNAs have been shown to influence the expression of the ER-α, ER-ß, Cyp19, KRAS 4A, KRAS 4B, and IL-6 genes [ 146 ]. Let-7b has a pleiotropic effect in the pathophysiology of endometriosis, affecting the regulation of estrogen levels and the receptor of these growth factors.
Liu et al. suggested that miR449b3p altered expression in endometriosis affects the development of the disease [ 159 ].
Altered expression levels of miR-139-5p and miR-375 were observed in the tissues of the ectopic endometrium. These microRNAs may regulate the expression of the transcription factors HOXA9 and HOXA10 and the endothelin 1 gene (EDN1) playing a role in vascular homeostasis, which may be related to the development of endometriosis [ 160 ].
Laudański et al. showed that the mammalian target of rapamycin (mTOR) and VEGF pathway genes can be regulated by abnormal miRNA expression in endometriosis [ 161 ].
The protein kinase mTOR regulates the process of cell growth, proliferation and movement, as well as translation and transcription processes, while VEGF factor intensifies the process of angiogenesis in endometriosis. A team of Polish researchers conducted miRNA profiling of the eutopic endometrium from women with endometriosis [ 162 ]. A sample of 667 human miRNAs were studied in patients with endometriosis compared to the control group. Two of the study miRNAs were elevated, while 13 miRNAs had reduced levels of expression in eutopic endometrium in endometriosis patients compared to control. In addition, hsa-miR-483-5p and miR-629 have been shown to be significantly reduced in expression in endometriosis patients [ 163 ].
Gu et al. identified 14 miRNAs that might be involved in the pathogenesis of ovarian endometriosis: hsa-let-7a-5p, hsa-let-7b-5p, hsa-let-7d-5p, hsa-let-7f-5p, hsa-let-7g-5p, has-let-7i-5p, hsa-miR-199a-3p, hsa-miR-320a, hsa-miR-320b, hsa-miR-320c, hsa-miR-320d, hsa-miR-328-3p, hsa-miR-331-3p and hsa-miR-320e.Among them, 10 miRNAs were reported for the first time to be associated with endometriosis [ 164 ]. The miRNA hsa-let-7i-5p and hub miRNAs including hsa-let-7a-5p, hsa-let-7b-5p, hsa-miR-320a, and hsa-miR-320d might be potential diagnostic biomarkers [ 164 ].
Recent studies showed that the levels of miRNAs 199b-3p, 224-5p, and Let-7d-3p in plasma are potential diagnostic biomarkers for endometriosis patients [ 165 ]. In addition, miR-146a, miR-149 and miR-499 may have a role in the pathogenesis of endometriosis [ 166 ].
Some miRNAs are associated with genetic, epigenetic and angiogenic factors, hormones, cytokines, chemokines, markers of oxidative stress, mediators of inflammation, hypoxia, angiogenesis, and altered immune systems, contributing to the pathogenesis of endometriosis. Hormonal imbalance occurs by reducing the levels of miRNA-23a and miRNA-23b and increasing miRNA-:135a, 135b, 29c and 194-3p [ 167 ]. Angiogenesis by vascular endothelial growth factor is attributed to an increase in miRNA-126, miRNA-210, miRNA-21, miRNA-199a-5p and miRNA 20A. OS upregulates miRNA-302a by increased levels of tumor necrosis factor (TNF)-α, TNF-β and interleukin-1β. Upregulation of miRNA-199a and miRNA-16 promotes inflammation and cell proliferation in endometrial lesions [ 168 ]. The gold standard in diagnosing endometriosis is laparoscopy; however, miRNA can be validated as a diagnostic tool for detection, progression and prevention of endometriosis in large, independent cohorts of women, with and without endometriosis.
Despite the identification of several miRNAs, the studies are investigatory in nature. To date, no specific miRNA has been validated for diagnostic purposes (systematic review conducted on PubMed ® , Latin American and Caribbean Health Sciences Literature (LILACS), MEDLINE ® and Web of Science databases using the search terms endometriosis (all fields) AND miRNA (all fields), evaluating all publications up to May 2019) [ 168 ].
In conclusion, studies of the diverse microRNA pool in endometriosis have shown that many biological processes are regulated by the above molecules and can have a significant impact on the development of lesions. However, the use of microRNA in the diagnosis of endometriosis is only at the initial stage of research [ 169 , 170 ].
Recently, in the context of endometriosis, long non-coding RNAs (lncRNAs) that cover more than 200 bp and are a subtype of non-coding RNAs (ncRNAs) have become the focus of interest [ 171 ].
Unlike a group of short, non-coding RNAs (sncRNAs) such as microRNAs, long non-coding RNAs (lncRNAs) tend to exhibit greater sequence matching and thus specificity of action relative to the target genes. Non-coding RNA molecules participate in the processes of regulation at virtually all stages of the transmission of genetic information: from DNA to protein. Particularly spectacular is the involvement of certain non-coding RNA molecules in the mechanisms leading to the switching on or off of the expression of individual genes.
Zhou et al. found that 388 transcripts of lncRNA tested showed overexpression and 188 showed decreased expression levels in the ectopic endometrium compared to eutopic endometrium [ 172 ].
Liu et al. showed that LncRNA H19 may be involved in the pathogenesis of endometriosis especially in the mechanism of recurrence and is a novel potential predictor of the recurrence of endometriosis. In this study LncRNA H19 expression in the ectopic and eutopic endometria of endometriosis patients was significantly higher than that in the normal endometrium [ 172 ].
AFAP1-AS1 (AFAP1 Antisense RNA 1) is an RNA Gene, and is affiliated with the lncRNA class. Recent studies have shown that AFAP1-AS1 may be a potential therapeutic target for controlling the progression of endometriosis [ 161 ]. AFAP1-AS1 silencing can inhibit cell proliferation and promote apoptosis by regulating the STAT3/TGF-β/Smad signaling pathway by targeting miR-424-5p in endometrial stromal cells [ 173 ].
A study by the Cui et al. team explains that LINC01116 promotes the progression of endometriosis through the miR-9-5p/FOXP1 axis. This discovery provides a new therapeutic target for endometriosis patients [ 174 ].
HOX antisense intergenic RNA (HOTAIR) is a recently discovered long non-coding RNA (lncRNA) that plays critical role in gene regulation and chromatin dynamics, which appears to be mis-regulated in endometriosis. HOTAIR interacts with key epigenetic regulators such as histone methyltransferase PRC2 and histone demethylase LSD1 and regulates gene silencing.
Research indicates a significant role for HOTAIR in promoting endometriosis [ 175 ]. HOTAIR can be used as a potential target for clinical applications. Higher levels of HOTAIR mRNA have been shown in endometrial patients with severe endometriosis.
It is suggested that lncRNAs are closely related to the process of endometriosis. Nevertheless, the molecular mechanisms by which lncRNAs bind to endometriosis need to be explained in more detail. In summary, lncRNAs show potential as biomarkers of endometriosis [ 176 ]. However, the clinical significance and biological mechanism of lncRNA in the development of endometriosis remain largely unknown.
11. Endometriosis—Genetics
11.1. hereditary genetic polymorphism.
The concept of the genetic basis for the development of endometriosis derives from many years of observations, in which the family occurrence of this disease was indicated. Since the 1940s, a much higher incidence among women from families in which women suffered from endometriosis has been described, as well as a high incidence among siblings, especially monozygotic twins, where the percentage of co-occurrence exceeds 80% [ 177 ]. On the basis of statistical analyses of the family history of endometriosis, it has been proven that a genetic factor is responsible for about 50% of the predisposition to the disease [ 178 ]. It is believed that genetic and epigenetic conditions are rather conducive to the development of endometriosis, which is caused by environmental factors, or genetic and epigenetic changes caused by environmental factors are necessary to move from subtle changes in endometriosis to the disease stage [ 179 ]. The different phenotypic traits observed among humans are conditioned by millions of polymorphisms scattered throughout the genome, of which single nucleotide polymorphism (SNPs) account for about 90% of the total phenotypic variability. Polymorphic variants sometimes occur in a large percentage of the population and are associated with a variety of phenotypic traits, including susceptibility to various conditions. It has been postulated that certain SNPs systems, perhaps related to jointly inherited haplotypes, may promote the development of endometriosis. In a study on coupling analysis conducted on thousands of families burdened with endometriosis (GWAS–genome-wide association study) from different populations, a number of potential regions associated with the inheritance of this disease were indicated.
The epidemiology of endometriosis can be better understood thanks to the work on the human genome, especially the development of the genome-wide association study (GWAS) technique, i.e., associative studies of the entire genome.
The first GWAS study on endometriosis was published between 2010 and 2011—two papers on the Japanese population and one study of European women [ 180 , 181 , 182 ].
Studies of the Japanese population showed the relationship of polymorphism rs10965235, in the CDKN2BAS gene at locus 9p21 and rs16826658, in the area of the WNT4 gene at locus 1p36 [ 183 ].
Extensive GWAS research was conducted by the International Endo Gene Consortium (IEC) in populations of British and Australian women with endometriosis [ 165 ]. On the basis of these studies, loci associated with the development of the disease–7p15.2–was established. This place is among the genes known so far responsible for the development of the uterus and placenta [ 182 ].
Of lesser importance in the mentioned populations was the loci of chromosome 1p36 previously announced by Japanese studies [ 180 ].
In 2012, an international team of scientists conducted the largest genome-wide association study to date, the first among European women comparing DNA from 5586 women with endometriosis and 9331 people free of the disease [ 183 ].
The team identified two regions of the genome associated with an increased risk of endometriosis. The first is located on chromosome 7. This region may be involved in the regulation of nearby genes involved in the development of the uterus and its lining. The second variant is located near the WNT4gene, which is involved in hormone metabolism and the development and functioning of the woman’s genital tract. The important role of the WNT4, CDKN2BAS and FN1 genes was confirmed by the research of the team of Pagliardini et al. [ 184 ].
Research indicates a link between the 2p25.1 region, located near the GREB1 gene, and the risk of endometriosis [ 185 ].
Literature data on GWAS studies suggest that certain genetic variants, significantly more common in endometriosis, appear to be good functional candidates for the genetic factors responsible for the onset of this disease [ 185 ].
GWAS research in endometriosis is still ongoing, providing new results. Literature reports on 998 Belgian patients with endometriosis and 783 controls showed that the polymorphisms rs7521902, rs13394619 and rs6542095 may be associated with this disease [ 170 ].
It is worth noting that three genetic variants in the area of GREB1 (close to rs13394619) and CDKN2B-AS1 (close to rs1537377) also showed nominally significant associations with endometriosis [ 186 ].
In the paper of Mafra et al., it has been suggested that analysis of genetic variants in the WNT4 gene area (rs3820282, rs16826658) may help identify patients at high risk of developing this disease [ 187 ].
Albertsen et al. conducted a GWAS study in the European population of 2019 surgically confirmed cases of endometriosis and 14,471 controls [ 188 ].
Three of the single nucleotide polymorphisms (SNPs) associated with the disease have been identified: LINC00339-WNT4 at locus 1p36.12 (rs2235529) and RND3-RBM43 at locus 2q23.3 (rs1519761 and rs6757804). The meta-analysis identified two additional loci that were associated with endometriosis: RNF144B-ID4 at 6p22.3 (rs6907340) and HNRNPA3P1-LOC100130539at 10q11.21 (rs10508881).
In the work of the Polish team Sobalska et al., GWAS analysis found statistically significant links between new SNPs not previously described in the literature and endometriosis [ 189 ]. In these studies, a relationship was observed between the rs10129516 polymorphism, located on chromosome 14 in the PARP1P2 and RHOJ intergenic region, and endometriosis. Gen RHOJ encodes one of many small proteins of the Rho family that bind GTP (guanosine-5′-triphosphate), which acts as an energy transporter in the cell. Rho proteins regulate the dynamic assembly of cytoskeleton components in several physiological processes, such as cell proliferation and motility. Rho are also involved in cancer transformation and metastasis. The protein encoded by the RHOJ gene is activated by vascular endothelial growth factor and can regulate angiogenesis and also plays an important role in adipocyte differentiation, endothelial motility, and cytoskeletal formation [ 190 ]. Overexpression of the RHOJ gene has been shown in ectopic endometrium [ 191 ]. Endometriosis can be the cause of ectopic pregnancy. The risk of such a pregnancy is therefore increased in women with endometriosis. It can therefore be assumed that the RHOJ gene may be involved in the development of endometriosis.
Genetic analyses of the Bylińska et al. confirm the role of polymorphisms of the HLA-G gene and its receptors LILRB1 and LILRB2 for the development of endometriosis [ 192 ].
Human leukocyte antigen G (HLA-G) is recognized by KIR2DL4, LILRB1 and LILRB2 receptors on NK cells, antigen-presenting cells, T cells and others. Expression of HLA-G molecules in the ectopic endometrium has been demonstrated. The genes for the receptors KIR2DL4, LILRB1 and LILRB2 are polymorphic, which may affect their activity. The study of Polish scientists analyzed whether polymorphisms of HLA-G, KIR2DL4, LILRB1 and LILRB2 genes may affect susceptibility to endometriosis and disease progression. It was shown that the GG genotype of the rs1632947 polymorphism of the HLA-G gene played a protecting role against disease and its severe stages; similarly, the CT genotype of the polymorphism rs1233334 HLA-G played a protective role against disease progression. The AA genotypes of the rs41308748 polymorphism of the LILRB1 gene and AG rs383369 of the LILRB2 gene predisposed to endometriosis and its progression. No association of polymorphism of the KIR2DL4 gene with endometriosis was observed [ 193 ].
The work of another team of Polish researchers showed the relationship of polymorphisms rs12700667 and rs4141819 of the RAF gene with infertility in women with advanced endometriosis [ 193 ].
Recent research points to new genes and their polymorphisms related to endometriosis. Christofolini et al. showed a correlation of the polymorphisms rs10928050 of the KAZN gene and the rs2427284 of the LAMA5 gene with endometriosis [ 194 ].
Genetic studies provide evidence that changes in DNA increase the likelihood of some women developing endometriosis. Genetic contribution appears to be particularly large in more serious forms of the disease. Many years of GWAS molecular studies have resulted in the selection of several candidate genes as potential markers of endometriosis [ 195 ].
The study, which is a systematic review of GWAS studies published by PubMed until December 31, 2019, indicates that variants of the genes WNT4 rs7521902, GREB1 rs13394619, FN1 rs1250248, IL1A rs6542095 and VEZT rs10859871 may affect the development of endometriosis. However, replication and validation of these variants in different populations are essential for a better understanding of endometriosis etiopathogenesis, to optimize diagnosis, and to improve the effectiveness of clinical treatment of the disease [ 196 ].
Quite high hopes were associated with the possibility of determining the operation of polymorphisms of genes responsible for the metabolism of hormones and xenobiotics. Most of the variants described in the context of changes in drug metabolism were found to have no effect on the risk of endometriosis. The few polymorphisms associated with this disease include rs12248560 and rs4244285 that alter the expression of CYP2C19, which is involved in the breakdown of estrogens and xenobiotics. Other significant changes concern the deletion variant of the glutathione transferase (GSTM1) gene and the glutathione transferase (GSTP1) variant rs1695, which as phase II detoxification enzymes participate in the final removal of drugs and toxins. The presence of glutathione transferase polymorphisms combined with significant dioxin exposure was associated with the development of endometriosis [ 197 ].
A recently published meta-analysis showed that in addition to the above-mentioned variants, gene variants for interferon γ (IFNG CA repeat) and variants of rs16826658 and rs2235529 of the WNT4 gene may be relevant. An unknown trend was also confirmed for the progesterone receptor (PGR) gene variant, the rs1799969 variants of the ICAM1 molecule, rs2292596 of the aromatic hydrocarbon receptor repressor gene (AHRR), the CYP17A1 rs743572 gene and the rs1801282 gene for the peroxisome γ proliferator-activated receptor (PPARγ–peroxisome proliferator-activated receptor γ) [ 198 ].
Estrogen receptors, acting as transcription factors, play a significant role in endometrial growth and differentiation, as well as in numerous biological functions in eutopic endometrium and endometriosis. The ERβ receptor encoded by the ESR2 gene is the dominant isoform in those with endometriosis [ 198 ].
A different expression of aromatase in endometriosis foci has been demonstrated compared to eutopic endometrium. CYP19A1 is a gene encoding aromatase–an enzyme involved in the biosynthesis of estrogens. The exact basis of the observed changes is not yet known.
Literature data indicate that studies draw attention to the significant role of estrogen receptor genes, especially the ESR2 gene and the CYP19A1 gene for the susceptibility and occurrence of endometriosis [ 199 , 200 , 201 ]. The study identified statistically significant correlations between the new, previously unseen, two SNPs and endometriosis: rs4986938 and rs928554 [ 200 , 202 ]. In the case of rs4986938, the AA genotype was found to reduce the risk of endometriosis. A similar effect was demonstrated in the presence of the AG genotype of rs928554. The results obtained during the analysis indicate that the polymorphisms rs4986938 and rs928554 of the ESR2 gene are associated with the occurrence of endometriosis. Recent studies have shown that the polymorphisms rs17179740 of the ESR2 gene and rs2899470 of the CYP19A1 gene are associated with the occurrence of endometriosis [ 202 ]. In the studied group of women with endometriosis, a significant increase in the expression of the ESR2 gene was found, which may indicate the participation of this factor in the pathogenesis of endometriosis [ 200 ]. The results obtained in the work contribute to the broadening of knowledge in the subject of molecular mechanisms conducive to the development of endometriosis. Due to the small amount of research on the analysis of CYP19A1 and ESR2 gene expression and polymorphisms in patients with endometriosis, the research makes a significant contribution to expanding the knowledge about the impact of genetic factors on the development of endometriosis.
Understanding the relationship between gene expression and polymorphism and endometriosis may contribute to the development of new therapeutic strategies in the context of this disease. Molecular studies may be a target for personalized therapy in the future.
11.2. Somatic Mutations
The association of endometriosis with cancer, which appears as a result of genetic mutations, contributed to the search for pathogenetic mutations occurring in cells during the development of the disease. The concept of this research is based on the hypothesis that at least some of the changes arise as a result of somatic mutations absent in the germline and occur during the development of an individual organism. The occurrence of de novo mutations in both the endometrial cyst epithelial and peritoneal foci as well as in DIE foci is described. The most frequently noted genes are: ARID1A, KRAS, PIK3CA and PPP2R1A [ 203 , 204 ].
Analyzing samples taken from DIE patients with the help of next generation sequencing (NGS), it was possible to describe 51 new mutations involving a large group of proto-oncogenes. Some of these mutations have previously been described as mutations that have an initiating effect on the formation of tumors. The new mutations can affect both the eutopic endometrium and endometriosis foci, and do not have to occur in the germline cell line. They can also selectively appear only in the foci of endometriosis themselves. The location of the mutation in the genome appears to be specific to the patient and is not repeated in others. It is emphasized that the impact of these mutations on the development of the disease is complex and requires further evaluation. However, it can be assumed that these patients will be burdened with a higher risk of cancer in the future [ 203 ].
12. Summary
Analyzing the research of recent years on endometriosis, it can be seen that the current therapeutic options available are not optimistic. Unfortunately, the treatment of this disease is still ineffective. However, the progress of knowledge, especially at the genetic level, which has taken place in recent years, allows the separation of certain molecular shields for new therapeutic methods. Much hope is associated with new directions of research, such as the use of miRNAs or lncRNAs, which regulate key cellular pathways in the development of the disease, as molecular markers in endometriosis. However, these studies must involve large groups of patients with their full clinical description in order to be able to draw conclusions about the use of these particles as markers of endometriosis. There are reports of the development of the possibility of using ncRNA as diagnostic tools [ 205 ]. It remains to be seen whether genetic research will allow for the establishment of new therapeutic regimens and significantly contribute to improving the treatment results of women suffering from endometriosis.
Author Contributions
Conceptualization, B.S., K.S. and H.R. writing—original draft preparation, B.S.; writing—review and editing B.S.; revision and proofreading B.S. All authors have read and agreed to the published version of the manuscript.
This research has received no external funding.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

COMMENTS
Scribd is the world's largest social reading and publishing site. ... woman with endometriosis: a case report," Fertility and Steril-ity, vol. 90, no. 5, ... Case Study About Rectal CA. Case Study About Rectal CA. Cantiga Vhadz. File. File. Alfa Febrianda. CPC case 2.
Patient Case Presentation. Background: Ms. L.C. is a 34 year old female presenting with concerns of infertility. She has been attempting a pregnancy over the past 16 months with no success. Patient reports that several times she thought she could be pregnant due to a cessation in her menses with accompanying constipation and some abdominal pain.
Endometriosis is a chronic, debilitating disease associated . with pelvic pain and infertility. 1,2. The most common types of pelvic endometriosis are superficial peritoneal lesions, deep-infiltrating endometriosis, and ovarian endometriotic cysts (endometriomas). 3. Deep infiltrating lesions are defined as lesions with more than 5 mm depth of ...
1. Introduction. Endometriosis is the presence of endometrial glands and stroma outside the endometrial cavity .It is a common and benign condition that affects about 10% of women of childbearing age and can present with dysmenorrhoa, dyspareuania and dyschezia .Endometriosis can affect the small bowel but symptomatic involvement is uncommon and it rarely manifests as an acute small bowel ...
We describe a case of cutaneous endometriosis and present a literature review, which may help reduce the emotional and physical distress of patients. ... In a study conducted by Lopez-Soto et al , out of 33 women who underwent cutaneous endometriosis treatment, only 3 (9%) had a recurrence. Our patient is in follow-up, and no recurrence has ...
Endometriosis is a chronic reproduction condition that still remains a mystery to the medical community. This paper starts off by providing the background information on what endometriosis is, the etiology, and risk factors associated with the condition. Following the introduction is a case study on a 20 year old female who currently suffers from the condition herself. Based on Patient X's ...
Case presentation. A 25-year-old nulliparous woman presented to the orthopaedic clinic with continuous right hip pain, exacerbated by hip adduction and flexion. Her medical and surgical history was unremarkable. She had discontinued combined oral contraceptive pill 18 months prior to presentation after 5 years of use.
Endometriosis is a chronic inflammatory disease that is defined as the presence of endometrium-like tissues outside the uterus causing pain symptoms (dysmenorrhea, dyspareunia, chronic pelvic pain) and infertility [].This gynecological disease affects approximately 2-10% of the reproductive-aged and 50% of infertile women, and women with endometriosis have increased risk of obstetric outcome [].
16 likes • 4,415 views. L. Lifecare Centre Follow. Endometriosis - Changing Perspective - Case based approach MODERATOR : Dr Sharda Jain Dr Meenakshi Sharma PANELIST : Dr. Rupam Arora Dr. Dipti Nabh Dr. Renu Chawla Dr. Vandana Gupta Dr. Jyoti Agarwal Dr. Poonam Goyal On 31st Oct 2018.
Pathophysiology. Endometriosis is a disorder characterized by the implantation of endometrial tissue in areas of the body outside of the uterus (Huether & McCance, 2019). The ectopic tissue is typically found in pelvic areas such as the ovaries and pelvic lining and within the abdominal cavity. This tissue is functional, so it proliferates and ...
Abstract. Endometriosis is a common clinical presentation for gynaecologists. Occasionally it can present to general surgeons as a swelling in the groin or abdominal wall. This condition should be included in the differential diagnosis in female patients. A 32-year-old woman with a 2-year history of a painful persistent lump in her right groin ...
Pelvic Pain - Endometriosis Symptoms. A 27-year-old woman presented with severe dysmenorrhoea and pain with intercourse (dyspareunia). She also complained of bowel-related pain during menstruation. Diagnosis. Laparoscopy revealed significant endometriosis behind the uterus (Pouch of Douglas) which extended right through to the vagina. Outcome
Endometriosis is defined as the presence of endometrial glands and stroma in ectopic locations, or locations other than the interior of the uterus. Primarily these locations are found to be the pelvic peritoneum, ovaries, and rectovaginal septum. Endometriosis is a chronic inflammatory, estrogen-dependent disease where ectopic implantations ...
Endometriosis is a female reproductive system disorder where the endometrium-like tissue develops outside of the uterus; it usually affects the ovaries and peritoneum, causing premenstrual discomfort and dysmenorrhea [ 13, 14 ]. The most widely recognized explanation of endometriosis is that endometrial tissue is implanted in the peritoneal ...
Endometriosis is a chronic gynecological disease characterized by the growth of functional ectopic endometrial glands and stroma outside the uterus. It causes pelvic pain, dysmenorrhea, dyspareunia, or infertility. Diagnosis requires a combination of clinical history, non-invasive and invasive techniques. The aim of the present review was to evaluate the contribution of imaging techniques ...
Patient Case Study. Mrs. M.D. is a 30 year old Caucasian female who was referred to the gynecology clinic by her PCP after complaining of pain in her pelvic area along with increased bleeding during her menstrual cycle. She states that she has experienced this pain for quite some time, but recently it seems to have been worsening with each cycle.
Endometriosis: A Case Study | PDF. Dokumen tersebut merangkum kasus seorang wanita berusia 32 tahun dengan keluhan utama nyeri haid yang berlangsung selama 6 bulan. Pasien juga mengeluh adanya benjolan di perut bawah selama 3 tahun ... by puja_antonius.
The second case study gives an indication of how the material was used in the lead author's teaching sessions, with the case study patient's full consent. Discover the world's research 25 ...
Deep infiltrative endometriosis (DIE) is a rare form of this condition, which mostly affects the uterosacral ligaments, the rectovaginal space, and the upper third of the posterior vaginal wall, the bowel, and the urinary tract. We present the case of a 29-year-old pregnant female who was diagnosed with infiltrative endometriosis during the ...
The first GWAS study on endometriosis was published between 2010 and 2011—two papers on the Japanese population and one study of European women [180,181,182]. Studies of the Japanese population showed the relationship of polymorphism rs10965235, in the CDKN2BAS gene at locus 9p21 and rs16826658, in the area of the WNT4 gene at locus 1p36 .